- 1College of Agronomy, Sichuan Agricultural University, Chengdu, China
- 2Sichuan Engineering Research Center for Crop Strip Intercropping System, Chengdu, China
- 3Key Laboratory of Crop Ecophysiology and Farming System in Southwest, Ministry of Agriculture, Chengdu, China
The genetic information and functional properties of plants have been further identified with the completion of the whole-genome sequencing of numerous crop species and the rapid development of high-throughput phenotyping technologies, laying a suitable foundation for advanced precision agriculture and enhanced genetic gains. Collecting phenotypic data from dicotyledonous crops in the field has been identified as a key factor in the collection of large-scale phenotypic data of crops. On the one hand, dicotyledonous plants account for 4/5 of all angiosperm species and play a critical role in agriculture. However, their morphology is complex, and an abundance of dicot phenotypic information is available, which is critical for the analysis of high-throughput phenotypic data in the field. As a result, the focus of this paper is on the major advancements in ground-based, air-based, and space-based field phenotyping platforms over the last few decades and the research progress in the high-throughput phenotyping of dicotyledonous field crop plants in terms of morphological indicators, physiological and biochemical indicators, biotic/abiotic stress indicators, and yield indicators. Finally, the future development of dicots in the field is explored from the perspectives of identifying new unified phenotypic criteria, developing a high-performance infrastructure platform, creating a phenotypic big data knowledge map, and merging the data with those of multiomic techniques.
Introduction
With the increasing popularity of sequencing technology and the scale of materials to be tested, a new issue has arisen: a lack of suitable high-throughput phenotype acquisition technology to obtain corresponding phenotypic information. In addition, based on a large amount of crop genome information, determining how to analyze the interaction mechanisms of gene function, plant phenotype, and environmental response efficiently and with a high resolution has become a new challenge (Furbank and Tester, 2011). In this context, genomics, corresponding to the phenomics concept, has arisen at a historic moment (Zhao et al., 2019). The essence of the phenotype is a plant genome sequence three-dimensional expression, and its regional differentiation characteristics and evolution are intergenerational (Tardieu et al., 2017; Zhao, 2019), so the plant phenotypic group contains information with a complexity far beyond the estimates. Therefore, the genotype–phenotype–environment relationship can be systematically and deeply explored from an omic perspective to reveal the response mechanism of structural and functional characteristics of plants to genetic information and environmental changes at multiple scales (Pan, 2015; Tardieu et al., 2017; Zhou et al., 2018).
Currently, crop phenotype research is primarily conducted in the United States, Germany, France, Australia, the United Kingdom, Italy, Japan, Canada, Mexico, India, and China (Xiao et al., 2021). Research objects have included maize (Souza and Yang, 2021; Xie et al., 2021; Shao et al., 2022), rice (Mishra et al., 2021; Muharam et al., 2021; Xiao et al., 2021), wheat (Prey and Schmidhalter, 2020; Furbank et al., 2021; Zelazny et al., 2021), and other monocotyledons. This focus on monocotyledons probably occurred because their morphological structure is relatively simple, and the difficulty of image acquisition and data analysis is relatively low. Leaf counting has been realized in maize, sorghum, and other monocotyledons over the entire growth period (Miao et al., 2021). However, studies on the leaves of dicotyledonous species such as soybean and cotton have focused only on comprehensive indicators such as canopy coverage and compactness due to the severe occlusion between leaves and complex plant types (Moreira et al., 2019; Li et al., 2020), which has led to the loss of many details. Leaves are closely related to plant photosynthesis, thus affecting biomass accumulation, which in turn is related to yield formation, so the loss of information is not conducive to the in-depth study of the phenotypes of dicotyledons. A significant positive correlation exists between the panicle number and the yield at maturity, and this number can be identified and counted directly at a certain regional scale in most monocotyledons. There was a significant positive correlation between panicle number and yield at the maturity stage, and it could be recognized and counted directly on a certain regional scale in most monocotyledons (Jun et al., 2021; Wanli et al., 2021). However, in many dicotyledons, researchers can predict yields using only a large number of other indicators or measure the yield by picking and laying out fruits at maturity (Casagrande et al., 2022) or by picking and spreading out fruits at maturity (Li et al., 2021; Xiaobin et al., 2022), which greatly increases labor costs and is not beneficial to the development of high-throughput phenotypes.
Branching is an important common feature of dicotyledons. The quantity of branches and their position influence yields and are connected to the lodging resistance. Studies have shown that by reducing the position of branches and increasing the number of effective branches in oilseed rape, the lodging resistance can be improved, and the yield per plant can be increased (Fan et al., 2021; Amoo et al., 2022). The branching ability guarantees the yield formation in soybean (Xiaobo et al., 2012; Yu-Shan et al., 2015). The major goal of breeders is to increase upland cotton yields by controlling the branch type and using appropriate mechanical picking methods. Therefore, Wu et al. (Wu et al., 2021; Zhan et al., 2021; Sun et al., 2022) carried out a series of studies on branching development. These studies have contributed to improved breeding by providing great genes for improving plant accessions. However, few studies involving the use of high-throughput phenotyping platforms have been conducted, which has severely slowed the breeding of dicotyledonous plants.
Dicotyledons account for 4/5 of the total number of angiosperms and play an important role in agricultural production (Chuanji, 1982). Soybean, broad bean, rape, cotton, and other dicotyledonous species are commonly cultivated and are all directly tied to human existence. According to imprecise statistics, the global demand for soybeans is ≈388 million tons per year. With an annual consumption of almost 600 million tons (searched in the U.S. Department of Agriculture data), rapeseed is the world's second-largest oil crop species. Therefore, high-throughput phenotypic studies on dicotyledons are highly important.
The current high-throughput phenotyping research environment mainly includes indoor potted plants (Bodner et al., 2021; Zea et al., 2022) because indoor imagining, which can swiftly and accurately obtain a large number of phenotype inages for later analysis and verification, faces fewer restrictive factors. Thousands of phenotypic experiments carried out in environmentally controlled growth facilities or fields each year can provide a vast amount of phenotypic data. Due to the impact of environmental variations, the replication of results by the same researcher and the repeatability of results in separate tests by other laboratories are frequently unsatisfactory (Poorter et al., 2012). Environmental aspects are critical and should be given at least as much attention as the characteristics being assessed, which leads to the next question: how does one quantify all environmental impacts? The phenotyping platform is systematically presented in this paper, and the determination of the phenotype of dicotyledons against the backdrop of the rapid development of the field phenotyping platform is discussed. This paper guides investigating high-throughput phenotypic application technology for dicotyledons in the field, enhancing precision agriculture and increasing genetic gains.
Overview of high-throughput phenotyping of dicotyledonous crops in the field
We collected statistics on field phenotyping facilities and the number of publications since 2010 to better understand how the high-throughput phenotyping of field dicot crops should be developed. In 2016,according to statistics from the International Plant Phenotype Network (IPPN), the phenotyping platform is used in the United States, Australia, China, Germany, and other countries. Nearly 200 large-scale phenotyping facilities are in operation around the world (the most notable being the Australian National Plant Phenotyping Facility “Plant Accelerator,” the British National Plant Phenotyping Center, the German Julich Phenotyping Research Center, the Netherlands Plant Eco-phenotyping Centre, and the German IPK Greenhouse Automation). There are ≈82 indoor mechanized phenotyping platforms and 81 European field mechanized phenotyping platforms (including 26 intensive and 55 barren types). Asian field mechanized phenotyping platforms are yet to be counted. However, over the last 5 years, many countries, led by the United States and China, have increased their investments in field mechanization platforms.
The development of phenotyping platforms has provided a solid foundation for crop phenotyping research. Only 25% of all global high-throughput phenotyping platforms are used for field research, and only 49% of the platforms are actually used to obtain high-throughput phenotyping information in the field (Figure 1A). The number of papers published on crop phenotypes has increased annually; in 2019, the number of papers published each year had surpassed 300. Additionally, among many countries and regions that are involved in high-throughput phenotyping studies of plants, the United States is ahead in terms of research results. We list only the top 10 countries or regions in Figure 1B. Dicotyledonous crops account for only 23% of the research results, which is substantially lower than monocotyledons. Arabidopsis is the most studied dicot crop, most likely because it is a commonly used model crop and using it to analyze new phenotypes can greatly decrease the difficulty of research. Furthermore, soybeans, cotton, tobacco, peanuts, rape, and other crops have gradually entered the academic research field. Finally, we compiled statistics on dicotyledonous crop research topics. According to the statistical findings, dicotyledonous plant research topics mainly focus on six aspects: yields, physiology and biochemistry, genes, biotic stress, abiotic stress, and growth dynamics. Genes were the most studied topic, followed by yield (Figure 1C). These research topics are covered in the following chapter.
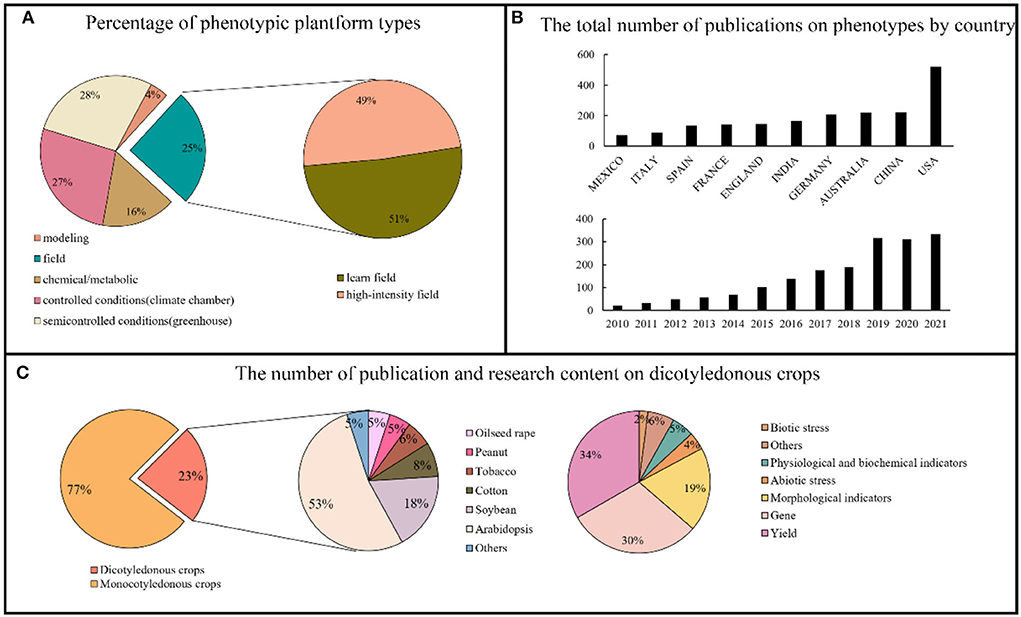
Figure 1. Overview of high-throughput phenotyping research. (A) The proportion of different phenotypic platforms and the composition of phenotypic platforms in the field (searched in IPPN, https://www.plant-phenotyping.org/ippn-survey_2016). (B) Ranking and annual number of phenotypic papers published by countries in the world (2010–2021) (searched in Web of Science). (C) Proportion of high-throughput phenotypes studied in monocotyledons and dicotyledons and the main subjects studied in dicotyledons (searched in Web of Science).
Research progress of field high-throughput phenotypic information platforms
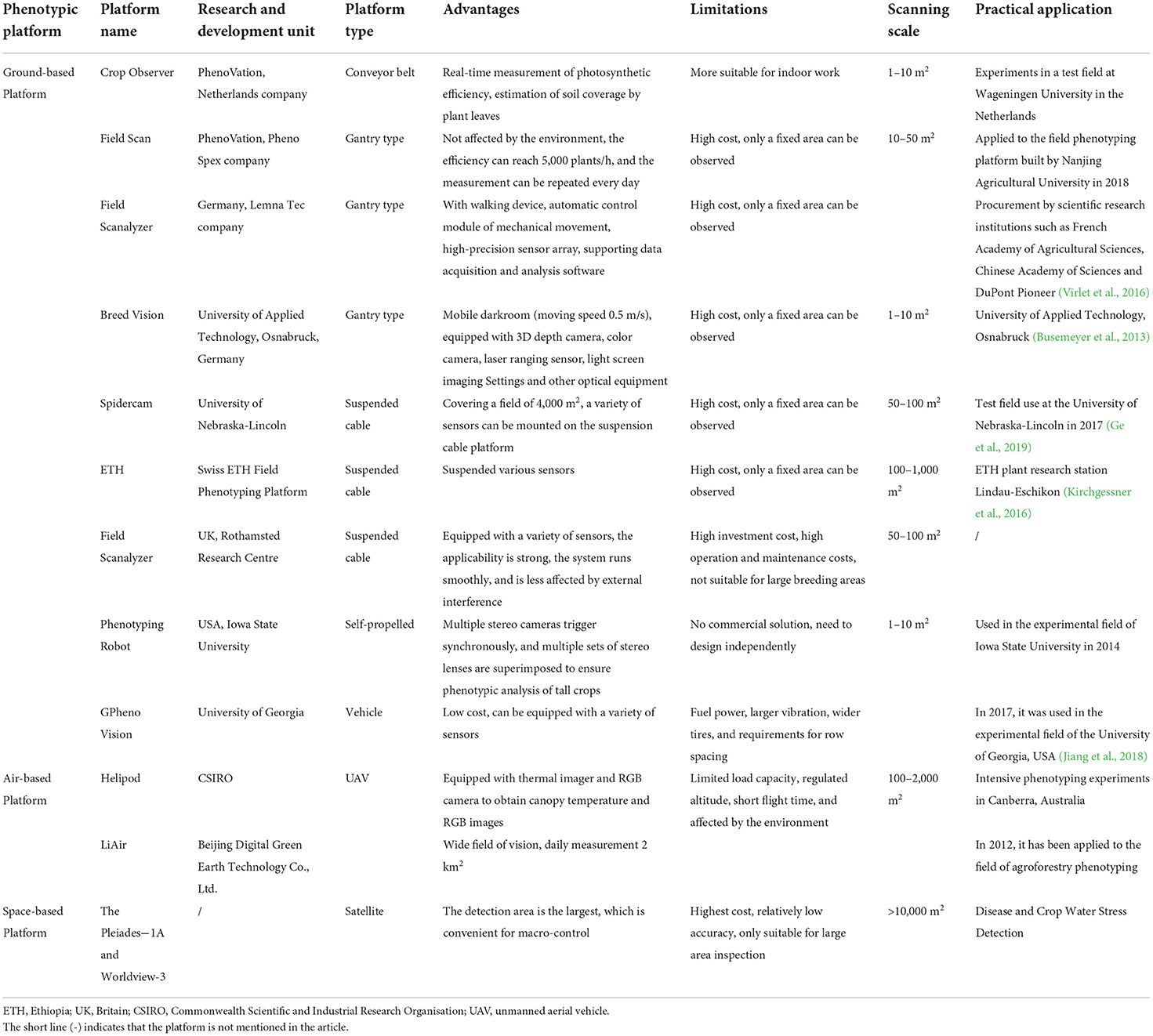
Platforms are generally classified into three types based on the different spatial areas in which they operate: ground-based platforms, air-based platforms, and space-based platforms (Huichun et al., 2020). Ground-based platforms encompass all plant phenotyping platforms that are in contact with the ground while being built or used. Based on their loading modes, sensors are classified into conveyor belt types, gantry types, suspension cable types, vehicle types, and self-propelled plant phenotyping platforms (Figure 2A). Air-based platforms include all platforms that collect phenotypic data in the air, which are classified as unmanned aerial vehicles (UAVs) or manned aircraft depending on whether or not a human pilot is present (Figure 2B). Space-based platforms collect phenotypic data using satellite remote sensing (Figure 2C).
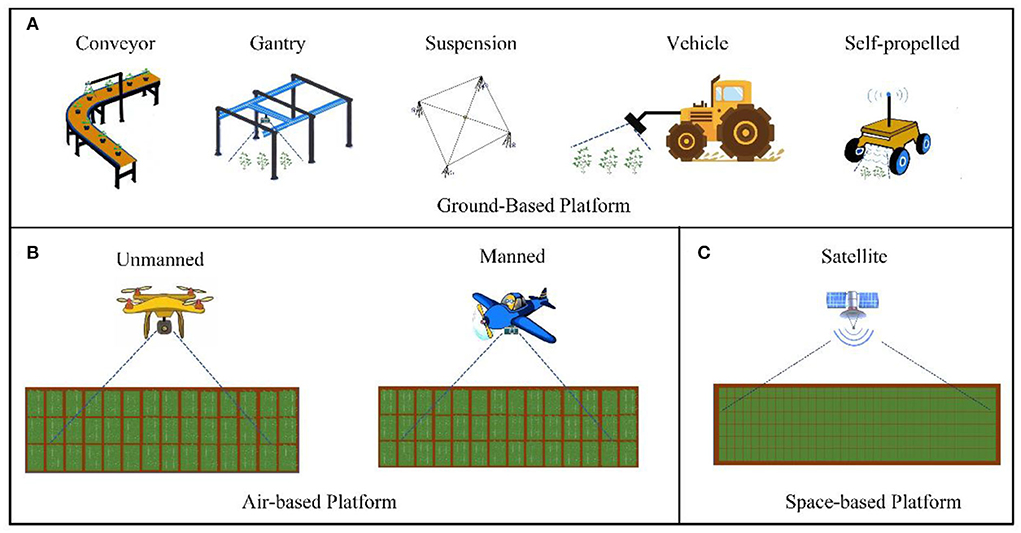
Figure 2. Field high-throughput phenotypic information platform. (A) Ground-based platforms include conveyor belt types, gantry types, suspension cable types, vehicle types, and self-propelled types. (B) Air-based platforms include UAVs or manned aircraft. (C) Space-based platforms include satellite remote sensing.
Ground-based platforms
Research has been conducted on ground-based platforms, with Crop Design in Belgium being the first company in the world to develop a commercialized large-scale phenotyping measurement platform (Reuzeau et al., 2006). Foundation platforms have advantages and disadvantages in their use (Table 1). The conveyor belt-type foundation platform can detect plant phenotypic indicators in real time, and the detection objects can be flexibly replaced based on individual needs. However, this device is better suited for indoor use. The gantry-type platform has a walking device, an automatic control module for mechanical motion, and a high-precision sensor array. It is not affected by the environment, has a low impact, and can take measurements repeatedly every day. However, the cost is high, and only a fixed area can be observed. Suspension-type platforms have the advantages of not requiring guards, continuous operation (such as at night), good repeatability, and high measurement accuracy, but they are typically expensive and can detect only a limited number of areas. Vehicle-mounted platforms are typically agricultural machinery platforms, such as tractors, that are outfitted with various sensors to form phenotype platforms. They can meet the application requirements of most researchers and small businesses to the greatest extent possible due to their low cost, constant perspective, easy installation, and simple operation. However, due to the wide wheel, low body height, and high vibration, agricultural machinery is primarily suitable for short plants and is limited by row spacing and plant space. Currently, the vehicle-borne phenotypic platform is being used to collect biomass (Busemeyer et al., 2013), plant height (Comar et al., 2012), leaf area, stem diameter, canopy temperature, and other phenotypic data for wheat (Andrade-Sanchez et al., 2013), cotton (Sun et al., 2018), and other crops. Researchers should examine the impact of the weight of agricultural machinery on the soil structure and the root system of plants. An increasing number of researchers are experimenting with compact self-propelled ground mobile platforms to carry phenotyping sensors to minimize costs, increase the measurement accuracy, and reduce environmental side effects (Bai et al., 2016; Young et al., 2018). Nonetheless, business solutions are lacking, making promotion extremely difficult. Furthermore, by using the chassis of commercial self-propelled ground rovers, several researchers have created phenotypic platforms for various applications (Shafiekhani et al., 2017), offering a novel approach to the creation of self-propelled phenotyping platforms.
Li et al. (2020) identified new I-trait indicators (plant density, relative frequencies, and entropy, among others) that accurately reflect the response of cotton to drought stress at the seedling stage. By merging high-throughput phenome, genome, and transcriptome data, researchers found that two unannotated genes, GhA040377 and GhA040378, were considerably upregulated in response to drought. Finally, this study advocated the use of phenomics to improve the genetics of cotton and was the first phenomics research publication on drought resilience in cotton.
Air-based platforms
Air-based platform research is still in its early stages, but it is progressing quickly. This type of platform has the advantage of scanning a large area of land in a short period of time, but there are also some drawbacks such as a low information accuracy, an insufficient payload, limited endurance, and weather vulnerability. Currently, air-based phenotyping platforms primarily include UAVs and manned helicopters. When compared to manned helicopters, UAVs have lower costs, lower flying altitudes, and superior information acquisition precision. As a result, numerous studies have been conducted on the acquisition of field phenotypic information via UAVs. The number of sensors that a UAV can carry is limited due to its low payload capacity compared to ground-based platforms. Remote sensing analysis of crop phenotypes (Liu et al., 2016) and maturity evaluation (Malambo et al., 2018) is performed using RGB cameras, infrared imaging, multispectral/hyperspectral cameras, and other sensors. Disease diagnosis (Sugiura et al., 2016), yield estimation (Chang et al., 2021), growth state monitoring and evaluation (Jin et al., 2017; Hu et al., 2018), and analysis of critical phenotypic features have been performed using this type of platform (Ding et al., 2019). Trevisan et al. (2020) used a 3D point cloud derived from UAV images to develop a method for detecting sorghum spikes. The correlation coefficients between the average panicle length and width assessed by UAVs and those measured on the ground were 0.61 and 0.83, respectively. Karthikeyan et al. (2020) used a space-based platform to collect images twice a week and then employed two complimentary convolutional neural networks (CNNs) to forecast soybean maturity. This method can detect the sources of mistakes in maturity forecasting, and its architecture overcomes earlier research limitations and can be used in large-scale commercial breeding initiatives.
When UAVs are employed, it is also important to consider its stability, safety, and controllability.
Space-based platforms
Satellite platforms for monitoring the status of large areas of crops are referred to as a “space-based platforms.” Because they have the highest data flux and lowest accuracy, space-based phenotyping platforms are suited only for a wide spectrum of detection. On the one hand, satellites have a massive payload, and onboard sensors can cover optical, thermal, microwave, and fluorescence frequencies, allowing for the collection of large amounts of data in a short period of time. On the other hand, satellites can provide recurrent information on agricultural conditions at different scales throughout the season (including yield forecasting, field preparation, crop health monitoring, irrigation, and site-specific management) (Zhang et al., 2020). Furthermore, improvements in the spatial (Weiss et al., 2020), spectral, and temporal resolutions of satellite measurements have increased their use in plant breeding (Prey et al., 2020; Weiss et al., 2020). Space-based platforms have become increasingly popular in recent years, and despite their high cost, they are being used in a limited number of agricultural applications. For example, NASA has created the Space Test Station for Thermal Radiation of Ecological Systems, which can be used to monitor the soil moisture content (Entekhabi et al., 2010), drought warnings, and water usage efficiency (Reynolds et al., 2019b). Soil Moisture Active Passive (SMAP) observations of soil moisture and freeze/thaw timing can reduce a major uncertainty in quantifying the global carbon balance by helping to resolve an apparent missing carbon sink on land over the boreal latitudes (Entekhabi et al., 2010). Pleiades-1a and World View-3 have been utilized to detect disease and agricultural water stress (Navrozidis et al., 2018; Salgadoe et al., 2018), promoting the advancement of precision agriculture. On the practical side, Jain and Balwinder-Singh (2019) demonstrated how microsatellite data management can have a substantial impact on agricultural sustainability in underdeveloped nations.
In summary, many crop phenotyping platforms in the field have distinct properties. Ground-based platforms can analyze the largest number of species and are well-established. Their price is reasonable; however, the vision height is limited, and the data throughput is modest. Two types of space-based platforms are available: manned and unmanned. Unmanned platforms can quickly acquire macrolevel information in a specific area, with broad vision and enormous data throughput, but their cost is significant. Space-based platforms, which offer the widest observation area, mostly rely on satellite remote sensing. However, they cannot perform small-scale or refined crop detection and are infrequently employed in agriculture due to their high cost. The use of space-based platforms in agriculture is projected to become more common as science and technology advance. The properties of many types of field phenotyping platforms are shown in Table 1. When employing phenotyping platforms, platforms that are reasonable for the circumstances must be chosen and developed, taking into account actual requirements such as mobility, ease of operation, data flux and accuracy, and costs (Lee et al., 2015).
Research status of high-throughput phenotypic information of dicots in the field
Morphological indicators
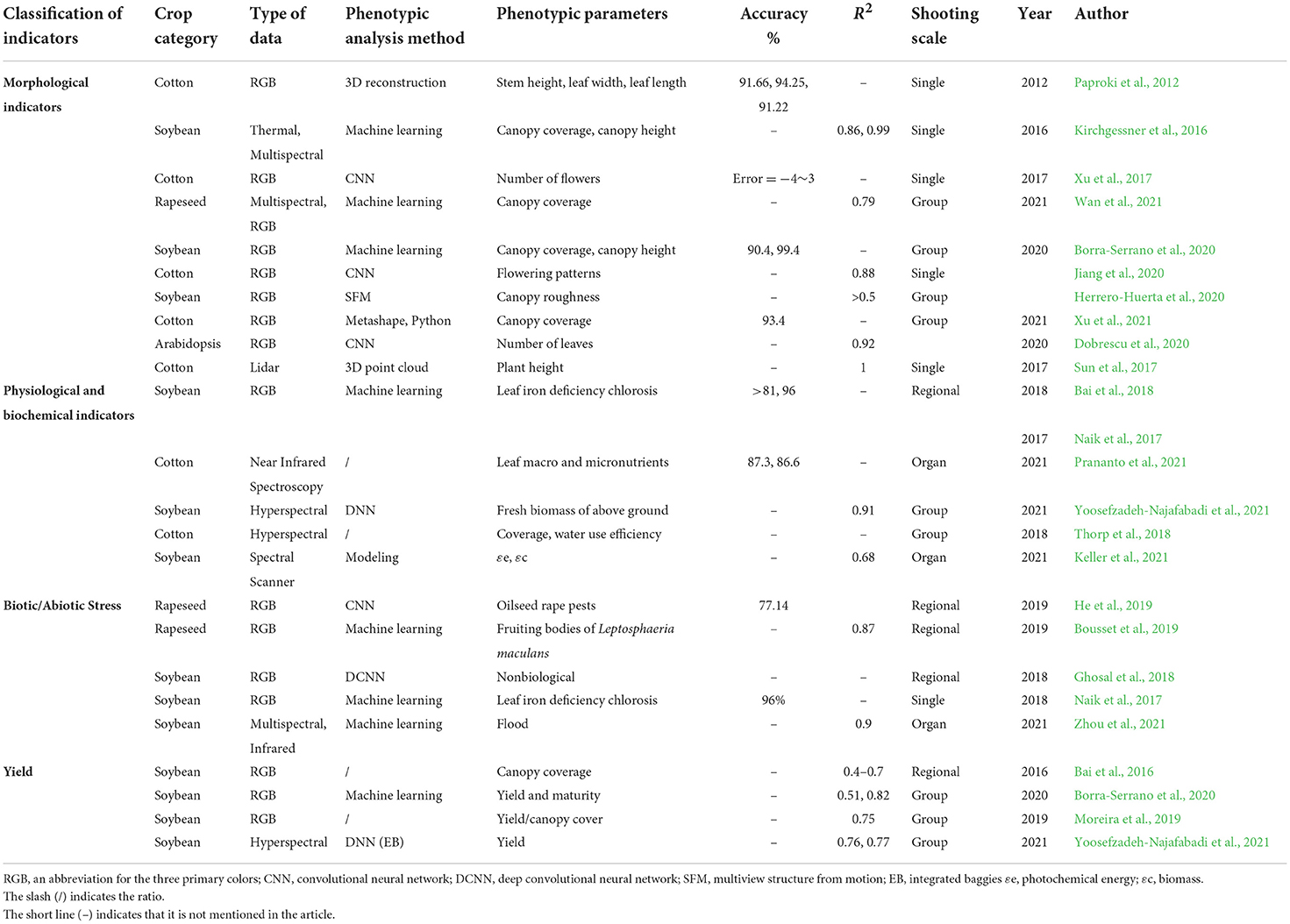
Plant morphology has essential biological implications in agricultural production, and it is a key component of plant science research. Traditional plant identification and classification approaches rely on professional experience, which is subjective and inaccurate, to examine plant morphological traits such as appearance, shape, texture, and color (Liu et al., 2016). Using machine vision, picture segmentation, and big data processing technologies to reliably gather and analyze crucial plant traits is an important technical means for the development of contemporary agriculture, with significant guiding value for crop management and genetic breeding (Granier and Vile, 2014; Li et al., 2021). Scholars have conducted field studies on the morphological indicators of dicotyledonous crops, including stem height (Paproki et al., 2012), plant height (Sun et al., 2017), leaf width (Paproki et al., 2012), leaf length (Paproki et al., 2012), number of leaves (Dobrescu et al., 2020), canopy coverage (Kirchgessner et al., 2016; Borra-Serrano et al., 2020; Wan et al., 2021; Xu et al., 2021), canopy height (Kirchgessner et al., 2016; Borra-Serrano et al., 2020), canopy roughness (Herrero-Huerta et al., 2020), and flowers (Xu et al., 2017; Jiang et al., 2020).
Stems and leaves are the most frequently utilized factors for crop morphological indication, and RGB values are commonly used by researchers to extract these parameters. For example, Paproki et al. (2012) created a cotton plant model by capturing RGB images of cotton plants and extracting indicators such as the cotton stem height, leaf width, and leaf length. When compared to manual measurements, the average absolute error was 9.34, 5.75, and 8.78%, respectively, while the correlation coefficients were 0.88, 0.96, and 0.95, respectively. Dobrescu et al. (2020) used deep learning to analyze the number of Arabidopsis leaves in RGB photos, and the R2 = 0.92 when compared to manual measurements. The approach of collecting crop morphological data from RGB images is quite accurate.
The extraction of crop canopy information is useful in the study of crop data. Previous research has demonstrated that thermal imaging, multispectral imaging, and RGB imaging can be utilized to monitor soybean canopy coverage and canopy height with an accuracy of >90% (Sun et al., 2017; Borra-Serrano et al., 2020), and an R2 > 0.5 was found after linear fitting using the values measured in the field (Herrero-Huerta et al., 2020). Moreover, similar research has been conducted on cotton, rape, and other crops. Xu et al. (2021) created a UAV system with three cameras (RGB, multispectral, and thermal) and a lidar sensor to identify cotton canopy coverage and canopy height, with an average relative error of only 6.6%. The approach of collecting crop morphological data from RGB images is quite accurate. Using a UAV platform fitted with an RGB and multispectral camera, Wan et al. (2021) obtained rape canopy images. The PROSAIL-GP model was used to invert rapeseed vegetation coverage and the R2 = 0.79. The resilience of the proposed method was confirmed in cotton (Gossypium hirsutum L.), and a better retrieval accuracy was obtained.
Many experiments have been conducted to analyze cotton flowering utilizing high-throughput phenotyping approaches. Color RGB images obtained by a UAV system and a CNN were used to detect the number of cotton blossoms in the original image, with an error of just −4~3 (Xu et al., 2017). Scanning cotton with a tractor-mounted lidar had an R2 = 0.98 compared with that of manual measurements (Sun et al., 2017). Moreover, cotton flowering status can be recognized using multiview color imaging and deep learning, with an R2 = 0.88 and an RMSE = 0.79 (Jiang et al., 2020).
Previous research has indicated that the use of a high-throughput phenotyping platform to obtain crop morphological indicators is nearing maturity. Researchers are more likely to use a ground-based platform equipped with an RGB camera as the primary research tool in the study of crop leaves and stalks. Canopy information can be extracted using UAVs equipped with RGB, hyperspectral, and radar sensors, but the accuracy is slightly lower than that obtained in leaf and stem studies. In terms of flower counting, UAVs and vehicle-mounted platforms outfitted with RGB cameras and lidar are commonly used, and the accuracy is acceptable. Thus, it is not surprising that the choice of a high-throughput phenotyping platform is closely related to the specific indicators being studied.
Physiological and biochemical indicators
Crop physiological and biochemical indices include chlorophyll, photosynthetic rate, water stress, biomass, salt tolerance, and leaf water content. These indices can accurately reflect crop growth, health, and resistance. Crop physiology and biochemistry studies involving the use of a high-throughput phenotyping platform have primarily focused on the leaf color (Bai et al., 2018), element content (Naik et al., 2017; Prananto et al., 2021), biomass (Yoosefzadeh-Najafabadi et al., 2021), and water use efficiency (Thorp et al., 2018).
To score iron deficiency chlorosis, RGB images of soybean plants in the field were collected, which revealed an overall accuracy of >81% (Naik et al., 2017; Bai et al., 2018). Prananto et al. (2021) used a ground-based platform equipped with a near-infrared spectrometer (wavelength range 1,350–2,500 nm) to estimate different macro- and micro-elements in cotton leaf tissues, with accuracies of 87.3 and 86.6%, respectively. The fitting degree of aboveground fresh biomass can be as high as 0.91 (Yoosefzadeh-Najafabadi et al., 2021) when combining hyperspectral photography with deep neural network (DNN) analysis for biomass acquisition and water use efficiency. Multispectral images can be used to determine the crop canopy coverage, which is then used to estimate the coefficient of basic crops to improve the crop water use efficiency (Thorp et al., 2018). Previous studies have shown that by acquiring high-throughput phenotypes, crop nutrients can be estimated in the field, farmers can proactively manage nutrition to avoid yield losses or environmental impacts, and evidence is provided for crop selection.
In short, physiological and biochemical indices have received less attention than morphological indices have. The platforms are mainly based on the ground and in space, and the sensor types are more complicated and varied. This research will help with nutrient decisions and the breeding of new varieties.
Biotic/abiotic stress indicators
Pests and diseases are the primary causes of crop yield reductions in terms of biological stress. Abiotic stress refers to all the factors that negatively affect crop growth and development as a result of an unsuitable external environment, which mainly includes light, temperature, water, and fertilizer. Crops are increasingly subjected to biotic/abiotic stress during growth as the global climate changes. The goal of smart agricultural plant protection is to locate the type of stress and determine the degree of stress through accurate identification before crops are stressed and irreparable damage is caused to protect plant operations.
The traditional method for evaluating the tolerance of crops to external stress in terms of the field conditions is to judge the damage level visually, but this method is labor intensive and susceptible to subjective error. This problem can be effectively resolved by utilizing high-throughput phenotyping. He et al. (2019) used RGB images of rapeseed to judge rapeseed insect pests with an accuracy of 77.14% using CNN processing and analysis. Naik et al. (2017) and Ghosal et al. (2018) used high-throughput phenotypes to evaluate soybean under abiotic stress and obtained promising results. Soybean field images were captured using a UAV equipped with a multispectral and infrared thermal imager, and five image features were extracted, including the canopy temperature, normalized difference vegetation index, canopy area, canopy width, and canopy length. The damage level was evaluated by a deep learning model, with an accuracy of 0.9 based on these features. The method proposed in this paper appears to be very promising for soybean breeding, and it is expected to replace an abundance of manual operations and more efficiently assess the level of waterlogging disasters (Zhou et al., 2021). High-throughput phenotypes have enormous potential for measuring crop traits and detecting crop responses to biological or nonbiological stresses.
Yield indicators
Crop yield estimation in the field is regarded as the foundation of food security. In recent years, remote sensing information and crop growth models have been coupled to resolve a variety of agricultural problems, such as crop growth detection and yield prediction.
Bai et al. (2016) collected soybean field traits via a self-propelled platform outfitted with five sensor modules (ultrasonic distance sensor, thermal infrared radiometer, normalized difference vegetation index (NDVI) sensor, portable spectrometer, and RGB network camera). The results of the analysis and processing revealed that the traits obtained by the sensors were highly correlated with the final grain yield in both the early and late seasons (r = 0.41–0.55, and r = 0.55–0.70). For example, Moreira et al. (2019) attached an RGB camera to a UAV to collect soybean production and canopy coverage data and continued the analysis to yield | ACC with an actual output correlation of 0.75. Yoosefzadeh-Najafabadi et al. (2021) used the hyperspectral vegetation index (HVI) collected by a UAV equipped with hyperspectral sensors to predict soybean yields in conjunction with two artificial intelligence algorithms integrated baggies (EB) and DNN and obtained determination coefficients (R2) of 0.76 and 0.77, respectively.
We suggest that to acquire a yield index, we must first acquire multi-index information. Because different researchers utilize different predictors, different high-throughput phenotypic platforms and sensors can be used. In general, the use of UAVs equipped with RGB cameras is the most common method. Yield prediction is beneficial for shortening the breeding time of varieties, reducing the cost of yield measurements, and enhancing the yield measurement efficiency, all of which are vital in crop research and development. Table 2 provides statistics on the field phenotypic information of dicotyledons.
High-throughput phenotyping and genetic breeding of dicotyledonous crops in the field
Crop breeding has gone through three generations, with the first being artificial screening as the primary method, the second being hybridization as the primary method, and the third being molecular markers and genome-wide association analysis as the primary and auxiliary methods. The fourth generation of intelligent-assisted breeding with big data, supported by multidisciplinary and multiomics data, is currently underway (Wallace et al., 2018). The incorporation of phenotypic and genomic data, as well as proteome, transcriptome, metabolome, and other omics data, is required for the fourth generation. Utilizing genome-wide association studies (GWASs), quantitative trait loci (QTL) analysis, and other technical methods, a large number of candidate genes and candidate molecular markers have been identified. Models, such as breeding information simulation, parental selection recommendation, breeding path recommendation, and breeding variety prediction, have been established to form the ultimate intelligent breeding decision system (Wang and Xu, 2019).
The high-throughput phenotyping platform, which enables the accurate assessment of a large number of field plots with a variety of measures in a short period of time, simplifies the routine quantification of crop development, physiology, and phenological characteristics (White et al., 2012; Araus and Cairns, 2014). These data provide a useful framework for addressing phenotypic bottlenecks in plant breeding (Furbank and Tester, 2011; Araus and Cairns, 2014; Kumar et al., 2015). Crop dwarfing has contributed to the growth of yields in the Green Revolution (Hammer et al., 2009; Swaminathan, 2014), and the fourth generation of intelligent-assisted breeding with big data could be the next breakthrough in accelerating the genetic harvest of crops in the field. Heritability and genetic gain potential will improve with high-throughput and precise phenotypes (Araus et al., 2018). Numerous successful results have been obtained in many crops by incorporating genomic and phenotypic data. The function of a large number of unknown genes, for example, has been quickly decoded, thereby improving the understanding of G-P maps (Raman, 2017). The water conditions of soybeans with different genotypes have been isolated (Braga et al., 2020), mutant and wild types have been effectively classified (Dobrescu et al., 2020), and flowering patterns in plants with complex canopy structures (such as cotton) have been identified (Jiang et al., 2020). Phenotypic indicators with strongly inherited traits are being investigated. Furthermore, phenotypic and genetic variation can be interpreted using optical sensing-based phenotyping (OSP) data analysis (Xavier et al., 2017; Sun et al., 2021).
High-throughput phenotyping and precise management of dicotyledonous crops in the field
Because agriculture is currently facing resource shortages and serious farmland environmental pollution, the implementation of precision agriculture demonstrations and research is critical. High-throughput phenotyping platforms can be used to obtain crop image traits and conduct modeling to estimate the yield and quality of a crop (Xu et al., 2021) and to analyze the relationship between crop growth and environmental factors, thereby enabling more precise management (Maimaitijiang et al., 2019).
In particular, high-throughput phenotypic data can be used to analyze the factors influencing yield differences among plots, treat different plots differently, and implement “prescription farming” based on positions, regulations, and needs. Making full use of information acquisition means analyzing the crop nutrition status and the spatial and temporal changes in pests and diseases to make tillage and field management decisions, as well as investing in agricultural resources such as water and fertilizer based on local conditions. This approach can ensure that the crop production potential is fully realized and avoid the serious consequences of the overuse of chemical fertilizers and pesticides, such as increased production costs, the pollution of farmland soil and water environments, and a decline in the quality of agricultural products. To achieve the best effect and the lowest cost, agricultural outputs can be increased, the quality can be enhanced, costs can be decreased, resources can be conserved, pollution can be reduced, and the environment can be protected. As a result, precision agriculture can produce significant economic and ecological benefits, which are vital for maximizing the production potential of cultivated land, efficiently using agricultural production factors, and preserving the farmland environment.
Development direction of high-throughput phenotypic information research on dicotyledonous field crops
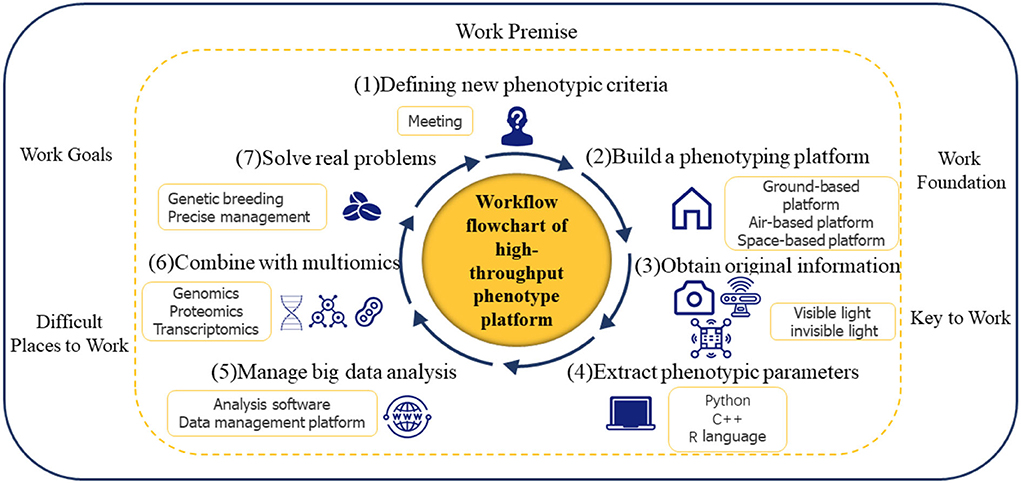
The development of a high-throughput phenotype must follow a certain workflow. As shown in Figure 3, from the determination of phenotypic concepts to the establishment of phenotypic platforms, the acquisition of original information, the extraction of phenotypic parameters, the analysis, processing and mining of big data, and joint analysis with multiple omics, practical problems can finally be solved. In fact, industry experts must decide on new phenotypic criteria because the step of “determination of unified new phenotypic criteria” is a premise for high-throughput phenotypic work; the demands of researchers for high-quality data, particularly in terms of resolution and accuracy, vary depending on the distinct objective features they are pursuing. Therefore, the key to collecting original information is to “develop a high-performance infrastructure platform,” but doing so is difficult. For better trait selection in breeding programs, more effective field management in agricultural operations, and the eventual augmentation of the germplasm of grain, the “construction of a knowledge map of phenotypic big data” stage is crucial but also difficult. The process of “combining with multiomics” is critical for finding functional genes and speeding up breeding.
Determination of new unified phenotypic criteria
Healthy, sustainable development must be based on consistent standards. Mendel, the father of genetics, began to define and evaluate phenotypes as early as 1866. He described seven pairs of relative characteristics of peas in his famous paper “Plant Hybridization Experiments,” including round and wrinkled seeds, tall stems vs. short stems, green pods vs. yellow pods, and so on. In 1911, Wilhelm Johannsen, a Danish geneticist, established the concept of a biological phenotype, claiming that an organism's phenotype was the consequence of a complex interaction between the genotype and environmental circumstances (Johannsen, 1911). In the 1990s, Nicholas Schoork, an epidemiology and biostatistics expert at Case Western Reserve University, was the first to propose the concept of physics as a counterpart to genomics (Zhao et al., 2019). Since then, studies on single phenotypes or series of phenotypes of humans, animals, and plants have piqued public interest (Siebner et al., 2009), and such studies have gradually evolved into an important branch of biology (Bilder et al., 2009; Houle et al., 2010; Tester and Langridge, 2010). Plant phenotyping research began at the end of the 20th century with the goals of obtaining high-quality and repeatable shape data and quantitatively analyzing the interactions between genotypes and environmental types and the effects on yields, quality, stress tolerance, and other related main traits (Ribaut et al., 2010). Fiorani and Schurr (2013) proposed a new definition of the plant phenotype in 2013, describing it as a collection of methods and protocols for accurately measuring the growth, structure, and composition of plants at various scales. In Zhao (2019) updated the definition of a phenotype, stating that it is a physical, physiological, and biochemical mechanism that can reflect the structural and functional characteristics of plant cells, tissues, organs, plants, and populations. In essence, a phenotype is the three-dimensional sequential expression of plant gene maps, regional differentiation characteristics, and intergenerational evolution. The phenotype concept is constantly being redefined. As a result, in the context of the rapid development of phenotyping platforms, the definition of new phenotypic concepts must be determined as soon as possible, and a consistent standard is necessary for correct phenotypic research by scientists.
Development of a high-performance infrastructure platform
With the widespread adoption of sequencing technology, an increasing number of plant genome sequences have been released, but few functional genes have been identified due to a lack of phenotypic data. Because of leaf occlusion, a substantial amount of information (such as the leaf number, main stem morphology, branch number, branch morphology, fruit number, and fruit morphology) has not been collected during the late growth stage of dicotyledons. The first step in achieving the comprehensive acquisition of high-throughput phenotypic information is to address the loss of original information, which is also the core challenge faced by the high-throughput phenotyping platform. How do you address the issue of lost data? Due to the nature of high-throughput phenotypic information gathering, all of the information that can be extracted is displayed in the original image. Some medical techniques, including computed tomography (CT), magnetic resonance imaging (MRI), and ultrasound, can be used to recover information that has been lost due to leaf occlusion. Unfortunately, because these technologies are both environmentally and financially demanding, they are rarely applied in agriculture.
After more than 10 years of development, crop phenotyping systems have the following characteristics: a high information acquisition efficiency, the use of non-invasive sensors, high-latitude information acquisition, and a resolution that decreases as the information acquisition area expands. As science and technology advance, future high-throughput phenotyping platforms will be able to combine the flux, resolution, dimension, load, robustness, and working height to obtain a large amount of original information efficiently and quickly. Resolution includes both temporal (from seconds to days to months) and spatial (very small, such as for cells, to large, such as for fields and natural environments) dimensions, denoting the variety of phenotypic features acquired by phenotyping systems under various time, space, and scale conditions. The load refers to the maximum weight that a phenotyping platform can carry; notably, in air-based platforms, the load capacity severely restricts the number and variety of sensors that can be used. Therefore, appropriately enhancing the load capacity of a phenotyping platform can promote the diversification of phenotypic data acquisition. The adaptability of phenotyping platforms to work in the field is referred to as robustness. Harsh field conditions pose significant challenges to the normal operation of phenotyping platforms, and enhancing the robustness of a platform can ensure that the platform operates properly. The working height is vital in obtaining phenotypic regions; the obtained regions increase as the working height increases, while the resolution decreases. The key issue for researchers to overcome is how to organically combine the aforementioned factors.
Furthermore, cost control is a critical link, and scientists have been working to determine how to achieve high performance at a low cost. Existing field phenotyping platforms are frequently unable to combine low cost and high performance, failing to meet the needs of most plant phenotype research institutes. As a result, obtaining fast and precise field phenotypic information at a low cost is a bottleneck in the development of a high-throughput phenotyping platform. This necessitates the gathering of talent from various fields, such as machinery, network communication, and sensors, to cross disciplines and contribute to the development of a high-throughput phenotyping platform in the field.
Construction of a knowledge map of phenotypic big data
Plants are dynamic, complex systems. Plant phenotypes, such as shape, size, color, posture, and texture, will change as they grow. Plants of different varieties have a wide range of appearances at the same time, resulting in the typical 3V characteristics of traditional big data in plant genome big data, that is, data volume, variety, and velocity. A large amount of data is mainly due to the rapid increase in phenotypic data obtained by advanced technology phenotyping equipment based on intelligent equipment and artificial intelligence technology. The diversity and heterogeneity of plant individuals and data types determine data polymorphism. The data are timely because of the dynamic and swift generation of large phenotypic data in the form of data flow.
At the same time, big plant phenomics data exhibit 3H characteristics: high dimension, high complexity, and high uncertainty primarily because plant genomic big data include text data and a large number of images, spectra, and cloud point data, resulting in a wide range of data. The high complexity of phenotypic information is determined by the diversity of genetic information and environmental differences. Phenotypic data have low repeatability and uncertainty because they are affected by many factors, and data acquisition criteria are not uniform. Dicotyledons have more complex plant types and higher 3V and 3H characteristics as a result of genetic diversity and geographical environmental resources, making dicotyledon phenotypic data analysis more difficult. How does one screen for key features under this premise? Big data analysis and in-depth mining must be improved.
After overcoming numerous obstacles to obtain phenotypic data, we are unable to extract such data in depth, resulting in a massive waste of data resources. Phenomic studies are more akin to point-like studies. On the one hand, phenotypic studies are carried out by organizations all over the world, but there is little cooperation between nations and institutions. Currently, only two national-level collaborations (with the US as the sole center and Germany, France, and the United Kingdom as common centers) and four institutional-level collaborations exist (with the United States Department of Agriculture and Cornell University as the centers; China Agricultural University, University of Queensland, Chinese Academy of Sciences, and Chinese Academy of Agricultural Sciences; University of Nottingham and University of Bonn; Wageningen University, French Agricultural Research Institute, and French Scientific Research Center). On the other hand, plant phenotypic databases are limited, and the main data contents vary greatly. For example, the Distributed Phenotypic Data Acquisition and Information Management System (Crop Sight), which primarily includes plant phenotypic data and environmental data (Reynolds et al., 2019a), the phenotypic mixed information system (PHIS), which primarily includes multisource and multiscale information in plant phenomics (Neveu et al., 2019), and the plant genome and phenotypic data sharing platform, primarily consists of data information on plant traits, phenotypes, gene functions, and gene expression of 95 plant taxa (Cooper et al., 2018); the Crop Phenotyping Center of Huazhong Agricultural University, which primarily consists of phenotype data and QTL data (Zhang et al., 2017); and the plant phenotype and genomics data publishing platform. Plant phenotypic data, genome data, mass spectrometry data, and data visualization and analysis software data are mainly included in the PGP Repository (Arend et al., 2016). This phenomenon will result in complex and variable phenotypic data formats, a lack of unified standards, and a significant reduction in the role of data. As a result, we urge countries and institutions to work together to strengthen collaborations, establish phenotype databases, share information, and hold joint discussions.
Combining with multiomics
The rapid development of high-throughput sequencing, mass spectrometry, and chromatography has facilitated the study of genomics, transcriptomics, proteomics, and metabolomics. Dicotyledons are characterized by complex plant types and a wealth of phenotypic data. When combined with multimers, dicotyledons can unlock more functional genes and facilitate plant genomics research. In breeding practice, high-throughput phenotyping combined with a variety of other omic techniques can be applied to crops in different growth periods and at different scales (cells, tissues, organs, groups) in research on the comprehensive analysis of the calculated crop regulation network of life activity, revealing the biological characteristics of crops. Certain studies on monocotyledons have been conducted in conjunction with multiple omics, such as the study by Leiboff et al. (2015), who used high-throughput image processing technology to determine the size of the shoot apical meristem (SAM) in a natural population of maize and discovered some new candidate genes controlling the SAM size through GWAS analysis. The link between the SAM morphology and trait-related SNP variants was verified after researchers looked into possible genes involved in hormone transport, cell division, and cell size. Xin et al. (2021) employed wild populations of 384 significant wheat varieties (lines) as the basis for a genome-wide association analysis that included phenotypes from three settings and 55K SNP chip typing data. The findings revealed that 142 SNPs were strongly related to the number of spikelets, with phenotypic variance ranging from 3.27 to 6.09%. Using the same strategy, Guo et al. (2018) discovered a novel drought tolerance gene in rice. For dicotyledons, few relevant studies have been conducted: Bac-Molenaar et al. (2015) used the PHENOPSIS phenotype platform to analyze high-throughput images of 324 Arabidopsis cultivars from the top view and, combined with genome-wide association analysis, identified some QTLs related to specific periods and growth rates, revealing a new perspective on the genetic structure of Arabidopsis dynamic development.
The ability to integrate metabolomics approaches into the current HTP phenotypic platform has significant potential to add value (Hall et al., 2022). Metabolites can be divided into volatile and nonvolatile categories, but they all play multiple roles in the plant life cycle. For example, they can be continuously present, having a protective function through antiinsect or antimicrobial activity (Lubes and Goodarzi, 2017; Maurya, 2020). The nonvolatile metabolome represents rich information reflecting past (e.g., slow turnover metabolites accumulated in response to past stress), present (e.g., high turnover metabolic intermediates), and future (e.g., precursors of biomass under construction) events. Accordingly, a growing number of top-down studies have shown that this metabolome can be correlated with performance in panels of genetic diversity (Meyer et al., 2007; Riedelsheimer et al., 2012). Until recently, cost issues have limited metabolomics applications in large-scale phenotyping. However, using high-resolution MS [TOF-MS, Orbitrap, and Fourier transform ion cyclotron resonance mass spectrometry (FT-ICR-MS)] to distinguish different structures with the same nominal mass alongside ultrafast chromatography now makes it possible to combine HTP with a high resolution (Fekete et al., 2014). Systems biology approaches and the use of large numbers of samples have become possible, and increased observations will enable the development of new prebreeding strategies based on predictive models (Fernandez et al., 2021).
We have reason to believe that plant genomics will advance faster with the establishment of relevant research institutions, the improvement of research facilities, the development of software, the convening of international academic conferences, and the formation of relevant academic teams.
The workflow of the high-throughput phenotyping platform is divided into seven steps. The first step is to determine the new phenotypic criteria. The second step is to build a phenotyping platform, including ground-based platforms, air-based platforms, and space-based platforms. The third step is to obtain raw information using visible and invisible sensors. The fourth and fifth steps are the extraction, analysis, and mining of phenotypic information. The next step is to combine phenotypic information with multi-omics. Finally, we aim to solve real problems (including genetic breeding and precision management).
Author contributions
XL, XX, and MC jointly wrote the article and prepared the figures and tables. MX, WW, CL, LY, and WY provided overall guidance and some references. WL helped to modify and improve the article. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Molecular mechanism of relay intercropping light environment regulating shade-tolerant plant architecture formation in soybean (3217150631), the Physiological mechanism of light regulating branch development in relay intercropping soybean (31871570), the physiology and regulation technology of high quality and high yield of soybean (2018YFD1000905), and the study and demonstration of corn—legume strip compound planting and mixed silage technology in Plateau Tibetan area (2020YFN0021).
Acknowledgments
We would like to thank Teachers MX and WW for their help in the work. We apologize to those colleagues who could not be quoted due to space limitations.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
3D, Three Dimensions; CNN, Convolutional Neural Network; CSIRO, Commonwealth Scientific and Industrial Research Organisation; CT, Computed Tomography; DCNN, Deep Convolutional Neural Network; EB, Integrated Baggies; ETH, Ethiopia; εc, Biomass; εe, Photochemical Energy; GWAS, Genome-Wide Association Studies; IPPN, International Plant Phenotype Network; MRI, Magnetic Resonance Imaging; NASA, National Aeronautics and Space Administration; OSP, Optical Sensing-based Phenotyping; PGP, Pretty Good Privacy; PHIS, Phenotypic mixed Information System; QTL, Quantitative Trait Loci; RGB, An abbreviation for three primary colors; SAM, Shoot Apical Meristem; SMAP, Soil Moisture Active Passive; SFM, Multiview Structure From Motion; SNP, Scottish National Party; UAVs, Unmanned Aerial Vehicles; UK, Britain.
References
Amoo, O., Li-Min, H., Yun-Gu, Z., Chu-Chuan, F., and Yong-Ming, Z. (2022). Regulation of shoot branching by BRANCHED1 in Brassica napus based on gene editing technology. Biotechnol. Bull. 38, 1–9. doi: 10.13560/j.cnki.biotech.bull.1985.2021-1344
Andrade-Sanchez, P., Gore, M. A., Heun, J. T., Thorp, K. R., Carmo-Silva, A. E., French, A. N., et al. (2013). Development and evaluation of a field-based high-throughput phenotyping platform. Funct. Plant Biol. 41, 68–79. doi: 10.1071/FP13126
Araus, J. L., and Cairns, J. E. (2014). Field high-throughput phenotyping: the new crop breeding frontier. Trends Plant Sci. 19, 52–61. doi: 10.1016/j.tplants.2013.09.008
Araus, J. L., Kefauver, S. C., Zaman-Allah, M., Olsen, M. S., and Cairns, J. E. (2018). Translating high-throughput phenotyping into genetic gain. Trends Plant Sci. 23, 451–466. doi: 10.1016/j.tplants.2018.02.001
Arend, D., Junker, A., Scholz, U., Schüler, D., Wylie, J., and Lange, M. (2016). PGP repository: a plant phenomics and genomics data publication infrastructure. Database. 33, 1–10. doi: 10.1093/database/baw033
Bac-Molenaar, J. A., Vreugdenhil, D., Granier, C., and Keurentjes, J. J. (2015). Genome-wide association mapping of growth dynamics detects time-specific and general quantitative trait loci. J. Exp. Bot. 66, 5567–5580. doi: 10.1093/jxb/erv176
Bai, G., Ge, Y., Hussain, W., Baenziger, P. S., and Graef, G. (2016). A multi-sensor system for high throughput field phenotyping in soybean and wheat breeding. Comput. Electron. Agric. 128, 181–192. doi: 10.1016/j.compag.2016.08.021
Bai, G., Jenkins, S., Yuan, W., Graef, G. L., and Ge, Y. (2018). Field-Based scoring of soybean iron deficiency chlorosis using RGB imaging and statistical learning. Front. Plant Sci. 9, 1002. doi: 10.3389/fpls.2018.01002
Bilder, R. M., Sabb, F., Cannon, T., London, E., Jentsch, J., Parker, D. S., et al. (2009). Phenomics: the systematic study of phenotypes on a genome-wide scale. Neuroscience. 164, 30–42. doi: 10.1016/j.neuroscience.2009.01.027
Bodner, G., Alsalem, M., and Nakhforoosh, A. (2021). “Root system phenotying of soil-grown plants via RGB and hyperspectral imaging,” in Crop Breeding (Springer), 245–268. doi: 10.1007/978-1-0716-1201-9_17
Borra-Serrano, I., De Swaef, T., Quataert, P., Aper, J., Saleem, A., Saeys, W., et al. (2020). Closing the phenotyping gap: high resolution UAV time series for soybean growth analysis provides objective data from field trials. Remote Sens. 12, 1644–1665. doi: 10.3390/rs12101644
Bousset, L., Palerme, M., Leclerc, M., and Parisey, N. (2019). Automated image processing framework for analysis of the density of fruiting bodies of Leptosphaeria maculans on oilseed rape stems. Plant Pathol. 68, 1749–1760. doi: 10.1111/ppa.13085
Braga, P., Crusiol, L. G. T., and Nanni, M. R. (2020). Vegetation indices and NIR-SWIR spectral bands as a phenotyping tool for water status determination in soybean. Precis. Agric. 22, 249–266. doi: 10.1007/s11119-020-09740-4
Busemeyer, L., Mentrup, D., Möller, K., Wunder, E., Alheit, K., Hahn, V., et al. (2013). BreedVision—a multi-sensor platform for non-destructive field-based phenotyping in plant breeding. Sensors. 13, 2830–2847. doi: 10.3390/s130302830
Casagrande, C. R., Sant'ana, G. C., Meda, A. R., Garcia, A., Souza Carneiro, P. C., Nardino, M., et al. (2022). Association between UAV high-throughput canopy phenotyping and soybean yield. Agron. J. 23, 1–18. doi: 10.1002/agj2.21047
Chang, A., Jung, J., Yeom, J., Maeda, M. M., Landivar, J. A., Enciso, J. M., et al. (2021). Unmanned aircraft system-(UAS-) based high-throughput phenotyping (HTP) for tomato yield estimation. J. Sensor. 2021, 1–14. doi: 10.1155/2021/8875606
Comar, A., Burger, P., De Solan, B., Baret, F. D. R., Daumard, F., and Hanocq, J. O. (2012). A semi-automatic system for high throughput phenotyping wheat cultivars in-field conditions: description and first results. Funct. Plant Biol. 39, 914–924. doi: 10.1071/FP12065
Cooper, L., Meier, A., Laporte, M.-A., Elser, J. L., Mungall, C., Sinn, B. T., et al. (2018). The Planteome database: an integrated resource for reference ontologies, plant genomics and phenomics. Nucleic Acids Res. 46, D1168–D1180. doi: 10.1093/nar/gkx1152
Ding, G., Xu, H., Wen, M., Chen, J., Wang, X., and Zhou, J. (2019). Developing cost-effective and low-altitude UAV aerial phenotyping and automated phenotypic analysis to measure key yield-related traits for bread wheat. J. Agric. Big Data. 1, 19–31. doi: 10.19788/j.issn.2096-6369.190202
Dobrescu, A., Giuffrida, M. V., and Tsaftaris, S. A. (2020). Doing more with less: a multitask deep learning approach in plant phenotyping. Front. Plant Sci. 11, 141. doi: 10.3389/fpls.2020.00141
Entekhabi, D., Njoku, E. G., O'neill, P. E., Kellogg, K. H., Crow, W. T., Edelstein, W. N., et al. (2010). The soil moisture active passive (SMAP) mission. Proc. IEEE. 98, 704–716. doi: 10.1109/jproc.2010.2043918
Fan, S., Zhang, L., Tang, M., Cai, Y., Liu, J., Liu, H., et al. (2021). CRISPR/Cas9-targeted mutagenesis of the BnaA03. BP gene confers semi-dwarf and compact architecture to rapeseed (Brassica napus L.). Plant Biotechnol. J. 19, 2383–2385. doi: 10.1111/pbi.13703
Fekete, S., Schappler, J., Veuthey, J.-L., and Guillarme, D. (2014). Current and future trends in UHPLC. TrAC Trends Anal. Chem. 63, 2–13. doi: 10.1016/j.trac.2014.08.007
Fernandez, O., Millet, E. J., Rincent, R., Prigent, S., Pétriacq, P., and Gibon, Y. (2021). “Plant metabolomics and breeding,” in Advances in Botanical Research (Elsevier), 207–235. doi: 10.1016/bs.abr.2020.09.020
Fiorani, F., and Schurr, U. (2013). Future scenarios for plant phenotyping. Annu. Rev. Plant Biol. 64, 267–291. doi: 10.1146/annurev-arplant-050312-120137
Furbank, R. T., Silva-Perez, V., Evans, J. R., Condon, A. G., Estavillo, G. M., He, W., et al. (2021). Wheat physiology predictor: predicting physiological traits in wheat from hyperspectral reflectance measurements using deep learning. Plant Methods 17, 1–15. doi: 10.1186/S13007-021-00806-6
Furbank, R. T., and Tester, M. (2011). Phenomics—technologies to relieve the phenotyping bottleneck. Trends Plant Sci. 16, 635–644. doi: 10.1016/j.tplants.2011.09.005
Ge, Y., Atefi, A., Zhang, H., Miao, C., Ramamurthy, R. K., Sigmon, B., et al. (2019). High-throughput analysis of leaf physiological and chemical traits with VIS-NIR-SWIR spectroscopy: a case study with a maize diversity panel. Plant Methods 15, 66–78. doi: 10.1186/s13007-019-0450-8
Ghosal, S., Blystone, D., Singh, A. K., Ganapathysubramanian, B., Singh, A., and Sarkar, S. (2018). An explainable deep machine vision framework for plant stress phenotyping. Proc. Natl. Acad. Sci. U. S. A. 115, 4613–4618. doi: 10.1073/pnas.1716999115
Granier, C., and Vile, D. (2014). Phenotyping and beyond: modelling the relationships between traits. Curr. Opin. Plant Biol. 18, 96–102. doi: 10.1016/j.pbi.2014.02.009
Guo, Z., Yang, W., Chang, Y., Ma, X., Tu, H., Xiong, F., et al. (2018). Genome-wide association studies of image traits reveal genetic architecture of drought resistance in rice. Mol. Plant. 11, 789–805. doi: 10.1016/j.molp.2018.03.018
Hall, R. D., D'auria, J. C., Ferreira, A. C. S., Gibon, Y., Kruszka, D., Mishra, P., et al. (2022). High-throughput plant phenotyping: a role for metabolomics? Trends Plant Sci. 27, 549–563. doi: 10.1016/j.tplants.2022.02.001
Hammer, G. L., Dong, Z., McLean, G., Doherty, A., Messina, C., Schussler, J., et al. (2009). Can changes in canopy and/or root system architecture explain historical maize yield trends in the US corn belt? Crop Sci. 49, 299–312. doi: 10.2135/cropsci2008.03.0152
He, Y., Zeng, H., Fan, Y., Ji, S., and Wu, J. (2019). Application of deep learning in integrated pest management: a real-time system for detection and diagnosis of oilseed rape pests. Mobile Inf. Syst. 2019, 1–14. doi: 10.1155/2019/4570808
Herrero-Huerta, M., Bucksch, A., Puttonen, E., and Rainey, K. M. (2020). Canopy roughness: a new phenotypic trait to estimate aboveground biomass from unmanned aerial system. Plant Phenomics. 2020, 6735967–6735983. doi: 10.34133/2020/6735967
Houle, D., Govindaraju, D. R., and Omholt, S. (2010). Phenomics: the next challenge. Nat. Rev. Genet. 11, 855–866. doi: 10.1038/nrg2897
Hu, P., Chapman, S. C., Wang, X., Potgieter, A., Duan, T., Jordan, D., et al. (2018). Estimation of plant height using a high throughput phenotyping platform based on unmanned aerial vehicle and self-calibration: example for sorghum breeding. Eur. J. Agron. 95, 24–32. doi: 10.1016/j.eja.2018.02.004
Huichun, Z., Hongping, Z., Jiaqiang, Z., Yufeng, G., and Yangxian, L. (2020). Research progress and prospect in plant phenotyping platform and image analysis technology. Nongye Jixie Xuebao/Trans. Chin. Soc. Agric. Mach. 51, 1–17 doi: 10.6041/j.issn.1000-1298.2020.03.001
Jain, M., and Balwinder-Singh, R. (2019). The impact of agricultural interventions can be doubled by using satellite data. Nat. Sustain. 2, 931–934. doi: 10.1038/s41893-019-0396-x
Jiang, Y., Li, C., Robertson, J. S., Sun, S., Xu, R., and Paterson, A. H. (2018). GPhenoVision: a ground mobile system with multi-modal imaging for field-based high throughput phenotyping of cotton. Sci. Rep. 8, 1213–1228. doi: 10.1038/s41598-018-19142-2
Jiang, Y., Li, C., Xu, R., Sun, S., Robertson, J. S., and Paterson, A. H. (2020). DeepFlower: a deep learning-based approach to characterize flowering patterns of cotton plants in the field. Plant Methods 16, 156–174. doi: 10.1186/s13007-020-00698-y
Jin, X., Liu, S., Baret, F., Hemerlé, M., and Comar, A. (2017). Estimates of plant density of wheat crops at emergence from very low altitude UAV imagery. Remote Sens. Environ. 198, 105–114. doi: 10.1016/j.rse.2017.06.007
Johannsen, W. (1911). The genotype conception of heredity. Am. Nat. 45, 129–159. doi: 10.1086/279202
Jun, S., Kaifeng, Y., Yuanqiu, L., Jifeng, S., Xiaohong, W., and Lei, Q. (2021). Method for the multiscale perceptual counting of wheat ears based on UAV images. Trans. Chin. Soc. Agric. Eng. 37, 136–144. doi: 10.11975/j.issn.1002-6819.2021.23.016
Karthikeyan, L., Chawla, I., and Mishra, A. K. (2020). A review of remote sensing applications in agriculture for food security: crop growth and yield, irrigation, and crop losses. J. Hydrol. 586, 124905. doi: 10.1016/j.jhydrol.2020.124905
Keller, B., Zimmermann, L., Rascher, U., Matsubara, S., Steier, A., and Muller, O. (2021). Towards predicting photosynthetic efficiency and biomass gain in crop genotypes over a field season. Plant Physiol. 188, 1–17. doi: 10.1093/plphys/kiab483
Kirchgessner, N., Liebisch, F., Yu, K., Pfeifer, J., Friedli, M., Hund, A., et al. (2016). The ETH field phenotyping platform FIP: a cable-suspended multi-sensor system. Funct. Plant Biol. 44, 154–168. doi: 10.1071/FP16165
Kumar, J., Pratap, A., and Kumar, S. (2015). Phenomics in Crop Plants: Trends, Options and Limitations. Springer, 195–205. doi: 10.1007/978-81-322-2226-2_13
Lee, C. M., Cable, M. L., Hook, S. J., Green, R. O., Ustin, S. L., Mandl, D. J., et al. (2015). An introduction to the NASA Hyperspectral InfraRed Imager (HyspIRI) mission and preparatory activities. Remote Sens. Environ. 167, 6–19. doi: 10.1016/j.rse.2015.06.012
Leiboff, S., Li, X., Hu, H.-C., Todt, N., Yang, J., Li, X., et al. (2015). Genetic control of morphometric diversity in the maize shoot apical meristem. Nat. Commun. 6, 1–10. doi: 10.1038/ncomms9974
Li, B., Chen, L., Sun, W., Wu, D., Wang, M., Yu, Y., et al. (2020). Phenomics-based GWAS analysis reveals the genetic architecture for drought resistance in cotton. Plant Biotechnol. J. 18, 2533–2544. doi: 10.1111/pbi.13431
Li, S., Yan, Z., Guo, Y., Su, X., Cao, Y., Jiang, B., et al. (2021). SPM-IS: an auto-algorithm to acquire a mature soybean phenotype based on instance segmentation. Crop J. 5, 1–22. doi: 10.1016/j.cj.2021.05.014
Liu, J., Zhao, C., Yang, G., Yu, H., Zhao, X., Xu, B., et al. (2016). Review of field-based phenotyping by unmanned aerial vehicle remote sensing platform. Trans. Chin. Soc. Agric. Eng. 32, 98–106. doi: 10.11975/j.issn.1002-6819.2016.24.013
Lubes, G., and Goodarzi, M. (2017). Analysis of volatile compounds by advanced analytical techniques and multivariate chemometrics. Chem. Rev. 117, 6399–6422. doi: 10.1021/acs.chemrev.6b00698
Maimaitijiang, M., Sagan, V., Sidike, P., Maimaitiyiming, M., Hartling, S., Peterson, K. T., et al. (2019). Vegetation index weighted canopy volume model (CVMVI) for soybean biomass estimation from unmanned aerial system-based RGB imagery. ISPRS J. Photogramm. Remote Sens. 151, 27–41. doi: 10.1016/j.isprsjprs.2019.03.003
Malambo, L., Popescu, S. C., Murray, S. C., Putman, E., Pugh, N. A., Horne, D. W., et al. (2018). Multitemporal field-based plant height estimation using 3D point clouds generated from small unmanned aerial systems high-resolution imagery. Int. J. Appl. Earth Obs. Geoinform. 64, 31–42. doi: 10.1016/j.jag.2017.08.014
Maurya, A. K. (2020). “Application of plant volatile organic compounds (VOCs) in agriculture,” in New Frontiers in Stress Management for Durable Agriculture (Springer), 369–388.
Meyer, R. C., Steinfath, M., Lisec, J., Becher, M., Witucka-Wall, H., Törjék, O., et al. (2007). The metabolic signature related to high plant growth rate in Arabidopsis thaliana. Proc. Nat. Acad. Sci. U. S. A. 104, 4759–4764. doi: 10.1073/pnas.0609709104
Miao, C., Guo, A., Thompson, A. M., Yang, J., Ge, Y., and Schnable, J. C. (2021). Automation of leaf counting in maize and sorghum using deep learning. Plant Phenome J. 4, 1–15. doi: 10.1002/ppj2.20022
Mishra, P., Angileri, M., and Woltering, E. (2021). Identifying the best rice physical form for non-destructive prediction of protein content utilising near-infrared spectroscopy to support digital phenotyping. Infrared Phys. Technol. 116, 103757. doi: 10.1016/J.INFRARED.2021.103757
Moreira, F. F., Hearst, A. A., Cherkauer, K. A., and Rainey, K. M. (2019). Improving the efficiency of soybean breeding with high-throughput canopy phenotyping. Plant Methods 15, 1–9. doi: 10.1186/s13007-019-0519-4
Muharam, F. M., Nurulhuda, K., Zulkafli, Z., Tarmizi, M. A., and Ismail, M. R. (2021). UAV- and Random-Forest-AdaBoost (RFA)-based estimation of rice plant traits. Agronomy 11, 915–943. doi: 10.3390/AGRONOMY11050915
Naik, H. S., Zhang, J., Lofquist, A., Assefa, T., Sarkar, S., Ackerman, D., et al. (2017). A real-time phenotyping framework using machine learning for plant stress severity rating in soybean. Plant Methods 13, 23–40. doi: 10.1186/s13007-017-0173-7
Navrozidis, I., Alexandridis, T. K., Dimitrakos, A., Lagopodi, A. L., Moshou, D., and Zalidis, G. (2018). Identification of purple spot disease on asparagus crops across spatial and spectral scales. Comput. Electron. Agric. 148, 322–329. doi: 10.1016/j.compag.2018.03.035
Neveu, P., Tireau, A., Hilgert, N., Nègre, V., Mineau-Cesari, J., Brichet, N., et al. (2019). Dealing with multi-source and multi-scale information in plant phenomics: the ontology-driven Phenotyping Hybrid Information System. New Phytol. 221, 588–601. doi: 10.1111/nph.15385
Pan, Y. (2015). Analysis of concepts and categories of plant phenome and phenomics. Acta Agron. Sin. 41, 175–186. doi: 10.3724/sp.J.1006.2015.00175
Paproki, A., Sirault, X., Berry, S., Furbank, R., and Fripp, J. (2012). A novel mesh processing based technique for 3D plant analysis. BMC Plant Biol. 12, 63. doi: 10.1186/1471-2229-12-63
Poorter, H., Fiorani, F., Stitt, M., Schurr, U., Finck, A., Gibon, Y., et al. (2012). The art of growing plants for experimental purposes: a practical guide for the plant biologist. Funct. Plant Biol. 39, 821–838. doi: 10.1071/FP12028
Prananto, J. A., Minasny, B., and Weaver, T. (2021). Rapid and cost-effective nutrient content analysis of cotton leaves using near-infrared spectroscopy (NIRS). PeerJ. 9, 11042–11066. doi: 10.7717/peerj.11042
Prey, L., Hu, Y., and Schmidhalter, U. (2020). High-throughput field phenotyping traits of grain yield formation and nitrogen use efficiency: optimizing the selection of vegetation indices and growth stages. Front. Plant Sci. 10, 1672. doi: 10.3389/fpls.2019.01672
Prey, L., and Schmidhalter, U. (2020). Deep phenotyping of yield-related traits in wheat. Agronomy. 10, 603. doi: 10.3390/agronomy10040603
Raman, R. (2017). The impact of Genetically Modified (GM) crops in modern agriculture: a review. GM Crops Food. 8, 195–208. doi: 10.1080/21645698.2017.1413522
Reuzeau, C., Frankard, V., Hatzfeld, Y., Sanz, A., Van Camp, W., Lejeune, P., et al. (2006). Traitmill™: a functional genomics platform for the phenotypic analysis of cereals. Plant Genet. Resour. 4, 20–24. doi: 10.1079/PGR2005104
Reynolds, D., Ball, J., Bauer, A., Davey, R., Griffiths, S., and Zhou, J. (2019a). CropSight: a scalable and open-source information management system for distributed plant phenotyping and IoT-based crop management. Gigascience 8, 1–11. doi: 10.1093/gigascience/giz009
Reynolds, D., Baret, F., Welcker, C., Bostrom, A., Ball, J., Cellini, F., et al. (2019b). What is cost-efficient phenotyping? Optimizing costs for different scenarios. Plant Sci. 282, 14–22. doi: 10.1016/j.plantsci.2018.06.015
Ribaut, J., De Vicente, M., and Delannay, X. (2010). Molecular breeding in developing countries: challenges and perspectives. Curr. Opin. Plant Biol. 13, 213–218. doi: 10.1016/j.pbi.2009.12.011
Riedelsheimer, C., Czedik-Eysenberg, A., Grieder, C., Lisec, J., Technow, F., Sulpice, R., et al. (2012). Genomic and metabolic prediction of complex heterotic traits in hybrid maize. Nat. Genet. 44, 217–220. doi: 10.1038/ng.1033
Salgadoe, A. S. A., Robson, A. J., Lamb, D. W., Dann, E. K., and Searle, C. (2018). Quantifying the severity of phytophthora root rot disease in avocado trees using image analysis. Remote Sens. 10, 226–343. doi: 10.3390/rs10020226
Shafiekhani, A., Kadam, S., Fritschi, F. B., and Desouza, G. N. (2017). Vinobot and vinoculer: two robotic platforms for high-throughput field phenotyping. Sensors. 17, 214. doi: 10.3390/s17010214
Shao, M., Nie, C., Cheng, M., Yu, X., Bai, Y., Ming, B., et al. (2022). Quantifying effect of tassels on near-ground maize canopy RGB images using deep learning segmentation algorithm. Precis. Agric. 23, 400–418. doi: 10.1007/S11119-021-09842-7
Siebner, H. R., Callicott, J. H., Sommer, T., and Mattay, V. S. (2009). From the genome to the phenome and back: linking genes with human brain function and structure using genetically informed neuroimaging. Neuroscience. 164, 1–6. doi: 10.1016/j.neuroscience.2009.09.009
Souza, A., and Yang, Y. (2021). High-throughput corn image segmentation and trait extraction using chlorophyll fluorescence images. Plant Phenomics 2021, 582–597. doi: 10.34133/2021/9792582
Sugiura, R., Tsuda, S., Tamiya, S., Itoh, A., Nishiwaki, K., Murakami, N., et al. (2016). Field phenotyping system for the assessment of potato late blight resistance using RGB imagery from an unmanned aerial vehicle. Biosyst. Eng. 148, 1–10. doi: 10.1016/j.biosystemseng.2016.04.010
Sun, D., Robbins, K., Morales, N., Shu, Q., and Cen, H. (2021). Advances in optical phenotyping of cereal crops. Trends Plant Sci. 27, 191–208. doi: 10.1016/j.tplants.2021.07.015
Sun, Q., Xie, Y., Li, H., Liu, J., Geng, R., Wang, P., et al. (2022). Cotton GhBRC1 regulates branching, flowering, and growth by integrating multiple hormone pathways. Crop J. 10, 75–87. doi: 10.1016/j.cj.2021.01.007
Sun, S., Li, C., and Paterson, A. (2017). In-field high-throughput phenotyping of cotton plant height using LiDAR. Remote Sens. 9, 377–398. doi: 10.3390/rs9040377
Sun, S., Li, C., Paterson, A. H., Jiang, Y., Xu, R., Robertson, J. S., et al. (2018). In-field high throughput phenotyping and cotton plant growth analysis using LiDAR. Front. Plant Sci. 9, 16. doi: 10.3389/fpls.2018.00016
Swaminathan, M. (2014). Norman Borlaug and a hunger-free world. Resonance 19, 109–115. doi: 10.1007/s12045-014-0014-1
Tardieu, F., Cabrera-Bosquet, L., Pridmore, T., and Bennett, M. (2017). Plant phenomics, from sensors to knowledge. Curr. Biol. 27, R770–R783. doi: 10.1016/j.cub.2017.05.055
Tester, M., and Langridge, P. (2010). Breeding technologies to increase crop production in a changing world. Science 327, 818–822. doi: 10.1126/science.1183700
Thorp, K., Thompson, A., Harders, S., French, A., and Ward, R. (2018). High-throughput phenotyping of crop water use efficiency via multispectral drone imagery and a daily soil water balance model. Remote Sens. 10, 1682–1701. doi: 10.3390/rs10111682
Trevisan, R., Pérez, O., Schmitz, N., Diers, B., and Martin, N. (2020). High-throughput phenotyping of soybean maturity using time series UAV imagery and convolutional neural networks. Remote Sens. 12, 214–214. doi: 10.3390/rs12213617
Virlet, N., Sabermanesh, K., Sadeghi-Tehran, P., and Hawkesford, M. J. (2016). Field Scanalyzer: an automated robotic field phenotyping platform for detailed crop monitoring. Funct. Plant Biol. 44, 143–153. doi: 10.1071/FP16163
Wallace, J. G., Rodgers-Melnick, E., and Buckler, E. S. (2018). On the road to breeding 4.0: unraveling the good, the bad, and the boring of crop quantitative genomics. Annu. Rev. Genet. 52, 421–444. doi: 10.1146/annurev-genet-120116-024846
Wan, L., Zhu, J., Du, X., Zhang, J., Han, X., Zhou, W., et al. (2021). A model for phenotyping crop fractional vegetation cover using imagery from unmanned aerial vehicles. J. Exp. Bot. 72, 4691–4707. doi: 10.1093/jxb/erab194
Wang, M., and Xu, S. (2019). Statistical power in genome-wide association studies and quantitative trait locus mapping. Heredity 123, 287–306. doi: 10.1038/s41437-019-0205-3
Wanli, Y., Lingfeng, D., and Wanneng, Y. (2021). Deep learning-based extraction of rice phenotypic characteristics and prediction of rice panicle weight. J. Huazhong Agric. Univ. 40, 227–235. doi: 10.13300/j.cnki.hnlkxb.2021.01.028
Weiss, M., Jacob, F., and Duveiller, G. (2020). Remote sensing for agricultural applications: a meta-review. Remote Sens. Environ. 236, 1–69. doi: 10.1016/j.rse.2019.111402
White, J. W., Andrade-Sanchez, P., Gore, M. A., Bronson, K. F., Coffelt, T. A., Conley, M. M., et al. (2012). Field-based phenomics for plant genetics research. Field Crops Res. 133, 101–112. doi: 10.1016/j.fcr.2012.04.003
Wu, H., Ren, Z., Zheng, L., Guo, M., Yang, J., Hou, L., et al. (2021). The bHLH transcription factor GhPAS1 mediates BR signaling to regulate plant development and architecture in cotton. Crop J. 9, 1049–1059. doi: 10.1016/j.cj.2020.10.014
Xavier, A., Hall, B., Hearst, A. A., Cherkauer, K. A., and Rainey, K. M. (2017). Genetic architecture of phenomic-enabled canopy coverage in Glycine max. Genetics 206, 1081–1089. doi: 10.1534/genetics.116.198713
Xiao, F., Li, W., Xiao, M., Yang, Z., Cheng, W., Gao, S., et al. (2021). A novel light interception trait of a hybrid rice ideotype indicative of leaf to panicle ratio. Field Crops Res. 274, 108338. doi: 10.1016/J.FCR.2021.108338
Xiaobin, Z., Baoliang, X., Yihang, Z., Kefeng, Z., and Qing, G. (2022). High-throughput phenotype collection and analysis of vegetablesoybean pod based on image processing technology. J. Nucl. Agric. Sci. 36, 602–612. doi: 10.11869/j.issn.100-8551.2022.03.0602
Xiaobo, Y., Mingrong, Z., Hsaiying, W., and Wenyu, Y. (2012). Agronomic characters and yield distribution of different shade tolerance soybean under monoculture and relay strip intercropping. Systems 05, 757–761. doi: 10.3969/j.issn.1000-9841.2012.05.014
Xie, J., Fernandes, S. B., Mayfield-Jones, D., Erice, G., Choi, M., Lipka, A. E., et al. (2021). Optical topometry and machine learning to rapidly phenotype stomatal patterning traits for maize QTL mapping. Plant Physiol. 187, 1462–1480. doi: 10.1093/PLPHYS/KIAB299
Xin, X., De-Hua, Z., Ji-Shun, Z., and Xiao-Jun, L. (2021). Genome-wide association study of grain number per spikelet in bread wheat. J. Plant Genet. Resour. 23, 1098–1110. doi: 10.13430/j.cnki.jpgr.20211222001
Xu, R., Li, C., and Bernardes, S. (2021). Development and testing of a UAV-based multi-sensor system for plant phenotyping and precision agriculture. Remote Sens. 13, 3517–3545. doi: 10.3390/rs13173517
Xu, R., Li, C., Paterson, A. H., Jiang, Y., Sun, S., and Robertson, J. S. (2017). Aerial images and convolutional neural network for cotton bloom detection. Front. Plant Sci. 8, 2235. doi: 10.3389/fpls.2017.02235
Yoosefzadeh-Najafabadi, M., Tulpan, D., and Eskandari, M. (2021). Using hybrid artificial intelligence and evolutionary optimization algorithms for estimating soybean yield and fresh biomass using hyperspectral vegetation indices. Remote Sens. 13, 2555–2577. doi: 10.3390/rs13132555
Young, S. N., Kayacan, E., and Peschel, J. M. (2018). Design and field evaluation of a ground robot for high-throughput phenotyping of energy sorghum. Precis. Agric. 20, 697–722. doi: 10.1007/s11119-018-9601-6
Yu-Shan, W., Wan-Zhuo, G., Qian-Jun, T., Feng, C., Yan-Ling, W., Wei-Guo, L., et al. (2015). Research on the relationship between agronomic traits and yield of soybean in relay strip intercropping. Soybean Sci. 03, 394–401. doi: 10.11861/j.issn.1000-9841.2015.03.0394
Zea, M., Souza, A., Yang, Y., Lee, L., Nemali, K., and Hoagland, L. (2022). Leveraging high-throughput hyperspectral imaging technology to detect cadmium stress in two leafy green crops and accelerate soil remediation efforts. Environ. Pollut. 292, 118405. doi: 10.1016/j.envpol.2021.118405
Zelazny, W. R., Chrpová, J., and Hamouz, P. (2021). Fusarium head blight detection from spectral measurements in a field phenotyping setting—a pre-registered study. Biosyst. Eng. 211, 97–113. doi: 10.1016/J.BIOSYSTEMSENG.2021.08.019
Zhan, J., Chu, Y., Wang, Y., Diao, Y., Zhao, Y., Liu, L., et al. (2021). The miR164-GhCUC2-GhBRC1 module regulates plant architecture through abscisic acid in cotton. Plant Biotechnol. J. 19, 1839–1851. doi: 10.1111/pbi.13599
Zhang, C., Marzougui, A., and Sankaran, S. (2020). High-resolution satellite imagery applications in crop phenotyping: an overview. Comput. Electron. Agric. 175, 1–10. doi: 10.1016/j.compag.2020.105584
Zhang, X., Huang, C., Wu, D., Qiao, F., Li, W., Duan, L., et al. (2017). High-throughput phenotyping and QTL mapping reveals the genetic architecture of maize plant growth. Plant Physiol. 173, 1554–1564. doi: 10.1104/pp.16.01516
Zhao, C. (2019). Big data of plant phenomics and its research progress. J. Agric. Big Data 1, 5–18. doi: 10.19788/j.issn.2096-6369.190201
Zhao, C., Zhang, Y., Du, J., Guo, X., Wen, W., Gu, S., et al. (2019). Crop phenomics: current status and perspectives. Front. Plant Sci. 10, 714. doi: 10.3389/fpls.2019.00714
Zhou, J., Mou, H., Zhou, J., Ali, M. L., Ye, H., Chen, P., et al. (2021). Qualification of soybean responses to flooding stress using UAV-based imagery and deep learning. Plant Phenomics 2021, 1–13. doi: 10.34133/2021/9892570
Keywords: high-throughput phenotyping platform, dicotyledonous crops, field, research progress, development direction
Citation: Li X, Xu X, Chen M, Xu M, Wang W, Liu C, Yu L, Liu W and Yang W (2022) The field phenotyping platform's next darling: Dicotyledons. Front. Plant Sci. 13:935748. doi: 10.3389/fpls.2022.935748
Received: 04 May 2022; Accepted: 21 July 2022;
Published: 24 August 2022.
Edited by:
Wei Guo, The University of Tokyo, JapanReviewed by:
Ravi Valluru, University of Lincoln, United KingdomHonglong Zhao, Center for Excellence in Molecular Plant Sciences (CAS), China
Copyright © 2022 Li, Xu, Chen, Xu, Wang, Liu, Yu, Liu and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiguo Liu, bHdnc3lAMTI2LmNvbQ==
 Xiuni Li
Xiuni Li Xiangyao Xu1,2,3
Xiangyao Xu1,2,3