- 1Shapotou Desert Research and Experiment Station, Northwest Institute of Eco-Environment and Resources, Chinese Academy of Sciences, Lanzhou, China
- 2University of Chinese Academy of Sciences, Beijing, China
Desert shrubs play important roles in desertification control and vegetation restoration, which are particularly affected by droughts caused by climate change. However, the hydraulic strategies associated with hydraulic functional traits of desert shrubs remain unclear. Here, eight desert shrub species with different life forms and morphologies were selected for a common garden experiment at the southeast edge of the Tengger Desert in northern China to study the hydraulic strategies mediated by leaf hydraulic functional traits. Diurnal leaf water potential change, leaf hydraulic efficiency and safety, hydraulic safety margin, hydraulic capacitance, and water potential and relative water content at the turgor loss point were observed to significantly differ among species, suggesting that leaf hydraulic functional traits were strongly associated with species even when living in the same environment. Additionally, shrubs with greater leaf hydraulic efficiency had lower midday leaf water potential and leaf hydraulic safety, suggesting that leaf hydraulic efficiency had a strong trade-off with hydraulic safety and minimum leaf water potential, whereas there was also a coordination between leaf hydraulic safety and the leaf minimal water potential. Moreover, shrubs with higher leaf hydraulic capacitance had greater hydraulic safety margins, indicating coordination between leaf hydraulic capacitance and hydraulic safety margin. Overall, this study indicated that minimal daily leaf water potential, as an easily measured parameter, may be used preliminarily to predict leaf hydraulic conductivity and the resistance to embolism of desert shrubs, providing critical insights into hydraulic trade-off and coordination strategies for native shrubs as priority species in desert vegetation restoration and reconstruction.
Introduction
Revegetation is one of the most effective ways to control desertification and to promote ecological restoration in arid and semiarid regions (Li et al., 2004). To ameliorate desertification, the Chinese government started a series of ecological construction programs in the 1950s (Chu et al., 2019). Among them, the Three-North Shelterbelt Program (TNSP) spanning about 4.07 × 106 km2 of Northern China plays an important role in restoring the environment, e.g., water and soil conservation, serving as a windbreak, and promoting sand fixation (Zhang et al., 2021). Generally, desert shrubs are often used as pioneer species in ecological restoration owing to their high resistance to extreme environments and their positive role in altering surface wind and improving soil fertility (Gómez‐Aparicio, 2009; Bai et al., 2019). In recent years, however, frequent droughts induced by climate change have triggered the widespread withering and death of woody plants, which had severe impacts on ecosystem patterns and processes (Breshears et al., 2009; McDowell and Allen, 2015). For example, woody plant mortality events induced by drought have been reported internationally, i.e., in Alaskan and Amazonian rainforests, Mediterranean Europe, Australia, boreal forests of North America, and semiarid forests of the Southwest United States (Phillips et al., 2009; Williams et al., 2013; McDowell and Allen, 2015). As a consequence of water resource shortages and climate change, the central areas of Inner Mongolia, the northwestern areas of Xinjiang, and the northern areas of Shaanxi within the TNSP have experienced degradation such as canopy withering and even plant death (Yu et al., 2021). Thus, it is very important to understand the drought tolerance and adaptability of replanted shrubs in changing environments to guide revegetation practices.
Revegetation with native shrubs has been one of the most effective ways to control desertification (Zhao et al., 2013; Tian et al., 2019). This is owing to the fact that native shrubs can physiologically adapt to local climates more quickly than non-native species, and they have a strong ability to resist sand burial because the adventitious buds of their branches can give rise to roots and seedlings after sand burial (Luo and Zhao, 2019; Ma et al., 2019). Desert shrubs have extensive geographical range and have evolved a variety of morphological and physiological characteristics and life-history strategies (Xu et al., 2007; De Micco and Aronne, 2012), e.g., desert shrubs vary in crown size, life form, and root system structures (Zhang et al., 2009; Venturas et al., 2016). Additionally, leaf morphology can vary such as the production of smaller-size, split, or degenerated leaves (Zou et al., 2010; Zhang et al., 2016a), which can reduce water consumption in arid habitats (Abd El-Ghani et al., 2017). Correspondingly, the physiological characteristics of desert shrubs also vary with the environmental conditions to adapt to limited water availability (Liu et al., 2021), such as lower water potential at the turgor loss point (Ψtlp) and less xylem hydraulic conductivity (Scholz et al., 2012; Zhou et al., 2013). Additionally, desert shrub species are more resistant to embolism (more negative P50) owing to their ability to tolerate very negative water potentials (Lopez et al., 2005). Thus, they have higher xylem hydraulic safety margins that can avoid mortality triggered by short-term drought (Xu et al., 2011; Li et al., 2020). Unquestionably, the variation in morphological and physiological characteristics of desert plants results from life history strategies shaped in long-term adaptation to drought conditions and are the main reason for their survival in harsh desert habitats. However, how desert shrubs physiologically adapt to arid habitats through hydraulic strategies remains unclear.
In recent years, numerous studies have found that various functional traits of plants are coordinated or exhibit trade-offs with each other during physiological adaption to environmental changes (Henry et al., 2019; Rosas et al., 2019). Among these studies, the trade-off between hydraulic efficiency (stem-specific hydraulic conductivity, Ks) and hydraulic safety (the water potential at 50% loss of Ks, P50) is the most widely studied (Ocheltree et al., 2016). Woody plants with more negative P50 lead to increased tolerance of drought and sustain hydraulic conductivity (Choat et al., 2018). However, many species have low hydraulic efficiency and low hydraulic safety, which might be associated with other traits, such as wood density or leaf-to-sapwood area (Gleason et al., 2016). A trade-off also exists between hydraulic safety and capacitance (De Guzman et al., 2017). Species with high hydraulic capacitance survive during drought even without high safety because capacitance buffers hydraulic failure (Santiago et al., 2018). Additionally, coordination between hydraulic traits and other traits plays an important role in drought response strategies of species (Santiago et al., 2018), e.g., xylem hydraulic conductance coordinated with leaf gas exchange (Rodríguez-Gamir et al., 2021). However, although desert plants have evolved a series of life history traits in response to frequent drought, including critical morphological and physiological characteristics (Xu et al., 2007; De Micco and Aronne, 2012), the role of hydraulics in their whole-plant life history strategies remains unclear. Therefore, identifying the hydraulic strategies of desert shrubs with various life forms and morphologies is important for understanding the drought tolerance and survival of desert shrubs under arid habitats.
In this study, eight desert shrub species that grow in the same arid habitat but differ in life form and morphology were used to study the hydraulic strategies of desert plants mediated by leaf hydraulic traits. Our aims included the following: (1) evaluating the difference in leaf hydraulic traits among species and (2) revealing the trade-off and coordination among leaf hydraulic traits to identify hydraulic strategies.
Materials and methods
Study site and species
The study was conducted at the Shapotou Desert Research and Experiment Station, Chinese Academy of Sciences. The station is located at the southeast edge of the Tengger Desert in northern China (37°33′N, 105°02′E). The area is covered by dense and continuous reticulate barchan dunes, and its gravimetric moisture content is about 3%–4%. The mean annual temperature is 9.6°C, the extreme minimum temperature is -25.1°C, and the maximum temperature is 38.1°C. The mean annual precipitation is 186.2 mm. There are about 50 days of precipitation exceeding 0.1 mm, and approximately 80% of the precipitation days were less than 5 mm of precipitation (Zhang et al., 2016b). The average air relative humidity is 40% with a minimum value of 10%. The mean annual wind speed is 2.9 m·s−1, mainly northwesterly. The potential evapotranspiration during the growing season (May–September) is 2,300 to 2,500 mm (Zhang et al., 2014).
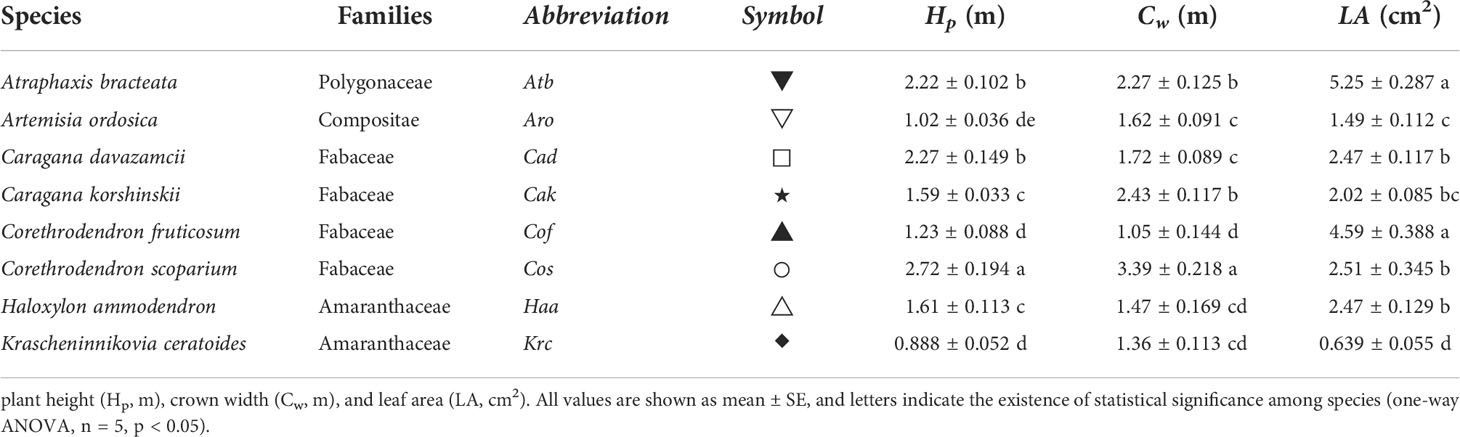
In 2010, the New Water Balance Experimental Fields (NWBEF; Supplementary Figure 1) were constructed by first leveling sand dunes and then erecting sand barriers using a 1 m × 1 m wheat-straw checkerboard pattern; next, 2-year-old seedlings introduced from the sandy areas of northern China were respectively planted at densities of 35 and 70 individuals per 100 m2 for shrubs and subshrubs, and each plot area was 600 m2. Subsequently, they grew in natural conditions without irrigation. This study was conducted from August to September in 2020, comprising eight species, namely, Atraphaxis bracteata, Artemisia ordosica, Caragana davazamcii (synonym: Caragana intermedia), Caragana korshinskii, Krascheninnikovia ceratoides (synonym: Ceratoides latens) , Haloxylon ammodendron, Corethrodendron fruticosum and Corethrodendron scoparium, which were selected from the NWBEF to study hydraulic traits (Figure 1). Among them, C. davazamcii, C. korshinskii, C. fruticosum, and C. scoparium are in the family Fabaceae, whereas K. ceratoides and H. ammodendron are in the family Amaranthaceae. The species A. ordosica and A. bracteata are from the families Compositae and Polygonaceae, respectively. These eight desert shrubs species are widely distributed in northern China (Supplementary Figure 2) and showed a larger difference in morphologies among genera (Figure 1; Supplementary Table 1). Two subshrub species A. ordosica and K. ceratoides have shallow root systems (mostly fine roots distributed in the upper 0.4 m of soil) with a broad lateral range to adequately absorb rainwater (Liu et al., 1991; Zhang et al., 2008); however, the other six shrub species have deep root systems (>3-m depth) that collect groundwater (Liu et al., 1991; Zhang et al., 2009; Feng et al., 2022). A. ordosica has full split needled leaves, whereas the leaves of C. davazamcii and C. korshinskii are pinnately compound with three to eight pairs of densely pilose leaflets (Zhang et al., 2016a). C. fruticosum and C. scoparium have linear oblong or narrowly lanceolate and small gray-green leaves. However, the leaves of H. ammodendron have been degenerated into squamous, and its succulent twigs perform photosynthesis using the C4 pathway (Zou et al., 2010). Moreover, the leaves of K. ceratoides are small (1–2 cm long), strip-lanceolate, lanceolate, or oblong, whereas A. bracteate leaves are leathery and oblong or oval. In this study, five individuals of each species were selected to investigate morphological traits (Table 1; Supplementary Table 2) and leaf hydraulic traits. During the experiment in August and September, there was no significant difference in soil water contents among different plots, and the precipitation in August and September accounts for 44% of the annual precipitation (Supplementary Figure 3).
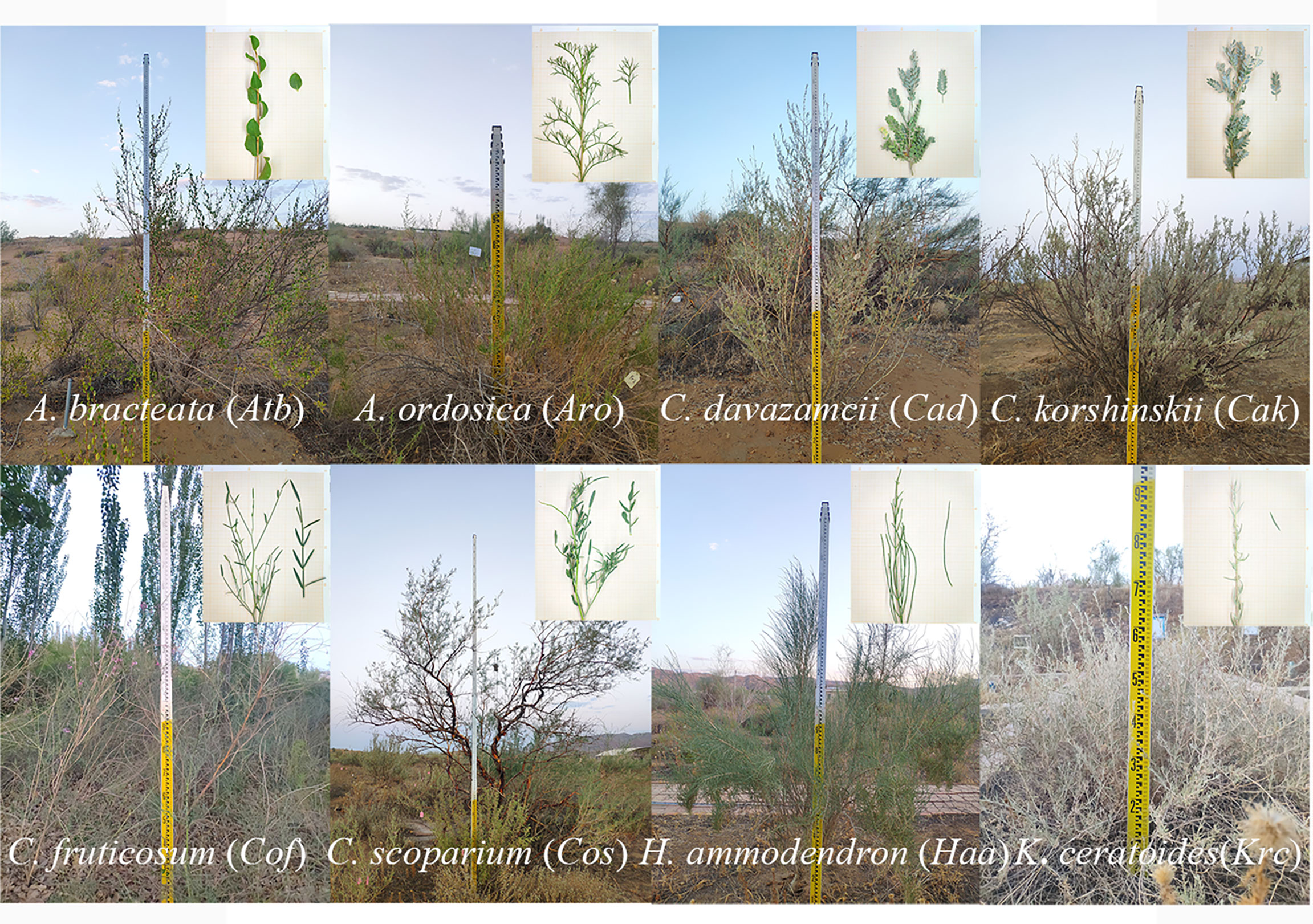
Figure 1 The shrubs and leaf morphology of eight desert shrubs in the southeast edge of the Tengger Desert in northern China.
Water potential measurements
The water potential (Ψ) of sun-exposed leafy shoots was measured using a pressure chamber (1505D-EXP, PMS Instrument Company, Albany, OR, USA) on five individuals per species (three leaves of each individual) before dawn (Ψpd, local time, 5:30–6:30) and at mid-afternoon (Ψmd, 13:00–14:00 when the air temperature is the highest, which is considered to correspond to the minimum daily water potential) on 28 August 2020. The daily maximum water potential difference (difference between Ψpd and Ψmd, ΔΨ = Ψpd - Ψmd) was also calculated.
Leaf hydraulic conductivity and vulnerability curves
For the vulnerability curves (VCs), we determined the leaf hydraulic conductance (Kl, mmol·m−2·s−1·MPa−1) using a timed rehydration method described by Brodribb and Holbrook (2003) and Johnson et al. (2018), which is based on an analogy between rehydrating a leaf and discharging a capacitor as Kl = Cl ln (Ψo/Ψf)/t, where Cl is the capacitance of leafy shoots (mmol·m−2·MPa−1), Ψo is the leaf water potential prior to partial rehydration (MPa), Ψf is the leaf water potential after partial rehydration, and t is the duration of rehydration (s). Briefly, the collected leafy shoots (10 cm) were placed in containers to rehydrate for at least 4 h and then dried on the bench top for different time periods to reach a range of leaf water potentials under room temperature. Leafy shoots were placed inside black plastic bags containing moist paper towels to allow them to equilibrate in dark conditions for at least 1 h. The Ψo value of leafy shoots was measured, and then, two adjacent leaves of the same shoots were cut under water and rehydrated for a time period of t (ranging from 10 to 60 s) before Ψf was measured. The VCs were plotted as Kl against Ψo using 10–20 shoots per species, and three VCs were established for each species based on three individuals. Maximum leaf hydraulic conductance (Kmax) was determined by averaging the five highest Kl values per species. The water potential at 50% loss of maximum leaf hydraulic conductance (P50, MPa) was determined using the P50 value of shoots, which were calculated by fitting a three-parameter sigmoidal regression function of the form Ψo = a/[1 + e-k (Kl -xc)] to the Kl versus Ψo data (Blackman et al., 2010; Johnson et al., 2018), where k and xc are constant terms of the equation. Additionally, in order to determine the leaf hydraulic safety margin of embolism occurring (LSMeo) and leaves wilting (LSMlw), the LSMs at 50% loss of conductivity and at the turgor loss point were calculated as the difference between Ψmd and P50 (LSMeo = Ψmd - P50, MPa) and the difference between Ψmd and Ψtlp (LSMlw = Ψmd- Ψtlp, MPa), respectively (Ziegler et al., 2019).
Pressure–volume curves
To establish pressure–volume (P–V) curves, we collected three 10-cm shoots from each shrub and immediately their measured fresh mass (FM, g) before placing them into a container with deionized water at room temperature for 6 h until complete saturation. After reaching full saturation, we removed the shoots from the containers, wiped away excess surface water, and measured the saturation mass (SM, g). We weighed the shoots using an analytical balance (AR2140, 1/10 000 accuracy; Ohaus International Trade (Shanghai) Co., Ltd., Shanghai, China) before measuring the balancing pressure. We then immediately placed shoots in the pressure chamber (1505D-EXP) to measure the initial balancing pressure of each sample. Then, chamber pressure was successively raised by 0.1- to 0.15-MPa increments at a speed of about 0.025 MPa s-1 and kept for 5 min under each target pressure at room temperature (Huo et al., 2021). The sample was reweighed after completing each measurement. The above operation was repeated more than 10 times until the maximum equilibrium pressure reached 4~5 MPa. Then, we measured the dry mass (DM, g) of samples after drying them in an oven at 75°C for 48 h.
P–V curves were established based on the relative water deficit (RWD; %) versus the reciprocal balance pressure (1/P) according to Nardini et al. (2013). All data points were connected to form a curve, and the entire curve was respectively fitted to a power function (1/P = αRWDβ) and a linear function (1/P = a + bRWD) with loss of turgor pressure as the transition point between the two functions (He et al., 2007), where α, β, a, and b are the equation coefficients. The traits, including osmotic potential at saturation (Ψπ, sat, MPa), water potential at the turgor loss point (Ψtlp, MPa), relative water content at the turgor loss point (RWCtlp, %), symplastic water content (SWC), and bulk tissue modulus of elasticity (ϵ, MPa), were estimated from the P–V curve (Tyree and Hammel, 1972; Nardini et al., 2013). Additionally, the sensitivity coefficients of water potential change before or after the turgor loss point, −1/β and −1/b, reflect the change in −1/Ψ for a unit change in the RWD of a species before and after the turgor loss point. We calculated these sensitivity coefficients according to Huo et al. (2021). The hydraulic capacitance (C, mmol · m−2 · MPa−1) values of both pre-turgor loss and post-turgor loss (Cpre-tlp and Cpos-tlp) were estimated from the P–V curves according to Brodribb and Holbrook (2003). The ratios of DM to LA and SM to DM were determined for each species and used in the following equation to calculate the leaf area normalized absolute capacitance: Cleaf = ΔRWC/ΔΨ × (DM/LA) × (SM/DM)/M. Here, ΔRWC and ΔΨ are the difference in leaf relative water content (%) and water potential (MPa) between before and after turgor loss points, respectively, DM is the leaf dry mass (g), LA is the projected leaf area (m2), SM is the mass of leaf water (g) at 100% RWC, and M is the molar mass of water (g·mol-1). The total hydraulic capacitance (Ctotal) is the sum of Cpre-tlp and Cpos-tlp.
Data analysis
One-way analysis of variance (ANOVA) followed by a Tukey post-hoc test was used to determine significant differences in leaf hydraulic functional traits among species and genera. Phylogenetic trees of the eight species in this study were built from the mega-tree ‘GBOTB.extended.tre’ using the R package ‘V.PhyloMaker’ (Jin and Qian, 2019). Principal component analysis (PCA) was performed to evaluate the multiple relationships among functional traits and species. The species scores comprising the first and second components of the PCA were extracted. The relationships among leaf hydraulic functional traits and drought traits were evaluated by linear regression analyses. Abbreviations of various functional traits are presented in Table 2. All statistical analyses, figure plotting, and curve fitting were performed using Origin version 2019b (OriginLab Corp., Northampton, MA, USA).
Results
Leaf water potential, hydraulic efficiency and safety, and hydraulic safety margins
For the eight species of desert shrubs, Ψpd value of leaves were relatively stable at -0.50 MPa (Figure 2A) except for H. ammodendron and K. ceratoides (which were significantly more negative than those of the other six species). However, Ψmd varied from -1.05 in A. bracteata to -2.48 MPa in H. ammodendron and was more negative than Ψpd. Among the Ψmd, those of K. ceratoides and H. ammodendron were significantly lower than those of other species, whereas A. ordosica and A. bracteata had significantly higher Ψmd than the other four species (Figure 2A). The ΔΨ values for K. ceratoides and H. ammodendron (1.80 and 1.64 MPa) were larger than those of the other species (Figure 2A). The larger Kmax were respectively found in A. bracteata and C. scoparium, which were 67.8 and 59.4 mmol·m-2·s-1·MPa-1, and H. ammodendron’s Kmax (31.6 mmol·m-2·s-1·MPa-1) was significantly lower than those of the other species (Figure 2B). Moreover, there was no significant difference among the other five species. The P50 estimated from VCs (Supplementary Figure 4) of H. ammodendron, K. ceratoides, and A. ordosica were -2.61, -2.34, and -2.17 MPa, respectively, which were more negative than those of the other species (Figure 2D). The other five species’ P50 had no obvious differences among them (Figure 2D). LSMeo and LSMlw values of A. bracteata and A. ordosica were highest (Figure 2C). However, H. ammodendron’s LSMeo was significantly lower than those of the other five species, which had LSMeo and LSMlw that were not significantly different (Figure 2C). Additionally, other differences in Ψpd, Ψmd, ΔΨ, Kmax, and P50 were also observed among the different genera (Supplementary Figure 5).
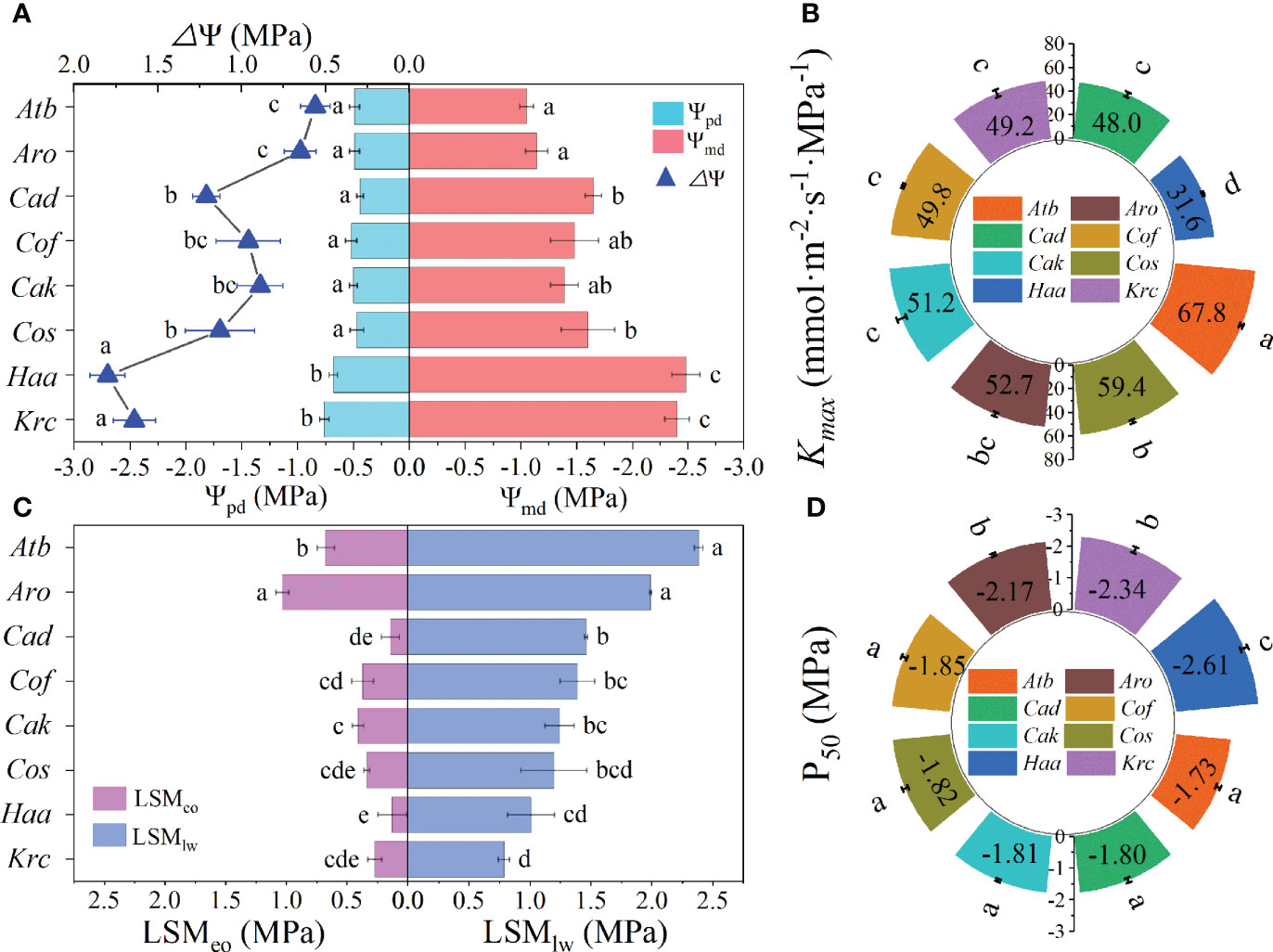
Figure 2 Leaf hydraulic functional traits of desert shrubs. (A) Leaf water potential at predawn (Ψpd) and at midday (Ψmd), and diurnal leaf water potential change (△Ψ). (B) Maximum leaf hydraulic conductance (Kmax). (C) Leaf hydraulic safety margins of embolism occurring (LSMeo) of leaves wilting (LSMlw). (D) Water potential at 50% loss of maximum leaf hydraulic conductance (P50). Abbreviations of species name and functional traits are shown in Tables 1, 2. All values are shown as mean ± SE, and different letters indicate the existence of statistical significance among species (one-way ANOVA, n = 5, p < 0.05).
The traits of shrubs from pressure–volume curves
The traits, including Ψπ, sat, Ψtlp, and ϵ, were estimated from the P–V curves (Supplementary Figure 6). More negative Ψtlp than Ψπ, sat was observed among all shrubs (Figure 3A). More negative Ψπ, sat was found in A. bracteata, A. ordosica, C. davazamcii, and C. korshinskii, whereas the Ψπ, sat of K. ceratoides and C. scoparium were larger than those of the other species (Figure 3A). The largest Ψtlp were observed in C. scoparium and C. korshinskii, but H. ammodendron had the smallest Ψtlp (Figure 3A). The difference between Ψπ, sat and Ψtlp (i.e., Ψπ, sat-Ψtlp) of C. korshinskii was significantly lower than those of others species, and this difference was obviously larger in H. ammodendron than in C. scoparium. However, the five other shrub species showed no significant difference in this value (the blue triangle in Figure 3A). The largest RWCtlp and smallest ϵ values were all found in H. ammodendron and K. ceratoides (Figures 3B, C). Regarding hydraulic capacitance, the minimum Cpre-tlp, Cpos-tlp, and Ctotal values were found in C. davazamcii, whereas the Cpos-tlp and Ctotal of A. ordosica were significantly larger than those of the other species (Figure 3D). Meanwhile, the Cpre-tlp of the other seven shrubs except for K. ceratoides were less than their respective Cpos-tlp values (Figure 3D). Moreover, higher -1/b were found in A. ordosica than in other shrubs, except for A. bracteate and C. davazamcii, whereas the -1/b value of H. ammodendron was obviously lower than those of the other species, apart from K. ceratoides (Figure 3E). The -1/β of C. korshinskii was only significantly larger than those of C. davazamcii and K. ceratoides, but there was no significant difference among the other species (Figure 3F). Additionally, differences in Ψπ, sat, Ψtlp, Cpre-tlp, Cpos-tlp, and Ctotal were also observed among genera (Supplementary Figure 5).
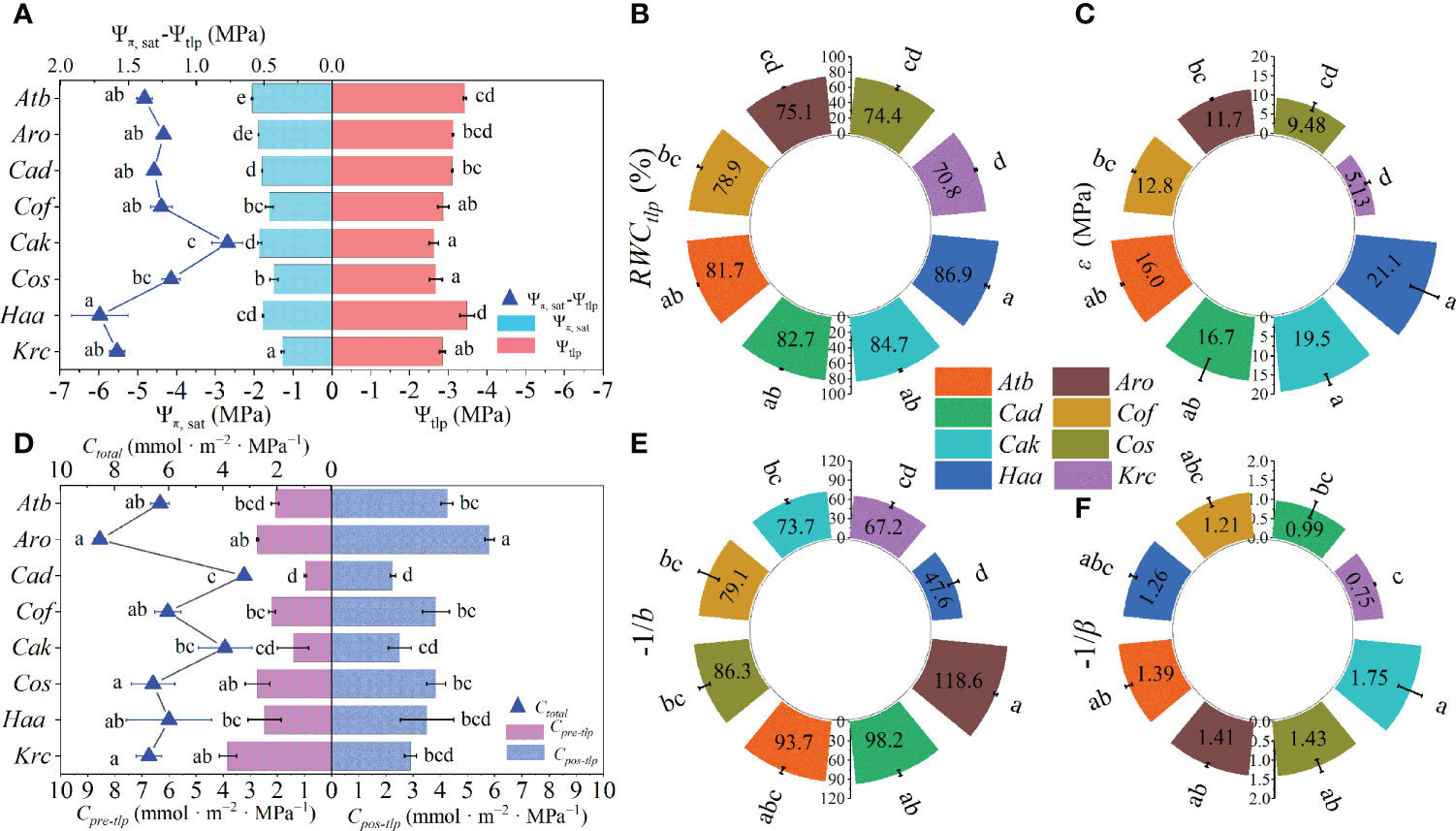
Figure 3 The traits of eight desert shrubs estimated from P–V curves. (A) Osmotic potential at saturation (Ψπ, sat), water potential at turgor loss (Ψtlp) and the difference value between them. (B) Relative water content at turgor loss (RWCtlp). (C) Bulk tissue modulus of elasticity (ε). (D) Leaf hydraulic capacitance of pre-turgor loss point (Cpre-tlp) and post-turgor loss point (Cpos-tlp), and total leaf hydraulic capacitance (Ctotal). (E, F) Water sensitivity coefficients before turgor loss point (−1/b) and after turgor loss point (−1/b). Abbreviations of species name and functional traits are shown in Tables 1, 2. All values are shown as mean ± SE, and different letters indicate the existence of statistical significance among species (one-way ANOVA, n = 5, p < 0.05).
Relationship among leaf hydraulic functional traits
Principal component analysis (PCA) results based on the 19 functional traits of the eight desert shrub species showed that the first and second the components accounted for 30.6% and 25.3% of the total variance, respectively (Figure 4; Supplementary Figure 7). The traits related to water status (i.e., Ψpd and Ψmd) and sensitivity to water potential changes (-1/b and -1/β) showed positive loading on the first PCA component, whereas only Ψπ, sat and △Ψ had negative loading on the first component. The second component was positively loaded by hydraulic capacitance (i.e., Cpre-tlp, Cpos-tlp, and Ctotal) and leaf hydraulic safety margin (LSMeo), whereas RWCtlp and ϵ had negative loadings on the second component (Figure 4A; Supplementary Table 3). However, morphological traits (Hp and Cw), leaf hydraulic efficiency (Kmax), and leaf hydraulic safety (P50) showed positive loading on the third component (Supplementary Table 3). Species appear to be separated among the PCA component by family (Figure 4B; Supplementary Figure 8). Artemisia and Atraphaxis were showed positively loading on first component, whereas Amaranthaceae family species tended to have negative first component values. However, Fabaceae family species had both negative and positive second component values (Figure 4B; Supplementary Figure 8).
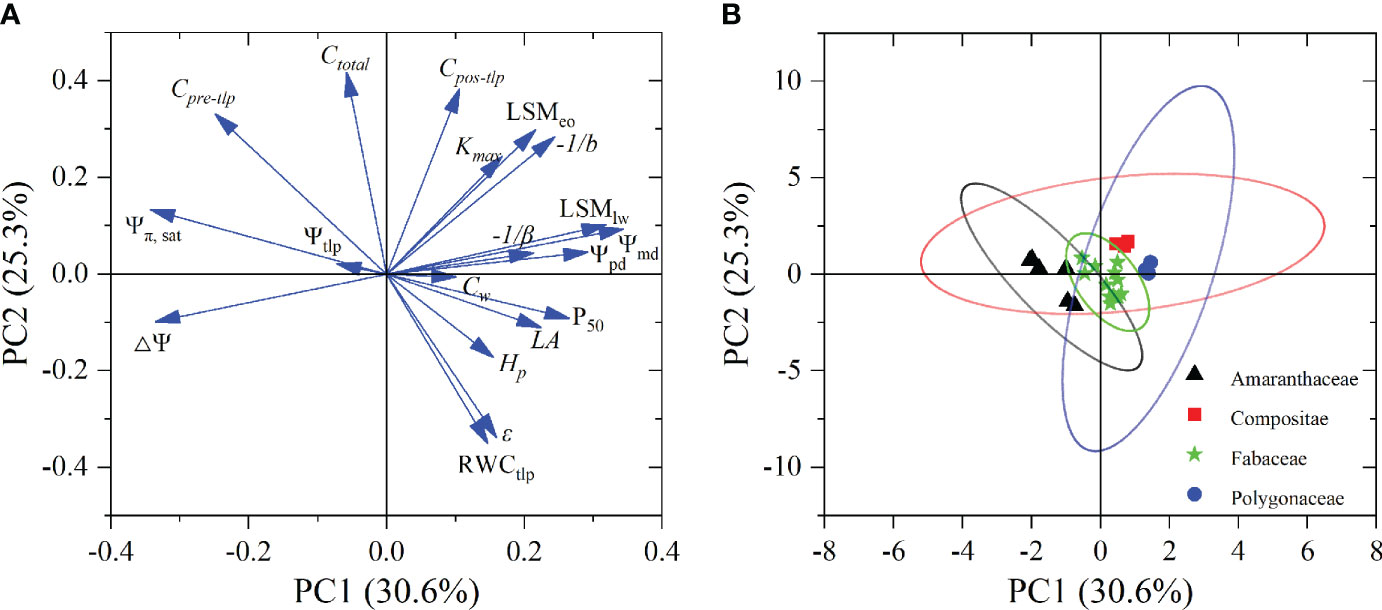
Figure 4 Factor loadings (A) and species scores (B) of PCA for 19 functional traits in eight desert shrubs species. Abbreviations of species name and functional traits are shown in Tables 1, 2. The ovals with different colored represent species belonging to different families in (B). Ellipses represent 90% confidence intervals for different families.
In this study, Kmax showed a strong significantly negative relationship with P50 (Figure 5A), Ψmd (Figure 5B), and ΔΨ (Figure 5C). Additionally, the sensitivity coefficient -1/b was significantly negatively related to Ψmd (Figure 5D) and ΔΨ (Figure 5E). However, a marginally negative correlation was observed between Ψpd and Cpre-tlp (Figure 5F). Importantly, a positive relationship was found between P50 with both Ψmd (Figure 6A) and ΔΨ (Figure 6B). Likewise, LSMeo exhibited a significant positive relationship with Cpos-tlp (Figure 6C), but a marginally positive relationship was observed between LSMeo and Ctotal (Figure 6F). The Ψπ, sat showed a significant negative correlation with ϵ (Figure 6D) and a marginally positively relationship with Cpre-tlp (Figure 6E). Conversely, RWCtlp showed a significant positive relationship with ϵ (Figure 6G) and a significant negative relationship with Cpre-tlp (Figure 6H). In addition, Cpre-tlp showed a significantly negative correlation with ϵ (Figure 6I). Unexpectedly, the morphological traits (Hp, Cw, LA, Ll, and Wl) exhibited no significant relationships with leaf hydraulic functional traits (Kmax, P50, LSMeo, ΔΨ, Ψπ, sat, RWCtlp, ϵ, and Cpre-tlp; Supplementary Figure 9 and 10), except for one; the linear relationship between Wl and ΔΨ was significantly negative (R2 = 0.707, p = 0.009).
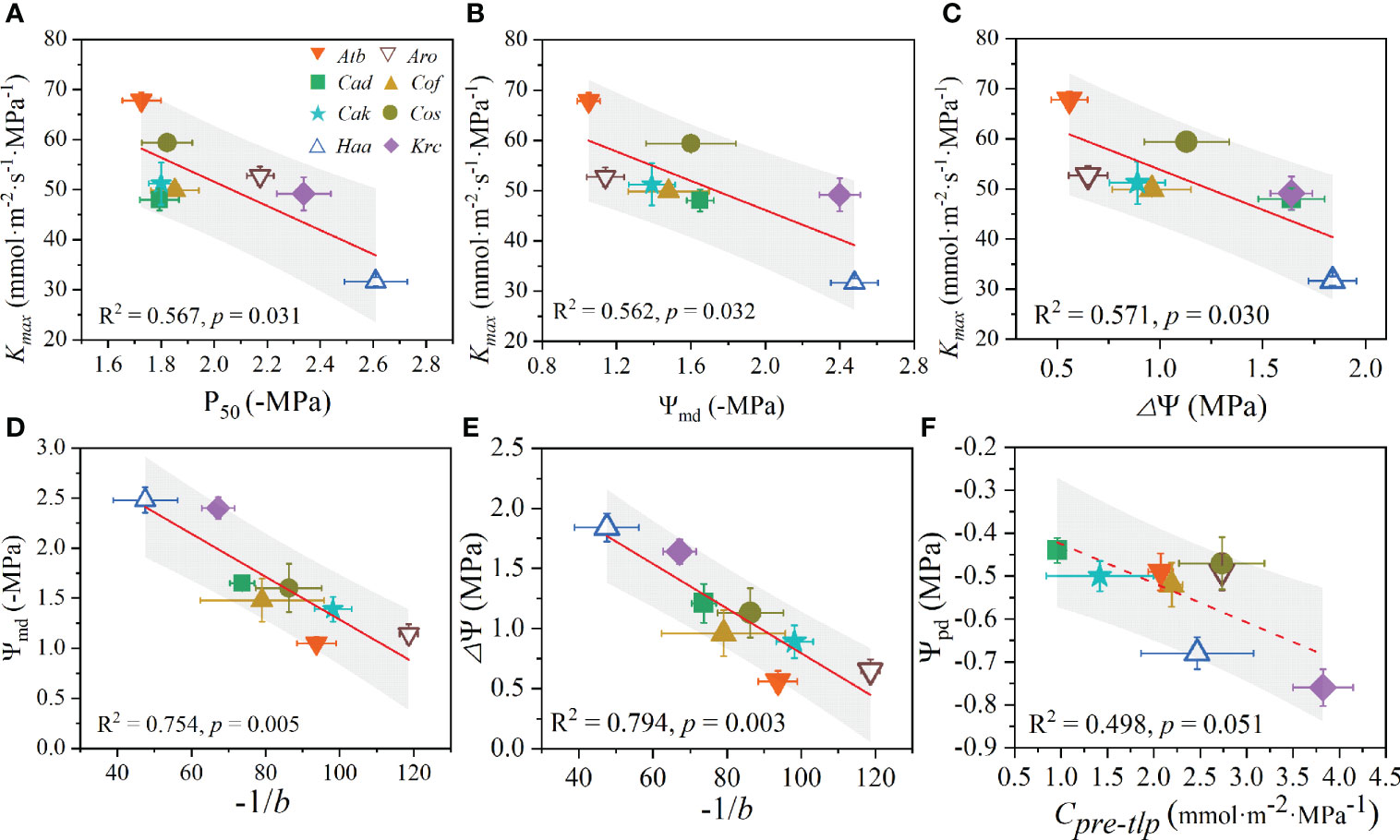
Figure 5 Trade-offs among leaf hydraulic functional traits of desert shrubs. The relationships between Kmax with P50 (A), Ψmd (B) and △Ψ (C). The relationships between −1/b with Ψmd (D) and △Ψ (E). The relationship between Ψpd and Cpre-tlp (F). All values are shown as mean ± SE. Abbreviations of species name and functional traits are shown in Tables 1, 2. The coefficients of determination (R2) and significance levels (p) of linear regression are shown. Shades of gray represent 90% confidence intervals.
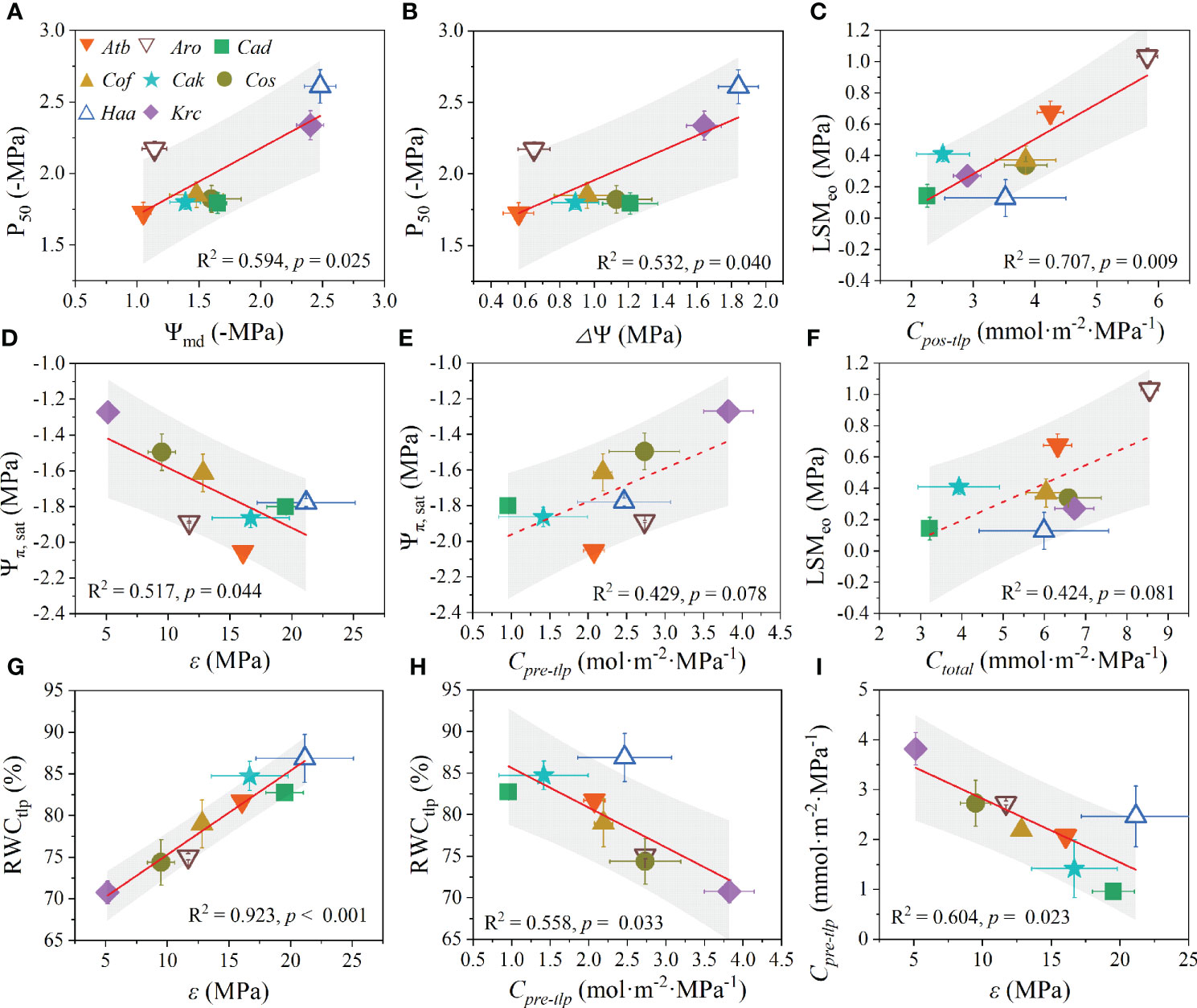
Figure 6 Coordination among leaf hydraulic functional traits of desert shrubs. The relationships between P50 with Ψmd (A) and △Ψ (B). The relationships between LSMeo with Cpos-tlp (C) and Ctotal (F). The relationships between Ψπ, sat with ε (D) and Cpre-tlp (E). The relationships between RWCtlp with ε (G) and Cpre-tlp (H). The relationship between ε and Cpre-tlp (I). All values are shown as mean ± SE. Abbreviations of species name and functional traits are shown in Tables 1, 2. The coefficients of determination (R2) and significance levels (p) of linear regression are shown. Shades of grey represent 90% confidence intervals.Tables.
Discussion
Differences in leaf functional traits among desert shrubs
Numerous studies have shown large interspecies differences in hydraulic functional traits (Chen et al., 2009; Fan et al., 2011; Borghetti et al., 2020). Our study found more negative Ψpd, Ψmd, and P50 values; larger ΔΨ; and smaller Kmax in H. ammodendron and K. ceratoides than in A. ordosica and A. bracteate. These results indicated that shrubs in the Amaranthaceae family with more negative Ψmd and P50 have strong tolerance and resistance against embolism, whereas shrubs in the Polygonaceae and Compositae families are more vulnerable to embolism due to their greater hydraulic conductance ensuring small water potential changes and less negative P50. Although the root systems play important roles in drought tolerance, the root distribution of A. ordosica (shallow root system) was not related to water potential changes and hydraulic safety in the present study, as was deep-rooted shrubs, which may be more related to other traits, e.g., morphological traits. For example, A. ordosica with less negative Ψpd, Ψmd, and P50 may be attributable to its full split needled and semi-succulent leaves but irrespective of root distribution (Supplementary Table 1; Liu et al., 1991; Zhang et al., 2008), highlighting that plant morphology can underlie differences in physiology (Possen et al., 2014). The leaf hydraulic safety margin could better explain mortality under continuous or severe droughts than P50 or other traits alone (Anderegg et al., 2016). Most angiosperm species with narrow hydraulic safety margin values (<1 MPa) suggest that species are highly vulnerable to increases in the frequency of droughts (Choat et al., 2012; Anderegg et al., 2016). Similarly, our results found narrow LSMeo (<1 MPa) among desert shrubs; however, the desert shrubs had wider LSMlw (Ψmd - Ψtlp; >1 MPa) than LSMeo. As Ψtlp is usually recognized as the key trait quantifying leaf wilting and plant drought tolerance most directly (Bartlett et al., 2012b), LSMlw shows the hydraulic safety margin of leaf wilting in this study. Therefore, our results suggested that desert shrubs were prone to embolism, but not quick to wilt during drought. This seems to suggest that embolism is not fatal to desert shrubs and that proper embolization of desert shrubs can indeed reduce water loss to support their basic survival. Finally, shrubs in the Amaranthaceae family have more narrow LSMeo and LSMlw relative to Polygonaceae and Compositae family shrubs, indicating that Amaranthaceae family shrubs are more prone to wilting than the latter species owing to their narrow hydraulic safety margins caused by larger daily water potential differences.
Recently, some studies have shown that Ψπ, sat and Ψtlp are key characteristics for predicting drought tolerance (Bartlett et al., 2012a). In the present study, we found more negative Ψtlp, Ψπ, sat and largest Ψπ, sat - Ψtlp in H. ammodendron relative to those in C. korshinskii and C. scoparium, which suggests that H. ammodendron has greater drought tolerance than the latter species because plants with more negative Ψtlp and Ψmd maintain stomatal and hydraulic conductance (Mitchell et al., 2008; Bartlett et al., 2012b) or because the leaves of H. ammodendron are degenerate to limit water loss (Zou et al., 2010). Meanwhile, the maximum and minimum RWCtlp and ϵ values were observed in H. ammodendron and K. ceratoides, showing that H. ammodendron with lower cell wall elasticity is prone to loss of cellular water, whereas K. ceratoides sacrifices cell wall elasticity to maintain tissue water content (Kozlowski and Pallardy, 2002; Bartlett et al., 2012b). However, RWCtlp and ϵ did not significantly differ among the other shrub species. The larger hydraulic capacitance (i.e., Cpre-tlp, Cpos-tlp, and Ctotal) of A. ordosica suggested that it has a strong water storage capacity and larger buffering effect during droughts (Meinzer et al., 2009; Huo et al., 2021), whereas the lower hydraulic capacitance of C. davazamcii and C. korshinskii makes them more sensitive to water deficit, perhaps owing to their greater water potential change and less negative P50. In addition, the water sensitivity coefficient (-1/β) was larger in C. korshinskii, also indicating that it is more sensitive to water deficit relative to other species. Overall, the significant difference in Ψtlp, Ψπ, sat RWCtlp, ϵ, Cpre-tlp, Cpos-tlp, and -1/β observed among different species suggested that change in these traits reflects differences in drought tolerance responses of species to water deficit (Touchette et al., 2007).
Taken together, there were significant differences in diurnal leaf water potential change, leaf hydraulic efficiency and safety, hydraulic safety margin, water potential and relative water content at the turgor loss point, and hydraulic capacitance among species and genera; however, these hydraulic functional traits among species did not show convergence under the same environment. The above results suggested that leaf hydraulic functional traits were more strongly associated with the species, rather than exhibiting convergence in the same environment where they live together. Overall, the difference in leaf hydraulic functional traits among shrubs species also provided important hydraulic-related insights for native shrubs as a priority species in desert vegetation restoration and reconstruction.
Trade-off among leaf hydraulic functional traits in desert shrubs
In the past decade, the trade-off between hydraulic safety and efficiency has been widely investigated (Nardini and Luglio, 2014; Bucci et al., 2019). Some studies across woody angiosperms and gymnosperms globally have revealed weak trade-offs between Kmax and P50 (Gleason et al., 2016; Yan et al., 2020; Liu et al., 2021). However, the evidence of a significant correlation between Kmax and P50 in this study indicated that the hydraulic safety and efficiency of desert shrubs exhibit a strong trade-off (R2 = 0.567, p = 0.031). For example, H. ammodendron has a low efficiency (lower Kmax) but strong resistance to embolism (i.e., more negative P50), whereas A. bracteata exhibits the opposite trend, which is likely owing to their leaf and canopy structure characteristics (e.g., large crown size and leaf degeneration in H. ammodendron, but larger leaf area and leathery leaves in A. bracteata) or differences in xylem anatomy, which merits further study. Our results are consistent with previous investigations that suggest strong trade-offs between hydraulic safety and efficiency within numerous single sites (Martınez-Vilalta et al., 2002; Pratt et al., 2007) and among particular taxa (Hacke et al., 2007; Yao et al., 2021). The relationships among traits at the global scale do not necessarily manifest similarly at specific sites or in a specific region—traits that appear closely coordinated at certain scales may have different sensitivities to scale-dependent drivers of variation (Messier et al., 2017), which may be attributed to species evolving different life history traits (physiological and morphological traits) in different environments (Xu et al., 2007; Gupta et al., 2020).
PCA indicated the diurnal leaf water potential change (ΔΨ) and leaf hydraulic efficiency (Kmax) show loading with opposite signs for the first component. This result implies that trade-offs exist between diurnal leaf water potential change and hydraulic efficiency. As the minimal leaf water potential (typically Ψmd) is the critical point for maintaining normal physiological metabolism and ΔΨ acts as an indicator of drought tolerance (Bhaskar and Ackerly, 2006), a strong negative correlation between Kmax and Ψmd indicated a hydraulic trade-off between hydraulic efficiency and the minimal leaf water potential. This trade-off indicated that less drought-tolerant shrubs (i.e., with less negative Ψmd and P50, and small ΔΨ) have higher hydraulic efficiency, like A. bracteate. This may be because A. bracteate with a particularly deep root system (Feng et al., 2022) can maintain higher xylem hydraulic efficiency through absorbing deep soil moisture or has a wide leaf hydraulic safety margin of embolism occurring (larger LSMeo) to avoid massive embolism formation. Additionally, the negative relationship between -1/b and both Ψmd and ΔΨ showed trade-offs, suggesting that shrubs with small diurnal leaf water potential change were more sensitive to drops in water potential after tissue turgor loss (Huo et al., 2021).
Overall, our study indicated that hydraulic efficiency (Kmax) exhibits a trade-off with both hydraulic safety (P50) and the minimal leaf water potential and water potential changes (Ψmd and ΔΨ), and the trade-off also existed between -1/b with Ψmd and ΔΨ. This means that desert shrub species with small diurnal leaf water potential change, less negative minimal leaf water potential, and low hydraulic safety were more sensitive to embolism and water deficit.
Coordination among leaf hydraulic functional traits in desert shrubs
Numerous studies have suggested that there is clear coordination among hydraulic traits (Pivovaroff et al., 2018). For instance, species with high resistance to embolism usually share the following characteristics: higher wood density, more negative leaf Ψtlp, and larger capacitance (Santiago et al., 2018). In the present study, the diurnal leaf water potential change (ΔΨ) and hydraulic safety (P50) had both positive and negative loadings on the principal component. Moreover, we found positive correlations between P50 with both ΔΨ and Ψmd, indicating that leaf hydraulic safety coordinated with the water potential changes and the minimal leaf water potential. That is, desert shrubs with more negative Ψmd and larger ΔΨ were more resistant to embolism, e.g., H. ammodendron. Moreover, the shrubs with lower ΔΨ and Ψmd had more negative P50 and higher Kmax, suggesting that the shrubs with smaller minimal leaf water potential and higher leaf hydraulic safety have lower leaf hydraulic efficiency, which was consistent with the results of previous studies (Nardini and Luglio, 2014; Yan et al., 2020; Liu et al., 2021). The minimal leaf water potential was coordinated with leaf hydraulic safety, whereas it exhibited a trade-off with leaf hydraulic efficiency; therefore, minimal leaf water potential seems to act as a mediator in the trade-off with leaf hydraulic efficiency and in coordination with leaf hydraulic safety. Additionally, the coordination between −1/b with Ψmd and ΔΨ suggested that desert shrubs with less negative Ψmd and smaller ΔΨ were more sensitive to drop in water potential after losing turgor pressure (Huo et al., 2021). Since Ψmd is easier to measure than leaf hydraulic safety and reflects the maximum water deficit that species must tolerate to maintain physiological activity (Bhaskar and Ackerly, 2006), we highlight that Ψmd could act as a convenient trait to preliminarily determine or predict leaf hydraulic conductivity and embolism resistance in shrub species.
Hydraulic safety margins are usually used to describe the degree of conservatism in a plant’s hydraulic strategies (Johnson et al., 2012). In our study, the hydraulic capacitance (i.e., Cpre-tlp, Cpos-tlp, and Ctotal) and leaf hydraulic safety margin (LSMeo) had loadings with the same sign for the second component of the PCA. Meanwhile, a significant positive or marginal correlation between LSMeo with Cpos-tlp and Ctotal indicated that there is coordination between hydraulic safety margins and hydraulic capacitance. That is, desert shrubs with larger hydraulic capacitance possess larger hydraulic safety margins, which may be because the buffering effect of hydraulic capacitance mitigates the drought-induced risks of reducing water potential (Ding et al., 2021). The significant coordination between LSMeo and -1/b suggested that desert shrubs with larger hydraulic safety margins to embolism are more sensitive to water deficit after losing turgor pressure. Additionally, the coordination in hydraulic capacitance of the pre-turgor loss point (Cpre-tlp) with other traits (i.e., Ψπ, sat, RWCtlp, and Ψpd) revealed that hydraulic capacitance plays an important buffering role in the drought tolerance of desert shrubs (Vergeynst et al., 2015). The bulk tissue modulus of elasticity (ϵ) had a trade-off with Ψπ, sat and Cpre-tlp but was coordinated with RWCtlp, indicating that desert shrubs with large cell wall elasticity (low ϵ) have large hydraulic capacitance and saturated osmotic potential and lose less water at the point of turgor loss (Bartlett et al., 2014; Al-Yasi et al., 2020).
Taken together, the minimal leaf water potential could preliminarily determine or predict leaf hydraulic conductivity and resistance to embolism in desert shrubs, because it is coordinated with leaf hydraulic safety and exhibits a trade-off with leaf hydraulic efficiency. Moreover, the coordination among leaf hydraulic capacitance with other traits (i.e., relative water content at the turgor loss point, osmotic potential at saturation, and bulk tissue modulus of elasticity) plays an important and indispensable role in the survival of shrubs in arid habitats. Critically, the coordination among leaf hydraulic functional traits provides an important strategy for the survival of desert shrubs.
Conclusion
In this study, the difference in the leaf functional traits and hydraulic strategies of eight desert shrub species were analyzed. Significant differences in leaf functional traits (i.e., diurnal leaf water potential change, hydraulic safety margin, leaf hydraulic efficiency and safety, water potential and relative water content at the turgor loss point, and hydraulic capacitance) were found to exist among species and genera, indicating that leaf hydraulic functional traits were more strongly associated with the species even when living in the same environment. Additionally, our results suggested that the minimal leaf water potential of desert shrubs was strongly coordinated with hydraulic safety and traded off with hydraulic efficiency; therefore, minimal leaf water potential may be used to preliminarily determine or predict leaf hydraulic conductivity and the resistance to embolism of desert shrubs. Leaf hydraulic capacitance was also coordinated with leaf hydraulic safety margins to embolism and other traits, suggesting that hydraulic capacitance plays an important buffering role in the drought tolerance of desert shrubs. In short, our study provides critical insight into hydraulic trade-off and coordination strategies for native shrubs as a priority species for desert vegetation restoration and reconstruction.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
ZZ and JH conceived the research and designed experiments. JH analyzed the data and wrote the manuscript. JH, YS, JC, HZ, and LF performed the experiments. ZZ and YZ revised the manuscript. All the authors read and approved the submission of the manuscript.
Funding
This work was supported by the Major Science and Technology Projects of Inner Mongolia Autonomous Region (2021ZD0008-3-2), the National Natural Science Foundation of China (Grant Nos. 31971529 and 32171630), the Chinese Academy of Sciences “Light of West China” Program and China Postdoctoral Science Foundation (2021M703465).
Acknowledgments
We greatly appreciate reviewers for their valuable and insightful comments on this manuscript. We would like to thank Dr. Larry Bowman at Yale University for his assistance with English language and grammatical editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2022.938758/full#supplementary-material
References
Abd El-Ghani, M. M., Huerta-Martínez, F. M., Hongyan, L., Qureshi, R. (2017). “The deserts of china,” in Plant responses to hyperarid desert environments (Springer International Publishing: Springer) 531–536.
Al-Yasi, H., Attia, H., Alamer, K., Hassan, F., Ali, E., Elshazly, S., et al. (2020). Impact of drought on growth, photosynthesis, osmotic adjustment, and cell wall elasticity in damask rose. Plant Physiol. Biochem. 150, 133–139. doi: 10.1016/j.plaphy.2020.02.038
Anderegg, W. R., Klein, T., Bartlett, M., Sack, L., Pellegrini, A. F., Choat, B., et al. (2016). Meta-analysis reveals that hydraulic traits explain cross-species patterns of drought-induced tree mortality across the globe. Proc. Natl. Acad. Sci. U. S. A. 113 (18), 5024–5029. doi: 10.1073/pnas.1525678113
Bai, Y., Zhang, Y., Michalet, R., She, W., Jia, X., Qin, S. (2019). Responses of different herb life-history groups to a dominant shrub species along a dune stabilization gradient. Basic Appl. Ecol. 38, 1–12. doi: 10.1016/j.baae.2019.06.001
Bartlett, M. K., Scoffoni, C., Ardy, R., Zhang, Y., Sun, S., Cao, K., et al. (2012a). Rapid determination of comparative drought tolerance traits: using an osmometer to predict turgor loss point. Methods Ecol. Evol. 3 (5), 880–888. doi: 10.1111/j.2041-210X.2012.00230.x
Bartlett, M. K., Scoffoni, C., Sack, L. (2012b). The determinants of leaf turgor loss point and prediction of drought tolerance of species and biomes: a global meta-analysis. Ecol. Lett. 15 (5), 393–405. doi: 10.1111/j.1461-0248.2012.01751.x
Bartlett, M. K., Zhang, Y., Kreidler, N., Sun, S., Ardy, R., Cao, K., et al. (2014). Global analysis of plasticity in turgor loss point, a key drought tolerance trait. Ecol. Lett. 17 (12), 1580–1590. doi: 10.1111/ele.12374
Bhaskar, R., Ackerly, D. D. (2006). Ecological relevance of minimum seasonal water potentials. Physiol. Plantarum 127 (3), 353–359. doi: 10.1111/j.1399-3054.2006.00718.x
Blackman, C. J., Brodribb, T. J., Jordan, G. J. (2010). Leaf hydraulic vulnerability is related to conduit dimensions and drought resistance across a diverse range of woody angiosperms. New Phytol. 188 (4), 1113–1123. doi: 10.1111/j.1469-8137.2010.03439.x
Borghetti, M., Gentilesca, T., Colangelo, M., Ripullone, F., Rita, A. (2020). Xylem functional traits as indicators of health in Mediterranean forests. Curr. For. Rep. 6 (3), 220–236. doi: 10.1007/s40725-020-00124-5
Breshears, D. D., Myers, O. B., Meyer, C. W., Barnes, F. J., Zou, C. B., Allen, C. D., et al. (2009). Tree die-off in response to global change-type drought: mortality insights from a decade of plant water potential measurements. Front. Ecol. Environ. 7 (4), 185–189. doi: 10.1890/080016
Brodribb, T. J., Holbrook, N. M. (2003). Stomatal closure during leaf dehydration, correlation with other leaf physiological traits. Plant Hysiol. 132 (4), 2166–2173. doi: 10.1104/pp.103.023879
Bucci, S. J., Carbonell Silletta, L. M., Garré, A., Cavallaro, A., Efron, S. T., Arias, N. S., et al. (2019). Functional relationships between hydraulic traits and the timing of diurnal depression of photosynthesis. Plant Cell Environ. 42 (5), 1603–1614. doi: 10.1111/pce.13512
Chen, J. W., Zhang, Q., Cao, K.-F. (2009). Inter-species variation of photosynthetic and xylem hydraulic traits in the deciduous and evergreen euphorbiaceae tree species from a seasonally tropical forest in south-western China. Ecol. Res. 24 (1), 65–73. doi: 10.1007/s11284-008-0482-4
Choat, B., Brodribb, T. J., Brodersen, C. R., Duursma, R. A., Lopez, R., Medlyn, B. E. (2018). Triggers of tree mortality under drought. Nature 558 (7711), 531–539. doi: 10.1038/s41586-018-0240-x
Choat, B., Jansen, S., Brodribb, T. J., Cochard, H., Delzon, S., Bhaskar, R., et al. (2012). Global convergence in the vulnerability of forests to drought. Nature 491 (7426), 752–755. doi: 10.1038/nature11688
Chu, X., Zhan, J., Li, Z., Zhang, F., Qi, W. (2019). Assessment on forest carbon sequestration in the three-north shelterbelt program region, China. J. Clean. Prod. 215, 382–389. doi: 10.1016/j.jclepro.2018.12.296
De Guzman, M. E., Santiago, L. S., Schnitzer, S. A., Álvarez-Cansino, L. (2017). Trade-offs between water transport capacity and drought resistance in neotropical canopy liana and tree species. Tree Physiol. 37 (10), 1404–1414. doi: 10.1093/treephys/tpw086
De Micco, V., Aronne, G. (2012). “Morpho-anatomical traits for plant adaptation to drought,” in Plant responses to drought stress, Springer, Berlin, Heidelberg, 37–61.
Ding, Y., Nie, Y., Chen, H., Wang, K., Querejeta, J. I. (2021). Water uptake depth is coordinated with leaf water potential, water-use efficiency and drought vulnerability in karst vegetation. New Phytol. 229 (3), 1339–1353. doi: 10.1111/nph.16971
Fan, D. Y., Jie, S. L., Liu, C. -C., Zhang, X. Y., Xu, X. W., Zhang, S. R., et al. (2011). The trade-off between safety and efficiency in hydraulic architecture in 31 woody species in a karst area. Tree Physiol. 31 (8), 865–877. doi: 10.1093/treephys/tpr076
Feng, X., Liu, R., Li, C., Li, M., Wang, Y., Li, Y. (2022). Multi-level physiological and morphological adjustment of haloxylon ammodendron related to groundwater drawdown in a desert ecosystem. Agr. For. Meteorol. 324, 109096. doi: 10.1016/j.agrformet.2022.109096
Gleason, S. M., Westoby, M., Jansen, S., Choat, B., Hacke, U. G., Pratt, R. B., et al. (2016). Weak tradeoff between xylem safety and xylem-specific hydraulic efficiency across the world's woody plant species. New Phytol. 209 (1), 123–136. doi: 10.1111/nph.13646
Gómez‐Aparicio, L. (2009). The role of plant interactions in the restoration of degraded ecosystems: a meta-analysis across life-forms and ecosystems. J. Ecol. 97 (6), 1202–1214. doi: 10.1111/j.1365-2745.2009.01573.x
Gupta, A., Rico-Medina, A., Caño-Delgado, A. I. (2020). The physiology of plant responses to drought. Science 368 (6488), 266–269. doi: 10.1126/science.aaz7614
Hacke, U. G., Sperry, J. S., Feild, T., Sano, Y., Sikkema, E., Pittermann, J. (2007). Water transport in vesselless angiosperms: conducting efficiency and cavitation safety. Int. J. Plant Sci. 168 (8), 1113–1126. doi: 10.1086/520724
He, X., Cong, P., Gao, Y., Lu, J., Wang, H., Xue, P., et al. (2007). Drought resistance of four grasses using pressure-volume curve. Front. Biol. China 2 (4), 425–430. doi: 10.1007/s11515-007-0065-8
Henry, C., John, G. P., Pan, R., Bartlett, M. K., Fletcher, L. R., Scoffoni, C., et al. (2019). A stomatal safety-efficiency trade-off constrains responses to leaf dehydration. Nat. Commun. 10 (1) 1-9. doi: 10.1038/s41467-019-11006-1
Huo, J., Shi, Y., Zhang, H., Hu, R., Huang, L., Zhao, Y., et al. (2021). More sensitive to drought of young tissues with weak water potential adjustment capacity in two desert shrubs. Sci. Total Environ. 790, 148103. doi: 10.1016/j.scitotenv.2021.148103
Jin, Y., Qian, H. (2019). V. PhyloMaker: an r package that can generate very large phylogenies for vascular plants. Ecography 42 (8), 1353–1359. doi: 10.1111/ecog.04434
Johnson, D. M., Berry, Z. C., Baker, K. V., Smith, D. D., McCulloh, K. A., Domec, J. C., et al. (2018). Leaf hydraulic parameters are more plastic in species that experience a wider range of leaf water potentials. Funct. Ecol. 32 (4), 894–903. doi: 10.1111/1365-2435.13049
Johnson, D. M., McCulloh, K. A., Woodruff, D. R., Meinzer, F. C. (2012). Hydraulic safety margins and embolism reversal in stems and leaves: why are conifers and angiosperms so different? Plant Sci. 195, 48–53. doi: 10.1016/j.plantsci.2012.06.010
Kozlowski, T. T., Pallardy, S. (2002). Acclimation and adaptive responses of woody plants to environmental stresses. botanical Rev. 68 (2), 270–334. doi: 10.1663/0006-8101(2002)068[0270:Aaarow]2.0.Co;2
Li, Y., Chen, J., Ai, S., Shi, H. (2020). Responses of leaf water potential and gas exchange to the precipitation manipulation in two shrubs on the Chinese loess plateau. J. Arid Land 12 (2), 267–282. doi: 10.1007/s40333-020-0008-7
Liu, Y. X., Li, Y. J., Yang, X. L. (1991). “Root system of psammpphytes,” in Research sand dunes control in shapotou area of tengger desert (in collection no. 2) (Yinchuan: Ningxia People’s Publishing House), 185–209.
Liu, H., Ye, Q., Gleason, S. M., He, P., Yin, D. (2021). Weak tradeoff between xylem hydraulic efficiency and safety: climatic seasonality matters. New Phytol. 229 (3), 1440–1452. doi: 10.1111/nph.16940
Li, X. R., Xiao, H. L., Zhang, J. G., Wang, X. P. (2004). Long-term ecosystem effects of sand-binding vegetation in the tengger desert, northern China. Restor. Ecol. 12 (3), 376–390. doi: 10.1111/j.1061-2971.2004.00313.x
Lopez, O. R., Kursar, T. A., Cochard, H., Tyree, M. T. (2005). Interspecific variation in xylem vulnerability to cavitation among tropical tree and shrub species. Tree Physiol. 25 (12), 1553–1562. doi: 10.1093/treephys/25.12.1553
Luo, W., Zhao, W. (2019). Adventitious roots are key to the development of nebkhas in extremely arid regions. Plant Soil 442 (1), 471–482. doi: 10.1007/s11104-019-04209-4
Ma, Q., Qian, J., Tian, L., Liu, Z. (2019). Responses of belowground bud bank to disturbance and stress in the sand dune ecosystem. Ecol. Indic. 106, 105521. doi: 10.1016/j.ecolind.2019.105521
Martınez-Vilalta, J., Piñol, J., Beven, K. (2002). A hydraulic model to predict drought-induced mortality in woody plants: an application to climate change in the Mediterranean. Ecol. Model. 155 (2-3), 127–147. doi: 10.1016/S0304-3800(02)00025-X
McDowell, N. G., Allen, C. D. (2015). Darcy's law predicts widespread forest mortality under climate warming. Nat. Clim. Change 5 (7), 669–672. doi: 10.1038/nclimate2641
Meinzer, F. C., Johnson, D. M., Lachenbruch, B., McCulloh, K. A., Woodruff, D. R. (2009). Xylem hydraulic safety margins in woody plants: coordination of stomatal control of xylem tension with hydraulic capacitance. Funct. Ecol. 23 (5), 922–930. doi: 10.1111/j.1365-2435.2009.01577.x
Messier, J., McGill, B. J., Enquist, B. J., Lechowicz, M. J. (2017). Trait variation and integration across scales: is the leaf economic spectrum present at local scales? Ecography 40 (6), 685–697. doi: 10.1111/ecog.02006
Mitchell, P. J., Veneklaas, E. J., Lambers, H., Burgess, S. S. (2008). Leaf water relations during summer water deficit: differential responses in turgor maintenance and variation in leaf structure among different plant communities in south-western Australia. Plant Cell Environ. 31 (12), 1791–1802. doi: 10.1111/j.1365-3040.2008.01882.x
Nardini, A., Luglio, J. (2014). Leaf hydraulic capacity and drought vulnerability: Possible trade-offs and correlations with climate across three major biomes. Funct. Ecol. 28 (4), 810–818. doi: 10.1111/1365-2435.12246
Nardini, A., Marchetto, A., Tretiach, M. (2013). Water relation parameters of six peltigera species correlate with their habitat preferences. Fungal Ecol. 6 (5), 397–407. doi: 10.1016/j.funeco.2013.05.004
Ocheltree, T. W., Nippert, J. B., Prasad, P. V. (2016). A safety vs efficiency trade-off identified in the hydraulic pathway of grass leaves is decoupled from photosynthesis, stomatal conductance and precipitation. New Phytol. 210 (1), 97–107. doi: 10.1111/nph.13781
Phillips, O. L., Aragão, L. E., Lewis, S. L., Fisher, J. B., Lloyd, J., López-González, G., et al. (2009). Drought sensitivity of the Amazon rainforest. Science 323 (5919), 1344–1347. doi: 10.1126/science.1164033
Pivovaroff, A. L., Cook, V. M. W., Santiago, L. S. (2018). Stomatal behaviour and stem xylem traits are coordinated for woody plant species under exceptional drought conditions. Plant Cell Environ. 41 (11), 2617–2626. doi: 10.1111/pce.13367
Possen, B. J., Anttonen, M. J., Oksanen, E., Rousi, M., Heinonen, J., Kostiainen, K., et al. (2014). Variation in 13 leaf morphological and physiological traits within a silver birch (Betula pendula) stand and their relation to growth. Can. J. For. Res. 44 (6), 657–665. doi: 10.1139/cjfr-2013-0493
Pratt, R. B., Jacobsen, A. L., Ewers, F. W., Davis, S. D. (2007). Relationships among xylem transport, biomechanics and storage in stems and roots of nine rhamnaceae species of the California chaparral. New Phytol. 174 (4), 787–798. doi: 10.1111/j.1469-8137.2007.02061.x
Rodríguez-Gamir, J., Xue, J., Meason, D. F., Clearwater, M., Clinton, P. W., Domec, J. C. (2021). Interclonal variation, coordination, and trade-offs between hydraulic conductance and gas exchange in pinus radiata: consequences on plant growth and wood density. J. Exp. Bot. 72 (7), 2419–2433. doi: 10.1093/jxb/eraa587
Rosas, T., Mencuccini, M., Barba, J., Cochard, H., Saura-Mas, S., Martinez-Vilalta, J. (2019). Adjustments and coordination of hydraulic, leaf and stem traits along a water availability gradient. New Phytol. 223 (2), 632–646. doi: 10.1111/nph.15684
Santiago, L. S., De Guzman, M. E., Baraloto, C., Vogenberg, J. E., Brodie, M., Herault, B., et al. (2018). Coordination and trade-offs among hydraulic safety, efficiency and drought avoidance traits in Amazonian rainforest canopy tree species. New Phytol. 218 (3), 1015–1024. doi: 10.1111/nph.15058
Scholz, F. G., Bucci, S. J., Arias, N., Meinzer, F. C., Goldstein, G. (2012). Osmotic and elastic adjustments in cold desert shrubs differing in rooting depth: coping with drought and subzero temperatures. Oecologia 170 (4), 885–897. doi: 10.1007/s00442-012-2368-y
Tian, L., Wu, W., Zhou, X., Zhang, D., Yu, Y., Wang, H., et al. (2019). The ecosystem effects of sand-binding shrub hippophae rhamnoides in alpine semi-arid desert in the northeastern qinghai–Tibet plateau. Land 8 (12), 183. doi: 10.3390/land8120183
Touchette, B. W., Iannacone, L. R., Turner, G. E., Frank, A. R. (2007). Drought tolerance versus drought avoidance: a comparison of plant-water relations in herbaceous wetland plants subjected to water withdrawal and repletion. Wetlands 27 (3), 656–667. doi: 10.1672/0277-5212(2007)27[656:DTVDAA]2.0.CO;2
Tyree, M., Hammel, H. (1972). The measurement of the turgor pressure and the water relations of plants by the pressure-bomb technique. J. Exp. Bot. 23 (1), 267–282. doi: 10.1093/jxb/23.1.267
Venturas, M. D., MacKinnon, E. D., Dario, H. L., Jacobsen, A. L., Pratt, R. B., Davis, S. D. (2016). Chaparral shrub hydraulic traits, size, and life history types relate to species mortality during california’s historic drought of 2014. PloS One 11 (7), e0159145. doi: 10.1371/journal.pone.0159145
Vergeynst, L. L., Dierick, M., Bogaerts, J. A., Cnudde, V., Steppe, K. (2015). Cavitation: a blessing in disguise? new method to establish vulnerability curves and assess hydraulic capacitance of woody tissues. Tree Physiol. 35 (4), 400–409. doi: 10.1093/treephys/tpu056
Williams, A. P., Allen, C. D., Macalady, A. K., Griffin, D., Woodhouse, C. A., Meko, D. M., et al. (2013). Temperature as a potent driver of regional forest drought stress and tree mortality. Nat. Clim. Change 3 (3), 292–297. doi: 10.1038/Nclimate1693
Xu, G.-Q., Li, Y., Xu, H. (2011). Seasonal variation in plant hydraulic traits of two co-occurring desert shrubs, tamarix ramosissima and haloxylon ammodendron, with different rooting patterns. Ecol. Res. 26 (6), 1071–1080. doi: 10.1007/s11284-011-0858-8
Xu, H., Li, Y., Xu, G., Zou, T. (2007). Ecophysiological response and morphological adjustment of two central Asian desert shrubs towards variation in summer precipitation. Plant Cell Environ. 30 (4), 399–409. doi: 10.1111/j.1365-3040.2006.001626.x
Yan, C. L., Ni, M. Y., Cao, K. F., Zhu, S. D. (2020). Leaf hydraulic safety margin and safety-efficiency trade-off across angiosperm woody species. Biol. Lett. 16 (11), 20200456. doi: 10.1098/rsbl.2020.0456
Yao, G. Q., Nie, Z. F., Zeng, Y. Y., Waseem, M., Hasan, M. M., Tian, X. Q., et al. (2021). A clear trade-off between leaf hydraulic efficiency and safety in an aridland shrub during regrowth. Plant Cell Environ. 44 (10), 3347-3357. doi: 10.1111/pce.14156
Yu, T., Liu, P., Zhang, Q., Ren, Y., Yao, J. (2021). Detecting forest degradation in the three-north forest shelterbelt in China from multi-scale satellite images. Remote Sens-Basel. 13 (6), 1131. doi: 10.3390/rs13061131
Zhang, Z.S., Chen, Y. L., Xu, B. X., Huang, L., Tan, H. J., Dong, X. J. (2014). Topographic differentiations of biological soil crusts and hydraulic properties in fixed sand dunes, tengger desert. J. Arid Land 7 (2), 205–215. doi: 10.1007/s40333-014-0048-y
Zhang, Z. S., Li, X. R., Liu, L. C., Jia, R. L., Zhang, J. G., Wang, T. (2009). Distribution, biomass, and dynamics of roots in a revegetated stand of caragana korshinskii in the tengger desert, northwestern China. J. Plant Res. 122 (1), 109–119. doi: 10.1007/s10265-008-0196-2
Zhang, Z. -S., Li, X. R., Wang, T., Wang, X. P., Xue, Q. W., Liu, L. C. (2008). Distribution and seasonal dynamics of roots in a revegetated stand of artemisia ordosica kracsh. in the tengger desert (North China). Arid Land Res. Manage. 22 (3), 195–211. doi: 10.1080/15324980802182980
Zhang, Y. F., Wang, X. P., Hu, R., Pan, Y. X. (2016a). Throughfall and its spatial variability beneath xerophytic shrub canopies within water-limited arid desert ecosystems. J. Hydrol. 539, 406–416. doi: 10.1016/j.jhydrol.2016.05.051
Zhang, Z., Zhao, Y., Li, X., Huang, L., Tan, H. (2016b). Gross rainfall amount and maximum rainfall intensity in 60-minute influence on interception loss of shrubs: A 10-year observation in the tengger desert. Sci. Rep.-UK 6 (1), 1-10. doi: 10.1038/srep26030
Zhang, D., Zuo, X., Zang, C. (2021). Assessment of future potential carbon sequestration and water consumption in the construction area of the three-north shelterbelt programme in China. Agr. For. Meteorol. 303, 108377. doi: 10.1016/j.agrformet.2021.108377
Zhao, L., Wang, L., Xiao, H., Liu, X., Cheng, G., Ruan, Y. (2013). The effects of short-term rainfall variability on leaf isotopic traits of desert plants in sand-binding ecosystems. Ecol. Eng. 60, 116–125. doi: 10.1016/j.ecoleng.2013.07.022
Zhou, H., Chen, Y., Li, W., Ayup, M. (2013). Xylem hydraulic conductivity and embolism in riparian plants and their responses to drought stress in desert of Northwest China. Ecohydrology 6 (6), 984–993. doi: 10.1002/eco.1412
Ziegler, C., Coste, S., Stahl, C., Delzon, S., Levionnois, S., Cazal, J., et al. (2019). Large Hydraulic safety margins protect Neotropical canopy rainforest tree species against hydraulic failure during drought. Ann. For. Sci. 76 (4), 1–18. doi: 10.1007/s13595-019-0905-0
Keywords: leaf hydraulic traits, trade-off, coordination, desert shrubs, leaf minimal water potential
Citation: Huo J, Shi Y, Chen J, Zhang H, Feng L, Zhao Y and Zhang Z (2022) Hydraulic trade-off and coordination strategies mediated by leaf functional traits of desert shrubs. Front. Plant Sci. 13:938758. doi: 10.3389/fpls.2022.938758
Received: 08 May 2022; Accepted: 11 October 2022;
Published: 31 October 2022.
Edited by:
Cristina Cruz, University of Lisbon, PortugalReviewed by:
Anna Lintunen, University of Helsinki, FinlandYang Gao, Farmland Irrigation Research Institute (CAAS), China
Copyright © 2022 Huo, Shi, Chen, Zhang, Feng, Zhao and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Zhao, emhhb3lhbmc2NkAxMjYuY29t; Zhishan Zhang, enN6aGFuZ0BsemIuYWMuY24=
 Jianqiang Huo
Jianqiang Huo Yafei Shi
Yafei Shi Jiajia Chen1,2
Jiajia Chen1,2 Hongxia Zhang
Hongxia Zhang Yang Zhao
Yang Zhao Zhishan Zhang
Zhishan Zhang