
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci. , 28 October 2022
Sec. Plant Pathogen Interactions
Volume 13 - 2022 | https://doi.org/10.3389/fpls.2022.1027004
This article is part of the Research Topic Chemical, Biological, and Biotechnological Control of Parasitic Weeds View all 6 articles
Strigolactones (SLs) are phytohormones that play an essential role in plant–microbe interactions. The instability of SLs makes it challenging to use them for application to agriculture. In this study, we successfully produced a large amount of the 4-deoxyorobanchol (4DO), one of SLs, in the leaves of Nicotiana benthamiana, using a transient expression system to express SL biosynthetic enzymes. Using this system, the yield of 4DO was 2.1 ± 0.3 μg/gFM (fresh mass). Treatment of leaves at 80°C for 16 h killed Agrobacterium and approximately half amount of 4DO was left in the leaves (1.0 μg/gFM (calculated based on the original FM) ± 0.3). Interestingly, incubation of dried leaves at room temperature for 1 month maintained an almost equal amount of 4DO (0.9 ± 0.2 μg/gFM) in the leaves. These results suggest that high accumulation of 4DO with stability for long periods can be achieved in plant leaves.
Strigolactones (SLs) are phytohormones that regulate plant architecture and development and are important signaling molecules in the rhizosphere (Gomez-Roldan et al., 2008; Umehara et al., 2008). SLs induce hyphal branching of arbuscular mycorrhizal fungi (AMF) (Akiyama et al., 2005) and seed germination of noxious root parasitic weeds such as Striga (witchweed), Orobanche (broomrape), and Phelipanche (Zwanenburg and Blanco-Ania, 2018). Hence, many potential applications of SLs have been proposed in agriculture and biomedicine. One of the applications of SLs is parasitic weed management, also known as suicidal germination, to kill Striga and Orobanche. Sphynolactone-7 has been developed as a highly active and specific germination stimulant for Striga seeds (Uraguchi et al., 2018). SLs can also improve nutrient assimilation, resulting in crop enhancement. SL levels are increased by nutrient stresses such as low phosphate, nitrogen, and sulfur conditions (Yoneyama et al., 2012; Shindo et al., 2018). Natural and synthetic SLs recruit beneficial microorganisms and AMF and promote hyphal branching (Akiyama et al., 2005), spore germination, mitochondrial biogenesis, and respiration (Besserer et al., 2006). AMF provide nutrients like phosphate to the host plant. Hence, enhancement of AMF hyphal branching and symbiosis with AMF are important for improving crop yield and for sustainable agriculture. SLs are also involved in legume nodulation processes, thereby playing an important role in nitrogen acquisition. The application of GR24, a synthetic SL, was found to increase nodulation in alfalfa (Soto et al., 2010) and soybean (Rehman et al., 2018). Mutations in the SL biosynthesis pathway reduced nodulation in Lotus japonica (Liu et al., 2013) and soybean (Haq et al., 2017).
Recent investigations have identified more than 30 SLs across the plant kingdom (Bürger and Chory, 2020; Yoneyama, 2020). The starting material of SL is all-trans-β-carotene, which is converted to 9-cis-β-carotene via isomerization of the C9-C10 double bond catalyzed by DWARF 27 (D27). Sequential carotenoid cleavage reactions are catalyzed by carotenoid cleavage dioxygenase 7 (CCD7) and CCD8 (Alder et al., 2012), leading to the production of carlactone (CL), a biosynthetic intermediate of SLs (Figure 1A). The MORE AXILLARY GROWTH1 (MAX1) gene in Arabidopsis encodes cytochrome P450 (CYP) monooxygenase CYP711A, which catalyzes the oxidation of CL at C-19 to produce carlactonoic acid (CLA) (Abe et al., 2014). OsCYP711A2 in rice converts CL to 4-deoxyorobanchol (4DO) via CLA and its ring closure reaction (Zhang et al., 2014; Yoneyama et al., 2018).
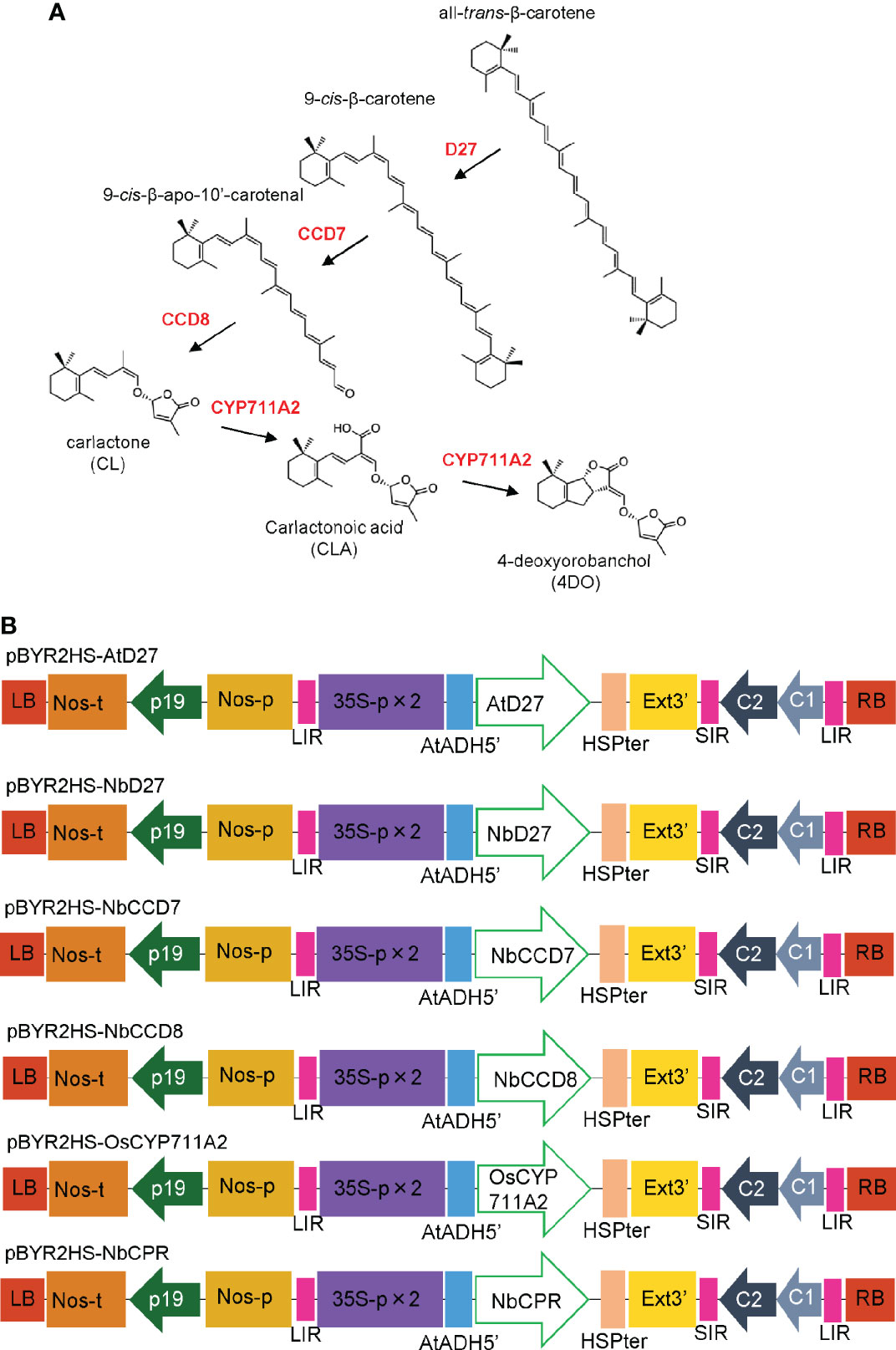
Figure 1 (A) Mechanism of biosynthesis of 4-deoxyorobanchol (4DO) from β-Carotene. β-carotene is converted into carlactone (CL) by D27, CCD7, and CCD8. OsCYP711A2 catalyzes the conversion of CL to 4DO in rice. (B) Schematic representation of the T-DNA regions of the plasmids, pBYR2HS-AtD27, pBYR2HS-NbD27, pBYR2HS-NbCCD7, pBYR2HS-NbCCD8, pBYR2HS-OsCYP711A2, or pBYR2HS-NbCPR. 35S-p x 2, CaMV 35S promoter with the double-enhanced element; AtADH5’, 5’-untranslated region (UTR) of Arabidopsis thaliana alcohol dehydrogenase gene; HSPter, heat shock protein gene terminator; Ext3’, tobacco extensin gene 3’ element; LIR, long intergenic region of the bean yellow dwarf virus (BeYDV) genome; SIR, short intergenic region of the BeYDV genome; C1/C2, BeYDV ORFs C1, and C2 encoding replication initiation protein (Rep) and RepA, respectively; LB and RB, left and right borders of the T-DNA region, respectively; Nos-p and Nos-t, NOS promoter and terminator, respectively; p19, a gene-silencing suppressor gene from the tomato bushy stunt virus.
It is estimated that very low quantities, in the range of 1–10 grams/hectare, of SLs are required for applications in agriculture (Screpanti et al., 2016). However, the chemical synthesis of SLs is an expensive procedure, thereby making them unsuitable for use in agricultural applications. SLs are endogenous rhizosphere signals with high affinity to receptors. Thus, plants produce a limited amount of SLs (pg/g or ng/g root fresh mass [FM]). Extraction of SLs from plants is also laborious and challenging. Furthermore, SLs are highly unstable. Even though the concentration of 5DS remained constant in acetone at 32°C for 21 days, its half-life at neutral pH was only 1.5 days (Akiyama et al., 2010). Moreover, plant cells could produce and release SLs into the culture media in suspension culture. Yet, the rapid degradation of SLs makes it challenging to collect large amounts of the compounds (Vurro et al., 2016). Therefore, no effective methods for producing and collecting large quantities of SLs have been developed to date.
We had previously developed one of the most efficient transient protein expression systems in plant cells, called as the Tsukuba system (Yamamoto et al., 2018; Nosaki et al., 2021a), that allowed the production of approximately 4 mg of GFP/gFM in Nicotiana benthamiana leaves. The vector for this system, pBYR2HS, consists of a geminiviral replication system and a double terminator. Although this system is applicable to several plant species (Hoshikawa et al., 2019; Suzaki et al., 2019), massive expression levels of target proteins have been particularly achieved in N. benthamiana. Transient expression of recombinant proteins such as hemagglutinin (Matsuda et al., 2017), swine hepatitis E ORF2 capsid proteins (Zahmanova et al., 2020), and human cullin (Nosaki et al., 2021b) in N. benthamiana leads to leaf necrosis and/or dehydration. We also discovered that foliar spray of high concentrations of sodium ascorbate (AsA) suppresses the necrosis of N. benthamiana leaves (Nosaki et al., 2021b).
In this study, we produced a large amount of 4DO in the leaves of N. benthamiana by applying foliar spray of high concentration of AsA. We also used our transient protein expression system to express D27, CCD7, CCD8, and OsCYP711A2 in N. benthamiana leaves. The yield of 4DO in the leaves was approximately 2.1 μg/gFM, which was approximately 42,000 times higher than that in the roots of wild-type rice plants (Seto et al., 2014). We then treated the 4DO-expressing leaves at 80°C for 16 h to kill Agrobacterium, followed by incubation of the dried leaves at room temperature for 1 month. The leaves were still able to maintain a certain amount of 4DO (0.9 μg/gFM). Our results suggest that high accumulation of 4DO can be achieved using our transient expression system, thereby allowing long-term storage of 4DO in plant leaves.
N. benthamiana codon-optimized AtD27 and OsCYP711A2 genes were synthesized using the GeneArt Strings DNA Fragments service (Thermo Fisher Scientific). The DNA fragments were inserted into SalI-digested pBYR2HS (Yamamoto et al., 2018) using an In-Fusion HD Cloning kit (Takara Bio). The resulting vectors were named pBYR2HS-AtD27 and pBYR2HS-OsCYP711A2, respectively (Figure 1B).
RT-PCR was performed as previously described to amplify the NbD27, NbCCD7, NbCCD8, and NbCPR genes (Miura et al., 2012). Briefly, total RNA was isolated from N. benthamiana leaves using TRIzol reagent (Thermo Fisher Scientific), according to the manufacturer’s instructions. First-strand cDNA was synthesized using SuperScript III Reverse Transcriptase (Thermo Fisher Scientific) with the primers NbD27-R2, NbCCD7-R2, NbCCD8-R2, and NbCPR-R2 (Supplemental Table S1). Each first-strand cDNA was used as a template, and PCR was performed with the primers pBYR2HS-NbD27-F and -R, pBYR2HS-NbCCD7-F and -R, pBYR2HS-NbCCD8-F and -R, and pBYR2HS-NbCPR-F and -R (Supplementary Table S1), respectively. The PCR product was then inserted into SalI-digested pBYR2HS with an In-Fusion HD Cloning kit (Takara Bio), and the resulting vectors were designated as pBYR2HS-NbD27, pBYR2HS-NbCCD7, pBYR2HS-NbCCD8, and pBYR2HS-NbCPR, respectively (Figure 1B).
Each vector was transformed into Agrobacterium tumefaciens GV3101. Pre-cultured A. tumefaciens harboring each vector was transferred to L-broth media containing 10 mM MES (pH 5.6), 20 μM acetosyringone, 50 mg/L kanamycin, 30 mg/L gentamycin, 30 mg/L rifampicin, and grown at 28°C overnight with shaking at 150 rpm on a rotary shaker (TAITEC Bio-Shaker BR-300LF) till stationary phase was reached. Subsequently, the Agrobacterium was centrifuged and resuspended in the infiltration buffer (Yamada et al., 2020). Each Agrobacterium solution was mixed at the same proportions. A 500 mL of the mixed Agrobacterium suspension was placed in a same volume glass beaker inside a vacuum desiccator. The 6-week-old N. benthamiana plants, grown in the cultivation room at 24°C under a 16-h light/8-h dark photoperiod, were immersed into the suspension and vacuum-infiltrated as described previously (Miura et al., 2020).
At 0, 2, and 4-days post agroinfiltration, 200 mM sodium ascorbate was applied to the leaves of N. benthamiana using a foliar spray (Nosaki et al., 2021b). At 7-day post agroinfiltration, the leaves were washed and immersed in acetone to measure the SL content. Alternatively, agroinfiltrated leaves were kept at 80°C overnight to kill the bacteria. After incubation at 80°C overnight, leaves were left on the bench for one month.
After agroinfiltration, 1 g of fresh agro-infiltrated leaves were cut into 1 cm squares. Five to 10 leaves were cut into 1 cm squares and randomly collected to 1 g in total. These leaves were immersed in 40 mL of acetone at 4°C. Ten ng of D6-4DO were spiked as an internal standard for quantification. The extraction and measurement of 4DO have been described previously (Abe et al., 2014; Yoda et al., 2021).
Leaves of N. benthamiana were ground in 200 μL of sterile water. After centrifugation, 50 μL of the supernatant was incubated in AB medium containing 50 μg/mL kanamycin for 5 days.
SLs are usually present in tiny amounts in plants (Xie et al., 2010), and are expensive to produce via chemical synthesis (Vurro et al., 2016). First, metabolic enzymes for biosynthesis of CL were transiently expressed in N. benthamiana leaves using the Tsukuba system (Figure 1B) (Yamamoto et al., 2018), a highly efficient expression system, to confirm whether the accumulation of plant secondary metabolites was enhanced by overexpression of metabolic enzymes. Because AtD27, AtCCD7, and AtCCD8 have been well-characterized in Arabidopsis and these proteins in N. benthamiana have not been identified, BLAST search was performed with the A. thaliana proteins against the SGN (Sol Genomics Network) database. Sequence alignment shows that NbCCD7 and NbCCD8 have 62% and 66% amino acid identity with AtCCD7 and AtCCD8, respectively. Thus, we assumed that NbCCD7 or NbCCD8 has activity to catalyze conversion of 9-cis-β-Carotene to 9-cis-β-apo-10’-carotenal or 9-cis-β-apo-10’-carotenal to CL, respectively. On the other hand, NbD27 contains only 43% amino acid identity with AtD27. Thus, both AtD27 and NbD27 were cloned into pBYR2HS to confirm whether NbD27 has metabolic activity to convert all-trans-β-carotene to 9-cis-β-carotene.
We cultured Agrobacterium harboring pBYR2HS-AtD27, pBYR2HS-NbD27, pBYR2HS-NbCCD7, or pBYR2HS-NbCCD8 (Figure 1) separately. The Agrobacterium solution was mixed in equal amounts, but either Agrobacterium harboring pBYR2HS-AtD27 or pBYR2HS-NbD27 was used for the experiment. Agrobacterium was resuspended in agroinfiltration buffer and infected with N. benthamiana leaves. Seven days after agroinfiltration, the leaves were collected to measure CL. Transient expression of AtD27, NbCCD7, and NbCCD8 as well as of NbD27, NbCCD7, and NbCCD8 exhibited clear expression peaks, even though no peak was observed in leaves infiltrated with pBYR2HS-EGFP (Figure 2A). Accumulation of CL with transient expression of AtD27, NbCCD7, and NbCCD8 was approximately 2-fold higher than that with transient expression of NbD27, NbCCD7, and NbCCD8 (Figure 2B). These results indicated that the metabolic enzyme expression led to the accumulation of CL in N. benthamiana. We also observed that A. thaliana D27 was better for accumulating CL than N. benthamiana D27.
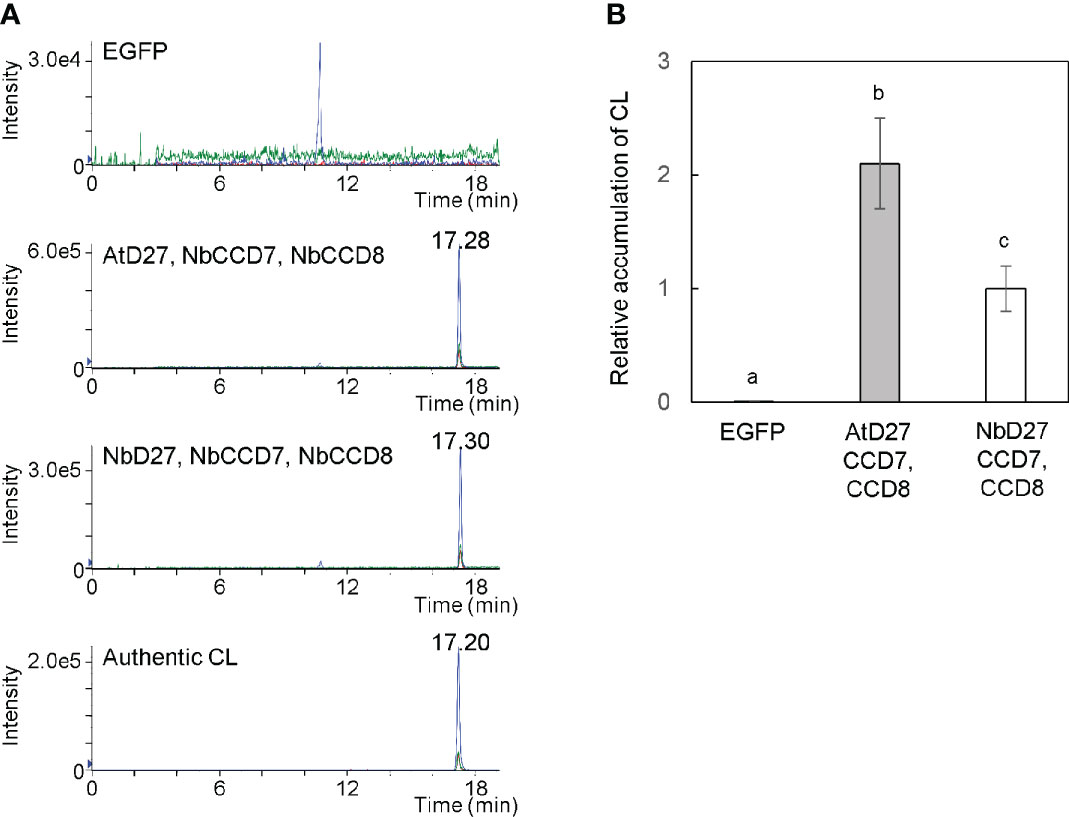
Figure 2 Detection of carlactone (CL) in Nicotiana benthamiana leaves. (A) N. benthamiana leaves were infiltrated with an Agrobacterium mixture containing pBYR2HS-EGFP; pBYR2HS-AtD27, -NbCCD7, and -NbCCD8; or pBYR2HS-NbD27, -NbCCD7, and -NbCCD8. Seven days after infiltration, the leaves were immersed into acetone, and the contents of CL were measured by LC-MS/MS. Multiple reaction monitoring chromatograms of CL (blue, 303.00/97.00; red, 303.00/189.00; green, 303.00/207.00; m/z in positive mode) are shown. (B) The relative accumulation of CL was calculated. The mean of CL accumulation in leaves infiltrated with pBYR2HS-NbD27, -NbCCD7, and -NbCCD8 was set to 1. The means ± SE (n = 3) from a representative of two biological replicates are indicated. The letters on the top of the error bars indicate the statistically significant differences according to Tukey-Kramer’s test (P < 0.05).
CL is an intermediate in SL biosynthesis. Next, we tried to accumulate one of SLs, 4DO. As shown in Figure 2, AtD27 is suitable for the accumulation of CL. OsCYP711A2 is an enzyme that converts CL to 4DO through the CLA intermediate in rice (Figure 1B) (Yoneyama et al., 2018). Moreover, NADPH-P450 reductase (CPR) is required for the activity of cytochrome P450 enzymes in plants (Pompon et al., 1996). For our study, we infiltrated a mixture of Agrobacterium solution containing equal amounts of pBYR2HS-AtD27, -NbCCD7, -NbCCD8, -OsCYP711A2, and -NbCPR into the N. benthamiana leaves and applied 200 mM AsA to leaves using foliar spray, which suppresses necrosis and enhances protein expression (Nosaki et al., 2021b) and increase accumulation of secondary metabolites (Romsuk et al., 2022). The peak corresponding to 4DO was detected when the mixture was infiltrated (Figure 3). The yield of 4DO in N. benthamiana leaves was 2.1 ± 0.3 μg/gFM (Figure 3C). Considering that the accumulation level of 4DO in nature is approximately 50 pg/gFM in roots of wild-type rice and 1.2 ng/gFM in the roots of the rice d14 mutants that defect in SL receptor (Seto et al., 2014), our observation suggested that the accumulation was approximately 42,000 and 1,750 times higher in N. benthamiana, respectively.
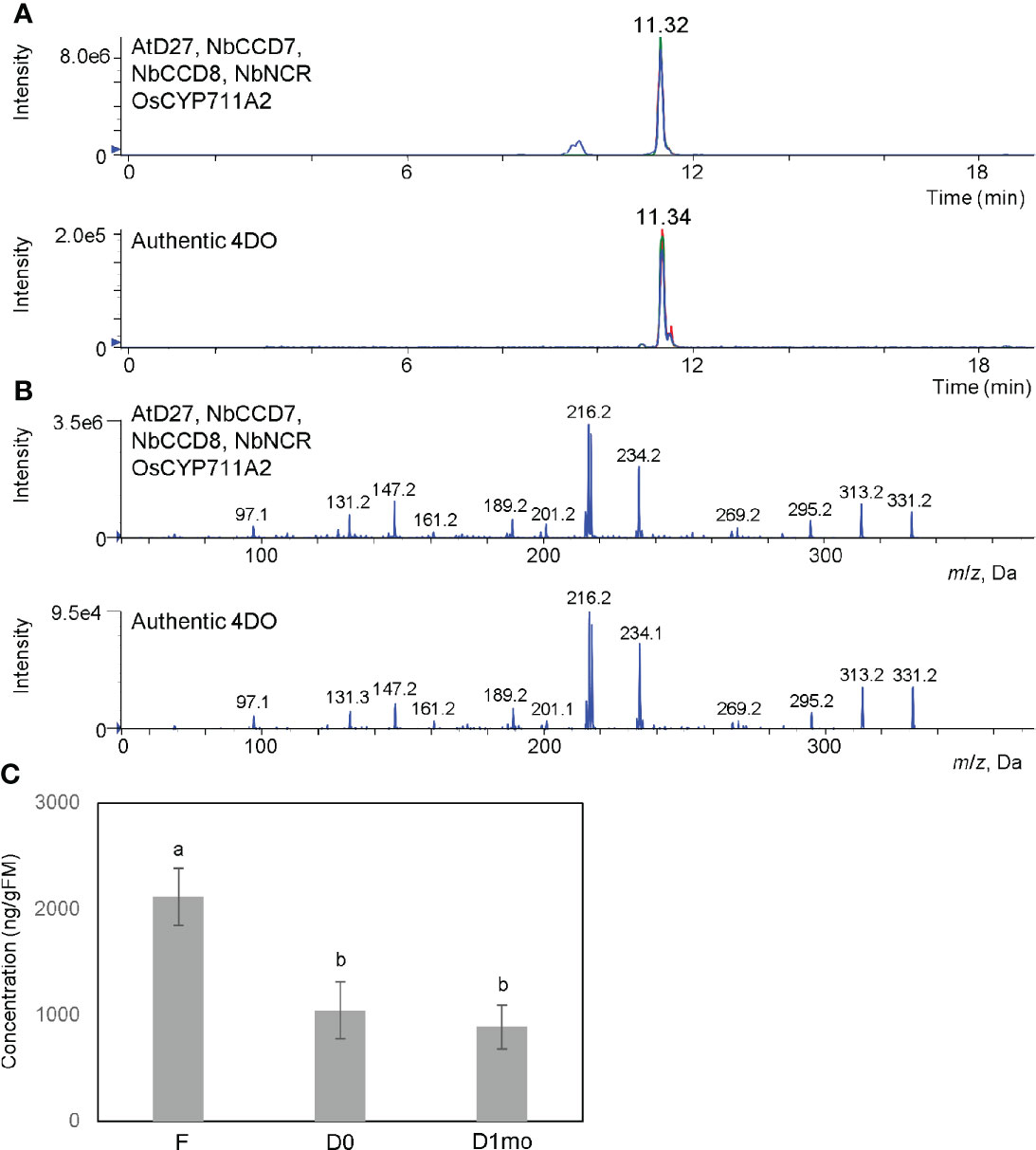
Figure 3 High accumulation of 4DO and suppression of degradation of 4DO in N. benthamiana. (A) Detection of 4DO in N. benthamiana leaves. Leaves were infiltrated with an Agrobacterium mixture containing pBYR2HS-AtD27, -NbCCD7, -NbCCD8, -OsCYP711A2, and -NbNCR. After infiltration, leaves were applied with 200 mM sodium ascorbate by a foliar spray. Seven days after infiltration, leaf extracts were analyzed by LC-MS/MS. Multiple reaction monitoring chromatograms of 4DO (blue, 331.15/97.00; red, 331.15/216.00; green, 331.15/234.00; m/z in positive mode) by LC-MS/MS are shown. (B) Product ion spectra of 4DO in N. benthamiana leaves expressing pBYR2HS-AtD27, -NbCCD7, -NbCCD8, -OsCYP711A2, and -NbNCR. Product ion spectra derived from the precursor ion [M+H]+ (m/z 331) of the peak at the retention time 11.32 min and authentic 4DO are shown. (C) The levels of 4DO production. Seven days after infiltration, one gFM of leaves was immersed into acetone and content of 4DO was measured (F). One gFM of leaves was incubated at 80°C for 16 h (D0). After incubation at 80°C, dried leaves were left at room temperature for 1 month (D1mo). These samples were analyzed by LC-MS/MS and area intensities of 4DO are calculated. Values represent the means ± SE (n = 3) from a representative of three biological replicates. Accumulation of 4DO in N. benthamiana was much higher than in rice d14 mutant (152 ng/gFM), and a high amount of 4DO still remained 1 month after infiltration. The letters on the top of the error bars indicate the statistically significant differences between F and D0 or D1mo according to Tukey-Kramer’s test (P < 0.05). The same letter indicates no significant difference.
The chemically unstable nature of SLs (Yoneyama and Brewer, 2021) make it challenging to apply SLs to the field. Tobacco dust can be used as a soil amendment (Shakeel, 2014; Mahmud et al., 2021). Thus, we hypothesized that if tobacco dust retains SLs for a long time, it would become a valuable amendment. Fresh leaves of N. benthamiana containing high amounts of 4DO were incubated at 80°C for 16 h to kill Agrobacterium and the plants (Figure 4A). The remaining powder contained 4DO (1.0 μg/gFM (calculated based on the original FM) ± 0.3) (Figure 3C), while the transgenic Agrobacterium was killed (Figure 4B). The dried powder, which was left at room temperature for 1 month, maintained a high level of 4DO (0.9 μg/gFM ± 0.2) (Figure 3C). These results collectively indicated that a high level of 4DO was retained in the dried leaves after a one-month incubation at room temperature.
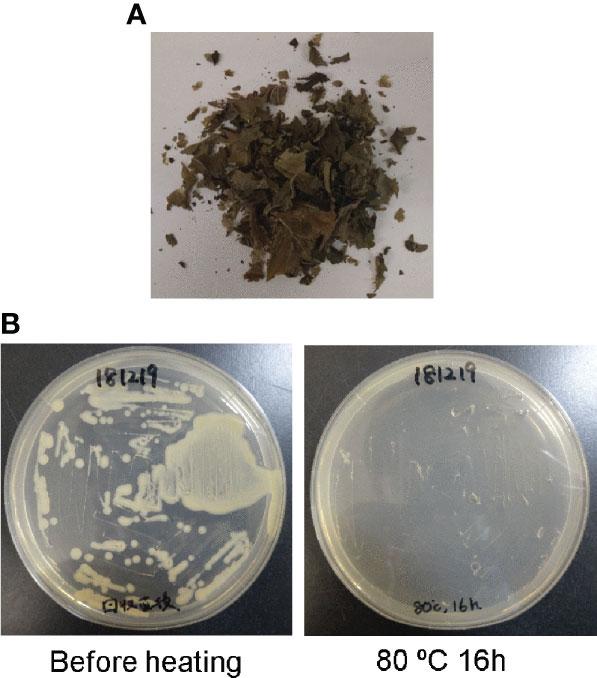
Figure 4 Agrobacterium was killed by heat treatment (80°C, 16 h). (A) After incubation at 80°C, the leaves were completely dried. (B) After agro-infiltration, Agrobacterium was alive in N. benthamiana leaves (before heating). After incubation of leaves at 80°C for 16 h, no bacterium survived in the AB medium with kanamycin.
Biosynthesis of SLs has been held with E. coli-yeast consortia, which led to the synthesis of several SLs like CL, 4DO, and 5DS, and the yield of 4DO and 5DS was 3.5 μg/L and 6.7 μg/L, respectively (Wu et al., 2021). Despite the inaccuracy of direct comparison, the amount of SLs is compared to that in 1 mL of liquid culture that is equivalent to 1 g. In N. benthamiana leaves, 2.1 μg/gFM of 4DO was accumulated (Figure 3), whereas 3.5 ng/mL of 4DO was accumulated in the E. coli-yeast consortia system. In plants, the biosynthesis enzymes D27, CCD7, and CCD8 are localized in the plastids for biosynthesis of CL. Then, CL is exported to the endoplasmic reticulum and oxidized by cytochrome P450s (Lopez-Obando et al., 2015). The E. coli-yeast consortia system used the bacteria instead of the plastids to synthesize CL. Moreover, the higher accumulation of 4DO in N. benthamiana leaves than in E. coli-yeast consortia may lead to an essential role of plastids in CL biosynthesis. Such observations collectively led us to hypothesize that the plant expression system is more efficient in producing phytohormones than the E. coli-yeast consortia system.
According to our results, NbD27 exhibited activity to catalyze conversion of all-trans-β-carotene to 9-cis-β-carotene and had less activity than AtD27 (Figure 2). Phylogenetic tree revealed that NbD27 has more similarity with SlD27 (70% amino acid identity) than AtD27 (Figure S1). D27 proteins of Solanaceae plants, such as tomato, potato, and tobacco, are included in one cluster, which is different from another cluster, in which A. thaliana D27 is included (Figure S1). It is possible that Solanacease D27 proteins has less activity than AtD27.
A bean yellow dwarf virus (BeYDV)-based replicon was used in this study. Co-expression with two plant viruses in a high virus load and stress for the plants. One major advantages of agroinfiltration is different Agrobacterium cultures can be expressed simultaneously. Tobacco mosaic virus (TMV) replication is sometimes combined with PVX (Poteto virus X)-based replication to co-express two proteins. Up to four proteins were simultaneously co-expressed in N. benthamiana, but the total amount of the four proteins, GFP, DsRed, CFP, and YFP, was similar to the amount of GFP (Diamos et al., 2020). According to our results, five enzymes, AtD27, NbCCD7, NbCCD8, OsCYP711A2, and NbCPR, were simultaneously co-expressed, and 4DO was successfully accumulated (Figure 3). Moreover, all-trans-β-carotene was used as a starting material to produce 4DO in this study (Figure 1). It is possible to increase more accumulation of 4DO by increasing level of all-trans-β-carotene accumulation. In plants, several enzymes, including phytoene desaturase, ζ-carotene isomerase, ζ-carotene desaturase, carotene isomerase, and lycopene β-cyclase, are required to synthesize all-trans-β-carotene from phytoene. On the other hand, the bacterial phytoene desaturase CRTI leads to the formation of lycopene, which is one step before β-carotene, directly from phytoene (Schaub et al., 2012). To increase accumulation of all-trans-β-carotene, the bacterial crtI gene is useful because it can be used in plants, as shown in golden rice (Al-Babili et al., 2006).
For application, stability of SLs is required. The rapid degradation of the natural SLs makes using these phytohormones as biostimulants difficult. Because 4DO is a diastereomer of 5DS, whose half-life at neutral pH was 1.5 days (Akiyama et al., 2010), the half-life of 4DO may be similar. 4DO in the dried leaves sustained for 1 month (the amount obtained was 0.9 μg/gFM) at room temperature (Figure 3C). Hence, we deduced that 1 kg of N. benthamiana would approximately yield 1 mg 4DO. It has been shown that 10 nM GR24, a synthetic SL, increased the root colonization of AMF Glomus intraradices, in pea plants grown under low phosphate conditions (Balzergue et al., 2010). Because SLs function at low levels, it is possible that this accumulation of 4DO may enhance the symbiosis of AMF in large fields. Sometimes, artificial synthetic chemicals are accumulated in soil for a very long time to cause negative effect (Bisht and Chauhan, 2020). 4DO is a natural SL, thus, it may not cause negative effect.
Recently, the beneficial effects of plant hormones on human health have been proposed. Anti-inflammatory activity for GR24, one of synthetic SLs, has been detected in RAW_263.7 cells and zebrafish larvae (Zheng et al., 2018). GR24 significantly inhibited the release of the pro-inflammatory mediator nitric oxide (NO) in lipopolysaccharide-stimulated cells. Furthermore, GR24 suppressed lipopolysaccharide-induced neuroinflammation in the SIM-A9 microglial cell line by regulating NF-κB, Nrf2, and PPARγ signaling (Kurt et al., 2020). Treatment with GR24 also led to the downregulation of COX-2, which is responsible for the production of prostaglandins in inflammatory processes (Kurt et al., 2020). There is a clear positive correlation between COX-2 expression and dementia severity in patients affected by dementia, Alzheimer’s disease, and Parkinson’s disease (Wang et al., 2014). The SLs regulating processes in anti-neuroinflammatory and neuroprotection may have potential against neurodegenerative disorders and the early events of Alzheimer’s disease. These results suggest that SLs have potential anti-inflammatory effects. The complications of SL synthesis requires biosynthetic technology using plants to cost-effectively obtain a large amount of SLs.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
TN and KM designed the original concept and project. AYa, SN, AYo, TN, and KM performed experiments and analyzed the data. TN and KM wrote the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by A JSPS Grant-in-Aid (19H04637), Program on Open Innovation Platform with Enterprise, Research Institute and Academia, Japan Science and Technology Agency (JST-OPERA, JPMJOP1851), a Cooperative Research Grant of Plant Transgenic Design Initiative (PTraD) by Tsukuba-Plant Innovation Research Center (T-PIRC), University of Tsukuba.
We thank Ms. Kazuko Ito, Ms. Yuri Nemoto, and Ms. Yuriko Nagai at Tsukuba-Plant Innovation Research Center (T-PIRC), University of Tsukuba, for technical support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2022.1027004/full#supplementary-material
Abe, S., Sado, A., Tanaka, K., Kisugi, T., Asami, K., Ota, S., et al. (2014). Carlactone is converted to carlactonoic acid by MAX1 in Arabidopsis and its methyl ester can directly interact with AtD14 in vitro. Proc. Natl. Acad. Sci. U.S.A. 111, 18084–18089. doi: 10.1073/pnas.1410801111
Akiyama, K., Matsuzaki, K.-I., Hayashi, H. (2005). Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435, 824–827. doi: 10.1038/nature03608
Akiyama, K., Ogasawara, S., Ito, S., Hayashi, H. (2010). Structural requirements of strigolactones for hyphal branching in AM fungi. Plant Cell Physiol. 51, 1104–1117. doi: 10.1093/pcp/pcq058
Al-Babili, S., Hoa, T. T., Schaub, P. (2006). Exploring the potential of the bacterial carotene desaturase CrtI to increase the beta-carotene content in golden rice. J. Exp. Bot. 57, 1007–1014. doi: 10.1093/jxb/erj086
Alder, A., Jamil, M., Marzorati, M., Bruno, M., Vermathen, M., Bigler, P., et al. (2012). The path from β-carotene to carlactone, a strigolactone-like plant hormone. Science 335, 1348–1351. doi: 10.1126/science.1218094
Balzergue, C., Puech-Pagès, V., Bécard, G., Rochange, S. F. (2010). The regulation of arbuscular mycorrhizal symbiosis by phosphate in pea involves early and systemic signalling events. J. Exp. Bot. 62, 1049–1060. doi: 10.1093/jxb/erq335
Besserer, A., Puech-Pagès, V., Kiefer, P., Gomez-Roldan, V., Jauneau, A., Roy, S., et al. (2006). Strigolactones stimulate arbuscular mycorrhizal fungi by activating mitochondria. PLoS Biol. 4, e226. doi: 10.1371/journal.pbio.0040226
Bisht, N., Chauhan, P. S. (2020). “Excessive and disproportionate use of chemicals cause soil contamination and nutritional stress,” in Soil contamination (London, UK: IntechOpen Limited). doi: 10.5772/intechopen.94593
Bürger, M., Chory, J. (2020). The many models of strigolactone signaling. Trends Plant Sci. 25, 395–405. doi: 10.1016/j.tplants.2019.12.009
Diamos, A. G., Hunter, J. G. L., Pardhe, M. D., Rosenthal, S. H., Sun, H., Foster, B. C., et al. (2020). High level production of monoclonal antibodies using an optimized plant expression system. Front. Bioeng. Biotechnol. 7. doi: 10.3389/fbioe.2019.00472
Gomez-Roldan, V., Fermas, S., Brewer, P. B., Puech-Pages, V., Dun, E. A., Pillot, J. P., et al. (2008). Strigolactone inhibition of shoot branching. Nature 455, 189–194. doi: 10.1038/nature07271
Haq, B. U. I., Ahmad, M. Z., Ur Rehman, N., Wang, J., Li, P., Li, D., et al. (2017). Functional characterization of soybean strigolactone biosynthesis and signaling genes in arabidopsis MAX mutants and GmMAX3 in soybean nodulation. BMC Plant Biol. 17, 259. doi: 10.1186/s12870-017-1182-4
Hoshikawa, K., Fujita, S., Renhu, N., Ezura, K., Yamamoto, T., Nonaka, S., et al. (2019). Efficient transient protein expression in tomato cultivars and wild species using agroinfiltration-mediated high expression system. Plant Cell Rep. 38, 75–84. doi: 10.1007/s00299-018-2350-1
Kurt, B., Ozleyen, A., Antika, G., Yilmaz, Y. B., Tumer, T. B. (2020). Multitarget profiling of a strigolactone analogue for early events of alzheimer’s disease: In vitro therapeutic activities against neuroinflammation. ACS Chem. Neurosci. 11, 501–507. doi: 10.1021/acschemneuro.9b00694
Liu, J., Novero, M., Charnikhova, T., Ferrandino, A., Schubert, A., Ruyter-Spira, C., et al. (2013). Carotenoid cleavage dioxygenase 7 modulates plant growth, reproduction, senescence, and determinate nodulation in the model legume lotus japonicus. J. Exp. Bot. 64, 1967–1981. doi: 10.1093/jxb/ert056
Lopez-Obando, M., Ligerot, Y., Bonhomme, S., Boyer, F. D., Rameau, C. (2015). Strigolactone biosynthesis and signaling in plant development. Development 142, 3615–3619. doi: 10.1242/dev.120006
Mahmud, A., Hossain, M. M., Hosasain, M. I., Bayazid, K. N., Islam, M. R. (2021). Tobacco dust combined with fertilizers improves the soil health and profit of rice cultivaation in silty loam soil of bangladish. J. Agr. Food Environ. 2, 45–49. doi: 10.47440/JAFE.2021.2108
Matsuda, R., Abe, T., Fujiuchi, N., Matoba, N., Fujiwara, K. (2017). Effect of temperature post viral vector inoculation on the amount of hemagglutinin transiently expressed in nicotiana benthamiana leaves. J. Biosci. Bioeng. 124, 346–350. doi: 10.1016/j.jbiosc.2017.04.007
Miura, K., Shiba, H., Ohta, M., Kang, S. W., Sato, A., Yuasa, T., et al. (2012). SlICE1 encoding a MYC-type transcription factor controls cold tolerance in tomato, Solanum lycopersicum. Plant Biotechnol. 29, 253–260. doi: 10.5511/plantbiotechnology.12.0303a
Miura, K., Yoshida, H., Nosaki, S., Kaneko, M. K., Kato, Y. (2020). And PMab-2 antibody: a tagging system for detecting and purifying proteins in plant cells. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.510444
Nosaki, S., Hoshikawa, K., Ezura, H., Miura, K. (2021a). Transient protein expression systems in plants and their applications. Plant Biotechnol. 25, 297–304. doi: 10.5511/plantbiotechnology.21.0610a
Nosaki, S., Kaneko, M. K., Tsuruta, F., Yoshida, H., Kato, Y., Miura, K. (2021b). Prevention of necrosis caused by transient expression in nicotiana benthamiana by application of ascorbic acid. Plant Physiol. 182, 832–835. doi: 10.1093/plphys/kiab102
Pompon, D., Louerat, B., Bronine, A., Urban, P. (1996). Yeast expression of animal and plant P450s in optimized redox environments. Methods Enzymol. 272, 51–64. doi: 10.1016/s0076-6879(96)72008-6
Rehman, N. U., Ali, M., Ahmad, M. Z., Liang, G., Zhao, J. (2018). Strigolactones promote rhizobia interaction and increase nodulation in soybean (Glycine max). Microb. Pathog. 114, 420–430. doi: 10.1016/j.micpath.2017.11.049
Romsuk, J., Yasumoto, S., Fukushima, E. O., Miura, K., Muranaka, T., Seki, H. (2022). High-yield bioactive triterpenoid production by heterologous expression in Nicotiana benthamiana using the tsukuba system. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.991909
Schaub, P., Yu, Q., Gemmecker, S., Poussin-Courmontagne, P., Mailliot, J., Mcewen, A. G., et al. (2012). On the structure and function of the phytoene desaturase CRTI from Pantoea ananatis, a membrane-peripheral and FAD-dependent oxidase/isomerase. PLoS One 7, e39550. doi: 10.1371/journal.pone.0039550
Screpanti, C., Fonné-Pfister, R., Lumbroso, A., Rendine, S., Lachia, M., De Mesmaeker, A. (2016). Strigolactone derivatives for potential crop enhancement applications. Bioorg. Med. Chem. Lett. 26, 2392–2400. doi: 10.1016/j.bmcl.2016.03.072
Seto, Y., Sado, A., Asami, K., Hanada, A., Umehara, M., Akiyama, K., et al. (2014). Carlactone is an endogenous biosynthetic precursor for strigolactones. Proc. Natl. Acad. Sci. U.S.A. 111, 1640–1645. doi: 10.1073/pnas.1314805111
Shakeeel, S. (2014). Consideration of tobacco dust as organic amendment for soil: A soil & waste management strategy. Earth Sci. 3, 117–121. doi: 10.11648/j.earth.20140305.11
Shindo, M., Shimomura, K., Yamaguchi, S., Umehara, M. (2018). Upregulation of DWARF27 is associated with increased strigolactone levels under sulfur deficiency in rice. Plant Direct 2, e00050. doi: 10.1002/pld3.50
Soto, M. J., Fernández-Aparicio, M., Castellanos-Morales, V., García-Garrido, J. M., Ocampo, J. A., Delgado, M. J., et al. (2010). First indications for the involvement of strigolactones on nodule formation in alfalfa (Medicago sativa). Soil Biol. Biochem. 42, 383–385. doi: 10.1016/j.soilbio.2009.11.007
Suzaki, T., Tsuda, M., Ezura, H., Day, B., Miura, K. (2019). Agroinfiltration-based efficient transient protein expression in leguminous plants. Plant Biotechnol. 36, 119–123. doi: 10.5511/plantbiotechnology.19.0220b
Umehara, M., Hanada, A., Yoshida, S., Akiyama, K., Arite, T., Takeda-Kamiya, N., et al. (2008). Inhibition of shoot branching by new terpenoid plant hormones. Nature 455, 195–200. doi: 10.1038/nature07272
Uraguchi, D., Kuwata, K., Hijikata, Y., Yamaguchi, R., Imaizumi, H., Am, S., et al. (2018). A femtomolar-range suicide germination stimulant for the parasitic plant Striga hermonthica. Science 362, 1301–1305. doi: 10.1126/science.aau5445
Vurro, M., Prandi, C., Baroccio, F. (2016). Strigolactones: how far is their commercial use for agricultural purposes? Pest Manage. Sci. 72, 2026–2034. doi: 10.1002/ps.4254
Wang, P., Guan, P. P., Wang, T., Yu, X., Guo, J. J., Wang, Z. Y. (2014). Aggravation of alzheimer's disease due to the COX-2-mediated reciprocal regulation of IL-1β and aβ between glial and neuron cells. Aging Cell 13, 605–615. doi: 10.1111/acel.12209
Wu, S., Ma, X., Zhou, A., Valenzuela, A., Zhou, K., Li, Y. (2021). Establishment of strigolactone-producing bacterium-yeast consortium. Sci. Adv. 7, eabh4048–eabh4048. doi: 10.1126/sciadv.abh4048
Xie, X., Yoneyama, K., Yoneyama, K. (2010). The strigolactone story. Annu. Rev. Phytopathol. 48, 93–117. doi: 10.1146/annurev-phyto-073009-114453
Yamada, Y., Kidoguchi, M., Yata, A., Nakamura, T., Yoshida, H., Kato, Y., et al. (2020). High-yield production of the major birch pollen allergen bet v 1 with allergen immunogenicity in Nicotiana benthamiana. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.00344
Yamamoto, T., Hoshikawa, K., Ezura, K., Okazawa, R., Fujita, S., Takaoka, M., et al. (2018). Improvement of the transient expression system for production of recombinant proteins in plants. Sci. Rep. 8, 4755. doi: 10.1038/s41598-018-23024-y
Yoda, A., Mori, N., Akiyama, K., Kikuchi, M., Xie, X., Miura, K., et al. (2021). Strigolactone biosynthesis catalyzed by cytochrome P450 and sulfotransferase in sorghum. New Phytol. 232, 1999–2010. doi: 10.1111/nph.17737
Yoneyama, K. (2020). Recent progress in the chemistry and biochemistry of strigolactones. J. Pestic. Sci. 45, 45–53. doi: 10.1584/jpestics.D19-084
Yoneyama, K., Brewer, P. B. (2021). Strigolactones, how are they synthesized to regulate plant growth and development? Curr. Opin. Plant Biol. 63, 102072. doi: 10.1016/j.pbi.2021.102072
Yoneyama, K., Mori, N., Sato, T., Yoda, A., Xie, X., Okamoto, M., et al. (2018). Conversion of carlactone to carlactonoic acid is a conserved function of MAX1 homologs in strigolactone biosynthesis. New Phytol. 218, 1522–1533. doi: 10.1111/nph.15055
Yoneyama, K., Xie, X., Kim, H. I., Kisugi, T., Nomura, T., Sekimoto, H., et al. (2012). How do nitrogen and phosphorus deficiencies affect strigolactone production and exudation? Planta 235, 1197–1207. doi: 10.1007/s00425-011-1568-8
Zahmanova, G. G., Mazalovska, M., Takova, K. H., Toneva, V. T., Minkov, I. N., Mardanova, E. S., et al. (2020). Rapid high-yield transient expression of swine hepatitis e ORF2 capsid proteins in Nicotiana benthamiana plants and production of chimeric hepatitis e virus-like particles bearing the M2e influenza epitope. Plants 9, 29. doi: 10.3390/plants9010029
Zhang, Y., Van Dijk, A. D., Scaffidi, A., Flematti, G. R., Hofmann, M., Charnikhova, T., et al. (2014). Rice cytochrome P450 MAX1 homologs catalyze distinct steps in strigolactone biosynthesis. Nat. Chem. Biol. 10, 1028–1033. doi: 10.1038/nchembio.1660
Zheng, J.-X., Han, Y.-S., Wang, J.-C., Yang, H., Kong, H., Liu, K.-J., et al. (2018). Strigolactones: a plant phytohormone as novel anti-inflammatory agents. Med. Chem. Commun. 9, 181–188. doi: 10.1039/C7MD00461C
Keywords: transient expression, Nicotiana benthamiana, biosynthesis, strigolactone, 4-deoxyorobanchol, tsukuba system
Citation: Yata A, Nosaki S, Yoda A, Nomura T and Miura K (2022) Production and stably maintenance of strigolactone by transient expression of biosynthetic enzymes in Nicotiana benthamiana. Front. Plant Sci. 13:1027004. doi: 10.3389/fpls.2022.1027004
Received: 24 August 2022; Accepted: 17 October 2022;
Published: 28 October 2022.
Edited by:
Atsushi Okazawa, Osaka Metropolitan University, JapanReviewed by:
Kosuke Fukui, Okayama University of Science, JapanCopyright © 2022 Yata, Nosaki, Yoda, Nomura and Miura. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kenji Miura, bWl1cmEua2VuamkuZ2FAdS50c3VrdWJhLmFjLmpw; Takahito Nomura, dG5vbXVyYUBjYy51dHN1bm9taXlhLXUuYWMuanA=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.