- Key Laboratory of Hangzhou City for Ecosystem Protection and Restoration, College of Life and Environmental Sciences, Hangzhou Normal University, Hangzhou, China
Plant intraspecific trait variation (ITV) including sex-dependent differences are matters of many ecological consequences, from individual to ecosystem, especially in endangered and rare species. Taxus fuana is an endangered dioecious species with small and isolated populations endemic to the Himalayas region. Little is known about its trait variation between sexes, and among populations. In this study, 18 leaf traits from 179 reproductive trees (males and females) along the altitude (2600-3200m a.s.l.) of the T. fuana populations distributed in Gyirong County, Tibet, China, were measured. ITV and sources of variation in leaf traits were assessed. The relationship between leaf traits of males and females and altitude was analyzed separately. Variations in leaf traits of T. fuana ranged from 3.1% to 24.2%, with the smallest in leaf carbon content and the largest in leaf thickness to area ratio. On average 78.13% of the variation in leaf traits was from within populations and 21.87% among populations. The trends in leaf width, leaf nitrogen to phosphorus ratio, leaf carbon to nitrogen ratio, leaf carbon isotope ratio, and leaf nitrogen isotope ratio in relation to altitude were the same for males and females. Leaf length to width ratio varied significantly with altitude only in males, while leaf phosphorus content, leaf nitrogen content, and leaf carbon to phosphorus ratio varied significantly with altitude only in females. The correlation coefficients of most leaf traits of females with altitude were larger than that of males. In the relationship between leaf traits, there was a high similarity among males and females, but the altitude accounted for more explanation in females than in males. Our results suggested that the variation in leaf traits of T. fuana was small and did not dominate the interspecific competition in the local communities. Adaptation to the altitude gradient of T. fuana might be through altering nutrient storage processes and water use efficiency. Adaptation of male and female T. fuana to environmental changes showed differences, where the males were more tolerant and the females responded greatly to altitude. The differences in adaptation strategies between male and female T. fuana may be detrimental to the maintenance of their populations.
Introduction
The earliest study of plant intraspecific trait variation (ITV) could be attributed to the notable novelist and naturalist Johann Wolfgang von Goethe (Stegmann, 2021). However, most early studies in trait-based ecology focused on interspecific variation and only used a mean trait value per species, which assumed smaller trait variation among conspecific individuals (Suding et al., 2008; Shipley et al., 2016). Recent studies found that ITV of some plants could range from 10% to 40% of total variation (Albert et al., 2010; Messier et al., 2010; Siefert et al., 2015; Burton et al., 2017; Gaudard et al., 2019), 25% on average based on a global meta-analysis (Siefert et al., 2015). Another recent meta-analysis showed that the ecological effects of ITV are comparable to species effects (Des Roches et al., 2018). The ecological consequences of ITV have received ever more renewed interest from ecologists (Bolnick et al., 2011), such as population dynamics, interspecific interactions, stability, coexistence, and diversity of ecological communities (Steinmetz et al., 2020). Those findings suggest ITV could not be simply ignored in trait-based ecology (Shipley et al., 2016; Westerband et al., 2021; Rixen et al., 2022).
ITV is thought to be correlated with population size (Steinmetz et al., 2020), which means that populations of endangered species usually have lower ITV compared to widely distributed or common species. Species’ trait variation and covariation were thought to contribute to explaining ecological strategies in response to environmental gradients or changes (Jung et al., 2014). For instance, the variability of plant traits with altitude gradient reflects the adaptation of plants to the environment (Mathiasen and Premoli, 2016; de Villemereuil et al., 2018; Rathee et al., 2021). Changes in altitude lead to variations in some other environmental factors (e.g., temperature, light, etc.) that ultimately affect plant trait variability (Körner, 2007; Graae et al., 2012; Hartl-Meier et al., 2014; Sidor et al., 2015). Such trait–environment relationships are important for predicting the responses of global environmental change on individuals and populations of plants (Niinemets et al., 2015; Meng et al., 2017). Meanwhile, higher ITV could stabilize populations from extreme temporal fluctuations in population density and decrease extinction risk (Bolnick et al., 2011). This implies that exploration of ITV of rare and endangered species could contribute to understanding population demography, dynamic, and adaptation to natural and/or anthropogenic disturbances of rare and endangered species in changing environments (Meng et al., 2017; Song et al., 2020a). Therefore, incorporating ITV of rare and endangered species helps to better understand the mechanism for being endangered or threatened and benefits conservation practice (Chown, 2012; Cochrane et al., 2015; Turner et al., 2017; Álvarez-Yépiz et al., 2019). ITV generally includes morphological traits, functional traits, and stoichiometric traits by which to reveal the adaptation strategies of species to heterogeneous environments (Cordell et al., 2001; Elser et al., 2010; Albert et al., 2011; de Bello et al., 2011; Álvarez-Yépiz et al., 2019).
Sexual dimorphism is rather common not only in angiosperm but also in gymnosperm plants (64.6%) based on the latest estimation (Walas et al., 2018), such as Cycadidae, Gnetidae, Ginkgo, and some species in Pinidae (e.g. Taxaceae) (Ohri and Rastogi, 2020). The rate of dioecious plants is much higher than monoecious plants in the temperate climate zone (Walas et al., 2018). As for altitude, it has been found that the proportion of dioecious plants decreases with increasing altitude (Lin et al., 2020). Moreover, high altitude tends to exhibit male-biased sexes, while females are more abundant in lower altitude regions, because the relative abundance of environmental conditions is more favorable for the reproduction and survival of females (Ortiz et al., 2002; Juvany and Munné-Bosch, 2015; Retuerto et al., 2018). Due to different reproductive demands and selective pressures, male and female plants usually expressed different physiological, morphological, phenological, and reproductive traits (Obeso, 2002; Song et al., 2016). Studies found that female plants generally have higher photosynthetic rates than males owing to a compensation mechanism for reproductive costs (Obeso, 2002; Wu et al., 2021). In addition, females usually are smaller and grow more slowly than males (Cipollini and Whigham, 1994; Li et al., 2007; Seyedi et al., 2019; Zhang et al., 2019). Accumulating studies on functional sex-related trait differences of dioecious angiosperm plants (e.g. Populus) responding to stressful biotic and abiotic environmental factors have been conducted under controlled systems (Retuerto et al., 2018; Xia et al., 2020). However, few studies on trait-based ecology incorporated trait variation between genders (Galfrascoli and Calviño, 2020), especially for gymnosperm plants in natural ecosystems across environmental gradients (e.g. altitude).
Taxus fuana Nan Li & R.R. Mill (syn. Taxus contorta), is an endangered and dioecious gymnosperm endemic to the Western Himalayas region and grows as a tree or large shrub in the understory of mixed or Pinus forests along an altitude gradient ranging from 2600 to 3200 m in Southwest Tibet (China), Nepal, North India, and Pakistan (Shah et al., 2008; Song et al., 2020b). Due to its timber production, traditional medicinal uses, and commercial production of Taxol (i.e. the cancer-inhibitory alkaloid Paclitaxel) like other species of Taxus, this species also suffered from heavy anthropologically disturbances (Poudel et al., 2013; Song et al., 2020b). Previous studies found males have higher height, larger diameter, shorter needles, smaller leaf area, and stomata density than females but no differences between sexes in specific leaf area (SLA), leaf nitrogen, and carbon concentration in T. baccata (Iszkuło et al., 2009; Cedro and Iszkuło, 2011; Stefanović et al., 2017). However, to date, few studies focused on ITV and sex-dependent variation responding to environmental changes in natural ecosystems, and little is known if there are sexual dimorphisms in terms of leaf functional traits of T. fuana.
In this study, we sampled leaf materials and measured 18 leaf traits of T. fuana along an altitude gradient (from 2600 m to 3200 m a.s.l.) in Gyirong County, aiming to quantify the ITV and their environmental explanation. Specifically, we address the following scientific questions: 1) What are intraspecific variations (including sex-dependent variations) of leaf traits in T. fuana? 2) How do leaf traits of T. fuana vary along the altitude gradient? 3) Whether males trees respond differently from female ones along an altitude gradient?
Materials and methods
Study site and sampling
This study was conducted at Gyirong County (28°21′–28°29′ N, 85°13′–85°21′ E, 2600-3200 m a.s.l.), southwest of Tibet in China. The region is a subtropical mountain monsoon climate, with a mean average temperature of 8 - 11°C, and a mean annual precipitation of 800 mm. Based on our field survey, we observed 6 main (sub)populations with total individuals of more than 5000 which showed significantly male-biased populations in this area (Song et al., 2020b).
Among six known populations (Tangbo, Kaire, Guofu, Jilong, Langjiu, and Jipu) (Song et al., 2020b; Li et al., 2022) (Figure 1), individuals of T. fuana were randomly selected along an altitude gradient, with the distance between trees ensured to be above 50 m as far as possible. Fresh, mature (fully developed), and healthy leaves (needles) of T. fuana were sampled during the growing season (July–August) in 2018. In total, 179 samples were collected, of which 85 were males and 94 were females (The sex of the trees was identified according to whether they had obvious female cone or staminate strobilus). For each tree, we measured diameter at breast height (DBH), tree height, and altitude of the locality.
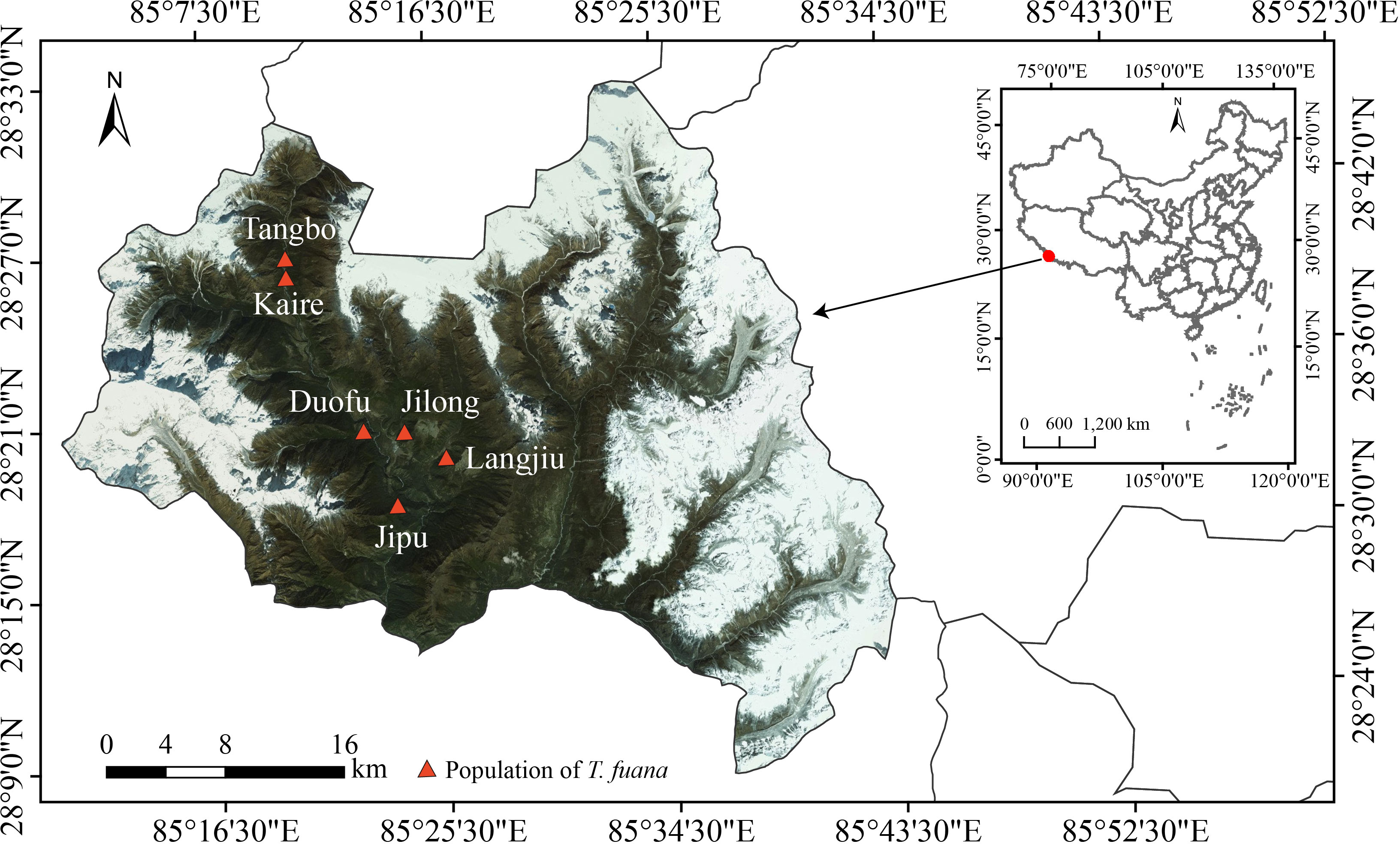
Figure 1 Geographical distribution of sampling sites (Li et al., 2022).
Leaf traits measurement
A total of 18 leaf traits were measured and categorized into morphological traits, functional traits, and stoichiometric traits (Pérez-Harguindeguy et al., 2013). For morphological traits, 10 fresh leaves from each tree were randomly selected and the thickness of each leaf was measured with a vernier caliper (0.01 mm), which was used to calculate the mean leaf thickness (LT) (mm) of each tree. Then, those leaves of each tree were separately immersed in water overnight, blotted up water, and measured for water-saturated weight. After that, the same leaf samples were scanned with a photo scanner and weighed after oven-dried at 60°C for 72h. Leaf area (LA) (cm2), leaf length (LL) (cm), leaf width (LW) (cm), leaf perimeter (LP) (cm) of each sample were accessed from scanned photo with ImageJ (http://imagej.nih.gov/ij/), which also was used to determine leaf length to width ratio [leaf length (cm)/leaf width(cm)] (LLWR) (cm cm-1), leaf thickness to area ratio [leaf thickness (cm)/leaf area (cm2)] (LTAR) (cm cm-2) and leaf profile index [leaf perimeter (cm)/ (cm)] (LPI) (cm cm-1) (Pérez-Harguindeguy et al., 2013).
For functional traits, the specific leaf area (SLA) (cm2 g-1) of each sample was calculated as the ratio of sample leaf area to oven-dry weight. Leaf dry matter content (LDMC) (g g-1) was determined by the ratio of leaf oven-dry weight to water-saturated weight. Leaf carbon (C) and nitrogen (N) content (mg g-1) was determined using an elemental analyzer (vario MICRO cube; Elemental, Germany) (Hu et al., 2015). Leaf phosphorus content (P) (mg g-1) was determined by digestion with HClO4-HNO3 and then measured by Prodigy7 ICP-OES Spectrometer (Leeman, US) (Hu et al., 2019).
For stoichiometric traits, leaf carbon to nitrogen ratio (C:N), leaf carbon to phosphorus ratio (C:P), and leaf nitrogen to phosphorus ratio (N:P) were calculated by the ratio of leaf C to leaf N, leaf C to leaf P and leaf N to leaf P, respectively. Leaf carbon isotope ratio (δ13C) (‰) and leaf nitrogen isotope ratio (δ15N) (‰) were determined using the elemental analyzer coupled to an isotope ratio mass spectrometer (Isoprime100; Isoprime Ltd, Germany) (Hu et al., 2017; Hu et al., 2022; Tan et al., 2022).
Statistical analyses
To describe the variation of T. fuana traits, the mean, minimum, maximum, and median values, as well as standard errors, and coefficients of variation (CV) were calculated for each leaf trait (Kumordzi et al., 2014). In order to analyze the intra-population variation of leaf traits, non-parametric multivariate analysis of variance with the “adonis” function in the “vegan” package was used to obtain among and within populations variance values for leaf traits, which were used as sources of variation among and within populations (Anderson, 2001). To investigate the overall response of T. fuana to environmental heterogeneity caused by altitude changes and the differential performance between males and females, a correlation analysis was conducted.
To explore the interrelationships among leaf traits of male and female T. fuana, and the differences between males and females, piecewise structural equation models (piecewiseSEM) were fitted to the leaf traits of male and female T. fuana, respectively (Lefcheck, 2016; Liu et al., 2020). Because the differences in light, water and heat conditions at different altitude (Körner, 2007; Moser et al., 2010) may have a greater impact on leaf traits of T. fuana, altitude was included as a random effect in the model, and the differences in marginal R2 (fixed factors only) and conditional R2 (all factors, including the random effect) were analyzed to assess the effect of altitude in T. fuana (Nakagawa and Schielzeth, 2013). Interrelationships among leaf traits were analyzed by standardized path coefficients. The fit of the model was confirmed using Fisher’s C test (when 0 ≤ Fisher’s C ≤ 2 and 0.05< P ≤ 1.00). The model was optimized by eliminating non-significant paths or factors (e.g., tree height, DBH, etc.), and the final model was obtained by comparison of models (Huang et al., 2020). The original model was constructed based on existing knowledge of the interrelationships of leaf traits (Navarro et al., 2010). A log10 transformation of the data was performed before model building. The main R packages involved in the model fitting process were “piecewiseSEM”, “nlme”, and “lme4” (Pinheiro et al., 2014; Lefcheck, 2016). All analyses were conducted using the statistical software R 4.2.1 (R Core Team, 2022).
Results
The variation of leaf traits in T. fuana
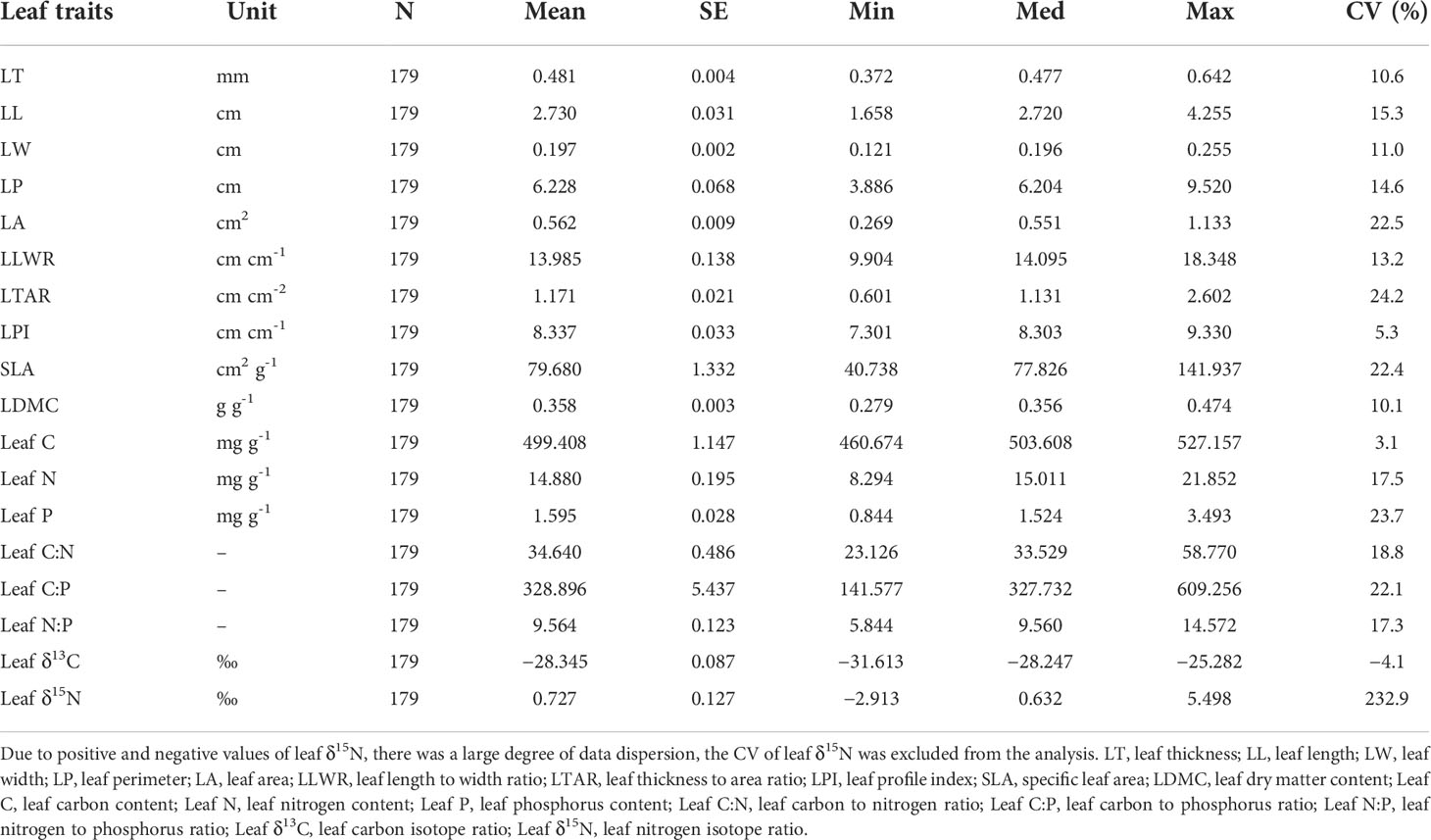
There was some variability among the 18 leaf traits of T. fuana (Table 1). The coefficients of variation were in the range of 3.1% to 24.2%. The larger variation occurred in leaf area, leaf thickness to area ratio, SLA, leaf P, and leaf C:P, respectively. The leaf thickness to area ratio was the most variable, ranging from 0.601-2.602, and the coefficient of variation was 24.2%. The smaller variation included leaf profile index, leaf C, and leaf δ13C, where the smallest variation was in leaf C, ranging from 460.674 to 527.157 and the coefficient of variation was 3.1%.
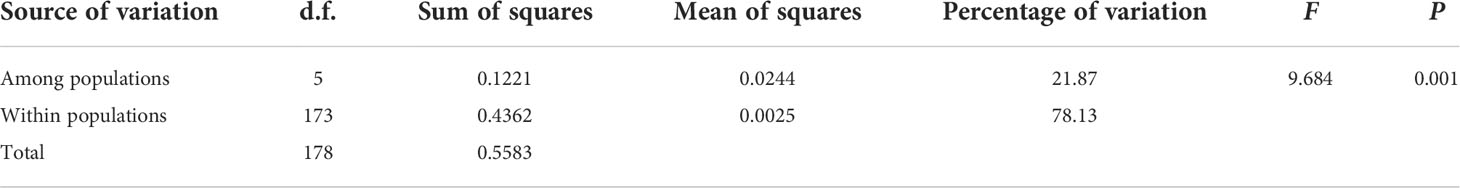
The results obtained from non-parametric analysis of variance showed that 78.13% of the 18 leaf traits variation for T. fuana could be attributed to differences within populations, while the remaining 21.87% explained variation among populations (Table 2) (F = 9.684, P = 0.001). The results indicated that the variation in leaf traits of T. fuana mainly occurred within populations.
Patterns of variation in leaf traits with altitude in T. fuana
On the whole, the leaf thickness (r = −0.149, P = 0.047) (Figure 2A), leaf width (r = −0.335, P< 0.001) (Figure 2C), leaf N (r = −0.205, P = 0.006) (Figure 2L), leaf C:P (r = −0.235, P = 0.002) (Figure 2O), leaf N:P (r = −0.572, P< 0.001) (Figure 2P) of T. fuana were significantly negatively correlated with altitude, whereas the leaf length to width ratio (r = 0.215, P = 0.004) (Figure 2F), leaf P (r = 0.283, P< 0.001) (Figure 2M), leaf C:N (r = 0.279, P< 0.001) (Figure 2N), leaf δ13C (r = 0.230, P = 0.002) (Figure 2Q), leaf δ15N (r = 0.243, P = 0.001) (Figure 2R) were significantly positively associated with altitude. The remaining leaf traits were not significantly related to altitude (Figures 2B, D, E, G–K).
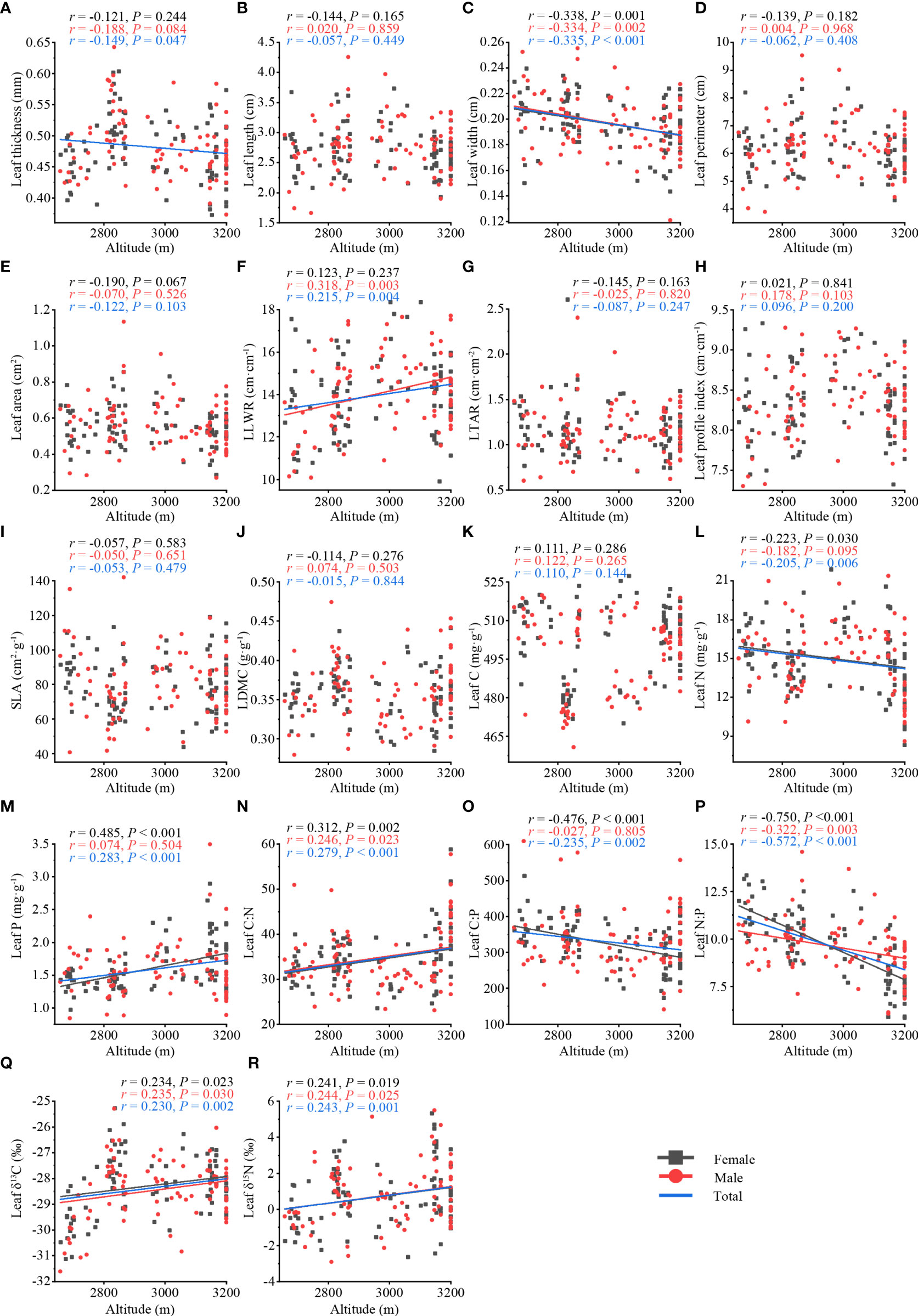
Figure 2 Relationship between leaf traits (total, female, male) along altitude in T. fuana. Morphological traits (A–H), functional traits (I–M), and stoichiometric traits (N–R). Regression lines were plotted for significant relationships with P < 0.05. LLWR, leaf length to width ratio; LTAR, leaf thickness to area ratio; SLA, specific leaf area; LDMC, leaf dry matter content; Leaf C, leaf carbon content; Leaf N, leaf nitrogen content; Leaf P, leaf phosphorus content; Leaf C:N, leaf carbon to nitrogen ratio; Leaf C:P, leaf carbon to phosphorus ratio; Leaf N:P, leaf nitrogen to phosphorus ratio; Leaf δ13C, leaf carbon isotope ratio; Leaf δ15N, leaf nitrogen isotope ratio.
In terms of sex, the leaf width (female: r = −0.338, P = 0.001; male: r = −0.334, P = 0.002) (Figure 2C) and leaf N:P (female: r = −0.750, P< 0.001; male: r = −0.322, P = 0.003) (Figure 2P) of female and male T. fuana were significantly negatively correlated with altitude, whereas leaf C:N (female: r = 0.312, P = 0.002; male: r = 0.246; P = 0.023) (Figure 2N), leaf δ13C (female: r = 0.234, P =0.023; male: r = 0.235, P = 0.030) (Figure 2Q), and leaf δ15N (female: r = 0.241, P = 0.019; male: r = 0.244, P = 0.025) (Figure 2R) were significantly positively correlated with altitude in both female and male. However, the leaf length to width ratio (male: r = 0.318, P = 0.003) (Figure 2F) showed a significant increase with altitude only in males. In addition, leaf P (female: r = 0.485, P< 0.001) (Figure 2M) increased significantly with altitude only in females, and leaf N (female: r = −0.223, P = 0.030) (Figure 2L) and leaf C:P (female: r = −0.476, P< 0.001) (Figure 2O) decreased significantly with altitude only in females. It was worth noting that the number of leaf traits significantly correlated with altitude in females was higher than that in males, as well as the magnitude of correlation coefficients.
Interrelations between male and female leaf traits in T. fuana
Results of the structural equation model revealed that there was a greater similarity in the relationship between male and female leaf traits of T. fuana (Figures 3A, B). However, some differences were shown between the two models. As far as the direct effects of leaf traits are concerned, the principal variation in leaf traits between male and female T. fuana was manifested by the intervention of the additional leaf length and the relationship between leaf area and leaf C with other leaf traits in males. On the other hand, the path coefficients between leaf traits of male T. fuana were relatively larger than those of females.
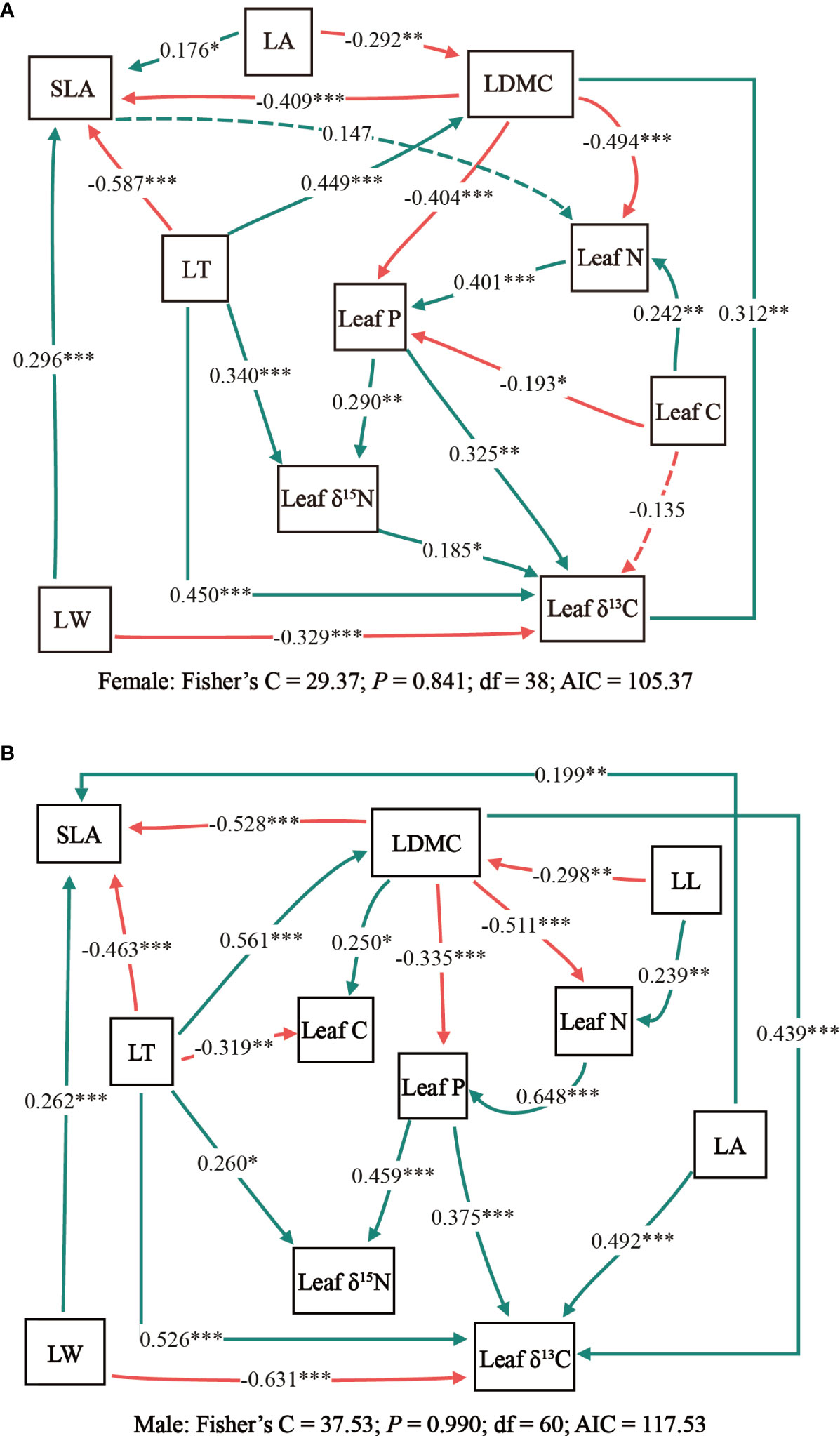
Figure 3 The piecewise structural equation models for testing the interrelations of leaf traits in female (A) and male (B) T. fuana. Solid arrows represent significant paths (P< 0.05) and dashed arrows represent non-significant paths (P > 0.05). The red arrows reflect negative relationships and the green arrows reflect positive relationships. Each path coefficient was standardized. *, **, *** indicated P < 0.05, P < 0.01, P < 0.001, respectively. LT, leaf thickness; LL, leaf length; LW, leaf width; LA, leaf area; SLA, specific leaf area; LDMC, leaf dry matter content; Leaf C, leaf carbon content; Leaf N, leaf nitrogen content; Leaf P, leaf phosphorus content; Leaf δ13C, leaf carbon isotope ratio; Leaf δ15N, leaf nitrogen isotope ratio.
The two structural equation models in this study differed significantly in the amount of explanation for leaf traits (Table 3). In female T. fuana, except for the LDMC, the conditional R2 (all factors) of the model for leaf traits was above 0.6. The variation of LDMC and leaf area explained by the model was minimum and maximum, respectively, and the conditional R2 (all factors) was 0.29 and 0.83. Correspondingly, the model of male T. fuana explained the variation of leaf δ15N was minimum, the conditional R2 (all factors) was 0.29. Among the other leaf traits, there were three leaf traits with conditional R2 (all factors) reaching 0.8, these were SLA, leaf P, and leaf δ13C. On the whole, the conditional R2 (all factors) of most leaf traits in male T. fuana was greater than that of females. Regarding marginal R2 (fixed effects), females had lower marginal R2 (fixed effects) than males except for leaf N. In contrast, the difference between marginal R2 (fixed effects) and conditional R2 (all factors) was higher in females than in males overall, which indicated that the random factor “altitude” accounted for more explanation in females than in males, namely, altitude had a higher effect on leaf traits in female T. fuana than in males.
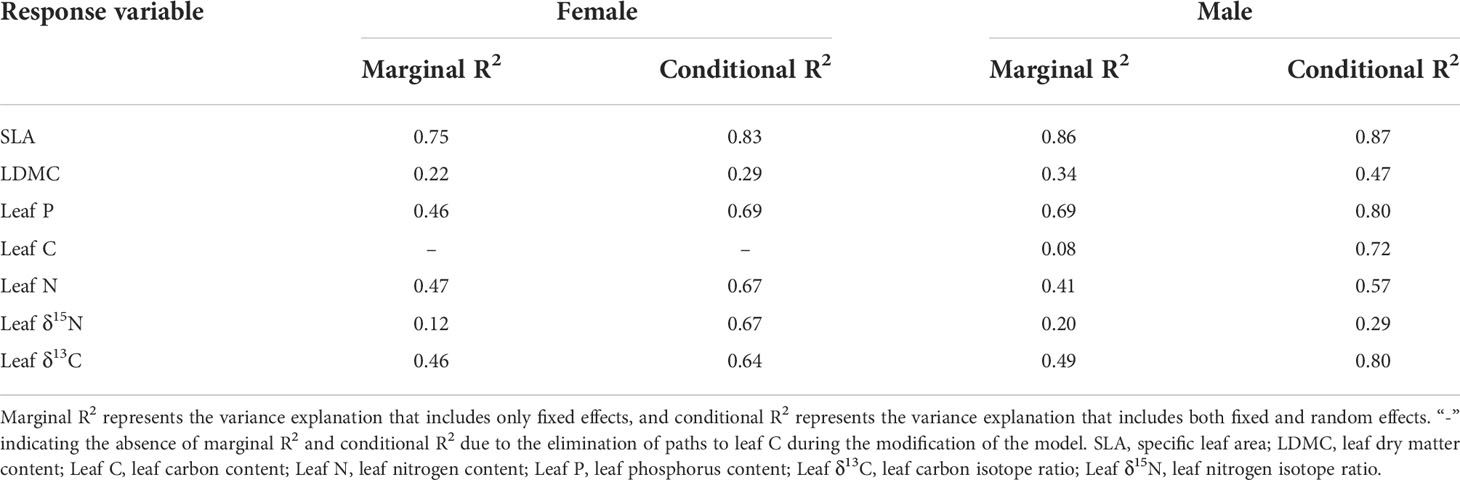
Table 3 The marginal R2 and conditional R2 of the structural equation model in functional traits of female and male T. fuana.
Discussion
Extent and sources of leaf trait variation in T. fuana
Generally, in most of the ecological studies on functional traits, the mean value of a trait was often taken to represent the whole species while ignoring ITV, which assumed there were few intraspecific variations compared to interspecific variations (Bolnick et al., 2011; de Bello et al., 2011; Shipley et al., 2016). However, numerous important functional traits showed considerable intraspecific variation with potential ecological effects similar to interspecific variation (Albert et al., 2011; Siefert et al., 2015; Moran et al., 2016; Laughlin et al., 2017; He et al., 2021; Rixen et al., 2022). The loss of ITV was even thought to be an important reason for its rareness in some wild species (Severns and Liston, 2008; González-Suárez and Revilla, 2013; Wang et al., 2022). Increasingly, studies found that ITV was probably an important ecological variable (Moran et al., 2016; Derroire et al., 2018; Mougi, 2020; Senthilnathan and Gavrilets, 2021), which played a non-negligible role in species coexistence (Ehlers et al., 2016; Hart et al., 2016; Zhang and Yu, 2018; Wang et al., 2022), interspecific competition (Kostikova et al., 2016; He et al., 2018; Xu et al., 2020), and population maintenance (Deepa, 2009; Bolnick et al., 2011; Raffard et al., 2019), etc. In this study, we found that leaf traits of six populations of T. fuana exhibited a certain degree of ITV, and the variation of leaf traits mostly originated from within populations and a small portion from among populations, which may be related to the distribution of T. fuana and local community features (Zhang et al., 2020; Li et al., 2022). It was shown that the importance of interspecific trait variation was likely to be greater than that of ITV at the large spatial scales of the study subjects. However, along with decreasing scales, for ITV, their relative importance would continually increase and possibly even emerge similar to that of interspecific trait variation (Messier et al., 2010; Albert et al., 2011; Kumordzi et al., 2014). Other studies had showed that the greater habitat heterogeneity, the more intraspecific and interspecific trait variation would be (Zhang and Yu, 2018; Homeier et al., 2021). The distribution area of T. fuana in the Gyirong region was relatively narrow (Song et al., 2020b), hence it showed a certain extent of ITV. Generally, the variation within populations reflects the plasticity and flexibility of the species to adapt to the environment, while the variation among populations reveals the influence of environmental selection (Siefert et al., 2015; Lajoie and Vellend, 2018; Moran et al., 2022). We found a small proportion of the ITV in T. fuana originated from among populations, perhaps because of the narrow distribution with little environmental heterogeneity among populations, and then failed to generate a large variation. In addition, the majority of trait variations originating from within populations may be associated with the position of T. fuana in the tree layer of the local community (Dan et al., 2020; Song et al., 2020b; Zhang et al., 2020). Plant communities in the Gyirong region were dominated by the tree layer, and strong interspecific competition led to a large plastic variation within the population. Therefore, the interactions between biotic factors in the community were perhaps the primary factors that determined the variation in T. fuana leaf traits.
Variation patterns of leaf traits along altitude in T. fuana
Plant trait variation is often influenced by climate and topography (Brousseau et al., 2013; Heilmeier, 2019; Dobbert et al., 2021; Joswig et al., 2022), which in general is mainly influenced by climatic factors at the global scale and by topographic factors at the small scale (van Bodegom et al., 2014; Bruelheide et al., 2018; Myers-Smith et al., 2019; Shao et al., 2019; Zheng et al., 2022). Altitude is a key topographic factor (Graae et al., 2012; Waigwa et al., 2020; Tang et al., 2022), and changes in altitude can lead to changes in temperature (Sidor et al., 2015; Chen et al., 2018), precipitation (Luce et al., 2013; Herrmann et al., 2016), light (Körner, 2007; Thakur et al., 2019), etc., resulting in hydrothermal differences that affect plant traits (Hartl-Meier et al., 2014; Zhang et al., 2022). In this study, we found that the leaf traits of T. fuana showed different trends with altitude. Among them, leaf width became significantly smaller with altitude, while the leaf length did not change significantly with altitude, thus leading to a larger leaf length to width ratio with altitude. This indicated that the leaves of T. fuana were more needle-shaped with increasing altitude. It was also found that the leaf thickness decreased slightly with altitude, indicating that the leaves became softer while needle-shaped, which was probably related to the adaptation of T. fuana to snowfall and resistance to the tearing by strong winds. As the altitude increases, the likelihood and amount of snowfall become greater (Šípek and Tesař, 2014; Deng et al., 2017). The more needle-shaped and softer leaves would better avoid excessive snow accumulation and reduce damage, as well as resist extreme wind with some advantages (Read and Sanson, 2003; Onoda et al., 2011; Hua et al., 2020). In other aspects, the variation of leaf carbon content with altitude was not significant, thus the significant variation of leaf carbon to nitrogen ratio and leaf carbon to phosphorus ratio with altitude was mainly determined by the change in leaf nitrogen content and leaf phosphorus content, which indicated that the leaf carbon content of T. fuana was highly stable in accordance with universality (McGroddy et al., 2004; Elser et al., 2010; Yang and Luo, 2011). The leaf nitrogen content was reduced with increasing altitude, while the leaf phosphorus content showed the opposite trend, which in turn contributed to the reduction of leaf nitrogen to phosphorus ratio with increasing altitude. The leaf nitrogen content, leaf phosphorus content, and leaf nitrogen to phosphorus ratio reflect the nutrient utilization status of plants (Thompson et al., 1997; Reich and Oleksyn, 2004; Chen et al., 2013). The mean value of leaf nitrogen to phosphorus ratio in this study was 9.564, indicating that the growth and development of T. fuana were principally limited by nitrogen (Koerselman and Meuleman, 1996; Güsewell et al., 2003; LeBauer and Treseder, 2008). The decrease in nitrogen mineralization rate with increasing altitude probably inhibited the uptake of nitrogen by plants and thus caused a decrease in leaf nitrogen content (Reich and Oleksyn, 2004). In addition, the reduced plant growth and metabolic activities may also lead to lower leaf nitrogen content as the temperature drops with increasing altitude (Elser et al., 2003). In general, plants prefer to accumulate excess nutrients in extreme environments, especially excess leaf phosphorus content to enhance survival (Chapin, 1990; Cordell et al., 2001). Therefore, as the habitat was progressively harsher with increasing altitude, T. fuana might improve leaf phosphorus to adapt to the habitat change, especially under nitrogen limitations. This suggested that the environmental heterogeneity caused by altitude change affected the nutrient storage process of T. fuana. It was at the same time found that both leaf δ13C and leaf δ15N were greater with increasing altitude, which seemed to be related to the temperature and precipitation variability caused by altitude changes (Zhou et al., 2011; Yang et al., 2013; Li et al., 2017). In general, both temperature and precipitation were reduced with increasing altitude, and the reduction of temperature would diminish the diffusion capacity of CO2 in leaves, which in turn reduced the CO2 conductance of stomata and increased δ13C in leaves (Hultine and Marshall, 2000; Diefendorf et al., 2010). Meanwhile, leaf δ13C was an indicator of plant water use efficiency, and the higher the leaf δ13C, the higher the plant water use efficiency (Pascual et al., 2013; Barbour, 2017). This indicated that the water use efficiency of T. fuana gradually improved with increasing altitude and precipitation reduction, which was a manifestation that T. fuana adapted to environmental changes. The relationship between leaf δ15N and altitude was complicated, and the correlation differed with the species and environment in the studies (Liu and Wang, 2009; Gavazov et al., 2016; Wang et al., 2019). It has been shown that plant δ15N relates more to climate globally, such as negative relation with precipitation and positively correlated with temperature (Amundson et al., 2003). In this study, leaf δ15N was positively correlated with altitude, and from another perspective, it could be considered to be indirectly negatively correlated with temperature and precipitation, i.e., it is consistent with the relationship of precipitation in the global pattern and opposite to that of temperature. The possible reason was that the increase in altitude would lead to a decrease in precipitation and temperature, but the Gyirong region was more influenced by the warm and humid airflow from the Indian Ocean (Adhikari and Mejia, 2021; Li et al., 2022), so that precipitation might have a more prominent effect on T. fuana. Therefore, the leaf δ15N of T. fuana showed a positive trend with altitude. It implied that environmental factors are important drivers affecting the variation of T. fuana leaf traits, and their specific roles should be further considered in future studies.
Interrelations and differences between male and female T. fuana leaf traits
Differences between dioecious plant traits and their response to environmental changes might detrimentally affect population maintenance (Melnikova et al., 2017; Hultine et al., 2018; LeRoy et al., 2020). In this study, leaf width, leaf nitrogen to phosphorus ratio, leaf carbon to nitrogen ratio, leaf δ13C, and leaf δ15N showed similar trends with altitude between male T. fuana and females, which reflected the similarity in adaptation to altitude changes between genders. Comparing the two structural equation models also exhibited such a situation, i.e., a greater similarity in the relationship between male and female leaf traits. This illustrated the coherence of adaptation strategies between male and female T. fuana (Ortiz et al., 2002; Munné-Bosch, 2015; Wang et al., 2018). However, in the correlation coefficients between leaf traits and altitude, females were mostly greater than males. Moreover, females exhibited remarkable performance in terms of stoichiometric characteristics. For instance, in leaf nitrogen content, leaf carbon to phosphorus ratio, and leaf phosphorus content, females reached significant levels with altitude only. Meanwhile, males showed relatively pronounced morphological characteristics, such as in leaf length to width ratio, where males reached significant levels with altitude only. These suggested that female T. fuana responded more strongly to altitude, and it can be inferred that the differences are mainly in nutrient utilization, while male T. fuana showed more stability to environmental heterogeneity caused by altitude changes (Lei et al., 2017). This might be related to the fact that males are more focused on vegetative growth, i.e. males may devote more resources to vegetative growth and thus have greater tolerance to corresponding environmental changes (Obeso, 2002; Meinzer et al., 2011). This was further illustrated in the performance of structural equation models, where the path coefficients among male leaf traits were mostly larger than the corresponding path coefficients for females (e.g., SLA, LDMC, leaf N, P, etc.). This may be related to the difference in resource absorption between the sexes. Studies showed that female trees allocated higher resources to reproduction than males, while males were likely to devote more resources to vegetative growth (Tognetti, 2012; Hultine et al., 2016; Lei et al., 2017). Therefore, this may result in the leaf traits of males being more closely linked and more tolerant to environmental changes, while females are more susceptible to multiple factors. A similar phenomenon was presented for the explained values (marginal R2 (fixed effect) and conditional R2 (all factors)). This suggested that the fixed factors could explain more variation in male T. fuana leaf traits than in females, while the random factor (altitude) accounted for a greater amount of explanation in females. Therefore, the inference seems to be that female T. fuana was more influenced by other factors or responded more strongly to environmental variability. Males, on the other hand, possess higher stability and could tolerate more environmental changes. This result supports previous studies that males were more adaptable than females and in a more favorable position in population development, which in turn possibly led to male-biased sex in T. fuana populations (Song et al., 2020b). Overall, it is inferred that male and female T. fuana exhibited greater similarity in their strategies for adapting to environmental changes, but there was also a considerable extent of variation between the male and female.
Conclusions
The analysis revealed that the variance of T. fuana leaf traits was at a low level and did not dominate the interspecific competition in the local communities. Altitude was an important factor affecting the variation of T. fuana leaf traits. Adaptation of T. fuana to altitude gradient probably through altered nutrient storage processes and water use efficiency. The growth and development of T. fuana was restricted mainly by nitrogen, and probably adapted to the altitude change by modifying the nutrient storage process and water use efficiency. Male and female T. fuana showed differences in their strategies for adapting to environmental variability, where the male T. fuana was more tolerant and the females responded more strongly to altitude changes and were more influenced by the environment. The differences in adaptation strategies between male and female T. fuana may be detrimental to the maintenance of their populations, besides the environment was an important aspect affecting T. fuana, which deserved further consideration in future studies.
Data availability statement
The original contributions presented in the study are included in the article/supplementary files. Further inquiries can be directed to Yao-Bin Song, eWJzb25nQGh6bnUuZWR1LmNu or Ming Dong, ZG9uZ21pbmdAaHpudS5lZHUuY24=.
Author contributions
Y-BS and MD contributed to the conception of the study. X-LS-T, T-XL, LX, W-JZ, J-PD performed the experiment and collected data. T-XL and X-LS-T analyzed the data. T-XL and Y-BS wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the National Key Research and Development Program of China, grant numbers 2016YFC0503100; and the National Natural Science Foundation of China, grant number 31670429 and 31400346.
Acknowledgment
We thank Yu-Lu Qin, Wang-Kai Shu, and Jun-Chao Ruan for their help in data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adhikari, P., Mejia, J. F. (2021). Influence of aerosols on clouds, precipitation and freezing level height over the foothills of the Himalayas during the Indian summer monsoon. Clim. Dyn. 57, 395–413. doi: 10.1007/s00382-021-05710-2
Albert, C. H., Grassein, F., Schurr, F. M., Vieilledent, G., Violle, C. (2011). When and how should intraspecific variability be considered in trait-based plant ecology? Perspect. Plant Ecol. Evol. Syst. 13, 217–225. doi: 10.1016/j.ppees.2011.04.003
Albert, C. H., Thuiller, W., Yoccoz, N. G., Soudant, A., Boucher, F., Saccone, P., et al. (2010). Intraspecific functional variability: Extent, structure and sources of variation. J. Ecol. 98, 604–613. doi: 10.1111/j.1365-2745.2010.01651.x
Álvarez-Yépiz, J. C., Búrquez, A., Martínez-Yrízar, A., Dovciak, M. (2019). A trait-based approach to the conservation of threatened plant species. Oryx 53, 429–435. doi: 10.1017/s003060531800087x
Amundson, R., Austin, A. T., Schuur, E. A. G., Yoo, K., Matzek, V., Kendall, C., et al. (2003). Global patterns of the isotopic composition of soil and plant nitrogen. Glob. Biogeochem. Cycle 17, 1031. doi: 10.1029/2002GB001903
Anderson, M. J. (2001). A new method for non-parametric multivariate analysis of variance. Aust. Ecol. 26, 32–46. doi: 10.1111/j.1442-9993.2001.01070.pp.x
Barbour, M. M. (2017). Understanding regulation of leaf internal carbon and water transport using online stable isotope techniques. New Phytol. 213, 83–88. doi: 10.1111/nph.14171
Bolnick, D. I., Amarasekare, P., Araújo, M. S., Bürger, R., Levine, J. M., Novak, M., et al. (2011). Why intraspecific trait variation matters in community ecology. Trends Ecol. Evol. 26, 183–192. doi: 10.1016/j.tree.2011.01.009
Brousseau, L., Bonal, D., Cigna, J., Scotti, I. (2013). Highly local environmental variability promotes intrapopulation divergence of quantitative traits: An example from tropical rain forest trees. Ann. Bot. 112, 1169–1179. doi: 10.1093/aob/mct176
Bruelheide, H., Dengler, J., Purschke, O., Lenoir, J., Jiménez-Alfaro, B., Hennekens, S. M., et al. (2018). Global trait-environment relationships of plant communities. Nat. Ecol. Evol. 2, 1906–1917. doi: 10.1038/s41559-018-0699-8
Burton, J. I., Perakis, S. S., McKenzie, S. C., Lawrence, C. E., Puettmann, K. J. (2017). Intraspecific variability and reaction norms of forest understory plant species traits. Funct. Ecol. 31, 1881–1893. doi: 10.1111/1365-2435.12898
Cedro, A., Iszkuło, G. (2011). Do females differ from males of European yew (Taxus baccata L.) in dendrochronological analysis? Tree-Ring Res. 67, 3–11. doi: 10.3959/2009-9.1
Chapin, F. (1990). The ecology and economics of storage in plants. Annu. Rev. Ecol. Syst. 21, 423–447. doi: 10.1146/annurev.es.21.110190.002231
Chen, Y. H., Han, W. X., Tang, L. Y., Tang, Z. Y., Fang, J. Y. (2013). Leaf nitrogen and phosphorus concentrations of woody plants differ in responses to climate, soil and plant growth form. Ecography 36, 178–184. doi: 10.1111/j.1600-0587.2011.06833.x
Chen, L., Huang, J. G., Ma, Q. Q., Hänninen, H., Rossi, S., Piao, S. L., et al. (2018). Spring phenology at different altitudes is becoming more uniform under global warming in Europe. Glob. Change Biol. 24, 3969–3975. doi: 10.1111/gcb.14288
Chown, S. L. (2012). Trait-based approaches to conservation physiology: Forecasting environmental change risks from the bottom up. Philos. Trans. R. Soc B. 367, 1615–1627. doi: 10.1111/1365-2656.13462
Cipollini, M. L., Whigham, D. F. (1994). Sexual dimorphism and cost of reproduction in the dioecious shrub Lindera benzoin (Lauraceae). Am. J. Bot. 81, 65–75. doi: 10.1002/j.1537-2197.1994.tb15410.x
Cochrane, A., Yates, C. J., Hoyle, G. L., Nicotra, A. B. (2015). Will among-population variation in seed traits improve the chance of species persistence under climate change? Glob. Ecol. Biogeogr. 24, 12–24. doi: 10.1111/geb.12234
Cordell, S., Goldstein, G., Meinzer, F. C., Vitousek, P. M. (2001). Morphological and physiological adjustment to N and P fertilization in nutrient-limited Metrosideros polymorpha canopy trees in Hawaii. Tree Physiol. 21, 43–50. doi: 10.1093/treephys/21.1.43
Dan, Z., Li, B. Z., Yin, Z. J. (2020). Analysis of the characteristics and floristic elements of communities containing Taxus contorta Griffith in Zhumulang-Mafeng National Nature Reserve, Tibet. Plant Sci. J. 38, 58–67. doi: 10.11913/PSJ.2095-0837.2020.10058
de Bello, F., Lavorel, S., Albert, C. H., Thuiller, W., Grigulis, K., Dolezal, J., et al. (2011). Quantifying the relevance of intraspecific trait variability for functional diversity. Methods Ecol. Evol. 2, 163–174. doi: 10.1111/j.2041-210X.2010.00071.x
Deepa, A. (2009). The stabilizing effect of intraspecific genetic variation on population dynamics in novel and ancestral habitats. Am. Nat. 174, 255–267. doi: 10.1086/600085
Deng, H. J., Pepin, N. C., Chen, Y. N. (2017). Changes of snowfall under warming in the Tibetan Plateau. J. Geophys. Res.-Atmos. 122, 7323–7341. doi: 10.1002/2017JD026524
Derroire, G., Powers, J. S., Hulshof, C. M., Varela, L. E. C., Healey, J. R., Sveriges, L. (2018). Contrasting patterns of leaf trait variation among and within species during tropical dry forest succession in Costa Rica. Sci. Rep. 8, 285. doi: 10.1038/s41598-017-18525-1
Des Roches, S., Post, D. M., Turley, N. E., Bailey, J. K., Hendry, A. P., Kinnison, M. T., et al. (2018). The ecological importance of intraspecific variation. Nat. Ecol. Evol. 2, 57–64. doi: 10.1038/s41559-017-0402-5
de Villemereuil, P., Mouterde, M., Gaggiotti, O. E., Till-Bottraud, I. (2018). Patterns of phenotypic plasticity and local adaptation in the wide elevation range of the alpine plant Arabis alpina. J. Ecol. 106, 1952–1971. doi: 10.1111/1365-2745.12955
Diefendorf, A. F., Mueller, K. E., Wing, S. L., Koch, P. L., Freeman, K. H. (2010). Global patterns in leaf 13C discrimination and implications for studies of past and future climate. Proc. Natl. Acad. Sci. U. S. A. 107, 5738–5743. doi: 10.1073/pnas.0910513107
Dobbert, S., Pape, R., Löffler, J. (2021). How does spatial heterogeneity affect inter- and intraspecific growth patterns in tundra shrubs? J. Ecol. 109, 4115–4131. doi: 10.1111/1365-2745.13784
Ehlers, B. K., Damgaard, C. F., Laroche, F. (2016). Intraspecific genetic variation and species coexistence in plant communities. Biol. Lett. 12, 20150853. doi: 10.1098/rsbl.2015.0853
Elser, J. J., Acharya, K., Kyle, M., Cotner, J., Makino, W., Markow, T., et al. (2003). Growth rate-stoichiometry couplings in diverse biota. Ecol. Lett. 6, 936–943. doi: 10.1046/j.1461-0248.2003.00518.x
Elser, J. J., Fagan, W. F., Kerkhoff, A. J., Swenson, N. G., Enquist, B. J. (2010). Biological stoichiometry of plant production: metabolism, scaling and ecological response to global change. New Phytol. 186, 593–608. doi: 10.1111/j.1469-8137.2010.03214.x
Galfrascoli, G. M., Calviño, A. (2020). Secondary sexual dimorphism in a dioecious tree: A matter of inter-plant variability? Flora 266, 151595. doi: 10.1016/j.flora.2020.151595
Gaudard, C. A., Robertson, M. P., Bishop, T. R. (2019). Low levels of intraspecific trait variation in a keystone invertebrate group. Oecologia 190, 725–735. doi: 10.1007/s00442-019-04426-9
Gavazov, K., Hagedorn, F., Buttler, A., Siegwolf, R., Bragazza, L. (2016). Environmental drivers of carbon and nitrogen isotopic signatures in peatland vascular plants along an altitude gradient. Oecologia 180, 257–264. doi: 10.1007/s00442-015-3458-4
González-Suárez, M., Revilla, E. (2013). Variability in life-history and ecological traits is a buffer against extinction in mammals. Ecol. Lett. 16, 242–251. doi: 10.1111/ele.12035
Graae, B. J., De Frenne, P., Kolb, A., Brunet, J., Chabrerie, O., Verheyen, K., et al. (2012). On the use of weather data in ecological studies along altitudinal and latitudinal gradients. Oikos 121, 3–19. doi: 10.1111/j.1600-0706.2011.19694.x
Güsewell, S., Koerselman, W., Verhoeven, J. (2003). Biomass N:P ratios as indicators of nutrient limitation for plant populations in wetlands. Ecol. Appl. 13, 372–384. doi: 10.1890/1051-0761(2003)013[0372:BNRAIO]2.0.CO;2
Hartl-Meier, C., Zang, C., Dittmar, C., Esper, J., Gottlein, A., Rothe, A. (2014). Vulnerability of Norway spruce to climate change in mountain forests of the European Alps. Clim. Res. 60, 119–132. doi: 10.3354/cr01226
Hart, S. P., Schreiber, S. J., Levine, J. M. (2016). How variation between individuals affects species coexistence. Ecol. Lett. 19, 825–838. doi: 10.1111/ele.12618
He, D., Biswas, S. R., Xu, M. S., Yang, T. H., You, W. H., Yan, E. R. (2021). The importance of intraspecific trait variability in promoting functional niche dimensionality. Ecography 44, 380–390. doi: 10.1111/ecog.05254
He, D., Chen, Y. F., Zhao, K. N., Cornelissen, J. H. C., Chu, C. J. (2018). Intra- and interspecific trait variations reveal functional relationships between specific leaf area and soil niche within a subtropical forest. Ann. Bot. 121, 1173–1182. doi: 10.1093/aob/mcx222
Heilmeier, H. (2019). Functional traits explaining plant responses to past and future climate changes. Flora 254, 1–11. doi: 10.1016/j.flora.2019.04.004
Herrmann, S. M., Didan, K., Barreto-Munoz, A., Crimmins, M. A. (2016). Divergent responses of vegetation cover in Southwestern US ecosystems to dry and wet years at different elevations. Environ. Res. Lett. 11, 124005. doi: 10.1088/1748-9326/11/12/124005
Homeier, J., Seeler, T., Pierick, K., Leuschner, C. (2021). Leaf trait variation in species-rich tropical Andean forests. Sci. Rep. 11, 9993. doi: 10.1038/s41598-021-89190-8
Hu, Y. K., Liu, G. F., Pan, X., Song, Y. B., Dong, M., Cornelissen, J. H. C. (2022). Contrasting nitrogen cycling between herbaceous wetland and terrestrial ecosystems inferred from plant and soil nitrogen isotopes across China. J. Ecol. 110, 1259–1270. doi: 10.1111/1365-2745.13866
Hu, Y. K., Pan, X., Liu, G. F., Li, W. B., Dai, W. H., Tang, S. L., et al. (2015). Novel evidence for within-species leaf economics spectrum at multiple spatial scales. Front. Plant Sci. 6, 901. doi: 10.3389/fpls.2015.00901
Hu, Y. K., Pan, X., Yang, X. J., Liu, G. F., Liu, X. Y., Song, Y. B., et al. (2019). Is there coordination of leaf and fine root traits at local scales? A test in temperate forest swamps. Ecol. Evol. 9, 8714–8723. doi: 10.1002/ece3.5421
Hu, Y. K., Zhang, Y. L., Liu, G. F., Pan, X., Yang, X., Li, W. B., et al. (2017). Intraspecific N and P stoichiometry of Phragmites australis: Geographic patterns and variation among climatic regions. Sci. Rep. 7, 43018. doi: 10.1038/srep43018
Hua, L., He, P., Goldstein, G., Liu, H., Yin, D., Zhu, S., et al. (2020). Linking vein properties to leaf biomechanics across 58 woody species from a subtropical forest. Plant Biol. 22, 212–220. doi: 10.1111/plb.13056
Huang, M. J., Liu, X., Zhou, S. R. (2020). Asynchrony among species and functional groups and temporal stability under perturbations: Patterns and consequences. J. Ecol. 108, 2038–2046. doi: 10.1111/1365-2745.13418
Hultine, K. R., Bush, S. E., Ward, J. K., Dawson, T. E. (2018). Does sexual dimorphism predispose dioecious riparian trees to sex ratio imbalances under climate change? Oecologia 187, 921–931. doi: 10.1007/s00442-018-4190-7
Hultine, K. R., Grady, K. C., Wood, T. E., Shuster, S. M., Stella, J. C., Whitham, T. G. (2016). Climate change perils for dioecious plant species. Nat. Plants 2, 16109. doi: 10.1038/nplants.2016.109
Hultine, K. R., Marshall, J. D. (2000). Altitude trends in conifer leaf morphology and stable carbon isotope composition. Oecologia 123, 32–40. doi: 10.1007/s004420050986
Iszkuło, G., Jasińska, A. K., Giertych, M. J., Boratyński, A. (2009). Do secondary sexual dimorphism and female intolerance to drought influence the sex ratio and extinction risk of Taxus baccata? Plant Ecol. 200, 229–240. doi: 10.1007/s11258-008-9447-5
Joswig, J. S., Wirth, C., Schuman, M. C., Kattge, J., Reu, B., Wright, I. J., et al. (2022). Climatic and soil factors explain the two-dimensional spectrum of global plant trait variation. Nat. Ecol. Evol. 6, 36–50. doi: 10.1038/s41559-021-01616-8
Jung, V., Albert, C. H., Violle, C., Kunstler, G., Loucougaray, G., Spiegelberger, T. (2014). Intraspecific trait variability mediates the response of subalpine grassland communities to extreme drought events. J. Ecol. 102, 45–53. doi: 10.1111/1365-2745.12177
Juvany, M., Munné-Bosch, S. (2015). Sex-related differences in stress tolerance in dioecious plants: A critical appraisal in a physiological context. J. Exp. Bot. 66, 6083–6092. doi: 10.1093/jxb/erv343
Koerselman, W., Meuleman, A. F. M. (1996). The vegetation N:P ratio: A new tool to detect the nature of nutrient limitation. J. Appl. Ecol. 33, 1441–1450. doi: 10.2307/2404783
Körner, C. (2007). The use of ‘altitude’ in ecological research. Trends Ecol. Evol. 22, 569–574. doi: 10.1016/j.tree.2007.09.006
Kostikova, A., Silvestro, D., Pearman, P. B., Salamin, N. (2016). Bridging inter- and intraspecific trait evolution with a hierarchical Bayesian approach. Syst. Biol. 65, 417–431. doi: 10.1093/sysbio/syw010
Kumordzi, B. B., Nilsson, M. C., Gundale, M. J., Wardle, D. A. (2014). Changes in local-scale intraspecific trait variability of dominant species across contrasting island ecosystems. Ecosphere 5, 26. doi: 10.1890/ES13-00339.1
Lajoie, G., Vellend, M. (2018). Characterizing the contribution of plasticity and genetic differentiation to community-level trait responses to environmental change. Ecol. Evol. 8, 3895–3907. doi: 10.1002/ece3.3947
Laughlin, D. C., Lusk, C. H., Bellingham, P. J., Burslem, D. F. R. P., Simpson, A. H., Kramer-Walter, K. R. (2017). Intraspecific trait variation can weaken interspecific trait correlations when assessing the whole-plant economic spectrum. Ecol. Evol. 7, 8936–8949. doi: 10.1002/ece3.3447
LeBauer, D. S., Treseder, K. K. (2008). Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 89, 371–379. doi: 10.1890/06-2057.1
Lefcheck, J. S. (2016). piecewiseSEM: Piecewise structural equation modelling in R for ecology, evolution, and systematics. Methods Ecol. Evol. 7, 573–579. doi: 10.1111/2041-210X.12512
Lei, Y. B., Jiang, Y. L., Chen, K., Duan, B. L., Zhang, S., Korpelainen, H., et al. (2017). Reproductive investments driven by sex and altitude in sympatric Populus and Salix trees. Tree Physiol. 37, 1503–1514. doi: 10.1093/treephys/tpx075
LeRoy, C. J., Ramstack Hobbs, J. M., Claeson, S. M., Moffett, J., Garthwaite, I., Criss, N., et al. (2020). Plant sex influences aquatic-terrestrial interactions. Ecosphere 11, e02994. doi: 10.1002/ecs2.2994
Li, M. X., Peng, C. H., Wang, M., Yang, Y. Z., Zhang, K. R., Li, P., et al. (2017). Spatial patterns of leaf δ13C and its relationship with plant functional groups and environmental factors in China. J. Geophys. Res.-Biogeosci. 122, 1564–1575. doi: 10.1002/2016JG003529
Li, T. X., Xu, L., Wang, F., Zhang, W. J., Duan, J. P., Shen-Tu, X. L., et al. (2022). Novel evidence from Taxus fuana forests for niche-neutral process assembling community. For. Ecosyst. 9, 100035. doi: 10.1016/j.fecs.2022.100035
Li, C. Y., Xu, G., Zang, R. G., Korpelainen, H., Berninger, F. (2007). Sex-related differences in leaf morphological and physiological responses in Hippophae rhamnoides along an altitudinal gradient. Tree Physiol. 27, 399–406. doi: 10.1093/treephys/27.3.399
Lin, H. Y., Tseng, Y. H., Hsieh, C. F., Hu, J. M. (2020). Geographical distribution of dioecy and its ecological correlates based on fine-scaled species distribution data from a subtropical island. Ecol. Res. 35, 170–181. doi: 10.1111/1440-1703.12068
Liu, W. G., Wang, Z. (2009). Nitrogen isotopic composition of plant-soil in the loess plateau and its responding to environmental change. Chin. Sci. Bull. 54, 272–279. doi: 10.1007/s11434-008-0442-y
Liu, S. G., Wang, H., Tian, P., Yao, X., Sun, H., Wang, Q. K., et al. (2020). Decoupled diversity patterns in bacteria and fungi across continental forest ecosystems. Soil Biol. Biochem. 144, 107763. doi: 10.1016/j.soilbio.2020.107763
Luce, C. H., Abatzoglou, J. T., Holden, Z. A. (2013). The missing mountain water: Slower westerlies decrease orographic enhancement in the Pacific Northwest USA. Science 342, 1360–1364. doi: 10.1126/science.1242335
Mathiasen, P., Premoli, A. C. (2016). Living on the edge: adaptive and plastic responses of the tree Nothofagus pumilio to a long-term transplant experiment predict rear-edge upward expansion. Oecologia 181, 607–619. doi: 10.1007/s00442-016-3568-7
McGroddy, M. E., Daufresne, T., Hedin, L. O. (2004). Scaling of C: N: P stoichiometry in forests worldwide: Implications of terrestrial Redfield-type ratios. Ecology 85, 2390–2401. doi: 10.1890/03-0351
Meinzer, F. C., Lachenbruch, B., Dawson, T. E. (2011). Size- and age-related changes in tree structure and function (Corvallis: Springer Netherlands). doi: 10.1007/978-94-007-1242-3
Melnikova, N. V., Borkhert, E. V., Snezhkina, A. V., Kudryavtseva, A. V., Dmitriev, A. A. (2017). Sex-specific response to stress in Populus. Front. Plant Sci. 8, 1827. doi: 10.3389/fpls.2017.01827
Meng, H., Wei, X., Franklin, S. B., Wu, H., Jiang, M. (2017). Geographical variation and the role of climate in leaf traits of a relict tree species across its distribution in China. Plant Biol. 19, 552–561. doi: 10.1111/plb.12564
Messier, J., McGill, B. J., Lechowicz, M. J. (2010). How do traits vary across ecological scales? A case for trait-based ecology. Ecol. Lett. 13, 838–848. doi: 10.1111/j.1461-0248.2010.01476.x
Moran, N. P., Caspers, B. A., Chakarov, N., Ernst, U. R., Fricke, C., Kurtz, J., et al. (2022). Shifts between cooperation and antagonism driven by individual variation: A systematic synthesis review. Oikos 2022, e08201. doi: 10.1111/oik.08201
Moran, E. V., Hartig, F., Bell, D. M. (2016). Intraspecific trait variation across scales: Implications for understanding global change responses. Glob. Change Biol. 22, 137–150. doi: 10.1111/gcb.13000
Moser, L., Fonti, P., Büntgen, U., Esper, J., Luterbacher, J., Franzen, J., et al. (2010). Timing and duration of European larch growing season along altitudinal gradients in the Swiss Alps. Tree Physiol. 30, 225–233. doi: 10.1093/treephys/tpp108
Mougi, A. (2020). Natural selection contributes to food web stability. PLoS One 15, e227420. doi: 10.1371/journal.pone.0227420
Munné-Bosch, S. (2015). Sex ratios in dioecious plants in the framework of global change. Environ. Exp. Bot. 109, 99–102. doi: 10.1016/j.envexpbot.2014.08.007
Myers-Smith, I. H., Thomas, H. J. D., Bjorkman, A. D. (2019). Plant traits inform predictions of tundra responses to global change. New Phytol. 221, 1742–1748. doi: 10.1111/nph.15592
Nakagawa, S., Schielzeth, H. (2013). A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 4, 133–142. doi: 10.1111/j.2041-210x.2012.00261.x
Navarro, T., El Oualidi, J., Taleb, M. S., Pascual, V., Cabezudo, B., Milla, R. (2010). Leaf patterns, leaf size and ecologically related traits in high Mediterranean mountain on the Moroccan High Atlas. Plant Ecol. 210, 275–290. doi: 10.1007/s11258-010-9756-3
Niinemets, U., Keenan, T. F., Hallik, L. (2015). A worldwide analysis of within-canopy variations in leaf structural, chemical and physiological traits across plant functional types. New Phytol. 205, 973–993. doi: 10.1111/nph.13096
Obeso, J. R. (2002). The costs of reproduction in plants. New Phytol. 155, 321–348. doi: 10.1046/j.1469-8137.2002.00477.x
Ohri, D., Rastogi, S. (2020). Sex determination in gymnosperms. Nucleus 63, 75–80. doi: 10.1007/s13237-019-00297-w
Onoda, Y., Westoby, M., Adler, P. B., Choong, A. M. F., Clissold, F. J., Cornelissen, J. H. C., et al. (2011). Global patterns of leaf mechanical properties. Ecol. Lett. 14, 301–312. doi: 10.1111/j.1461-0248.2010.01582.x
Ortiz, P. L., Arista, M., Talavera, S. (2002). Sex ratio and reproductive effort in the dioecious Juniperus communis subsp. alpina (Suter) Čelak. (Cupressaceae) along an altitudinal gradient. Ann. Bot. 89, 205–211. doi: 10.1093/aob/mcf028
Pascual, M., Lordan, J., Villar, J. M., Fonseca, F., Rufat, J. (2013). Stable carbon and nitrogen isotope ratios as indicators of water status and nitrogen effects on peach trees. Sci. Hortic. 157, 99–107. doi: 10.1016/j.scienta.2013.04.007
Pérez-Harguindeguy, N., Díaz, S., Garnier, E., Lavorel, S., Poorter, H., Jaureguiberry, P., et al. (2013). New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 61, 167–234. doi: 10.1071/BT12225
Pinheiro, J., Bates, D., Debroy, S., Sarkar, D., R Core Team (2014). nlme: Linear and nonlinear mixed effects model. R package version 3, 1–117. Available at: http://CRAN.R-project.org/package=nlme
Poudel, R. C., Gao, L. M., Möller, M., Baral, S. R., Uprety, Y., Liu, J., et al. (2013). Yews (Taxus) along the Hindu Kush-Himalayan region: Exploring the ethnopharmacological relevance among communities of Mongol and Caucasian origins. J. Ethnopharmacol. 147, 190–203. doi: 10.1016/j.jep.2013.02.031
R Core Team (2022). R: A language and environment for statistical computing (Vienna, Austria: R Foundation for Statistical Computing). Available at: https://www.R-project.org/.
Raffard, A., Santoul, F., Cucherousset, J., Blanchet, S. (2019). The community and ecosystem consequences of intraspecific diversity: A meta-analysis. Biol. Rev. 94, 648–661. doi: 10.1111/brv.12472
Rathee, S., Ahmad, M., Sharma, P., Singh, H. P., Batish, D. R., Kaur, S., et al. (2021). Biomass allocation and phenotypic plasticity are key elements of successful invasion of Parthenium hysterophorus at high elevation. Environ. Exp. Bot. 184, 104392. doi: 10.1016/j.envexpbot.2021.104392
Read, J., Sanson, G. D. (2003). Characterizing sclerophylly: The mechanical properties of a diverse range of leaf types. New Phytol. 160, 81–99. doi: 10.1046/j.1469-8137.2003.00855.x
Reich, P. B., Oleksyn, J. (2004). Global patterns of plant leaf N and P in relation to temperature and latitude. Proc. Natl. Acad. Sci. U. S. A. 101, 11001–11006. doi: 10.1073/pnas.0403588101
Retuerto, R., Sánchez Vilas, J., Varga, S. (2018). Sexual dimorphism in response to stress. Environ. Exp. Bot. 146, 1–4. doi: 10.1016/j.envexpbot.2017.12.006
Rixen, C., Wipf, S., Rumpf, S. B., Giejsztowt, J., Millen, J., Morgan, J. W., et al. (2022). Intraspecific trait variation in alpine plants relates to their elevational distribution. J. Ecol. 110, 860–875. doi: 10.1111/1365-2745.13848
Senthilnathan, A., Gavrilets, S. (2021). Ecological consequences of intraspecific variation in coevolutionary systems. Am. Nat. 197, 1–17. doi: 10.1086/711886
Severns, P. M., Liston, A. (2008). Intraspecific chromosome number variation: A neglected threat to the conservation of rare plants. Conserv. Biol. 22, 1641–1647. doi: 10.1111/j.1523-1739.2008.01058.x
Seyedi, N., Costa, C., Máguas, C., Correia, O. (2019). The contribution of leaf life span to sexual dimorphism in deciduous and evergreen Pistacia species under Mediterranean conditions. Flora 251, 114–121. doi: 10.1016/j.flora.2019.01.005
Shah, A., Li, D. Z., Gao, L. M., Li, H. T., Möller, M. (2008). Genetic diversity within and among populations of the endangered species Taxus fuana (Taxaceae) from Pakistan and implications for its conservation. Biochem. Syst. Ecol. 36, 183–193. doi: 10.1016/j.bse.2007.09.012
Shao, J. J., Yuan, T. F., Li, Z., Li, N., Liu, H. Y., Bai, S. H., et al. (2019). Plant evolutionary history mainly explains the variance in biomass responses to climate warming at a global scale. New Phytol. 222, 1338–1351. doi: 10.1111/nph.15695
Shipley, B., de Bello, F., Cornelissen, J. H. C., Laliberté, E., Laughlin, D. C., Reich, P. B. (2016). Reinforcing loose foundation stones in trait-based plant ecology. Oecologia 180, 923–931. doi: 10.1007/s00442-016-3549-x
Sidor, C. G., Popa, I., Vlad, R., Cherubini, P. (2015). Different tree-ring responses of Norway spruce to air temperature across an altitudinal gradient in the Eastern Carpathians (Romania). Trees 29, 985–997. doi: 10.1007/s00468-015-1178-3
Siefert, A., Violle, C., Chalmandrier, L., Albert, C. H., Taudiere, A., Fajardo, A., et al. (2015). A global meta-analysis of the relative extent of intraspecific trait variation in plant communities. Ecol. Lett. 18, 1406–1419. doi: 10.1111/ele.12508
Šípek, V., Tesař, M. (2014). Seasonal snow accumulation in the mid-latitude forested catchment. Biologia 69, 1562–1569. doi: 10.2478/s11756-014-0468-3
Song, Y. B., Li, W. B., Dai, W. H., Dong, M. (2016). Does sex constrain functional clonal traits and their responses to environmental heterogeneity in the stoloniferous herb Glechoma longituba? Flora 218, 18–23. doi: 10.1016/j.flora.2015.11.004
Song, Y. B., Shen-Tu, X. L., Dong, M. (2020a). Intraspecific variation of samara dispersal traits in the endangered tropical tree Hopea hainanensis (Dipterocarpaceae). Front. Plant Sci. 11, 599764. doi: 10.3389/fpls.2020.599764
Song, Y. B., Xu, L., Duan, J. P., Zhang, W. J., Shen-Tu, X. L., Li, T. X., et al. (2020b). Sex ratio and spatial pattern of Taxus fuana, a wild plant with extremely small populations in Tibet. Biodivers. Sci. 28, 269–276. doi: 10.17520/biods.2019102
Stefanović, M., Nikolić, B., Matić, R., Popović, Z., Vidaković, V., Bojović, S. (2017). Exploration of sexual dimorphism of Taxus baccata L. needles in natural populations. Trees 31, 1697–1710. doi: 10.1007/s00468-017-1579-6
Stegmann, U. E. (2021). A willow drawing from 1786: The earliest depiction of intraspecific trait variation in plants? Ann. Bot. 127, 411–412. doi: 10.1093/aob/mcaa091
Steinmetz, B., Kalyuzhny, M., Shnerb, N. M. (2020). Intraspecific variability in fluctuating environments: Mechanisms of impact on species diversity. Ecology 101, e3174. doi: 10.1002/ecy.3174
Suding, K. N., Lavorel, S., Chapin, F. S., Cornelissen, J. H. C., Díaz, S., Garnier, E., et al. (2008). Scaling environmental change through the community-level: A trait-based response-and-effect framework for plants. Glob. Change Biol. 14, 1125–1140. doi: 10.1111/j.1365-2486.2008.01557.x
Tang, L. L., Morris, W. K., Zhang, M., Shi, F. C., Vesk, P. A. (2022). Exploring how functional traits modulate species distributions along topographic gradients in Baxian Mountain, North China. Sci. Rep. 12, 994. doi: 10.1038/s41598-021-04210-x
Tan, L., Song, Y. B., Fu, R. H., Liu, M., Li, Y., Escudero, M., et al. (2022). Variation of stable carbon and nitrogen isotopes ratio in Ficus tikoua and their linkage to its specific pollinator. Flora 291, 152073. doi: 10.1016/j.flora.2022.152073
Thakur, D., Rathore, N., Chawla, A. (2019). Increase in light interception cost and metabolic mass component of leaves are coupled for efficient resource use in the high altitude vegetation. Oikos 128, 254–263. doi: 10.1111/oik.05538
Thompson, K., Parkinson, J. A., Band, S. R., Spencer, R. E. (1997). A comparative study of leaf nutrient concentrations in a regional herbaceous flora. New Phytol. 136, 679–689. doi: 10.1046/j.1469-8137.1997.00787.x
Tognetti, R. (2012). Adaptation to climate change of dioecious plants: Does gender balance matter? Tree Physiol. 32, 1321–1324. doi: 10.1093/treephys/tps105
Turner, S. R., Lewandrowski, W., Elliott, C. P., Merino-Martín, L., Miller, B. P., Stevens, J. C., et al. (2017). Seed ecology informs restoration approaches for threatened species in water-limited environments: A case study on the short-range Banded Ironstone endemic Ricinocarpos brevis (Euphorbiaceae). Aust. J. Bot. 65, 661–677. doi: 10.1071/BT17155
van Bodegom, P. M., Douma, J. C., Verheijen, L. M. (2014). A fully traits-based approach to modeling global vegetation distribution. Proc. Natl. Acad. Sci. U. S. A. 111, 13733–13738. doi: 10.1073/pnas.1304551110
Waigwa, A. N., Mwangi, B. N., Wahiti, G. R., Omengo, F., Zhou, Y. D., Wang, Q. F. (2020). Variation of morphological and leaf stoichiometric traits of two endemic species along the elevation gradient of Mount Kenya, East Africa. J. Plant Ecol. 13, 785–792. doi: 10.1093/jpe/rtaa067
Walas, Ł., Mandryk, W., Thomas, P. A., Tyrała-Wierucka, Ż, Iszkuło, G. (2018). Sexual systems in gymnosperms: A review. Basic Appl. Ecol. 31, 1–9. doi: 10.1016/j.baae.2018.05.009
Wang, X., Jiang, Y., Ren, H. Y., Yu, F. H., Li, M. H. (2019). Leaf and soil δ15N patterns along elevational gradients at both treelines and shrublines in three different climate zones. Forests 10, 557. doi: 10.3390/f10070557
Wang, J. Y., Wang, J. N., Guo, W. H., Li, Y. G., Wang, G. G., Wu, T. G. (2018). Stoichiometric homeostasis, physiology, and growth responses of three tree species to nitrogen and phosphorus addition. Trees 32, 1377–1386. doi: 10.1007/s00468-018-1719-7
Wang, S. T., Xu, Y. Z., Wei, X. Z., Jiang, M. X. (2022). Do leaf functional traits differ between 20-35-year-old transplanted and wild source populations? A case study involving five endangered tree species. Nat. Conserv. Res. 7, 32–41. doi: 10.24189/ncr.2022.016
Westerband, A. C., Funk, J. L., Barton, K. E. (2021). Intraspecific trait variation in plants: A renewed focus on its role in ecological processes. Ann. Bot. 127, 397–410. doi: 10.1093/aob/mcab011
Wu, J. M., Shi, Z. M., Liu, S., Centritto, M., Cao, X. W., Zhang, M. M., et al. (2021). Photosynthetic capacity of male and female Hippophae rhamnoides plants along an elevation gradient in eastern Qinghai-Tibetan plateau, China. Tree Physiol. 41, 76–88. doi: 10.1093/treephys/tpaa105
Xia, Z. C., He, Y., Yu, L., Lv, R. B., Korpelainen, H., Li, C. Y. (2020). Sex-specific strategies of phosphorus (P) acquisition in Populus cathayana as affected by soil P availability and distribution. New Phytol. 225, 782–792. doi: 10.1111/nph.16170
Xu, W. M., Tomlinson, K. W., Li, J. (2020). Strong intraspecific trait variation in a tropical dominant tree species along an elevational gradient. Plant Divers. 42, 1–6. doi: 10.1016/j.pld.2019.10.004
Yang, Y. H., Ji, C. J., Robinson, D., Zhu, B., Fang, H. J., Shen, H. H., et al. (2013). Vegetation and soil 15N natural abundance in alpine grasslands on the Tibetan Plateau: Patterns and implications. Ecosystems 16, 1013–1024. doi: 10.1007/s10021-013-9664-1
Yang, Y. H., Luo, Y. Q. (2011). Carbon: nitrogen stoichiometry in forest ecosystems during stand development. Glob. Ecol. Biogeogr. 20, 354–361. doi: 10.1111/j.1466-8238.2010.00602.x
Zhang, Y. Q., Li, Z. C., Song, L. G., Hou, L. Y., Sun, Q. W. (2020). Natural distribution and community ecological characteristics of Taxus fuana. Acta Ecol. Sin. 40, 1999–2009. doi: 10.5846/stxb201809282117
Zhang, R., Liu, J. Y., Liu, Q. S., He, H. G., Xu, X., Dong, T. F. (2019). Sexual differences in growth and defence of Populus yunnanensis under drought stress. Can. J. For. Res. 49, 491–499. doi: 10.1139/cjfr-2018-0270
Zhang, X. S., Wang, C., Zhou, C. N. (2022). The variation of functional traits in leaves and current-year twigs of Quercus aquifolioides along an altitudinal gradient in Southeastern Tibet. Front. Ecol. Evol. 10, 855547. doi: 10.3389/fevo.2022.855547
Zhang, Z. M., Yu, S. X. (2018). Potential tradeoffs between intraspecific and interspecific trait variations along an environmental gradient in a subtropical forest. J. Forest. Res. 29, 1731–1740. doi: 10.1007/s11676-018-0594-9
Zheng, J., Jiang, Y., Qian, H., Mao, Y. J., Zhang, C., Tang, X. X., et al. (2022). Size-dependent and environment-mediated shifts in leaf traits of a deciduous tree species in a subtropical forest. Ecol. Evol. 12, e8516. doi: 10.1002/ece3.8516
Keywords: altitude, endangered plant, intraspecific trait variation, leaf traits, sex-dependent variation, Taxus fuana
Citation: Li T-X, Shen-Tu X-L, Xu L, Zhang W-J, Duan J-P, Song Y-B and Dong M (2022) Intraspecific and sex-dependent variation of leaf traits along altitude gradient in the endangered dioecious tree Taxus fuana Nan Li & R.R. Mill. Front. Plant Sci. 13:996750. doi: 10.3389/fpls.2022.996750
Received: 18 July 2022; Accepted: 09 September 2022;
Published: 17 October 2022.
Edited by:
Runguo Zang, Chinese Academy of Forestry, ChinaCopyright © 2022 Li, Shen-Tu, Xu, Zhang, Duan, Song and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yao-Bin Song, eWJzb25nQGh6bnUuZWR1LmNu; Ming Dong, ZG9uZ21pbmdAaHpudS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Tian-Xiang Li
Tian-Xiang Li Xiao-Lu Shen-Tu
Xiao-Lu Shen-Tu Li Xu
Li Xu Yao-Bin Song
Yao-Bin Song Ming Dong
Ming Dong