
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci. , 14 July 2021
Sec. Plant Abiotic Stress
Volume 12 - 2021 | https://doi.org/10.3389/fpls.2021.708672
This article is part of the Research Topic Cellular Signaling Networks in Plant Heat Stress Responses View all 15 articles
We previously reported the involvement of cyclic nucleotide-gated ion channel 6 (CNGC6) and hydrogen peroxide (H2O2) in plant responses to heat shock (HS). To demonstrate their relationship with plant thermotolerance, we assessed the effect of HS on several groups of Arabidopsis (Arabidopsis thaliana) seedlings: wild-type, cngc6 mutant, and its complementation line. Under exposure to HS, the level of H2O2 was lower in the cngc6 mutant seedlings than in the wild-type (WT) seedlings but obviously increased in the complementation line. The treatment of Arabidopsis seeds with calcium ions (Ca2+) increased the H2O2 levels in the seedlings under HS treatment, whereas treatment with a Ca2+ chelator (EGTA) inhibited it, indicating that CNGC6 may stimulate the accumulation of H2O2 in a manner dependent on an increase in cytosolic Ca2+ ([Ca2+]cyt). This point was verified by phenotypic observations and thermotolerance testing with transgenic plants overexpressing AtRbohB and AtRbohD (two genes involved in HS-responsive H2O2 production), respectively, in a cngc6 background. Real-time reverse transcription-polymerase chain reactions and Western blotting suggested that CNGC6 enhanced the gene transcription of HS factors (HSFs) and the accumulation of HS proteins (HSPs) via H2O2. These upon results indicate that H2O2 acts downstream of CNGC6 in the HS signaling pathway, increasing our understanding of the initiation of plants responses to high temperatures.
Global warming is a serious environmental threat, and is an important limiting factor for normal plant growth and development. As fixed organisms, plants cannot escape from high temperature, but they have evolved methods and morphological variations to escape from its negative effects. As a countermeasure to heat shock (HS), plants can synthesize a series of HS proteins (HSPs) in the responses of cell to HS conditions. They act as molecular chaperones, ubiquitin, and certain proteases to counteract protein denaturation, aggregation, and degradation, which protect the plant cells from heat-damage (Lawas et al., 2018). Thus, the synthesis of HSP is especially important for plant survival under HS conditions. In eukaryotes, HSP induction is dependent on HS factors (HSFs), which act as transcription factors to be bound in HS elements in the promoter regions of HSP genes (Akerfelt et al., 2010).
Several reactive oxygen species (ROS) are constantly generated as by-products of aerobic metabolism at multiple locations in plant cells, including the photosynthetic electron transport chain in chloroplasts, NADPH oxidase in the plasma membrane (PM), and peroxidase in the cell wall (Gechev and Hille, 2005). They are always greatly toxic and swiftly detoxified by different cellular enzymatic and nonenzymatic mechanisms. In other situation, plants purposefully release ROS as signal molecules to initial various biological processes including stress defense, programmed cell death, and stomatal behavior. Hydrogen peroxide (H2O2), as the major and most stable type of ROS, plays a key role in resistance reactions in plant cells, and it primarily originates from PM NADPH oxidase. In Arabidopsis, NADPH oxidase is encoded by 10 genes, from AtRbohA to AtRbohJ, which have distinct and shared biological features (Macpherson et al., 2008).
For example, H2O2 generated from AtRbohD and AtRbohF acts as a signaling molecule in ABA-induced stomatal closure and is crucial for jasmonic acid-induced expression of genes controlled by the MYC2 transcription factor (Maruta et al., 2011; Iwai et al., 2019), but regulates lateral root development negatively by altering the localization of superoxide in primary roots of Arabidopsis (Li et al., 2015). Under Cd stress, the differential regulation of H2O2 metabolism, redox homeostasis, and nutrient balance by AtRbohC, AtRbohD, and AtRbohF is of potential interest for biotechnology applications for the phytoremediation of polluted soils (Gupta et al., 2017). AtRbohF is considered a key modulator of defense-associated metabolism and a crucial factor in the interplay between intracellular oxidative stress and pathogenesis responses in Arabidopsis (Chaouch et al., 2012). In addition, the level of H2O2 has been reported to increase following exposure to high temperatures, resulting in elevated HSF activation and HSP accumulation (Banti et al., 2010), whereas peroxide scavengers and inhibitors of H2O2 generation inhibited HSP expression in HS-exposed plants (Königshofer et al., 2008), implicating the involvement of H2O2 in the HS signaling pathway. Mutations in AtRbohB and AtRbohD, two isoforms of NADPH oxidase which contribute to H2O2 production, were reported to show weaker defects under HS (Larkindale et al., 2005). Our work further indicated that AtRbohB and AtRbohD-dependent H2O2 production acts upstream of nitric oxide (NO) in the HS signaling pathway, involving variations in HSF DNA-binding activity and HSP expression (Wang et al., 2014).
Calcium ions (Ca2+) mobilization is a core issue in various plant signaling pathways. Cyclic nucleotide-gated ion channels (CNGCs) are nonselective cation channels and the main entrances for Ca2+ influxes into cells (Jha et al., 2016). In Arabidopsis genome, there are 20 expressed CNGC genes, having both different and shared biological activities (Talke et al., 2003). For example, cyclic nucleotide-gated ion channel 6 (CNGC6), CNGC9, and CNGC14 fulfill part of redundant functions to generate and maintain tip focused Ca2+ oscillations, which are essential for proper root hair growth and polarity (Brost et al., 2019). CNGC2 and CNGC4-mediated Ca2+ entry is suggested to provide a vital link between the pattern-recognition receptor complex and Ca2+-dependent immunity programs in PAMP-triggered immunity signal pathways in plants (Tian et al., 2019). The pollen-tube-specific CNGC7, CNGC8, and CNGC18 together with calmodulin (CaM) constitute a molecular switch that control the open or close of the calcium channel depending on cellular Ca2+ levels (Pan et al., 2019). CNGC9 is reported to mediate the elevation of cytosolic Ca2+ ([Ca2+]cyt) to resist disease in rice (Wang et al., 2019). CNGCs are also believed to mediate Ca2+ signals in the HS pathway. We reported that CNGC6, a heat-responsive PM Ca2+-permeable channel, is associated with the expression of HSP genes and the acquisition of thermotolerance in Arabidopsis (Gao et al., 2012). CNGC6 via Ca2+ signaling initiates plant resistant reactions to heat stress, but its precise regulatory mechanisms remain obscure. Further investigations into HS signaling will enrich our understanding of the initial heat stress signaling processes.
Calcium ions and H2O2 are well known as two universal intracellular secondary messengers. Studies of plants have shown a close relationship between their individual pathways; however, there is controversy regarding which one is upstream of the other. Lots of studies implicate a specific role of H2O2 in regulating Ca2+ signaling. For example, H2O2 production regulates the elevation of [Ca2+]cyt in ABA signaling pathways in Arabidopsis guard cells (Jiang et al., 2013; Islam et al., 2019). On the contrary, some studies have pointed to the role of Ca2+ in influencing H2O2 signaling. For example, extracellular Ca2+ through H2O2 alleviates NaCl-induced stomatal openings in Vicia guard cells (Zhao et al., 2011). Also, crosstalk between Ca2+ signaling and H2O2 is required for some signaling networks, for example, their co-operation in the process of heavy metal stress resistance (Nazir et al., 2020). The relationship between Ca2+ and H2O2 is not yet fully understood in plants exposed to HS conditions.
In this investigation, we used the model plant Arabidopsis to explore the relationship between H2O2 and the Ca2+-permeable channel CNGC6 under heat stress conditions. Our results demonstrate the involvement of H2O2 in CNGC6 signaling as a downstream factor in the HS signaling pathway, by stimulating Hsf transcription and HSP accumulation.
The wild-type (WT) and mutant Arabidopsis were Col-0 ecotype. atrbohB and atrbohD mutant seeds were obtained from Dr. Miguel Angel Torress (University of North Carolina). The triple mutant cngc6/rbohB/D was obtained by crossing, while the transgenic lines cngc6/35S::RbohB-1, cngc6/35S::RbohB-2, cngc6/35S::RbohD-1, and cngc6/35S::RbohD-2 were obtained using the floral dip method.
The Arabidopsis seeds were surface sterilized in 2% (v/v) sodium hypochlorite for 1 min and then washed thoroughly with water. The sterilized seeds were placed on Murashige and Skoog (MS) medium containing 3% sucrose and 0.7% agar and kept at 4°C in the dark for 3 days. The plants were then transferred to a growth chamber set at 22°C and 120 μmol m−2s−1 on a 16-h daily light period.
For chemical treatment, 2 ml of H2O2 at various concentrations (0, 25, 50, 100, and 200 μM; Sigma-Aldrich, St. Louis, MO) were sprinkled onto the leaf surfaces of 8-day-old seedlings after filter sterilization. Sterilized water was used as a substitute for the control of seedlings. After 8 h of pre-treatment, the seedlings were subjected to HS conditions (Wang et al., 2014). In addition, 5 mM CaCl2 or 2 mM EGTA (these reagents were prepared with sterilized water) was used to pre-treat the WT, cngc6, and COM12 seeds for 30 min before their being placed on MS medium in the fluorescence experiment, with sterilized water as the control.
About 8-day-old seedlings, grown at 22°C, were incubated in sterilized 5 mM CaCl2 at 37°C for 30 min, returned to 22°C for 2 h, then challenged at 45°C for 100 min, and then returned to 22°C for 5 days of recovery (Lewis et al., 2016). The seedlings that were still green and continuing to produce new leaves were registered as survivors. For Western blotting, 10-day-old seedlings were kept at 37°C for 2 h and collected for the analyses of HSP accumulation. All the experiments were repeated at least three times, and there were three independent biological replicates in each repeat (Peng et al., 2019).
Hydrogen peroxide was visualized using the specific fluorescent probe 5-(and-6)-chloromethyl-29,79-dichlorodihydrofluorescein diacetate (CM-H2DCFDA; Invitrogen) as described previously (Wang et al., 2010) with some modifications. Wild-type and mutant seedlings were incubated in 1 ml of liquid MS medium (pH 5.8) with 10 μM CM-H2DCFDA for 20 min. Thereafter, the roots were washed three times for 15 min each in liquid MS medium prior to visualization with a fluorescence microscope (Eclipse TE 200, Nikon, Tokyo, Japan). The signal intensities were calculated using MetaMorph (Molecular Devices, Sunnyvale, CA).
To generate the 35S:6×Myc-RbohB construct, the full-length RbohB coding sequence was amplified using the primers 5'-CGGGATC-CATGCGGGAGGAAGAAATG-3' and 5'-TCCACAAGGAAAATTTCTAGCTGCAGTT-3'. To generate the 35S:6×Myc-RbohD construct, the full-length RbohD coding sequence was amplified with the primers 5'-CGGGATCCATGAAAATGAGACGAGGCAA-3' and 5'-CCACAAAGAGAACTTCTAGCTGCAGTT-3'. The products were cloned in the pCAMBIA1307-6×Myc vectors using the BamHI and PstI sites.
The transformation of the constructs into Arabidopsis (cngc6) was performed according to the floral dip method (Clough and Bent, 1998) with Agrobacterium tumefaciens (strain GV3101). Transformants were screened on plates containing 15 mg l−1 of Basta. Homozygous T3 transgenic lines were selected for further analysis.
Total RNA (500 ng) was isolated from 10-day-old seedlings at 37°C for 1 h with a PrimeScript RT Reagent Kit (Takara Bio Inc., Otsu, Japan) for first-stand cDNA synthesis, as per the manufacturer’s instructions. The program was as follows: initial polymerase activation for 10 s at 95°C followed by 40 cycles of 95°C for 5 s and 60°C for 31 s. The reactions were performed using an ABI Prism 7,000 sequence detection system (Applied Biosystems, Foster City, CA) with SYBR Premix Ex Taq (Takara Bio Inc.). Primer pairs were designed using Primer Express (Applied Biosystems). Detailed primer sequences are shown in Supplementary Table 1.
About 10-day-old seedlings were kept at 37°C for 2 h and then ground in liquid nitrogen. Total protein was extracted with an extraction buffer (10 mM HEPES, pH 7.9, containing 0.4 M NaCl, 0.5 mM dithiothreitol, 0.1 mM EDTA, 5% glycerol, and 0.5 mM phenylmethanesulfonyl fluoride), and the extracts were purified by centrifugation at 14,000 × g for 20 min at 4°C. The supernatants were transferred to fresh tubes, and the protein content was measured according to the description of Bradford (1976). Total proteins (50 μg) were analyzed by Western blotting, as described previously (Wang et al., 2014).
Protoplasts were isolated as described previously (Demidchik and Tester, 2002) from 1 cm long of root tips of Arabidopsis seedlings cultivated vertically at 22°C for 8 days. Whole-cell voltage patch-clamping was carried out as described previously (Gao et al., 2012; Peng et al., 2019; Niu et al., 2020) with minor modification. Patch-clamp pipettes were pulled on a vertical electrode puller. The electrode was filled with pipette solution [0.5 mM CaCl2, 2 mM Mg-ATP, 0.5 mM Tris-ATP, 4 mM Ca(OH)2, 10 mM EGTA and 15 mM HEPES/Tris, pH 7.2, adjusted to an osmolality of 300 mOsm/kg with sorbitol; free Ca2+ concentration 100 nM]. The basal external solution comprised 10 mM CaCl2 and 5 mM MES/Tris, pH 5.8, adjusted to an osmolality of 300 mOsm/Kg with sorbitol. The resistance of the electrode in the bath solution was approximately 20 MΩ. Seal resistances were up to 2 GΩ. After holding the whole-cell high seal resistances for 20 min, currents were recorded and data were sampled at 1 kHz and filtered at 200 Hz. Membrane potentials were corrected for liquid junction potentials and series resistance. An Axon 200B amplifier controlled by pCLAMP 9.0 software (Molecular Devices) was used to record the current signal. Basal currents were recorded at room temperature (20–22°C). HS treatment (37 ± 1°C) was performed using continuous bath perfusion.
In this work, we presented evidence for the involvement of H2O2 in Ca2+ signaling in plant thermotolerance. CNGC6, activated by HS and mediated Ca2+ influxes, functioned as a signal in the induction of H2O2 generation to stimulate the transcription of Hsfs and HSPs accumulation. Thus, CNGC6 was found to promote heat tolerance in Arabidopsis seedlings.
Hydrogen peroxide is a plant signaling molecule that plays a vital role in many environmental stress responses. Lots of studies suggest a key role for CNGCs in controlling H2O2 production (Walker and Berkowitz, 2013; Cui et al., 2020). To elucidate the relationship between H2O2 and CNGC6 in thermotolerance, we first determined the transcription levels of AtRbohB and AtRbohD at the seedling stage using the wild-type plants, a T-DNA insertion mutant (cngc6; SALK_042207), and a complementation line (COM12; cngc6 + CNGC6; Gao et al., 2012). The result showed that no clear difference existed between the expression levels in these seedlings under normal conditions; however, both of their expression levels were stimulated by high temperatures and varied depending on the expression level of CNGC6 (Supplementary Figure 1), implying that it had a role in the generation of H2O2. Thus, we examined endogenous H2O2 accumulations in these seedlings using the special fluorescent probe CM-H2DCFDA. This probe can be transported into cells, where its acetate groups are passively cleaved by intracellular esterases, producing the fluorescent compound dichlorodihydrofluorescein (DCF; Chozinski et al., 2016).
Fluorescence analysis indicated that under normal conditions (22°C), no clear difference in the abundance of H2O2 was observed among the seedlings. After HS treatment at 45°C for 30 min (Wang et al., 2014), the H2O2 level increased by 208% in the wild-type seedlings, higher than the increase observed in cngc6 (108%); however, it was nearly rescued in COM12 seedlings (187%; Figures 1A,B). We also found that not all the production of H2O2 responsive to HS was inhibited in ncgc6 mutant. Thus, these results suggest that the production of H2O2 observed after HS treatment was partially due to the activation of CNGC6.
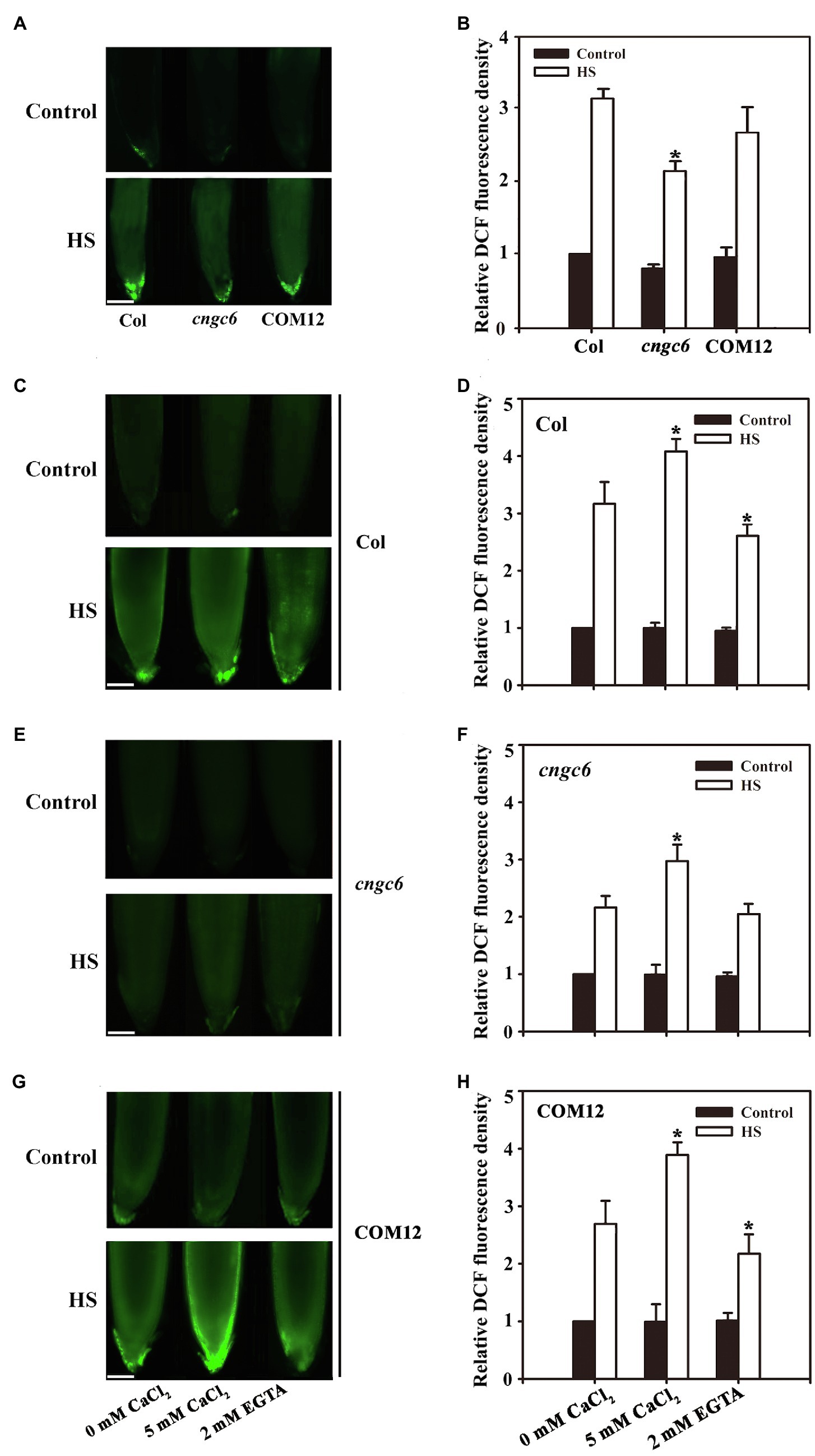
Figure 1. Effects of calcium ions (Ca2+) on hydrogen peroxide (H2O2) accumulation in Arabidopsis seedlings. (A) About 8-day-old wild-type (WT), cngc6, and COM12 seedlings grown at 22°C were exposed to 45°C (heat shock, HS) or maintained at 22°C (Control) for 30 min. The H2O2 levels in the seedlings were then examined by fluorescence microscopy using roots dyed with 5-(and-6)-chloromethyl-29,79-dichlorodihydrofluorescein diacetate (CM-H2DCFDA). Bar = 100 μm. (B) Relative dichlorodihydrofluorescein (DCF) fluorescence densities in the roots. The data presented are the means ± SE of measurements taken from five independent experiments with at least 10 roots for each treatment. *p < 0.05 vs. Col (Student’s t-test). (C,E,G) About 8-day-old seedlings of wild-type (C), cngc6 (E), and COM2 (G) were exposed to 45°C (HS) or maintained at 22°C (Control) for 30 min. The H2O2 levels in the plants were then examined by fluorescence microscopy using roots stained with CM-H2DCFDA. Bar = 100 μm. (D,F,H) The relative DCF fluorescence densities in the roots of wild-type (D), cngc6 (F), and COM2 (H). The data presented are the means ± SE of measurements taken from five independent experiments with at least 10 roots for each treatment. *p < 0.05 vs. 0 mM CaCl2 (Student’s t-test).
Cyclic nucleotide-gated ion channel 6 is a heat-responsive Ca2+-permeable channel in the PM of plant cells (Gao et al., 2012). Ca2+ is one of the most multifunctional ions existed in eukaryotes, and it has been confirmed to coordinate with H2O2 in many physiological processes (Ferreira et al., 2003). Thus, it is reasonable to consider that CNGC6 elevates the H2O2 level through Ca2+ to induce thermotolerance.
To test this hypothesis, the H2O2 levels were examined in the wild-type, cngc6, and COM12 seedlings pre-treated with 5 mM CaCl2 or 2 mM EGTA (a Ca2+ chelator) before germination as described previously (Liu et al., 2005; Peng et al., 2019). Fluorescence analysis showed that under normal growth conditions, the H2O2 levels in wild-type, cngc6, and COM12 seedlings were rather stable. However, under HS conditions, 5 mM Ca2+ treatment elevated the H2O2 level to 411, 303, and 389% of their individual controls in the wild-type, cngc6, and COM12 seedlings, respectively. Whereas 2 mM EGTA reduced the increase in H2O2 to 245 and 213% of the wild-type and COM12 controls, respectively, but there was no clear effect on the cngc6 mutant (Figures 1C–H).
Subsequently, a solution containing a series of concentrations of H2O2 was used to pre-treat the wild-type and cngc6 seedlings. Under HS conditions, the internal H2O2 level was higher in the wild-type seedlings than in the cngc6 seedlings. Exogenous application of H2O2 stimulated the internal H2O2 level in these seedlings depending on the H2O2 concentration, reaching a maximum value at 100 μM and decreasing slightly at 200 μM (Figures 2A,B). The survival ratios of the wild-type and cngc6 seedlings changed in the same manner as their internal H2O2 levels, reaching the maximum at 100 μM (Figures 2C,D).
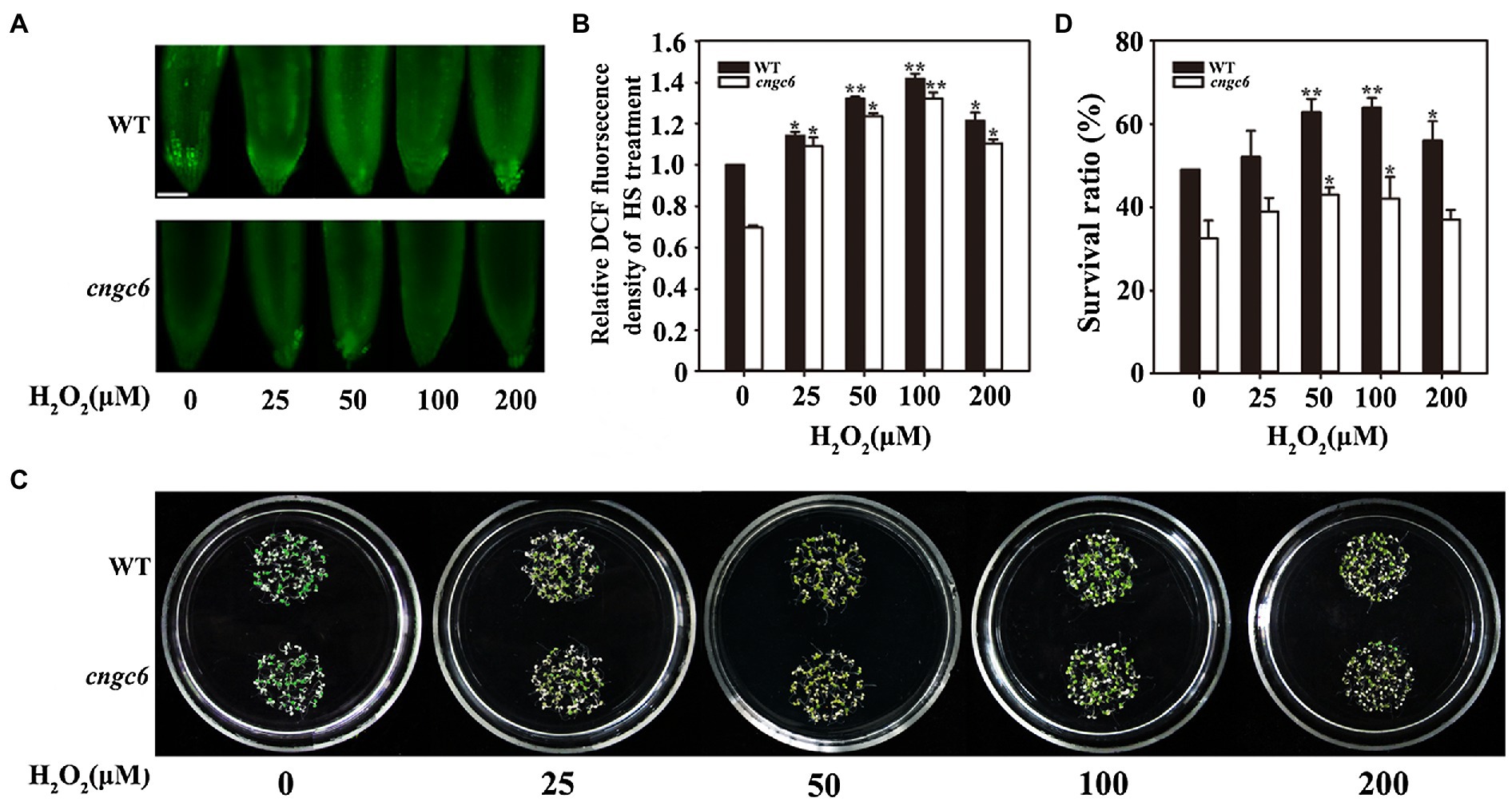
Figure 2. Effects of H2O2 on the thermotolerance of WT and cngc6 seedlings. (A) About 8-day-old WT and cngc6 seedlings grown at 22°C were pre-treated with 2 ml of 0, 25, 50, 100, or 200 mM H2O2 for 8 h and then exposed to 45°C (HS) for 30 min. The H2O2 levels were then assessed by fluorescence microscopy in roots stained with CM-H2DCFDA. Bar = 100 mm. (B) Relative DCF fluorescence densities in the roots. The data presented are means ± SE of measurements taken from at least 10 roots for each treatment. *p < 0.05 and **p < 0.01 vs. 0 mM H2O2 (Student’s t-test). (C) Seedlings were exposed to 45°C for 100 min, then returned to 22°C and photographed 5 days later. (D) Survival ratios of the seedlings after HS treatment. The data presented are means ± SE of at least five independent experiments with 50 seedlings per experiment. *p < 0.05 vs. 0 mM H2O2 (Student’s t-test).
Taken together, these results (Figures 1, 2) showed that heat-responsive Ca2+ channel CNGC6 regulated H2O2 production; however, an increased internal H2O2 level rescued the impaired thermotolerance of the CNGC6-deficient mutant, indicating H2O2 involvement in CNGC6 signaling as a downstream factor.
We even reported that H2O2 acts as a signal in heat tolerance using the mutants rbohB and rbohD, which show poor thermotolerance due to a deficiency in H2O2 (Wang et al., 2014). To further investigate the effect of CNGC6 on H2O2 signaling under HS conditions, we obtained two AtRbohB-overexpressing transgenic lines, cngc6/35S::RbohB-1 and cngc6/35S::RbohB-2, and two AtRbohD-overexpressing transgenic lines, cngc6/35S::RbohD-1 and cngc6/35S::RbohD-2, and examined the influences of excess internal H2O2 on CNGC6-deficient mutants under HS conditions. The increased expression of AtRbohB and AtRbohD was confirmed according to real-time quantitative PCR (RT-qPCR; Figures 3A, 4A).
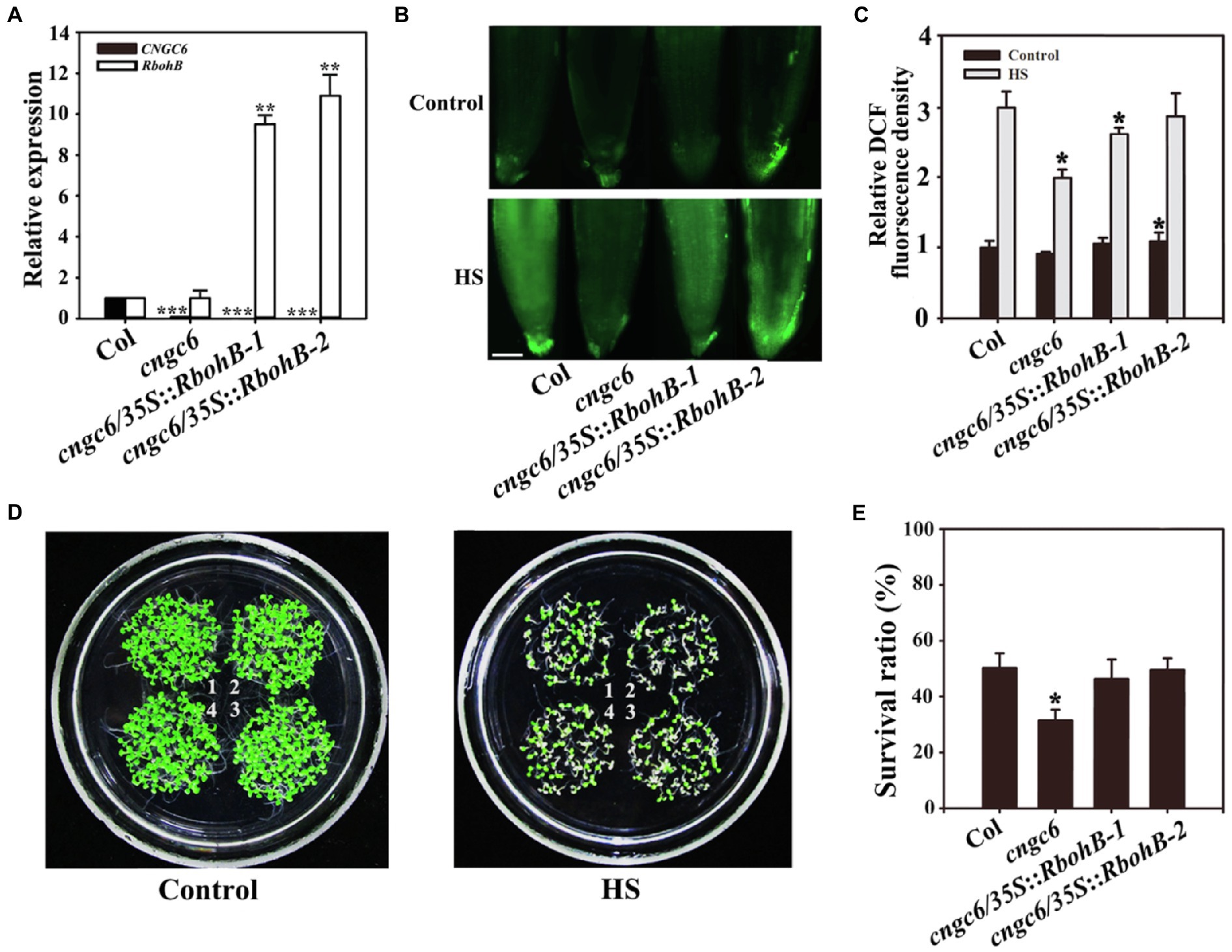
Figure 3. Improved thermotolerance through AtRbohB overexpression in a cngc6 background. (A) Real-time quantitative PCR (RT-qPCR) analysis of AtCNGC6 and AtRbohB transcription in wild-type, cngc6, cngc6/35S::RbohB-1, and cngc6/35S::RbohB-2 plants. The experiments were repeated three times with similar results. Each data point represents the mean ± SD (n = 3). Asterisks indicate a significant difference relative to Col (Student’s t-test: **p < 0.01 and ***p < 0.001). (B) About 8-day-old wild-type, cngc6, cngc6/35S::RbohB-1, and cngc6/35S::RbohB-2 seedlings grown at 22°C were exposed to 45°C (HS) or maintained at 22°C (Control) for 30 min. The H2O2 levels in the plants were then examined by fluorescence microscopy using roots stained with CM-H2DCFDA. Bar = 100 μm. (C) The relative DCF fluorescence densities in the roots. The data presented are the means ± SE of measurements taken from five independent experiments with at least 10 roots for each treatment. *p < 0.05 vs. Col. (D) Seedlings grown at 22°C were exposed to 45°C (HS) or maintained at 22°C (Control) for 100 min, then returned to 22°C and photographed 5 days later. The clusters are as follows: 1, wild-type; 2, cngc6; 3, cngc6/35S::RbohB-1; and 4, cngc6/35S::RbohB-2. (E) Survival ratios of the seedlings after HS treatment. The data presented are the means ± SE of at least five independent experiments with 50 seedlings per experiment. *p < 0.05 vs. Col (Student’s t-test).
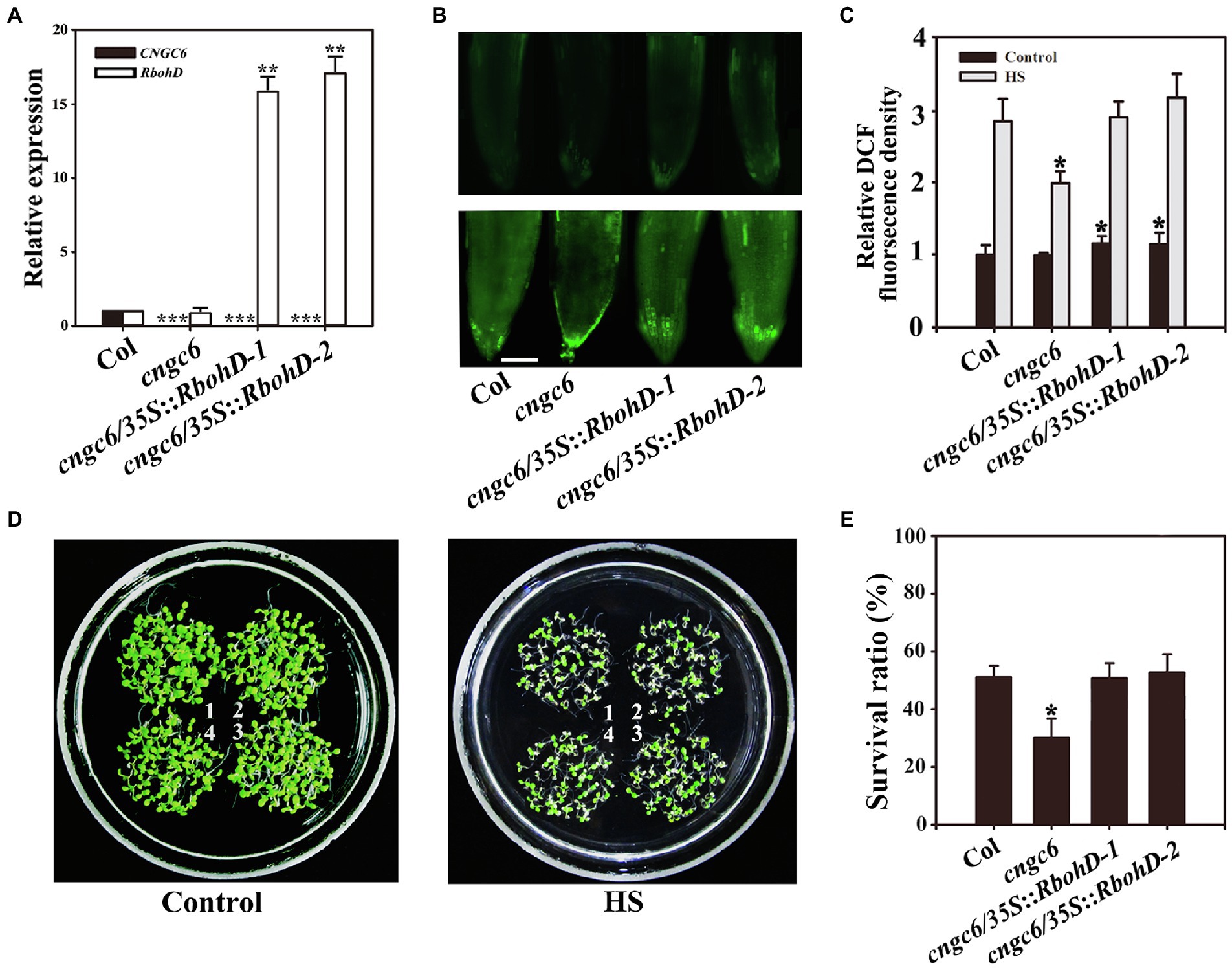
Figure 4. Improved thermotolerance through AtRbohD overexpression in a cngc6 background. (A) RT-qPCR analysis of AtCNGC6 and AtRbohD transcription in wild-type, cngc6, cngc6/35S::RbohD-1, and cngc6/35S::RbohD-2 plants. The experiments were repeated three times with similar results. Each data point represents the mean ± SD (n = 3). Asterisks indicate a significant difference relative to Col (Student’s t-test: **p < 0.01 and ***p < 0.001). (B) About 8-day-old wild-type, cngc6, cngc6/35S::RbohD-1, and cngc6/35S::RbohD-2 seedlings grown at 22°C were exposed to 45°C (HS) or maintained at 22°C (Control) for 30 min. The H2O2 levels in the plants were then examined by fluorescence microscopy using roots stained with CM-H2DCFDA. Bar = 100 μm. (C) The relative DCF fluorescence densities in the roots. The data presented are the means ± SE of measurements taken from five independent experiments with at least 10 roots for each treatment. *p < 0.05 vs. Col. (D) Seedlings grown at 22°C were exposed to 45°C (HS) or maintained at 22°C (Control) for 100 min, then returned to 22°C and photographed 5 days later. The clusters are as follows: 1, wild-type; 2, cngc6; 3, cngc6/35S::RbohD-1; and 4, cngc6/35S::RbohD-2. (E) Survival ratios of the seedlings after HS treatment. The data presented are the means ± SE of at least five independent experiments with 50 seedlings per experiment. *p < 0.05 vs. Col (Student’s t-test).
Dichlorodihydrofluorescein fluorescence analysis indicated that AtRbohB and AtRbohD overexpression enhanced the internal H2O2 levels in these transgenic plants under normal and HS conditions (Figures 3, 4). Under normal conditions, no clear phenotypic difference was observed between cngc6 mutant and these transgenic lines. However, under high temperature conditions, AtRbohB or AtRbohD overexpression greatly improved the survival ratio of the transgenic lines in comparison with their background cngc6 according to their individual transcriptional levels (Figures 3, 4).
These results showed that the overexpression of AtRbohB or AtRbohD restored heat tolerance in a CNGC6-deficient mutant, providing genetic proof for the relationship between CNGC6 and H2O2 in HS signaling.
To further examine the roles of CNGC6 and H2O2 in plant thermotolerance, we obtained the cngc6/rbohB/D triple mutant by crossing, which was deficient in CNGC6, RbohB, and RbohD transcription according to RT-qPCR analysis (Figure 5A). Under normal and HS conditions, the H2O2 level in the cngc6/rbohB/D seedlings was similar to that in the rbohB/D seedlings (Figures 5B,C), revealing that the deficiency of CNGC6 did not remarkably reduce H2O2 accumulation in the rbohB/D seedlings. Under normal conditions, cngc6/rbohB/D seedlings showed similar phenotypes with other seedlings (Figure 5D, Control). Under HS conditions, the survival ratio of the cngc6/rbohB/D seedlings was near to that of the rbohB/D seedlings (Figures 5D,E), showing that the deficiency of CNGC6 did not obviously aggravate the heat susceptibility of rbohB/D.
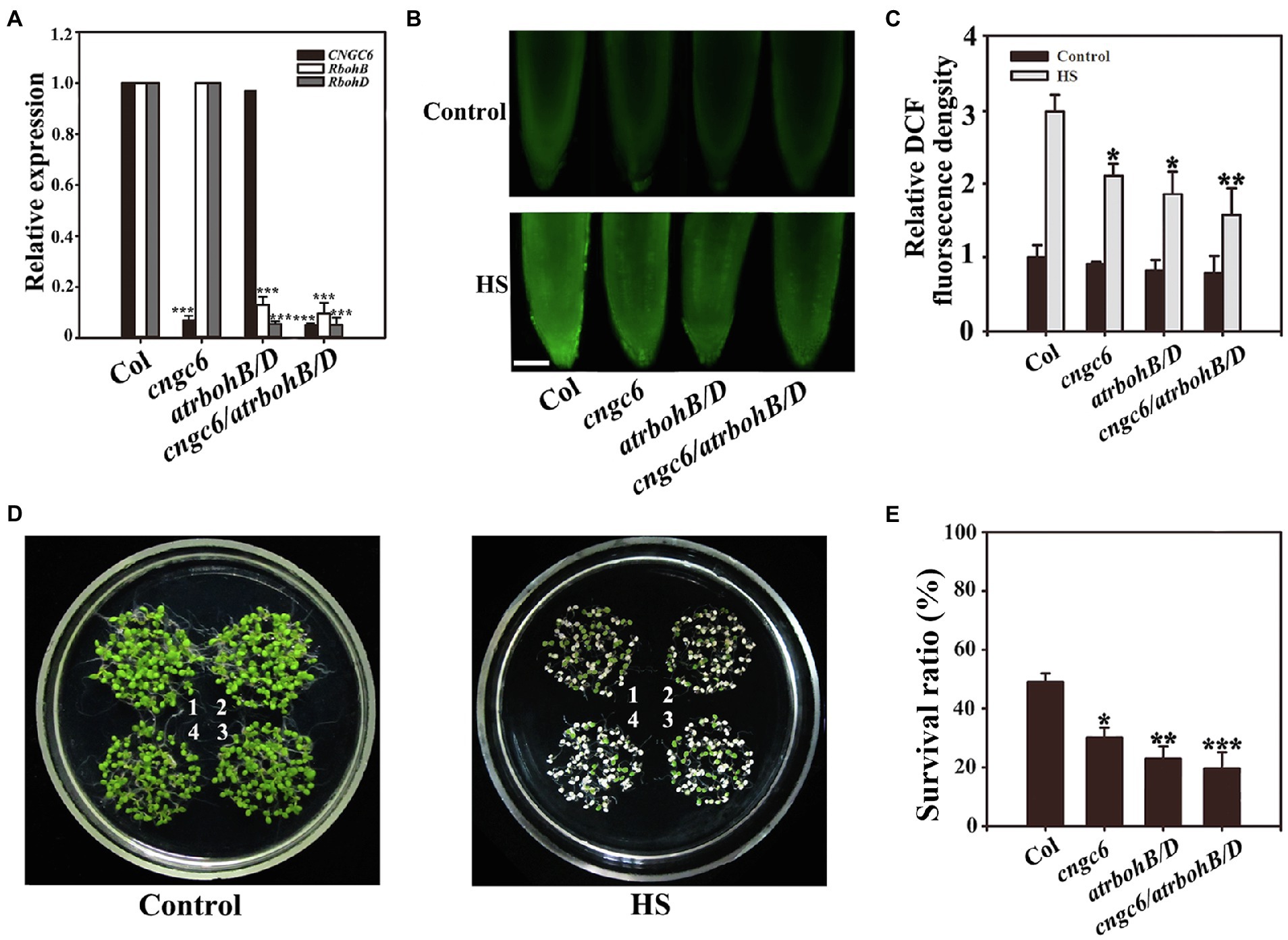
Figure 5. Survival status of the cngc6/rbohB/D triple mutant. (A) RT-qPCR analysis of cyclic nucleotide-gated ion channel 6 (CNGC6), RbohB and RbohD transcription in wild-type, cngc6, rbohB/D, and cngc6/rbohB/D seedlings. The experiments were repeated three times with similar results. Each data point represents the mean ± SD (n = 3). Asterisks indicate a significant difference relative to Col; ***p < 0.001 (Student’s t-test). (B) About 8-day-old wild-type, cngc6, rbohB/D, and cngc6/rbohB/D seedlings grown at 22°C were exposed to 45°C (HS) or maintained at 22°C (Control) for 30 min. The H2O2 levels in the seedlings were then examined by fluorescence microscopy using roots stained with CM-H2DCFDA. Bar = 100 μm. (C) Relative DCF fluorescence densities in the roots. The data presented are the means ± SE of measurements taken from five independent experiments with at least 10 roots for each treatment. *p < 0.05, and **p < 0.01 vs. Col (Student’s t-test). (D) About 8-day-old seedlings grown at 22°C were exposed to 45°C (HS) or maintained at 22°C (Control) for 100 min, then returned to 22°C and photographed 5 days later. The clusters are as follows: 1, wild-type; 2, cngc6; 3, rbohB/D; and 4, cngc6/rbohB/D. (E) Survival ratios of the seedlings after HS treatment. The data presented are the means ± SE of at least five independent experiments with 50 seedlings per experiment. *p < 0.05 and **p < 0.01 vs. Col (Student’s t-test).
The results provided evidence of the function of CNGC6 on the H2O2-mediated acquisition of heat tolerance. In Arabidopsis, a specific role for H2O2 in regulating Ca2+ mobilization has also been found (Islam et al., 2019).
To confirm whether H2O2 influences the action of heat-responsive Ca2+-permeable channels, we determined the effects of internal H2O2 on the function of CNCG6 in the PM of root protoplasts of Arabidopsis with the whole-cell patch-clamp technique (Gao et al., 2012; Peng et al., 2019). Under normal conditions at 22°C, the Ca2+ current in cngc6 (−136 pA) was lower than in the wild-type (−178 pA) at −200 mV. Under HS at 37°C, the inward Ca2+ current was swiftly elevated to −375 pA in the wild-type within 1 min. However, only a slight increase (to −171 pA) was observed in cngc6 (Figures 6A,B), in accordance with our previous reports (Gao et al., 2012; Peng et al., 2019; Niu et al., 2020). In the rbohB/D double mutant with low internal H2O2 levels, the Ca2+ currents exhibited similar changing trends to those in the wild-type under both of normal and HS conditions (Figure 6C). However, in the cngc6/rbohB/D triple mutant, the Ca2+ currents showed no clear difference with those in cngc6 under normal and HS conditions (Figure 6D). In two transgenic lines with high endogenous H2O2 levels, cngc6/35S::RbohB-1 and cngc6/35S::RbohD-1, the Ca2+ currents were similar to those of cngc6 (non-transgenic background; Figures 6E,F), indicating that H2O2 had no obvious affection on the activity of Ca2+ channel.
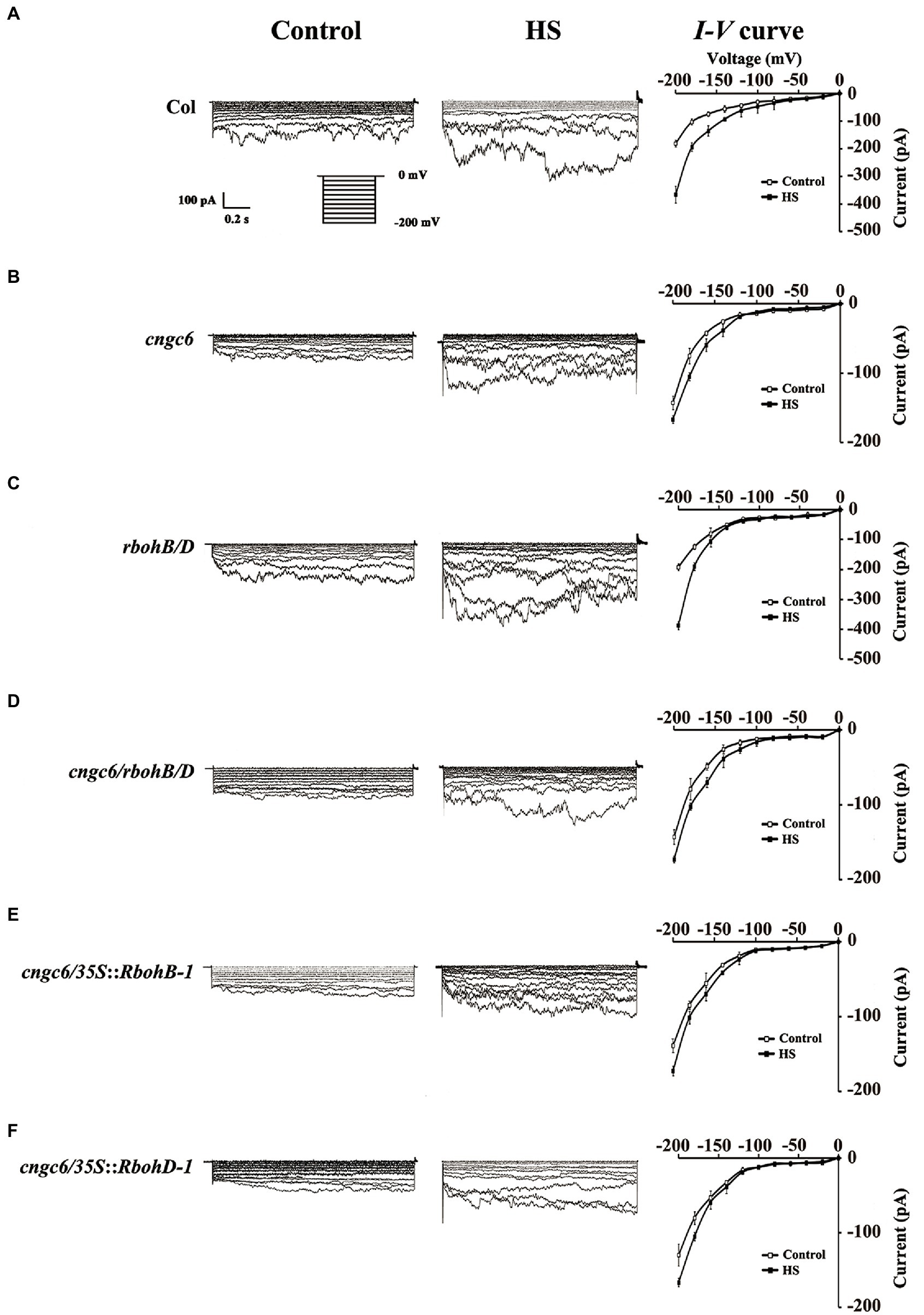
Figure 6. Patch-clamp analysis of Ca2+-permeable channels in wild-type, cngc6, rbohB/D, cngc6/rbohB/D, cngc6/35S::RbohB-1, and cngc6/35S::RbohD-1 seedlings. The Ca2+ current before HS (at 22°C, control) and after HS (at 37°C, HS) was compared in the root cell protoplasts of 8-day-old wild-type (A), cngc6 (B), rbohB/D (C), cngc6/rbohB/D (D), cngc6/35S::RbohB-1 (E), and cngc6/35S::RbohD-1 (F) plants. The Ca2+ current was recorded by step voltage clamp. Each trace is a representative current from six protoplasts. Currents in the protoplasts are shown in the left and middle columns, respectively. The I–V curve is shown in the right column (mean ± SD, n = 6).
To investigate the underlying mechanisms of CNGC6- and H2O2-induced plant thermotolerance, the mRNA level of Hsf in the wild-type, cngc6, rbohB/D, and cngc6/rbohB/D seedlings as well as in the two individual RbohB- and RbohD-overexpressing transgenic lines (cngc6/35S::RbohB-1 and cngc6/35S::RbohD-1) was analyzed using RT-qPCR. Under normal conditions, there was no clear difference among the levels in these seedlings (Figure 7, Control). After the HS treatment, Hsf (Hsf2A, HsfA7a, and HsfB2b) mRNA levels were dramatically elevated. However, in cngc6, rbohB/D, and cngc6/rbohB/D seedlings, they were lower than in the wild-type seedlings (and lowest for cngc6/rbohB/D) but they were significantly stimulated by 50 μM H2O2 and were activated in the two transgenic lines compared with their background cngc6 (Figure 7, HS).
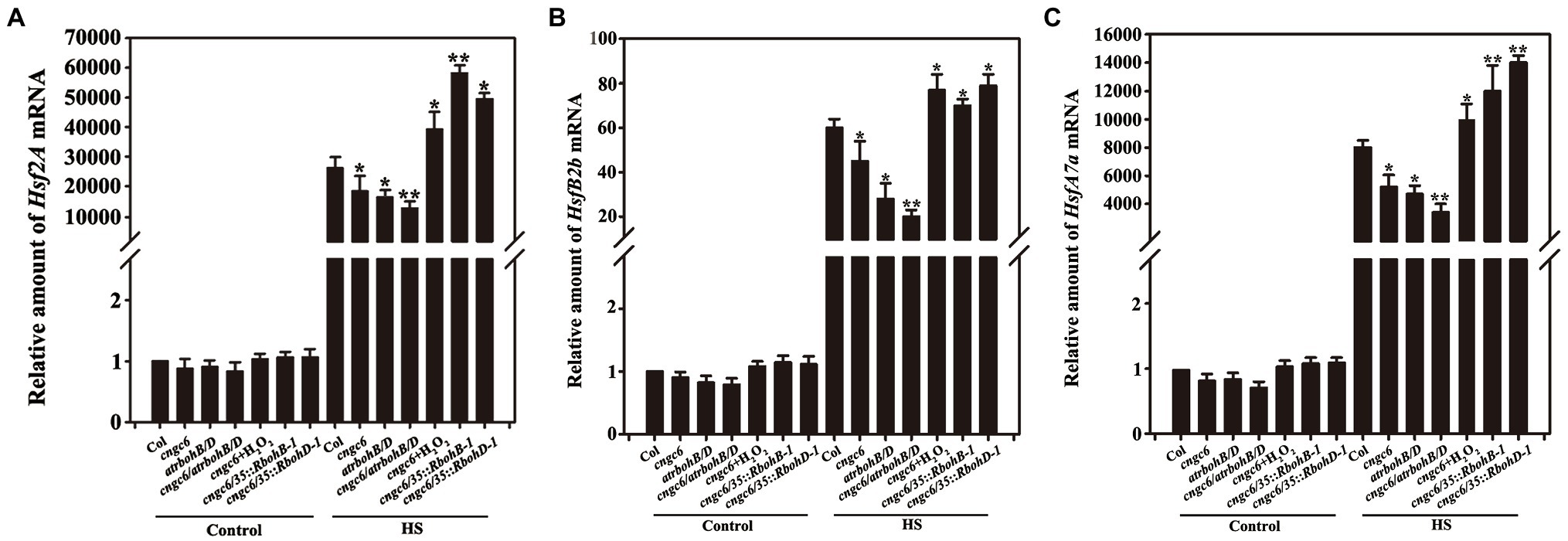
Figure 7. Analysis of the effects of CNGC6 on H2O2-induced Hsfs by RT-qPCR. About 10-day-old seedlings grown at 22°C was exposed to 37°C (HS) or maintained at 22°C (Control) for 60 min, then used for analysis of Hsf (A, Hsf2A; B, HsfA7a; and C, HsfB2b) mRNA expression. The data are the mean ± SE of at least five independent experiments. *p < 0.05 and **p < 0.01 vs. Col (Student’s t-test).
Heat shock proteins, as molecular chaperones, are crucial for all organisms to survive under severe stress through the maintenance of proteostasis (Akerfelt et al., 2010). Thus, we subsequently determined the influences of CNGC6 and H2O2 on the expression of AtHSP17.7 and AtHSP21 in these plants using Western blotting analysis. Neither AtHSP17.7 nor AtHSP21 was observed at 22°C; however, both of them accumulated at 37°C (Figure 8). The level of protein expression was lower in the mutants than in the wild-type (and lowest for cngc6/rbohB/D), and it was greatly elevated by 50 μM H2O2 in the cngc6 mutant. In addition, its accumulation was increased in the cngc6/35S::RbohB-1 and cngc6/35S::RbohD-1 plants in comparison with the cngc6 mutant (non-transformed background; Figure 8). In all these experiments, tubulin was adopted to ensure equal sample loading.
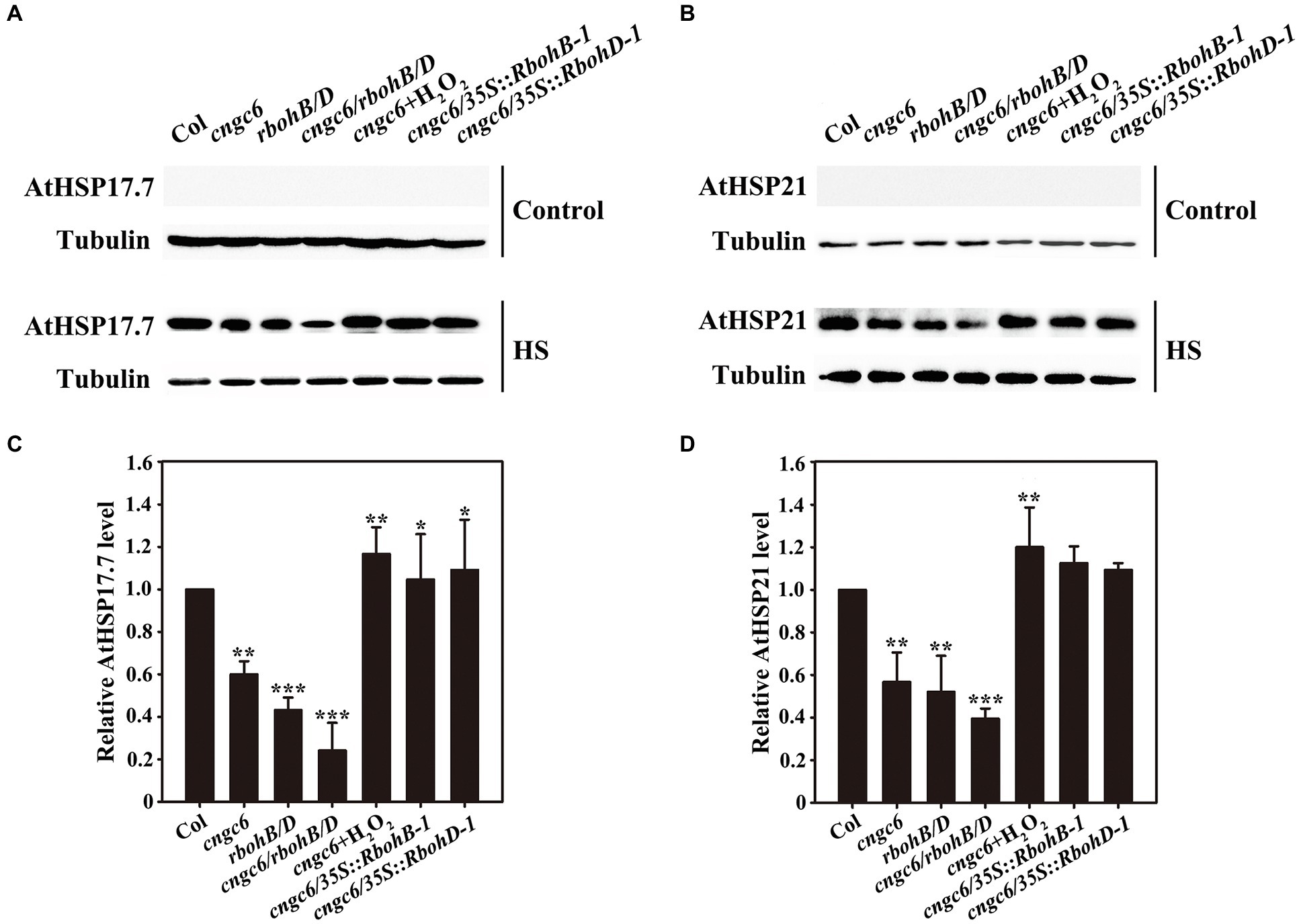
Figure 8. Effects of CNGC6 via H2O2 on AtHSP17.7 and AtHSP21 expression. (A,B) About 10-day-old seedlings grown at 22°C were exposed to 37°C (HS) or maintained at 22°C (Control) for 2 h. Total protein was then extracted, separated by SDS-PAGE, and analyzed by Western blotting. Tubulin was used as an internal control. Three independent experiments were carried out; the results indicate similar trends in protein accumulation. (C,D) The relative HSP17.7 (C) and HSP21 (D) level after HS treatment. The data presented are the means ± SE of measurements taken from three independent experiments and represents the relative intensity of each signal. *p < 0.05, **p < 0.01, and ***p < 0.001 vs. Col (Student’s t-test).
These results revealed that the application of H2O2 and the overexpression of AtRbohB or AtRbohD prompted HSP expression in a cngc6 mutant, providing further evidence that CNGC6 acts upstream of H2O2 in the HS pathway.
High external temperatures always result in elevated [Ca2+]cyt and the accumulation of H2O2 in plant cells, as they play crucial roles in the response of plant to HS (Liu et al., 2005; Sun and Guo, 2016). However, the relationship between H2O2 and Ca2+ signaling pathways in thermotolerance is unclear. Herein, our work showed that CNGC6, a heat-activated Ca2+-permeable channel, stimulates H2O2 accumulation to regulate the gene expression of Hsfs and HSPs accumulation to promote plant heat tolerance.
Hydrogen peroxide, an essential second messenger in a wide variety of biological processes, is stimulated by various factors to counteract exogenous stresses in plants. We previously reported that H2O2 acts as a signal in the induction of heat tolerance through NO (Wang et al., 2014). NO was even found to be associated with elevating intracellular levels of free Ca2+ under HS conditions (Peng et al., 2019). Recently, several studies have focused on the function of Ca2+ in initiating H2O2 accumulation in plants (Ferreira et al., 2003; Zhao et al., 2011). Therefore, we speculated that there should be a close relationship between Ca2+ and H2O2 in HS signaling pathway.
In plants, the CNGC proteins are expressed differentially in numerous tissues (Zelman et al., 2012). Molecular genetic studies have revealed that CNGCs frequently function in numerous biological processes, including plant growth and development, adaptations to increased Ca2+ concentration, and plant responses to abiotic and biotic stresses (Gao et al., 2016; Jha et al., 2016). Our prior work has demonstrated that AtCNGC6 is a heat-activated PM Ca2+-permeable channel that conducts Ca2+ into the cytoplasm to help regulate HS responses. A T-DNA insertion mutant cncg6 was used for those investigations due to its lower Ca2+ current than the wild-type, which is nearly totally restored in the transgenic line COM12 plants after HS treatment (Gao et al., 2012; Peng et al., 2019), indicating that CNGC6 regulates the influx of Ca2+ into plant cells. Thus, we used the cngc6 mutant and the COM12 plants to examine the relationship between H2O2 and CNGC6 in plant thermotolerance.
The mRNA level of AtRbohB/D is stimulated by HS depending on CNGC6 expression levels (Supplementary Figure 1), indicating that CNGC6 regulates H2O2 accumulation under HS conditions. Thus, we first examined H2O2 levels using the fluorescent probe CM-H2DCFDA. The results showed that high temperatures stimulated H2O2 accumulation according to their CNGC6 expression levels in the seedlings (Figures 1A,B), indicating an important role of CNGC6 in the regulation of H2O2 production in the HS pathway.
Because of the role of CNCG6 in conducting Ca2+ into the cytoplasm in HS-treated plants, we determined the effects of Ca2+ on H2O2 accumulations in the wild-type, cngc6, and COM12 seedlings. The results showed that Ca2+ increased H2O2 accumulation in the seedlings under high temperature, whereas the Ca2+ chelator EGTA clearly reduced H2O2 accumulations in the wild-type and COM12 seedlings (Figures 1C–H), indicating that CNGC6-mediated free Ca2+ is a crucial factor in promoting H2O2 signaling. Thus, we propose that CNGC6 participates in stimulating internal H2O2 levels via free Ca2+ in the HS pathway. However, EGTA had no clear effect on the H2O2 level in cngc6 seedlings, which might be due to the smaller increase in free Ca2+ under HS exposure (Figures 1E,F).
To interpret the effects of CNGC6 and H2O2 on thermotolerance, we determined the effects of H2O2 on the survival of wild-type and cngc6 seedlings exposed to HS conditions. Exogenous applications of H2O2 enhanced the internal H2O2 levels and the survival ratios of both of HS-treated wild-type and cngc6 seedlings (Figure 2). The overexpression of two HS-responsive H2O2 synthesis-related enzymes, RbohB and RbohD, simultaneously elevated the internal H2O2 levels and the survival ratios of these transgenic lines, in comparison with their non-transgenic background cngc6 under HS conditions (Figures 3, 4), respectively, indicating that an increase in internal H2O2 restored the heat sensitivity of the mutant plants because of the absence of CNGC6. We also identified a strange phenomenon in that a high H2O2 concentration (200 μM) could not produce a high internal H2O2 level under HS conditions (Figures 2A,B), which is likely due to plant self-protection against oxidative damage as discussed previously (Wang et al., 2014; Wu et al., 2015).
Next, we obtained the triple mutant cngc6/rbohB/D, which showed a phenotype similar to that of the rbohB/D double mutant under normal and HS conditions (Figure 5), revealing that deficiencies in CNGC6 and RbohB/D do not aggravate the heat susceptibility due to a deficiency in RbohB/D.
Collectively, the upon results provide physiological and genetic proof for the existence of a novel HS signaling pathway in which CNGC6 is activated by high temperatures to mediate H2O2 accumulation to confer plant thermotolerance.
Hydrogen peroxide is the especially stable one of ROS and regulates plant growth, development, and stress adaptations. It acts through increasing [Ca2+]cyt as a second messenger, by the activation of the PM Ca2+-permeable influx channels as a primary part of this process (Ordoñez et al., 2014; Richards et al., 2014; Shabala, 2019). However, only few studies have drawn the opposite conclusion that Ca2+ influx influences H2O2 generation. For example, the silencing of two tomato CNGC genes, SICNGC1 and SICNGC14, was reported to strikingly promote both pathogen-induced and flg22-elicited H2O2, revealing that two SICNGCs inhibit ROS production and attenuate non-host resistance and PAMP-triggered immunity (Zhang et al., 2018). Accordingly, we wondered whether H2O2 stimulates Ca2+ influxes to confer thermotolerance.
A marked elevation in Ca2+ current was presented in the response to a swift temperature increase from 22 to 37°C in the wild-type. However, the current was clearly inhibited in cngc6, cngc6/rbohB/D, cngc6/35S::RbohB-1, and cngc6/35S::RbohD-1 plants but not obviously varied in rbohB/D plants (Figure 6), showing no great effect of H2O2 on the activity of Ca2+-permeable channel. These results, in combination with those shown in Figures 2–5, proposed that the HS-induced alteration in Ca2+ unidirectionally stimulates H2O2 signaling in plants. A plausible interpretation for these data is that supplementation with H2O2, a downstream signal molecule, rescued the heat-susceptible phenotype of the CNGC6-deficient seedlings (Figures 2–5) but could not elevate the heat-responsive activity of CNGC6 (Figure 6).
To examine the mechanisms by which CNGC6 influences heat tolerance via H2O2, we determined the effects of CNGC6 and H2O2 on Hsf transcript and HSP expression under HS conditions.
Heat shock factors are known as downstream elements in the HS signaling pathway to regulate heat tolerance by deciding the expression of HSPs as the response to phosphorylation (Kotak et al., 2007). Our current data indicated that a reduction in the level of CNGC6 prohibits the transcript levels of Hsfs, whereas applications of H2O2 and overexpression of RbohB and RbohD elevates them in cngc6 plants (Figure 7). Therefore, H2O2 appears to restore the CNGC6 effects, thereby influencing the Hsfs transcription and inducing to thermotolerance.
Heat shock protein genes, stimulated by HSFs linking to promoter elements, are categorized depending on their molecular masses, for example, HSP110, HSP100, HSP90, HSP70, and small HSPs, which are the most important ones among them due to their irreplaceable role in plant tolerance against high temperatures (Carre et al., 2019). To interpret the relationship between CNGC6 and H2O2 in the HS signaling pathway, we used HSP21 and HSP17.7, two small HSPs, to examine how CNGC6 mediates thermotolerance through H2O2. Western-blot analysis revealed that the reduced CNGC6 level in cngc6 mutant decreased HSP21 and HSP17.7 expression under HS conditions, whereas application of H2O2 and the overexpression of RbohB or RbohD in cngc6 plants increased the accumulation of HSP21 and HSP17.7 (Figure 8), indicating that CNGC6 activated HSP expression via H2O2. Taken together, the mechanism through which CNGC6 influences thermotolerance via H2O2 involves variations in HSP gene expression.
These upon results suggest that CNGC6, the HS-responsive Ca2+-permeable channel, takes part in the initiation of HS signaling transduction through H2O2. We previously suggested a model for the HS signaling pathway in which the HS signal was received by an unknown receptor, resulting in an elevated H2O2 level and then stimulating NO production and AtCaM3 expression to initiate plant resistance against high temperatures (Xuan et al., 2010; Wang et al., 2014). Additionally, feedback inhibition existed between NO and H2O2 in the HS signaling pathway in Arabidopsis (Wu et al., 2015). AtCaM3 also inhibited excess NO accumulation and enhanced plant thermotolerance through stimulating S-nitrosoglutathione reductase by direct binding (Zhang et al., 2020). Recently, we found that CNGC6 through free Ca2+ acts upstream of NO in plant response to HS (Peng et al., 2019). In this work, CNGC6 was also proposed to act upstream of H2O2 through free Ca2+ in the HS pathway. Ca2+ and AtCaM3 are associated with HSP gene expression in Arabidopsis (Zhang et al., 2009). CaM, upon binding to Ca2+, attaches to specific targets, increasing their functions as part of a HS-responsive Ca2+ signaling pathway, for instance, CaM-binding protein kinase 3 (Liu et al., 2008) and PP7 (Liu et al., 2007). Thus, these findings suggest that interactions exist among Ca2+ channels, H2O2, NO, and the Ca2+/CaM-dependent target proteins to participate in regulating HSP expression in the HS pathway.
Sequence data from this article can be found in GenBank/EMBL under the following accession numbers: AtRbohB (At1G09090), AtRbohD (AT5G47910), CNGC6 (At2g23980), and Actin2 (At3g18780).
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
BL and LZo conceived the project and designed the research. WW and JZ carried out the phenotypic observation, RT-qPCR analysis, Arabidopsis transgenic experiments, and Western blot analysis. WW and LA carried out the whole-cell voltage patch-clamping. LZn and DW participated in the data analysis. LZo wrote the article with contributions from all authors and revised and proofread the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China Grants 31770297 (to LZo) and 31770261 (to LZn).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Miguel Angel Torress (Universidad Politécnica de Madrid) for providing the seeds and Yan Guo (China Agriculture University) for providing the pCAMBIA1307-6×Myc vector used in this research.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2021.708672/full#supplementary-material
Akerfelt, M., Morimoto, R. I., and Sistonen, L. (2010). Heat shock factors: integrators of cell stress, development and lifespan. Nat. Rev. Mol. Cell Biol. 11, 545–555. doi: 10.1038/nrm2938
Banti, V., Mafessoni, F., Loreti, E., Alpi, A., and Perata, P. (2010). The heat-inducible transcription factor HsfA2 enhances anoxia tolerance in Arabidopsis. Plant Physiol. 152, 1471–1483. doi: 10.1104/pp.109.149815
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254. doi: 10.1016/0003-2697(76)90527-3
Brost, C., Studtrucker, T., Reimann, R., Denninger, P., Czekalla, J., Krebs, M., et al. (2019). Multiple cyclic nucleotide-gated channels coordinate calcium oscillations and polar growth of root hairs. Plant J. 99, 910–923. doi: 10.1111/tpj.14371
Carre, S., Alberti, S., Benesch, J. L. P., Boelens, W., Buchner, J., Carver, J. A., et al. (2019). Small heat shock proteins: multifaceted proteins with important implications for life. Cell Stress Chaperones 24, 295–308. doi: 10.1007/s12192-019-00979-z
Chaouch, S., Queval, G., and Noctor, G. (2012). AtRbohF is a crucial modulator of defence-associated metabolism and a key actor in the interplay between intracellular oxidative stress and pathogenesis responses in Arabidopsis. Plant J. 69, 613–627. doi: 10.1111/j.1365-313X.2011.04816.x
Chozinski, T. J., Halpern, A. R., Okawa, H., Kim, H. J., Tremel, G. J., Wong, R. O. L., et al. (2016). Expansion microscopy with conventional antibodies and fluorescent proteins. Nat. Methods 13, 485–488. doi: 10.1038/nmeth.3833
Clough, S. J., and Bent, A. F. (1998). Floral dip: a simplified method for agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743. doi: 10.1046/j.1365-313x.1998.00343.x
Cui, Y., Lu, S., Li, Z., Cheng, J., Hu, P., Zhu, T., et al. (2020). Cyclic nucleotide-gated ion channels 14 and 16 promote tolerance to heat and chilling in rice. Plant Physiol. 183, 1794–1808. doi: 10.1104/pp.20.00591
Demidchik, V., and Tester, M. (2002). Sodium fluxes through nonselective cation channels in the PM of protoplasts from Arabidopsis roots. Plant Physiol. 128, 379–387. doi: 10.1104/pp.010524
Ferreira, A. C., de Carvalho Cardoso, L., Rosenthal, D., and de Carvalho, D. P. (2003). Share thyroid Ca2+/NADPH-dependent H2O2 generation is partially inhibited by propylthiouracil and methimazole. Eur. J. Biochem. 270, 2363–2368. doi: 10.1046/j.1432-1033.2003.03576.x
Gao, Q. F., Gua, L. L., Wang, H. Q., Fei, C. F., Fang, X., Hussain, J., et al. (2016). Cyclic nucleotide-gated channel 18 is an essential Ca2+ channel in pollen tube tips for pollen tube guidance to ovules in Arabidopsis. Proc. Natl. Acad. Sci. U. S. A. 113, 3096–3101. doi: 10.1073/pnas.1524629113
Gao, F., Han, X., Wu, J., Zheng, S., Shang, Z., Sun, D., et al. (2012). A heat-activated calcium-permeable channel—Arabidopsis cyclic nucleotide-gated ion channel 6—is involved in heat shock responses. Plant J. 70, 1056–1069. doi: 10.1111/j.1365-313X.2012.04969.x
Gechev, T. S., and Hille, J. (2005). Hydrogen peroxide as a signal controlling plant programmed cell death. J. Cell Biol. 168, 17–20. doi: 10.1083/jcb.200409170
Gupta, D. K., Pena, L. B., Romero-Puertas, M. C., Hernández, A., Inouhe, M., and Sandalio, L. M. (2017). NADPH oxidases differentially regulate ROS metabolism and nutrient uptake under cadmium toxicity. Plant Cell Environ. 40, 509–527. doi: 10.1111/pce.12711
Islam, M. M., Ye, W., Matsushima, D., Rhaman, M. S., Munemasa, S., Okuma, E., et al. (2019). Reactive carbonyl species function as signal mediators downstream of H2O2 production and regulate [Ca2+]cyt elevation in ABA signal pathway in Arabidopsis guard cells. Plant Cell Physiol. 60, 1146–1159. doi: 10.1093/pcp/pcz031
Iwai, S., Ogata, S., Yamada, N., Onjo, M., Sonoike, K., and Shimazaki, K. (2019). Guard cell photosynthesis is crucial in abscisic acid-induced stomatal closure. Plant Direct 3:e00137. doi: 10.1002/pld3.137
Jha, S. K., Sharma, M., and Pandey, G. K. (2016). Role of cyclic nucleotide gated channels in stress management in plants. Curr. Genomics 17, 315–329. doi: 10.2174/1389202917666160331202125
Jiang, Z., Zhu, S., Ye, R., Xue, Y., Chen, A., An, L., et al. (2013). Relationship between NaCl- and H2O2-induced cytosolic Ca2+ increases in response to stress in Arabidopsis. PLoS One 8:e76130. doi: 10.1371/journal.pone.0076130
Königshofer, H., Tromballa, H. W., and Löppert, H. G. (2008). Early events in signalling high-temperature stress in tobacco BY2 cells involve alterations in membrane flfluidity and enhanced hydrogen peroxide production. Plant Cell Environ. 31, 1771–1780. doi: 10.1111/j.1365-3040.2008.01880.x
Kotak, S., Larkindale, J., Lee, U., von Koskull-Döring, P., Vierling, E., and Scharf, K. D. (2007). Complexity of the heat stress response in plants. Curr. Opin. Plant Biol. 10, 310–316. doi: 10.1016/j.pbi.2007.04.011
Larkindale, J., Hall, J. D., Knight, M. R., and Vierling, E. (2005). Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance. Plant Physiol. 138, 882–897. doi: 10.1104/pp.105.062257
Lawas, L. M. F., Zuther, E., Jagadish, S. V. K., and Hincha, D. K. (2018). Molecular mechanism of combined heat and drought stress resilience in cereals. Curr. Opin. Plant Biol. 45, 212–217. doi: 10.1016/j.pbi.2018.04.002
Lewis, A. M., Matzdorf, S. S., and Rice, K. C. (2016). Fluorescent detection of intracellular nitric oxide in Staphylococcus aureus. Bio Protoc. 6:e1878. doi: 10.21769/BioProtoc.1878
Li, N., Sun, L., Zhang, L., Song, Y., Hu, P., Li, C., et al. (2015). AtrbohD and AtrbohF negatively regulate lateral root development by changing the localized accumulation of superoxide in primary roots of Arabidopsis. Planta 241, 591–602. doi: 10.1007/s00425-014-2204-1
Liu, H. T., Gao, F., Li, G. L., Han, J. L., Liu, D. L., Sun, D. Y., et al. (2008). The calmodulin-binding protein kinase 3 is part of heat-shock signal transduction in Arabidopsis thaliana. Plant J. 55, 760–773. doi: 10.1111/j.1365-313X.2008.03544.x
Liu, H. T., Li, G. L., Chang, H., Sun, D. Y., Zhou, R. G., and Li, B. (2007). Calmodulin binding protein phosphatase PP7 is involved in thermotolerance in Arabidopsis. Plant Cell Environ. 30, 156–164. doi: 10.1111/j.1365-3040.2006.01613.x
Liu, H. T., Sun, D. Y., and Zhou, R. G. (2005). Ca2+ and AtCaM3 are involved in the expression of heat shock protein gene in Arabidopsis. Plant Cell Environ. 28, 1276–1284. doi: 10.1111/j.1365-3040.2005.01365.x
Macpherson, N., Takeda, S., Shang, Z., Dark, A., Mortimer, J. C., Brownlee, C., et al. (2008). NADPH oxidase involvement in cellular integrity. Planta 227, 1415–1418. doi: 10.1007/s00425-008-0716-2
Maruta, T., Inoue, T., Tamoi, M., Yabuta, Y., Yoshimura, K., Ishikawa, T., et al. (2011). Arabidopsis NADPH oxidases, AtrbohD and AtrbohF, are essential for jasmonic acid-induced expression of genes regulated by MYC2 transcription factor. Plant Sci. 180, 665–660. doi: 10.1016/j.plantsci.2011.01.014
Nazir, F., Fariduddin, Q., and Khan, T. A. (2020). Hydrogen peroxide as a signalling molecule in plants and its crosstalk with other plant growth regulators under heavy metal stress. Chemosphere 252:126486. doi: 10.1016/j.chemosphere.2020.126486
Niu, W. T., Han, X. W., Wei, S. S., Shang, Z. L., Wang, J., Yang, S. W., et al. (2020). Arabidopsis cyclic nucleotide-gated channel 6 is negatively modulated by multiple calmodulin isoforms during heat shock. J. Exp. Bot. 71, 90–104. doi: 10.1093/jxb/erz445
Ordoñez, N. M., Marondedze, C., Thomas, L., Pasqualini, S., Shabala, L., Shabala, S., et al. (2014). Cyclic mononucleotides modulate potassium and calcium flux responses to H2O2 in Arabidopsis roots. FEBS Lett. 588, 1008–1015. doi: 10.1016/j.febslet.2014.01.062
Pan, Y., Chai, X., Gao, Q., Zhou, L., Zhang, S., Li, L., et al. (2019). Dynamic interactions of plant CNGC subunits and calmodulins drive oscillatory Ca2+ channel activities. Dev. Cell 48, 710–725. doi: 10.1016/j.devcel.2018.12.025
Peng, X., Zhang, X., Li, B., and Zhao, L. (2019). Cyclic nucleotide-gated ion channel 6 mediates thermotolerance in Arabidopsis seedlings by regulating nitric oxide production via cytosolic calcium ions. BMC Plant Biol. 19:368. doi: 10.1186/s12870-019-1974-9
Richards, S. L., Laohavisit, A., Mortimer, J. C., Shabala, L., Swarbreck, S. M., Shabala, S., et al. (2014). Annexin 1 regulates the H2O2-induced calcium signature in Arabidopsis thaliana roots. Plant J. 77, 136–145. doi: 10.1111/tpj.12372
Shabala, S. (2019). Linking ploidy level with salinity tolerance: NADPH-dependent ‘ROS-Ca2+ hub’ in the spotlight. J. Exp. Bot. 70, 1063–1067. doi: 10.1093/jxb/erz042
Sun, A. Z., and Guo, F. Q. (2016). Chloroplast retrograde regulation of heat stress responses in plants. Front. Plant Sci. 7:398. doi: 10.3389/fpls.2016.00398
Talke, I. N., Blaudez, D., Maathuis, F. J., and Sanders, D. (2003). CNGCs: prime targets of plant cyclic nucleotide signalling? Trends Plant Sci. 8, 286–293. doi: 10.1016/S1360-1385(03)00099-2
Tian, W., Hou, C., Ren, Z., Wang, C., Zhao, F., Dahlbeck, D., et al. (2019). A calmodulin-gated calcium channel links pathogen patterns to plant immunity. Nature 572, 131–135. doi: 10.1038/s41586-019-1413-y
Walker, R. K., and Berkowitz, G. A. (2013). Detection of reactive oxygen species downstream of cyclic nucleotide signals in plants. Methods Mol. Biol. 1016, 245–252. doi: 10.1007/978-1-62703-441-8_17
Wang, L., Guo, Y., Jia, L., Chu, H., Zhou, S., Chen, K., et al. (2014). Hydrogen peroxide acts upstream of nitric oxide in the heat shock pathway in Arabidopsis seedlings. Plant Physiol. 164, 2184–2196. doi: 10.1104/pp.113.229369
Wang, J., Liu, X., Zhang, A., Ren, Y., Wu, F., Wang, G., et al. (2019). A cyclic nucleotide-gated channel mediates cytoplasmic calcium elevation and disease resistance in rice. Cell Res. 29, 820–831. doi: 10.1038/s41422-019-0219-7
Wang, Y., Ries, A., Wu, K., Yang, A., and Crawford, N. M. (2010). The Arabidopsis prohibition gene PHB3 functions in nitric oxide-mediated responses and in hydrogen peroxide-induced nitric oxide accumulation. Plant Cell 22, 249–259. doi: 10.1105/tpc.109.072066
Wu, D., Chu, H., Jia, L., Chen, K., and Zhao, L. (2015). A feedback inhibition between nitic oxide and hydrogen peroxide in the heat shock pathway in Arabidopsis seedlings. Plant Growth Regul. 75, 503–509. doi: 10.1007/s10725-014-0014-x
Xuan, Y., Zhou, S., Wang, L., Cheng, Y., and Zhao, L. (2010). Nitric oxide functions as a signal and acts upstream of AtCaM3 in thermotolerance in Arabidopsis seedlings. Plant Physiol. 153, 1895–1906. doi: 10.1104/pp.110.160424
Zelman, A. K., Dawe, A., Gehring, C., and Berkowitz, G. A. (2012). Evolutionary and structural perspectives of plant cyclic nucleotide-gated cation channels. Front. Plant Sci. 3:95. doi: 10.3389/fpls.2012.00095
Zhang, X., Wang, W., Kang, X., and Zhao, L. (2020). Arabidopsis CaM3 inhibits nitric oxide accumulation and improves thermotolerance by promoting S-nitrosoglutathione reductase via direct binding. Plant Growth Regul. 90, 41–50. doi: 10.1007/s10725-019-00552-9
Zhang, X. R., Xu, Y. P., and Cai, X. Z. (2018). SICNGC1 and SICNGC14 suppress Xanthomonas oryzae pv. Oryzicola-induced hypersensitive response and non-host resistance in tomato. Front. Plant Sci. 9:285. doi: 10.3389/fpls.2018.00285
Zhang, W., Zhou, R. G., Gao, Y. J., Zheng, S. Z., Xu, P., Zhang, S. Q., et al. (2009). Molecular and genetic evidence for the key role of AtCaM3 in heat-shock signal transduction in Arabidopsis. Plant Physiol. 149, 1773–1784. doi: 10.1104/pp.108.133744
Keywords: heat shock, heat shock (stress) proteins, hydrogen peroxide, Arabidopsis, calcium ion
Citation: Wang W, Zhang J, Ai L, Wu D, Li B, Zhang L and Zhao L (2021) Cyclic Nucleotide-Gated Ion Channel 6 Mediates Thermotolerance in Arabidopsis Seedlings by Regulating Hydrogen Peroxide Production via Cytosolic Calcium Ions. Front. Plant Sci. 12:708672. doi: 10.3389/fpls.2021.708672
Received: 12 May 2021; Accepted: 22 June 2021;
Published: 14 July 2021.
Edited by:
Rosa M. Rivero, Center for Edaphology and Applied Biology of Segura, Spanish National Research Council (CSIC), SpainReviewed by:
Anshika Narang, Rutgers University, Newark, United StatesCopyright © 2021 Wang, Zhang, Ai, Wu, Li, Zhang and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liqun Zhao, emhhb2xxNzBAMTYzLmNvbQ==, orcid.org/0000-0001-6718-8130
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.