- 1Department of Environmental, Biological and Pharmaceutical Sciences and Technologies, University of Campania Luigi Vanvitelli, Caserta, Italy
- 2Department of Agricultural Sciences, University of Naples Federico II, Naples, Italy
Over the past five decades, nitrogen (N) fertilization has been an essential tool for boosting crop productivity in agricultural systems. To avoid N pollution while preserving the crop yields and profit margins for farmers, the scientific community is searching for eco-sustainable strategies aimed at increasing plants’ nitrogen use efficiency (NUE). The present article provides a refined definition of the NUE based on the two important physiological factors (N-uptake and N-utilization efficiency). The diverse molecular and physiological mechanisms underlying the processes of N assimilation, translocation, transport, accumulation, and reallocation are revisited and critically discussed. The review concludes by examining the N uptake and NUE in tandem with chloride stress and eustress, the latter being a new approach toward enhancing productivity and functional quality of the horticultural crops, particularly facilitated by soilless cultivation.
Introduction
For agricultural cropping systems, nitrogen (N) fertilization has been represented as a useful tool to improve plant growth, yield components, and quality. The high-energy cost for N fertilizer synthesis as well as its intrinsic mobility in the complex atmosphere–plant–soil system have highlighted the environmental drawbacks of the unsustainable N use (Keeney and Hatfield, 2001; Rothstein, 2007; Garnett et al., 2009; Chen K. E. et al., 2020; Bijay and Craswell, 2021). In this respect, there is a growing interest in the improvement of nitrogen use efficiency (NUE), especially for the horticulture crops, which are notoriously characterized by excessive and unjustified N “consumption” (Carillo et al., 2019a). NUE depends on the N-uptake efficiency, the amount of N consumed by the crop per unit of available N, the N utilization efficiency, and the harvestable product per unit of N uptake (Moll et al., 1982; Sisson et al., 1991). Nitrate (NO3–) is the main source of N in plants, but its concentration in the soil can fluctuate dramatically due to either time or space, thus becoming one of the main factors limiting the crop growth and development (Gojon et al., 2011). In fact, NO3– concentration is highly variable, ranging from 0.1 to 10 mM, depending on the soil process dominating at that time point, such as bursts of nitrification, leaching process intensification, or fertilization (Crawford and Glass, 1998; Miller et al., 2007). The importance of NO3– is attributed to its signaling role, which can trigger specific responses at different levels (cellular, biochemical, and molecular) and induce gene expression regulating its own uptake. NO3– assimilation, driven by the enzyme nitrate reductase (NR), involves the uptake of NO3–, its reduction to nitrite (NO2–) and then to ammonium (NH4+), and finally its incorporation into the organic compounds. Plants can adjust their capacity to acquire NO3– by reshaping the root architecture to enhance NO3– uptake and by modulating the NO3–-assimilation pathway. The comprehension of this nutrient acquisition response mechanism could help to improve the plants’ NUE. Significantly, if NO3– uptake exceeds the assimilative capacity of the plant, it can accumulate in the plant tissues, which in the case of leafy vegetables can be unsafe (Gupta et al., 2017). Human gastrointestinal metabolism reduces NO3– to NO2–, which, when reacting with N-based organic compounds, can form compounds with recognized carcinogenic action (Santamaria, 2006; Colla et al., 2018). As a preventive measure, the European Commission regulations n° 1881/2006 and 1258/2011 have de facto set a maximum NO3– threshold for leafy vegetables, such as spinach (Spinacia oleracea L.), lettuce (Lactuca sativa L.), and rocket (Eruca vesicaria L.). In this perspective, more and more studies have focused not only on the role of genetics, physiological status, timing, and the form of N application but also especially on the search for alternative horticultural and agronomic practices that can limit NO3– accumulation without compromising the product performance, such as the use of salinity as eustressor (Rouphael et al., 2018), the modified intermittent nutrient film technique (NFT) (Tabaglio et al., 2020), or the hydroponics to constantly monitor the nutrient solution (Ciriello et al., 2021). Therefore, it is not surprising that soilless systems, due to the potential to modulate and control a plant’s nutritional status, could be used to induce positive stress (eustress) that can limit the excessive accumulation of NO3– (Lucini et al., 2016; Rouphael and Kyriacou, 2018). NO3– uptake can be affected by Cl– that can indirectly interfere with the NO3–-uptake mechanisms by decreasing the internal demand for NO3– and consequently its utilization efficiency. However, although NO3–-uptake pathways and Cl–-inhibitory effects are well-documented, the possible implications of their interaction and the resulting impacts (negative or positive) on vegetables have not been clarified. Maximizing NUE in future agricultural systems requires an understanding of the diverse genetic and physiological mechanisms underlying the processes of N assimilation, translocation, transport, accumulation, and reallocation. In fact, a complete understanding of these processes will allow the implementation of efficient strategies. Our review aimed to elucidate these crucial mechanisms that are directly involved in N metabolism and also describe the possibility of using chloride eustress to improve NUE while reducing NO3– accumulation in the leafy vegetables.
Nitrogen Use Efficiency
The basic concept of NUE describes the yield of a harvestable product (dry matter) per unit N available or even the grain yield (kg) per unit (kg) of total available N (applied N + soil mineral N) (Hirose and Kitajima, 1986). However, NUE depends not only on the plant N uptake efficiency (NUpE, kg kg–1) but also on its assimilation and translocation and, in aged plants, on recycling and remobilization and therefore on N utilization efficiency (NUtE, kg kg–1) (Moll et al., 1982; Masclaux-Daubresse et al., 2010; Xu et al., 2012; Hawkesford et al., 2014). NutE, in particular, concerns the capacity of a certain species or cultivar to convert the assimilated/remobilized N in biomass (grain, leaves, and fruit) and, in the end, will be equal to the harvestable product per unit of N consumed by a crop (Todeschini et al., 2016). Indeed, an efficient N application helps decrease N losses from the soil–plant system, increasing NUpE and NutE, and therefore the amount of agricultural output (Li et al., 2018).
In the last decades, the increase of NUE (NUpE + NUtE) has been considered a focus to reduce the use of N fertilizers and minimize their cost and environmental impact (Hirose, 2011). In fact, plants can absorb only 30–50% of the approximately 110 million tons of N fertilizers spread over the fields every year, losing the rest due to surface runoff, leaching, volatilization, microbial consumption, and denitrification (Garnett et al., 2009; Chen K. E. et al., 2020).
In this scenario, the horticulture production of vegetable crops, which have high economic and nutritious value, entails the highest use of chemicals (in particular N) per unit area than any other agricultural system, causing high costs and environmental pollution (Carillo et al., 2019a). Moreover, the horticultural production systems are more prone to N losses than grain crop systems because of the higher rates of N fertilizer used and the shallow root systems of the horticultural plants compared to arable plants (Cameron et al., 2013). Therefore, there is a high interest in the field of horticultural science in reducing N inputs and improving NUE for the production of vegetable crops by selecting new genotypes, mostly by exploiting genetic variation in the available germplasm, understanding the physiological mechanisms involved in these mechanisms, and finding new management practices for the existing crops. The increase of NUE by only 1% may save USD$1.1 billion (Van Oosten et al., 2019) and can also reduce nitrous oxide emissions.
Nitrate Effect on the Root Development
Greenhouse horticulture is the best example of excessive NO3–/resource intensive agriculture, requiring the highest use of N/NO3– per unit area compared to other agricultural systems, resulting in high financial costs and environmental risks for the high N losses (Carillo et al., 2019a). However, until now, horticultural plants have been grown nearly under non-limiting N conditions, because the attempts to reduce N fertilization resulted in reduced plant growth and poor yield (Masclaux-Daubresse et al., 2010). N, in fact, is of pivotal importance in the plant’s metabolism. NR, the first enzyme in the NO3– assimilation pathway catalyzing the reduction of NO3– in NH4+, is strictly dependent on the plant NO3– flux from roots and in general on NO3– availability at the cellular level (Carillo et al., 2005; Annunziata et al., 2017). This enzyme represents the limiting step in the overall process of plant growth and productivity (Kaiser et al., 1999). NO3– is required for full levels of NR gene expression, as signals from N metabolism play an important role in inducing the expression of the NR gene Nia (Oaks, 1974).
Plants can modulate their NO3– uptake, storage, and assimilation according to the internal and external spatio-temporal changes in N status by modulating the type, number, spatial pattern, and affinity of hundreds of genes expressing NO3– transporters (Forde, 2002; Orsel et al., 2002; Bouguyon et al., 2012; Boer et al., 2020) in addition to extensively re-shaping the root system architecture (RSA) (Aibara and Miwa, 2014). In fact, low N status can upregulate NO3– uptake system (Nacry et al., 2013) and modify plant root architecture, increasing root length, density, and branching, thus resulting in a “nutrient acquisition response” improving NUE. Depending on its availability and distribution, NO3– can have both positive and negative effects on the development and growth of the lateral roots (Zhang et al., 1999; Nacry et al., 2013). In fact, it was demonstrated that, when Arabidopsis roots were exposed to a locally concentrated supply of NO3–, there was no increase in the lateral roots numbers but a 2-fold increase of elongation caused by an enhanced cell production in the lateral root meristem (Zhang et al., 1999). Other locally applied N sources, like NH4+, can promote lateral root branching but not elongation (Lima et al., 2010), proving that NO3 acts as a signal probably interacting and/or interfering with auxin response pathways (Zhang et al., 1999). The phenotypic plasticity of plants, which makes roots to grow preferentially toward NO3–-richer zones at low NO3–, is termed “root foraging”; whereas NO3– has been defined as an “environmental morphogen” for its ability to modulate the root architecture and root foraging (Giehl and von Wirén, 2014; Guan et al., 2014; Boer et al., 2020). The foraging response at low NO3– that entails root growth is exerted through the overexpression of the (i) TRYPTOPHAN AMINOTRANSFERASE-RELATED PROTEIN 2 (TAR2), involved in local auxin biosynthesis; (ii)WALL-ASSOCIATED KINASE 4 (WAK4); and iii) MULTIDRUG RESISTANCE4/P-GLYCOPROTEIN 4 (MDR4/PGP4), a downstream transporter of auxin (Giehl and von Wirén, 2014; Ma et al., 2014; Sun et al., 2017). Cytokinin signaling is also involved in the NO3– foraging response; in fact, this hormone is synthetized in a NO3–-dependent manner and is translocated to shoot, where it induces the expression of the genes involved in a complex long-distance root–shoot–root signaling network entailing root foraging (Poitout et al., 2018; Roy, 2018).
Giehl and von Wirén (2014) observed a continuous root growth response when plants grew in a homogeneous external NO3– concentration but a repressing surviving response at severely low NO3– concentration. This surviving response is regulated by the N-responsive CLAVATA3/ESR-related (CLE) signaling peptides and their receptor protein CLAVATA1 (CLV1) (Araya et al., 2014). Moreover, since there is upregulation of the dual-affinity NO3– transporter NRT1.1 in the lateral roots at a very low NO3– concentration, which acts as an auxin importer at low external NO3– levels, this determines a shootward movement of auxin that strongly decreases the concentration of this hormone in the lateral root tissues, consequently inhibiting the lateral root growth (Krouk et al., 2010; Giehl and von Wirén, 2014). At high NO3– concentrations, NRT1.1 is not able to transport auxin, thus does not decreasing the lateral root growth (Krouk et al., 2010); while it again starts to transport auxin at very high levels of NO3–, stimulated by a signaling pathway modulated by the (i) protein AUXIN SIGNALING F-BOX 3 (AFB3), (ii) NAC4 transcription factor, and (iii) OBF Binding Protein 4 (OBP4), exerting a repression response, which determines inhibition of root growth, cell elongation, and differentiation (Vidal et al., 2013).
On the contrary, NRT2.1 in rice regulates a NO3–-dependent root elongation involving auxin transport to roots; this mechanism appears related to the NO3–-dependent production of NO that upregulates PIN-FORMED 1 (PIN1), a key mediator of basipetal polar auxin transport in the cell roots, which promotes a reorientation of auxin transport toward the tip of the newly developing root (Naz et al., 2019).
Nitrate Transporters and Sensing
NO3– is consumed by the roots and mobilized to the other organs by NO3– transporters, which display a bi-phasic pattern (Crawford and Glass, 1998). In the low concentration range, a high-affinity transport system (HATS) can uptake NO3– from the soil at concentrations of 10-250 μM with an activity fitting the Michaelis-Menten kinetic model (Filleur et al., 2001). The HATS has both a constitutive component (cHATS) and a NO3–-inducible component (iHATS), whose Vmax was 30-fold higher than the cHATS one (Zhuo et al., 1999; Li et al., 2007; Gao et al., 2019). Whereas, starting from the concentration of about 0.5 mM, NO3– uptake is performed by two high-affinity transport systems (LATS) that substitute/superimpose the HATS: one is constitutive (cLATS), which does not show saturation even at 50 mM external NO3–, and the other is inducible (iLATS) (Liu et al., 1999; Zhuo et al., 1999; Forde, 2000). Both HATS and LATS proceed thermodynamically uphill since the uptake of NO3– is depressed or inhibited by processes that decrease or inhibit the synthesis of ATP and proteins (Peuke and Jeschke, 1998). NO3– uptake, in fact, is an energy-dependent transport consistent with NO3–: 2 H+ symport that requires the creation of an H+ electrochemical gradient, generated by a proton translocation coupled to ATP hydrolysis (Crawford and Glass, 1998; Forde, 2000). In addition, plants show an inducible NO3–-efflux system with a much slower turnover rate than the uptake system, which requires RNA and protein synthesis (Aslam et al., 1996).
The first NO3–-transporter gene identified in plants belonging to LATS was the AtNRT1.1 gene, originally named CHLORINA1 (CHL1) because it was associated with chloride (Cl–) sensitivity in Arabidopsis (Huang et al., 1996; Liu et al., 1999) but now known as AtNPF6.3. Subsequently, it was found that NRT1.1 also functions as a HAT at low NO3–levels; therefore, it is a dual-affinity transporter that can facilitate NO3– uptake at concentrations ranging from micromolar to millimolar (Liu et al., 1999). NRT1.1 has been demonstrated to contribute to over 75% of the high-affinity NO3– uptake in plants (Wang et al., 1998). It is involved in the NO3– uptake and transport (Liu et al., 1999), auxin transport activity (Krouk et al., 2010; Bouguyon et al., 2016), NO3–-modulated root development (Bouguyon et al., 2016; Albornoz et al., 2018), NO3– sensing (Miller et al., 2007), and growth improvement under N deficiency stress (Ho et al., 2009; Bouguyon et al., 2012, 2016). NRT1.1 has been defined as a moonlighting protein because it performs more than a single function (Fichtner et al., 2021) and also as a transceptor because it has transporter and receptor roles (Gojon et al., 2011). The intermediates of the oxidative pentose phosphate pathway regulate its expression and consequently root N levels (Lejay et al., 2008; Table 1).
AtNRT2.1 (ACH1) is another HAT regulated by external NO3– (Filleur et al., 2001), N starvation (Li et al., 2007), sucrose, and light (circadian or diurnal regulation) (Lejay et al., 1999; de Jong et al., 2013). It is downregulated by NH4+, amino acids, N-metabolites resulting from NO3– reduction, and dark (de Jong et al., 2013). It does not mediate transport on its own but functions as a dual-component transporter with NTR3.1 (Tong et al., 2005). It inhibits the lateral root initiation under high-sucrose/low-NO3– conditions (Little et al., 2005). It works as a central player in the integration of C- and N-metabolisms and is transcriptionally and post-transcriptionally regulated by C- and N-derived metabolites (de Jong et al., 2013). NRT2.1, NRT2.2, and NRT2.4 are required to ensure an optimal adaptation to N limitation (Kiba et al., 2012). OsNRT2.1 is involved in NO3–-dependent root elongation in Oryza sativa by regulating polar auxin transport to the roots (Naz et al., 2019; Table 1).
NRT2.2 (ACH2) acts as a dual-component transporter with NAR2.1 importing NO3– with high affinity; plants over-expressing NRT2.2 increase their growth under low NO3– conditions (Filleur et al., 2001). NRT2.3 acts as a dual-component transporter with NAR2.1 undergoing circadian regulation with a peak in the middle of the morning and at the end of the light period and downregulation by NH4+ and NH4+-derived metabolites (Feng et al., 2011). It has a key role in long-distance NO3– transport from roots to shoots, particularly at low external NO3– supply (Fu et al., 2015). Its co-overexpression with NAR2.1 may increase rice yield and NUE (Chen J. et al., 2020). OsNRT2.3a plays a key role in root to shoot NO3– translocation under N-limiting conditions (Tang et al., 2012). The overexpression of OsNRT2.3b has also been correlated with high grain yield and NUE in rice (Sandhu et al., 2021; Table 1).
NO3– is an important signal molecule that can trigger a range of responses at the molecular, biochemical, and cellular levels in the plant roots (Bouguyon et al., 2012). NO3– induces the expression and/or the transcription of the genes involved in its own uptake (e.g., HATS), whereas the addition of NH4+ or glutamine leads to a decrease in transcripts for the transporter system (Sanz-Luque et al., 2015). NO3– is also an important determinant for the induction of the NR genes NIA, and for the stability of the NR transcripts (Galangau et al., 1988; Foyer et al., 1998; Konishi and Yanagisawa, 2013).
In particular, the NIA1 encodes the cytosolic NADH-NR1, an enzyme present throughout the life cycle of plants being predominantly active in leaves in which it accounts for 10–15% of NO3– reductive assimilation (Olas and Wahl, 2019). When the NIA1 is mutated, it confers resistance to the herbicide chlorate (Wilkinson and Crawford, 1993). The biosynthesis of NADH-NR1 is activated by NO3– sumoylation (modulation by a small ubiquitin-related modifier, SUMO) and cytokinins (Yu et al., 1998; North et al., 2009; Park et al., 2011). NIA2 encodes for an NADH-NR 2 and is responsible for 90% of the total NR activity in seedlings. NIA2 complements NIA1 in the same organs and tissues and is involved in NO3– assimilation (Wilkinson and Crawford, 1991; Olas and Wahl, 2019), in response to light mediated by phytochrome and blue-light photoreceptors (Migge et al., 1998; Lillo and Appenroth, 2001), and in response to symbiotic fungi (Sherameti et al., 2005). Its transcript is present throughout the life cycle of plants being predominantly active in the meristematic tissues (Olas and Wahl, 2019). Both NIA1 and NIA2 are critical in nitric oxide (NO) production and are involved in the abscisic acid (ABA)-induced stomatal closure (Sun et al., 2015; Zhao et al., 2016; Costa-Broseta et al., 2020; Table 1). In rice, the NO produced by the NR pathway has a key role in improving the NUE by increasing the lateral roots initiation and inorganic N uptake rate, allowing plants to adjust plant NO3– acquisition to the fluctuating availability (Sun et al., 2015). The NR-dependent NO production is also critical for disease resistance; in fact, NO, in combination with H2O2, has a very efficient and cost-effective microbicidal effect that can reduce the energy expenditure associated with salicylic acid (SA)-mediated defense response (Vitor et al., 2013).
The supply of NO3– and/or metabolites formed during the NO3– assimilation can activate phosphoenolpyruvate carboxylase (PEPCase) and inactivate the sucrose phosphate synthase (SPS) activity (Scheible et al., 1997). Nitrate can also induce the expression of genes and enzymes involved in the NH4+ assimilation (e.g., root glutamine synthetase, GS) and increase the synthesis of organic acids which are useful as carbon skeletons for amino acids synthesis or as counter-anions (Scheible et al., 1997; Garnica et al., 2010; Sanz-Luque et al., 2015). Glutamine and NH4+ have roles in the feedback repression of NO3– uptake and assimilation (Stitt et al., 2002; Masclaux-Daubresse et al., 2010; Nacry et al., 2013). However, the presence of NR and/or its metabolism’s products are not required for NO3– sensing (Scheible et al., 1997). Fluctuations in the levels of NO3– may affect the biosynthesis of carbohydrates and vice versa; in fact, NO3– may inhibit the synthesis of starch (Foyer and Paul, 2001; Stitt et al., 2002; Fichtner et al., 2021) and modulate the carbohydrates allocation and development system (Wang et al., 2012; O’Brien et al., 2016).
Light may stimulate the root uptake of NO3– by a modulation effect exerted by recent photosynthates transported from shoots to roots, with a diurnal rhythm of NO3– peaking during the light period, while getting a minimum in the dark (Peuke and Jeschke, 1998; Lejay et al., 1999; Ruffel et al., 2014). Sucrose may replace the light-mediated response on NO3– uptake (Zhou et al., 2009). The extent of NO3– uptake and the modulation of the pH of the xylem sap may have a role in stomatal regulation by the delivery of ABA to guard cells (Gloser et al., 2020).
Nitrate Transport, Accumulation, and Re-Allocation
Nitrate can be accumulated or reduced and assimilated into amino acids in roots and/or in shoots, after being transported via xylem. If NO3– remains in the cytoplasm, it is rapidly reduced to NO3– and then assimilated; thus, the concentration of NO3– in plant tissues is modulated by the ratio of the distribution of NO3– between the cytoplasm and the vacuole (Liang and Zhang, 2020). Arabidopsis thaliana tonoplast Cl– channel an (AtCLCa) accumulation of NO3–, specifically in the vacuole and behaves as a NO3–/H+ exchanger, actively mediating the relative amounts of cytoplasm and vacuole NO3– reservoirs (De Angeli et al., 2006). Han et al. (2016) demonstrated that a decrease in the vacuolar sequestration capacity of NO3– in the roots of Brassica napus may enhance the transport to shoots contributing to the increase in NUE by promoting NO3– allocation to the aerial parts. Nitrate stored in the vacuole can be used for assimilation, serving as a reservoir to support the growth when the external N supply gets limited (Leij et al., 1998).
Nitrate remobilization from vacuoles to other plant tissues/organs is a key component of NUE (Chen K. E. et al., 2020). NPF5.11, NPF5.12, and NPF5.16 vacuolar NO3– efflux transporters in Arabidopsis may act for up taking NO3– from the vacuoles to the cytosol, thus functioning as important modulators of NO3– allocation between roots and shoots (He et al., 2017). Thus, the finding that the cytosolic concentration of NO3– is maintained constant and that surplus NO3– is accumulated in the vacuole implies that NO3– regulates the activity of the transport system on the tonoplast (Scheible et al., 1997). Moreover, since xylem transport is controlled by transpiration, expanded leaves that have a larger transpiration surface may obtain higher amounts of NO3– (Chen K. E. et al., 2020). The low-affinity NO3– transporters in Arabidopsis, NRT1.11 and NRT1.12 (also known as NPF1.2 and NPF1.1, respectively) expressed in the companion cells of the source leaves, are responsible for NO3– transport from the xylem to the phloem, thus lowering its concentration in the xylem stream and promoting nitrate transport to the younger leaves via the phloem (Hsu and Tsay, 2013).
The re-allocation of nitrate from source to sink tissues is of pivotal importance for improving the plant growth also under high nitrate concentration. NRT1.7, another NO3– transporter, is involved in the loading of excess NO3– present in the source leaves into the phloem, promoting NO3– re-allocation to sink leaves. Under low NO3–, the nrt1.7 mutant shows retardation of growth, demonstrating that NRT1.7-dependent NO3– remobilization from source to sink tissues is essential to sustain plants’ growth (Chen K. E. et al., 2020).
Indeed, efficient uptake, assimilation, and re-mobilization of NO3– are crucial for plant growth; however, at plant maturity, accumulation of NO3– in the vacuole of some plants, especially leafy vegetables supplied with nitrate exceeding plant demand, may be considered dangerous (Martinoia et al., 1981). Vegetables represent the main source of the dietary NO3– for humans, accounting for about 72–94% of the total intake (Dich et al., 1996). When NO3– accumulation in the edible plant tissues exceeds the maximum residue levels (MRLs), it exerts serious ill-effects on human health (Gupta et al., 2017). In fact, it can be reduced to NO2– by gastrointestinal microflora, leading to methemoglobinemia in children (Blue Baby Syndrome) (Aires et al., 2013; Colla et al., 2018; Kyriacou and Rouphael, 2018). Nitrite can react with amines and amides forming N-nitroso compounds (NOCs), categorized as “probably carcinogenic to humans” and linked to nasopharyngeal, esophageal, gastric, and colon cancers (Santamaria, 2006; Colla et al., 2018). Therefore, NO3– content must be accurately monitored in leafy vegetables and composed lower than the limits imposed by EU regulation no. 1258/2011 (Giro and Ferrante, 2016).
Chloride Interactions With Nitrate Uptake
Cl– in excess can strongly reduce NUE specifically interfering with its uptake, transport, and loading into the root xylem, since it uses the same anion channels used by NO3– (Diatloff et al., 2004; Carillo et al., 2005). The species’ sensitivity to salinity can be related to the Cl–-specific capacity of interference with their NO3– uptake systems (Leidi and Lips, 2004). The Cl–-dependent reduction of cellular concentrations of NO3– may indirectly downregulate the internal demand of NO3– and consequently its uptake (Glass et al., 2002; O’Brien et al., 2016). In fact, as mentioned above, NO3– may induce the expression and transcription of genes involved in its assimilation and transport, in addition to the genes involved in the energy and carbon metabolism (Galangau et al., 1988; Foyer et al., 1998; Goel et al., 2016; Zhao et al., 2018). Moreover, the decrease of NO3– levels may cause the proteolysis of plastid proteins and the remobilization of metabolites (including amino acids) from old to young leaves, quickening the yellowing and senescence of older leaves (Soltabayeva et al., 2018; Carillo et al., 2019a).
When Cl– decreases the NO3– transport to the root xylem, its loading to shoot is increased simultaneously, determining the presence of toxic Cl– levels that further impair the plant metabolism (Munns and Tester, 2008; Carillo et al., 2019a). Mild to moderate concentrations of Cl– may be toxic, exerting more severe ion imbalance and hyperosmotic stress than that of Na+ in several horticultural species, with consequent reduction of plant growth and yield (Colla et al., 2013; Cirillo et al., 2019). In fact, at a concentration of 4–7 mg g–1 DW, Cl– may be more toxic than sodium for Cl–-sensitive species, like herbaceous perennial plants (Cirillo et al., 2019), and at concentrations of 15–50 mg g–1 DW, it also proved to be toxic for Cl–-tolerant species if abruptly applied to the soil in a short time (Tavakkoli et al., 2010; Colmenero-Flores et al., 2019). Indeed, Cl–, as an essential micronutrient, at concentrations lower than 4 mg g–1, is involved in turgor and pH regulation and may act as a counteranion in the stabilization of membrane potential, a regulator of cytosolic enzymatic activities, and a co-factor of the photosynthetic water-splitting complex (White and Broadley, 2001; Geilfus, 2018). For this reason, under low Cl– levels, this ion is actively uptaken by a secondary active Cl–/2H+ symport (Felle, 1994). However, recent reports have shown that prolonged exposures to nutrient solutions containing Cl– at concentrations of 4–5 mM may cause a gradual non-toxic accumulation of Cl– at values ranging between 25 and 50 mg g–1 DW (macronutrient levels), which still allows plants to grow without apparent stress symptoms (Colmenero-Flores et al., 2019). Raven (2016) and Franco-Navarro et al. (2016) had already reported that the application of Cl– at 1–5 mM concentrations could help plants to maintain positive turgor pressure, regulate osmotic potential, and decrease stomatal conductance and transpiration, while improving water use and photosynthetic efficiency. Wege et al. (2017), reviewing the different routes taken by Cl– in plants, suggested that the energy costs associated with uptake and storage of Cl– in the vacuole for turgor maintenance are lower than those associated with NO3– because Cl– does not require the expense of ATP for proton gradient. Franco-Navarro et al. (2019) showed that Cl–, as a beneficial macronutrient, stimulated the formation of larger leaf cells with a lower stomatal density, thus indirectly decreasing the stomatal conductance and water consumption. At the same time, the increase in the surface area of chloroplasts exposed to the intercellular airspace of mesophyll cells facilitated CO2 exchanges and photosynthetic performance (Franco-Navarro et al., 2019). This new finding of Cl– as a beneficial macronutrient has therefore been confirmed by several studies and has been included in the fourth edition of the Marschner’s Mineral Nutrition of Higher Plants book (Rengel et al., 2022).
When Cl– is in excess, it is passively transported into the root cortical cells and the xylem by anion channels such as the NO3– transporter NPF7.3 (Lin et al., 2008) and the S-type anion heteromeric channel SLAH1/SLAH3 (Qiu et al., 2016). High Cl– concentrations at the leaf level turn out less controlled and more dangerous than those of sodium due to the lower capacity of leaf blades to exclude Cl– (Munns and Tester, 2008; Colla et al., 2013) and its limited basipetal phloem transport toward the roots (Munns, 2002; Geilfus, 2018). When Cl– is accumulated in high concentration in the leaf tissues, it initially decreases the apoplast osmotic potential interfering with the cellular water relations (Geilfus, 2018). Thereafter, it diffuses into the symplast by using anion (e.g., nitrate and phosphate) uptake symporters competing with these beneficial nutrients for the uptake within the cell (Carillo et al., 2005; Griffiths and York, 2020). High levels of cytosolic Cl– exceed the Cl– homeostatic control, causing a higher efflux of this ion into the chloroplasts and mitochondria, thus impairing the photosynthetic and mitochondrial electron transport chains and causing ROS formation (Tavakkoli et al., 2010). In these conditions, older leaves, at first, start showing necrosis symptoms at the leaf margins and tips (Ayers and Westcot, 1985; Geilfus, 2018). If the Cl– stress is prolonged, necrosis spreads toward the middle of the expanded leaves, which do not work anymore as a source of photosynthates with a consequent loss of younger leaves too (Goodrich et al., 2009).
Recently, it has been found that the addition of a small molecule like omeprazole (OMP), a selective proton pump inhibitor of human gastric parietal cells H+/K+-ATPase (Van Oosten et al., 2019), can alter NO3–/ Cl– homeostasis in the plant tissues under salinity, allowing plants to overcome the negative effects of Cl– stress. Rouphael et al. (2018) suggested that OMP in tomato plants could trigger signal transduction pathways mediated by endogenous phytohormone or calcium that can activate sub-traits conferring Cl– salinity tolerance. ABA, even when not able to regulate Cl– root uptake or its compartmentalization in vacuoles of root cortical cells (Li et al., 2017b), can interact with and/or be transported by a specific root NO3– transporter, encoded by the AtNPF2.5 gene, belonging to the Nitrate Excretion Transporter (NAXT) subfamily that can operate Cl– excretion from the root cortical cells plasma membrane under salinity (Li et al., 2017a). OMP could be responsible for regulating the expression of the AtNPF2.5 gene, thus modulating the root cell Cl– extrusion in the presence of ABA. Carillo et al. (2019b) have also hypothesized that OMP could be involved in a specific epigenetic single missense modification of a member of the family of the CLC anion transporters, CLCa, usually involved in the compartmentalization of NO3– in the vacuoles of the root cells (Wege et al., 2010). This mutation could change Cl– over NO3– selectivity of CLCa transporter, inducing Cl– compartmentalization in the root vacuoles while decreasing the loading of this toxic ion to leaves (Wege et al., 2010). In salt-stressed basil plants treated with OMP, an increase of NO3–, potassium levels and leaf area/expansion, and fresh yield were observed (Carillo et al., 2019b). It is possible that the exclusion of Cl– from the cytosol of the root cells and the consequent membrane depolarization may activate an outwardly rectifying non-selective cation channel (NORC), first identified in the xylem cells of barley roots (Wegner and Raschke, 1994), which enable the passive non-selective transport of NO3– and K+ to xylem, accelerating the transport of these ions to shoots.
Nitrate Accumulation and Chloride Eustress
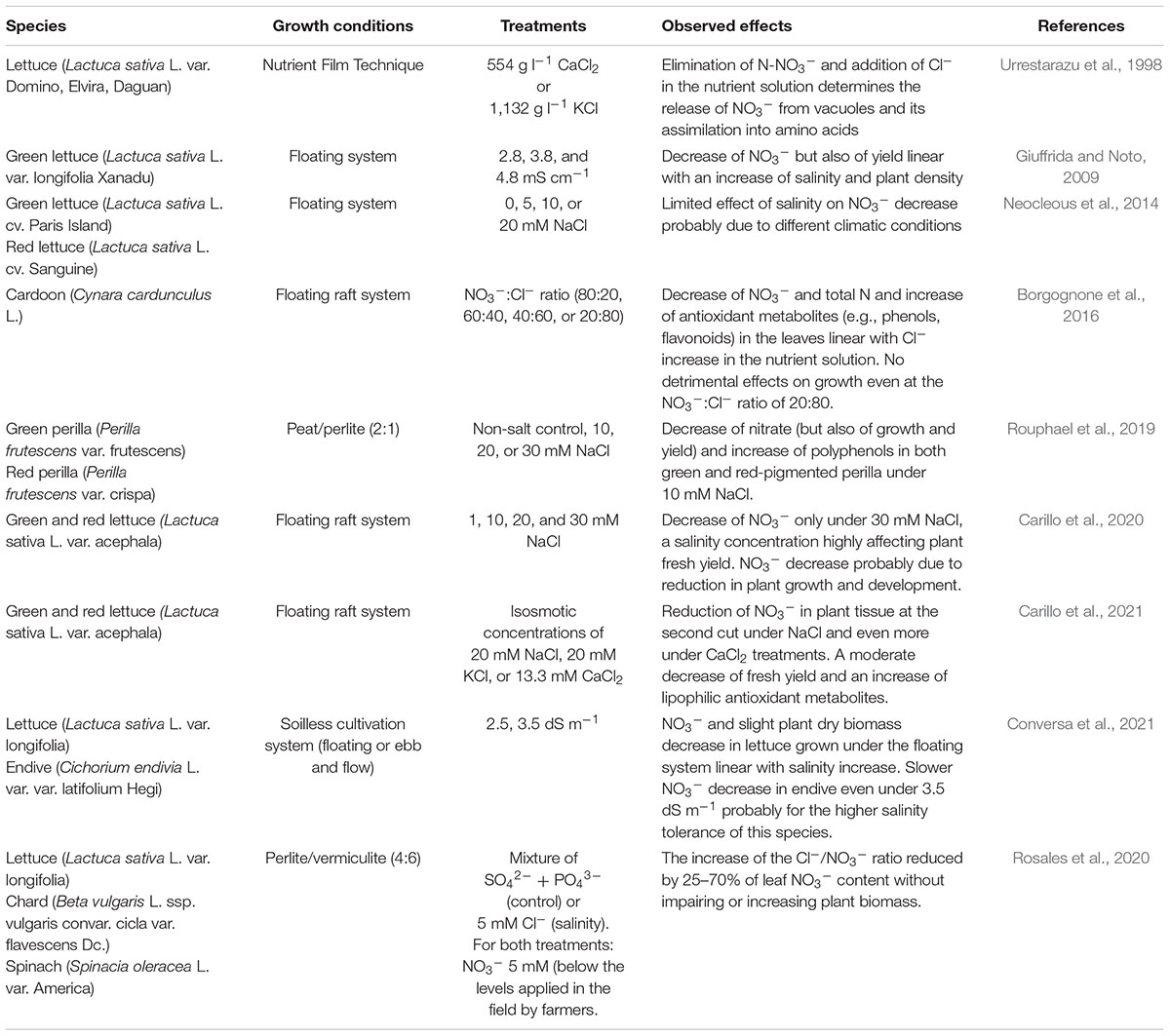
As mentioned above, NO3– accumulation in leafy vegetables at maturity should be avoided. Nitrate accumulation in leafy vegetables may depend on genetic material and plant physiological condition, cultivation practices, and amount, timing, and form of NO3– application [European farmers traditionally rely on NH4NO3 and Ca(NO3)2], as well as environmental conditions (light intensity, temperature, drought and/or salinity influencing water-use efficiency, and CO2 uptake) (Cantliffe, 1973; Escobar-Gutiérrez et al., 2002; Rouphael et al., 2018). Indeed, adopting practices to finely control/limit NO3– content in leafy vegetables without impairing the plant growth and yield could add value to the vegetable products and improve the use of N fertilizers while reducing or preventing pollution (Santamaria, 2006; Anjana and Iqbal, 2007). In particular, salinity eliciting has been considered an effective strategy to decrease NO3– accumulation in the leafy vegetables, thanks to the antagonism between Cl– and NO3– discussed above (Rubinigg et al., 2003; Colla et al., 2018; Rouphael and Kyriacou, 2018; Rouphael et al., 2018). The reduction and partial replacement of NO3– with Cl– in the nutrient solution may be also facilitated by using soilless/hydroponic cultivation, which allows to fine-tune the concentration of nutrients in the cultivation media (Rouphael and Kyriacou, 2018). In fact, decreasing the NO3–: Cl– ratio in growth media for several days or weeks before harvest may reduce NO3– accumulation in the edible plant parts (Rubinigg et al., 2003; Borgognone et al., 2016; Tabaglio et al., 2020). In particular, it has been found that accurately modulating the NO3–: Cl– ratio of the nutrient solution may allow in reducing the NO3– content in the leafy vegetables without abruptly modifying the ionic strength of the culture or fertigation media and therefore without causing N limitation or starvation (Carillo et al., 2019a; Table 2). Clearly, decreasing the NO3–: Cl– ratio may alter the morpho-physiological and qualitative features of salt-sensitive crops; however, a mild to moderate salinity stress (eustress) may decrease leaf NO3– accumulation, while also inducing the synthesis and accumulation of bioactive compounds (Akula and Ravishankar, 2011; Lucini et al., 2016; Woodrow et al., 2017; Kyriacou and Rouphael, 2018), and can increase the plant antioxidant response and hardening (Kim et al., 2008; Carillo et al., 2020). However, it has been suggested by Rosales et al. (2020) that Cl–, instead of impairing NO3– uptake and transport, facilitates its assimilation, improving NUE in tobacco. Probably, the efficient and inexpensive compartmentalization of Cl– in the vacuole prevents the storage of nitrate and promotes its reductive assimilation (Wege et al., 2017). Accordingly, Neocleous et al. (2021) found that replacing one-third of the standard recommended NO3– supply with Cl– in closed hydroponic systems determined a 2-fold increase of tomato NUE while decreasing NO3– losses to one-half without affecting the fruit biomass production. Therefore, regardless of whether Cl– is considered a nitrate antagonist or a beneficial macronutrient for NUE, it is important to finely modulate its dose for decreasing the NO3– accumulation in leaves or improving its uptake and assimilation without decreasing the growth and productivity of the plants, thus tuning up a critical equilibrium called sectio divina (Rouphael and Kyriacou, 2018; Giordano et al., 2019; Carillo et al., 2020). In fact, Giuffrida and Noto (2009) observed that NO3– in lettuce leaves decreased linearly with the increase of NaCl salinity (from 2.8 to 4.8 mS cm–1) and plant density, with negative effects on fresh yield. Borgognone et al. (2016) were able to reduce NO3– accumulation in cardoon leaves grown in floating raft culture by using a nutrient solution having a NO3–: Cl– ratio of 20:80 in the last 5, 10, and 15 days before harvest without negatively impacting the yield. Rouphael et al. (2019) obtained a decrease in accumulation of NO3– in leaves of green and red-pigmented perilla by applying a 10 mM NaCl eustress, and at the same time, this treatment enhanced polyphenols and therefore the antioxidant activity. Lettuce plants underwent a decrease in the leaf NO3– content between 20 and 35 mM NaCl, which determined an increase in polyphenols but also a decrease in the growth and yield proportional to the increase in the salinity (Carillo et al., 2020, 2021; Conversa et al., 2021). However, Conversa et al. (2021) found that the endive plants showed a decrease in the antinutrient nitrate without a simultaneous effect on the yield even at 35 mM NaCl (3.5 dS m–1), probably due to the higher salt tolerance of this plant. Rosales et al. (2020) proposed that only when Cl– is available at basal concentrations in soils, in the range of a micronutrient, nitrate is compartmentalized in tobacco leaf vacuoles to play an osmotic function instead of being assimilated.
Considering that the accumulation of NO3– is mainly responsible for the N oxides and nitrosamines in flue-cured tobacco during smoking, Cl– eustress may also help reduce nitrosamine levels in cigarettes, thus improving the quality of these crops and contributing to prevent a large proportion of deaths due to lung cancer (Mirvish, 2007; Rosales et al., 2020).
Conclusion and Future Perspectives
Enhancing the crop productivity and quality of the product together with taking care of environmental quality are urgent needs for the intermediate future. Meeting these two important goals presents a major sustainability challenge to growers, extension specialists, and researchers, which may be fostered by identifying the right source, rate, and time of N application. Such global NUE necessitates having a global view of the molecular and physiological basis of nitrate uptake, assimilation, and use in plants in the function of agricultural practices. Therefore, future attempts to modify and improve the plant productivity and/or quality through manipulation of the NUE will depend crucially on the knowledge that we gain from the new strategies of fertilization and management practices, that is timing, rate, and form of N application in relation with other nutrients and/or biostimulants. In addition, the combination of seed priming using novel, nitric oxide- and hydrogen sulfide-releasing (NOSH) hybrid molecules and foliar biostimulation using micro/macroalgae-derived extract (MAB), and vegetal-based protein hydrolysate can provide the required specific rapid induction responses since the early stage of cultivation and the wide-range long-term effects to improve NUE, profitability, and nutritional value of the vegetable crops. With regard to the nitrate accumulation and chloride eustress, the application of salinity eustress facilitated by hydroponics can reduce the accumulation of the anti-nutrient nitrate in the leafy vegetables. Finally, the comprehension of (i) genotype × management practices to enhance NUE and developing eco-friendly methods of cultivation with lower environmental impact and (ii) the molecular and physiological modes of actions responsible for the enhancement of NUE in vegetable crops under both open field and controlled conditions have to be encouraged.
Data Availability Statement
The original contributions presented in this study are included in the article, further inquiries can be directed to the corresponding author.
Author Contributions
Both authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
This research was funded by the Università degli Studi della Campania Luigi Vanvitelli (Grant No. VALERE: VAnviteLli pEr la RicErca) and by the Regione Campania Lotta alle Patologie Oncologiche progetto iCURE (CUP B21C17000030007–SURF 17061BP000000008).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aibara, I., and Miwa, K. (2014). Strategies for optimization of mineral nutrient transport in plants: multilevel regulation of nutrient-dependent dynamics of root architecture and transporter activity. Plant Cell Physiol. 55, 2027–2036.
Aires, A., Carvalho, R., Rosa, E. A. S., and Saavedra, M. J. (2013). Effects of agriculture production systems on nitrate and nitrite accumulation on baby-leaf salads. Food Sci. Nutr. 1, 3–7. doi: 10.1002/fsn3.1
Akula, R., and Ravishankar, G. A. (2011). Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 6, 1720–1731. doi: 10.4161/psb.6.11.17613
Albornoz, F., Gebauer, M., Ponce, C., and Cabeza, R. A. (2018). LeNRT1.1 improves nitrate uptake in grafted tomato plants under high nitrogen demand. Int. J. Mol. Sci. 19:3921. doi: 10.3390/ijms19123921
Almagro, A., Lin, S. H., and Tsay, Y. F. (2008). Characterization of the Arabidopsis nitrate transporter NRT1.6 reveals a role of nitrate in early embryo development. Plant Cell 20, 3289–3299. doi: 10.1105/tpc.107.056788
Anjana, S. U., and Iqbal, M. (2007). Nitrate accumulation in plants, factors affecting the process, and human health implications. A review. Agron. Sustain. Dev. 27, 45–57.
Annunziata, M. G., Ciarmiello, L. F., Woodrow, P., Maximova, E., Fuggi, A., and Carillo, P. (2017). Durum wheat roots adapt to salinity remodeling the cellular content of nitrogen metabolites and sucrose. Front. Plant Sci. 7:2035. doi: 10.3389/fpls.2016.02035
Araya, T., Miyamoto, M., Wibowo, J., Suzuki, A., Kojima, S., Tsuchiya, Y., et al. (2014). CLE-CLAVATA peptide-receptor signaling module regulates the expansion of plant root system in a nitrogen-dependent manner. Proc. Natl. Acad. Sci. U.S.A. 111, 2029–2034. doi: 10.1073/pnas.1319953111
Aslam, M., Travis, R. L., and Rains, D. W. (1996). Evidence for substrate induction of a nitrate efflux system in barley roots. Plant Physiol. 112, 1167–1175. doi: 10.1104/pp.112.3.1167
Ayers, R. S., and Westcot, D. W. (1985). Water Quality for Agriculture. Rome: FAO, Food and Agriculture Organization of the United Nations.
Becker, T. W., Foyer, C., and Caboche, M. (1992). Light-regulated expression of the nitrate-reductase and nitrite-reductase genes in tomato and in the phytochrome-deficient aurea mutant of tomato. Planta 188, 39–47. doi: 10.1007/BF00198937
Bijay, S., and Craswell, E. (2021). Fertilizers and nitrate pollution of surface and ground water: an increasingly pervasive global problem. SN Appl. Sci. 3:518.
Boer, M. D., Santos Teixeira, J., and Ten Tusscher, K. H. (2020). Modeling of root nitrate responses suggests preferential foraging arises from the integration of demand, supply and local presence signals. Front. Plant Sci. 11:708. doi: 10.3389/fpls.2020.00708
Borgognone, D., Rouphael, Y., Cardarelli, M., Lucini, L., and Colla, G. (2016). Changes in biomass, mineral composition, and quality of cardoon in response to NO3-:Cl- ratio and nitrate deprivation from the nutrient solution. Front. Plant Sci. 7:978. doi: 10.3389/fpls.2016.00978
Bouguyon, E., Brun, F., Meynard, D., Kubeš, M., Pervent, M., Leran, S., et al. (2015). Multiple mechanisms of nitrate sensing by Arabidopsis nitrate transceptor NRT1.1. Nat. Plants 1:15015. doi: 10.1038/nplants.2015.15
Bouguyon, E., Gojon, A., and Nacry, P. (2012). Nitrate sensing and signaling in plants. Semin. Cell Dev. Biol. 23, 648–654. doi: 10.1016/j.semcdb.2012.01.004
Bouguyon, E., Perrine-Walker, F., Pervent, M., Rochette, J., Cuesta, C., Benkova, E., et al. (2016). Nitrate controls root development through posttranscriptional regulation of the NRT1.1/NPF6.3 transporter/sensor. Plant Physiol. 172, 1237–1248. doi: 10.1104/pp.16.01047
Cameron, K. C., Di, H. J., and Moir, J. L. (2013). Nitrogen losses from the soil/plant system: a review. Ann. Appl. Biol. 162, 145–173. doi: 10.1111/j.1469-8137.2012.04300.x
Cantliffe, D. J. (1973). Nitrate accumulation in table beets and spinach as affected by nitrogen, Phosphorus, and Potassium nutrition and light intensity1. Agron. J. 65, 563–565.
Carillo, P., Colla, G., Fusco, G. M., Dell’aversana, E., El-Nakhel, C., Giordano, M., et al. (2019a). Morphological and physiological responses induced by protein hydrolysate-based biostimulant and nitrogen rates in greenhouse spinach. Agronomy 9:450.
Carillo, P., Giordano, M., Raimondi, G., Napolitano, F., Di Stasio, E., Kyriacou, M. C., et al. (2020). Physiological and nutraceutical quality of green and red pigmented lettuce in response to NaCl concentration in two successive harvests. Agronomy 10:1358.
Carillo, P., Mastrolonardo, G., Nacca, F., and Fuggi, A. (2005). Nitrate reductase in durum wheat seedlings as affected by nitrate nutrition and salinity. Funct. Plant Biol. 32, 209–219. doi: 10.1071/FP04184
Carillo, P., Soteriou, G. A., Kyriacou, M. C., Giordano, M., Raimondi, G., Napolitano, F., et al. (2021). Regulated salinity eustress in a floating hydroponic module of sequentially harvested lettuce modulates phytochemical constitution, plant resilience, and post-harvest nutraceutical quality. Agronomy 11:1040.
Carillo, P., Woodrow, P., Raimondi, G., El-Nakhel, C., Pannico, A., Kyriacou, M. C., et al. (2019b). Omeprazole promotes chloride exclusion and induces salt tolerance in Greenhouse Basil. Agronomy 9:355.
Chen, J., Liu, X., Liu, S., Fan, X., Zhao, L., Song, M., et al. (2020). Co-overexpression of OsNAR2.1 and OsNRT2.3a increased agronomic nitrogen use efficiency in transgenic rice plants. Front. Plant Sci. 11:1245. doi: 10.3389/fpls.2020.01245
Chen, K.-E., Chen, H.-Y., Tseng, C.-S., and Tsay, Y.-F. (2020). Improving nitrogen use efficiency by manipulating nitrate remobilization in plants. Nat. Plants 6, 1126–1135. doi: 10.1038/s41477-020-00758-0
Chiu, C. C., Lin, C. S., Hsia, A. P., Su, R. C., Lin, H. L., and Tsay, Y. F. (2004). Mutation of a nitrate transporter, AtNRT1:4, results in a reduced petiole nitrate content and altered leaf development. Plant Cell Physiol. 45, 1139–1148. doi: 10.1093/pcp/pch143
Chopin, F., Orsel, M., Dorbe, M. F., Chardon, F., Truong, H. N., Miller, A. J., et al. (2007). The Arabidopsis ATNRT2.7 nitrate transporter controls nitrate content in seeds. Plant Cell 19, 1590–1602. doi: 10.1105/tpc.107.050542
Ciriello, M., Formisano, L., El-Nakhel, C., Corrado, G., Pannico, A., De Pascale, S., et al. (2021). Morpho-physiological responses and secondary metabolites modulation by preharvest factors of three hydroponically grown genovese Basil cultivars. Front. Plant Sci. 12:671026. doi: 10.3389/fpls.2021.671026
Cirillo, C., De Micco, V., Arena, C., Carillo, P., Pannico, A., De Pascale, S., et al. (2019). Biochemical, physiological and anatomical mechanisms of adaptation of Callistemon citrinus and Viburnum lucidum to NaCl and CaCl(2) Salinization. Front. Plant Sci. 10:742. doi: 10.3389/fpls.2019.00742
Colla, G., Kim, H.-J., Kyriacou, M. C., and Rouphael, Y. (2018). Nitrate in fruits and vegetables. Sci. Hortic. 237, 221–238.
Colla, G., Rouphael, Y., Jawad, R., Kumar, P., Rea, E., and Cardarelli, M. (2013). The effectiveness of grafting to improve NaCl and CaCl2 tolerance in cucumber. Sci. Hortic. 164, 380–391.
Colmenero-Flores, J. M., Franco-Navarro, J. D., Cubero-Font, P., Peinado-Torrubia, P., and Rosales, M. A. (2019). Chloride as a beneficial macronutrient in higher plants: new roles and regulation. Int. J. Mol. Sci. 20:4686. doi: 10.3390/ijms20194686
Conversa, G., Bonasia, A., Lazzizera, C., La Rotonda, P., and Elia, A. (2021). Reduction of nitrate content in baby-leaf lettuce and Cichorium endivia through the soilless cultivation system, electrical conductivity and management of nutrient solution. Front. Plant Sci. 12:645671. doi: 10.3389/fpls.2021.645671
Costa-Broseta, Á., Castillo, M., and León, J. (2020). Nitrite reductase 1 is a target of nitric oxide-mediated post-translational modifications and controls nitrogen flux and growth in Arabidopsis. Int. J. Mol. Sci. 21:7270. doi: 10.3390/ijms21197270
Crawford, N. M., and Glass, A. D. M. (1998). Molecular and physiological aspects of nitrate uptake in plants. Trends Plant Sci. 3, 389–395.
De Angeli, A., Monachello, D., Ephritikhine, G., Frachisse, J. M., Thomine, S., Gambale, F., et al. (2006). The nitrate/proton antiporter AtCLCa mediates nitrate accumulation in plant vacuoles. Nature 442, 939–942. doi: 10.1038/nature05013
de Jong, F., Thodey, K., Lejay, L. V., and Bevan, M. W. (2013). Glucose elevates NITRATE TRANSPORTER2.1 protein levels and nitrate transport activity independently of its HEXOKINASE1-mediated stimulation of NITRATE TRANSPORTER2.1 expression. Plant Physiol. 164, 308–320. doi: 10.1104/pp.113.230599
Diatloff, E., Roberts, M., Sanders, D., and Roberts, S. K. (2004). Characterization of anion channels in the plasma membrane of Arabidopsis epidermal root cells and the identification of a citrate-permeable channel induced by phosphate starvation. Plant Physiol. 136, 4136–4149. doi: 10.1104/pp.104.046995
Dich, J., Järvinen, R., Knekt, P., and Penttilä, P. L. (1996). Dietary intakes of nitrate, nitrite and NDMA in the finnish mobile clinic health examination survey. Food Addit. Contam. 13, 541–552. doi: 10.1080/02652039609374439
Escobar-Gutiérrez, A. J., Burns, I. G., Lee, A., and Edmondson, R. N. (2002). Screening lettuce cultivars for low nitrate content during summer and winter production. J. Hortic. Sci. Biotechnol. 77, 232–237.
Fan, S. C., Lin, C. S., Hsu, P. K., Lin, S. H., and Tsay, Y. F. (2009). The Arabidopsis nitrate transporter NRT1.7, expressed in phloem, is responsible for source-to-sink remobilization of nitrate. Plant Cell 21, 2750–2761. doi: 10.1105/tpc.109.067603
Fan, X., Feng, H., Tan, Y., Xu, Y., Miao, Q., and Xu, G. (2016). A putative 6-transmembrane nitrate transporter OsNRT1.1b plays a key role in rice under low nitrogen. J. Integr. Plant Biol. 58, 590–599. doi: 10.1111/jipb.12382
Felle, H. H. (1994). The H+/Cl- symporter in root-hair cells of Sinapis alba (an electrophysiological study using ion-selective microelectrodes). Plant Physiol. 106, 1131–1136. doi: 10.1104/pp.106.3.1131
Feng, H., Yan, M., Fan, X., Li, B., Shen, Q., Miller, A. J., et al. (2011). Spatial expression and regulation of rice high-affinity nitrate transporters by nitrogen and carbon status. J. Exp. Bot. 62, 2319–2332. doi: 10.1093/jxb/erq403
Fichtner, F., Dissanayake, I. M., Lacombe, B., and Barbier, F. (2021). Sugar and nitrate sensing: a multi-billion-year story. Trends Plant Sci. 26, 352–374. doi: 10.1016/j.tplants.2020.11.006
Filleur, S., Dorbe, M.-F., Cerezo, M., Orsel, M., Granier, F., Gojon, A., et al. (2001). An Arabidopsis T-DNA mutant affected in Nrt2 genes is impaired in nitrate uptake. FEBS Lett. 489, 220–224. doi: 10.1016/s0014-5793(01)02096-8
Forde, B. G. (2000). Nitrate transporters in plants: structure, function and regulation. Biochim. Biophys. Acta (BBA) Biomembr. 1465, 219–235. doi: 10.1016/s0005-2736(00)00140-1
Forde, B. G. (2002). Local and long-range signaling pathways regulating plant responses to nitrate. Annu. Rev. Plant Biol. 53, 203–224. doi: 10.1146/annurev.arplant.53.100301.135256
Foyer, C. H., and Paul, M. J. (2001). Source–sink relationships. Plant Psychol. 78, 519–524. doi: 10.1038/npg.els.0001304
Foyer, C. H., Valadier, M.-H., Migge, A., and Becker, T. W. (1998). Drought-induced effects on nitrate reductase activity and mRNA and on the coordination of nitrogen and carbon metabolism in maize leaves. Plant Physiol. 117, 283–292. doi: 10.1104/pp.117.1.283
Franco-Navarro, J. D., Brumós, J., Rosales, M. A., Cubero-Font, P., Talón, M., and Colmenero-Flores, J. M. (2016). Chloride regulates leaf cell size and water relations in tobacco plants. J. Exp. Bot. 67, 873–891. doi: 10.1093/jxb/erv502
Franco-Navarro, J. D., Rosales, M. A., Cubero-Font, P., Calvo, P., Álvarez, R., Diaz-Espejo, A., et al. (2019). Chloride as a macronutrient increases water-use efficiency by anatomically driven reduced stomatal conductance and increased mesophyll diffusion to CO2. Plant J. 99, 815–831. doi: 10.1111/tpj.14423
Fu, Y., Yi, H., Bao, J., and Gong, J. (2015). LeNRT2.3 functions in nitrate acquisition and long-distance transport in tomato. FEBS Lett. 589, 1072–1079. doi: 10.1016/j.febslet.2015.03.016
Galangau, F., Daniel-Vedele, F., Moureaux, T., Dorbe, M.-F., Leydecker, M.-T., and Caboche, M. (1988). Expression of leaf nitrate reductase genes from tomato and tobacco in relation to light-dark regimes and nitrate supply. Plant Physiol. 88, 383–388. doi: 10.1104/pp.88.2.383
Gao, Z., Wang, Y., Chen, G., Zhang, A., Yang, S., Shang, L., et al. (2019). The Indica nitrate reductase gene OsNR2 allele enhances rice yield potential and nitrogen use efficiency. Nat. Commun. 10:5207. doi: 10.1038/s41467-019-13110-8
Garnett, T., Conn, V., and Kaiser, B. N. (2009). Root based approaches to improving nitrogen use efficiency in plants. Plant Cell Environ. 32, 1272–1283. doi: 10.1111/j.1365-3040.2009.02011.x
Garnica, M., Houdusse, F., Zamarreño, A. M., and Garcia-Mina, J. M. (2010). Nitrate modifies the assimilation pattern of ammonium and urea in wheat seedlings. J. Sci. Food Agric. 90, 357–369. doi: 10.1002/jsfa.3811
Geilfus, C.-M. (2018). Chloride: from nutrient to toxicant. Plant Cell Physiol. 59, 877–886. doi: 10.1093/pcp/pcy071
Giehl, R. F. H., and von Wirén, N. (2014). Root nutrient foraging. Plant Physiol. 166, 509–517. doi: 10.1104/pp.114.245225
Giordano, M., El-Nakhel, C., Pannico, A., Kyriacou, M. C., Stazi, S. R., De Pascale, S., et al. (2019). Iron biofortification of red and green pigmented lettuce in closed soilless cultivation impacts crop performance and modulates mineral and bioactive composition. Agronomy 9:290.
Girin, T., Lejay, L., Wirth, J., Widiez, T., Palenchar, P. M., Nazoa, P., et al. (2007). Identification of a 150 bp cis-acting element of the AtNRT2.1 promoter involved in the regulation of gene expression by the N and C status of the plant. Plant Cell Environ. 30, 1366–1380. doi: 10.1111/j.1365-3040.2007.01712.x
Giro, A., and Ferrante, A. (2016). Yield and quality of Corchorus olitorius baby leaf grown in a floating system. J. Horticult. Sci. Biotechnol. 91, 603–610. doi: 10.1080/14620316.2016.1200955
Giuffrida, F., and Noto, G. (2009). Effects of salinity and plant density on quality of lettuce grown in floating system for fresh-cut. Acta Hortic. 843, 219–226.
Glass, A. D., Britto, D. T., Kaiser, B. N., Kinghorn, J. R., Kronzucker, H. J., Kumar, A., et al. (2002). The regulation of nitrate and ammonium transport systems in plants. J. Exp. Bot. 53, 855–864. doi: 10.1093/jexbot/53.370.855
Gloser, V., Dvorackova, M., Mota, D. H., Petrovic, B., Gonzalez, P., and Geilfus, C. M. (2020). Early changes in nitrate uptake and assimilation under drought in relation to transpiration. Front. Plant Sci. 11:602065. doi: 10.3389/fpls.2020.602065
Goel, P., Bhuria, M., Kaushal, M., and Singh, A. K. (2016). Carbon: nitrogen interaction regulates expression of genes involved in N-uptake and assimilation in Brassica juncea L. PLoS One 11:e0163061. doi: 10.1371/journal.pone.0163061
Gojon, A., Krouk, G., Perrine-Walker, F., and Laugier, E. (2011). Nitrate transceptor(s) in plants. J. Exp. Bot. 62, 2299–2308. doi: 10.1093/jxb/erq419
Goodrich, B., Koski, R., and Jacobi, W. R. (2009). Condition of soils and vegetation along roads treated with magnesium chloride for dust suppression. Water Air Soil Pollut. 198, 165–188.
Griffiths, M., and York, L. M. (2020). Targeting root ion uptake kinetics to increase plant productivity and nutrient use efficiency. Plant Physiol. 182, 1854–1868. doi: 10.1104/pp.19.01496
Guan, P., Wang, R., Nacry, P., Breton, G., Kay, S. A., Pruneda-Paz, J. L., et al. (2014). Nitrate foraging by Arabidopsis roots is mediated by the transcription factor TCP20 through the systemic signaling pathway. Proc. Natl. Acad. Sci. U.S.A. 111, 15267–15272. doi: 10.1073/pnas.1411375111
Gupta, S. K., Gupta, A. B., and Gupta, R. (2017). “28 – Pathophysiology of nitrate toxicity in humans in view of the changing trends of the global nitrogen cycle with special reference to India,” in The Indian Nitrogen Assessment, eds Y. P. Abrol, T. K. Adhya, V. P. Aneja, N. Raghuram, H. Pathak, U. Kulshrestha, et al. (Amsterdam: Elsevier), 459–468.
Han, Y.-L., Song, H.-X., Liao, Q., Yu, Y., Jian, S.-F., Lepo, J. E., et al. (2016). Nitrogen use efficiency is mediated by vacuolar nitrate sequestration capacity in roots of Brassica napus. Plant Physiol. 170, 1684–1698. doi: 10.1104/pp.15.01377
Hawkesford, M., Kopriva, S., and De Kok, L. (2014). Nutrient Use Efficiency in Plants – Concepts and Approaches. Cham: Springer International Publishing.
He, Y.-N., Peng, J.-S., Cai, Y., Liu, D.-F., Guan, Y., Yi, H.-Y., et al. (2017). Tonoplast-localized nitrate uptake transporters involved in vacuolar nitrate efflux and reallocation in Arabidopsis. Sci. Rep. 7:6417. doi: 10.1038/s41598-017-06744-5
Hirose, T. (2011). Nitrogen use efficiency revisited. Oecologia 166, 863–867. doi: 10.1007/s00442-011-1942-z
Hirose, T., and Kitajima, K. (1986). Nitrogen uptake and plant growth: I. effect of nitrogen removal on growth of Polygonum cuspidatum. Ann. Bot. 58, 479–486.
Ho, C.-H., Lin, S.-H., Hu, H.-C., and Tsay, Y.-F. (2009). CHL1 functions as a nitrate sensor in plants. Cell 138, 1184–1194. doi: 10.1016/j.cell.2009.07.004
Hsu, P.-K., and Tsay, Y.-F. (2013). Two phloem nitrate transporters, NRT1.11 and NRT1.12, are important for redistributing xylem-borne nitrate to enhance plant growth. Plant Physiol. 163, 844–856. doi: 10.1104/pp.113.226563
Hu, B., Jiang, Z., Wang, W., Qiu, Y., Zhang, Z., Liu, Y., et al. (2019). Nitrate–NRT1.1B–SPX4 cascade integrates nitrogen and phosphorus signalling networks in plants. Nat. Plants 5, 401–413.
Huang, N. C., Chiang, C. S., Crawford, N. M., and Tsay, Y. F. (1996). CHL1 encodes a component of the low-affinity nitrate uptake system in Arabidopsis and shows cell type-specific expression in roots. Plant Cell 8, 2183–2191. doi: 10.1105/tpc.8.12.2183
Inoue, Y., Kobae, Y., Omoto, E., Tanaka, A., Banba, M., Takai, S., et al. (2014). The soybean mycorrhiza-inducible phosphate transporter gene, GmPT7, also shows localized expression at the tips of vein endings of senescent leaves. Plant Cell Physiol. 55, 2102–2111. doi: 10.1093/pcp/pcu138
Kaiser, W. M., Weiner, H., and Huber, S. C. (1999). Nitrate reductase in higher plants: a case study for transduction of environmental stimuli into control of catalytic activity. Physiol. Plant. 105, 384–389.
Kechid, M., Desbrosses, G., Rokhsi, W., Varoquaux, F., Djekoun, A., and Touraine, B. (2013). The NRT2.5 and NRT2.6 genes are involved in growth promotion of Arabidopsis by the plant growth-promoting rhizobacterium (PGPR) strain Phyllobacterium brassicacearum STM196. New Phytol. 198, 514–524. doi: 10.1111/nph.12158
Keeney, D. R., and Hatfield, J. (2001). “Chapter 1. The nitrogen cycle, historical perspective, and current and potential future concerns,” in Nitrogen in the Environment: Sources, Problems, and Management, eds J. L. Hatfield and R. F. Follett (Amsterdam: Academic Press).
Kiba, T., Feria-Bourrellier, A.-B., Lafouge, F., Lezhneva, L., Boutet-Mercey, S., Orsel, M., et al. (2012). The Arabidopsis nitrate transporter NRT2.4 plays a double role in roots and shoots of nitrogen-starved plants. Plant Cell 24, 245–258. doi: 10.1105/tpc.111.092221
Kim, H.-J., Fonseca, J. M., Choi, J.-H., Kubota, C., and Kwon, D. Y. (2008). Salt in irrigation water affects the nutritional and visual properties of romaine lettuce (Lactuca sativa L.). J. Agric. Food Chem. 56, 3772–3776. doi: 10.1021/jf0733719
Konishi, M., and Yanagisawa, S. (2010). Identification of a nitrate-responsive cis-element in the Arabidopsis NIR1 promoter defines the presence of multiple cis-regulatory elements for nitrogen response. Plant J. 63, 269–282. doi: 10.1111/j.1365-313X.2010.04239.x
Konishi, M., and Yanagisawa, S. (2013). Arabidopsis NIN-like transcription factors have a central role in nitrate signalling. Nat. Commun. 4:1617. doi: 10.1038/ncomms2621
Kotur, Z., and Glass, A. D. (2015). A 150 kDa plasma membrane complex of AtNRT2.5 and AtNAR2.1 is the major contributor to constitutive high-affinity nitrate influx in Arabidopsis thaliana. Plant Cell Environ. 38, 1490–1502. doi: 10.1111/pce.12496
Krouk, G., Lacombe, B., Bielach, A., Perrine-Walker, F., Malinska, K., Mounier, E., et al. (2010). Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants. Dev. Cell 18, 927–937. doi: 10.1016/j.devcel.2010.05.008
Kyriacou, M. C., and Rouphael, Y. (2018). Towards a new definition of quality for fresh fruits and vegetables. Sci. Hortic. 234, 463–469.
Leidi, E., and Lips, S. (2004). Effect of NaCl salinity on photosynthesis, 14C-translocation, and yield in wheat plants irrigated with ammonium or nitrate solutions. Irrig. Sci. 11, 155–161.
Leij, M., Smith, S., and Miller, A. (1998). Remobilization of vacuole stored nitrate in barley root cells. Planta 205, 64–72.
Lejay, L., Tillard, P., Lepetit, M., Olive, F., Filleur, S., Daniel-Vedele, F., et al. (1999). Molecular and functional regulation of two NO3- uptake systems by N- and C-status of Arabidopsis plants. Plant J. 18, 509–519. doi: 10.1046/j.1365-313x.1999.00480.x
Lejay, L., Wirth, J., Pervent, M., Cross, J. M.-F., Tillard, P., and Gojon, A. (2008). Oxidative pentose phosphate pathway-dependent sugar sensing as a mechanism for regulation of root ion transporters by photosynthesis. Plant Physiol. 146, 2036–2053. doi: 10.1104/pp.107.114710
Lezhneva, L., Kiba, T., Feria-Bourrellier, A.-B., Lafouge, F., Boutet-Mercey, S., Zoufan, P., et al. (2014). The Arabidopsis nitrate transporter NRT2.5 plays a role in nitrate acquisition and remobilization in nitrogen-starved plants. Plant J. 80, 230–241. doi: 10.1111/tpj.12626
Li, B., Tester, M., and Gilliham, M. (2017b). Chloride on the move. Trends Plant Sci. 22, 236–248. doi: 10.1016/j.tplants.2016.12.004
Li, B., Qiu, J., Jayakannan, M., Xu, B., Li, Y., Mayo, G. M., et al. (2017a). AtNPF2.5 modulates chloride (Cl-) efflux from roots of Arabidopsis thaliana. Front. Plant Sci. 7:2013. doi: 10.3389/fpls.2016.02013
Li, J., Zhao, C., Hu, S., Song, X., Lv, M., Yao, D., et al. (2020). Arabidopsis NRT1.2 interacts with the PHOSPHOLIPASE Dα1 (PLDα1) to positively regulate seed germination and seedling development in response to ABA treatment. Biochem. Biophys. Res. Commun. 533, 104–109. doi: 10.1016/j.bbrc.2020.08.025
Li, J.-Y., Fu, Y.-L., Pike, S. M., Bao, J., Tian, W., Zhang, Y., et al. (2010). The Arabidopsis nitrate transporter NRT1.8 functions in nitrate removal from the xylem sap and mediates cadmium tolerance. Plant Cell 22, 1633–1646. doi: 10.1105/tpc.110.075242
Li, T., Zhang, W., Yin, J., Chadwick, D., Norse, D., Lu, Y., et al. (2018). Enhanced-efficiency fertilizers are not a panacea for resolving the nitrogen problem. Glob. Chang. Biol. 24, e511–e521. doi: 10.1111/gcb.13918
Li, W., Wang, Y., Okamoto, M., Crawford, N. M., Siddiqi, M. Y., and Glass, A. D. M. (2007). Dissection of the AtNRT2.1:AtNRT2.2 inducible high-affinity nitrate transporter gene cluster. Plant Physiol. 143, 425–433. doi: 10.1104/pp.106.091223
Liang, G., and Zhang, Z. (2020). Reducing the nitrate content in vegetables through joint regulation of short-distance distribution and long-distance transport. Front. Plant Sci. 11:1079. doi: 10.3389/fpls.2020.01079
Lillo, C., and Appenroth, K.-J. (2001). Light regulation of nitrate reductase in higher plants: which photoreceptors are involved? Plant Biol. 3, 455–465. doi: 10.1074/jbc.M202924200
Lima, J. E., Kojima, S., Takahashi, H., and Von Wirén, N. (2010). Ammonium triggers lateral root branching in Arabidopsis in an AMMONIUM TRANSPORTER1;3-dependent manner. Plant Cell 22, 3621–3633. doi: 10.1105/tpc.110.076216
Lin, S.-H., Kuo, H.-F., Canivenc, G., Lin, C.-S., Lepetit, M., Hsu, P.-K., et al. (2008). Mutation of the Arabidopsis NRT1.5 nitrate transporter causes defective root-to-shoot nitrate transport. Plant Cell 20, 2514–2528. doi: 10.1105/tpc.108.060244
Little, D. Y., Rao, H., Oliva, S., Daniel-Vedele, F., Krapp, A., and Malamy, J. E. (2005). The putative high-affinity nitrate transporter NRT2.1 represses lateral root initiation in response to nutritional cues. Proc. Natl. Acad. Sci. U.S.A. 102, 13693–13698. doi: 10.1073/pnas.0504219102
Liu, K.-H., Huang, C.-Y., and Tsay, Y.-F. (1999). CHL1 is a dual-affinity nitrate transporter of Arabidopsis involved in multiple phases of nitrate uptake. Plant Cell 11, 865–874. doi: 10.1105/tpc.11.5.865
Lucini, L., Borgognone, D., Rouphael, Y., Cardarelli, M., Bernardi, J., and Colla, G. (2016). Mild potassium chloride stress alters the mineral composition, hormone network, and phenolic profile in artichoke leaves. Front. Plant Sci. 7:948. doi: 10.3389/fpls.2016.00948
Ma, W., Li, J., Qu, B., He, X., Zhao, X., Li, B., et al. (2014). Auxin biosynthetic gene TAR2 is involved in low nitrogen-mediated reprogramming of root architecture in Arabidopsis. Plant J. 78, 70–79. doi: 10.1111/tpj.12448
Martinoia, E., Heck, U., and Wiemken, A. (1981). Vacuoles as storage compartments for nitrate in barley leaves. Nature 289, 292–294.
Masclaux-Daubresse, C., Daniel-Vedele, F., Dechorgnat, J., Chardon, F., Gaufichon, L., and Suzuki, A. (2010). Nitrogen uptake, assimilation and remobilization in plants: challenges for sustainable and productive agriculture. Ann. Bot. 105, 1141–1157. doi: 10.1093/aob/mcq028
Migge, A., Carrayol, E., Hirel, B., Lohmann, M., Meya, G., and Becker, T. W. (1998). Two nitrite reductase isoforms are present in tomato cotyledons and are regulated differently by UV-A or UV-B light and during plant development. Planta 207, 229–234.
Miller, A. J., Fan, X., Orsel, M., Smith, S. J., and Wells, D. M. (2007). Nitrate transport and signalling. J. Exp. Bot. 58, 2297–2306.
Mirvish, S. (2007). Methods for lowering nitrosamine levels in cigarette smoke and likely effect on lung cancer rate. Cancer Epidemiol. Biomark. Prev. 16, B142–B142.
Moll, R. H., Kamprath, E. J., and Jackson, W. A. (1982). Analysis and interpretation of factors which contribute to efficiency of nitrogen utilization1. Agron. J. 74, 562–564.
Mounier, E., Pervent, M., Ljung, K., Gojon, A., and Nacry, P. (2014). Auxin-mediated nitrate signalling by NRT1.1 participates in the adaptive response of Arabidopsis root architecture to the spatial heterogeneity of nitrate availability. Plant Cell Environ. 37, 162–174. doi: 10.1111/pce.12143
Munns, R. (2002). Comparative physiology of salt and water stress. Plant Cell Environ. 25, 239–250. doi: 10.1046/j.0016-8025.2001.00808.x
Munns, R., and Tester, M. (2008). Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 59, 651–681.
Nacry, P., Bouguyon, E., and Gojon, A. (2013). Nitrogen acquisition by roots: physiological and developmental mechanisms ensuring plant adaptation to a fluctuating resource. Plant Soil 370, 1–29.
Naz, M., Luo, B., Guo, X., Li, B., Chen, J., and Fan, X. (2019). Overexpression of nitrate transporter OsNRT2.1 enhances nitrate-dependent root elongation. Genes 10:290. doi: 10.3390/genes10040290
Neocleous, D., Koukounaras, A., Siomos, A. S., and Vasilakakis, M. (2014). Assessing the salinity effects on mineral composition and nutritional quality of green and red “baby” lettuce. J. Food Qual. 37, 1–8.
Neocleous, D., Nikolaou, G., Ntatsi, G., and Savvas, D. (2021). Nitrate supply limitations in tomato crops grown in a chloride-amended recirculating nutrient solution. Agric. Water Manag. 258:107163.
North, K. A., Ehlting, B., Koprivova, A., Rennenberg, H., and Kopriva, S. (2009). Natural variation in Arabidopsis adaptation to growth at low nitrogen conditions. Plant Physiol. Biochem. 47, 912–918. doi: 10.1016/j.plaphy.2009.06.009
O’Brien, J. A., Vega, A., Bouguyon, E., Krouk, G., Gojon, A., Coruzzi, G., et al. (2016). Nitrate transport, sensing, and responses in plants. Mol. Plant 9, 837–856. doi: 10.1016/j.molp.2016.05.004
Oaks, A. (1974). The regulation of nitrate reductase in suspension cultures of soybean cells. Biochim. Biophys. Acta (BBA) Gen. Subj. 372, 122–126. doi: 10.1016/0304-4165(74)90078-6
Olas, J. J., and Wahl, V. (2019). Tissue-specific NIA1 and NIA2 expression in Arabidopsis thaliana. Plant Signal. Behav. 14:1656035. doi: 10.1080/15592324.2019.1656035
Orsel, M., Filleur, S., Fraisier, V., and Daniel-Vedele, F. (2002). Nitrate transport in plants: which gene and which control? J. Exp. Bot. 53, 825–833. doi: 10.1093/jexbot/53.370.825
Park, B. S., Song, J. T., and Seo, H. S. (2011). Arabidopsis nitrate reductase activity is stimulated by the E3 SUMO ligase AtSIZ1. Nat. Commun. 2:400. doi: 10.1038/ncomms1408
Peuke, A. D., and Jeschke, W. D. (1998). The effects of light on induction, time courses, and kinetic patterns of net nitrate uptake in barley. Plant Cell Environ. 21, 765–774.
Poitout, A., Crabos, A., Petřík, I., Novák, O., Krouk, G., Lacombe, B., et al. (2018). Responses to systemic nitrogen signaling in Arabidopsis roots involve trans-zeatin in shoots. Plant Cell 30, 1243–1257. doi: 10.1105/tpc.18.00011
Qiu, J., Henderson, S. W., Tester, M., Roy, S. J., and Gilliham, M. (2016). SLAH1, a homologue of the slow type anion channel SLAC1, modulates shoot Cl- accumulation and salt tolerance in Arabidopsis thaliana. J. Exp. Bot. 67, 4495–4505. doi: 10.1093/jxb/erw237
Raven, J. A. (2016). Chloride: essential micronutrient and multifunctional beneficial ion. J. Exp. Bot. 68, 359–367. doi: 10.1093/jxb/erw421
Rengel, Z., Cakmak, I., and White, P. (2022). Marschner’s Mineral Nutrition of Plants. London: Elsevier.
Rosales, M. A., Franco-Navarro, J. D., Peinado-Torrubia, P., Díaz-Rueda, P., Álvarez, R., and Colmenero-Flores, J. M. (2020). Chloride improves nitrate utilization and NUE in plants. Front. Plant Sci. 11:442. doi: 10.3389/fpls.2020.00442
Rothstein, S. J. (2007). Returning to our roots: making plant biology research relevant to future challenges in agriculture. Plant Cell 19, 2695–2699. doi: 10.1105/tpc.107.053074
Rouphael, Y., and Kyriacou, M. C. (2018). Enhancing quality of fresh vegetables through salinity eustress and biofortification applications facilitated by soilless cultivation. Front. Plant Sci. 9:1254. doi: 10.3389/fpls.2018.01254
Rouphael, Y., Kyriacou, M. C., Carillo, P., Pizzolongo, F., Romano, R., and Sifola, M. I. (2019). Chemical eustress elicits tailored responses and enhances the functional quality of novel food Perilla frutescens. Molecules 24:185.
Rouphael, Y., Petropoulos, S., Cardarelli, M., and Colla, G. (2018). Salinity as eustressor for enhancing quality of vegetables. Sci. Hortic. 234, 361–369.
Roy, S. (2018). Nitrate ahoy! shoot cytokinin signals integrate growth responses with nitrogen availability. Plant Cell 30, 1169–1170. doi: 10.1105/tpc.18.00453
Rubinigg, M., Posthumus, F., Ferschke, M., Elzenga, J. T. M., and Stulen, I. (2003). Effects of NaCl salinity on 15N-nitrate fluxes and specific root length in the halophyte Plantago maritima L. Plant Soil 250, 201–213.
Ruffel, S., Gojon, A., and Lejay, L. (2014). Signal interactions in the regulation of root nitrate uptake. J. Exp. Bot. 65, 5509–5517. doi: 10.1093/jxb/eru321
Sakuraba, Y., Chaganzhana Mabuchi, A., Iba, K., and Yanagisawa, S. (2021). Enhanced NRT1.1/NPF6.3 expression in shoots improves growth under nitrogen deficiency stress in Arabidopsis. Commun. Biol. 4:256. doi: 10.1038/s42003-021-01775-1
Sandhu, N., Sethi, M., Kumar, A., Dang, D., Singh, J., and Chhuneja, P. (2021). Biochemical and genetic approaches improving nitrogen use efficiency in cereal crops: a review. Front. Plant Sci. 12:657629. doi: 10.3389/fpls.2021.657629
Santamaria, P. (2006). Nitrate in vegetables: toxicity, content, intake and EC regulation. J. Sci. Food Agric. 86, 10–17.
Sanz-Luque, E., Chamizo-Ampudia, A., Llamas, A., Galvan, A., and Fernandez, E. (2015). Understanding nitrate assimilation and its regulation in microalgae. Front. Plant Sci. 6:899. doi: 10.3389/fpls.2015.00899
Scheible, W.-R., Gonzalez-Fontes, A., Lauerer, M., Muller-Rober, B., Caboche, M., and Stitt, M. (1997). Nitrate acts as a signal to induce organic acid metabolism and repress starch metabolism in tobacco. Plant Cell 9, 783–798. doi: 10.1105/tpc.9.5.783
Sherameti, I., Shahollari, B., Venus, Y., Altschmied, L., Varma, A., and Oelmüller, R. (2005). The endophytic fungus Piriformospora indica stimulates the expression of nitrate reductase and the starch-degrading enzyme glucan-water dikinase in tobacco and Arabidopsis roots through a homeodomain transcription factor that binds to a conserved motif in their promoters. J. Biol. Chem. 280, 26241–26247. doi: 10.1074/jbc.M500447200
Sisson, V. A., Rufty, T. W., and Williamson, R. E. (1991). Nitrogen-use efficiency among flue-cured tobacco genotypes. Crop Sci. 31, 1615–1620.
Soltabayeva, A., Srivastava, S., Kurmanbayeva, A., Bekturova, A., Fluhr, R., and Sagi, M. (2018). Early senescence in older leaves of low nitrate-grown Atxdh1 uncovers a role for purine catabolism in N supply. Plant Physiol. 178, 1027–1044. doi: 10.1104/pp.18.00795
Stitt, M., Müller, C., Matt, P., Gibon, Y., Carillo, P., Morcuende, R., et al. (2002). Steps towards an integrated view of nitrogen metabolism. J. Exp. Bot. 53, 959–970. doi: 10.1093/jexbot/53.370.959
Sun, C.-H., Yu, J.-Q., and Hu, D.-G. (2017). Nitrate: a crucial signal during lateral roots development. Front. Plant Sci. 8:485. doi: 10.3389/fpls.2017.00485
Sun, H., Li, J., Song, W., Tao, J., Huang, S., Chen, S., et al. (2015). Nitric oxide generated by nitrate reductase increases nitrogen uptake capacity by inducing lateral root formation and inorganic nitrogen uptake under partial nitrate nutrition in rice. J. Exp. Bot. 66, 2449–2459. doi: 10.1093/jxb/erv030
Tabaglio, V., Boselli, R., Fiorini, A., Ganimede, C., Beccari, P., Santelli, S., et al. (2020). Reducing nitrate accumulation and fertilizer use in lettuce with modified intermittent nutrient film technique (NFT) system. Agronomy 10:1208.
Tang, Z., Fan, X., Li, Q., Feng, H., Miller, A. J., Shen, Q., et al. (2012). Knockdown of a rice stelar nitrate transporter alters long-distance translocation but not root influx. Plant Physiol. 160, 2052–2063. doi: 10.1104/pp.112.204461
Tavakkoli, E., Rengasamy, P., and Mcdonald, G. K. (2010). High concentrations of Na+ and Cl– ions in soil solution have simultaneous detrimental effects on growth of faba bean under salinity stress. J. Exp. Bot. 61, 4449–4459. doi: 10.1093/jxb/erq251
Todeschini, M., Milioli, A., Trevizan, D., Bornhofen, E., Finatto, T., Storck, L., et al. (2016). Nitrogen use efficiency in modern wheat cultivars. Bragantia 75, 351–361.
Tong, W., Imai, A., Tabata, R., Shigenobu, S., Yamaguchi, K., Yamada, M., et al. (2016). Polyamine resistance is increased by mutations in a nitrate transporter gene NRT1.3 (AtNPF6.4) in Arabidopsis thaliana. Front Plant Sci 7:834. doi: 10.3389/fpls.2016.00834
Tong, Y., Zhou, J. J., Li, Z., and Miller, A. J. (2005). A two-component high-affinity nitrate uptake system in barley. Plant J. 41, 442–450. doi: 10.1111/j.1365-313X.2004.02310.x
Tsay, Y. F., Schroeder, J. I., Feldmann, K. A., and Crawford, N. M. (1993). The herbicide sensitivity gene CHL1 of Arabidopsis encodes a nitrate-inducible nitrate transporter. Cell 72, 705–713. doi: 10.1016/0092-8674(93)90399-b
Urrestarazu, M., Postigo, A., Salas, M., Sánchez, A., and Carrasco, G. (1998). Nitrate accumulation reduction using chloride in the nutrient solution on lettuce growing by NFT in semiarid climate conditions. J. Plant Nutr. 21, 1705–1714.
Van Oosten, M. J., Dell’aversana, E., Ruggiero, A., Cirillo, V., Gibon, Y., Woodrow, P., et al. (2019). Omeprazole treatment enhances nitrogen use efficiency through increased nitrogen uptake and assimilation in corn. Front. Plant Sci. 10:1507. doi: 10.3389/fpls.2019.01507
Vidal, E. A., Moyano, T. C., Riveras, E., Contreras-López, O., and Gutiérrez, R. A. (2013). Systems approaches map regulatory networks downstream of the auxin receptor AFB3 in the nitrate response of Arabidopsis thaliana roots. Proc. Natl. Acad. Sci. U.S.A. 110, 12840–12845. doi: 10.1073/pnas.1310937110
Vitor, S. C., Duarte, G. T., Saviani, E. E., Vincentz, M. G., Oliveira, H. C., and Salgado, I. (2013). Nitrate reductase is required for the transcriptional modulation and bactericidal activity of nitric oxide during the defense response of Arabidopsis thaliana against Pseudomonas syringae. Planta 238, 475–486. doi: 10.1007/s00425-013-1906-0
Wang, R., Liu, D., and Crawford, N. M. (1998). The Arabidopsis CHL1 protein plays a major role in high-affinity nitrate uptake. Proc. Natl. Acad. Sci. U.S.A. 95, 15134–15139. doi: 10.1073/pnas.95.25.15134
Wang, W., Li, A., Zhang, Z., and Chu, C. (2021). Posttranslational modifications: regulation of nitrogen utilization and signaling. Plant Cell Physiol. 62, 543–552. doi: 10.1093/pcp/pcab008
Wang, Y. Y., and Tsay, Y. F. (2011). Arabidopsis nitrate transporter NRT1.9 is important in phloem nitrate transport. Plant Cell 23, 1945–1957. doi: 10.1105/tpc.111.083618
Wang, Y.-Y., Hsu, P.-K., and Tsay, Y.-F. (2012). Uptake, allocation and signaling of nitrate. Trends Plant Sci. 17, 458–467.
Wege, S., Gilliham, M., and Henderson, S. W. (2017). Chloride: not simply a ‘cheap osmoticum’, but a beneficial plant macronutrient. J. Exp. Bot. 68, 3057–3069. doi: 10.1093/jxb/erx050
Wege, S., Jossier, M., Filleur, S., Thomine, S., Barbier-Brygoo, H., Gambale, F., et al. (2010). The proline 160 in the selectivity filter of the Arabidopsis NO3-/H+ exchanger AtCLCa is essential for nitrate accumulation in planta. Plant J. 63, 861–869. doi: 10.1111/j.1365-313X.2010.04288.x
Wegner, L. H., and Raschke, K. (1994). Ion channels in the xylem parenchyma of barley roots (A procedure to isolate protoplasts from this tissue and a patch-clamp exploration of salt passageways into xylem vessels. Plant Physiol. 105, 799–813. doi: 10.1104/pp.105.3.799
White, P. J., and Broadley, M. R. (2001). Chloride in soils and its uptake and movement within the plant: a review. Ann. Bot. 88, 967–988.
Wilkinson, J. Q., and Crawford, N. M. (1991). Identification of the Arabidopsis CHL3 gene as the nitrate reductase structural gene NIA2. Plant Cell 3, 461–471. doi: 10.1105/tpc.3.5.461
Wilkinson, J. Q., and Crawford, N. M. (1993). Identification and characterization of a chlorate-resistant mutant of Arabidopsis thaliana with mutations in both nitrate reductase structural genes NIA1 and NIA2. Mol. Gen. Genet. MGG 239, 289–297. doi: 10.1007/BF00281630
Woodrow, P., Ciarmiello, L. F., Annunziata, M. G., Pacifico, S., Iannuzzi, F., Mirto, A., et al. (2017). Durum wheat seedling responses to simultaneous high light and salinity involve a fine reconfiguration of amino acids and carbohydrate metabolism. Physiol. Plant. 159, 290–312. doi: 10.1111/ppl.12513
Xu, G., Fan, X., and Miller, A. J. (2012). Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 63, 153–182. doi: 10.1146/annurev-arplant-042811-105532
Yan, M., Fan, X., Feng, H., Miller, A. J., Shen, Q., and Xu, G. (2011). Rice OsNAR2.1 interacts with OsNRT2.1, OsNRT2.2 and OsNRT2.3a nitrate transporters to provide uptake over high and low concentration ranges. Plant Cell Environ. 34, 1360–1372. doi: 10.1111/j.1365-3040.2011.02335.x
Yokoyama, T., Kodama, N., Aoshima, H., Izu, H., Matsushita, K., and Yamada, M. (2001). Cloning of a cDNA for a constitutive NRT1 transporter from soybean and comparison of gene expression of soybean NRT1 transporters. Biochim. Biophys. Acta 1518, 79–86. doi: 10.1016/s0167-4781(01)00175-0
Yu, X., Sukumaran, S., and Mrton, L. (1998). Differential expression of the Arabidopsis nia1 and nia2 genes. cytokinin-induced nitrate reductase activity is correlated with increased nia1 transcription and mrna levels. Plant Physiol. 116, 1091–1096. doi: 10.1104/pp.116.3.1091
Zhang, H., Jennings, A., Barlow, P. W., and Forde, B. G. (1999). Dual pathways for regulation of root branching by nitrate. Proc. Natl. Acad. Sci. U.S.A. 96, 6529–6534. doi: 10.1073/pnas.96.11.6529
Zhang, J., Liu, Y.-X., Zhang, N., Hu, B., Jin, T., Xu, H., et al. (2019). NRT1.1B is associated with root microbiota composition and nitrogen use in field-grown rice. Nat. Biotechnol. 37, 676–684. doi: 10.1038/s41587-019-0104-4
Zhang, L., Yu, Z., Xu, Y., Yu, M., Ren, Y., Zhang, S., et al. (2021). Regulation of the stability and ABA import activity of NRT1.2/NPF4.6 by CEPR2-mediated phosphorylation in Arabidopsis. Mol. Plant 14, 633–646. doi: 10.1016/j.molp.2021.01.009
Zhao, C., Cai, S., Wang, Y., and Chen, Z.-H. (2016). Loss of nitrate reductases NIA1 and NIA2 impairs stomatal closure by altering genes of core ABA signaling components in Arabidopsis. Plant Signal. Behav. 11:e1183088. doi: 10.1080/15592324.2016.1183088
Zhao, L., Liu, F., Crawford, N. M., and Wang, Y. (2018). Molecular regulation of nitrate responses in plants. Int. J. Mol. Sci. 19:2039. doi: 10.3390/ijms19072039
Zhou, S., Gao, X., Wang, C., Yang, G., Cram, W. J., and He, G. (2009). Identification of sugar signals controlling the nitrate uptake by rice roots using a noninvasive technique. Z. Naturforsch. C 64, 697–703. doi: 10.1515/znc-2009-9-1015
Keywords: N fertilization, nitrate sensing, chloride toxicity, chloride beneficial macronutrient, salinity eustress
Citation: Carillo P and Rouphael Y (2022) Nitrate Uptake and Use Efficiency: Pros and Cons of Chloride Interference in the Vegetable Crops. Front. Plant Sci. 13:899522. doi: 10.3389/fpls.2022.899522
Received: 18 March 2022; Accepted: 20 May 2022;
Published: 16 June 2022.
Edited by:
Angelo Signore, University of Bari Aldo Moro, ItalyReviewed by:
Miguel A. Rosales, Institute of Natural Resources and Agrobiology of Seville (CSIC), SpainInderjot Chahal, University of Guelph, Canada
Copyright © 2022 Carillo and Rouphael. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Petronia Carillo, cGV0cm9uaWEuY2FyaWxsb0B1bmljYW1wYW5pYS5pdA==
 Petronia Carillo
Petronia Carillo Youssef Rouphael
Youssef Rouphael