
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci. , 03 October 2022
Sec. Plant Breeding
Volume 13 - 2022 | https://doi.org/10.3389/fpls.2022.999454
 Jing Zhang1
Jing Zhang1 Dajian Pan1
Dajian Pan1 Zhilan Fan1
Zhilan Fan1 Hang Yu1
Hang Yu1 Liqun Jiang1
Liqun Jiang1 Shuwei Lv1
Shuwei Lv1 Bingrui Sun1
Bingrui Sun1 Wenfeng Chen1
Wenfeng Chen1 Xingxue Mao1
Xingxue Mao1 Qing Liu*
Qing Liu* Chen Li*
Chen Li*Oryza rufipogon Griff. is a valuable germplasm resource for rice genetic improvement. However, natural habitat loss has led to the erosion of the genetic diversity of wild rice populations. Genetic diversity analysis of O. rufipogon accessions and development of the core collection are crucial for conserving natural genetic diversity and providing novel traits for rice breeding. In the present study, we developed 1,592 SNPs by multiplex PCR and next-generation sequencing (NGS) technology and used them to genotype 998 O. rufipogon accessions from 14 agroclimatic zones in Guangdong and Hainan Provinces, China. These SNPs were mapped onto 12 chromosomes, and the average MAF value was 0.128 with a minimum of 0.01 and a maximum of 0.499. The O. rufipogon accessions were classified into ten groups. The mean Nei’s diversity index and Shannon–Wiener index (I) were 0.187 and 0.308, respectively, in all populations, indicating that O. rufipogon accessions had rich genetic diversity. There were also differences in the genetic diversity of O. rufipogon resources in the 14 regions. Hainan populations possessed higher levels of genetic diversity, whereas the Guangzhou population had lower levels of genetic diversity than did the other populations. Phylogenetic analysis revealed that the genetic relationship among the distribution sites of O. rufipogon was closely related to geographical location. Based on genetic distance, a core collection of 299 accessions captured more than 99% of the genetic variation in the germplasm. This study provides insights into O. rufipogon conservation, and the constructed core collection provides valuable resources for future research and genomics-assisted breeding of rice.
In recent times, with the popularization of single varieties, pressure from environmental selection and the aggravation of pest and disease issues, the genetic basis of cultivated rice has inevitably narrowed (Swain et al., 2017). It is urgent that rice breeders enrich the genetic diversity of cultivated varieties and breed new varieties with better rice quality, higher yield and stronger resistance (Atwell et al., 2014). Crop wild relatives (CWRs) retain genetic diversity and thus represent a valuable genetic resource for modern agriculture (Zhang et al., 2017).
O. rufipogon Griff. (2n = 24, AA), an important CWR of cultivated rice, has accumulated many important agronomic characteristics over its long evolution, such as wide adaptability, resistance to insects, diseases and abiotic stresses, cytoplasmic male sterility, and better quality, which are desirable for the improvement of rice crops (Song et al., 2005; Henry, 2022). As one of the origins of cultivated rice in Asia, China has abundant wild rice resources, which are distributed in Guangdong, Guangxi, Hainan, Yunnan, Hunan, Jiangxi, Fujian and Taiwan (Liu et al., 2016). Guangdong and Hainan Provinces are in southern China and have favourable light and heat conditions. In this area, wild rice is widely distributed and exhibits abundant morphological and genetic diversity. Moreover, researchers have shown that South China is a centre of genetic diversity for wild rice (Li et al., 2006; Song et al., 2005). Therefore, O. rufipogon of Guangdong and Hainan Provinces play an important role in rice breeding improvement and basic research in China. However, the natural habitats of wild rice are rapidly shrinking due to urban and industrial occupation, and the large quantity of O. rufipogon resources also makes research and utilization difficult (Vaughan et al., 2008; Lakew et al., 2021). Hence, it is essential to identify the genetic diversity and develop efficient methods to conserve and make use of these natural O. rufipogon populations.
Molecular marker analysis is one of the most useful methods of investigating the genetic diversity of O. rufipogon because of its rapid, simple, environmentally friendly and reliable detection results (Sarif et al., 2020). In wild rice, DNA markers such as inter-simple sequence repeat (ISSR) (Qian et al., 2001; Girma et al., 2010) and microsatellite or simple sequence repeat (SSR) (Zhou et al., 2003; Melaku et al., 2013) markers are increasingly being used to identify and evaluate genetic variation. In addition, advanced NGS technology has enabled timely mining of high-density SNP information throughout the genome for genetic identification at a significantly reduced cost (Singh et al., 2016; Xu et al., 2016; Ab Razak et al., 2020).
Core collection (CC) construction is a favoured approach to the efficient exploration and conservation of novel variation in genetic resources (Zhang et al., 2011). A core germplasm is a fraction of resources representing the maximum genetic diversity of the total population (Liu et al., 2020). The research showed that the proportion of core germplasm was different for different crops, generally varying from 5 to 30%. At present, the core germplasm of cultivated rice has been established in most parts of the world (Abadie et al., 2005; Yan et al., 2007; Zhang et al., 2011; Kumar et al., 2020; Tanaka et al., 2020). In wild rice, the optimal primary core collection of O. rufipogon was acquired using 5571 accessions from the national genebank based on agronomic and morphological characters (Yu et al., 2003). Pan et al. (2018) explored the genetic diversity and population structure of 4173 O. rufipogon in Guangxi, China, and developed a core collection.
There are abundant O. rufipogon resources in Guangdong and Hainan Provinces, China. However, previous research was limited to individual populations from a few ecological regions. The genetic diversity of O. rufipogon in Gaozhou, Guangdong, China, was studied (Chen et al., 2009). A total of 110 O. rufipogon accessions from four subregions (Suixi, Enping, Lufeng, Fogang) in Guangdong Province was studied for preserving the genetic diversity (Zhang et al., 2021). In this study, 998 elite O. rufipogon accessions were selected from almost O. rufipogon distribution areas of Guangdong and Hainan Provinces and genotyped using multiplex PCR and NGS methods. Analyses were carried out as follows. First, the genetic diversity of the accessions was assessed. Then, a core collection of common wild germplasms was constructed. Finally, we evaluated the diversity of core germplasm resources.
The demonstration of genetic diversity and establishment of a CC would provide a scientific basis for the classification, conservation, and innovative utilization of these precious wild rice resources.
A total of 998 representative O. rufipogon accessions from fourteen regions of Guangdong and Hainan Provinces were used for this study. Of these accessions, 19 were from Dongguan, 48 were from Foshan, 60 were from Guangzhou, 38 were from Heyuan, 232 were from Huizhou, 128 were from Jiangmen, 74 were from Jieyang, 90 were from Maoming, 53 were from Qingyuan, 120 were from Shanwei, 15 were from Shenzhen, 22 were from Yangjiang, 52 were from Zhanjiang, and 47 were from Hainan (Figure 1). All materials were stored in the National Germplasm Guangzhou Wild Rice Nursery, China.
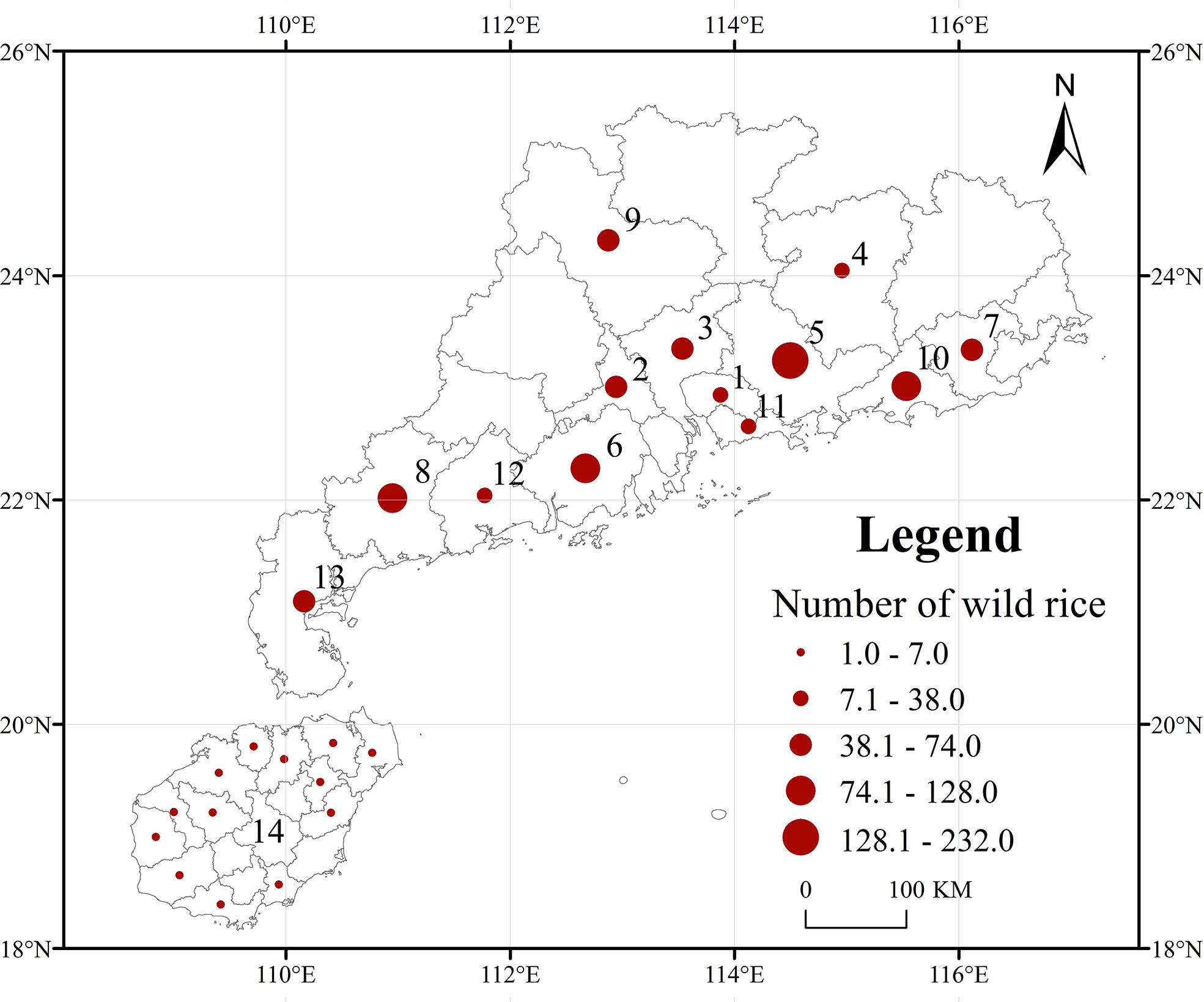
Figure 1 Ecogeographical distribution of 998 representative O. rufipogon accessions in Guangdong and Hainan Provinces, China. The circle size indicates the relative sampling number from each region. 1: Dongguan, 2: Foshan, 3: Guangzhou, 4: Heyuan, 5: Huizhou, 6: Jiangmen, 7: Jieyang, 8: Maoming, 9: Qingyuan, 10: Shanwei, 11: Shenzhen, 12: Yangjiang, 13: Zhanjiang; 14: Hainai.
Genomic DNA was isolated with the cetyltrimethylammonium bromide (CTAB) method from young leaves of each O. rufipogon accession (Allen et al., 2006). A total amount of 100 ng genomic DNA per sample was used as input material for the DNA sample preparation. Sequencing libraries were generated using a MultipSeq® Custom Panel (iGeneTech, Beijing, China) following the manufacturer’s recommendations, and index codes were added to each sample.
A total of 456 pairs of sample-specific multiplex PCR primers were designed and PCR amplification was performed at iGeneTech Bioscience Co., Ltd. The first round of multiplex PCR was performed as follows: 95̊ initial denaturation for 3 min 30 s, then 98̊ denaturation for 20 s, 60̊ primer annealing and extension for 2 min for 20 cycles, and finally extension for 5 min at 72̊. Products were purified using AMPure XP beads. The corresponding adapter oligonucleotides were joined by the second round of PCR, with the following procedure: 95°C initial denaturation for 3 min 30 s, 98°C denaturation for 20 s, 58°C primer annealing for 1 min, and 72°C extension for 30 s, with 10 cycles and finally extension for 5 min at 72°C. Products were purified using AMPure XP beads. Qubit® 3.0 was used to determine the concentration of the library. An Agilent 2100 Bioanalyzer system was used to determine the length of the library fragments. Qualified libraries were sequenced on an Illumina platform.
Raw reads were filtered to remove low-quality reads by using FastQC. After cutting primer sequences, clean reads were mapped to the reference genome of Oryza sativa MSU V7.0 (Kawahara et al., 2013) by using Bwa. The detailed reads information was listed in Supplementary Table S1. Then, duplications were removed, and SNPs and InDels were called and annotated by using GATK and SAMtools.
SNPs with a minor allele frequency (MAF) ≥ 1% and integrity ≥ 50% were used for phylogenetic and population structure analyses. The genetic diversity parameters of O. rufipogon were calculated using PowerMarker V3.25 (Liu and Muse, 2005). Principal component analysis (PCA) of the selected SNPs was performed with Cluster software (de Hoon et al., 2004). The ADMIXTURE program was used to assess population structure based on the maximum likelihood method with 10,000 iterations, and the number of clusters (K) was set from 1 to 10 (Alexander et al., 2009).
A subset of O. rufipogon accessions were selected based on the strategy of population priority and gradual clustering. First, according to the geographical distribution of O. rufipogon, the accessions from the same population were divided into a group, and only one O. rufipogon accession in the group was directly selected into the core germplasm. Then, the genetic diversity and cluster analysis of O. rufipogon germplasms in each group were carried out with the SNP data, and the core germplasm was screened according to different sampling ratios to capture most of the allelic diversity in the germplasm using Core Hunter II software (Thachuk et al., 2009). Finally, the core collection was evaluated for genetic diversity and gene coverage and PCA was performed. Additionally, a phylogenetic tree was constructed using MEGA X software with the neighbour-joining (NJ) method (Kumar et al., 2018).
A total of 998 O. rufipogon accessions were sequenced and genotyped by multiplex PCR and NGS. The SNPs were further filtered under the condition of MAF ≥ 1% and integrity ≥ 50%, and 1,592 SNPs were generated for genetic diversity analysis. These SNPs were mapped onto 12 chromosomes. The highest and lowest SNPs mapped per chromosome were 623 and 80 on chromosome 7 and chromosome 10, respectively (Figure 2A). To explain the total variability of each marker, the MAF, the expected heterozygosity (He), and polymorphic information content (PIC) were used. The average MAF value was 0.128, with a minimum of 0.01 and a maximum of 0.499 (Figure 2B). The He was in the range of 0.02-0.50 with an average of 0.187 (Figure 2C). The PIC values ranged from 0.02 to 0.375, with an average of 0.157 (Figure 2D).
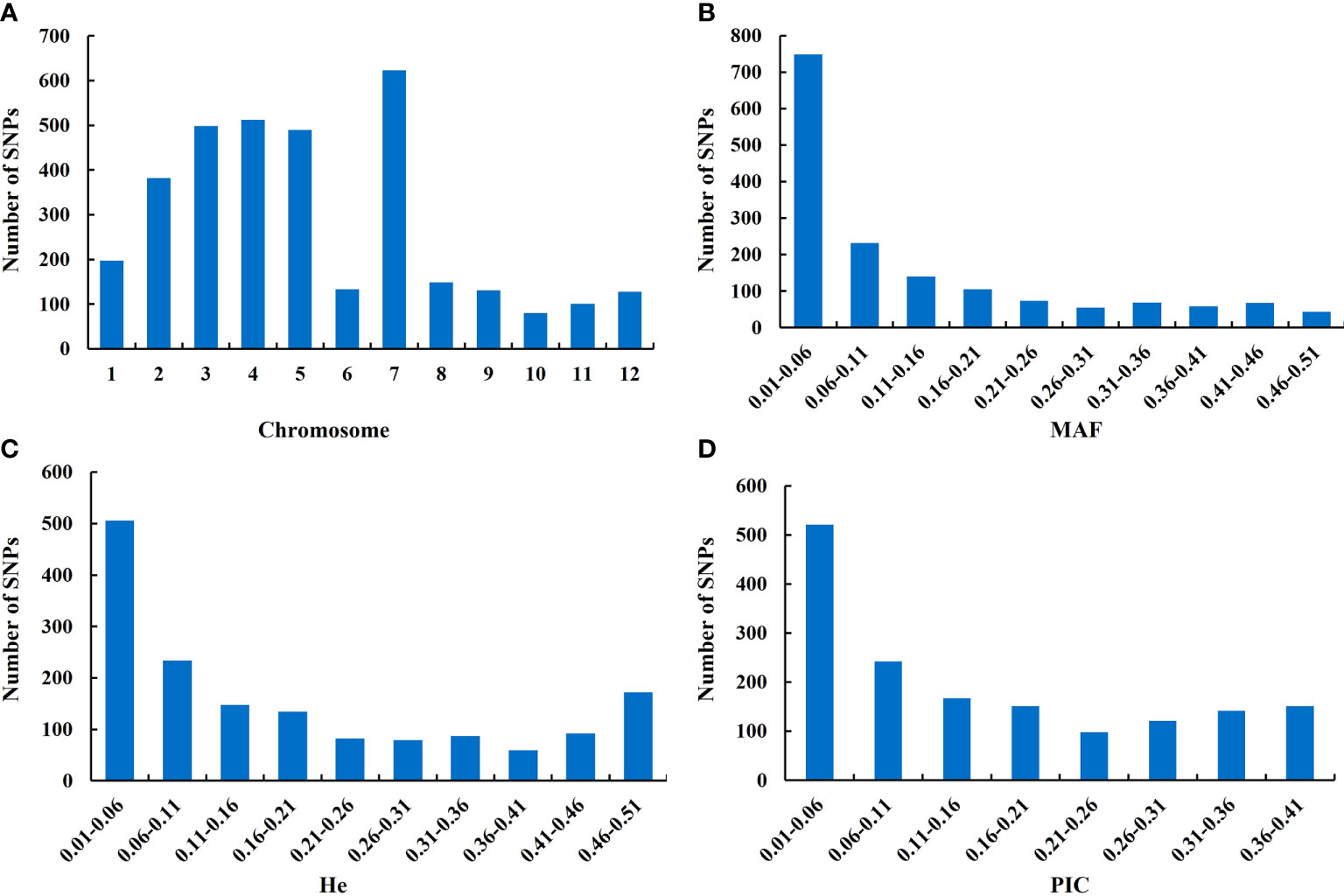
Figure 2 Characteristic statistics of SNPs. (A) SNP distribution on the rice scaffolds; (B) minor allele frequency (MAF); (C) expected heterozygosity (He); (D) polymorphism information content (PIC). The Y-axis represents the number of SNPs.
Population structure and genetic relationships were further examined to gain insight into the genetic diversity of O. rufipogon germplasm. The cross-validation errors (CVs) were examined under the models with K = 1-10 using admixture software. As suggested, a good K value will exhibit the lowest CV compared to other K values (Alexander et al., 2009). Here, the values of the CV continuously decreased with K from 1 to 9, and the minimum value was found when K = 10 (Figure 3A), which indicated that the 998 O. rufipogon accessions were classified into ten groups. The first and seventh groups both contained 75 accessions from 11 regions and six regions, respectively. The second group contained 65 accessions from seven regions. The 16, 131 and 55 wild rice accessions were divided into groups three, four and five, which came from four, fourteen and five regions. Groups six and eight contained 152 and 212 accessions, respectively, from 13 regions. The ninth group contained 124 O. rufipogon accessions from 12 regions, and the remaining 93 accessions were assigned to group 10 from 12 regions (Figure 3B). According to the geographical information of O. rufipogon, it was found that the germplasm resources of the ten different groups were geographically interspersed. The genetic relationship of some materials from different zones was close, indicating that there was mutual penetration between O. rufipogon accessions of different populations.
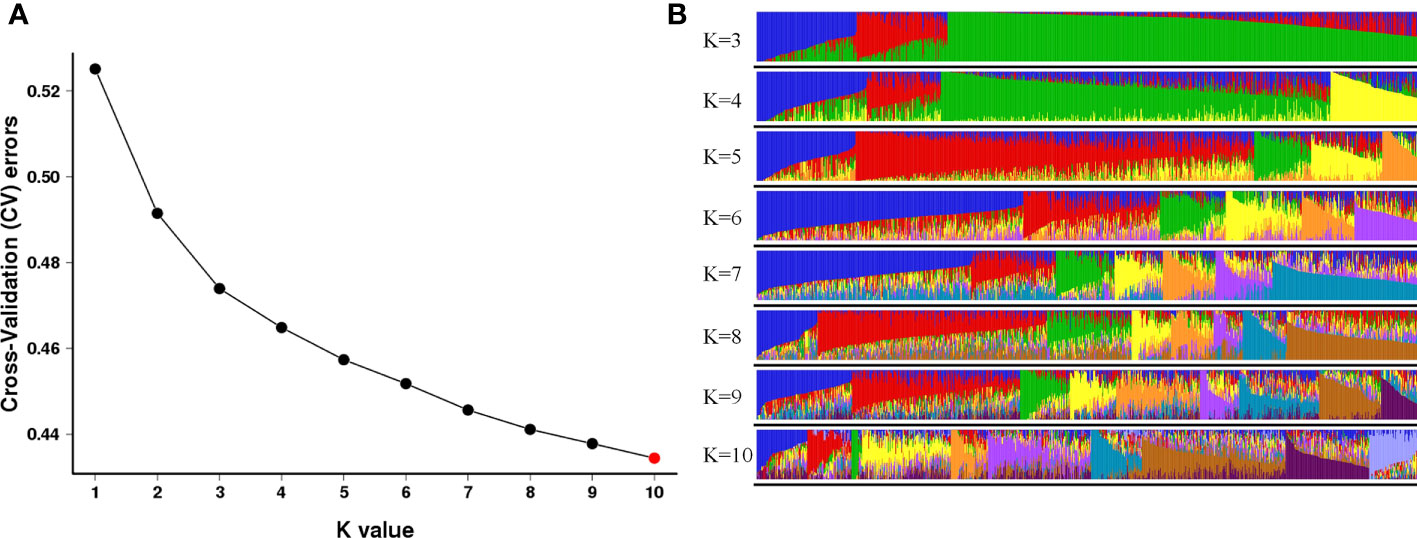
Figure 3 Population structure analyses of O. rufipogon accessions using ADMIXTURE. (A) Cross-validation plot for the number of population (K) values; (B) Model-based clustering with K values from 3 to 10. The colours represent different populations of the 998 wild rice accessions.
O. rufipogon accessions of Guangdong Province were distributed in 13 different regions, and all collections in Hainan were divided into one region separately, of which genetic diversity comparisons were conducted (Table 1). All the SNP markers were found to be polymorphic, and an average of 758 polymorphic markers in each region were identified. The MAF varied between 0.2139 and 0.2422 with a mean value of 0.2275. The observed heterozygosity (Ho) and expected heterozygosity (He) of O. rufipogon accessions in Hainan were higher than that of those from the other thirteen regions. The Shannon–Wiener index (I), representing the genetic diversity of populations, had a value of 0.5022 in the Hainan subgroup, while the I in the other subgroups was lower than 0.5. The range of the PIC was from 0.248 to 0.2674, with an average of 0.2576. Nei’s diversity index ranged from 0.3047 to 0.3347, with an average of 0.32.
With phylogenetic analysis, a neighbour-joining tree based on Nei’s genetic distance was also clearly constructed. The 998 O. rufipogon accessions of 14 regions were grouped into six main clusters when the tree scale was 0.005 (Figure 4). Cluster 1 contained accessions from Yangjiang, and the accessions from Maoming, Zhanjiang and Hainan tended to be grouped together in cluster 2. Cluster 3, 4 and 5 were characterized as independent groups of O. rufipogon containing accessions from Jiangmen, Foshan, and Guangzhou, respectively. All the accessions from seven regions (Dongguan, Huizhou, Shenzhen, Heyuan, Qingyuan, Jieyang and Shanwei) were restricted to the major cluster 6. When the tree scale was 0.01, all O. rufipogon accessions were clustered into 11 groups. The accessions from Dongguan and Huizhou were grouped together, and the accessions from Qingyuan was grouped separately. Moreover, another cluster containing accessions from Jieyang and Shanwei were characterized.
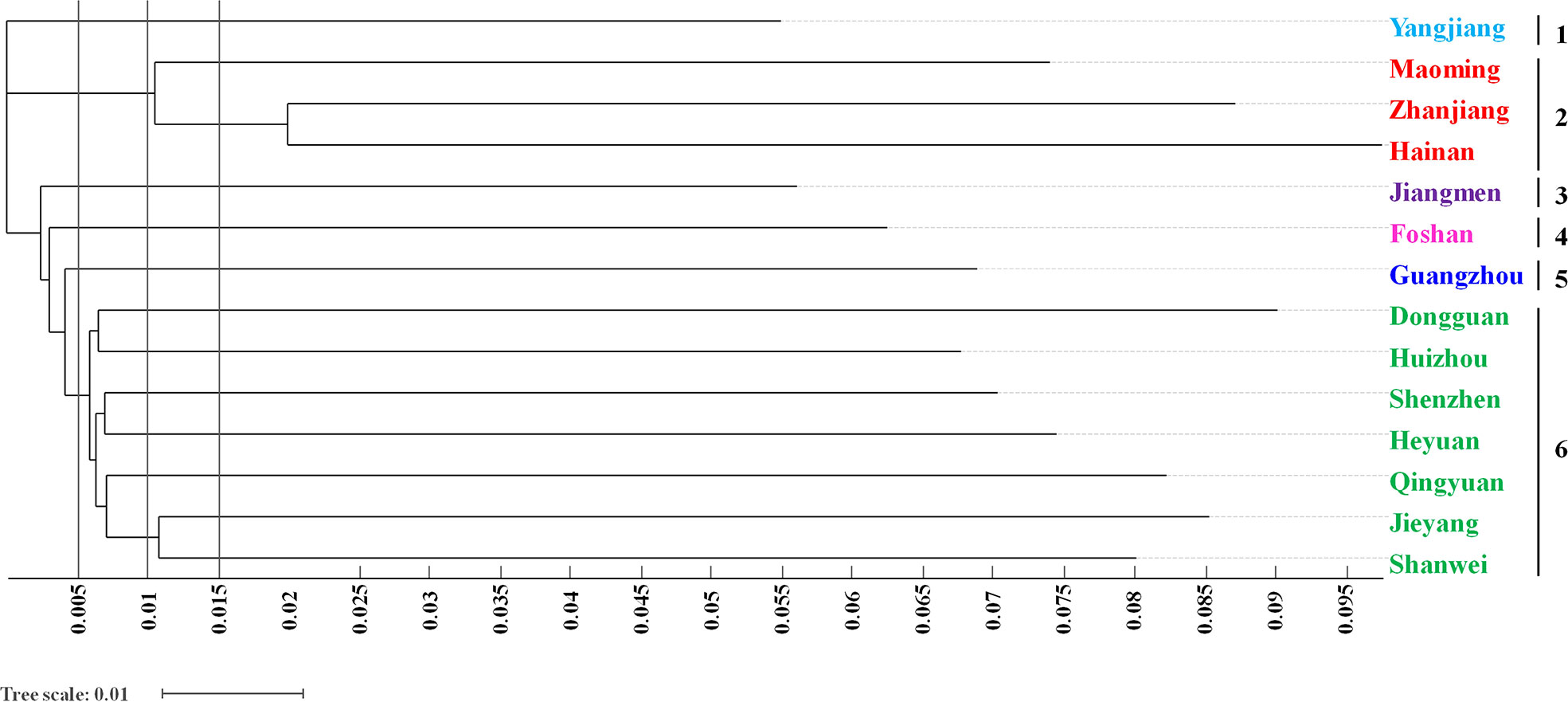
Figure 4 Dendrogram of O. rufipogon accessions in Guangzhou and Hainan using the NJ method. Different colours depict the population generated by the structure analysis.
Construction of a core collection provides a subset of representative accessions that captures most of the allelic diversity in the entire collection and can be used for genome-wide association studies (GWAS), gene cloning and marker development. According to the geographical distribution and genotype information of 998 O. rufipogon accessions, the core germplasm was developed using Core Hunter II software with a modified Rogers distance of 0.7 and Shannon diversity index of 0.3. Our analysis indicated that the 499 top-ranked accessions could capture 100% of the allelic diversity of the entire O. rufipogon resource collection (Figure 5A). A core set of 299 accessions was selected from 998 O. rufipogon accessions, capturing 99.906% of the entire allelic diversity. The top two groups with the highest proportions in the 299-accession core collection were from Huizhou (44; 14.7%) and Shanwei (37; 12.4%), while the proportion of core germplasm from Shenzhen and Yangjiang was relatively low, accounting for only 0.67% and 2.34% of the total core germplasm, respectively. The detailed information is listed in Table 2.
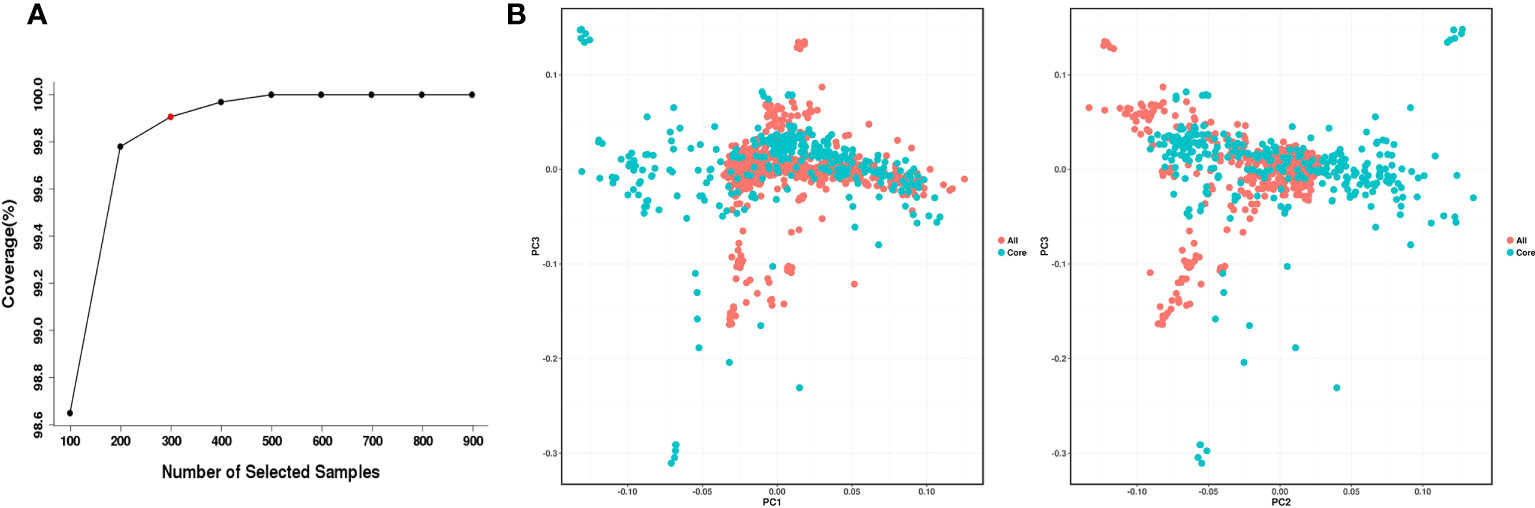
Figure 5 Development and evaluation of the O. rufipogon core collection. (A) Coverage of allelic diversity versus number of selected accessions. The red circle indicates the minimal number of samples (299) covering 99.906% allelic diversity. (B) PCA of O. rufipogon accessions. Red dots represent the accessions in the core collection; green dots represent the accessions not in the core collection.
To further verify the accuracy of the core accessions, the genetic diversity of 299 O. rufipogon resources was determined (Table 3). The coverage of polymorphic markers in the core collections was 95.35% of those detected in the whole germplasm. The Ne retained 1.336 and 1.291 in core collections and the whole germplasm. The mean Nei’s diversity index and PIC value of this core collection were 0.215 and 0.179, accounting for 114.97% and 114.01% of the total accessions, respectively, supporting the representativeness of the core collections. In addition, the Shannon–Wiener index, Ho and He between the representative set and the total collection were 1.12, 1.06 and 1.14, respectively, indicating a high degree of similarity.
PCA of the accessions in the O. rufipogon core collection exhibited a pattern nearly identical to that of the accessions in the entire collection (Figure 5B), further supporting the broad representation of the wild rice accessions in the core accessions from Guangdong and Hainan Provinces.
To infer phylogenetic relationships among the 299 O. rufipogon accessions, a neighbour-joining phylogenetic tree was constructed with the identified SNPs. Four major clades were identified in the core collections, with the red cluster tending to contain accessions from five regions in Guangdong Province. Similarly, the blue cluster mainly comprised accessions from ten regions in Guangdong and Hainan Provinces. The remaining two clades contained accessions from 13 and 14 regions, respectively (Figure 6). There was some overlap in the accessions from the 14 regions among the subgroups.
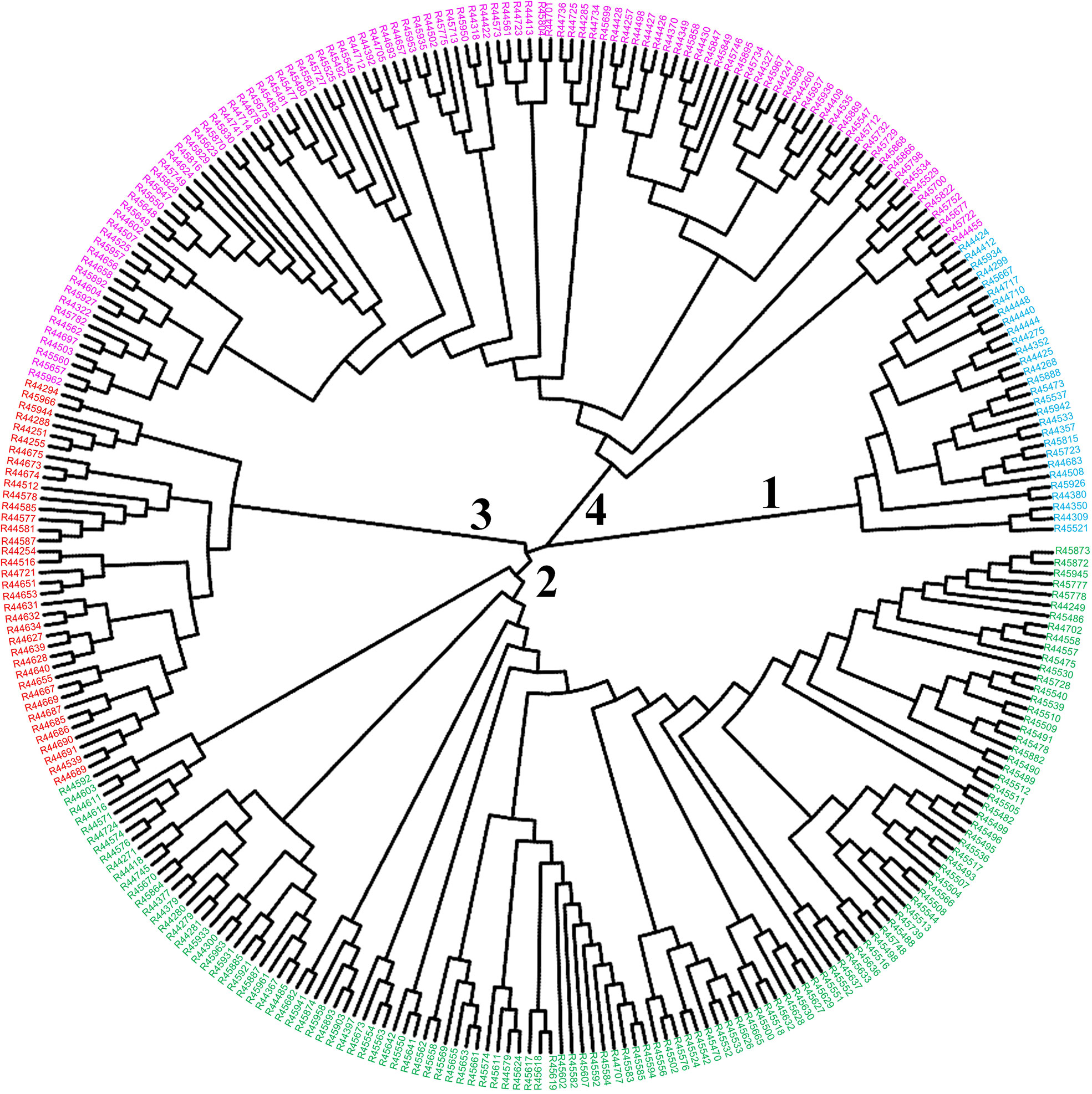
Figure 6 Neighbour-joining tree of the core collection of O. rufipogon. Colours represent different subpopulations of the core collection; blue colour = subpopulation 1, green = subpopulation 2, red = subpopulation 3, purple = subpopulation 4.
O. rufipogon is an important genetic resource for rice breeding. Guangdong and Hainan are important distribution areas of O. rufipogon in China. Due to increased industrialization and the expansion of economic production activities, the original habitat of wild rice has been destroyed, and the distribution area has been greatly reduced. Thus, there is an urgent need to study the genetic diversity of O. rufipogon resources. Currently, array-based SNP detection is one of the major high-throughput marker detection platforms and can be used to genotype multiple samples and study the genetic characteristics of plants within a short period (Zhu et al., 2019). In this study, a total of 998 O. rufipogon accessions from 14 regions of Guangdong and Hainan Provinces were sequenced and genotyped by multiplex PCR and NGS, and 1,592 SNPs were generated for genetic diversity analysis. The range of the Shannon–Wiener index (I) was from 0.056 to 0.693, with an average of 0.308. Nei’s diversity index ranged from 0.02 to 0.5, with an average of 0.187. The results revealed that O. rufipogon populations of Guangdong and Hainan Provinces had rich genetic diversity and that variations were widely distributed in the populations. However, if the number of SNPs would be further increased, the more perfect genetic relationships would be obtained. There were also differences in the genetic diversity of O. rufipogon resources in 14 regions, which suggested that the geographical environment might be responsible for the genetic background differences in O. rufipogon in different regions. It was consistent with previous research result (Zhang et al., 2021). We detected high levels of genetic diversity in O. rufipogon populations from Guangdong Province. For example, the Shenzhen and Heyuan populations had a higher Nei’s diversity index and Shannon–Wiener index, PIC, and He, respectively, while the Guangzhou population had the lowest level of diversity. Furthermore, O. rufipogon accessions from Hainan Province had the highest level of diversity compared to those from the other 13 regions, possibly because of the extensive habitat distribution of populations in Hainan. At the same time, due to the small amount of O. rufipogon accessions collected in each subregion of Hainan, the diversity comparison of populations within Hainan Province was limited.
O. rufipogon accessions were clustered according to the genetic similarity of each region. Populations geographically close to each other were often clustered into one group and were more closely related. As depicted in Figure 4, the accessions from Yangjiang tended to be grouped in cluster 1, while all the accessions from Jieyang and Shanwei were restricted to cluster 6. The results suggested that the genetic relationship among the O. rufipogon accessions from the distribution sites was closely related to geographical location. In addition, 998 O. rufipogon accessions had clear group divisions with K values from 2 to 10, and there was mutual infiltration between the germplasm resources of different groups in terms of geographical origin.
In Guangdong and Hainan Provinces, O. rufipogon accessions are widely distributed and difficult to protect. The results of genetic diversity analysis provide a scientific basis for the protection of these O. rufipogon resources. Conservation efforts should be made based on the geographical distribution characteristics of the genetic diversity of O. rufipogon. Accordingly, ex situ conservation in the Heyuan, Zhanjiang, Huizhou, Jiangmen and Hainan areas with high genetic diversity and large numbers of germplasms is a good strategy for preserving the genetic diversity of these important wild rice varieties. At the same time, the collected wild rice accessions should contain the accessions of each population during in situ conservation and core collection construction to maximize the genetic diversity of wild rice resources.
The construction of core collections could improve the efficiency of the utilization of germplasm resources. For instance, core collections can be used for the development of molecular markers, GWAS of important traits, rice domestication and diversity study (Agrama et al., 2009; Song et al., 2018; Wang et al., 2018; Zhang et al., 2019). Although there are multiple ways to construct core germplasms, the evaluation criteria established for core germplasms are largely similar, that is, that the diversity be representative of the original germplasm. A mini core collection consisting of 189 varieties of Oryza sativa was developed in China (Zhang et al., 2011), and a total of 701 accessions were developed that accounted for approximately 10% of accessions from the total North-Eastern region of India with the unweighted pair group method of arithmetic average (UPGMA) (Roy et al., 2014). Moreover, 130 wild rice accessions from China were selected as a core collection that retained over 90% of the alleles (Liu et al., 2016).
In this study, we used the NJ method and genetic distance in combination with population geographic distributions to develop the wild rice core collection. A core set of 299 accessions was established. Genetic diversity tests, PCA, and phylogenetic analysis were performed on the core and entire collection to ensure the genetic diversity of the core collection. The results showed that 29% of the sampling percentage selected from the entire germplasm retained 99.906% of the entire allelic diversity. In addition, the core collection contained accessions from all populations in Guangdong and Hainan Province. Briefly, the core germplasm maintained a high level of genetic diversity and was representative of the entire population.
In conclusion, we assembled a core collection of O. rufipogon with abundant genetic diversity from different ecological regions of Guangdong and Hainan Provinces, China, and our strategy was successful in selecting representative accessions from the entire population based on genotypic data. Furthermore, it is critical that ex situ and in situ conservation of the O. rufipogon germplasm be undertaken to avoid inter- and intrapopulation introgression. Finally, the core collections constructed in this study are valuable wild rice resources, laying a solid material foundation to promote the identification and utilization of superior genes of O. rufipogon resources in Guangdong and Hainan Provinces in the future.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
LC conceived the research. ZJ and LQ performed the research. ZJ wrote the paper. ZJ, PD, FZ, YH, JL, LS, SB CW and MX participated in the preparation of the reagents in this study. All authors contributed to the article and approved the submitted version.
This work was supported by grants from Guangdong Provincial Key Research and Development Project Program (2020B0202090003), the Special Fund for Science and Technology Innovation Strategy (Construction of High-Level Academy of Agricultural Sciences) (201926), the Subproject of National Key Research and Development Program (2021YFD1200101-05) and Guangdong Key Laboratory of New Technology in Rice Breeding (2020B1212060047).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2022.999454/full#supplementary-material
Abadie, T., Cordeiro, C. M. T., Fonseca, J. R., Alves, R. D. B. D. N., Burle, M. L., Magalhães, J. R., et al. (2005). Constructing a rice core collection for Brazil. Pesquisa Agropecuária Bras. 40, 129–136. doi: 10.1590/S0100-204X2005000200005
Ab Razak, S., Azman, N. H. E. N., Kamaruzaman, R., Saidon, S. A., Yusof, M. F. M., Abdullah, N., et al. (2020). Genetic diversity of released Malaysian rice varieties based on single nucleotide polymorphism markers. Czech J. Genet. Plant Breed. 56, 62–70. doi: 10.17221/58/2019-CJGPB
Agrama, H. A., Yan, W. G., Lee, F., Fjellstrom, R., Chen, M.-H., McClung, A., et al. (2009). Genetic assessment of a mini-core subset developed from the USDA rice genebank. Crop Sci. 49, 1336–1346. doi: 10.2135/cropsci2008.06.0551
Alexander, D. H., Novembre, J., Lange, K. (2009). Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 19, 1655–1664. doi: 10.1101/gr.094052.109
Allen, G. C., Flores-Vergara, M. A., Krasynanski, S., Kumar, S., Thompson, W. F. (2006). A modified protocol for rapid DNA isolation from plant tissues using cetyltrimethylammonium bromide. Nat. Protoc. 1, 2320–2325. doi: 10.1038/nprot.2006.384
Atwell, B. J., Wang, H., Scafaro, A. P. (2014). Could abiotic stress tolerance in wild relatives of rice be used to improve Oryza sativa? Plant Sci. 215, 48–58. doi: 10.1016/j.plantsci.2013.10.007
Chen, Y., Pan, D. J., Yang, Q. W., Liu, B., Fan, Z. L., Li, C., et al. (2009). Sampling strategy for an applied core collection of gaozhou wild rice (Oryza rufipogon griff.) in guangdong, China. Acta Agronomic Sin. 35, 459–466. doi: 10.3724/SP.J.1006.2009.00459
De Hoon, M. J. L., Imoto, S., Nolan, J., Miyano, S. (2004). Open source clustering software. Bioinformatics 20, 1453–1454. doi: 10.1093/bioinformatics/bth078
Girma, G., Tesfaye, K., Bekele, E. (2010). Inter simple sequence repeat (ISSR) analysis of wild and cultivated rice species from Ethiopia. Afr. J. Biotechnol. 9, 5048–5059. doi: 10.4314/AJB.V9I32
Henry, R. J. (2022). Wild rice research: Advancing plant science and food security. Mol. Plant 15, 563–565. doi: 10.1016/j.molp.2021.12.006
Kawahara, Y., de la Bastide, M., Hamilton, J. P., Kanamori, H., Wu, J. Z., Zhou, S. G., et al. (2013). Improvement of the Oryza sativa nipponbare reference genome using next generation sequence and optical map data. Rice 6, 1–10. doi: 10.1186/1939-8433-6-4
Kumar, A., Kumar, S., Singh, K. B. M., Prasad, M., Thakur, J. K. (2020). Designing a mini-core collection effectively representing 3004 diverse rice accessions. Plant Commun. 1, 1–14. doi: 10.1016/j.xplc.2020.100049
Kumar, S., Stecher, G., Li, M., Knyaz, C., Tamura, K. (2018). MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35, 1547–1549. doi: 10.1093/molbev/msy096
Lakew, T., Tanaka, K., Ishikawa, R. (2021). Genetic diversity of African wild rice (Oryza longistaminata chev. et roehr) at the edge of its distribution. Genet. Resour. Crop Ev. 68, 1769–1784. doi: 10.1007/s10722-020-01080-6
Li, C., Pan, D. J., Mao, X. X., Tu, C. Y., Zhou, H. Q., Fan, Z. L., et al. (2006). The genetic diversity of gaozhou wild rice analyzed by SSR. Chin. Sci. Bull. 51, 562–572. doi: 10.1007/s11434-006-0562-1
Liu, M., Hu, X., Wang, X., Zhang, J. J., Peng, X. B., Liu, Y. F., et al. (2020). Constructing a core collection of the medicinal plant angelica biserrata using genetic and metabolic data. Front. Plant Sci. 11, 600249. doi: 10.3389/fpls.2020.600249
Liu, K. J., Muse, S. V. (2005). PowerMarker: An integrated analysis environment for genetic marker analysis. Bioinformatics 21, 2128–2129. doi: 10.1093/bioinformatics/bti282
Liu, W., Shahid, M. Q., Bai., L., Lu, Z., Chen, Y., Lu, Y., et al. (2016). Evaluation of genetic diversity and development of a core collection of wild rice (Oryza rufipogon griff.) populations in China. PloS One 10, e0145990. doi: 10.1371/journal.pone.0145990
Melaku, G., Haileselassie, T., Feyissa, T., Kiboi, S. (2013). Genetic diversity of the African wild rice (Oryza longistaminata chev. et roehr) from Ethiopia as revealed by SSR markers. Genet. Resour. Crop Ev. 60, 1047–1056. doi: 10.1007/s10722-012-9900-0
Pan, Y. H., Xu, Z. J., Liang, Y. T. (2018). Genetic structure and core collection of common wild rice O. rufipogon (Oryza rufipogon griff.) in guangxi. J. Plant Genet. Resour. 19, 498–509. doi: 10.13430/j.cnki.jpgr.2018.03.015
Qian, W., Ge, S., Hong, D. Y. (2001). Genetic variation within and among populations of a wild rice Oryza granulata from China detected by RAPD and ISSR markers. Theor. Appl. Genet. 102, 440–449. doi: 10.1007/s001220051665
Roy, C. D., Singh, N., Singh, A. K., Kumar, S., Srinivasan, K., Singh, R., et al. (2014). Analysis of genetic diversity and population structure of rice germplasm from north-eastern region of India and development of a core germplasm set. PloS One 9 (11), e113094. doi: 10.1371/journal.pone.0113094
Sarif, H. M., Rafii, M. Y., Ramli, A., Oladosu, Y., Musa, H. M., Chukwu, S. C., et al. (2020). Genetic diversity and variability among pigmented rice germplasm using molecular marker and morphological traits. Biotechnol. Biotec. Eq. 34, 747–762. doi: 10.1080/13102818.2020.1804451
Singh, B. P., Singh, B., Mishra, S., Kumar, V., Singh, N. K. (2016). Genetic diversity and population structure in Indian wild rice accessions. Aust. J. Crop Sci. 10, 144–151. doi: 10.3316/informit.030724213830369
Song, Z. P., Li, B., Chen, J. K., Lu, B. R. (2005). Genetic diversity and conservation of common wild rice (Oryza rufipogon) in China. Plant Spec. Biol. 20, 83–92. doi: 10.1111/j.1442-1984.2005.00128.x
Song, J. Y., Li, J. Q., Sun, J., Hu, T., Wu, A. T., Zhao, M. H., et al. (2018). Genome-wide association mapping for cold tolerance in a core collection of rice (Oryza sativa l.) landraces by using high-density single nucleotide polymorphism markers from specific-locus amplified fragment sequencing. Front. Plant Sci. 9, 875. doi: 10.3389/fpls.2018.00875
Swain, R., Mohapatra, S., Roy, P., Swain, D., Singh, O. N., Subudhi, H. N., et al. (2017). Assessment of genetic diversity in wild rice of Eastern India using SSR markers. Thai J. Agric. Sci. 9, 239–250. doi: 10.5539/jas.v9n6p239
Tanaka, N., Shenton, M., Kawahara, Y., Kumagai, M., Sakai, H., Ishimoto, M., et al. (2020). Whole-genome sequencing of the NARO world rice core collection (WRC) as the basis for diversity and association studies. Plant Cell Physiol. 61, 922–932. doi: 10.1093/pcp/pcaa019
Thachuk, C., Crossa, J., Franco, J., Dreisigacker, S., Warburton, M., Davenport, G. F. (2009). Core hunter: An algorithm for sampling genetic resources based on multiple genetic measures. BMC Bioinf. 10, 1–13. doi: 10.1186/1471-2105-10-243
Vaughan, D. A., Lu, B. R., Tomooka, N. (2008). The evolving story of rice evolution. Plant Sci. 174, 394–408. doi: 10.1016/j.plantsci.2008.01.016
Wang, W. S., Mauleon, R., Hu, Z. Q., Chebotarov, D., Tai, S. S., Zhang, F., et al. (2018). Genomic variation in 3,010 diverse accessions of Asian cultivated rice. Nature 557, 43–49. doi: 10.1038/s41586-018-0063-9
Xu, Q., Yuan, X. P., Wang, S., Feng, Y., Yu, H. Y., Li, X. M., et al. (2016). The genetic diversity and structure of indica rice in China as detected by single nucleotide polymorphism analysis. BMC Genom. 17, 1–8. doi: 10.1186/s12863-016-0361-x
Yan, W. G., Rutger, J. N., Bryant, R. J., Bockelman, H. E., Fjellstrom, R. G., McClung, A. M., et al. (2007). Development and evaluation of a core subset of the USDA rice germplasm collection. Crop Sci. 47, 869–876. doi: 10.2135/cropsci2006.07.0444
Yu, P., Li, Z. C., Zhang, H. L., Cao, Y. S., Li, D. Y., Wang, X. K. (2003). Sampling strategy of primary core collection of common wild rice (Oryza rufipogon griff.) in China. J. China Agric. Univ. 8, 37–41.
Zhang, H. Y., Mittal, N., Leamy, L. J., Barazani, O., Song, B. H. (2017). Back into the wild-apply untapped genetic diversity of wild relatives for crop improvement. Evol. Appl. 10, 5–24. doi: 10.1111/eva.12434
Zhang, J., Sun, B. R., Li, C., Chen, W. F., Fan, Z. L., Pan, D. J., et al. (2021). Molecular diversity and genetic structure of wild rice accessions (Oryza rufipogon griff) in guangdong province, China, as revealed by SNP markers. Genet. Resour. Crop Evol. 68 (9), 1–10. doi: 10.1007/s10722-020-01038-8
Zhang, H. L., Zhang, D. L., Wang, M. X., Sun, J. L., Qi, Y. W., Li, Z. C., et al. (2011). A core collection and mini core collection of Oryza sativa l. in China. Theor. Appl. Genet. 122, 49–61. doi: 10.1007/s00122-010-1421-7
Zhang, P., Zhong, K. Z., Zhong, Z. Z., Tong, H. H. (2019). Genome-wide association study of important agronomic traits within a core collection of rice (Oryza sativa l.). BMC Plant Biol. 19, 1–12. doi: 10.1186/s12870-019-1842-7
Zhou, H. F., Xie, Z. W., Ge, S. (2003). Microsatellite analysis of genetic diversity and population genetic structure of a wild rice (Oryza rufipogon griff.) in China. Theor. Appl. Genet. 107, 332–339. doi: 10.1007/s00122-003-1251-y
Keywords: genetic diversity, SNP, O. rufipogon, core collection, multiplex PCR (mPCR)
Citation: Zhang J, Pan D, Fan Z, Yu H, Jiang L, Lv S, Sun B, Chen W, Mao X, Liu Q and Li C (2022) Genetic diversity of wild rice accessions (Oryza rufipogon Griff.) in Guangdong and Hainan Provinces, China, and construction of a wild rice core collection. Front. Plant Sci. 13:999454. doi: 10.3389/fpls.2022.999454
Received: 22 July 2022; Accepted: 02 September 2022;
Published: 03 October 2022.
Edited by:
Rodomiro Ortiz, Swedish University of Agricultural Sciences, SwedenReviewed by:
Sarla Neelamraju, Indian Institute of Rice Research (ICAR), IndiaCopyright © 2022 Zhang, Pan, Fan, Yu, Jiang, Lv, Sun, Chen, Mao, Liu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qing Liu, bGl1cWluZzE5ODUwNEAxMjYuY29t; Chen Li, MjM2OTUzODk3M0BxcS5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.