
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci. , 14 June 2022
Sec. Plant Symbiotic Interactions
Volume 13 - 2022 | https://doi.org/10.3389/fpls.2022.882255
This article is part of the Research Topic Rhizospheric Interactions: Integrating Plant-Microbe Signaling During Stresses View all 9 articles
The rhizospheric microbial community affects the population establishment of invasive plants in introduced areas, among which Bacillus has numerous functions in promoting plant growth. This study isolated and enriched the Bacillus community in the rhizospheric soil of the invasive plant Ageratina adenophora and the native accompanying plant Rabdosia amethystoides. The effects of these rhizospheric Bacillus communities on the growth and competition of A. adenophora and R. amethystoides were evaluated in pot experiments. The results showed that the number and diversity of Bacillus in the rhizospheric soil of A. adenophora were higher than those of R. amethystoides (A. adenophora: 122 strains in soil, 16 Bacillus taxa; R. amethystoides: 88 strains in soil, 9 Bacillus taxa). After Bacillus inoculation of A. adenophora in a pot experiment, Bacillus idriensis, Bacillus toyonensis and Bacillus cereus were accumulated in the rhizospheric of A. adenophora, which significantly increased the nitrate nitrogen (NO3–-N) content in the soil and the total carbon and nitrogen concentrations in A. adenophora in the mixed treatment. The selective accumulation of Bacillus enhanced the competitive advantage of A. adenophora over the native accompanying plant; the corrected index of relative competition intensity of A. adenophora-inoculated Bacillus reached double that of the uninoculated treatment, and the growth of native plants was greatly suppressed under mixed planting. Our study confirmed that invasion of A. adenophora can lead to the accumulation of specific Bacillus taxa in the rhizospheric soil, which in turn can increase the competitive advantage of A. adenophora.
Invasive weeds are a major threat to the structure and function of global ecosystems (van der Putten et al., 2007; D’Antonio et al., 2017) and cause huge economic losses (Duncan et al., 2004). Understanding the invasion mechanism is crucially important to effectively control and develop management strategies for invasive weeds. Modifications in the microbial community induced by invasive plants have the potential to initiate a self-promoting mechanism that facilitates the invasion process (Callaway and Ridenour, 2004; Massenssini et al., 2014; Fang et al., 2019). For instance, such changes in the microbiota associated with nutrient cycling induced by the exotic plant Conyza canadensis may have a beneficial effect that promotes its establishment and spread (Zhang et al., 2020). Beneficial microorganisms, such as arbuscular mycorrhizal (AM) fungi, plant growth-promoting rhizobacteria (PGPR), and rhizobia, were accumulated in the rhizosphere of invasive plants during their growth process, promoting their growth and competitiveness (Rodríguez-Echeverría, 2010; Philippot et al., 2013; Zhang et al., 2017). In the rhizospheric soil of the invasive plant Flaveria bidentis, the levels of diazotrophs, phosphorus-solubilizing bacteria, and silicate-solubilizing bacteria were much higher than those of native plants (Song et al., 2016, 2017). Compared with native plants, the invasive plants Avena barbata and Bromus secalinus increase the number of ammonia-oxidizing bacteria in soil, improve soil nitrogen cycling, and increase the soil nitrogen content, enhancing the growth and competitiveness of invasive plants (Hawkes et al., 2005). Consequently, invasive species can establish more positive feedback interactions with the soil microbiota and appear to have a greater dependence on these associations than noninvasive species (Massenssini et al., 2014; Trognitz et al., 2016).
The genus Bacillus has tremendous genetic and metabolic diversity, and they perform various ecological functions in the soil ecosystem, including nutrient cycling and endowing plants with stress tolerance (Choudhary and Johri, 2008; Dheeman et al., 2017). These bacteria can form endospores, allowing them to survive in hostile environments and to perform well under different environmental conditions (Gardner, 2004; Pesce et al., 2014). Bacillus strains can be used as plant growth promoters and biocontrol agents, promoting plant growth under various environmental conditions (Kumar et al., 2011; Saxena et al., 2020). The principal mechanism includes increasing the nutrient content and uptake by nitrogen fixation, phosphate, and potassium solubilization (Sharma et al., 2013; Ding et al., 2015; Verma et al., 2015), regulating plant hormone production (Alina et al., 2015; Asari et al., 2017), reducing abiotic and biotic stresses by biofilm formation, producing volatile organic compounds (VOCs), and inducing systemic resistance (Li et al., 2015; Burkhanova et al., 2017; Pandin et al., 2017). For example, Bacillus pumilus M8 significantly reduced the in vitro growth of Botrytis cinerea and Fusarium solani phytopathogens and induced systemic resistance of pepper to gray mold (Márquez et al., 2020). In both the presence and absence of salt stress, Bacillus licheniformis A2 increased peanut plant growth (Goswami et al., 2014). Several other Bacillus taxa such as Bacillus megaterium, Bacillus cereus, Bacillus subtilis, B. licheniformis, Bacillus mycoides, Bacillus idriensis, and Bacillus vietnamensis are known to promote plant growth by the main mechanisms described above (Goswami et al., 2016; Radhakrishnan and Lee, 2016; Yousuf et al., 2017; Fan et al., 2018). Therefore, studies on the relationship between Bacillus spp. and invasive plants are important to further elucidate the colonization and dominance of invasive plants in non-native habitats.
Ageratina adenophora (Sprengel, also known as Eupatorium adenophorum Sprengel) is a notorious weed that originated in Mexico and Costa Rica, invading more than 40 tropical and subtropical countries in Asia, Oceania, Africa, and Europe (Datta et al., 2019; Gu et al., 2021). It first invaded Yunnan Province, China, in the 1940s and is currently widely distributed in six provinces in Southwest China. It continues to spread to the east and north at a rate of approximately 20 km/year (Wang and Wang, 2006; Wan et al., 2010). It can reduce the diversity of native plant species, crop productivity, and forage production in pastures, resulting in severe economic losses for agricultural, forestry, and livestock industries (Gui et al., 2009; Kong et al., 2017; Poudel et al., 2019). An increasing number of evidence suggests that A. adenophora can alter the soil microbial community, promoting its growth and competitiveness while inhibiting neighboring native plants (Li et al., 2009; Xu et al., 2012; Zhao et al., 2019). For example, A. adenophora invasion leads to a decrease in actinomycetes but increases in aerobic, anaerobic bacteria, and nitrogen-fixing bacteria (Niu et al., 2007; Xu et al., 2012; Fang et al., 2019). The rhizospheric soil microbiomes of A. adenophora differed to varying degrees in the relative abundances of bacterial and fungal phyla and genera from those of two native plants, Artemisia indica and Imperata cylindrica, and were more metabolically active than both of these, as indicated by marked increases in the expression levels of genes associated with the cell wall, cell membrane, and envelope biogenesis, energy production and conversion, and the transport of carbohydrates, amino acids, coenzymes, nucleotides, and secondary metabolites (Xia et al., 2021). Previous studies have reported that A. adenophora can result in the accumulation of Bacillus, such as B. megaterium and B. cereus, which might significantly promote the growth of A. adenophora compared to native plants (Niu et al., 2007; Sun et al., 2021). Nonetheless, it remains unknown whether A. adenophora can selectively accumulate Bacillus from the local soil to promote its competitive growth. We hypothesized that the invasion of A. adenophora may involve the accumulation of Bacillus in its rhizosphere, increasing its competitiveness against native plants. To investigate this hypothesis, we analyzed and compared the composition and abundance of the culturable Bacillus community in the rhizospheric soil of the invasive plant A. adenophora and the native accompanying plant Rabdosia amethystoides and determined the effects of Bacillus on the growth of A. adenophora using a pot experiment.
Soil sampling was conducted at two sites in the same habitat at Yunnan Agricultural University in Yunnan Province, China, in the summer of 2020 (Lat. 25°08′30′′N, Lon. 102°45′13′′E, with an elevation of 1,940 m). Site I was dominated by A. adenophora, whereas site II was dominated by R. amethystoides. The coverage of the two plants was higher than 80%, and the abiotic factors such as light and soil texture were basically the same. Each site had three plots with a distance of approximately 5 m between plots. A number of ten plants were randomly collected from plots of 5 m × 5 m. Soil samples were collected from each plot as follows: 1 cm of litter was removed from the soil surface, and underlying soil within a 30-cm radius of each plant was loosened with a shovel. Then, plants were dug out, lightly shaken, and the soil remaining attached to the root surface was carefully collected with a brush; approximately 150–200 g of soil was extracted from each plot. Because the plants grew in a relatively high density and thus might have a large impact on the surrounding soil, these soil samples were operationally defined as rhizospheric soils (Batten et al., 2006). The collected samples from the same plots of the same site were mixed to form two soil samples, representing the rhizospheric soil of A. adenophora and R. amethystoides. The soil was sieved through a 2-mm sieve, transferred to sterile plastic bags, cooled immediately, transported to the laboratory, and stored at 4°C. Each sample was assigned to two groups: one group was used for chemical analysis and the other for Bacillus community isolation and analysis.
Bacillus was explicitly enriched in the soil, and a combination of heat-shock and serial dilution was used to isolate Bacillus, as Kumar et al. (2012) described. Briefly, soil samples (10 g) from the rhizosphere of A. adenophora and R. amethystoides were heat-treated (80°C), transferred to 90 ml sterile distilled water, and mixed by shaking (180 rpm) the triangular flask for 12 h at 30°C. After serial dilution suspension to 10–3, one part was used to isolate and identify strains, and the other part was used to prepare inoculants for pot experiments. For the isolated strains, the aseptic dilution involved the collection of a 0.1 ml suspension using a micropipette that was streaked on nutrient agar plates in triplicate. The plates were incubated at 30°C for 48 h. The number of Bacillus was obtained by counting the colony-forming units (CFUs), and the data were expressed as CFU per gram dry weight of each soil sample. All isolates were collected and purified based on the quadrant method until a single colony was obtained. The single colony was selected by an aseptic toothpick and was separately incubated in a 1.5-ml centrifuge tube (1 ml nutrient liquid medium culture in a 1.5-ml centrifuge tube) that was shaken at 37°C (200 rpm) for 12 h. Some of the Bacillus in the centrifuge tube were dissolved in 50% glycerin (v:v = 1:1) and were stored at –20°C for short-term storage. With respect to the inoculants, a 4 ml suspension was placed in a triangular flask containing 100 ml nutrient liquid medium (1% peptone, 0.3% beef extract, 0.5% NaCl) that was shaken (180 rpm) for 24 h at 37°C. The optical density of the suspension was adjusted to approximately 1.0 (optical density at 600 nm) by diluting it with sterile distilled water. The population count of Bacillus was maintained at 108 CFU/ml.
The isolated Bacillus culture suspension was centrifuged at 12,000 r/min for 10 min at 4°C. The suspension was agitated to homogeneity, and centrifugation was performed; the supernatant was removed, and 400 μl of 10 × TE and 25 μl of lysozyme (50 mg/L) were added to the tubes. The tubes were then shaken horizontally at 180 rpm for 12 h at 37°C. Then, 100 μl of 10% SDS was added, and the tubes were incubated in a 60°C water bath for 30 min and centrifuged as described above. Subsequently, the lysate was combined and mixed with an equal volume of phenol–chloroform–isoamyl alcohol (25:24:1). The supernatant was extracted with two times the volume of chloroform–isoamyl alcohol (24:1). The aqueous phase was precipitated with an equal volume of isopropanol at –20°C overnight. The aqueous phase was precipitated with an equal volume of isopropanol at –20°C overnight. The nucleic acid pellet was centrifuged at 12,000 g for 10 min at room temperature. The crude extracts were washed with 70% cold ethanol, resuspended in 50 μl of diethylpyrocarbonate-treated water, and stored at –20°C. The quality and quantity of DNA were detected by a NanoDrop 2000 ultramicro spectrophotometer (NanoDrop 2000, Thermo Scientific, United States).
Amplification of 16S rDNA by polymerase chain reaction (PCR) was carried out using the universal primers F27 (5′-AGAGTTTGATCCTGGCTCAG-3′) and R1492 (5′-GGTTACCTTGTTACGACTT-3′) (Weisburg et al., 1991). The polymerase chain reaction was performed in a 30-μl reaction volume containing approximately 20 ng of template DNA, 10 μm of each primer, 10 mM dNTPs, and 1.5 U Taq polymerase in 1 × PCR buffer. Reactions were cycled 30 times at 94°C for 1 min, 60°C for 45 s, and 72°C for 1 min, followed by a final extension at 72°C for 8 min. The amplified PCR product (1,500 bp) was separated by electrophoresis on 1% (w/v) agarose gels containing ethidium bromide and was visualized using a UV-transilluminator. Sequencing was conducted by Tsingke Biological Technology (Kunming, China). A BigDye Terminator kit (BDT v1.1) was used for the reaction; a 20 μl reaction volume contained 1 μl of DNA, 8 μl of BigDye, 1 μl of primer, and 10 μl of deionized water. Reactions were performed at 96°C for 1 min, (96°C for 10 s, 50°C for 5 s, and 60°C for 4 min) 25 times. Purification was performed by adding Magical Buffer and Ferrite Bead reagent followed by centrifugation. The DNA sequencing was performed using an automated DNA sequencer (ABI PRISM 3730XL Genetic Analyzer) (Applied Biosystems). Data were obtained from the sequencer and were analyzed by Sequencing Analysis 5.2 to form the final sequence.
The 16S rDNA sequence was assembled with DNAStar 6.0 and was added to the SeqMan program, where it was cut to eliminate 30 to 60-bp nucleotides at both ends of the sequence, and by clicking ‘‘Assemble,’’ homologous sequence linking was executed. Sequences were imported into the EZbiocloud software package1, and comparisons were carried out with BLAST homology compared with the known 16S rDNA sequence. 16S rDNA sequence similarity of ≥ 97% with a prototype strain sequence from the GenBank database was used for the identification of the species level. Multiple sequences and several near-margin sequences were aligned using the Clustal-W program in MEGA X software (version X, Mega Limited, Auckland, New Zealand). The phylogenetic tree was constructed using the neighbor-joining method (NJT), which was implemented in the MAGE X program (Kumar et al., 2018). The T92+G+1 (Tamura3-parameter and Gamma distributed) model was chosen as the best model for phylogenetic tree analysis and 1,000 bootstrap replications. One representative strain of each bacterium isolated and identified in this experiment was selected, and then, the nucleotide sequences of these strains were submitted to NCBI GenBank to obtain accession numbers (OM149778-OM149795). The relative abundance (RA) of Bacillus taxa in our total sample was calculated according to the following formula: RA = A/N × 100%. (Note: “A” represents the number of one Bacillus phylotype strains and “N” represents the total number of strains).
The Shannon (H’) index and Simpson’s diversity index (D) were calculated according to the formula as follows:
(“S” represents the total number of Bacillus phylotypes; Pi = Ni / N, “Ni” represents the number of the Bacillus phylotype i, “N” represents the number of all Bacillus phylotypes).
The collected soil samples from the same inoculation and plant growth treatment were mixed to form six soil samples, representing Am, A+R, and Rm of two inoculation treatments. This was done for each soil sample using heat-shock and serial dilution of isolated Bacillus. Colony separation 10–3 of the supernatant was used to count the colony-forming units. All isolates were collected and purified. The identification of Bacillus strains, alpha diversity, and the relative abundance of Bacillus strains were carried out according to the above method.
A greenhouse experiment was carried out to test the effect of Bacillus from the rhizosphere of A. adenophora or R. amethystoides on the competitive growth of A. adenophora at Yunnan Agricultural University. On three successive days, soil without plant cover was autoclaved for 2 h at 121°C to remove soil microbes. The basic properties of the soil were as follows: pH (w/v water = 1:5) was 6.25, the organic matter content was 15.502 g/kg, total nitrogen was 0.899 g/kg, total phosphorus was 0.351 g/kg, total potassium was 40.03 g/kg, available nitrogen was 20.28 μg/g, available phosphorus was 5.089 μg/g, and available potassium was 32.32 μg/kg. The sterilized soil was used for three treatments: monoculture of A. adenophora, monoculture of R. amethystoides, and an equal mixture of A. adenophora and R. amethystoides. Each treatment was further divided into three levels: uninoculated treatment (C), inoculated with Bacillus from A. adenophora (AB), and inoculated with Bacillus from R. amethystoides (RB). Seeds of A. adenophora and R. amethystoides were obtained from Yunnan Agricultural University and were surface-sterilized in 1.5% sodium hypochlorite (NaClO), rinsed five times with sterile distilled water, submerged in 70% ethanol for 1 min, and then washed five times with sterile distilled water. A number of ten seeds of A. adenophora or R. amethystoides (or five seeds of the two plants in a mixture) were added to 1 kg of soil in the pots (20 cm × 13 cm × 14 cm for length × width × height) and were covered by approximately 1 cm of soil, and the bacterial suspension of A. adenophora or R. amethystoides was inoculated (10 mL 108 CFU/ml). Bacillus suspension was added one time per month during the planting period for a total of four times. The plants germinated in approximately 5 days. Then, 10 days after germination, excess plants were removed, so that each pot contained only two of the same size plants (two monocultures, one from each of the mixed-cultures), with 10 replicates per treatment. Sterile water was applied every 2 days. The pots were placed on a shelf in the greenhouse for 4 months under a 14-h L:10-h D photoperiod at 28°C and were arranged in a completely randomized design.
All parameters were measured 4 months after sowing. Plants were dug out, and the soil attached to the root surface was collected. All plants were oven-dried at 80°C for 48 h before specific measurements were taken. Dry biomass was measured using the entire plant, including the above-ground biomass and root biomass. The corrected index of relative competition intensity (CRCI) was used to test the plant’s competitive ability. This index was calculated following the method of Oksanen et al. (2006).
where X is the average biomass without competition and Y is the average biomass of individual plants grown in competition. A CRCI value > 0 indicates that competition has a negative effect, and a CRCI value < 0 indicates that competition positively affects the target plant.
The concentration of C in the plants was measured by a CHN analyzer (LECO Corporation). The concentration of N was determined by the micro-Kjeldahl procedure (Nelson and Sommers, 1972). The concentrations of P and K were determined by inductively coupled plasma spectroscopy (Isaac and Johnson, 1983).
After the plants were harvested, the characteristics of the potting soil were assessed. A Chem II flow-injection analyzer (QC8500S2, Lachat, United States) was used to quantify the contents of ammonium nitrogen (NH4+-N) and nitrate-nitrogen (NO3–-N) in KCl extracts (Li and Li, 2013). Available phosphorus (AP) was determined according to the methods described by Olsen (Olsen, 1954). The burnt-luminosity method was used to determine the content of available potassium (AK) (Lu, 2000).
Before analyses, all data were tested for normality using the Shapiro–Wilk test. All data met the normality assumption. The variables in the treatments were expressed as the mean ± SE. One-way analysis of variance (ANOVA) was performed to determine the differences in biomass, nutrient concentrations of the plants (C, N, P, and K), and the available soil nutrient contents in different inoculated treatments and to determine the effect of Bacillus on the competitive potential (CRCI) of both species. Multiple comparisons between groups were performed using the least square differences (LSD). Student’s t-test was used to determine the effect of monoculture and the mixture on the plant growth parameters. Mean values and standard errors per treatment combination were presented (n = 5). All analyses were conducted using SPSS 19.0 (SPSS Inc., Chicago, IL, United States).
A total of 122 sequences were obtained from A. adenophora rhizospheric soil. A number of twelve phylotypes belonged to the genus Bacillus, and four others belonged to the phylotypes of other genera in the Bacillaceae family (Brevibacterium frigoritolerans, LysiniBacillus xylanilyticus, Sporosarcina aquimarina, and SoliBacillus isronensis). A total of 88 sequences were obtained from the R. amethystoides rhizospheric soil, of which eight phylotypes belonged to the genus Bacillus, and the others to the genus Brevibacterium (Supplementary Figure 1). The number and alpha diversity (Shannon–Wiener and Simpson’s diversity) index of Bacillus in rhizospheric soil of A. adenophora were significantly higher than those of R. amethystoides [numbers: F(1,4) = 29.952, p = 0.005; Shannon–Wiener index: F(1,4) = 28.491, p = 0.006; Simpson’s diversity index: F(1,4) = 18.424, p = 0.013; Table 1]. Among them, seven Bacillus phylotypes were found in the rhizospheric soil of both plants; their relative abundance (RA) in the A. adenophora rhizospheric soil was as follows: B. idriensis 35.25%, Bacillus toyonensis 18.03%, Bacillus thuringiensis 9.84%, B. cereus 7.38%, Bacillus mycoides 7.38%, Bacillus simples 2.46%, and B. frigoritolerans 5.74%, whereas the RA in the R. amethystoides rhizospheric soil was as follows: B. idriensis 4.55%, B. toyonensis 22.73%, B. thuringiensis 31.82%, B. cereus 4.55%, B. mycoides 2.27%, B. simples 15.91%, and B. frigoritolerans 15.91%. A number of nine Bacillus phylotypes were separated only from the rhizospheric soil of A. adenophora (B. pumilus 3.28%, B. licheniformis 0.82%, Bacillus tequilensis 3.28%, L. xylanilyticus 0.82%, S. aquimarina 0.82%, S. isronensis 0.82%, Bacillus subterraneus 0.82%, Bacillus firmus 2.46%, and Bacillus anthracis 0.82%). A number of two Bacillus phylotypes were separated only from the rhizospheric soil of R. amethystoides (Bacillus thioparans 1.14%, Bacillus wiedmannii 1.14%) (Figure 1). All of the above strains except Bacillus anthracis have been reported to have a strong plant growth-promoting ability.
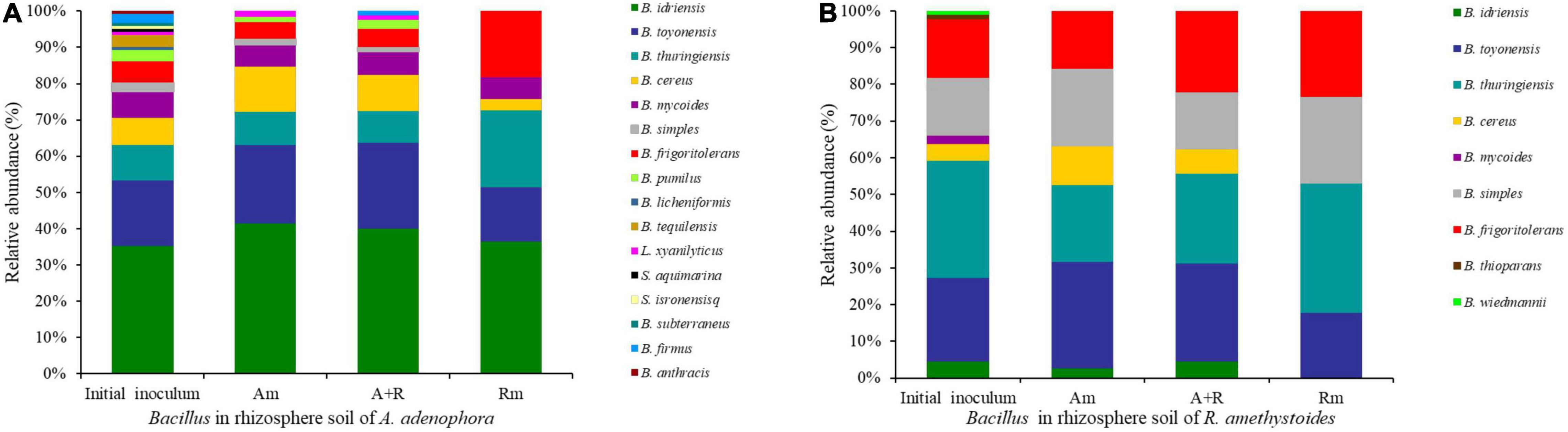
Figure 1. The relative abundance (RA) detected for each Bacillus phylotype in rhizospheric soil of A. adenophora (A) and (B) in different treatments. Am, A. adenophora monoculture; A+R, A. adenophora and R. amethystoides mixture; Rm, R. amethystoides monoculture.
Compared to the initial inoculum of A. adenophora and R. amethystoides, the number and Shannon–Wiener index of Bacillus in rhizospheric soil of different treatments were significantly decreased after the pot experiment [A. adenophora: numbers: F(3,8) = 100.179, p < 0.001; Shannon–Wiener index: F(3,8) = 28.558, p < 0.001; R. amethystoides: numbers: F(3,8) = 64.238, p < 0.001; Shannon–Wiener index: F(3,8) = 70.436, p < 0.001; Table 1]. For the inoculants of A. adenophora, Shannon–Wiener index of A+R was significantly higher than that of Rm (p < 0.05), whereas there was no significant difference in Simpson’s diversity index among the three treatments (Table 1). A total of three Bacillus taxa with high relative abundance were selected as the dominant bacteria. B. idriensis (41.54%), B. toyonensis (21.54%), and B. cereus (12.31%) were dominant in Am; B. idriensis (40.00%), B. toyonensis (23.75%), and B. cereus (10.00%) were dominant in A+R; and B. idriensis (36.36%), B. thuringiensis (21.21%), and B. frigoritolerans (18.18%) were dominant in Rm (Figure 1). For the inoculants of R. amethystoides, the alpha diversity index of Rm was lowest compared to Am and A+R (all p < 0.01; Table 1). B. toyonensis (28.95%), B. thuringiensis (21.05%), and B. simples (21.05%) were dominant in Am; B. toyonensis (26.67%), B. thuringiensis (24.44%), and B. frigoritolerans (22.22%) were dominant in A+R; and B. thuringiensis (35.29%), B. simples (23.53%), and B. frigoritolerans (23.53%) were dominant in Rm (Figure 1).
The effects of inoculation with Bacillus from A. adenophora (AB) and R. amethystoides (RB) were different on the biomasses of A. adenophora and R. amethystoides (Figure 2). Compared to the uninoculated treatment, the biomasses of A. adenophora and R. amethystoides were significantly increased by the two kinds of inoculum (A. adenophora, monoculture: F(2, 12) = 2366.343, p < 0.001; mixture: F(2, 12) = 666.172, p < 0.001; R. amethystoides, monoculture: F(2, 12) = 565.635, p < 0.001; mixture: F(2, 12) = 343.127, p < 0.001). For A. adenophora, the biomass values of inoculated AB treatments in the respective monoculture and mixture were 17.63 and 16.42 times those of the uninoculated treatments, and RB treatments in the respective monoculture and mixture were 6.51 and 6.29 times those of the uninoculated treatments. For R. amethystoides, the biomass values of inoculated AB treatments in the respective monoculture and mixture were 1.79 and 1.86 times those of the uninoculated treatments, and RB treatments in the respective monoculture and mixture were 2.46 and 2.97 times of those of the uninoculated treatments. The competition had a favorable effect on the biomass of A. adenophora but negatively affected the biomass of R. amethystoides. Specifically, the biomass of A. adenophora in the mixture was significantly higher than that in the monoculture (monoculture: C:0.121 ± 0.009, AB: 2.133 ± 0.056, RB: 0.788 ± 0.058; mixture: C:0.188 ± 0.006, AB: 3.087 ± 0.213, RB: 1.182 ± 0.057) [C: F(1, 8) = 167.773, p < 0.001, AB: F(1, 8) = 93.638, p < 0.001, RB: F(1, 8) = 115.269 p < 0.001]. In comparison, the biomass of R. amethystoides under mixed planting was substantially lower than that in the monoculture (monoculture: C:0.087 ± 0.001, AB: 0.156 ± 0.007, RB: 0.214 ± 0.007; mixture: C:0.065 ± 0.006, AB: 0.121 ± 0.011, RB: 0.193 ± 0.003) [C: F(1, 8) = 51.041, p < 0.001, AB: F(1, 8) = 36.207, p < 0.001, RB: F(1, 8) = 34.374, p < 0.001].
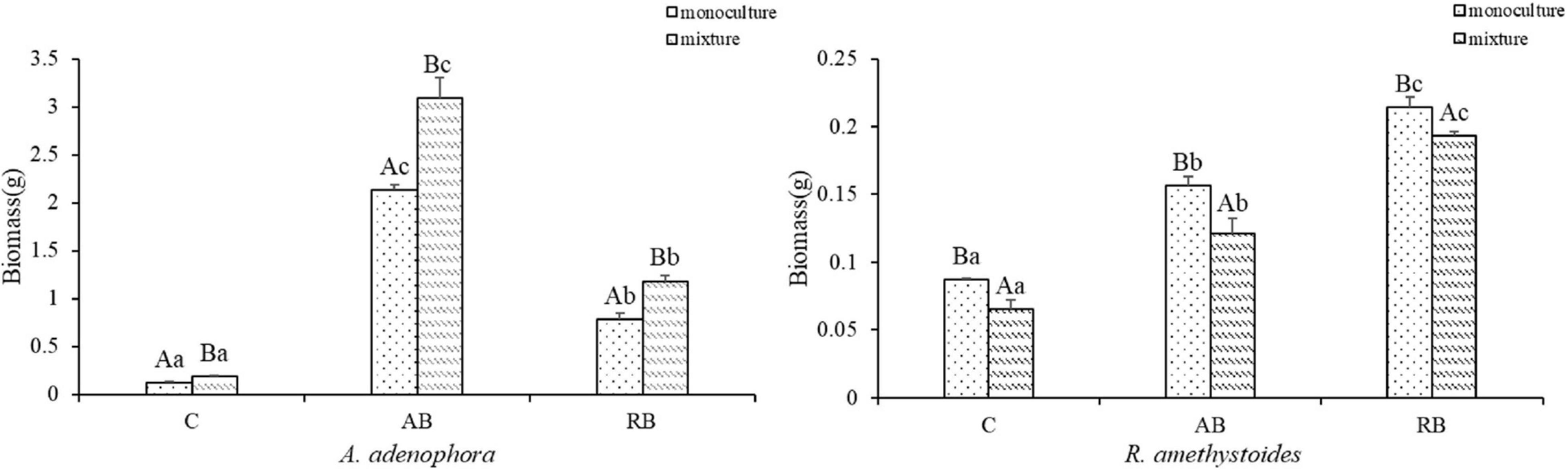
Figure 2. Effect of Bacillus on the plant dry biomass of invasive plant A. adenophora and native plants R. amethystoides when grown in monoculture or in mixture. C, control treatment; AB, Bacillus in A. adenophora rhizospheric soil; RB, Bacillus in R. amethystoides rhizospheric soil. Different lowercase letters indicate significant differences among the inoculation treatments at p < 0.05. Different capital letters indicate significant differences between the monoculture and mixture at p < 0.05. Error bars represent ± 1SE of the mean (n = 5).
The CRCI was determined to quantify the effects of Bacillus from the two types of rhizospheric soil on the growth of A. adenophora and R. amethystoides. In all treatments, the competition had a beneficial effect on A. adenophora growth and a detrimental effect on R. amethystoides growth. Distinct microbial inocula had different effects on the competitive growth of the two plants. The beneficial effect of AB on A. adenophora growth resulted in a significant improvement [F(2, 12) = 16.096, p = 0.001] in which the CRCI of the inoculated AB was two times as high as that of the uninoculated treatment, whereas RB inoculation had no significant effect on the competitive growth. The detrimental impact of RB on R. amethystoides growth resulted in a significant reduction [F(2,12) = 6.258, p = 0.013], whereas AB inoculation did not affect the competitive growth of R. amethystoides (Figure 3).
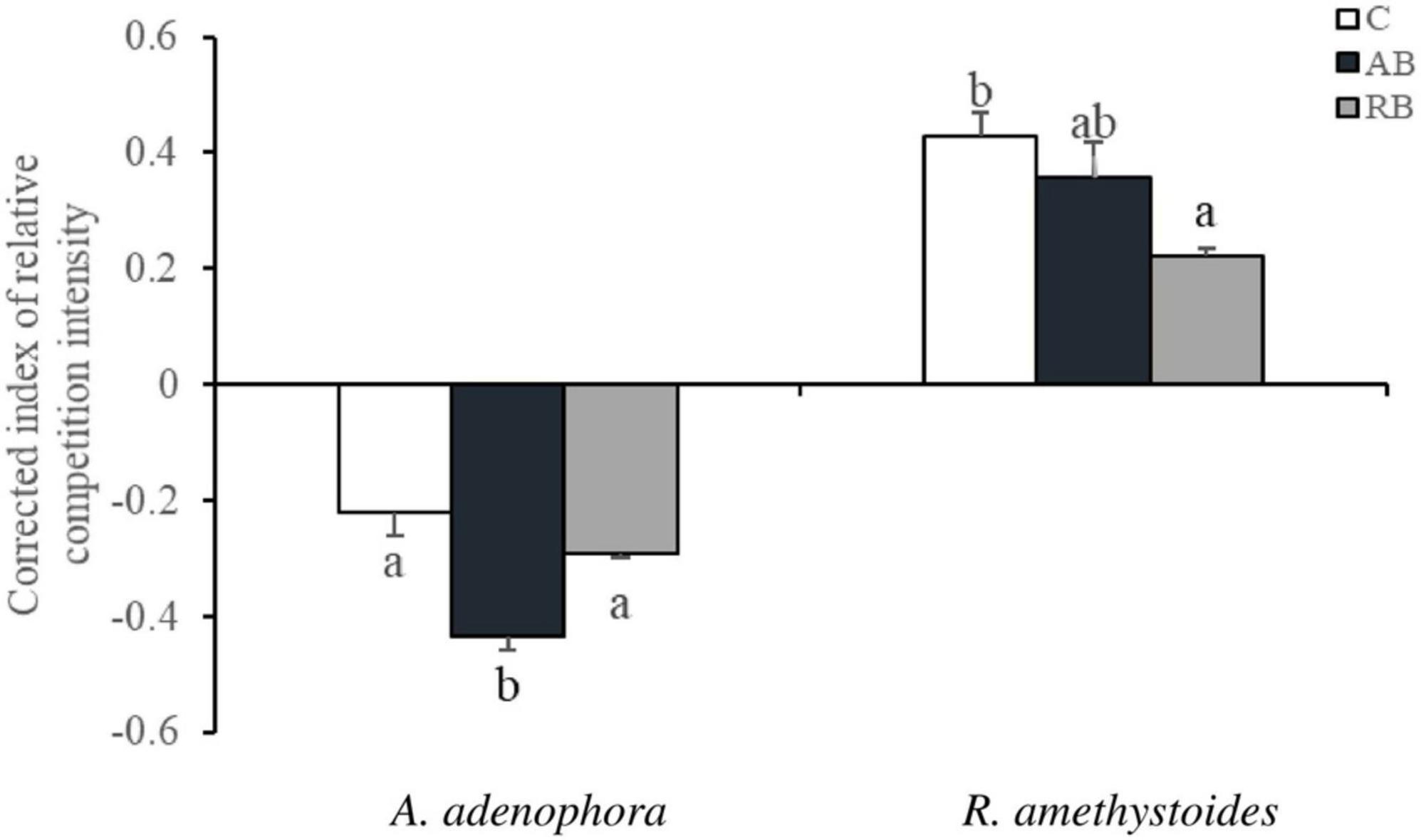
Figure 3. Effect of Bacillus on the corrected index of relative competition intensity (CRCI) of invasive plant A. adenophora and native plant R. amethystoides. Different lowercase letters indicate significant differences among the inoculation treatments at p < 0.05. Error bars represent ± 1SE of the mean (n = 5).
Inoculation with Bacillus from both rhizospheric soils significantly increased the total C, N, P, and K concentrations of A. adenophora and R. amethystoides in the monoculture and mixture [A. adenophora: total C: monoculture: F(2, 12) = 288.193, p < 0.001; mixture: F(2, 12) = 523.010, p < 0.001; total N: monoculture: F(2, 12) = 149.670, p < 0.001; mixture: F(2, 12) = 15.549, p < 0.001; total P: monoculture: F(2, 12) = 527.826, p < 0.001; mixture: F(2, 12) = 1758.326, p < 0.001; total K: monoculture: F(2, 12) = 355.287, P < 0.001; mixture: F(2, 12) = 560.950, p < 0.001; R. amethystoides: total C: monoculture: F(2, 12) = 108.751, p < 0.001; mixture: F(2, 12) = 143.196, p < 0.001; total N: monoculture: F(2, 12) = 120.554, P < 0.001; mixture: F(2, 12) = 288.873, p < 0.001; total P: monoculture: F(2, 12) = 305.177, p < 0.001; mixture: F(2, 12) = 317.306, p < 0.001; total K: monoculture: F(2, 12) = 507.227, p < 0.001; mixture: F(2, 12) = 414.302, p < 0.001; Figures 4, 5]. The total C, N, P, and K concentrations in A. adenophora in the monoculture and mixture were significantly higher in the AB treatment than in the other treatments. Similarly, the total C, N, P, and K concentrations of R. amethystoides in the monoculture and mixture with RB treatment were significantly higher than those in the other treatments. Competition had a significant effect on the total C and N concentrations of A. adenophora and R. amethystoides. However, the total P and K concentrations of the two plants were unaffected. Specifically, when A. adenophora was planted in a mixture with R. amethystoides, the total C and N concentrations of A. adenophora increased compared to monoculture [total C: C: F(1, 8) = 73.741, p < 0.001, AB: F(1, 8) = 44.380, p = 0.001, RB: F(1, 8) = 31.649, p < 0.001; total N: C: F(1, 8) = 32.268, p < 0.001, AB: F(1, 8) = 28.142, p = 0.001, RB: F(1, 8) = 34.088, p < 0.001], but those of R. amethystoides decreased [total C: C: F(1, 8) = 279.075, p < 0.001, AB: F(1, 8) = 76.003, p = 0.001, RB: F(1, 8) = 91.746, p < 0.001; total N: C: F(1, 8) = 31.588, p < 0.001, AB: F(1, 8) = 51.322, p < 0.001, RB: F(1, 18) = 37.579, p < 0.001, Figure 4]. However, in the mixed treatment, the total P and K concentrations of A. adenophora and R. amethystoides were similar to those in the monoculture treatment (Figure 5).
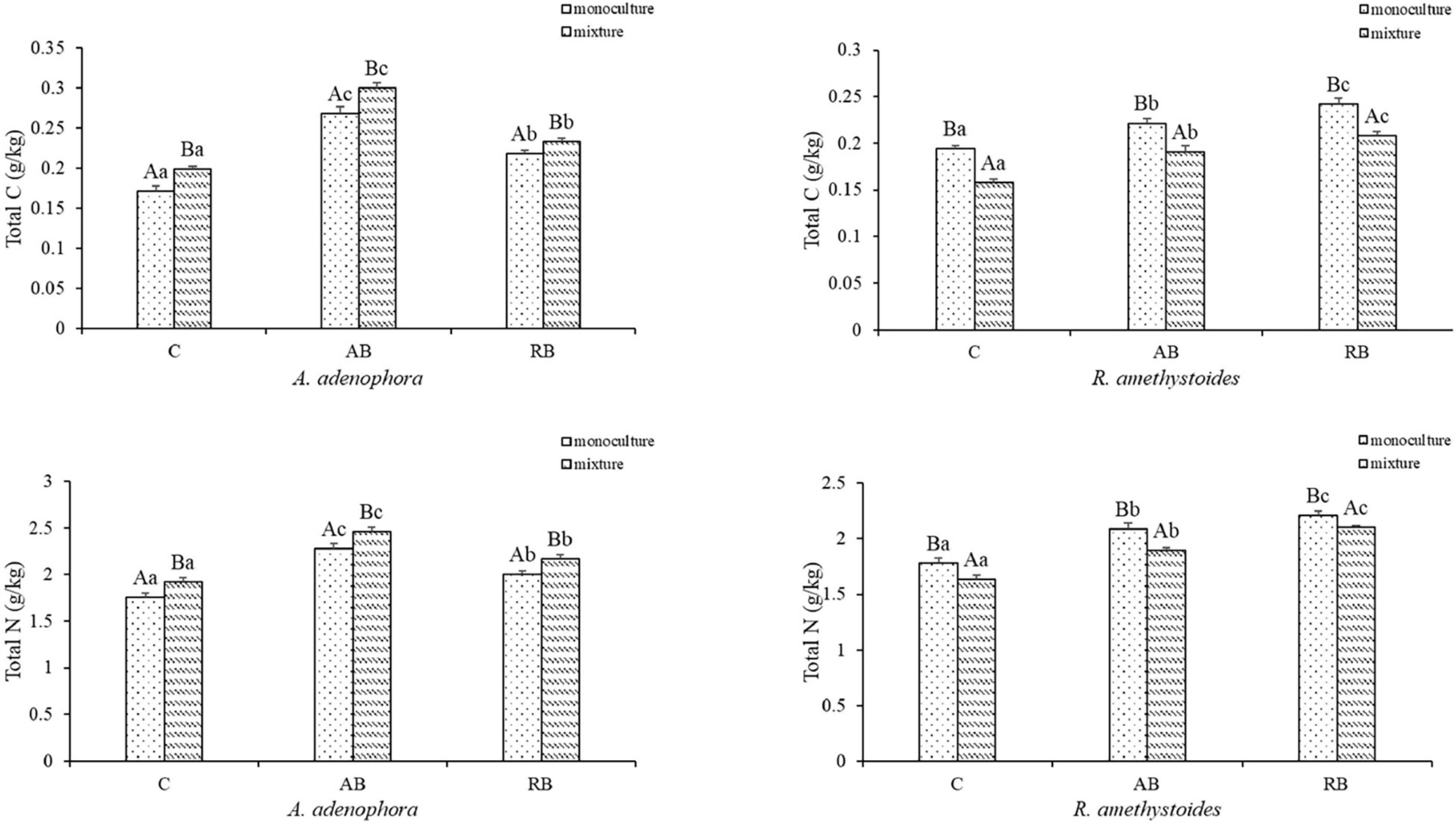
Figure 4. Effect of Bacillus on plant total carbon and nitrogen concentrations of invasive plant A. adenophora and native plant R. amethystoides when grown in monoculture or in mixture. Different lowercase letters indicate significant differences among the inoculation treatments at p < 0.05. Different capital letters indicate significant differences between the monoculture and mixture at p < 0.05. Error bars represent ± 1SE of the mean (n = 5).
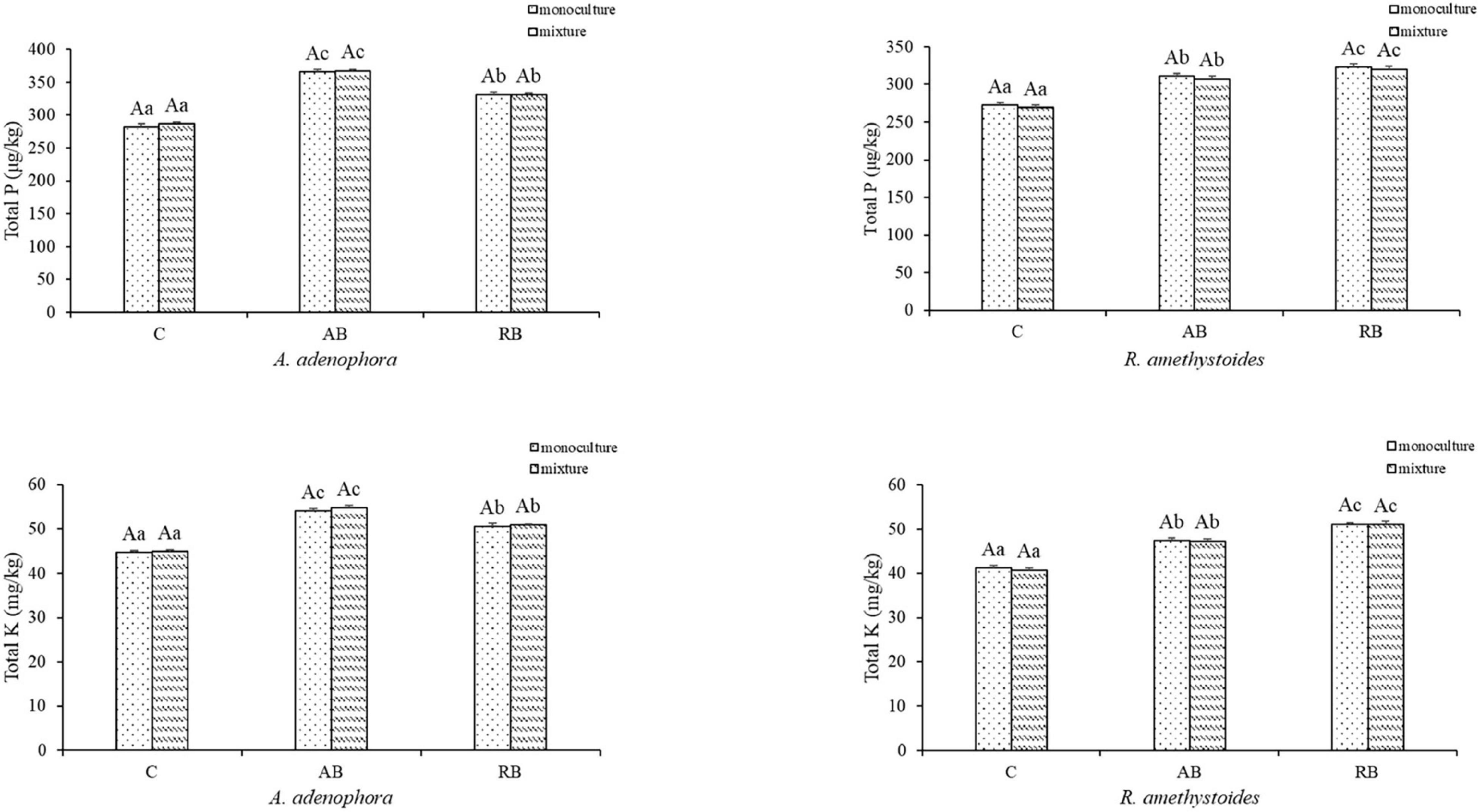
Figure 5. Effect of Bacillus on plant total phosphorus and potassium concentrations of invasive plant A. adenophora and native plant R. amethystoides when grown in monoculture or in mixture. Different lowercase letters indicate significant differences among the inoculation treatments at p < 0.05. Different capital letters indicate significant differences between the monoculture and mixture at p < 0.05. Error bars represent ± 1SE of the mean (n = 5).
For the monoculture treatment of A. adenophora and R. amethystoides, the nutrient contents including NO3–-N, NH4+-N, AP and AK in the soil of A. adenophora and R. amethystoides treated with two kinds of Bacillus were considerably higher than those of the control group [A. adenophora: NO3–-N: F(2, 12) = 255.633, p < 0.001; NH4+-N: F(2, 12) = 62.282, p < 0.001; AP: F(2, 12) = 65.245, p < 0.001; AK: F(2, 12) = 405.944, p < 0.001; R. amethystoides: NO3–-N: F(2, 12) = 317.878, p < 0.001; NH4+-N: F(2, 12) = 61.489, p < 0.001; AP: F(2, 12) = 94.169, p < 0.001; AK: F(2, 12) = 305.344, p < 0.001]. Between the rhizospheric soil Bacillus of the A. adenophora and R. amethystoides treatments, there was no significant difference in the NH4+-N and AP contents. However, the NO3–-N content of A. adenophora inoculated with AB was significantly higher than that of A. adenophora inoculated with RB (p < 0.001), whereas the NO3–-N and AK contents of R. amethystoides inoculated with RB were significantly higher than those of R. amethystoides inoculated with AB (all p < 0.001, Table 2).
Inoculation with the two kinds of Bacillus significantly increased the contents of available soil nutrients in the mixed treatment of A. adenophora and R. amethystoides compared to the control treatment [NO3–-N: F(2, 12) = 124.655, p < 0.001; NH4+-N: F(2, 12) = 74.726, p < 0.001; AP: F(2, 12) = 58.227, p < 0.001; AK: F(2, 12) = 166.378, p < 0.001] In the soil of R. amethystoides, no significant difference in the NH4+-N, AP, and AK contents was observed between the two kinds of Bacillus. However, the NO3–-N content of A. adenophora inoculated with AB was significantly higher than that of A. adenophora inoculated with RB (p < 0.001). The content of NO3–-N in the mixture was higher than that in the monoculture for both plants [F(2, 12) = 219.105, p < 0.001, Table 2]. This is consistent with the result of the all-strain function experiment. To determine the nitrogen-fixing ability, we performed a functional assay on all isolated strains that were able to survive on Ashby medium. The results showed that the proportion of nitrogen-fixing Bacillus in the rhizosphere of A. adenophora (the number of nitrogen-fixing strains/total strains, 20.217 ± 1.515%) was significantly higher than that in the rhizosphere of R. amethystoides [15.693 ± 1.585%, F(1, 4) = 0.004, p = 0.023].
The rhizospheric microbial community affects the population establishment of invasive plants in introduced areas (Sun et al., 2021). Our study revealed that the abundance and diversity of Bacillus in the rhizospheric soil of A. adenophora were more than those of native plants (Table 1 and Figure 1), and the Bacillus community in A. adenophora rhizospheric soil was beneficial to the competitive growth of A. adenophora (Figure 3). Previous study on A. adenophora and Bacillus has shown that A. adenophora could accumulate B. subtilis and B. megaterium in its invaded areas, whereas the abundance of the two bacteria taxa in the native plant areas is relatively lower (Niu et al., 2007). This study also revealed that the invasion of A. adenophora will promote the accumulation of B. cereus, which may in turn accelerate the growth of A. adenophora (Yang G. Q. et al., 2014; Sun et al., 2021). Here, we used monocultures and mixtures of the invasive plant A. adenophora with the native accompanying plant R. amethystoides, to demonstrate that inoculum with Bacillus could change their competitive growth. We found that the biomass of A. adenophora was higher when grown together with R. amethystoides in a mixture than grown in a monoculture, and this positive effect was enhanced by inoculating Bacillus. After the competitive experiment, the dominant Bacillus taxa in the rhizospheric soil of A. adenophora were B. idriensis, B. toyonensis, and B. cereus (Figure 1). The results showed that the number and abundance of Bacillus in rhizospheric soil of A. adenophora were higher than that of native accompanying plants, which promoted the competitive ability and invasion of A. adenophora.
The accumulation of beneficial soil bacteria in the rhizosphere of invasive plants increased their nutrient concentration and facilitated their invasion, which in turn provides an indirect advantage for the invasive plants to compete with the native species (Inderjit and van der Putten, 2010; Yu et al., 2021). It has been reported that Bacillus inoculated in the rhizospheric soil of plants has a significant growth-promoting function and can promote the absorption of nutrients by plants (Janarthine and Eganathan, 2012; Shameer et al., 2020). For example, inoculation with B. pumilus improved the plant N uptake, rhizobacterial population, and further improved plant growth (Marchut-Mikoajczyk et al., 2021). In our study, compared to the Bacillus community in rhizospheric soil of R. amethystoides, more functional Bacillus taxa, such as B. pumilus, B. licheniformis, B. tequilensis, B. firmus, B. subterraneus, and S. aquimarina, were found in rhizospheric soil of A. adenophora (Figure 1). The invasion of A. adenophora selected specific microbial taxa in soil, which may have mediated soil nutrient cycling and thus potentially improved plant nutrient acquisition (Xia et al., 2021; Li et al., 2022). Different Bacillus taxa vary in their ability to supply plants with nutrients (Pinyapach et al., 2018; Kang et al., 2019). Our results showed that Bacillus addition increased the C and N concentrations of A. adenophora while grown with R. amethystoides, whereas the C and N concentrations of R. amethystodies were significantly lower (Figure 4). The leaves of A. adenophora have higher CO2 fixation capacities, N concentration, and N-use efficiency than those of native accompanying species (Chen et al., 2016). Higher N content of A. adenophora improved its competitive ability over the native plant Lolium perenne (Zhao et al., 2007). N availability is critical for A. adenophora invasion under various soil conditions, and an increasing number of evidence suggests that higher N uptake by invasive plants provides them with a competitive advantage over native species (Jo et al., 2017; Zhang et al., 2020; Chen et al., 2021).
Invasive plants can outbreak in various environments and quickly establish populations as dominant species (Langmaier and Lapin, 2020; Wondafrash et al., 2021). One of the main reasons is that they interact with soil microorganisms to increase the availability of soil resources (Wang et al., 2019). Soil microorganisms have different effects on the nutrient absorption of different plants, thus affecting plant competitiveness (Yang G. W. et al., 2014; Sun et al., 2019). For the Bacillus in rhizospheric soil of A. adenophora, B. idriensis, B. toyonensis, and B. cereus were dominant in mixture treatment. B. idriensis was the most abundant Bacillus of A. adenophora, which have the potential abilities to promote seedling root growth, desorbing phosphorus and producing indoleacetic acid and ammonia (Afzal et al., 2016; Gholamalizadeh et al., 2017). B. cereus enhanced the soil microbial biomass, enzyme activity, N2-fixation, and P solubilization (Nayak et al., 2018; Azeem et al., 2021). B. toyonensis had direct and indirect plant growth-promoting traits and facilitated plant growth (Rojas-Solis et al., 2020; Zerrouk et al., 2020). The concentration of NO3–-N in A. adenophora soil was higher than that in R. amethystoides rhizospheric soil in our study, whether or not A. adenophora was grown with native species (Table 2). Among the 20 strains with nitrogen fixation ability, there were seven strains of B. idriensis, five strains of B. toyonensis, and five strains of B. cereus. This reflects the differences in Bacillus communities closely linked to soil N transformation (Kuypers et al., 2018). A. adenophora may induce higher N-fixation rates by enriching the higher abundance of functional microbes (Zhao et al., 2019; Li et al., 2022). Different nutrient availabilities of rhizospheric soil may lead to asymmetric competition between plant species (Yang G. W. et al., 2014). Therefore, the invasion of A. adenophora affected the growth of native plants by increasing or/and shifting its N uptake in the presence of specific Bacillus community.
In conclusion, we used the culture-dependent method to detect the positive feedback effect of the rhizospheric Bacillus community on A. adenophora growth promotion and competition function. Results showed that A. adenophora invasion increased the abundance and diversity of Bacillus in its rhizospheric soil compared to native accompanying plant rhizospheric soil. B. idriensis, B. toyonensis, and B. cereus might be recruited by A. adenophora, and increased the NO3–-N content in soil, enhanced C and N concentrations of plant to facilitate the growth and competitiveness of A. adenophora. In addition, the culture-dependent method was used in our investigation to detect the phylogenetic structure and diversity of Bacillus in the rhizospheric soil of the two plants. Based on our 16S rDNA results, quantity PCR can be performed to more accurately analyze the abundance of Bacillus taxa and elucidate the role of Bacillus in the invasion process of A. adenophora for further studies.
The original contributions presented in this study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
FG, ED, and YC designed the research. YL and YC collected the samples. ED performed the experiments. ZS and YL performed the bioinformatic and statistical analyses. ED and YC wrote the first draft. ZS and FG reviewed the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the Yunnan Eco-Friendly Food International Cooperation Research Center (YEFICRC) Project of Yunnan Provincial Key Program (Grant No. 2019ZG00910), National Key Research and Development Program of China (2021YFD1400200), and National Natural Science Foundation of China (NSFC) (31660546).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2022.882255/full#supplementary-material
Afzal, I., Iqrar, I., Shinwari, Z. K., and Yasmin, A. (2016). Plant growth-promoting potential of endophytic bacteria isolated from roots of wild Dodonaea viscosa L. Plant Growth Regul. 81, 1–10. doi: 10.1007/s10725-016-0216-5
Alina, S. O., Constantinscu, F., and Petruţa, C. C. (2015). Biodiversity of Bacillus subtilis group and beneficial traits of Bacillus species useful in plant protection. Rom. Biotechnol. Lett. 20, 10737–10750.
Asari, S., Tarkowská, D., Rolčík, J., Novák, O., Palmero, D. V., Bejai, S., et al. (2017). Analysis of plant growth-promoting properties of Bacillus amyloliquefaciens UCMB5113 using Arabidopsis thaliana as host plant. Planta 245, 15–30. doi: 10.1007/s00425-016-2580-9
Azeem, M., Hassan, T. U., Tahir, M. I., Ali, A., and Zhang, Z. (2021). Tea leaves biochar as a carrier of Bacillus cereus improves the soil function and crop productivity. Appl. Soil Ecol. 157:103732. doi: 10.1016/j.apsoil.2020.103732
Batten, K. M., Scow, K. M., Davies, K. F., and Harrison, S. P. (2006). Two invasive plants alter soil microbial community composition in serpentine grasslands. Biol. Invasions 8, 217–230. doi: 10.1007/s10530-004-3856-8
Burkhanova, G. F., Veselova, S. V., Sorokan, A. V., Blagova, D. K., Nuzhnaya, T. V., and Maksimov, I. V. (2017). Strains of Bacillus spp. regulate wheat resistance to Septoria nodorum Berk. Appl. Biochem. Microbiol. 53, 346–352. doi: 10.1134/S0003683817030048
Callaway, R. M., and Ridenour, W. M. (2004). Novel weapons: invasive success and the evolution of increased competitive ability. Front. Ecol. Environ. 2:436–443. doi: 10.1890/1540-92952004002[0436:NWISAT]2.0.CO;2
Chen, X., Li, Q., Wang, L. T., Meng, Y. L., Jiao, S. N., Yin, J. L., et al. (2021). Nitrogen uptake, not transfer of Carbon and Nitrogen by CMN, explains the effect of AMF on the competitive interactions between Flaveria bidentis and native species. Front. Ecol Evol 9:625519. doi: 10.3389/fevo.2021.625519
Chen, X. W., Wei, Z. S., Liu, H. M., Yang, D. L., Wang, H., and Huangfu, C. H. (2016). Comparison of photosynthetic characteristics between invasive and co-occuring native Asteraceae plants in Yunnan Province, China. Res. Environ. Sci. 29, 538–546. doi: 10.13198/j.issn.1001-6929.2016.04.10
Choudhary, D. K., and Johri, B. N. (2008). Interactions of Bacillus spp. and plants-with special reference to induced systemic resistance (ISR). Microbiol. Res. 164, 493–513. doi: 10.1016/j.micres.2008.08.007
D’Antonio, C. M., Yelenik, S. G., and Mack, M. C. (2017). Ecosystem vs. community recovery 25 years after grass invasions and fire in a subtropical woodland. J. Ecol. 105, 1462–1474. doi: 10.1111/1365-2745.12855
Datta, A., Schweiger, O., and Kühn, I. (2019). Niche expansion of the invasive plant species Ageratina adenophora despite evolutionary constraints. J. Biogeogr. 46, 1306–1315. doi: 10.1111/jbi.13579
Dheeman, S., Maheshwari, D. K., Agarwal, M., Dubey, R. C., Aeron, A., Kim, K., et al. (2017). Polyphasic and functional diversity of high altitude culturable Bacillus from rhizosphere of Eleusine coracana (l.) gaertn. Appl. Soil. Ecol. 110, 127–136. doi: 10.1016/j.apsoil.2016.10.005
Ding, X., Peng, X. J., Jin, B. S., Xiao, M., Chen, J. K., Li, B., et al. (2015). Spatial distribution of bacterial communities driven by multiple environmental factors in a beach wetland of the largest freshwater lake in China. Front. Microbiol. 6:129. doi: 10.3389/fmicb.2015.00129
Duncan, C. A., Jachetta, J. J., Brown, M. L., Carrithers, V. F., Clark, J. K., DiTomaso, J. M., et al. (2004). Assessing the economic, environmental, and societal losses from invasive plants on rangeland and wildlands. Weed Technol. 18, 1411–1416. doi: 10.1614/0890-037x(2004)018[1411:ateeas]2.0.co;2
Fan, B., Wang, C., Song, X., Ding, X., Wu, L., Wu, H., et al. (2018). Bacillus velezensis FZB42 in 2018: the gram-positive model strain for plant growth promotion and biocontrol. Front. Microbiol. 9:2491. doi: 10.3389/fmicb.2018.02491
Fang, K., Wang, Y. Z., and Zhang, H. B. (2019). Differential effects of plant growth-promoting bacteria on invasive and native plants. S. Afr. J. Bot. 124, 94–101. doi: 10.1016/j.sajb.2019.04.007
Gardner, B. B. M. (2004). Ecology of Bacillus and PaeniBacillus spp. in agricultural system. Phytopathology 94, 1252–1258. doi: 10.1094/PHYTO.2004.94.11.1252
Gholamalizadeh, R., Khodakaramian, G., and Ebadi, A. A. (2017). Assessment of rice associated bacterial ability to enhance rice seed germination and rice growth promotion. Braz. Arch. Biol. Technol. 60, 60–72. doi: 10.1590/1678-4324-2017160410
Goswami, D., Dhandhukia, P. C., Patel, P., and Thakker, J. N. (2014). Screening of PGPR from saline desert of Kutch: growth promotion in Arachis hypogea by Bacillus licheniformis A2. Microbiol. Res. 169, 66–75. doi: 10.1016/j.micres.2013.07.004
Goswami, D., Thakker, J. N., and Dhandhukia, P. C. (2016). Portraying mechanics of plant growth promoting rhizobacteria (PGPR): a review. Cogent Food Agric 2:1127500. doi: 10.1080/23311932.2015.1127500
Gu, C. J., Tu, Y. L., Liu, L. S., Wei, B., Zhang, Y. l, Yu, H. B., et al. (2021). Predicting the potential global distribution of Ageratina adenophora under current and future climate change scenarios. Ecol. Evol. 11, 12092–12113. doi: 10.1002/ece3.7974
Gui, F. R., Wan, F. H., and Guo, J. Y. (2009). Determination of the population genetic structure of the invasive weed Ageratina adenophora using ISSR-PCR markers. Russ. J. Plant Physiol. 56, 410–416. doi: 10.1134/S1021443709030157
Hawkes, C. V., Wren, I. F., Herman, D. J., and Firestone, M. K. (2005). Plant invasion alters nitrogen cycling by modifying the soil nitrifying community. Ecol. Lett. 8, 976–985. doi: 10.1111/j.1461-0248.2005.00802.x
Inderjit van der Putten, W. H. (2010). Impacts of soil microbial communities on exoticplant invasion. Trends Ecol. Evol. 25, 512–519. doi: 10.1016/j.tree.2010.06.006
Isaac, R. A., and Johnson, W. C. (1983). High speed analysis of agriculture samples using inductively coupled plasma-atomic emission spectroscopy. Spectrochim. Acta 38, 277–282. doi: 10.1016/0584-8547(83)80124-4
Janarthine, S., and Eganathan, P. (2012). Plant growth promoting of endophytic Sporosarcina aquimarina SjAM16103 isolated from the pneumatophores of Avicennia marina L. Int. J. Microbiol. 2012:532060. doi: 10.1155/2012/532060
Jo, I., Fridley, J. D., and Frank, D. A. (2017). Invasive plants accelerate nitrogen cycling: evidence from experimental woody monocultures. J. Ecol. 105, 1105–1110. doi: 10.1111/1365-2745.12732
Kang, S. M., Khan, A. L., Waqas, M., Asaf, S., and Lee, I. J. (2019). Integrated phytohormone production by the plant growth-promoting rhizobacterium Bacillus tequilensis SSB07 induced thermotolerance in soybean. J. Plant Interact. 14, 416–423. doi: 10.1080/17429145.2019.1640294
Kong, Y. H., Kong, J., Wang, D. K., Huang, H. P., Geng, K. Y., Wang, Y. X., et al. (2017). Effect of Ageratina adenophora invasion on the composition and diversity of soil microbiome. J. Gen. Appl. Micrbiol. 63, 114–123. doi: 10.2323/jgam.2016.08.002
Kumar, A., Prakash, A., and Johri, B. N. (2011). “Bacillus as PGPR in crop ecosystem,” in Bacteria in Agrobiology: Crop Ecosystems, ed. D. Maheshwari (Berlin: Springer), 123–125.
Kumar, P., Dubey, R. C., and Maheshwari, D. K. (2012). Bacillus strains isolated from rhizosphere showed plant growth promoting and antagonistic activity against phytopathogens. Microbiol. Res. 167, 493–499. doi: 10.1016/j.micres.2012.05.002
Kumar, S., Stecher, G., Li, M., Knyaz, C., and Tamura, K. (2018). MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35, 1547–1549. doi: 10.1093/molbev/msy096
Kuypers, M. M. M., Marchant, H. K., and Kartal, B. (2018). The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 16, 263–276. doi: 10.1038/nrmicro.2018.9
Langmaier, M., and Lapin, K. (2020). A systematic review of the impact of invasive alien plants on forest regeneration in European temperate forests. Front. Plant Sci. 11:524969. doi: 10.3389/fpls.2020.524969
Li, H. N., Liu, W. X., Dai, L., Wan, F. H., and Cao, Y. Y. (2009). Invasive impacts of Ageratina adenophora (Asteraceae) on the changes of microbial community structure, enzyme activity and fertility in soil ecosystem. Sci. Agric. Sin. 42, 3964–3971.
Li, Q., Wan, F., and Zhao, M. (2022). Distinct soil microbial communities under Ageratina adenophora invasions. Plant Biol. 24, 430–439. doi: 10.1111/plb.13387
Li, X. R., and Li, L. (2013). Research progress of plant allelopathic substances and soil microorganisms. Guangdong Agric. Sci. 40, 178–181.
Li, X. Y., Mao, Z. C., Wu, Y. X., Ho, H. H., and He, Y. Q. (2015). Comprehensive volatile organic compounds profiling of Bacillus species with biocontrol properties by head space solid phase microextraction with gas chromatography-mass spectrometry. Biocontrol Sci. Technol. 25, 132–143. doi: 10.1080/09583157.2014.960809
Marchut-Mikoajczyk, O., Drodyński, P., Polewczyk, A., Smulek, W., and Antczak, T. (2021). Biosurfactant from endophytic Bacillus pumilus 2A: physicochemical characterization, production and optimization and potential for plant growth promotion. Microb. Cell Fact. 20:40. doi: 10.1186/s12934-021-01533-2
Márquez, R., Blanco, E. L., and Aranguren, L. (2020). Bacillus strain selection with plant growth-promoting mechanisms as potential elicitors of systemic resistance to gray mold in pepper plants. Saudi J. Biol. Sci. 27, 1913–1922. doi: 10.1016/j.sjbs.2020.06.015
Massenssini, A. M., Bonduki, V. H. A., Melo, C. A. D., Tótola, M. R., Ferreira, F. A., and Costa, M. D. (2014). Soil microorganisms and their role in the interactions between weeds and crops. Planta Daninha. 32, 873–874. doi: 10.1590/S0100-83582014000400022
Nayak, A. K., Panda, S. S., Basu, A., and Dhal, N. K. (2018). Enhancement of toxic Cr(vi), Fe, and other heavy metals phytoremediation by the synergistic combination of native Bacillus cereus strain and Vetiveria zizanioides (L.). Int. J. Phytoremediation 20, 682–691. doi: 10.1080/15226514.2017.1413332
Nelson, D. W., and Sommers, L. E. (1972). Determination of total nitrogen in plant material. Agron J. 65, 109–112. doi: 10.2134/agronj1973.00021962006500010033x
Niu, H. B., Liu, W. X., Wan, F. H., and Liu, B. (2007). An invasive aster (Ageratina adenophora) invades and dominates forest understories in China: altered soil microbial communities facilitate the invader and inhibit natives. Plant Soil. 294, 73–85. doi: 10.1007/s11104-007-9230-8
Oksanen, L., Sammul, M., and Magi, M. (2006). On the indices of plant-plant competition and their pitfalls. Oikos 112, 149–155. doi: 10.1111/j.0030-1299.2006.13379.x
Olsen, S. R. (1954). Estimation of Available Phosphorus in soils by Extraction with Sodium Bicarbonate. Washington, DC: U.S. Dept. of Agriculture.
Pandin, C., Coq, D. L., Canette, A., Aymerich, S., and Briandet, R. (2017). Should the biofilm mode of life be taken into consideration for microbial biocontrol agents? Microb Biotechnol. 10, 719–734. doi: 10.1111/1751-7915.12693
Pesce, G., Rusciano, G., Sasso, A., Isticato, R., Sirec, T., and Ricca, E. (2014). Surface charge and hydrodynamic coefficient measurements of Bacillus subtilis spore by optical tweezers. Colloids Surface B. 116, 568–575. doi: 10.1016/j.colsurfb.2014.01.039
Philippot, L., Raaijmakers, J. M., Lemanceau, P., and Der Putten, W. H. (2013). Going back to the roots: the microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 11, 789–799. doi: 10.1038/nrmicro3109
Pinyapach, S., Andi, K., Tzu-Chuan, K., and Huey-Wen, C. (2018). A multifaceted rhizobacterium Bacillus licheniformis functions as a fungal antagonist and a promoter of plant growth and abiotic stress tolerance. Environ. Exp. Bot. 155, 541–551. doi: 10.1016/j.envexpbot.2018.08.005
Poudel, A. S., Jha, P. K., Shrestha, B. B., Muniappan, R., and Novak, S. (2019). Biology and management of the invasive weed Ageratina adenophora (Asteraceae): current state of knowledge and future research needs. Weed Res. 59, 79–92. doi: 10.1111/wre.12351
Radhakrishnan, R., and Lee, I. J. (2016). Gibberellins producing Bacillus methylotrophicus KE2 supports plant growth and enhances nutritional metabolites and food values of lettuce. Plant Physiol. Bioch. 109, 181–189. doi: 10.1016/j.plaphy.2016.09.018
Rodríguez-Echeverría, S. (2010). Rhizobial hitchhikers from down under: invasional meltdown in a plant-bacteria mutualism? J. Biogeogr. 37, 1611–1622. doi: 10.1111/j.1365-2699.2010.02284.x
Rojas-Solis, D., Vences-Guzmán, M. A., Sohlenkamp, C., and Santoyo, G. (2020). Bacillus toyonensis cope52 modifies lipid and fatty acid composition, exhibits antifungal activity, and stimulates growth of tomato plants under saline conditions. Curr. Microbiol. 77, 2735–2744. doi: 10.1007/s00284-020-02069-1
Saxena, A. K., Kumar, M., Chakdar, H., Anuroopa, N., and Bagyaraj, D. J. (2020). Bacillus species in soil as a natural resource for plant health and nutrition. J. Appl. Microbiol. 128, 1583–1594. doi: 10.1111/jam.14506
Shameer, S., Prasad, N. V. K. V., and Lian, B. (2020). Aspergillus and Fusarium control in the early stages of Arachis hypogaea (groundnut crop) by plant growth-promoting rhizobacteria (PGPR) consortium. Microbiol. Res. 240:126562. doi: 10.1016/j.micres.2020.126562
Sharma, S. B., Sayyed, R. Z., Trivedi, M. H., and Gobi, T. A. (2013). Phosphate solubilizing microbes: sustainable approach for managing phosphorus deficiency in agricultural soils. Springerplus 2:587. doi: 10.1186/2193-1801-2-587
Song, Z., Ji, Q. F., Fu, W. D., Zhang, R. H., Zhang, T., Yan, J., et al. (2016). Effects of Flaveria bidentis invasion on the diversity of functional bacteria in rhizosphere soil. Chin. J. Appl. Ecol. 27, 2636–2644. doi: 10.13287/j.1001-9332.201608.013
Song, Z., Zhang, R. H., Fu, W. D., Zhang, T., Yan, J., and Zhang, G. L. (2017). High-throughput sequencing reveals bacterial community composition in the rhizosphere of the invasive plant Flaveria bidentis. Weed Res. 57, 204–211. doi: 10.1111/wre.12250
Sun, F., Ou, Q. J., Yu, H. X., Li, N., and Peng, C. L. (2019). The invasive plant Mikania micrantha affects the soil foodweb and plant-soil nutrient contents in orchards. Soil Biol. Biochem. 139, 107630–107642. doi: 10.1016/j.soilbio.2019.107630
Sun, Y. Y., Zhang, Q. X., Zhao, Y. P., Diao, Y. H., Gui, F. R., and Yang, G. Q. (2021). Beneficial rhizobacterium provides positive plant–soil feedback effects to Ageratina adenophora. J. Integr. Agric 20, 1327–1335. doi: 10.1016/S2095-3119(20)63234-8
Trognitz, F., Hackl, E., Widhalm, S., and Sessitsch, A. (2016). The role of plant-microbiome interactions in weed establishment and control. FEMS Microbiol. Ecol. 92:fiw138. doi: 10.1093/femsec/fiw138
van der Putten, W. H., Klironomos, J. N., and Wardle, D. A. (2007). Microbial ecology of biological invasions. ISME J. 1, 28–37. doi: 10.1038/ismej.2007.9
Verma, P., Yadav, A. N., Khannam, K. S., Panjiar, N., Kumar, S., Saxena, A. K., et al. (2015). Assessment of genetic diversity and plant growth promoting attributes of psychrotolerant bacteria allied with wheat (Triticum aestivum) from the northern hills zone of India. Ann. Microbiol. 65, 1885–1899. doi: 10.1007/s13213-014-1027-4
Wan, F. H., Liu, W. X., Guo, J. Y., Qiang, S., Li, B. P., Wang, J. J., et al. (2010). Invasive mechanism and control strategy of Ageratina adenophora (Sprengel). Sci. China Life Sci. 53, 1291–1298. doi: 10.1007/s11427-010-4080-7
Wang, R., and Wang, Y. Z. (2006). Invasion dynamics and potential spread of the invasive alien plant species Ageratina adenophora (Asteraceae) in China. Divers. Distrib. 12, 397–408. doi: 10.1111/j.1366-9516.2006.00250.x
Wang, Y., Fan, Z. W., Shen, Y. D., Li, X. X., Liu, Y., and Huang, Q. Q. (2019). Comparative performance of the highly invasive Mimos invisa and its less invasive subspecies under different nutrient and light conditions. Weed Res. 59, 419–426. doi: 10.1111/wre.12379
Weisburg, W. G., Barns, S. M., Pelletier, D. A., and Lane, D. J. (1991). 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173, 697–703. doi: 10.1128/jb.173.2.697-703.1991
Wondafrash, M., Wingfield, M. J., Wilson, J. R. U., Hurley, B. P., Slippers, B., and Paap, T. (2021). Botanical gardens as key resources and hazards for biosecurity. Biodivers. Conserv. 30, 1929–1946. doi: 10.1007/s10531-021-02180-0
Xia, Y., Dong, M. H., Yu, L., Kong, L. D., Seviour, R., and Kong, Y. H. (2021). Compositional and functional profiling of the rhizosphere microbiomes of the invasive weed Ageratina adenophora and native plants. PeerJ 9:e10844. doi: 10.7717/peerj.10844
Xu, C. W., Yang, M. Z., Chen, Y. J., Chen, L. M., Zhang, D. Z., Mei, L., et al. (2012). Changes in non-symbiotic nitrogen-fixing bacteria inhabiting rhizosphere soils of an invasive plant Ageratina adenophora. Appl. Soil Ecol. 54, 32–38. doi: 10.1016/j.apsoil.2011.10.021
Yang, G. Q., Guo, J., and Gui, F. R. (2014). The effect of allelochemicals from Ageratina adenophorum on soil available phosphorus content and the growth of Bacillus megaterium. Jiangsu Agric. Sci. 42, 137–140.
Yang, G. W., Liu, N., Lu, W. J., Wang, S., Kan, H. M., Zhang, Y. J., et al. (2014). The interaction between arbuscular mycorrhizal fungi and soil phosphorus availability influences plant community productivity and ecosystem stability. J Ecol. 102, 1072–1082. doi: 10.1111/1365-2745.12249
Yousuf, J., Thajudeen, J., Rahiman, M., Krishnankutty, S., Alikunj, A. P., and Abdulla, M. H. A. (2017). Nitrogen fixing potential of various heterotrophic Bacillus strains from a tropical estuary and adjacent coastal regions. J. Basic Microbiol. 57, 922–932. doi: 10.1002/jobm.201700072
Yu, H. X., Le, J. J., Jiang, Z. Y., Sun, F., Peng, C. L., and Li, W. H. (2021). Soil nitrogen dynamics and competition during plant invasion: insights from Mikania micrantha invasions in China. New Phytol. 229, 3440–3452. doi: 10.1111/nph.17125
Zerrouk, I. Z., Rahmoune, B., Auer, S., Rsler, S., Lin, T., Baluska, F., et al. (2020). Growth and aluminum tolerance of maize roots mediated by auxin- and cytokinin-producing Bacillus toyonensis requires polar auxin transport. Environ. Exp. Bot. 176:104064. doi: 10.1016/j.envexpbot.2020.104064
Zhang, F. J., Li, Q., Chen, F. X., Xu, H. Y., and Inderjit Wan, F. H. (2017). Arbuscular mycorrhizal fungi facilitate growth and competitive ability of an exotic species Flaveria bidentis. Soil Biol. Biochem. 115, 275–284. doi: 10.1016/j.soilbio.2017.08.019
Zhang, H. Y., Goncalves, P., Copeland, E., Qi, S. S., Dai, Z. C., Li, G. L., et al. (2020). Invasion by the weed Conyza canadensis alters soil nutrient supply and shifts microbiota structure. Soil Biol. Biochem. 143:107739. doi: 10.1016/j.soilbio.2020.107739
Zhao, L., Meng, L., and Li, B. (2007). Effects of fertilization on the relative competitive ability of Eupatorium adenophorum and Lolium perenne at their seedling stage. Chin. J. Ecol. 26, 1743–1747.
Keywords: Ageratina adenophora, invasive plant, rhizosphere, Bacillus, competitive advantage, plant-soil feedback
Citation: Du E, Chen Y, Li Y, Sun Z and Gui F (2022) Rhizospheric Bacillus-Facilitated Effects on the Growth and Competitive Ability of the Invasive Plant Ageratina adenophora. Front. Plant Sci. 13:882255. doi: 10.3389/fpls.2022.882255
Received: 23 February 2022; Accepted: 27 April 2022;
Published: 14 June 2022.
Edited by:
Katharina Pawlowski, Stockholm University, SwedenReviewed by:
Josep Ramoneda, University of Colorado Boulder, United StatesCopyright © 2022 Du, Chen, Li, Sun and Gui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Furong Gui, ZnVyb25nZ3VpMThAc2luYS5jb20=
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.