- Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
A large amount of agro-industrial residues are produced from the planting, production and processing of traditional Chinese herbs. As a tonic, edible, and economical herb, Codonopsis pilosula root has been extensively developed into medicine and functional food. However, thousands of tons of aerial parts (stems, leaves, flowers and fruits) have been directly discarded after harvest each year. To utilise agro-wastes, Pleurotus ostreatus was cultivated on a basal substrate supplemented with C. pilosula stems and leaves (CSL). Physicochemical analyses revealed that the basal substrate mixed with CSL was more abundant in cellulose, hemicellulose, and most of micronutrients such as K, Ca, Mg, S, Fe, Zn and Mo. After the first flush, the fruit bodies in CSL group exhibited a higher fresh weight, a wider average pileus diameter and a lower moisture level. Nutrition analyses presented a higher protein content and a lower fat content in mushrooms from CSL group compared with control group. Interestingly, 14 amino acids (glutamine, arginine, valine, leucine, and etc.) and 3 micronutrients (Se, Fe and Zn) were increased after CSL addition to the substrate. Based on untargeted metabolomics, a total of 710 metabolites were annotated. Compared with control group, there were 142 and 117 metabolites significantly increased and decreased in the CSL group. Most of them were grouped into classes of amino acids and peptids, fatty acids, carbohydrates, terpenoids, and etc. Moreover, an abundance of phytometabolites from Codonopsis were detected in P. ostreatus from CSL group, including polyacetylenes or polyenes, flavonoids, alkaloids, terpenoids, organic acids, and etc. UPLC-MS/MS results demonstrated that lobetyolin content in the CSL group samples was 0.0058%. In summary, the aerial parts of C. pilosula processed for use in the production of edible mushroom is an emerging strategy to converting agricultural waste into functional foods.
1 Introduction
In East, Southeast, and central Asia, Codonopsis pilosula (Franch.) Nannf., a perennial herbaceous herb, is used for tonic production, edible herb and medicinal materials, and has been practiced on a considerable scale for hundreds of years (Gao et al., 2018; Luan et al., 2021). Radix Codonopsis (dried roots, ‘Dangshen’ in Chinese and ‘Tojin’ in Japanese) had a total yield of about 50,000 tons approximately, while thousands of tons of agro-wastes residues are produced after harvest each year. Most of the agro-residues, the withered stems and leaves of C. pilosula (CSL), were always discarded or incinerated directly (Zeng et al., 2022a). Resource waste, serious environmental pollution and enormous economic loss are the main problems.
Agro-industrial residues were widely investigated to generate value as food or health products. Recent studies have showed that agro-wastes from herbs contained a variety of functional substances (Madureira et al., 2020; Reguengo et al., 2022). Agro-waste residues of C. pilosula are also available and highly valuable resource. Previous studies reported that functional ingredients from the agro-waste residues of C. pilosula were similar to the constituents of the roots, including a variety of polyene and polyacetylene glycosides, polysaccharides, flavonoids, lignans and penoids (Chen et al., 2020). Approximately 230 phytometabolites have been isolated and identified from Radix Codonopsis, and 165 of them were detected in the C. pilosula stems and leaves (He et al., 2015; Zeng et al., 2022a). Moreover, some studies reported that C. pilosula stems and leaves were used as feed additives in the poultry and livestock industry to increase growth performance, strengthen immune system, improve antioxidative status, and regulate the intestinal microflora (Hong et al., 2019; Zeng et al., 2019). Fresh leaves and flowers of C. pilosula have also been explored as tea products. As we known, the agro-waste residues of C. pilosula are mainly lignocellulose materials. Edible fungi are common primary decomposers of woody residues. Thus, our study was designed to evaluate the potential application of C. pilosula stems and leaves in the edible fungus industry.
Pleurotus spp., commonly known as the oyster mushroom, is a kind of nutrient-rich food, which also presents a health care value to humans. The species of the genus Pleurotus occupy the third position in the production of edible mushrooms (Fernandes et al., 2015). Based on the data from China edible fungi association, the total yield of oyster mushrooms in 2019 reached 300,000 tons (Zeng et al., 2022b). Compared to other edible fungi, oyster mushrooms have shown several advantages in cultivation, including short growth cycle, few environmental controls, disease and pest resistance, and low producing cost (Wan-Mahari et al., 2020). Oyster mushrooms are highly nutritious food and provide a good source of protein, carbohydrates, vitamins, and micronutrients. In recent decades, fungal polysaccharides in Pleurotus spp. were widely reported to improve immunity, regulate gut microbiota, and exhibit antioxidation, antitumor and cholesterol-lowering activities (Jayakumar et al., 2011; Sharma et al., 2021). The metabolites in P. pulmonarius and P. ostreatus exhibited profound antileukemic potential and immunomodulatory effects (Ebrahimi et al., 2018). A recent pharmacological study also indicated that dietary fibers in P. sajor-caju could promote the abundance of small-chain fatty acids producing bacterial genera and inhibit the growth of intestinal pathogens (Maheshwari et al., 2021). Therefore, oyster mushrooms are important natural products for nutrient source and therapeutic diet.
In recent years, different agro-industrial residues have broad development prospects as substrates in mushroom production. The cultivation of Pleurotus spp. have been extensively studied in different steps, in particular the substrates from agro-industrial waste. In previous studies, coffee husks, blank and printed paper scraps, rice straw and banana straw have been supplemented into the formulation of the substrates ( (Ramos et al., 2011; Naozuka et al., 2012; Fernandes et al., 2015). Here, the cultivation of Pleurotus ostreatus with the substrates added C. pilosula stems and leaves (CSL, agro-waste residues) could promote the mycelial growth, shorten the producing period and increased mushroom yield. Moreover, nutritions and active ingredients in mushrooms cultured with CSL were investigated by chemometrics and metabolomics. Our study showed an emerging strategy to turning agricultural waste materials into functional foods.
2 Materials and methods
2.1 Samples
A commercial strain of P. ostreatus (ITS sequencing, Genbank accession OL905951, Figures 1A, B) was obtained from Zehai Edible Fungus Specialized Cooperative (Shandong province, China) and cultivated on the basal substrate.
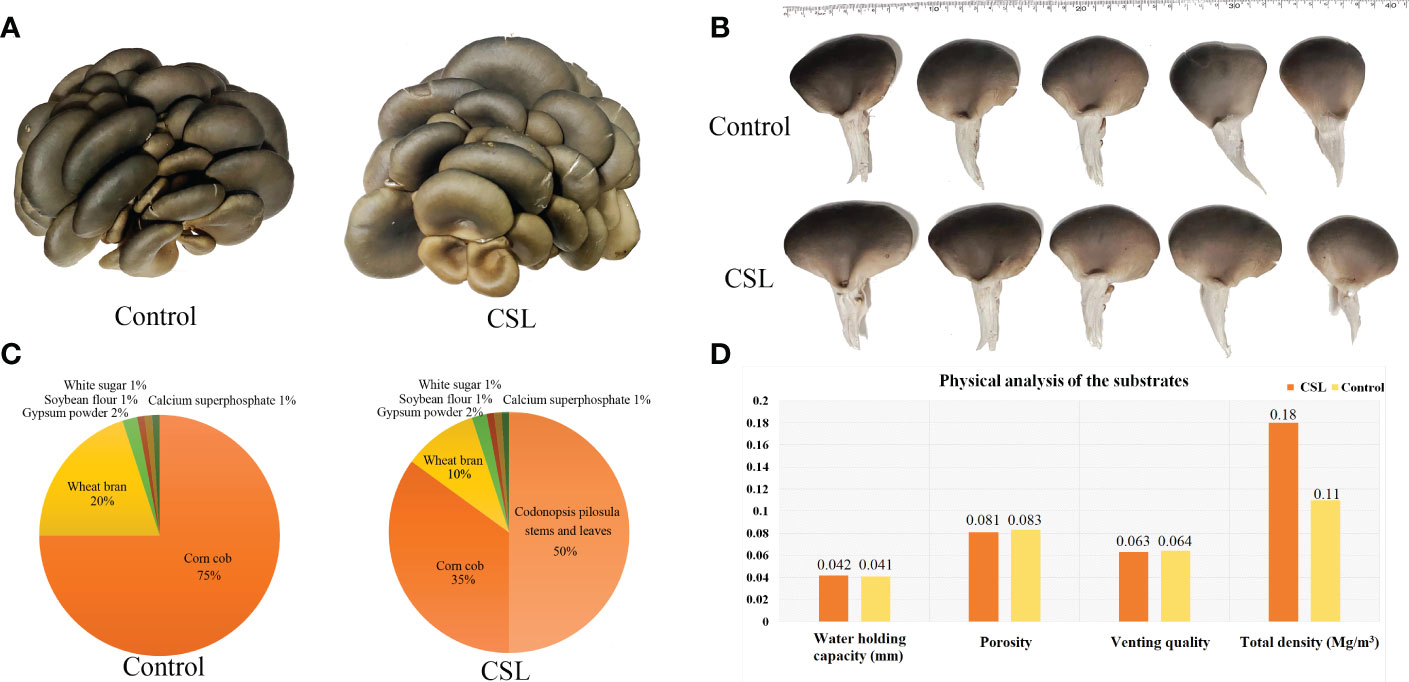
Figure 1 The fruiting body of P. ostreatus and physical parameter of the substrates. samples (A), pileus diameter (B), the formula of cultivation substrate (C), physical analyses of substrates (D).
Agro-waste residues were collected from in the major production areas of Radix Codonopsis in Changzhi, Shanxi province (Zhendong Pharmaceutical Co., Ltd.). After washing, C. pilosula stems and leaves (CSL) were dried and crushed to a coarse powder. The formula of cultivation substrate was shown in Figure 1C. The basal substrate is comprised of corn cob, wheat bran, gypsum powder, soybean flour, white sugar, and calcium superphosphate. The groups were designated the CSL and control groups. In the control group, 500 g of basal substrates were prepared, with the moisture content of 65% and the pH value of 6.5. In the CSL group, 250 g of CSL coarse powder and 250 g of basal substrate were used as cultivation substrate, with the moisture content of 65% and the pH value of 6.5. Then, 500 g of each substrates were placed in HDPE (high-density polyethylene) bags and autoclaved at approximately 120°C for 3 h. Moreover, physicochemical analyses of two substrates were performed by a qualified third institutions (Nanjing Convinced-Test Technology Co., Ltd). Next, each bag was inoculated with ~3 g of P. ostreatus grown on basal substrate and kept at 25°C in the dark to encourage mycelial growth within 25 days. Later, a hole (~3 cm diameter, one per bag) was made in the side of the bag. Each bag was placed at 10-15°C and 80-85% humidity, until mushroom formation. After the first fruiting, the fruiting bodies from control group and CSL group were collected, immediately chilled in liquid nitrogen and stored at -80°C.
2.2 Nutrient contents
The nutrient contents of mushrooms (six replicates of CSL and control groups) were detected according to the National Standards of the People’s Republic of China (NSPRC). The fat was determined by the acid hydrolysis method (NSPRC GB 5009.6-2016). The protein was estimated determined by Kjeldahl method (NSPRC GB 5009.5-2016). The total saccharide content was analyzed by the phenol-sulfuric acid method (NSPRC GB/T 15627-2009). The amino acid component was detected by an automatic amino acid analyzer (NSPRC GB/T 15627-2009). Se, Fe, Mn, and Zn were investigated by inductively coupled plasma mass spectrometry (ICP-MS, NSPRC 5009.268-2016) and inductively coupled plasma optical emission spectrometry (ICP-OES, NSPRC 5009.13-2017).
2.3 Metabolomics analysis
Referring to the method recorded in previous studies (Ma et al., 2021; Zeng et al., 2022b), six biological replicates of mushrooms from CSL and control group were assayed for sample extraction and metabolomics analysis. In brief, 50 mg of each sample was extracted with a methanol buffer with internal standard (0.4 mL 80% aqueous, and 20 μL 0.3 mg/mL 2-chloro-D-phenylalanine) for 30 min in an ultrasonic bath. The extraction was incubated for 30 min at -20°C, followed by centrifugation at 13,000 × g at 4°C for 15 min. After filtering by a microporous membrane (0.22-μm pore size), 200 μL of supernatant was used for UPLC-MS analysis. To monitor the stability and repeatability, 10 μL of supernatant from each test sample was pooled into a quality control (QC) sample and injected every six test samples throughout the analytical run.
Untargeted metabolomics analysis was performed on a UPLC system (Waters, Milford, USA) equipped with a Q Exactive HF-X Mass Spectrometer (Thermo Fisher Scientific, San Jose, CA). Acquity BEH C18 column (100 mm × 2.1 mm i.d., 1.7 μm; Waters, Milford, USA) was used for LC separation. The column temperature and injection volume were 40°C and 2.00 μL. The solvent consisted of water (phase A) and acetonitrile (phase B) with 0.1% (v/v) acetic acid respectively.
The following gradient was used for separation with flow rate at 0.40 mL/min: 5-20% B over 0-3 min, 20-95% B over 3-9 min, and 95% B over 9-13 min, 5% B over 13-16 min. Based on mass spectrometer equipped with an electrospray ionisation (ESI) source, MS-acquisition was performed in both positive and negative ion mode. All other parameters were as follows: spray voltage (+) and (−), 3500 and 2800 V; sheath gas, 40 arb; capillary temperature, 320°C; auxiliary gas, 10 arb; probe heater temperature, 400°C; normalised collision energy (NCE), 20-40-60 V; and mass spectrum scanning range, 70-1050 m/z.
The raw data were converted using Progenesis QI 2.3 (Waters Corporation, Milford, USA) for baseline filtration, peak identification, integration, retention time correction, peak alignment, and uniformisation. Subsequently those preprocessing data generated a matrix containing the retention time (RT), mass-to-charge ratio (m/z), and peak intensity. The data matrix was converted using SIMCA-P+ 14.0 to perform principal component analysis (PCA) and orthogonal partial least squares discriminate analysis (OPLS-DA) analyses. Differential metabolites were identified based on the combination of variable influence on projection (VIP) > 1, p < 0.05, and |Log2(fold_change)| > 1 in OPLS-DA analysis. Metabolites were annotated first using the experimental spectral data (m/z tolerance <10 ppm) and analysed for their biological functions from public databases: METLIN (https://metlin.scripps.edu/), Human Metabolome (http://www.hmdb.ca/), and KEGG databases (using MetaboAnalyst, https://www.metaboanalyst.ca/). For phytochemical compounds, more than 230 metabolites identified in C. pilosula from previous studies were also analysed separately in our data.
2.4 Determination of lobetyolin by LC-MS/MS
Lobetyolin is a standard composition to evaluate the quality of Radix Codonopsis. According to the Chinese Pharmacopoeia (2020) and the previous studies (Dong et al., 2021; Zeng et al., 2022b), lobetyolin in oyster mushrooms from CSL and control group was extracted by the ultrasound-assisted method. In brief, 10 g of each dried samples was precisely weighed and introduced into a conical flask with 50 mL of methanol. The flask was sealed and sonicated in a water bath operating at 40 kHz, 100 W and 30 min. After 10 minutes, the supernatant fluid was collected, diluted 100-fold, filtered through a 0.22-µm microporous membrane, and stored at 4°C until UPLC-MS/MS analysis.
The standard substance of lobetyolin was accurately weighed and dissolved in methanol to a concentration of 1 mg/mL. After dilution with methanol, the standard solutions with final concentrations of 0.5, 1, 5, 10, 50, 100, and 200 ng/mL were prepared.
Quantitative analysis was carried out using an Agilent 1290-6460 LC-MS/MS instrument (Agilent, USA), and an Agilent C18 column (2.1 mm × 100 mm, 3 μm) with column temperature at 35°C. Mobile phase was water containing 0.1% formic acid (A) and methanol (B) with with gradient conditions as follows: 0 min, 40% B; 0–1 min, 40–60% B; 1–3 min, 60–90% B; 3–4 min, 90% B; and 4.01–6 min, 40% B. The flow rate was 0.3 mL/min. The injection volume was 5 μL. The lobetyolin was achieved in negative ESI mode with dynamic Multiple Reaction Monitoring (MRM). The optimized conditions were listed as follows: ion spray voltage, 5500 V; collision energy, 30 V; and capillary temperature, 550°C. Lobetyolin produced a precursor ion of m/z 441.4, corresponding to the [M+COOH]-. Likewise, the mass transition patterns m/z 441.4 + 179.0 and 441.4 + 185.4 were selected for the identification and quantification. Data were analyzed with SPSS 19.0. Student’s t-tests were used and P-values ≤ 0.05 were considered significant.
3 Results and discussion
3.1 Physicochemical analysis of the substrates
In comparing substrates from CSL and control group (Figures 1D and 2), physicochemical analyses indicated that the basal substrate mixed with agro-waste residues was more abundant in cellulose (186.9 vs 137.9 mg/g), hemicellulose (308.9 vs 206.0 mg/g), and most of micronutrients K, Ca, Mg, S, Fe, Zn and Mo. However, the contents of Cu, Mn and B were decreased in the substrates of CSL group compared with control. In addition, there was no different in the amount of lignin, macronutrients C and N, and most of physical parameter, such as water holding capacity, porosity, venting quality and total density between the two substrates.
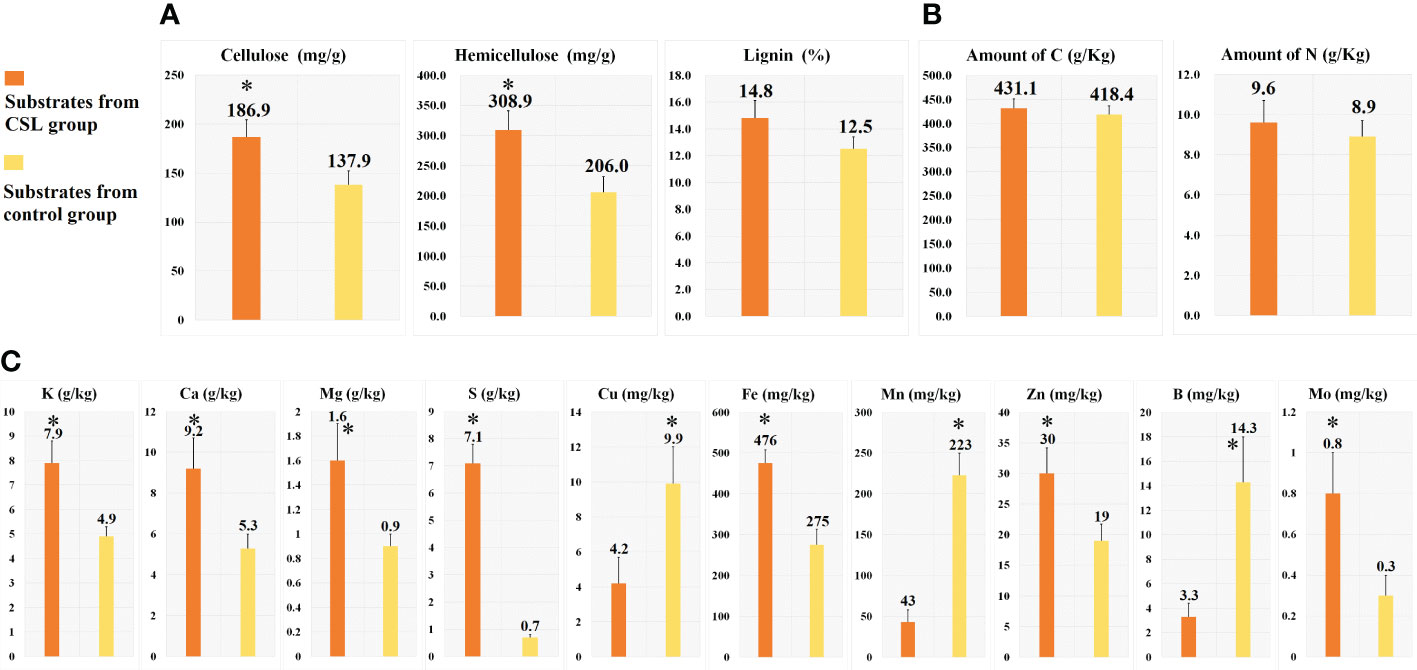
Figure 2 Chemical analyses of the substrates. cellulose, hemicellulose and lignin (A), macronutrients (B) and micronutrients (C). *, Statistically significant p < 0.05.
3.2 Nutrients in oyster mushroom samples
After the first flush, the fruit bodies obtained in the CSL group resulted in a higher fresh weight (90.23 ± 11.52 vs. 59.17 ± 7.22 g), a wider average pileus diameter (7.71 ± 0.79 vs. 6.96 ± 0.81 cm) and a lower moisture level (81.82 ± 1.25 vs. 86.24 ± 2.77 g/100 g) as compared to that of control group (Table 1). Previous studies have shown that oyster mushrooms grown on a variety of substrates present different levels of moisture, such as 88.06 and 85.64 g/100g for P. ostreatus cultivated in banana and rice straw, 83.17 and 88.08 g/100g for P. sajor-caju cultivated in banana and rice straw (Bonatti et al., 2004), 89.17 and 89.00 g/100 g for P. ostreatus and P. eryngii obtained in local supermarkets (Reis et al., 2012); and 90.3 and 91.0 g/100 g for P. ostreatus cultivated in blank and printed paper scraps (Fernandes et al., 2015).
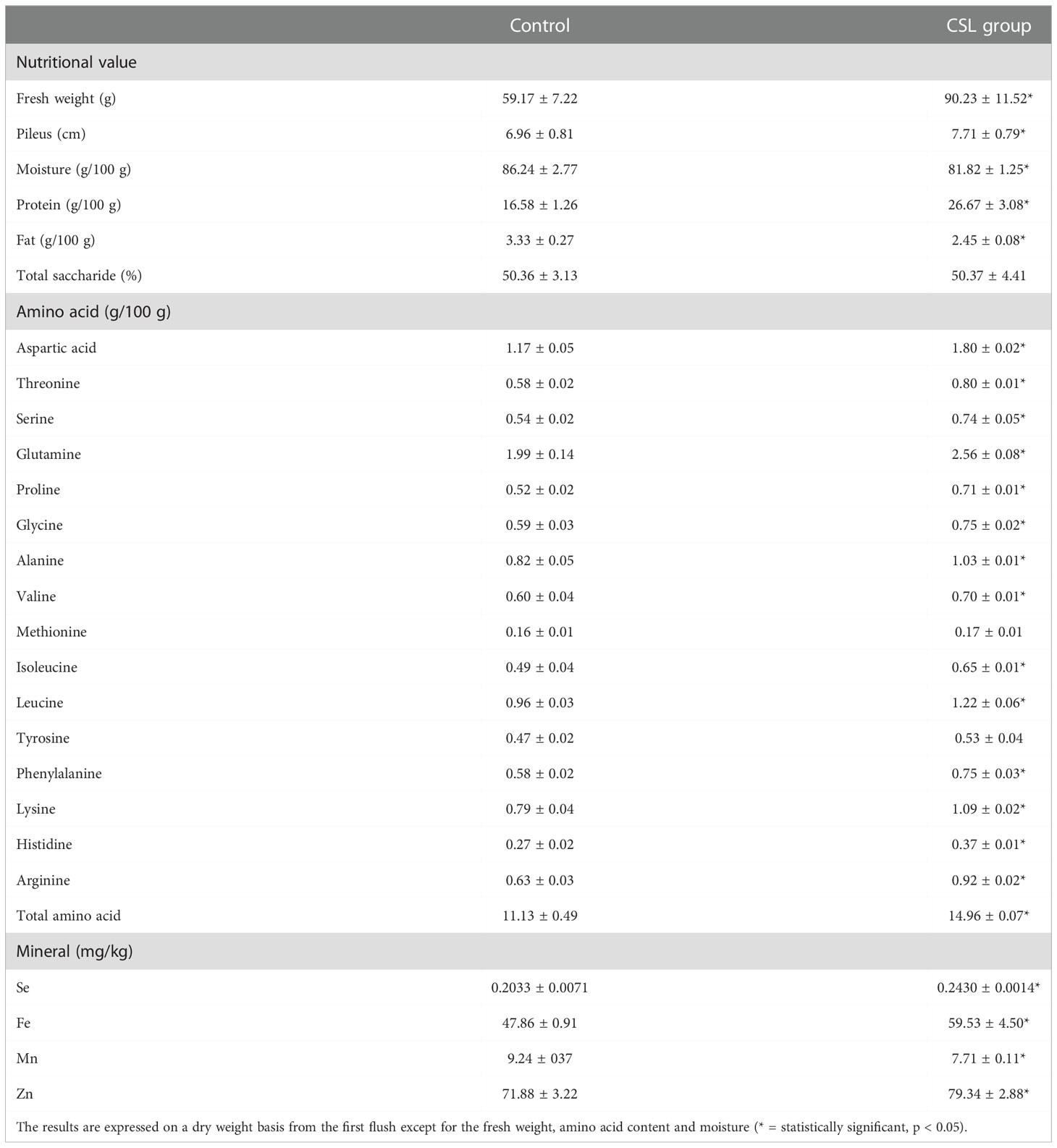
Table 1 Nutritional value, amino acid content and elemental concentrations of P. ostreatus (mean ± SD).
Nutrient composition was different between the two groups. Compared with control group, there was a higher protein content (26.67 ± 3.08 vs 16.58 ± 1.26 g/100g), a lower fat content (2.45 ± 0.08 vs 3.33 ± 0.27 g/100g), and a similar carbohydrate content were observed in the CSL group (shown in Table 1). In our study, the protein contents of oyster mushroom was similar with or higher than the values reported by previous studies: 19.9-34.7 g/100 g for P. ostreatus cultivated in wheat straw supplemented with sugar beet (Manzi et al., 1999), 21.0 g/100 g for P. ostreatus cultivated in wheat straw (Patil et al., 2010), 9.29 and 9.62 g/100g for oyster mushrooms cultivated on paper scraps and coffee husks, respectively (Fan et al., 2000; Aguilar-Rivera et al., 2012). Moreover, the total fat content of the oyster mushroom in our study (2.45 and 3.33 g/100g) was lower than the values obtained from samples cultivated on banana straw and rice straw (5.97 and 6.32 g/100 g), respectively (Bonatti et al., 2004); although higher than the values obtained from P. ostreatus grown on blank and printed paper scraps (1.18 and 1.68 g/100 g), respectively (Fernandes et al., 2015).
Subsequently, total amino acids contents of CSL group were obviously higher than those of control group (14.96 ± 0.07 vs. 11.13 ± 0.49 g/100 g). In terms of individual amino acids, 14 amino acids were more abundant in samples from CSL group than control group. Of these, the comparison between the two groups shows that the oyster mushroom in the CSL group contained more aspartic acid, threonine, serine, glutamine, glycine, alanine, valine, leucine, isoleucine, histidine, and arginine.
Meanwhile, the Se (0.2430 vs 0.2033 mg/kg), Fe (59.53 vs 47.86 mg/kg) and Zn (79.34 vs 71.88 mg/kg) concentrations in the CSL group were higher than those in the control group. However, the Mn (7.71 vs 9.24 mg/kg) concentrations were decreased after CSL addition to the substrate. Interestingly, similar results were investigated in the micronutrient analyses of the substrates (shown in Figure 2). Here, the concentrations of Se, Mn, and Zn (Table 1) from our results were similar to the previous reports (Kalac, 2009; Naozuka et al., 2012). For example, P. ostreatus were cultivated with coffee husks containing 0.12 mg/kg of Se, 70 mg/kg of Zn, and 10mg/kg of Mn. In addition, the concentrations of Fe (47.86 vs 59.53 mg/kg from control and CSL group) were lower than the data described in the previous reports (Koutrotsios et al., 2020). Their results were 80-130 mg/kg in P. ostreatus cultured on seven different substrates. However, according to the standard suggested by World Health Organization, the maximum limit of the Fe is 15 mg/kg. In the previous study (Karami et al., 2021), the Fe concentrations of many edible mushrooms (ranged from 97.2-3919 mg/kg) were higher than the WHO allowed limit, due to the ability of the mushroom to absorb Fe. Moreover, the recommended dietary intake (RDI) of Fe is 10-50 mg/day (Karami et al., 2021).
In summary, our results implied that the CSL addition to the substrate could contribute significantly to the overall nutritional value, amino acid content, and elemental concentration of oyster mushrooms.
3.3 Overview of metabolomics
Untargeted metabolomics of samples from the CSL group and the control (each group with six biological replicates) were carried out using the protocol with UPLC-MS and an integrated informatics pipeline. After removal of noise, redundancy, and poor quality signals, 6568 and 8584 spectral signals were collected by positive and negative ion modes, respectively. Subsequently, accurate m/z values were used for metabolite annotation. In total, 710 metabolites were annotated by our integrated bioinformatics pipeline (Supplementary Table S1).
The main differences in metabolite levels between the two groups were explored by OPLS-DA score plots (Figure 3A). The CSL group and the control group showed strong clustering, with no overlap. The permutation test of OPLS-DA model showed that R2X, R2Y (goodness-of-fit parameter) and Q2 (predictive ability parameter) were 0.576, 0.975, and 0.853, respectively, indicating the repeatability of the model (Figure 3B). Taken together, 17 of the 311 metabolites were annotated only in the CSL group (Figure 3C). The significantly differentially expressed metabolites (DEMs) were separated according to the criteria VIP > 1, p < 0.05 and |Log2(fold_change)| > 1 in the comparison between the CSL group and the control groups. There was a significant increase and decrease in the relative peak areas of 142 and 117 annotated metabolites in the CSL group, respectively (Figure 3D and Supplementary Table S2).
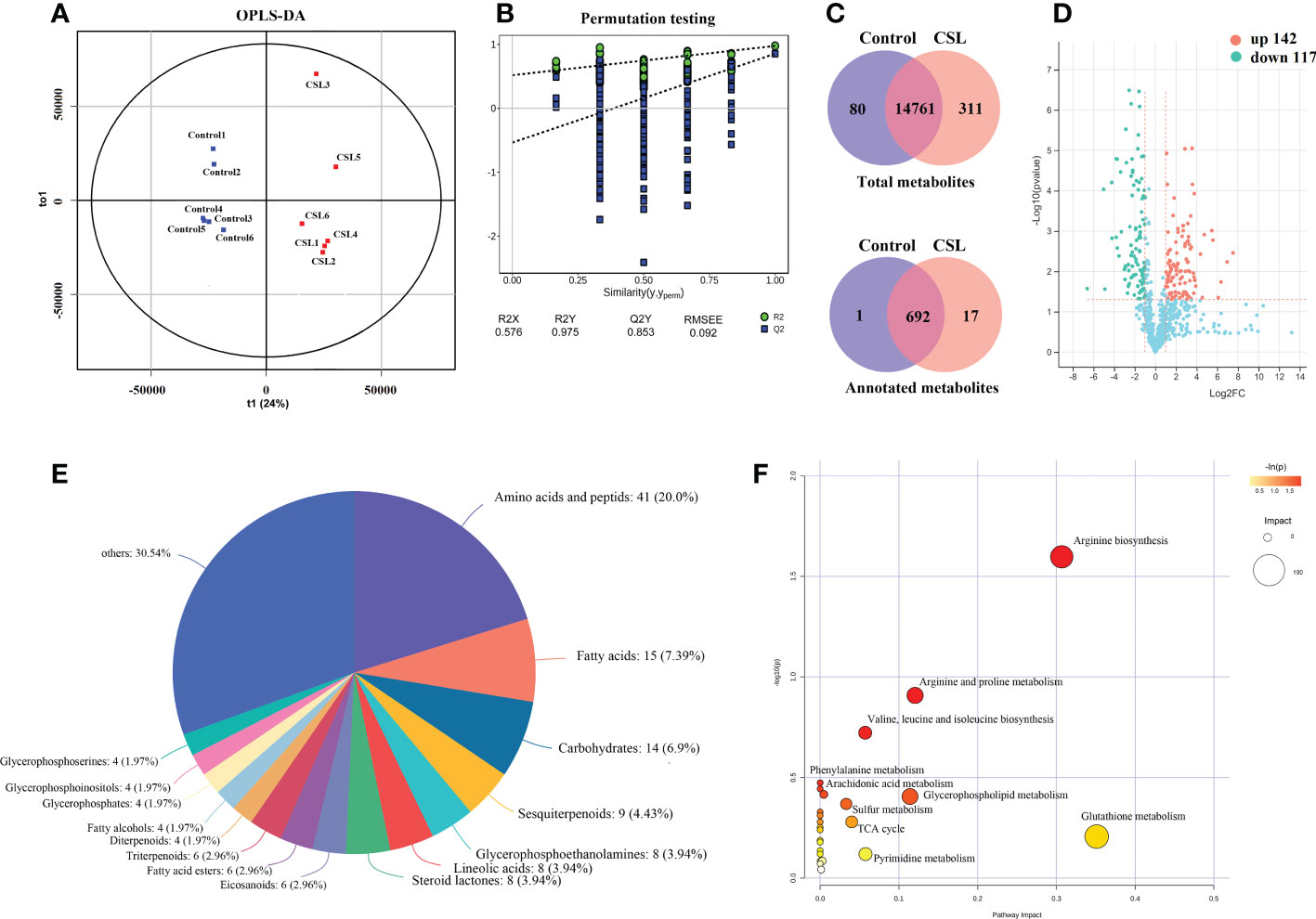
Figure 3 Overview of metabolomics in Codonopsis pilosula. OPLS-DA score plots (A), OPLS-DA model permutation test (B), Venn diagram (C), volcano plot (D), Classification of differential metabolites (E), KEGG enrichment analysis of differential metabolites (F).
A total of 259 DEMs were categorised into 16 classes based on the HMDB database (Figure 3E). The metabolites were grouped into classes of amino acids and peptids (41 metabolites), fatty acids (15), carbohydrates (14), sesquiterpenoids (9), and others, such as steroids, lineolic acids and glycerophosphoethanolamines. In addition, we mapped DEMs to KEGG pathways, most of which were enriched in amino acid biosynthesis and metabolism, such as arginine, valine, leucine, isoleucine, phenylalanine, pyrimidine, glutathione, glycerophospholipids, and aromatic metabolisms (observed in Figure 3F).
3.4 Overview of phytometabolites
More than 230 phytometabolites from Codonopsis have been described in previous studies (Gao et al., 2018). Currently, the metabolomics of C. pilosula have revealed a large number of phytometabolites, including polyacetylenes and polyenes, flavonoids, coumarins, lignans, alkaloids, terpenoids, steroids, organic acids, and other metabolites. In the present study, an abundance of phytometabolites were identified in P. ostreatus cultured on CSL (Figure 4A and Supplementary Table S3). In total, 16 polyacetylenes or polyenes, 6 flavonoids, 2 coumarins, 7 lignans, 23 alkaloids, 13 terpenoids, 5 steroids, 22 organic acids, and 22 other metabolites were detected from both of positive or negative mode. Differential phytometabolites were shown in Table 2.
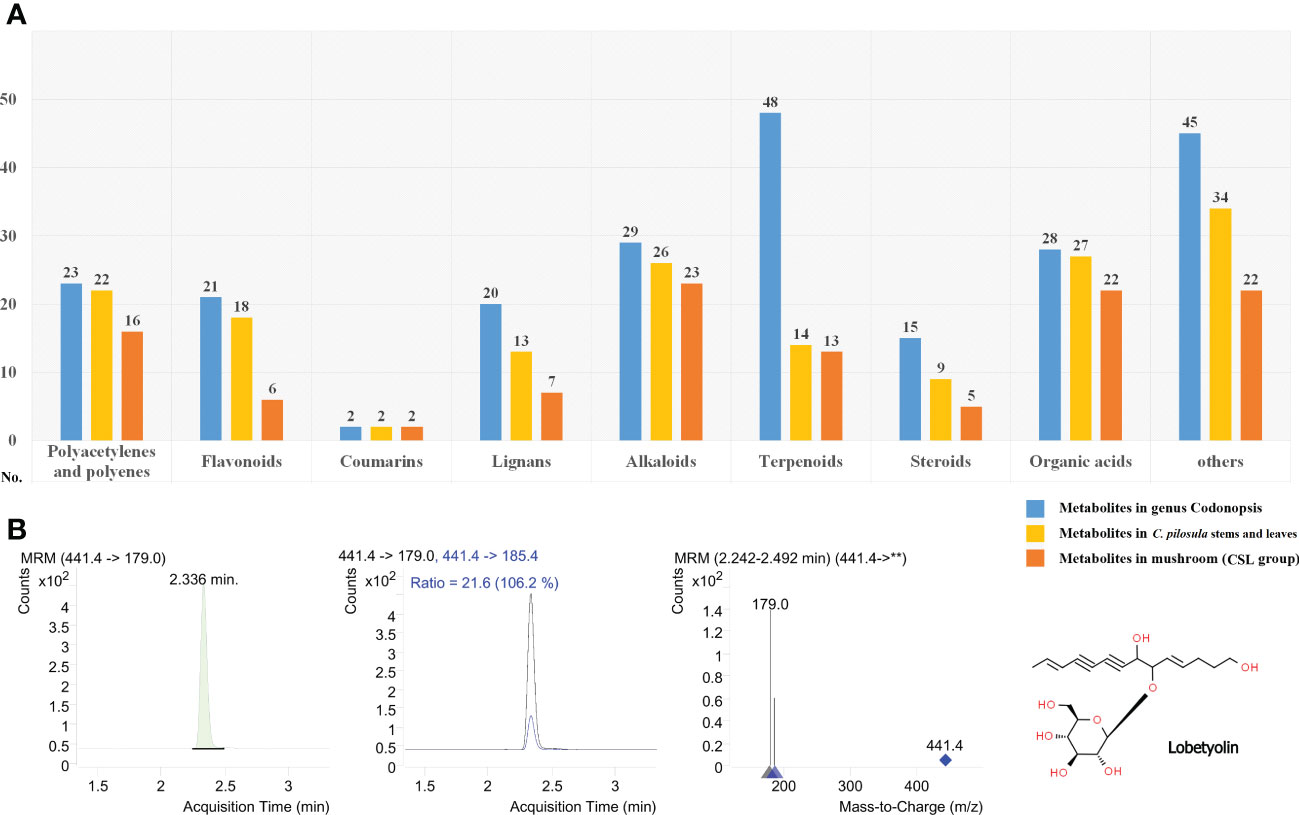
Figure 4 Phytometabolites identified in the fruiting body of P. ostreatus. (A) Annotation and classification of phytometabolites identified in CSL group; (B) Product ion mass spectra of [M+COOH]- ion of lobetyolin and chromatography peak of a representative CSL sample (retention time 2.336 min).
Overall, different types of pythometabolites were obviously accumulated (|Log2(fold_change)| > 1) in the fruit bodies obtained from the CSL group. In brief, 8 polyacetylenes or polyenes (lobetyol, lobetyolinin, pilosulyne and etc.), 2 flavonoids or coumarins (apigenin and angelicin), 5 lignans (syringin, lariciresinol and lanceolune), 7 alkaloids (codonopsine, codonopsinol, radicamine A and etc.), 3 terpenoids (atraetylenolide, atraetylenolide and etc.), 4 organic acids (nonadlenic acid, vanillic acid and etc.), and 7 other metabolites (glucopyranoside, fructofuranoside, sweroside, vanillin and etc.) have been reported in previous reports. Pharmacological evidences confirmed that most of them have different effects on health.
3.5 Active ingredients
The presence of polyacetylenes and polyenes, an important group of active ingredients in the roots of C. pilosula, is detected in other tissues, such as roots, stems, leaves and flowers. As shown in Table 2, 8 DEMs in the comparison of the CSL group and the control group were identified as polyacetylenes, polyenes and their glycosides. Lobetyol (metab_1740) is a major ingredient of C. pilosula that has multiple pharmacological effects, including anti-inflammation, antiviral and proliferation inhibition functions (Shen et al., 2016). Similarly, lobetyolinin (metab_14533), the bis-glucosylated forms of the lobetyol, has been shown various bioactive properties such as anti-inflammation, anticancer, antioxidative, and xanthine oxidase inhibitiory properties (Bailly, 2021). However, lobetyolin, the mono-glucosylated forms of lobetyol, was not detected in the metabolomics. In addition, pilosulynes A-G (metab_556, metab_520, metab_2005, metab_481 and metab_13480) were firstly identified as five polyynes and two polyenes from C. pilosula roots. Among them, pilosulyne F has been reported to possess specific bioactive properties, such as anti-HCV (hepatitis C virus) activity (Liao et al., 2015).
Flavonoids, coumarins and their glycosides are widely distributed among medicinal plants. A large number of studies have confirmed that flavonoids and coumarins have many health benefits for the human diet. Here, 4 DEMs were identified in the metabolomics dataset. Evidences have demonstrated that apigenin (metab_9173) has a variety of pharmacological effects on diabetes, amnesia, Alzheimer’s disease, depression, insomnia and cancer (Salehi et al., 2019). In addition, a recent study supported the efficacy of angelicin or psoralen (metab_8610) isolated from medicinal plant psoraleae, including anti-inflammatory antibacterial effects, and osteogenesis effects, as well as immune modulation (Li et al., 2018).
Lignans are widely found in the different tissues of plant such as roots, stems, rhizomes, leaves, flowers, seeds, fruits, xylem, and resins. Previous studies have demonstrated that lignans and their derivatives possess various bioactive properties, including anticancer, antioxidant, antiviral, and anti-asthmatic activities (Xu et al., 2022). In our study, 7 DEMs of lignans were shown in Table 2. In recent years, some studies have suggested that both syringin (metab_14820) and ethyl-syringin (metab_14115) may enhance humoral and cell-mediated immunity in immune-deficient mice (Gao et al., 2019).
Alkaloids are one of the largest group of phytometabolites found in many medicinal herbs, and have wide applications in tthe reatment, health care, and positive prevention fields. The pharmacological mechanism of bioactive alkaloids has been the subject of much research in recent years. For example, total alkaloids from C. pilosula roots may promote differentiation of neurite PC12 cells by strengthening the upstream phase of the MAPK-dependent signalling pathway (Liu et al., 2003). In this study, 9 DEMs in CSL versus control group comparison were identified as alkaloids. Codonopsine (metab_9797), codonopsinine (metab_12861), and codonopsinol (metab_6157, metab_8499 and metab_9124), are pyrrolidine and polyhydroxy alkaloids and exhibit antimicrobial activity against MRSA (El-Nezhawy et al., 2019). Additionally, radicamine A (metab_210), a pyrrolidine alkaloid, could markedly inhibit the absorption of glucose in the small intestine and would be developed as a new drug for diabetes treatment (Liu et al., 2010).
Terpenoids are effective and active components of many medicinal plant. In recent decades, a large amount of terpenoids from plants or fungi have been used extensively in products such as food, drug, health supplements, and biofuels. Our analysis resulted in 5 DEMs annotated as terpenoids. Among them, atraetylenolide (metab_4566 and metab_2636) is the main active ingredient that has been isolated from Atractylodes rhizomes with anti-inflammatory, gastroprotective and neuroprotective effects (Zhou et al., 2021). Meanwhile, oleanolic acid (metab_10523) and its derivatives possess different pharmacological properties, including anticancer, anti-osteoporosis, antibacterial, antidiabetic, and haemolytic effects (Ayeleso et al., 2017; Gupta, 2022). However, oleanolic acid (metab_10523) was down-regulated in the CSL group compared to the control group.
Steroids, organic acids and other phytometabolites are also effective ingredients of medicinal herbs and possess broad pharmacological activities for human health. More recently, organic acid derivatives have also been applied in plant protection against pathogens and insect pests (Izquierdovega et al., 2020). Here, 5 DEMs were found to be organic acids. In detail, multiple pharmacological studies have demonstrated that vanillic acid (metab_5507) and vanillin (metab_4847), two phenolic acid in numerous edible plants, have beneficial health effects on inflammation and oxidative stress (Bezerra-Filho et al., 2019). In addition, sweroside (metab_8913), a secoiridoid glycoside, has been discussed previously in some detail, including different effects such as antibacterial, antioxidant, anticancer, hepatoprotective, gastroprotective, sedative and and neuroprotective activities (Ouyang and Xu, 2019; Zhang et al., 2019).
3.6 Detection of lobetyolin
In the Chinese Pharmacopeia 2020 edition, lobetyolin (mono-glucosylated forms of lobetyol) used as the standard composition to assess the quality of Radix Codonopsis. With respect to previous studies, it is more common to increase the sensitivity for the detection of lobetyolin by applying electrospray ionization (ESI) in negative mode. As shown in Figures 4B, lobetyolin was identified from the standard reference by analysis for ion fragmentation patterns. None of this components was detected in any of the control group samples. In contrast, the content of lobetyolin in the CSL group samples was 58.66 ± 15.27 mg/kg (0.0058%).
Our previous study reported similar findings (Zeng et al., 2022b). In the case of C. pilosula, the content of lobetyolin in the agro-waste residues (dried stems and leave) were 0.142 ± 0.011 mg/g (0.0142%). Despite that the general content in fresh leaves (0.039%) and stems (0.027%) of C. pilosula was not high, in earlier studies, lobetyolin in roots was found to have a similar or slightly higher content (0.01-0.07%) as a result of the different detection method (Chen et al., 2020). Overall, our result showed that P. ostreatus grown with CSL might absorb a certain amount of lobetyolin and have potential health care value in improving immunity.
4 Conclusion
In this study, we analysed and compared chemical constituents derived from P. ostreatus cultured with the agricultural waste materials from C. pilosula. Metabolites were classified and identified by our integrated bioinformatic pipeline Based on UPLC-MS data, a total of 710 metabolites were identified; 259 were identified as DEMs and grouped into the following categories: amoni acids and peptids, fatty acids, carbohydrates, steroids, flavonoids, and others. Metabolic profiling of C. pilosula tissues implied that polyacetylenes, polyenes, flavonoids, alkaloids, steroids, terpenoids, and organic acids were accumulated in the P. ostreatus grown with CSL. In addition, lobetyol (a major ingredient of C. pilosula) and lobetyolinin (the bis-glucosylated forms of the lobetyol) were also found in the metabolomics dataset of the CSL group samples, and lobetyolin (the mono-glucosylated forms of the lobetyol) was quantified accurately in the same samples by UPLC-MS/MS. In light of our findings, many active compounds from C. pilosula can be absorbed and accumulate in the fruiting bodies of P. ostreatus. Oyster mushroom cultured with the agricultural waste materials of C. pilosula (stems and leaves) could have very high health care value in nutritional therapy.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
XZ, JL, XC, and SG discussed and plan the work. XZ, JL and XL conducted the experiments. XZ, TC, JC carried out the data analysis, created the figures, and drafted the initial manuscript. XZ, JL XC and SG wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the National Key Research and Development Program of China (Grant No. 2017YFC1701900) and CAMS Innovation Fund for medical Sciences (Grant No. 2021-I2M-1-031).
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2022.1085022/full#supplementary-material
Supplementary Figure 1 | The standard curve of lobetyolin.
Supplementary Table 1 | Overview of metabolomics data.
Supplementary Table 2 | Metabolites annotated with HMDB and KEGG databases.
Supplementary Table 3 | Phytometabolites in Codonopsis from metabolomics data.
References
Aguilar-Rivera, N., Moran, A. C., Lagunes, A. D. R., Gonzalez, J. M. (2012). “Chapter 4: Production of pleurotus ostreatus (oyster mushroom) grown on sugar cane biomass (trash, bagasse and pith),” in Mushrooms: Types, properties and nutrition. Eds. Andres, S., Baumann, N. (Hauppauge, NY: Nova Science Publishers, Inc), 77–103.
Ayeleso, T. B., Matumba, M. G., Mukwevho, E. (2017). Oleanolic acid and its derivatives: biological activities and therapeutic potential in chronic diseases. Molecules 22 (11), 1915. doi: 10.3390/molecules22111915
Bailly, C. (2021). Anticancer properties of lobetyolin, an essential component of radix Codonopsis (dangshen). Nat. Prod. Bioprospect. 11 (2), 143–153. doi: 10.1007/s13659-020-00283-9
Bezerra-Filho, C., Barboza, J. N., Souza, M., Sabry, P., Ismail, N., de Sousa, D. P. (2019). Therapeutic potential of vanillin and its main metabolites to regulate the inflammatory response and oxidative stress. Mini Rev. Med. Chem. 19 (20), 1681–1693. doi: 10.2174/1389557519666190312164355
Bonatti, M., Karnopp, P., Soares, H. M., Furlan, S. A. (2004). Evaluation of Pleurotus ostreatus and Pleurotus sajor-caju nutritional characteristics when cultivated in different lignocellulosic wastes. Food Chem. 88, 425–428. doi: 10.1016/j.foodchem.2004.01.050
Chen, M., Pi, W. X., Lu, T. L., Bai, D. T., Chen, J., Xiao, S. X., et al. (2020). Lobetyolin and polysaccharides in the aerial parts of Codonopsis pilosula. J. Chin. Med. Mater. 43, 1092–1098. doi: 10.13863/j.issn1001-4454.2020.05.009
Dong, J., Cheng, M., Xue, R., Deng, C., Liu, H., Zhang, T., et al. (2021). Comparative pharmacokinetic and bioavailability study of lobetyolin in rats after administration of lobetyolin and Codonopsis pilosula extract by ultra-performance LC-tandem mass spectrometry. Biomed. Chromatogr. 35, e5125. doi: 10.1002/bmc.5125
Ebrahimi, A., Atashi, A., Soleimani, M., Mashhadikhan, M., Barahimi, A., Maghari, A. (2018). Anti-invasive and antiproliferative effects of Pleurotus ostreatus extract on acute leukemia cell lines. J. Basic Clin. Physiol. Pharmacol. 29 (1), 95–102. doi: 10.1515/jbcpp-2017-0088
El-Nezhawy, A., Alrobaian, M., Khames, A., El-Badawy, M. F., Abdelwahab, S. F. (2019). Design and total synthesis of (-)-codonopsinine, (-)-codonopsine and codonopsinine analogues by O-(2-oxopyrrolidin-5-yl)trichloroacetimidate as amidoalkylating agent with improved antimicrobial activity via solid lipid nanoparticle formulations. Bioorgan. Med. Chem. 27 (7), 1263–1273. doi: 10.1016/j.bmc.2019.02.021
Fan, L., Soccol, C. R., Pandey, A. (2000). Producão de cogumelo comestıvel pleurotus em casca de cafe e avaliacão do grau de detoxificacão do substrato. Available at: https://www.sbicafe.ufv.br/handle/123456789/697 (Accessed September 29, 2000).
Fernandes, A., Barros, L., Martins, A., Herbert, P., Ferreira, I. (2015). Nutritional characterisation of Pleurotus ostreatus (Jacq. ex fr.) p. kumm. produced using paper scraps as substrate. Food Chem. 169, 396–400. doi: 10.1016/j.foodchem.2014.08.027
Gao, S. M., Liu, J. S., Wang, M., Cao, T. T., Qi, Y. D., Zhang, B. G., et al. (2018). Traditional uses, phytochemistry, pharmacology and toxicology of Codonopsis: A review. J. Ethnopharmacol. 219, 50–70. doi: 10.1016/j.jep.2018.02.039
Gao, S., Liu, J., Wang, M., Liu, Y., Meng, X., Zhang, T., et al. (2019). Exploring on the bioactive markers of Codonopsis radix by correlation analysis between chemical constituents and pharmacological effects. J. Ethnopharmacol. 236, 31–41. doi: 10.1016/j.jep.2019.02.032
Gupta, N. (2022). A review on recent developments in the anticancer potential of oleanolic acid and its analogs (2017-2020). Mini Rev. Med. Chem. 22 (4), 600–616. doi: 10.2174/1389557521666210810153627
He, J. Y., Ma, N., Zhu, S., Komatsu, K., Li, Z. Y., Fu, W. M. (2015). The genus Codonopsis (Campanulaceae): A review of phytochemistry, bioactivity and quality control. J.Nat Med. 69 (1), 1–21. doi: 10.1007/s11418-014-0861-9
Hong, Q. J., Hu, S. Q., Wang, C. L., Dong, S., Wang, J. H., Zhao, H. J. (2019). Reviewed on stems and leaves of Codonopsis pilosula. J. Trad. Chin. Vet. Med. 38 (6), 97–99. doi: 10.13823/j.cnki.jtcvm.2019.06.037
Izquierdovega, J. A., Arteagabadillo, D. A., Vargasmendoza, N., Castrorosas, J., Delgadoolivares, L., Madrigalbujaidar, E. (2020). Organic acids from roselle (Hibiscus sabdariffa l.) brief review of its pharmacological effects. Biomed. 8, 100. doi: 10.3390/biomedicines8050100
Jayakumar, T., Thomas, P. A., Sheu, J. R., Geraldine, P. (2011). In-vitro and in-vivo antioxidant effects of the oyster mushroom Pleurotus ostreatus. Food Res. Internat. 44, 851–861. doi: 10.1016/j.foodres.2011.03.015
Kalac, P. (2009). Chemical composition and nutritional value of European species of wild growing mushrooms: A review. Food Chem. 113, 9–16. doi: 10.1016/j.foodchem.2008.07.077
Karami, H., Shariatifar, N., Nazmara, S., Moazzen, M., Mahmoodi, B., Mousavi Khaneghah, A. (2021). The concentration and probabilistic health risk of potentially toxic elements (PTEs) in edible mushrooms (wild and cultivated) samples collected from different cities of Iran. Bio. Trace Elem. Res. 199 (1), 389–400. doi: 10.1007/s12011-020-02130-x
Koutrotsios, G., Danezis, G., Georgiou, C., Zervakis, G. I. (2020). Elemental content in Pleurotus ostreatus and Cyclocybe cylindracea mushrooms: Correlations with concentrations in cultivation substrates and effects on the production process. Molecules (Basel Switzerland) 25 (9), 2179. doi: 10.3390/molecules25092179
Liao, Y. R., Shih, H. C., Chao, C. H., Chan, H. H., Hung, H. Y. (2015). UV-Guided isolation of polyynes and polyenes from the roots of Codonopsis pilosula. RSC Adv. 52, 41324–41331. doi: 10.1039/c5ra02765a
Liu, J. H., Bao, Y. M., Song, J. J., An, L. J. (2003). Codonopsis pilosula (Franch) nannf total alkaloids potentiate neurite outgrowth induced by nerve growth factor in PC12 cells. Acta Pharmacol. Sin. 24, 913–917. doi: 10.1016/S0168-3659(03)00261-X
Liu, J. H., Bao, Y. M., Song, J. J., An, L. J. (2003). Codonopsis pilosula (Franch) nannf total alkaloids potentiate neurite outgrowth induced by nerve growth factor in PC12 cells. Acta Pharmacol. Sin. 24, 913–917. doi: 10.1016/S0168-3659(03)00261-X
Liu, C., Meng, A., Zhan, H. (2010). Study on radicamine A as a α-glucosidase inhabitor. Acta Acad. 4, 369–371.
Luan, F., Ji, Y., Peng, L., Liu, Q., Cao, H., Yang, Y., et al. (2021). Extraction, purification, structural characteristics and biological properties of the polysaccharides from Codonopsis pilosula: A review. Carbohyd. Polym. 261, 117863. doi: 10.1016/j.carbpol.2021.117863
Madureira, J., Barros, L., Cabo-Verde, S., Margaça, F., Santos-Buelga, C., Ferreira, I. (2020). Ionizing radiation technologies to increase the extraction of bioactive compounds from agro-industrial residues: A review. J. Agri Food Chem. 68 (40), 11054–11067. doi: 10.1021/acs.jafc.0c04984
Maheshwari, G., Gessner, D. K., Neuhaus, K., Most, E., Zorn, H., Eder, K., et al. (2021). Influence of a biotechnologically produced oyster mushroom (Pleurotus sajor-caju) on the gut microbiota and microbial metabolites in obese zucker rats. J. Agric. Food Chem. 69, 1524–1535. doi: 10.1021/acs.jafc.0c06952
Ma, Y., Ling, T. J., Su, X. Q., Jiang, B., Nian, B., Chen, L. J., et al. (2021). Integrated proteomics and metabolomics analysis of tea leaves fermented by Aspergillus niger, Aspergillus tamarii and aspergillus fumigatus. Food Chem. 334, 127560. doi: 10.1016/j.foodchem.2020.127560
Manzi, P., Gambelli, L., Marconi, S. (1999). Nutrients in edible mushrooms: An inter-species comparative study. Food Chem. 65, 477–482. doi: 10.1016/S0308-8146(98)00212-X
Naozuka, J., Luz, J., Assunção, L., Oliveira, P. V., Vanetti, M., Bazzolli, D., et al. (2012). Enrichment of Pleurotus ostreatus mushrooms with selenium in coffee husks. Food Chem. 131, 558–563. doi: 10.1016/j.foodchem.2011.09.023
Ouyang, Z., Xu, G. (2019). Antitumor effects of sweroside in human glioblastoma: its effects on mitochondrial mediated apoptosis, activation of different caspases, G0/G1 cell cycle arrest and targeting JNK/p38 MAPK signal pathways. J. BUON. 24 (5), 2141–2146.
Patil, S. S., Ahmed, S. A., Telang, S. M., Baig, M. (2010). The nutritional value of Pleurotus ostreatus (JACQ.:FR.) kumm cultivated on different lignocellulosic agrowastes. Innov. Roman. Food Biotech. 7, 66–76.
Ramos, C., Sapata, M., Ferreira, A., Andrada, L., Candeias, M. (2011). Production of three species of Pleurotus mushrooms and quality evaluation in modified atmosphere. Rev. Ciências Agrária 34, 57–64.
Reguengo, L. M., Salgaço, M. K., Sivieri, K., Maróstica Júnior, M. R. (2022). Agro-industrial by-products: Valuable sources of bioactive compounds. Food Res. Int. 152, 110871. doi: 10.1016/j.foodres.2021.110871
Reis, F. S., Barros, L., Martins, A., Ferreira, I. (2012). Chemical composition and nutritional value of the most widely appreciated cultivated mushrooms: An inter-species comparative study. Food Chem. Toxicol. 50, 191. doi: 10.1016/j.fct.2011.10.056
Salehi, B., Venditti, A., Sharifi-Rad, M., Kręgiel, D., Sharifi-Rad, J., Durazzo, A., et al. (2019). The therapeutic potential of apigenin. Int. J. Mol. Sci. 20 (6), 1305. doi: 10.3390/ijms20061305
Sharma, A., Sharma, A., Tripathi, A. (2021). Biological activities of Pleurotus spp. polysaccharides: A review. J. Food Biochem. 45 (6), e13748. doi: 10.1111/jfbc.13748
Shen, J., Lu, X., Du, W., Zhou, J., Qiu, H., Chen, J., et al. (2016). Lobetyol activate MAPK pathways associated with G1/S cell cycle arrest and apoptosis in MKN45 cells in vitro and in vivo. Biomed. Pharmacother. 81, 120–127. doi: 10.1016/j.biopha.2016.03.046
Wan-Mahari, W. A., Peng, W., Nam, W. L., Yang, H., Lee, X. Y., Lee, Y. K., et al. (2020). A review on valorization of oyster mushroom and waste generated in the mushroom cultivation industry. J. Hazard. Mate. 400, 123156. doi: 10.1016/j.jhazmat.2020.123156
Xu, X. Y., Wang, D. Y., Li, Y. P., Deyrup, S. T., Zhang, H. J. (2022). Plant-derived lignans as potential antiviral agents: A systematic review. Phytochem. Rev. 21 (1), 239–289. doi: 10.1007/s11101-021-09758-0
Zeng, X., Chen, J., Guo, S. X. (2019). The use of stems and leaves of Codonopsis pilosula to cultivate Pleurotus ostreatus. Acta Edulis Fungi 4 (27), 59–64. doi: 10.16488/j.cnki.1005-9873.2020.04.006
Zeng, X., Li, J. X., Lyu, X. K., Chen, J., Chen, X. M., Guo, S. X. (2022a). Untargeted metabolomics reveals multiple phytometabolites in the agricultural waste materials and medicinal materials of Codonopsis pilosula. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.814011
Zeng, X., Li, J. X., Lyu, X. K., Chen, X. M., Guo, S. X. (2022b). Nutritional characterization and untargeted metabolomics of oyster mushroom produced using astragalus membranaceus var. Mongolicus stems and leaves as substrates. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.802801
Zhang, R., Wang, C. M., Jiang, H. J., Tian, X. G., Li, W., Liang, W., et al. (2019). Protective effects of sweroside on il-1β-induced inflammation in rat articular chondrocytes through suppression of nf-κb and mtorc1 signaling pathway. Inflam. 42 (2), 496–505. doi: 10.1007/s10753-018-0906-4
Keywords: recycling of agro-waste, substrate formulation, edible fungi, nutritional composition, functional ingredients
Citation: Zeng X, Li J, Lyu X, Chen T, Chen J, Chen X and Guo S (2023) Utilization of functional agro-waste residues for oyster mushroom production: Nutritions and active ingredients in healthcare. Front. Plant Sci. 13:1085022. doi: 10.3389/fpls.2022.1085022
Received: 31 October 2022; Accepted: 05 December 2022;
Published: 04 January 2023.
Edited by:
Marta Olech, Medical University of Lublin, PolandReviewed by:
Anupam Barh, ICAR-Directorate of Mushroom Research, ItalyRocio Rodriguez Arcos, Spanish National Research Council (CSIC), Spain
Copyright © 2023 Zeng, Li, Lyu, Chen, Chen, Chen and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaomei Chen, eG1jaGVuQGltcGxhZC5hYy5jbg==; Shunxing Guo, c3hndW8xOTg2QDE2My5jb20=
 Xu Zeng
Xu Zeng Jiaxue Li
Jiaxue Li Xinkai Lyu
Xinkai Lyu Tongyao Chen
Tongyao Chen Juan Chen
Juan Chen Xiaomei Chen
Xiaomei Chen