- 1State Key Laboratory of Desert and Oasis Ecology, Xinjiang Institute of Ecology and Geography, Chinese Academy of Sciences, Urumqi, China
- 2Sino-Tajikistan Joint Laboratory for Conservation and Utilization of Biological Resources, Urumqi, China
- 3The Specimen Museum of Xinjiang Institute of Ecology and Geography, Chinese Academy of Sciences, Urumqi, China
- 4University of Chinese Academy of Sciences, Beijing, China
- 5Xinjiang Key Lab of Conservation and Utilization of Plant Gene Resources, Urumqi, China
Land degradation caused by soil salinization and wind erosion is the major obstruction to sustainable agriculture in the arid region. Salsola species have the potential to prevent land degradation. However, there is limited information about seed germination requirements and tolerance to salinity and drought for representative Salsola species. This study aimed to assess the effects of the winged perianth (seed structural features) and abiotic factors (light, temperature, salinity, and drought) on the seed germination of these species. These Salsola species varied considerably in seed germination characteristics. Compared with naked seeds, winged seeds had lower germination percentages for S. heptapotamica S. rosacea, and S. nitraria species. Darkness decreased the germination percentage of winged and naked seeds of S. rosacea, however, for S. heptapotamica and S. nitraria, decreased seed germination was only when the winged perianth existed. Germination of S. heptapotamica, S. rosacea, and S. nitraria seeds depended on the perianth and light conditions. The naked seeds of these three species could germinate at a wide range of temperatures, especially in light. The presence of perianth, light, and temperature did not significantly influence the germination of S. ruthenica seeds. When cultivating these species, it is beneficial to remove the winged perianth of seeds and sow it on the soil surface when the temperature is above 5/15°C. In addition, seed germination of Salsola displayed high tolerance to salinity and drought. Compared with winged seeds, naked seeds showed lower recovery germination under high salinity but had a similar recovery of germination under high PEG concentration. Our study provides detailed germination information for the cultivation of these four representative Salsola species in degraded saline soils of the arid zone.
Introduction
Land degradation refers to the succession process in which unfavorable natural factors or inappropriate land use leads to the gradual loss of production potential (Prince et al., 2009). The arid land in Central Asia is the largest dry area located in the temperate and warm temperate zone of the northern hemispheric earth, where land degradation is quite serious because of climatic variations and human activities resulting from population increase (Kuang et al., 2014). It is reported that the land degradation area in Central Asia is about 58.78 × 104 km2, accounting for 10.37% of the total land area (Kuang et al., 2014). Salinity and drought are two abiotic factors resulting in land degradation in the arid region in Central Asia, which increasingly threaten sustainable agriculture with global climate change (Negrão et al., 2017). Currently, ~10% of the total land is degraded by salinity (Ruan et al., 2010) and up to 41% by drought (D'Odorico et al., 2013). Therefore, it is not unexpected that a large effort is devoted to repairing or restoring the deteriorating land (Zhang et al., 2015; Wang et al., 2020).
Cultivation of native plant species could be a viable alternative to rehabilitating such degraded land (El-Keblawy and Ksiksi, 2005). The use of suitable candidate plants is a vital step in any recovery program (El-Keblawy, 2017). One of the most important selection criteria to choose plants for land rehabilitation is their capacity of seeds to germinate under complex conditions, especially under stress (Lu et al., 2016). Therefore, a thorough knowledge of seed germination requirements for light and temperature, and tolerance to salinity or drought stress of native plants is the key aspect to understanding the role of the plants in the restoration and rehabilitation strategies (Qian et al., 2016).
Temperature is a determining factor for seed germination of most plants, which can break seed dormancy and stimulate germination (Probert, 1992; Baskin and Baskin, 2014). Seed germination of native plants in arid regions is often limited by the temperature though the other conditions are suitable (Evans and Etherington, 1990). Light is another important regulatory environmental signal for seed germination of desert plants (Katerina et al., 2014). In the desert and semi-desert areas, plants vary in their requirement for light during germination. Some germinate strictly in need of light (Sen and Chatterji, 1968), while others can germinate well in light or dark (Huang et al., 2003), and some even germinate better in dark than in light (Sekmen et al., 2004). In addition, the actual germination requirements of seeds to light depend on the interaction with other environmental factors such as temperature (El-Keblawy et al., 2011). Many desert plants germinate only when the combinations of light and temperature are suitable for seedling establishment (Naidoo and Naicker, 1992).
In arid and semi-arid regions, reduction in water potential of soil caused by salinity or drought is common stress that affects seed imbibition and thereby germination (Rasheed et al., 2019). Seed germination percentage is generally decreased with the increase in salinity or PEG concentration (Khan and Gulzar, 2003; Xing et al., 2013). Most ungerminated seeds remain viable under harsh conditions (e.g., high salinity and drought) by entering a state of conditional or enforced dormancy and can recover germination on the provision of sufficient moisture (Rasheed et al., 2015). Such germination inhibition may be a survival strategy for plants in arid regions, which could reduce seedling mortality (Khan and Gul, 2006).
There are a large group of plants in nature, whose seed structure has additional appendages such as winged perianth or bracteole (Jurado et al., 1991). Seeds with winged perianth take the advantage in dispersal (Baskin et al., 2014). Moreover, the presence of perianth-inhibited seed germination is seen in many plants, such as Haloxylon stocksii (Rasheed et al., 2019), Salsola ikonnikovii (Xing et al., 2013), and S. schweinfurthii (Bhatt et al., 2016). In general, inhibition of perianth on seeds germination could occur through many different pathways, such as induction of a light requirement for germination, mechanical inhibition, chemical inhibitors, and specific ion effects (Wei et al., 2008). In addition, the presence of perianth affected the germination response of seeds to different environmental factors, such as increased germination requirements to light of S. rubescens seed (El-Keblawy et al., 2013); change optimal temperature ranges of H. stocksii seed (Rasheed et al., 2019); and aggravate the inhibitive effect of salinity on seed germination of S. ikonnikovii (Xing et al., 2013), and of drought on seed germination of S. ferganica (Maimaitijiang et al., 2019).
Salsola L. belongs to the family Amaranthaceae and includes ~130 species occurring in the arid desert of Africa, Asia, and Europe (Zhu et al., 2003). There are ~37 species in China, 33 species of which distribute in Xinjiang. The typical feature of this genus is that perianth segments are abaxially winged in fruit (Zhu et al., 2003). Many plants of this genus have good effects on soil and water conservation, diminish wind- and sand-shifting (Wang et al., 2008), and thus have great potential for restoration of deteriorated land in arid regions. Some scholars have studied germination response to different environmental factors of seeds for Salsola plants (e.g., El-Keblawy et al., 2013; Elnaggar et al., 2019). The effects of winged perianth on seed germination are also considered (Wei et al., 2008; Ma et al., 2017). However, most of the researches only consider the effects of one or at most two factors between perianth, temperature and light on seed germination (Chang et al., 2008; Wang et al., 2013), comprehensive evaluation of the effects of these three factors are absent, which is vital to assess the true germination condition of seed in the arid desert. In addition, studies on the effect of perianth on seed germination tolerance to salinity or drought of Salsola plants are also limited (Xing et al., 2013; Maimaitijiang et al., 2019).
In this study, we collected seeds of four representative Salsola species, including three endemic species S. heptapotamica Iljin, S. rosacea Linn., and S. nitraria Pall, mainly distributed in northern Xinjiang in China, and one widespread species S. ruthenica Iljin. distributed in many places in China (Zhu et al., 2003). We performed laboratory germination tests and aimed to provide detailed germination information for better screening of suitable Salsola plants in rehabilitating deteriorated lands in arid lands in Central Asia. We hypothesize that (1) the presence of winged perianth inhibits seed germination of these species; (2) the germination responses to light and temperature of endemic species are more sensitive than that of widespread species; (3) all these Salsola species have a high tolerance to salinity and drought.
Materials and Methods
Seed Collection and Habitat Characteristics
Freshly matured fruits of the four Salsola species were collected from natural populations growing at the edge of Junggar Basin in Xinjiang during September–October 2020. This area is arid to semi-arid with a typical temperate continental climate. Annual precipitation is around 167 mm with minimum precipitation in winter and maximum in summer. Annual potential evaporation is 2,300 mm. The mean annual temperature is 6.7°C with a minimum temperature of −34.4°C in January and a maximum temperature 41.7°C in August. The soil is saline soil and the pH is above 8. Fruits of each species were randomly collected from about 50 plants and taken to the laboratory. Fruits were air-dried under room temperature (18–25°C) for 2 weeks and then were stored at 4°C for about 1 month until used in this experiment. The specific habitat characteristics are shown in Table 1.
Seed Characteristics
The size, shape, and color of the four Salsola seeds with and without winged perianth (hereafter as winged seeds and naked seeds) were recorded using the stereomicroscope (Olympus SZX10, Tokyo, Japan). Winged perianth-enclosed seeds were removed manually. Seven groups of 100 winged seeds or naked seeds were weighed using an analytical balance (precision 0.001 g). The sizes of winged seeds or naked seeds were determined using Image J Analysis Software (National Institutes of Mental Health, America). Each determination had seven replicates.
Seed Germination
Winged Perianth, Light, and Temperature Effects
The winged seeds or naked seeds were incubated in five incubators adjusted to a temperature regime of 5/15, 5/20, 10/25, 15/30, and 20/35°C (common temperature regimes of the region) in both continuous darkness and alternating 12 h darkness/12 h light, hereafter referred as dark and light, respectively. Seeds were placed in 9-cm-diameter Petri dishes on two layers of Whatman no. 1 filter paper moistened with 7 ml of distilled water. The Petri dishes were sealed with parafilm. For the dark treatment, the Petri dishes were wrapped in two layers of aluminum foil to prevent any exposure to light. Each treatment had four replicates with 25 seeds. Radicle protrusion from the seed was the criterion for germination of winged seeds, and naked seeds were germinated when the radicle length was ≥ 2 mm. Seed germination was monitored every day, germinated seeds were discarded at each counting, and the experiment lasted for 14 d. The seeds incubated in dark were checked only after 14 d. The rate of germination was estimated by using a modified Timson's index of germination velocity = ΣG/t, where G is the percentage of seed germination every day and t is the total germination period (Khan and Ungar, 1984).
Winged Perianth and Salinity Effects
Due to the relatively higher germination percentages of the four Salsola species in light and at 10/25°C, winged seeds and naked seeds were sown in different NaCl concentrations (0, 50, 100, 300, 500, and 700 mM, based on preliminary results) and incubated at this condition. Each dish was wrapped with parafilm against loss of water by evaporation. Germination was monitored after 14 d of incubation. All the seeds that failed to germinate were rinsed with distilled water and then incubated in distilled water for another 7 d. Recovery percentages (RP) were calculated using the formula: RP = [(a – b)/c] × 100, where a is the sum of the number of seeds germinated in NaCl solutions plus those that recovered to germinate in the distilled water; b is the total number of seeds germinated in NaCl solutions, and c is the total number of seeds tested. The total germination percentage was recorded as (a/c) × 100.
Winged Perianth and Drought Effects
Winged seeds or naked seeds were germinated in Petri dishes with six levels of polyethylene glycol (PEG) concentrations (0, 50, 100, 150, 200, 300 g·L−1, based on preliminary results), which correspond to osmotic potential (ΨS) of 0, −0.05, −0.15, −0.3, −0.5 and −1.03 MPa (Michel and Kaufmann, 1973). The light and temperature conditions were the same as in the salinity experiment. Recovery percentage and total germination were also calculated.
Statistical Analysis
Seed germination data are of binomial type (i.e., germination is 1, not germination is 0). The forward stepwise (Wald) in binary logistic regression models was used to analyze the effect of winged perianth, light, temperature, and their interactions on seed germination. The same method was used to determine the effect of the winged perianth, salinity and their interactions, winged perianth, PEG and their interactions on seed germination and total germination. This method will automatically eliminate the parameters with a low probability of Wald statistics in the equation, to ensure the accuracy of the regression curve to a great extent (Li and Luo, 2003). In addition, for parameters that have significant effects on germination, Turkey's multiple comparison tests were used to test the differences that existed among groups. The same method was also used to determine the effect of winged perianth and temperature on the germination index of these Salsola species. Non-parametric tests were also performed to test the differences among the recovery percentages that did not meet the homogeneity of variance in different concentrations of NaCl or PEG solution. The difference between perianth treatments at the same temperature of seed germination was analyzed by Student's test at 95% confidence level. Data were expressed as mean ± standard error. All statistical tests were analyzed using the IBM SPSS Statistics 20 (IBM Corp., Armonk, New York, United States).
Results
Seed Characteristics
The winged seeds of the four Salsola plants are all utricle and surrounded by fan-shaped and membranous wings (Supplementary Figures 1B–K). The naked seeds of the four plants are slightly flattened to slightly conical and have the typical spiral embryo (Supplementary Figures 1C–L). Winged seeds and naked seeds of S. heptapotamica and S. rosacea are significantly larger and heavier than those of S. nitraria and S. ruthenica (Supplementary Figure 1; Table 2).
Winged Perianth, Light, and Temperature Effects
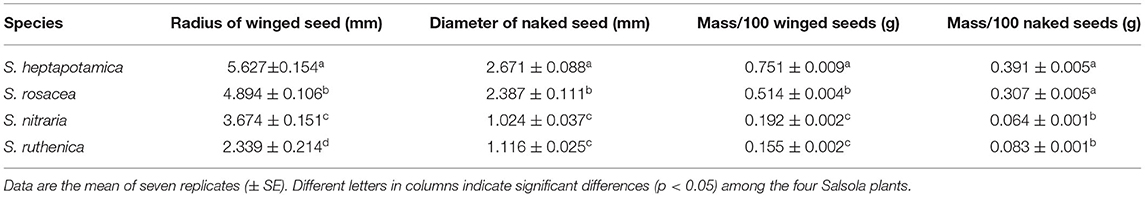
The effects of winged perianth, light, and temperature on seed germination of S. heptapotamica, S. rosacea, and S. nitraria were significant. The interactive effect of winged perianth, light, and temperature on seed germination of S. heptapotamica and S. nitraria were also significant but were not for S. rosacea (Supplementary Table 1). For the three Salsola plants, germinations of winged seeds were all significantly lower than those of naked seeds (Figures 1–3). Germination percentages of winged and naked seeds of S. rosacea were significantly lower in dark than those in the light (Figures 2A,B). While for S. heptapotamica and S. nitraria, only winged seeds performed significantly lower germination percentages in dark than in light (Figures 1A,B, 3A,B). At the five temperature ranges, germination percentages of naked seeds for the three Salsola plants were all up to 40% whether in light or dark. For winged seeds germinated in dark, germination percentages were all no more than 43%, and winged seeds of S. nitraria were even <5% at 15/30–20/35°C (Figures 1–3). For S. ruthenica, except winged perianth (p < 0.001), other factors and their interactions had no significant effect on seed germination (Supplementary Table 1). Besides, germination percentages of winged and naked seeds were both more than 90% at the five temperatures whether in light or dark (Figures 4A,B).
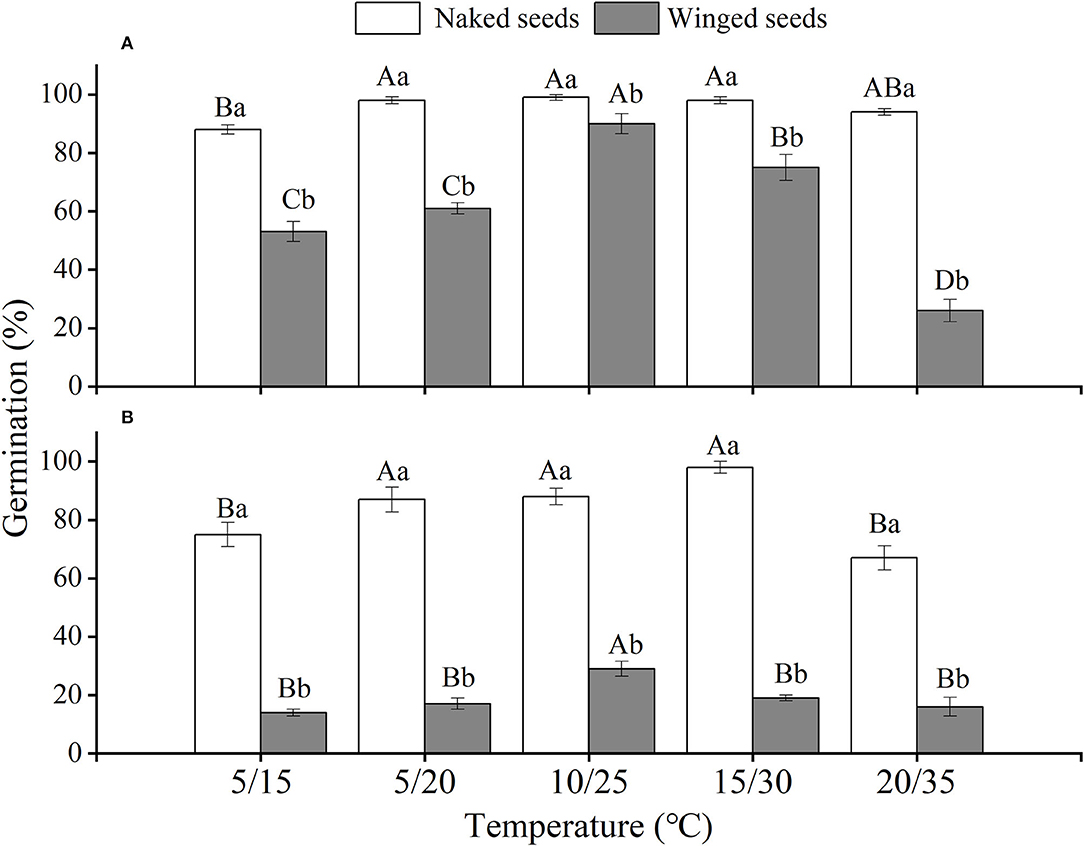
Figure 1. The effects of winged perianth, light, and temperature on seed germination percentage (mean ± SE) of Salsola heptapotamica. (A) germination in light, (B) germination in dark. Different uppercase letters denote a significant difference (p < 0.05) in germination percentage at different temperatures for the same perianth treatment, and different lowercase letters indicate a significant difference (p < 0.05) of germination percentage for different treatments of perianth at the same temperature.
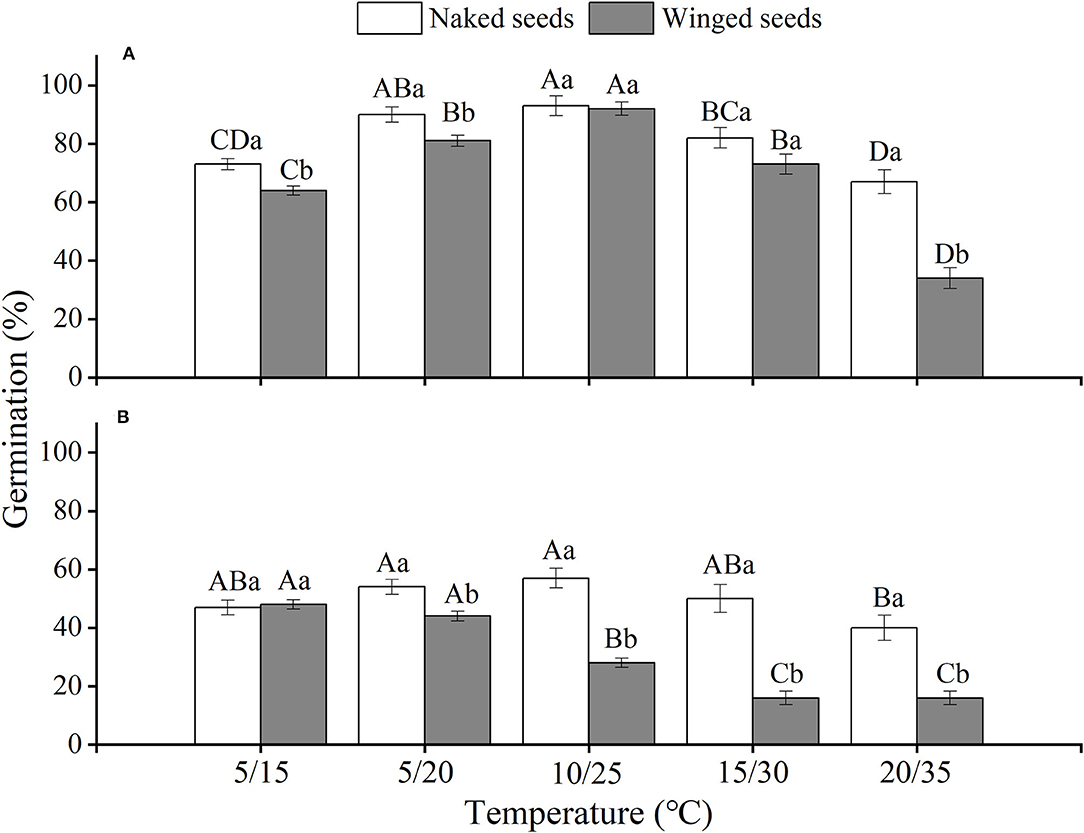
Figure 2. The effects of winged perianth, light, and temperature on seed germination percentage (mean ± SE) of Salsola rosacea. (A) germination in light, (B) germination in dark. Different uppercase letters denote a significant difference (p < 0.05) in germination percentage at different temperatures for the same perianth treatment, and different lowercase letters indicate a significant difference (p < 0.05) of germination percentage for different treatments of perianth at the same temperature.
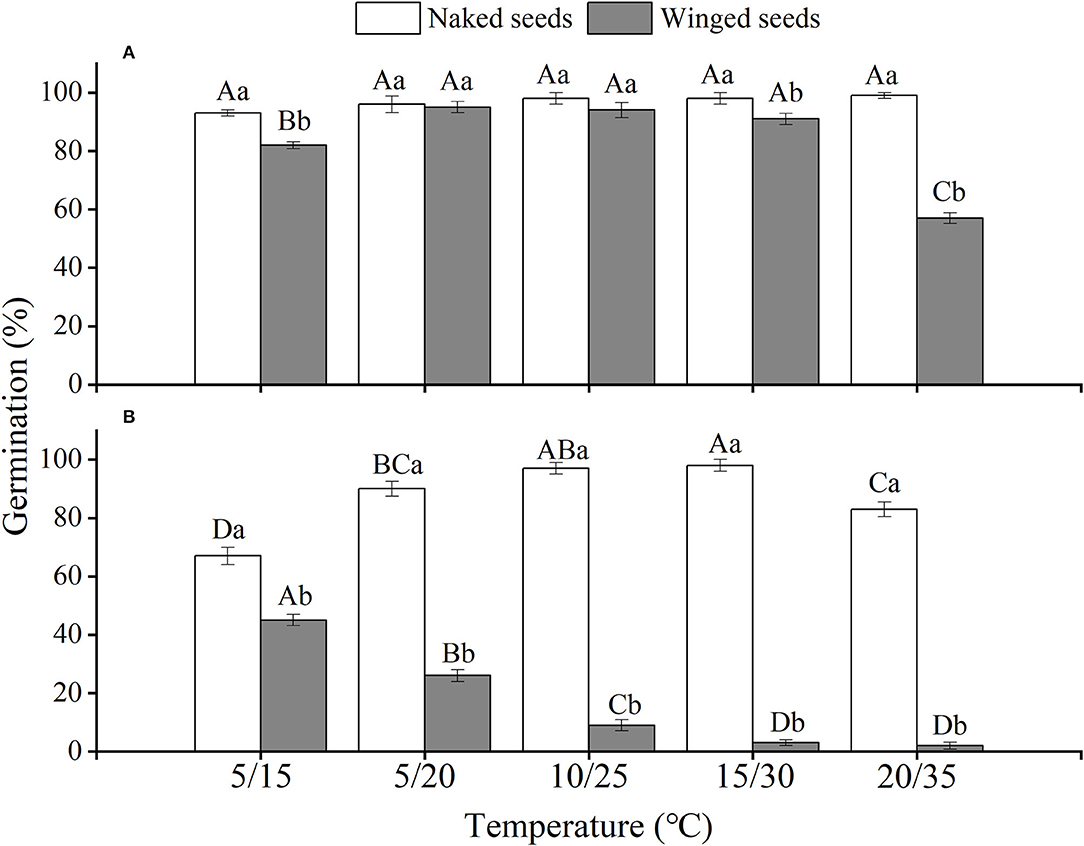
Figure 3. The effects of winged perianth, light, and temperature on seed germination percentage (mean ± SE) of Salsola nitraria. (A) germination in light, (B) germination in dark. Different uppercase letters denote a significant difference (p < 0.05) in germination percentage at different temperatures for the same perianth treatment, and different lowercase letters indicate a significant difference (p < 0.05) of germination percentage for different treatments of perianth at the same temperature.
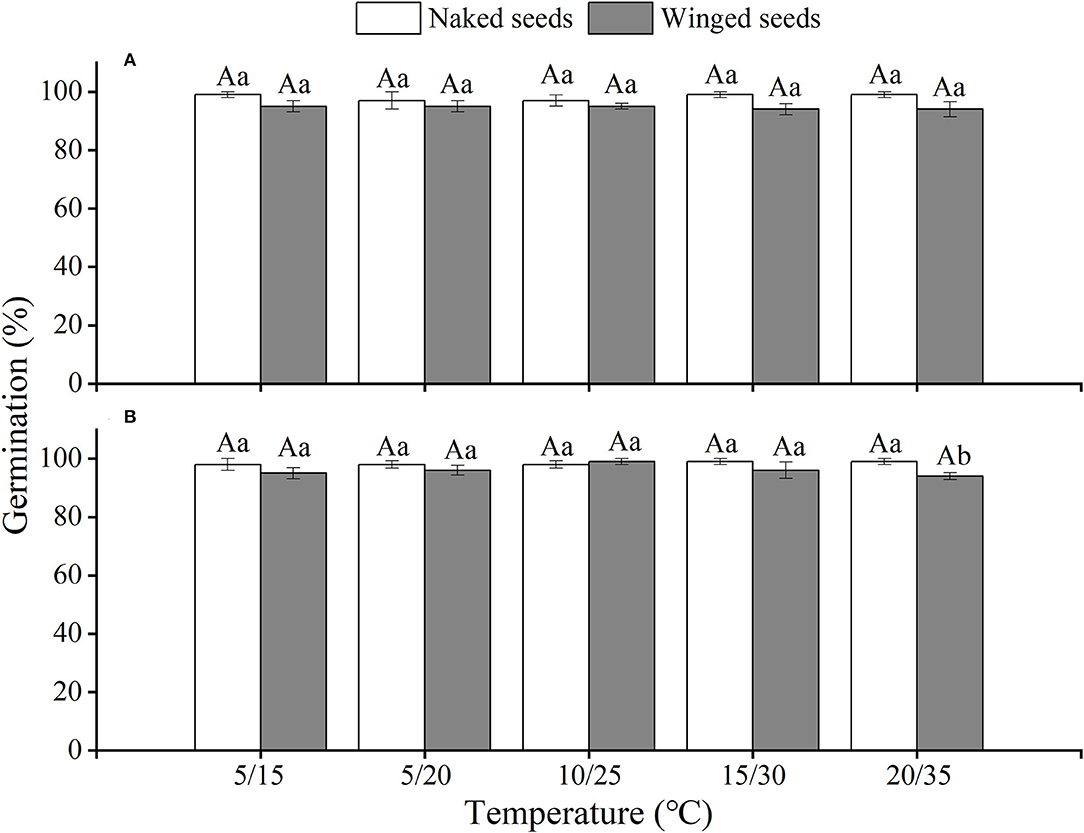
Figure 4. The effects of winged perianth, light, and temperature on seed germination percentage (mean ± SE) of Salsola ruthenica. (A) germination in light, (B) germination in dark. Different uppercase letters denote a significant difference (p < 0.05) of germination percentage at different temperatures for the same perianth treatment, and different lowercase letters indicate a significant difference (p < 0.05) of germination percentage for different treatments of perianth at the same temperature.
For S. heptapotamica, S. rosacea, and S. nitraria, germination indices of winged seeds were significantly lower than those of naked seeds (Supplementary Figures 2A–C). Germination indices of naked seeds for these three Salsola plants were increased with the raising of temperature, but those for winged seeds were increased initially and then decreased (Supplementary Figures 2A–C). For S. ruthenica, there was no significant difference in germination indices between winged and naked seeds at the five temperatures (Supplementary Figure 2D).
Winged Perianth and Salinity Effects
There was a significant (p < 0.01) interaction of winged perianth and salinity on seed germination and total germination for the four Salsola plants (Supplementary Table 2). Germination percentages and total germinations of winged and naked seeds of the four Salsola plants were all decreased with the increase of NaCl concentration (Figures 5–8). Germination percentages of naked seeds were all higher than those of winged seeds, except at 500 mM, in which germination percentages of naked seeds for S. heptapotamica, S. rosacea, and S. nitraria were lower than those of winged seeds (Figures 5–7). The recovery percentages of winged seeds for the four Salsola were all increased with the raising of NaCl concentration, while those of naked seeds were increased initially and then decreased. At 0–300 mM NaCl concentration, the total germinations of naked seeds for the four Salsola plants were higher than those of winged seeds or there were no significant differences, but at 500–700 mM, the results were opposite (Figures 5–8).
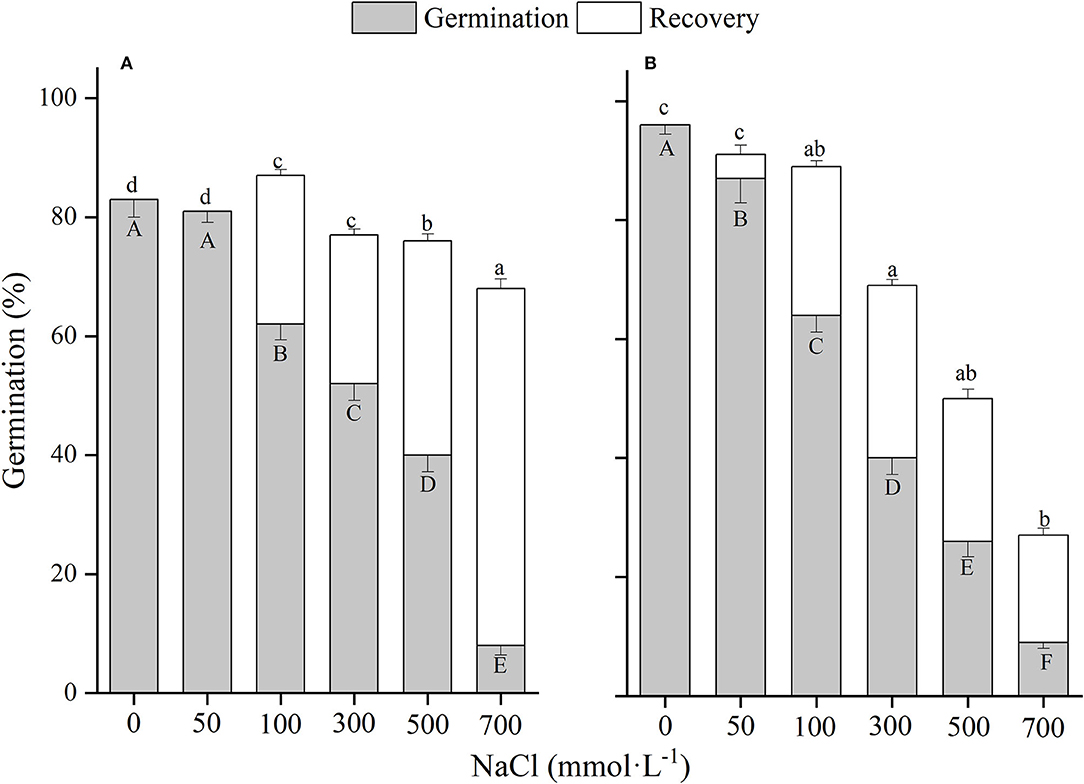
Figure 5. The effects of winged perianth and NaCl concentration on seed germination and recovery germination of Salsola heptapotamica. (A) winged seeds, (B) naked seeds. Different uppercase letters denote a significant difference (p < 0.05) in germination percentage among different NaCl concentration. Different lowercase letters indicate a significant difference (p < 0.05) in recovery germination among different NaCl concentration.
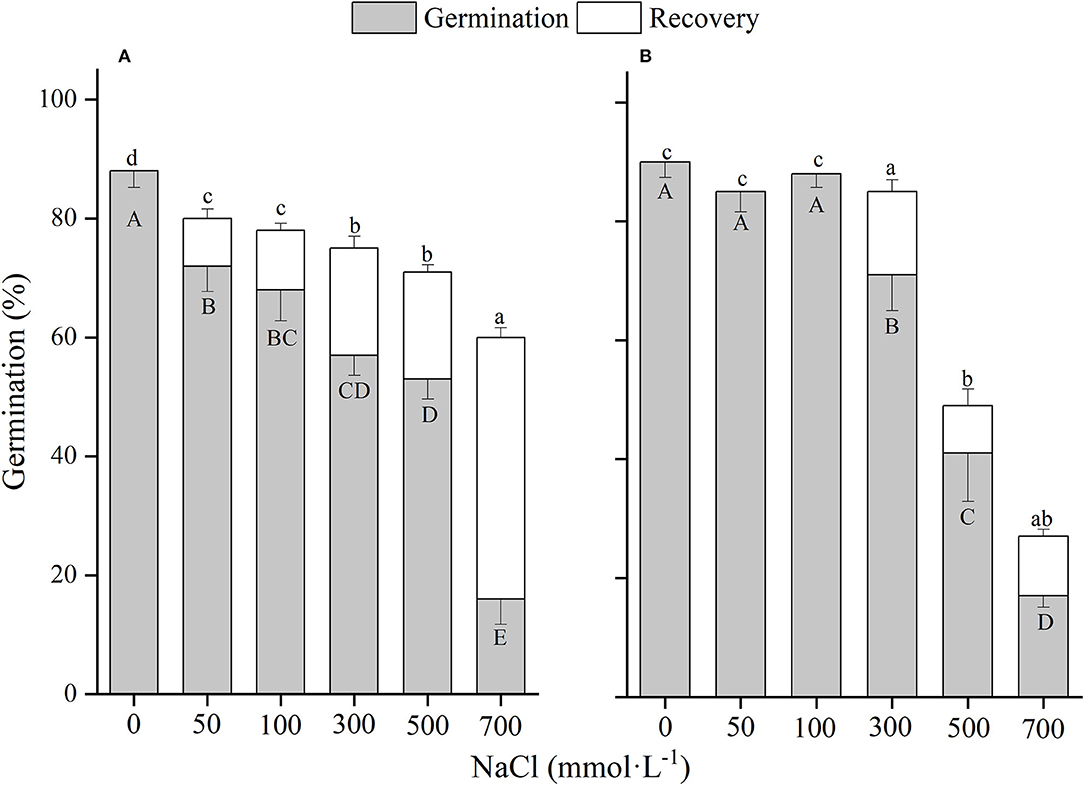
Figure 6. The effects of winged perianth and NaCl concentration on seed germination and recovery germination of Salsola rosacea. (A) winged seeds, (B) naked seeds. Different uppercase letters denote a significant difference (p < 0.05) in germination percentage among different NaCl concentrations. Different lowercase letters indicate a significant difference (p < 0.05) in recovery germination among different NaCl concentrations.
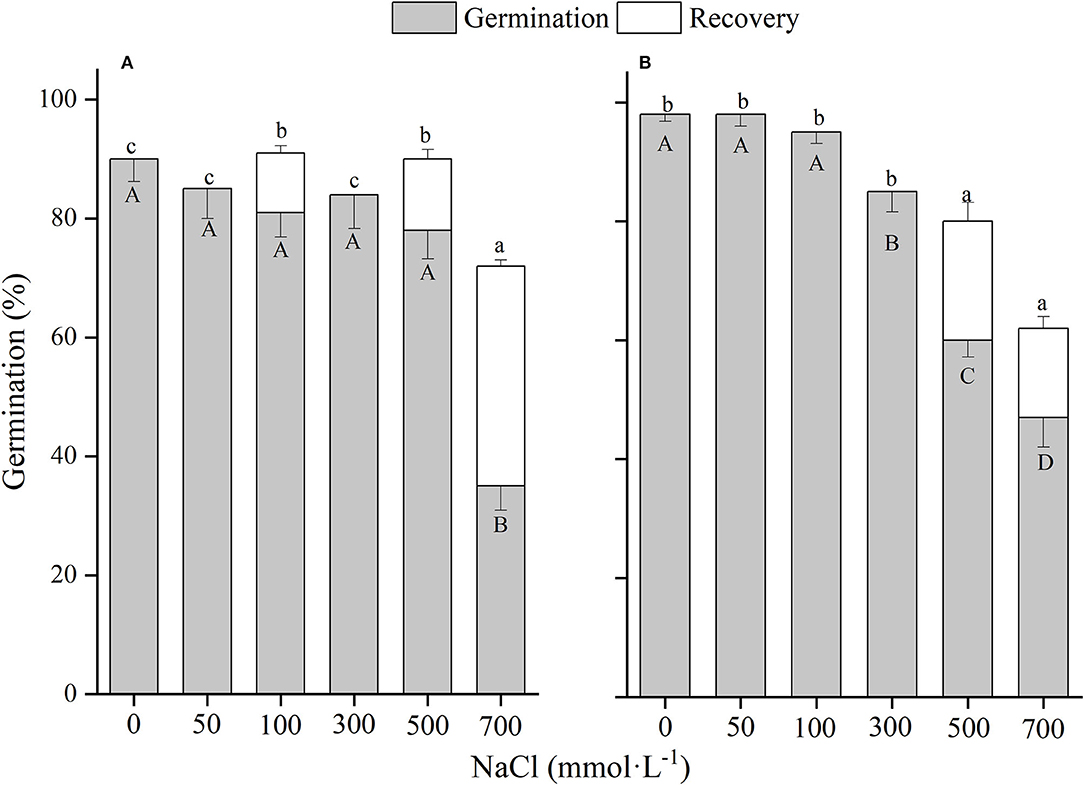
Figure 7. The effects of winged perianth and NaCl concentration on seed germination and recovery germination of Salsola nitraria. (A) winged seeds, (B) naked seeds. Different uppercase letters denote a significant difference (p < 0.05) in germination percentage among different NaCl concentrations. Different lowercase letters indicate a significant difference (p < 0.05) of recovery germination among different NaCl concentrations.
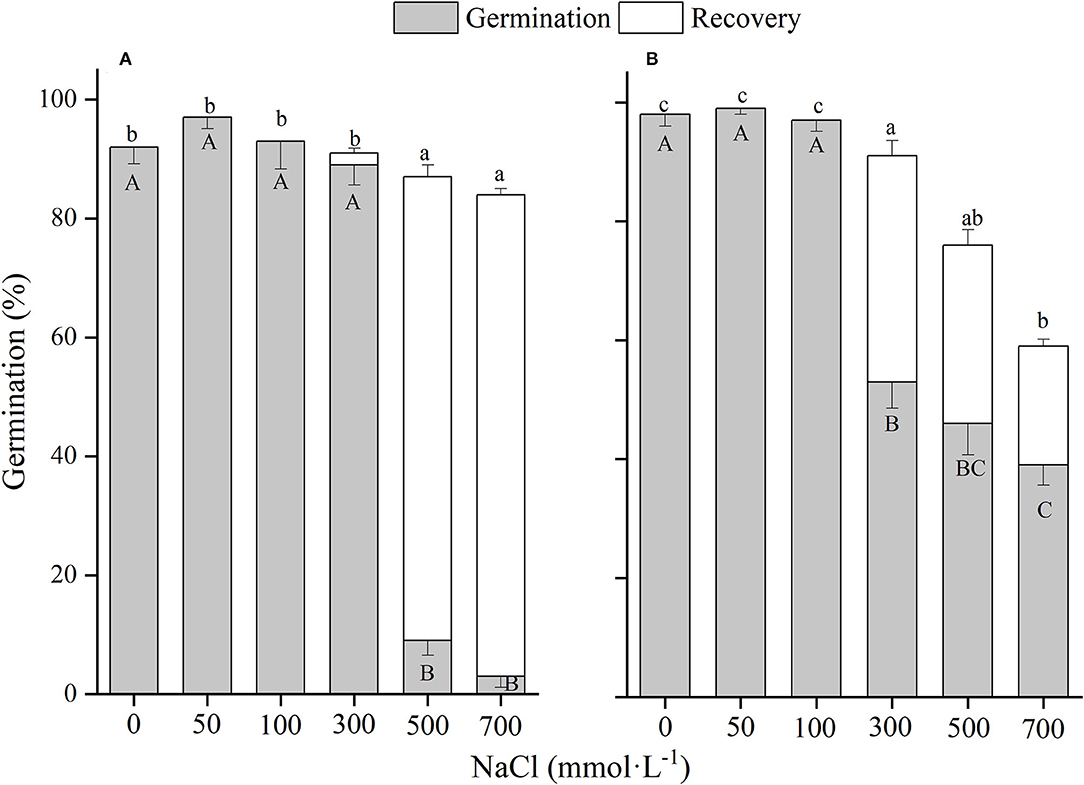
Figure 8. The effects of winged perianth and NaCl concentration on seed germination and recovery germination of Salsola ruthenica. (A) winged seeds, (B) naked seeds. Different uppercase letters denote a significant difference (p < 0.05) in germination percentage among different NaCl concentrations. Different lowercase letters indicate a significant difference (p < 0.05) of recovery germination among different NaCl concentrations.
Winged Perianth and Drought Effects
There were significant (p < 0.05) effects of winged perianth and drought on seed germination of the four Salsola species, but were not for their interactive effects. There was no significant interaction of winged perianth and drought on total germinations of the four Salsola plants either (Supplementary Table 3). Germination percentages and total germination of winged and naked seeds for the four Salsola plants were all decreased with the decline in ΨS of PEG solution (Figures 9–12). Germination percentages and total germinations of naked seeds were all higher or there were no significant differences than those of winged seeds. The winged seeds of S. heptapotamica even had no germination at ΨS of −1.03 MPa (Figure 9A). The recovery percentages of winged and naked seeds for S. heptapotamica, S. nitraria, and S. ruthenica were all increased with the decrease of ΨS, while those for S. rosacea seeds were increased initially and then decreased (Figure 10B).

Figure 9. The effects of winged perianth and PEG concentration on seed germination and recovery germination of Salsola heptapotamica. (A) winged seeds, (B) naked seeds. Different uppercase letters denote a significant difference (p < 0.05) in germination percentage among different PEG concentrations. Different lowercase letters indicate a significant difference (p < 0.05) of recovery germination among different PEG concentrations.

Figure 10. The effects of winged perianth and NaCl concentration on seed germination and recovery germination of Salsola rosacea. (A) winged seeds, (B) naked seeds. Different uppercase letters denote a significant difference (p < 0.05) in germination percentage among different PEG concentrations. Different lowercase letters indicate a significant difference (p < 0.05) in recovery germination among different PEG concentrations.
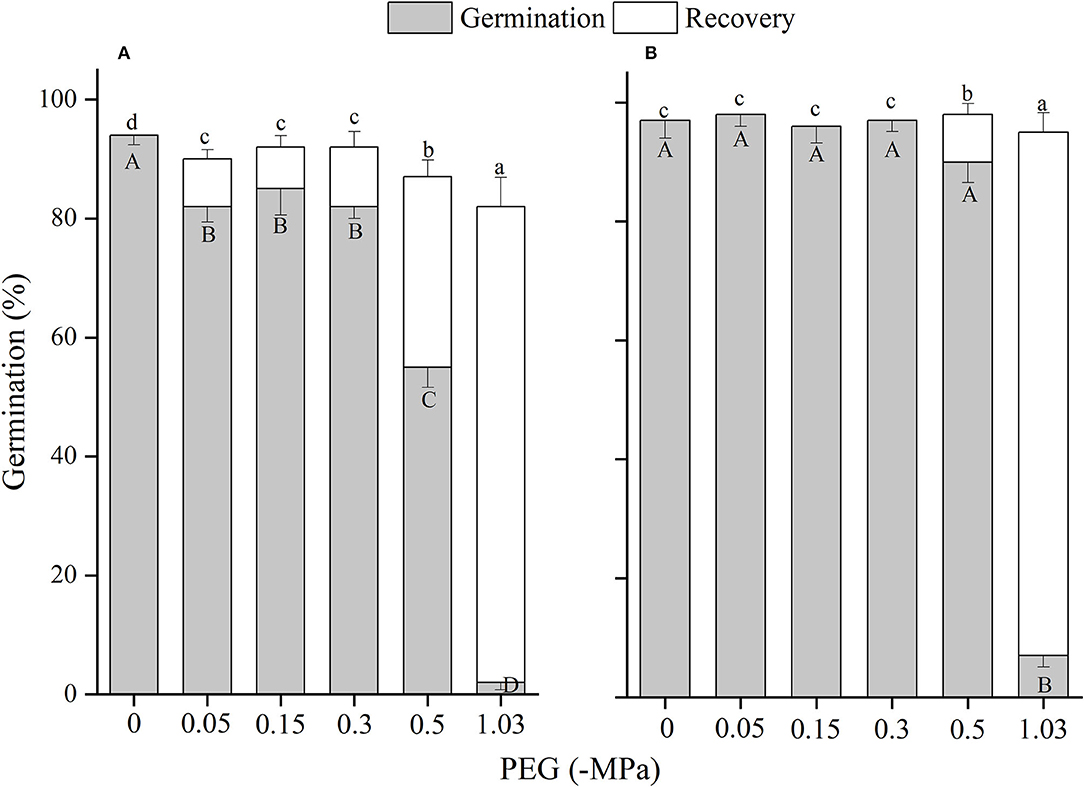
Figure 11. The effects of winged perianth and NaCl concentration on seed germination and recovery germination of Salsola nitraria. (A) winged seeds, (B) naked seeds. Different uppercase letters denote a significant difference (p < 0.05) in germination percentage among different PEG concentration. Different lowercase letters indicate a significant difference (p < 0.05) in recovery germination among different PEG concentrations.
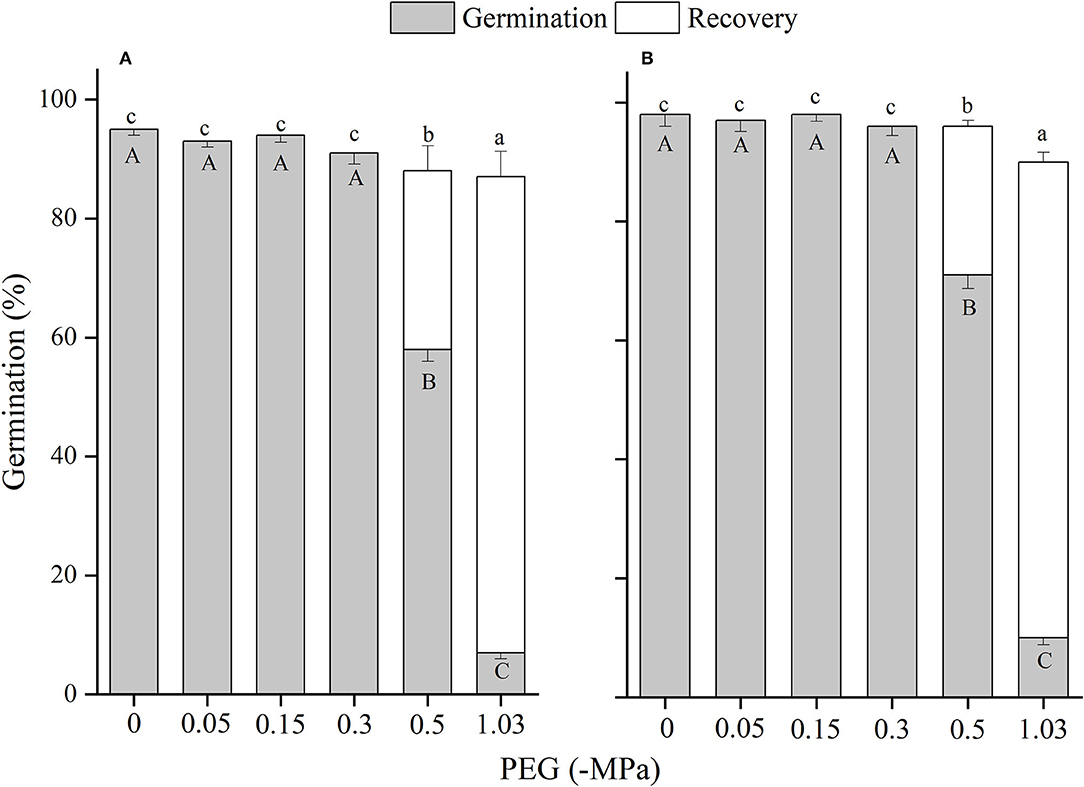
Figure 12. The effects of winged perianth and NaCl concentration on seed germination and recovery germination of Salsola ruthenica. (A) winged seeds, (B) naked seeds. Different uppercase letters denote a significant difference (p < 0.05) in germination percentage among different PEG concentrations. Different lowercase letters indicate a significant difference (p < 0.05) in recovery germination among different PEG concentrations.
Discussion
Although there is some information about the germination characteristics of Salsola seeds, our data provide a thorough knowledge of the seed germination ecology of four representative Salsola species to complex abiotic conditions and the potential interactions with the winged perianth. These data also indicate that the presence of winged perianth inhibits seed germination of S. heptapotamica, S. rosacea, and S. nitraria. In addition, all these Salsola species have a high tolerance to salinity and drought. The results indicate that the germination responses to light and temperature conditions of endemic species are more sensitive than that of widespread species.
Perianth-enclosed seeds are a characteristic feature of the Salsola species, which helps in the dispersal of seeds by wind. But the presence of winged perianth usually inhibit seed germination (El-Keblawy et al., 2013). In this study, germination percentages and germination indices of winged seeds of S. heptapotamica, S. rosacea, and S. nitraria were significantly inhibited. Removal of winged perianth significantly increased the germination percentages and germination indices of the three Salsola plants. A similar effect of perianth on seeds germination was also found in H. persicum (Wei and Wang, 2006), S. affinis (Wei et al., 2008), and H. stocksii (Rasheed et al., 2019). Previous studies reported that perianth-inhibited seed germination by acting as a mechanical barrier for radicle emergence or due to the presence of inhibitor substances, which caused low germinability of seeds (El-Keblawy et al., 2013; Xing et al., 2013). In addition, our results showed that inhibitory effects of winged perianth on seed germination could be alleviated at some specific combination of temperature and light, such as germinating at 10/25°C and light, indicating winged perianth has complex effects on germination requirements to light and temperature.
For S. rosacea, germination percentages of winged and naked seeds were all significantly lower in dark than in light at the five temperatures, which indicated the positive photoblastic nature of the seeds. Light requirement of seed ensures germination at or near soil surface that facilitates seedling survival and growth (Rasheed et al., 2019). For S. heptapotamica and S. nitraria, compared with naked seeds, only germinations of winged seeds showed high light requirement, indicating that presence of winged perianth enhanced seeds germination requirements to light for these two Salsola plants. Light-filtering properties of the winged perianth surrounding the seeds might be responsible for the sensitivity of germination to light (Cresswell and Grime, 1981). The color of the winged perianth of S. nitraria was yellow-green. The relative lower light absorbance by winged perianth of S. nitraria might be the reason for greater light requirement during seeds germination. However, the winged perianth of S. heptapotamica has a variety of colors in the wild (Zhu et al., 2003), and the absorption of pigments to light is not enough to explain the high light requirement for seed germination, which is needed to further research.
The naked seeds of S. heptapotamica, S. rosacea, and S. nitraria had up to 60% germination percentages in light, and had more than 40% germination percentages in dark at all tested temperature regimes. While winged seeds for these three Salsola plants were hard to germinate in dark, with <5% germination percentage for S. nitraria at high temperatures. These results indicated significant interaction among winged perianth, light, and temperature for seeds germination. In many species, interactions among different factors are often more inhibitory for seed germination than their individual effects (Rasheed et al., 2019). In this study, the inhibitory effect of winged perianth on seed germination aggravated further in dark at unsuitable temperatures.
Germination percentage of winged and naked seeds for S. ruthenica was almost 100% at wide temperature regimes from 5/15 to 20/35°C whether in light or dark. This indicated that S. ruthenica seeds may have an “opportunistic” germination strategy that allowed them to highly germinate in relatively wide environmental conditions (Wei et al., 2007), which might be the reason for its wide distribution in the Central Asia region (Zhu et al., 2003). In a changing climate, a wide adaptation range of environments confers an advantage for the species to be used in rehabilitation. Besides, germination indices of S. ruthenica seeds were more than 90% at the five temperatures, which allowed them to rapidly develop into seedlings in early spring when the soil is sufficiently moist, to occupy favorable habitats and complete colonization in arid deserts. By contrast, germination percentages and germination indices of S. heptapotamica, S. rosacea, and S. nitraria seeds were relatively low due to their interaction of winged perianth, light, and temperatures, which prevented germination of all the seeds at one time. Retention of a fraction of ungerminated seeds in the seed bank could be considered a bet-hedging strategy to share the risk of seed germination and improve chances for survival (Saatkamp et al., 2011). However, it also prevents these three species from being widely distributed as S. ruthenica in different habitats (Zhu et al., 2003).
These four Salsola plants decreased germination percentages with the increase of NaCl concentrations. Similar results are also found in other species, such as Suaeda salsa (Song et al., 2008) and S. iberica (Khan et al., 2002). This effect might be caused by salinity-induced osmotic stress and/or ion toxicity (Song et al., 2005). Seeds of the four Salsola plants can germinate at 700 mM NaCl solution with >10% germination percentages, which showed higher tolerance to salinity than other halophytes, such as S. vermiculata 600 mM (Guma et al., 2010), Atriplex triangularis 510 mM (Khan and Ungar, 1984), and Halogenton glomeratus 400 mM (Ahmed and Khan, 2010). Besides, El-Keblawy et al. (2020) found that seeds germination of S. drummondii collected in non-salty habitats had a lower germination percentage than those in salty habitats, indicating that maternal environment played an important role in seed tolerance to salinity. In our study, seeds of the four Salsola plants were all collected from non-salty habitats, which may be underestimated of their salt tolerance. In addition, the presence of winged perianth aggravated the inhibition effect of NaCl on germination percentage, especially for S. ruthenica, of which germination of naked seeds (39%) was significantly higher than that of winged seeds (3%) at 700 mM NaCl concentration. However, the recovery germinations and total germinations of winged seeds for the four Salsola plants at higher NaCl concentrations were significantly higher than those of naked seeds, suggesting that winged perianth also plays a positive role in the protection of seeds during exposure to high salinity (Xing et al., 2013).
Drought stress also caused a reduction in seed germination percentage and total germination of the four Salsola plants, which might be ascribed to a reduction in the osmotic potential of the solution that restricts sufficient imbibition of seeds (Tobe et al., 2000). At ΨS of −1.03 MPa, except for S. heptapotamica with the least germination percentage of 3%, the other three Salsola plants can germinate ~10%, which were more tolerant to drought than Agropyron mongolicum and Caragana korshinskii (Yu et al., 2021), S. vermiculata (Al-Shamsi et al., 2018), and Lachnoloma lehmannii (Mamut et al., 2019). Moreover, the inhibitive effect of drought was performed more significantly by the presence of winged perianth. The germination of winged seed for S. heptapotamica was no germination at ΨS of −1.03 MPa. It is reported that the perianth-enclosed seed confined germination occurrence only after sufficient rainfall, which will dilute soil salinity and can also soften the perianth and leach the inhibitors (Rasheed et al., 2019).
Conclusions
Seed germination of endemic species S. heptapotamica, S. rosacea, and S. nitraria were significantly inhibited by perianth and darkness and showed high sensitivity to different abiotic conditions. For better germination and seedling emergence, the perianth in these three species should be removed before sowing the seeds on the topsoil when the temperature is above 5/15°C. However, widespread species S. ruthenica seeds germinate equally well in light or dark in a wide range of temperatures. Considering the high tolerance of these Salsola species to salinity and drought, they could be cultivated for rehabilitating degraded arid-saline lands.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
ZW and LW conceived the topic. PC and LJ performed the experiments and analyzed all statistical data. PC, LJ, WY, ZW, and LW wrote the manuscript. ZW and LW revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Tianshan elite program of Xinjiang Uygur Autonomous Region (no. Y970000335), the National Natural Science Foundation of China (no. 31970354), and the Youth Innovation Promotion Association of Chinese Academy of Sciences (no. 2018479).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2022.892667/full#supplementary-material
References
Ahmed, M. Z., and Khan, M. A. (2010). Tolerance and recovery responses of playa halophytes to light, salinity and temperature stresses during seed germination. Flora 205, 764–771. doi: 10.1016/j.flora.2009.10.003
Al-Shamsi, N., El-Keblawy, A., Mosa, K. A., and Navarro, T. (2018). Drought tolerance and germination response to light and temperature for seeds of saline and non-saline habitats of the habitat-indifferent desert halophyte Suaeda vermiculata. Acta Physiol. Plant. 40, 200. doi: 10.1007/s11738-018-2771-z
Baskin, C. C., and Baskin, J. M. (2014). Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination. 2nd ed. San Diego, CA: Academic Press.
Baskin, J. M., Lu, J. J., Baskin, C. C., Tan, D. Y., and Wang, L. (2014). Diaspore dispersal ability and degree of dormancy in heteromorphic species of cold deserts of northwest China: a review. Perspect. Plant Ecol. Evol. Syst. 16, 93–99. doi: 10.1016/j.ppees.2014.02.004
Bhatt, A., Perez-Garcia, F., Caron, M. M., and Gallacher, D. (2016). Germination response of Salsola schweinfurthii (Chenopodiaceae) to salinity and winged perianth removal. Seed Sci. Technol. 44, 1–7. doi: 10.15258/sst.2016.44.2.14
Chang, S. J., Zuo, B., Wang, X. W., and Huang, J. H. (2008). Influence of light, temperature and salt on the germination of Salsola nitraria Pall. Arid Land Geogr. 31, 897–903. doi: 10.13826/j.cnki.cn65-1103/x.2008.06.002
Cresswell, E. G., and Grime, J. P. (1981). Induction of a light requirement during seed development and its ecological consequences. Nature 291, 583–585. doi: 10.1038/291583a0
D'Odorico, P., Bhattachan, A., Davis, K. F., Ravi, S., and Runyan, C. W. (2013). Global desertification: drivers and feedbacks. Adv. Water Resour. 51, 326–344. doi: 10.1016/j.advwatres.2012.01.013
El-Keblawy, A. (2017). Light and temperature requirements during germination of potential perennial grasses for rehabilitation of degraded sandy Arabian deserts. Land Degrad. Dev. 28, 1687–1695. doi: 10.1002/ldr.2700
El-Keblawy, A., Al-Ansari, F., and Al-Shamsi, N. (2011). Effects of temperature and light on salinity tolerance during germination in two desert glycophytic grasses, Lasiurus scindicus and Panicum turgidum. Grass Forage Sci. 66, 173–182. doi: 10.1111/j.1365-2494.2010.00773.x
El-Keblawy, A., Bhatt, A., and Gairola, S. (2013). Perianths color affects germination behaviour in wind-pollinated Salsola rubescens in Arabian deserts. Botany 92, 69–75. doi: 10.1139/cjb-2013-0183
El-Keblawy, A., Elnaggar, A., Tammam, A., and Mosa, K. A. (2020). Seed provenance affects salt tolerance and germination response of the habitat-indifferent Salsola drummondii halophyte in the arid Arabian deserts. Flora 266, 151592. doi: 10.1016/j.flora.2020.151592
El-Keblawy, A., and Ksiksi, T. (2005). Artificial forests as conservation sites for the native flora of the UAE. Forest Ecol. Manag. 213, 288–296. doi: 10.1016/j.foreco.2005.03.058
Elnaggar, A., El-Keblawy, A., Mosa, K. A., and Navarro, T. (2019). Adaptive drought tolerance during germination of Salsola drummondii seeds from saline and nonsaline habitats of the arid Arabian deserts. Botany 97, 122–123. doi: 10.1139/cjb-2018-0174
Evans, C. E., and Etherington, J. R. (1990). The effect of soil water potential on seed germination of some British plants. New Phytol. 115, 539–548. doi: 10.1111/j.1469-8137.1990.tb00482.x
Guma, I. R., Padron-Mederos, M. A., Santos-Guerra, A., and Reyes-Betancort, J. A. (2010). Effect of temperature and salinity on germination of Salsola vermiculata L. (Chenopodiaceae) from Canary Islands. J. Arid Environ. 74, 708–711. doi: 10.1016/j.jaridenv.2009.10.001
Huang, Z. Y., Zhang, X. S., Zheng, G. H., and Gutterman, Y. (2003). Influence of light, temperature, salinity and storage on seed germination of Haloxylon ammodendron. J. Arid Environ. 55, 453–464. doi: 10.1016/S0140-1963(02)00294-X
Jurado, E., Westoby, M., and Nelson, D. (1991). Diaspore weight, dispersal, growth form and perenniality of central Australian plants. J. Ecol. 79, 811–828. doi: 10.2307/2260669
Katerina, K., Daws, M. I., and Thanos, C. A. (2014). Campanulaceae: A family with small seeds that require light for germination. Ann. Bot-London 113, 135–143. doi: 10.1093/aob/mct250
Khan, M. A., and Gul, B. (2006). “Halophyte seed germination,” in Ecophysiology of High Salinity Tolerant Plants eds M. A. Khan, and D. J. Weber (Dordrecht: Springer Press), 11–30. doi: 10.1007/1-4020-4018-0_2
Khan, M. A., Gul, B., and Weber, D. J. (2002). Seed germination in the great basin halophyte Salsola iberica. Can. J. Bot. 80, 650–655. doi: 10.1139/b02-046
Khan, M. A., and Gulzar, S. (2003). Germination responses of Sporobolus ioclados: a saline desert grass. J. Arid Environ. 53, 387–394. doi: 10.1006/jare.2002.1045
Khan, M. A., and Ungar, I. A. (1984). The effect of salinity and temperature on the germination of polymorphic seeds and growth of Atriplex triangularis Willd. Am. J. Bot. 71, 481–489. doi: 10.1002/j.1537-2197.1984.tb12533.x
Kuang, W., Yonggang, M. A., Hong, L. I., and Liu, C. (2014). Analysis of land degradation intensity and trend in Central Asia from 1999 to 2012. Remote Sens. Environ., 26, 163–169. doi: 10.6046/gtzyyg.2014.04.26
Li, Z. H., and Luo, P. (2003). SPSS for Windows: Statistical Analysis Tutorial. 2nd ed. Beijing: Electronic Industry Press, 257–265.
Lu, Y. H., Ranjitkar, S., Xu, J. C., Ou, X. K., and Zhou, Y. Z. (2016). Propagation of native tree species to restore subtropical evergreen broad-leaved forests in SW China. Forests 7, 12. doi: 10.3390/f7010012
Ma, Y. L., Wang, J., Zhang, J. H., Zhang, S. Y., Liu, Y. X., and Lan, H. Y. (2017). Seed heteromorphism and effects of light and abiotic stress on germination of a typical annual halophyte Salsola ferganica in cold desert. Front. Plant Sci. 8, 2257. doi: 10.3389/fpls.2017.02257
Maimaitijiang, T., Ma, Y. L., and Lan, H. Y. (2019). Effect of saltm coupled with drought stress on seed germination and seedling growth of Salsola ferganica. Arid Zone Res. 36, 878–885. doi: 10.13866/j.azr.2019.04.11
Mamut, J., Tan, D. Y., Baskin, C. C., and Baskin, J. M. (2019). Effects of water stress and NaCl stress on different life cycle stages of the cold desert annual Lachnoloma lehmannii in China. J. Arid Land 11, 774–784. doi: 10.1007/s40333-019-0015-8
Michel, B. E., and Kaufmann, M. R. (1973). The osmotic potential of polyethylene glycol 6000. Plant Physiol. 51, 914–916. doi: 10.1104/pp.51.5.914
Naidoo, G., and Naicker, K. (1992). Seed germination in the coastal halophytes Triglochin bulbosa and Triglochin striata. Aquat. Bot. 42, 217–229. doi: 10.1016/0304-3770(92)90023-C
Negrão, S., Schmöckel, S. M., and Tester, M. (2017). Evaluating physiological responses of plants to salinity stress. Ann. Bot-London 119, 1–11. doi: 10.1093/aob/mcw191
Prince, S. D., Becker-Reshef, I., and Rishmawi, K. (2009). Detection and mapping of long-term land degradation using local net production scaling: application to Zimbabwe. Remote Sens. Environ. 113, 1046–1057. doi: 10.1016/j.rse.2009.01.016
Probert, R. J. (1992). “The role of temperature in germination ecophysiology,” in Seeds: the Ecology of Regeneration in plant Communities, ed M. Fenner (Wallingford: CABI Publishing), 410.
Qian, J. Q., Liu, Z. M., Hatier, J. H. B., and Liu, B. (2016). The vertical distribution of soil seed bank and its restoration implication in an active sand dune of Northeastern inner Mongolia, China. Land Degrad. Dev. 27, 305–315. doi: 10.1002/ldr.2428
Rasheed, A., Hameed, A., Gul, B., and Khan, M. A. (2019). Perianth and abiotic factors regulate seed germination of Haloxylon stocksii—a cash crop candidate for degraded saline lands. Land Degrad. Dev. 30, 1468–1478. doi: 10.1002/ldr.3334
Rasheed, A., Hameed, A., Khan, M. A., and Gul, B. (2015). Effects of salinity, temperature, light and dormancy regulating chemicals on seed germination of Salsola drummondii Ulbr. Pak. J. Bot. 47, 11–19.
Ruan, C. J., da Silva, J. A. T., Mopper, S., Qin, P., and Lutts, S. (2010). Halophyte improvement for a salinized world. Crit. Rev. Plant Sci. 29, 329–359. doi: 10.1080/07352689.2010.524517
Saatkamp, A., Affre, L., Baumberger, T., Dumas, P. J., Gasmi, A., Gachet, S., et al. (2011). Soil depth detection by seeds and diurnally fluctuating temperatures: different dynamics in 10 annual plants. Plant Soil 349, 331–340. doi: 10.1007/s11104-011-0878-8
Sekmen, A. H., Ozdemir, F., and Turkan, I. (2004). Effects of salinity, light, and temperature on seed germination in a Turkish endangered halophyte, Kalidiopsis wagenitzii (Chenopodiaceae). Isr. J. Plant Sci. 52, 21–30. doi: 10.1560/NXAR-71FB-CND5-E8FJ
Sen, D. N., and Chatterji, U. N. (1968). Ecology of desert plants and observations on their seedlings. II. germination behaviour of seeds in Asclepiadaceae. Plant Syst. Evol. 115, 18–27. doi: 10.1007/BF01373525
Song, J., Fan, H., Zhao, Y., Jia, Y., Du, X., and Wang, B. (2008). Effect of salinity on germination, seedling emergence, seedling growth and ion accumulation of a euhalophyte Suaeda salsa in an intertidal zone and on saline inland. Aquat. Bot. 88, 331–337. doi: 10.1016/j.aquabot.2007.11.004
Song, J., Feng, G., Tian, C. Y., and Zhang, F. S. (2005). Strategies for adaptation of Suaeda physophora, Haloxylon ammodendron and Haloxylon persicum to a saline environment during seed-germination stage. Ann. Bot-London 96, 399–405. doi: 10.1093/aob/mci196
Tobe, K., Li, X., and Omasa, K. (2000). Effects of sodium chloride on seed germination and growth of two Chinese desert shrubs, Haloxylon ammodendron and H. persicum (Chenopodiaceae). Aust. J. Bot. 48, 455–460. doi: 10.1071/BT99013
Wang, C., Wang, S., Fu, B. J., Lu, Y. H., Liu, Y. X., and Wu, X. (2020). Integrating vegetation suitability in sustainable revegetation for the Loess Plateau, China. Sci. Total Environ. 11, 559–564. doi: 10.1016/j.scitotenv.2020.143572
Wang, Y., Jiang, G. Q., Han, Y. N., and Liu, M. M. (2013). Effects of salt, alkali and salt–alkali mixed stresses on seed germination of the halophyte Salsola ferganica (Chenopodiaceae). Acta Ecol. Sini. 33, 354–360. doi: 10.1016/j.chnaes.2013.09.010
Wang, Y., Zhang, X. M., Li, L., and Li, H. X. (2008). Seed on response of germination of four species of Salsola L. to main ecology factors. Seed 27, 58–63, 67. doi: 10.16590/j.cnki.1001-4705.2008.08.061
Wei, Y., Dong, M., and Huang, Z. Y. (2007). Seed polymorphism, dormancy and germination of Salsola affinis (Chenopodiaceae), a dominant desert annual inhabiting the Junggar Basin of Xinjiang, China. Aust. J. Bot. 55, 464–470. doi: 10.1071/BT06016
Wei, Y., Dong, M., Huang, Z. Y., and Tan, D. Y. (2008). Factors influencing seed germination of Salsola affinis (Chenopodiaceae), a dominant annual halophyte inhabiting the deserts of Xinjiang, China. Flora 203, 134–140. doi: 10.1016/j.flora.2007.02.003
Wei, Y., and Wang, X. Y. (2006). Role of winged perianth in germination of Haloxylon (Chenopodiaceae) seeds. Acta Ecol. Sin. 26, 4014–4018.
Xing, J. J., Cai, M., Chen, S. S., Chen, L., and Lan, H. Y. (2013). Seed germination, plant growth and physiological responses of Salsola ikonnikovii to short-term NaCl stress. Plant Biosyst. 147, 285–297. doi: 10.1080/11263504.2012.731017
Yu, L., Guo, T. D., Sun, Z. C., Ma, Y. P., Li, Z. L., Zhao, Y. N., et al. (2021). The seed germination characteristics and thresholds of two dominant plants in desert grassland-shrubland transition of the eastern Ningxia, China. Acta Ecol. Sin. 41, 4160–4169. doi: 10.5846/stxb201910152147
Zhang, K., Zheng, H., Chen, F. L., Ouyang, Z. Y., Wang, Y., Wu, Y. F., et al. (2015). Changes in soil quality after converting Pinus to Eucalyptus plantations in southern China. Solid Earth 6, 115–123. doi: 10.5194/se-6-115-2015
Keywords: light, salinity, Salsola, seed germination, winged perianth, land rehabilitation
Citation: Chen P, Jiang L, Yang W, Wang L and Wen Z (2022) Seed Germination Response and Tolerance to Different Abiotic Stresses of Four Salsola Species Growing in an Arid Environment. Front. Plant Sci. 13:892667. doi: 10.3389/fpls.2022.892667
Received: 09 March 2022; Accepted: 15 April 2022;
Published: 19 May 2022.
Edited by:
Ali El-Keblawy, University of Sharjah, United Arab EmiratesReviewed by:
Sanjay Gairola, EPAA, United Arab EmiratesMohsin Tanveer, University of Tasmania, Australia
Salman Gulzar, University of Karachi, Pakistan
Copyright © 2022 Chen, Jiang, Yang, Wang and Wen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Wang, ZWdpd2FuZ0Btcy54amIuYWMuY24=; Zhibin Wen, emhpYmlud2VuQG1zLnhqYi5hYy5jbg==
†These authors have contributed equally to this work
 Pengyou Chen
Pengyou Chen Li Jiang1†
Li Jiang1† Lei Wang
Lei Wang