- 1College of Pharmacy, Guangxi University of Chinese Medicine, Nanning, China
- 2College of Horticulture, Hunan Agricultural University, Changsha, China
- 3College of Agriculture, Guangxi University, Nanning, China
The growth of Panax notoginseng (Burk.) F. H. Chen is frequently hindered due to replanting failure. In the present study, the objective is to determine whether root exudates from P. notoginseng have autotoxicity and identification of allelochemicals from root exudates or rhizosphere soil. We investigated autotoxicity in P. notoginseng using seedling emergence bioassays and hydroponic culture. The allelochemicals in the soils and root exudates were identified with GC-MS, and the autotoxicity of the identified key allelochemicals was investigated by bioassay. The results showed that the root exudates, and extracts from consecutively cultivated soils also showed significant autotoxicity against seedling emergence and growth. In the non-renewed culture solution without activated charcoal (AC), the fresh and dry mass of P. notoginseng tubers of roots was reduced by about half compared to the addition with AC. A total of 44 different components from all samples were defined by GC-MS analyses. Furthermore, the results of multiple statistical analysis showed a t the difference among cultivated soil, uncultivated soil and root exudates. Bioassay of the identified allelochemicals revealed that benzoic acid, phthalic acid, palmitic acid, and stearic acid significantly affected the root growth of P. notoginseng. These substances at 100 μM more significantly decreased the number of lateral roots. Our results demonstrated that autotoxicity results in replant failure of P. notoginseng.
1 Introduction
Panax notoginseng (Burk.) F. H. Chen is a valuable medicinal material in China. In China, P. notoginseng has more than 400 years of artificial cultivation (Qiao et al., 2018). Due to its unique geographical environment, P. notoginseng is mainly distributed in Yunnan and Guangxi Province, China (Dong et al., 2003). Demand for ginsenosides has rapidly increased due to their significant effects on cancer and cardiovascular disease (Xu et al., 2018; Liu et al., 2020). However, P. notoginseng on the market mainly relies on artificial cultivation, with limited land resources, production is often hindered by replanting failure, leading to lower yields and other difficulties when reestablishing plants in arable land (Yang et al., 2015; Dong et al., 2018). Generally, rotation is the preferred method to avoid failure of crop replanting (Wang et al., 2022). However, the successful replanting of P. notoginseng replanting requires more than 30 years of rotation (Yang et al., 2018), which limit the production of P. notoginseng.
Many factors can contribute to this problem, including deterioration of soil physicochemical properties, nutrient imbalance, soil-borne diseases, and autotoxicity (Qiao et al., 2020). Currently, the research on the continuous cropping obstacles of P. notoginseng is mostly focused on the treating and preventing pests and diseases. Diseases were caused by many types of soil-borne pathogens established, including fungi, oomycetes, bacteria, nematodes, and viruses (Jin et al., 2019; Fang et al., 2022; Wang et al., 2022). However, even using of specific fungicides, satisfactory results have not been achieved (Liu et al., 2019; Ye et al., 2019). Therefore, continuous cropping obstacles have become a major problem limiting the development of the P. notoginseng industry.
Previously, plant autotoxicity was reported to be one of the main factors causing continuous cropping obstacles (Singh et al., 1999). Autotoxicity is a common phenomenon in natural and agroecosystems, where toxins were released by living or decaying plants into their surroundings, thus lead to inhibition of the growth and development of the same species (Inderjit and Duke, 2003). In crop root exudates or rhizosphere soil, allelochemicals accumulate over years of cultiva, causing nutrient imbalances and microbial dysfunction in the soil, which cause continuous cropping obstacles (Li et al., 2019). The autotoxicity of Panax ginseng and Panax quinquefolius has been reported as a possible factor for transplant failure. (Zhang et al., 2020; Ji et al., 2021). Some phenolic acids in American ginseng’s root exudate and rhizosphere soil have been evaluated as potential allelochemicals (He et al., 2009). Ferulic acid, total saponin, and root extract of P. notoginseng could inhibit the plant’s growth (Yang et al., 2015). The crop can be grown continuously on the same land for years when the inhibitory chemicals are removed. (Gao and Wen, 2016; Xiang et al., 2020). Therefore, it is very important to understand the compositions of these inhibitory chemicals.
Studying on the autotoxicity of P. notoginseng would provide effective guidance for crop sustainable production. The objective of this study was to (i) confirm autotoxicity of root exudates in P. notoginseng, and (ii) identify allelochemicals therein.
2 Materials and methods
2.1 Determination of biological activities of soil extracts
Soil samples were collected in Baise city, Guangxi County (23°34 ′ 11 ″ N, 105°55 ′ 56 ″ E). The rhizosphere soil samples were obtained from fields that had been cultivated with continuous cropping for 1 to 3 years. Soil samples were collected from an adjacent uncultivated land as a control. At each collection site, nine samples were collected randomly and mixed thoroughly before being divided into three. Take 150 g of the air-dried and sieved soil samples, and put them in a triangular flask. After adding 100 mL of distilled water, ultrasonically extract for 30 min. The obtained solution was filtered with filter paper, and the extracts were collected. The above steps were repeated two times for the remaining samples, and all the extracts were mixed. The solvent was removed by vacuum rotary evaporation at 55°C to obtain the extracts. The extracts were dissolved in distilled water, and the volume was made up to 100 mL, and the concentration of 1.5 g/mL (that is, the extract containing 1.5 g of soil sample in 1 mL aqueous solution) was prepared as a stock solution. Two layers of filter paper were placed in Petri dishes (9 cm in diameter), and 5 mL of distilled water or soil extracts were added, respectively, and three replicates were set up for each treatment. Select the same size and disease-free P. notoginseng seeds, these seeds were sterilized with 1% NaClO for 15 min and then washed three times with sterilized water. Twenty seeds were placed in each dish and then put in a constant temperature incubator, cultivated at 25°C without light, ventilated once a day, ensured that the filter paper was wet and determine the germination rate after 30 d (Yang et al., 2015).
2.2 Plant cultivation either with or without AC
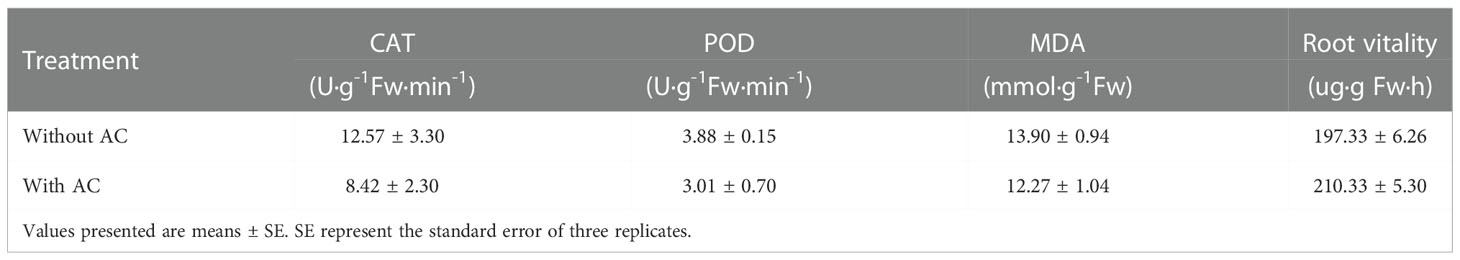
The autotoxicity of P. notoginseng was researched using hydroponics with or without activated charcoal (AC). The P. notoginseng used in the study was collected in Guangxi County (23°34 ′ 11 ″ N, 105°55 ′ 56 ″ E). The plants were transplanted into the seedling pots (50 cm × 25 cm × 15 cm) in a greenhouse of Guangxi University. Twelve plants were grown in each pot, with three replicates per treatment. Each pot was filled with an 8 L nutrient solution, and the nutrient solution was updated every 15 d. The nutrient solution uses the general formula of Hoagland and Arnon (Zandvakili et al., 2019). Each pot has two air pumps with filters for continuous aeration (3.5 L/min), and each filter contains 100 g AC. The control group was treated equally. The AC is used for chemicals secreted from plants, and at the same time, it is replaced with fresh AC for 2 weeks until the end of the experiment. The used AC was either immediately stored at 4°C for later extraction. Relevant agronomic traits were measured at the end of the experiment. Also, three plants from each treatment were taken to test the enzymatic activities of root viability, the enzyme activities of catalase (CAT), peroxidase (POD), and malondialdehyde (MDA) in leaves (Md. Asaduzzaman, 2012).
2.3 GC–MS analysis of root exudates adsorbed in AC and soil
Extracted components in AC or soils samples with reference to previous reports (Md. Asaduzzaman, 2012). One microliter of the concentrated sample was injected into a GC-MS system (Agilent GC7890A/MSD5975C, USA). The GC conditions were as follows: carrier gas, helium; splitless mode; temperature programming, 60°C (1 min), 60-180°C (10°C/min), and 180-280°C (20°C/min). Separation was achieved on a HP-5MS 5% Phenyl Methyl Silox column (30 mm × 0:25 mm × 0:25 μm). Mass spectra were obtained at an electron impact (EI) of 70 eV (Ren et al., 2017).
2.4 Autotoxicity bioassays
In order to further determine the autotoxicity of the precipitated chemical substances. Allelochemical aqueous solutions of different concentrations were prepared with 50% Hoagland nutrient solution (Md. Asaduzzaman, 2012). The selected plants were transplanted to seedling trays, each hole containing one plant, and an equal amount of vermiculite was added to each hole to hold the plants tight and upright. The trays were placed in a greenhouse at 25°C with a light intensity of 2000 Lux and 16 h photoperiod. Every 30 d, 20 mL of test solution was added to each hole, and each treatment was replicated 20 times. The plants were grown for two months and then the plant length, root length, and the number of lateral roots were measured.
2.5 Statistical analysis
The growth and yield data obtained from bioassay and hydroponics of plants were compiled and analyzed for statistical differences among the treatments and means were separated by the analysis of variation with Duncan’s test, LSD test, and t-test using SPSS Statistics 24. The multivariate data (GC-MS) analyses were conducted using MetaboAnalyst 4.0 (www.metaboanalyst.ca). To confirm an overview of clustering separation between different exposure groups, a partial least squares-discriminant analysis (PLS-DA), which is a supervised pattern recognition method, was conducted to distinguish between each treatment group, and R 2 Y and Q 2 parameters were also calculated. The VIP (variable importance in the projection) in PLS-DA was calculated to select metabolites with VIP scores > 1.
3 Results
3.1 Effects of aqueous extract from consecutively cultivated soil on seedling germination and growth
Compared with the control (aqueous extract from the uncultivated soil), the aqueous extract from different consecutively cultivated soils inhibited seed germination and seeding emergence to different degrees (Figure 1). Moreover, the germination rate declined significantly with the increase of successive planting years. The germination rate only reached 21.1% when the seeds were treated with aqueous extract from soil that was continuously cultivated for three years. Three months after added different aqueous extracts from soils in the seeding tray, the seedling survival rate in the control was 99.1%. However, the seeding survival rate treated with the aqueous extracts from soils that were consecutively cultivated for two and three years was reduced significantly to about 20.79% and 28.05%, with control, respectively.
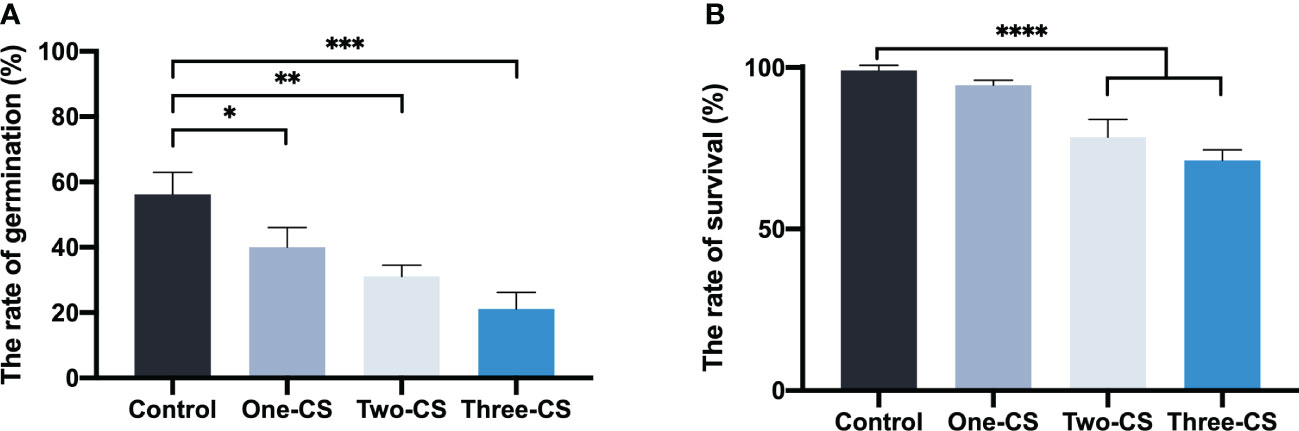
Figure 1 Effects of aqueous extract from consecutively cultivated soil on seedling germination rate (A), and seedling survival rate (B). Control represents the aqueous extract from the uncultivated soil, and One-CS, Two-CS, and Three-CS represent the aqueous extract from one, two, and three years of continuously cultivated soil. Values presented are means. Error bars represent the standard error of three replicates. Asterisks indicate statistically significant differences of treatment compared with control. *, p < 0.05; **, p < 0.01; ***, p < 0.005; ****, p < 0.001, LSD test.
3.2 Growth of P. notoginseng in hydroponics
Culture solutions containing AC had significant effects on the growth of P. notoginseng. The survival rate declined significantly(Log Rank P=0.0352)in the plants grown without AC compared with those grown with AC (Figure 2). After the fourth month of observation, in control (without AC) plants, the plant length, and their fresh and dry mass of aboveground in P. notoginseng were reduced to about 1.57%, 19.35%, and 17.95% of the values compared with AC, respectively. There was a significant difference in fresh and dry mass of roots between plants cultivated with and without AC addition, and the values increased by about 72.6% and 88.5% compared with the control, respectively (Table 1, Figure 3).
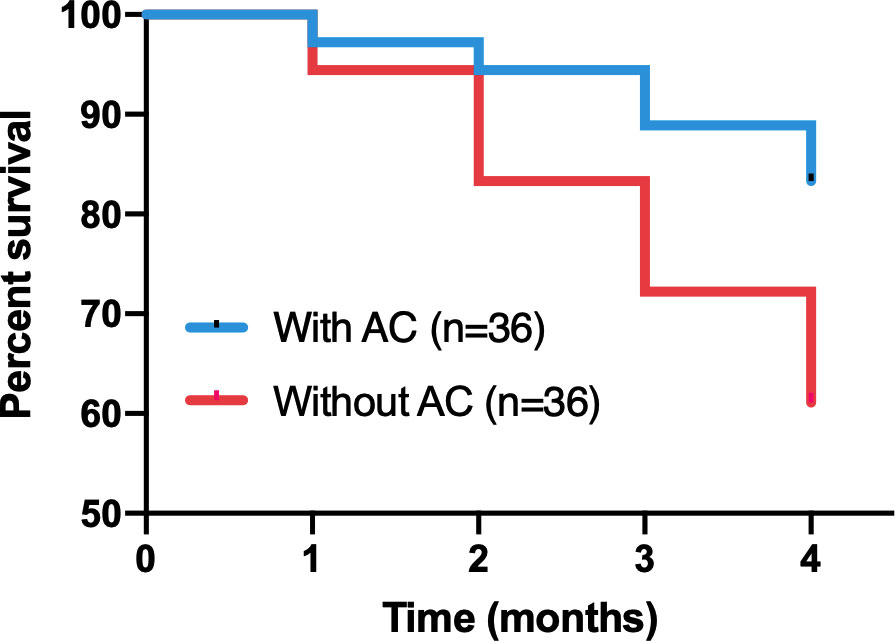
Figure 2 Survival curves of P. notoginseng either with or without AC addition in hydroponics. Through Kaplan-Merier with log-rank test (Prism).
3.3 Effects of root exudates on the physiological indexes of P. notoginseng
The results for antioxidant enzymes (CAT, POD, and MDA) activity and root activity of these plants were shown in Table 2, the activities of CAT, POD, and MDA were 33.02%, 22.42%, and 11.73% lower than those of the control group, and the root activity was 6.59% higher than that of the control group. However, the difference in these values between the two groups was not considered statistically significant (p<0,05).
3.4 Identification of allelochemicals from soils and root exudates
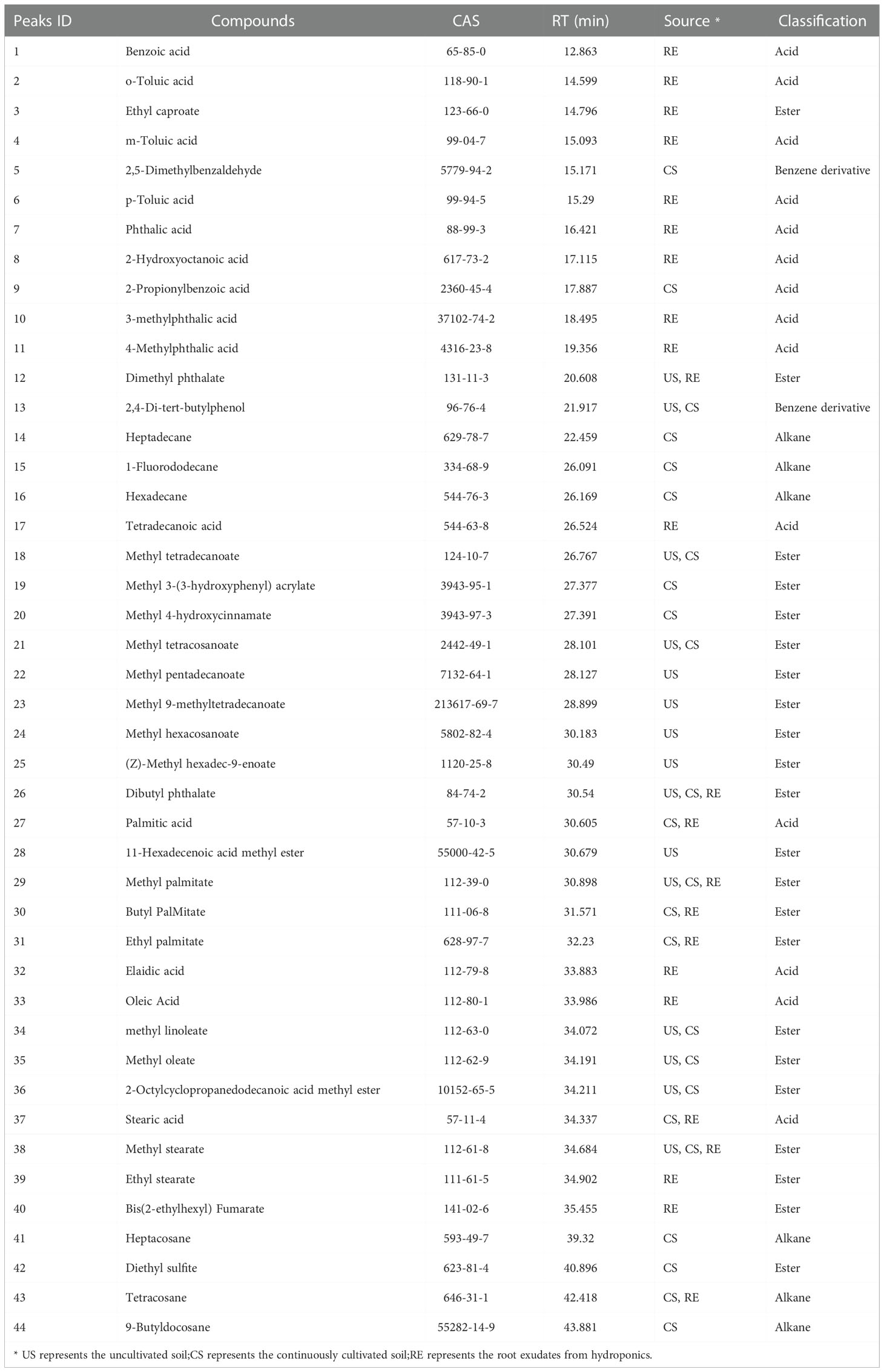
Herein, three rhizosphere soil samples (CS) were obtained from the continuously cultivated soil of P. notoginseng for three years, and three uncultivated soil samples (US) were obtained from the adjacent field. Moreover, three samples (RE) were extracted from exudates of P. notoginseng adsorbed on AC and added to the nutrient solution. A total of 44 different components with more than 80% similarity value were identified from all samples based on the GC-MS analysis (Table 3). The identified components were of four types: 14 acids (32%), 22 esters (50%), 6 alkanes (13%), and 2 benzene derivatives (5%). By comparing the types of compounds in the two soil extracts (Figure 4), continuous cropping soil contains more acid compounds than uncultivated soil. However, RE contains the most types of acids compounds.
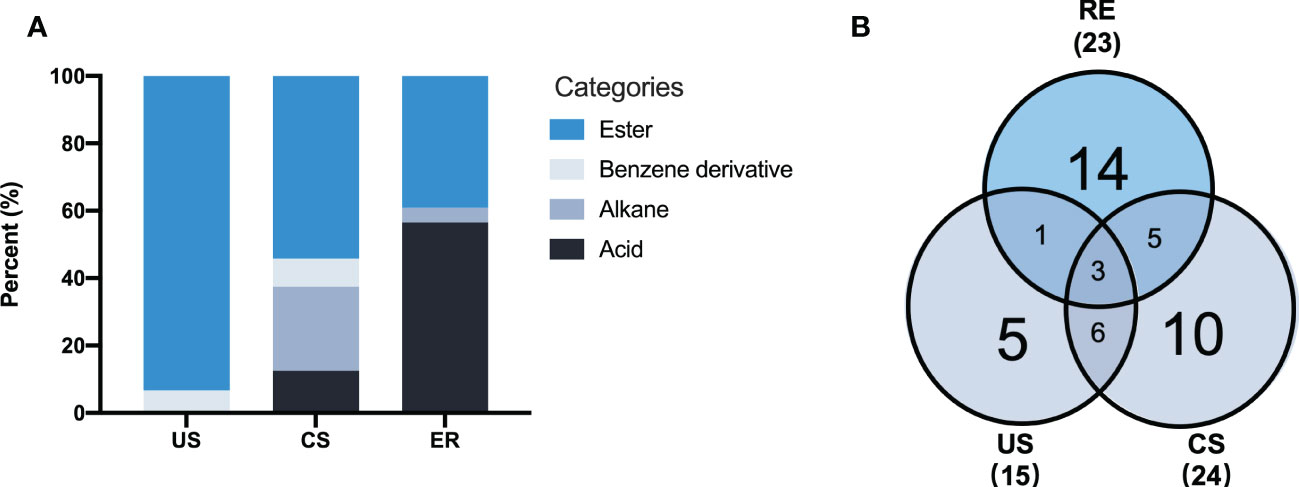
Figure 4 Categories of the metabolites identified from the soils and root exudates by GC-MS analysis (A), The Venn diagram shows the number of metabolites detected in soils and root exudates samples (B). The Numbers in brackets represent the total number of identified metabolites in each group. Additional CS-MS chart can be found in Supplementary Material.
As shown in Figure 5, the RE, CS, and US were clearly separated by PC1 and PC2 in the score plot. Based on the loading plot and the VIP value, 12 compounds, were found to play key roles in the classification. In the CS or RE grouping, the relative concentrations of the four compounds palmitic acid (27), stearic acid (37), benzoic acid (1), and phthalic acid (7) are higher than those in the US, and benzoic acid and phthalic acid are unique substances in the RE samples. Therefore, we selected these 4 compounds for activity testing to test their self-toxic activity against P. notoginseng.
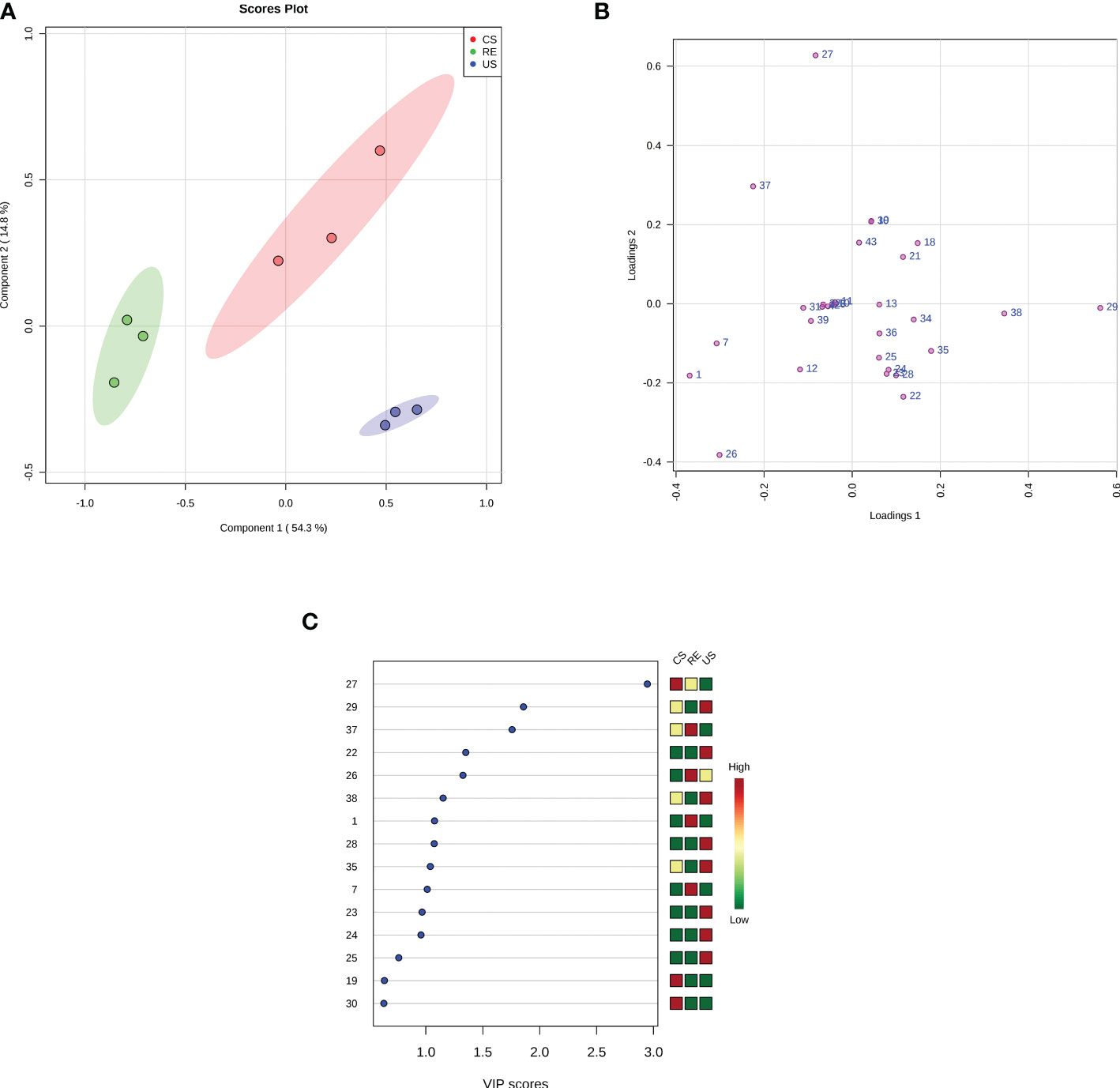
Figure 5 Multivariate analysis using PLS-DA. (A) PLS-DA score plots. The explained variances are shown in brackets; (B) PLS-DA loadings plot. The numbers in the loading plot of all the samples correspond to the Peak ID provided in Table 3; (C) Important features identified by PLS-DA. The colored boxes on the right indicate the relative concentrations of the corresponding metabolite in each group under study. The numbers on the left correspond to the Peak ID provided in Table 3.
3.5 Bioassay with the identified allelochemicals
Seedling growth bioassays were used to evaluate the allelopathic potential of the identified chemicals at different concentrations. The autotoxicity of the allelochemicals was assayed for growth parameters of P. notoginseng at several concentrations (Table 4). The benzoic acid, phthalic acid, palmitic acid, and stearic acid significantly inhibited the growth of P. notoginseng seedlings at a concentration of 1000 μM. The concentration of the test solution is basically positively correlated with inhibition. Benzoic acid at 1000 μM significantly reduced plant length, root length, and the number of lateral roots to 16, 31, and 42% of those of the control, respectively. When P.notoginseng was grown in the nutrient solution containing phthalic acid or palmitic acid, the number of lateral roots was significantly reduced to 24, and 18% compared with those of the control, respectively, even at a low concentration (10 μM). Obviously, these substances more significantly affect the growth of lateral roots. All compounds (at 100 and 1000 μM) decreased the number of lateral roots significantly.
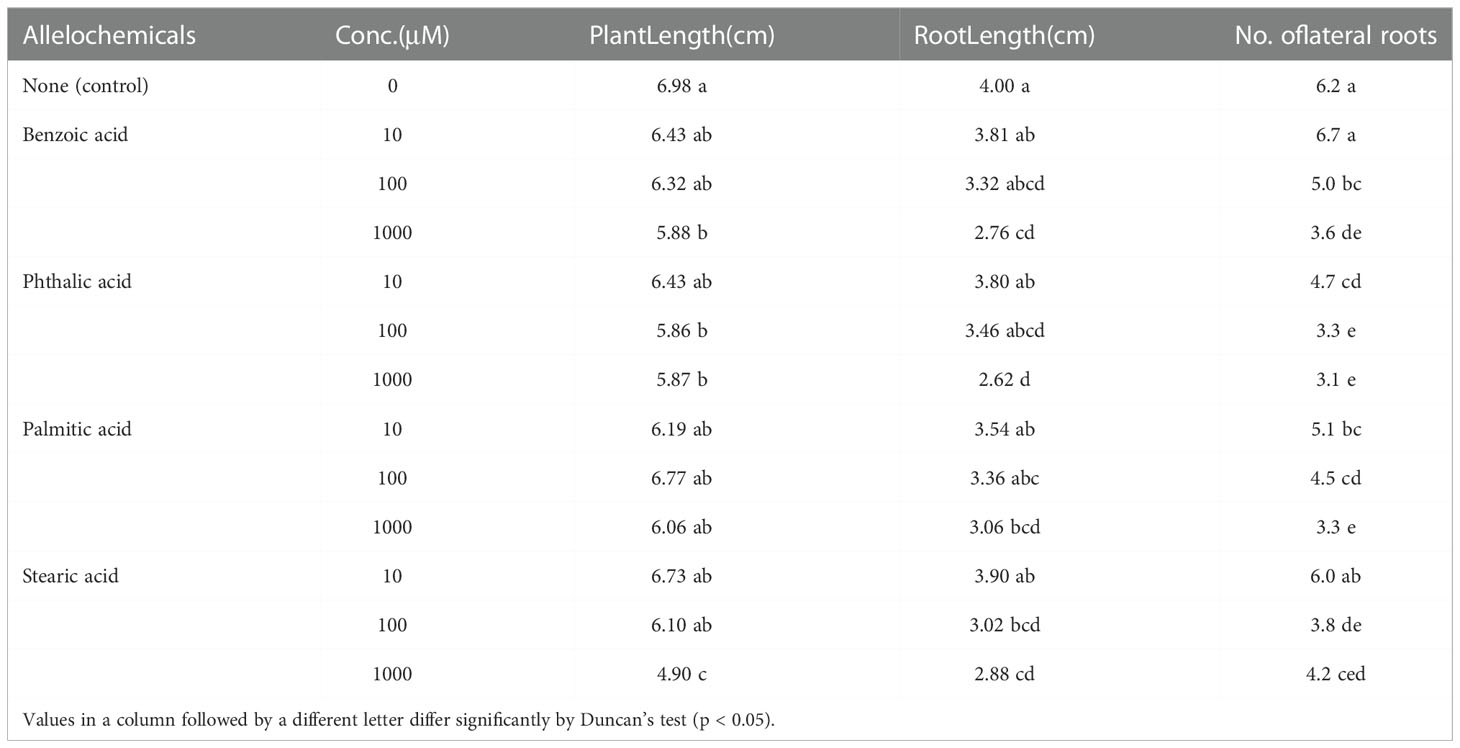
Table 4 Effects of the identified chemicals at different concentrations on the growth of P. notoginseng.
4 Discussion
Our results demonstrated that autotoxicity is a factor in the replant failure of P. notoginseng. Many factors are considered to be responsible for crop succession disorders (Bouhaouel et al., 2019; Yan et al., 2019). Autotoxicity was reported to be one of the main factors causing continuous cropping obstacles (Singh et al., 1999). Our results show that the water extract of continuous cropping soil has autotoxicity on P. notoginseng. Previous studies showed that P. notoginseng had a lower germination rate and index when replanted in heavy cropping soil. The EtOAc extract of P. notoginseng soil showed the most significant inhibition of germination rate, germination index, and root elongation of P. notoginseng seeds (PeiJin et al., 2009). The presence of allelochemicals in the soil affects the growth of plants (Guo et al., 2016; Xin et al., 2019). Besides, the extracts of P. notoginseng soil have been found to have chemosensitizing on other plants, such as cabbage, radish, and lettuce (Liu et al., 2019; Ye et al., 2019). In summary, this may be caused by the accumulation of certain allelochemicals in the soil (He et al., 2019; Meng et al., 2022).
In the hydroponic experiment, the addition of AC to the nutrient solution can significantly increase the weight of the roots of P. notoginseng, indicating that root exudates have a greater impact on the roots. The content of hydrogen peroxide, peroxidase activity, and malondialdehyde in the leaves of P. notoginseng cultured without the addition of activated carbon were all higher than those of the other, but the difference was not significant. It has been reported that allelochemicals can significantly reduce the enzyme activity of hydrogen peroxide and peroxide in tomato leaves (Staszek et al., 2021). In this study, the allelochemicals had no significant effect on the enzyme activity, which may be due to the insufficient concentration of root exudates in the hydroponic environment. However, it is inferred from the overall trend that with the increase in the concentration of root exudates of P. notoginseng, hydrogen peroxide, and peroxide the activity of bio-enzymes may also increase significantly, which can eliminate excessive hydrogen peroxide to protect the cell (Asgher et al., 2021; Zhang et al., 2021). These data demonstrate that the presence of potential autotoxic factors in the soil will affect the growth of P. notoginseng ginseng.Several types of chemicals have been associated with autotoxicity, including terpenoids, phenolics, steroids, alkaloids, and cyanogenic glycosides (Bruce and Richardson, 1988).In recent years, many of studies have proved that organic acids can significantly inhibit the germination of seeds and the growth of seedlings (Asao et al., 2003). Besides, some ginsenosides have been considered that cause the autotoxicity of P. notoginseng (Yang et al., 2015). This study used GC-MS to analyze the key allelochemicals in soil extracts and root exudates. The rhizosphere soil has many organic acids and their derivatives than uncultivated soil. Benzoic acid, phthalic acid, palmitic acid, and stearic acid were found in rhizosphere soil and hydroponic root exudates. These substances are similar to the allelochemicals reported in other plants (Md. Asaduzzaman, 2012; Qiao et al., 2019; Yang et al., 2019). It shows that these substances may also be self-toxic substances with significant self-toxic activity in the root exudates of P. notoginseng. In this study, it can be seen from the ion maps of soil extracts and root exudates that the substance dibutyl phthalate was detected in the three samples, and the relative content was more than 30%. Some researcher believe that this substance belongs to root exudates. Another part of the researchers believes these substances are pollutants (Deng et al., 2017; Ge et al., 2020). Whether dibutyl phthalate is notoginseng root exudates remains to be further verified.
Some identified chemicals showed autotoxicity against seed germination and seedling growth at a certain concentration. The benzoic acid, phthalic acid, palmitic acid, and stearic acid significantly affected the root growth of P. notoginseng. Previous studies have shown that allelochemicals exert autotoxic effects on P. notoginseng seed germination at a concentration of 10-1000μM. Therefore, we chose this concentration for the bioassay. In practice, some allelochemicals are present in lower concentrations and have no effect on plants, but allelopathy occurs (Cheng and Cheng, 2015). This experiment demonstrated the autotoxicity of a single allelochemical, which is in consistent with the actual field conditions. Although this concentration may not be related to natural conditions, our experiment was designed to test the causal relationship. Of course, our work has some limitations, and additional studies are needed to assess the interaction between autotoxicity and other factors of replanting failures, such as soil-borne pathogens, deterioration of soil physicochemical properties, and soil microbial community imbalance. These studies could be used to understand the mechanism of replantation failure of P. notoginseng.
5 Conclusions
The results showed that the growth of P. notoginseng was inhibited by the root exudates, and the AC has the function of adsorbing allelochemicals. The potential allelochemicals were detected as benzoic acid, phthalic acid, salicylic, palmitic acid, and stearic acid. In summary, allelochemicals accumulate in the rhizosphere during the course of root secretion or degradation, etc. and exhibit autotoxicity to P. notoginseng when they reach a certain concentration.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
WX designed the research. WX, JC, FZ, RH and LL performed the experiments and analyzed the data. WX wrote the draft of the manuscript. LL and RH reviewed the draft. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the National Natural Science Foundation of China (Grant No. 81860669), and the Scientific Research Foundation of Guangxi University of Chinese Medicine (2020BS014), and the Inheritance and innovation team of Guangxi Traditional Chinese Medicine (2022B005), and the Scientific Research Foundation of Hunan Education Department (21C0127).
Acknowledgments
The authors express their great thanks to reviewers and editorial staff for their time and attention.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2022.1020626/full#supplementary-material
References
Asaduzzaman, Md. (2012). Autotoxicity in beans and their allelochemicals. Sci. Hortic. 134, 26–31. doi: 10.1016/j.scienta.2011.11.035
Asao, T., Hasegawa, K., Sueda, Y., Tomita, K., Taniguchi, K., Hosoki, T., et al. (2003). Autotoxicity of root exudates from taro. Sci. Hortic. 97, 389–396. doi: 10.1016/S0304-4238(02)00197-8
Asgher, M., Ahmed, S., Sehar, Z., Gautam, H., Gandhi, S. G., Khan, N. A. (2021). Hydrogen peroxide modulates activity and expression of antioxidant enzymes and protects photosynthetic activity from arsenic damage in rice (Oryza sativa l.). J. Hazard. Mater. 401, 123365. doi: 10.1016/j.jhazmat.2020.123365
Bouhaouel, I., Richard, G., Fauconnier, M.-L., Ongena, M., Franzil, L., Gfeller, A., et al. (2019). Identification of barley (Hordeum vulgare l. subsp. vulgare) root exudates allelochemicals, their autoallelopathic activity and against bromus diandrus roth. germination. Agronomy 9, 345. doi: 10.3390/agronomy9070345
Bruce, W. G., Richardson, D. (1988). Bioassays for allelopathy: Measuring treatment responses with independent controls. J. Chem. Ecol. 14, 181–187. doi: 10.1007/BF01022540
Cheng, F., Cheng, Z. (2015). Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Front. Plant Sci. 6. doi: 10.3389/fpls.2015.01020
Deng, J., Zhang, Y., Hu, J., Jiao, J., Hu, F., Li, H., et al. (2017). Autotoxicity of phthalate esters in tobacco root exudates: Effects on seed germination and seedling growth. Pedosphere 27, 1073–1082. doi: 10.1016/S1002-0160(17)60374-6
Dong, T. T. X., Cui, X. M., Song, Z. H., Zhao, K. J., Ji, Z. N., Lo, C. K., et al. (2003). Chemical assessment of roots of panax notoginseng in china: Regional and seasonal variations in its active constituents. J. Agric. Food Chem. 51, 4617–4623. doi: 10.1021/jf034229k
Dong, L., Xu, J., Li, Y., Fang, H., Niu, W., Li, X., et al. (2018). Manipulation of microbial community in the rhizosphere alleviates the replanting issues in panax ginseng. Soil Biol. Biochem. 125, 64–74. doi: 10.1016/j.soilbio.2018.06.028
Fang, W., Liu, X., Song, Z., Jin, X., Yan, D., Wang, Q., et al. (2022). Mechanism of the antifungal action of chloropicrin fumigation against panax notoginseng root rot caused by fusarium solani. Physiol. Mol. Plant Pathol. 121, 101859. doi: 10.1016/j.pmpp.2022.101859
Gao, D.-W., Wen, Z.-D. (2016). Phthalate esters in the environment: A critical review of their occurrence, biodegradation, and removal during wastewater treatment processes. Sci. Total Environ. 541, 986–1001. doi: 10.1016/j.scitotenv.2015.09.148
Ge, J., Cheng, J., Li, Y., Li, Q. X., Yu, X. (2020). Effects of dibutyl phthalate contamination on physiology, phytohormone homeostasis, rhizospheric and endophytic bacterial communities of brassica rapa var. chinensis. Environ. Res. 189, 109953. doi: 10.1016/j.envres.2020.109953
Guo, K., He, X., Yan, Z., Li, X., Ren, X., Pan, L., et al. (2016). Allelochemicals from the rhizosphere soil of cultivated astragalus hoantchy. J. Agric. Food Chem. 64, 3345–3352. doi: 10.1021/acs.jafc.5b06093
He, C. N., Gao, W. W., Yang, J. X., Bi, W., Zhang, X. S., Zhao, Y. J. (2009). Identification of autotoxic compounds from fibrous roots of panax quinquefolium l. Plant Soil 318, 63–72. doi: 10.1007/s11104-008-9817-8
He, H., Zhu, W., Noor, I., Liu, J., Li, G. (2019). Pseudomonas putida WH-B3 degrades benzoic acid and alleviates its autotoxicity to peach (Prunus persica l. batsch) seedlings grown in replanted soil. Sci. Hortic. 255, 183–192. doi: 10.1016/j.scienta.2019.05.020
Inderjit, Duke, S. O. (2003). Ecophysiological aspects of allelopathy. Planta 217, 529–539. doi: 10.1007/s00425-003-1054-z
Jin, S. Y., Li, D. Q., Lu, S., Han, L. T., Liu, D. H., Huang, Z., et al. (2019). Ethanol extracts of panax notoginseng increase lifespan and protect against oxidative stress in caenorhabditis elegans via the insulin/IGF-1 signaling pathway. J. Funct. Foods 58, 218–226. doi: 10.1016/j.jff.2019.04.031
Ji, L., Tian, L., Nasir, F., Chang, J., Chang, C., Zhang, J., et al. (2021). Impacts of replanting American ginseng on fungal assembly and abundance in response to disease outbreaks. Arch. Microbiol. 203, 2157–2170. doi: 10.1007/s00203-021-02196-8
Liu, H., Lu, X., Hu, Y., Fan, X. (2020). Chemical constituents of panax ginseng and panax notoginseng explain why they differ in therapeutic efficacy. Pharmacol. Res. 161, 105263. doi: 10.1016/j.phrs.2020.105263
Liu, J. H., Yang, M., Zhu, S. S. (2019). Strategies to solve the problem of soil sickness of panax notoginseng (Family: Araliaceae). Allelopathy J. 47, 37–56. doi: 10.26651/allelo.j/2019-47-1-1218
Li, Y., Wang, B., Chang, Y., Yang, Y., Yao, C., Huang, X., et al. (2019). Reductive soil disinfestation effectively alleviates the replant failure of sanqi ginseng through allelochemical degradation and pathogen suppression. Appl. Microbiol. Biotechnol. 103, 3581–3595. doi: 10.1007/s00253-019-09676-4
Meng, L., Xia, Z., Lv, J., Liu, G., Tan, Y., Li, Q. (2022). Extraction and GC-MS analysis of phenolic acids in rhizosphere soil of pinellia ternate. J. Radiat. Res. Appl. Sci. 15, 40–45. doi: 10.1016/j.jrras.2022.05.015
PeiJin, Y., WenQuan, W., Yuan, Z., ZiLong, Z., YuXin, P., XiuMing, C. (2009). Allelopathic effects of extracts from root-zone soil of panax notoginseng on notoginseng’s seedlings. Southwest China J. Agric. Sci. 22, 308–310. doi: 10.16213/j.cnki.scjas.2009.02.035
Qiao, Y. J., Gu, C. Z., Zhu, H. T., Wang, D., Zhang, M. Y., Zhang, Y. X., et al. (2020). Allelochemicals of panax notoginseng and their effects on various plants and rhizosphere microorganisms. Plant Diversity 42, 323–333. doi: 10.1016/j.pld.2020.04.003
Qiao, Y. J., Shang, J. H., Wang, D., Zhu, H. T., Yang, C. R., Zhang, Y. J. (2018). Research of panax spp. in kunming institute of botany, CAS. Nat. Prod. Bioprospect. 8, 245–263. doi: 10.1007/s13659-018-0176-8
Qiao, Y. J., Zhang, J. J., Shang, J. H., Zhu, H. T., Wang, D., Yang, C. R., et al. (2019). GC-MS-based identification and statistical analysis of liposoluble components in the rhizosphere soils of panax notoginseng. RSC Adv. 9, 20557–20564. doi: 10.1039/C9RA02110H
Ren, X., Yan, Z., He, X., Li, X., Qin, B. (2017). Allelochemicals from rhizosphere soils of glycyrrhiza uralensis fisch: Discovery of the autotoxic compounds of a traditional herbal medicine. Ind. Crops Prod. 97, 302–307. doi: 10.1016/j.indcrop.2016.12.035
Singh, H. P., Batish, D. R., Kohli, R. K. (1999). Autotoxicity: Concept, organisms, and ecological significance. Crit. Rev. Plant Sci. 18, 757–772. doi: 10.1080/07352689991309478
Staszek, P., Krasuska, U., Ciacka, K., Gniazdowska, A. (2021). ROS metabolism perturbation as an element of mode of action of allelochemicals. Antioxidants 10, 1648. doi: 10.3390/antiox10111648
Wang, F., Zhang, X., Wei, M., Wang, Y., Liang, Z., Xia, P. (2022). Appropriate crop rotation alleviates continuous cropping barriers by changing rhizosphere microorganisms in panax notoginseng. Rhizosphere 23, 100568. doi: 10.1016/j.rhisph.2022.100568
Xiang, W., Wei, X., Tang, H., Li, L., Huang, R. (2020). Complete genome sequence and biodegradation characteristics of benzoic acid-degrading bacterium pseudomonas sp. SCB32. BioMed. Res. Int. 2020, e6146104. doi: 10.1155/2020/6146104
Xin, A., Li, X., Jin, H., Yang, X., Zhao, R., Liu, J., et al. (2019). The accumulation of reactive oxygen species in root tips caused by autotoxic allelochemicals – a significant factor for replant problem of angelica sinensis (Oliv.) diels. Ind. Crops Prod. 138, 111432. doi: 10.1016/j.indcrop.2019.05.081
Xu, Y., Tan, H. Y., Li, S., Wang, N., Feng, Y. (2018). Panax notoginseng for inflammation-related chronic diseases: A review on the modulations of multiple pathways. Am. J. Chin. Med. 46, 971–996. doi: 10.1142/S0192415X18500519
Yang, M., Chuan, Y., Guo, C., Liao, J., Xu, Y., Mei, X., et al. (2018). Panax notoginseng root cell death caused by the autotoxic ginsenoside Rg1 is due to over-accumulation of ROS, as revealed by transcriptomic and cellular approaches. Front. Plant Sci. 9. doi: 10.3389/fpls.2018.00264
Yang, F., Liu, X., Wang, H., Deng, R., Yu, H., Cheng, Z. (2019). Identification and allelopathy of green garlic (Allium sativum l.) volatiles on scavenging of cucumber (Cucumis sativus l.) reactive oxygen species. Molecules 24, 3263. doi: 10.3390/molecules24183263
Yang, M., Zhang, X., Xu, Y., Mei, X., Jiang, B., Liao, J., et al. (2015). Autotoxic ginsenosides in the rhizosphere contribute to the replant failure of panax notoginseng. PloS One 10 (2), e011855. doi: 10.1371/journal.pone.0118555
Yan, Z., He, X., Guo, K., Li, X., Yang, X., Jin, H., et al. (2019). Allelochemicals from the rhizosphere of lanzhou lily: Discovery of the autotoxic compounds of a bulb crop. Sci. Hortic. 250, 121–126. doi: 10.1016/j.scienta.2019.02.038
Ye, C., Fang, Y., H., J., Liu, H., Yang, M., Zhu, S. S. (2019). Current status of soil sickness research on panax notoginseng in yunnan, China. Allelopathy J. 47, 1–14. doi: 10.26651/allelo.j/2019-47-1-1216
Zandvakili, O. R., Barker, A. V., Hashemi, M., Etemadi, F., Autio, W. R. (2019). Comparisons of commercial organic and chemical fertilizer solutions on growth and composition of lettuce. J. Plant Nutr. 42, 990–1000. doi: 10.1080/01904167.2019.1589505
Zhang, J., Fan, S., Qin, J., Dai, J., Zhao, F., Gao, L., et al. (2020). Changes in the microbiome in the soil of an American ginseng continuous plantation. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.572199
Keywords: Panax notoginseng, continuous cropping obstacle, root exudates, autotoxicity, allelochemicals
Citation: Xiang W, Chen J, Zhang F, Huang R and Li L (2022) Autotoxicity in Panax notoginseng of root exudatesand their allelochemicals. Front. Plant Sci. 13:1020626. doi: 10.3389/fpls.2022.1020626
Received: 03 October 2022; Accepted: 06 December 2022;
Published: 20 December 2022.
Edited by:
Hua Shao, Xinjiang Institute of Ecology and Geography (CAS), ChinaCopyright © 2022 Xiang, Chen, Zhang, Huang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rongshao Huang, aHJzaGFvODAyQDE2My5jb20=; Liangbo Li, bGxiMTAwQDEyNi5jb20=
 Wei Xiang
Wei Xiang Jianhua Chen3
Jianhua Chen3 Liangbo Li
Liangbo Li