- Department of Grassland Resources and Ecology, College of Grassland Science and Technology, China Agricultural University, Beijing, China
The majority of terrestrial plants can form symbiotic associations on their roots with arbuscular mycorrhizal fungi (AMF) in the soil to stimulate the growth and nutrient uptake of the host plant and to improve plant resistance to insects and disease. However, the use of AMF for insect control on gramineous forages requires further study. Here, we evaluated the effects of AMF (Funneliformis mosseae) inoculation on the defense against Locusta migratoria attack in Elymus nutans. Inoculation assays showed that mycorrhizal plants had a higher resistance than non-inoculated plants, as evidenced by plants having more plant biomass, a higher nitrogen and phosphorus content, and greater lipoxygenase (LOX) activity. The results of insect damage showed that in addition to a decrease in the enzyme phenylalanine-ammonia-lyase, the activities of other plant defense-related enzymes (including polyphenol oxidase and β-1,3-glucanase) were increased. A key enzyme, LOX, belonging to the jasmonic acid (JA) signaling pathway was notably increased in mycorrhizal treatment. Volatile organic compounds (VOCs) were identified using gas chromatography mass spectrometry and the results showed that several metabolites with insect-resistant properties, including D-Limonene, p-Xylene, 1,3-Diethylbenzene were detected in mycorrhizal plants. These findings suggest that mycorrhizal inoculation has potential applications in insect management on forage grasses and demonstrates that the JA signaling pathway is essential for insect resistance in Elymus nutans.
Introduction
Plants have evolved a series of complex, protective strategies in order to enhance their fitness and survival under herbivore attack and biotic stresses (Sharma et al., 2017; Frew et al., 2022). One important strategy is the formation of symbiotic relationships between plant roots and some specific fungi, such as arbuscular mycorrhiza (Begum et al., 2019; Dowarah et al., 2021).
Arbuscular mycorrhizal fungi (AMF) belong to the subphylum Glomeromycotina of the phylum Mucoromycota (Spatafora et al., 2016). They are important beneficial fungi that form symbiotic relationships with over 90% of plant roots including ferns, herbaceous plants, and some economically important crop species (Begum et al., 2019; Selvaraj et al., 2020). AMF are obligate biotrophs that ingest plant photosynthetic products (Dowarah et al., 2021) and lipids to support their life cycle (Jiang et al., 2017). AMF symbiosis can help plants to obtain essential nutrients from the soil, such as nitrogen, phosphorus, and potassium, absorb other trace nutrients from resource-deficient soils, and assist plant roots to take-up more water from the soil (Kayama and Yamanaka, 2014; Bhantana et al., 2021; Jiang et al., 2021). In return, plants supply AMF with carbohydrates and lipids that can be used to develop extensive mycelial networks (Bernaola et al., 2018). These mycelial networks act as conduits for carbon and mineral nutrient transmission (Jiang et al., 2017; Luginbuehl et al., 2017); they also signal transmission channels between plants to initiate an early warning for disease and herbivore attack (Basu et al., 2018; Begum et al., 2019).
Arbuscular mycorrhizal fungi confer benefits to their host plant in many ways, including enhancement in nutrient uptake and protection against stressors (Dowarah et al., 2021). Most importantly, AMF inoculation is known to induce the activation of plant resistance and protect plants from phytopathogenic fungi, bacteria, viruses, and herbivores (Hao et al., 2019; Miozzi et al., 2019; Jiang et al., 2021). Mycorrhizal symbiosis have been reported to respond variably to above-ground insect damage, including positive (Babikova et al., 2013; Meier and Hunter, 2018), negative (Bernaola et al., 2018; Bolin et al., 2018), and neutral (Gehring and Bennett, 2009) effects on plant defense. In addition, the defense response of mycorrhizal plants to herbivorous insects has been found to vary with the species involved and the growth stage of the plant (Malik et al., 2018) and the AMF (Selvaraj et al., 2020). Specifically, mycorrhizal inoculation improves plant resistance to generalist herbivores and insects that are sensitive to jasmonic acid (JA)-associated defenses (Frew et al., 2022).
Plants can release distinct blends of volatile organic compounds (VOCs) following an external attack, including alkenes, terpenes, and aldehydes (Sharma et al., 2017; Ye et al., 2018). VOCs can be classified according to their metabolic pathway: terpenoids [mevalonate/2-methyl-D-erythritol-4-phosphate (MVA/MEP) pathway], volatile fatty acid derivatives [lipoxygenase (LOX) pathway], and benzenic compounds and amino acid derivatives [shikimate (SK) pathway] (Dudareva et al., 2004; Velásquez et al., 2020a). Plants can communicate with both distant plants (Heil and Ton, 2008), and neighboring plants (Karban et al., 2016) via VOCs, and this airborne signal allows receptor plants to invoke defense systems prior to the threat. Specifically, VOCs can induce the expression and production of defense-related enzymes (polyphenol oxidase, PPO; β-1,3-glucanase, phenylalanine-ammonia-lyase, PAL; and lipoxygenase, LOX), hormones (JA, and salicylic acid, SA), protease inhibitor I and II genes (PI-I and PI-II) and other related secondary metabolites in recipient plants, activating the defense system (Jung et al., 2012; Song et al., 2013). Recent studies have found that plant volatile emissions are strongly influenced by environmental stimuli, including plant-microorganism interaction which plays a key role in plant development (Valenzuela et al., 2019; Velásquez et al., 2020a). For one thing, the impact of mycorrhizal symbiosis extends beyond plant growth, enhancing plant defenses by promoting the synthesis of secondary metabolites such as VOC. For example, milkweeds inoculated with AMF release more VOC after being fed on by insects (Meier and Hunter, 2019). For another, AMF affect the diversity of plant primary and secondary metabolites by altering plant nutrient uptake and defensive signaling pathways (Schweiger et al., 2014; Begum et al., 2019), including the SA and JA pathways (Frew et al., 2022), which are essential defense systems to herbivore feeding (Meier and Hunter, 2019; Dowarah et al., 2021). Song Y. Y. et al. (2015) showed that JA-related genes and defense enzymes (LOX and PPO) are notably up-regulated in receptor tomato plants through common mycorrhizal networks, when neighboring plants are eaten by herbivores. However, these mycorrhiza-related studies mainly focused on cultivated crops (Babikova et al., 2013; Song Y. Y. et al., 2015; Selvaraj et al., 2020) and less information is available regarding the tripartite interactions among AM fungi, gramineous forage, and phytophagous insects.
Elymus nutans (Elymus nutans Griseb.) is a perennial gramineous grass that grows in alpine regions (Xu et al., 2018). It is highly resistant to drought, cold, and salt, and has become a preferred forage species for planting in high altitude areas (Tan et al., 2020; Quan et al., 2021). As the forage livestock industry expands at high altitudes, the areas of cultivated grasslands growing E. nutans will continue to increase; therefore, the stability of E. nutans swards in alpine regions needs to be addressed. Grasshopper feeding can cause necrotic spots or even plant mortality, thus reducing the palatability of E. nutans and affecting livestock grazing (Hao H. et al., 2019). Furthermore, E. nutans can form a symbiotic relationship with AMF (Gai et al., 2009). However, whether this symbiosis enhances E. nutans resistance to grasshopper attack and what types of signaling substances are induced by AMF inoculation with Elymus spp., transduction pathways, physiological effects, and mechanisms of action have not been systematically explored. Therefore, we selected E. nutans in an alpine meadow as host plants to investigate the response of inoculation with F. mosseae to insect attack. We sought to (i) determine the response and main defense pathways of E. nutans to grasshopper feeding and (ii) elucidate the role of AMF on plant growth and defense against insects. The hypotheses were (i) E. nutans will produce large amounts of defense-related enzymes to activate relevant defense pathways. (ii) AMF-colonized plants will have higher nutrient content, increased defense enzyme activity, and will release more VOCs.
Materials and Methods
Plant, Fungal, and Insect Materials
Elymus nutans seeds were collected from natural alpine meadows in Haiyan County, Haibei Tibetan Autonomous Prefecture, Qinghai Province, China (36°55′ N, 100°57′ E, 3029 m a.s.l.) in August 2021.
The mycorrhizal fungus Funneliformis mosseae (BGCYN05), isolated from white clover and maize, was obtained from the Beijing Academy of Agricultural and Forestry Sciences and propagated by the Guizhou Academy of Agricultural Sciences. These AMF inocula contained root fragments, rhizosphere soil, and 133 spores per gram of dry soil.
The grasshoppers Locusta migratoria (Orthoptera, Locusta Linnaeus) were collected from a Qinghai Haibei alpine meadow. As the dominant locust species, the density of L. migratoria was 10 grasshopper/m2 (Hao et al., 2019). They were reared on gramineous plants in the laboratory (28 ± 2°C; 18 h day:8 h night; 30–50% relative humidity) (Babikova et al., 2013; Veenstra et al., 2021).
Growth Medium
Field soil was obtained from natural alpine grassland in Haiyan County, Haibei Tibetan Autonomous Prefecture, Qinghai Province, China (36°55′ N, 100°57′ E, 3029 m a.s.l.). All soils were sieved with a 2-mm sieve, autoclaved at 121°C for 1 h twice within 3 days and dried at 110°C for 36 h (Li et al., 2021). The physical and chemical properties were: 4.1 g⋅kg–1 total nitrogen, 48 g⋅kg–1 total carbon, 0.69 g⋅kg–1 total phosphorus, and pH 8.2. Each pot was filled with 2.5 kg of sterilized soil.
Experimental Design
The pot trials took place in a greenhouse at the China Agricultural University from December 2020 to April 2021. F. mosseae was used for mycorrhizal inoculation of E. nutans, and the grasshopper L. migratoria was used for insect feeding. Experiment designs consisted of one AMF inoculation factor (inoculate with F. mosseae, F), one insect feeding factor (attack by grasshopper L. migratoria, L) and their interaction. The whole experiment had four treatments with five replications each to exclude possible effects of sole AMF inoculation and grasshopper feeding; CK (plants without AMF inoculation and grasshopper feeding), Fm (plants only inoculated with F. mosseae), Lm (non-inoculated plants with grasshopper feeding), and Fm + Lm (plants inoculated with AMF plus grasshopper feeding). There were 20 experimental pots, and each pot was placed randomly.
Seeds were surface sterilized with 10% H2O2 and rinsed five times with sterile distilled water before sowing in autoclaved soil (121°C; 2 h). Forty to fifty plants from the original 80 were chosen randomly per pot and watered regularly four times a week to maintain soil moisture. One week after germination, 40 healthy seedlings with good growth were kept.
Thirty grams of AMF inoculum (fungal: soil mixture) were spread on the soil to a depth of 2 cm. For non-AMF treatments, an equal amount of soil containing autoclaved fungi was added (121°C; 2 h). Insect feeding treatments took place when E. nutans plants were 14 weeks old. Before the formal experiment, all plants were placed in polyethylene terephthalate (PET) bags to prevent plant-to-plant communication via aerial volatiles, and grasshoppers were starved for 2 h. Subsequently, 12 fifth-instar grasshoppers were placed in each pot of Lm and Fm + Lm treatments to feed on the plants.
After 24 h of grasshopper feeding, we collected VOCs and removed the insects (all grasshoppers were alive). The plants continued growing for 48 h and were then harvested for the measurement of plant nutrient content, biomass, and AMF colonization of roots.
Mycorrhizal Colonization Analysis
Mycorrhizal colonization was examined following the procedure of Koske and Gemma (1989). After harvesting of plant roots, they were washed with distilled water to remove soil particles, and approximately 0.5–1.0 g of roots were cut into 1 cm segments. Root segments were soaked in KOH (10%, w/v) and boiled until the roots were transparent. Then root segments were acidified in HCl (2%, v/v), and stained with trypan blue (0.05%, w/v). After 30 min, these roots were immersed in destaining solution (glycerol: lactic = 1:1). Finally, stained roots were mounted on slides and AMF colonization was calculated using the magnified gridline intersect method (McGonigle et al., 1990) with a compound microscope under 40× magnification. A root intersection was considered colonized if hyphae, arbuscules, or vesicles were present.
Enzyme Assays
The kits for assaying the PPO, PAL, β-1,3-glucanase, and LOX were obtained from Solarbio Science & Technology Co., Ltd. (Beijing, China). All the chemicals used were analytical (Li et al., 2019). Leaf samples (0.1 g) were ground in liquid nitrogen with different 1 ml extractive solutions; they were then centrifuged and homogenated, and the supernatants were used for enzyme assays. A Microplate reader (SpectraMax iD5, Molecular Devices, San Jose, CA, United States) and 96-well plates were used. PPO enzyme absorbance was recorded at 410 nm for the measurement and control tubes; a change in absorbance at 410 nm of 0.005/min was defined as a unit of enzyme activity. PAL enzyme absorbance was read at 290 nm; a change in absorbance at 290 nm of 0.05/min was defined as a unit of enzyme activity. β-1,3-glucanase enzyme was measured at 540 nm for the measurement and control tubes, then a standard curve was built to calculate enzyme activity. LOX enzyme catalyzed the oxidation of linolenic acid, and the oxidation product had a characteristic absorption peak at 234 nm; a change in absorbance at 234 nm of 0.0006/min was defined as a unit of enzyme activity.
Collection of Plant Volatile Organic Compounds
Plants and grasshoppers were placed in PET bags (100 cm × 111 cm, low volatility at high temperatures and high light intensities). After 24 h of insect feeding, volatiles were collected using an aerated kit (Babikova et al., 2013). The air was purified by activated carbon through a dry glass sorbent tube (0.5 cm diameter, 8.0 cm long) and the ends of this tube were plugged with clean glass fiber, containing 50 mg of sorbent (Porapak Q, 80–100 mesh, Waters Corporation, Ireland). After the air had been extracted from the bag for a short time, sampling began after 30 min. During this process, the gas in the bag was adsorbed by the sorbent. The flow rate was 300 ml min–1 and the extraction was continuous for 6 h. The sorbent was eluted with 4 ml of chromatographic n-hexane into 2 ml sample bottles and the samples were then stored at −20 °C in a deep freezer for later use.
GC-MS Analysis of Plant VOCs We analyzed a 2 μl aliquot of a VOC sample by gas chromatography mass spectrometry (GC-MS, Agilent Technologies, Santa Clara, CA, United States) using the following GC method: Injector maintained at 220°C, initial column temperature maintained at 50°C for 10 min, ramped to 200°C at 5°C min–1, and held for 10 min, with a helium carrier gas flow rate of 1 ml min–1. We used a DB-5MS column (30 m × 0.25 mm × 0.25 μm film; J&W Scientific, Folsom, CA, United States) with a 0.25-μm film thickness (Velásquez et al., 2020b). Then VOCs were initially identified by using the mass spectra with a library of authentic standards or databases (NIST 08, National Institute of Standards and Technology, Gaithersburg, MD, United States, 2008). The relative concentrations of VOCs were determined by comparing each peak area with the total peak area in each treatment.
We selected random harvested plant material (roots, stems, and leaves) to measure nutrient content. They were dried at 65 °C for 24 h and milled with a ball mill (Retsch MM400, Retsch, Haan, Germany) Samples of 0.15-g sieved plant material were weighed and placed into tin cups. Plant total nitrogen and total carbon contents were determined using an elemental analyzer (Elementar, Hanau, Germany); total phosphorus was determined by the HClO4-H2SO4 method (Han et al., 2021).
Statistical Analysis
All available data were analyzed by SPSS 19.0 (Inc., Armonk, NY, United States). Two-way analysis of variance (ANOVA) was used to examine the effects of inoculation and insect feeding on plant biomass, defense-related enzyme activities and plant nutrient content. Differences among treatments were performed at P < 0.05 by Duncan’s multiple range test (DMRT). The results were given as means with standard errors (mean ± SE). Origin2018 (OriginLab, United States) was used for plotting.
Results
Mycorrhizal Colonization Rate and Plant Biomass
Mycorrhizal colonization structures such as vesicles and mycelium were observed in all inoculation treatments, which indicated successful inoculation with AMF (Figure 1A). Mycorrhizal colonization rates were similar between the two inoculation treatments (Figure 1B). Grasshopper feeding resulted in a small amount of aboveground biomass (Supplementary Figure 1). Mycorrhizal inoculation and insect feeding significantly affected plant biomass (P < 0.05). The Fm treatment had the greatest aboveground biomass, 53.53% higher than non-inoculated CK treatment (F = 13.82; P < 0.05). Insect attack caused a decrease in the aboveground biomass of plants, the Fm + Lm treatment was still higher than that of the Lm treatment, similar to the aboveground biomass of plants that were not inoculated (Figure 2A). The belowground biomass of Fm treatment was significantly higher than other treatments (Figure 2B). After the plants were attacked by insects, inoculated plants showed higher belowground biomass, at 43.46% higher in Fm + Lm than in the Lm treatment (F = 96.64; P < 0.05).
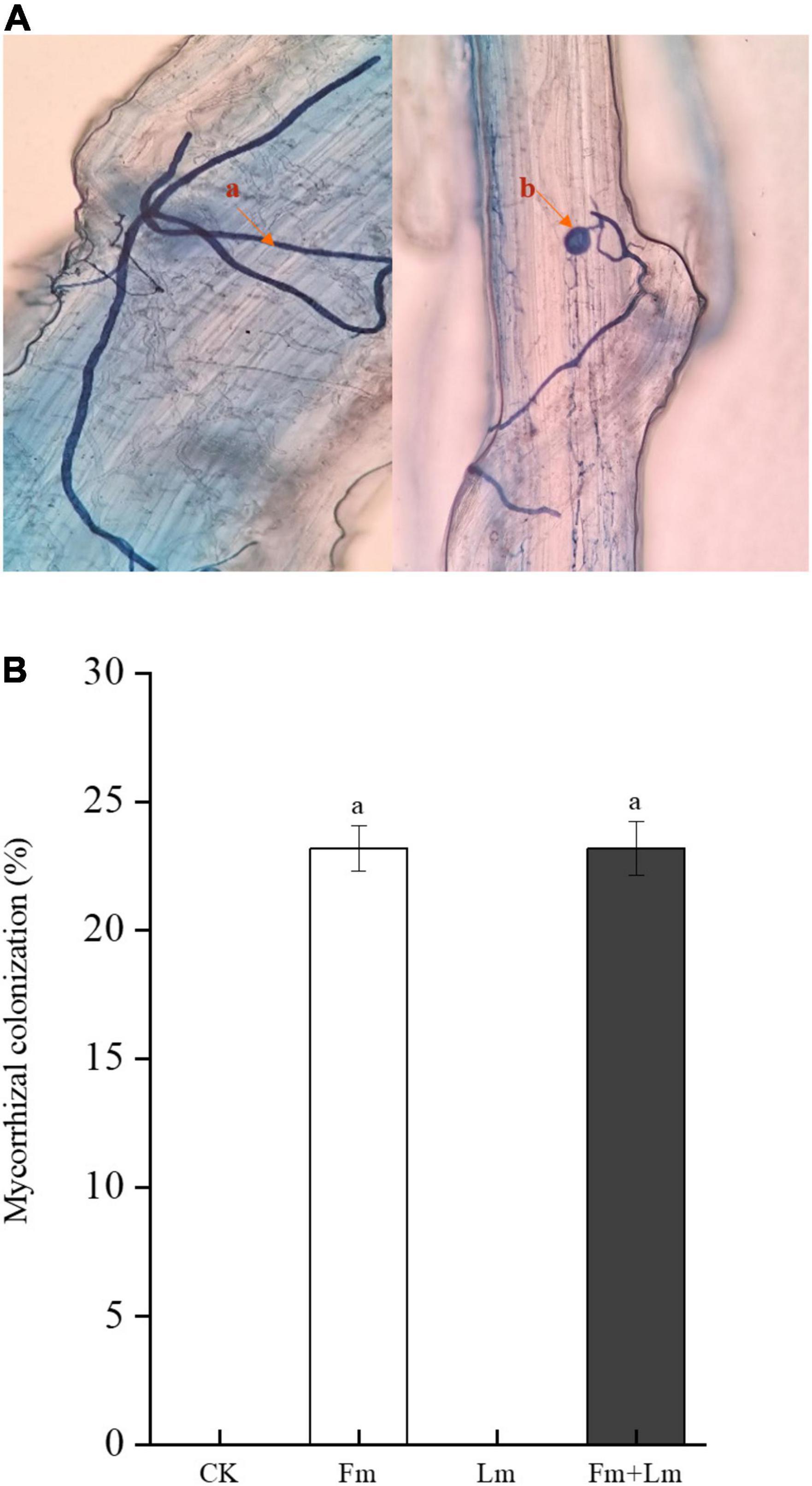
Figure 1. The mycorrhizal colonization structure of AMF observed under 40× magnification after (A) the root segments of E. nutans were stained with trypan blue (B) (0.05%, w/v) and the percentage of root mycorrhizal colonization in plants. The four treatments were (1) CK: no inoculation; (2) Fm: inoculated with F. mosseae only; (3) Lm: feeding with L. migratoria only; (4) Fm + Lm: inoculated with F. mosseae and L. migratoria feeding. (a) Internal hyphae; (b) Vesicles.
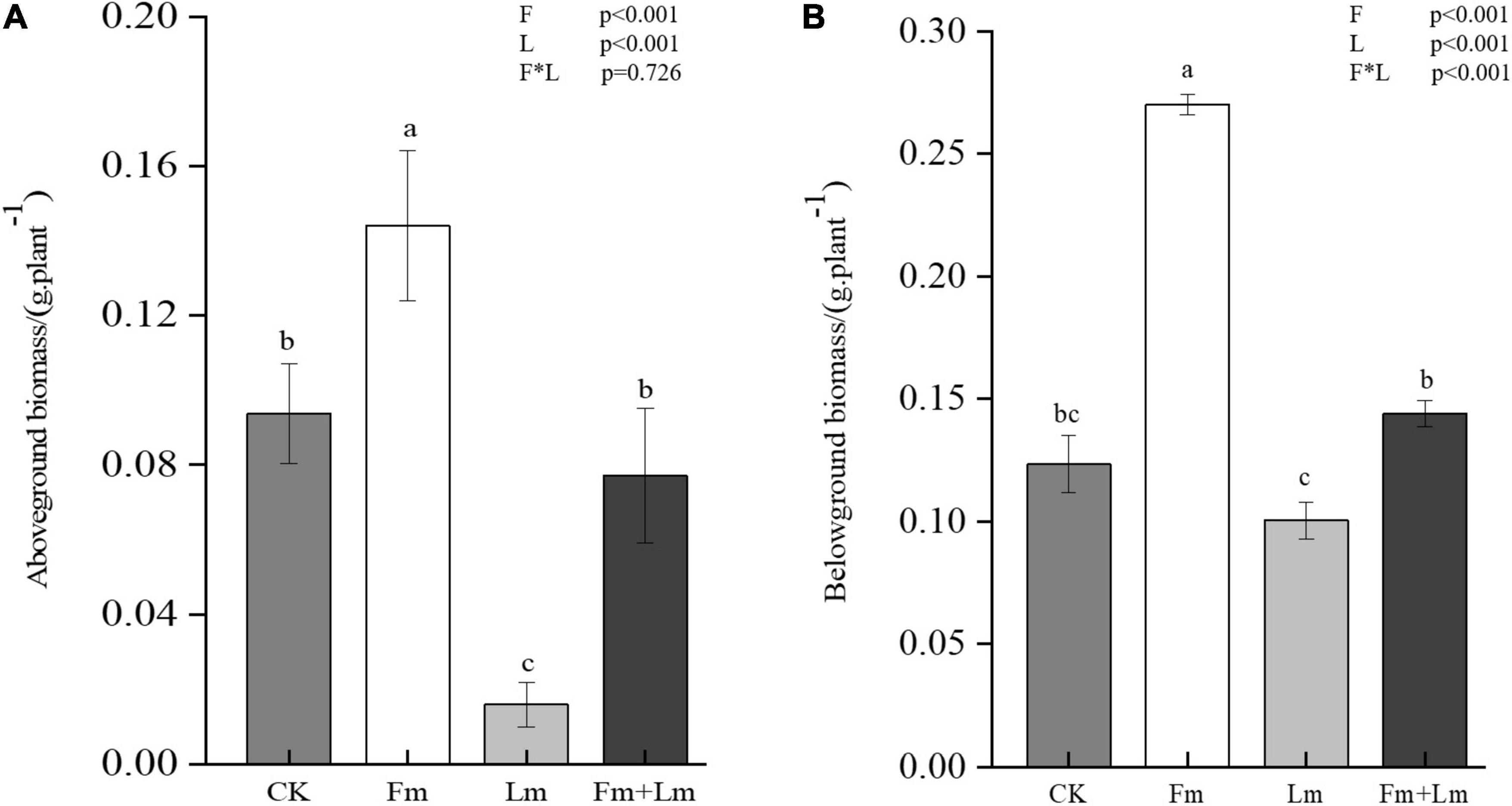
Figure 2. Plant aboveground biomass (A) and belowground biomass (B) under different treatments. The four treatments were (1) CK: no inoculation; (2) Fm: inoculated with F. mosseae only; (3) Lm: feeding with L. migratoria only; (4) Fm + Lm: inoculated with F. mosseae and L. migratoria feeding. Values are means ± standard error. P-values for inoculation and insect feeding effects were based on two-way ANOVA, F: inoculated AMF; L: insect feeding. Mean values followed by the same letter do not differ significantly at P ≤ 0.05 by DMRT.
Plant Nutrient Content
Arbuscular mycorrhizal fungi inoculation promoted the uptake of nutrients by E. nutans, the inoculated plants contained more nitrogen and phosphorus than non-inoculated plants (Supplementary Table 1). Total nitrogen content in Fm plants was 16.07 g⋅kg–1 (Figure 3A), a significant increase of 30.11% compared to CK (F = 102.42; P < 0.05); But, insect attack significantly affected plant nutrient content (P < 0.001). After grasshopper feeding, all insect treatments showed a reduced nutrient content (Figure 3B and Supplementary Table 1). Plant total phosphorus content decreased by 26.70% in Fm + Lm and 18.57% in Lm compared to Fm and CK treatments, respectively. Plant carbon content decreased after AMF inoculation, the total carbon content of CK treatment was 395.98 g⋅kg–1, which was notably higher than that of Fm (Figure 3C). Insect feeding further reduced the total carbon content of plants; Fm + Lm decreased by 2.22% compared to Fm and Lm treatments, which decreased by 4.17% (F = 15.932; P < 0.05).
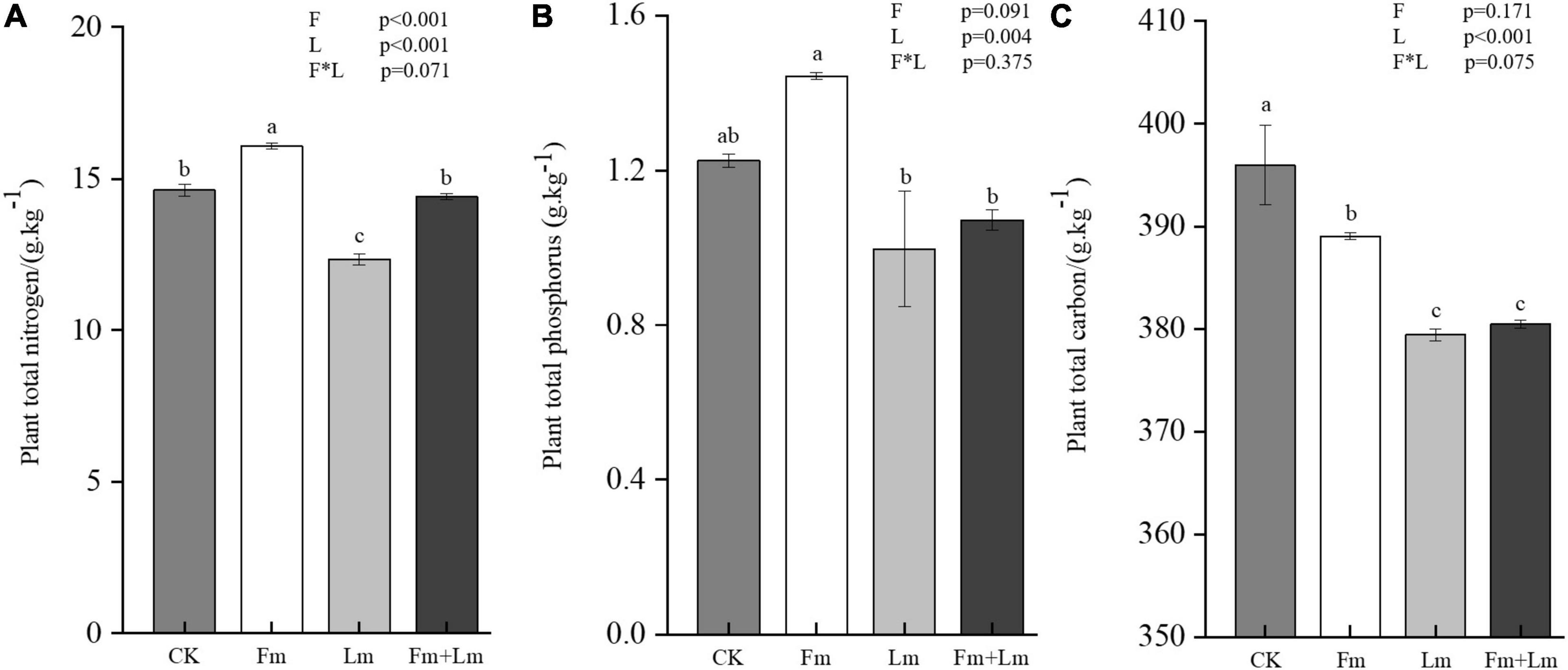
Figure 3. The effects of different treatments on plant nutrition. Total nitrogen, (A) total phosphorus, (B) and total carbon (C) of plant leaves. Four treatments (CK, Fm, Lm, and Fm + Lm) were established as described in Figure 2. Values are means ± standard error. P-values for inoculation and insect feeding effects were based on two-way ANOVA. Mean values followed by the same letter do not differ significantly at P ≤ 0.05 by DMRT.
Induction of Defense-Related Enzymes by Mycorrhizal Inoculation
Both inoculation and insect feeding significantly influenced the expression of plant defense-related enzyme activities (P < 0.05) (Table 1). PPO and β-1,3-glucanase enzyme activities were significantly lower in the Fm treatment compared to the CK treatment (P < 0.05). The two enzyme activities were increased in Fm + Lm and Lm treatments after insect attack, with 65.79 and 19.83% increase in Lm treatment compared to CK treatment, respectively. However, mycorrhizal inoculation decreased the expression of the two enzyme activities in insect treatments (Fm + Lm, Lm), PPO, β-1,3-glucanase enzyme activities in the Fm + Lm treatment were decreased by 54.38 and 17.92% compared to the Lm treatment, respectively. No matter inoculation or insect attack, PAL enzyme activity was decreased, but there was no significant differences among treatments.

Table 1. The activity of four defense-related enzymes in leaves of Elymus nutans plants in response to mycorrhizal colonization by F. mosseae and herbivorous feeding by fifth-instar grasshoppers L. migratoria.
After plants were inoculated with AMF, the LOX enzyme activity of mycorrhizal plants was significantly higher compared to CK treatment (Table 1). And grasshopper feeding caused an increase in LOX enzyme activity in the Lm treatment, which was about 2.5 times higher than that in CK treatment, but still lower than that in the Fm treatment. Moreover, inoculation further increased the expression of LOX enzyme activity in plants, and the LOX enzyme activity in the Fm + Lm treatment was significantly increased by 57.25% (F = 15.268; P < 0.01) compared with Fm treatment, which was about twice as much as that in the Lm treatment. Two-way ANOVA showed no significant effects of the interaction between inoculation and insect feeding on LOX enzyme activity expression.
Composition and Concentration of Volatile Organic Compounds
Grasshopper feeding resulted in VOCs released from E. nutans. A total of 24 VOCs were produced after plants were attacked by insect, and these metabolites include ketones, aldehydes and benzenic compounds (Table 2). The Lm treatment detected 18 VOCs, of which were “benzenic compounds” (6) (1-methylpropyl Benzene, p-ethyl-Cumene, 4-Ethyltoluene, 1,2,3-Trimethylbenzene, 1,4-Diethylbenzene, Cumene), “ketones” (4) (2′-Methylacetophenone, 2,4-Dimethylacetophenone, 2,5-Dimethylacetophenone, 4′-Ethylacetophenone), “alkenes” (2), “alkanes” (2), “aldehydes” (2), “alcohols” (1), and ethyl acetate. The VOCs with high relative concentrations in the Lm treatment were 2,4-Dimethylacetophenone, β-Cymene, and 1,4-Diethylbenzene. Ethyl acetate was only released from non-AMF plants after grasshopper feeding. There were 19 VOCs detected in Fm + Lm treatment, including “benzenic compounds” (9), “ketones” (3), “alkenes” (3), “alkanes” (2), “aldehydes” (1), “alcohols” (1). Mycorrhizal inoculation led plants to release some different, insect-resistant compounds, like 1,3-Diethylbenzene, D-Limonene, p-Xylene, p-Methylcumene. The VOCs with high relative concentrations in the Fm + Lm treatment were 2,4-dimethylacetophenone, D-Limonene and p-Xylene. Regardless of whether AMF was inoculated or not, 13 common VOCs were detected in E. nutans after the insect feeding and the relative concentration of 2,4-dimethylacetophenone was the greatest; however, mycorrhiza slightly reduced emissions.
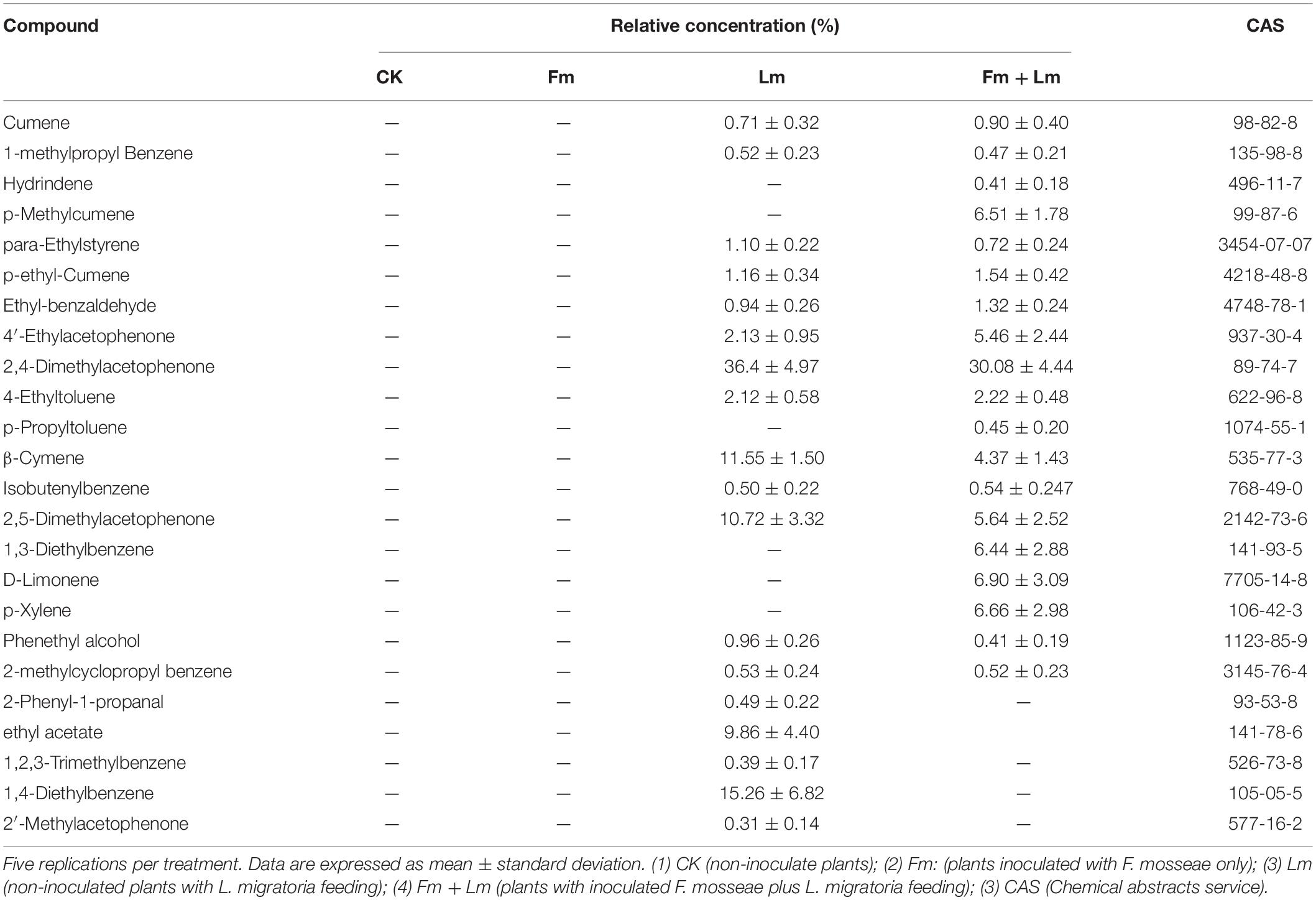
Table 2. The composition and concentration of volatile organic compounds (VOCs) collected from plants through GC-MS analysis, included four treatments.
Discussion
Mycorrhizal symbiosis as a potential pest control strategy may be an effective alternative to some chemical pesticides in contemporary agriculture (Jiang et al., 2021). However, the applicability of this insect resistance mechanism in alpine forage plants is unknown. In the present study, we investigated the results of insect–plant–microbe interactions, the first report of a tripartite interaction between Elymus nutans, AMF, and a chewing insect.
It has been commonly reported in both laboratory and field experiments that plant inoculation with AMF promote growth and increase biomass, a phenomenon considered as the positive mycorrhizal growth response (MGR) (Bernaola et al., 2018; Zhang et al., 2019). Positive MGR was previously found to be due to the improved uptake and transfer of nutrients (usually phosphorus and nitrogen) (Jiang et al., 2021), which is consistent with the findings of this study. E. nutans inoculated with F. mosseae significantly increased plant biomass compared to the non-inoculated treatment, in addition to increasing the uptake of nitrogen and phosphorus. This improvement is probably because a common mycorrhizal network can greatly improve the efficiency of use of soil nutrients by increasing the contact area between the root system and soil via a greater number of extraradical hyphae (Begum et al., 2019). There is a consequent increase in concentration of various macro-nutrients and micro-nutrients in plants, which increases the production of photosynthetic products, and leads to greater biomass accumulation (Balliu et al., 2015; Chen et al., 2017). Therefore, AMF assist plant development under normal as well as stressful circumstances and improve plant tolerance to biotic and abiotic factors (Plassard and Dell, 2010; Begum et al., 2019).
The soil used in this study was native soil from alpine meadows where the F. mosseae had a good symbiotic relationship with E. nutans (Gai et al., 2009), but we used commercial AMF inocula whose host plants were maize and clover, the experiment results revealed a low mycorrhizal colonization rate (Jin et al., 2011; Xu et al., 2018). The reason may be explained by the fact when E. nutans inoculated with AMF from the other plants rhizosphere soil, the other plants produced secondary metabolites which cause negative effects on plant growth (Wang et al., 2019), and possibly because of the lignification of plant roots intensified—it was not favorable for AMF colonization (Xu et al., 2018). However, AMF could still promote the nutrient uptake and utilization of E. nutans, probably because the small amount of AMF inocula diluted the potential allelopathy effects of the host plant (Wang et al., 2019). As Bati et al. (2015) showed, irrespective of mycorrhizal inoculation (commercial or native), AMF promoted nutrient uptake and plant growth. Therefore, the low colonization rate of E. nutans in the current experiment was acceptable. The total carbon in plants is an important physiological parameter reflecting their carbon metabolism and an important indicator of a plant’s physiological condition, growth, vitality, and disease resistance (Jiang et al., 2017; Charters et al., 2020). Extra disturbance will affect the carbon allocation of plants and could then influence AMF activity (Walder and van der Heijden, 2015; Charters et al., 2020). We found that after AMF inoculation, the total carbon content of plants (Fm, Fm + Lm) decreased compared with the non-inoculated treatment (CK), while AMF inoculation (Fm) increased the phosphorus content of plants. However, after grasshopper feeding, the total carbon and phosphorus content decreased in the Fm + Lm treatment. These results suggested that the insects reduced belowground plant carbon allocation and ultimately aboveground phosphorus, possibly as AMF resulted in a lower transfer of phosphorus to the host plant (Frew, 2021). Girousse et al. (2005) found that aphid feeding had a profound effect on plant carbon allocation, which was detrimental to mycorrhizal fungi. The result indicated that herbivore insects could drive asymmetry in the nutrient exchange between mycorrhizal symbionts (Walder and van der Heijden, 2015). We therefore speculated that short-term changes in external biological carbon sinks may have altered the nutrient supply of E. nutans to AMF; this finding was similar to that of Charters et al. (2020), but there was insufficient evidence to prove this. Greater attention could be paid to this point in future experiments.
Mycorrhizal inoculation enhances direct and indirect plant defense systems (Jung et al., 2012; Velásquez et al., 2020b). When inoculated plants are eaten by herbivores, mycorrhizal symbionts increase the release of defense metabolites, through interactions with phytohormone signaling pathways (Meier and Hunter, 2018). Study have demonstrated that the JA signaling pathway plays a critical role in mediating plant defense in response to herbivorous insects (Frew et al., 2022). Therefore, we tested defense-related enzyme activities including PPO, which catalyzes the formation of lignin and other oxidative phenols (Song et al., 2010). PAL is involved in the biosynthesis of SA signal molecules and phytoalexin or phenolic compounds (Selvaraj et al., 2020). LOX, a key enzyme of the JA signaling pathway, catalyzes the initial reaction in the JA biosynthesis pathway, and β-1,3-glucanase is able to degrade fungal cell walls, causing lysis of fungal cells to participate in the SA defense pathway (Zhao et al., 2009; Song Y. Y. et al., 2015). Song et al. (2013) found that mycorrhizal colonization could initiate the JA signaling defense pathway in tomatoes and upregulate the expression of genes that synthesize LOX enzymes in response to caterpillar feeding. This could explain our result that LOX was significantly increased in mycorrhizal treatments. The LOX enzyme activity of Fm + Lm was approximately double that of the non-inoculated Lm treatment. Thus, despite the low levels of colonization in our experiments, we could still detect significant impacts of AMF on insect attack (Bernaola et al., 2018). However, the activities of PAL and β-1,3-glucanase decreased after AMF inoculation and grasshopper feeding did not notably increase these plant enzyme activities, suggesting that E. nutans may be predominantly protected against adverse external factors through the JA pathway and that the SA pathway does not play a dominant role. Previous studies found that once the JA defense pathway was up-regulated, the SA pathway was inhibited (Song Y. Y. et al., 2015; Schoenherr et al., 2019).
Previous research has also demonstrated that AMF could change the concentration and composition of VOCs (Sharma et al., 2017; Meier and Hunter, 2018), which alter plant attractiveness to insect behavior (Babikova et al., 2014; Meier and Hunter, 2019). The genus Glomus can be seen as a potent AMF in imparting biotic stress tolerance in a wide range of plants that are eaten by herbivores (Dowarah et al., 2021). Our results, in addition to findings from previous studies, clearly show that some of the genus Glomus fungi could induce plants to produce large amounts of volatile compounds, and these VOCs included terpenes, alcohols, esters, and small amounts of alkanes (Roger et al., 2013). In the present study, insect feeding led E. nutans to produce more terpenoids such as 2,4-Dimethylacetophenone and 2,5-Dimethylacetophenon, which have been shown to deter herbivores (Sharma et al., 2017). Ethyl acetate was only detected in the non-inoculated treatment, with low relative concentrations of other VOCs. The Fm + Lm treatment produced a greater variety of VOCs and metabolites with insect-repelling properties. AMF-inoculated plants increased the relative concentration of benzenic compounds compared to non-AMF plants. Benzenic compounds are the main components of various essential oils and are involved in plant reproduction and defense (Dudareva et al., 2004; Velásquez et al., 2020a). D-limonene and 1,3-diethylbenzene could be produced in other insect-infested plants and it has an important role in improving plant defenses and the repellence of insects (Agut et al., 2015; Kigathi et al., 2019; Mitra et al., 2021). p-Xylene mainly acts as an attractant to natural enemies of the feeding insect (Li et al., 2022). Taken together, these results suggest that AMF F. mosseae colonization can improve the chemical defense of E. nutans.
In conclusion, inoculation of E. nutans with F. mosseae improved the uptake of nutrients and induced resistance to grasshopper attack. AMF promote the expression of defense-related enzymes and the types of insect-resistant VOCs increased (Figure 4). The JA pathway was also found to be the main insect resistance pathway in E. nutans, with mycorrhizal colonization further inducing to strength the defense response. Furthermore, insect feeding could reduce the nutrient content of plants. Thus, the initiation of defense by common symbiotic organisms may be an important evolutionary strategy for plant defense against herbivores and other biotic stresses. Nevertheless, additional experiments are needed to assess whether E. nutans under AMF colonization in the field will initiate the same defense pathways and produce similar VOCs. This will help to clarify the mode and mechanism of AMF-grasshopper interactions and analyze the complex relationship of AMF-plant-herbivore interaction.
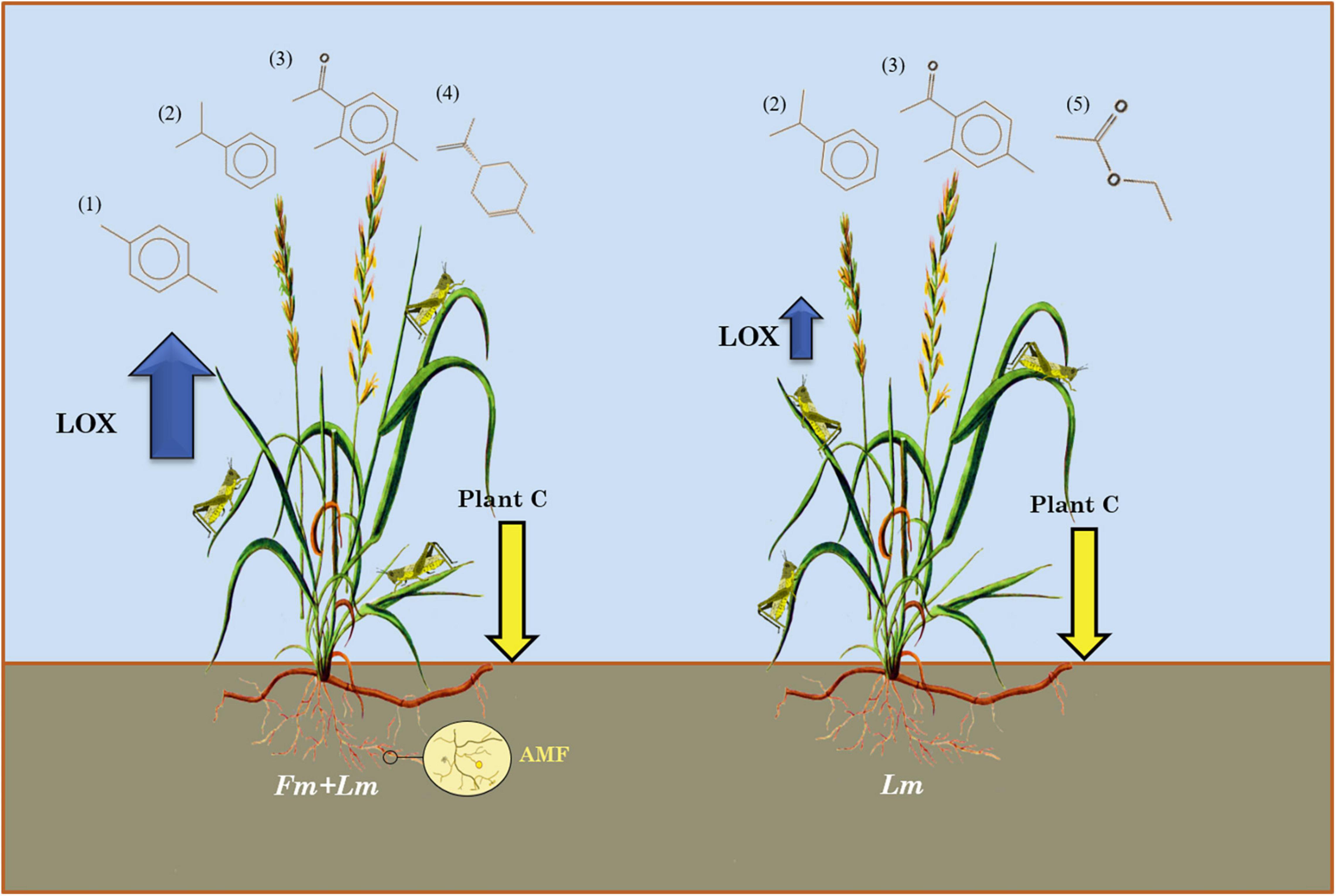
Figure 4. Diagrammatic representation of the various means of E. nutans defense response induction by AMF. (1) p-Xylene, (2) Cumene, (3) 2,4-dimethylacetophenone, (4) D-Limonene, and (5) ethyl acetate.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
XS and KL conceived the study, supervised the writing, and revised the manuscript. WZ led the writing. LY and BH contributed sections to the manuscript. All authors read and approved the final submission.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Nos. 31971746 and 32171685) and Qilian Mountain National Park Qinghai Area Biodiversity Conservation Project (QHTX-2021-009).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2022.898969/full#supplementary-material
References
Agut, B., Gamir, J., Jaques, J. A., and Flors, V. (2015). Tetranychus urticae-triggered responses promote genotype-dependent conspecific repellence or attractiveness in citrus. N. Phytol. 207, 790–804. doi: 10.1111/nph.13357
Babikova, Z., Gilbert, L., Bruce, T. J. A., Birkett, M., Caulfield, J. C., Woodcock, C., et al. (2013). Underground signals carried through common mycelial networks warn neighbouring plants of aphid attack. Ecol. Lett. 16, 835–843. doi: 10.1111/ele.12115
Babikova, Z., Gilbert, L., Randall, K. C., Bruce, T. J. A., and Pickett, J. A. (2014). Increasing phosphorus supply is not the mechanism by which arbuscular mycorrhiza increase attractiveness of bean (Vicia faba) to aphids. J. Exp. Bot. 65, 5231–5241. doi: 10.1093/jxb/eru283
Balliu, A., Sallaku, G., and Rewald, B. (2015). AMF inoculation enhances growth and improves the nutrient uptake rates of transplanted, salt-stressed tomato seedlings. Sustainability 7, 15967–15981. doi: 10.3390/su71215799
Basu, S., Rabara, R. C., and Negi, S. (2018). Amf: the future prospect for sustainable agriculture. Physiol. Mol. Plant Pathol. 102, 36–45. doi: 10.1016/j.pmpp.2017.11.007
Bati, C. B., Santilli, E., and Lombardo, L. (2015). Effect of arbuscular mycorrhizal fungi on growth and on micronutrient and macronutrient uptake and allocation in olive plantlets growing under high total Mn levels. Mycorrhiza 25, 97–108. doi: 10.1007/s00572-014-0589-0
Begum, N., Qin, C., Ahanger, M. A., Raza, S., and Khan, M. I. (2019). Role of arbuscular mycorrhizal fungi in plant growth regulation: implications in abiotic stress tolerance. Front. Plant Sci. 10:1068. doi: 10.3389/fpls.2019.01068
Bernaola, L., Cosme, M., Schneider, R. W., and Stout, M. (2018). Belowground inoculation with arbuscular mycorrhizal fungi increases local and systemic susceptibility of rice plants to different pest organisms. Front. Plant Sci. 9:747. doi: 10.3389/fpls.2018.00747
Bhantana, P., Rana, M. S., Sun, X., Moussa, M. G., and Saleem, M. H. (2021). Arbuscular mycorrhizal fungi and its major role in plant growth, zinc nutrition, phosphorous regulation and phytoremediation. Symbiosis 84, 19–37. doi: 10.1007/s13199-021-00756-6
Bolin, L. G., Benning, J. W., and Moeller, D. A. (2018). Mycorrhizal interactions do not influence plant–herbivore interactions in populations of Clarkia xantiana ssp.xantiana spanning from center to margin of the geographic range. Ecol. Evol. 8, 10743–10753. doi: 10.1002/ece3.4523
Charters, M. D., Sait, S. M., and Field, K. J. (2020). Aphid herbivory drives asymmetry in carbon for nutrient exchange between plants and an arbuscular mycorrhizal fungus. Curr. Biol. 30, 1801–1808. doi: 10.1016/j.cub.2020.02.087
Chen, S., Zhao, H., Zou, C., Li, Y., and Chen, Y. (2017). Combined inoculation with multiple arbuscular mycorrhizal fungi improves growth, nutrient uptake and photosynthesis in cucumber seedlings. Front. Microbiol. 8:2516. doi: 10.3389/fmicb.2017.02516
Dowarah, B., Gill, S. S., and Agarwala, N. (2021). Arbuscular mycorrhizal fungi in conferring tolerance to biotic stresses in plants. J. Plant Growth Regul. 110:999. doi: 10.1007/s00344-021-10392-5
Dudareva, N., Pichersky, E., and Gershenzon, J. (2004). Biochemistry of plant volatiles. Plant Physiol. 135, 1893–1902. doi: 10.1104/pp.104.049981
Frew, A. (2021). Aboveground herbivory suppresses the arbuscular mycorrhizal symbiosis, reducing plant phosphorus uptake. Appl. Soil Ecol. 168:104133. doi: 10.1016/j.apsoil.2021.104133
Frew, A., Antunes, P. M., Cameron, D. D., Hartley, S. E., and Johnson, S. N. (2022). Plant herbivore protection by arbuscular mycorrhizas: a role for fungal diversity? N. Phytol. 233, 1022–1031. doi: 10.1111/nph.17781
Gai, J. P., Christie, P., Cai, X. B., Fan, J. Q., and Zhang, J. L. (2009). Occurrence and distribution of arbuscular mycorrhizal fungal species in three types of grassland community of the Tibetan Plateau. Ecol. Res. 24, 1345–1350. doi: 10.1007/s11284-009-0618-1
Gehring, C., and Bennett, A. (2009). Mycorrhizal fungal-plant-insect interactions: the importance of a community approach. Environ. Entomol. 38, 93–102. doi: 10.1603/022.038.0111
Girousse, C., Moulia, B., Silk, W., and Bonnemain, J. (2005). Aphid infestation causes different changes in carbon and nitrogen allocation in alfalfa stems as well as different inhibitions of longitudinal and radial expansion. Plant Physiol. 137, 1474–1484. doi: 10.1104/pp.104.057430
Han, B., Li, J., Liu, K., Zhang, H., and Wei, X. (2021). Variations in soil properties rather than functional gene abundances dominate soil phosphorus dynamics under short-term nitrogen input. Plant Soil 469, 227–241. doi: 10.1007/s11104-021-05143-0
Hao, H., Bao, M., Ke, J., Li, L., and Ma, C. (2019). Fauna elements and eco-geographical distribution of locus in Qinghai province. J. Biol. 36, 62–68.
Hao, Z., Xie, W., and Chen, B. (2019). Arbuscular mycorrhizal symbiosis affects plant immunity to viral infection and accumulation. Viruses 11:534. doi: 10.3390/v11060534
Heil, M., and Ton, J. (2008). Long-distance signalling in plant defence. Trends Plant Sci. 13, 264–272. doi: 10.1016/j.tplants.2008.03.005
Jiang, D., Tan, M., Wu, S., Zheng, L., and Wang, Q. (2021). Defense responses of arbuscular mycorrhizal fungus-colonized poplar seedlings against gypsy moth larvae: a multiomics study. Hortic. Res. 8:245. doi: 10.1038/s41438-021-00671-3
Jiang, Y., Wang, W., Xie, Q., Liu, N., and Liu, L. (2017). Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. Science 356, 1172–1175. doi: 10.1126/science.aam9970
Jin, L., Zhang, G., Wang, X., Dou, C., and Chen, M. (2011). Arbuscular mycorrhiza regulate inter-specific competition between a poisonous plant, Ligularia virgaurea, and a co-existing grazing grass, Elymus nutans, in Tibetan Plateau Alpine meadow ecosystem. Symbiosis 55, 29–38. doi: 10.1007/s13199-011-0141-3
Jung, S. C., Martinez-Medina, A., Lopez-Raez, J. A., and Pozo, M. J. (2012). Mycorrhiza-induced resistance and priming of plant defenses. J. Chem. Ecol. 38, 651–664. doi: 10.1007/s10886-012-0134-6
Karban, R., Wetzel, W. C., Shiojiri, K., Pezzola, E., and Blande, J. D. (2016). Geographic dialects in volatile communication between sagebrush individuals. Ecology 97, 2917–2924. doi: 10.1002/ecy.1573
Kayama, M., and Yamanaka, T. (2014). Growth characteristics of ectomycorrhizal seedlings of Quercus glauca, Quercus salicina, and Castanopsis cuspidata planted on acidic soil. Trees 28, 569–583. doi: 10.1007/s00468-013-0973-y
Kigathi, R. N., Weisser, W. W., Reichelt, M., Gershenzon, J., and Unsicker, S. B. (2019). Plant volatile emission depends on the species composition of the neighboring plant community. BMC Plant Biol. 19:58. doi: 10.1186/s12870-018-1541-9
Koske, R. E., and Gemma, J. N. (1989). A modified procedure for staining roots to detect VA mycorrhizas. Mycol. Res. 92, 486–488. doi: 10.1016/S0953-7562(89)80195-9
Li, B., Ding, Y., Tang, X., Wang, G., and Wu, S. (2019). Effect of L-arginine on maintaining storage quality of the white button mushroom (Agaricus bisporus). Food Bioproc. Technol. 12, 563–574. doi: 10.1007/s11947-018-2232-0
Li, M., Xia, S., Zhang, T., Williams, R. L., and Xiao, H. (2022). Volatiles from cotton plants infested by Agrotis segetum (Lep.: Noctuidae) attract the larval parasitoid Microplitis mediator (Hym.: Braconidae). Plants (Basel) 11:863. doi: 10.3390/plants11070863
Li, Y., Duan, T., Nan, Z., and Li, Y. (2021). Arbuscular mycorrhizal fungus alleviates alfalfa leaf spots caused by Phoma medicaginis revealed by RNA-seq analysis. J. Appl. Microbiol. 130, 547–560. doi: 10.1111/jam.14387
Luginbuehl, L. H., Menard, G. N., Kurup, S., Van Erp, H., and Radhakrishnan, G. V. (2017). Fatty acids in arbuscular mycorrhizal fungi are synthesized by the host plant. Science 356, 1175–1178. doi: 10.1126/science.aan0081
Malik, R. J., Ali, J. G., and Bever, J. D. (2018). Mycorrhizal composition influences plant anatomical defense and impacts herbivore growth and survival in a life-stage dependent manner. Pedobiologia 66, 29–35. doi: 10.1016/j.pedobi.2017.12.004
McGonigle, T. P., Miller, M. H., Evans, D. G., Fairchild, G. L., and Swan, J. A. (1990). A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. N. Phytol. 115, 495–501. doi: 10.1111/j.1469-8137.1990.tb00476.x
Meier, A. R., and Hunter, M. D. (2018). Arbuscular mycorrhizal fungi mediate herbivore-induction of plant defenses differently above and belowground. Oikos 127, 1759–1775. doi: 10.1111/oik.05402
Meier, A. R., and Hunter, M. D. (2019). Mycorrhizae alter constitutive and Herbivore-Induced volatile emissions by milkweeds. J. Chem. Ecol. 45, 610–625. doi: 10.1007/s10886-019-01080-6
Miozzi, L., Vaira, A. M., Catoni, M., Fiorilli, V., and Accotto, G. P. (2019). Arbuscular mycorrhizal symbiosis: plant friend or foe in the fight against viruses? Front. Microbiol. 10:1238. doi: 10.3389/fmicb.2019.01238
Mitra, P., Das, S., Debnath, R., Mobarak, S. H., and Barik, A. (2021). Identification of Lathyrus sativus plant volatiles causing behavioral preference of Aphis craccivora. Pest Manag. Sci. 77, 285–299. doi: 10.1002/ps.6018
Plassard, C., and Dell, B. (2010). Phosphorus nutrition of mycorrhizal trees. Tree Physiol. 30, 1129–1139. doi: 10.1093/treephys/tpq063
Quan, X., Qiao, Y., Chen, M., Duan, Z., and Shi, H. (2021). Comprehensive evaluation of the allelopathic potential of Elymus nutans. Ecol. Evol. 11, 12389–12400. doi: 10.1002/ece3.7982
Roger, A., Gétaz, M., Rasmann, S., and Sanders, I. R. (2013). Identity and combinations of arbuscular mycorrhizal fungal isolates influence plant resistance and insect preference. Ecol. Entomol. 38, 330–338. doi: 10.1111/een.12022
Schoenherr, A. P., Rizzo, E., Jackson, N., Manosalva, P., and Gomez, S. K. (2019). Mycorrhiza-induced resistance in potato involves priming of defense responses against cabbage looper (Noctuidae: Lepidoptera). Environ. Entomol. 48, 370–381. doi: 10.1093/ee/nvy195
Schweiger, R., Baier, M. C., Persicke, M., and Müller, C. (2014). High specificity in plant leaf metabolic responses to arbuscular mycorrhiza. Nat. Commun. 5, 3886. doi: 10.1038/ncomms4886
Selvaraj, A., Thangavel, K., and Uthandi, S. (2020). Arbuscular mycorrhizal fungi (Glomus intraradices) and diazotrophic bacterium (Rhizobium BMBS) primed defense in blackgram against herbivorous insect (Spodoptera litura) infestation. Microbiol. Res. 231:126355. doi: 10.1016/j.micres.2019.126355
Sharma, E., Anand, G., and Kapoor, R. (2017). Terpenoids in plant and arbuscular mycorrhiza-reinforced defence against herbivorous insects. Ann. Bot. 119, 791–801. doi: 10.1093/aob/mcw263
Song, Y., Chen, D., Lu, K., Sun, Z., and Zeng, R. (2015). Enhanced tomato disease resistance primed by arbuscular mycorrhizal fungus. Front. Plant Sci. 6:786. doi: 10.3389/fpls.2015.00786
Song, Y. Y., Ye, M., Li, C., He, X., and Zhu-Salzman, K. (2015). Hijacking common mycorrhizal networks for herbivore-induced defence signal transfer between tomato plants. Sci. Rep. 4:3915. doi: 10.1038/srep03915
Song, Y. Y., Ye, M., Li, C. Y., Wang, R. L., and Wei, X. C. (2013). Priming of anti-herbivore defense in tomato by arbuscular mycorrhizal fungus and involvement of the jasmonate pathway. J. Chem. Ecol. 39, 1036–1044. doi: 10.1007/s10886-013-0312-1
Song, Y. Y., Zeng, R. S., Xu, J. F., Li, J., and Shen, X. (2010). Interplant communication of tomato plants through underground common mycorrhizal networks. PLoS One 5:e13324. doi: 10.1371/journal.pone.0013324
Spatafora, J. W., Chang, Y., Benny, G. L., Lazarus, K., and Smith, M. E. (2016). A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia 108, 1028–1046. doi: 10.3852/16-042
Tan, X., Huang, Y., Xiong, D., Lv, K., and Chen, F. (2020). The effect of Elymus nutans sowing density on soil reinforcement and slope stabilization properties of vegetation–concrete structures. Sci. Rep. 10, 20462. doi: 10.1038/s41598-020-77407-1
Valenzuela, M., Méndez, V., Montenegro, I., Besoain, X., and Seeger, M. (2019). Streptomycin resistance in Clavibacter michiganensis subsp. michiganensis strains from Chile is related to anrpsL gene mutation. Plant Pathol. 68, 426–433. doi: 10.1111/ppa.12971
Veenstra, J. A., Leyria, J., Orchard, I., and Lange, A. B. (2021). Identification of gonadulin and insulin-like growth factor from migratory locusts and their importance in reproduction in Locusta migratoria. Front. Endocrinol. 12:693068. doi: 10.3389/fendo.2021.693068
Velásquez, A., Valenzuela, M., Carvajal, M., Fiaschi, G., and Avio, L. (2020a). The arbuscular mycorrhizal fungus Funneliformis mosseae induces changes and increases the concentration of volatile organic compounds in Vitis vinifera cv. Sangiovese leaf tissue. Plant Physiol. Biochem. 155, 437–443. doi: 10.1016/j.plaphy.2020.06.048
Velásquez, A., Vega-Celedón, P., Fiaschi, G., Agnolucci, M., and Avio, L. (2020b). Responses of Vitis vinifera cv. Cabernet Sauvignon roots to the arbuscular mycorrhizal fungus Funneliformis mosseae and the plant growth-promoting rhizobacterium Ensifer meliloti include changes in volatile organic compounds. Mycorrhiza 30, 161–170. doi: 10.1007/s00572-020-00933-3
Walder, F., and van der Heijden, M. G. A. (2015). Regulation of resource exchange in the arbuscular mycorrhizal symbiosis. Nat. Plants 1:15159. doi: 10.1038/nplants.2015.159
Wang, X., Wang, Q., Jin, L., Sun, L., and Wang, Q. (2019). Arbuscular mycorrhizal fungi in the rhizosphere soil of poisonous plants depressed the growth of pasture grasses in the Tibetan Plateau Alpine meadow. Ecosyst. Health Sustain. 5, 226–236.
Xu, Y. F., Chu, X. T., Zhang, X. H., Liu, Q., and Miao, Y. J. (2018). The forms of nitrogen source influence the interaction between Elymus nutans Griseb and arbuscular mycorrhizal fungi. S. Afr. J. Bot. 119, 37–44. doi: 10.1016/j.sajb.2018.08.007
Ye, M., Veyrat, N., Xu, H., Hu, L., and Turlings, T. (2018). An herbivore-induced plant volatile reduces parasitoid attraction by changing the smell of caterpillars. Sci. Adv. 4, r4767. doi: 10.1126/sciadv.aar4767
Zhang, S., Lehmann, A., Zheng, W., You, Z., and Rillig, M. C. (2019). Arbuscular mycorrhizal fungi increase grain yields: a meta-analysis. N. Phytol. 222, 543–555. doi: 10.1111/nph.15570
Keywords: arbuscular mycorrhizal fungi, Elymus nutans, plants defense, volatile organic compounds, Locusta migratoria, Funneliformis mosseae
Citation: Zhang W, Yu L, Han B, Liu K and Shao X (2022) Mycorrhizal Inoculation Enhances Nutrient Absorption and Induces Insect-Resistant Defense of Elymus nutans. Front. Plant Sci. 13:898969. doi: 10.3389/fpls.2022.898969
Received: 18 March 2022; Accepted: 10 May 2022;
Published: 31 May 2022.
Edited by:
Jin-Lin Zhang, Lanzhou University, ChinaReviewed by:
Yinglong Chen, University of Western Australia, AustraliaKai Xue, University of Chinese Academy of Sciences, China
Copyright © 2022 Zhang, Yu, Han, Liu and Shao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kesi Liu, a2xpdUBjYXUuZWR1LmNu; Xinqing Shao, c2hhb3hpbnFpbmdAMTYzLmNvbQ==
 Wantong Zhang
Wantong Zhang Lu Yu
Lu Yu Bing Han
Bing Han Kesi Liu
Kesi Liu Xinqing Shao
Xinqing Shao