- 1Tropical Crops Genetic Resources Institute, Chinese Academy of Tropical Agricultural Sciences, Danzhou, China
- 2Zhanjiang Experimental Station, Chinese Academy of Tropical Agricultural Sciences, Zhanjiang, China
- 3Key Laboratory of Ministry of Education for Genetics and Germplasm Innovation of Tropical Special Trees and Ornamental Plants, Key Laboratory of Germplasm Resources of Tropical Special Ornamental Plants of Hainan Province, College of Forestry, Hainan University, Danzhou, China
The dynamics and correlations of chlorophyll and phytol content with silage bacterial of different growth heights Pennisetum sinese were investigated. The results demonstrated that the chlorophyll and phytol content of P. sinese before and after ensiled decreased with the increase of growth height. Ensiling significantly reduced pigment content but had no significant effect on phytol. In addition, P. sinese pigment yield before and after ensiled increased with growth heights increasing, and the yield at 150 or 180 cm was obviously higher. Moreover, the higher silage quality V-Score were at 150 or 180 cm growth heights. Furthermore, the silage microbial diversity were varied by growth heights, and some specific undesirable microorganisms (Acinetobacter, Cellvibrio, Sphingobacterium, etc.) were negatively correlated with pigment and phytol content. Therefore, with comprehensive consideration of pigment, phytol yield, and silage quality, the optimum harvest growth height of P. sinese was 150 cm. Furthermore, precise reduction of particular undesirable microorganisms maybe helps to preserve pigments and phytol.
Introduction
In recent years, consumers are becoming more and more concerned about the healthy functions of animal products (Eggersdorfer and Wyss, 2018; Liu et al., 2020). Phytanic acid is an important functional component, which could regulate the oxidation of fatty acids, reduce the incidence of some types of cancer, and relieve fatty liver and diabetes (Wright et al., 2014; Kwan et al., 2015; Roca-Saavedra et al., 2017; Lv et al., 2020). The main sources of phytanic acid are milk and beef; hence, the concentration of phytanic acid is one of the most important indexes to evaluate functional animal products, especially for ruminants (Vetter and Schroder, 2010). In addition, phytol in chlorophyll is a synthetic precursor of phytanic acid, which directly determines phytanic acid concentration (Lv et al., 2017). Moreover, phytol has positive effects on fat metabolism, meat quality, muscle development, and regulation of animals, which further affects animal health and animal product quality (Yang et al., 2017; Bobe et al., 2020; Ding et al., 2021). In general, the content of functional components in animal products is mainly associated with feed and animal digestion and absorption (Liu et al., 2020). Therefore, phytol or chlorophyll content in forage will affect the accumulation of phytanic acid in animal products, and providing ruminants with higher level of phytol or chlorophyll diet will help to produce high-quality animal products (Santra et al., 2021).
According to previous reports, the contents of phytol or chlorophyll in forage were affected by many factors, for instance, forage variety and growth period (Lv et al., 2021). In addition, cultivation measures affect these pigment content of forage, Hák et al. (1993) and Lv et al. (2017) reported that the chlorophyll and phytol contents increased when applied nitrogen fertilizer and harvested earlier. Moreover, the forage processing and modulation method also affects the pigment content; Lv et al. (2021) reported that drying can obviously reduce the content of chlorophyll and phytol in the harvest grass. On the contrary, ensiling could preserve the phytol in temperate grasses Italian ryegrass well (Lv et al., 2017; Lv et al., 2020). Phytol is produced by chlorophyll degradation in acidic environments, and the acidity of silage may be the key factor of phytol conversion. During the ensiling, pH value was mainly affected by the silage microbial community (Du et al., 2022; Zi et al., 2022). Therefore, the resolving of silage microbial community structure may help to improve the efficiency of phytol conversion.
Pennisetum sinese is typical tropical grass and important ruminant feed, which also was C4 plant with high photosynthetic efficiency (Sanborn et al., 2021). Due to multiple cutting, P. sinese is hard to judge its nutritional value through its growth period. To some extent, the growth heights reflect the growth and development stages of plants, which influenced chemical composition and feed quality (Dong et al., 2013; Zi et al., 2017). Therefore, we speculate that P. sinese contains more photosynthetic pigments, and the impact of growth heights on the phytol or chlorophyll level also should be clarified. However, the contents of phytol or chlorophyll in P. sinese were still unknown. Meanwhile, the role of silage microorganisms in the preservation of phytol was also unclear. The purpose of this study was to clarify these doubts; thus, we investigated the dynamics and correlations of chlorophyll and phytol content with silage bacterial of different growth heights P. sinese.
Materials and methods
Location, grass production, and sample preparation
Pennisetum sinese (P. purpureum × P. glaucum cv. Reyan No.4) was planted at the experimental base of Chinese Academy of Tropical Agricultural Sciences (CATAS) in Danzhou, Hainan, China (longitude of 109°30′ E, latitude of 19°30′ N, altitude of 149 m). The average annual rainfall in this area is 1,815 mm with a mean annual temperature of 23.3°C. A completely randomized design was used in the present study, Pennisetum sinese of four plant height (approximately 90, 120, 150, and 180 cm) were harvested, each plant height was assigned into three randomly allocated plots with each plot measuring an area of 5 m × 5 m, and each plot was 0.5 m apart. Pennisetum sinese were planted on February 2018 and planting distance was 40 cm between rows, which was fertilized at a rate of 120-kg Urea, 150-kg P2O2, and 120-kg K2O ha-1, and watering was carried out 1 day each week except that when natural precipitation occurred during these days. Weed, pest, and disease control were conducted once a month. Pennisetum sinese was cut 3 cm above the ground on 28 February 2018 (plant height 90 cm, H1), 16 March 2018 (plant height 120 cm, H2), 29 March 2018 (plant height 150 cm, H3) and 23 April 2018 (plant height 180 cm, H4), respectively. Weighed the cut grass and calculated the yield of each height (three plots), and the harvested grass was chopped into 2 cm using a grass chopper (9Z-2.5, Zhengzhou Jinhongxing
Industrial Co., Ltd., Zhengzhou China). Then, the mixed well grass spread out in the shade about 4h and wilted to approximately 80% moisture content. Then, 100 g of above sample was stored at −80°C for analysis of pigment analysis and 100 g sample was dried at 65°C for 48h for chemical composition analysis, each height had three replicates (AOAC, 2000).
Silage preparation
Above wilted Pennisetum sinese samples (200 g) was blended and vacuumed (Chunze Vacuum Sealer, Chunze Machinery Technology Co. Ltd, Weifang, China) in plastic bags (Synthetic resin, 30 cm × 10 cm × 4 cm; Henghou Packing Co. Ltd, Jiangmen, China). A total of 12 bags (four heights × three replicates) were prepared and incubated at room temperature (25°C–30°C). After 60 days ensiling, the bags were opened, 50 g of silage was stored at −80°C for analysis of pigment analysis and 100 g silage was dried at 65°C for 48h for chemical composition analysis (AOAC, 2000). In addition, 50 g silage was blended with 200 ml of distilled water, followed by incubation at 4°C for 24h and then filtration (Zi et al., 2022). Half of each extract sample was stored at −80°C for analysis of the microbiota diversity and the rest of extract sample stored at 4°C for 24h for fermentation quality analysis (Zi et al., 2022).
Chemical analysis
Specimens were heated at 65°C for 72h, and dried materials were ground for chemical analysis. The contents of dry matter (DM), crude protein (CP), neutral detergent fiber (NDF), and acid detergent fiber (ADF) were measured as previously described (Van Soest et al., 1991; AOAC, 2000). CP values were calculated used nitrogen value multiplied by 6.25. Heat-stable amylase and sodium sulfite were used in the NDF procedure, and the results were expressed without residual ash. The pigments and phytol was determined as reported previously by Lv et al. (2017), the above frozen grass and silage samples were freeze-dried for 72h and extracted with 80% acetone, the chlorophyll a and chlorophyll b in the acetone extract were analyzed by HPLC, and the phytol was analyzed by gas chromatography (GC) after saponification and purification of the acetone extracts. The silage fermentation quality was determined using above extracts. The pH was measured with a glass electrode pH meter. The levels of lactic acid, acetic acid, propionic acid, butyric acid, and NH3 -N/total N were determined as previously established (Liu et al., 2012). V-Score was calculated from the NH3-N/total N and organic acid concentrations (Chen et al., 2016).
Microbial community analysis
The extracts described above were used to analyze the microbial communities. Total DNA was isolated using the Stool DNA Kit (OMEGA Bio-Tek, Norcross, GA, USA). The V3–V4 regions of 16S rDNA were amplified using the thermocycler PCR system (GeneAmp 9700, ABI, Los Angeles, CA, USA) and primer sequence: 338F (5′- ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). Then, the high-quality PCR products sequencing was carried out using an Illumina MiSeq 2500 platform (Illumina, Inc., San Diego, CA, USA). The microbial diversity was assessed using the alpha diversity indices: Shannon and Simpson index. Beta diversity: principal components analysis (PCA) and Unweighted Pair-group Method with Arithmetic Mean (UPGMA) were conducted. The linear discriminant analysis (LDA) effect size (LEfSe) method was employed to identify the bacterial strains with different abundances among groups. The P. sinese silage microbial (genus) and pigments and phytol content were used R software to generate a heat map. Above methods details were as previously described (Zi et al., 2021; Zi et al., 2022). The data were analyzed using the free online BMKCloud Platform (www.biocloud.net). The sequencing data were deposited in the Sequence Read Archive (SRA) with the accession number of PRJNA637294.
Statistics
The effects of plant heights were evaluated by one-way analysis of variance using the general linear model procedure of SAS 9.3 software (SAS Institute Inc., Cary, NC, USA). Differences were compared using Duncan’s multiple range test, and differences with P < 0.05 were considered statistically significant.
Results
Dynamics of chemical composition, chlorophyll, phytol, and biomass yield of Pennisetum sinese before ensiled
The dynamics of chemical composition, chlorophyll, phytol, and biomass yield of Pennisetum sinese at different plant heights are shown in Table 1. The contents of NDF and ADF significant increased (P < 0.01) with the increase of plant height, whereas the CP content and RFV decreased significantly (P < 0.01). Meanwhile, the plant height also affects pigment content. The biomass yield increased with plant height increasing (P < 0.01), but the chlorophyll and phytol contents decreased with plant height increasing (P < 0.01).
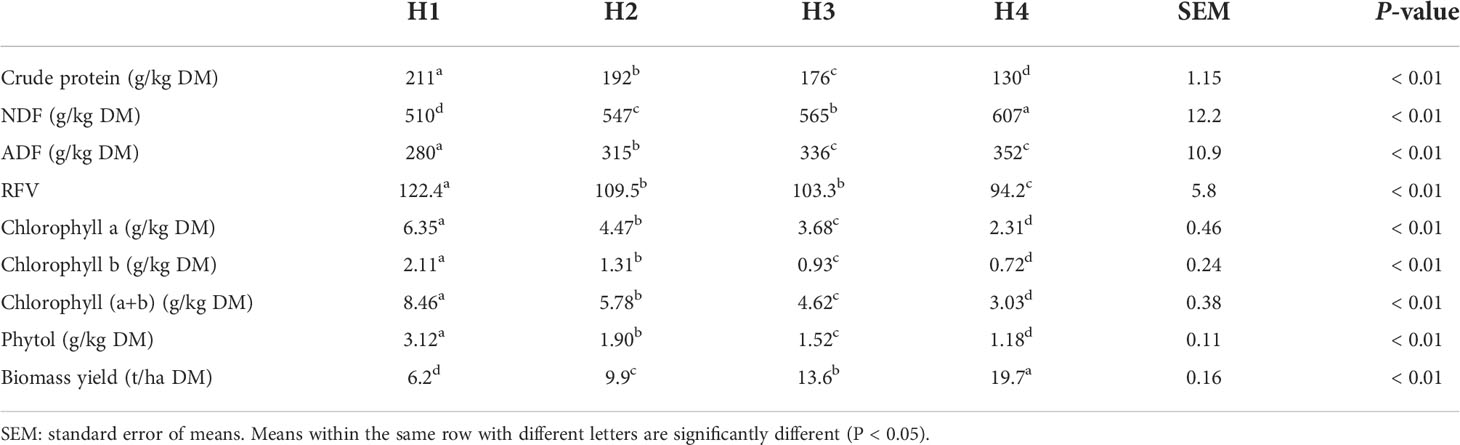
Table 1 The chemical composition, chlorophyll, phytol, and biomass yield of Pennisetum sinese at different heights.
Comparation of the content and yield of chlorophyll and phytol of Pennisetum sinese at different heights before and after ensiling
The content of chlorophyll and phytol of Pennisetum sinese at different heights before and after ensiling were presented in Figure 1. The contents of chlorophyll a, chlorophyll b, and chlorophyll a+b significant decreased with the increase of plant height (P < 0.05), and the similar tendency also found in Pennisetum sinese silage (P < 0.05). Meanwhile, silage significantly reduced the chlorophyll a, chlorophyll b and chlorophyll a+b content (P < 0.05). Furthermore, there was no significant difference in phytol content before and after ensiling.
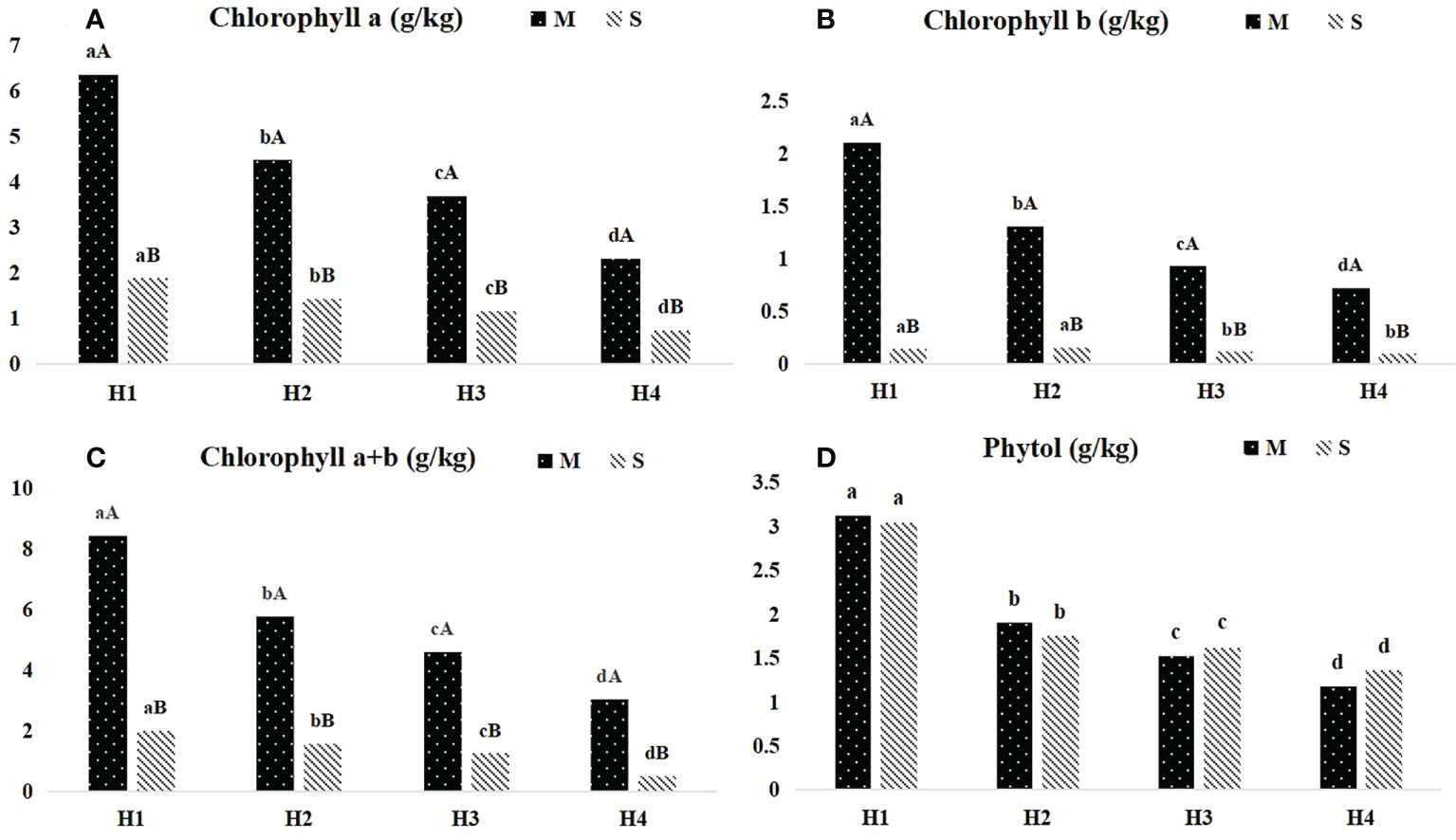
Figure 1 Dynamics of chlorophyll and phytol content in different height Pennisetum sinese and silage. The contents of chlorophyll a, chlorophyll b, chlorophyll a+b and phytol (A–D). M: Pennisetum sinese material; S: Pennisetum sinese silage. Boxes with a different letters above the error bars are significantly different between different height (a–d) or material and silage (A, B) at P < 0.05.
The dynamics of chlorophyll and phytol yield of Pennisetum sinese at different heights before and after ensiling were presented in Figure 2. The yield of chlorophyll a and chlorophyll a+b Pennisetum sinese before and after ensiling shown the inverted “V” trend, and H3 group had the highest yield (P < 0.05). Meanwhile, the highest chlorophyll b and phytol yield were in H4 group (P < 0.05). Furthermore, there was also no significant difference in phytol yield before and after ensiling.
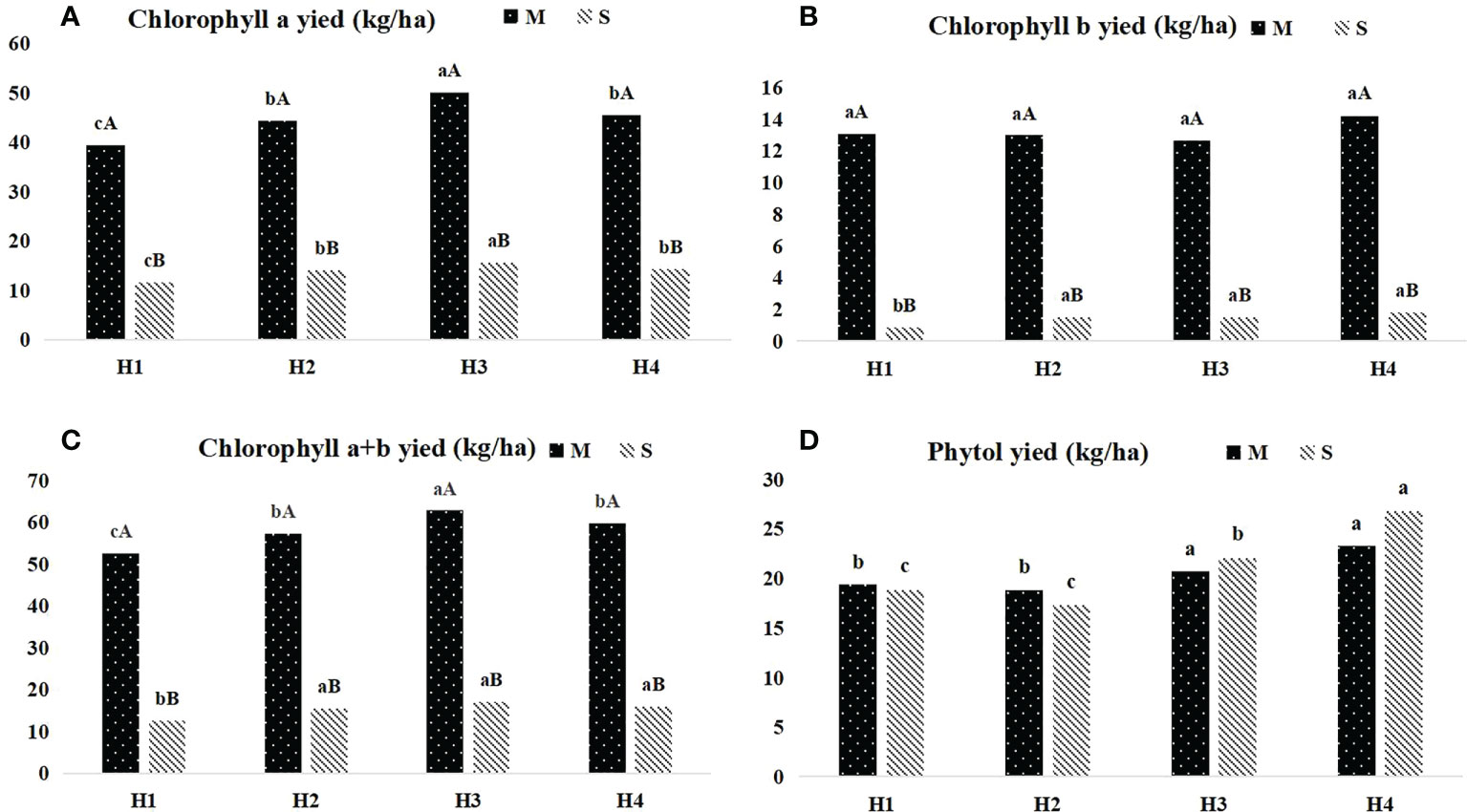
Figure 2 Dynamics of chlorophyll and phytol yield in different height Pennisetum sinese and silage. The yied of chlorophyll a, chlorophyll b, chlorophyll a+b and phytol (A–D) .M: Pennisetum sinese material; S: Pennisetum sinese silage. Boxes with a different letters above the error bars are significantly different between different height (a–c) or material and silage (A, B) at P < 0.05.
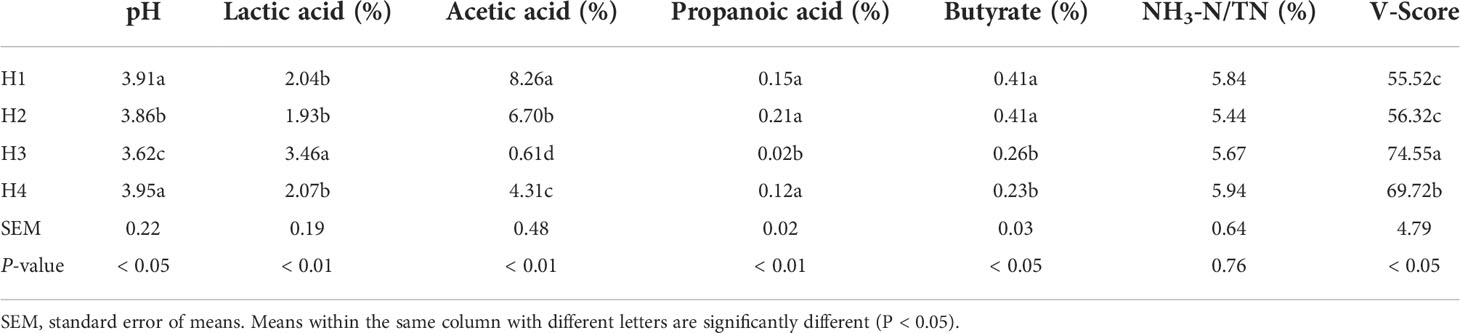
Silage fermentation quality of Pennisetum sinese in different heights
The dynamics of different height Pennisetum sinese silage fermentation characteristics list in Table 2. The plant height affect the fermentation characteristics of Pennisetum sinese silage, the highest lactic acid (P < 0.01), and lowest pH, acetic acid and propanoic acid (P < 0.05) were found in H3 group. The lowest and highest V-Score value were 55.52 in H1 group (P < 0.05) and 74.55 in H3 group (P < 0.05), respectively. Pennisetum sinese silage quality in H3 group was the best.
Silage microbial communities of Pennisetum sinese in different heights
The silage microbial diversity of Pennisetum sinese was shown in Figure 3. The significant differences were observed in Shannon and Simpson indices between four groups (Figures 3A, B). The highest Shannon and lowest Simpson indices were in H3 group, which indicated H3 group had the highest microbial diversity. The PCA and cluster tree of the silage microbial community structures were conducted, and the clear separation and differences in bacterial community were found in all four groups (Figures 3C, D), which indicating that the microbial composition was changed by growth height, and microbial community structure was similar when the growth height was closer. Overall, above results demonstrated that growth height affects the microbial diversity and community structure of Pennisetum sinese silage.
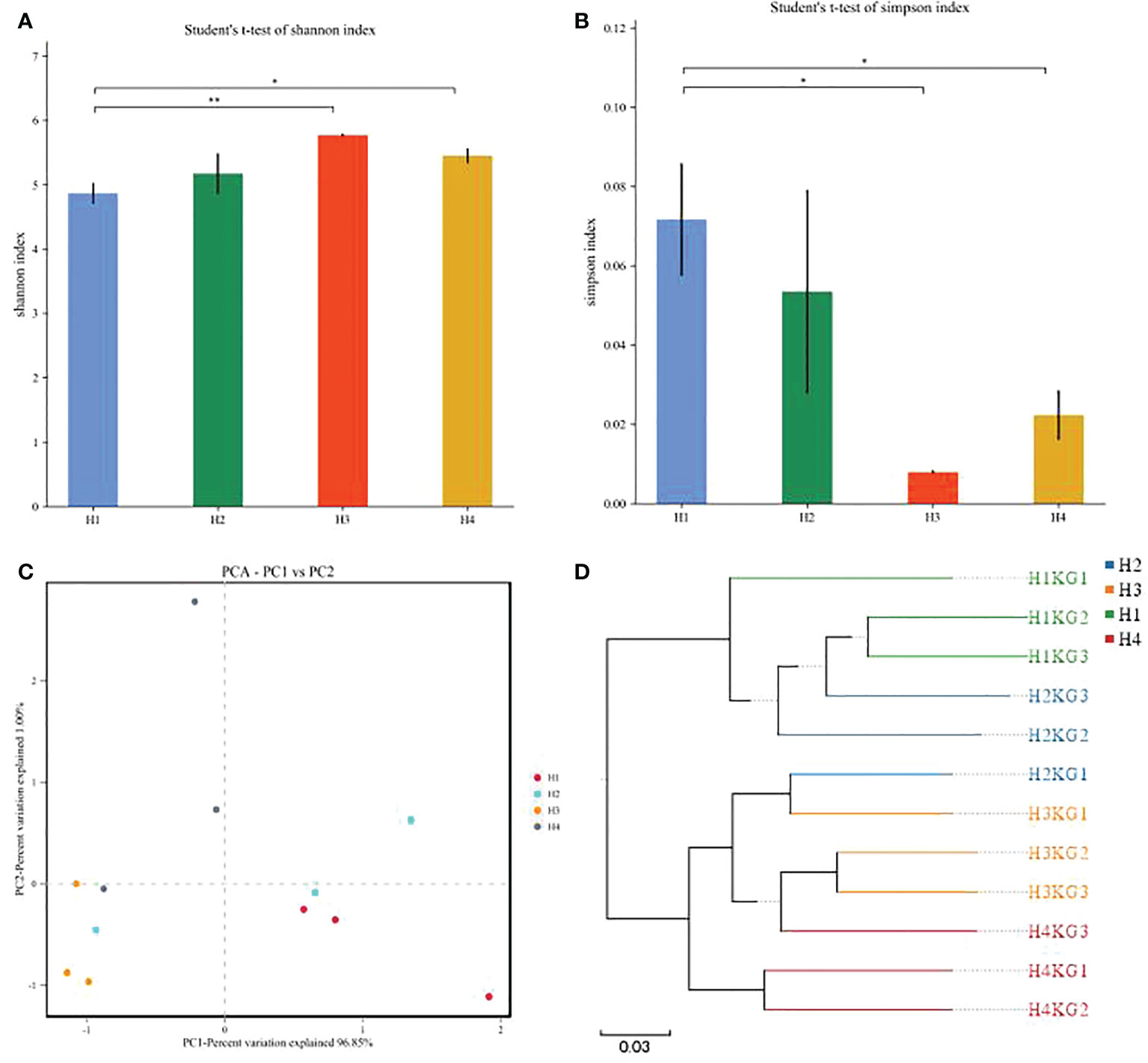
Figure 3 The α diversity and β diversity of different height Pennisetum sinese silage bacterial community. The Shannon and Simpson index (A, B) of the silage bacterial community, PCA and UPGMA analysis (C, D) of silage bacterial community. P-values are shown as *P < 0.05, **P < 0.01.
Figures 4A, B shows the effects of growth height on the relative abundance of bacteria in the most dominant phylum and genus in Pennisetum sinese silage. The community was shifted along with different growth height: The abundances of Proteobacteria, Bacteroidetes, and Actinobacteria were increased, whereas the abundance of Firmicutes was decreased with rise of growth height. The H3 group was the highest abundance of Proteobacteria, Bacteroidetes, and Actinobacteria, and the lowest of Firmicutes (P < 0.05). Furthermore, the abundances of Enterococcus and Lactobacillus were decreased with rise of growth height, while the abundances of Sphingobacterium and Pseudomonas were increased. Meanwhile, the H3 group was the highest abundance of Sphingobacterium and Pseudomonas and the lowest of Enterococcus and Lactobacillus (P < 0.05).
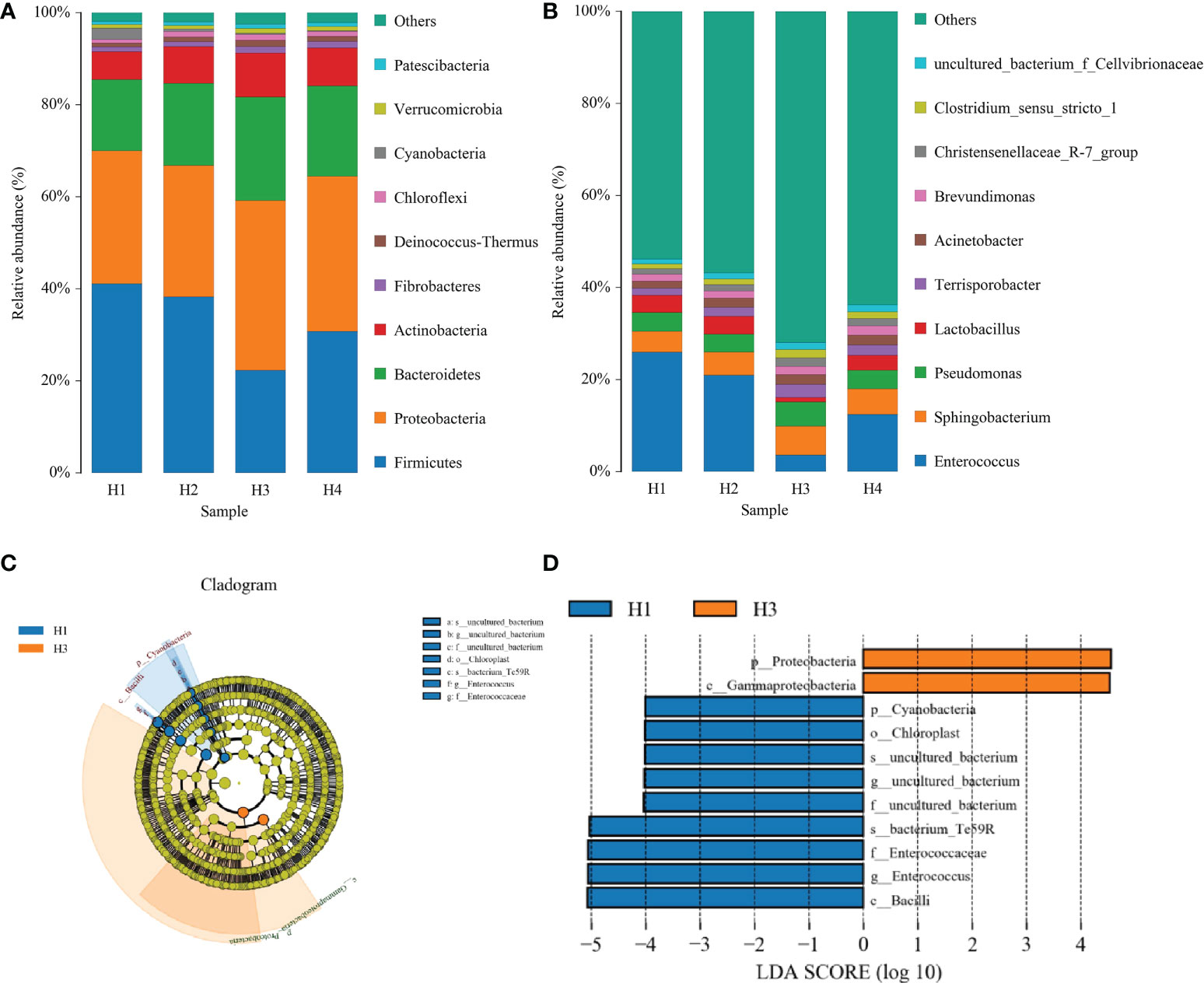
Figure 4 The composition (phylum, A and genus, B) and comparison using the LEfSe online tool (C, D) of the bacterial community of different height Pennisetum sinese silage.
The specific bacterial community of different growth height Pennisetum sinese silage was explored by the LDA effect size (LEfSe) method (LDA score > 4.0). Figures 4C, D shows that the different height of Pennisetum sinese used to produce the silage exerted a dramatic impact on the resultant microbial community. Proteobacteria and Gammaproteobacteria were the most abundant phylum and class in H3 group, respectively. In addition, Cyanobacteria, Bacilli, Chloroplast, Enterococcaceae, and Enterococcus were the most abundant phylum, class, order, family, and genus in H1 group, respectively. Moreover, these microorganisms could be used as biomarkers of the different height of Pennisetum sinese silage.
Correlations between chlorophyll, phytol content and microbial communities of Pennisetum sinese silage
The correlation between chlorophyll, phytol content, and microbial communities (the genus level) of Pennisetum sinese silage were assessed (Figure 5). The chlorophyll a, chlorophyll b, chlorophyll a+b, and phytol were no significant positively associated with Enterococcus, Lactobacillus, Pseudomonas, and weissella, whereas it was significant negatively associated with Acinetobacter, Cellvibrio, Sphingobacterium, Christensenellaceae_R-7_group, Microbacterium, Sphingopyxis, Truepera, Turicibacter, uncultured_bacterium_f_Cellvibrionaceae, uncultured_bacterium_f_Enterobacteriaceae, and uncultured_bacterium_f_Fibrobacteraceae. The result revealed that silage microbial maybe influence the degradation of chlorophyll and phytol during ensiling process.
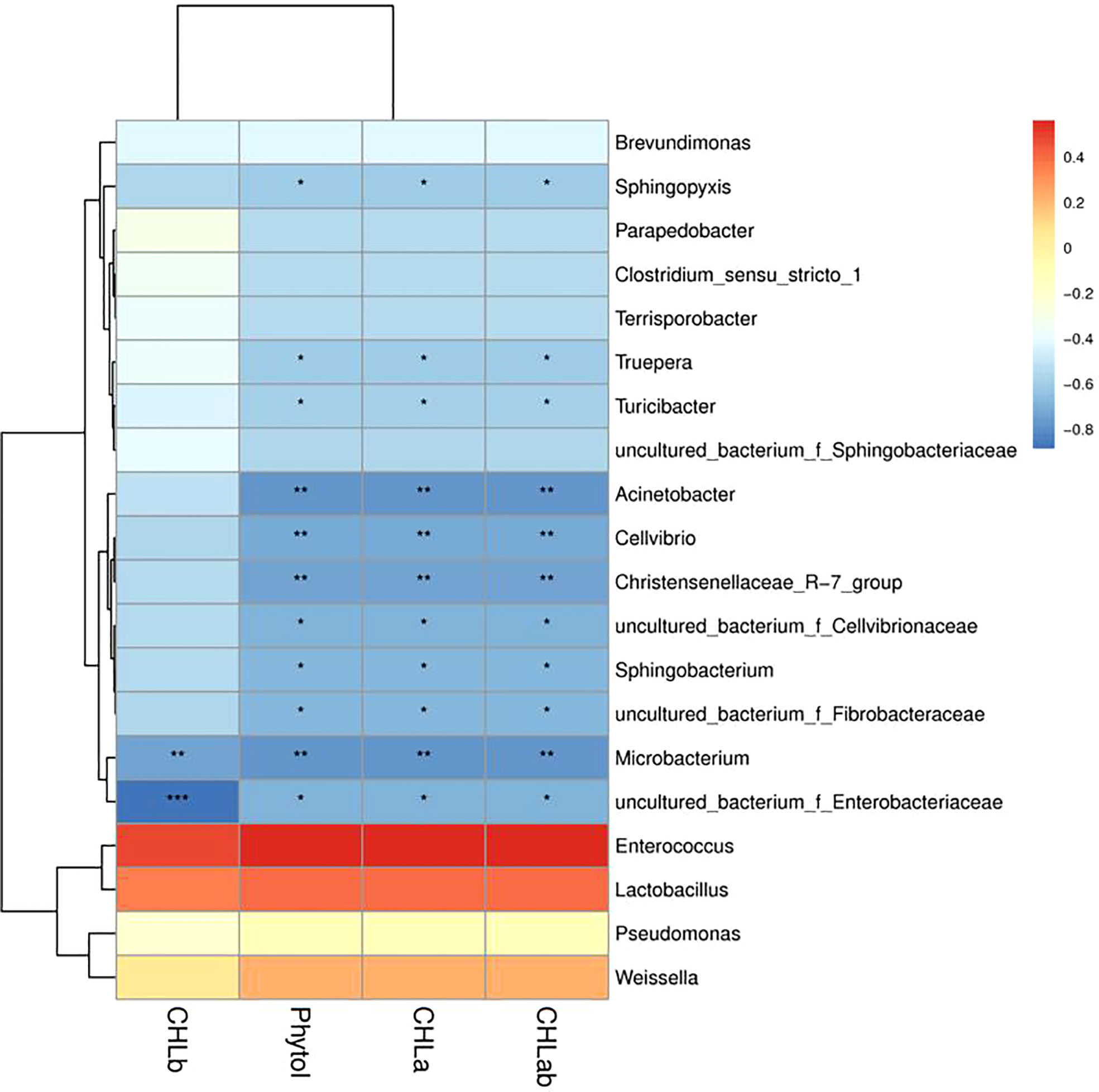
Figure 5 Correlations analysis between chlorophyll and phytol content and silage bacterial of Pennisetum sinese. Positive correlations are shown in red, and negative correlations are indicated in blue. Color intensity is proportional to the correlation values [r]. P-values are shown as *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
In this study, the growth heights affected the chemical composition, chlorophyll, phytol, and biomass yield of P. sinese, and this result has been verified in several studies (King et al., 2013; Lv et al., 2017; Zi et al., 2017). The concentration of pigment decreased gradually with the increase of the growth height of P. sinese. On the contrary, DM yield increased with growing height. That result in pigment yield was higher in H3 or H4. In addition, the pigment content of P. sinese was reported for the first time in this study, the content were significantly higher than Italian ryegrass (Lv et al., 2017; Lv et al., 2020; Lv et al., 2021). Besides the cultivation conditions, the plants characteristics (variety or harvesting stage) also determine the differences in photosynthetic pigment content (Lv et al., 2017; Lv et al., 2021). As a kind of typical plant, the photosynthesis would be more effective under full sunlight, thus P. sinese produce the photosynthetic pigments more beneficial. However, Italian ryegrass is native to temperate zone with low photosynthetic efficiency and affected pigment synthesis. Furthermore, after ensiling the pigment in P. sinese markedly decrease, but not in phytol. Therefore, silage is an ideal way to preserve phytol compare with hay. Lv et al. (2017; 2020) reported the similar phenomenon in Italian ryegrass silage. Moreover, many studies have shown that forage at different growth stages has different silage qualities (Neylon and Kung, 2003; Lv et al., 2017). This could be explained by chemical differences at different growth stages. In this study, the best silage quality were found in H3 group ,which shown the highest V-Score value. Therefore, with comprehensive consideration of forage value, pigment yield, and silage quality, the optimum growth height of P. sinese was H3 (150 cm).
In this study, the PCA and cluster tree results showed separation and difference of microbial communities of P. sinese silage. The specific changes were reflected by the differences in bacterial composition at the genus level among different growth heights treatments silage. In general, lactobacillus is the dominant organism in well-preserved forage silage (Zi et al., 2021; Du et al., 2022). However, in the present study, Enterococcus, Sphingobacterium, and Pseudomonas were predominant, and Enterococcus has the highest abundance, that means the fermentation quality is not ideal enough. Then, we found an interesting phenomenon, the abundances of Enterococcus were reduce significantly, whereas Sphingobacterium and Pseudomonas were increased with rise of growth height, and then the fermentation quality was also improved. Enterococcus was aerobic fermentative Gram-positive coccus, which can utilize wide range of fermentable carbohydrates, mainly produced L(+) -lactic acid (Murray, 1990). It is a common microorganism in silage, especially at the beginning of ensiling, so it belongs to silage beneficial microorganisms at some extent (Bal et al., 2012; Wang et al., 2019). Sphingobacterium can consume carbohydrates and produce acid (Yabuuchi et al., 1983; Palleroni and Bradbury, 1993; Li et al., 2021). Pseudomonas is generally regarded as undesirable microorganisms for silage due to its possibility decreased protein content (Santos Silla, 1996; Dunière et al., 2013; Li et al., 2021). However, Ogunade et al. (2018) reported that these microorganisms were negatively correlated with pH, ammonium nitrogen, yeast, and mold and thought that they may contribute to silage fermentation. Moreover, in this study, Sphingobacterium and Pseudomonas have relative lower abundances, and which significantly increased in higher growth heights groups and improved the silage quality. Therefore, it is necessary to conduct in-depth studies on the characteristics and functions of these uncommon silage microorganisms.
In order to resolve the regulation mechanism of silage fermentation, some previous studies explored the correlation between silage microbial and fermentation characteristics, forage chemical composition or silage metabolites, and some key chemical components or metabolites were obtained, which could be used for precise control of silage fermentation quality (Xu et al., 2019; Wu et al., 2020; Zi et al., 2021; Li et al., 2021; Du et al., 2022). The variation of pigment and phytol content in silage were reported, which was consistent with this study, that was silage significantly reduced the pigment content but had no significant effect on phytol content (Lv et al., 2017; Lv et al., 2020). Spearman correlation analysis between silage microbial and pigment and phytol content was conducted; some specific undesirable microorganisms were significant negatively associated with pigment and phytol content. The results suggest that it is possible to increase pigment and phytol content by reducing the specific undesirable microorganisms. Nevertheless, studies have shown that phytol can be preserved well in silage, and the fermentation quality did not affect its content (Lv et al., 2017; Lv et al., 2020). However, the role of silage microorganisms in pigment degradation is also unclear. Usually, it is certain that the forage with good fermentation quality is more conducive to long-term preservation. Therefore, in order to reduce the pigment degradation and silage well storage, it is of great significance to control the specific undesirable microorganisms in silage.
Conclusions
This study explored the dynamics of chlorophyll and phytol contents in different growth heights of P. sinese before and after silage and their relationship with silage microbial. The pigment content of P. sinese decreased with the increase of growth height and the difference of pigment content before and after silage was significant (except phytol). In addition, the pigment yield before and after silage at different growth heights were of significant differences, and yield of height H3 or H4 were obviously higher. Moreover, there were diverse fermentation quality and microbial community of P. sinese silage at different growth heights, and some undesirable microorganisms were negatively correlated with pigment content. Therefore, comprehensive consideration of pigment, phytol yield and silage quality, the optimum harvest growth height of P. sinese was 150 cm.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Author contributions
ML, RL, XZ, and HZ did the experimental design work. ML and RL conducted the experiments. ML, RL, XZ, and HZ analyzed the data. ML and XZ wrote and revised the manuscript. All authors read and approved the manuscript.
Funding
This study was funded by the Natural Science Foundation of Hainan Province, China (322RC774), the Key Research and Development Program of Hainan Province (GHYF2022004), the Ministry of Agriculture and Rural Affairs of the People’s Republic of China (16220078) and the Central Public-Interest Scientific Institution Basal Research Fund for Chinese Academy of Tropical Agricultural Sciences (1630032022011).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bal, E., Isevi, T., Bal, M. A. (2012). Characterization of an anti-listerial enterocin from wheat silage based enterococcus faecium. J. Basic Microbiol. 52 (5), 496–503. doi: 10.1002/jobm.201100235
Bobe, G., Zhang, Z., Kopp, R., Garzotto, M., Shannon, J., Takata, Y. (2020). Phytol and its metabolites phytanic and pristanic acids for risk of cancer: current evidence and future directions. Eur. J. Cancer Prev. 29 (2), 191–200. doi: 10.1097/CEJ.0000000000000534
Chen, L., Guo, G., Yuan, X., Zhang, J., Li, J., Shao, T. (2016). Effects of applying molasses, lactic acid bacteria and propionic acid on fermentation quality, aerobic stability and in vitro gas production of total mixed ration silage prepared with oat-common vetch intercrop on the Tibetan plateau. J. Sci. Food Agric. 96, 1678–1685. doi: 10.1002/jsfa.7271
Ding, L. L., Matsumura, M., Obitsu, T., Sugino, T. (2021). Phytol supplementation alters plasma concentrations of formate, amino acids, and lipid metabolites in sheep. Animal 15 (3), 100174. doi: 10.1016/j.animal.2021.100174
Dong, C. F., Shen, Y. X., Ding, C. L., Xu, N. X., Cheng, Y. H., Gu, H. R. (2013). The feeding quality of rice (Oryza sativa l.) straw at different cutting heights and the related stem morphological traits. Field Crop Res. 141, 1–8. doi: 10.1016/j.fcr.2012.11.003
Dunière, L., Sindou, J., Chaucheyras-Durand, F., Chevallier, I., Sergentet, D. (2013). Silage processing and strategies to prevent persistence of undesirable microorganisms. Anim. Feed Sci. Technol. 182, 1–15. doi: 10.1016/j.anifeedsci.2013.04.006
Du, Z., Sun, L., Lin, Y., Yang, F., Cai, Y. (2022). Using PacBio SMRT sequencing technology and metabolomics to explore the microbiota-metabolome interaction related to silage fermentation of woody Plant.Front. Microbiol. 13, 857431. doi: 10.3389/fmicb.2022.857431
Eggersdorfer, M., Wyss, A. (2018). Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 652, 18–26. doi: 10.1016/j.abb.2018.06.001
Hák, R., Rinderle- Zimmer, U., Lichtenthaler, H. K., Nátr, L. (1993). Chlorophyll a fluorescence signatures of nitrogen deficient barley leaves. Photosynthetica 28, 151–159. doi: 10.1007/BF02185415
King, C., McEniry, J., Richardson, M., O'Kiely, P. (2013). Silage fermentation characteristics of grass species grown under two nitrogen fertilizer inputs and harvested at advancing maturity in the spring growth. Grassl Sci. 59, 30–43. doi: 10.1111/grs.12011
Kwan, H. Y., Chao, X., Su, T., Fu, X. Q., Liu, B., Tse, A. K., et al. (2015). Dietary lipids and adipocytes: potential therapeutic targets in cancers. J. Nutr. Biochem. 26, 303–311. doi: 10.1016/j.jnutbio.2014.11.001
Li, M., Lv, R., Zhang, L., Zi, X., Zhou, H., Tang, J. (2021). Melatonin is a promising silage additive: Evidence from microbiota and Metabolites.Front. Microbiol. 12, 1232. doi: 10.3389/fmicb.2021.670764
Liu, Q., Chen, M., Zhang, J., Shi, S., Cai, Y. (2012). Characteristics of isolated lactic acid bacteria and their effectiveness to improve stylo (Stylosanthes guianensis sw.) silage quality at various temperatures. Anim. Sci. J. 83, 128–135. doi: 10.1111/j.1740-0929.2011.00937.x
Liu, Q. H., Wu, J. X., Dong, Z. H., Wang, S. R., Shao, T. (2020). Effects of overnight wilting and additives on the fatty acid profile, α-tocopherol and β-carotene of whole plant oat silages. Anim. Feed Sci. Technol. 260, 114370. doi: 10.1016/j.anifeedsci.2019.114370
Lv, R., El-Sabagh, M., Obitsu, T., Sugino, T., Kurokawa, Y., Kawamura, K. (2017). Effects of nitrogen fertilizer and harvesting stage on photosynthetic pigments and phytol contents of Italian ryegrass silage. Anim. Sci. J. 88, 1513–1522. doi: 10.1111/asj.12810
Lv, R., Elsabagh, M., Obitsu, T., Sugino, T., Kurokawa, Y., Kawamura, K. (2020). Effect of varying fermentation conditions with ensiling period and inoculum on photosynthetic pigments and phytol content in Italian ryegrass (Lolium multiflorum lam.) silage. Anim. Sci. J. 91, e13309. doi: 10.1111/asj.13309
Lv, R., Elsabagh, M., Obitsu, T., Sugino, T., Kurokawa, Y., Kawamura, K. (2021). Changes of photosynthetic pigments and phytol content at different levels of nitrogen fertilizer in Italian ryegrass fresh herbage and hay. Grassl Sci. 00, 1–7. doi: 10.1111/grs.12335
Murray, B. E. (1990). The life and times of the Enterococcus. Clin. Microbiol. Rev. 3 (1), 46–65. doi: 10.1128/CMR.3.1.46
Neylon, J. M., Kung, L. (2003). Effects of cutting height and maturity on the nutritive value of corn silage for lactating cows. J. Dairy Sci. 86 (6), 2.163–2.169. doi: 10.3168/jds.S0022-0302(03)73806-5
Ogunade, I. M., Jiang, Y., Pech Cervantes, A. A., Kim, D. H., Oliveira, A. S., Vyas, D., et al. (2018). Bacterial diversity and composition of alfalfa silage as analyzed by illumina MiSeq sequencing: Effects of, Escherichia coli, O157:H7 and silage additives. J. Dairy Sci. 101, 2048–2059. doi: 10.3168/jds.2017-12876
Palleroni, N. J., Bradbury, J. F. (1993). Stenotrophomonas, a new bacterial genus for Xanthomonas maltophilia (Hugh 1980) swings et al. 1983. Int. J. Syst. Bacteriol. 43, 606–609. doi: 10.1099/00207713-43-3-606
Roca-Saavedra, P., Mariño-Lorenzo, P., Miranda, J. M., Porto-Arias, J. J., Lamas, A., Vazquez, B. I., et al. (2017). Phytanicacid consumption and human health, risks, benefits and futuretrends: A review. Food Chem. 221, 237–247. doi: 10.1016/j.foodchem.2016.10.074
Sanborn, L. H., Reid, R., Bradley, A. S., Liu, X. (2021). The effect of water availability on the carbon and nitrogen isotope composition of a C4 plant (pearl millet, Pennisetum glaucum). J. Archaeol Sci. Rep. 38 (2), 103047. doi: 10.1016/j.jasrep.2021.103047
Santos Silla, M. H. (1996). Biogenic amines: their importance in foods. Int. J. Food Microbiol. 29, 213–231. doi: 10.1016/0168-1605(95)00032-1
Santra, K., Song, A., Petrich, J. W., Rasmussen, M. A. (2021). The degradation of chlorophyll pigments in dairy silage: the timeline of anaerobic fermentation. J. Sci. Food Agric. 101, 2863–2868. doi: 10.1002/jsfa.10917
Van Soest, P. J., Robertson, J. B., Lewis, B. A. (1991). Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J.Dairy Sci. 74, 3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2
Vetter, W., Schroder, M. (2010). Concentrations of phytanic acid and pristanic acid are higher in organic than in conventional dairy products from the German market. Food Chem. 119 (2), 746–752. doi: 10.1016/j.foodchem.2009.07.027
Wang, Y., He, L., Xing, Y., Zheng, Y., Zhou, W., Pian, R., et al. (2019). Dynamics of bacterial community and fermentation quality during ensiling of wilted and unwilted moringa oleifera leaf silage with or without lactic acid bacterial inoculants. mSphere 4, e00341–e00319. doi: 10.1128/mSphere.00341-19
Wright, M. E., Albanes, D., Moser, A. B., Weinstein, S. J., Snyder, K., Männistö, S., et al. (2014). Serum phytanic and pristanic acid levels and prostate cancer risk in Finnish smokers. Cancer Med. 3 (6), 1562–1569. doi: 10.1002/cam4.319
Wu, Z., Luo, Y., Bao, J., Luo, Y., Yu, Z. (2020). Additives affect the distribution of metabolic profile, microbial communities and antibiotic resistance genes in high-moisture sweet corn kernel silage. Bioresour. Technol. 315, 123821. doi: 10.1016/j.biortech.2020.123821
Xu, D., Ding, W., Ke, W., Li, F., Zhang, P., Guo, X. (2019). Modulation of metabolome and bacterial community in whole crop corn silage by inoculating homofermentative Lactobacillus plantarum and heterofermentative Lactobacillus buchneri. Front. Microbiol. 9, 3229. doi: 10.3389/fmicb.2018.03299
Yabuuchi, E., Kaneko, T., Yano, I., Moss, C. W., Miyoshi, N. (1983). Sphingobacterium gen. nov. Sphingobacterium spiritivorum comb. nov. Sphingobacterium multivorum comb. nov. sphingobacterium mizutae sp. nov. and Flavobacterium indologenes sp. nov.: Glucose-nonfermenting gram-negative rods in CDC groups IIK-2 and IIb. Int. J. Syst. Bacteriol. 33, 580–598. doi: 10.1099/00207713-33-3-580
Yang, K., Wang, L., Zhou, G., Lin, X., Peng, J., Wang, L., et al. (2017). Phytol promotes the formation of slow-twitch muscle fibers through PGC-1α/mi RNA but not mitochondria oxidation. J. Agric. Food Chem. 65 (29), 5916–5925. doi: 10.1021/acs.jafc.7b01048
Zi, X., Li, M., Chen, Y., Lv, R., Zhou, H., Tang, J. (2021). Effects of citric acid and Lactobacillus plantarum on silage quality and bacterial diversity of king grass silage. Front. Microbiol. 12, 362. doi: 10.3389/fmicb.2021.631096
Zi, X., Liu, Y., Chen, T., Li, M., Zhou, H., Tang, J. (2022). Effects of sucrose, glucose and molasses on fermentation quality and bacterial community of stylo silage. Fermentation. 8, 191. doi: 10.3390/fermentation8050191
Keywords: Pennisetum sinese, growth height, chlorophyll, phytol, silage microbial
Citation: Li M, Lv R, Zhou H and Zi X (2022) Dynamics and correlations of chlorophyll and phytol content with silage bacterial of different growth heights Pennisetum sinese. Front. Plant Sci. 13:996970. doi: 10.3389/fpls.2022.996970
Received: 18 July 2022; Accepted: 23 September 2022;
Published: 13 October 2022.
Edited by:
Siran Wang, Nanjing Agricultural University, ChinaReviewed by:
Cheng Wang, South China Agricultural University, ChinaLin Sun, Inner Mongolia Academy of Agricultural & Animal Husbandry Sciences, China
Copyright © 2022 Li, Lv, Zhou and Zi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hanlin Zhou, emhvdWhhbmxpbkBjYXRhcy5jbg==; Xuejuan Zi, eml4dWVqdWFuQDE2My5jb20=
†These authors have contributed equally to this work
 Mao Li
Mao Li Renlong Lv1,2†
Renlong Lv1,2†