- 1Department of Biological Sciences, University of Eldoret, Eldoret, Kenya
- 2Department of Biotechnology, GLA University, Mathura, Uttar Pradesh, India
- 3Department of Biological and Physical Sciences, Moi University, Eldoret, Kenya
- 4Department of Sustainable Agriculture and Biodiversity Conservation, Nelson Mandela African Institution of Science and Technology, Arusha, Tanzania
Recent decades have witnessed increased agricultural production to match the global demand for food fueled by population increase. Conventional agricultural practices are heavily reliant on artificial fertilizers that have numerous human and environmental health effects. Cognizant of this, sustainability researchers and environmentalists have increased their focus on other crop fertilization mechanisms. Biofertilizers are microbial formulations constituted of indigenous plant growth-promoting rhizobacteria (PGPR) that directly or indirectly promote plant growth through the solubilization of soil nutrients, and the production of plant growth-stimulating hormones and iron-sequestering metabolites called siderophores. Biofertilizers have continually been studied, recommended, and even successfully adopted for the production of many crops in the world. These microbial products hold massive potential as sustainable crop production tools, especially in the wake of climate change that is partly fueled by artificial fertilizers. Despite the growing interest in the technology, its full potential has not yet been achieved and utilization still seems to be in infancy. There is a need to shed light on the past, current, and future prospects of biofertilizers to increase their understanding and utility. This review evaluates the history of PGPR biofertilizers, assesses their present utilization, and critically advocates their future in sustainable crop production. It, therefore, updates our understanding of the evolution of PGPR biofertilizers in crop production. Such information can facilitate the evaluation of their potential and ultimately pave the way for increased exploitation.
Introduction
The earth will be home to about 10 billion people by 2050 and a lot of pressure will be mounted on the existing food resources (United Nations, 2015). Although global crop production can be achieved through agricultural intensification, this will escalate reliance on chemical agro-inputs like fertilizers that pose several environmental effects (Vassilev et al., 2015; Abhilash et al., 2016b). For instance, chemical fertilizers are extensively associated with greenhouse gas emissions that fuel global warming and climatic changes (e.g., Kahrl et al., 2010; Mapanda et al., 2011; Carmo et al., 2013). Similarly, the eutrophication of several water bodies and the destabilization of aquatic ecosystems have several times been attributed to fertilizer runoffs from agricultural fields (Melo et al., 2012; Deepa and Venkateswaran, 2018; Zhang et al., 2018). Ironically, long-term artificial fertilization can also include the overall deterioration of soil productivity and quality through acidification (Neog, 2018; Bai et al., 2020; Yan et al., 2020).
Owing to the aforementioned challenges, the exploration of alternative crop fertilization mechanisms is mounting worldwide in an attempt to develop sustainable food production systems. The exploitation of plant microbiomes has particularly gathered surmountable interest in this regard. Among the most interesting plant microbiomes are the plant growth-promoting rhizobacteria (PGPR) that present several advantageous functions in plant rhizospheres, from nutrients solubilization (Ibarra-Galeana et al., 2017; Borgi et al., 2020; Verma et al., 2020), to suppression of plant diseases (e.g., Rizvi et al., 2017; Agisha et al., 2019; Bektas and Kusek, 2021; Jayakumar et al., 2021), nitrogen (N2) fixation (Bahulikar et al., 2014; Hara et al., 2020), and improved phytochemical composition (Rizvi et al., 2022a), among others. Biofertilizers are microbial formulations of PGPR strains that can either be immobilized or trapped on inert carrier materials to enhance plant growth and soil fertility (Aloo et al., 2022a). Over the decades, considerable strides have been made to understand, investigate and formulate various PGPR as alternative crop fertilization tools (e.g., Htwe et al., 2019; Paliya et al., 2019; Bangash et al., 2021; Barin et al., 2022; Aloo et al., 2022b). The yield of various crops can be increased by about 25% and the use of inorganic N and P fertilizers be reduced by about 25–50 and 25% through biofertilizer application (Khan and Chattopadhyay, 2009; Saber et al., 2012).
The utilization of biofertilizers dates back to the 1980s when the first Rhizobium formulations were patented and marketed in Germany (Nobbe and Hiltner, 1986). Several developments have been made through the decades and today, biofertilizer formulations are applied for the production of several crops entirely or with reduced usage of artificial fertilizers as presented in Section 4. Despite these developments, biofertilizer technology is yet to be exploited to its maximum potential. It is important to increase our knowledge of biofertilizers and their massive potential in the sustainability of our food production systems to increase their utilization. Herein, we evaluate the history of PGPR biofertilizers, assess their present utilization status from a global perspective, and critically propound on their future in sustainable crop production. This can update our understanding of the evolution of PGPR biofertilizers in crop production. We believe that such information will provide a good starting point for debate, and intensive global efforts to harness these bio-resources as biotechnological-based solutions for sustainable crop production systems. This work has been modified from a previous preprint (Aloo et al., 2020).
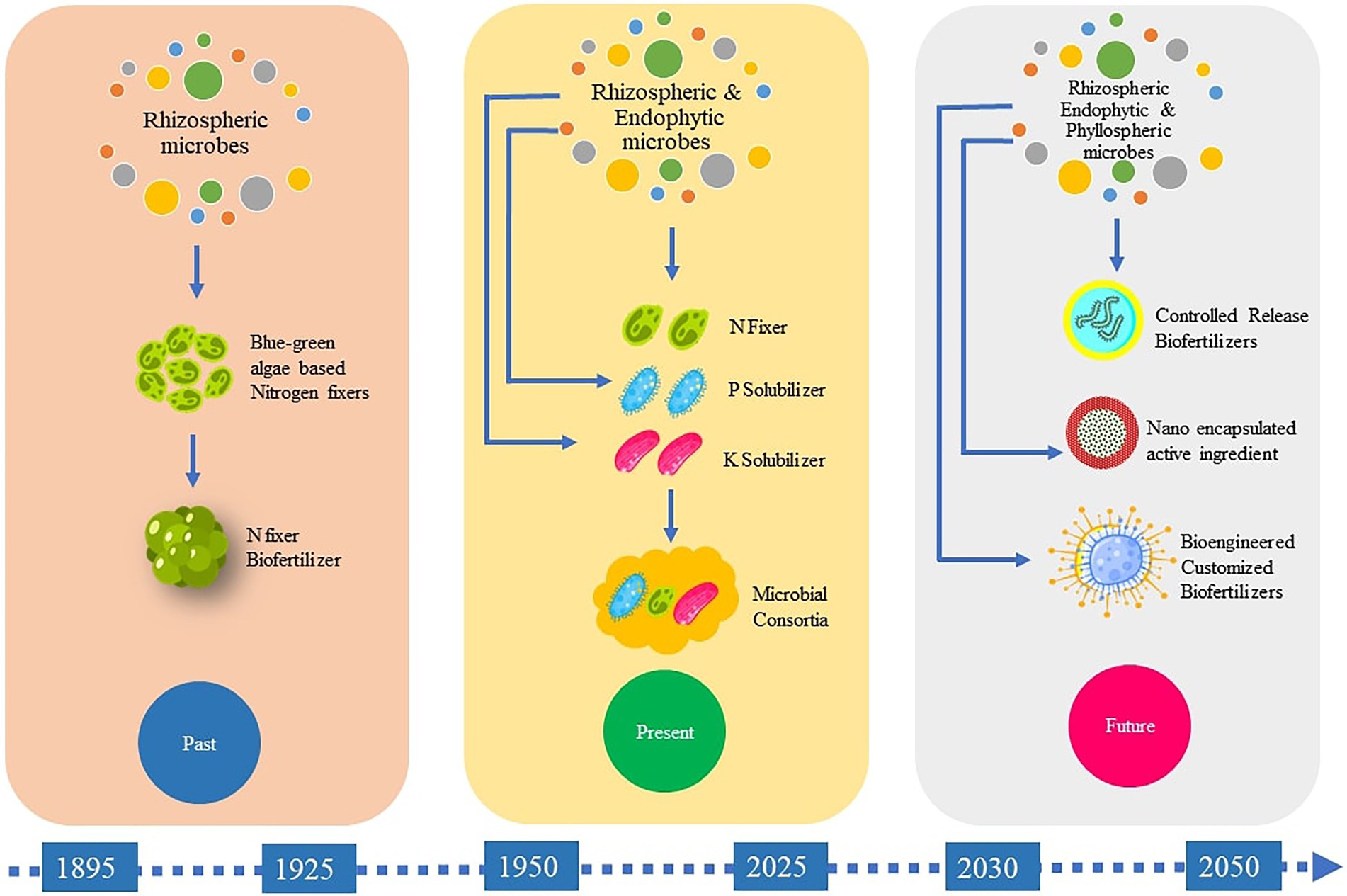
Overview of rhizobacterial biofertilizers and types
The meaning of biofertilizers has evolved for several decades, with many interpretations. The term has therefore received several different definitions over time (Table 1), reflecting the development of our comprehension of them. Most scholars consider PGPR as a biofertilizer because of their positive influences on the plant rhizospheres that can generally stimulate plant growth. However, Riaz et al. (2020) advance that PGPR and biofertilizers should not be used interchangeably since not all PGPR are biofertilizers.
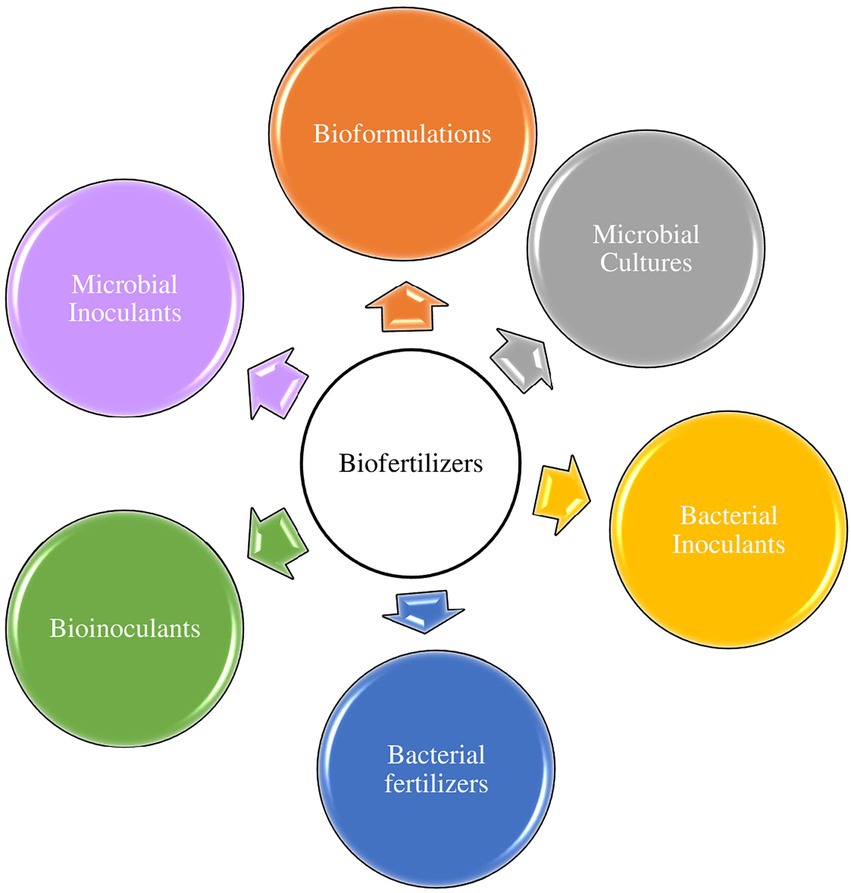
This is probably because the efficient PGPR must be formulated into products that can be applied to plants/soils to stimulate plant growth to qualify as biofertilizers. Nevertheless, the major components of biofertilizers are PGPR whose activities generally contribute to the overall increment, concentration, and accessibility of plant nutrients in plant rhizospheres. Herein, we adopt the definition of biofertilizers as active microbial agents that stimulate plant growth by improving nutrient availability in plant rhizosphere(s). Other synonymous terminologies with biofertilizers are microbial inoculants or bioformulations, bioinoculants, microbial cultures, and bacterial fertilizers or inoculants (Figure 1).
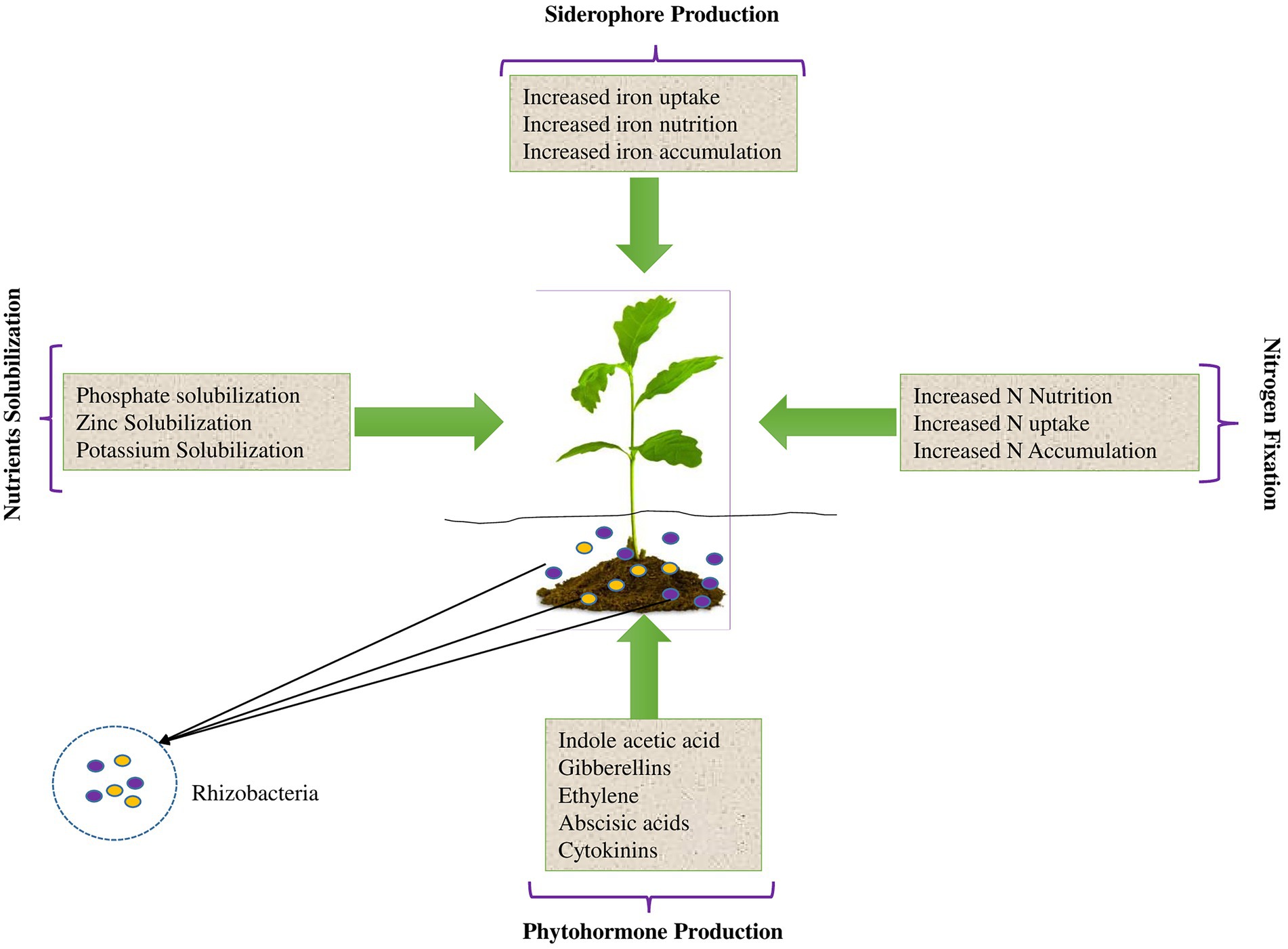
There are several types of biofertilizers depending on their functions in plant rhizospheres. Notably, a single biofertilizer can consist of a single PGPR strain with single or multiple PGP traits, or microbial consortia with multifarious PGP traits. A simulation of the various functions of biofertilizers in PGP is shown in Figure 2 and subsections 2.1 to 2.5 highlight the various types of PGPR biofertilizers. Nevertheless, the different types of biofertilizers normally function synergistically and offer an effective and environmentally-friendly solution for achieving food security while minimizing environmental impacts. Consequently, biofertilizers and PGPR are largely documented as significant factors in integrated soil nutrient management for sustainable crop production as discussed throughout this review.
Nitrogen-fixing biofertilizers
Nitrogen-fixing biofertilizers are currently the most common in the global market and their demand is anticipated to grow by a further 11.9% compounding annual growth rate (CAGR) to reach about USD 4.5 billion by 2026 up from the current USD 2.1 billion (Markets and Markets, 2020). Nitrogen-fixing biofertilizers comprise bacteria that carry out biological N2 fixation (BNF) and boost soil N supply to crops. The legume N2-fixing rhizobia have been researched for decades and shown to increase the quantity of fixed N in inoculated plants relative to un-inoculated ones (Koskey et al., 2017; Sanyal et al., 2020; Gedamu et al., 2021; Ketema and Tefera, 2022). Previous inputs of fixed N for red clover, alfalfa, soybean, pea, and cowpea were estimated to range from 23 to 335 kg ha−1 year−1 (Thies et al., 1995; Wani et al., 1995). The variabilities in terms of quantities of fixed N depend much on the type of legume-rhizobia symbiosis which is dictated by several factors like the legume cultivars and genotypes (Gunnabo et al., 2020; Riah et al., 2021), as well as the geographical distributions (Ramoneda et al., 2020).
According to Herridge et al. (2008), rhizobial inoculants can reduce the annual N fertilization costs by approximately USD 29 ha−1. This scenario demonstrates the importance of N2-fixing rhizobacteria as biofertilizers. Nevertheless, there is a need to perform field trials of new strains for suitability and adaptability before application as inoculants. Besides, N2-fixing biofertilizers are widely investigated for leguminous plants, and more efforts are required to demonstrate their potential in non-leguminous crops using asymbiotic diazotrophs like Azospirillum, Azotobacter, Gluconaceotobacter, and Burkholderia. Earlier studies by Melchiorre et al. (2011) and Hungria et al. (2006) both established that the yield of grains in Brazil, and Argentina, respectively, could reach close to 5 t ha−1 each season through rhizobia-mediated BNF. Similarly, annual N2 fixation rates of approximately 40 kg N ha−1 are documented in Australian soils (Unkovich and Baldock, 2008). Nevertheless, the contribution of asymbiotically fixed N in crop fields largely remains unestablished. More research is necessary, especially for crops like cereals, vegetables, and tubers, considering they contribute to the bulk of human food.
Some efficient N-fixing strains such as Rhizobium and Azotobacter spp. have successfully been formulated into commercial biofertilizers (Adeleke et al., 2019). However, the commercially available N biofertilizers mostly consist of Rhizobium and a few other bacteria such as Azotobacter, and Azospirillum species and are widely applicable to legume crops as presented in Section 4 (Vassilev et al., 2015; Adeleke et al., 2019). Nevertheless, inoculating crops and farms with such biofertilizers can meet the required N levels by plants and substantially reduce the application of artificial fertilizers (Aloo et al., 2022a).
Phosphorus and potassium solubilizing biofertilizers
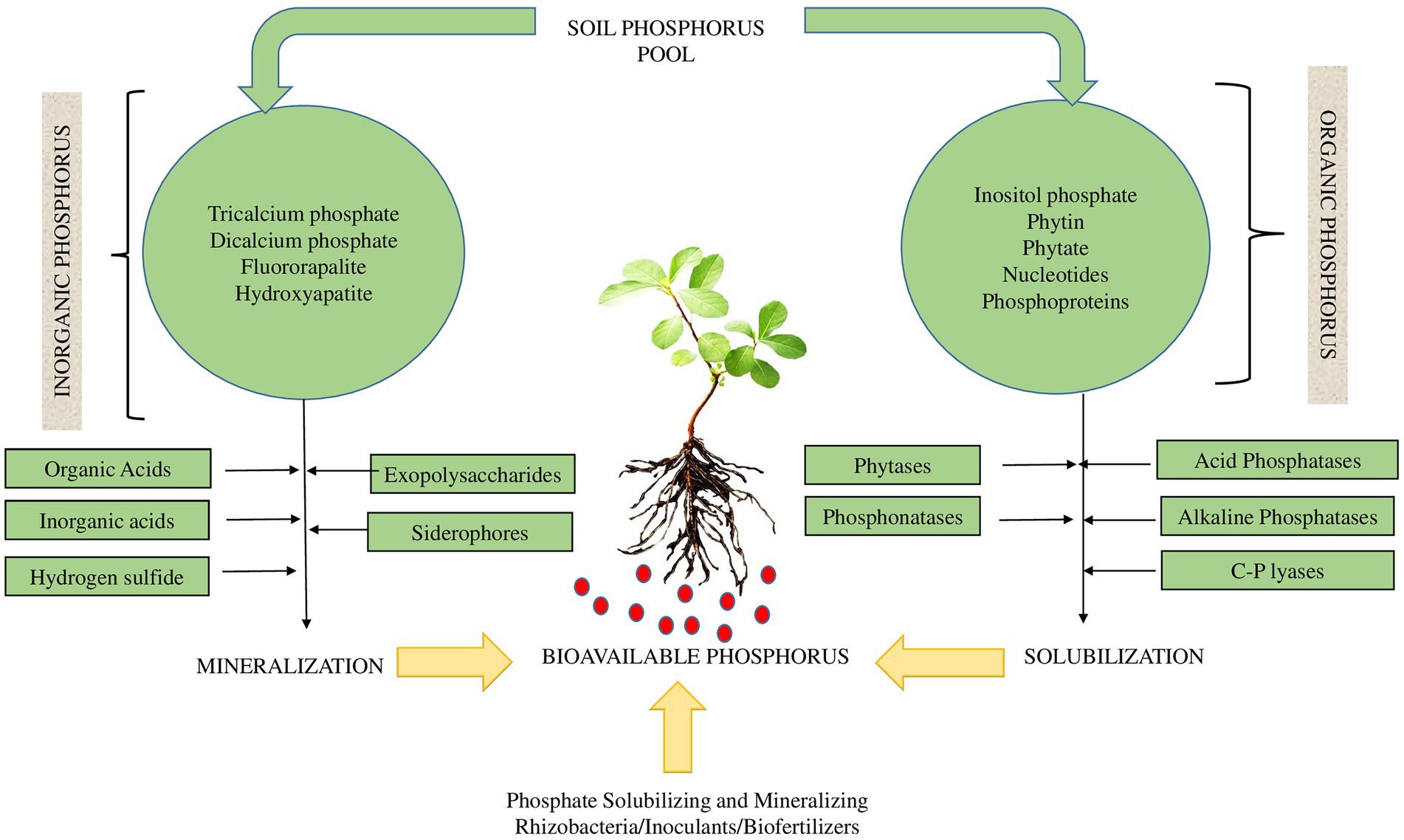
Apart from N-fixation, biofertilizers can also solubilize plant nutrients in soil and facilitate their bioavailability and crop uptake (e.g., Ahmad et al., 2019; Abdelmoteleb and Gonzalez-Mendoza, 2020; El-Deen et al., 2020). Recent biofertilizer forecasts have favored the increased uptake of phosphatic biofertilizers owing to their ability to increase soil P availability and their biocontrol attributes for crop pests (Soumare et al., 2020). The solubilization of P is however dependent on the P forms in soil, whether organic or inorganic (Figure 3).
Since P deficiency is inherent in numerous agricultural soils, such organisms are largely proposed as potential P biofertilizers. Despite the growing literature, research concerning their application as biofertilizers is still limited and generally inconsistent. Since the economically-mineable P deposits are limited (Cordell et al., 2009; Vassilev et al., 2015), it is doubtless that phosphatic biofertilizers can significantly enhance crop yields, and that the use of P solubilizing biofertilizers (PSB) as bioinoculants can open up a new horizon for sustaining soil P levels and by large, sustainable crop production (Rizvi et al., 2021a).
Similarly, potassium solubilizing biofertilizers (KSB) are equally important in crop production since these are also often limiting in agricultural soils. The K-solubilizing capacity of PGPR from K-bearing rocks through acidification has widely been investigated, thus KSB have a significant role in enhancing crop growth and productivity, for instance, wheat (Laxita and Shruti, 2020), maize (Akintokun et al., 2019; Imran et al., 2020), tomatoes (Reyes-Castillo et al., 2019; Raji and Thangavelu, 2021), and many others. These reports show that these bacteria can significantly improve germination, uptake of nutrients, growth, and crop yields under both controlled and uncontrolled conditions. Although K solubilization may not entirely fulfill plant K requirements like chemical fertilizers, studies show that this novel approach may significantly improve K availability in croplands (Huda et al., 2007; Imran et al., 2020; Laxita and Shruti, 2020). Furthermore, the application of KSB to agricultural soils as biofertilizers can greatly cut the use of artificial fertilizers and are eco-friendly approaches to crop production. Native KSB are especially emerging as a viable technology for mitigating K deficits in agricultural soils. The diversity, solubilizing abilities, and mechanisms of KSB are extensively reviewed by Sattar et al. (2019) and Ahmad et al. (2016). Despite the burgeoning literature, little is still known about the efficacy of KSB and how they can stimulate plant growth in different climates. Meena et al. (2018) advance that KSB are valuable resources for mitigating K-deficiencies in agricultural farms but experimental results on their field efficacy are still grossly inadequate. More research is needed to enhance their usability. This, and related knowledge will undoubtedly help in comprehending their value as bioinoculants for practical field applications.
Zinc solubilizing biofertilizers
Zinc solubilizing biofertilizers (ZSB) are equally important in crop production owing to worldwide Zn deficiency in soils. Such deficiency is prevalent in most arable lands caused by nutrient mining due to crop harvesting (Cakmak et al., 2017). Although chemical Zn fertilizers are often employed to augment these deficits at the recommended rates of approximately 5 kg ha−1 Zn, However, synthetic fertilizers are costly and do not readily get converted into plant-usable forms (Montalvo et al., 2016). Recent literature advances in rhizobacterial Zn solubilization (e.g., Joshi et al., 2013; Hussain et al., 2015; Kamran et al., 2017; Perumal et al., 2019) suggest that the field application of ZSB in the can increase Zn uptake by plants, and subsequently, improve their growth and yields. In an investigation by Naz et al. (2016), Pseudomonas, Azotobacter, Azospirillum, and Rhizobium species were shown to significantly enhance Zn uptake in wheat. Similarly, Sharma et al. (2012) studied 134 bacilli from the soybean (G. max) rhizosphere for Zn solubilization and established that the isolates greatly enhanced the concentration of Zn in the inoculated crops relative to the un-inoculated ones. Similarly, several ZSB like Pantoea dispersa, P. fragi, P. agglomerans, Rhizobium sp., and E. cloacae from the sugarcane and wheat rhizospheres were recently shown to improve the Zn contents and growth of potted wheat (Kamran et al., 2017). A more recent greenhouse trial by Dinesh et al. (2018) that evaluated several rhizospheric ZSB for their effects on soil and plant Zn contents revealed that the concentration in soil and plants was greater in treated plants than in non-treated ones. In India, Goteti et al. (2013) bacterized potted maize seeds with Zn-solubilizing Pseudomonas that significantly enhanced Zn uptake and concentration. Reports also exist for the Zn solubilizing abilities and increased Zn uptake following inoculation of wheat by Pseudomonads (Joshi et al., 2013), maize by Bacillus (Hussain et al., 2015), wheat and soybean by B. aryabhattai (Ramesh et al., 2014), and rice by several ZSB (Perumal et al., 2019).
Iron sequestering biofertilizers
Some biofertilizers can sequester iron (Fe) through special mechanisms using metabolites called siderophores with a high affinity for Fe in low-Fe environments (Wang et al., 2022). After the formation of the Fe3 + −microbial siderophores complexes formed in the microbial membrane, the former is reduced to Fe2+ which is subsequently freed into the cell through an input mechanism. In this process, plants access and directly assimilate the Fe2+ from bacterial siderophores from the Fe-siderophore complexes or through ligand exchange reactions (Yehuda et al., 1996). Siderophore production is a typical example of Fe nutrition enhancement by rhizobacterial inoculants in biofertilizers and owing to its indisputable significance, should be given more attention (Aloo et al., 2019).
Phytostimulators
Still, other biofertilizers can promote plant growth through phytohormone production and plant growth stimulation in many ways (Figure 4). Such biofertilizers are largely known as phytostimulators owing to the various roles they play in stimulating the growth of crops through the production of phytohormones. The most common phytohormones are auxins, gibberellins, and cytokinin. Although very small amounts of phytohormones are produced by PGPR, they are still very crucial for plant metabolic processes, including those that modulate plant growth (e.g., Kalimuthu et al., 2019; Haerani et al., 2021) and plant tolerance to various abiotic stresses (Mahmoody and Noori, 2014; Santhi et al., 2021). Among the most potential PGPR that can function as biofertilizers due to phytohormone production are Azospirillum (e.g., Coniglio et al., 2019) and Bacillus spp. (Kang et al., 2019; Bandopadhyay, 2020), and many others. Owing to the importance of phytostimulation, such PGPR are viable candidates for PGP as biofertilizers, especially if they can also solubilize plant nutrients and/or fix N to improve plant nutrition.
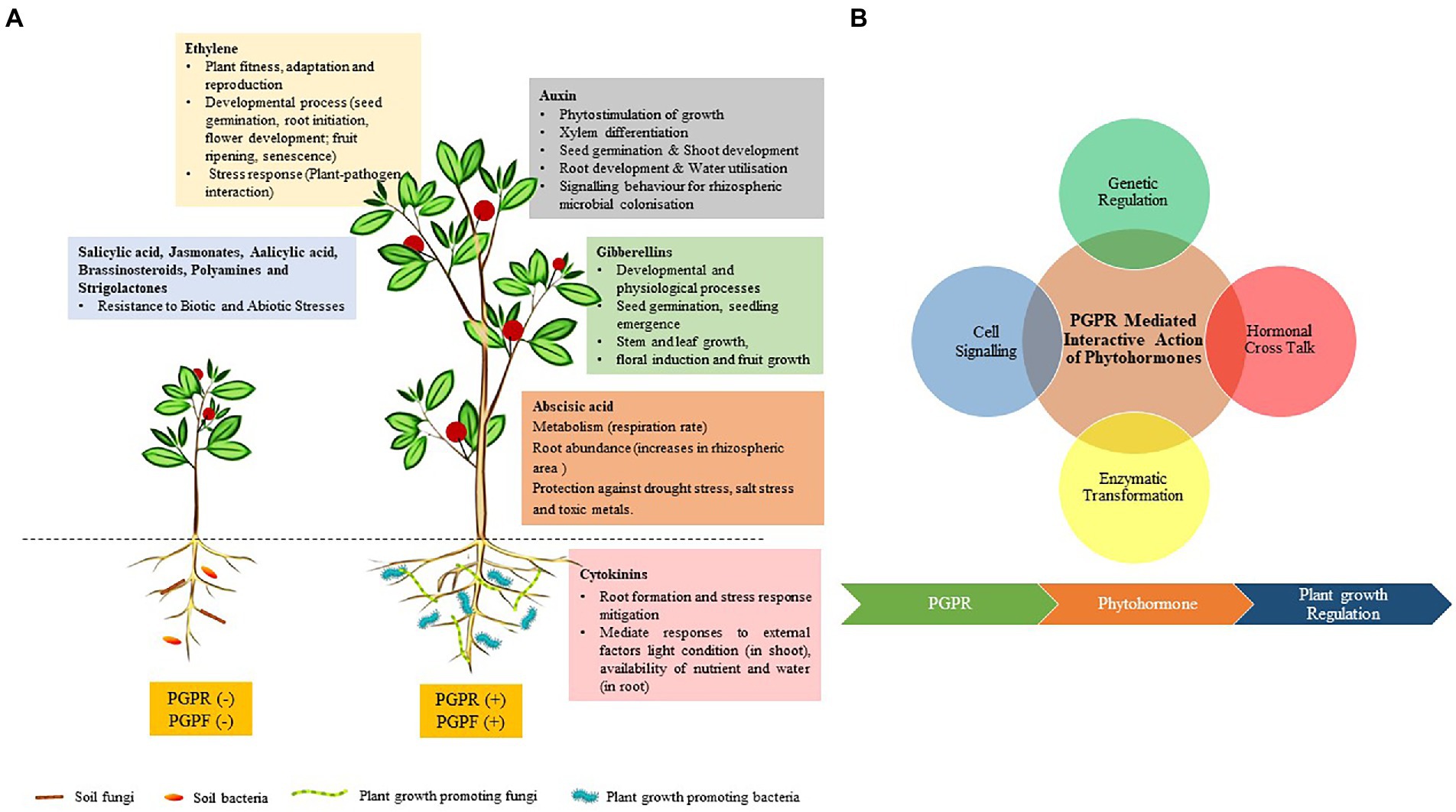
Figure 4. (A) Plant growth promotion through the production of different phytohormones and (B) PGPR-mediated plant growth promotion is governed through a complex network of cell signaling, genetic regulation, hormonal cross-talk, and enzymatic transformation. The PGPR generates multiple stimuli through the synthesis of phytohormones. These phytohormones interact through phosphorylation cascade or activating a secondary messenger which leads to the regulation of genes affecting hormone biosynthesis and developmental process in plants (Khan et al., 2020).
History of rhizobacterial biofertilizers
Whereas the application of microbial formulations is generally considered a modern and novel biotechnological agricultural approach in, crop inoculation with efficient PGPR for yield improvement is a century-old practice. The 1st attempts at rhizobacterial formulation date back to the late 18th century when a French scientist called Jean-Baptiste Boussingault (1801–1887) recognized that plant growth was proportional to N quantities. This observation was later linked to the reduction of N2 to ammonium and the 1st commercial biofertilizer Nitragin® made from Rhizobium was produced (Nobbe and Hiltner, 1986). These were the first commercial formulations of PGPR that were patented and marketed over a century ago (Nobbe and Hiltner, 1986).
Due to the inconsistent performance of bioformulations relative to artificial fertilizers, the use of biofertilizers slowed down but picked up after subsequent decades of research that produced encouraging greenhouse results using Pseudomonas spp. (Kloepper et al., 1980). Large-scale field trials were performed using Azotobacter and Bacillus spp. on more than 35 million ha of land in the former Soviet Union in 1958 (Cooper, 1959), but the impact of bacterization was relatively unsatisfactory. Nevertheless, the commercialization of Rhizobium formulations continued in the 19th century (Fages, 1992), and extended globally thereafter (Deaker et al., 2004). A lot of biofertilizers have since been formulated and marketed worldwide attempts have also been made to formulate bacterial soil-fertilizing preparations for non-legume crops. The 1st preparation “Alinit” based on B. ellenbachensis was introduced in Germany to promote cereal growth (Caron, 1897).
Rhizobacterial inoculations in parts of Southern Africa can be traced back to 1963 after successful soybean nodulation efficiency by native Bradyrhizobium and Rhizobium inoculants (Shurtleff and Aoyagi, 2018). Thereafter, a natural soybean nodulating variety called Nitrozam was formulated for use in Zambia and other African countries (Raimi et al., 2021). These concerted efforts massively increased soybean cultivation by 48% from 6,550 ha in 1984 to 22,780 ha in 1992. Additionally, about US$100,000 worth of Nitrozam was sold during this period. In South Africa, the biofertilizer market rapidly expanded in 1952, and to date, the country has one of the most established biofertilizer markets and regulations in the whole of Africa. The marketing and application of N2-fixing rhizobial biofertilizers in legume production have since been practiced for years. Globally, the total area of legumes under treatment with biofertilizers yearly was over 40 million ha by the year 2000 (Phillips, 2004), half of which was used for soybean production (Catroux et al., 2001). There are more success stories of legume inoculants in different parts of the world (El-Wakeil and El-Sebai, 2009; Ngakou et al., 2009; Gomare et al., 2013). The production and marketing of rhizobial inoculants for legume production have thus been practiced for decades, somewhat decreasing the need for chemical fertilizers in several countries around the world. The development of new biofertilizer bioformulations continues to expand, and the future of the technology seems bright.
The current state of rhizobacterial biofertilizers
There is a burgeoning literature on the current application of microbial products as biofertilizers and agricultural inputs. Nearly 170 establishments in 24 countries commercialize biofertilizers and possess factories that produce, and market microbe-based fertilizers at both small and large scales (Bharti et al., 2017). The marketing of rhizobial inoculants has particularly been practiced for several decades now to partially eliminate the application of artificial fertilizers (Paudyal and Gupta, 2018). However, the full potential of many potential biofertilizers is largely untapped. Likewise, biofertilizer commercialization remains low globally, albeit steadily increasing.
In developed countries where artificial agricultural inputs are fairly cheap, the use of PGPR is less prioritized but is albeit growing. In 2013, the highest demand for biofertilizers was highest in North America and projections were that the entire Asia-Pacific biofertilizer market would show the maximum growth from 2014 to 2019 and lead in biofertilizer consumption worldwide (Markets and Markets, 2014). The consumption of biofertilizers is reportedly growing in countries such as Canada, Argentina, China, India, Europe, and the United States of America (USA) due to tax exemptions, and input subsidies, among other incentives (Markets and Markets, 2019). Such approaches have generally served to expand the global biofertilizer market, but more efforts are still required.
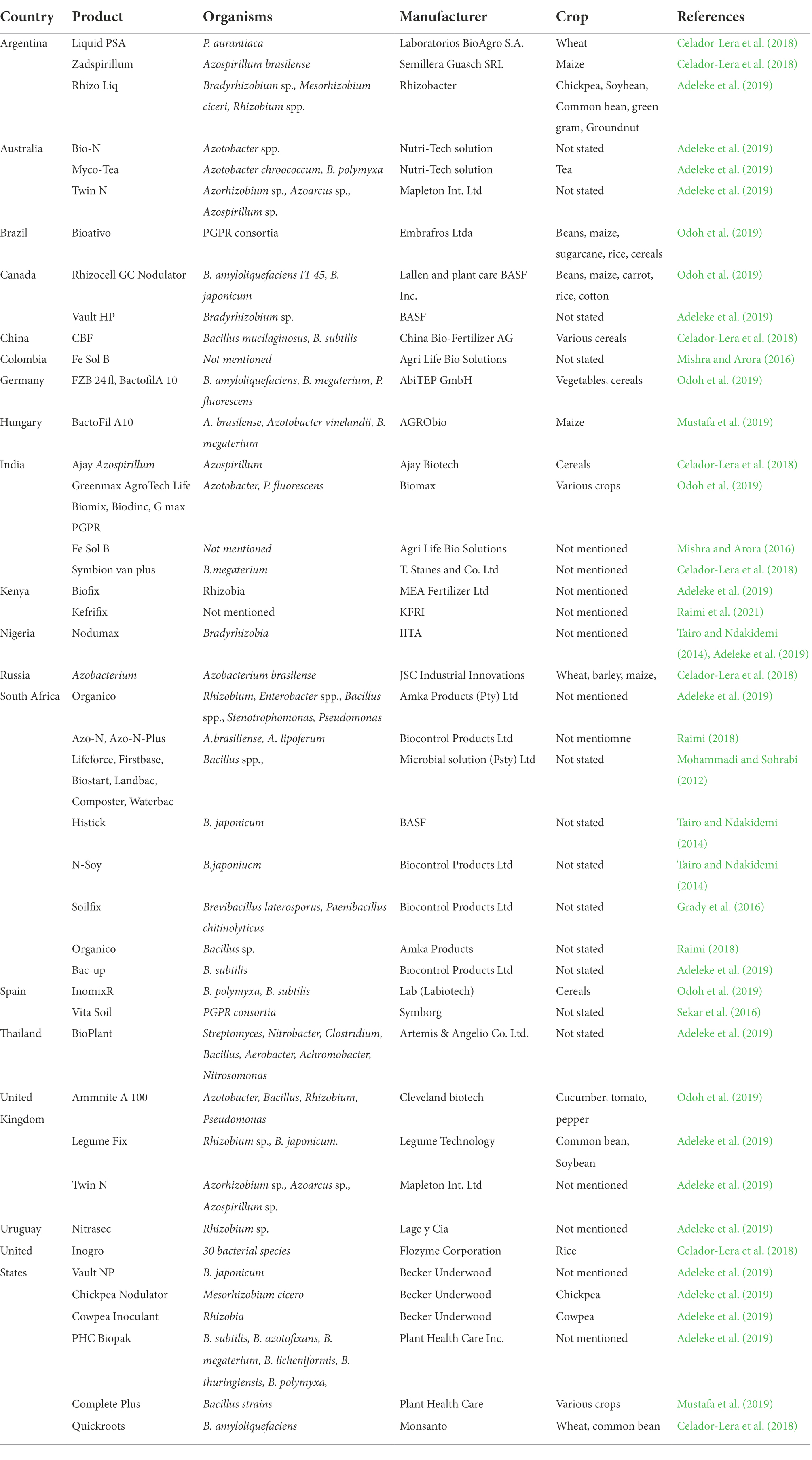
The advancement of research around the globe on the diversity, functions, and potentials of native rhizobacteria has stimulated the selection and isolation of efficient PGPR, and several biofertilizer formulations are already produced and commercialized for use in different countries across the globe (Table 2). The most progressive and dominant biofertilizer market in the world is Europe, where biofertilizer demand has grown at a CAGR of 12.3% from approximately US$2566 million in 2012 to US$4582 million in 2017 (Chandrasekhar, 2014). The global biofertilizer market was worth US$1.06 million in 2016 and was estimated to hit US$2 billion in 2019 and over US$3.8 billion in 2026, at a CAGR of 11.2% (Markets and Markets, 2020). The global increase in demand for biofertilizers has greatly been influenced by the growing demand for organic food products.
Although there exist many reports on the formulation and/or commercialization and application of rhizobacterial biofertilizers in several parts of the world, only a few reports indicate their application and commercialization in Africa. The PGPR inoculant technology has little or no impact on crop productivity in developing countries since it is either not practiced or the poor quality of available inoculants (Bashan, 1998). According to Aloo et al. (2021), the potential benefits of biofertilizers have largely been untapped in Africa due to inadequate regulatory frameworks and several other challenges. Most biofertilizers are commercialized for use in Asia, Europe, and the USA but in Africa, most commercialization and application occur only in South Africa. In East Africa, the production and use of biofertilizers are pronounced in Kenya which is the manufacturer of Biofix which can effectively inoculate 15 kg of common bean seeds per ha at approximately US$1.25 in comparison to 90 kg of artificial N fertilizer required for the same number of seeds per ha at US$12.50 (Raimi et al., 2021). However, Biofix and other biofertilizers are still largely underutilized in Kenya, probably due to a lack of awareness and other technology adoption challenges. Current and future initiatives are anticipated to improve the uptake of biofertilizers in Africa (Raimi et al., 2021). However, more efforts are needed to boost the consumption of these microbial products and promote the sustainability of global food production systems (Aloo et al., 2021).
The worldwide market for biofertilizers is presently largely dominated by legume and N2-fixing inoculants (Vassilev et al., 2015). While rhizobial inoculants currently dominate the global biofertilizer market, PSB, and other bioinoculants occupy less than 30% altogether (Transparency Market Research, 2022). Nevertheless, P-, K-, and Zn-based biofertilizers are now developing into significant bioinoculants to address soil nutrient deficiencies, and KSM are already commonly used as inoculants in some countries with K-deficient croplands (Teotia et al., 2016). India is reportedly the 4th largest consumer of K bioinoculants globally while countries like Brazil, the USA, and China come first in the overall consumption of these microbial products (Matich, 2016).
Unlike rhizobial biofertilizers, PSB like Pseudomonas, Bacillus, and diazotrophs like Azospirillum have neither been used as much nor at a large scale (Lesueur et al., 2016). Most of the commercially-available non-rhizobial PGPR inoculants consist of Azospirillum as free-living N2 fixers or Bacilli as PSB (Herrmann and Lesueur, 2013). The application of non-rhizobial biofertilizers has a less significant impact on global food production probably because of several bottlenecks that exist in biofertilizer uptake and use relative to well-documented PGP functions. Yet, PGPR like PSB are essential candidates for improving legume P nutrition for efficient nodulation (Zaidi et al., 2017). The global biofertilizers market for crop production is projected to grow from US$2.02 billion in 2022 to US$4.47 billion by 2029, at a CAGR of 12.04% from 2022 to 2029 (Fortune Business Insights, 2022). Still, there is a need for more efforts for adequate market infiltration and application.
The future of biofertilizers
The incorporation of biofertilizers as fundamental components of agricultural practices is quickly gathering momentum globally. These microbial products are already in use in some countries and are expected to become more popular in the future. The global and future expansion of the biofertilizer market will largely be driven by the need to increase food production sustainably. With an ever-increasing demand for organic food products, growing awareness of sustainable agricultural practices, and promotion of cleaner production methods for reducing soil contamination, land degradation, and water pollution the market growth for biofertilizers will continue to grow over the coming years (Abhilash et al., 2016a; Anand et al., 2022; Vaishnav et al., 2022).
Forecasts are that the present global market for biofertilizers which was approximated at US$396 million in 2018 will grow at a CAGR of 10.9% to escalate to US$4448.97 million by 2028 (Vintage Market Research, 2022). Further indications are that the global biofertilizer market which was valued at close to US$ 3.0 billion in 2020 will grow at a CAGR of 12.2% and reach about US$5 billion by 2031 (Transparency Market Research, 2022). The number of investigations targeting the isolation, identification, and evaluation of the capacity of PGPR with the potential of being transformed into inoculants for a variety of crops is equally expanding (Vatsyayan and Ghosh, 2013; Datta et al., 2015; Tan et al., 2015; Koskey et al., 2017; Anand et al., 2022; Aloo et al., 2022b). It is, therefore, realistic to expect that widespread biofertilizer usage will soon offer countless approaches to the progression of sustainable crop production systems.
For the extensive utilization of biofertilizers, proper regulatory and legal frameworks will be required in place of the existing ones that are currently stringent and hinder their proper utilization. Fortunately, regulatory authorities are increasingly encouraging the implementation of alternative crop fertilization mechanisms to promote the development of sustainable agricultural technologies. Recognizing the need for a specific legislative framework for biofertilizers in Europe, the European Commission has proposed to amend existing regulations (European Parliament and Council of the European Union, 2016). Such initiatives will eventually relax the stringent regulatory frameworks and enable the widespread adoption of these microbial resources.
While a number of the existing biofertilizers are mainly composed of natural rhizobacterial strains chosen for their PGP qualities, the development of genetically-modified inoculants that are likely to be more efficient at plant growth stimulation is required. Still, the biggest hurdle will be for scientists to convince society and regulatory authorities worldwide that such genetically-engineered organisms are harmless. Our current ability to manipulate and exploit the plant microbiome in situ similarly remains limited, and more investigations are required to facilitate their large-scale application and commercialization. The inoculant industry is faced with various challenges in making formulations with prolonged shelf lives. Progress into developing formulations with improved shelf lives, broad spectra of action, and reliable field performance will therefore hasten the commercialization of this technology (Nakkreen et al., 2005). In this regard, new approaches should be evaluated to develop formulations with longer shelf lives. Micro-encapsulation is one viable approach but most experiments in this regard have been restricted to laboratories and the standardization of this technology for industrial and field applications should be pursued. The future of biofertilizer technology depends a lot on developing efficient PGP strains. This is quite challenging but continued research in this area will eventually pave the way for this (Figure 5).
Investigation on N-fixing and PSB is developing fairly well, unlike for K solubilizers despite K being among the important macronutrients for plant development. More research in this regard will promote their application and utilization as bioinoculants in the future. Future research should additionally focus on the development of inoculants that can tolerate unfavorable environmental conditions for applicability in stressful environments, especially for inoculants that have been shown to relieve plants of metal stress (Rizvi et al., 2019, 2020, 2022b), salinity stress (Shahid et al., 2021), and cold stress (Rizvi et al., 2021b). More research is needed on the practical aspects of large-scale formulation and production to develop stable, effective, and state-of-the-art bioformulations. Microbial consortia offer multiple PGP traits for producing novel biofertilizer formulations as substitutes for artificial inputs (Singh et al., 2020; Cakmaksi et al., 2021; Vaishnav and Singh, 2021).
The interactions among plants and biofertilizer inoculants will require further studies and new approaches. Future research should additionally include careful selection of rhizosphere microbiota, and their in-situ testing for use as plant inoculants. It is expected that the identification of effective microbiomes in different soil types and climates will be extremely helpful in this regard. To improve this strategy, establishing a global database of effective plant microbiomes will be an important milestone toward successful translational research. A lot of obstacles remain to be overcome before this can fully be realized. For instance, several formulations based on such microbes have been developed for applications to different crops worldwide. However, inoculation results are often inconsistent and dependent on the prevailing local soil and plant-related properties, altogether necessitating the optimization of each system.
The application of biotechnology and the improvement of biofertilizer regulations will facilitate in designing of more effective and reliable rhizobial bioformulations as biofertilizers. To design suitable rhizobial formulations, we must use modern technologies to increase our understanding of plant-microbe interactions (Jain et al., 2021). For example, multi-omics approaches can greatly help us to comprehend complex plant-microbial symbioses to design suitable bioformulations for particular soils and crops (Kaul et al., 2016; López-Mondéjar et al., 2017; Ding et al., 2021; Yamazaki et al., 2021). These novel approaches will with time enhance the complete characterization of PGPR and their influence on plant nutrient acquisition and other PGP traits to facilitate their application. Thus, these should be prioritized for research.
Finally, it will be important to identify the challenges in the production and application of biofertilizers and strategies to address such problems. For example, the field efficiency of biofertilizers is dependent on crop species, soil complexity, and climatic conditions. Research on suitable biofertilizers should in the future be handled by agronomists that understand the nexus between crops, climatic conditions, and nutrients in various parts of the world. Besides, genomic engineering can be necessary for manipulating indigenous PGPR with suitable genes for enhanced expression of biofertilization functions for field applications. Additionally, particular additives could improve product stability, shelf life, and field efficiency.
Conclusion
The greatest global challenge in the 21st century is to invent and implement sustainable agricultural practices. This can only be achieved if we accommodate changing and advanced technologies such as the use of efficient rhizobacterial biofertilizers. The present discussion is useful for the development of sustainable agricultural systems. The use of these bio-resources though has been practiced in several parts of the globe is still low but the results are encouraging and there is room for development to boost their efficiency. With time, the practice will certainly grow and projections are that biofertilizers will have massive market potential soon. Researchers, agricultural institutions, and universities can fast-track biofertilizer development and promote their usage and adaptation for sustainable agricultural practices. If issues linked to regulation, policy development, and social acceptability of biofertilizer products can simultaneously be addressed, these bio-based tools can potentially and significantly contribute to sustainable agricultural productivity.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Acknowledgments
This research was originally published as a preprint: doi: 10.20944/preprints202009.0650.v1.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdelmoteleb, A., and Gonzalez-Mendoza, D. (2020). Isolation and identification of phosphate solubilizing bacillus spp. from Tamarix ramosissima rhizosphere and their effect on growth of Phaseolus vulgaris under salinity stress. Geomicrobiol J. 37, 901–908. doi: 10.1080/01490451.2020.1795321
Abhilash, P., Dubey, R. K., Tripathi, V., Gupta, V. K., and Singh, H. B. (2016a). Plant growth-promoting microorganisms for environmental sustainability. Trends Biotechnol. 34, 847–850. doi: 10.1016/j.tibtech.2016.05.005
Abhilash, P., Tripathi, V., Edrisi, S. A., Dubey, R. K., Bakshi, M., Dubey, P. K., et al. (2016b). Sustainability of crop production from polluted lands. Energy Ecol. Environ. 1, 54–65. doi: 10.1007/s40974-016-0007-x
Adeleke, R. A., Raimi, A. R., Roopnarain, A., and Mokubedi, S. M. (2019). “Status and prospects of bacterial inoculants for sustainable Management of Agroecosystems” in Biofertilizers for Sustainable Agriculture and Environment. eds. B. Giri, R. Prasad, Q. S. Wu, and A. Varma (Cham: Springer International Publishing), 137–172.
Agisha, V. N., Kumar, A., Eapen, S. J., Sheoran, N., and Suseelabhai, R. (2019). Broad-spectrum antimicrobial activity of volatile organic compounds from endophytic pseudomonas putida BP25 against diverse plant pathogens. Biocontrol Sci. Technol. 29, 1069–1089. doi: 10.1080/09583157.2019.1657067
Ahmad, M., Adil, Z., Hussain, A., Mumtaz, M. Z., Nafees, M., Ahmad, I., et al. (2019). Potential of phosphate solubilizing bacillus strains for improving growth and nutrient uptake in mungbean and maize crops. Pak. J. Agric. Sci. 56, 283–289. doi: 10.21162/PAKJAS/19.7455
Ahmad, M., Nadeem, S. M., Naveed, M., and Zahir, Z. A. (2016). “Potassium-solubilizing microorganisms and their application in agriculture” in Potassium Solubilizing Microorganisms for Sustainable Agriculture. eds. V. Meena, B. Maurya, J. Verma, and R. Meena (New Delhi: Springer), 293–313.
Akintokun, A. K., Ezaka, E., Akintokun, P. O., Shittu, O. B., and Taiwo, L. B. (2019). Isolation, screening and response of maize to plant growth promoting. Sci. Agric. Bohem. 50, 181–190. doi: 10.2478/sab-2019-0025
Aloo, B. N., Makumba, B. A., and Mbega, E. R. (2019). The potential of bacilli rhizobacteria for sustainable crop production and environmental sustainability. Microbiol. Res. 219, 26–39. doi: 10.1016/j.micres.2018.10.011
Aloo, B. N., Makumba, B. A., and Mbega, E. R. (2020). Plant growth promoting rhizobacterial biofertilizers for sustainable crop production: The past, present, and future. Preprints 2020090650.
Aloo, B. N., Mbega, E. R., Makumba, B. A., and Tumuhairwe, J. B. (2022b). Effects of carrier materials and storage temperatures on the viability and stability of three biofertilizer inoculants obtained from potato (Solanum tuberosum L.) Rhizosphere. Agriculture 12:140. doi: 10.3390/agriculture12020140
Aloo, B. N., Mbega, E. R., Tumuhairwe, J. B., and Makumba, B. A. (2021). Advancement and practical applications of rhizobacterial biofertilizers for sustainable crop production in sub-Saharan Africa. Agric. Food Secur. 10, 1–12. doi: 10.1186/s40066-021-00333-6
Aloo, B. N., Mbega, E., Tumuhairwe, J., and Makumba, B. (2022a). “Microbial biostimulants for crop production: industry advances, bottlenecks, and future prospects” in Microbial biostimulants for Sustainable Agriculture and Environmental Bioremediation. eds. C. O. A. Inamuddin, M. I. Ahamed, and T. Altalhi (Boca Raton: CRC Press), 177–198.
Anand, U., Vaishnav, A., Sharma, S. K., Sahu, J., Ahmad, S., Sunita, K., et al. (2022). Current advances and research prospects for agricultural and industrial uses of microbial strains available in world collections. Sci. Total Environ. 842:156641. doi: 10.1016/j.scitotenv.2022.156641
Atieno, M., Herrmann, L., Nguyen, H. T., Phan, H. T., Nguyen, N. K., Srean, P., et al. (2020). Assessment of biofertilizer use for sustainable agriculture in the great Mekong region. J. Environ. Manage. 275:111300. doi: 10.1016/j.jenvman.2020.111300
Bahulikar, R. A., Torres-Jerez, I., Worley, E., Craven, K., and Udvardi, M. (2014). Diversity of nitrogen-fixing bacteria associated with switchgrass in the native tallgrass prairie of northern Oklahoma. Appl. Environ. Microbiol. 80, 5636–5643. doi: 10.1128/AEM.02091-14
Bai, Y. C., Chang, Y. Y., Hussain, M., Lu, B., Zhang, J. P., Song, X. B., et al. (2020). Soil chemical and microbiological properties are changed by long-term chemical fertilizers that limit ecosystem functioning. Microorganisms 8:694. doi: 10.3390/microorganisms8050694
Bandopadhyay, S. (2020). Application of plant growth promoting bacillus thuringiensis as biofertilizer on Abelmoschus esculentus plants under field condition. J. Pure Appl. Microbiol. 14, 1287–1294. doi: 10.22207/JPAM.14.2.24
Bangash, N., Mahmood, S., Akhtar, S., Hayat, M. T., Gulzar, S., and Khalid, A. (2021). Formulation of biofertilizer for improving growth and yield of wheat in rain dependent farming system. Environ. Technol. Innov. 24:101806. doi: 10.1016/j.eti.2021.101806
Barin, M., Asadzadeh, F., Hosseini, M., Hammer, E. C., Vetukuri, R. R., and Vahedi, R. (2022). Optimization of biofertilizer formulation for phosphorus solubilizing by Pseudomonas fluorescens Ur21 via response surface methodology. PRO 10:650. doi: 10.3390/pr10040650
Bashan, Y., Puente, M. E., Myrold, D. D., and Toledo, G. (1998). In vitro transfer of fixed nitrogen from diazotrophic filamentous cyanobacteria to black mangrove seedlings. FEMS Microbiol. Ecol. 26, 165–170. doi: 10.1111/j.1574-6941.1998.tb00502.x
Bektas, I., and Kusek, M. (2021). Biological control of onion basal rot disease using phosphate solubilising rhizobacteria. Biocontrol Sci. Technol. 31, 190–205. doi: 10.1080/09583157.2020.1839381
Bhardwaj, D., Ansari, M. W., Sahoo, R. K., and Tuteja, N. (2014). Biofertilizers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. Microb. Cell Fact. 13, 1–10. doi: 10.1186/1475-2859-13-66
Bharti, N., Sharma, S. K., Saini, S., Verma, A., Nimonkar, V., and Prakash, O. (2017). “Microbial plant probiotics: problems in application and formulation” in Probiotics and Plant Health. eds. V. Kumar, M. Kumar, S. Sharma, and R. Prasad (Singapore: Springer), 317–335.
Bisen, K., Keswani, C., Mishra, S., Saxena, A., Rakshit, A., and Singh, H. (2015). “Unrealized potential of seed biopriming for versatile agriculture” in Nutrient Use Efficiency: From Basics to Advances. eds. A. Rakshit, H. B. Singh, and A. Sen (New Delhi: Springer), 193–206.
Borgi, M. A., Saidi, I., Moula, A., Rhimi, S., and Rhimi, M. (2020). The attractive Serratia plymuthica BMA1 strain with high rock phosphate-solubilizing activity and its effect on the growth and phosphorus uptake by Vicia faba L. Plants Geomicrobiol. J. 37, 437–445. doi: 10.1080/01490451.2020.1716892
Brahmaprakash, G., and Sahu, P. K. (2012). Biofertilizers for sustainability. J. Indian Inst. Sci. 92, 37–62.
Cakmak, I., McLaughlin, M. J., and White, P. (2017). Zinc for better crop production and human health. Plant Soil 411, 1–4. doi: 10.1007/s11104-016-3166-9
Cakmaksi, R., Akcura, S., and Mustafa, E. (2021). Effect of co-inoculation of multi-traits bacteria based bio-formulations on the growth, yield and enzyme activities of tea. J. Agric. Nat. Resour. 8, 594–604. doi: 10.30910/turkjans.807411
Carmo, J. B. D., Filoso, S., Zotelli, L. C., De Sousa Neto, E. R., Pitombo, L. M., Duarte-Neto, P. J., et al. (2013). Infield greenhouse gas emissions from sugarcane soils in Brazil: effects from synthetic and organic fertilizer application and crop trash accumulation. Glob. Change Biol. Bioenergy 5, 267–280. doi: 10.1111/j.1757-1707.2012.01199.x
Caron, A. (1897). Culture of Bacteria. U.S. patent no. 679600. Washington, DC: U.S. Patent and Trademark Office
Catroux, G., Hartmann, A., and Revellin, C. (2001). Trends in rhizobial inoculant production and use. Plant Soil 230, 21–30. doi: 10.1023/A:1004777115628
Celador-Lera, L., Jiménez-Gómez, A., Menéndez, E., and Rivas, R. (2018). “Biofertilizers based on bacterial Endophytes isolated from cereals: potential solution to enhance these crops” in Stress Management and Agricultural Sustainability. ed. V. S. Meena (Singapore: Springer), 175–203.
Chandrasekhar, K. (2014). Europe bio fertilizer market is expected to reach $4,582.2 million in 2017 new report. MicroMarket monitor. Available at: http://www.micromarketmonitor.com/market/europe-bio-fertilizer-4637178345.html (Accessed July 2, 2022).
Coniglio, A., Mora, V., Puente, M., and Cassán, F. (2019). “Azospirillum as biofertilizer for sustainable agriculture: Azospirillum brasilense AZ39 as a model of PGPR and field traceability,” in Microbial Probiotics for Agricultural Systems. Sustainability in Plant and Crop Protection, eds. D. Zúñiga-Dávila, F. González-Andrés, and E. Ormeño-Orrillo, (Cham, Springer) pp. 45–70.
Cordell, D., Drangert, J. O., and White, S. (2009). The story of phosphorus: global food security and food for thought. Glob. Environ. Change 19, 292–305. doi: 10.1016/j.gloenvcha.2008.10.009
Datta, A., Singh, R. K., and Tabassum, S. (2015). Isolation, characterization and growth of rhizobium strains under optimum conditions for effective biofertilizer production. Int. J. Pharm. Sci. Rev. Res. 32, 199–208.
Deaker, R., Roughley, R. J., and Kennedy, I. R. (2004). Legume seed inoculation technology—a review. Soil Biol. Biochem. 36, 1275–1288. doi: 10.1016/j.soilbio.2004.04.009
Deepa, S., and Venkateswaran, S. (2018). Appraisal of groundwater quality in upper Manimuktha sub basin, Vellar river, Tamil Nadu, India by using water quality index (WQI) and multivariate statistical techniques. Model. Earth Syst. Environ. 4, 1165–1180. doi: 10.1007/s40808-018-0468-3
Dinesh, R., Srinivasan, V., Hamza, S., Sarathambal, C., Anke Gowda, S. J., Ganeshamurthy, A. N., et al. (2018). Isolation and characterization of potential Zn solubilizing bacteria from soil and its effects on soil Zn release rates, soil available Zn and plant Zn content. Geoderma 321, 173–186. doi: 10.1016/j.geoderma.2018.02.013
Ding, Y., Yi, Z., Fang, Y., He, S., Li, Y., He, K., et al. (2021). Multi-Omics reveal the efficient phosphate-solubilizing mechanism of bacteria on rocky soil. Front. Microbiol. 12:761972. doi: 10.3389/fmicb.2021.761972
El-Deen, S. R. O., El-Azeem, A., Samy, A., Abd Elwahab, A. F., and Mabrouk, S. S. (2020). Effects of phosphate solubilizing microorganisms on wheat yield and phosphatase activity. Egypt. J. Med. Microbiol. 55, 71–86. doi: 10.21608/ejm.2020.20675.1137
El-Wakeil, N. E., and El-Sebai, T. N. (2009). Role of biofertilizer on faba bean growth, yield, and its effect on bean aphid and the associated predators. Arch. Phytopathol. Plant Prot. 42, 1144–1153. doi: 10.1080/03235400701650882
European Parliament and Council of the European Union (2016). Proposal for a regulation of the European Parliament and of the council laying down rules on the making available on the market of CE marked Fertilising products and amending regulations (ec) no 1069/2009 and (ec) no 1107/2009 (regulation no. COM(2016)157). Available at: https://www.eumonitor.eu/9353000/1/j9vvik7m1c3gyxp/vk2hefenu2z2 (Accessed July 3, 2022).
Fages, J. (1992). An industrial view of Azospirillum inoculants: formulation and application technology. Symbiosis 13, 15–26.
Fortune Business Insights (2022). Biofertilizer market size, share & COVID-19 impact analysis, by type (nitrogen fixing, phosphate Solubilizers, others), by microorganism (rhizobium, Azotobacter, Azospirillum, pseudomonas, bacillus, VAM, others), by application (seed treatment, soil treatment, others), by crop type (cereals, Pulses & Oilseeds, Fruits & Vegetables, others), and regional forecast, 2022-2029. Available at: https://www.fortunebusinessinsights.com/industry-reports/biofertilizers-market-100413 (Accessed July 3, 2022).
Gedamu, S. A., Tsegaye, E. A., and Beyene, T. F. (2021). Effect of rhizobial inoculants on yield and yield components of faba bean (Vicia fabae L.) on vertisol of Wereillu District, South Wollo. Ethiop. CABI Agric. Biosci. 2, 1–10. doi: 10.1186/s43170-021-00025-y
Gomare, K., Mese, M., and Shetkar, Y. (2013). Isolation of rhizobium and cost effective production of biofertilizer. Indian J. Life Sci. 2:49.
Goteti, P. K., Emmanuel, L. D. A., Desai, S., and Shaik, M. H. A. (2013). Prospective zinc Solubilising bacteria for enhanced nutrient uptake and growth promotion in maize (Zea mays L.). Int. J. Microbiol. 2013:869697. doi: 10.1155/2013/869697
Grady, E. N., MacDonald, J., Liu, L., Richman, A., and Yuan, Z. C. (2016). Current knowledge and perspectives of Paenibacillus: a review. Microb. Cell Fact. 15, 1–18. doi: 10.1186/s12934-016-0603-7
Gunnabo, A., van Heerwaarden, J., Geurts, R., Wolde-Meskel, E., Degefu, T., and Giller, K. (2020). Symbiotic interactions between chickpea (Cicer arietinum L.) genotypes and Mesorhizobium strains. Symbiosis 82, 235–248. doi: 10.1007/s13199-020-00724-6
Haerani, N., Syam’Un, E., Rasyid, B., and Haring, F. (2021). Isolation and characterization of N-fixing and IAA producing rhizobacteria from two rice field agro-ecosystems in South Sulawesi. Indonesia Biodivers J. Biol. Divers. 22, 2497–2503. doi: 10.13057/biodiv/d220506
Hara, S., Takashi, M., Sawa, W., Yasuhiro, K., Taichi, K., Kiyoshi, Y., et al. (2020). Identification of nitrogen-fixing Bradyrhizobium associated with roots of field-grown sorghum by Metagenome and proteome. Front. Microbiol. 10:47. doi: 10.3389/fmicb.2019.00407
Herridge, D. F., Peoples, M. B., and Boddey, R. M. (2008). Global inputs of biological nitrogen fixation in agricultural systems. Plant Soil 311, 1–18. doi: 10.1007/s11104-008-9668-3
Herrmann, L., and Lesueur, D. (2013). Challenges of formulation and quality of biofertilizers for successful inoculation. Appl. Microbiol. Biotechnol. 97, 8859–8873. doi: 10.1007/s00253-013-5228-8
Htwe, A. Z., Moh, S. M., Soe, K. M., Moe, K., and Yamakawa, T. (2019). Effects of biofertilizer produced from Bradyrhizobium and Streptomyces griseoflavus on plant growth, nodulation, nitrogen fixation, nutrient uptake, and seed yield of Mung bean, cowpea, and soybean. Agronomy 9:77. doi: 10.3390/agronomy9020077
Huda, S., Sujauddin, M., Shafinat, S., and Uddin, M. (2007). Effects of phosphorus and potassium addition on growth and nodulation of Dalbergia sissoo in the nursery. J. Forest Res. 18, 279–282. doi: 10.1007/s11676-007-0056-2
Hungria, M., Franchini, J. C., Campo, R. J., Crispino, C. C., Moraes, J. Z., Sibaldelli, R. N., et al. (2006). Nitrogen nutrition of soybean in Brazil: contributions of biological N2 fixation and N fertilizer to grain yield. Can. J. Plant Sci. 86, 927–939. doi: 10.4141/P05-098
Hussain, A., Arshad, M., Zahir, Z. A., and Asghar, M. (2015). Prospects of zinc solubilizing bacteria for enhancing growth of maize. Pak. J. Agric. Sci. 52, 915–922.
Ibarra-Galeana, J. A., Castro-Martínez, C., Fierro-Coronado, R. A., Armenta-Bojórquez, A. D., and Maldonado-Mendoza, I. E. (2017). Characterization of phosphate-solubilizing bacteria exhibiting the potential for growth promotion and phosphorus nutrition improvement in maize (Zea mays L.) in calcareous soils of Sinaloa. Mexico. Ann. Microbiol. 67, 801–811. doi: 10.1007/s13213-017-1308-9
Imran, M., Shahzad, S. M., Arif, M. S., Yasmeen, T., Ali, B., and Tanveer, A. (2020). Inoculation of potassium solubilizing bacteria with different potassium fertilization sources mediates maize growth and productivity. Pak. J. Agric. Sci. 57, 1045–1055.
Jain, A., Singh, H. B., and Das, S. (2021). Deciphering plant-microbe crosstalk through proteomics studies. Microbiol. Res. 242:126590. doi: 10.1016/j.micres.2020.126590
Jayakumar, V., Sundar, A. R., and Viswanathan, R. (2021). Biocontrol of Colletotrichum falcatum with volatile metabolites produced by endophytic bacteria and profiling VOCs by headspace SPME coupled with GC–MS. Sugar Tech 23, 94–107. doi: 10.1007/s12355-020-00891-2
Joshi, D., Negi, G., Vaid, S., and Sharma, A. (2013). Enhancement of wheat growth and Zn content in grains by zinc solubilizing bacteria. Int. J. Environ. Agric. Biotechnol. 6, 363–370. doi: 10.5958/j.2230-732X.6.3.004
Kahrl, F., Li, Y., Su, Y., Tennigkeit, T., Wilkes, A., and Xu, J. (2010). Greenhouse gas emissions from nitrogen fertilizer use in China. Environ. Sci. Pol. 13, 688–694. doi: 10.1016/j.envsci.2010.07.006
Kalimuthu, R., Suresh, P., Varatharaju, G., Balasubramanian, N., Rajasekaran, K. M., and Shanmugaiah, V. (2019). Isolation and characterization of Indole acetic acid [IAA] producing tomato Rhizobacterium pseudomonas sp VSMKU4050 and its potential for plant growth promotion. Int. J. Curr. Microbiol. Appl. Sci. 8, 443–455. doi: 10.20546/ijcmas.2019.806.050
Kamran, S., Shahid, I., Baig, D. N., Rizwan, M., Malik, K. A., and Mehnaz, S. (2017). Contribution of zinc solubilizing bacteria in growth promotion and zinc content of wheat. Front. Microbiol. 8:2593. doi: 10.3389/fmicb.2017.02593
Kang, S.-M., Khan, A. L., Waqas, M., Asaf, S., Lee, K.-E., Park, Y.-G., et al. (2019). Integrated phytohormone production by the plant growth-promoting rhizobacterium bacillus tequilensis SSB07 induced thermotolerance in soybean. J. Plant Interact. 14, 416–423. doi: 10.1080/17429145.2019.1640294
Kaul, S., Sharma, T., and Dhar, K. (2016). “Omics” tools for better understanding the plant–endophyte interactions. Front. Plant Sci. 7:955. doi: 10.3389/fpls.2016.00955
Ketema, P., and Tefera, T. (2022). Effectiveness of rhizobium strains on faba bean (Vicia fabae L.) at Gumer district, highland area of Southern Ethiopia. Ukraine J. Ecol. 12, 13–18.
Khan, N., Bano, A., Ali, S., and Babar, M. (2020). Crosstalk amongst phytohormones from planta and PGPR under biotic and abiotic stresses. Plant Growth Regul. 90, 189–203. doi: 10.1007/s10725-020-00571-x
Khan, S., and Chattopadhyay, N. (2009). Effect of inorganic and biofertilizers on chilli. J. Crop Weed 5, 191–196.
Kloepper, J. W., Leong, J., Teintze, M., and Schroth, M. N. (1980). Enhanced plant growth by siderophores produced by plant growth-promoting rhizobacteria. Nature 286, 885–886. doi: 10.1038/286885a0
Koskey, G., Mburu, S. W., Njeru, E. M., Kimiti, J. M., Ombori, O., and Maingi, J. M. (2017). Potential of native rhizobia in enhancing nitrogen fixation and yields of climbing beans (Phaseolus vulgaris L.) in contrasting environments of eastern Kenya. Front. Plant Sci. 8:443.
Kumar, S., Bauddh, K., Barman, S., and Singh, R. P. (2014). Amendments of microbial biofertilizers and organic substances reduces requirement of urea and DAP with enhanced nutrient availability and productivity of wheat (Triticum aestivum L.). Ecol. Eng. 71, 432–437. doi: 10.1016/j.ecoleng.2014.07.007
Laxita, L., and Shruti, S. (2020). Isolation and characterization of potassium solubilizing microorganisms from South Gujarat region and their effects on wheat plant. Mukta Shabad 9, 7483–7496.
Lesueur, D., Deaker, R., Herrmann, L., Bräu, L., and Jansa, J. (2016). “The production and potential of biofertilizers to improve crop yields” in Bioformulations: For Sustainable Agriculture. eds. N. K. Arora, S. Mehnaz, and R. Balestrini (New Delhi: Springer), 71–92. doi: 10.1007/978-81-322-2779-3_4
López-Mondéjar, R., Kostovčík, M., Lladó, S., Carro, L., and García-Fraile, P. (2017). “Exploring the plant microbiome through multi-omics approaches” in Probiotics in Agroecosystem. eds. V. Kumar, M. Kumar, S. Sharma, and R. Prasad (Singapore: Springer), 233–268.
Mahmoody, M., and Noori, M. (2014). Effect of gibberellic acid on growth and development plants and its relationship with abiotic stress. Int. J. Farming Allied Sci. 3, 717–721.
Malusá, E., Sas-Paszt, L., and Ciesielska, J. (2012). Technologies for beneficial microorganisms inocula used as biofertilizers. Sci. World J. 2012:491206, 1–12. doi: 10.1100/2012/491206
Mapanda, F., Wuta, M., Nyamangara, J., and Rees, R. M. (2011). Effects of organic and mineral fertilizer nitrogen on greenhouse gas emissions and plant-captured carbon under maize cropping in Zimbabwe. Plant Soil 343, 67–81. doi: 10.1007/s11104-011-0753-7
Markets and Markets (2019). Biofertilizer market by form (liquid, carrier-based), mode of application (soil treatment, seed treatment), crop type, type (nitrogen-fixing, phosphates solubilizing and mobilizing, potash solubilizing and mobilizing), region-global forecast to 2025, 2019. Available at: https://www.marketsandmarkets.com/Market-Reports/compound-biofertilizers-customized-fertilizers-market-856.html (Accessed September 1, 2020).
Markets and Markets (2020). Markets and markets, biofertilizer market by form (liquid, carrier-based), mode of application (soil treatment, seed treatment), crop type, type (nitrogen-fixing, phosphates solubilizing and mobilizing, potash solubilizing and mobilizing), region-global forecast to 2026. Available at: https://www.researchandmarkets.com/reports/5334050/global-biofertilizers-market-by-form-liquid (Accessed May17, 2022).
Markets and Markets (2014). Global biofertilizer markets by types, application and geography-trends and forecast. Available at: MarketResearch.com (Accessed July 3, 2022).
Matich, T. (2016). Potash investing. What is potash. Investing news network. Available at: https://investingnews.com/daily/resource-investing/agriculture-investing/potash-investing/what-is-potash/ (Accessed July11, 2022).
Mazid, M., and Khan, T. A. (2015). Future of bio-fertilizers in Indian agriculture: an overview. Int. J. Agric. Res. 3, 10–23.
Meena, V. S., Maurya, B. R., Meena, S. K., Mishra, P. K., Bisht, J. K., and Pattanayak, A. (2018). Potassium solubilization: Strategies to mitigate potassium deficiency in agricultural soils. Glob. J. Biol. Agric. Health Sci. 7, 1–3. doi: 10.24105/2319-5584.100003
Melchiorre, M., De Luca, M. J., Gonzalez Anta, G., Suarez, P., Lopez, C., Lascano, R., et al. (2011). Evaluation of Bradyrhizobia strains isolated from field-grown soybean plants in Argentina as improved inoculants. Biol. Fertil. Soils 47, 81–89. doi: 10.1007/s00374-010-0503-7
Melo, A., Pinto, E., Aguiar, A., Mansilha, C., Pinho, O., and Ferreira, I. M. P. L. V. O. (2012). Impact of intensive horticulture practices on groundwater content of nitrates, sodium, potassium, and pesticides. Environ. Monit. Assess. 184, 4539–4551. doi: 10.1007/s10661-011-2283-4
Mishra, J., and Arora, N. K. (2016). “Bioformulations for plant growth promotion and combating phytopathogens: a sustainable approach” in Bioformulations: For sustainable agriculture. eds. N. Arora, S. Mehnaz, and R. Balestrini (New Delhi: Springer), 3–33.
Mohammadi, K., and Sohrabi, Y. (2012). Bacterial biofertilizers for sustainable crop production: a review. ARPN J. Agric. Biol. Sci. 7, 307–316.
Montalvo, D., Degryse, F., da Silva, R. C., Baird, R., and McLaughlin, M. J. (2016). “Agronomic effectiveness of zinc sources as micronutrient fertilizer” in Advances in Agronomy. ed. D. L. Sparks (Cambridge, MA: Academic Press)
Mustafa, S., Kabir, S., Shabbir, U., and Batool, R. (2019). Plant growth promoting rhizobacteria in sustainable agriculture: from theoretical to pragmatic approach. Symbiosis 78, 115–123. doi: 10.1007/s13199-019-00602-w
Nair, S. S., and Brahmaprakash, G. (2017). Development and standardization of effervescent biofertilizer consortial tablets for french bean (Phaseolus vulgaris L.). Mysore J. Agric. Sci. 51, 373–384.
Nakkreen, S., Fernando, D. W. G., and Siddiqui, Z. A. (2005). “Plant growth promoting rhizobacteria formulations and its scope in commercialization for the management of pests and diseases” in Biocontrol and biofertilization. ed. Z. A. Siddiqui (Dordrecht: Springer), 257–296.
Naz, I., Ahmad, H., Khokhar, S. N., Khan, K., and Shah, A. H. (2016). Impact of zinc solubilizing bacteria on zinc contents of wheat. Am. Euras. J. Agric. Environ. Sci. 16, 449–454.
Neog, R. (2018). Assessing the impact of chemical fertilizers on soil acidification: a study on Jorhat district of Assam. India. Agric. Sci. Digest 38, 270–274. doi: 10.18805/ag.D-4220
Ngakou, A., Megueni, C., Ousseni, H., and Massai, A. (2009). Study on the isolation and characterization of rhizobia strains as biofertilizer tools for growth improvement of four grain legumes in Ngaoundéré-Cameroon. Int. J. Biol. Chem. 3, 1078–1089. doi: 10.4314/ijbcs.v3i5.51086
Nobbe, F., and Hiltner, L. (1986). Inoculation of the soil for cultivating leguminous plants. U.S. patent no. 570:813. Washington, DC: U.S. Patent and Trademark Office.
Odoh, C. K., Eze, C. N., Akpi, U. K., and Unah, V. U. (2019). Plant growth promoting rhizobacteria (PGPR): a novel agent for sustainable food production. Am. J. Agric. Biol. Sci. 14, 35–54. doi: 10.3844/ajabssp.2019.35.54
Paliya, S., Mandpe, A., Kumar, S., and Kumar, M. S. (2019). Enhanced nodulation and higher germination using sludge ash as a carrier for biofertilizer production. J. Environ. Manag. 250:109523. doi: 10.1016/j.jenvman.2019.109523
Paudyal, S. P., and Gupta, V. (2018). Substitution of chemical fertilizer nitrogen through rhizobium inoculation technology. Our Nat. 16, 43–47. doi: 10.3126/on.v16i1.22121
Perumal, M. D., Selvi, D., Chitdeshwari, T., and Balachandar, D. (2019). Enhanced zinc nutrient and enzyme activity of rice crop by zinc solubilizing bacteria with Zn sources in Zn deficient Rice soil. Madras Agric. J. 106, 171–177. doi: 10.29321/MAJ.2019.000242
Phillips, P. W. (2004). An economic assessment of the global inoculant industry. Crop Manage. 3, 1–10. doi: 10.1094/CM-2004-0301-08-RV
Raimi, A. (2018). Quality assessment of commercial Biofertilisers and the awareness of smallholder farmers in Gauteng province, South Africa. Dissertation/master's thesis. Gauteng (South Africa): University of South Africa.
Raimi, A., Roopnarain, A., and Adeleke, R. (2021). Biofertilizer production in Africa: current status, factors impeding adoption and strategies for success. Sci. Afr. 11:e00694. doi: 10.1016/j.sciaf.2021.e00694
Raji, M., and Thangavelu, M. (2021). Isolation and screening of potassium solubilizing bacteria from saxicolous habitat and their impact on tomato growth in different soil types. Arch. Microbiol. 203, 3147–3161. doi: 10.1007/s00203-021-02284-9
Ramesh, A., Sharma, S. K., Sharma, M. P., Yadav, N., and Joshi, O. P. (2014). Inoculation of zinc solubilizing bacillus aryabhattai strains for improved growth, mobilization and biofortification of zinc in soybean and wheat cultivated in Vertisols of Central India. Appl. Soil Ecol. 73, 87–96. doi: 10.1016/j.apsoil.2013.08.009
Ramoneda, J., Roux, J. J. L., Frossard, E., Frey, B., and Gamper, H. A. (2020). Geographical patterns of root nodule bacterial diversity in cultivated and wild populations of a woody legume crop. FEMS Microbiol. Ecol. 96:fiaa145. doi: 10.1093/femsec/fiaa145
Reyes-Castillo, A., Gerding, M., Oyarzúa, P., Zagal, E., Gerding, J., and Fischer, S. (2019). Plant growth-promoting rhizobacteria able to improve NPK availability: selection, identification and effects on tomato growth. Chil. J. Agric. Res. 79, 473–485. doi: 10.4067/S0718-58392019000300473
Riah, N., de Lajudie, P., Béna, G., Heulin, K., and Djekoun, A. (2021). Variability in symbiotic efficiency with respect to the growth of pea and lentil inoculated with various rhizobial genotypes originating from sub-humid and semi-arid regions of eastern Algeria. Symbiosis 85, 371–384. doi: 10.1007/s13199-021-00821-0
Riaz, U., Mehdi, S. M., Iqbal, S., Khalid, H. I., Qadir, A. A., Anum, W., et al. (2020). “Bio-fertilizers: eco-friendly approach for plant and soil environment” in Bioremediation and Biotechnology. eds. K. Hakeem, R. Bhat, and H. Qadri (Cham: Springer), 189–213.
Rizvi, A., Ahmed, B., Khan, M. S., El-Beltagi, H. S., Umar, S., and Lee, J. (2022a). Bioprospecting plant growth promoting rhizobacteria for enhancing the biological properties and phytochemical composition of medicinally important crops. Molecules 27:1407. doi: 10.3390/molecules27041407
Rizvi, A., Ahmed, B., Khan, B., Rajput, V. D., Umar, S., Minkina, T., et al. (2022b). Maize associated bacterial microbiome linked mitigation of heavy metal stress: a multidimensional detoxification approach. Environ. Exp. Bot. 200:104911. doi: 10.1016/j.envexpbot.2022.104911
Rizvi, A., Ahmed, B., Khan, M. S., Umar, S., and Lee, J. (2021a). Sorghum-phosphate solubilizers interactions: crop nutrition, biotic stress alleviation, and yield optimization. Front. Plant Sci. 12:746780. doi: 10.3389/fpls.2021.746780
Rizvi, A., Ahmed, B., Khan, M. S., Umar, S., and Lee, J. (2021b). Psychrophilic bacterial phosphate-biofertilizers: a novel extremophile for sustainable crop production under cold environment. Microorganisms 9:2451. doi: 10.3390/microorganisms9122451
Rizvi, A., Ahmed, B., Zaidi, A., and Khan, M. (2019). Bioreduction of toxicity influenced by bioactive molecules secreted under metal stress by Azotobacter chroococcum. Ecotoxicology 28, 302–322. doi: 10.1007/s10646-019-02023-3
Rizvi, A., Zaidi, A., Ameen, F., Ahmed, B., AlKahtani, M. D., and Khan, M. S. (2020). Heavy metal induced stress on wheat: phytotoxicity and microbiological management. RSC Adv. 10, 38379–38403. doi: 10.1039/D0RA05610C
Rizvi, A., Zaidi, A., Khan, M., Saif, S., Ahmed, B., and Shahid, M. (2017). Growth Improvement and Management of Vegetable Diseases by Plant Growth-promoting Rhizobacteria in Microbial Strategies for Vegetable Production, (Cham, Springer)
Saber, Z., Pirdashti, H., Esmaeili, M., Abbasian, A., and Heidarzadeh, A. (2012). Response of wheat growth parameters to co-inoculation of plant growth promoting rhizobacteria (PGPR) and different levels of inorganic nitrogen and phosphorus. World Appl. Sci. J. 16, 213–219.
Sahu, P. K., and Brahmaprakash, G. P. (2016). “Formulations of biofertilizers – approaches and advances” in Microbial Inoculants in Sustainable Agricultural Productivity: Functional Applications. eds. D. P. Singh, H. B. Singh, and R. Prabha (New Delhi: Springer), 179–198.
Santhi, C., Rajesh, M., Ramesh, S., Muralikrishna, K., Gangaraj, K., and Payal, G. (2021). Genome-wide exploration of auxin response factors (ARFs) and their expression dynamics in response to abiotic stresses and growth regulators in coconut (Cocos nucifera L.). plant. Gene 28:100344. doi: 10.1016/j.plgene.2021.100344
Sanyal, D., Osorno, J. M., and Chatterjee, A. (2020). Influence of rhizobium inoculation on dry bean yield and symbiotic nitrogen fixation potential. J. Plant Nutr. 43, 798–810. doi: 10.1080/01904167.2020.1711946
Sattar, A., Naveed, M., Ali, M., Zahir, Z. A., Nadeem, S. M., Yaseen, M., et al. (2019). Perspectives of potassium solubilizing microbes in sustainable food production system: a review. Appl. Soil Ecol. 133, 146–159. doi: 10.1016/j.apsoil.2018.09.012
Sekar, J., Raj, R., and Prabavathy, V. (2016). “Microbial consortial products for sustainable agriculture: commercialization and regulatory issues in India” in Agriculturally Important Microorganisms. eds. H. Singh, B. Sarma, and C. Keswani (Singapore: Springer), 107–132.
Shahid, M., Ameen, F., Maheshwari, H. S., Ahmed, B., AlNadhari, S., and Khan, M. S. (2021). Colonization of Vigna radiata by a halotolerant bacterium Kosakonia sacchari improves the ionic balance, stressor metabolites, antioxidant status and yield under NaCl stress. Appl. Soil Ecol. 158:103809. doi: 10.1016/j.apsoil.2020.103809
Sharma, S. K. M. P., Ramesh, A., and Joshi, O. P. (2012). Characterization of zinc-solubilizing bacillus isolates and their potential to influence zinc assimilation in soybean seeds. J. Microbiol. Biotechnol. 22, 352–359. doi: 10.4014/jmb.1106.05063
Shurtleff, W., and Aoyagi, A. (2018). History of Research on Nitrogen Fixation in Soybeans (1887–2018). Lafayette, CA: Soyinfo Center.
Simarmata, T., Turmuktini, T., Fitriatin, B. N., and Setiawati, M. R. (2016). Application of bioameliorant and biofertilizers to increase the soil health and rice productivity. HAYATI J. Biosci. 23, 181–184. doi: 10.1016/j.hjb.2017.01.001
Singh, J., Singh, A. V., Upadhayay, V. K., and Khan, A. (2020). Comparative evaluation of developed carrier based bio-formulations bearing multifarious PGP properties and their effect on shelf life under different storage conditions. Environ. Ecol. 38, 96–103.
Soumare, A., Boubekri, K., Lyamlouli, K., Hafidi, M., Ouhdouch, Y., and Kouisni, L. (2020). From isolation of phosphate solubilizing microbes to their formulation and use as biofertilizers: status and needs. Front. Bioeng. Biotechnol. 7:425. doi: 10.3389/fbioe.2019.00425
Tairo, E. V., and Ndakidemi, P. A. (2014). Macronutrients uptake in soybean as affected by Bradyrhizobium japonicum inoculation and phosphorus (P) supplements. Am. J. Plant Sci. 5, 488–496. doi: 10.4236/ajps.2014.54063
Tan, K., Radziah, O., Halimi, M., Khairuddin, A., and Shamsuddin, Z. (2015). Assessment of plant growth-promoting rhizobacteria (PGPR) and rhizobia as multi-strain biofertilizer on growth and N2 fixation of rice plant. Aust. J. Crop. Sci. 9, 1257–1264.
Teotia, P., Kumar, V., Kumar, M., Shrivastava, N., and Varma, A. (2016). “Rhizosphere microbes: potassium solubilization and crop productivity–present and future aspects” in Potassium Solubilizing Microorganisms for Sustainable Agriculture. eds. V. Meena, B. Maurya, J. Verma, and R. Meena (New Delhi: Springer), 315–325. doi: 10.1007/978-81-322-2776-2_22
Thies, J., Woomer, P., and Singleton, P. (1995). Enrichment of Bradyrhizobium spp populations in soil due to cropping of the homologous host legume. Soil Biol. Biochem. 27, 633–636. doi: 10.1016/0038-0717(95)98643-3
Tomer, S., Suyal, D. C., and Goel, R. (2016). “biofertilizers: a timely approach for sustainable agriculture” in Plant-microbe Interaction: an Approach to Sustainable Agriculture. ed. D. K. Choudhary (Singapore: Springer)
Transparency Market Research (2022). Biofertilizers market (product: nitrogen fixing, phosphate mobilizing, and potassium mobilizing; and application: Cereals & Grains, Fruits & Vegetables, oil Seeds & Pulses, and others [nursery and turf]) – global industry analysis, size, share, growth, trends, and forecast, 2021–2031. Available at: https://www.transparencymarketresearch.com/pressrelease/globalbiofertilizersmarket.htm (Accessed July 11, 2022).
United Nations (2015). Department of Economic and Social Affairs, Population Division. World population prospects: the 2015 revision, key findings and advance tables (ESA/P/WP.241; p. 66). United Nations. Available at: https://population.un.org/wpp/publications/files/key_findings_wpp_2015.pdf (Accessed July 13, 2022).
Unkovich, M., and Baldock, J. (2008). Measurement of asymbiotic N2 fixation in Australian agriculture. Soil Biol. Biochem. 40, 2915–2921. doi: 10.1016/j.soilbio.2008.08.021
Vaishnav, A., Kumar, R., Singh, H. B., and Sarma, B. K. (2022). Extending the benefits of PGPR to bioremediation of nitrile pollution in crop lands for enhancing crop productivity. Sci. Total Environ. 826:154170. doi: 10.1016/j.scitotenv.2022.154170
Vaishnav, A., and Singh, H. B. (2021). New and Future Developments in Microbial Biotechnology and Bioengineering. Amsterdam: Elsevier.
Vassilev, N., Vassilev, M., Lopez, A., Martos, V., Reyes, A., Maksimovic, I., et al. (2015). Unexploited potential of some biotechnological techniques for biofertilizer production and formulation. Appl. Microbiol. Biotechnol. 99, 4983–4996. doi: 10.1007/s00253-015-6656-4
Vatsyayan, N., and Ghosh, A. K. (2013). Isolation and characterization of microbes with biofertilizer potential. J. Environ. Sci. 7, 5–9.
Verma, M., Singh, A., Dwivedi, D. H., and Arora, N. K. (2020). Zinc and phosphate solubilizing rhizobium radiobacter (LB2) for enhancing quality and yield of loose leaf lettuce in saline soil. Environ. Sustain. 3, 209–218. doi: 10.1007/s42398-020-00110-4
Vessey, J. K. (2003). Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 255, 571–586. doi: 10.1023/A:1026037216893
Vintage Market Research (2022). Global biofertilizers market to witness a CAGR growth of 10.9% market is projected to worth of USD 4448.97 million by 2028 key players change the view of the global face of industry. Available at: https://www.globenewswire.com/news-release/2022/04/01/2414570/0/en/Global-Biofertilizers-Market-to-witness-a-CAGR-Growth-of-10-9-Market-is-Projected-to-Worth-of-USD-4448-97-Million-by-2028-Key-Players-Change-the-View-of-the-Global-Face-of-Industry.html (Accessed July 3, 2022).
Wang, Y., Zhang, G., Huang, Y., Guo, M., Song, J., Zhang, T., et al. (2022). A potential biofertilizer-Siderophilic bacteria isolated from the Rhizosphere of Paris polyphylla var. yunnanensis. Front. Microbiol. 13:870413. doi: 10.3389/fmicb.2022.870413
Wani, S., Rupela, O., and Lee, K. (1995). “Sustainable agriculture in the semi-arid tropics through biological nitrogen fixation in grain legumes” in Management of Biological Nitrogen Fixation for the Development of more Productive and Sustainable Agricultural Systems. eds. J. K. Ladha and M. B. Peoples (Singapore: Dordrecht)
Yamazaki, S., Mardani-Korrani, H., Kaida, R., Ochiai, K., Kobayashi, M., Nagano, A. J., et al. (2021). Field multi-omics analysis reveals a close association between bacterial communities and mineral properties in the soybean rhizosphere. Sci. Rep. 11, 1–16. doi: 10.1038/s41598-021-87384-8
Yan, P., Wu, L., Wang, D., Fu, J., Shen, C., Li, X., et al. (2020). Soil acidification in Chinese tea plantations. Sci. Total Environ. 715:136963. doi: 10.1016/j.scitotenv.2020.136963
Yehuda, Z., Shenker, M., Romheld, V., Marschner, H., Hadar, Y., and Chen, Y. (1996). The role of ligand exchange in the uptake of iron from microbial siderophores by gramineous plants. Plant Physiol. 112, 1273–1280. doi: 10.1104/pp.112.3.1273
Zaidi, A., Khan, M. S., Rizvi, A., Saif, S., Ahmad, B., and Shahid, M. (2017). “Role of phosphate-solubilizing bacteria in legume improvement” in Microbes for Legume Improvement. eds. A. Zaidi and M. S. Khan (Musarrat: Springer International Publishing), 175–197.
Keywords: biofertilizers, sustainable agriculture, plant growth-promoting rhizobacteria, microbial stimulants, microbial formulations
Citation: Aloo BN, Tripathi V, Makumba BA and Mbega ER (2022) Plant growth-promoting rhizobacterial biofertilizers for crop production: The past, present, and future. Front. Plant Sci. 13:1002448. doi: 10.3389/fpls.2022.1002448
Edited by:
Yassine Mabrouk, National Center for Nuclear Science and Technology, TunisiaReviewed by:
Stefan Shilev, Agricultural University–Plovdiv, BulgariaBilal Ahmed, Yeungnam University, South Korea
Copyright © 2022 Aloo, Tripathi, Makumba and Mbega. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vishal Tripathi, dmlzaGFsYmlvdGVjaGJodUBnbWFpbC5jb20=; Becky N. Aloo, YWxvb2JlY2t5QHlhaG9vLmNvbQ==
 Becky N. Aloo
Becky N. Aloo Vishal Tripathi
Vishal Tripathi Billy A. Makumba3
Billy A. Makumba3