- 1Institute of Wheat Research, Shanxi Agricultural University, Linfen, China
- 2School of Life Sciences, Shanxi University, Taiyuan, China
- 3Institute of Cotton Research, Shanxi Agricultural University, Yuncheng, China
Flag-leaf-related traits including length (FLL), width (FLW), area (FLA), thickness (FLT), and volume (FLV) of flag leaves are the most important determinants of plant architecture and yield in wheat. Understanding the genetic basis of these traits could accelerate the breeding of high yield wheat varieties. In this study, we constructed a doubled haploid (DH) population and analyzed flag-leaf-related traits in five experimental locations/years using the wheat 90K single-nucleotide polymorphism array. It’s worth noting that a novel method was used to measure FLT and FLV easily. Leaf thickness at two-thirds of the leaf length from tip to collar represented the average leaf thickness as measured with freehand sections and was used to calculate the leaf volume. In addition, flag-leaf-related traits showed positive correlations with yield related traits under two different water regimes. A total of 79 quantitative trait loci (QTL) controlling the five traits were detected among all chromosomes except 4D and 5A, explaining 3.09–14.52% of the phenotypic variation. Among them, 15 stable QTL were identified in more than three environments, including two major QTL for FLT, six for FLW, three for FLA, two for FLT and two for FLV. DH lines with positive alleles at both QTL regions had an average FLL (9.90%), FLW (32.87%), FLT (6.62%), FLA (18.47%), and FLV (20.87%) greater than lines with contrasting alleles. QFLT-2B, QFLV-2A, and QFLV-7D were co-located with yield-related traits. The 15 QTL were validated by tightly linked kompetitive allele specific PCR (KASP) markers in a recombinant inbred line (RIL) population derived from a different cross. QFLL-4A, QFLW-4B, QFLA-5D.1, QFLA-7A, QFLA-7D.1, QFLT-2B, QFLT-6A, QFLV-2A, and QFLV-7D are likely novel loci. These results provide a better understanding of the genetic basis underlying flag-leaf-related traits. Also, target regions for fine mapping and marker-assisted selection were identified and these will be valuable for breeding high yielding bread wheat.
Introduction
Wheat (Triticum aestivum L.) is one of the most important food crops worldwide. Wheat production must be increased sustainably to meet the food demand of a growing human population. The flag leaf is the main organ for photosynthesis. It is regarded as the “functional leaf” in wheat production and contributes 45–58% of plant photosynthate during grain-filling (Duncan, 1971; Xu and Zhao, 1995; Sharma et al., 2003; Khaliq et al., 2008). Also, flag-leaf-related traits are important determinants of plant architecture and grain yield. Donald (1968) regarded upright leaves as an “ideotype” for wheat. Having vertical leaves improves sunlight capture, thus enhancing photosynthesis and the production of dry matter. Moreover, flag-leaf-related traits showed a significant correlation with traits associated with yield and quality, such as thousand grain weight (TGW), grain number per spike (GNS), grain weight per spike (GWS), grain hardness, and grain yield (Guitman et al., 1991; Sakamoto et al., 2006). Therefore, to better breed for high yield it is necessary to understand the genetic basis of flag-leaf-related traits.
Flag-leaf-related traits, including flag leaf length (FLL), flag leaf width (FLW), flag leaf area (FLA), flag leaf thickness (FLT), and flag leaf volume (FLV), are complex quantitative traits controlled by multiple genes and are strongly influenced by environmental factors (Simón, 1999; Coleman et al., 2001; Kobayashi et al., 2003). Many quantitative trait loci (QTL) for flag-leaf-related traits have been reported in wheat. A QTL for FLW on chromosome 5A and 5B was fine mapped in previous studies (Xue et al., 2013; Zhao et al., 2022). A total of 38 QTL for flag-leaf-related traits was identified in a recombinant inbred line (RIL) population, among which were three stable QTL on chromosomes 4B, 5B, and 6B (Fan et al., 2015). Another study detected 61 QTL for flag-leaf-related traits in a RIL population using a genetic linkage map that integrated high-density simple sequence repeat (SSR) and single-nucleotide polymorphism (SNP) markers (Wu et al., 2016). Twenty-three putative QTL for flag-leaf-related traits were detected in another study, among which 15 were detected in at least two environments (Liu et al., 2018). A total of 43 QTL for traits related to flag leaves and yield were identified using four RIL populations (Hu et al., 2020). Using the wheat 90K SNP array and both genome-wide association study (GWAS) analysis and bi-parental linkage mapping, 23 QTL regulating flag-leaf-related traits were detected (Yan et al., 2020). Eight stable QTL in six populations were reported by using a 55K SNP array and SSR markers (Ma et al., 2020) and eight major QTL were identified in 11 environments (Tu et al., 2021). Although QTL for FLL, FLW, and FLA have been found in previous studies, the genetic basis of FLT and FLV in common wheat has not been studied in detail.
The amount of light absorbed by the leaves and the diffusion of CO2 through the leaf tissue depends in part on leaf thickness (Givnish, 1979; Agustí et al., 1994; Syvertsen et al., 1995). Some studies have shown that leaf thickness is related to photosynthesis and growth rate (Poorter, 1990; Enriquez et al., 1996; Nielsen et al., 1996; Garnier et al., 1999). The number of chloroplasts can be increased by increasing leaf thickness, thus improving photosynthetic capacity (Araus et al., 1986; Devika et al., 2018). Thicker leaves can increase leaf water content under dry conditions (Wang et al., 2011). Increasing leaf mass area, leaf thickness, and stomatal closure were used to reduce water loss and achieve higher yield under drought and high temperature stress (Cellier et al., 2000; Peña-Rojas et al., 2005). In addition, leaf thickness is closely related to the vertical shape of leaves. When plant density is high, canopy structure can be changed to reduce shade and improve the photosynthetic efficiency of lower leaves (Pendleton et al., 1968; Stewart et al., 2003). QTL for leaf thickness have been reported in rice. The major QTL qSLW-7 for flag leaf specific leaf weight was located on chromosome 7, which was very close to a major QTL for flag leaf dry weight and flag specific leaf area (Kanbe et al., 2008). A major QTL for flag specific leaf area in rice was identified and overlapped with a major QTL for chlorophyll content (Takai et al., 2010). FLT and FLV are important determinants of yield in cereal crops, yet there are few studies of these traits in wheat.
Given the importance of flag-leaf-related traits to wheat yield, it is essential to identify and pyramid major and stably expressed loci for flag-leaf-related traits from diverse wheat germplasm resources. In the present study, a simple yet accurate detection method for FLT was developed and used to calculate FLV. Using the new method and conventional methods, QTL for five flag-leaf-related traits were detected in a DH population derived from a cross between the common wheat cultivars Jinchun 7 and Jinmai 919. Results provide a basis for selecting ideal flag leaves to increase yield by improving wheat plant architecture.
Materials and methods
Plant materials and field trials
Two populations with Jinmai 919 as a common parent were used, comprised of 180 DH lines from a cross of Jinchun 7 × Jinmai 919 and 165 F10 RILs from cross of DH118 × Jinmai 919. Bred by the Institute of Maize Research, Shanxi Academy of Agricultural Sciences, Jinchun 7 is a high-yielding variety for irrigated conditions. Jinmai 919 has strong drought resistance and was developed by the Institute of Wheat Research, Shanxi Agricultural University. DH118 is also a high-yielding variety for irrigated conditions selected by the Institute of Wheat Research, Shanxi Agricultural University. Also, five wheat varieties with various plant habit and adaptation were selected for leaf thickness measurements, including Liangxing 99 (released for the National Huanghai winter wheat area), Jimai 22 (released by Shandong Province), Nongda 211 (released for the Northern Winter wheat area), Taichang 29 (a spring wheat released by Sichuan Province), and a line selected from Jinmai 919. Three flag leaves were collected for measurements 20 days after flowering from each of the five wheats.
The two populations were planted at the Yaodu Experimental Station (36°08′N, 111°52′E, altitude 450 m), the Hancun Experimental Station (36°25′N, 111°67′E, altitude 450 m), and the Yuncheng Experimental Station (34°35′ N, 110°15′ E, altitude 450 m) in Shanxi Province in 2018–2019, 2019–2020, and 2020–2021. Plants were grown under well-watered (WW) and rainfed conditions, providing five environments designated as E1 (WW, 19YD), E2 (WW, 20YD), E3 (WW 20YC), E4 (rainfed, 21HC), and E5 (rainfed, 21YD). The field design was a randomized complete block with three replications. Each plot consisted of two 1.5 m rows spaced 0.3 m apart at 21 seeds per row. Field management used standard wheat production practices.
Testing flag-leaf-related traits
FLL, FLW, FLT, FLA, and FLV were measured on the main tiller of 10 randomly selected plants. FLL (cm) was measured from leaf collar to the tip. FLW (cm) was measured at the widest part of the leaf. The derived trait FLA (cm2) was calculated as FLA = FLL × FLW × 0.83 after Xue et al. (2013).
To develop a convenient way to measure FLT, the flag leaves of the five wheat varieties were cut into 20 equal segments and the cross-sectional area of each cut was calculated using the image processing software Image J.1 To calculate the thickness of the flag leaf the formula h = S/W was used where h is the flag leaf thickness, S is the cross-sectional area, and W leaf is leaf width at the cut. The thickness of each of the 20 segments was then compared with their average.
FLV (mm3) was calculated as FLV = FLT × FLA. The accuracy of the calculated FLV was verified by measuring FLV with the drainage method. A volume of water (V1) was placed into beakers, and a lead weight with volume V2 was used to sink a flag leaf segment into the water. The volume after immersion (V3) was recorded. FLV was calculated as FLV = V3 – V2 – V1 and the measurement replicated three times for each variety. Additionally, grain-yield-related traits were measured for all lines, including spikelet number per spike (SN), GNS, spikelet length (SL), grain length (GL), grain width (GW), grain thickness (GT), TGW, and plant height (PH) (Zheng et al., 2019).
Statistical analysis
Basic statistics and Pearson’s correlation analysis were performed on the phenotypic data from each environment. Analysis of variance (ANOVA) was done using the SPSS (V22.0) statistical package (IBM SPSS, Armonk, NY, United States). SAS V8.0 (SAS Institute, Cary, North Carolina, United States)2 was used to calculate the best linear unbiased predictions (BLUP) and broad-sense heritability (H2) (Smith et al., 1998; Qin et al., 2016).
Genetic linkage map construction and quantitative trait loci mapping
DNA was extracted from each line in the DH population and the respective parents using the CTAB method (Vijayalakshmi et al., 2010). The DH population and parents were genotyped with the Infinium wheat SNP 90K iSelect assay (Illumina Inc., San Diego, CA, United States) developed by the International Wheat SNP Consortium (Wang et al., 2014). IciMapping v4.03 was used to construct a high-density genetic linkage map (Li et al., 2021). SNP markers with no recombination were placed into a single bin using the “BIN” function in IciMapping V4.0. The final markers were chosen with a minimum percentage of missing data and sorted into different groups with LOD (logarithm of odds) thresholds ≥ 8 using the “Grouping” function in JoinMap 4.0 (Li et al., 2021). QTL were detected using WinQTLCart version 2.54 and composite interval mapping. The minimal LOD score to accept the presence of a QTL was set at 2.5. Stable QTL were stably detected in at least three datasets including the BLUP dataset. Major QTL were those that met these criteria in at least three environments (including the BLUP dataset as an environment) with more than 10% of explained phenotypic variation in at least one environment. QTL that were either within 1 cM of one another or shared common flanking markers were considered identical. The naming of QTL followed the International Rules of Genetic Nomenclature.5
To determine the physical position of identified QTL regions, a BLAST search in WheatOmics2.16 was performed to align the QTL-associated peak and flanking SNP marker sequences (Ma et al., 2021).
Validation for the major quantitative trait loci identified
To develop KASP tags from the peak marker SNP sequence of the major QTL, two specific primers (F1/F2) and a universal primer (R) were designed for each SNP. An F1 tail that could bind to induce FAM fluorescence and an F2 tail that could bind to induce HEX fluorescence were added to the specific sequences. KASP primers were designed by Polymarker7 and synthesized by Beijing Jiacheng Biotechnology Co., Ltd. The developed KASP markers were used to detect previously identified QTL in the DH118 × Jinmai 919 population as a means of validation. Following genotyping, the validation population was divided into two groups and differences in flag-leaf-related traits between the groups were assessed by t-tests in SAS V8.0 (Yang et al., 2022).
Candidate gene analysis for the major quantitative trait loci identified
The genes within the target region were obtained using the genome browser (JBrowse) on the Triticeae Multi-omics website.8 Functional annotation and enrichment analysis of the genes were carried out in the GO (gene ontology) database using the R package cluster Profiler.
Results
A simple method to measure flag leaf thickness
For each of the five wheat cultivars, the thickness of each of the 20 segments was compared with the average of all segments. The leaf thickness of segments taken at two-thirds of the leaf length from tip to collar was 0.207–0.233 mm, which was close to the average thickness of the whole leaf (0.204–0.226 mm) (Supplementary Table 1). Thus, the leaf thickness from a single segment could be used as a measure of FLT (Table 1).
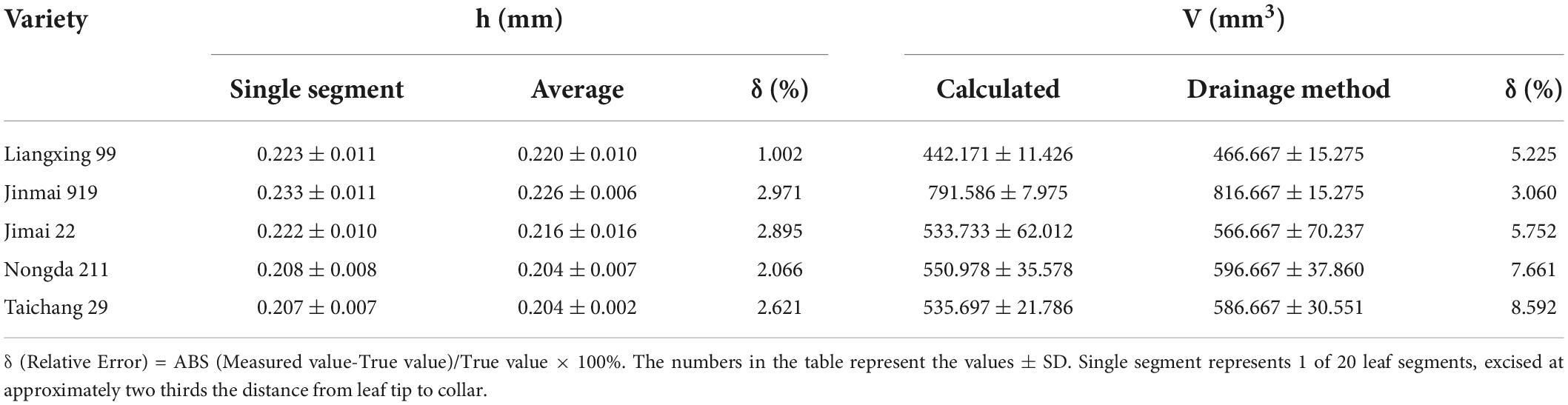
Table 1. Leaf thickness from a single segment compared with the average of 20 segments and calculated leaf volume compared to volume measured with the drainage method.
The new FLT assessment method was used to calculate FLV. The calculated FLV values were verified with the drainage method for assessing FLV. The average relative error was 3.060–8.592%, indicating that the method to calculate FLV in the present study was reliable (Supplementary Tables 2, 3). Also, the standard error for the calculated FLV was less than that of the drainage method, indicating the method was more precise.
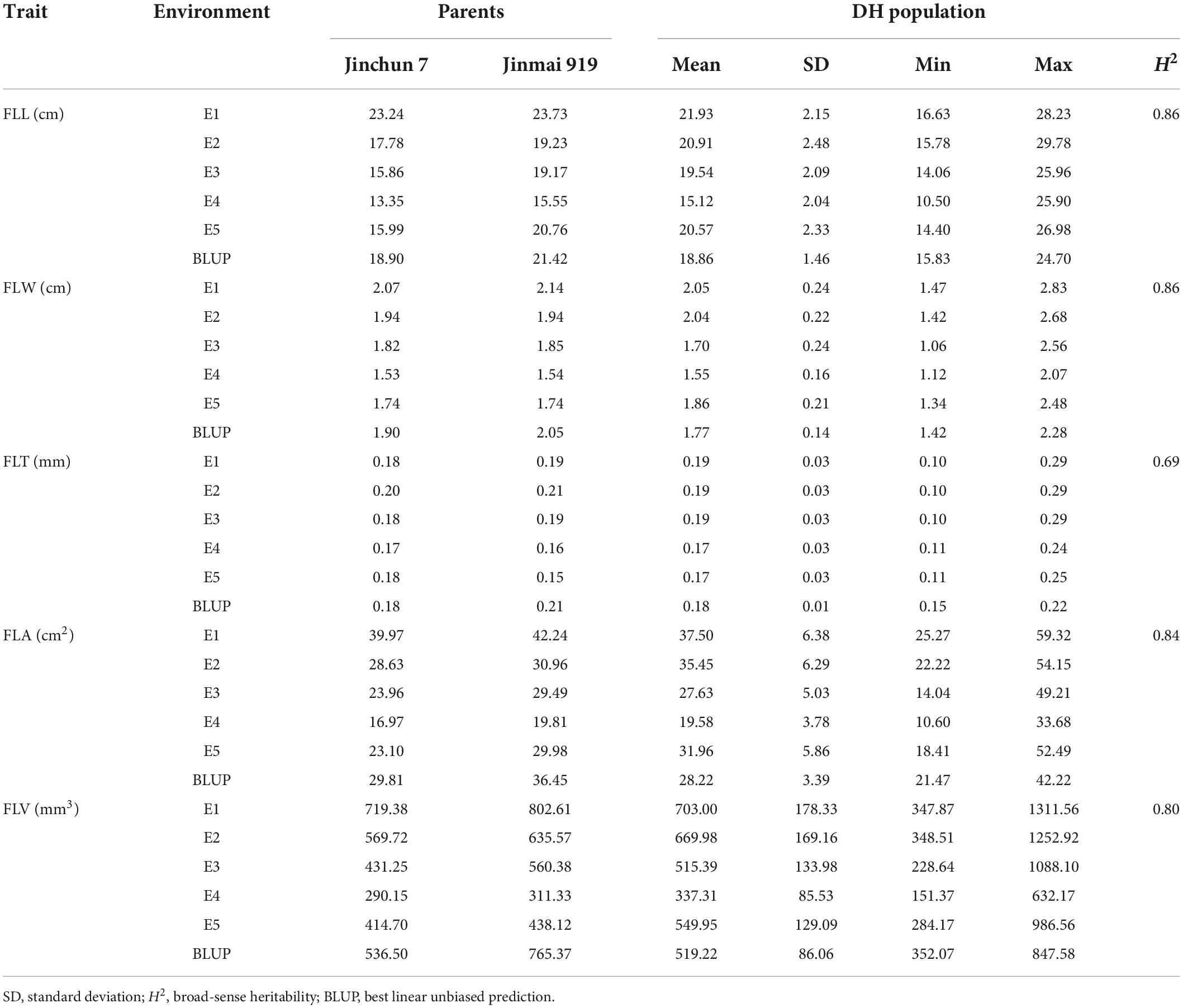
Phenotypic variation of flag-related traits
The phenotypic variation of the DH population and the parental lines was evaluated in five environments. Flag leaves of Jinmai 919 were larger than those of Jinchun 7 as measured by FLL, FLW, FLT, FLA, and FLV (Table 2) and the BLUP data. The flag-leaf-related traits of the DH population ranged from 10.50 to 29.78 cm for FLL, 1.06 to 2.83 cm for FLW, 0.10 to 0.29 mm for FLT, 10.60 to 59.32 cm2 for FLA, and 151.37 to 1311.56 mm3 for FLV (Figure 1). The H2 of five flag-leaf-related traits ranged from 0.69 to 0.86, indicating that these traits were significantly affected by genetic factors. The Pearson correlations among different environments were significant (Supplementary Table 4). All traits were normally distributed in the DH population (Supplementary Figure 1).
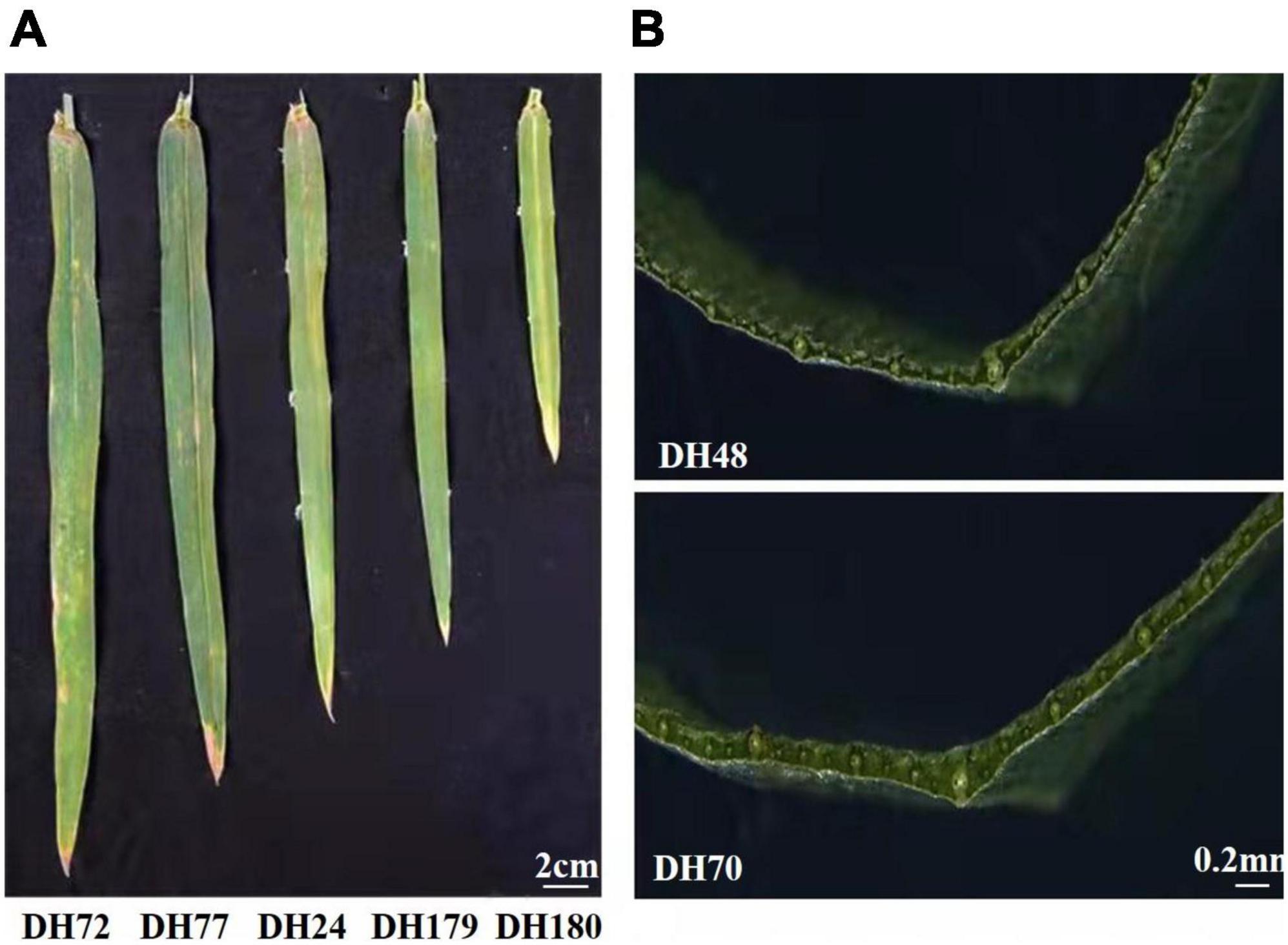
Figure 1. Phenotypes of selected DH lines. (A) The flag leaf size of lines from the Jinchun 7 × Jinmai 919 DH population. (B) Lines with different flag leaf thickness from the Jinchun 7 × Jinmai 919 DH population.
Correlation analysis for traits related to flag leaves and yield
The relationship between traits related to flag leaves and traits related to yield was analyzed under two different water regimes. For flag-leaf-related traits, FLL had a significant positive correlation with FLW (Figure 2 and Supplementary Table 5). Significant positive correlations were observed among FLL, FLW, and FLA. All four traits showed a significant positive correlation with FLV. However, FLL and FLW showed no correlations with FLT.
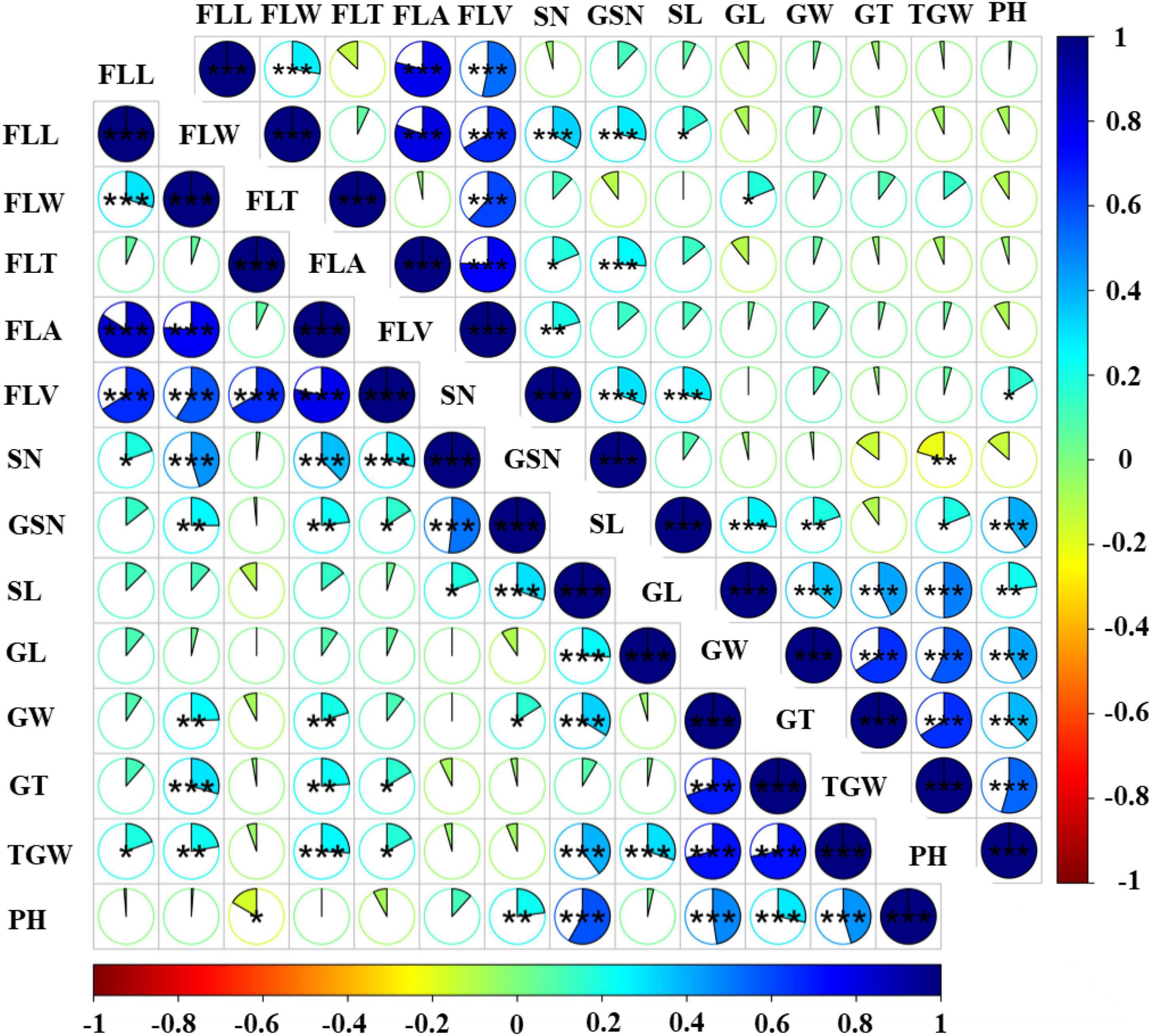
Figure 2. Phenotypic correlation coefficients between flag-leaf-related traits and yield related traits in the DH population grown under two water regimes. The lower left triangular matrix represents rainfed conditions, the upper right triangular matrix represents WW conditions, the sector area represents the correlation coefficients. *Significant at P < 0.05, **significant at P < 0.01, ***significant at P < 0.001.
The relationship between flag-leaf-related traits and yield related traits was also evaluated. FLL showed highly significant positive correlations with SN and TGW in rainfed conditions, but FLL was negatively correlated with SN, GL, GT, TGW in WW conditions. Furthermore, FLW, FLA, and FLV had highly significant positive correlations with SN, GNS, GT, and TGW in both water regimes, and FLA and FLV showed significant positive correlations with GW in rainfed conditions. FLW, FLA, and FLV also had highly significant positive correlations with SN in both rainfed and WW conditions. However, different results were obtained for some pairs of traits under WW conditions. For example, in contrast to rainfed conditions, FLV and GNS were not correlated under WW conditions Also, although negative correlations were observed among FLT and GNS, SL, GW, GT, TGW, and PH in rainfed conditions, in WW conditions such correlations were only identified between FLT and GNS and PH.
Linkage map construction
A high-density genetic linkage map for the DH population was constructed using marker data from the Wheat 90k SNP array. The total length of the map was 2,474 cM with an average genetic distance of 0.92 cM, including 2,700 SNP markers and covering all 21 chromosomes (Table 3). Respectively, the numbers of SNP markers in the A, B, and D genomes were 1,012, 1,128, and 560, the linkage lengths 789.59, 960.78, and 723.66 cM, and the average distances between markers 0.78, 0.85, and 1.29 cM. The D genome had the lowest marker coverage, the longest linkage group was 169.66 cM for chromosome 3B, and the shortest was 52.33 cM for chromosome 1A.
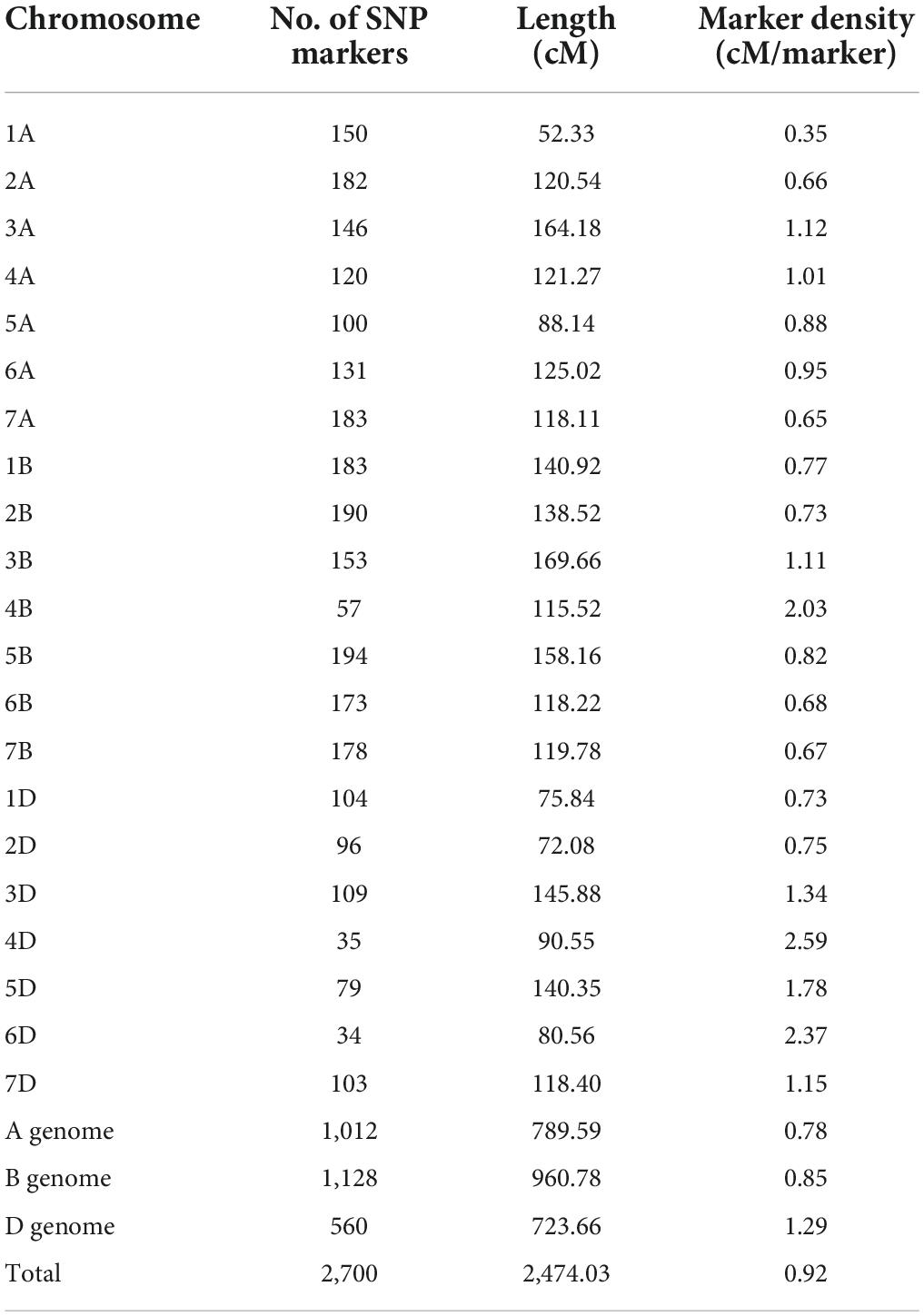
Table 3. Summary of linkage groups and marker statistics obtained from a 90K SNP array analysis of the Jinchun 7 × Jinmai 919 DH population.
Quantitative trait loci mapping
Among all chromosomes except 4D and 5A, 79 QTL for flag-leaf-related traits were detected (Table 4 and Supplementary Table 6). These QTL explained 3.09–14.52% of the phenotypic variation in different environments. Favorable alleles of 33 QTL were derived from Jinchun 7 and favorable alleles of 46 QTL were derived from Jinmai 919.
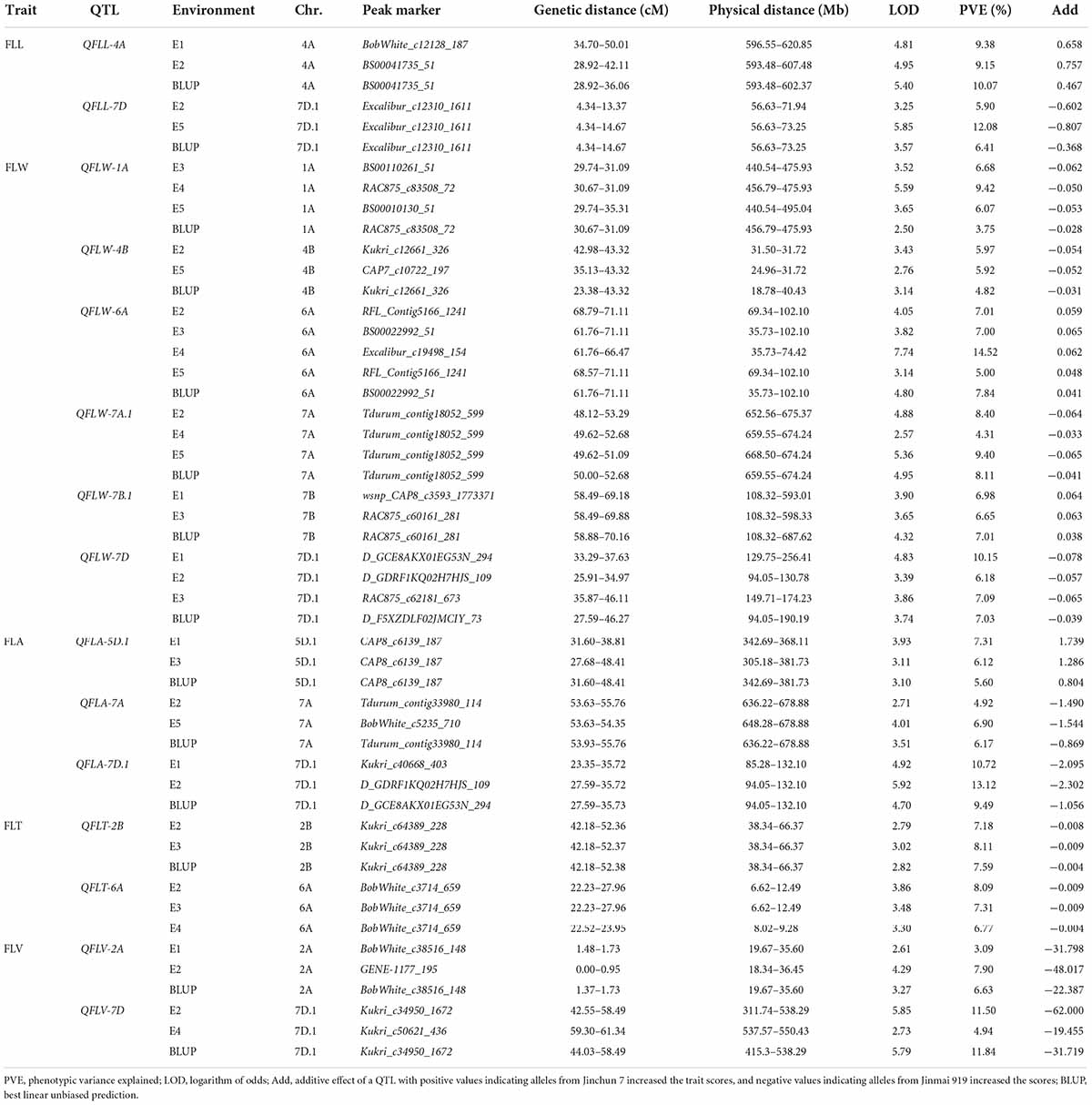
Table 4. Stable quantitative trait loci (QTL) for flag-leaf-related traits detected in the DH population derived from Jinchun 7 × Jinmai 919.
For FLL, 11 QTL were mapped to chromosomes 1B, 1D (2), 2B, 3B, 4A, 4B (2), 6B, 7B, and 7D, with individual QTL explaining 5.19–12.08% of the phenotypic variance. Among them, QFLL-4A and QFLL-7D were major QTL detected in three environments as well as with BLUP values, explaining 9.15–10.07% and 5.90–12.08% of the phenotypic variance, respectively. The additive effect values indicated that the positive alleles at QFLL-4A were from Jinchun 7 and those at QFLL-7D were from Jinmai 919. For the remaining 9 QTL, Jinchun 7 contributed five positive alleles and Jinmai 919 contributed four, and the range of phenotypic variation was 5.19–10.45%.
For FLW, 23 QTL were identified on chromosomes 1A, 2A, 2B (5), 2D (2), 3D, 4A (2), 4B, 5D, 6A, 7A (5), 7B (2), and 7D. These QTL explained 3.75–14.52% of the phenotypic variation in different environments. QFLW-1A and QFLW-7A.1 were detected in four environments and their phenotypic variance ranged from 3.75 to 9.42% and 4.31 to 9.40%, respectively. QFLW-4B and QFLW-7B.1 were detected in three environments and their phenotypic variance ranged from 4.82 to 5.97% and 6.65 to 7.01%, respectively. QFLW-6A was detected in five environments with phenotypic variance ranging from 5.00 to 14.52% and QFLW-7D was detected in four environments with phenotypic variance ranging from 6.18 to 10.15%. Among the 23 QTL, in 10 instances alleles that that increased FLW were from Junchun 7 and in 13 the positive alleles were contributed by Jinmai 919.
For FLA, 23 QTL were mapped to chromosomes 1D, 2A, 2B (3), 3A, 3B (2), 4A (2), 5D (2), 6A, 6D (2), 7A, 7B (2), and 7D (5), with individual QTL contributing 4.32–13.12% of the phenotypic variance. QFLA-5D.1 and QFLA-7A were stably detected in three environments, explaining 5.60–7.31% and 4.92–6.90% of the phenotypic variance, respectively. QFLA-7D.1, a major QTL, was stably detected in three environments, explaining 9.49–13.12% of the phenotypic variance. For the 23 QTL, 11 of FLA-increase alleles were contributed by Jinchun 7 and 12 by Jinmai 919.
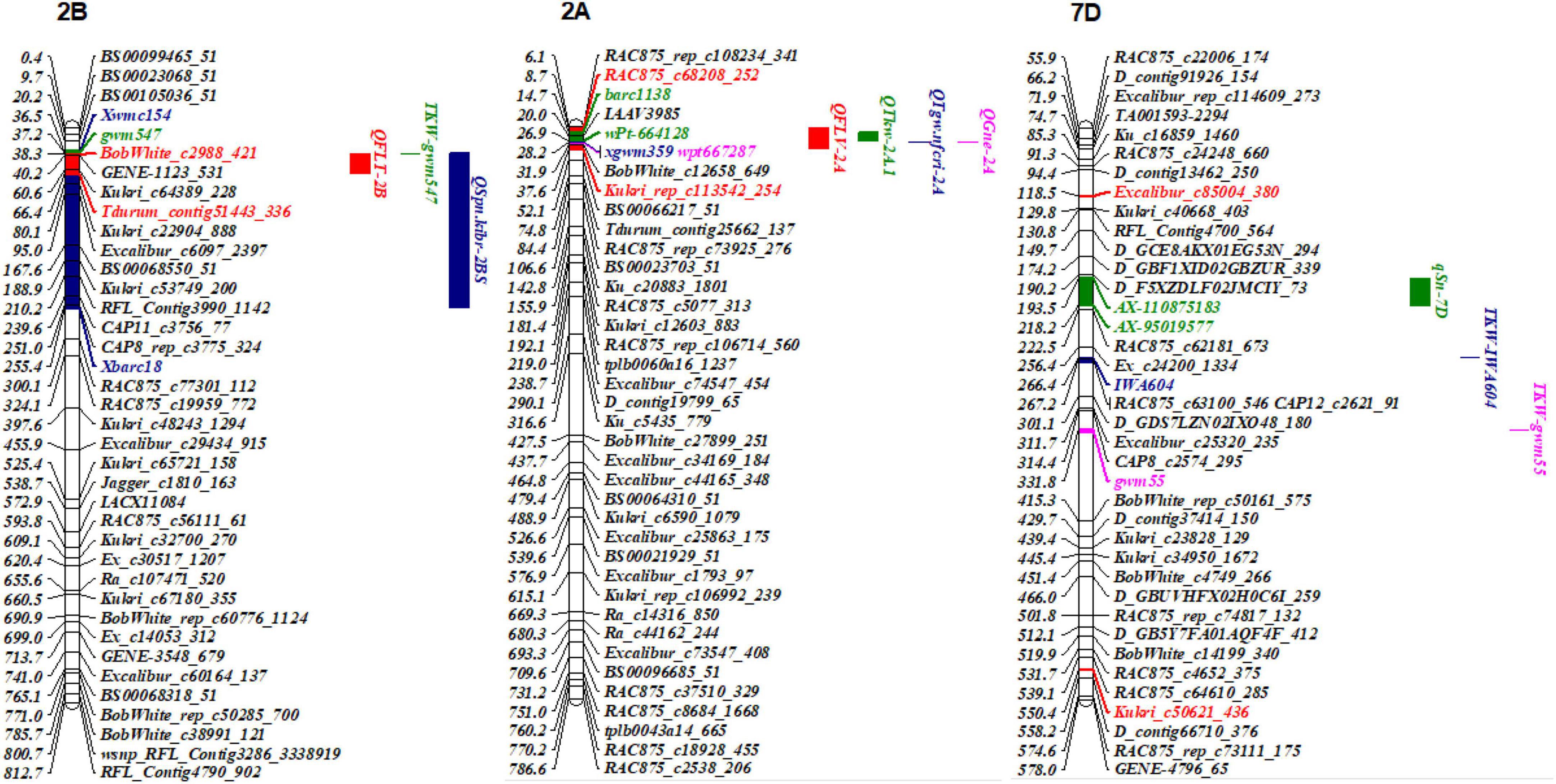
For FLT, six QTL were found on chromosomes 2A, 2B, 2D, 6A, 6B, and 7A, with the degree of phenotypic variation contribution ranging from 4.69 to 8.31%. The QTL QFLT-2B and QFLT-6A were observed in three environments and explained phenotypic variance from 7.18 to 8.11% and 6.77 to 8.09%, respectively. Additive effects indicated that, except for QFLT-7A, the QTL contributing to decreased FLA all came from Jinmai 919 alleles.
For FLV, 16 additive effect QTL were detected on chromosomes 1B, 1D, 2A, 2B, 2D, 4A, 5B, 5D, 6A (3), 7A (3), 7B, and 7D, and the phenotypic variance explained by individual QTL ranged from 3.09 to 11.84%. The QTL QFLV-2A was identified in three environments and explained phenotypic variance from 3.09 to 7.90%. QFLV-7D was a major QTL, which was stably detected in three environments and explained 4.94–11.84% of the phenotypic variance. The additive effect indicated that the allele contributing to the increase of FLV was from Jinmai 919. For the other 14 QTL, five of the FLV-increasing QTL were contributed by Jinchun 7.
Remarkably, there were two co-localized regions for flag-leaf-related traits. QFLW-7A.1 for FLW and QFLA-7A.1 for FLA were co-located in the region of 636.22–678.88 Mb (Table 4 and Figure 3). Similarly, QFLW-7D for FLW, QFLA-7D.1 for FLA, and QFLV-7D for FLV were co-located between markers Ku_c16859_1460 and Kukri_c50621_436. To further validate these major QTL, we used the peak SNPs for the five QTL to evaluate their effects on the corresponding traits. The effect of QFLW-7A.1 and QFLA-7A.1 were significant (P < 0.05) in all six environments as well as BLUP values. The effect of QFLW-7D, QFLA-7D.1, and QFLV-7D were significant (P < 0.05) in all environments except for E4.
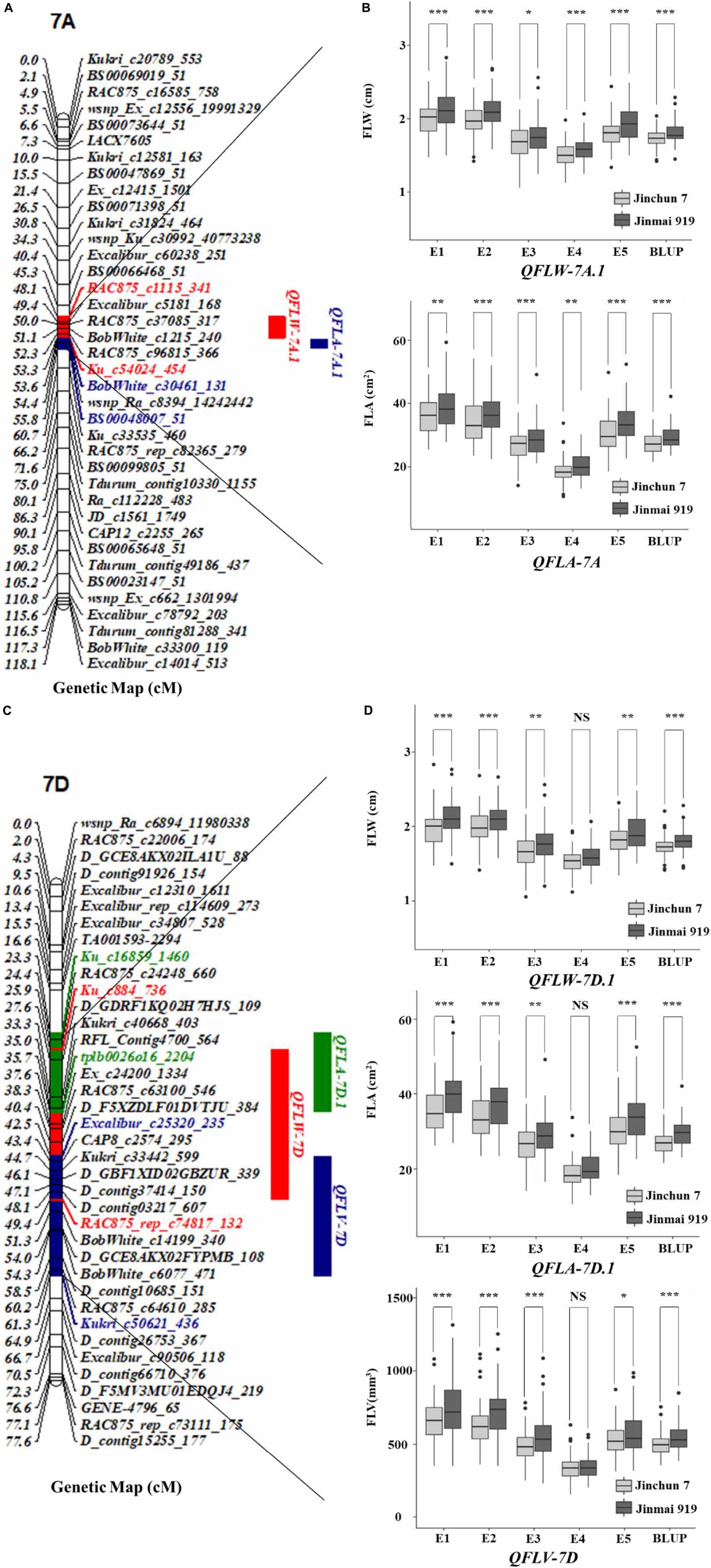
Figure 3. Genetic map of the major QTL QFLW-7A.1, QFLA-7A.1, QFLW-7D, QFLA-7D.1, QFLV-7D, and their effects. (A,C) Genetic map of 7A and 7D; (B,D) box plots of the effects of QTL on FLW, FLA, and FLV calculated after grouping the Jinchun 7 × Jinmai 919 DH population into two classes based on the allele of the flanking marker. *, **, and *** represent significance at P < 0.05, P < 0.01, and P < 0.001, respectively.
Analyses of additive effects of the major quantitative trait loci
Generally, the higher the number of positive alleles, the larger the leaf. The linear relationship between phenotypic data and the number of positive alleles was analyzed.
We detected 15 stable QTL including two QTL for FLL (QFLL-4A and QFLL-7D), six QTL for FLW (QFLW-1A, QFLW-4B, QFLW-6A, QFLW-7A.1, QFLW-7B.1, and QFLW-7D), three QTL for FLA (QFLA-5D.1, QFLA-7A, and QFLA-7D.1), two QTL for FLT (QFLT-2B and QFLT-6A), and two QTL for FLV (QFLV-2A and QFLV-7D). The additive effects for each trait were further analyzed based on linked peak markers. The average traits value increased as the number of positive alleles increased (Figure 4 and Supplementary Table 7). DH lines with positive alleles at both QTL regions had an average FLL 1.78 cm (9.90%) greater than lines with contrasting alleles. Similarly, lines with more than two positive alleles showed significantly increased FLW. DH lines with positive alleles at all six QTL regions had an average FLW 0.49 cm (32.87%) greater than lines with contrasting alleles. DH lines with positive alleles at three QTL regions had an average FLA 0.52 cm2 (18.47%) greater than lines with contrasting alleles. DH lines with positive alleles at two QTL regions had an average FLT 0.01 mm (6.62%) thicker than lines with contrasting alleles. DH lines with positive alleles at two QTL regions had an average FLV 99.19 mm3 (20.87%) greater than lines with contrasting alleles.
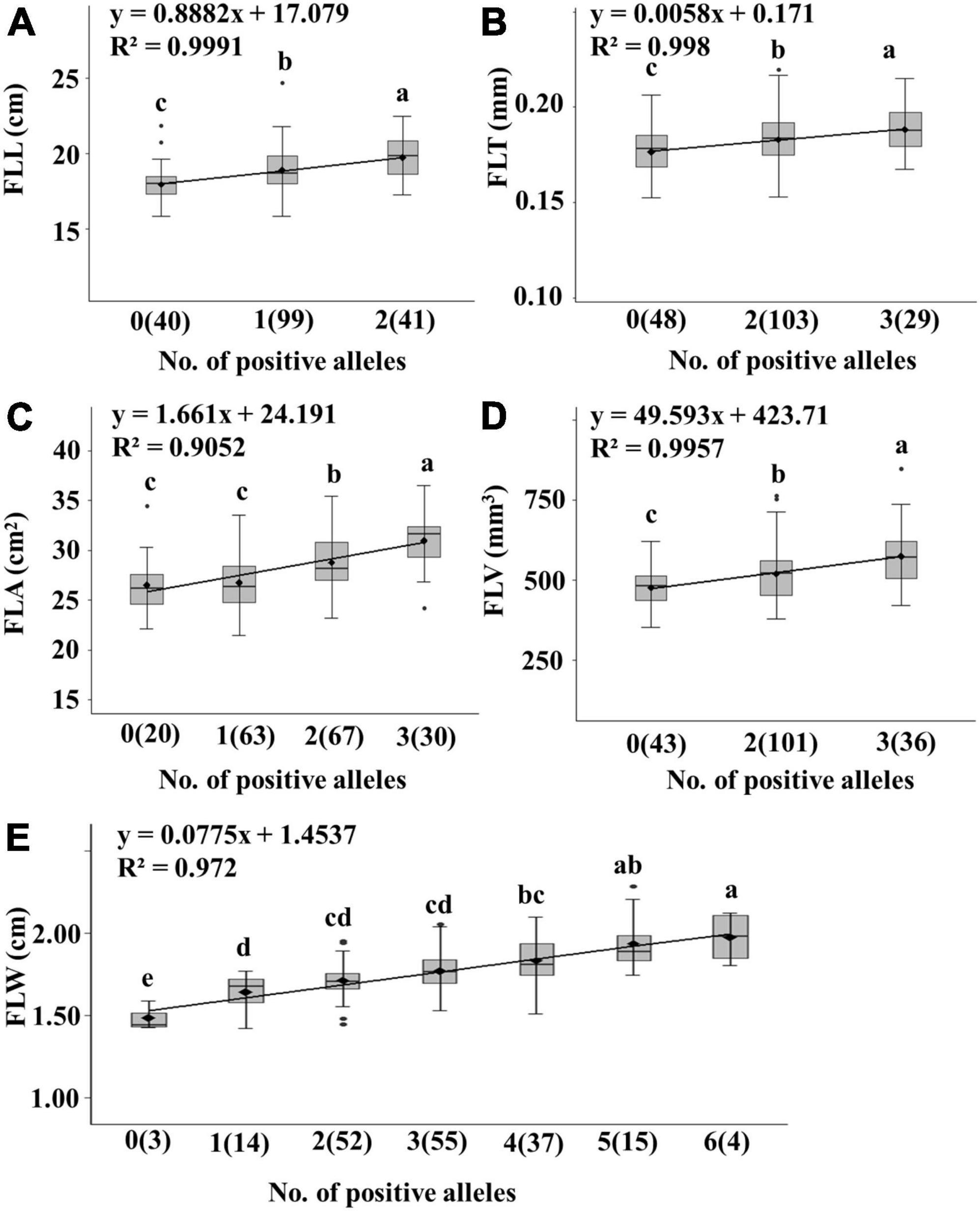
Figure 4. Linear regressions between number of positive alleles and trait values for five flag related traits in the Jinchun 7 × Jinmai 919 DH population (A–E). The number of lines carrying the corresponding number of positive alleles are shown in brackets. The letter above the bars indicated comparisons result at the significant level 0.05.
Co-location of for flag leaf thickness and flag leaf volume with yield related traits
Hundreds of yield-related QTL have been mapped on all wheat chromosomes (Cao et al., 2020). QFLT-2B was mapped between BobWhite_c2988_421 and Tdurum_contig51443_336 and physically located between 38.34 and 66.37 Mb on chromosome 2B. It overlapped with loci TKW-gwm547 for thousand kernel weight on 2B reported by Wang et al. (2012; Figure 5) QFLT-2B was near QSpn.kibr-2BS for SN that mapped between Xwmc154 and Xbarc18 and was physically located between 36.45 and 255.35 Mb on chromosome 2B, suggesting they may be allelic (Katkout et al., 2014). QFLV-2A mapped between AC875_c68208_252 and Kukri_rep_c113542_254 and was physically located between 8.71 and 37.61 Mb on chromosome 2A. It overlapped with QTkw-2A.1 (Katkout et al., 2014) and QTgw.nfcri-2A (Wang et al., 2009) for TGW, and QGne-2A (Narjesi et al., 2015) for GNS, which were in the markers barc1138-wPt664128, xgwm359, and wpt667287-Xgwm359, respectively. In addition, QFLV-7D mapped between BobWhite_rep_c50161_575 and D_contig10685_151 and was physically located between 118.53 and 550.43 Mb on chromosome 7D, which indicated that this region may consist of several different QTL. In addition, TKW-IWA604 (Zhang et al., 2018) and TKW-gwm55 (Wang et al., 2012) were also located in the same interval. qSn-7D for SN mapped between AX-110875183 and AX-95019577 and was physically located between 193.47 and 218.18 Mb on chromosome 7D (Fan et al., 2019), which also overlapped with QFLV-7D.
Quantitative trait loci validation
To further validate the fifteen major QTL, the KASP markers for each QTL were used to evaluate their effects on chlorophyll content in the DH118 × Jinmai 919 population. The KASP markers for QFLW-4B, QFLW-6A, QFLW-7D, and QFLA-5D were not polymorphic between DH118 and Jinmai 919, and thus the effects could not be evaluated. The remaining eleven QTL were evaluated. The effect of QFLW-7B, QFLT-6A, QFLV-2A, and QFLV-7D did not differ significantly between two groups in DH118 × Jinmai 919 population (Figure 6). The effect of other seven QTL, QFLL-4A, QFLL-7D, QFLW-1A, QFLW-7A, QFLT-2B, QFLA-7A, and QFLA-7D, were highly significant (P < 0.05) in more than three environments. According to marker profiles of QFLL-7D, QFLW-1A, QFLW-7A, QFLT-2B, QFLA-7A, and QFLA-7D, the lines with homozygous alleles from Jinmai919 had significantly higher (P < 0.05) values than those from Jinchun 7. The QFLL-4A lines homozygous for the Jinchun 7 alleles had significantly higher phenotypic values than those with the Jinmai919 alleles irrespective of QTL region, with differences in FLL ranging from 3.24 to 7.13%. The QFLL-4A lines homozygous for the Jinmai919 alleles had significantly higher phenotypic values than those with the Jinchun 7 alleles irrespective of QTL region, with differences in FLL ranging from 0.71 to 7.02%. Lines with the positive allele from QFLW-1A and QFLW-7A had significantly greater FLW ranging from 2.29 to 3.52% and 0.45 to 4.93%, respectively. The FLT of lines with the QFLT-2B allele was significantly higher than for lines lacking the QFLT-2B allele, and the difference was between 1.07 and 7.30%. Lines with the positive allele for QFLA-7A and QFLA-7D had significantly greater FLA.
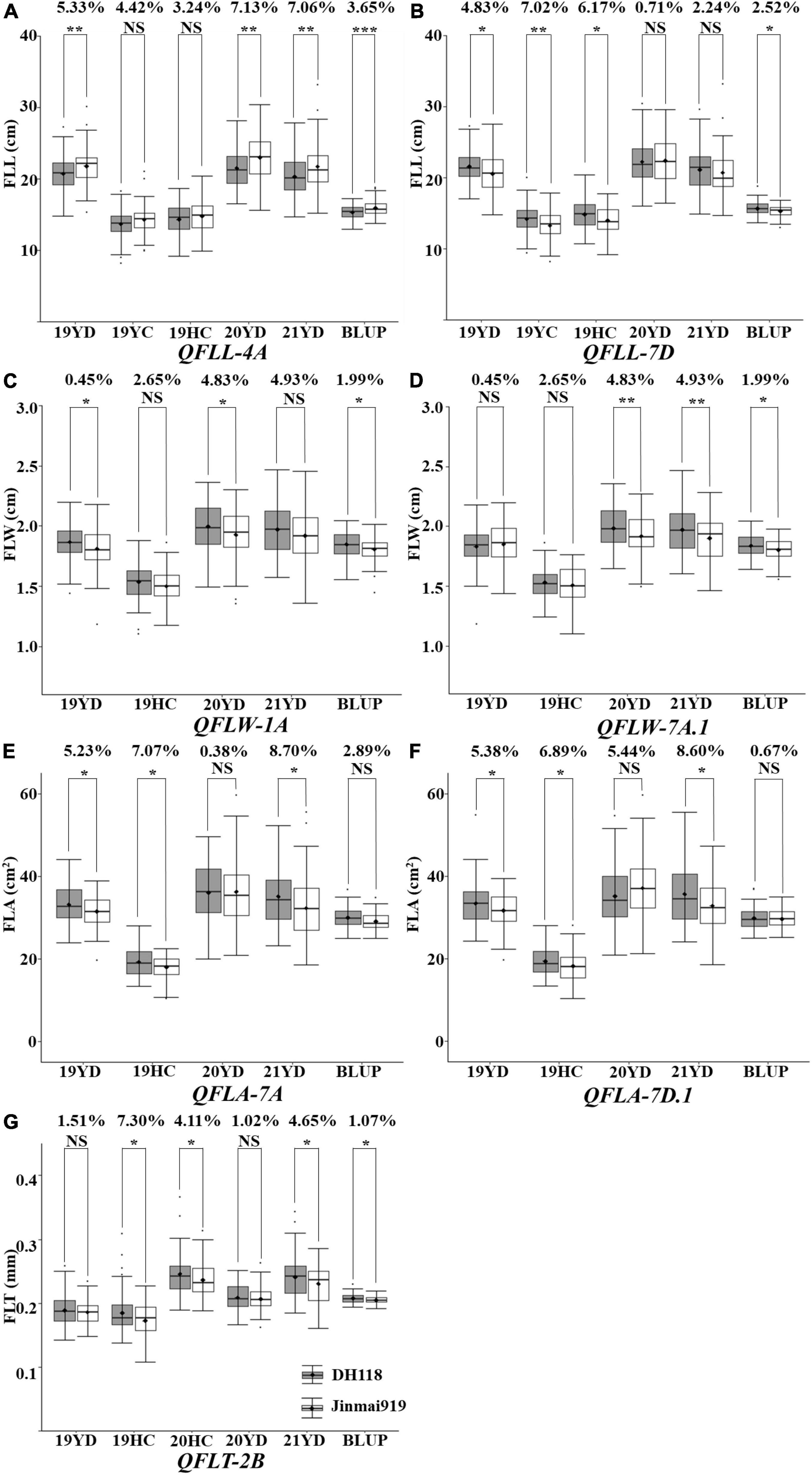
Figure 6. Validation of 11 stable quantitative trait loci (QTL) in the DH118 × Jinmai 919 population. Effects of QFLL-4A (A), QFLL-7D (B) on flag leaf length and effects of QFLW-1A (C), QFLW-7A (D) on flag leaf width and effects of QFLT-2B (E) on flag leaf thickness and effects of QFLA-7A (F) and QFLA-7D (G) on flag leaf area. Designations *, **, *** and NS represent P < 0.05, P < 0.01, P < 0.001 and no significant difference, respectively.
Marker development
To apply important QTL associated with flag-leaf-related traits to wheat breeding and gene cloning, seven SNPs were identified, wsnp_Ex_c19773_28772235 (4A) linked to QFLL-4A and D_contig26694_169 (7D) linked to QFLL-7D for FLL, wsnp_Ex_c572_1138770 (1A) linked to QFLW-1A and wsnp_BQ160404A_Ta_1_1 (7A) linked to QFLW-7A.1 for FLW, wsnp_Ra_c265_560747 (2B) linked to QFLT-2B for FLT, wsnp_BQ160404A_Ta_1_1 (7A) linked to QFLA-7A and IAAV2530 (7D) linked to QFLA-7D.1 for FLA (Supplementary Table 8).
Putative candidate genes
A total of 6,007 genes were identified within the seven major QTL, 1,059 genes in QFLL-4A, 503 genes in QFLL-7D, 1,036 genes in QFLW-1A, 516 genes in QFLW-7A, 767 genes in QFLT-2B, 1,071 genes in QFLA-7A, and 1,055 genes in QFLA-7D (Supplementary Table 9). Among these genes in each interval, some were related to leaf growth and development. A cluster of 226 genes harboring an F-box domain may be of particular interest because they were reported to participate in hormone signal transduction, which affects leaf development (Song et al., 2012). TraesCS4A03G0838400LC and TraesCS7D03G0218200 encode purple acid phosphatase, which are involved in leaf senescence in Arabidopsis (Robinson et al., 2012; Tu et al., 2021). Seventeen ethylene-responsive transcription factors were reported to regulate leaf development (Koyama, 2014).
Discussion
A Simple and accurate method to measure flag leaf thickness
Previous studies generally used the drainage method to measure leaf volume. However, since wheat leaf volume is relatively small and difficult to measure the results using this method are variable. Leaf thickness is the main parameter of flag leaf volume. At present, leaf thickness is mainly measured through a plant leaf parameter instrument with the help of modern sensing technology and a direct contact method. Leaf thickness can change during extrusion measurement, which introduces errors. Wheat flag leaves gradually thins from base to tip, further complicating the measurement of wheat flag leaf volume. In the present study, leaf volume was measured by incorporating the use of a Leica microscope and the image processing software Image J to measure leaf thickness. Leaf thickness at two-thirds of the leaf length from tip to collar was found to represent the average leaf thickness, and this discovery enabled a more accurate calculation of leaf volume. Leaf volume as measured with the more laborious drainage method was consistent with results from the new method. This new high-throughput method for measuring leaf volume will have value in future research on leaf volume in cereal crops.
Nine novel stable quantitative trait loci for flag-leaf-related traits were detected
Fifteen major QTL for flag-leaf-related traits were detected in the present study. To compare the intervals of the QTL detected with those identified previously, these QTL were physically mapped on target chromosomes in Chinese Spring. QFLL-7D mapped between D_GCE8AKX02ILA1U_88 and RAC875_c44223_285 and was physically located between 56.63 and 73.25 Mb on chromosome 7D. It overlapped with QFLL-7D.2, which mapped between 72.95 and 93.50 Mb (Hu et al., 2020). QFLW-1A mapped between Excalibur_c9662_70 and Tdurum_contig44112_1609 and was physically located between 102.14 and 556.49 Mb on chromosome 1A. It overlapped with QFLW-1A, which mapped to 511.45 Mb (Yan et al., 2020). QFLW-6A.1 overlapped with QFlw.sicau-6A.2 as reported by Ma et al. (2020) and was close to QFLW-6A (Yan et al., 2020). QFLW-7A.1 overlapped with a genetic region reported by Yang et al. (2016). QFLW-7B.1 was located near QFlw.sicau-7B (Ma et al., 2020). QFLW-7D.1 was located near QTL QFlw.cau-7D, which was detected in all four environments tested (Wu et al., 2016). Comparison of physical intervals suggested that no previously reported QTL overlapped with QFLL-4A, QFLW-4B, QFLA-5D.1, QFLA-7A, and QFLA-7D.1. Thus, these five QTL are likely novel loci. There are few reports of QTL for FLT and FLV in wheat, so QFLT-2B, QFLT-6A, QFLV-2A, and QFLV-7D are also likely novel.
Leaf thickness is of great significance to gain yield
Leaf thickness is an important leaf trait as it affects light energy utilization and yield. Two QTL for FLT, QFLT-2B and QFLT-6A, were detected in the Jinchun 7 × Jinmai 919 DH population. One of the QTL for FLT was co-located with QTL for yield related traits. QFLT-2B overlapped with the previously identified loci TKW-gwm547 for TKW and QSpn.kibr-2BS for SN, indicating that these loci have either a pleiotropic effect or are closely linked (Wang et al., 2012; Katkout et al., 2014). Breeding for optimum flag leaf size is thought to be a good approach to improve grain yield by reducing shading and disease conduciveness within the plant population. However, there is an upper limit to increasing leaf area for increased grain yield. Therefore, increasing leaf thickness through breeding offers another approach to increase the photosynthetic potential and grain yield. In addition, the SNPs for FLT identified in the present study could be converted to KASP markers and used in molecular marker-assisted breeding in the future.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
YW, LQ, CY, XL, JJZ, and BW: data curation. LQ, XZ, PL, and JZ: methodology. YW and LQ: writing—original draft. XZ, PL, and JZ: writing—review and editing. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Research Program Sponsored by State Key Laboratory of Integrative Sustainable Dryland Agriculture (in preparation), Shanxi Agricultural University (No. 202102-1), and Research Program of Shanxi Province (YDZJSX2021C012 and 201903D221074).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2022.990287/full#supplementary-material
Footnotes
- ^ https://imagej.nih.gov/ij/
- ^ https://www.sas.com
- ^ https://isbreedingen.caas.cn/software/qtllcimapping/294605.htm
- ^ https://brcwebportal.cos.ncsu.edu/qtlcart/WQTLCart.htm
- ^ https://wheat.pw.usda.gov/ggpages/wgc/98/Intro.htm
- ^ http://wheatomics.sdau.edu.cn/blast/blast.html
- ^ http://www.polymarker.info/
- ^ http://wheatomics.sdau.edu.cn
References
Agustí, S., Enriquez, S., Frostchristensen, H., Sandjensen, K., and Duarte, C. M. (1994). Light harvesting among photosynthetic organisms. Funct. Ecol. 8, 273–279. doi: 10.2307/2389911
Araus, J. L., Alegre, L., Tapia, L., Calafell, R., and Serret, M. D. (1986). Relationships between photosynthetic capacity and leaf structure in several shade plants. Am. J. Bot. 73, 1760–1770. doi: 10.1002/j.1537-2197.1986.tb09708.x
Cao, S. H., Xu, D. G., Hanif, M., Xia, X. C., and He, Z. H. (2020). Genetic architecture underpinning yield component traits in wheat. Theo. Appl. Gene. 133, 1811–1823. doi: 10.1007/s00122-020-03562-8
Cellier, F., Conejero, G., and Casse, F. (2000). Dehydrin transcript fluctuations during a day/night cycle in drought-stressed sunflower. J. Exper. Bot. 51, 299–304. doi: 10.1093/jexbot/51.343.299
Coleman, R. K., Gill, G. S., and Rebetzke, G. J. (2001). Identification of quantitative trait loci for traits conferring weed competitiveness in wheat (Triticum aestivum L.). Aust. J. Agric. Res. 52, 1235–1246. doi: 10.1071/AR01055
Devika, S., Ravichandran, V., and Boominathan, P. (2018). Physiological analyses of nitrogen use efficiency and yield traits of rice genotypes. Indian J. Plant Physiol. 23, 100–110. doi: 10.1007/s40502-018-0358-8
Duncan, W. G. (1971). Leaf angles, leaf area, and canopy photosynthesis. Crop Sci. 11, 482–485. doi: 10.2135/cropsci1971.0011183X001100040006x
Enriquez, S., Duarte, C. M., Sand-Jensen, K., and Nielsen, S. L. (1996). Broad-scale comparison of photosynthetic rates across phototropic organisms. Oecologia 108, 197–206. doi: 10.1007/BF00334642
Fan, X. L., Cui, F., Ji, J., Zhang, W., Zhao, X. Q., Liu, J. J., et al. (2019). Dissection of Pleiotropic QTL Regions Controlling Wheat Spike Characteristics Under Different Nitrogen Treatments Using Traditional and Conditional QTL Mapping. Front. Plant Sci. 10:187. doi: 10.3389/fpls.2019.00187
Fan, X. L., Cui, F., Zhao, C. H., Zhang, W., Yang, L. J., Zhao, X. Q., et al. (2015). QTLs for flag leaf size and their influence on yield-related traits in wheat (Triticum aestivum L.). Mol. Breed. 35:24. doi: 10.1007/s11032-015-0205-9
Garnier, E., Salager, J. L., Laurent, G., and Sonié, L. (1999). Relationships between photosynthesis, nitrogen and leaf structure in 14 grass species and their dependence on the basis of expression. New Phytol. 143, 119–129. doi: 10.1046/j.1469-8137.1999.00426.x
Givnish, T. J. (1979). “On the adaptive significance of leaf form,” in Topics in Plant Population Biology, eds O. T. Solbrig, S. Jain, G. B. Johnson, and P. H. Raven (London: Palgrave), 375–407. doi: 10.1007/978-1-349-04627-0_17
Guitman, M. R., Arnozis, P. A., and Barneix, A. J. (1991). Effect of source-sink relations and nitrogen nutrition on senescence and N remobilization in the flag leaf of wheat. Physiol. Plant. 82, 278–284. doi: 10.1111/j.1399-3054.1991.tb00094.x
Hu, J. M., Wang, X. Q., Zhang, G. X., Jiang, P., Chen, W. Y., Hao, Y. C., et al. (2020). QTL mapping for yield-related traits in wheat based on four RIL populations. Theo. Appl. Gene. 133, 917–933. doi: 10.1007/s00122-019-03515-w
Kanbe, T., Sasaki, H., Aoki, N., Yamagishi, T., Ebitani, T., Yano, M., et al. (2008). Identification of QTLs for improvement of plant type in rice (Oryza sativa L.) Using Koshihikari/Kasalath chromosome segment substitution lines and backcross progeny F2 population. Plant Produc. Sci. 11, 447–456. doi: 10.1626/pps.11.447
Katkout, M., Kishii, M., Kawaura, K., Mishina, K., Sakuma, S., Umeda, K., et al. (2014). QTL analysis of genetic loci affecting domestication-related spike characters in common wheat. Genes Genet. Syst. 89, 121–131. doi: 10.1266/ggs.89.121
Khaliq, I., Irshad, A., and Ahsan, M. (2008). Awns and flag leaf contribution towards grain yield in spring wheat (Triticum aestivum L.). Cereal Res. Commun. 36, 65–76. doi: 10.1556/CRC.36.2008.1.7
Kobayashi, S., Fukuta, Y., Morita, S., Sato, T., Osaki, M., and Khush, G. S. (2003). Quantitative trait loci affecting flag leaf development in rice (Oryza sativa L.). Breed. Sci. 53, 255–262. doi: 10.1270/jsbbs.53.255
Koyama, T. (2014). The roles of ethylene and transcription factors in the regulation of onset of leaf senescence. Front. Plant Sci. 5:650. doi: 10.3389/fpls.2014.00650
Li, F. J., Wen, W. E., Liu, J. D., Zhai, S. N., Cao, X. Y., and Liu, C. (2021). Genome-wide linkage mapping for canopy activity related traits using three RIL populations in bread wheat. Euphytica 217:67. doi: 10.1007/s10681-021-02797-w
Liu, K. Y., Xu, H., Liu, G., Guan, P. F., Zhou, X. Y., Peng, H. R., et al. (2018). QTL mapping of flag leaf-related traits in wheat (Triticum aestivum L.). Theo. Appl. Genet. 131, 839–849. doi: 10.1007/s00122-017-3040-z
Ma, J., Tu, Y., Zhu, J., Luo, W., Liu, H., Li, C., et al. (2020). Flag leaf size and posture of bread wheat: Genetic dissection, QTL validation and their relationships with yield-related traits. Theo. Appl. Genet. 133, 297–315. doi: 10.1007/s00122-019-03458-2
Ma, S. W., Wang, M., Wu, J. H., Guo, W. L., Chen, Y. M., and Li, G. W. (2021). WheatOmics: A platform combining multiple omics data to accelerate functional genomics studies in wheat. Mol. Plant 14, 1965–1968. doi: 10.1016/j.molp.2021.10.006
Narjesi, V., Mardi, M., Hervan, E. M., Azadi, A., Naghavi, M. R., Ebrahimi, M., et al. (2015). Analysis of Quantitative Trait Loci (QTL) for Grain Yield and Agronomic Traits in Wheat (Triticum aestivum L.) Under Normal and Salt-Stress Conditions. Plant Mol. Biol. Rep. 33, 2030–2040. doi: 10.1007/s11105-015-0876-8
Nielsen, S. L., Enriquez, S., Duarte, C. M., and Sand-Jensen, K. (1996). Scaling maximum growth rates across photosynthetic organisms. Funct. Ecol. 10, 167–175. doi: 10.2307/2389840
Peña-Rojas, K., Aranda, X., Joffre, R., and Fleck, I. (2005). Leaf morphology, photochemistry and water status changes in resprouting Quercus ilex during drought. Funct. Plant Biol. 32, 117–130. doi: 10.1071/fp04137
Pendleton, J. W., Smith, G. E., Winter, S. R., and Johnston, T. J. (1968). Field investigations of the relationships of leaf angle in corn (Zea mays L.) to grain yield and apparent photosynthesis. Agron. J. 60, 422–424. doi: 10.2134/agronj1968.00021962006000040027x
Poorter, H. (1990). “Interspecific variation in relative growth rate: On ecological causes and physiological consequences,” in Causes and Consequences of Variation in Growth Rate and Productivity in Higher Plants, eds H. Lambers, M. L. Cambridge, H. Konings, and T. L. Pons (The Hague: SPB Academic Publishing), 45–68. doi: 10.1007/BF00029062
Qin, P., Lin, Y., Hu, Y. D., Liu, K., Mao, S. S., Li, Z. Y., et al. (2016). Genome-wide association study of drought-related resistance traits in Aegilops tauschii. Genet. Mol. Biol. 39, 398–407. doi: 10.1590/1678-4685-GMB-2015-0232
Robinson, W. D., Carson, I., Ying, S., Ellis, K., and Plaxton, W. C. (2012). Eliminating the purple acid phosphatase at PAP 26 in Arabidopsis thaliana delays leaf senescence and impairs phosphorus remobilization. New Phytol. 196, 1024–1029. doi: 10.1111/nph.12006
Sakamoto, T., Morinaka, Y., Ohnishi, T., Sunohara, H., Fujioka, S., and Ueguchi-Tanaka, M. (2006). Erect leaves caused by brassinosteroid deficiency increase biomass production and grain yield in rice. Nat. Biotechnol. 24:105. doi: 10.1038/nbt1173
Sharma, S. N., Sain, R. S., and Sharma, R. K. (2003). The genetic control of flag leaf length in normal and late sown durum wheat. J. Agric. Sci. 141, 323–331. doi: 10.1017/S0021859603003642
Simón, M. R. (1999). Inheritance of flag-leaf angle, flag-leaf area and flag-leaf area duration in four wheat crosses. Theo. Appl. Genet. 98, 310–314. doi: 10.1007/s001220051074
Smith, S. E., Kuehl, R. O., Ray, I. M., Hui, R., and Soleri, D. (1998). Evaluation of simple methods for estimating broad-sense heritability in stands of randomly planted genotypes. Crop Sci. 38, 1125–1129. doi: 10.2135/cropsci1998.0011183X003800050003x
Song, J., Huang, S., Dalmay, T., and Yang, Z. (2012). Regulation of leaf Morphology by MicroRNA394 and its target LEAF CURLING RESPONSIVENESS. Plant Cell Physiol. 53, 1283–1294. doi: 10.1093/pcp/pcs080
Stewart, D. W., Costa, C., Dwyer, L. M., Smith, D. L., Hamilton, R. I., and Ma, B. L. (2003). Canopy structure, light interception, and photosynthesis in maize. Agron. J. 95, 1465–1474. doi: 10.2134/agronj2003.1465
Syvertsen, J. P., Lloyd, J., McConchie, C., Kriedemann, P. E., and Farquhar, G. D. (1995). On the relationship between leaf anatomy and CO2 diffusion through the mesophyll of hypostomatous leaves. Plant Cell Environ. 18, 149–157. doi: 10.1111/j.1365-3040
Takai, T., Kondo, M., Yano, M., and Yamamoto, T. (2010). A Quantitative Trait Locus for Chlorophyll Content and its Association with Leaf Photosynthesis in Rice. Rice 3, 172–180. doi: 10.1007/s12284-010-9047-6
Tu, Y., Liu, H., Liu, J. J., Tang, H. P., Mu, Y., Deng, M., et al. (2021). QTL mapping and validation of bread wheat flag leaf morphology across multiple environments in different genetic backgrounds. Theo. Appl. Genet. 134, 261–278. doi: 10.1007/s00122-020-03695-w
Vijayalakshmi, K., Fritz, A. K., Paulsen, G. M., Bai, G., Pandravada, S., and Gill, B. S. (2010). Modeling and mapping QTL for senescence-related traits in winter wheat under high temperature. Mol. Breed. 26, 163–175. doi: 10.1007/s11032-009-9366-8
Wang, L. F., Ge, H. M., Hao, C. Y., Dong, Y. S., and Zhang, X. Y. (2012). Identifying loci influencing 1,000-kernel weight in wheat by microsatellite screening for evidence of selection during breeding. PLoS One 7:e29432. doi: 10.1371/journal.pone.0029432
Wang, R. X., Hai, L., Zhang, X. Y., You, G. X., Yan, C. S., and Xiao, S. H. (2009). QTL mapping for grain filling rate and yield-related traits in RILs of the Chinese winter wheat population Heshangmai×Yu8679. Theo. Appl. Genet. 118, 313–325. doi: 10.1007/s00122-008-0901-5
Wang, R. Z., Huang, W. W., Chen, L., Ma, L. N., Guo, C. Y., and Liu, X. Q. (2011). Anatomical and physiological plasticity in Leymus chinensis (Poaceae) along large-scale longitudinal gradient in northeast China. PLoS One 6:e26209. doi: 10.1371/journal.pone.0026209
Wang, S., Wong, D., Forrest, K., Allen, A., Chao, S., Huang, B. E., et al. (2014). Characterization of polyploid wheat genomic diversity using a high-density 90 000 single nucleotide polymorphism array. Plant Biotechnol. J. 12, 787–796. doi: 10.1111/pbi.12183
Wu, Q. H., Chen, Y. X., Lin, F., Zhou, S. H., Chen, J. J., and Zhao, X. J. (2016). QTL mapping of flag leaf traits in common wheat using an integrated high-density SSR and SNP genetic linkage map. Euphytica 208, 337–351. doi: 10.1007/s10681-015-1603-0
Xu, H. Y., and Zhao, J. S. (1995). Canopy photosynthesis capacity and the contribution from different organs in high-yielding winter wheat. Acta Agron. Sinica 2, 204–209.
Xue, S. L., Xu, F., Li, G. Q., Zhou, Y., Lin, M. S., and Gao, Z. X. (2013). Fine mapping TaFLW1, a major QTL controlling flag leaf (Triticum aestivum L.). Theo. Appl. Genet. 126, 1941–1949. doi: 10.1007/s00122-013-2108-7
Yan, X. F., Zhao, L., Ren, Y., Zhang, N., Dong, Z. D., and Chen, F. (2020). Identification of genetic loci and a candidate gene related to flag leaf traits in common wheat by genome-wide association study and linkage mapping. Mol. Breed. 40:58. doi: 10.1007/s11032-020-01135-7
Yang, B., Wen, X. J., Wen, H. W., Feng, Y. R., Zhao, J. J., and Wu, B. B. (2022). Identification of Genetic Loci Affecting Flag Leaf Chlorophyll in Wheat Grown under Different Water Regimes. Front. Genet. 13:832898.
Yang, D. L., Liu, Y., Cheng, H. B., Chang, L., Chen, J. J., Chai, S. X., et al. (2016). Genetic dissection of flag leaf morphology in wheat (Triticum aestivum L.) under diverse water regimes. BMC Genet. 17:94. doi: 10.1186/s12863-016-0399-9
Zhang, J., Gizaw, S. A., Bossolini, E., Hegarty, J., Howell, T., Carter, A. H., et al. (2018). Identification and validation of QTL for grain yield and plant water status under contrasting water treatments in fall-sown spring wheats. Theor. Appl. Genet. 131, 1741–1759. doi: 10.1007/s00122-018-3111-9
Zhao, C. H., Liu, X. J., Liu, H. X., Kong, W. C., Zhao, Z. C., Zhang, S. R., et al. (2022). Fine mapping of QFlw-5B, a major QTL for flag leaf width in common wheat (Triticum aestivum L.). Theo. Appl. Genet. 135, 2531–2541. doi: 10.1007/s00122-022-04135-7
Keywords: wheat, flag leaf, flag leaf volume measurement, quantitative trait loci, grain
Citation: Wang Y, Qiao L, Yang C, Li X, Zhao J, Wu B, Zheng X, Li P and Zheng J (2022) Identification of genetic loci for flag-leaf-related traits in wheat (Triticum aestivum L.) and their effects on grain yield. Front. Plant Sci. 13:990287. doi: 10.3389/fpls.2022.990287
Received: 09 July 2022; Accepted: 08 August 2022;
Published: 08 September 2022.
Edited by:
Shuaifeng Geng, Institute of Crop Sciences (CAAS), ChinaCopyright © 2022 Wang, Qiao, Yang, Li, Zhao, Wu, Zheng, Li and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Zheng, c3hua3l6akAxMjYuY29t; Pengbo Li, bGlwZW5nYm9Ac3hhdS5lZHUuY24=
†These authors have contributed equally to this work
 Ying Wang1,2†
Ying Wang1,2† Ling Qiao
Ling Qiao Xingwei Zheng
Xingwei Zheng Jun Zheng
Jun Zheng