- 1Department of Biochemistry, Indian Institute of Science, Bengaluru, India
- 2Department of Pharmaceutics, Unaizah College of Pharmacy, Qassim University, Unaizah, Saudi Arabia
- 3Smart-Health Initiative (SHI) and Red Sea Research Center (R.S.R.C.), Division of Biological and Environmental Sciences and Engineering (B.E.S.E.), King Abdullah University of Science and Technology (K.A.U.S.T.), Thuwal, Saudi Arabia
- 4Department of Plant Biology, Rutgers University, The State University of New Jersey, New Brunswick, NJ, United States
- 5Division of Microbiology, Indian Council of Agricultural Research (ICAR), New Delhi, India
- 6Centre of Advanced Study in Botany, Banaras Hindu University, Varanasi, India
- 7Campus Law Centre, Faculty of Law, University of Delhi, New Delhi, India
- 8Instituto de Investigaciones Químico Biológicas, Universidad Michoacana de San Nicolás de Hidalgo, Morelia, Mexico
- 9Center Agriculture Food Environment, University of Trento, Trentino, TN, Italy
As endophytes are widely distributed in the plant’s internal compartments and despite having enormous potential as a biocontrol agent against postharvest diseases of fruits, the fruit–endophyte–pathogen interactions have not been studied detail. Therefore, this review aims to briefly discuss the colonization patterns of endophytes and pathogens in the host tissue, the diversity and distribution patterns of endophytes in the carposphere of fruits, and host–endophyte–pathogen interactions and the molecular mechanism of the endophytic microbiome in postharvest disease management in fruits. Postharvest loss management is one of the major concerns of the current century. It is considered a critical challenge to food security for the rising global population. However, to manage the postharvest loss, still, a large population relies on chemical fungicides, which affect food quality and are hazardous to health and the surrounding environment. However, the scientific community has searched for alternatives for the last two decades. In this context, endophytic microorganisms have emerged as an economical, sustainable, and viable option to manage postharvest pathogens with integral colonization properties and eliciting a defense response against pathogens. This review extensively summarizes recent developments in endophytic interactions with harvested fruits and pathogens—the multiple biocontrol traits of endophytes and colonization and diversity patterns of endophytes. In addition, the upscale commercial production of endophytes for postharvest disease treatment is discussed.
Introduction
In the recent era of climate change and the rising global population, food security is one of the most critical issues worldwide. At the same time, postharvest losses of fresh products, including fruits, vegetables, or horticultural crops, accelerate food security challenges. Currently, it has been estimated that approximately 50%–60% of the total agricultural production (Kumar and Kalita, 2017) and 30%–50% of the total fruit production are lost after harvesting due to improper storage, attack of pathogens, or the incidence of diseases (Zhang et al., 2017). However, on the broad industrial scale or even a laboratory scale, various chemical pesticides or fungicides have been broadly employed to prevent postharvest loss caused by phytopathogens or diseases. Nevertheless, the undistributed use of chemical pesticides adversely affects the nutrient constituents, texture, flavor, and quality of the fruits and negatively impacts consumer health. Furthermore, the emergence of resistant pathogen varieties against existing pesticides is a severe problem (Hahn, 2014; Nicolopoulou-Stamati et al., 2016). Therefore, the negative consequences of chemical pesticides on fruit quality, human health, and the environment urgently need the development of a reliable and sustainable approach to replace toxic agrochemicals with suitable microbial antagonists.
Utilizing the endophytic microbiome as a biocontrol agent (BCA) during preharvest or postharvest storage conditions has emerged as a suitable alternative to chemical pesticides in the last few years (Singh et al., 2019; Kumar et al., 2021; Ahmad et al., 2022). Endophytes are the microbes that colonize intercellular/intracellular spaces of plants without causing any apparent sign of infection (Bacon and White, 2016; Pathak et al., 2022). Endophytes are well known for inducing plant growth-promoting traits and ameliorating biotic and abiotic stresses (Glassner et al., 2015). In addition, it synthesizes a plethora of bioactive compounds that enhance the host’s immune response and protect the plant from pathogen attacks or disease incidence (Nair and Padmavathy, 2014; Singh et al., 2017). For practical biocontrol efficacy, the most challenging task is the administration and establishment of microorganisms inside the host plant. An endophytic microbiome is a suitable option in this context due to better colonization and proliferation efficacy (Busby et al., 2016; O’Brien, 2017). Nevertheless, there is still a need to explore the endophytic microbiome for its practical application as microbial antagonistic agents against various phytopathogens or plant diseases during postharvest storage conditions.
Furthermore, the diversity of endophytic microbiome in the fruits, its role in biotic stress amelioration, and an insight into the mechanistic aspects are still under investigation (Aiello et al., 2019; Chaouachi et al., 2021). Therefore, research on the endophytic microbiome and its role in minimizing postharvest loss of horticultural crops, including fruits, needs special attention with an in-depth discussion regarding their prospects and their transition from lab to field or industry. This review summarizes the molecular interaction of plant endophytes, the diversity of endophytic microbiome, the screening of BCAs, and the technological aspect of endophytic microbiome postharvest management. This review also focuses on the literature and discussion on the modes of application, the future aspects, and the hurdles to be overcome for converting endophytes into the success stories of postharvest management of fruits in a sustainable manner.
An overview of microbial endophytes
Plants host diverse communities of microorganisms as epiphytes (on the surface) or endophytes (inside the plant tissue) and share a complex relationship. These host–microbe interactions play significant roles in maintaining the plant normal physiology under biotic and abiotic stress conditions (Khalaf and Raizada, 2018; Verma et al., 2021). The term endophyte was firstly introduced by De Bary (1866) as the fungal species living inside the host tissue. However, Petrini (1991) considered endophytes, of either fungal or bacterial strains, as those that reside in the host tissue or plant for at least some part of their life cycle without causing any disease or apparent sign of infection. With technological advancement or next-generation sequencing (NGS), it has been estimated that each plant species harbors multiple endophytic microbes during its life cycle (Senthilkumar et al., 2011; Verma et al., 2021). The latest NGS revealed that Proteobacteria is the most prominent endophytic bacterial phylum, followed by Actinobacteria, Firmicutes, and Bacteroidetes. In contrast, Glomeromycota is the major fungal phylum followed by Ascomycota and Basidiomycota; however, Pseudomonas, Pantoea, Acinetobacter, and Enterobacter members of Gamma-Proteobacteria are the commonly found bacterial genera. Arbuscular mycorrhizal fungi (AMF) are the most prominent fungal taxa among endophytic fungi in plant tissues (Hardoim et al., 2015; Kumar et al., 2020; Verma et al., 2021).
The endophytic microbes within plant tissue interact with plants and modulate the plant’s growth, fitness, and physiology. The mutualistic endophytes live inside the host and mutually benefit each other; for example, endophytes produce phytohormones, solubilize nutrients, and modulate bioactive compounds of the host, all resulting in the growth and development of the plant, and in return, the plant provides shelter and nutrients to the endophytes (Papik et al., 2020; Khalaf and Raizada, 2020).
Colonization by microbial endophytes
The host–endophyte share a complex relationship that is driven by various intrinsic and extrinsic factors (White et al., 2019; White et al., 2021). However, the entry or establishment of microorganisms in the host tissue is the primary step for any strain to be an endophyte (White et al., 2019; Micci et al., 2022). According to Kandel et al. (2017), endophytic colonization refers to the entry, growth, and multiplication of endophytes within the internal compartments of the plant host. However, colonization is a complex process regulated by different signaling molecules in several consecutive steps (Kumar et al., 2020). Firstly, the plant species attract the microbes by the specific components of their exudates, which are generally composed of sugars, organic acids, amino acids, lipopolysaccharides (LPSs), flavonoids, and proteins and may be specific for each microbial strain (White et al., 2019). The microbes showed a chemotactic response toward the specific components of the exudates and facilitated effective colonization (Oku et al., 2012). The motility of the microbial strain/s toward the host surface is facilitated by appendages that protrude from the cell surface, such as flagella, or through type IV pili (Knights et al., 2021). Several reports reinforce the importance of lateral appendages during this movement (Sauer and Camper, 2001; Zheng et al., 2015). For instance, flagella were reported to have direct involvement in adhering to Azospirillum brasilense with wheat roots (Pinski et al., 2019). Böhm et al. (2007) reported type IV pili and their direct role in the colonization of Azoarcus sp. BH72 to the surface and root interior of rice. However, attachment of the endophyte on the host surface is facilitated through secretory products such as exopolysaccharides (EPSs), LPSs, cell surface saccharides, and cellulase of the microbial strain. For example, Meneses et al. (2011) reported that the inactivation of gene gumD, which is responsible for EPS synthesis, decreased the colonization rate of the endophytic strain Gluconacetobacter diazotrophicus in rice roots.
Similarly, Monteiro et al. (2012) observed that inactivation of gene wssD, bcsZ, which are responsible for the synthesis of beta-1,4, glucanase (cellulose), decreased the colonization rate of Herbaspirillum rubrisubalbicans M1 in Zea mays. The endophytic microorganism, before its entry or colonization, confronts the challenges of oxidative environments of the host tissue. This situation is similar to the one the pathogens face during infection of the host. The host plant provides a barrier to oxidative burst, resulting in only a few microorganisms that can enter plant cells (White et al., 2019; White et al., 2021). Experiments have shown that this initial oxidative burst can be reduced by treating seedlings with low concentrations of humic substances, resulting in increased entry of bacteria into root cells at root tips (White et al., 2021). To be an endophyte, microbial strains must be able to survive in the oxidative environment within plant cells (Di Pietro and Talbot, 2017; White et al., 2019). In this context, several authors reported the successful acclimation potential of endophytic strains; for example, Enterobacter spp. encodes antioxidant enzymes during the colonization of poplar plants (Balsanelli et al., 2016).
Additionally, Malfanova et al. (2013) reported genes responsible for antioxidative enzymes used by Klebsiella to protect the host plant from reactive oxygen species (ROS). Similarly, strain G. diazotrophicus showed the expression of antioxidant enzyme genes during the early stage of colonization in rice plants (Meneses et al., 2017). In addition, the colonization efficacy of the endophyte depends upon several factors; host genotype, nutrient status, and specificity of microbial strain are the prime factors (Hardoim et al., 2015).
Colonization patterns of endophytes and pathogens in the host tissue
The colonization patterns of the pathogens and endophytes are similar to some extent. However, the response of plant defense systems differs and depends upon the nature of the microorganisms. Similarly, the expression patterns against oxidative stress are also different. Chen et al. (2020a) reported the colonization patterns of endophytic strain Azoarcus olearius and the pathogen Xanthomonas oryzae in rice plants and observed differential expression patterns of genes. The pathogen followed the salicylate pathway; however, the Azoarcus used the jasmonate signaling pathway during colonization. The colonization patterns of symbiotic endophytes and pathogenic strains are also dissimilar regarding secretions. Pathogenic strains secrete comparatively higher amounts of cell wall-degrading enzymes at the infection sites. In contrast, a lower amount of cell wall-degrading enzymes was reported during endophyte colonization, which could not elicit the plant immune system and make easy access to endophytes inside the host tissue (Elbeltagy et al., 2000; Reinhold-Hurek et al., 2006; Naveed et al., 2014). The overview of endophytic dynamics, entry, colonization, transmission, and interacted factors is presented in Figure 1.
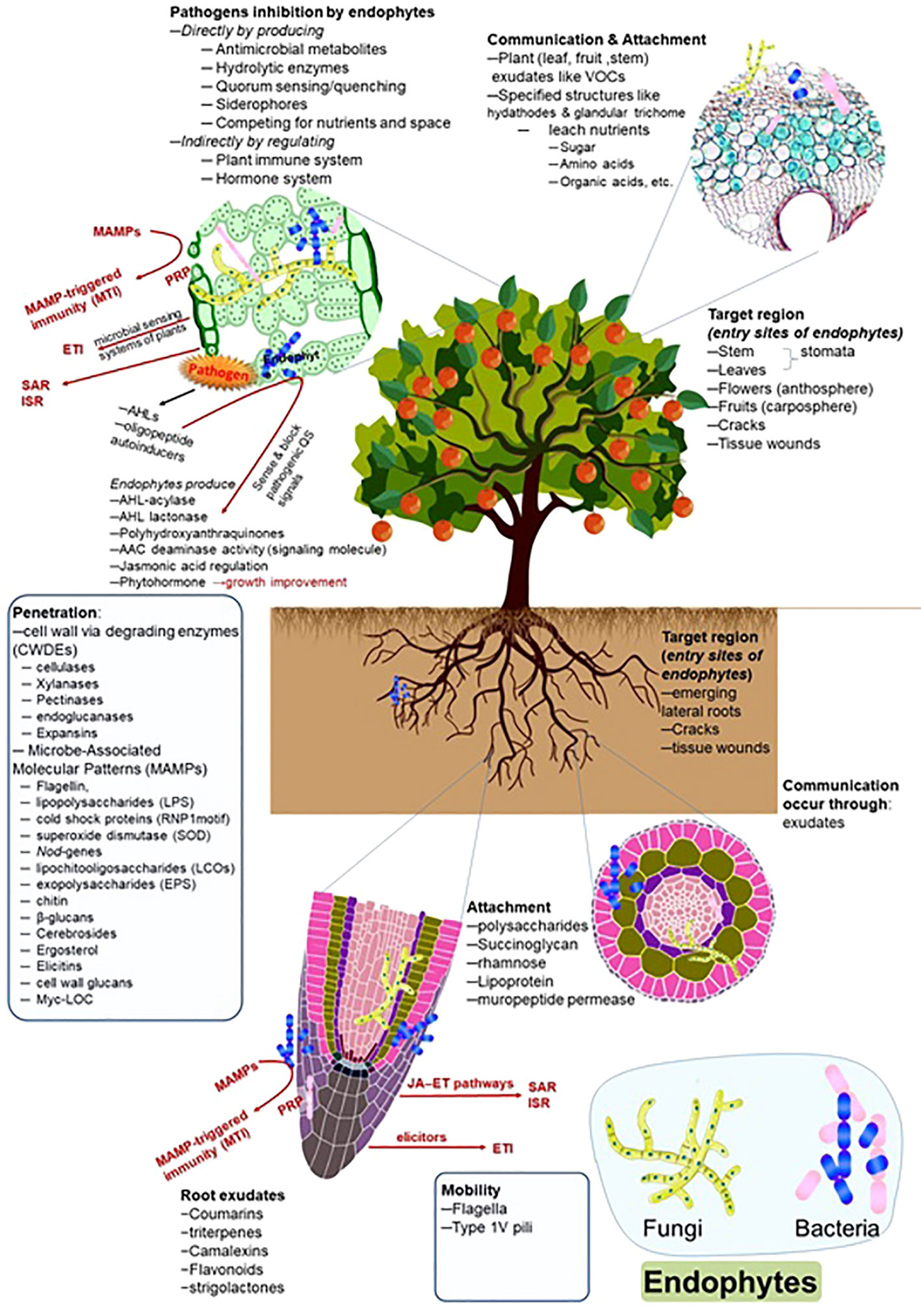
Figure 1 Endophytes and their interaction with the host plants. The figure describes the detailed role and approach of root exudates, communication, mobility, attachment, penetration, and target region (entry site) during endophyte colonization.
Diversity of endophytic microbiota in the fruit
The physiology and biochemistry of the plant depend upon the surrounding biotic and abiotic factors, which ultimately affect the diversity and composition of the microbiota, either epiphytes or endophytes. For instance, seasonal variations affect the number of plant exudates, which are a determining factor in rhizospheric microbial population and endophytic colonization (Wang et al., 2009; Kuffner et al., 2012). The genotype (Mocali et al., 2003), cultivars (Pettersson and Bååth, 2003), and host plant’s age influence endophytic microbial compositions.
Recently published reports reinforce the variation in the endophytic populations among the plant organs. For example, Ren et al. (2019a) reported variations in the endophytic bacterial microbiome among the different organs of the same Jingbai pear (Pyrus ussuriensi Maxim.) plant. Maximum richness and diversity were observed in the root tissue, followed by flower, stem, and fruit, and the lowest were in the leaf tissue. This report illustrates that each plant organ has a specific richness or diversity.
Furthermore, in another study, Ren et al. (2019b) reported variations in fungal richness or diversity in the different plant organs of the Jingbai pear forest. They observed that the root tissue had maximum fungal richness and diversity, followed by stem, fruit, and leaf, and the lowest were observed in the flower tissue. Thus, the diversity patterns of both bacteria and fungi are different in the same plants. Finally, Dong et al. (2019) reported a similar observation of bacterial distribution patterns among the root zone, rhizosphere, phyllosphere, and endosphere of roots, stems, leaves, fruits, and seeds of tomatoes under greenhouse conditions. They observed that the root zone and rhizospheric soil had the highest diversity and richness, followed by stem, flowers, and fruits; however, the lowest diversity and richness were observed in the phyllosphere tissue.
Abdelfattah et al. (2015) also reported that leaves contain higher diversity than flowers or olive fruits (Olea europaea), and the fungal diversity consequentially decreased from fruitlets to mature stages of the olive. However, the trends of the fungal community were very similar from fruitlets to the flowering stage, which later changed. However, the microbial diversity in the flower or fruit section is similar to the diversity of some other parts. Therefore, the uniqueness and diversity of endophytic microbiota may vary among the different compartments of the fruits (Ottesen et al., 2013). The uniqueness may be due to the ovaries, which turn into flesh and create a new environment that harbors specific microbiota or microbial strains (Tadych et al., 2012; Aleklett et al., 2014).
Host–endophyte interaction in terms of biocontrol agents
It is well known that during plant–microbe interactions, microbial strains showed neutral, commensalism, mutualistic, or pathogenic interaction with the host plants. The establishment depends upon several factors, including the genotype of microorganisms or host plants and the surrounding environment (Brader et al., 2017). Plants rely on their sophisticated defense systems to counteract attacks of phytopathogens (Jones and Dangl, 2006), as the pathogenic strains secrete numerous biomolecules inside the host during infection. The host plant responds accordingly after recognizing conserved structure and elicits its immune behavior as the first line of defense to control the pathogen by the present pattern recognition receptors (PRRs). The PRRs sense the nature of microbes through the perception of microbe-associated molecular patterns (MAMPs) or pathogen-associated molecular patterns (PAMPs) (Plett and Martin, 2018). Bacterial flagellin, elongation factor Tu (EF-Tu), fungal chitin, and yeast mannans are the most commonly reported PAMPs/MAMPs (Newman et al., 2013).
During co-evolution with the host plant, pathogenic strains improved the strategies to suppress the MAMP/PAMP-triggered immunity. In response, the host plant developed a second line of defense known as effector-triggered immunity. The plant system develops receptors that sense or recognize the pathogen’s constituents. For instance, for the pathogenic microbes (biotrophic) that depend upon the nutrient uptake of living cells, a hypersensitive response may be activated, which leads to the programmed cell death of plants under attack (de Wit, 2007). However, this response must be suppressed in the case of necrotrophic pathogens or endophytes or symbiotic microorganisms (Liu et al., 2017). However, to cope with the plant immune system, the endophytic microorganisms produce their MAMPs, which do not significantly elicit the host immune or defense system. However, there is significant variation between the cell surface components (flagellin proteins in the endophytic microbes) of endophytic/symbiotic or pathogenic microbial strains (Trdá et al., 2014), which show differential patterns at the time of recognition by the receptors (Figure 2).
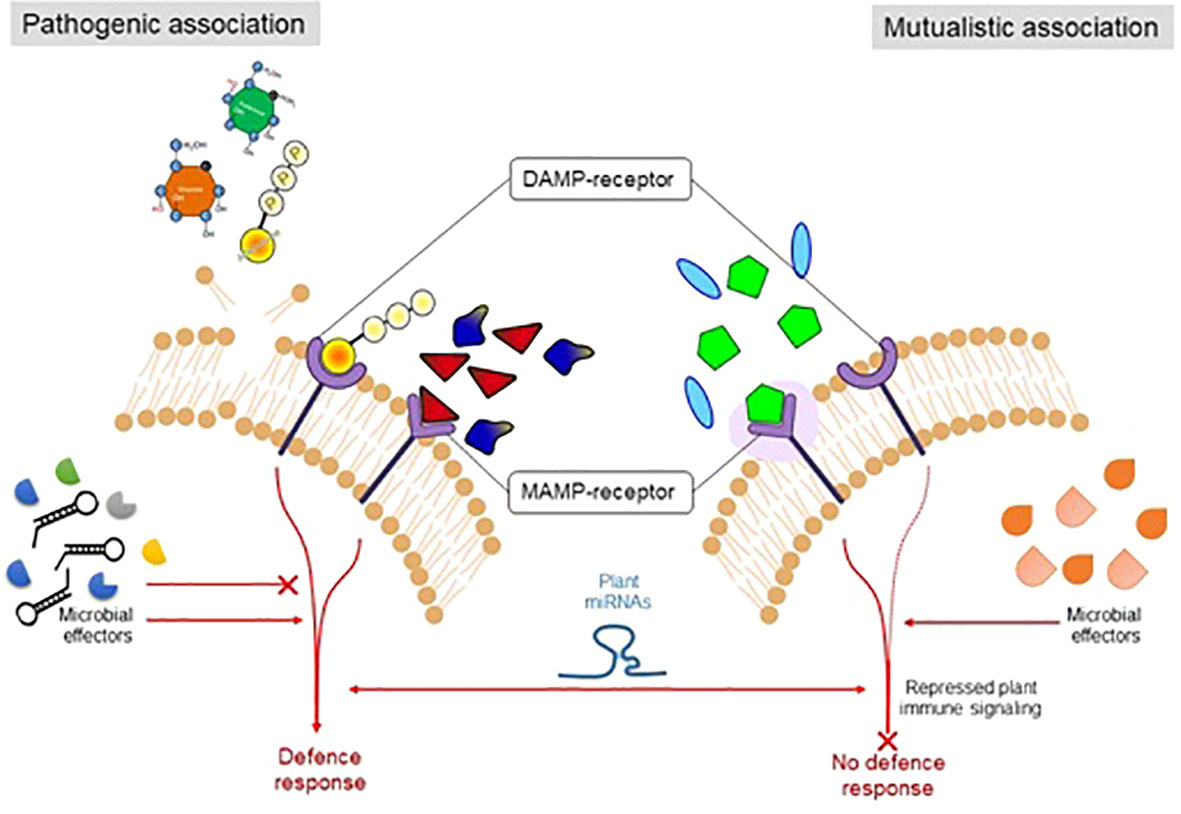
Figure 2 The figure illustrates the mechanism by which plants sense to differentiate symbiotic and pathogenic microorganisms.
Endophytes as biocontrol agents
To explore endophytes as biological control agents, several factors have been considered relevant, including survival, stability, storage, application, and marketability. Despite the massive exploration of various microbial strains as BCAs in vivo or in vitro, only a limited number of strain/s, bacteria, fungi, or yeast, have been commercialized, and the possible reason is the survivability or stability of BCAs. The endospore formation of Bacillus subtilis or chlamydospore structure of Trichoderma makes them most suitable compared to other microbial strains because of stability or survivability under unfavorable conditions to fulfill the requirement of commercial exploitation. However, the endophytic microbiome can easily be administered, penetrating and colonizing the host tissue, unlike other microorganisms where colonization is a complex process. However, the effectiveness of BCAs against the pathogen may also depend upon various factors, including the growth or physiological state of the plant, genotype, colonization pattern, population dynamics, and the surrounding environmental conditions (Card et al., 2016; Bolívar-Anillo et al., 2020).
Recent studies have reported the antagonistic activities of a diverse range of endophytes, which is present on the fruit surface. A number of bacterial, actinomycetes, and fungal species are present on the fruit surface that can impact the growth of postharvest pathogens (Huang et al., 2021). Similar to field conditions, Pseudomonas, Citrobacter, Paenibacillus, Burkholderia, and Bacillus sp. are some of the most prevalent biocontrol bacteria found on fruit surfaces (Shi et al., 2013; Huang et al., 2021). The use of endophytic yeast Metschnikowia pulcherrima along with chitosan prevented the growth of Alternaria alternata in table grapes (Stocco et al., 2019). Aureobasidium pullulans prevented the growth of Botrytis cinerea and Monilinia laxa in sweet cherries and table grapes, decreasing the decomposition rate of fruits between 10% and 100% (Schena et al., 2003). Pantoea dispersa controlled the black rot of sweet potato by exhibiting antibiosis (Jiang et al., 2019). Trichoderma and Nodulisporium are some of the most found fungal BCAs on the carposphere. Recently, mycofumigation with the fungal volatile organic compounds (VOCs) has also gained attention to inhibit the growth of postharvest pathogens (Zhi-Lin et al., 2012). Suwannarach et al. (2013) reported on biofumigation with the Nodulisporium spp. CMU-UPE34, an endophytic fungus, to prevent the postharvest decay of citrus fruits. The endophytic fungal stain Nodulisporium sp. strain GS4d2II1 produced six different VOCs, which inhibited Fusarium oxysporum growth in cherry tomato fruits after their harvest (Medina-Romero et al., 2017). Details of endophytic microbial strains and their utilization in postharvest disease or pathogen control of fruits have been discussed in Table 1.
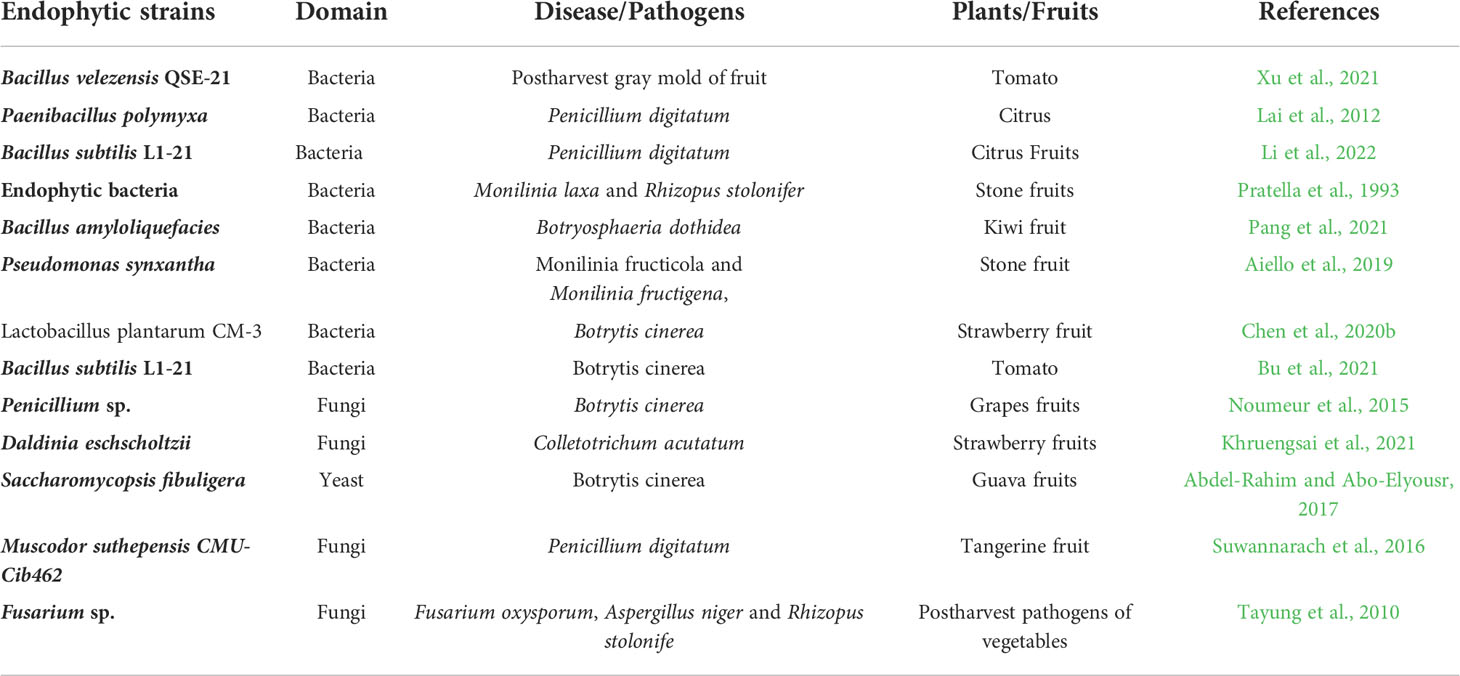
Table 1 Endophytic microbial strains used for the postharvest disease or pathogen management in fruits.
Screening of endophytic biocontrol agents
The search for endophyte agents with biocontrol capacities is imperative in detecting those agents with excellent antagonistic capacities against potential pathogens. Detecting these characteristics depends on having better chances of generating microbial endophyte-based biocontrols with good chances of being successful in open field application and not just showing good actions in the laboratory. Next, we detail some tools for detecting and selecting endophytic BCAs. Screening microbial antagonists against various phytopathogens is one of the most crucial steps. The BCAs are generally screened on the basis of some specific characteristics such as parasitism, in which BCAs live together with the host plant, resulting in antagonistic effects (Mukherjee et al., 2012). Furthermore, strains having the capability to synthesize antimicrobial or volatiles compounds and enzymes such as pectinases and cutinases, which can interfere with pathogenicity factors or reduce the virulence of pathogens, are preferred for BCA screening (Zimand et al., 1996; Kapat et al., 1998).
However, other direct or indirect mechanisms have been employed to screen suitable BCAs for particular or broad-scale phytopathogens causing plant diseases. Dual-culture assay is one of the standard phenotype-based direct screening methods for identifying microbial antagonists during in vitro identification. In this assay, BCAs and pathogens were cocultivated on semisolid media. The pathogen’s antagonistic behavior toward BCAs and pathogenicity are evaluated by measuring the lesion diameter (Shi et al., 2014). During the evaluation, both the BCAs and the pathogen were grown together on the plates at different locations, and a significant decrease in mycelium growth and fungal spores was observed (Comby et al., 2017). In another case, the pathogen has been evenly spread over the plate, and BCA was spotted over the medium. The clear zone around the spotted BCA was measured to evaluate biocontrol activity. The larger the clear zone, the higher the biocontrol potential (Shehata et al., 2016).
Synthesis of antimicrobial compunds, either diffusible or volatile, by the microbial endophytic strain is also one of the parameters for biocontrol screening. During in vitro volatile analysis, the BCA and the pathogen grow on an agar base plate, which is grown under physically separated conditions and sealed with parafilm or tape to avoid VOC escape (Stinson et al., 2003). However, screening of BCAs in liquid media has also been done under which both the BCAs and pathogen were grown either simultaneously or consecutively, and their impact has been evaluated either by measuring the optical density or by the microscopic evaluation of pathogen spore or germination tube of mycelia tube (Omar and Abd-Alla, 1998).
However, in vivo screening is the standard method for evaluating potential BCAs under natural or greenhouse conditions through several parameters such as measuring lesion diameter, disease severity, or defined disease index (Lecomte et al., 2016). In vivo screening not only is based on antagonistic activity but also includes the physiological status of the plant by measuring water status (e.g., transpiration, stomatal conductance), variation in antioxidant activity (e.g., enzymatic activity levels), production of plant defense molecules (e.g., phytoalexins), morphological growth parameters such as plant height, the dry or fresh weight of certain plant parts, or the flowering date (Lecomte et al., 2016). The antagonistic potential of the BCAs varies with plant genotype or species; differences in host genotypes differentially regulate the physiological functions that may modulate the rate of infections and response of host immune systems. Similarly, the colonization potential of the endophytes, which depends upon the various physiochemical nature of plant exudates, also impacts the biocontrol potential against the pathogen more efficiently and effectively (Martin et al., 2015).
Postharvest factors that affect the quality of food and disease incidence
Postharvest diseases can result from incorrect postharvest practices and faulty preharvest management. The significant postharvest factors that affect the storage of food are as follows.
Fruit storage conditions
Fruits are generally transported to supermarkets and cold chains before reaching customers’ hands. Temperature, pH, and humidity conditions in cold chains significantly affect the growth of pathogens and endophytes (Carmona-Hernandez et al., 2019). Low pH due to fruit metabolism and high humidity support the growth of fungal pathogens (Arah et al., 2015). In addition, temperature and pH conditions also influence the production of volatile secondary metabolites (VOCs) from the microbes (Lazazzara et al., 2017; Fadiji and Babalola, 2020). In a study, a lower pH condition of the fermentation medium significantly influenced the production of phloroglucinol and gallic acid from isolated endophytic fungus Colletotrichum gloeosporioides (Gasong and Tjandrawinata, 2016).
Physical handling and gaseous treatments
The rough handling of already ripened fruits invites the attack of pathogens on soft and brushed surfaces. In addition, mechanical injuries to the fruits due to improper handling can increase the metabolism and ethylene production, which can cause adverse biotic stresses on the stored fruits (Miller, 2003). The stored fruit’s carbon monoxide (CO) treatment increases ripening and decreases pathogen infestation. The Alternaria rot in jujube fruits was effectively controlled by CO application in fruit storage conditions (Zhang et al., 2020). High carbon dioxide concentration around fruits also reduced the respiratory activities and consumption of soluble solids, which results in a reduction in pathogen infection (Huyskens-Keil and Herppich 2013). Apart from the growth of pathogens, physical handling and food storage conditions can also play a significant role in the growth and secondary metabolite production of endophytes.
Postharvest management strategies by endophytes: Action mechanisms
Endophytes are known to show a myriad of mechanisms against pathogens ranging from direct competition to change in the molecular architecture of the host plants. Endophytes against postharvest pathogens, being a relatively new field, require an in-depth literature review to understand the possible mechanisms employed against postharvest pathogens. Following are the possible mechanisms that endophytes employ to combat pathogenic attacks on the harvested fruits.
Direct competition for space and nutrients
In the tripartite system of fruit–pathogen–endophyte interaction, the nutrition and space of the host are limited. Nitrogen, carbon, macronutrients, and micronutrients are essential for the survival of both endophytes and pathogens (Kumari et al., 2020a, b). Endophytes, being fast in growth and colonization, quickly occupy the exposed fruit surface and outnumber pathogens in the space competition and utilization of nutritional resources (Adame-Álvarez et al., 2014; Spadaro and Droby, 2016). Different studies have demonstrated the utilization of carbon resources by endophytic Bacillus spp., inhibiting spore germination of the pathogens; however, bacterial dosage needs to be optimized according to the fruit (Carmona-Hernandez et al., 2019). A phenotypic and gene transcription study revealed the increased expression of genes involved in nutrition uptake by the bacterium Lactobacillus plantarum when cocultivated with the pathogen Aspergillus carbonarius isolated from grape berries (Lappa et al., 2018). The L. plantarum culture effectively inhibited the growth of four fungal pathogens isolated from the grape berries. A 32%–90% inhibition in mycotoxin produced by A. carbonarius was also observed after coculturing with L. plantarum. Successful in vivo application of this bacterium not only may help in controlling postharvest pathogens but also will act as a source of probiotics for modulating gut microflora.
Production of siderophores (iron-chelating compounds)
Iron is one of the essential minerals required for the growth, survival, and virulence of pathogens. Siderophores are the secondary microbial metabolites produced by many endophytes, which can form a tight and stable octahedral Fe(H2O6)3+ complex with available iron (Miethke and Marahiel, 2007). The exposed fruit surface is an adverse niche, where the bioavailability of nutrients, especially iron, is relatively low. In the competition for survival, endophytes are known to colonize faster than pathogens, chelating the available iron by producing several types of siderophores and thus depriving the postharvest pathogen of any iron source (Chowdappa et al., 2020). Genome mining of the endophytic Pseudomonas fluorescens BRZ63 has revealed siderophore production by the bacterium, protecting against several postharvest pathogens, including Colletotrichum dematium K, Sclerotinia sclerotiorum K2291, and Fusarium avenaceum (Chlebek et al., 2020). Many endophytic Bacillus sp. produce bacilibactin type of siderophore-protecting bacterial wilt in banana (Carmona-Hernandez et al., 2019). Trichoderma spp. has been known to produce hydroxamate siderophore, which can deplete iron and inhibit the growth of postharvest pathogens in apples and citrus fruits (Sood et al., 2020). Though the endophytic Trichoderma spp. is still in the nascent stage for controlling postharvest diseases of fruits, it can pave a new and sustainable path for the disease control of fruits after harvest. However, optimizing the concentration of endophytes and factors affecting siderophore production should not be neglected to increase endophytic efficiency against postharvest pathogens.
Production of bioactive antimicrobial compounds and antibiosis
Endophytic microbiomes have recently emerged as potent and novel sources of secondary metabolites, many of which are antimicrobial. They are known to produce alkaloids, flavonoids, phenolics, terpenoids, steroids, non-ribosomal peptides, and VOCs (Kumari et al., 2018). For example, endophytic Trichoderma sp. produced antifungal epipolythiodioxopiperazines, peptaibols, koninginins, and pyrenes, which combat postharvest diseases in kiwi fruit, apple, and banana (Khan et al., 2020). The recently published review article by Huang et al. (2021) briefly covered the bioactive compounds produced by endophytes and how they enhance the resistance against postharvest diseases of fruit and vegetables. Similarly, Carmona-Hernandez et al. (2019) also covered the bioactive compounds, volatiles produced by the endophytic strains, and their role in postharvest disease management. The details of bioactive metabolites produced by endophytes, which can potentially be used against postharvest pathogens of fruits, are described in Table 2.
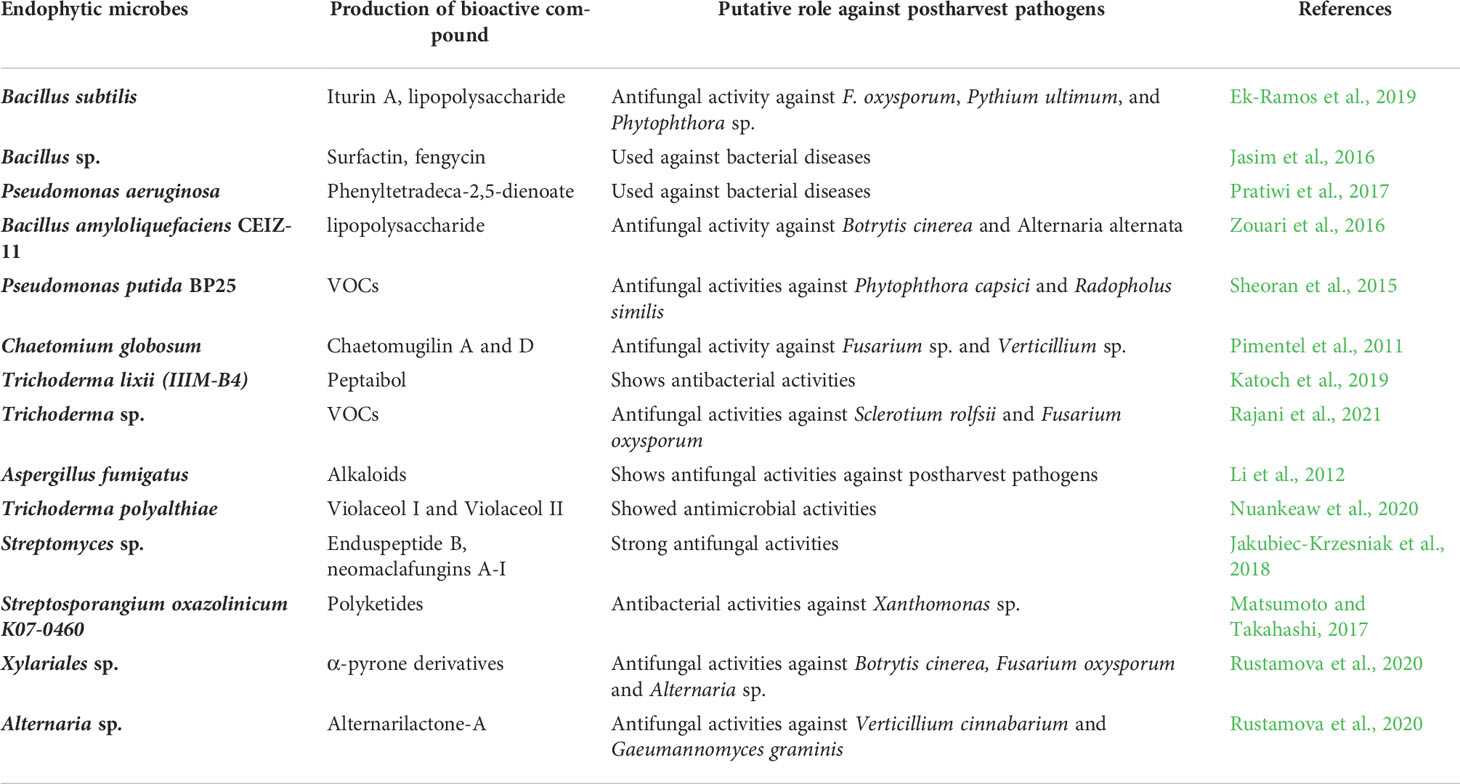
Table 2 Bioactive compounds produced by endophytic microbes used in the management of postharvest diseases of fruits.
Though the potential of bioactive secondary metabolites is enormous in postharvest disease control of fruits, the low quantity produced, in planta pressure, and influence of the culture conditions are some of the factors that need optimization.
Mycoparasitism and production of lytic enzymes
One of the essential mechanisms employed by endophytic fungi against pathogenic fungi is mycoparasitism by the production of cell wall-degrading enzymes and direct parasitism. The lytic enzymes, including glucanase, chitinase, and cellulose produced by endophytes, can degrade the pathogenic cell wall. For example, Talaromyces acidophilus a fungal strain AUN-1 emerged as a novel mycoparasite of postharvest pathogen B. cinerea by producing lytic enzyme chitinase, lipase, and protease (Abdel-Rahim and Abo-Elyousr, 2018). Endophytic fungus Choiromyces aboriginum inhibited postharvest pathogen Pythium sp. by producing β-1,3-glucanases and degraded the pathogenic cytoplasm coiling around the hyphae (Cao et al., 2009). In the same sense, plant beneficial fungus Trichoderma spp. can inhibit the growth of several pathogens through parasitism, for example, a Trichoderma sp. strain inhibited the fungal pathogen F. oxysporum by producing a lytic enzyme and coiling around the pathogenic fungal hyphae (Rajani et al., 2021).
Some bacterial strains are also prolific producers of lytic enzymes, making them suitable candidates for postharvest disease management, though endophytes specifically have not been explored much. For example, endophytic Bacillus sp. are known to produce β-1,3-glucanase, chitinase, and protease, which can disrupt fungal cell walls (Carmona-Hernandez et al., 2019). The hydrolytic enzymes produced by B. subtilis 739 caused the lysis of phytopathogenic fungi A. alternata, B. sorokiniana, F. culmorum, and R. solani. The cocktail of cold-adapted lytic enzymes produced by archaea and cold-adapted bacteria has also shown their potential against antagonistic fungal pathogens (de Oliveira et al., 2020), which provides an excellent opportunity to explore endophytes from extreme conditions.
Production of endotoxins and lipopolysaccharides
Endophytes are being developed as prolific producers of LPSs of several lengths of fatty acids. For example, phengicines and iturins produced by B. subtilis GA1 inhibited the growth of B. cinerea in apple fruits (Toure et al., 2004). Thus, the optimized media conditions for synthesizing LPSs from endophytes can pave a sustainable path for the biological control of postharvest fruit diseases. The toxin Leu7-surfactin was produced from the endophytic bacterium Bacillus mojavensis RRC 101 against antagonistic fungus Fusarium verticillioides (Snook et al., 2009). Several mycotoxins produced by endophytic fungi can also be explored for their efficacy against the antagonistic pathogens to control postharvest disease, though their safety also needs to be analyzed thoroughly (Lacava and Azevedo, 2013).
Modulating the redox homeostasis of harvested fruits and pathogens
Many postharvest pathogens overcome the fruit defense system by manipulating their redox potential. For example, Penicillium digitatum, the causative agent of green mold in citrus fruits, produces catalase that decomposes hydrogen peroxide to establish an infection (Macarisin et al., 2007). Endophytes provide oxidative stress protection to plants (Hamilton et al., 2012; White et al., 2019). However, their role in modulating stress in postharvest disease management is not much explored. Endophytes help plants combat biotic stress by lowering lipid peroxidation and accumulation of proline (Spadaro and Droby, 2016). As an example, endophytic fungus Paraburkholderia phytofirmans strain PsJN increased the expression of genes involved in reactive oxygen species (ROS)-scavenging pathways, resulting in detoxification of ROS and modulating the signaling pathways (Pacifico et al., 2019). The plant–pathogen and endophytic relation has been documented well in literature, but the research on the role of endophytes in modulating redox homeostasis of stored fruits needs special attention.
Quorum sensing and biofilm formation and disruption by endophytes
Bacterial endophytes, including Bacillus spp. and Pseudomonas spp., are known to colonize exposed fruit areas by quorum sensing (QS) and biofilm formation. The ability of endophytic bacteria to secrete small molecules such as tyrosol, farnesol, and phenethyl alcohol to regulate colonization helps them outnumber the pathogenic microbes in the competition for space and nutrients (Carmona-Hernandez et al., 2019). Recently, endophytes were also found to produce anti-QS molecules, which can help combat the biofilm established by pathogenic bacteria on fruit surfaces. For example, endophytic fungi Fusarium graminearum and Lasiodiplodia sp. isolated from the plant Ventilago madraspatana produced secondary metabolites with anti-QS potential (Mookherjee et al., 2017). Furthermore, the isolated fungi produced QS inhibitors that were quantified spectrophotometrically by their ability to inhibit the production of violacein in wild and mutants of Chromobacterim violaceum (Rajesh and Rai, 2013). Whether it is biofilm formation or the production of anti-QS molecules by endophytes, both properties can be exploited in postharvest disease management in fruits, as this field of research remains unexplored.
Modulation and synthesis of phytohormones
Endophytic microbes can synthesize phytohormones, including auxin, gibberellins, cytokines, ethylene, nitric oxide, and 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase, which provide additional immunity to postharvested plants to cope up with biotic and abiotic stresses (Ali et al., 2017). The increased phytohormone synthesis helps to overcome the stress-induced wilting. Not only are the endophytes capable of synthesizing plant hormones themselves, but they can also modulate the plant–hormone metabolic pathways for enhanced stress tolerance. For example, the interaction of endophytic fungus Piriformospora indica in the synthesis of auxins, cytokinin, gibberellins, abscisic acid, ethylene, salicylic acid (SA), jasmonates, and brassinosteroids resulted in better efficiency of stress tolerance in higher plants (Xu et al., 2018).
Induction of disease resistance in fruits
In response to a pathogenic attack, plants develop two kinds of disease resistance mechanisms: 1) systemic acquired response (SAR) and 2) induced systemic resistance (ISR). Many endophytic microbes have been known to elicit ISR, thereby providing solid immunity against biotic stress (Pacifico et al., 2019). Endophytes activate ISR pathways by synthesizing pathogen-related proteins, enhancing the synthesis of phenolic compounds, and activating signaling pathways by jasmonate/SA and ethylene (Jacob et al., 2020) (Figure 3).
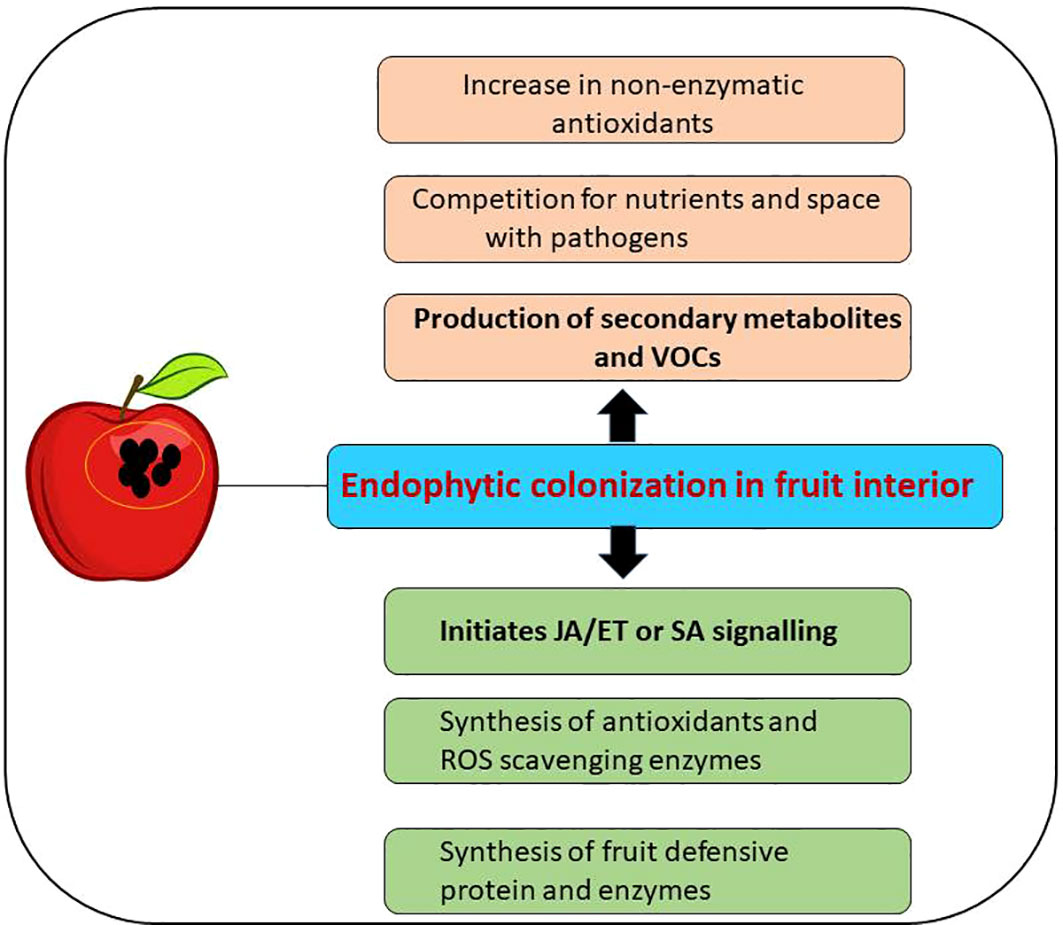
Figure 3 Activation of induced systemic resistance (ISR) signaling pathway and production of bioactive secondary metabolites after colonization of endophytes in the host (postharvested fruits).
The endophytic bacterial strain Pseudomonas putida MGY2 was able to control anthracnose caused by C. gloeosporioides in harvested papaya fruit (Shi et al., 2011). It was found that the endophyte induced ISR by increasing the gene expression of phenylalanine ammonia-lyase (PAL), catalase (CAT), and peroxidase (POD), increasing the phenolic content and decreasing the production of ethylene. The same group demonstrated the control of Phytophthora nicotianae disease in papaya fruits by induction of the pathogenesis-related protein 1 gene (PR1) and non-expression of PR1 gene (NPR1) after inoculation of P. putida MGP1 strain (Shi et al., 2013). Louarn et al. (2013) demonstrated a significant change in the endophytic community in organically and conventionally grown carrots. Endophytic Bacillus amyloliquefaciens YTB1407 strain elicited ISR by activating the expression of SA-responsive PR1 gene, thus inhibiting pathogenic fungus Fusarium solani. The literature is insufficient regarding the elicitation of molecular responses of fruits in postharvest conditions. Furthermore, in-depth mechanistic studies are required to understand the disease resistance of fruits after endophytic microbe application.
Modulating the native microbiota and ecological effects
The endophytic microbial population modulates the native microbiota of fruits, roots, leaves, and soil, promoting a sustainable crop production system. Therefore, it is of great economic relevance (Sturz et al., 2010; Baghel et al., 2020). However, its interference with the native population of harvested fruit microbiota is still waiting to be explored. Endophytes bear the potential to shift the native bacterial population toward favorable conditions for plant growth and stress amelioration (Baghel et al., 2020). It has been found that healthy fruits tend to have a diverse microbial community, whereas diseased fruits have a limited microbial growth dominated by pathogen microorganisms (Huang et al., 2021). In their study, Diskin et al. (2017) found that colonization of endophytic communities was much less prevalent in mango fruits suffering from stem-end rot disease than that in their healthier counterpart. By utilizing multiple mechanisms, including parasitism, production of bioactive compounds, lytic enzymes, and siderophores against postharvest pathogens, endophytes can modulate the native microbiota of the harvested fruits to increase their resistance against biotic stresses.
Controlling mycotoxins
Mycotoxins are a major cause of qualitative and quantitative loss in stored fruits. Deoxynivalenol, alternariol, aflatoxin, and patulin, produced by antagonistic fungi, can impact fruit and human health negatively (Bartholomew et al., 2021). Many endophytes and their secondary metabolites have shown the effectiveness of controlling mycotoxins in vitro and in planta (Abdallah et al., 2018) in maize and other crops, although studies on their impact on postharvested fruits are limited. Sarrocco and Vannacci (2018) emphasized preharvest application of endophytes for controlling postharvest damage caused by mycotoxins. The VOCs produced by endophytic fungi can be incorporated in edible biofilms or can be an ingredient during packaging to effectively control mycotoxins in store fruits (Mari et al., 2016).
As biocontrol strategies usually rely on a single or mixture of antagonists, endophytic microbial strains have been suggested as antagonistic microorganisms against various diseases in various crops. The additional effect of endophytic microbiota as BCAs is the phytohormone synthesis, metabolites, and nutrients utilized for growth promotion and stress management in host plants (Lodewyckx et al., 2002; Suhandono et al., 2016).
In the recent past, various BCAs, including bacteria, yeast, and fungi, have been frequently applied for effective management of postharvest pathogens, while practices with endophytes are very limited. Endophytes’ properties appear superior to those of epiphytic microorganisms due to their better colonization and tolerance potential against various biotic and abiotic stresses (Shi et al., 2010). In recent years, several pieces of literature regarding utilizing the endophytic microbiome for screening BCAs against postharvest pathogens have been reported. Shimizu et al. (2009) reported on the endophytic actinomycete Streptomyces sp., which showed effective biocontrol potential against the pathogen Colletotrichum orbiculare, the causal agent of anthracnose disease in cucumber. Similarly, Shi et al. (2010) reported on P. putida biovar isolated from the pericarp of papaya with strong colonization potential and showed potent inhibition against several pathogens.
Additionally, the strain effectively inhibits the growth of P. nicotianae just after a short period of treatment. Lai et al. (2012) screened the endophytic strain Paenibacillus polymyxa isolated from the root tissue of Sophora tonkinensis and showed antagonistic potential against P. digitatum, one of the most devastating pathogens causing postharvest diseases in citrus fruit. The application of endophytic strains effectively reduces postharvest decay by inhibiting conidia germination in a fungal cell suspension. Additionally, the unwashed cell suspension of the strain was found to be more effective than the washed cell suspension and culture filtrate in the in vivo trials.
Ji et al. (2008) isolated 45 endophytic bacterial strains from the mulberry leaves (Morus alba L.) and reported the strong inhibitory potential of B. subtilis Lu144 against Ralstonia solanacearum, the causal agent of bacterial wilt of mulberry fruits. Furthermore, Furuya et al. (2011) utilized the strain B. subtilis KS1 isolated from the skin part of grape berry and applied it as a potential antagonistic agent against fungal grapevine diseases. In vitro screening showed that the strain effectively suppressed the growth of B. cinerea and C. gloeosporioides. Furthermore, after applications in the vineyards, the strains significantly reduce the incidence of downy mildew from the leaves and skin of the berry. Chen et al. (2016) screened the B. amyloliquefaciens PG12 strain isolated from apple fruits as a potential BCA against apple ring rot disease. The strain significantly suppressed the Botryosphaeria dothidea growth during in vivo and in vitro screening and showed a potent antagonistic effect against different fungal pathogens. Madbouly et al. (2020) evaluated the biocontrol potential of endophytic yeast strains Schwanniomyces vanrijiae, Galactomyces geotrichum, Pichia kudriavzevii, isolated from apple fruits, against the pathogen Monilinia fructigena, the causal agent of apple fruit brown rot of golden delicious apples. During in vitro test analysis, all three endophytic yeast strains showed inhibitory potential against M. fructigena and significantly inhibited conidial germination by 67.6%–89.2%. In the last few years, rapid enhancement can be seen in the use of endophytic microorganisms in postharvest disease management in fruits. However, still, most of the experiments are limited to the laboratory scale. Furthermore, we need to study how the fruit microbiome affects the fruit’s physiology and disease resistance and how the fruit-associated microbial communities shifted during the postharvest stages and after applying BCAs.
Commercial upscale production and hurdles ahead
Antagonistic endophytic application against postharvest diseases, especially in fruits, has emerged as a new generation of pesticides. Though the mechanisms are still to be deciphered completely, many endophytes have paved their path to commercial applications. B. subtilis strain B-3 has been patented, and pilot experiments have been conducted against the peach brown rot disease. It was observed that after the application of the endophyte in either powder or paste form, it was as effective as traditional pesticide benomyl in Clemson, SC, USA (Pusey et al., 1988). Products based on B. subtilis QST713 with the trade name Serenade™ are produced commercially by AgraQuest Inc., USA, against powdery mildew, brown rot, and late blight of apple, pear, and grapes (Punjia et al., 2016). Multiple formulations in many countries with trade names, including Candifruit™, Shemer™, and Boni-protect™, have been successfully used against postharvest pathogens (Fenta et al., 2019). The endophytes, a new concept, have to face many hurdles for their successful commercialization. In addition to the agricultural giants such as Dupont, Monsanto, and Bayer, many small startup companies such as Indigo and NewLeaf Symbiotics have entered the microbial domain with promising contributions. The following hurdles need to be overcome to achieve economically and sustained commercial-scale production of antagonistic endophytes or their products.
Increased shelf life and multiple stress-tolerant endophytic microbes
In the niche of postharvest fruits, endophytes have to overcome several biotic and abiotic stresses (Diskin et al., 2017). For the successful application and upscale production of antagonistic endophytes against postharvest diseases of fruits, the endophytes must be stress-tolerant to prolong their shelf life and sustain antipathogenic activities. Many stress-tolerant endophytic microbes are already studied for plant growth promotion in adverse conditions (Giauque et al., 2019; Singh et al., 2022). Furthermore, the synergistic application of endophytes can also help increase the shelf life of endophytes in their battle against postharvest pathogens (Huang et al., 2021). Therefore, exhaustive screening of stress-tolerant endophytes and their in vitro and in vivo stress amelioration potential should be conducted for the endophytes to go from lab to field.
Some endophytes are deeply associated with their host for stress tolerance and the production of the desired natural products (Khare et al., 2018). Therefore, their ability to cope up with the stress condition in the absence of their host plants and the niche of postharvest fruits should also be assessed before their commercialization.
Optimizing the modes of endophyte application
The modes of application of endophytes to the surface of postharvest fruits also play a crucial role in plant disease management and increasing the shelf life of the endophytes. Therefore, the application of endophytes on fruit surfaces should be optimized on a case-by-case basis. Generally, the formulations are applied as liquid or powder/paste formulations. Though the dry form provides a longer shelf life, it can cause a loss of viability of microbes through repeated rehydration-dehydration processes (Kumari et al., 2020a, b). Many rehydration agents, including whey proteins and maltodextrins, have been suggested to coat dry formulations (Martin et al., 2017). For sustained release of endophytes, their secondary metabolites, and VOCs, nanoencapsulation of the products and nanoemulsions can also be studied (Pandey et al., 2020). Recently, Ghazy et al. (2021) studied the role of anise extract oil nanoemulsion against different postharvest antagonistic bacteria for their sustained release. A combination of SA with endophytic B. subtilis was used to treat postharvest diseases by F. oxysporum and P. infestans (Lastochkina et al., 2020). Preharvest and postharvest modes of endophytic application should also be considered for their antagonistic application. For the upscale production of endophytes as postharvest disease management in fruits, the mode of application is an important parameter, whose optimization should be carried out in detail.
Sustained release and cost-effective production of microbial metabolites
The commercialization of secondary metabolites and VOCs derived from endophytes faces hurdles in sustainable release and economic upscale production. Media optimization, selection of potent microbial strains, and metabolic engineering are some of the parameters that can be employed (Sah et al., 2020; Kamat et al., 2020; Taritla et al., 2021) for the sustained production of desired antimicrobial secondary metabolites from endophytes. The addition of some of the precursors from the host system has also been studied during media optimization for continuous upscale production of the antimicrobial metabolites from endophytes during the fermentation process.
The second hurdle faced during their commercialization includes the hydrophobicity of natural products. To overcome the solubility issue, several solutions, including their encapsulation in non-toxic and biodegradable polymers, have been proposed (Soh and Lee, 2019), which provide solubility and the slow release of the active ingredient. Chitosan, carrageenan, starch, and alginate nanopolymers have been used to encapsulate natural products, including polyphenols, alkaloids, and terpenoids with increased water solubility and bioactivity (Detsi et al., 2020).
Overcoming the in planta pressure for survival and stress amelioration
The biggest hurdle in successfully applying endophytic microbes in the fruit microbiome is overcoming their host pressure. Endophytes have always lived as symbionts with their host, sharing many physical and chemical attributes with their host plants (Spadaro and Droby, 2016). Several hypotheses, including the defensive mutualism hypothesis, xenohormesis hypothesis, and trait-specific endophytic infallibility (TSEI) hypothesis, have been shared among the research community to describe the co-evolution of the host and the endophytes (Kusari et al., 2015; Pathak et al., 2022). Their isolation and survival without their hosts may alter their growth cycle and physiological performance in the competition of the new fruit microbiome. The question of replacement dynamics with the preexisting microbiome of fruits is always relevant while introducing a new endophytic strain. The mode of application and the growth and production of secondary metabolites in vitro should be monitored before their in vivo application in postharvested fruit microbiomes.
Genome mining and metagenomics
Getting the superior strains of endophytes required digging deep into the unexplored wealth of endophytes and exploring the biosynthetic pathways to synthesize beneficial secondary metabolites, siderophores, and phytohormones. To bypass the tedious process of endophyte isolation and screening for postharvest disease management, genome mining and metagenomic studies can be performed to select the right strain economically (Kusari et al., 2015). For example, genome mining of the endophytic fungus Penicillium dangeardii revealed a cluster of 43 biosynthetic genes demonstrating their strong ability to synthesize secondary metabolites (Wei et al., 2021) exploited in postharvest disease management. Thus, genome mining and metagenomics can provide better endophytic strains that can be commercially produced for the desired secondary metabolites.
Change in policymaking and awareness regarding the use of antagonistic endophytes
The most critical parameter for introducing endophytes as substitutes for conventional pesticides in postharvest disease management is to increase the awareness of the end-users and people involved in the distribution chain. Therefore, outreach programs and workshops related to these new ideas should constantly be organized to bring awareness and benefits of using endophyte-based biopesticides.
Any effort is not fruitful without governments, policymaking, and funding agencies to implement new technologies in agri-business sectors. Earlier, the Department of Biotechnology (DBT), India, launched the National Biocontrol Network Programme (NBNP) to popularize and commercialize more than 30 biopesticides (Kumari et al., 2020a, b). Similar programs should be launched and funded to popularize financial, most effective, and eco-friendly products for managing postharvest diseases of fruits.
Safety of endophytes and their secondary metabolites for consumers and the environment
Endophytes, a new aspect of BCAs in postharvest disease management in fruits, need thorough scrutiny regarding their safety for consumers and the environment. Endophytes themselves or their products should not be opportunistic pathogens or should not pose any harm to the environment. Unfortunately, many of the earlier studied rhizobacteria or their secondary metabolites have acted as opportunistic human pathogens or environmental contaminants in certain conditions (Keswani et al., 2019). To avoid similar conditions with the endophytes, their safety in animal models and their effect on the environment due to higher dosage should also be assessed.
Conclusion
Endophytic microorganisms can colonize different organ tissues of the host plant and interact in multiple ways to regulate physiological and metabolic pathways, which can further be utilized in the effective management of postharvest diseases. Endophytic bacterial, actinomycetes, and fungal strains have been broadly utilized as BCAs against various plant pathogens during preharvest and postharvest stages. Currently, it is estimated that approximately 30% of the total fruit production is lost annually due to various diseases. Therefore, the potential colonization efficacy of endophytes is a crucial characteristic for disease management.
In addition, next-generation omics may be applied to identify the gene(s) responsible for disease management. Thus, during the application, consortia of mixed microbial agents (bacteria-bacteria; bacteria-fungus; fungus-fungus) showed a practical approach in disease management, but the survival and better adaptability of both strains together are reasons for further investigation, particularly under diverse environmental conditions. Endophytes have reported multiple mechanisms that are used to inhibit pathogenic growth and increase fruit health. Though there are numerous examples of successful bioformulations of microbial endophytic strains capable of controlling the pathogenicity of the pest or pathogens during preharvest conditions, their application in postharvest pathogen control is in the nascent stage. Further application of endophytic microbiome can further reduce, or at some point will eliminate, the harmful dependence on chemical pesticides and fungicides in postharvest disease management.
Author contributions
MK and AK designed the study. MK, KQ ,SS, VS, KS, and AK wrote the manuscript. KQ and MJ acquired funding. KQ, MJ, JW, GS, and GP reviewed and provided valuable feedback to this study. All the authors contributed to the article and agreed to the published version of the manuscript.
Funding
The research is financially supported by King Abdullah University of Science and Technology, Thuwal, Jeddah, Saudi Arabia.
Acknowledgments
The authors are thankful to the Agriculture Research Organization for providing lab facilities.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer SP declared a shared affiliation with the author SS to the handling editor at the time of review.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdallah, F. M., De Boevre, M., Landschoot, S., De Saeger, S., Haesaert, G., Audenaert, K. (2018). Fungal endophytes control fusarium graminearum and reduce trichothecenes and zearalenone in maize. Toxins 10 (12), .493. doi: 10.3390/toxins10120493
Abdelfattah, A., Nicosia, M. G. L. D., Cacciola, S. O., Droby, S., Schena, L. (2015). Metabarcoding analysis of fungal diversity in the phyllosphere and carposphere of olive (Olea europaea). PloS One 10 (7), e0131069. doi: 10.1371/journal.pone.0131069
Abdel-Rahim, I. R., Abo-Elyousr, K. A. (2017). Using of endophytic saccharomycopsis fibuligera and thyme oil for management of gray mold rot of guava fruits. Biol. Control. 110, 124–131. doi: 10.1016/j.biocontrol.2017.04.014
Abdel-Rahim, I. R., Abo-Elyousr, K. A. M. (2018). Talaromyces pinophilus strain AUN-1 as a novel mycoparasite of botrytis cinerea, the pathogen of onion scape and umbel blights. Microbiol. Res. 212-213, 1–9. doi: 10.1016/j.micres.2018.04.004
Adame-Álvarez, R. M., Mendiola-Soto, J., Heil, M. (2014). Order of arrival shifts endophyte–pathogen interactions in bean from resistance induction to disease facilitation. FEMS microbial. Let. . 355 (2), 100–107. doi: 10.1111/1574-6968.12454
Ahmad, T., Farooq, S., Mirza, D. N., Kumar, A., Mir, R. A., Riyaz-Ul-Hassan, S. (2022). Insights into the endophytic bacterial microbiome of crocus sativus: functional characterization leads to potential agents that enhance the plant growth, productivity, and key metabolite content. Microbial Ecol. 83 (3), 669–688.
Aiello, D., Restuccia, C., Stefani, E., Vitale, E., Cirvilleri, G. (2019). Postharvest biocontrol ability of pseudomonas synxantha against Monilinia fructicola and Monilinia fructigena on stone fruit. Postharvest Biol. Technol. 149, 83–89. doi: 10.1016/j.postharvbio.2018.11.020
Al-Ani, L. K. T. (2019). “Recent patents on endophytic fungi and their international market,” in Intellectual property issues in microbiology. Eds. Singh, H., Keswani, C., Singh, S. (Singapore: Springer). doi: 10.1007/978-981-13-7466-1_14
Aleklett, K., Hart, M., Shade, A. (2014). The microbial ecology of flowers: an emerging frontier in phyllosphere research 1. Botany 92 (4), 253–266. doi: 10.1139/cjb-2013-0166
Ali, S., Charles, T. C., Glick, B. R. (2017). “Endophytic phytohormones and their role in plant growth promotion,” in Functional importance of the plant microbiome. Ed. Doty, S. (Cham: Springer). doi: 10.1007/978-3-319-65897-1_6
Ali, S., Duan, J., Charles, T. C., Glick, B. R. (2014). A bioinformatics approach to the determination of genes involved in endophytic behavior in burkholderia spp. J. Theor. Biol. 343, 193–198. doi: 10.1016/j.jtbi.2013.10.007
Amann, R. I., Ludwig, W., Schleifer, K. H. (1995). Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 59 (1), 143–169. doi: 10.1128/mr.59.1.143-169.1995
Anaya, P., Onofre, J., Torres-Quintero, M. C., Sánchez, J., Gill, S. S., Bravo, A., et al. (2020). Oligomerization is a key step for bacillus thuringiensis Cyt1Aa insecticidal activity but not for toxicity against red blood cells. Insect Biochem. Mol. Biol. 119, 103317. doi: 10.1016/j.ibmb.2020.103317
Arah, I., Amaglo, H. K., Kumah, E. K., Ofori, H. (2015). Preharvest and postharvest factors affecting the quality and shelf life of harvested tomatoes: A mini review Int. J. Agron, Vol. 2015. doi: 10.1155/2015/478041
Aravind, R., Kumar, A., Eapen, S. J., Ramana, K. V. (2009). Endophytic bacterial flora in root and stem tissues of black pepper (Piper nigrum l.) genotype: isolation, identification and evaluation against phytophthora capsici. Lett. Appl. Microbiol. 48 (1), 58–64. doi: 10.1111/j.1472-765X.2008.02486.x
Bacon, C. W., White, J. F. (2016). Functions, mechanisms and regulation of endophytic and epiphytic microbial communities of plants. Symbiosis 68, 87–98. doi: 10.1007/s13199-015-0350-2
Baghel, V., Thakur, J. K., Yadav, S. S., Manna, M. C., Mandal, A., Shirale, A. O., et al. (2020). Phosphorus and potassium solubilization from rock minerals by endophytic burkholderia sp. strain FDN2-1 in soil and shift in diversity of bacterial endophytes of corn root tissue with crop growth stage. Geomicrobiol. J. 37, 550–563. doi: 10.1080/01490451.2020.1734691
Balsanelli, E., Tadra-Sfeir, M. Z., Faoro, H., Pankievicz, V. C., de Baura, V. A., Pedrosa, F. O., et al. (2016). Molecular adaptations of h erbaspirillum seropedicae during colonization of the maize rhizosphere. Environm. Microbiol. 18 (8), 2343–2356. doi: 10.1111/1462-2920.12887
Bartholomew, H. P., Bradshaw, M., Jurick, W. M., Fonseca, J. M. (2021). The good, the bad, and the ugly: Mycotoxin production during postharvest decay and their influence on tritrophic host–Pathogen–Microbe interactions. Front. Microbiol. 12, 611881. doi: 10.3389/fmicb.2021.611881
Besset-Manzoni, Y., Joly, P., Brutel, A., Gerin, F., Soudière, O., Langin, T., et al. (2019). Does in vitro selection of biocontrol agents guarantee success in planta? a study case of wheat protection against fusarium seedling blight by soil bacteria. PloS One 14 (12), e0225655. doi: 10.1371/journal.pone.0225655
Böhm, M., Hurek, T., Reinhold-Hurek, B. (2007). Twitching motility is essential for endophytic rice colonization by the N2-fixing endophyte azoarcus sp. strain BH72. Mol. Plant Microbe Interact. . 20 (5), 526–533. doi: 10.1094/MPMI-20-5-0526
Bolívar-Anillo, H. J., Garrido, C., Collado, I. G. (2020). Endophytic microorganisms for biocontrol of the phytopathogenic fungus botrytis cinerea. Phytochem. Rev. 19, 721–740. doi: 10.1007/s11101-019-09603-5
Brader, G., Compant, S., Vescio, K., Mitter, B., Trognitz, F., Ma, L. J., et al. (2017). Ecology and genomic insights into plant-pathogenic and plant-nonpathogenic endophytes. Annu. Rev. Phytopathol. 55, 61–83. doi: 10.1146/annurev-phyto-080516-035641
Bu, S., Munir, S., He, P., Li, Y., Wu, Y., Li, X., et al. (2021). Bacillus subtilis L1-21 as a biocontrol agent for postharvest gray mold of tomato caused by botrytis cinerea. Biol. Control 157, 104568. doi: 10.1016/j.biocontrol.2021.104568
Busby, P. E., Ridout, M., Newcombe, G. (2016). Fungal endophytes: modifiers of plant disease. Plant Mol. Biol. 90 (6), 645–655. doi: 10.1007/s11103-015-0412-0
Cabello-Olmo, M., Oneca, M., Torre, P., Díaz, J. V., Encio, I. J., Barajas, M., et al. (2020). Influence of storage temperature and packaging on bacteria and yeast viability in a plant-based fermented food. Foods 9 (3), 302. doi: 10.3390/foods9030302
Cao, R., Liu, X., Gao, K., Mendgen, K., Kang, Z., Gao, J., et al. (2009). Mycoparasitism of endophytic fungi isolated from reed on soilborne phytopathogenic fungi and production of cell wall-degrading enzymes in vitro. Curr. Microbiol. 59, 584–592. doi: 10.1007/s00284-009-9477-9
Card, S., Johnson, L., Teasdale, S., Caradus, J. (2016). Deciphering endophyte behaviour: The link between endophyte biology and efficacious biological control agents. FEMS Microbiol. Ecol. 92, 1–44. doi: 10.1093/femsec/fiw114
Carmona-Hernandez, S., Reyes-Pérez, J. J., Chiquito-Contreras, R. G., Rincon-Enriquez, G., Cerdan-Cabrera, C. R., Hernandez-Montie, L. G. (2019). Biocontrol of postharvest fruit fungal diseases by bacterial antagonists: a review. Agronomy 9, 121. doi: 10.3390/agronomy9030121
Chaouachi, M., Marzouk, T., Jallouli, S., Elkahoui, S., Gentzbittel, E., Ben, C., et al. (2021). Activity assessment of tomato endophytic bacteria bioactive compounds for the postharvest biocontrol of botrytis cinerea. Postharvest Biol. Technol. 101, 161–170. doi: 10.1016/j.postharvbio.2020.111389
Chen, C., Cao, Z., Li, J., Tao, C., Feng, Y., Han, Y. (2020b). A novel endophytic strain of lactobacillus plantarum CM-3 with antagonistic activity against botrytis cinerea on strawberry fruit. Biol. Cont. 148, 104306. doi: 10.1016/j.biocontrol.2020.104306
Chen, X., Marszałkowska, M., Reinhold-Hurek, B. (2020a). Jasmonic acid, not salicyclic acid restricts endophytic root colonization of rice. Front. Plant Sci. 10, 1758. doi: 10.3389/fpls.2019.01758
Chen, X., Zhang, Y., Fu, X., Li, Y., Wang, Q. (2016). Isolation and characterization of Bacillus amyloliquefaciens PG12 for the biological control of apple ring rot. Postharvest Biol. Technol. 115, 113–121.
Chlebek, D., Pinski, A., Zur, J., Michalska, J., Hupert-Kocurek, K. (2020). Genome mining and evaluation of the biocontrol potential of pseudomonas fluorescens BRZ63, a new endophyte of oilseed rape (Brassica napus l.) against fungal pathogens. Int. J. Mol. Sci. 21, 8740. doi: 10.3390/ijms21228740
Chowdappa, S., Jagannath, S., Konappa, N., Udayashankar, A. C., Jogaiah, S. (2020). Detection and characterization of antibacterial siderophores secreted by endophytic fungi from cymbidium aloifolium. Biomol 10, 1412. doi: 10.3390/biom10101412
Comby, M., Gacoin, M., Robineau, M., Rabenoelina, F., Ptas, S., Dupont, J., et al. (2017). Screening of wheat endophytes as biological control agents against fusarium head blight using two different in vitro tests. Microbiol. Res. 202, 11–20. doi: 10.1016/j.micres.2017.04.014
Compant, S., Mitter, B., Colli-Mull, J. G., Gangl, H., Sessitsch, A. (2011). Endophytes of grapevine flowers, berries, and seeds: identification of cultivable bacteria, comparison with other plant parts, and visualization of niches of colonization. Microbial Ecol. 62 (1), 188–197. doi: 10.1007/s00248-011-9883-y
Cruz, A. F., Barka, G. D., Blum, L. E. B., Tanaka, T., Ono, N., Kanaya, S., et al. (2019). Evaluation of microbial communities in peels of Brazilian tropical fruits by amplicon sequence analysis. Braz. J. Microbiol. 50, 739–748. doi: 10.1007/s42770-019-00187-y
De Bary, A. (1866). “Morphologie und physiologie der pilze, flechten, und myxomyceten,” in Hofmeister’s handbook of physiological botany. Ed. Engelmann, W.(Leipzig).
de Oliveira, T. B., de Lucas, R. C., Scarcella, A. S. D. A., Pasin, T. M., Contato, A. G., Polizeli, M.D.L.T.D.M. (2020). Cold-active lytic enzymes and their applicability in the biocontrol of postharvest fungal pathogens. J. Agric. Food. Chem. 68 (24), 6461–6463. doi: 10.1021/acs.jafc.0c03085
Detsi, A., Kavetsou, E., Kostopoulou, I., Pitterou, I., Pontillo, A. R. N., Tzani, A., et al. (2020). Nanosystems for the encapsulation of natural products: the case of chitosan biopolymer as a matrix. Pharmaceutics 12, 669. doi: 10.3390/pharmaceutics12070669
Di Pietro, A., Talbot, N. J. (2017). Fungal pathogenesis: Combatting the oxidative burst. Nat. Microbiol. 2 (7), 1–2.
de Wit, P. J. (2007). How plants recognize pathogens and defend themselves. Cell. Mol. Life Sci. 64 (21), 2726–2732. doi: 10.1007/s00018-007-7284-7
Diskin, S., Feygenberg, O., Feygenberg, D., Droby, S., Prusky, D., Alkan, N. (2017). Microbiome alterations are correlated with occurrence of postharvest stem-end rot in mango fruit. Phytobiomes 1, 117–127. doi: 10.1094/PBIOMES-05-17-0022-R
Dong, C.-J., Wang, L.-L., Li, Q., Qing-Mao, S. (2019). Bacterial communities in the rhizosphere, phyllosphere and endosphere of tomato plants. PloS One 14 (11), e0223847. doi: 10.1371/journal.pone.0223847
Ek-Ramos, M. J., Gomez-Flores, R., Orozco-Flores, A. A., Rodríguez-Padilla, C., González-Ochoa, G., Tamez-Guerra, P. (2019). Bioactive products from plant-endophytic gram-positive bacteria. Front. Microbiol. 10, 463. doi: 10.3389/fmicb.2019.00463
Elbeltagy, A., Nishioka, K., Suzuki, H., Sato, T., Sato, Y. I., Morisaki, H., et al. (2000). Isolation and characterization of endophytic bacteria from wild and traditionally cultivated rice varieties. Soil Sci. Plant Nutr. 46 (3), 617–629. doi: 10.1080/00380768.2000.10409127
Fadiji, A. E., Babalola, O. O. (2020). Elucidating mechanisms of endophytes used in plant protection and other bioactivities with multifunctional prospects. Front. Bioeng. Biotechnol. 8, 467. doi: 10.3389/fbioe.2020.00467
Félix, C., Meneses, R., Gonçalves, M. F. M., Tilleman, L., Duarte, A.S., Jorrín-Novo, J.V., et al. (2019). A multi-omics analysis of the grapevine pathogen Lasiodiplodia theobromae reveals that temperature affects the expression of virulence- and pathogenicity-related genes. Sci. Rep. 9, 13144. doi: 10.1038/s41598-019-49551-w
Fenta, L., Mekonnen, H., Gashaw, T. (2019). Biocontrol potential of trichoderma and yeast against postharvest fruit fungal diseases: A review. Int J. Life Sci. 8 (1), 15–27. Available at: https://ijlsci.in/ls/index.php/home/article/view/144.
Frank, A. C., Saldierna Guzmán, J. P., Shay, J. E. (2017). Transmission of bacterial endophytes. Microorganisms 5 (4), 70. doi: 10.3390/microorganisms5040070
Furuya, S., Mochizuki, M., Aoki, Y., Kobayashi, H., Takayanagi, T., Shimizu, M., et al. (2011). Isolation and characterization of bacillus subtilis KS1 for the biocontrol of grapevine fungal diseases. Biocontrol Sci. Technol. 21 (6), 705–720. doi: 10.1080/09583157.2011.574208
Gasong, B. T., Tjandrawinata, R. R. (2016). Production of secondary metabolite E2. 2 from phaleria macrocarpa endophytic fungus. Asian Pac. J. Trop. Biomed. 6 (10), 881–885. doi: 10.1016/j.apjtb.2016.01.005
Ghazy, O. A., Fouas, M. T., Saleh, H. H., Kohli, A. E., Morsy, T. A. (2021). Ultrasound-assisted preparation of anise extract nanoemulsion and its bioactivity against different pathogenic bacteria. Food Chem. 341, 128259. doi: 10.1016/j.foodchem.2020.128259
Giauque, H., Connor, E. W., Hawkes, C. V. (2019). Endophyte traits relevant to stress tolerance, resource use and habitat of origin predict effects on host plants. New Phytol. 221 (4), 2239–2249. doi: 10.1111/nph.15504
Glassner, H., Zchori-Fein, E., Compant, S., Sessitsch, A., Katzir, N., Portnoy, V., et al. (2015). Characterization of endophytic bacteria from cucurbit fruits with potential benefits to agriculture in melons (Cucumis melo l.). FEMS Microbiol. Ecol. 91 (7). doi: 10.1093/femsec/fiv074
Gouda, S., Das, G., Sen, S. K., Shin, H. S., Patra, J. K. (2016). Endophytes: a treasure house of bioactive compounds of medicinal importance. Front. Microbiol. 7, 1538. doi: 10.3389/fmicb.2016.01538
Granada, D., Lopez-Lujan, L., Ramírez-Restrepo, S., Morales, J., Peláez-Restrepo, C., Andrade, G., et al. (2020). Bacterial extracts and bioformulates as a promising control of fruit body rot and root rot in avocado cv. Hass. J. Integrat. Agricult. 19 (3), 748–775. doi: 10.1016/S2095-3119(19)62720-6
Hahn, M. (2014). The rising threat of fungicide resistance in plant pathogenic fungi: Botrytis as a case study. J. Chem. Biol. 7 (4), 133–141. doi: 10.1007/s12154-014-0113-1
Hallmann, J., Quadt-Hallmann, A., Mahaffee, W., Kloepper, J. (1997). Bacterial endophytes in agricultural crops. Can. J. Microbiol. 43, 895–914. doi: 10.1139/m97-131
Hamilton, C. E., Gundel, P. E., Helander, M., Saikkonen, K. (2012). Endophytic mediation of reactive oxygen species and antioxidant activity in plants: a review. Fungal Divers. 54 (1), 1–10. doi: 10.1007/s13225-012-0158-9
Hardoim, P. R., Van Overbeek, L. S., Berg, G., Pirttilä, A. M., Compant, S., Campisano, A., et al. (2015). The hidden world within plants: ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 79 (3), 293–320. doi: 10.1128/MMBR.00050-14
Huang, X., Ren, J., Li, P., Feng, S., Dong, P., Ren, M. (2021). Potential of microbial endophytes to enhance the resistance to postharvest diseases of fruit and vegetables. J. Sci. Food Agric. 101 (5), 1744–1757. doi: 10.1002/jsfa.10829
Huyskens-Keil, S., Herppich, W. B. (2013). High CO2 effects on postharvest biochemical and textural properties of white asparagus (Asparagus officinalis l.) spears. Postharvest Biol. Technol. 75, 45–53. doi: 10.1016/j.postharvbio.2012.06.017
Jacob, J., Krishnan, G. V., Thankappan, D., Bhaskaran, N.S.A.D. K. (2020). Endophytic bacterial strains induced systemic resistance in agriculturally important crop plants. Microbial Endophytes, 75–105. doi: 10.1016/B978-0-12-819654-0.00004-1
Jakubiec-Krzesniak, K., Rajnisz-Mateusiak, A., Guspiel, A., Ziemska, J., Solecka, J. (2018). Secondary metabolites of actinomycetes and their antibacterial, antifungal and antiviral properties. Pol. J. Microbiol. 67 (3), 259–272. doi: 10.21307/pjm-2018-048
Jasim, B., Sreelakshmi, K. S., Mathew, J., Radhakrishnan, E. K. (2016). Surfactin, iturin, and fengycin biosynthesis by endophytic bacillus sp. from Bacopa monnieri. Microb. Ecol. 72 (1), 106–119. doi: 10.1007/s00248-016-0753-5
Jiang, L., Jeong, J. C., Lee, J. S., Park, J. M., Yang, J. W., Lee, M. H., et al. (2019). Potential of pantoea dispersa as an effective biocontrol agent for black rot in sweet potato. Sci. Rep. 9 (1), 1–13. doi: 10.1038/s41598-019-52804-3
Ji, X., Lu, G., Gai, Y., Zheng, C., Mu, Z. (2008). Biological control against bacterial wilt and colonization of mulberry by an endophytic bacillus subtilis strain. FEMS Microbiol. Ecol. 65 (3), 565–573. doi: 10.1111/j.1574-6941.2008.00543.x
Kaewkla, O., Franco, C. M. M. (2021). Genome mining and description of streptomyces albidus sp Nov., an endophytic actinobacterium with antibacterial potential. Antonie van Leeuwenhoek 114 (5), 539–551. doi: 10.1007/s10482-021-01539-1.
Kamat, S., Kumari, M., Jayabaskaran, C. (2020). Endophytic fungus, Chaetomium globosum, associated with marine green alga, a new source of chrysin. Sci. Rep. 10, 18726. doi: 10.1038/s41598-020-72497-3
Kandel, S. L., Joubert, P. M., Doty, S. L. (2017). Bacterial endophyte colonization and distribution within plants. Microorganisms 5 (4), 77. doi: 10.3390/microorganisms5040077
Kapat, A., Zimand, G., Elad, Y. (1998). Effect of two isolates ofTrichoderma harzianumon the activity of hydrolytic enzymes produced byBotrytis cinerea. Physiol. Mol. Plant Pathol. 52 (2), 127–137. doi: 10.1006/pmpp.1997.0140
Katoch, M., Singh, D., Kapoor, K. K., Vishwakarma, R.A. (2019). Trichoderma lixii (IIIM-B4), an endophyte of Bacopa monnieri l. producing peptaibols. BMC Microbiol. 19, 98. doi: 10.1186/s12866-019-1477-8
Keswani, C., Prakash, O., Bharti, N., Vílchez, J. I., Sansinenea, E., Lally, R. D., et al. (2019). Re-addressing the biosafety issues of plant growth promoting rhizobacteria. Sci. Total Environ. 10, 841–852. doi: 10.1016/j.scitotenv.2019.07.046
Khalaf, E. M., Raizada, M. N. (2018). Bacterial seed endophytes of domesticated cucurbits antagonize fungal and oomycete pathogens including powdery mildew. Front. Microbiol 9, 42. doi: 10.3389/fmicb.2018.00042
Khalaf, E. M., Raizada, M. N. (2020). Draft genome sequences of bacillus and paenibacillus species isolated from seeds of citrullus lanata (watermelon), cucurbita moschata (butternut squash), and cucurbita pepo l. var. pepo l.(pumpkin). Microbiol. Resour. Announc. 9 (34), e00727–e00720. doi: 10.1128/MRA.00727-20
Khan, R. A. A., Najeeb, S., Hussain, S., Xie, B., Li, Y. (2020). Bioactive secondary metabolites from trichoderma spp. against phytopathogenic fungi. Microorganisms 8 (6), 817. doi: 10.3390/microorganisms8060817
Khare, E., Mishra, J., Arora, N. K. (2018). Multifaceted interactions between endophytes and plant: developments and prospects. Front. Microbiol. 9, 2732. doi: 10.3389/fmicb.2018.02732
Khruengsai, S., Pripdeevech, P., Tanapichatsakul, C., Srisuwannapa, C., D’Souza, P. E., Panuwet, P. (2021). Antifungal properties of volatile organic compounds produced by daldinia eschscholtzii MFLUCC 19-0493 isolated from barleria prionitis leaves against colletotrichum acutatum and its post-harvest infections on strawberry fruits. Peer J. 9, e11242. doi: 10.7717/peerj.11242
Knights, H. E., Jorrin, B., Haskett, T. L., Poole, P. S. (2021). Deciphering bacterial mechanisms of root colonization. Environ. Microbiol. Rep. 13 (4), 428–444. doi: 10.1111/1758-2229.12934
Kuffner, M., Hai, B., Rattei, T., Melodelima, C., Schloter, M., Zechmeister-Boltenstern, S., et al. (2012). Effects of season and experimental warming on the bacterial community in a temperate mountain forest soil assessed by 16S rRNA gene pyrosequencing. FEMS Microbiol. Ecol. 82, 551–562. doi: 10.1111/j.1574-6941.2012.01420.x
Kumar, A., Droby, S., Singh, V. K., Singh, S. K., White, J. F. (2020). “Entry, colonization, and distribution of endophytic microorganisms in plants,” in Microbial endophytes (Cambridge, MA, USA: Woodhead Publishing), 1–33. doi: 10.1016/B978-0-12-819654-0.00001-6
Kumari, M., Kamat, S., Dixit, R., Pandey, S., Giri, V. P., Mishra, A. (2020a). “Microbial formulation approaches in postharvest disease management,” In Food Security and Plant Disease Management Eds. , A, K., Dobry, S. (Cambridge, UK: Woodhead Publishing), 279–305.
Kumari, M., Pandey, S., Mishra, S. K., Giri, V. P., Agarwal, L., Dwivedi, et al. (2020b). Omics-based mechanistic insight into the role of bioengineered nanoparticles for biotic stress amelioration by modulating plant metabolic pathways. Front. Bioeng. Biotechnol. 17, 242. doi: 10.3389/fbioe.2020.00242
Kumari, M., Taritla, S., Sharma, S., Jayabaskaran, C. (2018). Antiproliferative and antioxidative bioactive compounds in extracts of marine-derived endophytic fungus Talaromyces purpureogenus. front. Microbiol 9, 1777. doi: 10.3389/fmicb.2018.01777
Kumar, D., Kalita, P. (2017). Reducing postharvest losses during storage of grain crops to strengthen food security in developing countries. Foods. 6 (1), 8. doi: 10.3390/foods6010008
Kumar, A., Singh, R., Yadav, A., Giri, D. D., Singh, P. K., Pandey, K. D. (2016). Isolation and characterization of bacterial endophytes of curcuma longa l. 3. Biotech 6 (1), 60. doi: 10.1007/s13205-016-0393-y
Kumar, A., Zhimo, Y., Biasi, A., Salim, S., Feygenberg, O., Wisniewski, M., et al. (2021). “Endophytic microbiome in the carposphere and its importance in fruit physiology and pathology,” in Postharvest pathology: Plant pathology in the 21st century, vol. 11 . Eds. Spadaro, D., Droby, S., Gullino, M. L. (Schwitzerland: Springer: Cham), 73–88.
Kusari, P., Kusari, S., Spiteller, M., Kayser, O. (2015). Implications of endophyte-plant crosstalk in light of quorum responses for plant biotechnology. Appl. Microbiol. Biot 99 (13), 5383–5390. doi: 10.1007/s00253-015-6660-8
Lacava, P. T., Azevedo, J. L. (2014). “Biological control of insect-pest and diseases by endophytes,” in Advances in endophytic research (New Delhi: Springer), 231–256.
Lacava, P. T., Azevedo, J. L. (2013). Endophytic bacteria: A biotechnological potential in agrobiology system. In Bacteria agrobiology: Crop productivity (Berlin, Heidelberg) pp, 1–44). doi: 10.1007/978-3-642-37241-4_1
Lai, K., Chen, S., Hu, M., Hu, Q., Geng, P., Weng, Q., et al. (2012). Control of postharvest green mold of citrus fruit by application of endophytic Paenibacillus polymyxa strain SG-6. Postharvest Biol. Tech. 69, 40–48. doi: 10.1016/j.postharvbio.2012.03.001
Lappa, I. K., Mparampouti, S., Lanza, B., Panagou, E. Z. (2018). Control of Aspergillus carbonarius in grape berries by Lactobacillus plantarum: A phenotypic and gene transcription study. Int. J. Food Microbiol. 275, 56–65. doi: 10.1016/j.ijfoodmicro.2018.04.001
Laskowski, R. A., MacArthur, M. W., Moss, D. S., Thornton, J. M. (1993). PROCHECK - a program to check the stereochemical quality of protein structures. J. App. Cryst. 26, 283–291. doi: 10.1107/S0021889892009944
Lastochkina, O., Baymiev, A., Shayahmetova, A., Garshina, D., Koryakov, I., Irina Shpirnaya, I., et al. (2020). Effects of endophytic Bacillus subtilis and salicylic acid on postharvest diseases (Phytophthora infestans, fusarium oxysporum) development in stored potato tubers. Plants 9, 76. doi: 10.3390/plants9010076
Lazazzara, V., Perazzolli, M., Pertot, I., Biasioli, F., Puopolo, G., Cappellin, L. (2017). Growth media affect the volatilome and antimicrobial activity against phytophthora infestans in four lysobacter type strains. Microbiol. Res. 201, 52–62. doi: 10.1016/j.micres.2017.04.015
Lecomte, C., Alabouvette, C., Edel-Hermann, V., Robert, F., Steinberg Adame-Álvarez, C. (2016). Biological control of ornamental plant diseases caused by fusarium oxysporum: a review. Biol. Control. 101, 17–30. doi: 10.1016/j.biocontrol.2016.06.004
Liu, H., Carvalhais, L. C., Crawford, M., Singh, E., Dennis, P. G., Pieterse, C. M. J., et al. (2017). Inner plant values: Diversity, colonization and benefits from endophytic bacteria. Front. Microbiol. 8, 2552. doi: 10.3389/fmicb.2017.02552
Liu, C., Wang, X., Zhao, J., Liu, Q., Wang, L., Guan, X., et al. (2013). Streptomycesharbinensis sp. nov., an endophytic, ikarugamycin-producing actinomycete isolated from soybean root [Glycine max (L.) merr.]. Int. J. Syst. Evol. 63 (10), 3579–3584. doi: 10.1099/ijs.0.050088-0
Li, Y., Xia, M., He, P., Yang, Q., Wu, Y., He, P., et al. (2022). Developing penicillium digitatum management strategies on post-harvest citrus fruits with metabolic components and colonization of Bacillus subtilis L1-21. J. Fungi 8 (1), 80. doi: 10.3390/jof8010080
Li, X. J., Zhang, Q., Zhang, A. L., Gao, J. M. (2012). Metabolites from Aspergillus fumigatus, an endophytic fungus associated with melia azedarach, and their antifungal, antifeedant, and toxic activities. J. Agric. Food Chem. 60, 3424–3431.
Lodewyckx, C., Vangronsfeld, J., Portcous, F., Moore, E. R. B., Taghavi, S., Mergeay, M., et al. (2002). Endophytic bacteria and their potential applications. Crit. Rev. Plant Sci. 21, 583–606. doi: 10.1080/0735-260291044377
Louarn, S., Nawrocki, A., Thorup-Kristensen, K., Lund, O. S., Jensen, O. N., Collinge, D., et al. (2013). Proteomic changes and endophytic micromycota during storage of organically and conventionally grown carrots. Postharvest Biol. Technol. 76, 26–33. doi: 10.1016/j.postharvbio.2012.08.011
Macarisin, D., Cohen, L., Eick, A., Rafael, G., Belausov, E., Wisniewski, M., et al. (2007). Penicillium digitatum suppresses production of hydrogen peroxide in host tissue during infection of citrus fruit. Phytopathol 97, 1491–1500. doi: 10.1094/PHYTO-97-11-1491
Madbouly, A. K., Elyousr, K. A. A., Ismail, I. M. (2020). Biocontrol of monilinia fructigena, causal agent of brown rot of apple fruit, by using endophytic yeasts. Biol. Control. 144, 104239. doi: 10.1016/j.biocontrol.2020.104239
Malfanova, N., Lugtenberg, B. J., Berg, G. (2013). Bacterial endophytes: who and where, and what are they doing there. Mol. microbial Ecol. rhizosphere 1, 393–403. doi: 10.1002/9781118297674.ch36
Mari, M., Bautista-Banos, S., Sivakumar, D. (2016). Decay control in the postharvest system: Role of microbial and plant volatile organic compounds. Postharvest Biol. Technol. 122, 70–81. doi: 10.1016/j.postharvbio.2016.04.014
Martin, A., Atares, L., Chiralt, A. (2017a). Improving function of biocontrol agents incorporated in antifungal fruit coatings: a review. Biocon. Sci. Technol. 27, 10. doi: 10.1080/09583157.2017.1390068
Martin, F. M., Uroz, S., Barker, D. G. (2017b). Ancestral alliances: plant mutualistic symbioses with fungi and bacteria. Science 356 (6340), eaad4501. doi: 10.1126/science.aad4501
Martín, J. A., Macaya-Sanz, D., Witzell, J. (2015). Strong in vitro antagonism by elm xylem endophytes is not accompanied by temporally stable in planta protection against a vascular pathogen under field conditions. Eur. J. Plant Pathol. 142, 185–196. doi: 10.1007/s10658-015-0602-2
Matsumoto, A., Takahashi, Y. (2017). Endophytic actinomycetes: promising source of novel bioactive compounds. J. Antibiot. 70, 514–519. doi: 10.1038/ja.2017.20
Medina-Romero, Y. M., Roque-Flores, G., Macías-Rubalcava, M. L. (2017). Volatile organic compounds from endophytic fungi as innovative postharvest control of fusarium oxysporum in cherry tomato fruits. Appl. Microbiol. Biotechnol. 101 (22), 8209–8222. doi: 10.1007/s00253-017-8542-8
Meneses, C., Gonçalves, T., Alquéres, S., Rouws, L., Serrato, R., Vidal, M., et al. (2017). Gluconacetobacter diazotrophicus exopolysaccharide protects bacterial cells against oxidative stress in vitro and during rice plant colonization. Plant Soil 416 (1), 133–147. doi: 10.1007/s11104-017-3201-5
Meneses, C. H., Rouws, L. F., Simões-Araújo, J. L., Vidal, M. S., Baldani, J. I. (2011). Exopolysaccharide production is required for biofilm formation and plant colonization by the nitrogen-fixing endophyte gluconacetobacter diazotrophicus. Mol. Plant Microbe Interact. 24 (12), 1448–1458. doi: 10.1094/MPMI-05-11-0127
Micci, A., Zhang, Q., Chang, X., Kingsley, K., Park, L., Chiaranunt, P., et al. (2022). Histochemical evidence for nitrogen-transfer endosymbiosis in non-photosynthetic cells of leaves and inflorescence bracts of angiosperms. Biology 11, 876. doi: 10.3390/biology11060876
Miethke, M., Marahiel, M. A. (2007). Siderophore-based iron acquisition and pathogen control. Microbiol. Mol. Biol. Rev. 71 (3), 413–451. doi: 10.1128/MMBR.00012-07
Miguel, P. S. B., Delvaux, J. C., Oliveira, M. N. V., Monteiro, L. C. P., Freitas, F. D. S., Costa, M. D., et al. (2013). Diversity of endophytic bacteria in the fruits of coffea canephora. Afr. J. Microbiol. Res. 7, 586–594. doi: 10.5897/AJMR12.2036
Miller, R. A. (2003). “Harvest and handling injury: physiology biochemistry and detection,” in Postharvest physiol. pathol. veg. Eds. Bartz, J. A., Brecht, J. K. (New York, NY, USA: Marcel Dekker), 177–208.
Mocali, S., Bertelli, E., Cello, F. D., Mengoni, A., Sfalanga, A., Viliani, F., et al. (2003). Fluctuation of bacteria isolated from elm tissues during different seasons and from different plant organs. Res. Microbiol. 154, 105–114. doi: 10.1016/S0923-2508(03)00031-7
Monteiro, R. A., Balsanelli, E., Tuleski, T., Faoro, H., Cruz, L. M., Wassem, R., et al. (2012). Genomic comparison of the endophyte herbaspirillum seropedicae SmR1 and the phytopathogen herbaspirillum rubrisubalbicans M1 by suppressive subtractive hybridization and partial genome sequencing. FEMS Microbiol. Ecol. 80 (2), 441–451. doi: 10.1111/j.1574-6941.2012.01309.x
Mookherjee, A., Singh, S., Maiti, M. K. (2017). Quorum sensing inhibitors: can endophytes be prospective sources? Arch. Microbiol. 200 (2), 355–369. doi: 10.1007/s00203-017-1437-3.
Mukherjee, P. K., Horwitz, B. A., Kenerley, C. M. (2012). Secondary metabolism in trichoderma–a genomic perspective. Microbiol 158 (1), 35–45. doi: 10.1099/mic.0.053629-0
Nair, D. N., Padmavathy, S. (2014). Impact of endophytic microorganisms on plants, environment and humans. Sci. World J.,2014 1–12. doi: 10.1155/2014/250693
Naveed, M., Mitter, B., Reichenauer, T. G., Wieczorek, K., Sessitsch, A. (2014). Increased drought stress resilience of maize through endophytic colonization by Burkholderia phytofirmans PsJN and enterobacter sp. FD17. Environ. Exp. Bot. 97, 30–39. doi: 10.1016/j.envexpbot.2013.09.014
Newman, M.-A., Sundelin, T., Nielsen, J. T., Erbs, G. (2013). M.A.M.P. (microbe-associated molecular pattern) triggered immunity in plants. Front. Plant Sci. 4, 139. doi: 10.3389/fpls.2013.00139
Nicolopoulou-Stamati, P., Maipas, S., Kotampasi, C., Stamatis, P., Hens, L. (2016). Chemical pesticides and human health: The urgent need for a new concept in agriculture. Front. Public Health 4, 148. doi: 10.3389/fpubh.2016.00148
Nigora Rustamova, N., Bozorov, K., Efferth, T.n, Yili, A. (2020). Novel secondary metabolites from endophytic fungi: Synthesis and biological properties. Phytochem. Rev. 19, 425–448. doi: 10.1007/s11101-020-09672-x
Noumeur, S. R., Mancini, V., Romanazzi, G. (2015). “Activity of endophytic fungi from artemisia absinthium on botrytis cinerea,” in III international symposium on postharvest pathology: Using science to increase food availability, vol. 1144. , 101–104. doi: 10.17660/ActaHortic.2016.1144.14
Nuankeaw, K., Chaiyosang, B., Suebrasri, T., Kanokmedhakul, S., Lumyong, S., Boonlue, S. (2020). First report of secondary metabolites, violaceol I and violaceol II produced by endophytic fungus, trichoderma polyalthiae and their antimicrobial activity. Mycosci 61, 16–21. doi: 10.1016/j.myc.2019.10.001
O’Brien, P. A. (2017). Biological control of plant diseases. Australas. Plant Pathol. 46 (4), 293–304. doi: 10.1007/s13313-017-0481-4
Oku, S., Komatsu, A., Tajima, T., Nakashimada, Y., Kato, J. (2012). Identification of chemotaxis sensory proteins for amino acids in pseudomonas fluorescens Pf0-1 and their involvement in chemotaxis to tomato root exudate and root colonization. Microbes Environ. 27 (4), ME12005. doi: 10.1264/jsme2.ME12005
Oliveira, M. N., Santos, T. M., Vale, H. M., Delvaux, J. C., Cordero, A. P., Ferreira, A. B., et al. (2013). Endophytic microbial diversity in coffee cherries of Coffea arabica from southeastern Brazil. Can. J. Microbiol. 59, 221–230. doi: 10.1139/cjm-2012-0674
Ottesen, A. R., Peña, A. G., White, J. R., Pettengill, J. B., Li, C., Allard, S., et al. (2013). Baseline survey of the anatomical microbial ecology of an important food plant: Solanum lycopersicum (tomato). BMC Microbiol. 13 (1), 114. doi: 10.1186/1471-2180-13-114
Omar, S. A., Abd-Alla, M. H. (2021). Biocontrol of fungal root rot diseases of crop plants by the use of rhizobia and bradyrhizobia. Folia Microbiol. 43 (4), 431–437.
Pacifico, D., Squartini, A., Crucitti, D., Barizza, E., Lo Schiavo, F., Muresu, R., et al. (2019). The role of the endophytic microbiome in the grapevine response to environmental triggers. Front. Plant Sci. 10, 1256. doi: 10.3389/fpls.2019.01256
Pandey, S., Giri, V. P., Tripathi, A., Kumari, M., Narayan, S., Bhattacharya, A., et al. (2020). Early blight disease management by herbal nanoemulsion in Solanum lycopersicum with bio-protective manner. Indust. Crop Products. 150, 112421. doi: 10.1016/j.indcrop.2020.112421
Pang, L., Xia, B., Liu, X., Yi, Y., Jiang, L., Chen, C., et al. (2021). Improvement of antifungal activity of a culture filtrate of endophytic bacillus amyloliquefaciens isolated from kiwifruit and its effect on postharvest quality of kiwifruit. J. Food Biochem. 45 (1), e13551. doi: 10.1111/jfbc.13551
Papik, J., Folkmanova, M., Polivkova, M., Suman, J., Uhlik, O. (2020). The invisible life inside plants: Deciphering the riddles of endophytic bacterial diversity. Biotechnol. Adv. 44, 107614. doi: 10.1016/j.biotechadv.2020.107614
Parmar, S., Li, Q., Wu, Y., Li, X., Yan, J., Sharma, V. K., et al. (2018). Endophytic fungal community of dysphania ambrosioides from two heavy metal-contaminated sites: evaluated by culture-dependent and culture-independent approaches. Microbial Biotechnol. 11 (6), 1170–1183. doi: 10.1111/1751-7915.13308
Pathak, P., Rai, V. K., Can, H., Singh, S. K., Kumar, D., Bhardwaj, N., et al. (2022). Plant-endophyte interaction during biotic stress management. Plants 11 (17), 2203. doi: 10.3390/plants11172203
Pettersson, M., Bååth, E. (2003). Temperature-dependent changes in the soil bacterial community in limed and unlimed soil. FEMS Microbiol. Ecol. 45 (1), 13–21. doi: 10.1016/S0168-6496(03)00106-5
Petrini, O. (1991). Fungal endophytes of tree leaves. In Microbial Ecol. Leaves; Andrews J. H. Hirano S. S. Eds. SpringerVerlag: New York NY pp, 179–197.
Pimentel, M. R., Molina, G., Dionísio, A. P., Maróstica, J. M. R., Pastore, G. M. (2011). The use of endophytes to obtain bioactive compounds and their application in biotransformation process. Biotechnol. Res. Int. 1–11. doi: 10.4061/2011/576286.
Pinski, A., Betekhtin, A., Hupert-Kocurek, K., Mur, L. A. J., Hasterok, R. (2019). Defining the genetic basis of Plant⁻Endophytic bacteria interactions. Int. J. Mol. Sci. 20 (8), 1947. doi: 10.3390/ijms20081947.
Pinto, C., Pinho, D., Sousa, S., Pinheiro, M., Egas, C., Gomes, A. C. (2014). Unravelling the diversity of grapevine microbiome. PloS One 9, e85622. doi: 10.1371/journal.pone.0085622
Plett, J. M., Martin, F. M. (2018). Know your enemy, embrace your friend: using omics to understand how plants respond differently to pathogenic and mutualistic microorganisms. Plant J. 93 (4), 729–746. doi: 10.1111/tpj.13802
Pratella, G. C., Mari, M., Guizzardi, M., Folchi, A. (1993). Preliminary studies on the efficiency of endophytes in the biological control of the postharvest pathogens monilinia laxa and rhizopus stolonifer in stone fruit. Postharvest Biol. Technol. 3 (4), 361–368. doi: 10.1016/0925-5214(93)90016-V
Pratiwi, R. H., Hidayat, I., Hanafi, M., Mangunwardoyo, W. (2017). Antibacterial compound produced by Pseudomonas aeruginosa strain UICC b-40, an endophytic bacterium isolated from Neesia altissima. J. Microbiol. 55, 289–295. doi: 10.1007/s12275-017-6311-0
Punjia, Z. K., Rodriguez, G., Tirajoh, A. (2016). Effects of Bacillus subtilis strain QST 713 and storage temperatures on postharvest disease development on greenhouse tomatoes. Crop Prot. 84, 98–104. doi: 10.1016/j.cropro.2016.02.011
Pusey, P. L., Hotchkiss, M. W., Dulmage, H. T., Banumgardner, R. A., Zehr, E. I. (1988). Pilot tests for commercial production and application of Bacillus subtilis (B-3) for postharvest control of peach brown rot. Plant Dis. 72, 622–626. doi: 10.1094/PD-72-0622
Rajani, P., Rajasekaran, C., Vasanthakumari, M. M., Olsson, S. B., Ravikanth, G., Uma Shaanker, R. (2021). Inhibition of plant pathogenic fungi by endophytic trichoderma spp. through mycoparasitism and volatile organic compounds. Microbiol. Res. 242, 126595. doi: 10.1016/j.micres.2020.126595
Rajesh, P. S., Rai, V. R. (2013). Hydrolytic enzymes and quorum sensing inhibitors from endophytic fungi of Ventilago madraspatana gaertn. Biocatal. Agric. Biotechnol. 2 (2), 120–124. doi: 10.1016/j.bcab.2013.01.002
Reinhold-Hurek, B., Maes, T., Gemmer, S., Van Montagu, M., Hurek, T. (2006). An endoglucanase is involved in infection of rice roots by the not-cellulose-metabolizing endophyte azoarcus sp. strain BH72 19 (2), 181–188. doi: 10.1094/MPMI-19-0181
Ren, F., Dong, W., Sun, H., Yan, D. H. (2019b). Endophytic mycobiota of jingbai pear trees in north China. Forests 10 (3), 260. doi: 10.3390/f10030260
Ren, F., Dong, W., Yan, D. H. (2019a). Endophytic bacterial communities of jingbai pear trees in north China analyzed with illumina sequencing of 16S rDNA. Arch. Microbiol. 201 (2), 199–208. doi: 10.1007/s00203-018-1597-9
Ren, F., Dong, W., Yan, D. H. (2019c). Organs, cultivars, soil, and fruit properties affect structure of endophytic mycobiota of pinggu peach trees. Microorganisms 7 (9), 322.4.1. doi: 10.3390/microorganisms7090322
Rosenblueth, M., Martínez-Romero, E. (2006). Bacterial endophytes and their interactions with hosts. Mol. Plant Microbe Interact. 19 (8), 827–837. doi: 10.1094/MPMI-19-0827
Rustamova, N., Bozorov, K., Efferth, T., Yili, A. (2020). Novel secondary metabolites from endophytic fungi: synthesis and biological properties. Phytochem. Rev. 19 (2), 425–448. doi: 10.1007/s11101-020-09672-x
Sah, B., Kumari, M., Subban, K., Jayabaskaran, C. (2020). Evaluation of anticancer activity of enzymatically synthesized baccatin III: An intermediate precursor of taxol®. 3 Biotech. 10 (11), 465. doi: 10.1007/s13205-020-02457-1
Saijo, Y., Loo, E.-i., Yasuda, S. (2018). Pattern recognition receptors and signaling in plant–microbe interactions. Plant J. 93 (4), 592–613. doi: 10.1111/tpj.13808
Santos, S. F. D., Cardoso, R. C. V., Borges, Í.M.P., Almeida, A. C. E., Andrade, E. S., Ferreira, I. O., et al. (2020). Postharvest losses of fruits and vegetables in supply centers in Salvador, Brazil: Analysis of determinants, volumes and reduction strategies. Waste Manage. 101, 161–170. doi: 10.1016/j.wasman.2019.10.007
Sarhan, M. S., Hamza, M. A., Youssef, H. H., Patz, S., Becker, M., ElSawey, H., et al. (2019). Culturomics of the plant prokaryotic microbiome and the dawn of plant-based culture media–a review. J. Adv. Res. 19, 15–27. doi: 10.1016/j.jare.2019.04.002
Sarrocco, S., Vannacci, G. (2018). Preharvest application of beneficial fungi as a strategy to prevent postharvest mycotoxin contamination: A review. Crop Prot. 110, 160–170. doi: 10.1016/j.cropro.2017.11.013
Sauer, K., Camper, A. K. (2001). Characterization of phenotypic changes in Pseudomonas putida in response to surface-associated growth. J. Bacteriol. 183 (22), 6579–6589. doi: 10.1128/JB.183.22.6579-6589.2001
Schena, L., Nigro, F., Pentimone, I., Ligorio, A., Ippolito, A. (2003). Control of postharvest rots of sweet cherries and table grapes with endophytic isolates of aureobasidium pullulans. Postharvest Biol. Technol. 30 (3), 209–220. doi: 10.1016/S0925-5214(03)00111-X
Sengupta, P., Sen, S., Mukherjee, S., Acharya, K. (2020). Postharvest diseases of Indian gooseberry and their management: a review. Int. J. Fruit Sci. 20 (2), 178–190. doi: 10.1080/15538362.2019.1608889
Senthilkumar, M., Anandham, R., Madhaiyan, M., Venkateswaran, V., Sa, T. (2011). “Endophytic bacteria: perspectives and applications in agricultural crop production,” in Bacteria in agrobiology: crop ecosystems (Berlin, Heidelberg: Springer), 61–96.
Shaoying Zhang, S., Wang, Q., Guo, Y., Kang, L., Yu., Y. (2020). Carbon monoxide enhances the resistance of jujube fruit against postharvest alternaria rot. Postharvest Biol. Technol. 168, 111268. doi: 10.1016/j.postharvbio.2020.111268
Sharma, V. K., Kumar, J., Singh, D. K., Mishra, A., Verma, S. K., Gond, S. K., et al. (2017). Induction of cryptic and bioactive metabolites through natural dietary components in an endophytic fungus colletotrichum gloeosporioides (Penz.) sacc. Front. Microbiol. 8, 1126. doi: 10.3389/fmicb.2017.01126
Shehata, M. G., El Sohaimy, S. A., El-Sahn, M. A., Youssef, M. M. (2016). Screening of isolated potential probiotic lactic acid bacteria for cholesterol lowering property and bile salt hydrolase activity. Ann. Agric. Sci. 61 (1), 65–75. doi: 10.1016/j.aoas.2016.03.001
Sheoran, N., Nadakkakath, A. V., Munjal, V., Kundu, A., Subaharan, K., Venugopal, V., et al. (2015). Genetic analysis of plant endophytic pseudomonas putida BP25 and chemo-profiling of its antimicrobial volatile organic compounds. Microbiol.Res 173, 66–78. doi: 10.1016/j.micres.2015.02.001
Shi, Y. W., Kai, L., Chun, L., Zhao, Z. Y., Zhao, S., Tian, C.Y., et al. (2015a). Illumina-based analysis of bacterial diversity related to halophytes salicornia europaea and Sueada aralocaspica. J. Microbiol 53, 678–685. doi: 10.1007/s12275-015-5080-x
Shi, J., Liu, A., Li, X., Chen, W. (2013). Control of Phytophthora nicotianae disease, induction of defense responses and genes expression of papaya fruits treated with Pseudomonas putida MGP1. J. Sci. Food Agric. 93 (3), 568–574. doi: 10.1002/jsfa.5831
Shi, J., Liu, A., Li, X., Feng, S., Chen, W. (2010). Identification of endophytic bacterial strain MGP1 selected from papaya and its biocontrol effects on pathogens infecting harvested papaya fruit. J. Sci. Food Agric. 90 (2), 227–232. doi: 10.1002/jsfa.3798
Shi, J., Liu, A., Li, X., Feng, S., Chen, W. (2011). Inhibitory mechanisms induced by the endophytic bacterium MGY2 in controlling anthracnose of papaya. Biol. Contr. 56, 2–8. doi: 10.1016/j.biocontrol.2010.09.012
Shi, X., Liu, Q., Ma, J., Liao, H., Xiong, X., Zhang, K., et al. (2015b). An acid-stable bacterial laccase identified from the endophyte pantoea ananatis sd-1 genome exhibiting lignin degradation and dye decolorization abilities. Biotechnol. Lett. 37 (11), 2279–2288. doi: 10.1007/s10529-015-1914-1
Shimizu, M., Yazawa, S., Ushijima, Y. (2009). A promising strain of endophytic streptomyces sp. for biological control of cucumber anthracnose. J. Gen. Plant Pathol. 75 (1), 27–36. doi: 10.1007/s10327-008-0138-9
Shi, Y. W., Yang, H. M., Zhang, T., Sun, J., Lou, K. (2014). Illumina-based analysis of endophytic bacterial diversity and space-time dynamics in sugar beet on the north slope of tianshan mountain. Appl. Microbiol. Biotechnol. 98, 6375–6385. doi: 10.1007/s00253-014-5720-9
Shi, Y., Yang, H., Zhang, T., Sun, J., Lou, K. (2014). Illumina-based analysis of endophytic bacterial diversity and space-time dynamics in sugar beet on the north slope of tianshan mountain. Appl. Microbiol.Biotechnol. 98 (14), 6375–6385. doi: 10.1007/s00253-014-5720-9
Shi, C., Yan, P., Li, J., Wu, H., Li, Q. (2014). Guan s. biocontrol of Fusarium graminearum growth and deoxynivalenol production in wheat kernels with bacterial antagonists. Int. J. Environ. Res. Public Health 11, 1094–1105. doi: 10.3390/ijerph110101094
Singh, R., Pandey, K. D., Singh, M., Singh, S. K., Hashem, A., Al-Arjani, A. B. F., et al. (2022). Isolation and characterization of endophytes bacterial strains of Momordica charantia l. and their possible approach in stress management. Microorganisms 10 (2), 290. doi: 10.3390/microorganisms10020290
Singh, D. K., Sharma, V. K., Kumar, J., Mishra, A., Verma, S. K., Sieber, T. N., et al. (2017). Diversity of endophytic mycobiota of tropical tree tectona grandis linn. f.: Spatiotemporal and tissue type effects. Sci. Rep. 7 (1), 1–14. doi: 10.1038/s41598-017-03933-0
Snook, M. E., Mitchell, T., Hinton, D. M., Bacon, C. W. (2009). Isolation and characterization of Leu7-surfactin from the endophytic bacterium Bacillus mojavensis RRC 101, a biocontrol agent for Fusarium verticillioides. J Agric. Food Chem. 57 (10), 4287–4292. doi: 10.1021/jf900164h
Soh, S. H., Lee, L. Y. (2019). Microencapsulation and nanoencapsulation using supercritical fluid (scf) techniques. Pharmaceutics 11 (1), 21. doi: 10.3390/pharmaceutics11010021
Sood, M., Kapoor, D., Kumar, V., Sheteiwy, M. S., Ramakrishnan, M., Landi, M., et al. (2020). Trichoderma: the “secrets” of a multitalented biocontrol agent. Plants 9, 762. doi: 10.3390/plants9060762
Spadaro, D., Droby, S. (2016). Development of biocontrol products for postharvest diseases of fruit: The importance of elucidating the mechanisms of action of yeast antagonists. Trends Food Sci. Technol. 47, 39–49. doi: 10.1016/j.tifs.2015.11.003
Stinson, M., Ezra, D., Hess, W. M., Sears, J., Strobel, G. (2003). An endophytic gliocladium sp. of eucryphia cordifolia producing selective volatile antimicrobial compounds. Plant Sci. 165 (4), 913–922. doi: 10.1016/S0168-9452(03)00299-1
Stocco, A. F., Diaz, M. E., Romera, M. R., Mercado, L. A., Rivero, M. L., Ponsone, M. L. (2019). Biocontrol of postharvest alternaria decay in table grapes from Mendoza province. Biol. Control. 134, 114–122. doi: 10.1016/j.biocontrol.2019.03.019
Sturz, A. V., Christie, B. R. (1996). Endophytic bacteria of red clover as causal agents of allelopathic clover–maize syndromes. Soil Biol. Biochem. 28, 583–588. doi: 10.1016/0038-0717(95)00168-9
Sturz, A. V., Christie, B. R., Nowak, J. (2010). Bacterial endophytes: Potential role in developing sustainable systems of crop production. Crit. Rev. Plant Sci. 19, 1–30. doi: 10.1080/07352680091139169
Suárez-Moreno, Z. R., Devescovi, G., Myers, M., Hallack, L., Mendonça-Previato, L., Caballero-Mellado, J., et al. (2010). Commonalities and differences in regulation of n-acyl homoserine lactone quorum sensing in the beneficial plant-associated Burkholderia species cluster. Appl. Environ. Microbiol. 76 (13), 4302–4317. doi: 10.1128/AEM.03086-09
Suhandono, S., Kusumawardhani, M. K., Aditiawati, P. (2016). Isolation and molecular identification of endophytic bacteria from rambutan fruits (Nephelium lappaceum l.) cultivar binjai. H.A.Y.A.T.I. J.Biosci. 23 (1), 39–44. doi: 10.1016/j.hjb.2016.01.005
Suwannarach, N., Kumla, J., Bussaban, B., Nuangmek, W., Matsui, K., Lumyong, S. (2013). Biofumigation with the endophytic fungus nodulisporium spp. CMU-UPE34 to control postharvest decay of citrus fruit. Crop Prot. 45, 63–70. doi: 10.1016/j.cropro.2012.11.015
Suwannarach, N., Bussaban, B., Nuangmek, W., Pithakpol, W., Jirawattanakul, B., Matsui, K., et al. (2016). Evaluation of muscodor suthepensis strain CMU-Cib462 as a postharvest biofumigant for tangerine fruit rot caused by penicillium digitatum. J. Sci. Food Agric. 96 (1), 339–345. doi: 10.1002/jsfa.7099
Tadych, M., Bergen, M. S., Johnson-Cicalese, J., Polashock, J. J., Vorsa, N., White, J. F. (2012). Endophytic and pathogenic fungi of developing cranberry ovaries from flower to mature fruit: diversity and succession. Fungal Diversity 54 (1), 101–116. doi: 10.1007/s13225-012-0160-2
Taritla, S., Kumari, M., Kamat, S., Bhat, S. G., Jayabaskaran, C. (2021). Optimization of physico-chemical parameters for production of cytotoxic secondary metabolites and apoptosis induction in the culture extract of a marine algal-derived endophytic fungus aspergillus sp. Front. Pharmacol. 12, 542891. doi: 10.3389/fphar.2021.542891
Tayung, K., Barik, B. P., Jha, D. K. (2010). Antifungal activity and biocontrol potential of metabolite produced by an endophytic fusarium (MTCC-9622) against some postharvest pathogens. J.Agri. Technol. 6 (3), 409–419.
Toure, Y., Ongena, M. A. R. C., Jacques, P., Guiro, A., Thonart, P. (2004). Role of lipopeptides produced by bacillus subtilis GA1 in the reduction of grey mould disease caused by botrytis cinerea on apple. J. Appl. Microbiol. 96, 1151–1160. doi: 10.1111/j.1365-2672.2004.02252.x
Trdá, L., Fernandez, O., Boutrot, F., Héloir, M. C., Kelloniemi, J., Daire, X., et al. (2014). The grapevine flagellin receptor VvFLS2 differentially recognizes flagellin-derived epitopes from the endophytic growth-promoting bacterium burkholderia phytofirmans and plant pathogenic bacteria. New Phytol. 201, 1371–1384. doi: 10.1111/nph.12592
Trikurnia, B., Raymond, G., Tjandrawinata, R. (2016). Production of secondary metabolite E2.2 from Phaleria macrocarpa endophytic fungus. Asian Pacific J. Trop. Biomed. 6, 881–885. doi: 10.1016/j.apjtb.2016.01.005
Verma, H., Kumar, D., Kumar, V., Kumari, M., Singh, S. K., Sharma, V. K., et al. (2021). The potential application of endophytes in management of stress from drought and salinity in crop plants. Microorganisms 9, 1729. doi: 10.3390/microorganisms9081729
Wang, G. H., Xu, Y. X., Jin, J., Liu, J. D., Zhang, Q. Y., Liu, X. (2009). Effect of soil type and soybean genotype on fungal community in soybean rhizosphere during reproductive growth stages. Plant Soil 317, 135–144. doi: 10.1007/s11104-008-9794-y
Wei, Q., Bai, J., Yan, D., Bao, X., Li, W., Liu, B., et al. (2021). Genome mining combined metabolic shunting and OSMAC strategy of an endophytic fungus leads to the production of diverse natural products. Acta Pharm. Sin. B 11 (2), 572–587. doi: 10.1016/j.apsb.2020.07.020
White, J. F., Chang, X., Kingsley, K. L., Zhang, Q., Chiaranunt, P., Micci, A., et al. (2021). Endophytic bacteria in grass crop growth promotion and biostimulation. Grass Res. 1, 5. doi: 10.48130/GR-2021-0005
White, J. F., Kingsley, K. L., Zhang, Q., Verma, R., Obi, N., Dvinskikh, S., et al. (2019). Review: Endophytic microbes and their potential applications in crop management. Pest. Manage. Sci. 75, 2558–2565. doi: 10.1002/ps.5527
Xu, Y., Wang, L., Liang, W., Liu, M. (2021). Biocontrol potential of endophytic bacillus velezensis strain QSE-21 against postharvest grey mould of fruit. Biol. Control. 161, 104711. doi: 10.1016/j.biocontrol.2021.104711
Xu, L., Wu, C., Oelmüller, R., Zhang, W. (2018). Role of phytohormones in Piriformospora indica-induced growth promotion and stress tolerance in plants: More questions than answers. Front. Microbiol. 9, 1646. doi: 10.3389/fmicb.2018.01646
Zhang, H., Mahunu, G. K., Castoria, R., Apaliya, M. T., Yang, Q. (2017). Argumentation of biocontrol agents with physical methods against postharvest diseases of fruits and vegetables. Trends Food Sci. Technol. 69, 36–45. doi: 10.1016/j.tifs.2017.08.020
Zhang, X., Wu, F., Gu, N., Yan, X., Wang, K., Dhanasekaran, S., et al. (2020). Postharvest biological control of rhizopus rot and the mechanisms involved in induced disease resistance of peaches by Pichia membranefaciens. Postharvest Biol. Technol. 163, 111146. doi: 10.1016/j.postharvbio.2020.111146
Zheng, H., Mao, Y., Teng, J., Zhu, Q., Ling, J., Zhong, Z. (2015). Flagellar-dependent motility in Mesorhizobium tianshanense is involved in the early stage of plant host interaction: study of an flgE mutant. Curr. Microbiol. 70 (2), 219–227. doi: 10.1007/s00284-014-0701-x
Zhi-Lin, Y., Yi-Cun, C., Bai-Ge, X., Chu-Long, Z. (2012). Current perspectives on the volatile-producing fungal endophytes. Crit. Rev. Biotechnol. 32 (4), 363–373. doi: 10.3109/07388551.2011.651429
Zimand, G., Elad, Y., Chet, I. (1996). Effect of trichoderma harzianum on botrytis cinerea pathogenicity. Phytopathol 86 (11), 1255–1260. doi: 10.1094/Phyto-86-1255
Keywords: endophytes, molecular interactions, biocontrol screening, commercial hurdles, postharvest management, fruits
Citation: Kumari M, Qureshi KA, Jaremko M, White J, Singh SK, Sharma VK, Singh KK, Santoyo G, Puopolo G and Kumar A (2022) Deciphering the role of endophytic microbiome in postharvest diseases management of fruits: Opportunity areas in commercial up-scale production. Front. Plant Sci. 13:1026575. doi: 10.3389/fpls.2022.1026575
Received: 24 August 2022; Accepted: 13 October 2022;
Published: 17 November 2022.
Edited by:
Mamoona Rauf, Abdul Wali Khan University Mardan, PakistanReviewed by:
Julio Cesar Polonio, State University of Maringá, BrazilSangeeta Paul, Indian Agricultural Research Institute (ICAR), India
Walaa K. Mousa, Mansoura University, Egypt
Copyright © 2022 Kumari, Qureshi, Jaremko, White, Singh, Sharma, Singh, Santoyo, Puopolo and Kumar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ajay Kumar, YWpheWt1bWFyX2JodUB5YWhvby5jb20=
†These authors have contributed equally to this work
 Madhuree Kumari
Madhuree Kumari Kamal A. Qureshi
Kamal A. Qureshi Mariusz Jaremko
Mariusz Jaremko James White
James White Sandeep Kumar Singh
Sandeep Kumar Singh Vijay Kumar Sharma
Vijay Kumar Sharma Kshitij Kumar Singh
Kshitij Kumar Singh Gustavo Santoyo
Gustavo Santoyo Gerardo Puopolo
Gerardo Puopolo Ajay Kumar
Ajay Kumar