- 1Department of Archaeology and Anthropology, University of Chinese Academy of Sciences, Beijing, China
- 2Key Laboratory of Vertebrate Evolution and Human Origins, Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences, Beijing, China
- 3Center for Excellence in Life and Paleoenvironment, Chinese Academy of Sciences, Beijing, China
Tibetan Plateau is the “third pole” of Earth and significantly influences the world’s ecosystems. However, limited work on phytolith analysis has been done due to its harsh environment, and no study on phytolith production and morphotypes in modern plants on the Tibetan Plateau has been carried out yet. In this study, we investigated 73 modern plant samples collected on the Tibetan Plateau to study phytolith production and morphology. The results showed that the major phytolith producers are Poaceae and Cyperaceae plants, the production of phytolith is higher than 0.4 million grains/g in most samples. We found one new morphotype, BILOBATE SADDLE, which could be the diagnostic type for Tribe Stipeae and phytoliths morphotypes might indicate different hydrological conditions on the Tibetan Plateau. Our findings add new information about phytoliths on the Tibetan Plateau and will aid the future phytolith analysis in this region.
Introduction
The Tibet Plateau (TP) is well known as the “Earth’s Third Pole,” with an average altitude of 4,000 m (Zheng and Yao, 2004). The TP has a unique but vulnerable ecosystem due to the anoxic and strong ultraviolet radiation environment, which is highly sensitive to climate change and human activities (Yao and Zhu, 2015). Therefore, it is crucial to understand the past environmental changes for policy-making and address future climate changes (Chen D.L. et al., 2015). The TP also played an important role in understanding the dispersal of modern humans in Asia (Bae et al., 2017). Meanwhile, the time humans appeared on the TP (Zhang et al., 2018; Chen et al., 2019) and their permanent occupation of the TP (Chen F.H. et al., 2015) are fascinating topics in archeology. However, much more details of how and when humans conquered the TP remain to be studied.
In TP, fossil pollen assemblages and charred seeds are most used as indicators for the past environment (Tang et al., 2021) and agricultural activities (Gao et al., 2020; Ren et al., 2020; Wang et al., 2020), respectively. However, less attention has been paid to phytolith analysis (Chen et al., 2008). Phytolithsr are micro silica bodies originating from plant cells (Piperno, 1988), which are of high taxonomical value and have been applied in reconstructing the paleoenvironment (Barboni et al., 2010; Gu et al., 2012; Stromberg et al., 2013; Zhang et al., 2020; Li et al., 2021) and prehistory agricultural activities (Piperno et al., 2009; Deng et al., 2017; Wang et al., 2017; Zhang et al., 2017; Dunseth et al., 2019; Huan et al., 2022) in many regions. In contrast with pollen and charred seeds, the in situ deposition of phytoliths could provide more local information (Rovner, 1971) and phytoliths are more durable in fire pits or environments where organic matter is hard to preserve (Piperno, 2006). Previous studies implied that regional modern phytoliths references are crucial for the better interpretation of sedimental phytoliths assemblages (Fredlund and Tieszen, 1997; Prebble et al., 2002; Lu and Liu, 2005; Lu et al., 2006; Liu et al., 2018). Thus, phytolith analysis could be a promising tool for reconstructing TP’s paleoenvironment and past agricultural activities.
The primary vegetation type on the TP is alpine grassland and alpine meadow, and the dominant species are mainly from Poaceae and Fabaceae (Tsering et al., 2021), which are typical phytoliths producers and non-producers. The most cultivated crops on the TP are naked barley (Hordeum vulgare var. nudum), which is also a phytolith producer. However, no study on modern plant phytoliths has been published yet, which resulted in a lack of knowledge on diagnostic phytolith types and the relationship between phytoliths and environmental factors on the TP. Thus, the study on modern plants could provide insight into the phytolith assemblages and further aid the studies on reconstructing the regional paleoenvironment and past agricultural activities on the TP.
Materials and Methods
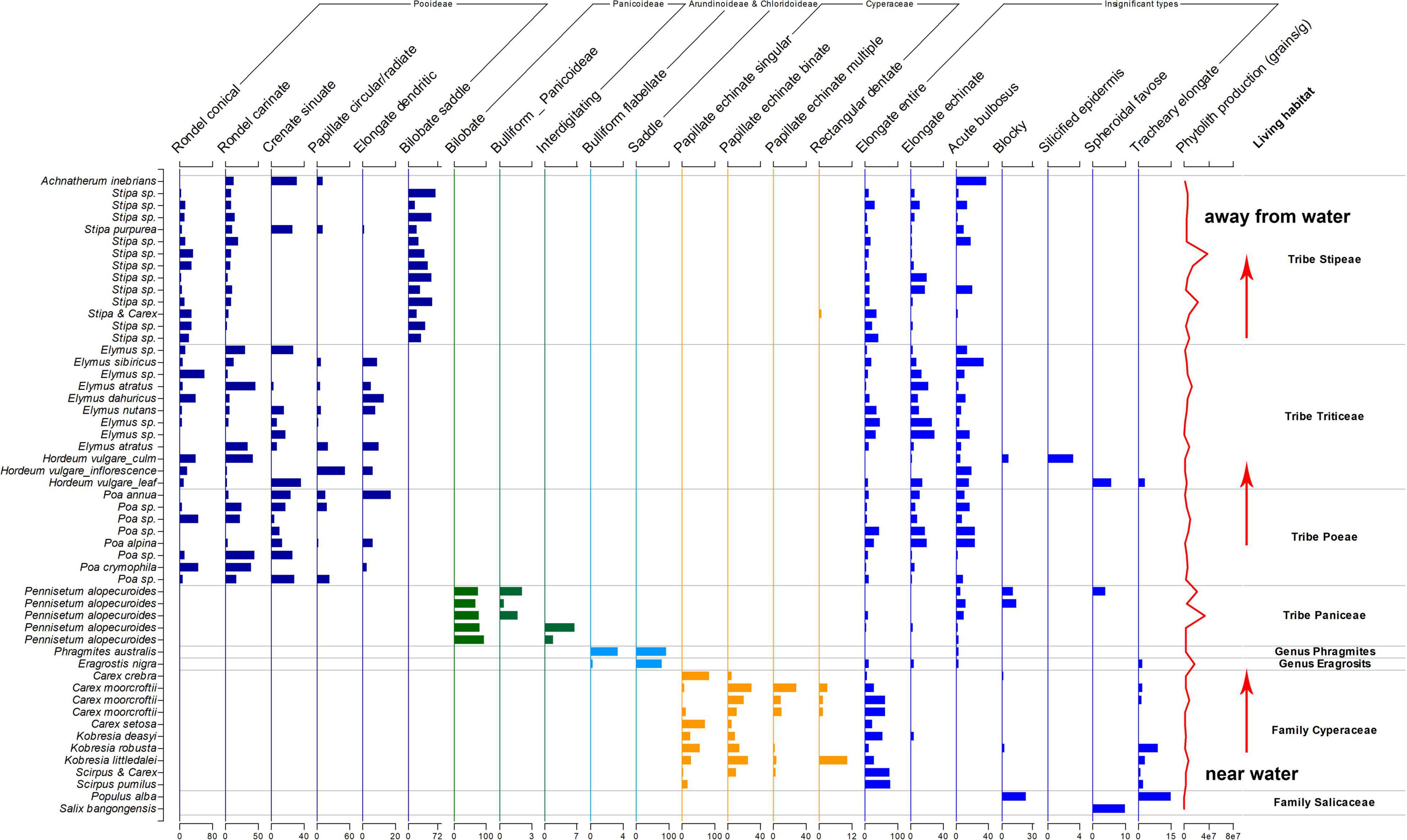
The samples for this study mainly were collected on the northern high plateau of Tibet (above 4,000 m) in July and August 2018, where the vegetation type is the alpine meadow (Tsering et al., 2021). The growing season is from June to August in the northern high plateau of Tibet, when the monthly temperature and precipitation could be above 10°C and 20 mm, respectively, (Hu et al., 2022); the sampling sites in this region (Figure 1) shared the same climate. Plant samples collected in this study were fully ripened and could be found with seeds (if not eaten by animals). However, most plant samples were no higher than 20 cm due to the grazing in these regions, and inflorescences were mostly eaten by animals. Thus, the identification of plant samples was limited to genera level and sometimes to species level (if applicable). A total of 73 plant specimens were involved in this study and ultrasonically cleaned with distilled water to clean the surface of collected aerial parts; all samples were dried in a drier and then weighted before phytolith extraction.
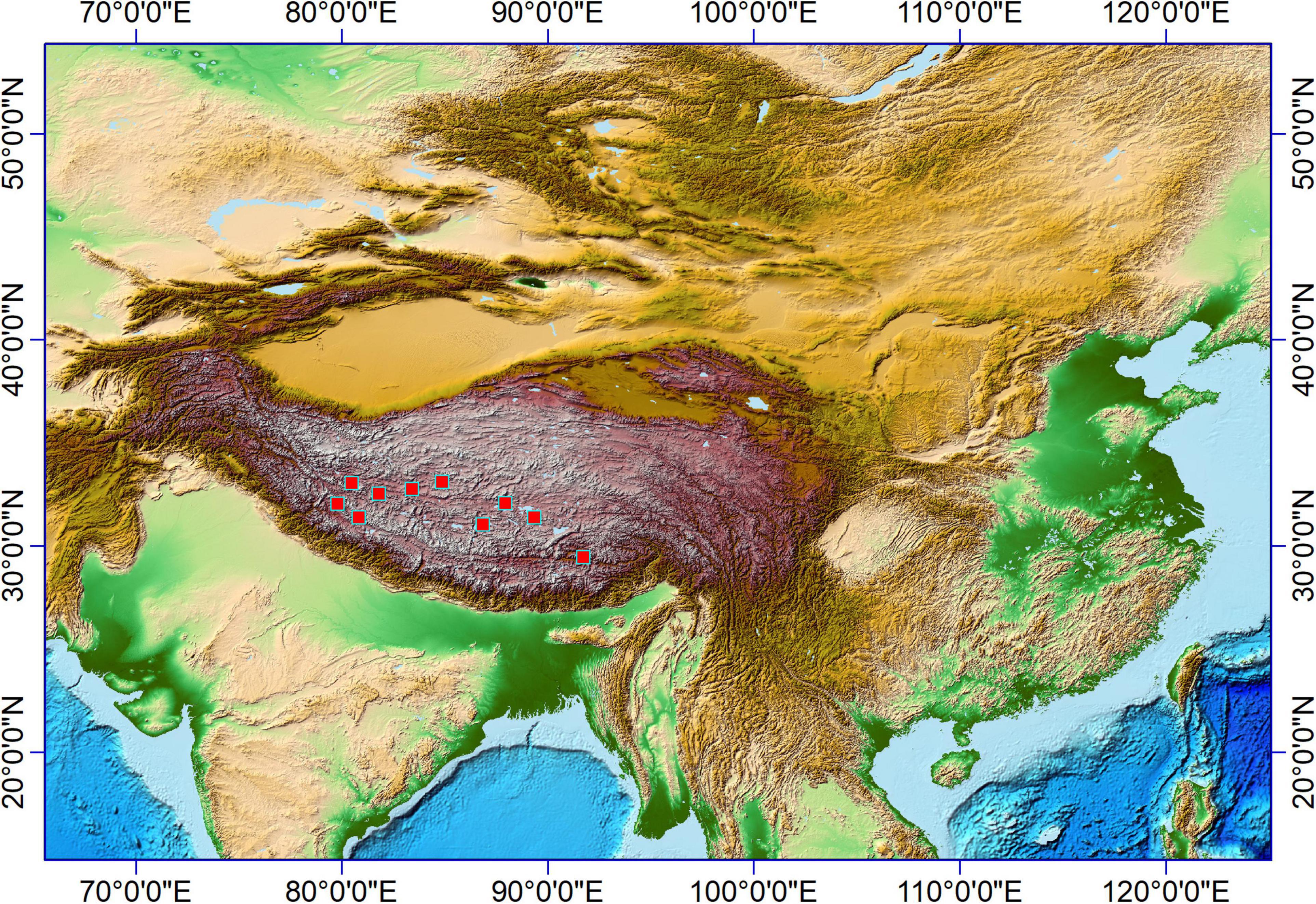
Figure 1. A map showed the Tibet Plateau and the surrounding regions. The red box shows the sampling sites.
Phytolith extraction followed the previously published wet oxidation method (Ge et al., 2020b). Additional polypodium tablet (ca. 27560 grains per tablet) is added to estimate the phytolith yield in each specimen (one tablet for one specimen). Phytolith count in each sample was above 300 grains, except for Salix bangongensis, in which we observed only one phytolith in two slides. As we knew that some taxa might not produce phytoliths, 21 plant specimens from Fabaceae, Asteraceae, Polygonaceae, and Primulaceae were identified at the family level, and after phytolith extraction, they were examined to be non-phytolith producers. Thus, only 52 samples (Table 1) were applied for further analysis. The identification and imaging of phytoliths were under 400× with a Leica DM750 microscope. Phytolith nomenclature follows the ICPN 2.0 rules (Neumann et al., 2019) and a previous study (Ge et al., 2020a).
Results
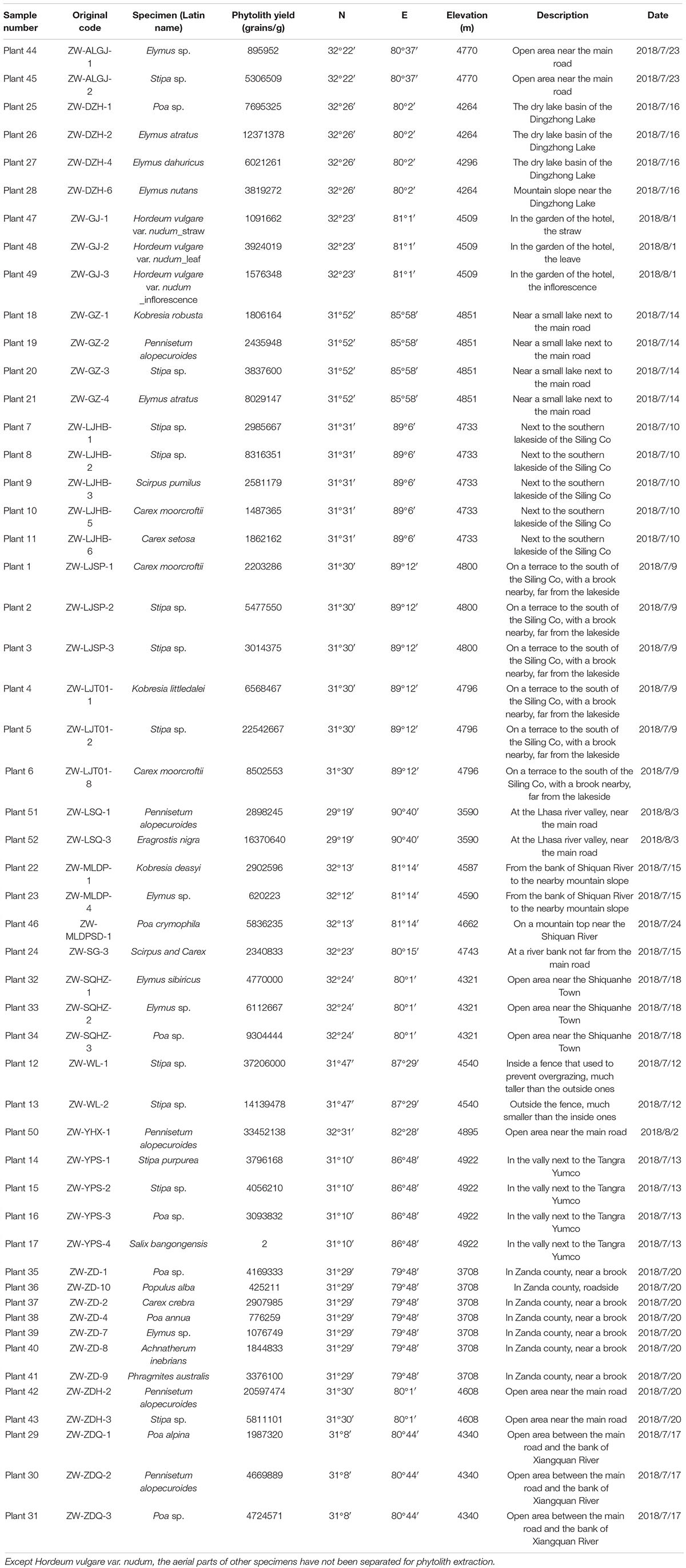
Phytoliths are found in three families: Poaceae, Cyperaceae, and Salicaceae. The highest production is 37 million grains/g in a Stipa sp. sample, while the lowest is two grains/g in S. bangongensis, and all other samples are higher than 0.4 million grains/g (Table 1). In Poaceae plants, phytolith production range from 0.6 million to 37.2 million grains/g, with an average of 7.2 million grains/g; in Cyperaceae plants, phytolith production range from 1.4 million to 8.5 million grains/g, with an average of 3.3 million grains/g; in Salicaceae plants, S. bangongensis barely produces phytoliths while Populus alba produces 0.4 million grains/g. Phytolith production is lowest in Salicaceae and highest in Poaceae; the production of Poaceae plants is twice higher than that of Cyperaceae with a larger SD (8258549.7 for Poaceae and 2316185.8 for Cyperaceae).
There are 22 morphotypes of phytoliths observed in the studied species, including diagnostic morphotypes produced in specific subfamilies and common morphotypes found in different families.
In Pooideae plants, RONDEL CONICAL (Figure 2-1), RONDEL CARINATE (Figure 2-2), CRENATE SINUATE (Figure 2-3), BILOBATE SADDLE (Figure 2-4), ELONGATE DENDRITIC (Figure 2-6,7) and PAPILLATE CIRCULAR/RADIATE (Figure 2-9,10) are diagnostic phytolith types; BLOCKY (Figure 2-8) and SILICIFIED EPIDERMIS (Figure 2-5) are insignificant types. ELONGATE DENDRITIC and PAPILLATE CIRCULAR/RADIATE originated from the inflorescence, SILICIFIED EPIDERMIS originated from the straw, and other types originated from leaves. BILOBATE SADDLE (Figure 2-4) from Tribe Stipae has a dumbbell-shaped base with one bracket-shaped ridge on each lobe; the two bracket-shaped ridges on each lobe together form the saddle top. On the other hand, the typical BILOBATE (Figure 3-4,5) from Panicoideae has a dumbbell-shaped base with two bracket-shaped ridges on each lobe. The morphological difference: one or two bracket-shaped ridges on top of the dumbbell base could be the discriminate criteria for distinguishing BILOBATE SADDLE from Tribe Stipae with BILOBATE from Panicoideae.
Figure 2. Phytoliths morphotypes in Pooideae plants. 1. RONDEL CONICAL; 2. RONDEL CARINATE; 3. CRENATE SINUATE; 4. BILOBATE SADDLE; 5. SILICIFIED EPIDERMIS; 6 and 7. ELONGATE DENDRITIC; 8. BLOCKY; 9. PAPILLATE CIRCULAR; and 10. PAPILLATE RADIATE.
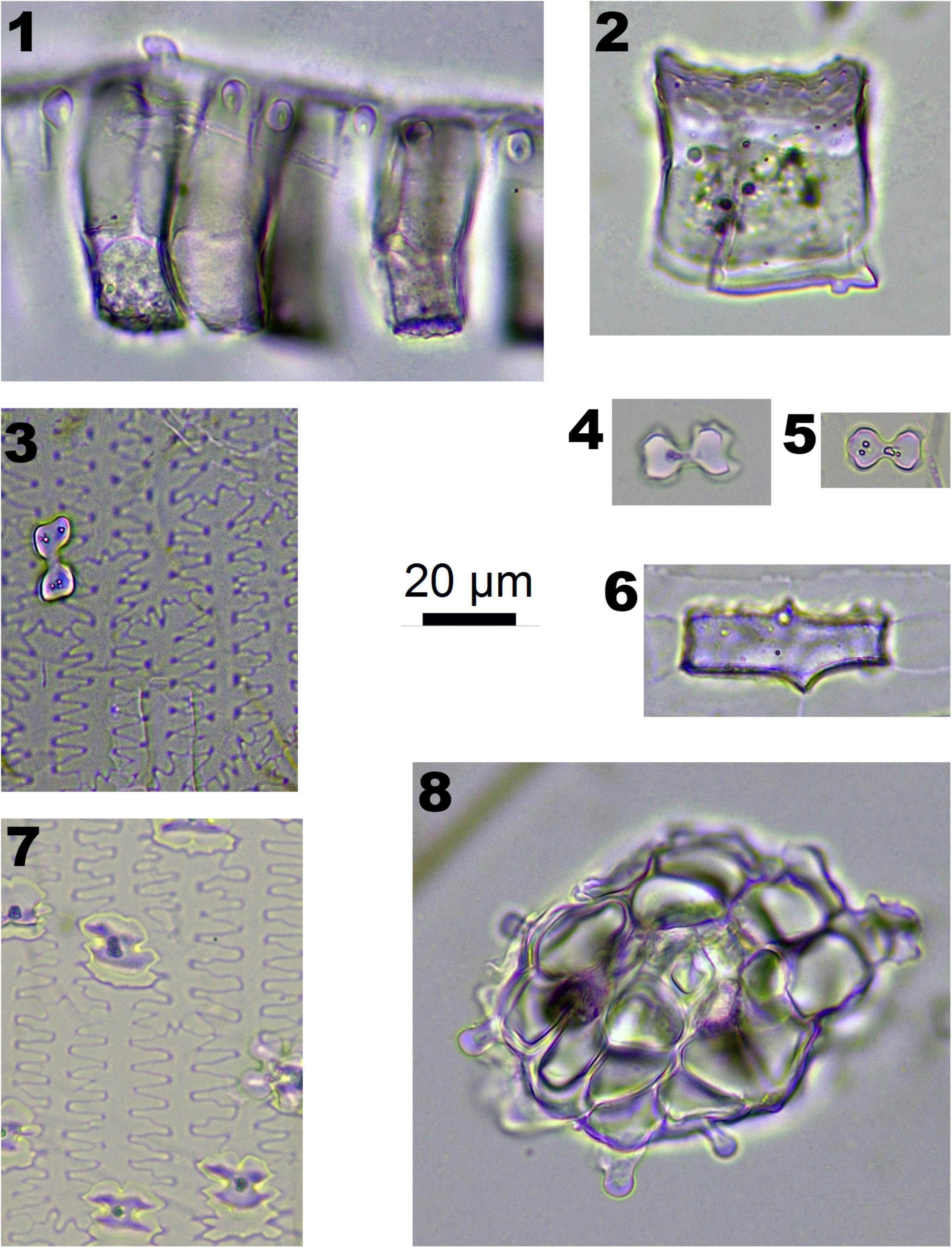
Figure 3. Phytoliths morphotypes in Panicoideae plants. 1 and 2. Bottom and side view of BULLIFORM; 3 and 7. INTERDIGITATING; 4 and 5. BILOBATE; 6. BLOCKY; and 8. SPHEROIDAL FAVOSE.
In Panicoideae plants (one species, Pennisetum alopecuroides), BILOBATE (Figure 3-4,5) and INTERDIGITATING (Figure 3-3,7) are diagnostic phytolith types. BULLIFORM (Figure 3-1,2), BLOCKY (Figure 3-1,2) and SPHEROIDAL FAVOSE (Figure 3-8) are insignificant types. BULLIFORM was found in three specimens, which originated from leaves. INTERDIGITATING was found in two specimens, which originated from the inflorescence.
In Arundinoideae (one species, Phragmites australis) and Chloridoideae (one species, Eragrostis nigra), Bulliform flabellate (Figure 4-6) and Saddle (Figure 4-4,7) are diagnostic types. Although these two specimens were collected at the lower elevation basin area (around 3,700 m), they were not observed in the high elevation area (over 4,000 m).
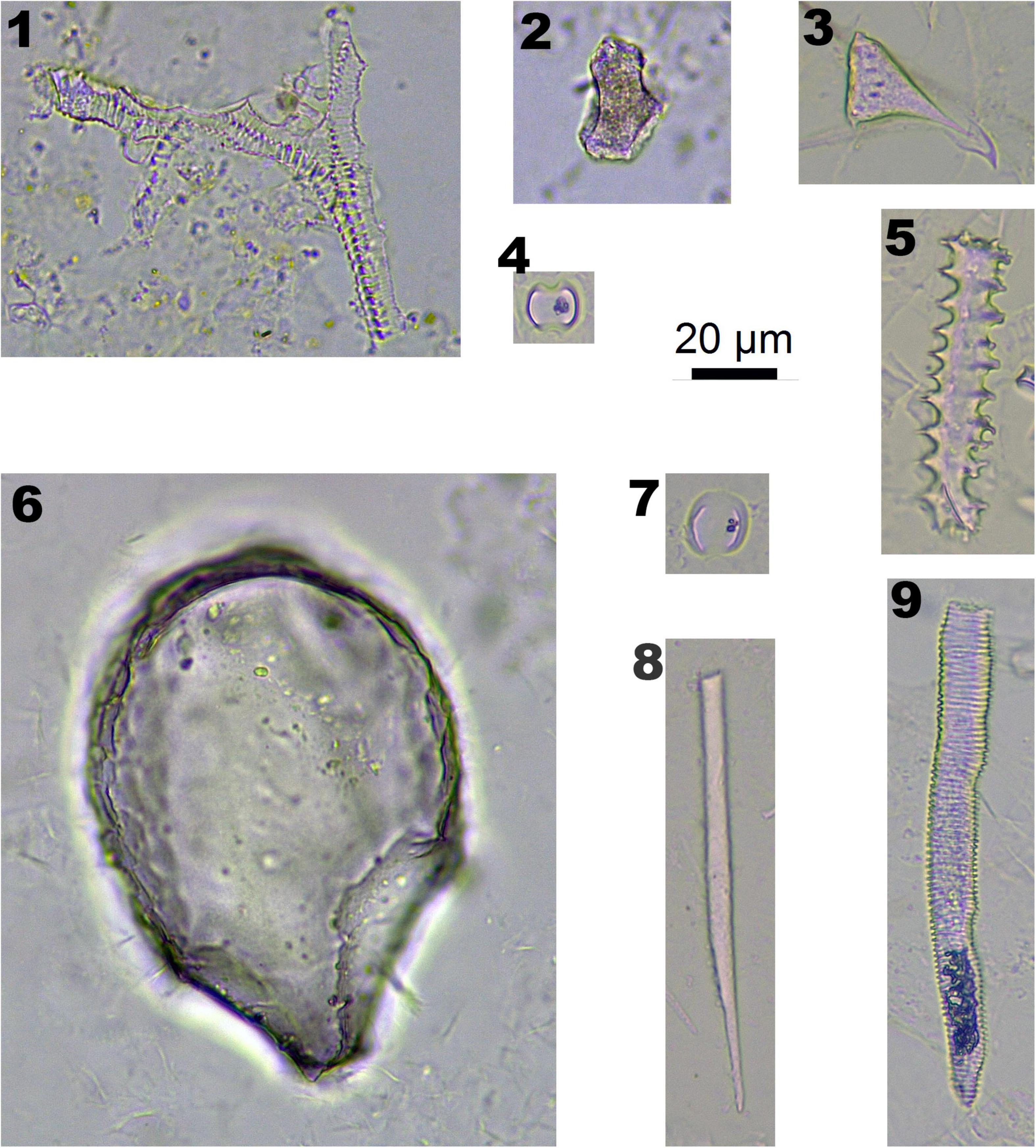
Figure 4. Phytoliths morphotypes in Arundinoideae, Chloridoideae, and Salicaceae. 1. TRACHEARY, in Populus alba; 2. BLOCKY, in Populus alba; 3. ACUTE BULBOSUS, in Poaceae and Cyperaceae plants; 4. SADDLE, in Eragrostis nigra; 5. ELONGATE ECHINATE, in Poaceae and Cyperaceae plants; 6. BULLIFORM FLABELLATE, in Phragmites australis; 7. SADDLE, in Phragmites australis; 8. ELONGATE ENTIRE, in Poaceae and Cyperaceae plants; and 9. TRACHEARY ELONGATE, in Poaceae and Cyperaceae plants.
In Cyperaceae, PAPILLATE phytolith is the dominant and diagnostic type. PAPILLATE phytoliths have three major morphotypes, PAPILLATE SINGULAR (Figure 5-1,4,6) with one conical papilla, PAPILLATE BINATE (Figure 5-7,9) with two conical papillae and PAPILLATE MULTIPLE (Figure 5-2,5,8) with multiple conical papillae. Another potential diagnostic type is RECTANGULAR DENTATE (Figure 5-3) which originated from the inflorescence. It has a rectangular shape with dentate long sides and smooth short sides.

Figure 5. Phytoliths morphotypes in Cyperaceae plants. 1. PAPILLATE SINGULAR; 2,5, and 8. PAPILLATE MULTIPLE; 3. RECTANGULAR DENTATE; 4 and 6. PAPILLATE SINGULAR; 7 and 9. PAPILLATE BINATE; and 10. BLOCKY.
In Salicaceae, TRACHEARY (Figure 4-1) and BLOCKY (Figure 4-2) were observed in P. alba, and only one SPHEROIDAL FAVOS phytolith was observed in S. bangongensis. However, these types of phytoliths could also be found in other plants.
Some phytolith types could be observed in many species, which are defined as insignificant types, including ACUTE BULBOSUS (Figure 4-3), ELONGATE ECHINATE (Figure 4-5), ELONGATE ENTIRE (Figure 4-8), and TRACHAERY ELONGATE (Figure 4-9). These phytolith types are common in both Poaceae and Cyperaceae plants.
The assemblages of phytoliths types showed significant differences on the family level, tribe level or genus level, as shown in Figure 6. It is possible to distinguish different taxon using phytoliths assemblages on the TP.
Discussion
In Tibetan Plateau, overgrazing is the primary biotic stress during the growing season, and grassland degradation has become a major threat to the sustainable development of livestock raising (Niu et al., 2019; Zhan, 2020). As a result, most samples collected in this study have been eaten by herbivores, the aerial parts are no higher than 20 cm. Only one specimen (Stipa sp.) was collected inside a closed fence, which grew 50 cm higher, and the phytolith production is the highest of all samples (37 million grains per gram of dry weight). While the specimen collected outside the fence grew only no higher than 10 cm, and the phytolith production was much lower (14 million grains per gram of dry weight). It has been known that phytoliths deposit while plants grow (Ma and Yamaji, 2006) and increase with the evapotranspiration (Madella et al., 2009): inside the closed fence, plants could grow better and have higher evapotranspiration; thus, they accumulate more phytoliths. In recent years, phytoliths have been considered a long term carbon sink (Carter, 2009; Song et al., 2022). However, in Tibet, overgrazing influenced the biomass of plants and decreased the production of phytoliths, which might eventually influence the sustainable development of grassland and carbon sink in these regions.
On the northern Tibetan Plateau, most plants grow during the growing season (Che et al., 2014). Thus, the temperature and precipitation are the same during the phytolith accumulation in the sampling sites (Hu et al., 2022). However, humidity showed to bea more critical factor in the growth of plants (Fu and Shen, 2016). During the exploration and sampling, we found that Cyperaceae plants only grew in or near the open water and formed sedge communities; away from the open water, Poaceae plants became dominant species and formed grass communities. Within the Poaceae plants, P. australis and E. nigra grew near water, P. alopecuroides grew near, or not far from open water, Stipa sp. grew better in drier areas than Poa sp. Thus, the phytoliths producers in this study showed that the hydrological conditions influenced their distribution; in other words, the vegetation changed along the hydrological gradient. In many cases, phytolith types have been considered to represent particular climate and environment types (Fredlund and Tieszen, 1997; Boyd, 2005; Gu et al., 2007; Li et al., 2017; Zuo et al., 2020). In China, RONDEL and CRENATE phytoliths from Pooideae plants indicated a cooler climate, BILOBATE phytoliths from Panicoideae plants indicated a warmer climate and PAPILLATE phytoliths from Cyperaceae plants were found to indicate a wetter environment (Wang and Lu, 1993). In contrast, the phytoliths types in Tibet might reflect the hydrological condition: PAPILLATE, BULLIFORM FLABELLATE, and SADDLE might indicate a strong humid environment, BILOBATE might indicate a less strong humid environment, RONDEL and CRENATE might indicate a medium humid environment, and BILOBATE SADDLE might indicate a relative drier environment. Such differences in the relationship between phytoliths types and environmental factors would lead to the different explanations of phytoliths combination in soil profiles on Tibetan Plateau, which also emphasizes the importance of regional study before the implication of phytolith analysis.
Conclusion
This study explored the phytolith production and types in modern plants on the Tibetan Plateau. The results showed that the major phytolith producers are Poaceae and Cyperaceae plants, and we have found that BILOBATE SADDLE might be the new diagnostic type for the discrimination of Tribe Stipeae. Furthermore, phytoliths morphotypes and aseemblages on the TP respond to the environmental hydrological gradients rather than temperature compare with other regions in China: PAPILLATE, BULLIFORM FLABELLATE, SADDLE, BILOBATE represented a more humid environment, RONDEL, CRENATE, and BILOBATE SADDLE represented a relative dryer environment. These new data on modern phytoliths morphotypes and assemblages, as well as their response to the hydrological conditions could help future studies on the identification of phytoliths origins and the reconstruction of paleohydrological conditions.
Data Availability Statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
YG and XZ designed the research. YG, YJ, and XZ collected the samples. YG performed the experiment, and carried out the image process and data analysis. All authors were involved in the writing and discussion of the manuscript, read and approved the final manuscript.
Funding
This study was jointly supported by the National Natural Science Foundation of China (Grant Nos. 42072033 and 41802021) and the China Postdoctoral Science Foundation (Grant Nos. 2021T140654 and 2018M641480).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Junyi Ge, Fang Han, Yuchao Zhao, and Wei He for their assistance during the field trip and sample collection.
References
Bae, C. J., Douka, K., and Petraglia, M. D. (2017). On the origin of modern humans: asian perspectives. Science 358:eaai9067.
Barboni, D., Ashley, G. M., Dominguez-Rodrigo, M., Bunn, H. T., Mabulla, A. Z. P., and Baquedano, E. (2010). Phytoliths infer locally dense and heterogeneous paleovegetation at FLK North and surrounding localities during upper Bed I time, Olduvai Gorge, Tanzania. Quat. Res. 74, 344–354. doi: 10.1016/j.yqres.2010.09.005
Boyd, M. (2005). Phytoliths as paleoenvironmental indicators in a dune field on the northern Great Plains. J. Arid Environ. 61, 357–375. doi: 10.1016/j.jaridenv.2004.09.015
Carter, J. A. (2009). Atmospheric carbon isotope signatures in phytolith-occluded carbon. Quat. Int. 193, 20–29. doi: 10.1016/j.quaint.2007.11.013
Che, M. L., Chen, B. Z., Innes, J. L., Wang, G. Y., Dou, X. M., Zhou, T. M., et al. (2014). Spatial and temporal variations in the end date of the vegetation growing season throughout the Qinghai–Tibetan Plateau from 1982 to 2011. Agric. For. Meteorol. 189-190, 81–90. doi: 10.1016/j.agrformet.2014.01.004
Chen, D. L., Xu, B. Q., Yao, T. D., Guo, Z. T., Cui, P., Chen, F. H., et al. (2015). Assessment of past, present and future environmental changes on the Tibetan Plateau. Chin. Sci. Bull. 60, 3021–3022.
Chen, F. H., Dong, G. H., Zhang, D. J., Liu, X. Y., Jia, X., An, C. B., et al. (2015). Agriculture facilitated permanent human occupation of the Tibetan Plateau after 3600 B.P. Science 347, 248–250. doi: 10.1126/science.1259172
Chen, F., Welker, F., Shen, C.-C., Bailey, S. E., Bergmann, I., Davis, S., et al. (2019). A late middle pleistocene denisovan mandible from the Tibetan Plateau. Nature 569, 409–412. doi: 10.1038/s41586-019-1139-x
Chen, L. K., Guo, J. Q., and Gu, Y. S. (2008). Characteristics of phytolith aseemblages from sediments of modern river floodplain and first terraces in Lhasa River, Tibet. Acta Sedimentol. Sin. 26:8.
Deng, Z. H., Hung, H. C., Fan, X. C., Huang, Y. M., and Lu, H. Y. (2017). The ancient dispersal of millets in southern China: new archaeological evidence. Holocene 28, 34–43.
Dunseth, Z. C., Fuks, D., Langgut, D., Weiss, E., Melamed, Y., Butler, D. H., et al. (2019). Archaeobotanical proxies and archaeological interpretation: a comparative study of phytoliths, pollen and seeds in dung pellets and refuse deposits at Early Islamic Shivta, Negev, Israel. Quat. Sci. Rev. 211, 166–185. doi: 10.1016/j.quascirev.2019.03.010
Fredlund, G. G., and Tieszen, L. L. (1997). Calibrating grass phytolith assemblages in climatic terms: application to late pleistocene assemblages from Kansas and Nebraska. Palaeogeogr. Palaeoclimatol. Palaeoecol. 136, 199–211.
Fu, G., and Shen, Z. X. (2016). Environmental humidity regulates effects of experimental warming on vegetation index and biomass production in an alpine meadow of the Northern Tibet. PLoS One 11:e0165643. doi: 10.1371/journal.pone.0165643.g001
Gao, Y., Yang, J. S., Ma, Z. K., Tong, Y., and Yang, X. Y. (2020). New evidence from the Qugong site in the central Tibetan Plateau for the prehistoric highland silk road. Holocene 31, 230–239. doi: 10.1177/0959683620941144
Ge, Y., Lu, H. Y., Wang, C., and Gao, X. (2020b). Phytoliths in selected broad-leaved trees in China. Sci. Rep. 10:15577. doi: 10.1038/s41598-020-72547-w
Ge, Y., Lu, H., Zhang, J., Wang, C., and Gao, X. (2020a). Phytoliths in inflorescence bracts: preliminary results of an investigation on common panicoideae plants in China. Front. Plant Sci. 10:1736. doi: 10.3389/fpls.2019.01736
Gu, Y. S., Qiu, Y., and Wang, Z. X. (2007). Mid-late Pleistocene palaeovegetation and palaeoclimate changes: evidence from phytolith and molecular fossil records in vermicular red paleosols in Changxing, Zhejiang. Water Rock Interaction 1, 1485–1489.
Gu, Y. S., Wang, H. L., Huang, X. Y., Peng, H. X., and Huang, J. H. (2012). Phytolith records of the climate change since the past 15000 years in the middle reach of the Yangtze River in China. Front. Earth Sci. 6, 10–17. doi: 10.1007/s11707-012-0302-6
Hu, X. F., Wei, L. F., Cheng, Q., Wu, X. L., and Ni, J. (2022). A climate diagram atlas of Qingzang Plateau. Chin. J. Plant Ecol. 46, 1–9. doi: 10.17521/cjpe.2021.0360
Huan, X. J., Wei, X. T., Zhang, J. P., Li, J. D., Zhang, X. H., Shao, K. L., et al. (2022). Discovery of the earliest rice paddy in the mixed rice&–millet farming area of China. Land 11:831.
Li, D. H., Jie, D. M., Wang, Y., Liu, L. D., Liu, H. Y., Gao, G. Z., et al. (2017). Holocene climate reconstruction based on herbaceous phytolith indices from an AMS 14 C-dated peat profile in the Changbai Mountains, northeast China. Quat. Int. 447, 144–157.
Li, N., Sharifi, A., Chambers, F. M., Ge, Y., Dubois, N., Gao, G., et al. (2021). Linking holocene east asian monsoon variability to solar forcing and ENSO activity: multi-proxy evidence from a peatland in Northeastern China. Holocene 31, 966–982. doi: 10.1177/0959683621994662
Liu, H. Y., Gu, Y. S., Lun, Z. J., Qin, Y. M., and Cheng, S. G. (2018). Phytolith-inferred transfer function for paleohydrological reconstruction of Dajiuhu peatland, central China. Holocene 28, 1623–1630. doi: 10.1177/0959683618782590
Lu, H. Y., and Liu, K. B. (2005). Phytolith assemblages as indicators of coastal environmental changes and hurricane overwash deposition. Holocene 15, 965–972. doi: 10.1191/0959683605hl870ra
Lu, H. Y., Wu, N. Q., Yang, X. D., Jiang, H., Liu, K. B., and Liu, T. S. (2006). Phytoliths as quantitative indicators for the reconstruction of past environmental conditions in China I: phytolith-based transfer functions. Quat. Sci. Rev. 25, 945–959. doi: 10.1016/j.quascirev.2005.07.014
Ma, J. F., and Yamaji, N. (2006). Silicon uptake and accumulation in higher plants. Trends Plant Sci. 11, 392–397. doi: 10.1016/j.tplants.2006.06.007
Madella, M., Jones, M. K., Echlin, P., Powers-Jones, A., and Moore, M. (2009). Plant water availability and analytical microscopy of phytoliths: implications for ancient irrigation in arid zones. Quat. Int. 193, 32–40. doi: 10.1016/j.quaint.2007.06.012
Neumann, K., Stromberg, C. A. E., Ball, T., Albert, R. M., Vrydaghs, L., and Cummings, L. S. (2019). International code for phytolith nomenclature (ICPN) 2.0. Ann. Bot. 124, 189–199. doi: 10.1093/aob/mcz064
Niu, Y. J., Zhu, H. M., Yang, S. W., Ma, S. J., Zhou, J. W., Chu, B., et al. (2019). Overgrazing leads to soil cracking that later triggers the severe degradation of alpine meadows on the Tibetan Plateau. Land Degrad. Dev. 30, 1243–1257. doi: 10.1002/ldr.3312
Piperno, D. R. (1988). Phytolyth Analysis: An Archaeological and Geological Perspective. San Diego, CA: Academic Press, Inc.
Piperno, D. R. (2006). Phytoliths: A Comprehensive Guide for Archaeologists and Paleoecologists. Lanham, MA: AltaMira Press.
Piperno, D. R., Ranere, A. J., Holst, I., Iriarte, J., and Dickau, R. (2009). Starch grain and phytolith evidence for early ninth millennium B.P. maize from the Central Balsas River Valley, Mexico. Proc. Natl. Acad. Sci. U.S.A. 106, 5019–5024. doi: 10.1073/pnas.0812525106
Prebble, M., Schallenberg, M., Carter, J., and Shulmeister, J. (2002). An analysis of phytolith assemblages for the quantitative reconstruction of late quaternary environments of the Lower Taieri Plain, otago, South Island, New Zealand I. Modern assemblages and transfer functions. J. Paleolimnol. 27, 393–413. doi: 10.1023/A:1020318803497
Ren, L. L., Dong, G. H., Liu, F. W., d’Alpoim-Guedes, J., Flad, R. K., Ma, M. M., et al. (2020). Foraging and farming: archaeobotanical and zooarchaeological evidence for neolithic exchange on the Tibetan Plateau. Antiquity 94, 637–652. doi: 10.15184/aqy.2020.35
Rovner, I. (1971). Potential of opal phytoliths for use in paleoecological reconstruction. Quat. Res. 1, 343–359. doi: 10.1016/0033-5894(71)90070-6
Song, Z., Wu, Y., Yang, Y., Zhang, X., Van Zwieten, L., Bolan, N., et al. (2022). High potential of stable carbon sequestration in phytoliths of China’s grasslands. Global Change Biol. 28, 2736–2750. doi: 10.1111/gcb.16092
Stromberg, C. A. E., Dunn, R. E., Madden, R. H., Kohn, M. J., and Carlini, A. A. (2013). Decoupling the spread of grasslands from the evolution of grazer-type herbivores in South America. Nat. Commun 4, 1–8. doi: 10.1038/Ncomms2508
Tang, L., Shen, C., Lu, H., Li, C., and Ma, Q. (2021). Fifty years of quaternary palynology in the Tibetan Plateau. Sci. China Earth Sci. 64:20.
Tsering, L., Dekyi, P., Gao, Y., Phur, T., and Penpa, D. (2021). Analysis on vegetation type and dominant species in the Midwest Tibetan Plateau based on field investigation. Tibet Sci. Technol. 3:3.
Wang, C., Lu, H. Y., Gu, W. F., Zuo, X. X., Zhang, J. P., Liu, Y. F., et al. (2017). Temporal changes of mixed millet and rice agriculture in neolithic-bronze age central plain, China: archaeobotanical evidence from the Zhuzhai site. Holocene 28:0959683617744269.
Wang, Y. J., and Lu, H. Y. (1993). The Study of Phytolith and Its Application. Beijing: China Ocean Press.
Wang, Y. R., Gao, Y., Yang, J. S., Tan, Y. Y., Shargan, W. D., Zhang, S., et al. (2020). New evidence for early human habitation in the Nyingchi region, Southeast Tibetan Plateau. Holocene 31, 240–246. doi: 10.1177/0959683620970255
Yao, T. D., and Zhu, L. P. (2015). The response of environmental changes on Tibetan Plateau to gobal changes and adaptation strategy. Adv. Earth Sci. 21:6.
Zhan, T. Y. (2020). Response of Alpine Grassland Ecosystem to Grazing and Fencing in Tibetan Plateau. Master, Chengdu University of Technology, China.
Zhang, J. P., Lu, H. Y., Jia, J. W., Shen, C. M., Wang, S. Y., Chu, G. Q., et al. (2020). Seasonal drought events in tropical East Asia over the last 60,000 y. Proc. Natl. Acad. Sci. U.S.A. 117, 30988–30992. doi: 10.1073/pnas.2013802117
Zhang, J. P., Lu, H. Y., Jia, W. M., Flad, R., Wu, N. Q., and Betts, A. (2017). Cultivation strategies at the ancient Luanzagangzi settlement on the easternmost Eurasian steppe during the late Bronze Age. Veget. Hist. Archaeobot. 26, 505–512. doi: 10.1007/s00334-017-0608-0
Zhang, X. L., Ha, B. B., Wang, S. J., Chen, Z. J., Ge, J. Y., Long, H., et al. (2018). The earliest human occupation of the high-altitude Tibetan Plateau 40 thousand to 30 thousand years ago. Science 362, 1049–1051. doi: 10.1126/science.aat8824
Zheng, D., and Yao, T. D. (2004). Progress in research on fomation and evolution of tibetan plateau with its environment and resource effects. China Basic Sci. 2:7.
Keywords: phytolith production, phytolith morphology, Poaceae, Cyperaceae, Tibetan Plateau
Citation: Ge Y, Jin Y and Zhang X (2022) Phytolith Production and Morphotypes in Modern Plants on the Tibetan Plateau. Front. Plant Sci. 13:950322. doi: 10.3389/fpls.2022.950322
Received: 22 May 2022; Accepted: 22 June 2022;
Published: 11 July 2022.
Edited by:
Jianping Zhang, Institute of Geology and Geophysics (CAS), ChinaReviewed by:
Hongye Liu, China University of Geosciences Wuhan, ChinaZhenwei Qiu, National Museum of China, China
Xinxin Zuo, Fujian Normal University, China
Copyright © 2022 Ge, Jin and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoling Zhang, emhhbmd4aWFvbGluZ0BpdnBwLmFjLmNu
 Yong Ge
Yong Ge Yingshuai Jin2,3
Yingshuai Jin2,3 Xiaoling Zhang
Xiaoling Zhang