- 1Institute of Biotechnology and Food Science, Hebei Academy of Agriculture and Forestry Sciences, Shijiazhuang, China
- 2Key Laboratory of Hebei Plant Genetic Engineering Center, Shijiazhuang, China
- 3Institute of Quality Standard and Testing Technology, Beijing Academy of Agriculture and Forestry Sciences, Beijing, China
Superficial scald is a serious physiological disorder in “Yali” pear (Pyrus bretschneideri Rehd. cv. Yali) after long-term cold storage. Changes in superficial scald, ethylene production, α-farnesene and phenylpropane metabolism with associated gene expression in “Yali” pear treated with and without (control) 1-methylcyclopropene (1-MCP) were investigated. Compared with the control group (without 1-MCP), 1-MCP (1.0 μl L–1) significantly lowered the superficial scald index after 180 days of cold storage. During cold storage and shelf life, the contents of α-farnesene, conjugated trienols, chlorogenic acid, and epicatechin in the peel were reduced, while quercetin was enhanced in 1-MCP-treated fruit, and the expression of genes associated with ethylene synthesis (ACS1, ACO1), receptors (ETR2, ERS1) and signal transduction (ERF1), α-farnesene metabolism (AFS1, HMGR2, GST7), phenolic biosynthesis (PAL1, C4H1, C4H2, HCT3, 4CL2, C3H), and oxidases (PPO1, PPO5, and LAC7) were significantly downregulated by 1-MCP. These results suggested that the onset and development of superficial scald was closely related to the ethylene receptor, conjugated trienols, chlorogenic acid and epicatechin and related genes expression in “Yali” pear.
Introduction
After long-term cold storage, pears appear to develop superficial scald, which is similar to that in apples. Previous investigation have suggested that this phenomenon is related to the metabolism of α-farnesene and especially the accumulation of conjugated trienols (Whitaker et al., 2000, Whitaker, 2007, 2013; Lurie and Watkins, 2012; Zhou et al., 2017), and reactive oxygen species (ROS; Marc et al., 2020) as well as phenolic acids and flavonoids (Piretti et al., 1996; Zhi and Dong, 2018; Cebulj et al., 2020). 3-Hydroxy-3-methylglutaryl-CoA reductase (HMGR) and α-farnesene synthase (AFS) are key synthesis enzymes (Lurie and Watkins, 2012), and the HMGR and AFS genes expression is associated with α-farnesene and scald development (Gapper et al., 2006; Zhou et al., 2017). Furthermore, glutathione peroxidase (GPX) and glutathione S-transferase (GST) are suggested to promote the oxidation of α-farnesene to conjugated trienols (Whitaker, 2013), and the GST gene is involved in the development of superficial scald of pear (Zhou et al., 2017; Wang et al., 2018).
The phenolic acids and flavonoids are belonged to phenylpropanoid metabolites (Lurie and Watkins, 2012; Cebulj et al., 2020). Phenolic compounds are substrates of phenolic oxidation catalyzed by polyphenol oxidase (PPO), which is associated with the development of the scald (Busatto et al., 2014; Gao et al., 2015; Feng et al., 2018; Niu et al., 2018). Recent studies suggested that epicatechin oxidation catalyzed by laccase (LAC) is closely related to the development of scald in apples and pears (Gong et al., 2018; Zhai et al., 2021). However, the mechanism of the phenylpropanoid metabolism involved in the scald development has been less studied.
As an ethylene action inhibitor, 1-methylcyclopropene (1-MCP) could significantly inhibit the development of superficial scald by controlling the metabolism of ethylene, α-farnesene, phenolic acids, and flavonoids in apples and pears (Watkins, 2006; Wang, 2016; Busatto et al., 2018; Zhi and Dong, 2018; Larrigaudière et al., 2019), and 1-MCP suppress the expression of AFS, HMGR, GPX, and GST (Gapper et al., 2006; Xie et al., 2014, 2016; Zhou et al., 2017; Busatto et al., 2018; Karagiannis et al., 2018, 2020; Honaas et al., 2019; Marc et al., 2020) and the accumulation of ROS in fruit peel (Sabban-Amin et al., 2011). However, the correlation of the synthesis and signaling of ethylene with the α-farnesene accumulation, phenolic acids, and flavonoids should be fully explained, and the mechanism of 1-MCP controlling superficial scald in pear needs to be clarified in depth.
“Yali” pear is a famous cultivar in China, which has a large cultivation area and yield. However, it may appear superficial scald after long-term cold storage (more than 6 months), which severely affects the quality of the fruit’s appearance. The main aim of the present study was to investigate the development of scald in “Yali” pear treated with 1-MCP after cold storage under ethylene inhibition condition, and try to explore the correlation of ethylene synthesis, receptors and signal transduction with α-farnesene and main phenolic compounds metabolism in peels during the development of superficial scald in order to further reveal the roles of the main target substances associated with the α-farnesene, phenylpropane metabolism and the related genes in the onset and development of scald in “Yali” pear.
Materials and methods
Materials
“Yali” pear (Pyrus bretschneideri Rehd. cv. Yali) trees were selected in a commercial orchard located in Jinzhou, Hebei Province, China (N: 38°01′20.35′′, E: 115°04′23.94′′). Fruits were hand-harvested at September 27, 2017 and transported to the laboratory within 2 h and then uniformly sized fruit (fruit weight 286.76 ± 34.41 g) without damage or fungal infection were selected. After being stored overnight at room temperature (25 ± 1°C), one portion of the fruit was exposed to a final concentration of 1.0 μL L–1 1-MCP (SmartFresh™, AgroFresh, Spring House, PA, United States) in an airtight container (60 L) with a circulation fan at 25°C for 24 h. Another portion of the fruit without 1-MCP was used as control group under the same condition. After treatment, pears were placed in paper boxes and stored in a fruit storage chamber (0 ± 0.5°C, RH = 90 ± 5%). After 60, 90, 120, 150, and 180 days of cold storage, pears were transferred to 20 ± 1°C for 1, 3, and 7 days shelf life experiment. Attributes such as fruit quality, superficial scald, ethylene production, and respiration rates were evaluated in triplicate, and ten fruit per replicate. Peel tissue was sampled and flash-frozen in liquid nitrogen (N2) and then stored at −80°C.
Superficial scald index
Superficial scald index was classified according to the method reported by Calvo et al. (2015), the proportion of peel browning to total peel area was divided into four grades: grade 0 indicated no browning; grade 1 indicated 0% < browning area ≤ 25%; grade 2 indicated 25% < browning area ≤ 50%; and grade 3 indicated a browning area >50%.
The calculation formula is as follows: superficial scald index = Σ (browning grade × number of fruit per grade)/(total number of fruit × the highest grade).
Fruit firmness and soluble solids content
Fruit firmness was determined using a digital fruit penetrometer (Model: GY-4, Top Instruments Co., Ltd., Hangzhou, China) at two equidistant points on the equatorial region with the skin removed. The firmness was expressed as N. Soluble solids content (SSC) was measured by a PAL-1 pocket digital refractometer (Atago Co., Ltd., Tokyo, Japan) for flesh juice squeezing from two equidistant points.
Respiration and ethylene production rates
Respiration and ethylene production rates were measured in triplicate, and ten fruits were sealed in a 6 L container at 20°C as one replicate. After the fruit sealed for 1 h, 10 ml of gas was withdrawn from the container and injected into an HFY-1a CO2 infrared analyzer (Kexi Instrument Co., Ltd., Jiangsu, China) to measure the content of CO2. Subsequently, the respiration rate was calculated and expressed as the release rate for CO2 (ng kg–1 s–1). To measure ethylene production rate, 1 ml of gas was withdrawn from the container after the fruit sealed for 3 h, and then injected into the gas chromatograph (Model: GC9790II, Fuli Instruments Technology Co., Ltd., Wenling, China) equipped with a GDX-502 column and a flame ionization detector (FID). The temperatures of the column, vaporization oven and FID were set as 78, 120, and 200°C, respectively. N2 was used as the carrier gas with a rate of 40 ml min–1. The ethylene production rate was expressed as ng kg–1 s–1.
Contents of α-farnesene and conjugated trienols
The contents of α-farnesene and conjugated trienols (CTols) were determined based on the method reported by Feng et al. (2018). A peel disk (1 cm in diameter) was formed along the equatorial part of the fruit, and 20 disks from each group were placed in a 25 ml graduated test tube. Afterward, 15 ml n-hexane was added and stored in the dark for 2 h. The extract was filtered by a clean Florisil SPE column, and the absorbance was recorded at 232, 281, and 290 nm. The concentration of α-farnesene and CTols were calculated using the molar extinction coefficients ε232 = 27740 for α-farnesene and ε281–290 = 25000 for the CTols, and the contents of α-farnesene and CTols expressed as nmol cm–2.
Phenolic acid extraction
Extraction of phenolic acids was carried out according to the procedure reported by Wang et al. (2017). After liquid nitrogen grinding, 1 g of freeze-dried peel powder was added with 10 ml of 80% methanol containing 0.5% hydrochloric acid solution. The mixture was then ultrasonicated for 30 min and centrifuged at 10,000 × g for 10 min at 4°C, and the supernatant was collected finally. The extraction was repeated for three times. All the supernatants were combined and evaporated at 50°C under a gentle flow of nitrogen until completely dry. The residue was redissolved in 5 ml of 50% (v/v) methanol/ultrapure water and filtered through a 0.22 μm PTFE filter (Pall, MI, United States).
Flavonoid extraction
Extraction of flavonoids was performed according to the method reported by Gao et al. (2019) with slight modifications. Two grams of peel powder mixing with 30 ml of 80% methanol was kept in the dark for 24 h at −20°C. Afterward, the mixture was centrifuged at 10,000 × g at 4°C for 10 min. Afterward the supernatant was filtered through a 0.22 μm PTFE filter (Pall, MI, United States) and collected for analysis immediately.
UPLC–MS/MS analysis
The contents of the phenolic acids and flavonoids were performed according to the method described by Gao et al. (2019). The extracts were identified via an Acquity UPLC system (Waters, Milford, MA, United States) with a triple quadrupole mass spectrometer (TQ-S, Waters Micromass, Manchester, United Kingdom). The column used was an Acquity HSS C18 column (1.8 μm particle size; 2.1 mm × 150 mm; Waters, Milford, MA, United States). The column and sample managers were maintained at 40 and 10°C, respectively. Mobile phase A consisted of 0.1% (v/v) formic acid in water and mobile phase B consisted of 0.1% (v/v) formic acid in acetonitrile. The gradient used was as follows: 0.5–4.5 min, 5–30% B; 4.5–9.0 min, 30–90%; 9.0–10.0 min, 0.5% B. The mass spectrometer was operated in both positive and negative ionization modes depending on the structure and properties of compounds. The parameters were set as follows: capillary voltage, +2.5 kV/−1.0 kV; source temperature, 150°C; desolvation temperature, 500°C; cone gas flow, 150 L h–1; and desolvation gas flow, 1000 L h–1. Detection was conducted in multiple reaction monitoring (MRM) mode. The MS data were collected and analyzed by MassLynx™ 4.1 software (Waters, Milford, MA, United States). Quantification of the phenolic acids and flavonoids were performed using the standard curves with authentic standards in serial dilutions (1–500 ng mL–1).
RNA isolation and real-time quantitative polymerase chain reaction analysis
After liquid nitrogen grinding, 100 mg peel powder was used for total RNA isolation by the RNA-prep Pure Plant Plus Kit (polysaccharides and polyphenolics-rich) (Tiangen Biotech Co., Ltd., Beijing, China). A total of 500 ng of RNA after elimination genomic DNA was used to generate the first strand cDNA via reverse transcription using a PrimeScript RT Reagent Kit with gDNA Eraser (Perfect Real Time) (TaKaRa Bio Inc., Dalian, China).
Real-time quantitative polymerase chain reaction analysis was performed on a Real-time System (ABI7500, Applied Biosystems, United States) using a TB Green™ Premix ex Taq II quantitative PCR kit (TaKaRa Bio Inc., Dalian, China). The sequences and references of primers for qRT–PCR are shown in Supplementary Table 1 (Fischer et al., 2007; Jugdé et al., 2008; Qian et al., 2014; Cheng et al., 2015; He et al., 2017; Gong et al., 2018; Li et al., 2018; Zhou et al., 2020; Zhai et al., 2021). The relative gene expression amount was calculated with the formula 2–ΔΔCt in Microsoft Excel 2007 (Livak and Schmittgen, 2001) by using PbACTIN2 as the internal reference gene (Cheng et al., 2015).
Statistical analysis
Duncan’s multiple comparison test was used to compare the significant differences between control and 1-MCP treatment (P < 0.05). Statistical analysis was performed via SPSS Statistics 23 (IBM Co., Armonk, NY, United States). Figures were generated by GraphPad Prism 8.0 (GraphPad Software, San Diego, CA, United States), heatmaps with clusters and PCA loading plots were created by Origin 9.0 (OriginLab Co., Northampton, MA, United States).
Results
Fruit quality and superficial scald index
Firmness and SSC were the main quality indicators, in which the firmness indicates the degree of fruit softening. During long-term cold storage, the firmness of the control fruit decreased, and the SSC increased. There was no obvious difference in the firmness and SSC between control and 1-MCP treatment fruit, except a higher firmness at Day 120 and a lower SSC at Day 150 in the 1-MCP treatment pears (Figures 1A,B). The superficial scald appeared after 180 days of cold storage, however, the scald index increased significantly in the control group during shelf life (Figures 1C,D). Nevertheless, the scald index was significantly reduced by 1-MCP and maintained at a much lower level (Figure 1C). 1-MCP had no significant effect on fruit firmness and SSC during cold storage and shelf life.
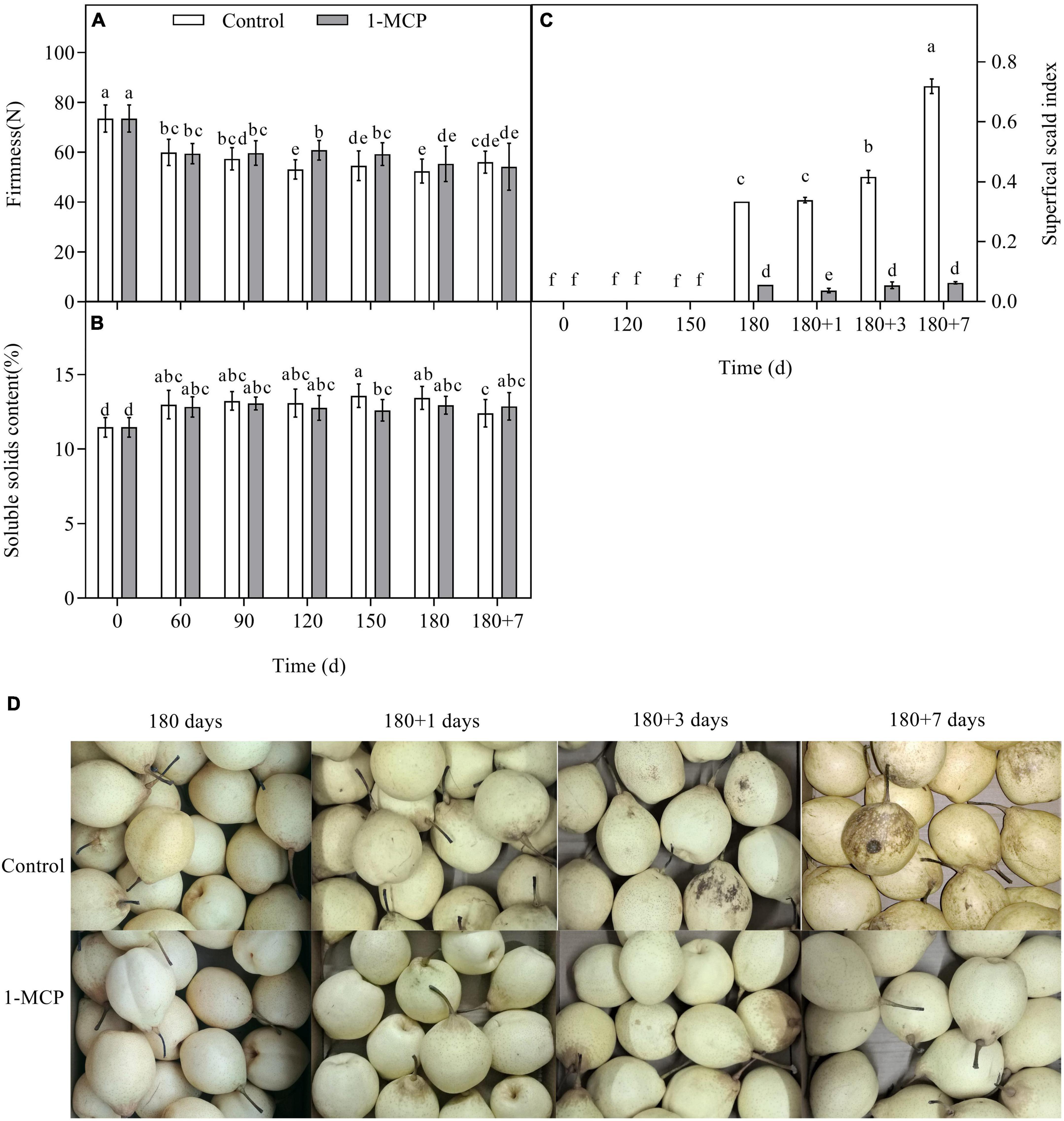
Figure 1. Effect of 1-MCP on firmness (A), SSC (B), superficial scald index (C), and symptom (D) in “Yali” pear during cold storage and shelf life. 180 + 1, 180 + 3, 180 + 7 indicated Days 1, 3, 7 at shelf life after 180 days of cold storage, respectively. All data are expressed as means ± standard errors of triplicate samples. Different letters indicate significant differences (P < 0.05) by Duncan’s multiple comparison test.
Respiration and ethylene production rates
The respiration and ethylene production rates during cold storage were lower than their initial values, but increased markedly when transferred to shelf life. 1-MCP had no significant impact on the respiration rate during cold storage, however, it dramatically reduced the respiration rate during shelf life (Figure 2A). In comparison with control, 1-MCP apparently reduced the ethylene production rate during cold storage and shelf life (Figure 2B).

Figure 2. Effect of 1-MCP on respiration (A) and ethylene production (B) of “Yali” pear during cold storage and shelf life. 180 + 1, 180 + 3, 180 + 7 indicated Days 1, 3, 7 at shelf life after 180 days of cold storage, respectively. All data are expressed as means ± standard errors of triplicate samples. Different letters indicate significant differences (P < 0.05) by Duncan’s multiple comparison test.
Expression of ethylene biosynthesis, receptor and response factor genes
During cold storage, the expression of ethylene synthesis genes (PbACS1, PbACO1), ethylene receptors (PbETR2, PbERS1) increased significantly, while ethylene response factor (PbERF1) increased initially and then decreased in control. During shelf life, the expression of PbACS1, PbACO1, PbETR2, and PbERS1 decreased to some extent, but that of PbERF1 increased slightly. Cluster analysis showed that the gene expression patterns of PbACS1, ethylene receptors PbETR2 and PbERS1 were similar and that the downstream signals PbERF1 were the same (Figure 3).
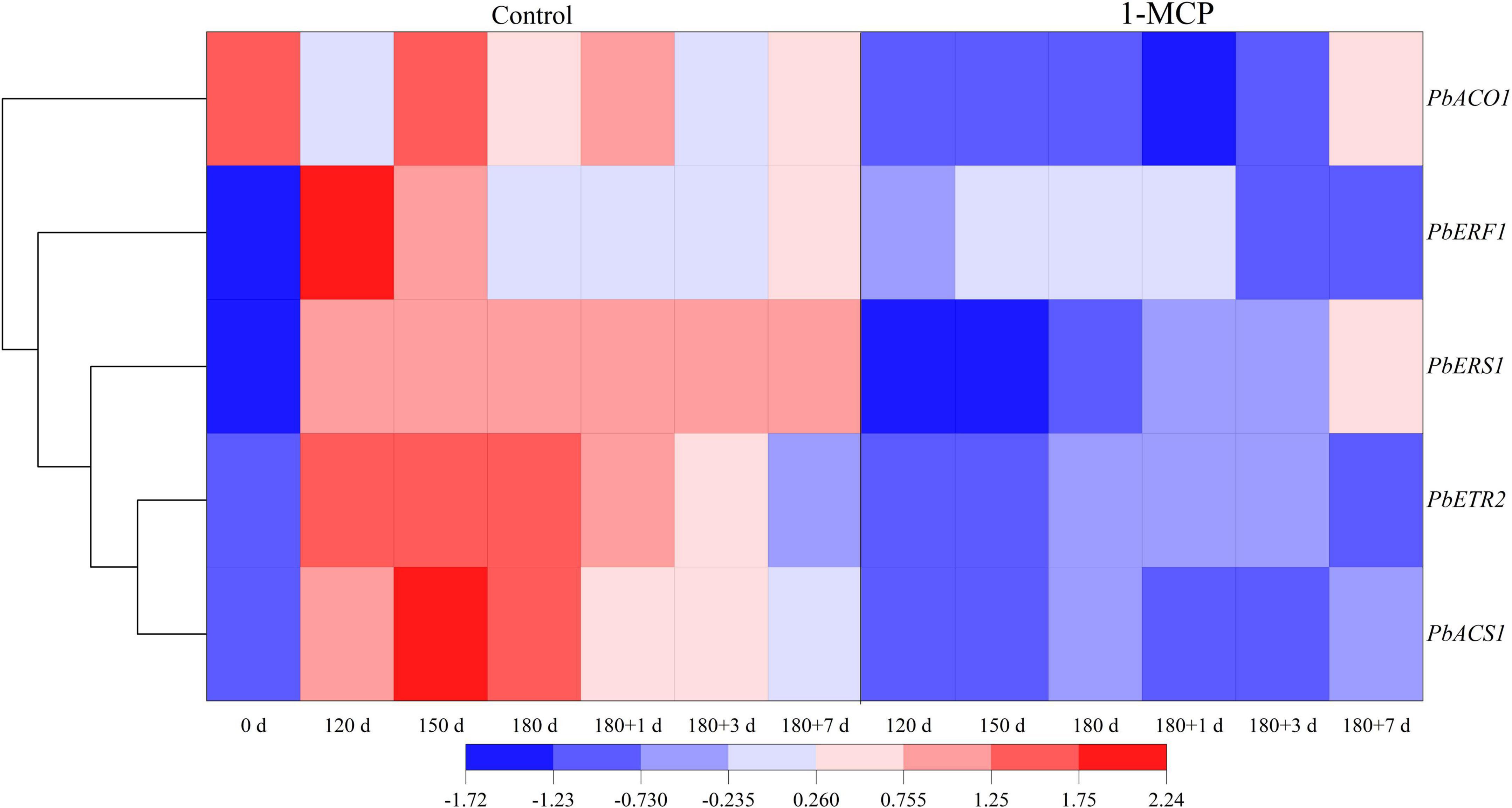
Figure 3. Effect of 1-MCP on the expression of ethylene synthesis genes (PbACS1, PbACO1), receptor genes (PbETR2, PbERS1), and ethylene response factor genes (PbERF1) of the peels in “Yali” pear during cold storage and shelf life. 180 + 1, 180 + 3, 180 + 7 indicated Days 1, 3, 7 at shelf life after 180 days of cold storage, respectively.
Contents of α-farnesene, conjugated trienols and the related gene expression
The contents of α-farnesene and conjugated trienols in the fruit peel of control group reached a peak after 150 days of cold storage and then decreased. After 7 days of shelf life, the content of α-farnesene increased, but conjugated trienols decreased significantly. The trends of α-farnesene and conjugated trienols were similar between the 1-MCP treatment and the control group, while the contents of α-farnesene and conjugated trienols were markedly reduced for 1-MCP treatment group (Figures 4A,B).
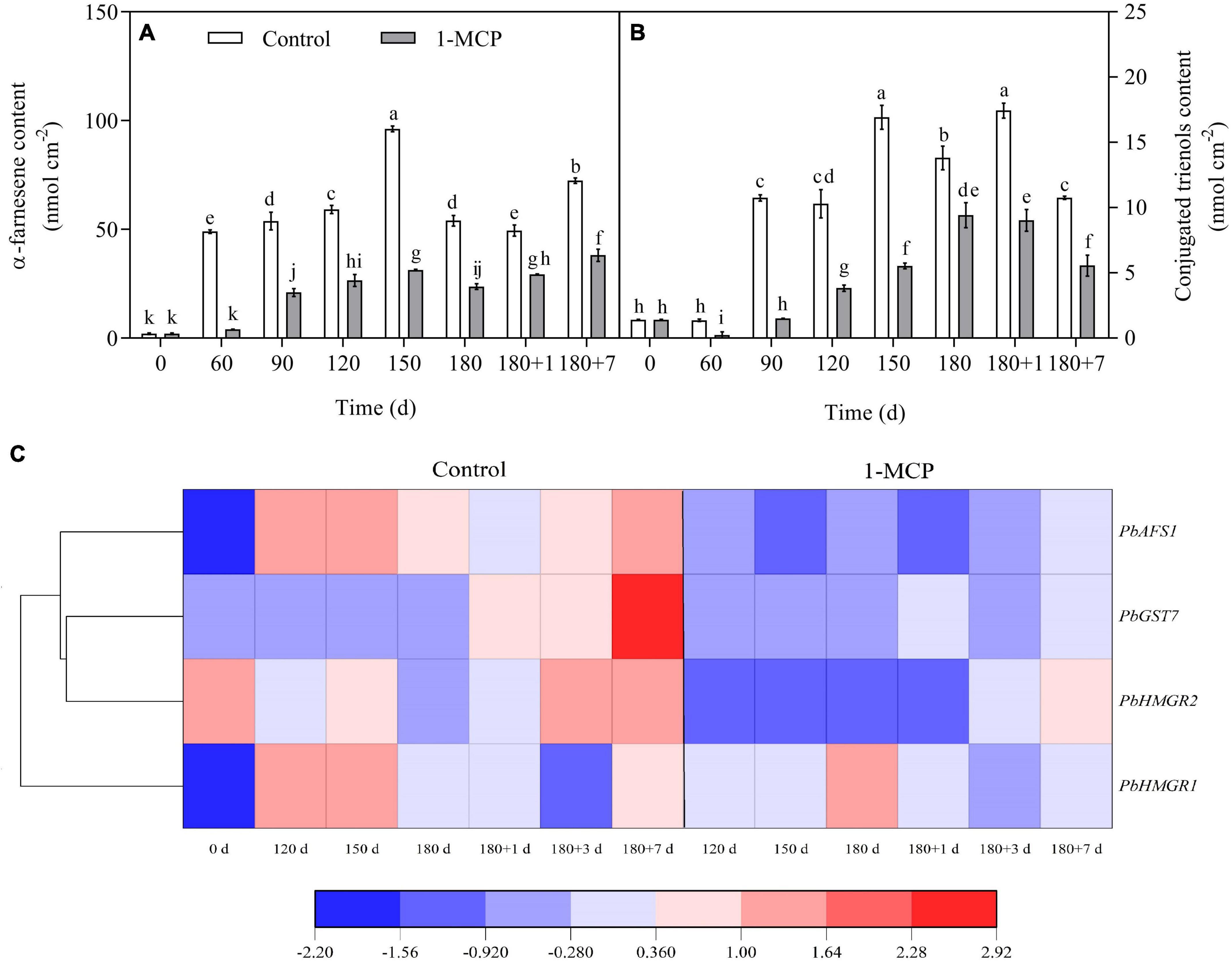
Figure 4. Effect of 1-MCP on α-farnesene (A) and conjugated trienols (B) contents and related gene expression (C) of the peels in “Yali” pear. 180 + 1, 180 + 7 indicated Days 1, 7 at shelf life after 180 days of cold storage, respectively. All data are expressed as means ± standard errors of triplicate samples. Different letters indicate significant differences (P < 0.05) by Duncan’s multiple comparison test.
During cold storage, the expression of PbHMGR1 increased initially, then decreased at Day 180, and lastly increased at the end of shelf life. The expression amount of PbHMGR1 was significantly upregulated by 1-MCP at Day 180 but decreased at other stages. The expression level of PbHMGR2 showed clear variation during cold storage and decreased by 1-MCP. The expression of PbGST7 changed slightly during cold storage, and was markedly upregulated during shelf life, and was significantly decreased by 1-MCP. The expression of PbAFS1 decreased in the later stage of cold storage but then increased during shelf life, moreover, it was significantly inhibited by 1-MCP (Figure 4C).
Contents of phenolic acids and flavonoids and the related synthesis genes expression
The results showed that phenolic acids of the peel mainly included chlorogenic acid, arbutin, and neochlorogenic acid. Flavonoids mainly included epicatechin, quercetin and proanthocyanidin B2. During cold storage stage, the phenolic acid content in the peels increased gradually, reached to the peak on the first day of shelf life, and then decreased. Treatment with 1-MCP significantly reduced the content of chlorogenic acid at Day 150, 180, and 180 + 1. Similarly, the content of epicatechin was significantly reduced by 1-MCP at Day 120, 150, 180, and 180 + 1. Unlike the above changes, the content of quercetin maintained at a higher level for the group treated with 1-MCP. The changes of other phenolic acids (e.g., arbutin) and flavonoids (e.g., kaempferol-3-o-glucoside) had fewer or no significant differences between the two groups (Table 1). During cold storage stage, except for PbPAL2, the expression levels of other genes (PbPAL1, PbC4H1, PbC4H2, PbHCT3, Pb4CL2, PbC3H, PbHCT1) were enhanced to different degrees, in which PbPAL1 showed the greatest variation. At shelf life, the expression of PbC4H1, PbC4H2, and PbHCT3 increased significantly, but was downregulated by 1-MCP. In contrast, the expression of the PbHCT1 gene was inhibited by 1-MCP during cold storage but promoted at Day 180 and 180 + 1 (Figure 5).
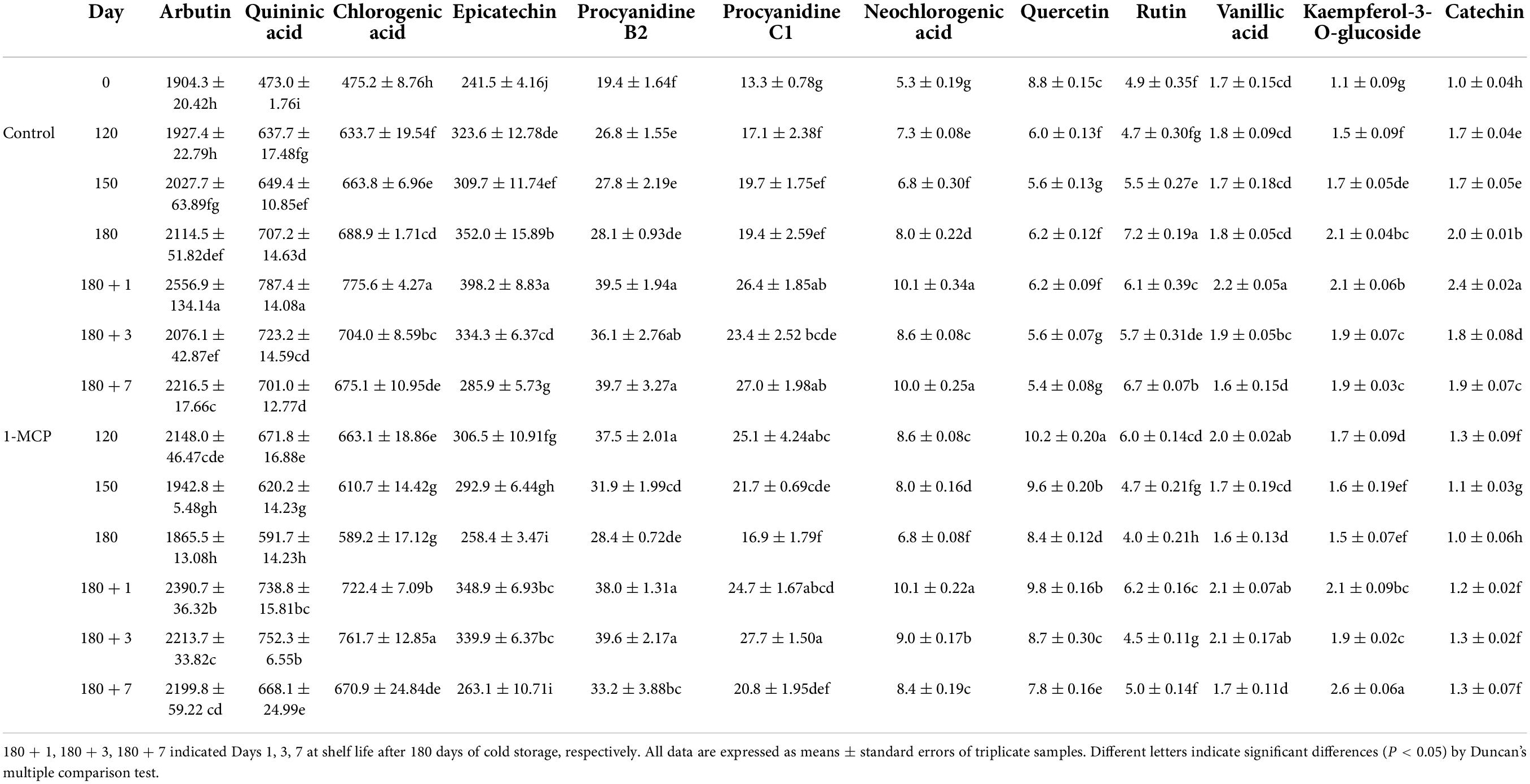
Table 1. Effect of 1-MCP on contents of phenolic acids and flavonoids of the peels in “Yali” pear (mg kg–1).
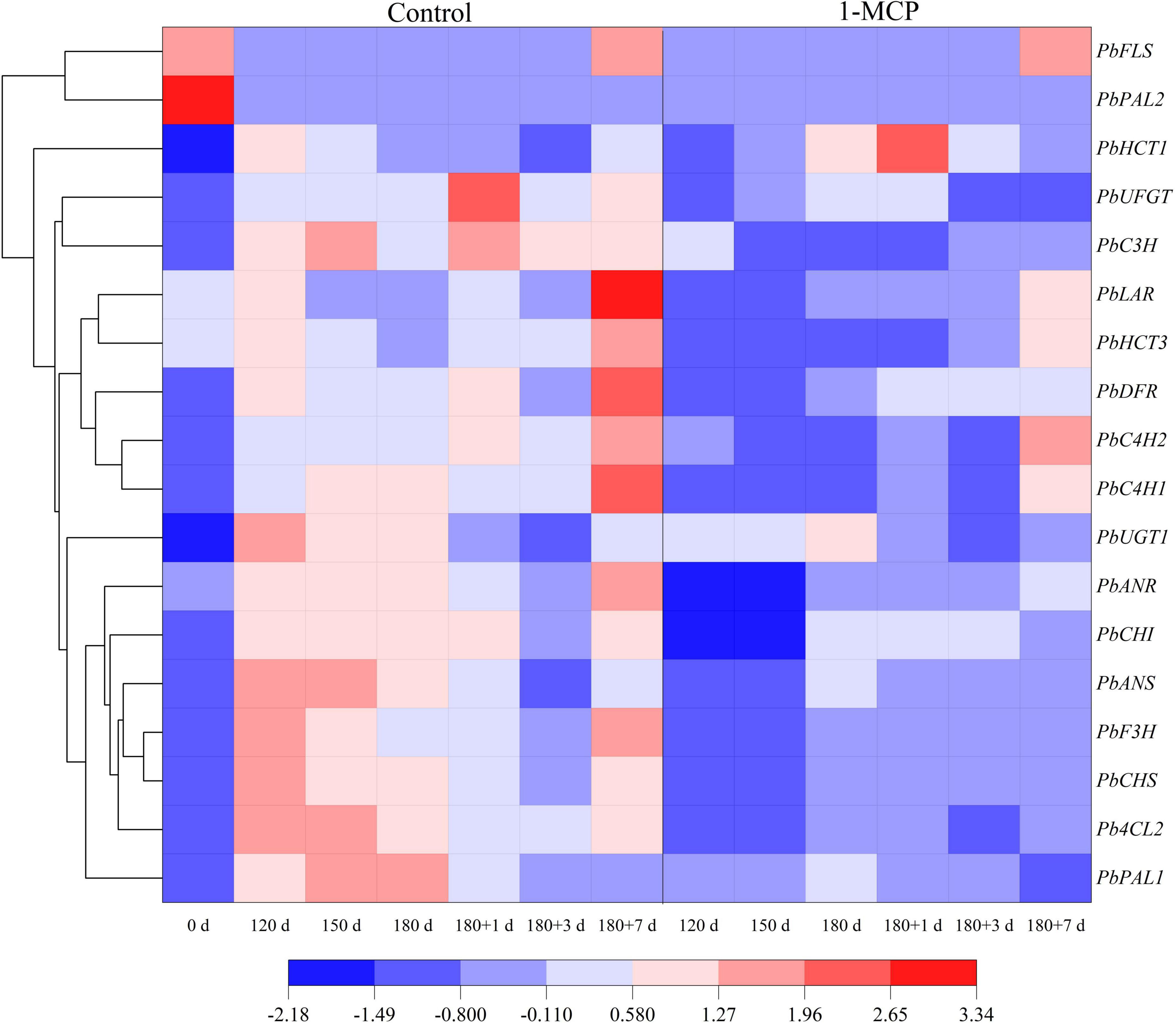
Figure 5. Effect of 1-MCP on phenolic acid and flavonoid synthesis-related genes expression of the peels in “Yali” pear. 180 + 1, 180 + 3, and 180 + 7 indicated Days 1, 3, and 7 at shelf life after 180 days of cold storage, respectively.
The expression levels of PbCHS, PbUGT1, PbCHI, PbF3H, PbANS, and PbANR were initially increased and then decreased during cold storage and at the end of shelf life, while those of PbLAR, PbFLS, PbDFR, and PbUFGT were increased to different degrees during cold storage and then decreased, but showed a huge increase at the end of shelf life (Day 180 + 7), especially PbLAR. And obviously, 1-MCP displayed effective inhibition to the expression of those genes (Figure 5). Nevertheless, the expression level of PbFLS was significantly enhanced by 1-MCP, while other genes were inhibited (Figure 5).
Expression of PbPPOs and PbLACs
During cold storage, the expression of PbPPO1 and PbPPO5 was significantly enhanced initially and then decreased until the end of shelf life. Differently, the expression levels of PbLAC7 and PbLAC15 did not show obvious changes, and even decreased to some extent during cold storage, but they were enhanced to different degrees during shelf life in the control group. However, it was observed that the expression levels of PbPPO1, PbPPO5, PbLAC7, and PbLAC15 of 1-MCP-treated fruit were significantly lower than those in the control group at the same stage (Figure 6).
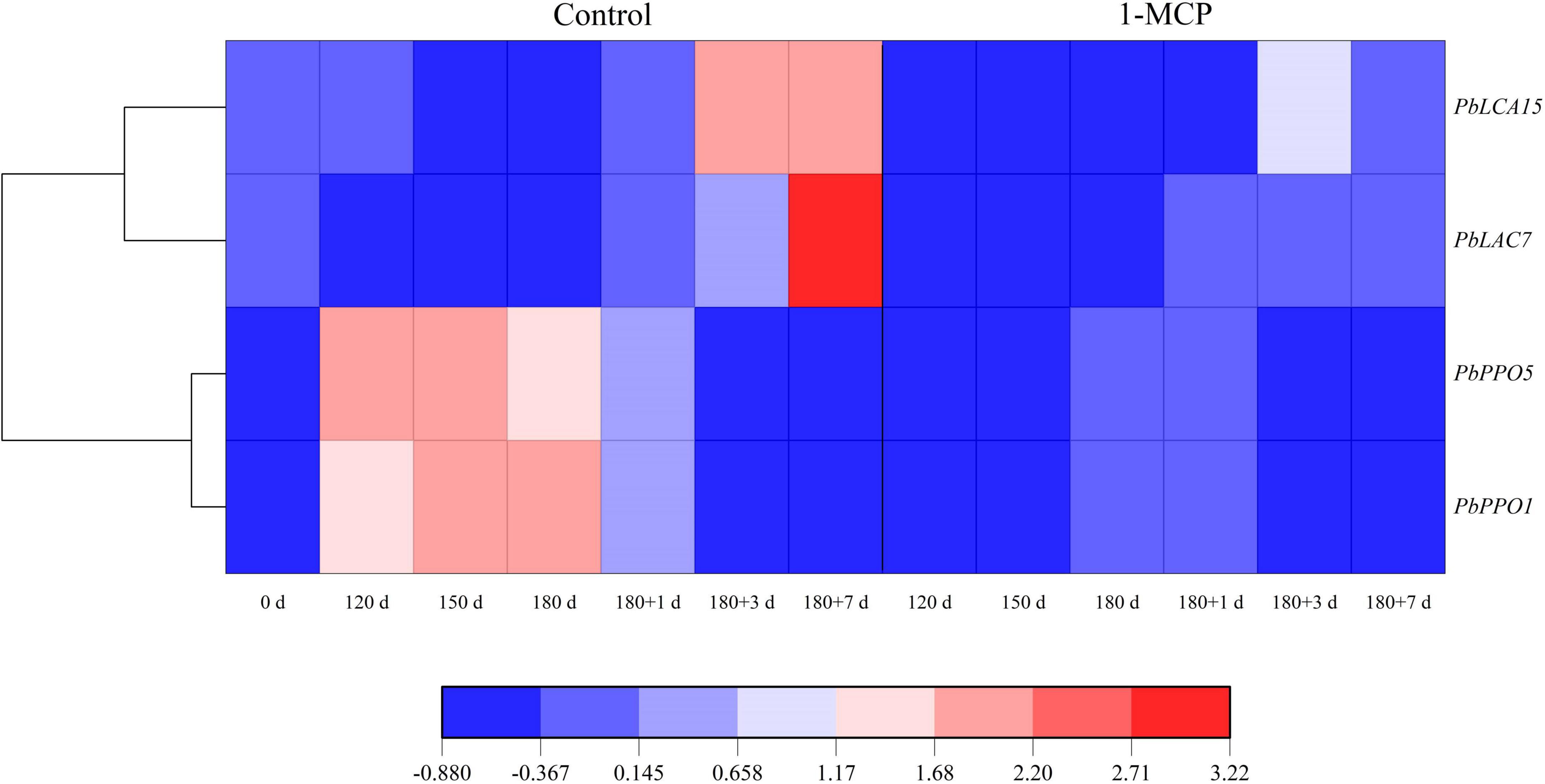
Figure 6. Effect of 1-MCP on the expression profiles of PbPPOs and PbLACs in the peels of “Yali” pear. 180 + 1, 180 + 3, and 180 + 7 indicated Days 1, 3, and 7 at shelf life after 180 days of cold storage, respectively.
Correlation and principal component analysis
The correlation analysis (Supplementary Figure 1) reveals that the superficial scald index had a significantly positive correlation with the contents of arbutin (r = 0.536*), quininic acid (r = 0.621*), chlorogenic acid (r = 0.650*), procyanidine B2 (r = 0.614*), neochlorogenic acid (r = 0.582*), kaempferol-3- o-glucoside (r = 0.708**), catechin (r = 0.552*), and conjugated trienols (r = 0.589*), and also with the expression levels of PbERS1 (r = 0.627*), PbGST7 (r = 0.820**), PbC4H2 (r = 0.717**), and PbUFGT (r = 0.650), but had a significantly negative correlation with PbPAL2 (r = −0.625*).
Principal component analysis indicated that the total variability was explained by the first two principal components (PCs) with PC1 and PC2 accounting for 45.4 and 17.8% of the variation, respectively. It was shown that the contents of α-farnesene, CTols and catechin, and the expression levels of PbERF1, PbC3H, PbERS1, PbDFR, and PbCHI were clustered in the same interval with superficial scald index. The content of rutin, and the expression levels of PbUGT1, PbUFGT, PbHMGR, PbAFS1, PbETR2, and PbC4H2, also included the expression levels of PbLAC7, PbPAL1, PbPPO1, PbPPO5, PbF3H, PbACS1, PbANS, Pb4CL2, PbCHS, and PbC4H1 were scatted in a cluster, this further confirmed the contribution of above-mentioned genes to the development of superficial scald (Figure 7).
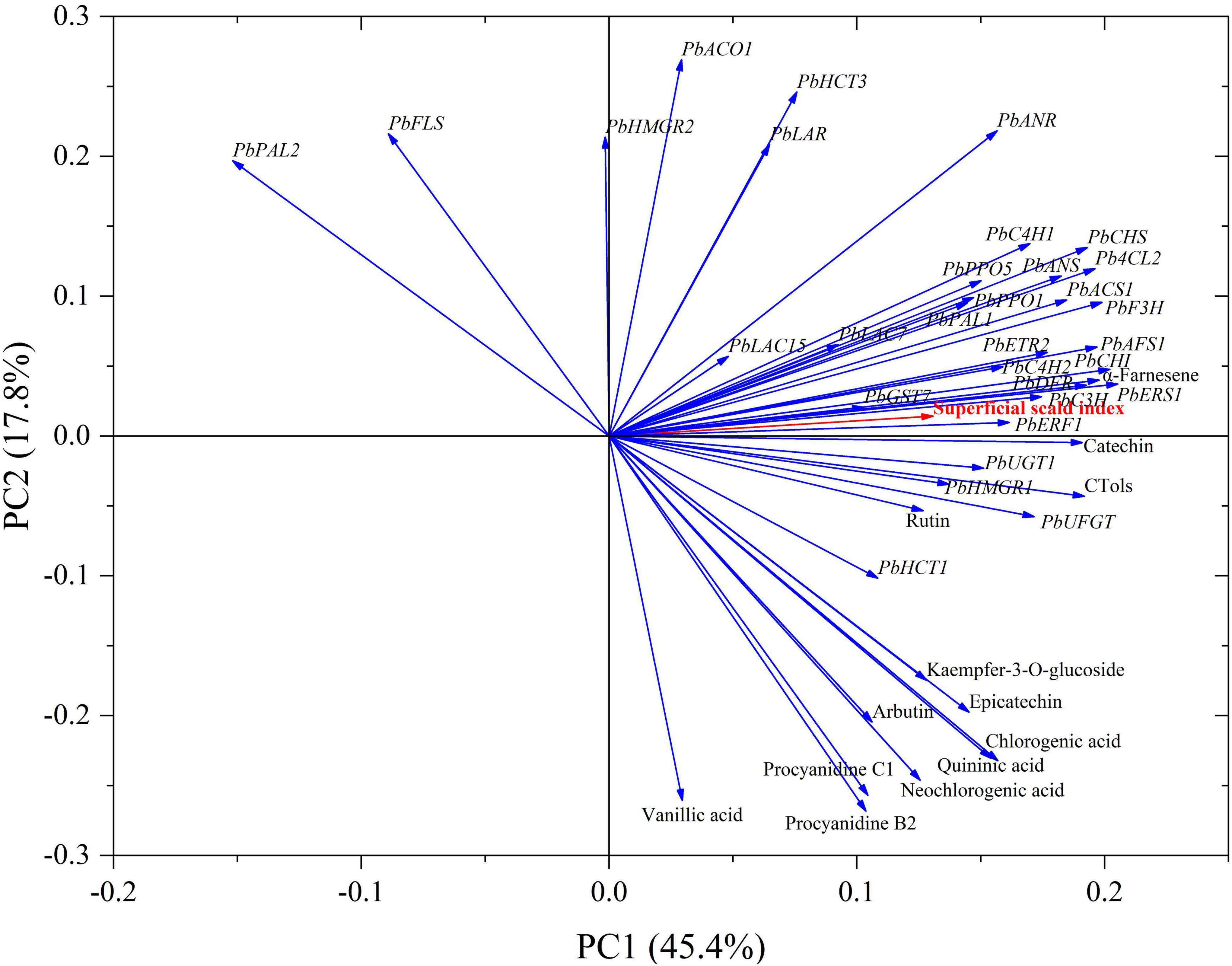
Figure 7. Loading plot of principal component analysis (PCA) of scald index, phenolics contents, and the related genes expression levels in the peels of the control group and 1-MCP-treated “Yali” pears. Arrows indicated the different variables.
Discussion
1-Methylcyclopropene did not clearly affect the firmness and SSC of “Yali” pear (Figures 1A,B), which may be closely related to the slower softening characteristic of “Yali” pear (Wei et al., 2015). The ethylene production rate of the “Yali” pear was lower during cold storage and increased when they were transferred to shelf life. 1-MCP significantly reduced the respiration rate and ethylene production rate (Figure 2) and lowered the expression of the ethylene synthesis genes (PbACS1, PbACO1), receptor and signal transduction genes (such as PbETR2, PbERS1, and PbERF1) (Figure 3). The result is in line with previous studies (El-Sharkawy et al., 2003; Xie et al., 2016, 2017; Zhou et al., 2017), therefore, it was further demonstrated that 1-MCP could inhibit the expression of ethylene signal related genes in “Yali” pear. The correlation coefficient and loading plot (Figure 7 and Supplementary Figure 1) further confirmed that the superficial scald index had a significantly positive correlation with the expression level of PbERS1. Thus, the ethylene receptor PbERS1 plays an essential role in the development of superficial scald.
The metabolism of α-farnesene is involved in the development of superficial scald, especially due to the accumulation of conjugated trienols (Lurie and Watkins, 2012; Ding et al., 2019). In the present study, we have showed that 1-MCP could significantly repress the accumulation of α-farnesene and conjugated trienols in the peels (Figures 4A,B). The scald showed serious symptoms after the reaching of peak level for α-farnesene and conjugated trienols (Figures 1C,D, 4A,B), which indicated that the accumulation of α-farnesene and conjugated trienols after long-term cold storage (180 days) was closely related to the onset of the scald. The HMGR and AFS1 genes were strongly associated with the α-farnesene and development of scald (Zhou et al., 2017). Thus, the variation in α-farnesene was consistent with the expression of PbHMGR and PbAFS1 in peels. Additionally, GST and GPX play a role in oxidative damage and cell senescence (Chen et al., 2004; Gill and Tuteja, 2010; Hui et al., 2016). The present study further revealed that the expression of PbGST7 was enhanced with the development of scald in the “Yali” pear, which might affect the change of the content of conjugated trienol. In addition, the scald index was significantly correlated with the expression level of PbGST7 (r = 0.820, Supplementary Figure 1). The results indicate that PbHMGR, PbAFS, and PbGST are closely related to the development of scald. According to the PCA results (Figure 7), the contents of α-farnesene, conjugated trienols and the expression levels of PbERF1, PbERS1 were clustered together, suggesting that ethylene had a regulatory effect on the accumulation of α-farnesene and conjugated trienols.
Phenolics in cells are generally recognized to be oxidized to quinones and cause tissue browning. Phenolic oxidation catalyzed by PPO contributed to the development of superficial scald (Busatto et al., 2014; Niu et al., 2018; Lindo-García et al., 2020). It has been reported that the accumulation of chlorogenic acid and epicatechin was closely related to the development of the superficial scald (Busatto et al., 2014; Gong et al., 2018; Cebulj et al., 2020). This study further demonstrated that the development of the scald in “Yali” pear was accompanied by the accumulation of chlorogenic acid and epicatechin (Table 1). Additionally, the development of the scald was accompanied by the increase in expression levels of PbPPO1, PbPPO5, PbLAC7, and PbLAC15, in which were lowered by 1-MCP (Figure 6). In addition, the expression of PbETR2 and PbACS1 was clustered in a same interval with PbLAC7, PbPPO1, and PbPPO5 (Figure 7), indicating that the expression of PbETR2 and PbACS1 was closely related to that of PbLAC7, PbPPO1, and PbPPO5, and therefore, the enzymatic reaction catalyzed by PPO and LAC was reduced and the occurrence of the scald was inhibited subsequently through ethylene inhibition by 1-MCP.
Previous studies have shown that 1-MCP could restrain the expression of PAL and C3H, and reduce the occurrence of superficial scald (Busatto et al., 2014, 2018; Du et al., 2017), which are in consistent with our results (Figures 1C, 4). C3H and ANR are the key enzymes that promote the synthesis of chlorogenic acid and epicatechin, respectively (Henry-Kirk et al., 2012). 1-MCP markedly reduced the transcription of PbC3H and PbANR (Figure 5), which might result in the lower level of chlorogenic acid and epicatechin in peels (Table 1). We also noticed that treatment by 1-MCP enhanced the content of quercetin (Table 1), since FLS is the key enzyme for quercetin synthesis (Henry-Kirk et al., 2012). Therefore, the upregulation of the expression of PbFLS by 1-MCP may be an important factor in accelerating the synthesis of quercetin. In contrast, the content of flavonoids decreased at the onset and development of scald in apples (Cebulj et al., 2020). This further indicated that quercetin might play a role at the beginning of scald, which should be fully studied later.
Conclusion
In conclusion, superficial scald was found after 180 days of cold storage, and the symptoms became serious at shelf life, which was accompanied by higher respiration and ethylene production rates, in combination with the accumulation of conjugated trienols, chlorogenic acid and epicatechin in the peels of “Yali” pear after a long-term of cold storage. 1-MCP significantly decreased superficial scald index with the lower contents of α-farnesene, conjugated trienols, chlorogenic acid, epicatechin, catechin and rutin in the peels, and meanwhile reduced the expression levels of genes associated with ethylene biosynthesis (ACS1, ACO1), receptors and signal transduction (ETR2, ERS1, ERF1), α-farnesene metabolism (AFS1, HMGR2, GST7), and phenolic synthesis (PAL1, C4H1, C4H2, HCT3, 4CL2, C3H) as well as PPO1, PPO5, and LAC7, except that increased the expression of FLS gene. In addition, PPO1 and PPO5 were associated with the onset of superficial scald, and the LAC7 gene was closely related to the development of superficial scald in “Yali” pear.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
JG, JH, and YC conceived the work. JH, YF, YC, and MW conducted the experiments. JH, YF, YC, MW, and JG contributed to analyses and interpretation. JH, MW, and JG wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the HAAFS Agriculture Science and Technology Innovation Project (2019-2-1).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2022.987240/full#supplementary-material
References
Busatto, N., Farneti, B., Commisso, M., Bianconi, M., Iadarola, B., Zago, E., et al. (2018). Apple fruit superficial scald resistance mediated by ethylene inhibition is associated with diverse metabolic processes. Plant J. 93, 270–285. doi: 10.1111/tpj.13774
Busatto, N., Farneti, B., Tadiello, A., Vrhovsek, U., Cappellin, L., Biasioli, F., et al. (2014). Target metabolite and gene transcription profiling during the development of superficial scald in apple (malus × domestica borkh). BMC Plant Biol. 14:193. doi: 10.1186/s12870-014-0193-7
Calvo, G., Candan, A. P., Civello, M., Giné-Bordonaba, J., and Larrigaudière, C. (2015). An insight into the role of fruit maturity at harvest on superficial scald development in ‘BeurréD’Anjou’ pear. Sci. Hortic. 192, 173–179. doi: 10.1016/j.scienta.2015.05.032
Cebulj, A., Halbwirth, H., Mikulic-Petkovsek, M., Veberic, R., and Slatnar, A. (2020). The impact of scald development on phenylpropanoid metabolism based on phenol content, enzyme activity, and gene expression analysis. Hortic. Environ. Biotechnol. 61, 849–858. doi: 10.1007/s13580-020-00268-0
Chen, S., Vaghchhipawala, Z., Li, W., Asard, H., and Dickman, M. B. (2004). Tomato phospholipid hydroperoxide glutathione peroxidase inhibits cell death induced by bax and oxidative stresses in yeast and plants. Plant Physiol. 135, 1630–1641. doi: 10.1104/pp.103.038091
Cheng, Y., Liu, L., Zhao, G., Shen, C., Yan, H., Guan, J., et al. (2015). The effects of modified atmosphere packaging on core browning and the expression patterns of PPO and PAL genes in ‘Yali’ pears during cold storage. LWT Food Sci. Technol. 60, 1243–1248. doi: 10.1016/j.lwt.2014.09.005
Ding, R., Du, B., and Zhang, Y. (2019). Conjugated trienols and programmed cell death are more closely related to superficial scald than reactive oxygen species in apple fruit stored at low temperature. Sci. Hortic. 246, 597–603. doi: 10.1016/j.scienta.2018.11.053
Du, L., Song, J., Palmer, L. C., Fillmore, S., and Zhang, Z. Q. (2017). Quantitative proteomic changes in development of superficial scald disorder and its response to diphenylamine and 1-MCP treatments in apple fruit. Postharvest Biol. Technol. 123, 33–50. doi: 10.1016/j.postharvbio.2016.08.005
El-Sharkawy, I., Jones, B., Li, Z. G., LelieÁvre, J. M., Pech, J. C., and Latche, A. (2003). Isolation and characterization of four ethylene perception elements and their expression during ripening in pears (Pyrus communis L.) with/without cold requirement. J. Exp. Bot. 54, 1615–1625. doi: 10.1093/jxb/erg158
Feng, Y., Cheng, Y., He, J., Li, L., and Guan, J. (2018). Effects of 1-methylcyclopropene and modified atmosphere packaging on fruit quality and superficial scald in Yali pears during storage. J. Int. Agric. 17, 1667–1675. doi: 10.1016/S2095-3119(18)61940-9
Fischer, T. C., Gosch, C., Pfeiffer, J., Halbwirth, H., Halle, C., Stich, K., et al. (2007). Flavonoid genes of pear (Pyrus communis). Trees 21, 521–529.
Gao, M., Zhou, S., Guan, J., and Zhang, Y. (2015). Effects of 1-methylcyclopropene on superficial scald and related metabolism in ‘Wujiuxiang’ pears during cold storage. J. Appl. Bot. Food Quality 88, 102–108.
Gao, Y., Wang, M., Jiang, N., Wang, Y., and Feng, X. (2019). Use of ultra-performance liquid chromatography-tandem mass spectrometry on sweet cherries to determine phenolic compounds in peel and flesh. J. Sci. Food Agric. 99, 3555–3562. doi: 10.1002/jsfa.9576
Gapper, N. E., Bai, J., and Whitaker, B. D. (2006). Inhibition of ethylene-induced α-farnesene synthase gene PcAFS1 expression in ‘d’Anjou’ pears with 1-MCP reduces synthesis and oxidation of α-farnesene and delays development of superficial scald. Postharvest Biol. Technol. 41, 225–233. doi: 10.1016/j.postharvbio.2006.04.014
Gill, S. S., and Tuteja, N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 48, 909–930. doi: 10.1016/j.plaphy.2010.08.016
Gong, Y., Jun, S., Lina, D., Mindy, V., Leslie, P., and Zhang, Z. (2018). Characterization of laccase from apple fruit during postharvest storage and its response to diphenylamine and 1-methylcyclopropene treatments. Food Chem. 253, 314–321. doi: 10.1016/j.foodchem.2018.01.142
Henry-Kirk, R. A., Mcghie, T. K., Andre, C. M., Hellens, R. P., and Allan, A. C. (2012). Transcriptional analysis of apple fruit proanthocyanidin biosynthesis. J. Exp. Bot. 63, 5437–5450.
He, J., Cheng, Y., Guan, J., Ge, W., and Zhao, Z. (2017). Changes of chlorogenic acid content and its synthesis-associated genes expression in Xuehua pear fruit during development. J. Int. Agric. 16, 471–477. doi: 10.1016/S2095-3119(16)61496-X
Honaas, L. A., Hargarten, H. L., Ficklin, S. F., Hadish, J. A., Wafula, E., de Pamphilis, C. W., et al. (2019). Co-expression networks provide insights into molecular mechanisms of postharvest temperature modulation of apple fruit to reduce superficial scald. Postharvest Biol. Technol. 149, 27–41. doi: 10.1016/j.postharvbio.2018.09.016
Hui, W., Niu, J., Xu, X., and Guan, J. (2016). Evidence supporting the involvement of MHO in the formation of superficial scald in ‘Dangshansuli’ pears. Postharvest Biol. Technol. 121, 43–50. doi: 10.1016/j.postharvbio.2016.07.005
Jugdé, H., Nguy, D., Moller, I., Cooney, J. M., and Atkinson, R. G. (2008). Isolation and characterization of a novel glycosyltransferase that converts phloretin to phlorizin, a potent antioxidant in apple. FEBS J. 275, 3804–3814. doi: 10.1111/j.1742-4658.2008.06526.x
Karagiannis, E. E., Tanou, G. G., Scossa, F. F., Samiotaki, M. M., Michailidis, M. M., Manioudaki, M. M., et al. (2020). Systems-based approaches to unravel networks and individual elements involved in apple super?cial scald. Front. Plant Sci. 111:8. doi: 10.3389/fpls.2020.00008
Karagiannis, E., Michailidis, M., Tanou, G., Samiotaki, M., Karamanoli, K., Avramidou, E., et al. (2018). Ethylene-dependent and -independent superficial scald resistance mechanisms in ‘Granny Smith’ apple fruit. Sci. Rep. 8:11436. doi: 10.1038/s41598-018-29706-x
Larrigaudière, C., Lindo-García, V., Ubach, D., and Giné-Bordonaba, J. (2019). 1-Methylcyclopropene and extreme ULO inhibit superficial scald in a different way highlighting the physiological basis of this disorder in pear. Sci. Hortic. 250, 148–153. doi: 10.1016/j.scienta.2019.02.049
Li, D., Cheng, Y., and Guan, J. (2018). Effects of 1-methylcyclopropene on surface wax and related genes expression in cold-stored “Hongxiangsu” pear. J. Sci. Food Agric. 99, 2438–2446. doi: 10.1002/jsfa.9452
Lindo-García, V., Giné-Bordonaba, J., Leclerc, C., Ubach, D., and Larrigaudière, C. (2020). The relationship between ethylene- and oxidative-related markers at harvest with the susceptibility of pears to develop superficial scald. Postharvest Biol. Technol. 163:111135. doi: 10.1016/j.postharvbio.2020.111135
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCt method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Lurie, S., and Watkins, C. B. (2012). Superficial scald, its etiology and control. Postharvest Biol. Technol. 65, 44–60. doi: 10.1016/j.postharvbio.2011.11.001
Marc, M., Cournol, M., Hanteville, S., Poisson, A. S., Guillou, M. C., Pelletier, S., et al. (2020). Pre-harvest climate and post-harvest acclimation to cold prevent from superficial scald development in granny smith apples. Sci. Rep. 10:6180. doi: 10.1038/s41598-020-63018-3
Niu, J., Hou, Z., Ou, Z., and Hui, W. (2018). Comparative study of effects of resveratrol, 1-MCP and DPA treatments on postharvest quality and superficial scald of ‘Starkrimson’ apples. Sci. Hortic. 240, 516–521. doi: 10.1016/j.scienta.2018.06.037
Piretti, M. V., Gallerani, G., and Brodnik, U. (1996). Polyphenol polymerisation involvement in apple superficial scald. Postharvest Biol. Technol. 8, 11–18. doi: 10.1016/0925-5214(95)00056-9
Qian, M., Yu, B., Li, X., Sun, Y., Zhang, D., and Teng, W. (2014). Isolation and expression analysis of anthocyanin biosynthesis genes from the red Chinese sand pear, Pyrus pyrifolia Nakai cv. mantianhong, in response to methyl jasmonate treatment and UV-B/VIS conditions. Plant Mol. Biol. Rep. 32, 428–437. doi: 10.1007/s11105-013-0652-6
Sabban-Amin, R., Feygenberg, O., Belausov, E., and Pesis, E. (2011). Low oxygen and 1-MCP pretreatments delay superficial scald development by reducing reactive oxygen species (ROS) accumulation in stored ‘granny smith’ apples. Postharvest Biol. Technol. 62, 295–304. doi: 10.1016/j.postharvbio.2011.06.016
Wang, L., Qian, M., Wang, R., Wang, L., and Zhang, S. (2018). Characterization of the glutathione S-transferase (GST) gene family in Pyrus bretschneideri and their expression pattern upon superficial scald development. Plant Growth Regulat. 86, 211–222. doi: 10.1007/s10725-018-0422-4
Wang, M., Jiang, N., Wang, Y., Jiang, D., and Feng, X. (2017). Characterization of phenolic compounds from early and late ripening sweet cherries and their antioxidant and antifungal activities. J. Agric. Food Chem. 65, 5413–5420. doi: 10.1021/acs.jafc.7b01409
Wang, Y. (2016). Storage temperature, controlled atmosphere, and 1-methylcyclopropene effects on alpha-farnesene, conjugated trienols, and peroxidation in relation with superficial Scald, pithy brown core, and fruit quality of ‘d’anjou’ pears during long-term storage. J. Am. Soc. Hortic. Sci. 141, 177–185. doi: 10.21273/JASHS.141.2.177
Watkins, C. B. (2006). The use of 1-methylcyclopropene (1-MCP) on fruits and vegetables. Biotechnol. Adv. 24, 389–409. doi: 10.1016/j.biotechadv.2006.01.005
Wei, J., Qi, X., Cheng, Y., and Guan, J. (2015). Difference in activity and gene expression of pectin-degrading enzymes during softening process in two cultivars of Chinese pear fruit. Sci. Hortic. 197, 434–440. doi: 10.1016/j.scienta.2015.10.002
Whitaker, B. D. (2007). Oxidation products of alpha-farnesene associated with superficial scald development in d’anjou pear fruits are conjugated trienols. J. Agric. Food Chem. 55, 3708–3712. doi: 10.1021/jf063710i
Whitaker, B. D. (2013). Genetic and biochemical bases of superficial scald storage disorder in apple and pear fruits. Acta. Hort. 989, 47–60. doi: 10.17660/ActaHortic.2013.989.3
Whitaker, B. D., Nock, J. F., and Watkins, C. B. (2000). Peel tissue α-farnesene and conjugated trienol concentrations during storage of ‘white angel’ × ‘rome beauty’ hybrid apple selections susceptible and resistant to superficial scald. Postharvest Biol. Technol. 20, 231–241. doi: 10.1016/S0925-5214(00)00139-3
Xie, X., Fang, C., and Wang, Y. (2017). Inhibition of ethylene biosynthesis and perception by 1-methylcyclopropene and its consequences on chlorophyll catabolism and storage quality of ‘bosc’ pears. J. Am. Soc. Hortic. Sci. Am. Soc. Hortic. Sci. 142, 92–100. doi: 10.21273/JASHS04017-16
Xie, X., Song, J., Wanga, Y., and Sugar, D. (2014). Ethylene synthesis, ripening capacity, and superficial scald inhibition in 1-MCP treated ‘d’Anjou’ pears are affected by storage temperature. Postharvest Biol. Technol. 97, 1–10. doi: 10.1016/j.postharvbio.2014.06.002
Xie, X., Zhao, J., and Wang, Y. (2016). Initiation of ripening capacity in 1-MCP treated green and red ‘anjou’ pears and associated expression of genes related to ethylene biosynthesis and perception following cold storage and post-storage ethylene conditioning. Postharvest Biol. Technol. 111, 140–149. doi: 10.1016/j.postharvbio.2015.08.010
Zhai, R., Xiang, F., Song, J., Wang, Z., Yang, C., and Xu, L. (2021). Roles of laccase and cultivar-specific phenolic composition in scald-like disorder development in pears. Postharvest Biol. Technol. 181:111651. doi: 10.1016/j.postharvbio.2021.111651
Zhi, H., and Dong, Y. (2018). Effect of 1-methylcyclopropene on superficial scald associated with ethylene production, α-farnesene catabolism, and antioxidant system of over-mature ‘d’anjou’ pears after long-term storage. Food Bio. Technol. 11, 1775–1786. doi: 10.1007/s11947-018-2141-2
Zhou, S., Cheng, Y., and Guan, J. (2017). The molecular basis of superficial scald development related to ethylene perception and α-farnesene metabolism in ‘wujiuxiang’ pear. Sci. Hortic. 216, 76–82. doi: 10.1016/j.scienta.2016.12.025
Keywords: pear, superficial scald, phenolics, α-farnesene, ethylene
Citation: He J, Feng Y, Cheng Y, Wang M and Guan J (2022) A comprehensive insight on the main physiological biochemical and related genes expression changes during the development of superficial scald in “Yali” pear. Front. Plant Sci. 13:987240. doi: 10.3389/fpls.2022.987240
Received: 06 July 2022; Accepted: 05 August 2022;
Published: 02 September 2022.
Edited by:
Zhihui Chen, University of Dundee, United KingdomReviewed by:
Athanassios Molassiotis, Aristotle University of Thessaloniki, GreeceYang Bi, Gansu Agricultural University, China
Li Zhengguo, Chongqing University, China
Copyright © 2022 He, Feng, Cheng, Wang and Guan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junfeng Guan, anVuZmVuZy1ndWFuQDI2My5uZXQ=
 Jingang He
Jingang He Yunxiao Feng
Yunxiao Feng Yudou Cheng
Yudou Cheng Meng Wang
Meng Wang Junfeng Guan
Junfeng Guan