- 1International Research Center for Environmental Membrane Biology and Department of Horticulture, Foshan University, Foshan, China
- 2Laboratory of Insect Ecology, South China Agricultural University, Guangzhou, China
- 3Guangdong Provincial Key Laboratory of High Technology for Plant Protection, Plant Protection Institute, Guangdong Academy of Agricultural Sciences, Guangzhou, China
The Asian citrus psyllid, Diaphorina citri Kuwayama (Hemiptera: Liviidae), is a key vector of the causal agents of Huanglongbing (HLB), a devastating disease affecting citrus almost worldwide. Nicotiana tabacum L. is an important commercial crop in China. Field observations suggested that D. citri adults die on N. tabacum leaves when grown nearby citrus orchards. In this study, the preference for and survivorship of D. citri adults on N. tabacum and their feeding behavior were investigated. The results showed that D. citri adults were attracted to N. tabacum and to the green leaf volatiles (GLVs) (Z)-3-hexenol and (E)-2-hexenol. The survival of D. citri adults on N. tabacum was less than 30 h, which was shorter than that for adults without food (35 h) and on a suitable host Murraya exotica L. (29 days). Electrical penetration graph (EPG) recordings revealed that the pathway phase of D. citri on N. tabacum leaves consisted of four waveforms—the non-probing phase (NP), the pathway phase (PP, including intercellular probing of activity in the phloem (C) and phloem penetration (D)), phloem salivation (E1), and phloem ingestion (E2). Diaphorina citri only secreted saliva and ingested sap from phloem on N. tabacum leaves and spent the longest duration in phloem sap ingestion (E2). Moreover, L-nicotine, an important defense compound against insects in N. tabacum plants, was highly toxic to D. citri. These results suggested that N. tabacum plants could help to sustainably control the spread of D. citri and HLB when growing in and around citrus orchards.
Introduction
Huanglongbing (HLB) is a disease caused by the phloem-inhabiting bacterium Candidatus Liberibacter asiaticus (CLas) or Ca. L. americanus (CLam) (Bové, 2006). It is the most devastating citrus disease and a worldwide threat to the citrus industry (Bové, 2006; da Graça et al., 2016) because of the lack of a cure or/and an effective control method (Chin et al., 2014). The most important and effective measure to control the spread of HLB in citrus is the management of the key vector, the Asian citrus psyllid, Diaphorina citri Kuwayama (Hemiptera: Liviidae) (Stansly et al., 2014; Qureshi et al., 2014; Monzo and Stansly, 2015). Diaphorina citri nymphs and adults can acquire CLas by feeding on infected plants (Inoue et al., 2009; Pelz-Stelinski et al., 2010). If the pathogen is acquired at the nymphal stage, adults are able to transmit it immediately after emergence (Inoue et al., 2009). Previous studies have demonstrated that feeding on infected plants could increase the fecundity and produce more offspring of D. citri (Pelz-Stelinski et al., 2010; Pelz-Stelinski and Killiny, 2016). Diaphorina citri has highly efficient CLas transmission, and even an individual adult can successfully transmit the pathogen to healthy plants within a short time period (Pelz-Stelinski et al., 2010). Currently, D. citri and HLB have spread to almost all citrus-growing regions worldwide (Ghanim et al., 2017).
Diaphorina citri is an oligophagous insect with a host range restricted to plants in the Rutaceae family (Li, 2011) that includes more than 50 species (Halbert and Manjunath, 2004). However, D. citri also tends to feed on weeds of non-Rutaceae surrounding citrus orchards, such as Bidens alba L. (Asteraceae), Eupatorium capillifolium (Lam.) Small ex Porter & Britton (Asteraceae), Ludwigia octovalvis (Jacq.) P.H. Raven (Onagraceae) (Johnston et al., 2019; George et al., 2020), Solanum nigrum L. (Solanaceae), Ageratum conyzoides L. (Asteraceae), and Praxelis clematidea R.M. King & Robinson (Asteraceae) (Lu et al., 2021). These weeds have been suggested as alternative or secondary host plants for D. citri and allow the insect to survive in the absence of suitable hosts, although they are unable to reproduce and survive for an extended period on these hosts (Tiwari et al., 2010; Johnston et al., 2018; Johnston et al., 2019). Thus, these alternative hosts could play an important role in area-wide management of D. citri and affect control measures such as insecticide applications. Diaphorina citri can utilize hosts in the Rutaceae, Asteraceae, Onagraceae, Solanaceae, Moraceae (Thomas and de León, 2011; Felisberto et al., 2019), and Boraginaceae (Arshad et al., 2019), suggesting that their olfactory system can sense chemical signals emitted by these hosts. It also suggests that these signals might not be specific but common to several plant species such as green leaf volatiles (GLVs) (Riddiford, 1967).
GLVs are a series of six-carbon aldehydes and alcohols, their esters emitted by plants and produced from polyunsaturated fatty acids through the lipoxygenase (LOX) pathway (Holopainen and Gershenzon, 2010), such as (Z)-3-hexenol, 1-hexanol, (Z)-3-hexenyl acetate, and (±)-2-hexanol (Frati et al., 2008; Zheng et al., 2014). They usually serve as signal molecules to herbivorous insects in host finding. Although D. citri has a relatively simple olfactory system (Onagbola et al., 2008; Zheng et al., 2020), there are rhinarial plates known as the principal odorant sensors (Park and Hardie, 2002; Couthinho-Abreu et al., 2014) found on their antennae (Onagbola et al., 2008; Zheng et al., 2020), containing plant volatile-sensing olfactory neurons (Chapman, 1982; Kristoffersen et al., 2008). Couthinho-Abreu et al., 2014 demonstrated that the antennal neurons in D. citri strongly respond to odorants found in citrus. Several studies with D. citri revealed that stimuli emitted by flushing shoots of the host are implicated in host location (Patt and Sétamou, 2010; Sétamou et al., 2012; Sule et al., 2012). Therefore, these studies point to the feasibility of developing an odorant-based approach for improving D. citri surveillance and control.
Nicotiana tabacum is an important commercial crop in China that serves as a natural source of active compounds with important applications in medicine and agriculture for centuries (Peng et al., 2022). For instance, nicotine (a natural compound from N. tabacum) and its synthetic derivatives were used as insecticides against sucking insect pests (Feyereisen, 2018). Fu et al. (2008) found that (E)-2-hexenal and (Z)-3-hexenyl acetate (GLVs from N. tabacum) significantly attracted Helicoverpa assulta Guenée and H. armigera Hübner to N. tabacum plants. Several studies have demonstrated that D. citri adults may move to surrounding non-host plants (at least temporarily) when host conditions are unfavorable and insecticide sprays induce their dispersal (Lewis-Rosenblum et al., 2015; Johnston et al., 2018; Johnston et al., 2019; Lu et al., 2021). In South China, it is a common practice to plant N. tabacum around citrus orchards, and our field survey found that D. citri adults on N. tabacum leaves that moved from nearby citrus orchards were all dead (Supplementary Figure 1). While the applied significance of this finding for D. citri and HLB management can be significant, the mechanistic basis of this process remains unclear. We hypothesized that the GLVs from N. tabacum plants are involved in D. citri host location, especially when adults move to N. tabacum plants due to unfavorable host conditions. Following, adults on N. tabacum are killed by the plant’s secondary metabolites such as nicotine. Therefore, the main objective of this study was to determine the preference and performance of D. citri on N. tabacum that could offer some practical methods to control the spread of D. citri and HLB.
Materials and methods
Insects
Adult D. citri were collected from a colony established in 2019 at Foshan University that was initiated from insects collected from disease-free, ornamental plants of Murraya exotica L. (Rutaceae) in South China Agriculture University. They were kept without exposure to insecticides in a nylon net room with dimensions of 2.5 m (length, L) × 1.5 m (width, W) × 2.5 m (height, H) at 28 ± 1°C, 70 ± 5% relative humidity, and 14:10 L:D photoperiod.
Plants
The plants used for the trials were potted seedlings. For the behavioral and survivorship assays and electrical penetration graph (EPG) experiments, potted seedlings of 3-year-old M. exotica (~40 cm) and N. tabacum (~30 cm, Yunyan 87) were used. They were grown with nutrient soil in a temperature-controlled greenhouse (28 ± 1°C) under natural sunlight conditions. Selected plants were washed and watered 24 h prior to the experiments.
Chemicals
According to Yi et al. (2017), the main GLVs from N. tabacum are (Z)-3-hexenal (CAS: 929-96-1, purity of 98%), (E)-2-hexenol (CAS: 928-95-0, purity of 97%), (E)-2-hexenal (CAS: 85761-70-2, purity of 98%), (Z)-3-hexenyl acetate (CAS: 3681-71-8, purity of 98%), and (E)-2-hexenyl acetate (CAS: 2497-18-9, purity of 98%), and all were purchased from Sigma-Aldrich (Shanghai, China). These chemicals were tested individually in a Y-tube experiment.
Behavioral response of D. citri adults
An H-tube olfactometer was used to test the preference of adult psyllids to N. tabacum plants. The H-tube olfactometer (Supplementary Figure 2) in this study was modified according to Khan and Saxena (1986). The H-tube olfactometer was made of odorless polypropylene plastic and consisted of two cylinders (arms) that were connected to a transverse tube (10-cm diameter, 30-cm long with a hole of 1 cm in the middle for releasing psyllids). The entire olfactometer was covered with an opaque cloth to protect psyllids from light. The N. tabacum plants served as an odor source, whereas the other (empty) arm was used as a control. Thereafter, starved adult psyllids were introduced individually in the middle of the transverse tube. If the tested individual crossed more than one-third of the choice area within 30 min after introduction, it was recorded as a positive response to the treatment or the control. Failure to move within this timeframe was recorded as no response. After 10 insects were tested, the samples at the two sides were exchanged to randomize any positional effects, and the H-tube olfactometer was washed with 75% alcohol. Each psyllid was tested only once, and 100 female and male D. citri were tested for each choice test. The adult psyllids were placed in glass tubes and deprived of food for 4 h prior to the experiments. Bioassays were performed under controlled conditions at 28 ± 1°C, 70 ± 5% relative humidity.
A Y-tube glass olfactometer was used to test the preferences of adult psyllids to the main GLVs from N. tabacum. The Y-tube olfactometer consisted of a 30-cm-long, 3-cm-diameter central tube and two 15-cm-long, 2-cm-diameter lateral arms, which were individually connected to the odor stimuli and a control through a Teflon connection. An atmospheric sample collector (model QC-1S, Beijing Labor Protection Institute Co. Ltd., Beijing, China) was used to draw the air to the apparatus. Air from the atmospheric sample collector was divided into two ways and passed through charcoal filters and humidifiers. Then, the humidified and purified air at 160 ml/min was introduced into the odor source bottles and each arm of the olfactometer (Supplementary Figure 3). The Y-tube was also covered with an opaque cloth to protect psyllids from light. In the tests, a 20-µl liquid paraffin applied on a filter paper (20 mm in diameter) was used as control, and 20 µl of the diluted test odor (20 µl/ml) applied on a filter paper (20 mm in diameter) served as an odor source. The test method and environment were the same as in the H-tube olfactometer assays.
Survival duration of D. citri adults
The young twigs of N. tabacum and M. exotica potted plants were covered with an 80-mesh nylon net, and 20 (3–6-day-old) mated D. citri females and males were released into the net. Each N. tabacum potted plant was a replicate. Adults released in an empty box with a mesh cover were treated as the negative control (starvation treatment), and M. exotica plants were used as a positive control. The mortality of D. citri adults in each treatment was observed and recorded once a day until they were all dead. In addition, the number of eggs laid on N. tabacum and M. exotica leaves was also recorded once a day until all adults were dead. The trials were conducted at 28 ± 1°C, 70 ± 5% relative humidity. There were six replicates for each treatment.
EPG analysis
A Giga-8 DC EPG system (Wageningen, the Netherlands) (Tjallingii, 1978) was used to record the feeding activities of adult D. citri on N. tabacum leaves. The D. citri adults were fixed using a negative pressure device. One end of a 3-cm length of gold wire (12.5-μm diameter) was connected to an amplifier through the EPG probe. The other end was connected to the dorsum of the D. citri adults with conductive silver glue (Wageningen Agricultural University). To complete the circuit, a copper electrode (10-cm length, 2-mm diameter) was inserted into the soil of the N. tabacum plants. There was a 30-min starvation period before the adult D. citri was placed on N. tabacum plants. All EPG experiments were recorded for 6 h and conducted inside a Faraday cage under 28 ± 1°C, 70 ± 5% relative humidity in a climate-controlled room. The EPG data were analyzed using EPG Stylet + software (Wageningen Agricultural University, 2012), and the waveforms recorded for D. citri probing behavior were characterized according to prior histological studies (Bonani et al., 2010; Yang et al., 2011; George et al., 2017). Windows Dataq Waveform Browser (Dataq Instruments Inc., Akron, OH, USA) was used to annotate waveforms. For the purposes of this study, five main feeding phases were distinguished by EPG, which were described based on their morphology (amplitude, frequency) and electrical (voltage level, electrical origin) characteristics and their correlations with stylet activities in the plant tissues (Supplementary Figure 4): (1) the non-probing phase (NP), (2) the pathway phase (PP, including intercellular probing of activity in the phloem (C) and phloem penetration (D)), (3) phloem salivation (E1), (4) phloem ingestion (E2), and (5) xylem ingestion (G) (Bonani et al., 2010; Yang et al., 2011; George et al., 2017). A total of 20 adult psyllids (10 males and 10 females) were recorded feeding on the N. tabacum potted plant.
Toxicological bioassays
The toxicity of L-nicotine to psyllids was tested using a leaf-dip method. L-Nicotine (CAS: 54-11-5, purity of 98.57%) was supplied by Beijing Putian Tongchuang Biotechnology Co., Ltd. (Beijing, China). Stock solutions (250, 500, 1,000, 1,500, 2,000, and 4,000 mg/L) of L-nicotine were prepared with acetone. Young citrus leaves were collected and dipped into the test solutions for 10 s and dried by natural air ventilation. The leaves, with the leaf petiole covered with moist cotton, were placed into Petri dishes containing moistened filter papers to avoid desiccation of the leaves. Mock controls used citrus leaves treated with acetone only. Thirty adults were introduced into each Petri dish for a total of three Petri dishes (replicates) per L-nicotine concentration and held at 28 ± 1°C, 70 ± 5% relative humidity, and a photoperiod of 14:10 h (L:D). The mortality of D. citri adults in each treatment was observed and recorded after 12, 24, 48, and 72 h of exposure.
Statistical analyses
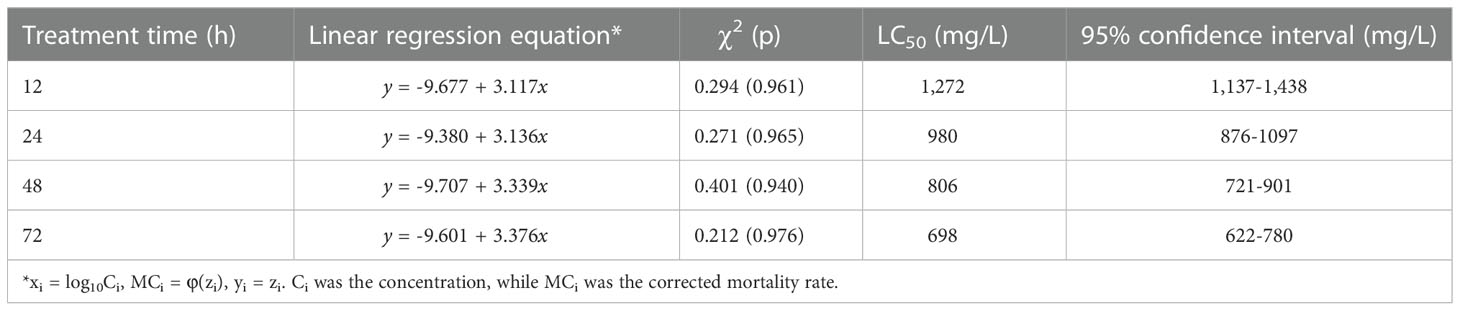
Statistical analysis was performed using IBM SPSS Statistics 25.0. The H-tube and Y-tube olfactometer data were analyzed with the likelihood ratio statistic test (Kleinbaum and Klein, 2010). The survival rate of adult psyllids feeding on N. tabacum leaves was calculated using Kaplan–Meier’s survival analysis and was subjected to log-rank (Mantel–Cox) coefficient. EPG data were subjected to analysis of variance (ANOVA), and means were compared by the least significant difference (LSD) test. For toxicological bioassays, the corrected mortality rate (MC) of each treatment was calculated by the Abbot formula MC = (M2-M1)/(1-M1) × 100, where M1 is the control mortality and M2 is the mortality of each treatment. LC50 was estimated using Probit analysis (Bliss, 1934).
Results
Behavioral response of adult D. citri to N. tabacum and its main GLVs
Diaphorina citri females and males were attracted to N. tabacum plants, (Z)-3-hexenol, and (E)-2-hexenol compared with the control (all P < 0.05) (Table 1). In addition, males were attracted to (E)-2-hexenal and (Z)-3-hexenyl acetate (Table 1). There were no significant differences between the numbers of female D. citri adults choosing (E)-2-hexenal, (Z)-3-hexenyl acetate, and (E)-2-hexenyl acetate and male D. citri adults choosing (E)-2-hexenyl acetate when compared with the control (all P > 0.05) (Table 1).
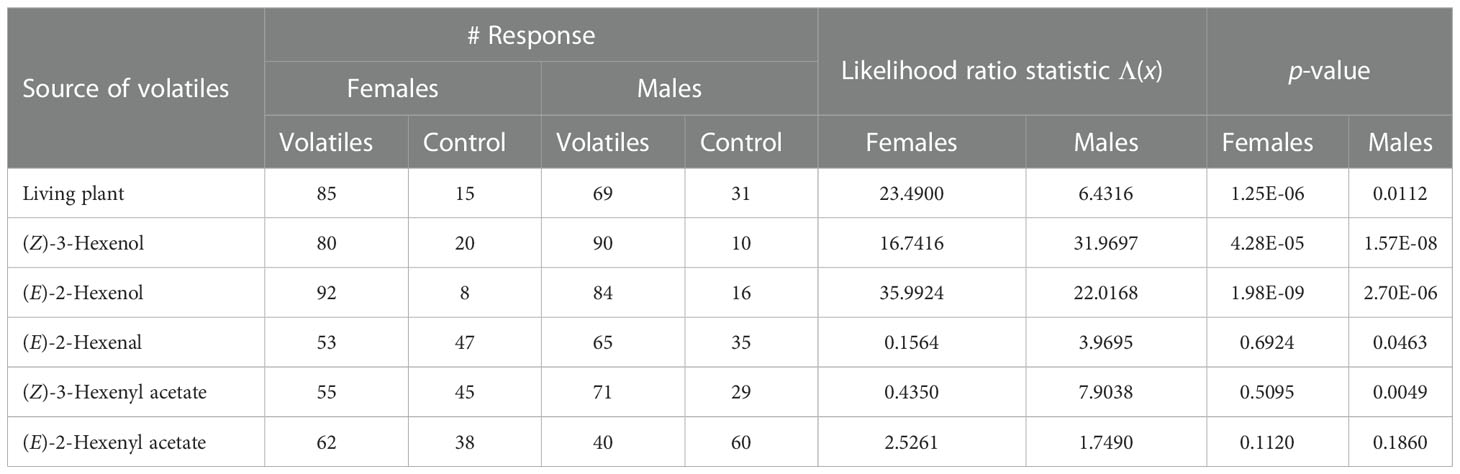
Table 1 Responses of Diaphorina citri adults to tobacco plant and synthetic standards of green leaf volatiles (GLVs).
Survival duration of D. citri adults
Survival of D. citri females and males on M. exotica was similar and less than 29 days, with high survival until 15 days of feeding (Figures 1A, B). Moreover, females laid eggs on M. exotica leaves from days 5 to 20. The number of eggs laid on M. exotica peaked on day 15 (Figure 1B).
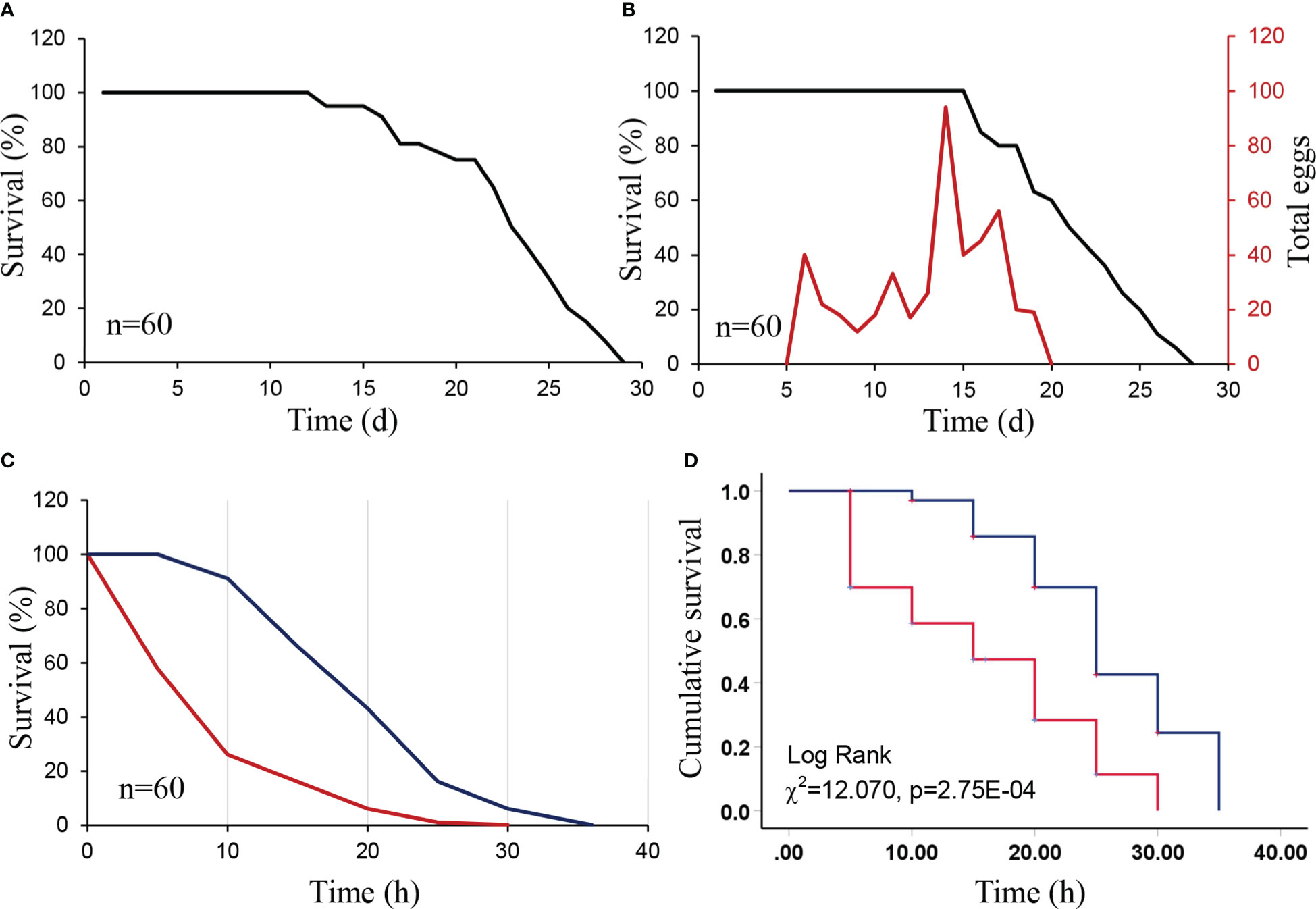
Figure 1 Survival curve and cumulative survival functions of Diaphorina citri adults over time when feeding on Nicotiana tabacum and a preferred host Murraya exotica (positive treatment). We also included a starvation treatment as a negative control. (A) Survival curve of male D. citri adults feeding on M. exotica. (B) Survival and fecundity curves of female D. citri adults feeding on M. exotica. (C) Survival curves of D. citri adults feeding on N. tabacum (red line) and starved (blue line). (D) Cumulative survival functions of D. citri adults on N. tabacum (red line) and the starved (control, blue line).
Survival of D. citri adults on N. tabacum lasted less than 30 h and was less than the starvation treatment (35 h) (Figures 1C, D). There were significant differences in the survival of adult D. citri between N. tabacum and the starvation treatment (χ2 = 12.070, P = 2.75E-04). In addition, the mean and median survival of adult D. citri on N. tabacum was 15.77 and 15 h, which are much shorter than those in the starvation treatment (25.976 and 25 h, respectively). Additionally, there were no eggs laid on N. tabacum leaves.
Diaphorina citri feeding behavior on N. tabacum
Figure 2 shows a representative EPG trace of the different feeding phases for D. citri on N. tabacum leaves. Diaphorina citri rarely had a waveform of xylem ingestion (G) on N. tabacum. The EPG waveform characteristics of pathway and phloem probing events on N. tabacum, involving the waveforms of NP (non-probing), PP (C and D), E1, and E2, are shown in Figure 2. Waveform C was the first waveform event, with an average amplitude of 40% and a frequency of 14–18 Hz. Waveform D possessed a lower frequency range of 3–7 Hz, whereas waveform E2 showed the highest frequency (14 Hz) (Table 2). As shown in Figure 3, during 6 h of feeding, the phloem ingestion phase (E2) was 52.5% of the total feeding time, and its duration was twice as long as the NP waveform (P = 0.000599), indicating that D. citri could feed on N. tabacum leaves.
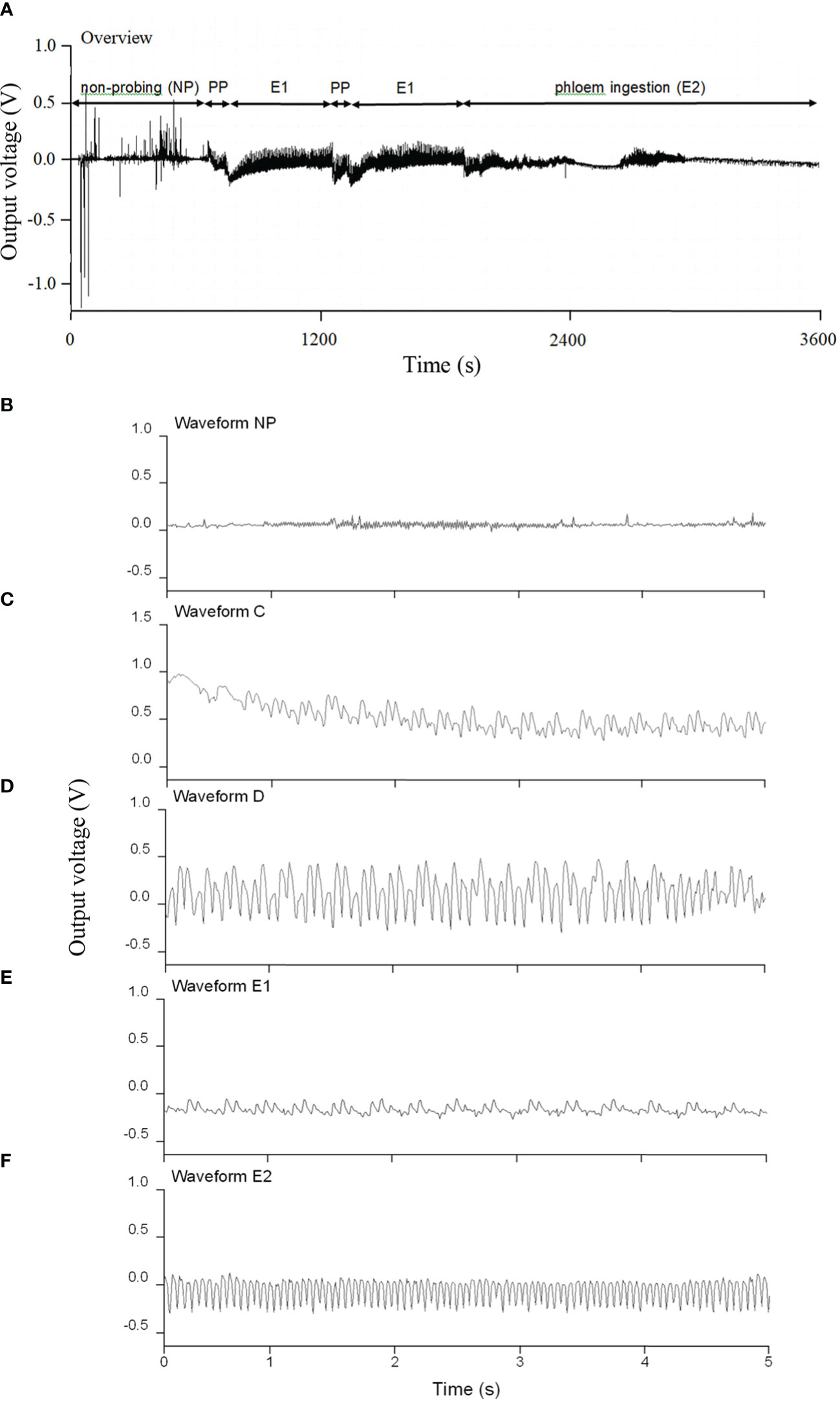
Figure 2 Typical electrical penetration graph (EPG) of an adult Diaphorina citri feeding on Nicotiana tabacum leaves. (A) EPG trace for the first 1 h after the start of assessment (NP = non-probing phase; PP = pathway phase (C + D), including intercellular probing of activity in the phloem (C) and phloem penetration (D); E1 = phloem salivation; E2 = phloem ingestion). (B) Enlargement of waveform NP. (C) Enlargement of waveform C. (D) Enlargement of waveform D. (E) Enlargement of waveform E1. (F) Enlargement of waveform E2.
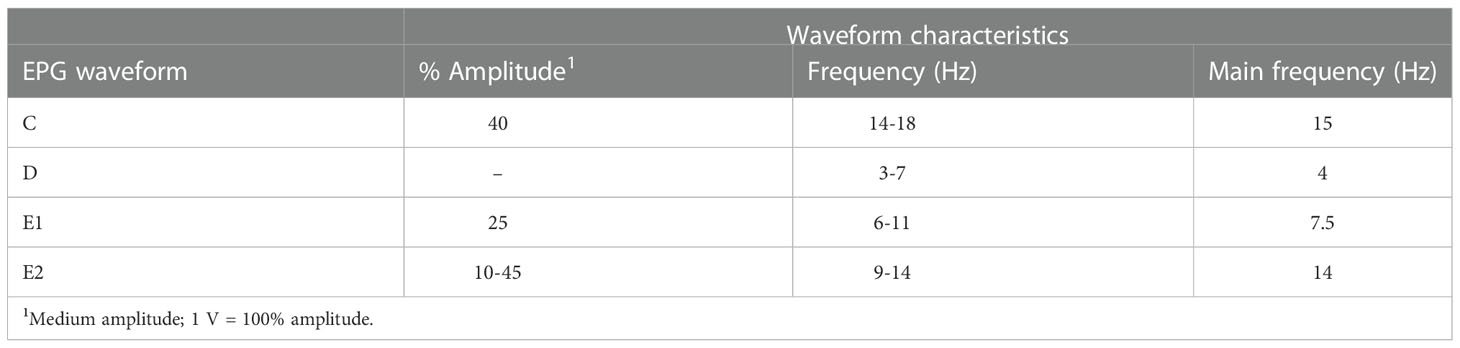
Table 2 Main characteristics of Diaphorina citri electrical penetration graph (EPG) waveforms on tobacco leaves.
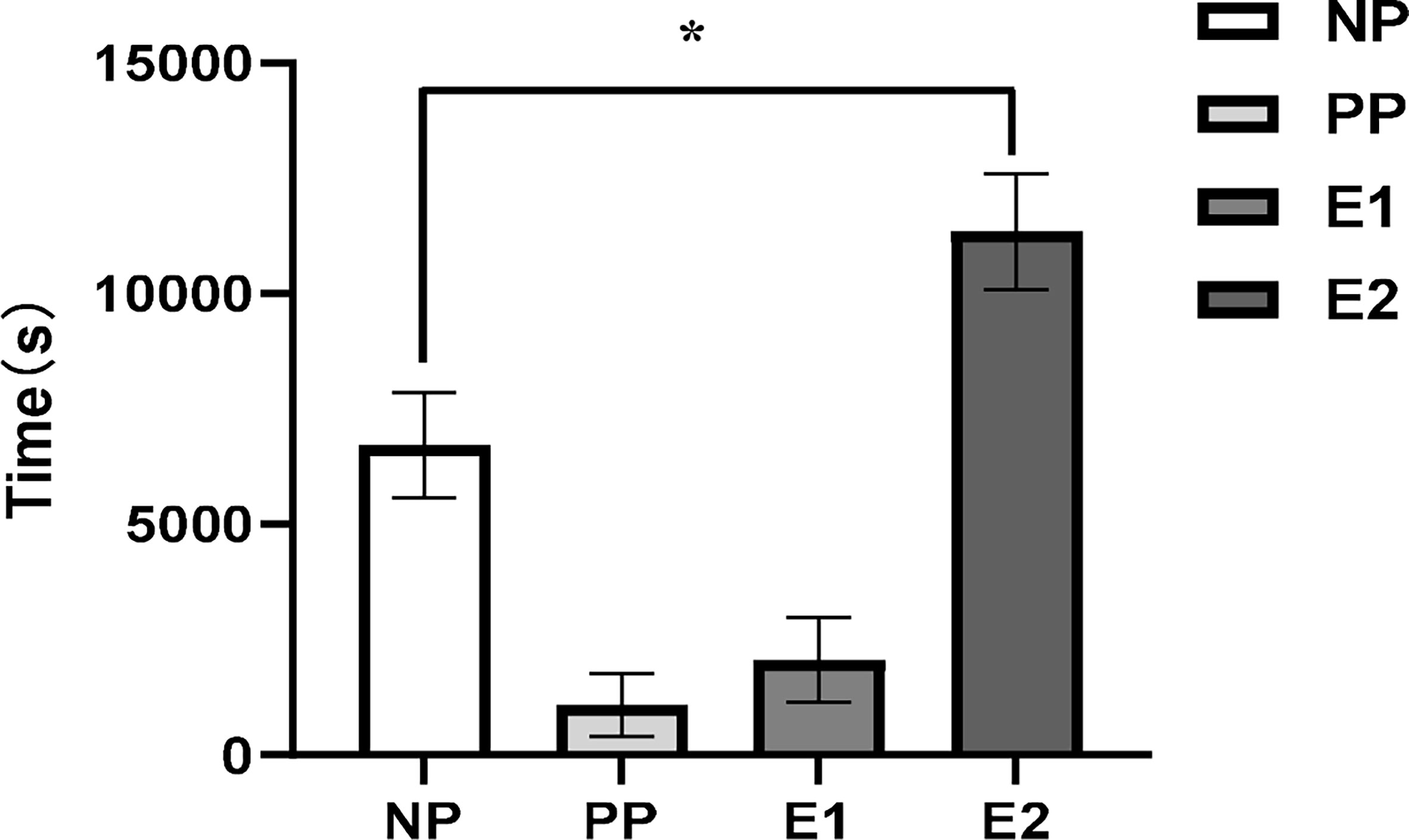
Figure 3 Mean ( ± SE, n = 5) duration of each feeding waveforms of adult Diaphorina citri feeding on Nicotiana tabacum leaves (ANOVA, LSD, *P < 0.05). (NP, non-probing phase; PP, pathway phase; E1, phloem salivation; E2, phloem ingestion).
Toxicity of L-nicotine on adult D. citri
The toxicity of L-nicotine on adult D. citri increased with increasing concentrations, as well as the treatment time (Figure 4). The corrected mortality rates of 2,000 and 4,000 mg/l were much higher than those of other concentrations, which was more than 70 and 95%, respectively. At the concentration of 1,000 mg/l, the corrected mortality rate (66.6%) at 72 h after exposure was twice than that of 12 h (33.3%). In addition, the LC50 for adult D. citri was 1,272 and 698 mg/l at 12 and 72 h after exposure, respectively. In general, the toxicity of L-nicotine on adult D. citri increased in time and was the highest at 72 h of exposure (Table 3).
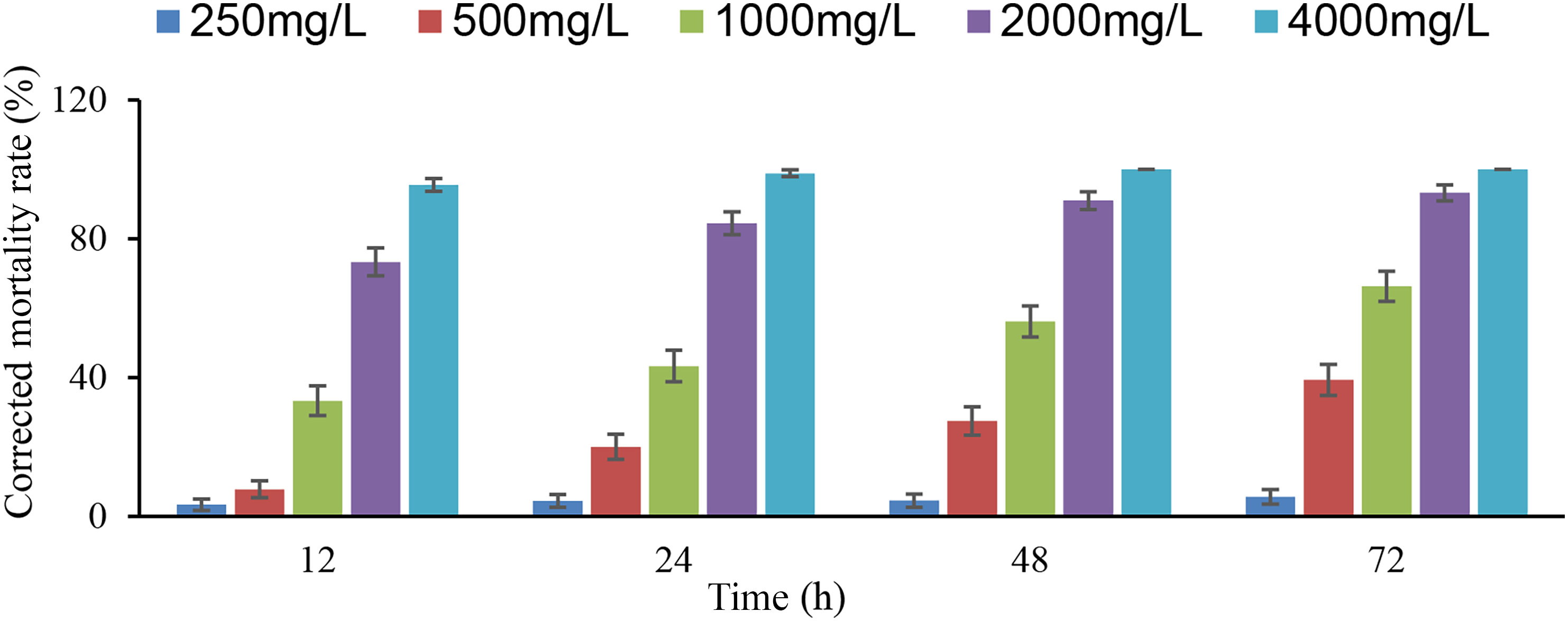
Figure 4 Corrected mortality rate of different concentrations of L-nicotine on Diaphorina citri adults.
Discussion
Our study revealed that N. tabacum and synthetic GLVs (Z)-3-hexenol and (E)-2-hexenol are attractive to adult D. citri, supporting the notion that this insect relies on olfaction to locate its host plants. GLVs, especially leaf alcohols, play an important role in host location for other phytophagous insects, for example (Z)-3-hexen-1-ol and 1-hexanol for Lygus rugulipennis Poppius (Frati et al., 2008) and (±)-2-hexanol for Aleurodicus dispersus Russell (Zheng et al., 2014). (E)-2-hexenal and (Z)-3-hexenyl acetate, the synthetic GLVs from N. tabacum, were also attractive to male D. citri adults (Table 3), and (Z)-3-hexenyl acetate was reported to synergize with the sex pheromone of H. armigera (Kvedaras et al., 2007). Xia et al. (2017) found that D. citri adults prefer N. tabacum plants rather than Murcott tangor (host plant). In addition, previous studies have reported that D. citri adults may be able to use some non-host plants opportunistically as secondary host plants for acquiring water or alternate food when optimal hosts are scarce or absent (George et al., 2020; Lu et al., 2021). Thus, D. citri adults are likely to move from citrus orchards to N. tabacum if they grow next to each other.
Our EPG results showed that D. citri adults only secreted saliva and ingested sap from phloem in N. tabacum leaves and spent the longest duration in phloem sap ingestion (E2) (Figure 2), which is evidence of a phloem-feeding habit in this psyllid species (Bonani et al., 2010). Diaphorina citri adults performed more phloem feeding activities (D, E1, E2) than other stylet activities (NP, C) on N. tabacum leaves, which agree with observations that adults more readily feed on phloem from citrus to obtain nutrition (Bonani et al., 2010; Zhu et al., 2010). Moreover, CLas can occur in N. tabacum (Francischini et al., 2007), and Xia et al. (2017) reported that D. citri adults can also transfer the bacteria to N. tabacum after feeding. The survival time of D. citri on N. tabacum was much shorter than that under starvation conditions, suggesting that the death of adults on N. tabacum was not caused by starvation but could be related to the toxic components of N. tabacum. Nicotine is the predominant alkaloid accumulating in the leaves of most N. tabacum varieties and represents 90–95% of the total alkaloid content (Wang et al., 2000; Shoji et al., 2010). Several studies have demonstrated that neonicotinoid insecticides derived from nicotine, such as thiamethoxam and imidacloprid, are highly toxic to D. citri (Boina and Bloomquist, 2015; Miranda et al., 2016).
Previous studies have reported that D. citri may disperse from citrus orchards and utilize secondary host plants as a reservoir, facilitating their survival when primary host conditions are unfavorable or when these hosts are sprayed with insecticides (Lewis-Rosenblum et al., 2015; Johnston et al., 2018; Johnston et al., 2019; Lu et al., 2021). However, our study showed that D. citri adults that move to N. tabacum from citrus orchards would die after feeding on these secondary plants. Thus, N. tabacum could be used as a dead-end trap for adult D. citri.
For N. tabacum to kill adult D. citri, two conditions must be met. (i) Nicotiana tabacum should be grown nearby citrus crops. Diaphorina citri is critical for the spread of CLas due to their strong capacity to move between infected and healthy trees within an orchard (Wu et al., 2018). Insecticide applications to control D. citri are the primary management strategy currently recommended for CLas (Stansly et al., 2012) and could promote this movement. (ii) Diaphorina citri adults must be pushed away from citrus trees by insecticide applications or as a result of poor host conditions. Basically, adult D. citri dispersal requires citrus trees to either lack citrus flushes or to be sprayed with pesticides. However, while moving to N. tabacum, D. citri are unable to survive (Figure 1, Xie et al., 2015; Xia et al., 2017). In this context, N. tabacum could be used as a dead-end trap plant, which has considerable potential in integrated pest management (IPM) for D. citri. Several kinds of dead-end trap plants have been suggested and used in IPM, such as N. tabacum and Barbarea vulgaris R. Br. for Plutella xylostella (L.) larvae (Gupta and Thorsteinson, 1960; Shelton and Nault, 2004; Møldrup et al., 2012) and Vetiveria zizanioides (L.) Nash for Chilo suppressalis Walker larvae (Lu et al., 2019).
Diaphorina citri eggs were only observed on M. exotica plants (Figure 1B). The pre-oviposition period of D. citri females ranged from 7 to 20 days (Li et al., 2019); thus, it is likely that females on N. tabacum died before laying eggs. Furthermore, adults on N. tabacum died within 30 h of feeding, whereas they initiated oviposition 5 days after feeding on M. exotica. In other words, N. tabacum is very effective as a dead-end trap because D. citri females were attracted to the plants, fed on them, and died shortly prior to laying any eggs. Our results suggest that it is worthwhile to grow N. tabacum in and around citrus orchards. To optimize area-wide management of D. citri, field studies are needed to evaluate the use of N. tabacum surrounding citrus orchards as dead-end traps.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
LZ, WW, and WC designed the study. LZ performed the majority of experiments with the help of QX, GG, and YL. GG and WW analyzed the data, and LZ wrote the first version of the manuscript. MY, SS, and WC revised the manuscript and approved the submitted version. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Nos. 32202277 and 31660517).
Acknowledgments
We would like to express our sincere gratitude to the editors and reviewers who have put considerable time and effort into their comments on this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2022.1081663/full#supplementary-material
References
Arshad, M., Ullah, M. I., Çağatay, N. S., Dikmen, F., Abdullah, A., Afzal, M. (2019). Cordia myxa l., a new host plant record for Asian citrus psyllid, Diaphorina citri kuwayama. Southwest Entomol. 44, 331–334. doi: 10.3958/059.044.0137
Boina, D. R., Bloomquist, J. R. (2015). Chemical control of the Asian citrus psyllid and of huanglongbing disease in citrus. Pest Manage. Sci. 71 (6), 808–823. doi: 10.1002/ps.3957
Bonani, J. P., Fereres, A., Garzo, E., Miranda, M. P., Appezzato-Da-Gloria, B., Lopes, J. R. S. (2010). Characteriztion of electrical penetration graphs of the Asian citrus psyllid, Diaphorina citri, in sweet orange seedlings. Entomol. Experimentalis Applicata 134, 35–49. doi: 10.1111/j.1570-7458.2009.00937.x
Bové, J. M. (2006). Huanglongbing: A destructive, newly emerging, century-old disease of citrus. J. Plant Pathol. 88, 7–37. doi: 10.2307/41998278
Chapman, R. F. (1982). Chemoreception-the significance of receptor numbers. Adv. Insect Physiol. 16, 247–356. doi: 10.1016/S0065-2806(08)60155-1
Chin, E., Mishchuk, D., Bruce, J., Cilia, M., Coaker, G., Davis, C., et al. (2014). An interdisciplinary approach to combat HLB. Citrograph 5, 28–34. www.citrusresearch.org/citrograph-winter-2014
Coutinho-Abreu, I. V., Forster, L., Guda, T., Ray, A. (2014). Odorants for surveillance and control of the Asian citrus psyllid (Diaphorina citri). PLoS ONE 9(10), e109236. doi: 10.1371/journal.pone.0109236
da Graça, J. V., Douhan, G. W., Halbert, S. E., Keremane, M. L., Lee, R. F., Vidalakis, G., et al. (2016). Huanglongbing: An overview of a complex pathosystem ravaging the world’s citrus. J. Integr. Plant Biol. 58, 373–387. doi: 10.1111/jipb.12437
Felisberto, P. A., Girardi, E. A., Peña, L., Felisberto, G., Beattie, G. A., Lopes, S. A. (2019). Unsuitability of indigenous south American rutaceae as potential hosts of Diaphorina citri. Pest Manage. Sci. 75, 1911–1920. doi: 10.1002/ps.5304
Feyereisen, R. (2018). Toxicology: Bee P450s take the sting out of cyanoamidine neonicotinoids. Curr. Biol. 28, 560–562. doi: 10.1016/j.cub.2018.03.013
Francischini, F. J. B., Oliveira, K. D. S., Astú-Monge, G., Novelli, A., Lorenzino, R., Matiolli, C., et al. (2007). First report on the transmission of ‘Candidatus liberibacter asiaticus’ from citrus to Nicotiana tabacum cv. xanthi. Plant Dis. 91, 631. doi: 10.1094/pdis-91-5-0631b
Frati, F., Salerno, G., Conti, E., Bin, F. (2008). Role of the plant-conspecific complex in host location and intra-specific communication of Lygus rugulipennis. Physiol. Entomol 33, 129–137. doi: 10.1111/j.1365-3032.2008.00614.x
Fu, X. W., Guo, X. R., Luo, M. H., Yuan, G. H., Zheng, L. W., Wu, S. Y. (2008). Electrophysiological and behavioral responses of Helicoverpa assulta (Guenée) and H. armigera (Hübner) (Lepidoptera: Noctuidae) to tobacco volatile compounds of high concentration. Acta Entomol. Sin. 51, 902–909. doi: 10.3779/j.issn.1009-3419.2005.05.09
George, J., Ammar, E. D., Hall, D. G., Lapointe, S. L. (2017). Sclerenchymatous ring as a barrier to phloem feeding by Asian citrus psyllid: Evidence from electrical penetration graph and visualization of stylet pathways. PloS One 12 (3), e0173520. doi: 10.1371/journal.pone.0173520
George, J., Kanissery, R., Ammar, E. D., Cabral, I., Markle, L. T., Patt, J. M., et al. (2020). Feeding behavior of Asian citrus psyllid [Diaphorina citri (Hemiptera: Liviidae)] nymphs and adults on common weeds occurring in cultivated citrus described using electrical penetration graph recordings. Insects 11, 1–17. doi: 10.3390/insects11010048
Ghanim, M., Achor, D., Ghosh, S., Kontsedalov, S., Lebedev, G., Levy, A. (2017). ‘Candidatus liberibacter asiaticus’ accumulates inside endoloasmic reticulum associated vacuoles in the gut cells of Diaphorina citri. Sci. Rep. 7, 16945. doi: 10.1038/s41598-017-16095-w
Gupta, P. D., Thorsteinson, A. J. (1960). Food plant relationships of the diamondback moth (Plutella maculipennis (Curt.)). Entomol. Exp. Appl. 3, 241–250. doi: 10.1111/j.1570-7458.1960.tb00454.x
Halbert, S. E., Manjunath, K. L. (2004). Asian Citrus psyllids (Sternorrhyncha: Psyllidae) and greening disease of citrus: a literature review and assessment of risk in Florida. Florida Entomol. 87, 330–353. doi: 10.1653/0015-4040(2004)087[0330:acpspa]2.0.co;2
Holopainen, J. K., Gershenzon, J. (2010). Multiple stress factors and the emission of plant VOCs. Trends Plant Sci 15(3), 176–184. doi: 10.1016/j.tplants.2010.01.006
Inoue, H., Ohnishi, J., Ito, T., Tomimura, K., Miyata, S., Iwanami, T., et al. (2009). Enhanced proliferation and efficient transmission of Candidatus liberibacter asiaticus by adult Diaphorina citri after acquisition feeding in the nymphal stage. Ann. Appl. Biol. 155, 29–36. doi: 10.1111/j.1744-7348.2009.00317.x
Johnston, N., Stansly, P. A., Stelinski, L. L. (2019). Secondary hosts of the Asian citrus psyllid, Diaphorina citri kuwayama: Survivorship and preference. J. Appl. Entomol. 143, 921–928. doi: 10.1111/jen.12673
Johnston, N., Stelinski, L. L., Stansly, P. A. (2018). Dispersal patterns of Diaphorina citri (Kuwayama) as influenced by citrus grove management and abiotic factors. Fla. Entomol. 102, 168–173. doi: 10.1653/024.102.0127
Khan, Z. R., Saxena, R. C. (1986). Effect of steam distillate extracts of resistant and susceptible rice cultivars on behavior of Sogatella furcifera (Homoptera: Delphacidae). J. Econ. Entomol. 79, 928–935. doi: 10.1093/jee/79.4.928
Kristoffersen, L., Larsson, M. C., Anderbrant, O. (2008). Functional characteristics of a tiny but specialized olfactory system: olfactory receptor neurons of carrot psyllids (Homoptera: Triozidae). Chem. Sens. 33, 759–769. doi: 10.1093/chemse/bjn034
Kvedaras, O. L., Del Socorro, A. P., Gregg, P. C. (2007). Effects of phenylacetaldehyde and z-3-hexenyl acetate on male response to synthetic sex pheromone in Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Aust. J. Entomol. 46, 224–230. doi: 10.1111/j.1440-6055.2007.00596.x
Lewis-Rosenblum, H., Martini, X., Tiwari, S., Stelinski, L. L. (2015). Seasonal movement patterns and long-range dispersal of Asian citrus psyllid in Florida citrus. J. Econom. Entomol. 108, 3–10. doi: 10.1093/jee/tou008
Li, H. L., Zheng, X. L., Wang, X. Y., Lu, W. (2019). Prophase of reproductive behavior and activity rhythm in adults of Diaphorina citri (Kuwayama). J. South. Agric. 50 (9), 2009–2014. doi: 10.3969/j.issn.2095-1191.2019.09.16
Lu, H. L., Fang, X. D., Wu, F. N., Ouyang, G. C. (2021). Adaptability and ‘Candidatus liberibacter asiaticus’ titres of Diaphorina citri adults on three weed species in China. Pest Manage. Sci. 77, 3261–3223. doi: 10.1002/ps.6360
Lu, Y. H., Zheng, X. S., Lu, Z. X. (2019). Application of vetiver grass Vetiveia zizanioides: Poaceae (L.) as a trap plant for rice stem borer Chilo suppressalis: Crambidae (Walker) in the paddy fields. J. Integr. Agric. 18 (4), 797–804. doi: 10.1016/s2095-3119(18)62088-x
Møldrup, M. E., Geu-Flores, F., de Vos, M., Olsen, C. E., Sun, J., Jander, G., et al. (2012). Engineering of benzylglucosinolate in tobacco provides proof-of-concept for dead-end trap crops genetically modified to attract Plutella xylostella (diamondback moth). Plant Biotechnol. J. 10, 435–442. doi: 10.1111/j.1467-7652.2011.00680.x
Miranda, M. P., Yamamoto, P. T., Garcia, R. B., Lopes, J. P. A., Lopes, J. R. S. (2016). Thiamethoxam and imidacloprid drench applications on sweet orange nursery trees disrupt the feeding and settling behavior of Diaphorina citri (Hemiptera: Liviidae). Pest Manage. Sci. 72 (9), 1785–1793. doi: 10.1002/ps.4213
Monzo, C., Stansly, P. A. (2015). Thresholds for vector control and compatibility with beneficial fauna in citrus with high incidence of huanglongbing. Acta Hortic. 1065, 1137–1143. doi: 10.17660/actahortic.2015.1065.14
Onagbola, E. O., Meyer, W. L., Boina, D. R., Stelinski, L. L. (2008). Morphological characterization of the antennal sensilla of the Asian citrus psyllid, Diaphorina citri kuwayama (Hemiptera: Psyllidae), with reference to their probable functions. Micron 39, 1184–1191. doi: 10.1016/j.micron.2008.05.002
Park, K. C., Hardie, J. (2002). Functional specialization and polyphenism in aphid olfactory sensilla. J. Insect Physiol. 48, 527–535. doi: 10.1016/s0022-1910(02)00082-3
Patt, J. M., Sétamou, M. (2010). Responses of the Asian citrus payllid to volatiles emitted by the flushing shoots of its rutaceous host plants. Environ. Entomol. 39, 618–624. doi: 10.1603/en09216
Pelz-Stelinski, K. S., Brlansky, H. R., Ebert, T. A., Rogers, M. E. (2010). Transmission parameters for Candidatus liberibacter asiaticus by Asian citrus psyllid. J. Econ. Entomol. 103, 1531–1541. doi: 10.1603/ec10123
Pelz-Stelinski, K. S., Killiny, N. (2016). Better together: association with ‘Candidatus liberibacter asiaticus’ increases the reproductive fitness of its insect vector, Diaphorina citri (Hemiptera: Liviidae). Ann. Entomol. Soc Am. 109, 371–376. doi: 10.1093/aesa/saw007
Peng, M. J., Zhao, H. M., Zhao, C. Q., Lv, F., Li, G. D., Wang, G., et al. (2022). Diterpenes from oriental tobacco Nicotiana tabacum ‘YNOTBS1’ and their bioactivities. Natural Product Res. 24, 1–8. doi: 10.1080/14786419.2021.2025367
Qureshi, J. A., Kostyk, B. C., Stansly, P. A. (2014). Insecticidal suppression of Asian citrus psyllid Diaphorina citri (Hemiptera: Liviidae) vector of huanglongbing pathogens. PloS One 9, e112331. doi: 10.1371/journal.pone.0112331
Riddiford, L. M. (1967). Trans-2-hexenal: mating stimulant for polyphemus moths. Science 158, 139–140. doi: 10.2307/1722403
Sétamou, M., Sanchez, A., Patt, J. M., Nelson, S. D., Jifon, J., Louzada, E. S. (2012). Diurnal patterns of flight activity and effects of light on host finding behavior of the Asian citrus psyllid. J. Insect Behav. 25, 264–276. doi: 10.1007/s10905-011-9295-3
Shelton, A. M., Nault, B. A. (2004). Dead-end trap cropping: a technique to improve management of the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). Crop Prot. 23, 497–503. doi: 10.1016/j.cropro.2003.10.005
Shoji, T., Kajikava, M., Hashimoto, T. (2010). Clustered transcription factor genes regulate nicotine biosynthesis in tobacco. Plant Cell 22, 3390–3409. doi: 10.1105/tpc.110.078543
Stansly, P. A., Arevalo, H. A., Qureshi, J. A., Jones, M. M., Hendricks, K., Roberts, P. D., et al. (2014). Vector control and foliar nutrition to maintain economic sustainability of bearing citrus in Florida groves affected by huanglongbing. Pest Manage. Sci. 70, 415–425. doi: 10.1002/ps.3577
Stansly, P., Qureshi, J., Kostyk, B. (2012). Effectiveness ranking for insecticides against Asian citrus psyllid. Citrus Industry 93, 6–9. doi: swfrec.ifas.ufl.edu/docs/pdf/entomology/tja_0002.pdf
Sule, H., Muhamad, R., Omar, D., Hee, A. K. W. (2012). Response of Diaphorina citri kuwayama (Hemiptera: Psyllidae) to volatiles emitted from leaves of two rutaceous plants. J. Agric. Sci. 4, 152. doi: 10.5539/jas.v4n6p152
Thomas, D. B., de León, J. H. (2011). Is the old world fig. Ficus carica l. (Moraceae), an alternative host for the asian citrus psyllid, Diaphorina citri (Kuwayama) (Homoptera: Psyllidae)? Fla Entomol 94, 1081–1084. doi: 10.1653/024.094.0455
Tiwari, S., Lewis-Rosenblum, H., Pelz-Stelinski, K., Stelinski, L. L. (2010). Incidence of ‘Candidatus liberibacter asiaticus’ infection in abandoned citrus occurring in proximity to commercially managed groves. J. Econ. Entomol. 103, 1972–1978. doi: 10.1603/ec10149
Tiwari, S., Mann, R. S., Rogers, M. E., Stelinski, L. L. (2011). Insecticide resistance in field populations of Asian citrus psyllid in Florida. Pest Manage. Sci. 67, 1258–1268. doi: 10.1002/ps.2181
Tjallingii, W. (1978). Electronic recording of penetration behavior by aphids. Entomol. Exp. Appl. 24, 721–730. doi: 10.1111/j.1570-7458.1978.tb02836.x
Wang, J. M., Sheehan, M., Brookman, H., Timko, M. P. (2000). Characterization of cDNAs differentially expressed in roots of tobacco (Nicotiana tabacum cv burley 21) during the early stages of alkaloid biosynthesis. Plant Sci. 158, 19–32. doi: 10.1016/s0168-9452(00)00293-4
Wu, L. H., Chen, R. C., Han, R. C., Wei, H. Y., Zheng, L. X. (2018). Study on the preference of Diaphorina citri kuwayama to different colors. J. Fruit Sci. 35, 1509–1515. doi: 10.13925/j.cnki.gsxb.20180173
Xia, C. X., Meng, L. X., Yan, X., Zhang, H. Y., Zhao, Z., Zhang, Y. B., et al. (2017). Study on the relationship between Diaphorina citri and Nicotiana tabacum. South China Fruits 46, 14–16. doi: 10.13938/j.issn.1007-1431.20170077
Xie, X. T., Liu, W. D., Lai, J. F., Peng, L., Lai, H. R. (2015). A preliminary study on the feeding and survival of Diaphorina citri kuwayama (Hemiptera: Liviidae). South China Fruits 44, 41–42. doi: 10.13938/j.issn.1007-1431.20150370
Yang, C. L., Cen, Y. J., Liang, G. W., Chen, H. Y. (2011). Study on the electrical penetration graph of Diaphorina citri. J. South China Agric. Univ. 32, 49–51. doi: 10.7671/j.issn.1001-411X.2011.01.011
Yi, S. Y., Ku, S. S., Sim, H.-J., Kim, S. K., Park, J. H., Lyu, J. I., et al. (2017). An alcohol dehydrogenase gene from synechocystis sp. confers salt tolerance in transgenic tobacco. Front. Plant Sci., 8, 1965. doi: 10.3389/fpls.2017.01965
Zheng, L. X., Liang, Q. C., Yu, M., Cao, Y., Chen, W. S. (2020). Morphological characterization of antennae and antennal sensilla of Diaphorina citri kuwayama (Hemiptera: Liviidae) nymphs. PloS One 15, e0234030. doi: 10.1371/journal.pone.0234030
Zheng, L. X., Wu, W. J., Fu, Y. G. (2014). (±)-2-Hexanol from Pterocarpus indicus leaves as attractant for female Aleurodicus dispersus (Hemiptera: Aleyrodidae). Afr. Entomol. 22, 267–272. doi: 10.4001/003.022.0224
Keywords: Asian citrus psyllid, Nicotiana tabacum, Huanglongbing, nonhost plant, EPG, L-nicotine
Citation: Zheng L, Xu Q, Gong G, Liao Y, Yu M, Shabala S, Chen W and Wu W (2023) Nicotiana tabacum as a dead-end trap for adult Diaphorina citri: A potential biological tactic for protecting citrus orchards. Front. Plant Sci. 13:1081663. doi: 10.3389/fpls.2022.1081663
Received: 27 October 2022; Accepted: 13 December 2022;
Published: 06 January 2023.
Edited by:
Gen-ichiro Arimura, Tokyo University of Science, JapanReviewed by:
Cesar Rodriguez-Saona, Rutgers, The State University of New Jersey, United StatesYooichi Kainoh, University of Tsukuba, Japan
Copyright © 2023 Zheng, Xu, Gong, Liao, Yu, Shabala, Chen and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weijian Wu, d2Vpand1QHNjYXUuZWR1LmNu; Wensheng Chen, Y2pmMDAwMEAxNjMuY29t
 Lixia Zheng
Lixia Zheng Qianqian Xu2
Qianqian Xu2 Sergey Shabala
Sergey Shabala