- 1Department of Plant Science, Department of Agriculture, Forestry and Bioresources, Research Institute of Agriculture and Life Sciences, College of Agriculture and Life Sciences, Seoul National University, Seoul, South Korea
- 2Crop Protection Division, National Institute of Agricultural Sciences, Rural Development Administration, Jeonju, South Korea
- 3Integrated Field Science, Corteva Agriscience, Indianapolis, IN, United States
Echinochloa species is one of the most problematic weed species due to its high competitiveness and increasing herbicide resistance. Florpyrauxifen-benzyl, a new auxin herbicide, was recently introduced for Echinochloa management; however, the potential risk for the development of herbicide resistance in Echinochloa species has not been well-investigated. Thus, this study was conducted to evaluate the baseline sensitivity of Echinochloa species to florpyrauxifen-benzyl to estimate the risk of future resistance development. A total of 70 and 71 accessions of Echinochloa crus-galli and Echinochloa oryzicola were collected from paddy fields in Korea, respectively. These two Echinochloa species were grown in plastic pots up to the 5-leaf stage, and treated with florpyrauxifen-benzyl at a range of doses from 2.2 g to 70.0 g a.i. ha–1. Nonlinear regression analyses revealed that GR50 values for E. oryzicola ranged from 4.54 g to 29.66 g a.i. ha–1, giving a baseline sensitivity index (BSI) of 6.53, while those for E. crus-galli ranged from 6.15 g to 16.06 g a.i. ha–1, giving a BSI of 2.61. Our findings suggest that E. oryzicola has a greater potential risk than E. crus-galli for the development of metabolism-based resistance to florpyrauxifen-benzyl.
Introduction
The genus Echinochloa includes over 50 plant species, most of which are considered weeds in agricultural fields (Holm et al., 1977; Michael, 1983). These species are distributed in temperate to tropical regions and inhabit paddy and upland fields; therefore, they are important weeds for rice and upland crops (Barrett, 1983; Yabuno, 1983). Two Echinochloa species, E. crus-galli (barnyardgrass) and E. oryzicola (late watergrass), are predominantly found in Korean rice paddy fields and cause significant yield losses in rice cultivation (Gibson et al., 2002; Moon et al., 2010). Echinochloa crus-galli var. crus-galli inhabits paddy fields but mainly along the edge of paddy fields where the water depth is shallower. Similarly, E. oryzicola inhabits paddy fields but mainly inside paddy fields where the water depth is deeper. Echinochloa crus-galli var. crus-galli (Echinochloa crus-galli hereinafter) prefers wet soil rather than flooded water conditions, while E. oryzicola prefers flooded paddy fields due its high flooding adaptability (Kim, 1993; Nah et al., 2015). Their high competitiveness against rice (Moon et al., 2010, 2014) and dominance in paddy fields (Ha et al., 2014) have made Echinochloa species the most troublesome weed in paddy fields of rice cropping countries, including Korea.
Since the introduction of bensulfuron-methyl in 1987, acetolactate synthase (ALS) inhibitors have widely been used for paddy weed management due to their broad weed control spectrum and wide application window. Although azimsulfuron, bispyribac-sodium, imazosulfuron, and pyrazosulfuron-ethyl have herbicidal activity against Echinochloa species, flucetosulfuron and penoxsulam are the first ALS inhibitors claimed to have full activity against Echinochloa species. Acetyl CoA carboxylase (ACCase) inhibitors, particularly cyhalofop-butyl, have also been introduced to control Echinochloa species. However, the continuous and frequent use of ACCase and ALS inhibitors has resulted in herbicide-resistant Echinochloa species in Korea (Im et al., 2009; Kang et al., 2010; Kim, 2016; Song et al., 2017). Currently, herbicide-resistant Echinochloa species are distributed nationwide in Korean paddy fields (Lee et al., 2017; Lim et al., 2018). Herbicide resistance in Echinochloa species is not limited to Korea, but becomes a global issue in many rice cropping countries, such as the United States (Fischer et al., 2000; Norsworthy et al., 2014), Italy (Panozzo et al., 2013), Greece (Kaloumenos et al., 2013), Brazil (Matzenbacher et al., 2015), Japan (Iwakami et al., 2014), and China (Chen et al., 2016). Alternative herbicides with different modes of action are urgently required to manage the spread of herbicide-resistant Echinochloa species. Florpyrauxifen-benzyl (RinskorTM Active, developed by Dow AgroSciences) is one of the alternatives. Florpyrauxifen-benzyl is a synthetic auxin herbicide of the arylpicolinate family (WSSA Group 4) that inhibits auxin action by binding to different sites than many existing auxin herbicides (Epp et al., 2016). Unlike general synthetic auxins, florpyrauxifen-benzyl has a broad herbicidal spectrum ranging from broadleaf weeds to grass and sedge weeds, with a particular activity against problematic weeds such as Echinochloa species, Palmer amaranth, and yellow nutsedge (Miller and Norsworthy, 2018). Due to its strong activity against Echinochloa species, it is also expected to control currently resistant Echinochloa species such as ALS and ACCase inhibitor-resistant Echinochloa, as well as quinclorac-resistant Echinochloa (Miller et al., 2018). However, the continuous and repeated use and sole reliance on this herbicide may eventually result in resistance to florpyrauxifen-benzyl. It is essential to estimate the potential risk of the development of resistance to a new herbicide. The response of each weed species to a specific herbicide differs between populations. The variation in genetic diversity and sensitivity is closely related to the possibility of the development of herbicide resistance in the species (Blows and Hoffmann, 2005; Moss, 2017). The baseline sensitivity test is mainly used to investigate the variation in sensitivity. As a definition of the EPPO standard PP1/213(2) (EPPO, 2003), baseline sensitivity data consider the variation in sensitivity among weed populations that have never been exposed to the herbicide or to related active substances with the same mode of action. The main objective of the baseline test is to investigate the natural variation in response to a specific chemical compound in a specific area or period. Nonetheless, a limited number of baseline sensitivity studies were conducted to evaluate the natural variations in response to new herbicides. The natural sensitivity variation of Echinochloa species was assessed for penoxsulam and other herbicides (Vidotto et al., 2004, 2007). Although a baseline sensitivity study requires substantial effort and resources, it is a useful tool to evaluate natural sensitivity variation in a particular weed species using populations from different regions and times and to provide us with an estimation of the potential risk for the development of herbicide resistance (Espeby et al., 2011).
Florpyrauxifen-benzyl is a new herbicide that was introduced to Korea in 2018. It is necessary to estimate the potential risk of resistance development, which is essential information to set up a strategy for sustainable use of the herbicide by delaying or minimizing resistance development. Therefore, this study was conducted to evaluate the natural variation in sensitivity to florpyrauxifen-benzyl among populations of E. crus-galli and E. oryzicola collected in Korean paddy fields before the introduction of florpyrauxifen-benzyl.
Materials and Methods
Plant Materials and Growing Conditions
Among seeds collected from paddy fields in eight provinces of Korea between 2009 and 2016, a total of 70 accessions of E. crus-galli and 71 accessions of E. oryzicola were selected to represent 8–10 counties in each province (Figure 1). Echinochloa crus-galli and E. oryzicola were identified based on morphological traits (Lee et al., 2016). Echinochloa crus-galli accessions collected from Suwon and Seosan were previously confirmed to be susceptible and resistant to cyhalofop-butyl, respectively (Im et al., 2009), and E. oryzicola accessions collected from Suwon and Gimje were susceptible and resistant to penoxsulam, respectively (Kang et al., 2010; Song et al., 2017). Therefore, these accessions were used as reference accessions for this study. Collected accessions were stored in the cold chamber (4°C) to break dormancy until the seed was used. To harmonize seed germination, a priming treatment was made by keeping immersed seeds with distilled water in a cold chamber maintained at 4°C with no light for 48 h. The primed seeds of Echinochloa spp. were germinated in a 90 mm petri dish that was placed in a growth incubator under a 14-hour photoperiod with a 35/25°C day/night temperature for 72 h. Pregerminated seeds were transplanted into plastic pots (7 cm × 7 cm × 8 cm) filled with artificial paddy soil (Punong Co. Ltd., Gyeongju, South Korea) at a density of four plants per pot. All plants were placed in the glasshouse maintained at approximately 35/25°C day/night temperature until the final assessment at 30 days after treatment (DAT). Experiments were conducted in the glasshouse located at the Experimental Farm Station of Seoul National University, Suwon, South Korea.
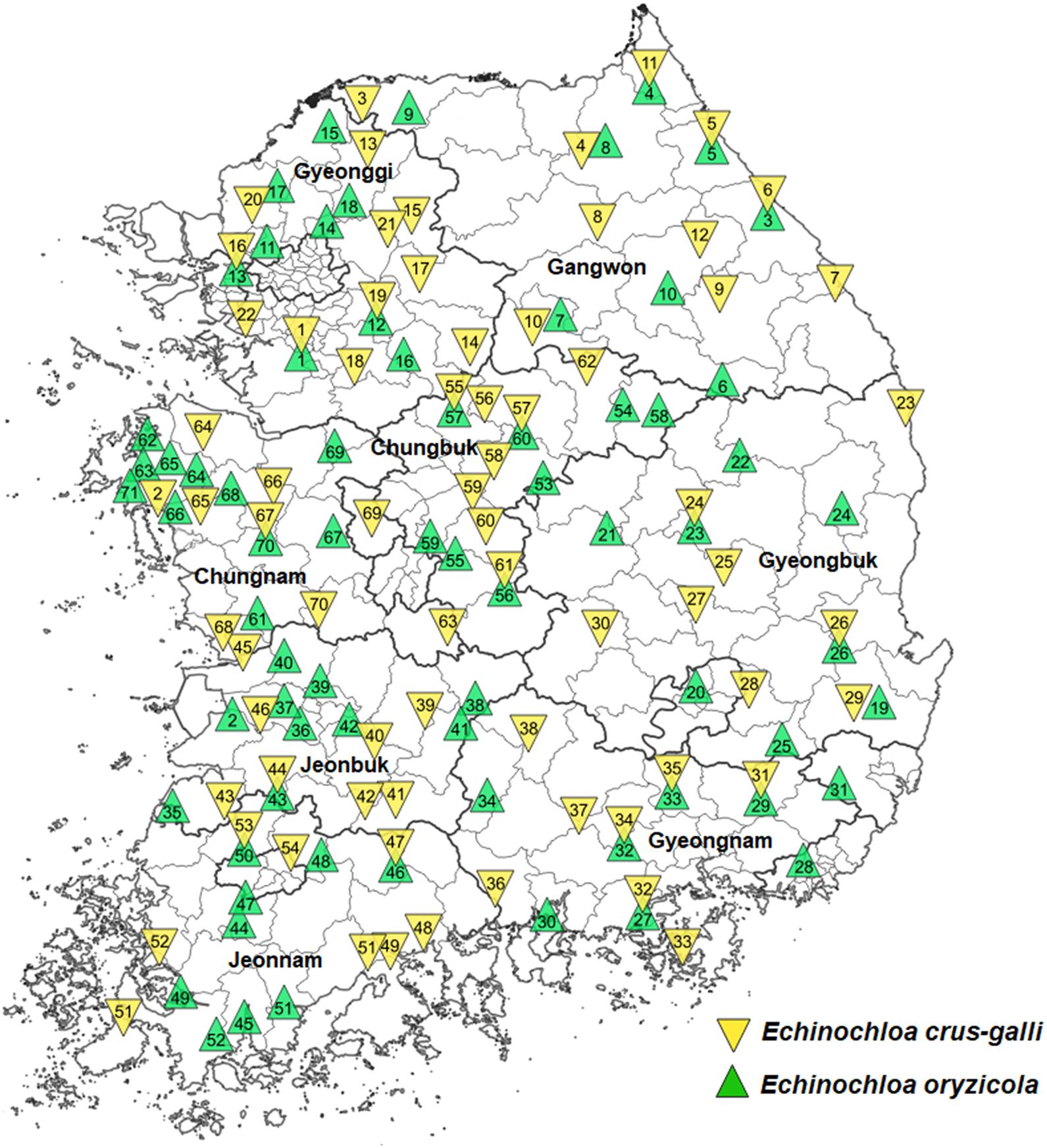
Figure 1. Collection sites of 70 Echinochloa crus-galli and 71 Echinochloa oryzicola accessions in Korea.
Dose-Response Study of Echinochloa Species to Florpyrauxifen-Benzyl (Whole-Plant Assay)
To investigate the baseline sensitivity of Echinochloa spp. to florpyrauxifen-benzyl, a dose-response study was conducted. Florpyrauxfen-benzyl (3.75% EC; LoyantεTM, KyungNong Co. Ltd., Seoul, South Korea) was applied to the foliage of Echinochloa accessions at the 5-leaf stage (plant height: 20–25 cm) at a range of doses from x1/16 to x2 the recommended dose of 35 g a.i. ha–1, and an untreated control was included. Herbicide application was completed using a compressor-pressurized belt-driven track sprayer (R&D Sprayers, United States) equipped with an 8002E flat-fan nozzle (Spraying Systems Company, United States) adjusted to deliver 500 L ha–1. After herbicide treatment, all the plants were placed in the glasshouse and arranged in a randomized block design with four replications. The shoot fresh weight after harvesting all the plants in a pot was measured at 30 days after treatment (DAT).
Statistical Analysis
The shoot fresh weight data were examined using analysis of variance (ANOVA), and nonlinear regression was conducted to fit the data to the three-parameter log-logistic dose-response curve (Streibig, 1980) described as follows:
where Y is the shoot fresh weight of Echinochloa spp., x is the herbicide dose, Yo is the shoot fresh weight of untreated control, B is the slope of the curve, and the GR50 is the dose required for 50% shoot fresh weight reduction compared with untreated control. Using the parameter estimates, the GR80 values were also calculated. The baseline sensitivity index (BSI) was calculated by dividing the greatest GR50 and GR80 values (GR50 max and GR80 max, respectively.) by the smallest values (GR50 min and GR80 min).
The skewness and kurtosis were analyzed from the distribution of GR50 and GR80 values of all accessions (Pearson, 1905). The skewness is defined as follows:
where n is the number of values, Xi is the ith GR50 or GR80 value, is the mean of GR50 or GR80 value, and s is the standard deviation. The kurtosis is defined as follows:
where n is the number of values, Xi is the ith GR50 or GR80 value, is the mean of GR50 or GR80 value, and s is the standard deviation. All statistical analyses were conducted using Prism 7.04 (GraphPad Software, United States).
Results
Dose-Response of Echinochloa Species to Florpyrauxifen-Benzyl
Whole-plant assays with Echinochloa accessions and nonlinear regression analysis revealed dose responses of E. crus-galli and E. oryzicola to florpyrauxifen-benzyl, and showed sensitivity differences between E. crus-galli and E. oryzicola (Figure 2 and Supplementary Tables 1, 2). At the recommended dose (35 g a.i. ha–1) of florpyrauxifen-benzyl, 63 out of 70 tested E. crus-galli accessions (90%) were well-controlled with over 90% growth reduction in fresh weight compared to the untreated control, while 48 out of 71 tested E. oryzicola accessions (68%) were controlled (data not shown). At the double rate of the recommended dose, 8 E. oryzicola accessions (11%) were not well-controlled with less than 80% growth reduction, while all the tested E. crus-galli accessions were well-controlled with greater than 90% growth reduction. In E. crus-galli, Miryang accession from Gyeongnam province showed the greatest sensitivity with 6.15 g a.i. ha–1 of GR50, while Pyongchang accession from Gangwon province showed the lowest sensitivity with 16.06 g a.i. ha–1 of GR50 (Figure 2A and Supplementary Table 1), resulting in a 2.6-fold difference between them. Interestingly, two E. crus-galli reference accessions, Suwon and Seosan, previously confirmed susceptible and resistant, respectively, to both ACCase and ALS inhibitors (Im et al., 2009; Song et al., 2017), showed contrasting sensitivity to the herbicide. Suwon accession (susceptible reference) showed high sensitivity, similar to Miryang accession, with 6.81 g a.i. ha–1 of GR50, while Seosan accession (resistant reference) showed relatively low sensitivity with 13.83 g a.i. ha–1 of GR50 (Supplementary Table 1). In the case of E. oryzicola, Pohang accession from Gyeongbuk province showed the greatest sensitivity with 4.54 g a.i. ha–1 of GR50, while Seosan accession from Chungnam province showed the lowest sensitivity with 29.66 g a.i. ha–1 of GR50 (Figure 2B and Supplementary Table 2), resulting in a 6.5-fold difference between them. Similar to the case of E. crus-galli, two reference accessions, Suwon and Gimje previously confirmed susceptible and resistant, respectively, to both ACCase and ALS inhibitors (Zhang and Kim, 2016; Song et al., 2017), also showed contrasting sensitivity to the herbicide. Suwon accession (susceptible reference) showed greater sensitivity with 7.49 g a.i. ha–1 of GR50, than Gimje accession (resistant reference) with 24.62 g a.i. ha–1 of GR50 (Supplementary Table 2).
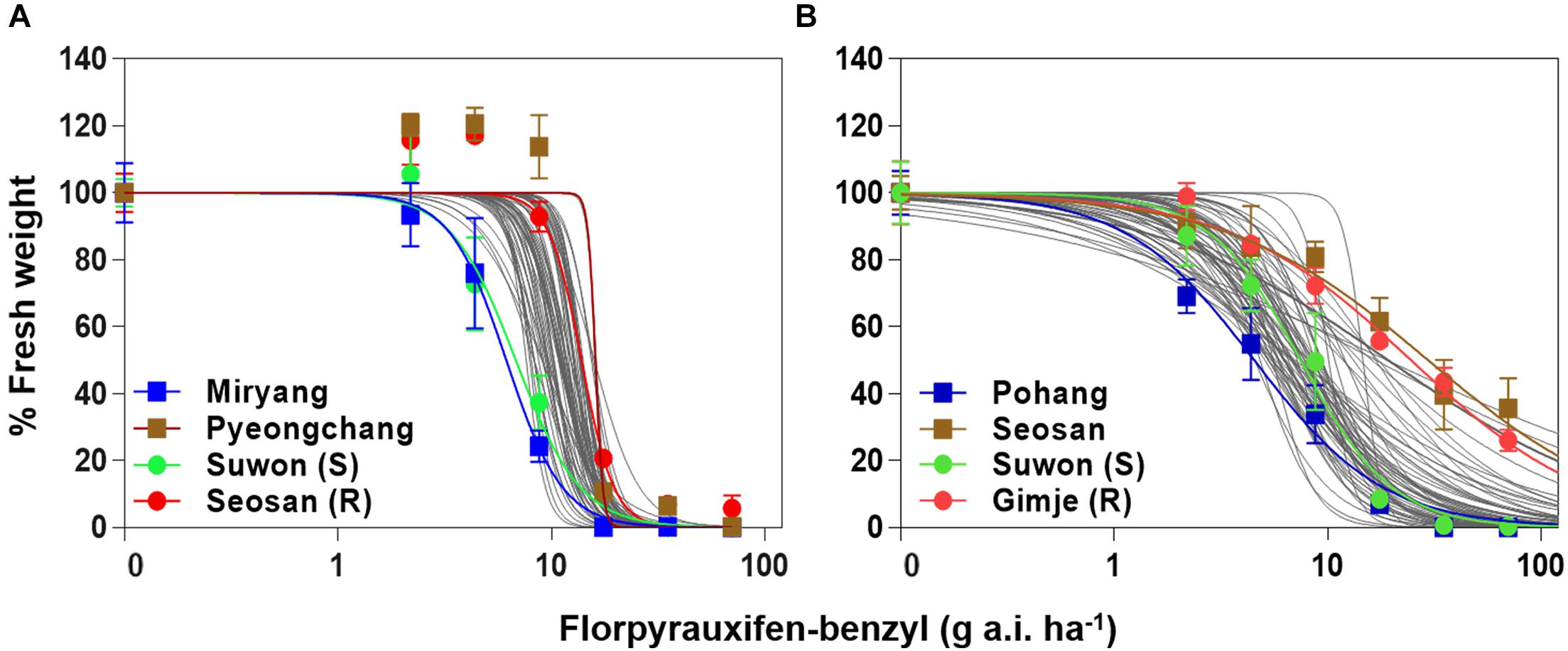
Figure 2. Dose-response curves in fresh weight (% control) of (A) Echinochloa crus-galli accessions and (B) Echinochloa oryzicola accessions measured at 30 days after florpyrauxifen-benzyl treatment.
Variation in Sensitivity of Echinochloa Species to Florpyrauxifen-Benzyl
To compare variation in sensitivity of Echinochloa species to florpyrauxifen-benzyl, the GR50 values of E. crus-galli and E. oryzicola were arranged from the lowest to highest (Figure 3). In addition, the GR80 values were estimated and arranged because the GR50 value is derived from nonlinear regression to fit the log-logistic model and does not always represent the effective weed control dose, while the GR80 value is close to the effective weed control dose (Figure 4). The GR50 values of E. crus-galli ranged from 6.15 g to 16.06 g a.i. ha–1, while those of E. oryzicola ranged from 4.54 g to 29.66 g a.i. ha–1 (Figure 4), indicating that E. oryzicola has a wider variation in the GR50 value than E. crus-galli. In the case of GR80 values, variation in E. crus-galli ranging from 8.81 g to 22.32 g a.i. ha–1 was similar to that observed in GR50 values, while variation in E. oryzicola ranging from 7.17 g to 204.90 g a.i. ha–1 was greater than that in GR50 values, resulting in much larger variation in GR80 values in E. oryzicola than in E. crus-galli (Table 1).
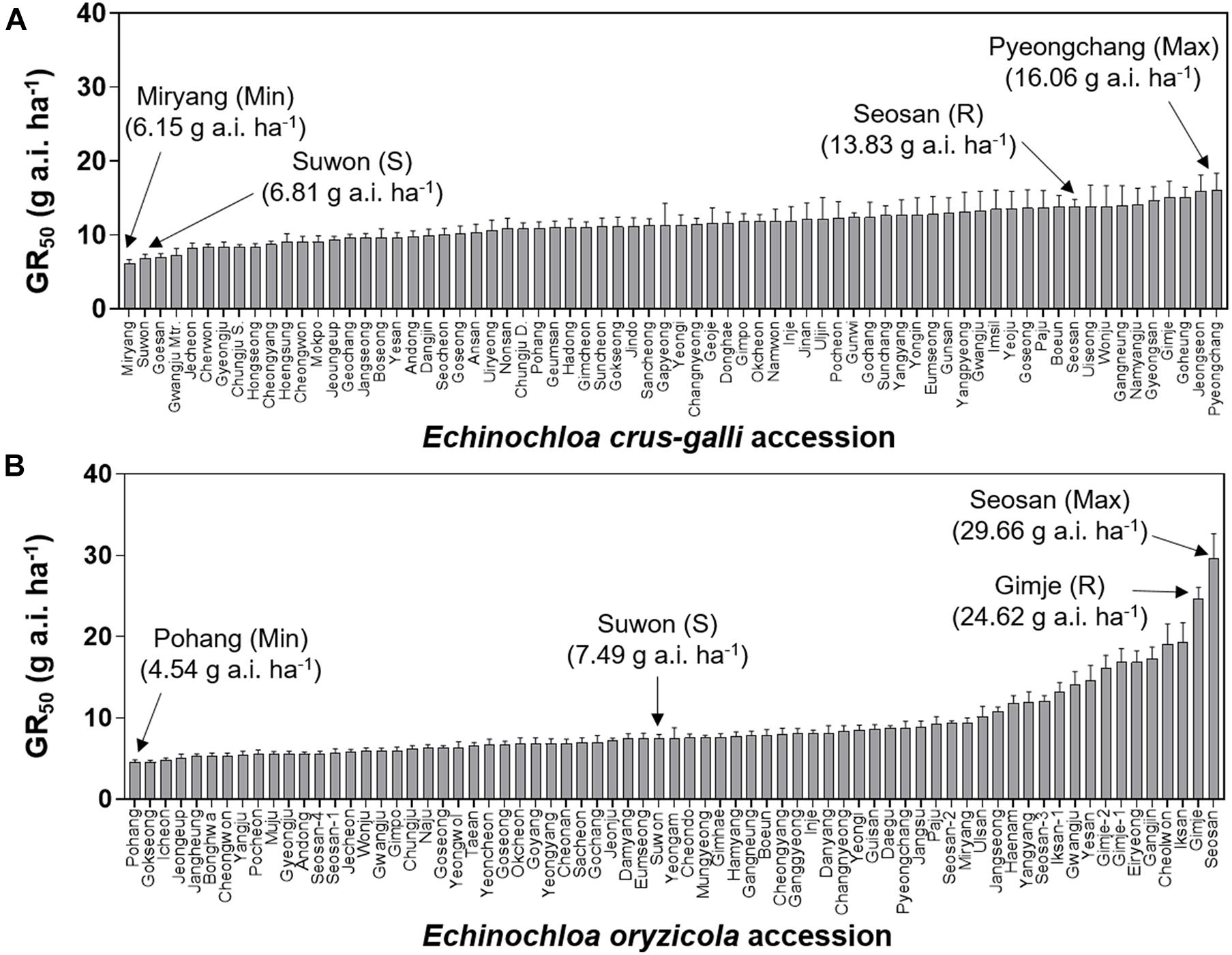
Figure 3. GR50 values of florpyrauxifen-benzyl for (A) Echinochloa crus-galli and (B) Echinochloa oryzicola accessions.
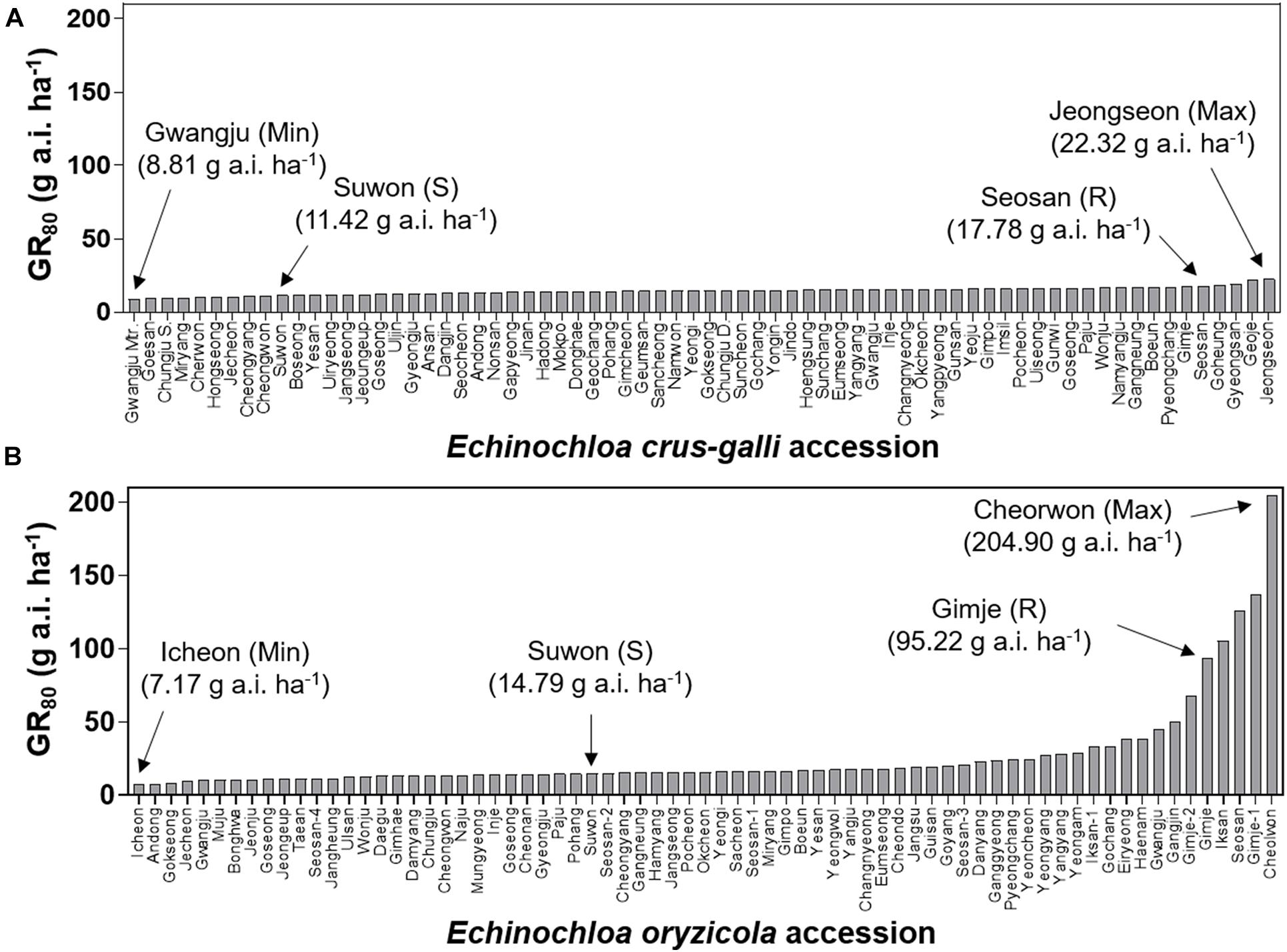
Figure 4. GR80 values of florpyrauxifen-benzyl for (A) Echinochloa crus-galli and (B) Echinochloa oryzicola accessions.
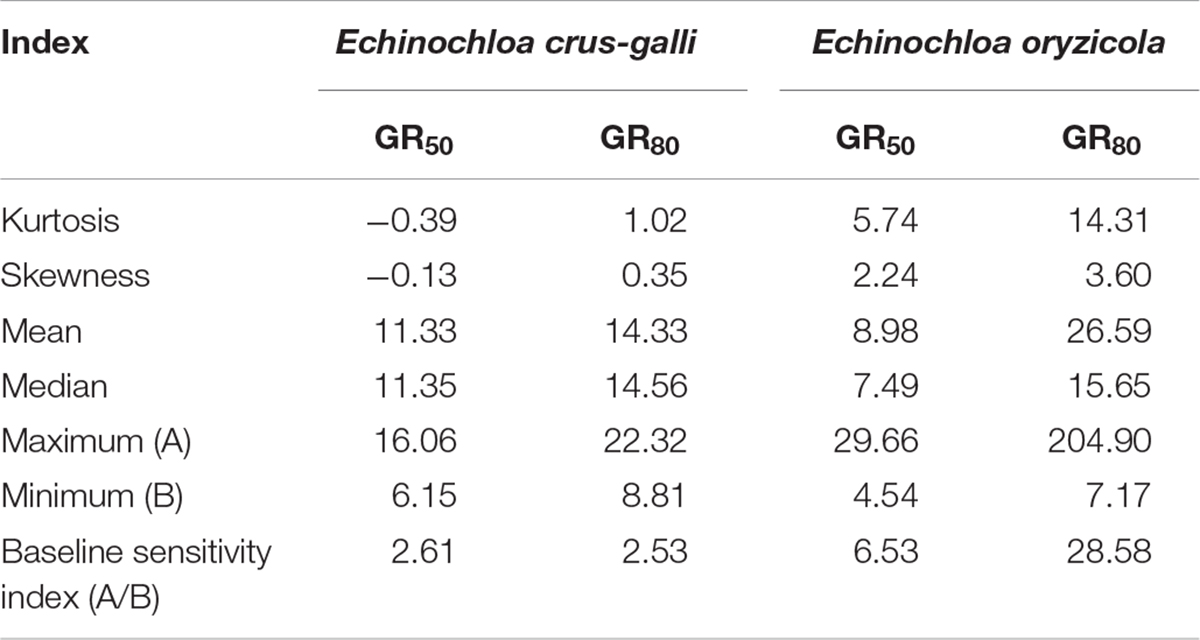
Table 1. Summary of distribution analysis and baseline sensitivity in GR50 and GR80 values (g a.i. ha–1) of Echinochloa species in response to florpyrauxifen-benzyl.
When looking at individual accessions, the greatest GR50 value was 16.06 g a.i. ha–1 observed in the Pyeongchang accession of E. crus-galli, while the lowest value was 6.15 g a.i. ha–1 in the Miryang accession (Figure 3A). The highest GR80 value was 22.32 g a.i. ha–1 observed in the Jeongseon accession, and the lowest value was 8.81 g a.i. ha–1 in the Gwangju accession (Figure 4A). In the case of E. oryzicola, the greatest GR50 value was 29.66 g a.i. ha–1 observed in the Seosan accession, and the lowest value was 4.54 g a.i. ha–1 in the Pohang accession (Figure 3B). The highest GR80 value was 204.90 g a.i. ha–1 in the Cheorwon accession, and the lowest value was 7.17 g a.i. ha–1 in the Icheon accession (Figure 4B). These results suggest that E. crus-galli has no difference in variations between GR50 and GR80 values, while variation in E. oryzicola increased from 6.5 times in GR50 values to 28.6 times in GR80 values (Table 1). Additional dose response study of E. oryzicola accessions, Suwon, Gimje, and Cheorwon, at a range of florpyrauxifen-benzyl up to 140 g a.i. ha–1 also showed a similar sensitivity variation to florpyrauxifen-benzyl, GR50 ranging from 13.43 g to 85.59 g a.i. ha–1 and GR80 ranging from 18.15 g to 244.64 g a.i. ha–1 (Supplementary Table 3). Interestingly, GR50 and GR80 values were observed in resistant and susceptible resistant reference E. crus-galli and E. oryzicola accessions. In all cases, the GR50 and GR80 values of the resistant reference accession were always greater than those of the susceptible reference accession. All of the tested reference accessions were originally collected much earlier than the introduction of florpyrauxifen-benzyl and confirmed to be resistant to cyhalofop-butyl and penoxsulam.
Baseline Sensitivity of Echinochloa Species to Florpyrauxifen-Benzyl
Frequency distribution analysis of E. crus-galli and E. oryzicola GR50 and GR80 values was conducted to compare the baseline sensitivity of Echinochloa species to florpyrauxifen-benzyl (Figures 5, 6). The frequency distribution for the GR50 and GR80 values of E. crus-galli accessions followed a normal distribution with a narrow distribution range (Figures 5A, 6A), while those of E. oryzicola appeared to be a bimodal distribution with a much wider distribution range, almost 2 times the value and 8 times wider than E. crus-galli in GR50 and GR80 values, respectively (Figures 5B, 6B). The frequency distribution analysis based on normal distribution showed that E. crus-galli accessions were distributed following a normal distribution with mean and median values of 11.33 g and 11.35 g a.i. ha–1 for GR50, respectively, and values of 14.33 g and 14.56 g a.i. ha–1 for GR80, respectively (Table 1). In contrast, E. oryzicola did not follow a normal distribution considering its high kurtosis and skewness values for both GR50 and GR80 values. For example, the high E. oryzicola kurtosis values of 5.74 and 14.31 for GR50 and GR80 values, respectively, indicate a much wider range of distribution in GR50 and GR80 values of E. oryzicola accessions. Additionally, the high skewness values of 2.24 and 3.60 for GR50 and GR80 values, respectively, indicate right-skewed distribution and suggest that E. oryzicola has a wider sensitivity range than E. crus-galli.
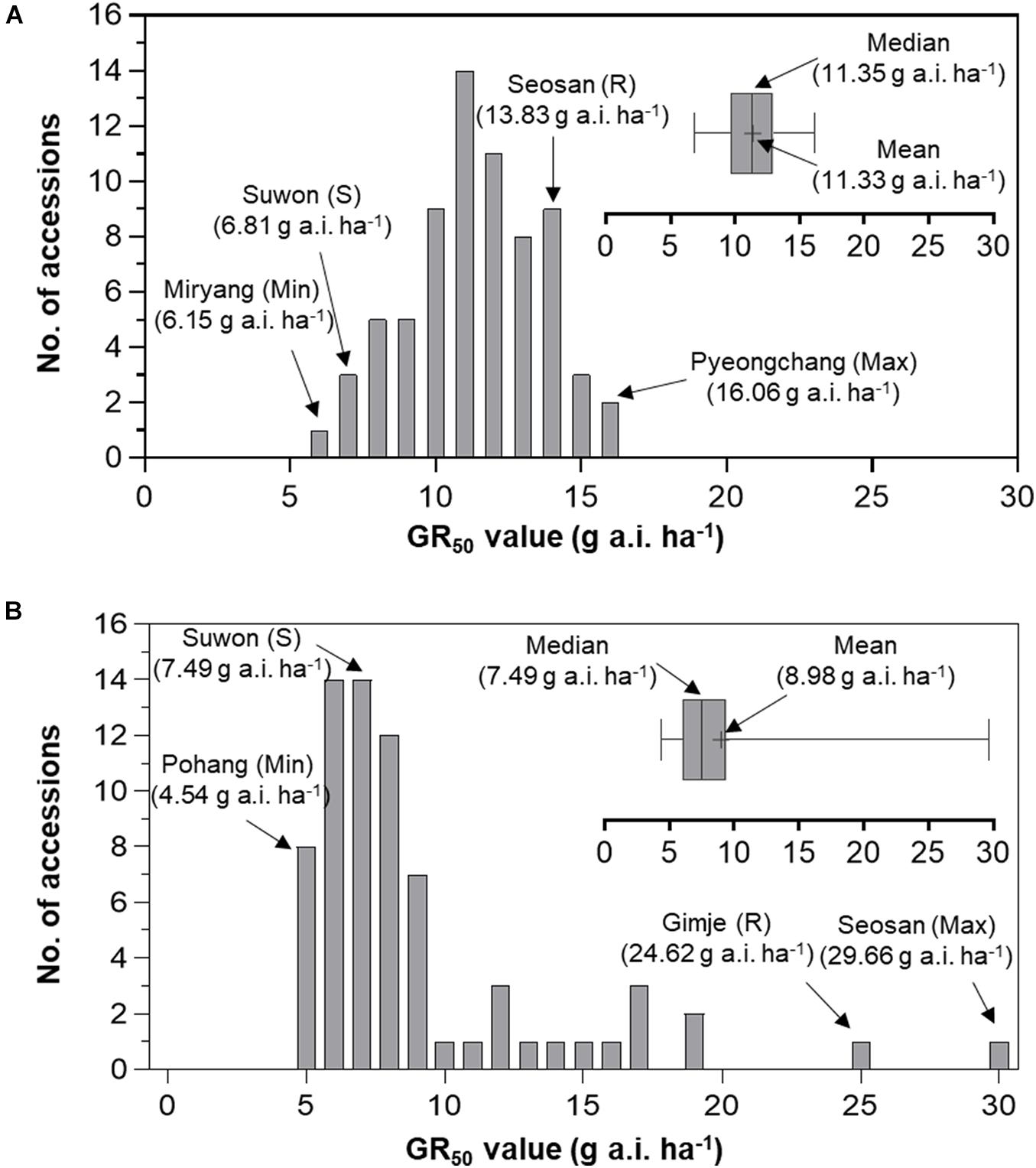
Figure 5. Distribution of GR50 values of florpyrauxifen-benzyl for (A) Echinochloa crus-galli and (B) Echinochloa oryzicola accessions.
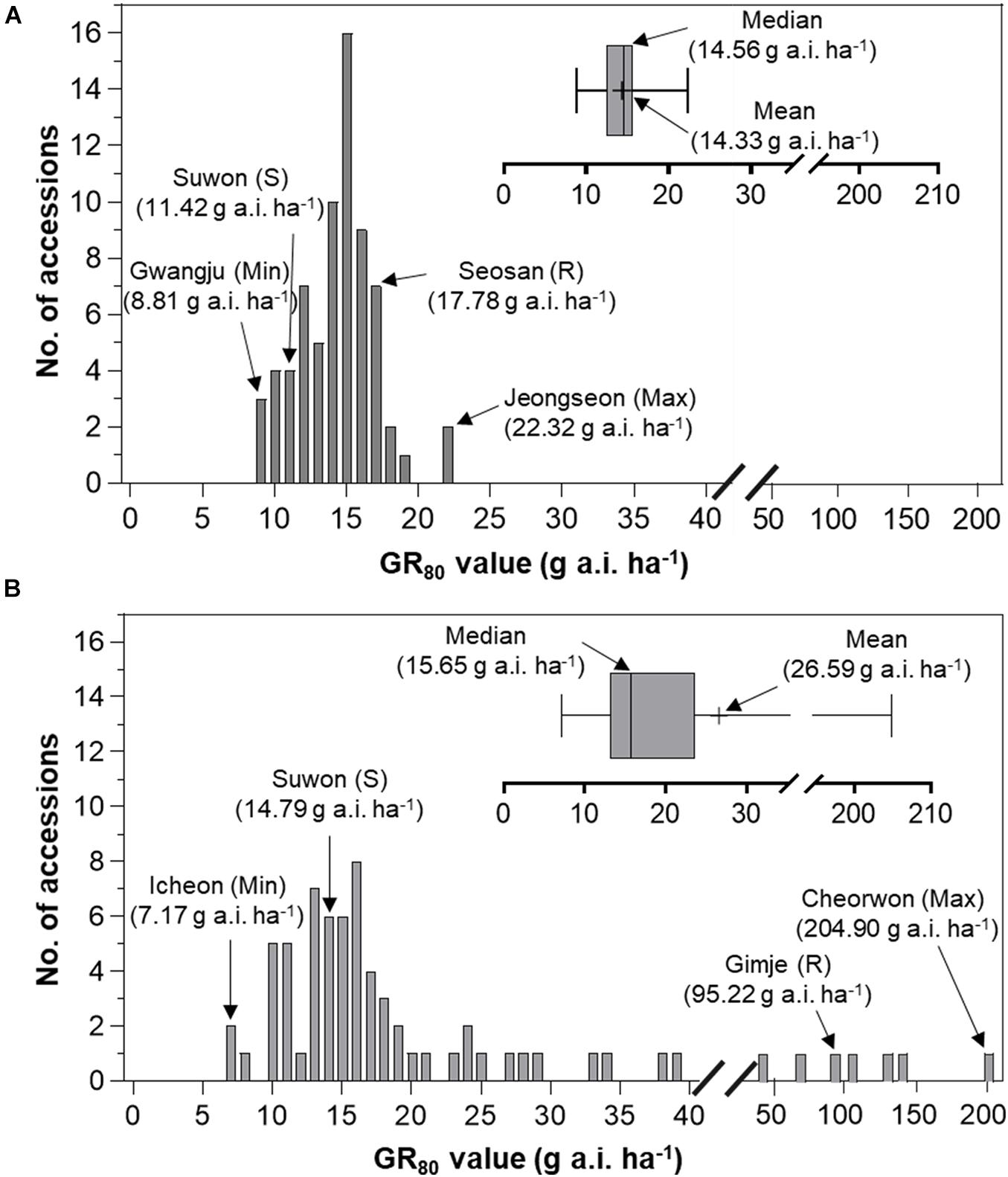
Figure 6. Distribution of GR80 values of florpyrauxifen-benzyl to (A) Echinochloa crus-galli and (B) Echinochloa oryzicola accessions.
To estimate the potential risk of development of florpyrauxifen-benzyl resistance in Echinochloa species, the BSI was calculated by dividing the greatest GR50 and GR80 values by the lowest GR50 and GR80 values, respectively. The BSI results for GR50 and GR80 values of E. crus-galli were 2.61 and 2.53, respectively, while those of E. oryzicola were 6.53 and 28.58, respectively (Table 1). In the case of BSI estimated based on the GR50 value, the difference between E. crus-galli and E. oryzicola was 2.50 times. As seen in Figure 5, both E. crus-galli and E. oryzicola accessions showed GR50 values less than 35 g a.i. ha–1, which is the recommended dose rate of florpyrauxifen-benzyl. However, when comparing the GR80 values, 9 accessions of E. oryzicola showed GR80 values greater than 35 g a.i. ha–1, suggesting that these accessions cannot be fully controlled by the recommended dose of florpyrauxifen-benzyl.
Discussion
Comparison and Implication of Baseline Sensitivity Between Echinochloa Species
The BSI of E. oryzicola was 2.5 times greater than that of E. crus-galli when estimated based on GR50 values, and it became 11.3 times greater when estimated based on GR80 values (Table 1). Therefore, E. oryzicola inhabiting paddy fields in Korea has a much greater potential of resistance development to florpyrauxifen-benzyl than E. crus-galli. The difference between BSI values estimated by GR50 and GR80 values suggests that the BSI should take into account both GR50 and GR80 values with greater importance in GR80 value, as it is more related to the recommended dose rate used in paddy fields. In addition, the frequency distribution of GR50 and GR80 values of E. oryzicola showed a right-skewed bimodal distribution, which is typical of creeping resistance (Gressel, 2009), with 9 accessions surviving above the recommended dose of florpyrauxifen-benzyl (Figure 6), while E. crus-galli showed a normal distribution with all of the E. crus-galli accessions effectively controlled by florpyrauxifen-benzyl. A similar right-skewed bimodal distribution was also observed in the GR50 frequency distribution of E. crus-galli and E. oryzicola in their dose responses to cyhalofop-butyl and penoxsulam, which confirmed that resistance to herbicides had already developed in Echinochloa species (Kim, 2016). Other studies also reported that E. oryzicola showed lower sensitivity to herbicides than E. crus-galli (Vidotto et al., 2007; Damalas et al., 2008; Song et al., 2017). Our findings, thus, suggest that E. oryzicola has a greater potential risk of resistance development for florpyrauxifen-benzyl due to its genetic difference; E. oryzicola is tetraploid (2n = 4X = 36), while E. cru-galli is hexaploid (2n = 6X = 54) (Gould et al., 1972).
The higher BSI and the right-skewed frequency distributions of GR50 and GR80 values observed in E. oryzicola might be due to the difference in the habitat and the selection pressure given. In general, Echinochloa crus-galli inhabits both dry and wet lands, while E. oryzicola inhabits flooded paddy fields, suggesting that the adaptability to submerged conditions is higher in E. oryzicola than E. crus-galli (Yabuno, 1960; Yamasue et al., 1989; Kim, 1993; Nah et al., 2015; Im, 2016). Since weed management for rice in Korea has been carried out by treating pre- or post-emergence herbicides directly to flooded paddy fields, it is likely that the entire population of E. oryzicola inhabiting flooded paddy fields has been well-exposed to herbicides for a long period of time, giving them a high selection pressure. However, many E. crus-galli plants inhabiting along the edge of paddy fields or banks of paddy fields have not been well-exposed to herbicides, giving them a low selection pressure. Yu and Powles (2014) suggested that NTSR may exhibit low sensitivity to herbicides with different modes of action, even first-time herbicides. Considering that the resistance mechanism of ACCase and ALS inhibitor-resistant Echinochloa species found in Korea was related to CYP450s-involved metabolism (Kim, 2016; Song et al., 2017), the low sensitivity (or insensitivity) to the new auxin herbicide florpyrauxifen-benzyl observed in some accessions of E. oryzicola in this study may also be related to CYP450s-involved metabolism. CYP450s-involved metabolism has been preselected by other herbicides, particularly ALS inhibitors, which have been used in rice fields in Korea for a long period of time. Other studies showed that florpyrauxifen-benzyl at the recommended standard dose could well control existing herbicide-resistant E. crus-galli populations in the Mekong delta of Vietnam (Duy et al., 2018) and in Arkansas, United States (Miller et al., 2018). However, our findings imply that Echinochloa populations with enhanced CYP450s-involved metabolism due to existing herbicides will become resistant to florpyrauxifen-benzyl eventually, depending on the use scenario. However, this should not exclude the involvement of reduced herbicide absorption and translocation.
Sustainable Use of Florpyrauxifen-Benzyl for Echinochloa Management
Our study revealed that florpyrauxifen-benzyl could control Echinochloa species up to the 5-leaf stage and showed reasonably high activity against some ACCase and ALS inhibitor-resistant Echinochloa accessions, while was not effective against others. However, the baseline sensitivity study revealed that E. oryzicola has a relatively high BSI of 6.7 and many E. oryzicola accessions showed GR80 values greater than the recommended dose of florpyrauxifen-benzyl. Although the tested Echinochloa accessions have never been previously exposed to florpyrauxifen-benzyl, the high BSI suggests that E. oryzicola has a high potential risk of resistance development to florpyrauxifen-benzyl. If florpyrauxifen-benzyl is used solely and continuously for Echinochloa control, herbicide resistance will rapidly evolve due to the high selection pressure.
Although further studies are required to clearly elucidate the mechanism of this insensitivity, metabolism-based herbicide resistance might be a main mechanism for the insensitivity to florpyrauxifen-benzyl found in some Echinochloa accessions. Among the low sensitive or insensitive accessions tested in our study, some of them, including Seosan and Gimje, have already shown multiple resistances to ACCase and ALS inhibitors and cross-resistance to ALS inhibitors with different chemistries (Kim, 2016; Song et al., 2017). If florpyrauxifen-benzyl is intensively used with no rotation and/or mixtures with other herbicide modes of action, the risk of resistance development would increase. Sole reliance on a specific mode of action has resulted in the rapid development of herbicide resistance in Echinochloa species. Intensive use of penoxsulam in Gimje, South Korea, resulted in the rapid development of resistant E. oryzicola within 4 years of the commercial release of penoxsulam single a.i. product (SalchodaechupTM, Hankook Samgong, Korea) in 2004 (Kang et al., 2010; Park et al., 2010). Continuous use of limited herbicides such as propanil (PSII inhibitor) and bispyribac-sodium (ALS inhibitor) resulted in resistant Echinochloa oryzicola in the United States (Fischer et al., 2000). Echinochloa colona became resistant to propanil and fenoxaprop-P-ethyl due to the intensive use of these herbicides in Colombia and Costa Rica (Garro et al., 1991; Garita et al., 1995; Riches et al., 1996).
According to Busi et al. (2012), long-term selection pressure by a high dose of herbicide induces target site resistance, while that by a low dose of herbicide induces metabolic resistance. In our case, it is presumed that the insensitivity of Echinochloa species to florpyrauxifen-benzyl involves CYP450s-mediated metabolism although reduced herbicide absorption and translocation should also be another reason for the insensitivity. To avoid further resistance development and control resistant weeds, florpyrauxifen-benzyl needs to be used in a mixture or in rotation with other herbicides with different modes of action. Florpyrauxifen-benzyl applied in a mixture with acifluorfen, bentazon, carfentrazone, saflufencil, or propanil showed no antagonistic effect in Korean paddy fields, and shows good compatibility with other herbicides (Miller and Norsworthy, 2018). A recent study revealed that a florpyrauxifen-benzyl mixture with cyhalofop-butyl controlled paddy weeds well without residual toxicity to other crops or antagonistic effects (Sreedevi et al., 2020). Therefore, to maintain the sustainability of florpyrauxifen-benzyl in managing Echinochloa species in paddy fields, integrated weed management (IWM), including appropriate dosage (Busi et al., 2012), appropriate application timing, alternation of various modes of action (Evans et al., 2016), and cultural and physical methods (Bajwa, 2014; Weisberger et al., 2019), is required.
Conclusion
The baseline sensitivity test for florpyrauxifen-benzyl against E. crus-galli and E. oryzicola demonstrated that E. oryzicola has a larger sensitivity variation and, thus, a greater potential for resistance development than E. crus-galli. The right-skewed frequency distributions of GR50 and GR80 values in E. oryzicola suggest that E. oryzicola can become resistant to florpyrauxifen-benzyl earlier than E. crus-galli if the herbicide is continuously and solely used for paddy weed management in Korea. Although the risk of resistance might be lower and the speed of resistance development slower in E. crus-galli, the continuous and sole use of the herbicide could also eventually lead to resistance. This potential risk of resistance development for florpyrauxifen-benzyl may not be limited to Korea because Echinochloa species have long been exposed to various herbicides in many rice cropping countries, particularly ACCase and ALS inhibitors, and ACCase and ALS inhibitor-resistant Echinochloa species are mostly involved with CYP450s-mediated metabolism. Although further studies are required to elucidate the mechanism of such baseline sensitivity differences between E. oryzicola and E. crus-galli, our results strongly support the diversity strategy of florpyrauxifen-benzyl, such as use in mixtures, in rotation with other herbicides with different modes of action, or in combination with diverse alternative nonchemical methods. The integrated use of florpyrauxifen-benzyl with other methods will be useful to maintain the sustainability of florpyrauxifen-benzyl as a rice herbicide.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Author Contributions
S-HL and HK conceived, conducted the experiments, and drafted and revised the manuscript. T-KN and J-SL helped to conduct the experiments. M-JY and J-WK helped to analyze the data and revised the manuscript. J-HY helped the funding acquisition and revised the manuscript. D-SK conceived, supervised the whole process of experiments, and wrote the manuscript. All authors critically reviewed the manuscript and approved the final version of the manuscript.
Funding
This work was carried out with the financial support of the Corteva Agriscience. This work was also carried out with the support of “Cooperative Research Program for Agricultural Science & Technology Development (Project No. PJ012457),” Rural Development Administration, South Korea.
Conflict of Interest
J-HY was employed by the company Corteva Agriscience.
The authors declare that this study received funding from Corteva Agrisciecne. The funder had the following involvement in the study: study design and the decision to submit it for publication.
Acknowledgments
We thank the members of Crop Molecular Physiology and Weed Science Lab for their support during the study. We are also grateful to Corteva Agriscience, United States and Rural Development Administration, South Korea for their support.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2021.656642/full#supplementary-material
Abbreviations
a.i., active ingredient; GRxx, xx % of growth reduction; BSI, baseline sensitivity index; ALS, acetolactate synthase; ACCase, acetyl CoA carboxylase; EPPO, European and Mediterranean plant protection organization; DAT, days after treatment; ANOVA, analysis of variance; NTSR, non-target site resistance; CYP450s, cytochrome P450; IWM, integrated weed management.
References
Bajwa, A. A. (2014). Sustainable weed management in conservation agriculture. Crop Protec. 65, 105–113. doi: 10.1016/j.cropro.2014.07.014
Blows, M. W., and Hoffmann, A. A. (2005). A reassessment of genetic limits to evolutionary change. Ecology 86, 1371–1384. doi: 10.1890/04-1209
Busi, R., Gaines, T. A., Walsh, M. J., and Powles, S. B. (2012). Understanding the potential for resistance evolution to the new herbicide pyroxasulfone: field selection at high doses versus recurrent selection at low doses. Weed Res. 52, 489–499. doi: 10.1111/j.1365-3180.2012.00948.x
Chen, G., Wang, Q., Yao, Z., Zhu, L., and Dong, L. (2016). Penoxsulam-resistant barnyardgrass (Echinochloa crus-galli) in rice fields in China. Weed Biol. Manag. 16, 16–23. doi: 10.1111/wbm.12086
Damalas, C. A., Dhima, K. V., and Eleftherohorinos, I. G. (2008). Morphological and physiological variation among species of the genus Echinochloa in Northern Greece. Weed Sci. 56, 416–423. doi: 10.1614/ws-07-168.1
Duy, L., Chon, N. M., Mann, R. K., Kumar, B. V., and Morell, M. A. (2018). Efficacy of RinskorTM (florpyrauxifen-benzyl ester) on herbicide resistant barnyardgrass (Echinochloa crus-galli) in rice fields of Mekong Delta, Vietnam. J. Crop Sci. Biotechnol. 21, 75–81. doi: 10.1007/s12892-017-0142-0
Epp, J. B., Alexander, A. L., Balko, T. W., Buysse, A. M., Brewster, W. K., Bryan, K., et al. (2016). The discovery of ArylexTM active and RinskorTM active: two novel auxin herbicides. Bioorg. Med. Chem. Lett. 24, 362–371. doi: 10.1016/j.bmc.2015.08.011
EPPO (2003). Efficacy evaluation of plant protection products: resistance risk analysis. OEPP/EPPO Bull. 33, 37–63. doi: 10.1046/j.1365-2338.2003.00627.x
Espeby, L. Å, Fogelfors, H., and Milberg, P. (2011). Susceptibility variation to new and established herbicides: examples of inter-population sensitivity of grass weeds. Crop Prot. 30, 429–435. doi: 10.1016/j.cropro.2010.12.022
Evans, J. A., Tranel, P. J., Hager, A. G., Schutte, B., Wu, C., Chatham, L. A., et al. (2016). Managing the evolution of herbicide resistance. Pest Manag. Sci. 72, 74–80.
Fischer, A. J., Ateh, C. M., Bayer, D. E., and Hill, J. E. (2000). Herbicide-resistant Echinochloa oryzoides and E. phyllopogon in California Oryza sativa fields. Weed Sci. 48, 225–230.
Garita, I., Valverde, B. E., Vargas, E., Chacon, L. A., and Cruz, R. D. L. (1995). “Occurrence of propanil resistance in Echinochloa colona in Central America,” in Proceedings of Brighton Crop Protection Conference – Weed, 1995 Nov 20-23 (Farnham: British Crop Protection Council), 193–196.
Garro, J. E. D., La Cruz, R., and Shannon, P. J. (1991). “Propanil resistance in Echinochloa colona populations with different herbicide use histories,” in Proceedings of Brighton Crop Protection Conference – Weed, 1991 Nov 18-21 (Farnham: British Crop Protection Council), 1079–1083.
Gibson, K. D., Fischer, A. J., Foin, T. C., and Hill, J. E. (2002). Implications of delayed Echinochloa spp. germination and duration of competition for integrated weed management in water-seeded rice. Weed Res. 42, 351–358. doi: 10.1046/j.1365-3180.2002.00295.x
Gould, F. W., Ali, M. A., and Fairbrothers, D. E. (1972). A revision of Echinochloa in the United States. Am. Midl. Nat. 87, 36–59. doi: 10.2307/2423880
Gressel, J. (2009). Evolving understanding of the evolution of herbicide resistance. Pest Manag. Sci. 65, 1164–1173. doi: 10.1002/ps.1842
Ha, H. Y., Hwang, K. S., Suh, S. J., Lee, I. Y., Oh, Y. J., Park, J., et al. (2014). A survey of weed occurrence on paddy field in Korea. Weed Turf. Sci. 3, 71–77. doi: 10.5660/wts.2014.3.2.71
Holm, L. G., Plucknett, D. L., Pancho, J. V., and Herberger, J. P. (1977). Echinochloa crus-galli (L.) Beauv., The World’s Worst Weeds. Honolulu, HI: University Press of Hawaii, 32–40.
Im, J. H. (2016). Physiological and Molecular Analyses of Flooding Adaptability in Echinochloa species. M. Sc. Thesis. Seoul: Seoul National University.
Im, S. H., Park, M. W., Yook, M. J., and Kim, D. S. (2009). Resistance to ACCase inhibitor cyhalofop-butyl in Echinochloa crus-galli var. crus-galli collected in Seosan, Korea. Korean J. Weed Sci. 29, 178–184.
Iwakami, S., Endo, M., Saika, H., Okuno, J., Nakamura, N., Yokoyama, M., et al. (2014). Cytochrome P450 CYP81A12 and CYP81A21 are associated with resistance to two acetolactate synthase inhibitors in Echinochloa phyllopogon. Plant Physiol. 165, 618–629. doi: 10.1104/pp.113.232843
Kaloumenos, N. S., Chatzilazaridou, S. L., Mylona, P. V., Polidoros, A. N., and Eleftherohorinos, I. G. (2013). Target-site mutation associated with cross-resistance to ALS-inhibiting herbicides in late watergrass (Echinochloa oryzicola Vasing.). Pest Manag. Sci. 69, 865–873. doi: 10.1002/ps.3450
Kang, S. W., Yook, M. J., Kim, J. W., Lim, S. H., and Kim, D. S. (2010). “Dose-responses of Echinochloa oryzicola to ALS inhibitors applied to flooded soil,” in Proceedings of Korean Weed Science Society Symposium, 2010 Oct 28-29 (Iksan: Korean Society of Weed Science), 55–56.
Kim, D. S. (1993). Characterization of Emergence and Early Growth of Echinochloa spp. and Rice Under the Condition of Direct-Seeding Rice Culture. M. Sc. Thesis. Seoul: Seoul National University.
Kim, J. W. (2016). Geographical Distribution and Mechanism of ALS Inhibitor Herbicide Resistant Echinochloa spp. in Korea. Ph. D. Dissertation. Seoul: Seoul National University.
Lee, E. J., Nah, G., Yook, M. J., Lim, S. H., Park, T. S., Lee, D. K., et al. (2016). Phylogenetic relationship of Echinochloa species based on simple sequence repeat and phenotypic marker analyses. Weed Sci. 64, 441–454. doi: 10.1614/ws-d-15-00187.1
Lee, J., Kim, J. W., and Lee, I. Y. (2017). Distribution of cyhalofop-butyl and penoxsulam resistant Echinochloa spp. in Korean paddy fields. Weed Turf. Sci. 6, 345–349.
Lim, S. H., Yook, M. J., Park, Y. H., Yu, H., Kim, J. W., Lee, J., et al. (2018). Geographical distribution of acetolactate synthase inhibitor resistant paddy weeds in Gyeonggi and Gangwon provinces of Korea. Weed Turf. Sci. 7, 331–341.
Matzenbacher, F. O., Bortoly, E. D., Kalsing, A., and Merotto, A. (2015). Distribution and analysis of the mechanisms of resistance of barnyardgrass (Echinochloa crus-galli) to imidazolinone and quinclorac herbicides. J. Agric. Sci. 153, 1044–1058. doi: 10.1017/s0021859614000768
Michael, P. W. (1983). “Taxonomy and distribution of Echinochloa species with special reference to their occurrence as weeds of rice,” in Proceedings of Conference on Weed Control in Rice, 1983 Aug 31 - Sep 4 (Los Baños, PH: International Rice Research Institute), 291–306.
Miller, M. R., and Norsworthy, J. K. (2018). Florpyrauxifen-benzyl weed control spectrum and tank-mix compatibility with other commonly applied herbicides in rice. Weed Technol. 32, 319–325. doi: 10.1017/wet.2017.107
Miller, M. R., Norsworthy, J. K., and Scott, R. C. (2018). Evaluation of Florpyrauxifen-benzyl on herbicide-resistant and herbicide-susceptible barnyardgrass accessions. Weed Technol. 32, 126–134. doi: 10.1017/wet.2017.100
Moon, B. C., Cho, S. H., Kwon, O. D., Lee, S. G., Lee, B. W., and Kim, D. S. (2010). Modelling rice competition with Echinochloa crus-galli and Eleocharis kuroguwai in transplanted rice cultivation. J. Crop Sci. Biotechnol. 13, 121–126. doi: 10.1007/s12892-010-0066-z
Moon, B. C., Kim, J. W., Cho, S. H., Park, J. E., Song, J. S., and Kim, D. S. (2014). Modelling the effects of herbicide dose and weed density on rice-weed competition. Weed Res. 54, 484–491. doi: 10.1111/wre.12102
Moss, S. (2017). “Herbicide resistance in weeds,” in Weed Research: Expanding Horizons, eds E. H. Paul and J. F. W. Robert (Hoboken, NJ: John Wiley & Sons Ltd), 181–214.
Nah, G., Im, J. H., Kim, J. W., Park, H. R., Yook, M. J., Yang, T. J., et al. (2015). Uncovering the differential molecular basis of adaptive diversity in three Echinochloa leaf transcriptomes. PLoS One 10:e0134419. doi: 10.1371/journal.pone.0134419
Norsworthy, J. K., Wilson, M. J., Scott, R. C., and Gbur, E. E. (2014). Herbicidal activity on acetolactate synthase-resistant barnyardgrass (Echinochloa crus-galli) in Arkansas, USA. Weed Biol. Manag. 14, 50–58. doi: 10.1111/wbm.12032
Panozzo, S., Scarabel, L., Tranel, P. J., and Sattin, M. (2013). Target-site resistance to ALS inhibitors in the polyploid species Echinochloa crus-galli. Pestic. Biochem. Physiol. 105, 93–101. doi: 10.1016/j.pestbp.2012.12.003
Park, T. S., Ku, B. I., Kang, S. K., Choi, M. K., Park, H. K., Lee, K. B., et al. (2010). Response of the resistant biotype of Echinochloa oryzoides to ACCase and ALS inhibitors, and effect of alternative herbicides. Korean J. Weed Sci. 30, 291–299. doi: 10.5660/kjws.2010.30.3.291
Pearson, K. (1905). Das fehlergesetz und seine verallgemeiner-ugen durch Fechner und pearson, a rejoinder. Biometrika 4, 169–212. doi: 10.1093/biomet/4.1-2.169
Riches, C. R., Caseley, J. C., Valverde, B. E., and Down, V. M. (1996). “Resistance of Echinochloa colona to ACCase inhibiting herbicides,” in Proceedings of the International Symposium on Weed and Crop Resistance to Herbicides, 1995 Apr 3-6 (Cordoba: European Weed Research Society), 14–16.
Song, J. S., Lim, S. H., Yook, M. J., Kim, J. W., and Kim, D. S. (2017). Cross-resistance of Echinochloa species to acetolactate synthase inhibitor herbicides. Weed Biol. Manag. 17, 91–102. doi: 10.1111/wbm.12123
Sreedevi, B., Singh, A., Thakur, C., Kumar, M. P., Mehra, V., Mahenderkumar, R., et al. (2020). Weed control by single post-emergence combination herbicide florpyrauxifen-benzyl plus cyhalofop butyl in aerobic rice. Curr. J. Appl. Sci. Technol. 39, 109–122. doi: 10.9734/cjast/2020/v39i330522
Streibig, J. C. (1980). Models for curve-fitting herbicide dose response data. Acta Agric. Scand. 30, 59–64. doi: 10.1080/00015128009435696
Vidotto, F., Tesio, F., Ferrero, A., and Cavanna, S. (2004). “Baseline sensitivity of penoxsulam to Echinochloa spp. accessions from Italian rice fields,” in Proceedings on Challenges and Opportunities for Sustainable Rice-based Production Systems, 2004 Sep 13-15, Vol. 4, (Vercelli: Edizioni Mercurio), 257–267.
Vidotto, F., Tesio, F., Tabacchi, M., and Ferrero, A. (2007). Herbicide sensitivity of Echinochloa spp. accessions in Italian rice fields. Crop Prot. 26, 285–293. doi: 10.1016/j.cropro.2005.07.016
Weisberger, D., Nichols, V., and Liebman, M. (2019). Does diversifying crop rotations suppress weeds? A meta-analysis. PLoS One 14:e0219847. doi: 10.1371/journal.pone.0219847
Yabuno, T. (1983). “Biology of Echinochloa species,” in Proceedings of Conference on Weed Control in Rice, 1983 Aug 31 - Sep 4 (Los Baños, PH: International Rice Research Institute), 291–318.
Yamasue, Y., Hamada, M., and Kusanagi, T. (1989). Anaerobic seed germination of Echinochloa weeds: the inherent mode and relationship with isozymes of alcohol dehydrogenase. Mem. Col. Agric. Kyoto Univ. 135, 43–52.
Yu, Q., and Powles, S. (2014). Metabolism-based herbicide resistance and cross-resistance in crop weeds: a threat to herbicide sustainability and global crop production. Plant Physiol. 166, 1106–1118. doi: 10.1104/pp.114.242750
Keywords: baseline sensitivity, Echinochloa species, florpyrauxifen-benzyl, herbicide resistance, Rinskor
Citation: Lim S-H, Kim H, Noh T-K, Lim J-S, Yook M-J, Kim J-W, Yi J-H and Kim D-S (2021) Baseline Sensitivity of Echinochloa crus-gall and E. oryzicola to Florpyrauxifen-Benzyl, a New Synthetic Auxin Herbicide, in Korea. Front. Plant Sci. 12:656642. doi: 10.3389/fpls.2021.656642
Received: 21 January 2021; Accepted: 10 May 2021;
Published: 09 June 2021.
Edited by:
Aldo Merotto Junior, Federal University of Rio Grande do Sul, BrazilReviewed by:
Ilias Eleftherohorinos, Auckland University of Technology, New ZealandLauren M. Lazaro, Louisiana State University Agricultural Center, United States
Copyright © 2021 Lim, Kim, Noh, Lim, Yook, Kim, Yi and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Do-Soon Kim, ZG9zb29ua2ltQHNudS5hYy5rcg==
†ORCID: Soo-Hyun Lim, orcid.org/0000-0002-9578-4658; Do-Soon Kim, orcid.org/0000-0002-7388-4308
‡These authors have contributed equally to this work
 Soo-Hyun Lim
Soo-Hyun Lim Harim Kim
Harim Kim Tae-Kyeong Noh
Tae-Kyeong Noh Ji-Soo Lim
Ji-Soo Lim Min-Jung Yook
Min-Jung Yook Jin-Won Kim
Jin-Won Kim Jee-Hwan Yi
Jee-Hwan Yi Do-Soon Kim
Do-Soon Kim