- 1Zhejiang Provincial Key Laboratory of Resources Protection and Innovation of Traditional Chinese Medicine, Hangzhou, Zhejiang, China
- 2College of Food and Health, Zhejiang Agriculture and Forestry University, Hangzhou, Zhejiang, China
- 3State Key Laboratory of Subtropical Silviculture, Zhejiang Agriculture and Forestry University, Hangzhou, Zhejiang, China
- 4Botanical Garden, Zhejiang Agricultural and Forestry University, Zhejiang, China
Tetrastigma hemsleyanum Diels et Gilg is a folk herb in Zhejiang Province with anti-inflammatory, antineoplastic, and anti-oxidation effects. Given its pharmacological activity, T. hemsleyanum is known as New “Zhebawei” and included in the medical insurance system of Zhejiang and other provinces. Flavonoids are the most important components of T. hemsleyanum, and their contents are mainly regulated by ultraviolet (UV) radiation. In this study, the total flavonoid contents, flavonoid monomer contents, and flavonoid synthesis related enzyme activities (phenylalanine ammonia–lyase, chalcone synthase, and chalcone isomerase), anti-oxidant enzyme activities (catalase, peroxidase, and superoxide dismutase), and biochemical indicators (malondialdehyde, free amino acid, soluble protein, and soluble sugar) in the leaves (L) and root tubers (R) of T. hemsleyanum with UV treatments were determined. Three kinds of UV radiation (UV-A, UV-B, and UV-C) and six kinds of radiation durations (15 and 30 min, 1, 2, 3, and 5 h) were used. Appropriate doses of UV-B and UV-C radiation (30 min to 3 h) induced eustress, which contributed to the accumulation of flavonoids and improve protective enzyme system activities and bioactive compound contents. Especially, certain results were observed in several special structures of the flavonoid monomer: quercetin contents in L increased by nearly 20 times, isoquercitrin contents in R increased by nearly 34 times; most of flavonoids with glycoside content, such as quercitrin (19 times), baicalin (16 times), and apigenin-7G (13 times), increased multiple times. Compared with the CK group, the flavonoid synthase activities, anti-oxidant enzyme activities, and biochemical substance contents in L and R all increased with UV treatments. This study provides a theoretical foundation for regulating flavonoids by light factors and improving the quality of T. hemsleyanum in production and medical industries.
Introduction
Tetrastigma hemsleyanum Diels et Gilg is one of the folk herbs in Zhejiang Province; it mainly grows in mountains and under woods and is distributed in Zhejiang, Fujian, Guangxi, etc., in China. According to ancient records, T. hemsleyanum is widely used in folk herbs and treatment of children with high fever, convulsion, and dysentery (Ji et al., 2021); thus, it is also called a plant antibiotic. Modern studies showed that T. hemsleyanum has anti-inflammatory, antineoplastic, and anti-oxidant effects (Ye and Liu, 2015; Chen et al., 2019) and is widely used in clinical practice. Given its powerful pharmacological activity, T. hemsleyanum is known as New “Zhebawei” and included in the medical insurance system of Zhejiang and other provinces. In addition, T. hemsleyanum has been used as the main raw material to produce Chinese patent medicines with tumor prevention and treatment effects and the main material in the anti-COVID-19 prescription (He et al., 2020; Zhang et al., 2022) in Zhejiang Province.
The main components of T. hemsleyanum are flavonoids, terpenoids, steroids, polysaccharides, and phenolic acids, among which flavonoids are the most important (Liu et al., 2022). To date, numerous flavonoid compounds have been found in T. hemsleyanum (Zhu et al., 2020); these compounds include kaempferol, isoquercetin, polygonin, and vitexin, and they have important functions. Flavonoids are ubiquitous functional factors in plants, showing a variety of biological activities, including anti-oxidant (Sakina et al., 2015), anti-inflammatory (Zaragozá et al., 2020), and immunomodulatory (Jasso-Miranda et al., 2019) effects. Numerous studies (Maan et al., 2020; Bondonno et al., 2021) found the correlation between flavonoid consumption and reduced incidence of diseases, such as diabetes, asthma, hypertension, cardiovascular disease, age-related diseases, and neurodegenerative diseases (such as Parkinson’s and Alzheimer’s).
Therefore, finding a way to increase the flavonoid contents in T. hemsleyanum has drawn our attention. At present, the common methods for increasing flavonoid contents are stress treatments, including ultraviolet (UV) (Rao et al., 2019), oxidative (Nakabayashi et al., 2014), and drought stresses (Davies et al., 2018). UV radiation constitutes 7% of all sunlight that reaches the Earth (Müller-Xing et al., 2014). UV-A (320–400 nm), UV-B (280–320 nm), and UV-C (100–280 nm) are the three primary regions of the UV spectrum, which extends from 100 to 400 nm. Rao et al. (2019) reported that one of the most important mechanisms of plants exposed to radiation to resist damage is their reliance on flavonoids (flavonoids, flavonols, isoflavones, and anthocyanins), which play a protective role by providing a strong antioxidant capacity. UV radiation can boost the accumulation of flavonoids in numerous plants. Szopa et al. (2018) showed that UV-A increased the contents of rutin, luteolin, and quercetin in Aronia melanocarpa, A. arbutifolia, and A. prunifolia. UV-B can increase the contents of multein, oleuropein, and luteonin-7-O-glucoside in Olea europaea (Dias et al., 2020). Guajardo-Flores et al. (2014) discovered that UV-C treatment mainly improved the levels of quercetin-3-O-glucoside by about two times relative to those in the control group of Phaseolus vulgaris.
Our previous studies showed that the total flavonoid contents of forest-undergrowth T. hemsleyanum were higher than those in field cultivation. Given that short-wave light dominates the understory spectrum, we attempted to increase the flavonoid content of T. hemsleyanum with UV radiation. A limited number of studies focused on the effects of UV treatment on flavonoids in T. hemsleyanum. Thus, this study aimed to evaluate the effects of different UV wavelengths and doses on the accumulation of various flavonoids in T. hemsleyanum. On the basis of relationships among accumulated flavonoids, flavonoid synthesis related enzymes, and substances related to anti-stress under different radiation wavelengths and doses, the regulation of flavonoids by UV radiation was explored. Our results will be helpful to improve the quality of T. hemsleyanum and beneficial for its development and utilization in the future.
Materials and methods
Materials
This study was conducted in Zhejiang Provincial Key Laboratory of Resource Protection and Innovative Utilization of Traditional Chinese Medicine, the State Key Laboratory of Subtropical Forest Cultivation. T. hemsleyanum plants were purchased from Suichang Planting Base (Lishui City, Zhejiang Province) and identified by Dr. Aicun Zhou of Zhejiang A&F University.
All plants grew in the Pingshan Practice base (Lin’an, Zhejiang province, 30° 15′ 30.39″ N, 119° 43′ 26.92″ E) in 18 cm diameter plastic pots. Each plant was cultivated in a pot containing a soil mixture with peat:pastoral soil:perlite:cow dung ratio of 4:4:4:1.
Ultraviolet treatment
Tetrastigma hemsleyanum with the same growth vigor (3 year growths, vegetative growth phase) were transplanted to the laboratory. The aboveground plant parts were treated with UV irradiation in a shelf with an opaque black cloth. After UV irradiation for 15 and 30 min and 1, 2, 3, and 5 h, all plants were treated with darkness for 24 h. Leaves (L) and root tubers (R) were harvested as experimental materials. The details are shown in Table 1. The L treated with UV-A for 15 min and dark treatment for 23 h and 45 min were denoted as L-UV-A (15 min). Three kinds of UV light (Huaqiang Electronics Co., Ltd., China) were used: 40 W UV-A (320–400 nm), UV-B (280–320 nm), and UV-C (200–280 nm). The light intensity range was 1,630–1,660 Lx. Each treatment group comprised five biological replicates.
Metabolome analysis of flavonoids
The determination of flavonoid monomers was performed as described by Bai et al. (2021). Approximately 0.1 g freeze-dried L or R were ground into fine powder (80-mesh sieve) and placed in a 2 ml Eppendorf tube. Approximately 1.5 ml 85% methanol solution was added. The solution was ultrasonicated for 30 min, cooled and stood at room temperature, shaken uniformly, and left to stand. The supernatant was filtered with 0.22 μm membranes, and the subsequent filtrate was collected as the test solution.
The optimal chromatographic conditions were ACQUITY UPLCTM I-Class (Waters, Milford, MA, United States), ACQUITY UPLC BEH C18 column (2.1 mm × 100 mm, 1.8 μm) and 40°C. The mobile phase consisted of 0.1% formic acid–water (A) and 0.1% formic acid–acetonitrile (B) with a gradient elution of 5% B (0 min), 25% B (1 min), 40% B (3.5 min), and 60% B (4.5 min). The flow rate was 0.6 ml/min, and the injection volume was 1 μl. The mass spectrometry parameters were as follows: quadrupole ion trap mass spectrometer API 6500 (AB SCIEX, Framingham, MA, United States); ion source, Turbo V; ionization mode, ESI-; curtain gas flow rate, 30 L/min; spray voltage, −4500 V; atomized gas (GS1) flow rate, 55 L/min; auxiliary gas (GS2) flow rate, 55 L/min; collection mode, multiple reaction monitoring mode; ionization temperature, 550°C.
Supplementary Table 1 shows the optimized conditional parameters. The linear relationship results are shown in Supplementary Table 2.
Determination of total flavonoid content
Total flavonoid contents in L and R of T. hemsleyanum were determined by a UV–visible (UV-VIS) spectrophotometer (UNICO 3802, Shanghai, China) in accordance with the method of Bai et al. (2021). Supplementary Method 1 provides more details on the experiment.
Determination of flavonoid synthesis related enzymes activity
The extraction method of crude enzyme solution was as follows: 0.5 g fresh L or R were ground with 9 ml buffer solution into a homogenate and centrifuged at 10,000 r/min for 20 min at 4°C, and the supernatant was used as the extract.
Phenylalanine ammonia–lyase (PAL) enzyme activity was determined following the method of Bai et al. (2021). We obtained 1 ml crude enzyme solution, 1 ml 0.33% phenylalanine solution, and 2 ml distilled water and water bathed the mixture for 30 min at 30°C. A total of 0.2 ml 6 mol/L hydrochloric acid solution was added to terminate the reaction. The absorbance was measured by a UV-VIS spectrophotometer at 290 nm.
Chalcone synthase (CHS), chalcone isomerase (CHI), flavanone 3-hydroxylase (F3H), flavonol synthase (FLS), flavone synthase (FNS), flavonoid-3′-hydroxylase (F3′H), and flavonoid-3′,5′-hydroxylase (F3′5′H) enzyme activities were determined using the CHI, CHS, F3H, FLS, FNS, F3′H, and F3′5′H kits, respectively (Wanxiang Hengyuan Co., Ltd., Tianjin, China).
Determination of biochemical indexes
Catalase (CAT), peroxidase (POD), and superoxide dismutase (SOD) activities and malondialdehyde (MDA) contents were determined in accordance with the method described by Ma et al. (2019). More experimental details are provided in Supplementary Method 2.
The contents of free amino acid, soluble proteins, and soluble sugars were determined using the ninhydrin, Coomassie bright blue, and anthrone methods, respectively (Mao et al., 2021). More experimental details are provided in Supplementary Method 3.
Statistical analysis
Analyses were performed with SPSS 19.0 software. The results were statistically analyzed by using one-way analysis of variance, followed by least significant difference test at a probability level of 0.05.
Multivariate principal component analysis (PCA), variable importance in projection (VIP) analysis, and correlation chart analysis were performed with MetaboAnalyst (Chong et al., 2018). A cluster heatmap was generated with TBtools software (Chen et al., 2018).
Results
Flavonoid monomers content in leaves and root tubers were significantly different
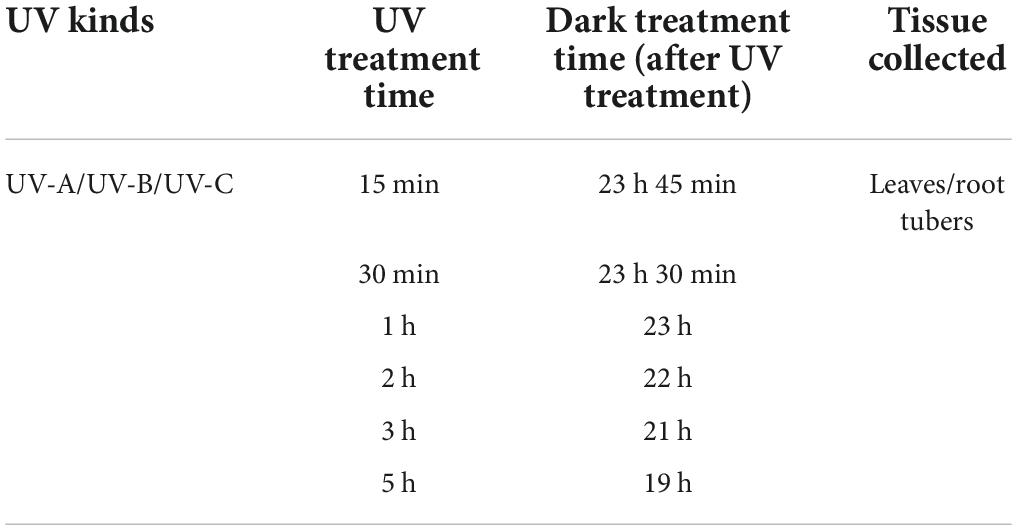
The flavonoid contents and flavonoid synthesis related enzyme activities of T. hemsleyanum were detected (Figure 1). The total flavonoid contents in L were slightly higher than those in R, but the changes were not significant. As for flavonoid synthesis related enzymes, PAL enzyme activities in L and R showed no significant difference, CHS and CHI enzyme activities in R were significantly higher than those in L.
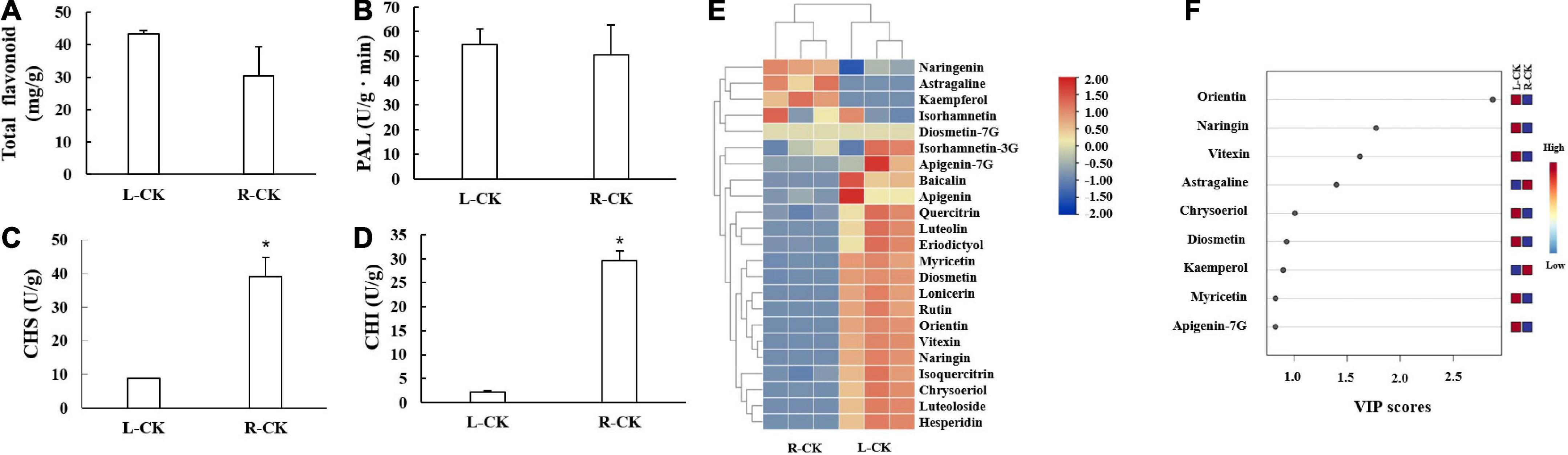
Figure 1. Flavonoid contents and flavonoid synthesis related enzymes activities in control group of Tetrastigma hemsleyanum. (A) Total flavonoids. (B) Phenylalanine ammonia-lyase (PAL). (C) Chalcone synthase (CHS). (D) Chalcone isomerase (CHI). (E) Heatmap of flavonoid metabolites. (F) VIP scores. Metabolites with VIP scores higher than 1.0 were obtained through PLS-DA. *P < 0.05.
The heatmap showed that most of flavonoid monomers in L were higher than those in R (Figure 1E). Six components were identified as differential metabolites (criteria: VIP > 1.0, t-test P < 0.05). The contents of orientin, naringin, vitexin, chrysoeriol, and diosmetin in L were significantly higher than those in R, but the contents of astragaline were lower (Figure 1F).
Content of glycosylated flavonoids increased significantly with ultraviolet treatments
The flavonoid contents in L and R all increased with UV treatments. In L (Figure 2A), the total flavonoid contents with UV-C treatment were significantly higher than those in other groups, especially in the 2, 3, and 5 h groups, whose total flavonoid contents were 3.8, 3.7, and 3.5 times (164.98, 161.51, and 153.77 mg/g), respectively, those of the CK group (43.41 mg/g). Compared with the control group (1.060 mg/g), the total flavonoid contents obtained with UV-B treatment were always higher than those of other treatment groups. However, the contents of the UV-A group were lower than those of the CK group. In R (Figure 2B), the total flavonoid contents increased under UV-B exposure. Especially, the total flavonoid content of UV-B 1 h group reached the highest level (123.89 mg/g) and was considerably higher (4.1 times) than that of the CK group.
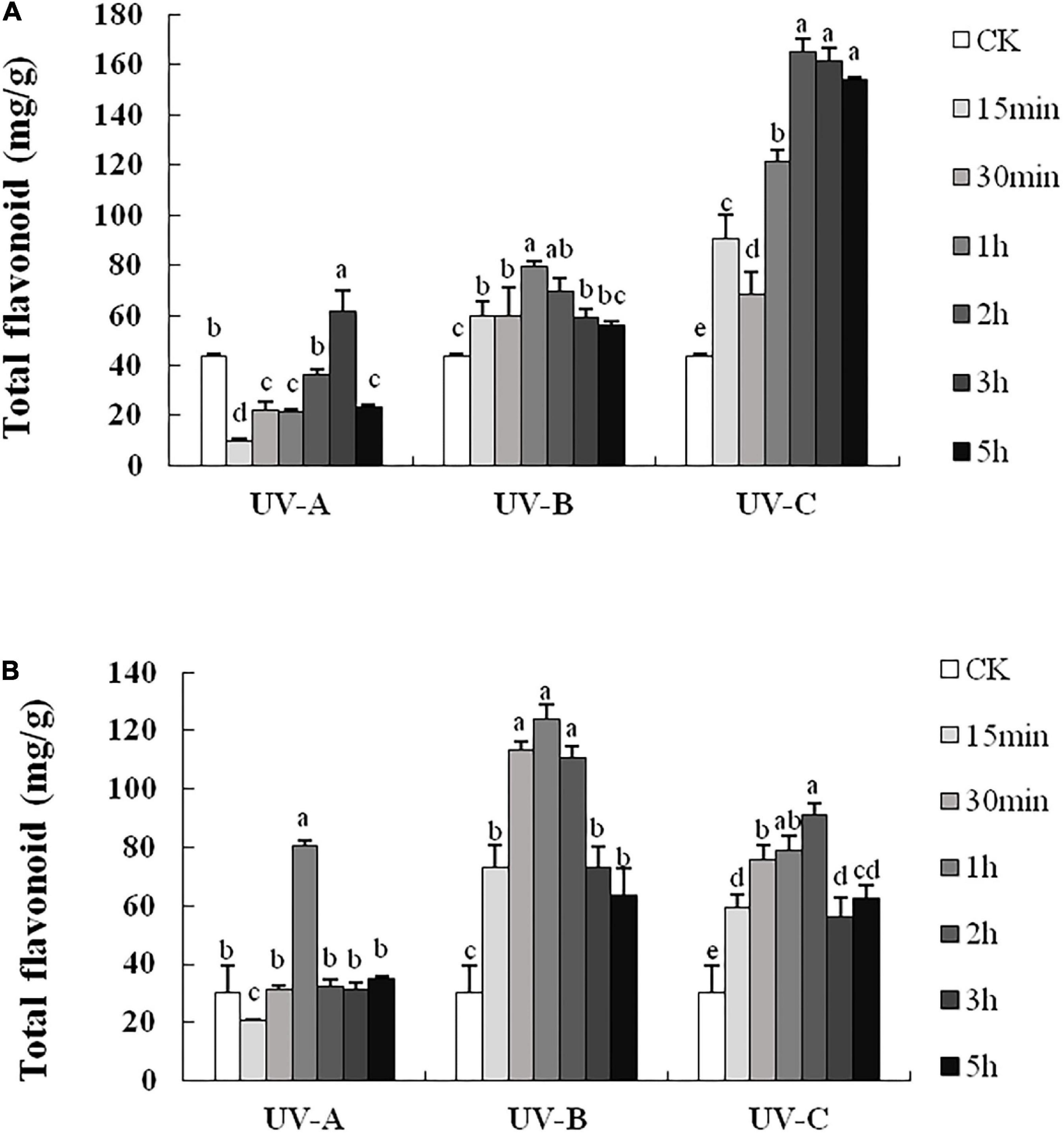
Figure 2. Total flavonoid contents in leaves (A) and root tubers (B) of Tetrastigma hemsleyanum with UV-A, UV-B, and UV-C treatments. Different lowercase letters indicate significant difference at 0.05 level (P < 0.05).
Supplementary Tables 3, 4 show the influences of radiation wavelength, radiation type, and radiation time on the contents of different flavonoid monomers. Based on targeted metabolomics, PCA was performed to evaluate the variations in the levels of flavonoid metabolites. The PCA score plot results indicated that the effect of UV radiation on L was more evident than that on R. In L (Figure 3A), the CK group was separated from the other treatment groups. Especially, the UV-C group was significantly separated with other groups, and the UV-A group was located close to the UV-B group. In R (Figure 4A), four groups were located close together and could not be separated. In addition, the effect of UV-C treatment on flavonoids in L was different from those of UV-A and UV-B.
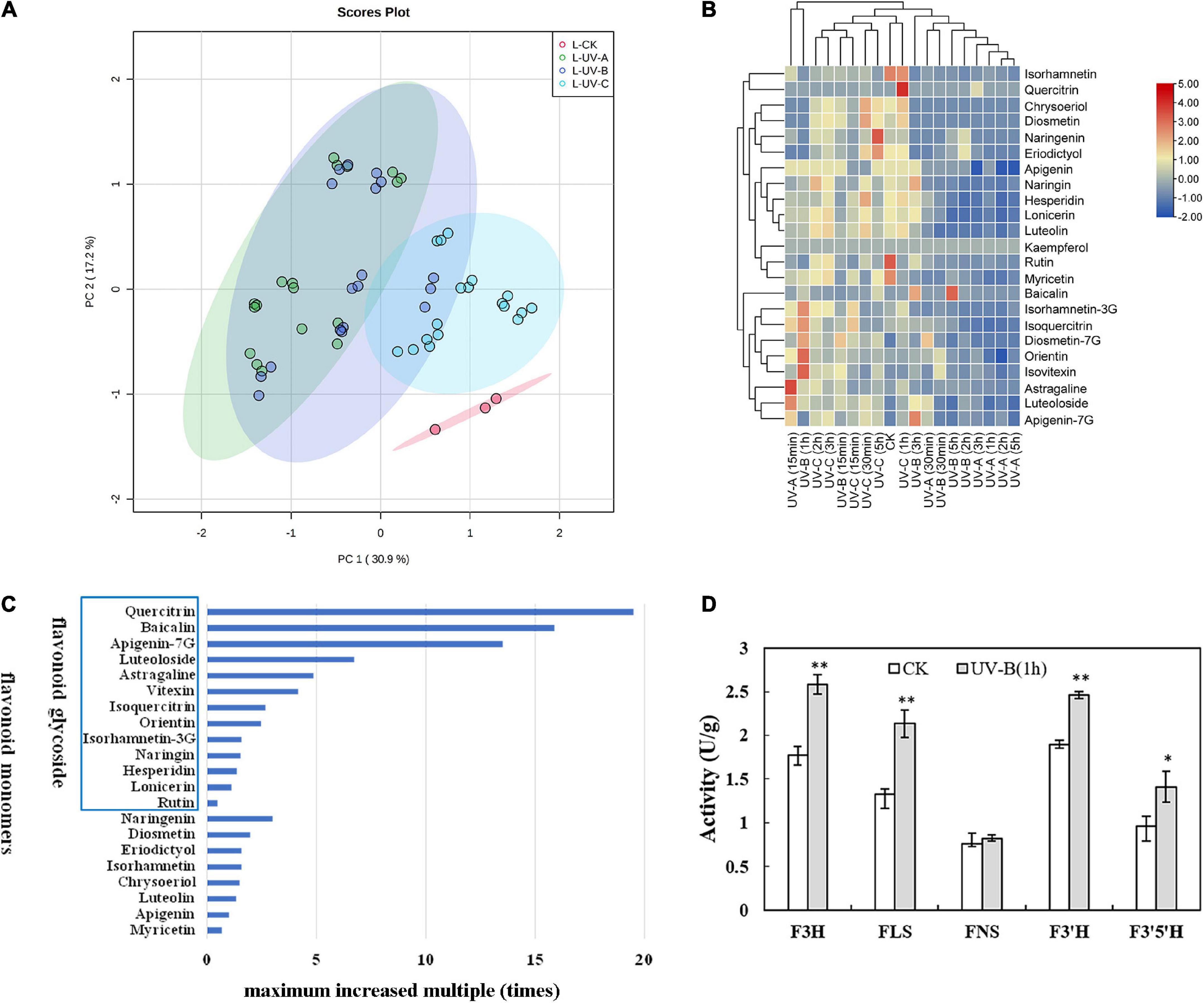
Figure 3. Flavonoid metabolites in leaves of Tetrastigma hemsleyanum with UV treatments. (A) PCA score plot of metabolites in leaves. (B) Heatmap of flavonoid metabolites in leaves. (C) The maximum increased multiple of flavonoid monomers with different structural characteristics in leaves. (D) Activities of flavanone 3-hydroxylase (F3H), flavonol synthase (FLS), flavone synthase (FNS), flavonoid-3′-hydroxylase (F3′H), flavonoid-3′,5′-hydroxylase (F3′5′H) enzyme with UV-B 1 h treatment in leaves. *P < 0.05, **P < 0.05.
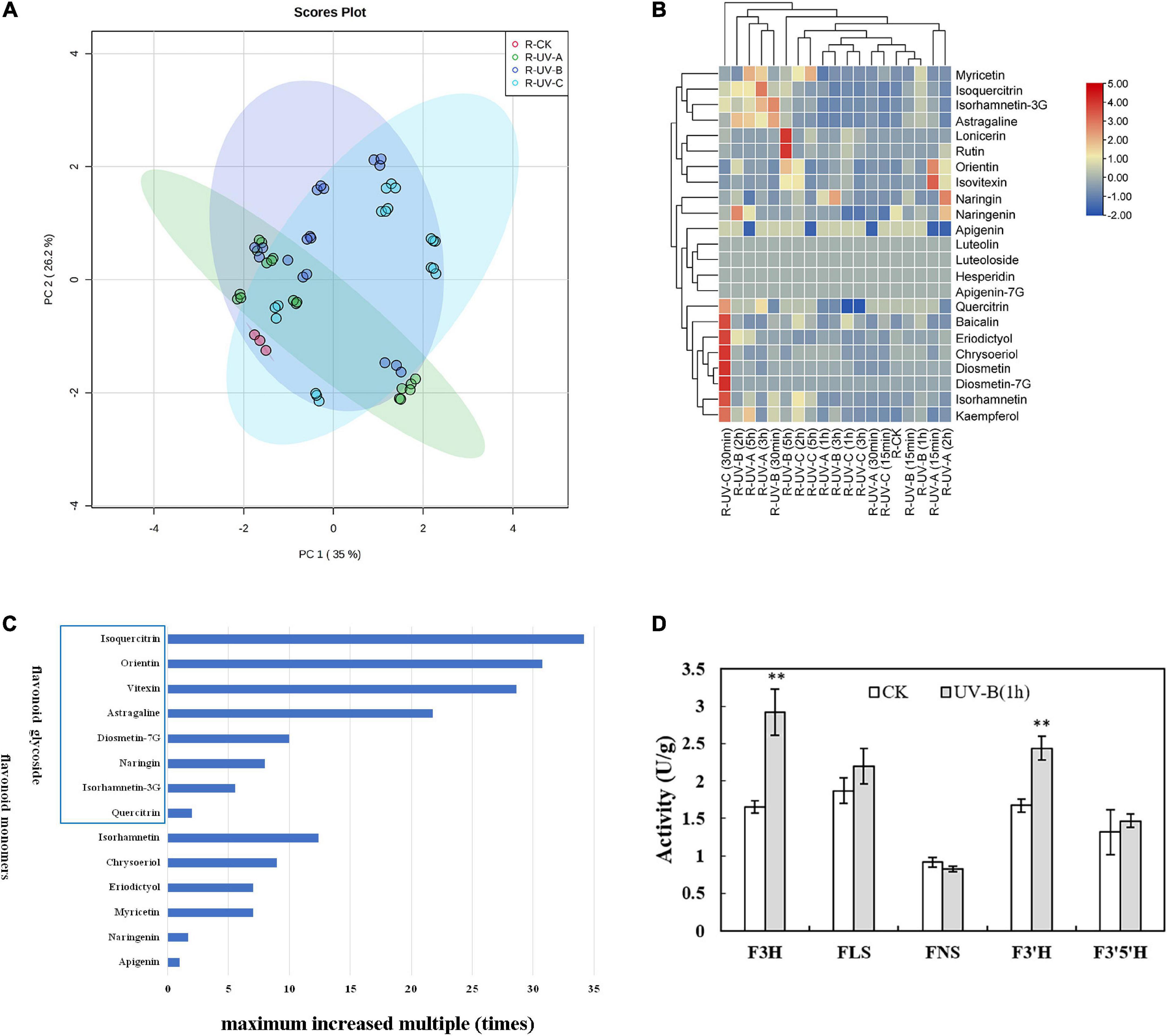
Figure 4. Flavonoid metabolites in root tubers of Tetrastigma hemsleyanum with UV treatments. (A) PCA score plot of metabolites in root tubers. (B) Heatmap of flavonoid metabolites in root tubers. (C) The maximum increased multiple of flavonoid monomers with different structural characteristics in root tubers. (D) Activities of flavanone 3-hydroxylase (F3H), flavonol synthase (FLS), flavone synthase (FNS), flavonoid-3′-hydroxylase (F3′H), flavonoid-3′,5′-hydroxylase (F3′5′H) enzyme with UV-B 1 h treatment in root tubers. *P < 0.05, **P < 0.05.
Heatmap analysis provided an integrated view of UV radiation effect on L (Figure 3B) and R (Figure 4B). In L, the flavonoid contents increased under the three UV treatments. Especially under UV-B and UV-C radiation, the flavonoid content increased compared with those in the control group. Notably, short-time (15 min) UV-A treatment increased the levels of most flavonoid monomers, especially the contents of apigenin-7G, luteoloside, astragaline, and isoquercitrin, whereas more than 30 min UV-A treatment had less effect on flavonoid accumulation. Long-time UV-B radiation reduced the flavonoid contents, but less than 1 h of radiation improved the contents of most flavonoids (isovitexin, orientin, diosmetin-7G, isoquercitrin, and isorhamnetin-3G). UV-C radiation at all durations (15 min to 5 h) promoted the enhancement of flavonoid contents (nearly all flavonoid monomers were detected in our study). In R, the flavonoid contents increased slightly with UV in general, except under certain radiation conditions. Under UV-C radiation for 30 min, the contents of isorhamnetin, baicalin, kaempferol, quercitrin, chrysoeriol, eriodictyol, diosmetin, and diosmetin-7G increased dramatically. Under UV-B radiation for 5 h, the contents of lonicerin and rutin increased significantly. In general, UV-B and UV-C treatments are optimal methods for promoting the accumulation of flavonoid contents.
Combined with the above results, the flavonoid monomers were further classified. Compared with the control group in L (Figure 3C), most of flavones with glycoside content increased multiple times. These flavones included quercitrin (19 times), baicalin (16 times), apigenin-7G (13 times), luteoloside (7 times), astragalin (5 times), vitexin (4 times), isoquercitrin (3 times), and orientin (2 times). The same phenomenon was found in R (Figure 4C). The contents of isoquercitrin (34 times), orientin (30 times), vitexin (29 times), astragalin (22 times), diosmetin-7G (10 times), naringin (8 times), and isorhamnetin-3G (6 times) were substantially higher than those in the control group.
To gain more information on the variation in the flavonoid monomer contents with UV radiation, we randomly selected the materials treated with UV-B for 1 h and determined the activities of specific enzymes involved in the increase in flavonoid metabolites in R and L. In L (Figure 3D), the F3H, FLS, F3′H, and F3′5′H activities were significantly higher than those of the control group. In R (Figure 4D), F3H and F3′H activities were significantly higher than those of the control group.
Activities of flavonoid synthesis related enzymes in the leaves and root tubers all increased with ultraviolet treatments
As presented in Figure 5, the activities of flavonoid synthesis related enzymes in L and R increased with UV treatments first and then decreased as the radiation time increased. UV-A radiation reduced PAL enzyme activities at all time points in L (Figure 5A). Under UV-B or UV-C conditions, PAL enzyme activities were higher than those of the CK group, especially at UV-C 3 h (108.43 U/g min). In terms of CHS and CHI, UV-C treatments increased sharply the enzyme activities, which reached the highest values (62.55 and 89.06 U/g min, respectively) at UV-C 2 h. In R (Figure 5B), UV-B and UV-C treatments resulted in evident enzyme activities. Under UV-B exposure for 1 h, PAL and CHS enzyme activities reached the highest values (87.90 and 72.23 U/g min, respectively). Under UV-C exposure 3 h, CHI enzyme activities reached the highest value (56.83 U/g min).
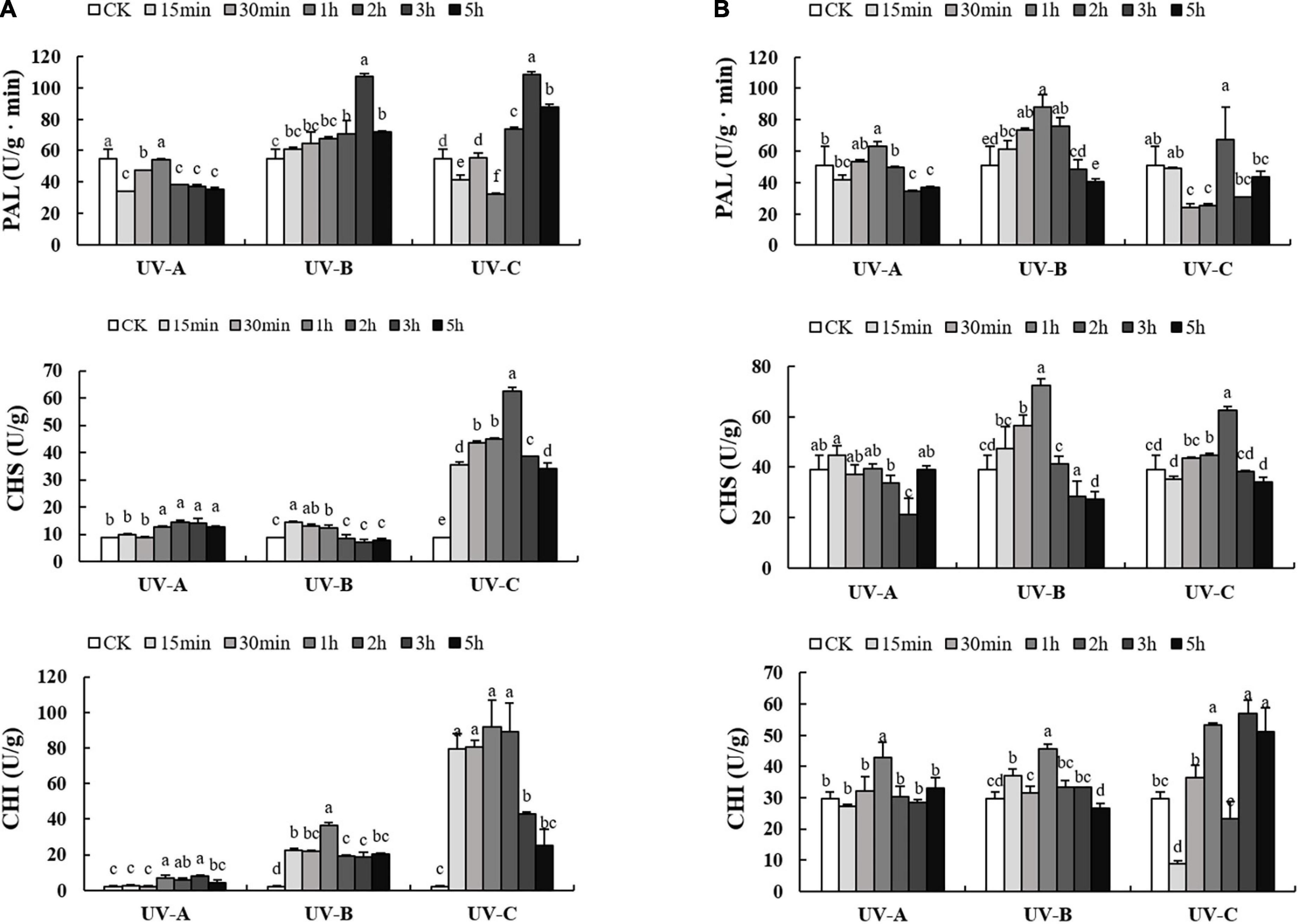
Figure 5. Activities of phenylalanine ammonia-lyase (PAL), chalcone synthase (CHS), chalcone isomerase (CHI) enzyme in leaves (A) and root tubers (B) of Tetrastigma hemsleyanum with UV-A, UV-B, and UV-C treatments. Different lowercase letters indicate significant difference at 0.05 level (P < 0.05).
Most of substances related to anti-stress first increased and then decreased with radiation time
Soluble substances are the basic nutrients in plants and can reflect plant growth and development status. Most of the soluble substance increased first and then decreased with stress duration (Figure 6A). Compared with the control group, the UV-B treatments significantly increased the total soluble sugar contents. UV-A and UV-B treatments increased the soluble amino acid contents under various treatment periods, whereas the soluble amino acid contents in the UV-C treatment group were lower than that in the CK group in each treatment period. UV-A, UV-B, and UV-C radiation increased the soluble protein content in each treatment group. The highest levels of soluble amino acid, protein, and sugar were obtained with UV-B treatments, with values of 82.74 (2 h), 2.26 (1 h), and 0.40 mg/g (30 min), respectively.
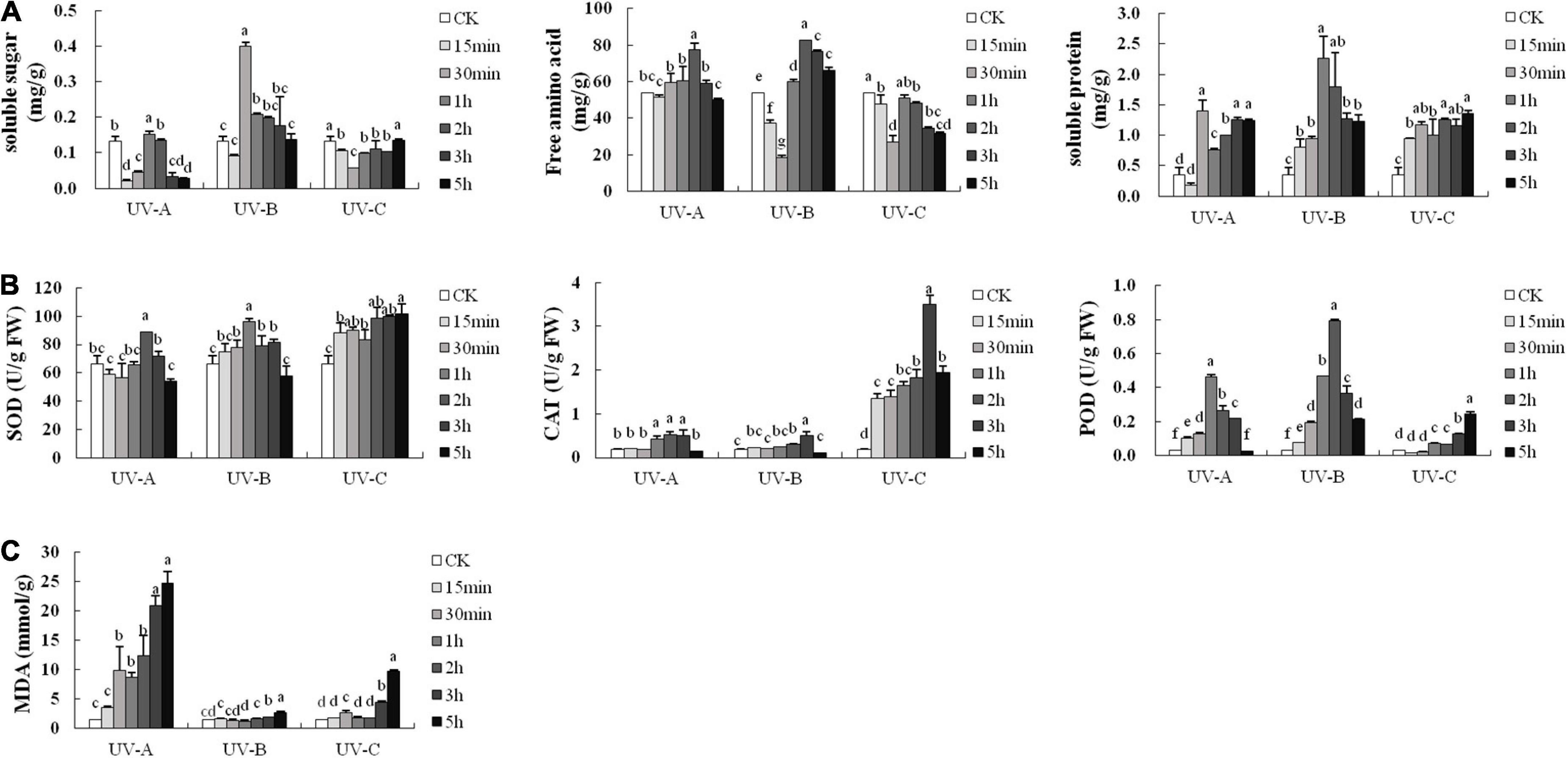
Figure 6. Soluble substance contents (A), antioxidant enzyme activities (B), and malondialdehyde (MDA) contents (C) of leaves in Tetrastigma hemsleyanum with UV treatments. Different lowercase letters indicate a significant difference at 0.05 level (P < 0.05).
Superoxide dismutase, CAT, and POD are the main members of the protective enzyme system. Figure 6B shows the activities of these enzymes with UV treatment. UV-B and UV-C radiation improved their protective activities. The highest values of SOD, CAT, and POD activities were 101.85 (UV-C 5 h), 3.50 (UV-C 3 h), and 0.793 U/g (UV-B 2 h), respectively, which were considerably higher than those of the control group. These results indicated that L and R are in adversity under UV exposure.
Figure 6C shows that the MDA contents were maintained at low levels and close to that of the CK group with UV-B treatment at different times. The MDA contents exhibited minimal changes when the UV-C radiation time was less than 2 h. Compared with the MDA contents in UV-B and UV-C groups, those of the UV-A groups showed an upward trend and reached a maximum value (24.71 nmol/g FW) with 5 h exposure.
Tetrastigma hemsleyanum is a known source of phytotherapeutics. As an edible plant, the R can be used for medicinal purposes, whereas the L are consumed as functional tea or dietary supplement because of their health benefits. An increasing number of studies on T. hemsleyanum have been published in recent years, and its flavonoids have attracted considerable interest. Fan et al. (2017) identified orientin, vitexin, isovitexin, etc., and eight kinds of flavonoid monomers from the L of T. hemsleyanum. Zhu et al. (2020) reported more than 30 kinds of flavonoid monomers from whole plants (T. hemsleyanum). Most of these substances were detected in our study. A total of 21 flavonoids were detected in the L of T. hemsleyanum, and 15 flavonoids were detected in the R (Figure 1E). Shi et al. (2022) observed that the flavonoids in T. hemsleyanum were affected by external environment, similar to our results. We employed UV treatments to regulate the flavonoids of T. hemsleyanum (naringenin, astragaline, kaempferol, isorhamnetin, diosmetin-7G, isorhamnetin-3G, apigenin-7G, baicalin, apigenin, quercitrin, luteolin, eriodictyol, myricetin, diosmetin, lonicerin, rutin, orientin, vitexin, naringin, isoquercitrin, chrysoeriol, luteoloside, and hesperidin) and increased the flavonoid contents and varieties to further improve the quality of T. hemsleyanum (Figure 1E).
Based on the research results and analysis, UV radiation had various effects on flavonoid monomers. Several studies indicated that flavonoid monomers with diverse structures have different levels of sensitivity to UV radiation, especially those with specific structures (Murai et al., 2009). Under UV exposure, the contents of flavanol and flavonoid subclasses increased more than that of the dihydroflavonoid subclass in L and R, particularly the flavanol subclass. Zoratti et al. (2014) suggested that the flavanol subclass is the main group of flavonoids induced by UV exposure. When the flavanol subclass possessed an ortho-dihydroxy substitution at the B-ring (hydroxyl groups at C3′ and C4′), quercetin (flavonol) contents in L increased nearly 20 times with UV-C exposure for 1 h (Figure 3C). The isoquercitrin contents in R increased nearly 34 times under UV-A exposure for 1 h (Figure 4C). Numerous studies (Ryan et al., 1998; Agati et al., 2011) demonstrated that ortho-dihydroxylated B-ring flavonoids, such as quercetin and its derivatives, accumulated in plants with UV treatment. In addition, the hydroxyl groups of flavonoids exhibited an anti-oxidant activity in vitro and in vivo, promoting the radical scavenging potential (Rice-Evans et al., 1996).
Ultraviolet radiation particularly increased the content of glycoside flavonoids. Recently, abundant studies indicated that UV radiation promotes the accumulation of flavonoid glycosides in plants, especially certain functional food raw materials. In Rhodiola rosea L. (Dong et al., 2020), flavonoids (characterized by glycosides) were significantly up-regulated with the increasement of elevation. In Astragalus membranaceus (Liu et al., 2018), the contents of isoflavones, especially flavonoid glycosides, increased under UV-B radiation. In Glycyrrhiza uralensis (Zhang et al., 2018), the contents of glycosides in L increased under UV-B radiation. Flavonoids with glycoside perform numerous functions in plants. Modifications provide extra structural stability to flavonoids during storage in vacuoles and chloroplasts (Marinova et al., 2007). These substances confer structural complexity, molecular solubility and stability, subcellular transportability, and biological activity, which play important roles in plant growth, hormone balance, and elimination of toxicity of endogenous and exogenous substances (Xin et al., 2017). Furthermore, flavonoids with glycosides strongly enhance water solubility and thus increase bioavailability, improving anti-oxidation and exerting beneficial effects on human health (Hollman et al., 2000; Lewandowska et al., 2013).
The accumulations of these flavonoid compounds are closely related to the activation of enzymes. PAL, CHS, and CHI play important roles in flavonoid synthesis. UV exposure enhances PAL, CHS, and CHI activities, particularly in Brassica oleracea (Lee et al., 2019) and Dendrobium officinale (Chen Y. et al., 2020). Our results indicated similar effects (Figure 5), that is, significant positive correlations were found among total flavonoid content, partial flavonoid monomers, and enzyme activities. Notably, flavonoid monomer contents barely increased upstream of the pathway (naringin, apigenin, and eriodictyol). We observed that numerous flavonoid monomers were secondary metabolite products but consumed as upstream substrates simultaneously. This high consumption was probably caused by a large increase in the glycoside flavonoid content. Several studies (Wu et al., 2017; Zhang et al., 2018) showed that specific downstream flavonoid synthetases, such as FLS and UGT(), are highly sensitive to UV treatment, which further explains the increased contents of downstream flavonoids. Referring to metabolic pathways in other studies (Zhang et al., 2017; Xu et al., 2019), a close relationship was observed between the increase in eriodictyol, kaempferol, quercitrin, and luteoloside contents in Zea mays subsp. mays, Fagopyrum tataricum, and activities of flavonoid synthesis related enzymes, including F3H, FLS, F3’H, F3’5’H, and FNS. Correlation analysis (Supplementary Figure 1) showed that these five enzymes were significantly positively correlated with lonicerin, diosmetin-7G, isorhamnetin, quercitrin, orientin, vitexin, isoquercitrin, astragaline, luteoloside, and baicalin in the L of T. hemsleyanum. This finding prompted us to focus on these enzymes. We will explore further the relationship between flavonoid synthase activity and flavonoid metabolome regulated by UV treatment.
The synthesis of flavonoids is a complicated process, and although various studies have shown that flavonoid content is related to enzyme activities, a wide range of other factors affect this process. According to our results, CAT and SOD and most soluble substances have a significant positive correlation with the total flavonoid content, whereas a significant negative correlation was observed among MDA content (lipid peroxidation), the content of total flavonoids, and 70% of flavonoid monomers. In addition, soluble substance contents and protective enzyme activities showed an upward and subsequent downward trend (Figure 6A). Plants synthesize a large number of soluble substances (soluble sugars, amino acids, and proteins) with short-term UV radiation and provide basic substances for plant metabolism and osmotic adjustment to protect macromolecules and membranes. Soluble substances are the basic nutrients in plants and can reflect plant growth and development status (Ibrahim et al., 2020). Soluble sugars in plants include glucose, fructose, sucrose, and other monosaccharides and oligosaccharides (Malejane et al., 2018). Soluble amino acids comprise alanine, arginine, asparagine, and about 20 kinds of amino acids (Sun et al., 2017). Soluble proteins are proteins that can be dissolved in water or other solvents as small molecules. A variety of plant studies (Chen L. et al., 2020; Wang et al., 2022) have shown that under adverse conditions, accumulated soluble substances can regulate cell fluid concentration to regulate osmotic pressure, indicating stress resistance. In this study, we aimed to determine the state of T. hemsleyanum under UV stress by measuring these three indicators. The flavonoid contents must be increased through UV stress to improve the quality of T. hemsleyanum. We also aimed to maintain its normal growth state while giving stress. The soluble substances mentioned are very important for the growth of T. hemsleyanum and serve as substrates for the generation of secondary metabolites (including flavonoids). However, the correlation between flavonoid content and these substances has not been explored. Meanwhile, SOD, CAT, and POD can remove intracellular reactive oxygen species (ROS) and free radicals. SOD is the first defense line against ROS and converts highly reactive O2– into less toxic H2O2 and O2, whereas CAT and POD convert H2O2 into H2O (Jing et al., 2020). As the final decomposition product of lipid peroxidation and due to the combined mechanisms above, MDA contents reflect the lipid peroxidation level and cellular lipid peroxidation damage to a certain extent (Zeng et al., 2019). Our results were similar to those of other research (Tripathi et al., 2021), short-term UV radiation significantly enhanced the anti-oxidant enzyme systems. Other studies (Li et al., 2019) showed that with further stress, the peroxidation of the membrane system was enhanced, and the MDA content increased. The MDA content can be used as an indicator to assess the resistance. Similar to our results, T. hemsleyanum was irreversibly damaged, and MDA content increased continuously with radiation dose (Figure 6C).
Conclusion
Ultraviolet exposure with different durations or wavelengths can promote the increase in the contents of flavonoids with different structures, which is beneficial to the accurate regulation of special flavonoids in T. hemsleyanum. Appropriate UV-B and UV-C radiation doses (30 min to 3 h) can induce eustress, which can improve bioactive compound contents and protective enzyme system activities for the regulation of physiological state and enhancement of flavonoid accumulations.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
YB and YG contributed equally to this work and contributed to the writing of manuscript. YB designed the research and revised the manuscript. YG, SL, LJ, MH, and DG performed the experiments and disposal the data. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by the Key Research and Development Program of Zhejiang Province (Grant No. 2021C02043), Scientific Research Project of Zhejiang Education Department (Grant No. Y202147328), Forestry Special Fund of Zhejiang Provincial Forestry Bureau (Grant No. lqly2020-03), and Forestry Science and Technology Extension Foundation of Central Government Financial Project (Grant No. 2019TS08).
Acknowledgments
Sincere thanks to Zhe Li, Wen Chen, Dan Chen, Hongxia Sang, and Lijia Chen. They assisted with the part of test and data analysis. Shan Li participated in some revisions of the thesis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2022.926197/full#supplementary-material
References
Agati, G., Biricolti, S., Guidi, L., Ferrini, F., Fini, A., and Tattini, M. (2011). The biosynthesis of flavonoids is enhanced similarly by UV radiation and root zone salinity in L. vulgare leaves. J. Plant Physiol. 168, 204–212.
Bai, Y., Chen, W., Liu, S. Z., Xu, L. Y., and Liu, B. (2021). Physiological Responses of the Tetrastigma hemsleyanum Plant under Different Color Films. HortScience 56, 1–6.
Bondonno, N., Dalgaard, F., Murray, K., Davey, R., Bondonno, C., Cassidy, A., et al. (2021). Higher Habitual Flavonoid Intakes Are Associated with a Lower Incidence of Diabetes. J. Nutr. 151, 3533–3542. doi: 10.1093/jn/nxab269
Chen, C., Chen, H., He, Y., and Xia, R. (2018). TBtools, a toolkit for biologists integrating various biological data handling tools with a user-friendly interface. BioRxiv 289660(10.1101) [preprint].
Chen, L., Liu, L., Lu, B., Ma, T., Jiang, D., Li, J., et al. (2020). Exogenous melatonin promotes seed germination and osmotic regulation under salt stress in cotton (Gossypium hirsutum L.). PLoS One 15:e0228241. doi: 10.1371/journal.pone.0228241
Chen, X., Tao, L., Ru, Y., Weng, S., and Qiu, B. (2019). Antibacterial mechanism of Tetrastigma hemsleyanum Diels et Gilg’s polysaccharides by metabolomics based on HPLC/MS. Int. J. Biol. Macromole. 140, 206–215.
Chen, Y., Shen, Q., Lv, P., and Sun, C. (2020). Dendrobium officinale Kimura et Migo Comparative metabolomic analyses of responding to UV-B radiation reveal variations in the metabolisms associated with its bioactive ingredients. PeerJ 8:e9107. doi: 10.7717/peerj.9107
Chong, J., Othman, S., Li, C., Iurie, C., Li, S., Guillaume, B., et al. (2018). MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 46, W486–W494.
Davies, K. M., Albert, N. W., Zhou, Y., and Schwinn, K. E. (2018). Functions of Flavonoid and Betalain Pigments in Abiotic Stress Tolerance in Plants. Annu. Plant Rev. 1, 21–62.
Dias, M., Pinto, D., Freitas, H., Santos, C., and Silva, A. (2020). The antioxidant system in Olea europaea to enhanced UV-B radiation also depends on flavonoids and secoiridoids. Phytochemistry 170:112199. doi: 10.1016/j.phytochem.2019.112199
Dong, X., Guo, Y., Xiong, C., and Sun, L. (2020). Evaluation of Two Major Rhodiola Species and the Systemic Changing Characteristics of Metabolites of Rhodiola crenulata in Different Altitudes by Chemical Methods Combined with UPLC-QqQ-MS-Based Metabolomics. Molecules 25:4062. doi: 10.3390/molecules25184062
Fan, S., Xie, X., Zeng, F., Zhou, X., Cai, B., Xu, W., et al. (2017). Identification of chemical components and determination of flavonoids in Tetrastigma hemsleyanum leaves. Chin. J. Pharmaceut. Analysis 37, 1481–1488.
Guajardo-Flores, D., Serna-Guerrero, D., Serna-Saldívar, S., and Jacobo-Velázquez, D. (2014). Effect of Germination and UV-C Radiation on the Accumulation of Flavonoids and Saponins in Black Bean Seed Coats. Cereal Chem. 91:276.
He, Q., Ye, X. X., and Xu, B. (2020). Two cases of intergrative medicine treatment of new COVID-19. Chin. J. Int. Tradit. West. Med. 40, 378–379.
Hollman, P. C. H., Bijsman, M. N. C. P., Gameren, Y. V., Cnossen, E. P. J., and Katan, M. B. (2000). The sugar moiety is a major determinant of the absorption of dietary flavonoid glycosides in man. Free Rad. Res. 31, 569–573.
Ibrahim, M. F. M., Elbar, O. H. A., Farag, R., Hikal, M., and El-Gawad, H. G. A. (2020). Melatonin Counteracts Drought Induced Oxidative Damage and Stimulates Growth, Productivity and Fruit Quality Properties of Tomato Plants. Plants 9:1276.
Jasso-Miranda, C., Herrera-Camacho, I., Flores-Mendoza, L. K., Dominguez, F., Vallejo-Ruiz, V., Sanchez-Burgos, G. G., et al. (2019). Antiviral and immunomodulatory effects of polyphenols on macrophages infected with dengue virus serotypes 2 and 3 enhanced or not with antibodies. Infect. Drug Resist. 12, 1833–1852.
Ji, T., Ji, W. W., Wang, J., Chen, H. J., Peng, X., Cheng, K. J., et al. (2021). A comprehensive review on traditional uses, chemical compositions, pharmacology properties and toxicology of Tetrastigma hemsleyanum. J. Ethnopharmacol. 264:113247. doi: 10.1016/j.jep.2020.113247
Jing, J., Guo, S., Li, Y., and Li, W. (2020). The alleviating effect of exogenous polyamines on heat stress susceptibility of different heat resistant wheat (Triticum aestivum L.) varieties. Scient. Rep. 10:7467.
Lee, J. H., Oh, M. M., and Son, K. H. (2019). Short-Term Ultraviolet (UV)-A Light-Emitting Diode (LED) Radiation Improves Biomass and Bioactive Compounds of Kale. Front. Plant Sci. 10:1042. doi: 10.3389/fpls.2019.01042
Lewandowska, U., Szewczyk, K., Hrabec, E., Janecka, A., and Gorlach, S. (2013). Overview of Metabolism and Bioavailability Enhancement of Polyphenols. J. Agricult. Food Chem. 61, 12183–12199. doi: 10.1021/jf404439b
Li, H., Huang, X. Y., Jiang, S. Y., Xiang, Q. Y., and Gan, J. J. (2019). Effects of drought stress on physiological indicators and biomass of Rosa laevigata seedlings. Chin. Tradit. Herbal Drugs 50, 4455–4460.
Liu, J. Q., Gao, Y. F., Zhang, J. Y., Zhou, Y. C., et al. (2022). Chemical Compositions and Anti-tumor Effect of Tetrastigma hemsleyanum:A Review. Chin. J. Exp. Tradit. Med. Formulae 28, 233–241.
Liu, Y., Liu, J., Wang, Y., Abozeid, A., Tian, D., Zhang, X., et al. (2018). The Different Resistance of Two Astragalus Plants to UV-B Stress is Tightly Associated with the Organ-specific Isoflavone Metabolism. Photochem. Photobiol. 94, 115–125. doi: 10.1111/php.12841
Ma, M., Wang, P., Yang, R., Zhou, T., and Gu, Z. (2019). UV-B mediates isoflavone accumulation and oxidative-antioxidant system responses in germinating soybean. Food Chem. 275, 628–636. doi: 10.1016/j.foodchem.2018.09.158
Maan, G., Sikdar, B., Kumar, A., Shukla, R., and Mishra, A. (2020). Role of Flavonoids in Neurodegenerative Diseases: Limitations and Future Perspectives. Curr. Top. Med. Chem. 20, 1169–1194. doi: 10.2174/1568026620666200416085330
Malejane, D., Tinyani, P., Soundy, P., Sultanbawa, Y., and Sivakumar, D. (2018). Deficit irrigation improves phenolic content and antioxidant activity in leafy lettuce varieties. Food Sci. Nutr. 6, 334–341. doi: 10.1002/fsn3.559
Mao, P., Duan, F., Zheng, Y., and Yang, Q. (2021). Blue andUV-Alight wavelengths positively affected accumulation profiles of healthy compounds in pak-choi. J. Sci. Food Agricult. 101, 1676–1684. doi: 10.1002/jsfa.10788
Marinova, K., Kleinschmidt, K., Weissenböck, G., and Klein, M. (2007). Flavonoid biosynthesis in barley primary leaves requires the presence of the vacuole and controls the activity of vacuolar flavonoid transport. Plant Physiol. 144:432.
Müller-Xing, R., Xing, Q., and Goodrich, J. (2014). Footprints of the sun: memory of UV and light stress in plants. Front. Plant Sci. 5:474. doi: 10.3389/fpls.2014.00474
Murai, Y., Takemura, S., Takeda, K., Kitajima, J., and Iwashina, T. (2009). Altitudinal variation of UV-absorbing compounds in Plantago asiatica. Biochem. Systemat. Ecol. 37, 378–384.
Nakabayashi, R., Yonekura-Sakakibara, K., Urano, K., Suzuki, M., Yamada, Y., Nishizawa, T., et al. (2014). Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. Plant J. 77, 367–379.
Rao, M. J., Xu, Y., Huang, Y., Tang, X., and Xu, Q. (2019). Ectopic expression of citrus UDP-GLUCOSYL TRANSFERASE gene enhances anthocyanin and proanthocyanidins contents and confers high light tolerance in Arabidopsis. BMC Plant Biol. 19:603. doi: 10.1186/s12870-019-2212-1
Rice-Evans, C. A., Miller, N. J., Bolwell, P. G., Bramley, P. M., and Pridham, J. B. (1996). The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Rad. Res. Commun. 22, 375–383.
Ryan, K. G., Markham, K. R., Bloor, S. J., Bradley, J. M., Mitchell, K. A., and Jordan, B. R. (1998). UVB Radiation Induced Increase in Quercetin: Kaempferol Ratio in Wild-Type and Transgenic Lines of Petunia. Photochem. Photobiol. 68, 323–330.
Sakina, R., Kandikattu, K., Ilaiyaraja, N., Mahadeva, N., and Farhath, K. (2015). Antioxidant, Biomolecule Oxidation Protective Activities of Nardostachys jatamansi DC and Its Phytochemical Analysis by RP-HPLC and GC-MS. Antioxidants 4, 185–203.
Shi, Y., Yang, L., Yu, M., Li, Z., Ke, Z., Qian, X., et al. (2022). Seasonal variation influences flavonoid biosynthesis path and content, and antioxidant activity of metabolites in Tetrastigma hemsleyanum Diels & Gilg. PLoS One 17:e0265954. doi: 10.1371/journal.pone.0265954
Sun, L., Liu, Q., Bao, C., and Fan, J. (2017). Comparison of Free Total Amino Acid Compositions and Their Functional Classifications in 13 Wild Edible Mushrooms. Molecules 22:350.
Szopa, A., Starzec, A., and Ekiert, H. (2018). The importance of monochromatic lights in the production of phenolic acids and flavonoids in shoot cultures of Aronia melanocarpa, Aronia arbutifolia and Aronia × prunifolia. J. Photochem. Photobiol. B 179, 91–97.
Tripathi, D., Meena, R. P., and Pandey-Rai, S. (2021). Short term UV-B radiation mediated modulation of physiological traits and withanolides production in Withania coagulans (L.) Dunal under in-vitro condition. Physiol. Mole. Biol. Plants 27, 1823–1835.
Wang, G., Wang, X., Ma, H., Fan, H., Lin, F., Chen, J., et al. (2022). PcWRKY11, an II-d WRKY Transcription Factor from Polygonum cuspidatum, Enhances Salt Tolerance in Transgenic Arabidopsis thaliana. Int. J. Mol. Sci. 23:4357. doi: 10.3390/ijms23084357
Wu, B., Gao, L., Gao, J., Xu, Y., Liu, H., Cao, X., et al. (2017). Genome-Wide Identification, Expression Patterns, and Functional Analysis of UDP Glycosyltransferase Family in Peach (Prunus persica L. Batsch). Front. Plant Sci. 8:389. doi: 10.3389/fpls.2017.00389
Xin, W., Li, C., Chen, Z., Jia, L., and Zhang, Y. (2017). Molecular characterization of the C-glucosylation for puerarin biosynthesis in Pueraria lobata. Plant J. 90, 535–546.
Xu, G., Cao, J., Wang, X., Chen, Q., Jin, W., Li, Z., et al. (2019). Evolutionary Metabolomics Identifies Substantial Metabolic Divergence between Maize and Its Wild Ancestor Teosinte. Plant Cell 31, 1990–2009.
Ye, C., and Liu, X. (2015). Extraction of Flavonoids from Tetrastigma hemsleyanum Diels Et Gilg and Their Antioxidant Activity. J. Food Proc. Preservat. 39, 2197–2205.
Zaragozá, C., Villaescusa, L., Monserrat, J., Zaragozá, F., and Álvarez-Mon, M. J. M. (2020). Potential Therapeutic Anti-Inflammatory and Immunomodulatory Effects of Dihydroflavones, Flavones, and Flavonols. Molecules 25:1017. doi: 10.3390/molecules25041017
Zeng, W., Peng, Y., Zhao, X., Wu, B., and Ding, Y. (2019). Comparative Proteomics Analysis of the Seedling Root Response of Drought-sensitive and Drought-tolerant Maize Varieties to Drought Stress. Int. J. Mol. Sci. 20:2793.
Zhang, L., Li, B., Wang, M., Lin, H., Peng, Y., Zhou, X., et al. (2022). Genus Tetrastigma: A review of its folk uses, phytochemistry and pharmacology. Chin. Herbal Med. 14, 210–233.
Zhang, L., Li, X., Ma, B., Gao, Q., Du, H., Han, Y., et al. (2017). The Tartary Buckwheat Genome Provides Insightsinto Rutin Biosynthesis and Abiotic StressTolerance. Mol. Plant 10, 1224–1237.
Zhang, X., Ding, X., Ji, Y., Wang, S., Chen, Y., Luo, J., et al. (2018). Measurement of metabolite variations and analysis of related gene expression in Chinese liquorice (Glycyrrhiza uralensis) plants under UV-B irradiation. Scientif. Rep. 8:6144. doi: 10.1038/s41598-018-24284-4
Zhu, R., Xu, X., Ying, J., Cao, G., and Wu, X. (2020). The Phytochemistry, Pharmacology, and Quality Control of Tetrastigma hemsleyanum Diels & Gilg in China: A Review. Front. Pharmacol. 11:550497. doi: 10.3389/fphar.2020.550497
Keywords: Tetrastigma hemsleyanum Diels et Gilg, flavonoid, ultraviolet radiation, metabolic expression, physiological response
Citation: Bai Y, Gu Y, Liu S, Jiang L, Han M and Geng D (2022) Flavonoids metabolism and physiological response to ultraviolet treatments in Tetrastigma hemsleyanum Diels et Gilg. Front. Plant Sci. 13:926197. doi: 10.3389/fpls.2022.926197
Received: 22 April 2022; Accepted: 15 August 2022;
Published: 15 September 2022.
Edited by:
Marco Landi, University of Pisa, ItalyReviewed by:
Kollipara P. M. S. V. Padmasree, University of Hyderabad, IndiaJaime Barros-Rios, University of Missouri, United States
Copyright © 2022 Bai, Gu, Liu, Jiang, Han and Geng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Bai, aHpiYWl5YW5AemFmdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Yan Bai1,2,3*†
Yan Bai1,2,3*† Yiwen Gu
Yiwen Gu