
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci. , 05 December 2022
Sec. Technical Advances in Plant Science
Volume 13 - 2022 | https://doi.org/10.3389/fpls.2022.1059197
This article is part of the Research Topic Wild Plant Genetic Resources: A Hope for Tomorrow View all 9 articles
Japonica rice (Oryza sativa L.) is an important staple food in high-latitude regions and is widely distributed in northern China, Japan, Korea, and Europe. However, the genetic diversity of japonica rice is relatively narrow and poorly adapted. Weedy rice (Oryza sativa f. spontanea) is a semi-domesticated rice. Its headings are earlier than the accompanied japonica rice, making it a potential new genetic resource, which can make up for the defects of wild rice that are difficult to be directly applied to japonica rice improvement caused by reproductive isolation. In this study, we applied a natural population consisting of weedy rice, japonica landrace, and japonica cultivar to conduct a genome-wide association study (GWAS) of the heading date and found four loci that could explain the natural variation of the heading date in this population. At the same time, we developed recombinant inbred lines (RILs) crossed by the early-heading weedy rice WR04-6 and its accompanied japonica cultivar ShenNong 265 (SN265) to carry out a QTL mapping analysis of the heading date and mapped four quantitative trait locus (QTLs) and three epistatic effect gene pairs. The major locus on chromosome 6 overlapped with the GWAS result. Further analysis found that two genes, Hd1 and OsCCT22, on chromosome 6 (Locus 2 and Locus 3) may be the key points of the early-heading character of weedy rice. As minor effect genes, Dth7 and Hd16 also have genetic contributions to the early heading of weedy rice. In the process of developing the RIL population, we introduced fragments of Locus 2 and Locus 3 from the weedy rice into super-high-yielding japonica rice, which successfully promoted its heading date by at least 10 days and expanded the rice suitable cultivation area northward by about 400 km. This study successfully revealed the genetic basis of the early heading of weedy rice and provided a new idea for the genetic improvement of cultivated rice by weedy rice.
Japonica rice (Oryza sativa L.) is a staple food for most people in China, particularly in the northeast. It is crucial for economic development and ensuring global and national food security (Mao et al., 2017). Superior japonica varieties have been applied over a wide range for a long time. However, their long-term application will reduce genetic diversity (Wang et al., 2014). Due to genetic bottlenecks, new improvement strategies have encountered challenges.
Although wild rice resources have been widely suggested for rice improvement, the unfavorable characteristics of the hybrid offerings of wild rice and japonica rice far exceed the favorable characteristics due to reproductive isolation and geographic and genetic distance. Thus, wild rice is difficult to apply in breeding programs (Brambilla and Fornara, 2013; Brar and Khush, 2018; Xie et al., 2019).
As a semi-domesticated genetic resource, weedy rice (Oryza sativa f. spontanea) has no reproductive isolation with accompanied cultivar and adapts to the current paddy field ecosystem. In addition, weedy rice also possesses some adaptive alleles that cultivars lack. Among these alleles, the early-heading genes have great application potential for improving japonica cultivars, while the underlying molecular genetic mechanisms of the early heading in weedy rice are still not revealed, which hinders its use in breeding applications (Zhao et al., 2018; Sun et al., 2019).
With the rapid development of next-generation high-throughput sequencing technology and multi-omics, many genes related to the rice heading have been identified. The identification of genes related to photoperiodic pathway and analysis of their molecular regulatory network mechanisms can not only reveal the genetic variation in the rice heading but also guide the genetic improvement of rice breeding, which is of great significance and necessary to the high-yield breeding of rice (Ebana et al., 2011; Brambilla and Fornara, 2013; Shrestha et al., 2014; Hori et al., 2015; Onogi et al., 2016). Rice has two known relatively conserved photoperiodic flowering pathways: the Hd1-centered gene and its related genes under short-day (SD) conditions and the Ehd1-centered gene and its regulatory genes under long-day (LD) conditions (Li et al., 2015; Chen et al., 2022).
So far, many heading genes have been identified; however, the reason for weedy rice heading earlier than their accompanied cultivars is still largely unknown. We performed a genome-wide associated study (GWAS) and QTL mapping to reveal this genetic basis in this study. Meanwhile, we provide new genetic materials and gene resources for breeding early-heading and widely adapted japonica cultivated rice.
A total of 274 accessions, including 154 modern japonica cultivars, 87 japonica landrace, and 33 japonica weedy rice at Asian high latitudes (WRAH), were planted in the paddy field in three consecutive years from 2019 to 2021 for GWAS of the heading date. Another recombinant inbred line (RIL) population of 165 individuals derived from the weedy rice WR04-6 and japonica cultivar ShenNong 265 (SN265 called super rice) was used for QTL mapping of the heading date. All materials were planted at Shenyang Agricultural University (123°25′E, 41°48′N, Liaoning Province, China, temperate semi-humid continental climate, under natural long day-length conditions, average day length >14 h from May to September, average temperature of 23.5°C, fertilizer applied in the field with the following standards: urea 300 kg/hm2, diammonium 220 kg/hm2, potassium chloride 220 kg/hm2). Each variety was planted in three rows with 1.2-m row length, 30 cm apart between rows, and 12-cm space in rows, with one plant per hill. Field management followed normal agricultural practices. The list of accessions used in this study is displayed in Supplementary Tables S1, S2.
The first panicle beyond flag leaf 1 cm was recorded as the heading. The heading date was calculated as the date from sowing to the heading of half of the accession. The phenotype data are displayed in Supplementary Tables S1 and S2. Microsoft Excel 2019 and GraphPad Prism 9.0 were used for statistical analysis. The heading date difference of three ecotypes was tested by one-way ANOVA multiple comparisons. The D’Agostino–Pearson test method was used for the normal distribution test of the RIL population.
The genotype of the GWAS panel for the heading date has been effectively used to study the genetic basis of agronomic traits in weedy rice (Sun et al., 2022). All raw reads were screened for high quality with Q20 quality scores >95% and Guanine and cytosine (GC) content <50%. The reads of each accession were aligned and then mapped to the reference genome (IRGSP1.0) using BWA software. We used samtools v0.1.19 and GATK v4.0 for population SNP variant data, and potential PCR duplicates were removed. It is also necessary to control the single nucleotide polymorphism (SNP) quality with a minor allele frequency >0.05 and no more than 50% missing data. Finally, we got a high-quality SNP haplotype map with an average coverage depth of 23.2 times for each sample to proceed with follow-up analysis (Supplementary Figure S1). We performed the GWAS based on 1,311,445 genetic markers and a heading date of 3 years by using EMMAX software to fit a linear mixed model. To control the population structure during the GWAS, the first two principal components of the principal component analysis (PCA) were used as covariates. The Manhattan plots of GWAS results were drawn by R package CMplot. The threshold for genome-wide significance was determined by 10-5 and 10-6.
Based on the result of the GWAS, we performed linkage disequilibrium (LD) block analysis for the 2MB region surrounding the leading SNP. The LD block was defined by LDblockShow software. The genes located within the high LD blocks were selected for checking the gene annotation (https://rapdb.dna.affrc.go.jp/). Then, a haplotype-based association analysis for the genes within the LD block was conducted by candihap software (Li et al., 2020).
DNA was extracted from fresh leaves of RILs by the Cetyltrimethylammonium Bromide (CTAB) method. The genotypes were obtained using a 50K liquid-phase sequence capture chip (Guo et al., 2021). According to the genotyping results, 13,097 polymorphic sites were used for genetic map construction. Finally, a linkage map containing 2,275 bins was constructed based on the 13,097 polymorphic sites, spanning a total genetic distance of 2,068.6 cM, and the average genetic distance between adjacent markers was 0.91 cM. Marker information for the genetic map constructed is listed in Supplementary Table S3. This linkage map was used for QTL mapping of the heading date (Supplementary Figure S2).
The heading date of the RIL population was used as phenotype data combined with a high-quality bin marker map to proceed with QTL mapping by IciMapping software with a threshold Likelihood of Odd (LOD) value of 2.5. Two models were applied in this study: inclusive composite interval mapping with an additive effect (ICIM-ADD) and inclusive composite interval mapping with an epistasis effect (ICIM-EPI).
In developing RILs derived from WR04-6 and SN265, individuals with the early heading and other elite characteristics were selected. We extracted these individuals’ DNA by the CTAB method and designed primers by https://primer3.ut.ee/to detect the locus responsible for the early heading. Primers used in this study are displayed in Supplementary Table S6.
In order to further reveal the domestication and evolution relationship of Hd1 in different rice ecotypes, we used the sequencing data of 1,293 accessions to perform haplotype network analysis including five ecotypes, temperate japonica, tropical japonica, japonica intermediate, weedy rice, and wild rice [Oryza rufipogon (Or-IIIa)]. The sequence data of wild rice and weedy rice were from Sun et al. (2022), and the sequence of the japonica population was obtained from the database http://ricevarmap.ncpgr.cn/. The haplotype network was built based on 14 SNP variations of the Hd1 Coding sequence (CDS) region with Or-IIIa as an outgroup by using the “Median Joining Network” approach implemented in Popart software (Version 1.7).
In high-latitude paddy ecosystems, weedy rice tends to heading earlier than cocultivated rice for rapid completion of reproduction. In the present study, we collected 274 accessions from three japonica rice ecotypes as a GWAS panel: the high-latitude modern japonica cultivar and its accompanied japonica weedy rice and japonica landrace. The heading date of this panel was investigated for three consecutive years (2019, 2020, and 2021) as the phenotype for GWAS. In three ecotypes, weedy rice (average 85 days) exhibited significantly earlier heading than cultivated rice (average 96 days) and landrace (average 106 days) (Figures 1A–C).
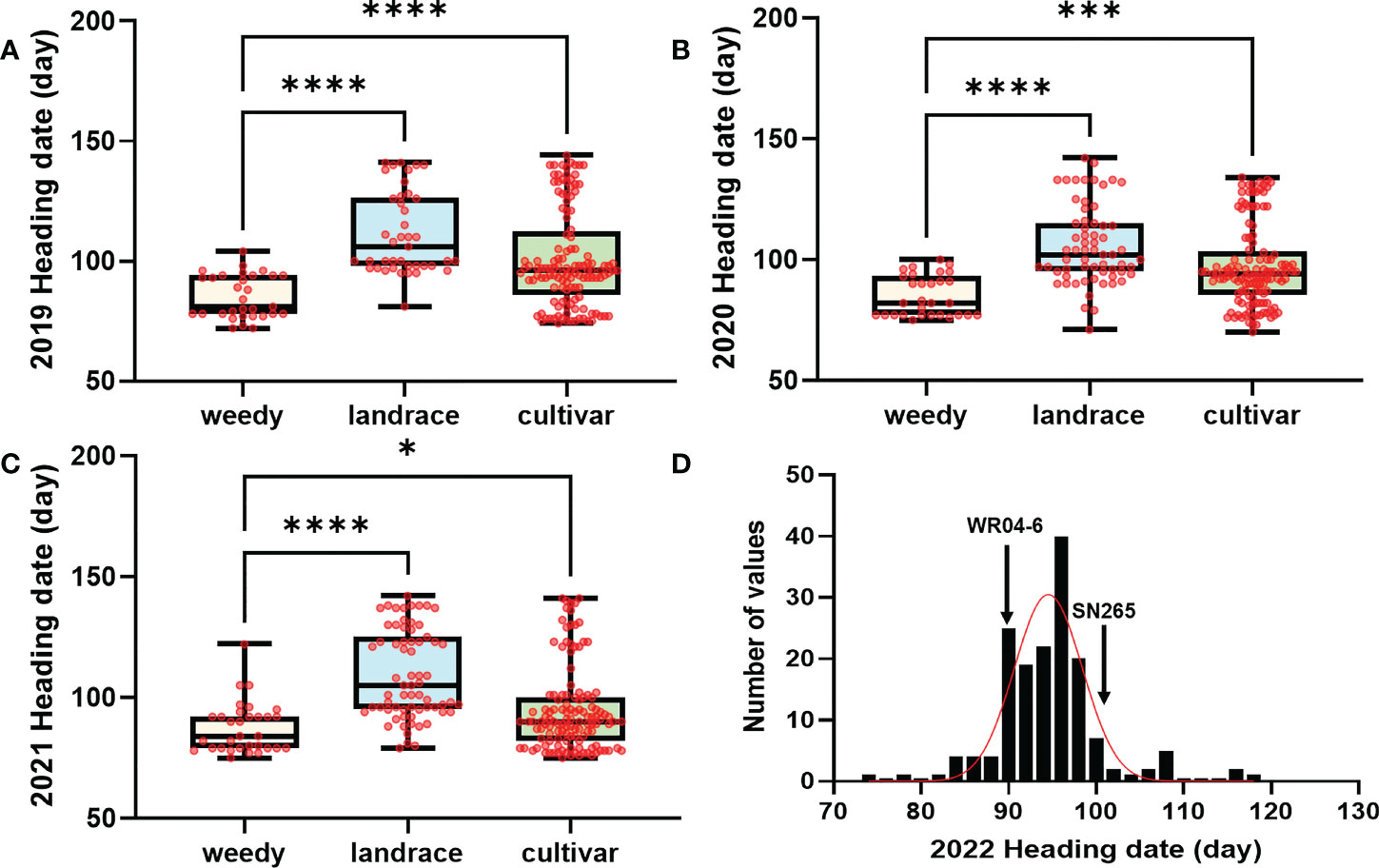
Figure 1 The heading date in the GWAS and RIL population. (A–C) The heading date of weedy rice, landrace, and japonica cultivar for 2019, 2020, and 2021 in the GWAS population. (D) Distribution of the RIL progenies of the weedy rice WR04-6 and japonica cultivar SN265. Bars are the maximum and minimum values of samples. Data were analyzed by one-way ANOVA multiple comparisons. ****, ***, and * represent significant differences at P = 0.0001, P = 0.001, and P = 0.05, respectively; red dots indicate samples. The D’Agostino–Pearson test method was used for the normal distribution test. weedy, weedy rice; landrace, japonica landrace; cultivar, modern japonica cultivar.
In the present japonica rice panel, the heading date exhibits significant variations. To find quantitative trait nucleotides (QTNs) that are significantly associated with this phenotypic variation, the heading date was investigated for three consecutive years (2019, 2020, and 2021). The Manhattan plots of the GWAS (using a linear mixed model and the first two principal components of PCA as covariates) showed that the four strongest associated signals (Locus 1, Locus 2, Locus 3, and Locus 4) were stable and detected for at least 2 years (Figures 2A–C). The specific GWAS results and the locus distribution of ecotype information are displayed in Table 1.

Figure 2 Results of the genome-wide association study. (A–C) Manhattan plot of 274 accessions in 2019, 2020, and 2021. Gray lines indicate threshold lines by 10-5 and 10-6; red and green dots mean SNPs above the threshold line. (D–F) Annotation of genes that are in highly linkage disequilibrium block (G) linkage disequilibrium block analysis of 2MB region surrounding the lead SNPs, highly linkage disequilibrium blocks are displayed in bold lines.
Considering the following QTL analysis in this study and the background knowledge on the known heading date genes in rice, Locus 2 and Locus 3 on chromosome 6 were the main objects of interest for further analysis. We expanded the 2MB physical distance surrounding the lead SNP of Locus 2 and Locus 3 to find highly LD regions. We finally detected two LD blocks in Locus 2 and one LD block in Locus 3 that involved 361 and 85 genes, respectively (Figure 2G). Based on the candidate gene association analysis, we further narrowed down the candidate genes to 24 in Locus 2 and 38 in Locus 3 (Supplementary Table S4). On the other hand, three genes according to the annotation (http://rice.uga.edu/), OsCCT20, OsCCT21 (Hd1), and OsCCT22, within the three LD blocks were considered as candidate causal genes due to all of them being CCT family members that were reported to have important roles in flowering regulation (Zhang et al., 2021) (Figures 2D–F). However, considering the candidate gene association analysis, different haplotypes of OsCCT20 did not show significant differences in the heading date. Finally, OsCCT21 (Hd1) and OsCCT22 were speculated to be the causal genes for responding to the natural variation of the heading date in Locus 2 and Locus 3 (Figures 3A–C).
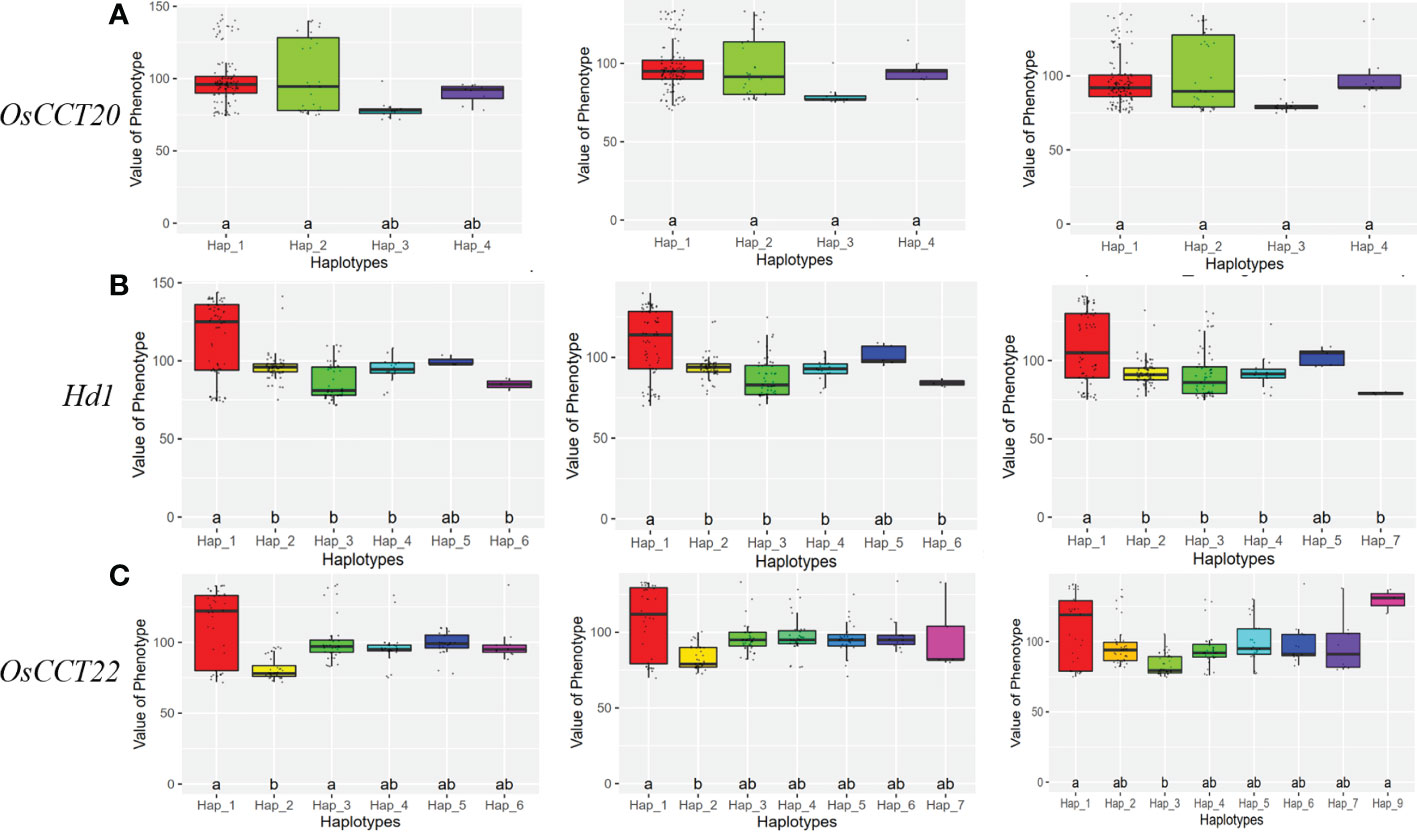
Figure 3 Boxplots of the heading date phenotype for different haplotypes of candidate genes. (A) A 3-year heading date box for the different haplotypes of OsCCT20. (B) A 3-year heading date box for the different haplotypes of Hd1. (C) A 3-year heading date box for the different haplotypes of OsCCT22. Duncan’s test at p ranks the phenotypic differences. The letter a and b are ranked by Duncan’s test at p<0.05.
Japonica rice SN265 with a high yield advantage was recognized as a “super rice” by the Chinese Ministry of Agriculture, and its accompanied weedy rice (named WR04-6) showed 10–15 days earlier heading than SN265. The heading date showed continuous variation in the RIL population from 74 days to 123 days, but not following a normal distribution (k2 = 30.19) (Figure 1D). We crossed SN265 with WR04-6 to develop an RIL population for detecting the genetic basis of these differences in the heading date by QTL mapping. We applied the ICIM-ADD model for additive-effects QTL mapping and found four QTLs above the threshold, as shown in Figure 4A; they were distributed on chromosomes 3, 6, 7, and 12 (Table 2). Among them, we noticed that three genes within the three QTL genomic regions were reported before: Hd1/OsCCT21 (Chr6), Dth7 (Chr7), and Hd16 (Chr3). Among them, Hd1 can explain the largest phenotypic variation (PVE = 35.9%); the phenotypic variance explained (PVE) of Hd16 is 8.3% and that of Dth7 is 6.1%. Moreover, the additive effect of Hd1 is 6.3111, while the additive effect of Hd16 and Dth7 is -2.1566 and -1.83, respectively. We further analyzed the genotype of the three genes between the two parents, weedy rice WR04-6 and japonica cultivar SN265, to confirm the specific variants (Figures 5A–C). We found that the genotype of Hd1 in WR04-6 in the coding region is different from that of SN265 and Nipponbare (reference genome). The changed base located in 134 coding regions in the zinc finger domain of Hd1 caused a non-synonymous substitution from valine to glycine, which may cause the functional variation of Hd1 between SN265 and WR04-6 (Figure 5A). Considering the GWAS results of the heading date in the japonica panel of the present study, we have reason to believe that OsCCT21 (Hd1) is the common cause of the early-heading feature in weedy rice.
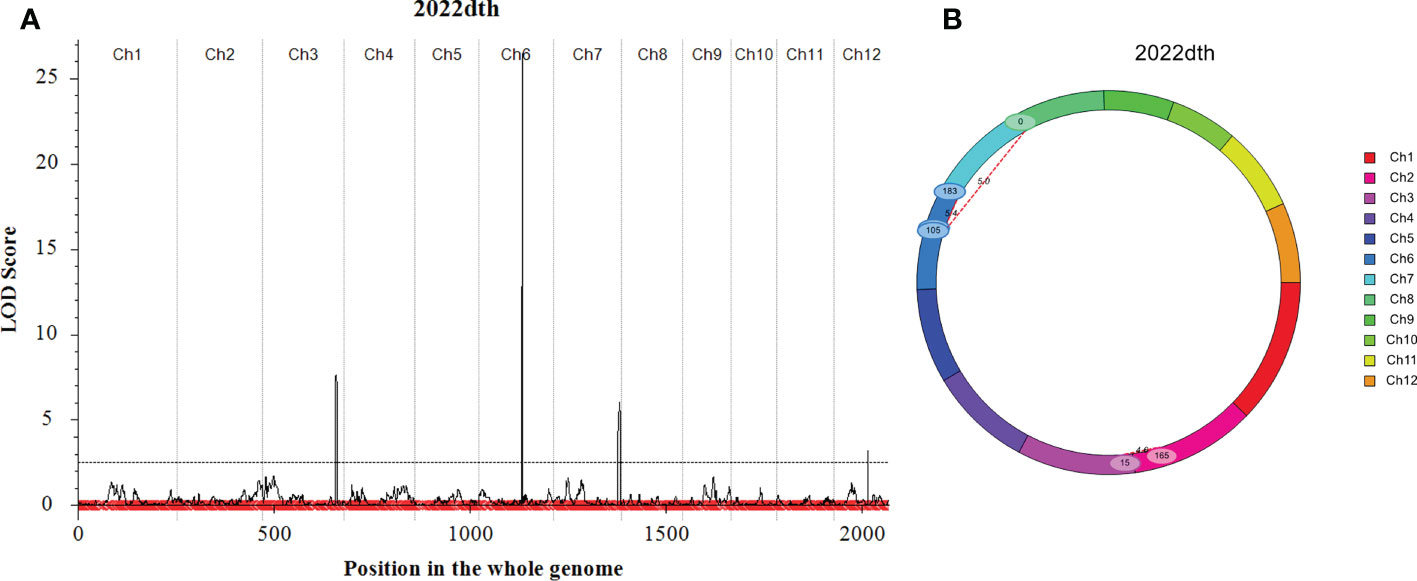
Figure 4 QTL mapping results of the heading date. (A) Additive effect for the heading date calculated by the ICIM-ADD model. (B) The epistatic effect for the heading date calculated by the ICIM-EPI model. The threshold line was set with an LOD score ≥ 2.5.
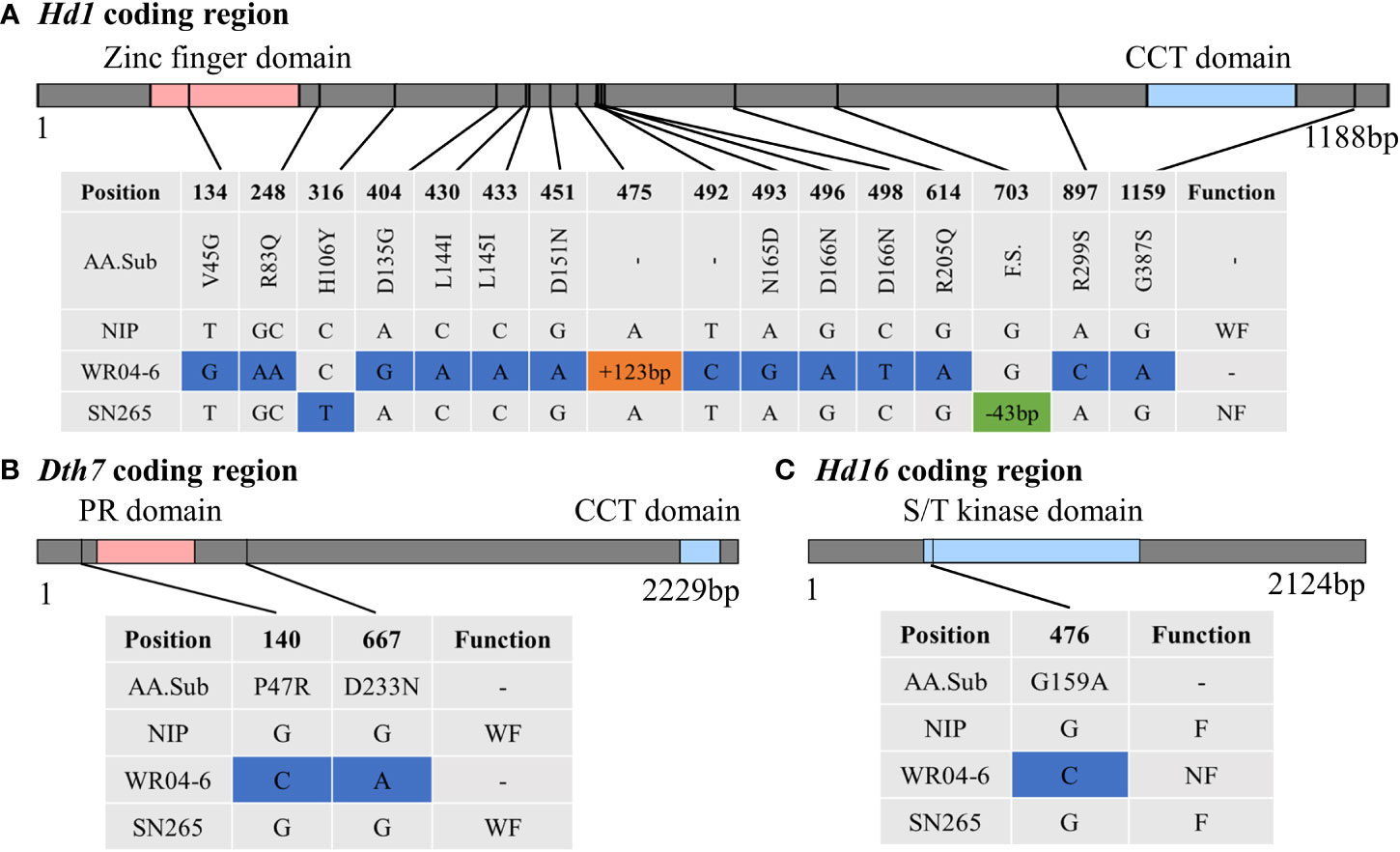
Figure 5 Genotype analysis between the weedy rice WR04-6 and japonica cultivar SN265. (A) The variation of Hd1 between WR04-6 and SN265 with Nipponbare genotype as a reference in the coding region. (B) The variation of Dth7 between WR04-6 and SN265 with Nipponbare genotype as a reference in the coding region. (C) The variation of Hd16 between WR04-6 and SN265 with Nipponbare genotype as a reference in the coding region. The blue box indicates a single base variation; the orange and green boxes represent insertion and deletion, respectively. F, Functional; WF, Weak functional; NF, Non-functional. The gray top bar represents the coding region; the colorful region means the domain of the gene.
How Hd1 of weedy rice evolves is another important scientific issue. Therefore, we established the genetic relationship between weedy rice and other ecotypes of japonica through the haplotype network. As shown in Supplementary Figure S4, weedy rice has four haplotypes, Hd1:Hap2, Hap3, Hap6, and Hap7. Most of the weedy rice (76.9%) is Hap7, which is exclusive to weedy rice and derived from Hap4, the major haplotype shared by temperate japonica, tropical japonica, and japonica intermediate. This result implies that selection pressure on weedy rice resulted in its Hd1 diverging from cultivated rice under long-term natural selection, which is further evidence of the genetic contribution of Hd1 in the early heading of weedy rice.
The genotype of Dth7 and Hd16 in minor effect QTL genomic regions also showed functional variations between WR04-6 and SN265. Dth7 has two non-synonymous substitutions in the WR04-6 coding region nearby the PR domain; although this haplotype of Dth7 has been reported before, its function is still unknown (Gao et al., 2014; Li et al., 2015; Li et al., 2018). A non-synonymous substitution in the WR04-6 S/K kinase domain caused the loss of function of Hd16 (Kwon et al., 2014) (Figures 5B, C). The other QTLs located on chromosome 12 were new unknown QTLs for the rice heading date reported by this study. In addition, we found three gene pairs that may regulate the heading date by epistasis effect interaction based on the ICIM-EPI (Figure 4B). Interestingly, one epistasis gene pair occurred between Hd1 and another locus; the specific details are displayed in Supplementary Table S5.
In developing the RIL population using the weedy rice WR04-6 and the super-high-yielding japonica rice SN265, we also performed genetic improvement practices of early-heading lines. From the F3 to the F8 generation, individuals with early heading and good comprehensive traits were selected as the selection targets in each generation. Finally, six genetically stable lines with different genetic backgrounds were successfully bred, and their heading dates ranged from 81 to 92 days, at least 10 days earlier than that of the japonica parent SN265. After detecting the genotype, we found that all of the fragments of Locus 2 and Locus 3 on chromosome 6 of these six lines were derived from the weedy rice parent WR04-6 (Supplementary Figure S3). We then cultivated the six lines in higher latitudes, Wuchang, Heilongjiang province (127°16′E, 44°93′N), and confirmed that they could safely head. Thus, our practice of this genetic improvement using weedy rice as a genetic resource has successfully expanded the planting range of super japonica rice northward by about 400 km. We also developed molecular markers for this early-heading interval that combined with the early-heading genetic resources of weedy rice will greatly contribute to breeding the early-heading japonica rice.
In high-latitude paddy ecosystems, weedy rice tends to head early than cocultivated rice for rapid completion of reproduction. In this study, we performed a GWAS and QTL mapping to reveal the genetic basis of the early-heading feature of weedy rice. Our research has found that Hd1 and OsCCT22 may be responsible for the heading date variations in the natural population by GWAS (Takahashi et al., 2009; Zhang et al., 2021). Furthermore, Hd1, Dth7, and Hd16 were colocated using the ICIM-ADD model in the RIL population, and the three genes do have variations between parents. We also found the epistatic effect of Hd1 for another QTL by the ICIM-EPI model. Considering the GWAS results with QTL mapping, we believe that Hd1 is the common cause of the early-heading feature in weedy rice. Our findings provide a new perspective for studying the molecular genetic mechanisms and regulatory networks governing the heading date in rice.
Hd1 contains the B-box zinc finger domain and CCT domain, which is the first cloned gene that can regulate the heading in rice from Nipponbare (Yano et al., 1997); it may be a selection target in the process of domestication of flowering diversity of cultivated rice (Takahashi and Shimamoto, 2011). In our study, we found a new haplotype of Hd1 in WR04-6, which contains a new non-synonymous substitution in the B-box binding domain. Based on available data, we speculate that this new haplotype is functional because the conserved CCT domain has no variation in WR04-6, and the additive effect of Hd1 is 6.311 in the RIL population, which means that the early-heading characteristic comes from the weedy rice WR04-6 (Takahashi et al., 2009). In addition, this substitution in the B-box binding domain may change the protein interaction and affect the other aspect of rice growth. More importantly, this single base substitution variation only exists in weedy rice and is not detected in other accessions, but we did not validate the activity of the Hd1 protein of weedy rice.
The flowering time of rice is regulated by plenty of genes; different combinations of these allelic genes determine the rice adaptability and regional distribution (Ebana et al., 2011; Guo et al., 2013; Lee and An, 2015; Brambilla et al., 2017). Hd1 has a basic function of promoting the expression of florigen genes Hd3a/RFT1 and heading regardless of the day length (Zong et al., 2021). Hd1 interacts with the Ghd7 (OsCCT26) CCT domain to form a complex that represses the rice heading. The Dth8/Hd1 complex binds to the promoter of Ghd7 (OsCCT26) to form a ternary complex (Lin et al., 2003; Nemoto et al., 2016; Du et al., 2017; Wang et al., 2019; Zhang B. et al., 2019; Zhang Z. et al., 2019; Zong et al., 2021). Dth7 (OsCCT28) is a major gene regulating the flowering time in the northeast of China, which also regulates the plant height and grains per panicle. Dth7 acts downstream of phyB, inhibiting the expression of Ehd1 (upstream regulator of rice florigen genes Hd3a and RFT1), thereby delaying flowering under day length (Liu et al., 2013; Gao et al., 2014; Liu et al., 2021). We speculate that the Dth7 haplotype found in WR04-6 is non-functional because the additive effect of Dth7 is -1.8338 in the RIL population (Table 2); the non-functional Dth7 leads to the early heading of weedy rice by reduced suppression of Ehd1 and promotes the expression of florigen genes Hd3a/RFT1 in the shoot apical meristem. The non-functional Dth7 reduced the competition for Hd1, which means that Hd1 will play a greater role in promoting the heading under long day-length conditions. Hd16 can phosphorylate Ghd7 (OsCCT26), which contributes to the later heading in japonica rice under LD conditions. Hd16 can also interact with and phosphorylate Dth7, although the underlying mechanism is still unknown (Kwon et al., 2015). The genetic interactions between Ghd7 and Dth7 and between Dth7 and Hd16 are also involved in the heading under LD conditions (Shibaya et al., 2011). The non-functional Hd16 in weedy rice will reduce the expression of Dth7 and Ghd7, thereby enhancing the expression of Ehd1 and promoting the heading. Variations found in weedy rice change the complex regulatory network of the heading date and other aspects of rice growth. Based on the above research, we can conclude that the early heading of weedy rice results from natural selection and competition with cultivated rice; however, how selections shape the early-heading loci in the weedy rice genome is still unknown. We uncovered this scientific question through the GWAS and QTL mapping in this study.
As an important ecological characteristic, the heading date plays a decisive role in the rice breeding practice. It affects cultivation environments, yield, rice quality, nutritional value, and other traits; suitable heading dates will maximize the use of light, heat, and other ecological resources in the local ecological environment (Brambilla et al., 2017). The early heading is a necessary feature for weedy rice to survive. Under long-term natural selection, the elite dominant allele of the early heading in weedy rice has been fixed. It is easy to reproduce naturally in the paddy field and breed offspring for weedy rice (Xia et al., 2011; Sun et al., 2013). Therefore, exploring early-heading gene resources of weedy rice is of great value for cultivating the early-heading cultivated rice.
Weedy rice can be an excellent genetic resource for its large number of elite characteristics, the most important is that weedy rice has no reproductive isolation with cultivars. Thus, these elite genes can be applied in the breeding practice and improvement programs for cultivars by Molecular marker-assisted selection (MAS). The genetic diversity of cultivated rice has decreased yearly due to the single breeding objective, so the genetic resource from weedy rice is very precious (Ebana et al., 2011). Applying the early-heading genes from weedy rice makes more elite cultivated varieties that can be planted in more expansive areas (Imaizumi et al., 2021). In the present study, we also developed several molecular markers to assist the selection of the early-heading varieties and expand the rice-suitable cultivation area northward.
Besides the early-heading characteristics, weedy rice still has many other fine biological characteristics that can be used in breeding. All these make weedy rice more competitive than the cultivated rice. Weedy rice is a precious genetic material for cultivated rice that is reported to be a hidden gold mine in the paddy field (Tang et al., 2011; Qiu et al., 2017; Sun et al., 2019; Wu et al., 2022). Especially in high latitudes where wild rice is difficult to apply, weedy rice might be the best choice to expand the genetic diversity of japonica rice.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
JS and WC designed the study. ZL, RG, JC, and XZ investigated the heading date trait. ZL and RG performed data statistical analysis. XY and JS performed GWAS analyses. ZL, XY, and CL carried out candidate gene association analysis. XmZ and PY performed searching candidate genes/QTLs. ZL wrote the paper. JS revised the manuscript. All authors read and approved the final manuscript.
This work was supported by the Liaoning Applied Basic Research Program (2022JH2/101300172), and the Support Program for Young Scientific and Technological Innovation Talents of Shenyang, China (No. RC210408).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2022.1059197/full#supplementary-material
Brambilla, V., Fornara, F. (2013). Molecular control of flowering in response to day length in rice. J. Integr. Plant Biol. 55 (5), 410–418. doi: 10.1111/jipb.12033
Brambilla, V., Gomez-Ariza, J., Cerise, M., Fornara, F. (2017). The importance of being on time: Regulatory networks controlling photoperiodic flowering in cereals. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.00665
Brar, D. S., Khush, G. S. (2018). “Wild relatives of rice: A valuable genetic resource for genomics and breeding research,” in The wild oryza genomes. Eds. Mondal, T. K., Henry, R. J. (Cham: Springer International Publishing), 1–25.
Chen, R., Deng, Y., Ding, Y., Guo, J., Qiu, J., Wang, B., et al. (2022). Rice functional genomics: decades' efforts and roads ahead. Sci. China Life. Sci. 65 (1), 33–92. doi: 10.1007/s11427-021-2024-0
Du, A., Tian, W., Wei, M., Yan, W., He, H., Zhou, D., et al. (2017). The DTH8-Hd1 module mediates day-Length-Dependent regulation of rice flowering. Mol. Plant 10 (7), 948–961. doi: 10.1016/j.molp.2017.05.006
Ebana, K., Shibaya, T., Wu, J., Matsubara, K., Kanamori, H., Yamane, H., et al. (2011). Uncovering of major genetic factors generating naturally occurring variation in heading date among Asian rice cultivars. Theor. Appl. Genet. 122 (6), 1199–1210. doi: 10.1007/s00122-010-1524-1
Gao, H., Jin, M., Zheng, X. M., Chen, J., Yuan, D., Xin, Y., et al. (2014). Days to heading 7, a major quantitative locus determining photoperiod sensitivity and regional adaptation in rice. Proc. Natl. Acad. Sci. U. S. A. 111 (46), 16337–16342. doi: 10.1073/pnas.1418204111
Guo, Z., Yang, Q., Huang, F., Zheng, H., Sang, Z., Xu, Y., et al. (2021). Development of high-resolution multiple-SNP arrays for genetic analyses and molecular breeding through genotyping by target sequencing and liquid chip. Plant Commun. 2 (6), 100230. doi: 10.1016/j.xplc.2021.100230
Guo, L., Zhang, Z.-h., Zhuang, J.-y. (2013). Quantitative trait loci for heading date and their relationship with genetic control of yield traits in rice (Oryza sativa). Rice Sci. 20 (1), 1–12. doi: 10.1016/s1672-6308(13)60101-8
Hori, K., Nonoue, Y., Ono, N., Shibaya, T., Ebana, K., Matsubara, K., et al. (2015). Genetic architecture of variation in heading date among Asian rice accessions. BMC Plant Biol. 15 (1), 115. doi: 10.1186/s12870-015-0501-x
Hori, K., Ogiso-Tanaka, E., Matsubara, K., Yamanouchi, U., Ebana, K., Yano, M. (2013). Hd16, a gene for casein kinase I, is involved in the control of rice flowering time by modulating the day-length response. Plant J. 76 (1), 36–46. doi: 10.1111/tpj.12268
Imaizumi, T., Ebana, K., Kawahara, Y., Muto, C., Kobayashi, H., Koarai, A., et al. (2021). Genomic divergence during feralization reveals both conserved and distinct mechanisms of parallel weediness evolution. Commun. Biol. 4 (1), 952. doi: 10.1038/s42003-021-02484-5
Kwon, C. T., Koo, B. H., Kim, D., Yoo, S. C., Paek, N. C. (2015). Casein kinases I and 2α phosphorylate oryza sativa pseudo-response regulator 37 (OsPRR37) in photoperiodic flowering in rice. Mol. Cells 38 (1), 81–88. doi: 10.14348/molcells.2015.2254
Kwon, C. T., Yoo, S. C., Koo, B. H., Cho, S. H., Park, J. W., Zhang, Z., et al. (2014). Natural variation in early flowering1 contributes to early flowering in japonica rice under long days. Plant Cell. Environ. 37 (1), 101–112. doi: 10.1111/pce.12134
Lee, Y. S., An, G. (2015). Complex regulatory networks of flowering time in rice. J. Rice Res. 3, 141. doi: 10.4172/2375-4338.1000141
Li, X., Liu, H., Wang, M., Liu, H., Tian, X., Zhou, W., et al. (2015). Combinations of Hd2 and Hd4 genes determine rice adaptability to heilongjiang province, northern limit of China. J. Integr. Plant Biol. 57 (8), 698–707. doi: 10.1111/jipb.12326
Lin, H., Liang, Z. W., Sasaki, T., Yano, M. (2003). Fine mapping and characterization of quantitative trait loci Hd4 and Hd5 controlling heading date in rice. Breed. Sci. 53 (1), 51–59. doi: 10.1270/jsbbs.53.51
Li, X., Shi, Z., Qie, Q., Gao, J., Wang, X., Han, Y. (2020). CandiHap: a toolkit for haplotype analysis for sequence of samples and fast identification of candidate causal gene(s) in genome-wide association study. BioRxiv, 967539. doi: 10.1101/2020.02.27.967539
Li, X., Sun, Y., Tian, X., Ren, Y., Tang, J., Wang, Z., et al. (2018). Comprehensive identification of major flowering time genes and their combinations, which determined rice distribution in northeast China. Plant Growth Regul. 84 (3), 593–602. doi: 10.1007/s10725-017-0364-2
Liu, X., Liu, H., Zhang, Y., He, M., Li, R., Meng, W., et al. (2021). Fine-tuning flowering time via genome editing of upstream open reading frames of heading date 2 in rice. Rice 14 (1), 59. doi: 10.1186/s12284-021-00504-w
Liu, T., Liu, H., Zhang, H., Xing, Y. (2013). Validation and characterization of Ghd7.1, a major quantitative trait locus with pleiotropic effects on spikelets per panicle, plant height, and heading date in rice (Oryza sativa l.). J. Integr. Plant Biol. 55 (10), 917–927. doi: 10.1111/jipb.12070
Mao, T., Li, X., Jiang, S.-k., Tang, L., Wang, J.-y., Xu, H., et al. (2017). Discussion on strategy of grain quality improvement for super high yielding japonica rice in northeast China. J. Integ. Agr. 16 (5), 1075–1083. doi: 10.1016/S2095-3119(16)61563-0
Nemoto, Y., Nonoue, Y., Yano, M., Izawa, T. (2016). Hd1, a CONSTANS ortholog in rice, functions as an Ehd1 repressor through interaction with monocot-specific CCT-domain protein Ghd7. Plant J. 86 (3), 221–233. doi: 10.1111/tpj.13168
Onogi, A., Watanabe, M., Mochizuki, T., Hayashi, T., Nakagawa, H., Hasegawa, T., et al. (2016). Toward integration of genomic selection with crop modelling: the development of an integrated approach to predicting rice heading dates. Theor. Appl. Genet. 129 (4), 805–817. doi: 10.1007/s00122-016-2667-5
Qiu, J., Zhou, Y., Mao, L., Ye, C., Wang, W., Zhang, J., et al. (2017). Genomic variation associated with local adaptation of weedy rice during de-domestication. Nat. Commun. 8 (1). doi: 10.1038/ncomms15323
Shibaya, T., Nonoue, Y., Ono, N., Yamanouchi, U., Hori, K., Yano, M. (2011). Genetic interactions involved in the inhibition of heading by heading date QTL, Hd2 in rice under long-day conditions. Theor. Appl. Genet. 123 (7), 1133–1143. doi: 10.1007/s00122-011-1654-0
Shrestha, R., Gómez-Ariza, J., Brambilla, V., Fornara, F. (2014). Molecular control of seasonal flowering in rice, arabidopsis and temperate cereals. Ann. Bot. 114 (7), 1445–1458. doi: 10.1093/aob/mcu032
Sun, J., Ma, D., Tang, L., Zhao, M., Zhang, G., Wang, W., et al. (2019). Population genomic analysis and De novo assembly reveal the origin of weedy rice as an evolutionary game. Mol. Plant 12 (5), 632–647. doi: 10.1016/j.molp.2019.01.019
Sun, J., Qian, Q., Ma, D.-R., Xu, Z.-J., Liu, D., Du, H.-B., et al. (2013). Introgression and selection shaping the genome and adaptive loci of weedy rice in northern China. New. Phytol. 197 (1), 290–299. doi: 10.1111/nph.12012
Sun, J., Zhang, G., Cui, Z., Kong, X., Yu, X., Gui, R., et al. (2022). Regain flood adaptation in rice through a 14-3-3 protein OsGF14h. Nat. Commun. 13 (1), 5664. doi: 10.1038/s41467-022-33320-x
Takahashi, Y., Shimamoto, K. (2011). Heading date 1 (Hd1), an ortholog of arabidopsis CONSTANS, is a possible target of human selection during domestication to diversify flowering times of cultivated rice. Genes. Genet. Syst. 86 (3), 175–182. doi: 10.1266/ggs.86.175
Takahashi, Y., Teshima, K. M., Yokoi, S., Innan, H., Shimamoto, K. (2009). Variations in Hd1 proteins, Hd3a promoters, and Ehd1 expression levels contribute to diversity of flowering time in cultivated rice. Proc. Natl. Acad. Sci. U. S. A. 106 (11), 4555–4560. doi: 10.1073/pnas.0812092106
Tang, L., Ma, D. R., Xu, Z. J., Deng, H. F., Chen, W. F., Yuan, L. P. (2011). Utilization of weedy rice for development of japonica hybrid rice (Oryza sativa l.). Plant Sci. 180 (5), 733–740. doi: 10.1016/j.plantsci.2011.02.002
Wang, P., Gong, R., Yang, Y., Yu, S. (2019). Ghd8 controls rice photoperiod sensitivity by forming a complex that interacts with Ghd7. BMC Plant Biol. 19 (1), 462. doi: 10.1186/s12870-019-2053-y
Wang, J., Jiang, T., Zou, D., Zhao, H., Li, Q., Liu, H., et al. (2014). Genetic diversity and genetic relationships of japonica rice varieties in northeast Asia based on SSR markers. Biotechnol. Biotechnol. Equip. 28 (2), 230–237. doi: 10.1080/13102818.2014.908019
Wu, D., Qiu, J., Sun, J., Song, B. K., Olsen, K. M., Fan, L. (2022). Weedy rice, a hidden gold mine in the paddy field. Mol. Plant 15 (4), 566–568. doi: 10.1016/j.molp.2022.01.008
Xia, H. B., Xia, H., Ellstrand, N. C., Yang, C., Lu, B. R. (2011). Rapid evolutionary divergence and ecotypic diversification of germination behavior in weedy rice populations. New. Phytol. 191 (4), 1119–1127. doi: 10.1111/j.1469-8137.2011.03766.x
Xie, Y., Shen, R., Chen, L., Liu, Y. G. (2019). Molecular mechanisms of hybrid sterility in rice. Sci. China Life. Sci. 62 (6), 737–743. doi: 10.1007/s11427-019-9531-7
Yan, W., Liu, H., Zhou, X., Li, Q., Zhang, J., Lu, L., et al. (2013). Natural variation in Ghd7.1 plays an important role in grain yield and adaptation in rice. Cell. Res. 23 (7), 969–971. doi: 10.1038/cr.2013.43
Yano, M., Harushima, Y., Nagamura, Y., Kurata, N., Minobe, Y., Sasaki, T. (1997). Identification of quantitative trait loci controlling heading date in rice using a high-density linkage map. Theor. Appl. Genet. 95 (7), 1025–1032. doi: 10.1007/s001220050658
Yano, M., Katayose, Y., Ashikari, M., Yamanouchi, U., Monna, L., Fuse, T., et al. (2000). Hd1, a major photoperiod sensitivity quantitative trait locus in rice, is closely related to the Arabidopsis flowering time gene CONSTANS. Plant Cell 12 (12), 2473–2484. doi: 10.1105/tpc.12.12.2473
Zhang, J., Fan, X., Hu, Y., Zhou, X., He, Q., Liang, L., et al. (2021). Global analysis of CCT family knockout mutants identifies four genes involved in regulating heading date in rice. J. Integr. Plant Biol. 63 (5), 913–923. doi: 10.1111/jipb.13013
Zhang, B., Liu, H., Qi, F., Zhang, Z., Li, Q., Han, Z., et al. (2019). Genetic interactions among Ghd7, Ghd8, OsPRR37 and Hd1 contribute to Large variation in heading date in rice. Rice 12 (1), 48. doi: 10.1186/s12284-019-0314-x
Zhang, Z., Zhang, B., Qi, F., Wu, H., Li, Z., Xing, Y. (2019). Hd1 function conversion in regulating heading is dependent on gene combinations of Ghd7, Ghd8, and Ghd7.1 under long-day conditions in rice. Mol. Breed. 39 (7). doi: 10.1007/s11032-019-1001-8
Zhao, C., Xu, W., Song, X., Dai, W., Dai, L., Zhang, Z., et al. (2018). Early flowering and rapid grain filling determine early maturity and escape from harvesting in weedy rice. Pest. Manage. Sci. 74 (2), 465–476. doi: 10.1002/ps.4730
Keywords: GWAS, genetic resources, heading date, weedy rice, QTL mapping
Citation: Li Z, Gui R, Yu X, Liang C, Cui J, Zhao X, Zhang X, Yu P, Chen W and Sun J (2022) Genetic basis of the early heading of high-latitude weedy rice. Front. Plant Sci. 13:1059197. doi: 10.3389/fpls.2022.1059197
Received: 01 October 2022; Accepted: 11 November 2022;
Published: 05 December 2022.
Edited by:
Mohd. Kamran Khan, Selcuk University, TurkeyReviewed by:
Deyong Ren, China National Rice Research Institute (CAAS), ChinaCopyright © 2022 Li, Gui, Yu, Liang, Cui, Zhao, Zhang, Yu, Chen and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenfu Chen, d2ZjaGVuQHN5YXUuZWR1LmNu; Jian Sun, c3VuamlhbjgxMTExOUBzeWF1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.