- 1Horta s.r.l., Piacenza, Italy
- 2Department of Sustainable Crop Production (DI.PRO.VES.), Università Cattolica del Sacro Cuore, Piacenza, Italy
- 3Department of Agricultural, Food, Environmental and Forestry Science and Technology (DAGRI), Plant Pathology and Entomology Section, University of Florence, Firenze, Italy
- 4Biotechnical Faculty, University of Montenegro, Podgorica, Montenegro
Phomopsis cane and leaf spot (PCLS), known in Europe as “excoriose,” is an important fungal disease of grapevines caused by Diaporthe spp., and most often by Diaporthe ampelina (synonym Phomopsis viticola). PCLS is re-emerging worldwide, likely due to climate change, changes in the management of downy mildew from calendar- to risk-based criteria that eliminate early-season (unnecessary) sprays, and the progressive reduction in the application of broad-spectrum fungicides. In this study, a mechanistic model for D. ampelina infection was developed based on published information. The model accounts for the following processes: (i) overwintering and maturation of pycnidia on affected canes; (ii) dispersal of alpha conidia to shoots and leaves; (iii) infection; and (iv) onset of disease symptoms. The model uses weather and host phenology to predict infection periods and disease progress during the season. Model output was validated against 11 independent PCLS epidemics that occurred in Italy (4 vineyards in 2019 and 2020) and Montenegro (3 vineyards in 2020). The model accurately predicted PCLS disease progress, with a concordance correlation coefficient (CCC) = 0.925 between observed and predicted data. A ROC analysis (AUROC>0.7) confirmed the ability of the model to predict the infection periods leading to an increase in PCLS severity in the field, indicating that growers could use the model to perform risk-based fungicide applications.
Introduction
Phomopsis cane and leaf spot (PCLS; known in Europe as “excoriose”) is an important fungal disease that is widely distributed throughout the viticultural regions of Europe (Phillips, 2000; Guarnaccia et al., 2018), United States (Schilder et al., 2005; Nita et al., 2008; Urbez-Torres et al., 2013; Wilcox et al., 2015), South Africa (Mostert, 2000), and Australia (Merrin et al., 1995). PCLS mainly affects shoots and leaves, although all green parts of the grapevines can be infected. On shoots, PCLS forms small, black cortical necrotic tissue mostly in the three to four basal internodes; these necrotic tissues develop into elliptical lesions that may grow together to form irregular, black, crusty, longitudinal cankers; when shoots lignify during the dormant season, infected canes become grayish; and numerous black pycnidia emerge from their surfaces. On leaves, the disease consists of small light-green lesions with irregular margins that develop early in the season, which later on turn dark with surrounding yellow halos. On rachises, symptoms are similar to those on shoots, and berries turn brown and shrivel during ripening (Erincik et al., 2002; Nita et al., 2008; Wilcox et al., 2015). Damage to grapevines includes weakened canes (which may break and be susceptible to frost damage), reduced photosynthesis of leaves, poor fruit development, and premature fruit drop because of rachis infection and fruit rot. Fruit and rachis infections are sporadic and occur only under very high disease pressure but if present can be responsible for important losses (Erincik et al., 2002; Nita et al., 2008; Wilcox et al., 2015).
PCLS was traditionally known to be caused by Phomopsis viticola. Phomopsis viticola has also been described as a wood-canker pathogen, causal agent of dead-arm symptoms (Baumgartner et al., 2013; Urbez-Torres et al., 2013). Species of the genus Phomopsis were considered to be host specific, and more than 1,000 species have been described based on the assumption of host specificity; this assumption is no longer considered valid (Gomes et al., 2013; Urbez-Torres et al., 2013). In the last decade, the genus has been redefined based on a combination of morphological, cultural, phytopathological, and mating type data, and the teleomorph stage Diaporthe became the only name broadly accepted and adopted (Udayanga et al., 2011; Gomes et al., 2013; Guarnaccia et al., 2018). As a consequence of this revision, multiple species of Diaporthe have been associated with PCLS and trunk diseases on Vitis vinifera, including D. ampelina, D. amygdali, D. eres, D. ambigua, and D. foeniculina. Among these species, D. ampelina is the most prevalent (Baumgartner et al., 2013; Urbez-Torres et al., 2013; Lawrence et al., 2015; Guarnaccia et al., 2018; González-Domínguez et al., 2021).
Diaporthe ampelina overwinters in lesions on dormant canes that had been infected during the previous growing seasons (Anco et al., 2012; González-Domínguez et al., 2021). In spring, mature flask-shaped pycnidia produce two types of conidia, i.e., alpha and beta conidia, that are different in shape (Sergeeva et al., 2003; Gomes et al., 2013). The primary inoculum of PCLS consists of the alpha conidia produced by D. ampelina pycnidia from budbreak until shortly after the end of bloom (Anco et al., 2012; González-Domínguez et al., 2021); these conidia are responsible for infection of leaves and shoots during cool, wet weather (Erincik et al., 2003; Anco et al., 2013). The function of beta conidia in the disease epidemiology is unclear (Pezet, 1974; Sergeeva et al., 2003; González-Domínguez et al., 2021). The disease is known to be monocyclic, because several primary infections occur through the season but no secondary infections contribute to disease progress (Anco et al., 2012; Wilcox et al., 2015; González-Domínguez et al., 2021). PCLS usually increases in a vineyard over a number of years, leading to a general decline in vine vigor and yield (Hewit and Pearson, 1988).
PCLS is re-emerging worldwide (Phillips, 2000; Wilcox et al., 2015; Caffi et al., 2020), because of three probable factors: climate change, which has advanced seasonal grapevine growth, including the time of budbreak (Caffarra and Eccel, 2011; Molitor and Junk, 2019); changes in the management of downy mildew from calendar- to risk-based criteria that eliminate early-season, unnecessary sprays (Rossi et al., 2019); and the progressive reduction in the application of broad-spectrum fungicides, which although highly effective (Nita et al., 2006a), may negatively affect human health and the environment (Wightwick et al., 2010; Epstein, 2014; Mostafalou and Abdollahi, 2017). At present, PCLS control is still mainly based on repeated treatments with protective broad-spectrum fungicides (mancozeb, dithianon, captan, or metiram), which are more effective than QoI or DMI fungicides (Hewit and Pearson, 1988; Nita et al., 2006a), starting from budbreak or at 2.5 cm shoot growth (Pscheidt and Pearson, 1991; Rawnsley, 2012; Wilcox et al., 2015).
To improve the timing of fungicide applications for control of PCLS, Nita et al. (2006b) developed a simple warning system based on the effect of weather conditions on infection by alpha conidia and on the assumption that inoculum is always present in sufficient amount to initiate the disease (Erincik et al., 2003). However, the production and dispersal of D. ampelina alpha conidia have a within-season temporal pattern (Anco et al., 2012; González-Domínguez et al., 2021) that may also affect infection occurrence and severity. The latter finding suggests that the timing of fungicide application should be based not only on weather conditions but also on the production and dispersal of D. ampelina alpha conidia.
In the current study, we developed a model for predicting the risk of shoot and leaf infection by alpha conidia of D. ampelina. The model was developed with a mechanistic approach, which accounted for the processes resulting in the availability of D. ampelina conidia during the season and in repeated infections and disease progress over the season. We evaluated the model in four Italian vineyards during two growing seasons (2019 and 2020), and in three Montenegrin vineyards during one growing season (2020).
Model Development
The Biological Processes Addressed by the Model
Diaporthe ampelina overwinters as pycnidia that are produced on canes and rachises and that remain viable for more than one season (Anco et al., 2012; González-Domínguez et al., 2021). In spring, pycnidia mature, emerge through the periderm of canes, and exude conidia in a gelatinous mass (the cirrus; Nita et al., 2006b). Alpha conidia in pycnidia are produced at temperatures ranging from 4 to 36°C, with an optimum at 21°C, and their production increases with increasing wetness duration (Anco et al., 2013). Dispersal of alpha conidia is mainly associated with rain events, from budbreak to shortly after the end of bloom (Anco et al., 2012; Wilcox et al., 2015; González-Domínguez et al., 2021). No secondary inoculum is produced during the season (Anco et al., 2012; Wilcox et al., 2015; González-Domínguez et al., 2021).
Alpha conidia can germinate at temperatures ranging from 8 to 30°C (temperatures out of this range have not been evaluated); at 20 to 30°C, 100% of the conidia germinate within 28 h (Sergeeva et al., 2003). Infection of grape leaves and shoots occurs at 5 to 35°C; at optimum temperatures (16 to 20°C), infection occurs with a minimum of 5 h of wetness; and wet periods longer than 10 h are necessary at higher and lower temperatures (Erincik et al., 2003). Rachis, berries, and leaves are susceptible through the growing season, from pre-bloom (GS = 15 of the scale of Lorenz et al., 1995) to veraison (GS = 81; Erincik et al., 2001). Symptoms first appear on leaves and shoots at 7 to 11 days after infection, although the effect of the environment and growth stage on the incubation period is not well understood (Erincik et al., 2001, 2003).
Model Description
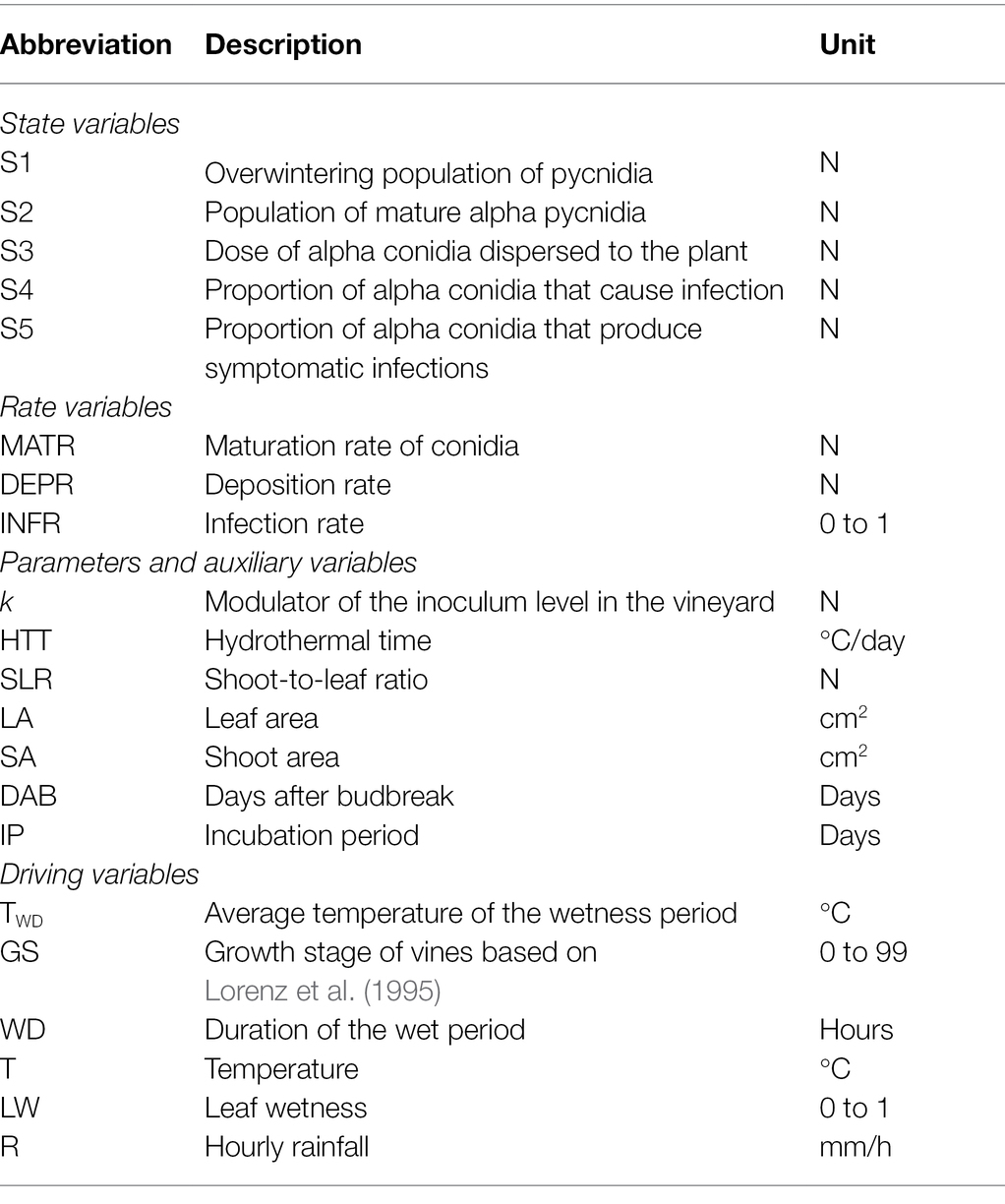
The model was developed according to the principles of “systems analysis” (Leffelaar, 1993; Rossi et al., 2010). The biological processes described above were organized into different state variables, and changes from one state to another were determined by rate variables depending on host growth stage and environmental conditions, i.e., temperature (T, °C), rainfall (R, mm), and leaf wetness (LW, min), as shown in a relational diagram (Figure 1; Table 1). The model calculations begin at budbreak (GS = 01) and end at harvest (GS = 90), and the time step is 1 day.
Figure 1. Relational diagram of a model simulating Phomopsis cane and leaf spot (PCLS) dynamics. Boxes are state variables; line arrows show fluxes and direction of changes from one state variable to the next one; valves define rates regulating these fluxes; diamonds show switches (i.e., conditions that open or close a flux); segments with circles indicate external variables; circles indicate auxiliary variables; broken arrows link external or auxiliary variables to diamonds or circles that they influence; and clouds indicate state variables that enter or exit the system (and are not quantified). All variables are listed in Table 1.
The first state variable in Figure 1 consists of a population of pycnidia (S1, which is not defined in size) that overwinters in the vineyard and produces mature alpha conidia (S2, which is the second state variable in Figure 1). The mature alpha conidia are produced during the grape-growing season starting at budbreak and at a maturation rate (MATR).
The maturation rate of conidia is calculated as the first derivative of the equation of González-Domínguez et al. (2021):
in which k is a modulator of the inoculum level in the vineyard, and HTTi is the hydrothermal time accumulated on each ith day after budbreak (Lovell et al., 2004). HTT is calculated as follows:
in which Teqi is an equivalent of temperature calculated as Teqi = (Ti – Tmin)/(Tmax – Tmin), where Ti the average temperature of the day i, and Tmin and Tmax are the minimal and maximal temperatures for the production of conidia (5 and 35°C, respectively; González-Domínguez et al., 2021); WDi is the wetness duration (i.e., the number of wet hours in a day).
Alpha conidia in S2 are dispersed when rain is ≥0.2 mm/h and a minimum of 24 h have passed since the previous rain (Nita et al., 2006b; González-Domínguez et al., 2021); in the model, this is considered a dispersal event. The model assumes that the dose of conidia that is deposited on shoots or leaves depends on the shoot and leaf area (the auxiliary variables SA and LA, respectively), and a deposition rate (DEPR) that in turn depends on the shoot-to-leaf ratio (the auxiliary variable SLR, calculated as SA/(LA + SA)). Both SA and LA depend on the number of days after budbreak (DAB; Supplementary Material).
The conidia in S3 cause infection when there is a wet period of at least 5 h with a temperature from 5 to 35°C (Erincik et al., 2003). This is an infection event in the model; two infection events are separated by a dry period that is >4 h (Nita et al., 2006b). The severity of any infection event, i.e., the proportion of S3 that causes infection (S4, the fourth state variables in Figure 1), is calculated based on an infection rate for leaves (INFRL) and shoots (INFRS) as follows:
in which Twd is the average temperature of the wetness period; Teqwd is as in equation (2), with T = Twd; WD is the consecutive number of hours of wetness; and WDmax is the number of wet hours for maximum disease development on shoots (i.e., 20 h). Equations (3) and (4) were developed as described in Supplementary Material.
S4 flows to S5 (the fifth state variable in Figure 1) after an incubation period of 12 days (range 7–18 days) for leaves and 18 days (range 11–25 days) for shoots. Because clear information has not been published (Erincik et al., 2002; Nita et al., 2006b), we defined the incubation period empirically, as shown in Supplementary Material.
Model Output
Starting from budbreak of the vines (GS = 01), the model produces the following daily outputs: the proportion of the seasonal dose of conidia that (i) is not yet mature (and will mature later in the season), i.e., k-S2; (ii) is dispersed to plants at each dispersal event, i.e., S3; and (iii) causes infection on leaves and shoots at each infection event, i.e., S4. The accumulation of S5 over the season provides an estimate of disease progress.
Model Validation
Vineyards and Disease Assessment
Disease assessments for model validation were carried out in four Italian vineyards during two growing seasons (2019 and 2020) and in three Montenegrin vineyards during one growing season (2020), for a total of 11 epidemics (Table 2).
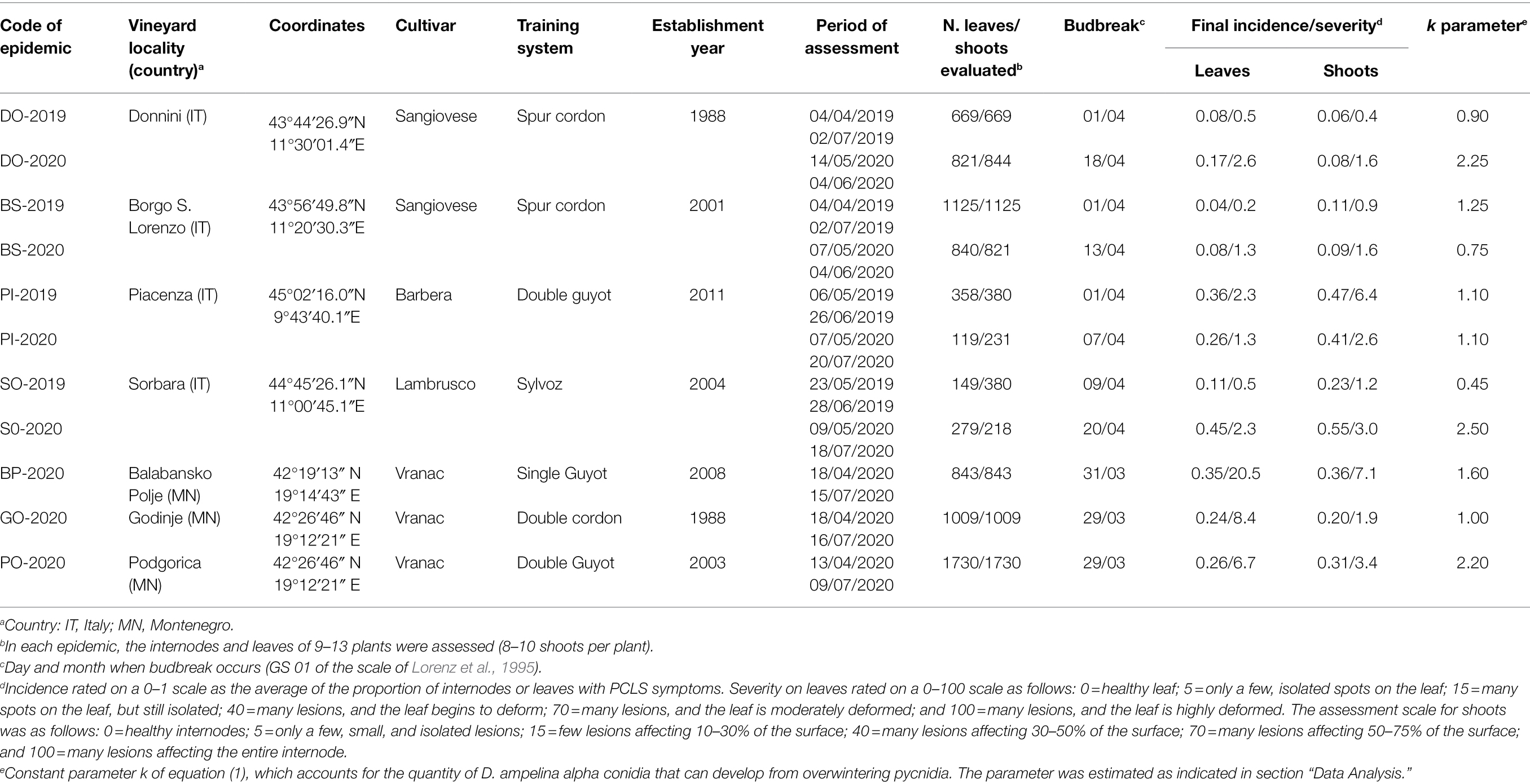
Table 2. Summary of the characteristics of the vineyards used to validate the Diaporthe ampelina model.
All of the vineyards had a history of PCLS symptoms and positive detection of D. ampelina (Guarnaccia et al., 2018; González-Domínguez et al., 2021). The vineyards were managed following standard practices, and no treatments for control of PCLS were applied. The weather stations were located at 2 m above the ground in the vineyards and provided hourly records of air temperature (T, °C), relative humidity (RH, %), rainfall (R, mm), and leaf wetness duration (WD, min). iMeteos stations (Pessl Instruments GmbH, Weiz, Austria) were used in Italy, and Vantage Pro2 stations (Davis Instruments, Hayward, CA, United States) were used in Montenegro.
Starting from budbreak, the vineyards were inspected to determine (i) the prevalent growth stage of the vines based on the scale of Lorenz et al. (1995) and (ii) the incidence and severity of PCLS on leaves and shoots. In 2019, the assessments were performed until the end of June (80–90 days after budbreak, DAB); based on the observations of 2019, the assessments were extended until late July (100–110 DAB) in 2020 (Table 2). Each year, 8 to 10 shoots were tagged on 9 to 13 plants per vineyard, and all internodes and leaves were observed at 7- to 14-day intervals; in total, 2,554 and 2,114 internodes, and 2,059 and 2,301 leaves were assessed in 2019 and 2020, respectively (Table 2). Disease severity on leaves was rated on a 0–100 scale with interval continuity as follows: 0 = disease-free leaf; 5 = only a few, isolated spots on the leaf; 15 = many spots on the leaf, but still isolated; 40 = many lesions, and the leaf has begun to deform; 70 = many lesions, and the leaf is moderately deformed; and 100 = many lesions, and the leaf is heavily deformed. Similarly, disease severity on shoot internodes was assessed as follows: 0 = disease-free; 5 = a few, small, and isolated lesions; 15 = a few lesions affecting 10–30% of the internode surface; 40 = many lesions affecting 30–50% of the internode surface; 70 = many lesions affecting 50–75% of the internode surface; and 100 = many lesions affecting the entire internode.
Observed disease incidence on leaves (DIL) and shoots (DIS) was calculated on a 0–1 scale at each assessment date as the average proportion of internodes or leaves with PCLS symptoms, respectively, irrespective of disease severity (Madden et al., 2007). Observed disease severity was calculated by averaging the ratings of leaves (DSL) and all internodes (DSS).
Data Analysis
A first analysis was conducted to evaluate the ability of the model to correctly predict the progress of PCLS incidence in the different epidemics. For this purpose, the model was operated by using the weather data starting from grapevine budbreak (i.e., GS = 01). Because we lacked information on the inoculum dose in the different vineyards and years, the modulator k in equation (1) was estimated empirically based on observed PCLS incidence at the end of the season (Table 2). In a first step, we ran the model with k = 1 so as to obtain a final value of S5 (k = 1) for leaves and shoots; in a second step, we rescaled this final value to the final PCLS incidence in the vineyard (DIL and DIS). For instance, for epidemic PI-2019 (Table 2), the sum of final disease incidence for leaves (0.370) and shoots (0.470) was 0.830 and S5(k = 1) = 0.742; therefore, k was estimated as: 0.830/0.742 = 1.10. The root mean square deviation (RMSD), the coefficient of residual mass (CRM), and the concordance correlation coefficient (CCC) were calculated (Nash and Sutcliffe, 1970; Lin, 1989; Madden et al., 2007; Piñeiro et al., 2008). For CCC calculation, the epi.ccc function of the “epiR” package in R v. 4.0.4 (R Core Team, 2022; Stevenson, 2022) was used. The squared sum of predictive error of a linear regression of observed PCLS incidence on leaves or shoots (DIL or DIS) versus predicted data (S5 on leaves or S5 on shoots) was decomposed by calculating Theil’s partial inequality (Theil’s U statistic) coefficients, which distinguish between different sources of predictive error: Ubias is the proportion of predictive error associated with the mean differences between the observed and predicted; Uslope is the proportion of predictive error associated with the deviations from the 1:1 line; and Uerror is the proportion of predictive error associated with the unexplained variance. The sum of these coefficients is 1 (Smith and Rose, 1995; Piñeiro et al., 2008).
A second analysis was conducted (i) to evaluate the model’s ability to predict D. ampelina infection events and (ii) to determine the best thresholds of model output for predicting infection events. For this purpose, the model was operated as indicated before, with k = 1 for any epidemic. A receiver operating characteristic (ROC) curve analysis was conducted using the roc function of the “pROC” package (Robin et al., 2011). For either leaves or shoots, field assessments were categorized as either 0 (no increase of PCLS severity relative to the previous assessment) or 1 (increase in PCLS severity relative to the previous assessment). Model outputs were in turn categorized as 0 or 1 depending on whether or not the accumulated values of INFRL or INFRS in an incubation period between two assessments were > 0, with the incubation periods of 12 days for leaves and 18 days for shoots. The ROC analysis measures the accuracy of different cut-off values as predictors; in this work, the cut-off values were the different values of the model outputs associated to all the field assessment performed in the 11 epidemics. ROC curves plot the specificity values (true negative proportion of cases; i.e., the cases in which no infection was predicted by the model and no disease was observed in the field) and 1-sensitivity values (true positive proportion of cases; i.e., the cases in which disease was predicted by the model and was observed in the field) associated with all of the possible cut-off thresholds of the model output (Madden, 2006; Madden et al., 2007). The best threshold is the point of the ROC curve closest to the upper left corner, i.e., the threshold with the combination of highest specificity and sensitivity. The area under the ROC curve (AUROC) and its confidence interval (CI) were also calculated; AUROC values between 0.5 and 1 indicate that the model output is a good predictor of the increase in PCLS in the field.
Results of Model Validation
In the Italian vineyards, the spring of 2019 was mild and rainy, with an average temperature ranging from 15.1 to 17.0°C (minimum 7.7–8.5°C and maximum 26.4–33.2°C), and with >250 mm of rain accumulated from April to June. Hours of wetness accumulated in spring ranged from 426 h in PI-2019 to 759 h in SO-2019 (Supplementary Figure 1). Observed PCLS incidence was higher in PI-2019 (the proportion of leaves and shoots affected on a 0–1 scale was 0.36 and 0.47, respectively) than in SO-2019 (0.11 and 0.23), BS-2019 (0.04 and 0.11), or DO-2019 (0.08 and 0.06). Similarly, observed PCLS severity was higher in PI-2019 (2.3 and 6.4% of the leaf and shoot area with symptoms, respectively) than in SO-2019 (0.5 and 1.2%), BS-2019 (0.2 and 0.9%), or DO-2019 (0.5 and 0.4%; Table 2).
In 2020, the weather was drier in Piacenza than in the other Italian vineyards; in PI-2020, there were 172 mm of rain and only 276 h of accumulated wetness; and in the other vineyards, there were > 190 mm of rain and > 500 h of wetness. In BS-2020, there were > 800 h of wetness. Consequently, the observed PCLS incidence and severity were lower in 2020 than in 2019 in PI-2020 but were higher in 2020 than in 2019 for the other vineyards (Table 2).
In the Montenegrin vineyards, the spring of 2020 was less rainy than in the Italian vineyards, with 100–140 mm rain and 445–694 h of wetness; temperatures were mild and similar in vineyards from both countries (Supplementary Figure 2). PCLS was overall higher in the Montenegrin than in the Italian vineyards, probably due to a higher level of D. ampelina inoculum in the former vineyards. PCLS incidence ranged from 0.26 to 0.35 for leaves and from 0.20 to 0.36 for shoots; PCLS severity ranged from 7.0 to 3.9% for leaves and shoots, respectively, in PO-2020; from 8.4 to 1.9% in GO-2020; and from 20.5 to 7.0% in BP-2020 (Table 2).
Figures 2–4 show examples of the model output for the epidemics of PI-2020, DO-2020, and GO-2020, respectively. In PI-2020, budbreak occurred in early April, and the inoculum was predicted to be present between April and the end of July (Figure 2B); in this period, 19 infection events were predicted (orange bars in Figure 2B). In DO-2020, budbreak of the vines occurred in mid-April, and most of the inoculum was dispersed before mid-June; infections were mainly predicted in mid-May and mid-June (Figure 3B). In GO-2020, budbreak of the vines occurred in early April, and most of the inoculum was dispersed between mid-April and mid-May; infections were predicted in early May and early June (Figure 4B).
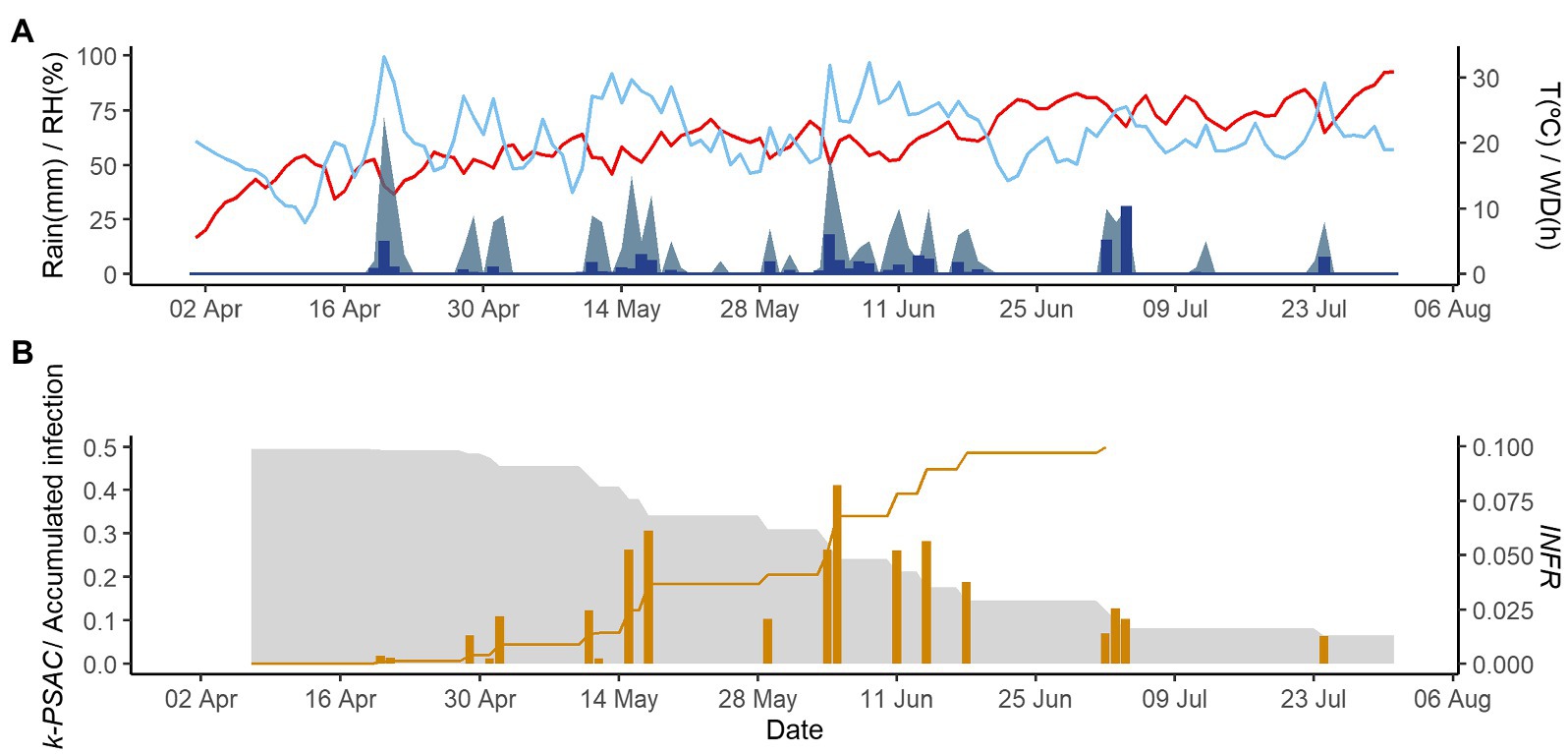
Figure 2. Model output for the vineyard in Piacenza in 2020. (A) Weather data: air temperature (T, red line in °C), rain (blue bars in millimeters), wetness duration (WD, light blue area in hours), and relative humidity (RH, blue line in %). (B) The gray is represent the inoculum dose as k-MATR (gray area), daily values of predicted infection (orange bars; average value of INFRL and INFRS), and accumulated infection during the season (orange line).
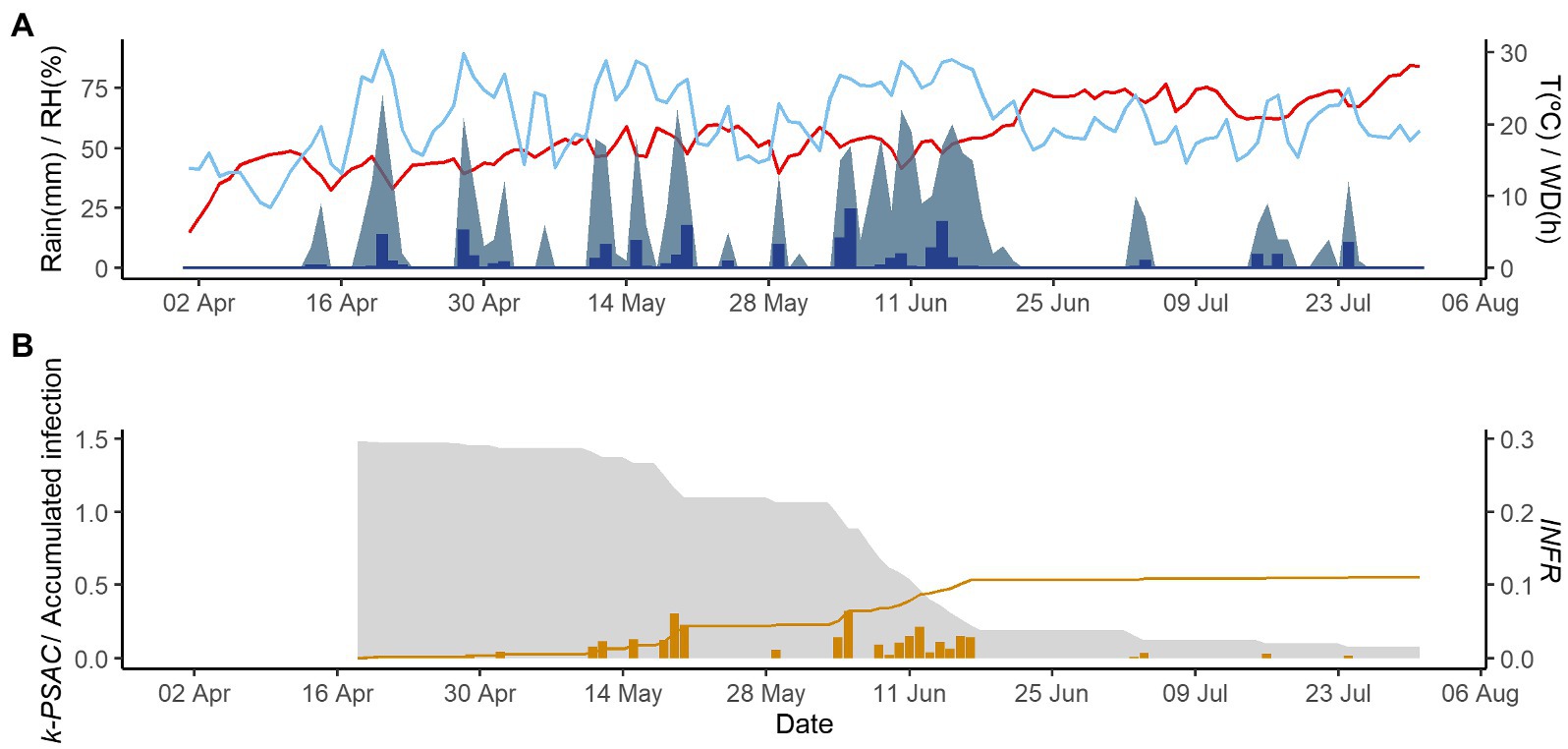
Figure 3. Model output of PCLS for the vineyard in Domini in 2020. (A) Weather variables: air temperature (T, red line in °C), rain (blue bars in millimeters), leaf wetness duration (WD, light blue area in hours), and relative humidity (RH, blue line in %). (B) Inoculum dose as k-MATR (gray area), daily values of predicted infection (orange bars; average value of INFRL and INFRS), and accumulated infection during the season (orange line).
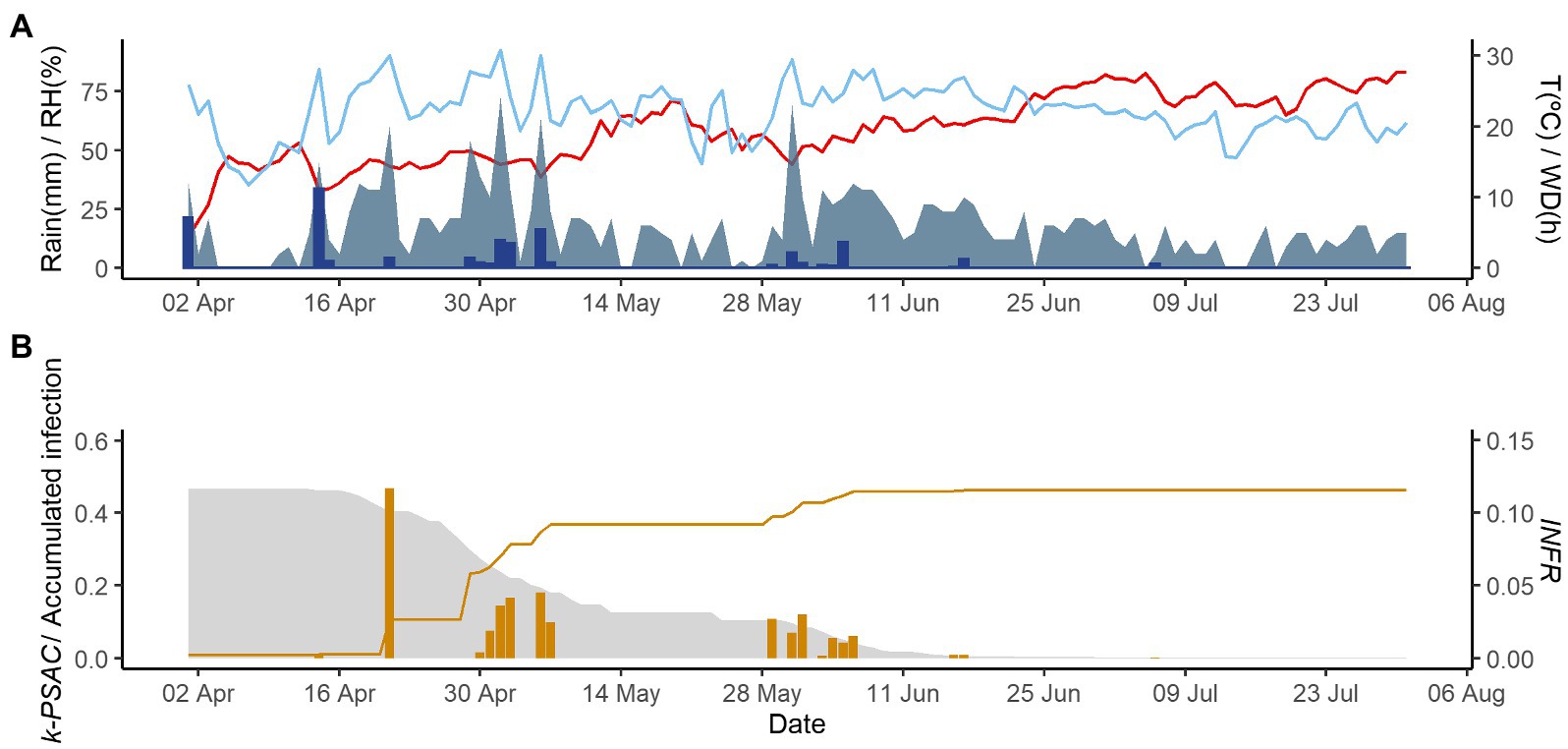
Figure 4. Model output of PCLS for the vineyard in Godijne in 2020. (A) Weather variables: air temperature (T, red line in °C), rain (blue bars in millimeters), leaf wetness duration (WD, light blue area in hours), and relative humidity (RH, blue line in %). (B) Inoculum dose as k-MATR (gray area), daily values of predicted infection (orange bars; average value of INFRL and INFRS), and accumulated infection during the season (orange line).
Examples of model validation for two epidemics are provided in Figure 5; gray dots show disease assessments, lines are model outputs for disease progress (as S5 on leaves and shoots), and dotted lines show the prediction interval based on the variability in the incubation length. Most of the observed PCLS incidence was within the prediction range of the model. In BP-2020, the model correctly predicted the substantial increase of PCLS incidence in shoots with values ranging from 0.11 at the beginning of May, to 0.21 at mid-May, and to 0.31 at the beginning of June; after that, the model correctly predicted the slow increase of PCLS incidence to a value of 0.36 at the end of July (Figure 5A). For leaves, disease increase in BP-2020 in early May was underestimated, but then, the model correctly predicted the PCLS incidence from mid-May to mid-July (Figure 5B). In PI-2020, the pattern of observed PCLS incidence differed on leaves vs. shoots, and the model correctly predicted these differences. For shoots, the model predicted an increase from the beginning of June (PCLS incidence of 0.104) to mid-July (PCLS incidence of 0.407), and this pattern was consistent with observed data (Figure 5C). For leaves, the model predicted the increase of PCLS symptoms mostly on June (PCLS incidence ranging from 0.084 to 0.220), and this pattern was also consistent with observed data (Figure 5D); although the model underestimate PLCS incidence predictions from mid-June to the end of the season, this underestimation was always <0.05, with average of 0.012 (on a 0 to 1 scale).
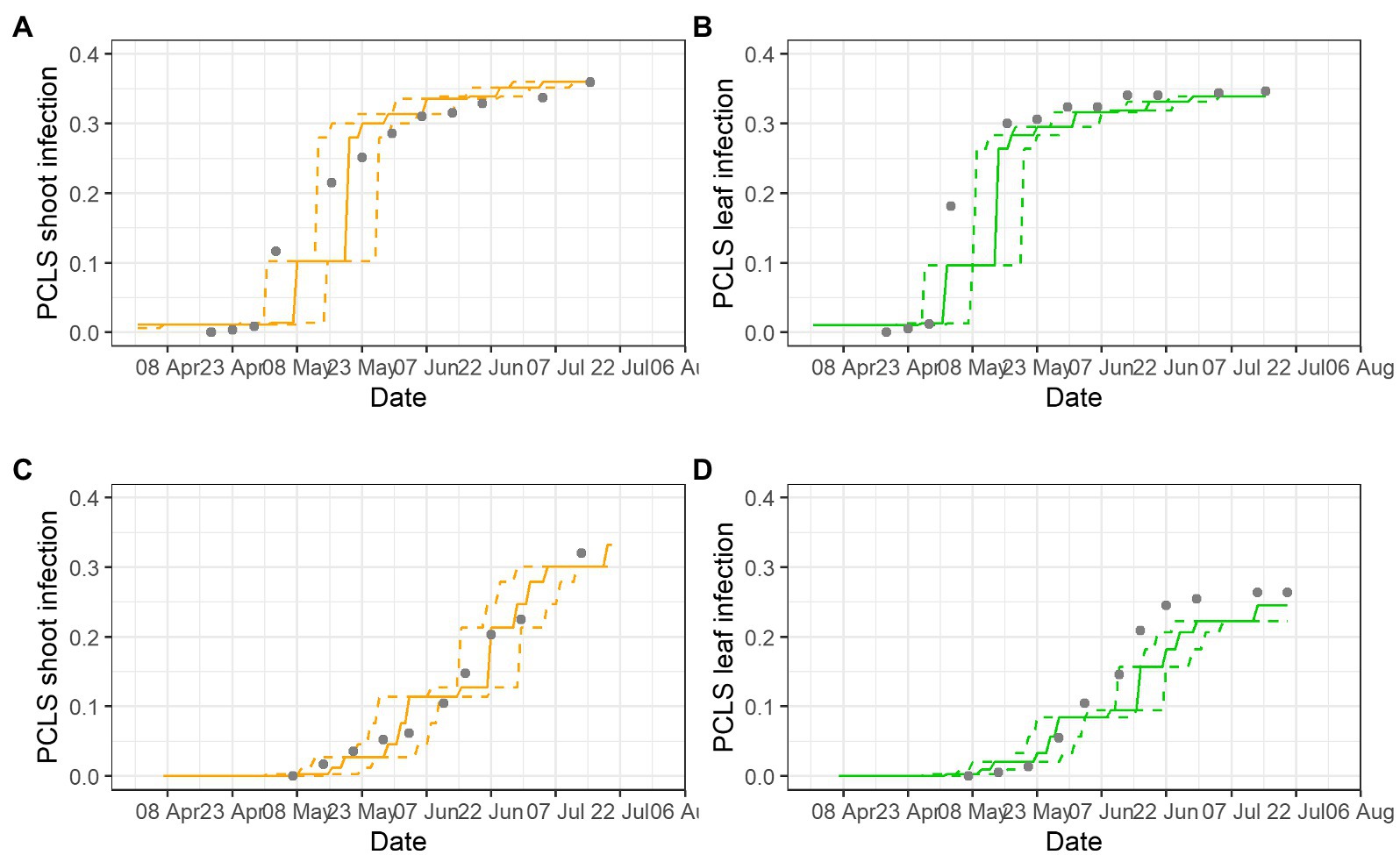
Figure 5. Comparison of model output and PCLS incidence observed on shoots (A,C) and leaves (B,D) in epidemics BP-2020 (A,B) and PI-2020 (C,D). Dots represent the observed PCLS incidence; lines represent the predicted disease progress on leaves (unbroken green lines) or shoots (unbroken orange lines), and their prediction interval based on the variability of the incubation length (dotted lines).
In our comparisons between observed and predicted data for PLCS infection in all 11 epidemics, the values of CCC were 0.913 for leaves and 0.937 for shoots, and values for RMSD and CRM were always <0.09 (Table 3). As indicated by Theil’s statistic, most of the errors were associated with unexplained variance (73% for leaves and 90% for shoots), although a higher proportion of errors was attributed to bias in the case of leaves (26%) than in the case of shoots (7%). When both datasets (leaves and shoots) were merged, the CCC was 0.925, RMSD was 0.055, and CRM was 0.005. The Theil’s statistic showed that 88% of the errors corresponded to unexplained variance, indicating that the model did not have a pattern of over- or underestimating predictions (Table 3).
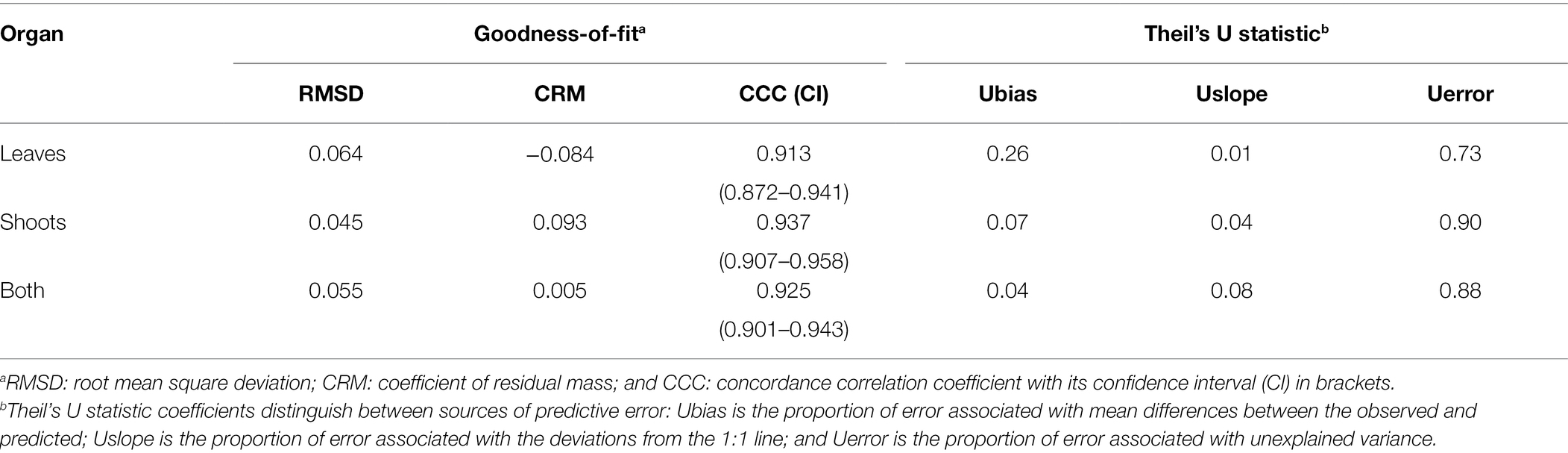
Table 3. Overall goodness-of-fit and Theil’s U statistic for the PCLS infection (of leaves, shoots, and both leaves and shoots) predicted by the model and the disease incidence observed in the vineyards of Table 2.
Regarding the ROC analysis, the use of the model for predicting D. ampelina infection events resulted in an AUROC value of 0.716 (CI 0.6110–0.822) for leaves and 0.749 (CI 0.655–0.843) for shoots, in both cases with CI values >0.5. For both shoots and leaves, the best cutting point of the curve (the part of the curve that is closest to the upper left corner, i.e., the point with the highest specificity and sensitivity) was at INFRL or INFRS = 0.013, which can then be considered the best predictor of D. ampelina infection.
Discussion
In this study, we developed a mechanistic model of D. ampelina infection by using previously reported data and knowledge obtained by in planta experiments conducted under controlled conditions (Erincik et al., 2001, 2003) and by field experiments conducted in multiple years and locations (Anco et al., 2012, 2013; González-Domínguez et al., 2021). The model is dynamic, because its predictions are driven by weather and other external variables (Teng et al., 1980; Leffelaar, 1993; Rossi et al., 2010).
Our model can also be considered to be pathogen-focused, because it uses systems analysis (Leffelaar, 1993; Rossi et al., 2010) to describe and simulate the main stages of the D. ampelina life cycle that lead to disease onset and progress during the grape-growing season. These stages include (i) the overwintering and maturation of pycnidia on affected canes and rachises, (ii) the dispersal of alpha conidia to leaves and shoots, (iii) the infection, and (iv) the onset of disease symptoms.
The model was validated with an independent dataset (i.e., data not used in model development) of 11 PCLS epidemics. In this validation, we compared the observed versus the predicted PCLS incidence; the high CCC values (>0.91) and the low weight of errors (RMSD<0.06 and CRM < 0.09) indicate that the model correctly predicts PCLS development in vineyards. The decomposition of the errors indicated that most errors could be attributed to unexplained variance rather than to model bias (Smith and Rose, 1995; Piñeiro et al., 2008). Because the dataset used for validation included multiple locations and years, with a wide range of climatic conditions, grapevine cultivars, agronomical management, and disease incidence and severity, the model may be considered accurate (i.e., it provides predictions close to reality) and robust (i.e., it provides accurate predictions under a range of environmental, agronomical, and epidemiological conditions; Rossi et al., 2010). The ROC curve analysis confirmed the ability of the model to predict the increase of PCLS severity in the field, indicating that it could be used by growers for risk-based decision making for disease control (Zadoks and Rabbinge, 1989; Madden, 2006).
Although the model was mainly developed based on well-documented data and a well-documented understanding of the disease, we needed to make assumptions because of knowledge gaps regarding the quantification of primary inoculum and the effect of the environment on the length of the incubation period.
For the primary inoculum, the model modulates the primary inoculum dose in a vineyard through the k parameter of equation (1). This parameter depends on several difficult-to-estimate factors, including the incidence and severity of PCLS on shoots in the previous season, the proportion of affected shoots remaining on the trellis after pruning, and the effect of the environment on the production of new pycnidia during fall and winter (Anco et al., 2013; Wilcox et al., 2015; González-Domínguez et al., 2021). For a precise parametrization of k, the effect of disease, environment, and agronomical practices on the dose of inoculum inherited from the previous season should be studied and incorporated in the model (Leffelaar, 1993). Alternatively, methods should be developed for a precise quantification of the potential inoculum, as has been done for ascospores of Venturia inaequalis in apple leaf residue (parameter “PAD” on MacHardy and Jeger, 1983) or for oospores of Plasmopara viticola in grape leaf residue (Si Ammour et al., 2019). To validate the model’s ability to predict disease progress in the current research, we estimated k empirically in the different vineyards and years, with values ranging 0.45–2.25 (Table 2). The magnitude of k should affect the magnitude of model predictions, but not the shape of the predicted disease progress; therefore, the model validation in terms of predicted versus observed PCLS incidence is valid regardless the magnitude of k. In addition, the model’s ability to correctly predict infection periods was validated by setting k = 1 in a ROC analysis, as has been previously done for other models (Rossi et al., 2008; Ji et al., 2021). Therefore, in the absence of more precise information about the inoculum dose in a vineyard, the model can be used to predict PCLS infection events during the season by using k = 1.
For the length of the incubation period, the model assumes a number of days from infection to the appearance of symptoms on leaves or shoots (Erincik et al., 2002; Nita et al., 2006b). It is well known that D. ampelina growth and incubation length are influenced by environmental conditions (Wilcox et al., 2015); for instance, the growth of the fungus on potato dextrose agar is influenced by temperature, with cardinal temperatures of 5–35°C and an optimum of 20°C (González-Domínguez et al., 2021). In a preliminary analysis, however, we found that the number of days provided a better estimate of the incubation length in vineyards than temperature-dependent equations (see Supplementary Material). For a better estimate of the temperature-dependent length of incubation, studies should be conducted with artificial inoculation of D. ampelina alpha conidia in planta and with subsequent incubation at different temperatures. For decision making for the control of a monocyclic disease like PCLS, however, a precise estimation of the incubation length may be much less important than the correct prediction of infection; this is because the onset of a monocyclic disease is not related to the production of secondary inoculum that can generate new infections during the season.
The model was validated in vineyards with no fungicide treatments against PCLS; fungicides were, however, used to control other diseases; and for example, azoxystrobin, cymoxanil, folpet, metiram, or zimoxanile were used to control downy mildew in some cases. These treatments should have had only minor effects or no effect on our model validation for three reasons: (i) these fungicides are not able to completely control D. ampelina (Nita et al., 2006b; Wilcox et al., 2015); (ii) the possible effects of these fungicides were to some extent accounted for in the empirical estimation of the k parameter; and (iii) our prediction of infection events was qualitative (infected or not infected) rather than quantitative (i.e., we did not predict the severity of disease severity resulting from an infection).
In conclusion, by using the threshold of the model output that minimizes the proportion of false positives/false negatives (Madden, 2006), the model presented here may correctly predict D. ampelina infection events in vineyards. It follows that the model has the potential to guide the scheduling of fungicide applications for the control of PCLS in vineyards (Rossi et al., 2019). The practical use of the model should be confirmed by specific experiments that compare risk-based (model-based) application of fungicides with the usual calendar-based application of fungicides (Rossi et al., 2019, 2021). Experiments should also be conducted to find new ways to control PCLS; the ban of mancozeb in the EU and the increasing worldwide restrictions on the use of the broad-spectrum fungicides that are commonly used against PCLS represent new challenges in the management of the disease (Topping et al., 2020; Duarte et al., 2021; Rossi et al., 2021). QoI and DMI fungicides have been tested in only a few experiments, with inconsistent results, and almost no information exists about biological control agents (Wilcox et al., 2015).
Once it practically is evaluated, the model could be easily integrated into vite.net,1 a decision support system (DSS) that already includes models of downy and powdery mildews, Botrytis bunch rot, and black rot (González-Domínguez et al., 2020). The integration of the model into this DSS could greatly improve IPM in the vineyard by helping growers determine when a fungicide application or other treatment is needed based on the processes (predicted by epidemiological models) that lead to the development of multiple diseases (Rossi et al., 2019).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
EG-D, TC, and VR contributed to conception and design of the model. AP, LM, LL, NL, and JL obtained the field and weather data and organized the database for model validation. EG-D and VR wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The Project “Share web-based Decision Support Systems to increase the sustainability of the viticultural sector in Montenegro and Italy (VITISUST)” started in August 2018 and is supported by Foreign Affairs Ministry (IT) and Ministry of Science (ME) within the International collaboration framework between Italy and Montenegro.
Conflict of Interest
EG-D was employed by Horta s.r.l., a spin-off company of the Università Cattolica del Sacro Cuore (Piacenza, Italy).
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2022.872333/full#supplementary-material
Footnotes
References
Anco, D. J., Madden, L. V., and Ellis, M. A. (2012). Temporal patterns of sporulation potential of Phomopsis viticola on infected grape shoots, canes, and rachises. Plant Dis. 96, 1297–1302. doi: 10.1094/PDIS-09-11-0806-RE
Anco, D. J., Madden, L. V., and Ellis, M. A. (2013). Effects of temperature and wetness duration on the sporulation rate of Phomopsis viticola on infected grape canes. Plant Dis. 97, 579–589. doi: 10.1094/PDIS-07-12-0666-RE
Baumgartner, K., Fujiyoshi, P. T., Castlebury, L. A., Wilcox, W. F., and Rolshausen, P. E. (2013). Characterization of species of Diaporthe from wood cankers of grape in eastern north american vineyards. Plant Dis. 97, 912–920. doi: 10.1094/PDIS-04-12-0357-RE
Caffi, T., Latinović, N., Latinović, J., Languasco, L., Gonzalez-Dominguez, E., and Rossi, V. (2020). A dynamic mechanistic model for Diaporthe ampelina for enhancing IPM in Montenegrin and Italian viticulture, VITISUST Project. WPRS Bull. 154, 106–112.
Caffarra, A., and Eccel, E. (2011). Projecting the impacts of climate change on the phenology of grapevine in a mountain area. Australian Journal of Grape and Wine Research 17, 52–61. doi: 10.1111/j.1755-0238.2010.00118.x
Duarte, D., Gaspar, C., Galhano, C., and Castro, P. (2021). “Biopesticides and sustainability in a land use context,” in Sustainability in Natural Resources Management and Land Planning. eds. W. Leal Filho, U. M. Azeiteiro, and A. F. F. Setti (Berlin: Springer-World Sustainability Series), 111–133.
Epstein, L. (2014). Fifty years Since silent spring. Annu. Rev. Phytopathol. 52, 377–402. doi: 10.1146/annurev-phyto-102313-045900
Erincik, O., Madden, L. V., Ferree, D. C., and Ellis, M. A. (2001). Effect of growth stage on susceptibility of grape berry and rachis tissues to infection by Phomopsis viticola. Plant Dis. 85, 517–520. doi: 10.1094/PDIS.2001.85.5.517
Erincik, O., Madden, L. V., Ferree, D. C., and Ellis, M. A. (2002). Infection of grape berry and rachis tissues by Phomopsis viticola. Plant. Health. Prog. 3:8. doi: 10.1094/PHP-2002-0702-01-RS
Erincik, O., Madden, L. V., Ferree, D. C., and Ellis, M. A. (2003). Temperature and wetness-duration requirements for grape leaf and cane infection by Phomopsis viticola. Plant Dis. 87, 832–840. doi: 10.1094/PDIS.2003.87.7.832
Gomes, R. R., Glienke, C., Videira, S. I. R., Lombard, L., Groenewald, J. Z., and Crous, P. W. (2013). Diaporthe, a genus of endophytic, saprobic and plant pathogenic fungi. Persoonia 31, 1–41. doi: 10.3767/003158513X666844
González-Domínguez, E., Caffi, T., Languasco, L., Latinovic, N., Latinovic, J., and Rossi, V. (2021). Dynamics of Diaporthe ampelina conidia released from grape canes that overwintered in the vineyard. Plant Dis. 105, 3092–3100. doi: 10.1094/PDIS-12-20-2639-RE
González-Domínguez, E., Legler, S. E., Reyes-Aybar, J., Giralt, L., Val, C., and Rossi, V. (2020). The experience of application of a web-based decision support system in the Iberian Peninsula for the sustainable management of vineyards. IOBC WPRS Bull. 154, 88–92.
Guarnaccia, V., Groenewald, J. Z., Woodhall, J., Armengol, J., Cinelli, T., Eichmeier, A., et al. (2018). Diaporthe diversity and pathogenicity revealed from a broad survey of grapevine diseases in Europe. Persoonia 40, 135–153. doi: 10.3767/persoonia.2018.40.06
Hewit, W. B., and Pearson, R. C. (1988). “Phomopsis cane and leaf spot,” in Compendium of Grape Diseases. eds. R. C. Pearson and A. C. Goheen (United States: The American Phytopathological Society), 17–18.
Ji, T., Salotti, I., Dong, C., Li, M., and Rossi, V. (2021). Modeling the effects of the environment and the host plant on the ripe rot of grapes, caused by the Colletotrichum species. Plan. Theory 10:2288. doi: 10.3390/plants10112288
Lawrence, D. P., Baumgartner, K., Travadon, R., and Baumgartner, K. (2015). Diversity of Diaporthe species associated with wood cankers of fruit and nut crops in northern California. Mycologia 107, 926–940. doi: 10.3852/14-353
Leffelaar, P.A. (1993). On Systems Analysis and Simulation of Ecological Processes with Examples in CSMP and FORTRAN. Berlin: Springer.
Lin, L. (1989). A concordance correlation coefficient to evaluate reproducibility. Biometrics 45, 255–268.
Lorenz, D. H., Eichhorn, K. W., Bleiholder, H., Klose, R., Meier, U., and Weber, E. (1995). Phenological growth stages of the grapevine (Vitis vinifera L. ssp. vinifera)- codes and descriptions according to the extended BBCH scale. Aust. J. Grape Wine Res. 1, 100–103. doi: 10.1111/j.1755-0238.1995.tb00085.x
Lovell, D. J., Powers, S. J., Welham, S. J., and Parker, S. R. (2004). A perspective on the measurement of time in plant disease epidemiology. Plant Pathol. 53, 705–712. doi: 10.1111/j.1365-3059.2004.01097.x
MacHardy, W. E., and Jeger, M. J. (1983). Integrating control measures for the management of primary apple scab, Venturia inaequalis (Cke.). Wint. Prot. Ecol. 5, 103–125.
Madden, L. V. (2006). Botanical epidemiology, some key advances and its continuing role in disease management. Eur. J. Plant Pathol. 115, 3–23. doi: 10.1007/s10658-005-1229-5
Madden, L.V., Hughes, G., and Van den Bosch, F. (2007). The Study of Plant Disease Epidemics. United States: The American Phytopathological Society.
Merrin, S. J., Nair, N. G., and Tarran, J. (1995). Variation in Phomopsis recorded on grapevine in Australia and its taxonomic and biological implications. Aust. Plant Pathol. 24, 44–56. doi: 10.1071/APP9950044
Molitor, D., and Junk, J. (2019). Climate change is implicating a two-fold impact on air temperature increase in the ripening period under the conditions of the Luxembourgish grapegrowing region. Oeno One 53, 409–422. doi: 10.20870/oeno-one.2019.53.3.2329
Mostafalou, S., and Abdollahi, M. (2017). Pesticides, an update of human exposure and toxicity. Arch. Toxicol. 91, 549–599. doi: 10.1007/s00204-016-1849-x
Mostert, L. (2000). The Characterization and Control of Phomopsis Cane and Leaf Spot on Vine. M.S. Thesis. SudAfrica: University of Stellenbosch.
Nash, J., and Sutcliffe, J. (1970). River flow forecasting through conceptual models part I. J. Hidrol. 10, 282–290. doi: 10.1016/0022-1694(70)90255-6
Nita, M., Ellis, M. A., and Madden, L. V. (2008). Variation in disease incidence of Phomopsis cane and leaf spot of grape in commercial vineyards in Ohio. Plant Dis. 92, 1053–1061. doi: 10.1094/PDIS-92-7-1053
Nita, M., Ellis, M. A., Wilson, L. L., and Madden, L. V. (2006a). Effects of application of fungicide during the dormant period on Phomopsis cane and leaf spot of grape disease intensity and inoculum production. Plant Dis. 90, 1195–1200. doi: 10.1094/PD-90-1195
Nita, M., Ellis, M. A., Wilson, L. L., and Madden, L. V. (2006b). Evaluation of a disease warning system for Phomopsis cane and leaf spot of grape, a field study. Plant Dis. 90, 1239–1246. doi: 10.1094/PD-90-1239
Pezet, P. R. (1974). Methode favorisant la production de spores de type beta chez Phomopsis viticola Sacc. J. Phytopathol. 79, 67–76. doi: 10.1111/j.1439-0434.1974.tb02690.x
Phillips, A. J. L. (2000). Excoriose, cane blight and related diseases of grapevines, a taxonomic review of the pathogens. Phytopathol. Mediterr. 39, 341–356. doi: 10.14601/Phytopathol_Mediterr-1583
Pscheidt, J., and Pearson, R. (1991). Phomopsis cane and leaf spot of grape. Cornell Cooperative Extension 6, 1–2.
Piñeiro, G., Perelman, S., Guerschman, J., and Paruelo, J. (2008). How to evaluate models, observed vs. predicted or predicted vs. observed? Ecol. Model. 216, 316–322. doi: 10.1016/j.ecolmodel.2008.05.006
R Core Team (2022). R, A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Robin, X., Turck, N., Hainard, A., Tiberti, N., Lisacek, F., Sanchez, J., et al. (2011). pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 12:77. doi: 10.1186/1471-2105-12-77
Rossi, V., Caffi, T., Giosuè, S., and Bugiani, R. (2008). A mechanistic model simulating primary infections of downy mildew in grapevine. Ecol. Model. 212, 480–491. doi: 10.1016/j.ecolmodel.2007.10.046
Rossi, V., Caffi, T., Legler, S. E., and Fedele, G. (2021). A method for scoring the risk of fungicide resistance in vineyards. Crop Prot. 143:105477. doi: 10.1016/j.cropro.2020.105477
Rossi, V., Giosuè, S., and Caffi, T. (2010). “Modelling plant diseases for decision making in crop protection,” in Precision Crop Protection–The Challenge and Use of Heterogeneity. eds. E. C. Oerke, R. Gerhards, G. Menz, and R. A. Sikora (Dordrecht: Springer), 241–258.
Rossi, V., Sperandio, G., Caffi, T., Simonetto, A., and Gianni, G. (2019). Critical success factors for the adoption of decision tools in IPM. Agronomy 9:710. doi: 10.3390/agronomy9110710
Schilder, A. M. C., Erincik, O., Castlebury, L., Rossman, A., and Ellis, M. A. (2005). Characterization of Phomopsis spp. infecting grapevines in the Great Lakes region of North America. Plant Dis. 89, 755–762. doi: 10.1094/PD-89-0755
Sergeeva, V., Nair, N. G., Barchia, I., Priest, M., and Spooner-Hart, R. (2003). Germination of β conidia of Phomopsis viticola. Austral. Plant Pathol. 32, 105–107. doi: 10.1071/AP02072
Si Ammour, M., Fedele, G., Morcia, C., Terzi, V., and Rossi, V. (2019). Quantification of Botrytis cinerea in grapevine bunch trash by real-time PCR. Phytopathology 109, 1312–1319. doi: 10.1094/PHYTO-11-18-0441-R
Smith, E. P., and Rose, K. A. (1995). Model goodness-of-fit analysis using regression and related techniques. Ecol. Model. 77, 49–64. doi: 10.1016/0304-3800(93)E0074-D
Stevenson, M. (2022). Epir, an R package for the analysis of epidemiological data. R package version 2.0.41. Available at: https://cran.r-project.org/web/packages/epiR/index.html (Accessed February 09, 2022).
Teng, P. S., Blackie, M. J., and Close, R. C. (1980). Simulation of barley leaf rust epidemic, structure and validation of BARSIM-I. Agric. Syst. 5, 85–103. doi: 10.1016/0308-521X(80)90001-3
Topping, C. J., Aldrich, A., and Berny, P. (2020). Overhaul environmental risk assessment for pesticides. Science 367, 360–363. doi: 10.1126/science.aay1144
Udayanga, D., Liu, X., McKenzie, E. H. C., Chukeatirote, E., Bahkali, A. H., and Hyde, K. D. (2011). The genus Phomopsis, biology, applications, species concepts and names of common phytopathogens. Fungal Divers. 50, 189–225. doi: 10.1007/s13225-011-0126-9
Urbez-Torres, J. R., Peduto Hand, F., Smith, R. J., and Gubler, W. D. (2013). Phomopsis dieback, a grapevine trunk disease caused by Phomopsis viticola in California. Plant Dis. 97, 1571–1579. doi: 10.1094/PDIS-11-12-1072-RE
Wightwick, A., Walters, R., Allinson, G., Reichman, S., and Menzies, N. (2010). “Environmental risks of fungicides used in horticultural production systems,” in Fungicides (Germany: Books on Demand), 273–304.
Wilcox, W. F., Ellis, M. A., Rawnsley, B., Rossman, A., and Pscheidt, J. (2015). “Phomopsis cane and leaf spot of grape,” in Compendium of Grape disease, Disorders, and Pets. eds. W. F. Wilcox, W. D. Gubler, and J. K. Uyemoto (United States: The American Phytopathological Society), 68–72.
Keywords: excoriose, Vitis vinifera, alpha conidia, systems analyses, process-based model
Citation: Gonzalez-Dominguez E, Caffi T, Paolini A, Mugnai L, Latinović N, Latinović J, Languasco L and Rossi V (2022) Development and Validation of a Mechanistic Model That Predicts Infection by Diaporthe ampelina, the Causal Agent of Phomopsis Cane and Leaf Spot of Grapevines. Front. Plant Sci. 13:872333. doi: 10.3389/fpls.2022.872333
Edited by:
David Gramaje, Spanish National Research Council (CSIC), SpainReviewed by:
Dan Anco, Clemson University, United StatesAles Eichmeier, Mendel University in Brno, Czechia
Copyright © 2022 Gonzalez-Dominguez, Caffi, Paolini, Mugnai, Latinović, Latinović, Languasco and Rossi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vittorio Rossi, dml0dG9yaW8ucm9zc2lAdW5pY2F0dC5pdA==
 Elisa Gonzalez-Dominguez
Elisa Gonzalez-Dominguez Tito Caffi
Tito Caffi Aurora Paolini3
Aurora Paolini3 Laura Mugnai
Laura Mugnai Nedeljko Latinović
Nedeljko Latinović Luca Languasco
Luca Languasco Vittorio Rossi
Vittorio Rossi