
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci., 04 July 2022
Sec. Plant Nutrition
Volume 13 - 2022 | https://doi.org/10.3389/fpls.2022.881561
This article is part of the Research TopicHeavy Metal Toxicity in Plants: Recent Insights on Physiological and Molecular Aspects, Volume IIView all 16 articles
 Anis Ali Shah1*†
Anis Ali Shah1*† Adnan Noor Shah2†
Adnan Noor Shah2† Muhammad Bilal Tahir3
Muhammad Bilal Tahir3 Asad Abbas4
Asad Abbas4 Sumera Javad5
Sumera Javad5 Sajid Ali6
Sajid Ali6 Muhammad Rizwan7
Muhammad Rizwan7 Saqer S. Alotaibi8
Saqer S. Alotaibi8 Hazem M. Kalaji9,10
Hazem M. Kalaji9,10 Arkadiusz Telesinski11
Arkadiusz Telesinski11 Talha Javed12
Talha Javed12 Hamada AbdElgawad13
Hamada AbdElgawad13This study explains the scarce information on the role of harzianopyridone (HZRP) in the alleviation of chromium (Cr) stress alleviation in Vigna radiata (L.). To this end, V. radiata seedlings primed with HZRP at 1 and 2 ppm were exposed to 50 mg kg–1 Cr for 30 days. Cr stress reduced growth, chlorophyll (Chl) content, net photosynthetic rate, gas-exchange attributes along with enhanced oxidative damages, i.e., electrolyte leakage (EL), hydrogen peroxide (H2O2), and malondialdehyde (MDA). Application of HZRP enhanced intercellular carbon dioxide (CO2) concentration, stomatal conductance, and net photosynthetic rate with decreased activity of the chlorophyllase (Chlase) enzyme in V. radiata seedlings exposed to Cr stressed conditions. To maintain Cr-induced oxidative damages, HZRP treatment increased the levels of antioxidant metabolites (phenolic and flavonoids) and the activity of antioxidative enzymes [superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD)] in V. radiata seedlings grown in normal and Cr-polluted potted soil. In addition to this, glycine betaine content was also increased in plants grown in Cr-contaminated soil. It is proposed the potential role of supplementation of HZRP in mitigating Cr stress. Further research should be conducted to evaluate the potential of HZRP in the mitigation of abiotic stresses in plants.
Excessive deposition of heavy metal toxicants in agricultural soils leads to growth retardation and hindrance of normal physiological processes in plants. Heavy metals accumulation in edible parts of plants poses various damages to animals and human health (Ihtisham et al., 2021; Zaynab et al., 2022). Chromium (Cr) is the 21st most abundant heavy metal and is regarded as one of the most potent pollutants in the environment. Cr is a transition metal and exists in two common oxidation states, i.e., hexavalent Cr (Cr6+) and trivalent Cr (Cr3+) (Singh et al., 2021). Cr6+ is more toxic, mobile, and exerts carcinogenic effects on living organisms (Bharagava and Mishra, 2018). Cr6+ uptake in plants is facilitated through sulfate and phosphate pathways and is easily transported to various parts of plants (Devi and Kumar, 2020). Chromates and dichromates are hexavalent Cr compounds and are mostly used in Cr stress tolerance mechanisms (Shah et al., 2020).
The accumulation of increased Cr content in soil and subsequently in different portions of plants affects plants and human health (Sharma et al., 2020). Moreover, increased Cr concentration in the soil affects the growth, photosynthesis, metabolism, biomass production, and yield of several crops (Anjum et al., 2017; Singh P. et al., 2020). For instance, higher Cr levels disturb physiological and biochemical changes in plants, leading to reduced yield and productivity (Anjum et al., 2017; Singh P. et al., 2020). In addition, the growth of mung beans is severely hampered by Cr stressed conditions (Jabeen et al., 2016; Husain et al., 2021). Cr6+ toxicity disturbs homeostasis in plants due to the enhanced accumulation of reactive oxygen species (ROS). Increased accumulation of Cr results in oxidative damage in plant tissues through enhanced production of hydrogen peroxide (H2O2), malondialdehyde (MDA) content, electrolyte leakage (EL), and ROS levels (Singh P. et al., 2020). ROS production causes oxidative stress in plants and results in oxidative modification of nucleic acids, proteins, and lipids (Huang et al., 2019). At the cellular level, Cr accumulation results in increased ROS production, as proved by an enhanced level of MDA, EL, and H2O2 in plants exposed to Cr toxicity (Yu et al., 2018; Patra et al., 2019). Cr (VI) toxicity causes more damage as compared with Cr (III) toxicity in plants (Beyersmann and Hartwig, 2008). Cr (VI) accumulation disturbs ROS homeostasis in plants grown in Cr polluted soil (Maqbool et al., 2018; Zaheer et al., 2019; Tirry et al., 2021). The accumulation of ROS enhances lipid peroxidation besides the oxidation of crucial biomolecules (Wakeel et al., 2019; Askari et al., 2021). ROS accumulation changes the morpho-physiology and architecture of plants facing Cr stressed conditions (Sharma et al., 2022).
To mitigate heavy metal-induced oxidative stress, plants regulate the activities of various enzymatic and non-enzymatic antioxidants. In the case of severe heavy metal toxicity, plant metabolomics is negatively affected resulting in disruption of some biomolecules, which leads to oxidative stress (Paithankar et al., 2021; Sarraf et al., 2022). However, Cr toxicity in plants depends on the concentration of Cr uptake from the rhizospheric region (Wakeel et al., 2018, 2019; Farid et al., 2019). This results in disturbed nutrient translocation in plants due to Cr binding with membranous H+-ATPase and other carrier channels (Shahid et al., 2017). Antioxidant enzymes in plants reverse the deleterious effect of ROS produced by various mechanisms. Crucial antioxidant enzymes in plants include CAT, POD, and SOD (Zaheer et al., 2020). Cr stress affects the activity of antioxidant enzymes in plants (Zaheer et al., 2022).
Vigna radiata is a short-duration legume crop, cultivated predominately in Asia and other regions of the world (Nair et al., 2019). V. radiata is rich in nutritional content such as proteins, vitamins, dietary fibers, minerals, and a huge number of bioactive compounds (Hou et al., 2019).
Harzianopyridone (HZRP) is a Trichoderma harzianum secondary metabolite containing a penta-substituted pyridine ring system with a 2,3-dimethoxy-4-pyridinyl pattern. It is a volatile organic compound that has been reported to have active defensive mechanisms in plants and regulates growth in tomato, canola, and pea plants (Vinale et al., 2013; Stewart and Hill, 2014). In the study of Hermosa et al. (2012), HZRP may promote plant growth via auxin-like activity at low doses, but confer an antimicrobial effect at higher concentrations. Despite the utilization of HZRP as a promising metabolite to promote plant growth, its potential role in improving plant growth under heavy metal toxicity, e.g., Cr, is not yet evaluated. Thus, the current research was conducted to test the potential of HZRP in the alleviation of Cr toxicity and regulation of growth in V. radiata seedlings. To our knowledge, this study exploits the effect of HZRP on the growth and morpho-physiological characteristics of V. radiata.
The experiment was conducted in the wirehouse of the Department of Botany, University of Education. A V. radiata cultivar, Inqalab Mung, was used during the experiment. Seeds of V. radiata were surface sterilized with 0.01% mercuric chloride for 5 min, followed by washing with double-distilled H2O. For Cr toxification, K2Cr2O7 was used during the experiment. Then, 50 mg kg–1 was added to the potting soil. This toxic Cr concentration refers to agricultural contaminated sites near District Lahore, Pakistan. Agricultural contaminated sites were irrigated with toxic effluents from the Hudiara drain. In the case of control (C) treatment, only distilled H2O was added to the soil. HZRP was purchased from Sigma Aldrich. Two concentrations of HZRP were used during the experiment, i.e., 1.0 and 2.0 ppm. Seeds of V. radiata were primed in HZRP solutions for 2 h. A completely randomized design (CRD) was used during the experiment. After 3 weeks, the root and shoot length of V. radiata were determined.
Photosynthesis pigment and other photosynthetic factors were determined. The chlorophyll (Chl) content of leaves was evaluated in a non-destructive manner throughout the experiment with a Chl meter, SPAD 402 PLUS (Minolta, Japan). Using an infrared gas analyzer (LI-6400XT, Portable Photosynthesis System, LI-COR, NE, United States), the net photosynthetic rate, intercellular carbon dioxide (CO2) concentration, and stomatal conductance of the topmost fully developed leaves of V. radiata plants were recorded.
The methodology described by Velikova et al. (2000) was used to determine the H2O2 content in the leaves of V. radiata plants. Supernatant (0.5 ml) was mixed with 0.5 ml of 10 mM phosphate buffer (pH 7.0) having 1 ml potassium iodide following extraction in 5 ml of 1 M TCA (0.1 w/v) to determine H2O2. Using the extinction coefficient of 0.28 M1 cm1 and the content expressed as nmol g1 fresh weight (FW), the content of H2O2 was determined after taking the absorbance at 390 nm (FW).
Fresh leaf samples from V. radiata plants were collected and mashed at 4°C in a pre-chilled pestle mortar for the enzyme assays. The 0.5-g powder was added to three volumes (w/v) of cold extraction medium buffer, which contained potassium phosphate buffer (100 mM) pH 7.0, 0.5% Triton X-100, and 1% polyvinylpyrrolidone. Following 20-min centrifugation at 15,000 g at 4°C, the supernatants were utilized in the experiments afterward. The extraction buffer was spiked with 2 mM ascorbate to estimate ascorbate peroxidase (APX). Filtered homogenates were centrifuged for 20 min at 4°C at 15,000 g. Protein and enzyme activity tests were done with the supernatants.
The activity of the enzyme chlorophyllase (Chlase) was evaluated according to the methods of McFeeters et al. (1971) and Fang et al. (1998). Using the method described by Jain and Gadre (2004), the activity of aminolevulinic acid dehydratase (ALAD) in V. radiata leaves was measured by quantifying the production of porphobilinogen (PBG) spectrophotometric value at 553 nm for 15 min against a zero-time control. Using this definition, one unit of enzyme activity was defined as 1 nmol of PBG produced per hour per gram (h–1g–1) of freshly harvested leaf weight (LW).
Spectrophotometric analysis at 560 nm was used to calculate the activity of SOD (Giannopolitis and Ries, 1977). The activity of APX was determined using the Nakano and Asada (1981) procedure.
The Folin-Ciocalteau method was used for the estimation of total phenolic content (Ordon et al., 2006). Then, a 0.5-ml plant sample was mixed with Folin-Ciocalteau reagent (0.2 N) for 5 min and sodium carbonate (2.0 ml of 75 g/L). After 2 h, the absorbance of the reaction was carried out at 760 nm at room temperature.
The method of Sarker and Oba (2018) was used for the determination of flavonoid content in the leaf extract. Leaf extract (500 μl), methanol (1.5 ml), potassium iodide (1 M), and aluminum chloride (1 ml of 10%) were allowed to stand for half an hour in the test tube at room temperature. Absorbance was calibrated at 415 nm using a spectrophotometer (Hitachi, Tokyo, Japan).
Root and shoot samples of V. radiata seedlings were dried in an oven and digested with the help of HCLO4. The Cr content in the study samples was determined with the help of an atomic absorption spectrophotometer (SpectraAA-220FS).
The SPSS software version 20.0 was applied for the analysis of the variance of the obtained data. The mean values obtained during the research were compared by employing Duncan’s multiple range test (DMRT) at p ≤ 0.05. The data depicted are mean ± SE, where n = 5.
Table 1 shows that Cr stress reduced root and shoot length by 57 and 38%, respectively, as compared with C-treated V. radiata seedlings. In contrast, HZRP priming increased the root, and shoot length of treated seedlings in normal as well as Cr-polluted soil. In the case of soil spiked with Cr, HZRP, mainly at a concentration of 2 ppm, enhanced root and shoot length by 85 and 33% as compared to Cr-only treatment.
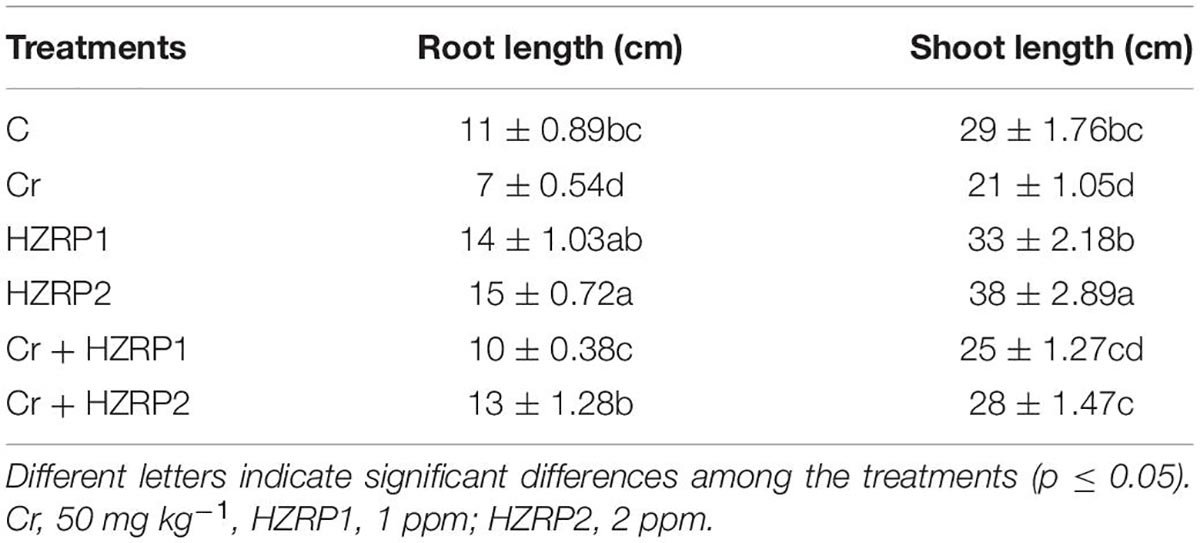
Table 1. Effect of harzianopyridone (HZRP) on root and shoot length of Vigna radiata exposed to chromium (Cr) stress.
Harzianopyridone priming reduced Cr uptake in V. radiata seedlings exposed to Cr stress. A high Cr value was found in the roots of V. radiata seedlings exposed to Cr-alone treatment (Table 2). Priming with HZRP at 2 ppm reduced Cr content in the root and shoot of V. radiata seedlings by 80.7 and 78.9%, respectively, in comparison with Cr-treatment.
Chromium stress reduced the net photosynthetic rate in V. radiata seedlings by 29% as compared with the control treatment. On the other hand, HZRP supplementation increased the net photosynthetic rate in V. radiata seedlings grown in normal and Cr-toxificated soil. In contrast, 2 ppm of HZRP increased the net photosynthetic rate by 47.05% in V. radiata seedlings grown in Cr-toxic soil, in comparison with Cr-only treatment. To understand the bases of increased photosynthesis, stomatal, and non-stomatal parameters were measured. At the stomatal level, HZRP at 2 ppm significantly enhanced stomatal conductance and intercellular CO2 concentration in V. radiata seedlings grown in normal and Cr-toxificated soil, as compared with the Cr-only treatment (Figure 1). Regarding the non-stomatal parameters, Cr toxicity reduced Chl content in V. radiata seedlings. However, 1 and 2 ppm of HZRP enhanced Chl content by 35 and 45%, respectively. In the case of V. radiata seedlings grown in Cr-toxic conditions, HZRP (2 ppm) enhanced Chl content by more than onefold in comparison with Cr-only treatment. Contrarily, Cr stress significantly increased the Chlase activity in V. radiata seedlings. Both applied concentrations of HZRP decreased the Chlase activity in V. radiata seedlings grown in Cr-contaminated soil.
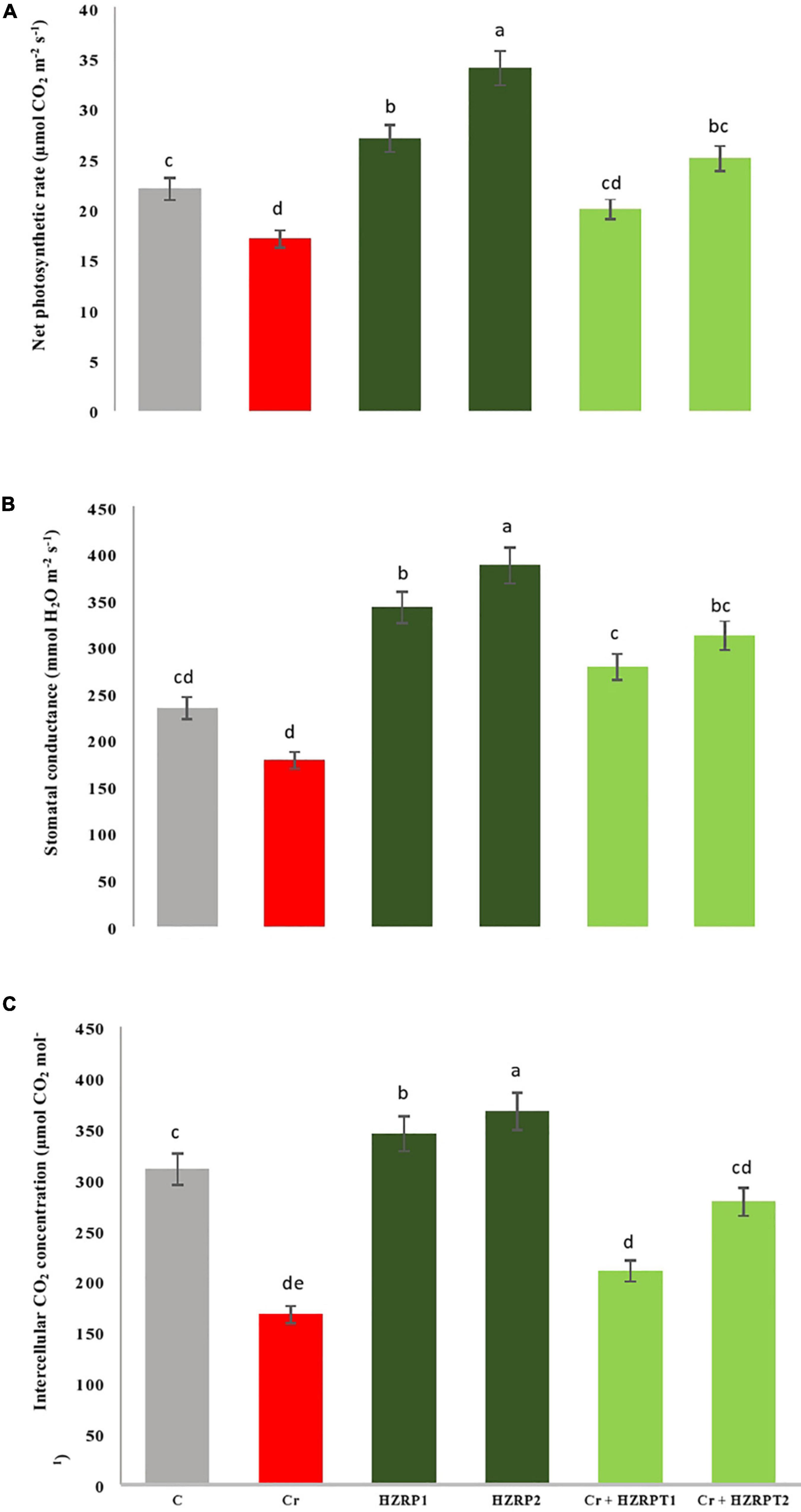
Figure 1. Effect of harzianopyridone on net photosynthetic rate (A), stomatal conductance (B) and intercellular CO2 concentration (C) in V. radiata seedlings grown in Cr toxificated soil. Different letters indicate significant difference among the treatments (p < 0.05). Cr, 50 mg kg–1, HZRP1, 1 p.p.m; HZRP2, 2 p.p.m.
Chromium can induce oxidative stress on the plant by the generation of ROS. Consequently, the destruction of membrane lipids under cobalt stress could increase the MDA content and EL. Here, Cr toxicity increased MDA content, EL, and H2O2 content by 36, 60, and 52%, respectively, as compared with control-treated V. radiata seedlings. Interestingly, HZRP supplementation reduced MDA content and EL in V. radiata seedlings grown in normal and Cr-exposed soil. A high concentration of HZRP (2 ppm) significantly reduced MDA content in V. radiata as compared with Cr-only treatment. In the case of V. radiata seedlings grown in Cr-toxificated soil, HZRP (2 ppm) treatment reduced MDA content by > 1-fold in comparison with Cr-only treatment. HZRP (2 ppm) treatment also reduced EL and H2O2 content in V. radiata seedlings grown in Cr-polluted soil (Figure 2).
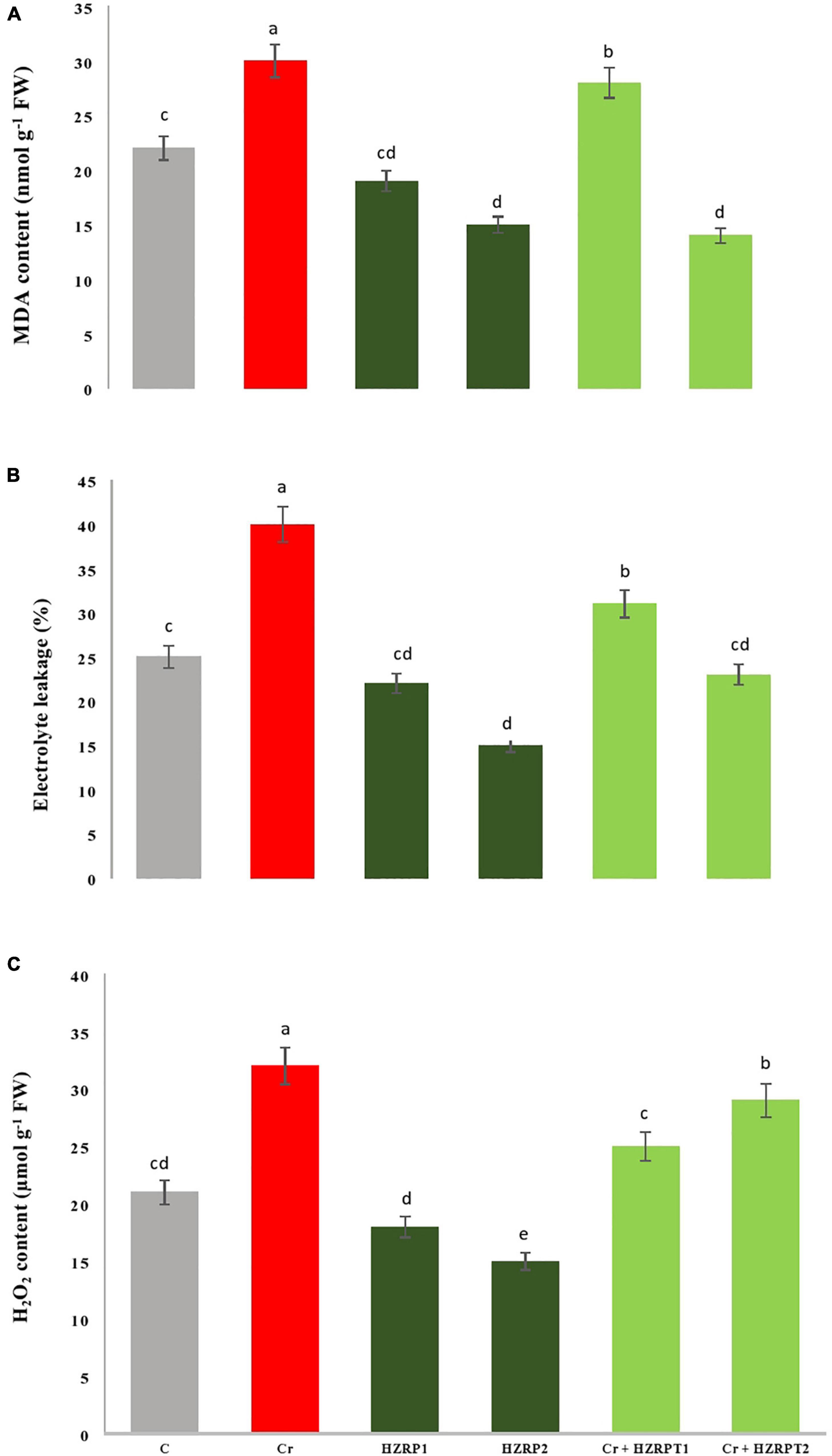
Figure 2. Effect of HZRP on malondialdehyde content (A), electrolyte leakage (B) and hydrogen peroxide content (C) in V. radiata seedlings grown in Cr toxificaled soil. Different letters indicate significant difference among the treatments (p < 0.05). Cr, 50 mg kg–1, HZRP1, 1 p.p.m; HZRP2, 2 p.p.m.
To cope with stress conditions, plants might induce antioxidants, which could play a role in mitigating the detrimental effects of heavy metal stress. In addition, HZRP might contribute to increasing the antioxidant metabolites levels and antioxidant enzyme activities to reduce the oxidative stress under Cr stress. Our results indicated that Cr stress escalated the activity of antioxidant enzymes (SOD, CAT, and POD) in V. radiata seedlings. The two priming concentrations of HZRP (1 and 2 ppm) significantly increased SOD, CAT, and POD activities in V. radiata seedlings grown in Cr-toxificated soil, in comparison to the Cr-only treatment. In the case of normal soil, priming of 2 ppm HZRP increases SOD activity by 42% in V. radiata seedlings grown in normal soil. It also increased SOD activity by 34% in V. radiata seedlings grown in Cr-polluted soil. Both the two priming concentrations of HZRP (1 and 2 ppm) also escalated POD activity in V. radiata seedlings grown in normal and Cr-contaminated potted soil. In the case of V. radiata seedlings grown in Cr-toxificated soil, HZRP increased the POD activity by 44% in comparison with the Cr-only treatment. Furthermore, they increased the activity of the CAT enzyme in V. radiata seedlings grown in normal and Cr-polluted potted (Figure 3).
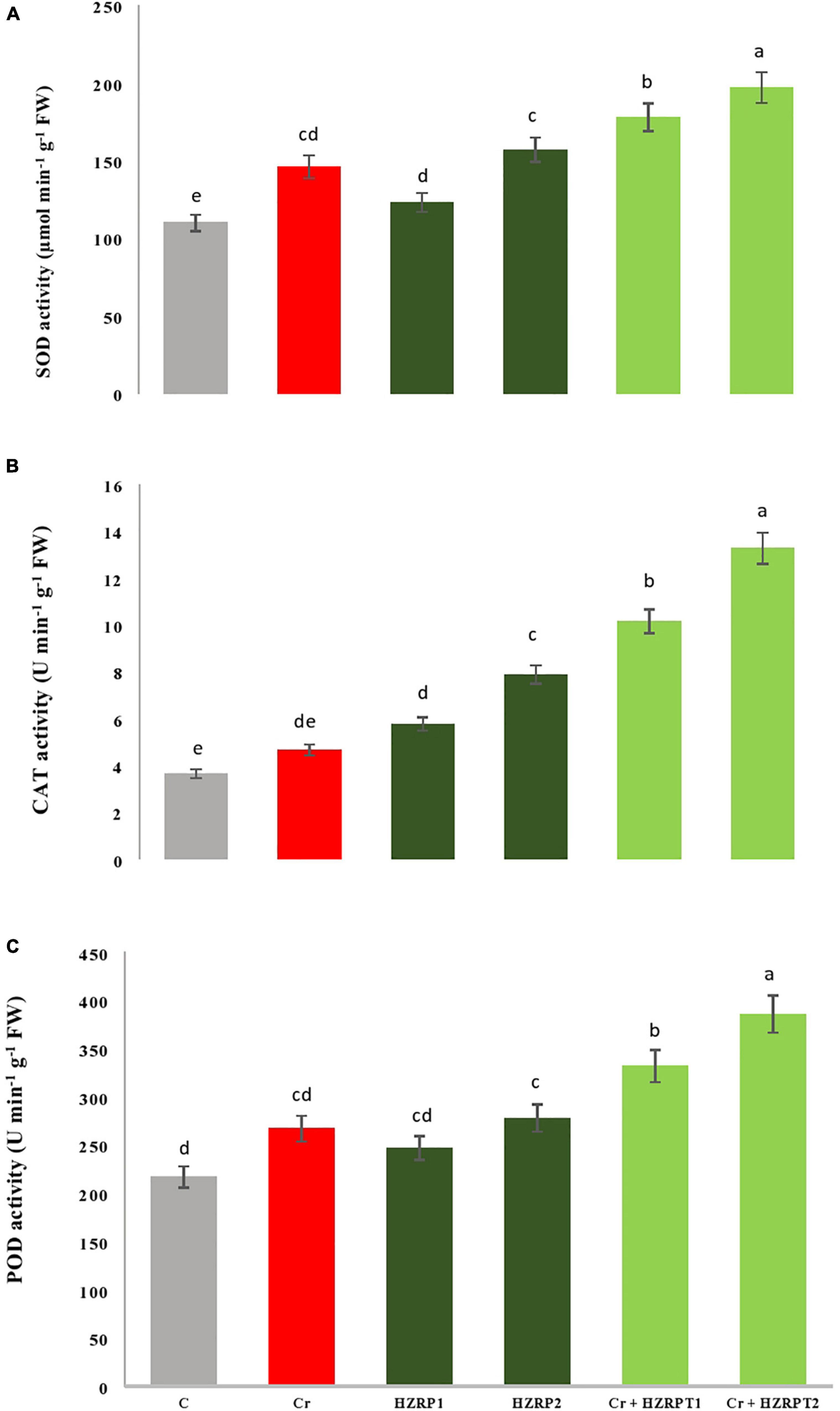
Figure 3. Effect of HZRP on SOD (A), CAT (B) and POD (C) activity in V. radiata seedlings grown in Cr toxificaled soil. Different letters indicate significant difference among the treatments (p < 0.05). Cr. 50 mg kg1, HZRP1, 1 p.p.m; HZRP2. 2 p.p.m.
At the antioxidant metabolic level, Cr stress decreased total phenolic content (33%) in V. radiata seedlings as compared to C-treatment. Contrarily, Cr stress enhanced flavonoid content by 51% as compared to V. radiata seedlings grown in the control treatment. Priming with 2 ppm of HZRP significantly enhanced total phenolic content in normal and Cr-contaminated soil as compared with C-treatment. It also increased flavonoid content by 56% in V. radiata seedlings grown in Cr-polluted potted soil. Similarly, priming with both concentrations of HZRP significantly increased glycine betaine content in V. radiata seedlings grown in normal and Cr-polluted potted soil (Figure 4).
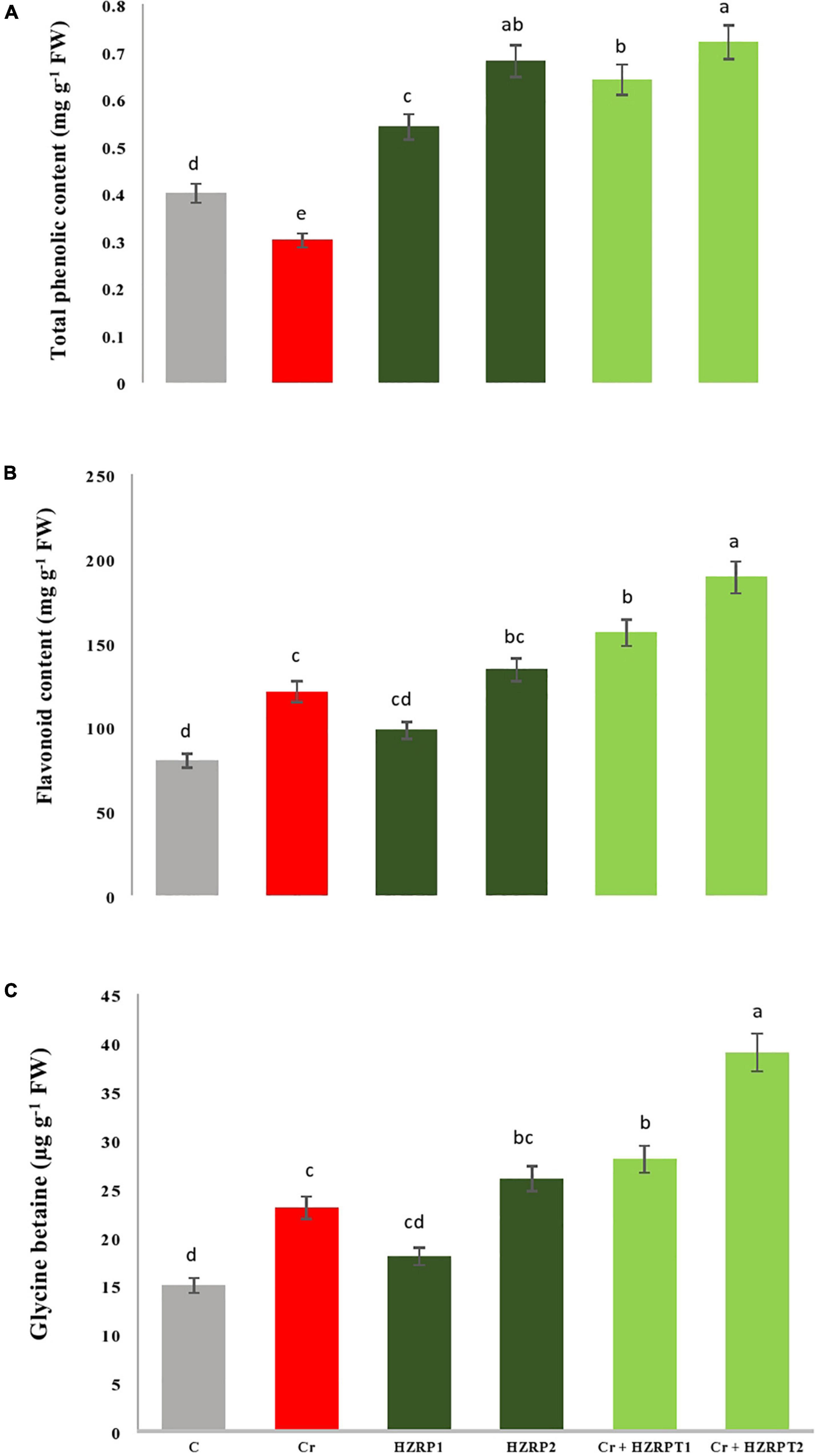
Figure 4. Effect of HZRP on total phenolic content (A), flavonoids (B) and glycine betaine (C) content in V. radiata seedlings grown in CT toxificated soil. Different letters indicate significant difference among the treatments (p < 0.05). Cr, 50 mgkg–1, H7.RP1, 1 p.p.m; HZRP2. 2 p.p.m.
Globally, Cr pollution in the environment is one of the key reasons for the deterioration in ecosystem sustenance. Cr is one of the toxic heavy metals with hazardous effects on plants and human health. During this study, the effect of Cr on growth and physicochemical parameters was also investigated. Amin et al. (2013) reported that Cr stress reduced seed germination in Hibiscus esculentus and other legume crops.
Chromium toxicity reduced growth, net photosynthetic rate, and gas exchange attributes in V. radiata seedlings. Alamri et al. (2020) also reported an increase in the activation of Chl degrading enzyme Chlase in tomato seedlings exposed to hexavalent Cr stress. During this study, Figure 5 shows that Cr toxicity enhanced Chlase activity, which reduced Chl content, leading to a decrease in net photosynthetic rate and photosynthate production in V. radiata seedlings.
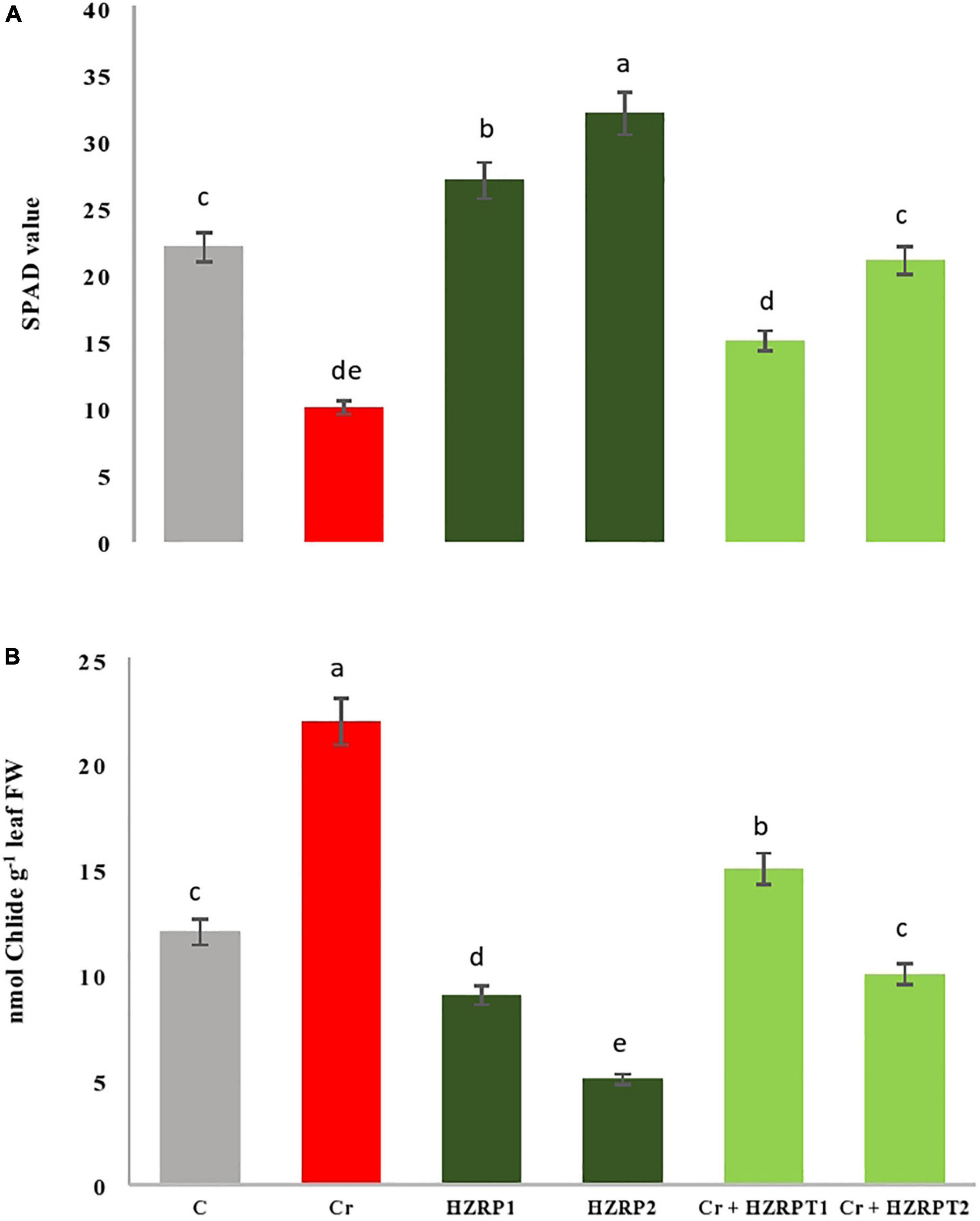
Figure 5. Effect of harzianopyridone on Ch1 content (A) and chlorophyllase activity (B) in Vigna radiata seedlings grown in Cr toxificated soil. Different letters indicate significant difference among the treatments (p < 0.05). Cr, 50 mg kg–1, HZRP1, 1 p.p.m; HZRP2, 2 p.p.m.
Increased levels of MDA, EL, and H2O2 advocated oxidative stress in mung beans. Increased content of these oxidative stress markers disturbed the equilibrium between the antioxidative defensive approach and ROS accumulation. Similar results are reported in Zea mays (Anjum et al., 2017), Brassica napus (Gill et al., 2016), and Cicer arietinum (Singh D. et al., 2020).
Accumulation of H2O2 in plants is a crucial stress marker that results in oxidative stress in plants (Sharma et al., 2012). At higher concentrations, H2O2 disturbs the crucial physiological processes in plants, such as photosynthesis, respiration, stomatal conductance, and cellular development (Noctor et al., 2002). Current research showed an increase in H2O2 content in V. radiata seedlings exposed to Cr stress. This increase in H2O2 content resulted in a disturbance in normal physiological processes in V. radiata seedlings. Contrarily, HZRP treated seedlings showed reduced H2O2 levels.
Figure 3 shows that HZRP treatment enhanced the activities of SOD, CAT, and POD in V. radiata seedlings in normal and Cr-toxic conditions. Exogenous application of 2 ppm HZRP significantly increased the activity of the antioxidant enzyme, which reduced MDA, EL, and H2O2 content in treated V. radiata seedlings. SOD is an important line of defense in plants against stresses. SOD converts O⋅–2 into O2 and H2O2. This conversion reduces OH⋅ formation. The activity of SOD is found to be upregulated in plants facing stressed conditions (Das and Roychoudhury, 2014). Current research reveals that HZRP application increased the activity of antioxidative enzymes (SOD, CAT, and POD) in V. radiata seedlings exposed to Cr stress.
Flavonoids are crucial for stress responses in plants. Flavonoids are involved in the scavenging of ROS produced in plants exposed to stress (Di Ferdinando et al., 2012). These secondary metabolites are involved in the stabilization of photosynthetic apparatus in plants (Stefanov et al., 2021). Glycine betaine is a crucial organic osmolyte that plays a pivotal role in mediating osmotic balance in plants facing stressed conditions (Ashraf and Foolad, 2007). This study showed HZRP-treated seedlings showed an increase in flavonoid content, which might have reduced ROS, thereby leading to Cr stress alleviation in V. radiata seedlings exposed to Cr stress.
Glycine betaine is reported to alleviate numerous abiotic stresses in sorghum (Kumar, 2021). The present study revealed that HZRP treatment alleviated Cr toxicity in V. radiata seedlings. This alleviation in Cr stress might be due to a reduction in Cr uptake in treated seedlings. It means that MDA and oxidative stress markers were not increased, which maintained Chl content, net photosynthetic rate, stomatal conductance, and intercellular CO2 concentration in V. radiata seedlings exposed to Cr stress. Aamer et al. (2018) reported that foliar application of glycine betaine alleviated Cd stress in Spinacia oleraceae through a reduction in Cd uptake and increased the activity of the antioxidative defensive system.
The plant has developed various adaptations to detoxify Cr content, such as Cr uptake (Shahid et al., 2017). Similar results have been reported in Oryza sativa (Chen et al., 2017), Arabidopsis thaliana (Wakeel et al., 2019), and Brassica juncea (Handa et al., 2018). Current studies depicted that HZRP supplementation reduced Cr uptake in V. radiata seedlings. This reduced Cr uptake regulated the growth and physiochemical properties of treated seedlings.
Chromium stress reduced the growth of V. radiata seedlings in potting soil. Cr stress decreased Chl content, net phostosynthetic rate, besides an increase in Chlase activity. Increased levels of EL, MDA, and H2O2 were observed in V. radiata seedlings exposed to Cr-toxificated soil. Contrarily, HZRP increased the activities of SOD, CAT, and POD in V. radiata seedlings. Apart from this, HZRP increased total phenolic content, flavonoids, and glycine betaine level in treated seedlings. Moreover, HZRP supplementation reduced Cr uptake in V. radiata exposed seedlings exposed to Cr stress. It is proposed that HZRP and other volatile organic compounds may be exploited for abiotic stress tolerance in plants (Figure 6).
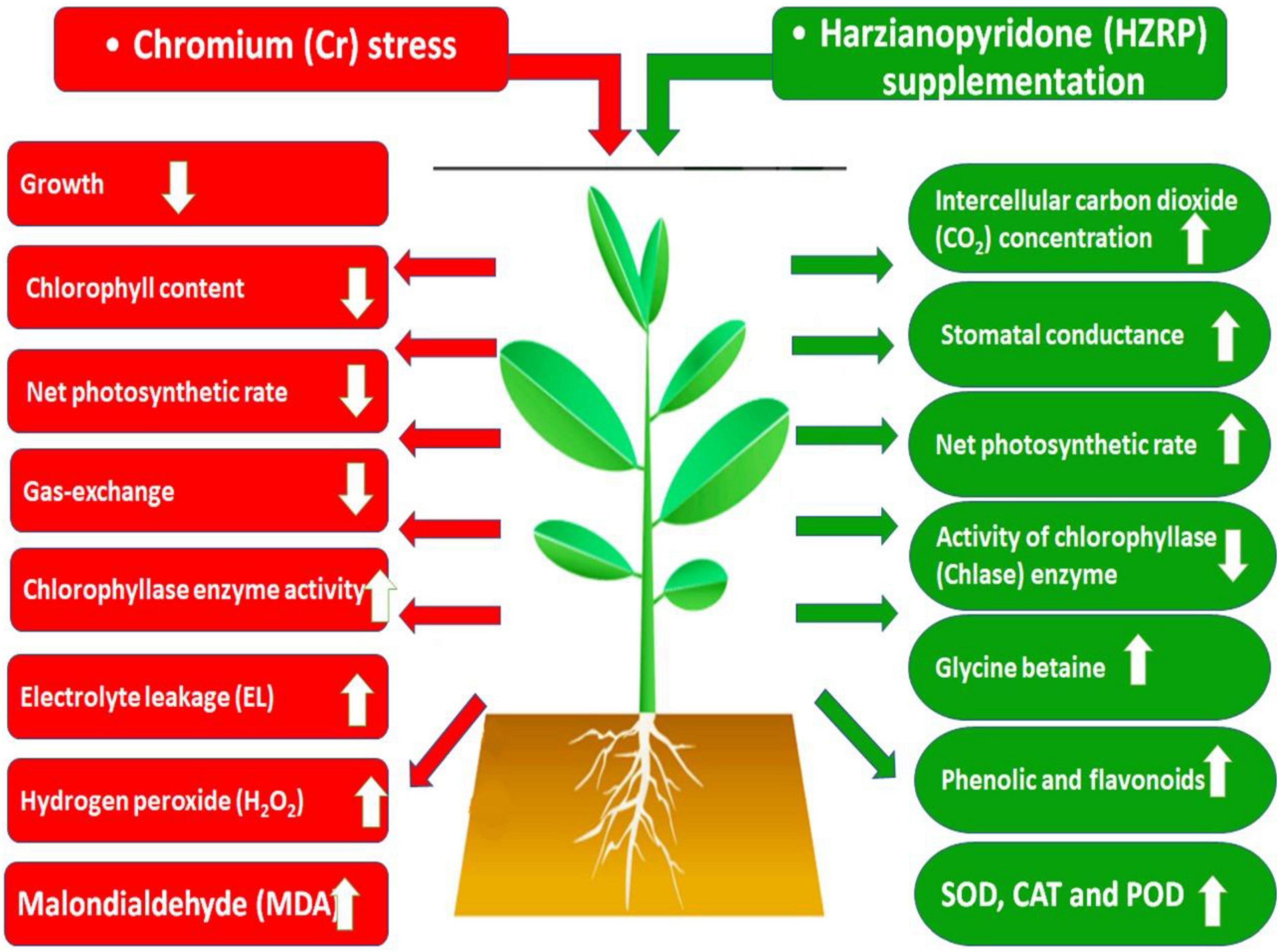
Figure 6. Schematic model explaining the role of HZRP in alleviation of Cr stress in V. radiata seedlings.
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
AAS and ANS: experimentation and validation. MB: research design. AA and SJ: statistical analysis. MR: validation. SA, HA, and TJ: Research design and review and drafting. SSA, AT, and HMK: Funding acquisition and review and drafting. All authors contributed to the article and approved the submitted version.
Taif University researchers supporting project number (TURSP-2020/38), Taif University, Taif, Saudi Arabia.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors extend their sincere appreciation to Taif University Researchers Supporting Project Number (TURSP-2020/38), Taif, Saudi Arabia for financially supporting the current research.
Aamer, M., Muhammad, U. H., Li, Z., Abid, A., Su, Q., Liu, Y., et al. (2018). Foliar application of glycinebetaine (GB) alleviates the cadmium (Cd) toxicity in spinach through reducing Cd uptake and improving the activity of anti-oxidant system. Appl. Ecol. Environ. Res. 16, 7575–7583. doi: 10.15666/aeer/1606_75757583
Alamri, S., Ali, H. M., Khan, M. I. R., Singh, V. P., and Siddiqui, M. H. (2020). Exogenous nitric oxide requires endogenous hydrogen sulfide to induce the resilience through sulfur assimilation in tomato seedlings under hexavalent chromium toxicity. Plant Physiol. Biochem. 155, 20–34. doi: 10.1016/j.plaphy.2020.07.003
Amin, H., Arain, B., Amin, F., and Surhio, M. (2013). Phytotoxicity of chromium on germination, growth and biochemical attributes of Hibiscus esculentus L. Am. J. Plant Sci. 4, 2431–2439.
Anjum, S. A., Ashraf, U., Imran, K. H. A. N., Tanveer, M., Shahid, M., Shakoor, A., et al. (2017). Phyto-toxicity of chromium in maize: oxidative damage, osmolyte accumulation, anti-oxidative defense and chromium uptake. Pedosphere 27, 262–273. doi: 10.1016/s1002-0160(17)60315-1
Ashraf, M. F. M. R., and Foolad, M. R. (2007). Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 59, 206–216. doi: 10.1016/j.envexpbot.2005.12.006
Askari, S. H., Ashraf, M. A., Ali, S., Rizwan, M., and Rasheed, R. (2021). Menadione sodium bisulfite alleviated chromium effects on wheat by regulating oxidative defense, chromium speciation, and ion homeostasis. Environ. Sci. Pollut. Res. 28, 36205–36225. doi: 10.1007/s11356-021-13221-0
Beyersmann, D., and Hartwig, A. (2008). Carcinogenic metal compounds: recent insight into molecular and cellular mechanisms. Arch. Toxicol. 82:493. doi: 10.1007/s00204-008-0313-y
Bharagava, R. N., and Mishra, S. (2018). Hexavalent chromium reduction potential of Cellulosimicrobium sp. isolated from common effluent treatment plant of tannery industries. Ecotoxicol. Environ. Saf. 147, 102–109. doi: 10.1016/j.ecoenv.2017.08.040
Chen, Q., Zhang, X., Liu, Y., Wei, J., Shen, W., Shen, Z., et al. (2017). Hemin-mediated alleviation of zinc, lead and chromium toxicity is associated with elevated photosynthesis, antioxidative capacity; suppressed metal uptake and oxidative stress in rice seedlings. Plant Growth Regul. 81, 253–264. doi: 10.1007/s10725-016-0202-y
Das, K., and Roychoudhury, A. (2014). Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2:53. doi: 10.3389/fenvs.2014.00053
Devi, P., and Kumar, P. (2020). Enhancement effect of biofertilizers on germination percentage and plant height in maize grown under chromium toxic soil. J. Pharmacogn. Phytochem. 9, 702–707.
Di Ferdinando, M., Brunetti, C., Fini, A., and Tattini, M. (2012). “Flavonoids as Antioxidants in Plants Under Abiotic Stresses,” in Abiotic Stress Responses in Plants, eds P. Ahmad and M. Prasad (New York, NY.: Springer).
Fang, Z., Bouwkamp, J. C., and Solomos, T. (1998). Chlorophyllase activities and chlorophyll degradation during leaf senescense in non-yellowing mutant and wild type of Phaseolus vulgaris L. J. Exp. Bot. 49, 503–510.
Farid, M., Ali, S., Saeed, R., Rizwan, M., Bukhari, S. A. S., Abbasi, G. H., et al. (2019). Combined application of citric acid and 5-aminolevulinic acid improved biomass, photosynthesis and gas exchange attributes of sunflower (Helianthus annuus L.) grown on chromium contaminated soil. Int. J. Phytoremediation 161, 166–179. doi: 10.1080/15226514.2018.1556595
Giannopolitis, C. N., and Ries, S. K. (1977). Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 59, 309–314.
Gill, R. A., Zhang, N., Ali, B., Farooq, M. A., Xu, J., Gill, M. B., et al. (2016). Role of exogenous salicylic acid in regulating physio-morphic and molecular changes under chromium toxicity in black- and yellow- seeded Brassica napus L. Environ. Sci. Pollut. Res. 23, 20483–20496. doi: 10.1007/s11356-016-7167-2
Handa, N., Kohli, S. K., Thukral, A. K., Bhardwaj, R., Alyemeni, M. N., Wijaya, L., et al. (2018). Protective role of selenium against chromium stress involving metabolites and essential elements in Brassica juncea L. seedlings. 3Biotech 8, 1–14. doi: 10.1007/s13205-018-1087-4
Hermosa, R., Viterbo, A., Chet, I., and Monte, E. (2012). Plant-beneficent effects of Trichoderma and of its genes. Microbiology 158, 17–25.
Hou, D., Yousaf, L., Xue, Y., Hu, J., Wu, J., Hu, X., et al. (2019). Mung bean (Vigna radiata L.): bioactive polyphenols, polysaccharides, peptides, and health benefits. Nutrients 11:1238. doi: 10.3390/nu11061238
Huang, H., Ullah, F., Zhou, D. X., Yi, M., and Zhao, Y. (2019). Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 10:800. doi: 10.3389/fpls.2019.00800
Husain, T., Suhel, M., Prasad, S. M., and Singh, V. P. (2021). Ethylene and hydrogen sulphide are essential for mitigating hexavalent chromium stress in two pulse crops. Plant Biol. [Ebup ahead of print]. doi: 10.1111/plb.13324
Ihtisham, M., Noori, A., Yadav, S., Sarraf, M., Kumari, P., Brestic, M., et al. (2021). Silver nanoparticle’s toxicological effects and phytoremediation. Nanomaterials 11:2164. doi: 10.3390/nano11092164
Jabeen, N., Abbas, Z., Iqbal, M., Rizwan, M., Jabbar, A., Farid, M., et al. (2016). Glycinebetaine mediates chromium tolerance in mung bean through lowering of chromium uptake and improved antioxidant system. Arch. Agric. Soil Sci. 62, 648–662.
Jain, M., and Gadre, R. (2004). Inhibition of 5-amino levulinic acid dehydratase activity by arsenic in excised etiolated maize leaf segments during greening. J. Plant Physiol. 161, 251–255. doi: 10.1078/0176-1617-00879
Kumar, P. (2021). Stress amelioration response of glycine betaine and Arbuscular mycorrhizal fungi in sorghum under Cr toxicity. PLoS One 16:e0253878. doi: 10.1371/journal.pone.0253878
Maqbool, A., Ali, S., Rizwan, M., Ishaque, W., Rasool, N., Bashir, A., et al. (2018). Management of tannery wastewater for improving growth attributes and reducing chromium uptake in spinach through citric acid application. Environ. Sci. Pollut. Res. 25, 10848–10856. doi: 10.1007/s11356-018-1352-4
McFeeters, R. F., Chichester, C. O., and Whitaker, J. R. (1971). Purification and properties of chlorophyllase from Ailanthus altissima (Tree-of-Heaven). Plant Physiol. 47, 609–618. doi: 10.1104/pp.47.5.609
Nair, R. M., Pandey, A. K., War, A. R., Hanumantharao, B., Shwe, T., Alam, A. K. M. M., et al. (2019). Biotic and abiotic constraints in mungbean production—progress in genetic improvement. Front. Plant Sci. 10:1340. doi: 10.3389/fpls.2019.01340
Nakano, Y., and Asada, K. (1981). Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 22, 867–880.
Noctor, G., Veljovic-Jovanovic, S., Driscoll, S., Novitskaya, L., and Foyer, C. H. (2002). Drought and oxidative load in the leaves of C3 plants: a predominant role for photorespiration? Ann. Bot. 89, 841–850. doi: 10.1093/aob/mcf096
Ordon, J. D., Gomez, J. D., Vattuone, M. A., and Isla, M. I. (2006). Antioxidant activities of Sechium edule (Jacq.) Swart extracts. Food Chem. 97, 452–458. doi: 10.1016/j.foodchem.2005.05.024
Paithankar, J. G., Saini, S., Dwivedi, S., Sharma, A., and Chowdhuri, D. K. (2021). Heavy metal associated health hazards: An interplay of oxidative stress and signal transduction. Chemosphere 262:128350. doi: 10.1016/j.chemosphere.2020.128350
Patra, D. K., Pradhan, C., and Patra, H. K. (2019). Chromium bioaccumulation, oxidative stress metabolism and oil content in lemon grass Cymbopogon flexuous (Nees ex Steud.) W. Watson grown in chromium rich over burden soil of Sukinda chromite mine, India. Chemosphere 218, 1082–1088. doi: 10.1016/j.chemosphere.2018.11.211
Sarker, U., and Oba, S. (2018). Response of nutrients, minerals, antioxidant leaf pigments, vitamins, polyphenol, flavonoid and antioxidant activity in selected vegetable amaranth under four soil water content. Food Chem. 252:72–83. doi: 10.1016/j.foodchem.2018.01.097
Sarraf, M., Vishwakarma, K., Kumar, V., Arif, N., Das, S., Johnson, R., et al. (2022). Metal/Metalloid-Based Nanomaterials for Plant Abiotic Stress Tolerance: An Overview of the Mechanisms. Plants 11:316. doi: 10.3390/plants11030316
Shah, A. A., Bibi, F., Hussain, I., Yasin, N. A., Akram, W., Tahir, M. S., et al. (2020). Synergistic effect of bacillus thuringiensis IAGS 199 and putrescine on alleviating cadmium-induced phytotoxicity in capsicum annum. Plants 9:1512. doi: 10.3390/plants9111512
Shahid, M., Shamshad, S., Rafiq, M., Khalid, S., Bibi, I., Niazi, N. K., et al. (2017). Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: a review. Chemosphere 178, 513–533. doi: 10.1016/j.chemosphere.2017.03.074
Sharma, A., Kapoor, D., Wang, J., Shahzad, B., Kumar, V., Bali, A. S., et al. (2020). Chromium bioaccumulation and its impacts on plants: an overview. Plants 9:100. doi: 10.3390/plants9010100
Sharma, P., Jha, A. B., Dubey, R. S., and Pessarakli, M. (2012). Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012:217037.
Sharma, A., Vishwakarma, K., Singh, N. K., Prakash, V., Ramawat, N., Prasad, R., et al. (2022). Synergistic action of silicon nanoparticles and indole acetic acid in alleviation of chromium (CrVI) toxicity in Oryza sativa seedlings. J. Biotech. 343, 71–82. doi: 10.1016/j.jbiotec.2021.09.005
Singh, D., Sharma, N. L., Singh, C. K., Sarkar, S. K., Singh, I., and Dotaniya, M. L. (2020). Effect of chromium (VI) toxicity on morpho-physiological characteristics, yield, and yield components of two chickpea (Cicer arietinum L.) varieties. PLoS One 15:e0243032. doi: 10.1371/journal.pone.0243032
Singh, D., Sharma, N. L., Singh, C. K., Yerramilli, V., Narayan, R., Sarkar, S. K., et al. (2021). Chromium (VI)-Induced Alterations in Physio-Chemical Parameters, Yield, and Yield Characteristics in Two Cultivars of Mungbean (Vigna radiata L.). Front. Plant Sci. 12:735129. doi: 10.3389/fpls.2021.735129
Singh, P., Itankar, N., and Patil, Y. (2020). Biomanagement of hexavalent chromium: current trends and promising perspectives. J. Environ. Manage. 279:111547. doi: 10.1016/j.jenvman.2020.111547
Stefanov, M., Yotsova, E., Gesheva, E., Dimitrova, V., Markovska, Y., Doncheva, S., et al. (2021). Role of flavonoids and proline in the protection of photosynthetic apparatus in Paulownia under salt stress. S. Afr. J. Bot. 139, 246–253. doi: 10.1016/j.sajb.2021.02.008
Stewart, A., Hill, R. (2014). Applications of Trichoderma in plant growth promotion. In: Gupta V.K., Schmoll M., Herrera-Estrella A., Upadhyay R.S., Dru-zhinina I., Tuohy M.G. (Eds.), Biotechnology and Biology of Trichoderma. Amsterdam: Elsevier.
Tirry, N., Kouchou, A., El Omari, B., Ferioun, M., and El Ghachtouli, N. (2021). Improved chromium tolerance of Medicago sativa by plant growth-promoting rhizobacteria (PGPR). J. G. E. B. 19, 1–14. doi: 10.1186/s43141-021-00254-8
Velikova, V., Yordanov, I., and Edreva, A. (2000). Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci. 151, 59–66. doi: 10.1016/s0168-9452(99)00197-1
Vinale, F., Nigro, M., Sivasithamparam, K., Flematti, G., Ghisalberti, E. L., Ruocco, M., et al. (2013). Harzianic acid: a novel siderophore from Trichoderma harzianum. FEMS Microbiol. Lett. 347, 123–129. doi: 10.1111/1574-6968.12231
Wakeel, A., Ali, I., Upreti, S., Azizullah, A., Liu, B., Khan, A. R., et al. (2018). Ethylene mediates dichromate-induced inhibition of primary root growth by altering AUX1 expression and auxin accumulation in Arabidopsis thaliana. Plant Cell Environ. 41, 1453–1467. doi: 10.1111/pce.13174
Wakeel, A., Ali, I., Wu, M., Kkan, A. R., Jan, M., Ali, A., et al. (2019). Ethylene mediates dichromate-induced oxidative stress and regulation of the enzymatic antioxidant system-related transcriptome in Arabidopsis thaliana. Environ. Exp. Bot. 161, 166–179.
Yu, X., Yu, R. Q., Gui, D., Zhang, X., Zhan, F., Sun, X., et al. (2018). Hexavalent chromium induces oxidative stress and mitochondria-mediated apoptosis in skin fibroblasts of Indo-Pacific humpback dolphin. Aquat. Toxicol. 203, 179–186. doi: 10.1016/j.aquatox.2018.08.012
Zaheer, I. E., Ali, S., Rizwan, M., Bareen, F. E., Abbas, Z., Bukhari, S. A. H., et al. (2019). Zinc-lysine prevents chromium-induced morphological, photosynthetic, and oxidative alterations in spinach irrigated with tannery wastewater. Environ. Sci. Pollut. Res. 26, 28951–28961. doi: 10.1007/s11356-019-06084-z
Zaheer, I. E., Ali, S., Saleem, M. H., Imran, M., Alnusairi, G. S. H., Alharbi, M. B., et al. (2020). Role of iron–lysine on morpho-physiological traits and combating chromium toxicity in rapeseed (Brassica napus L.) plants irrigated with different levels of tannery wastewater. Plant Physiol. Biochem. 155, 70–84. doi: 10.1016/j.plaphy.2020.07.034
Zaheer, I. E., Ali, S., Saleem, M. H., Yousaf, H. S., Malik, A., Abbas, Z., et al. (2022). Combined application of zinc and iron-lysine and its effects on morpho-physiological traits, antioxidant capacity and chromium uptake in rapeseed (Brassica napus L.). PLoS One 17:e0262140. doi: 10.1371/journal.pone.0262140
Keywords: mung bean, chromium, stress, growth, harzianopyridone
Citation: Shah AA, Shah AN, Bilal Tahir M, Abbas A, Javad S, Ali S, Rizwan M, Alotaibi SS, Kalaji HM, Telesinski A, Javed T and AbdElgawad H (2022) Harzianopyridone Supplementation Reduced Chromium Uptake and Enhanced Activity of Antioxidant Enzymes in Vigna radiata Seedlings Exposed to Chromium Toxicity. Front. Plant Sci. 13:881561. doi: 10.3389/fpls.2022.881561
Received: 22 February 2022; Accepted: 25 April 2022;
Published: 04 July 2022.
Edited by:
Rafaqat Ali Gill, Oil Crops Research Institute (CAAS), ChinaReviewed by:
Mona F. A. Dawood, Assiut University, EgyptCopyright © 2022 Shah, Shah, Bilal Tahir, Abbas, Javad, Ali, Rizwan, Alotaibi, Kalaji, Telesinski, Javed and AbdElgawad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anis Ali Shah, YW5pc2FsaWJvdEBnbWFpbC5jb20=, YW5zLjc4NkB5YWhvby5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.