- 1State Key Laboratory of Protein and Plant Gene Research, School of Life Sciences and School of Advanced Agricultural Sciences, Peking University, Beijing, China
- 2China Tobacco Gene Research Center, Zhengzhou Tobacco Research Institute of China National Tobacco Corporation (CNTC), Zhengzhou, China
- 3Technology Center, China Tobacco Hunan Industrial Co., Ltd., Changsha, China
Carboxylesterases (CXE) are a class of hydrolytic enzymes with α/β-folding domains that play a vital role in plant growth, development, stress response, and activation of herbicide-active substances. In this study, 49 Nicotiana tabacum L. CXE genes (NtCXEs) were identified using a sequence homology search. The basic characteristics, phylogenetic evolution, gene structure, subcellular location, promoter cis-elements, and gene expression patterns of the CXE family were systematically analyzed. RNA-seq data and quantitative real-time PCR showed that the expression level of CXEs was associated with various stressors and hormones; gene expression levels were significantly different among the eight tissues examined and at different developmental periods. As a new class of hormones, strigolactones (SLs) are released from the roots of plants and can control the germination of axillary buds.NtCXE7, NtCXE9, NtCXE22, and NtCXE24 were homologous to Arabidopsis SLs hydrolase AtCXE15, and changes in their expression levels were induced by topping and by GR24 (a synthetic analogue of strigolactone). Further examination revealed that NtCXE22-mutant (ntcxe22) plants generated by CRISPR-Cas9 technology had shorter bud outgrowth with lower SLs content. Validation of NtCXE22 was also performed in NtCCD8-OE plants (with fewer axillary buds) and in ntccd8 mutant plants (with more axillary buds). The results suggest that NtCXE22 may act as an efficient SLs hydrolase and affects axillary bud development, thereby providing a feasible method for manipulating endogenous SLs in crops and ornamental plants.
Introduction
Carboxylesterases (CXEs) are a class of hydrolytic enzymes with α/β-folded domains that are found in many animals, plants, and microorganisms, which can influence the hydrolysis of esters and amides (Hatfield et al., 2016). The active sites of CXEs include nucleophilic serine, acidic amino acids (arginine or glutamic acid), and histidine (Marshall et al., 2003). Hydrolysis of natural compounds may cause changes in the biological activity and transport of CXEs, which play important roles in plants. At present, 20 CXE genes have been identified in Arabidopsis thaliana (Marshall et al., 2003), 16 in Malus domestica (Schaffer et al., 2007), 33 in Prunus persica (Cao et al., 2019), and 72 in Gossypium barbadense (Rui et al., 2022). Plant CXE isoenzymes are found in multiple organs, at various developmental stages, and in various parts of cells (Nomura et al., 2015; Abdel-Daim et al., 2018). The expression of CXE genes in plants shows certain tissue specificity (Kamatham et al., 2017). For example, among the 20 AtCXE genes identified in A. thaliana, AtCXE13 is only expressed in flowers and fruits, whereas AtCXE1 is expressed in multiple organs but not in leaves, while other genes are expressed in all plant tissues (Marshall et al., 2003). Furthermore, CXE genes are constitutively expressed in plants. The expression of MdCXE1 is low in the early stages of fruit development but increases sharply after 146 d of flowering (Schaffer et al., 2007). Expression is induced by hormones and pathogens, and in Vitis flexuosa, infection with Botrytis cinerea upregulates VfCXE12827, VfCXE5585, and VfCXE13132 (Islam and Yun, 2016).
Plant CXE proteins have extensive substrate catalytic activities and take part in plant growth and development, secondary metabolism, and biological stress response (Mindrebo et al., 2016). CXE genes are also involved in ester metabolism. MdCXE1 may affect apple flavor by hydrolyzing the 4-methyl umbelliferyl ester substrates in apple fruit at harvest maturity (Souleyre et al., 2011), and Di-n-butyl phthalate (DnBP), commonly used as a plasticizer, is easily absorbed by plants and contributes to the metabolism of rice (Zhu et al., 2019). The expression of PpCXE1 is related to the catabolic activity of volatile acetate in peach fruits (Cao et al., 2019). CXEs also participate in the regulation of plant tolerance to both biotic and abiotic stresses. For example, GBCXE49 regulates the tolerance of cotton to alkali stress (Rui et al., 2022); AtCXE8 enhances plant resistance to gray mold, with the knockout of this gene increasing plant susceptibility (Lee et al., 2013); and NbCXE is a novel resistance-related gene that inhibits the accumulation of tobacco mosaic virus (TMV) in tobacco plants (Guo and Wong, 2020). CXEs participate in the activation of plant hormone-signaling substances, regulating IAA metabolism in immature maize endosperm tissues (Kowalczyk et al., 2003). CXE genes also regulate strigolactones (SLs) metabolism in A. thaliana (Xu et al., 2021) and are involved in herbicide activation. For example, in A. thaliana, AtCXE12 shares the properties of the hydrolytic herbicide precursor methyl-2, 4-dichlorophenoxyacetic acid (Gershater et al., 2007a). Jasmonic acid (JA) seed treatment also influences the expression level of CXE genes and promotes the detoxification of mustard seed insecticides (Sharma et al., 2018).
SLs and their derivatives are novel plant hormones derived from β-carotene, which play a crucial role in axillary bud outgrowth (Luo et al., 2019), root elongation (Sun et al., 2021), abiotic stress response (Marzec et al., 2020), and plant-fungi symbiosis (Akiyama et al., 2005). For example, the interference of SLs synthesis gene CCD8 results in increased branching of potato plants, and its gene editing was found to increase the branching of grapevines (Pasare et al., 2013; Ren et al., 2020). At present, the identified plant endogenous (natural) SLs contain a tricyclic lactone (ABC ring) and monocyclic lactone linked together by an enol ether bond (Yoneyama et al., 2018). The sensory mechanism of SLs is characteristic compared with other phytohormones, as the SLs receptor is an α/β hydrolase folding protein, which is regulated by ligand binding ability and hydrolysability (Toh et al., 2015; Seto et al., 2019). ShHTL7, a SLs receptor, enhances binding ability, having a large binding-pocket volume (Chen et al., 2021). Another SLs receptor, D14, is a member of the hydrolase family with α/β-folding characteristics, but its binding effect is greater than that of hydrolysis and may not be the key mechanism in SLs hydrolysis (Hamiaux et al., 2012; Zhao et al., 2013). In A. thaliana, AtCXE15 and its homologues have been identified as highly hydrolytic enzymes for SLs; AtCXE15 catalyzes the hydrolysis of various SLs analogs, and overexpression of AtCXE15 induces bud branching by SLs (Xu et al., 2021). Ectopic expression of AtCXE20 in A. thaliana and maize also results in increased plant branching and tillering (Roesler et al., 2021).
Tobacco (Nicotiana tabacum L.) is an economically important commercial crop and a model plant for genetic studies. Axillary bud germination and lateral branch growth in tobacco plants are also regulated by SLs. However, the CXE gene family has not yet been thoroughly evaluated in tobacco. Therefore, in this study, we examine the CXE gene family in tobacco and identify those CXE genes responsible for environmental stress tolerance and tissue specificity using bioassays. In addition, we verify a CXE gene that regulates axillary bud development in tobacco via genetic transformation. Our results not only provide a valuable reference for further research into the functional mechanisms of this gene family and the biological functions of CXEs in plant growth and development, but also suggest that CXEs may regulate SLs.
Materials and methods
Acquisition and sequence analysis of NtCXE family
Tobacco CXE (NtCXE) genes were found in the tobacco genome database (unpublished) based on the conserved domain (accession PF07859) and protein sequences of A. thaliana using “HMMER” software. In addition, the gene length, protein molecular weight, and the theoretical potential of the members of the CXE family of tobacco plants were analyzed using the software “ExPASy” (http://www.expasy.org/tools/). The subcellular localization of 49 NtCXE gene family members was carried out using the “Genscript” tool (https://www.genscript.com/psort.html) for prediction. To analyze the evolutionary relationships, the amino acid sequences of CXE genes in Arabidopsis, tomato, peach, apple and tobacco. were aligned using “CLUSTALX” and “MEGA 7.0” (Kumar et al., 2018).
Chromosomal location and gene duplication analyses
All the NtCXE genes were mapped onto their corresponding chromosomes. “TBtools” (Chen et al., 2020) was adopted to display the positions of chromosome locations and draw the chromosome distribution map of the NtCXE genes family. “KaKs_Calculator” was used to calculate the non-synonymous replacement rate (Ka), synonymous replacement rate (Ks), and their ratio (Ka/Ks) (Zhang et al., 2006).
Gene structure and conserved domain analysis
The “MEME” tool (http://meme.sdsc.edu/meme) was adopted to detect the NtCXE conserved motif in members of the gene family. For this, the number of conserved radicals detected was 15, and the length of the motifs was a minimum of six and a maximum of 50 amino acids. The coding sequence (CDS) and genome sequences of the CXE family members were uploaded to the Gene Structure Display Server program (http://gsds.cbi.pku.edu.cn/) to generate an intron-exon structure map.
Cis-acting element analysis and regulatory network prediction
The upstream 3,000-base-pair (bp) sequence of the CXE genes were adopted as the promoter region, and the promoter sequence was downloaded from the tobacco genome database. The PlantCare website (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/search_CARE.html) (Lescot et al., 2002) was used to identify cis-elements in the promoter regions of the NtCXEs. Regulatory elements of promoters were then be classified according to hormones, light, stress, etc. miRNAs downloaded from the miRBase database were used to build the miRNA-NtCXE regulatory relationships (http://plantgrn.noble.org/psRNATarget/) (Dai and Zhao, 2011; Kozomara et al., 2019). Transcription factors (TFs) screened from the Plant Transcription Factor Database (PlantTFDB; http://planttfdb.cbi.pku.edu.cn) were used to build the TF-NtCXE regulatory relationship network (http://plantgrn.noble.org/psRNATarget/) (Jin et al., 2016), and “Cytoscape” was used to map the regulatory networks (Smoot et al., 2011).
Plant growth conditions
The common tobacco variety K326 was cultured at the Zhengzhou Tobacco Research Institute in April 2022. Seedlings were grown in a greenhouse with a 14-h light at 28°C/10-h dark at 25°C cycle and a relative humidity of 50-60%. Uniformly growing tobacco (four-week-old seedlings) was screened for hormonal treatment.The tobacco seedlings were planted in 1/2 Hoagland nutrient solution with IAA (10 μM), MeJA (50 μM), ABA (10 μM), SA (10 μM), GA (10 μM), 6-BA (10 μM), GR24 (10 μM), and sucrose (10 mM) for 6 h. Uniformly growing tobacco (six-week-old seedlings) was screened for abiotic stress treatment. Tobacco seedlings were exposed to in 1/2 Hoagland nutrient solution at high temperature (35°C), low temperature (4 °C), salty (150 mM NaCl), dark, cadmium (10 μM), and drought (40% polyethylene glycol, PEG) conditions for 3 d. Roots, stems, leaves, axillary buds, and flowers were subsequently collected during the flowering stage. All collected examples were frozen in liquid nitrogen quickly and stored at -80°C in the refrigerator.
NtCXE gene expression in different tissues exposed to different stress treatments
The transcriptome data was adopted to reveal the expression patterns of CXE genes in tobacco in various tissue types and under the different stress conditions. Organizational data including leaves, roots, stems, veins, axillary buds, blades, calluses, and seeds were obtained from the tobacco genome database (unpublished). The sampling method is described in detail in Supplementary Table 1. Data on stress, including cold, drought, cadmium (Cd), topping, and CMV and Phytophthora nicotianae infection were obtained from the Sequence Read Archive (SRA) (Leinonen et al., 2011; He et al., 2016; Jin et al., 2017; Yang H. et al., 2017; Yang J. K. et al., 2017; Chen et al., 2019). These data were mapped to the tobacco reference genome using “HISAT2.2.1” with default parameters (Kim et al., 2019).
RNA isolation and expression analysis
Total RNA from each sample was extracted using Trizol reagent. RNA quality and purity were determined using 2% agarose gel electrophoresis and ultraviolet spectrophotometry, respectively. The reverse-transcribed cDNA was synthesized using the Prime Script RT Reagent Kit and stored at -20°C. Primers were designed using the Primer 3.0 online program based on the CDS sequence of NtCXE genes for quantitative real-time (qRT)-PCR. An Applied Biosystems CFX96 machine was used for the qRT-PCR with the SYBR qPCR kit (TaKaRa). The tobacco ribosomal protein gene, L25 (GenBank No. L18908), was used as an internal reference, and three biological replicates were performed (Schmidt and Delaney, 2010). The gene primers used in this study are listed in Supplementary Table 2.
Subcellular localization and GUS staining assay
The Open Reading Frame of the target gene was fused downstream of the PC1300s-GFP vector using EcroRI enzyme digestion. The enzymatic digestion product was purified and recombined with the amplified products (ClonExpress-II One Step Cloning Kit). The recombinant plasmid was then transferred into Agrobacterium tumefaciens (LBA4404). The monoclonal cells were coated with kanamycin resistant plates and cultured in yeast extract broth liquid medium on a 28 °C shaking table for 2 d. The bacteria were centrifuged at 4,000 rpm/min for 4 min. After supernatant removal, the bacteria were re-suspended in 10 mM MgCl2 (including 120 µM AS) suspension, and the OD600 was adjusted to approximately 0.6. The Agrobacterium solution was then injected into the lower epidermis (back side) of the tobacco leaves. The injected tobacco plants were then cultured under low light for 2 d and observed using laser confocal microscopy. The empty vector-transformed A. tumefaciens was used as a control. The vector map of PC1300s-NtCEX22-GFP was shown in Supplementary Figure 1.
The plasmid PBI121 was digested with BamHI enzyme and SacI enzyme at 37 °C for 3h. The reaction system included 15 μL of PBI121 plasmid, 1μL of BamHI enzyme, and 1 μL of SacI enzyme. The digested product was analyzed by electrophoresis, recovered, and purified. The promoter sequence of NtCXE22 was subcloned into the vector pBI121 by clonEZ homologous recombination, and 35S in the vector was replaced. The vector map of proCXE22-GUS was shown in Supplementary Figure 2. The proCXE22-GUS vector was then transformed into the tobacco plants (Horsch et al., 1985). The plant materials were placed into β-glucuronidase (GUS) staining solution and then stained for 12 h in the dark at 37°C. After staining, the GUS staining solution was recovered. Plant tissues were immersed in 75% ethanol for decolorization, and after chlorophyll removal, the staining results were photographed for analysis. The gene primers used in this study are listed in Supplementary Table 2.
Gene mutation plasmid construction, plant transformation and mutant analysis
A Cas9/sgRNA vector was constructed as previously described (Gao et al., 2015). According to the mRNA sequence information of NtCXE22, two CRISPR target sites of 20 nucleotides were designed to produced small guide RNA (sgRNA). Plasmid Cas9/gRNA was digested by BsaI. enzyme at 37 °C for 4 h. The target site sequence was ligated into pORE-Cas9 binary vector using T4 ligase. The connected carriers were transformed into DH5α competent cells and coated onto lysogeny broth (LB) solid medium. The cells were cultured overnight at 37 °C until positive plaques grew (approximately 16 h). After sequencing, the plasmid was extracted using the OMEGA plasmid extraction kit and transformed into Agrobacterium tumefaciens (LBA4404). The vectors were subsequently transformed into tobacco plants using the A. tumefaciens-mediated leaf disk method (Horsch et al., 1985). The design method of PCR primers and detection methods of mutation efficiency were carried out accoring to previous literature (Xie et al., 2017).
Plant tissue safranin O-fast green staining and scanning electron microscope
For plant tissue safranin O-fast green staining, axillary buds were selected as samples and fixed in FAA soution. The sections of samples were rehydrated in BioDewax and put into the safranin O staining solution for 3-8s. The sections were then decolorized and put into plant solid green staining solution for 6-20s. The last, the sections was transparent and sealed for microscope observation.
For the scanning electron microscope, samples (axilalry buds) were quickly taken and fixed with SEM fixation solution for 2h. Then the post-fixation was performed (PBS washing; fixed with OsO4 for 2h; PBS washing). The sample was dehydrated in alcohol and isoamyl acetate. The dehydrated samples were dried and treated with conductive metal coating. Finally, the samples were observed and photographed under a scanning electron microscope.
Extraction and detection of strigolactone
SL was determined using a plant SL ELISA kit (RJ21771, Shanghai; China). The chemical formula for SL is C17H14O5, the molecular weight is 298.29. Samples were collected from the roots of the wild type and NtCXE22 mutant plants following the manufacturer’s instructions.
Statistical analyses
Excel 2016, SPSS 26.0 and GraphPad Prism 9.0 software were used for data analysis and visualization. All treatments and sample assays were performed with at least three replicates, and each biological replicate included at least three uniformly grown plants.
Results
Genome-wide identification of the NtCXE family in N. tabacum
To identify members of the CXE family in tobacco, gene annotation and the hidden Markov model-based profile of the CXE domain (accession PF07859) were used as query conditions, and 49 CXE genes were identified in the N. tabacum genome database. To understand the evolutionary relationships of CXEs, a phylogenetic tree was constructed using full-length deduced amino acid sequences from Arabidopsis, tobacco, peach, apple and tobacco (Figure 1). The 49 tobacco CXE genes were divided into seven subfamilies according to their sequence homology. Group three contains 12 tobacco CXE members, accounting for 24% of the entire gene family, and was the subgroup with the largest number of members. The CDS length of the NtCXE genes ranged from 411 to 1,479 bp, and the protein length ranged from 136 to 492 amino acids. The protein molecular weights (MWs) of the NtCXE proteins were between 15.55 and 53.98 kDa, with the isoelectric points of members of the CXE gene family ranging from 4.58 to 5.93. The predicted locations of the NtCXE proteins in the cell were mostly in the cytoplasm and mitochondria based on subcellular localization prediction. The CDS sequences, physical and chemical properties of the 49 identified NtCXE genes are listed in Supplementary Table 3.

Figure 1 Phylogenetic analysis of carboxylesterase (CXE) families. The Neighbour-Joining (NJ) phylogenetic tree was constructed according to amino acid sequences of CXE genes in Arabidopsis, tomato, peach, apple and tobacco by MEGA 7.0. The CXE proteins were divided into seven groups (marked as groups 1-7), distinguished by different colors. The standard value of nodes was derived using bootstrapping, with 1000 replicates.
Chromosomal locations, duplication, and multiple sequence alignment
The chromosome analysis of NtCXE genes is presented in Figure 2A. We found that 33 NtCXE genes were present on the following 14 chromosomes: Chr01, Chr02, Chr03, Chr5, Chr6, Chr8, Chr11, Chr12, Chr13, Chr14, Chr16, Chr17, Chr20, and Chr21. The largest gene cluster (13 members) was observed on Chr6 (Figure 2A). There were five NtCXEs on Chr13 (NtCXE5, NtCXE6, NtCXE30, NtCXE31, and NtCXE32), four on Chr11 (NtCXE26, NtCXE33, NtCXE34, and NtCXE35), and three on Chr20 (NtCXE15, NtCXE16, and NtCXE45). Chr01, Chr02, Chr03, Chr5, and Chr8 contained the fewest NtCXE genes, with only one each. In addition, 16 NtCXE genes were not located on a chromosome but were mapped onto certain scaffolds (Figure 2A). The nucleotide sequences of the NtCXE genes were subsequently compared in a gene replication analysis. A total of 27 gene replication events occurred in the NtCXE gene family, including two tandem replication events and 25 segmental replication events. The Ka and Ks values of the gene replication pairs were used to evaluate the factors affecting gene evolution in tobacco. The same type of duplicated gene showed different Ka and Ks distributions; whole genome duplication (WGD)-type repeat gene pairs showed a smaller Ka/Ks ratio, revealing slower sequencing or functionalization over a longer period of time (Supplementary Table 4). The CXE family belongs to the α/β sheet hydrolase superfamily, and contain a conserved core-a HGGGF-and-GXSXG-motif-associated with catalysis and degradation (Ueguchi-Tanaka et al., 2005). The NtCXE protein sequence alignment showed that this motif was highly conserved (Figure 2B). This warrants further study regarding the degradation of NtCXEs.
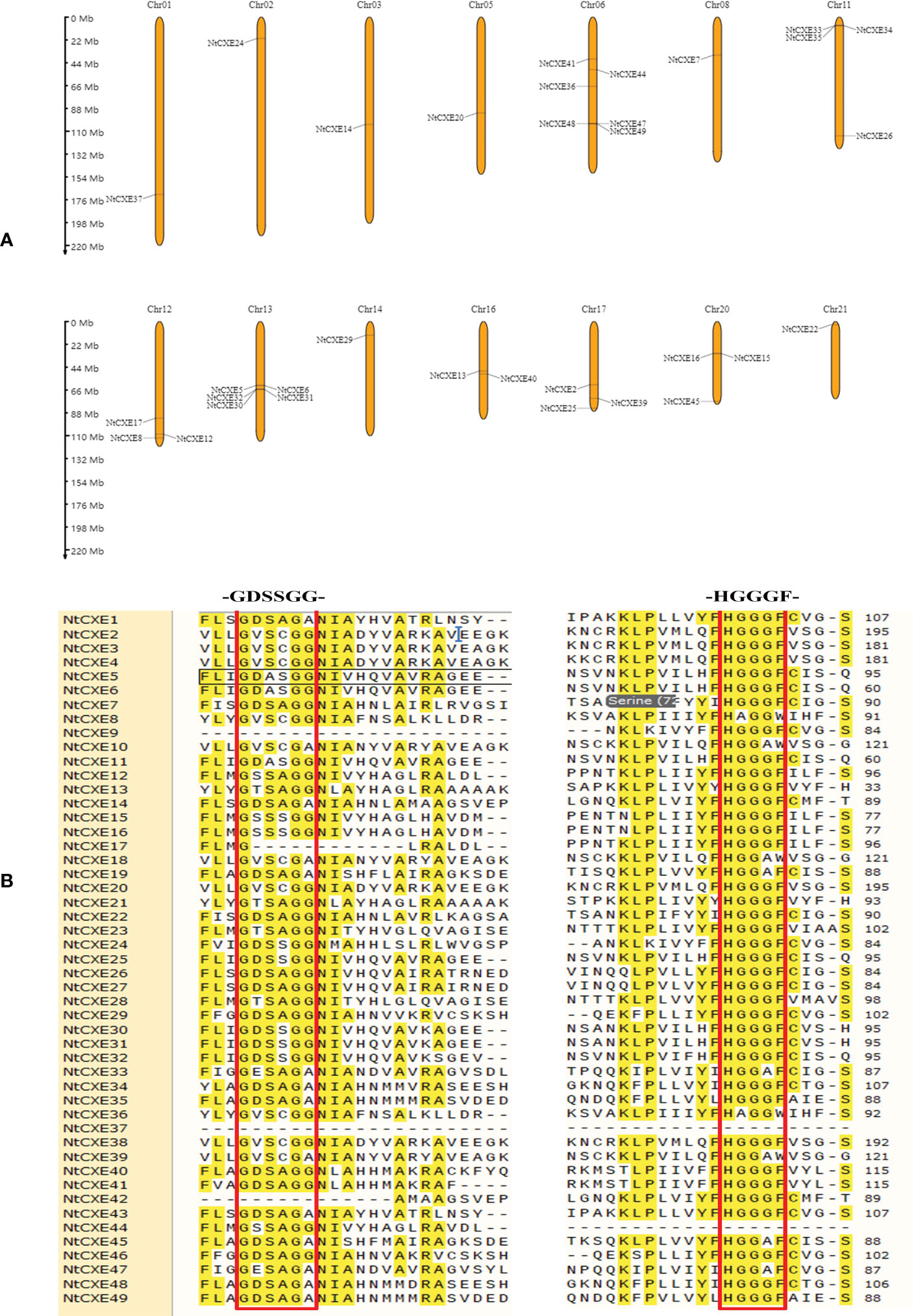
Figure 2 Analysis of genomic location, duplicated gene pairs, and sequence comparison of tobacco NtCXE proteins. (A) Chromosomal positions of the CXE genes. The chromosomal names are in red and are shown at the top, and the gene names are shown on the chromosome. The length of chromosomes is shown to scale. (B) Multiple sequence alignment of conserved domain of NtCXE proteins. The amino acid sequences were aligned using ClustalX.
Gene structure and conserved motif analysis
The “MEME” suite tool was chosen to analyze the conserved motifs, 10 of which were identified (Supplementary Table 5; Figure 3A). Analysis of these genes suggested that the motif of the NtCXE gene family had a certain conserved type. Introns are an important component of eukaryotic genes that can participate in the post-transcriptional re-splicing of structural genes. Some introns also participate in the regulation of promoter activity and the activity of response elements contained in promoter introns (Hoshida et al., 2017). The CDS of the NtCXE genes was more deeply analyzed with the genome sequences; intron and exon analyses were performed using GSDS 2.0 (Figure 3B). The 26 NtCXEs were all intron-containing genes, and exons were separated by introns. However, the gene structures were very different, and the number of exons ranged from one to four. Twenty (40.81%) genes had one intron, whereas NtCXE6 had three introns. Notably, paralogous NtCXEs genes shared similar exon/intron distribution rules.
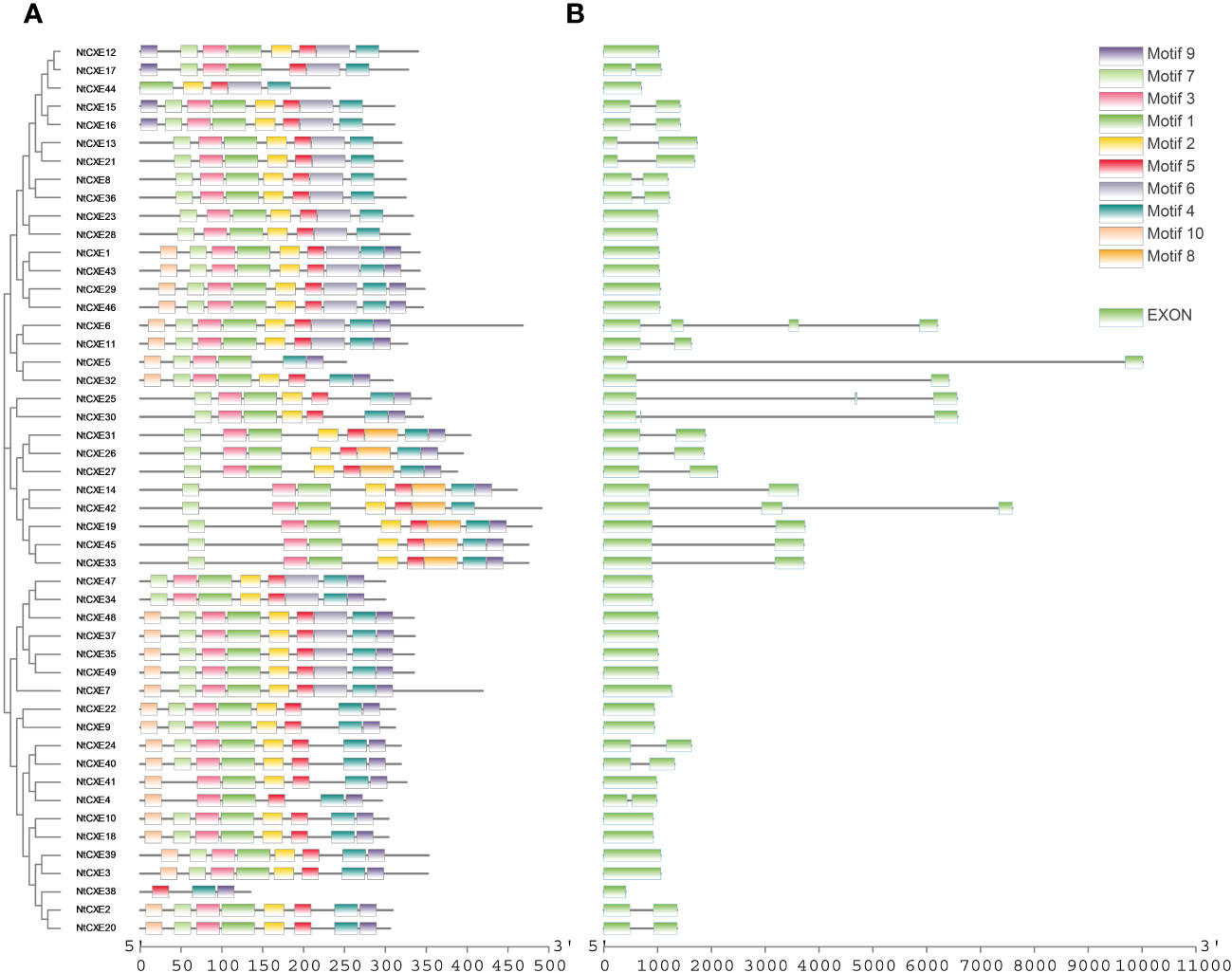
Figure 3 Phylogenetic relationship, gene structures, and protein conserved motif of NtCXE genes. (A) Analysis of conserved motif in the amino acid sequences of NtCXEs. The differently colored boxes in the upper right corner represent different conserved motifs with the number 1 to 10. The sequence information corresponding to different motifs is provided in Supplementary Table 5. (B) Exon-intron structure of NtCXEs in tobacco.
Cis-acting elements and interaction networks of the CXE family
In view of the potential regulatory mechanisms of various cis-acting elements in the CXE family, the 3 kb upstream region of the transcription start site was detected, and the putative functions were identified in seven groups. Of these, light-responsive and promoter-related elements were the most abundant (Figure 4A). Elements related to the environment include low temperature, defense and stress responsiveness, and anaerobic induction. The plant hormone-responsive category includes auxin, MeJA, abscisic acid, salicylic acid, zein, and gibberellin (Figure 4B). Notably, the promoter regions of 44 NtCXE members (89.8%) included ABA response elements (ABRE), 35 genes (89.8%) with MeJA response elements, 33 genes (89.8%) with gibberellin response elements, and 30 genes (89.8%) with auxin response elements (Figure 4C) (Supplementary Table 6). In addition, the promoter regions of the 25 NtCXE genes comprised meristem expression related components. Analysis of promoter elements revealed that NtCXE genes may take part in many plant growth and developmental processes.
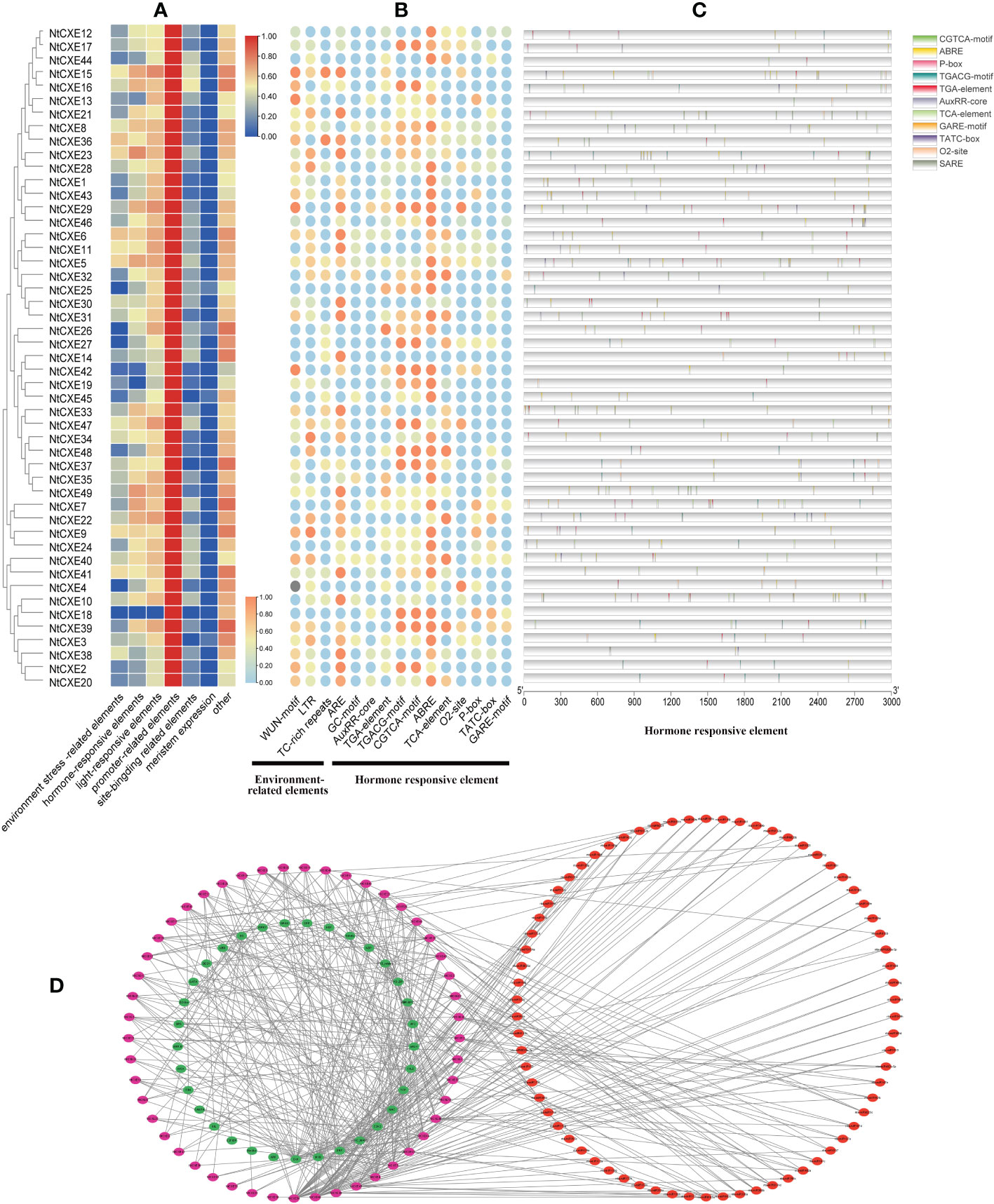
Figure 4 Analysis of promoters and interaction network of the tobacco NtCXE genes. (A) Predicted cis-acting elements divided into six types for each NtCXE promoter using PlantCARE software. Heat maps were drawn by TBtools using FPKM mean values. Color represents the gene expression levels (red, high expression level; and blue, low expression level). (B) Prediction of the magnitude of elements related to hormone and environmental stress in NtCXE promoters. The heat map is drawn in the same way as figure A (orange, high expression level; and blue, low expression level). (C) Position, type, and number of hormone-related elements in NtCXE promoters using “TBtools”. (D) Regulation network of NtCXEs in tobacco plants. The purple, green, and red circles denote NtCXE genes, transcription factors (TFs), and miRNAs, respectively.
Promoter cis-element-binding transcription factors (TFs) can regulate genetic expression. Here, TFs were predicted using “PlantTFDB” and regulatory relationships were displayed using “Cytoscape” (Jin et al., 2016). We predicted 731 TF members binding to the NtCXE promoters, divided into 33 TF families including WRKY, TCP, and NAC. Potential miRNA-binding sites for NtCXEs were subsequently identified using “PsRNATarget” (Dai and Zhao, 2011). In total, 138 miRNA members from 23 miRNA families were screened, implying their complex and potentially important roles in the regulation of NtCXEs. Some of miRNAs have several NtCXE targets; nta-miR167a, for example, targeted three NtCXE genes. In addition, some NtCXEs could be targeted by multiple miRNAs; for instance, CXE2 could be regulated by 20 miRNAs. The regulatory network of NtCXEs with transcription factors and miRNAs is shown in Figure 4D. Notably, the relationships between NtCXEs and TFs/miRNAs require further study. Specific regulatory information is presented in Supplementary Table 7.
Expression patterns of NtCXE genes in different tissues
To further explore the possible roles of NtCXE genes, their expression in eight different tissues (seeds, veins, axillary buds, blades, calluses, roots, stems, and leaves) were screened and analyzed. Of these genes, six were not expressed, and the remaining genes were expressed in five of the screened tissues (Figure 5A; Supplementary Table 8). NtCXE35 and NtCXE49 had higher expression levels in seeds; NtCXE13 and NtCXE35 had higher expression levels in seeding roots, NtCXE21 and NtCXE35 in seeding leaves, NtCXE26 and NtCXE47 in axillary buds, NtCXE25 and NtCXE35 in stems, NtCXE13 and NtCXE35 in veins, NtCXE35 and NtCXE49 in blades, and NtCXE35 and NtCXE44 in calluses. Thus, the NtCXE genes have distinct expression profiles in different tissues, underlying their potential functions in various physiological processes. In addition, the expression of NtCXE genes was significantly affected by the developmental stage (i.e., seedling, maturity, and 2 d after topping). Topping promoted NtCXE gene expression in roots but decreased expression in leaves and stems, including NtCXE5 and NtCXE28. Topping increased the expression level of NtCXE7/9/22 in the leaves. NtCXE gene expression at maturity was significantly different from that at the seedling stage. For example, NtCXE38 was not expressed in seedling roots but was expressed in roots at the mature stage, and NtCXE29 was highly expressed in seeding leaves but was significantly decreased in mature leaves. Eight NtCXE genes (NtCXE 3, 5, 8, 13, 22, 25, 33, and 44) were randomly selected to validate the transcriptome results using qRT-PCR analysis, which showed similar expression patterns (Figure 5B).
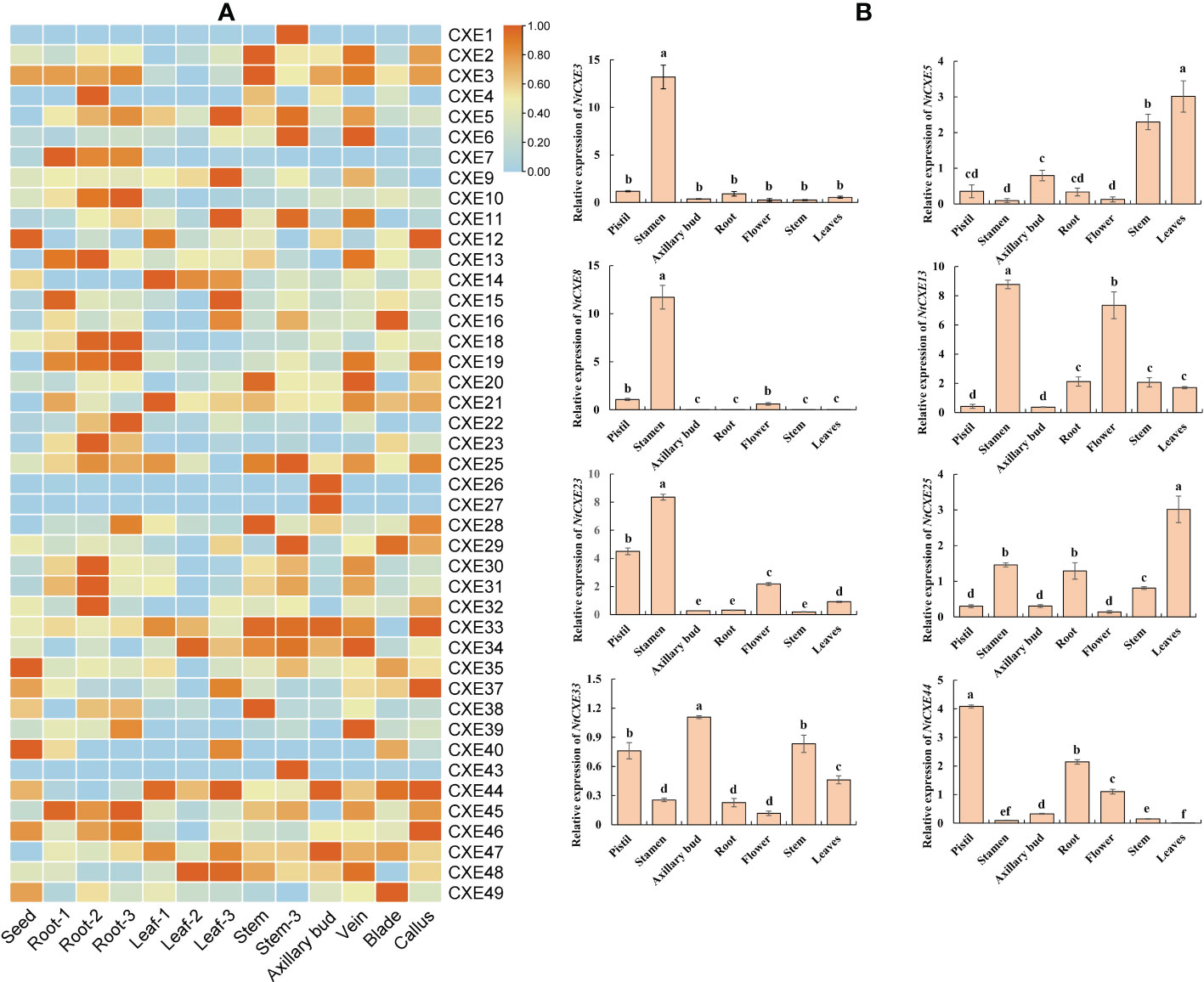
Figure 5 Analysis of tobacco NtCXE genes in various plant tissues. (A) Expression specificity of all NtCXE genes in various plant tissues, some with three developmental stages. ‘1’, ‘2’, and ‘3’ represent the seedling stage, mature stage, and 2 d after topping, respectively. Heat maps were drawn by TBtools using FPKM mean values, scaled by rows. Color represents the gene expression levels (orange, high expression level; and blue, low expression level). (B) qRT-PCR analysis of NtCXE3, NtCXE5, NtCXE8, NtCXE13, NtCXE23, NtCXE25, NtCXE33, NtCXE44 in axillary buds, flowers, leaves, roots, terminal buds, and veins. Data are presented as means ± SDs (n = 3). Different letters indicate significant differences between various tissues, based on one-way ANOVA.
Expression of NtCXE genes under stress
To further analyze the NtCXE genes involved in stress response, we used publicly available transcriptome data to assess their expression levels (Supplementary Table 9). As shown in Figure 6A, NtCXE14 and NtCXE42 showed a decreasing trend in response to cold stress, while NtCXE27 showed an increasing trend. NtCXE7, NtCXE47, and NtCXE48 were significantly upregulated in response to drought, whereas eight NtCXEs were downregulated. NtCXE5, NtCXE25, and seven other NtCXEs were upregulated in response to cadmium, while NtCXE22, NtCXE34, and seven other NtCXEs were downregulated. The expression of NtCXE6 and NtCXE7 increased in response to CMV treatment relative to that under normal nutritional conditions, while NtCXE9, NtCXE24, and NtCXE28 expression decreased. Sixteen NtCXEs showed an increasing trend upon inoculation with P. nicotianae, with the expression level of NtCXE14 increasing by a factor of 5.47 compared to the control. In contrast, the expression levels of six NtCXEs decreased upon inoculation with P. nicotianae, with NtCXE9 decreasing by a factor of 6.16 relative to the control. Finally, topping promoted the expression of NtCXE4 and NtCXE28 but inhibited the expression of NtCXE37 and NtCXE46. Subsequently, nine NtCXE genes (NtCXE 5, 10, 14, 22, 27, 30, 39, 42, and 47) were randomly selected to validate the transcriptome results using qRT-PCR analysis, which showed similar expression patterns. CXE genes were also found to respond to salt, high temperature, and darkness (Figure 6B).
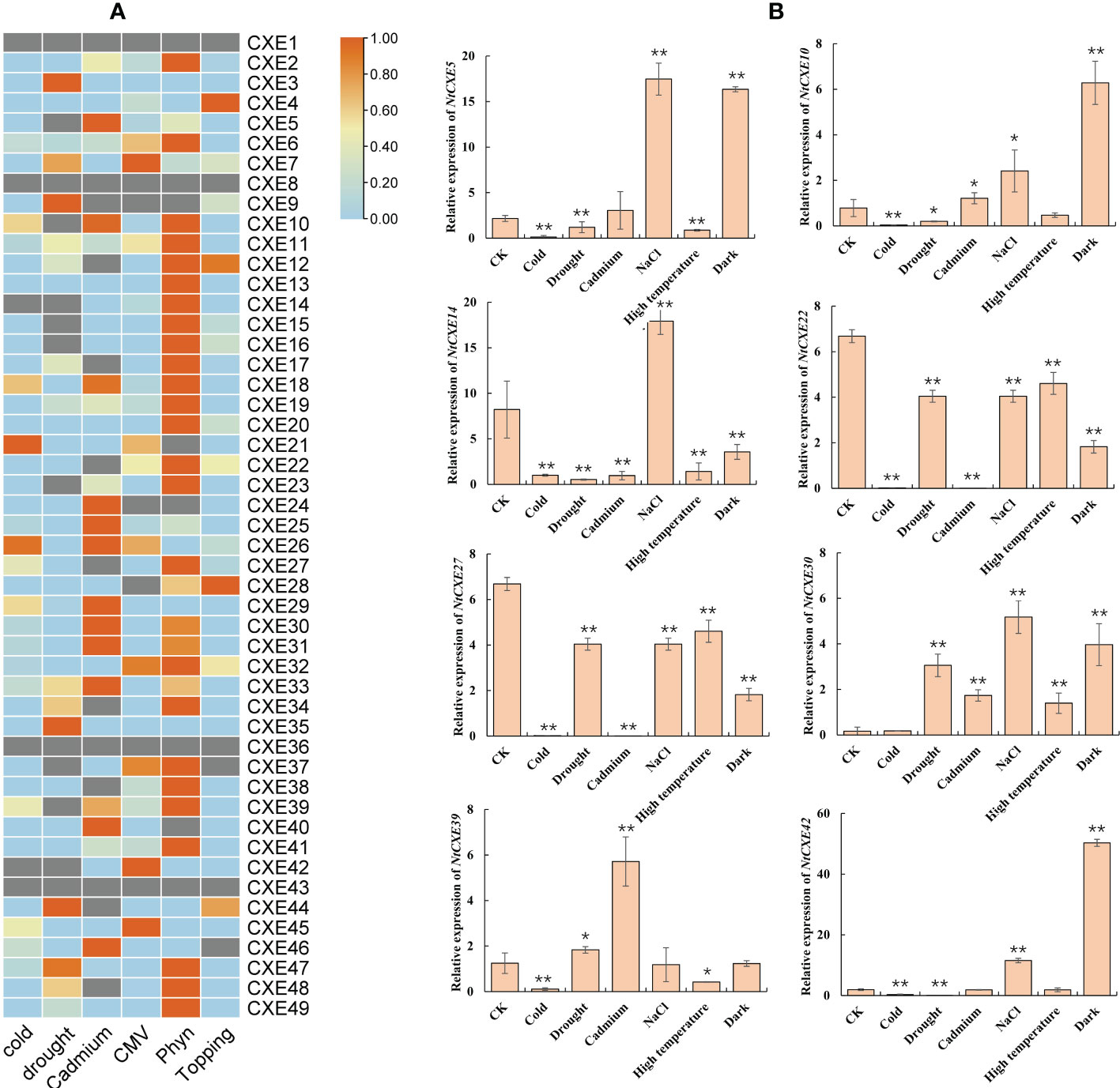
Figure 6 Analysis of tobacco NtCXE genes under various stress states. (A) Expression profiles of all NtCXE genes under cold, cadmium, topping, P. nicotiana infection, drought, and cucumber mosaic virus stresses, compared to the control treatment. Heat maps were drawn by TBtools using FPKM mean values, scaled by rows. Color represents the gene expression levels (orange, high expression level; and blue, low expression level). (B) qRT-PCR quantitative analysis of NtCXE5, NtCXE10, NtCXE14, NtCXE22, NtCXE27, NtCXE30, NtCXE39, NtCXE42 in response to NaCl, cold, cadmium, drought, high temperature, cold, darkness stressors. Data are presented as means ± SDs (n = 3). *P < 0.05, **P < 0.01 (significant difference between the stress treatment and control, based on Student’s t-test).
Expression of NtCXE genes under the influence plant hormones
Phytohormones are small-molecule organic substances produced during plant metabolism that move from their production sites to action sites to perform regulatory functions. These hormones play key roles in regulating almost all processes of plant growth and development, and response to environmental stress. To analyze the response of NtCXE genes to these hormones, tobacco seedlings were treated with ABA, 6-BA, GA, GR24, IAA, SA, and MeJA, which we found to differentially induce different CXE genes. Some genes responded to multiple hormones, such as NtCXE2, which responded to ABA, 6-BA, GA, GR24, IAA, SA, and MeJA, while some responded to only one, such as NtCXE2, which responded to ABA, and NtCXE5, which responded to GA. Notably, for the group-five genes, NtCXE9/22/24 were all induced by GA, GR24, IAA, SA, and MeJA. NtCXE7/22/24 were inhibited by ABA and 6-BA(Figure 7).
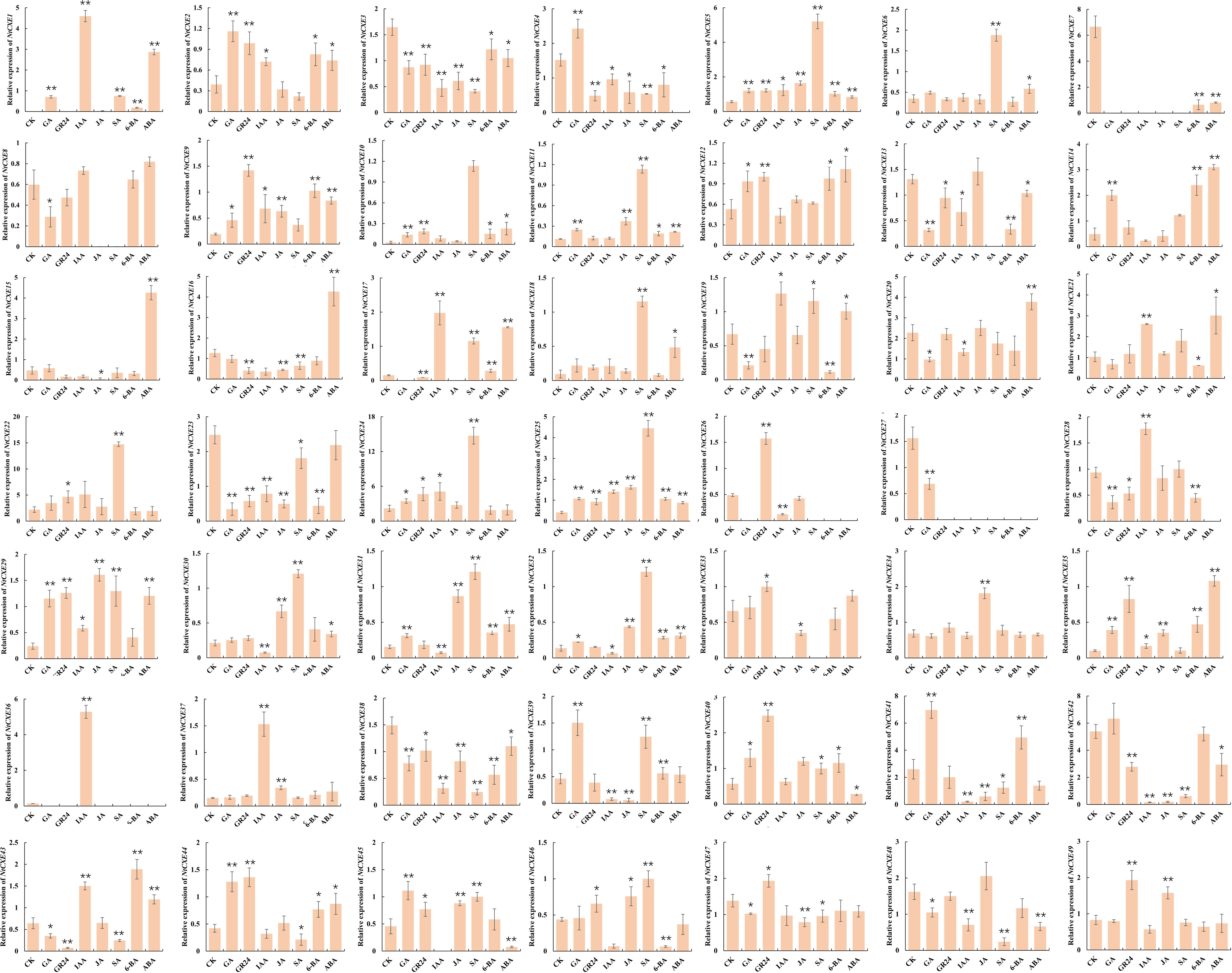
Figure 7 Expression profiles of 49 NtCXE genes under diverse hormone treatments. The expression patterns of all NtCXE genes in response to ABA, 6-BA, GA, GR24, IAA, SA, and JA were analyzed by qRT-PCR. Seedlings grown under normal conditions were used as controls. Data are presented as means ± SDs (n = 3). *P < 0.05, **P < 0.01 (significant difference between the hormone treatment and control, based on Student’s t-test).
Identification of the SLs hydrolase genes in tobacco
In A. thaliana, AtCXE15 participates in hydrolysis of SLs and affects axillary bud development. Through homology comparison, we found that in tobacco, NtCXE7, NtCXE9, NtCXE22, and NtCXE24 are homologous to AtCXE15, being significantly promoted in the axillary buds at different time points after topping (Figure 8A). Among them, the expression level of NtCXE7 and NtCXE22 was also increased in the roots after topping (Figure 6A). According to the induction of GR24 and its highest homology of AtCXE15, NtCXE22 was selected further verify its function in axillary bud development. NtCXE22 was expressed in different tissues, and its expression level in dormant axillary buds was higher than that in the other two types of axillary bud (Figure 8B). The constructed plant expression vector with GUS gene fusion of the NtCXE22 promoter was used to infect tobacco seedlings via Agrobacteria-mediated transient transformation, and GUS staining was adopted to verify the tissue expression pattern of NtCXE22. We found that the GUS gene driven by the NtCXE22 promoter was expressed in all tissues, which was similar to the qRT-PCR results (Figure 8C). To determine the subcellular localization of NtCXE22, a PC1300s-NtCEX22-GFP construct was introduced into the tobacco leaf protoplasts. As shown in Figure 8D, the GFP was predominantly localized in the nucleus and cytoplasm (The original pictures were shown in Supplementary Figure 3-12). These results are consistent with the network predictions and confirm the location of NtCXE22 in the cytoplasm (Figure 8D).
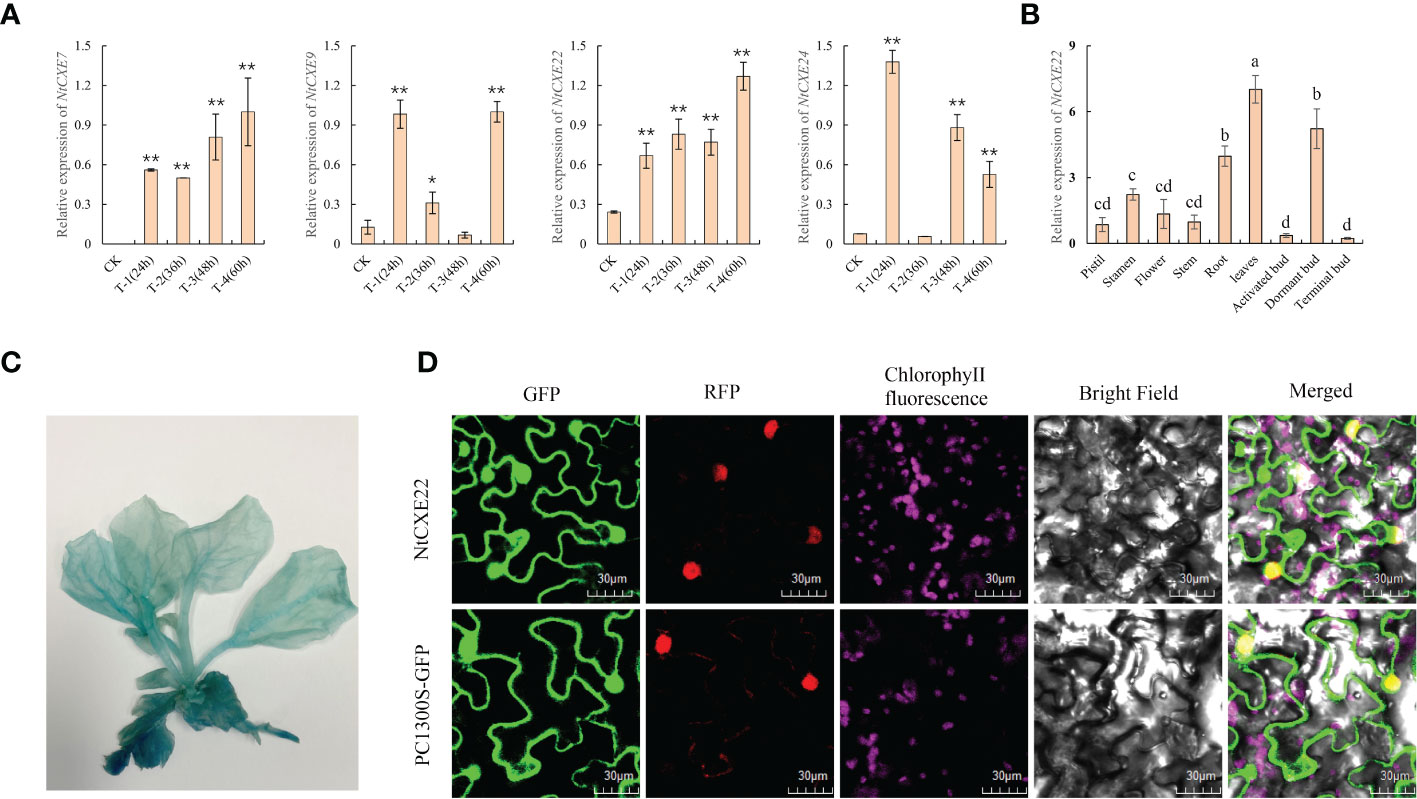
Figure 8 Expression patterns of NtCXE22 and subcellular localization of the NtCXE22 protein. (A) qRT-PCR quantitative analysis of NtCXE7, NtCXE9, NtCXE22, and NtCXE24 genes at different time points after topping, compared with that before topping. Data are presented as means ± SDs (n = 3). *P < 0.05, **P < 0.01 (significant difference between the topping treatment and control, based on Student’s t-test). (B) qRT-PCR quantitative analysis of NtCXE22 in different tissues. Data are presented as means ± SDs (n = 3). Different letters indicate significant differences between various tissues, based on one-way ANOVA. (C) Histochemical analysis of GUS expression driven by proCXE22. (D) Subcellular localization of NtCXE22 protein.
Knockout of NtCXE22 inhibits tiller bud outgrowth in tobacco plants
To further understand the function of NtCXE22 in axillary buds, targeted NtCXE22 mutants were built using the CRISPR/Cas9 technology. The two 20 bp target sequences were introduced into a Cas9 vector and transformed into tobacco using A. tumefaciens-mediated transformation. Ten T0 transgenic lines were obtained from the two editing sites, which were evaluated for mutants. Among the ten plants, six mutants were identified in this study with a ratio of 60%. NtCXE22 mutants (ntcxe22) resulted in the smaller axillary buds than in the wild type tobacco plants (Figure 9A). The axillary bud length of wild-type and ntcxe22 plants were also quantified in the Figure 9B. Sections and electron microscope images of the axillary buds in wild-type and ntcxe22-g1 plants are shown in Figures 9C-D (The original pictures were shown in Supplementary Figures 15-18). Cells in the wild type plants divided more rapidly than those in the NtCXE plants. The mutation sites of the two mutated materials (ntcxe22-g1-2, ntcxe22-g2-3) are shown in Figure 9E, which were determined to be putative homozygous genotype.
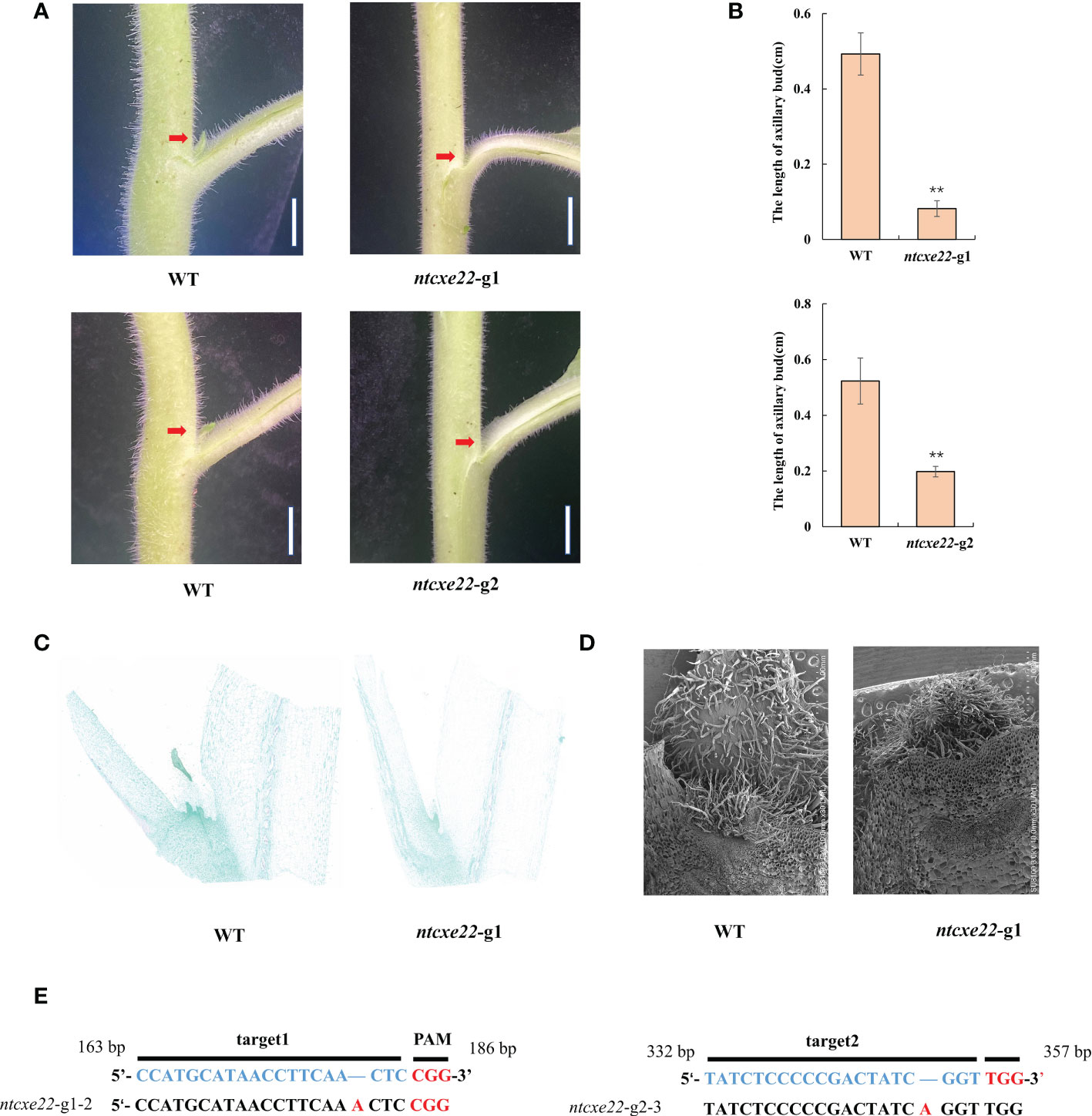
Figure 9 CRISPR/Cas9-mediated gene editing of NtCXE22 in tobacco. (A) Appearance of visible axillary buds in the wild type and NtCXE22 mutant (ntcxe22) tobacco seedings. Scale bar = 1 cm. Phenotypes of the whole plants were shown in Supplementary Figures 13, 14. (B) Quantitative analysis of axillary bud length in the wild type and ntcxe22 tobacco seedings. Data are presented as means ± SDs (n = 3). *P < 0.05, **P < 0.01 (significant difference relative to controls based on Student’s t-test). (C) Paraffin section of axillary bud in the wild type and ntcxe22 tobacco seedings. (D) Scanning electron micrograph of axillary bud in the wild type and ntcxe22 tobacco seedings. (E) Target locations in NtCXE22 are marked with blue letters, the protospacer adjacent motif (PAM) with red letters, and mutations in ntcxe22 -g1 and ntcxe22 -g2 are also shown.
NtCXE22 participates in SLs regulation
The SLs-affected NtCXE22 plants had axillary bud phenotypes similar to those of plants over-expressing NtCCD8, a synthetic gene that inhibits the axillary bud development (Pasare et al., 2013; Ren et al., 2020). Therefore, we hypothesized that NtCXE22 might affect axillary bud development through SLs signaling. NtTB1, a SLs downstream target gene, that inhibits axillary bud outgrowth (Braun et al., 2012; Dun et al., 2013). To investigate further, we first monitored the expression levels of NtCXE22 and NtTB1 in the axillary buds of tobacco lines exposed to GR24. The exogenous application of GR24 induced an increase in NtCXE22 and NtTB1 expression (Figure 10A). Moreover, in the ntcxe22 plants, we found the SL content was increased in the roots, and the expression levels of NtTB1 was increased in the axillary bud relative to that in the wild type, similar to the phenotypic changes in the NtCCD8-overexpression (NtCCD8-OE) plants (Figure 10B). These results preliminary verify that NtCXE22 has a regulatory effect on axillary bud development via SL catabolism or impaired signaling. Moreover, the expression level of NtCXE22 was determined in transgenic plants with a distinct axillary bud phenotype. For this, NtCCD8-OE plants with smaller axillary buds were cultivated, and CRISPR/Cas9-mediated gene editing of NtCCD8 (ccd8) were cultivated, with more axillary buds than in the wild type plants. The relative expression of NtCXE22 was increased in the roots of the NtCCD8-OE and reduced in the ntccd8 plants (Figure 10C). The regulatory network between the CXE22, CCD8, SL, and TB1 in the tobacco were shown as follow (Figure 10D). These results strongly suggest that NtCXE22 regulates axillary bud development not only by mediating SL signaling but also through other pathways, which require further study.
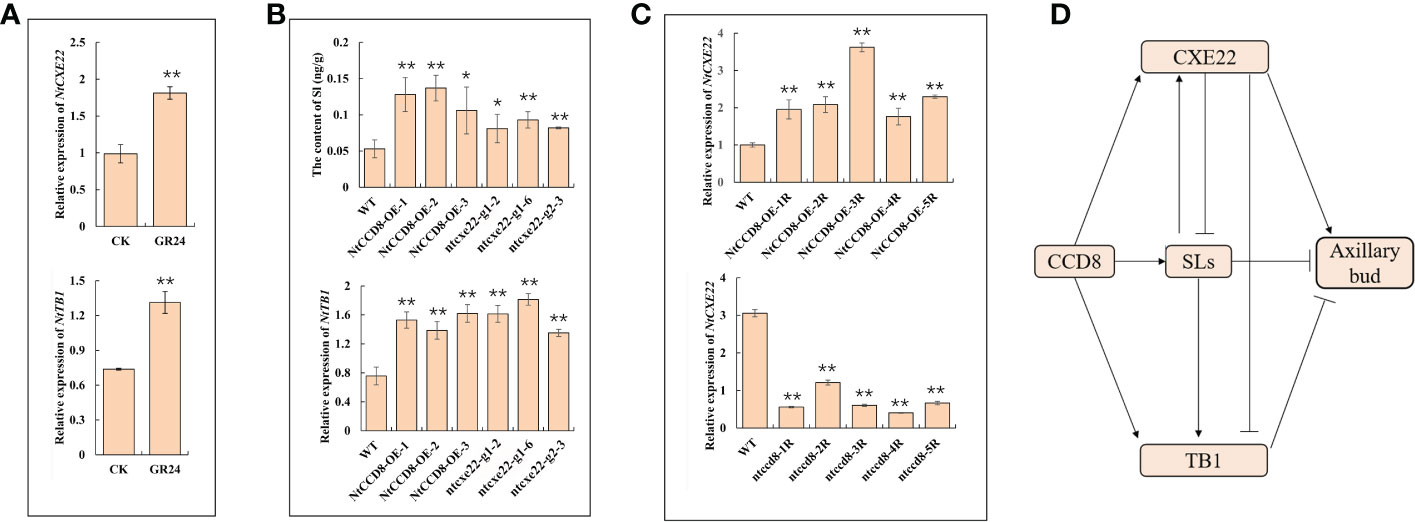
Figure 10 The verification of NtCXE22 involved in regulating SLs. (A) Transcriptional response of NtCXE22 and NtTB1 to GR24 (strigolactone analog) by qRT-PCR. (B) Expression level of NtTB1 and the content of SL in the axillary buds and roots of the wild type, NtCCD8-OE, and ntcxe22 plants by qRT-PCR. (C) Expression level of NtCXE22 were monitored in the roots of NtCCD8 mutant (ntccd8) and NtCCD8-OE plants by qRT-PCR. (D) The regulatory network between the CXE22, CCD8, SL, and TB1. Data are presented as means ± SDs (n = 3). *P < 0.05, **P < 0.01 (significant difference relative to controls based on Student’s t-test).
Discussion
Characteristics of the NtCXE gene family
CXEs are enzymes with α/β-folding domains that catalyze the hydrolysis of esters and amides and play a role in many physiological reactions in plants (Mindrebo et al., 2016; Lin et al., 2017). The functions of CXE genes have been extensively investigated in Arabidopsis, cotton, peaches, and other plants, but there are few studies on tobacco, despite it being a model plant (Yang et al., 2008; Cao et al., 2019; Rui et al., 2022). Based on our results, 49 CXE genes were identified and characterized in tobacco, which is more than in Arabidopsis (20), peach (33), and apple (16) (Schaffer et al., 2007; Mindrebo et al., 2016; Lin et al., 2017). The molecular weight of the NtCXE gene family was varied from 15.56 to 53.98 kDa, and most NtCXEs have pI values of <7, which indicates that most NtCXE proteins are acidic. The phylogenetic tree divided CXE genes into seven groups, which is similar to other plants. Members of the same subfamily are similar in CDS length, molecular weight, and motifs, suggesting that they may have similar functions.
Gene duplication and subsequent functional divergence are important drivers of genome and species evolution. Tandem replication, genome-wide replication, and fragment replication play major roles in the expansion of individual gene families (Panchy et al., 2016; Kong et al., 2019). In our study, 49 tobacco CXE genes were found to be distributed on 14 chromosomes, and two replicas containing five gene clusters were distributed across 13 chromosomes. All of these patterns suggest that gene duplication may benefit gene expansion in the tobacco CXE gene family. According to the motif and gene structure analyses, the tobacco CXE gene family is relatively conserved. Among them, all members contained motif 4, which can be used to explore the putative origin of CXEs. Introns are not directly involved in the proteome, but introns usually contain regulatory elements. Thus, the number and length of introns can affect the protein-coding potential of the genome (Jacob and Smith, 2017; Morgan et al., 2019; Parenteau et al., 2019). Based on previous research, we assume that the long intron of NtCXE11 may be used to further explore the vital regulatory roles of these genes. The introns and exons of the coding regions of eukaryotic genes control gene transcription and can, therefore, be used to further study the evolution of CXEs.
The analysis of promoter cis-elements can help understand the tissue specificity and regulatory functions of genes. Numerous environmental stress- and hormonal-response elements are widely distributed, which suggests crucial functions in plant bio/abiotic stress resistance (Rui et al., 2022). Furthermore, TFs, miRNAs, and enzymes can form a complex network that influences plant biological processes (Ibraheem et al., 2010; Chen et al., 2018). TFs interact with cis-acting elements on downstream gene promoters to regulate the expression of target genes and induce a series of responses, thereby enhancing plant growth and development (Priest et al., 2009). In our study, accoring to the cis-elements, 731 TFs from 33 TF families may be associated with NtCXE gene regulation. miRNAs regulate various biological functions by controlling the expression of target genes (Begum, 2022; Guo et al., 2022). In our study, 138 miRNA members predicated may have regulatory relationships with NtCXE genes. Specifically, we found that three NtCXEs (NtCXE2, NtCXE38, and NtCXE20) are targeted by miRNA167, which is involved in the regulation of Arabidopsis flowering time (Yao et al., 2019). NAC, a well-known flower-development-related TF (Wang J et al., 2022), may also be involved in regulating NtCXE38 expression. miR156-NtCXE45-ERF was another regulation module, and previous studies indicate that both miR156 and ERF are involved in drought tolerance (González-Villagra et al., 2017; Yu et al., 2022). Our interaction network can further contribute to the functional research of NtCXEs. Notably, 36 NtCXEs can be targeted by miR169, which is involved in plant disease and abiotic stress regulation (Luan et al., 2015; Hanemian et al., 2016), and NtCXE22 can be targeted by miR482, a miRNA superfamily that is critical for both disease resistance and plant development (Zhang et al., 2022).
Expression of the NtCXE gene family
Plant CXE isoenzymes are found in many plants including in different tissues, organs, and at different developmental stages (Pontier et al., 1998; Ichinose et al., 2001). The NtCXE genes are expressed in a wide range of tissues similar to the finding of AtCXE genes in A. thaliana (Marshall et al., 2003). In apples, MdCXE1 expression is low during early fruit development and peaks at harvest ripening (Souleyre et al., 2011). In our study, the expression of NtCXE12 and NtCXE23 was low in the leaves of seedlings but increased in mature leaves. Topping is an important agronomic procedure in tobacco cultivation, which can promote the development of axillary buds (Wang L et al., 2022) and the accumulation of secondary metabolites in leaves (Zhao et al., 2018). In our results, topping increased the expression of NtCXE22 in the stems and roots of tobacco and decreased the expression of NtCXE22 in leaves. We speculate that NtCXE22 may be involved in the regulation of axillary buds and the secondary metabolism of leaves in tobacco plants. Analysis of NtCXE genes expression in different tissues and at different stages should prove helpful in further clarifying the different functions of NtCXE genes.
CXEs are highly specific and only act on certain substrates with very high efficiency, whereas other enzymes are able to hydrolyze a wide range of substrates. The functions of CXE genes in plants include the activation of plant hormone signaling substances and responses to biotic stresses (Griffiths et al., 2006). The expression of many CXE genes is upregulated under abiotic stress, such as alkaline stress (Rui et al., 2022) and V. flexuosa infection (Islam and Yun, 2016). In our study, the expression levels of NtCXE6 and NtCXE7 were significantly increased after CMV infection, which is consistent with NbCXE expression in tobacco infected with TMV (Guo and Wong, 2020). Overexpression of the AtCXE8 gene in A. thaliana enhances resistance to Botrytis cinerea (Lee et al., 2013), and in our study, NtCXE14 expression was significantly increased upon inoculation with P. nicotianae. Furthermore, NtCXE22 was responsive to the cadmium and Phyn infection treatments, and NtCXE5 was responsive to the drought treatment. These results suggest that CXE genes are responsive to a wide range of biotic and abiotic stresses.
Hormone-signaling molecules may control plant physiological processes through conversion between inactive esters and active molecules, and these signaling molecules are released through the selective hydrolysis of esterases (Westfall et al., 2013; Kamatham et al., 2017). Plant CXEs can demethylate inactive methyl salicylate and methyl jasmonate to produce active salicylate and jasmonate (Kumar and Klessig, 2003; Stuhlfelder et al., 2004). In our study, RT-qPCR experiments were performed using multi-hormone treatments, which showed that the expression levels of many NtCXEs were significantly enhanced by MeJA and SA, such as NtCXE5 and NtCXE22. CXEs have been reported to control IAA metabolism in immature maize endosperm tissues, and these genes also regulate GA20 glycogroup metabolism in maize (Schneider et al., 1992; Kowalczyk et al., 2003). Similarly, we found that CXE gene expression was also induced by IAA, ABA, 6-BA, GA, and GR24. However, the types of hormone-induced genes were inconsistent, indicating that different CXE genes participate in different biological processes in response to different hormones, which requires further exploration.
NtCXE22 is involved in axillary bud development via SL
SLs are newly identified hormones with important applications in agriculture, being notably involved in tillering regulation (Wang et al., 2018). SLs biosynthesis and signaling have been extensively studied in the regulation of axillary bud development (Lin et al., 2009; Vogel et al., 2010; Pasare et al., 2013; Wen et al., 2016; Ren et al., 2020). In particular, CCD8 (a synthetic SLs gene) mutation caused increased branching in tobacco (Gao et al., 2018), and CpCCD8 overexpression reduces the branching in the Arabidopsis CCD8 mutant (Wang et al., 2021). On account of low abundance of SLs, little is known about their inactivation at the catabolic level (Snowden et al., 2005; Arite et al., 2007; Simons et al., 2007). In Arabidopsis, AtCXE15 has been identified as a functionally important SLs hydrolase (Xu et al., 2021), and the ectopic expression of CXE20 effectively reduces the concentration of free SLs and increases the number of branches (Roesler et al., 2021). These studies indicate a new mechanism of SLs degradation regulation in plants. In our study, NtCXE22 was identified in tobacco and was homologous with AtCXE15. NtCXE22 is located in the cytoplasm and nucleus, which is consistent with observations in peach and cotton (Cao et al., 2019; Rui et al., 2022). SLs are involved in the regulation of apical dominance in plants, and play a direct inhibitory role in branching (Cheng et al., 2013). Our results imply that topping (i.e., removal of apical dominance) increases the expression of the NtCXE22 gene in roots and leaves, which may be involved in the regulation of SLs degradation. CRISPR/Cas9-mediated gene editing of NtCXE22 (ntcxe22) in tobacco also inhibited axillary bud development with increased SLs content, which is consistent with the phenotype of AtCXE15. In addition, altered expression levels of NtCXE22 in CCD8 (the SLs synthesis gene) overexpression also indirectly confirms its regulatory effect on SLs. CXEs belong to the ABH superfamily, whose members function as carboxylic ester hydrolases of both xenobiotics and endogenous metabolites in plants (Gershater and Edwards, 2007b). We hypothesize that CXE22 might mediate SLs catabolism and, thereby, affect axillary bud development, which provides the basis for further research into the molecular mechanisms of CXE genes in plant growth and development. In addition, as SLs are distributed in both roots and axillary buds (Gomez-Roldan et al., 2008; Xie, 2016), the targeted degradation of SLs content in different tissues by CXE gene can be regarded as a research direction, which can be used to specifically regulate plant architecture or root system.
Conclusion
In summary, we explored the evolutionary relationships, functional information, and regulatory networks of the CXE gene family in tobacco plants. We successfully revealed the details of 49 genes, including gene structures, chromosomes, promoter cis-elements, associated transcription factors, and miRNAs. In addition, the expression levels of CXE genes in various tissues, under various abotic stresses, and in response to a range of plant hormones were determined. We found that NtCXE7, NtCXE9, NtCXE22, and NtCXE24 are regulated by topping and GR24. Furthermore, knockout of NtCXE22 inhibited axillary bud development and increased SLs content and the expression level of NtTB1, which was consistent with NtCCD8 (the SLs synthesis gene) overexpression lines. Overall, our work provides a solid foundation for the functional study of CXE genes as well as new understanding of the regulation of plant architecture.
Data availability statement
The datasets presented in this article are not readily available because the China tobacco genome database is not publicly available . Requests to access the datasets should be directed to Peijian Cao, cGVpamlhbmNhb0AxNjMuY29t.
Author contributions
LW, WP, and PC conceived the experiments. LW drafted the manuscript. LW and XX conducted the experiments with assistance from JY and GX. YX, ZL, and LC processed and analyzed the data. LL revised the manuscript. All authors contributed to the manuscript and approved the submitted version.
Funding
This work was supported by grants from the CNTC Research Program (No. 110202001020 [JY-03]), the Joint Laboratory of HNTI and ZTRI for Tobacco Gene Research and Utilization, and the Science and Technology Planning Project of Henan Province, China (No. 202102110147, 222102110347).
Acknowledgments
The authors are grateful for the software support provided by OmicShare’s work platform. We also thank Editage for their assistance with language editing.
Conflict of interest
Author WP was employed by China Tobacco Hunan Industrial Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2022.1019538/full#supplementary-material
References
Abdel-Daim, A., Ohura, K., Imai, T. (2018). A novel quantification method for serine hydrolases in cellular expression system using fluorophosphonate-biotin probe. Eur. J. Pharm. Sci. 114, 267–274. doi: 10.1016/j.ejps.2017.12.016
Akiyama, K., Matsuzaki, K., Hayashi, H. (2005). Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435, 824–827. doi: 10.1038/nature03608
Arite, T., Iwata, H., Ohshima, K., Maekawa, M., Nakajima, M., Kojima, M., et al. (2007). DWARF10, an RMS1/MAX4/DAD1 ortholog, controls lateral bud outgrowth in rice. Plant J. 51 (6), 1019–1029. doi: 10.1111/j.1365-313X.2007.03210.x
Begum, Y. (2022). Regulatory role of microRNAs (miRNAs) in the recent development of abiotic stress tolerance of plants. Gene 5 (821), 146283. doi: 10.1016/j.gene.2022.146283
Braun, N., de Saint Germain, A., Pillot, J. P., Boutet-Mercey, S., Dalmais, M., Antoniadi, I., et al. (2012). Te Pea TCP transcription factor PsBRC1 acts downstream of strigolactones to control shoot branching. Plant Physiol. 158, 225–238. doi: 10.1104/pp.111.182725
Cao, X., Duan, W., Wei, C., Chen, K., Grierson, D., Zhang, B. (2019). Prunus persicagenome-wide identification and functional analysis of carboxylesterase and methylesterase gene families in peach (L. batsch). Front. Plant Sci. 10, 1511–1523. doi: 10.3389/fpls.2019.01511
Chen, C., Chen, H., Zhang, Y., Thomas, H. R., Frank, M. H., He, Y., et al. (2020). TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 13 (8), 1194–1202. doi: 10.1016/j.molp.2020.06.009
Cheng, X., Ruyter-Spira, C., Bouwmeester, H. (2013). The interaction between strigolactones and other plant hormones in the regulation of plant development. Front. Plant Sci. 4, 199. doi: 10.3389/fpls.2013.00199
Chen, X., Sun, S., Liu, F., Shen, E., Liu, L., Ye, C., et al. (2019). A transcriptomic profile of topping responsive non-coding RNAs in tobacco roots (Nicotiana tabacum). BMC Genomics 20 (1), 856. doi: 10.1186/s12864-019-6236-6
Chen, J. M., White, A., Nelson, D. C., Shukla, D. (2021). Role of substrate recognition in modulating strigolactone receptor selectivity in witchweed. J. Biol. Chem. 297 (4), 101092. doi: 10.1016/j.jbc.2021.101092
Chen, D., Yan, W., Fu, L. Y., Kaufmann, K. (2018). Architecture of gene regulatory networks controlling flower development in arabidopsis thaliana. Nat. Commun. 9 (1), 4534. doi: 10.1038/s41467-018-06772-3
Dai, X., Zhao, P. X. (2011). PsRNATarget: a plant small rNA target analysis server. Nucleic. Acids Res. 39 (Suppl. l_2), W155–W159. doi: 10.1093/nar/gkr319
Dun, E. A., de Saint Germain, A., Rameau, C., Beveridge, C. A. (2013). Dynamics of strigolactone function and shoot branching responses in pisum sativum. Mol. Plant 6, 128–140. doi: 10.1093/mp/sss131
Gao, J., Wang, G., Ma, S., Xie, X., Wu, X., Zhang, X., et al. (2015). CRISPR/cas9-mediated targeted mutagenesis in Nicotiana tabacum. Plant Mol. Biol. 87, 99–110. doi: 10.1007/s11103-014-0263-0
Gao, J., Zhang, T., Xu, B., Jia, L., Xiao, B., Liu, H., et al. (2018). CRISPR/Cas9-mediated mutagenesis of carotenoid cleavage dioxygenase 8 (CCD8) in tobacco affects shoot and root architecture. Int. J. Mol. Sci. 19, 1062. doi: 10.3390/ijms19041062
Gershater, M. C., Cummins, I., Edwards, R. (2007a). Role of a carboxylesterase in herbicide bioactivation in arabidopsis thaliana. J. Biol. Chem. 282 (29), 21460–21466. doi: 10.1074/jbc.M701985200
Gershater, M. C., Edwards, R. (2007b). Regulating biological activity in plants with carboxylesterases. Plant Sci. 173 (6), 579–588. doi: 10.1016/j.plantsci.2007.08.008
Gomez-Roldan, V., Fermas, S., Brewer, P., Puech-Pagès, V., Dun, E., Pillot, J. P., et al. (2008). Strigolactone inhibition of shoot branching. Nature 455, 189–194. doi: 10.1038/nature07271
González-Villagra, J., Kurepin, L. V., Reyes-Díaz, M. M. (2017). Evaluating the involvement and interaction of abscisic acid and miRNA156 in the induction of anthocyanin biosynthesis in drought-stressed plants. Planta 246, 299–312. doi: 10.1007/s00425-017-2711-y
Griffiths, J., Murase, K., Rieu, I., Zentella, R., Zhang, Z. L., Powers, S. J., et al. (2006). Genetic characterization and functional analysis of the GID1 gibberellin receptors in Arabidopsis. Plant Cell 18 (12), 3399–414. doi: 10.1105/tpc.106.047415
Guo, Z., Kuang, Z., Zhao, Y., Deng, Y., He, H., Wan, M., et al. (2022). PmiREN2.0: from data annotation to functional exploration of plant microRNAs. Nucleic. Acids Res. 50 (D1), 1475–D1482. doi: 10.1093/nar/gkab811
Guo, S., Wong, S. M. (2020). A conserved carboxylesterase inhibits tobacco mosaic virus (TMV) accumulation in nicotiana benthamiana plants. Viruses 12 (2), 195. doi: 10.3390/v12020195
Hamiaux, C., Drummond, R. S., Janssen, B. J., Ledger, S. E., Cooney, J. M., Newcomb, R. D., et al. (2012). DAD2 is an α/β hydrolase likely to be involved in the perception of the plant branching hormone, strigolactone. Curr. Biol. 22 (21), 2032–2036. doi: 10.1016/j.cub.2012.08.007
Hanemian, M., Barlet, X., Sorin, C., Yadeta, K. A., Keller, H., Favery, B., et al. (2016). Arabidopsis CLAVATA1 and CLAVATA2 receptors contribute to ralstonia solanacearum pathogenicity through a miR169-dependent pathway. New Phytol. 211 (2), 502–515. doi: 10.1111/nph.13913
Hatfield, M., Umans, R., Hyatt, J., Edwards, C., Wierdl, M., Tsurkan, L., et al. (2016). Carboxylesterases: general detoxifying enzymes. Chem. Biol. Interact. 259, 327–331. doi: 10.1016/j.cbi.2016.02.011
He, X., Zheng, W., Cao, F., Wu, F. (2016). Identification and comparative analysis of the microRNA transcriptome in roots of two contrasting tobacco genotypes in response to cadmium stress. Sci. Rep. 6 (1), 32805–32814. doi: 10.1038/srep32805
Horsch, R. B., Fry, J. E., Hoffmann, N. L., Eichholtz, D., Rogers, S. G., Fraley, R. T. (1985). A simple and general-method for transferring genes into plants. Science 227, 1229–1231. doi: 10.1126/science.227.4691.1229
Hoshida, H., Kondo, M., Kobayashi, T., Yarimizu, T., Akada, R. (2017). 5´-UTR introns enhance protein expression in the yeast saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 101 (1), 241–251. doi: 10.1007/s00253-016-7891-z
Ibraheem, O., Botha, C. E., Bradley, G. (2010). In silico analysis of cis-acting regulatory elements in 5′ regulatory regions of sucrose transporter gene families in rice (Oryza sativa japonica) and arabidopsis thaliana. Comput. Biol. Chem. 34 (5-6), 268–283. doi: 10.1016/j.compbiolchem.2010.09.003
Ichinose, Y., Hisayasu, Y., Sanematsu, S., Ishiga, Y., Seki, H., Toyoda, K., et al. (2001). Molecular cloning and functional analysis of pea cDNA E86 encoding homologous protein to hypersensitivity-related hsr203J. Plant Sci. 160 (5), 997–1006. doi: 10.1016/S0168-9452(01)00343-0
Islam, M. Z., Yun, H. K. (2016). Identification and expression profiles of six transcripts encoding carboxylesterase protein in vitis flexuosa infected with pathogens. Plant Pathol. J. 32 (4), 347–356. doi: 10.5423/PPJ.OA.11.2015.0241
Jacob, A. G., Smith, C. W. J. (2017). Intron retention as a component of regulated gene expression programs. Hum. Genet. 136, 1043–1057. doi: 10.1007/s00439-017-1791-x
Jin, J., Tian, F., Yang, D. C., Meng, Y. Q., Kong, L., Luo, J., et al. (2016). PlantTFDB 4.0: toward a central hub for transcription factors and regulatory interactions in plants. Nucleic. Acids Res. 45 (D1), D1040–D1045. doi: 10.1093/nar/gkw982
Jin, J., Zhang, H., Zhang, J., Liu, P., Chen, X., Li, Z., et al. (2017). Integrated transcriptomics and metabolomics analysis to characterize cold stress responses in nicotiana tabacum. BMC Genomics 18 (1), 496. doi: 10.1186/s12864-017-3871-7
Kamatham, S., Pallu, R., Pasupulati, A., Singh, S., Gudipalli, P. (2017). Benzoylsalicylic acid derivatives as defense activators in tobacco and arabidopsis. Phytochemistry 143 (1), 160–169. doi: 10.1016/j.phytochem.2017.07.014
Kim, D., Paggi, J. M., Park, C., Bennett, C., Salzberg, S. L. (2019). Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 37 (8), 907–915. doi: 10.1038/s41587-019-0201-4
Kong, W. L., An, B. G., Zhang, Y., Yang, J., Li, S. M., Sun, T., et al. (2019). Sugar transporter proteins (STPs) in gramineae crops: comparative analysis, phylogeny, evolution, and ex-pression profiling. Cells 8 (6), 560. doi: 10.3390/cells8060560
Kowalczyk, S., Jakubowska, A., Zielinska, E., Bandurski, R. (2003). Bifunctional indole-3-acetyl transferase catalyses synthesis and hydrolysis of indole-3-acetyl-myo-inositol in immature endosperm of zea mays. Physiol. Plant 119 (2), 165–174. doi: 10.1034/j.1399-3054.2003.00158.x
Kozomara, A., Birgaoanu, M., Griffiths-Jones, S. (2019). MiRBase: from microRNA sequences to function. Nucleic. Acids Res. 47 (D1), D155–D162. doi: 10.1093/nar/gky1141
Kumar, D., Klessig, D. F. (2003). High-affinity salicylic acid-binding protein 2 is required for plant innate immunity and has salicylic acid-stimulated lipase activity. Proc. Natl. Acad. Sci. 100 (26), 16101–16106. doi: 10.1073/pnas.0307162100
Kumar, S., Stecher, G., Li, M., Knyaz, C., Tamura, K. (2018). MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35 (6), 1547–1549. doi: 10.1093/molbev/msy096
Lee, S., Hwang, S., Seo, Y. W., Jeon, W. B., Oh, B. J. (2013). Molecular characterization of the AtCXE8 gene, which promotes resistance to botrytis cinerea infection. Plant Biotechnol. Rep. 7, 109–119. doi: 10.1007/s11816-012-0253-0
Leinonen, R., Sugawara, H., Shumway, M. (2011). The international nucleotide sequence database collaboration. the sequence read archive. Nucleic. Acids Res. 39 (1), D19–D21. doi: 10.1093/nar/gkr1006
Lescot, M., Dehais, P., Thijs, G., Marchal, K., Moreau, Y., Van de Peer, Y., et al. (2002). PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic. Acids Res. 30, 325–327. doi: 10.1093/nar/30.1.325
Lin, Q., Chen, S., Chao, Y., Huang, X., Wang, S., Qiu, R. (2017). Carboxylesterase-involved metabolism of di-n-butyl phthalate in pumpkin (Cucurbita moschata) seedlings. Environ. pollut. 220 (Pt A), 421–430. doi: 10.1016/j.envpol.2016.09.084
Lin, H., Wang, R., Qian, Q., Yan, M., Meng, X., Fu, Z., et al. (2009). DWARF27, an iron-containing protein required for the biosynthesis of strigolactones, regulates rice tiller bud outgrowth. Plant Cell. 21 (5), 1512–1525. doi: 10.1105/tpc.109.065987
Luan, M., Xu, M., Lu, Y., Zhang, L., Fan, Y., Wang, L. (2015). Expression of zma-miR169 miRNAs and their target ZmNF-YA genes in response to abiotic stress in maize leaves. Gene 555 (2), 178–185. doi: 10.1016/j.gene.2014.11.001
Luo, L., Takahashi, M., Kameoka, H., Qin, R., Shiga, T., Kanno, Y., et al. (2019). Developmental analysis of the early steps in strigolactone-mediated axillary bud dormancy in rice. Plant J. 97 (6), 1006–1021. doi: 10.1111/tpj.14266
Marshall, S. D., Putterill, J. J., Plummer, K. M., Newcomb, R. D. (2003). The carboxylesterase gene family from arabidopsis thaliana. J. Mol. Evol. 57, 487–500. doi: 10.1007/s00239-003-2492-8
Marzec, M., Daszkowska-Golec, A., Collin, A., Melzer, M., Eggert, K., Szarejko, I. (2020). Barley strigolactone signaling mutant hvd14.d reveals the role of strigolactones in abscisic acid-dependent response to drought. Plant Cell Environ. 43 (9), 2239–2253. doi: 10.1111/pce.13815
Mindrebo, J., Nartey, C., Seto, Y., Burkart, M., Noel, J. (2016). Unveiling the functional diversity of the alpha/beta hydrolase superfamily in the plant kingdom. Curr. Opin. Struct. Biol. 41 (1), 233–246. doi: 10.1016/j.sbi.2016.08.005
Morgan, J. T., Fink, G. R., Bartel, D. P. (2019). Excised linear introns regulate growth in yeast. Nature 565, 606–611. doi: 10.1038/s41586-018-0828-1
Nomura, T., Murase, T., Ogita, S., Kato, Y. (2015). Molecular identification of tuliposide b-converting enzyme:a lactone-forming carboxylesterase from the pollen of tulip. Plant J. 83 (2), 252–262. doi: 10.1111/tpj.12883
Panchy, N., Lehti-Shiu, M., Shiu, S. H. (2016). Evolution of gene duplication in plants. Plant Physiol. 171 (4), 2294–2316. doi: 10.1104/pp.16.00523
Parenteau, J., Maignon, L., Berthoumieux, M., Catala, M., Gagnon, V., Abou Elela, S. (2019). Introns are mediators of cell response to starvation. Nature 565, 612–617. doi: 10.1038/s41586-018-0859-7
Pasare, S. A., Ducreux, L. J. M., Morris, W. L., Campbell, R., Sharma, S. K., Roumeliotis, E., et al. (2013). The role of the potato (Solanum tuberosum) CCD8 gene in stolon and tuber development. New Physiol. 198 (4), 1108–1120. doi: 10.1111/nph.12217
Pontier, D., Tronchet, M., Rogowsky, P., Lam, E., Roby, D. (1998). Activation of hsr203, a plant gene expressed during incompatible plant-pathogen interactions, is correlated with programmed cell death. Mol. Plant Microbe Interact. 11 (6), 544–554. doi: 10.1094/MPMI.1998.11.6.544
Priest, H. D., Filichkin, S. A., Mockler, T. C. (2009). Cis-regulatory elements in plant cell signaling. Curr. Opin. Plant Biol. 12 (5), 643–649. doi: 10.1016/j.pbi.2009.07.016
Ren, C., Guo, Y. C., Kong, J. H., Lecourieux, F., Dai, Z. W., Li, S. H., et al. (2020). Knockout of VvCCD8 gene in grapevine affects shoot branching. BMC Plant Biol. 20 (1), 47. doi: 10.1186/s12870-020-2263-3
Roesler, K., Lu, C., Thomas, J., Xu, Q., Vance, P., Hou, Z., et al. (2021). Arabidopsis carboxylesterase 20 binds strigolactone and increases branches and tillers when ectopically expressed in arabidopsis and maize. Front. Plant Sci. 12, 639401. doi: 10.3389/fpls.2021.639401
Rui, C., Peng, F., Fan, Y., Zhang, Y., Zhang, Z., Xu, N., et al. (2022). Genome-wide expression analysis of carboxylesterase (CXE) gene family implies GBCXE49 functional responding to alkaline stress in cotton. BMC Plant Biol. 22 (1), 194. doi: 10.1186/s12870-022-03579-9
Schaffer, R., Friel, E., Souleyre, E., Bolitho, K., Thodey, K., Ledger, S., et al. (2007). A genomics approach reveals that aroma production in apple is controlled by ethylene predominantly at the final step in each biosynthetic pathway. Plant Physiol. 144 (4), 1899–1912. doi: 10.1104/pp.106.093765
Schmidt, G. W., Delaney, S. K. (2010). Stable internal reference genes for normalization of real-time RT-PCR in tobacco (Nicotiana tabacum) during development and abiotic stress. Mol. Genet. Genomics 283, 233–241. doi: 10.1007/s00438-010-0511-1
Schneider, G., Jensen, E., Spray, C. R., Phinney, B. O. (1992). Hydrolysis and reconjugation of gibberellin A20 glucosyl ester by seedlings of zea mays l. Proc. Natl. Acad. Sci. U. S. A. 89 (17), 8045–8048. doi: 10.1073/pnas.89.17.8045
Seto, Y., Yasui, R., Kameoka, H., Tamiru, M., Cao, M., Terauchi, R., et al. (2019). Strigolactone perception and deactivation by a hydrolase receptor DWARF14. Nat. Commun. 10 (1), 191. doi: 10.1038/s41467-018-08124-7
Sharma, A., Kumar, V., Yuan, H., Kanwar, M. K., Bhardwaj, R., Thukral, A. K., et al. (2018). Jasmonic acid seed treatment stimulates insecticide detoxification in brassica juncea l. Front. Plant Sci. 9, 1609. doi: 10.3389/fpls.2018.01609
Simons, J. L., Napoli, C. A., Janssen, B. J., Plummer, K. M., Snowden, K. C. (2007). Analysis of the DECREASED APICAL DOMINANCE genes of petunia in the control of axillary branching. Plant Physiol. 143, 697–706. doi: 10.1104/pp.106.087957
Smoot, M. E., Ono, K., Ruscheinski, J., Wang, P. L., Ideker, T. (2011). Cytoscape 2.8: new features for data integration and network visualization. Bioinformatics 27 (3), 431–432. doi: 10.1093/bioinformatics/btq675
Snowden, K. C., Simkin, A. J., Janssen, B. J., Templeton, K. R., Loucas, H. M., Simons, J. L., et al. (2005). The decreased apical dominance1/Petunia hybrida CAROTENOID CLEAVAGE DIOXYGENASE8 gene affects branch production and plays a role in leaf senescence, root growth, and flower development. Plant Cell. 17 (3), 746–759. doi: 10.1105/tpc.104.027714
Souleyre, E. J., Marshall, S. D., Oakeshott, J. G., Russell, R. J., Plummer, K. M., Newcomb, R. D. (2011). Biochemical characterisation of MdCXE1, a carboxylesterase from apple that is expressed during fruit ripening. Phytochemistry 72 (7), 564–571. doi: 10.1016/j.phytochem.2011.01.020
Stuhlfelder, C., Mueller, M. J., Warzecha, H. (2004). Cloning and expression of a tomato cDNA encoding a methyl jasmonate cleaving esterase. Eur. J. Biochem. 271 (14), 2976–2983. doi: 10.1111/j.1432-1033.2004.04227.x
Sun, H., Guo, X., Qi, X., Feng, F., Xie, X., Zhang, Y., et al. (2021). SPL14/17 act downstream of strigolactone signaling to modulate rice root elongation in response to nitrate supply. Plant J. 106 (3), 649–660. doi: 10.1111/tpj.15188
Toh, S., Holbrook-Smith, D., Stogios, P. J., Onopriyenko, O., Lumba, S., Tsuchiya, Y., et al. (2015). Structure-function analysis identifies highly sensitive strigolactone receptors in striga. Science 350 (6257), 203–212. doi: 10.1126/science.aac9476
Ueguchi-Tanaka, M., Ashikari, M., Nakajima, M., Itoh, H., Katoh, E., Kobayashi, M., et al. (2005). GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin. Nature 437 (7059), 693–698. doi: 10.1038/nature04028
Vogel, J. T., Walter, M. H., Giavalisco, P., Lytovchenko, A., Kohlen, W., Charnikhova, T., et al. (2010). SlCCD7 controls strigolactone biosynthesis, shoot branching and mycorrhiza-induced apocarotenoid formation in tomato. Plant J. 61 (2), 300–311. doi: 10.1111/j.1365-313X.2009.04056.x
Wang, L., Gao, J. P., Wang, C., Xu, Y. L., Li, X. X., Yang, J., et al. (2022). Comprehensive analysis of long non-coding RNA modulates axillary bud development in tobacco (Nicotiana tabacum l.). Front. Plant Sci. 13. doi: 10.3389/fpls.2022.809435
Wang, X., Liu, D., Lin, J., Zhu, T., Liu, N., Yang, X., et al. (2021). Carotenoid cleavage dioxygenase genes of chimonanthus praecox, CpCCD7 and CpCCD8, regulate shoot branching in arabidopsis. Int. J. Mol. Sci. 22, 8750. doi: 10.3390/ijms22168750
Wang, B., Smith, S. M., Li, J. (2018). Genetic regulation of shoot architecture. Annu. Rev. Plant Biol. 69, 437–468. doi: 10.1146/annurev-arplant-042817-040422
Wang, J. L., Wang, H. B., Yang, H. Q., Hu, R. L., Wei, D. Y., Tang, Q. L., et al. (2022). The role of NAC transcription factors in flower development in plants. Chin. J. Biotechnol. 38 (8), 2687–2699. doi: 10.13345/j.cjb.210943
Wen, C., Zhao, Q., Nie, J., Liu, G. Q., Shen, L., Cheng, C. X., et al. (2016). Physiological controls of chrysanthemum DgD27 gene expression in regulation of shoot branching. Plant Cell Rep. 35, 1053–1070. doi: 10.1007/s00299-016-1938-6
Westfall, C. S., Muehler, A. M., Jez, J. M. (2013). Enzyme action in the regulation of plant hormone responses. J. Biol. Chem. 288 (27), 19304–19311. doi: 10.1074/jbc.R113.475160
Xie, X. (2016). Structural diversity of strigolactones and their distribution in the plant kingdom. J. Pestic. Sci. 41, 175–180. doi: 10.1584/jpestics.J16-02
Xie, X., Qin, G., Si, P., Luo, Z., Gao, J., Chen, X., et al. (2017). Analysis of nicotiana tabacum PIN genes identifies NtPIN4 as a key regulator of axillary bud growth. Physiol. Plant. 160, 222–239. doi: 10.1111/ppl.12547
Xu, E., Chai, L., Zhang, S., Yu, R., Zhang, X., Xu, C., et al. (2021). Catabolism of strigolactones by a carboxylesterase. Nat. Plants. 7 (11), 1495–1504. doi: 10.1038/s41477-021-01011-y
Yang, J. K., Tong, Z. J., Fang, D. H., Chen, X. J., Zhang, K. Q., Xiao, B. G. (2017). Transcriptomic profile of tobacco in response to phytophthora nicotianae infection. Sci. Rep. 7 (1), 401–407. doi: 10.1038/s41598-017-00481-5
Yang, Y., Xu, R., Ma, C. J., Vlot, A. C., Klessig, D. F., Pichersky, E. (2008). Inactive methyl indole-3-acetic acid ester can be hydrolyzed and activated by several esterases belonging to the AtMES esterase family of arabidopsis. Plant Physiol. 147, 1034–1045. doi: 10.1104/pp.108.118224
Yang, H., Zhao, L., Zhao, S., Wang, J., Shi, H. (2017). Biochemical and transcriptomic analyses of drought stress responses of LY1306 tobacco strain. Sci. Rep. 7 (1), 17442. doi: 10.1038/s41598-017-17045-2
Yao, X. Z., Chen, J. L., Zhou, J., Yu, H. C., Ge, C. N., Zhang, M., et al. (2019). An essential role for miRNA167 in maternal control of embryonic and seed development. Plant Physiol. 180 (1), 453–464. doi: 10.1104/pp.19.00127
Yoneyama, K., Xie, X., Yoneyama, K., Kisugi, T., Nomura, T., Nakatani, Y., et al. (2018). Which are the major players, canonical or non-canonical strigolactones? J. Exp. Bot. 69 (9), 2231–2239. doi: 10.1093/jxb/ery090
Yu, Y., Yu, M., Zhang, S., Song, T., Zhang, M., Zhou, H., et al. (2022). Transcriptomic identification of wheat AP2/ERF transcription factors and functional characterization of TaERF-6-3A in response to drought and salinity stresses. Int. J. Mol. Sci. 23, 3272. doi: 10.3390/ijms23063272
Zhang, Z., Li, J., Zhao, X. Q., Wang, J., Wong, G. K., Yu, J. (2006). KaKs_Calculator: calculating ka and ks through model selection and model averaging. Genomics Proteomics Bioinf. 4 (4), 259–263. doi: 10.1016/S1672-0229(07)60007-2
Zhang, Y., Waseem, M., Zeng, Z., Xu, J., Chen, C., Liu, Y., et al. (2022). MicroRNA482/2118, a miRNA superfamily essential for both disease resistance and plant development. New Phytol. 233 (5), 2047–2057. doi: 10.1111/nph.17853
Zhao, J., Li, L., Zhao, Y., Zhao, C. X., Chen, X., Liu, P. P., et al. (2018). Metabolic changes in primary, secondary, and lipid metabolism in tobacco leaf in response to topping. Anal. Bioanal. Chem. 410, 839–851. doi: 10.1007/s00216-017-0596-z
Zhao, L. H., Zhou, X. E., Wu, Z. S., Yi, W., Xu, Y., Li, S., et al. (2013). Crystal structures of two phytohormone signal-transducing α/β hydrolases: karrikin-signaling KAI2 and strigolactone-signaling DWARF14. Cell Res. 23 (3), 436–439. doi: 10.1038/cr.2013.19
Zhu, T. K., Du, P. P., Zeng, L. J., Lü, H., Zhao, H. M., Li, Y. W., et al. (2019). Variation in metabolism and degradation of di-n-butyl phthalate (DBP) by high- and low-DBP accumulating cultivars of rice (Oryza sativa l.) and crude enzyme extracts. Sci. Total. Environ. 10 (668), 1117–1127. doi: 10.1016/j.scitotenv.2019.03.047
Keywords: carboxylesterase, tobacco, differential expression, axillary bud, strigolactone, NtCXE22
Citation: Wang L, Xie X, Xu Y, Li Z, Xu G, Cheng L, Yang J, Li L, Pu W and Cao P (2022) Comprehensive analysis of the carboxylesterase gene reveals that NtCXE22 regulates axillary bud growth through strigolactone metabolism in tobacco. Front. Plant Sci. 13:1019538. doi: 10.3389/fpls.2022.1019538
Received: 15 August 2022; Accepted: 24 November 2022;
Published: 12 December 2022.
Edited by:
Ruifeng Yao, Hunan University, ChinaReviewed by:
Yuzhou Zhang, Institute of Science and Technology Austria (IST Austria), AustriaPeipei Xu, Shanghai Institutes for Biological Sciences (CAS), China
Copyright © 2022 Wang, Xie, Xu, Li, Xu, Cheng, Yang, Li, Pu and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peijian Cao, cGVpamlhbmNhb0AxNjMuY29t; Wenxuan Pu, cHV3eDA2MDVAaG5neXRvYmFjY28uY29t
 Lin Wang
Lin Wang Xiaodong Xie
Xiaodong Xie Yalong Xu
Yalong Xu Zefeng Li2
Zefeng Li2 Guoyun Xu
Guoyun Xu Lingtong Cheng
Lingtong Cheng Jun Yang
Jun Yang Lei Li
Lei Li Peijian Cao
Peijian Cao