- Qinghai Academy of Animal Husbandry and Veterinary Sciences, Qinghai Provincial Key Laboratory of Adaptive Management on Alpine Grassland, Key Laboratory of Superior Forage Germplasm in the Qinghai–Tibetan Plateau, Qinghai University, Xining, China
Grazing rest during the spring regreening period is the most economical and feasible measure for the ecological restoration of degraded alpine meadows and has been widely popularized and applied in China. The aim of the present study was to undertake a comparative analysis of the effects of grazing rest on the ecological restoration of degraded alpine meadows by plant photosynthesis and respiration. Coverage, height, ground biomass, belowground biomass of degraded alpine meadow vegetation, net photosynthetic rate, stomatal conductance, transpiration rate, intercellular CO2 concentration, chlorophyll fluorescence parameters, relative chlorophyll content, respiration rate, metabolite content, leaf relative water content, and related mineral element content of the dominant grass Elymus nutans Griseb. were measured in degraded alpine grassland with different grazing rest years. The results show that grazing rest during the spring regreening period promoted the ecological restoration of degraded alpine meadows by enhancing the photosynthesis and respiration of the dominant grass E. nutans Griseb. Grazing rest enhanced photosynthesis in dominant grass by increasing metabolites related to the Calvin cycle, chlorophyll content, leaf relative water content, and related mineral element content. Grazing at rest enhanced the respiration of dominant grass by increasing metabolites related to the TCA cycle, leaf relative water content, and related mineral element content. This positive effect gradually became stable with increasing years of grazing rest. Our results provide a fundamental basis for the popularization and application of grazing rest during the spring regreening period on degraded Tibetan Plateau grasslands.
Introduction
The Qinghai–Tibet Plateau is a sensitive and ecologically fragile zone of global climate change. Because of global climate change and human activities, alpine grasslands on the Qinghai–Tibet Plateau continue to degrade, and the structure and function of the ecosystem are seriously disturbed (Fan et al., 2010). Overgrazing is the main reason for the degradation of alpine grasslands (Zimmer et al., 2010; Ash et al., 2011; Selemani et al., 2013; Li et al., 2016; Shang et al., 2017); therefore, short-term grazing rest has become an effective way to control degraded grasslands and perform natural restoration (Wu et al., 2017). The forage spring regreening period refers to the stage at which plants end their dormant state and begin to recover with an increase in temperature and moisture conditions after forage overwintering. This is the most important stage for the initial growth of grassland vegetation in a year (Zhang et al., 2011). Consequently, the implementation of grazing rest during the spring regreening period is an effective approach for the natural restoration and rational utilization of degraded grasslands. How grazing rest during the spring regreening period promotes the restoration of degraded grasslands remains a research hotspot (Mavromihalis et al., 2013; Li et al., 2017; Fedrigo et al., 2018). The answer to this scientific question can not only fill in the mechanism of the significant restoration of degraded grassland by grazing rest during spring regreening period but also provide a strong theoretical basis for the promotion of grazing rest during spring regreening period measures and further provide guidance for grassland management policies in China.
Carbon assimilation and utilization by plants play an important role in the restoration of degraded alpine grassland vegetation, and the main processes of carbon metabolism are photosynthesis and respiration (Liu et al., 2008; Chamizo et al., 2021). Photosynthesis and respiration are also sensitive processes in response to environmental changes (Martínkov et al., 2021; Crous et al., 2022). These can directly reflect the growth status of grassland plants (Chen et al., 2005). Zhao et al. (2009) and Zheng et al. (2011) reported that overgrazing significantly reduces the photosynthetic and transpiration rates of forage plants, and chlorophyll fluorescence parameters are appropriate for detecting the effect of environmental factors (Simkó et al., 2020). Chlorophyll is essential for plant photosynthesis. Livestock grazing can affect the chlorophyll content in steppe plants of Tuva (Zvereva, 2004). Plant respiration refers to the process by which plants absorb O2 or release CO2 per unit time, which provides most of the energy required for plant life activities (Noguchi and Yoshida, 2008). Shen et al. (2013) reported that grazing affects the respiration rate of grassland plants. In addition, photosynthesis and respiration are inseparable from water and mineral elements—for example, nitrogen (N), phosphorus (P), and potassium (K) are essential elements in photosynthesis and respiration (Brooks, 1986; Nobuyuki et al., 2008; Zhang et al., 2017). Magnesium (Mg)-containing chelatase is the first enzyme in the chlorophyll biosynthetic pathway (Rissler et al., 2002). Copper (Cu) is vital for photosynthetic and respiratory electron transport processes and other cellular redox reactions (Biswas et al., 2013), and manganese (Mn) is an essential component of chloroplasts (Anja and Sébastien, 2018). However, there is a dearth of information regarding the reasons underlying this grazing rest-induced effect on forage photosynthesis and respiration. Metabolomics is an emerging approach in the post-genome era, which can comprehensively analyze the changes in metabolite content in plants and their dynamic responses to exogenous environmental factors (Ning et al., 2013). Therefore, this approach is a good choice to explore the response mechanism of photosynthesis and respiration processes to grazing rest.
The objectives of this study were (a) to identify the effects on the physiological characteristics of degraded alpine meadow vegetation after varying years of grazing rest during the spring regreening period and (b) to determine how priming of forage with grazing rest during the spring regreening period affects photosynthesis and respiration in a dominant grass (E. nutans Griseb.) for the degraded alpine meadow vegetation.
Materials and methods
Plant materials and treatments
The study site is located in Wariga Village, Mole Town, Qilian County, Qinghai Province, with a geographical location of 37°56′ N, 100°13′ E and an altitude of 3,650 m mainly containing alpine meadow soil. The grassland is a typical alpine meadow vegetation. The main species were Kobresia pygmaea, Kobresia humilis, E. nutans Griseb., and Poa crymophila. A relatively uniform natural alpine meadow was selected as the test area, with 24 hm2 as the treatment area and the other 6 hm2 as the control. The grassland utilization patterns were winter and spring pastures. The grassland degradation level in the experimental area was moderate (i.e., the proportion of edible grass in the grassland was 5–15%). The mean value of the treatment area was divided into four parts. Grazing rest was implemented during the green-up period from 2015, 2016, 2017, and 2018 (the green-up period was from May 10 to July 10): treatment 1, grazing rest during the green-up period for 1 year (2018); treatment 2, grazing rest during the green-up period for 2 years (2017 and 2018); treatment 3, grazing rest during the green-up period for 3 years (2016, 2017, and 2018); and treatment 4, grazing rest during the green-up period for 4 years (2015, 2016, 2017, and 2018). The control area was free grazing according to local traditional (with moderately severe grazing intensity and utilization rate of forage grass above 50%). The control and treatment areas were three replicates, each with 2 hm2. The total grassland coverage, ground biomass, belowground biomass and height, photosynthetic characteristics, chlorophyll fluorescence parameters, respiration rate, relative chlorophyll content, and leaf relative water content of the dominant species’ leaves were measured at nine representative points in each replicate, and the indices were measured in August 2018. Based on the preliminary test results of our research group, the dominant species selected in this study was E. nutans Griseb.
Quantitative characteristics of alpine meadow
A quadrate method was used to determine the quantitative characteristics of grassland vegetation, and the quadrate area was 50 cm × 50 cm. The specific method was as follows: the total coverage of vegetation in the quadrate was evaluated by visual measurement. A steel tape was used to select three plants of E. nutans Griseb. in each square to measure the natural plant height, which was calculated as the average plant height of the dominant herbage species in the quadrate. All plants in the quadrate square on the ground were cut, put in an envelope bag, and brought to the laboratory to be dried to constant weight at 75°C. Their dry weight was the ground biomass. Five samples (0–15 cm) of the plant underground root system were taken from the quadrate with a root drill (inner diameter: 5 cm) and dried to constant weight after being washed with clean water, which was converted into the belowground biomass of vegetation in the quadrate.
Leaf water and chlorophyll contents
Ten representative E. nutans plants were selected, of which the leaves were cut and weighed as fresh weight and then brought to the laboratory for drying to a constant weight at 75°C. The dry weight was obtained, and the leaf water content was calculated. Relative chlorophyll content was measured using a chlorophyll meter (SPAD-502).
Photosynthesis and respiration parameters
The photosynthetic characteristics, chlorophyll fluorescence parameters, and respiration rate of Elymus nutans were measured using li-COR 6400XT (LI-COR, Lincoln, NE). The photosynthetic characteristics included the net photosynthetic rate (Pn), transpiration rate (Tr), intercellular CO2 concentration (Ci), stomatal conductance (Gs), respiration rate (R), and fluorescence parameters. The chlorophyll fluorescence parameters included photochemical quantum efficiency (Fv/Fm), (Fv′/Fm′), actual photochemical quantum efficiency (φPSII), photochemical quenching coefficient (qP), and electron transfer rate (ETR). After being induced by natural light for 1.5–2 h, an open air path was adopted. According to the average temperature of 09:00–12:00 during the measurement period, the temperature of the measuring chamber (T-block) was set to 25°C, and the flow rate was 500 mol S-1. After the gas exchange parameters were measured, the light source was closed. The leaves were maintained in the dark for 30 min for adaptation before measuring the minimum (F0) and maximum (Fm) fluorescence.
Metabolite content analysis
Representative and healthy two-leaf pots from each treatment replicate were selected as the six replicates for metabolite content analysis. Fresh leaves (0.1 g) were collected from each replicate (pot) for each treatment, immediately frozen in liquid nitrogen, and stored at −80°C for subsequent analysis. The extraction protocol used was modified from that described by Du et al. (2012). The frozen leaves were ground to a fine powder with liquid N2, and then 100 mg of the powdered leaves was weighed in a 2-ml centrifuge tube. Then, 750 μl of methanol, 250 μl of chloroform, and 100 μl of aqueous chlorphenylalanine solution (3 mg ml–1, as the internal standard solution) were added to the tube, and the solution was extracted at 60 Hz for 5 min in an ultrasonic water bath. The extraction solution was centrifuged at 12,000 × g for 10 min at 4°C, and then 400 μl of the polar phase was decanted and dried in a Centrivap benchtop centrifugal vacuum concentrator (Labconco, Kansas City, MI, USA). The dried polar phase was incubated for 90 min at 37°C with 100 μl methoxyamine hydrochloride (20 mg ml–1) in pyridine and then incubated with 100 μl bis (trimethylsilyl) trifluoroacetamide for 1 h at 70°C. After methoximation and trimethylsilylation, the extracts were analyzed according to Du et al. (2013) using a gas chromatograph–mass spectrometer (TurboMass-Autosystem XL; PerkinElmer, Waltham, MA, USA). The metabolites detected were identified using the Turbomass 4.1.1 software (PerkinElmer) coupled with commercially available compound libraries: NIST 2005 (PerkinElmer, Waltham, MS) and Wiley 7.0 (John Wiley & Sons, Hoboken, NJ).
Mineral element content analysis
Fresh and healthy leaves were dried at 60°C until a constant weight was achieved. The dried leaves were ground, and 1 g of powdered sample was weighed. Then, the weighed particulates were digested with a mixture of H2SO4 and H2O2 for further N and P determination. Total N was analyzed using a Kjeltec 2300 analyzer (Foss Tecator AB, Hoeganaes, Sweden), and the vanadium molybdate yellow colorimetric method was used to determine the total leaf P content.
The powdered samples (1 g) were placed in a silica crucible for K, Mg, Cu, and Mn determination (25 ml). The silica crucible was heated at 550°C for 3 h, and 2 ml of double-distilled water was added when the silica crucible was cooled. Then, 10 ml of 6.00 mol/L muriatic acid was added to the silica crucible at 25°C and was heated again until dry. Subsequently, 5 ml of 6.00 mol L–1 muriatic acid was added to the silica crucible and then dissolved in double-distilled water up to 50.00 ml for further determination of K, Mg, Cu, and Mn. The blank control group was subjected to the same procedure. K, Mg, Cu, and Mn were determined using an atomic absorption spectrophotometer (SOLAAR, Thermo Elemental) at 766.5, 285.2, 324.8, and 279.5 nm, respectively. The detection limits (micrograms per milliliter) of the four elements were 0.2474, 0.1650, 0.0633 and 0.0306. The instruments were calibrated using standard solutions (0.20–100 μg ml–1) for the above-mentioned elements.
Statistical analysis
Data were preliminarily sorted and statistically analyzed using Excel 2010, and an independent sample t-test was conducted using SPSS 20.0, with a significance level of 0.05. Plot analysis was performed using Sigma Plot 12.5, and correlation analysis was conducted using R language.
Results
Effects of grazing rest during the spring regreening period on the quantitative characteristics of degraded alpine meadow vegetation
The quantitative characteristics of degraded alpine meadow vegetation included coverage, height, ground biomass, and belowground biomass. In this study, grazing rest during the spring regreening period significantly increased the coverage, height, ground biomass, and belowground biomass of degraded alpine meadow vegetation compared with the control (Figure 1). The coverage significantly increased from grazing rest during the spring regreening period of 1 year. There was no significant difference in coverage between grazing rests for one to four years. The height was significantly increased from grazing rest during the spring regreening period for 2 years, but then it decreased for grazing rest for 3 or 4 years. There was no significant difference in height between grazing rests for 3 and 4 years. The ground biomass significantly increased from grazing rest during the spring regreening period for 1 year and then approached the peak level for grazing for the remaining 2 years. The ground biomass did not differ significantly between grazing rests for 3 and 4 years. The belowground biomass significantly increased from grazing rest during the spring regreening period for 1 year but then decreased for grazing rest for 3 or 4 years. The belowground biomass did not differ significantly between grazing rests for 3 and 4 years.
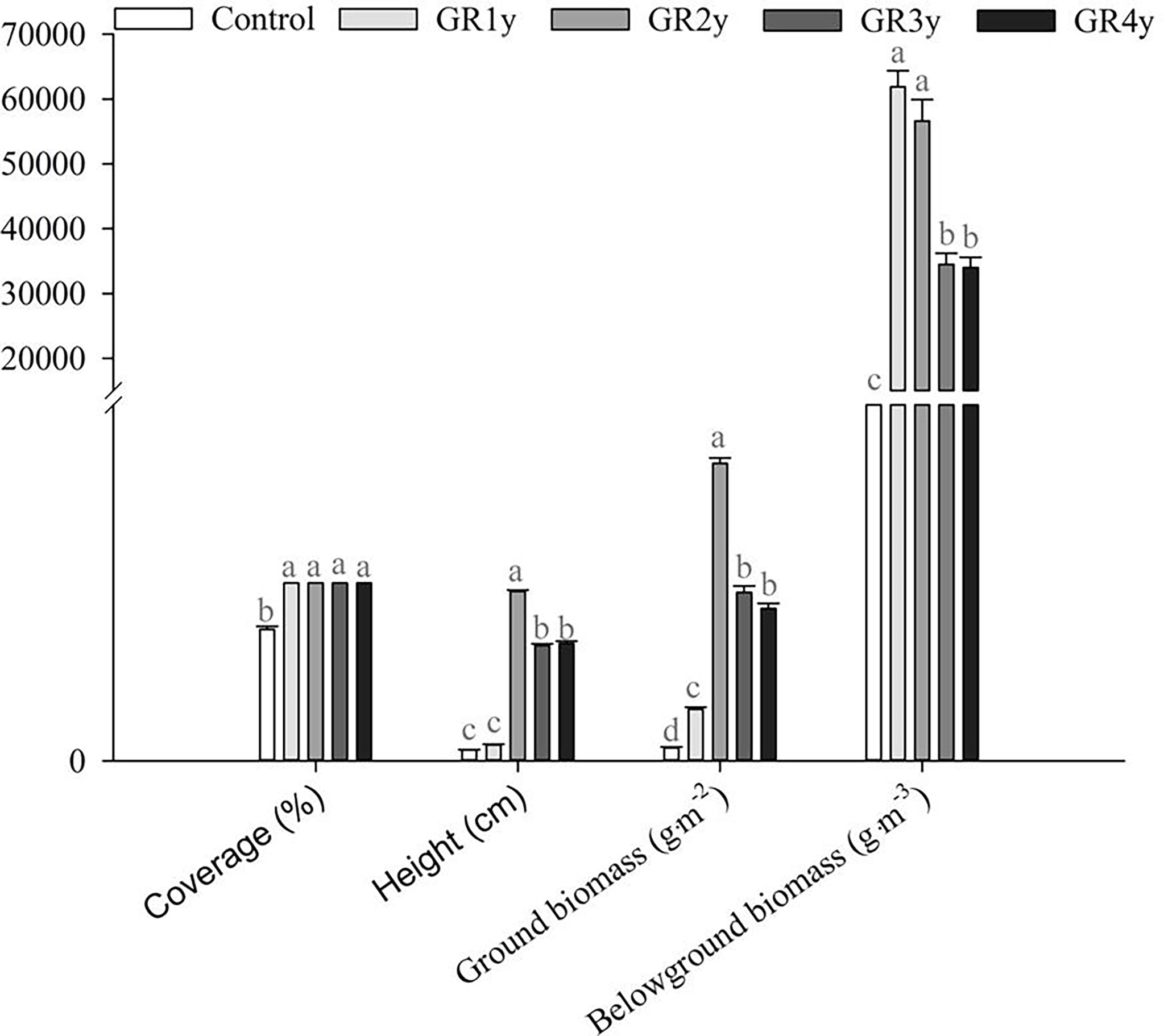
Figure 1 Effect of grazing rest during the spring regreening period on the quantity characteristics of the degraded alpine meadow vegetation. Bars represent SEs. Common letters above columns indicate no significant difference at P = .05. GR 1y, GR 2y, GR 3y, and GR 4y mean grazing rest for 1, 2, 3, and 4 years, respectively.
Effects of grazing rest during the spring regreening period on the photosynthesis of degraded alpine meadow vegetation’s dominant grass E. nutans Griseb.
All photosynthetic characteristics were significantly increased by grazing rest during the spring regreening period for several years compared with the control (Figure 2). The Pn, Cond, and Tr approached peak levels for grazing for the remaining 2 years. The Ci significantly increased for grazing rest during the spring regreening period of 2, 3, or 4 years, and there was no significant difference between them.
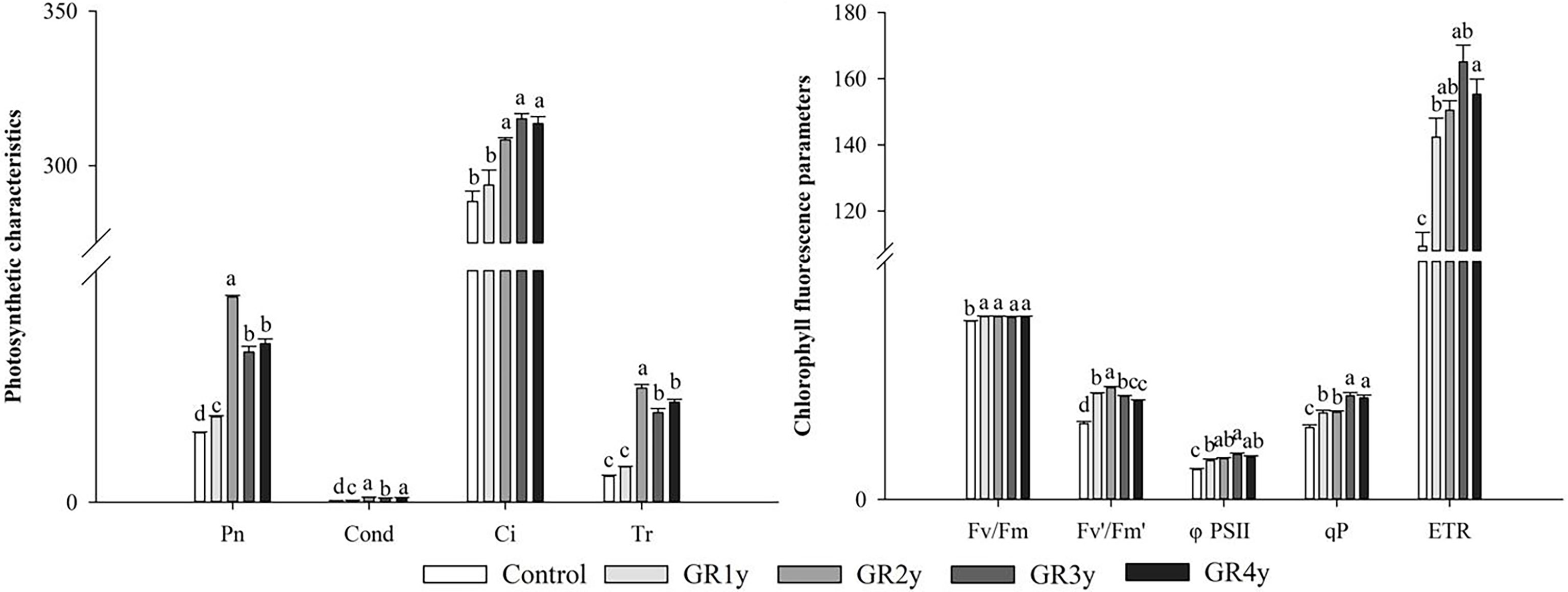
Figure 2 Effect of grazing rest during the spring regreening period on the photosynthesis of the dominant grass Elymus nutans Griseb of the degraded alpine meadow vegetation. Bars represent SEs. Common letters above columns indicate no significant difference at P = .05. GR 1y, GR 2y, GR 3y, and GR 4y mean grazing rest for 1, 2, 3, and 4 years, respectively.
All chlorophyll fluorescence parameters were significantly increased by grazing rest during the spring regreening period for several years compared with the control (Figure 2). The maximal quantum yield of PSII (Fv/Fm) was not significantly different between grazing rests for 1, 2, 3, or 4 years. The photochemical efficiency of PSII in the light (Fv′/Fm′), actual photochemical quantum efficiency (φPS II), and ETR tended to increase first and then decrease. The photochemical quenching coefficient (qP) approached a peak level for grazing rest of 3 or 4 years.
The Calvin cycle is an important photosynthetic process. In this study, there were eight metabolites associated with this cycle, which were significantly changed by grazing rest during the spring regreening period for several years (Figure 3). The contents of all eight metabolites were significantly increased by grazing rest during the spring regreening period for 2, 3, and 4 years compared with the control, and the value of log2FC reached a maximum at grazing rest for 2 years.
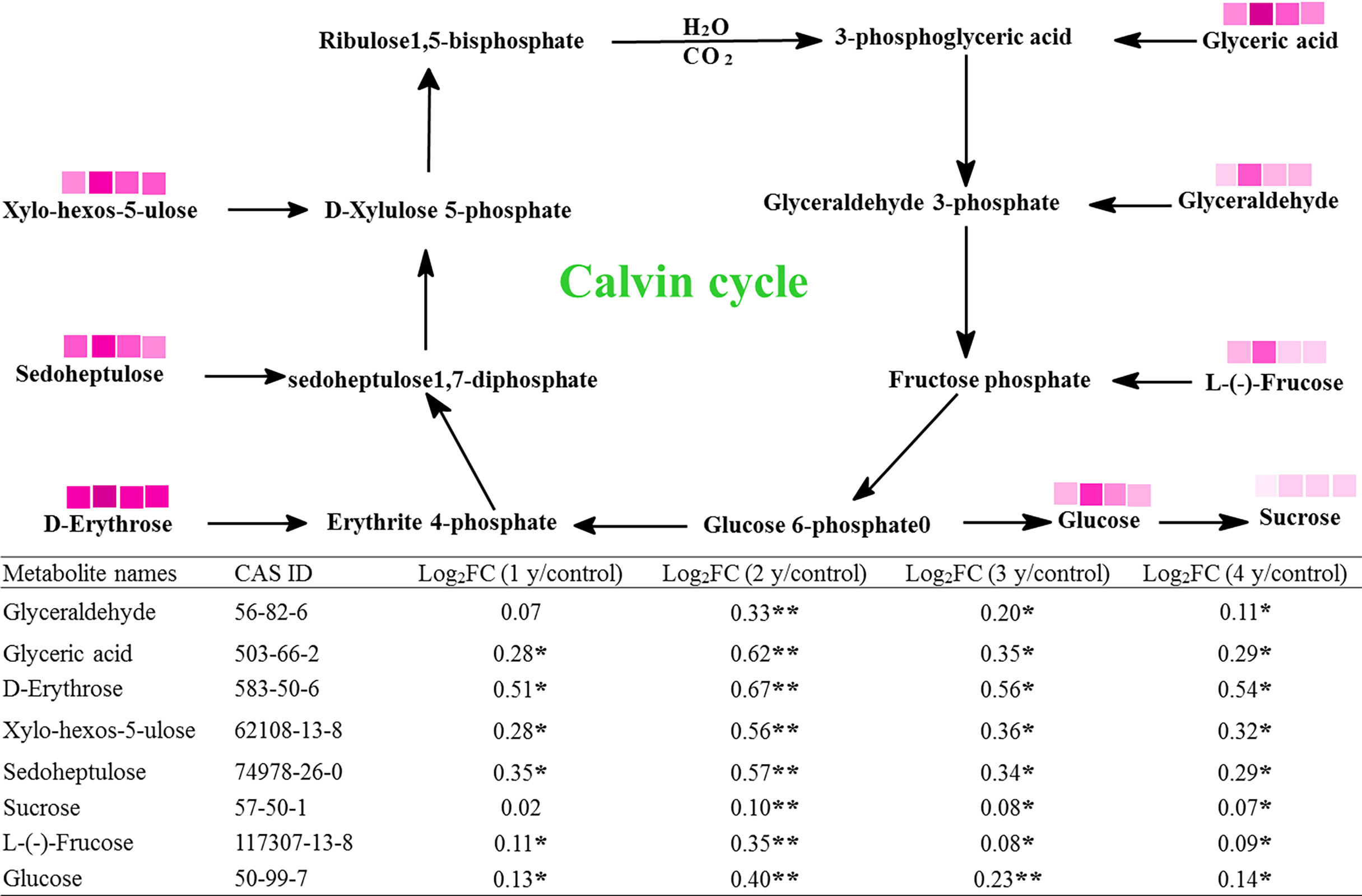
Figure 3 Effect of grazing rest during the spring regreening period on the Calvin cycle of the dominant grass Elymus nutans Griseb of the degraded alpine meadow vegetation. FC, fold change of relative content; *significant difference at P = .05; **significant difference at P = .01  represent log2FC ≤0.7, ≤0.6, ≤0.5, ≤0.4, ≤0.3, ≤0.2, ≤0.1, and ≤0.05, respectively.
represent log2FC ≤0.7, ≤0.6, ≤0.5, ≤0.4, ≤0.3, ≤0.2, ≤0.1, and ≤0.05, respectively.
Effects of grazing rest during the spring regreening period on the chlorophyll synthesis of degraded alpine meadow vegetation’s dominant grass E. nutans Griseb.
Grazing rest during the spring regreening period significantly increased the relative chlorophyll content for several years compared with the control (Figure 4A). The relative chlorophyll content approached the peak level for grazing for the remaining 2 years, and there was no significant difference between grazing rest for 3 or 4 years. There were two metabolites associated with the chlorophyll synthesis pathway, which were significantly changed by grazing rest during the spring regreening period (Figure 4B). The contents of the two metabolites were significantly increased by grazing rest for 1, 2, 3, and 4 years compared with the control, and the value of log2FC reached a maximum after 2 years of grazing rest.
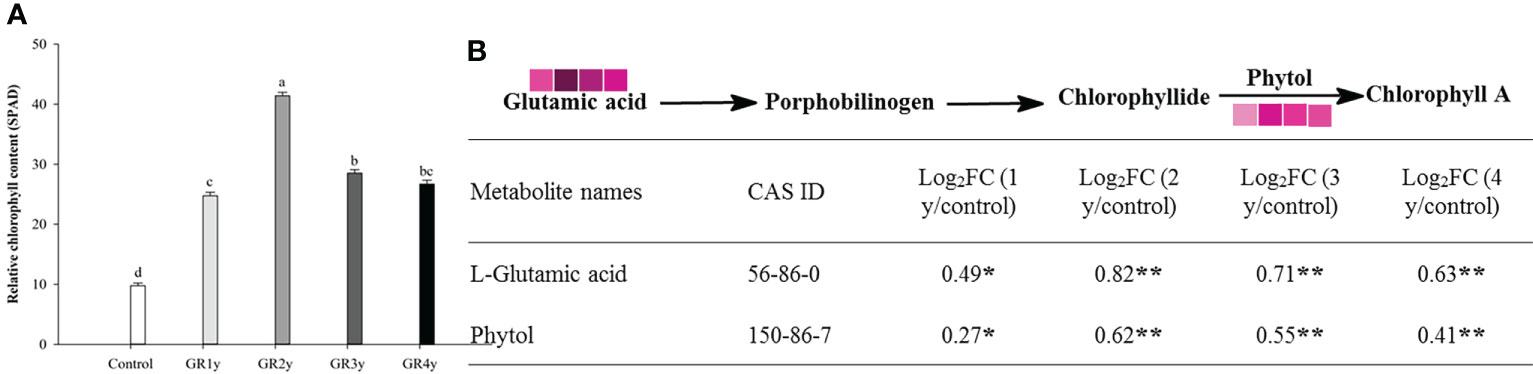
Figure 4 Effect of grazing rest during the spring regreening period on chlorophyll synthesis of degraded alpine meadows vegetation dominant grass Elymus nutans Griseb. (A) Effect of grazing rest during the spring regreening period on relative chlorophyll content of Elymus nutans Griseb. (B) Effect of grazing rest during the spring regreening period on metabolite contents in chlorophyll synthesis pathway of Elymus nutans Griseb. Bars represent SEs. Common letters above columns indicate no significant difference at P = .05. FC means fold change of relative content. * indicate significant difference at P = .05. ** indicate significant difference at P = .01. GR 1y, GR 2y, GR 3y and GR 4y mean grazing rest for 1 year, 2 years, 3 years and 4 years respectively  represent log2FC ≤0.7, ≤0.6, ≤0.5, ≤0.4, ≤0.3, ≤0.2, ≤0.1, and ≤0.05, respectively.
represent log2FC ≤0.7, ≤0.6, ≤0.5, ≤0.4, ≤0.3, ≤0.2, ≤0.1, and ≤0.05, respectively.
Effects of grazing rest during the spring regreening period on the respiration of degraded alpine meadow vegetation’s dominant grass E. nutans Griseb.
The respiration rate first increased gradually to the highest levels and then reduced owing to grazing rest during the spring regreening period, with the peak at grazing rest for 2 years. The TCA cycle is a critical link in the entire respiration process (Figure 5A). We found six metabolites, the contents of which significantly increased in this cycle caused by grazing rest during the spring regreening period for several years compared with the control (Figure 5B). The value of log2FC decreased after the peak at grazing rest for 2 years, and there was no significant difference between grazing rest for 3 and 4 years.
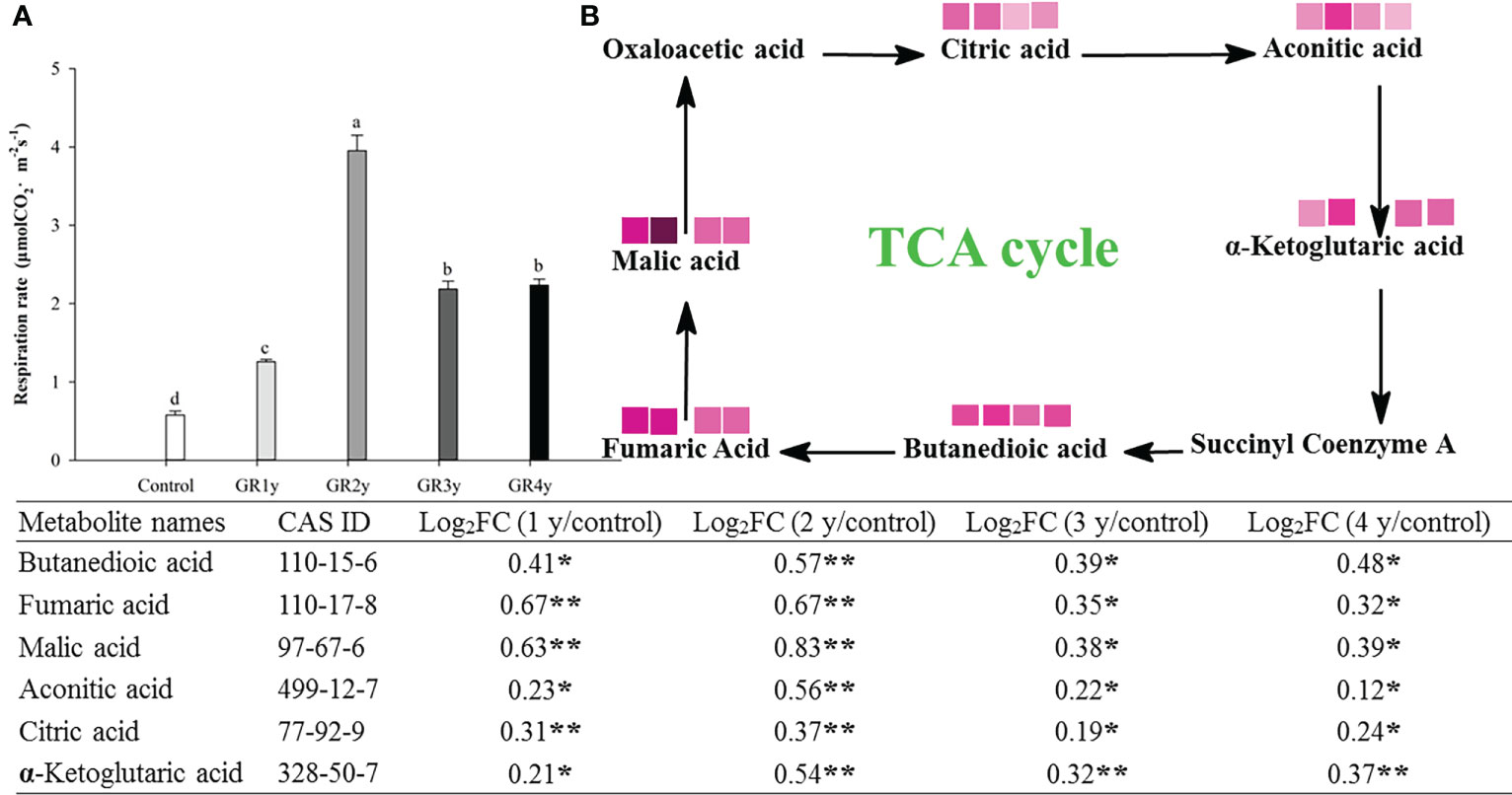
Figure 5 Effect of grazing rest during the spring regreening period on respiration of degraded alpine meadows vegetation dominant grass Elymus nutans Griseb. (A) Effect of grazing rest during the spring regreening period on respiration rate of Elymus nutans Griseb. (B) Effect of grazing rest during the spring regreening period on metabolite contents in TCA Cycle of Elymus nutans Griseb. Bars represent SEs. Common letters above columns indicate no significant difference at P = .05. FC means fold change of relative content. * indicate significant difference at P = .05. ** indicate significant difference at P = .01. GR 1y, GR 2y, GR 3y and GR 4y mean grazing rest for 1 year, 2 years, 3 years and 4 years respectively. represent Log2FC ≤0.7, ≤0.6, ≤0.5, ≤0.4, ≤0.3, ≤0.2, ≤0.1 and ≤0.05 respectively.  represent log2FC ≤0.7, ≤0.6, ≤0.5, ≤0.4, ≤0.3, ≤0.2, ≤0.1, and ≤0.05, respectively.
represent log2FC ≤0.7, ≤0.6, ≤0.5, ≤0.4, ≤0.3, ≤0.2, ≤0.1, and ≤0.05, respectively.
Effects of grazing rest during the spring regreening period on the leaf water and mineral contents of degraded alpine meadow vegetation’s dominant grass E. nutans Griseb.
Grazing rest during the spring regreening period significantly increased the leaf relative water content in all rest periods. The leaf water content was not significantly different between grazing rests for 1 to 4 years (Figure 6). The macro elements (N, P, and K) showed the same variation tendency that increased first and approached the peak at grazing rest for 2 years and then decreased. There was no significant difference between grazing rest for 3 and 4 years. Mg showed an increasing trend year by year and was significantly increased by grazing rest for 2, 3, and 4 years. The Cu content of grass leaves significantly increased by grazing rest during the spring regreening period, and there was no difference between the years of grazing rest. Grazing rest during the spring regreening period significantly increased the Mn content of the leaves. The Mn content under grazing rest for 2, 3, and 4 years was more than 1 year, and under the latter 3 years, the content tended to be stable (Figure 7).
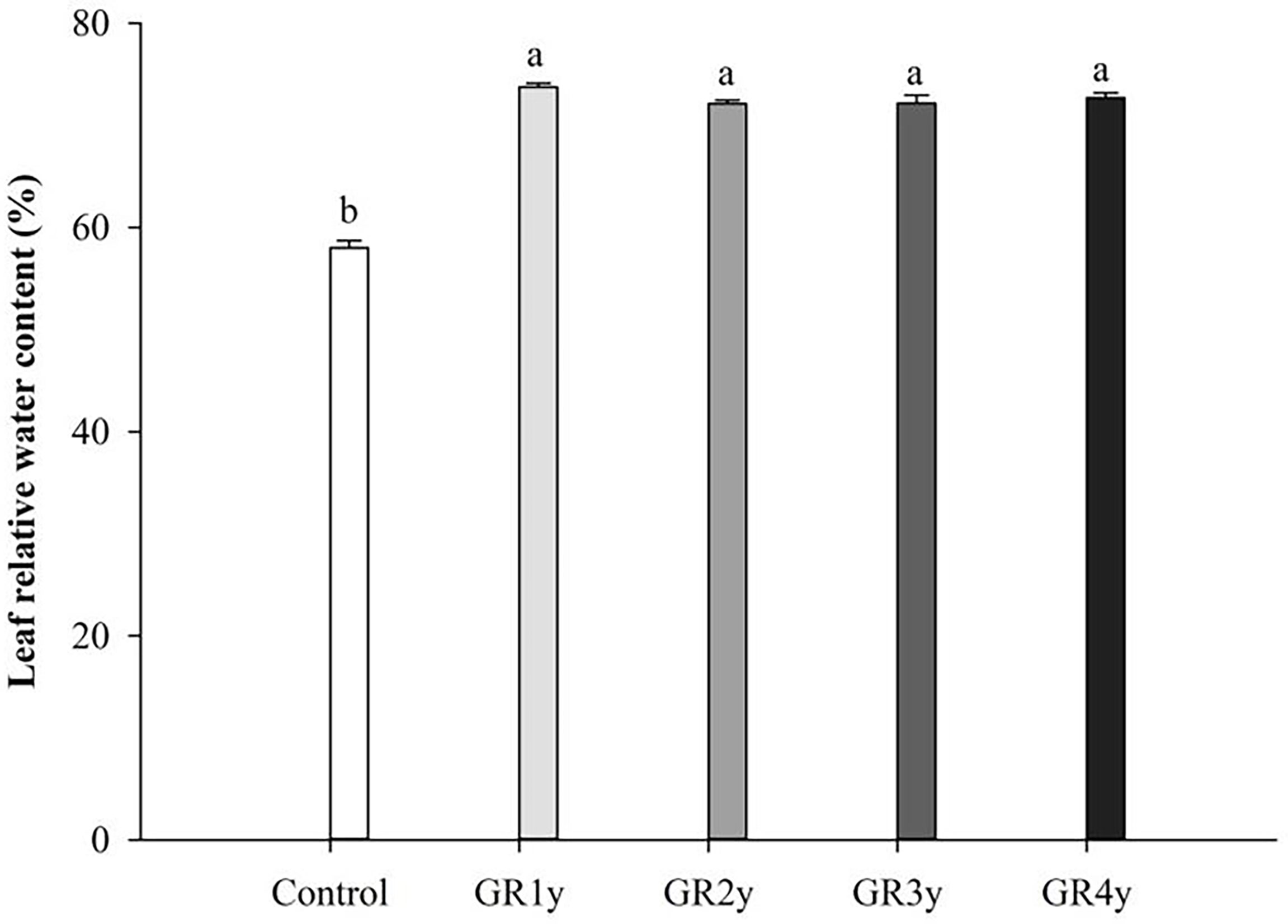
Figure 6 Effect of grazing rest during the spring regreening period on the leaf water content of the degraded alpine meadow vegetation. Bars represent SEs. Common letters above columns indicate no significant difference at P = .05. GR 1y, GR 2y, GR 3y, and GR 4y mean grazing rest for 1, 2, 3, and 4 years, respectively.
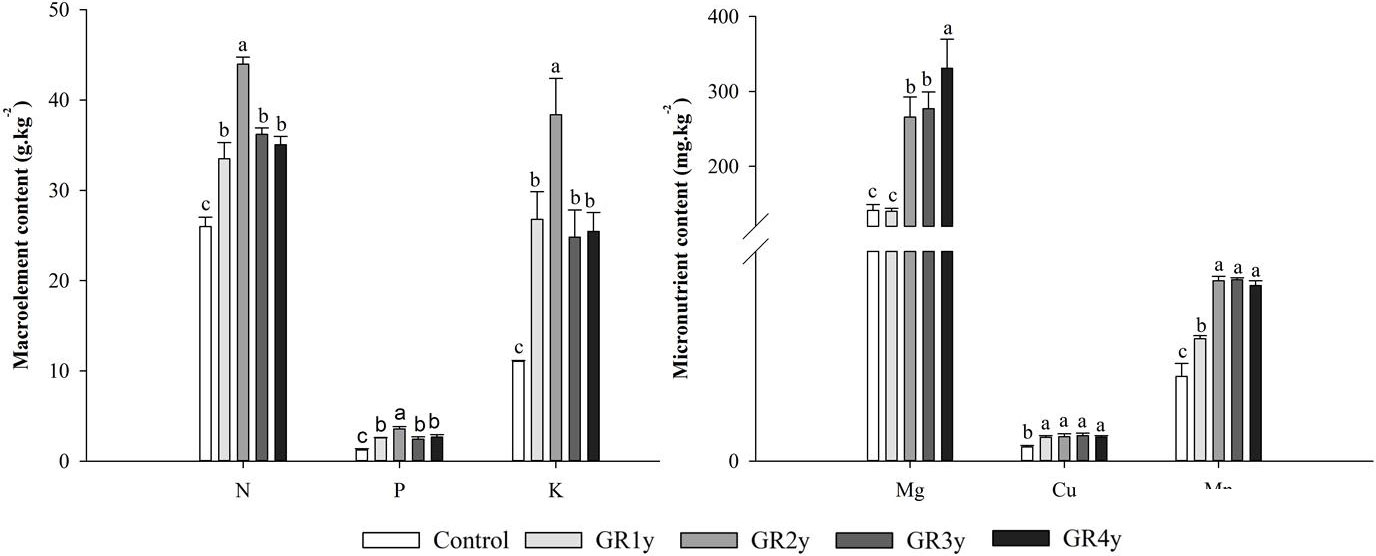
Figure 7 Effect of grazing rest during the spring regreening period on the mineral element content of the degraded alpine meadow vegetation. Bars represent SEs. Common letters above columns indicate no significant difference at P = .05. GR 1y, GR 2y, GR 3y, and GR 4y mean grazing rest for 1, 2, 3, and 4 years, respectively.
Correlation analysis of vegetation quantitative characteristics, photosynthetic characteristics, respiratory characteristics, and key influencing factors of degraded alpine meadows
In this study, all indicators of degraded alpine grassland vegetation were significantly correlated with each other (P < 0.05). The correlation coefficients between aboveground biomass and Pn, height and Pn, aboveground biomass and respiration rate, and height and respiration rate were 0.89, 0.91, 0.83, and 0.80, respectively (Figure 8). This indicated that the restoration of degraded meadow vegetation by grazing rest during the spring regreening period was significantly correlated with photosynthesis and respiration. Cond, α-ketoglutaric acid, Tr, sucrose, glutamic acid, phytol, and N were highly correlated with Pn, except vegetation quantitative characteristics. In addition, the correlation coefficient between Fv′/Fm′ and erythrose was 0.82. These indicated that the key factors for increased photosynthesis were the supply of reactants involved in the Calvin cycle and the synthesis of chlorophyll. Meanwhile, glyceric acid, α-ketoglutaric acid, glucose, and aconitic acid were highly correlated with respiration rate, except vegetation quantitative characteristics. This suggests that the increase in respiration was due to an increase in the direct reactant content of the TCA cycle.
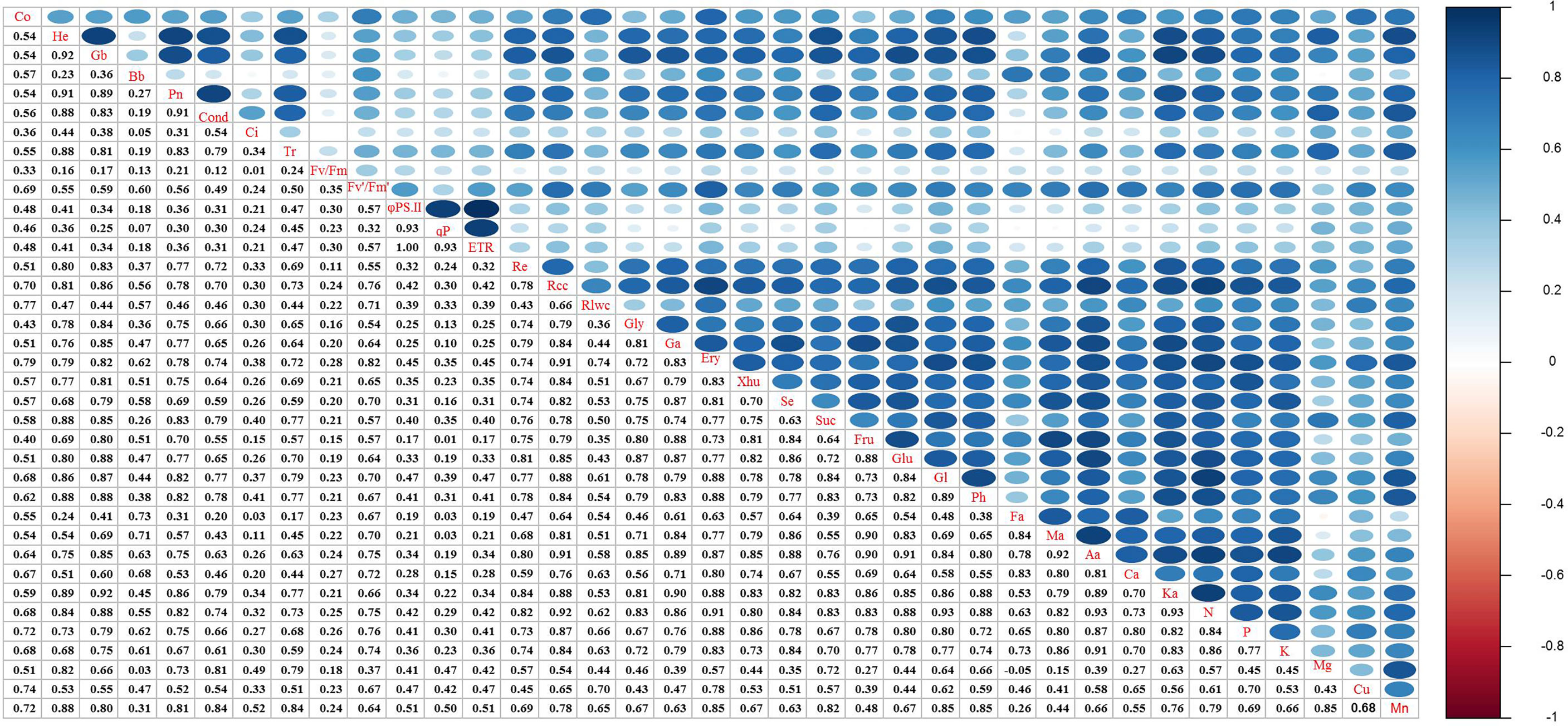
Figure 8 Effects of grazing rest during the spring regreening period on quantitative characteristics of degraded alpine meadow vegetation. Pn, Net photosynthetic rate; Cond, Stomatal conductance; Ci, Intercellular CO2 concentration; Tr, Transpiration rate; Fv/Fm, Photochemical quantum efficiency; Fv’/Fm’, Effective photochemical quantum efficiency; φPSII, Actual photochemical quantum efficiency; qP, Photochemical quenching coefficient; ETR, Electron transfer rate; Re, Respiratory rate; Co, Coverage; He, Height; Gb, Ground biomass; Bb, Belowground biomass; Rcc, Relative chlorophyll content; Rlwc, Relative leaf water content; Gly, DL-Glyceraldehyde; Ga, Glyceric acid; Ery, D-Erythrose; Xhu, Xylo-hexos-5-ulose; Se, Sedoheptulose; Suc, Sucrose; Fru, Frucose; Glu, Glucose; Gl, Glutamic acid; Ph, Phytol; Ba, Butanedioic acid; Fa, Fumaric acid; Ma, Malic acid; Aa, Aconitic acid; Ca, Citric acid; Ka, α-Ketoglutaric acid.
Discussion
Effects of grazing rest during the spring regreening period on the quantitative characteristics of degraded alpine meadow vegetation
Scholars generally agree that grazing rest can improve the vegetation growth of degraded grasslands (Zimmer et al., 2010; Ash et al., 2011; Selemani et al., 2013; Wu et al., 2017). In our study, grazing rest significantly increased the coverage, height, ground biomass, and belowground biomass of the degraded alpine meadow vegetation. Zhao et al. (2016) obtained a similar result in that grazing exclusion enhances plant height, total cover, aboveground biomass, and belowground biomass in the alpine steppe, alpine meadow, and swamp meadow. Bai et al. (2015); Qin et al. (2021), and Chen et al. (2007) also reported that grazing significantly decreases the aboveground biomass of the Inner Mongolia temperate steppe. Bai et al. (2015) indicated that grazing reduces root production. The most important reasons for the improvement of degraded grassland vegetation during grazing rest are to avoid trampling and feeding by livestock and to change the grassland ecosystem environment (Zhao et al., 2016). In addition, the improvement in vegetation quantitative characteristics gradually stabilized with the increase in rest years, which might be caused by the gradual adaptation to the environment without trampling and feeding by livestock. In order to understand the reason why the grazing rest during the spring regreening period promoted the restoration of the degraded alpine meadow vegetation, the photosynthesis and respiration processes were explored in this study. The correlation analysis showed that the quantitative characteristics of the alpine meadow vegetation were significantly correlated with the parameters of photosynthesis and respiration.
Effects of grazing rest during the spring regreening period on the photosynthesis of degraded alpine meadow vegetation’s dominant grass E. nutans Griseb.
The photosynthesis of grass leaves is the primary determinant of carbohydrate sources for plant growth and development (Ellsworth et al., 2004). Our results show that all photosynthetic functions (Pn, Cond, Tr, and Ci) in E. nutans were significantly increased by grazing rest. Several researchers have obtained similar results. They found that overgrazing by livestock dramatically restricts leaf photosynthetic capacity and function (such as Pn, Cond, Tr, and Ci) under field conditions (Chen et al., 2005; Zhao et al., 2009; Zheng et al., 2011; Shen et al., 2013; Ren et al., 2017). Chlorophyll fluorescence parameters are highly sensitive to changes in external environmental conditions and can be used to evaluate the effects of external disturbances on plants (Maxwell and Johnson, 2000). Wang et al. (2022) found that chlorophyll fluorescence shows a good correlation with grassland productivity. Our results show that all chlorophyll fluorescence parameters (Fv/Fm, Fv′/Fm′, φPS II, ETR, and qP) of E. nutans were significantly increased by grazing rest. Zhang et al. (2022) obtained similar results in that the regulation of chlorophyll fluorescence is constrained under grazing. In addition, Li et al. (2018) found that Fv/Fm, φPSII, and qP are not significantly changed by grazing for 1 year. This may be caused by different climates, grassland types, and dominant grasses. Weng and Lai (2005) and Wang et al. (2022) found that the chlorophyll fluorescence parameters are decreased by heat and drought tolerance. These results indicate that the increase in chlorophyll fluorescence parameters caused by grazing rest resulted from the elimination of grazing stress. However, there have been no detailed reports on why grazing rest promotes photosynthesis in herbage. By using metabolomic techniques, our study demonstrated that grazing rest significantly promoted the Calvin cycle in herbage. We found that the levels of eight metabolites related to the Calvin cycle significantly increased under grazing rest, and sucrose and erythrose had a higher correlation with related parameters of photosynthesis among the eight enriched metabolites. At present, the relationship between grazing rest and the Calvin cycle has not been reported.
Moreover, the photosynthetic responses in different grazing rest years were compared in this study. We found that all photosynthetic functions and chlorophyll fluorescence parameters significantly increased first and then gradually stabilized. These results might be caused by the similar pattern of changes in the eight metabolites in the Calvin cycle, chlorophyll content, leaf relative water content, and mineral element content. Chlorophyll, a photosynthetic pigment, plays an important role in the absorption and utilization of light energy by green plants. Chlorophyll content is closely related to the level of plant photosynthesis. Many other researchers have reported similar results in that grazing rest induces more chlorophyll than grazing (Thomas, 2012; Ren et al., 2017). Furthermore, we used metabolomics techniques and found that the increased chlorophyll content resulted from the increased levels of glutamic acid and phytol, which are metabolites related to chlorophyll biosynthesis, and the correlation coefficient between glutamic acid and relative chlorophyll content was 0.87. However, this may result from the decrease in plant leaf water and soil water contents after grazing disturbance, leading to some degradation of chlorophyll in forage leaves (Sohrabi et al., 2017). In this study, not only was leaf relative water content absolutely increased by grazing rest but also the N, Mg, and Cu contents in leaves. N and Mg are essential elements for chlorophyll biosynthesis (Rissler et al., 2002; Eckhardt et al., 2004). The Cu supply significantly increases the Chl-a concentration (Biswas et al., 2013). In addition to promoting chlorophyll biosynthesis, mineral elements play important roles in other photosynthetic processes. Nobuyuki et al. (2008) pointed out that N, P, and K deficiencies significantly decrease photosynthesis and RuBP carboxylase–oxygenase activity in rice (Oryza sativa) plants. Tanwar et al. (2013) indicated that phosphate application significantly increases photosynthesis and stomatal conductance in bell pepper (Capsicum annuum). Mn is indispensable for water splitting during photosynthesis (Eisenhut et al., 2018). Our results show that the N, P, K, Mg, Cu, and Mn contents were significantly increased by grazing rest. Yin and Lu (2013) obtained a similar result; Leymus chinensis displays increasing leaf N and P concentrations over time after grazing exclusion. Harrison et al. (2010) stated that grazing may affect photosynthesis as a consequence of changes in leaf water status, nitrogen content, and photosynthetic enzymes. Moreover, the variation trends of mineral elements and leaf relative water content with grazing rest years are consistent with the variation trends of the parameters related to underground biomass and photosynthesis. Therefore, it was reasonable to infer that grazing rest could enhance the grass absorption of mineral elements and water by increasing the underground biomass of herbage, thereby promoting chlorophyll biosynthesis and photosynthesis.
Effects of grazing rest during the spring regreening period on the respiration of degraded alpine meadow vegetation’s dominant grass E. nutans Griseb.
Plant respiration is a basic process of plant physiological metabolism that provides energy for plant life activities (Noguchi and Yoshida, 2008). Respiration and photosynthesis cooperate closely in plant energy metabolism (Archontoulis et al., 2012). Similar to the net photosynthetic rate, the respiration rate of the degraded alpine meadows vegetation’s dominant grass E. nutans Griseb. significantly increased by grazing rest during the spring regreening period. There are very few reports on the effects of grazing rest or grazing on plant leaf respiration rate. Shen et al. (2013) indicated that the dark respiration rate of Gentiana straminea in un-grazed regimes is higher than that in grazed regimes under ambient conditions. Furthermore, we used metabolomics to determine which metabolic process of respiration responds to grazing rest, and the results show that six key metabolites in the TCA cycle were significantly enriched and that aconitic acid and ketoglutaric acid had a higher correlation with respiration rate among the six enriched metabolites. In addition, a significant increase in respiration factors was also detected owing to grazing rest in this study. Water is necessary for respiration (Galmes et al., 2007). The rate of respiration is lower in P-deficient plants than in P-fertilized plants (Bahar et al., 2018). K enhances the respiration of Nicotiana tabacum by increasing the O2 uptake (Wakhloo and Ruppenthal, 1989). Copper oxidase is involved in the reduction of oxygen molecules in plants and has significant effects on plant respiration (Patterson, 2013). Mn increases the respiration intensity of plants and regulates redox processes (Hsieh, 2011). This may be because grazing rest could enhance the respiration of the degraded alpine meadow vegetation’s dominant grass E. nutans Griseb.
Conclusion
Grazing rest during the spring regreening period promoted the ecological restoration of degraded alpine meadows by enhancing the photosynthesis and respiration of the dominant grass E. nutans Griseb. Grazing rest enhanced photosynthesis in dominant grass by increasing the metabolites related to the Calvin cycle, chlorophyll content, leaf relative water content, and related mineral element content. Grazing rest also enhanced the respiration of dominant grass by increasing the metabolites related to the TCA cycle, leaf relative water content, and related mineral element content.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: according to state policies, requests to access these datasets should be directed to YL, liuying_yanhong@sina.com.
Author contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Funding
This research was supported by the National Natural Science Foundation of China (U21A20241) and the Key Laboratory Project of Qinghai Province, China (2020-ZJ-Y03).
Acknowledgments
The author would like to thank Yushou Ma and Shixiong Li for providing the experimental field, and Wenhui Liu for providing the instruments facility. The author is grateful to all editors and reviewers for their valuable suggestions on the manuscript.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Anja, K. L., Sébastien, T. (2018). Importing manganese into the chloroplast: many membranes to cross. Mol. Plant 11, S1674205218302405. doi: 10.1016/j.molp.2018.07.006
Archontoulis, S. V., Yin, X., Vos, J., Danalatos, N. G., Struik, P. C. (2012). Leaf photosynthesis and respiration of three bioenergy crops in relation to temperature and leaf nitrogen: how conserved are biochemical model parameters among crop species? J. Exp. Bot. 63 (2), 895–911. doi: 10.1093/jxb/err321
Ash, A. J., Corfield, J. P., Mcivor, J. G., Ksiksi, T. S. (2011). Grazing management intropical savannas: utilization and rest strategies to manipulate rangeland condition. Rangeland Ecol. Manage. 64 (3), 223–239. doi: 10.2111/REM-D-09-00111.1
Bahar, N., Gauthier, P., O'Sullivan, O. S., Thomas, B., Evans, J. R., Atkin, O. K. (2018). Phosphorus deficiency alters scaling relationships between leaf gas exchange and associated traits in a wide range of contrasting Eucalyptus species. Funct. Plant Biol. 45 (8), 813–826. doi: 10.1071/FP17134
Bai, W., Fang, Y., Zhou, M., Xie, T., Li, L., Zhang, W. H., et al. (2015). Heavily intensified grazing reduces root production in an inner Mongolia temperate steppe. Agriculture Ecosyst. Environ. 200, 143–150. doi: 10.1016/j.agee.2014.11.015
Biswas, H., Bandyopadhyay, D., Waite, A. (2013). Copper addition helps alleviate iron stress in a coastal diatom: Response of Chaetoceros gracilis from the bay of bengal to experimental Cu and fe addition. Mar. Chem. 157 (20), 224–232. doi: 10.1016/j.marchem.2013.10.006
Brooks, A. (1986). Effects of phosphorus nutrition on ribulose-1,5-bisphosphate carboxylase activation, photosynthetic quantum yield and amounts of some calvin-cycle metabolites in spinach leaves. Funct. Plant Biol. 13 (2), 221–237. doi: 10.1071/PP9860221
Chamizo, S., Rodríguez-Caballero, E., Moro, M. J., Cantón, Y. (2021). Non-rainfall water inputs: A key water source for biocrust carbon fixation. Sci. Total Environ. 792, 148299. doi: 10.1016/j.scitotenv.2021.148299
Chen, S. P., Bai, Y. F., Lin, G. H., Liang, Y., Han, X. G. (2005). Effects of grazing on photosynthetic characteristics of major steppe species in the xilin river basin, inner Mongolia, China. Photosynthetica 43 (4), 559–565. doi: 10.1007/s11099-005-0088-9
Chen, Y., Lee, G., Lee, P., Oikawa, T. (2007). Model analysis of grazing effect on above-ground biomass and above-ground net primary production of a Mongolian grassland ecosystem. J. Hydrology 333 (1), 155–164. doi: 10.1016/j.jhydrol.2006.07.019
Crous, K. Y., Uddling, J., Kauwe, M. (2022). Temperature responses of photosynthesis and respiration in evergreen trees from boreal to tropical latitudes. New Phytol. 234, 353–374. doi: 10.1111/nph.17951
Du, H., Wang, Z., Yu, W., Huang, B. (2012). Metabolic responses of hybrid bermudagrass to short-term and long-term drought stress. J. Am. Soc. Hortic. Sci. Am. Soc. Hortic. Sci. 137 (6), 411–420. doi: 10.21273/JASHS.137.6.411
Du, H., Zhou, P., Huang, B. (2013). Antioxidant enzymatic activities and gene expression associated with heat tolerance in a cool-season perennial grass species. Environ. Exp. Bot. 87, 159–166. doi: 10.1016/j.envexpbot.2012.09.009
Eckhardt, U., Grimm, B. H., Rtensteiner, S. (2004). Recent advances in chlorophyll biosynthesis and breakdown in higher plants. Plant Mol. Biol. 56 (1), 1–14. doi: 10.1007/s11103-004-2331-3
Eisenhut, M., Weber, A., Leister, D., Schneider, A., Hoecker, N., Schmidt, S. B., et al. (2018). The plastid envelope chloroplasr manganese transporter1 is essential for manganese homeostasis in Arabidopsis. Mol. Plant 11 (7), 955–969. doi: 10.1016/j.molp.2018.04.008
Ellsworth, D. S., Reich, P. B., Naumburg, E. S., Koch, G. W., Kubiske, M. E., Smith, S. D. (2004). Photosynthesis, carboxylation and leaf nitrogen responses of 16 species to elevated pCO2 across four free air CO2 enrichment experiments in forest, grassland and desert. Global Change Biol. 10 (12), 2121–2138. doi: 10.1111/j.1365-2486.2004.00867.x
Fan, J. W., Shao, Q. Q., Liu, J. Y., Wang, J. B., Harris, W., Chen, Z. Q., et al. (2010). Assessment of effects of climate change and grazing activity on grassland yield in the three rivers headwaters region of qinghai–Tibet plateau, China. Environ. Monit. Assess. 170 (1), 571–584. doi: 10.1007/s10661-009-1258-1
Fedrigo, J. K., Ataide, P. F., Azambuja Filho, J., Oliveira, L. V., Jaurena, M., Laca, E. A., et al. (2018). Temporary grazing exclusion promotes rapid recovery of species richness and productivity in a long-term overgrazed Campos grassland. Restor. Ecol. 26 (4), 677–685. doi: 10.1111/rec.12635
Galmes, J., Ribas-Carbo, M., Medrano, H., Flexas, J. (2007). Response of leaf respiration to water stress in Mediterranean species with different growth forms. J. Arid Environments 68 (2), 206–222. doi: 10.1016/j.jaridenv.2006.05.005
Harrison, M. T., Kelman, W. M., Moore, A. D., Evans, J. R. (2010). Grazing winter wheat relieves plant water stress and transiently enhances photosynthesis. Funct. Plant Biol. 37 (8), 726–736. doi: 10.1071/FP10040
Hsieh, S. I. (2011). Metal-responsive proteomes: Copper, iron, zinc, and manganese micronutrient deficiency in chlamydomonas reinhardtii[D] (Los Angeles: University of California).
Li, W., Cao, W., Wang, J., Li, X., Xu, C., Shi, S. (2017). Effects of grazing regime on vegetation structure, productivity, soil quality, carbon and nitrogen storage of alpine meadow on the qinghai-Tibetan plateau. Ecol. Engeering 98 (Complete), 123–133. doi: 10.1016/j.ecoleng.2016.10.026
Li, X., Huang, Q., Xue, M. I., Bai, Y., Zhang, M., Li, X. (2018). Grazing every month minimizes size but boosts photosynthesis in stipa grandis in the steppe of inner Mongolia, China. J. Arid Land 10, 4, 11. doi: 10.1007/s40333-018-0011-4
Li, X., Perry, G., Brierley, G. J. (2016). “Grassland ecosystems of the yellow river source zone: degradation and restoration[C],” in Landscape and ecosystem diversity, dynamics and management in the yellow river source zone (Switzerland, Springer International Publishing), 137–165.
Liu, X., Duan, S., Li, A., Xu, N., Cai, Z., Hu, Z. (2008). Effects of organic carbon sources on growth, photosynthesis, and respiration of Phaeodactylum tricornutum. J. Appl. Phycology 21, 239–246. doi: 10.1007/s10811-008-9355-z
Martínkov, J., Hajek, T., Adamec, L., Klimešová, J. (2021). Growth, root respiration and photosynthesis of a root-sprouting short-lived herb after severe biomass removal. Flora 284, 151915. doi: 10.1016/j.flora.2021.151915
Mavromihalis, J. A., Dorrough, J., Clark, S. G., Turner, V., Moxham, C. (2013). Manipulating livestock grazing to enhance native plant diversity and cover in native grasslands. Rangeland J. 35 (1), 95–108. doi: 10.1071/RJ12074
Maxwell, K., Johnson, G. N. (2000). Chlorophyll fluorescence–a practical guide. J. Exp. Bot. 51 (345), 659–668. doi: 10.1093/jexbot/51.345.659
Ning, Z., Lu, C., Zhang, Y., Zhao, S., Liu, B., Xu, X., et al. (2013). Application of plant metabonomics in quality assessment for large-scale production of traditional Chinese medicine. Planta Med. 79 (11), 897–908. doi: 10.1055/s-0032-1328656
Nobuyuki, K., Hitoshi, S., Shigemi, A. (2008). Effects of nitrogen, phosphorus and potassium deficiencies on photosynthesis and RuBP carboxylase-oxygenase activities in rice plants. Japanese J. Crop Sci. 48 (3), 378–384. doi: 10.1626/jcs.48.378
Noguchi, K., Yoshida, K. (2008). Interaction between photosynthesis and respiration in illuminated leaves. Mitochondrion 8 (1), 87–99. doi: 10.1016/j.mito.2007.09.003
Patterson, E. (2013). Analyzing genetic response mechanisms associated with copper homeostasis in populus trichocarpa using a bioinformatics approach[D] (Fort Collins, Colorado State University).
Qin, Q., Xu, D., Hou, L., Shen, B., Xin, X. (2021). Comparing vegetation indices from sentinel-2 and landsat 8 under different vegetation gradients based on a controlled grazing experiment. Ecol. Indic. 133, 108363. doi: 10.1016/j.ecolind.2021.108363
Ren, W., Hu, N., Hou, X., Zhang, J., Guo, H., Liu, Z., et al. (2017). Long-term overgrazing-induced memory decreases photosynthesis of clonal offspring in a perennial grassland plant. Front. Plant Sci. 8, 419. doi: 10.3389/fpls.2017.00419
Rissler, H. M., Collakova, E., DellaPenna, D., Pogson, W. (2002). Chlorophyll biosynthesis. expression of a second chllgene of magnesium chelatase in Arabidopsis supports only limited chlorophyll synthesis. Plant Physiol. 128 (2), 770–779. doi: 10.1104/pp.010625
Selemani, I. S., Eik, L. O., Holand, Ø., Ådnøy Mtengeti, T E., Mushi, D. (2013). The effects of a deferred grazing system on rangeland vegetation in a north-western, semi-arid region of Tanzania. Afr. J. Range Forage Sci. 30 (3), 141–148. doi: 10.2989/10220119.2013.827739
Shang, Z. H., Cao, J. J., Guo, R. Y., Henkin, Z., Ding, L. M., Long, R. J., et al. (2017). Effect of enclosure on soil carbon, nitrogen and phosphorus of alpine desert rangeland. Land Degradation Dev. 28 (4), 1166–1177. doi: 10.1002/ldr.2283
Shen, H., Wang, S., Tang, Y. (2013). Grazing alters warming effects on leaf photosynthesis and respiration in Gentiana straminea, an alpine forb species. J. Plant Ecol. 6 (5), 418–427. doi: 10.1093/jpe/rtt010
Simkó, A., Gáspár, G. S., Kiss, L., Makleit, P., Veres, S. (2020). Evaluation of nitrogen nutrition in diminishing water deficiency at different growth stages of maize by chlorophyll fluorescence parameters. Plants 9 (6), 676–692. doi: 10.3390/plants9060676
Sohrabi, S., Ebadi, A., Jalali, S., Salami, S. A. (2017). Enhanced values of various physiological traits and VvNACI, gene expression showing better salinity stress tolenrance in some grapevine cultivars as well as rootstocks. Scientia Horticulture 225, 317–326. doi: 10.1016/j.scienta.2017.06.025
Tanwar, A., Aggarwal, A., Kadian, N., Gupta, A. (2013). Arbuscular mycorrhizal inoculation and super phosphate application influence plant growth and yield of Capsicum annuum. J. Soil Sci. Plant Nutr. 13 (1), 55–66. doi: 10.4067/S0718-95162013005000006
Thomas, A. D. (2012). Impact of grazing intensity on seasonal variations in soil organic carbon and soil CO2 efflux in two semiarid grasslands in southern Botswana. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 367, 3076–3086. doi: 10.1098/rstb.2012.0102
Wakhloo, J. L., Ruppenthal, H. (1989). Respiration in developing leaves in relation to vertical profiles in concentration of potassium in shoots of Nicotiana tabacum. J. Plant Physiol. 135 (4), 501–504. doi: 10.1016/S0176-1617(89)80111-7
Wang, X., Pan, S., Pan, N., Pan, P. (2022). Grassland productivity response to droughts in northern China monitored by satellite-based solar-induced chlorophyll fluorescence. Sci. Total Environ. 830, 154550. doi: 10.1016/j.scitotenv.2022.154550
Weng, J. H., Lai, M. F. (2005). Estimating heat tolerance among plant species by two chlorophyll fluorescence parameters. Photosynthetica 43 (3), 439–444. doi: 10.1007/s11099-005-0070-6
Wu, G. L., Dong, W., Yu, L., Ding, L. M., Liu, Z. H. (2017). Warm-season grazing benefits species diversity conservation. Land Degradation Dev. 28 (4), 1311–1319. doi: 10.1002/ldr.2536
Yin, J., Lu, X. (2013). “Opposite responses in leaf n and p concentrations and resorption of two dominant grass species along a 30-yr temperate steppe restoration chronosequence[C],” in Esa convention. (Minneapolis: ResearchGate).
Zhang, Z., Gong, J., Shi, J., Li, X., Song, L., Zhang, W., et al. (2022). Multiple herbivory pressures lead to different carbon assimilation and allocation strategies: Evidence from a perennial grass in a typical steppe in northern China. Agriculture Ecosyst. Environ. 326, 107776. doi: 10.1016/j.agee.2021.107776
Zhang, R. Z., Huang, D., Wang, K., Zhang, Y. J., Wang, C. J. (2011). Effect of mowing and grazing on ramet emergence of, leymus racemosus, in the inner Mongolia steppe during the spring regreening period. Afr. J. Biotechnol. 10 (10), 2216–2222. doi: 10.1186/1472-6750-11-24
Zhang, G., Johkan, M., Hohjo, M., Tsukagoshi, S., Maruo, T. (2017). Plant growth and photosynthesis response to low potassium conditions in three lettuce (Lactuca sativa) types. Horticulture J. 86 (2), 229–237. doi: 10.2503/hortj.OKD-008
Zhao, W., Chen, S. P., Lin, H. G.H. (2009). Effects of long-term grazing on the morphological and functional traits of Leymus chinensis in the semiarid grassland of inner Mongolia, China. Ecol. Res. 24, 99–108. doi: 10.1007/s11284-008-0486-0
Zhao, J., Li, X., Li, R., Tian, L., Zhang, T. (2016). Effect of grazing exclusion on ecosystem respiration among three different alpine grasslands on the central Tibetan plateau. Ecol. Eng. 94, 599–607. doi: 10.1016/j.ecoleng.2016.06.112
Zheng, S. X., Lan, Z. C., Li, W. H., Shao, R. X., Shan, Y. M., Wan, H. W., et al. (2011). Differential responses of plant functional trait to grazing between two contrasting dominant C3 and C4 species in a typical steppe of inner Mongolia, China. Plant Soil 340, 141–155. doi: 10.1007/s11104-010-0369-3
Zimmer, H. C., Turner, V. B., Mavromihalis, J., Dorrough, J., Moxham, C. (2010). Forb responses to grazing and rest management in a critically endangered Australian native grassland ecosystem. Rangeland J. 32 (2), 187–195. doi: 10.1071/RJ09069
Keywords: photosynthesis, Calvin cycle, respiration, TCA cycle, grazing rest during spring regreening period
Citation: Liu Y (2022) Grazing rest during spring regreening period promotes the ecological restoration of degraded alpine meadow vegetation through enhanced plant photosynthesis and respiration. Front. Plant Sci. 13:1008550. doi: 10.3389/fpls.2022.1008550
Received: 31 July 2022; Accepted: 07 September 2022;
Published: 03 October 2022.
Edited by:
Jing Zhang, Nanjing Agricultural University, ChinaReviewed by:
Yanfu Bai, Sichuan Agricultural University, ChinaJing Meiling, Qinghai Nationalities University, China
Copyright © 2022 Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Liu, liuying_yanhong@sina.com
 Ying Liu
Ying Liu