
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Plant Sci., 02 September 2022
Sec. Plant Metabolism and Chemodiversity
Volume 13 - 2022 | https://doi.org/10.3389/fpls.2022.980854
This article is part of the Research TopicChemistry and Biology of Plant Natural ProductsView all 9 articles
Paeoniflorin, a monoterpene glucoside, is increasingly used in the clinical treatment of many diseases because it has a variety of pharmacological activities. Besides, paeoniflorin has been considered the characteristic chemical constituent of Paeoniaceae plants since it was first reported in 1963. Therefore, how to better develop and utilize paeoniflorin in Paeoniaceae has always been a research hotspot. We reviewed the current knowledge on paeoniflorin in Paeoniaceae, with particular emphasis on its distribution and influencing factors. Moreover, the limited understanding of the biosynthesis pathway has restricted the production of paeoniflorin by synthetic biology. This review provides insights into the post-modification pathway of paeoniflorin biosynthesis and proposes directions for further analysis in the future.
Paeoniaceae is a single genus family, and Paeonia consists of 34 species, comprising shrubs and perennial herbs mainly distributed in temperate Eurasia, northwest Africa, and western North American (Hong, 2010, 2021). The shrubs, called tree peonies, belong to Section Moutan DC., and the perennials, called herbaceous peonies, are classified into Sect. Paeonia or Sect. Onaepia Lindley (Hong, 2011). China has the most concentrated distribution of Paeoniaceae plants, and it is also one of the centers of differentiation and development of existing Paeoniaceae primitive groups (Zhou et al., 2021). Since the Eastern Han Dynasty (25–220 A.D.), Paeoniaceae plants have been widely cultivated and used in China because of their special medicinal effects (Li et al., 2011). Currently, there are three traditional Chinese medicines in China, Mudanpi, Baishao, and Chishao, which are processed from the roots of Paeoniaceae plants (Chinese Pharmacopoeia Committee, 2020). These three medicines have the functions of clearing heat, cooling blood, promoting blood circulation, and removing blood stasis (Tan et al., 2020; Wu et al., 2020).
Although there are many active components in Paeoniaceae plants, paeoniflorin, a monoterpene bicyclic glycoside, is considered the most important medicinal ingredient (Xiu et al., 2021). Modern medical studies have shown that paeoniflorin has immunoregulatory, antidepressant, anti-arthritis, antithrombosis, anti-tumor, hepatoprotective, cerebral ischemic injury protective, and neuroprotective effects (Zhang et al., 2019a; Zhang, 2020). Moreover, paeoniflorin, found only in Paeoniaceae plants in nature, is considered the characteristic constituent of Paeoniaceae (Yu and Xiao, 1987). Therefore, numerous studies have focused on the distribution of paeoniflorin in Paeoniaceae to better develop and utilize these plants for medicinal purposes (He, 2010; Yang et al., 2020; Yan et al., 2021). As a secondary metabolite, the content of paeoniflorin is affected by many factors, including biological and abiotic factors (Chen et al., 2009; Feng et al., 2012; Shen et al., 2019; Zhang and Wei, 2020). The influence of these factors on the content of paeoniflorin has become a topic of research interest. Our research group has also carried out several studies on paeoniflorin in Paeoniaceae plants (Zhang et al., 2019b). Thus, it is necessary to systematically review this research and establish expectations for future studies.
Currently, paeoniflorin is mainly extracted from the roots of Paeoniaceae plants, but this has many disadvantages, such as low yield, difficulty in separation of extracts, and wastage of plant resources (Nie et al., 2005). Although paeoniflorin has been synthesized by chemical methods for a long time, it is not used in actual production because of its complicated process, high production cost, and contaminated production process (Corey and Wu, 1993; Hatakeyama et al., 1994). As an active natural product with important medicinal value, paeoniflorin is increasingly required for drug preparation; for this reason, it is important to realize its large-scale production economically. The biosynthesis of natural products is one of the most important research directions in synthetic biology. Compared with the two abovementioned methods, biosynthesis has many advantages, such as a single product, high yield, an environmentally friendly process, and no restriction of raw materials (Sun et al., 2017). However, the biosynthesis pathway of paeoniflorin has not been fully elucidated, especially the modification stage that determines its structural and functional specificity. There are few studies on the enzymes involved in this stage, which fundamentally limit the production of paeoniflorin by synthetic biology.
In this review, we summarized the distribution characteristics of paeoniflorin and the effects of different factors on paeoniflorin content in Paeoniaceae. More importantly, we predicted the post-modification pathway of paeoniflorin biosynthesis and analyzed the paeoniflorin biosynthesis pathway.
The secondary metabolites in Paeoniaceae are complex, with more than 300 chemical components identified, mainly including monoterpene glycosides, triterpenoids, flavonoids, tannins, phenolic acids, sugars, steroids, and volatile oils (He et al., 2010). Paeoniflorin was first isolated from the roots of Paeonia albiflora and named by Shibata et al. (1963), who pointed out that paeoniflorin was D-glucoside with a benzoylated C10-compound (C10H14O5). Further studies showed that paeoniflorin is a monoterpene glucoside whose basic skeleton is a pinane derivative (Shibata et al., 1964; Aimi et al., 1969; Kaneda et al., 1972). Subsequently, an increasing number of species belonging to Paeoniaceae have been reported to contain paeoniflorin in their roots (Egon and Cooper, 1969). Paeoniflorin has always only been found in Paeoniaceae plants (Yu and Xiao, 1987). Therefore, paeoniflorin was naturally considered to be the characteristic constituent of Paeoniaceae until 2008, when it was isolated from the water fern Salvinia molesta (Choudhary et al., 2008), which was the first report that paeoniflorin occurred in plants other than Paeoniaceae. In our previous study (Zhang et al., 2019b), we did not detect paeoniflorin in S. molesta by UPLC-MS, nor did we detect it in any of the 16 plants from different families. In addition to the roots of Paeoniaceae plants, other organs, including flowers, stems, leaves, fruits, seeds, and rhizomes, have also been investigated, and the results show that paeoniflorin is distributed throughout the plant. These results have thus confirmed that paeoniflorin is a characteristic constituent of Paeoniaceae without tissue specificity.
Although paeoniflorin occurs widely in the roots of species belonging to Paeoniaceae, its contents were quite different (Table 1; Supplementary Table 1). He et al. (1980) determined the paeoniflorin content in the roots of 11 species and varieties, and the content ranged from 0.09 to 10.72%. Yu and Xiao (1985, 1987) showed that the paeoniflorin content of 19 species and 6 varieties ranged from 0.40 to 4.36%, and a study by Jin et al. (1989) on 13 species and varieties found paeoniflorin contents ranging from 0.76 to 7.78%. Recently, our study included 16 species that exhibited a wide range of paeoniflorin content, from 0.22 to 5.12% (Zhang et al., 2019b). Many studies with a few samples (Han et al., 2008; Yang et al., 2020; Yan et al., 2021; Li et al., 2021a) that were not representative have shown that the paeoniflorin content in the roots of different species differs. In all the above studies, the roots varied by sampling time and location, and the detection methods of paeoniflorin were different; therefore, there is no agreement on which species have the highest and lowest paeoniflorin content. However, the consistent conclusion was that the paeoniflorin content of herbaceous peony roots was higher than that of tree peony roots. Furthermore, most studies have concluded that the paeoniflorin content in the roots of species collected from the wild habitat was higher than that in the roots of species collected from the cultivated habitat (Hu et al., 2000; Guo et al., 2002; Zhou et al., 2003; Wang et al., 2012).
Some studies have suggested that older Paeonia lactiflora plants have a higher paeoniflorin content in their roots (Huang et al., 2000; Zhang, 2000; Jian et al., 2010). However, if the roots are more than 5 years old, the paeoniflorin content decreases (Jian et al., 2010; Kang, 2011). Other studies have reported contrasting results, that is, the paeoniflorin content in the roots is negatively correlated with plant age (Xue et al., 1992; Li et al., 2008; Yan et al., 2019). The reason for this may be that the starch in the roots increases with the growth of the plant, and paeoniflorin decomposes in vivo (Xue et al., 1992). Our studies have shown that there is no significant correlation between paeoniflorin content and plant age in either the roots of Paeonia ostii or in the roots of P. lactiflora (Zhang et al., 2019b). In conclusion, plant ages affect paeoniflorin content in the roots (Supplementary Table 2), but there is no unified conclusion on this effect.
Paeoniaceae are perennial herbs or woody shrubs, with flower buds and leaf buds sprouting in early spring and falling into dormancy in autumn. Their development stages can be divided into budding, leaf unfolding, buds, flowering, fruiting, and withering (Li et al., 2011). Numerous studies have confirmed that the paeoniflorin contents in the roots at different developmental stages are significantly different (Supplementary Table 3). With the development of plants, the paeoniflorin content of the roots first increased and then decreased. The highest content was usually observed in spring (budding or flowering stages; He et al., 1980; Yu and Xiao, 1985; Jin et al., 1991, 2010; Huang et al., 2000; Fu et al., 2020). It seems best to harvest roots in spring to produce Mudanpi, Baishao, and Chishao. However, the roots are often harvested in autumn during the actual production in consideration of the dry matter accumulation. In addition, the level of paeoniflorin in the roots also varies throughout the day, increasing with temperature (Chen et al., 2002). This founding shows that it is better to harvest the roots at noon. Unlike the change of paeoniflorin in the roots, paeoniflorin in the leaves regularly decreased with plant development (Jian et al., 2010; Zhang et al., 2019b; Fu et al., 2020). The roots of Paeoniaceae plants usually can be used as medicines after four to five years of cultivation. However, the leaves can be harvested every year, so they can be developed as a new resource of paeoniflorin. Besides, removing some leaves at an early stage can reduce the incidence of plant disease, which is also much more conducive to the healthy growth of Paeoniaceae plants.
Paeoniflorin has been widely found in various organs of Paeoniaceae plants, but its contents were significantly different (Supplementary Table 4). The paeoniflorin content in P. lactiflora, from high to low, was in the following order: rhizomes, roots, leaves, stems, and flowers (Hu et al., 2000). Yang et al. (2001) determined that the paeoniflorin content in Paeonia rockii was in the following order: branch bark, root phloem, fibrous root, leaf, and petiole. However, among the organs of Paeonia delavayi var. lutea, the highest paeoniflorin content is found in the fruit, followed by the leaf, cork, cortex, stem, and xylem (Feng et al., 2012). The paeoniflorin distribution in P. ostii differs from these results, with the highest paeoniflorin content in the leaves, followed by stems, petioles, petals, xylem, and phloem (Zhang et al., 2019b). During the production of Mudanpi, Baishao, and Chishao, only the roots are harvested, while the other organs of Paeoniaceae plants are discarded. These studies indicate that the other organs also have potential medicinal value due to the considerable amounts of paeoniflorin.
Paeoniflorin content was also different in different root tissues. The paeoniflorin content in the rhizome of P. lactiflora is close to or even higher than that in the root, and the content in the branch roots and fine roots is higher than that in the main roots and thick roots, respectively (Hu et al., 2000; Chen et al., 2002; Kang, 2011; Ji et al., 2014). The paeoniflorin content in the phloem (2.69%) of P. lactiflora is similar to that in the xylem (2.90%), but it is significantly lower than that in the cork periderm (4.65%; Wu and Zhao, 2005). Based on high mass resolution matrix-assisted laser desorption/ionization MS imaging, Li et al. (2021b) reported that paeoniflorin was observed mainly in the cork and phloem region of P. suffruticosa and in the xylem rays of P. lactiflora, showing a great contrast with wedge-shaped xylem patches. The distribution of paeoniflorin in the roots of most wild species belonging to Sect. Moutan is similar to that of P. suffruticosa. In contrast, paeoniflorin is mainly distributed in the xylem of P. ostii, regardless of whether the roots are from plants grown in wild or cultivated habitats (Zhang et al., 2019b). This finding also indicates that the root xylem should be fully utilized for medicinal purposes instead of being discarded.
In addition to biological factors, some abiotic factors, including the annual average temperature, annual precipitation, annual sunshine duration, annual total solar radiation, and soil type of the plant habitat, also affected the paeoniflorin content (Hu et al., 2000; Han et al., 2008; Yuan et al., 2020; Zhang, 2020). There is a significant positive correlation between the paeoniflorin content in the roots and annual precipitation (Zhou et al., 2007), but a significant negative correlation has been observed between the paeoniflorin content in the roots and the annual average temperature (Yuan et al., 2020). Moreover, the elements zinc and potassium in the soil also play positive roles in paeoniflorin synthesis (Chen et al., 2009). These results reveal that the ecological and environmental factors of habitat should be carefully considered in order to produce the traditional Chinese medicines with high paeoniflorin content. Boiling and drying are usually necessary steps in the production of Mudanpi, Baishao, and Chishao, and these measures are considered to have effects on paeoniflorin content. High temperatures and long-term heating account for the decrease in paeoniflorin content due to its poor thermostability (Jin et al., 2015; Huo et al., 2017; Shen et al., 2019; Zhang et al., 2019b), and thermostability is negatively correlated with the water content of the roots (Yan et al., 2003). These studies show that the freeze-drying is better suited for increasing paeoniflorin contents in the production of traditional Chinese medicines.
Paeoniflorin is a monoterpene glycoside, and its biosynthesis pathway can be divided into three stages (Figure 1). The universal precursor, isopentenyl pyrophosphate (IPP), and its isomer, dimethylallyl diphosphate (DMAPP), are synthesized in the first stage. Plants have two pathways for producing IPP and DMAPP. One is the mevalonate (MVA) pathway located in the cytoplasm with acetyl CoA as the raw material (Bekkering et al., 2018), and the other is the 2-C-methyl-D-erythritol4-phosphate (MEP) pathway, or 1-deoxy-D-xylulose5-phosphate (DXP) pathway, which is located in plastids and uses pyruvate and glyceraldehyde-3-phosphate as raw materials (Shi et al., 2019). These two pathways do not exist in isolation, and there is a universal interaction between them (Laule et al., 2003). The paeoniflorin content has been determined in different organs of P. lactiflora (Yuan et al., 2013), and the relative expression of 24 genes encoding enzymes involved in MEP/DXP and MVA pathways has been revealed. Correlation analysis indicates that hydroxymethylglutaryl-CoA synthase and phosphomevalonate kinase in the MVA pathway play important roles in the biosynthesis of paeoniflorin. In the second stage, geranyl diphosphate (GPP), the monoterpene precursor, is formed by head-to-tail condensation of one IPP and one DMAPP catalyzed by geranyl diphosphate synthase in the plastids (Zebec et al., 2016).
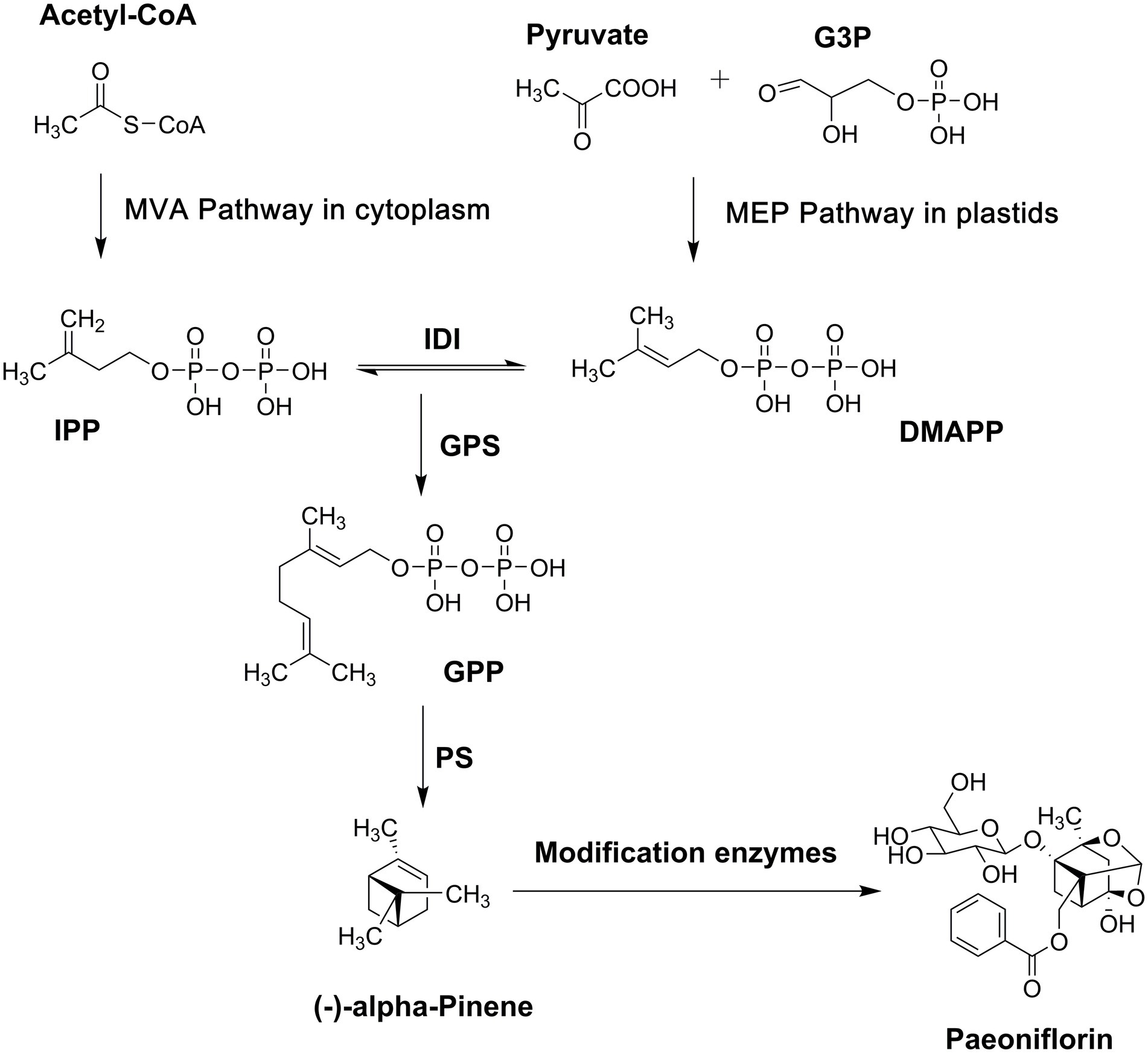
Figure 1. Biosynthesis pathway of paeoniflorin. G3P, glyceraldehyde-3-phosphate; MVA, mevalonate; MEP, 2-C-methyl-D-erythritol4-phosphate; IPP, isopentenyl pyrophosphate; DMAPP, dimethylallyl diphosphate; IDI, isopentenyl diphosphate isomerase; GPS, geranylgeranyl pyrophosphate synthase; GPP, geranyl diphosphate; PS, pinene synthase.
Then, the formation and post-modification of the basic pinene skeleton occur in the final stage. Terpenoid synthase (TPS), located at the branch point of the isoprenoid pathway, catalyzes GPP to form the monoterpenoid skeleton directly. Therefore, TPS is not only the key enzyme for terpenoid synthesis but also the main inducer for the structural diversity of terpenoids (Chen et al., 2011). A TPS gene named PlPIN, which encodes α-pinene synthase, was isolated from P. lactiflora (Ma et al., 2016). An in vitro enzyme assay showed that PlPIN converted GPP into α-pinene as a single product. Terpenoids in plants are often not the direct products obtained by the one-step catalysis of TPS, and most of them must be modified by special groups, even if their skeleton structures are rearranged. These post-modifications, including glycosylation, acylation, epoxidation, hydroxylation, halogenation, and addition and reduction reactions, substantially increase the variety of terpenoids and their structural diversity (Rivas et al., 2013; Zhao et al., 2014; Li et al., 2015). Unfortunately, no studies have addressed the post-modification of paeoniflorin biosynthesis.
The basic skeleton of paeoniflorin is α-pinane, which is also connected to the benzoyl group, β-D-glucopyranosyl group, and hemiketal–acetal linkage. Based on previous literature (Chedgy et al., 2015; Xu et al., 2016), we speculated that the β-D-glucopyranosyl group is connected to the skeleton through glycosylation, which is catalyzed by glycosyltransferase, while the benzoyl group is connected to the skeleton through acylation, which is catalyzed by benzoyltransferase (Figure 2).
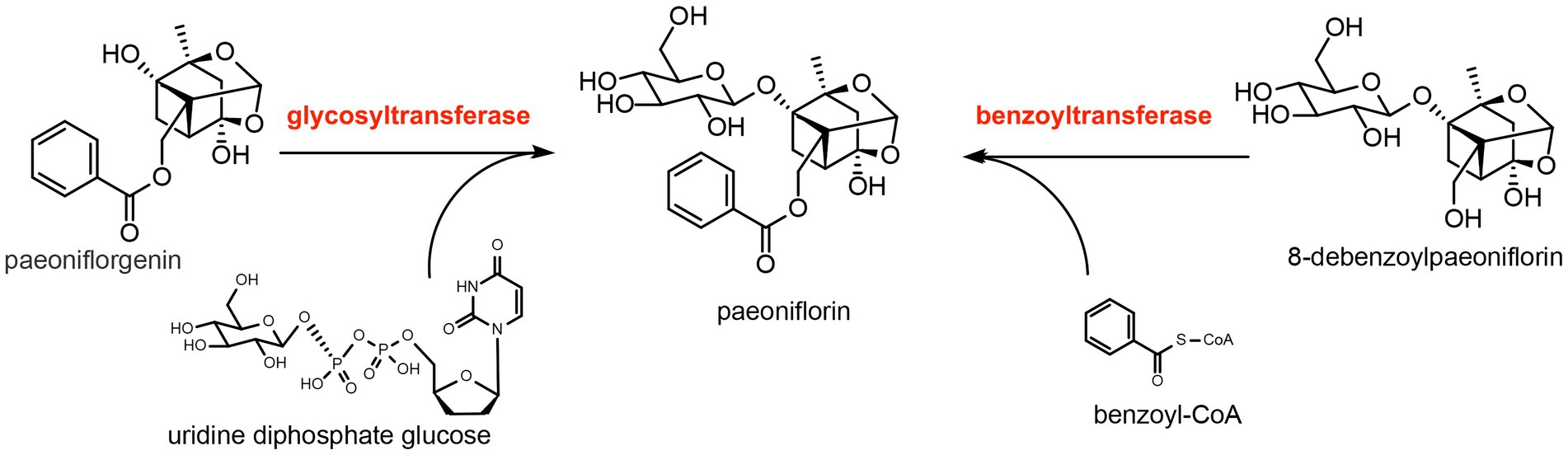
Figure 2. Predicted synthesis of paeoniflorin catalyzed by glycosyltransferase and benzoyltransferase. The substrate of glycosyltransferase is paeoniflorgenin and the glycosyl donor is uridine diphosphate glucose. The substrate of benzoyltransferase is 8-debenzoylpaeoniflorin and the benzoyl donor is benzoyl-CoA.
Unfortunately, there is a lack of sufficient understanding of the biosynthetic mechanism of the hemiketal–acetal linkage. Based on the compounds with structures similar to paeoniflorin in Paeoniaceae (He et al., 2010; Wu et al., 2010), the biosynthesis pathway of hemiketal-acetal linkage is proposed for the first time in the present study (Figure 3). First, the C-4 of α-pinene is hydroxylated by hydroxylase and then oxidized to the ketone group by dehydrogenase. Second, C-2 and C-9 are both hydroxylated by hydroxylases, and then, the two hydroxyl groups dehydrate to form a cyclic ether linkage. Finally, C-9 continues to be added to the hydroxyl group under the catalysis of hydroxylase, at which time the hemiacetal linkage is formed. Then, the hydroxyl group of C-9 reacts with the ketone group to form the hemiketal, while the hemiacetal is converted into acetal, eventually forming the hemiketal–acetal linkage. In these reaction steps, the hydroxylation of C-2, C-4, and C-9 is the basis.
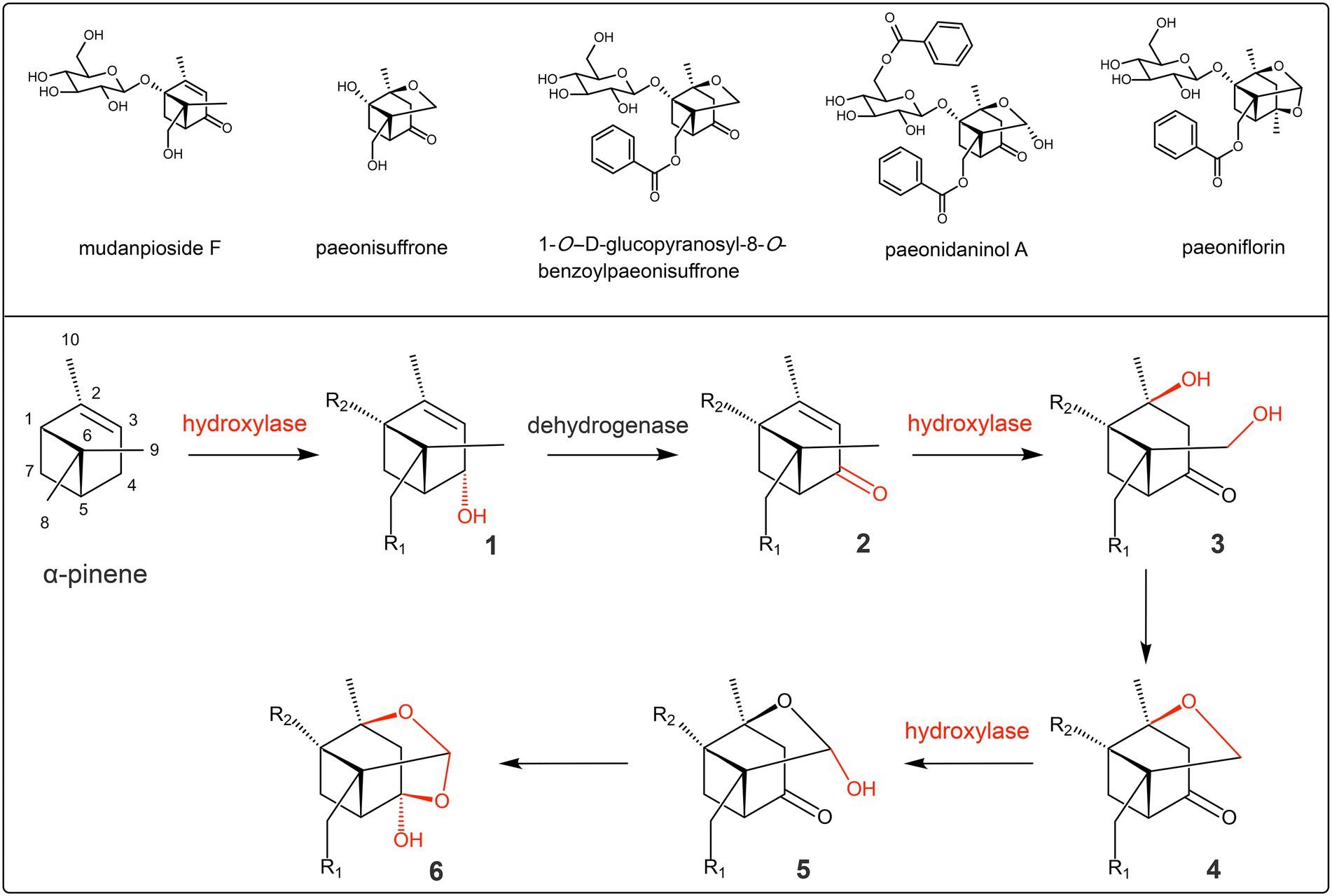
Figure 3. Predicted biosynthesis pathway of the hemiketal–acetal linkage of paeoniflorin. Compounds 1 and 3 are possible intermediates; the representative of compound 2 is mudanpioside F; the representatives of compound 4 are paeonisuffrone and 1-O-D-glucopyranosyl-8-O-benzoylpaeonisuffrone; the representative of compound 5 is paeonidaninol A; the representative of compound 6 is paeoniflorin; R1 and R2 represent the possible modification groups.
Combining the reaction process in Figure 2 and the structure of α-pinene (Figure 3), it can be shown that the glycosylation modification is the reaction between the glycosyl donor (uridine diphosphate glucose) and the hydroxyl group at C-1, and the acylation modification is the reaction between the benzoyl donor (benzoyl-CoA) and the hydroxyl group at C-8. In these reaction steps, the hydroxylation of C-1 and C-8 is the basis. In summary, the hydroxylation of C1, C2, C4, C8, and C9 is the basic and necessary step in the modification stages of paeoniflorin biosynthesis, and hydroxylases play crucial roles in these stages.
High-throughput sequencing has been increasingly applied to the mining of key enzyme genes for natural product biosynthesis. By using plant tissues with large differences in natural product content as materials, many differentially expressed genes (DEGs) can be obtained by transcriptome sequencing, and the candidate enzyme genes can be further obtained based on a comparison with online databases, such as non-redundant protein sequences, Kyoto Encyclopedia of Genes and Genomes, and Gene Ontology. Plant materials are crucial throughout the sequencing process and directly determine the number of DEGs under the established analysis process. The plant materials for sequencing should preferably be based on the univariate principle, for example, the same tissues with different developmental stages, and whether the same tissues experience hormone induction or adversity stress. PlPIN, the α-pinene synthase gene, was isolated using the transcriptome data of P. lactiflora roots (Ma et al., 2016). Paeoniflorin content in the leaves of P. ostii is significantly correlated with developmental stage, with the paeoniflorin content being higher at earlier developmental stages. These differences in paeoniflorin content make the leaf a more suitable sequencing material for a detailed investigation of the biosynthesis pathway (Zhang et al., 2019b).
The functions of candidate genes obtained by transcriptome sequencing can be quickly identified by enzyme assays in vitro. However, for plant studies, it is usually insufficient to verify the functions of candidate genes through enzyme assays in vitro, and the functions of candidate genes in plants need to be further verified. Paeoniflorin is the characteristic constituent of Paeoniaceae; thus, it is almost impossible to verify the biological functions of candidate genes in model plants (e.g., Arabidopsis and Petunia). Additionally, at least at present, the long-life cycle of Paeoniaceae and the immature genetic transformation system make it difficult to overexpress candidate genes in herbaceous peony or tree peony to verify their biological functions. Fortunately, the virus-induced gene silencing system has been established in tree peony (Xie et al., 2019; Wang et al., 2020), which can be used to verify their biological functions by transiently silencing the candidate genes in vivo.
Analysis of the paeoniflorin biosynthesis pathway is still at the preliminary stage, and many scientific questions are yet to be answered. At present, the most urgent task is the identification and functional analysis of key modification enzyme genes. There are several studies on the generation of β-D-glucopyranosyl and benzoyl groups in natural products, which can provide a reference for paeoniflorin biosynthesis. It is believed that the identification and functional analysis of glycosyltransferase and benzoyltransferase genes will be completed in a short time. Nevertheless, the biosynthesis of the hemiketal–acetal linkage has not yet been reported, and we speculate that there are several enzymes involved in this pathway, including multi-step reactions, which may be the key restricting the analysis of paeoniflorin biosynthesis. With the development of technology, some new techniques, such as a target identification strategy based on biosynthetic intermediate probes, have been successfully used to discover key enzymes (Gao et al., 2020). We speculate that a breakthrough will be achieved in the elucidation of paeoniflorin biosynthesis in the near future. This will not only provide technical support to produce paeoniflorin using synthetic biology but will also lay the theoretical foundation for the use of biotechnology to improve the quality of Paeoniaceae.
X-XZ, Y-HH, and J-HY conceived and designed this review. X-XZ wrote the manuscript. J-QZ, Y-TW, and H-YD performed the literature collection and made classification. J-HY and Y-HH edited and revised the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (32000240), Key project at central government level, the ability establishment of sustainable use for valuable Chinese medicine resources (2060302), Chinese Universities Scientific Fund (2452020213), Open Project of Shanghai Key Laboratory of Plant Functional Genomics and Resources (PFGR202203), and Special Fund for Shanghai Landscaping Administration Bureau Program (G222415, G192418, G192419).
We thank Yan-Long Zhang (Northwest A&F University) for supporting this work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2022.980854/full#supplementary-material
Aimi, N., Inaba, M., Watanabe, M., and Shibata, S. (1969). Chemical studies on the oriental plant drugs—XXIII: Paeoniflorin, a glucoside of Chinese paeony root. Tetrahedron 25, 1825–1838. doi: 10.1016/S0040-4020(01)82804-0
Bekkering, S., Arts, R. J., Novakovic, B., Kourtzelis, I., van der Heijden, C. D., Li, Y., et al. (2018). Metabolic induction of trained immunity through the mevalonate pathway. Cell 172, 135–146. doi: 10.1016/j.cell.2017.11.025
Chedgy, R. J., Köllner, T. G., and Constabel, C. P. (2015). Functional characterization of two acyltransferases from Populus trichocarpa capable of synthesizing benzyl benzoate and salicyl benzoate, potential intermediates in salicinoid phenolic glycoside biosynthesis. Phytochemistry 113, 149–159. doi: 10.1016/j.phytochem.2014.10.018
Chen, B. L., Hang, Y. Y., Zhou, Y. F., Shi, Y. Y., Guo, K. Y., and Chen, B. E. (2002). The dynamic change of paeoniaflorin content from Paeonia lactiflora pall root in harvest time. J. Plant Resour. Environ. 11, 25–28. doi: 10.3969/j.issn.1674-7895.2002.02.006
Chen, F., Tholl, D., Bohlmann, J., and Pichersky, E. (2011). The family of terpene synthases in plants: a mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. Plant J. 66, 212–229. doi: 10.1111/j.1365-313x.2011.04520.x
Chen, X., Zhang, X. Y., Zhang, R. R., and Wang, K. C. (2009). Effects of Mn, Fe, Zn and Cu on growth and paeoniflorin content of Paeonia lactiflora. China J. Chin. Mater. Medica. 34, 961–964.
Chinese Pharmacopoeia Committee. (2020). The Pharmacopoeia of the People’s Republic of China. Beijing: China Medical Science Press.
Choudhary, M. I., Naheed, N., Abbaskhan, A., Musharraf, S. G., and Siddiqui, H. (2008). Phenolic and other constituents of fresh water fern Salvinia molesta. Phytochemistry 69, 1018–1023. doi: 10.1016/j.phytochem.2007.10.028
Corey, E. J., and Wu, Y. J. (1993). Total synthesis of (±)-paeoniflorigenin and paeoniflorin. J. Am. Chem. Soc. 115, 8871–8872. doi: 10.1021/ja00072a063
Egon, S., and Cooper, S. (1969). Untersuchungen über Inhaltsstoffe von Paeonia-Arten. Arch. Pharm. 302, 685–690. doi: 10.1002/ardp.19693020907
Feng, Y., Wang, Y., Zhou, N., Jiang, B., and Yu, Y. (2012). Determination of paeoniflorin in different parts of Paeonia delavayi var. lutea by HPLC. Chin. J. Exp. Tradit. Med. Form. 18, 139–142. doi: 10.3969/j.issn.1005-9903.2012.09.041
Fu, S. P., Shen, H. W., Wang, Q. B., Li, J. P., Wang, C., Guo, S. L., et al. (2020). Study on content determination of 6 active ingredients in Paeonia lactiflora during different harvesting periods and their variation. China Pham. 31, 441–446. doi: 10.6039/j.issn.1001-0408.2020.04.10
Gao, L., Su, C., Du, X., Wang, R., Chen, S., Zhou, Y., et al. (2020). FAD-dependent enzyme-catalysed intermolecular [4+2] cycloaddition in natural product biosynthesis. Nat. Chem. 12, 620–628. doi: 10.1038/s41557-020-0467-7
Guo, B. L., Basang, D., Xiao, P. G., and Hong, D. Y. (2002). Research on the quality of original plants and material medicine of cortex Paeoniae. China J. Chin. Mater. Medica. 27, 654–657. doi: 10.3321/j.issn:1001-5302.2002.09.004
Han, X. Y., Liu, Z. A., and Wang, L. S. (2008). Comparison of the content of effective components between tree peony wild species and main cultivars. J. Chin. Med. Mater. 31, 327–331. doi: 10.3321/j.issn:1001-4454.2008.03.002
Hatakeyama, S., Kawamura, M., and Takano, S. (1994). Total synthesis of (−)-paeoniflorin. J. Am. Chem. Soc. 116, 4081–4082. doi: 10.1021/ja00088a055
He, C. N., Peng, Y., Zhang, Y., Xu, L., Gu, J., and Xiao, P. (2010). Phytochemical and biological studies of Paeoniaceae. Chem. Biodivers. 7, 805–838. doi: 10.1002/cbdv.200800341
He, C. N. (2010). Study on the Pharmacophylogenetic Significance of the Paeonia in China. Beijing: Chinese Academy of Medical Sciences and Peking Union Medical College.
He, L. Y., Feng, R. Z., and Xiao, P. G. (1980). The occurrence of paeoniflorine in the genus Paeonia. Acta Pharm. Sin. 15, 429–433.
Hong, D. Y. (2010). Peonies of the World: Taxonomy and Phytogeography. London: Royal Botanic Gardens Kew.
Hong, D. Y. (2011). Peonies of the World: Polymorphism and Diversity. London: Royal Botanic Gardens Kew.
Hong, D. Y. (2021). Peonies of the World: Phylogeny and Evolution. London: Royal Botanic Gardens Kew.
Hu, S. L., Lan, G. L., Feng, X. F., Tang, X. J., and He, X. R. (2000). Determination of paeoniflorin content in different origins and parts of Paeoniae lactiflora. China J. Chin. Mater. Medica. 25, 714–416. doi: 10.3321/j.issn:1001-5302.2000.12.003
Huang, M. Y., Wu, Z. W., and Zhang, X. G. (2000). Influence of harvesting periods and cultivation years on the quality of Paeoniae lactiflora cultivated in Zhongjiang. J. Chin. Med. Mater. 23, 435–436. doi: 10.3321/j.issn:1001-4454.2000.08.001
Huo, X. G., Hu, X. T., Chen, L. X., Feng, X. C., Ding, L. Q., and Qiu, F. (2017). Study on the stability of paeoniflorin. China Sci. Pap. 12, 2092–2097. doi: 10.3969/j.issn.2095-2783.2017.18.010
Ji, K. M., Fang, C. W., Guan, Y. Y., Guan, D. P., and Wu, J. H. (2014). Content comparison of the paeonol and paeoniflorin on different part of one Feng-cortex Moutan. Chin. J. Exp. Tradit. Med. Form. 20, 54–56. doi: 10.13422/j.cnki.syfjx.2014180054
Jian, Z. Y., Yu, J. B., and Wang, W. Q. (2010). RP-HPLC determination of main chemical components in different parts and different harvest periods of Paeonia lactiflora. Acta Pharm. Sin. 45, 489–493. doi: 10.16438/j.0513-4870.2010.04.009
Jin, C. D., Xu, G. J., Jin, R. L., and Xu, L. S. (1989). Quantitative analysis of glucosides and benzoic acid in peony root from 13 species by RP-HPLC. J. China Pharm. Univ. 3, 139–142. doi: 10.3321/j.issn:1000-5048.1989.03.003
Jin, C. D., Xu, G. J., Jin, R. L., Xu, L. S., and Cong, X. D. (1991). Quantitative analysis of the glucosides and benzoic acid in peony root collected in different seasons by RP-HPLC. J. China Pharm. Univ. 5, 279–281. doi: 10.3321/j.issn:1000-5048.1991.05.005
Jin, C. S., Cai, Y. J., and Wu, D. L. (2010). Changes in the contents of paeoniflorin and total glycosides of paeony in Paeoniae lactiflora of Bozhou at different harvesting periods. J. Chin. Med. Mater. 33, 1548–1550. doi: 10.3969/j.issn.1001-1528.2000.04.012
Jin, L., Zhao, W. S., Guo, Q. S., Zhang, W. S., and Ye, Z. L. (2015). Study on chemical components distribution in Paeoniae radix Alba and its processing methods. China J. Chin. Mater. Medica. 40, 1953–1959. doi: 10.4268/cjcmm20151021
Kaneda, M., Iitaka, Y., and Shibata, S. (1972). Chemical studies on the oriental plant drugs—XXXIII: the absolute structures of paeoniflorin, albiflorin, oxypaeoniflorin and benzoylpaeoniflorin isolated from chinese paeony root. Tetrahedron 28, 4309–4317. doi: 10.1016/S0040-4020(01)88953-5
Kang, X. F. (2011). Study on Active and Nutritional Components of Paeonia lactiflora Pall. Taian: Shandong Agricultural University. doi: 10.7666/d.d143830
Laule, O., Fürholz, A., Chang, H. S., Zhu, T., Wang, X., Heifetz, P. B., et al. (2003). Crosstalk between cytosolic and plastidial pathways of isoprenoid biosynthesis in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 100, 6866–6871. doi: 10.1073/pnas.1031755100
Li, B., Ge, J. Y., Liu, W., Hu, D. J., and Li, P. (2021b). Unveiling spatial metabolome of Paeonia suffruticosa and Paeonia lactiflora roots using MALDI MS imaging. New Phytol. 231, 892–902. doi: 10.1111/nph.17393
Li, J. J., Zhang, X. F., and Zhao, X. Q. (2011). Tree Peony of China. Beijing: Encyclopedia of China Publishing House.
Li, R. R., Liu, H. X., Peng, P. H., and Hu, X. R. (2021a). Study on UPLC fingerprint of Paeonia decomposita cortex and simultaneous determination of four components. China Meas. Test. 47, 77–82. doi: 10.11857/j.issn.1674-5124.2021040089
Li, W., Zhang, F., Chang, Y., Tao, Z., and Wang, G. (2015). Nicotinate O-Glucosylation is an evolutionarily metabolic trait important for seed germination under stress conditions in Arabidopsis thaliana. Plant Cell 27, 1907–1924. doi: 10.1105/tpc.15.00223
Li, Y. F., Yan, X. K., Shen, F., and Li, T. L. (2008). Study on qualities of different growing time of radix Paeoniae Alba. J. US–China Med. Sci. 5, 23–25.
Ma, X., Guo, J., Ma, Y., Jin, B., Zhan, Z., Yuan, Y., et al. (2016). Characterization of a monoterpene synthase from Paeonia lactiflora producing α-pinene as its single product. Biotechnol. Lett. 38, 1213–1219. doi: 10.1007/s10529-016-2098-z
Nie, X. Y., Shang, C., and Wang, Y. L. (2005). Study on separation of paeoniflorin from Paeonia lactiflora by macroporous adsorption resin. Chin. Tradit. Pat. Med. 27, 350–351. doi: 10.3969/j.issn.1001-1528.2005.03.037
Rivas, F., Parra, A., Martinez, A., and Garcia-Granados, A. (2013). Enzymatic glycosylation of terpenoids. Phytochem. Rev. 12, 327–339. doi: 10.1007/s11101-013-9301-9
Shen, M. L., Yan, B. J., and Qin, L. P. (2019). Research progress on different geographical areas, processing methods and collecting time of the effective compounds in Paeoniae radix (Shaoyao). J. Zhejiang Chin. Med. Univ. 43, 622–630. doi: 10.16466/j.issn1005-5509.2019.06.023
Shi, M., Huang, F., Deng, C., Wang, Y., and Kai, G. (2019). Bioactivities, biosynthesis and biotechnological production of phenolic acids in salvia miltiorrhiza. Crit. Rev. Food Sci. 59, 953–964. doi: 10.1080/10408398.2018.1474170
Shibata, S., Aimi, N., and Watanabe, M. (1964). Paeoniflorin, a monoterpene glucoside of Chinese paeony root. Tetrahedron Lett. 5, 1991–1995. doi: 10.1016/S0040-4039(00)75129-X
Shibata, S., Nakahara, M., and Aimi, N. (1963). Studies on the constituents of Japanese and Chinese crude drugs. VIII. Paeoniflorin, a glucoside of Chinese paeony root. Chem. Pharm. Bull. 11, 372–378. doi: 10.1248/cpb.11.372
Sun, L. C., Li, S. Y., Wang, F. Z., and Xin, F. J. (2017). Research progresses in the synthetic biology of terpenoids. Biotechnol. Bull. 33, 64–75. doi: 10.13560/j.cnki.biotech.bull.1985.2017.01.007
Tan, Y. Q., Chen, H. W., Li, J., and Wu, Q. J. (2020). Efficacy, chemical constituents, and pharmacological actions of radix Paeoniae Rubra and radix Paeoniae Alba. Front. Pharmacol. 11, 1054. doi: 10.3389/fphar.2020.01054
Wang, Q. L., Wang, W. Q., Wei, S. L., Yu, F. L., Peng, F., and Fang, Y. Q. (2012). Study on effect of different processing methods on seven main chemical components of wild and cultivated Paeonia lactiflora. China J. Chin. Materia. Medica. 37, 920–924. doi: 10.4268/cjcmm20120711
Wang, X., Li, J., Guo, J., Qiao, Q., Guo, X., and Ma, Y. (2020). The WRKY transcription factor PlWRKY65 enhances the resistance of Paeonia lactiflora (herbaceous peony) to Alternaria tenuissima. Hortic. Res. 7, 57. doi: 10.1038/s41438-020-0267-7
Wu, C. C., Guo, X. H., Liu, X., Yang, M., and Wu, W. H. (2020). Advances in modern pharmaceutical action of Moutan cortex in recent five years. Chin. J. New Drugs 29, 281–284. doi: 10.3969/j.issn.1003-3734.2020.03.008
Wu, J., and Zhao, L. (2005). Determination the change of paeoniflorin contents in different parts of cultivated Paeonia lactiflora before and after wilting by HPLC. Chin. Tradit. Herb. Drug 36, 122–123. doi: 10.7501/j.issn.0253-2670.2005.1.058
Wu, S. H., Wu, D. G., and Chen, Y. W. (2010). Chemical constituents and bioactivities of plants from the genus Paeonia. Chem. Biodivers. 7, 90–104. doi: 10.1002/cbdv.200800148
Xie, L., Zhang, Q., Sun, D., Yang, W., Hu, J., Niu, L., et al. (2019). Virus-induced gene silencing in the perennial woody Paeonia ostii. Peer J. 7:e7001. doi: 10.7717/peerj.7001
Xiu, J. X., Xu, J., Zhang, T. J., and Liu, C. X. (2021). Modern research progress of traditional Chinese medicine Paeoniae radix Alba and prediction of its Q-markers. China J. Chin. Materia. Medica. 46, 5486–5495. doi: 10.19540/j.cnki.cjcmm.20210818.201
Xu, G., Cai, W., Gao, W., and Liu, C. (2016). A novel glucuronosyltransferase has an unprecedented ability to catalyse continuous two-step glucuronosylation of glycyrrhetinic acid to yield glycyrrhizin. New Phytol. 212, 123–135. doi: 10.1111/nph.14039
Xue, J. H., Xiao, T. H., Wang, X. H., and Hu, H. X. (1992). A study on the quality of Bozhou radix Paeoniae Alba in Anhui. J. Chin. Med. Materia. 15, 37–40. doi: 10.13863/j.issn1001-4454.1992.12.014
Yan, A. D., Song, S., Sun, Y. H., and Xie, J. (2019). Characterization of the temporal changes in the content of paeonol and paeoniflorin components in Fengdan cortex. J. Chin. Med. Materia. 42, 541–544. doi: 10.13863/j.issn1001-4454.2019.03.012
Yan, X. L., Zhang, J. J., Li, X. Y., Du, S. Y., and Li, W. (2003). Influence of temperature on the stability of paeoniflorin in radix Paeonia, extract and Qiankunqing granules. Chin. Tradit. Herb. Drug 34, 131–133.
Yan, Z. G., Xie, L. H., Li, M. C., Yuan, M., Tian, Y., Sun, D. Y., et al. (2021). Phytochemical components and bioactivities of novel medicinal food–peony roots. Food Res. Int. 140:109902. doi: 10.1016/j.foodres.2020.109902
Yang, S. S., Song, P. S., Luo, X. P., Zhang, B. C., and Mu, X. H. (2001). Analysis of paeonol and paeoniflorin in Paeonia rockii (S.G.haw et L.A.Laeuner) T.Hong et j.j.Li. Northwest. Pharm. J. 16, 108–109. doi: 10.3969/j.issn.1004-2407.2001.03.007
Yang, Y., Li, S. S., Silva, J. A. T. D., Yu, X. N., and Wang, L. S. (2020). Characterization of phytochemicals in the roots of wild herbaceous peonies from China and screening for medicinal resources. Phytochemistry 174:112331. doi: 10.1016/j.phytochem.2020.112331
Yu, J., and Xiao, P. G. (1985). Ontogenetic chemical changes of the active constituents in mudan (Paeonia suffruticosa) and shaoyao (P. lactiflora). Acta Pharm. Sin. 20, 782–784. doi: 10.16438/j.0513-4870.1985.10.011
Yu, J., and Xiao, P. G. (1987). A preliminary study of the chemistry and systematics of Paeoniaceae. Acta Pharm. Sin. 25, 172–179.
Yuan, M., Yan, Z. G., Sun, D. Y., Luo, X. N., Xie, L. H., Li, M. C., et al. (2020). New insights into the impact of ecological factor on bioactivities and phytochemical composition of Paeonia veitchii. Chem. Biodivers. 17:e2000813. doi: 10.1002/cbdv.202000813
Yuan, Y., Yu, J., Jiang, C., Li, M., Lin, S., Wang, X., et al. (2013). Functional diversity of genes for the biosynthesis of paeoniflorin and its derivatives in Paeonia. Int. J. Mol. Sci. 14, 18502–18519. doi: 10.3390/ijms140918502
Zebec, Z., Wilkes, J., Jervis, A. J., Scrutton, N. S., Takano, E., and Breitling, R. (2016). Towards synthesis of monoterpenes and derivatives using synthetic biology. Curr. Opin. Chem. Biol. 34, 37–43. doi: 10.1016/j.cbpa.2016.06.002
Zhang, L. L., and Wei, W. (2020). Anti-inflammatory and immunoregulatory effects of paeoniflorin and total glucosides of paeony. Pharmacol. Ther. 207:107452. doi: 10.1016/j.pharmthera.2019.107452
Zhang, M. (2020). Study on Genetic Diversity of Medicinal Plant Paeoniae radix Rubra and Correlation Between Paeoniforin and Climatic Factors. Huhhot: Inner Mongolia University.
Zhang, X. R. (2000). Determination of paeoniflorin in Paeoniae lactiflora at different growth ages by TCL. Chin. Tradit. Herb. Drug 31, 832–833. doi: 10.7501/j.issn.0253-2670.2000.11.2000011526
Zhang, Y. G., Zhang, S. J., Bian, T. T., Si, X. L., Niu, J. T., Xin, E. D., et al. (2019a). New progress in pharmacological action of paeoniflorin. Chin. Tradit. Herb. Drug 50, 3735–3740. doi: 10.7501/j.issn.0253-2670.2019.15.033
Zhang, X. X., Zhai, Y. H., Yuan, J. H., and Hu, Y. H. (2019b). New insights into Paeoniaceae used as medicinal plants in China. Sci. Rep. 9, 18469. doi: 10.1038/s41598-019-54863-y
Zhao, Y. J., Cheng, Q. Q., Su, P., Chen, X., Wang, X. J., Gao, W., et al. (2014). Research progress relating to the role of cytochrome P450 in the biosynthesis of terpenoids in medicinal plants. Appl. Microbiol. Biotechnol. 98, 2371–2383. doi: 10.1007/s00253-013-5496-3
Zhou, H. T., Luo, Y. Q., Hu, S. L., Li, R. K., Liu, H. W., and Feng, X. F. (2003). A comparative study on content of major constituents between radix Paeoniae Rubra and radix Paeoniae Alba by HPCE. Chin. Pharm. J. 38, 654–656. doi: 10.3321/j.issn:1001-2494.2003.09.005
Zhou, S. L., Xu, C., Liu, J., Yu, Y., Wu, P., and Cheng, T. (2021). Out of the pan-Himalaya: evolutionary history of the Paeoniaceae revealed by phylogenomics. J. Syst. Evol. 59, 1170–1182. doi: 10.1111/jse.12688
Keywords: Paeoniaceae, Paeoniflorin, influencing factors, biosynthesis, post-modification enzyme, Monoterpene
Citation: Zhang X-X, Zuo J-Q, Wang Y-T, Duan H-Y, Yuan J-H and Hu Y-H (2022) Paeoniflorin in Paeoniaceae: Distribution, influencing factors, and biosynthesis. Front. Plant Sci. 13:980854. doi: 10.3389/fpls.2022.980854
Received: 29 June 2022; Accepted: 02 August 2022;
Published: 02 September 2022.
Edited by:
Zhiping Che, Henan University of Science and Technology, ChinaReviewed by:
Xiaobo Huang, Henan University of Science and Technology, ChinaCopyright © 2022 Zhang, Zuo, Wang, Duan, Yuan and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun-Hui Yuan, eXVhbmp1bmh1aWdzbHlAMTI2LmNvbQ==; Yong-Hong Hu, aHV5b25naG9uZ0Bjc25iZ3NoLmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.