- 1Department of Pharmaceutical Sciences, Maharshi Dayanand University, Rohtak, Haryana, India
- 2Department of Zoology, College of Science, King Saud University, Riyadh, Saudi Arabia
- 3Chrono-Environnement Laboratory, UMR CNRS 6249, Bourgogne, Franche-Comté University, CEDEX, Besançon, France
- 4Department of Medicine and Infectious Diseases, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt
- 5Pharmacology Department, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt
Nardostachys jatamansi (D. Don) DC is a highly valued medicinal herb that has been used in traditional medicinal systems for its remedial effects. Owing to the over-exploitation and unethical trade of N. jatamansi, the accelerating global demand of herbal products from this plant cannot be satisfied by the conventional extraction approach. In view of the progressive demand and incredible biological potential of herb, the present research was designed to optimize various extraction parameters for microwave-assisted extraction (MAE). The extracts obtained from the traditional and green approach were also assessed for the recovery of secondary metabolites and anti-Alzheimer’s potential. Various parameters like microwave power, temperature, and time of irradiation were optimized for MAE using Box Behkhen Design (BBD) The scanning electron microscopy of different plant samples was also done to observe the effect of microwave radiations. Further, the metabolite profiling of different extracts was also done by gas chromatography-mass spectrometry (GC-MS) analysis. Also the different behavioral and biochemical parameters along with acetylcholinesterase (AChE) inhibitory potential were assessed to evaluate the anti-Alzheimer’s potential. Optimized parameters for MAE were found to be as microwave power 187.04 W, temperature 90°C, and irradiation time 20 min. The extract yield in MAE was significantly enhanced as compared to the conventional method. Also, the total phenolic content and total flavonoid content (TFC) were improved pointedly from 32.13 ± 0.55 to 72.83 ± 1.1 mg of GAE/g of extract and 21.7 ± 0.85 to 39.21 ± 0.7 mg of RUE/g of extract respectively. Later, the GC-MS analysis of various extracts confirmed the enhancement in the concentration of various sesquiterpenes like jatamansone, spirojatamol, valerenal, valeric acid, globulol, nootkatone and steroidal compounds such as sitosterol, ergosterol, stigmastanone, etc. in the optimized extract. A significant improvement in anti-Alzheimer’s potential was also observed owing to the better concentration of secondary metabolites in the optimized microwave extract. From the current findings, it could be concluded that the MAE could be a successful and green alternative for the extraction and recovery of secondary metabolites from the selected medicinal herb.
1 Introduction
A vast range of secondary metabolites is produced by plants which may act as the compounds of defense against herbivores, microbes, and other plants or may act as the signal molecules, UV protectants, and antioxidants (Pagare et al., 2015; Wink, 2015; Badyal et al., 2020). Secondary metabolites from plants, by and large, owe an impressive range of biological and therapeutic possessions. Consequently, plants and their extracts have thus been and are still utilized as remedies to treat various infections, health disorders, and ailments or as flavors, perfumes, dyes, dietary supplements, toxins, and pesticides (Wink, 2015; Teoh, 2016; Alamgir, 2018; Velu et al., 2018). Importantly, the plants are renewable assets for providing raw materials and phytochemicals (precisely, secondary metabolites) for various industrial and pharmaceutical applications. For these reasons, plants are supposed as vital to favor the evolution to a low-cost bio-economy that is not fossil-resource dependent. Moreover, currently, the global market for herbal medicines is steadily growing and is estimated to achieve 7.2% CAGR for the next five years. Such considerable economic, as well as remedial potential of secondary metabolites from plants or their extracts, has triggered the hunt for novel or improved ways to extract the plants. Moreover, the exploration of innovative methods for the extraction of plants for secondary metabolites is needed to meet the growing demand of the herbal industry (Guerriero et al., 2018).
Further, it has been observed that most of the herbal products coming from India are prepared by using Himalayan plants. As the Himalayan flora found to possess an immense collection of phytochemicals with well-known pharmacological potential, and are exceptionally appropriate for all herbal sectors including healthcare, foods, beverages, nutraceuticals, and personal care (Dhiman and Bhattacharya, 2020). The selected medicinal plant, Nardostachys jatamansi (D. Don) DC (Family- Capriofoliaceae) is also one of the commercially important herbs from the Himalayan region. The plant is mainly collected from wild sources as the cultivation of this herb is not so successful. However, the altitude of 2200-4800 m and sandy loamy soil with sufficient moisture is favorable for its growth (Nautiyal et al., 2003; Ghimire and Aumeeruddy-Thomas, 2009). This species bears an extended history of use and has been extensively mentioned in Unani, Ayurveda, Nepalese, Bhutanese, Japanese, and Chinese systems of medicine. The herb was also used as a seasoning in a sweet and spiced wine drink called “Hippocras” (Bose et al., 2019; Kaur et al., 2020). In traditional medicine systems, this species is considered a mind rejuvenator or brain tonic, stimulant, antidiabetic, cardio tonic, antipyretic, and antiflatulent, and is used in treating various neurological disorders like epilepsy, insomnia, anxiety, depression, and hysteria. It also claimed to exhibit antioxidant, antimicrobial, hepatic- and cardio-protective, and anticancer properties (Rao et al., 2005; Ahmad et al., 2006; Subashini et al., 2007; Sharma and Singh, 2012; Sahu et al., 2016; Kapoor et al., 2017; Saroya and Singh, 2018). The pharmacological potential of the plant is attributed to the presence of various secondary metabolites of different classes like sesquiterpenes, phenolics, flavonoids, steroids, lignans, coumarins, etc (Bose et al., 2019; Singh, 2020). Moreover, a large number of herbal pharmaceutical companies are commercially marketing single or polyherbal preparations from this plant globally (Dandagi et al., 2008; Thorat et al., 2009). Also, Ved and Goraya (2007) estimated the annual trade for its rhizomes and extract in domestic markets has increased to 200-500 metric tons. But the great demand for the plant actives and extract from this herb cannot be fulfilled by the conventional techniques alone. Traditional methods such as maceration, percolation, and distillation were generally applied for the extraction of herbs. However, these processes are not environmentally friendly and require a lot of time and solvent for exhaustive extraction of herbs (Wang and Weller, 2006). Literature indicated that extraction of herbs such as ginseng by conventional methods takes several hours (12 h) (Kwon et al., 2003). Also, the usage of organic solvents could reach up to 100 mL per g of sample for the extraction of leaves of Iochroma gesneriodes (Kaufmann et al., 2001).
Therefore, novel green methods of extraction such as microwave-assisted extraction (MAE) can be utilized to extract the plant to meet the rising demand for extracts for commercial application. In this non-conventional method, electromagnetic radiation was applied to enhance the kinetics of extraction, and could also improve the quality of extracts. Microwave energy is absorbed by the polar compounds according to their dielectric constant and is dissipated as heat to the surroundings. Thus the heating of the specific metabolites can be targeted in the plant samples for better extraction (Eskilsson and Bjorklund, 2000). In past, the yield of secondary metabolites from various herbs has also improved by using the MAE (Chan et al., 2011; Zhang et al., 2011; Arya et al., 2022). For example, the recovery of triterpenoid saponins from Ganoderma artrum enhanced significantly for 15 min of MAE at 800W and 70°C as compared to conventional methods of extraction (Chen et al., 2007). Similarly, MAE of Radix astragali at 700W and 70°C for 15 min enhanced the concentration of astragalosides-I to more than twice as compared to the maceration process (Yan et al., 2010).
A broad investigation has been designed and executed to extract the selected herb by green methods such as microwave-assisted extraction along with conventional techniques like maceration. Further, the extraction conditions in MAE are also optimized by using box-behnken design coupled response surface methodology. The extracts were also analyzed by GC-MS to confirm the presence of various secondary metabolites. Also, the anti-Alzheimer’s potential of different extracts was assessed on the various behavioral and biochemical parameters along with acetylcholinesterase (AChE) inhibitory potential.
2 Materials and methods
2.1 Plant sample and solvents
Dried roots of Nardostachys jatamansi (NJ) were procured from the market near Hisar, Haryana, India, and identified by a botanist before extraction by different techniques. The chemicals and solvents utilized for extraction and other investigations were of AR grade. Standard drugs like Donpezil and scopolamine were purchased from Sigma Aldrich, USA.
2.2 Extraction by maceration
The conventional extraction method, maceration was chosen to carry out the extraction of the selected herb. Briefly, in maceration, the plant part taken was coarsely powdered, sieved (40 size), and transferred to a conical flask containing ethanol: water (80:20) as solvent. After keeping it undisturbed for eight days, the sample solution was then filtered and kept in a water bath to get a concentrated product. Later, the extract yield was calculated and the extract was kept in an airtight container until further analysis was performed (Devgan et al., 2013).
2.3 Microwave-assisted extraction (MAE)
Microwave synthesis apparatus (CEM, Model: Discover system, 908010, Matthewis, NC; USA) was used to perform the microwave treatment on the powdered roots. Throughout the study, the solid/solvent ratio (1:15) was kept constant for hydroalcoholic solvent (80:20) to carry out the novel extraction procedure. About 0.4 g of sample powder was irradiated using varying operating conditions of microwave power, temperature, and irradiation period as proposed by the software used. During the experiments, the microwave power and the temperature were adjusted automatically to a particular set of conditions and displayed on the screen of the instrument. After the completion of the extraction process, the extract was kept at room temperature and filtered after centrifuging it for 5 min at 2000 rpm. Subsequently, a rotary evaporator was used to concentrate the extract and weighed it for percentage yield (w/w) calculation (Mittal and Nanda, 2017).
2.4 Experimental design
In this study, various parameters such as microwave power (X1: 100-300 watts), temperature (X2: 50-90°C), and irradiation time (X3: 2-20 min) were optimized using box-behkhen design (BBD) (Table 1). The design expert software (7.0.3, Statease Inc, Minneapolis, USA) was used for experimental design which suggested 17 trial runs and was carried out accordingly. Responses were expressed in terms of dependent variables like extraction yield (Y1), total phenolic content (TPC; Y2), and total flavonoid content (TFC; Y3) to study the effect of independent factors (Filip et al., 2017).
2.4.1 Total phenolic content (TPC)
The TPC for obtained extracts was measured using the Folin-Ciocalteu method. Shortly, in the method adopted, the extract was dissolved in methanol (10mg/ml). Then, 1.5 ml Folin-Ciocalteu reagent was added to the above-prepared solution. After 10 min, 1.5ml of 25% aqueous Na2CO3 was mixed and the mixture was stirred. Later, the mixture was incubated in a water bath for 30 min at 45°C. UV- visible spectrophotometer (UV-1800, Shimadzu Scientific Instruments Private Limited) was used to analyze the resultant samples against the blank sample at 760nm. To obtain the straight-line equation, a standard solution of Gallic acid was prepared and analyzed for the concentrations ranging between 50-250 μg/ml. To calculate the TPC quantitatively, readings for absorbance were taken in triplicate and expressed as mg GAE/g of the extract while the fallouts were stated as mean ± standard deviation (S.D.) (Sharma and Singh, 2012).
2.4.2 Total flavonoid content (TFC)
To evaluate the total flavonoid content (TFC) aluminum chloride method was used. A required amount of plant extract was mixed with methanol (10 mg/mL). Then, 1.5 mL 2% aluminum chloride solution was prepared and 1.5 mL was added to the mixture and kept aside for 1 h at room temperature. Later, the absorbance was estimated with the help of a UV-visible spectrophotometer at a wavelength of 415 nm against the blank. The TFC values were expressed in mg rutin equivalent/g of extract. The observations were observed in the triad and shown as mean ± standard deviation (S.D.) (Sharma and Singh, 2012).
2.5 Scanning electron microscopy (SEM)
SEM was used to analyze the possible modification in the microstructure of powdered plant samples before and after the extraction process. To prepare the samples for SEM, the residuals obtained after the extraction process were dried under vacuum at 40°-50°C for at least 2 h and coated properly with gold before microscopical analysis (Zhou and Liu, 2006; Zhang et al., 2008).
2.6 GC-MS analysis
The analysis of extracts obtained by chosen extraction methods (maceration and MAE) was carried out by Gas chromatography coupled with mass spectra (Shimadzu QP-2010 having Thermal Desorption System TD 20; fitted with MS capillary column). Electron ionization system with the chosen parameters,70 eV energy; helium as carrier gas; injector temperature at 260°C with 1.5 mL/min flow rate were the chosen parameters used for GC-MS analysis. Initially, the column temperature was 70°C for 2 min and later accelerated to 150°C at a rate of 3°C/min for 10 min and lastly increased to 250°C at the rate of 4°C/min. Then, the required volume of 1 μL sample was injected manually in a split manner to obtain the chromatogram and identify the constituents present (Razack et al., 2015).
2.7 In-vivo anti-alzheimer’s evaluation
2.7.1 Animals
Institutional Animal Ethical Committee, IAEC, MDU, Rohtak granted approval for our research protocol presented against vide reference number 1767/RE/S/14/CPCSEA/CAH/76-85 dated 26.02.2021. The mice were Swiss albino weighing between 25-30g, procured from an aseptic animal house, Lala Lajpat Rai University of Veterinary and Animal Sciences, (LLRU-VAS), Hisar, India. Polypropylene cages were used to retain the animals throughout the study. All the mice had an appropriate approach to water ad-libitum along with the dried feed and 12 h light/dark sequence at 25 ± 2°C. House conditions were set and sustained consistently in reference to the CPCSEA guidelines.
2.7.2 Experimental protocol
Swiss albino mice were equally divided into six groups (n=5) and provided with dry feed along with water for seven days prior to dosing. The first group, the control, was given normal saline. Scopolamine and donepezil in doses of 0.4mg/kg and 3mg/kg, i.p were administered to the 2ndand 3rdgroup respectively while the animals of groups fourth and fifth were administered orally with various extracts prepared (200 mg/kg) for the next fourteen days. On the 15th day of dosing, test group animals were injected with scopolamine (0.4 mg/kg, i.p.) to evaluate the protective effect of different extracts against amnesia induced by scopolamine with the help of below-mentioned parameters.
2.7.2.1 Evaluation of behavioral parameters
The behavioral studies were performed using (i) an elevated plus maze and (ii) passive avoidance apparatus to analyze the learning and memory potential found in various groups of mice. As the name suggested plus maze is a + (plus) shaped wooden block consisting of open and closed arms in counter directions and is placed at an elevated height of 50 cm from the floor. In this model, one mouse at a time was placed towards the open arm of the instrument and its movement was observed for 5 min to estimate transfer latency (TL) i.e., the time the mouse takes to move inside the covered arm (Vasudevan and Parle, 2006). A passive avoidance test is commonly employed to describe the investigational practice in particular behavioral suppression to sidestep noxious circumstances, learned by the chosen animals. In this apparatus, 60V electric current at a frequency of 1Hz for 0.5sec was applied to the steel grid on the floor to determine the step-down latency (SDL), i.e. the time taken by the mice to reach the wooden floor from the steel grid floor). During training, each mouse was kept on the wooden floor and an electric current was passed into the steel grid floor. The time that the mouse takes to come back to the floor deprived of current i.e., the wooden floor, from the steel grid floor was noted. All mice were trained for 24 h and SDL was recorded for five minutes to evaluate passive avoidance behavior characteristics in mice (Joshi and Parle, 2006).
2.7.2.2 Estimation of biochemical parameters
On the 17th day of the experimental procedure, complete mice brain was isolated from each group and cleaned thoroughly with standard ice cold saline. The phosphate buffer with pH 7.4 was used to homogenize the brain samples and centrifuged at 10000 rpm for 15min. The brain homogenates were further analyzed for several biochemical parameters. Catalase activity was carried out according to Aebi’s method (Aebi, 1974). To carry out the process, 0.1 mM phosphate buffer (pH 7.4) and 1 mL of 30 mM hydrogen peroxide were mixed with 100 μL brain homogenate, and this reaction mixture was analyzed at a wavelength of 240 nm using UV- spectrophotometer. Absorbance variation was noted and stated as nmol of H2O2 consumed/min/mg/protein.
Glutathione activity was performed using Ellman’s method (Ellman, 1959). To perform this, an equal quantity of brain homogenate was dissolved in an equal quantity of 10% of trichloroacetic acid. Then, for 15 min centrifugation was carried out to isolate the proteins present in the blend. After centrifugation, 0.01 mL of supernatant was dissolved in 2 mL phosphate buffer having pH 8.4 and with vigorous stirring added 0.5 mL 5’5-dithiobis (2-nitrobenzoic acid) to water. The absorbance of the reaction mix was noted using UV- spectrophotometer at 412 nm within 15 min and dropped glutathione concentration was expressed in μmol/mg tissue. Brain homogenate is evaluated to evaluate the content of nitric oxide present using Griess Reagent and calculated as total nitrate/nitrite (NOx) (Lieben and Blokland, 2005). The chemical reaction of nitrate to nitrite is the result of vanadium trichloride. Briefly, in this mechanism, sulfanilamide diazotization is responsible for the formation of chromophores as the binding of acidified nitrite with N-(1-naphthyl) ethylenediamine lead to the formation of a colored derivative of azo which was later analyzed using spectrophotometer at a wavelength 540 nm and stated as μmol/mg tissue. In various groups, mice brain homogenates were used to determine the concentration of superoxide dismutase (SOD). To perform this procedure, brain homogenate was mixed with n-butanol with continuous stirring. The obtained reaction mix was kept uninterrupted for some time and after 15 min of centrifugation, n-butanol was separated. UV-Visible spectrophotometer at a wavelength of 560 nm was employed to take the absorbance of the solution (Kakkar et al., 1984).
2.7.2.3 Acetylcholinesterase (AChE) inhibitory potential
Ellman’s method was used to evaluate the inhibitory potential of AChE in brain homogenates for extracts obtained by maceration and optimized microwave-assisted extraction (Ellman et al., 1961). To carry out this method, brain homogenate, phosphate buffer (0.1M; pH 8) and dithiobisnitro-benzoic acid (0.01M) were mixed and the reaction mixture was incubated at room temperature for the next 5 min. Acetylthiocholine iodide substrate was added to the mixture and the absorbance was recorded at 412 nm.
2.8 Statistical analysis
Graphpad Prism 9.0 was applied to evaluate the data significance using analysis of variance (ANOVA). The results observed were taken in triplicate throughout the study.
3 Results and discussion
Nardostachys jatamansi (NJ), commonly known as ‘Jatamansi’ possesses an exclusive role in Ayurvedic medicinal system and is conventionally consumed in preventing mental illness and also to improve learning and memory power (Dhiman and Bhattacharya, 2020). In the preparation of traditional herbal formulations, the powdered rhizomes and the roots of the plant, Nardostachys jatamansi, were utilized. The extraction was generally carried out using various conventional techniques such as maceration and percolation. But, considering the remedial and economical worth of the selected plant together with the global demand these traditional methods do not seem to be sufficient. Also, the limitations associated with conventional methods of extraction made us implement one of the modern, time-saving, and green extraction methods i.e., Microwave Assisted Extraction (MAE). Various extraction parameters in novel extraction techniques were also optimized using CCD coupled with response surface methodology.
Our investigation intended to comparatively assess the various extracts for probable enhancement in the recovery of secondary metabolites along with improved pharmacological potential. Further, it is well established that the collection of plant samples from the correct source dictates the quality and quantity of plant actives (Sutivisedsak et al., 2010). Moreover, the Jatamansi roots are highly confused with the Valerian species, thus the procured roots of the selected plant were first identified and authenticated by a botanist (Mabberley and Noltie, 2014). The morphological characters of the collected roots were compared with the standard sample present in the Raw Material Herbarium and Museum by Dr. Sunita Garg, Scientist, CSIR, NISCAIR, Delhi. On the basis of a comparative morphological study, the collected sample of NJ roots was confirmed as Nardostachys jatamansi (D. Don) DC vide reference number NISCAIR/RHMD/Consult/2018/3157-06-2 dated 19/03/2018. After authentication of collected NJ roots, these were powdered coarsely and extracted with conventional (maceration) and non-conventional (microwave-assisted extraction) methods of extraction. The extraction yield (%) for the maceration process was found to be 5.35 ± 0.14% w/w and this extract is termed MCNJ in this manuscript.
3.1 Microwave-assisted extraction
Among various promising green and effective modern technologies of extraction, microwave-assisted extraction is quite prevalent and works with electromagnetic radiation. In this method, the solvent and the plant material are heated up rapidly by high-speed energy radiations which leads to the efficient and time-saving extraction of constituents (Wang and Weller, 2006). Improved dipole rotation accompanied by better ionic conduction in the solvent particles enables the efficient separation of desired molecules from the plant matrix (Wang and Weller, 2006; Chemat et al., 2009; Chemat et al., 2012). Numerous factors such as microwave power, solvent nature and concentration, time of irradiation, solid-to-solvent ratio, temperature for extraction, and particle size need to be optimized as these parameters can alter the phytoconstituent yield and extraction efficiency significantly (Wang and Weller, 2006). In this study, for the microwave extraction of the selected herb, based on some preliminary investigations, hydroalcoholic solvent (80:20) was selected. The literature also suggests that polar solvents like water and ethanol are best suited for microwave irradiation. The addition of water to the ethanol improves the dielectric constant of the mixture to some extent (Alara et al., 2018). Thus as the water is added (upto20%) to the organic solvent, it enhances the polarity indices of the solvent and resulted in improved microwave energy absorption along with the solubilization of plant actives. Further, it also elevated the temperature of the inner wall of the particles of plant sample and leads to the cell wall rupture, and accelerated the phytoconstituents recovery (Eskilsson and Bjorklund, 2000; Chan et al., 2011; Bouras et al., 2015; Moreira et al., 2017). Likewise, solvent-to-solid ratio is also a critical factor in the extraction of plant samples by MAE. A sufficient volume of solvent is required for the absorption of microwaves and also to solubilize the bio-actives (Wang et al., 2008; Zhang and Yang, 2009). Therefore the ratio (15:1) was also selected on the basis of the results of some initial experiments. Further increasing the amount of solvent did not improve the extract, or the TPC and TFC yields. The microwave energy dissipated in heating the higher amount of solvent and did not impact the plant particles (Gao et al., 2007). Consequently, the more parameters which can affect the concentration of plant actives along with extraction kinetics are also optimized using box-behnken design (BBD).
3.2 Model fitting for MAE
The effect of various selected independent variables like microwave power, temperature, and irradiation time on different dependent variables (EY, TPC, and TFC) was calculated using BBD coupled response surface methodology Further the design proposed a run of a total of 17 experiments which were carried out accordingly and the observations were represented in Table 2. Also, the statistical analysis of the data was carried out and it confirmed the significance of all the linear (X1, X2, and X3), interactive (X1X2, X2X3), and quadric terms ( , and ) for EY, TPC, and TFC (Table 3). To verify, if the model is significant or not statically, an analysis of variance was performed on the experimental data obtained. The higher F value indicated by the developed model for Y1 (~99), Y2(~980), andY3 (~145) proved that the model chosen was significant. Also, the probability value was found lower, i.e., p<0.0001; signifying the extremely significant behavior of the model while the regression equation can describe the response difference clearly. The degree of fitness was estimated by R2 values of EY, TPC, and TFC (0.99) and basically it is the explained to total variance ratio. The vicinity of R2 to unity showed the well-fitted developed model in reference to the actual data. Similarly, predicted R2 values of (EY 0.91, TPC 0.99, and TFC 0.96) presented a good correlation of the selected response with the independent variables. Moreover, a small value of the coefficient of variance of EY (~4), TPC (1.42), and TFC (4.2) validates the dependability of data and accuracy to noise proportion justifying the developed model fitness to direct the design space. Further on applying multiple regression analysis, a second-order polynomial mathematical equation was also derived. The magnitude of the effects of different variables on the responses like EY (Y1), TPC (Y2), and TFC (Y3) were also represented by Equations 1,2,3.
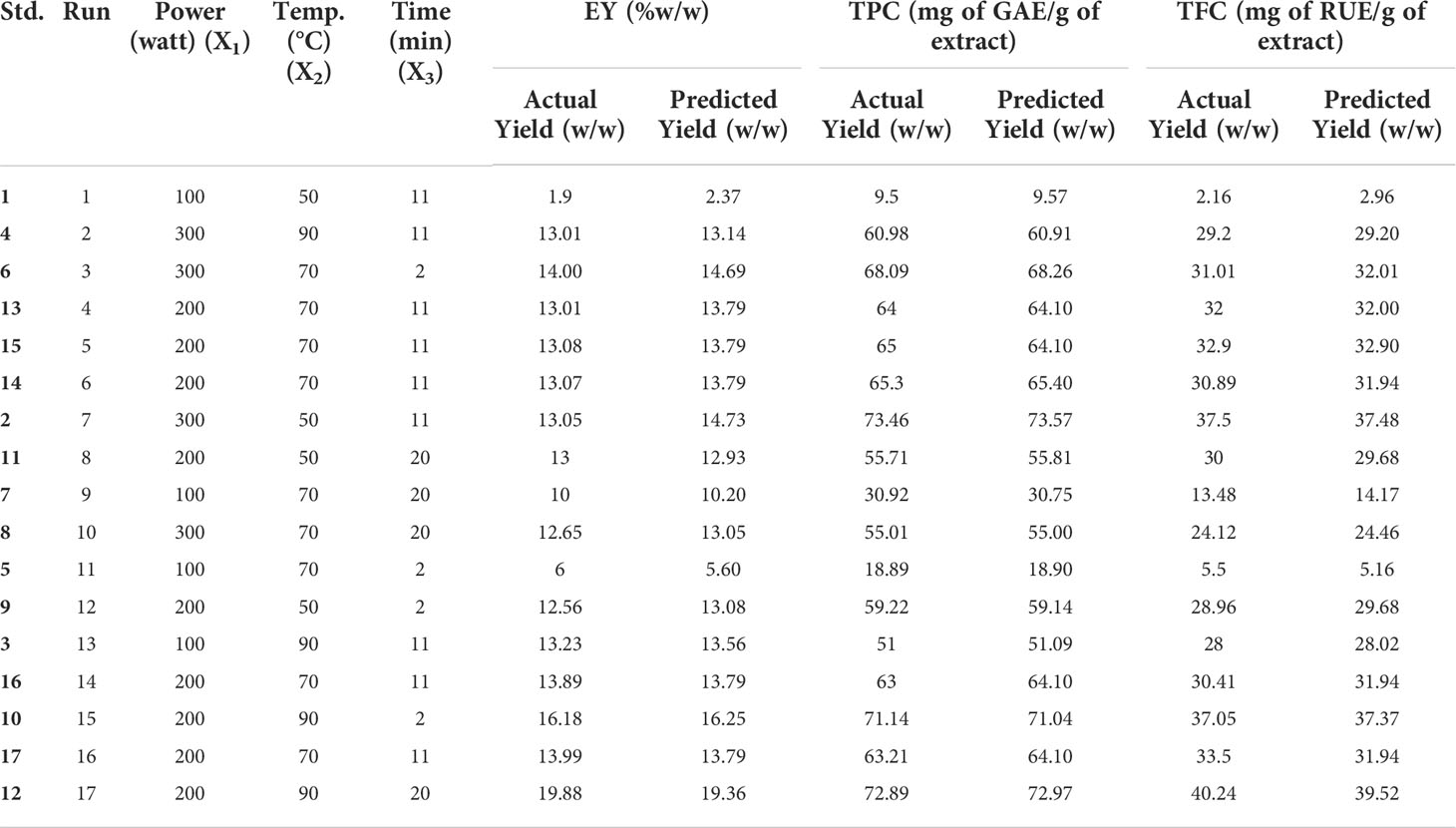
Table 2 Actual and Predicted values for extraction yield, TPC and TFC for different parameters (MAE).
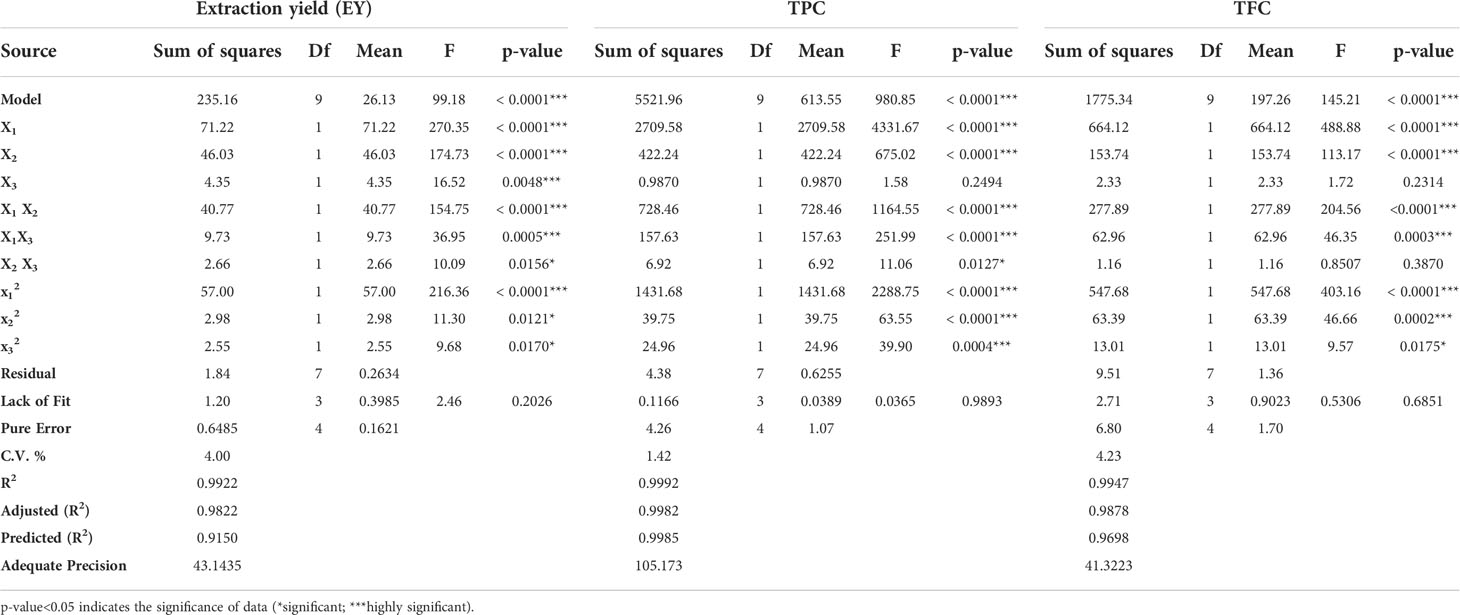
Table 3 ANOVA applied on obtained data for significance and suitability of design and different variables.
Equation 1
Equation 2
Equation 3
3.2.1 Diagnostic of model adequacy
Diagnostic plots were also used to estimate the adequacy of the developed model. The residual plot between normal percentage probability and internal residual was found normal without any significant variance proving the developed model fitness (Figures 1A, 2A, 3A). The plot between actual and predicted values evidently depicts that predicted values are situated close to a straight line and are in conformity with real values for EY, TPC, and TFC (Figures 1B, 2B, 3B). Also, the λ value of the obtained data is near to 1 as indicated in box-cox plot (Figures 1C, 2C, 3C) and no power transform is required for the dependent variables (EY, TPC, and TFC).
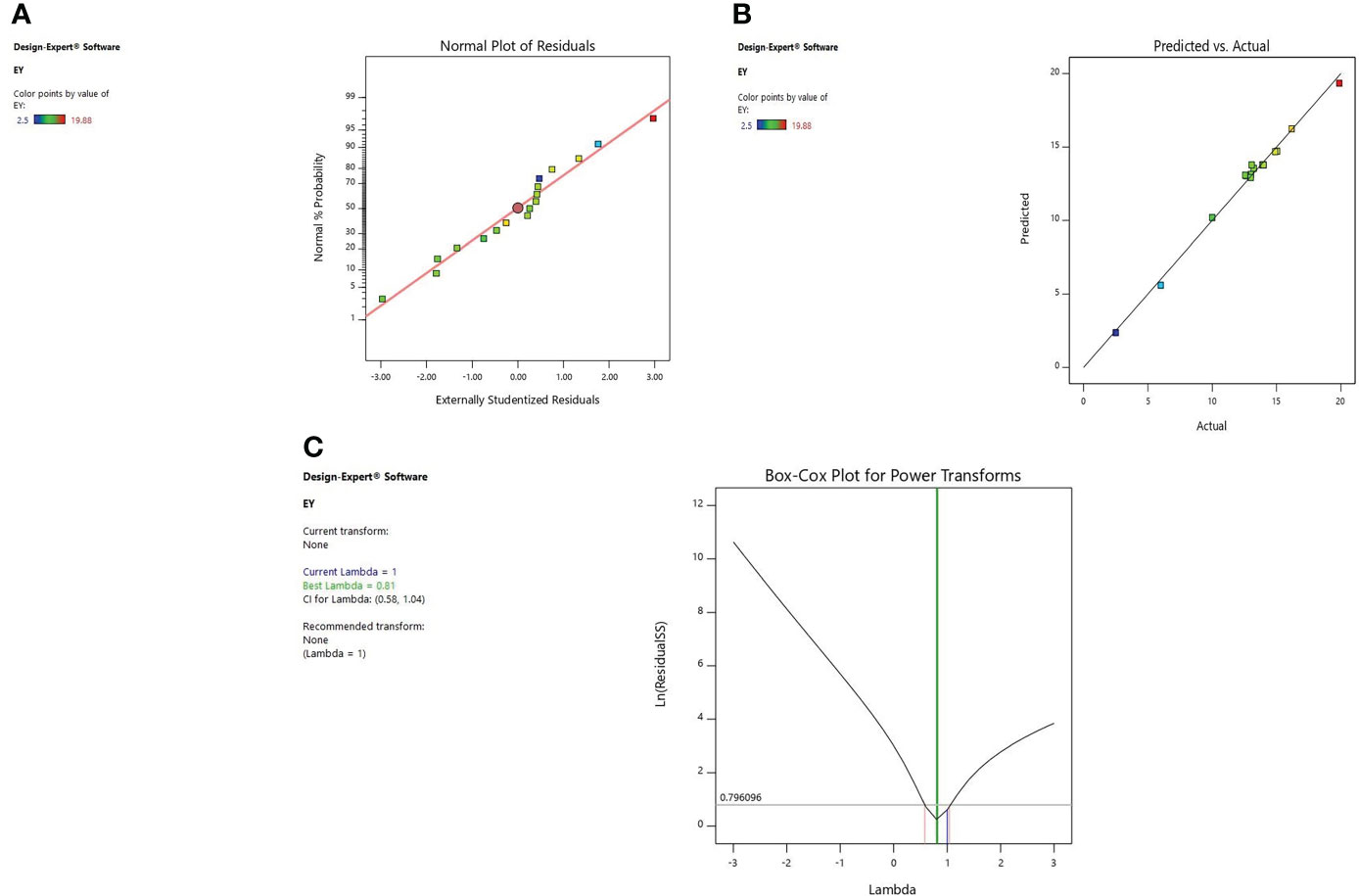
Figure 1 Diagnostic plot of Extraction yield (EY); (A) Normal % probability versus internal residual, (B) Actual versus predicted values, (C) Box-cox plot.
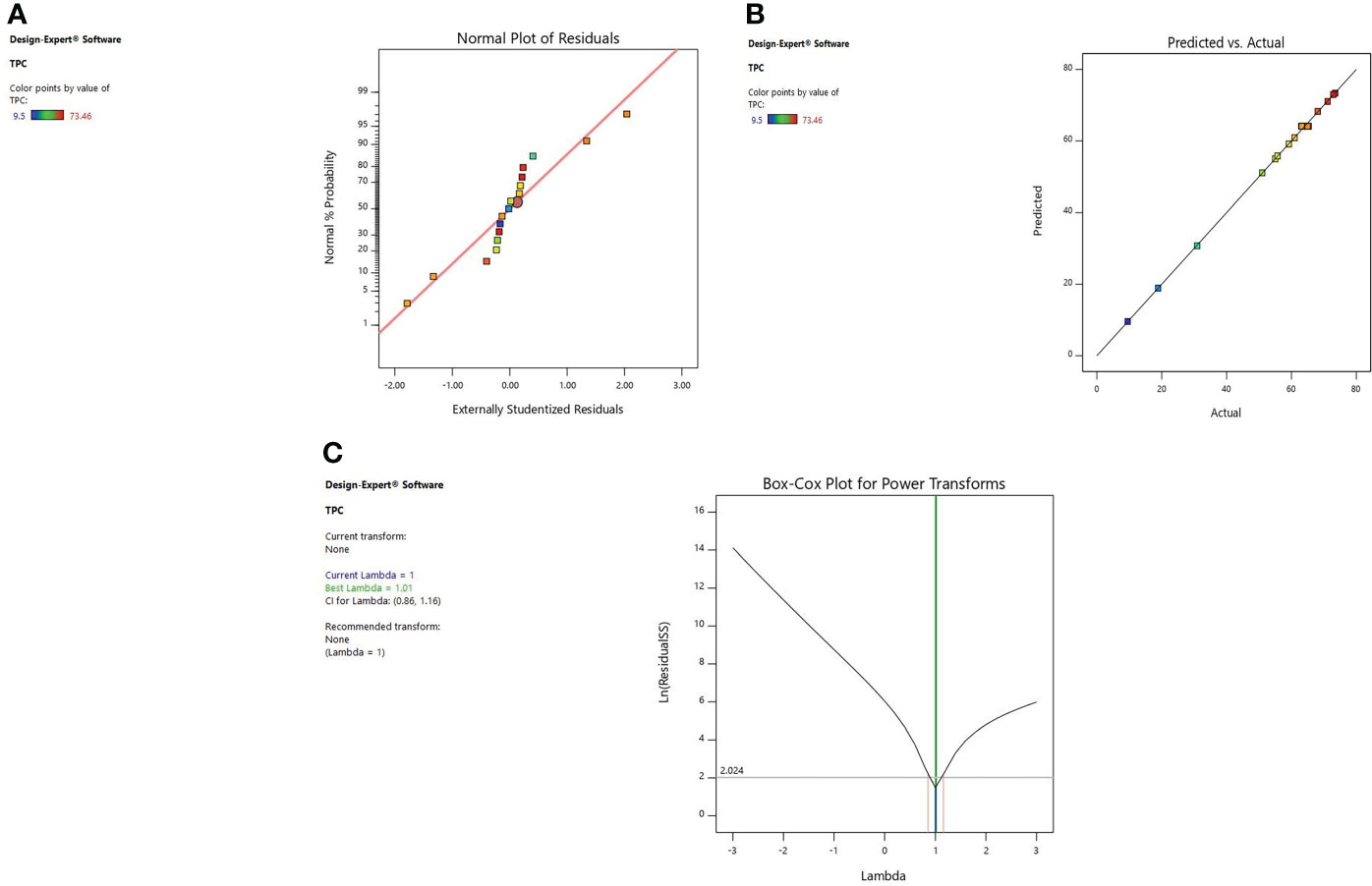
Figure 2 Diagnostic plot of TPC; (A) Normal % probability versus internal residual, (B) Actual versus predicted values, (C) Box-cox plot.

Figure 3 Diagnostic plot of TFC; (A) Normal % probability versus internal residual, (B) Actual versus predicted values, (C) Box-cox plot.
3.2.2 Response surface analysis
Response surface analysis Figure 4 revealed that microwave power (X1) along with the temperature (X2) at a constant time factor (11 min) impart an encouraging significant effect on extraction yield, TPC, and TFC. Extraction yield has been influenced by microwave power significantly. A higher value for EY was observed at 200 W and after that, a decrease was noticed in the extraction yields up to 300 watts. A decrease in extraction yield has been observed because of the thermal degradation of phytoconstituents for higher values of microwave power at which the potent heat produced can reduce the number/quantity of phenolic components present in plant material (Spigno and De Faveri, 2009; Dahmoune et al., 2014; Xiong et al., 2016). Microwave power increases as the irradiation time is elevated due to which the heat produced within the cellular part of the plant and obtained extract causes a faster release of phytoconstituents. In a dispersion medium, the dipole rotation along with the ionic conduction of molecular particles generally relies on heat intensity. The dipoles present in solvent go through an oscillatory motion and later are arranged in the electronic field direction which happens at a higher rate so as to cause heat generation within the medium (Eskilsson and Bjorklund, 2000). Furthermore, the molecules in the solvent start to dissociate into ions also known as charged particles, and the increased flow rate of ions is detected in the vessel due to the applied electronic field. The excessive ion movement results in an increased ionic collision and friction energy is generated which accelerates the temperature in the medium (Gfrerer and Lankmayr, 2005; Nadagouda et al., 2011; Ahmad et al., 2017). The moisture existing in the plant material is vaporized due to the heat and the cracks on the cell walls of the plants are produced because of the thermal effect of microwaves. These cracks cause solvent penetration within the cells which in turn leads to solubilization and oozing out of the active phytoconstituents in the surrounding liquid (Rostagno et al., 2009). The concentration of the phytoconstituents was not affected by the microwave power after the total exhaustion of plant material and if further exposure of power is given to the plant it may degrade the chemical structure of polyphenolic compounds and would lead to decreased extraction yield (Hao et al., 2002). Xiong et al. (2016) developed the extraction conditions for efficient microwave-assisted extraction of Lotus plumue using a central composite design of RSM and selected 200 W as optimized microwave power.
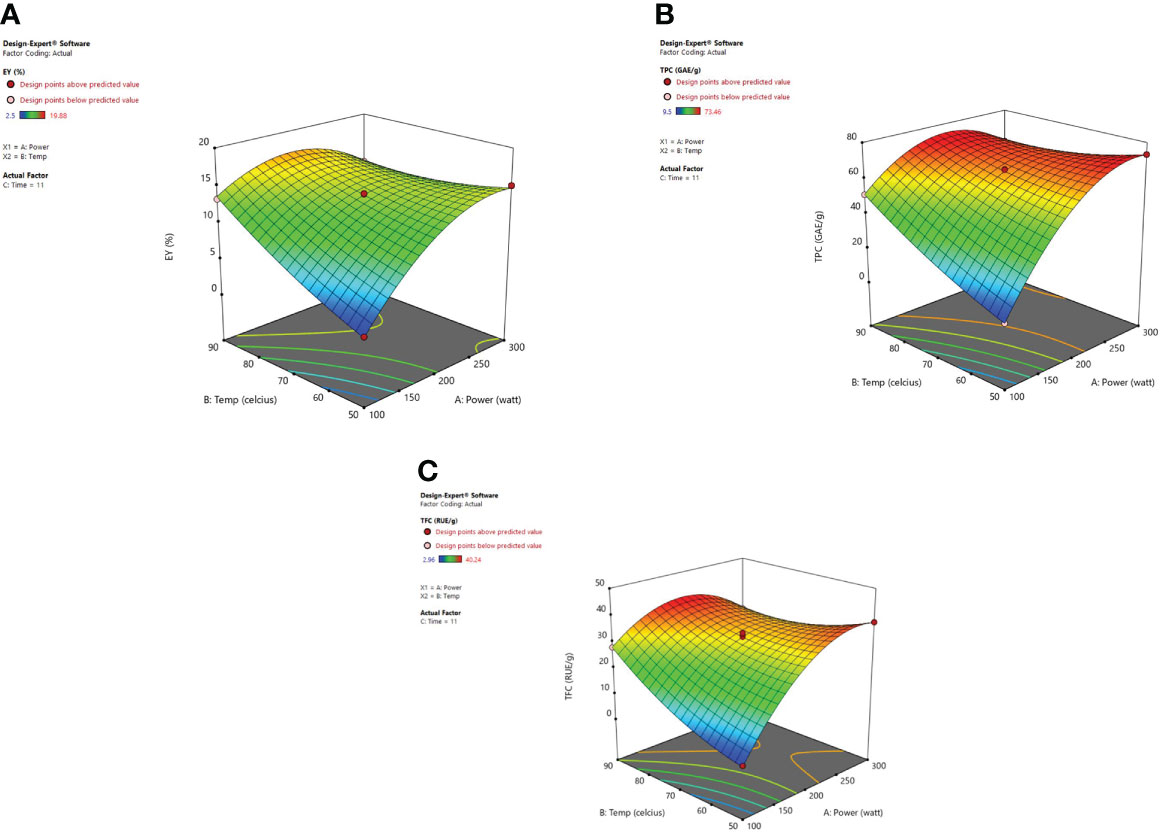
Figure 4 3-D diagrams indicating the effect of different variables (X1, X2) on response, (A) EY (Y1), (B) TPC (Y2), (C) TFC (Y3) in MAE.
The irradiation time of microwaves is also an important factor in the extraction of desired phytoconstituents from herbal plants. From response surface analysis Figure 5, it was revealed that microwave power (X1) along with the time (X3) at a fixed temperature of 70°C imparts an encouraging effect on extraction yield, TPC, and TFC. At 200W, it was observed that the recovery of phenolic compounds and extraction yield of the extract increased steadily up to 20 minutes, and further a decrease in the extraction yield was noticed beyond 20 minutes. Also, the higher power applied for a stretched irradiation time can affect the chemical structure of the targeted compounds adversely (Xiong et al., 2016). In various MAE studies, it has been indicated that both the factors (i) high microwave power and (ii) prolonged irradiation time can degrade the plant metabolites significantly (Gfrerer and Lankmayr, 2005; Rostagno et al., 2009; Nadagouda et al., 2011; Ahmad et al., 2017). If the solution resists the electrophoretic movement of ions, then it would lead to fast heating of sample material within the solvent mix. Because of this rapid increase of temperature in the solvent mix, the cell walls of the plant matrix got ruptured owing to boosted cavitations and turbulence possessions that results in enhanced mass transfer (Hao et al., 2002; Routray and Orsat, 2014; Fernandez-Pastor et al., 2017). After these observed parameters, no substantial alteration was detected in the response factor (EY). Moreover, in the MAE technique, if the plant material is exposed to irradiations for a prolonged period, the concentration of desired compounds could decrease due to overheating of the plant sample at elevated temperatures. Besides, literature corroborates the same that when irradiation time increases continuously, then after a certain time period extraction yield begins to drop. Extended time duration of microwave irradiation exposure can degrade the active constituents present in the matrix and also reduce the extraction yields considerably (Qiu et al., 2012; Ahmad et al., 2017).
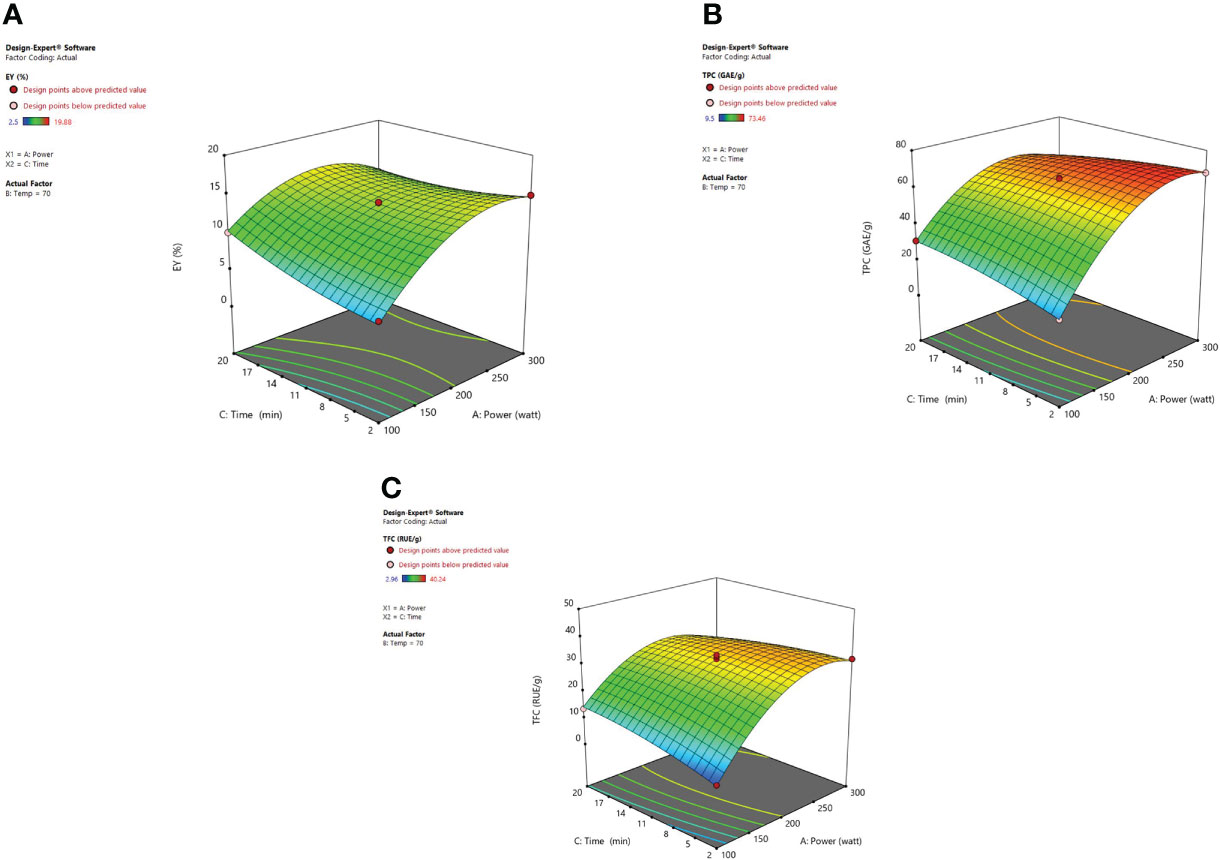
Figure 5 3-D diagrams indicating the effect of different variables (X1, X3) on response, (A) EY (Y1), (B) TPC (Y2), (C) TFC (Y3) in MAE.
Another imperative factor is the temperature, which influences the extraction yield while using MAE. Figure 6 shows the significant effect of temperature (X2) and irradiation time (X3) at a constant microwave power of 200 W on the EY, TPC, and TFC. As heating enhanced the diffusivity of the solvent mixture in the plant material so solubility for the recovery of phenolic content from plant material was also improved (Spigno and De Faveri, 2009; Chew et al., 2011; Dahmoune et al., 2014; Alara et al., 2018). Higher temperatures were found to affect the extraction yields positively though cannot be enhanced indefinitely. Various studies showed that 60-80°C temperature range is quite suitable for the recovery of phytoconstituents using microwave-assisted extraction. (Chan et al., 2011; Shang et al., 2016). Ahmad et al. (2017) demonstrated that when very high temperatures are used to carry out MAE then the sample matrix can be degraded and lead to alteration in the structure of desired compounds (Gfrerer and Lankmayr, 2005). Also, Routray and Orsat (2012) observed an increase in extraction efficiency with the elevated temperatures until an optimum temperature was attained. Later, efficiency begins to decrease with the continual increase in temperature, this occurs as a consequence of the selected ideal extraction temperature which is directly associated with the stability and yield of the targeted compound(s). To an extent, the factors such as irradiation time and microwave power influence each other. A low to moderate power combination with longer irradiation exposure is usually selected to optimize the MAE procedure. Our results were clearly in correlation with the study of Gandhi et al. (2019) and Alara et al. (2018). According to their studies, an increase in extraction temperature up to 90°C not only increased the extraction efficiency but also the extraction yield of betulic acid was enhanced. In MAE closed system, temperature escalation results in the vapor pressure rise so as to improve the efficiency of the extract because the chemical compounds are desorbed from the sample matrix. The rise in temperature leads to a decrease in surface tension along with the lowering of solvent viscosity and that results in improved sample wetting and solvent diffusion power respectively. With the increase in temperature the solute starts releasing from the active sites of the matrix and as a result, improved extraction efficiency is achieved (Eskilsson and Bjorklund, 2000). The energy provided to the plant matrix from microwave power is converted into heat energy and hence the temperature in the vessel can be controlled (Mandal et al., 2007; Khajeh et al., 2010; Li et al., 2010).
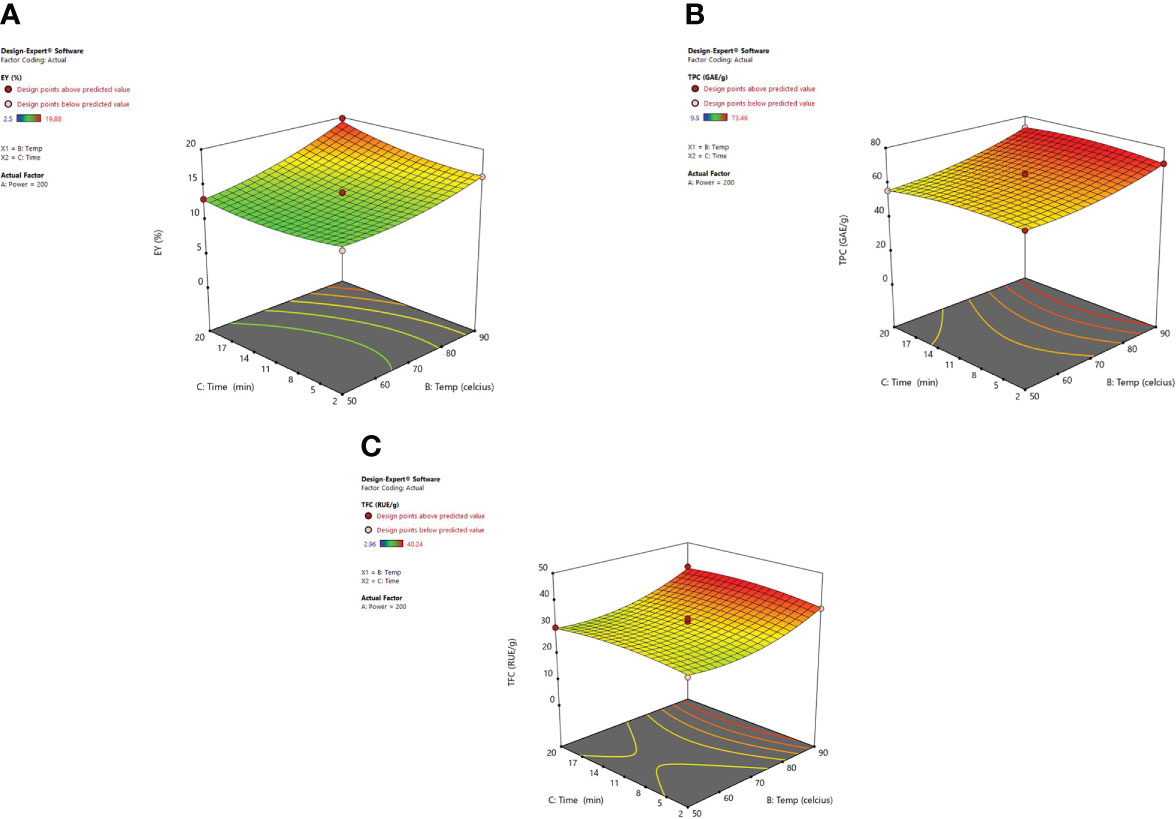
Figure 6 3-D diagrams indicating the effect of different variables (X2, X3) on response, (A) EY (Y1), (B) TPC (Y2), (C) TFC (Y3) in MAE.
3.2.3 Optimization and validation of the predictive model
In this current study, various parameters were optimized for MAE using BBD-coupled RSM to attain maximum extraction yield (%), TPC, and TFC. A numerical optimization method was adopted and optimized extraction conditions for MAE were predicted (microwave power 187.04 W, temperature 90°C, and irradiation time 20 min) to achieve the maximum EY, TPC, and TFC. The root of the NJ was also extracted at the optimized conditions of MAE (extract termed as OMNJ) and all responses were observed to lie within 95% confidence and thus validated the established model. The comparative extraction conditions and responses in different methods (OMNJ and MCNJ) are presented in Table 4.
3.3 Scanning Electron Microscopy (SEM)
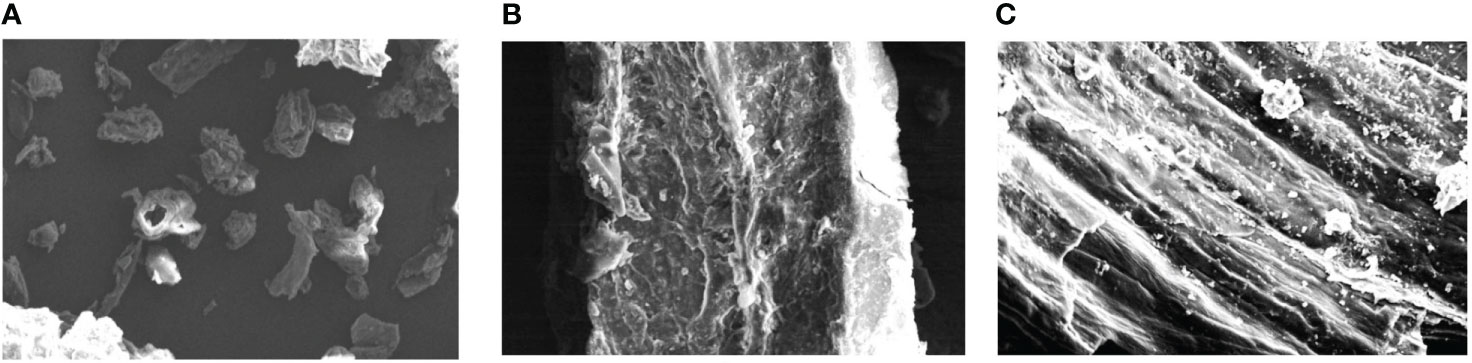
To confirm the structural changes in the powdered plant samples after the extraction with different methods (maceration and MAE), scanning electron microscopy (SEM) was generally employed (Liu et al., 2006; Lucchesi et al., 2007). The powder of roots of selected medicinal herb obtained after drying of the marc (after maceration and MAE) was also subjected to SEM. The intact powder of the plant sample (without extraction) was also analyzed by SEM to further compare the micrographs of different samples. The photomicrograph of untreated powder of selected herb showed uniform and consistent cellular surface of particles (Figure 7A). Likewise, on maceration treatment, the particles lost uniformity in shape and the surface became somewhat flat (Figure 7B). Plant tissues were significantly disturbed and crumbled on treatment with microwave radiations (Figure 7C). Absorption of microwave energy by moisture present in the plant particles and further vaporization of this created high pressure inside the sample which causes the disruption and crumbling of plant samples (Rostagno et al., 2009). Similar results were also reported by the previous researchers for the samples of Erigeron breviscapus and tobacco leaves, where the microwave treatment significantly causes the change in the microstructure of the plant samples (Zhou and Liu, 2006; Gao et al., 2007).
3.4 GC-MS analysis
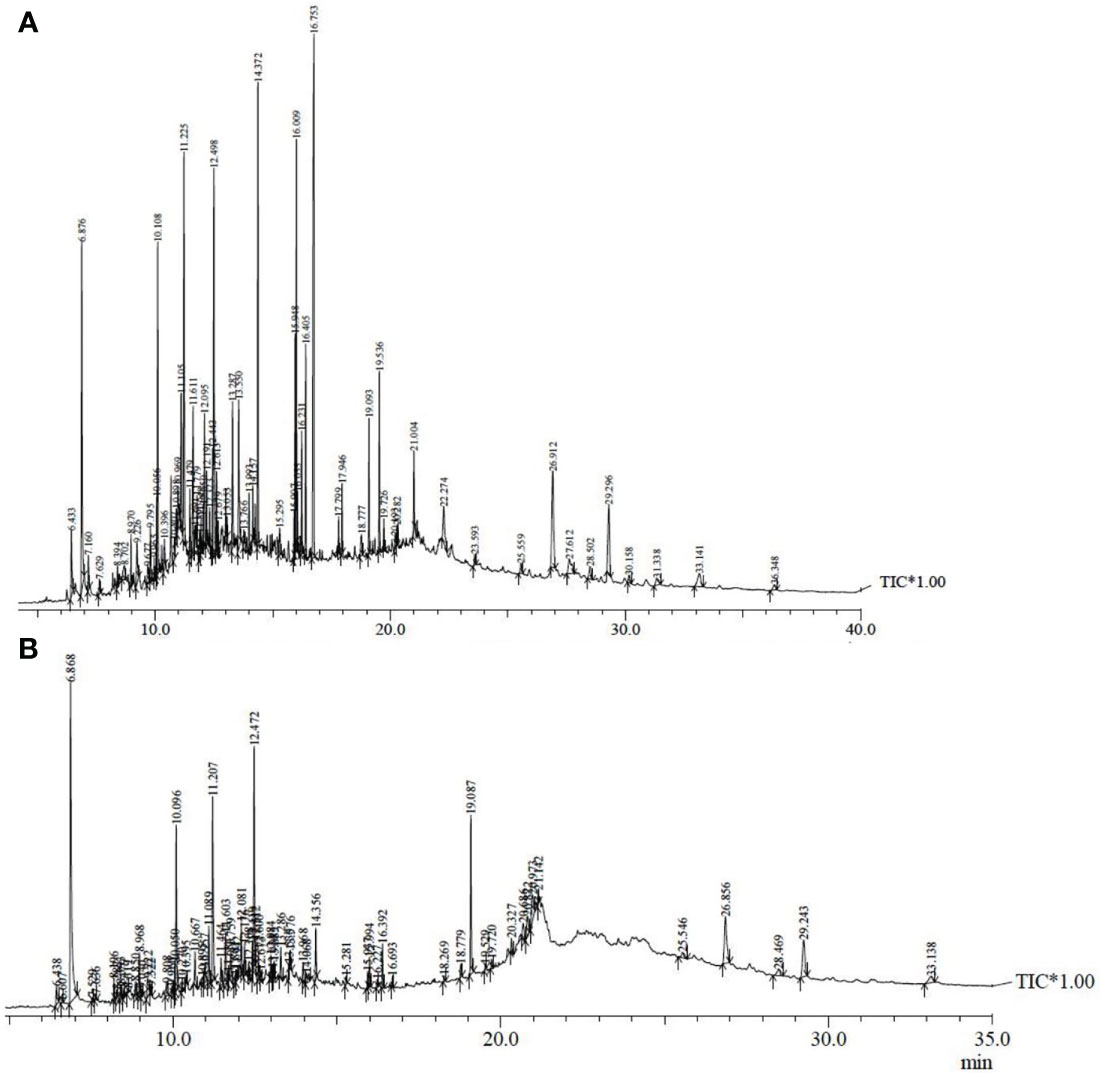
A literature study revealed that MAE significantly enhanced the concentration of secondary metabolites from plants (MAE review reference). Hence the extract obtained at optimized conditions of green approach (OMNJ) was also subjected to GC-MS analysis for the tentative confirmation of plant actives present in it. Retention time (RT) and percentage (%) area metabolites is also compared in the two extracts (Table 5). The GC-MS chromatogram of MCNJ and OMNJ is also presented in Figure 8.
Phytochemical analysis showed the presence of quite a lot of phytoconstituents including sesquiterpenes, sesquiterpenoids, esters, and steroids. The outcomes of the analysis confirmed that most of the phytoconstituents present in MCNJ were also reported in OMNJ.
The concentration of pharmacologically important sesquiterpenes such as spirojatamol (4.29%), jatamansone (7.86%), valeric acid (8.06%), valerenal (3.02%), globulol (6.71%), and steroidal compounds like sitosterol (4.81%), sitostenone (3.87%), stigmastane-3,6-dione (1.13%) stigmasta-3,5-diene-7-one (0.78%), and ergost-5-en-3-ol (0.55%) was found significantly higher in the extract obtained by using green approach (OMNJ). Also the concentration of cyclopentanoid monoterpene alkaloid, actinidine increased to more than twice in the OMNJ extract (~18%) as compared to the conventional extract, MCNJ (~7%). The possible justification for the increase in the quantity of these plant actives could be attributed to rupturing of the cell wall of the plant particles by microwave radiation. Further, it has also been reported that phytoconstituents like sesquiterpenes, steroids, ubiquinone are generally biosynthesized in the cytoplasm (Bick and Lange, 2003). Therefore disruption of the cell wall by microwave radiation causes the enhanced outward movement of accumulated secondary metabolite in the cytoplasm. Moreover, the monotepenes are generally presented in epidermal cells or trichome (Kutchan, 2005) so the significant increase in the concentration of actinidine by MAE is also justified. Because the microwave radiations vaporize the moisture present in the cell and resulted in rupturing of the cell membrane (Rostagno et al., 2009).
3.5 In-Vivo estimation of anti-alzheimer’s potential
Nardostachys jatamansi is a highly significant medicinal plant genera bearing wonderful memory enhancer, stimulant, and mind rejuvenator properties and is used in preventing various cognitive deficits and neurological disorders like Alzheimer’s disease (AD) (Bose et al., 2019). The majority of secondary metabolites present in the root extract of NJ are identified as antioxidants in nature and found to be tremendously effective in cognition enhancement (Saroya and Singh, 2018). Hence the optimized extract, OMNJ, along with the conventional extract, MCNJ, is also evaluated for anti-Alzheimer’s potential.
3.5.1 Estimation of behavioral parameters
The parameters such as cognitive impairment, retention in learning, and memory power were analyzed using an elevated plus maze (EPM) and passive avoidance apparatus. Short-term memory impairment was observed by scopolamine administration in experimental animals. On the 15th day, the transfer latency (TL) was noted for 5 min after drug administration for 24 h. The different extracts of NJ showed reduced effects on the TL in different groups for which the results are represented in Figure 9A. It was observed that TL for the scopolamine group was significantly increased in comparison to the control group (p<0.005). On treatment with extracts, the MCNJ and OMNJ reduced the TL significantly (p<0.05 and p<0.01 respectively) as compared to the negative control group. Also, the step-down latency (SDL) was found significantly decreased in the scopolamine-induced group as compared to the control group (p<0.005). Figure 9B indicated that SDL significantly improved by MCNJ (p<0.01) and OMNJ (p<0.01).
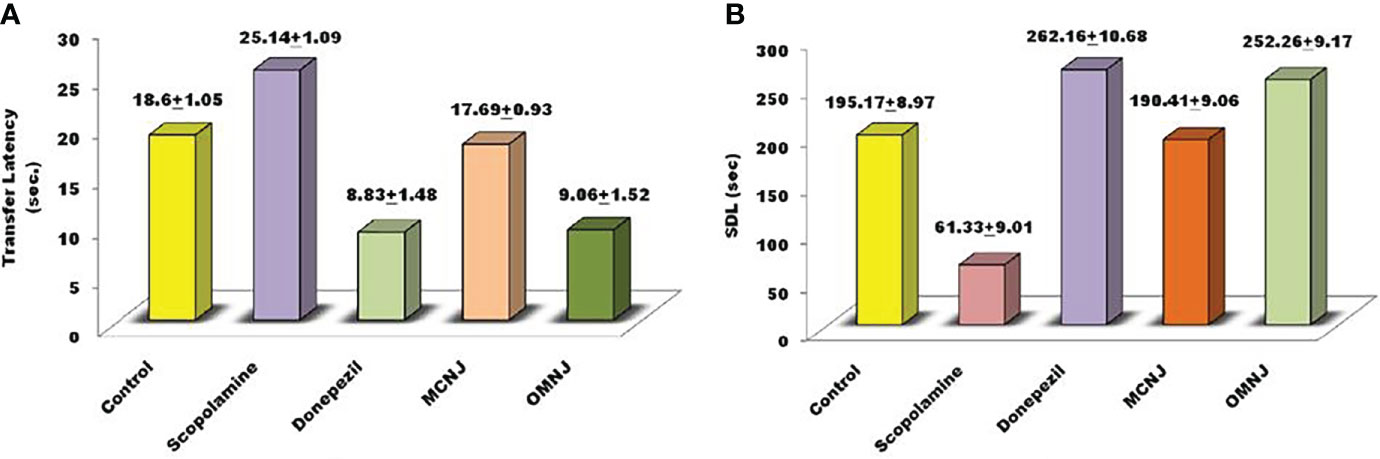
Figure 9 Effect of NJ extracts (MCNJ and OMNJ) on behavioral parameters (A) Transfer latency (TL) and (B). Step down latency (SDL).
3.5.2 Biochemical estimation of antioxidant parameters
Several studies have shown the involvement of oxidative stress in various pathogenesis mechanisms related to neurodegenerative disorders like AD. Also, the therapeutic strategies are based on preventing or reducing oxidative damage. Hence, in the present research, the effects of maceration and optimized MAE extracts on different biochemical parameters such as catalase and glutathione activity, nitric oxide content, and the superoxide dismutase concentration were also evaluated and the results were represented in Figure 10. Scopolamine significantly enhanced the oxidative stress level and also altered the concentration of glutathione, catalase, SOD, and nitric oxide content significantly as compared to the control group (p<0.005). The concentration of catalase, nitrite content (p<0.01), glutathione, and SOD (p<0.05) was significantly altered by OMNJ as compared to the negative control group. Conventional extract, MCNJ also reduced the catalase, SOD, and glutathione concentration but the change was not significant in the case of SOD. Various secondary metabolites including the phenolic and flavonoid compounds having antioxidant properties have been recognized as a part of therapeutic approaches beneficial in the management of AD (Devore et al., 2010; Feng and Wang, 2012). Antioxidants are linked with the decreased incidence of AD and a slower rate of cognitive impairment occurrence has been reported in patients of AD who are on high doses of antioxidants (93). The majority of phytoconstituents reported in roots of NJ comprises of sesquiterpenes like spirojatamaol, jatamansone, valeric acid, and globulol and steroidal compounds, and these compounds have been reported to exhibit anti-oxidant potential (Rice-Evans et al., 1995; Gilgun-Sherki et al., 2003; Melo et al., 2003; Bouayed, 2010; Neganova et al., 2012; Sharififar et al., 2012; Sharma and Singh, 2012; Hasnat et al., 2013; Razack et al., 2015; Goula et al., 2016; Saroya and Singh, 2018). The improved concentration of these metabolites could be responsible for the better anti-oxidant potential of OMNJ. Also, previous studies indicated that the scavenging of free radicals in the brain improves cognitive activities, prevents damage caused by oxidative stress, and shields CNS via BBB crossing (Bouayed, 2010). Thus, it can be interpreted from the data that this improvement anti-Alzheimer’s potential of the optimized extract could be a consequence of improved concentrations of various plant metabolites together with the TPC and TFC in OMNJ.
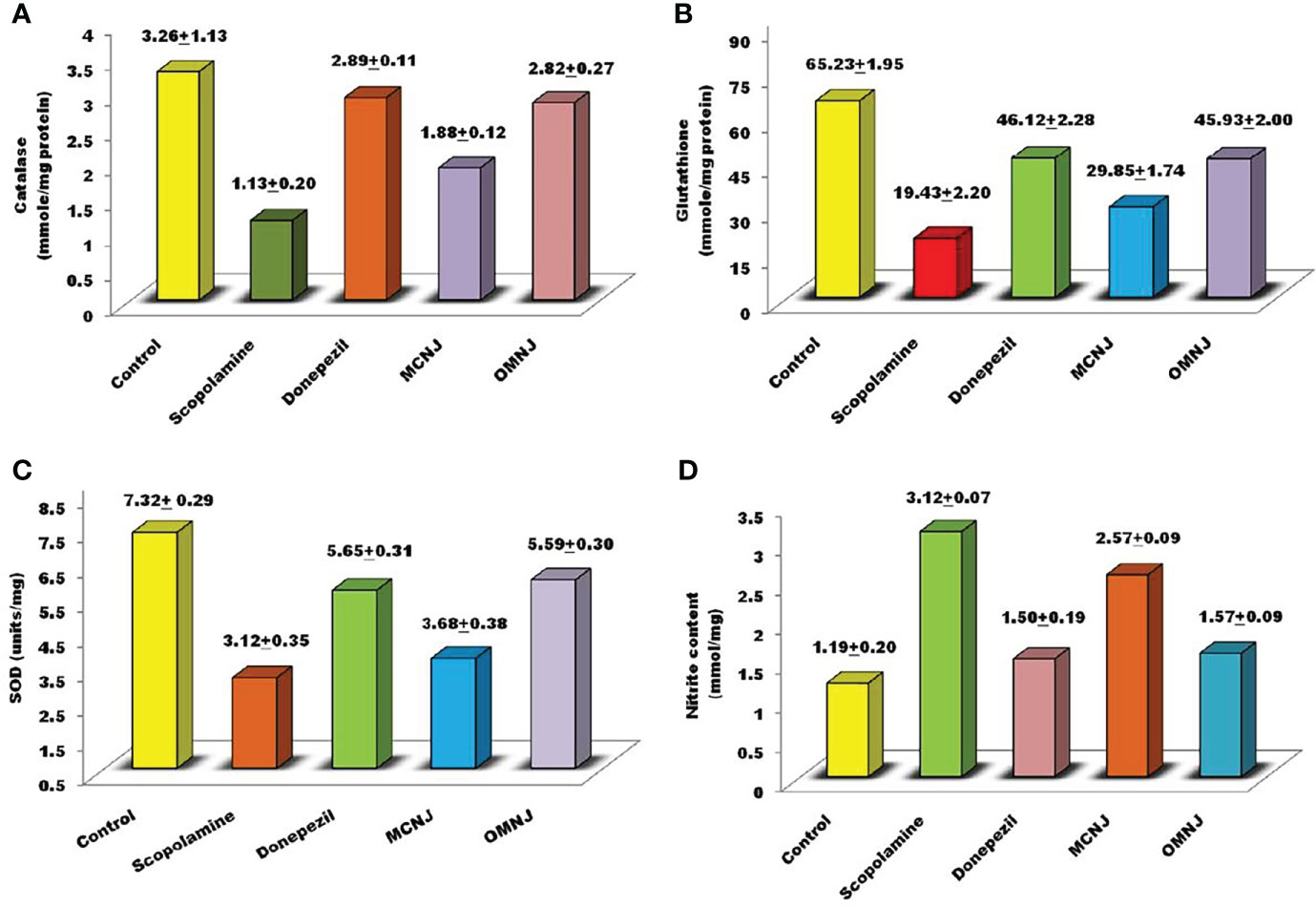
Figure 10 Biochemical evaluation of various extracts related to anti-oxidant stress (A) catalase (B) reduced glutathione (C) SOD (D) nitrite content.
3.5.3 AChE inhibitory potential of different extracts
Acetylcholine neurotransmitter reduction was found to be associated with the degradation of cholinergic neurons which are present in the basal forebrain (Selkoe et al., 1994; Hamlin et al., 2013). The reduction of acetylcholine level leads to dementia which is found in patients who are critically suffering from AD (Nagele et al., 2002; Arya et al., 2021). Recent advancements in research emphasize the alteration of acetylcholinesterase (AChE) activity for AD management. As per the literature, AChE inhibitors reduce the symptoms associated with dementia by enhancing the acetylcholine release at cholinergic synapses, and also stimulate cognitive performance in humans and animals (Davidsson et al., 2001; Nagele et al., 2002; Devore et al., 2010; Rockwood, 2011). The different extracts were administered for fourteen succeeding days and it was observed that AChE activity significantly declined in all the extracts (Figure 11) when compared to the control (p<0.05 for MCNJ and p<0.01 for OMNJ).
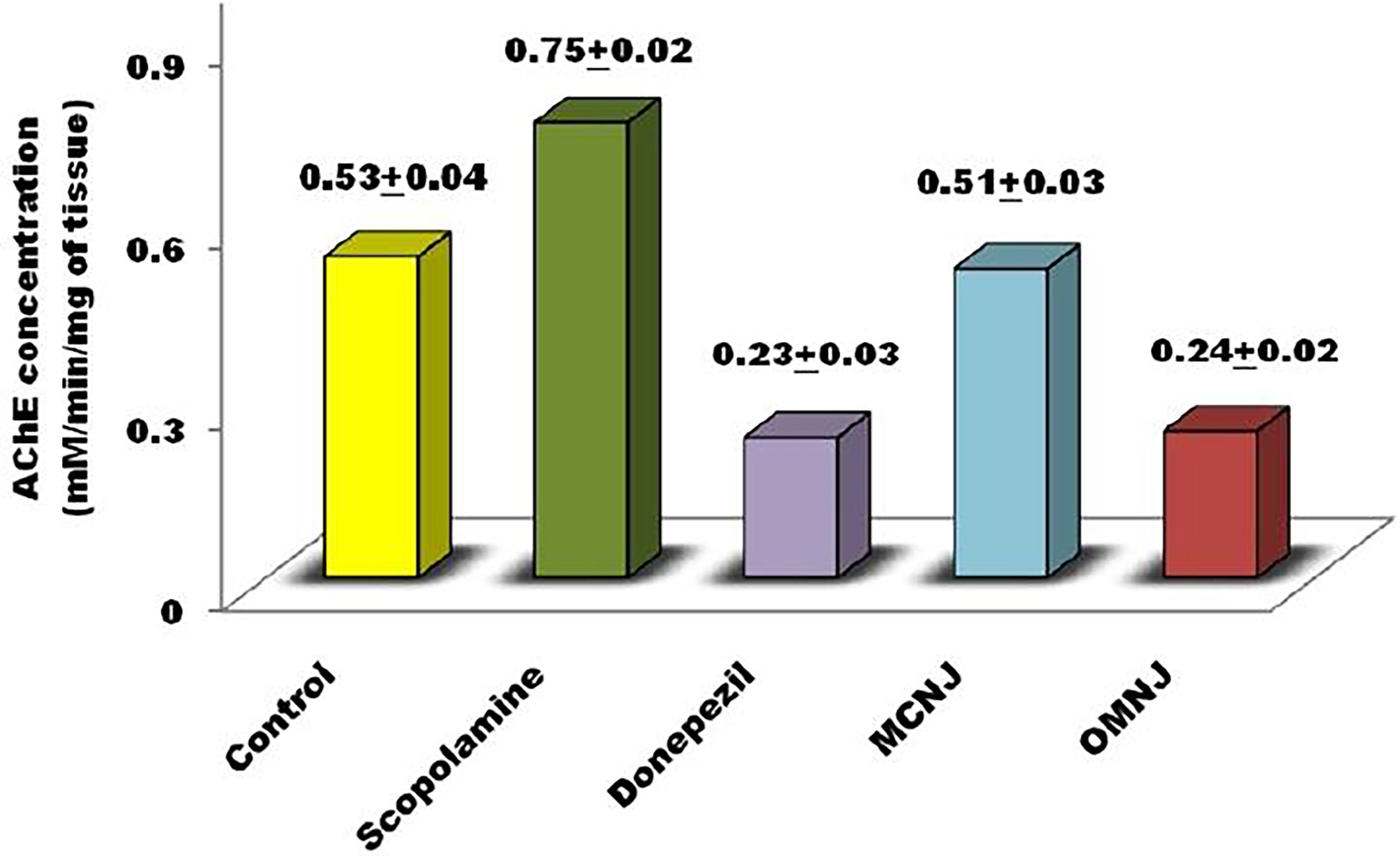
Figure 11 AChE inhibitory activity exerted by various extracts and standard drugs in different groups.
With the help of data obtained from the experiments, we can easily predict that the optimized extract prepared by green methods (OMNJ) firmly possesses a higher potential of AChE inhibition in comparison to extracts obtained from conventional method owing to the presence of an improved concentration of secondary metabolites, mainly sesquiterpenes including TPC and TFC. These results are also concomitant with past studies which stated that various sesquiterpenes including that jatamansone inhibits AChE and can be used for the management of AD (Arya et al., 2021).
4 Conclusion
Worldwide an extreme inclination towards the use of herbal formulations has been observed over the last few decades. The Nardostachys jatamansi (D. Don) DC extracts or its polyherbal preparations hold a great demand in the global market. Its root extract has been used in several ayurvedic formulations like Mentat and Intellimax for the improvement of learning and memory power. Previously, the conventional methods were used to extract the herbs but now due to various limitations associated with these methods, the non-conventional and green techniques have been put forward to carry out the extraction of selected herbs. In the present study, roots of the NJ are extracted by a green method, MAE, at optimized conditions. MAE not only improves the kinetics of extraction but also enhanced the pharmacologically important sesquiterpenes (jatamansone, spirojatamol, globulol, valeric acid, etc) concentration as compared to conventional methods. Hence, it can be concluded that MAE could be employed for the extraction of roots of Nardostachys jatamansi at commercial scales after suitable modifications to meet the rising industrial demand.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: Nil.
Ethics statement
The animal study was reviewed and approved by Institutional Animal Ethical Committee, IAEC, Maharshi Dayanand University, Rohtak granted approval for our research protocol presented against vide reference number 1767/RE/S/14/CPCSEA/CAH/76-85 dated 26.02.2021.
Author contributions
AA- Writing the original manuscript and conduct experiments. RC- Manuscript writing and conduct experiments. MA- Revise the manuscript and analyze the data. DK- Revise the manuscript and application of design software. LA- Manuscript revision and analyse the data. MK- Manuscript revision and data analysis. MA-D- Conceptualization, revision and analysis of data. VM- Conceptualization, Supervision, analysis and revision of manuscript.
Acknowledgments
The authors want to sincerely acknowledge Maharshi Dayanand University, Rohtak, Haryana, India, and the Researchers Supporting Project number (RSP-2021/191), King Saud University, Riyadh, Saudi Arabia for their support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aebi, H. (1974). Catalase in methods of enzymatic analysis (Academic press Inc. New York), 673–684. Available at: doi.org/10.1016/B978-0-12-091302-250032-3.
Ahmad, I., Yanuar, A., Mulia, K., Munim, A. (2017). Optimization of ionic liquid-based microwave-assisted extraction of polyphenolic content from peperomia pellucida (L) kunth using response surface methodology. Asian Pac. J. Trop. Biomed. 7, 660–665. doi: 10.1016/j.apjtb.2017.06.010
Ahmad, M., Yousuf, S., Khan, M. B., Hoda, M. N., Ahmad, A. S., Ansari, M. A., et al. (2006). Attenuation by nardostachys jatamansi of 6-hydroxydopamine-induced parkinsonism in rats: Behavioral neurochemical and immunohistochemical studies. Pharmacol. Biochem. Behav. 83, 150–160. doi: 10.1016/jpbb200601005
Alamgir, A. N. M. (2018). “Biotechnology, in vitro production of natural bioactive compounds, herbal preparation, and disease management (treatment and prevention),” in Therapeutic use of medicinal plants and their extracts (Cham: Springer). doi: 10.1007/978-3-319-92387-1_7
Alara, O. R., Abdurahman, N. H., Olalere, O. A. (2018). Optimization of microwave-assisted extraction of flavonoids and antioxidants from vernonia amygdalina leaf using response surface methodology. Food Bioprod. Process. 107, 36–48. doi: 10.1016/j.fbp.2017.10.007
Arya, A., Chahal, R., Rao, R., Rahman, M., Kaushik, D., Akhtar, M. F., et al. (2021). Acetylcholinesterase inhibitory potential of various sesquiterpene analogues for alzheimer’s disease therapy. Biomolecules 11, 1–27. doi: 10.3390/biom11030350
Arya, A., Kaushik, D., Almeer, R., Bungau, S. G., A.A. Sayed, A. A., Abdel-Daim, M. M., et al. (2022). Application of green technologies in design-based extraction of celastrus paniculatus (Jyotishmati) seeds, SEM, GC-MS analysis, and evaluation for memory enhancing potential. Front. Nutr. 9. doi: 10.3389/fnut.2022.871183
Badyal, S., Singh, H., Yadav, A. K., Sharma, S., Bhushan, I. (2020). Plant secondary metabolites and their uses. Plant Arch. 20, 3336–3340.
Bick, J. A., Lange, ,. B. M. (2003). Metabolic cross talk between cytosolic and plastidial pathways of isoprenoid biosynthesis: Unidirectional transport of intermediates across the chloroplast envelope membrane. Arch. Biochem. Biophys. 415, 146–154. doi: 10.1016/S0003-9861(03)00233-9
Bose, B., Tripathy, D., Chatterjee, A., Tandon, P., Kumaria, S. (2019). Secondary metabolite profiling, cytotoxicity, anti-inflammatory potential and in vitro inhibitory activities of nardostachys jatamansi on key enzymes linked to hyperglycemia, hypertension and cognitive disorders. Phytomedicine 55, 58–69. doi: 10.1016/j.phymed.2018.08.010
Bouayed, J. (2010). Polyphenols: A potential new strategy for the prevention and treatment of anxiety and depression. Curr. Nutr. Food Sci. 6, 13–18. doi: 10.2174/157340110790909608
Bouras, M., Chadni, M., Barba, F. J., Grimi, N., Bals, O., Vorobiev, E. (2015). Optimization of microwave-assisted extraction of polyphenols from quercus bark. Ind. Crops Prod. 77, 590–601. doi: 10.1016/j.indcrop.2015.09.018
Chan, C., Yusoff, R., Ngoh, G., Kung, F. W. (2011). Microwave-assisted extractions of active ingredients from plants. J. Chromatogr. A. 1218, 6213–6225. doi: 10.1016/j.chroma.2011.07.040
Chemat, F., Abert-Vian, M., Zill-e-Huma, Y. J. (2009). “Microwave assisted separations: green chemistry in action,” in Green chemistry research trends (New York NY: Nova Science Publishers), 33–62.
Chemat, F., Vian, M. A., Cravotto, G. (2012). Green extraction of natural products: concept and principles. Int. J. Mol. Sci. 13, 8615–8627. doi: 10.3390/ijms13078615
Chen, ,. Y., Xie, M. Y., Gong, X. F. (2007). Microwave-assisted extraction used for the isolation of total triterpenoid saponins from Ganoderma atrum. J. Food Engg. 81, 162–170. doi: 10.1016/j.jfoodeng.2006.10.018
Chew, K. K., Khoo, M. Z., Ng, S. Y., Thoo, Y. Y., Aida, W. M. W., Ho, C. W. (2011). Effect of ethanol concentration, extraction timeand extraction temperature on the recovery of phenoliccompounds and antioxidant capacity of Orthosiphon stamineus extracts. Int. Food Res. J. 18, 1427–1435.
Dahmoune, F., Spigno, G., Moussi, K., Remini, H., Cherbal, A., Madani, K. (2014). Pistacia lentiscus leaves as a source of phenolic compounds: Microwave-assisted extractionoptimized and compared with ultrasound-assisted and conventional solvent extraction. Ind. Crops Prod. 61, 31–40. doi: 10.1016/j.indcrop.2014.06.035
Dandagi, P. M., Patil, M. B., Mastiholimath, V. S., Gadad, A. P., Dhumansure, R. H. (2008). Development and evaluation of hepatoprotective polyherbal formulation containing some indigenous medicinal plants. Indian J. Pharm. Sci. 70, 265–268. doi: 10.4103/0250-474X.41474
Davidsson, P., Blennow, K., Andreasen, N., Eriksson, B., Minthon, L., Hesse, C. (2001). Differential increase in cerebrospinal fluid-acetylcholinesterase after treatment withacetylcholinesterase inhibitors in patients with alzheimer’s disease. Neurosci. Lett. 300, 157–160. doi: 10.1016/S0304-3940(01)01586-5
Devgan, M., Nanda, A., Ansari, S. H. (2013). Comparative evaluation of the anti-diabetic activity of Pterocarpus marsupium roxb. heartwood in alloxan induced diabetic rats using extracts obtained by optimized conventional and non conventional extraction methods. Pak. J. Pharm. Sci. 26, 973–976.
Devore, E. E., Grodstein, F., Van Rooij, F. J., Hofman, A., Stampfer, M. J., Witteman, J. C., et al. (2010). Dietary antioxidants and long-term risk of dementia. Arch. Neurol. 67, 819–825. doi: 10.1001/archneurol2010144
Dhiman, N., Bhattacharya, A. (2020). Nardostachysjatamansi (D. don) DC.-challenges and opportunities of harnessing the untapped medicinal plant from the Himalayas. J. ethnopharmaco. 246, 112211. doi: 10.1016/j.jep.2019.112211Get
Ellman, G. L. (1959). Tissue sulfhydryl groups. Arch. Biochem. Biophy. 82, 70–77. doi: 10.1016/0003-9861(59)90090-6
Ellman, G. L., Courtney, D., Andres, V., Feathersone, R. M. (1961). A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 7, 88–95. doi: 10.1016/0006-2952(61)90145-9
Eskilsson, C. S., Bjorklund, E. (2000). Analytical-scale microwave-assisted extraction. J. Chromatogr. A. 902, 227–250. doi: 10.1016/S0021-9673(00)00921-3
Feng, Y., Wang, X. (2012). Antioxidant therapies for alzheimer’s disease. Oxid. Med. Cell. Longev., 1–18. doi: 10.1155/2012/472932
Fernandez-Pastor, I., Fernandez-Hernandez, A., Perez-Criado, S., Rivas, F., Martinez, A., Garcia-Granados, A., et al. (2017). Microwave-assisted extraction versus soxhlet extraction to determine triterpene acids in olive skins. J. Sep. Sci. 40, 1209–1217. doi: 10.1002/jssc.201601130
Filip, S., Pavlić, B., Vidović, S., Vladić, J., Zeković, Z. (2017). Optimization of microwave-assisted extraction of polyphenolic compounds from ocimumbasilicum by response surface methodology. Food Anal. Methods 10, 2270–2280. doi: 10.1007/s12161-017-0792-7
Gandhi, D. M., Patel, H., Patel, N., Mehta, P. (2019). Optimization of microwave-assisted extraction technique for isolation of betulinic acid from Dillenia indica linn. and its quantification using developed HPTLC method. J. Appl. Pharm. Sci. 9, 101–110. doi: 10.7324/JAPS.2019.90814
Gao, M., Huang, W., Moytri, R. C., Liu, C. (2007). Microwaveassisted extraction of scutellarin from erigeron breviscapus hand- mazz and its determination by high-performance liquid chromatography. Analytic. Chimica. Acta 591, 161–166. doi: 10.1016/j.aca.2007.04.004
Gfrerer, M., Lankmayr, E. (2005). Screening optimization and validation of microwave-assisted extraction for the determination of persistent organochlorine pesticides. Anal. Chim. Acta 533, 203–211. doi: 10.1016/j.aca.2004.11.016
Ghimire, S. K., Aumeeruddy-Thomas, Y. (2009). Ethnobotanical classification and plant nomenclature system of high altitude agro-pastoralists in dolpo, Nepal. Bot. Orient. J. Plant Sci. 6, 56–68. doi: 10.3126/botor.v6i0.2912
Gilgun-Sherki, Y., Melamed, E., Offen, D. (2003). Antioxidant treatment in alzheimer’s disease. J. Mol. Neurosci. 21, 1–11. doi: 10.1385/JMN:21:1:1
Goula, A. M., Thymiatis, K., Kaderides, K. (2016). Valorization of grape pomace: Drying behavior and ultrasound extraction of phenolics. Food Bioprod. Process 100, 132–144. doi: 10.1016/j.fbp.2016.06.016
Guerriero, G., Berni, R., Muñoz-Sanchez, J. A., Apone, F., Abdel-Salam, E. M., Qahtan, A. A., et al. (2018). Production of plant secondary metabolites: Examples, tips and suggestions for biotechnologists. Genes 9, 309. doi: 10.3390/genes9060309
Hamlin, A. S., Windels, F., Boskovic, Z., Sah, P., Coulson, E. J. (2013). Lesions of the basal forebrain cholinergic system in mice disrupt idiothetic navigation. PLoS One. 8(1):e53472, 1–8. doi: 10.1371/journalpone005347
Hao, J., Han, W., Huang, S., Xue, B., Deng, X. (2002). Microwave-assisted extraction of artemisinin from artemisis annua l. Sep. Purif. Technol. 28, 191–196. doi: 10.1016/S1383-5866(02)00043-6
Hasnat, M., Pervin, M., Lim, B. O. (2013). Acetylcholinesterase inhibition and in vitro and in vivo antioxidant activities of ganoderma lucidum grown on germinated brown rice. Molecules 18, 6663–6678. doi: 10.3390/molecules18066663
Joshi, H., Parle, M. (2006). Nardostachys jatamansi improves learning and memory in mice. J. Med. Food. 9, 113–118. doi: 10.1089/jmf20069113
Kakkar, P., Das, B., Viswanathan, P. N. (1984). A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys. 21, 130–132.
Kapoor, H., Yadav, N., Chopra, M., Mahapatra, S. C., Agrawal, V. (2017). Strong anti-tumorous potential of nardostachys jatamansi rhizome extract on glioblastoma and in silico analysis of its molecular drug targets. Curr. Cancer Drug Targets. 17, 74–88. doi: 10.2174/1570163813666161019143740
Kaufmann, B., Christen, P., Veuthey, J. L. (2001). Parameters affecting microwave-assisted extraction of withanolides. Phytochem. Anal. 12, 327–331. doi: 10.1002/pca.599
Kaur, H., Lekhak, M. M., Chahal, S., Goutam, U., Jha, P., Naidoo, D., et al. (2020). Nardostachys jatamansi (D. don) DC.: An invaluable and constantly dwindling resource of the Himalayas. S. Afr. J. Bot. 135, 252–267. doi: 10.1016/j.sajb.2020.08.010
Khajeh, M., Akbari, M. A. R., Sanchooli, E. (2010). Application of doehlert design in the optimization of microwave-assisted extraction for determination of zinc and copper in cereal samples using FAAS. Food Anal. Methods 3, 133–137. doi: 10.1007/s12161-009-9099-7
Kutchan, T. M. (2005). A role for intra- and intercellular translocation in natural product biosynthesis. Curr. Opin. Plant Biol. 8, 292–300. doi: 10.1016/j.pbi.2005.03.009
Kwon, J. H., Belanger, J. M. R., Jocelyn Pare, J. R., Yaylayan, V. A. (2003). Application of microwave-assisted process (MAP TM) to the fast extraction of ginseng saponins. Food Res. Int. 36, 491–498. doi: 10.1016/S0963-9969(02)00197-7
Lieben, C. K., Blokland, A. (2005). The selective 5-HT6 receptor antagonist Ro4368554 restores memory performance in cholinergic and serotonergic models of memory deficiency in the rat. Neuropsycho. Pharmacol. 30, 2169–2179. doi: 10.1038/sj.npp.1300777
Liu, Z., Wei, G., Guo, Y., John, F. K. (2006). Image study of pectin extraction from orange skin assisted by microwave. Carbohydr. Polym. 64, 548–552. doi: 10.1016/j.carbpol.2005.11.006
Li, J., Zu, Y. G., Fu, Y. J., Yang, Y. C., Li, S. M., Li, Z. N. (2010). Optimization of microwave-assisted extraction of triterpene saponins from defatted residue of yellow horn (Xanthoceras sorbifolia bunge.) kernel and evaluation of its antioxidant activity. Innov. Food Sci. Emerg. Technol. 11, 637–643. doi: 10.1016/j.ifset.2010.06.004
Lucchesi, M. E., Smadja, J., Bradshaw, S., Louw, W., Chemat, F. (2007). Solvent free microwave extraction of elletaria cardamomum l.: a multivariate study of a new technique for the extraction of essential oil. J. Food Eng. 79, 1079–1086. doi: 10.1016/j.jfoodeng.2006.03.029
Mabberley, D. J., Noltie, H. J. (2014). A note on valeriana jatamansi Jones (Caprifoliaceae s.l.). Blumea 59, 37–41. doi: 10.3767/000651914X683476
Mandal, V., Mohan, Y., Hemalath, S. (2007). Microwave-assisted extraction–an innovative and promising extraction tool for medicinal plant research. Pharmacogn. Rev. 1, 7–18.
Melo, J. B., Agostinho, P., Oliveira, C. R. (2003). Involvement of oxidative stress in the enhancement of acetylcholinesterase activity induced by amyloid beta-peptide. Neurosci. Res. 45, 117–127. doi: 10.1016/S0168-0102(02)00201-8
Mittal, V., Nanda, A. (2017). Intensification of marrubiin concentration by optimization of microwave-assisted (low CO2 yielding) extraction process for marrubium vulgare using central composite design and antioxidant evaluation. Pharm. Biol. 55, 1337–1347. doi: 10.1080/1388020920171297837
Moreira, M. M., Barroso, M. F., Boeykens, A., Withouck, H., Morais, S., Delerue-Matos, C. (2017). Valorization of apple tree wood residues by polyphenols extraction: Comparison between conventional and microwave-assisted extraction. Ind. Crops Prod. 1, 210–220. doi: 10.1016/j.indcrop.2017.04.038
Nadagouda, M. N., Speth, T. F., Varma, R. S. (2011) Microwave-assisted green synthesis of silver nanostructures. Acc. Chem. Res. 44, 469–478. doi: 10.1021/ar1001457
Nagele, R. G., D’andrea, M. R., Anderson, W. J., Wang, ,. H. Y. (2002). Intracellular accumulation of β-amyloid1–42 in neurons is facilitated by the α7 nicotinic acetylcholine receptor in alzheimer’s disease. Neuroscience 110, 199–211. doi: 10.1016/S0306-4522(01)00460-2
Nautiyal, B. P., Chauhan, R. S., Prakash, V., Purohit, H., Nautiyal, M. C. (2003). Population studies for the evaluation of germplasm and threat status of the alpine medicinal herb Nardostachys jatamansi. plant Genet. Resour. Newsl. 136, 34–39.
Neganova, M. E., Afanas’eva, S. V., Klochkov, S. G., Shevtsovs, E. F. (2012). Mechanisms of antioxidant effect of natural sesquiterpene lactone and alkaloid derivatives. Bull. Exp. Biol. Med. 152, 720–722. doi: 10.1007/s10517-012-1615-x
Pagare, S., Bhatia, M., Tripathi, N., Pagare, S., Bansal, Y. K. (2015). Secondary metabolites of plants and their role: Overview. Curr. Trends Biotechnol. Pharm. 9, 293–304.
Qiu, H., Xiao, X., Li, G. (2012). Separation and purification of furanocoumarins from toddalia asiatica (L.) lam. using microwave-assisted extraction coupled with high-speed counter-current chromatography. J. Sep. Sci. 35, 901–906. doi: 10.1002/jssc.201100995
Rao, V. S., Rao, A., Karanth, K. S. (2005). Anticonvulsant and neurotoxicity profile of nardostachys jatamansi in rats. J. Ethnopharmacol. 102, 351–356. doi: 10.1016/jjep200506031
Razack, S., Kumar, K. H., Nallamuthu, I., Naika, M., Khanum, F. (2015). Antioxidant biomolecule oxidation protective activities of nardostachysjatamansi DC and its phytochemical analysis by RP-HPLC and GC-MS. Antioxidants 124, 185–203. doi: 10.3390/antiox4010185
Rice-Evans, C. A., Miller, N. J., Bramley, P. M., Pridham, J. B. (1995). The relative antioxidant activity of plant derived polyphenolic flavonoids. Free Radic. Res. 22, 375–383. doi: 10.3109/10715769509145649
Rockwood, K. (2011). Biomarkers to measure treatment effects in alzheimer’s disease: What should we look for? Int. J. Alzheimers Dis. 598175, 1–14. doi: 10.4061/2011/598175
Rostagno, M. A., Villares, A., Guillamon, E., Garcia-Lafuente, A., Martinez, J. A. (2009). Sample preparation for the analysis of isoflavones from soybeans and soy foods. J. Chromatogr. A. 1216, 2–29. doi: 10.1016/j.chroma.2008.11.035
Routray, W., Orsat, V. (2012). Microwave-assisted extraction of flavonoids: a review. Food Bioproc. Tech. 5, 409–424. doi: 10.1007/s11947-011-0573-z
Routray, W., Orsat, V. (2014). MAE of phenolic compounds from blueberry leaves and comparison with other extraction methods. Ind. Crop Prod. 58, 36–45. doi: 10.1016/j.indcrop.2014.03.038
Sahu, R., Dhongade, H. J., Pandey, A., Sahu, P., Sahu, V., Patel, D., et al. (2016). Medicinal properties of nardostachys jatamansi (a review). Orient. J. Chem. 32, 859–866. doi: 10.13005/ojc/320211
Saroya, A. S., Singh, J. (2018). “Neuropharmacology of nardostachys jatamansi DC,” in Pharmacotherapeutic potential of natural products in neurological disorders (Singapore: Springer). Available at: doi.org/10.1007/978-981-13-0289-3_17.
Selkoe, D. J. (1994). Alzheimer's disease: A central role for amyloid. J Neuropathol Exp Neurol. 53(5), 438–447. doi: 10.1097/00005072-199409000-00003
Shang, X., Guo, X., Li, B., Pan, H., Zhang, J., Zhang, Y., et al. (2016). Microwave-assisted extraction of three bioactive alkaloids from peganum harmala l. and their acaricidal activity against psoroptes cuniculi in vitro. J. Ethnopharmacol. 92, 350–361. doi: 10.1016/j.jep.2016.07.057
Sharififar, F., Moshafi, M. H., Shafazand, E., Koohpayeh, A. (2012). Acetyl cholinesterase inhibitory antioxidant and cytotoxic activity of three dietary medicinal plants. Food Chem. 130, 20–23. doi: 10.1016/j.foodchem.2011.06.034
Sharma, S. K., Singh, A. P. (2012). In vitro antioxidant and free radical scavenging activity of nardostachys jatamansi DC. J. Acupunct. Meridian. Stud. 5, 112–118. doi: 10.1016/jjams201203002
Singh, T. G. (2020). Phytochemical activities and pharmacology of herbal drug: Nardostachys jatamansi. Plant Arch. 20, 3842–3848. doi: 10.1155/2021/5901653
Spigno, G., De Faveri, D. M. (2009). Microwave-assisted extraction of tea phenols: a phenomenological study. J. Food Eng. 93, 210–217. doi: 10.1016/j.jfoodeng.2009.01.006
Subashini, R., Yogeeta, S., Gnanapragasam, A., Devaki, T. (2007). Protective effect of nardostachys jatamansi on oxidative injury and cellular abnormalities during doxorubicin-induced cardiac damage in rats. J. Pharm. Pharmacol. 58, 257–262. doi: 10.1211/jpp5820014
Sutivisedsak, N., Cheng, H. N., Willett, J. L., Lesch, W. C., Tangsrud, R. R., Biswas, A. (2010). Microwave-assisted extraction of phenolics from bean (Phaseolus vulgaris l.). Food Res. Int. 43, 516e519. doi: 10.1016/j.foodres.2009.09.014
Teoh, E. S. (2016). “Secondary metabolites of plants,” in Medicinal orchids of Asia (Cham: Springer), 59–73. doi: 10.1007/978-3-319-24274-3_5
Thorat, R. M., Jadhav, V. M., Kadam, V. J. (2009). Development and evaluation of polyherbal formulations for hair growth-promoting activity. Int. J. Pharm. Tech. Res. 1, 1251–1254. doi: 10.1111/j.1473-2165.2007.00305.x
Vasudevan, M., Parle, M. (2006). Pharmacological actions of thespesia populnea relevant to alzheimer’s disease. Phytomedicine 13, 677–687. doi: 10.1016/j.phymed.2006.01.007
Ved, D. K., Goraya, G. S. (2007). Demand and supply of medicinal plants in India. NMPB New Delhi FRLHT Bangalore India 18, 210–252.
Velu, G., Palanichamy, V., Rajan, A. P. (2018). “Phytochemical and pharmacological importance of plant secondary metabolites in modern medicine,” in Bioorganic phase in natural food: an overview (Cham: Springer), 135–156. doi: 10.1007/978-3-319-74210-6_8
Wang, L., Li, D., Bao, C., You, J., Wang, Z., Shi, Y., et al. (2008). Ultrasonic extraction and separation of anthraquinones from rheum palmatum l. Ultrason. Sonochem. 15, 738–746. doi: 10.1016/j.ultsonch.2007.12.008
Wang, L., Weller, C. L. (2006). Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 17, 300–312. doi: 10.1016/j.tifs.2005.12.004
Wink, M. (2015). Modes of action of herbal medicines and plant secondary metabolites. Medicines 2, 251–286. doi: 10.3390/medicines2030251
Xiong, W., Chen, X., Lv, G., Hu, D., Zhao, J., Li, S. (2016). Optimization of microwave-assisted extraction of bioactive alkaloids from lotus plumule using response surface methodology. J. Pharm. Anal. 6, 382–388. doi: 10.1016/j.jpha.2016.05.007
Yan, M. M., Liu, W., Fu, Y. J., Zu, Y. G., Chen, C. Y., Luo, M. (2010). Optimization of the microwave-assisted extraction process for four main astragalosides in Radix astragali. Food Chem. 119, (2010)1663–1670. doi: 10.1016/j.foodchem.2009.09.021
Zhang, H. F., Yang, X. H. (2009). Problems and countermeasure for quality control of herba epimedii. Chin. Tradit. Herb. 40, 160e163.
Zhang, B., Yang, R., Liu, C. (2008). Microwave-assisted extraction of chlorogenic acid from flower buds of lonicera japonica thunb. Sep. Purif. Technol. 62, 480–483. doi: 10.1016/j.seppur.2008.02.013
Zhang, H. F., Yang, X. H., Wang, Y. (2011). Microwave assisted extraction of secondary metabolites from plants: Current status and future directions. Trends Food Sci. Technol. 22, 672–688. doi: 10.1016/j.tifs.2011.07.003
Keywords: Nardostachys jatamansi, antioxidant, scanning electron microscopy, microwave assisted extraction, gas chromatography, response surface methodology
Citation: Arya A, Chahal R, Almutairi MH, Kaushik D, Aleya L, Kamel M, Abdel-Daim MM and Mittal V (2022) Green approach for the recovery of secondary metabolites from the roots of Nardostachys Jatamansi (D. Don) DC using microwave radiations: Process optimization and anti-alzheimer evaluation. Front. Plant Sci. 13:987986. doi: 10.3389/fpls.2022.987986
Received: 06 July 2022; Accepted: 23 September 2022;
Published: 01 November 2022.
Edited by:
Maura Ferri, University of Bologna, ItalyReviewed by:
Kamel Msaada, Center of Biotechnology of Borj Cedria (CBBC), TunisiaÓscar Rodríguez, SL, Spain
Copyright © 2022 Arya, Chahal, Almutairi, Kaushik, Aleya, Kamel, Abdel-Daim and Mittal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vineet Mittal, ZHIudmluZWV0MTIzQHJlZGlmZm1haWwuY29t; Mohamed M. Abdel-Daim, YWJkZWxkYWltLm1AdmV0LnN1ZXouZWR1LmVn
 Ashwani Arya1
Ashwani Arya1 Mikhlid H. Almutairi
Mikhlid H. Almutairi Deepak Kaushik
Deepak Kaushik Lotfi Aleya
Lotfi Aleya Mohamed Kamel
Mohamed Kamel Mohamed M. Abdel-Daim
Mohamed M. Abdel-Daim Vineet Mittal
Vineet Mittal