
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci. , 22 July 2022
Sec. Aquatic Photosynthetic Organisms
Volume 13 - 2022 | https://doi.org/10.3389/fpls.2022.929368
 Juveria Samar1*
Juveria Samar1* Ghazala Yasmeen Butt2
Ghazala Yasmeen Butt2 Anis Ali Shah3*
Anis Ali Shah3* Adnan Noor Shah4*
Adnan Noor Shah4* Sajad Ali5
Sajad Ali5 Basit Latief Jan6
Basit Latief Jan6 Nader R. Abdelsalam7
Nader R. Abdelsalam7 Muhammad Hussaan8
Muhammad Hussaan8Seaweeds are non-vascular, photosynthetic that inhabit the coastal regions commonly within rocky intertidal or submerged reef-like habitats and have been one of the richest and most promising sources of bioactive primary and secondary metabolites with antimicrobial properties. They selectively absorb elements like Na, K, Ca, Mg, I, and Br from the seawater and accumulate them in their thalli. Padina antillarum (Kützing) Piccone is a member of Phaeophycota and has remarkable phycochemistry as well as bioactivity. The phycochemical tests of the different extracts showed the presence of alkaloids, terpenoids, saponins, tannins, steroids, and phenols. The relative percentage of Oxirane, tetradecyl (C16H32O), and Cyclononasiloxane (C18H54O9Si9) are higher while Tetrasiloxane (C16H50O7Si8) is lowest in Gas Chromatography – Mass Spectrometry analysis. FRAP, %inhibition, the total antioxidant value of P. antillarum was higher in methanolic extract. Hexane, chloroform extracts showed no zone of inhibition against Bacillus subtilis, Escherichia coli, Klebsiella pneumonia, Staphylococcus aureus, and Staphylococcus epidermidis. The methanolic extract of P. antillarum exhibits a maximum zone of inhibition against S. epidermidis (18.66 ± 0.09). Antifungal activity of the P. antillarum in hexane extract exhibited no zone of inhibition against Aspergillus niger and Penicillium notatum while the chloroform extract yields maximum zone (37 ± 0.012, 21.66 ± 0.03). Diabetes mellitus is one of the most familiar chronic diseases associated with carbohydrate metabolism. It is also an indication of co-morbidities such as obesity, hypertension, and hyperlipidemia which are metabolic complications of both clinical and experimental diabetes. The treatment of P. antillarum methanol extract in mice reduced the body weight loss, low level of triglycerides, and elevated HDL cholesterol level as compared to diabetic mice.
The Karachi Coast (100 km) touches the Arabian Sea. It includes beaches and numerous islands. The coastal waters around Manora, Sandspit, Hawkesbay, Buleji, Paradise Point, Pacha, Nathiagali, and Cape Monze are inhabited by a variety of marine benthic algae (Shameel and Tanaka, 1992). Although a lot of work has been done on their taxonomy and distribution, as well as morpho-ecological and phycochemistry studies (Hameed et al., 2000, 2001; Shameel et al., 2000), little data are available in the literature related to their bioactivity and elemental composition (Rizvi and Shameel, 2005). A total of 234 species and 110 genera of seaweeds are reported from the coast of Balochistan, demonstrating a great biological diversity, distributed among 57 families, 33 orders, 12 classes and 6 divisions (Shameel, 2001). Many studies have been made on the standing crop and biomass of seaweeds like Shameel, 1987 served seaweed from the coast of Lasbella, Pakistan. Saleem (1965), Saifullah (1973), and Saifullah (2009) studied the distribution and biomass of marine algae along Karachi. Qari and Qasim (1994) investigated the Seasonal change in the standing crop of intertidal seaweeds from the Buleji and Manora coasts of Karachi. Qari et al. (2014) studied Phytomass on a natural bed of seaweed at Paradise Point, Karachi coast. Qari (2017) assessed the seaweed diversity and distribution at the beach of Nathia gali, Karachi, Pakistan. Seaweeds are non-vascular, photosynthetic that inhabit the coastal regions commonly within rocky intertidal or submerged reef-like habitats and have been one of the richest and most promising sources of bioactive primary and secondary metabolites with antimicrobial properties (Chapman, 1950; Jebasingh et al., 2011). Seaweeds were consumed as whole food since ancient times, and they still have great economic importance. Seaweeds are considered a nutrient-rich food as they are a good source of minerals, vitamins (A, B1, B2, B9, B12, C, D, E, and K), essential minerals (calcium, iron, iodine, magnesium, phosphorus, potassium, zinc, copper, manganese, selenium, and fluoride), dietary fibers (Rajapakse and Kim, 2011; Dhargalkar, 2015; Pereira, 2018; Shannon and Abu-Ghannam, 2019), protein, essential amino acids, and polyphenols, which exhibit antioxidant and anti-inflammatory properties (Panzella and Napolitano, 2017). Seaweeds possess a low lipid content, nonetheless enriched in polyunsaturated fatty acids. This characteristic makes them even more attractive, as they are a healthy, nutritive, and low-caloric food (Pereira, 2018). The accumulated elements vary from species to species. Different types of dietary fibers exist depending on the seaweed phyla. For brown seaweeds (Phaeophyta) the soluble fibers are alginates, fucans, and laminarians (Peñalver et al., 2020). A plague of concern is the biological effects of natural antioxidants, encompassed in the attack against oxidative trauma that grounds aging, the release, and progress of numerous diseases such as cancer, cardiovascular accidents, inflammatory diseases, and neurodegenerative diseases (Martemucci et al., 2022). Macroalgae are available, safe, cheap, and due to their bioactive properties, with positive effects on human health have received considerable attention. Padina can be utilized as food, fodder, and bio-fertilizer. The brown alga is well known and is utilized for its antimicrobial, insecticidal, antioxidants, antibiotics, anti-inflammatory, hypo-allergenic, hepatoprotective, and antidiabetic activities. The macroalgae like Padina play important role in environmental monitoring and management of coastal marine ecosystems. They can be considered biological indicators and can also be utilized in the phytoremediation, for the management of contaminants in coastal marine ecosystems (Ansari et al., 2019). A few species of Padina have been used traditionally as a food source in many coastal parts of the world. Padina is also reported as a substitute for salts for patients with high blood pressure (Novaczek and Athy, 2001). Seaweed distribution and bioactive compounds from the coastline of Pakistan have already been studied (Yasmeen et al., 2021) and in spite of some scattered studies in this area are not completely known yet. Padina antillarum ((Kützing) Piccone was found as a drifted material or benthic in bulk amount at Karachi coast. This type of work is not done on this species in Pakistan. Objectives are following:
(1) Extraction and analysis of phycochemicals from Padina antillarum.
(2) Evaluation of antimicrobial, antioxidant, antidiabetic, and anthelmintic potential of Padina antillarum.
Padina antillarum (Kützing) Piccone was collected from the intertidal and subtidal habitat of the Manora, Hawkesbay and Buleji between longitude 66° 59″ E and latitude 24° 48″ N were the suitable place for the collection of drifted seaweed at the coast of Karachi, Pakistan. Collection was performed during the April 2018 to November 2020. The drifted seaweeds were collected from the rocks by hands, forceps, and scrapers. Collection bags of different sizes and plastic containers were used to keep them preserved. Zipper bags of different sizes were used for preservation. The collected healthy seaweeds were brought to the laboratory for cleaning and washed thoroughly with tap water with an adequate amount of salt. The washed algal samples were dried under shade on bloating paper and made some herbarium. The herbarium of collected algal species is kept in Kashyap Botany Museum, GC University, Lahore, Pakistan. Seaweeds were identified by using standard literature and verified by the Department of Botany, University of Karachi, Karachi. Systematic positions of these algal species were followed according to the classification of Shameel and Tanaka (1992) and Shameel (2001, 2008). Powder algal material soaking was performed based on the standard static state maceration technique using n hexane, chloroform, and methanol solvents.
The morphological analysis includes the appearance of the P. antillarum according to Shameel (2008) and Abbas (2014).
Moisture content algal samples were determined according to the standard procedure of Association of Official Analytical Chemists [AOAC] (1997). For this purpose, porcelain crucibles were dried and placed in an oven for 3 h at 105°C, and then allowed to cool. After cooling, crucibles were weighed and 1 g each algal powdered material of the sample was placed in the crucible and placed in an oven at 105°C for 3 h. Crucibles were then allowed to cool and weighed afterward.
Ash content was determined according to the standard protocol of Association of Official Analytical Chemists [AOAC] (1997). For the process, crucibles were placed in a furnace at 550°C overnight in order to get rid of any impurities if present. Crucibles were then allowed to cool in a desiccator after cooling crucibles were weighed up to 3 decimal points. About 1 g of the equally weighed powdered algal material sample under examination was then placed in crucibles and heated at 550°C in a furnace overnight. Crucibles along with ash were then weighed after cooling.
The porosity test examined dry weight. The material was inserted into the water and examined the mass. The porosity value could be known by comparing the dry weight material and the wet material mass. The procedure was done by mixing 0.3 g of material with 150 μ L of water, after setting measured the dry weight sample material was dipped, into 6 ml water and measured the wet sample weight. The dry and wet weight difference was calculated (Widiyanti and Siswanto, 2012).
The qualitative determination of extracts was carried out according to Harbone (1973) and Ayoola et al. (2008). These are as follows Glycosides, cardic glycosides (Keller Killani test) anthroquinones (Chloroform, Borntager test), alkaloids (Dragendroff reagent, Mayer’s reagent, Wanger test), flavonoids (Sodium hydroxide test, Ammonia test, Ferric chloride test), saponins (Frothing test), terpenoids (Salkowski Test), phenols (Ferric Chloride Test), amino acids (Ninhydrin Test), proteins (Biuret Test), steroids, coumarins, beta cyanins, reducing sugar (Fehling Test, Iodine Test, Tollen’s Reagent), oils and resins, phlobatanins, quinones, and tannins (Ferric Chloride Test, Match test).
A sample of 3 g was taken in a beaker, and 200 ml of 20% ethanolic acetic acid was added. The beaker was covered and left for 4 h. The extract was filtered and volume was reduced to one-quarter of the initial, using a water bath. Then concentrated NH4OH was added slowly dropwise to the extract until the precipitation get complete. The precipitates were left for settling then filtered, dried, and weighed (Poornima and Ravishankar, 2009). Total fat was extracted by Soxhlet apparatus 10 g sample was taken into a thimble. The empty extraction flask was weighed. And 250 ml of n-hexane was used as an extraction solvent. The refluxing was continued for 8 h at the boiling point of n-hexane. After extraction, the solvent was evaporated from the flask and weighed the flask with extracted crude oil. The percentage of the extracted oil was calculated by using the equation (Association of Official Analytical Chemists [AOAC], 1997).
The protein content was estimated by the Biuret method (Raymont et al., 1964). Incubate the mixture prepared, using 5 mg of dried powdered sample, 1 ml of distilled water followed by addition of 4 ml biuret reagent, for 30 min at room temperature. Then the mixture was centrifuged for 10 min at 4,000 rpm. The supernatant was collected and the optical density was measured in a Spectrophotometer at 540 nm. The protein content was calculated using Bovine Serum Albumin as standard and expressed as mg/g protein.
The carbohydrate content was estimated by the Dubois method (Dubois et al., 1956). Add 20 mg of dried seaweed powder and 1 ml of 4% phenol solution and 5 ml of concentrated sulfuric acid. After that, they were kept in a dark room for 30 min. The color intensity developed was read in a spectrophotometer at 490 nm. Sugar content was calculated by referring to a standard D-Glucose and the results have were expressed as mg/g sugar.
The lipid content was estimated using chloroform–methanol mixture as described by Folch et al. (1957). Add 400 mg of sample to 5 ml chloroform–methanol (2:1) mixture. The mixture was incubated at room temperature for 24 h. After incubation, the mixture was filtered using filter paper. The filtrate was collected in a 10 ml pre-weighed beaker. The chloroform–methanol mixture was evaporated on a hot plate leaving a residue at the bottom of the beaker. The beaker with the residue and the weight of the empty beaker was calculated to know the weight of the lipid present in the sample.
Oven dried samples were accurately weighed in 0.2 g quantities in a dry conical flask and 10 ml of diacid mixture (2:5 of nitric and perchloric acid) were added. The contents of the conical flask were allowed to stand for a few hours for cold digestion. The mixture was then kept on a hot plate, and the contents were digested by increasing the temperature. The digestion continued until the content became colorless. The digestion material was filtered through Whatmann No. 40 filter paper, and the filtrate collected was diluted to a suitable volume and fed into an ICP–Perkin Elmer Mayer Optical Emission Spectrophotometer (Association of Official Analytical Chemists [AOAC], 1995).
Fourier Transform Infrared Analysis (FTIR) analysis was performed using Perk in Elmer Spectrophotometer system, which was used to detect the characteristics of peaks and their functional groups. The peak values of the FTIR were recorded. Each and every analysis was repeated twice and confirmed the spectrum (Janakiraman et al., 2011).
Column chromatography is one of the most useful methods for the separation and purification of crude extract. The methanolic crude extracts were applied in a silica gel column (230–400 mech) packed with chloroform and eluted with a mixture of chloroform and methanol after purification, the fractions were stored at a temperature of 20°C. The compound separated from the column was analyzed using Gas Chromatography – Mass Spectrometry (GC-MS). Chemical compounds in the sample were analyzed using Shimadzu GC-MSQP-2010A. Helium (He) gas was used in it. The flow rate of He gas was 32 ml/min. The column temperature was about 70–250°C with an increasing rate of 8°C per minute.
ABTS++ assay was performed as described by Re et al. (1999) for the estimation of antioxidant potential. It was produced by reacting ABTS++ aqueous solution with 2.45 mM potassium persulfate at room temperature for 16 h. The ABTS++ solution was diluted with Phosphate buffered saline (PBS), pH 7.0 to an absorbance of 0.70 at 734 nm. Afterward, 2.9 ml of this solution (with adjusted and recorded reading) was dispensed in a test tube followed by the addition of 10 μl of algal extracts. After precisely noting at an interval of 8 min, absorbance was measured at 734 nm. A dose response curve of Trolox was organized by plotting its absorbance at 734 nm.
DPPH radical scavenging activity was evaluated according to the method of Brand-Williams et al. (1995). Prepare four dilutions of each algal extract. Each DPPH reaction test tube was prepared by adding 1 ml of algal extract and 3 ml of freshly prepared DPPH solution. The test tubes were then incubated for 45–60 min in dark at room temperature. During the incubation period, reduction of the DPPH mixture occurs which is evident by the change in color from purple to yellow. The absorbance of samples was recorded at 517 nm by spectrophotometer. Ascorbic acid was used as a standard whereas methanol was used as a blank. The remaining DPPH radical percentage was calculated.
Ferric reducing the antioxidant ability of different algal extracts was evaluated according to the protocol of Benzie and Strain (1996). For this evaluation, 1 ml of each seaweed extract was treated with a 3 ml working solution of FRAP reagent followed by keeping it under 30 min dark period. The absorbance was chronicled at 593 nm. Standard Trolox was recorded as micromoles of Trolox equivalent (TE) per ml which were evaluated by a standard curve-derived equation.
FTC was predicted by the scheme developed by Valento et al. (2002). The investigation solution was formulated by the supplementation of 0.1 ml (500 μg/ml) seaweed macerate into 2 ml phosphate buffer and 2.5 ml linoleic and incubated for 24 h at 40°C. After the accomplishment of incubation period, 0.1 ml of stemmed mixture was dissolved into 0.1 ml (20 mM) FeCl2, 0.1 mL of (30%) ammonium thiocyanate and 5 ml of (75%) ethanol. The acquired mixtures were retained for 3 min at room temperature and checked the optical density at 500 nm, while butylated hydroxyl toluene (BHT) was affianced as standard. The percentage of inhibition is calculated using the following formula:
Dinis et al. (1994) guidelines for metal chelating activity were espoused for estimating the seaweed extracts. Prepare mixture using 1 ml of seaweed macerated extract, add 50 μl of 2 mM FeCl2 and 0.2 ml of 5 mM ferrozine solution. This mixture was vigorously shaken followed by incubation at room temperature for 10 min. Absorbance was measured at 562 nm. Percentage inhibition of ferrozine-Fe2+ complex development was estimated as:
Total antioxidant potential of algal extracts was determined by phosphomolybdenum method of Prieto et al. (1999). For each test tube of 1 ml seaweed extract add 4 ml of reagent. Test tubes were incubated at 95°C for 80–90 min in a water bath and cool them in a desiccator at room temperature. The absorbance of samples is recorded at a 695 nm UV spectrophotometer. Ascorbic acid was used as a standard and results were documented as AA μg/ml.
Aluminum chloride colorimetric techniques were evaluated on different extracts of P. antillarum samples for determination of the total flavonoid contents according to Zhishen et al. (1999). About 1 ml of each seaweed extract was taken in a test tube, 0.2 ml of distilled water was added to each tube, by further addition of 0.15 ml of 5% NaNO2 solution and this mixture was then incubated for 5 min at room temperature. After incubation 0.15 ml of 10%, AlCl3 solution was added to the mixture and the solution was allowed to stand at room temperature for 6 min. Then 2 ml of 4% NaOH was added to the mixture. The final volume of this mixture in the test tube was made 5 ml with distilled water, the mixture was shaken well and left to stand at room temperature for 15 min. Absorbance was measured at 510 nm and total flavonoid content was evaluated as mg equivalent of Rutin (mg Ru/g).
Total phenolic content was estimated following the procedure of Makkar et al. (1993). For this determination quantified amounts of Folin-Ciocalteu (FC) reagent were commercially prepared and sodium carbonate was taken into account. Take 1 ml of each seaweed extract and add 2.8 ml of 10% Na2CO3 and 0.1 ml of 2N FC reagent. This mixture was then incubated at 25°C for 40 min. After incubation, the absorbance was recorded at 725 nm. Gallic acid was taken as standard and results were documented as GAE μg/ml.
Total carotenoid contents were estimated by using Hess et al. (1991) protocol. In the test tube, 300 μl (500 μg/ml) algal extract was placed in 1.2 ml n-hexane and 300 μl deionized water, followed by centrifugation at 2000 rpm for 5 min. Ultimately, the supernatant was separated and its absorbance was taken at 35 nm using UV-vis spectrophotometer. Results were represented in terms of reference curve of β -carotene as mg β -carotene/100 g dried sample (mgBC/100 g).
Antibacterial activity of different extracts was performed according to Jorgensen et al. (2007). About 3 g positive bacteria (Staphylococcus aureus (ATCC 14923), Bacillus subtilis (ATCC 15029) S. epidermidis (ATCC14990)) and 2 g negative bacteria (Klebsiella pneumonia (ATCC700721) Escherichia coli (ATCC 14962) while two fungal strains (Aspergillus niger and Penicillium notatum) were used. For the bacterial strains sterilized Nutrient Agar and for fungal strains, Potato dextrose agar was used as media. The bacterial cultures used for determining the antibacterial activity were spread on the Nutrient Agar medium, formulated under the directions given by American Public Health Association [APHA] (1948) following Cruickshank et al. (1975) protocol. As per Mc- Farland turbidity standard 1.5 × 108 CFU/ml was the maintained for inoculum.
Agar well diffusion method was formulated by Kirby et al. (1957) for estimation of antimicrobial activity. The procedure was later modified as per Jorgensen et al. (2007). Crude seaweed extract inhibits bacterial growth and forms a zone. The inoculum was homogeneously spread on the solidified medium. A well was made at the center of the Petri plate by 0.1–0.3 Cork borer followed by injected the algal material into the well. Petri plates were incubated at 27 ± 2°C for 48 h for fungal activity and at 37 ± 2°C for bacterial growth. The entire experiment was run in triplicates for accurate results.
The zone of inhibition was scanned using Agar well diffusion method. Antibiotics disk taken as positive control while n-hexane, chloroform, and methanol were used as negative control. Once the incubation period was completed the zone of inhibition molded by the algal extract was measured in mm. The area with the apparent stroke of the algal extract was recorded as a zone; no progress was discernable as 0 mm zone. Sometimes, the zone shaped by the algal extract isn’t symmetrical. In such situations zone is measured from center to side from different angles.
The determination of MIC was accessed by the broth dilution method (Jorgensen et al., 2007). Different concentrations of 0.625–10 mg/ml of the different extracts were made. About 20 μl of growing inoculum of tested bacteria/fungal species adjusted to 107 CFU/ml, was added to each well and incubated for 24 h at 29 and 37°C. The lowest sample concentration showing no visible antimicrobial growth was considered as the MIC value expressed in mg/ml.
According to Selvi and Santhanam (2016), the seaweed extract was evaluated for anthelmintic inquiry. The contemplation on the anthelmintic activity of the seaweed extracts was piloted against the gastro-intestinal nematode Haemonchus contortus at the desired concentrations (10, 20, 50, and 100 mg/ml). The tested worms were considered as six groups for evaluation of the anthelmintic potential of seaweed extracts. Seaweed extract of 10 ml (saline solution used as control and Piperazine citrate as standard) was imperiled to each plate monitored by the addition of 5 uniform-sized nematodes in it. The paralysis to the death time period was noted in minutes.
According to Radhika and Priya (2015) the activity was divided into two phases, i.e., toxicity test and hyperglycemic activity. Ethical conditions regarding the use of laboratory animals were considered before the start of the experiment. The institutional bioethical committee of Government College University approved ethical conditions (No. GCU-IIB-333) to be as per standards.
Healthy swiss albino male mice (22–27 g) approximately 6–7 weeks old were purchased from The University of Lahore animal house. Before the commencement of the experiment animals were acclimatized (Hong et al., 2011). Animal house temperature was maintained between 19 and 25°C and humidity 30–37%. The facility was provided with 12-h light and dark. Standard polypropylene mice cages with stainless steel top grill were used. The cages were cleaned with alcohol on regular basis and a clean paddy husk was used as bedding material. Standard mice pellet feed and water ad libitum were provided to the mice except for the fasting period during which only water was provided (Banu and Umamageswari, 2011).
In the experiment, a total of 28 swiss albino mice were used for the administration of seaweed extracts with a test compound at the highest dose of 2,000 mg/Kg. The dose of each extract was administrated orally to the overnight fasted mice. The sign of possible toxicity was observed every 3 h during the first 24 h and every day for 14 days. Individual animal weight was noted down and any sign or symptoms of toxicity, changes in fur, skin, eyes, and mortality were observed for 14 days (Lee et al., 2002; Mohapatra et al., 2016). The groups are as follows Group-I: Vehicle control (distilled water) Group-II: Methanolic extract of P. antillarum.
Swiss albino mice (20–30 Kgs) 6–7 weeks old were selected for the antihyperglycemic activity. Before commencement of the experiment the mice were acclimatized to laboratory conditions for 1 week under standard conditions.
Alloxan monohydrate was used as a diabetogenic agent. Alloxan selectively destroys beta cells of the pancreas with irreversible necrosis and it can be generating chronic hyperglycemia. Before the induction of diabetes, the mice were fasted overnight (had free access to water). A freshly prepared dose of 200 mg/Kg Alloxan monohydrate dissolved in 10 mg/Kg body weight distilled water was injected intraperitoneally. Prevention from hypoglycemia the animal is immediately provided with the standard diet. On the fifth day of induction, diabetes was checked by a small incision on the tail for 6 h fasted animal, and blood glucose level was monitored to confirmation of diabetes. Mice with 300 mg/kg blood glucose levels were considered diabetic (Mohapatra et al., 2016).
In the experiment, a total of 36 mice (27 diabetic mice and 9 normal mice in group) were used. The diabetic mice were divided into four groups after the induction of alloxan diabetes (Radhika and Priya, 2015).
The body weight of each mouse was measured regularly after the induction of diabetes.
Blood glucose levels of all experimental groups was measured after the induction of diabetes on the 5th day prior to 6 h fasting. Blood glucose level was also determined on 7th and 14th days by using a standard glucometer (Trinder, 1969).
At the end of 14th days study, an oral glucose tolerance test was performed to monitor the clearance of oral glucose load from the body (Mohapatra et al., 2016). Mice were kept overnight fast before administration of 2 g dextrose/kg body weight. About 2 g of dextrose was injected into the mice by glucose gavage through an oral route. 10 × body weight(g) = 20% dextrose. Blood glucose levels were measured at 0, 30, 60, and 120 min after the administration of glucose. Blood glucose level was checked by a glucometer.
Blood samples were assessed for various hematological and biochemical analyses as red blood cell, white blood cell, etc.
All the readings were recorded in triplicate and an excel sheet was used to statistically analyze data. All the data were expressed as Mean ± SEM. ANOVA was applied where it was necessary. Differences were considered to be statistically significant if p < 0.05.
Thalli olive green or dark green in color (Figure 1) irregularly branched; margins smooth or slightly undulate, apex enrolled, surface smooth; sporangia present in double sporangial lines, sporangial lines and hair lines alternate to each other; attached with the help of a small compact (Shameel and Tanaka, 1992) hold fast, 0.5–1.5 cm broad and 0.7–2.0 cm long; thallus divided into many lobes up to 3/4 part of the thallus, many clefts present on the thallus; thalli 7–15 cm long, 7–12 cm broad at the apex, 10–12 cm broad to the middle and 7.5–10.0 cm broad at the base (Abbas and Shameel, 2011; Abbas, 2014).
The ash content, moisture content, and porosity of P. antillarum were 73.8, 5.8, and 5.79 g, respectively.
The qualitative phycochemical tests were executed to ascertain the presence of different chemicals in the sample, e.g., glycosides, alkaloids, flavonoids, reducing sugar, tannins, saponins, terpenoids, phenols, amino acids, protein, oil and resins, phlobatanins, quinones, steroids, coumarins and β cyanine. The presence of the different chemicals may vary as in the different extraction solvents for maceration. The qualitative analysis of phycochemistry (Table 1) was assessed according to the standard protocols.
Investigation for total fats in P. antillarum was performed by standard Association of Official Analytical Chemists [AOAC] (1997) soxhlet extraction method while the total alkaloid contents were determined according to Poornima and Ravishankar (2009). Most of the algae had fat less than 4% of dry weight but as compared to this several marine algae have high values (Mcdermid and Stuercke, 2003). The total fat contents and total alkaloid of P. antillarum are 14.4% and 3.12 g, respectively (Figure 2).
The nutritive value of algae can be determined by carbohydrates, protein, and lipids in the sample. The assessment of carbohydrates was expressed in mg/g as referred to as the standard curve of D-glucose. A total of 46.14 mg/g sugar, 107.6 mg/g protein, and 4% lipids contents were estimated.
Padina antillarum has calcium in the highest amount 258.943 while cadmium is present in the lowest amount –0.0279. Other minerals such as potassium 14.972, sodium 17.6543, magnesium 10.7506, iron 4.3142, zinc 0.3940, and lead 0.0151.
The active components of the functional group in a sample could be identified by the peak value in a region by Fourier transform infrared spectroscopy (FT-IR). The peak ratios separated functional groups of the components as passed the P. antillarum crude powder into the FT-IR. The FT-IR spectra (Figure 3) showed different peaks at 676.87 functional group is alkynes, 853.86 functional group is aromatic, 1002.31 functional group is alkenes, 1240.54 functional group is amine, 1405.68 functional group is sulfonyl chloride, 1456.14 functional group is Alkane methyl group, 1742.65 functional group is aldehyde, 2359.71 functional group is carbon dioxide, 2980.89 functional group is amine salt, 3282.04 functional group is alcohol (Table 2).
Approximately, 257 g of dry seaweed powder was macerated in the methanol and 20.12 g of crude extract was obtained. According to Ghazala et al. (2004a,b) esterification process was performed and run the column chromatography. The fractions obtained from the column chromatography were directly injected into the GC-MS. The GC-MS of Lipids (Figure 4). Some of the prominent radicals are given in Table 3.
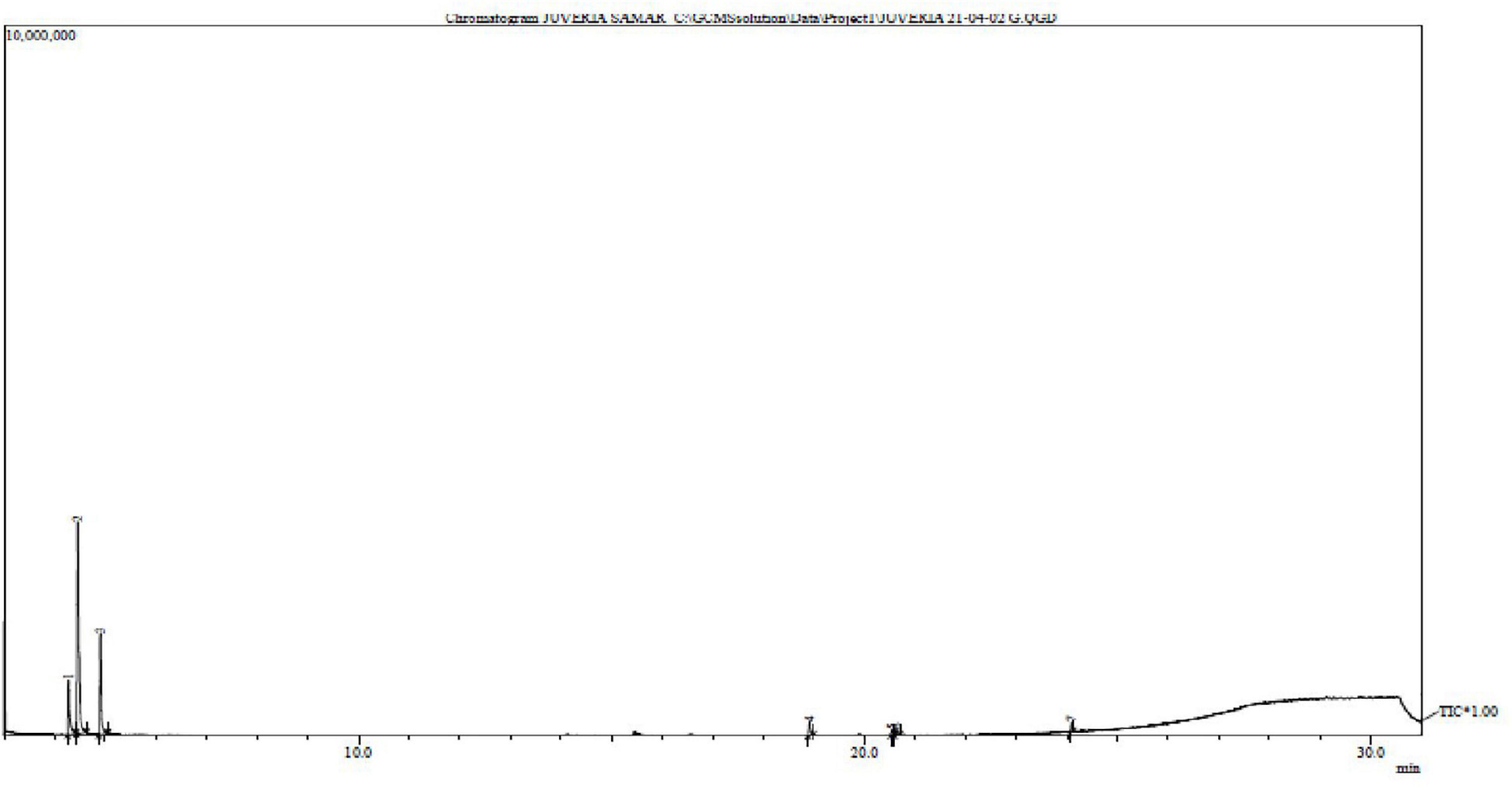
Figure 4. Gas chromatography – mass spectrometry chromatogram of Padina antillarum (Kützing) Piccone.
ABTS++ radical cation was produced in the stable form using potassium persulphate. The maximum ABTS++ value was 600.47 from the methanol extract while the minimum value was 293.63 extracted from chloroform extract (Figure 5). The relative antioxidant ability to scavenge the radical ABTS++ has been compared with the standard curve Trolox mg/ml.
Methanol, n-hexane, and chloroform exhibited good FRAP values, i.e., 1985.78, 929.42, and 767.57 TE μM/ml. The crude methanol extract exhibited the highest FRAP value showing a synergic effect of antioxidant constituents while chloroform exhibited the lowest value (Figure 5). Higher FRAP values were obtained for samples in more polar solvents. The highest value of FRAP exhibited the highest level of phenolic and flavonoid contents. The FRAP assay was used to estimate the reduction potential in the solvent. The standard curve Trolox μM/ml.
The Percentage Lipid Peroxidation Inhibition (IP %) of various seaweed extracts (Figure 5) was found between 1216.93 and 1456.6% inhibition of peroxidation. The FTC mg/ml value of seaweeds can also be determined on the basis of butylated hydroxyl toluene (BHT) standard curve. The minimum FTC mg/ml was shown by chloroform extracts at 0.165 mg/ml BHT and the maximum by methanol extract at 8.5 mg/ml BHT.
The metal chelating potential in terms of % inhibition of ferrozine-Fe2 of P. antillarum various extracts varied from 48.82 to 92.42%. The minimum potency was indicated by methanol extract and the maximum by chloroform extract (Figure 5).
The total antioxidant activity of different crude extracts of P. antillarum was investigated by Phosphomolybdenum assay (Figure 5). The maximum antioxidant potential was exhibited by methanol extract, i.e., 911.88 mg/ml of ascorbic acid, while the minimum effectiveness was shown by chloroform, i.e., 277.88 mg/ml ascorbic acid. The total antioxidant value was calculated according to the ascorbic acid standard curve.
The carotenoid concentration in extracts of P. antillarum ranged between 45.24 and 326 β-cE mg/100 g (β-carotene Equivalent mg per 100 g). The least contents were observed in methanol macerates in addition to higher concentrations reported in n-hexane extracts (Figure 5). Results were calculated with reference to the β-carotene standard curve.
Flavonoids are responsible for many biological activities such as anti-cancer, antimicrobial, antiviral, anti-inflammatory. The antimicrobial activity of flavonoids is commonly used in Chinese traditional medicine. Adjuvant arthritis has been cured by Rutin (Guardia et al., 2001). The flavonoids from P. antillarum were found in methanol extract 11.07 while the minimum value in chloroform 2.935 (Figure 5). The values were referred to in the standard curve of Rutin as RE μg/g.
A major source of bioactive compounds is seaweeds. Approximately 850 phenolic compounds are known, mostly belonging to marine algae. The maximum amount of phenolics was extracted from methanol 235.68 mg/ml GAE while the minimum was from n-hexane 20.78 mg/ml GAE extract of P. antillarum (Figure 5). The values were calculated in comparison with the gallic acid standard curve and expressed in terms of GAE mg/ml
DPPH potential obtained by different fractions was compared with the standard Ascorbic acid curve (Figure 6). The methanol extract of the P. antillarum yields a maximum value (84.97) at a concentration of 1,000 μl. The DPPH potential ranged from 50.99 to 84.97% at various concentrations of different extracts. The DPPH percentage was dependent on the concentration of the sample, i.e., more the concentration more the % scavenging. Inhibition concentration 50% of the sample was determined to check the greater antioxidant potential and vice versa. The methanol extract showed the least IC50 value, i.e., 1.173 μg/ml followed by n-hexane 10.89 μg/ml and chloroform 40.66 μg/ml (Figure 6).
A number of seaweed species have been already reported for antimicrobial activity. The zone of inhibition of selected seaweed extracts is shown in Figure 7. The methanol extract exhibits the maximum zone against Staphylococcus epidermidis (18.66 ± 0.234 mm) while the n-hexane extract inhibits the zone against Bacillus subtilis, Escherichia coli, and Klebsiella pneumonia. There was no zone formation of n-hexane extract against B. subtilis, E. coli, and K. pneumonia while no zone formed of chloroform extract against B. subtilis, E. coli, S. aureus, and S. epidermidis. The zone of inhibition of methanol extract was 15.00 ± 0.087, 15.33 ± 0.28, 15 ± 0.0045, 16.09 ± 0.0032, and 18.66 ± 0.09 against B. subtilis, K. pneumonia, E. coli, S. aureus, and S. epidermidis, respectively (Table 4). The results of MIC were noticed to have the broadest activity of the extract against the bacteria was 0.125 mg/ml.
The chloroform extract inhibits the maximum zone against Aspergillus niger (37.00 ± 0.012 mm) and methanol extract showed 12.33 ± 0.004 mm while the n-hexane extract gives no zone against A. niger (Figure 7). The zone of inhibition of chloroform and methanol extract was 21.66 ± 0.03, 18.66 ± 0.43 against Penicillium notatum, respectively.
Helminthes are the main disease causing in the human being and animals. The treatments available in market never remained up to the mark or have developed resistance causing recurrent attacks. For this purpose, the anthelmintic activity was taken into consideration. The anthelmintic potential of various extracts of P. antillarum was evaluated at four concentrations (10, 20, 50, and 100 μg/ml). In 4 h treatment period, the paralysis and death time was calculated for various working concentrations. The paralysis time duration in P. antillarum extracts ranged from 1.1 to 4.1 min. The maximum time was taken by n-hexane extracts indicating its least anthelmintic activity. The most potent results were shown by methanol extracts which paralyzed the Haemonchus contortus within 1 min. The overall examination of various concentrations indicated that methanol extract had the highest potential followed by chloroform and hexane. The n-hexane extract was documented as the least efficient. The treatment period for death varied between 1.5 and 7.4 min at various concentrations (Figure 8). The shortest death period was shown by methanol extract while the highest time duration was taken using n-hexane extract.
Antidiabetic activity of P. antillarum extracts is divided into two phases.
The methanolic extracts of P. antillarum were observed to be non-significant which did not produce any change in autonomic or behavioral responses during the study. No mortality was observed up to the 7th day of monitoring in any group of mice.
The mean body weight of mice was significantly p < 0.05 increased as compared to the normal mice (Figure 9). The blood glucose level was also checked on fasted mice and compared the values with control mice. The blood glucose level of each mouse was checked prior to the Alloxan administration the basal level of plasma glucose was not significantly higher than 300 in the mice selected for the study. The blood glucose level was checked on 0, 7, and 14 days of the study. P. antillarum extract decreases the blood glucose level as compared to other groups (Figure 10).
On the 14th-day, a complete blood screening of the mice have done. Table 5 shows the hematological parameters of the study period P. antillarum. This extract has a high Hb level as compared to Glipizide, with positive control and negative control. RBC, WBC, Hb, and platelet count showed a significant increase was seen in the mean count (Table 6). In diabetes, elevated levels of serum urea and creatinine are observed which may be due to renal damage caused by abnormal glucose regulation. In the present study, a significant increase in serum urea and creatinine levels were observed in diabetic mice compared to normal control mice. The treatment with P. antillarum methanolic extract lower the above parameters compared to diabetic control mice and it showed a protective effect on kidney. Under diabetic conditions, the occurrence of reduction of protein and albumin may be due to increased protein catabolism which is clinical markers. After the treatment with P. antillarum extract, the albumin and protein levels were seen to be increased.
Oral glucose test is used extensively for the diagnosis of type 2 diabetes. This test displays the competence of the diabetic individual to effectively utilize glucose after a meal. The oral glucose test resembles the glucose and insulin dynamics of physiological conditions closely (Figure 11). Results of the oral glucose test revealed that P. antillarum methanolic extract curve was significantly p < 0.05 attenuated at both low and high doses.
Algal specimens were collected in drifted conditions from various sites (Buleji, Haji Ghot, Chasma Ghot, Ali Ghot French beach, Hawks bay, and Manora) on the Karachi coast. All these coasts have a lavish amount of sea weed growth at the intertidal zone of the sea, which comes out mostly and collected as drifted conditions as well as scratched from the exposed rocks during the low tides (Khan, 2017). The different extracts of P. antillarum revealed the presence of alkaloids, terpenoids, saponins, tannins, steroids, and phenols in phycochemical tests. All these secondary metabolites have been extensively used in the pharmaceutical industry (Ravi et al., 2017). The ash, moisture, and porosity of the sample are similar to the recent publication of Anis et al. (2018). Ash content of P. antillarum powder was high if compared with P. australis only 34.58%. It is also different from other species such as P. minor 30.53% (Felix and Brindo, 2014). Ash contents in a material could be related to a number of mineral components (Ratana-arporn and Chirapart, 2006). The mineral components of macroalgae could be affected by the processing method as well (Ruperez, 2002). Tannins bind to proline-rich protein and interfere with protein synthesis. Tannins have anthelmintic, antioxidant properties and are used in remedies as an antimicrobial and antiviral agent. Terpenoids have antioxidant activity for cancer treatment. Antimicrobial, cytotoxic, and antiplasmodic properties are commonly found in alkaloids (Nugraha et al., 2019). Steroids isolated from marine algae have medicinal value, saponins have unique residues like 2,3-dihydro-2, 5-dihydroxy-6-methyl-4H-pyran-4-one (DDMP) which allows saponins to scavenge superoxides by forming hydroperoxide intermediates which prevent bio-molecular damage (Kumar et al., 2015). Even though the bioactive compounds have different contents, all the active compounds of Padina species can be used for pharmaceutical properties such as inhibiting the growth of the pathogenic organisms (Salosso et al., 2020). The highest mineral contents of P. antillarum were calcium 258.943 followed by sodium 17.6543 and the lowest was cadmium-0.0279. Other minerals such as potassium 14.972, magnesium 10.7506, iron 4.3142, zinc 0.3940, and lead 0.0151. A similar result is also reported by Manteu et al. (2018) which has the highest mineral contents of P. minor calcium (32.91 mg/g) and potassium (26.9 mg/ml). Although the highest mineral content is found in the same mineral in three Padina species. It means that the mineral contents of the Padina species are affected by species and habitat (Salosso et al., 2020).
Fourier Transform Infrared Analysis spectra were used to identify the active components based on the peak value in the region of infrared radiation. The P. antillarum spectra absorption peaks near the Ponnanikajamideen et al. (2014) results on P. tetrastromatica. The peaks were identified as alcohols, phenols, secondary amine, halide group, and nitriles. An increasing number of investigations of volatile compounds from non-essentials to essential oils from marine algae have been published in recent years (Kamenarska et al., 2002). The volatile compounds were investigated by GC/MS and the result is summarized in Table 3. Most of the fatty acids are identified as common for marine organisms and are normally found as esters in different groups of lipids. The main part of the polar compound as free fatty acids. Their composition was typical of the genus with palmitic acid being the main fatty acid followed by oleic acid similar to Kamenarska et al. (2002) findings. Palmitic acid appears to be the most abundant fatty acid in brown algae (Aknin et al., 1992).
Seaweeds produce varied and versatile biomass useful for multiple applications. They can be used in a broad variety of formats (e.g., fresh, dried, powder or flakes, salted, canned, liquid extracts or as prepared foods) for direct human consumption or processed into food additives and nutraceuticals, feeds, fertilizers, biofuels, cosmetics and medicines, amongst others (Anis et al., 2017).
Algae produce bioactive compounds with rich pharmacological potential. They generate these compounds as a response to environmental conditions or characteristics (competition for space, maintenance of unfolded surfaces, repulsion of predators, etc.). According to a recent article (Chingizova et al., 2017) isolated chemical compounds from marine seaweed have been shown to owe bioactivities such as antimicrobial, antioxidant, and anti-inflammatory properties, as well as anticoagulant and apoptotic effects.
Flavonoids are proved to have antitumor and antioxidant properties (Kopustinskiene et al., 2020). Flavonoids were present in all samples. These are the major disease fighters on human health. Phenolic compounds have specific physical, chemical, and biological properties which make them a superior drug constituent against disease. These are the main fighter for antimicrobial, anti-inflammatory, antiviral, and anticancer diseases. Phenolics, flavonoids, and carotenoid contents may be varied according to the extraction solvents. The phenolic compounds that have been isolated from seaweeds are scarce, and further research will enlarge the biochemical library and improve the chance to discover new potential compounds for different industries or areas, so this area is still evolving along the road from isolation to application. The major problem of these compounds to be inserted in real commercial applications is mainly the compound concentration in seaweed. Most of the seaweed phenolic pharmaceutical and biomedical bioavailability studies have been supported in mouse-model systems. Phenolic compounds are the most researched seaweed compounds and are already applied in commercial solutions, e.g., cosmetic products (Cotas et al., 2020). The highest phenolic contents were observed in P. antillarum methanolic extract. These findings are similar to the finding of Sameeh et al. (2016) as Padina extract has higher phenolic values among the Enteromorpha.
Antioxidants are considered to be effective protecting tools against oxygen species and their derivatives, produce inside living cells during biochemical processes. Specifically, natural antioxidants are very important for the last three decades because of health issues with synthetic antioxidants available on the market. Natural antioxidants can be extracted from terrestrial as well as aquatic sources, e.g., phenolics, terpenoids, and flavonoids (Yan et al., 1999). Antioxidant compounds play an important role to prevent health from harmful factors. It is known that seaweeds contain several bioactive compounds with potential/higher antioxidant activity as compared to the terrestrial plants due to the presence of up to eight interconnected polyphenols rings (O’sullivan et al., 2011). Antioxidant activity of seaweeds is due to the presence of pigments chlorophylls, xanthophylls (fucoxanthin), carotenoids, vitamins (vitamins B1, B3, C, and E), and vitamin precursors such as α-tocopherol, β-carotene, lutein, and zeaxanthin, phenolics such as polyphenols (gentisic acid, phloroglucinol, gallic acid, protocatechuic acid), flavonoids (i.e., rutin, quercetin, myricetin, flavones, flavonols, flavanones, chalcones, hesperidin and flavan-3-ols, isoflavones, methylated flavones), lignins, tocopherols, tannins, and phenolic acids and hydroquinones, phospholipids, particularly phosphatidylcholine, terpenoids, peptides, and other antioxidative substances, which directly or indirectly contribute to the inhibition or suppression of oxidation processes (Farvin and Jacobsen, 2013). The FRAP, % inhibition, and total antioxidant activity value of P. antillarum methanolic extract were the highest similar to the results of Chia et al. (2015). An excessive amount of reactive oxygen species may result in lipid peroxidation, which changes the structure of body biomolecules, causing cellular disorders, premature aging, mutations, or cell death. Different researches have demonstrated seaweed antioxidant capacity in vitro, attributed to the presence of new antioxidant compounds like carotenoids, certain polysaccharides, and polyphenols, which show scavenger activity, being able to neutralize those reactive oxygen species through their own oxidation, since their affinity to those oxidative compounds is very high (Vieira et al., 2018).
The antimicrobial activity of seaweeds may be influenced by some factors such as the habitat and the season of algal collection, different growth stages of algae, experimental methods, etc. Although a variety of solvents have been employed in screening seaweeds for antimicrobial activity (Manivannan et al., 2011). The potential of seaweed as a source of compounds active against pathogenic microorganisms has been confirmed in different studies. Padmakumar and Ayyakkannu (1997) screened 80 species against bacterial and fungal pathogens. Of the algae, 70% exhibited antibacterial activity but only 27.5% showed antifungal activity (Pérez et al., 2016). Rajasekar et al. (2019) have reported that seaweeds are an excellent source of components such as polysaccharides, tannins, flavonoids, phenolic acids, bromophenols, and carotenoids has exhibits different biological activities. Depending upon their solubility and polarity, different solvents show different antimicrobial activity. According to Rajasekar et al. (2019), P. gymonospora exhibit maximum activity to B. cereus and minimum activity against C. albicans, D. delicatula showed better activity toward P. aeruginosa and lower activity against S. aureus. In the current study Padina antillarum n-hexane, chloroform extract showed no zone of inhibition against B. subtilis, E. coli, K. pneumonia, S. aureus, and S. epidermidis. The methanolic extract of P. antillarum exhibits a maximum zone of inhibition against S. epidermidis (18.66 ± 0.09) similar to the findings of Madkour et al. (2019).
Seaweeds have been recognized as potential sources of antibiotic substances. The results of the study clearly reveal that the higher the concentration the extract became faster due to the paralytic effect and shorter due to the death. The anthelmintic activity of all the extract has a wide range of chemical classes as per the result of GC-MS. The anthelmintic results can be compared to the Selvi and Santhanam (2016) findings. All the anthelmintic findings compare with the standard drug piperazine citrate available on the market. The results showed dose-dependent anthelmintic activity. It was found that higher concentrations of the extract became faster due to the paralytic effect and shorter due to the death time of all the helminthes, respectively. The current investigation leads to the conclusion that all the subjected seaweed extract have potent anthelmintic activity which may be due to the phytochemicals compounds in the seaweed.
Seaweeds are rich in dietary fibers, unsaturated fatty acids, and polyphenolic compounds. Many of these seaweed compositions have been reported to be beneficial to human health including in managing diabetes. Polysaccharides and dietary fibers from seaweed may improve post-prandial satiety feeling, thereby they assist in the reduction of blood glucose levels and improved insulin sensitivity. Seaweed dietary fibers are also helpful in reducing body weight or weight maintenance; hence they are beneficial in attenuating the risk of obesity (Sharifuddin et al., 2015). Diabetes mellitus is one of the most familiar chronic diseases associated with carbohydrate metabolism. It is also an indication of co-morbidities such as obesity, hypertension, and hyperlipidemia which are metabolic complications of both clinical and experimental diabetes. Despite the fact that diabetes mellitus has a high prevalence, morbidity, and mortality globally; it is regarded as a non-curable but controllable disease. Different synthetic drugs, remedies, and dietary modification play an efficient role in the reduction of the suffering that it causes (Balamurugan et al., 2014). According to Radhika and Priya (2015), fucoxanthin which was found in the brown seaweed may inhibit the accumulation of fats causing obesity and can be used as an antidiabetic drug. In general, increased hepatic glucose production plus decreased hepatic glycogen synthesis and glycolysis are the major symptoms of type II diabetes that results in hyperglycemia. In the current study diabetic condition elevated blood glucose reduced the body weight but by the seaweed extract treatments, the loss of weight is less which is similar to the Balamurugan et al. (2014). A diabetic patient has specific type of blood lipid profile by elevated serum, triglycerides, total cholesterol, and low HDL cholesterol. The mice treated with P. antillarum methanolic extract resulted in a low level of triglycerides, total cholesterol, and elevated HDL cholesterol level.
Marine alga P. antillarum is an important resource and industrial applications of these algae drive continuous research to reveal new findings notably for commercial interest as well as versatile sustainable ecological development. The extracts of P. antillarum are an important rich source for the development of compounds with medicinal properties. The algal extracts comprising macronutrients, ashes, carotenoids, carbohydrates, lipids, and protein composition were also recorded. The present studies related to algal extracts are important as they can serve as new drug therapeutics with novel activity, as well as a possible solution for the treatment of diseases like diabetes. It is important to develop a strategy to use local algae resources.
The finding of this study suggests that P. antillarum extracts have a treasured phycochemistry and high antioxidant activity. The antimicrobial activity of the methanolic extract is good as compared to others while the use of this seaweed may minimize the complication of diabetes II (more insulin production in the body) but further studies are necessary to corroborate these results for the dietary recommendation for patients.
Padina antillarum extract will be used as a dietary supplement due to its high nutritional value. It is economical to use P. antillarum extracts for the isolation of compounds that are used in biofuel, dying, pharmaceutical, and cosmetic industries. The high antioxidant value of seaweed may lead to the treatment of different diseases like cardiovascular, diabetes, and cancer treatment.
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
JS performed and conducted the experiments. GB supervised the manuscript and contributed the research design and validation. AAS contributed to the review, writing, and drafting the manuscript. ANS contributed to the review design of research and funding. SA drafted and funded the manuscript. BLJ guided the statistical analysis NRA shaped the revised manuscript. MH helped with the analysis of experimental results and funding. All authors contributed to the article and approved the submitted version.
Researchers Supporting Project Number (RSP-2021/168), King Saud University, Riyadh, Saudi Arabia.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP-2021/168), King Saud University, Riyadh, Saudi Arabia.
Abbas, A., and Shameel, M. (2011). Anatomy of Dictyopteris divaricate (Phaeophycota) from the coast of Karachi. Pak. J. Bot. 43, 2207–2210.
Aknin, M., Dogbevi, K., Samb, A., Kornprobst, J. M., Gaydou, E. M., and Miralles, J. (1992). Fatty acid and sterol composition of eight algae from the Senegalese coast. Comp. Biochem. Physiol. B Comp. Biochem. 102, 841–843. doi: 10.1016/0305-0491(92)90089-A
American Public Health Association [APHA] (1948). Standard Methods for the Examination of Dairy Products. Washington, DC: American Public Health Association.
Anis, M., Ahmed, S., and Hasan, M. (2017). Algae as nutrition, medicine and cosmetic: the forgotten history, present status and future trend. World J. Pharm. Pharmaceut. Sci. 6, 1934–1959. doi: 10.20959/wjpps20176-9447
Anis, M., Yasmeen, A., Baig, S. G., Ahmed, S., Rasheed, M., and Hasan, M. (2018). Phycochemical and pharmacological studied on Ulva fasciata Delile. Pak. J. Pharm. Sci. 31, 875–883.
Ansari, A. A., Alghanem, S. M., and Naeem, M. (2019). Brown alga Padina: a review. Int. J. Bot. Stud. 4, 01–03.
Association of Official Analytical Chemists [AOAC] (1995). Official Method of Analysis. Washington, DC: Association of Official Analytical Chemists, 4–15.
Association of Official Analytical Chemists [AOAC] (1997). AOAC-Official Method of Analysis. Washington, DC: Association of Official Analytical Chemists, 6–18.
Ayoola, G. A., Cooker, H. A. B., Adessegun, S. A., depoju-Bello, A. A. A., Obaweya, K., Ezennia, E. C., et al. (2008). Phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in southwestern Nigeria. Trop. J. Pharma. Res. 7, 1019–1024. doi: 10.4314/tjpr.v7i3.14686
Balamurugan, K., Nishanthini, A., and Mohan, V. R. (2014). Antidiabetic and antihyperlipidaemic activity of ethanol extract of Melastoma malabathricum Linn. leaf in alloxin induced diabetic rats. Asian Pac. J. Trop. Biomed. 4, S442–S448. doi: 10.12980/APJTB.4.2014C122
Banu, T. A., and Umamageswari, S. (2011). Toxicity study of seaweeds in rats. J. Food Sci. Tech. 5, 23–31.
Benzie, I. F. F., and Strain, J. J. (1996). The Ferric Reducing Ability of Plasma (FRAP) as a Measure of Antioxidant Power: The FRAP Assay. Anal. Biochem. 239, 70–76. doi: 10.1006/abio.1996.0292
Brand-Williams, W., Cuvelier, M. E., and Berset, C. (1995). Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. 28, 25–30. doi: 10.1016/S0023-6438(95)80008-5
Chia, Y. Y., Kanthimathi, M. S., Rajarajeswaran, J., Khoo, K. S., and Cheng, H. M. (2015). Antioxidant, antiproliferative, genotoxic and cytoprotective effects of the methanolic extract of Padina tetrastromatica on human breast adenocarcinoma and embryonic fibroblast cell lines. Front. Life Sci. 8, 411–418. doi: 10.1080/21553769.2015.1051245
Chingizova, E. A., Skriptsova, A. V., Anisimov, M. M., Aminin, D. L., and Chingizova, E. A. (2017). Antimicrobial activity of marine algal extracts. Int. J. Phytomed. 9, 113–122.
Cotas, J., Leandro, A., Monteiro, P., Pacheco, D., Figueirinha, A., Gonçalves, A. M. M., et al. (2020). Seaweed Phenolics: From Extraction to Applications. Mar. Drugs 18, 384–431. doi: 10.3390/md18080384
Cruickshank, R., Duguid, J. P., Marmion, B. R., and Swain, R. H. A. (1975). Medical Microbiology, 12th Edn. London, UK: Living stone, 812–825.
Dhargalkar, V. (2015). Uses of seaweeds in the Indian diet for sustenance and well-being. Sci. Cult. 80, 192–202.
Dinis, T. C. P., Madeira, V. M. C., and Almeida, L. M. (1994). Action of phenolic derivates (acetoaminophen, salycilate and 5-aminosalycilate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys. 315, 161–169. doi: 10.1006/abbi.1994.1485
Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A., and Smith, F. (1956). Colorimetric method for determination of sugar and related substances. Anal. Chem. 28, 350–356. doi: 10.1021/ac60111a017
Farvin, K. S., and Jacobsen, C. (2013). Phenolic compounds and antioxidant activities of selected species of seaweeds from Danish coast. Food Chem. 138, 1670–1681. doi: 10.1016/j.foodchem.2012.10.078
Felix, N., and Brindo, R. A. (2014). Effects of raw and fermented seaweed, Padina tetrastomatica on the growth and food conversion of giant freshwater prawn Microbrachium rosenbergii. Int. J. Fish. Aquat. Stud. 1, 108–113.
Folch, J., Lees, M., and Sloane-Stanley, G. (1957). A simple method for isolation and purification of total lipids from animal’s tissues. J. Biol. Chem. 226, 497–509. doi: 10.1016/S0021-9258(18)64849-5
Ghazala, B., Shameel, M., Choudhary, M. I., Shahzad, S., and Legari, S. M. (2004a). Phycochemistry and bioactivity of two microalgae (Volvocophyta) from Sindh. Int. J. Biol. Biotech. 1, 343–350.
Ghazala, B., Naila, B., Shameel, M., Shahzad, S., and Legari, S. M. (2004b). Phycochemistry and bioactivity of two stonewort algae (Charophyta) of Sindh. Pak. J. Bot. 36, 733–743.
Guardia, T., Rotelli, A. E., Juarez, A. O., and Pelzer, L. E. (2001). Anti-inflammatory properties of plant flavonoids. Effect of rutin, quercetin and hesperidin on adjuvant arthritis in rats. Farmaco 56, 683–687. doi: 10.1016/S0014-827X(01)01111-9
Hameed, S., Ahmad, M., and Shameel, M. (2000). An ecological study on the tide pools of the rocky ledge at Pacha, near Karachi (Pakistan). Pak. J. Mar. Biol. 6, 179–197.
Hameed, S., Ahmad, M., and Shameel, M. (2001). Common structure and species composition of macroorganisms at the rocky bench at Pacha, near Karachi, Pakistan. Pak. J. Mar. Biol. 7, 135–146.
Harbone, J. B. (1973). Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis, 13th Edn. London, UK: Chapman and Hall, Ltd, 5–15.
Hess, D., Keller, H. E., and Oberlin, B. (1991). Simultaneous determination of retinol, tocopherols, carotenes, and lycopene in plasma by means of high-performance liquid chromatography on reversed-phase. Int. J. Vitam. Nutr. Res. 61, 232–238.
Hong, D. D., Hien, H. M., and Anh, H. T. L. (2011). Studies on analgesic and anti-inflammatory activities of Sargassum swartzii (Turner) C. Agardh (Phaeophyta) and Ulva reticulate Forsskal (chlorophyte) in experiment animal models. J. Biotech. 10, 2308–2314.
Jebasingh, E. J. S., Rosenmary, R., Elaiyaraja, S., Sivaraman, K., Lakshmikandan, K., Murugan, A., et al. (2011). Potential antibacterial activity of selected green and red seaweeds. J. Pharma. Biomed. Sci. 5, 1–7.
Jorgensen, P., Edgington, N. P., Schneider, B. L., Rupes, I., Tyers, M., and Futcher, B. (2007). The size of the nucleus increases as yeast cells grow. Mol. Biol. Cell 18, 3523–3532. doi: 10.1091/mbc.e06-10-0973
Kamenarska, Z., Gasicb, M. J., Zlatovicb, M., Rasovicd, A., Sladicb, D., Kljajicd, Z., et al. (2002). Chemical Composition of the Brown Alga Padina pavonia (L.) Gaill. from the Adriatic Sea. Bot. Mar. 45, 339–345. doi: 10.1515/BOT.2002.034
Khan, A. (2017). Flora of brown seaweeds from Pakistan (Part Ectocarpales). Pak. J. Mar. Sci. 26, 15–49. doi: 10.25301/JPDA.261.15
Kirby, W. M. M., Yoshihara, G. M., Sundsted, K. S., and Warren, J. H. (1957). Clinical usefulness of a single disc method for antibiotic sensitivity testing. Antibiot. Annu. 892, 1956–1957.
Kopustinskiene, D. M., Jakstas, V., Savickas, A., and Bernatoniene, J. (2020). Flavonoids as anticancer agents. Nutrients 12, 457–482. doi: 10.3390/nu12020457
Kumar, V., Murugesan, R., and Bhuvaneswari, S. (2015). Phytochemical analysis of red alga Champia parvula (C. Agardh) collected from Mandapam coast of tamil Nadu, India. Int. J. Adv. Pharm. 4, 15–20.
Lee, J. N., Park, C. S., Kim, H. P., Hwang, S. Y., and Chung, W. G. (2002). Single dose toxicity of Hwangjaegongjinbo, an ivigorator, in mice and rats. J. Toxicol. Public Health 18, 73–77.
Madkour, F. F., El-Shoubaky, G. A., and Ebada, M. A. (2019). Antibacterial activity of some seaweeds from the red sea coast of Egypt. Egypt. J. Aquat. Biol. Fish. 23, 265–274. doi: 10.21608/ejabf.2019.31016
Makkar, H. P. S., Blummel, M., Borowy, N. K., and Becker, K. (1993). Gravimetric determination of tannins and their correlations with chemical and protein precipitation methods. J. Sci. Food Agric. 61, 161–165. doi: 10.1002/jsfa.2740610205
Manivannan, K., Devi, G. K., Anantharaman, P., and Balasubramanian, T. (2011). Antimicrobial potential of selected brown seaweeds from vedalai coastal water, Gulf of Mannar. Asia Pac. J. Trop. Biomed. 1, 114–120. doi: 10.1016/S2221-1691(11)60007-5
Manteu, S. H., Nurjanah, N., and Nurhayati, T. (2018). Characteristics of brown algae (Sargassum policystum and Padina minor) from Pohuwato waters, Gorontalo Province. JPHPI 21, 396–405. doi: 10.17844/jphpi.v21i3.24709
Martemucci, G., Costagliola, C., Mariano, M., D‘andrea, L., Napolitano, P., and D’Alessandro, A. G. (2022). Free Radical Properties, Source and Targets, Antioxidant Consumption and Health. Oxygen 2, 48–78. doi: 10.3390/oxygen2020006
Mcdermid, K. J., and Stuercke, B. (2003). Nutritional composition of edible hawaaiian seaweeds. J. Appl. Phycol. 15, 513–524. doi: 10.1023/B:JAPH.0000004345.31686.7f
Mohapatra, L., Bhattamisra, S. K., Panigrahy, R. C., and Parida, S. K. (2016). Evaluation of the antioxidant, hypoglycaemic and antidiabetic activities of some seaweed collected from the east coast of India. Biomed. Pharmacol. J. 9, 365–375. doi: 10.13005/bpj/948
Novaczek, I., and Athy, A. (2001). Sea Vegetable Recipes for the Pacific Islands. Suva: USP marine studies programme, 36.
Nugraha, A. S., Damayanti, Y. D., Wangchuk, P., and Keller, P. A. (2019). Antiinfective and anti-cancer properties of the Annona species: their ethnomedicinal uses, alkaloid diversity, and pharmacological activities. Molecules 24, 4419–4440. doi: 10.3390/molecules24234419
O’sullivan, A. M., O’callaghan, Y. C., and O’grady, M. N. (2011). In vitro and cellular antioxidant activities of seaweed extracts prepared from five brown seaweeds harvested in spring from the west coast of Ireland. Food Chem. 126, 1064–1070. doi: 10.1016/j.foodchem.2010.11.127
Padmakumar, K., and Ayyakkannu, K. (1997). Seasonal variation of antibacterial and antifungal activities of the extracts of marine algae from southern coasts of India. Bot. Mar. 40, 507–515. doi: 10.1515/botm.1997.40.1-6.507
Panzella, L., and Napolitano, A. (2017). Natural phenol polymers: recent advances in food and health applications. Antioxidants 6:30. doi: 10.3390/antiox6020030
Peñalver, R., Lorenzo, J. M., Ros, G., Amarowicz, R., Pateiro, M., and Nieto, G. (2020). Seaweeds as functional ingredient for a healthy diet. Mar.Drugs 18, 301–328. doi: 10.3390/md18060301
Pereira, L. (2018). Therapeutic and Nutritional Uses of Algae. Boca Raton, FL: CRC Press. doi: 10.1201/9781315152844
Pérez, M. J., Flaqué, E., and Dominguez, H. (2016). Antimicrobial action of compounds from marine seaweeds. Mar. Drugs 14:52. doi: 10.3390/md14030052
Ponnanikajamideen, M., Malini, M., Malarkodi, C., and Rajeshkumar, S. (2014). Bioactivity and phytochemical constituents of marine brown seaweeds (Padina tetrastromatica) extract from various organic solvents. Int. J. Pharm. Ther. 5, 108–112.
Poornima, G. N., and Ravishankar, R. (2009). Evaluation of phytonutrients and vitamin contents in a wild yam, Dioscorea belophylla (Prain) Haines. Afr. J. Biotech. 8, 23–35.
Prieto, P., Pineda, M., and Aguilar, M. (1999). Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal. Biochem. 269, 337–341. doi: 10.1006/abio.1999.4019
Qari, R. (2017). An assessment of seaweeds diversity and distribution at the beach of Nathia Gali, Karachi, Pakistan. J. Mar. Sci. Res. Dev. 7, 228–239. doi: 10.4172/2155-9910.1000228
Qari, R., and Qasim, R. (1994). “Seasonal change in the standing crop of intertidal seaweeds from Manora coast Karachi,” in Proc Nat Sem Fish Policy and Plan, eds A. Majid, M. Y. Khan, M. Moazzam, and J. Ahmed (Karachi: Marine Fisheries Department), 279–286.
Qari, R., Qureshi, N. A., and Siddiqui, S. A. (2014). Phytomass Studies on Natural bed of Seaweed at Paradise Point, Karachi Coast. Int. J. Econ. Environ. Geol. 5, 11–17.
Radhika, D., and Priya, R. (2015). Assessment of anti-diabetic activity of some selected seaweeds. Eur. J. Biomed. Pharm. Sci. 2, 151–154.
Rajapakse, N., and Kim, S. K. (2011). Nutritional and Digestive Health Benefits of Seaweed, 1st ed. Amsterdam: Elsevier Inc, 64. doi: 10.1016/B978-0-12-387669-0.00002-8
Rajasekar, T., Shamya, M. A., and Joseph, J. (2019). Screening of phytochemical, antioxidant activity and anti-bacterial activity of marine seaweeds. Int. J. Pharm. Pharm. Sci. 11, 61–66. doi: 10.22159/ijpps.2019v11i1.29119
Ratana-arporn, P., and Chirapart, A. (2006). Nutritional evaluation of tropical green seaweeds Caulerpa lentillifera and Ulva reticulate. Kasetsart J. 40, 75–83.
Ravi, C. R., Lakshmanan, R., and Thiyagarajan, A. (2017). Invitro bioactivity and phytochemical analysis of two marine macroalgae. J. Coast. Life Med. 5, 427–432. doi: 10.12980/jclm.5.2017J7-124
Raymont, T. J. E. G., Austin, J., and Linford, E. (1964). Biochemical studies on marine zooplankton. I. The biochemical composition of Neomysis integer. J. Cons. Perm. Int. Explor. Mer. 28, 354–363. doi: 10.1093/icesjms/28.3.354
Re, R., Pellegrini, N., Proteggente, A., Pannnala, A., Yang, M., and Rice-Evans, C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 26, 1231–1237. doi: 10.1016/S0891-5849(98)00315-3
Rizvi, M. A., and Shameel, M. (2005). Pharmaceutical biology of seaweeds from the Karachi coast of Pakistan. Pharm. Biol. 43, 97–107. doi: 10.1080/13880200590919366
Ruperez, P. (2002). Mineral content of edible seaweed. Food Chem. 79, 23–26. doi: 10.1016/S0308-8146(02)00171-1
Saifullah, S. M. (1973). A preliminary survey of the standing crop of seaweeds from Karchi coast. Bot. Mar. 16, 139–144. doi: 10.1515/botm.1973.16.3.139
Saifullah, S. M. (2009). A Preliminary survey of the standing crop of seaweeds from Karachi coast. Bot. Mar. 16, 139–144.
Saleem, S. M. (1965). The distribution of marine algae along Karachi. Bot. Mar. 8, 183–195. doi: 10.1515/botm.1965.8.2-4.183
Salosso, Y., Aisiah, S., Toruan, L. N. L., and Pasaribu, W. (2020). Nutrient content, Active Compound, and Antibacterial Activity of Padina australis against Aeromonas hydropilla. Pharmacogn. J. 12, 771–776. doi: 10.5530/pj.2020.12.110
Sameeh, M. Y., Mohamed, A. A., and Elazzazy, A. M. (2016). Polyphenolic contents and antimicrobial activity of different extracts of Padina boryana Thivy and Enteromorpha sp marine algae. J. Appl. Pharm. Sci. 6, 087–092. doi: 10.7324/JAPS.2016.60913
Selvi, B. C., and Santhanam, A. (2016). Evaluation of anthelmintic activity using solvent extract of Padina tetrastromatica in Indian earthworm (Pheretima posthuma). Int. J. Ther. Appl. 32, 77–80. doi: 10.20530/IJTA_32_77-80
Shameel, M. (1987). A preliminary survey of seaweed from the coast of Lasbella, Pakistan. Bot. Mar. 30, 511–515. doi: 10.1515/botm.1987.30.6.511
Shameel, M. (2001). An approach to the classification of algae in the new millennium. Pak. J. Mar. Biol. 7, 233–250.
Shameel, M. (2008). Change of divisional nomenclature in the Shameelian Classification of algae. Int. J. Phycol. Phycochem. 4, 225–232.
Shameel, M., and Tanaka, J. (1992). “A preliminary checklist of marine algae from the coast and inshore waters of Pakistan,” in Cryptogamic Flora of Pakistan, eds T. Nakaike and S. Malik (Tokyo: National Science Museum), 1–64.
Shameel, M., Khan, S. H., and Afaq-Husain, S. (2000). Biodiversity of marine benthic algae along the coast of Balochistan, Pakistan. Pak. J. Mar. Biol. 6, 69–100.
Shannon, E., and Abu-Ghannam, N. (2019). Seaweeds as nutraceuticals for health and nutrition. Phycologia 58, 563–577. doi: 10.1080/00318884.2019.1640533
Sharifuddin, Y., Chin, Y. X., Lim, P. E., and Phang, S. M. (2015). Potential bioactive compounds from seaweed for diabetes management. Mar. Drugs 13, 5447–5491. doi: 10.3390/md13085447
Trinder, P. (1969). Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann. Clin. Biochem. 6, 24–26. doi: 10.1177/000456326900600108
Valento, P., Fernandes, E., Carvalho, F., Andrade, P. B., Seabra, R. M., and Bastos, M. L. (2002). Antioxidant activity of Hypericum androsaemum infusion: scavenging activity against superoxide radical, hydroxyl radical and hypochlorous acid. Biol. Pharm. Bull. 25, 1320–1323. doi: 10.1248/bpb.25.1320
Vieira, V., Prieto, M. A., Barros, L., Coutinho, J. A. P. I, Ferreira, C. F. R., and Ferreira, O. (2018). Enhanced extraction of phenolic compounds using choline chloride based deep eutectic solvents from Juglans regia L. Ind. Crop Prod. 115, 261–271. doi: 10.1016/j.indcrop.2018.02.029
Widiyanti, P., and Siswanto, S. (2012). Physical characteristic of brown algae (Phaeophyta) from madura strait as irreversible hydrocolloid impression material. Dent. J. 45, 177–180. doi: 10.20473/j.djmkg.v45.i3.p177-180
Yan, X., Chuda, Y., Suzuki, M., and Nagata, T. (1999). Fucoxanthin as the major antioxidant in Hijikia fusiformis, a common edible seaweed. Biosci. Biotechnol. Biochem. 63, 605–607. doi: 10.1271/bbb.63.605
Yasmeen, A., Ibrahim, M., Hasan, M. M. U., Jilani, T., Shafique, S., and Rasheed, M. (2021). Phycochemical analyses and pharmacological activities of seven macroalgae of Arabian sea (Northern coast line). Pak. J. Pharm. Sci. 34, 963–969.
Keywords: antioxidant, antimicrobial, anthelmintic, antidiabetic, Padina antillarum
Citation: Samar J, Butt GY, Shah AA, Shah AN, Ali S, Jan BL, Abdelsalam NR and Hussaan M (2022) Phycochemical and Biological Activities From Different Extracts of Padina antillarum (Kützing) Piccone. Front. Plant Sci. 13:929368. doi: 10.3389/fpls.2022.929368
Received: 26 April 2022; Accepted: 16 June 2022;
Published: 22 July 2022.
Edited by:
Johann Lavaud, Centre National de la Recherche Scientifique (CNRS), FranceReviewed by:
Nathalie Bourgougnon, Université Bretagne Sud, FranceCopyright © 2022 Samar, Butt, Shah, Shah, Ali, Jan, Abdelsalam and Hussaan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juveria Samar, SnV2LnNhbWFyQHlhaG9vLmNvbQ==; Anis Ali Shah, YW5pc2FsaWJvdEBnbWFpbC5jb20=; Adnan Noor Shah, YW5zLjc4NkB5YWhvby5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.