- State Key Laboratory of Crop Stress Biology for Arid Areas/Shaanxi Key Laboratory of Apple, College of Horticulture, Northwest A&F University, Yangling, China
Improved water use efficiency (WUE) promotes plant survival and crop yield under water deficit conditions. Although the plant-specific HD-Zip I transcription factors have important roles in plant adaptation to various abiotic stresses, including water deficit, their functions in regulating WUE of apple (Malus domestica) are poorly understood. We characterized the role of MdHB-7 in WUE regulation by subjecting MdHB-7 transgenic plants to long-term moderate soil water deficit. The long-term WUE (WUEL) of transgenic apple plants with MdHB-7 overexpression or MdHB-7 RNA interference (RNAi) differed significantly from that of control plants. Upregulation of MdHB-7 caused reduced stomatal density, whereas the suppression of MdHB-7 increased stomatal density under both normal and long-term moderate soil water deficit conditions. Moderate reduction in stomatal density helped to improve the WUE of MdHB-7 overexpression transgenic plants, especially under water deficit conditions. MdHB-7 overexpression plants maintained high rates of photosynthesis that were conducive to the accumulation of biomass and the improvement of WUEL. MdHB-7 overexpression also alleviated the inhibition of root growth caused by long-term moderate soil water deficit and improved root vitality and hydraulic conductivity, which were essential for improving plant WUEL. By contrast, MdHB-7 RNA interference reduced the WUEL of transgenic plants by inhibiting these factors under normal and long-term moderate soil water deficit conditions. Taken together, our results provide solid evidence for a crucial role of MdHB-7 in the regulation of apple WUEL and provide new insights for improving the WUE of apple plants under moderate soil water deficit.
Introduction
Global climate change and the increasing use of groundwater for agriculture have caused severe water shortages for crops in many parts of the world (Shao et al., 2008; Falkenmark, 2013; Wang et al., 2018). Water use efficiency (WUE) is defined as the carbon fixed or biomass produced per unit of water used. It is a comprehensive indicator used to assess plant growth under water deficit. Improvement of WUE is an effective way to reduce production losses due to soil water deficit (Condon et al., 2004; Ali and Talukder, 2008). Apple (Malus domestica) is one of the most widely grown and economically important fruits in temperate regions (Zhao et al., 2020b). The Loess Plateau of Shaanxi Province is one of China’s main apple growing regions (Liu et al., 2019). However, as a semi-arid area, the annual rainfall in the Loess Plateau is unevenly distributed. Plants face continuous moderate soil water deficit, which limit the sustainable development of apples in this area (Yan et al., 2015). Therefore, genes related to WUE regulation whose manipulation could improve apple WUE under soil water deficit conditions are important targets for apple breeding.
Many studies have shown the complexity of WUE regulation in plants. The WUE of plants is closely related to two physiological processes, photosynthesis and transpiration, and all factors that affect crop photosynthesis and transpiration have an impact on WUE (Condon et al., 2004; Karaba et al., 2007). Stomata affect WUE by controlling the rates of evapotranspiration and CO2 absorption (Yoo et al., 2010; Guo et al., 2019b), and the regulation of stomatal density is a strategy that can improve plant WUE (Yoo et al., 2010; Franks et al., 2015). For example, the overexpression of EPF genes in various plant species greatly improved long-term WUE (WUEL) by altering stomatal development and density (Franks et al., 2015; Wang et al., 2016; Hughes et al., 2017; Caine et al., 2019). Similarly, the expression of the Arabidopsis HARDY gene improved WUE in rice by enhancing photosynthesis and reducing transpiration (Karaba et al., 2007). MhYTP1 enhanced WUEL of transgenic apple by increasing ABA levels under soil water deficit (Guo et al., 2019a), and MhYTP2 enhanced WUEL of transgenic apples by activating ABA and ethylene signaling (Liu et al., 2019). Roots are the main interface through which plants absorb water from the surrounding soil, and the root system is therefore considered to be a key determinant of WUE under various levels of soil water deficit (Coudert et al., 2010; Wang et al., 2020).
The homeodomain–leucine zipper (HD-Zip) transcription factor (TF) family is unique to plants and plays an important role in stress response regulation (Schena and Davis, 1992; Ariel et al., 2007; Gong et al., 2019). HD-Zip TFs have been identified in multiple species and divided into four subfamilies (Ariel et al., 2007). Many HD-Zip I members have been shown to function in the adaptive response to abiotic stress. For example, ATHB7 and ATHB12 were strongly induced by water deficiency and ABA treatment (Ré et al., 2014). Overexpression of ATHB13 or HaHB1 stabilized cell membrane integrity in transgenic Arabidopsis and increased plant tolerance of drought and salt stress (Cabello and Chan, 2012). Overexpression of the maize HD-Zip I genes ZmHDZ4 and ZmHDZ10 reduced relative electrolyte leakage (REL) and malondialdehyde (MDA) content, thereby conferring drought tolerance on transgenic rice (Zhao et al., 2014; Wu et al., 2016). TaHDZipI-5 conferred freezing and drought tolerance on transgenic wheat plants (Yang et al., 2018). Overexpression of HaHB4 improved the water deficit tolerance, yield, and WUE of transgenic soybean plants (Ribichich et al., 2020). Although the roles of several HD-Zip I TFs in the regulation of abiotic stress response and tolerance have been characterized, the roles of most HD-Zip I TFs in regulating WUE remain unclear, especially in woody plants.
In our recent work, we found that the HD-Zip I TF MdHB-7 positively regulated apple drought tolerance. MdHB-7 overexpressing (OE) transgenic apple plants were more tolerant of drought treatment, whereas MdHB-7 RNA interference (RNAi) plants were more sensitive (Zhao et al., 2020a). It is important to note that short-term drought produced by withholding irrigation differs from the long-term moderate soil water deficit that is common during apple production in the Loess Plateau. Furthermore, the increased tolerance of MdHB-7 transgenic plants to drought does not necessarily mean that the gene positively regulates WUE, especially under long-term moderate soil water deficit. In order to study the effect of MdHB-7 on WUE and to explore the possible mechanisms by which MdHB-7 regulates WUE in apple plants, such as affecting stomatal density and root water uptake capacity, we cultivated MdHB-7 transgenic apple plants under long-term (60days) moderate soil water deficits and evaluated their performance.
Materials and Methods
Plant Materials, Growth Conditions, and Treatments
GL-3 (“Royal Gala”) plants were obtained from Dai et al. (2013). MdHB-7 OE and MdHB-7 RNAi transgenic lines were generated in our previous studies (Zhao et al., 2020b) and were subcultured according to the method of Sun et al. (2018). GL-3 and MdHB-7 transgenic plants were rooted as described in Zhou et al. (2019). Rooted GL-3 plants and MdHB-7 transgenic lines were transplanted into plastic pots filled with nutrient soil, vermiculite, and perlite (3:1:1; v:v:v), then grown in an artificial climate chamber under a 16/8-h light/dark photoperiod at a temperature of 23–25°C. After 1month of adaptation, plants of similar size were transplanted into pots (38cm×23cm) filled with loess soil, sand, and organic matter (5:1:1; v:v:v) and grown in the greenhouse of Northwest A & F University in Yangling (34°20'N, 108°24'E), Shaanxi Province, China. The weight of soil in each pot was 13.5±0.1kg. When the plants had grown to about 60cm in height, they were divided into a well-watered control group and a moderate soil water deficit treatment group. Forty plants were used from each genotype and were divided into two groups, one group for sampling and one group for the final biomass statistics. Seedlings were irrigated so that the control pots were maintained at 75–85% of maximum field capacity and the soil water deficit-treated pots were maintained at 45–55% of maximum field capacity (Geng et al., 2018). Maximum water field capacity was defined as (W1−W2)/W2, where W1 is the saturated soil weight, and W2 is the dry soil weight. The soil texture and weight of all pots were the same, so the maximum field capacity of all pots was also the same. Therefore, the weight of each pot at 75–85 or 45–55% of maximum field capacity could be calculated. GL-3 and transgenic plants were irrigated every 2days. And, all pots were weighed before each watering in order to calculate the amount of water to be added, and this amount was recorded. At the end of the experiments, the total water consumption was calculated.
Physiological Analyses
All photosynthetic measurements were obtained using a Li-6,400 portable photosynthesis system (Li-Cor, Inc., Lincoln, NE, United States) with 1,000μmol photons m−2 s−1 and a cuvette CO2 concentration of 400μmol CO2 mol−1 air. Net photosynthetic rate (Pn), stomatal conductance (gs), transpiration rate, and instantaneous WUE (WUEi) were measured on at least five plants from each genotype. Chlorophyll content, leaf relative water content, and water loss were measured as described by Hu et al. (2018) and Jiang et al. (2019).
The triphenyltetrazolium chloride (TTC) method was used to assess the effect of long-term soil water deficit on the root activity of GL-3 and MdHB-7 transgenic plants (Huo et al., 2020). Root hydraulic conductance was measured using a pressure chamber (Model 1505D, PMS Instrument Company, Albany, OR, United States) as described by Zhu et al. (2015). At the end of the experiment, the roots of the apple plants were too large to directly measure the root hydraulic conductivity at the whole-root level. For each plant, five similarly sized lateral roots were selected for the measurement of root hydraulic conductivity. The average root hydraulic conductivity value of the five lateral roots was used to represent the root hydraulic conductivity of the plant. For each genotype, six plants were randomly selected for measurement of root hydraulic conductivity.
The plant height and stem diameter of GL-3 and MdHB-7 transgenic plants were measured using a tape measure and vernier caliper, respectively.
Biomass Accumulation and Long-Term Water Use Efficiency
At the beginning and end of the moderate soil water deficit treatment, the dry weights of roots, stems, and leaves of GL-3 and transgenic plants were measured. At the end of the experiment, 18 plants from each line that had not previously been sampled during the experiment were used for biomass statistics. The relative growth rate (RGR) was calculated as described previously (Radford, 1967). RGR=(Ln DW2−Ln DW1)/(T2−T1). DW2 is the plant dry weight at the final harvest time (T2) of the moderate soil water deficit treatment, and DW1 is the plant dry weight at the initial time (T1) of the moderate soil water deficit treatment. Long-term water use efficiency (WUEL) was calculated as the ratio of the accumulation of total dry mass produced to total water used (Ehdaie and Waines, 1993). WUEL=(DW2−DW1)/total water consumption.
Stomatal Characteristics
Leaf stomata were observed under an EX30 microscope (SDPTOP). At least 10 fully expanded leaves from the same stem position were harvested from each genotype after 60days of moderate soil water deficit. The number of stomata in each image was recorded using Image J software and used to calculate the final stomatal density.
RNA Extraction and qRT-PCR Analysis
Total RNA was extracted from leaves using the Plant RNA Isolation Kit from Wolact [Wolact, Vicband Life Sciences Company (Hk) Limited]. First-strand cDNA was synthesized using a RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific), and the reaction products were diluted to 100ngμl−1 with sterile water. Real time qRT-PCR analysis was performed as previously described by Zhao et al. (2020b), and all the primers used are listed in Supplementary Table S1.
Statistical Analysis
SPSS Version 17.0 (SPSS Inc., Chicago, IL, United States) was used for statistical analysis. Data were analyzed by one-way ANOVA followed by Tukey’s multiple range test, and experimental data were presented as mean±SD. Differences were considered as statistically significant at p<0.05.
Results
MdHB-7 Promotes Plant Growth Under Long-Term Moderate Soil Water Deficit
At the beginning of the experiment, there were no significant differences in plant height, stem diameter, and dry weight between GL-3 and MdHB-7 transgenic plants (Supplementary Figure S1). Under well-watered conditions, the plant height and stem diameter of GL-3 and MdHB-7 OE lines (OE #2 and OE #3) showed no significant difference throughout the experimental period (Figure 1; Supplementary Figure S2). The height of MdHB-7 RNAi lines was significantly lower than that of the GL-3 and MdHB-7 overexpression lines after more than 40days of growth under normal conditions (Figure 1; Supplementary Figure S2). Sixty days of long-term moderate soil water deficit inhibited the growth of GL-3 and transgenic plants compared with the control group. At increasing treatment durations, the growth of the MdHB-7 overexpression transgenic lines gradually became better than that of the GL-3 and RNAi plants (Figure 1; Supplementary Figure S2). Compared with GL-3 plants, MdHB-7 OE lines had taller shoots and thicker stems under moderate soil water deficit, whereas MdHB-7 RNAi lines had shorter shoots and thinner stems (Figures 1A–C). To eliminate the possibility that MdHB-7 affected plant growth by regulating the expression of other HD-Zip genes, we examined the expression of MdHD-Zips in GL-3 and MdHB-7 transgenic plants. These MdHD-Zips have high sequence similarity to MdHB-7, and their expression was not significantly altered in MdHB-7 transgenic plants (Supplementary Figure S3). These results indicated that the weak growth of MdHB-7 RNAi plants was due directly to MdHB-7 suppression, rather than the influence of MdHB-7 on other MdHD-Zips. MdHB-7 therefore had a positive effect on plant growth under long-term moderate soil water deficit.
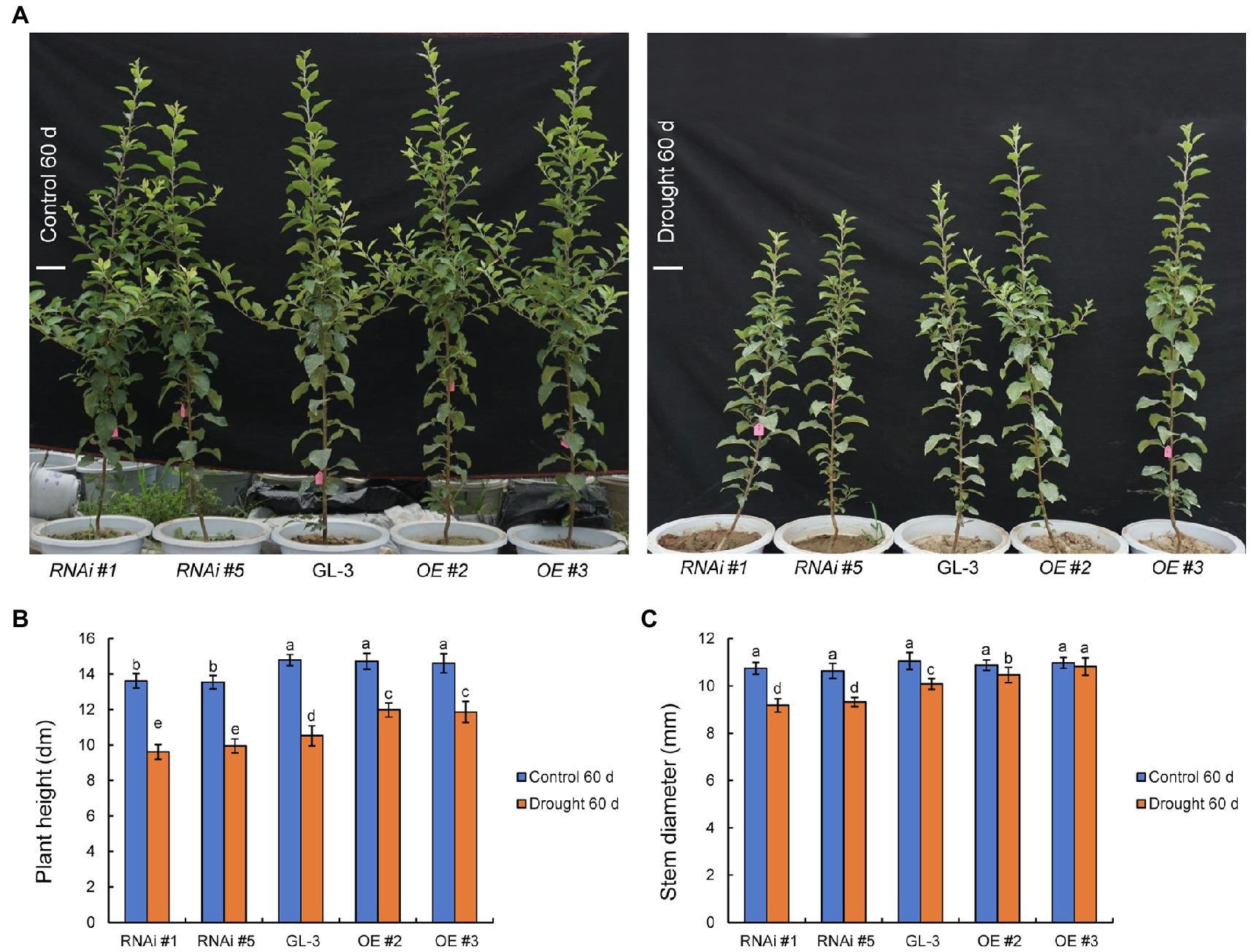
Figure 1. Comparison of the growth status of GL-3 and transgenic apple plants under long-term moderate soil water deficit. (A) Phenotypic comparison of GL-3 and MdHB-7 transgenic apple plants grown in well-watered and moderate soil water deficit conditions for 60days. RNAi and OE represent the MdHB-7-RNAi and MdHB-7-overexpressing apple plants, respectively. Scale bars, 10cm. (B) Plant height. (C) Stem diameter. Data are means±SD (n=18 for B,C; 18 plants from each line). Different letters indicate significant differences between GL-3 and transgenic apple plants based on one-way ANOVA and Tukey’s multiple range test (p<0.05).
MdHB-7 Regulates Biomass Accumulation, RGR, and WUEL Under Long-Term Moderate Soil Water Deficit
There were no significant differences in biomass accumulation and RGR between GL-3 and MdHB-7 OE lines under normal conditions, whereas the MdHB-7 RNAi lines accumulated significantly lower biomass and had a lower RGR. Compared with the control group, moderate soil water deficit significantly inhibited the biomass accumulation and RGR of GL-3 and MdHB-7 transgenic plants. Under long-term moderate soil water deficit, MdHB-7 OE lines accumulated more biomass and had higher RGR than GL-3, whereas MdHB-7 RNAi lines accumulated less biomass and had the lowest RGR (Figures 2A,B). There were no differences in WUEL between GL-3 and MdHB-7 OE lines after 60days under normal conditions. Under long-term moderate soil water deficit, the WUEL of MdHB-7 OE lines was significantly higher than that of GL-3. The WUEL of the RNAi lines was lower than that of GL-3 under both normal and drought conditions (Figure 2C). Interestingly, compared with normal conditions, moderate soil water deficit inhibited the WUEL of GL-3 and RNAi lines but increased the WUEL of OE plants (Figure 2C). These results indicated that MdHB-7 promoted biomass accumulation and RGR and improved WUEL under long-term moderate soil water deficit.
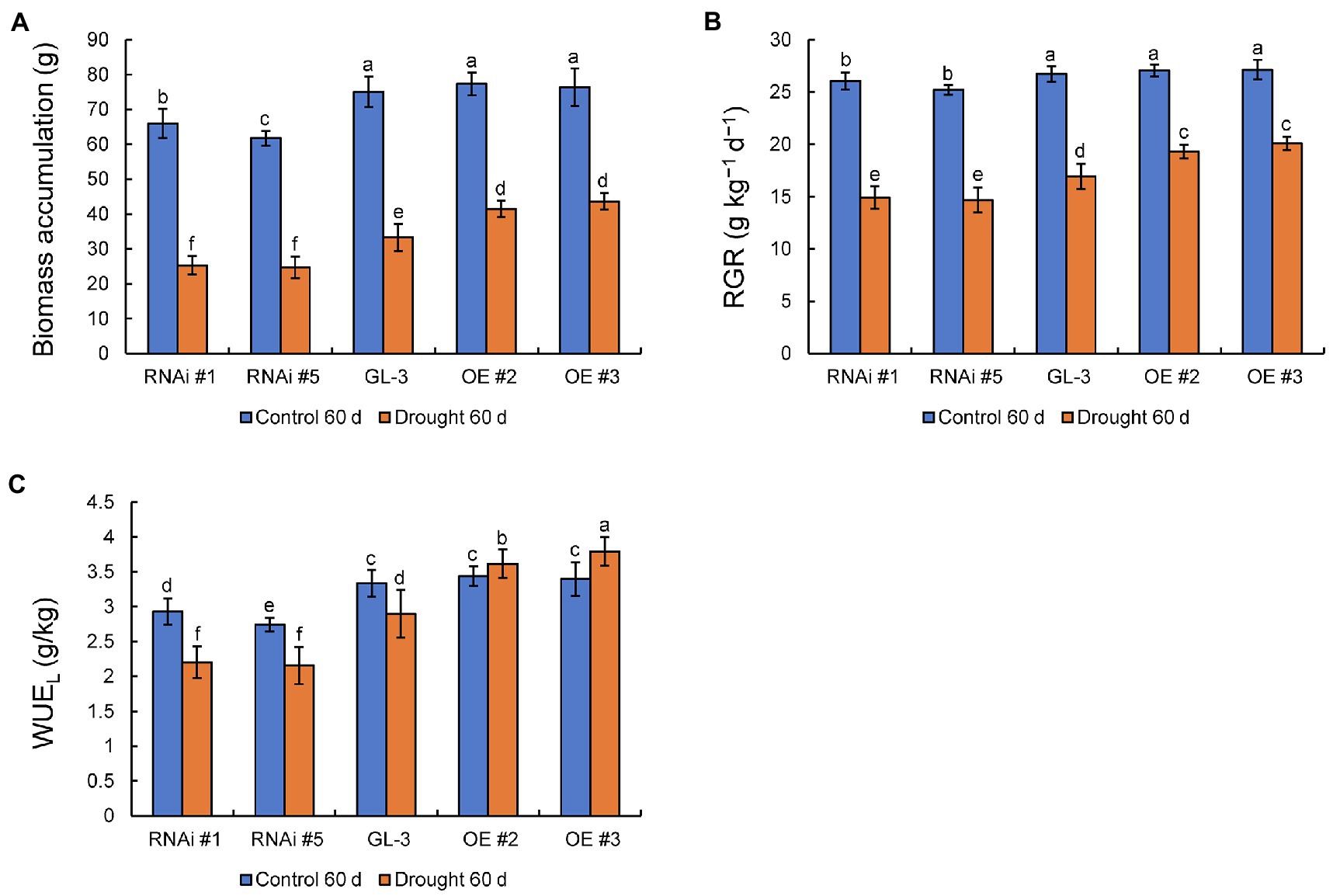
Figure 2. Biomass accumulation, RGR, and long-term WUE (WUEL) of GL-3 and MdHB-7 transgenic plants after 0 and 60days under moderate soil water deficit conditions. (A) Biomass (plant dry weight). (B) Relative growth rate (RGR). (C) WUEL. Data are means±SD (n=18; 18 plants from each line). Different letters indicate significant differences between GL-3 and transgenic apple plants based on one-way ANOVA and Tukey’s multiple range test (p<0.05).
MdHB-7 Affects Stomatal Density
Stomatal density plays an important role in the regulation of WUE, and members of the HD-Zip family are known to regulate plant stomatal density (Mishra et al., 2012). We therefore examined the stomatal density of GL-3 and MdHB-7 transgenic plants. Under normal conditions, stomatal density was lower in MdHB-7 OE lines and higher in MdHB-7 RNAi lines than in GL-3 plants (Figures 3A,B). Long-term moderate soil water deficit increased the stomatal density of all genotypes. Nonetheless, the stomatal density of OE lines was still lower than that of GL-3 plants, and the stomatal density of RNAi lines was higher. Stomatal density affects leaf water loss, which in turn affects plant adaptability to water deficit and WUE (Jiang et al., 2019). We found that relative leaf water loss was lower in MdHB-7 OE lines than in GL-3, whereas MdHB-7 RNAi lines had the highest relative water loss (Figure 3C). Changes in stomatal density also influence leaf relative water content (Yoo et al., 2010), thereby affecting the adaptability of plants to water deficit. After 60days of long-term moderate soil water deficit, the relative water content was higher in MdHB-7 OE lines than in GL-3, whereas the relative water content of MdHB-7 RNAi lines was lower (Figure 3D). These results suggest that MdHB-7 inhibits water loss under soil water deficit conditions by reducing stomatal density, thereby improving WUEL.
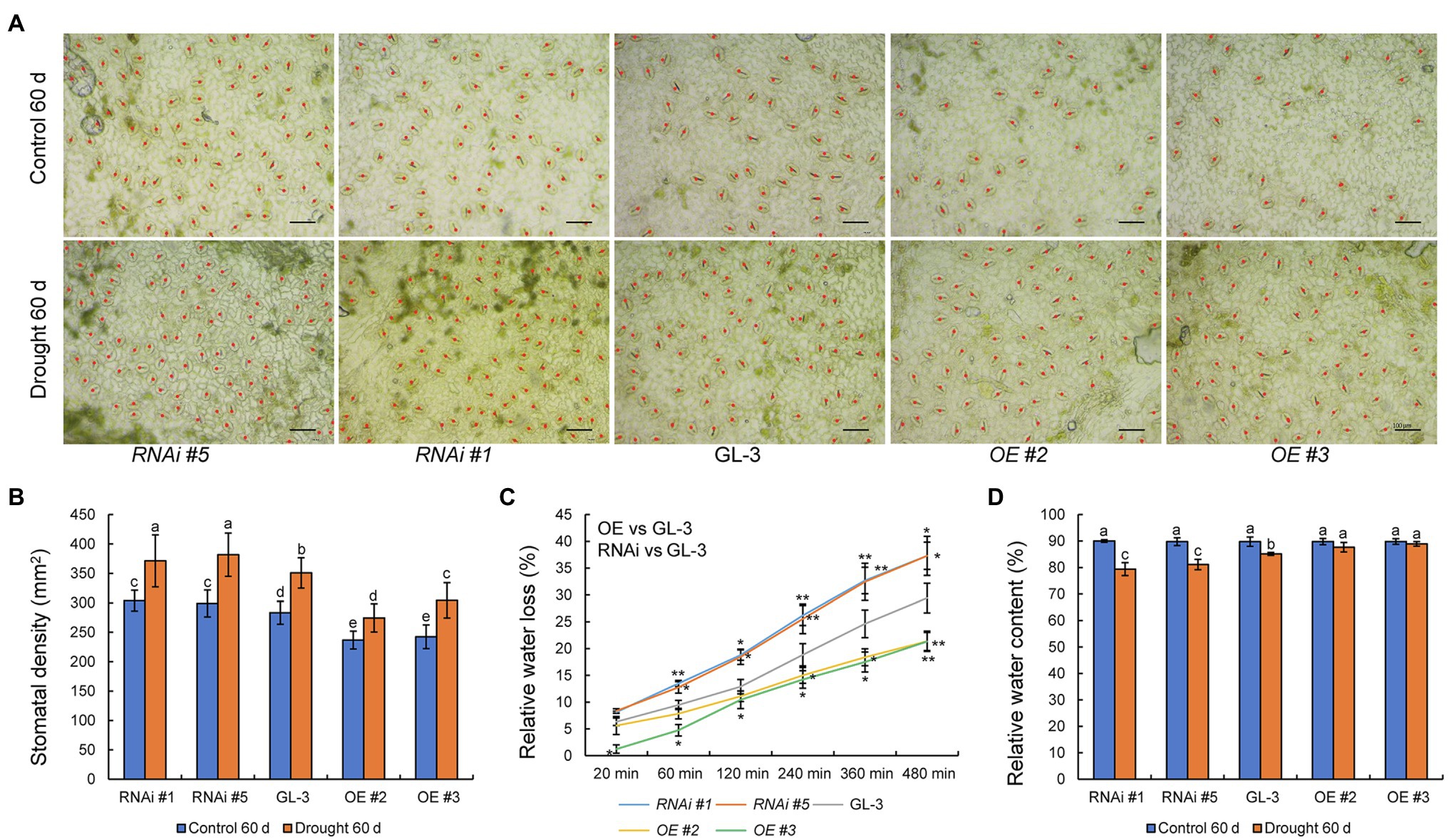
Figure 3. Effect of MdHB-7 expression on stomatal density, leaf water loss, and relative water content. (A) Micrographs of abaxial leaf epidermis from GL-3 and MdHB-7 transgenic plants. Scale bars, 50μm. (B) Stomatal density of the leaf abaxial epidermis of GL-3 and MdHB-7 transgenic plants. (C) Relative water loss from detached leaves measured at the indicated time points. (D) Relative water content. Data are means±SD (n≥50 for B, at least 50 stomatal densities were measured per genotype; n=5 for C,D). Different letters indicate significant differences among genotypes based on one-way ANOVA and Tukey’s multiple range test (p<0.05).
The epidermal patterning factors (EPF) encode a secreted peptide family (EPF1 and EPF2) that play a vital role in stomatal development process (Wang et al., 2016). The ectopic expression of MdEPF2 in tomato reduced the stomatal density of transgenic plants (Jiang et al., 2019). As shown in Figure 4, the expression levels of MdEPF1 and MdEPF2 were higher in MdHB-7 OE lines than in GL-3, and their expression was lower in MdHB-7 RNAi lines. These results suggested that the effect of MdHB-7 on stomatal density may depend on its direct or indirect regulation of MdEPF1 and MdEPF2.
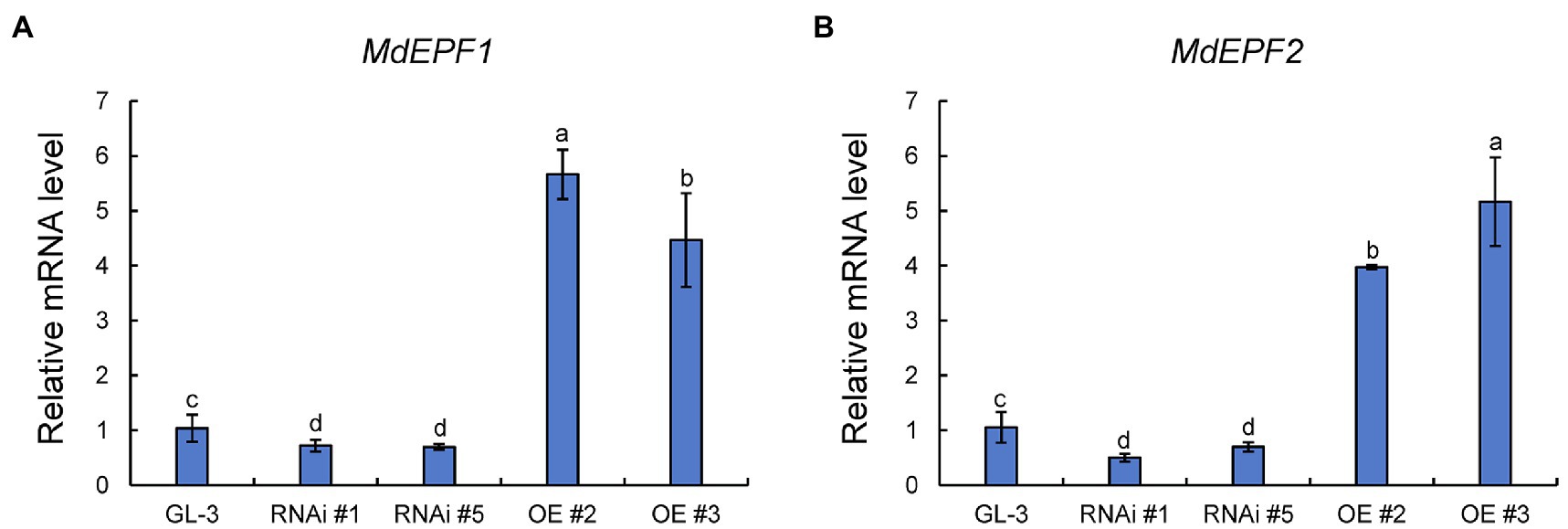
Figure 4. Relative expression of MdEPF1 (A) and MdEPF2 (B) in the leaves of GL-3 and MdHB-7 transgenic plants. Data are presented as means±SD (n=3). Different letters indicate significant differences based on one-way ANOVA and Tukey’s multiple range tests (p<0.05).
MdHB-7 Affects Photosynthetic Rate and WUEi Under Long-Term Moderate Soil Water Deficit
Water deficits affect photosynthetic efficiency, and we found that the Pn of all genotypes decreased under long-term moderate soil water deficit. The decline in Pn under moderate soil water deficit was lowest in the MdHB-7 OE lines and greatest in the RNAi lines. There were no significant differences in Pn between GL-3 and MdHB-7 transgenic plants under well-watered conditions (Figure 5A). The efficiency of photosynthesis is closely related to chlorophyll accumulation, and we therefore measured the chlorophyll content of all genotypes. Chlorophyll content was reduced in all lines under long-term moderate soil water deficit, but the chlorophyll content of MdHB-7 OE lines decreased less than that of GL-3 and RNAi plants (Figure 5B).
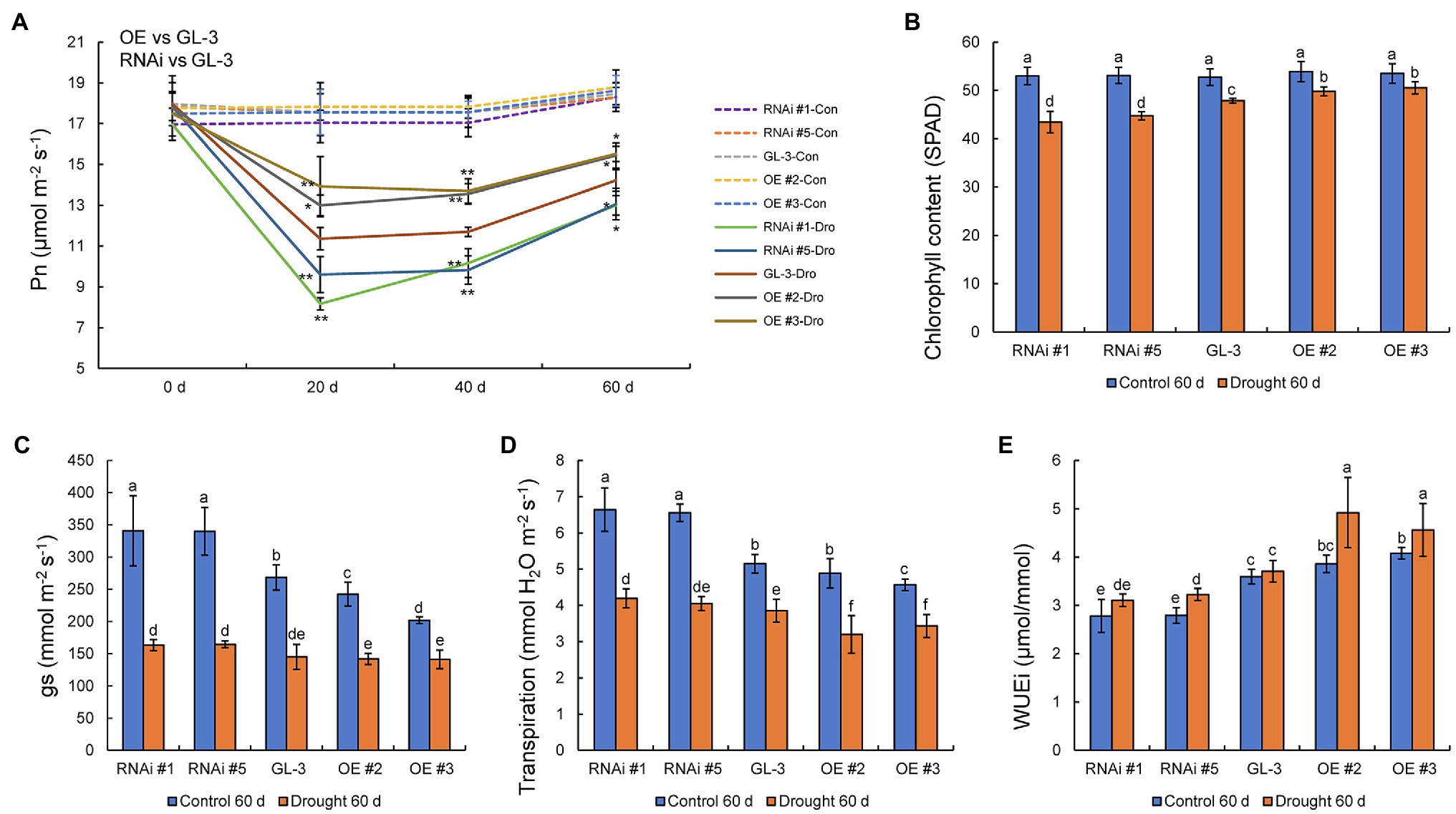
Figure 5. Physiological indices of GL-3 and MdHB-7 transgenic apple plants under well-watered and soil water deficit conditions. (A) Photosynthetic rate (Pn). (B) Chlorophyll content. (C) Stomatal conductance (gs). (D) Transpiration. (E) WUEi. Data are means±SD (n=5 for A–E). In comparison with GL-3, *p<0.05 and **p<0.01. Different letters indicate significant differences between lines based on one-way ANOVA and Tukey’s multiple range test (p<0.05).
Stomatal density affects plant transpiration rate and leaf gas exchange, which are crucial determinants of photosynthesis. Under normal conditions, values of gs were significantly lower in MdHB-7 OE lines than in GL-3 and RNAi lines. Long-term moderate soil water deficit reduced gs values in GL-3 and transgenic plants. Under long-term moderate soil water deficit, gs values were slightly lower in MdHB-7 OE lines than in GL-3, and gs values were higher in RNAi lines than in GL-3, but there were no significant differences (Figure 5C). Under well-watered conditions, the transpiration rate was significantly lower in OE #3 and slightly lower in OE #2 compared with GL-3. By contrast, the transpiration rate of the RNAi lines was significantly higher than that of GL-3 (Figure 5D). Under long-term moderate soil water deficit, the transpiration rate was significantly lower in the two OE lines than in GL-3. The transpiration rate of RNAi #1 was significantly higher than that of GL-3; that of RNAi #5 was also higher, but this difference was not significant (Figure 5D). Under well-watered and long-term moderate soil water deficit, WUEi was higher in MdHB-7 OE lines and lower in RNAi lines compared with GL-3 (Figure 5E). Interestingly, the WUEi of OE plants under long-term moderate soil water deficit was significantly higher than that of OE plants under normal conditions (Figure 5E). These results suggest that the overexpression of MdHB-7 enhanced the photosynthetic ability and WUEi of plants under long-term moderate soil water deficit.
MdHB-7 Affects Root Activity and Hydraulic Conductivity Under Long-Term Moderate Soil Water Deficit
In addition to aboveground plant parts, the growth of plant roots and their absorption and transport of water also influence WUE under moderate soil water deficit conditions. We previously demonstrated that MdHB-7 was highly expressed in apple roots (Zhao et al., 2020a). Here, we examined the roots of GL-3 and MdHB-7 transgenic plants under normal and long-term moderate soil water deficit conditions. Under normal conditions, there were no differences in root growth and dry weight between GL-3 and MdHB-7 OE lines. By contrast, root growth and dry weight were significantly lower in MdHB-7 RNAi lines than in GL-3 (Figures 6A,B). Root growth of all genotypes was inhibited by long-term moderate soil water deficit. Nonetheless, the root dry weights of MdHB-7 OE and RNAi lines were significantly higher and lower, respectively, than those of GL-3 (Figures 6A,B). Likewise, root activity was higher in MdHB-7 OE lines and lower in MdHB-7 RNAi lines under moderate soil water deficit (Figure 6C). Root hydraulic conductivity decreased significantly in all genotypes under long-term moderate soil water deficit, but it was higher in MdHB-7 OE lines and lower in RNAi lines compared with GL-3 (Figure 6D). These results indicated that MdHB-7 overexpression promotes the root growth and helps to maintain greater water transport capacity in transgenic apple plants under long-term moderate soil water deficit.
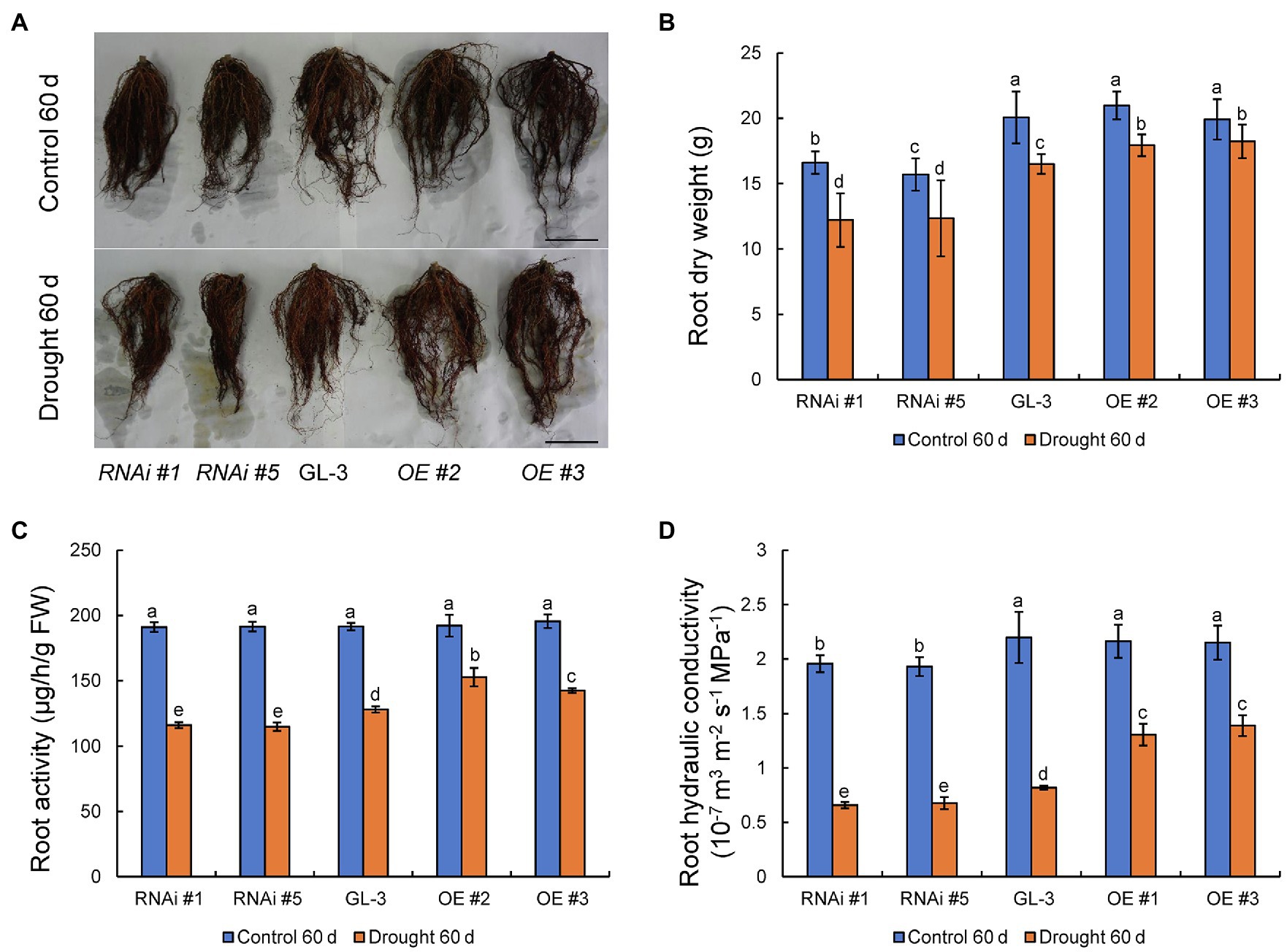
Figure 6. Root growth and physiology of GL-3 and MdHB-7 transgenic apple plants under long-term moderate soil water deficit. (A) Root morphology. Scale bars, 5cm. (B) Root dry weight. (C) Root activity measured by the TTC method. (D) Root hydraulic conductivity. Data are expressed as means±SD (n=18 for B, 18 plants from each line; n=3 for C, three biological replicates; n=6 for D, six plants from each line). Within a sampling date, different letters indicate significant differences based on one-way ANOVA and Tukey’s multiple range test (p<0.05).
Discussion
Stomata are two-celled valves that control epidermal pores and play a central role in leaf transpiration and CO2 absorption (Le et al., 2014; Lee et al., 2017). Here, we showed that the overexpression of MdHB-7 reduced stomatal density (Figures 3A,B) and decreased transpiration rate and water loss rate (Figures 3C, 5D). The decrease in stomatal density decreases leaf water loss and increases leaf relative water content (Yoo et al., 2010; Jiang et al., 2019). Interestingly, the difference between the relative water content of leaves of each genotype after long-term moderate drought treatment was not as large as the difference in relative water loss of isolated leaves of each genotype. It might be that the root system of the plant was able to continuously absorb water to replenish the leaves during the long-term moderate drought treatment, while the isolated leaves were not supplied with water. Moreover, the reduced stomatal density caused by MdHB-7 overexpression did not affect CO2 assimilation and biomass accumulation and increased WUE. Previous studies have shown that the net CO2 assimilation rate reached saturation with increasing stomatal conductance, while the increase in transpiration rate remained linear (Yoo et al., 2009). Therefore, a moderate reduction in stomatal density can significantly reduce the transpiration rate and leaf water loss without simultaneously affecting CO2 assimilation and improving WUE (Yoo et al., 2010; Guo et al., 2019b; Li et al., 2020). Previous studies have shown that long-term moderate drought treatment increased stomatal density in newly developed leaves of apple plants (Liang et al., 2018a,b; Jia et al., 2021). The leaf area of the plant decreased after long-term moderate drought treatment, which might be responsible for the increase in stomatal density (Jiang et al., 2019). Besides, altering the stomatal density of plants is a strategy for plants to adapt to long-term moderate water deficit (Li et al., 2020; Jia et al., 2021).
Stomatal density is influenced by stomatal development (Xiang et al., 2021). Peptides play an important role in stomatal development. EPF1 and EPF2 belong to the EPF family of secretory peptides and participate in multiple aspects of stomatal development (Wang et al., 2016). Overexpression of PdEPF1 has been shown to reduce stomatal density on the back of the leaf, thereby reducing transpiration, maintaining leaf relative water content, and enhancing WUEL (Wang et al., 2016). The leaves of AtEPF2 OE Arabidopsis plants showed significantly lower stomatal density and greater WUEi and WUEL, whereas epf1epf2 mutants exhibited higher stomatal density and lower WUEi and WUEL (Franks et al., 2015). Here, the expression of MdEPF1 and MdEPF2 was higher in MdHB-7 OE lines and lower in MdHB-7 RNAi lines (Figure 4). This result, combined with the fact that overexpression of MdEPF2 leads to reduced stomatal density in transgenic plants and improved WUEL under long-term water deficit (Jiang et al., 2019), suggests that MdHB-7 may affect stomatal density by influencing the expression of EPF family genes, such as MdEPF1 and MdEPF2, and thus WUEL. Further studies are needed to verify this regulatory mechanism, including studies on the direct or indirect regulatory effect of MdHB-7 on the expression of EPF family genes.
The maintenance of a high photosynthetic rate is important for improving WUE (Karaba et al., 2007; Condon, 2020), especially under stress conditions. The regulation of photosynthesis contributed significantly to higher WUEL in apple plants under long-term moderate water deficit (Zhou et al., 2015). Here, the overexpression of MdHB-7 improved WUEL partly by maintaining a high photosynthetic rate under long-term moderate soil water deficit (Figure 5A). Photosynthesis is affected by multiple mechanisms, including stomatal restriction, and water deficit leads to a decrease in stomatal conductance (Warren et al., 2011). Another mechanism is the limitation associated with non-stomatal factors, such as decreases in leaf chlorophyll content (Pagter et al., 2005). Under long-term moderate soil water deficit, there was no significant difference in gs between MdHB-7 transgenic lines and GL-3 plants (Figure 5C). Therefore, we speculated that Pn may have been limited primarily by non-stomatal factors such as ribulose 1,5-bisphosphate regeneration and chlorophyll content. Chlorophyll is the main photosynthetic pigment and has an important role in light absorption, transmission, and transformation (Zuo et al., 2014). If chlorophyll levels are reduced, the absorption of light energy by the chloroplast will also be reduced (Mafakheri et al., 2010). Water deficit induces the production of reactive oxygen species in leaves and promotes the degradation of chlorophyll (Liang et al., 2018b). The chlorophyll content of all genotypes decreased under long-term moderate soil water deficit, but that of MdHB-7 OE lines decreased least (Figure 5B), allowing them to maintain a higher Pn (Figure 5A). These results indicated that the overexpression of MdHB-7 could help to maintain high chlorophyll content in apple leaves under long-term moderate soil water deficit, thereby enabling MdHB-7 OE lines to maintain a higher Pn, which in turn promoted biomass accumulation and improved WUEL.
Roots play a key role in water and nutrient absorption and in sensing dehydration stress signals and transferring them to shoots. Therefore, roots are the key to solving problems caused by water shortage (Wang et al., 2020). Root vitality refers to the absorption, synthesis, oxidation, and reduction capabilities of the root system; it can be used as a physiological indicator to objectively quantify root system activity (Lin and Fan, 2013). The ability of the root system to transport water from the surrounding soil can be evaluated by the root hydraulic conductivity, and higher root hydraulic conductivity usually indicates a greater potential for water transfer from soil to roots (Geng et al., 2018; Jia et al., 2020). Overexpression of MdMYB88 or MdMYB124 positively regulated root architecture and increased root hydraulic conductivity of transgenic apple plants relative to GL-3 under long-term moderate soil water deficit, promoting greater adaptation of transgenic plants to moderate water deficit (Geng et al., 2018). Our results indicated that the overexpression of MdHB-7 alleviated the inhibition of root growth caused by long-term moderate soil water deficit. Compared with GL-3, MdHB-7 OE plants had greater root vitality and hydraulic conductivity under long-term moderate soil water deficit (Figure 6), and this may explain why MdHB-7 overexpression improved WUEL.
In conclusion, overexpression of MdHB-7 improved WUEL under long-term moderate soil water deficit by reducing stomatal density and water loss and promoting high photosynthetic rates. Overexpression of MdHB-7 also minimized the root growth inhibition caused by long-term moderate soil water deficit and increased root vitality and hydraulic conductivity. Our findings provide new evidence for a role of HD-Zip TFs in improving the WUEL of apple plants under long-term moderate soil water deficit.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Author Contributions
FM, KM, and SZ conceived and designed the study. SZ, HG, XJ, and JW performed the analyses. SZ drafted the manuscript. FM and KM supervised the process of this research and provided financial support for the study. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Key Research and Development Program of China (2018YFD1000303), the National Natural Science Foundation of China (31972391), and the earmarked fund for the China Agricultural Research System (CARS-27).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors are grateful to Zhihong Zhang from Shenyang Agricultural University for providing tissue-cultured GL-3 plants.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2021.740492/full#supplementary-material
References
Ali, M. H., and Talukder, M. S. U. (2008). Increasing water productivity in crop production: a synthesis. Agric. Water Manag. 95, 1201–1213. doi: 10.1016/j.agwat.2008.06.008
Ariel, F. D., Manavella, P. A., Dezar, C. A., and Chan, R. L. (2007). The true story of the HD-zip family. Trends Plant Sci. 12, 419–426. doi: 10.1016/j.tplants.2007.08.003
Cabello, J. V., and Chan, R. L. (2012). The homologous homeodomain-leucine zipper transcription factors HaHB1 and AtHB13 confer tolerance to drought and salinity stresses via the induction of proteins that stabilize membranes. Plant Biotechnol. J. 10, 815–825. doi: 10.1111/j.1467-7652.2012.00701.x
Caine, R. S., Yin, X., Sloan, J., Harrison, E. L., Mohammed, U., Fulton, T., et al. (2019). Rice with reduced stomatal density conserves water and has improved drought tolerance under future climate conditions. New Phytol. 221, 371–384. doi: 10.1111/nph.15344
Condon, A. G. (2020). Drying times: plant traits to improve crop water use efficiency and yield. J. Exp. Bot. 7, 2239–2252. doi: 10.1093/jxb/eraa002
Condon, A. G., Richards, R. A., Rebetzke, G. J., and Farquhar, G. D. (2004). Breeding for high water use efficiency. J. Exp. Bot. 55, 2447–2460. doi: 10.1093/jxb/erh277
Coudert, Y., Périn, C., Courtois, B., Khong, N. G., and Gantet, P. (2010). Genetic control of root development in rice, the model cereal. Trends Plant Sci. 15, 219–226. doi: 10.1016/j.tplants.2010.01.008
Dai, H., Li, W., Han, G., Yang, Y., Ma, Y., Li, H., et al. (2013). Development of a seedling clone with high regeneration capacity and susceptibility to agrobacterium in apple. Sci. Hortic. 164, 202–208. doi: 10.1016/j.scienta.2013.09.033
Ehdaie, B., and Waines, J. G. (1993). Variation in water-use efficiency and its components in wheat: I. well-watered pot experiment. Crop Sci. 33, 294–299. doi: 10.2135/cropsci1993.0011183X003300020016x
Falkenmark, M. (2013). Growing water scarcity in agriculture: future challenge to global alter security. Philos. Trans. R. Soc. A 371:20120410. doi: 10.1098/rsta.2012.0410
Franks, P. J., Doheny-Adams, T. W., Britton-Harper, Z. J., and Gray, J. E. (2015). Increasing water-use efficiency directly through genetic manipulation of stomatal density. New Phytol. 207, 188–195. doi: 10.1111/nph.13347
Geng, D., Chen, P., Shen, X., Zhang, Y., Li, X., Jiang, L., et al. (2018). MdMYB88 and MdMYB124 enhance drought tolerance by modulating root vessels and cell walls in apple. Plant Physiol. 178, 1296–1309. doi: 10.1104/pp.18.00502
Gong, S., Ding, Y., Hu, S., Ding, L., Chen, Z., and Zhu, C. (2019). The role of HD-zip class I transcription factors in plant response to abiotic stresses. Physiol. Plant. 167, 516–525. doi: 10.1111/ppl.12965
Guo, T., Wang, N., Xue, Y., Guan, Q., Nocker, S., Liu, C., et al. (2019a). Overexpression of the RNA binding protein MhYTP1 in transgenic apple enhances drought tolerance and WUE by improving ABA level under drought condition. Plant Sci. 280, 397–407. doi: 10.1016/j.plantsci.2018.11.018
Guo, X., Wang, Y., Zhao, P., Xu, P., Yu, G., Zhang, L., et al. (2019b). AtEDT1/HDG11 regulates stomatal density and water use efficiency via ERECTA and E2Fa. New Phytol. 223, 1478–1488. doi: 10.1111/nph.15861
Hu, L., Zhou, K., Li, Y., Chen, X., Liu, B., Li, C., et al. (2018). Exogenous myo-inositol alleviates salinity-induced stress in Malus hupehensis Rehd. Plant Physiol. Biochem. 133, 116–126. doi: 10.1016/j.plaphy.2018.10.037
Hughes, J., Hepworth, C., Dutton, C., Dunn, J. A., Hunt, L., Stephens, J., et al. (2017). Reducing stomatal density in barley improves drought tolerance without impacting on yield. Plant Physiol. 174, 776–787. doi: 10.1104/pp.16.01844
Huo, L., Guo, Z., Jia, X., Sun, X., Wang, P., Gong, X., et al. (2020). Increased autophagic activity in roots caused by overexpression of the autophagy-related gene MdATG10 in apple enhances salt tolerance. Plant Sci. 294:110444. doi: 10.1016/j.plantsci.2020.110444
Jia, J., Liang, Y., Gou, T., Hu, Y., Zhu, Y., Huo, H., et al. (2020). The expression response of plasma membrane aquaporins to salt stress in tomato plants. Environ. Exp. Bot. 17:104190. doi: 10.1016/j.envexpbot.2020.104190
Jia, X., Mao, K., Wang, P., Wang, Y., Jia, X., Huo, L., et al. (2021). Overexpression of MdATG8i improves water use efficiency in transgenic apple by modulating photosynthesis, osmotic balance, and autophagic activity under moderate water deficit. Hortic. Res. 8:81. doi: 10.1038/s41438-021-00521-2
Jiang, Q., Yang, J., Wang, Q., Zhou, K., Mao, K., and Ma, F. (2019). Overexpression of MdEPF2 improves water use efficiency and reduces oxidative stress in tomato. Environ. Exp. Bot. 162, 321–332. doi: 10.1016/j.envexpbot.2019.03.009
Karaba, A., Dixit, S., Greco, R., Aharon, A., Trijatmiko, K. S., Marsch-Martinez, N., et al. (2007). Improvement of water use efficiency in rice by expression of HARDY, an Arabidopsis drought and salt tolerance gene. PNAS 104, 15270–15275. doi: 10.1073/pnas.0707294104
Le, J., Zou, J., Yang, K., and Wang, M. (2014). Signaling to stomatal initiation and cell division. Front. Plant Sci. 5:297. doi: 10.3389/fpls.2014.00297
Lee, J. H., Jung, J. H., and Park, C. M. (2017). Light inhibits COP1-mediated degradation of ICE transcription factors to induce stomatal development in Arabidopsis. Plant Cell 29, 2817–2830. doi: 10.1105/tpc.17.00371
Li, S., Zhang, J., Liu, L., Wang, Z., Li, Y., Guo, L., et al. (2020). SlTLFP8 reduces water loss to improve water-use efficiency by modulating cell size and stomatal density via endoreduplication. Plant Cell Environ. 43, 2666–2679. doi: 10.1111/pce.13867
Liang, B., Gao, T., Zhao, Q., Ma, C., Chen, Q., Wei, Z., et al. (2018a). Effects of exogenous dopamine on the uptake, transport, and resorption of apple ionome under moderate drought. Front. Plant Sci. 9:755. doi: 10.3389/fpls.2018.00755
Liang, B., Ma, C., Zhang, Z., Wei, Z., Gao, T., Zhao, Q., et al. (2018b). Long-term exogenous application of melatonin improves nutrient uptake fluxes in apple plants under moderate drought stress. Environ. Exp. Bot. 155, 650–661. doi: 10.1016/j.envexpbot.2018.08.016
Lin, Y., and Fan, H. (2013). Effects of salt stress on root activity of Limonium bicolor (Bunge) Kuntze. Agric. Sci. Technol. 14, 832–834. doi: 10.16175/j.cnki.1009-4229.2013.06.020
Liu, C., Guo, T., Wang, N., Wang, Q., Xue, Y., Zhan, M., et al. (2019). Overexpression of MhYTP2 enhances apple water-use efficiency by activating ABA and ethylene signaling. Environ. Exp. Bot. 157, 260–268. doi: 10.1016/j.envexpbot.2018.10.018
Mafakheri, A., Siosemardeh, A., Bahramnejad, B., Struik, P. C., and Sohrabi, Y. (2010). Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Aust. J. Crop. Sci. 4, 580–585. doi: 10.1007/s12230-010-9149-0
Pagter, M., Bragato, C., and Brix, H. (2005). Tolerance and physiological responses of Phragmites australis to water deficit. Aquat. Bot. 81, 285–299. doi: 10.1016/j.aquabot.2005.01.002
Radford, J. P. (1967). Growth analysis formulae—their use and abuse1. Crop Sci. 7, 171–175. doi: 10.2135/cropsci1967.0011183X000700030001x
Ré, D. A., Capella, M., Bonaventure, G., and Chan, R. L. (2014). Arabidopsis AtHB7 and AtHB12 evolved divergently to fine tune processes associated with growth and responses to water stress. BMC Plant Biol. 14:150. doi: 10.1186/1471-2229-14-150
Ribichich, K. F., Chiozza, M., Ávalos-Britez, S., Cabello, J. V., Arce, A. L., Watson, G., et al. (2020). Successful field performance in warm and dry environments of soybean expressing the sunflower transcription factor HaHB4. J. Exp. Bot. 71, 3142–3156. doi: 10.1093/jxb/eraa064
Schena, M., and Davis, R. W. (1992). HD-zip proteins: members of an Arabidopsis homeodomain protein superfamily. Proc. Natl. Acad. Sci. 89, 3894–3898. doi: 10.2307/2359772
Shao, H. B., Chu, L. Y., Jaleel, C. A., and Zhao, C. X. (2008). Water-deficit stress-induced anatomical changes in higher plants. C. R. Biol. 331, 215–225. doi: 10.1016/j.crvi.2008.01.002
Sun, X., Wang, P., Jia, X., Huo, L., Che, R., and Ma, F. (2018). Improvement of drought tolerance by overexpressing MdATG18a is mediated by modified antioxidant system and activated autophagy in transgenic apple. Plant Biotechnol. J. 16, 545–557. doi: 10.1111/pbi.12794
Wang, L., Li, Z., Wen, S., Wang, J., Zhao, S., and Lu, M. (2020). WUSCHEL-related homeobox gene PagWOX11/12a responds to drought stress by enhancing root elongation and biomass growth in poplar. J. Exp. Bot. 71, 1503–1513. doi: 10.1093/jxb/erz490
Wang, C., Liu, S., Dong, Y., Zhao, Y., Geng, A., Xia, X., et al. (2016). PdEPF1 regulates water-use efficiency and drought tolerance by modulating stomatal density in poplar. Plant Biotechnol. J. 14, 849–860. doi: 10.1111/pbi.12434
Wang, H., Zhao, S., Mao, K., Dong, Q., Liang, B., Li, C., et al. (2018). Mapping QTLs for water-use efficiency reveals the potential candidate genes involved in regulating the trait in apple under drought stress. BMC Plant Biol. 18:136. doi: 10.1186/s12870-018-1308-3
Warren, C. R., Aranda, I., and Cano, F. J. (2011). Responses to water stress of gas exchange and metabolites in Eucalyptus and Acacia spp. Plant Cell Environ. 34, 1609–1629. doi: 10.1111/j.1365-3040.2011.02357.x
Wu, J., Zhou, W., Gong, X., and Cheng, B. (2016). Expression of ZmHDZ4, a maize homeodomain-leucine zipper I gene, confers tolerance to drought stress in transgenic rice. Plant Mol. Biol. Report. 34, 845–853. doi: 10.1007/s11105-015-0970-y
Xiang, Y., Sun, X., Bian, X., Wei, T., Han, T., Yan, J., et al. (2021). The transcription factor ZmNAC49 reduces stomatal density and improves drought tolerance in maize. J. Exp. Bot. 72, 1399–1410. doi: 10.1093/jxb/eraa507
Yan, W., Deng, L., Zhong, Y., and Shangguan, Z. (2015). The characters of dry soil layer on the loess plateau in China and their influencing factors. PLoS One 10:e0134902. doi: 10.1371/journal.pone.0134902
Yang, Y., Luang, S., Harris, J., Riboni, M., Li, Y., Bazanova, N., et al. (2018). Overexpression of the class I homeodomain transcription factor TaHDZipI-5 increases drought and frost tolerance in transgenic wheat. Plant Biotechnol. J. 16, 1227–1240. doi: 10.1111/pbi.12865
Yoo, C. Y., Pence, H. E., Hasegawa, P. M., and Mickelbart, M. V. (2009). Regulation of transpiration to improve crop water use. Crit. Rev. Plant Sci. 28, 410–431. doi: 10.1080/07352680903173175
Yoo, C. Y., Pence, H. E., Jin, J. B., Miura, K., Gosney, M. J., Hasegawa, P. M., et al. (2010). The Arabidopsis GTL1 transcription factor regulates water use efficiency and drought tolerance by modulating stomatal density via transrepression of SDD1. Plant Cell 22, 4128–4141. doi: 10.1105/tpc.110.078691
Zhao, S., Gao, H., Jia, X., Wang, H., Mao, K., and Ma, F. (2020a). The HD-zip I transcription factor MdHB-7 regulates drought tolerance in transgenic apple (Malus domestica). Environ. Exp. Bot. 180:104246. doi: 10.1016/j.envexpbot.2020.104246
Zhao, S., Gao, H., Luo, J., Wang, H., Dong, Q., Wang, P., et al. (2020b). Genome-wide analysis of the light-harvesting chlorophyll a/b-binding gene family in apple (Malus domestica) and functional characterization of MdLhcb4.3, which confers tolerance to drought and osmotic stress. Plant Physiol. Biochem. 154, 517–529. doi: 10.1016/j.plaphy.2020.06.022
Zhao, Y., Ma, Q., Jin, X., Peng, X., Liu, J., Deng, L., et al. (2014). A novel maize homeodomain-leucine zipper (HD-zip) I gene, ZmHDZ10, positively regulates drought and salt tolerance in both rice and Arabidopsis. Plant Cell Physiol. 55, 1142–1156. doi: 10.1093/pcp/pcu054
Zhou, K., Hu, L., Li, Y., Chen, X., Zhang, Z., Liu, B., et al. (2019). MdUGT88F1-mediated phloridzin biosynthesis regulates apple development and Valsa canker resistance. Plant Physiol. 180, 2290–2305. doi: 10.1104/pp.19.00494
Zhou, S., Li, M., Guan, Q., Liu, F., Zhang, S., Chen, W., et al. (2015). Physiological and proteome analysis suggest critical roles for the photosynthetic system for high water-use efficiency under drought stress in Malus. Plant Sci. 236, 44–60. doi: 10.1016/j.plantsci.2015.03.017
Zhu, Y., Xu, X., Hu, Y., Han, W., Yin, J., Li, H., et al. (2015). Silicon improves salt tolerance by increasing root water uptake in Cucumis sativus L. Plant Cell Rep. 34, 1629–1646. doi: 10.1007/s00299-015-1814-9
Keywords:MdHB-7, water use efficiency, stomatal density, root, long-term moderate soil water deficit, Malus domestica
Citation: Zhao S, Gao H, Jia X, Wei J, Mao K and Ma F (2021) MdHB-7 Regulates Water Use Efficiency in Transgenic Apple (Malus domestica) Under Long-Term Moderate Water Deficit. Front. Plant Sci. 12:740492. doi: 10.3389/fpls.2021.740492
Edited by:
Sachin Teotia, Sharda University, IndiaReviewed by:
Da-Gang Hu, Shandong Agricultural University, ChinaGuang Chen, Zhejiang Academy of Agricultural Sciences, China
Copyright © 2021 Zhao, Gao, Jia, Wei, Mao and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ke Mao, bWFva2UyMDAyQDE2My5jb20=; Fengwang Ma, ZndtNjRAc2luYS5jb20=
 Shuang Zhao
Shuang Zhao Fengwang Ma
Fengwang Ma