
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci. , 16 September 2022
Sec. Plant Metabolism and Chemodiversity
Volume 13 - 2022 | https://doi.org/10.3389/fpls.2022.988352
This article is part of the Research Topic Exploring Complex Biosphere Molecular Signaling Networks: Plant-Microbes Symbiosis at Microscopic to Macroscopic Levels View all 8 articles
 Sirajudheen Anwar1*
Sirajudheen Anwar1* Muhammad Faisal Nadeem2
Muhammad Faisal Nadeem2 Irfan Pervaiz3
Irfan Pervaiz3 Umair Khurshid4*
Umair Khurshid4* Nimra Akmal4
Nimra Akmal4 Khurram Aamir5
Khurram Aamir5 Muhammad Haseeb ur Rehman5,6
Muhammad Haseeb ur Rehman5,6 Khaled Almansour7
Khaled Almansour7 Farhan Alshammari7
Farhan Alshammari7 Mohd Farooq Shaikh8
Mohd Farooq Shaikh8 Marcello Locatelli9
Marcello Locatelli9 Nafees Ahemad10
Nafees Ahemad10 Hammad Saleem2*
Hammad Saleem2*This study was designed to seek the phytochemical analysis, antioxidant, enzyme inhibition, and toxicity potentials of methanol and dichloromethane (DCM) extracts of aerial and root parts of Crotalaria burhia. Total bioactive content, high-performance liquid chromatography-photodiode array detector (HPLC-PDA) polyphenolic quantification, and ultra-high performance liquid chromatography-mass spectrometry (UHPLC-MS) analysis were utilized to evaluate the phytochemical composition. Antioxidant [including 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH)], 2,2′-azino-bis[3-ethylbenzothiazoline-6-sulfonic acid (ABTS), ferric reducing antioxidant power assay (FRAP), cupric reducing antioxidant capacity CUPRAC, phosphomolybdenum, and metal chelation assays] and enzyme inhibition [against acetylcholinesterase (AChE), butyrylcholinesterase (BChE), α-glucosidase, α-amylase, and tyrosinase] assays were carried out for biological evaluation. The cytotoxicity was tested against MCF-7 and MDA-MB-231 breast cell lines. The root-methanol extract contained the highest levels of phenolics (37.69 mg gallic acid equivalent/g extract) and flavonoids (83.0 mg quercetin equivalent/g extract) contents, and was also the most active for DPPH (50.04 mg Trolox equivalent/g extract) and CUPRAC (139.96 mg Trolox equivalent /g extract) antioxidant assays. Likewise, the aerial-methanol extract exhibited maximum activity for ABTS (94.05 mg Trolox equivalent/g extract) and FRAP (64.23 mg Trolox equivalent/g extract) assays. The aerial-DCM extract was noted to be a convincing cholinesterase (AChE; 4.01 and BChE; 4.28 mg galantamine equivalent/g extract), and α-glucosidase inhibitor (1.92 mmol acarbose equivalent/g extract). All of the extracts exhibited weak to modest toxicity against the tested cell lines. A considerable quantities of gallic acid, catechin, 4-OH benzoic acid, syringic acid, vanillic acid, 3-OH-4-MeO benzaldehyde, epicatechin, p-coumaric acid, rutin, naringenin, and carvacrol were quantified via HPLC-PDA analysis. UHPLC-MS analysis of methanolic extracts from roots and aerial parts revealed the tentative identification of important phytoconstituents such as polyphenols, saponins, flavonoids, and glycoside derivatives. To conclude, this plant could be considered a promising source of origin for bioactive compounds with several therapeutic uses.
Plants are genetically very diverse and vital to human existence, shelter, food, and medicine. Among plants, the study of medicinal plants has gained worldwide attention in recent years. A substantial amount of research demonstrates the intriguing potential of medicinal plants employed in traditional, complementary, and alternative methods of treating human ailments (Fitzgerald et al., 2020; Erdinc et al., 2021; Tamer et al., 2021). The investigation of medicinal plants as a unique source of enzyme inhibitors, natural antioxidant components, and treatments for a variety of common illnesses has attracted considerable interest (Phumthum et al., 2018). Phytochemicals, also known as secondary metabolites, are bioactive plant molecules and the source of the majority of currently accessible pharmaceuticals. 77% of antibiotics and 547 medicines approved by the FDA by the end of 2013 were derived from natural products, according to a survey (Patridge et al., 2016). Natural products play a major role in medication development; therefore, screening plants for substantial active ingredients can be viewed as a first step toward producing more effective treatments against a broader range of ailments (Bibi Sadeer et al., 2022). Herbal applications are now a rapidly expanding market, with the goal of creating new pharmaceutical and nutraceutical materials with herbal ingredients. Lifestyle diseases such as obesity, cancer, and diabetes mellitus are to blame for the current state of affairs (Ceylan et al., 2016; Yener et al., 2018).
Crotalaria belongs to the family Fabaceae. Approximately 700 species are make up this family disseminated throughout the world’s tropical and subtropical regions (Lewis, 2005). In the desert regions of West Pakistan, India, and Afghanistan, C. burhia, or Khip, is found as a shrub and fibrous plant. The ancient Indian Ayurvedic system, identified this plant as having great medicinal potential. Anticancer and soothing properties are found in the leaves, roots, and branches of C. burhia, while fresh plant juice can be used to treat eczema, gout, hydrophobia, pain, and edema. Roots extract with sugar is used to alleviate chronic kidney pain and to treat typhoid fever. It has a wide range of medical properties (Talaviya et al., 2018), Cooling medication can be made from the plant’s leaves, branches, and roots. Gout, eczema, hydrophobia, pain and swelling, wounds and cuts, infection, renal pain, stomach disorders, rheumatism, and joint pain can all be treated using plant juice in traditional medicine (Katewa and Galav, 2006; Sandeep et al., 2010; Bibi et al., 2015). There are several active compounds in this plant, including triterpenoids, flavonoids, anthraquinones, phenols, polyphenols, steroids, alkaloids, and tannins (Kataria et al., 2011; Kumar et al., 2011; Bibi et al., 2015). Additionally, C. burhia’s antibacterial, anti-inflammatory, and antinociceptive properties are supported by its traditional applications (Kataria et al., 2010; Kataria et al., 2012; Soni, 2014; Talaviya et al., 2014; Bibi et al., 2015). Crotalaria burhia is a highly important medicinal plant used to treat different ailments. Some researchers also mentioned that the whole plant, as well as its different parts like its branches, roots, leaves, and stem applied for the cure of diseases (Talaviya et al., 2018). Fresh plant juices have magical ethnobotanical values and are reported to treat different disorders. Crotalaria burhia is a valuable plant used to treat cancer, infections, pain, swelling, inflammation, hydrophobia, and skin diseases (Kataria et al., 2010). This plant is well known for the useful cure of general contaminations in the Thal Desert of Punjab (Niaz et al., 2013). Previous literature exposed that it is also utilized as a good soil binder, as food for goats, and in the desert to make sheds for animals and ropes (Soni, 2014). Some phytochemical studies reported the isolation of secondary metabolites from Crotalaria burhia are identified as toxicarol, elliptone, rotenone, sumatrol, deguelin, and tephrosin (Uddin and Khanna, 1979), crotalarine (Ali and Adil, 1973), crosemperine (Ahmad and Fatima, 1986), quercetin, β-sitosterol (Soni, 2014). However, many species of the Crotalaria genus are yet to be explored scientifically.
Polyphenol compounds, which include flavonoids and phenolic acids, are widely distributed throughout the plant kingdom. Over 6,000 different flavonoid species have been discovered so far. In the fight against microbial and insect attacks, they play an important role (Boǧa et al., 2016; Bouhafsoun et al., 2018; Bakir et al., 2020). The biological activities of C. burhia, a species of the Crotalaria genus, was examined in this study with regard to enzymes targeted for the treatment of diabetes type II, Alzheimer’s disease, and skin hyperpigmentation problems. Methanol and DCM were used to extract the aerial and root sections of C. burhia, and ultra-high performance liquid chromatography-mass spectrometry (UHPLC-MS) profiling, HPLC poly-phenolic quantification, and total bioactive contents were used to determine the phytochemical composition of each extract. Several in vitro bio-assays were used to measure the antioxidant capacity of each extract, including the phosphomolybdenum assay, DPPH and ABTS assays for radical scavenging, FRAP and CUPRAC for reducing power, and total antioxidant capacity. The inhibition potential of all the extracts was studied against a panoply of clinically important enzymes, including AChE, BChE, glucosidase, amylase, and tyrosinase. Furthermore, statistical correlation of all the activities by principal component analysis (PCA) was also studied.
Dr. H. Waris, Taxonomist of the Cholistan Institute of Desert Studies, The Islamia University of Bahawalpur, recognized C. burhia aerial and root parts obtained from Bahawalpur, Pakistan. For future reference, the herbarium of the Department of Pharmacy and Alternative Medicine, also deposited a voucher specimen number. For 15 days, the plant material was kept in the shade to dry. Using a combination of DCM and methanol, the powdered dried plant was extracted over the course of 72 h and further concentrated using rotary evaporator.
Standard Folin-Ciocalteu and aluminum chloride techniques (Slinkard and Singleton, 1977; Zengin et al., 2016) with minor modifications were used to assess the total phenolic (TPC) and flavonoid (TFC) concentrations. Gallic acid equivalents (mg GAE/g extract) and quercetin equivalents (mg QE/g extract) were used to measure phenolic and flavonoid content, respectively.
High-performance liquid chromatography-photodiode array detector (HPLC-PDA) analysis was used to determine the presence of 22 distinct polyphenolic standards in each sample. Waters liquid chromatograph with a model 600 solvent pump and a 2996 PDA detector was used for the analysis. The data was collected using Empower v.2 Software (Waters Spa, Milford, MA, United States) (Locatelli et al., 2017). The details of HPLC instrumentation are provided in “Supplementary Material” section. The gradient profiles and calibration parameters of the quantified phenolic standards are provided in Supplementary Tables 1, 2, respectively.
RP-UHPLC-MS was used to profile secondary metabolites. An Agilent 6,520 was used to perform UHPLC-MS analysis of methanolic extracts of aerial and root portions (negative ionization mode) on the Agilent 1,290 Infinity LC system (Khurshid et al., 2019). In order to make some tentative predictions about the presence of various secondary metabolites in the samples, we turned to the METLIN database. The details of UHPLC-MS instrumentation are provided in “Supplementary Material” section.
According to already adopted methods by Grochowski et al. (2017), DPPH and ABTS radical scavenging, reducing power (FRAP, CUPRAC), total antioxidant capacity (phosphomolybdenum), and metal chelating power of the investigated extracts were evaluated. The antioxidant activity of all assays was measured in terms of Trolox equivalents (mg TE/g extract) while the metal chelating activity was assessed in terms of mg EDTAE/g extract. The details of antioxidant assays are provided in “Supplementary Material” section.
The enzyme inhibition potential of plant extracts against cholinesterases (AChE and BChE), tyrosinase, α-amylase, and α-glucosidase was evaluated using previously established in vitro standard methods (Grochowski et al., 2017; Mollica et al., 2017). Galantamine equivalents per gram of extract (GALAE/g) were used to measure AChE and BChE inhibitory activities. On the other hand, millimoles of acarbose equivalents (ACAE/g) and milligram of kojic acid equivalents (KAE/g) were used to measure inhibition of α-amylase, α-glucosidase, and tyrosinase, respectively. The details of enzyme inhibition assays are provided in “Supplementary Material” section.
Using the previously published approach, the cytotoxicity of the tested products was assessed against two breast cancer cell lines, MDA-MB 231 and MCF-7 cells, using the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay (Nemudzivhadi and Masoko, 2014). The cell viability percentage (%) was calculated.
Three separate experiments were conducted for each of the assays. Mean standard deviation was used to express results (SD). SPSS v.17.0 was employed for data analysis. ANOVA and Tukey’s test were used to examine the differences between the means. Statistical significance was defined as a p-value of 0.05 or less. A link between bioactive content and evaluated biological assays was obtained using PCA and Pearson linear correlation.
When it comes to plant secondary metabolites, phytochemicals, such as phenols and flavonoids, are regarded to be the most bioactive secondary metabolites (Rahman et al., 2018). Table 1 lists the TPC and TFC values of methanol and DCM extracts of C. burhia’s aerial and root portions, respectively. The methanolic root extract had the highest TPC concentration (37.69 mg GAE/g), whilst the DCM aerial extract had the lowest (27.62 mg GAE/g). The flavonoid content determination followed a similar trend to that of the TPC, with TFC values of 83.11 and 12.64 mg QE/g extract for both methanol root and DCM aerial extracts, respectively.
Similarly, HPLC-PDA polyphenolic quantification was performed in order to quantify the phenolic standards in the studied extracts and the results are presented in Table 2, while, the HPLC-PDA chromatograms of the quantified phenolics in the tested extracts are given in Supplementary Figures 1, 2. In comparison to the other extracts, C. burhia methanol root extract comprised a significant quantity of phenolics (4.28 μg/mg), with the highest amounts of epicatechin (0.71 μg/mg extract) and p-coumaric acid (0.68 μg/mg extract), while rutin (0.33 μg/mg extract) was quantified in lesser amount. Likewise, aerial methanol extract presented the highest quantities of epicatechin (1.89 μg/mg extract), while DCM root extract displayed the lowest amounts of carvacrol (0.65 μg/g extract). Both roots and aerial DCM extracts accounted for the least amounts of phenolic standards (0.65 and 0.36 μg/g extract, respectively), which could be due to the extracts being nonpolar. Further investigations of plant extracts/fractions can be done to separate bioactive compounds with potentially important functions as a result of this phenolic profiling.
Additionally, methanolic extracts of C. burhia roots and aerial parts were subjected to UHPLC-MS analysis in order to get thorough profiles of individual secondary metabolites. Figures 1A,B depict standard total ion chromatograms with mass spectrometric peaks for both extracts. Tables 3, 4 give a preliminary list of secondary metabolites found in aerial and root extracts, respectively. A total of 36 distinct secondary metabolites were detected in the methanolic aerial extract. A preliminary analysis of the root extract identified 53 distinct chemicals. Majority of the compounds belonged to phytoconstituents’ phenols, flavonoid, saponin, coumarin, and glycoside classes. Polyphenols, notably flavonoids and coumarins, have been discovered to possess a wide range of health benefits, including antibacterial, enzyme inhibitory and antioxidant capabilities (Dilworth et al., 2017), whereas glycosides, tannins, alkaloids, and resins have been shown to have antibacterial activities (Rascon-Valenzuela et al., 2017). According to our research, this is the first time this plant has been profiled in such detail.
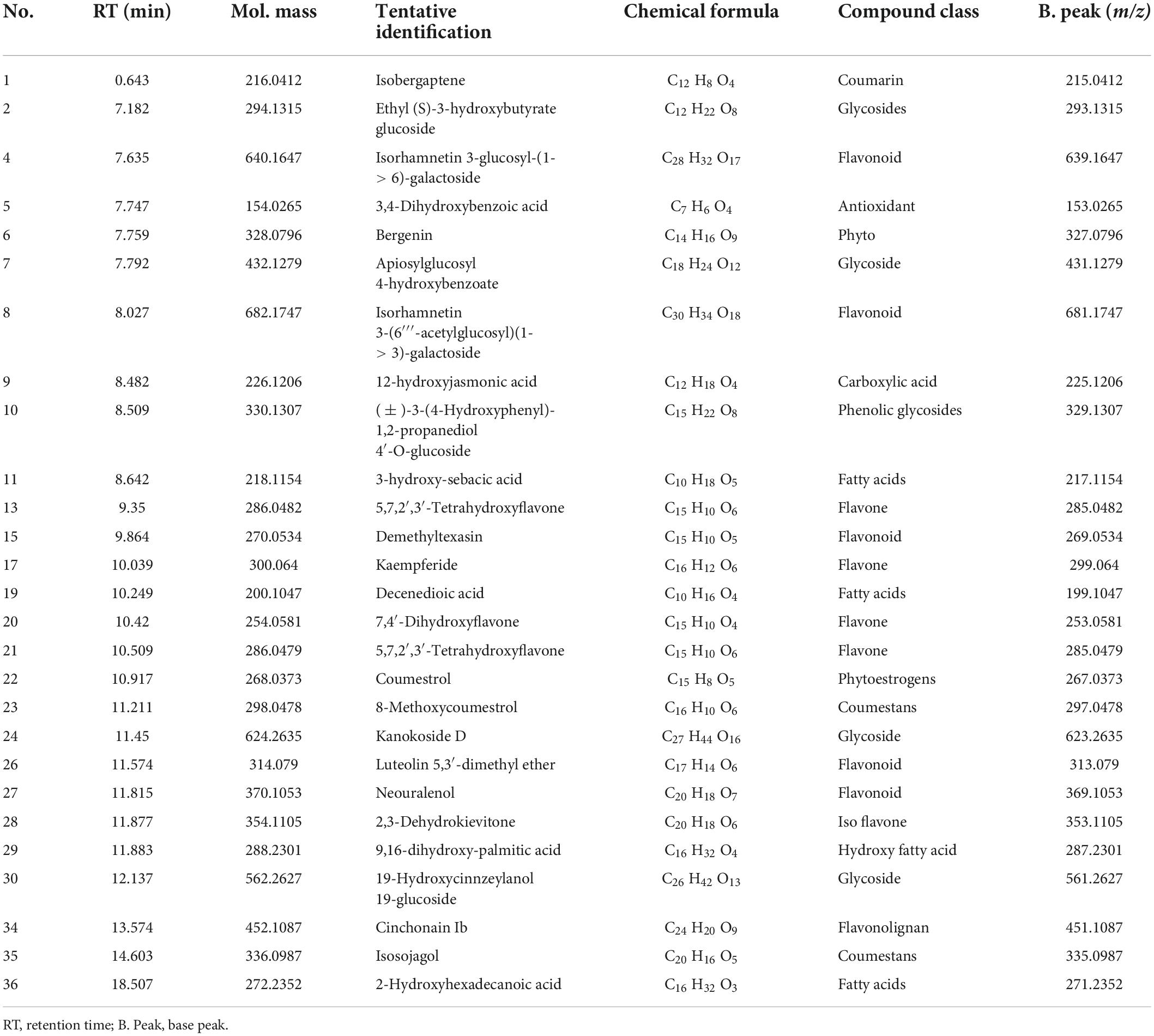
Table 3. UPHLC-MS analysis tentative identification of the secondary metabolites from C. burhia aerial methanol extract (negative ionization mode).
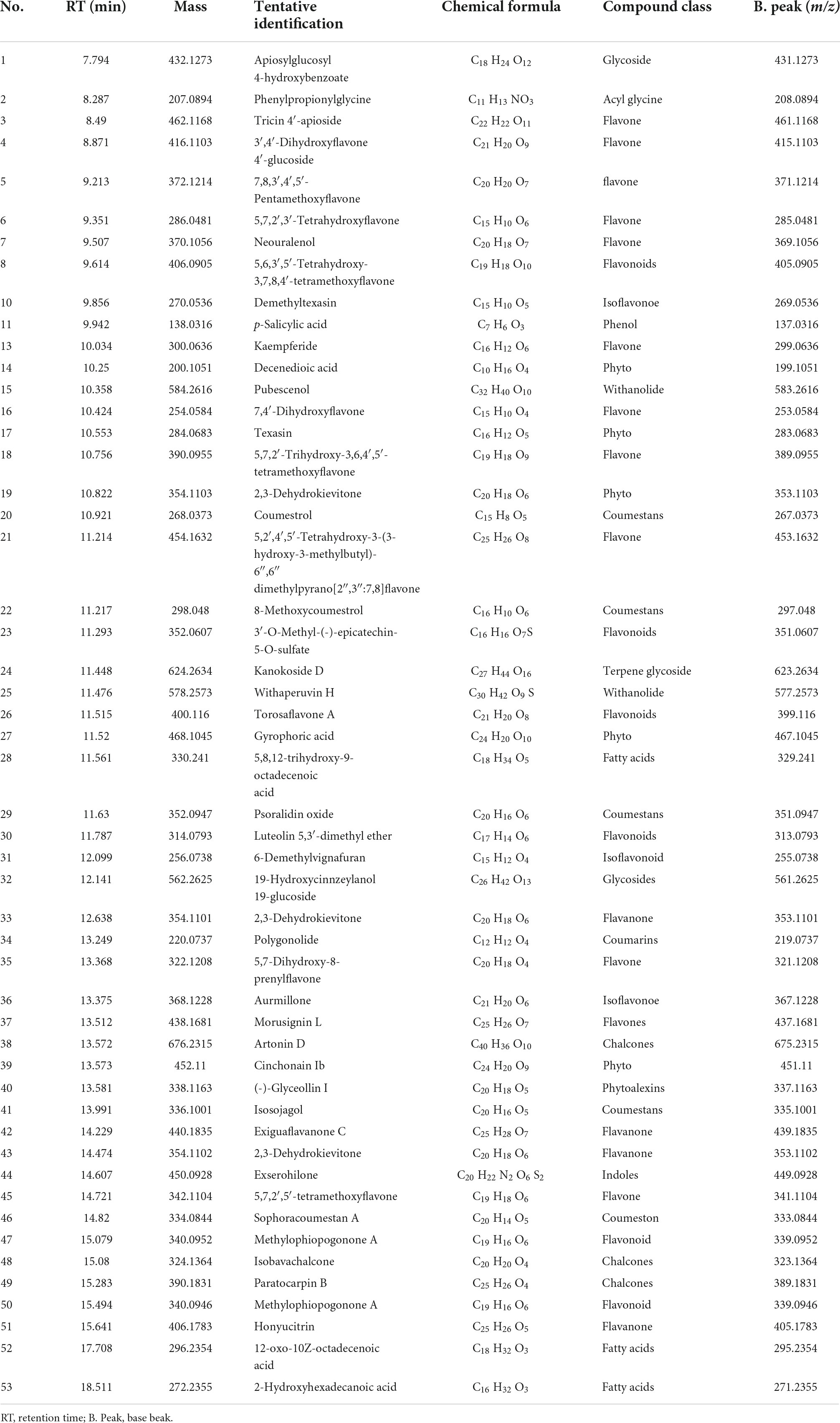
Table 4. UPHLC-MS analysis tentative identification of the secondary metabolites from C. burhia root methanol extract (negative ionization mode).
Metabolic processes typically produce reactive oxygen species (ROS). Excessive accumulation of ROS causes tissue injury and inflammation by damaging fatty acids, DNA, and proteins. As a result of these illnesses, plant extracts have been examined for their possible function in reducing the oxidative stress burden (Zengin et al., 2022).
Antioxidant activity of C. burhia extracts was tested using six different assays, the findings of which may be found in Table 1. To sum up, it was shown that the roots and aerial methanolic extracts had the highest radical scavenging and reducting power assays’ maximum values. Bioactive components with reducing power and anti-oxidant activity have been shown to have a favorable correlation with the amount of phenols and flavonoids found in this extract (Khan et al., 2019). Antioxidant activity was found in phenolic compounds quantified through HPLC-PDA, including 4-OH benzoic acid, vanillic acid, syringaldehyde, p-coumaric acid, and carvacrol (Verma et al., 2008). As mentioned in Table 1, the root-methanol extract was the most active for DPPH radical scavenging (50.04 mg TE/g extract) and CUPRAC reducing power potential (139.96 mg TE/g extract). Likewise, the aerial-methanol extract exhibited maximum ABTS radical scavenging (94.05 mg TE/g extract) and FRAP reducing power potential (64.23 mg TE/g extract). The DCM aerial extract exhibited the highest potential for phosphomolybdenum assay at 60.46 mg TE/g and metal chelation activity at 2.24 mg EDTAE/g. Previous studies have shown that this plant has significant antioxidant activity which validates our current findings (Talaviya et al., 2014; Ahmed, 2018). Rutin and naringenin, two important flavonoids with antioxidant potential, were also found in the current study’s HPLC polyphenol quantification and UHPLC-MS analysis (Yang et al., 2008; Cavia-Saiz et al., 2010).
Enzyme inhibition is gaining popularity as a therapeutic technique for various global health challenges, including type 2 diabetes, neurodegenerative diseases, and dermatological disorders. This phenomenon illustrates the strategy of inhibiting certain enzymes from treating specific diseases. Neurodegenerative diseases like Alzheimer’s and Parkinson’s have been linked to butyrylcholinesterase (BChE) and Acetylcholinesterase (AChE) (Zengin et al., 2018). Some research has shown that isolated compounds and plant extracts can both inhibit cholinesterase activity (Ballard et al., 2005). Galantamine, an alkaloid extracted from the Galanthus woronowii plant, is one example. Treatment for mild to moderate Alzheimer’s disease with the AChE inhibitor galantamine (Colovic et al., 2013). Previously, significant AChE inhibition potential has been reported in ethanolic extract of C. hebecarpa leaves (IC50: 208.6 μg/mL) (Rao et al., 2017). As presented in Table 5, the aerial DCM aerial showed maximum inhibition for AChE (4.01 mg GALAE/g extract) and BChE (4.28 mg GALAE/g extract). While, DCM root extract and methanolic aerial extract displayed the lowest inhibition potential against AChE and BChE (2.07 and 2.93 mg GALAE/g extract), respectively.
The enzyme tyrosinase catalyzes human melanin biosynthesis, also known as melanogenesis, a physiological process that results in the production of melanin (Muddathir et al., 2017). Considering that the inhibition of tyrosinase activity can control melanin formation, dermatological conditions, such as those characterized by excessive melanin pigmentation, could benefit from tyrosinase inhibitor treatment (Jdey et al., 2017). Tyrosinase inhibition can also be used in the food industry. Fruits and vegetables can gain a lot from the inhibition of tyrosinase. Enzyme tyrosine catalyzes the decomposition of phenolic compounds, which results in undesirable color and taste (Zaidi et al., 2014). C. burhia methanol aerial extract showed maximum tyrosinase inhibition, i.e., 131.72 mg KAE/g extract. In comparison, the methanolic root extract showed inhibition of 128.51 mg KAE/g extract, followed by DCM aerial and DCM root extracts124.95 and 120.76 mg KAE/g extract, respectively (Table 5). According to previous studies, different phenolics and flavonoids have been shown to have anti-tyrosinase properties, which may explain why the methanolic extract rich in phenolic and flavonoid compounds was found active against mushroom tyrosinase (Zielinska et al., 2017; Choi et al., 2021). Significant tyrosinase inhibition potential of ethanolic extract of another Crotalaria species C. hebecarpa (IC50: 40.15 μg/mL), has been reported previously (Rao et al., 2017). Similarly, another study reported the methanol and aqueous extracts of C. juncea shoots to show moderated tyrosinase inhibition (16.12 and 22.45%) at 1 mg/mL (Ketprayoon and Chaicharoenpong).
Hyperglycemia occurs when the pancreas produces less insulin or the cells’ insulin sensitivity decreases. According to the World Health Organization, approximately 422 million individuals worldwide have been diagnosed with diabetes. Although synthetic medications have advanced, the number of people with diabetes continues to rise at an alarming rate. Several medicinal herbs, including curcumin, have been demonstrated to be beneficial in the diabetes (Choudhury et al., 2018; Obih et al., 2019). The alpha-amylase and alpha-glucosidase inhibitors acarbose, miglitol, and viglibose have been established. Acarbose is derived from plants. Bloating, flatulence, and other gastrointestinal discomforts have been linked to an excess inhibition of -amylase (Figueiredo-González et al., 2016). As a result, the mild inhibition of α-amylase and the significant inhibition of α-glucosidase were preferred (Kazeem et al., 2013).
In light of these findings, the enzyme inhibition capability of C. burhia extract and fractions was assessed against the clinically significant enzymes involved in diabetes, namely α-glucosidase and α-amylase. The current investigations have revealed (Table 5) that C. burhia extracts a mild inhibitor of α-glucosidase and α-amylase enzymes. The DCM root extract displayed the highest inhibitory potential against α-amylase (0.70 mmol ACAE/g extracts) while DCM aerial extract presented maximum potential against α-glucosidase (1.92 mmol ACAE/g extracts). The α-amylase inhibition results of C. burhia extracts were ordered as follows: CbR-D > CbA-D > CbA-M > CbR-M.
Two breast cancer cell lines, MCF-7 and MDA-MD-231, were tested for cytotoxicity of C. burhia extracts, as shown in Table 6. The results show that none of the extracts presented significant toxicity to the breast cell line used in the study. For MCF-7 and MDA-MB-231 cell lines, the CbA-M extract was found to be the most effective, with a percentage viability of 74.29 and 70%, respectively. Likewise, the CbR-M extract was also found to be considerably active against the MDA-MB-231 cell line, likewise, the CbA-D extract was also active against this cell line. The CbR-D extract was less toxic to either of the cell lines that were tested. In-vivo toxicity studies are recommended following this preliminary toxicity testing of the plant extract studied.
Data from multiple tests can be analyzed using PCA. To accomplish this, we used PCA to analyze the tested extracts. Correlation, clustering, and PCA were used to show how aerial and root extracts interacted with the biological assays. The results are summarized in Figure 2. Three dimensions summarizing, respectively, 50.6, 32.3, and 11.1% of the biological activities variability were obtained (Figure 2A1). It was noted that the two principal components were built by PCA, explaining 88.9% of the total variability, with dimension 1 (56.6%) and dimension 2 (32.3%) (Figure 2A2). Moreover, it was seen that the variables DPPH, ABTS, CUPRAC, tyrosinase, glucosidase, and AChE were strongly associated with the origination of axis 1 (56.6%), whereas, the variables inclusive of amylase, phosphomolybdenum, and BChE were strongly contributed to the formation of axis 2 (32.3%). The TPC was noted to be highly positive co-related with the CUPRAC, while a positive moderate co-relation was noted for the DPPH, and ABTS activities, whereas, a weak positive relationship was observed for the tyrosinase and glucosidase. Likewise, a moderate to weak negative correlation was observed among TPC and FRAP, PPBD, MCA, AChE, and BChE, while a strong negative co-relation occurred for the TPC and amylase. Similarly, the TFC presented a considerable positive relationship for CUPRAC, DPPH, ABTS, moderate to weak positive correlation for the tyrosinase, glucosidase, and FRAP, and a weak relationship for the PPBD, MCA, and amylase. These results are further verified from the heatmap.
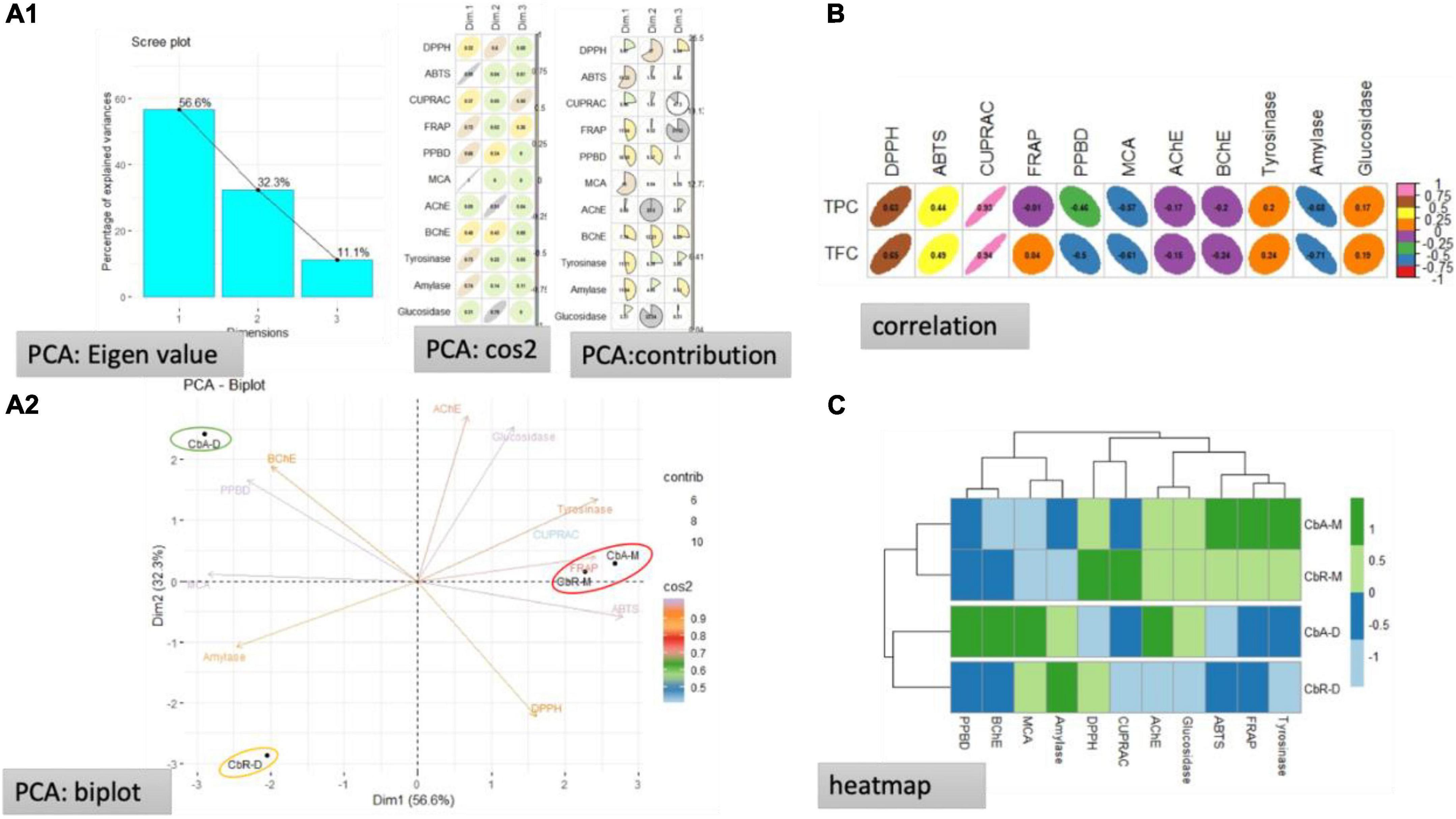
Figure 2. Statistical evaluations, (A1) eigenvalues and percentage of variability expressed by the factors; (A2) representation of biological activities on the correlation circle based on PCA; (B) correlation coefficients between total bioactive compounds and biological activities [Pearson Correlation Coefficient (R), p < 0.05]; (C) heat map of extracts in according to bioactive compounds and biological activities.
The specific phytochemical and biological composition of several extracts of the C. burhia plant has emphasized the possible consequences of these extracts. Secondary metabolites in the phenolic, flavonoid, and glycoside classes were identified through HPLC-PDA and UHPLC-MS analysis. It was found that the most polar solvent extracts had the highest bioactive content. All of the tested extracts had varying antioxidant and enzyme-inhibiting potential. In addition, statistical studies confirm the link between the contents and the apparent biological activities. C. burhia plant extracts can be used as a natural source of bioactive compounds, according to the findings of this comprehensive report. However, more exploration is required for better insight in terms of isolation and characterization studies.
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
SA, HS, and UK: writing and editing. MF, IP, NAk, KAm, and MH: data curation. KAl, FA, MS, ML, and NAh: supervision. All authors contributed to the article and approved the submitted version.
The authors extended their appreciation to the Research Deanship Project Fund number (RG-21 0131), University of Hail, Hail, Saudi Arabia.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2022.988352/full#supplementary-material
Ahmad, V., and Fatima, I. (1986). Isolation and c-13 nmr of crosemperine from crotalaria-burhia buch-ham. J. Chem. Soc. Pakistan 8, 89–90.
Ahmed, A. B. (2018). Phytochemical and Biological Studies on Crotalaria burhia (Fabaceae). Paderborn: DSpace.
Ali, M., and Adil, G. (1973). Isolation and structure of crotalarine, a new alkaloid from Crotalaria burhia. Pak. J. Sci. Indust. Res. 16, 227–229.
Bakir, D., Akdeniz, M., Ertas, A., Yilmaz, M. A., Yener, I., Firat, M., et al. (2020). A GC–MS method validation for quantitative investigation of some chemical markers in Salvia hypargeia Fisch. & CA Mey. of Turkey: enzyme inhibitory potential of ferruginol. J. Food Biochem. 44:e13350. doi: 10.1111/jfbc.13350
Ballard, C. G., Greig, N. H., Guillozet-Bongaarts, A. L., Enz, A., and Darvesh, S. (2005). Cholinesterases: roles in the brain during health and disease. Curr. Alzheimer Res. 2, 307–318. doi: 10.2174/1567205054367838
Bibi, Y., Arshad, M., Ahmad, N., Riaz, I., and Chaudhari, S. K. (2015). An insight into medicinal and ethnopharmacological potential of Crotalaria burhia. Asian Pacific J. Trop. Disease 5, 511–514. doi: 10.1016/S2222-1808(15)60826-X
Bibi Sadeer, N., Sinan, K. I., Cziáky, Z., Jekõ, J., Zengin, G., Jeewon, R., et al. (2022). Towards the pharmacological validation and phytochemical profiling of the decoction and maceration of Bruguiera gymnorhiza (L.) lam.—a traditionally used medicinal halophyte. Molecules 27:2000. doi: 10.3390/molecules27062000
Boǧa, M., Ertaş, A., Yılmaz, M. A., Kızıl, M., Çeken, B., Haşimi, N., et al. (2016). UHPLC-ESI-MS/MS and GC-MS analyses on phenolic, fatty acid and essential oil of Verbascum pinetorum with antioxidant, anticholinesterase, antimicrobial and DNA damage protection effects. Iran. J. Pharmaceutical Res. IJPR 15:393.
Bouhafsoun, A., Yilmaz, M. A., Boukeloua, A., Temel, H., and Harche, M. K. (2018). Simultaneous quantification of phenolic acids and flavonoids in Chamaerops humilis L. using LC–ESI-MS/MS. Food Sci. Technol. 38, 242–247. doi: 10.1590/fst.19917
Cavia-Saiz, M., Busto, M. D., Pilar-Izquierdo, M. C., Ortega, N., Perez-Mateos, M., and Muñiz, P. (2010). Antioxidant properties, radical scavenging activity and biomolecule protection capacity of flavonoid naringenin and its glycoside naringin: a comparative study. J. Sci. Food Agriculture 90, 1238–1244. doi: 10.1002/jsfa.3959
Ceylan, R., Kataniæ, J., Zengin, G., Matiæ, S., Aktumsek, A., Boroja, T., et al. (2016). Chemical and biological fingerprints of two Fabaceae species (Cytisopsis dorycniifolia and Ebenus hirsuta): are they novel sources of natural agents for pharmaceutical and food formulations? Industrial Crops Products 84, 254–262. doi: 10.1016/j.indcrop.2016.02.019
Choi, J. Y., Lee, J. W., Jang, H., Kim, J. G., Lee, M. K., Hong, J. T., et al. (2021). Quinic acid esters from Erycibe obtusifolia with antioxidant and tyrosinase inhibitory activities. Nat. Prod. Res. 35, 3026–3032. doi: 10.1080/14786419.2019.1684285
Choudhury, H., Pandey, M., Hua, C. K., Mun, C. S., Jing, J. K., Kong, L., et al. (2018). An update on natural compounds in the remedy of diabetes mellitus: a systematic review. J. Traditional Complementary Med. 8, 361–376. doi: 10.1016/j.jtcme.2017.08.012
Colovic, M. B., Krstic, D. Z., Lazarevic-Pasti, T. D., Bondzic, A. M., and Vasic, V. M. (2013). Acetylcholinesterase inhibitors: pharmacology and toxicology. Curr. Neuropharmacol. 11, 315–335. doi: 10.2174/1570159X11311030006
Dilworth, L., Riley, C., and Stennett, D. (2017). “Plant constituents: carbohydrates, oils, resins, balsams, and plant hormones,” in Pharmacognosy, eds S. Badal and R. Delgoda (Amsterdam: Elsevier), 61–80. doi: 10.1016/B978-0-12-802104-0.00005-6
Erdinc, C., Ekincialp, A., Turan, S., Kocak, M., Baloch, F. S., and Şensoy, S. (2021). The first report about genetic diversity analysis among endemic wild rhubarb (Rheum ribes L.) populations through iPBS markers. Turkish J. Agriculture Forestry 45, 784–796. doi: 10.3906/tar-2102-12
Figueiredo-González, M., Grosso, C., Valentão, P., and Andrade, P. B. (2016). α-Glucosidase and α-amylase inhibitors from Myrcia spp.: a stronger alternative to acarbose? J. Pharmaceutical Biomed. Anal. 118, 322–327. doi: 10.1016/j.jpba.2015.10.042
Fitzgerald, M., Heinrich, M., and Booker, A. (2020). Medicinal plant analysis: a historical and regional discussion of emergent complex techniques. Front. Pharmacol. 10:1480. doi: 10.3389/fphar.2019.01480
Grochowski, D. M., Uysal, S., Aktumsek, A., Granica, S., Zengin, G., Ceylan, R., et al. (2017). In vitro enzyme inhibitory properties, antioxidant activities, and phytochemical profile of Potentilla thuringiaca. Phytochem. Lett. 20, 365–372. doi: 10.1016/j.phytol.2017.03.005
Jdey, A., Falleh, H., Jannet, S. B., Hammi, K. M., Dauvergne, X., Ksouri, R., et al. (2017). Phytochemical investigation and antioxidant, antibacterial and anti-tyrosinase performances of six medicinal halophytes. South African J. Botany 112, 508–514. doi: 10.1016/j.sajb.2017.05.016
Kataria, S., Shrivastava, B., Kaur, D., and Sharma, P. (2012). Anti-inflammatory and antinociceptive activities of Crotalaria burhia Buch.-Ham. whole plant. Indian J. Nat. Products Resources 3, 189–196.
Kataria, S., Shrivastava, B., Khajuria, R., Suri, K., and Sharma, P. (2010). Antimicrobial activity of Crotalaria burhia Buch.-Ham. root. Ind. J. Nat. Products Resources 1, 481–484.
Kataria, S., Shrivastava, B., Khajuria, R., Suri, K., and Sharma, P. (2011). Pharmacognostic evaluation of Crotalaria burhia buch.-Ham. Ind. J. Traditional Knowledge 10, 629–635.
Katewa, S., and Galav, P. (2006). Additions to the traditional folk herbal medicines from Shekhawati region of Rajasthan. Ind. J. Traditional Knowledge 5, 494–500.
Kazeem, M., Adamson, J., and Ogunwande, I. (2013). Modes of inhibition of α-amylase and α-glucosidase by aqueous extract of Morinda lucida Benth leaf. BioMed. Res. Int. 2013:527570. doi: 10.1155/2013/527570
Provide the complete details for the following reference “Ketprayoon and Chaicharoenpong”.. Ketprayoon, T., and Chaicharoenpong, C. “Tyrosinase inhibitory activity of some edible plants,” in Proceedings of the international conference on biochemistry and molecular biology.
Khan, S., Nazir, M., Raiz, N., Saleem, M., Zengin, G., Fazal, G., et al. (2019). Phytochemical profiling, in vitro biological properties and in silico studies on Caragana ambigua stocks (Fabaceae): a comprehensive approach. Industrial Crops Products 131, 117–124. doi: 10.1016/j.indcrop.2019.01.044
Khurshid, U., Ahmad, S., Saleem, H., Nawaz, H. A., Zengin, G., Locatelli, M., et al. (2019). Phytochemical composition and in vitro pharmacological investigations of Neurada procumbens L. (Neuradaceae): a multidirectional approach for industrial products. Industrial Crops Products 142:111861. doi: 10.1016/j.indcrop.2019.111861
Kumar, G. G., Gali, V., and Dwiwedi, S. (2011). Phytochemical investigation of Crotalaria burhia Hamilt. Int. J. Res. Pharmaceut. Biomed. Sci. 2, 1721–1724.
Locatelli, M., Zengin, G., Uysal, A., Carradori, S., De Luca, E., Bellagamba, G., et al. (2017). Multicomponent pattern and biological activities of seven Asphodeline taxa: potential sources of natural-functional ingredients for bioactive formulations. J. Enzyme. Inhib. Med. Chem. 32, 60–67. doi: 10.1080/14756366.2016.1235041
Mollica, A., Zengin, G., Locatelli, M., Stefanucci, A., Mocan, A., Macedonio, G., et al. (2017). Anti-diabetic and anti-hyperlipidemic properties of Capparis spinosa L.: in vivo and in vitro evaluation of its nutraceutical potential. J. Funct. Foods 35, 32–42. doi: 10.1016/j.jff.2017.05.001
Muddathir, A., Yamauchi, K., Batubara, I., Mohieldin, E., and Mitsunaga, T. (2017). Anti-tyrosinase, total phenolic content and antioxidant activity of selected Sudanese medicinal plants. South African J. Botany 109, 9–15. doi: 10.1016/j.sajb.2016.12.013
Nemudzivhadi, V., and Masoko, P. (2014). In vitro assessment of cytotoxicity, antioxidant, and anti-inflammatory activities of Ricinus communis (Euphorbiaceae) leaf extracts. Evidence-Based Complementary Alternative Med. 2014:625961. doi: 10.1155/2014/625961
Niaz, S., Bokhari, T., Sherwani, S., Younis, U., and Dasti, A. (2013). Ethnobotanical study of some medicinal plants of thal desert Punjab. Pakistan. Int. J. Pharm. Res. Biosci. 2, 31–41. doi: 10.4314/ajtcam.v11i3.39
Obih, P., Obih, J.-C., and Arome, O. (2019). Is alpha-glucosidase inhibition a mechanism of the antidiabetic action of garlic (Allium sativum)? J. Biosci. Med. 7, 42–49. doi: 10.4236/jbm.2019.710004
Patridge, E., Gareiss, P., Kinch, M. S., and Hoyer, D. (2016). An analysis of FDA-approved drugs: natural products and their derivatives. Drug Discov. Today 21, 204–207. doi: 10.1016/j.drudis.2015.01.009
Phumthum, M., Srithi, K., Inta, A., Junsongduang, A., Tangjitman, K., Pongamornkul, W., et al. (2018). Ethnomedicinal plant diversity in Thailand. J. Ethnopharmacol. 214, 90–98. doi: 10.1016/j.jep.2017.12.003
Rahman, M. J., Ambigaipalan, P., and Shahidi, F. (2018). Biological activities of camelina and sophia seeds phenolics: Inhibition of LDL oxidation, DNA damage, and pancreatic lipase and α-glucosidase activities. J. Food Sci. 83, 237–245. doi: 10.1111/1750-3841.14007
Rao, A. S., Saheb, S. B., and Mallikarjuna, K. (2017). Pharmacological evaluation of leaf ethanol extract of Crotalaria hebecarpa (DC) Rudd. Curr. Trends Biotechnol. Pharmacy 11, 34–42.
Rascon-Valenzuela, L., Torres Moreno, H., Velazquez, C., Garibay-Escobar, A., and Robles-Zepeda, R. (2017). Triterpenoids: synthesis, uses in cancer treatment and other biological activities. Adv. Med. Biol. 106:41.
Sandeep, K., Birendra, S., Khajuria, R., Suri, K., and Piush, S. (2010). Antimicrobial activity of Crotalaria burhia Buch.-Ham. root. Indian J. Nat. Products Resources 1, 481–484.
Slinkard, K., and Singleton, V. L. (1977). Total phenol analysis: automation and comparison with manual methods. Am. J. Enol. Viticulture 28, 49–55.
Soni, B. (2014). Preliminary phytochemical screening and antimicrobial activity of methanol extract of Crotalaria burhia. Pharma Tutor 2, 115–118.
Talaviya, P. A., Vyas, B. M., Rao, S. K., Patel, V., and Ghadiya, S. (2018). Evaluation of antitumor activity of Crotalaria burhia buch.-ham. roots against ehrlich’s ascites carcinoma treated mice. Indian J. Physiol. Pharmacol. 62, 259–266.
Talaviya, P. A., Vyas, B. M., Sharma, D., Indoria, S. P., and Suman, R. K. (2014). Anti-inflammatory activity of four fractions of ethanolic extract of Crotalaria burhia buch.-ham. root in rats. Natl. J. Physiol. Pharmacy Pharmacol. 4, 213–217. doi: 10.5455/njppp.2014.4.120420141
Tamer, C. E., Temel, ŞG., Suna, S., Karabacak, A. Ö, Özcan, T., Ersan, L. Y., et al. (2021). Evaluation of bioaccessibility and functional properties of kombucha beverages fortified with different medicinal plant extracts. Turkish J. Agriculture Forestry 45, 13–32. doi: 10.3906/tar-2003-75
Uddin, A., and Khanna, P. (1979). Rotenoids in tissue cultures of Crotalaria burhia. Planta Med. 36, 181–183. doi: 10.1055/s-0028-1097261
Verma, B., Hucl, P., and Chibbar, R. N. (2008). Phenolic content and antioxidant properties of bran in 51 wheat cultivars. Cereal Chem. 85, 544–549. doi: 10.1094/CCHEM-85-4-0544
Yang, J., Guo, J., and Yuan, J. (2008). In vitro antioxidant properties of rutin. LWT-Food Sci. Technol. 41, 1060–1066. doi: 10.1016/j.lwt.2007.06.010
Yener, Ý, Ölmez, ÖT., Ertas, A., Yilmaz, M. A., Firat, M., Kandemir, S. Ý, et al. (2018). A detailed study on chemical and biological profile of nine Euphorbia species from Turkey with chemometric approach: remarkable cytotoxicity of E. fistulasa and promising tannic acid content of E. eriophora. Industrial Crops Products 123, 442–453. doi: 10.1016/j.indcrop.2018.07.007
Zaidi, K. U., Ali, A. S., Ali, S. A., and Naaz, I. (2014). Microbial tyrosinases: promising enzymes for pharmaceutical, food bioprocessing, and environmental industry. Biochem. Res. Int. 2014:854687. doi: 10.1155/2014/854687
Zengin, G., Ak, G., Ceylan, R., Uysal, S., Llorent-Martínez, E., Di Simone, S. C., et al. (2022). Novel perceptions on chemical profile and biopharmaceutical properties of mentha spicata extracts: adding missing pieces to the scientific puzzle. Plants 11:233. doi: 10.3390/plants11020233
Zengin, G., Nithiyanantham, S., Locatelli, M., Ceylan, R., Uysal, S., Aktumsek, A., et al. (2016). Screening of in vitro antioxidant and enzyme inhibitory activities of different extracts from two uninvestigated wild plants: Centranthus longiflorus subsp. longiflorus and Cerinthe minor subsp. auriculata. Eur. J. Int. Med. 8, 286–292. doi: 10.1016/j.eujim.2015.12.004
Zengin, G., Uysal, A., Diuzheva, A., Gunes, E., Jekõ, J., Cziáky, Z., et al. (2018). Characterization of phytochemical components of Ferula halophila extracts using HPLC-MS/MS and their pharmacological potentials: a multi-functional insight. J. Pharm. Biomed. Anal. 160, 374–382. doi: 10.1016/j.jpba.2018.08.020
Keywords: Crotalaria burhia, secondary metabolites, antioxidant, enzyme inhibition, toxicity
Citation: Anwar S, Faisal Nadeem M, Pervaiz I, Khurshid U, Akmal N, Aamir K, Haseeb ur Rehman M, Almansour K, Alshammari F, Shaikh MF, Locatelli M, Ahemad N and Saleem H (2022) A comprehensive phytochemical, biological, and toxicological studies of roots and aerial parts of Crotalaria burhia Buch.-Ham: An important medicinal plant. Front. Plant Sci. 13:988352. doi: 10.3389/fpls.2022.988352
Received: 09 July 2022; Accepted: 28 July 2022;
Published: 16 September 2022.
Edited by:
Sezai Ercisli, Atatürk University, TurkeyReviewed by:
Gülçe Ilhan, Atatürk University, TurkeyCopyright © 2022 Anwar, Faisal Nadeem, Pervaiz, Khurshid, Akmal, Aamir, Haseeb ur Rehman, Almansour, Alshammari, Shaikh, Locatelli, Ahemad and Saleem. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sirajudheen Anwar, c2kuYW53YXJAdW9oLmVkdS5zYQ==; Umair Khurshid, dW1haXIua2h1cnNoaWRAaXViLmVkdS5waw==; Hammad Saleem, aGFtbWFkLnNhbGVlbUB1dmFzLmVkdS5waw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.