
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci., 09 December 2022
Sec. Plant Abiotic Stress
Volume 13 - 2022 | https://doi.org/10.3389/fpls.2022.999051
This article is part of the Research TopicAn Update on Brassinosteroids: Homeostasis, Crosstalk, and Adaptation to Environmental Stress, Volume IIView all 7 articles
Tip-burn has seriously affected the yield, quality and commodity value of mini Chinese cabbage. Calcium (Ca2+) deficiency is the main cause of tip-burn. In order to investigate whether exogenous brassinosteroids (BRs) can alleviate tip-burn induced by calcium (Ca2+) deficiency and its mechanism, in this study, Ca2+ deficiency in nutrient solution was used to induced tip-burn, and then distilled water and BRs were sprayed on leaves to observe the tip-burn incidence of mini Chinese cabbage. The tip-burn incidence and disease index, leaf area, fluorescence parameters (Fv/Fm, NPQ, qP andφPSII) and gas exchange parameters (Tr, Pn, Gs and Ci), pigment contents, cell wall components, mesophyll cell ultrastructure and the expression of genes related to chlorophyll degradation were measured. The results showed that exogenous BRs reduced the tip-burn incidence rate and disease index of mini Chinese cabbage, and the tip-burn incidence rate reached the highest on the ninth day after treatment. Exogenous BRs increased the contents of cellulose, hemifiber, water-soluble pectin in Ca2+ deficiency treated leaves, maintaining the stability of cell wall structure. In addition, BRs increased photosynthetic rate by increasing the activities of Ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) and fructose 1,6-bisphosphatase (FBPase) related to Calvin cycle, maintaining relatively complete chloroplast structure and higher chlorophyll content via down-regulating the expression of BrPPH1 and BrPAO1 genes related to chlorophyll degradation. In conclusion, exogenous BRs alleviated calcium deficiency-induced tip-burn by maintaining cell wall structural stability and higher photosynthesis.
Mini Chinese cabbage (Brassica rapa L. ssp. pekinensis) is one of the main cultivated summer vegetables in plateaus, as well as an important export vegetable in China. In recent years, with global climate change, tip-burn has become exceptionally common in mini Chinese cabbage, which is generally considered a calcium-associated physiological disorder (Thibodeau, 1969; Bangerth, 1979), and negatively affect the quality and yield of mini Chinese cabbage (Everaarts and Blom-Zandstra, 2001). Tip-burn mostly occurs at the rosette stage, but can also occur at the seedling stage if the growth environment is not suitable or if the variety has weak resistance to the disease. The occurrence of tip-burn shows that the edges of plant leaves shrink, chlorosis and finally dry into brown paper, which it causes the structure and function destruction of cell membrane and cell wall, leading to meristem necrosis, and even the infection of pathogenic bacteria in the late stage of the disease (Su et al., 2016), which seriously affects the appearance quality, taste and commodity value of vegetables (Saure, 1998). Therefore, it is necessary to find an effective strategy to prevent and control the occurrence of tip-burn key problems to be solved urgently in mini Chinese cabbage production.
Calcium (Ca2+) is an important regulator of plant growth and development, which will have a great impact on plant quality (Lecourieux et al., 2006), as well as, it is a component of plant cell wall and cell membrane, maintaining the stability of cell wall structure and normal physiological functions in the process of maintaining plants from abiotic stresses (Zhu, 2016). Calcium is also an important structural, metabolic and signaling element (Tuteja, 2009). The physiological function of Ca2+ is realized through the orderly transport across cell membrane mediated by Ca2+ osmotic ion channel, Ca2+-ATPase and Ca2+/H+ exchanger (Straltsova et al., 2015). The occurrence of tip-burn is particularly serious in the facility environment (Lee et al., 2013). This may occur because facility conditions promote plant growth and faster growth rate, the formation and expansion of new tissue cell walls require large amounts of calcium, and the calcium demand becomes much higher than the actual absorption, thus increasing the incidence of tip-burn (Borkowski et al., 2016). Although its pathogenesis is complex and controversial, it is generally accepted that the inability of plants to provide sufficient calcium for rapidly developing leaves is the main reason for tip-burn (Kuronuma et al., 2019; Su et al., 2019; Wang et al., 2019).
In 1970, Mitchell and his team discovered a substance in the extract of rapeseed pollen that promoted plant cell division and elongation (Mitchell et al., 1970), subsequently Grove et al. (1979) defined this substance as brassinosteroid (BRs). In 1980, Fung and Siddall (1980) used their previous studies as a guide to successfully synthesize BRs for the first time. BRs constitute the sixth major class of plant hormones, and they are also recognized as highly efficient, broad-spectrum, non-toxic plant growth regulators, with strong penetration and fast internal absorption (Kurepin et al., 2016). BRs has concentration dose effect, at very low concentrations, they can significantly increase the growth of plants and regulate their various developmental processes (J Ahammed et al., 2015; Ahammed et al., 2020), including root and hypocotyl elongation, seed germination, flowering, and fruiting. In addition, they can mediate various stimuli in response to the environment, improve abiotic stress resistance in crops such as water stress (Li and Feng, 2011), high temperature stress (Cao and Hua, 2008),chilling damage (Sun et al., 2020), salinity stress (Su et al., 2020), heavy metal stress (Bajguz and Botany, 2010), and drought stress (Naveen et al., 2021), and significantly reduce the occurrence of diseases such as Botrytis cinerea infection (Li et al., 2020), rice blast and bacterial blight diseases (Nakashita et al., 2003). Fariduddin et al. (2009) showed that there is a concentration effect of BRs application in plants, and that the appropriate concentration BRs enhanced chlorophyll content and photo-synthetic efficiency in cucumbers. Spraying BRs at appropriate concentrations on tomato crops can enhance the activity of RuBP carboxylation/oxygenase (Rubisco), increase stomatal opening of tomato leaves and improve photosynthesis (Ogweno et al., 2008). Yamamoto et al. (1997) demonstrated the role of BRs on secondary cell wall formation and cell death during tracheary element differentiation in Zinnia system.
The above literatures showed that BR had certain effects on plant growth and development (photosynthetic characteristics, cell wall homeostasis, as so on) as well as various stresses. However, there are few reports on the relationship between BR and Ca2+ deficiency induced tip-burn. Therefore, we explored the effects of BRs on the growth and development, photosystem II (Fv/Fm, qP and Y (II)) and photosynthetic segment content, cell wall components of mini Chinese cabbage during Ca2+ deficiency induced tip-burn in this study. The results revealed the mitigation mechanism of BRs on the Ca2+ deficiency induced tip-burn in mini Chinese cabbage, which provided a new strategy and theoretical basis for overcoming the occurrence of tip-burn in mini Chinese cabbage cultivation.
The seeds of mini Chinese cabbage (Brassica pekinensis cv. Qiu Yu Huang) were surface sterilized in 5% sodium hypochlorite for 10 min, placed in a wet towel covered with double-layer filter paper, and then germinated for 2 d at 25°C in the dark. The germinated seeds were transplanted into planting cotton (30 cm × 20 cm × 3 cm) and placed at 25 ± 1°C and 17 ± 1°C (day and night, respectively) for 12 d with a 14 h photoperiod (photosynthetically active radiation = 200 µmol m-2 s-1) in an incubator. At the two-leaf stage, the uniform growth seedlings were transplanted into a hydroponic box (20 cm × 11 cm × 8 cm) filled with 700 mL improved Hoagland’s solution at pH 6.0 for 12 d of cultivation and each hydroponic box contained two seedlings. Analytical grade chemicals used in this study were obtained from Chinese companies [Sinopharm Chemical Reagent Co., Ltd)].
At the five-leaf stage, the plants were transplanted to different formulae of Hoagland’s nutrient solution (Wang et al., 2019), improved Hoagland’s solution and Hoagland’s nutrient solution without Ca2+. In preliminary experiment, the suitable concentration of BR (0.5µM) was screened out, which could reduce the incidence rate and disease index of tip-burn in mini Chinese cabbage seedlings. In the present experiment, the seedlings were treated as follows:
(a) (+Ca) Con + H2O: Normal Ca2+-containing nutrient solution + foliar spray distilled water.
(b) (+Ca) Con + 0.5 µM BR: Normal Ca2+-containing nutrient solution + foliar spray 0.5 µM BR.
(c) (−Ca) Con + H2O: Ca2+-free nutrient solution + foliar spray distilled water.
(d) (−Ca) Con + 0.5 µM BR: Ca2+-free nutrient solution + foliar spray 0.5 µM BR.
The seedlings were sprayed with BRs every 2 d and the amount of BRs (0.3-0.5 mL) was used to wet each leaf, subject to no dripping. The symptoms of tip-burn initially appeared at 3 d after treatment and the seedlings were treated for 12 d. For each treatment, 1 cm of leaf margin tissue was obtained from three individual plants and equally homogenized as one sample, which was immediately frozen with liquid nitrogen and stored in an ultra-low temperature refrigerator at −80°C. Each treatment had three biological replicates.
Whole leaves were scored using an arbitrary tip-burn severity index, with a ranking from 0-9. The classified criterion of tip-burn of mini Chinese cabbage was presented as follow:
0: Asymptomatic.
0.5: Only small spots on the edge of true leaves.
1: The edge of a leaf is chlorosis.
3: The edges of two leaves are chlorosis and wrinkled.
5: The edges of more than two leaves are slightly tip-burn, and the tip-burn area accounts for less than 25% of the leaves.
7: The edges of more than two leaves are moderately tip-burn, and the tip-burn area accounts for 25%–50% of the leaf area.
9: The edges of more than two leaves are severely tip-burn or the whole plant dies, and the tip-burn area accounts for more than 50% of the leaf area.
The severity of tip-burn in each plant was defined as follows:
Tip-burn disease index = Σ {(severity index × leaf number)/total number of leaves per pot}× 100 (Corriveau et al., 2012)
The incidence of tip-burn was expressed as the percentage of plants exhibiting tip-burn symptoms in all cultivation plants.
The leaves of mini Chinese cabbage on the ninth day of treatment were selected as samples. The functional leaves near the same growth point (The fourth functional leaf of mini Chinese cabbage seedlings counted from inside to outside), determination using root scanner (win rhizo Pro la2400, Canada).
The cell wall components (cellulose, hemicellulose, water-soluble pectin) were determined according to the methods of Fishman et al. (1993); Brummell et al. (2004) and Thygesen et al. (2005), respectively.
After 30 min of full dark reaction, the chlorophyll fluorescence parameters of seedlings on the ninth day of treatment were measured by the Chl fluorescence imaging (imaging WinGigE, Walz, Effeltrich, Germany) (Hu et al., 2017). The calculation formula is as follows:
The fourth functional leaf of mini Chinese cabbage seedlings counted from inside to outside on the ninth day after treatment was selected to measure gas exchange parameters with photosynthetic apparatus (CIRAS-2, UK).
RuBP carboxylation/oxygenase (Rubisco) and fructose 1,6-bisphosphatase (FBPase) activity detection kits (Suzhou Keming Biotechnology Co., Ltd., China) were used to determine the activities of Rubisco and FBPase in mini Chinese cabbage leaves, respectively. Refer to the instructions of the kit for specific operation steps.
The fresh leaves of mini Chinese cabbage treated for the ninth day were used as samples. Take about 1cm2 small pieces with a punch, fix them in 3% glutaraldehyde, and conduct vacuum pumping to sink the leaf tissue to the bottom. Rinse with 0.1 M phosphate buffer (pH 7.4) for 3 times, soak for 10 min (elute malondialdehyde), and place at 4 °C for 24 h. Then soak it in 1% Russian acid for 5 h, rinse it with phosphoric acid buffer for 3 times, then dehydrate it with ethanol of different concentration gradient, and then wash it with acetone, then penetrate the acetone and embed it into EPON812 epoxy resin for embedding. Ultrathin sample sections were cut on the ultramicro body (LeicaEMUC6 ultramicro, Japan), and then stained with uranyl acetate and lead citrate for 15 min. The ultrathin sections of mini Chinese cabbage leaves were observed and photographed by transmission electron microscope (TEM, JoelJem-1230, Japan).
The leaves of mini Chinese cabbage seedlings on the ninth day of treatment were randomly perforated and sampled from the same part leaves, then put into a test tube filled with 80% acetone and placed in the dark for 24 h. The absorbance values of the extract at 665, 649 and 470 nm were measured by UV-1780 spectrophotometer (Shimadzu, Japan), and the contents of chlorophyll a (Chla), chlorophyll b (Chlb), total chlorophyll (Chla+b) and carotenoid (Car) were calculated as described by Anwar et al. (2018).
Total RNA was isolated from 100 mg (fresh weight) of excised mini Chinese cabbage seedling leaves by grinding with a mortar and pestle in liquid nitrogen to obtain a fine paste using TaKaRa reagent (TaKaRa Bio, Japan) according to the manufacturer’s instructions. Each treatment was performed in triplicate. Primers designed for genes and reference genes were listed in Table 1. Each reaction (20 µL total volume) consisted of SYBR Premix Ex Taq II (10 µL), diluted cDNA (2 µL), forward and reverse primers (0.8 µL each), and nuclease-free water (6.4 µL). PCR amplification conditions were as follows: 1 min at 95°C followed by 40 cycles of 5 s at 95°C and 20 s at 60°C with data collection at the annealing step. After 40 cycles, the dissociation/melting curve stage at 95°C, 65°C for 15 s, 95°C for 0 s, and 50°C for 30 s. The relative quantification of mRNA was based on the method described by Livak and Schmittgen (2001). The expression level relative to the control for each sample was expressed as 2-ΔΔCt.
Data analysis was performed using Duncan’s multiple tests (P< 0.05) using SPSS software (version 19.0; IBM SPSS, Chicago, USA). Each experiment was performed in triplicate and the data are expressed as mean values ± SE (n=3).
As shown in Figure 1A, the tip-burn incidence rates of seedling grown under different treatments from 0 d to 12 d were recorded every 3 days. The tip-burn incidence rate of seedling treated with (-Ca) Con+H2O increased continuously from 0 d to 9 d, and reached the maximum on 9th day. On the 12th day, the number of seedlings died from tip-burn, which led to a decrease in the incidence rate. Therefore, the 9th day was chosen for the following indexes measurements.
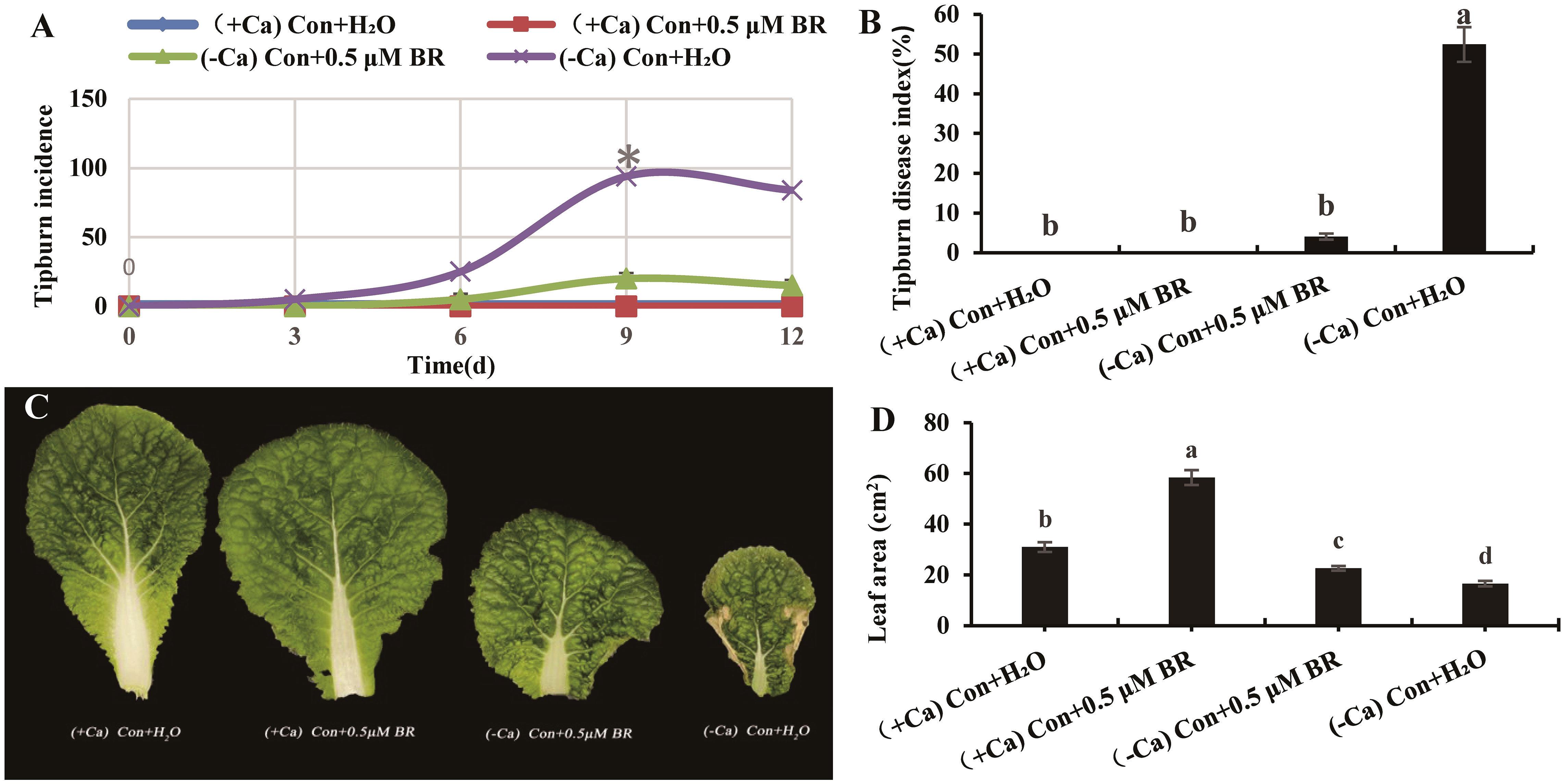
Figure 1 Effects of exogenous BRs on tip-burn incidence rate, disease index and leaf area of mini Chinese cabbage induced by Ca2+ deficiency. (A) the incidence rate of tip-burn at 0, 3, 6, 9 and 12 d after treatment. (B) the disease index of tip-burn on 9th day; (C) the fourth functional leaf counted from inside to outside of mini Chinese Cabbage seedlings; (D) Leaf area. The different letters above the bars showed significantly different among treatments according to Duncan’s multiple test (P< 0.05).
The tip-burn disease index on the ninth day between different treatments was shown in Figure 1B. Ca2+ deficiency significantly increased the tip-burn disease index, while foliar spray 0.5 µM BR significantly reduced the disease index of tip-burn by 92.36% compared to (- Ca) Con + H2O. Therefore, it can be speculated that exogenous BRs (0.5 µM) can reduce the tip-burn incidence rate and disease index of mini Chinese cabbage caused by Ca2+deficiency (Figures 1A, B). As shown in Figures 1C, D, the tip-burn of mini Chinese cabbage seriously affects the leaf area. Compared with (- Ca) Con + H2O treatment, exogenous spraying BRs significantly increased the leaf area by 36.38%. Similarly, under (+ Ca) nutrient solution treatment, exogenous BRs significantly increased the leaf area by 88.2%, compared with the treatment of exogenous spraying distilled water. In conclusion, exogenous BRs could significantly reduce the incidence rate and disease index of tip-burn induced by Ca2+ deficiency, as well as promote the leaf area of cabbage.
Plant cell wall as the first defense system will be affected after stress. The content of cell wall components will change with the time of stress. Compared with (+ Ca) nutrient solution culture, Ca2+ deficiency induced tip-burn significantly reduces the contents of cellulose (Figure 2A), hemicellulose (Figure 2B) and water soluble pectin (Figure 2C), which proves that tip-burn seriously destroys the components of cell wall, destroys the structure of cell wall, weakens the defense system of cell wall and hinders plant growth. Under the culture condition of (+ Ca), Compared with the treatment of spraying distilled water, spraying BRs increased the contents of cellulose and hemicellulose by 43.1% and 27.8%, respectively. Similarly, under (- Ca) environmental conditions, compared with the treatment of spraying distilled water, spraying exogenous BRs significantly increased the contents of cellulose, hemicellulose and pectin by 30.4%, 44.68% and 59.8%, respectively. In conclusion, exogenous BRs enhanced the contents of cell wall components, maintaining cell wall structure stability in calcium deficiency leaves.
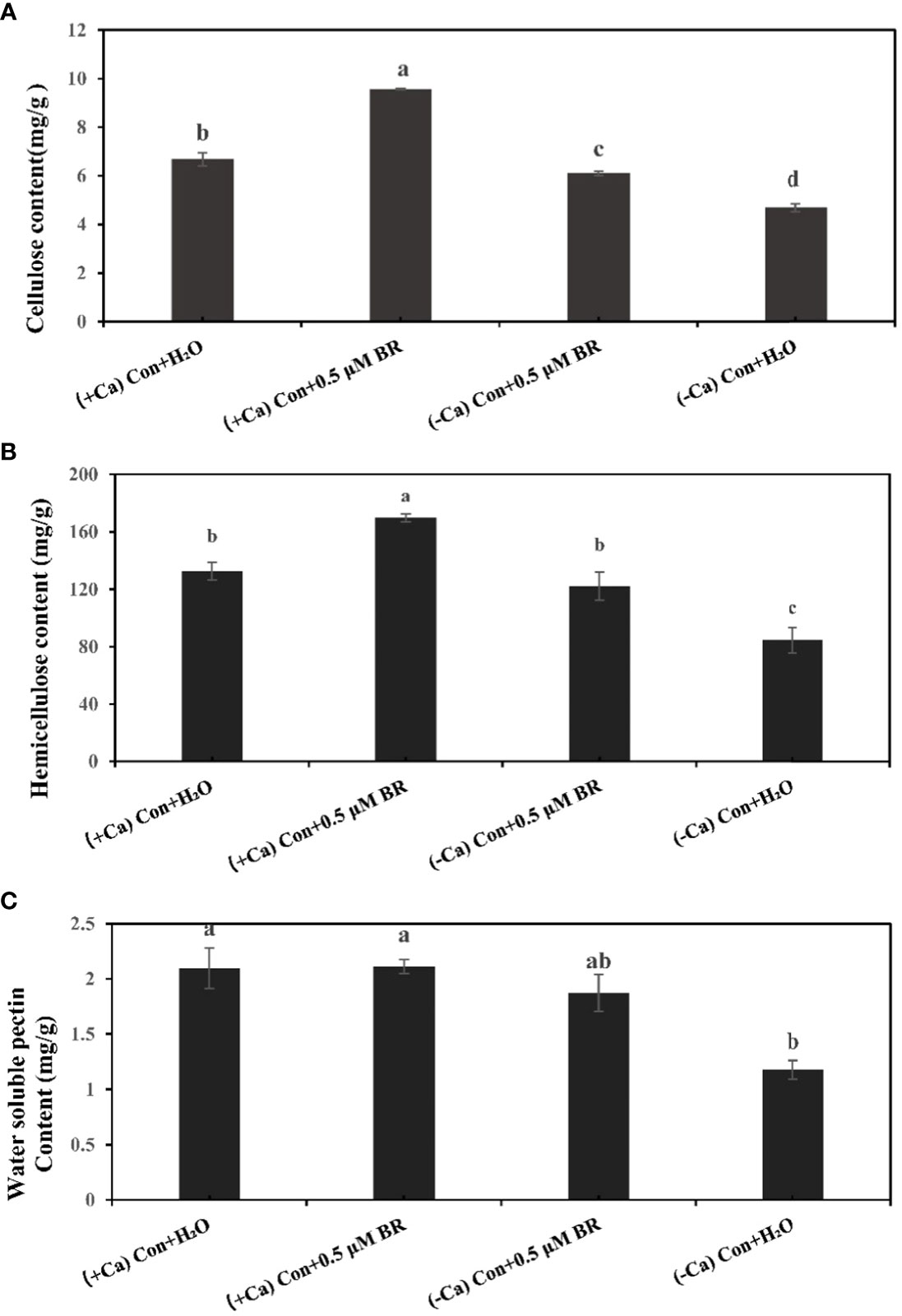
Figure 2 Effect of exogenous BRs on cell wall components of mini Chinese cabbage leaves. (A) cellulose. (B) hemicellulose. (C) water-soluble pectin. The different letters above the bars showed significantly different among treatments according to Duncan’s multiple test (P< 0.05).
As shown in Table 2, compared with calcium exist treatment, calcium deficiency significantly reduced the Fv/Fm, NPQ, qP, and φPSII values of mini Chinese Cabbage seedlings. After exogenous spraying BRs, the above values increased significantly. The excitation pressure of PSII (1-qP) and the excess excitation energy of reaction center [(1-qP)/NPQ] significantly increased under calcium deficiency condition, while leaf spraying BRs significantly decreased the two values. The above results suggested that BRs reduced the excess excitation energy of PSII through heat dissipation, so as to reduce the excitation pressure of leaf reaction center, to resist the photoinhibition induced by calcium deficiency in mini Chinese cabbage.
Under normal nutrient solution condition, exogenous BRs significantly increased the net photosynthetic rate (Pn), stomatal conductance (Gs) and transpiration rate (Tr) values. However, calcium deficiency significantly decreased these values, while foliar spraying BRs significantly reversed the negative effects of calcium deficiency on these parameters, which increased Tr, Gs and Pn by 27.4%, 34% and 170.9%, respectively (Table 3). Moreover, calcium deficiency significantly increased the intercellular carbon dioxide concentration (Ci), while exogenous BRs significantly decreased Ci under calcium deficiency conditions (Table 3). In conclusion, exogenous BRs can alleviate calcium deficiency induced tip-burn by maintaining higher photosynthesis.
As shown in Figure 3, Compared with (+ Ca) treatment, (- Ca) significantly inhibited the activities of Rubisco and FBPase by 187.6% and 18.98%, respectively. Exogenous spraying of BRs significantly increased the activity of photosynthetic enzyme, compared with (- Ca) Con + H2O treatment, the (- Ca) Con + 0.5 μM BR treatment significantly increased the activity of Rubisco and FBPase by 107.7% and 19.17%, respectively. In conclusion, exogenous BRs enhanced the photosynthesis of leaves by promoting the activity of Rubisco and FBPase.
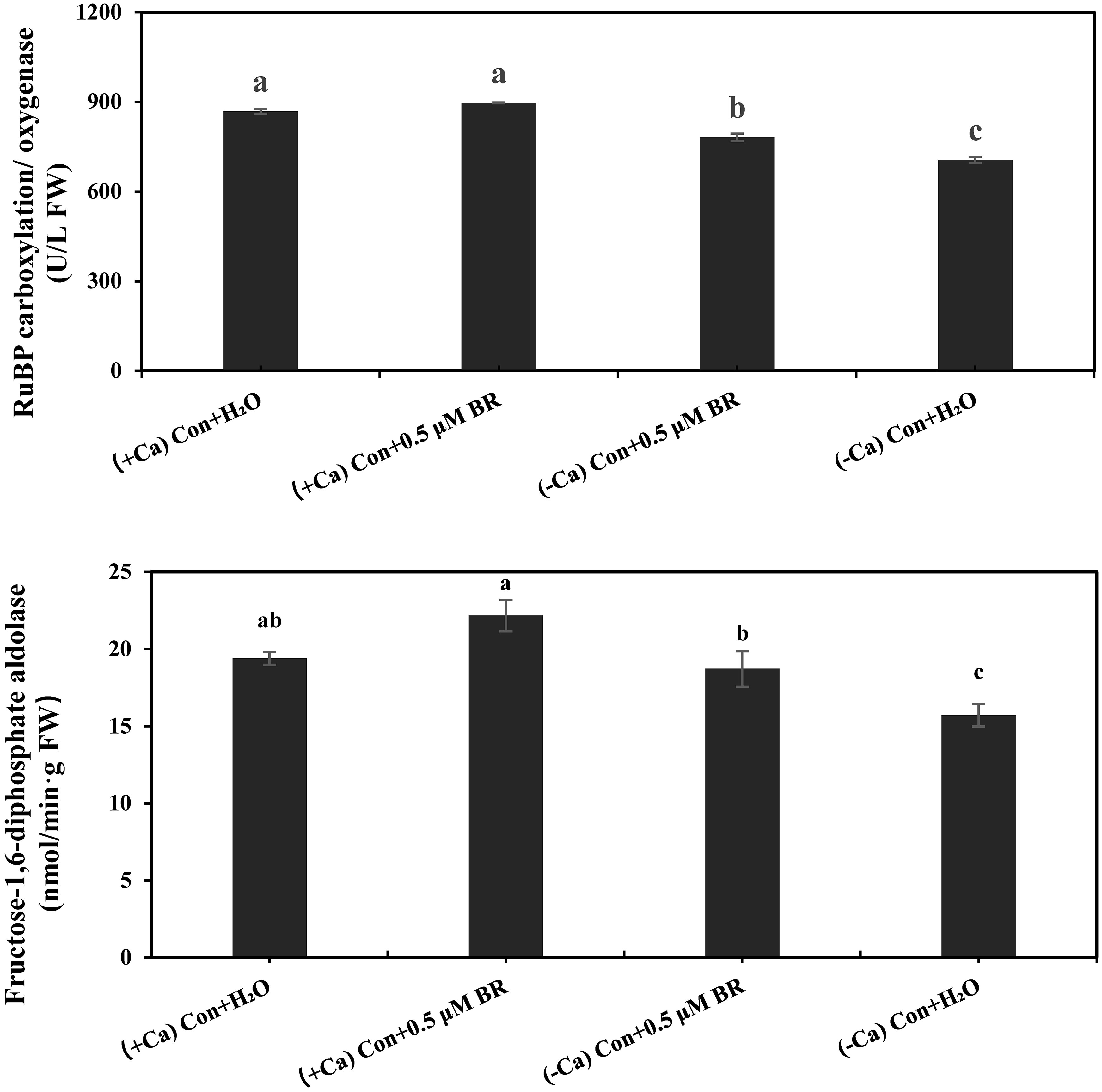
Figure 3 Effects of exogenous BRs on the activities of Rubisco and FBPase in mini Chinese cabbage leaves. Data are presented as mean ± SE (n=3). The different letters above the bars showed significantly different among treatments according to Duncan’s multiple test (P< 0.05).
As shown in Figure 4, in normal nutrient solution (+ Ca) treated leaf, most cells had complete structure. In individual cells, chloroplasts are arranged neatly close to the cell wall in the shape of spindle. The edge of chloroplasts is smooth, the thylakoid matrix lamella is clearly visible, arranged tightly and orderly, there are many and obvious starch particles, and osmiophilic particles do not appear. Compared with the treatment of (+ Ca) culture, Ca2+ deficiency reduced the number of chloroplasts, the stromal lamella was chaotic. The cell wall was prone to plasmolysis, the starch granule in the cell were reduced, the osmiophilic granule were increased, the chloroplasts were not close to the cell wall, the membrane structure was fuzzy, and some chloroplasts even differentiated into irregular structures. However, in (-Ca) Con+0.5μM BR treated leaf, the cell wall did not separate, the cell membrane structure and the chloroplast structure were relatively intact, and it have a relatively good thylakoid structure. There were less osmiophilic granule in the chloroplast, but also a small amount of starch granule. In conclusion, exogenous BRs had a certain alleviating effect on the damage of chloroplast ultrastructure caused by calcium deficiency.
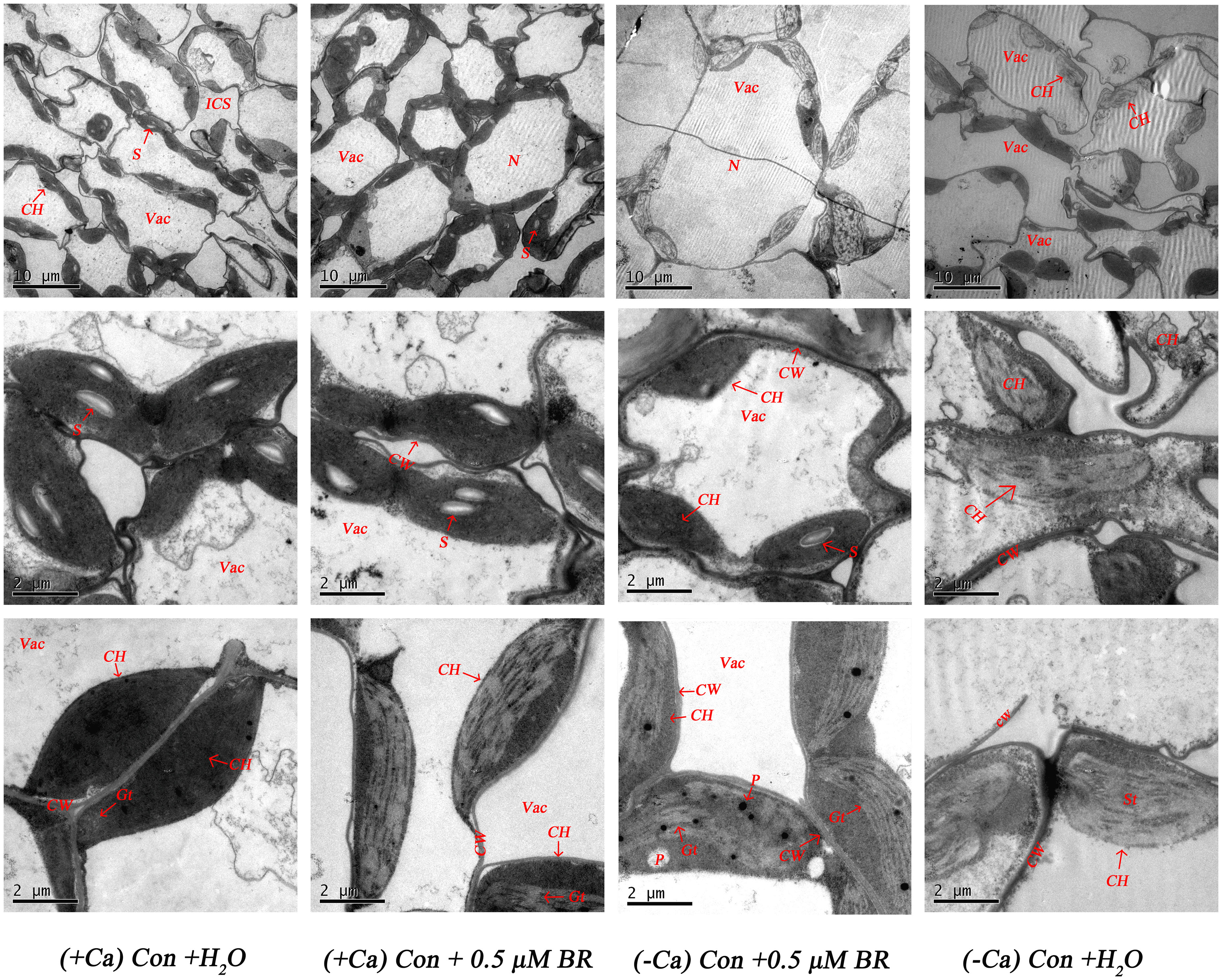
Figure 4 Effect of exogenous BRs on mesophyll cells ultrastructure of mini Chinese cabbage. CW, cell wall; Vac, vacuole; ICS, intercellular space; S, starch granule; ch, chloroplast; N, cell; P, plastid globule; OG, osmiophilic granule; ST, stromal lamella. The scale of chloroplasts was 10 μ m and 2 μ m.
Photosynthetic pigments are involved in absorbing and transmitting light energy or causing primary photochemical reactions in photosynthesis. As shown in Figure 5, in (+ Ca) nutrient treated leaf, exogenous BRs increased the content of Chla, Chlb, and Chla+b. However, when plants were exposed to the calcium deficiency nutrient solution, the contents of Chla, Chlb, Chla+b and Car can be significantly reduced by 50.5%, 30.9%, 45.2% and 63.02%, respectively. while Chla, Chlb and Chla+b contents of seedlings sprayed with exogenous BRs increased significantly compared with those sprayed with distilled water. As well as, exogenous BRs treatment increased the content of Car, but it was not significant, indicating that the effect of BRs on the content of Car was also regulated by other factors. In conclusion, these results suggested that exogenous BRs alleviated calcium deficiency induced tip-burn by increasing photosynthetic pigment contents, thus delaying calcium deficiency caused leaf senescence and maintaining higher photosynthesis.
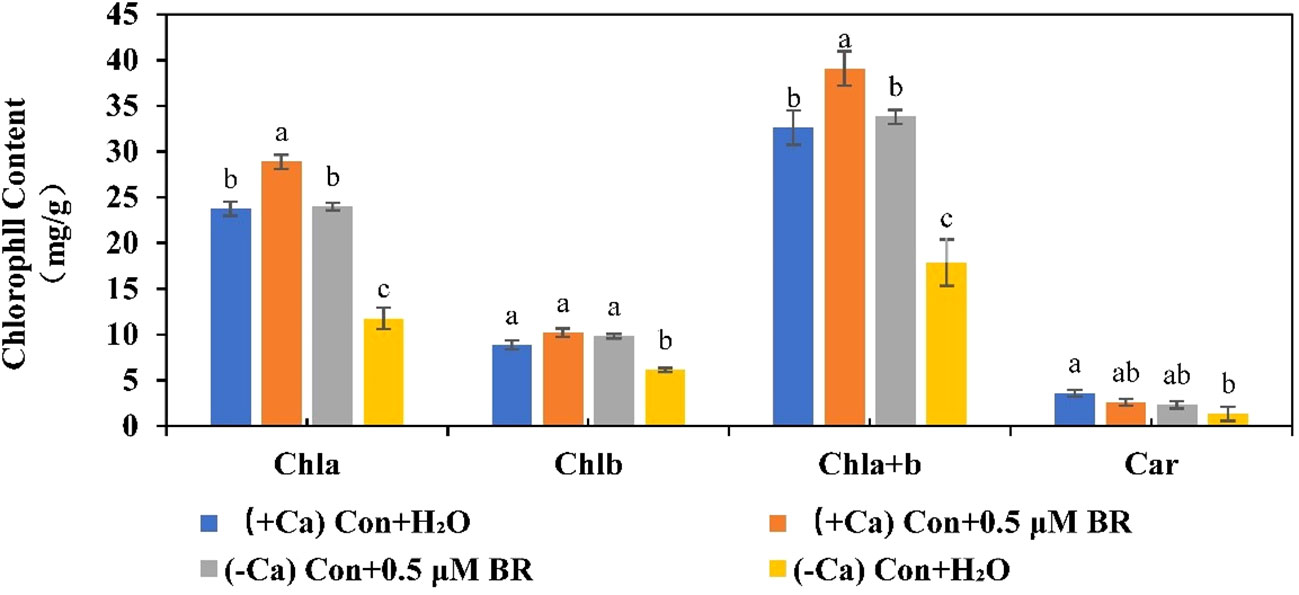
Figure 5 Effect of exogenous BRs on chlorophyll content in mini Chinese cabbage. Data are presented as mean ± SE of three replicates. The different letters above the bars showed significantly different among treatments according to Duncan’s multiple test (P< 0.05).
As shown in Figure 6, compared with (+ Ca) treatment, calcium deficiency significantly up-regulated the gene expression of BrPPH1 and BrPAO1, which proved that mini Chinese cabbage with tip-burn could promote the degradation of leaf chlorophyll and accelerate leaf aging. Compared with (- Ca) Con + H2O treatment, exogenous BRs could down-regulate the expression of BrPPH1 and BrPAO1 by 35.97% and 49.02%, respectively. In conclusion, exogenous BRs can inhibit the expression of chlorophyll degradation related genes in mini Chinese cabbage leaves, increase the content of chlorophyll, enhance the photosynthesis and promote the recovery of normal growth of mini Chinese cabbage.

Figure 6 Effect of exogenous BRs on the expression levels of genes related to chloroplast degradation in mini Chinese cabbage leaves. The relative expression level of BrPPH1 and BrPAO1 on ninth day were expressed as mean ± SE (n =3). The different letters above the bars showed significantly different among treatments according to Duncan’s multiple test (P< 0.05).
Calcium deficiency is the main factor inducing the occurrence of tip-burn. Meanwhile, tip-burn is the main disease restricting the yield and quality of mini Chinese cabbage (Aloni, 1986). How to alleviate and prevent the occurrence of tip-burn is an urgent problem to be solved in the cultivation of cabbage. Since the plant hormone brassinosteroids (BR) was discovered in the extract of rape pollen by Mitchell et al. (1970) and Grove et al. (1979), more and more experts have found that it can promote various processes of plant growth and development (Aghdam et al., 2014; Kurepin et al., 2016). In addition, BRs also has a certain relationship with plant disease resistance (Nakashita et al., 2003; Ding et al., 2021). In this experiment, Ca2+ deficiency in nutrient solution was used to induced the occurrence of tip-burn, and exogenous spraying of a certain concentration of BRs was used to alleviate the occurrence of tip-burn. We found that 0.5 μM BR reduced the tip-burn incidence rate and disease index of mini Chinese cabbage (Figures 1A and B). Among the 12 d of treatment, we recorded the incidence rate and disease index daily. The data showed that the incidence rate reached maximum values on the ninth day after treatment (Figure 1A). Therefore, the samples on 9th day after treatment were used to measure the following indices. Leaf area is related to the efficiency of leaf photosynthesis. It had been reported that the plant photosynthetic rate and leaf area size were reduced under external abiotic stress conditions such as water, salinity stress, and drought (He and Cramer, 1996; Dalirie et al., 2010; Slabbert and Krüger, 2014). The leaf area of mini Chinese cabbage in which tip-burn occurred was significantly reduced, while exogenous BRs application could increase leaf area and maintain normal plant growth (Figures 1C, D). This finding is consistent with those of the study of Ali et al. (2021). Plant cell wall is involved in maintaining a certain shape of cells, enhancing the mechanical strength of cells, and is also related to the physiological activities of cells. When plants are subjected to stress, the cell wall is the first to undergo changes, and the content of cell wall components decreases significantly (Braidwood et al., 2014; Tenhaken, 2015). In our study, calcium deficiency significantly decreased the levels of cellulose, hemicellulose and pectin in mini Chinese cabbage (Figure 2), while exogenous BRs increased these cell wall components, thus maintaining the stability of the cell wall structure. The regulation of BRs on the content of cell wall components may be due to the signal cascade mediated by BRs to regulate the gene expression involved in the synthesis of cell wall biological components. For example, in Arabidopsis, the BR-activated transcription factor BZR1 and its homologous gene BZR2/BES1 have been shown to directly bind to promoter regions of a large number of cell wall-related genes (Jiang et al., 2015), including the majority of cellulose synthase genes (Xie et al., 2011). Wolf et al. (2012) reported that BRs signaling in Arabidopsis is coupled with the modification of methyl-esterified HGs to control pectin-dependent cell wall integrity (Wolf et al., 2012).
In the process of photosynthesis, solar energy, carbon dioxide, and water are utilized to produce oxygen and energy in the form of sugar, thus maintaining the life activities of the organism (Simkin et al., 2019). It is widely believed that the stronger the photosynthesis of a plant, the more organic matter is synthesized, and the more adequate the energy supply is, contributing to the robust growth of the crop and a higher final yield. The chlorophyll fluorescence parameters are important photosynthesis indicators and reflect the effect of stress on the photosystem (Deell et al., 1999). Tip-burn is also a physiological disease of mini Chinese cabbage caused by Ca2+ deficiency stress. As our experimental results showed, under Ca2+ deficiency treatment, chlorophyll fluorescence parameters (Fv/Fm, NPQ, and qP) of mini Chinese cabbage seedlings decreased significantly, while exogenous BRs application alleviated these effects and restored the chlorophyll fluorescence parameters to near normal levels (Table 2). Our results are basically consistent with the research results of Zhao et al. (2017), who found that application of exogenous EBR reversed the suppressive effects on photosynthesis, antioxidant enzyme activity, Rubisco activase (RCA), and gene expression induced by combined drought and heat stress in common wheat. Exogenous EBR can mitigate the adverse effects of high temperatures on plant growth by improving the maximum quantum yield of leaves, the excitation capture efficiency of PSII centers, and the effective quantum yield of photochemistry (Zhang et al., 2013). The photochemical quenching coefficient reflects the redox state of the primary electron acceptor and the number of open centers in photosystem II. The larger the qP, the greater the amount of QA reoxidation to form QA, indicating greater PSII electron transfer activity (Deell et al., 1999). The differences in chlorophyll fluorescence properties of plant leaves also reflected the differences in their photosynthetic capacity. Photosynthetic gas exchange parameters are the basis for measuring the exchange between plants and the outside world, and are the indicators of photosynthetic intensity. Lawlor (2002) reported that since CO2 enters leaves through stomata, the change of stomatal opening is very important for the study of photosynthesis. BRs can improve the drought tolerance of maize by improving the photosynthetic gas exchange parameters (Anjum et al., 2011). BRs are also able to promote photosynthesis and growth by positively regulating the synthesis and activity of several photosynthetic enzymes in cucumber, including Rubisco, as well as increasing photosynthesis by regulating the expression of P450 and MRP (Xia et al., 2009), these results are basically consistent with our results. In our present study, calcium deficiency decreased stomatal conductance but increased the accumulation of intercellular CO2, suggesting that non-stomatal factor was the main reason for reduced photosynthesis caused by calcium deficiency. At the same time, BRs increased the activity of Rubisco and FBPase in Ca2+ deficiency treated leaves (Figure 3). The above results suggested that the application of exogenous BRs effectively mitigated the damage degree of photosynthesis in mini Chinese cabbage caused by calcium deficiency.
When plants are subjected to stress, chloroplasts are affected to some extent, with changes in their shape, detachment from the cell membrane, disappearance of starch granules, increases in osmiophilic granules, vesicle expansion, loosening and distortion, and significant deformation of palisade and spongy tissues. Exogenous phytohormone treatment causes chloroplasts in plant leaves to exhibit an intact internal lamellar system with normal chloroplast shape, intact cell membrane structure, and reduced osmiophilic granules, and mitigates cell structural damage and destruction (Meng et al., 2014). This is basically consistent with our findings, which Ca2+ deficiency-induced tip-burn in mini Chinese cabbage resulted in reduced numbers of cellular chloroplasts and grana, disorganized stromal lamellae, stromal wall separation, increased osmiophilic granules, lack of chloroplast adherence to the cell wall, and blurred chloroplast membrane structure, with some chloroplasts even differentiating into irregular structures. In contrast, exogenous BRs spraying reduced the aforementioned cell damage, leaving the cell membrane structure intact and the chloroplast structure relatively intact, and thus maintaining a relatively good vesicle-like structure (Figure 4). These findings demonstrated that the application of exogenous BRs mitigated calcium deficiency induced tip-burn by improving photosynthesis via increasing the activities of Rubisco and FBPase and protecting the leaf mesophyll cells and chloroplast structure of mini Chinese cabbage.
Photosynthetic pigments are the important material basis of leaf photosynthesis: Chla is the photosynthetic pigment for energy conversion, and Chlb and Car capture and transmit light energy. As shown in Figure 5, calcium deficiency significantly reduced the Chla and Chlb content in mini Chinese cabbage leaves, while exogenous BRs increased the content of photosynthetic pigments and somewhat alleviate calcium deficiency -induced attenuation of photosynthesis. These findings are consistent with those of the study by Yang et al. (2018), who reported that the application of BRs on leaves could promote the photosynthesis and chlorophyll fluorescence characteristics of Leymus chinensis under varying levels of shade. During leaf senescence, chlorophyll is removed from thylakoid membranes and converted in a multistep pathway to colorless breakdown products that are stored in vacuoles, and removal of Mg to form pheophytin is likely the first step, followed by removal of the phytol tail, catalyzed by pheophytinase (PPH), production of amphetamine (Eckardt, 2009). PPH, a chloroplast-located and senescence-induced hydrolase, is widely distributed in algae and land plants. In the process of plant senescence, PPH1 gene can activate PPH and accumulate pheophytin (Schelbert et al., 2009). Pheophorbide a oxygenase (PAO) activity is found only during senescence, hence PAO seems to be a key regulator of chl catabolism (Pružinská et al., 2003), which is positively correlated with senescence. In our experiments, exogenous BRs application increased chlorophyll content, enhanced photosynthesis, and promoted the return to normal growth of mini Chinese cabbage by down-regulating the expression of BrPPH1 and BrPAO1 genes related to chlorophyll degradation in mini Chinese cabbage leaves (Figure 6). These findings are largely consistent with those presented by Liu et al. (2016). They found that BRs antagonistically regulated rice flag leaf senescence by mediating chlorophyll degradation, membrane degeneration, and senescence-related gene expression. These findings suggested that the application of exogenous BRs mitigated calcium deficiency induced tip-burn by maintaining a higher chlorophyll content via down-regulating the expression of chlorophyll degradation related genes, finally delay the leaf senescence caused by calcium deficiency.
Exogenous BRs alleviated calcium deficiency induced tip-burn via 1): maintaining cell wall structure stability by increasing the contents of cell wall components, 2) improving photosynthesis by increasing the activities of Rubisco and FBPase enzymes, maintaining a relatively complete chloroplast structure and 3) as well as maintaining a higher chlorophyll content by down-regulating the expression of chlorophyll degradation related genes. Our results will provide theoretical basis and technical reference for prevention strategies of tip-burn in mini Chinese cabbage in future.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
YL: methodology, validation, formal analysis, writing-original draft, and writing - review & editing. JM and XG: writing - review & editing. JT, YW, and ZT: analyzed the data and prepared the figures and illustrations. JY: funding acquisition. LH: methodology, conceptualization, funding acquisition, and writing - review & editing. All authors read and approved the submission of the manuscript.
This work was supported by Gansu Provincial Key Laboratory of Aridland Crop Science, Gansu Agricultural University (GSCS-2021-04), Education Science and Technology Innovation Project of Gansu Province (GSSYLXM-02), Agriculture Research System of China (CARS-23-C-07), Key Project of Gansu Provincial Natural Science Foundation (21JR7RA803), and Gansu Top Leading Talent Plan (GSBJLJ-2021-14).
We thank Dr. Li-xiang Cheng (Gansu Provincial Key Laboratory of Arid Land Crop Science, Gansu Agricultural University, Lanzhou 730070, P.R. China) for providing the method support. Moreover, we thank to editage company (https://www.editage.cn) for editing the English language of our paper.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aghdam, M. S., Mohammadkhani, N. J. F., Technology, B. (20142014). Enhancement of chilling stress tolerance of tomato fruit by postharvest brassinolide treatment. Food Bioprocess Technol. 7 (3), 909–914. doi: 10.1007/s13197-012-0757-1
Ahammed, G. J., Li, X., Liu, A., Chen, S. (2020). Brassinosteroids in plant tolerance to abiotic stress. J. Plant Growth Regul. 39 (4), 1451–1464. doi: 10.1007/s00344-020-10098-0
Ahammed, J., Xia, X. J., Li, X., Shi, K., Yu, J. Q., Zhou, Y. H. (2015). Role of brassinosteroid in plant adaptation to abiotic stresses and its interplay with other hormones. Curr. Protein Pept. Sci. 16 (5), 462–473. doi: 10.2174/1389203716666150330141427
Ali, M. M., Anwar, R., Malik, A. U., Khan, A. S., Ahmad, S., Hussain, Z., et al. (2021). Plant growth and fruit quality response of strawberry is improved after exogenous application of 24-epibrassinolide. J. Plant Growth Regul. 41 (4), 1786–1799. doi: 10.1007/s00344-021-10422-2
Aloni, B. (1986). Enhancement of leaf tipburn by restricting root growth in Chinese cabbage plants. J. Hortic. Sci. 61 (4), 509–513. doi: 10.1080/14620316.1986.11515733
Anjum, S., Wang, L., Farooq, M., Hussain, M., Xue, L., Zou, C., et al. (2011). Brassinolide application improves the drought tolerance in maize through modulation of enzymatic antioxidants and leaf gas exchange. J. Agron. Crop Sci. 197 (3), 177–185. doi: 10.1111/j.1439-037X.2010.00459.x
Anwar, A., Bai, L., Miao, L., Liu, Y., Li, S., Yu, X., et al. (2018). 24-epibrassinolide ameliorates endogenous hormone levels to enhance low-temperature stress tolerance in cucumber seedlings. Int. J. Mol. Sci. 19 (9), 2497. doi: 10.3390/ijms19092497
Bajguz, A. J. E., Botany, E. (2010). An enhancing effect of exogenous brassinolide on the growth and antioxidant activity in chlorella vulgaris cultures under heavy metals stress. Environ. Exp. Bot. 68 (2), 175–179. doi: 10.1016/j.envexpbot.2009.11.003
Bangerth, F. (1979). Calcium-related physiological disorders of plants. Annu. Rev. Phytopathol. 17, 97–122. doi: 10.1146/annur ev. py.17.090179.000525
Borkowski, J., Dyki, B., Oskiera, M., Machlańska, A., Felczyńska, A. (2016). The prevention of tipburn on Chinese cabbage (L.(Lour.) olson) with foliar fertilizers and biostimulators. J. Hortic. Res. 24 (1), 47–56. doi: 10.1515/johr-2016-0006
Braidwood, L., Breuer, C., Sugimoto, K. J. N. P. (2014). My body is a cage: mechanisms and modulation of plant cell growth. New Phytol. 201, 388–402. doi: 10.1111/nph.12473
Brummell, D. A., Dal Cin, V., Crisosto, C. H., Labavitch, J. (2004). Cell wall metabolism during maturation, ripening and senescence of peach fruit. J Exp Botany 55, 2029–2039. doi: 10.1093/jxb/erh227
Cao, Y.-Y., Hua, Z. J. R. S. (2008). Protective roles of brassinolide on rice seedlings under high temperature stress. Rice Sci. 15, 63–68. doi: 10.1016/S1672-6308(08)60021-9
Corriveau, G., Guilbault, R., Tahan, A., Sabourin, R. (2012). Review and study of genotypic diversity measures for real-coded representations. IEEE Trans. Evolutionary Comput. 16, 695–710. doi: 10.1109/TEVC.2011.2170075
Dalirie, M. S., Sharifi, R. S., Farzaneh, S. (2010). Evaluation of yield, dry matter accumulation and leaf area index in wheat genotypes as affected by terminal drought stress. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 38, 182–186. doi: 10.15835/nbha3814583
Deell, J. R., Kooten, O. V., Prange, R. K., Murr, D. P. (1999). Applications of chlorophyll fluorescence techniques in postharvest physiology. Hortic. Rev. 23, 69–107. doi: 10.1002/9780470650752.ch2
Ding, Y., Sheng, J., Cheng, F. (2021). Assessment of the role of brassinosteroid in regulating the disease resistance of postharvest tomato fruit by proteomic analysis. J. Food Process. Preservation. 45, e15708. doi: 10.1111/jfpp.15708
Eckardt, N. A. (2009). A new chlorophyll degradation pathway. Plant Cell. 21, 700–700. doi: 10.1105/tpc.109.210313
Everaarts, A., Blom-Zandstra, M. (2001). Review ArticleInternal tipburn of cabbage (Brassica oleracea var. capitata). J. Hortic. Sci. Biotechnol. 76, 522–528. doi: 10.1080/14620316.2001.11511402
Fariduddin, Q., Yusuf, M., Hayat, S., Ahmad, A. J. E., Botany, E. (2009). Effect of 28-homobrassinolide on antioxidant capacity and photosynthesis in Brassica juncea plants exposed to different levels of copper. Environ. Exp. Bot. 66, 418–424. doi: 10.1016/j.envexpbot.2009.05.001
Fishman, M., Levaj, B., Gillespie, D., Scorza, R. (1993). Changes in the physico-chemical properties of peach fruit pectin during on-tree ripening and storage. J. Am. Soc. Hortic. Science. 118, 343–349. doi: 10.21273/JASHS.118.3.343
Fung, S., Siddall, J. (1980). Stereoselective synthesis of brassinolide: a plant growth promoting steroidal lactone. J. Am. Chem. Soc. 102 (21), 6580–6581. doi: 10.1021/ja00541a045
Grove, M. D., Spencer, G. F., Rohwedder, W. K., Mandava, N., Worley, J. F., Warthen, J. D., et al. (1979). Brassinolide, a plant growth-promoting steroid isolated from brassica napus pollen. Food Agric. Organ. United Nations. 281, 216–217. doi: 10.1038/281216a0
He, T., Cramer, G. R. J. P. (1996). Abscisic acid concentrations are correlated with leaf area reductions in two salt-stressed rapid-cycling brassica species. Plant Soil. 179, 25–33. doi: 10.1007/BF00011639
Hu, L., Liao, W., Dawuda, M. M., Yu, J., Lv, J. (2017). Appropriate : ratio improves low light tolerance of mini Chinese cabbage seedlings. BMC Plant Biol. 17 (1), 1–14. doi: 10.1186/s12870-017-0976-8
Jiang, J., Zhang, C., Wang, X. (2015). A recently evolved isoform of the transcription factor BES1 promotes brassinosteroid signaling and development in Arabidopsis thaliana. Plant Cell. 27, 361–374. doi: 10.1105/tpc.114.133678
Kurepin, L. V., Bey, M. A., Back, T. G., Pharis, R. P. (2016). Structure–function relationships of four stereoisomers of a brassinolide mimetic on hypocotyl and root elongation of the brassinosteroid-deficient det2-1 mutant of arabidopsis. J. Plant Growth Regulation. 35, 215–221. doi: 10.1007/s00344-015-9523-8
Kuronuma, T., Watanabe, Y., Ando, M., Watanabe, H. (2019). Relevance of tipburn incidence to the competence for Ca acquirement and Ca distributivity in lisianthus [Eustoma grandiflorum (Raf.) shinn.] cultivars. Scientia Horticulturae. 246, 805–811. doi: 10.1016/j.scienta.2018.10.043
Lawlor, D. (2002). Limitation to photosynthesis in water-stressed leaves: stomata vs. metabolism and the role of ATP. Ann. Botany. 89, 871–885. doi: 10.1093/aob/mcf110
Lecourieux, D., Ranjeva, R., Pugin, A. (2006). Calcium in plant defence-signalling pathways. New phytologist. 171, 249–269. doi: 10.1111/j.1469-8137.2006.01777.x
Lee, J. G., Choi, C. S., Jang, Y. A., Jang, S. W., Lee, S. G., Um, Y. (2013). Effects of air temperature and air flow rate control on the tipburn occurrence of leaf lettuce in a closed-type plant factory system. Horticulture Environment Biotechnol. 54 (4), 303–310. doi: 10.1007/s13580-013-0031-0
Li, K. R., Feng, C. (2011). Effects of brassinolide on drought resistance of xanthoceras sorbifolia seedlings under water stress. Acta Physiologiae Plantarum. 33, 1293–1300. doi: 10.1007/s11738-010-0661-0
Liu, L., Li, H., Zeng, H., Cai, Q., Zhou, X., Yin, C. (2016). Exogenous jasmonic acid and cytokinin antagonistically regulate rice flag leaf senescence by mediating chlorophyll degradation, membrane deterioration, and senescence-associated genes expression. J. Plant Growth Regulation. 35, 366–376. doi: 10.1007/s00344-015-9539-0
Livak, K. J., Schmittgen, T. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods: A Companion to Methods Enzymology. 25 (4), 402–408. doi: 10.1006/meth.2001.1262
Li, T.-T., Zhang, J.-D., Tang, J.-Q., Liu, Z.-C., Li, Y.-Q., Chen, J., et al. (2020). Combined use of trichoderma atroviride CCTCCSBW0199 and brassinolide to control botrytis cinerea infection in tomato. Plant Disease. 104, 1298–1304. doi: 10.1094/pdis-07-19-1568-re
Meng, J. F., Xu, T. F., Wang, Z. Z., Fang, Y. L., Xi, Z. M., Zhang, Z. (2014). ). the ameliorative effects of exogenous melatonin on grape cuttings under water-deficient stress: antioxidant metabolites, leaf anatomy, and chloroplast morphology. J. Pineal Res. 57 (2), 200–212. doi: 10.1111/jpi.12159
Mitchell, J., Mandava, N., Worley, J., Plimmer, J., Smith, M. J. N. (1970). Brassins–a new family of plant hormones from rape pollen. Nature. 225 (5237), 1065–1066. doi: 10.1038/2251065a0
Nakashita, H., Yasuda, M., Nitta, T., Asami, T., Fujioka, S., Arai, Y., et al. (2003). Brassinosteroid functions in a broad range of disease resistance in tobacco and rice. Plant J. 33 (5), 887–898. doi: 10.1046/j.1365-313X.2003.01675.x
Naveen, N., Kumari, N., Avtar, R., Jattan, M., Ahlawat, S., Rani, B., et al. (2021). Evaluation of Effect of Brassinolide in Brassica juncea Leaves under Drought Stress in Field Conditions. Horticulturae 7 (11), 514. doi: 10.3390/horticulturae7110514
Ogweno, J. O., Song, X. S., Shi, K., Hu, W. H., Mao, W. H., Zhou, Y. H., et al. (2008). Brassinosteroids alleviate heat-induced inhibition of photosynthesis by increasing carboxylation efficiency and enhancing antioxidant systems in Lycopersicon esculentum. J. Plant Growth Regulation. 27 (1), 49–57. doi: 10.1007/s00344-007-9030-7
Pružinská, A., Tanner, G., Anders, I., Roca, M., Hörtensteiner, S. (2003). ). chlorophyll breakdown: pheophorbide a oxygenase is a rieske-type iron–sulfur protein, encoded by the accelerated cell death 1 gene. Proc. Natl. Acad. Sci. 100 (25), 15259–15264. doi: 10.1073/pnas.2036571100
Saure, M. (1998). Causes of the tipburn disorder in leaves of vegetables. Scientia horticulturae 1998 76 (3-4), 131–147. doi: 10.1016/S0304-4238(98)00153-8
Schelbert, S., Sylvain, A., Burla, Bo, Agne, B., Kessler, Cell, F. J. P. (2009). Pheophytin pheophorbide hydrolase (pheophytinase) is involved in chlorophyll breakdown during leaf senescence in Arabidopsis. Plant Cell 21 (3), 767–785. doi: 10.1105/tpc.108.064089
Simkin, A. J., López-Calcagno, P. E., Raines, C. (2019). Feeding the world: improving photosynthetic efficiency for sustainable crop production. J. Exp. Bot. 70 (4), 1119–1140. doi: 10.1093/jxb/ery445
Slabbert, M., Krüger, G. (2014). Antioxidant enzyme activity, proline accumulation, leaf area and cell membrane stability in water stressed amaranthus leaves. South Afr. J. Bot. 95, 123–112. doi: 10.1016/j.sajb.2014.08.008
Straltsova, D., Chykun, P., Subramaniam, S., Sosan, A., Kolbanov, D., Sokolik, A., et al. (2015). Cation channels are involved in brassinosteroid signalling in higher plants. Steroids. 97, 98–106. doi: 10.1016/j.steroids.2014.10.008
Su, T., Li, P., Wang, H., Wang, W., Zhao, X., Yu, Y., et al. (2019). Natural variation in a calreticulin gene causes reduced resistance to Ca2+ deficiency-induced tipburn in Chinese cabbage (Brassica rapa ssp. pekinensis). Plant Cell Environment. 42 (11), 3044–3060. doi: 10.1111/pce.13612
Su, Q., Zheng, X., Tian, Y., Wang, C. (2020). Exogenous brassinolide alleviates salt stress in Malus hupehensis Rehd. by regulating the transcription of NHX-Type Na+ (K+)/H+ antiporters. Frontiers in plant science 11, 38. doi: 10.3389/fpls.2020.00038
Sun, Y., He, Y., Irfan, A. R., Liu, X., Yu, Q., Zhang, Q., et al. (2020). Exogenous brassinolide enhances the growth and cold resistance of maize (Zea mays l.) seedlings under chilling stress. Angronomy. 10 (4), 488. doi: 10.3390/agronomy10040488
Su, T., Yu, S., Yu, R., Zhang, F., Yu, Y., Zhang, D., et al. (2016). Effects of endogenous salicylic acid during calcium deficiency-induced tipburn in Chinese cabbage (Brassica rapa l. ssp. pekinensis). Plant Mol. Biol. Reporter. 34 (3), 607–617. doi: 10.1007/s11105-015-0949-8
Tenhaken, R. (2015). Cell wall remodeling under abiotic stress. Front. Plant Science. 5. doi: 10.3389/fpls.2014.00771
Thibodeau, P. (1969). The influence of calcium on the development of lettuce tipburn. j.am.soc.hortic.sci. 94, 372–376. doi: 10.1007/BF01158284
Thygesen, A., Oddershede, J., Lilholt, H., Thomsen, A. B., Ståhl, K. (2005). On the determination of crystallinity and cellulose content in plant fibres. Cellulose 12 (6), 563–576. doi: 10.1007/s10570-005-9001-8
Tuteja, N. (2009). “Integrated calcium signaling in plants,” in Signaling in plants (Berlin, Heidelberg: Springer), 29–49. doi: 10.1007/978-3-540-89228-1_2
Wang, W., Wang, J., Wei, Q., Li, B., Zhong, X., Hu, T., et al. (2019). Transcriptome-wide identification and characterization of circular RNAs in leaves of Chinese cabbage (Brassica rapa l. ssp. pekinensis) in response to calcium deficiency-induced tip-burn. Signaling Plants. 9 (1), 1–9. doi: 10.1038/s41598-019-51190-0
Wolf, S., Mravec, J., Greiner, S., Mouille, G., Höfte, H. (2012). Plant cell wall homeostasis is mediated by brassinosteroid feedback signaling. Curr. Biol. 22, 1732–1737. doi: 10.1016/j.cub.2012.07.036
Xia, X. J., Zhang, Y., Wu, J. X., Wang, J. T., Zhou, Y. H., Shi, K., et al. (20098406). Brassinosteroids promote metabolism of pesticides in cucumber. J. Agric. Food Chem. 57. doi: 10.1021/jf901915a
Xie, L., Yang, C., Wang, X. (2011). Brassinosteroids can regulate cellulose biosynthesis by controlling the expression of CESA genes in Arabidopsis. J. Exp. Botany. 62 (13), 4495–4506. doi: 10.1093/jxb/err164
Yamamoto, R., Demura, T., Fukuda, H. J. P., Physiology, C. (1997). Brassinosteroids induce entry into the final stage of tracheary element differentiation in cultured zinnia cells. Plant Cell Physiol. 38 (8), 980–983. doi: 10.1093/oxfordjournals.pcp.a029262
Yang, A., Anjum, S., Wang, L., Song, J., Zong, X., Lv, J., et al. (2018). Effect of foliar application of brassinolide on photosynthesis and chlorophyll fluorescence traits of leymus chinensis under varying levels of shade. Photosynthetica. 56 (3), 873–883. doi: 10.1007/s11099-017-0742-z
Zhang, Y., Zhu, X., Ding, H., Yang, S., Chen, Y. J. P. (2013). Foliar application of 24-epibrassinolide alleviates high-temperature-induced inhibition of photosynthesis in seedlings of two melon cultivars. Photosynthetica. 51 (3), 341–349. doi: 10.1007/s11099-013-0031-4
Zhao, G., Xu, H., Zhang, P., Su, X., Zhao, H. (2017). Effects of 2, 4-epibrassinolide on photosynthesis and rubisco activase gene expression in Triticum aestivum l. seedlings under a combination of drought and heat stress. Plant Growth Regulation. 81 (3), 77–384. doi: 10.1007/s10725-016-0214-7
Keywords: Tip-burn, deficiency calcium, brassinosteroids, cell wall structural, photosynthesis
Citation: Li Y, Ma J, Gao X, Tie J, Wu Y, Tang Z, Hu L and Yu J (2022) Exogenous brassinosteroids alleviate calcium deficiency-induced tip-burn by maintaining cell wall structural stability and higher photosynthesis in mini Chinese Cabbage. Front. Plant Sci. 13:999051. doi: 10.3389/fpls.2022.999051
Received: 20 July 2022; Accepted: 31 October 2022;
Published: 09 December 2022.
Edited by:
Iftikhar Ali, State Key Laboratory of Molecular Developmental Biology, Chinese Academy of Sciences (CAS), ChinaReviewed by:
Basharat Ali, Khwaja Fareed University of Engineering and Information Technology (KFUEIT), PakistanCopyright © 2022 Li, Ma, Gao, Tie, Wu, Tang, Hu and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jihua Yu, eXVqaWh1YWdnQDE2My5jb20=; Linli Hu, aHVsbEBnc2F1LmVkdS5jbg==
†ORCID: Jihua Yu, orcid.org/0000-0001-5849-7636
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.