
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci. , 14 October 2021
Sec. Crop and Product Physiology
Volume 12 - 2021 | https://doi.org/10.3389/fpls.2021.738247
 Rahul Dev Gautam1,2*
Rahul Dev Gautam1,2* Ajay Kumar1,2
Ajay Kumar1,2 Ravi Kumar1
Ravi Kumar1 Ramesh Chauhan1
Ramesh Chauhan1 Satbeer Singh1
Satbeer Singh1 Manish Kumar3
Manish Kumar3 Dinesh Kumar3
Dinesh Kumar3 Ashok Kumar1
Ashok Kumar1 Sanatsujat Singh1,2*
Sanatsujat Singh1,2*Valeriana jatamansi Jones (Syn. V. wallichii DC.) is an aromatic, medicinal herb used as a tranquilizer and in treating sleep disorders. Rhizome is mainly used to extract essential oil (EO) and valepotriates. High quality and economic yield of rhizomes are available in the third year of growth. Therefore, the cultivation of V. jatamansi is not picking up, and over-exploitation of this plant from wild habitats to meet the increasing demand of the pharmaceutical industry is the cause of threat to the genetic diversity of the species. Further, collections from the wild are heterogeneous, resulting in variable produce. The development of clonal lines can ensure uniform quality and yield of rhizome biomass. An effective clonal propagation method was standardized using different hormonal concentrations of naphthalene acetic acid (NAA) on apical shoot cuttings from the selected clone CSIR-IHBT-VJ-05 for different time durations and raised over various planting media. NAA treatment of 50 ppm concentration for 30 min was found optimum for root induction in apical shoots of V. jatamansi. Variations for EO composition within the clone were non-significant, while samples of the control population were variable. The best quality EO (patchouli alcohol ∼62%) was available during the third year of plant growth. A propagation technique for large-scale quality plant material (QPM) production has been standardized to reduce the stress over natural resources and promote V. jatamansi for use in the aromatic and pharmaceutical industry.
Valeriana jatamansi Jones, commonly known as “Indian Valerian” (English name) and “Mushkbala” (Hindi name), is a perennial aromatic medicinal herb belonging to the family Valerianaceae. It is a perennial and shade-loving plant found at elevations ranging from 1200 to 4000 m amsl in the temperate Himalayas from Kashmir to Bhutan and between 1200 and 2000 m amsl in Khasi and Jaintia hills in North East India (Anonymous, 1976). This species generally grows on shady slopes, moist places, and near streams (Jugran et al., 2013). Important constituents from rhizomes and roots of V. jatamansi are sesquiterpenoids (Willis et al., 2000) bakkenoloids type sesquiterpenoids (Xu et al., 2011), EO and phenolics (Bhatt et al., 2012). Iridoids from V. jatamansi possess anti-inflammatory and antiproliferative activity (Liu et al., 2021). EO of such herbs are the traditional natural resources which exhibit antibacterial and antioxidant properties (Mahmoudi and Nosratpour, 2013; Mahmoudi et al., 2013a,b; Jafari et al., 2014; Mohammadzadeh et al., 2018). Major interest in Valeriana species is focused on two groups; valepotriates and EO sesquiterpenes (Bhardwaj et al., 2021). Valepotriates chemically belong to the group of iridoids and can be extracted from the roots, rhizomes, or from whole plant (Lin et al., 2010). Extraction of iridoids is performed using powdered air dried roots with ethanol alcohol (Wang et al., 2017; Quan et al., 2019), while EO is extracted through the hydrodistillation method using Clevenger type apparatus (Thusoo et al., 2014; Jugran et al., 2020). The plant has a vital role in curing many diseases and disorders, including epilepsy, hysteria, leprosy, asthma, snakebite, and scorpion stings in Unani and Ayurvedic systems of medicine (Mukherjee, 2015). It also has important use in the preparation of Havan and Dhoop in the Indian local markets. Dua et al. (2008) reported extracts of V. jatamansi to be effective against the larvicidal and adulticidal activities of mosquitoes.
Due to its high-value EO, V. jatamansi is uprooted extensively from the wild. Increasing demand for its EO in perfumery has imposed pressure on the plant’s natural populations, leading to population loss. Many patches of its natural habitat where it was present in abundance have now disappeared (Maurya et al., 2017). Further, the quality of constituents in medicinal and aromatic plants depends on several factors, viz., age of the plants, stage of harvest, climatic variations from one location to the other, and uniformity of the population’s germplasm been raised. Conventionally, this plant is propagated through seeds and also vegetatively through the division of rhizome. Flowers of V. jatamansi are easily visible to pollinators due to their crowding effect favoring cross-pollination (Rather et al., 2014). In natural conditions, it is difficult to maintain uniformity in the progeny on account of uncontrolled pollination which leads to a high degree of outcrossing and development of heterogeneous populations, showing variations for chemical constituents and cause quality degradation. Poor germination of seeds (Mukherjee and Chakraborty, 2014) may affect crop stand and reduce overall production. Also, due to the slow rate of rhizome formation as crop is harvested after 2 years of transplantation (Singh et al., 2010), the vegetative propagation through the Rhizome becomes a time-consuming process. Therefore, it is vital to have elite germplasm on a large scale which can attain through an efficient clonal propagation method to maintain uniformity in the quality and yield of raw produce.
Various techniques have been studied for the propagation of V. jatamansi. For instance, micro-propagation in V. jatamansi took 5 months to develop a plant from culture initiation (Purohit et al., 2015). In Valeriana officinalis (a related species of V. jatamansi), cultured leaf explants were used for plant regeneration through direct shoot organogenesis (Abdi and Khui, 2007). Likewise, Chen et al. (2014) used leaves of V. jatamansi as explants for the shoot and somatic embryo induction from embryogenic callus using different concentrations of PGRs. Successful plantlet development has also been reported under in vitro conditions by apical and axial shoot buds of Valeriana wallichii (Mathur et al., 1989). Clonal propagation can be successfully achieved through optimal use of phytohormones such as naphthalene acetic acid (NAA) which are extensively used for root induction. It was used in micropropagation protocols of Zingiber spectabile (Faria and Illg, 1995), sugarcane (Tolera, 2016), and gerberas (Singh et al., 2016). Sarropoulou and Maloupa (2019) recorded maximum root numbers with 0.5 mg/l NAA while propagating Satureja thymbra. On similar lines, Firoz et al. (2015) recorded high root number, and root length in Cucumis sativus shoots growing on media containing 0.5 mg/L NAA. Also, rooting in Solanum nigrum was found better in NAA as compared to indole-acetic acid (IAA) and indole-butyric acid (IBA) (Jabeen et al., 2005). However, in vitro propagation methods may take 5–6 months to establish plantlets. They may not be economically feasible on a commercial scale at the farmers’ level as they involve high initial costs.
Naphthalene acetic acid has also been successfully used for inducing roots in cuttings of Valeriana carnosa (Nagahama et al., 2019) and other plant species such as Hemarthria compressa (Yan et al., 2014) and Andrographis paniculata (Hossain and Urbi, 2016) for achieving clonal propagation. Currently, there are no reports on the exogenous application of NAA for root induction in apical shoots of V. jatamansi. Therefore, the present study focuses on the potential of clonal propagation of V. jatamansi through the treatment of apical shoots with different concentrations and durations of NAA in various growing media (soil mixture, sand, and coco-peat) for root induction and variation among the EO composition in mother plant and clonally raised plant. Findings will help to establish a cost-effective propagation method for the multiplication of this plant species.
An elite selection ‘Him Surbhit’ (CSIR-IHBT-VJ-05), with high root production potential compared to control variety ‘Himbala’ (Anonymous, 2018), was used for clonal propagation studies suitable indigenous germplasm and is being maintained in situ as mother stock through division of rhizomes. The EO yield of CSIR-IHBT-VJ-05 is 0.31% as compared to 0.29% in control. CSIR-IHBT-VJ-05 (IC0630604, INGR20096) is registered with the Plant Germplasm Registration Committee, National Bureau of Plant Genetic Resources (NBPGR), ICAR, New Delhi. NAA [C10H7CH2CO2H, 2-(naphthalen-1-yl)acetic acid, molecular weight = 186.2066 g mol–1) was procured from Himedia (India), dissolved in ethanol, and concentrations of 50, 100, 200, and 300 ppm were prepared with distilled water. Apical shoots of CSIR-IHBT-VJ-05 with single nodes were used for raising cuttings. These cuttings are treated with different doses of NAA to evaluate the effect of NAA treatments in comparison to control (no treatment) based on the performance of apical buds for root development parameters viz., the number of roots, root length (cm), and root diameter (mm). Different planting media (soil mix, sand, and coco-peat) were used as replicates to assess the influence of the soil environment over the root development parameters.
The experiment was conducted in 2018 under controlled environmental conditions in a propagation chamber (temperature variations from 20 to 25°C) at the experimental farm of CSIR-Institute of Himalayan Bioresource Technology, Palampur (1320 m amsl; 32°68′N, 76°38′E), situated in the mid-hills of Himachal Pradesh, with humid sub-temperate climate and high mean annual rainfall (∼2500 mm). The cuttings were raised during the onset of monsoon season (end of June) in root trainers under 50% shade (using the agro-shade net) conditions, and light irrigation was done. Light irrigation was performed using watering daily for the first week of the experiment and subsequently when the soil media on the surface started drying.
A comparative study on the multiplication rate of different accessions was also undertaken in terms of new plant generation through the splitting rhizomes (after 3 years) and the number of apical shoots formed (in a year). It will help estimate quality plant material (QPM) production on a large scale to meet commercial cultivation requirements.
The experiment was carried out using factorial design to study the effect of four different hormonal (NAA) doses (ppm) and four duration of treatments (time in minutes) in comparison to control (no treatment) on clonal propagation of V. jatamansi replicated over different planting media viz., (a) mixture of soil, sand, and farmyard manure (1:1:1), (b) medium-fine river sand, and (c) coco-peat. The different doses and durations of NAA (α-naphthaleneacetic acid) used for the treatments are presented in Table 1.
Five cuttings were raised in each treatment. However, data recording was done on three of the most competitive rooted plants, and the mean value was generated for each treatment to satisfy the condition of homogeneity of variance. The data of root growth parameters were recorded after 28 days of planting the cuttings in the root trainers. The average number of roots per plant was counted manually, while root length (cm) and root diameter (mm) were measured using a geometrical scale and vernier calipers.
Thirty clonal plants of CSIR-IHBT-VJ-05 were raised in the field under partial shade conditions (using 50% agro-shade net) for 3 years (2018–2021) along with thirty plants of control variety ‘Himbala.’ The EO content was determined by hydrodistillation (Agnihotri et al., 2011; Thusoo et al., 2014; Kumar et al., 2020, 2021) of 500 g of cleaned fresh rhizomes of the mother plant, cloned plants and control plants during the third year in Clevenger-type apparatus for 6 h in October 2020. The EO was dried over anhydrous sodium sulfate and placed in amber-colored vials at 4°C.
Gas chromatography (GC) and GC–MS analysis was performed through flame ionization detector (FID) on Shimadzu QP2010 equipped with AOC-5000 auto-injector and SH-RX-5Si/MS capillary column Shimadzu Asia pacific, United States (30 M × 0.25 mm × 0.25 μm). Helium was the carrier gas (1.28 mL min–1 flow rate). The oven temperature was set to 70°C for 3 min and then to 220°C for 5 min with a constant rate (4°C min). The temperatures of MS source and interface were 240 and 250°C, respectively. The mass was scanned at 70 eV from 40 to 800 amu. The sample injection was 2 μL with a split ratio of 1:10. The retention indices of compounds were calculated by using homologous series of n-alkanes (C8–C24). The RI value for each peak of GC–MS spectra was calculated and compared with Adams tabulated indexes (not exceeding ±10) (Adams, 1995) stored at the New York mass spectral (MS) library, National Institute of Standards and Technology (NIST) (Stein, 2005).
The experiment involved the analysis of simultaneous variations for more than one factor. Using randomized block design (RBD), we get information for a single factor at a time. The relevance of following the RBD approach depends on the condition that responds to different levels of one factor is independent of varying levels of the second factor. However, such an assumption may not work in all situations. Notably, there will be interaction among the levels of the two factors under study in the present experiment. Therefore, a practical approach is to investigate the effect of two factors together by comparing the possible combinations of different levels of both the factors in the same experiment. Accordingly, factorial analysis of variance was done to identify significant variations among the treatments (both for doses and durations) for root parameters. The significance of differences was tested by the least significant difference (LSD) at P ≤ 0.05. Bar plots were generated using PAST software based on doses and duration of treatments to present the trend of root parameters in response to treatment effects.
The composition of EO samples obtained from mother plants and clonal plants of CSIR-IHBT-VJ-05 was analyzed using Student’s t-test. Pooled standard deviations were used for the test to compare mean values for significant components of EO. Similarly, clonal plants of CSIR-IHBT-VJ-05 were compared with seed-raised plants of Himbala to understand the variations among the two diverse selections.
Overall results about NAA-induced root growth in apical shoots of V. jatamansi indicated significant variations for different doses and durations of treatments (Figure 1). Induction of a higher number of roots in apical shoots provides easy maintenance of clonal stocks and ensures higher chances of early establishment and field survival of the rooted plants. Seed-raised nurseries of V. jatamansi require great care in the initial stages of growth due to small seed size and may take up to 4 months to harden the plants before transplanting to field conditions. Propagation through the division of rhizomes takes up to 3 years in V. jatamansi and is not feasible as rhizomes and roots are the parts utilized for the production of EO and valepotriates in this species.

Figure 1. Apical buds of Valeriana jatamansi (A), single-node cuttings (B), and root formation in cuttings (C), treated with NAA 50 ppm (left) and control (right).
Observations concerning root number, length, and diameter in different NAA treatments reflected the plants’ vigor resulting from dosage and duration of treatments. Based on factorial analysis of data, significant variations were observed for doses of NAA treatments for the number of roots formed, while durations of treatment showed non-significant variations (Table 2). Maximum root number was observed using 50 ppm NAA solution (T1 = 5.58), and it was at par with the number of roots formed under 100 ppm (T2 = 4.96) and 200 ppm (T3 = 4.68) treatments (Figure 2) but significantly higher than 300 ppm treatment (T4 = 3.74) as well as control (T0 = 3.33). Number of roots formed were statistically at par for different durations of treatment (Figure 2) with maximum root numbers in 60 minutes’ duration (D2 = 4.61), followed by treatments for 30 min (D1 = 4.54), 120 min (D3 = 4.46), and 180 min (D4 = 4.23).
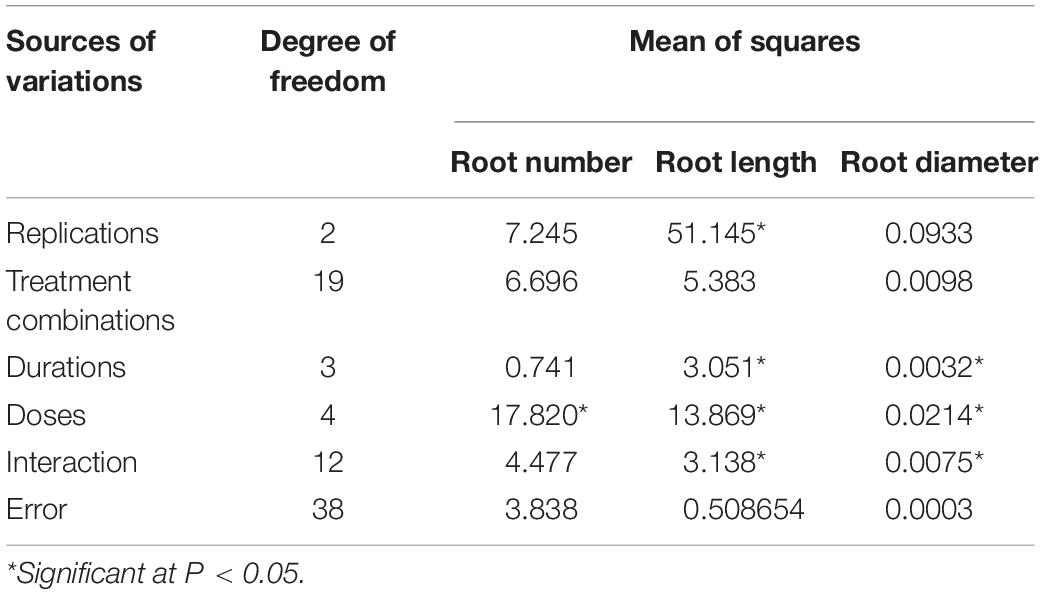
Table 2. Factorial analysis of data for root parameters on varying doses and durations of NAA treatments in apical cuttings of CSIR-IHBT-VJ-05 (Valeriana jatamansi).
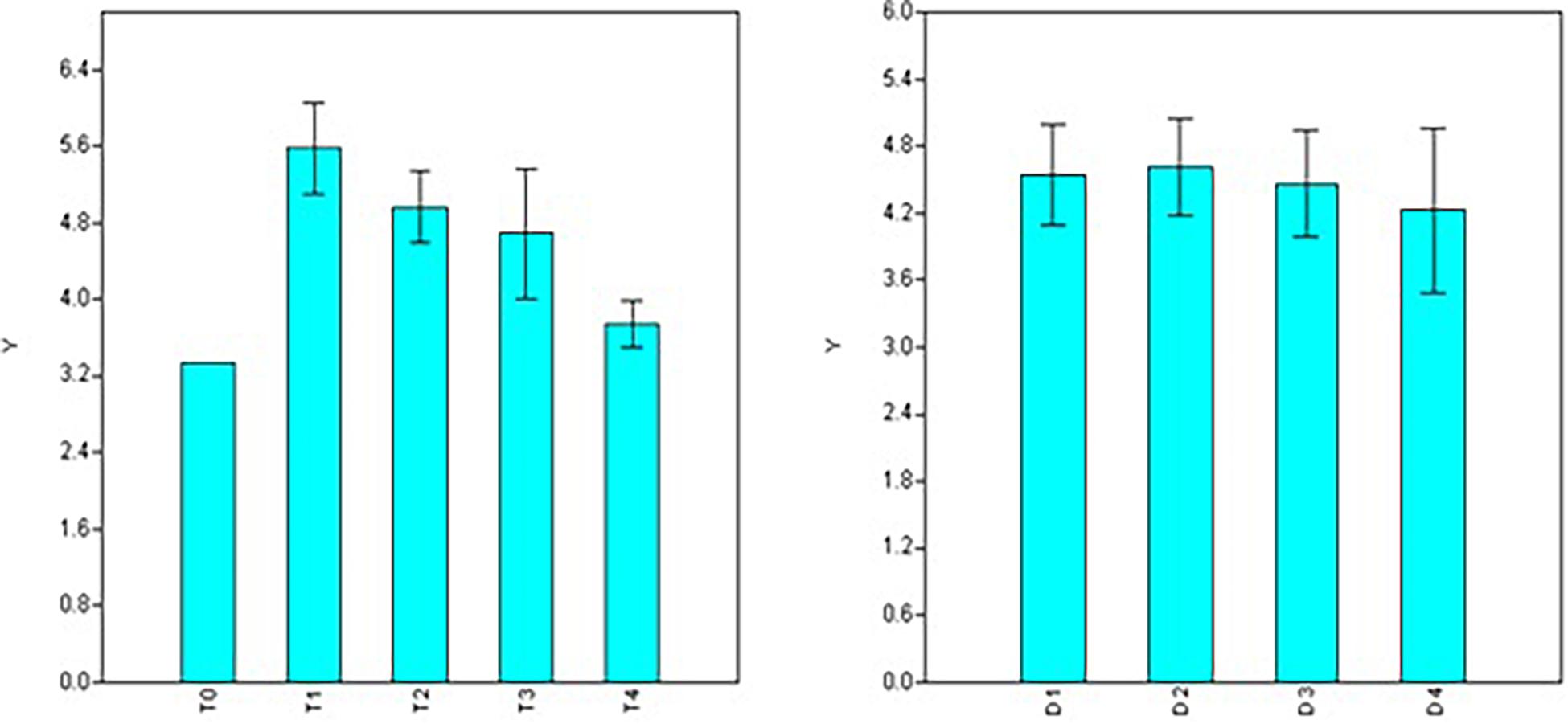
Figure 2. Effect of NAA doses (left) and durations (right) on number of roots formed in apical shoots of Valeriana jatamansi.
In the case of root length, significant variations were observed for doses, durations, and interaction between the two factors (dose × duration) involved in the treatments (Table 2). Maximum root length was observed using 50 ppm NAA solution (T1 = 5.06 cm) and it was at par with number of roots formed under 100 ppm treatment (T2 = 4.76 cm) and 200 ppm (T3 = 4.51 cm) but significantly higher than 300 ppm (T4 = 3.32 cm) treatments (Figure 3) as well as control (T0 = 2.51 cm). Significant variations for root length were also observed for different durations of treatment with maximum root length in duration of 120 min (D3 = 5.18 cm), followed by treatments for 30 min (D1 = 4.50 cm) and 180 min (D4 = 4.09 cm) while a significant decline was observed in root length in treatments of 60 min (D2 = 3.88 cm) duration (Figure 3).
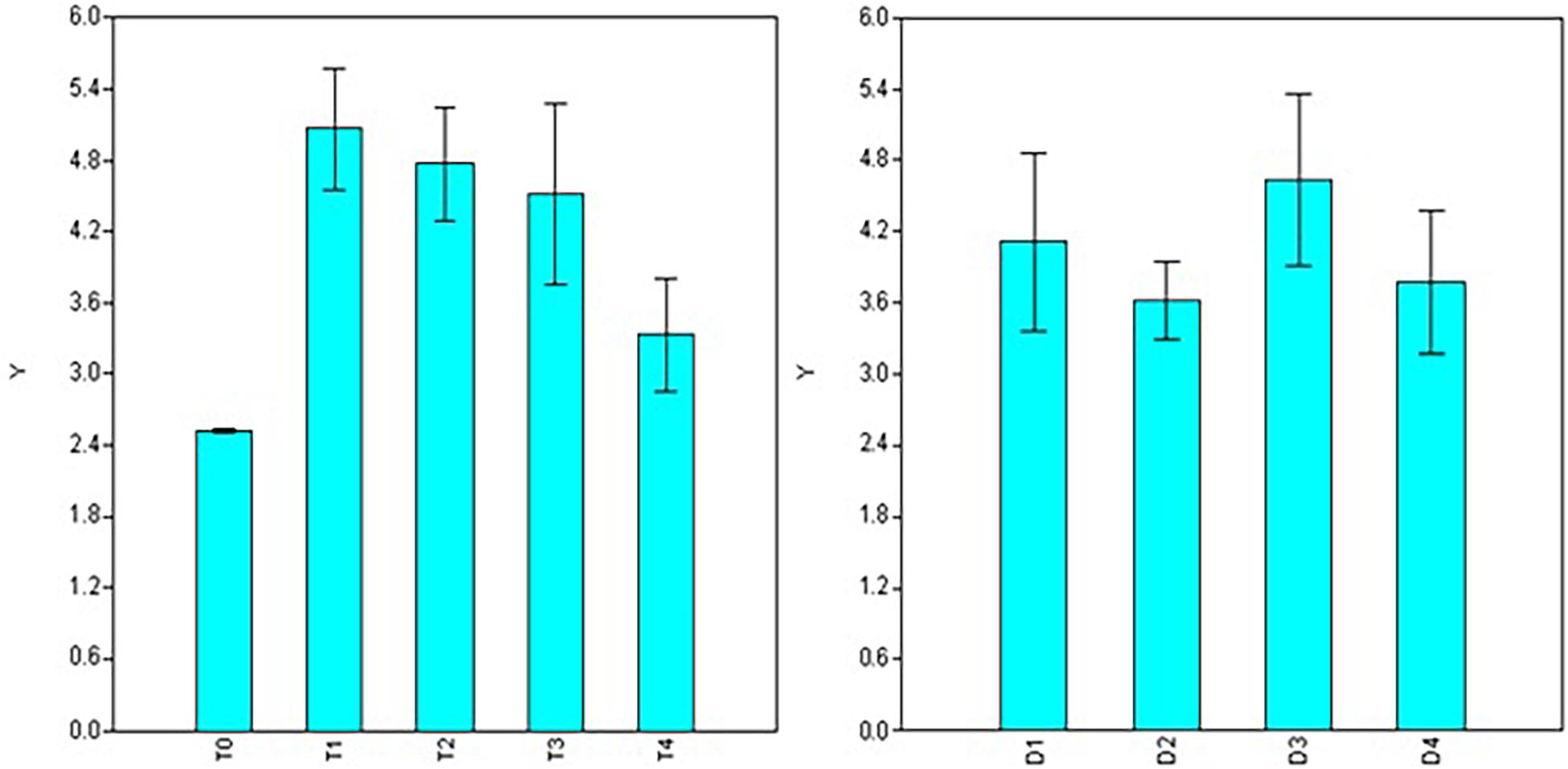
Figure 3. Effect of NAA doses (left) and durations (right) on root length (cm) in apical shoots of Valeriana jatamansi.
Significant variations were also observed for doses, durations, and interaction (dose × duration) when analyzing root diameter (Table 2). Maximum root diameter was observed using 50 ppm NAA solution (T1 = 0.282 mm) and it was at par with root diameter under 100 ppm treatment (T2 = 0.277 mm), 200 ppm (T3 = 0.264 mm), and 300 ppm (T4 = 0.234 mm) treatments (Figure 4), while it was significantly higher than root diameter in control (T0 = 0.179 mm). Root diameter was maximum in 30 min (0.266 mm) and was significantly higher than all the other durations of treatments (Figure 4) viz., 60 min (0.245 mm), 120 min (0.238 mm), and 180 min (0.237 mm).
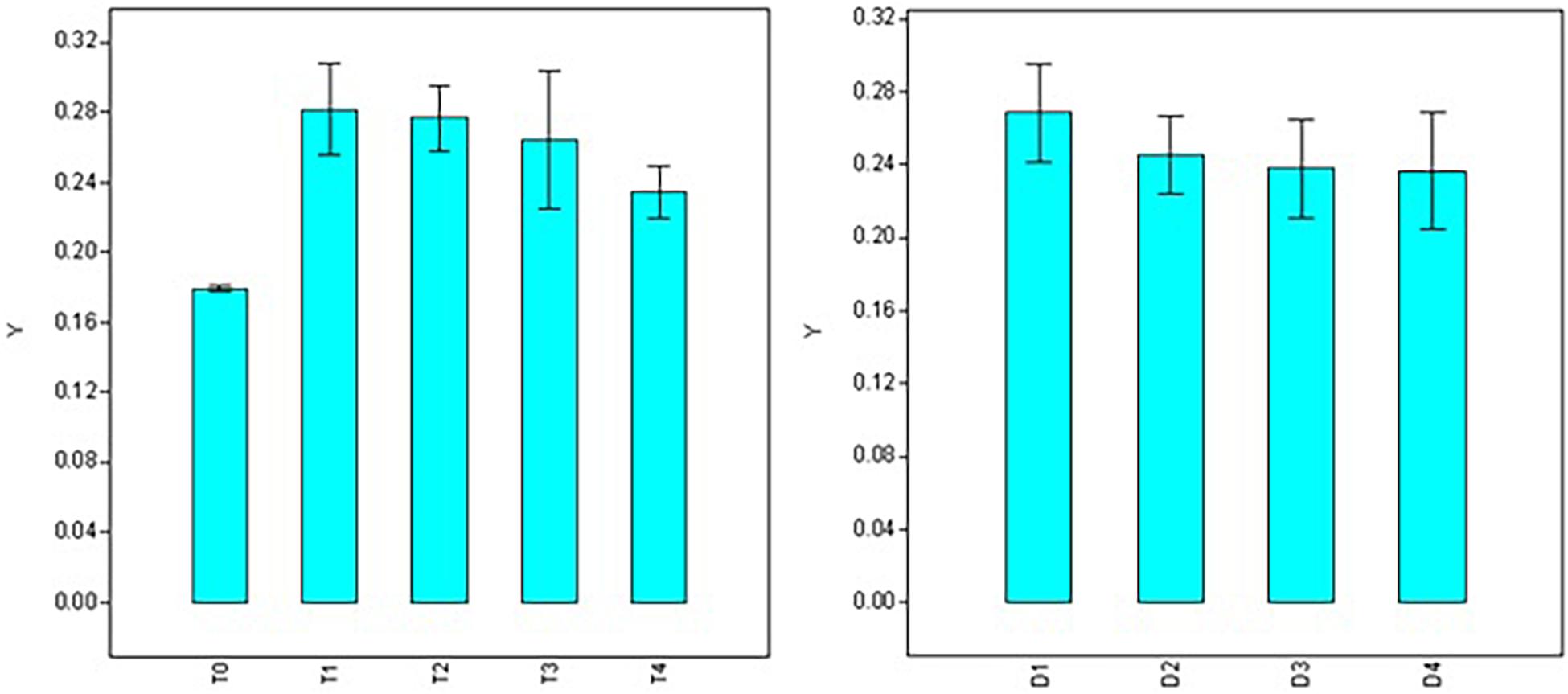
Figure 4. Effect of NAA doses (left) and durations (right) on root diameter (mm) in apical shoots of Valeriana jatamansi.
The most important physiological effect of auxin is stimulating elongation in stems and coleoptile through cell division and cell elongation. The proper formation of adventitious roots at the base of apical shoots/stem cuttings is an essential developmental phenomenon in the growth and survival of cuttings. It involves initiating several new meristematic areas in different tissues of stem cuttings (Kaur et al., 2002). Auxin perception is believed to be mediated by receptors [Auxin Binding Protein 1 (ABP1) that physically bind auxin, allowing it to travel from outside the cell into the cell cytoplasm, where it then initiates signal transduction cascades that trigger specific physiological auxin responses. As a synthetic auxin, NAA is commonly used at a relatively low dose to elicit auxin-type responses in cell growth, cell division, fruit set, and rooting (Srivastava, 2002). Earlier studies support the observation, whereby the adventitious root production increased rapidly at lower NAA concentration, while the number of roots decreased at higher concentrations (Raju and Prasad, 2010; Sun and Hong, 2010). However, the dosage requirements of different species vary to a vast extent. Rooting in softwood cuttings of Chimonanthus praecox was successfully promoted using 100 mg/L NAA (Hu et al., 2020). The use of 400 mg/L NAA resulted in maximum rooting in Couroupita guianensis (Shekhawat and Manokari, 2016). The root development by applying NAA was more effective for root development against IBA in V. carnosa (Nagahama et al., 2019).
The 750 and 1000 ppm concentrations of IBA were best for rooting rosemary and geranium cuttings (Rawat et al., 2020). In our study, maximum root numbers in V. jatamansi were obtained at 50 ppm concentration with a decline at higher concentrations.
Contrary to dosage effects, durations of treatments do not show significant variations for root numbers. However, the analysis revealed that root length is significantly affected by the duration of treatments that peak at 120 minutes’ treatment (Figure 3). Root diameter was maximum at 30 min duration (D1) and declined at higher durations of treatments. The higher number of roots during the initial growth phase favors the early establishment of apical shoots/cuttings, while longer roots are prone to damage during transplantation and may turn out to be an impediment. Therefore, NAA treatment of 50 ppm concentration for 30 min may be considered optimum for root induction in apical shoots of V. jatamansi.
Variations were also studied for the performance of rooting parameters in different planting media. Non-significant variations were observed in terms of the number of roots formed in various planting media, and based on mean values, maximum root formation was observed in the sand (6.56) followed by coco-peat (5.93) and soil mix (5.35). Root length was significantly affected under different planting media (Table 3), with maximum length (4.52 cm) under coco-peat followed by sand (4.35 cm) and soil mix (3.24 cm). Variations observed for root diameter were non-significant under different planting media used, with maximum root diameter observed in coco-peat (0.26 mm) followed by sand (0.23 mm) and soil mix (0.23 mm).
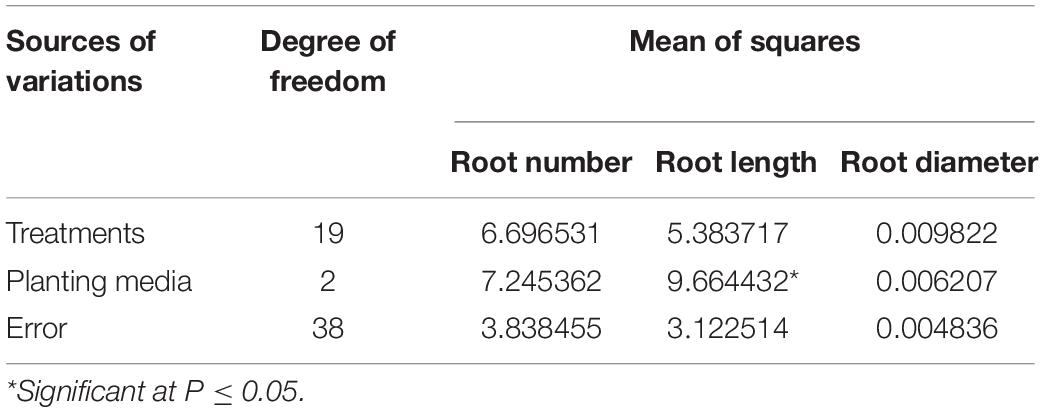
Table 3. Analysis of variance for root parameters in apical cuttings of CSIR-IHBT-VJ-05 under different planting media.
Sand and coco-peat have high porosity than soil mix resulting in easy elongation of roots in such media. Abigaba et al. (2020) found that sterilized sand as propagating medium for softwood cuttings of Carissa edulis resulted in better propagation, while Nurhayati et al. (2020) successfully propagated Cinnamomum zeylanicum plants in the coco-peat medium. Overall, in the present study, both sand and coco-peat gave better propagation results. However, significant variations in comparison to soil mix were not evident except for the root length parameter.
A comparative study of EO was performed to understand the variations of EO metabolites in mother plants, clonally propagated CSIR-IHBT-VJ-05 plants, and control plants (Himbala seed raised plants) an open-pollinated plant population variety. Overall, 38 compounds were identified in the EO using GC–MS analysis, which accounted for 98.1–100.0% of the total EO profile of V. jatamansi samples (Table 4).
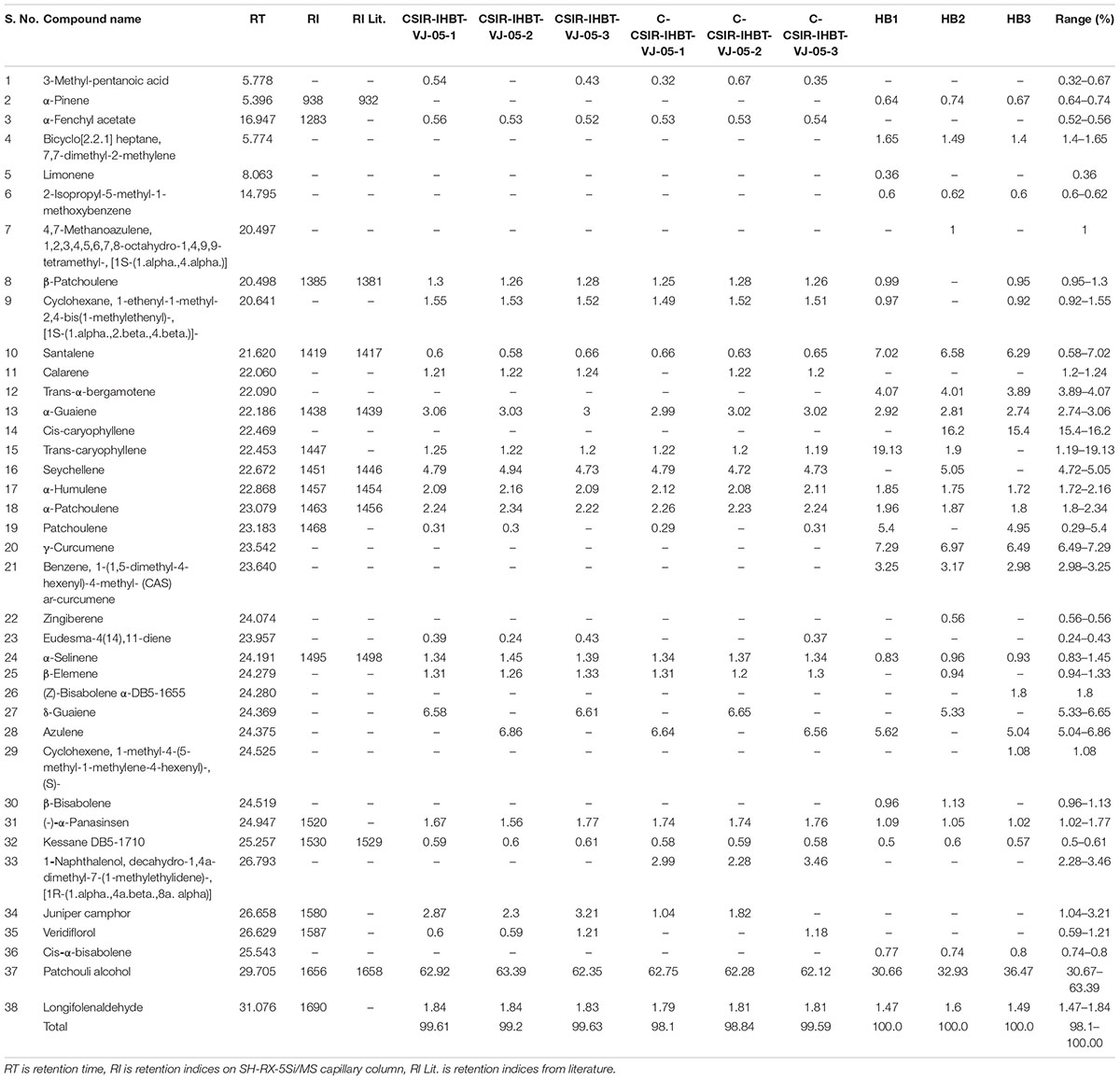
Table 4. Essential oil composition of mother plants, clonally propagated plants of CSIR-IHBT-VJ-05, and seed raised plants of Himbala.
The EO metabolites such as 3-methyl-pentanoic acid (0.32–0.67%), α-fenchyl acetate (0.32–0.67%), calarene (0.32–0.67%), eudesma (0.32–0.67%), naphthalenone (0.32–0.67%), juniper camphor (0.32–0.67%), and veridiflorol (0.32–0.67%) were detected in samples of mother plant and clones of CSIR-IHBT-VJ-05 but absent in samples of Himbala, while the β-patchoulene (0.95–1.3%), cyclohexane1-ethenyl-1-methyl-2,4-bis (1-methylethenyl) (0.92–1.55%), trans-caryophyllene (1.19–19.13%), seychellene (4.72–5.05%), and β-elemene (0.94–1.33%) were present among samples of mother plant and clones of CSIR-IHBT-VJ-05 but not detected in all samples of control. On the other hand, α-pinene (0.64–0.74%), bicycloheptane (1.4–1.65%), limonene (0.36%), 2 isopropyl methoxy benzene (0.6–0.62%), methanoazulene (1.0%), trans-α-berganotene (3.89–4.07%), cis-caryophyllene (15.4–16.2%), γ-curcumene (6.49–7.29%), benzene curcumene (2.98–3.25%), zingiberene (0.56%), α-bisabolene (1.8%), cyclohexene (1.08%), β-bisabolene (0.96–1.13%), and cis-α-bisabolene (0.74–0.8%) were detected exclusively in samples of Himbala, while these were absent in samples of clone CSIR-IHBT-VJ-05. Some of the compounds viz., patchoulene (0.29–5.4%), δ-guaiene (5.33–6.65%), and azulene (5.04–6.86%) were identified exclusively in Himbala and CSIR-IHBT-VJ-05, but not in all the samples analyzed, while 1-naphthalenol, decahydro-1,4a-dimethyl-7-(1-methylethylidene)-, [1R-(1.α.,4a.β.,8a.α.)] (2.28–3.46%) was identified in clones of CSIR-IHBT-VJ-05.
Fifteen compounds were detected of EO of mother and clonal plants ofCSIR-IHBT-VJ-05, which accounted for 86.39% of EO (Table 5). Based on the t-test using pooled standard deviations, variations for all these components were statistically non-significant among the mother plants and clonal plants of CSIR-IHBT-VJ-05 except for longifolenaldehyde. Based on mean values the components of EO in both mother and clonal plants of CSIR-IHBT-VJ-05 were patchouli alcohol (62.89% in mother plant/62.38% in clones), seychellene (4.82/4.74%), α-guaiene (3.03/3.01%), α-patchoulene (2.27/2.24%), α-humulene (2.11/2.10%), longifolenaldehyde (1.84/1.80%), α-panasinsen (1.67/1.75%), cyclohexane (1.53/1.51%), α-selinene (1.39/1.35%), β-elemene (1.3/1.27%), β-patchoulene (1.28/1.26%), trans-caryophyllene (1.22/1.20%), santalene (0.61/0.65%), kessane (0.60/0.58%), and α-fenchyl acetate (0.54/0.53%), respectively (Figure 5).
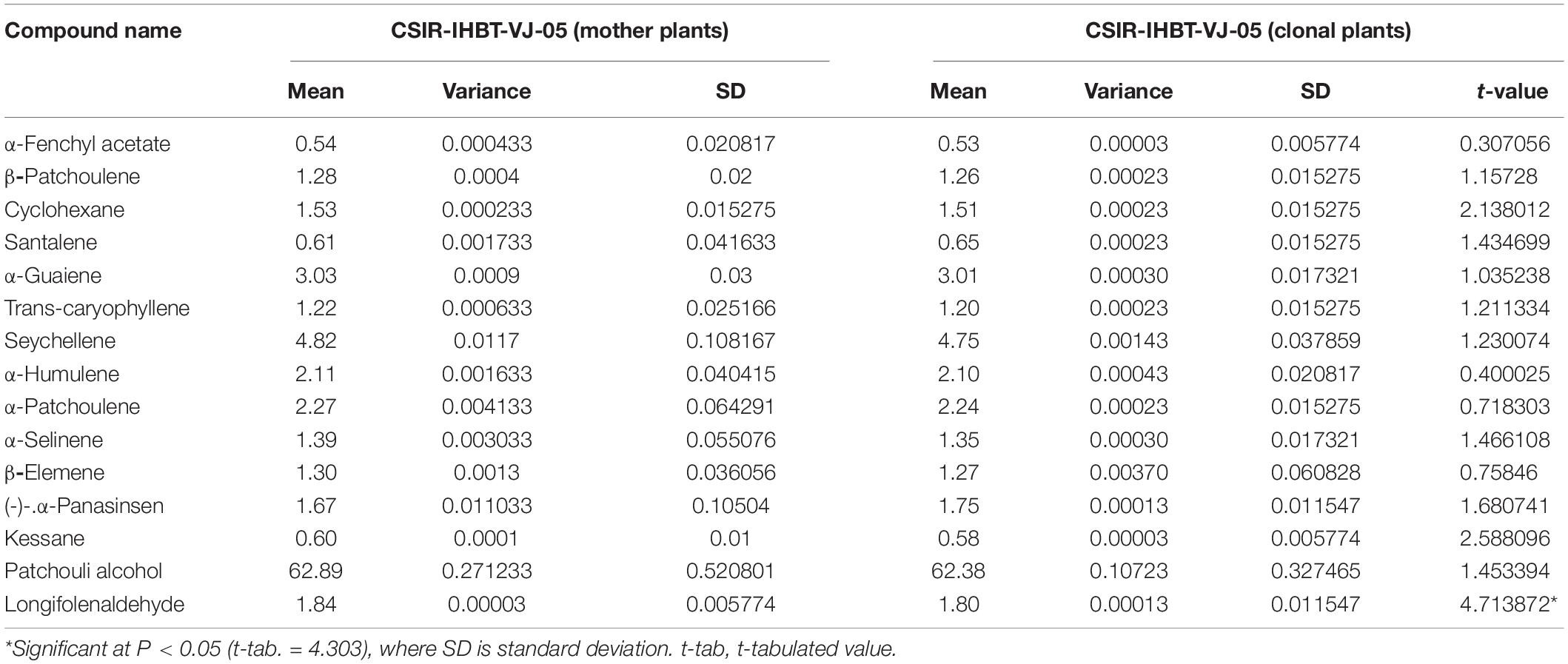
Table 5. Variations of essential oil compounds in mother plant and vegetatively propagated clone of CSIR-IHBT-VJ-05.
Overall, patchouli alcohol is the major component of V. jatamansi EO which forms the largest fraction (∼ 62%). Earlier studies on EO have revealed populations which exhibited about 63.7 and 65.04% of patchouli alcohol, respectively (Singh et al., 2013; Raina and Negi, 2015). The present study on GC–MS analysis reveals that clonal plants of a high yielding selection can produce a uniform grade of EO, which is a primary requirement of the aroma industry.
Nine components of EO were detected in mother and clonal plants of CSIR-IHBT-VJ-05 and EO from plants of ‘Himbala’ (Table 6 and Figure 6). Based on t-test using pooled standard deviations, significant variations were obtained for patchouli alcohol (62.38% in clone of VJ05/33.35% in seed raised plants of Himbala), α-patchoulene (2.24/1.88%), α-humulene (2.10/1.77%), longifolenaldehyde (1.80/1.52%), α-panasinsen (1.75/1.05%), α-selinene (1.35–0.91%), and santalene (0.65/6.63%). Only α-guaiene (3.01/2.82%) and kessane (0.64–0.92%) were found statistically non-significant components among the EO samples of clonal plants of CSIR-IHBT-VJ-05 and Himbala. Samples of Himbala plants varied from CSIR-IHBT-VJ-05 and each other for different EO components (Table 6), as is evident from high variance and standard deviation values CSIR-IHBT-VJ-05 clone.
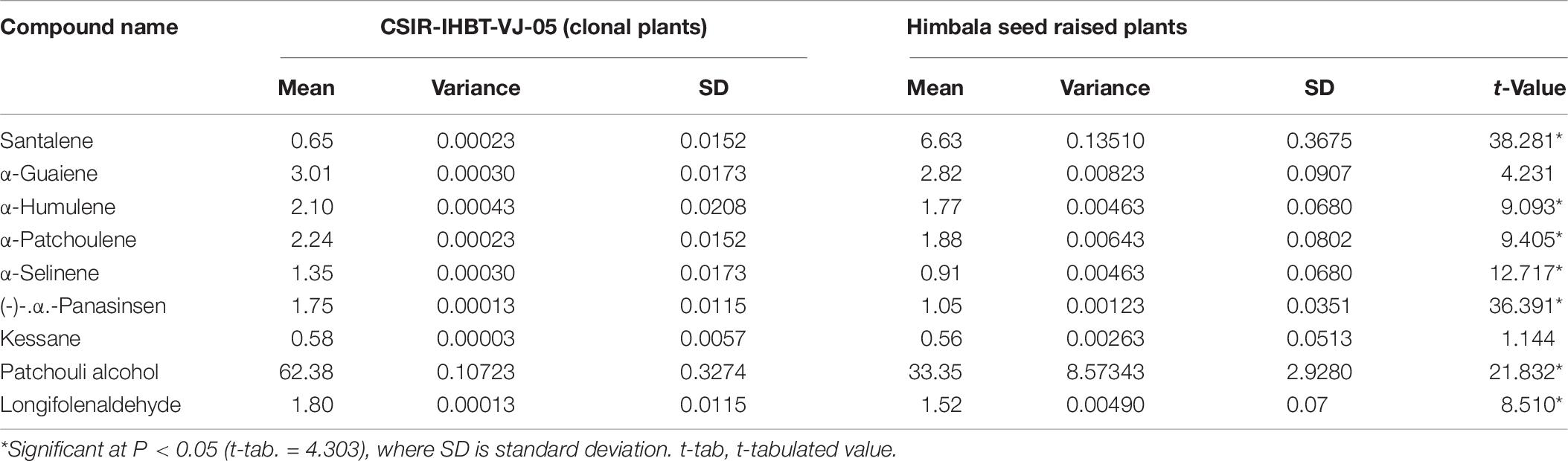
Table 6. Variation of essential oil compounds in a vegetatively propagated clone of CSIR-IHBT-VJ-05 and seed raised plants of Himbala.
Variations in EO profile of clonal line CSIR-IHBT-VJ-05 and open-pollinated plant population of variety ‘Himbala’ reveal chemotypic distinction among the two. CSIR-IHBT-VJ-05 (collection from Salooni region in district Chamba, Himachal Pradesh) and Himbala (selection from Palampur region, district Kangra, Himachal Pradesh) have diverse characteristics geographic origin and accordingly are expected to differ for their EO composition. Earlier studies based on the EO composition of V. jatamansi from Himalayan regions of India (Singh et al., 2010, 2013; Verma et al., 2013; Raina and Negi, 2015) confirm chemotypic and seasonal variations among accessions collected from different regions. The pre-flowering stage is reported to have high concentrations of phenols, flavonoids, and higher antioxidant activity while the decrease in concentration of monoterpenes and sesquiterpenes with increase in oxygenated sesquiterpenes due to qualitative and quantitative variation in EO was observed among different phenological stages across altitude gradient (Jugran et al., 2020). In our study, EO was extracted during month of October from all the samples studied to ensure homogeneity. Open-pollinated varieties derived through mass selection tend to be more variable on phenotypic and chemotypic basis with time, owing to random mating among the individuals of the population leading to generation of new variations on account of genetic reconstitution resulting in heterogenous genetic makeup. The flower size of V. jatamansi is small with plants having either hermaphrodite or pistillate flowers. The high degree of entomophilous pollination is evident during the flowering stage in V. jatamansi, ensuring cross-fertilization. However, experiments on plants with hermaphrodite flowers in isolation also result in some seed-set due to selfing. Studies on genetic diversity and population structure of natural populations from the western Himalayan region using amplified fragment length polymorphism (Rajkumar et al., 2011) suggest high genetic variation (93%) within populations. Natural populations during evolution acquire such adaptive floral mechanisms to ensure cross-fertilization and achieve fitness of the populations. However, from a cultivation point of view, clonal selection presents the advantage of producing a homogenous genetic background and thereby control at least one of the factors (uniform planting material) contributing to the quality of produce in this commercially important herb.
The multiplication rate in V. jatamansi through rhizome splits per plant is 17.07 plants after 3 years of the growth period, whereas 22.47 apical shoots are available per plant right in the first year of growth (Table 7).
With a start from 10 plants, three multiplication cycles are required using apical shoots to produce around 80,000 plants to cover a 1-ha cultivation area. About 74,074 plants ha–1 are required for plantation at 30 × 45 cm spacing, which maximizes underground mass (Mukherjee, 2015). No mortality of clonally raised plants using apical shoots was observed, and its multiplication can be undertaken very well using sand or coco-peat as the planting media. Plants can be grown from apical shoots in root trainers, and space requirements for raising 80,000 plants is 167 m2, which can be reduced to half, one-third, or even further following two-tier, three-tier, and multi-tier planting systems. Thus, large-scale QPM production of V. jatamansi can be ensured through the improvised clonal propagation method even under the limitation of mother plants’ improved selections (Figure 7). Also, the EO obtained from clonal plants is of uniform quality, similar to the mother plant’s chemical profile, leading to higher returns.
Based on the results, significant variations among treatments of NAA were observed for root growth parameters in apical shoots of V. jatamansi. Factorial analysis of variance revealed a substantial influence of NAA dosage on the number of roots formed, while treatment durations were non-significant. On the other hand, both root length and diameter were significantly affected due to doses, durations, and interaction between them (dose × duration). NAA treatment of 50 ppm concentration for 30 min was found optimal for root induction in apical shoots of V. jatamansi. Comparisons of EO from mother plants, clonally propagated plants of CSIR-IHBT-VJ-05, and control plants using GC–MS analysis revealed statistically non-significant variations for major oil components among the mother plants and clonal plants of CSIR-IHBT-VJ-05 highlighting the potential of clonal plants to produce the uniform grade of EO. Selection CSIR-IHBT-VJ-05 was a unique chemotype rich in patchouli alcohol (∼62%) and distinct from the existing open-pollinated variety Himbala. This selection has potential utility in breeding to improve rhizome biomass and EO quality of V. jatamansi. NAA-induced rooting in apical cuttings of V. jatamansi led to the establishment of a simple and fast propagation method that can be utilized for efficient large-scale QPM production to ensure uniformity of produce.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
RG: clonal propagation experiment. AjK: performed oil extraction. RK: data recording. RC: implemented standard agronomic practices in experiments. StS: data analysis. MK: chemical profiling of essential oils. DK: chemical evaluation of essential oil and manuscript editing. AsK: germplasm collection from diverse locations and manuscript editing. SnS: planning and monitoring of experiment, important suggestions, and the manuscript writing. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors are grateful for the support of Sanjay Kumar, Director, CSIR-Institute of Himalayan Bioresource Technology, Palampur (HP) India, for providing the necessary facility for this study. It is IHBT Communication no. 4859.
Abdi, G., and Khui, K. M. (2007). Shoot regeneration via direct organogenesis from leaf segments of Valerian (Valerina officinalis L.). Int. J. Agric. Res. 2, 877–882. doi: 10.3923/ijar.2007.877.882
Abigaba, G., Mujuni, D. B., Kamusiime, E., Kazigaba, D., and Oluk, R. (2020). Propagation of Carissa edulis using stem cuttings for domestication and conservation in Uganda. GSC Biol. Pharm. Sci. 12, 24–30. doi: 10.30574/gscbps.2020.12.3.0230
Adams, P. R. (1995). Identification of Essential oil Components by Gas Chromatography/Mass Spectroscopy, 4th Edn. (Carol Stream, IL: Allured Publishing Corporation), 60188–62403.
Agnihotri, S., Wakode, S., and Ali, M. (2011). Chemical composition, antimicrobial and topical anti-inflammatory activity of Valeriana jatamansi Jones. essential oil. J. Essent. Oil Bear. Plants 14, 417–422.
Anonymous (1976). The Wealth of India, Vol. X: Sp-D (Raw Material). (New Delhi: CSIR Publication), 424–426.
Bhardwaj, P., Rattan, S., Naryal, A., Bhardwaj, A., and Warghat, A. R. (2021). “Valeriana jatamansi,” in Himalayan Medicinal Plants, eds N. Malhotra and M. Singh (Cambridge, MA: Academic Press), 259–271.
Bhatt, I. D., Dauthal, P., Rawat, S., Gaira, K. S., Jugran, A., Rawal, R. S., et al. (2012). Characterization of essential oil composition, phenolic content, and antioxidant properties in wild and planted individuals of Valeriana jatamansi Jones. Sci. Hortic. 136, 61–68. doi: 10.1016/j.scienta.2011.12.032
Chen, R., Zhang, M., Lü, J., Zhang, X., Teixeira, da Silva, J. A., et al. (2014). Shoot organogenesis and somatic embryogenesis from leaf explants of Valeriana jatamansi Jones. Sci. Hortic. 165, 392–397. doi: 10.1016/j.scienta.2013.11.036
Dua, V. K., Alam, M. F., Pandey, A. C., Rai, S., Chopra, A. K., Kaul, V. K., et al. (2008). Insecticidal activity of Valeriana jatamansi (valerianaceae) against mosquitoes. J. Am. Mosq. Control Assoc. 24, 315–318. doi: 10.2987/5642.1
Faria, R. T., and Illg, R. D. (1995). Micropropagation of Zingiber spectabile Griff. Sci. Hortic. 62, 135–137. doi: 10.1016/0304-4238(94)00759-9
Firoz, A. M., Ruhul, A., Uddin, E. M., Biswas, S. K., and Monirul, I. M. (2015). Regeneration of shoot from nodal explants of Cucumuis sativus considering different hormonal concentration. Int. Res. J. Biol. Sci. 4, 48–52.
Hossain, M. S., and Urbi, Z. (2016). Effect of Naphthalene Acetic Acid on the Adventitious Rooting in Shoot Cuttings of Andrographis paniculata (Burm.f.) Wall. ex Nees: an important Therapeutical Herb. Int. J. Agron. 2016:1617543. doi: 10.1155/2016/1617543
Hu, H., Chai, N., Zhu, H., Li, R., Huang, R., Wang, X., et al. (2020). Factors affecting vegetative propagation of Wintersweet (Chimonanthus praecox) by softwood cuttings. HortScience 55, 1853–1860. doi: 10.21273/HORTSCI15289-20
Jabeen, F. T. Z., Venugopal, R. B., Kiran, G., Kaviraj, C. P., and Rao, S. (2005). Plant regeneration and in vitro flowering from leaf and nodal explants of Solanum nigrum (L.) - An important medicinal plant. Plant Cell Biotechnol. Mol. Biol. 6, 17–22.
Jafari, A. A., Shohrati, M., Mahmoudi, R., Hoseini, R. H., Nosratpour, S., Pajohi, A. M., et al. (2014). Chemical composition and biological activities of Scrophularia striata extracts. Minerva Biotecnol. 26, 183–189.
Jugran, A. K., Bhatt, I. D., Rawal, R. S., Nandi, S. K., and Pande, V. (2013). Patterns of morphological and genetic diversity of Valeriana jatamansi Jones in different habitats and altitudinal range of West Himalaya, India. Flora Morphol. Distrib. Funct. Ecol. Plants 208, 13–21. doi: 10.1016/j.flora.2012.12.003
Jugran, A. K., Rawat, S., Bhatt, I. D., and Rawal, R. S. (2020). Essential oil composition, phenolics and antioxidant activities of Valeriana jatamansi at different phenological stages. Plant Biosyst. 155, 891–898. doi: 10.1080/11263504.2020.1810803
Kaur, S., Cheema, S. S., Chhabra, B. R., and Talwar, K. K. (2002). Chemical induction of physiological changes during adventitious root formation and bud break in grapevine cuttings. Plant Growth Regul. 37, 63–68. doi: 10.1023/A:1020355505105
Kumar, A., Gautam, R. D., Kumar, A., Bisht, A., and Singh, S. (2020). Floral biology of wild marigold (Tagetes minuta L.) and its relation to essential oil composition. Ind. Crops Prod. 145:111996. doi: 10.1016/j.indcrop.2019.111996
Kumar, A., Gautam, R. D., Kumar, R., Chauhan, R., Kumar, M., Singh, S., et al. (2021). Floral studies of palmarosa [Cymbopogon martinii (Roxb.) W. Watson] and chemical insights during inflorescence development. Ind. Crops Prod. 171:113960. doi: 10.1016/j.indcrop.2021.113960
Lin, S., Chen, T., Liu, X. H., Shen, Y. H., Li, H. L., Shan, L., et al. (2010). Iridoids and lignans from Valeriana jatamansi. J. Nat. Prod. 73, 632–638.
Liu, H., Liu, D., Jiang, M. Y., Zhao, X. D., Li, R. T., and Li, H. M. (2021). Iridoids from Valeriana jatamansi with anti-inflammatory and antiproliferative properties. Phytochemistry 184:112681. doi: 10.1016/j.phytochem.2021.112681
Mahmoudi, R., Ehsani, A., Tajik, H., and Pajohi, A. M. (2013a). Evaluation of phytochemical and antibacterial properties of some medicinal plants from Iran. J. Biol. Act. Prod. Nat. 3, 310–322.
Mahmoudi, R., Kosari, M., and Barati, S. (2013b). Phytochemical and biological properties of Ferula sharifi essential oil. J. Biol. Act. Prod. Nat. 3, 331–338. doi: 10.1080/22311866.2013.867664
Mahmoudi, R., and Nosratpour, S. (2013). Teucrium polium L. essential oil: phytochemiacl component and antioxidant properties. Int. Food Res. J. 20, 1697–1701.
Mathur, J., Ahuja, P. S., Lal, N., and Mathur, A. K. (1989). Propagation of Valeriana wallichii DC. using encapsulated apical and axial shoot buds. Plant Sci. 60, 111–116. doi: 10.1016/0168-9452(89)90050-2
Maurya, A. K., Meena, R. L., Kumar, A., Prasad, R., and Gopichand (2017). Current status of Valeriana jatamansi: an endangered species. Glob. J. Res. Med. Plants Indig. Med. 6, 95–102.
Mohammadzadeh, M., Mahmoudi, R., and Ghajarbeygi, P. (2018). Evaluation of chemical composition and antibacterial properties of Froriepia subpinnta essential oils from guilan region: before and after flowering. J. Essent. Oil Bear. Plants 21, 1119–1127. doi: 10.1080/0972060X.2018.1505555
Mukherjee, D. (2015). Studies on seed germination pattern and effect of sowing date with plant prototype on Valeriana jatamansi (Jones): a rare medicinal plant of eastern Himalaya. J. Crop Weed. 11, 9–13.
Mukherjee, D., and Chakraborty, S. (2014). Studies on ecology, habitats diversification and seed germination behavior of Valeriana jatamansi Jones: a critical endangered plant. Int. J. Agric. Sci. 4, 203–209.
Nagahama, N., Manifesto, M. M., and Fortunato, R. H. (2019). Vegetative propagation and proposal for sustainable management of Valeriana carnosa Sm., a traditional medicinal plant from Patagonia. J. Appl. Res. Med. Aromat. Plants 14, 1–5. doi: 10.1016/j.jarmap.2019.100218
Nurhayati, H., Supriatna, N., Setyono, Syukur, C., and Pitono, J. (2020). The effect of cutting material and planting medium to the growth of cinnamon (Cinnamomum zeylanicum Blume) seedling. IOP Conf. Ser. Earth Environ. Sci. 418:012024.
Purohit, S., Rawat, V., Jugran, A. K., Singh, R. V., Bhatt, I. D., and Nandi, S. K. (2015). Micropropagation and genetic fidelity analysis in Valeriana jatamansi Jones. J. Appl. Res. Med. Aromat. Plants 2, 15–20. doi: 10.1016/j.jarmap.2015.01.001
Quan, L. Q., Su, L. H., Qi, S. G., Xue, Y., Yang, T., Liu, D., et al. (2019). Bioactive 3, 8-epoxy iridoids from Valeriana jatamansi. Chem. Biodivers. 16:e1800474.
Raina, A. P., and Negi, K. S. (2015). Essential oil composition of Valeriana jatamansi Jones from Himalayan Regions of India. Indian J. Pharm. Sci. 77, 218–222.
Rajkumar, S., Singh, S. K., Nag, A., and Ahuja, P. S. (2011). Genetic structure of Indian Valerian (Valeriana jatamansi) populations in western Himalaya revealed by AFLP. Biochem. Genet. 49, 674–681. doi: 10.1007/s10528-011-9448-2
Raju, N. L., and Prasad, M. N. V. (2010). Influence of growth hormones on adventitious root formation in semi-hardwood cuttings of Celasturs paniculatus Willd.: a contribution for rapid multiplication and conservation management. Agrofor. Syst. 79, 249–252. doi: 10.1007/s10457-009-9251-9
Rather, A. M., Nawchoo, A. I., Aijaz, H. G., and Wani, A. G. (2014). Inflorescence architecture and stamina movement as contrivance measures in reproductive assurance and survival of Valeriana jatamansi Jones (Valeriana wallichii DC). Curr. Sci. 107, 568–570.
Rawat, R., Vasishth, A., Kumar, V., and Guleria, V. (2020). Effect of rooting hormones on growth performance of two important aromatic crops of Garhwal Himalaya. Pharma Innov. J. 9, 650–655.
Sarropoulou, V., and Maloupa, E. (2019). In vitro propagation of Satureja thymbra L.(Lamiaceae): a valuable aromatic-medicinal native plant of the Mediterranean region. GSC Biol. Pharm. Sci. 9, 009–020. doi: 10.30574/gscbps.2019.9.2.0190
Shekhawat, M. S., and Manokari, M. (2016). Impact of Auxins on vegetative propagation through stem cuttings of Couroupita guianensis Aubl.: A conservation approach. Scientifica 2016:6587571. doi: 10.1155/2016/6587571
Singh, R. D., Gopichand, Meena, R. L., Sharma, B., Singh, B., Kaul, V. K., et al. (2010). Seasonal variation of bioactive components in Valeriana jatamansi from Himachal Pradesh, India. Ind. Crops Prod. 32, 292–296. doi: 10.1016/j.indcrop.2010.05.006
Singh, S., Ram, R., Kaundal, S., Sharma, A., Kumar, A., and Dhyani, D. (2016). Field performance and differential response of micro-propagated potential F1 Genotypes of Gerbera jamesonii. Am. J. Exp. Agric. 10, 1–11. doi: 10.9734/ajea/2016/20653
Singh, S. K., Katoch, R., and Kapila, R. K. (2013). Chemotypic variation for essential oils in Valeriana jatamansi jones populations from Himachal Pradesh. J. Essent. Oil Res. 25, 154–159. doi: 10.1080/10412905.2013.767757
Srivastava, L. M. (2002). Plant Growth and Development: Hormones and Environment. Oxford: Academic Press.
Stein, S. E. (2005). Mass Spectral Database and Software, version 3.02. Gaitherburg, MD: National Institute of Standard and Technology (NIST).
Sun, Y. L., and Hong, S. K. (2010). Effects of plant growth regulators and l-glutamic acid on shoot organogenesis in the halophyte Leymus chinensis (Trin.). Plant Cell Tissue Organ. Cult. 100, 317–328. doi: 10.1007/s11240-009-9653-4
Thusoo, S., Gupta, S., Sudan, R., Kour, J., Bhagat, S., Hussain, R., et al. (2014). Antioxidant activity of essential oil and extracts of Valeriana jatamansi roots. Biomed Res. Int. 2014:614187. doi: 10.1155/2014/614187
Tolera, B. (2016). Effects of Naphthalene Acetic Acid (NAA) and Indole -3- Butyric Acid (IBA) on in vitro rooting of sugarcane (Saccharum officinarum L.) Micro- Shoots. J. Biotechnol. Biomater. 06:215. doi: 10.4172/2155-952x.1000215
Verma, R. S., Padalia, R. C., and Chauhan, A. (2013). Chemical differentiation of rhizome and root essential oils of Indian valerian (Valeriana jatamansi Jones). J. Essent. Oil Bear. Plants 16, 835–840. doi: 10.1080/0972060X.2013.862082
Wang, R. J., Chen, H. M., Yang, F., Deng, Y., Hui, A. O., Xie, X. F., et al. (2017). Iridoids from the roots of Valeriana jatamansi Jones. Phytochemistry 141, 156–161.
Willis, R. B., Bone, K., and Morgan, M. (2000). Herbal products: active constituents, mode of action and quality control. Nutr. Res. Rev. 13, 47–77. doi: 10.1079/095442200108729007
Xu, J., Zhao, P., Guo, Y. Q., Xie, C. F., Jin, D. Q., Ma, Y. G., et al. (2011). Iridoids from the roots of Valeriana jatamansi and their neuroprotective effects. Fitoterapia 82, 1133–1136. doi: 10.1016/j.fitote.2011.07.013
Keywords: Valeriana jatamansi, vegetative propagation, clone, naphthalene acetic acid, essential oil, patchouli alcohol, QPM
Citation: Gautam RD, Kumar A, Kumar R, Chauhan R, Singh S, Kumar M, Kumar D, Kumar A and Singh S (2021) Clonal Propagation of Valeriana jatamansi Retains the Essential Oil Profile of Mother Plants: An Approach Toward Generating Homogenous Grade of Essential Oil for Industrial Use. Front. Plant Sci. 12:738247. doi: 10.3389/fpls.2021.738247
Received: 08 July 2021; Accepted: 27 September 2021;
Published: 14 October 2021.
Edited by:
Spyridon Alexandros Petropoulos, University of Thessaly, GreeceReviewed by:
Raazagh Mahmoudi, Qazvin University of Medical Sciences, IranCopyright © 2021 Gautam, Kumar, Kumar, Chauhan, Singh, Kumar, Kumar, Kumar and Singh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sanatsujat Singh, c2FuYXRzdWphdEBpaGJ0LnJlcy5pbg==; Rahul Dev Gautam, Z2F1dGFtcmFodWxkZXY3QGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.