
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci., 13 January 2023
Sec. Aquatic Photosynthetic Organisms
Volume 13 - 2022 | https://doi.org/10.3389/fpls.2022.1069842
This article is part of the Research TopicAquatic Photosynthetic Organisms under Global ChangeView all 10 articles
 Weronika A. Makuch1,2,3*†
Weronika A. Makuch1,2,3*† Stefan Wanke3,4†
Stefan Wanke3,4† Barbara Ditsch5
Barbara Ditsch5 Frank Richter6
Frank Richter6 Veit Herklotz7†
Veit Herklotz7† Julian Ahlborn7†
Julian Ahlborn7† Christiane M. Ritz7,8†
Christiane M. Ritz7,8†Information provided by population genetic studies is often necessary to effectively protect endangered species. In general, such data is scarce for aquatic plants and this holds also for Luronium natans, an aquatic macrophyte endemic to northwestern and western Europe. It is threatened across its whole distribution range due to human influences, in particular due to eutrophication and intensive fish farming. In spite of habitat protection populations continue to decline and re-introductions are one possibility to prevent the species’ extinction. Therefore, insights in genetic diversity and relatedness of source populations is warranted.
Thus, we performed Amplified Fragment-Length Polymorphism (AFLP) on two large populations in Saxony, Germany (Großenhainer Pflege and Niederspree), complemented with numerous additional occurrences from Europe. In addition, we conducted experiments on plant growth to assess optimal conditions for ex-situ cultivation taking water temperature, water level and substrate into account.
We revealed considerably high levels of genetic diversity within populations (Shannon Indices ranged from 0.367 to 0.416) implying that populations are not restricted to clonal growth only but reproduce also by open-pollinated flowers. Remarkably, the two geographically close Saxon populations were genetically distant to each other but subpopulations within a locality were completely intermingled. Concerning optimal cultivation conditions, longest roots were obtained at temperatures >14°C and saturated, but not submerging water levels.
Thus, our findings advocate for a re-introduction scheme from nearby source populations and provide detailed information on successful ex-situ cultivation.
The loss of aquatic vegetation has been accelerating in the last four decades (Zhang et al., 2017). Oligotrophic waterbodies such as lakes and ponds are among the most threatened habitats in Europe and they harbor many endangered plant and animal species. However, changes in land use, nutrient inputs, and water pollution represent the main causes of the reduction in species diversity and abundance (Jamin et al., 2020). The dramatic decline in the number of such wetlands and resulting fragmentation is putting further pressure on their flora and fauna, thus these habitats have been protected by European law (Habitats Directive, 1992). In particular, mainly due to eutrophication, aquatic macrophytes belong to the most endangered groups (Preston and Croft, 2001). Anthropogenic increases in nutrients allow plant species adapted to this condition to grow faster and displace rare pioneer species (Kozlowski et al., 2009). Indeed, wetlands are rarely managed to protect threatened species, and it remains incompletely understood which conservation measures are likely to stabilize populations of endangered aquatic plants (Doust and Doust, 1995).
Luronium natans (monotypic genus within Alismataceae) is a typical example of such an aquatic pioneer plant (Greulich et al., 2000; Szańkowski and Kłosowski, 2001) because it can hardly compete with more nitrophilic aquatic and semi-terrestrial plants (Szmeja, 2004). Throughout its distribution area covering the Atlantic and sub-Atlantic climatic zones of western and northwestern Europe, population trends are ‘declining’ (Preston and Hill, 1997; Greulich, 1999; IUCN, 2021). In Germany, L. natans is found mainly in northern lowlands. Since many populations have been lost since 1950, it has been classified as ‘critically endangered’ and became even extinct in some parts of Germany (Metzing et al., 2018). In the German Federal state Saxony, L. natans has suffered a significant population decline during recent decades (Schulz, 2013). Today, only a few populations remain in large, mostly extensively managed ponds in eastern Saxony (Hanspach, 2007), and botanical surveys report a continuous decline in populations (Figure 1). Although historical distribution is not fully documented, it is very likely that isolated populations have existed for the last 100 years. Population declines until 1990 are probably due to loss of suitable habitats, while more recent declines from 1990 onwards are not fully understood and may be caused by changes in water chemistry.
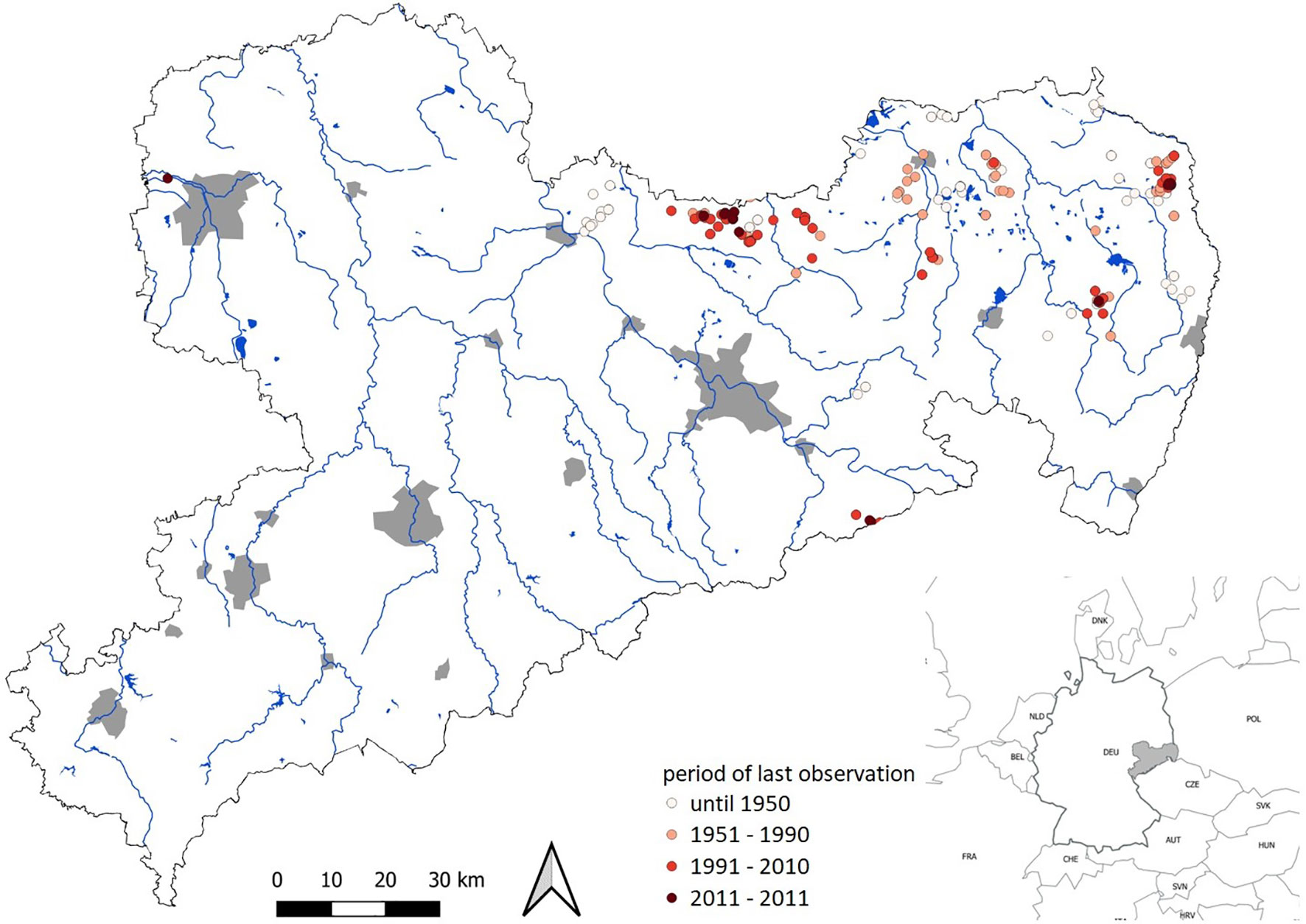
Figure 1 Occurrences of Luronium natans in Saxony (Germany) based on records from herbaria in Dresden (DR), Görlitz (GLM), Halle (HAL), Jena (JE) and Leipzig (LZ). Main rivers and water reservoirs are marked in blue, cities in grey.
Luronium natans is a perennial herb hibernating by submerged rosettes adapted to both aquatic and semi-terrestrial environments with changing water levels (Szmeja, 2004). The species grows on muddy, usually peaty substrates and is part of the endangered oligotrophic standing water plant communities (Littorelletea uniflorae Br.-Bl. et R. Tx.; Greulich et al., 2000; Reißmann and Dieter, 2015). Luronium natans is morphologically highly variable because it develops distinct phenotypes adapted to both aquatic and semi-terrestrial environments (Kay et al., 1999; Szmeja, 2004). These phenotypic adaptations are highly plastic, may occur several times during an individual’s life time and these transformations need usually a few weeks only (Glück, 1905; Kay et al., 1999). The aquatic form grows in waters up to about 3 m in depth and is characterized by rosettes of linear submerged leaves and bundled roots. Individuals growing in shallower waters of up to 1.5 m depth may additionally develop floating leaves consisting of long petioles and leathery elliptical to oval blades (Szmeja, 2004). The semi-terrestrial form has well-developed, thread-like, mostly unbranched fine roots and its leaves shape resembles that of floating leaves, however, petioles are wider and shorter (Glück, 1905).
In addition to insect-pollinated flowers above the water level, plants can develop submerged cleistogamous flowers and reproduce vegetatively by stolons and fragmentation (Kay et al., 1999; Szmeja, 2004). However, the relative importance of these reproductive modes has been controversially discussed (Kay et al., 1999; Nielsen et al., 2006; Cox et al., 2014). Nuts do not have special adaptations, but both dispersal by water or birds seems to be likely (Kay et al., 1999; Halvorsen and Grostad, 2002). The species forms a considerable seed bank with seeds germinating after long periods of stasis at considerable rates (Glück, 1905; Nielsen et al., 2006) and the seedbank is considered to play a key role for surviving periods of inadequate conditions (Kaplan et al., 2014). Particularly in clonal populations, seeds are the major carrier of long-distance spread (Eckert et al., 2016).
Because sufficient genetic variation is the basis for the adaptability to the ever-changing environment (Hoban et al., 2020) and thus for the survival of a species (Hughes et al., 1997; Luck et al., 2003), assessing the extent of genetic diversity in endangered plant species has become a fundamental tool for conservation efforts. Still, the role of genetic diversity for conservation is often under-appreciated in plants and explicit goals for genetic diversity are undeveloped or focus mostly on species of agricultural relevance (Hoban et al., 2020). The importance of intraspecific genetic diversity for the resilience of ecosystems and survival of species is crucial and confirmed by a large body scientific evidence (Laikre et al., 2020). However, estimation of the effective population size (Ne) in natural plant populations with a polymorphic reproductive strategy and a considerable seed bank is not straight-forward at least using easily available dominant marker systems, so most studies rely on simple estimators of genetic diversity (Allendorf et al., 2013). Moreover, dramatic habitat losses due to land use changes may happen so rapidly that they are often not mirrored by lagging changes in genetic diversity (Reichel et al., 2016; Aavik et al., 2019).
Ex-situ collections can be effective measures in conserving plant species (Abeli et al., 2020) by preventing extinction and restoring a species’ historic range by re-introductions (He et al., 2016). Therefore, the knowledge of the genetic status and relatedness of source populations can significantly influence re-introduction efforts by maximizing genetic variation in target populations (He et al., 2016; Robinson et al., 2021).
As with many other endangered aquatic plants, neither population genetics nor ecology of L. natans has been intensively studied and data are rather anecdotal across its distribution area. So far, population genetic studies have been carried out in the Czech Republic, Germany, Wales and Ireland using isozymes (Kay et al., 1999; Bartuška, 2009) as well as in Belgium and Denmark using Amplified Fragment Length Polymorphism (AFLP; Nielsen et al., 2006; Cox et al., 2014). Bartuška (2009) found little genetic diversity within populations from the Czech Republic and Germany (Saxony: Großenhainer Pflege and Niederspree). Furthermore, Belgian populations were found to have a high degree of clonal reproduction (Cox et al., 2014).
However, effective reintroduction requires knowledge not only of the genetic status of populations, but ecological experiments on plant growth and survival, which is particularly important for a species with diverse growth forms such as Luronium.
To get fundamental genetic and ecological knowledge for successful re-introduction efforts of Luronium natans, we investigated population genetics of the remaining Saxon populations using AFLPs. Although this method relies on presence/absence data of anonymous fragments and may have disadvantages compared to sequence-based population genomic methods it still provides reliable and cost-effective data for population genetics in species without prior knowledge on the genome (Woodhead et al., 2005; Allendorf et al., 2013). These markers yield a genome-wide overview about population genetic patterns and robust data can be retrieved when a rigorous scoring and replication scheme is applied (Ley and Hardy, 2013, see below). In addition, previous studies on other European populations of Luronium natans were also performed with AFLPs (Cox et al., 2014; Nielsen et al., 2006), allowing for direct comparisons of population genetic estimators.
In particular, we determined the genetic diversity within populations to estimate the degree of clonality and thus the influence of sexual versus vegetative reproduction. We also studied the genetic distance of these populations in comparison to samples covering the species’ distribution area and with material from ex-situ cultures from various botanical gardens. Since all previous publications indicated a low genetic variability within populations in L. natans (Kay et al., 1999; Nielsen et al., 2006; Bartuška, 2009; Cox et al., 2014) and such a pattern seems to hold in general for aquatic plants (Santamaría, 2002) because clonal propagation appears to be common in endangered and aquatic species populations (Silvertown, 2008), we hypothesize low levels of genetic diversity within Saxonian populations but a considerable genetic variance between them.
In addition, we conducted a greenhouse experiment to investigate the influence of temperature, substrate, and water level on plant growth in order to find optimum growing conditions for ex-situ cultures potentially used for re-introduction experiments.
Our sampling of L. natans focused on Saxony (Germany), where the species is found in two pond areas approximately 90 km distant from each other: Niederspree (DE_Ni) and Großenhainer Pflege (DE_Gr; Hanspach, 2007; Hanspach et al, 2016). The occurrences in the Niederspree pond area have been known at least since 1899 and in Großenhainer Pflege since 1840.
Both pond areas contain a number of distinct populations in the respective single ponds (Table 1). Within the pond areas several thousand shoots have been detected, in which high fluctuation rates up to 50% were observed (Hanspach et al., 2016, own observations). However, despite historical records (personal communication L. Runge) no occurrences of Luronium were found at Goldgrubenteiche. The material was collected from the end of September to the beginning of October 2020. In accordance with the collection permit issued by the relevant authorities leaves from 1−28 plants per pound (depending on the size of the subpopulation; minimum distance between samples – 0.8 m) were collected and dried in silica gel. To ensure that the harvested material came from one plant, only a single leaf per plant was harvested. In many cases plants occurred in rather dense patches, from which we sampled only one leaf to avoid collecting clonal plants.
Additionally, leaves from plants of two other localities in Germany (Mecklenburg-Vorpommern: near Rostock; Bavaria: Bad Alexandersbad), as well as several samples from the Czech Republic, Poland, Great Britain, and Norway were investigated (Table 1). Furthermore, leaves from plants cultivated in the Botanical Garden of the TU Dresden were also studied (Table 1).
DNA was isolated from silica gel dried leaf material using innuPREP Plant DNA Kit (Analytic Jena, Germany) following the manufacturer’s instructions except for the elution of DNA, which was eluted in two steps: 1. with 70 µl HPLC grade water, 2. with 30 µl HPLC grade water. The quality of isolates was checked with agarose gel electrophoresis (1%) and DNA quantity was estimated using Qubit Fluorometer (Thermo Fisher Scientific). Extracted DNA was stored at -22°C until further processing.
To investigate genetic diversity within Saxon populations and their relationships to other European populations, AFLP (Vos et al., 1995) was used with minor modifications. One hundred nanograms of isolated DNA were digested with the restriction enzymes PstI and MseI. Restriction and ligation were performed in a single reaction for 10 hours at 37°C. For selective PCR, we followed Schuelke (2000). We first tested 15 primer combinations based on previous AFLP studies on L. natans (Cox et al., 2014; Nielsen et al., 2006) and selected four combinations for further analyses: S7 (PstI-ACG/MseI-CAC), S8 (PstI-ACG/MseI-CCG), S11 (PstI-ACT/MseI-CTC), S12 (PstI-ACT/MseI-GCT). Samples were randomly distributed on 96-well plates to avoid position effects. Sixty-six of 253 samples were analyzed twice for quality control. In addition, five identical samples were repeated on each of the five 96-well plates, and negative controls for restriction/ligation, pre-selective PCR, and selective PCR were included. Automated detection of AFLP fragments was performed by the Senckenberg Biodiversity and Climate Research Center (SBik-F; Frankfurt am Main, Germany) with an ABI 3730 sequencer (ABI Life Technologies, Darmstadt, Germany) using the LIZ-600 size standard (ABI Life Technologies).
Fragment scoring was processed as described in Ley and Hardy (2013). First, scoring was automatically done using PeakScanner 1.0 (Applied Biosystems, ThermoFisher Scientific, Berlin, Germany) on a size range from 100–500 bp, the minimal peak height of 30, and maximal peak width of 1. The program TinyFLP v1.30 (Arthofer, 2010) was then used for a pre-choice of markers. Finally, the software SPAGeDi (Hardy and Vekemans, 2002) was used to estimate the reproducibility of bands by calculating fragment-wise Fst values across repeated samples by combining all combinations within one matrix (see details in Ley and Hardy, 2013). We retained a final data matrix consisting of 151 samples from 22 (sub)populations with 48 fragments with a Fst ≥0.25.
To estimate the genetic diversity within populations (for those with >5 samples) we calculated Expected Heterozygosity (He); Shannon’s Index of Diversity (I), and the Percentage of Polymorphic Loci (%P) with GenAIEx v. 6.5 (Peakall and Smouse, 2012). Population structure was analyzed using GenAIEx by performing Analysis of Molecular Variance (AMOVA) and Principal Coordinate Analysis (PCoA) based on Jaccard distances with the R-Package vegan (Oksanen et al., 2020). In addition, we performed Mantel tests (Mantel, 1967) with GenAIEx to check for a correlation between geographic and genetic distances. Furthermore, Bayesian clustering was conducted with the R-package ParallelStructure (Besnier and Glover, 2013) with 10 iterations for every K from 1 to 10, with a burn-in of 500,000 generations followed by 1,000,000 generations. The best-fitting model was chosen according to the method described by Evanno et al. (2005) using the software Structure Harvester v.0.6.94 (Earl and vonHoldt, 2012). Results were visualized with the program DISTRUCT v.1.1 (Rosenberg, 2003).
The experiment started on October 27, 2020 and ended on March 12, 2021. Plant material for the experiment originated from the population Kleiner Tiergartenteich (DE_Gr_Tier; Table 1) and had been cultivated since 2019 in the Botanical Garden of the TU Dresden. The plants used for the experiment were propagated by runners from mother plants and were grown after separation from the mother plants for 7−8 weeks in submerged pots in the outside area of the botanical garden.
Conditions tested in this experiment were selected based on observations from habitat and previous publications (Glück, 1905; Barrat-Segretain and Bornette, 2000; Nielsen et al., 2006). Plants were potted into four types of substrates: clay, sand, mixed, i.e. clay/sand mixture (1:1), and layered, i.e. sand as the top layer and clay as the bottom layer (1:1) to check whether lack of nutrients (especially sand as top layer) will influence root length. Pots were subsequently placed in three different water levels: (1) semi-terrestrial condition (saturated): water up to about 3 cm below the rim of the pot; (2) aquatic condition I: water about 0.5 – 1.0 cm above the rim of the pot; (3) aquatic condition II: water about 7 cm above the rim of the pot. Since seasonal differences in plant growth have been observed previously which are likely related to water temperature (Barrat-Segretain and Bornette, 2000; Nielsen et al., 2006), plants were exposed to two different water temperatures: cold: ~ 5 °C and warm: ~ 14 °C. Four plants each, i.e. biological replications were used for each combination of substrate, water level, and temperature (Supplementary Figure 1), thus the experiment constituted a full-factorial design. Water level (rainwater) was regularly checked and replenished when necessary. The plants were illuminated with assimilation lighting from 7 to 10 a.m. Since not all plants used for the experiments were of the same size, their size classes were recorded in the beginning of the experiment: small (s), medium without floating leaves (m), and medium with floating leaves (wfl). After completion of the experiment roots of each plant were rinsed with water to remove remaining substrate. Then, the length of the longest root and of the longest leaf (floating leaves were excluded) were measured. Typically, roots are cut and weighted, but this procedure could not be performed since the plants had to be kept alive for further ex situ conservation.
The effects of the substrate, water level, and temperature on root and leaf length were analyzed using linear regressions. Additionally, we analyzed the effect of the plant size at the onset of the experiment by including the variable as a covariate. For each response variable, we calculated 27 different models (Supplementary Tables 1, 3) with the predictor variables in plausible combinations. For model selection, we used Akaike’s information criterion (AIC). The 27 models were ranked via AIC and the model with the lowest AIC and a difference of at least 2 to the next model was then considered as the best-fitting model (Burnham and Anderson, 2002). All analyses were performed using the R environment (R Core Team, 2020).
Genetic diversity of L. natans populations was reasonably high Table 2; (grand means He = 0.25; Shannon-Index = 0.387) and differed only little between populations, with highest values found in the population Raschützteich (DE_Gr_Ras: He = 0.268) and lowest diversity detected in the Norwegian population (NO: He = 0.234).
The Principal Coordinate Analyses including all 151 samples across Europe separated mainly samples from Eastern Europe (Czech Republic = CZ and Poland =PL) and those from Eastern Saxony (pond area Niederspree = DE_Ni) from the remaining samples along the first axis (Figure 2). In the left part of the plot samples from the Central Saxon area Großenhainer Pflege (DE_Gr) were clustered, whereas samples from Northern and Southern Germany (DE_Ro, DE_By), Great Britain (UK), and Norway (NO) were found in a rather intermediate position. Note that samples obtained from ex situ collections of Botanical Gardens clustered according to their geographical origin (Table 1). The PCoA presented in Figure 2 was based on Saxon samples only. Accordingly, samples from Niederspree (Eastern Saxony) were clearly separated along the first axis. We observed no genetic structure among the populations within both pond areas, respectively.
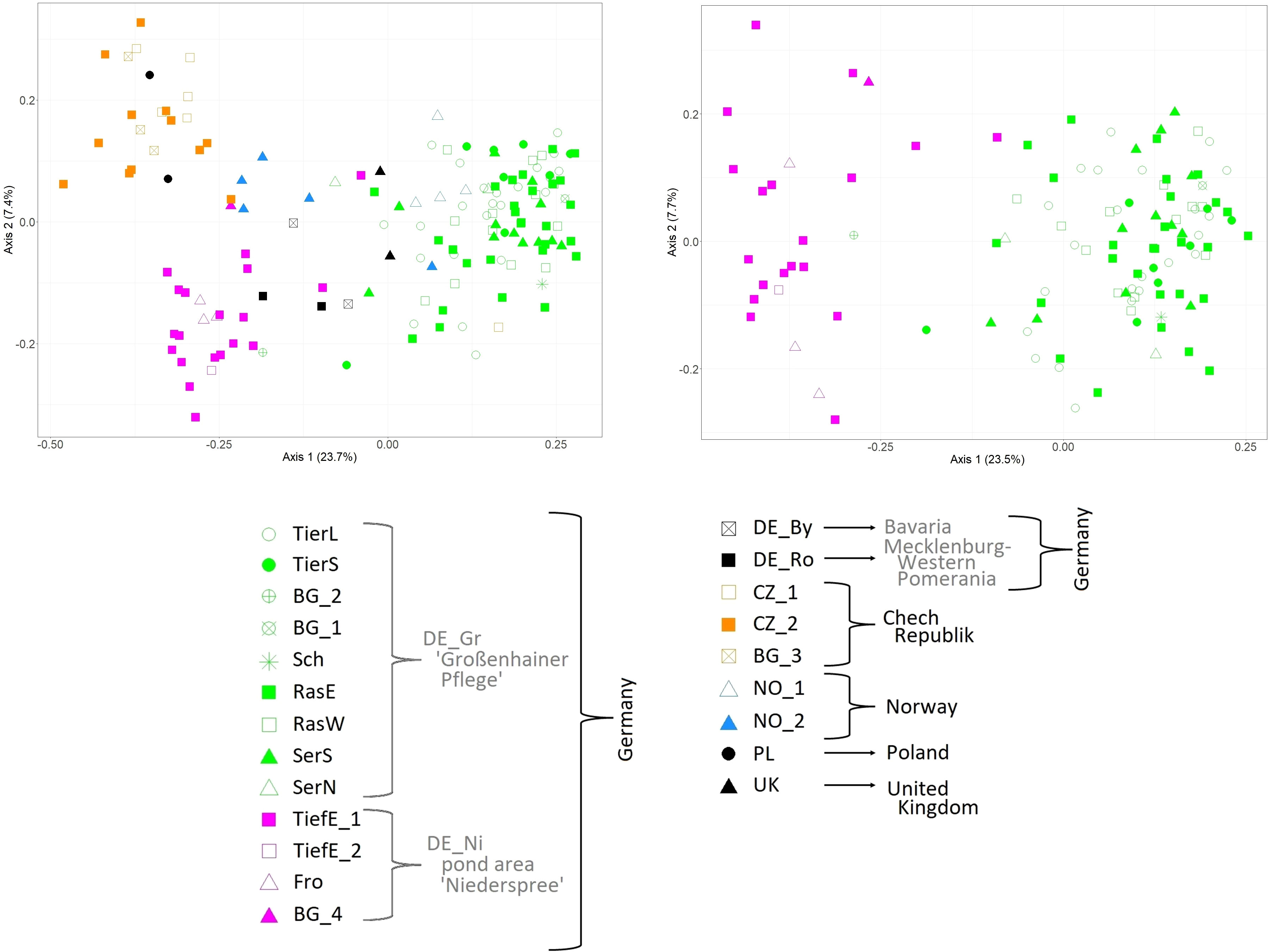
Figure 2 Principal Coordinate Analysis based on 48 AFLP loci of 151 individuals from European L. natans populations (left), and 113 individuals from Saxon populations only (right). Abbreviations for populations are according to Table 1.
Analyses of Molecular Variance (Table 3) revealed a moderate differentiation (ΦPT = 0.334, P ≥ 0.001) between both Saxon localities (DE_Gr and DE_Ni). Results from Bayesian clustering (Figure 3) with two clusters (K = 2) roughly corresponded to the PCoA analyses of all samples. Accessions from the area Großenhainer Pflege (DE_Gr) were assigned to the green cluster, whereas populations from Niederspree were found in the same cluster containing most of the Czech (CZ), Polish (PL), and Norwegian (NO) samples. Admixture between both clusters was detected for a few samples from various ponds in Central Saxony and for plants originating from Norway (NO) and Wales (UK). Mantel test did not detect a significant correlation between geographic and genetic distances (all populations: R2 = 0.0271; p = 0.100, Saxon populations: R2 = 0.9974 and p = 0.078).
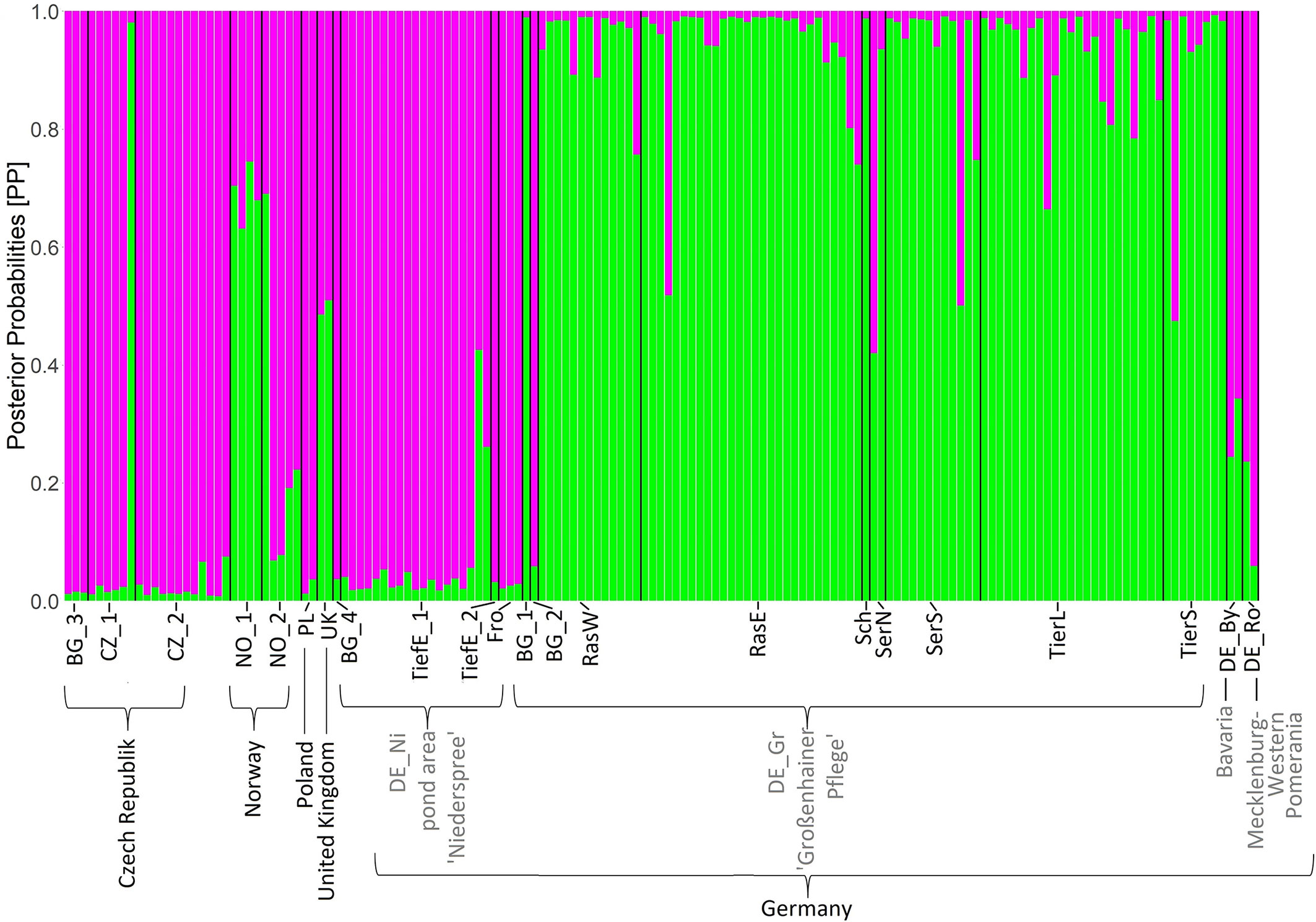
Figure 3 Bayesian clustering based on 48 loci AFLP for the model K = 2. Length of the colored bars indicates posterior probabilities (PP) for belonging to one of the two clusters (pink and green). Abbreviations for populations are according to Table 1.
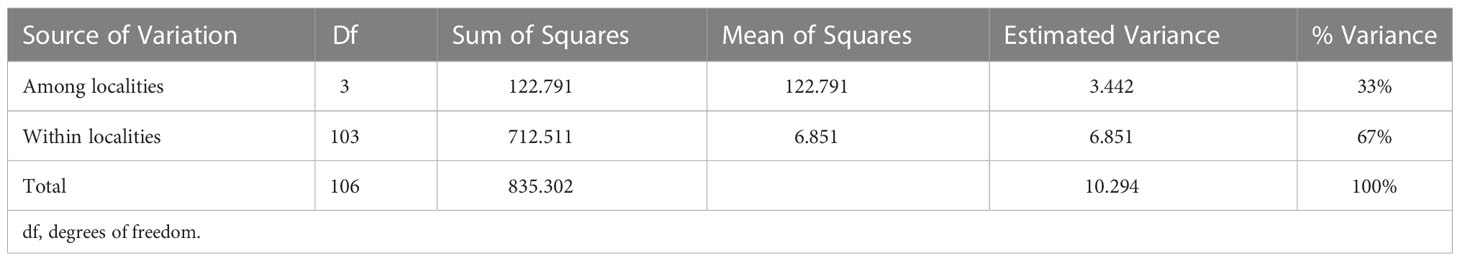
Table 3 Analysis of Molecular Variance based on 48 AFLP loci among and within two Saxon localities of Luronium natans (Niederspree: DE_Ni and Großenhain: DE_Gr).
The best-fitting model for root length (adjusted r-squared 0.65, p < 0.001) included an interaction between temperature and water level (Supplementary Tables 1, 2). The size of the plants at the beginning of the experiment did not have remarkable effects on root length. Roots were longer in plants grown under high water levels than under low water levels. The effect of temperature on the root length was restricted to plants grown under saturated water conditions: here, warm temperature led to roots three times longer than those of plants grown under cold temperatures (Figure 4).
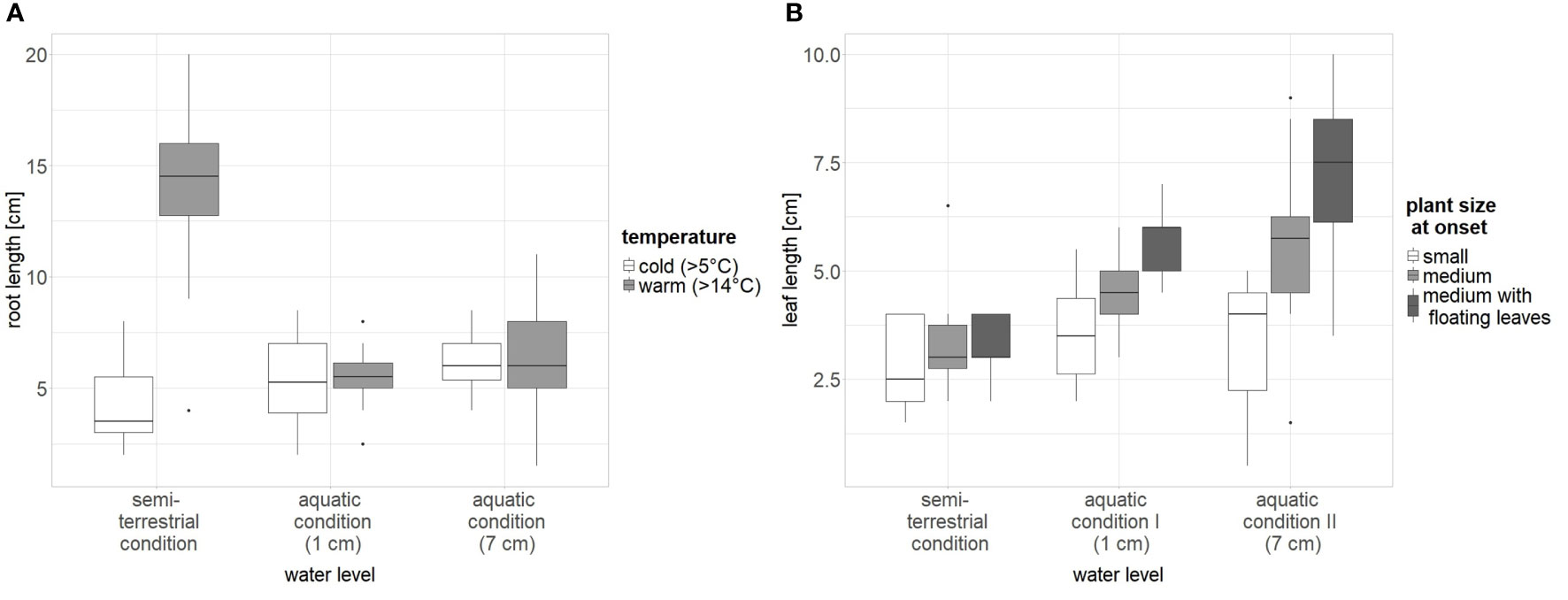
Figure 4 The influence of significant factors on plant growth (A: root length and B: leaf length) of L. natans. Water level: “1 cm”: water about 0.5 − 1 cm above the rim of the pot; “7 cm”: water about 7 cm above the rim of the pot; saturated: semi-terrestrial form, water only up to about 3 cm below the rim of the pot; temperature: cold ~ 5°C, warm ~ 14°C.
The best-fitting model for leaf length (adjusted r-squared 0.47, p < 0.001) included an interaction between plant size at the onset of the experiment and water level. According to the best-fitting model (Supplementary Tables 3, 4), leaf length increased with the water level. Plants of medium size and with already developed floating leaves had the longest leaves, and this effect tended to increase with increasing water depths (Figure 4). However, the selected model did not fit much better than the model with plant size at onset and water level lacking interaction, and the model including plant size at onset, water level, and temperature (Δ AIC of the first three models was <2).
In contrast to our initial hypothesis of low genetic variation within populations, the values of the indices of genetic diversity were quite high (I = 0.367−0.416; Table 2), and these results suggest that Saxon populations are not genetically impoverished and that clonal reproduction does not cover the effect of the sexual reproduction. This finding was rather unexpected and contradicts the results from Bartušek (2009), who reported a moderate to very low (sometimes absent) genetic diversity in Czech and Saxon populations but applied possibly less polymorphic isozyme markers compared to AFLPs (Ipek et al., 2003; Mondini et al., 2009). Similarly, Kay et al. (1999) suggested the dominance of clonal propagation in the United Kingdom and Ireland based on isozyme analyses, and also previous studies from Belgium reported considerably low values (I = 0.0996) based on AFLPs (Cox et al., 2014). In addition, Szmeja (2004) assumed that L. natans populations in Poland are mainly represented by multigenerational clones reproducing primarily by fragmentation. However, he observed plants in water reservoirs with deeper water bodies, where cleistogamy occurs more often (Kay et al., 1999). Thus, the high genetic diversity observed in the Saxon populations may be due to marked outcrossing from insect-pollinated chasmogamic flowers above the water surface, which we observed in all ponds studied. Moreover, L. natans produces a substantial seed bank and is characterized by high rates of seed germination (average 51-60%; Nielsen et al., 2006). Lansdown and Wade (2003) assumed the seeds to have a capacity for extended dormancy over many years. Germination might take place preferably in shallow waters or on naked mud in empty ponds. The extreme fluctuations of observed shoots (up to 50%) between years (Hanspach et al., 2016, own observations) can be caused by pronounced clonal growth or by high proportion of regeneration from seeds. A much denser sampling design in consecutive years may help to investigate the different ways of population growth. Moreover, estimators of genetic diversity respond often delayed compared to rapid changes in habitats and thus in strong declines in population size (Reichel et al., 2016; Aavik et al., 2019).
In contrast to our results, low values of genetic diversity based on AFLPs (I = 0.025−0.140) were reported within three species of the closely related genus Baldellia (Alismataceae) in Europe (Arrigo et al., 2011). However, these authors found varying values depending on the geographic origin of the samples with higher values detected at Iberian Peninsula compared to France and Switzerland, which were explained by a recent post-glacial re-colonization to the North. Thus, considering a species’ biogeographic history might also be crucial regarding conservation approaches.
According to our hypothesis of a pronounced genetic structure between Saxon populations, L. natans samples clustered mainly according to their geographic origin, whereby we detected three groups of samples: (1) Czech populations, (2) Saxon populations from Niederspree and (3) Saxon populations from the pond area Großenhainer Pflege, with remaining samples from Germany and Norway in between (Figure 2). This partially coincides with the results reported by Bartuška (2009), who showed that the populations from the Czech Republic, and the two Saxon populations Großenhainer Pflege and Niederspree constituted three separate groups. Remarkably, sites from Saxony, which are only separated by a distance of approximately 90 km appeared to be rather distantly related because samples from the area Großenhainer Pflege formed a separate cluster and those from Niederspree were close to remaining samples from various sites across Europe (Figure 3). Most accessions were assigned with high posterior probabilities to one of the two clusters (Figure 3), thus admixture between both Saxon areas played a minor role. Given the rather low geographic distance between Saxon sites the ΦPT value of 0.334 suggests a moderate level of genetic differentiation between populations (Table 3), which was comparable to the values observed in Belgian populations ranging between 0.226 and 0.455 (Cox et al., 2014). Samples from neighboring ponds within each of the respective Saxon areas appeared to be completely intermingled, thus, no genetic structuring on very short distances was observed (Figure 2). While the lack of genetic structure between sub-populations per population (Table 1) may be an effect of water-mediated dispersal (Kay et al., 1999), it does not explain the situation between the three populations in Großenhainer Pflege (DE_Gr_Tier, DE_Gr_Ras, and DE_Gr_Ser) as these areas are not connected by ditches but rather suggests zoochoric dispersal, e.g. by birds. Although, Mikkelsen (1943) argued that Luronium nuts lack adaptations to bird dispersal, more recent studies reported likely dispersals of 50 to 100 km (Fritz, 1989; Halvorsen and Grostad, 2002; Lansdown and Wade, 2003; Nielsen et al., 2006). Here, bird dispersal would only explain the genetic similarity at the local scale (Table 1) but not between areas, because both areas in Saxony (DE_Gr and DE_Ni) were clearly separated (Figures 2, 3). However, we detected no significant pattern of isolation by distance (Mantel tests for European populations: R2 = 0.0271; P = 0.100, for Saxony R2 = 0.9974 and P = 0.078) but due to the restricted availability of plant material our sampling was rather unbalanced.
Remarkably the genetic similarity of Polish (PL) and Czech (CZ) samples (Figure 2) is surprising, given the geographic distance between the populations. We would have rather expected a close relationship of the Polish samples with the German population from Rostock (DE_Ro). In Poland, two big geographically separated occurrences, i.e. Pomerania (current populations; closer to Rostock) and Lower Silesia (historical populations; closer to the Czech Republic) are present (Dajdok and Proćków, 2003; Szmeja, 2004). Our findings are in line with the observation of Nischkowsky and Schube (1908) and Suda et al. (2000), who referred to the Lower Silesian occurrences as part of the Jizera Foothills meta-population, which also include the oldest Czech populations. The Czech populations examined in the present study are about 120 km away from these historical occurrences (Nischkowsky and Schube, 1908) but may represent together with scattered populations from Wielkopolska, Poland (Szmeja, 2007; Szmeja, 2013) remnants of a former widespread distribution in Poland and the Czech Republic. The closer relationship of samples from Niederspree (DE_Ni) to Czech populations rather than to the more western Saxon samples from Großenhainer Pflege (DE_Gr) implies a geographic separation of L. natans between Eastern and Western Europe, whose border might cross Saxony.
Our experiment showed that Luronium plants developed longer roots under semi-terrestrial (saturated) conditions at higher temperature (Figure 4). This is in line with the assumption of Glück (1905) who argued that a more extensive root system of semi-terrestrial forms may be caused by their restricted access to water. The here observed longer roots at higher temperatures fit also with Luronium’s Ellenberg Indicator Value for temperature (T6; Ellenberg et al., 2001) indicating its adaptation to moderate warm to warm temperatures. In addition, we found longer leaves in plants growing at higher water level, which can be explained by the fact that the aquatic form of Luronium has longer leaves than the semi-terrestrial form. In addition, plants which were larger at the onset also produced longer leaves, however, plant size at onset was an uncontrolled factor, and the plants were randomly selected according to size. Thus, the best results for obtaining robust plants for potential reintroduction are obtained under semi-terrestrial conditions and at higher temperatures, as a strong root system can facilitate active planting.
Our study revealed moderate levels of genetic diversities in Saxon populations suggesting that the observed decline in populations is not caused by lacking genetic diversity. We assume that populations in ponds rely strongly on regeneration by seeds emerged from outcrossing rather than by clonal reproduction or cleistogamous flowers. While we found a considerable genetic differentiation among Saxon populations our results showed no genetic differentiation within pond systems indicating sufficient genetic exchange between neighboring ponds probably mediated by birds or water. Thus, our results imply to use rather nearby populations from the same area as source for reintroductions. Our plant growth experiments demonstrated that possible reintroductions of plants are facilitated by optimal growing conditions in gardens since we obtained plants with longest roots when grown under saturated water conditions and higher temperatures, whereas the tested soil type seems to have less impact.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
CR, SW, BD, and FR planned the study, WM and BD collected the samples, WM did the laboratory work, WM, VH, and CR performed population genetic analyses, JA performed statistical analyses on plant growth, WM, JA, CR, and SW wrote the manuscript. All authors read, contributed and approved the final manuscript.
The work was financed by the core budget of Senckenberg Natural History Museum Görlitz and the Institute of Botany of the TU Dresden, third-party funds were not included.
Special thanks go to M. Striese (Boxberg, Germany), L. Runge (Linz, Germany) and F. Ditsch (TU Dresden) who helped with fieldwork, to A. Sembdner (TU Dresden) for the help in the botanical garden and to M. Schwager (Senckenberg Museum für Naturkunde Görlitz) and A. Wenke (TU Dresden) who helped with laboratory work. We also thank the following persons sending plant material from all over Europe, namely M. Čtvrtlíková (Institute of Hydrobiology of the Czech Academy of Sciences), M. Lauerer (Botanical Garden University of Bayreuth), D. Götze (Botanical Garden University of Rostock), Ø. Lofthus (Oslo Botanical Garden), K. Reczek (Botanical Garden of the University of Wrocław) and R. Blackhall-Miles (FossilPlants Gwynedd/UK). We thank P. Gebauer (GLM), J. Mueller (JE), F. Mueller (DR) and P. Otto (LZ) for summarizing herbarium records of the respective collections. In addition, we would also like to thank ‘Untere Naturschutzbehörden of Landratsamt Görlitz’ and ‘Landratsamt Meißen’ for granting permission to collect Luronium samples. We thank the two reviewers for their thoughtful comments on the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2022.1069842/full#supplementary-material
Aavik, T., Thetloff, M., Träger, S., Hernández-Agramonte, I. M., Reinula, I., Pärtel, M. (2019). Delayed and immediate effects of habitat loss on the genetic diversity of the grassland plant Trifolium montanum. Biodivers. Conserv. 28 (12), 3299–3319. doi: 10.1007/s10531-019-01822-8
Abeli, T., Dalrymple, S., Godefroid, S., Mondoni, A., Müller, J. V., Rossi, G., et al. (2020). Ex situ collections and their potential for the restoration of extinct plants. Conserv. Biol. 34 (2), 303–313. doi: 10.1111/cobi.13391
Allendorf, F. W., Aitken, S. N., Luikart, G. (2013). Conservation and the genetics of populations (John Wiley & Sons). doi: 10.1111/mec.13948
Arrigo, N., Buerki, S., Sarr, A., Guadagnuolo, R., Kozlowski, G. (2011). Phylogenetics and phylogeography of the monocot genus Baldellia (Alismataceae): Mediterranean refugia, suture zones and implications for conservation. Mol. Phylogenet. Evol. 58 (1), 33–42. doi: 10.1016/j.ympev.2010.11.009
Arthofer, W. (2010). tinyFLP and tinyCAT: software for automatic peak selection and scoring of AFLP data tables. Mol. Ecol. Resour. 10 (2), 385–388. doi: 10.1111/j.1755-0998.2009.02751.x
Barrat-Segretain, M. H., Bornette, G. (2000). Regeneration and colonization abilities of aquatic plant fragments: effect of disturbance seasonality. Hydrobiologia 421 (1), 31–39. doi: 10.1023/A:1003980927853
Bartuška, M. (2009). Genetická variabilita kriticky ohroženého žabníčku vzplývavého (Luronium natans (L.) Raf. Alismataceae) na okraji areálu a její význam pro cílenou druhovou ochranu. [master’s thesis] (Prague: Univerzita Karlova v Praze).
Besnier, F., Glover, K. A. (2013). ParallelStructure: A R package to distribute parallel runs of the population genetics program STRUCTURE on multi-core computers. PloS One 8 (7), e70651. doi: 10.1371/journal.pone.0070651
Burnham, K.P., Anderson, D.R. (2002). Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. New York, Springer.
Cox, K., Leyssen, A., Mergeay, J., Ronse, A., Packet, J., Denys, L. (2014). Genetic assessment of Luronium natans in lower Belgium. Analysis of population connectivity in an aquatic perennial 2014 (5021339). (Brussel: Instituut voor Natuur- en Bosonderzoek).
Dajdok, Z., Proćków, J. (2003). Flora wodna i błotna dolnego Śląska na tle zagrożeń i możliwości ochrony. Ed. Kącki, W. Z. (Wrocław: Zagrożone gatunki flory naczyniowej Dolnego Śląska), 131–150.
Doust, L. L., Doust, J. L. (1995). Wetland management and conservation of rare species. Can. J. Bot. 73 (7), 1019–1028. doi: 10.1139/b95-11
Earl, D. A., vonHoldt, B. M. (2012). STRUCTURE HARVESTER: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 4 (2), 359–361. doi: 10.1007/s12686-011-9548-7
Eckert, C. G., Dorken, M. E., Barrett, S. C. (2016). Ecological and evolutionary consequences of sexual and clonal reproduction in aquatic plants. Aquat. Bot. 135, 46–61. doi: 10.1016/j.aquabot.2016.03.006
Ellenberg, H., Weber, H. E., Duell, R., Wirth, V., Werner, W., Paulissen, D. (2001). Zeigerwerte von Pflanzen in Mitteleuropa. 3. edition (Goltze. Scripta Geobotanica: 18 Göttingen).
Evanno, G., Regnaut, S., Goudet, J. (2005). Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol. Ecol. 14 (8), 2611–2620. doi: 10.1111/j.1365-294X.2005.02553.x
Fritz, O. (1989). A find of Luronium natans in the province of Halland, SW Sweden. Svensk. Botanisk. Tidskrift. 83 (2), 135–136.
Glück, H. (1905). Biologische und morphologische Untersuchungen über Wasser- und Sumpfgewächse. 118–133.
Greulich, S. (1999). Compétition, perturbations et productivité potentielle dans la définition de l'habitat d'espèces rares: étude expérimentale du macrophyte aquatique Luronium natans (L) Raf. (Doctoral dissertation, Lyon 1). Lyon.
Greulich, S., Bornette, G., Amoros, C., Roelofs, J. G. (2000). Investigation on the fundamental niche of a rare species: an experiment on establishment of Luronium natans. Aquat. Bot. 66 (3), 209–224. doi: 10.1016/S0304-3770(99)00073-X
Habitats Directive (1992). Council directive 92/43/EEC of 21 may 1992 on the conservation of natural habitats and of wild fauna and flora. Off. J. Eur. Union. 206, 7–50.
Halvorsen, R., Grostad, T. (2002). Kinnhalvoya i brunlanes, larvik i vestfold og et funn av flytegro Luronium natans (L.) Raf. Blyttia 60 (2), 117–121.
Hanspach, D. (2007). Zur Bestandsentwicklung des Froschkrautes, Luronium natans (L.) Raf. im Niederspreer Teichgebiet. Ber. Naturforsch. Ges. Oberlausitz. 15, 149–161.
Hanspach, D., Landgraf, K., Richter, F. (2016). Erstellung von Artenschutzkonzepten und Aktionsplänen für Pflanzenarten und wirbellose Tierarten mit besonderer landesweiter Bedeutung, Los 5 (Froschkraut (Luronium natans), Abschlussbericht, Landgraf & Richter GbR).
Hardy, O. J., Vekemans, X. (2002). SPAGeDi: a versatile computer program to analyse spatial genetic structure at the individual or population levels. Mol. Ecol. Notes 2 (4), 618–620. doi: 10.1046/j.1471-8286.2002.00305.x
He, X., Johansson, M. L., Heath, D. D. (2016). Role of genomics and transcriptomics in selection of reintroduction source populations. Conserv. Biol. 30 (5), 1010–1018. doi: 10.1111/cobi.12674
Hoban, S., Campbell, C., da Silva, J., Ekblom, R., Funk, W. C., Garner, B., et al. (2020). An analysis of genetic diversity actions, indicators and targets in 114 national reports to the Convention on Biological Diversity. BioRxiv. doi: 10.1101/2020.08.28.254672
Hughes, J. B., Daily, G. C., Ehrlich, P. R. (1997). Population diversity: its extent and extinction. Science 278 (5338), 689–692. doi: 10.1126/science.278.5338.689
Ipek, M., Ipek, A., Simon, P. W. (2003). Comparison of AFLPs, RAPD markers, and isozymes for diversity assessment of garlic and detection of putative duplicates in germplasm collections. J. Am. Soc. Hortic. Sci. 128 (2), 246–252. doi: 10.21273/JASHS.128.2.0246
IUCN (2021) The IUCN Red List of Threatened Species. version 2020-3. Available at: https://www.iucnredlist.org.
Jamin, A., Peintinger, M., Gimmi, U., Holderegger, R., Bergamini, A. (2020). Evidence for a possible extinction debt in Swiss wetland specialist plants. Ecol. Evol. 10 (3), 1264–1277. doi: 10.1002/ece3.5980
Kay, Q. O. N., John, R. F., Jones, R. A. (1999). Biology, genetic variation and conservation of Luronium natans (L.) Raf. in Britain and Ireland. Watsonia. 22 (4), 301–316.
Kaplan, Z., Šumberová, K., Formanová, I., Ducháček, M. (2014). Re-establishment of an extinct population of the endangered aquatic plant Potamogeton coloratus. Aquat. Bot., 119, 91–99. doi: 10.1016/j.aquabot.2014.08.005
Kozlowski, G., Rion, S., Python, A., Riedo, S. (2009). Global conservation status assessment of the threatened aquatic plant genus Baldellia (Alismataceae): challenges and limitations. Biodivers. Conserv. 18 (9), 2307–2325. doi: 10.1007/s10531-009-9589-3
Laikre, L., Hoban, S., Bruford, M. W., Segelbacher, G., Allendorf, F. W., Gajardo, G., et al. (2020). Post-2020 goals overlook genetic diversity. Science 367 (6482), 1083–1085. doi: 10.1126/science.abb2748
Lansdown, R. V., Wade, P. M. (2003). Ecology of the floating water-plantain Luronium natans. Conserving Natura 2000 rivers. Ecol. Ser. 9.
Ley, A. C., Hardy, O. J. (2013). Improving AFLP analysis of large-scale patterns of genetic variation –a case study with the central African lianas Haumania spp. (Marantaceae) showing interspecific gene flow. Mol. Ecol. 22 (7), 1984–1997. doi: 10.1111/mec.12214
Luck, G. W., Daily, G. C., Ehrlich, P. R. (2003). Population diversity and ecosystem services. Trends Ecol. Evol. 18 (7), 331–336. doi: 10.1016/S0169-5347(03)00100-9
Mantel, N. (1967). The detection of disease clustering and a generalized regression approach. Cancer Res. 27 (2 Part 1), 209–220.
Metzing, D., Hofbauer, N., Ludwig, G., Matzke-Hajek, G. (2018). Rote Liste gefährdeter Tiere, Pflanzen und Pilze Deutschlands. Band 70 (7).
Mondini, L., Noorani, A., Pagnotta, M. A. (2009). Assessing plant genetic diversity by molecular tools. Diversity 1 (1), 19–35. doi: 10.3390/d1010019
Nielsen, U. N., Riis, T., Brix, H. (2006). The importance of vegetative and sexual dispersal of Luronium natans. Aquat. Bot. 84 (2), 165–170. doi: 10.1016/j.aquabot.2005.09.002
Nischkowsky, R., Schube, T. (1908). Ergebnisse der Durchforschung der schlesischen Gefässpflanzenwelt im Jahre 1907. Jahresber. Schles. Gesellsch. Vaterl. Cult. 73, 43–61.
Oksanen, J., Blanchet, F. G., Friendly, M., Kindt, R., Legendre, P., McGlinn, D., et al. (2020) Vegan: Community ecology package. R package version 2.5-7. Available at: https://CRAN.R-project.org/package=vegan.
Peakall, R. O. D., Smouse, P. E. (2012). GenAlEx 6.5: Genetic analysis in excel. population genetic software for teaching and research-an up-date. Bioinformatics 28, 2537–2539. doi: 10.1111/j.1471-8286.2005.01155.x
Preston, C. D., Croft, J. M. (2001). Aquatic plants in Britain and Ireland (Colchester, Essex, England: Harley Books).
Preston, C. D., Hill, M. O. (1997). The geographical relationships of British and Irish vascular plants. Botanical. J. Linn. Soc. 124 (1), 1–120. doi: 10.1111/j.1095-8339.1997.tb01785.x
R Core Team (2020). R: A language and environment for statistical computing (Vienna, Austria: R Foundation for Statistical Computing).
Reißmann, K., Dieter, F. (2015). 3130 Oligo- bis mesotrophe stehende gewässer mit Vegetation Littorelletea uniflorae und/oder der Isoëto-Nanojuncetea. Available at: http://www.lau.sachsen-anhalt.de/naturschutz/natura-2000/arten-und-lebensraumtypen/lrt-anhang-i-ffh-rl/
Reichel, K., Richter, F., Eichel, L., Kącki, Z., Wesche, K., Welk, E., et al. (2016). Genetic diversity in the locally declining Laserpitium prutenicum L. and the more common Selinum carvifolia (L.) L.: a “silent goodbye”? Conserv. Genet. 17 (4), 847–860. doi: 10.1007/s10592-016-0827-4
Robinson, N. M., Rhoades, C., Pierson, J., Lindenmayer, D. B., Banks, S. C. (2021). Prioritising source populations for supplementing genetic diversity of reintroduced southern brown bandicoots Isoodon obesulus obesulus. Conserv. Genet. 22 (3), 341–353. doi: 10.1007/s10592-021-01341-6
Rosenberg, N. A. (2003). Distruct: A program for the graphical display of population structure: PROGRAM NOTE. Mol. Ecol. Notes 4 (1), 137–138. doi: 10.1046/j.1471-8286.2003.00566.x
Santamaría, L. (2002). Why are most aquatic plants widely distributed? Dispersal, clonal growth and small-scale heterogeneity in a stressful environment. Acta Oecol. 23 (3), 137–154. doi: 10.1016/S1146-609X(02)01146-3
Schuelke, M. (2000). An economic method for the fluorescent labeling of PCR fragments. Nat. Biotechnol. 18 (2), 233–234. doi: 10.1038/72708
Schulz, D. (2013) Rote Liste und Artenliste Sachsens- Farn- und Samenpflanzen. Available at: https://publikationen.sachsen.de/bdb/artikel/19031.
Silvertown, J. (2008). The evolutionary maintenance of sexual reproduction: evidence from the ecological distribution of asexual reproduction in clonal plants. Int. J. Plant Sci. 169 (1), 157–168. doi: 10.1086/523357
Suda, J., Bauer, P., Brabec, J., Hadinec, J. (2000). Znovunalezené druhy naší květeny–žabníček vzplývavý. Živa, 48, 205–207.
Szańkowski, M., Kłosowski, S. (2001). Habitat conditions of the phytocoenoses dominated by Luronium natans (L.) Raf. in Poland. Hydrobiologia 455 (1), 213–222. doi: 10.1023/A:1011914607379
Szmeja, J. (2007). Monitoring gatunków i siedlisk przyrodniczych ze szczególnym uwzględnieniem specjalnych obszarów ochrony siedlisk Natura 2000 (Główny Inspektorat Ochrony Środowiska).
Szmeja, J. (2013). Monitoring gatunków i siedlisk przyrodniczych ze szczególnym uwzględnieniem specjalnych obszarów ochrony siedlisk Natura 2000 (Główny Inspektorat Ochrony Środowiska).
Vos, P., Hogers, R., Bleeker, M., Reijans, M., Lee, T. V. D., Hornes, M., et al. (1995). AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res. 23 (21), 4407–4414. doi: 10.1093/nar/23.21.4407
Woodhead, M., Russell, J., Squirrell, J., Hollingsworth, P. M., Mackenzie, K., Gibby, M., et al. (2005). Comparative analysis of population genetic structure in Athyrium distentifolium (Pteridophyta) using AFLPs and SSRs from anonymous and transcribed gene regions. Mol. Ecol. 14 (6), 1681–1695. doi: 10.1111/j.1365-294X.2005.02543.x
Keywords: aquatic plant, Alismatales, Luronium natans, endangered species, population genetics, conservation, growth form
Citation: Makuch WA, Wanke S, Ditsch B, Richter F, Herklotz V, Ahlborn J and Ritz CM (2023) Population genetics and plant growth experiments as prerequisite for conservation measures of the rare European aquatic plant Luronium natans (Alismataceae). Front. Plant Sci. 13:1069842. doi: 10.3389/fpls.2022.1069842
Received: 14 October 2022; Accepted: 14 December 2022;
Published: 13 January 2023.
Edited by:
Justine Marchand, Le Mans Université, FranceReviewed by:
Gudasalamani Ravikanth, Ashoka Trust for Research in Ecology and the Environment (ATREE), IndiaCopyright © 2023 Makuch, Wanke, Ditsch, Richter, Herklotz, Ahlborn and Ritz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weronika A. Makuch, d2Vyb25pa2EtYWduaWVzemthLm1ha3VjaEBib3RhbmlrLnVuaS1oYWxsZS5kZQ==
†ORCID: Weronika A. Makuch, orcid.org/0000-0001-5833-5004
Stefan Wanke, orcid.org/0000-0001-5405-5216
Veit Herklotz, orcid.org/0000-0003-4335-0193
Julian Ahlborn, orcid.org/0000-0002-4406-9654
Christiane M. Ritz, orcid.org/0000-0002-8728-6974
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.