
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci. , 06 October 2022
Sec. Plant Abiotic Stress
Volume 13 - 2022 | https://doi.org/10.3389/fpls.2022.997475
This article is part of the Research Topic Costs and Tradeoffs in Plant Adaptation and Acclimation to Metals View all 5 articles
 Samira A. F. El-Okkiah1
Samira A. F. El-Okkiah1 Amira M. El-Tahan2*
Amira M. El-Tahan2* Omar M. Ibrahim2
Omar M. Ibrahim2 Mohamed A. Taha3
Mohamed A. Taha3 Shereen Magdy Korany4
Shereen Magdy Korany4 Emad A. Alsherif5
Emad A. Alsherif5 Hamada AbdElgawad6
Hamada AbdElgawad6 Esmaeel Z. F. Abo Sen7
Esmaeel Z. F. Abo Sen7 Mohamed A. Sharaf-Eldin8
Mohamed A. Sharaf-Eldin8Soil pollution with cadmium (Cd) is a serious threat to plant growth and development. On the other hand, silicon (Si) can support plants to cope with Cd stress. However, the Cd stress mitigating impact of Si reduction in pea (Pisum sativum L.) is not known. The objective of this study is to see if and how Si can reduce Cd toxicity. To the end, a greenhouse pot experiment was performed twice during the 2018/2019 and 2019/2020 seasons to investigate the effect of Si on the growth, anatomy, and biochemistry of Cd stressed peas plants. Cd exposure increased the contents of Cd ions in the root and shoot of pea plants. Consequentially, Cd accumulation in pea tissue significantly reduced plant growth i.e., plant height, leaf area, and shoot and root dry weights. The effect of Cd was concentration-dependent, where at low concentration (50 mg/kg soil), the plant height was 94.33 and 97.33cm and at high concentration (100 mg/kg soil), it was 89.0 and 91.0 cm in the two seasons, respectively. This growth reduction can be explained by the decrease in plants’ photosynthesis, whereas plants exposed to Cd toxicity had lower chlorophyll levels. At the anatomy level, high Cd concentrations resulted in anatomical abnormalities such as an unusual vascular system, abnormal lignification in the pith parenchyma, and enlarged cortical cells. Moreover, all Cd concentrations resulted in a highly significant decrease in stomatal area and stomatal density (the number of stomata per mm2). In addition to growth inhibition, Cd-induced oxidative damage to pea plants as indicated by increased hydrogen peroxide (H2O2) and Malondialdehyde (MDA) levels. To reduce stress toxicity, plants treated with Cd at 50 and 100 (mg/kg) showed a significant increase in antioxidant capacity. Peroxidase (POD) enzyme activity was significantly increased by 41.26%, 28.64%, 77.05%, and 60.77% in both seasons, respectively. Si at 300 ppm under Cd (100 mg/kg) stress conductions considerably reduced (MDA) contents by 29.02% and 29.12%, in the two seasons, respectively. The findings pointed out that Si’s ability to protect pea against the oxidative stress caused by Cd toxicity.
Pisum sativum L. (pea) is an annual plant that grows in the winter, and it is one of the most important energetic crops in the Leguminosae family (Fabaceae). The immature seeds of this crop are eaten as fresh, frozen, or canned vegetables. Pea, starchy vegetable with high protein, has long been regarded as a low-cost, easily available source of protein due to their high levels of key amino acids such as tryptophan and lysine (Orzoł et al., 2022). Carbohydrates (fiber and starch) are also the primary components of peas, accounting for 20 and 46% of dry matter in seeds. Pea seeds are also rich in vitamins (A, B6, C, and K), minerals (P, Mg, Cu, Fe, and Zn) and lutein content (Eid et al., 2019). Pea seeds are extensively farmed including Egypt’s Nile Delta. In Egypt, the total area planted with pea was 4326.9 (ha), with a total yield of 14 t/ha of green legumes (FAO, 2014). However, different sorts of soil contaminants [including heavy metals mercury (Hg), lead (Pb), and cadmium (Cd)] have an impact on vegetable growth of the West Nile Delta soil (Khalifa and Gad, 2018). Therefore, the development of agriculture in a low-input and sustainable way is urgently needed to maintain pea growth under heavy metal contaminations.
Plants are naturally susceptible to various stress factors, including abiotic and biotic stressors. In this context, excess irrigation from drawing wells, agricultural chemical fertilizers, and quick industrial expansion has increased the number of toxic metals in agricultural soils that negatively impact the soil-plant environment system (Gadallah and Sayed, 2014; Jali et al., 2016). For instance, heavy metals-contaminated water for crop irrigation survived cadmium-rich rocks, smelting, mining, sewage sludge application, or excessive use of phosphate fertilizers (Hooda et al., 2007; Kabta-Pendias, 2011; Alloway, 2013). Because heavy metal stress negatively impacts crop development and productivity, it is of great interest to researchers (Gill, 2014). In recent years, there has been a lot of focus on heavy metals and trace elements in the environment, specifically in soil and water, as well as their effect on plant nutrition and productivity. (Gaafar et al., 2012) who reported that carbohydrate content in white radish and Triticum aestivum L. significant decline as the heavy metal (cadmium and lead) concentration increased. For example, using polluted soil or water for agricultural cultivation reduces productivity and produces contaminated food grains and vegetables, both of which are harmful to human health (Galal and Shehata, 2015a). (Ayangbenro and Babalola, 2017) and (Zhang et al., 2018) also found that trace metals (TM) influence public health and the ecological system. Crops and vegetables that grow in heavy metal-polluted soil accumulated a higher heavy metal concentration. Using this contaminated land for crop cultivation has a negative impact on plant cultivations, leading to lower production efficiency and contaminated food grains and vegetables, both of which are harmful to plant and human health (Singh and Agrawal 2010). Because of its water solubility and movement within the ecosystem, Cd induces necrosis and chlorophyll degradation and alters nutrient absorption, cellular metabolism, carbon fixation, and membrane function (Ahmad et al., 2015; Jali et al., 2016). Cd stimulates the production of ROS, which causes severe damage to various cell organelles and, as a result, inhibits plant growth (Ahmad et al., 2016). Moreover, Cd increases the antioxidant defense systems of stressed plants as a defense strategy to cope with induced oxidative stress (Peng et al., 2017). Furthermore, Cd stress can alter gene expression in a variety of ways (Farooq et al., 2016). Thus the phytotoxic impact of such compounds on the growth, development, and productivity of economical crops should be deeply investigated.
Silicon (Si), is not an essential element for higher plant growth, but it has improved the growth of several crops including rice, wheat, barley, and cucumber. Thus, Si is widely used as a fertilizer in numerous nations to boost productivity and ensure long-term yield (Ma et al., 2015). It has been shown to mitigate abiotic stress (e.g., salt stress, metal toxicity, drought stress, radiation damage, high temperature, and freezing), biotic stress (pests and fungi and bacteria infections), and nutritional imbalance (Ma and Yamaji, 2006). Particularly, multiple studies have recently demonstrated that Si represents a significant alternative to provide tolerance against the negative effects of various metals such as Cd2+ (Shi et al., 2017) and Ni2+ (Abd-Allah et al., 2019). Nonetheless, the role of Si in sustaining plant growth in the presence of toxicants remains largely unknown. According to (Rizwan et al., 2016), Si-mediated Cd toxicity mitigation includes external and internal mechanisms, such as increased nutrient uptake and decreased metal uptake and translocation. Furthermore, the formation of Cd–Si co-complexation in the cell wall reduces Cd transport to plant tissues (Ma et al., 2015). Again, (Ribera-Fonseca et al., 2018) demonstrated that reduced heavy metal uptake by Si could be attributed to root exudation of secondary metabolites such as phenolic compounds and organic acids. Secretions of these compounds chelate with metal cations and limit their entry into plant roots. Furthermore, Si’s protective effect against heavy metals may be related to the activation of enzymatic antioxidant processes (Farooq et al., 2016; Abd-Allah et al., 2019). According to (Hussain et al., 2015), Si application increased CAT and GPx activity in Cd-treated wheat plants compared to control plants. In this context, (Shi et al., 2017) found that increasing antioxidant activities, particularly those involved in H2O2 eradication, can reduce ROS generation in crops subjected to biotic stress Our study’s objectives were to (1) determine how Cd toxicity affects pea growth, anatomical features, and biochemistry (antioxidant enzyme activities and non-enzymatic antioxidants) of Pea plants, which is one of the most important crops in Egypt, (2) gain insight into the possible mechanisms involved in Si-mediated Cd detoxification. We investigated the hypothesis that less Cd accumulation and changes improved physiological and antioxidant defense systems underlie the Cd stress mitigating effect of Si treatment.
Pisum sativum L. cv. Master-B seeds were obtained from the Ministry of Agriculture in Egypt. Pot experiments were carried out in the greenhouse of the Department of Agricultural Botany, Faculty of Agriculture, Kafr El-Sheikh University, Egypt, during the two successive seasons of 2018/2019 and 2019/2020. Plastic pots (30 cm in diameter) were filled with 10 kg of clay loam soil (Table 1). After five minutes of seed surface sterilization with 2% sodium hypochlorite, the seeds were carefully washed and rinsed multiple times with sterile water. Before planting, the soil used in this experiment was fertilized with calcium super phosphate fertilizer (P2O5 15.5%) that was added at the rate of 5 g/kg. Six healthy seeds were planted in each pot on the 19th of September 2018/2019 and on the 17th of September 2019/2020 seasons respectively. Under ideal conditions, in aerated full-nutrient media in plastic pots (Sandalio et al., 2001). The treatments were: control soil or Cadmium (CdCl2) treated soil at low concentration (50 mg/kg soil) and high concentration (100 mg/kg soil). Then silicon or Si(OH)4 (Sigma Chemical Company, St. Louis, MO, USA). Si treatments were introduced when seedlings were 25 days old at three concentrations of 100, 200, and 300 ppm Si. Plant foliar application was carried out two times using a one-hand pressure sprayer; the first application was 25 days after planting and the second after 45 days from sowing (at vegetative and flowering stages, respectively). The application was in the early morning when the stomata were open, allowing for better foliar penetration. Tween-20 was added at 0.1% (v/v) to applications as a surfactant to ensure optimal penetration into leaf tissues. The pots were then distributed into 12 treatments/groups, each treatment of 3 pots. A split-split plot design was used in this experiment with three replications.
The roots and shoots of control plants, Cd, and/or Si-treated plants were harvested at the early flowering stage. The vegetative growth indicators such as plant height (which was measured from the soil surface to the top of the main stem), as well as shoot and root dry weights, were measured. Shoots were removed from the plant organs and oven-dried at 70°C until a constant weight was attained as (g) per plant, leaf area (cm2) as measured with a leaf area meter LI-3100 Area Meter).
At harvest, 80 days after sowing, the number of pods per plant and the number of seeds per plant were counted. Total seed yield per plant (g) by gathering all seed weight per plant at the moisture content of 10-12%, weights of 100 seeds were determined.
Some physiological and biochemical parameters were assessed during the early flowering stage as follows:
Cd was determined using an atomic absorption spectrophotometer in the digest acid solution of the root and shoot samples, according to (Chapman and Pratt, 1982.)
The photosynthetic pigments (chlorophyll a, b, and total chlorophyll) were determined using the fourth leaf from the tip of the pea plant after 50 days from sowing during both seasons. The concentrations of chlorophyll (Chl.) were calculated as g/0.5 g fresh weight from the leaves. The pigments were extracted with 5 mL of N-N Dimethyl formamide before being stored in the refrigerator for 24 hours in the dark. The absorbency of the samples was measured using a spectrophotometer at 664 and 647 nm wavelengths. The concentration pigments was determined according to Moran, 1982.
The total nitrogen content of pea seeds was determined using the micro-Kjeldahl method, as described by (A.O.A.C., 1995). The proportion of protein in the seeds was computed by multiplying the total N % by 6.25 to get the percentage of protein in the seeds. Total soluble carbohydrates (TSC) were determined according to (Yemm and Willis, 1954).
The contents of malondialdehyde (MDA) and H2O2 were measured. A plant tissue sample (shoot) of 0.2 g was grounded in 5 ml of 0.1% TCA and centrifuged at 10,000g for 5 minutes. To 1 ml of the supernatant aliquot, 4 ml of 20% TCA containing 0.5% thiobarbituric acid (TBA) was added. The concentration of MDA (mol g 1 FW) was measured using (= 155 mM 1 cm1).
H2O2 levels were determined by grounding 0.5 g of fresh plant tissues (root) with 5 ml of trichloroacetic acid (TCA 0.1%) and centrifuging at 12,000g for 15 minutes at 4°C. A total of 0.5 ml of supernatant was mixed with 0.5 ml of 10 mM KPO4 buffer (pH = 7.0). Absorbance was measured at 390 nm using spectrophotometer (Velikova et al., 2000).
pt?>At 4°C, pulverized shoot samples (100 mg) were blended in 1 mL of 0.2 M potassium phosphate buffer (pH 7.0) containing 0.1 mM EDTA to estimate antioxidant enzymatic tests. The filtrates were utilized to assess the antioxidant enzymes (catalase (CAT) and peroxidase (POD) activities after centrifugation at 15 000 g for 20 minutes. Measuring enzyme activity methods were according to Rios-Gonzalez et al. (2002). CAT activity was measured spectrophotometrically by looking for a decrease in H2O2 absorbance at 240 nm. The potassium phosphate reaction buffer was 50 mM. A phosphate buffer and 15 mM H2O2 were used (pH 7.0). One minute after the reaction began, the absorbance change was measured (e = 43.6 mM1 cm1). The guaiacol substrate was used to determine POD activity. The greatest absorption of the tetraguaiacol produced in the reaction was at 470 nm. In a 50 mM phosphate buffer (pH 7.0) solution with 0.3% H2O2 and guaiacol, the enzyme was evaluated (1%).
A minimum of 5 samples of pea plant stem were randomly collected after 30 and 50 days from sowing (specimens 1cm long were taken from the fourth upper internode). For 48 hours, the sampled material was fixed in FAA (50% ethanol + 5% formaldehyde + 10% glacial acetic acid in water). Two washes in 70% ethyl alcohol were performed. Dehydration was achieved by passing the samples through a series of ethyl alcohol concentrations (75–100%). Each sample was passed through a mixture of xylol and absolute ethyl alcohol at the following percentages: 25%, 50%, 75%, and pure xylol in the final two changes for each dilution. Within 12 hours, the paraffin shavings reagent containing samples was saturated. To remove all traces of xylol, two changes of paraffin were performed. Samples were immersed in melted Paraffin in embedding paper trays, then quickly cooled in cold water. Rotary Microtome (Leica RM 2125 apparatus) sections (10-12 microns thick) were cut, and paraffin sections were fixed to the slides with Albumin. Slides were dried completely in a dry oven at 50°C for 24 hours. The slides were first immersed in two changes of xylol for about 10 seconds before being transferred to a jar containing equal parts absolute ethyl alcohol and xylol for 5 minutes. The sections were immersed in a series of descending ethyl alcohol dilutions ranging from absolute to 5%. After that, the sections were stained for 10 minutes in a jar containing 1% safranin, and the excess stain was washed away. Sections were stained in a jar containing 1% light green for 1 minute, then cleared in xylol, mounted in Canda Balsam, and ready for microscopic examination (Ruzin, 1999). Each slide was photographed after five readings were examined with an electric microscope (Leica DM LS) and a digital camera (Leica DC300).
From the fourth upper internode, a series of hand sections of stems were prepared at 1 mm intervals. The free hand sections were stained with 001% Fluorol yellow 088 dissolved in lactic acid for 30 minutes and then washed in distilled water for suberin visualization in fluorescence microscopy (Lux et al., 2005). Prior to observation, the samples were placed in a drop of 1% FeCl3 dissolved in 50% glycerin. Toluidine blue was used to stain the sections for bright-field observations. The sections were photographed with an Olympus DP 72 digital camera and examined with a Zeiss Axioskop2 plus epifluorescence microscope.
We looked at the primary and lateral roots of pea that had been exposed to Cd for seven days. The approach of (Thordal-Christensen et al., 2000) was modified for H2O2 localization. Fresh, hand-cut samples were incubated for 30 minutes at room temperature in a 1 mg/mL solution of 3,3′-DAB-HCl, pH 3.8 (Sigma). H2O2 production was represented by a reddish-brown hue. After washing the slides for 10 minutes, we fixed the DAB-stained tissues in 2.5% glutaraldehyde and Nikon microscope was used to examine the results.
Leaf stomatal density was calculated (Radoglou and Jarvis, 1992). The leaf’s abaxial epidermis was cleaned with a degreased cotton ball before being carefully smeared with the nail varnish for about 20 minutes. The thin film was peeled off the leaf surface and under a photomicroscope system with computer attachment, the number of stomata (s) and epidermal cells for each film strip were counted.
Before sowing pea seeds, soil samples were taken from the major root zone. The soil samples were airdried, crushed, and passed through a 2 mm sieve before being analyzed for physicochemical properties. According to (Bouyoucos, 1965), each sample was prepared to form soil texture and used the hydrometer method. pH and EC meters were used to measure soil pH and electrical conductivity (EC) in a 1:2 soil: water suspension (Hati et al., 2007). A calcimeter was used to measure calcium carbonate (CaCO3). Heavy metals (Pb and Cd) in soil were extracted and measured using an Atomic Absorption Spectrophotometer (Gasco and Lobo, 2007).
Using SPSS for Windows, an analysis of variance (ANOVA) with post hoc Duncan (1955) Multiple comparison test was performed (Ver. 13.0, SPSS Inc., USA). The significance of the means among controls and treatments was estimated using the probability level of P > 0.05.
The effects of Cd and or Si treatments on plant height, leaf area, shoot dry weight, and root dry weight were measured (Table 2) Cd reduced plant height, leaf area/plant, shoot dry weight, and root dry weight of pea plants. Increasing cadmium concentrations progressively reduced the average plant height, where it reached 94.33 and 97.33 cm, at the low concentration while at the high concentration, it reached 89.0 and 91.0 cm compared to controlled plants in both seasons. Similarly, the average leaf area was decreased (Table 2) and at high concentrations of Cd, the maximum reduction in leaf area was achieved. A significant decrease in the average values of both the dry weight of shoot and root compared to the control was recorded. Under high concentrations, the average weights of shoot dry weight reached 4.57 g, 5.65 g, 1.35 g, and 1.80 g for dry weight of root, respectively. Si treatment reduced Cd’s negative effects on growth parameters. At 300 ppm exogenous Si to Cd (100mg/k) treated plants increased plant height by 37.45% and 36.26%, leaf area by 33.99% and 31.87%, dry weight of shoot by 31.48% and 37.16, and root dry weight by 36.49% and 29.97%, in the two seasons respectively.
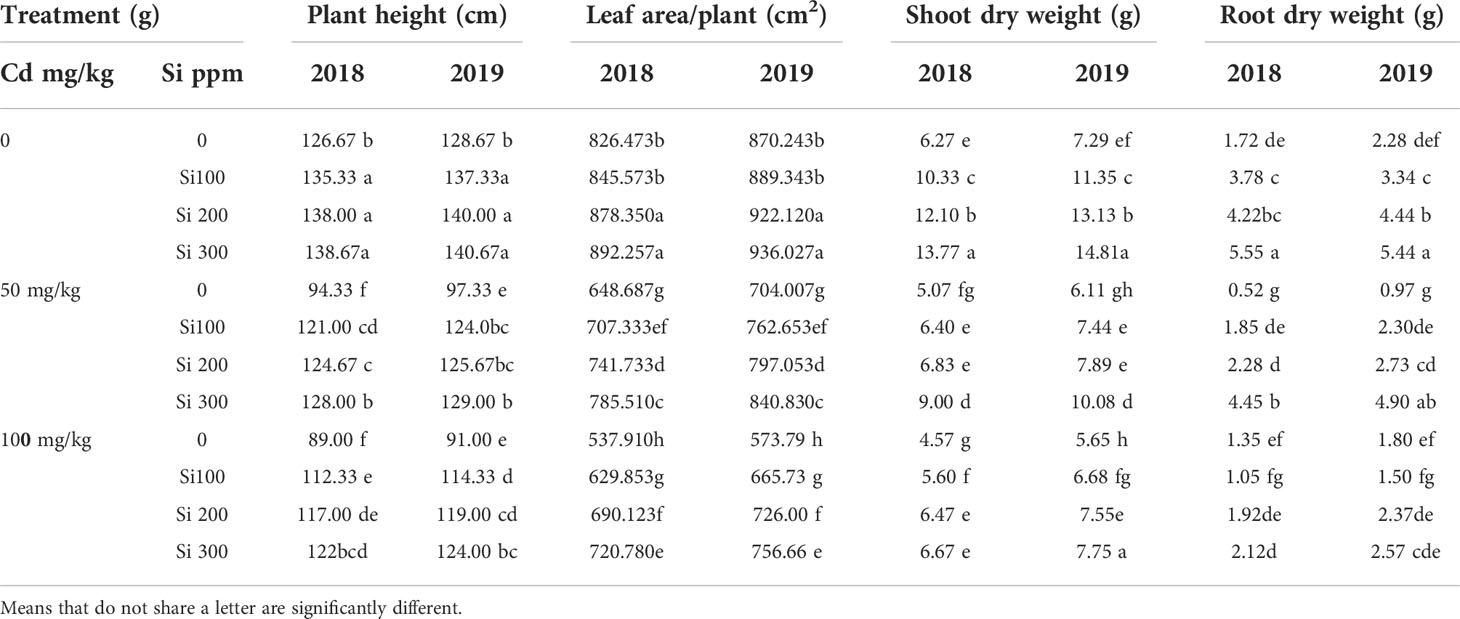
Table 2 Effect of foliar application of silicon on pea (plant height, leaf area, shoot and root dry weight) grown in different concentrations of cadmium (Cd) during 20182019 and 2019/2020 growing seasons.
Cd treatment resulted in a significant decline in seed yield (Table 3). Reduced number of pods per plant, fewer seeds per pod, and lower seed weight were among the factors shown to be responsible for lower seed yield in Cd-treated plants in this study. With increasing Cd concentrations, the number of pods and weight of 100 seeds declined progressively. The foliar spray of Si improved yield and yield components characteristics in Cd-stressed plants in both seasons.
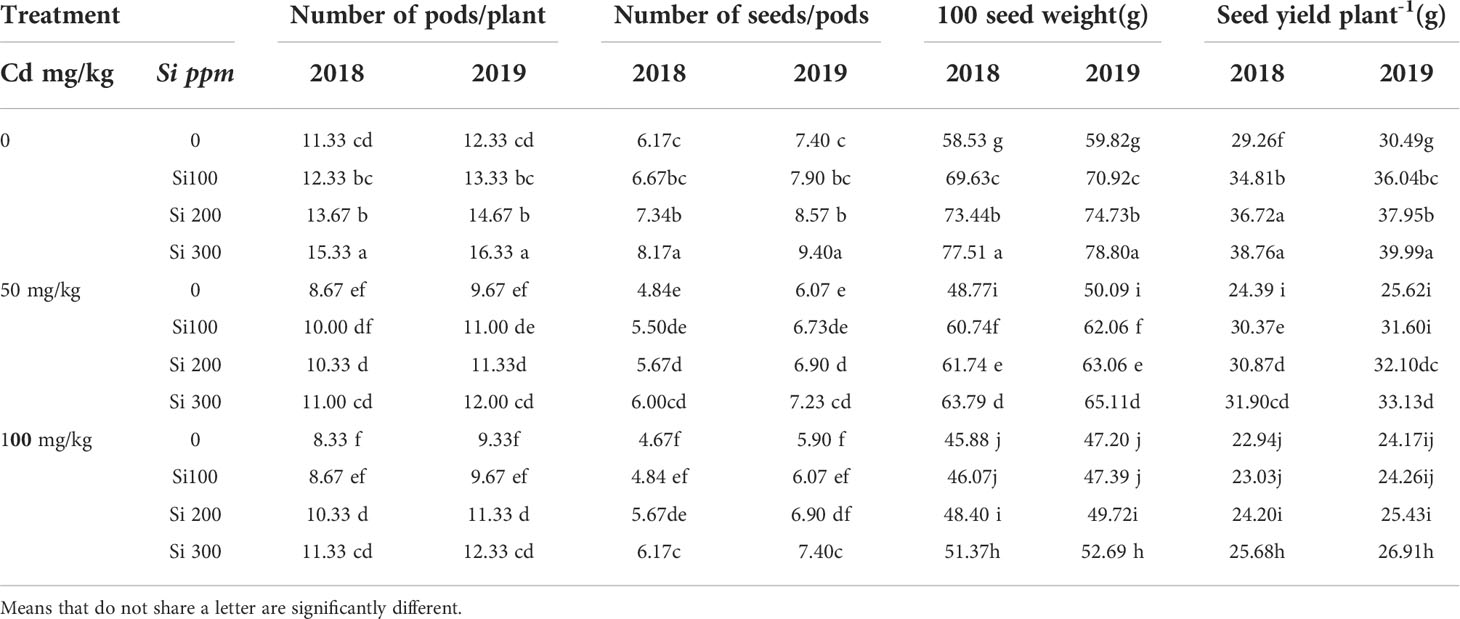
Table 3 Effect of foliar application of silicon on pea (yield and yield components characteristics) grown in different concentrations of cadmium (Cd) during 2018/2019 and 2019/2020 growing seasons.
Data in Table 4 illustrates that the content of chlorophylls (a and b) steadily decreased as Cd concentrations increased. The Si foliar treatment, on the other hand, may mitigate the deleterious effects of Cd imposed on chlorophyll content. Over a Cd dosage range of 50 to 100 (mg/kg), Si addition increased the levels of chlorophylls (a and b) in Cd-stressed plants.
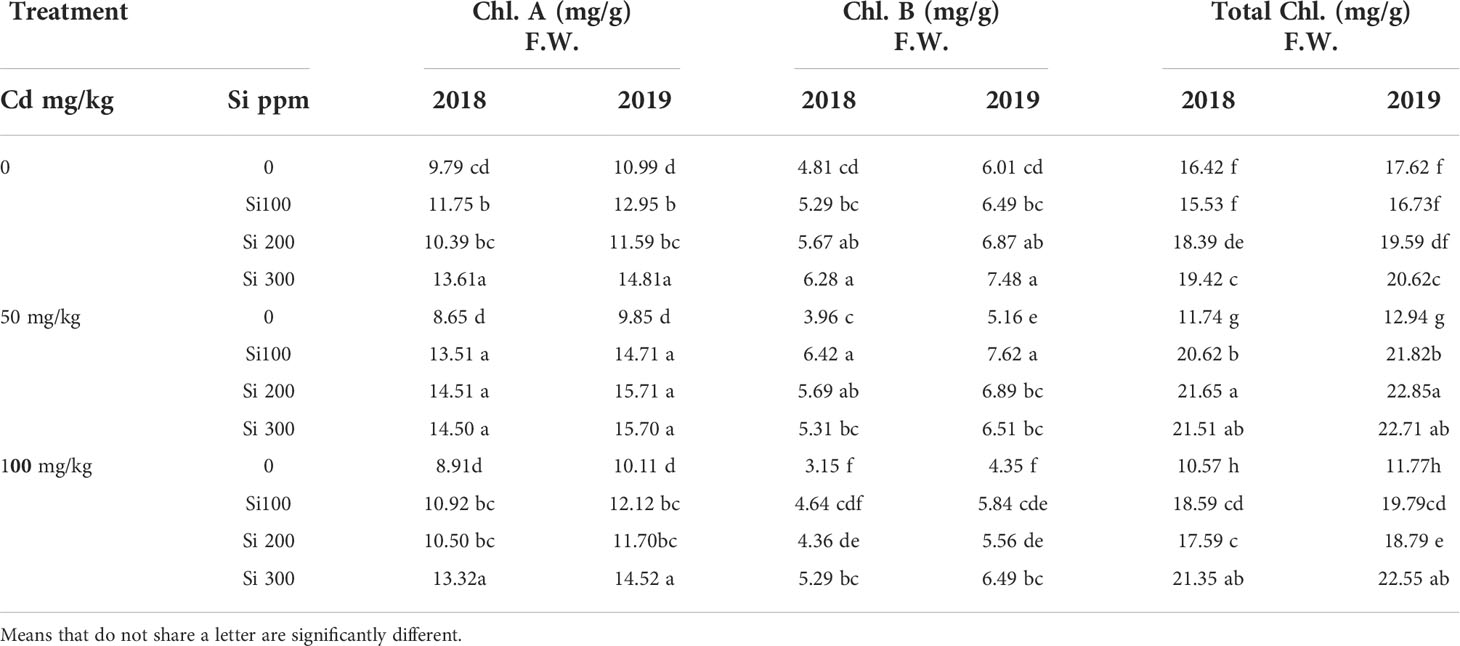
Table 4 Effect of foliar application of silicon on pea (chlorophyll pigments) grown in different concentrations of cadmium (Cd) during 2018/2019 and 2019/2020 growing seasons.
Table 5 shows that Cd accumulation in the root was much greater than in the shoot. The rise in Cd concentration was given intravenously in all plant parts, including the shoot and root. With increasing cadmium concentrations, the level of proteins in pea seeds decreased significantly. Total protein content was also found to be significantly higher in pea plants treated with Si under control conditions. In contrast, Si treatments resulted in a significant increase under Cd stress conditions when compared to the control.
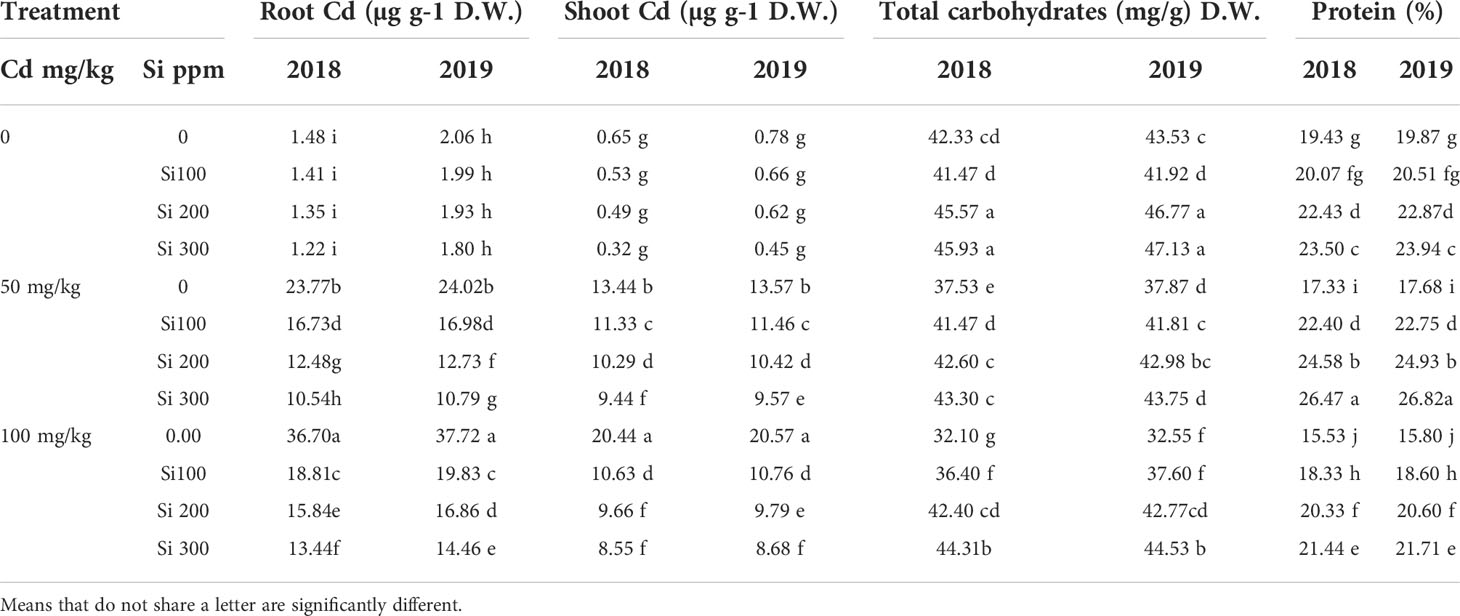
Table 5 Effect of foliar application of silicon on pea (Chemical composition of plant organs) grown in different concentrations of cadmium (Cd) during 2018/2019 and 2019/2020 growing seasons.
With increasing Cd concentrations, there is a significant decrease in total carbohydrate content in pea seedlings. The total carbohydrate content of pea shoots was significantly higher in treated plants with 200 or 300 ppm Si. However, when combined with 50mg of Cd, it had no significant effect.
Cd at 50 and 100 (mg/kg) concentration dramatically increased the levels of ROS in pea leaves (Figures 1A, B). In comparison to the control, Cd concentrations of 50 and 100 (mg/kg) increased MDA by 38.20 and 44.34% for season one, 36.78%, and 42.11% for the second season, respectively. In the line with increased MDA level, the H2O2 level increased by 404.9% and 523.37% for the first season and 294.42% and 380.5% for the second season, respectively in the pea shoot. The use of Si dramatically reduced Cd toxicity by lowering ROS levels in pea shoot. Si at 300 ppm considerably reduced MDA contents by 29.02% and 29.12%, and H2O2 contents by 56.43% and 35.07% under in combination with Cd100(mg/kg) compared to Cd (100 mg/kg) treatment in the two seasons, respectively. In this study, under Cd stress, Si application at 300 ppm increased the activities of POD and CAT (Figure 1), and the increased antioxidant activated the defiance mechanism in plants, thus increasing the Cd resistance of pea plants.
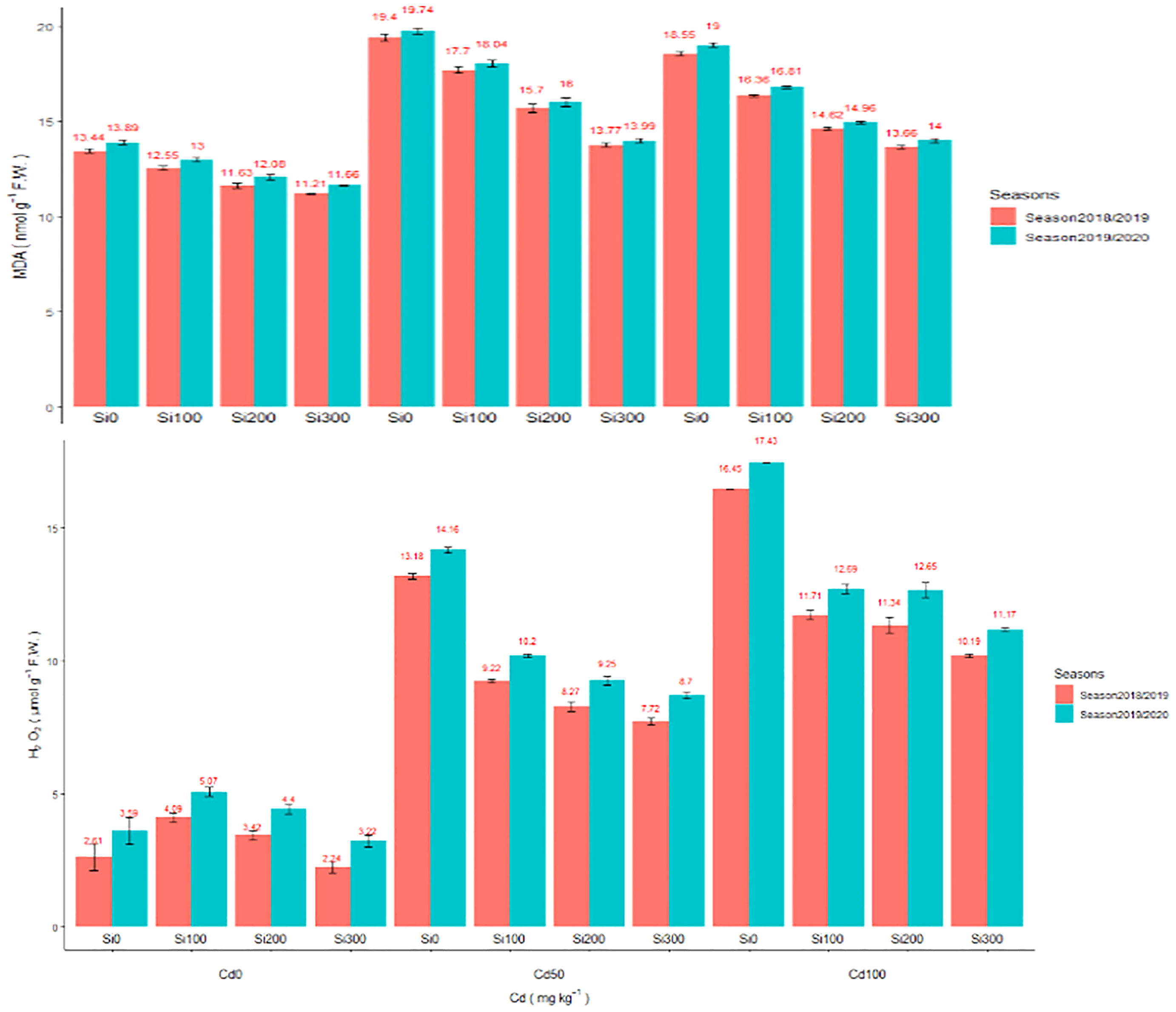
Figure 1 (A) Effect of foliar application of Silicon on pea MDA (nmol g-1 F.W.) grown in different concentrations of cadmium (Cd) during 2018/2019 and 2019/2020 growing seasons. (B) Effect of foliar application of Silicon on H2O2µmol g–1 FW grown in different concentrations of cadmium (Cd) during 2018/2019 and 2019/2020 growing seasons.
Assimilation of Cd elevated the enzymatic activity of oxidoreductase enzymes such as CAT and POD (Figures 2A, B). Compared to the control, Cd concentrations of 50 and 100 (mg/kg) significantly increased antioxidant capacity in the pea plants. In the shoot of pea plants, Cd at 50 and 100 mg/kg increased the activity of CAT enzyme by 70.09% and 186.82%, 62.43%, and 173.33% in both seasons, respectively. POD enzyme activity significantly increased by 41.26%, 28.64%, 77.05%, and 60.77% in the two seasons, respectively. Adding Si at a concentration of 300 ppm under Cd concentrations dramatically reduced Cd heights by increasing enzymatic antioxidant capacity in pea shoots.
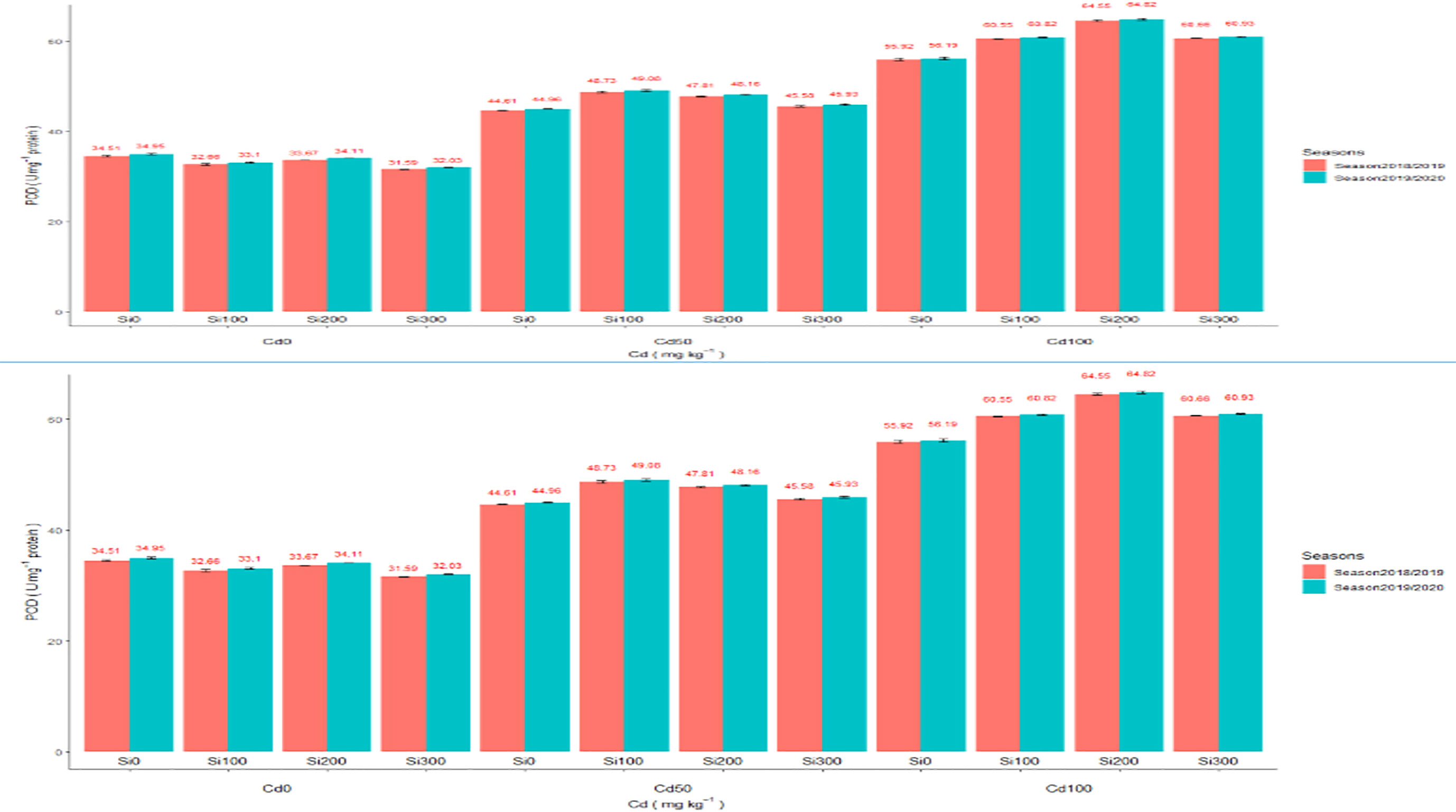
Figure 2 (A) Effect of foliar application of Silicon on pea (CAT U mg–1 protein) grown in different concentrations of cadmium (Cd) during 2018/2019 and 2019/2020 growing seasons. (B) Effect of foliar application of Silicon on pea POD U mg–1 protein grown in different concentrations of cadmium (Cd) during 2018/2019 and 2019/2020 growing seasons.
In Cd-treated plants, root diameter decreased when compared to control plants (Figures 3, 4 and Table 6). In control plant roots, 14 – 16 layers of the cortical cell were present, whereas, in Cd-treated plant roots, it was 7 – 10 layers (Figures 3A, B). In the more external region, refer to specific not exposed to Cd had uniseriate epidermis and hypoderm, whereas Cd increased two or three cell layers in the hypoderm, irregular and loosely arranged cortical cells (Figures 3B). Endodermal cells in treated roots were smaller and thicker walled than those in control plant roots (Figures 4B). A modified DAB-dependent method was used to examine the H2O2 in the roots of control and Cd-treated pea plants (Figure 3B). A distinctive reddish-brown coloration, indicating H2O2 occurrence, was found in root tissues. Furthermore, the H2O2 coloration in the Cd-treated roots’ cells and cell walls was noticeably stronger than in the control roots.
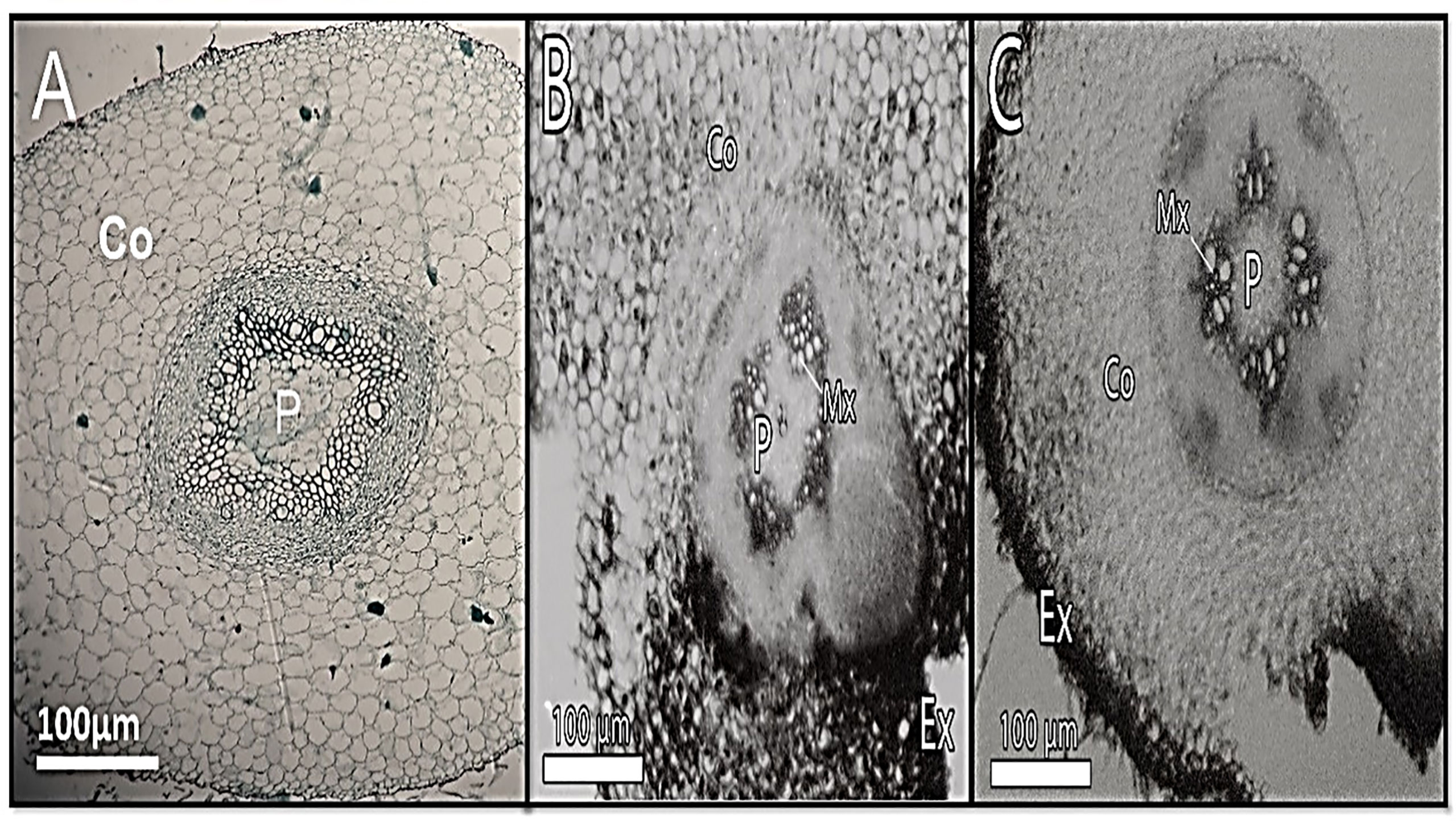
Figure 3 Show transverse section of a pea root. (A) - control, (B) - 100 mg/kg Cd-treated plant (C) - Cd100+Si 300 ppm displaying cortex (co)), pith (p), and meta xylem (Mx), localization of hydrogen peroxide (H2O2) (panel B).

Figure 4 Shows a transverse section of a pea root. (A) - control, (B) - 100 mg/kg Cd-treated plant (C) - Cd100+Si 300 ppm pith (p), and metaxylem (Mx).
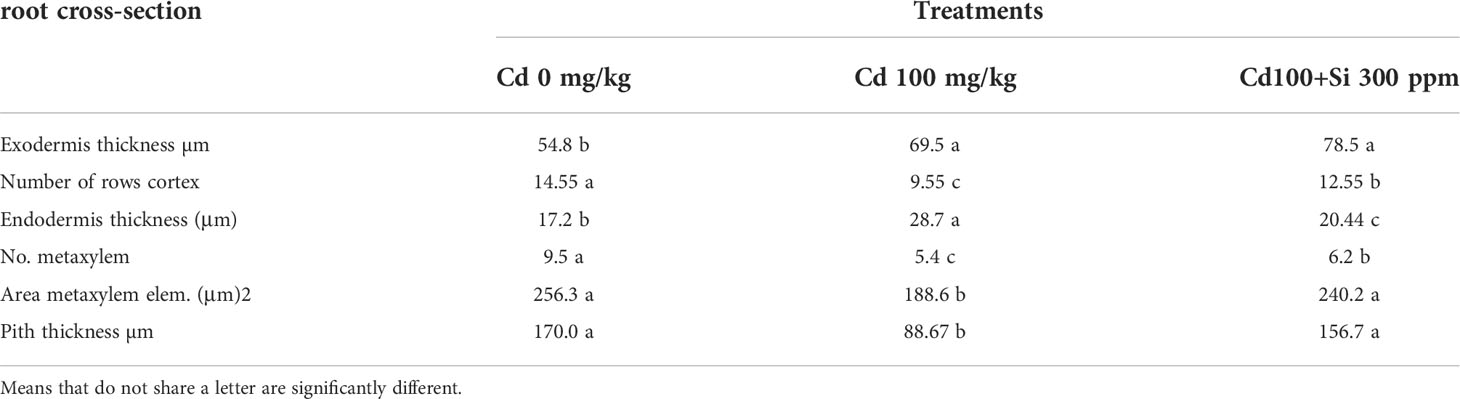
Table 6 Effect of foliar application of silicon on pea root cross-section grown in different concentrations of cadmium.
The diameter of the Cd-treated stem shrank (Figure 5 and Table 7). The epidermal stem cells were thicker and the cortical cells in the Cd-treated stem occupied a smaller area (Figure 5 and Table 7). The number of cortical layers in the experimental plant was 10-12, whereas it was 14 -16 in the control plant. The cambium ring of the Cd-treated stem was thin and the number of vessels was reduced in Cd-treated stems (Micrographs3-B). In comparison to control samples, stem xylem and phloem elements had a more subdued appearance. Phenolic compounds were discovered in higher concentration vessels of Cd-treated stem (Figure 5B).
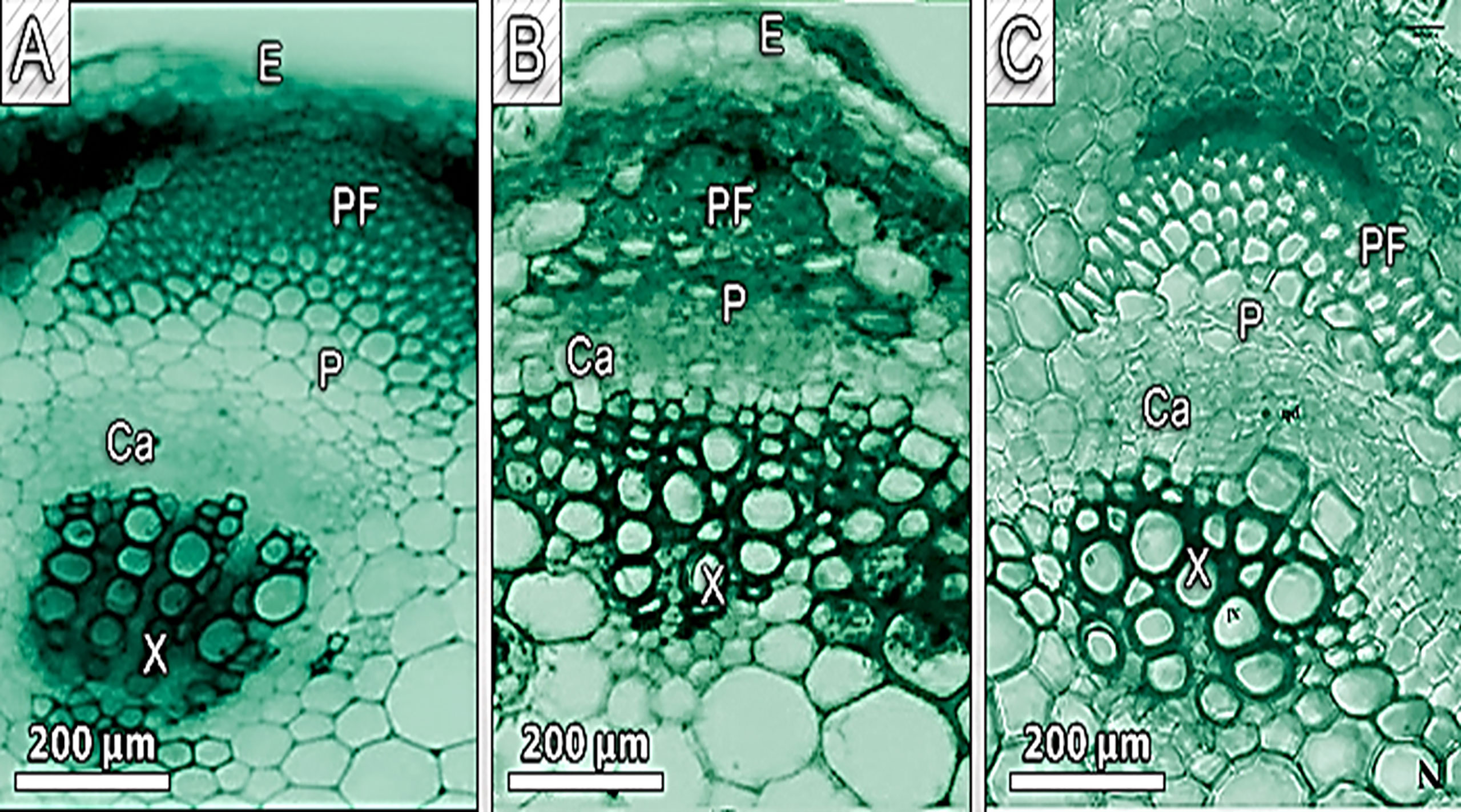
Figure 5 Show a transverse section of a pea stem. (A) - control, (B) - 100 mg/kg Cd-treated plant (C) - Cd100+Si 300 ppm pith (p), and meta vessel (E, epidermal; X, xylem; P, phloem; Ca, cambiumand;Pf, phloemfiber.

Table 7 Effect of foliar application of silicon on pea stem cross-section grown in different concentrations of cadmium.
All Cd concentrations resulted in a highly significant decrease in stomatal area, and stomatal density (the number of stomata per mm2 leaf area) decreased as Cd concentration increased when compared to control plants (Figures 6 and Table 8). Si treatment, in general, resulted in significant increases in the stomatal area when compared to Cd-treated plants.
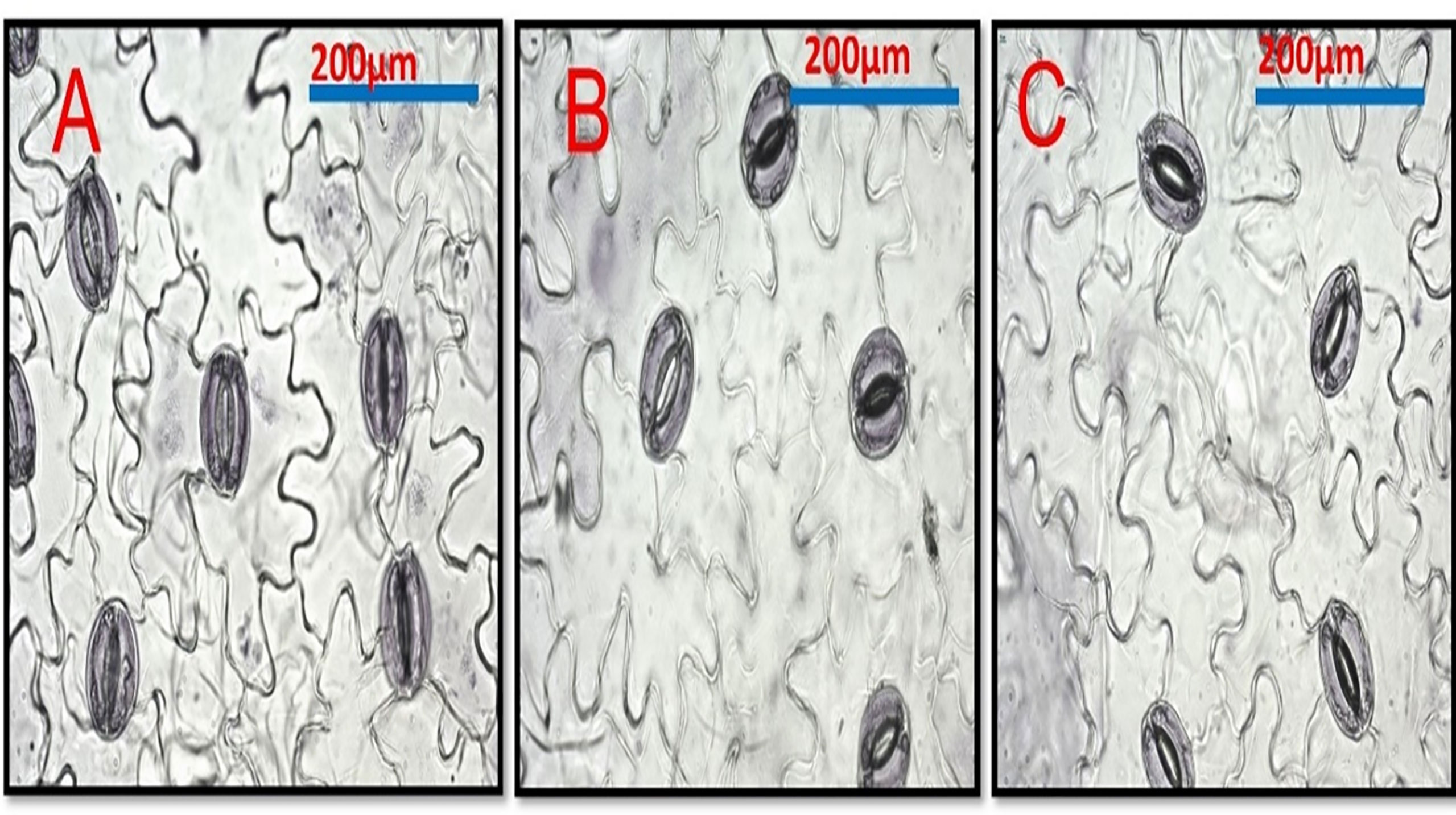
Figure 6 Shows the effect of Cd and Si on the stomata in pea leaf cells. Pea plants were grown with Cd 100 mg/kg. (A) Control leaf. (B) Pea leaf from Cd 100 mg/kg plants (C) Cd 100 mg/kg +Si 300ppm.
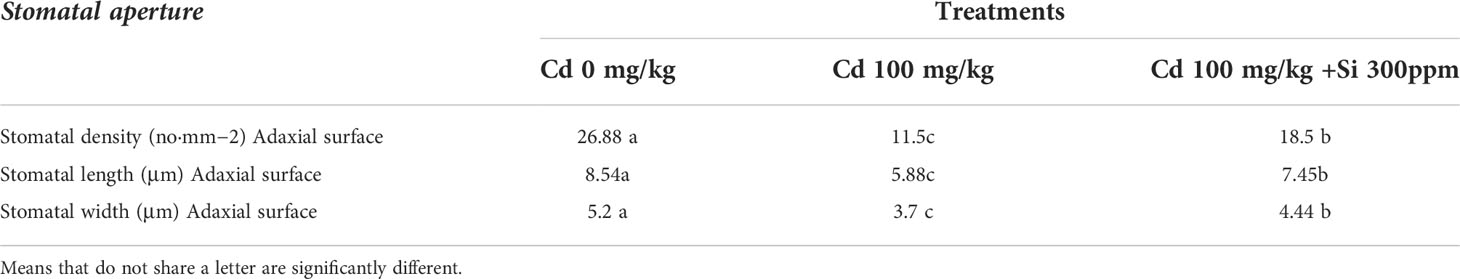
Table 8 Effect of foliar application of silicon on number of stomata and size of stomata guard cell grown in different concentrations of cadmium.
(Figure 6B), compared to control plant roots (Figures 6A). Lignin detection in the endodermis and parenchymatous pith. Also. Lignin deposits in the xylem walls appeared yellow under brightfield microscopy (Figures 6B). Cd-treated plant roots had less parenchymatous pith. (Figures 7) the thicker endodermis is caused by a higher Cd concentration.
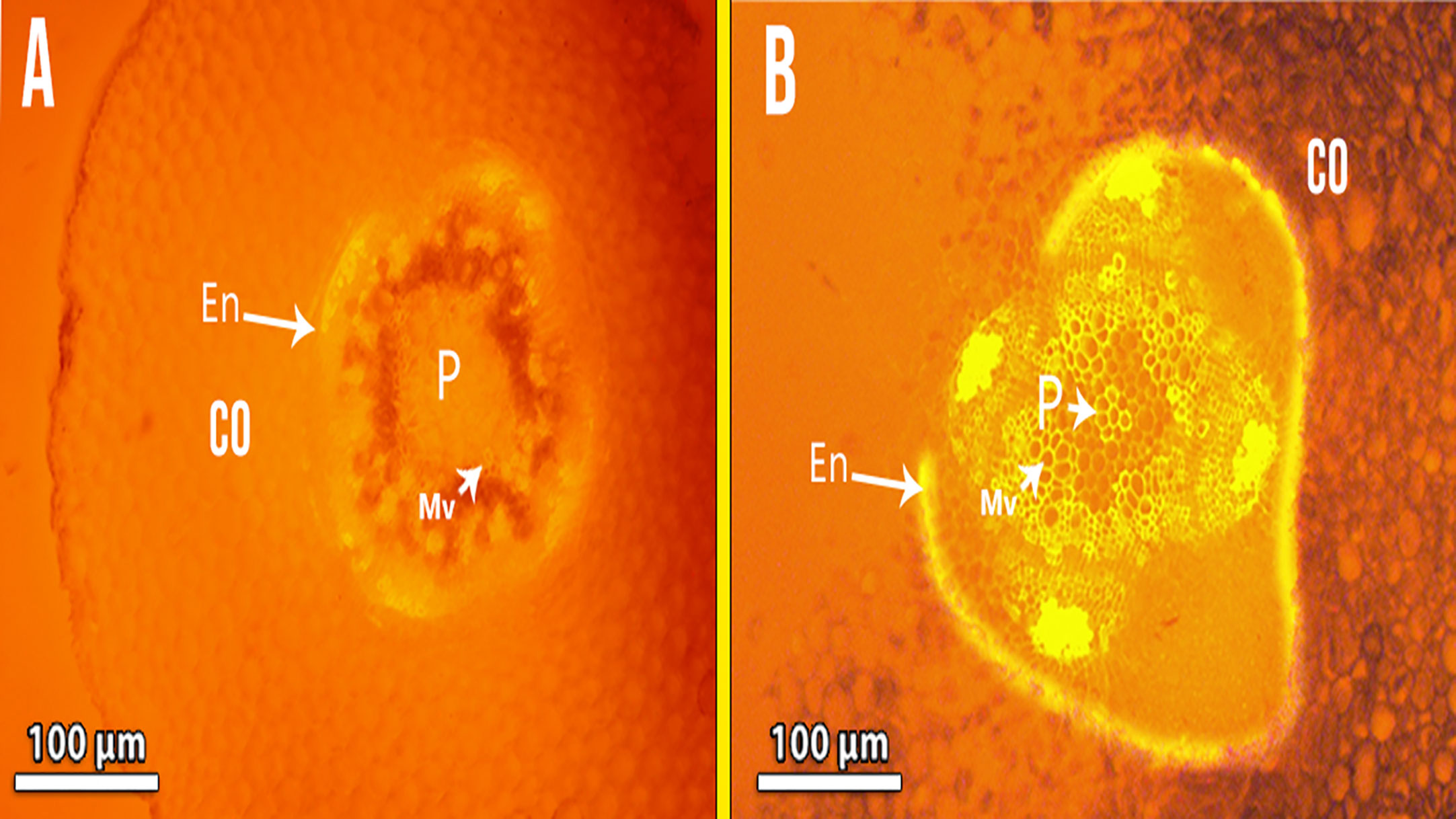
Figure 7 Showing Fluorescence photomicrography for lignin detection in the endodermis and parenchymatous pith in Cd-treated plant roots (B) compared with control (A) Co, cortex; En, endodermis; P, pith; Mv, meta xylem vessels.
Figure 8 show pea stems in normal conditions, where the epidermis’ outer wall has a thin cuticle layer. The epidermis has only one layer and the epidermal cells are tubular and small. Fluorescence findings indicate a thicker cuticle layer found at the outer wall of the epidermis. Pea stem responds to Cd stress by increasing lignification of endodermal cells and xylem vessels (Figure 8B). The highest concentration of Cd caused a lignin deposit in the stem epidermis cells. Cortical parenchyma of stem and pith of pea plants exposed to higher concentrations of Cd had thicker walls than the control. The pith parenchyma metaxylem development was found to be developed in the pith region (Figure 8B).
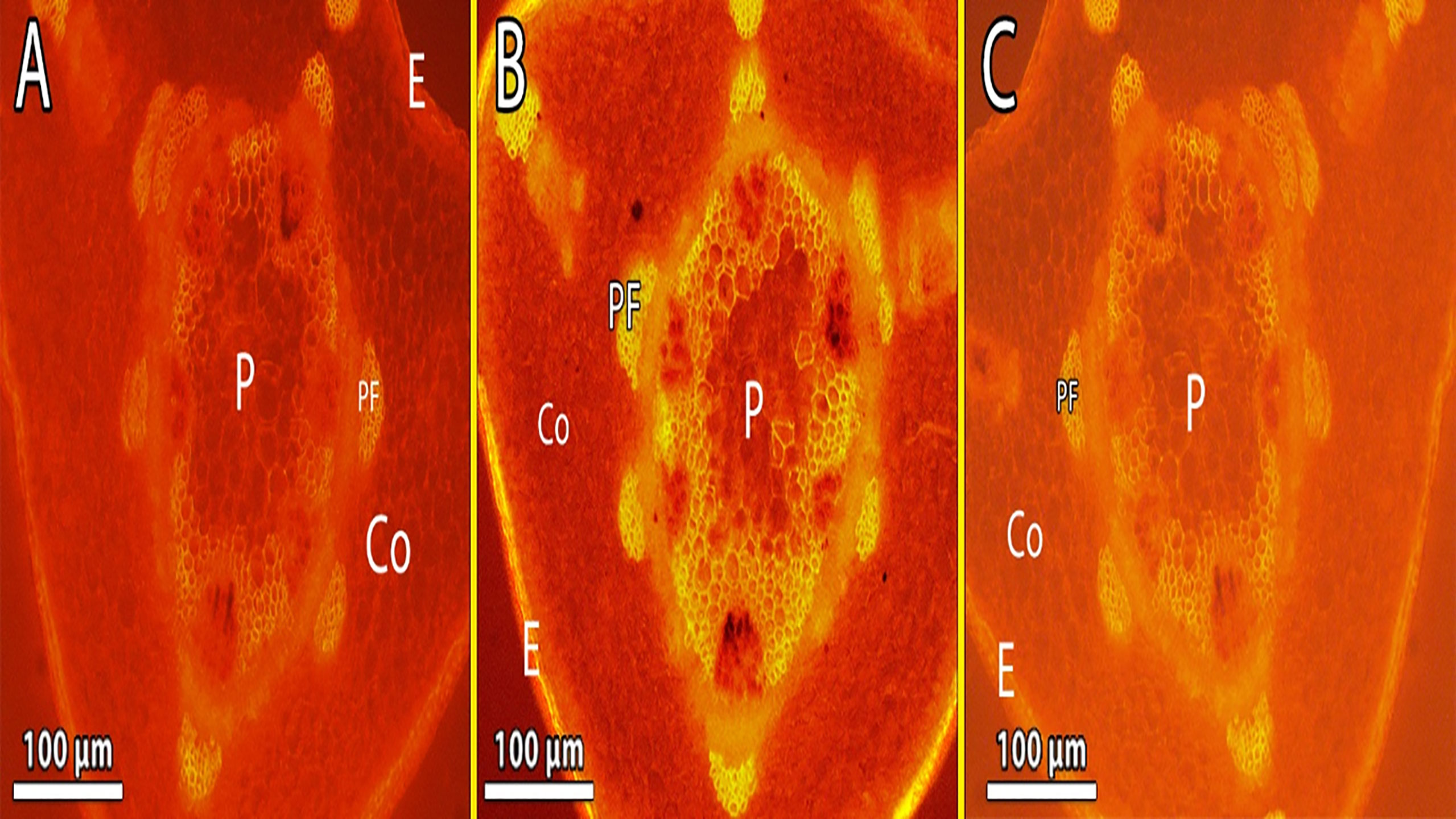
Figure 8 (A–C) Showing the lignin deposit in the stem epidermis cells was observed during the treatment, and Metaxylem development was found to be developed in the pith region exposed to the highest level of Cd (B) lignin deposits in the xylem walls appeared yellow under brightfield microscopy. E, epidermis; Co, cortex; P, pith; Ca, cambium and Pf, phloem fiber.
Pearson correlation matrix among the studied traits was performed and indicated by the color scale (Figure 9). Seed yield per plant (SY) was negatively and significantly correlated with MAD, POD, CAT, H2O2, root Cd, and shoot Cd. However, there was a weak correlation between SY and Chl a. On the other hand, SY was positively correlated with the agronomic characters (Ch. b, root dry weight (RDW), shoot dry weight (SDW), PH, LA, SI, NPP, and NSP) which were positively and significantly correlated with each other. Cadmium content in both root and shoot was positively and significantly correlated with MAD, POD, CAT, and H2O2.

Figure 9 Spearman correlation matrix among malondialdehyde (MDA), antioxidant enzymes peroxidase (POD), antioxidant enzymes catalase (CAT), chlorophyll a (Chl.A), chlorophyll b (Chl.B), total carbohydrates (T.Carb.), Hydrogen peroxide (H2O2), root cadmium (Root Cd), shoot cadmium (Shoot Cd), plant height (PH), root dry weight (RDW), shoot dry weight (SDW), leaf area (LA), weight of 100 seeds (SI), number of pods/plant (NPP), number of seeds/pod (NSP), seed yield/plant (SY).
Heatmap presented a color scale comparison among treatments with respect to the studied traits based on scaled or standardized data (Figure 10). Cells with red color in the heatmap represent high values of the traits, while cells with blue color represent low values of the traits. Before constructing the heatmap, the data was standardized by subtracting the mean of each trait from every single value and dividing the result by the standard deviation of that trait. The standardization process is important to make the comparison feasible as the studied traits were measured in different measuring units. The results reveal that the treatment of Si at 300 under control conditions was the highest in seed yield (SY) represented by red color. The highest SY was positively associated with higher values (red color) of SI, NPP, NSP, LA, PH, SDW, and RDW. While it was negatively associated with lower values (blue color) of root Cd, shoot Cd, MAD, CAT, and POD. The highest values of root Cd, shoot Cd, MAD, and H2O2 occurred in the treatment Cd100_Si0 which own the lowest values (blue color) of PH, LA, Chl. a, Chl. b, total Chl., protein, and T. carbohydrates.
Artificial neural networks (ANNs) are robust mathematical models. They consist of numerous and simple processing interconnected units known as neurons with a structure that is similar to the biological neurons in human brains (Zhongheng, 2016). Neurons in ANNs are arranged in layers such as the input layer, hidden layer, and output layer (Figure 11). The neurons in one layer are connected to the neurons in the next layer, but not to the neurons in the same layer. The strength of the connection weights among two neurons between the layers is used to estimate the relative importance of the inputs to the output and can be expressed as a fraction or %. In the present experiment, a three-layer feed-forward multilayer perceptron neural network (MLP) was used using a back propagation algorithm to model the relationship between yield components and seed yield/plant. The relative importance of yield components to seed yield/plant using three different methods Figure 12 (Ibrahim et al., 2022). The seed index or weight of 100 seeds was the most important yield component to seed yield followed by the number of seeds per pod (NSP), while the number of pods/plant was the least important yield component to seed yield/plant.
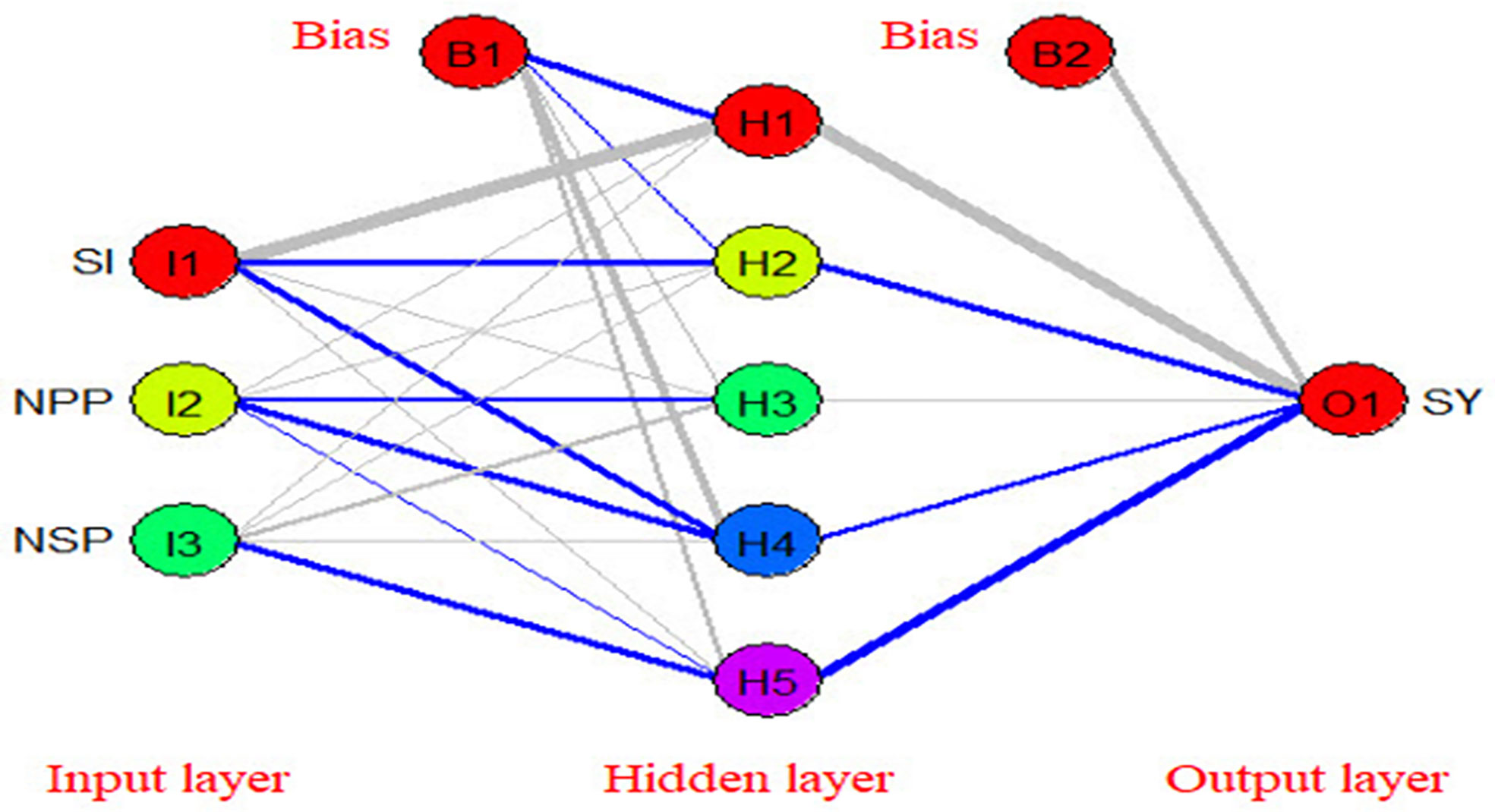
Figure 11 The structure of the used artificial neural network in the present study (blue color represents positive effect while grey color represents negative effect).
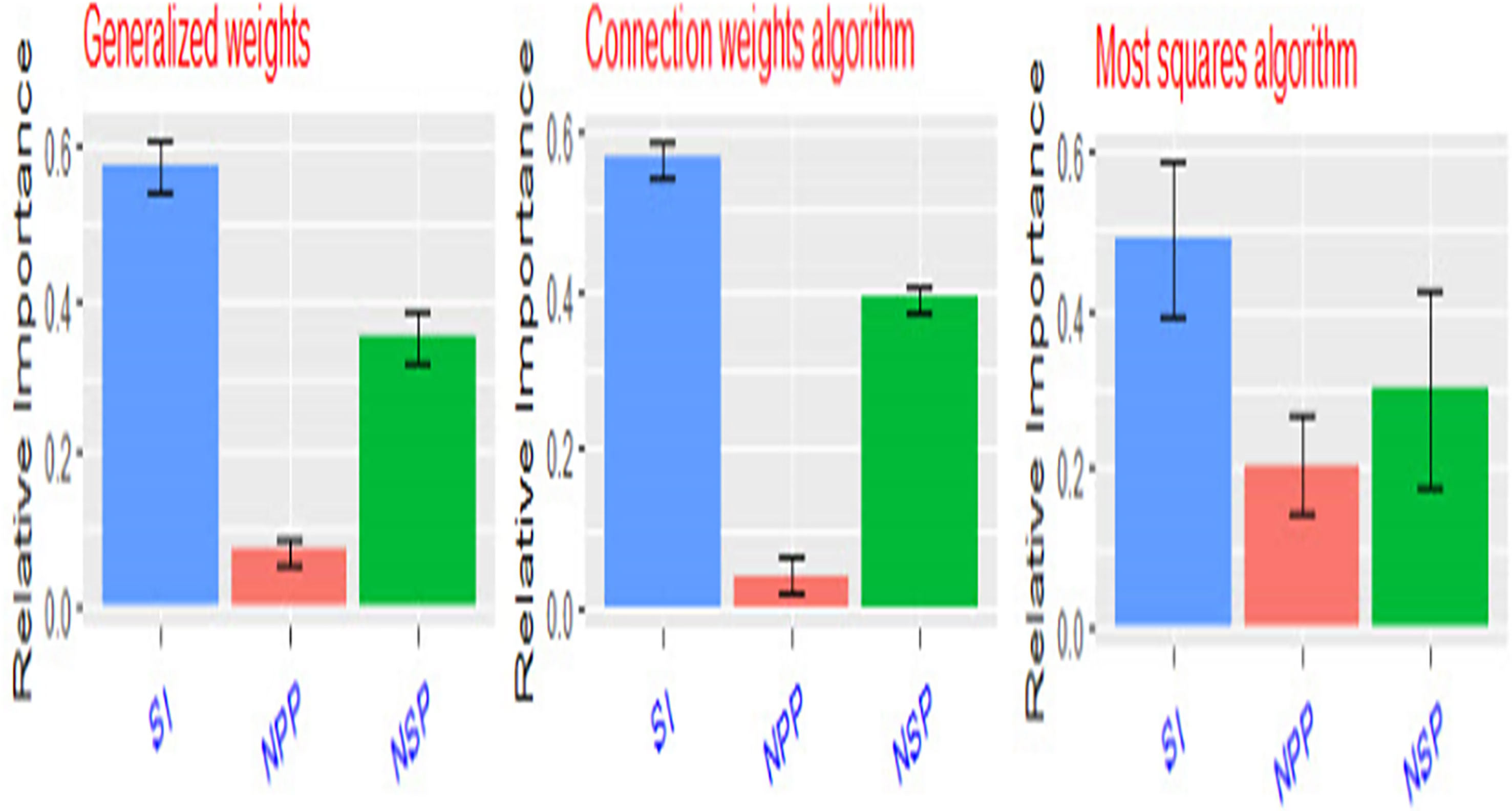
Figure 12 Relative importance of seed yield components (SI, weight of 100 seeds – NPP, number of pods/plant – NSP, number of seeds/pod) to seed yield/plant using three different methods.
Cd toxicity significantly reduced the growth characteristics of pea plants including plant height, leaf area, and shoot, root dry weight. Indeed, the inhibitory effects varied according to plant age, duration of exposure, heavy metal concentration, and type of heavy metal (Sytar et al., 2019; Ahmad et al., 2020). In line with our findings, Cd also reduced the growth of other plant species (EL-Okkiah, 2015; Perveen et al., 2016; Sun et al., 2016; Chen et al., 2019). In this regard, Cd stress inhibits root and stem cell growth by inhibiting cell elongation and cell division via an inhibition activity of the glycolysis pathway (Dalla Vecchia et al., 2005). Cd toxicity minimizes smitotic division of meristematic cells, which leads to reduced root length and dry biomass, and enhanced root diameter (Seth et al., 2008; Gratao et al., 2009; Abbas et al., 2017). Cd exposure also negatively affects multifactorial levels such as decreased nutrient uptake, photosynthetic efficiency, amino acids, protein metabolism, and water relations (Haider et al., 2021). In many agronomic crops such as pea, maize, barley (Popova et al., 2012), mungbean (Wahid et al., 2008), and wheat (Abbas et al., 2017), short- and long-term exposure to Cd toxicity inhibits photosynthetic activity. In this context, a 35.9% reduction in photosynthetic pigments was achieved by (Mayonde et al., 2021).
On the other hand, Si concentration in plants positively correlated with plant growth. Hussain et al., 2019 considered Si to be a growth regulator and played a defensive role against various biotic and abiotic stresses in previous research. Furthermore, Si application may have contributed to improved pea growth performance under stress and non-stress conditions by increasing photosynthesis as indicated by increased chlorophyll levels (Galal et al., 2021). The photosynthetic pigments (Chl a and Chl b) were significantly reduced by Cd toxicity. Cd stress causes a decrease in chlorophyll content (Gaballah and; Rady, 2012 and EL-Okkiah, 2015). Chlorophyll value is diminished in cadmium-treated plants due to inhibition of its biosynthesis as well as Cd-induced lower Mg and Fe concentrations (Gill et al., 2013). It is known that Si increases cellulose, hemicellulose, and lignin contents in rice cell walls in rice plants (Zhang et al., 2015). Higher cellulose and hemicellulose depositions due to Si result in forming a cell wall and consequentially inhibit heavy metal absorption (Zhang et al., 2020). Sugars are the most important soluble constituent that assists plants with osmotic adjustment. Sugars also provide energy to rapidly growing cells as well as the carbon skeletons needed to synthesize organic compounds (Taiz and Zeiger, 2010).
It is well known that heavy metal including Cd stress causes oxidative damage to plants, which can be exacerbated both directly and indirectly by increasing cellular ROS concentrations and decreasing cellular antioxidant capacity (Livingstone, 2001). Here we also reported increased lipid peroxidation (MDA), consistently, high MDA levels in pea shoots from Cd-treated plants were recorded (Ahmad et al., 2016; Chen et al., 2018). The observed increases were correlated with increased H2O2 levels. In this regard, Cd toxicity caused cell death in tobacco cells due to the accumulation of NADPH-oxidase and the over-production of H2O2 in fatty acids (Gill and Tuteja, 2010). Cadmium toxicity may stimulate ROS development in the mitochondrial electron transfer chain (Heyno et al., 2008).
Regardless of the fact that Cd is not an oxidative metal and cannot produce ROS directly through Fenton and Haber-Weiss reactions, indirect ROS production is common in Cd-exposed plants (Shahid et al., 2016). ROS can react with proteins, lipids, and nucleic acids at high concentrations, causing structural and functional changes including lipid peroxidation, membrane leakage, DNA breakage, and mutation (Apel and Hirt, 2004; Andresen and Küpper, 2013). They have the ability to oxidize proteins, fatty acids, and nucleic acids, which frequently results in cell structure changes and mutagenesis (Younis et al., 2016). Cd stress also results in damage to plant membranes and the destruction of cell biomolecules and organelles (Abbas et al., 2017).
To cope with Cd-induced oxidative stress, Pea plants increased the activities of antioxidant enzymes like POD and CAT. Interestingly, foliar treatment with Si further increased these activities under Cd stress conditions. Similarly, Cd-induced antioxidant enzyme activation, possibly through gene expression modulation or Cd-induced inhibition of enzyme inhibitors (Rahman et al., 2017). Another mechanism by which Si alleviated Cd stress in plants was stimulating antioxidant systems in plants (Shi et al., 2005). These antioxidants worked together to alleviate oxidative injury in Si treatment (Shen et al., 2010).
Therefore, Cd may indirectly contribute to the production of ROS through disruption in the chloroplasts of leaves (Gallego et al., 2012).
Cd accumulation in the root was much greater than in the shoot. This finding is consistent with previous research on various plant species (Gaballah and Rady, 2012; Mostofa et al., 2015; Ahmad et al., 2016). Because Cd accumulated in the root is incapacitated by the cell wall and extracellular carbohydrates, higher Cd content in the root as compared to the shoot is one of the most important defense mechanisms of plants against Cd toxicity (Rahman et al., 2017). (Ahmad et al., 2016). Si application may reduce Cd accumulation in shoots; this indicates that silicon significantly negatively impacted Cd transport from roots to shoots, limiting it to root tissues. Similar findings have been reported in other monocotyledon species, including rice (Shi et al., 2005 and Zhang and Ge 2008). Kastori et al. (1992) discovered that the content of soluble proteins in Helianthus annus decreased with increasing heavy metal concentration. Protein content may be affected by heavy metals due to: (i) increased protein hydrolysis, resulting in a lower concentration of soluble proteins (ii) Lead’s catalytic activity (Bhattacharyya and Choudhuri, 1997); (iii) Protein synthesis decreasing under all stress conditions. (El-Beltagi et al., 2010; Ibrahim et al., 2012). Sugar levels in pea shoots were significantly higher in Si-treated plants, but not under Cd stress (50 mg/Kg soil).
Heavy metal can induce alterations in anatomical parameters. Furthermore, changes in anatomical traits were rather highly reliant on Cd concentration, with the changes becoming more noticeable as Cd concentration increased. Thus, the current study found that increasing concentrations of Cd had a negative impact on the anatomical structures of the pea, despite the plant’s preventing and mitigating such effects by modifying cellular structures to some extent. Among anatomical changes, degraded and smaller mesophyll tissue, the disintegration of parenchymatous tissues, cortical tissue loosening in roots of Phaseolus vulgaris L. (Talukdar, 2013), shriveling and cell breakdown, which resulted in cortical cell shape loss in Phaseolus aureus (Singh and Agrawal 2007) and reduced cortical thickness in maize roots (Gowayed and Almaghrabi, 2013). Similarly, Cd accumulation in Pteris vittata caused root cortical and endodermal cell breakdown or reduction (Sridhar et al., 2007; Armendariz et al., 2016). Heavy metals can affect the balance of root hormones, affecting cellular tissue differentiation and the number of cells in these tissues (Sandalio et al., 2001). Furthermore, the increased number of tracheary elements and decreased metaxylem area alter water storage capacity. Larger diameter vessels are more productive but less safe, leading to an increased risk of blisters (Dickison, 2000).
Moreover, Thalassia hemprichii exposed to Pb had wider cortical air spaces, and rising Pb concentration increased exodermal and endodermal cell wall thickness (Tupan and Azrianingsih, 2016). Cu promoted endodermal cell wall thickening in the bean (Bouazizi et al., 2010) and exposure separated the vascular bundles and pith regions (Talukdar, 2013).
Even though xylem conduits are tight and less susceptible to damage due to lignification, breakdown, loss of shape, or change in diameter have been observed in Cicer arietinum roots after Cr stress (Mondal et al., 2013). Lignin deposition in cortical cell walls can aid in the repairs of root turgescence by reducing water loss from the root via refluxas an adaptive mechanism that aids in root architecture stability (Hose et al., 2001).
Here, Cd strongly induced stomatal closure and decreased stomatal conductance in pea leaves. In line with these findings, several studies have found that Cd strongly induces stomatal closure in different plant species (Sun et al., 2016; Sadeghipour, 2018). Stomata closed individually under Cd stress, regardless of water status. Furthermore, Cd caused stomatal closure as a result of Cd entry into the guard cells, and it reduced the number of stomata per unit area (Ismael et al., 2019). Cd toxicity reduced stomatal conductance and cell division (Abbas et al., 2017). The following mechanisms of Si inhibition of metal transport in plants have been proposed: (1) thickening Casparian strips (2) depositing lignin in the cell walls and (3) depositing Si in the cell (Shi et al., 2005; Da Cunha and Do Nascimento, 2009).
Cd has a toxic effect on pea plants, according to our research. Cd toxicity altered root and stem growth, anatomy, and biochemistry. Plants exposed to Cd toxicity showed reduced growth, which was correlated with Cd accumulation and reduced chlorophyll and primary metabolites such as sugars and protein. Additionally, Cd significantly oxidative damage (MDA and H2O2), and to cope with stress plants increased their enzyme activity (CAT, POD) compared to controls. Higher Cd concentrations appear to cause anatomical abnormalities such as a distinct vascular system, abnormal lignification in the pith parenchyma, and enlarged cortical cells, according to our findings. The use of Si effectively reduced the oxidative burst caused by Cd toxicity. Therefore, we recommend using Si at 300 ppm as a promising approach to reduce Cd toxicity
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
All authors have contributed equally to the research and analysis of the various results sections within the review. All have corrected and modified the different versions of the manuscript as prepared by the corresponding and senior authors. All authors read and approved the final manuscript.
Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R214), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
The authors are grateful to the Researchers Supporting Project number (PNURSP2022R214), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbas, T., Rizwan, M., Ali, S., Adrees, M., Zia-ur-Rehman, M., Qayyum, M. F., et al. (2017). Effect of biochar on alleviation of cadmium toxicity in wheat (Triticum aestivum l.) grown on cd-contaminated saline soil. Environ. Sci. pollut. Res. 25, 25668–25680. doi: 10.1007/s11356-017-8987-4
Abd-Allah, E. F., Hashem, A., Alam, P., Ahmad, P. (2019). Silicon alleviates nickel-induced oxidative stress by regulating antioxidant defense and glyoxalase systems in mustard plants. J. Plant Growth Regul. 38, 1–14.
Ahmad, P., Abdel Latef, A. A., Abd_Allah, E. F., Hashem, A., Sarwat, M., Anjum, N. A., et al. (2016). Calcium and potassium supplementation enhanced growth, osmolyte secondary metabolite production, and enzymatic antioxidant machinery in cadmium-exposed chickpea (Cicer arietinum l.). Front. Plant Sci. 7, 513. doi: 10.3389/fpls.2016.00513
Ahmad, R., Ali, S., Rizwan, M., Dawood, M., Farid, M., Hussain, A., et al. (2020). Hydrogen sulfide alleviates chromium stress on cauliflower by restricting its uptake and enhancing antioxidative system. Physiol. Plant 168, 289–300. doi: 10.1111/ppl.13001
Ahmad, I., Akhtar, M. J., Zahir, Z. A., Mitter, B. (2015). Organic amendments: effects on cereals growth and cadmium remediation. Int. J. Environ. Sci. Technol. 12, 2919–2928.
Alloway, B. J. (2013). “Sources of heavy metals and metalloids in soils,” in Alloway BJ (ed) heavy metals in soils–trace metals and metalloids in soils and their bioavailability (Netherlands: Springer), p 11–p 50.
Andresen, E., Küpper, H. (2013). Cadmium toxicity in plants. cadmium: from toxicity to essentiality (Dordrecht: Springer), p 395–p 413.
A.O.A.C (1995). Official methods of analysis 16 the Washington D.C (USA: Association of Official AgriculturalChemists).
Apel, K., Hirt, H. (2004). Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55, 373–399. doi: 10.1146/annurev.arplant.55.031903.141701
Armendariz, A. L., Talano, M. A., Travaglia, C., Reinoso, H., Wevar Oller, A. L., Agostini, E. (2016). Arsenic toxicity in soybean seedlings and their attenuation mechanisms. Plant Physiol. Biochem. 98, 119–127. doi: 10.1016/j.plaphy.2015.11.021
Ayangbenro, A. S., Babalola, O. O. (2017). A new strategy for heavy metal polluted environments: a review of microbial bio sorbents. Int. J.Environ. Res. Public Health 14, 94–109. doi: 10.3390/ijerph14010094
Bhattacharyya, M., Choudhuri, M. A. (1997). Effect of Pb and cd on the biochemical changes in the leaves of terrestrial (Vigna) and aquatic (Hydrilla) plants under solution culture. Indian J. Plant Physiol. 32, 99–103.
Bouyoucos, G. J. (1965). Hydrometer method for making particle size analysis of soils. Agron. J. 54, 464–465. doi: 10.2134/agronj1962.00021962005400050028x
Bouazizi, H., Jouili, H., Geitmann, A., Ferjani, E. E. I. (2010). Copper toxicity in expanding leaves of Phaseolus vulgaris L.: antioxidant enzyme response and nutrient element uptake. Ecotox. Environ. Safe. 73, 1304–1308.
Chapman, H. D., Pratt, P. F. (1982). Method and of analysis of soil, plant and water. 2nd Ed (California: California University Agricultural Division), p 170.
Chen, D., Chen, D., Xue, R., Long, J., Lin, X., Lin, Y., et al. (2019). Effects of boron, silicon and their interactions on cadmium accumulation and toxicity in rice plants. J. Hazard. Mater. 367, 447–455. doi: 10.1016/j.jhazmat.2018.12.111
Da Cunha, K. P. V., Do Nascimento, C. W. A. (2009). Silicon effects on metal tolerance and structural changes in maize (Zea mays l.) grown 210 f. guntzer et. al. on a cadmium and zinc enriched soil. Water Air SoilPollution 197, 323–330. doi: 10.1007/s11270-008-9814-9
Dalla Vecchia, F., Rocca, N. L., Moro, I., De Faveri, S., Andreoli, C., Rascio, N. (2005). Morphogenetic, ultrastructural and physiological damages suffered by submerged leaves of Elodea canadensis exposed to cadmium. Plant Sci. 168, 329–338. doi: 10.1016/j.plantsci.2004.07.025
Dickison, W. C. (2000). “Ecological anatomy,” in Integrative plant anatomy. Ed. Dicksison, W. C. (San Diego, CA, USA: Academic Press).
Duncan, D. B. (1955). Multiple ranges and multiple f-test. Biometrics 11, 1–42. doi: 10.2307/3001478
Eid, E. M., Shaltout, K. H., Alamri, S. A. M., Sewelam, N. A., Galal, T. M., Brima, E. I. (2019). Prediction models for evaluating heavy metal uptake by pisum sativum l. @ in soil amended with sewage sludge. J. Environ. Sci. Health Part A 55, 151–160 1–10. doi: 10.1080/10934529.2019.1668217
El-Beltagi, H. S., Mohamed, A. A., Rashed, M. M. (2010). Response of antioxidative enzymes to cadmium stress in leaves and roots of radish. Not. Sci. Biol. 2 (4), 76–82. doi: 10.15835/nsb245395
EL-Okkiah, Samira A.F. (2015) Phytotoxic effects sewage water on growth, yield, physiological, biochemical and anatomical parameters of faba bean (Vicia faba L.). Annals of Agric. Sci., Moshtohor 53 (4) 597–614.
Farooq, M. A., Detterbeck, A., Clemens, S., et al. (2016). Silicon-induced reversibility of cadmium toxicity in rice. J. Exp. Bot. 67, 3573–3585. doi: 10.1093/jxb/erw175
Food and Agriculture Organization of United Nations FAO (2014) Stat Database Internet. Available at: http://faostat.fao.org/default.aspx.
Gaafar, A. Z., Ghdan, A. A., Siddiqui, M. H., Al-Whaibi, M. H., Basalah, M. O., Ali, H. M., et al. (2012). Influence of sulfur on cadmium (Cd) stress tolerance in triticum aestivum l. Afr. J. Biotechnol. 11 (43), 10108–10114. doi: 10.5897/AJB11.4206
Gaballah, M. S., Rady, M. M. (2012). Salicylic acid mitigated cadmium toxicity by attenuating theoxidative stress in pea (Pisum sativum l.) plants. Int. J. Biological Ecol. Environ. Sci. 1 (4), 159–165.
Gadallah, M. A. A., Sayed, S. A. (2014). Impacts of different water pollution sources on antioxidant defense ability in three aquatic macrophytes in assiut province, Egypt. J. Stress Physiol. Biochem. 10, 47–61.
Galal, T. M., Hassan, L. M., Ahmed, D. A., Alamri, S. A. M., Alrumman, S. A., Eid, E. M. (2021). Heavy metals uptake by the global economic crop (Pisum sativum l.) grown in contaminated soils and its associated health risks. PloS One 16 (6), e0252229. doi: 10.1371/journal.pone.0252229
Galal, T. M., Shehata, H. S. (2015a). Impact of nutrients and heavy metals capture by weeds on the growth and production of rice (Oryza sativa l.) irrigated with different water sources. Ecol. Indic. 54, 108–115. doi: 10.1016/j.ecolind.2015.02.024
Galal, T. M., Shehata, H. S. (2015b). Bioaccumulation and translocation of heavy metals by plantago major l. grown in contaminated soils under the effect of traffic pollution. Ecol. Ind. 48, 244–251. doi: 10.1016/j.ecolind.2014.08.013
Gallego, S. M., Pena, L. B., Barcia, R. A., Azpilicueta, C. E., Iannone, M. F., Rosales, E. P., et al. (2012). Unravelling cadmium toxicity and tolerance in plants: insight into regulatory mechanisms. Environ. Exp. Bot. 83, 33–46. doi: 10.1016/j.envexpbot.2012.04.006
Gasco, G., Lobo, M. C. (2007). Composition of Spanish sewage sludge and effects on treated soil and olive trees. Waste Manage 27, 1494–1500. doi: 10.1016/j.wasman.2006.08.007
Gill, S. S., Anjum, N. A., Hasanuzzaman, M., Gill, R., Trivedi, D. K. (2013). Glutathione and glutathione reductase: a boon in disguise for plant abiotic stress defense operations. Plant Physiol. Biochem. 70, 204–212. doi: 10.1016/j.plaphy.2013.05.032
Gill, S. S., Tuteja, N. (2010). Reactive oxygen species and antioxidant machinery in abioticstress tolerance in crop plants. Plant Physiol. Biochem. 48, 909–930. doi: 10.1016/j.plaphy.2010.08.016
Gowayed, S. M. H., Almaghrabi, O. A. (2013). Effect of copper and cadmium on germination and anatomical structure of leaf and root seedling in maize (Zea mays l.). Aust. J. Basic Appl. Sci. 7 (1), 548–555.
Gratao, P. L., Monteiro, C. C., Rossi, M. L., Martinelli, A. P., Peres, L. E. P., Medici, L. O., et al. (2009). Differential ultrastructural changes in tomato hormonal mutants exposed to cadmium. Environ. Exp. Bot. 67, 387–394. doi: 10.1016/j.envexpbot.2009.06.017
Haider, F. U., Liqun, C., Coulter, J. A., Cheema, S. A., Wu, J., Zhang, R., et al. (2021). Cadmium toxicity in plants:Impacts and remediation strategies. Ecotoxicol. Environ. Saf. 211, 111887. doi: 10.1016/j.ecoenv.2020.111887
Hati, K. M., Biswas, A. K., Bandyopadhyay, K. K., Misra, A. K. (2007). Soil properties and crop yields on a vertisol in India with application of distillery effluent. Soil Till. Res. 92, 60–68. doi: 10.1016/j.still.2006.01.011
Heyno, E., Klose, C., Krieger-Liszkay, A. (2008). Origin of cadmium induced reactive oxygen species production: mitochondrial electron transfer versus plasma membrane NADPH oxidase. New Phytol. 179, 687–699. doi: 10.1111/j.1469-8137.2008.02512.x
Hooda, P. S., Miller, A., Edwards, A. C. (2007). The distribution of automobile catalysts-cast platinum, palladium and rhodium in soils adjacent to roads and their uptake by grass, Sci. Total Environ. 384, 384–392.
Hose, E., Clarkson, D. T., Steudle, L., Hartung, W. (2001). The exodermis: a variable apoplástica barrier. J. Exp. Bot. 52, 2245–2264. doi: 10.1093/jexbot/52.365.2245
Hussain, I., Ashraf, M. A., Rasheed, R., Asghar, A., Sajid, M. A., Iqbal, M. (2015). Exogenous application of silicon at the boot stage decreases accumulation of cadmium in wheat (Triticum aestivum l.) grains. Braz. J. Bot. 38, 223–234. doi: 10.1007/s40415-014-0126-6
Hussain, A., Ali, S., Rizwan, M., Zia-ur-Rehman, M., Yasmeen, T., Hayat, M. T., et al. (2019). Morphological and physiological responses of plants to cadmium toxicity. In Cadmium Toxicity Tolerance Plants pp, 47–72. doi: 10.1016/B978-0-12-814864-8.00003-6
Ibrahim, N. M., Eweis, E. A., El-Beltagi, H. S., Abdel-Mobdy, Y. E. (2012). The effect of lead acetate toxicity on experimental male albino rat. Asian Pac. J. Trop. BioMed. 2 (1), 41–46. doi: 10.1016/S2221-1691(11)60187-1
Ibrahim, O. M., El-Gamal, E. H., Darwish, K. M., et al. (2022). Modeling main and interactional effects of some physiochemical properties of Egyptian soils on cation exchange capacity via artificial neural networks. Eurasian Soil Sc 55, 1052–1063. doi: 10.1134/S1064229322080051
Ismael, M. A., Elyamine, A. M., Moussa, M. G., Cai, M., Zhao, X., Hu, C. (2019). Cadmium in plants: Uptake, toxicity, and its interactions with selenium fertilizers. Metallomics 11, 255–277. doi: 10.1039/C8MT00247A
Jali, P., Pradhan, C., Das, A. B. (2016). Effects of cadmium toxicity in plants: a review article. Sch. Acad. J. Biosci. 4 (12), 1074–1081.
Kabta-Pendias, A. (2011). Trace elements in soils and plants (Boca Raton: CRC Press, Taylor and Francis Group).
Kastori, K., Petrovic, M., Petrovic, N. (1992). Effect of excess Pb, cd, Cu and zn on water relations in sunflower. J. Plant Nutr. 15, 24–27. doi: 10.1080/01904169209364485
Khalifa, M., Gad, A. (2018). Assessment of heavy metals contamination in agricultural soil of southwestern Nile delta, Egypt. Soil Sediment Contam. 27, 619–642. doi: 10.1080/15320383.2018.1498445
Livingstone, D. R. (2001). Contaminant-stimulated reactive oxygen species production and oxidative damage in aquatic organisms. Mar. pollut. Bull. 42, 656–666. doi: 10.1016/S0025-326X(01)00060-1
Lux, A., Morita, S., Abe, J., Ito, K. (2005). Improvedmethod for clearing and staining free-hand sections and whole-mount samples. Ann. Bot. 96, 989–996. doi: 10.1093/aob/mci266
Ma, J., Cai, H., He, C., Zhang, W., Wang, L. A. (2015). Hemicellulose-bound form of silicon inhibits cadmium ion uptake in rice (Oryza sativa) cells. New Phytol. 206, 1063–1074. doi: 10.1111/nph.13276
Ma, J. F., Yamaji, N. (2006). Silicon uptake and accumulation in higher plants. Trends Plant Sci. 11, 392–397. doi: 10.1016/j.tplants.2006.06.007
Mayonde, S., Cron, G. V., Glennon, K. L., Byrne, M. J. (2021). Effects of cadmium toxicity on the physiology and growth of a halophyticplant, tamarix usneoides (E. mey. ex bunge). Int. J. Phytoremediat. 23, 130–138. doi: 10.1080/15226514.2020.1801573
Mondal, N. K., Das, C., Roy, S., Datta, J. K., Banerje (2013). Effect of varying cadmium stress on chickpea (Cicer arietinum l) seedlings: An ultrastructural study. Ann. Environ. Sci. 7, 59–70.
Moran, R. (1982). Formula for determination of chlorophyllous pigment extracted with n-n-dimethyl formamide. Plant Physiol. 69, 1376–1381. doi: 10.1104/pp.69.6.1376
Mostofa, M. G., Rahman, A., Ansary, M. M. U., Watanabe, A., Fujita, M., Tran, L. S. P. (2015). Hydrogen sulfide modulates cadmium-induced physiological and biochemical responses to alleviate cadmium toxicity in rice. Sci. Rep. 5, 14078. doi: 10.1038/srep14078
Orzoł, A., Gołębiowski, A., Szultka-Młyńska, M., Głowacka, K., Pomastowski, P., Buszewski, B. (2022). ICP-MS analysis of cadmium bioaccumulation and its effect on pea plants (Pisum sativum l.). Polish. J. Environ. Stud 31(5):1–9. doi: 10.15244/pjoes/149259
Peng, Q., Chen, W., Wu, L., Bai, L. (2017). The uptake, accumulation, and toxic effects of cadmium in barnyard grass (Echinochloa crus–galli). Pol. J. Environ. Stud. 26 (2), 779–784. doi: 10.15244/pjoes/65780
Perveen, S., Shahbaz, M., Iqbal, M., Akram, M.S, Parveen, A., Ali, H.M.M (2016)Triticum aestivum. Appl. Ecol. Environ. Res. 14. doi: 10.15666/aeer/1405_121136
Popova, L. P., Maslenkova, L. T., Ivanova, A., Stoinova, Z.. (2012). Role of salicylic acid in alleviating heavy metal stress. doi: 10.1007/978-1-4614-0815-4_21
Rahman, M. F., Ghosal, A., Alam, M. F., Kabir, A. H. (2017). Remediation of cadmium toxicity in field peas (Pisum sativum l.) through exogenous silicon. Ecotoxicol. Environ. Saf. 135, 165–172. doi: 10.1016/j.ecoenv.2016.09.019
Radoglou, K. M., Jarvis, P. G. (1992). Effects of CO2 enrichment and nutrient supply on growth morphology and anatomy of Phaseolus vulgaris L. seedlings. Ann. Bot. 70 (3), 245–256.
Ribera-Fonseca, A., Rumpel, C., Mora, M. L., Nikolic, M., Cartes, P. (2018). Sodium silicate and calcium silicate differentially affect silicon and aluminium uptake, antioxidant performance and phenolics metabolism of ryegrass in an acid andisol. Crop &Pasture Sci. 69, 205–215. doi: 10.1071/CP17202
Rios-Gonzalez, K., Erdei, L., Herman, S. (2002). The activity of antioxidant enzymes in maize and sunflower seedlings as affected by salinity and different nitrogen sources. Plant Sci. 162 (6), 923–930. doi: 10.1016/S0168-9452(02)00040-7
Rizwan, M., Meunier, J. D., Davidian, J. C., Pokrovsky, O. S., Bovet, N., Keller, C. (2016). Silicon alleviates cd stress of wheat seedlings (Triticum turgidum lcv. Claudio) grown in hydroponics. Environ. Sci. pollut. Res. 23, 1414–1427. doi: 10.1007/s11356-015-5351-4
Sadeghipour, O. (2018). Enhancing cadmium tolerance in common bean plants by potassium application. Philippine Agric. Scientist 101 (2), 167–175.
Sandalio, L. M., Dalurzo, H. C., Gómes, M., Romero-Puertas, M. C., Del Rio, L. A. (2001). Cadmium-induced changes in the growth and oxidativemetabolism of pea plants. J. Exp. Bot. 52, 2115–2126. doi: 10.1093/jexbot/52.364.2115
Seth, C. S., Misra, V., Chauhan, L. K. S., Singh, R. R. (2008). Genotoxicity of cadmium on root meristem cells of allium cepa: cytogenetic and comet assay approach. Ecotoxicol. Environ. Saf. 71, 711–716. doi: 10.1016/j.ecoenv.2008.02.003
Shahid, M., Dumat, C., Khalid, S., Niazi, N. K., Antunes, P. M. C. (2016). Cadmium bioavailability, uptake, toxicity and detoxification in soilplant system. Rev. Environ. Contam Toxicol. 241, 73–137. doi: 10.1007/398_2016_8
Shen, X., Zhou, Y., Duan, L., Li, Z., Eneji, A. E., Li, J. (2010). Silicon effects on photosynthesis and antioxidant parameters of soybean seedlings under drought and ultraviolet-b radiation. J. Plant Physiol. 167, 1248–1252. doi: 10.1016/j.jplph.2010.04.011
Shi, Q., Bao, Z., Zhu, Z., He, Y., Qian, Q., Yu, J. (2005). Silicon-mediated alleviation of Mn toxicity in cucumis sativus in relation to activities of superoxide dismutase and ascorbate peroxidase. Phytochemistry 66, 1551–1559. doi: 10.1016/j.phytochem.2005.05.006
Shi, G. R., Zhang, Z., Liu, C. F. (2017). Silicon influences cadmium translocation by altering sub-cellular distribution and chemical forms of cadmium in peanut roots. Arch. Agron. Soil Sci. 63, 117–123. doi: 10.1080/03650340.2016.1189075
Singh, R. P., Agrawal, M. (2007). Effects of sewage sludge amendment on heavy metalaccumulation and consequent responses of Beta vulgaris plants. Chemosphere. 67, 2229–2240.
Singh, R. P., Agrawal, M. (2010). Variations in heavy metal accumulation, growth andyield of rice plants grown at different sewage sludge amendment rates. Ecotoxicology and Environmental Safety. doi: 10.1016/j.ecoenv.2010.01.020
Sridhar, B. B. M., Han, F. X., Diehl, S. V., Monts, D. I., SU, Y. (2007). Effects of zn and cd accumulation on structural and physiological characteristics of barley plants. Braz. J. Plant Physiol. 19, 15–22. doi: 10.1590/S1677-04202007000100002
Sun, H., Wang, X., Wang, Y., Wei, Y., Wang, G. (2016). Alleviation of cadmium toxicity in cucumber (Cucumis sativus) seedlings by the application of selenium. Spanish J. AgriculturalResearch 14 (4), 12, e1105. doi: 10.5424/sjar/2016144-10008
Sytar, O., Kumari, P., Yadav, S., Brestic, M., Anshu Rastogi, A. (2019). Phytohormone priming: regulator for heavy metal stress in plants. J. Plant Growth Regul. 38, 739–752. doi: 10.1007/s00344-018-9886-8
Talukdar, D. (2013). Arsenic-induced oxidative stress in the common bean legume, phaseolus vulgaris l. seedlings and its amelioration by exogenous nitric oxide. Physiol. Mol. Biol. Plants 19, 69–79. doi: 10.1007/s12298-012-0140-8
Thordal-Christensen, H., Gregersen, P. L., Collinge, D. B. (2000). The barley/Blumeria (syn. erysiphe) graminis interaction. in mechanisms of resistance to plant diseases. Eds. Slusarenko, A., Fraser, R., van Loon, L. C. (Dordrecht, The Netherlands: Kluwer AcademicPublishers), 77–100.
Tupan, C. I., Azrianingsih, R. (2016). Accumulation and deposition of lead heavy metal in the tissues of roots, rhizomes and leaves of seagrassThalassia hemprichii (Monocotyledoneae, hydrocharitaceae). AACL Bioflux 9 (3), 580–589.
Velikova, V., Yordanov, I., Edreva, A. (2000). Oxidative stress and some antioxidant system in acid rain treated bean plants: Protective role of exogenouspolyamines. Plant Sci. 151 (1), 59–66. doi: 10.1016/S0168-9452(99)00197-1
Wahid, A., Ghani, A., Javed, F. (2008). Effect of cadmium on photosynthesis, nutrition and growth of mungbean. Agron. Sustain Dev. 28, 273–280. doi: 10.1051/agro:2008010
Yemm, E. W., Willis, A. J. (1954). The estimation of carbohydrates in plant extracts byanthrone. Biochem. J. 57, 508e514. doi: 10.1042/bj0570508
Younis, U., Malik, S. A., Rizwan, M., Qayyum, M. F., Ok, Y. S., Shah, M. H. R., et al. (2016). Biochar enhances the cadmium tolerance in spinach (Spinaciaoleracea) through modification of cd uptake and physiological and biochemical attributes. Environ. Sci. pollut. Res. 23, 21385–21394. doi: 10.1007/s11356-016-7344-3
Zhang, C. H., Ge, Y. (2008). Response of glutathione and glutathione s-transferase in rice seedlings exposed to cadmium stress. Rice Sci. 15, 73–76. doi: 10.1016/S1672-6308(08)60023-2
Zhang, L., Pei, Y., Wang, H., Jin, Z., Liu, Z., Qiao, Z., et al. (2015). Hydrogen sulfide alleviates cadmium-induced cell death through restraining ROS accumulation in roots of Brassica rapa L. ssp. pekinensis. Oxid. Med. Cell. Longev. doi: 10.1155/2015/804603
Zhang, S., Geng, L., Fan, L., Zhang, M., Zhao, Q., Xue, P., et al. (2020). Spraying silicon to decrease inorganic arsenic accumulation in rice grain from arsenic-contaminated paddy soil. Sci. Total. Environ. 704, 135239. doi: 10.1016/j.scitotenv.2019.135239
Keywords: cadmium, Pea, oxidative stress, silicon, MDA
Citation: El-Okkiah SAF, El-Tahan AM, Ibrahim OM, Taha MA, Korany SM, Alsherif EA, AbdElgawad H, Abo Sen EZF and Sharaf-Eldin MA (2022) Under cadmium stress, silicon has a defensive effect on the morphology, physiology, and anatomy of pea (Pisum sativum L.) plants. Front. Plant Sci. 13:997475. doi: 10.3389/fpls.2022.997475
Received: 18 July 2022; Accepted: 31 August 2022;
Published: 06 October 2022.
Edited by:
Bhumi Nath Tripathi, Indira Gandhi National Tribal University, IndiaReviewed by:
Bilal Ahmad Mir, University of Ladakh, IndiaCopyright © 2022 El-Okkiah, El-Tahan, Ibrahim, Taha, Korany, Alsherif, AbdElgawad, Abo Sen and Sharaf-Eldin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amira M. El-Tahan, YWVsdGFoYW5Ac3J0YWNpdHkuc2NpLmVn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.