- 1State Key Laboratory for Biology of Plant Diseases and Insect Pests, Institute of Plant Protection, Chinese Academy of Agricultural Sciences, Beijing, China
- 2Institute of Zoology, Chinese Academy of Sciences, Beijing, China
Ecological niche is a key concept that links species distributions. Ecological niche shifts are expected to affect the potential invasive risk of alien species. Rapistrum rugosum is an invasive agricultural weed in many countries. Wild populations of R. rugosum have been recorded in China, representing a great threat to the regional crops. Based on distribution records from different regions and relevant environmental variables, the present study predicted the potential distribution and estimated the invasive risk of R. rugosum in China. Ecological niche shifts strongly affected the potential invasive risk of R. rugosum in China. The two most important variables were annual temperature range (Bio7) and mean temperature of the coldest quarter (Bio11). The total suitable habitat for the species covered an area of 287.53 × 104km2 and was mainly distributed in Southwest, Southeast, and Central China. Australia, Canada, Brazil, the United States, and Argentina accounted for over 90% of the inspection records of R. rugosum from Chinese entry ports during 2015–2018. The intercepted R. rugosum was frequently mixed in Glycine max (L.) Merr., Hordeum vulgare L., linseed, Triticum aestivum L., and Sorghum bicolor (L.) Moench. Moreover, 80% interceptions were recorded from Tianjin, Guangdong, Nanjing, and Chengdu customs. Climatic conditions do not limit the establishment capability of R. rugosum in China. Our results provide a theoretical reference for the development of monitoring and control measures for this invasive weed.
Introduction
Biological invasions, as part of global change, are considered to be one of the important factors contributing to the decline in global biodiversity as well as high economic losses to the society (Mačić et al., 2018; Essl et al., 2020; Pyšek et al., 2020; Diagne et al., 2021; Pisani et al., 2021; Stinca et al., 2021). In recent years, China has become one of the countries that are most seriously affected by biological invasions worldwide (Yan et al., 2017). To date, more than 600 Invasive Alien Species (IAS) have been identified in China, of which more than 45% were invasive alien plants (Wan et al., 2017). Rapistrum rugosum (L.) All., a new reported invasive alien plant in China, belongs to the Brassicaceae family, and it is a relatively common weed in agricultural fields in wheat- and corn-growing regions (Pardo et al., 2019; Ali et al., 2020). R. rugosum is profusely branched and possesses a well-developed root system. In plots where wheat was mixed with R. rugosum, the former was at a competitive disadvantage, and its growth and yield were severely affected. In Australia, competition from R. rugosum led to 72–78% yield reduction in wheat (Manalil and Chauhan, 2019). R. rugosum can grow up to 1–5-feet-tall and bears a taproot that can become rather large (Lemke and Worthington, 1991). It can successfully outcompete native plant species, forming a vegetative cover of a single species (Manalil et al., 2018). R. rugosum originated in Central Europe, the Mediterranean, northern Africa, and western and temperate Asia (Brown, 1878), from where it has dispersed to the Americas, Oceania, and East Asia (Global Biodiversity Information Facility, 2021). The oval, dark brown, smooth, and minute seeds of R. rugosum can be dispersed over long distances aboard logs, other seeds, and contaminated materials. China has 306 international ports of entry (Cao, 2020). Information from different plant quarantine Customs revealed that over 100 interceptions of R. rugosum have been made each year since 2016. R. rugosum has been detected in the containers and seeds of incoming shipments to China.
Recently, wild populations of R. rugosum were discovered in China, representing a great potential threat to the regional crops. On Chinese mainland, R. rugosum was recorded for the first time in the Xi’an City (Shaanxi Province, northwestern China); it was found growing in patches and showed the tendency to disperse rapidly (Xun et al., 2020). Majority of the recent studies on R. rugosum mainly focused on its biological characteristics (Hichri et al., 2019), herbicide resistance (Hatami et al., 2016), and control measures (Simmons, 2005); however, only a few studies have assessed its global invasive risk in habitats through species distribution modeling. Risk assessment and early warning are the most effective strategies to prevent the introduction and dispersal of IAS (Greenberg et al., 2012). For IAS, a stable ecological niche is an invasive area that is identical to the region of origin (Liu et al., 2020). Meanwhile, a shifted ecological niche is different from the region of origin. However, it is impossible to accurately predict the distribution of IAS in the invasive area based on habitat information of its origin (Fernández and Hamilton, 2015). Although predictions of species distribution modeling are reliable in the model-fitted area, the model simulation capacity must be interpreted cautiously when switching to a new prediction area (Broennimann and Guisan, 2008; Beaumont et al., 2009; Tang et al., 2021). When IAS invade new habitats, they gradually adapt to the given conditions, expand their ecological niche, and adversely affect agroecosystems and biodiversity (Hejda et al., 2015). Thus, ecological niche models cannot predict ecological niches based solely on the information of species origin.
Species distribution models (SDMs) have been playing an increasingly important role in predicting the potential geographic distribution of species, particularly IAS (Srivastava et al., 2019; Thomas et al., 2021). The MaxEnt model uses species distribution records and the corresponding environmental variables in a given habitat, and this model is suitable for predicting the potential geographic distribution of species (Elith et al., 2011; Liu et al., 2017; Adhikari et al., 2019). In recent years, the application of the MaxEnt model has expanded not only to the examination of ecological degradation processes, such as biological invasions (Yeh et al., 2021) and ecological damage (Venne and Currie, 2021), but also to the potential risk assessment of IAS (Simpson and Prots, 2013). “kuenm,” an R package in the ecological niche model (ENM), uses MaxEnt as the modeling algorithm to automate the calibration of models, creation of optimized models and their transfer and evaluations, as well as assessment of extrapolation risks (Cobos et al., 2019). The MaxEnt model, combined with ArcGIS, has been widely used to identify areas at a high risk of IAS invasion (Kariyawasam et al., 2019) and to predict the impacts of climate change on IAS, enabling scientists and policymakers to establish effective and early warning strategies.
To this end, based on the optimized MaxEnt model, related environmental variables, and distribution records of R. rugosum in native, invasive, and native + invasive regions, the present study simulated the invasion risk habitats of R. rugosum in China. For the simulated risk area in China, we speculated that the ecological niche of R. rugosum would shift based on the distribution records of native, invasive, or native + invasive. Therefore, the risk area identified based on the native and invasive distribution records of R. rugosum was integrated as the final result of the invasion risk habitat in China. Further, the environmental variables that significantly affect the invasion risk habitats of R. rugosum in China were clarified. Finally, the specific distribution range in the invasion risk habitats of R. rugosum in China was explored to predict its dispersal risk and propose early warning measures.
Materials and Methods
Distribution Records of Rapistrum rugosum
The distribution records of R. rugosum were collected from the Global Biodiversity Information Facility (GBIF1) and Invasive Species Compendium (ISC) of the Center for Agriculture and Bioscience International (CABI2). A total of 31.585 distribution records were obtained. Duplicate records and distribution points without detailed geographic information were removed using ENMtools (Warren et al., 2010). Regarding the resolution of the environmental variables, only one distribution point was retained within each 5 km × 5 km raster. Finally, a total of 8.938 valid distribution records of R. rugosum were retained. Among these, respectively, 6.259 and 2.679 distribution records were in native and invasive areas (Figure 1).
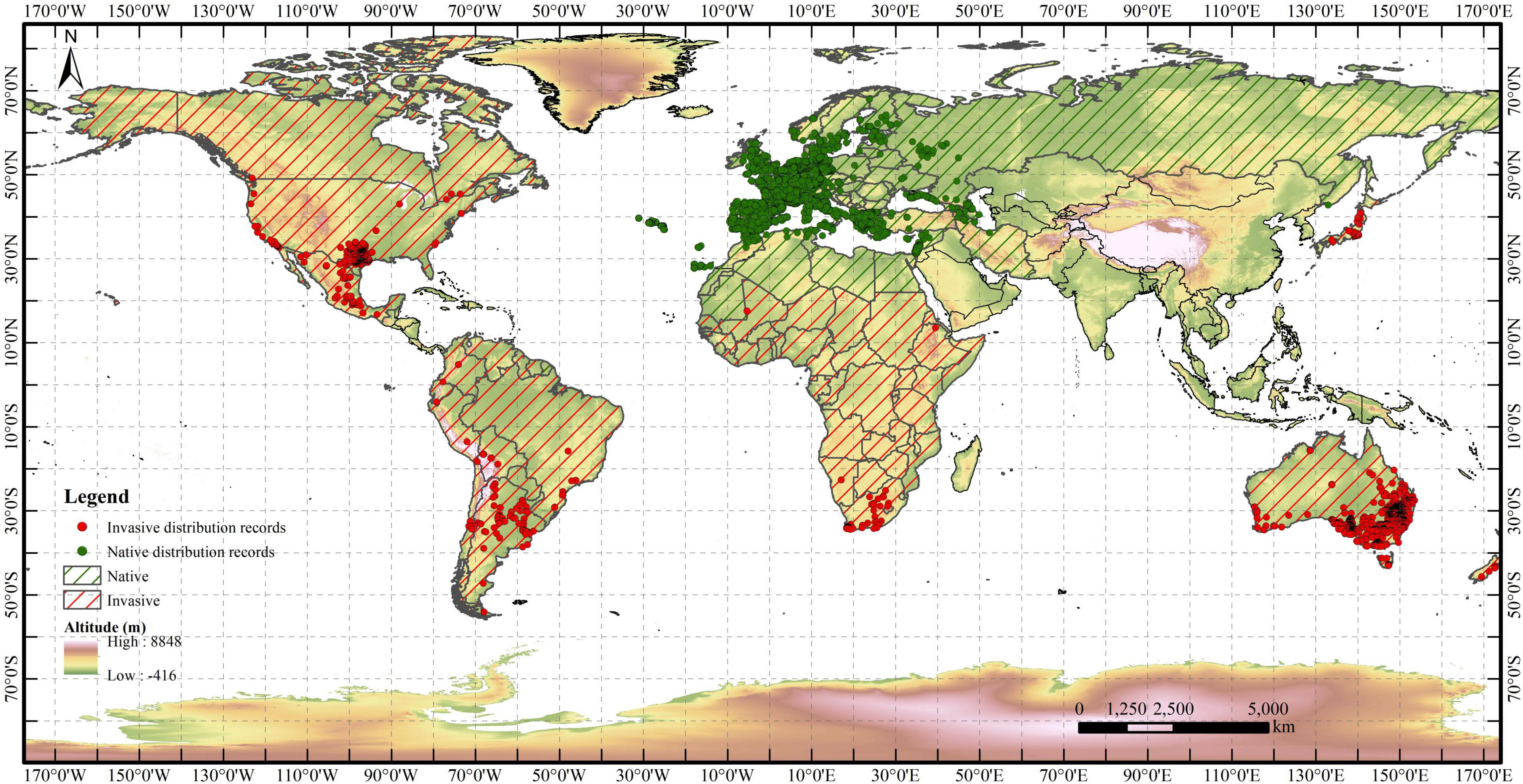
Figure 1. World distribution records of Rapistrum rugosum. Red and green points indicate invasive and native distribution records, respectively.
Environmental Variables, Mapping, and Modeling
Raster files for 19 bioclimatic variables and elevation at a resolution of 2.5′ were downloaded from the World Climate Database Version 2.13. This database includes detailed meteorological information from meteorological stations around the world during 1970–2000. Soil data were obtained from the Harmonized World Soil Database v1.2 (Fischer et al., 2008). Land use data for China in 2020 were downloaded from the Resource and Environment Science and Data Center4. All the data were converted to a resolution of 2.5′ (the same as that of the bioclimatic variables; Supplementary Table 1). The world administrative map was downloaded from the National Earth System Science Data Center, National Science and Technology Infrastructure of China5. MaxEnt 3.4.4 is freely available online6.
Correlation analysis of the 19 bioclimatic variables was performed using ENMtools to eliminate multivariate collinearity (Yang et al., 2013). The bioclimatic variables were selected through two steps: (1) the bioclimatic variables were imported into the MaxEnt model three times, and the bioclimatic variables with zero contribution were removed; and (2) all bioclimatic variables with contribution rates greater than zero were selected for correlation analysis using ENMtools. When the correlation coefficient of two bioclimatic variables was greater than or equal to 0.8 (Supplementary Figure 1), the one with the highest contribution rate was retained. The final environmental variables were retained for MaxEnt modeling (Supplementary Tables 2–4).
MaxEnt Model Calibration
MaxEnt is an ecological niche model based on the theory of maximum entropy based on the Java platform (Phillips et al., 2006). The most important parameters of the MaxEnt model are the feature classes (FCs) and regularization multiplier (RM). FC and RM calibration can significantly improve the prediction accuracy of the MaxEnt model. In the present study, the MaxEnt model calibrated by setting different combinations of FCs and incremental RMs. FCs include five basic parameters, namely linear (L), quadratic (Q), product (P), threshold (T), and hinge (H), and there are 31 different combinations of FCs (Radosavljevic and Anderson, 2014). Generally, RM is set from 0.1 to 4 with an interval of 0.1. A total of 40 RM values were used in the present study. The “kuenm” package in R was used to create 1.240 candidate models. Finally, using R, significant models with the omission rate of >5% and delta Akaike Information Criterion (AICc) value of <2 (Cobos et al., 2019) were selected. The candidate model with the smallest delta AICc was selected for the final analysis.
Model Settings and Evaluation
Following model calibration, 25% of the distribution records were used to test the MaxEnt model, and the remaining 75% were used to train the optimized MaxEnt model. In total, maximum 500 iterations and 10.000 background points were used. The importance of the environmental variables limiting R. rugosum distribution was assessed using the contribution rates and the Jackknife method. The receiver operating characteristic (ROC) curve and area under the curve (AUC) were used to test the accuracy of the model results. The ROC curve is an acceptance curve with the horizontal coordinate indicating the false positive rate (1 - specificity) and the vertical coordinate indicating the true positive rate (1 - omission rate) (Fan et al., 2006). Because the AUC values are not affected by the thresholds, it is an objective assessment of the model. An AUC value closer to 1 indicates that the model results are better. The evaluation criteria of model simulation accuracy were classified into three levels: poor (AUC ≤ 0.50), acceptable (0.5 < AUC ≤ 0.80), and excellent (0.80 < AUC ≤ 1.00) (Swets, 1988).
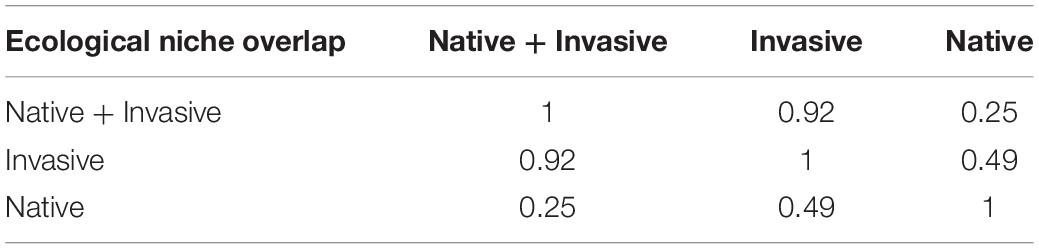
The maximum value of 10 replications was selected as the final MaxEnt model result in the present study. The ASCII raster layers were generated based on the logical value (P) of the presence probability of R. rugosum, ranging from 0 to 1. A higher P values indicates a higher probability of the presence of R. rugosum. The results were converted to a raster file and extracted using the administrative division map of China in ArcGIS. Then, the suitable habitats were ranked and visualized. The suitable areas were classified into four classes: highly suitable habitat (0.5 < P ≤ 1.0), moderately suitable habitat (0.3 < P ≤ 0.5), slightly suitable habitat (0.1 < P ≤ 0.3), and unsuitable habitat (0.0 ≤ P ≤ 0.1). Grids in each class were counted, and the proportion of suitable habitats in each class was calculated. Next, the precise invasion risk area of R. rugosum was determined by removing the suitable habitats of R. rugosum in water and unused land as the final result. The ecological niche overlap of R. rugosum was expressed in terms of the Schoener’s D (D) value in ENMtools. A higher Schoener’s D indicates a greater overlap of the ecological niches (Warren et al., 2010).
Results
Feature Classes and Regularization Multiplier of the Optimized Model
The results of R analysis revealed that 1.160 of the 1.240 selected candidate models were statistically significant. The optimized model was selected based on the smallest delta AICc value. Based on the native distribution records of R. rugosum, the FCs were L and Q and the RM was 0.7 in the optimized model. Based on the invasive distribution records of R. rugosum, the FCs were L, P, T, and H and the RM was 0.4 in the optimized model. Based on the native + invasive distribution records of R. rugosum, the FCs were L, P, T, and H and the RM was 1.3 in the optimized model (Figure 2). Based on the native, invasive, and native + invasive distribution records of R. rugosum, the suitable habitats of R. rugosum were simulated using the MaxEnt model under current climatic conditions and the mean AUC values of, respectively, 0.813, 0.910, and 0.789 were obtained for the MaxEnt models Supplementary Figure 2). Model fitting based on the native or invasive distribution records of R. rugosum was excellent and that based on both native and invasive distribution records was acceptable.
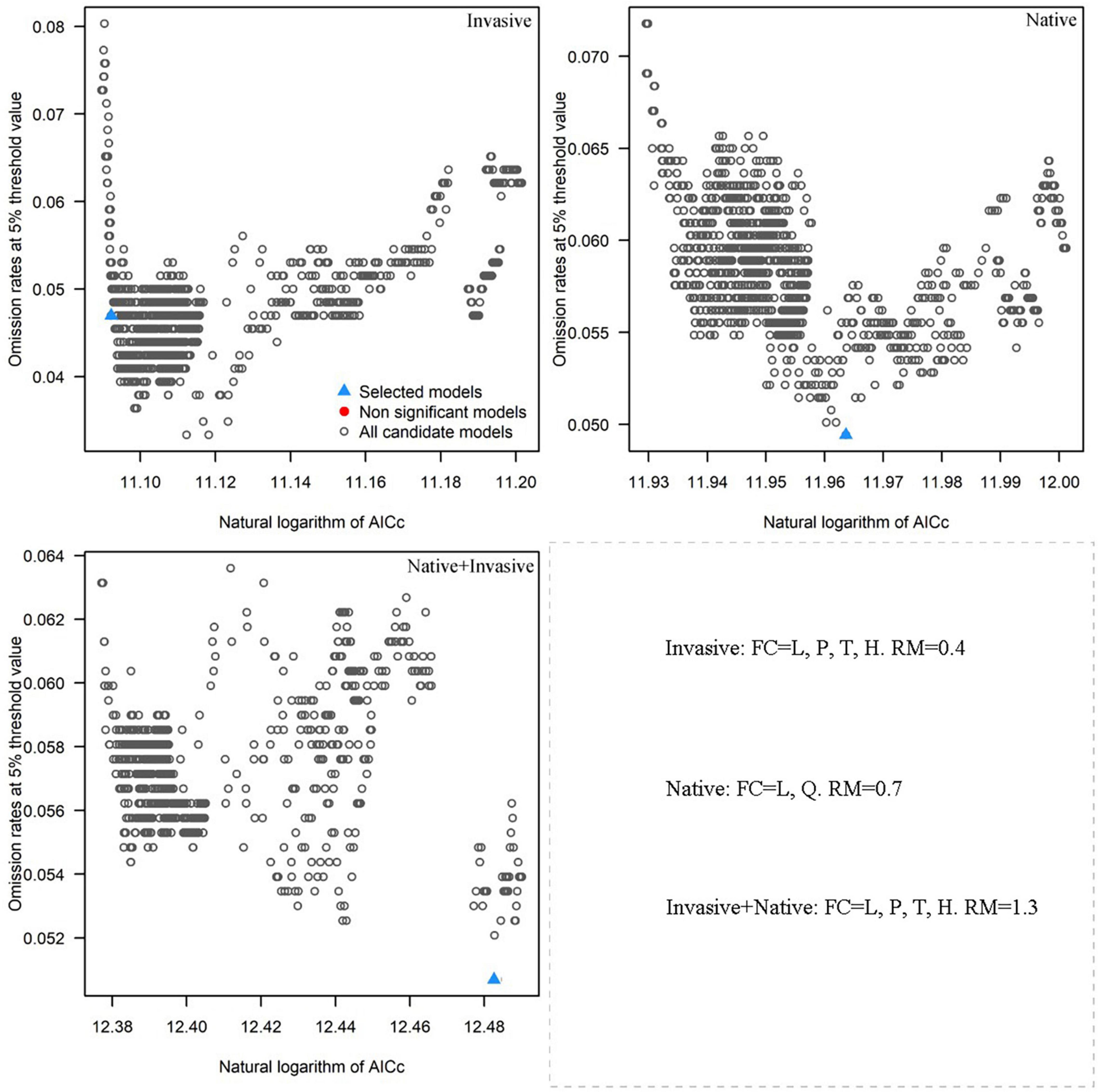
Figure 2. Omission rates and AICc values for all, non-significant, and selected “best” candidate models based on the invasive, native, and native + invasive records of Rapistrum rugosum.
Significant Environmental Variables
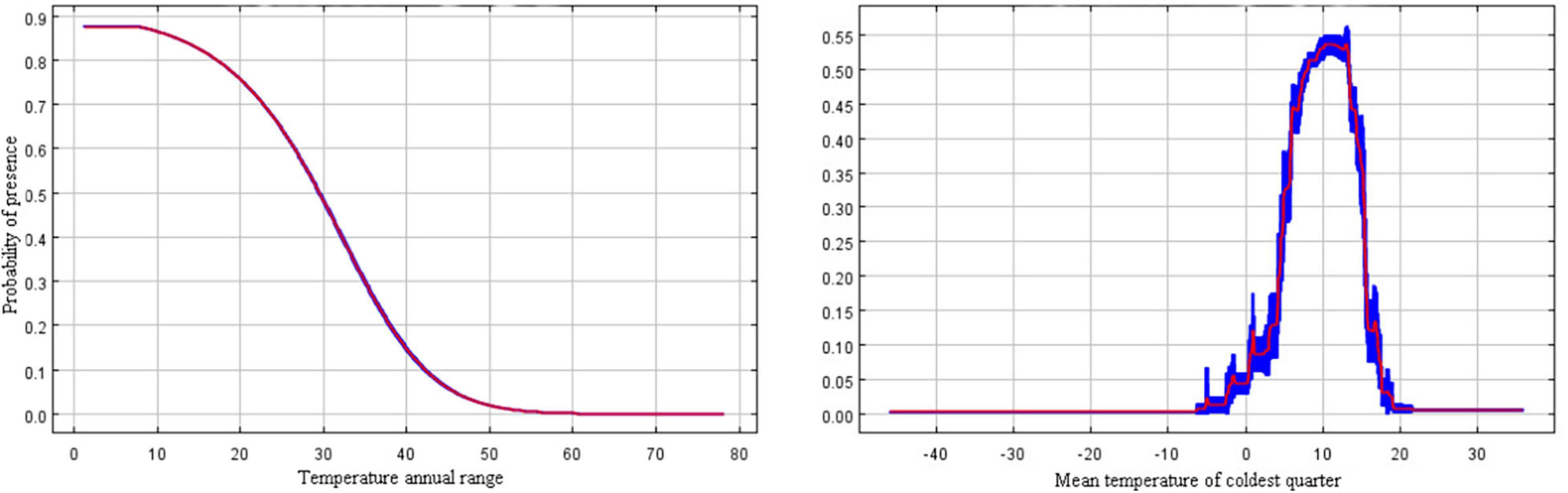
Regularized training gain was used to determine the significant environmental variables, which were modeled in MaxEnt based on native and invasive distribution records of R. rugosum. Jackknife analysis revealed that the two most significant influencing factors for regularized training gain with a single variable were annual temperature range (Bio7) and mean temperature of the coldest quarter (Bio11). These two environmental variables provided information that the other environmental variables did not (Figure 3).
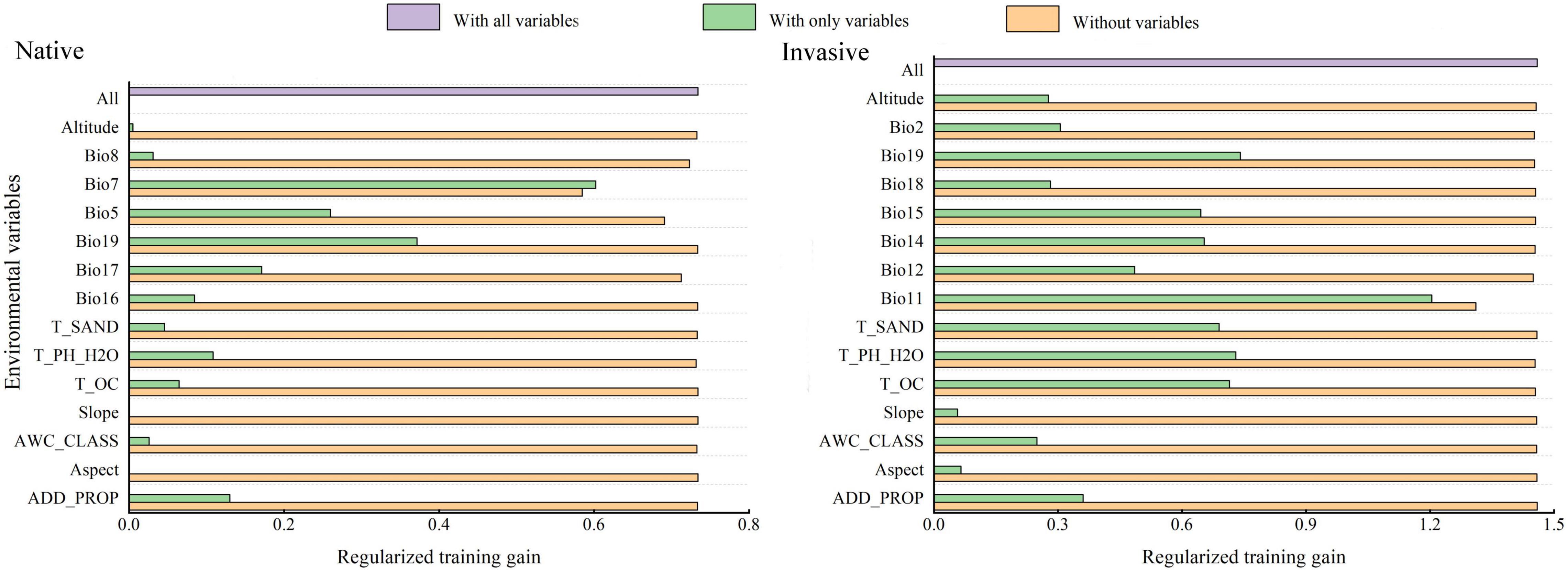
Figure 3. Regularized training gains of environmental variables using the Jackknife method in MaxEnt based on the native and invasive distribution records of Rapistrum rugosum.
In the present study, the relationship between the presence probability of R. rugosum and environmental variables was determined based on the response curves of environmental variables to the presence probability (Figure 4). When the presence probability of R. rugosum was greater than the threshold of highly suitable habitat classification (0.5), the corresponding interval was suitable for the growth of R. rugosum. The annual temperature range suitable for the growth of R. rugosum was 1.2–29.5°C, and the mean temperature of the coldest quarter suitable for the growth of R. rugosum was 8.1–13.4°C.
Ecological Niche Shifts of Rapistrum rugosum
The suitable habitats and ecological niches of R. rugosum differed based on invasive, native, and native + invasive distribution records (Figure 5; Table 1 and Supplementary Table 5). Based on the invasive distribution records (Figure 5A), the area of the highly suitable habitat was 80.55 × 104 km2, accounting for 8.39% of Chinese mainland, and these habitats were mainly distributed in southern and southeastern China. The area of the moderately suitable habitat was 60.35 × 104 km2, accounting for 8.39% of Chinese mainland, and these habitats were mainly distributed around highly suitable habitats.
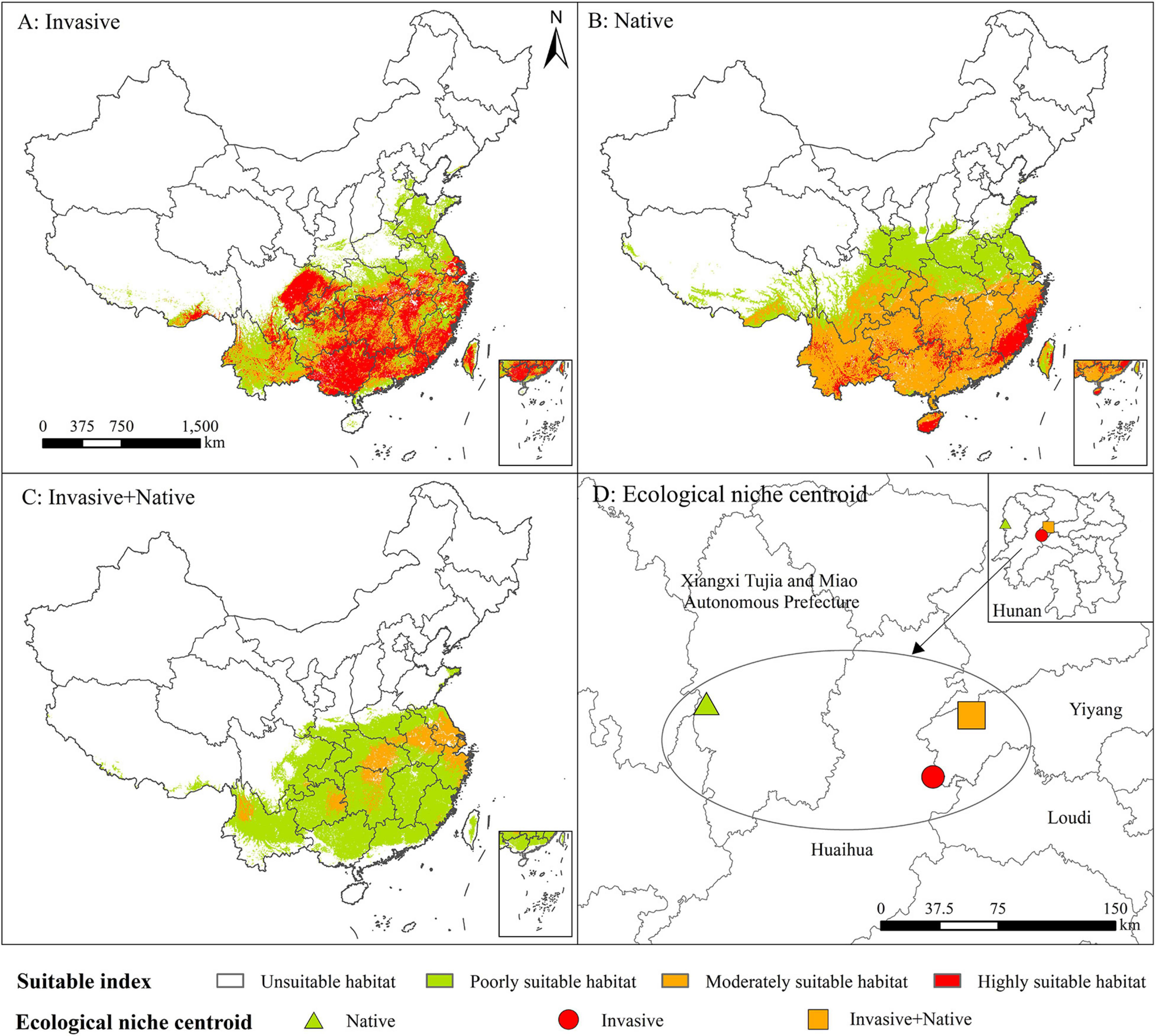
Figure 5. Potential geographic distribution of Rapistrum rugosum based on the native (A), invasive (B), and native + invasive (C) distribution records and the ecological niche centroid of R. rugosum (D).
Based on the native distribution records (Figure 5B), the area of the highly suitable habitat was 21.56 × 104km2, accounting for 2.25% of Chinese mainland, and these habitats were mainly distributed in Zhejiang, Fujian, and Hainan provinces. The area of the moderately suitable habitat was 153.69 × 104 km2, accounting for 16.01% of Chinese mainland, and these habitats were mainly distributed in Southwest, Southeast, and Central China.
Based on the native + invasive distribution records (Figure 5C), the area of the highly suitable habitat was 0.02 × 104 km2, and these habitats were only distributed in Shanghai City. The area of the moderately suitable habitat was 31.33 × 104 km2, accounting for 3.26% of Chinese mainland, and these habitats were mainly distributed in Jiangsu, Anhui, Hubei, Hunan, Guizhou, and Yunnan provinces.
The ecological niche centroid of R. rugosum was located in Hunan Province (Figure 5D). The highest ecological niche overlap (Schoener’s D = 0.92) was observed in the simulation results based on the invasive and native + invasive distribution records of R. rugosum, while the lowest overlap (Schoener’s D = 0.25) was between the native distribution records of R. rugosum (Table 1).
In summary, the results based on the invasive and native distribution records were consistent with the invasion risk habitats of R. rugosum in China, while the results based on the native + invasive distribution records were less consistent. Overall, the prediction of the risk habitats based on different distribution records revealed that the ecological niches of R. rugosum have shifted.
Interception Records of Rapistrum rugosum at Chinese Ports
There have been 1.061 inspection records of R. rugosum from Chinese entry ports during 2015–2018 (Figure 6). The top five countries of origin associated with over 50 interceptions were Australia, Canada, Brazil, the United States, and Argentina, together accounting for >90% of all records. Of the R. rugosum interceptions recorded from imported commodities, 37.5% were from Glycine max (L.) Merr., 20.9% from Hordeum vulgare L., 17.3% from linseed, 9.5% from Triticum aestivum L., 7.2% from Sorghum bicolor (L.) Moench, and the remaining 7.6% from other goods. The frequent R. rugosum interceptions were recorded from 17 of 42 customs directly under the General Administration of Customs, China. Overall, 80% interception records were primarily from Tianjin, Guangdong, Nanjing, and Chengdu customs (more than 50 records each). Given the diversity of the countries of origin and commodities of R. rugosum interceptions at Chinese customs, the risk areas of this IAS in China must be first identified based on the invasive or native distribution records and then subjected to overlap analysis.
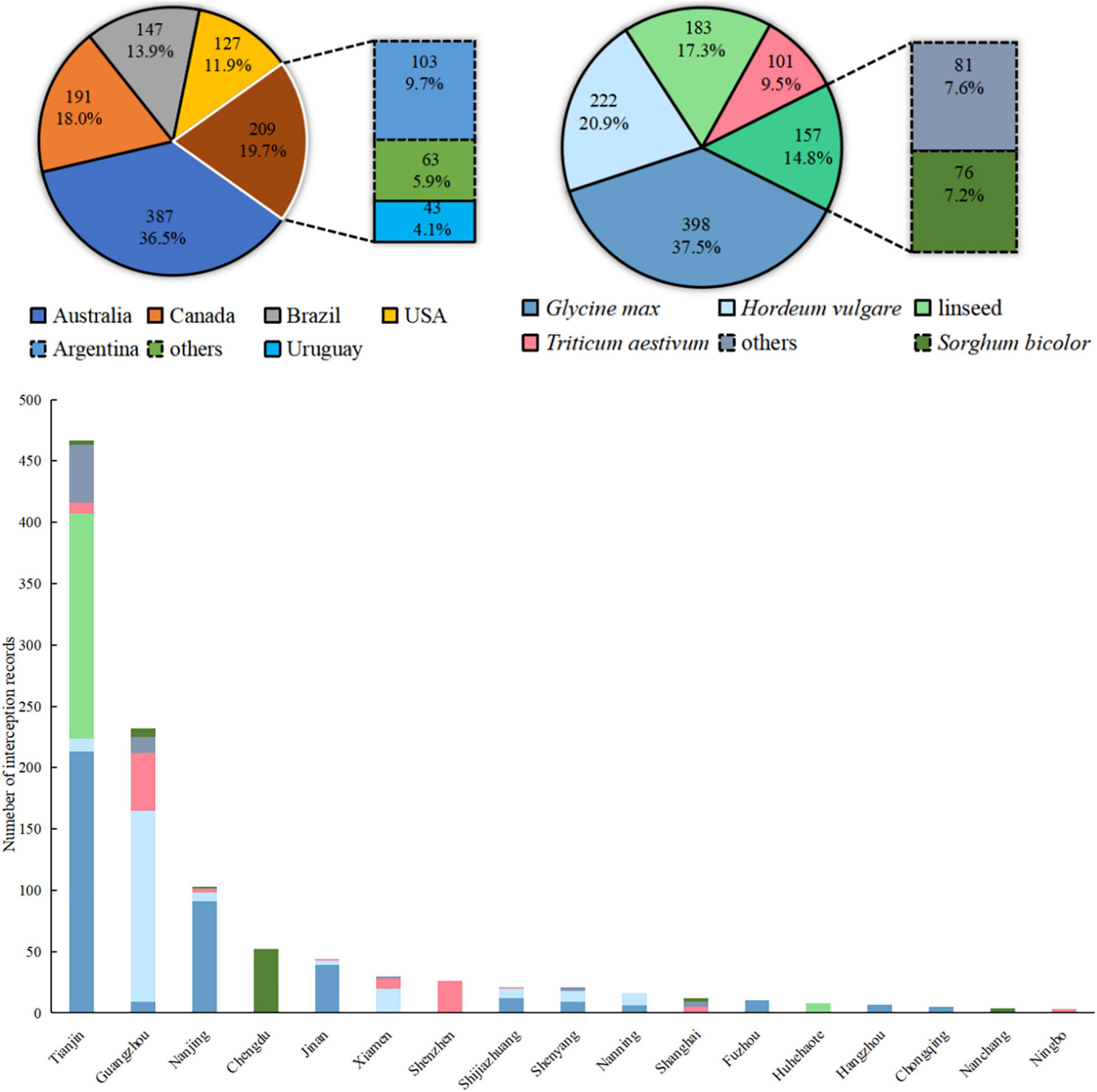
Figure 6. Imported commodities, countries of origin, and customs with interception records of Rapistrum rugosum in China during 2015–2018.
Potential Invasive Risk Areas of Rapistrum rugosum in China
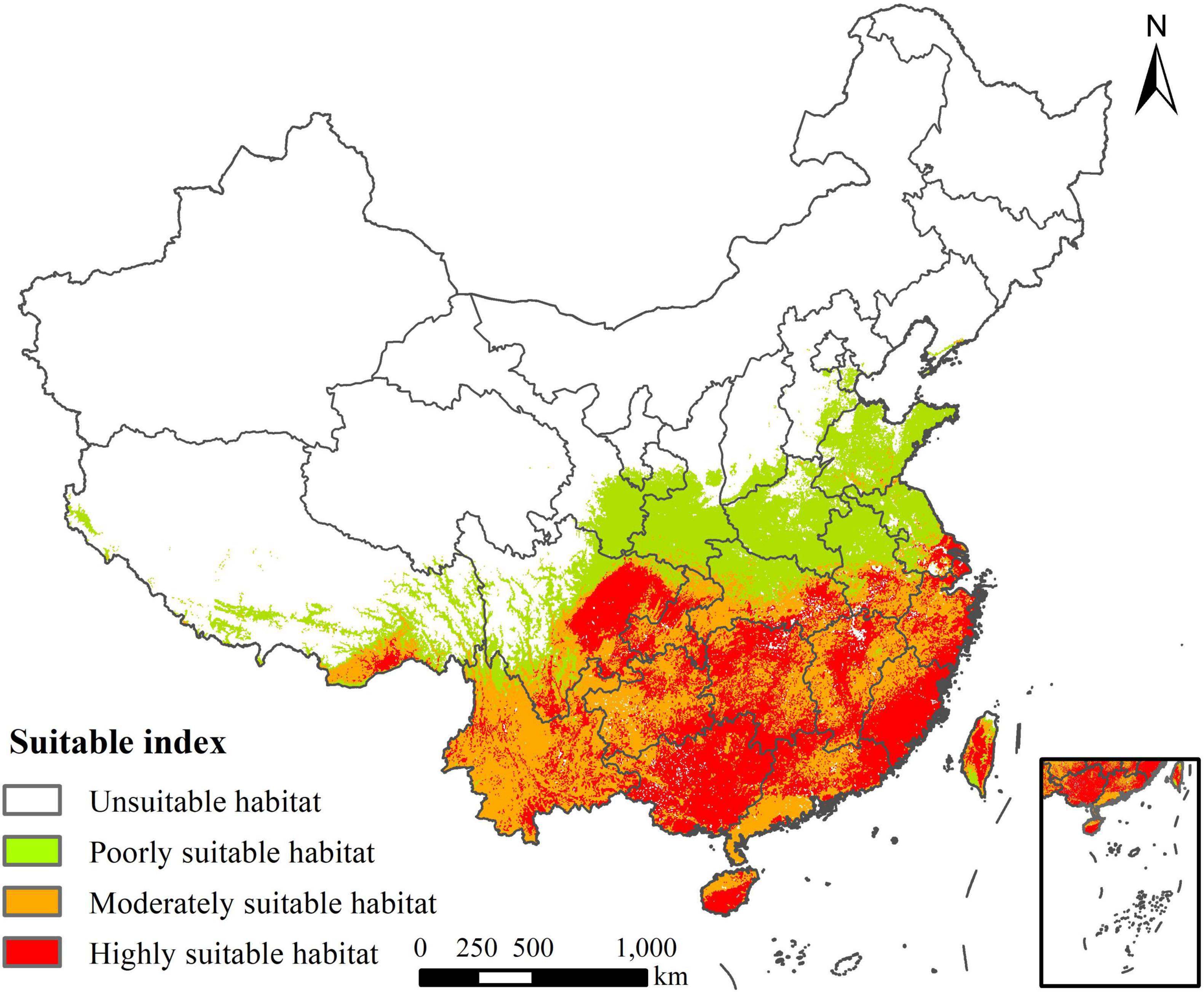
The area of the highly suitable habitat of R. rugosum in China was 91.09 × 104 km2, accounting for 9.49% of Chinese mainland, and these habitats were mainly distributed in Anhui, Zhejiang, Fujian, Jiangxi, Hunan, Guangdong, Hainan, Sichuan, Yunnan, Guizhou, Guangxi, Xizang, Shanghai, Chongqing, and Taiwan (Figure 7). The area of the moderately suitable habitat was 101.79 × 104 km2, accounting for 10.6% of Chinese mainland, and these habitat was mainly distributed around the highly suitable habitat. The area of the total suitable habitat was 287.53 × 104 km2, accounting for 29.95% of Chinese mainland.
Discussion
MaxEnt Model Development Using “kuenm”
Model calibration determines the combination of parameters that best represents the result by finding the best fit with the distribution records and environmental variables. In the present study, the R package “kuenm” was used to create optimized models based on model significance, performance, and simplicity. This approach helps prevent the overinterpretation of model outcomes (Cobos et al., 2019). “kuenm” has been previously used to optimize the MaxEnt model and predict the distribution of Zanthoxylum bungeanum in China; the optimized model reduced the overfitting degree, and the MaxEnt model fit was excellent (Zhuo et al., 2020). In the present study, the mean AUC values of the optimized MaxEnt models based on native, invasive, and native + invasive distribution records of R. rugosum were 0.813, 0.910, and 0.789, respectively, and the MaxEnt model fit was acceptable to excellent. Model optimization significantly improved the accuracy of the results.
Ecological Niche Shifts for the Native and Invasive Populations of Rapistrum rugosum
Ecological niches play pivotal roles in understanding the patterns of species distribution (Liu et al., 2020). The native ecological niche of a species does not encompass all suitable habitats for its growth. The ecological niche of a species, particularly an IAS, is expected to shift if it is allowed to disperse freely (David and Menges, 2011). In 1785, R. rugosum was first reported in France, and thereafter, it rapidly spread over Europe and the Mediterranean region (Bruno and Solène, 2016). R. rugosum invaded the United States in 1883 and rapidly expanded throughout the Americas. Thus, it is considered a problematic agricultural weed worldwide. In the 2000s, R. rugosum was recorded from North and South America, southern Africa, East Asia, and Oceania (Staten Island Museum, 2021). In 2019, R. rugosum newly invaded northwestern China, without any information on its origin. The interception records indicated that the source countries of R. rugosum were home to both native and invasive populations of this weed. Invasive species can adapt to new environments in various ways and expand their ecological niches spatially, leading to inconsistencies between the ecological niches of the invasive and native populations (Zenni et al., 2014). The differences in phenology between the invasive and native populations of plants lead to a shift in the ecological niche of IAS (Wolkovich and Cleland, 2011). R. rugosum occupies widely variable climatic and ecological niches. The MaxEnt model cannot predict the potential invasive risk of IAS if they disperse beyond their native range (Sillero et al., 2021). Thus, ecological niche shifting should be considered when predicting the invasive risk habitats of IAS based on species distribution modeling.
Comprehensive multi-angle considerations are fundamental to predict the invasion risk areas of R. rugosum in China. Based on native, invasive, native + invasive distribution records of R. rugosum, our results showed that when modeled with the distribution records of native or invasive, the ecological niche had shifted. Ecological niche shifts during biological invasion have been proven in many case studies of IAS (Broennimann and Guisan, 2008; Manzoor et al., 2020). MaxEnt models consistently provided qualitatively different predictions based on native and invasive distribution records, mainly because SDMs fit on native range data poorly predict introduced range occupancy or because ecological niche shifts significantly reduce the transferability of MaxEnt SDMs (Atwater and Barney, 2021). Meanwhile, SDMs can only predict the initial spread of the introduced population but cannot accurately predict its future spread trend. When an IAS first invades a new habitat, the ecological niche does not change. However, the phenology, thermal tolerance, and life history of invasive plants may change after they gradually adapt to the new habitats and expand their ecological niche (Colautti et al., 2017; Atwater et al., 2018). Shifts in the realized niche are common during plant bioinvasion. Ecological niche shifts are practically important, as they alter the predicted geographical distribution of IAS (Pearman et al., 2008). Therefore, ecological niche shifts play a key role in predicting the spread of IAS. Studies on the identification of the invasive risk areas of IAS have mainly focused on a combination of native and invasive data, regardless of whether the species has spread to a broad ecological niche (Qin et al., 2015; Saranya et al., 2021). Our predictions of the invasive risk areas of R. rugosum based on specific climate variables together with a combination of native and invasive records may be less or more one-sided than predictions based on native and invasive records considered separately, which may have affected model accuracy. R. rugosum is a new IAS in China that has been intercepted from many countries (including native and invasive ranges). Therefore, multiple invasions of mixed populations are expected to contribute to the further spread of R. rugosum in China. Therefore, in the present study, the native and invasive records of R. rugosum were separately used to model the potential invasive risk areas of this IAS in China. Overall, the ecological niche of R. rugosum has shifted, affecting its potential invasion risk in China.
Colonization and Dispersal Risk of Rapistrum rugosum in China and Significant Environmental Variables
Rapistrum rugosum is a globally important IAS. All customs in China should implement strict plant quarantine regulations on imported grain from Australia, Canada, Brazil, the United States, and Argentina to prevent the introduction of this species to new areas in the country. R. rugosum poses a great risk of invasion in China. The number of interception records from Tianjin Customs was the highest. Even though Tianjin customs is located in a slightly suitable habitat, it was also associated with the greatest risk of potential introduction. Moreover, Guangzhou Customs is associated with a high risk of potential introduction, because it is located in a highly suitable habitat and recorded many interceptions. Thus, Tianjin and Guangzhou customs should be closely monitored in terms of the quarantine of imported grain to prevent the introduction of R. rugosum mixed in imported grain. A wild population of R. rugosum has been discovered in Xi’an, and this population continues to spread in the surrounding areas. American countries accounted for over 50% of the total interception records of R. rugosum. In general, China, Europe, and North America have comparable climatic conditions. North America, Europe, and China have climate zones located south of the 40th parallel; therefore, most North American species can readily adapt to new habitats following their introduction into China and can effectively colonize in a relatively short time (Peel et al., 2007). According to the China Environment Report 2019, presented by the Ministry of Ecology and Environment, there are over 660 IAS in China; plants account for a majority of them, and over 50% originated from the United States. Therefore, climatic conditions cannot limit the ability of R. rugosum to establish populations in China.
Many Brassicaceae weeds germinate in a similar manner under different temperature conditions (Long et al., 2011). Temperature is an important variable affecting the germination of R. rugosum seeds (Ohadi et al., 2011; Hasanfard et al., 2021). Our analysis revealed that the most significant variables shaping R. rugosum distribution were annual temperature range (Bio7) and mean temperature of the coldest quarter (Bio11). These results further proved that temperature was an important factor limiting the survival of this weed. R. rugosum is a fast-growing weed, and its invasiveness is facilitated by its ability to germinate under a wide range of temperatures. In Australia, the seeds of R. rugosum could germinate at most temperatures ranging from 5 to 30°C (Ali et al., 2020). Our results showed that the annual temperature range and the mean temperature of the coldest quarter suitable for the growth of R. rugosum were 1.2–29.5°C and 8.1–13.4°C, respectively. We found that when the mean temperature of the coldest quarter was between 0 and 20°C, the survival probability of R. rugosum showed a fluctuating increase, followed by a fluctuating decrease. Our results are consistent with previous reports. Moreover, the mean annual temperature of suitable habitats for R. rugosum in China was >10°C, and the mean annual temperature of highly suitable habitats was >15°C. Overall, the mean annual temperature and mean temperature of the coldest quarter in southern China are suitable for the germination of R. rugosum seeds; thus, this region faces a risk of colonization and dispersal of R. rugosum.
In recent years, R. rugosum was frequently detected in the commodities and containers of importing grains and seeds, indicating that this weed can spread over long distances aboard other seeds, logs, and other contaminated materials. Early warning and control measures are essential to prevent and reduce R. rugosum invasion in China. Tianjin, Guangdong, Nanjing, and Chengdu customs have reported frequent interceptions of R. rugosum, underscoring the need for strict quarantine measures to prevent and reduce the introduction and survival of this IAS in the surrounding areas of entry ports and imported grain processing factories. Specific attention should be paid to the imported grains, including Glycine max, Hordeum vulgare, and linseed, from Australia, Canada, and Brazil. For wild populations of R. rugosum, chemical control tends to be problematic because of the potential risk of development of resistance to a number of specific herbicides (Hatami et al., 2016; Ntoanidou et al., 2019). Manual removal of the plant and its taproot and seed disposal are successful but time-consuming.
Conclusion
The present study used the R package “kuenm” to develop comprehensive MaxEnt models. The overall model fit was excellent. Through the simulation of risk areas based on distribution records from different regions in China, we predicted that the ecological niche of R. rugosum would shift, and our model results confirmed this assumption. The two most significant variables shaping R. rugosum distribution were annual temperature range and mean temperature of the coldest quarter. Invasion risk assessment revealed that the area of the total suitable habitat for R. rugosum in China is 287.53 × 104 km2, and these habitats are mainly distributed in Southwest, Southeast, and Central China. The potential habitats of R. rugosum accounted for a large proportion of Chinese mainland. Furthermore, climatic conditions will not limit the ability of R. rugosum to establish populations in China, and it has already successfully colonized specific regions within the country. Australia, Canada, Brazil, the United States, and Argentina are the five major source countries of R. rugosum in China. Meanwhile, Glycine max, Hordeum vulgare, linseed, Triticum aestivum, and Sorghum bicolor are the major grain sources of R. rugosum. Tianjin, Guangzhou, Nanjing, and Chengdu customs are the high-risk regions for the introduction of R. rugosum into China. Our results can serve as the reference to develop effective control measures against this IAS. Information about latitudinal clines in defense and joint clinical evolution of growth and defense in R. rugosum is essential for its adaptive evolution. Our further investigations will primarily focus on the re-establishment of heritable latitudinal clines in growth-related traits of R. rugosum.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
XX, HZ, and WL: conception and design of the research. XX and HZ: acquisition of data, analysis and interpretation of data, statistical analysis, and drafting the manuscript. RW, HQ, JG, GZ, and FW: manuscript revision. All authors contributed to the article and approved the submitted version.
Funding
This project was funded by the National Key R&D Program of China (grant no. 2021YFC2600400) and Technology Innovation Program of Chinese Academy of Agricultural Sciences (grant no. caascx-2017-2022-IAS).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2022.827497/full#supplementary-material
Footnotes
- ^ https://www.gbif.org/
- ^ https://www.cabi.org/isc
- ^ http://www.worldclim.org
- ^ https://www.resdc.cn/
- ^ http://www.geodata.cn
- ^ https://biodiversityinformatics.amnh.org/open_source/maxent/
References
Adhikari, P., Jeon, J.-Y., Kim, H. W., Shin, M.-S., Adhikari, P., and Seo, C. (2019). Potential impact of climate change on plant invasion in the Republic of Korea. J. Ecol. Environ. 43:36. doi: 10.1186/s41610-019-0134-3
Ali, H. H., Kebaso, L., Manalil, S., and Chauhan, B. S. (2020). Emergence and germination response of Sonchus oleraceus and Rapistrum rugosum to different temperatures and moisture stress regimes. Plant Species Biol. 35, 16–23. doi: 10.1111/1442-1984.12254
Atwater, D. Z., and Barney, J. N. (2021). Climatic niche shifts in 815 introduced plant species affect their predicted distributions. Glob. Ecol. Biogeogr. 30, 1671–1684. doi: 10.1111/geb.13342
Atwater, D. Z., Ervine, C., and Barney, J. N. (2018). Climatic niche shifts are common in introduced plants. Nat. Ecol. Evol. 2, 34–43. doi: 10.1038/s41559-017-0396-z
Beaumont, L. J., Gallagher, R. V., Thuiller, W., Downey, P. O., Leishman, M. R., and Hughes, L. (2009). Different climatic envelopes among invasive populations may lead to underestimations of current and future biological invasions. Divers. Distrib. 15, 409–420. doi: 10.1111/j.1472-4642.2008.00547.x
Broennimann, O., and Guisan, A. (2008). Predicting current and future biological invasions: both native and invaded ranges matter. Biol. Lett. 4, 585–589. doi: 10.1098/rsbl.2008.0254
Brown, A. (1878). Plants introduced with ballast and on made land. Bull. Torrey Bot. Club 6, 255–258. doi: 10.2307/2476788
Bruno, D., and Solène, R. (2016). INPN - Données Flore Des CBN Agrégées Par La FCBN. Version 1.2. PatriNat, U. (OFB-CNRS-MNHN), Paris. Paris: UMS PatriNat (OFB-CNRS-MNHN). doi: 10.15468/omae84
Cobos, M. E., Peterson, A. T., Barve, N., and Osorio-Olvera, L. (2019). Kuenm: an R package for detailed development of ecological niche models using Maxent. PeerJ. 7:e6281. doi: 10.7717/peerj.6281
Colautti, R. I., Ågren, J., and Anderson, J. T. (2017). Phenological shifts of native and invasive species under climate change: insights from the Boechera–Lythrum model. Philos. Trans. R. Soc. Lond. B Biol. Sci. 372:20160032. doi: 10.1098/rstb.2016.0032
David, A. S., and Menges, E. S. (2011). Microhabitat preference constrains invasive spread of non-native natal grass (Melinis repens). Biol. Invas. 13, 2309–2322. doi: 10.1007/s10530-011-0044-5
Diagne, C., Leroy, B., Vaissière, A. C., Gozlan, R. E., Roiz, D., Jarić, I., et al. (2021). High and rising economic costs of biological invasions worldwide. Nature 592, 571–576. doi: 10.1038/s41586-021-03405-6
Elith, J., Phillips, S. J., Hastie, T., Dudík, M., Chee, Y. E., and Yates, C. J. (2011). A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 17, 43–57. doi: 10.1111/j.1472-4642.2010.00725.x
Essl, F., Lenzner, B., Bacher, S., Bailey, S., Capinha, C., Daehler, C., et al. (2020). Drivers of future alien species impacts: an expert-based assessment. Glob. Change Biol. 26, 4880–4893. doi: 10.1111/gcb.15199
Fan, J., Upadhye, S., and Worster, A. (2006). Understanding receiver operating characteristic (ROC) curves. Can. J. Emerg. Med. 8, 19–20. doi: 10.1017/s1481803500013336
Fernández, M., and Hamilton, H. (2015). Ecological niche transferability using invasive species as a case study. PLoS One 10:e0119891. doi: 10.1371/journal.pone.0119891
Fischer, G., Nachtergaele, F., Prieler, S., Teixeira, E., Tóth, G., Velthuizen, H., et al. (2008). Global Agro-ecological Zones Assessment for Agriculture (GAEZ 2008. Laxenburg: FAO.
Global Biodiversity Information Facility (2021). Rapistrum Rugosum (L.) All. Available online at: https://www.gbif.org/species/5373368 (accessed December 22, 2021).
Greenberg, M., Haas, C., Cox, A. Jr., Lowrie, K., McComas, K., and North, W. (2012). Ten most important accomplishments in risk analysis, 1980–2010. Risk Anal. 32, 771–781. doi: 10.1111/j.1539-6924.2012.01817.x
Hasanfard, A., Rastgoo, M., Izadi Darbandi, E., Nezami, A., and Chauhan, B. S. (2021). Regeneration capacity after exposure to freezing in wild oat (Avena ludoviciana Durieu.) and turnipweed (Rapistrum rugosum (L.) All. in comparison with winter wheat. Environ. Exp. Bot. 181:104271. doi: 10.1016/j.envexpbot.2020.104271
Hatami, Z. M., Gherekhloo, J., Rojano-Delgado, A. M., Osuna, M. D., Alcántara, R., Fernández, P., et al. (2016). Multiple mechanisms increase levels of resistance in Rapistrum rugosum to ALS herbicides. Front. Plant Sci. 7:169. doi: 10.3389/fpls.2016.00169
Hejda, M., Chytrý, M., Pergl, J., and Pyšek, P. (2015). Native-range habitats of invasive plants: are they similar to invaded-range habitats and do they differ according to the geographical direction of invasion? Divers. Distrib. 21, 312–321. doi: 10.1111/ddi.12269
Hichri, A. O., Hichri, F., Mastouri, M., Brahmia, A., Flamini, G., and Selmi, B. (2019). Study of chemical composition, antibacterial and antioxidant activities of Rapistrum rugosum L. essential oils from flowers, leaves, and stems. J. Essent. Oil Bear. Plants 22, 1416–1426. doi: 10.1080/0972060X.2019.1682682
Kariyawasam, C. S., Kumar, L., and Ratnayake, S. S. (2019). Invasive plant species establishment and range dynamics in Sri Lanka under climate change. Entropy 21:571. doi: 10.3390/e21060571
Lemke, D. E., and Worthington, R. D. (1991). Brassica and Rapistrum (Brassicaceae) in Texas. Southwest. Nat. 36, 194–197. doi: 10.2307/3671920
Liu, C., Wolter, C., Xian, W., and Jeschke, J. M. (2020). Most invasive species largely conserve their climatic niche. Proc. Natl Acad. Sci. U.S.A. 117, 23643–23651. doi: 10.1073/pnas.2004289117
Liu, X., Liu, H., Gong, H., Lin, Z., and Lv, S. (2017). Appling the one-class classification method of Maxent to detect an invasive plant Spartina alterniflora with time-series analysis. Remote Sens. 9:1120. doi: 10.3390/rs9111120
Long, R. L., Stevens, J. C., Griffiths, E. M., Adamek, M., Gorecki, M. J., Powles, S. B., et al. (2011). Seeds of Brassicaceae weeds have an inherent or inducible response to the germination stimulant karrikinolide. Ann. Bot. 108, 933–944. doi: 10.1093/aob/mcr198
Mačić, V., Albano, P. G., Almpanidou, V., Claudet, J., Corrales, X., Essl, F., et al. (2018). Biological invasions in conservation planning: a global systematic review. Front. Mar. Sci. 5:178. doi: 10.3389/fmars.2018.00178
Manalil, S., and Chauhan, B. S. (2019). Interference of turnipweed (Rapistrum rugosum) and Mexican pricklepoppy (Argemone mexicana) in wheat. Weed Sci. 67, 666–672. doi: 10.1017/wsc.2019.42
Manalil, S., Haider Ali, H., and Chauhan, B. S. (2018). Germination ecology of turnip weed (Rapistrum rugosum (L.) All.) in the northern regions of Australia. PLoS One 13:e0201023. doi: 10.1371/journal.pone.0201023
Manzoor, S. A., Griffiths, G., Obiakara, M. C., Esparza-Estrada, C. E., and Lukac, M. (2020). Evidence of ecological niche shift in Rhododendron ponticum (L.) in Britain: hybridization as a possible cause of rapid niche expansion. Ecol. Evol. 10, 2040–2050. doi: 10.1002/ece3.6036
Ntoanidou, S., Madesis, P., and Eleftherohorinos, I. (2019). Resistance of Rapistrum rugosum to tribenuron and imazamox due to Trp574 or Pro197 substitution in the acetolactate synthase. Pestic. Biochem. Physiol. 154, 1–6. doi: 10.1016/j.pestbp.2018.12.001
Ohadi, S., Mashhadi, H. R., and Tavakol-Afshari, R. (2011). Effects of storage and burial on germination responses of encapsulated and naked seeds of turnipweed (Rapistrum rugosum) to light. Weed Sci. 59, 483–488. doi: 10.1614/WS-D-10-00153.1
Pardo, G., Marí, A. I., Aibar, J., Vilaplana, L., and Cirujeda, A. (2019). Bastard cabbage (Rapistrum rugosum L.) resistance to Tribenuron-methyl and Iodosulfuron-methyl-sodium in Spain and alternative herbicides for its control. Agronomy 9:492. doi: 10.3390/agronomy9090492
Pearman, P. B., Guisan, A., Broennimann, O., and Randin, C. F. (2008). Niche dynamics in space and time. Trends Ecol. Evol. 23, 149–158. doi: 10.1016/j.tree.2007.11.005
Peel, M. C., Finlayson, B. L., and McMahon, T. A. (2007). Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 11, 1633–1644. doi: 10.5194/hess-11-1633-2007
Phillips, S. J., Anderson, R. P., and Schapire, R. E. (2006). Maximum entropy modeling of species geographic distributions. Ecol. Modell. 190, 231–259. doi: 10.1016/j.ecolmodel.2005.03.026
Pisani, D., Pazienza, P., Perrino, E. V., Caporale, D., and De Lucia, C. (2021). The economic valuation of ecosystem services of biodiversity components in protected areas: a review for a framework of analysis for the gargano national park. Sustainability 13:11726. doi: 10.3390/su132111726
Pyšek, P., Hulme, P. E., Simberloff, D., Bacher, S., Blackburn, T. M., Carlton, J. T., et al. (2020). Scientists’ warning on invasive alien species. Biol. Rev. Camb. Philos. Soc. 95, 1511–1534. doi: 10.1111/brv.12627
Qin, Z., Zhang, J. E., DiTommaso, A., Wang, R. L., and Wu, R. S. (2015). Predicting invasions of Wedelia trilobata (L.) Hitchc. with Maxent and GARP models. J. Plant Res. 128, 763–775. doi: 10.1007/s10265-015-0738-3
Radosavljevic, A., and Anderson, R. P. (2014). Making better Maxent models of species distributions: complexity, overfitting and evaluation. J. Biogeogr. 41, 629–643. doi: 10.1111/jbi.12227
Saranya, K. R. L., Lakshmi, T. V., and Reddy, C. S. (2021). Predicting the potential sites of Chromolaena odorata and Lantana camara in forest landscape of Eastern Ghats using habitat suitability models. Ecol. Inform 66:101455. doi: 10.1016/j.ecoinf.2021.101455
Sillero, N., Arenas-Castro, S., Enriquez-Urzelai, U., Vale, C. G., Sousa-Guedes, D., Martínez-Freiría, F., et al. (2021). Want to model a species niche? A step-by-step guideline on correlative ecological niche modelling. Ecol. Modell. 456:109671. doi: 10.1016/j.ecolmodel.2021.109671
Simmons, M. T. (2005). Bullying the bullies: the selective control of an exotic, invasive annual (Rapistrum rugosum) by oversowing with a competitive native species (Gaillardia pulchella). Restor. Ecol. 13, 609–615. doi: 10.1111/j.1526-100X.2005.00078.x
Simpson, M., and Prots, B. (2013). Predicting the distribution of invasive plants in the Ukrainian Carpathians under climatic change and intensification of anthropogenic disturbances: implications for biodiversity conservation. Environ. Conserv. 40, 167–181. doi: 10.1017/S037689291200032X
Srivastava, V., Lafond, V., and Griess, V. C. (2019). Species distribution models (SDM): applications, benefits and challenges in invasive species management. CAB Rev. 14, 1–13. doi: 10.1079/PAVSNNR201914020
Staten Island Museum (2021). Staten Island museum. Occurrence Dataset. Staten Island, NY: Staten Island Museum. doi: 10.15468/ctqpb5
Stinca, A., Musarella, C. M., Rosati, L., Laface, V. L. A., Licht, W., Fanfarillo, E., et al. (2021). Italian vascular flora: new findings, updates and exploration of floristic similarities between regions. Diversity 13:600. doi: 10.3390/d13110600
Swets, J. A. (1988). Measuring the accuracy of diagnostic systems. Science 240, 1285–1293. doi: 10.1126/science.3287615
Tang, X., Yuan, Y., Liu, X., and Zhang, J. (2021). Potential range expansion and niche shift of the invasive Hyphantria cunea between native and invasive countries. Ecol. Entomol. 46, 910–925. doi: 10.1111/een.13028
Thomas, S. M., Verhoeven, M. R., Walsh, J. R., Larkin, D. J., and Hansen, G. J. A. (2021). Species distribution models for invasive Eurasian watermilfoil highlight the importance of data quality and limitations of discrimination accuracy metrics. Ecol. Evol. 11, 12567–12582. doi: 10.1002/ece3.8002
Venne, S., and Currie, D. J. (2021). Can habitat suitability estimated from MaxEnt predict colonizations and extinctions? Divers. Distrib. 27, 873–886. doi: 10.1111/ddi.13238
Wan, F., Jiang, M., and Zhan, A. (2017). “Biological invasions and its management in China,” in Biological Invasion and Its Research in China: An Overview, Vol. 1, eds Y. Yan, X. Xian, M. Jiang, and F. Wang (Dordrecht: Springer), 3–20. doi: 10.1007/978-94-024-0948-2
Warren, D. L., Glor, R. E., and Turelli, M. (2010). ENMTools: a toolbox for comparative studies of environmental niche models. Ecography 33, 607–611. doi: 10.1111/j.1600-0587.2009.06142.x
Wolkovich, E. M., and Cleland, E. E. (2011). The phenology of plant invasions: a community ecology perspective. Front. Ecol. Environ. 9, 287–294. doi: 10.1890/100033
Xun, L., Li, S., Li, W., Zhou, Y., Lu, Y., Mao, S., et al. (2020). Newly recorded plants of Brassicaceae from Shaanxi Province. Acta Bot. Boreali Occidentalia Sin. 40, 1425–1435. doi: 10.7606/.j.issn.100-4025.2020.05.1428
Yan, Y., Xian, X., Jiang, M., and Wan, F. (2017). “Biological invasion and its research in China: an overview,” in Biological Invasions And Its Management In China: 2, eds F. Wan, M. Jiang, and A. Zhan (Dordrecht: Springer Netherlands), 3–19. doi: 10.1371/journal.pone.0001208
Yang, X., Kushwaha, S. P. S., Saran, S., Xu, J., and Roy, P. S. (2013). Maxent modeling for predicting the potential distribution of medicinal plant. Justicia adhatoda L. in lesser himalayan foothills. Ecol. Eng. 51, 83–87. doi: 10.1016/j.ecoleng.2012.12.004
Yeh, H. T., Cheah, H. Y., Chiu, M. C., Liao, J. R., and Ko, C. C. (2021). Assessment of potential invasion for six phytophagous quarantine pests in Taiwan. Sci. Rep. 11:10666. doi: 10.1038/s41598-021-89914-w
Zenni, R. D., Bailey, J. K., and Simberloff, D. (2014). Rapid evolution and range expansion of an invasive plant are driven by provenance–environment interactions. Ecol. Lett. 17, 727–735. doi: 10.1111/ele.12278
Keywords: Rapistrum rugosum, ecological niche, suitable habitat, MaxEnt model, invasive risk
Citation: Xian X, Zhao H, Wang R, Qiao H, Guo J, Zhang G, Liu W and Wan F (2022) Ecological Niche Shifts Affect the Potential Invasive Risk of Rapistrum rugosum (L.) All. in China. Front. Plant Sci. 13:827497. doi: 10.3389/fpls.2022.827497
Received: 02 December 2021; Accepted: 17 March 2022;
Published: 15 April 2022.
Edited by:
Lars Hendrik Wegner, Foshan University, ChinaReviewed by:
Enrico Vito Perrino, International Centre for Advanced Mediterranean Agronomic Studies, ItalyLefort François, University of Applied Sciences and Arts of Western Switzerland, Switzerland
Copyright © 2022 Xian, Zhao, Wang, Qiao, Guo, Zhang, Liu and Wan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wanxue Liu, bGl1d2FueHVlQGNhYXMuY24=
†These authors have contributed equally to this work
 Xiaoqing Xian
Xiaoqing Xian Haoxiang Zhao
Haoxiang Zhao Rui Wang1
Rui Wang1 Jianyang Guo
Jianyang Guo Wanxue Liu
Wanxue Liu Fanghao Wan
Fanghao Wan