
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci. , 23 September 2021
Sec. Plant Breeding
Volume 12 - 2021 | https://doi.org/10.3389/fpls.2021.719394
 Rajbir Yadav1*†
Rajbir Yadav1*† Soma Gupta2†
Soma Gupta2† Kiran B. Gaikwad1
Kiran B. Gaikwad1 Naresh Kumar Bainsla1
Naresh Kumar Bainsla1 Manjeet Kumar1
Manjeet Kumar1 Prashanth Babu1
Prashanth Babu1 Rihan Ansari1
Rihan Ansari1 Narain Dhar3
Narain Dhar3 Palaparthi Dharmateja1
Palaparthi Dharmateja1 Rajender Prasad4
Rajender Prasad4Knowledge about the yield gain over the years due to associated changes in the yield component traits is essential for a critical understanding of yield-limiting factors. To estimate genetic gain in grain yield (GY) and component agronomic traits of wheat varieties released between 1900 and 2016 for northwestern plain zone (NWPZ) of India and to identify agronomic and/or genetic basis of the realized gains, two sets of wheat varieties comprising mega varieties and two recently developed varieties were evaluated under timely sown, tilled, and early sown conservation agriculture (CA) conditions for four consecutive years under irrigated conditions. The average annual genetic gain in GY since 1,905 under timely sown irrigated conditions was found to be 0.544% yr−1 over the average of all varieties and 0.822% yr−1 (24.27 kg ha−1 yr−1) over the first released variety, NP4. The realized mean yield increased from 2,950 kg ha−1 of the variety NP4 released in 1,905–5,649 kg ha−1 of HD3086 released in 2014. Regression analysis revealed a linear reduction in height and peduncle length (PL) over the years with a simultaneous and linear increase in biomass at the rate of 43.9 kg ha−1 yr−1 or relatively at 0.368% yr−1 mainly because of delayed heading and increased crop duration. Regression analysis showed no linear trend for tiller number and thousand-grain weight (TGW). Though harvest index (HI) was found to linearly increase relatively at the rate of 0.198% per annum, polynomial regression improved the fitness of data with the indication of no increase in HI since 1982. Interestingly, genetic gain evaluation under early sown CA conditions for 4 years showed similar relative gain (RG) [a relative improvement in varieties across breeding periods (BP)] (0.544% yr−1) but with a higher absolute value (29.28 kg ha−1 yr−1). Major mega varieties like Kalyan Sona, HD2009, PBW 343, HD2967, and HD3086, which occupied a comparatively larger area, were found highly plastic to the improvements in the production environment under timely sown conditions.
Wheat (Triticum aestivum L.), being an important source of carbohydrates, protein, vitamins, and many other essential mineral elements, is the most widely grown crop in the world. Global wheat consumption is likely to be 834.8 MT by 2028 (OECD/FAO, 2019). Despite impressive growth in global wheat production in the recent past, it will be still challenging to meet this demand due to increased competition for alternate use of land, depleting natural resources, degrading soil health, biotic stresses, and changing climatic conditions (Yadav et al., 2010, 2017). To feed the still increasing world population, though, at an unequal rate in different parts of the world, the global average productivity of wheat has to be increased at the rate of 1.3% yr−1 (Rosegrant and Agcaoili, 2010). Stagnating productivity in Europe (Brisson et al., 2010) that relatively lowers gain in the high yielding environments (Maureen, 2019) and slowing yield gains in other countries (Fischer and Edmeades, 2010; Matus et al., 2012; Beche et al., 2014; Maureen, 2019) are worrying concerns. However, the recent estimates of genetic gain, the improvement in average phenotypic value due to selection within a population over cycles of breeding (Crespo-Herrera et al., 2017) for a mega wheat-growing environment in the northwestern plains of India are quite assuring. The difference in genetic gain realized across the world can be due to many factors including the available crop growth duration, agronomic practices followed, and prevailing weather and soil conditions. However, breeding improved varieties by nicking various agronomic traits has always been the main path to maximize the genetic gain through per unit area yield increments (Zhang et al., 2016; Gao et al., 2017; Mingliang et al., 2020).
Identification of yield-limiting factors is one of the crucial steps in designing any future breeding strategies. Genetic gains have largely been assessed by systematically evaluating the performance of historical varieties released over different points of breeding time (Sadras and Lawson, 2011; Sanchez-Garcia et al., 2013; Beche et al., 2014; Morgounov et al., 2014; Wu et al., 2014). Studies limited to a shorter period have reported more than 1% genetic gain per annum for wheat yield (Waddington et al., 1986; Sayre et al., 1997; Underdahl et al., 2008) whereas, gain assessment spread over a longer period (Perry and D'Antuono, 1989; Siddique et al., 1989; Royo et al., 2007) have reported around 0.5% gain annually. In winter wheat, maximum gain in yield (around 1%) was realized during 1960–2000 (Abeledo et al., 2003; Brancourt-Hulmel et al., 2003; Zhou et al., 2007). However, century-long analysis highly subdued the gain (Cox et al., 1988; Austin et al., 1989; Berzonsky and Lafever, 1993; Donmez et al., 2001). Since the beginning of the twenty-first century, progress in increasing the potential yield began to slow down in winter wheat or even reached to plateau in some of the countries (Barutçular et al., 2006; Peltonen-Sainio et al., 2009a; Graybosch and Peterson, 2010) and therefore, increased world wheat demand is most likely to be met by the South and East Asian countries including India. Periodic evaluation of released cultivars for associated changes in physiological and agronomic traits can provide the much-needed cue for future breeding strategies. In China, for example, grain weight and spike weight along with HI and biomass production were found to be the most exploited traits by wheat breeders since mid twentieth century (Zhou et al., 2007). In Australia, on the other hand, it was mainly the improvement of HI, which was responsible for yield gain (Sadras and Lawson, 2011). The increased spikes number (@ 0.30% yr−1) and grains per spike (GPS) (@ 0.60% yr−1) have mainly brought the yield gain in Spain (Sanchez-Garcia et al., 2012). A major jump in yield gain in the past has been realized with the introduction of dwarfing genes, which by reducing the size of vegetative plant organs resulted in better availability of assimilates to reproductive organs and thereby leading to higher yields through improved harvest index (HI) (Brancourt-Hulmel et al., 2003; Álvaro et al., 2008). Recent studies, however, indicate that future gain in yield will come through improved biomass by integrating modern genomic tools and a better understanding of physiological processes (Reynolds et al., 2011; Yadav et al., 2017) along with structural and agronomic adjustment for lodging resistance (Fischer and Edmeades, 2010; Yadav et al., 2017; Bainsla et al., 2018, 2020). India had a remarkable run of wheat production since 2011 with some intermittent hiccups due to climatic uncertainty. However, India cannot afford to be complacent on wheat production because of the likely increased demand due to a still-growing population and widening food security net by the Government of India. Wheat being central to food security in India is cultivated throughout the country; however, the northwestern plain zone (NWPZ) with about 11.59 Mha area, largely under irrigated condition (more than 95%), and accounting for 50% of Indian wheat production is the most important environment available in the country. It was, therefore felt necessary to examine the pattern of yield gain through changes in agronomic traits so that future breeding strategies can be formulated through the identification of important yield-limiting factors in modern cultivars.
High fluctuation in realized yield among many important states of northern India, such as Haryana, Rajasthan, Uttar Pradesh, and Bihar (Yadav et al., 2019) is frequently observed largely because of a sudden and abrupt rise in temperature toward the terminal growth stage of the crop, management constrains (Peltonen-Sainio et al., 2009a,b; Peltonen-Sainio and Hakala, 2011) and no time for compensation in short-growing varieties particularly under late-sown conditions. Trait plasticity, a response of different traits to environmental changes and an environmental contingent trait expression (Sadras and Rebetzke, 2013) have remained as important adaptive genetic traits and are highly relevant under changing climatic conditions. With no limitation of land in ancient agriculture, the success of agriculture was measured in terms of produce harvested from the quantity of seed sown and therefore, generally resulting in highly competitive plant type with a large sink and profuse tillering (Dewitt et al., 2004). Restricted availability of agricultural land for crop production has shifted the focus toward communal plant type, yielding more per unit of sown area. Besides this, the tradeoff between different yield contributing traits is the biggest stumbling block for future gain and for maximizing the fitness of genotype for better yield realization, the plasticity of different traits needs to be better understood (Peltonen-Sainio and Hakala, 2011).
In this study, we took the historical mega varieties, varieties covered the maximum growing area of their respective released time and recently released high yielding varieties for NWPZ, and breeding period (BP) for this study spanning over the last century and one and half decades of the current twenty-first century. Besides trait per se improvement, we were more interested to know the plant traits which were responsible for increment in plasticity resulting in their large-scale adaptation in Indian wheat-growing areas. We have also tried to analyze to know whether the recent gain in wheat yields was largely due to the development of communal or competitive genotypes by the wheat breeders.
The experimental material consisted of two sets of bread wheat varieties (14 varieties in Experiment 1 and 10 varieties in Experiment 2). These varieties included the mega wheat varieties predominantly grown in NWPZ of India during the twentieth and in the first decade of the twenty-first century (1900–2016) and high yielding recently released varieties along with one variety registered with the protection of plant variety and farmer's rights authority (PPVFRA), Delhi, India for its suitability to conservation agriculture (CA) condition (CA, growing crop under zero tillage with previous crop residue retention). The list of varieties, their pedigree, developing institute, and the year of identification/release are detailed in Table 1. Experiment 1 was conducted under irrigated timely sown conditions, while, Experiment 2 was conducted in early sown irrigated CA condition. Experiment 2 was conducted to provide sufficient growth duration to long-duration varieties as in India we witness a high temperature toward the terminal stage of crop growth which forces each variety to mature fast. The varieties were chosen as they are assumed to cover the maximum wheat-grown area during their period of cultivation except for two recent varieties i.e., HDCSW 16 and HDCSW 18 in NWPZ (a prominent wheat-growing region with more than 50% contribution to the total wheat production of a country) of India since 1905 till 2016.
The first experiment with 14 widely adapted bread wheat varieties was conducted during 2012–13, 2013–14, 2016–17, and 2018–19 crop seasons in the experimental farm of Indian Agricultural Research Institute (IARI), New Delhi under irrigated (5–6 irrigations, each at 20 days interval until physiological maturity to maintain optimal moisture conditions) timely sown conditions (≈ November 10). During 2012–13 and 2013–14, the material was raised in a randomized block design of two replications in a plot of six rows of 5.0 m length spaced at 0.20 m apart with a seeding density of 350–400 seed m−2. Excluding two border rows, all the data points were collected on only the central four rows, to avoid any border effects. During 2016–17 and 2018–19, the plot size was increased to 12 rows with similar spacing and row length, and data were collected on the central 10 rows keeping the same experimental conditions. A uniform population was maintained in each plot by seeding rate equivalent to 100 kg/ha for an approximate 36 g/1,000 seed test weight. The soil at the experimental site is alluvial with slightly alkaline characteristics and clay loam texture having low organic matter. The area has a semi-arid and sub-tropical climate with an average annual rainfall of 700 mm and the crop was irrigated as and when required. Fertilizer dose equivalent to 120 kg of N, 60 kg P2O5, 60 kg of K2O, 25 kg of ZnSO4 per ha was applied during 2012–13, 2013–14, and 2016–17 whereas, during 2018–19, the dose of nitrogenous fertilizer was increased to 150 kg ha−1 under conventional tillage. Half the dose of urea and a full dose of P2O5 and K2O were incorporated in the soil before seeding as basal fertilizers. The remaining half of the urea was applied as a top dressing after the first and second irrigation. As crop lodging has been a more common phenomenon in these years in India, a non-shading net was used to avoid the lodging of the crop under conventionally tilled conditions.
The second set of experiment was carried out with two assumptions: (i) that pregreen revolution cultivars in the absence of irrigation condition used to be sown in mid-October under conserved moisture conditions (Yadav et al., 2017), and their sowing in November might not give them an environment for their potential expression and, (ii) as the duration of the many high-yielding varieties have increased over the years, which exposes them to heat stress toward the terminal stage and therefore, might not be able to realize their full yield potential in November seeding (Figures 6A–D). To make the evaluation more competitive, we added C306, a variety released for early sown conditions with comparatively long duration, and replaced HDCSW 16 with HDCSW 18, a variety released for early sown conditions. Some of the other varieties like Kalyan Sona, Sonora 64, DBW 17, and WH542 were dropped due to their less strong or adverse response to early seeding. This experiment with 10 released varieties was carried out during 2015–16, 2016–17, 2017–18, and 2019–20 under CA condition by seeding around October 25, an early sowing condition for wheat in India. In this experiment, the dose of fertilizer was the same except for 120 kg N ha−1 during the first 2 years and 150 kg N ha−1 in the last 2 years, and the mode of fertilizer application was kept the same as in Experiment 1. Under the no-till condition, no support was provided with the non-shade net as CA generally supports high biomass without the incidence of lodging even for tall varieties. In the case of the experiment under CA conditions, glyphosate was used before seeding of wheat crop to kill all germinated weeds.
In both experiments, the incidence of wheat rusts and aphid infestation were controlled with a prophylactic spray of a fungicide, propiconazole 25 EC, and pesticide, imidacloprid @ 20 g active ingredient (a.i.) per ha. Weed infestation was controlled either manually or by the application of selective herbicide as per need arisen. In all growing seasons, efforts were made to create a non-yield-limiting environment. Both the border rows and the remaining four central rows were hand-harvested, threshed, dried, and weighed separately to record grain yield (GY) as tons ha−1 at a maximum of 12% seed moisture content. The traits, 50% days to heading (DH), days to physiological maturity of dry matter (DM), plant height (PH), ear length (EL), number of tillers, number of GPS, thousand-grain weight (TGW), peduncle length (PL), coleoptile length (CL), spikelets per spike (SPS), total biomass, and GY were measured in different trials. The data on DH and DM were recorded as per Zadocks stage 59 and Zadoks stage 89, respectively, when more than half of the tillers exhibited heads out and the day when more than half of the spikes in a plot showed yellowing (Zadok et al., 1974).
Plant height was recorded at the time of physiological maturity by measuring the length from the base of the plant to the tip of the main spike excluding awns. The number of florets was counted 5–10 days after anthesis on 10 randomly selected spikes as the number of florets differentiated. The GPS was counted after harvesting and threshing the 10 random spikes from each plot at maturity. After harvesting and threshing, a random sample was taken from each plot and 250 grains were counted, dried in an oven at 65°C for 48 h to measure TGW. The GY and plant biomass were measured after harvesting the central four rows and drying in the field for 4 days with due care to avoid the humidity at night.
Analysis of variance was performed by assuming the effect of varieties, replicate, year, and BP as a fixed effect, characters were taken as a random effect and the interactions of varieties with year, the BP for a particular trait as the mixed effect. The analysis was done using R software (RStudio Team, 2016). Linear, quadratic, and cubical polynomial regression equations were drawn to decipher the effect of the date of release by using the mean data over 4 years in both experiments. The linear equation yi = a + bxi + e was used to estimate the absolute or relative gain (RG) (%) for yield and its component traits, where, yi is the dependent variable (character mean value) and xi is the independent variable (year of variety release), a is the intercept, and e indicates the residual error. Genetic gain, the improvement in average phenotypic value due to selection within a population over cycles of breeding was calculated as a mean improvement in average yield throughout the BP.
A stepwise regression analysis was performed to further identify the key traits responding to yield realization using the backward regression approach by eliminating the least contributing factors. The varieties × environment interaction was estimated and elaborated using additive main effect and multiplicative interaction (AMMI) and genotype main effect and genotype by environment (GGE) interaction models. The best linear unbiased prediction (BLUP)-based factor analysis interaction was used to identify the pattern of gain in yield in the varieties released during the different BPs. All the multivariate analysis was done in R software using metan R-package (Olivoto et al., 2019; Package “metan”, 2020). The correlation and principal component analysis (PCA) were also performed using R-software using ggcorplot (Kassambara, 2019) and factoextra (Kassambara and Mundt, 2019) packages.
To calculate phenotypic or trait plasticity separately in Experiment 1, each trait and variety was quantified by calculating the ratio of the standard deviation of the trait for each variety to the overall phenotypic standard deviation of the population of varieties. Response to competition (RC), which is the ability of plants to respond to available space, was calculated as per the procedure mentioned in earlier publications (Reynolds et al., 1994; Slafer and Savin, 1994).
Analysis of variance revealed significant differences between environment (year), varieties released in different years, and the BPs in India for most of the agronomic traits across years under testing for Experiment 1. The varieties were highly significant for all the phenological and component traits except for the number of tillers (Supplementary Table 1). Similarly, the year or environment was also significant for all the traits except GPS, PL, and CL. The variety × year was non-significant for yield, the number of tillers, and TGW and significant was for all other traits. The contribution of the BP toward the genotypic sum of squares was the largest for yield, biomass, PH, PL, and CL, and quite large for DH, DM, and TGW. The BP could significantly explain the variation in all the traits except the number of tillers. The BP × year was significant for biomass, PH, PL, and CL. The varieties within the period were found significant for DM, TGW, GPS, PH, SPS, and CL.
Yield averaged over 4 years showed consistent improvement since the beginning of the twentieth century when the initial variety NP4 was released for cultivation. The BLUPs estimated over 4 years of replicated GY data also indicated the same trend (Figure 1). The average GY of 2,949 and 3,253 kg ha−1, respectively, of the varieties NP4 (1905) and C591(1936) bred in the first breeding period (BP-I, Table 1) shows a remarkable gain in yield within the BP-I representing pregreen revolution breeding activities. The next gain was brought by the introduction of dwarf wheat varieties like Sonora 64 (3,618 kg ha−1) and Kalyan Sona (3,650 kg ha−1), representing the second breeding period (BP-II), and the gain in GY was equal to what we observed from NP4 to C 591. However, a quantum jump in productivity potential was brought about by indigenously bred wheat varieties like HD 2009 (4,419 kg ha−1) and WL 711 (4,446 kg ha−1), representing the third breeding period (BP-III). In the next decade and during the fourth breeding period (BP-IV), the variety HD 2329 was released for cultivation, whose average productivity realized in our experiments was 4,586 kg ha−1. This variety was highly tolerant to lodging because of its comparatively short stature and solid stem at the base. The lodging resistance provided opportunities to the farmers for increasing fertilizer application and realizing better yield. HD 2329 became the first indigenously bred wheat mega varieties occupying a very large area in NWPZ. In the subsequent years representing the fifth breeding period (BP-V), HD 2329 was replaced by Veery lines (IBL/IRS translocation) like PBW 343 and WH 542 with nominal gain in the yield. The yield gain of PBW 343 and WH 542 over HD 2329 was more apparent during 2018–19 when the urea application was increased to 150 kg ha−1. PBW 343 and WH 542 are of comparatively longer duration and their yield gain is probably more reflected in the crop year with the comparatively longer winter season. Farmers of Haryana and Punjab states in NWPZ realized a quantum jump in yield by seeding PBW 343 at the end of October or the first week of November against the middle of November seeding practiced for HD 2329. Varieties PBW 550 and DBW 17 released during the sixth breeding period (BP-VI) were not able to completely replace PBW 343 and WH 542, thus giving a scope for the next quantum jump. The biggest gain in GY was thus realized through recent mega varieties like HD 2967 and HD 3086 released during the seventh breeding period (BP-VII), which simultaneously replaced three varieties namely PBW 343, DBW 17, and PBW 550. Across varieties, biomass has ranged between 8,766 kg ha−1in NP4 to 14,235 kg ha−1in HDCSW 16 and it has increased linearly over the years at the rate of 43.60 kg ha−1 yr−1.
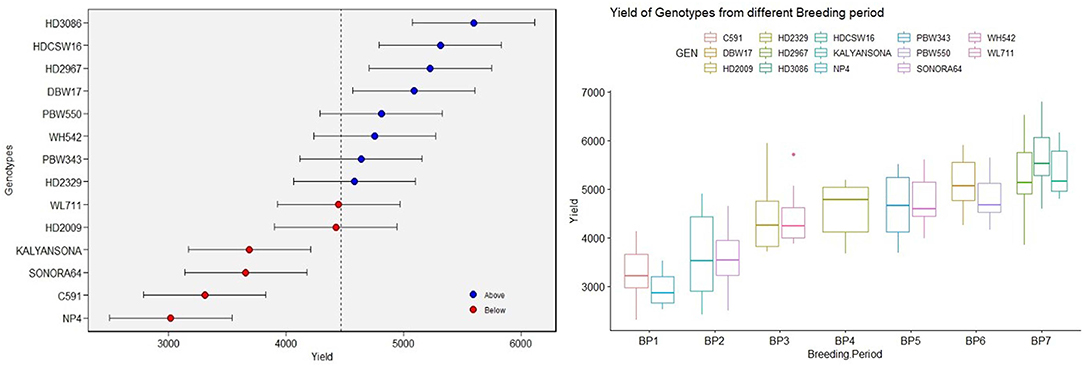
Figure 1. The box plot [based on the best linear unbiased prediction (BLUP)] of yield over varieties under timely sown conditions and yield of varieties from different breeding periods.
The Pearson's correlation coefficients (Figure 2) between the traits under study indicated a very strong positive correlation of yield with DM and biomass followed by TGW, DH, and GPS. The negative correlation of the yield was observed with PL, PH, and CL.
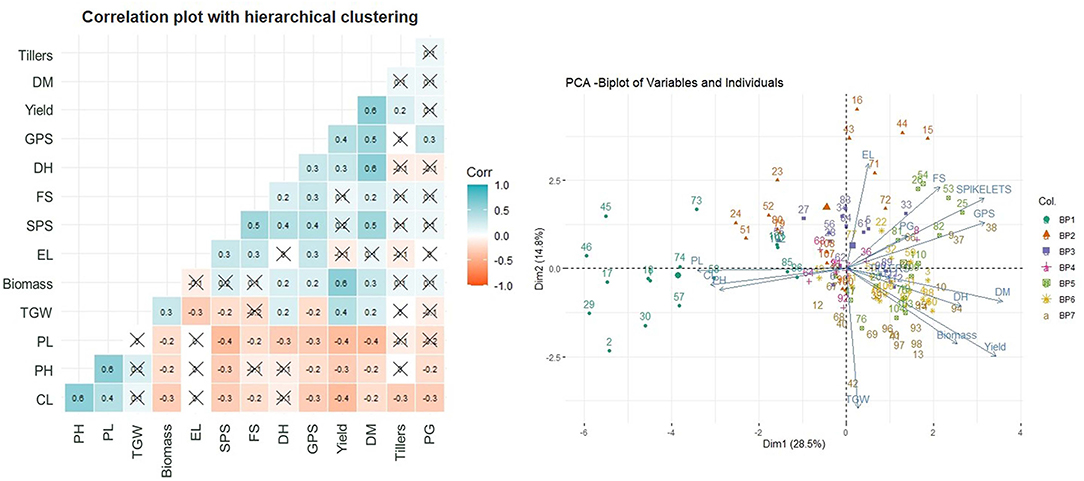
Figure 2. Correlation plot with hierarchical clustering of traits and principal component analysis (PCA).
The acute angle between the Eigenvector of yield with DM, biomass, and DH and also between themselves, indicate the relatedness in these traits through PCA (Figure 2). The TGW and GPS though have an acute angle of Eigenvectors with yield but have an obtuse angle with each other indicating exclusiveness of these traits. The data points of varieties bred in a given BP are largely following the grouping within the BP with some overlapping from BP-V to BP-VII.
The AMMI analysis showed a significant genotype and G × E interaction for GY (Supplementary Table 2). The AMMI biplot analysis could differentiate among the environments and the varieties as it combines the analysis of variance of genotypes and the environment main effects with PCA of the genotype environment interaction (GEI) into a unified approach.
The multiple regression analysis models identified DH, DM, TGW, PH, GPS, and biomass as highly significant traits while the number of tillers and CL were found non-significant. BP as a variable explained the larger amount of variance with an R2-value of 69% largely because of compounded inclusion effect of DH, PH, biomass, EL, and number tillers. The stepwise regression analysis (Supplementary Table 3) suggested for DM, biomass, TGW, CL, GPS, PH, and EL traits and thus explaining variance up to the tune of 65%.
Analysis of regression detected a significant association between phenological stages and the year of release. Slopes of DH and DM vs. year of release were significantly >0 (Figures 3A,B). Association was much stronger between DM vs. year of release than with DH. The DH and DM have increased linearly since 1905 in Indian wheat cultivars. In the last 100 years, mean heading has been delayed at the rate of 0.09 days per year or 0.09% per year and the recent varieties head 4–12 days later than the initial variety, NP4. On the other hand, crop duration increased linearly at the rate of 0.010 days per year delaying the maturity by 5–13 days in the recent varieties. Polynomial regression shows a declining trend in both DH and DM after HD 2967, released in 2011. Both of these traits, however, are highly influenced by the environment and the interaction of varieties × year was very strong.
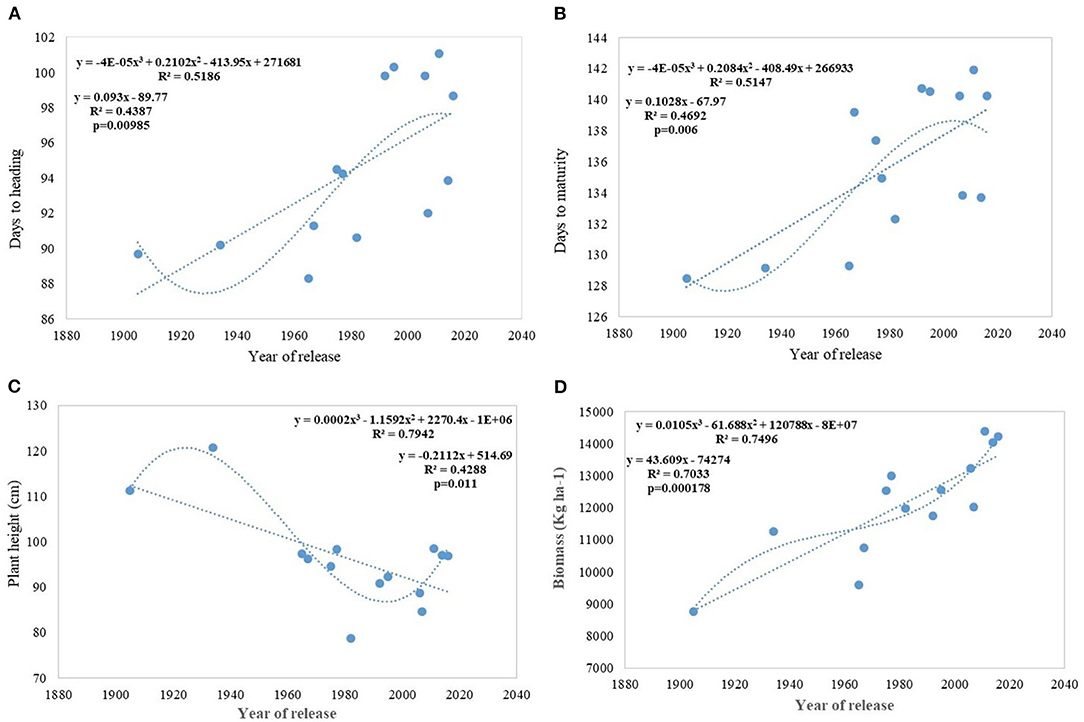
Figure 3. Regression equation between phenological traits and yield component traits against the year of release. (A) Days to heading, (B) Days to maturity, (C) Plant height (cm), (D) Biomass (Kg ha−1).
Regression analysis showed that the year of release accounted for 90% of the variation in mean gain yield data of varieties released in different years and the increase in yield was linear over the years (Figure 4D). An absolute genetic gain for GY in the last 115 years is around 24.27 kg ha−1. Relative genetic gain has been found around 0.544% per year (over the average of all varieties) (Figure 5A) and 0.822% per year if calculated by taking NP4 as a base (Figure 5B). PH (Figure 3C) was also affected by both varieties and year; however, there was no significant varieties × year interaction. plant height ranged from 79 cm for HD 2329 to 121 cm of C 591 and the linear regression revealed a linear reduction in height with the year (p = 0.005); however, the cubical polynomial regression indicating increase in height of recent varieties significantly improved the relationship with R2-value as high as 0.7942.
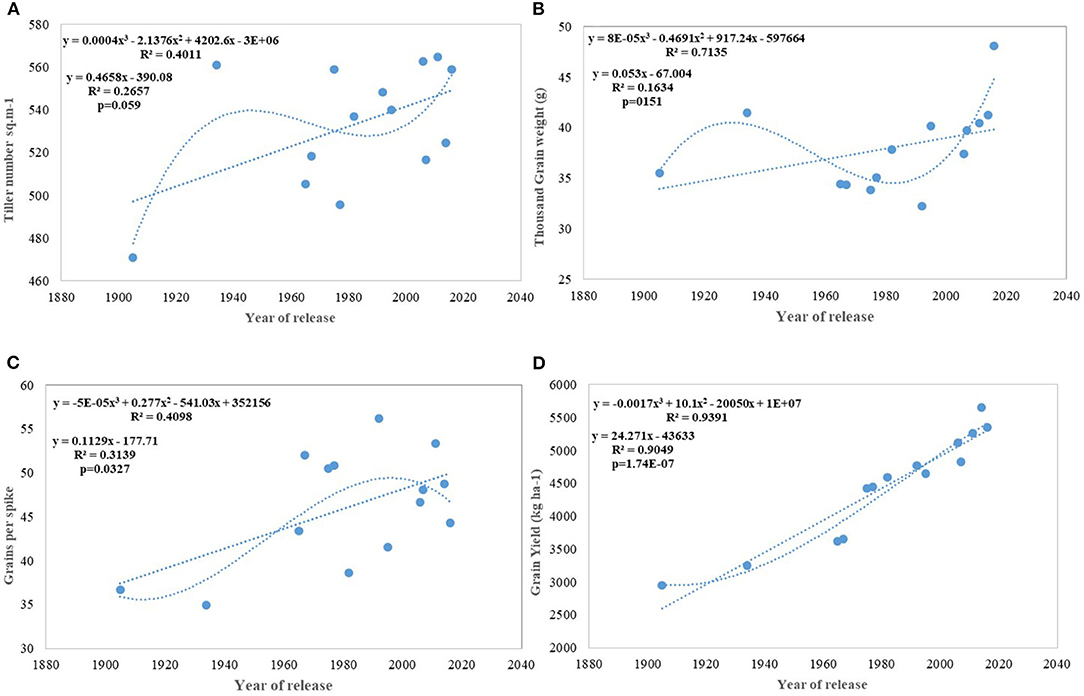
Figure 4. Regression equation between yield and its component traits with the year of release of wheat cultivars. (A) Tiller number per sq m−1, (B) Thousand grain weight (g), (C) Grains per spike, (D) Grain yield (Kg ha−1).
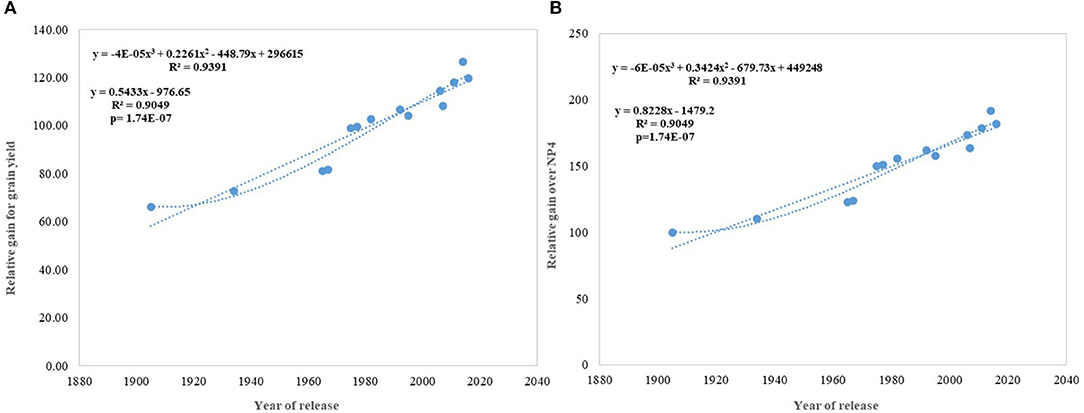
Figure 5. Relative genetic gain over the average of all varieties and NP4 as a base variety. (A) Relative gain for grain yield, (B) Relative gain over NP4.
Results for productive tillers m−2 shows that there has been a continuous linear improvement in spike m−2; however at a very slow pace of 0.46 tillers per year and with comparatively larger standard error (±25.78 tillers) (Figure 4A). Similarly, GPS (Figure 4C) also showed a linear increase over years at the rate of 0.112 GPS (p = 0.037) with the RG being 0.245% in the last 115 years. Flowering nodes have no linear relationship; however, polynomial regression shows that the flowering nodes first increase and then slightly drops. Interestingly, TGW (Figure 4B) showed no linear relationship but cubical polynomial significantly improved the relationship between TGW and the year of release (p = 0.01). In a curvilinear relationship, the year of release accounted for 71.3% of the variation in TGW.
The relative annual rate of growth for biomass was 0.358%. Like GY, the biomass production also does not indicate any saturation and the biggest gain (0.737) was realized after 1992 (WH 542). It was interesting to note that, both linear and cubical polynomial showed a significant relationship between biomass (Figure 3D) and the year of release. The HI has also increased linearly with the year of release from 0.35 in NP4 and 0.29 in C 591 to 0.41 in WH 542 released in 1992 and since then, there is no improvement in HI. The absolute and RG per year for HI was found to be 0.0007 and 0.373%.
We carried out this experiment with two assumptions: (i) that pregreen revolution cultivars in the absence of irrigation condition used to be sown in mid-October under conserved moisture conditions (Yadav et al., 2017), and their sowing in November might not give them an environment for their potential expression and (ii) as the duration of the many high yielding varieties have increased over the years, which exposes them to heat stress toward the terminal stage and therefore, might not be able to realize their full yield potential in November seeding (Figures 6A–D). To make the evaluation more competitive, we added C 306, a variety released for early sown rainfed conditions, and replaced HDCSW16 with HDCSW18, a variety released for early sown conditions. Some of the other varieties like Kalyan Sona, Sonora 64, DBW17, and WH542 were dropped. The absolute genetic gain realized in the experiment from 1905 till 2016 was 29.28 kg ha−1 yr−1, at least, 5 kg higher than that realized under timely sown conditions. However, the relative genetic gain measured over average value was almost similar in both sets of the experiment. The trend for a linear increase in the biomass is stronger under early sown conditions (Figure 6D) and seems to be a major factor besides the GPS for yield gain (Figure 6B). The TGW which was showing a slight increasing trend in timely sown condition clearly shows a declining trend in early sown condition (Figure 6C), mainly because the GPS in the varieties requiring mild vernalization is strongly increased leading to some compromise in TGW due to interfloret competition. The duration of stem elongation phase under the early sown condition in mild vernalization requiring variety is prolonged, resulting in a higher GPS. The absolute (Figure 7A) and relative genetic gain (Figure 7B) over the years showed a significant relationship with the GY and the year of release under early sown conditions.
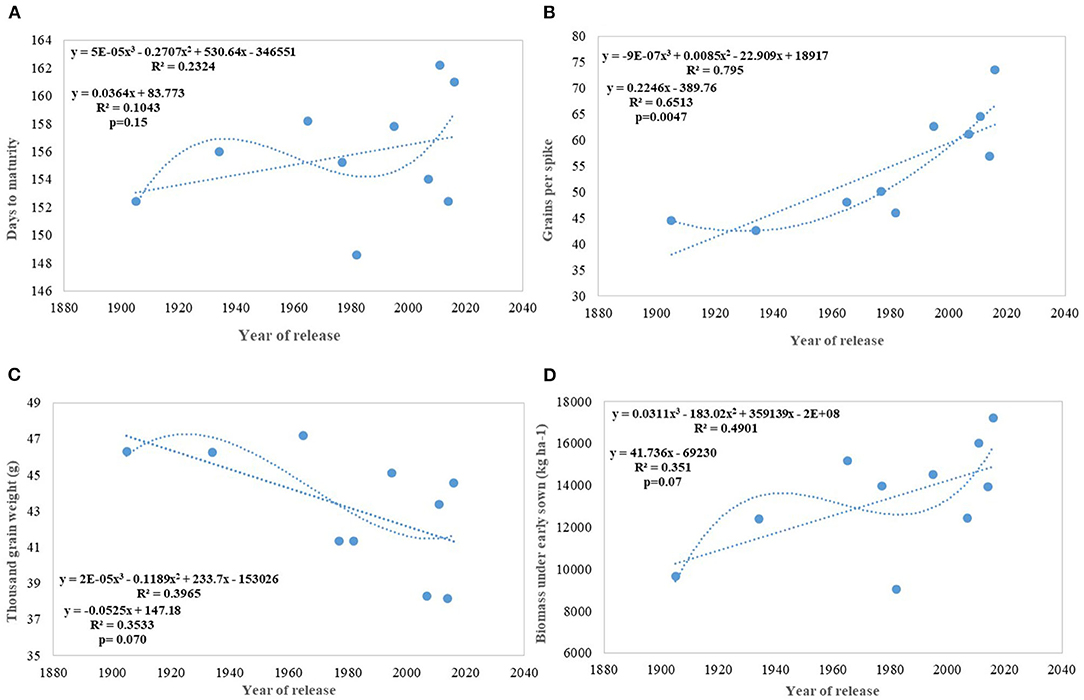
Figure 6. Regression equation between phenological and yield component traits against the year of release under early sown conditions. (A) Days to maturity, (B) Grains per spike, (C) Thousand grain weight (g), (D) Biomass under early sown (Kg ha−1).
On average, border rows yielded 15% more than central rows of the plot largely because of more space availability between two adjacent plots. No trend was observed for the RC in the released cultivars for GY (Figure 8A). However, among the early cultivars, C 591 was highly competitive and dwarf wheat introductions were highly non-competitive for yield. Comparatively broader leaves in WL 711 and HDCSW 16, earlier canopy cover in PBW 550, compact plant type in HD 2329, and more ground cover in WH 542, and DBW 17 were probably providing them with a competitive advantage. In the present experiment, the response to the competition was calculated to assess whether we are moving toward communal varieties or not (Table 2). Though there is no trend, most of the dwarf varieties are showing comparatively lesser RC for GY. There is no linear trend for any of the component traits for RC except for biomass and tiller number (Figures 8B–D). Compilation and analysis of information related to the plasticity of various GY forming traits can enhance our understanding of why a particular cultivar becomes a mega cultivar. In Indian wheat cultivation history, probably four real mega cultivars can be defined during different period and these are Kalyan Sona in the 70s, HD 2329 in the 80s, PBW 343 in the 1990s, and now HD 2967 and HD 3086 in the second decade of the twenty-first century. All these cultivars except HD 2329 have comparatively higher GY plasticity. Plasticity has come from different traits in different cultivars i.e., from biomass, tiller number, and grain number in Kalyan Sona, from grain weight and grain number in PBW 343, and GPS in HD 2967 (Table 3). Overall, important varieties like Kalyan Sona, HD2009, HD2967, HD3086, and PBW 343 which occupied a comparatively larger area among the mega varieties, are highly plastic and are able to respond well to the improvement in the production environment.
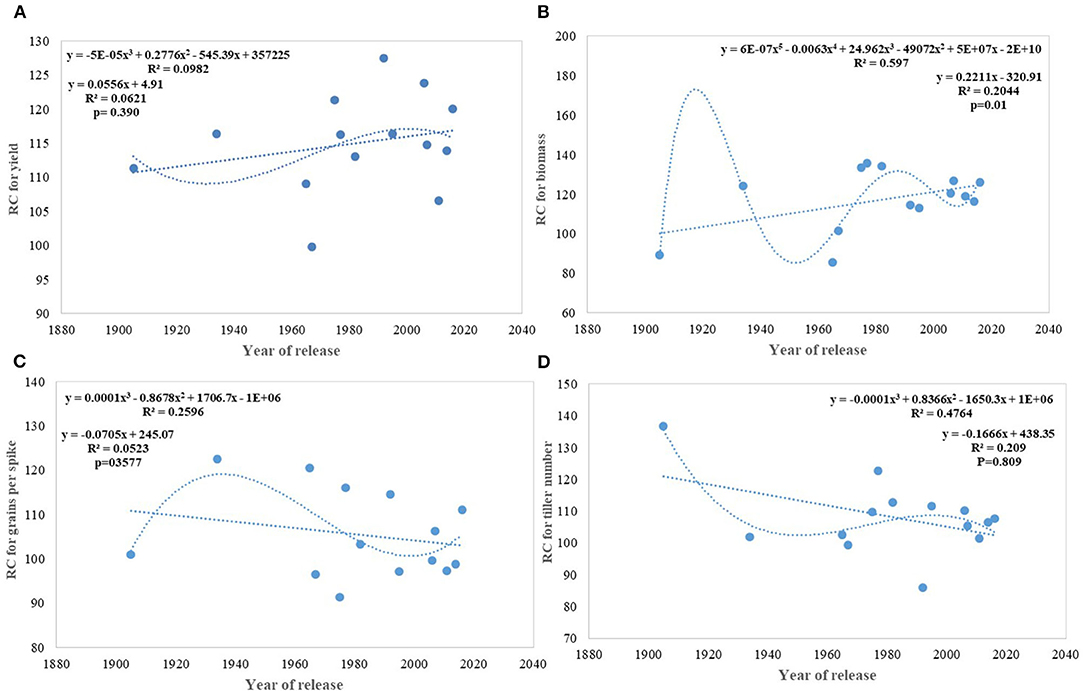
Figure 8. Relative competitiveness for yield and component traits against the year of release. (A) yield, (B) biomass, (C) grains per spike, (D) tiller number.
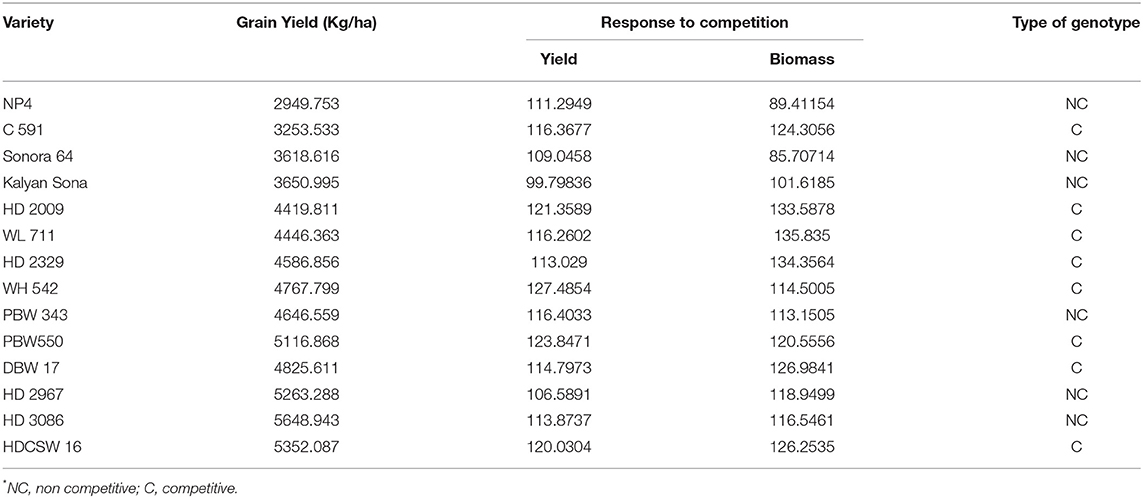
Table 2. Grain yield and response to competition for yield and biomass in cultivars released over the years.
Our study, like many other earlier studies (Sanchez-Garcia et al., 2013; Zhang et al., 2016), shows a significant difference in GY and related yield component traits in the varieties released in different years and BPs. Having each BP represented by at least one variety, a variance analysis revealed little or no differences among the varieties within each BP for yield, biomass, tillers per plant, and the number of spikelets. Varieties within the BP were though differing for DM, PH, CL, GPS, and TGW indicating that no single phenological criterion was adopted by the breeders for developing varieties in a specific BP.
It is a common perception among the breeders of the current era that yield gain in wheat started with the introduction of dwarf varieties in 1964–66; however, the yield of C 591 over NP4 and also of C 306 in early sown experiment refutes this assumption and shows a remarkable gain within the first BP, representing the potential impact of pregreen revolution breeding activities. Current analysis shows that the next quantum jump in productivity potential was brought about by the release of the first-generation indigenous breeding products like WL 711 and HD 2329 by involving dwarf introduction in the crossing. Yield plateau imposed by HD2329, which remained dominating for 10–15 years was subsequently broken by the release of Veery lines like PBW 343 and WH 542 with IB/IR translocation. Farmers in the two major states of NWPZ amplified the realized gain by seeding PBW 343 slightly early because of its mild vernalization requirement and using a slightly higher dose of nitrogenous fertilizer. PBW 343 comprising Yr23 and Yr9 genes for yellow rust resistance became susceptible to yellow rust pathotype 78S84 and incidence and losses due to yellow rust started building up in the first decade of the twenty-first century. Subsequent releases like DBW 17 and PBW 550, could not completely replace these varieties. The formal release of HD 2967 in 2011 was a great relief to the farmers and within a short span of 3–4 years, it simultaneously replaced PBW 343, PBW 550, and DBW 17. HD 2967 became immensely popular among the farmers with its area crossing over 10 M ha in the Northern Plains and received unprecedented breeder seeds indents of 4,000 q for the year 2019–20 (ICAR-IIWBR, 2019). Yield gain in the Northern Plains was further consolidated by the subsequent release of HD 3086.
In contrast to studies on winter wheat (Barutçular et al., 2006; Peltonen-Sainio et al., 2009a; Graybosch and Peterson, 2010) and many other studies on spring wheat (Fischer and Edmeades, 2010; Matus et al., 2012; Beche et al., 2014; Maureen, 2019), where from the beginning of the twenty-first century, yield gain has either started to slow down or even plateaued, we found a strong linear increase in wheat yield since 1905 with no indication of yield saturation (Figures 4D, 5A,B). An absolute genetic gain of 24.27 kg ha−1 y−1 with a relative value of around 0.544% per year in a century-long period is equal or better than many other breeding programs in the world (Fischer and Edmeades, 2010; Sadras and Lawson, 2011). Early sown experiment revealed a comparatively higher absolute gain of 29.28 kg ha−1 yr−1 (Figure 6D) but with a similar relative value (0.544 %).
Regression analysis indicates DM and DH (Figures 3A,B) in Indian wheat varieties have linearly increased over the years significantly. This is in contrast to earlier reports that the yield gain in the Mediterranean environment has been due to earlier heading and other studies showing no chronological trend (Slafer et al., 2009). Analysis of these varieties for physiological traits like chlorophyll content (Gupta et al., 2017) shows no change or chronological trend and higher capturing of radiation due to increased duration rather than improved radiation use efficiency (Shearman et al., 2005; Sadras and Lawson, 2011) is the major reason for the increased biomass and yield gain. Linear increasing trend for leaf area index (LAI) along with near-perfect LAI 5.94 with semi-erect leaves observed in all-time mega variety HD 2967 (Gupta et al., 2017) indicated the importance of physiological traits, plant architecture, and crop canopy beside yield component in yield maximization. Polynomial regression fitting quadratic function of crop duration vs. year of variety release shows a declining trend in both DH and DM after the release of HD 2967 in 2011. Increased duration, however, introduced instability in the production over the years due to sudden rise in temperature toward the terminal stages (Yadav et al., 2019), and therefore further increase in the duration without adjusting the sowing time will be highly unrewarding. Highly significant environment and varieties × year interaction for both of these traits (DH and DM) indicated the same. The higher realization of yield gain in the early sown experiment was also largely because of the longer duration available to all varieties along with delay in the heading because of optimum combination of vernalization alleles with no reduction in grain filling duration in the adapted varieties. It is also realized that the weak linear trend for the increased duration and delayed heading (DH) in the early sown experiment was because of the presence of both mild vernalizations requiring and non-vernalization requiring genotypes in the study. However, still, DH accounted for 54% of yield variation in the early sown experiment and interestingly polynomial regression shows no saturation of relationship between delayed DH and higher yield.
Biomass has been one of the most important parameters influenced by wheat breeding activities in India during the twentieth and twenty-first century as the year of release alone explained 70% (p = 0.000178) of biomass variation in the released varieties tested under timely sown condition. Biomass has increased linearly at the rate of 43.60 kg ha−1 yr−1 in century-long analysis; however, fractured analysis shows the biggest gain (97.12 kg ha−1 yr−1) after 1992. As discussed in the earlier section, biomass-like GY increased linearly over the years due to a linear increase in the duration along with DH. Delayed heading explained comparatively more variation in biomass than days to maturity, probably because of the conflict introduced by forced maturity in longer duration varieties (Figures 9A,B). Quadratic equation fitted for both phenological traits vs. biomass, not only improved the level of fitness but also explained more variation.
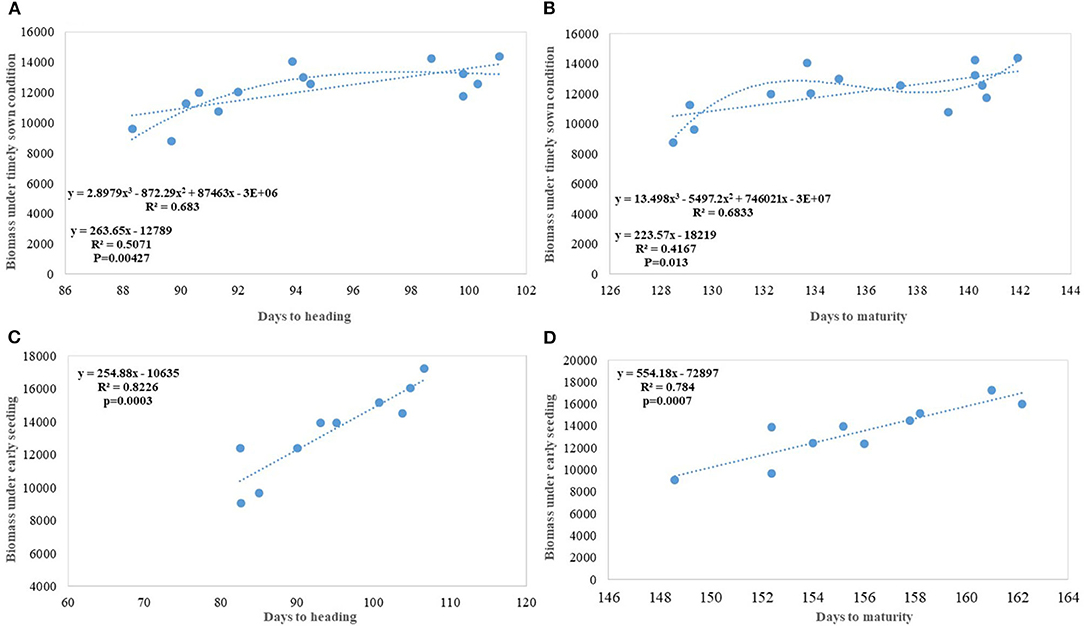
Figure 9. Increase in biomass due to increased duration and delayed heading (A) Days to heading (DH) vs. biomass under timely sown condition; (B) Days to maturity vs. biomass under timely sown condition; (C) DH vs. biomass under early seeding; (D) Days to maturity vs. biomass under early seeding.
In earlier studies, it was reported that biomass has increased due to improved photosynthesis, better stomatal conductance, higher leaf chlorophyll content (Maureen, 2019), and improved radiation-use efficiency (Bustos et al., 2013); however, in our earlier study, we found no trend for total leaf chlorophyll content and declining trend for stomata number (Gupta et al., 2016). In the present study, an increase in the biomass has been largely attributed to increased duration with DH (Figures 9A–D) and to some extent improved higher LAI (Gupta et al., 2016). The contribution of biomass toward higher yield realization has earlier been reported by several studies (Slafer et al., 2009; Sadras and Lawson, 2011; Bustos et al., 2013; Aisawi et al., 2015; Gao et al., 2017) and our study further corroborate that. New cultivars in the Indian wheat breeding program have biomass gain parallel to Chinese wheat cultivars (Gao et al., 2017) (@ 62.6 kg ha−1 yr−1). The role of duration in increasing the biomass becomes more evident in early sown CA experiment where the absolute genetic gain for biomass in the varieties released after 1982 happened @ 175 kg ha−1 yr−1 and the percent variation in biomass explained by DH and DM increased significantly (Figures 9C,D).
For HI, our results are in contrast to many other studies showing that yield gain was largely because of the increase in HI with no change in biomass production (Austin et al., 1989; Sharma et al., 2012; Wu et al., 2014). Researchers also feel that probably HI has already reached a theoretical limit and a further increase in HI is not feasible (Slafer and Andrade, 1993). Our results are, therefore, clearly in agreement with the hypothesis that HI played an important role for many years after the green revolution, however an increase in biomass is mainly responsible for yield gain in recent varieties (Foulkes et al., 2009). Harvest index in Indian wheat varieties reached a maximum value of 0.419, which is well below the theoretical limit of 0.60 as proposed (Foulkes et al., 2009). The HI-value observed in our experiment under the Indian conditions has shown an almost similar increase as in many other countries like around 0.25–0.55 in China (Zhang et al., 2016; Gao et al., 2017), 0.26–0.42 in Spain (Royo et al., 2007), 0.21–0.43 in Australia (Flohr et al., 2018). In most of the varieties, the trade-off between heavy head and lodging in high biomass wheat crops along with forced maturity due to rise in temperature toward terminal stages generally led to the poor realization of HI under Indian condition like many other studies (Fischer and Edmeades, 2010; Foulkes et al., 2011; Yadav et al., 2017).
Productive tillers m−2 under timely sown conditions show a continuous linear improvement in spike m2, though at a very slow pace of 0.46 tillers per year and with comparatively larger standard error (±25.78 tillers) (Figure 4A). Similarly, GPS (Figure 4C) also showed a linear increase over years at the rate of 0.112 GPS (p = 0.037) with the RG of 0.245% per annum in the last 115 years. Wheat GY is largely sink-limited and yield gain particularly in winter wheat came through increased grain number against larger grain size (Shearman et al., 2005). In contrast, in spring wheat varieties of India, cubical polynomial regression improve the grain weight (Figure 4B) in recent years (p = 0.01). In a curvilinear relationship, the year of release accounted for 71.3% of the variation in seed weight. It is interesting to note that trend for yield component traits under early sown CA conditions is not consistent with timely sown tilled conditions for the number of spikes m−2 and grain weight, largely because of the conflict introduced by vernalization gene, and probably by the role of stem reserve mobilization under stress induced by a rise in temperature toward terminal stage under timely sown condition. Biomass and GY potential in wheat is largely set by stem weight and the number of tillers produced per unit area (Tausz-Posch et al., 2015) particularly under high yielding environment (Sadras and Rebetzke, 2013). The trend in tillering potential under early sowing conditions was probably thwarted by the inclusion of C 306, a high tillering variety released in 1965, and HDCSW18, a moderate tillering variety released in 2016. The strongest component for yield increase under both sets of production conditions is GPS like many other studies (Aisawi et al., 2015; Flohr et al., 2018; Liu et al., 2018) besides biomass and phenological trait. The GPS under early sown condition shows more strong linear increase than timely sown condition largely because of the increased spikelet fertility under early sown condition. The negative correlation between the number of GPS and TGW suggested as a stumbling block (Sadras and Lawson, 2011; Bustos et al., 2013) for further gain in yield was not apparent in our study as a negative correlation was offset by a prolonged vegetative phase in recent varieties supporting more number of grains and longer duration providing optimum grain-filling period. The number of GPS has increased linearly because of the increase in the number of spikelet and floret fertility, though with no change in spike length in contrast to the hypothesis proposed (Lopes et al., 2012). It indicates that both source and sink are at optimum, at least in the recently developed varieties under early sown condition. The declining linear trend for grain weight under early sown condition, however, was mainly because earlier varieties with lesser fruiting sites and enough reserve were able to fully exploit the available grain capacity to imbibe stem reserve and limitation of individual grain capacity in the recently released variety HD 3086. No strong difference in grain weight under early sown and timely sown condition in one of the recent variety like HD 3086 indicates its limitation about individual grain sink strength .Another probable reason for the declining trend for grain weight in early sown condition (Figure 6C) in contrast to timely sown can be due to prolonged stem elongation phase induced by mild vernalization requirement in some of the varieties resulted in a higher number of grains through increased number of spikelets and higher number of florets per node and interfloret competition at each node reduced the grain weight in such varieties.
The linear increase in peduncle girth under timely sown condition also supports our earlier assumption that recent varieties have probably no limitation of source and increase in biomass besides tiller number is also being contributed by increased stem girth. Similarly, PH, CL, and PL above the flag leaf has declined linearly over the years largely because of dwarfing alleles like Rht-B1b, Rht-D1b, Rht-D1c, and Rht8 being used just like another international breeding program (Zheng et al., 2011; Green et al., 2012; Lopes et al., 2012; Joudi et al., 2014; Zhang et al., 2016; Chairi et al., 2018) and their associated effect on CL, PL, and PH (Rebetzke et al., 2011, 2012) and better resource partitioning. The quadratic equation further improved the relationship (R2 = 0.7942) and it indicates an increasing trend for PH in the recently released varieties. We, therefore, assume that the biggest jump in yield gain achieved in the recent years was largely through an increase in biomass contributed by an increase in height, stem girth, and more tillering. Reduction in CL, however, introduced uncertainty in crop production because of its unsuitability for deeper seeding in early sown crop under comparatively higher mean temperature (Yadav et al., 2017) as high temperature depletes the soil moisture quickly in upper profile even under irrigated condition. Generating information on the association of other dwarfing genes with agronomically relevant traits like CL and their utilization in the breeding program can resolve these conflicts (Yadav et al., 2010), as only a limited number of dwarfing genes have been exploited in wheat breeding (Chen et al., 2015).
A very strong positive correlation of yield with DM and biomass followed by TGW, DH, and GPS and, a negative correlation with PL, PH, and CL were found. Historical studies have shown that traits, such as GPS, biomass, HI, and reduced PH are positively associated with the yield (Shearman et al., 2005; Xiao et al., 2012). The PCA divided the whole dataset into 10 principal components and the first two principal components together represent only 43.3% variation indicating the strong importance of traits explained by other principal components. DH, DM, TGW, PH, GPS, and biomass were identified through the multiple regression analysis models as highly significant traits. Based upon the multiple regression analysis, the traits were selected to establish the relationship between the average productivity of the varieties released in NWPZ since 1900 and the year of release of the variety.
Under this study, we targeted to evaluate whether yield gain has any association with the competitive ability (better performance of varieties in border rows when compared to central rows) of the varieties as proposed under the ideotype breeding concept (Donald, 1981; Reynolds et al., 1994; Sadras et al., 2000; Denison, 2009) due to impaired detection and response to neighbors along with an inability to imbibe the increased available resources (Aphalo and Ballare, 1995; Ugarte et al., 2010). Response to competition was high in older tall varieties like C591 and C306, giving them more scope for weed suppression and a positive response to the availability of space. Over the years, we found no trend in RC particularly for yield and biomass; however, the component traits like GPS and tiller number show a declining trend. Polynomial regression shows that recent varieties are either neutral phenotype or with slightly higher competitive ability than their immediate predecessor for biomass production and yield. Under changing climatic conditions, it is becoming highly important that cultivars should have the plasticity to accommodate environmental variation. It indicates the ability of the varieties to respond to the improvement or deterioration in the production environment. Four out of five real mega cultivars, Kalyan Sona, PBW 343, HD 2967, and HD 3086 except HD 2329 showed comparatively higher yield plasticity, which conclusively came from different component traits among these cultivars. Polynomial regression analysis shows that an increase in yield plasticity in the recently released varieties comes largely through grain number and seed weight (Figure 10).
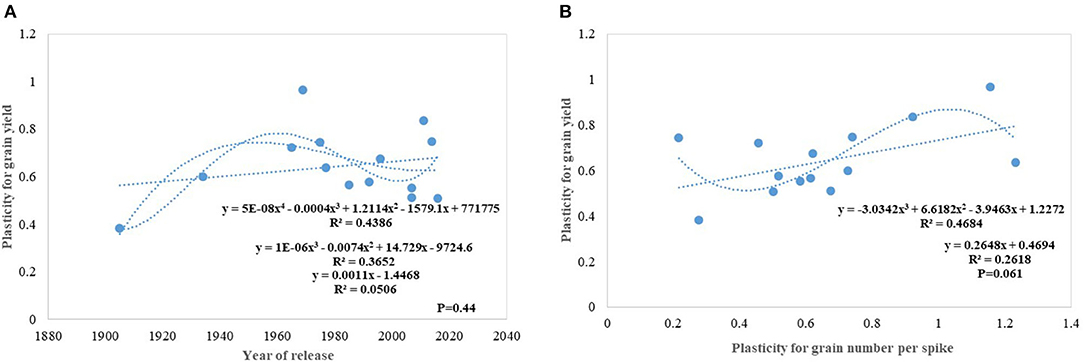
Figure 10. Trait plasticity for grain yield (A) and its linear relation with grain number (B) in wheat genotypes.
The present study establishes the strong contribution of plant breeding activities carried out in the last 115 years in increasing the wheat productivity in the northern plains of India and there is no indication of yield plateauing in the near future. The yield gain rate has improved in the last three decades. It is very interesting to note that, the yearly gain is robust, linear, and equal to the gain achieved in other parts of the world. Under the timely sown condition, which occupies the maximum area in India, the yield gain came largely through increased crop duration with the delayed heading and biomass. Moreover, an early sown experiment showed nearly 20% higher yield gain per year largely through higher biomass and a greater number of GPS. This has proved an immense impact of phenological traits by indirectly improving the overall genetic gain in terms of many agronomic traits. In a developing country like India, where large trained manpower is available, investment in traditional plant breeding can still be highly rewarding. Though the introduction of dwarf wheat leads to a gain in productivity, the major gain comes through subsequent rounds of breeding involving these varieties and indigenous local strains in the crossing programs. The next yield jump came through Veery-derived varieties, particularly PBW 343 utilizing mild vernalization requiring genes for higher biomass production. Yield being a function of biomass and its subsequent partitioning to grain, a linear increase in HI and biomass is expected. However, our results show that HI during the last three decades has remained almost stagnant and yield gain came through improved biomass. Tiller number shows a linear increase under timely seeding condition but under early sown, it remained almost unchanged. Among the yield component, grain numbers make a contribution toward yield improvement both in the early sown and timely sown conditions. The GPS improved because of a pleiotropic effect of dwarfing gene on spike fertility (He et al., 2012) as well as due to the improved availability of photosynthate to the developing florets resulting in lesser floret abortion and its selection by the breeder (Álvaro et al., 2008). Phenological manipulation, which seems to be saturating under timely sown condition, the trend of latest varieties utilizing mild vernalization genes provide some more scope for yield gain through its manipulation under early sown condition. Modern varieties are showing either neutral behavior or a slight increase in RC and therefore more in-depth study is required to understand the role of varieties, resources, and non-resource environmental cues in modulating plant–plant interactions and their consequences for plant development and crop yield. An increase in biomass and HI happen not simultaneously but in separate phases and therefore, with a strong production of biomass in some of the recent varieties, further improvement in HI can lead to the next jump in GY in wheat. It was also proposed that yield gain can be furthered by crossing complementary varieties exhibiting high biomass and HI (Reynolds et al., 2017). We, hereby propose, in the targeted cross, further gain in yield can be realized by fully exploiting the advantage of crop duration by adjusting the time of seeding, exploring the alternate dwarfing genes that do not reduce CL, optimize the biomass production along with structural changes for lodging resistance, increasing the grain weight through optimized spike morphology without compromising grain number and increasing sugar reserve mobilization toward sink in case of a sudden rise in temperature toward the terminal stage.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
RY and SG conceived the idea. All authors contributed to conducting the experiments and article edits. All authors contributed to the article and approved the submitted version.
The authors would like to thank the Indian Agricultural Research Institute (IARI), New Delhi for facilitating finance to undertake the current research work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
SG is thankful to the Indian Council of Agricultural Research, New Delhi, India for providing fellowship for the master's degree program.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2021.719394/full#supplementary-material
Abeledo, L. G., Calderini, D. F., and Slafer, G. A. (2003).Genetic improvement of yield responsiveness to nitrogen fertilization and its physiological determinants in barley. Euphytica 133, 291–298. doi: 10.1023/A:1025796527208
Aisawi, K. A. B., Reynolds, M. P., Singh, R. P., and Foulkes, M. J. (2015). The physiological basis of the genetic progress in yield potential of CIMMYT spring wheat cultivars from 1966 to 2009. Crop Sci. 55, 1749–1764. doi: 10.2135/cropsci2014.09.0601
Álvaro, F., Royo, C., García del Moral, L. F., and Villegas, D. (2008). Grain filling and dry matter translocation responses to source-sink modifications in a historical series of durum wheat. Crop Sci. 48, 1523–1531. doi: 10.2135/cropsci2007.10.0545
Aphalo, P. J., and Ballare, C. L. (1995). On the importance of information-acquiring systems in plant-plant interactions. Funct. Ecol. 9, 5–14. doi: 10.2307/2390084
Austin, R. B., Ford, M. A., and Morgan, C. L. (1989). Genetic improvement in the yield of winter wheat: a further evaluation. J. Agric. Sci. 112, 295–301. doi: 10.1017/S0021859600085749
Bainsla, N. K., Yadav, R., Sharma, R. K., Sharma, A., Gaikwad, K. B., Kumar, A., et al. (2018). Mechanistic understanding of Lodging in spring wheat (Triticum aestivum): an Indian perspective. Indian J. Agric. Sci. 88, 1483–1495.
Bainsla, N. K., Yadav, R., Singh, G. P., and Sharma, R. K. (2020). Additive genetic behavior of stem solidness in wheat (Triticum aestivum L.). Sci. Rep. 10, 1–9. doi: 10.1038/s41598-020-64470-x
Barutçular, C., Koç, M., Tiryakioglu, M., and Yazar, A. (2006). Trends in performance of Turkish durum wheats derived from the International Maize and Wheat Improvement Center in an irrigated West Asian and North African environment. J. Agric. Sci. 144, 317–326. doi: 10.1017/S0021859606006150
Beche, E., Benin, G., da Silva, C. L., Munaro, L. B., and Marchese, J. A. (2014). Genetic gain in yield and changes associated with physiological traits in Brazilian wheat during the 20th century. Eur. J. Agron. 61, 49–59. doi: 10.1016/j.eja.2014.08.005
Berzonsky, W. A., and Lafever, H. N. (1993). Progress in Ohio soft red winter wheat breeding: grain yield and agronomic traits of cultivars released from 1871 to 1987. Crop Sci. 33, 1382–1386. doi: 10.2135/cropsci1993.0011183X003300060050x
Brancourt-Hulmel, M., Doussinault, G., Lecomte, C., Bérard, P., Le Buanec, B., and Trottet, M. (2003). Genetic improvement of agronomic traits of winter wheat cultivars released in France from 1946 to 1992. Crop Sci. 43, 37–45. doi: 10.2135/cropsci2003.3700
Brisson, N., Gate, P., Gouache, D., Charmet, G., Oury, F. X., and Huard, F. (2010). Why are wheat yields stagnating in Europe? A comprehensive data analysis for France. Field Crops Res. 119, 201–212. doi: 10.ff10.1016/j.fcr.2010.07.012
Bustos, D. V., Hasan, A. K., Reynolds, M. P., and Calderini, D. F. (2013). Combining high grain number and weight through a DH-population to improve grain yield potential of wheat in high-yielding environments. Field Crops Res. 145, 106–115. doi: 10.1016/j.fcr.2013.01.015
Chairi, F., Vergara-Diaz, O., Vatter, T., Aparicio, N., Nieto-Taladriz, M. T., Kefauver, S. C., et al. (2018). Post-green revolution genetic advance in durum wheat: the case of Spain. Field Crops Res. 228, 158–169. doi: 10.1016/j.fcr.2018.09.003
Chen, S., Gao, R., Wang, H., Wen, M., Xiao, J., Bian, N., et al. (2015). Characterization of a novel reduced height gene (Rht23) regulating panicle morphology and plant architecture in bread wheat. Euphytica 203, 583–594. doi: 10.1007/s10681-014-1275-1
Cox, T. S., Shroyer, J. P., Ben-Hui, L., Sears, R. G., and Martin, T. J. (1988). Genetic improvement in agronomic traits of hard red winter wheat cultivars 1919 to 1987. Crop Sci. 28, 756–760. doi: 10.2135/cropsci1988.0011183X002800050006x
Crespo-Herrera, L. A., Crossa, J., Huerta-Espino, J., Autrique, E., Mondal, S., Velu, G., et al. (2017). Genetic yield gains in CIMMYT's International Elite Spring Wheat Yield Trials by modeling the genotype × environment interaction. Crop Sci. 57, 789–801. doi: 10.2135/cropsci2016.06.0553
Denison, R. F. (2009). “Darwinian agriculture: real, imaginary and complex trade-offs as constraints and opportunities,” in Crop Physiology: Applications for Genetic Improvement and Agronomy, eds V. Sadras and D. Calderini (Burlington VT: Academic Press), 215–234.
Dewitt, T. J., DeWitt, T. J., and Scheiner, S. M., (eds.). (2004). Phenotypic Plasticity: Functional and Conceptual Approaches. Oxford University Press. Available online at: https://www.researchgate.net/publication/229190614 (accessed February 25, 2019).
Donald, C. M. (1981). Competitive Plants, Communal Plants, and Yield in Wheat Crops. Cambridge: Cambridge University Press.
Donmez, E., Sears, R. G., Shroyer, J. P., and Paulsen, G. M. (2001). Genetic gain in yield attributes of winter wheat in the Great Plains. Crop Sci. 41, 1412–1419. doi: 10.2135/cropsci2001.4151412x
Fischer, R. A., and Edmeades, G. O. (2010). Breeding and cereal yield progress. Crop Sci. 50, S-85–S-98. doi: 10.2135/cropsci2009.10.0564
Flohr, B. M., Hunt, J. R., Kirkegaard, J. A., Evans, J. R., Swan, A., and Rheinheimer, B. (2018). Genetic gains in NSW wheat cultivars from 1901 to 2014 as revealed from synchronous flowering during the optimum period. Eur. J. Agron. 98, 1–13. doi: 10.1016/j.eja.2018.03.009
Foulkes, M. J., Reynolds, M. P., and Sylvester-Bradley, R. (2009). “Genetic improvement of grain crops: yield potential,” in Crop Physiology: Applications for Genetic Improvement and Agronomy, eds V. Sadras and D. Calderini (Burlington VT: Academic Press), 355–385.
Foulkes, M. J., Slafer, G. A., Davies, W. J., Berry, P. M., Sylvester-Bradley, R., Martre, P., et al. (2011). Raising yield potential of wheat. III. Optimizing partitioning to grain while maintaining lodging resistance. J. Exp. Bot. 62, 469–486. doi: 10.1093/jxb/erq300
Gao, F., Ma, D., Yin, G., Rasheed, A., Dong, Y., Xiao, Y., et al. (2017). Genetic progress in grain yield and physiological traits in Chinese wheat cultivars of southern yellow and Huai Valley since 1950. Crop Sci. 57, 760–773. doi: 10.2135/cropsci2016.05.0362
Graybosch, R. A., and Peterson, C. J. (2010). Genetic improvement in winter wheat yields in the Great Plains of North America, 1959–2008. Crop Sci. 50, 1882–1890. doi: 10.2135/cropsci2009.11.0685
Green, A. J., Berger, G., Griffey, C. A., Pitman, R., Thomason, W., Balota, M., et al. (2012). Genetic yield improvement in soft red winter wheat in the Eastern United States from 1919 to 2009. Crop Sci. 52, 2097–2108. doi: 10.2135/cropsci2012.01.0026
Gupta, S., Yadav, R., Gaikwad, K., Kushwah, A., Singh, A. M., and Bainsla, N. K. (2016). Genetic improvement trend analysis for end-use quality characteristics among wheat cultivars of North-Western India. Indian J. Genet. Plant Breed. 76, 137–143. doi: 10.5958/0975-6906.2016.00030.4
Gupta, S., Yadav, R., Gaikwad, K. B., Arora, A., Kumar, A., and Kushwah, A. (2017). Deciphering physiological basis of yield gain in indian wheat cultivars. Cereal Res. Commun. 45, 512–524. doi: 10.1556/0806.45.2017.023
He, J., Le Gouis, J., Stratonovitch, P., Allard, V., Gaju, O., Heumez, E., et al. (2012). Simulation of environmental and genotypic variations of final leaf number and anthesis date for wheat. Eur. J. Agron. 42, 22–33. doi: 10.1016/j.eja.2011.11.002
ICAR-IIWBR (2019). Director's Report of AICRP on Wheat and Barley 2018–19, ed G. P. Singh. Karnal: ICAR-Indian Institute of Wheat and Barley Research. 72.
Joudi, M., Ahmadi, A., Mohammadi, V., Abbasi, A., and Mohammadi, H. (2014). Genetic changes in agronomic and phenologic traits of Iranian wheat cultivars grown in different environmental conditions. Euphytica 196, 237–249. doi: 10.1007/s10681-013-1027-7
Kassambara, A. (2019). ggcorrplot: Visualization of a Correlation Matrix using'ggplot2. 2016. Available online at: http://www.sthda.com/english/wiki/ggcorrplot-visualization-of-a-correlation-matrix-using-ggplot2
Kassambara, A., and Mundt, F. (2019). factoextra: Extract and Visualize the Results of Multivariate Data Analyses R Package Version 1.0. 5.999.
Liu, D., Zhang, L., Hao, M., Ning, S., Yuan, Z., Dai, S., et al. (2018). Wheat breeding in the hometown of Chinese Spring. Crop J. 6, 82–90. doi: 10.1016/j.cj.2017.08.009
Lopes, M. S., Reynolds, M. P., Manes, Y., Singh, R. P., Crossa, J., and Braun, H. J. (2012). Genetic yield gains and changes in associated traits of CIMMYT spring bread wheat in a “historic” set representing 30 years of breeding. Crop Sci. 52, 1123–1131. doi: 10.2135/cropsci2011.09.0467
Matus, I., Mellado, M., Pinares, M., Madariaga, R., and del Pozo, A. (2012). Genetic Progress in Winter Wheat Cultivars released in Chile from 1920 to 2000. Chil. J. Agric. Res. 72, 303–308. doi: 10.4067/S0718-58392012000300001
Maureen, T. N. (2019). Evaluation of Elite Heat and Drought Tolerant Wheat (Triticum aestivum) Genotypes Based on Drought Tolerance and Water-Use Efficiency parameters. Thesis submitted to University of KwaZulu-Natal Pietermaritzburg, South Africa.
Mingliang, D., Asim, M., Mingju, L., Abdelkhalik, S., Manore, D., Shaoxiang, L., et al. (2020). Wheat genetic gains for two distinct management schemes in China: an analysis of elite spring type genotypes. PLoS ONE 15:e0228823. doi: 10.1371/journal.pone.0228823
Morgounov, A., Abugalieva, A., and Martynov, S. (2014). Effect of climate change and variety on long-term variation of grain yield and quality in winter wheat in Kazakhstan. Cereal Res. Commun. 42, 163–172. doi: 10.1556/CRC.2013.0047
OECD/FAO. (2019). OECD-FAO Agricultural outLook 2019-2028. OECD Publishing, Paris/Food and Agriculture Organization of the United Nations, Rome.
Olivoto, T., Lúcio, A. D., da Silva, J. A., Marchioro, V. S., de Souza, V. Q., and Jost, E. (2019). Mean performance and stability in multi-environment trials I: combining features of AMMI and BLUP techniques. Agron. J. 111, 2949–2960. doi: 10.2134/agronj2019.03.0220
Package “metan” (2020). Type Package Title Multi Environment Trials Analysis. doi: 10.2135/cropsci2013.04.0241
Peltonen-Sainio, J. L., and Hakala, K. (2011). Crop responses to temperature and precipitation according to long-term multi-location trials at high-latitude conditions. J. Agric. Sci. 149, 49–62. doi: 10.1017/S0021859610000791
Peltonen-Sainio, P., Jauhiainen, L., Hakala, K., and Ojanen, H. (2009b). Climate change and prolongation of growing season: changes in regional potential for field crop production in Finland. Agric. Food Sci. 18, 171–190. doi: 10.2137/145960609790059479
Peltonen-Sainio, P., Jauhiainen, L., and Laurila, I. P. (2009a). Cereal yield trends in northern European conditions: changes in yield potential and its realisation. Field Crops Res. 110, 85–90. doi: 10.1016/j.fcr.2008.07.007
Perry, M. W., and D'Antuono, M. F. (1989). Yield improvement and associated characteristics of some Australian spring wheat cultivars introduced between 1860 and 1982. Aust. J. Agric. Res. 40, 457–472. doi: 10.1071/AR9890457
Rebetzke, G. J., Ellis, M. H., Bonnett, D. G., Condon, A. G., Falk, D., and Richards, R. A. (2011). The Rht13 dwarfing gene reduces peduncle length and plant height to increase grain number and yield of wheat. Field Crops Res. 124, 323–331. doi: 10.1016/j.fcr.2011.06.022
Rebetzke, G. J., Ellis, M. H., Bonnett, D. G., Mickelson, B., Condon, A. G., and Richards, R. A. (2012). Height reduction and agronomic performance for selected gibberellin-responsive dwarfing genes in bread wheat (Triticum aestivum L.). Field Crops Res. 126, 87–96. doi: 10.1016/j.fcr.2011.09.022
Reynolds, M., Bonnett, D., Chapman, S. C., Furbank, R. T., Manès, Y., Mather, D. E., et al. (2011). Raising yield potential of wheat. I. Overview of a consortium approach and breeding strategies. J. Exp. Bot. 62, 439–452. doi: 10.1093/jxb/erq311
Reynolds, M. P., Acevedo, E., Sayre, K. D., and Fischer, R. A. (1994). Yield potential in modern wheat varieties: its association with a less competitive ideotype. Field Crops Res. 37, 149–160. doi: 10.1016/0378-4290(94)90094-9
Reynolds, M. P., Pask, A. J., Hoppitt, W. J., Sonder, K., Sukumaran, S., Molero, G., et al. (2017). Strategic crossing of biomass and harvest index—source and sink—achieves genetic gains in wheat. Euphytica 213, 1–23. doi: 10.1007/s10681-017-2040-z
Rosegrant, M. W., and Agcaoili, M. (2010). Global Food Demand, Supply, and Price Prospects to 2010. Washington, DC: Int. Food Policy Res. Institute.
Royo, C., Alvaro, F., Martos, V., Ramdani, A., Isidro, J., Villegas, D., et al. (2007). Genetic changes in durum wheat yield components and associated traits in Italian and Spanish varieties during the 20th century. Euphytica 155, 259–270. doi: 10.1007/s10681-006-9327-9
Sadras, V. O., and Lawson, C. (2011). Genetic gain in yield and associated changes in phenotype, trait plasticity and competitive ability of South Australian wheat varieties released between 1958 and 2007. Crop Pasture Sci. 62, 533–549. doi: 10.1071/CP11060
Sadras, V. O., and Rebetzke, G. J. (2013). Plasticity of wheat grain yield is associated with plasticity of ear number. Crop Pasture Sci. 64, 234–243. doi: 10.1071/CP13117
Sadras, V. O., Trápani, N., Pereyra, V. R., Pereira, M. L., Quiroz, F., and Mortarini, M. (2000). Intraspecific competition and fungal diseases as sources of variation in sunflower yield. Field Crops Res. 67, 51–58. doi: 10.1016/S0378-4290(00)00083-6
Sanchez-Garcia, M., Álvaro, F., Martín-Sánchez, J. A., Sillero, J. C., Escribano, J., and Royo, C. (2012). Breeding effects on the genotype × environment interaction for yield of bread wheat grown in Spain during the 20th century. Field Crops Res. 126, 79–86. doi: 10.1016/j.fcr.2011.10.001
Sanchez-Garcia, M., Royo, C., Aparicio, N., Martín-Sánchez, J. A., and Álvaro, F. (2013). Genetic improvement of bread wheat yield and associated traits in Spain during the 20th century. J. Agric. Sci. 151, 105–118. doi: 10.1017/S0021859612000330
Sayre, K. D., Rajaram, S., and Fischer, R. A. (1997).Yield potential progress in short bread wheats in northwest Mexico. Crop Sci. 37, 36–42. doi: 10.2135/cropsci1997.0011183X003700010006x
Sharma, R. C., Crossa, J., Velu, G., Huerta-Espino, J., Vargas, M., Payne, T. S., et al. (2012). Genetic gains for grain yield in CIMMYT spring bread wheat across international environments. Crop Sci. 52, 1522–1533. doi: 10.2135/cropsci2011.12.0634
Shearman, V., Sylvester-Bradley, R., and Scott, R. (2005). Physiological processes associated with wheat yield progress in the UK. Crop Sci. 45, 175–185. doi: 10.2135/cropsci2005.0175a
Siddique, K. M., Belfbrd, R. K., Perry, M. W., and Tennant, D. (1989). Growth, development and light interception of old and modern wheat cultivars in a mediterranean-type environment. Aust. J. Agric. Res. 40, 473–487. doi: 10.1071/Ar9890473
Slafer, G., and Savin, R. (1994). Source—sink relationships and grain mass at different positions within the spike in wheat. Field Crops Res. 37, 39–49. doi: 10.1016/0378-4290(94)90080-9
Slafer, G. A. A. G, Kantolic, M. L., Appendino, D. J., Miralles, and Savin, R. (2009). “Crop development: genetic control, environmental modulation and relevance for genetic improvement of crop yield,” in Crop Physiology: Applications for Genetic Improvement and Agronomy, Vol. 19, eds V. Sadras and D. Calderini (Burlington VT: Academic Press), 277–308. doi: 10.1016/B978-0-12-374431-9.00012-8
Slafer, G. A., and Andrade, F. H. (1993). Physiological attributes related to the generation of grain yield in bread wheat cultivars released at different eras. Field Crops Res. 31, 351–367. doi: 10.1016/0378-4290(93)90073-V
Tausz-Posch, S., Dempsey, R. W., Seneweera, S., Norton, R. M., Fitzgerald, G., and Tausz, M. (2015). Does a freely tillering wheat cultivar benefit more from elevated CO2 than a restricted tillering cultivar in a water-limited environment? Eur. J. Agron. 64, 21–28. doi: 10.1016/j.eja.2014.12.009
Ugarte, C. C., Trupkin, S. A., Ghiglione, H., Slafer, G., and Casal, J. J. (2010). Low red/far-red ratios delay spike and stem growth in wheat. J. Exp. Bot. 61, 3151–3162. doi: 10.1093/jxb/erq140
Underdahl, J. L., Mergoum, M., Ransom, J. K., and Schatz, B. G. (2008). Agronomic traits improvement and associations in hard red spring wheat cultivars released in North Dakota from 1968 to 2006. Crop Sci. 48, 158–166. doi: 10.2135/cropsci2007.01.0018
Waddington, S. R., Ransom, J. K., Osmanzai, M., and Saunders, D. A. (1986). Improvement in the yield potential of bread wheat adapted to northwest mexico. Crop Sci. 26, 698–703. doi: 10.2135/cropsci1986.0011183X002600040012x
Wu, W., Li, C., Ma, B., Shah, F., Liu, Y., and Liao, Y. (2014). Genetic progress in wheat yield and associated traits in China since 1945 and future prospects. Euphytica 196, 155–168. doi: 10.1007/s10681-013-1033-9
Xiao, Y. G., Qian, Z. G., Wu, K., Liu, J. J., Xia, X. C., Ji, W. Q., et al. (2012). Genetic gains in grain yield and physiological traits of winter wheat in Shandong Province, China, from 1969 to 2006. Crop Sci. 52, 44–56. doi: 10.2135/cropsci2011.05.0246
Yadav, R., Gaikwad, K., Bhattacharyya, R., Bainsla, N. K., Kumar, M., and Yadav, S. S. (2019). “Breeding new generation genotypes for conservation agriculture in maize-wheat cropping systems under climate change,” in Food Security and Climate Change, eds S. S. Yadav, R. J. Redden, J. L. Hatfield, A. W. Ebert, and D. Hunter (Chichester: John Wiley and Sons Ltd.), 189–228. doi: 10.1002/9781119180661.ch10
Yadav, R., Gaikwad, K. B., and Bhattacharyya, R. (2017). Breeding wheat for yield maximization under conservation agriculture. Indian J. Genet. Plant Breed. 77, 185–198.doi: 10.5958/0975-6906.2017.00026.8
Yadav, R., Singh, S. S., Jain, N., Singh, G. P., and Prabhu, K. V. (2010). Wheat production in India: technologies to face future challenges. J. Agric. Sci. 2:164. doi: 10.5539/jas.v2n2p164
Zadok, J. C., Chang, T. T., and Konzak, C. F. (1974). A decimal code for the growth stages of cereals. Weed Res. 14, 415–421
Zhang, Y., Xu, W., Wang, H., Dong, H., Qi, X., Zhao, M., et al. (2016). Progress in genetic improvement of grain yield and related physiological traits of Chinese wheat in Henan Province. Field Crops Res. 199, 117–128. doi: 10.1016/j.fcr.2016.09.022
Zheng, T. C., Zhang, X. K., Yin, G. H., Wang, L. N., Han, Y. L., Chen, L., et al. (2011). Genetic gains in grain yield, net photosynthesis and stomatal conductance achieved in Henan Province of China between 1981 and 2008. Field Crops Res. 122, 225–233. doi: 10.1016/j.fcr.2011.03.015
Keywords: bread wheat, genetic gain, mega cultivars, phenotypic plasticity, response to the competition
Citation: Yadav R, Gupta S, Gaikwad KB, Bainsla NK, Kumar M, Babu P, Ansari R, Dhar N, Dharmateja P and Prasad R (2021) Genetic Gain in Yield and Associated Changes in Agronomic Traits in Wheat Cultivars Developed Between 1900 and 2016 for Irrigated Ecosystems of Northwestern Plain Zone of India. Front. Plant Sci. 12:719394. doi: 10.3389/fpls.2021.719394
Received: 02 June 2021; Accepted: 18 August 2021;
Published: 23 September 2021.
Edited by:
Manuel Enrique Pinto, University of O'Higgins, ChileReviewed by:
Ivan A. Matus, Instituto de Investigaciones Agropecuarias, ChileCopyright © 2021 Yadav, Gupta, Gaikwad, Bainsla, Kumar, Babu, Ansari, Dhar, Dharmateja and Prasad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rajbir Yadav, cmFqYmlyeWFkYXZAeWFob28uY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.