- 1Center for Agricultural Water Research in China, China Agricultural University, Beijing, China
- 2Institute of Tibet Plateau Ecology, Tibet Agriculture and Animal Husbandry University, Nyingchi, China
- 3Key Laboratory of Forest Ecology in Tibet Plateau (Tibet Agriculture and Animal Husbandry University), Ministry of Education, Nyingchi, China
Carbon metabolism in higher plants is a basic physiological metabolism, and carbon allocation and conversion require the activity of various enzymes in metabolic processes that alter the content and overall composition of sugars in the sink organ. However, it is not known how various enzymes affect carbon metabolism when tomato plants are subjected to water stress or treated with potassium. Although the process of carbon metabolism is very complex, we used the carbon conversion rate to compare and analyze the enzyme activities related to sugar metabolism and find out which carbon conversion rate are the most important. Results showed that water stress and potassium increased carbon import flux in the fruit, which was beneficial to carbon accumulation. Water deficit increased the activity of sucrose synthase (SuSy) and starch phosphorylase (SP) and decreased the activity of sucrose phosphate synthase (SPS) and adenosine diphosphate glucose pyrophosphorylase (AGPase) in the source. Water stress increased the activity of acid invertase (AI), SuSy and SP but decreased the activity of AGPase in the sink. Potassium modified the balance of enzymes active in sugar and starch metabolism by increasing the activity of AI, SuSy, SPS and SP and significantly decreasing the activity of AGPase, resulting in increase of hexose. Canonical correlational analysis revealed that the carbon conversion rate was mainly affected by the relative rate of conversion of sucrose to fructose and glucose [p1(t)] and glucose to starch [p5m(t)]. SuSy and AGPase had the greatest effect on enzyme activity in the fruit; respectively regulated p1(t) and p5m(t).
Introduction
Carbon metabolism is a basic physiological metabolism in high plants, but its process is complicated (Winter and Huber, 2000). In most plants, assimilate is transported from source to sink in the form of sucrose, and the ability of a plant organ to obtain assimilate depends to a large extent on its sink strength (Seehuber et al., 2011). Sink strength depends on enzyme activity in sucrose metabolism (Lunn and Macrae, 2003; Du et al., 2020). Enzyme activity, which affects the accumulation of sugar in the fruit, is regulated by external factors such as water and nutrients (Patan and Saita, 2015). It has been found that water stress increases the accumulation of hexose and thus improves fruit quality (Terry et al., 2009; Chen et al., 2013). Sugar metabolism is regulated by key enzymes, and water stress affects the activity of key enzymes in the metabolism of carbon assimilates (Mafakheri et al., 2011). Nutrients, such as potassium, promote the conversion and transport of photosynthesis products, which include sugars (Deeken et al., 2002; Walter and Difonzo, 2007). Thus plant respiration, enzyme activity, and sugar metabolism are all influenced by potassium (Philippe et al., 2006; Almeselmani et al., 2009; Zahoor et al., 2017b; Omondi et al., 2019), and so both water and mineral nutrients are key factors in determining carbon allocation (Reynolds and Tuberosa, 2008; Rosa et al., 2009; Witt et al., 2012).
As the “source” organ, the leaf is the only resource depend on for existence in plants (Büchi et al., 1998). Sugars and starch are important carbon metabolites in plants. Sucrose metabolism is critical to a plant’s success because it regulates enzyme activity in photoassimilates transported to sink tissue, thereby affecting the accumulation and type of various sugars in the fruit (Chopra et al., 2005; Jain et al., 2013). The enzymes that catalyze sucrose metabolism are mainly invertase β-fructofuranosidase (EC 3.2.1.26, Inv), sucrose synthase (EC 2.4.1.13, SuSy), and sucrose phosphate synthase (EC 2.4.1.14, SPS) (Schaffer and Petreikov, 1997; Tymowska-Lalanne and Kreis, 1998; Binh et al., 1999; Roitsch and Mari, 2004). They regulate the distribution of carbohydrates in source and sink organs, control the rate of sucrose absorption, and govern the storage of sucrose and hexose (Ruan, 2014). Starch metabolism is an accurate, systematic and complex process that includes starch synthesis and conversion. Starch phosphorylase (EC 2.4.1.1, SP) and ADP-glucose pyrophosphorylase (EC 2.7.7.27, AGPase) are the principal enzymes in starch metabolism (Vardy et al., 2002; Rathore et al., 2009; Subasinghe et al., 2014).
Carbon metabolism had been well studied. The SUGAR model was developed to represent the partitioning of carbon in peaches and to calculate the rates of carbon conversion; the model has been improved since its introduction (Génard and Souty, 1996). Subsequently, the model was used to predict the variation of different sugar concentrations in peach fruit with development and relative fruit growth rate under different environmental or management conditions (e.g., water deficit, thinning and different light interception) (Génard et al., 2003) or to simulate the sugar accumulation process in grape during the veraison-maturation stage (Dai et al., 2009). On this basis, Prudent et al. (2011) combined the two variables of genotype and sink-source ratio to describe the sugar accumulation in tomato fruits from the perspective of physiology and ecology, to provide a basis for understanding the physiological process of sugar accumulation in tomato.
However, there are few studies of how enzymes regulate carbon allocation in tomato under different water and nutrient supply condition, or of the relationship between enzymes and the carbon conversion rate. We analyzed and compared changes in enzyme activity in sucrose metabolism and starch metabolism while controlling water stress and potassium supply. We also investigated the relationship between enzymes and carbon conversion to identify the most important factors. We verified our model of the effects of water and potassium on enzyme activity and sugar metabolism, thus providing a theoretical basis for improving fruit quality by controlling sugar accumulation in the fruit.
Materials and Methods
Plant Materials and Growth Conditions
The experiments were conducted in a greenhouse at the Shiyanghe Experimental Station (37°52′N, 102°50′E, 1581 m elevation), Gansu Province, Northwest China, from April to August 2017. The greenhouse, 76 m × 8 m, was a steel frame construction covered with 0.2 mm thick polyethylene. A ventilation system on the roof controlled the interior daytime temperature in summer. Temperatures in the greenhouse from April-August 2017 ranged from 14.83 to 30.98°C and humidity from 25.98 to 91.42 RH. The research plant was an indeterminate pink tomato (Lycopersicon esculentum Miller cv. Jinpeng 11), a cultivar that is commonly planted by local farmers. Therefore, after the fifth fruit trusses appearance, the plants were pruned by removing the apex to stop the vegetative growth.
Plants in all treatments were fully irrigated at the seedling stage to ensure plant survival. At the third to fourth leaf stage, the single seedlings were transplanted into each plastic containers (top diameter 33 cm, bottom diameter 25 cm, depth 28 cm) and the container was buried in the ground up to its top edge to maintain a soil temperature in the container similar to that in the surrounding field. Cheesecloth and 1 kg of small gravel were packed at the bottom of each container to prevent soil loss, and the containers were filled with 17 kg of air-dried sandy loam soil (particle size < 5 mm) with bulk density 1.3 ± 0.5 g⋅cm–3. Planting was carried out in a single hole and single plant, with a row spacing of 80cm and a plant spacing of 60cm at the experimental site, with one drip irrigation belt controlling one crop row. The plants in each treatment were arranged in six north–south rows of 10 plants, a total of 240 plants. For each treatment, flowers of the first and fourth trusses in ten plants were marked with their pollination date. Due to the small north-south span in the greenhouse, the crops planted close to the underside of the vents and the edges of the greenhouse film are affected by the boundary effect to a certain extent, the experimental sites should be as far away from the inner greenhouse boundary as possible. In order to measure the fruit various indicators more accurately and to avoid errors in the results due to sampling, random sampling was concentrated in 2nd to 8th row. The experiment layout and sampling diagram were shown in Supplementary Figure 1. The basic physical properties of the soil were volumetric field capacity 0.258 (cm3⋅cm–3), saturated paste extract electrical conductivity 0.205 dS⋅m–1, available potassium 88 mg⋅kg–1, and pH 7.96.
During entire growth period, the tomato growth stage was divided into flowering and fruit-bearing stage (stage I: 2017-05-14–2017-06-15), fruit-swelling stage (stage II: 2017-06-16–2017-07-13), fruit maturation stage (stage III: 2017-07-14–2017-08-15). These three growth stages represent the stages of cell division (0–15 days after anthesis), cell expansion (15–48 days after anthesis) and maturation (more than 48 days after anthesis) of tomato fruit, according to Guichard et al. (2001) and Ripoll et al. (2015).
Treatments
Two levels of irrigation, full irrigation (W) and deficit irrigation (W/2) in four water treatments were created: CK (irrigation quantity W in every stage), T1 (stage I: W/2), T2 (stage II: W/2), T3 (stage III: W/2) and the full irrigation quantity W in the other stages in the experiment. Each water treatments was equally divided into 2 subgroups: potassium addition (K1) and without potassium (K0), the additional potassium treatment was the same for all treatments. Plants that were treated with potassium were identified as a subgroup by appending K to the group label: plants in group CK that were treated with potassium were identified as CKK and plants in the treatment group Ti that were treated with potassium were identified as TiK. The amount of potassium to be applied for optimum fruit development was determined from previous literature to be 0.46 g/kg (K2O:soil) per application (Han et al., 2012; Feng et al., 2017). Thus half of the plants was treated with a total of 15.64 g K2O in application twice (Table 1).
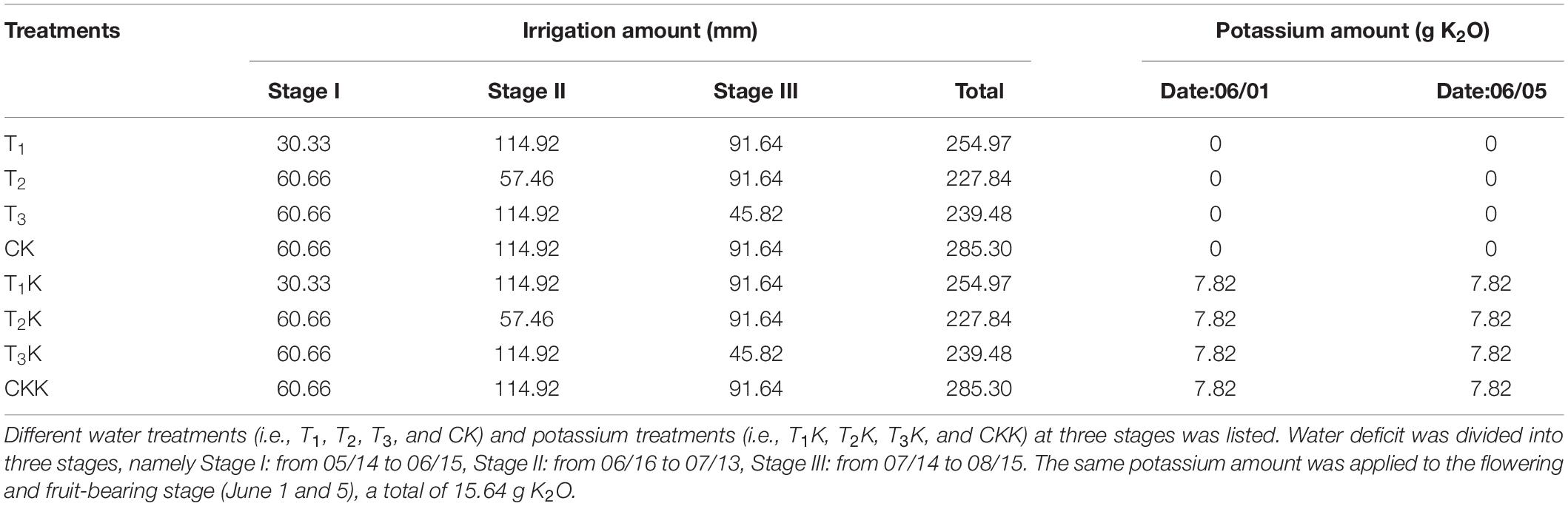
Table 1. Details of irrigation amount and potassium application in different water and potassium treatments in the three stages.
Test Items and Methods
Irrigation Amount
A 5TE sensor (Decagon Devices, Inc., United States) was installed at 15 cm depth in three randomly selected containers in every treatment to measure soil water content (SWC; cm3/cm3). The data were collected every 30 min by an EM50 data logger (Decagon Devices, Inc., United States). The sensors were calibrated gravimetrically using sensor-measured data for volumetric water content. When the water content in the containers decreased to 70% of field capacity θf (Agbenin and Tiessen, 1995), which was determined using the cutting ring method (Hu et al., 2011), the pots were irrigated to about 95% of field capacity. The amount of irrigation water was calculated using the equation:
where W (cm3) is the irrigation amount; θt1 and θt2 (cm3⋅cm–3) are, respectively, the upper limits of soil water content and the measured soil water content before irrigation; and V (cm3) is the pot soil volume. To prevent irrigation water leakage from the containers, irrigation occurred over a short period, and the irrigation quantity did not exceed field capacity. Irrigation quantities and potassium amounts applied during all growth stages are given in Table 1.
Index Measurement
Fruits were picked at 34 days after anthesis (DAA) from the first truss; 37, 48, and 57 DAA from the second truss; 58 and 65 DAA from the third truss; and 66 and 73 DAA from the fourth truss; the sampling of the leaves corresponds to the first leaf under each truss of fruits that has been picked, and each treatment was replicated three times. After picking, the fruit and leaves were quickly transferred to the laboratory, washed in distilled water and left to dry in a cool place. The fruit was cut open and a portion with pulp was weighed, then ground and mixed in a juicer for the determination of glucose, fructose, sucrose, starch content and enzyme activities related to sucrose and starch metabolism. Sugar content and related enzyme activities were measured every 5–7 days in fruit, and the whole growth stage was measured 8 times in total.
Sugar and Potassium Determination
Soluble sugars were extracted using the procedures described in Gomez et al. (2002) and assayed by HPLC analysis. Starch content was determined enzymatically using the method described in Gomez et al. (2003). The potassium content was determined by employing atomic absorption spectrophotometry (Xue et al., 2006).
Enzyme Extraction and Assays
SPS, SuSy and AI: leaf and fruit samples (0.2 g fresh weight) and 10 mL of extraction buffer (50 mM Hepes-NaOH, pH 7.5, 10 mM MgCl2, 2 mM EDTA, 5 Mm DTT, 2% (w/v) PVP) were ground into a homogenate in an ice bath. The samples were centrifuged subsequently at 12,000 × g for 20 min at 4 °C. The supernatant was gradually added with ammonium sulfate to 80% saturation and then centrifuged at 12,000 × g for 20 min at 4°C. The supernatant was discarded and the precipitate was dissolved with 3 mL of extraction buffer and then dialyzed for 20 h with 10-fold dilution of extraction buffer (without PVPP).
SPS activity was determined by the method of Keller and Ludlow (1993) with minor modifications. 50 μL enzyme solution was added to 50 μL 100 mM Hepes-NaOH buffer, 20 μL 50 mM MgCl2, 20 μL 100 mM UDPG, and 20 μL 100 mM fructose 6-phosphate. After 30 min, the reaction was terminated by the addition of 200 μL 40% NaOH solution, followed by 1.5 mL 30% HCl and 0.5 mL 1% resorcinol to determine the sucrose production, the unit of enzyme activity was expressed as μmol Suc⋅g–1⋅FW⋅h–1. SuSy activity (synthetic direction) was determined by replacing fructose 6-phosphate with fructose in the same way as SPS activity, the unit of enzyme activity was expressed as μmol Suc⋅g–1⋅FW⋅h–1. The AI activity was determined by the methods of Merlo and Passera (1991), 0.2 mL enzyme solution was added into 0.8 mL reaction solution (pH 4.8 0.1M Na2HPO4-0.1M sodium citrate, 0.1M sucrose), and reacted at 37 °C for 30min, the unit of enzyme activity was expressed as μmol Glu⋅g–1⋅FW⋅h–1.
SP: 0.3 g of fresh samples was taken into a pre-cooled mortar, enzyme extraction medium was added at w:v = 1:5 and ground into a homogenate in a rapid ice bath. The grindings were filtered through 4 layers of gauze, centrifuged at 4°C for 10 min at 12,000 × g and the supernatant was poured out as the crude enzyme solution. SP was determined according to the method of Merlo and Passera (1991), the reaction was started by adding 0.2 mL of reaction medium, 0.65 mL of distilled water, 0.05 mL of enzyme solution and finally 0.1 mL of Glu-1-P in a water bath at 30°C for 10 min. 0.5 mL of 5% TCA was added to terminate the reaction. For CK, TCA was added before the enzyme solution, and the other steps were performed as above. Centrifuged the reacted solution at 4,000 × g for 10 min to discard the precipitate, taken the supernatant for inorganic phosphorus determination. 0.3 mL supernatant and 2.7 mL of distilled water were added into the test tube, and then 3 mL of phosphate reagent was added. The liquid was shaken well and kept warm in 45°C water bath for 25 min. The absorbance was measured at 660nm wavelength and calculated, the unit of enzyme activity was expressed as μg Pi⋅g–1⋅FW⋅min–1.
AGPase: a fresh sample of 0.3 g was peeled and placed in a mortar after an ice bath and ground into a homogenate by adding 3 mL of extraction medium (100 mM Hepes-NaOH with pH 7.6, 2 mM EDTA, 5 mM DTT, 8 mM MgCl2, 12.5% (v/v) propanetriol and 5% (w/v) PVP), and then centrifuged at 12,000 × g for 10 min. Two milliliter of supernatant was taken into a 5 mL centrifuge tube and used for enzyme activity determination. AGPase was determined according to the method of Fernie et al. (2001), 100 μL 5 mM ADPG, 50 μL 50 mM MgCl2, 100 μL buffer, 50 μL enzyme extract, 100 μL 20 mM PPi was added to start the reaction for 15 min, and the reaction was terminated by boiling water bath for 1 min. Cool, add 100 μL 6 mM NADP+, 1.5U phosphoglucose metatase, 50 μL 5UL–1 6-P-G dehydrogenase, 0.3 mL buffer, total volume 1.5 mL, react at 30°C for 10min then colorimetric at 340 nm, use 1-P-G for standard curve, the unit of enzyme activity was expressed as nmol Glu⋅g–1⋅FW⋅min–1.
Related Model Parameters and Metabolism
The main physiological processes of carbon metabolism in tomato plants are shown in Figure 1. Sucrose is converted into glucose and fructose by sucrose invertase and sucrose synthase in fruits (María et al., 2000). Glucose and fructose are converted to sucrose by sucrose-phosphate synthase (Galtier et al., 1993). Glucose and fructose are also converted into each other (Makkee et al., 1984). Starch is synthesized from glucose by adenosine diphosphate glucose pyrophosphorylase (Schaffer and Petreikov, 1997); starch is also converted to glucose by amylase and inorganic pyrophosphatase (Nettancourt and Gösta, 1968). Starch compartment in tomato fruit is explicitly described in detail in Chen (2016); which followed principle of carbon balance (Walker and Thornley, 1977). Carbon allocation and transformation in fruit is closely related to the activity of metabolic enzymes during growth and development (Sharkey et al., 1991; Coleman et al., 2009). The carbon conversion rate can be calculated using Wu’s equations for peach fruit (Wu et al., 2012). The equations that describe diurnal carbon variation are the following.
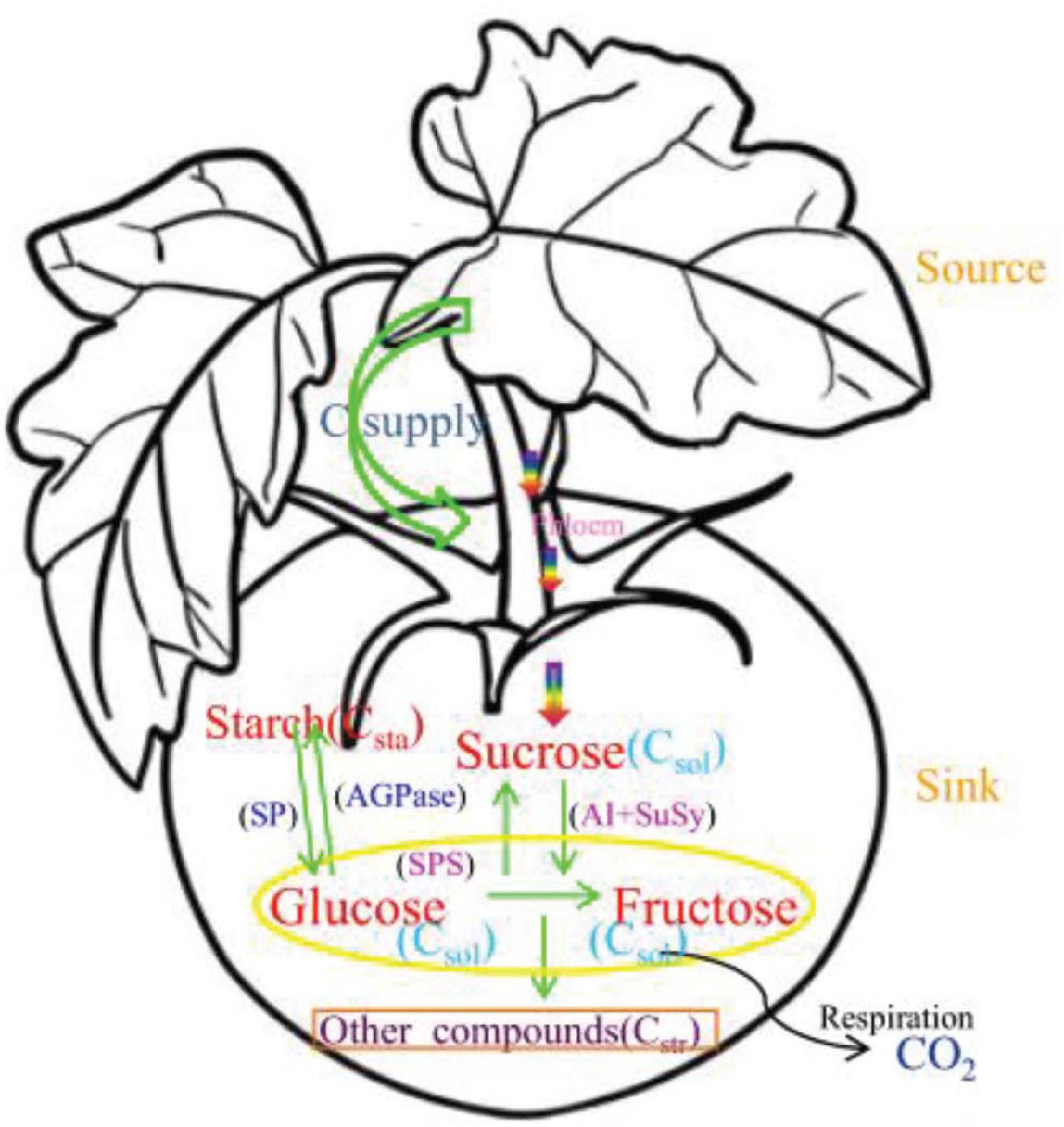
Figure 1. Carbon metabolism and related enzymes in tomato fruit. Arrows represent carbon flows, and the parameters p1(t), p2(t), p3(t), p4(t), p4m(t), and p5m(t) are the relative rates of carbon conversion for fructose, glucose, sucrose, starch and other compounds. The source is the leaf, which supplies carbon; the sink is the fruit, to which carbon is transported. Green arrows show the interconversion of different forms of carbon catalyzed by enzymes in the fruit. The yellow ellipse contains glucose and fructose, which are the main sugars in the tomato, orange rectangle denotes other carbon-containing compounds and the black curved line indicates carbon lost through respiration.
The parameters in the equations are defined in Table 2. Parameter values were found using the nls() and optim() functions in the computer language R. The process of solving for the parameters has been explained in detail by Luo et al. (2020) and is not shown here. Previous research have shown that glucose and fructose content in tomato fruits are almost equal (Islam et al., 1996; Levin et al., 2000; Qi et al., 2005), thus the parameters p4(t) and p4m(t) between glucose and fructose can be assumed to be a constant. The parameter p5(t) was considered to be a constant in previous studies (Nguyen-Quoc and Foyer, 2001; Chen et al., 2020) and is not discussed further. In this study, we focus on the relationship between enzymes active in sucrose and starch metabolism and the carbon conversion rates p1(t), p2(t), p3(t), and p5m(t).
Canonical Correlation Analysis of Enzyme and Carbon Conversion Rate
The vector composed of the parameters for the carbon conversion rate and enzyme activity measured in the fruit was used for canonical correlation analysis. The results were calculated according to Equations (10) and (11).
where U1 represents the linear combination of various enzyme activities in the fruit, and V1 represents the linear combination of the rates of carbon conversion. The significance of canonical variables is determined mainly by the variables with greater load.
Statistical Analysis
Three-way analysis of variance was performed using R studio version 3.6.1 (Robert, 2016) to evaluate the individual effects, and any interactive effects, of the three factors irrigation, potassium, and growth stage on source–sink enzyme activity (Tables 3, 5). Mean values were used for water treatments (shown by different letters), and the least significant difference (LSD) and multiple range tests were used to calculate differences between treatments at confidence level P < 0.05. Canonical correlational analysis, Spearman correlation analysis, multiple linear regression, nonlinear regression and the Kruskal–Wallis test were carried out using R, and the ggplot2-based plots were drawn using R packages ggpubr, ggthemes and PerformanceAnalytics (Alboukadel, 2017).
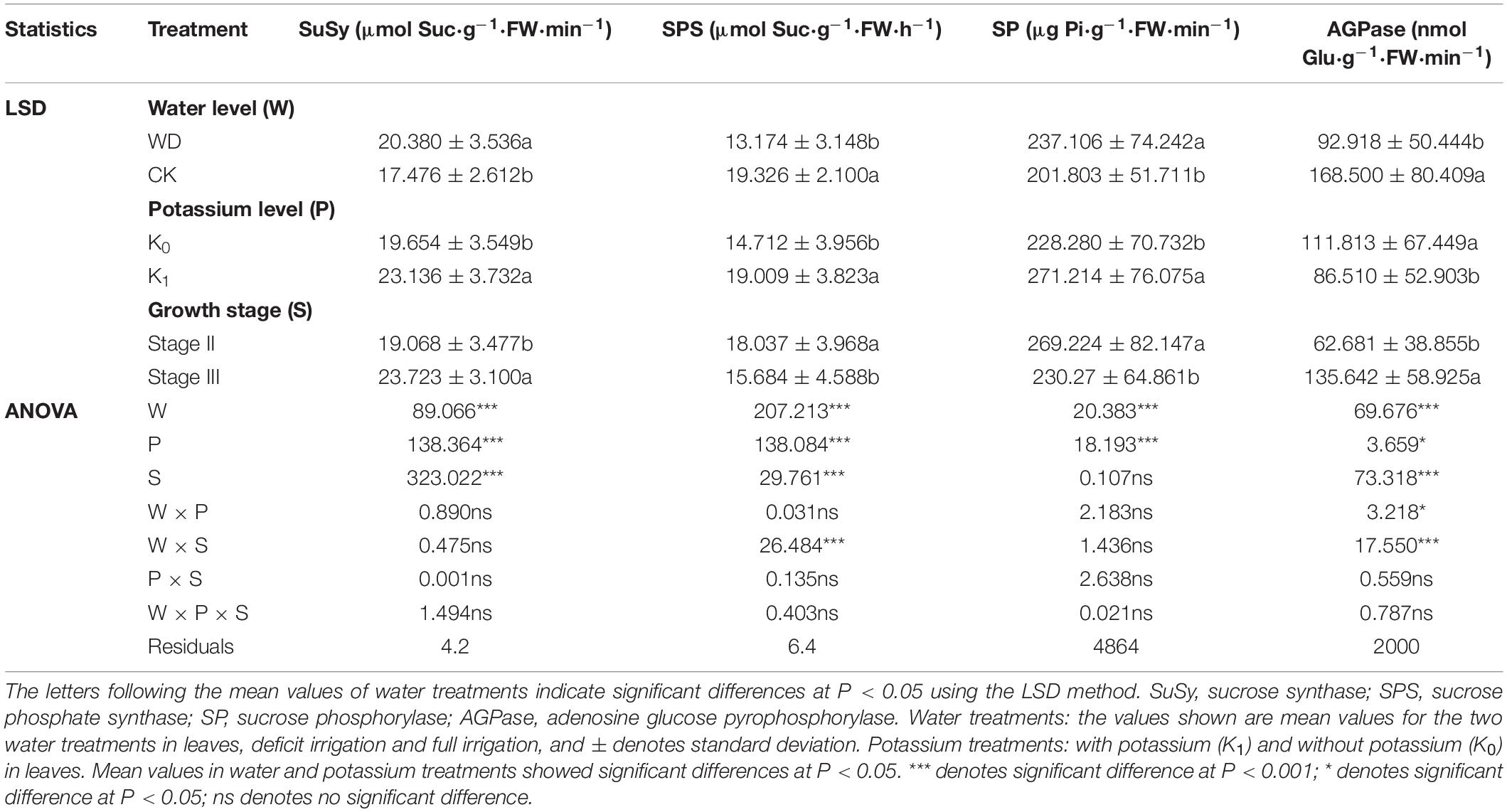
Table 3. Three-way analysis of variance of leaves SuSy, SPS, SP, and AGPase were performed to identify individual and interactive effects of water (2 levels: water deficit and CK), potassium (2 levels: K0, K1) and growth stage (2 levels: stage II and stage III) during all growth stages.
Results
Activity of Metabolic Enzymes Related to Source Leaves
Figure 2 showed the activity of sucrose synthase (SuSy), sucrose phosphate synthase (SPS), starch phosphorylase (SP), and adenosine glucose pyrophosphorylase (AGPase) in tomato leaves for different treatments; K0 denotes without potassium, and K1 denotes with potassium. CK denotes full irrigation, and WD denotes water deficit treatments (T1, T2, and T3).
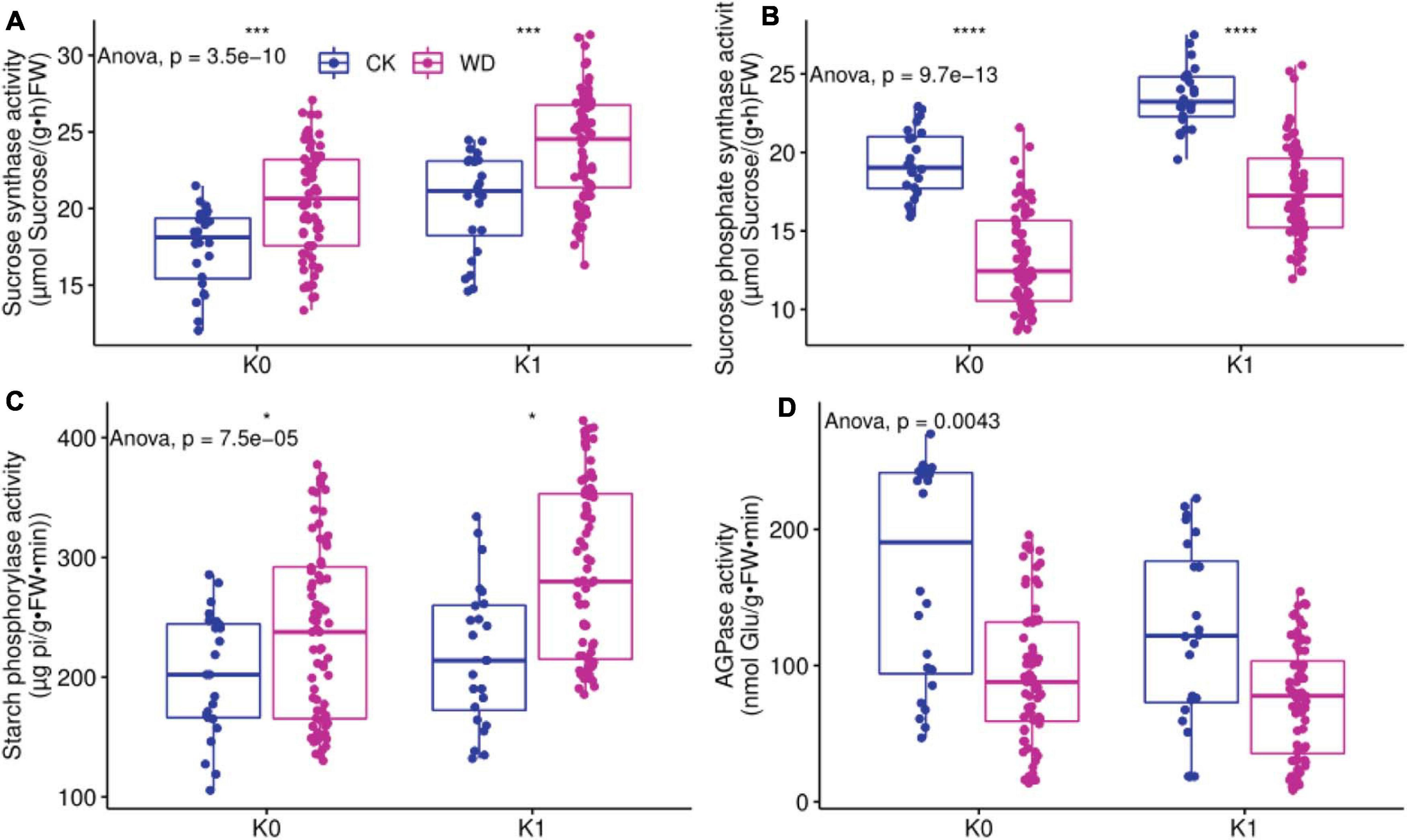
Figure 2. The sucrose synthase (SuSy), sucrose phosphate synthase (SPS), starch phosphorylase (SP), and starch synthase (AGPase) activity in source leaf. Water include 2 level: water deficit (WD) and CK, and potassium level contain without potassium (K0) and with potassium (K1). (A) SuSy activity in leaf under deficit irrigation and potassium application condition. (B) SPS activity in leaf under deficit irrigation and potassium application condition. (C) SP activity in leaf under deficit irrigation and potassium application condition. (D) AGPase activity in leaf under deficit irrigation and potassium application condition. * denotes significant difference at P < 0.05, *** denotes significant difference at P < 0.001, **** denotes significant difference at P < 0.0001.
The effects of water deficit and potassium on SuSy activity in leaves were considerable (Table 3). SuSy activity was greater in water deficit treatments (WD) than in full irrigation (CK) regardless of whether potassium was applied or not; regardless of water deficit or full irrigation, the SuSy activity of potassium treatment (K1) was significantly greater than without potassium treatment (K0), with was greatest in the water deficit and potassium treatments but least in CK.
The SPS activity of WD was significantly less than CK regardless of whether potassium was applied or not; whether full irrigation or water deficit conditions, K1 was greater than K0. The interactive effect of water deficit and stage had a significant effect on SPS, and WD was the least (Figure 2B).
The effects of water deficit and potassium application on SP activity were very noticeable (Table 3). Whether potassium was applied or not, the SP activity of WD was greater than CK; whether full irrigation or water deficit conditions, K1 was significantly greater than K0, of which water deficit and potassium condition was the greatest, and CK was the lowest (Figure 2C).
The effect of water deficit and potassium application on AGPase activity was highly significant, the interaction of water and potassium application had a significant impact on AGPase, and the interactive effect of water deficit and stage was considerable (Table 3). AGPase activity for WD was significantly less than for CK whether or not potassium was applied; AGPase activity was less for K1 than for K0 both under full irrigation or water deficit conditions and greatest in CK (Figure 2D).
Carbon Flux in the Sink Under Water and Potassium Supply
According to equation (7), the dCsup/dt was the carbon import to the fruit through the phloem, including the total carbon amount of dry matter and the carbon consumption through respiration, which can directly reflect the carbon flows into the fruit; and equation (9) showed that dCrep/dt was only related to dry matter, and the others are constants. Carbon flux (dCsup/dt) for the water deficit treatments (T1, T2 and T3) was greater than CK, compared with CK, T1 was significantly difference, both T2 and T3 were an extremely significant under water treatments (Figure 3A). However, in the potassium treatments (T1K, T2K, T3K, and CKK), the difference between T1K and CKK was not significant, T2K and CKK was very significant, T3K and CKK was significant (Figure 3B), in addition, all potassium treatments were greater than CK.
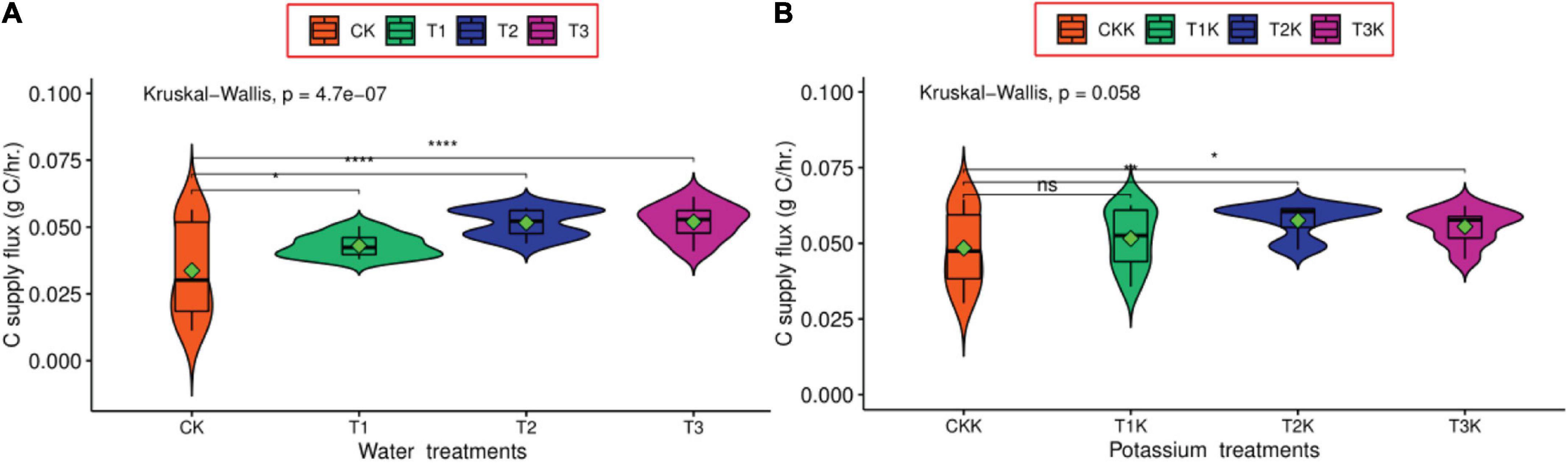
Figure 3. Diurnal variation of carbon supply flux between K0 and K1 treatments during the all growth stages. K0 indicates no potassium and K1 indicates potassium addition. The Wilcoxon signed rank test was used, ns denotes no significant difference, * denotes significant difference at the confidence level P < 0.05, *** denotes significant difference at the confidence level P < 0.001, **** denotes significant difference at P < 0.0001. (A) Carbon supply for different water treatments. (B) Carbon supply for different water and potassium treatments.
Sucrose-Metabolizing Enzyme Activity in the Sink
Figure 1 showed that the enzymes related to sucrose metabolism were SuSy, AI and SPS, and the sugars involved were hexose (glucose+fructose) and sucrose, sugars and enzymes activity over the entire fruit growth period were shown in Figure 4. Hexose increased with the fruit age, of which T2 was the highest and CK was the lowest, the difference between water treatments was significant. Compared with CK, T1, T2, and T3 increased by 13.18, 44.96, and 22.48%, respectively. The sucrose tended to decrease gradually with fruit age, but the range of variation was not large.
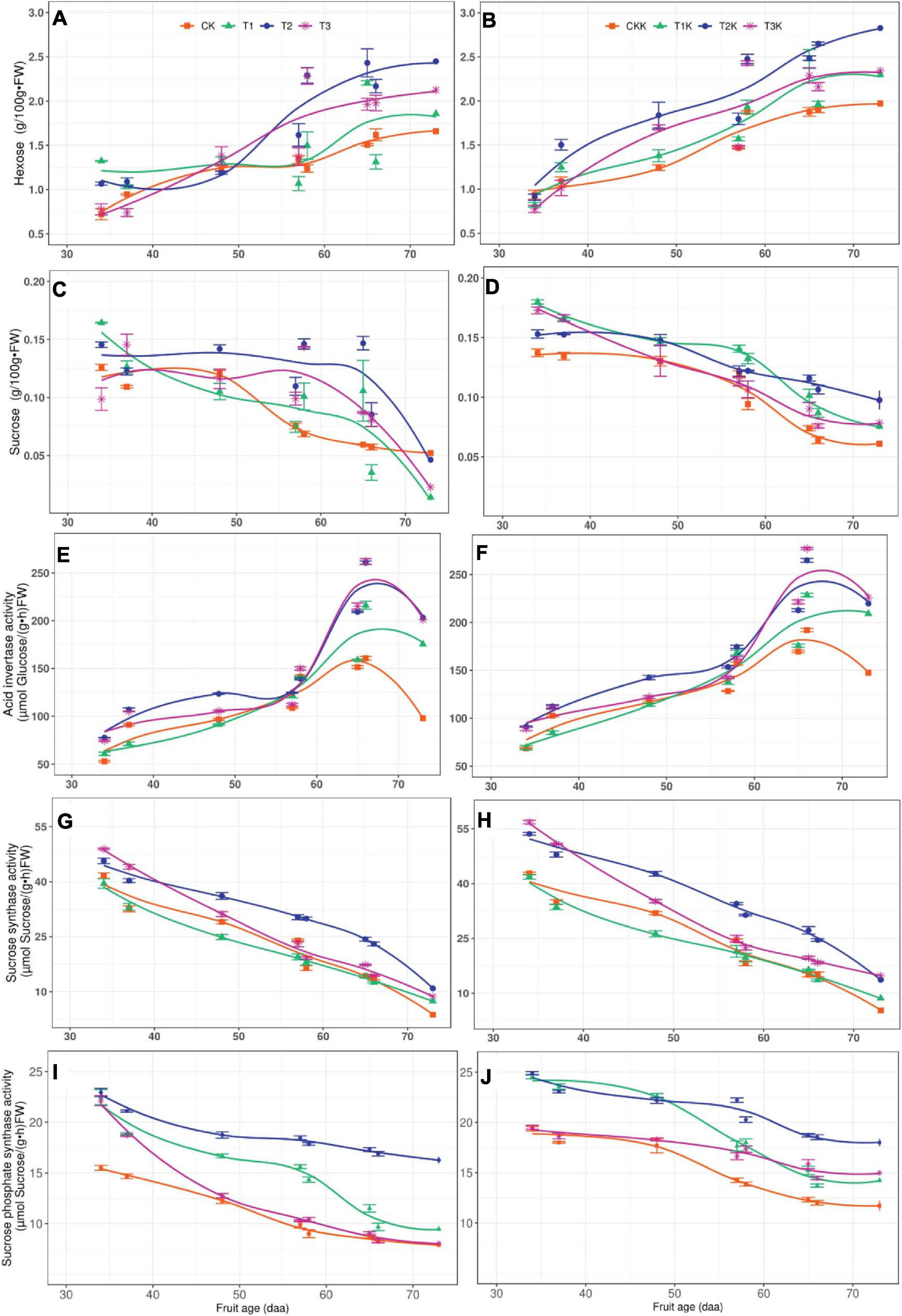
Figure 4. Related sugars and sucrose metabolic enzymes activity in fruit. Measured hexose (points), sucrose (points), acid invertase (points), sucrose synthase (point), sucrose phosphate synthase (point) and their corresponding fitted curves (lines) for the different water and potassium treatments as a function of days after anthesis (DAA). (A) Variation of Hexose with fruit age for different water treatments. (B) Variation of Hexose with fruit age for different water and potassium treatments. (C) Variation of Sucrose with fruit age for different water treatments. (D) Variation of Sucrose with fruit age for different water and potassium treatments. (E) Variation of AI activity with fruit age for different water treatments. (F) Variation of AI activity with fruit age for different water and potassium treatments. (G) Variation of SuSy activity with fruit age for different water treatments. (H) Variation of SuSy activity with fruit age for different water and potassium treatments. (I) Variation of SPS activity with fruit age for different water treatments. (J) Variation of SPS activity with fruit age for different water and potassium treatments.
Mean sucrose was ordered by treatment T2 > T3 > T1 > CK and water deficit had a significant on sucrose. The trends in sucrose for potassium treatments were the same as for the water treatments and potassium application had a significant effect on sucrose. It was found that both hexose and sucrose were greater for K1 (T1K, T2K, T3K, and CKK) than for K0 (T1, T2, T3, and CK) (Table 4).
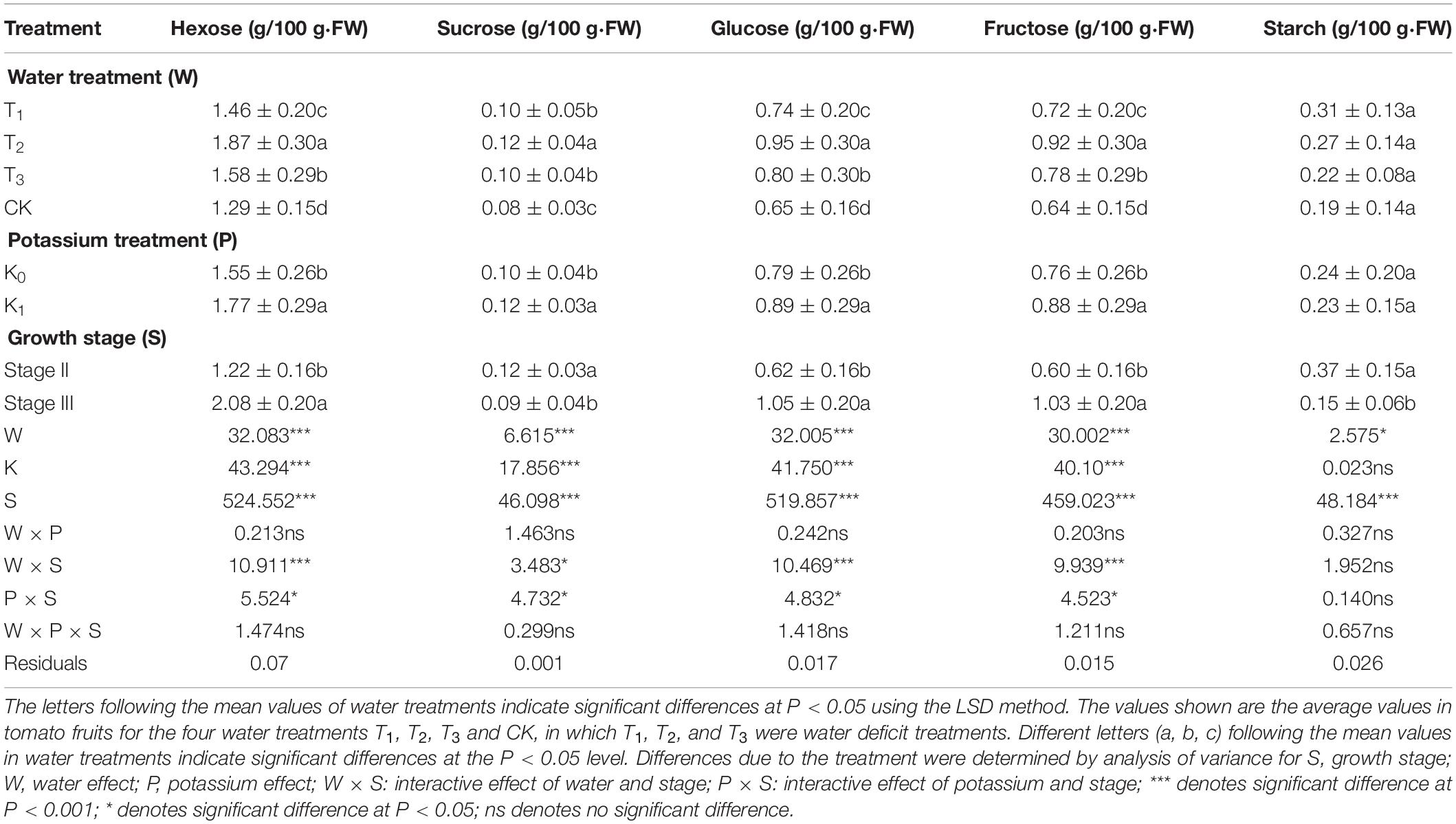
Table 4. Three-way analysis of variance of fruits Hexose, Sucrose, Glucose, Fructose and Strach were performed to identify individual and interactive effects of water (4 levels: T1,T2,T3, and CK), potassium (2 levels: K0, K1) and growth stage (2 levels: stage II and stage III) on the tomato fruits.
AI activity increased gradually as DAA increased and reached a maximum at 66 DAA, and then began to decrease (Figure 4E). The order of the AI mean was: T2 > T3 > T1 > CK, the AI for the water deficit treatments was higher than for CK. Compared with CK, T1, T2, and T3 increased by 15.07, 38.24, and 36.03%, but the difference between T2 and T3 was not notable. The results of F-test showed that water and potassium application had significant effect on AI, and the AI activity of Stage III was greater than Stage II. The variation trend of AI in potassium treatments was the same as that of water treatments (Figure 4F).
SuSy activity gradually decreased as DAA increased and reached a very low level at maturity (Figures 4G,H). The mean of SuSy activity under different water conditions was: T2 > T3 > T1 > CK, with water deficit treatments displayed significantly greater than CK. T1, T2, and T3 showed increases of 5.52, 37.12, and 17.88%, respectively, compared to CK. The potassium addition resulted in a very significant change in SuSy activity, SuSy was greater for K1 than K0 (Table 5).
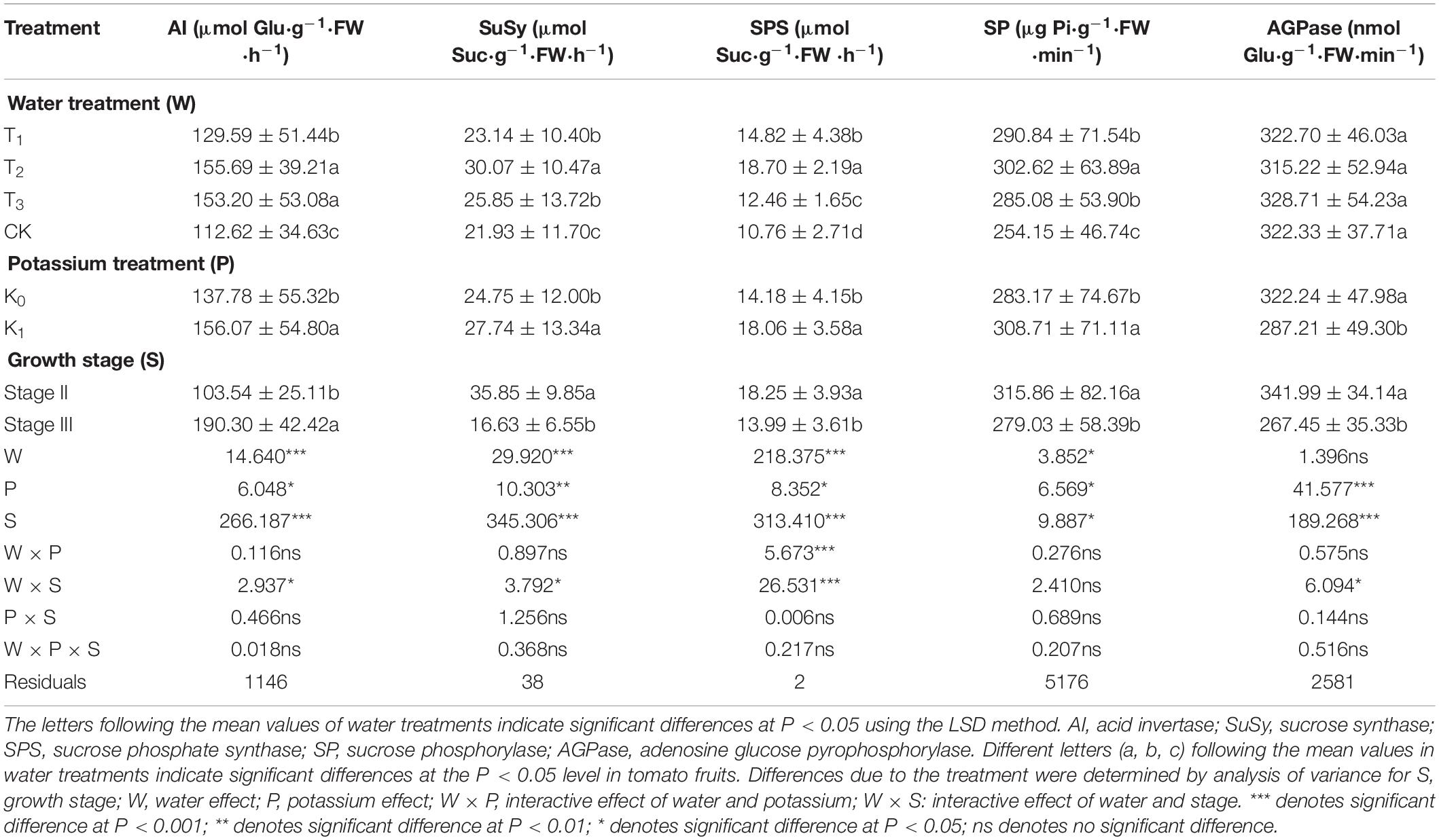
Table 5. Three-way analysis of variance of fruits AI, SuSy, SPS, SP, and AGPase, were performed to identify individual and interactive effects of water, potassium and growth stage on the tomato fruits.
SPS activity was greatest at the early stage and tended to decrease gradually with fruit age, but increased slightly at harvest stage, such as 73DAA (Figures 4I,J). The order of the SPS mean was: T2 > T1 > T3 > CK, with significant differences among treatments. Compared with CK, SPS increased by 18.94, 50.08, and 15.80% in T1, T2, and T3, respectively. SPS activity was greater for K1 than K0, showed that potassium had a great effect on SPS. The SPS activity of Stage II was greater than Stage III (Table 5).
Starch Metabolism Enzymes in the Sink
The main enzymes involved in starch synthesis and decomposition were SP and AGPase, and the sugars involved were starch and glucose (Figure 1). Glucose tended to increase gradually with fruit age, reaching a peak at maturity. The order of mean glucose was: T2 > T3 > T1 > CK (Figure 5A), with highly significant differences between different water treatments. Compared with CK, T1, T2, and T3 increasing by 13.85, 46.15, and 23.08%, respectively. Glucose displayed the same variation trend in potassium treatments, and T2K was the greatest (Table 4).
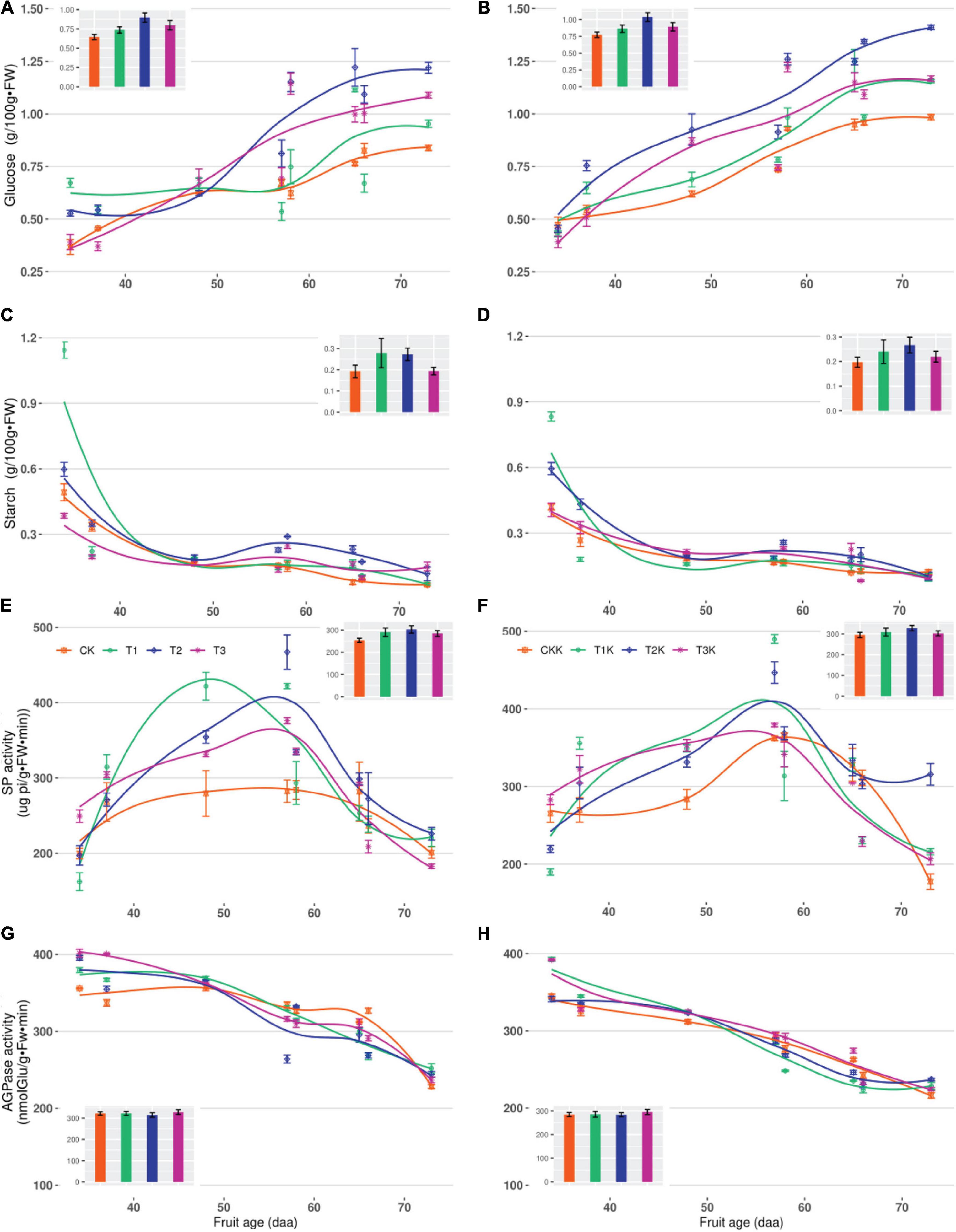
Figure 5. Related sugars and starch metabolic enzymes activity in fruit during the growth stages. Measured glucose (points), starch (points), starch phosphorylase (points), starch synthase (points) and their corresponding fitted curves (lines) for different water and potassium treatments are shown as functions of DAA. The bar chart showed the average value of each treatment. (A) Variation of Glucose with fruit age for different water treatments. (B) Variation of Glucose with fruit age for different water and potassium treatments. (C) Variation of Starch with fruit age for different water treatments. (D) Variation of Starch with fruit age for different water and potassium treatments. (E) Variation of SP activity with fruit age for different water treatments. (F) Variation of SP activity with fruit age for different water and potassium treatments. (G) Variation of AGPase activity with fruit age for different water treatments. (H) Variation of AGPase activity with fruit age for different water and potassium treatments.
Starch tended to decrease gradually with fruit growth and development, reached a minimum at maturity. The order of the mean starch for different water treatments was: T1 > T2 > T3 > CK, however, there was no significant difference among the treatments (Table 4).
The variation of SP and AGPase activity with fruit age (daa) for different treatments during the whole growth stage was shown in Figure 5. SP activity gradually increased with fruit age at the early stage and reached maximum at 48–57 daa, with the T1 treatment peaked 453μg pi/g⋅FW⋅min, and then gradually decreased until the harvest stage. The SP mean was: T2 > T1 > T3 > CK from bar chart (Figure 5E). Compared with CK, SP activity in water deficit treatment (T1, T2, and T3) was increased by 14.44, 19.07, and 12.17%, respectively. Change in SP for the potassium treatments showed a parabolic trend, SP increased as fruit age increased, reached a peak at 57 daa and then declined rapidly at during maturation. SP activity was greater for K1 than for K0, displayed that potassium had a significant effect on SP activity (Table 5).
AGPase activity gradually decreased as fruit age (daa) increased, from the initial value of 410 nmol Glu⋅g–1⋅FW⋅min–1 to 122 nmol Glu⋅g–1⋅FW⋅min–1 at maturity (Figure 5G). There was no notable difference in AGPase activity in the water deficit treatments (T1, T2, and T3) compared to CK, and the effect of water on AGPase was not significant. Variation in AGPase activity for potassium treatments (T1K, T2K, T3K, and CKK) showed a similar trend to water treatments (Figure 5H); also AGPase activity was less than for water treatments. This showed that potassium had a notable effect on AGPase activity, which varied significantly at different stages, Stage III was greater than Stage III in AGPase (Table 5).
Carbon Conversion Rate
We discussed the rate of carbon conversion between sugar and starch and other structural carbohydrates in fruit, but did not involve the enzymes associated with glucose and fructose, such as glucose isomerase (GI), and without including p4(t) and p4m(t) (Figure 1). The rate of carbon conversion p1(t), p2(t), p3(t), and p5m(t) were shown in Figure 6. Whether or not potassium was applied, p1(t) did not change significantly during the whole growth stage, and there was almost stable (Figure 6A). The p2(t) gradually decreased with the DAA in the K0 treatment, but in the K1 treatment, it showed a different trend, increasing first and then decreasing, and was lower than that K0 before harvest (Figure 6B). The p3(t) reached a maximum in the early stage and then decreased to near zero at maturity. The p3(t) values of the K1 were noticeably less than K0 at the early stage (Figure 6C). The p5m(t) reached the peak at early stage, gradually dropped with the DAA, and decreased the minimum at harvest under both K0 and K1 treatments, the p5m(t) of K0 was greater than K1 (Figure 6D).
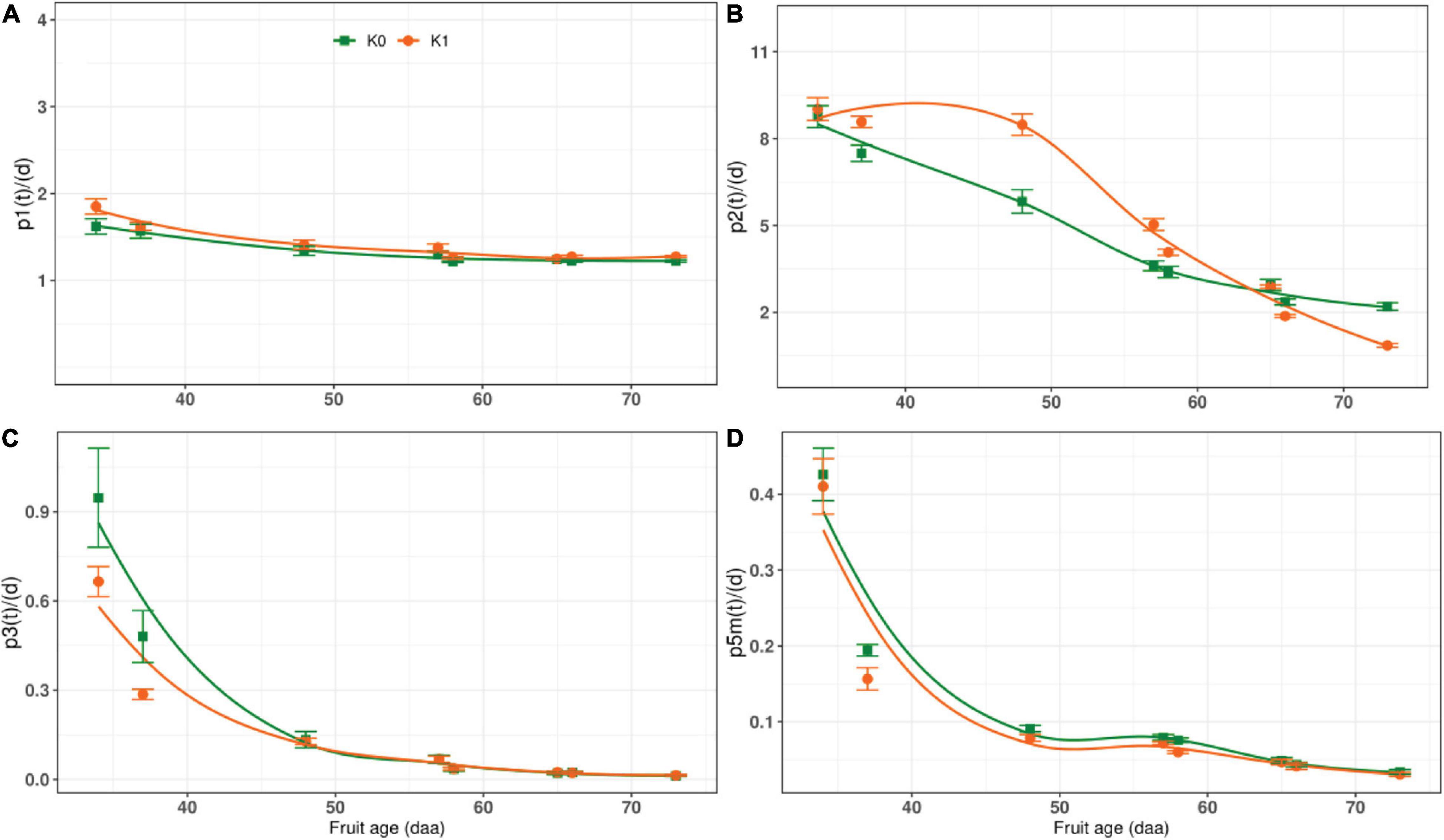
Figure 6. The rate of carbon conversion during the whole growth stage. K0 indicates no potassium and K1 indicates potassium application. (A) The change of carbon conversion rate of sucrose to hexose with fruit age under different potassium condition. (B) The change of carbon conversion rate of hexose to sucrose with fruit age under different potassium condition. (C) The change of carbon conversion rate of hexose to other compounds with fruit age under different potassium condition. (D) The change of carbon conversion rate of glucose to starch with fruit age under different potassium condition.
Correlation and Canonical Correlation Analyses of Enzymes and Parameters
According to Equations (10) and (11), SuSy and AGPase had the greatest load in the first canonical variable U1, indicating that overall enzyme activity in the fruit is mainly determined by SuSy activity and AGPase activity; p1(t) and p5m(t) have the greatest load in V1, indicating that the conversion rate is mainly determined by p1(t) and p5m(t). SuSy, AGPase and p1(t), p5m(t) can therefore be used as significance indicators in the analysis of correlations between enzyme activity and the carbon conversion coefficients.
The Spearman correlation coefficient matrix is shown in Figure 7. The coefficients of correlation between AI and the rates of conversion of fructose and glucose to sucrose [p2(t)], of fructose and glucose to other compounds [p3(t)], and of glucose to starch [p5m(t)] were negative in the range from −0.66 to −0.83. The coefficients of correlation between SuSy and the rates of conversion of sucrose to fructose and glucose, p1(t), p2(t), p3(t), and p5m(t), were in the range 0.65–0.85, which shows significant positive correlations. SPS showed significant weak positive correlations with p1(t), p2(t), p3(t), and p5m(t); the coefficients were in the range 0.48–0.54. SP was not correlated with the rate of carbon conversion. The coefficients of correlation between AGPase and p2(t) and p5m(t) were 0.77, showing significant positive correlations.
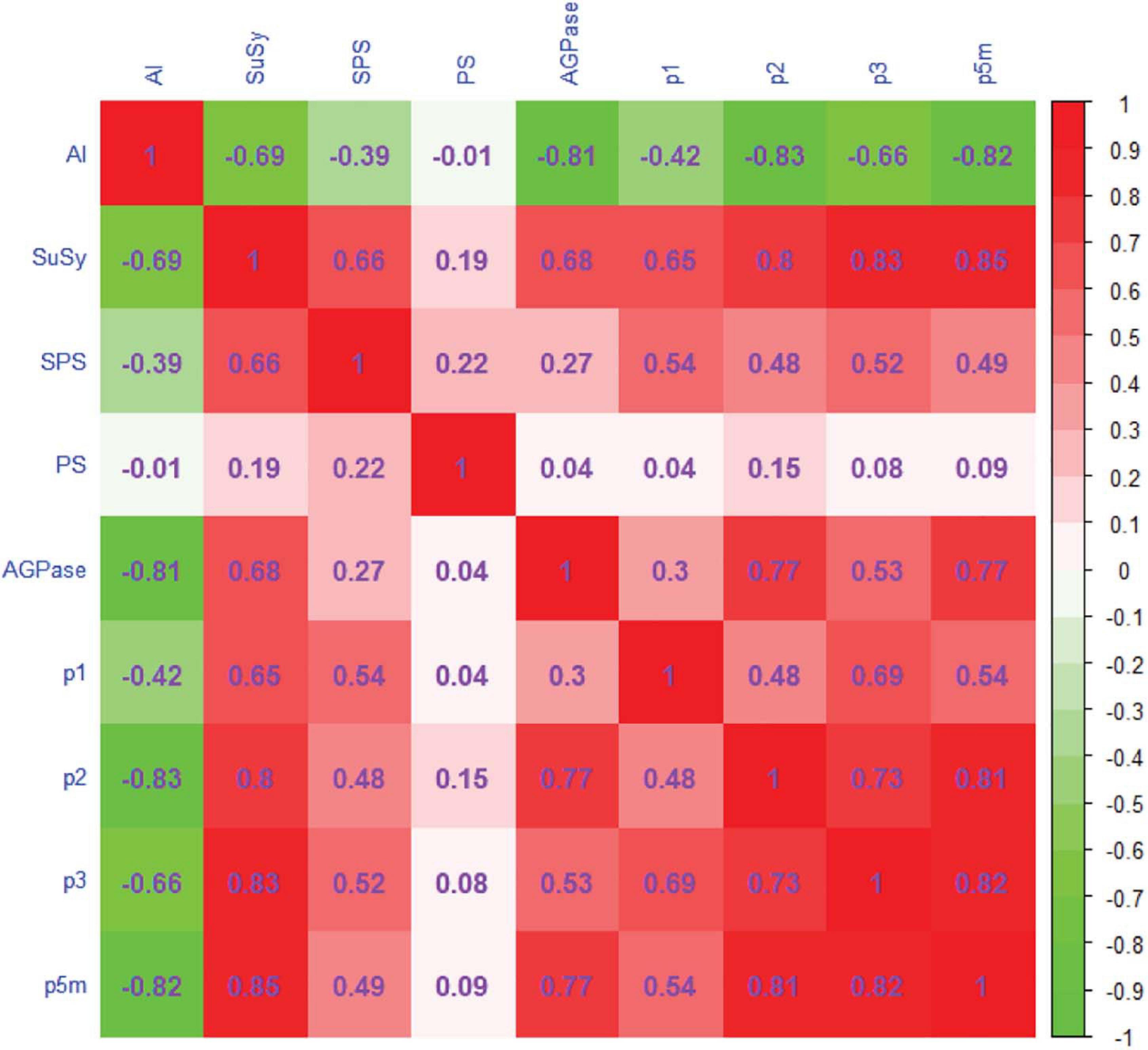
Figure 7. Correlation matrix relating enzyme activity and the rate of carbon conversion in tomatoes. The cell containing the Spearman correlation coefficient (positive or negative) is colored according to the color key (red positive, green negative, white zero). Color intensity increases as the absolute value of the correlation increases.
Discussion
Carbon metabolism is closely related to plant growth. Carbohydrates transported from the source (leaves) provide metabolic substrates for fruit growth and non-photosynthetic tissue maintenance (Osorio et al., 2014), and the types of carbohydrate in the sink and their quantities determine fruit quality (Jorquera-Fontena et al., 2018). Sucrose is the principal carbohydrate assimilate in the source. Source loading and leaf metabolism, as well as assimilate transport, are closely related to enzyme activity in the source and sink tissues and significantly affect sugar accumulation in the fruit (Sachdeva et al., 2003). External environmental factors (such as: light, temperature, water, mineral nutrients) also influence carbon metabolism (Merlo and Passera, 1991; Hanson and Roje, 2001).
Sugar is first synthesized in plant mesophyll cells, and transported to the companion cell, and then transported to the phloem via the sucrose transporter, and transported to the sink tissue, thereby regulating the activity of enzymes to promote plant growth (Kühn and Grof, 2010). Therefore, sugar transporters play an important role in sugar transport, such as sucrose transporters (SUT) and hexose and sucrose transporters (SWEET) proteins, which are involved in the transport and distribution of photosynthates, thus regulating physiological processes such as plant response to environmental stresses and seed formation and development (Rae and Grof, 2005).
Sucrose transporter is not only precisely regulated at the transcription and protein level, but also regulated by several external environmental factors, such as light, circadian rhythm, and stress conditions, the presence of which modulates the expression of sucrose transporters (Davies et al., 1999). For example, drought can cause an increase in the phytohormone ABA, which regulates the gene expression of sucrose transporters, thereby enhancing the sensitivity to drought resistance (Yoshida et al., 2010). The transport activity of sucrose transporters gene expression is blocked by sucrose, for example, OsSUT1 expression is enhanced when endogenous sugar levels are increased (Matsukura et al., 2000), and there is bound to be a correlation with sucrose transporters as potassium can enhance transport efficiency of the phloem, increase carbon supply (Figure 3) and promote protein synthesis. In addition, almost 90–97% of the total transpiration occurs through the stomata. It is well known that under drought stress conditions, potassium regulates stomatal opening and helps plants adapt to water deficits (Hasanuzzaman et al., 2018), and enhance the cellular osmotic regulation, which in turn alters carbon allocation. Therefore, it is easy to find that the activities of SuSy, SPS, and SP were greater in leaves under potassium application condition, which is conducive to soluble sugar accumulation (Table 3).
Carbon metabolism cannot be achieved without the involvement of enzymes. Our results show that in the water deficit treatments, SuSy activity was significantly higher than in CK, and SPS activity was significantly lower than in CK (Figures 2A,B). The results indicate that water stress inhibited sucrose synthesis and intensified hydrolysis, resulting in more hexose being available to participate in osmotic adjustment (Wu et al., 2007). Whether or not water deficit, SPS activity and SuSy activity were both noticeably greater in the potassium treatments than in the non-potassium treatments, with the two enzyme activities increasing by 15.05 and 29.21%, respectively (Table 3), favoring more soluble sugars in the leaves, which is consistent with previous results (Qi et al., 2005). Moreover, the result also indicates that potassium increases the synthesis of SPS and SuSy in leaves (Table 3) and increases the transport of carbohydrates into the fruit (Figure 3).
The regulation of photosynthesized carbon and its distribution between sucrose and starch in the leaves is dependent on the activity of enzymes active in starch metabolism (Schaffer and Petreikov, 1997; Rathore et al., 2009). Our results showed that water deficit increased SP activity by 17.49% and decreased AGPase activity by 44.86% compared with CK, resulting in a blocked starch synthesis and an enhanced decomposition, which was benefit to starch hydrolysis, which was consistent with previous result (Luengwilai et al., 2010). It was increased SP activity by 18.81% and decreased AGPase activity by 22.63% in K1 compared to K0 (Table 3), indicating that potassium enhanced amylolytic enzyme activity and inhibited starch synthase activity, which was consistent with the findings of Guo et al. (2019).
Carbon allocation changed in response to environmental changes during fruit development (Geigenberger, 2005; Hermans et al., 2006; Zhang et al., 2014). For example, water stress led to changes in plant growth through reduced photosynthesis (Vu et al., 1999) and increased enzyme activity in sucrose metabolism, which altered carbon allocation in the fruit (Reynolds and Tuberosa, 2008; Witt et al., 2012). Mineral nutrients such as potassium increase enzyme activity in sugar metabolism in the fruit, thus increasing sink strength and promoting assimilation transport and dry matter accumulation (Qi et al., 2005; Gerardeaux et al., 2010). The result displayed that water deficit and potassium application could increase carbon import flux in fruit, which were beneficial to carbon accumulation, and dCsup/dt were significantly higher than CK (Figure 3). Water and potassium have an important effect on sucrose and starch metabolism, which determine carbon allocation in the fruit, by regulating enzyme activity in Table 4 (Reynolds and Tuberosa, 2008; Rosa et al., 2009; Witt et al., 2012; Jákli et al., 2018).
Acid invertase is important in sucrose metabolism (Tang et al., 1999); it is a biochemical marker that indicates and regulates sink strength in fruit (Sturm, 1999). AI activity remains at a high level during fruit development, which promotes sucrose conversion and hexose accumulation. AI activity has an important effect on assimilate transportation and conversion and maintains the sucrose concentration gradient from source to sink (María et al., 2000). Compared with CK, the AI activity of water deficit treatments (T1, T2, and T3) increased by 15.07, 38.24, and 36.03%, respectively, indicating that water deficit can increase AI activity and promote hexose accumulation (Tables 4, 5), which was the same as the findings of Qi et al. (2005). The study showed that the AI activity increased significantly after potassium application at maturity in tomato fruit (Han et al., 2012) and the results of our research led to similar conclusion, such as K1 > K0 (Table 5). Both water deficit and potassium individually raised AI activity (Nicole and Paul, 2004; Zahoor et al., 2017a).
Sucrose synthase is an indicator of sink strength. It is important in sink accumulation (Gupta et al., 2001; Sung et al., 2006). SuSy activity is greater in the early stage of fruit development than in later stages, and no energy is consumed in the synthesis or conversion of sucrose (Koch, 2004). Compared with CK, SuSy in water deficit treatment (T1, T2, and T3) increased by 5.51, 37.12, and 17.88%, respectively, indicating that water deficit resulted in increased SuSy activity. It was found that AI activity was greater for K1 than for K0 (Figures 4G,H), correspondingly, the hexose was also greater for K1 than K0 in the fruit (Figures 4A,B), which were consistent with the results of previous studies (Almeselmani et al., 2009; Cui et al., 2011; Cao et al., 2011).
Distribution of photosynthate in sink between sucrose and starch is directly affected by SPS activity, the lower the SPS, the less sucrose accumulation (Hubbard et al., 1991). The SPS activity showed a gradual downward trend with fruit development, but increased slightly at 73DAA, on account of the AI activity decreased at this time (Figures 4E,F), resulting in sucrose accumulation in the experiment. Especially in K1 treatments, sucrose increased before harvest due to the enhanced SPS activity by potassium (Figure 4D).
Interestingly, SPS behaves differently in leaves and fruits under water deficit conditions. When plants are subjected to water stress, leaves stomata are closed and osmoregulation is enhanced (Dai et al., 2007), large amounts of monosaccharides, soluble proteins, proline accumulate, and the osmotic potential is significantly reduced (Mirás-Avalos et al., 2013), which helps maintain high cell turgor (Kobashi et al., 2000) and prevents cell dehydration, which is an internal mechanism for plants to resist water stress (Srinivasa et al., 2001). Glucose and fructose, as the most important osmotic adjustment substances, were significantly increased (Lu et al., 2009). SPS activity was reduced and catabolic enzyme activity (AI) was increased due to substrate feedback inhibition, which was in line with the findings of Wu et al. (2007) on tomato leaves.
Studies have shown that tomato fruit with 8 days interval irrigation treatment had higher sucrose content than those with 6 days interval treatment, and water deficit increased SuSy and SPS activities (Han et al., 2012). Zhang et al. (2009) showed that with the intensification of water stress, the activities of SPS and SuSy showed an increasing trend in wolfberry fruit, the more significant the degree of water stress, the more favorable the sucrose accumulation. Liu et al. (2012) found that the activities of AI, SuSy, and SPS were greater for water stress than full irrigation at the late stages of litchi fruit development, which favored sugar accumulation. The results of our experiment were the same as previous studies (Figures 4C,I).
Starch maintains sink strength, ensures carbohydrate is imported from source to sink, and sustains normal development in fruit (Mohapatra et al., 2009; Jonik et al., 2012). The enzymes principally active in starch metabolism are SP and AGPase (Schaffer and Petreikov, 1997). Our results showed that in both water deficit and applying potassium treatments, SP activity was noticeably greater than in CK and that potassium enhanced starch hydrolase activity (Figures 5E,F), resulting in an increase in glucose in the fruit (Figures 5A,B), which was the same as the study of Guo et al. (2019). There was no significant difference in AGPase activity in water treatments (Table 4), but previous studies showed that enzyme activity was greater in water deficit treatments than in full irrigation (Su et al., 2015), which may be related to the later sampling time (34–73 daa). In addition, compared with K0 treatments, the AGPase activity in K1 was reduced by 10.87%, indicating that potassium application can significantly reduce the AGPase activity in the fruit, thereby inhibiting the starch synthesis and accumulation (Vardy et al., 2002).
The rate of carbon conversion indicates carbon allocation and corresponds to metabolic activity during fruit development (Alonso et al., 2007); carbon allocation is a direct measure of sugar content in the source and the sink (Gomathi and Thandapani, 2004; Dumschott et al., 2017). Sugar conversion and sugar content depend on enzyme activity and are related to the rate of carbon conversion. Our results show that greater AI activity increased sucrose hydrolysis and decreased p2(t) (Figure 6B), thus maintaining the sucrose concentration gradient between sink and source to ensure sugar accumulation in the fruit (Lalonde et al., 2003). The structure of the fruit stabilized as the fruit developed, and metabolic activity in the synthesis of starch [p5m(t)] and other compounds [p3(t)] decreased (Figure 6). AI was significantly negatively correlated with p2(t), p3(t), and p5m(t) (Figure 7).
SuSy converts sucrose into fructose and uridine diphosphate glucose (UDPG) in a reversible reaction. In the early stage, when AI activity was low, SuSy was most active in sucrose conversion (Winter and Huber, 2000). Decreased SuSy activity resulted in sucrose synthesis being blocked and a decrease in p2(t) (Figure 6B). SPS maintained a low level of activity throughout all growth stages and therefore has a low correlation with the rate of carbon conversion (Figure 7).
AGPase is a rate-limiting enzyme in starch synthesis (Sweetlove et al., 1999). Decrease in AGPase activity inevitably leads to a decrease in the rate of starch synthesis. Decreased AGPase activity reduces the synthesis of UDPG from glucose-1-phosphate and uridine triphosphate (Janhendrik et al., 2000). UDGP is a precursor of sucrose synthesis: UDPG and fructose-6-phosphoric acid are converted to sucrose, catalyzed by SPS (Black et al., 2006; Xu et al., 2017). Insufficient substrate eventually decreases the rate of sucrose synthesis (Figures 6B,D). Thus AGPase was positively correlated with p2(t) and p5m(t) (Figure 7).
Canonical correlational analysis showed that SuSy and AGPase are critical enzymes and that carbon conversion was influenced mainly by p1(t) in sucrose metabolism and by p5m(t) in starch metabolism. There is a one-to-one correspondence between the two enzymes in maintaining carbon balance in tomato fruit (Figure 1). SuSy regulates p1(t), and AGPase regulates p5m(t), as shown by canonical correlational analysis.
Conclusion
This study shows that water stress and potassium could increase carbon supply flux in the fruit, which is beneficial to carbon accumulation. The addition of potassium modified the balance between enzymes active in sugar and starch synthesis, further reducing AGPase activity, resulted in the increase of hexose. Canonical correlational analysis showed that the carbon conversion rate was principally affected by p1(t) and p5m(t). Sucrose synthase and AGPase had the greatest effects on enzyme activity in tomato fruit, which showed they were critical to regulating the rate of carbon conversion. Spearman correlation analysis showed that acid invertase was significantly negatively correlated with p2(t) and p5m(t). Sucrose synthase was noticeably positively correlated with p2(t), p3(t), and p5m(t). AGPase was significantly positively correlated with p2(t) and p5m(t).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
AL conducted the experiment and finished the first manuscript. CZ supervised the manuscript. JC assisted in the experiment. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (31960256).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2021.681145/full#supplementary-material
Supplementary Figure 1 | Details of the experiment site and plant layout in the greenhouse. The greenhouse used in the experiment was a non-heated naturally ventilated solar greenhouse oriented lengthwise east–west (length 76 m, width 8 m), constructed with a PVC film canopy (0.2 mm thick) supported by a steel and bamboo strip framework. Tomato plants were spaced 60 cm between rows and 80 cm between plants within a row.
References
Agbenin, J. O., and Tiessen, H. (1995). Phosphorus sorption at field capacity and soil ionic strength: kinetics and transformation. Soil Sci. Soc. Am. J. 59, 998–1005. doi: 10.2136/sssaj1995.03615995005900040006x
Almeselmani, M., Pant, R. C., and Singh, B. (2009). Potassium level and physiological response and fruit quality in hydroponically grown tomato. J. Veg. Sci. 16, 85–99. doi: 10.1080/19315260903271526
Alonso, A. P., Goffman, F. D., Ohlrogge, J. B., and Shachar, H. Y. (2007). Carbon conversion efficiency and central metabolic fluxes in developing sunflower (Helianthus annuus L.) embryos. Plant J. 52, 296–308. doi: 10.1111/j.1365-313X.2007.03235.x
Binh, N. Q., Hyacinthe, N., Foyer, C. H., and Serge, Y. (1999). Overexpression of sucrose phosphate synthase increases sucrose unloading in transformed tomato fruit. J. Exp. Bot. 50, 785–791. doi: 10.1093/jxb/50.335.785
Black, C. C., Mustardy, L., Sung, S. S., Kormaník, P. P., and Paz, N. (2006). Regulation and roles for alternative pathways of hexose metabolism in plants. Physiol. Plant. 69, 387–394. doi: 10.1111/j.1399-3054.1987.tb04305.x
Büchi, R., Bachmann, M., and Keller, F. (1998). Carbohydrate metabolism in source leaves of sweet basil (Ocimum basilicum L.), a starch-storing and stachyose-translocating labiate. J. Plant Physiol. 153, 308–315. doi: 10.1016/S0176-1617(98)80156-9
Cao, Y. J., Zhao, H. W., Wang, X. H., and Wei, W. W. (2011). Effects of potassium fertilization on yield, quality and sucrose metabolism of sweet maize. Plant Nutr. Fert. Sci. 17, 881–887. doi: 10.11674/zwyf.2011.0401
Chen, J. L. (2016). Modeling Fruit Growth and Sugar Accumulation and Optimizing Irrigation Scheduling for Improving Water Use Efficiency and Fruit Quality of Tomato. Ph.D. dissertation. (Beijing: China Agricultural University), 74–75.
Chen, J. L., Gilles, V., Kang, S. Z., Nadia, B., Hélène, G., and Génard, M. (2020). Fruit water content as an indication of sugar metabolism improves simulation of carbohydrate accumulation in tomato fruit. J. Exp. Bot. 71, 5010–5026. doi: 10.1093/jxb/eraa225
Chen, J. L., Kang, S. Z., Du, T. S., Qiu, R. J., Guo, P., and Chen, R. Q. (2013). Quantitative response of greenhouse tomato yield and quality to water deficit at different growth stages. Agric. Water Manage. 129, 152–162. doi: 10.1016/j.agwat.2013.07.011
Chopra, J., Kaur, N., and Gupta, A. K. (2005). Role of enzymes of sucrose-starch conversion in seed sink strength in mung bean. Biol. Plant. 49, 561–566. doi: 10.1007/s10535-005-0050-5
Coleman, H. D., Yan, J., and Mansfield, S. D. (2009). Sucrose synthase affects carbon partitioning to increase cellulose production and altered cell wall ultrastructure. Proc. Natl. Acad. Sci. U.S.A. 106, 13118–13123. doi: 10.1073/pnas.0900188106
Cui, L. N., Xu, Z., and Dong, S. T. (2011). Effects of potassium fertilization on enzyme activities associated with sucrose metabolism in the grain development of maize. Plant Nutr. Fert. Sci. 17, 869–880. doi: 10.11674/zwyf.2011.0398
Dai, Z. W., Vivin, P., Robert, T., Milin, S., Li, S. H., and Génard, M. (2009). Model-based analysis of sugar accumulation in response to source-sink ratio and water supply in grape(Vitis vinifera) berries. Funct. Plant Biol. 36, 527–540. doi: 10.1071/FP08284
Dai, Z. W., Wang, L. J., Zhao, J. Y., Fan, P. G., and Li, S. H. (2007). Effect and after-effect of water stress on the distribution of newly-fixed 14C-photoassimilate in micropropagated apple plants. Environ. Exp. Bot. 60, 484–494. doi: 10.1016/j.envexpbot.2007.02.001
Davies, C., Wolf, T., and Robinson, S. P. (1999). Three putative sucrose transporters are differentially expressed in grapevine tissues. Plant Sci. 147, 93–100. doi: 10.1016/S0168-9452(99)00059-X
Deeken, R., Geiger, D., and Fromm, J. (2002). Loss of the akt2/3 potassium channel affects sugar loading into the phloem of Arabidopsis. Planta 216, 334–344. doi: 10.1007/s00425-002-0895-1
Du, Y. L., Zhao, Q., Chen, L. R., Yao, X. D., Zhang, W., Zhang, B., et al. (2020). Effect of drought stress on sugar metabolism in leaves and roots of soyben seedlings. Plant Physiol. Biochem. 146, 1–12. doi: 10.1016/j.plaphy.2019.11.003
Dumschott, K., Richter, A., Loescher, W., and Merchant, A. (2017). Post photosynthetic carbon partitioning to sugar alcohols and consequences for plant growth. Phytochemistry 144, 243–252. doi: 10.1016/j.phytochem.2017.09.019
Feng, P. Y., Chen, S., Zhou, Z. J., and Hu, T. T. (2017). Effect of soil water content on titratable acid content in tomato fruits based on rotatable design. J. Northwest A F Univ. 45, 67–84. doi: 10.13207/j.cnki.jnwafu.2017.11.009
Fernie, A. R., Roessner, U., Leisse, A., Lubeck, J., Trethewey, R. N., and Willmitzer, L. (2001). Simultaneous antagonistic modulation of enzyme activities in transgenic plants through the expression of a chimeric transcript. Plant Physiol. Biochem. 39, 825–830. doi: 10.1016/s0981-9428(01)01312-2
Galtier, N., Foyer, C. H., Huber, J., Voelker, T. A., and Huber, S. C. (1993). Effects of elevated sucrose-phosphate synthase activity on photosynthesis, assimilate partitioning, and growth in tomato (Lycopersicon esculentum var uc82b). Plant Physiol. 101, 535–543. doi: 10.1104/pp.101.2.535
Geigenberger, P. (2005). Redox regulation of carbon storage and partitioning in response to light and sugars. J. Exp. Bot. 56, 1469–1479. doi: 10.1093/jxb/eri178
Génard, M., Lescourret, F., Gomez, L., and Habib, R. (2003). Changes in fruit sugar concentrations in response to assimilate supply, metabolism and dilution: a modeling approach applied to peach fruit (Prunus persica). Tree Physiol. 23, 373–385. doi: 10.1093/treephys/23.6.373
Génard, M., and Souty, M. (1996). Modeling the peach sugar contents in relation to fruit growth. J. Am. Soc. Hortic. Sci. 121, 914–923. doi: 10.21273/JASHS.121.6.1122
Gerardeaux, E., Jordan, M. L., Constantin, J., Pellerin, S., and Dingkuhn, M. (2010). Changes in plant morphology and dry matter partitioning caused by potassium deficiency in Gossypium hirsutum (L.). Environ. Exp. Bot. 67, 451–459. doi: 10.1016/j.envexpbot.2009.09.008
Gomathi, R., and Thandapani, P. V. (2004). Sugar metabolism and carbon partitioning of sugarcane genotypes under salinity stress condition. Sugar Tech 6, 151–158. doi: 10.1007/bf02942716
Gomez, L., Rubio, E., and Auge, M. (2002). A new procedure for extraction and measurement of soluble sugars in ligneous plants. J. Sci. Food Agric. 82, 360–369. doi: 10.1002/jsfa.1046
Gomez, L., Rubio, E., and Lescourret, F. (2003). Critical study of a procedure for the assay of starch in ligneous plants. J. Sci. Food Agric. 83, 1114–1123. doi: 10.1002/jsfa.1512
Guichard, S., Bertin, N., Leonardi, S., and Gary, C. (2001). Tomato fruit quality in relation to water and carbon fluxes. Agronomie 21, 385–392. doi: 10.1051/agro:2001131
Guo, A., Lin, X. J., and Gao, H. H. (2019). Effects of potassium fertilization on sugar metabolism and related activities in ficus carica. Fujian J. Agric. Sci. 34, 1388–1396. doi: 10.19303/j.issn.1008-0384.2019.12.005
Gupta, A. K., Singh, J., and Kaur, N. (2001). Sink development, sucrose metabolising enzymes and carbohydrate status in turnip (Brassica rapa L.). Acta Physiol. Plant. 23, 31–36. doi: 10.1007/s11738-001-0019-8
Han, Q. H., Jiang, W. J., Yu, H. J., and Wang, M. (2012). Effects of potash applied at different growth phases on tomato yield and quality in greenhouse. Acta Hortic. 944, 45–49. doi: 10.17660/ActaHortic.2012.944.5
Hanson, A. D., and Roje, S. (2001). One-carbon metabolism in higher plants. Annu. Rev. Plant Biol. 52, 119–137. doi: 10.1146/annurev.arplant.52.1.119
Hasanuzzaman, M., Bhuyan, M., Nahar, K., Hossain, M. S., and Fujita, M. (2018). Potassium: a vital regulator of plant responses and tolerance to abiotic stresses. Agronomy 8, 31–60. doi: 10.3390/agronomy8030031
Hermans, C., Hammond, J. P., White, P. J., and Verbruggen, N. (2006). How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci. 11, 610–617. doi: 10.1016/j.tplants.2006.10.007
Hu, S. J., Tian, C. Y., Song, Y. D., and Gan, Y. D. (2011). Determination and calculation of soil permeability coefficient. Trans. Chin. Soc. Agric. Eng. 27, 68–72. doi: 10.3969/j.issn.1002-6819.2011.05.011
Hubbard, N. L., Pharr, D. M., and Huber, S. C. (1991). Sucrose phosphate synthase and other sucrose metabolizing enzymes in fruits of various species. Physiol. Plant. 82, 191–196. doi: 10.1111/j.1399-3054.1991.tb00080.x
Islam, M. S., Matsui, T., and Yoshida, Y. (1996). Carbohydrate content and the activities of sucrose synthase, sucrose phosphate synthase and acid invertase in different tomato cultivars during fruit development. Sci. Hortic. 65, 125–136. doi: 10.1016/0304-4238(95)00871-3
Jain, R., Chandra, A., and Solomon, S. (2013). Impact of exogenously applied enzymes effectors on sucrose metabolizing enzymes (SPS, SS and SAI) and sucrose content in sugarcane. Sugar Tech 15, 370–378. doi: 10.1007/s12355-013-0211-3
Jákli, B., Jákli, H. F., Böttcher, J., Müdehorst, M. Z., and Dittert, K. (2018). Leaf, canopy and agronomic water-use efficiency of field-grown sugar beet in response to potassium fertilization. J. Agron. Crop Sci. 204, 99–110. doi: 10.1111/jac.12239
Janhendrik, K., Dick, V., and Linus, H. W. (2000). Uptake and phosphorylation of glucose and fructose in Daucus carota cell suspensions are differently regulated. Plant Physiol. Biochem. 38, 603–612. doi: 10.1016/s0981-9428(00)00776-2
Jonik, C., Sonnewald, U., Hajirezaei, M. R., Flügge, U. I., and Ludewig, F. (2012). Simultaneous boosting of source and sink capacities doubles tuber starch yield of potato plants. Plant Biotechnol. J. 10, 1088–1098. doi: 10.1111/j.1467-7652.2012.00736.x
Jorquera-Fontena, F. E., Pastenes, C., Meriño, G. C., and Franck, N. (2018). Effect of source/sink ratio on leaf and fruit traits of blueberry fruiting canes in the field. Sci. Hortic. 241, 51–56. doi: 10.1016/j.scienta.2018.06.041
Keller, F., and Ludlow, M. M. (1993). Carbohydrate metabolism in drought-stressed leaves of pigeonpea (Cajanus cajan). J. Exp. Bot. 44, 1351–1359. doi: 10.1093/jxb/44.8.1351
Kobashi, K., Gemma, H., and Iwahori, S. (2000). Abscisic acid content and sugar metabolism of peaches grown under water stress. J. Am. Soc. Hortic. Sci. 125, 425–428. doi: 10.1023/A:1008738130592
Koch, K. (2004). Sucrose metabolism: regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr. Opin. Plant Biol. 7, 235–246. doi: 10.1016/j.pbi.2004.03.014
Kühn, C., and Grof, C. P. (2010). Sucrose transporters of higher plants. Curr. Opin. Plant Biol. 13, 287–297. doi: 10.1016/j.pbi.2010.02.001
Lalonde, S., Tegeder, M., Throne, M., Frommer, W. B., and Patrick, J. W. (2003). Phloem loading and unloading of sugars and amino acids. Plant Cell Environ. 26, 37–56. doi: 10.1046/j.1365-3040.2003.00847.x
Levin, I., Gilboa, N., Yeselson, E., Shen, S., and Schaffer, A. A. (2000). Fgr, a major locus that modulates the fructose to glucose ratio in mature tomato fruits. Theor. Appl. Genet. 100, 256–262. doi: 10.1007/s001220050034
Liu, X. Y., Qiu, Y. P., Chen, J. Z., and Yuan, P. Y. (2012). Effects of water stress on litch fruit development and sugar metabolism. J. Fruit Sci. 29, 620–624. doi: 10.13925/j.cnki.gsxb.2012.04.025
Lu, S. W., Li, T. L., and Jiang, J. (2009). Tomato key sucrose metabolizing enzyme activities and gene expression under Nacl and PEG iso-osmotic stresses. Sci. Agric. Sin. 8, 1046–1052. doi: 10.1016/S1671-2927(08)60312-0
Luengwilai, K., Tananuwong, K., Shoemaker, C. F., and Beckles, D. M. (2010). Starch molecular structure shows little association with fruit physiology and starch metabolism in tomato. J. Agric. Food Chem. 58, 1275–1282. doi: 10.1021/jf9032393
Lunn, J. E., and Macrae, E. (2003). New complexities in the synthesis of sucrose. Curr. Opin. Plant Biol. 6, 208–214. doi: 10.1016/S1369-5266(03)00033-5
Luo, A. R., Kang, S. Z., and Chen, J. L. (2020). SUGAR model-assisted analysis of carbon allocation and transformation in tomato fruit under different water along with potassium conditions. Front. Plant Sci. 11:712. doi: 10.3389/fpls.2020.00712
Mafakheri, A., Siosemardeh, A., Bahramnejad, B., Struik, P. C., and Sohrabi, Y. (2011). Effect of drought stress and subsequent recovery on protein, carbohydrate contents, catalase and peroxidase activities in three chickpea (cicer arietinum) cultivars. Aust. J. Crop Sci. 5, 1255–1260. doi: 10.1684/agr.2011.0511
Makkee, M., Kieboom, A. G., and Bekkum, H. V. (1984). Glucose-isomerase-catalyzed D-glucose-D-fructose interconversion: mechanism and reactive species. Recl. Trav. Chim. 103, 361–364. doi: 10.1002/recl.19841031206
María, E. B., José, D. A., Maríaa, C. B., and Francisco, P. A. (2000). Carbon partitioning and sucrose metabolism in tomato plants growing under salinity. Physiol. Plant. 110, 503–511. doi: 10.1111/j.1399-3054.2000.1100412
Matsukura, C. A., Saitoh, T., Hirose, T., Ohsugi, R., Perata, P., and Yamaguchi, J. (2000). Sugar uptake and transport in rice embryo. expression of companion cell-specific sucrose transporter (OsSUT1) induced by sugar and light. Plant Physiol. 124, 85–93. doi: 10.1104/pp.124.1.85
Merlo, L., and Passera, C. (1991). Changes in carbohydrate and enzyme levels during development of leaves of Prunus persica, a sorbitol synthesizing species. Physiol. Plant. 83, 621–626. doi: 10.1111/j.1399-3054.1991.tb02478.x
Mirás-Avalos, J. M., Alcobendas, R., Alarcón, J. J., Valsesia, P., Génard, M., and Nicolás, E. (2013). Assessment of the water stress effects on peach fruit quality and size using a fruit tree model, QualiTree. Agric. Water Manage. 128, 1–12. doi: 10.1016/j.agwat.2013.06.008
Mohapatra, P. K., Sarkar, R. K., and Kuanar, S. R. (2009). Starch synthesizing enzymes and sink strength of grains of contrasting rice cultivars. Plant Sci. 176, 256–263. doi: 10.1016/j.plantsci.2008.11.001
Nettancourt, D. D., and Gösta, E. (1968). Effects of irradiation upon starch formation and starch hydrolysis in tomato microspores. Hereditas 60, 167–176. doi: 10.1111/j.1601-5223.1968.tb02200.x
Nguyen-Quoc, B., and Foyer, C. (2001). A role for ‘futile cycles’ involving invertase and sucrose synthase in sucrose metabolism of tomato fruit. J. Exp. Bot. 52, 881–889. doi: 10.1093/jexbot/52.358.881
Nicole, S., and Paul, Z. (2004). The relation of starch phosphorylases to starch metabolism in wheat. Plant Cell Physiol. 45, 1471–1484. doi: 10.1093/pcp/pch170
Omondi, J. O., Naftali, L., Shimon, R., Kukew, T., and Yasuor, H. (2019). Potassium and storage root development: focusing on photosynthesis, metabolites and soluble carbohydrates in cassava. Physiol. Plant. 64, 71–80. doi: 10.1111/ppl.13060
Osorio, S., Ruan, Y. L., and Fernie, A. R. (2014). An update on source-to-sink carbon partitioning in tomato. Front. Plant Sci. 5:516. doi: 10.3389/fpls.2014.00516
Patan, C., and Saita, A. (2015). Biomass, fruit yield, water productivity and quality response of processing tomato to plant density and deficit irrigation under a semi-arid Mediterranean climate. Crop Pasture Sci. 66, 224–234. doi: 10.1071/CP14152
Philippe, L., Michel, G., Patrick, S., and Robert, H. (2006). Modelling malic acid accumulation in fruits: relationships with organic acids, potassium, and temperature. J. Exp. Bot. 57, 1471–1483. doi: 10.1093/jxb/erj128
Prudent, M., Lecomte, A., Bouchet, J. P., Bertin, N., Causse, M., and Genard, M. (2011). Combining ecophysiological modelling and quantitative trait locus analysis to identify key elementary processes underlying tomato fruit sugar concentration. J. Exp. Bot. 62, 907–919. doi: 10.1093/jxb/erq318
Qi, H. Y., Li, T. L., and Chen, Y. H. (2005). Effects of foliage applications of KH2PO4 and glucose on photosynthesis and sucrose metabolism of tomato. Trans. Chin. Soc. Agric. Eng. 21, 137–142. doi: 10.3321/j.issn:1002-6819.2005.z2.035
Rae, A. L., and Grof, P. (2005). Sucrose partitioning between vascular bundles and storage parenchyma in the sugarcane stem: a potential role for the ShSUT1 sucrose transporter. Planta 220, 817–825. doi: 10.1007/s00425-004-1399-y
Rathore, R. S., Garg, N., Garg, S., and Kumar, A. (2009). Starch phosphorylase: role in starch metabolism and biotechnological applications. Crit. Rev. Biotechnol. 29, 214–224. doi: 10.1080/07388550902926063
Reynolds, M., and Tuberosa, R. (2008). Translational research impacting on crop productivity in drought-prone environments. Curr. Opin. Plant Biol. 11, 171–179. doi: 10.1016/j.pbi.2008.02.005
Ripoll, J., Urban, L., Brunel, B., and Bertin, N. (2015). Water deficit effects on tomato quality depend on fruit developmental stage and genotype. J. Plant Physiol. 190, 26–35. doi: 10.1016/j.jplph.2015.10.006
Roitsch, T., and Mari, G. (2004). Function and regulation of plant invertases: sweet sensations. Trends Plant Sci. 9, 613–618. doi: 10.1016/j.tplants.2004.10.009
Rosa, M., Prado, C., Podazza, G., Interdonato, R., González, J. A., Hilal, M., et al. (2009). Soluble sugars – metabolism, sensing and abiotic stress. Plant Signal. Behav. 4, 388–393. doi: 10.4161/psb.4.5.8294
Ruan, Y. L. (2014). Sucrose metabolism: gateway to diverse carbon use and sugar signaling. Annu. Rev. Plant Biol. 65, 33–67. doi: 10.1146/annurev-arplant-050213-040251
Sachdeva, M., Mann, A. P. S., and Batta, S. K. (2003). Sucrose metabolism and expression of key enzyme activities in low and high sucrose storing sugarcane genotypes. Sugar Tech 5, 265–271. doi: 10.1007/bf02942483
Schaffer, A. A., and Petreikov, M. (1997). Inhibition of fructokinase and sucrose synthase by cytosolic levels of fructose in young tomato fruit undergoing transient starch synthesis. Physiol. Plant. 101, 800–806. doi: 10.1111/j.1399-3054.1997.tb01066.x
Seehuber, C., Damerow, L., and Blanke, M. (2011). Regulation of source: sink relationship, fruit set, fruit growth and fruit quality in european plum (Prunus domestica L.)—using thinning for crop load management. Plant Growth Regul. 65, 335–341. doi: 10.1007/s10725-011-9606-x
Sharkey, T. D., Vassey, T. L., and Vierstra, V. D. (1991). Carbon metabolism enzymes and photosynthesis in transgenic tobacco (Nicotiana tabacum L.) having excess phytochrome. Planta 185, 287–296. doi: 10.2307/23381293
Srinivasa, K. N., Rao, M. R., Bhatt, T., and Sadashiva, A. T. (2001). Tolerance to water stress in tomato cultivars. Photosynthetica 38, 465–467. doi: 10.1023/A:1010902427231
Sturm, A. (1999). Invertases. Primary structures, functions, and roles in plant development and sucrose partitioning. Plant Physiol. 121, 1–8. doi: 10.1104/pp.121.1.1
Su, L. W., Li, S., Ma, S. Y., Wang, Y. M., and Cao, B. C. (2015). Physiological response of non-structural carbothydrates to water stress in wheat. Chin. J. Appl. Ecol. 26, 1759–1764. doi: 10.13287/j.1001-9332.20150331.008
Subasinghe, R. M., Liu, F., Polack, U. C., Lee, E. A., and Tetlow, I. J. (2014). Multimeric states of starch phosphorylase determine protein-protein interactions with starch biosynthetic enzymes in amyloplasts. Plant Physiol. Biochem. 83, 168–179. doi: 10.1016/j.plaphy.2014.07.016
Sung, S. S., Sheih, W. J., Geiger, D. R., and Black, C. C. (2006). Growth, sucrose synthase, and invertase activities of developing Phaseolus vulgaris L. fruits. Plant Cell Environ. 17, 419–426. doi: 10.1111/j.1365-3040.1994.tb00310.x
Sweetlove, L. J., Bernd, M. R., and Hill, W. S. A. (1999). The contribution of adenosine 5’-diphosphoglucose pyrophosphorylase to the control of starch synthesis in potato tubers. Planta 209, 330–337. doi: 10.2307/23385792
Tang, G. Q., Lüscher, M., and Sturm, A. (1999). Antisense repression of vacuolar and cell wall invertase in transgenic carrot alters early plant development and sucrose partitioning. Plant Cell 11, 177–189. doi: 10.2307/3870849
Terry, L. A., Chope, G. A., and Bordonaba, J. G. (2009). Effect of water deficit irrigation on strawberry (Fragaria × ananassa) fruit quality. Acta Hortic. 842, 839–842. doi: 10.17660/ActaHortic.2009.842.185
Tymowska-Lalanne, Z., and Kreis, M. (1998). The plant invertases: physiology, biochemistry and molecular biology. Adv. Bot. Res. 28, 71–117. doi: 10.1016/S0065-2296(08)60294-3
Vardy, K. A., Emes, M. J., and Burrell, M. M. (2002). Starch synthesis in potato tubers transformed with wheat genes for adp-glucose pyrophosphorylase. Funct. Plant Biol. 29, 975–985. doi: 10.1071/pp01161
Vu, J. V., Gesch, R. W., Allen, L. H., Boote, K. J., and Bowes, G. (1999). CO2 enrichment delays a rapid, drought induced decrease in Rubisco small subunit transcript abundance. J. Plant Physiol. 155, 139–142. doi: 10.1016/S0176-1617(99)80156-4
Walker, A. J., and Thornley, J. M. (1977). The tomato fruit: import, growth, respiration and carbon metabolism at different fruit sizes and temperatures. Ann. Bot. 41, 977–985. doi: 10.1093/oxfordjournals.aob.a085395
Walter, A. J., and Difonzo, C. D. (2007). Soil potassium deficiency affects soybean phloem nitrogen and soybean aphid populations. Environ. Entomol. 36, 26–33.
Winter, H., and Huber, S. C. (2000). Regulation of sucrose metabolism in higher plants: localization and regulation of activity of key enzymes. Crit. Rev. Biochem. Mol. Biol. 35, 253–289. doi: 10.1080/10409230008984165
Witt, S., Galicia, L., Lisec, J., Tiessen, J., Araus, J. L., Palacios, N., et al. (2012). Metabolic and phenotypic responses of greenhouse grown maize hybrids to experimentally controlled drought stress. Mol. Plant 5, 401–417. doi: 10.1093/mp/ssr102
Wu, B. H., Quilot, B., Génard, M., Li, S. H., Zhao, J. B., Yang, J., et al. (2012). Application of a sugar model to analyze sugar accumulation in peach cultivars that differ in glucose–fructose ratio. J. Agric. Sci. 150, 53–63. doi: 10.1017/s0021859611000438
Wu, Z. J., Cheng, Z. H., Huan, W., Meng, H. W., Zhang, Y. L., and Xu, Q. (2007). Diurnal variation of invertase activities and sugar content in tomato leaves. Acta Bot. Boreali Occident. Sin. 27, 705–709. doi: 10.1016/S1872-2075(07)60055-7
Xu, X., Dees, D., Dechesne, A., Huang, X. F., and Trindade, L. M. (2017). Starch phosphorylation plays an important role in starch biosynthesis. Carbohydr. Polym. 157, 1628–1637. doi: 10.1016/j.carbpol.2016.11.043
Xue, G. Q., Liu, Q., and Qi, H. Y. (2006). Determination of thirteen metal elements in the plant foeniculum vulgare mill.by flame atomic absorption spectrophotometry. Spectrosc. Spectr. Anal. 26, 1935–1938. doi: 10.1109/INFOCOM.2006.241
Yoshida, T., Fujita, Y., Sayama, H., Kidokoro, S., Maruyama, K., Mizoi, J., et al. (2010). AREB1, AREB2, and ABF3 are master transcription factors that cooperatively regulate abre-dependent aba signaling involved in drought stress tolerance and require ABA for full activation. Plant J. 61, 672–685. doi: 10.1111/j.1365-313X.2009.04092.x
Zahoor, R., Dong, H., Abid, M., Zhao, W., Wang, Y., and Zhou, Z. (2017a). Potassium fertilizer improves drought stress alleviation potential in cotton by enhancing photosynthesis and carbohydrate metabolism. Environ. Exp. Bot. 137, 73–83. doi: 10.1016/j.envexpbot.2017.02.002
Zahoor, R., Zhao, W., Dong, H., Snider, J. L., and Zhou, Z. (2017b). Potassium improves photosynthetic tolerance to and recovery from episodic drought stress in functional leaves of cotton (Gossypium hirsutum L.). Plant Physiol. Biochem. 119, 21–32. doi: 10.1016/j.plaphy.2017.08.011
Zhang, P., Zheng, G. Q., Zheng, G. B., Zhang, Y. P., and Xu, Y. (2009). Effects of deficit irrigation on accumulation of sugar and activity of sugar metabolism-related enzymes of Lycium barbarum L. Agric. Res. Arid Areas. 27, 160–163.
Keywords: sucrose-metabolizing enzymes, starch metabolism enzymes, carbon conversion rate, tomato, potassium, water stress
Citation: Luo A, Zhou C and Chen J (2021) The Associated With Carbon Conversion Rate and Source–Sink Enzyme Activity in Tomato Fruit Subjected to Water Stress and Potassium Application. Front. Plant Sci. 12:681145. doi: 10.3389/fpls.2021.681145
Received: 16 March 2021; Accepted: 21 May 2021;
Published: 16 June 2021.
Edited by:
María Serrano, Miguel Hernández University of Elche, SpainReviewed by:
Ece Turhan, Eskişehir Osmangazi University, TurkeyBambang Sugiharto, University of Jember, Indonesia
Copyright © 2021 Luo, Zhou and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chenni Zhou, Y2hlbm5pMjAxOEAxMjYuY29t
 Anrong Luo1
Anrong Luo1 Chenni Zhou
Chenni Zhou