
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci. , 25 November 2022
Sec. Functional Plant Ecology
Volume 13 - 2022 | https://doi.org/10.3389/fpls.2022.1041742
This article is part of the Research Topic Dispersal Ecology of Land Plants: Striving Towards a More Universal Understanding View all 5 articles
Environmental filtering (EF) and dispersal filtering (DF) are widely known to shape plant community assembly. Particularly in arid and semi-arid mountainous regions, however, it remains unclear whether EF or DF dominate in the community assembly of different life forms or how they interact along elevational gradients. This research aims to reveal how different ecological processes influence herbaceous and woody community assembly and how they respond to various environmental drivers and elevational gradients. Here we integrated taxonomic diversity (TD), phylogenetic diversity (PD), and ecological drivers across an elevational gradient of 1,420 m in the Helan Mountain Nature Reserve, in typical arid and semi-arid areas of China. This study showed that the TD and PD of herbaceous communities significantly increase linearly with changing elevation gradients, while woody ‘TD’ showed a unimodal pattern, and there was little relationship between woody ‘PD’ and elevation. Herbaceous species exhibited significant phylogenetic clustering at low elevations, where they were influenced by climate, aspect, and tree cover. However, woody species exhibited random patterns across elevations. Herbaceous and woody species’ taxonomic and phylogenetic beta diversity is governed primarily by spatial turnover rather than nestedness. Spatial turnover is caused primarily by EF and DF’s combined influence, but their relative importance differs between herbaceous and woody communities. Therefore, we conclude that the responses of herbaceous and woody plants along elevation gradients in the Helan Mountains are decoupled due to their different adaptation strategies to climate factors in the drylands. These findings are important for understanding the assembly mechanisms driving plant communities in dryland under the context of dramatic increases in drought driven by climate warming.
Mountains are hotspots of biodiversity, covering 24% of the total geographical area and supporting about 50% of the planet’s biodiversity (Körner et al., 2011) They provide refuge for many plants and animals due to the various conditions created by the complex topography and could play an important role in protecting biodiversity (Becker et al., 2007; Körner et al., 2011; Yuancai et al., 2022). Mountains often establish unique local climates along elevation gradients (Wang et al., 2022). Continuous change in landscape and climate factors across elevation leads to variations in resource availability (i.e., light and moisture), creating different microenvironmental conditions within short distances (Becker et al., 2007; Ahmad et al., 2020; Behzad et al., 2022). It leads to a sharp transformation in the ecophysiological adaptation of plants (Jiang et al., 2016) and ultimately alters their diversity patterns along elevation gradients (Boscutti et al., 2018). Because of their steep climatic gradients, mountains are considered sentinels of global warming and, therefore, offer a unique field laboratory for understanding the mechanisms driving the evolution and maintenance of biodiversity along elevation gradients (Körner et al., 2016).
Elevation-diversity relationships have been popular in ecology, biogeography, and biodiversity conservation in recent decades (Körner et al., 2011; Shahriari et al., 2019; Montano-Centellas et al., 2021). Previous studies about biodiversity patterns along elevation gradients found an evident disparity in elevation-richness relationships worldwide (Berhanu et al., 2016; Zhang et al., 2016; Mayor et al., 2017). The maximum species diversity occurs in the mid-elevation range (Grytnes, 2003), but decreases or increases in species richness along elevation have also been documented (Zhu et al., 2009; Berhanu et al., 2016; Qianwen et al., 2022). Yet, contemporary changes in biodiversity across the globe have been investigated based largely on taxonomic diversity (TD; i.e., species identity) (Li et al., 2020), which does not necessarily reflect changes in phylogenetic diversity (PD) (Faith, 1992). To solve this problem, Webb et al. (2002) developed the basis for using phylogenetic data to discover the influence of deterministic processes on community assembly. The method based on PD, considering the phytogeographical affinities that drive community assemblages (Webb et al., 2002), has been used to reveal species diversity in elevation gradients (Dainese et al., 2015; Wang et al., 2022). Combining TD and PD has been increasingly recommended to reveal the underlying driving mechanisms of community assemblages (Li et al., 2020; Macheroum et al., 2021) because it provides more comprehensive information on community assembly from ecological and evolutionary processes at the α and β diversity scales (Li et al., 2020; Du et al., 2021). Approaches that relate local processes to regional and evolutionary processes based on PD or spatial turnover will quantify the elevation-diversity relationship from an ecological and evolutionary perspective (Webb et al., 2002). However, to date, the assembly mechanisms driving plant communities along elevational gradients remain well elusive (Luo et al., 2019).
Theories of community assemblages, including niche-based processes and stochastic processes (Montano-Centellas et al., 2021), considered processes of species dispersal, species persistence, and species coexistence, were used to illustrate the species assembly (Webb et al., 2002; Grigoropoulou et al., 2022). Niche theory highlights the role of environmental filtering (EF; species with specific traits coexist under specific environmental stresses) and biological interactions (competitive exclusion causes limiting similarity) (Wang et al., 2021). Webb et al. (2002) claimed that interspecific competition might result in a community pattern of phylogenetic overdispersion. It might also result in phylogenetic clustering if certain clades have stronger competitiveness than others. Some evidence suggests that high-elevation habitats are generally under harsher conditions (low temperatures, dramatic temperature fluctuations) (Wang et al., 2021), in which plant community compositions are more sensitive to climate change than those of low-elevation habitats (Sproull et al., 2015). In contrast, the neutral theory holds that stochastic fluctuations and dispersal filtering (DF) independently determine the patterns of community assembly (Tilman, 2004; Hubbell, 2005). It is now commonly accepted that community assembly is determined by both processes (Farjalla et al., 2012; Grigoropoulou et al., 2022). Recent studies demonstrate that EF could become more critical for shaping plant community structure in stressful environmental conditions, while DF might dominate under benign conditions (Pouteau et al., 2019). Thus, a shift in the community assembly from overdispersal in low elevations to clustering in high elevations appears to occur. For heterogeneous mountains, however, the species assemblage along elevation gradients may be very complex and show various trends (Jarzyna et al., 2021; Montano-Centellas et al., 2021) for different life-form plants because they have diverged from adaptation strategies to specific environmental factors (Lee and Chun, 2015; Loidi et al., 2021; Macheroum et al., 2021). Moreover, due to different spatial scales and life forms in terms of evolutionary relationships and dispersal ability (Liu et al., 2015; Shahriari et al., 2019), quantifying the relative importance of the EF and DF along elevation gradients remains challenging (Du et al., 2021). Despite knowing that communities are being formed, it remains unclear whether EF or DF dominate in the community assembly of different life forms or how they interact along elevational gradients, especially in arid areas (Macheroum et al., 2021).
Drylands are defined as places with an aridity index of less than 0.65. They encompass over 45% of the planet’s land surface and inhabit over 38% of the Earth’s population (Peng et al., 2020). The drylands of China account for about 10.8% of the world’s drylands, and China is the most drought-affected country in Asia (Huang et al., 2015). The dramatic increase in climate warming-driven drought is considered a potential threat to worldwide biodiversity (Dai, 2010; Jiao et al., 2021). Climate change may aggravate dryland degradation through the alteration of spatial and temporal patterns in temperature, rainfall, solar radiation, and winds, leading to vegetation degradation and ecosystem service loss in the drylands (Macheroum et al., 2021; Gul et al., 2022). Nevertheless, plant diversity patterns and assembly mechanisms in dryland mountain ranges are not well documented. The Helan Mountains, a typical arid and semi-arid region of China with particularly fragile habitats exposed to extreme climate change, are one example (Pang et al., 2013; Cheng et al., 2020). The Helan Mountains are also the boundary line between the grassland and desert areas of Northwest China (Jiang et al., 2007). The vegetation types can be classified as desert grassland, sparse mountain grassland, montane coniferous forest, subalpine scrub meadow or alpine meadow. The vegetation distribution patterns in these regions are strongly influenced by climatic and topographical factors (Cheng et al., 2020; Arif et al., 2022a; Chen et al., 2022). However, to our knowledge, elevation-diversity relationships have not been well revealed in the drylands of China, especially when considering the different life forms of plants.
This study investigated herbaceous and woody communities along elevational gradients in the Helan Mountains of arid and semi-arid regions in Northwest China. Here we integrate taxonomic and phylogenetic diversity and environmental drivers (climatic and topographic variables; Cheng et al., 2020) to elucidate how various ecological mechanisms shape herbaceous and woody community assemblages and to reveal how these communities respond to different environmental drivers along elevational gradients. Specifically, we aimed to answer the following key scientific questions:
(1) How do the TD and PD of herbaceous and woody communities vary with changing elevation gradients in the Helan Mountains?
(2) Which factors in the environment impact the TD and PD of herbaceous and woody communities in this area?
(3) How and to what extent do EF and DF affect the spatial turnover of herbaceous and woody species in this region?
This research was performed in the Helan Mountains (38°13′ N, 105°41′ E; Figure 1), which are situated in arid to semi-arid regions in Northwest China. The climate in this region is primarily subject to the influence of the summer and winter monsoons, with mean annual temperatures ranging from 8.2°C to 8.6°C (-8.54°C in January and 21.43°C in August) and mean annual precipitation of 209.2 ± 57.2 mm, of which around 44% of precipitation occurs during the July-August growing season (Pang et al., 2013). High-elevation areas had lower temperatures and higher precipitation than low-elevation areas (Jiang et al., 2007). Gray cinnamonic soils prevail in this area (Wang and Yang, 2021). It is robust evidence of local vegetation evolution in northern China and its relationship with East Asian summer winds (Cheng et al., 2020). Trees (such as Picea crassifolia, Pinus tabuliformis, and Ajania fruticulose), shrubs, and grasses are the main land cover types across this region. However, due to the long lifespan and limited distribution of trees, it is difficult to detect tree distribution patterns with changing elevation gradients. Specifically, an interspersed distribution of continuous woody (shrubs and small trees) and grassland dominates in this area. Considering accessibility, we identified 23 study sites with the peak growing season of 2021, from 1,169 m to 2,589 m in the Helan Mountains. At each site, we established a permanent plot (20 m × 20 m) (Lopez-Angulo et al., 2018), and its vegetation structure and composition were assessed by systematic sampling by using the quadrat method, as it has less bias and covers the entire plant composition (Ahmad et al., 2020). Within each permanent plot, five quadrats of 25 m2 (5 m × 5 m for woody) and 1 m2 (1 m × 1 m for herbaceous) were sampled (Shi et al., 2020). Each 1 m2 quadrat was nested within each 25 m2 quadrat. A total of 230 quadrats were sampled. We recorded and measured all the species’ compositions and abundance in each quadrangle. We found 120 species that came from 90 genera and 41 families.
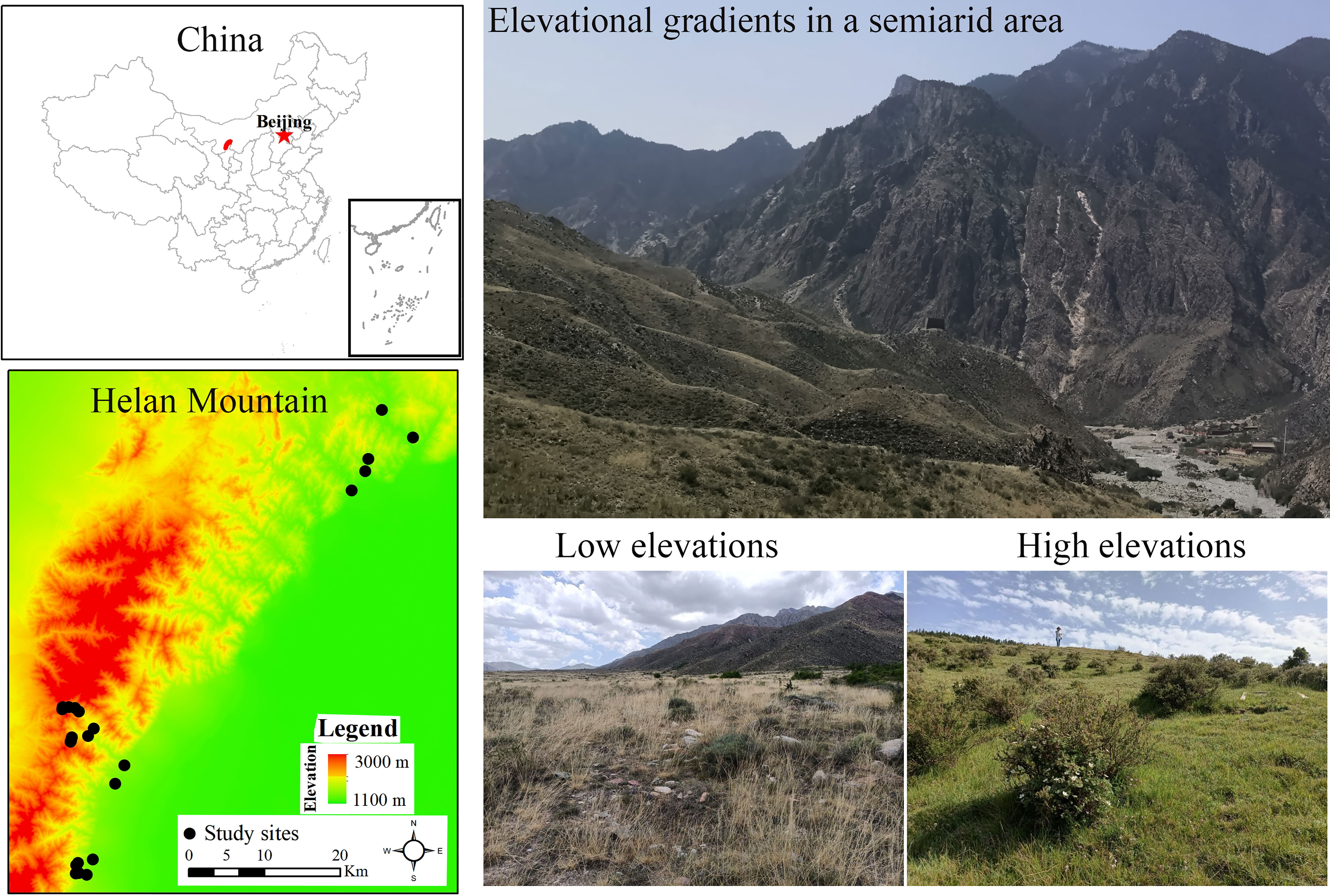
Figure 1 Map of the study site and plots on Helan Mountain of arid and semi-arid regions in Northwest China.
We classified the plants into 44 woody (including all shrubs and trees) and 76 herbaceous species according to the Flora of China (http://www.iplant.cn/) and field observations to understand how different species responded to elevational gradients. To compare the TD and PD of herbaceous and woody communities, species-level phylogenetic trees based on the mega-tree approach were constructed using the “V.PhyloMaker” package (Smith and Brown, 2018; Jin and Qian, 2019). The package is the largest dated phylogeny for vascular plants and includes 74,533 species of extant vascular plants (Jin and Qian, 2019). We then used the pruned versions of the tree for calculating the phylogenetic structure of both herbaceous and woody species in each of the 23 communities. We then calculated TD using species richness and PD using the mean pairwise distance (MPD) and the mean nearest taxon distance (MNTD) (Qian et al., 2019). To disentangle the assembly mechanisms of herbaceous and woody communities (Luo et al., 2019), we converted their MPD and MNTD metrics into standardized effect values (SESmpd and SESmntd indices) in the ‘Picante’ package (Kembel et al., 2010). Positive and negative values of SESmpd and SESmntd respectively indicate overdispersal and clustering (Yakimov et al., 2020). Their absolute values are higher than 1.96, meaning that the community structure is significantly overdispersed or clustered compared to null expectations (Webb et al., 2002; Yakimov et al., 2020). In addition, to distinguish TD and PD of herbaceous and woody communities at the beta scale, in the “betapart” package, we calculated three pairwise dissimilarity indices (Baselga, 2010; Baselga and Orme, 2012), in which the Sørensen dissimilarity (βsor) index measures the overall beta diversity, the Simpson dissimilarity (βsim) index measures the turnover component, and the nestedness dissimilarity (βnes) index measures the nestedness component derived from nestedness-related richness differences.
Temperature, precipitation, and their extremes will remarkably influence species’ ecological and evolutionary processes (Qian et al., 2019). Consequently, seven indicators of temperature and precipitation (Table S1) from the WorldClim database (Hijmans et al., 2005) were used to represent the climatic characteristics of the study area. Before the analyses, a principal component analysis (PCA; Table S1) was used to prevent the effects of collinearity (Figure S1) (Dormann et al., 2013), and the PCA axes with an interpretation rate higher than 10% were retained for subsequent analysis (Slik et al., 2013), resulting in two axes of climate variables (PC1 and PC2; Figure S2). Besides, the slope and aspect (a proxy for topographic heterogeneity; Shiferaw et al., 2018) of each plot were determined and closely related to the amount of incoming solar radiations and the construction of different microclimatic conditions (Pang et al., 2013), which is a significant factor that promotes diversification in plant community composition. In addition, the altitude, longitude, and latitude of each plot were recorded with GPS.
We analyzed the relationship between TD, SESmpd, SESmntd and elevation using simple linear and quadratic regression models (without significant spatial autocorrelation based on Morans’I index, Table S2). The analysis considered both linear and quadratic terms but only retained the results for the linear term when the quadratic term was not significant. A linear multiple regression model involving the ordinary least squares (OLS) method was used to identify the role of climate (climate PC1, climate PC2), topographic (slope and aspect), and biotic (woody cover for herbaceous) drivers (Figure S3) in determining a regional disparity in the TD and PD of herbaceous and woody species. Prior to the OLS analyses, all predictors were centered and scaled (Chun and Lee, 2017). To understand the relative contribution of predictor variables to the diversity of herbaceous and woody species, model averaging methods based on Akaike information criterion weights were used in the “MuMIn” package (Barton, 2019). We estimated the coefficients by averaging over all possible models and weighting them according to the probability associated with each model.
We investigated the correlation between taxonomic and phylogenetic turnover based on the βsim index with spatial and environmental distance to evaluate the species turnover along altitudinal gradients by employing the “ecodist” package (Goslee and Urban, 2007). A variance partitioning approach was performed to quantify the relative contribution of EF and DF in taxonomic and phylogenetic turnover. Prior to variation partitioning, the redundancy analysis and the forward selection were executed to reduce redundant components (Zheng et al., 2021). In this analysis, the ‘vegan’ package partitions the total explained variance (R2) into three parts (Oksanen et al., 2020; Shi et al., 2021), including their unexplained, joint, and independent effects. The Euclidean distances of five environmental variables (PC1, PC2, elevation, aspect, slope) between 23 sites were chosen to reflect EF, and spatial distances between study sites were selected to reflect DF (Li et al., 2021; Shi et al., 2021). We use the adjusted R2 to estimate the explanatory power of each component because of the different number of explanatory variables in our models.
In addition, to further examine clustering or overdispersion of plant communities, we performed a co-occurrence analysis by calculating the C-score (Checkerboard score; Stone and Roberts, 1990), which is a widely used index to measure associations between species pairs. We used null model analyses to quantify whether species co-occurrence patterns deviated from the expectations of a random (stochastic) assembly process (Ulrich et al., 2012). The values obtained were standardized to allow comparisons among assemblages using the standardized effect size (SES). The observed C-score was higher than randomized expectations, indicating that pairs of species co-occurred to a lesser extent than expected at random. The magnitude of SES was interpreted as the strength of the effect of deterministic processes on the assemblage. The C-score was evaluated based on 10,000 simulations and using the sequential swap randomization algorithm with the package “EcoSimR” (Gotelli et al., 2015).
The TD (species richness) and PD (SESmpd and SESmntd) of herbaceous communities significantly increased linearly along the elevational gradient (Figure 2A, Figures 3A, C). The nonlinear model was remarkable for woody communities with relatively low richness at the ends of the elevation range and peak richness in the 1500 ~ 1600 m range (Figure 2B). However, the PD (SESmpd and SESmntd) of woody communities did not significantly change as elevation increased (Figures 3B, D). The SESmpd and SESmntd of herbaceous communities showed random patterns at high elevations (above 2000 m) and were characterized by significant phylogenetic clustering (negative values below ‐1.96) at low elevational sites (Figures 3A, C). Surprisingly, all woody communities showed random patterns for SESmpd and SESmntd, except for two communities at low elevations that showed significant phylogenetic clustering for SESmntd (Figures 3B, D). Overall, most phylogenetic SESmpd and SESmntd values of herbaceous and woody communities were less than zero, indicating that phylogenetic clustering was driven by deterministic processes. This result was further supported by the co-occurrence analysis, where the observed C-score was higher than the simulated C-score values (Figure S4), indicating a non-random co-occurrence pattern. In addition, the C-score showed a higher standardized effect size (SES) for woody plants compared to herbaceous plants, indicating the greater importance of deterministic processes for woody plant assemblages (Figure S4).
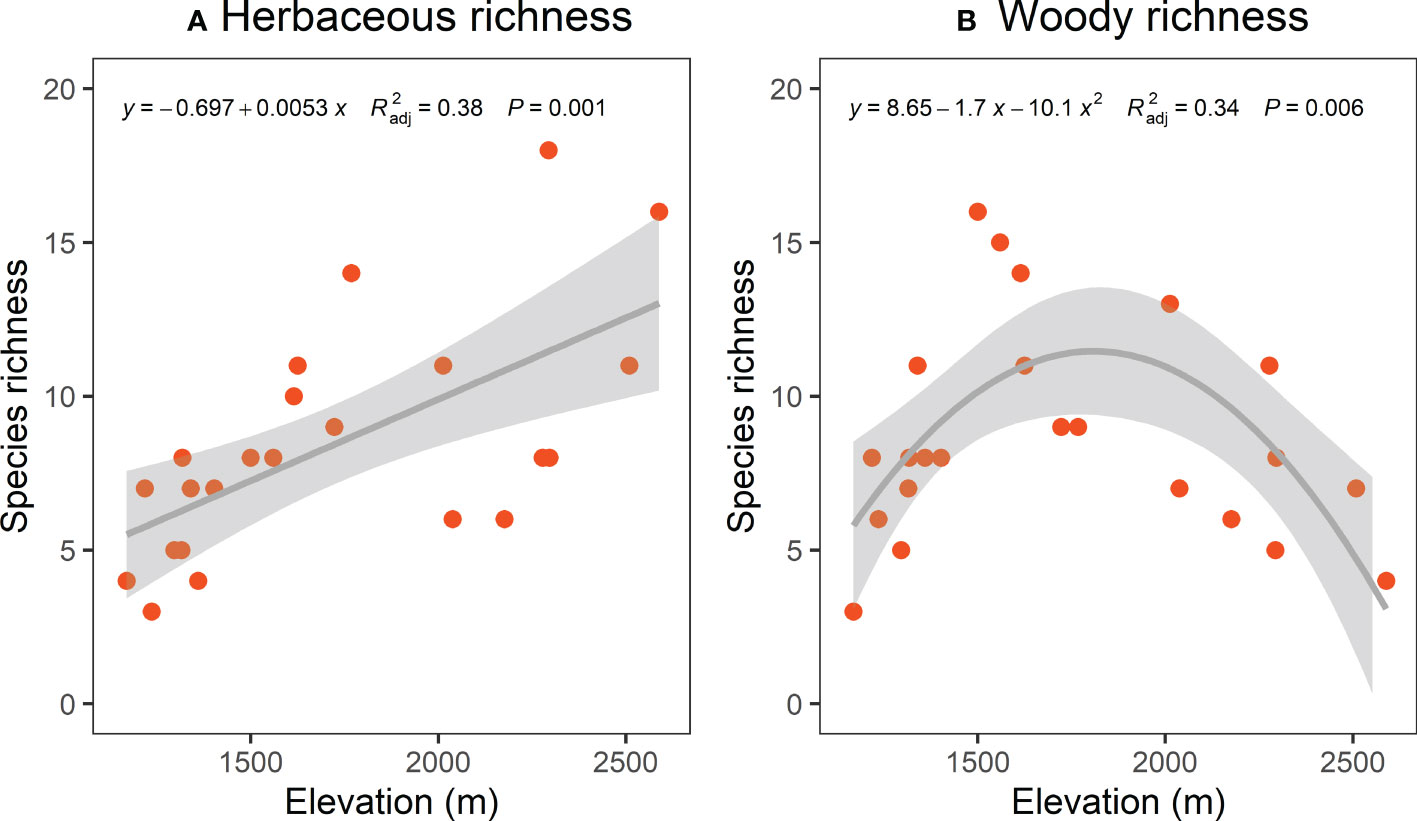
Figure 2 Elevational patterns of species richness of herbaceous (A) and woody (B). Trend lines and shaded areas represent the fitted values from linear regression with a linear term (A) and quadratic (B) and their 95% confidence intervals, respectively.
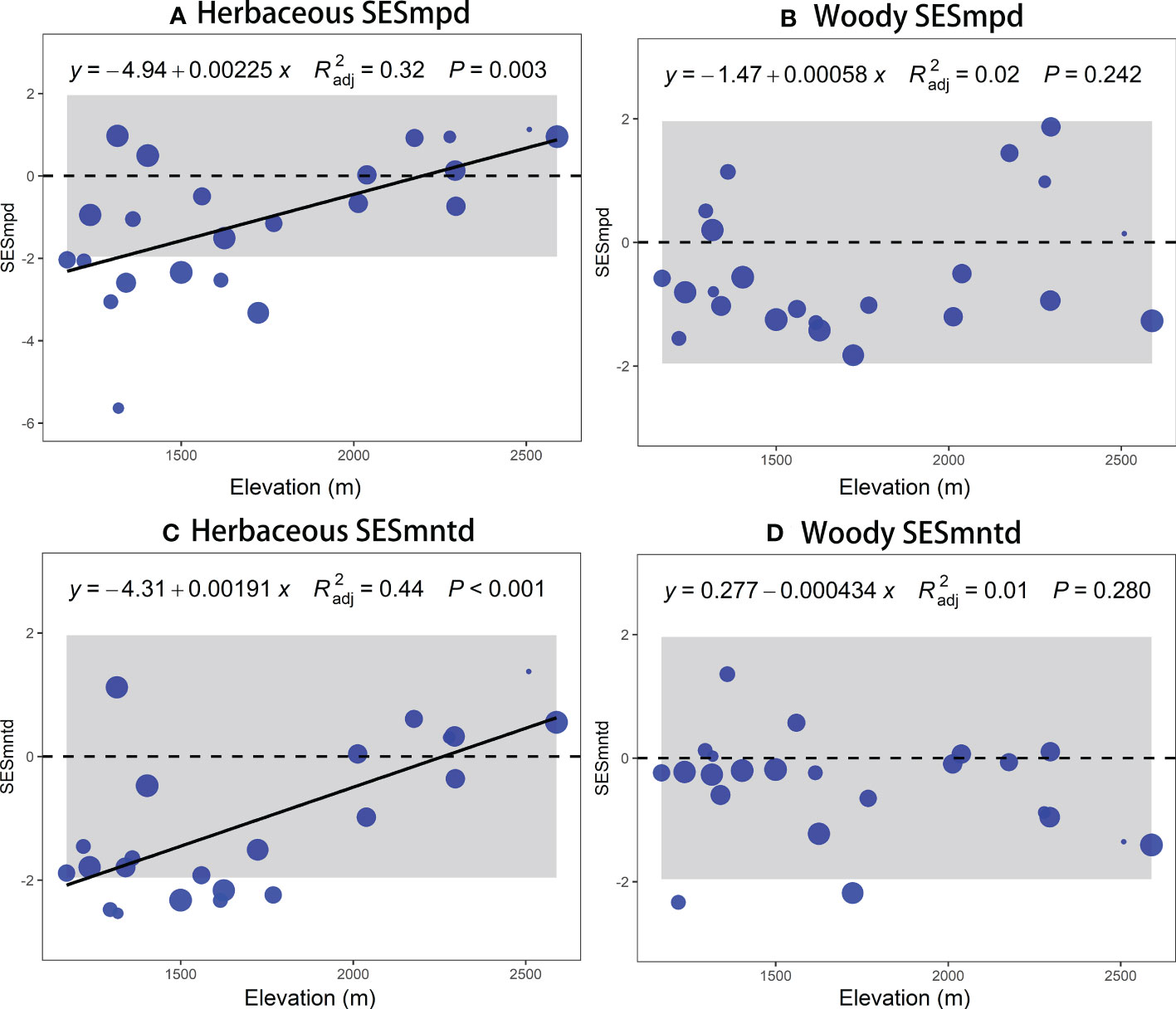
Figure 3 Elevational patterns of standardized values of mean phylogenetic structure (SESmpd and SESmntd) of plant communities of herbaceous (A, C) and woody (B, D). The size of the circle indicates the relative species richness associated with each plot.
For herbaceous communities, climate PC1 and woody cover had significantly negative effects on species richness (Figure 4A), whereas SESmntd indices showed significant negative relationships with climate PC1 and aspect, and no relationship between SESmpd and multi-dimensional variables (Figures 4C, E). Surprisingly, regional (climatic PC1 and PC2) and local (aspect and slope) variables were not significantly correlated with the TD and PD of woody communities (Figures 4B, D, F).
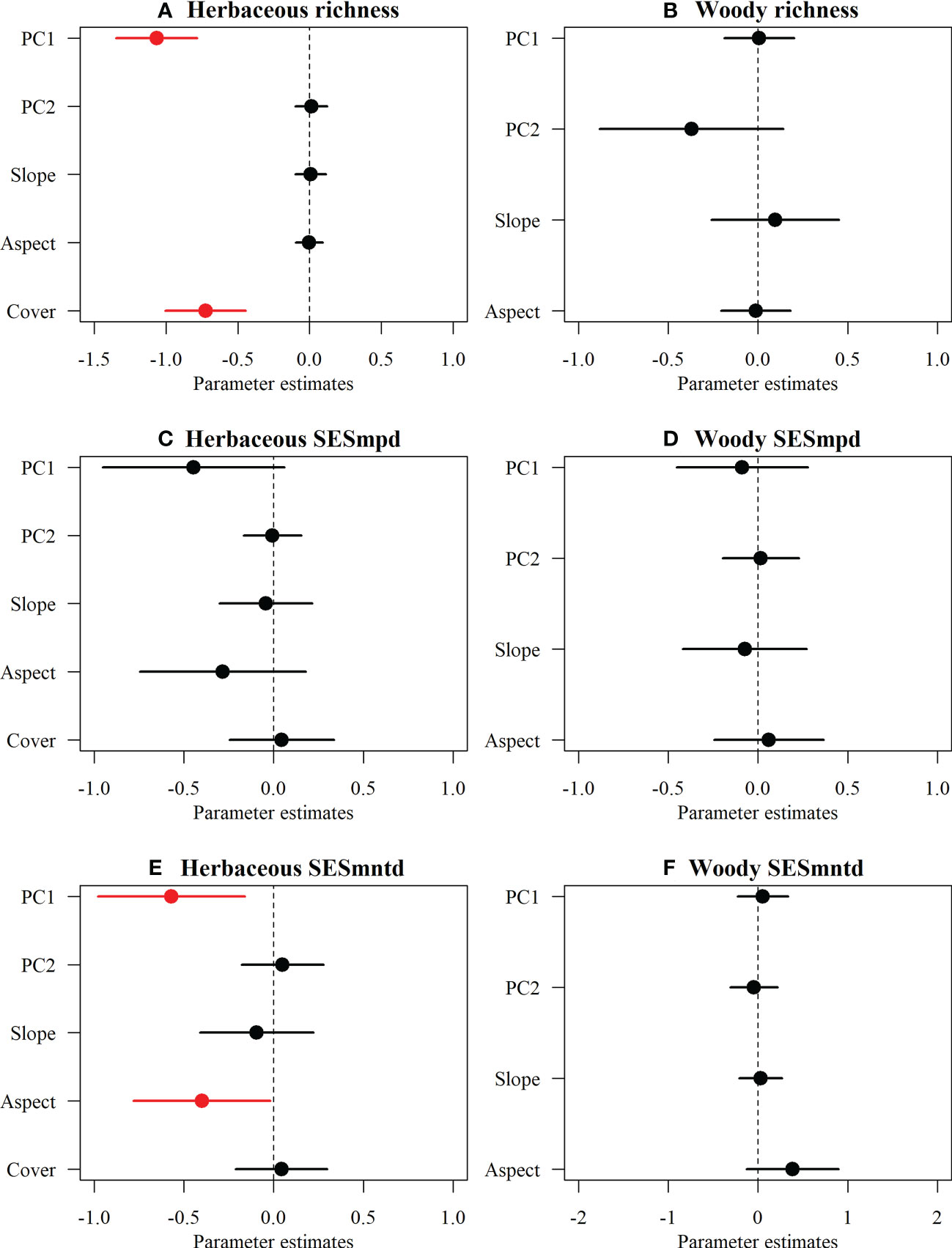
Figure 4 The effects of multi-dimensional variables on the species richness and phylogenetic structure (SESmpd, SESmntd) of plant communities of herbaceous (A, C, E) and woody (B, D, F). The parameter estimates (standardized model-averaged coefficients for variables) and the associated 95% confidence intervals are shown. Coefficient > 0 represents a positive effect, while coefficient < 0 indicates a negative effect (red). All predictors were centered and scaled to make the coefficients directly comparable.
Overall variation in the taxonomic and phylogenetic β-diversity of the herbaceous and the woody resulted mainly from turnover rather than nestedness (Figure 5). In taxonomic β-diversity, the species turnover of herbaceous and woody were 0.842 (94.08%) and 0.825 (94.83%), whereas species nestedness accounted for only 0.053 (5.92%) and 0.045 (5.17%), respectively (Figure 5A). In phylogenetic β-diversity, the species turnover of herbaceous and woody were 0.912 (97.33%) and 0.875 (96.05%), whereas species nestedness accounts for only 0.025 (2.67%) and 0.036 (3.95%), respectively (Figure 5B).
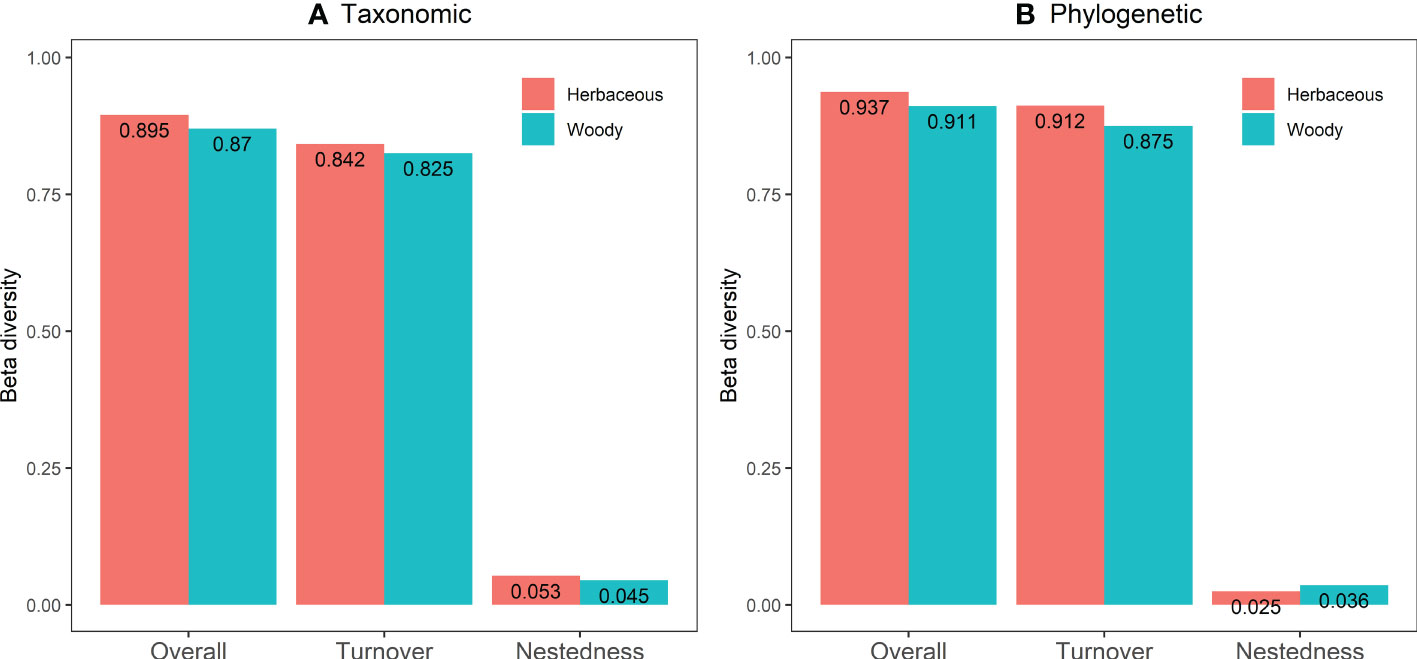
Figure 5 The taxonomic (A) and phylogenetic (B) β-diversity and its composition of the herbaceous and the woody community in the arid and semiarid areas of China.
For herbaceous communities, the taxonomic turnover was related to environmental distance after accounting for spatial effects (Mantel’s r = 0.42, p = 0.001), but it did not depend on the spatial distances when considering the environmental influences (Mantel’s r = -0.14, p = 0.12). Phylogenetic turnover displayed a similar trend, but it was reduced in prominence when considering spatial influences (Mantel’s r = 0.34, p = 0.001) or environmental effects (Mantel’s r = -0.20, p = 0.06). For woody communities, the taxonomic turnover was related to environmental (Mantel’s r = 0.53, p = 0.001) but it was not associated significantly with spatial distance (Mantel’s r = -0.16, p = 0.06). Phylogenetic turnover was significantly correlated with the environment (Mantel’s r = 0.55, p = 0.001) and spatial distance (Mantel’s r = -2.4, p = 0.011).
Environmental and spatial variables explained 33% to 77% of the variation in species turnover (Figure 6). The independent effects of environmental variables accounted for 3% and 7% of the overall variances of taxonomic turnover and phylogenetic turnover, respectively. Independent spatial effects accounted for 3% and 2% of overall variances of taxonomic turnover and phylogenetic turnover (Figures 6A, C). In comparison, environmental variables (24% for taxonomic turnover and 12% for phylogenetic turnover) were drastically higher than that spatial variables (4% and 3%) (Figures 6B, D). Compared to the pure effects, their combined effects accounted for most of the turnover variation in herbaceous (27% for taxonomic turnover and 36% for phylogenetic turnover) and woody (50% for taxonomic turnover and 53% for phylogenetic turnover) species (Figure 6).
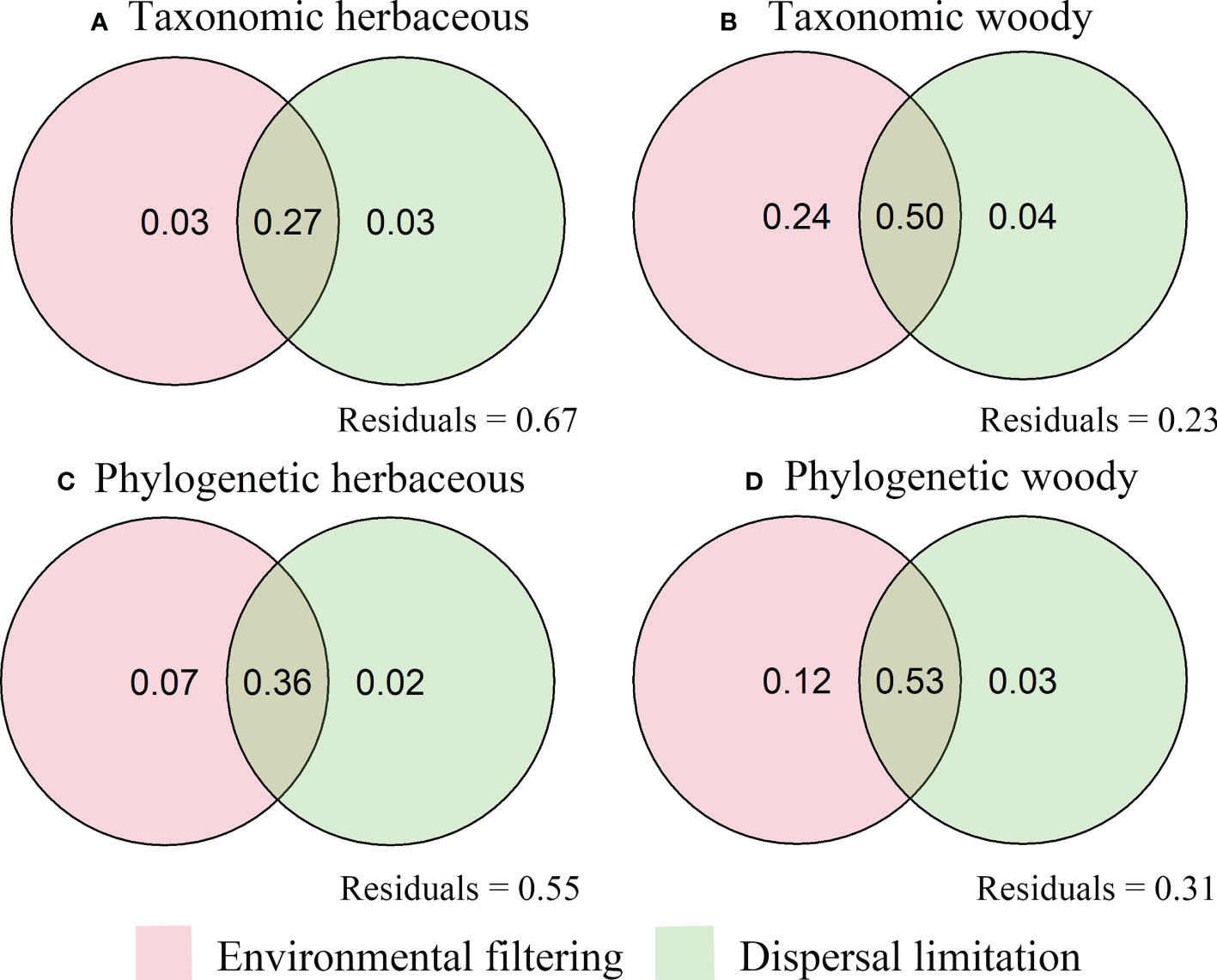
Figure 6 Venn diagram displaying the relative importance of environmental filtering and dispersal filtering on herbaceous (A, C) and woody (B, D) species turnover in the arid and semiarid areas of China.
The species richness of the woody community peaked at mid-elevation areas and declined toward both sides of elevation gradients (Figure 2B), supporting the earlier studies in the Helan Mountains (Zhu et al., 2009). The authors attributed the findings to serious drought (low altitude) and lower temperatures (high altitude) with low species richness, and highlighted that the humped pattern of species richness was explained by the moderate climatic situation in the mid-elevations (Zhu et al., 2009). Meanwhile, this result is consistent with previous research on elevational gradients in other mountain habitats (Zhou et al., 2019), which revealed a unimodal relationship. The low richness at lower altitudes might be explained by anthropogenic disturbances and the extreme competition for resources (Sproull et al., 2015; Zhang et al., 2016). On the contrary, the poorer richness at higher altitudes might be explained by the ecophysiological limitations of the harsh environment (Zhu et al., 2009; Luo et al., 2019), in which their growth was hampered and led to poor biodiversity (Ding et al., 2022; Hu et al., 2022). Another possible reason was that abiotic filtration imposed a limit on the seed settlement or species persistence (Montano-Centellas et al., 2021; Wang et al., 2021). In other words, water scarcity reduces species richness at lower altitudes, and low temperatures limit species richness at higher altitudes. In comparison, the greater number of species in mid-elevation regions might be mainly related to favorable climatic conditions (e.g., water availability, optimal temperature) in these regions. This means that pressures are strong at both ends of the elevational gradient, but environmental conditions are milder at mid-elevations and therefore species richness is higher at mid-elevations (Lopez-Angulo et al., 2018).
Unlike woody richness, herbaceous richness showed an increasing tendency along elevational gradients. A study in a subtropical forest supported the pattern that plant species richness increased with elevation (Zhang et al., 2021). They suggest that both human activities and the elevation range described by the study are two key factors leading to the observed diverse patterns (Macheroum et al., 2021; Zhang et al., 2021; Muhammad et al., 2022). In the Helan Mountains, plant communities at high elevations are predominantly herbaceous, whereas those at low elevations are mainly composed of woody plants (Jiang et al., 2007; Pang et al., 2013). The study reported that environmental conditions were generally more beneficial for alpine plants, which seem to have essential roles in herbaceous plants (Yakimov et al., 2020). When compared to species at lower elevations, alpine meadow plants without woody constraints use different survival strategies in harsh environments, such as investing more resources in reproductive and below-ground components and expanding display areas to attract pollinators (Wang et al., 2021). Therefore, the shorter life cycle of herbaceous plants and the higher potential for seed dispersal and germination strategies that favor survival may be the main contributors to changes in herbaceous richness across elevational gradients in the Helan Mountains. Furthermore, our findings demonstrate the combined effect of biotic and abiotic stresses on herbaceous species distribution and support the inconsistent trends of herbaceous and woody richness along altitudinal gradients. Notably, our results appear contrary to the widely reported view that elevational trends of species richness should decrease in a unimodal or monotonic pattern (Lee and Chun, 2015; Sproull et al., 2015; Zhou et al., 2019). However, when drought is exacerbated at lower elevations, it might reduce species diversity and likely produce unexpected biodiversity patterns (Lopez-Angulo et al., 2018). In addition, given the short lifespan of herbaceous plants, especially annual herbs, we may have overlooked species coexistence patterns associated with them due to the fact that we used only one field survey. Therefore, such limitations should be taken into account when interpreting our results. Huang et al. (2016) emphasize that it is essential to pay more attention to herbaceous plants in biodiversity conservation. So, we suggested that more research be done to discover their general distribution in the dryland mountains.
In addition, this study found that both the SESmpd and SESmntd of herbaceous communities significantly increased linearly along the elevational gradient. Still, there is no clear pattern in the phylogenetic structure of woody communities. This difference can be traced to disparities in the responses of various plant functional groups toward climate change, topographic heterogeneity, soil nutrients, and disturbances along the altitudinal gradient in mountainous areas (Paz et al., 2021). In the Helan Mountains, elevation, local conditions, slope, and aspect were reported to strongly influence spatial patterns of plant biodiversity (Jiang et al., 2007; Pang et al., 2013). Compared to woody species, herbaceous plants were significantly constrained by multi-dimensional variables (e.g., climatic, aspect, and woody cover), indicating that the effects of various environmental factors on different groups of life forms were inconsistent (Loidi et al., 2021). Such variations probably relate to differences in longevity and tolerance to weather (Qian et al., 2014). Large differences in life cycles and tolerance to climatic stress between herbaceous and woody plants may result in inconsistencies in diversity distribution patterns across elevations (Qian et al., 2014). Herbaceous plants are generally able to adjust to novel climate situations two to ten times quicker compared to woody plants due to their shorter reproductive cycles and faster growth rates (Smith and Beaulieu, 2009; Zhou et al., 2019). Moreover, their seed germination, dispersal, and establishment may be tightly controlled by climate and other environmental factors (Wang et al., 2021). In our study, after controlling for other variables, herbaceous richness showed a negative correlation with woody cover, which could be that woody species act as a limiting factor in the species composition of herbaceous communities through the reduction of available resources such as light and soil (Sproull et al., 2015). Therefore, herbaceous and woody assembly processes may be driven by different mechanisms (Luo et al., 2019). We suggest that the herbaceous communities are radically distinct from woody communities concerning the phylogenetic structure (Qian et al., 2014), mainly attributed to abiotic and biotic factors (Gao et al., 2017). In mountainous areas that have been grazed and logged for a long time, different results may be found (Dainese et al., 2015; Wang et al., 2019). Therefore, more field experiments in the mountain region are required to validate the above hypothesis.
Although the taxonomic and phylogenetic patterns of elevation distribution of herbaceous and woody plants were not consistent, our study indicated (Figure 3) that most herbaceous and woody communities showed phylogenetic clustering (both SESmpd and SESmntd < 0) throughout elevation gradients. Another important finding was that the phylogenetic structure of most communities did not differ significantly from that expected from the expected null model. We believe that this phylogenetic pattern might stem from deterministic and stochastic processes. Environmental filters played a big role in the phylogenetic clustering of SESmpd, which had a big impact on the whole lineage (Zobel and Scheiner, 2016). However, the SESmntd might result from the decentralization capability of contemporary species (Volis and Bohrer, 2013). In the Helan Mountains, drought has likely filtered out species that lack drought tolerance mechanisms. Preliminary research has shown that strong environmental filtering procedures are more likely to form community assemblages at high altitudes than competitive exclusion procedures (Chun and Lee, 2017). Other studies support that herbaceous species may be subject to assembly processes (Luo et al., 2019), such as dispersal limitation due to their smaller body and fruit size (Jiang et al., 2015). Moreover, the stochasticity may promote the early assembly of plant communities due to the limitations of seeds (Farjalla et al., 2012). Apparently, niche-based and random processes may play a big role in how plants are put together in the Helan Mountains.
Here we found a comparatively high taxonomic and phylogenetic species turnover of herbaceous and woody communities in the Helan Mountains flora (Figure 5), which is consistent with research in Donglingshan Mountain (Yakimov et al., 2020). Although herbaceous and woody plants exhibit a higher taxonomic and phylogenetic turnover, they correlated poorly with spatial distance after controlling for environmental effects. As previous studies have shown, an environmentally heterogeneous ecosystem generally has a relatively high turnover rate (Coelho et al., 2018). Species turnovers tend to increase with environmental heterogeneity due to amplified variations in community composition and species richness (Ahmad et al., 2020). There is an evident species substitution phenomenon in this highly heterogeneous mountain ecosystem due to regional environmental differences (Bergamin et al., 2017). In addition, turnover is related to elevation and may also be influenced by soil fertility, water resources, meteorology, and light conditions (Dainese et al., 2015; Nascimbene and Spitale, 2017).
This study found that independent contributions of spatial and environmental factors differentially influenced taxonomic and phylogenetic turnovers of herbaceous and woody species (Figure 6). The reason could be that herbaceous and woody plants differ in their evolutionary relationships and dispersion patterns (Liu et al., 2015). Studies show that woody plants are both less plastic in their basic niche and more dispersal-selected than herbaceous plants (Farjalla et al., 2012). Our study area’s topographic structure may have resulted in significant geographic isolation, limiting the migration, dispersal, and assemblage of many of the species distributed in the current forest. Further, canopy structure might more intensely influence understory plant (herbaceous) composition (Luo et al., 2019). Consequently, they probably followed relatively independent pathways in terms of evolution, dispersal filtering, and species interactions. We also found that the joint contribution of spatial and environmental factors had the highest proportion in explaining the turnover of herbaceous and woody plants (Figure 6). This result means that the spatial turnover of plant communities in our study area is mainly shaped by a combination of EF and DF (Chun and Lee, 2017; Grigoropoulou et al., 2022). As emphasized by previous studies, our results support the view that EF and DF jointly control plant community assembly, although the relative contribution of these two processes at different areas and scales is still inconclusive (Shi et al., 2021). It is worth noting that herbaceous and woody species are constantly interacting. Woody species can act as a filter in the structuring of understory plants (herbaceous) by reducing resource availability (Luo et al., 2019), but the sampling design of this study did not proceed according to plant-plant interactions, which would not directly capture the effect of woody plants on herbaceous plants. Nevertheless, integrating the taxonomic and phylogenetic diversity of herbaceous and woody species might provide a novel insight into the flora assemblage in the arid and semi-arid mountains.
As a crucial dimension of biodiversity, beta diversity could offer important evidence to predict ecosystem functioning and improve protected priorities (Martinez-Almoyna et al., 2019; Scriven et al., 2015). Furthermore, knowledge of beta diversity patterns may effectively shape preservation policies (Swenson, 2011; Bergamin et al., 2017). For example, if species turnover is the dominant pattern, more protected areas are needed for biodiversity conservation (Arif et al., 2022b; Hira et al., 2022). However, when nestedness is the prevailing pattern, there is a need to establish a sufficiently large protected area with high species richness (Bergamin et al., 2017). Given that species turnover in the Helan Mountains is the dominant factor in driving the β-diversity pattern (Figure 5), conservations must focus on a vast number of protected areas distributed to provide maximum protection for the life forms of species (Bergamin et al., 2017; Muhammad and Changxiao, 2022). Increasing evidence suggests that phylogenetic diversity is a key to understanding species assemblages and might lead to a fundamental understanding of ecosystem function (Li et al., 2020). Phylogenetic diversity represents the lineage evolution relationships among species and it can help form conservation strategies, it may be more important to conserve a phylogenetically unique species, compared to a more redundant one. Furthermore, our results suggest that both environmental and spatial factors play an important role in shaping plant community assembly, and therefore, it is desirable to combine environmental filtering with dispersal limitation in the practical process of biodiversity conservation in drylands. As a consequence of climate change, the extent of global dryland area is projected to increase particularly in developing countries, which further increases dryland degradation and biodiversity loss (Huang et al., 2015). The global decline in biodiversity has driven calls for ambitious targets for biodiversity conservation and protected areas coverage (Peng et al., 2021). Thus, our findings could have a big impact on the conservation of biodiversity in drylands as the climate warms and causes more drought (Zhu et al., 2020; Jiao et al., 2021).
In summary, this study integrates TD, PD, and environmental drivers to evaluate herbaceous and woody species’ general patterns and assembly mechanisms along elevation gradients in arid and semi-arid regions. Our study demonstrated that the responses of herbaceous and woody plants along elevation gradients in the Helan Mountains are decoupled mostly due to the inconsistent influence of biotics and abiotics on them. The TD and PD of herbaceous communities substantially increase linearly along an elevational gradient, while a nonlinear model was remarkable for woody communities with relatively low richness at the ends of the elevation range and peaked richness at mid-altitudes. In addition, there was no relationship between woody PD and elevation. We found TD and PD were mainly dominated by species turnover with fewer contributions from nestedness. The turnover process is primarily caused by a combination of environmental and spatial variables. We also showed that environmental and dispersal filtering jointly shapes the plant community assemblages along elevational gradients in arid and semi-arid areas. These findings highlight that conservationists and policymakers should focus on the different adaptation strategies of herbaceous and woody plants to the drought that continues to increase with global warming.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Resources, Data curation, Visualization, Writing - original draft, Writing - review, and editing were performed by JZ. Methodology, Software, Validation, Formal analysis, Investigation, Writing - review, and editing was performed by MA. Investigation, Writing - review, and editing were performed by XH and DD. Validation, Writing - review, and editing were performed by SZ. Investigation, Formal analysis, Data curation was performed by XN. Investigation, Resources, Writing - review and editing, Supervision, Project administration, Funding acquisition were performed by CL. All authors contributed to the article and approved the submitted version.
This work was supported by Ningxia Key Research and Development Project (No. 2020BFG03006, 2021BEG02005); Ningxia Natural Science Foundation Project (No. 2020AAC03107); Chongqing Municipality Key Forestry Research Project (No. 2021-9); Chongqing Municipality Housing and Urban Construction Committee (No. Chengkezi 2019-1-4-2); Forestry Extension Project of China Central Finance (No. Yulinketui 2020-2); Science Foundation of College of Life Sciences of Southwest University (No. 20212005406201).
We are grateful to Mr. Zhu Qiang and Mr. Wang Jifei for their technical and field support during data collection. Finally, we want to express our profound gratitude to Mr. Yan Lingbing for his immeasurable support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2022.1041742/full#supplementary-material
Ahmad, M., Uniyal, S. K., Batish, D., Singh, H. P., Jaryan, V., Kohli, R. K. (2020). Patterns of plant communities along vertical gradient in dhauladhar mountains in lesser Himalayas in north-Western India. Sci. Total Environ. 716, 136919. doi: 10.1016/j.scitotenv.2020.136919
Arif, M., Jiajia, L., Dongdong, D., Xinrui, H., Qianwen, G., Fan, Y., et al. (2022a). Effect of topographical features on hydrologically connected riparian landscapes across different land-use patterns in colossal dams and reservoirs. Sci. Total Environ. 851, 158131. doi: 10.1016/j.scitotenv.2022.158131
Arif, M., Jie, Z., Tahir, M., Xin, H., Changxiao, L. (2022b). The impact of stress factors on riparian and drawdown zones degradation around dams and reservoirs. Land Degrad. Dev. 33 (12), 2127–2141. doi: 10.1002/ldr.4310
Barton, K. (2019). MuMIn: Multi-model inference. R package version 1.43.15. Available at: https://cran.r-project.org/package=MuMIn.
Baselga, A. (2010). Partitioning the turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 19, 134–143. doi: 10.1111/j.1466-8238.2009.00490.x
Baselga, A., Orme, C. D. L. (2012). Betapart: An r package for the study of beta diversity. Methods Ecol. Evol. 3, 808–812. doi: 10.1111/j.2041-210X.2012.00224.x
Becker, A., Körner, C., Brun, J.-J., Guisan, A., Tappeiner, U. (2007). Ecological and land use studies along elevational gradients. Mt. Res. Dev. 27, 58–65. doi: 10.1659/0276-4741(2007)27[58:Ealusa]2.0.Co;2
Behzad, H. M., Jiang, Y., Muhammad, A., Wu, C., He, Q., Zhao, H., et al. (2022). Tunneling-induced groundwater depletion limits long-term growth dynamics of forest trees. Sci. Total Environ. 811, 152375. doi: 10.1016/j.scitotenv.2021.152375
Bergamin, R. S., Bastazini, V. A. G., Vélez-Martin, E., Debastiani, V., Zanini, K. J., Müller, S. C. (2017). Linking beta diversity patterns to protected areas: Lessons from the Brazilian Atlantic rainforest. Biodivers. Conserv. 26, 1557–1568. doi: 10.1007/s10531-017-1315-y
Berhanu, A., Woldu, Z., Demissew, S. (2016). Elevation patterns of woody taxa richness in the evergreen afromontane vegetation of Ethiopia. J. Forestry Res. 28, 787–793. doi: 10.1007/s11676-016-0350-y
Boscutti, F., Casolo, V., Beraldo, P., Braidot, E., Zancani, M., Rixen, C. (2018). Shrub growth and plant diversity along an elevation gradient: Evidence of indirect effects of climate on alpine ecosystems. PloS One 13, e0196653. doi: 10.1371/journal.pone.0196653
Cheng, Y., Liu, H., Dong, Z., Duan, K., Wang, H., Han, Y. (2020). East Asian Summer monsoon and topography co-determine the Holocene migration of forest-steppe ecotone in northern China. Glob. Planet Change. 187, 103135. doi: 10.1016/j.gloplacha.2020.103135
Chen, Z., Song, H., Arif, M., Li, C. (2022). Effects of hydrological regime on Taxodium ascendens plant decomposition and nutrient dynamics in the three gorges reservoir riparian zone. Front. Environ. Sci. 10. doi: 10.3389/fenvs.2022.990485
Chun, J. H., Lee, C. B. (2017). Disentangling the local-scale drivers of taxonomic, phylogenetic and functional diversity in woody plant assemblages along elevational gradients in south Korea. PloS One 12, e0185763. doi: 10.1371/journal.pone.0185763
Coelho, M. S., Carneiro, M. A. A., Branco, C. A., Borges, R. A. X., Fernandes, G. W. (2018). Species turnover drives beta-diversity patterns across multiple spatial scales of plant-galling interactions in mountaintop grasslands. PloS One 13, e0195565. doi: 10.1371/journal.pone.0195565
Dai, A. (2010). Drought under global warming: A review. Wires. Clim. Change. 2, 45–65. doi: 10.1002/wcc.81
Dainese, M., Lepš, J., de Bello, F. (2015). Different effects of elevation, habitat fragmentation and grazing management on the functional, phylogenetic and taxonomic structure of mountain grasslands. Perspect. Plant Ecol. 17, 44–53. doi: 10.1016/j.ppees.2014.09.002
Ding, D., Arif, M., Liu, M., Li, J., Hu, X., Geng, Q., et al. (2022). Plant-soil interactions and C:N:P stoichiometric homeostasis of plant organs in riparian plantation. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.979023
Dormann, C. F., Elith, J., Bacher, S., Buchmann, C., Carl, G., Lautenbach, S., et al. (2013). Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 36, 27–46. doi: 10.1111/j.1600-0587.2012.07348.x
Du, Y., Fan, L., Xu, Z., Wen, Z., Cai, T., Qiao, H., et al. (2021). A multi-faceted comparative perspective on elevational beta-diversity: The patterns and their causes. Proc. Biol. Sci. 288, 20210343. doi: 10.1098/rspb.2021.0343
Faith, D. P. (1992). Conservation evaluation and phylogenetic diversity. Biol. Conserv. 61, 1–10. doi: 10.1016/0006-3207(92)91201-3
Farjalla, V. F., Srivastava, D. S., Marino, N. A. C., Azevedo, F. D., Dib, V., Bozelli, R. L., et al. (2012). Ecological determinism increases with organism size. Ecology 93, 1752–1759. doi: 10.2307/23225239
Gao, N., Zhou, J., Zhang, X., Cai, W., Guan, T., Zheng, Y., et al. (2017). Correlation between vegetation and environment at different levels in an arid, mountainous region of China. Ecol. Evol. 7, 5482–5492. doi: 10.1002/ece3.3088
Goslee, S. C., Urban, D. L. (2007). The ecodist package for dissimilarity-based analysis of ecological data. J. Stat. Software 22, 1–19. doi: 10.1080/09298210701859362
Gotelli, N. J., Hart, E., Ellison, A. M. (2015). EcoSimR: Null model analysis for ecological data. R package version 0.1.0.
Grigoropoulou, A., Schmidt-Kloiber, A., Múrria, C., Qiao, H. (2022). Incongruent latitudinal patterns of taxonomic, phylogenetic and functional diversity reveal different drivers of caddisfly community assembly across spatial scales. Glob. Ecol. Biogeogr. 31, 1006–1020. doi: 10.1111/geb.13479
Grytnes, J. A. (2003). Species-richness patterns of vascular plants along seven altitudinal transects in Norway. Ecography 26, 291–300. doi: 10.1034/j.1600-0587.2003.03358.x
Gul, Z., Tang, Z.-H., Arif, M., Ye, Z. (2022). An insight into abiotic stress and influx tolerance mechanisms in plants to cope in saline environments. Biology 11 (4), 1–24. doi: 10.3390/biology11040597
Hijmans, R. J., Cameron, S. E., Parra, J. L., Jones, P. G., Jarvis, A. (2005). Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978. doi: 10.1002/joc.1276
Hira, A., Muhammad, A., Zarif, N., Gul, Z., Liu, X., Cao, Y. (2022). Impacts of stressors on riparian health indicators in the upper and lower indus river basins in Pakistan. Int. J. Environ. Health Res. 19 (20), 13239. doi: 10.3390/ijerph192013239
Huang, J., Huang, J., Liu, C., Zhang, J., Lu, X., Ma, K. (2016). Diversity hotspots and conservation gaps for the Chinese endemic seed flora. Biol. Conserv. 198, 104–112. doi: 10.1016/j.biocon.2016.04.007
Huang, J., Yu, H., Guan, X., Wang, G., Guo, R. (2015). Accelerated dryland expansion under climate change. Nat. Clim. Change. 6, 166–171. doi: 10.1038/nclimate2837
Hu, X., Arif, M., Ding, D., Li, J., He, X., Li, C. (2022). Invasive plants and species richness impact litter decomposition in riparian zones. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.955656
Hubbell, S. P. (2005). Neutral theory in community ecology and the hypothesis of functional equivalence. Funct. Ecol. 19, 166–172. doi: 10.1111/j.0269-8463.2005.00965.x
Jarzyna, M. A., Quintero, I., Jetz, W. (2021). Global functional and phylogenetic structure of avian assemblages across elevation and latitude. Ecol. Lett. 24, 196–207. doi: 10.1111/ele.13631
Jiang, Y., Kang, M., Zhu, Y., Xu, G. (2007). Plant biodiversity patterns on helan mountain, China. Acta Oecol. 32, 125–133. doi: 10.1016/j.actao.2006.12.003
Jiang, Z., Ma, K., Anand, M. (2016). Can the physiological tolerance hypothesis explain herb richness patterns along an elevational gradient? a trait-based analysis. Community Ecol. 17, 17–23. doi: 10.1556/168.2016.17.1.3
Jiang, Z., Ma, K., Anand, M., Zhang, Y. (2015). Interplay of temperature and woody cover shapes herb communities along an elevational gradient in a temperate forest in Beijing, China. Community Ecol. 16, 215–222. doi: 10.1556/168.2015.16.2.9
Jiao, L., Wang, S., Chen, K., Liu, X. (2021). Dynamic response to climate change in the radial growth of Picea schrenkiana in western tien shan, China. J. Forestry Res. 33, 147–157. doi: 10.1007/s11676-021-01336-6
Jin, Y., Qian, H. (2019). V.PhyloMaker: An r package that can generate very large phylogenies for vascular plants. Ecography 42, 1353–1359. doi: 10.1111/ecog.04434
Kembel, S. W., Cowan, P. D., Helmus, M. R., Cornwell, W. K., Morlon, H., Webb, C. O., et al. (2010). Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26, 1463–1464. doi: 10.1093/bioinformatics/btq166
Körner, C., Jetz, W., Paulsen, J., Payne, D., Rudmann-Maurer, K., Spehn, E. M. (2016). A global inventory of mountains for bio-geographical applications. Alp. Bot. 127, 1–15. doi: 10.1007/s00035-016-0182-6
Körner, C., Paulsen, J., Spehn, E. M. (2011). A definition of mountains and their bioclimatic belts for global comparisons of biodiversity data. Alp. Bot. 121, 73–78. doi: 10.1007/s00035-011-0094-4
Lee, C.-B., Chun, J.-H. (2015). Patterns and determinants of plant richness by elevation in a mountain ecosystem in south Korea: area, mid-domain effect, climate and productivity. J. Forestry Res. 26, 905–917. doi: 10.1007/s11676-015-0115-z
Li, D., Olden, J. D., Lockwood, J. L., Record, S., McKinney, M. L., Baiser, B. (2020). Changes in taxonomic and phylogenetic diversity in the anthropocene. Proc. Biol. Sci. 287, 20200777. doi: 10.1098/rspb.2020.0777
Liu, Y. N., Tang, Z. Y., Fang, J. Y. (2015). Contribution of environmental filtering and dispersal limitation to species turnover of temperate deciduous broad-leaved forests in China. Appl. Veg Sci. 18, 34–42. doi: 10.1111/avsc.12101
Li, F., Yan, Y., Zhang, J., Zhang, Q., Niu, J. (2021). Taxonomic, functional, and phylogenetic beta diversity in the inner Mongolia grassland. Glob. Ecol. Conserv. 28, e01634. doi: 10.1016/j.gecco.2021.e01634
Loidi, J., Chytrý, M., Jiménez-Alfaro, B., Alessi, N., Biurrun, I., Marcenò, C., et al. (2021). Life-form diversity across temperate deciduous forests of Western Eurasia: A different story in the understory. J. Biogeogr. 48, 2932–2945. doi: 10.1111/jbi.14254
Lopez-Angulo, J., Pescador, D. S., Sanchez, A. M., Mihoc, M. A. K., Cavieres, L. A., Escudero, A. (2018). Determinants of high mountain plant diversity in the Chilean Andes: From regional to local spatial scales. PloS One 13, e0200216. doi: 10.1371/journal.pone.0200216
Luo, Y. H., Cadotte, M. W., Burgess, K. S., Liu, J., Tan, S. L., Gao, L. M., et al. (2019). Forest community assembly is driven by different strata-dependent mechanisms along an elevational gradient. J. Biogeogr. 46, 2174–2187. doi: 10.1111/jbi.13669
Macheroum, A., Kadik, L., Neffar, S., Chenchouni, H. (2021). Environmental drivers of taxonomic and phylogenetic diversity patterns of plant communities in semi-arid steppe rangelands of north Africa. Ecol. Indic. 132, 108279. doi: 10.1016/j.ecolind.2021.108279
Martinez-Almoyna, C., Thuiller, W., Chalmandrier, L., Ohlmann, M., Foulquier, A., Fox, C., et al. (2019). Multi-trophic β-diversity mediates the effect of environmental gradients on the turnover of multiple ecosystem functions. Funct. Ecol. 33, 2053–2064. doi: 10.1111/1365-2435.13393
Mayor, J. R., Sanders, N. J., Classen, A. T., Bardgett, R. D., Clement, J. C., Wardle, D. A., et al. (2017). Elevation alters ecosystem properties across temperate treelines globally. Nature 542, 91–95. doi: 10.1038/nature21027
Montano-Centellas, F. A., Loiselle, B. A., Tingley, M. W. (2021). Ecological drivers of avian community assembly along a tropical elevation gradient. Ecography 44, 574–588. doi: 10.1111/ecog.05379
Muhammad, A., Behzad, H. M., Tahir, M., Li, C. (2022). The impact of ecotourism on ecosystem functioning along main rivers and tributaries: Implications for management and policy changes. J. Environ. Manage. 320, 1–11. doi: 10.1016/j.jenvman.2022.115849
Muhammad, A., Changxiao, L. (2022). Impacts of environmental literacy on ecological networks in the three gorges reservoir, China. Ecol. Indic. 145, 109571. doi: 10.1016/j.ecolind.2022.109571
Nascimbene, J., Spitale, D. (2017). Patterns of beta-diversity along elevational gradients inform epiphyte conservation in alpine forests under a climate change scenario. Biol. Conserv. 216, 26–32. doi: 10.1016/j.biocon.2017.09.021
Oksanen, J., Blanchet, F. G., Friendly, M., Kindt, R., Legendre, P., Wagner, H. (2020). Vegan: Community ecology package. version 2.5-7. Available at: https://cran.r-project.org/web/packages/vegan/index.html
Pang, Y., Zhang, B. P., Zhao, F., Yao, Y. H., Zhang, S., Qi, W. W. (2013). Omni-directional distribution patterns of montane coniferous forest in the helan mountains of China. J. Mt. Sci. 10, 724–733. doi: 10.1007/s11629-013-2670-0
Paz, A., Brown, J. L., Cordeiro, C. L. O., Aguirre-Santoro, J., Assis, C., Lei, F., et al. (2021). Environmental correlates of taxonomic and phylogenetic diversity in the Atlantic forest. J. Biogeogr. 48, 1377–1391. doi: 10.1111/jbi.14083
Peng, Y., Fu, B., Zhang, L., Yu, X., Fu, C., Li, F., et al. (2020). Global dryland ecosystem programme (G-DEP): Africa consultative meeting report. J. Arid. Land. 12, 538–544. doi: 10.1007/s40333-020-0056-z
Peng, Q., Yang, R., Cao, Y., Wang, F., Hou, S., Locke, H., et al. (2021). One-third of lands face high conflict risk between biodiversity conservation and human activities in China. J. Environ. Manage. 299, 113449. doi: 10.1016/j.jenvman.2021.113449
Pouteau, R., Munoz, F., Birnbaum, P. (2019). Disentangling the processes driving tree community assembly in a tropical biodiversity hotspot (New Caledonia). J. Biogeogr. 46, 796–806. doi: 10.1111/jbi.13535
Qian, H., Hao, Z., Zhang, J. (2014). Phylogenetic structure and phylogenetic diversity of angiosperm assemblages in forests along an elevational gradient in changbaishan, China. J. Plant Ecol. 7, 154–165. doi: 10.1093/jpe/rtt072
Qianwen, G., Arif, M., Zhongxun, Y., Jie, Z., Xinrui, H., Dongdong, D., et al. (2022). Plant species composition and diversity along successional gradients in arid and semi-arid regions of China. For. Ecol. Manage. 524, 120542. doi: 10.1016/j.foreco.2022.120542
Qian, H., Zhang, J., Sandel, B., Jin, Y. (2019). Phylogenetic structure of angiosperm trees in local forest communities along latitudinal and elevational gradients in eastern north America. Ecography 43, 419–430. doi: 10.1111/ecog.04873
Scriven, S. A., Hodgson, J. A., McClean, C. J., Hill, J. K. (2015). Protected areas in Borneo may fail to conserve tropical forest biodiversity under climate change. Biol. Conserv. 184, 414–423. doi: 10.1016/j.biocon.2015.02.018
Shahriari, H., Abrari Vajari, K., Pilehvar, B., Heydari, M. (2019). Diversity and biomass of different functional groups of herbaceous species along an altitudinal gradient in the semi-arid zagros mountain forests of Iran. J. Forestry Res. 31, 1723–1731. doi: 10.1007/s11676-019-00947-4
Shiferaw, W., Bekele, T., Demissew, S. (2018). Anthropogenic effects on floristic composition, diversity and regeneration potential of the debrelibanos monastery forest patch, central Ethiopia. J. Forestry Res. 30, 2151–2161. doi: 10.1007/s11676-018-0782-7
Shi, Y., Su, C., Wang, M., Liu, X., Liang, C., Ma, W., et al. (2020). Modern climate and soil properties explain functional structure better than phylogenetic structure of plant communities in northern China. Front. Ecol. Evol. 8. doi: 10.3389/fevo.2020.531947
Shi, W., Wang, Y. Q., Xiang, W. S., Li, X. K., Cao, K. F. (2021). Environmental filtering and dispersal limitation jointly shaped the taxonomic and phylogenetic beta diversity of natural forests in southern China. Ecol. Evol. 11, 8783–8794. doi: 10.1002/ece3.7711
Slik, J. W. F., Paoli, G., McGuire, K., Amaral, I., Barroso, J., Zweifel, N. (2013). Large Trees drive forest aboveground biomass variation in moist lowland forests across the tropics. Glob. Ecol. Biogeogr. 22, 1261–1271. doi: 10.1111/geb.12092
Smith, S. A., Beaulieu, J. M. (2009). Life history influences rates of climatic niche evolution in flowering plants. Proc. Biol. Sci. 276, 4345–4352. doi: 10.1098/rspb.2009.1176
Smith, S. A., Brown, J. W. (2018). Constructing a broadly inclusive seed plant phylogeny. Am. J. Bot. 105, 302–314. doi: 10.1002/ajb2.1019
Sproull, G. J., Quigley, M. F., Sher, A., Gonzalez, E. (2015). Long-term changes in composition, diversity and distribution patterns in four herbaceous plant communities along an elevational gradient. J. Veg. Sci. 26, 552–563. doi: 10.1111/jvs.12264
Stone, L., Roberts, A. (1990). The checkerboard score and species distributions. Oecologia 85, 74–79. doi: 10.1007/BF00317345
Swenson, N. G. (2011). Phylogenetic beta diversity metrics, trait evolution and inferring the functional beta diversity of communities. PloS One 6, e21264. doi: 10.1371/journal.pone.0021264
Tilman, D. (2004). Niche tradeoffs, neutrality, and community structure: A stochastic theory of resource competition, invasion, and community assembly. P. Natl. Acad. Sci. U.S.A. 101, 10854–10861. doi: 10.1073/pnas.0403458101
Ulrich, W., Piwczyński, M., Maestre, F. T., Gotelli, N. J. (2012). Null model tests for niche conservatism, phylogenetic assortment and habitat filtering. Methods Ecol. Evol. 3, 930–939. doi: 10.1111/j.2041-210X.2012.00217.x
Volis, S., Bohrer, G. (2013). Joint evolution of seed traits along an aridity gradient: Seed size and dormancy are not two substitutable evolutionary traits in temporally heterogeneous environment. New Phytol. 197, 655–667. doi: 10.1111/nph.12024
Wang, X. J., Alvarez, M., Donohue, K., Ge, W. J., Cao, Y. Q., Bu, H. Y., et al. (2021). Elevation filters seed traits and germination strategies in the eastern Tibetan plateau. Ecography 44, 242–254. doi: 10.1111/ecog.04972
Wang, Y., Wang, J., Chen, C., Li, J., Chu, J. (2019). Grazing plays an important role in structuring alpha and beta components of taxonomic, functional, and phylogenetic diversity in semi-arid sandy land of northern China. Glob. Ecol. Conserv. 20, e00790. doi: 10.1016/j.gecco.2019.e00790
Wang, X., Yang, B. (2021). Divergent tree radial growth at alpine coniferous forest ecotone and corresponding responses to climate change in northwestern China. Ecol. Indic. 121, 107052. doi: 10.1016/j.ecolind.2020.107052
Wang, X. Y., Zhong, M. J., Zhang, J., Si, X. F., Yang, S. N., Hu, J. H., et al. (2022). Multidimensional amphibian diversity and community structure along a 2600 m elevational gradient on the eastern margin of the qinghai-Tibetan plateau. Zool Res. 43, 40–51. doi: 10.24272/j.issn.2095-8137.2021.166
Webb, C. O., Ackerly, D. D., Mcpeek, M. A., Donoghue, M. J. (2002). Phylogenies and community ecology. Annu. Rev. Ecol. Syst. 33, 475–505. doi: 10.1146/annurev.ecolsys.33.010802.150448
Yakimov, B. N., Gerasimova, A. S., Zhang, S., Ma, K., Zhang, Y. (2020). Phylogenetic α- and β-diversity elevational gradients reveal consistent patterns of temperate forest community structure. Acta Oecol. 109, 103657. doi: 10.1016/j.actao.2020.103657
Yuancai, Q., Arif, M., Dong, Z., Ting, W., Qin, Y., Bo, P., et al. (2022). The effect of hydrological regimes on the concentrations of nonstructural carbohydrates and organic acids in the roots of Salix matsudana in the three gorges reservoir, China. Ecol. Indic. 142, 109176. doi: 10.1016/j.ecolind.2022.109176
Zhang, W., Huang, D., Wang, R., Liu, J., Du, N. (2016). Altitudinal patterns of species diversity and phylogenetic diversity across temperate mountain forests of northern China. PloS One 11, e0159995. doi: 10.1371/journal.pone.0159995
Zhang, R., Zhang, Z., Shang, K., Zhao, M., Kong, J., Wang, Z. (2021). A taxonomic and phylogenetic perspective on plant community assembly along an elevational gradient in subtropical forests. J. Plant Ecol. 14, 702–716. doi: 10.1093/jpe/rtab026
Zheng, J., Arif, M., Zhang, S., Yuan, Z., Zhang, L., Dong, Z., et al. (2021). The convergence of species composition along the drawdown zone of the three gorges dam reservoir, China: implications for restoration. Environ. Sci. pollut. Res. 28, 42609–42621. doi: 10.1007/s11356-021-13774-0
Zhou, Y., Ochola, A. C., Njogu, A. W., Boru, B. H., Mwachala, G., Wang, Q. (2019). The species richness pattern of vascular plants along a tropical elevational gradient and the test of elevational rapoport's rule depend on different life-forms and phytogeographic affinities. Ecol. Evol. 9, 4495–4503. doi: 10.1002/ece3.5027
Zhu, Y., Jiang, Y., Liu, Q., Kang, M., Spehn, E. M., Körner, C. (2009). Elevational trends of biodiversity and plant traits do not converge–a test in the helan range, NW China. Plant Ecol. 205, 273–283. doi: 10.1007/s11258-009-9616-1
Zhu, H., Yi, X., Li, Y., Duan, Y., Wang, X., Zhang, L. (2020). Limiting climatic factors in shaping the distribution pattern and niche differentiation of prunus dielsiana in subtropical China. J. Forestry Res. 32, 1467–1477. doi: 10.1007/s11676-020-01194-8
Keywords: dryland, community assembly, taxonomic diversity, phylogenetic diversity, Helan Mountain Nature Reserve
Citation: Zheng J, Arif M, He X, Ding D, Zhang S, Ni X and Li C (2022) Plant community assembly is jointly shaped by environmental and dispersal filtering along elevation gradients in a semiarid area, China. Front. Plant Sci. 13:1041742. doi: 10.3389/fpls.2022.1041742
Received: 11 September 2022; Accepted: 14 November 2022;
Published: 25 November 2022.
Edited by:
Yuanrun Zheng, Institute of Botany (CAS), ChinaCopyright © 2022 Zheng, Arif, He, Ding, Zhang, Ni and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Changxiao Li, bGljaGFuZ3hAc3d1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.