- 1Molecular Crop Research Unit, Department of Biochemistry, Chulalongkorn University, Bangkok, Thailand
- 2Amity Institute of Biotechnology, Amity University, Lucknow, India
- 3Botany and Microbiology Department, College of Science, King Saud University, Riyadh, Saudi Arabia
- 4Mycology and Plant Disease Survey Department, Plant Pathology Research Institute, ARC, Giza, Egypt
- 5Plant Production Department, College of Food and Agricultural Sciences, King Saud University, Riyadh, Saudi Arabia
- 6Department of Botany, University of Lucknow, Lucknow, India
Plants are subjected to a plethora of environmental cues that cause extreme losses to crop productivity. Due to fluctuating environmental conditions, plants encounter difficulties in attaining full genetic potential for growth and reproduction. One such environmental condition is the recurrent attack on plants by herbivores and microbial pathogens. To surmount such attacks, plants have developed a complex array of defense mechanisms. The defense mechanism can be either preformed, where toxic secondary metabolites are stored; or can be inducible, where defense is activated upon detection of an attack. Plants sense biotic stress conditions, activate the regulatory or transcriptional machinery, and eventually generate an appropriate response. Plant defense against pathogen attack is well understood, but the interplay and impact of different signals to generate defense responses against biotic stress still remain elusive. The impact of light and dark signals on biotic stress response is one such area to comprehend. Light and dark alterations not only regulate defense mechanisms impacting plant development and biochemistry but also bestow resistance against invading pathogens. The interaction between plant defense and dark/light environment activates a signaling cascade. This signaling cascade acts as a connecting link between perception of biotic stress, dark/light environment, and generation of an appropriate physiological or biochemical response. The present review highlights molecular responses arising from dark/light fluctuations vis-à-vis elicitation of defense mechanisms in plants.
Introduction
Plants are prone to a number of biotic stress conditions. The suite of molecular and cellular processes is triggered once the plant senses stress (Rejeb et al., 2014; Lamers et al., 2020), which in turn activates a cross-wired mesh of morphological, physiological, and biochemical mechanisms (Nejat and Mantri, 2017; Saijo and Loo, 2020). Plants have developed complex sensory mechanisms to identify biotic invasion and overcome the detriment of growth, yield, and survival (Rizhsky et al., 2004; Lamers et al., 2020). Consequently, plants have evolved a surfeit of responses to defend themselves against attacks by a broad spectrum of pests and pathogens, including viruses, nematodes, bacteria, fungi, and herbivorous insects (Hammond-Kosack and Jones, 2000). Thus, plants tend to strike a balance between their response and biotic stress to combat the deleterious effect on their survival (Peck and Mittler, 2020). The molecular mechanisms contributing toward plant defense responses had been elucidated to a great depth (Cheng et al., 2012; Wang Z. et al., 2019). But how and why different signaling pathways converge to biotic stress responses still remain obscure. The light signaling pathway is one such area of interest amongst the research community.
Dark and light alterations are fundamental to plant survival. It affects all aspects of plant growth and development. The light signals are perceived by photoreceptors, which are capable of discriminating various wavelengths of light (Franklin et al., 2004). Photoreceptors, namely, phytochromes (sense red and far-red light), phototropins, and cryptochromes (sense blue light and UV light), develop cues from qualitative and quantitative light alterations (Christie, 2007; Yu et al., 2010; Tilbrook et al., 2013). This sensing activates several signal transduction pathways, which in turn regulate plant growth, physiology, morphology, and immunity (Kami et al., 2010; Moreno and Ballaré, 2014; Mawphlang and Kharshiing, 2017; Tripathi et al., 2019). In addition, photosynthetic reactions themselves regulate biochemical machinery in plant tissues (Lu and Yao, 2018). This is evident by the point that a number of genes are transcriptionally induced by the circadian clock in Arabidopsis thaliana and other plants (Harmer et al., 2000; Creux and Harmer, 2019). Circadian clock has been reported to meticulously regulate the defense machinery in plants (Sharma and Bhatt, 2015).
There are two developmental fates of seedling upon germination that are primarily dependent upon the presence or absence of light. In the presence of light, seedlings develop a shorter hypocotyl and open green cotyledons. This default pathway of plant development is termed photomorphogenesis (Bae and Choi, 2008; Pham et al., 2018). On the contrary, plants grown in dark conditions undergoes skotomorphogenesis (plant development under dark conditions), allocating the resources toward hypocotyl elongation rather than on cotyledon or root development (Josse and Halliday, 2008). Elongated hypocotyls, closed cotyledons, and an apical hook at the shoot meristem are characteristic to skotomorphogenetic plant development (Pham et al., 2018). Skotomorphogenesis is accomplished by repressing genes implicated in de-etiolation and photomorphogenic development (Josse and Halliday, 2008). Additionally, the effect of dark/light alteration is not only limited to plant growth and development, but it also impacts other responses to the environment such as defense against pests and pathogens (Ballaré, 2014). Extensive research exists to vindicate the effect of dark/light alterations on plant defense responses, extending from biological to ecological scales (Huner et al., 1998; Roberts and Paul, 2006; Kazan and Manners, 2011; Ballare et al., 2012; Kangasjärvi et al., 2012; Hua, 2013; Garcia-Guzman and Heil, 2014; Saijo and Loo, 2020). But the in-depth mechanistic details with regard to the complex regulatory networks are yet to be explored. The basic research in this direction can assist the idea of sustainable agriculture to ensure food security for the ever-growing world population (Sánchez-Muros et al., 2014; González de Molina et al., 2017; Iqbal et al., 2020a; Saiz-Rubio and Rovira-Más, 2020). The present review recapitulates biochemical, physiological, and molecular aspects of biotic stress and plant defense responses operating in light/dark scenarios.
Biotic Stress and Plant Defense Responses
A number of pests, parasites, and pathogens are responsible for infecting plants and inciting biotic stress. Fungal parasites can be either necrotrophic (kill host cell by toxin secretion) or biotrophic (feed on living host cell). They are capable of inducing vascular wilts, leaf spots, and cankers in plants (Laluk and Mengiste, 2010; Doughari, 2015; Sobiczewski et al., 2017). Nematodes feed on plant parts and primarily cause soil-borne diseases leading to nutrient deficiency, stunted growth, and wilting (Lambert and Bekal, 2002; Bernard et al., 2017; Osman et al., 2020). Similarly, viruses are also capable of local and systemic damage resulting in chlorosis and stunting (Pallas and García, 2011). On the contrary, mites and insects impair plants by either feeding (piercing and sucking) on them or laying eggs. The insects might also act as carriers of other viruses and bacteria (Schumann and D’Arcy, 2006). Plants have developed an elaborate immune system to combat such stresses (Taiz and Zeiger, 2006; Saijo and Loo, 2020). Plants have a passive first line of defense, which includes physical barriers such as cuticles, wax, and trichomes to avert pathogens and insects. Plants are also capable of producing chemical compounds to defend themselves from infecting pathogens (Taiz and Zeiger, 2006) (discussed in section “Effect of Dark/Light on Plant–Pathogen Interaction and Associated Mechanisms”). Additionally, plants trigger defense against biotic agents by two levels of pathogen recognition (Dangl and McDowell, 2006).
The first level of pathogen recognition encompasses pattern recognition receptors (PRRs), which identify pathogen-associated molecular patterns (PAMPs). Such plant immunity is categorized as PAMP-triggered immunity (PTI) (Monaghan and Zipfel, 2012). Phytophagous pests respond by identification of herbivore-associated elicitors (HAEs), herbivore-associated molecular patterns (HAMPs), or PRR herbivore effectors (Santamaria et al., 2013). The second level of pathogen recognition encircles plant resistance (R) proteins, which identify specific receptors from a pathogen (Avr proteins) (Dangl and McDowell, 2006; Gouveia et al., 2017; Abdul Malik et al., 2020). It is considered an effective mechanism of plant resistance to pests and involves effector-triggered immunity (ETI) (Kaloshian, 2004; Mur et al., 2008; Spoel and Dong, 2012). ETI stimulates hypersensitive responses (HRs) and triggers programmed cell death (PCD) in infected and surrounding cells (Mur et al., 2008). The proteins encoded by a majority of R genes have a specific domain with conserved nucleotide-binding site (NBS). The second next important domain is leucine-rich repeat (LRR). Pathogen effectors are recognized directly (physical association) or indirectly (association of an accessory protein) by NB-LRR receptors (Dodds and Rathjen, 2010). Sometimes, R gene-mediated plant response toward invading pathogen provokes a higher degree of defense, termed as systemic acquired resistance (SAR). SAR generates whole-plant systemic resistance against a broad spectrum of pathogens. In SAR, a local encounter results in the stimulation of resistance to the other plant organs through intraplant communication (Fu and Dong, 2013). Generally, both categories of plant immune responses induce the same reaction, but ETI is considered more rigorous to pathogen infection (Tao et al., 2003).
Perturbations in cytosolic calcium (Ca2+) concentrations are the earliest signaling events occurring upon the exposure of plants to biotic stress. Ca2+ signals are the center to plant immune signaling pathways (Seybold et al., 2014; Aldon et al., 2018). Rapid and transient perturbations in Ca2+ concentrations are crucial to gene reprogramming required to generate an adequate response (Reddy et al., 2011). The plant immune responses differ in their Ca2+ signatures. For example, Ca2+ transients upon PTI activation returns to basic levels within a few minutes (Lecourieux et al., 2005), while ETI involves a prolonged increase in cytosolic Ca2+ levels lasting for several hours (Grant et al., 2000). Lanthanum, a known Ca2+ channel blocker, is reported to hinder the immune responses associated with both PTI and ETI (Grant et al., 2000; Boudsocq et al., 2010). Precisely, in response to the biotic invasion, PTI and ETI activate the Ca2+ ion channels, resulting in an increase of cytoplasmic Ca2+ concentrations (Figure 1). In A. thaliana, cyclic nucleotide-gated channels (CNGCs), glutamate receptor-like channels (GLRs), stretch-activated Ca2+ channels (OSCAs), and the MID1-complementing activity (MCA) families are the four main plasma membrane Ca2+-permeable channels (Dodd et al., 2010; Yuan et al., 2014; Liu et al., 2018). Twenty distinct members of the CNGC family of plasma membrane Ca2+-permeable channels have been identified in A. thaliana (Meena and Vadassery, 2015; DeFalco et al., 2016). CNGCs are extensively linked to plant development and biotic stress responses (Meena and Vadassery, 2015; DeFalco et al., 2016; Breeze, 2019). In response to fungal and bacterial pathogens, the Ca2+-permeable channels CNGC2, CNGC4, CNGC11, and CNGC12 are reported to play critical roles in the entry of Ca2+ ions inside the plant cell (Yoshioka et al., 2001; Ahn, 2007). The role of CNGC2, CNGC4 (Ma et al., 2012; Chin et al., 2013), CNGC11, and CNGC12 (Yoshioka et al., 2006; Moeder et al., 2011) has been well established in plant immune responses. Very recently, the function of CNGC19 Ca2+ channel was also extended to herbivory-induced Ca2+ flux, plant defense responses against pathogen Spodoptera litura (Meena et al., 2019), and basal defense signaling to regulate colonization of Piriformospora indica on A. thaliana roots (Jogawat et al., 2020). The first CNGC from plants was identified nearly two decades ago in barley as a calmodulin (CaM)-binding protein (Schuurink et al., 1998). CNGCs from plants and animals are reported to possess one or more CaM-binding domains at their cytosolic N- and C-termini, but the gating of CNGCs from plants is not well deduced (DeFalco et al., 2016; Fischer et al., 2017; James and Zagotta, 2018). The progress of plant CNGC research has been relatively low due to the difficulties in electrophysiological studies encircling CNGCs. However, the recent technological advances and reliability on reverse genetics using cngc mutants have resulted in few successful studies (Gao et al., 2016; Chiasson et al., 2017; Wang et al., 2017; Zhang et al., 2017). CNGC7, CNGC8, and CNGC18 have been specifically reported to act together with CaM2 as a molecular switch that operates in response to cellular Ca2+ concentrations (Pan et al., 2019). Additionally, CNGC18 is co-expressed with CPK32, indicating the regulation of its activity by phosphorylation (Zhou et al., 2014). Similarly, GLRs, which are systematically classified into three clades—clade I (GLRs 1.1–1.4), clade II (GLRs 2.1–2.9), and clade III (GLRs 3.1–3.7) (Lacombe et al., 2001)—are linked to plant defense against Botrytis cinerea (Sun et al., 2019) and Hyaloperonospora arabidopsidis (Manzoor et al., 2013). As such, the role of AtGLR3.3 and AtGLR3.6 in aphid-elicited cytosolic Ca2+ elevation is also well established (Vincent et al., 2017). In-vitro kinase assay confirmed that AtGLR3.7 is phosphorylated by CDPK3, CDPK16, and CDPK34 at serine-860 site (Wang P.-H. et al., 2019). CDPKs have been extensively associated with plant stress management and development (Singh et al., 2017). The other plasma membrane localized Ca2+-permeable channels, namely, OSCAs (phosphorylation of OSCA1.3 by BIK1) and MCAs (MCA1 and MCA2), are reported to regulate plant stomatal immunity (Thor et al., 2020) and manage hypergravity in A. thaliana hypocotyls under dark conditions, respectively (Hattori et al., 2020). Apart from the Ca2+ channels localized in the plasma membrane, several other Ca2+ channels are known to exist in the endoplasmic reticulum, mitochondria, golgi body, and plant vacuole (Singh et al., 2014; Xu et al., 2015a; Costa et al., 2018; Pandey and Sanyal, 2021). For example, autoinhibited Ca2+-ATPases (ACAs), ER-type Ca2+-ATPases (ECAs), mitochondrial Ca2+ uniporter (MCU), P1-ATPases (e.g., HMA1), Ca2+ exchangers (CAX), two-pore channel (TPC), 1,4,5-trisphosphate receptor-like channel (InsP3R), 1,4,5-trisphosphate (IP3), cyclic ADP-ribose (cADPR)-activator ryanodine receptor-like channel (RyR), slow-activating vacuolar channel (SV), and sodium-calcium exchanger (NCX) represents the organellar Ca2+ machinery (Figure 1). Many of these channels are reported to play pivotal roles in plant immunity (Bose et al., 2011; Pittman, 2011; Spalding and Harper, 2011; Kiep et al., 2015; Costa et al., 2017; Teardo et al., 2017; Yang et al., 2017; Demidchik et al., 2018; Taneja and Upadhyay, 2018; Pandey and Sanyal, 2021).
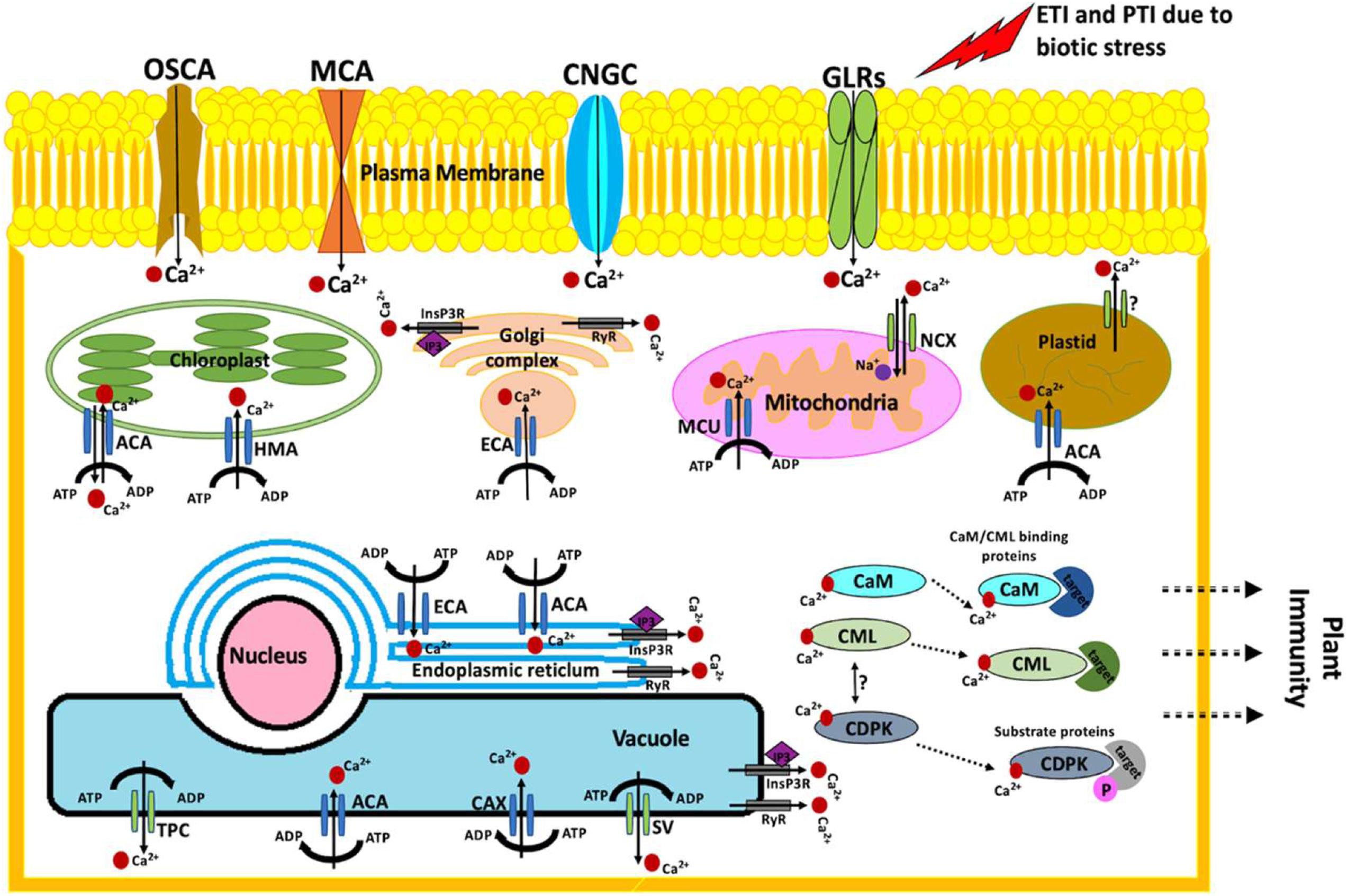
Figure 1. Schematic representation of biotic stress perception and Ca2+ signaling for the regulation of plant immune responses. The cytosolic Ca2+ levels increase (>200 nM) upon perceiving biotic stress: calcium (Ca2+), Ca2+–dependent protein kinases (CDPKs), calmodulin (CaM), calmodulin-like protein (CML), autoinhibited Ca2+-ATPases (ACAs), ER-type Ca2+-ATPases (ECAs), mitochondrial Ca2+ uniporter (MCU), P1-ATPases (e.g., HMA1), Ca2+ exchangers (CAX), two-pore channel (TPC), cyclic nucleotide-gated channels (CNGCs), glutamate receptor-like channels (GLRs), stretch-activated Ca2+ channels (OSCAs), MID1-complementing activity (MCA), phosphate (P), adenosine triphosphate (ATP), adenosine diphosphate (ADP), 1,4,5-trisphosphate receptor-like channel (InsP3R), 1,4,5-trisphosphate (IP3), cyclic ADP-ribose (cADPR)-activator ryanodine receptor-like channel (RyR), slow-activating vacuolar channel (SV), and sodium-calcium exchanger (NCX).
Once the Ca2+ ion enters the cell, it is sensed by an array of Ca2+-binding proteins. The Ca2+-binding proteins work as Ca2+ sensors decoding complex Ca2+ signatures (Kudla et al., 2018). Ca2+ sensors are highly conserved proteins and are classified into (a) CaM and CaM-like proteins (CMLs), (b) calcineurin-B-like proteins (CBLs), and (c) Ca2+-dependent protein kinases (CPKs) and Ca2+ and Ca2+/CaM-dependent protein kinase (CCaMK) (Cheng et al., 2002; Luan, 2009; Bender and Snedden, 2013; Ranty et al., 2016). CaM, CMLs, CBLs, and CPKs are comprehensively involved in the cross-talk of various biotic and abiotic stress signals (Ranty et al., 2016; Aldon et al., 2018). Many Ca2+ and Ca2+ sensor-associated transcription factors (TFs) are implicated in stress signaling in plants (Carrion et al., 1999; Singh and Virdi, 2013; Ranty et al., 2016; Chung et al., 2020; Shen et al., 2020). The largest and best characterized family of Ca2+/CaM-dependent TFs are CAMTAs (Iqbal et al., 2020b). CAMTA3 has been reported enormously as a suppressor of plant biotic defense responses (Benn et al., 2016; Jacob et al., 2018; Kim et al., 2020). It works downstream to MAP kinase (Bjornson et al., 2014) and is directly phosphorylated and degraded by flg22-responsive mitogen-activated protein kinases (MAPKs) (Jiang et al., 2020). Precisely, MPK3 and MPK6 activate CAMTA3 nuclear export and destabilization (Jiang et al., 2020). Similarly, NAC TF, upon interaction with Ca2+/CaM, positively regulates various biotic stress responses in Solanum lycopersicum (Wang G. et al., 2016). NAC is also responsive to Colletotrichum gloeosporioides and Ralstonia solanacearum infection in woodland strawberry (Zhang et al., 2018). WRKY is another Ca2+/CaM-dependent TF (Park et al., 2005; Yan et al., 2018) implicated in pathogen incursion (Park et al., 2005; Bai et al., 2018). WRKY7, WRKY45, WRKY43, WRKY53, and WRKY50 in a Ca2+-driven manner bind to various isoforms of CaM (Park et al., 2005; Popescu et al., 2007). MYB TF is also well characterized as a Ca2+-dependent TF. MYB functions upstream in a vast majority of defense-responsive and abiotic stress-receptive genes (Stracke et al., 2001; Chezem et al., 2017; Li et al., 2019). Similarly taking CMLs into consideration, AtCML9 works as positive regulator of plant immune response. It was found to be induced by Pseudomonas syringae and phytohormones including abscisic acid (ABA) and salicylic acid (SA) (Magnan et al., 2008; Leba et al., 2012). Further, AtCML9 interacts with WRKY53 and TGA3 TFs, both of which are known to mediate biotic stress responses (Popescu et al., 2007). In concurrence, AtCML37 and AtCML42 are associated with defense against herbivorous insects (Spodoptera littoralis) (Vadassery et al., 2012; Scholz et al., 2014). Very recently, 17 AcoCPK genes from Ananas comosus (pineapple) were analyzed for their effect under biotic stress. AcoCPK1, AcoCPK3, and AcoCPK6 were shown to render susceptible disease resistance in A. thaliana against Sclerotinia sclerotiorum (Zhang et al., 2020). Another class of Ca2+ sensors, CBLs, are known to specifically interact with a family of plant-specific CBL-interacting protein kinases (CIPKs). CBL interacts with Ca2+ and binds with CIPK, resulting in kinase activation. The CBL–CIPK complex actively regulates downstream target proteins by phosphorylation (reviewed by Ma et al., 2020; Tang et al., 2020).
The other initial responses of pathogen attack on plants include the generation of reactive oxygen species (ROS) and activation of mitogen-activated protein kinases (MAPKs) (Muthamilarasan and Prasad, 2013). ROS and MAPKs overlap with other signaling pathways, including light pathways (Goldsmith and Bell-Pedersen, 2013; Foyer, 2018). Furthermore, pest attack on plants activates local or systemic defense responses involving oligogalacturonoids (OGAs), jasmonic acid (JA), and hydrogen peroxide (H2O2) signaling pathways (Fürstenberg-Hägg et al., 2013). Plants are also capable of producing volatile compounds that repel attacking pests (discussed in section “Effect of Dark/Light on Plant–Pathogen Interaction and Associated Mechanisms”). These compounds are part of lipoxygenase (LOX) and terpenoid signaling pathways (Pichersky and Gershenzon, 2002; Dudareva et al., 2006). Another pivotal downstream defense mechanism by plants include the generation of defensive proteins and universal stress proteins. These proteins comprise protein inhibitors, lectins, chitinases, α-amylase inhibitors, and polyphenol oxidases (Fürstenberg-Hägg et al., 2013; Lee et al., 2019). Additionally, the role of pathogenesis-related (PR) genes in plant defense responses has been considerably explored (Ali et al., 2018). PR genes translate into proteins that are induced in plants only upon pathological or similar conditions (conditions of non-pathogenic origin) (Jain and Khurana, 2018). They are considered as an important component of plant innate immune response and are implicated in HR and SAR responses (Jain and Khurana, 2018). PR proteins are grouped into 17 families, depending upon their biochemical and molecular properties (van Loon et al., 2006). In A. thaliana, five PR genes (PR-1, PR-2, PR-3, PR-4, and PR-5) are routinely explored for their involvement in plant biotic interactions (Hamamouch et al., 2011). PR-1, PR-2, and PR-5 are implicated in SA-dependent SAR response, while PR-3 and PR-4 are involved in JA-dependent SAR response (Thomma et al., 1998; Hamamouch et al., 2011). An important aspect associated with PR proteins is their simultaneous indulgence in biotic and abiotic stress (Ali et al., 2018). To substantiate this, the 1,000-bp upstream region of all five PR genes from A. thaliana were analyzed bioinformatically to determine the presence of different motifs associated with a variety of environmental stresses. Intriguingly, all the PR genes contained multiple light-responsive motifs (AE-box, GAP-box, GT-1 motif, G-box, GATA-motif, box-4, and chs-CMA2a). The presence of light-responsive motifs in the promoter region of PR genes probably implies the binding of light-dependent genes to these conserved sequences (Figure 2). This notion itself supports the idea of intense cross-talks between biotic stress responses and light signaling pathways.
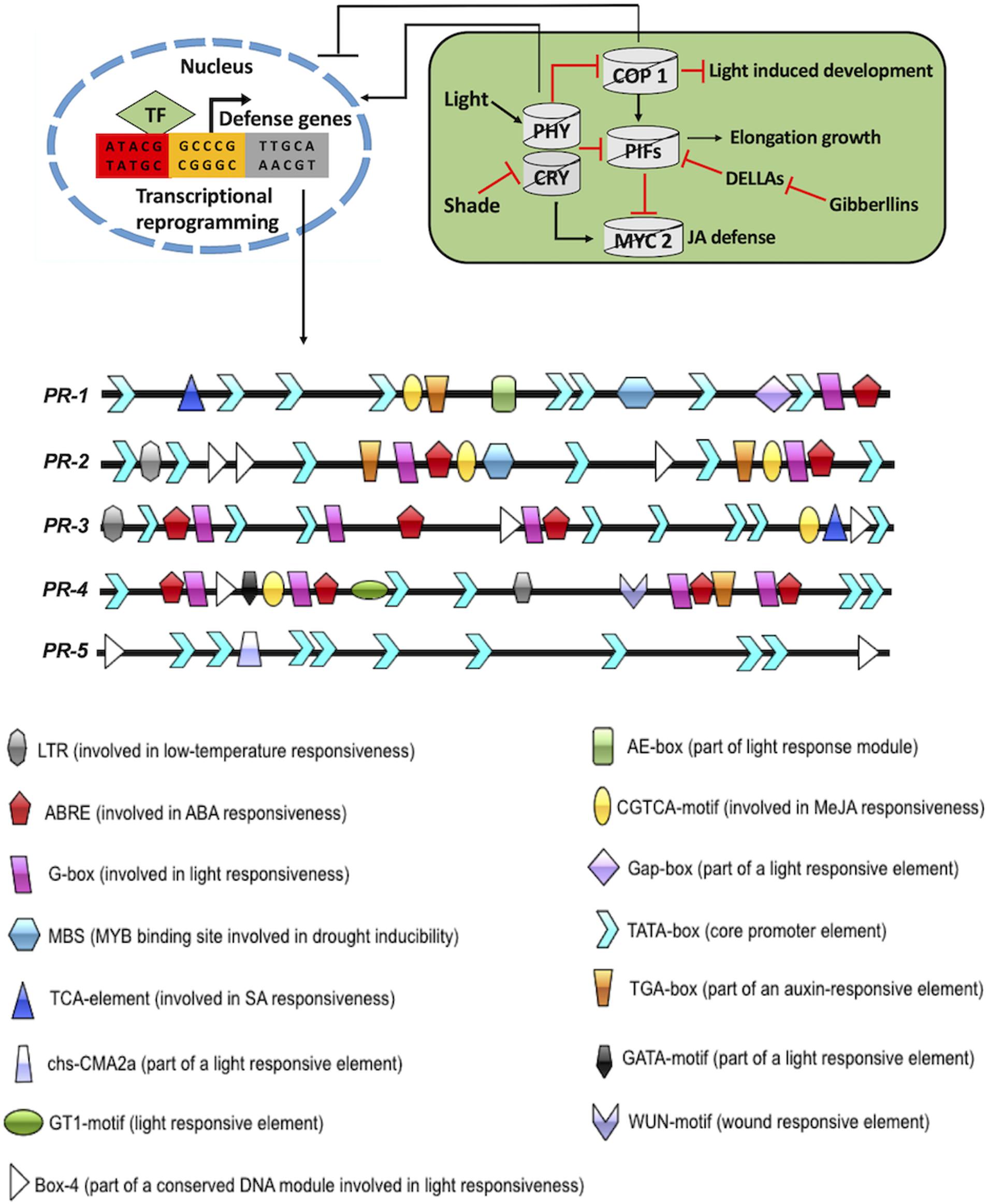
Figure 2. Intersection of plant defense and light signaling. The 1,000-bp upstream sequence of PR-1, PR-2, PR-3, PR-4, and PR-5 were fetched from TAIR10 (https://www.arabidopsis.org/). The motif analysis was done by PLACE database (https://www.dna.affrc.go.jp/PLACE/?action=newplace) and PlantCARE database (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/). The motif structures were drawn using Illustrator for Biological sequences software (http://ibs.biocuckoo.org/).
Finally, the involvement of phytohormones in regulating plant biotic defense responses cannot be ruled out. ETI and PTI induces specific downstream signaling pathways, in which three phytohormones are crucial, namely, SA, JA, and ethylene (ET). SA regulatory pathways are responsive to biotrophic and hemi-biotrophic pathogenic agents. Similarly, JA and ET pathways are responsive to necrotrophic agents and chewing pests (Bari and Jones, 2009; De Vleesschauwer et al., 2014). SA stimulates the SAR pathway promoting the expression of PR genes, which in-turn renders tolerance against a wide range of pathogens (Grant and Lamb, 2006; Fu and Dong, 2013; Ádám et al., 2018). SA, JA, and ET regulatory pathways for plant defense exhibit significant divergence, but they overlap to render defense against pathogenic agents (Glazebrook, 2005; Ku et al., 2018). Additionally, ABA, auxin, brassinosteroids (BRs), cytokinin (CK), gibberellic acid (GA), and peptide hormones also have vital significance in regulating the immune responses of the plants (Bari and Jones, 2009; Ku et al., 2018; Islam et al., 2019; Chen et al., 2020). Amongst all the phytohormones, JA is critical in triggering the plant defense system and cross-talks with other phytohormonal pathways to stimulate the plant immune responses (Yang et al., 2019).
Light as an Environmental Cue
Plants are exposed to variable light intensities that encompass light perception and signaling pathways responsible for growth, development, and immune responses (Hua, 2013; Ballaré, 2014). Nevertheless, plants often confront light intensities that exceed their photosynthetic capacity, inducing light stress (Mishra et al., 2012). Mechanisms encompassing light/dark alteration under stress conditions have been comprehensively studied (Mittler, 2002; Cerdán and Chory, 2003; Jiao et al., 2007; Koussevitzky et al., 2007; Mühlenbock et al., 2008; Alabadí and Blázquez, 2009; Chory, 2010; Kami et al., 2010; Lau and Deng, 2010; Trotta et al., 2014; Kaiserli et al., 2015; Saijo and Loo, 2020). Given the extreme importance of light for survival, immunity, growth, and development, plants have evolved the capability to sense and respond to different spectra of light (visible, infrared, ultraviolet, etc.) through photoreceptors. In A. thaliana, five distinctive genes (PHYA–PHYE) encode phytochrome protein (Clack et al., 1994; Li et al., 2011). They potentially act as receptors for red and far-red lights (Takano et al., 2009). Similarly, in A. thaliana, cryptochromes encoded by CRY1 and CRY2 dedicatedly sense blue (∼400 nm) and green (500–600 nm) lights and UV-A (Folta and Maruhnich, 2007; Jiao et al., 2007; Bae and Choi, 2008; Jenkins, 2009).
As previously discussed, plants undergo skotomorphogenesis in the absence of light while photomorphogenesis in the presence of light (see section “Introduction”). Repressor proteins such as constitutive photomorphogenic/de-etiolated1/fusca (COP/DET/FUS) inhibit photomorphogenesis under dark conditions (Hardtke and Deng, 2000; Dong et al., 2014). Mutants with defects in any of these repressor proteins display constitutive photomorphogenic (COP) phenotypes under dark conditions (Lau and Deng, 2012). The repressor proteins are characterized into four categories with overlapping functions and have been studied extensively (Deepika et al., 2020; Pham et al., 2020). The first one is COP1, which is a RING-finger-type ubiquitin E3 ligase (Deng et al., 1992). Under dark conditions, it acts as a repressor of light signaling and accumulates in the nucleus (Xu D. et al., 2014). On the contrary, COP1 is exported out of the nucleus, facilitating photomorphogenesis under light conditions (von Arnim et al., 1997; Hardtke et al., 2000; Seo et al., 2003; Duek et al., 2004; Xu et al., 2016a; Podolec and Ulm, 2018). COP1 acts as a central repressor and facilitates ubiquitination and degradation of various positive regulators of light, namely, long hypocotyl in far-red 1 (HFR1), long hypocotyl 5 (HY5), and long after far-red light 1 (LAF1) (Hardtke et al., 2000; Osterlund et al., 2000; Jang et al., 2005; Yang et al., 2005). The degradation of positive regulators of light by COP1 is constrained under light by prohibiting COP1 protein from the nucleus. This triggers the initiation event of photomorphogenesis. The function of COP1 has been extensively linked to light signaling (Figure 2). However, it is also implicated in the regulation of flowering time, circadian rhythm, and temperature signaling (Ma et al., 2002; Yu et al., 2008; Jeong et al., 2010; Catalá et al., 2011; Menon et al., 2016; Wang W.-X. et al., 2016; Xu et al., 2016b; Hoecker, 2017). COP1 is also known to interact with the suppressor of PHYA 1–4 (SPA 1–4). This interaction results in tetrameric complexes comprising two COP1 and two SPA proteins (COP1/SPA complex) (Zhu et al., 2008). SPA proteins are reported to positively enhance COP1 function (Ordoñez-Herrera et al., 2015). Skotomorphogenesis is accomplished by suppressing the expression of genes involved in photomorphogenic development in the dark (Josse and Halliday, 2008). This is tightly regulated by the COP1–SPA1E3 ligase complex (Osterlund et al., 2000; Josse and Halliday, 2008; Ordoñez-Herrera et al., 2015; Holtkotte et al., 2016; Paik et al., 2019). COP1–SPA1E3 ligase targets HY5 TF for degradation by the proteasome (Osterlund et al., 2000). COP1–SPA complex interacts with CULLIN4 (CUL4) to form CUL4–COP1–SPA complex. CUL4–COP1–SPA complex acts as CULLIN ring E3 ligase (CRL) and degrades positively acting TFs under dark conditions to suppress photomorphogenesis (Chen et al., 2010). Interestingly, CUL4–COP1–SPA complex has a dual function in dark/light-induced photomorphogenesis (Zhu et al., 2015; Paik et al., 2019). CUL4–COP1–SPA complex activates early ubiquitin-mediated degradation of phytochrome interacting factor 1 (PIF1) to trigger light-induced seed germination (Zhu et al., 2015; Paik et al., 2019). The second group of repressor protein is COP9 signalosome (CSN). It is highly conserved and comprises eight subunits (Serino and Deng, 2003). CSN had been reported to be implicated in deneddylation/derubylation of CRLs (Schwechheimer et al., 2001). The third group of repressor protein is de-etiolated1 (DET1), COP10, DNA damage-binding protein 1 (DDB1), and CUL4. DET1 is known to bind histone H2B (Benvenuto et al., 2002). It also regulates PIFs and HFRs to suppress seed germination and photomorphogenesis under dark conditions (Dong et al., 2014; Shi et al., 2015). Finally, the fourth group of repressor protein is PIFs (PIF1–PIF8) that belong to basic helix-loop-helix (bHLH) family of TFs and suppresses photomorphogenesis under dark conditions (Leivar et al., 2008; Shin et al., 2009; Leivar and Quail, 2011; Pham et al., 2018). They bind to the G-box consensus sequence in the 1,000-bp upstream region of light-responsive genes. Under dark conditions, phytochromes physically interact with PIFs to repress light response. The activation of photoreceptors suppresses COP1/SPA E3 ubiquitin ligase complexes and PIFs (Martínez et al., 2018b). This eventually activates HY5 to modulate the expression of light-inducible genes and disrupts PIF function (Chen et al., 2013; Toledo-Ortiz et al., 2014; Gangappa and Kumar, 2017). Upon plant exposure to dark conditions, photoreceptor inactivation enables COP1/SPA- and PIF-mediated disruption of light signaling (Xu X. et al., 2014; Xu et al., 2015b, 2017). This signaling cascade promotes plant growth by involving phyto-hormones (such as BR, auxins, and GA) at the cost of plant immunity (Lozano-Durán and Zipfel, 2015; Martínez et al., 2018b).
Photoreceptors are also responsible to determine the quality of light (R:FR ratios). Upon excitation by R light, phytochromes are transformed into FR light-absorbing state (biologically active “Pfr”). Since red light is absorbed by chlorophyll and carotenoids, its quantity is significantly decreased when penetrating through a dense canopy (Slattery et al., 2017; Walker et al., 2018). Shade-intolerant plants (such as A. thaliana) perceive and respond to such conditions by elongating stems and promoting flowering (Fiorucci and Fankhauser, 2017). This is an evolutionary phenomenon developed in plants and is termed shade-avoidance syndrome (SAS). Plants exhibit SAS, which is represented by the elongation of plant parts such as hypocotyls, stems, and petioles (Casal, 2013). Both PHYA and PHYB proteins contribute towards SAS. PHYB restrains SAS under R-enriched light (R:FR > 1), while PHYA restrains SAS under FR-enriched light (R:FR < 1) (Franklin, 2008; Lorrain et al., 2008; Franklin and Quail, 2010; Jaillais and Chory, 2010; Martinez-Garcia et al., 2010; Stamm and Kumar, 2010). This also result in the inactivation of PIF to promote BR and auxin production (Martínez et al., 2018a).
The amalgamation of photochemical and non-photochemical processes (NPQ) dissipates excess excitation energy (EEE) of plants as heat. Photochemical- and NPQ-dissipated EEE maintenance is facilitated by the acidification of the chloroplast lumen, involving PSII-associated proteins (Niyogi, 2000; Müller et al., 2001; Li et al., 2004; Niyogi et al., 2005; Ciszak et al., 2015). EEE eventually results in the formation of ROS, H2O2, superoxide (), and singlet oxygen (1O2), which overlaps with biotic stress signaling. Light/dark alterations induce plant resistance to pathogen infection and oxidative damage in systemic tissues. This indicates a cross-wired signaling between dark/light conditions and biotic stress (Rossel et al., 2007; Mühlenbock et al., 2008; Szechyńska-Hebda et al., 2010; Zhao et al., 2014). EEE induces SAR and basal response to pathogenic biotrophic bacteria. This response alters ROS and redox signals and thus induces SA, ET, and glutathione (Mühlenbock et al., 2008; Szechyńska-Hebda et al., 2010).
Effect of Dark/Light on Plant–Pathogen Interaction and Associated Mechanisms
Accumulating evidences indicate that plant response to biotic stress cannot be fully deciphered by studying discrete stress response (Suzuki et al., 2014; Dworak et al., 2016). Such notions support comprehensive study in connection with plant responses to simultaneously appearing stresses. Both qualitative and quantitative changes occur in the intensity of light during dark/light alterations. The majority of invertebrate herbivores with few exceptions (Kreuger and Potter, 2001; VanLaerhoven et al., 2003) are more active at night in comparison with day because of parasitism or predation constraints during the day (Hassell and Southwood, 1978). The emission of volatiles also affects herbivory with respect to diurnal variation. There are even qualitative and quantitative disparities during day/night in wound-induced volatiles (De Moraes et al., 2001; Gouinguené and Turlings, 2002; Martin et al., 2003). Taking into account the effect of dark/light on pathogen attack upon plants, the number of airborne fungal spores is significantly high at night (dark) in comparison with day (Schmale and Bergstrom, 2004; Gilbert and Reynolds, 2005; Zhang et al., 2005). On the contrary, few fungal spores peak at day (light) time (Gadoury et al., 1998; Su et al., 2000). Along with dark/light alterations, the plant–pathogen interaction is also influenced by an array of factors such as temperature fluctuations, humidity changes, and leaf surface water content resulting from dew conditions at night (Meijer and Leuchtmann, 2000; Koh et al., 2003). The presence of light also reduces germ tube growth and spore germination in plant pathogenic fungi (Mueller and Buck, 2003; Beyer et al., 2004). A number of studies have revealed that pathogen infection is influenced by light/dark conditions before inoculation happens. Tolerance to aphid infestation was also confirmed by high-light pre-exposures in wild-type plants and mutants impaired in protein phosphatase 2A (PP2A) (Rasool et al., 2014). Similarly, inoculation of Puccinia striiformis in wheat (Triticum aestivum) seedlings was more at low light intensity than dark-grown seedlings (De Vallavieille-Pope et al., 2002). In a few other instances, inoculation irradiances have been found to be inversely proportional to infection (Shafia et al., 2001), indicating a direct impact of dark/light on host tolerance. Recently, nucleotide-binding NLR Rpi-vnt1.1 proteins have been shown to require light for imparting disease resistance against races of the Irish potato famine pathogen Phytophthora infestans, which discharge the effector protein AVRvnt1 (Gao et al., 2020). Glycerate 3-kinase (GLYK), which is a nuclear-encoded chloroplast protein, is necessary for the activation of Rpi-vnt1.1. Under light conditions, AVRvnt1 binds to the full-length chloroplast targeted GLYK isoform triggering of Rpi-vnt1.1. However, under the dark scenario, plants generate a shorter truncated GLYK that is devoid of the intact chloroplast transit peptide, thus compromising Rpi-vnt1.1-mediated resistance. The conversion between full-length and short-length GLYK transcripts is governed by light-dependent promoter selection mechanism. In plants that are devoid of Rpi-vnt1.1, the occurrence of AVRvnt1 decreases GLYK accumulation in chloroplasts, hence reducing GLYK contribution to basal immunity. The findings are thus clearly depictive of the fact that the pathogen-driven functional alteration of the chloroplast results in a light-dependent immune response (Gao et al., 2020). Plausibly, plants are more prone to pathogen attack in the dark than during the day. However, it cannot be held true for all pathogens attacking the plant systems.
There occur two mechanisms that contribute to the regulation of plant defense responses during dark/light fluctuations: first, the energetic significance of light-dependent chemical reactions (depends on the capacity of photosynthetic electron transport to produce ATP and reducing power); and second, perception of light (shade and R:FR exposure conditions) and regulation of downstream light-dependent signaling pathways (Roberts and Paul, 2006). The following subsections highlight both the mechanisms with respect to photosynthesis, ROS accumulation, and light signaling.
Photosynthetic Processes and Reactive Oxygen Species Accumulation in Biotic Stress
Photosynthesis captures light energy via electron transport chain (ETC) for assimilation of carbon dioxide as well as repair and growth of plant body. The vital metabolites so produced from photosynthesis are utilized in carbon fixation, fatty acid biosynthesis, assimilation of nitrogen into amino acids, etc. (Nunes-Nesi et al., 2010). These light-driven pathways occurring in chloroplast can impact short term-induced plant defense responses (Delprato et al., 2015). Intriguingly, some part of the biosynthetic pathways of ABA, JA, and SA (plant defense hormones) also occur in the plastids (Bobik and Burch-Smith, 2015). This might impact plant defense in the dark due to the hormonal cross-talk in plant–microbe interaction. Moreover, chloroplast acts as a site for ROS generation upon stress perception. Leaves get acclimatized to light fluctuations during growth and development, as calvin cycle enzymes and light-harvesting complexes are adjusted to efficiently manage the available light. However, photosynthetic electron transport produces more electrons when carbon fixation is halted or light fluctuations occur. This helps in the generation of more electrons for the electron acceptor NADP+. Under such circumstances, free electrons from ETC are transferred to oxygen leading to ROS generation. Additionally, the light-dependent events and pathways occurring in the chloroplast impact short and long-term-induced plant defense responses via photorespiration resulting in the generation of H2O2 in the peroxisomes (Lu and Yao, 2018). Under acute light stress conditions, impairment in chlorophyll synthesis and disruption of chloroplast can also lead to the accumulation of ROS. This might surpass the potential of the antioxidant system in the chloroplast (Apel and Hirt, 2004). Nevertheless, ROS has also been very well implicated in plant defense against pathogens (Torres, 2010; Nath et al., 2017; Huang et al., 2019), and any deviation of the redox balance in the chloroplast can impact ROS regulated plant defense (Figure 3). For instance, lipid peroxidation occurs when ROS accumulates upon biotic stress perception (De Dios Alché, 2019). The repercussions of the requisite of light/dark fluctuations for chloroplast-derived ROS goes far beyond direct signaling functions of ROS.
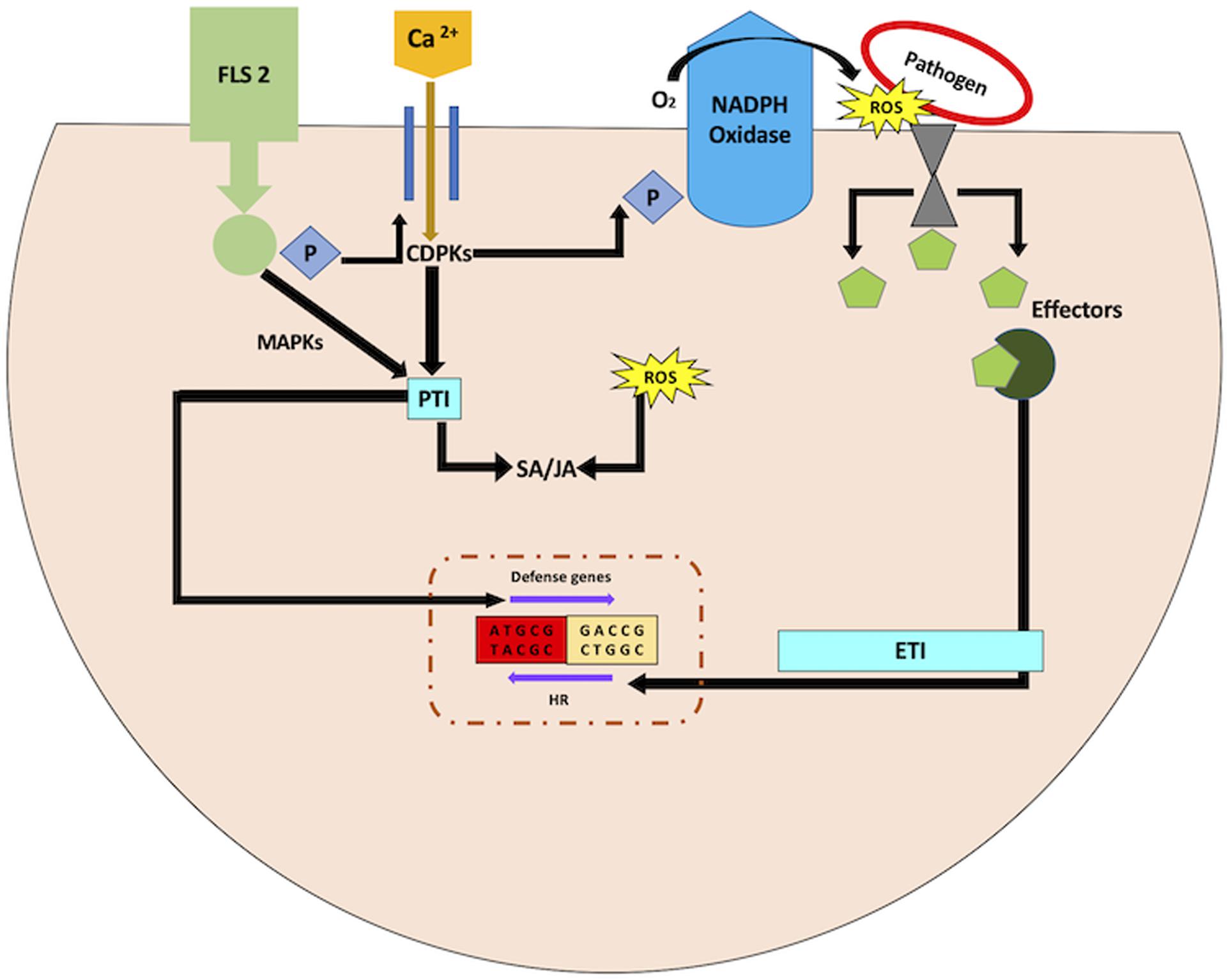
Figure 3. ROS modulation of biotic stress responses. FLS2 receptor kinase triggers the Ca2+ flux, followed with mitogen-activated protein kinase (MAPK) and Ca2+–dependent protein kinase (CDPK) cascades. These initial signals contribute to pathogen-associated molecular pattern (PAMP)-triggered immunity (PTI). Effector molecules are synthesized by host-adapted microbes, which suppress PTI. Plant system under such circumstances specifically identifies effector molecules to activate effector-triggered immunity (ETI). This eventually initiates the hypersensitive response (HR).
As for post pathogen attack, some of the products of lipid peroxidation are reactive electrophiles with a carbonyl group (Vollenweider et al., 2000). These electrophiles are a consequence of ROS impact on membrane lipids or are products arising from lipoxygenase enzyme activity. Many amongst these electrophiles are imperative signaling molecules implicated in the regulation of cell death and defense gene expression (Vollenweider et al., 2000; Alméras et al., 2003; Thoma et al., 2003; Cacas et al., 2005). Hence, light/dark fluctuations impact the production of ROS-derived electrophiles. Such cases are reported in interactions amongst plants and pathogens or their elicitors. Taking into consideration the response of cryptogein (a known elicitor), cell death was mediated by ROS accumulation in light conditions (Montillet et al., 2005). On the contrary, when plants are subjected to dark conditions, cell death is independent of ROS accumulation and correlates with specific lipoxygenase activity (Montillet et al., 2005).
The primary source of ROS during biotic stress response is not the chloroplast. It is rather NADPH oxidase (respiratory burst oxidase) that is localized in the plasma membrane (Apel and Hirt, 2004; Figure 3). This implies that chloroplast-derived ROS in the presence of light may not help with pathogen defense. Nonetheless, this may or may not hold true, since NADPH oxidase does not impede the production of chloroplast-derived ROS. More so, lesion mimic mutants with random necrotic lesions are characterized to comprehend the underlying mechanisms involved in signaling of biotic stress tolerance (Lorrain et al., 2003). These necrotic lesions on the leaves are comparable with those generated in response to HR. Lesion mimic mutants have higher expression of PR genes and enhanced resistance against pathogen attack. These mutants highlight the common nexus between biotic stress response and chloroplast ROS based on two observations (Karpinski et al., 2003; Bechtold et al., 2005). First, the formation of lesions in lesion mimic mutants are light-dependent (Brodersen et al., 2002). Second, the functional characterization of these mutants highlights genes implicated in chlorophyll biosynthesis or degradation (Ishikawa et al., 2001; Mach et al., 2001; Pružinská et al., 2003; Wang F. et al., 2016; Lv et al., 2019). Additionally, the change in expression profiles of genes implicated in chlorophyll biosynthesis also leads to light-dependent lesion mimic phenotypes, eventually resulting in enhanced disease tolerance (Molina et al., 1999; Lv et al., 2019). This may be due to the formation of ROS generated by the effect of light on chlorophyll intermediates acting as photosensitizers. The electrons are excited by the absorption of light energy by photosensitizers. The ROS thus produced acts as signals for pathogen resistance responses. Hence, it is evident that light-derived ROS from either free photosensitive pigments or photosynthetic light-harvesting complexes can influence plant defense signaling.
Plants have decentralized well-defined mechanisms for light-derived ROS in tissues subjected to biotic stress. For instance, the A. thaliana chlorophyllase 1 (AtCHL1) gene is implicated in chlorophyll degradation and removal of photosensitive porphyrin ring intermediates. AtCHL1 functions to preclude ROS accumulation due to damaged chloroplast (Kariola et al., 2005). This particular gene has been established to be triggered upon necrotrophic infections (Kariola et al., 2005). Plants with impaired AtCHL1 gene display enhanced tolerance to Erwinia carotovora (necrotrophic bacterial pathogen) but reduced tolerance to Alternaria brassicicola (a fungal necrotroph) (Kariola et al., 2005). SA-dependent pathway is involved in E. carotovora resistance, while JA-dependent pathway is involved in A. brassicicola resistance. SA- and JA-mediated plant defense responses are antagonistic in nature (Figure 4). As such, AtCHL1 mediates the equilibrium between SA- and JA-dependent plant–pathogen resistance pathways by adjusting ROS accumulation from chlorophyll metabolites. Similarly, the A. thaliana ACD2 gene decreases the accumulation of photosensitizers. This results in an increased resistance to P. syringae (Mach et al., 2001). It is also noteworthy that several plants generate photosensitizers, which directly play a prominent role in imparting biotic stress tolerance. Phototoxins produce ROS in the presence of white or UV light that directly prevents herbivore or pathogen infection (Downum, 1992; Flors and Nonell, 2006). On the contrary, few fungal pathogens themselves generate photosensitive toxins (namely, cercosporin) leading to plant cell necrosis (Daub and Ehrenshaft, 2000). An entire range of various levels of interaction amongst light, dark, and biotic stress constitutes induced defenses in plants. These levels of interaction include ROS generation, phytochrome signaling, and activation of biotic stress-related genes. Taken together, different biotic agents deploy overlapping signaling pathways with ROS as the key modulator molecule (Figure 4). Thus, comprehending the significance and pathways involved in these overlapping responses may be useful in deciphering the overall involvement of light/dark alterations on biotic stress tolerance and resistance mechanisms.
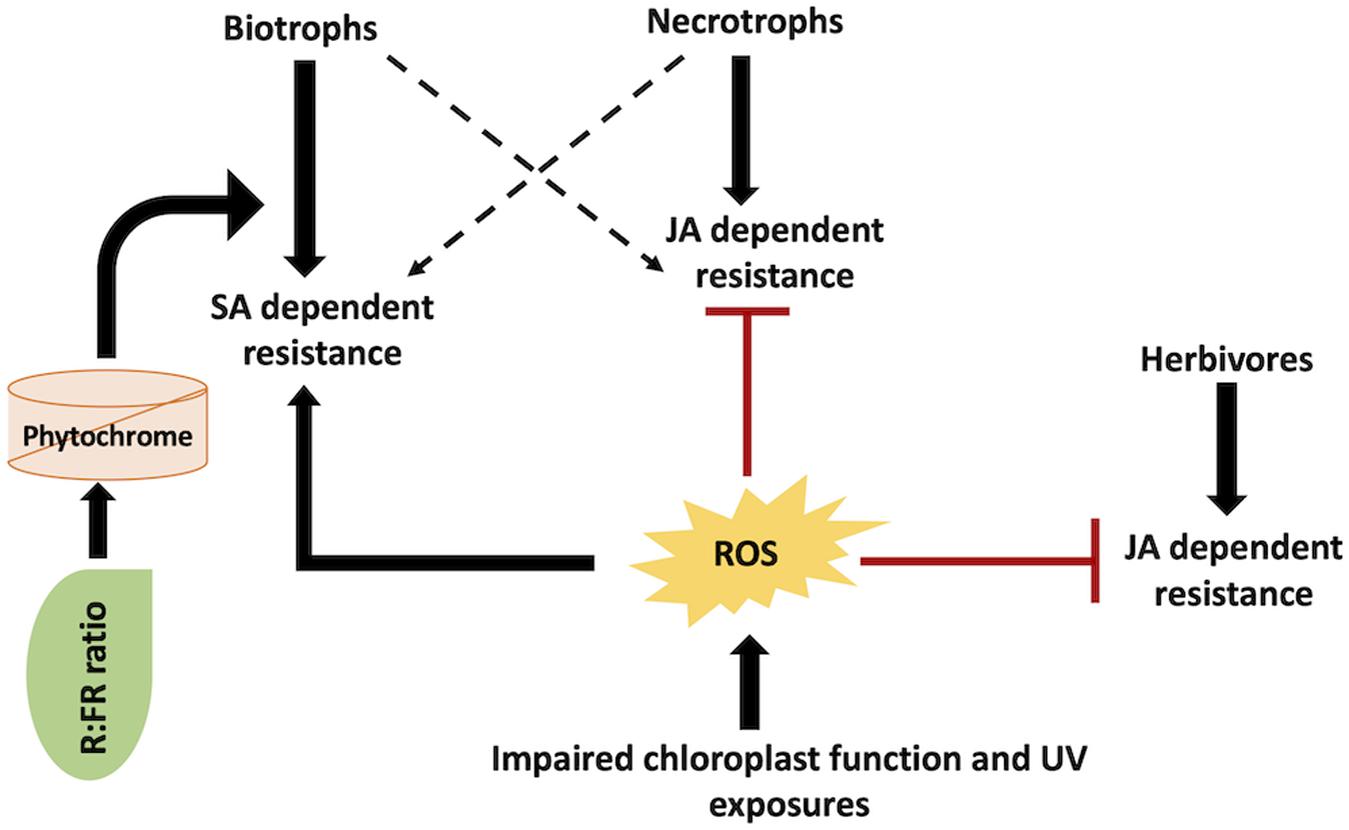
Figure 4. SA/JA mediated cross-talk in light signaling and defense responses against different pathogenic agents: various biotic agents activate different mechanisms. Reactive oxygen species (ROS), salicylic acid (SA), and jasmonic acid (JA).
Perception of Light With Respect to Shade and R:FR Exposures; and Regulation of Downstream Light-Dependent Signaling Pathways
The second key mechanism by which light/dark alterations regulate biotic stress responses engages direct light-responsive signaling pathways. The Genoud et al. (1998) has elegantly unraveled this mechanism in A. thaliana. The group has identified psi2 light signaling defective mutant that develops light-dependent random necrotic lesions and has an increased expression of PR1 gene (Genoud et al., 1998). Further characterization of psi2 mutant reveals that the biotic stress responses are governed by light at various levels. For example, PSI2 regulates the responses associated with phytochrome. Moreover, PHYA and PHYB are essential for PR gene expression and light-dependent HR lesion formation (Genoud et al., 1998, 2002). Hence, the phytochrome mutants have decreased resistance to P. syringae, while the psi2 mutants have enhanced resistance to P. syringae. This is a clear evidence where light signals play a pivotal role in the regulation of induced biotic resistance. However, why and how phytochrome signaling modulate biotic stress responses still remain obscure. On the contrary, the dark conditions or high light stress also operates molecular pathways that are common with pathogen responses (Mackerness et al., 1999; Rossel et al., 2002; Izaguirre et al., 2003; Kimura et al., 2003; Stratmann, 2003; Zhao et al., 2014). Enormous literature exists on the physiological basis of light dependency in relation to biotic defense, but the in-depth basis of dark/light effect on induced resistance remains elusive. A vital question, therefore, is to ascertain the mechanistic details for such observations. Undoubtedly, light is indispensable for plant growth and development, meaning that there is no unambiguous explanation to connect various observations across distinctive scales of organization. However, there are two modules that can be taken into account: first, resistance, which decreases the rigor of pathogen attack by restricting the activity of pathogen; and second, tolerance, which decreases the adverse effects of pathogen attack on the host plant. The demarcation between resistance and tolerance is critical for comprehension of interaction mechanisms between dark/light and plant defense.
In the field, the shade affects the cumulative radiation balance with plausible influence on biotic environment of the host. The temperature of the surrounding air and organisms is usually lower in shade, influencing a wide range of biological processes including biotic stress. For instance, tree canopies influence the species richness of insectivorous birds that affects herbivory (Strong et al., 2000; Van Bael and Brawn, 2005; Nell et al., 2018). Similarly, canopy shade has varying effects on photosynthetically active radiation (PAR) and UV wavelength (Grant and Heisler, 2001; Heisler et al., 2003; Grant et al., 2005). Additionally, the shade also results in either infestation by many pathogens or protection from the others. Pathogenic infestation is more stern in shade, for example, anthracnose (C. gloeosporioides) of Euonymus fortunei (Ningen et al., 2005), powdery mildew (Microsphaera alphitoides) on oak (Quercus petraea) (Kelly, 2002), and coffee rust (Hemileia vastatrix) (Soto-Pinto et al., 2002). Nonetheless, very often, plants develop a symbiotic relationship with beneficial microbes to enhance their defense responses and obtain nutrients under deficit conditions. The intense interplay between light signaling and defense mechanisms against beneficial and harmful microorganisms might be imperative for plant growth on high planting densities. Taking into account the beneficial interactions, the best-studied example is the nitrogen-fixing rhizobium bacteria and the leguminous plants (Ferguson et al., 2010). Rhizobium colonizes plant roots to form nodules that fix atmospheric nitrogen into mineral nitrogen for efficient usage by the leguminous plants. In return, the bacteria get carbon sources from the plant, which is essential for their survival (Ferguson et al., 2010). The Lotus japonicus PhyB mutant displays a shade-avoidance phenotype (similar to Arabidopsis mutant) with lesser number of root nodules in contrast to control plants (Suzuki et al., 2011; Sessa et al., 2018). Experimental validation reveals that the nodulation is decreased in grafted plants with phyB shoots and control roots. This is indicative of the fact that the mutations in the shoot tissue decrease nodulation in the roots (Suzuki et al., 2011; Shigeyama et al., 2012). The decreased nodulation in phyB mutants can be linked to downregulation of JA-responsive gene expression leading to lower JA levels in roots (Suzuki et al., 2011; Shigeyama et al., 2012). Next, taking into account the impact of R:FR exposures, the plant defense mechanisms against herbivores and pathogens are downregulated under low R:FR conditions (Ballaré, 2014; Ballaré and Austin, 2019; Figure 5). This probably implies that the interplay between beneficial interactions and light signaling is species-specific. In yet another example, plants establish a symbiotic relationship with arbuscular mycorrhizal fungi (AMF). These phosphate-acquiring fungi form “arbuscules” to enable phosphate and nitrogen uptake in plants, and in return, they derive carbon sources from plants (Keymer et al., 2017). The exposure of low R:FR ratios to L. japonicus roots decreases hyphal development of the AMF Rhizophagus irregularis. This is tightly regulated by the downregulation of JA-responsive genes resulting in decreased JA levels in root exudates (Nagata et al., 2015, 2016). At high plant density area, symbiotic relationship with rhizobium and AMF may be under scrutiny during low R:FR light conditions. However, the relationship between plant–microbe beneficial interactions and light signaling is still unclear and requires further investigation to improve plant growth and immunity.
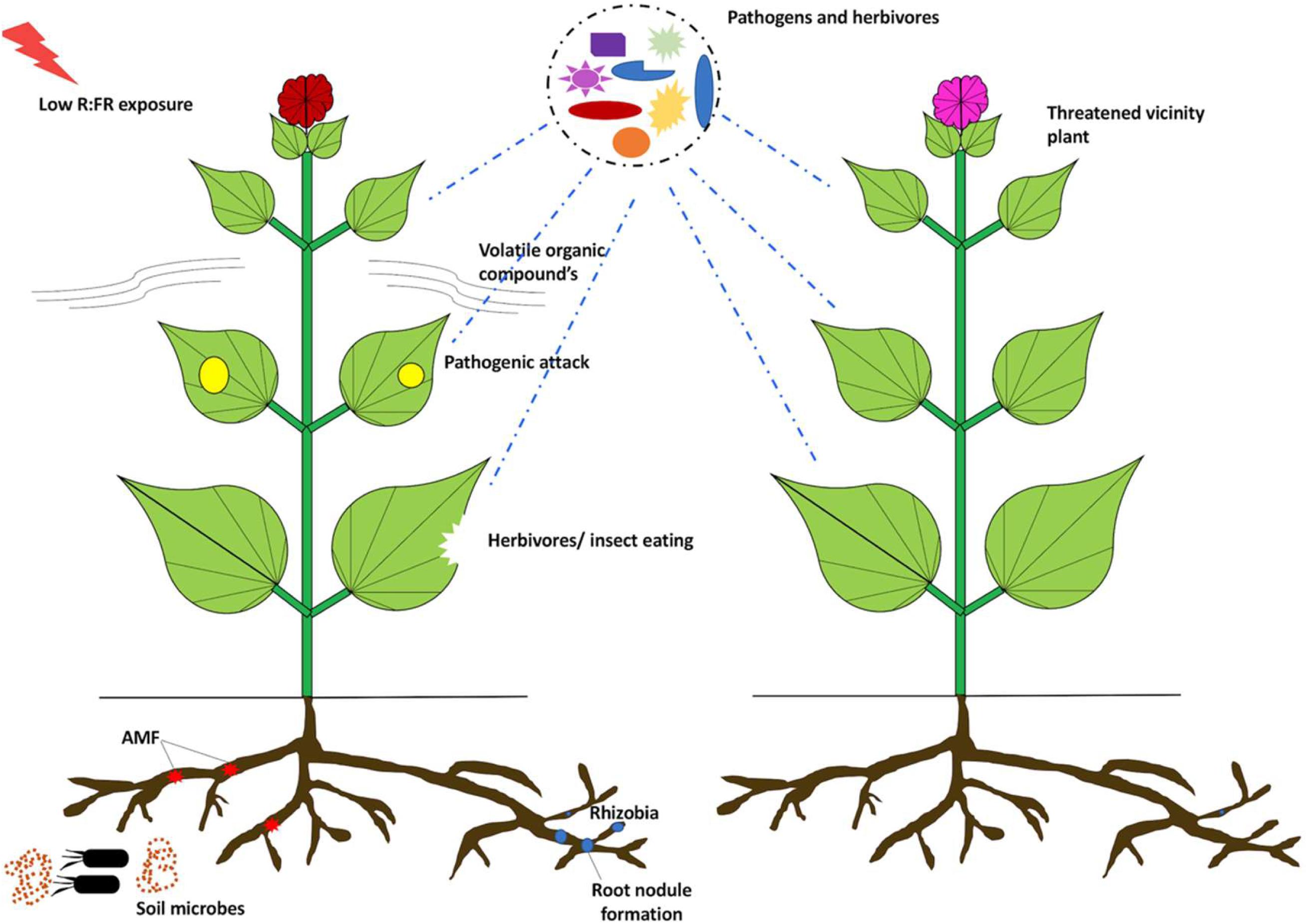
Figure 5. Low R:FR modulation of plant immunity: low R:FR makes the plants more susceptible to pathogens and insects. Low R:FR exposure modulates VOC compositions, exposing plants to herbivory attack. Also, the formation of nodules and arbuscules is impacted by R:FR ratios. Arbuscular mycorrhizal fungi (AMF), volatile organic compounds (VOCs).
Plants possess a continuous ever-evolving armor of defense mechanisms to prevent the colonization of harmful pathogens (Jones and Dangl, 2006; Nishad et al., 2020). Plants identify the signatures from the impeding pathogens and microbes via PAMPs, HAMPs, ETI, and PTI (Zipfel, 2014; Cui et al., 2015; Peng et al., 2018) (see section “Biotic Stress and Plant Defense Responses”). As already discussed, the antagonistic relationship between JA and SA modulates defense responses against biotrophic and necrotrophic pathogens (Glazebrook, 2005). JA is the central regulatory phytohormone coordinating the defense responses against pathogens and insects (Turner et al., 2002; Santino et al., 2013; Yang et al., 2019). Initial studies indicated that plants exposed to low R:FR or with impaired PHYB gene function exhibit reduced resistance to herbivores that is associated with declined sensitivity to JA (McGuire and Agrawal, 2005; Izaguirre et al., 2006; Moreno et al., 2009). Upon herbivory attack, volatile organic compound (VOC) emissions and methyl jasmonate (MeJA)-associated gene expression decreases in A. thaliana under low R:FR exposures (Kegge et al., 2013; Figure 5). A similar observation has been reported in barley where low R:FR exposure modifies constitutive VOC emissions to regulate the responses associated with plant–plant interactions (Kegge et al., 2015). This is further confirmed in Solanum (Cortés et al., 2016). In tomato, a low R:FR ratio affects MeJA-mediated VOC composition. This in turn influences the indirect defense response by enticing the insects (Cortés et al., 2016). Additionally, an intricate regulation of light signaling pathways maintains a balance of the constructive or destructive effects of light on plant growth and immunity. In contrast to the above observations, under low R:FR conditions, Geranium robertianum (a shade adapted forest understory plant) does not display downregulation of its JA-related plant defenses (Gommers et al., 2017). It also exhibits a slight increase in resistance against B. cinerea. Transcriptome analysis of G. robertianum and Geranium pyrenaicum (a shade-avoiding plant) reveals a number of genes with an opposite mode of regulation upon encountering shade conditions. Under low R:FR conditions, receptors like kinases FER and THE1 (responsible for shade induced elongation growth) are induced in G. pyrenaicum. FER and THE1 may be directly involved in regulating plant immunity and growth under shade. Conversely, in G. robertianum, exposure to low R:FR ratios leads to suppression of JAZ genes, which confer immunity under shade conditions. This establishes a classical example of the plasticity of light signaling in modulating plant growth and defense responses (Hématy et al., 2007; Kessler et al., 2010; Stegmann et al., 2017). Phenotypic and transcriptomic studies unravel a link between SAS- and SA-based defense components in shade-unresponsive Arabidopsis mutants (Nozue et al., 2018). JA, SA, and auxin-related signaling pathways are stimulated under low R:FR conditions and contribute strongly toward SAS (Nozue et al., 2018). Prolonged photoperiods positively regulate SA production, SA-related defenses, systemic immunity, and autoimmunity in lesion-mimic mutants (Griebel and Zeier, 2008; Gangappa and Kumar, 2017). Shade-avoidance mechanism under low light conditions restrains defense via a number of mechanisms (Cipollini, 2004). The swing in the distribution of resources to growth under shade may compete with the allocation of resources to plant defense. There might also be an intersection between light signaling and defense signaling. Under shade, stem elongation is regulated by auxin and gibberellins (Vandenbussche and Van Der Straeten, 2004). Auxin is known to interact with defense signaling pathways via a cross-wired mesh involving indole acetic acid (IAA). IAA also decreases JA-regulated generation of defense compounds (Baldwin et al., 1997; Yang et al., 2019). Contrariwise, the expression and concentration of auxins are altered upon wounding and herbivory (Cheong et al., 2002; Schmelz et al., 2003; Machado et al., 2016). Even the stiffening of the cell wall is an antagonistic mechanism between plant defense and shade (Cipollini, 2004), where gibberellin causes cell wall loosening resulting in cell expansion in shade. This can be attributed as an imperative component of plant defense.
Extensive research has been devoted to the mechanistic details as to how phytochromes regulate JA responses in relation to biotic defense responses (Hou et al., 2010; Ballaré, 2014; Leone et al., 2014; Pieterse et al., 2014; Campos et al., 2016). The described mechanism involves the interaction between DELLA proteins (growth repressor) and JAZ proteins (negative defense regulator) (Ballaré, 2014; Pieterse et al., 2014). MYC2 has been very well implicated to activate downstream defense responses (Hou et al., 2010; Verhage et al., 2012; Woldemariam et al., 2013; Liu et al., 2019). The DELLA proteins are degraded to sequester JAZ, resulting in inhibition of MYC2 TF (Hou et al., 2010). JAZ10 protein has been observed to be highly stable in A. thaliana phyB mutant. This could probably be due to the degradation associated with DELLA proteins (Leone et al., 2014). Again, the lower sensitivity of the jaz10 phyB double mutant than the phyB mutant to B. cinerea highlights the importance of JAZ10 in relation to light signaling and biotic stress responses (Cerrudo et al., 2017). Particularly, inactivation of PHYB suppresses JA-related plant defense responses exclusive of shade-avoiding morphological changes (Moreno et al., 2009). In contrast, the JAZ absence reinforces JA-related plant defenses without compromising plant growth in phyB (Campos et al., 2016). Thus, plant defense activation or suppression is not dependent upon growth promotion or inhibition. This is suggestive of the fact that growth, light signaling, and defense trade-off are effective adaptive responses. Both JA- and SA-dependent defense responses are downregulated under low R:FR conditions. This also overlaps with NPR1 phosphorylation inhibition leading to reduced defense induction (de Wit et al., 2013). Also, for JA-related defense responses, prolonged photoperiods require the involvement of PHYA, cryptochromes, DELLAs, and the JA-regulating TF MYC2 (Cagnola et al., 2018). Conversely, short photoperiods result in PIF4-mediated growth elevation and immunity suppression. This is in concert with the fact that the elevated PIF4 accumulation and activation in the dark are dependent upon COP1/DET1 (Gangappa et al., 2017; Gangappa and Kumar, 2017). The COP1/DET1–PIF4 complex is also essential for autoimmunity suppression at high temperatures in snc1 and cpr5 mutants (Gangappa and Kumar, 2017). These studies are indicative of crucial involvement of the COP1/DET1-PIF module in prioritizing growth over plant immunity.
In addition, BR signaling apart from being involved in growth responses also plays a vital role in biotic stress responses (Planas-Riverola et al., 2019). BR signaling is linked with flagellin (a well-known PAMP) recognition upon pathogen attack. This is accomplished by the interaction between the BR receptor kinase BRI1 and its coreceptor BAK1 (Chinchilla et al., 2007). BR inhibits the defense machinery of plants by inducing Brassinazole-resistant 1 (BZR1) gene (Lozano-Durán et al., 2013; Lozano-Durán and Zipfel, 2015). BZR1 is an important component of the BAP/D module, which is very well implicated in plant growth and development (Bouré et al., 2019). Under low R:FR conditions, BR responses may be involved in growth via the BAP/D module that can supersede flagellin-mediated plant defense response. It is also pertinent to mention that low R:FR affects the primary metabolism of plants (Yang et al., 2016; de Wit et al., 2018). Upon infecting plants, pathogens target carbohydrates as the key source of carbon for their survival. The enhanced susceptibility under low R:FR or in the phytochrome mutants may be due to higher accessibility of carbohydrates by the pathogens in plant tissues. Secondary metabolite production and defense-related gene expression (viz. MAPK and PR genes) are usually correlated with high concentrations of sugar accumulation in plant tissues (Bolouri Moghaddam and Van Den Ende, 2012). Reduced plant defense has been observed for B. cinerea under low R:FR conditions (Cargnel et al., 2014). This obstructed plant defense is a result of declined defense-related gene expression and metabolite production (Cargnel et al., 2014). Thus, low R:FR exposure declines defense-related pathways and enriches soluble sugars in plants, eventually inducing lesion formation in infected plant tissue (Figure 5). Taken together, plant growth responses to shade conditions are intricately cross-wired with the immune response generated by the plants upon pathogen exposure.
Conclusion and Future Prospects
Exposure of plants to a combination of adverse environmental cues such as biotic stresses and light fluctuations coerces the efforts to meet enormous food demand. Despite the massive usage of pesticides and insecticides in the last few decades, the overall crop losses due to pathogen attack have not been reduced significantly. Monitoring infection time, plant growth, and other important parameters such as light/dark conditions can result in a better understanding of plant defense toward pathogens, particularly when extrapolated to field conditions. The present review provides an elaborate information on how plants perceive and respond to multiple dark/light alterations and biotic stresses. Light and dark conditions together or independently modulate a diverse range of signaling pathways to control pivotal plant growth and defense regulators. The function of multi-faceted dark/light signaling intermediates such as COP, CRY, PHY, and PIF has been extensively covered to highlight the impact of dark and light modulations on plant biotic defense responses. Even though significant efforts have been made to deep dive into the plant–microbe interactions and their association with light signaling, the mechanistic details encircling this complex intersection are obscure. Thus, the basic research to comprehend the mechanisms involved in the integrated circuitry of plant immunity and dark/light interactions, at both biological and ecological scales, will pave the way to overcome the limitations associated with crop losses globally.
Author Contributions
MIA conceptualized and designed the study. ZI, MSI, AH, and EFA compiled the data and wrote the manuscript. All authors have read the manuscript and agreed for publication.
Funding
King Saud University, Saudi Arabia, research group (No. RG-1435-014).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We acknowledge the past and present members of our laboratory as well as our scientific collaborator in the field of plant stress physiology. We would like to extend our sincere appreciation to the Deanship of Scientific Research at King Saud University for funding this research group (No. RG-1435-014).
References
Abdul Malik, N. A., Kumar, I. S., and Nadarajah, K. (2020). Elicitor and receptor molecules: orchestrators of plant defense and immunity. Intern. J. Mol. Sci. 21:963. doi: 10.3390/ijms21030963
Ádám, A. L., Nagy, Z. Á, Kátay, G., Mergenthaler, E., and Viczián, O. (2018). Signals of systemic immunity in plants: progress and open questions. Intern. J. Mol. Sci. 19:1146. doi: 10.3390/ijms19041146
Ahn, I. P. (2007). Disturbance of the Ca2+/calmodulin-dependent signalling pathway is responsible for the resistance of Arabidopsis dnd1 against Pectobacterium carotovorum infection. Mol. Plant Pathol. 8, 747–759. doi: 10.1111/j.1364-3703.2007.00428.x
Alabadí, D., and Blázquez, M. A. (2009). Molecular interactions between light and hormone signaling to control plant growth. Plant Mol. Biol. 69:409. doi: 10.1007/s11103-008-9400-y
Aldon, D., Mbengue, M., Mazars, C., and Galaud, J.-P. (2018). Calcium signalling in plant biotic interactions. Intern. J. Mol. Sci. 19:665. doi: 10.3390/ijms19030665
Ali, S., Ganai, B. A., Kamili, A. N., Bhat, A. A., Mir, Z. A., Bhat, J. A., et al. (2018). Pathogenesis-related proteins and peptides as promising tools for engineering plants with multiple stress tolerance. Microbiol. Res. 212, 29–37. doi: 10.1016/j.micres.2018.04.008
Alméras, E., Stolz, S., Vollenweider, S., Reymond, P., Mène-Saffrané, L., and Farmer, E. E. (2003). Reactive electrophile species activate defense gene expression in Arabidopsis. Plant J. 34, 205–216. doi: 10.1046/j.1365-313X.2003.01718.x
Apel, K., and Hirt, H. (2004). Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55, 373–399. doi: 10.1146/annurev.arplant.55.031903.141701
Bae, G., and Choi, G. (2008). Decoding of light signals by plant phytochromes and their interacting proteins. Annu. Rev. Plant Biol. 59, 281–311. doi: 10.1146/annurev.arplant.59.032607.092859
Bai, Y., Sunarti, S., Kissoudis, C., Visser, R. G., and Van Der Linden, C. (2018). The role of tomato WRKY genes in plant responses to combined abiotic and biotic stresses. Front. Plant Sci. 9:801. doi: 10.3389/fpls.2018.00801
Baldwin, I. T., Zhang, Z.-P., Diab, N., Ohnmeiss, T. E., Mccloud, E. S., Lynds, G. Y., et al. (1997). Quantification, correlations and manipulations of wound-induced changes in jasmonic acid and nicotine in Nicotiana sylvestris. Planta 201, 397–404. doi: 10.1007/s004250050082
Ballaré, C. L. (2014). Light regulation of plant defense. Annu. Rev. Plant Biol. 65, 335–363. doi: 10.1146/annurev-arplant-050213-040145
Ballaré, C. L., and Austin, A. T. (2019). Recalculating growth and defense strategies under competition: key roles of photoreceptors and jasmonates. J. Exper. Bot. 70, 3425–3434. doi: 10.1093/jxb/erz237
Ballare, C. L., Mazza, C. A., Austin, A. T., and Pierik, R. (2012). Canopy light and plant health. Plant Physiol. 160, 145–155. doi: 10.1104/pp.112.200733
Bari, R., and Jones, J. D. (2009). Role of plant hormones in plant defence responses. Plant Mol. Biol. 69, 473–488. doi: 10.1007/s11103-008-9435-0
Bechtold, U., Karpinski, S., and Mullineaux, P. M. (2005). The influence of the light environment and photosynthesis on oxidative signalling responses in plant-biotrophic pathogen interactions. Plant Cell Environ. 28, 1046–1055. doi: 10.1111/j.1365-3040.2005.01340.x
Bender, K. W., and Snedden, W. A. (2013). Calmodulin-related proteins step out from the shadow of their namesake. Plant Physiol. 163, 486–495. doi: 10.1104/pp.113.221069
Benn, G., Bjornson, M., Ke, H., De Souza, A., Balmond, E. I., Shaw, J. T., et al. (2016). Plastidial metabolite MEcPP induces a transcriptionally centered stress-response hub via the transcription factor CAMTA3. Proc. Natl. Acad. Sci. U.S.A. 113, 8855–8860. doi: 10.1073/pnas.1602582113
Benvenuto, G., Formiggini, F., Laflamme, P., Malakhov, M., and Bowler, C. (2002). The photomorphogenesis regulator DET1 binds the amino-terminal tail of histone H2B in a nucleosome context. Curr. Biol. 12, 1529–1534. doi: 10.1016/S0960-9822(02)01105-3
Bernard, G. C., Egnin, M., and Bonsi, C. (2017). “The impact of plant-parasitic nematodes on agriculture and methods of control,” in Nematology-Concepts, Diagnosis and Control, eds M. M. Shah and M. Mahamood (London: IntechOpen). doi: 10.5772/intechopen.68958
Beyer, M., Röding, S., Ludewig, A., and Verreet, J. A. (2004). Germination and survival of Fusarium graminearum macroconidia as affected by environmental factors. J. Phytopathol. 152, 92–97. doi: 10.1111/j.1439-0434.2003.00807.x
Bjornson, M., Benn, G., Song, X., Comai, L., Franz, A. K., Dandekar, A. M., et al. (2014). Distinct roles for mitogen-activated protein kinase signaling and CALMODULIN-BINDING TRANSCRIPTIONAL ACTIVATOR3 in regulating the peak time and amplitude of the plant general stress response. Plant Physiol. 166, 988–996. doi: 10.1104/pp.114.245944
Bobik, K., and Burch-Smith, T. M. (2015). Chloroplast signaling within, between and beyond cells. Front. Plant Sci. 6:781. doi: 10.3389/fpls.2015.00781
Bolouri Moghaddam, M. R., and Van Den Ende, W. (2012). Sugars and plant innate immunity. J. Exper. Bot. 63, 3989–3998. doi: 10.1093/jxb/ers129
Bose, J., Pottosin, I., Shabala, S. S. S., Palmgren, M. G., and Shabala, S. (2011). Calcium efflux systems in stress signaling and adaptation in plants. Front. Plant Sci. 2:85. doi: 10.3389/fpls.2011.00085
Boudsocq, M., Willmann, M. R., Mccormack, M., Lee, H., Shan, L., He, P., et al. (2010). Differential innate immune signalling via Ca 2+ sensor protein kinases. Nature 464, 418–422. doi: 10.1038/nature08794
Bouré, N., Kumar, S. V., and Arnaud, N. (2019). The BAP module: a multisignal integrator orchestrating growth. Trends Plant Sci. 24, 602–610. doi: 10.1016/j.tplants.2019.04.002
Breeze, E. (2019). State of (in) flux: Action of a CNGC Ca2+ channel in defense against herbivory. Plant Cell 31, 1423–1424. doi: 10.1105/tpc.19.00372
Brodersen, P., Petersen, M., Pike, H. M., Olszak, B., Skov, S., Ødum, N., et al. (2002). Knockout of Arabidopsis accelerated-cell-death11 encoding a sphingosine transfer protein causes activation of programmed cell death and defense. Genes Dev. 16, 490–502. doi: 10.1101/gad.218202
Cacas, J.-L., Vailleau, F., Davoine, C., Ennar, N., Agnel, J. P., Tronchet, M., et al. (2005). The combined action of 9 lipoxygenase and galactolipase is sufficient to bring about programmed cell death during tobacco hypersensitive response. Plant Cell Environ. 28, 1367–1378. doi: 10.1111/j.1365-3040.2005.01369.x
Cagnola, J. I., Cerdan, P. D., Pacin, M., Andrade, A., Rodriguez, V., Zurbriggen, M. D., et al. (2018). Long-day photoperiod enhances jasmonic acid-related plant defense. Plant Physiol. 178, 163–173. doi: 10.1104/pp.18.00443
Campos, M. L., Yoshida, Y., Major, I. T., De Oliveira Ferreira, D., Weraduwage, S. M., Froehlich, J. E., et al. (2016). Rewiring of jasmonate and phytochrome B signalling uncouples plant growth-defense tradeoffs. Nat. Commun. 7, 1–10. doi: 10.1038/ncomms12570
Cargnel, M. D., Demkura, P. V., and Ballaré, C. L. (2014). Linking phytochrome to plant immunity: low red: far-red ratios increase Arabidopsis susceptibility to B otrytis cinerea by reducing the biosynthesis of indolic glucosinolates and camalexin. New Phytol. 204, 342–354. doi: 10.1111/nph.13032
Carrion, A. M., Link, W. A., Ledo, F., Mellström, B., and Naranjo, J. R. (1999). DREAM is a Ca 2+-regulated transcriptional repressor. Nature 398, 80–84. doi: 10.1038/18044
Casal, J. J. (2013). Photoreceptor signaling networks in plant responses to shade. Annu. Rev. Plant Biol. 64, 403–427. doi: 10.1146/annurev-arplant-050312-120221
Catalá, R., Medina, J., and Salinas, J. (2011). Integration of low temperature and light signaling during cold acclimation response in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 108, 16475–16480. doi: 10.1073/pnas.1107161108
Cerdán, P. D., and Chory, J. (2003). Regulation of flowering time by light quality. Nature 423, 881–885. doi: 10.1038/nature01636
Cerrudo, I., Caliri-Ortiz, M. E., Keller, M. M., Degano, M. E., Demkura, P. V., and Ballaré, C. L. (2017). Exploring growth-defence trade-offs in Arabidopsis: phytochrome B inactivation requires JAZ10 to suppress plant immunity but not to trigger shade-avoidance responses. Plant Cell Environ. 40, 635–644. doi: 10.1111/pce.12877
Chen, D., Xu, G., Tang, W., Jing, Y., Ji, Q., Fei, Z., et al. (2013). Antagonistic basic helix-loop-helix/bZIP transcription factors form transcriptional modules that integrate light and reactive oxygen species signaling in Arabidopsis. Plant Cell 25, 1657–1673. doi: 10.1105/tpc.112.104869
Chen, H., Huang, X., Gusmaroli, G., Terzaghi, W., Lau, O. S., Yanagawa, Y., et al. (2010). Arabidopsis CULLIN4-damaged DNA binding protein 1 interacts with CONSTITUTIVELY PHOTOMORPHOGENIC1-SUPPRESSOR OF PHYA complexes to regulate photomorphogenesis and flowering time. Plant Cell 22, 108–123. doi: 10.1105/tpc.109.065490
Chen, Y. L., Fan, K. T., Hung, S. C., and Chen, Y. R. (2020). The role of peptides cleaved from protein precursors in eliciting plant stress reactions. New Phytol. 225, 2267–2282. doi: 10.1111/nph.16241
Cheng, S.-H., Willmann, M. R., Chen, H.-C., and Sheen, J. (2002). Calcium signaling through protein kinases. The Arabidopsis calcium-dependent protein kinase gene family. Plant Physiol. 129, 469–485. doi: 10.1104/pp.005645
Cheng, X., Tian, C., Li, A., and Qiu, J. (2012). Advances on molecular mechanisms of plant-pathogen interactions. Yi Chuan Hereditas 34, 134–144. doi: 10.3724/SP.J.1005.2012.00134
Cheong, Y. H., Chang, H.-S., Gupta, R., Wang, X., Zhu, T., and Luan, S. (2002). Transcriptional profiling reveals novel interactions between wounding, pathogen, abiotic stress, and hormonal responses in Arabidopsis. Plant Physiol. 129, 661–677. doi: 10.1104/pp.002857
Chezem, W. R., Memon, A., Li, F.-S., Weng, J.-K., and Clay, N. K. (2017). SG2-type R2R3-MYB transcription factor MYB15 controls defense-induced lignification and basal immunity in Arabidopsis. Plant Cell 29, 1907–1926. doi: 10.1105/tpc.16.00954
Chiasson, D. M., Haage, K., Sollweck, K., Brachmann, A., Dietrich, P., and Parniske, M. (2017). A quantitative hypermorphic CNGC allele confers ectopic calcium flux and impairs cellular development. eLife 6:e25012. doi: 10.7554/eLife.25012.036
Chin, K., Defalco, T. A., Moeder, W., and Yoshioka, K. (2013). The Arabidopsis cyclic nucleotide-gated ion channels AtCNGC2 and AtCNGC4 work in the same signaling pathway to regulate pathogen defense and floral transition. Plant Physiol. 163, 611–624. doi: 10.1104/pp.113.225680
Chinchilla, D., Zipfel, C., Robatzek, S., Kemmerling, B., Nürnberger, T., Jones, J. D., et al. (2007). A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 448, 497–500. doi: 10.1038/nature05999
Chory, J. (2010). Light signal transduction: an infinite spectrum of possibilities. Plant J. 61, 982–991. doi: 10.1111/j.1365-313X.2009.04105.x
Christie, J. M. (2007). Phototropin blue-light receptors. Annu. Rev. Plant Biol. 58, 21–45. doi: 10.1146/annurev.arplant.58.032806.103951
Chung, J.-S., Koo, S. C., Jin, B. J., Baek, D., Yeom, S.-I., Chun, H. J., et al. (2020). Rice CaM-binding transcription factor (OsCBT) mediates defense signaling via transcriptional reprogramming. Plant Biotechnol. Rep. 14, 309–321. doi: 10.1007/s11816-020-00603-y
Cipollini, D. (2004). Stretching the limits of plasticity: can a plant defend against both competitors and herbivores? Ecology 85, 28–37. doi: 10.1890/02-0615
Ciszak, K., Kulasek, M., Barczak, A., Grzelak, J., Maćkowski, S., and Karpiński, S. (2015). PsbS is required for systemic acquired acclimation and post-excess-light-stress optimization of chlorophyll fluorescence decay times in Arabidopsis. Plant Signal. Behav. 10:e982018. doi: 10.4161/15592324.2014.982018
Clack, T., Mathews, S., and Sharrock, R. A. (1994). The phytochrome apoprotein family inArabidopsis is encoded by five genes: the sequences and expression ofPHYD andPHYE. Plant Mol. Biol. 25, 413–427. doi: 10.1007/BF00043870
Cortés, L. E., Weldegergis, B. T., Boccalandro, H. E., Dicke, M., and Ballaré, C. L. (2016). Trading direct for indirect defense? Phytochrome B inactivation in tomato attenuates direct anti-herbivore defenses whilst enhancing volatile-mediated attraction of predators. New Phytol. 212, 1057–1071. doi: 10.1111/nph.14210
Costa, A., Luoni, L., Marrano, C. A., Hashimoto, K., Köster, P., Giacometti, S., et al. (2017). Ca2+-dependent phosphoregulation of the plasma membrane Ca2+-ATPase ACA8 modulates stimulus-induced calcium signatures. J. Exper. Bot. 68, 3215–3230. doi: 10.1093/jxb/erx162
Costa, A., Navazio, L., and Szabo, I. (2018). The contribution of organelles to plant intracellular calcium signalling. J. Exper. Bot. 69, 4175–4193. doi: 10.1093/jxb/ery185
Creux, N., and Harmer, S. (2019). Circadian rhythms in plants. Cold Spring Harb. Perspect. Biol. 11:a034611. doi: 10.1101/cshperspect.a034611
Cui, H., Tsuda, K., and Parker, J. E. (2015). Effector-triggered immunity: from pathogen perception to robust defense. Annu. Rev. Plant Biol. 66, 487–511. doi: 10.1146/annurev-arplant-050213-040012
Dangl, J. L., and McDowell, J. M. (2006). Two modes of pathogen recognition by plants. Proc. Natl. Acad. Sci. U.S.A. 103, 8575–8576. doi: 10.1073/pnas.0603183103
Daub, M. E., and Ehrenshaft, M. (2000). The photoactivated Cercospora toxin cercosporin: contributions to plant disease and fundamental biology. Annu. Rev. Phytopathol. 38, 461–490. doi: 10.1146/annurev.phyto.38.1.461
De Dios Alché, J. (2019). A concise appraisal of lipid oxidation and lipoxidation in higher plants. Redox Biol. 23:101136. doi: 10.1016/j.redox.2019.101136
De Moraes, C. M., Mescher, M. C., and Tumlinson, J. H. (2001). Caterpillar-induced nocturnal plant volatiles repel conspecific females. Nature 410, 577–580. doi: 10.1038/35069058
De Vallavieille-Pope, C., Huber, L., Leconte, M., and Bethenod, O. (2002). Preinoculation effects of light quantity on infection efficiency of Puccinia striiformis and P. triticina on wheat seedlings. Phytopathology 92, 1308–1314. doi: 10.1094/PHYTO.2002.92.12.1308
De Vleesschauwer, D., Xu, J., and Höfte, M. (2014). Making sense of hormone-mediated defense networking: from rice to Arabidopsis. Front. Plant Sci. 5:611. doi: 10.3389/fpls.2014.00611
de Wit, M., George, G. M., Ince, Y. Ç, Dankwa-Egli, B., Hersch, M., Zeeman, S. C., et al. (2018). Changes in resource partitioning between and within organs support growth adjustment to neighbor proximity in Brassicaceae seedlings. Proc. Natl. Acad. Sci. U.S.A. 115, E9953–E9961. doi: 10.1073/pnas.1806084115
de Wit, M., Spoel, S. H., Sanchez-Perez, G. F., Gommers, C. M., Pieterse, C. M., Voesenek, L. A., et al. (2013). Perception of low red: far-red ratio compromises both salicylic acid-and jasmonic acid-dependent pathogen defences in Arabidopsis. Plant J. 75, 90–103. doi: 10.1111/tpj.12203
Deepika, A., Sagar, S., and Singh, A. (2020). Dark-induced hormonal regulation of plant growth and development. Front. Plant Sci. 11:581666. doi: 10.3389/fpls.2020.581666
DeFalco, T. A., Marshall, C. B., Munro, K., Kang, H.-G., Moeder, W., Ikura, M., et al. (2016). Multiple calmodulin-binding sites positively and negatively regulate Arabidopsis CYCLIC NUCLEOTIDE-GATED CHANNEL12. Plant Cell 28, 1738–1751. doi: 10.1105/tpc.15.00870
Delprato, M. L., Krapp, A. R., and Carrillo, N. (2015). Green light to plant responses to pathogens: the role of chloroplast light-dependent signaling in biotic stress. Photochem. Photobiol. 91, 1004–1011. doi: 10.1111/php.12466
Demidchik, V., Shabala, S., Isayenkov, S., Cuin, T. A., and Pottosin, I. (2018). Calcium transport across plant membranes: mechanisms and functions. New Phytol. 220, 49–69. doi: 10.1111/nph.15266
Deng, X.-W., Matsui, M., Wei, N., Wagner, D., Chu, A. M., Feldmann, K. A., et al. (1992). COP1, an Arabidopsis regulatory gene, encodes a protein with both a zinc-binding motif and a Gβ homologous domain. Cell 71, 791–801. doi: 10.1016/0092-8674(92)90555-Q
Dodd, A. N., Kudla, J., and Sanders, D. (2010). The language of calcium signaling. Annu. Rev. Plant Biol. 61, 593–620. doi: 10.1146/annurev-arplant-070109-104628
Dodds, P. N., and Rathjen, J. P. (2010). Plant immunity: towards an integrated view of plant-pathogen interactions. Nat. Rev. Genet. 11, 539–548. doi: 10.1038/nrg2812
Dong, J., Tang, D., Gao, Z., Yu, R., Li, K., He, H., et al. (2014). Arabidopsis DE-ETIOLATED1 represses photomorphogenesis by positively regulating phytochrome-interacting factors in the dark. Plant Cell 26, 3630–3645. doi: 10.1105/tpc.114.130666
Doughari, J. (2015). An overview of plant immunity. J. Plant Pathol. Microbiol. 6:104172. doi: 10.4172/2157-7471.1000322
Downum, K. R. (1992). Tansley review no. 43. Light-activated plant defence. New Phytol. 122, 401–420. doi: 10.1111/j.1469-8137.1992.tb00068.x
Dudareva, N., Negre, F., Nagegowda, D. A., and Orlova, I. (2006). Plant volatiles: recent advances and future perspectives. Crit. Rev. Plant Sci. 25, 417–440. doi: 10.1080/07352680600899973
Duek, P. D., Elmer, M. V., Van Oosten, V. R., and Fankhauser, C. (2004). The degradation of HFR1, a putative bHLH class transcription factor involved in light signaling, is regulated by phosphorylation and requires COP1. Curr. Biol. 14, 2296–2301. doi: 10.1016/j.cub.2004.12.026
Dworak, A., Nykiel, M., Walczak, B., Miazek, A., Szworst-Łupina, D., Zagdańska, B., et al. (2016). Maize proteomic responses to separate or overlapping soil drought and two-spotted spider mite stresses. Planta 244, 939–960. doi: 10.1007/s00425-016-2559-6
Ferguson, B. J., Indrasumunar, A., Hayashi, S., Lin, M. H., Lin, Y. H., Reid, D. E., et al. (2010). Molecular analysis of legume nodule development and autoregulation. J. Integr. Plant Biol. 52, 61–76. doi: 10.1111/j.1744-7909.2010.00899.x
Fiorucci, A.-S., and Fankhauser, C. (2017). Plant strategies for enhancing access to sunlight. Curr. Biol. 27, R931–R940. doi: 10.1016/j.cub.2017.05.085
Fischer, C., Defalco, T. A., Karia, P., Snedden, W. A., Moeder, W., Yoshioka, K., et al. (2017). Calmodulin as a Ca2+-sensing subunit of Arabidopsis cyclic nucleotide-gated channel complexes. Plant Cell Physiol. 58, 1208–1221. doi: 10.1093/pcp/pcx052
Flors, C., and Nonell, S. (2006). Light and singlet oxygen in plant defense against pathogens: phototoxic phenalenone phytoalexins. Acc. Chem. Res. 39, 293–300. doi: 10.1021/ar0402863
Folta, K. M., and Maruhnich, S. A. (2007). Green light: a signal to slow down or stop. J. Exper. Bot. 58, 3099–3111. doi: 10.1093/jxb/erm130
Foyer, C. H. (2018). Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ. Exper. Bot. 154, 134–142. doi: 10.1016/j.envexpbot.2018.05.003
Franklin, K. A. (2008). Shade avoidance. New Phytol. 179, 930–944. doi: 10.1111/j.1469-8137.2008.02507.x
Franklin, K. A., Larner, V. S., and Whitelam, G. C. (2004). The signal transducing photoreceptors of plants. Intern. J. Dev. Biol. 49, 653–664. doi: 10.1387/ijdb.051989kf
Franklin, K. A., and Quail, P. H. (2010). Phytochrome functions in Arabidopsis development. J. Exper. Bot. 61, 11–24. doi: 10.1093/jxb/erp304
Fu, Z. Q., and Dong, X. (2013). Systemic acquired resistance: turning local infection into global defense. Annu. Rev. Plant Biol. 64, 839–863. doi: 10.1146/annurev-arplant-042811-105606
Fürstenberg-Hägg, J., Zagrobelny, M., and Bak, S. (2013). Plant defense against insect herbivores. Intern. J. Mol. Sci. 14, 10242–10297. doi: 10.3390/ijms140510242
Gadoury, D. M., Stensvand, A., and Seem, R. C. (1998). Influence of light, relative humidity, and maturity of populations on discharge of ascospores of Venturia inaequalis. Phytopathology 88, 902–909. doi: 10.1094/PHYTO.1998.88.9.902
Gangappa, S. N., Berriri, S., and Kumar, S. V. (2017). PIF4 coordinates thermosensory growth and immunity in Arabidopsis. Curr. Biol. 27, 243–249. doi: 10.1016/j.cub.2016.11.012
Gangappa, S. N., and Kumar, S. V. (2017). DET1 and HY5 control PIF4-mediated thermosensory elongation growth through distinct mechanisms. Cell Rep. 18, 344–351. doi: 10.1016/j.celrep.2016.12.046
Gao, C., Xu, H., Huang, J., Sun, B., Zhang, F., Savage, Z., et al. (2020). Pathogen manipulation of chloroplast function triggers a light-dependent immune recognition. Proc. Natl. Acad. Sci. U.S.A. 117, 9613–9620. doi: 10.1073/pnas.2002759117
Gao, Q.-F., Gu, L.-L., Wang, H.-Q., Fei, C.-F., Fang, X., Hussain, J., et al. (2016). Cyclic nucleotide-gated channel 18 is an essential Ca2+ channel in pollen tube tips for pollen tube guidance to ovules in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 113, 3096–3101. doi: 10.1073/pnas.1524629113
Garcia-Guzman, G., and Heil, M. (2014). Life histories of hosts and pathogens predict patterns in tropical fungal plant diseases. New Phytol. 201, 1106–1120. doi: 10.1111/nph.12562
Genoud, T., Buchala, A. J., Chua, N.-H., and Métraux, J.-P. (2002). Phytochrome signalling modulates the SA-perceptive pathway in Arabidopsis. Plant J. 31, 87–95. doi: 10.1046/j.1365-313x.2002.01338.x
Genoud, T., Millar, A. J., Nishizawa, N., Kay, S. A., Schäfer, E., Nagatani, A., et al. (1998). An Arabidopsis mutant hypersensitive to red and far-red light signals. Plant Cell 10, 889–904. doi: 10.1105/tpc.10.6.889
Gilbert, G. S., and Reynolds, D. R. (2005). Nocturnal fungi: Airborne spores in the canopy and understory of a tropical rain forest 1. Biotropica J. Biol. Conserv. 37, 462–464. doi: 10.1111/j.1744-7429.2005.00061.x
Glazebrook, J. (2005). Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 43, 205–227. doi: 10.1146/annurev.phyto.43.040204.135923
Goldsmith, C. S., and Bell-Pedersen, D. (2013). Diverse roles for MAPK signaling in circadian clocks. Adv. Genet. 84, 1–39. doi: 10.1016/B978-0-12-407703-4.00001-3
Gommers, C. M., Keuskamp, D. H., Buti, S., Van Veen, H., Koevoets, I. T., Reinen, E., et al. (2017). Molecular profiles of contrasting shade response strategies in wild plants: differential control of immunity and shoot elongation. Plant Cell 29, 331–344. doi: 10.1105/tpc.16.00790
González de Molina, M., Soto Fernández, D., Infante-Amate, J., Aguilera, E., Vila Traver, J., and Guzmán, G. I. (2017). Decoupling food from land: the evolution of Spanish agriculture from 1960 to 2010. Sustainability 9:2348. doi: 10.3390/su9122348
Gouinguené, S. P., and Turlings, T. C. (2002). The effects of abiotic factors on induced volatile emissions in corn plants. Plant Physiol. 129, 1296–1307. doi: 10.1104/pp.001941
Gouveia, B. C., Calil, I. P., Machado, J. P. B., Santos, A. A., and Fontes, E. P. (2017). Immune receptors and co-receptors in antiviral innate immunity in plants. Front. Microbiol. 7:2139. doi: 10.3389/fmicb.2016.02139
Grant, M., Brown, I., Adams, S., Knight, M., Ainslie, A., and Mansfield, J. (2000). The RPM1 plant disease resistance gene facilitates a rapid and sustained increase in cytosolic calcium that is necessary for the oxidative burst and hypersensitive cell death. Plant J. 23, 441–450. doi: 10.1046/j.1365-313x.2000.00804.x
Grant, M., and Lamb, C. (2006). Systemic immunity. Curr. Opin. Plant Biol. 9, 414–420. doi: 10.1016/j.pbi.2006.05.013
Grant, R. H., Apostol, K., and Gao, W. (2005). Biologically effective UV-B exposures of an oak-hickory forest understory during leaf-out. Agric. For. Meteorol. 132, 28–43. doi: 10.1016/j.agrformet.2005.06.008
Grant, R. H., and Heisler, G. M. (2001). Multi-waveband solar irradiance on tree-shaded vertical and horizontal surfaces: cloud-free and partly cloudy skies. Photochem. Photobiol. 73, 24–31. doi: 10.1562/0031-8655(2001)073<0024:MWSIOT>2.0.CO;2
Griebel, T., and Zeier, J. (2008). Light regulation and daytime dependency of inducible plant defenses in Arabidopsis: phytochrome signaling controls systemic acquired resistance rather than local defense. Plant Physiol. 147, 790–801. doi: 10.1104/pp.108.119503
Hamamouch, N., Li, C., Seo, P. J., Park, C. M., and Davis, E. L. (2011). Expression of Arabidopsis pathogenesis-related genes during nematode infection. Mol. Plant Pathol. 12, 355–364. doi: 10.1111/j.1364-3703.2010.00675.x
Hammond-Kosack, K., and Jones, J. D. G. (2000). Responses to plant pathogens. Biochem. Mol. Biol. Plants 1, 1102–1156.
Hardtke, C. S., and Deng, X.-W. (2000). The cell biology of the COP/DET/FUS proteins. Regulating proteolysis in photomorphogenesis and beyond? Plant Physiol. 124, 1548–1557. doi: 10.1104/pp.124.4.1548
Hardtke, C. S., Gohda, K., Osterlund, M. T., Oyama, T., Okada, K., and Deng, X. W. (2000). HY5 stability and activity in Arabidopsis is regulated by phosphorylation in its COP1 binding domain. EMBO J. 19, 4997–5006. doi: 10.1093/emboj/19.18.4997
Harmer, S. L., Hogenesch, J. B., Straume, M., Chang, H.-S., Han, B., Zhu, T., et al. (2000). Orchestrated transcription of key pathways in Arabidopsis by the circadian clock. Science 290, 2110–2113. doi: 10.1126/science.290.5499.2110
Hassell, M., and Southwood, T. (1978). Foraging strategies of insects. Annu. Rev. Ecol. Syst. 9, 75–98. doi: 10.1146/annurev.es.09.110178.000451
Hattori, T., Otomi, Y., Nakajima, Y., Soga, K., Wakabayashi, K., Iida, H., et al. (2020). MCA1 and MCA2 are involved in the response to hypergravity in Arabidopsis hypocotyls. Plants 9:590. doi: 10.3390/plants9050590
Heisler, G. M., Grant, R. H., and Gao, W. (2003). Individual-and scattered-tree influences on ultraviolet irradiance. Agric. For. Meteorol. 120, 113–126. doi: 10.1016/j.agrformet.2003.08.024
Hématy, K., Sado, P.-E., Van Tuinen, A., Rochange, S., Desnos, T., Balzergue, S., et al. (2007). A receptor-like kinase mediates the response of Arabidopsis cells to the inhibition of cellulose synthesis. Curr. Biol. 17, 922–931. doi: 10.1016/j.cub.2007.05.018
Hoecker, U. (2017). The activities of the E3 ubiquitin ligase COP1/SPA, a key repressor in light signaling. Curr. Opin. Plant Biol. 37, 63–69. doi: 10.1016/j.pbi.2017.03.015
Holtkotte, X., Dieterle, S., Kokkelink, L., Artz, O., Leson, L., Fittinghoff, K., et al. (2016). Mutations in the N-terminal kinase-like domain of the repressor of photomorphogenesis SPA 1 severely impair SPA 1 function but not light responsiveness in Arabidopsis. Plant J. 88, 205–218. doi: 10.1111/tpj.13241
Hou, X., Lee, L. Y. C., Xia, K., Yan, Y., and Yu, H. (2010). DELLAs modulate jasmonate signaling via competitive binding to JAZs. Dev. Cell 19, 884–894. doi: 10.1016/j.devcel.2010.10.024
Hua, J. (2013). Modulation of plant immunity by light, circadian rhythm, and temperature. Curr. Opin. Plant Biol. 16, 406–413. doi: 10.1016/j.pbi.2013.06.017
Huang, H., Ullah, F., Zhou, D.-X., Yi, M., and Zhao, Y. (2019). Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 10:800. doi: 10.3389/fpls.2019.00800
Huner, N. P., Oquist, G., and Sarhan, F. (1998). Energy balance and acclimation to light and cold. Trends Plant Sci. 3, 224–230. doi: 10.1016/S1360-1385(98)01248-5
Iqbal, Z., Iqbal, M. S., Ahmad, A., Memon, A. G., and Ansari, M. I. (2020a). New prospects on the horizon: genome editing to engineer plants for desirable traits. Curr. Plant Biol. 24, 100171. doi: 10.1016/j.cpb.2020.100171
Iqbal, Z., Iqbal, M. S., Singh, S. P., and Buaboocha, T. (2020b). Ca2+/calmodulin complex triggers CAMTA transcriptional machinery under stress in plants: signaling cascade and molecular regulation. Front. Plant Sci. 11:598327. doi: 10.3389/fpls.2020.598327
Ishikawa, A., Okamoto, H., Iwasaki, Y., and Asahi, T. (2001). A deficiency of coproporphyrinogen III oxidase causes lesion formation in Arabidopsis. Plant J. 27, 89–99. doi: 10.1046/j.1365-313x.2001.01058.x
Islam, W., Naveed, H., Zaynab, M., Huang, Z., and Chen, H. Y. (2019). Plant defense against virus diseases; growth hormones in highlights. Plant Signal. Behav. 14:1596719. doi: 10.1080/15592324.2019.1596719
Izaguirre, M. M., Mazza, C. A., Biondini, M., Baldwin, I. T., and Ballaré, C. L. (2006). Remote sensing of future competitors: impacts on plant defenses. Proc. Natl. Acad. Sci. U.S.A. 103, 7170–7174. doi: 10.1073/pnas.0509805103
Izaguirre, M. M., Scopel, A. L., Baldwin, I. T., and Ballaré, C. L. (2003). Convergent responses to stress. Solar ultraviolet-B radiation and Manduca sexta herbivory elicit overlapping transcriptional responses in field-grown plants of Nicotiana longiflora. Plant Physiol. 132, 1755–1767. doi: 10.1104/pp.103.024323
Jacob, F., Kracher, B., Mine, A., Seyfferth, C., Blanvillain-Baufumé, S., Parker, J. E., et al. (2018). A dominant-interfering camta3 mutation compromises primary transcriptional outputs mediated by both cell surface and intracellular immune receptors in Arabidopsis thaliana. New Phytol. 217, 1667–1680. doi: 10.1111/nph.14943
Jaillais, Y., and Chory, J. (2010). Unraveling the paradoxes of plant hormone signaling integration. Nat. Struct. Mol. Biol. 17, 642–645. doi: 10.1038/nsmb0610-642
Jain, D., and Khurana, J. P. (2018). “Role of pathogenesis-related (PR) proteins in plant defense mechanism,” in Molecular Aspects of Plant-Pathogen Interaction, eds A. Singh and I. Singh (Singapore: Springer), 265–281. doi: 10.1007/978-981-10-7371-7_12
James, Z. M., and Zagotta, W. N. (2018). Structural insights into the mechanisms of CNBD channel function. J. Gen. Physiol. 150, 225–244. doi: 10.1085/jgp.201711898
Jang, I.-C., Yang, J.-Y., Seo, H. S., and Chua, N.-H. (2005). HFR1 is targeted by COP1 E3 ligase for post-translational proteolysis during phytochrome A signaling. Genes Dev. 19, 593–602. doi: 10.1101/gad.1247205
Jenkins, G. I. (2009). Signal transduction in responses to UV-B radiation. Annu. Rev. Plant Biol. 60, 407–431. doi: 10.1146/annurev.arplant.59.032607.092953
Jeong, R.-D., Chandra-Shekara, A., Barman, S. R., Navarre, D., Klessig, D. F., Kachroo, A., et al. (2010). Cryptochrome 2 and phototropin 2 regulate resistance protein-mediated viral defense by negatively regulating an E3 ubiquitin ligase. Proc. Natl. Acad. Sci. U.S.A. 107, 13538–13543. doi: 10.1073/pnas.1004529107
Jiang, X., Hoehenwarter, W., Scheel, D., and Lee, J. (2020). Phosphorylation of the CAMTA3 transcription factor triggers its destabilization and nuclear export. Plant Physiol. 184, 1056–1071. doi: 10.1104/pp.20.00795
Jiao, Y., Lau, O. S., and Deng, X. W. (2007). Light-regulated transcriptional networks in higher plants. Nat. Rev. Genet. 8:217. doi: 10.1038/nrg2049
Jogawat, A., Meena, M. K., Kundu, A., Varma, M., and Vadassery, J. (2020). Calcium channel CNGC19 mediates basal defense signaling to regulate colonization by Piriformospora indica in Arabidopsis roots. J. Exper. Bot. 71, 2752–2768. doi: 10.1093/jxb/eraa028
Jones, J. D., and Dangl, J. L. (2006). The plant immune system. Nature 444, 323–329. doi: 10.1038/nature05286
Josse, E.-M., and Halliday, K. J. (2008). Skotomorphogenesis: the dark side of light signalling. Curr. Biol. 18, R1144–R1146. doi: 10.1016/j.cub.2008.10.034
Kaiserli, E., Paldi, K., O’donnell, L., Batalov, O., Pedmale, U. V., Nusinow, D. A., et al. (2015). Integration of light and photoperiodic signaling in transcriptional nuclear foci. Dev. Cell 35, 311–321. doi: 10.1016/j.devcel.2015.10.008
Kaloshian, I. (2004). Gene-for-gene disease resistance: bridging insect pest and pathogen defense. J. Chem. Ecol. 30, 2419–2438. doi: 10.1007/s10886-004-7943-1
Kami, C., Lorrain, S., Hornitschek, P., and Fankhauser, C. (2010). Light-regulated plant growth and development. Curr. Top. Dev. Biol. 91, 29–66. doi: 10.1016/S0070-2153(10)91002-8
Kangasjärvi, S., Neukermans, J., Li, S., Aro, E.-M., and Noctor, G. (2012). Photosynthesis, photorespiration, and light signalling in defence responses. J. Exper. Bot. 63, 1619–1636. doi: 10.1093/jxb/err402
Kariola, T., Brader, G., Li, J., and Palva, E. T. (2005). Chlorophyllase 1, a damage control enzyme, affects the balance between defense pathways in plants. Plant Cell 17, 282–294. doi: 10.1105/tpc.104.025817
Karpinski, S., Gabrys, H., Mateo, A., Karpinska, B., and Mullineaux, P. M. (2003). Light perception in plant disease defence signalling. Curr. Opin. Plant Biol. 6, 390–396. doi: 10.1016/S1369-5266(03)00061-X
Kazan, K., and Manners, J. M. (2011). The interplay between light and jasmonate signalling during defence and development. J. Exper. Bot. 62, 4087–4100. doi: 10.1093/jxb/err142
Kegge, W., Ninkovic, V., Glinwood, R., Welschen, R. A., Voesenek, L. A., and Pierik, R. (2015). Red: far-red light conditions affect the emission of volatile organic compounds from barley (Hordeum vulgare), leading to altered biomass allocation in neighbouring plants. Ann. Bot. 115, 961–970. doi: 10.1093/aob/mcv036
Kegge, W., Weldegergis, B. T., Soler, R., Eijk, M. V. V., Dicke, M., Voesenek, L. A., et al. (2013). Canopy light cues affect emission of constitutive and methyl jasmonate-induced volatile organic compounds in Arabidopsis thaliana. New Phytol. 200, 861–874. doi: 10.1111/nph.12407
Kelly, D. L. (2002). The regeneration of Quercus petraea (sessile oak) in southwest Ireland: a 25-year experimental study. For. Ecol. Manag. 166, 207–226. doi: 10.1016/S0378-1127(01)00670-3
Kessler, S. A., Shimosato-Asano, H., Keinath, N. F., Wuest, S. E., Ingram, G., Panstruga, R., et al. (2010). Conserved molecular components for pollen tube reception and fungal invasion. Science 330, 968–971. doi: 10.1126/science.1195211
Keymer, A., Pimprikar, P., Wewer, V., Huber, C., Brands, M., Bucerius, S. L., et al. (2017). Lipid transfer from plants to arbuscular mycorrhiza fungi. eLife 6:e29107. doi: 10.7554/eLife.29107.051
Kiep, V., Vadassery, J., Lattke, J., Maaß, J. P., Boland, W., Peiter, E., et al. (2015). Systemic cytosolic Ca2+ elevation is activated upon wounding and herbivory in Arabidopsis. New Phytol. 207, 996–1004. doi: 10.1111/nph.13493
Kim, Y., Gilmour, S. J., Chao, L., Park, S., and Thomashow, M. F. (2020). Arabidopsis CAMTA transcription factors regulate pipecolic acid biosynthesis and priming of immunity genes. Mol. Plant 13, 157–168. doi: 10.1016/j.molp.2019.11.001
Kimura, M., Yamamoto, Y. Y., Seki, M., Sakurai, T., Sato, M., Abe, T., et al. (2003). Identification of Arabidopsis genes regulated by high light-stress using cDNA microarray. Photochem. Photobiol. 77, 226–233. doi: 10.1562/0031-8655(2003)077<0226:IOAGRB>2.0.CO;2
Koh, K., Bell, G., Martin, D., and Walker, N. (2003). Shade and airflow restriction effects on creeping bentgrass golf greens. Crop Sci. 43, 2182–2188. doi: 10.2135/cropsci2003.2182
Koussevitzky, S., Nott, A., Mockler, T. C., Hong, F., Sachetto-Martins, G., Surpin, M., et al. (2007). Signals from chloroplasts converge to regulate nuclear gene expression. Science 316, 715–719. doi: 10.1126/science.1140516
Kreuger, B., and Potter, D. A. (2001). Diel feeding activity and thermoregulation by Japanese beetles (Coleoptera: Scarabaeidae) within host plant canopies. Environ. Entomol. 30, 172–180. doi: 10.1603/0046-225X-30.2.172
Ku, Y.-S., Sintaha, M., Cheung, M.-Y., and Lam, H.-M. (2018). Plant hormone signaling crosstalks between biotic and abiotic stress responses. Intern. J. Mol. Sci. 19:3206. doi: 10.3390/ijms19103206
Kudla, J., Becker, D., Grill, E., Hedrich, R., Hippler, M., Kummer, U., et al. (2018). Advances and current challenges in calcium signaling. New Phytol. 218, 414–431. doi: 10.1111/nph.14966
Lacombe, B., Becker, D., Hedrich, R., Desalle, R., Hollmann, M., Kwak, J. M., et al. (2001). The identity of plant glutamate receptors. Science 292, 1486–1486. doi: 10.1126/science.292.5521.1486b
Laluk, K., and Mengiste, T. (2010). Necrotroph attacks on plants: wanton destruction or covert extortion? Am. Soc. Plant Biol. 8:e0136. doi: 10.1199/tab.0136
Lambert, K., and Bekal, S. (2002). Introduction to plant-parasitic nematodes. Plant Health Instruct. 10, 1094–1218. doi: 10.1094/PHI-I-2002-1218-01
Lamers, J., Van Der Meer, T., and Testerink, C. (2020). How plants sense and respond to stressful environments. Plant Physiol. 182, 1624–1635. doi: 10.1104/pp.19.01464
Lau, O. S., and Deng, X. W. (2010). Plant hormone signaling lightens up: integrators of light and hormones. Curr. Opin. Plant Biol. 13, 571–577. doi: 10.1016/j.pbi.2010.07.001
Lau, O. S., and Deng, X. W. (2012). The photomorphogenic repressors COP1 and DET1: 20 years later. Trends Plant Sci. 17, 584–593. doi: 10.1016/j.tplants.2012.05.004
Leba, L. J., Cheval, C., Ortiz-Martín, I., Ranty, B., Beuzón, C. R., Galaud, J. P., et al. (2012). CML9, an Arabidopsis calmodulin-like protein, contributes to plant innate immunity through a flagellin-dependent signalling pathway. Plant J. 71, 976–989. doi: 10.1111/j.1365-313X.2012.05045.x
Lecourieux, D., Lamotte, O., Bourque, S., Wendehenne, D., Mazars, C., Ranjeva, R., et al. (2005). Proteinaceous and oligosaccharidic elicitors induce different calcium signatures in the nucleus of tobacco cells. Cell Calc. 38, 527–538. doi: 10.1016/j.ceca.2005.06.036
Lee, S. Y., Chi, Y. H., Koo, S. S., Oh, H. T., Lee, E. S., Park, J. H., et al. (2019). The physiological functions of universal stress proteins and their molecular mechanism to protect plants from environmental stresses. Front. Plant Sci. 10:750. doi: 10.3389/fpls.2019.00750
Leivar, P., Monte, E., Oka, Y., Liu, T., Carle, C., Castillon, A., et al. (2008). Multiple phytochrome-interacting bHLH transcription factors repress premature seedling photomorphogenesis in darkness. Curr. Biol. 18, 1815–1823. doi: 10.1016/j.cub.2008.10.058
Leivar, P., and Quail, P. H. (2011). PIFs: pivotal components in a cellular signaling hub. Trends Plant Sci. 16, 19–28. doi: 10.1016/j.tplants.2010.08.003
Leone, M., Keller, M. M., Cerrudo, I., and Ballaré, C. L. (2014). To grow or defend? Low red: far-red ratios reduce jasmonate sensitivity in Arabidopsis seedlings by promoting DELLA degradation and increasing JAZ10 stability. New Phytol. 204, 355–367. doi: 10.1111/nph.12971
Li, J., Han, G., Sun, C., and Sui, N. (2019). Research advances of MYB transcription factors in plant stress resistance and breeding. Plant Signal. Behav. 14:1613131. doi: 10.1080/15592324.2019.1613131
Li, J., Li, G., Wang, H., and Deng, X. W. (2011). Phytochrome signaling mechanisms. Arabidopsis Book Am. Soc. Plant Biol. 9:e0148. doi: 10.1199/tab.0148
Li, X.-P., Gilmore, A. M., Caffarri, S., Bassi, R., Golan, T., Kramer, D., et al. (2004). Regulation of photosynthetic light harvesting involves intrathylakoid lumen pH sensing by the PsbS protein. J. Biol. Chem. 279, 22866–22874. doi: 10.1074/jbc.M402461200
Liu, X., Wang, J., and Sun, L. (2018). Structure of the hyperosmolality-gated calcium-permeable channel OSCA1. 2. Nat. Commun. 9, 1–9. doi: 10.1038/s41467-018-07564-5
Liu, Y., Du, M., Deng, L., Shen, J., Fang, M., Chen, Q., et al. (2019). MYC2 regulates the termination of jasmonate signaling via an autoregulatory negative feedback loop. Plant Cell 31, 106–127. doi: 10.1105/tpc.18.00405
Lorrain, S., Allen, T., Duek, P. D., Whitelam, G. C., and Fankhauser, C. (2008). Phytochrome-mediated inhibition of shade avoidance involves degradation of growth-promoting bHLH transcription factors. Plant J. 53, 312–323. doi: 10.1111/j.1365-313X.2007.03341.x
Lorrain, S., Vailleau, F., Balagué, C., and Roby, D. (2003). Lesion mimic mutants: keys for deciphering cell death and defense pathways in plants? Trends Plant Sci. 8, 263–271. doi: 10.1016/S1360-1385(03)00108-0
Lozano-Durán, R., Macho, A. P., Boutrot, F., Segonzac, C., Somssich, I. E., and Zipfel, C. (2013). The transcriptional regulator BZR1 mediates trade-off between plant innate immunity and growth. eLife 2:e00983. doi: 10.7554/eLife.00983.023
Lozano-Durán, R., and Zipfel, C. (2015). Trade-off between growth and immunity: role of brassinosteroids. Trends Plant Sci. 20, 12–19. doi: 10.1016/j.tplants.2014.09.003
Lu, Y., and Yao, J. (2018). Chloroplasts at the crossroad of photosynthesis, pathogen infection and plant defense. Intern. J. Mol. Sci. 19:3900. doi: 10.3390/ijms19123900
Luan, S. (2009). The CBL-CIPK network in plant calcium signaling. Trends Plant Sci. 14, 37–42. doi: 10.1016/j.tplants.2008.10.005
Lv, R., Li, Z., Li, M., Dogra, V., Lv, S., Liu, R., et al. (2019). Uncoupled expression of nuclear and plastid photosynthesis-associated genes contributes to cell death in a lesion mimic mutant. Plant Cell 31, 210–230. doi: 10.1105/tpc.18.00813
Ma, L., Gao, Y., Qu, L., Chen, Z., Li, J., Zhao, H., et al. (2002). Genomic evidence for COP1 as a repressor of light-regulated gene expression and development in Arabidopsis. Plant Cell 14, 2383–2398. doi: 10.1105/tpc.004416
Ma, X., Li, Q.-H., Yu, Y.-N., Qiao, Y.-M., and Gong, Z.-H. (2020). The CBL-CIPK pathway in plant response to stress signals. Intern. J. Mol. Sci. 21:5668. doi: 10.3390/ijms21165668
Ma, Y., Walker, R. K., Zhao, Y., and Berkowitz, G. A. (2012). Linking ligand perception by PEPR pattern recognition receptors to cytosolic Ca2+ elevation and downstream immune signaling in plants. Proc. Natl. Acad. Sci. U.S.A. 109, 19852–19857. doi: 10.1073/pnas.1205448109
Mach, J. M., Castillo, A. R., Hoogstraten, R., and Greenberg, J. T. (2001). The Arabidopsis-accelerated cell death gene ACD2 encodes red chlorophyll catabolite reductase and suppresses the spread of disease symptoms. Proc. Natl. Acad. Sci. U.S.A. 98, 771–776. doi: 10.1073/pnas.98.2.771
Machado, R. A., Robert, C. A., Arce, C. C., Ferrieri, A. P., Xu, S., Jimenez-Aleman, G. H., et al. (2016). Auxin is rapidly induced by herbivore attack and regulates a subset of systemic, jasmonate-dependent defenses. Plant Physiol. 172, 521–532. doi: 10.1104/pp.16.00940
Mackerness, A.-H. S., Surplus, S., Blake, P., John, C., Buchanan-Wollaston, V., Jordan, B., et al. (1999). Ultraviolet-B-induced stress and changes in gene expression in Arabidopsis thaliana: role of signalling pathways controlled by jasmonic acid, ethylene and reactive oxygen species. Plant Cell Environ. 22, 1413–1423. doi: 10.1046/j.1365-3040.1999.00499.x
Magnan, F., Ranty, B., Charpenteau, M., Sotta, B., Galaud, J. P., and Aldon, D. (2008). Mutations in AtCML9, a calmodulin-like protein from Arabidopsis thaliana, alter plant responses to abiotic stress and abscisic acid. Plant J. 56, 575–589. doi: 10.1111/j.1365-313X.2008.03622.x
Manzoor, H., Kelloniemi, J., Chiltz, A., Wendehenne, D., Pugin, A., Poinssot, B., et al. (2013). Involvement of the glutamate receptor A t GLR 3.3 in plant defense signaling and resistance to H yaloperonospora arabidopsidis. Plant J. 76, 466–480. doi: 10.1111/tpj.12311
Martin, D. M., Gershenzon, J., and Bohlmann, J. (2003). Induction of volatile terpene biosynthesis and diurnal emission by methyl jasmonate in foliage of Norway spruce. Plant Physiol. 132, 1586–1599. doi: 10.1104/pp.103.021196
Martínez, C., Espinosa-Ruíz, A., De Lucas, M., Bernardo-García, S., Franco-Zorrilla, J. M., and Prat, S. (2018a). PIF 4-induced BR synthesis is critical to diurnal and thermomorphogenic growth. EMBO J. 37:e99552. doi: 10.15252/embj.201899552
Martínez, C., Nieto, C., and Prat, S. (2018b). Convergent regulation of PIFs and the E3 ligase COP1/SPA1 mediates thermosensory hypocotyl elongation by plant phytochromes. Curr. Opin. Plant Biol. 45, 188–203. doi: 10.1016/j.pbi.2018.09.006
Martinez-Garcia, J. F., Galstyan, A., Salla-Martret, M., Cifuentes-Esquivel, N., Gallemi, M., and Bou-Torrent, J. (2010). Regulatory components of shade avoidance syndrome. Adv. Bot. Res. 53, 65–116. doi: 10.1016/S0065-2296(10)53003-9
Mawphlang, O. I., and Kharshiing, E. V. (2017). Photoreceptor mediated plant growth responses: implications for photoreceptor engineering toward improved performance in crops. Front. Plant Sci. 8:1181. doi: 10.3389/fpls.2017.01181
McGuire, R., and Agrawal, A. (2005). Trade-offs between the shade-avoidance response and plant resistance to herbivores? Tests with mutant Cucumis sativus. Funct. Ecol. 19, 1025–1031. doi: 10.1111/j.1365-2435.2005.01047.x
Meena, M. K., Prajapati, R., Krishna, D., Divakaran, K., Pandey, Y., Reichelt, M., et al. (2019). The Ca2+ channel CNGC19 regulates Arabidopsis defense against Spodoptera herbivory. Plant Cell 31, 1539–1562. doi: 10.1105/tpc.19.00057
Meena, M. K., and Vadassery, J. (2015). Channels hold the key: cyclic nucleotide gated channels (CNGC) in plant biotic stress signaling. J. Endocytob. Cell Res. 581, 25–30.
Meijer, G., and Leuchtmann, A. (2000). The effects of genetic and environmental factors on disease expression (stroma formation) and plant growth in Brachypodium sylvaticum infected by Epichloë sylvatica. Oikos 91, 446–458. doi: 10.1034/j.1600-0706.2000.910305.x
Menon, C., Sheerin, D. J., and Hiltbrunner, A. (2016). SPA proteins: SPAnning the gap between visible light and gene expression. Planta 244, 297–312. doi: 10.1007/s00425-016-2509-3
Mishra, Y., Jänkänpää, H. J., Kiss, A. Z., Funk, C., Schröder, W. P., and Jansson, S. (2012). Arabidopsis plants grown in the field and climate chambers significantly differ in leaf morphology and photosystem components. BMC Plant Biol. 12:6. doi: 10.1186/1471-2229-12-6
Mittler, R. (2002). Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 7, 405–410. doi: 10.1016/S1360-1385(02)02312-9
Moeder, W., Urquhart, W., Ung, H., and Yoshioka, K. (2011). The role of cyclic nucleotide-gated ion channels in plant immunity. Mol. Plant 4, 442–452. doi: 10.1093/mp/ssr018
Molina, A., Volrath, S., Guyer, D., Maleck, K., Ryals, J., and Ward, E. (1999). Inhibition of protoporphyrinogen oxidase expression in Arabidopsis causes a lesion-mimic phenotype that induces systemic acquired resistance. Plant J. 17, 667–678. doi: 10.1046/j.1365-313X.1999.00420.x
Monaghan, J., and Zipfel, C. (2012). Plant pattern recognition receptor complexes at the plasma membrane. Curr. Opin. Plant Biol. 15, 349–357. doi: 10.1016/j.pbi.2012.05.006
Montillet, J.-L., Chamnongpol, S., Rustérucci, C., Dat, J., Van De Cotte, B., Agnel, J.-P., et al. (2005). Fatty acid hydroperoxides and H2O2 in the execution of hypersensitive cell death in tobacco leaves. Plant Physiol. 138, 1516–1526. doi: 10.1104/pp.105.059907
Moreno, J. E., and Ballaré, C. L. (2014). Phytochrome regulation of plant immunity in vegetation canopies. J. Chem. Ecol. 40, 848–857. doi: 10.1007/s10886-014-0471-8
Moreno, J. E., Tao, Y., Chory, J., and Ballaré, C. L. (2009). Ecological modulation of plant defense via phytochrome control of jasmonate sensitivity. Proc. Natl. Acad. Sci. U.S.A. 106, 4935–4940. doi: 10.1073/pnas.0900701106
Mueller, D., and Buck, J. (2003). Effects of light, temperature, and leaf wetness duration on daylily rust. Plant Dis. 87, 442–445. doi: 10.1094/PDIS.2003.87.4.442
Mühlenbock, P., Szechyńska-Hebda, M., Płaszczyca, M., Baudo, M., Mateo, A., Mullineaux, P. M., et al. (2008). Chloroplast signaling and LESION SIMULATING DISEASE1 regulate crosstalk between light acclimation and immunity in Arabidopsis. Plant Cell 20, 2339–2356. doi: 10.1105/tpc.108.059618
Müller, P., Li, X.-P., and Niyogi, K. K. (2001). Non-photochemical quenching. A response to excess light energy. Plant Physiol. 125, 1558–1566. doi: 10.1104/pp.125.4.1558
Mur, L. A., Kenton, P., Lloyd, A. J., Ougham, H., and Prats, E. (2008). The hypersensitive response; the centenary is upon us but how much do we know? J. Exper. Bot. 59, 501–520. doi: 10.1093/jxb/erm239
Muthamilarasan, M., and Prasad, M. (2013). Plant innate immunity: an updated insight into defense mechanism. J. Biosci. 38, 433–449. doi: 10.1007/s12038-013-9302-2
Nagata, M., Yamamoto, N., Miyamoto, T., Shimomura, A., Arima, S., Hirsch, A. M., et al. (2016). Enhanced hyphal growth of arbuscular mycorrhizae by root exudates derived from high R/FR treated Lotus japonicus. Plant Signal. Behav. 11:e1187356. doi: 10.1080/15592324.2016.1187356
Nagata, M., Yamamoto, N., Shigeyama, T., Terasawa, Y., Anai, T., Sakai, T., et al. (2015). Red/far red light controls arbuscular mycorrhizal colonization via jasmonic acid and strigolactone signaling. Plant Cell Physiol. 56, 2100–2109. doi: 10.1093/pcp/pcv135
Nath, M., Bhatt, D., Prasad, R., and Tuteja, N. (2017). “Reactive oxygen species (ROS) metabolism and signaling in plant-mycorrhizal association under biotic and abiotic stress conditions,” in Mycorrhiza-Eco-Physiology, Secondary Metabolites, Nanomaterials, eds A. Varma, R. Prasad, and N. Tuteja (Cham: Springer), 223–232. doi: 10.1007/978-3-319-57849-1_12
Nejat, N., and Mantri, N. (2017). Plant immune system: crosstalk between responses to biotic and abiotic stresses the missing link in understanding plant defence. Curr. Issues Mol. Biol. 23, 1–16. doi: 10.21775/cimb.023.001
Nell, C. S., Abdala-Roberts, L., Parra-Tabla, V., and Mooney, K. A. (2018). Tropical tree diversity mediates foraging and predatory effects of insectivorous birds. Proce. R. Soc. B 285:20181842. doi: 10.1098/rspb.2018.1842
Ningen, S. S., Cole, J. C., Smith, M. W., Dunn, D. E., and Conway, K. E. (2005). Increased shade intensity and afternoon irrigation decrease anthracnose severity on three Euonymus fortunei cultivars. Hortscience 40, 111–113. doi: 10.21273/HORTSCI.40.1.111
Nishad, R., Ahmed, T., Rahman, V. J., and Kareem, A. (2020). Modulation of plant defense system in response to microbial interactions. Front. Microbiol. 11:1298. doi: 10.3389/fmicb.2020.01298
Niyogi, K. K. (2000). Safety valves for photosynthesis. Curr. Opin. Plant Biol. 3, 455–460. doi: 10.1016/S1369-5266(00)00113-8
Niyogi, K. K., Li, X.-P., Rosenberg, V., and Jung, H.-S. (2005). Is PsbS the site of non-photochemical quenching in photosynthesis? J. Exper. Bot. 56, 375–382. doi: 10.1093/jxb/eri056
Nozue, K., Devisetty, U. K., Lekkala, S., Mueller-Moulé, P., Bak, A., Casteel, C. L., et al. (2018). Network analysis reveals a role for salicylic acid pathway components in shade avoidance. Plant Physiol. 178, 1720–1732. doi: 10.1104/pp.18.00920
Nunes-Nesi, A., Fernie, A. R., and Stitt, M. (2010). Metabolic and signaling aspects underpinning the regulation of plant carbon nitrogen interactions. Mol. Plant 3, 973–996. doi: 10.1093/mp/ssq049
Ordoñez-Herrera, N., Fackendahl, P., Yu, X., Schaefer, S., Koncz, C., and Hoecker, U. (2015). A cop1 spa mutant deficient in COP1 and SPA proteins reveals partial co-action of COP1 and SPA during Arabidopsis post-embryonic development and photomorphogenesis. Mol. Plant 8, 479–481. doi: 10.1016/j.molp.2014.11.026
Osman, H. A., Ameen, H. H., Mohamed, M., and Elkelany, U. S. (2020). Efficacy of integrated microorganisms in controlling root-knot nematode Meloidogyne javanica infecting peanut plants under field conditions. Bull. Natl. Res. Centre 44, 1–10. doi: 10.1186/s42269-020-00366-0
Osterlund, M. T., Hardtke, C. S., Wei, N., and Deng, X. W. (2000). Targeted destabilization of HY5 during light-regulated development of Arabidopsis. Nature 405, 462–466. doi: 10.1038/35013076
Paik, I., Chen, F., Pham, V. N., Zhu, L., Kim, J.-I., and Huq, E. (2019). A phyB-PIF1-SPA1 kinase regulatory complex promotes photomorphogenesis in Arabidopsis. Nat. Commun. 10, 1–17. doi: 10.1038/s41467-019-12110-y
Pallas, V., and García, J. A. (2011). How do plant viruses induce disease? Interactions and interference with host components. J. Gen. Virol. 92, 2691–2705. doi: 10.1099/vir.0.034603-0
Pan, Y., Chai, X., Gao, Q., Zhou, L., Zhang, S., Li, L., et al. (2019). Dynamic interactions of plant CNGC subunits and calmodulins drive oscillatory Ca2+ channel activities. Dev. Cell 48, 710–725. doi: 10.1016/j.devcel.2018.12.025
Pandey, G. K., and Sanyal, S. K. (2021). “Ca 2+-ATPase and Ca 2+/cation antiporters,” in Functional Dissection of Calcium Homeostasis and Transport Machinery in Plants, eds G. K. Pandey and S. K. Sanyal (Cham: Springer), 89–104. doi: 10.1007/978-3-030-58502-0_9
Park, C. Y., Lee, J. H., Yoo, J. H., Moon, B. C., Choi, M. S., Kang, Y. H., et al. (2005). WRKY group IId transcription factors interact with calmodulin. FEBS Lett. 579, 1545–1550. doi: 10.1016/j.febslet.2005.01.057
Peck, S., and Mittler, R. (2020). Plant signaling in biotic and abiotic stress. J. Exper. Bot. 71, 1649–1651. doi: 10.1093/jxb/eraa051
Peng, Y., Van Wersch, R., and Zhang, Y. (2018). Convergent and divergent signaling in PAMP-triggered immunity and effector-triggered immunity. Mol. Plant Microb. Interact. 31, 403–409. doi: 10.1094/MPMI-06-17-0145-CR
Pham, V. N., Kathare, P. K., and Huq, E. (2018). Phytochromes and phytochrome interacting factors. Plant Physiol. 176, 1025–1038. doi: 10.1104/pp.17.01384
Pham, V. N., Paik, I., Hoecker, U., and Huq, E. (2020). Genomic evidence reveals SPA-regulated developmental and metabolic pathways in dark-grown Arabidopsis seedlings. Physiol. Plant. 169, 380–396. doi: 10.1111/ppl.13095
Pichersky, E., and Gershenzon, J. (2002). The formation and function of plant volatiles: perfumes for pollinator attraction and defense. Curr. Opin. Plant Biol. 5, 237–243. doi: 10.1016/S1369-5266(02)00251-0
Pieterse, C. M., Pierik, R., and Van Wees, S. C. (2014). Different shades of JAZ during plant growth and defense. New Phytol. 204, 261–264. doi: 10.1111/nph.13029
Pittman, J. K. (2011). Vacuolar Ca2+ uptake. Cell Calc. 50, 139–146. doi: 10.1016/j.ceca.2011.01.004
Planas-Riverola, A., Gupta, A., Betegón-Putze, I., Bosch, N., Ibañes, M., and Cańo-Delgado, A. I. (2019). Brassinosteroid signaling in plant development and adaptation to stress. Development 146:151894. doi: 10.1242/dev.151894
Podolec, R., and Ulm, R. (2018). Photoreceptor-mediated regulation of the COP1/SPA E3 ubiquitin ligase. Curr. Opin. Plant Biol. 45, 18–25. doi: 10.1016/j.pbi.2018.04.018
Popescu, S. C., Popescu, G. V., Bachan, S., Zhang, Z., Seay, M., Gerstein, M., et al. (2007). Differential binding of calmodulin-related proteins to their targets revealed through high-density Arabidopsis protein microarrays. Proc. Natl. Acad. Sci. U.S.A. 104, 4730–4735. doi: 10.1073/pnas.0611615104
Pružinská, A., Tanner, G., Anders, I., Roca, M., and Hörtensteiner, S. (2003). Chlorophyll breakdown: pheophorbide a oxygenase is a Rieske-type iron-sulfur protein, encoded by the accelerated cell death 1 gene. Proc. Natl. Acad. Sci. U.S.A. 100, 15259–15264. doi: 10.1073/pnas.2036571100
Ranty, B., Aldon, D., Cotelle, V., Galaud, J.-P., Thuleau, P., and Mazars, C. (2016). Calcium sensors as key hubs in plant responses to biotic and abiotic stresses. Front. Plant Sci. 7:327. doi: 10.3389/fpls.2016.00327
Rasool, B., Karpinska, B., Konert, G., Durian, G., Denessiouk, K., Kangasjärvi, S., et al. (2014). Effects of light and the regulatory B-subunit composition of protein phosphatase 2A on the susceptibility of Arabidopsis thaliana to aphid (Myzus persicae) infestation. Front. Plant Sci. 5:405. doi: 10.3389/fpls.2014.00405
Reddy, A. S., Ali, G. S., Celesnik, H., and Day, I. S. (2011). Coping with stresses: roles of calcium-and calcium/calmodulin-regulated gene expression. Plant Cell 23, 2010–2032. doi: 10.1105/tpc.111.084988
Rejeb, I. B., Pastor, V., and Mauch-Mani, B. (2014). Plant responses to simultaneous biotic and abiotic stress: molecular mechanisms. Plants 3, 458–475. doi: 10.3390/plants3040458
Rizhsky, L., Liang, H., Shuman, J., Shulaev, V., Davletova, S., and Mittler, R. (2004). When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiol. 134, 1683–1696. doi: 10.1104/pp.103.033431
Roberts, M. R., and Paul, N. D. (2006). Seduced by the dark side: integrating molecular and ecological perspectives on the influence of light on plant defence against pests and pathogens. New Phytol. 170, 677–699. doi: 10.1111/j.1469-8137.2006.01707.x
Rossel, J. B., Wilson, I. W., and Pogson, B. J. (2002). Global changes in gene expression in response to high light in Arabidopsis. Plant Physiol. 130, 1109–1120. doi: 10.1104/pp.005595
Rossel, J. B., Wilson, P. B., Hussain, D., Woo, N. S., Gordon, M. J., Mewett, O. P., et al. (2007). Systemic and intracellular responses to photooxidative stress in Arabidopsis. Plant Cell 19, 4091–4110. doi: 10.1105/tpc.106.045898
Saijo, Y., and Loo, E. P. I. (2020). Plant immunity in signal integration between biotic and abiotic stress responses. New Phytol. 225, 87–104. doi: 10.1111/nph.15989
Saiz-Rubio, V., and Rovira-Más, F. (2020). From smart farming towards agriculture 5.0: a review on crop data management. Agronomy 10:207. doi: 10.3390/agronomy10020207
Sánchez-Muros, M.-J., Barroso, F. G., and Manzano-Agugliaro, F. (2014). Insect meal as renewable source of food for animal feeding: a review. J. Clean. Product. 65, 16–27. doi: 10.1016/j.jclepro.2013.11.068
Santamaria, M. E., Martínez, M., Cambra, I., Grbic, V., and Diaz, I. (2013). Understanding plant defence responses against herbivore attacks: an essential first step towards the development of sustainable resistance against pests. Transgen. Res. 22, 697–708. doi: 10.1007/s11248-013-9725-4
Santino, A., Taurino, M., De Domenico, S., Bonsegna, S., Poltronieri, P., Pastor, V., et al. (2013). Jasmonate signaling in plant development and defense response to multiple (a) biotic stresses. Plant Cell Rep. 32, 1085–1098. doi: 10.1007/s00299-013-1441-2
Schmale, D. G., and Bergstrom, G. C. (2004). Spore deposition of the ear rot pathogen, Gibberella zeae, inside corn canopies. Can. J. Plant Pathol. 26, 591–595. doi: 10.1080/07060660409507179
Schmelz, E. A., Engelberth, J., Alborn, H. T., O’donnell, P., Sammons, M., Toshima, H., et al. (2003). Simultaneous analysis of phytohormones, phytotoxins, and volatile organic compounds in plants. Proc. Natl. Acad. Sci. U.S.A. 100, 10552–10557. doi: 10.1073/pnas.1633615100
Scholz, S. S., Vadassery, J., Heyer, M., Reichelt, M., Bender, K. W., Snedden, W. A., et al. (2014). Mutation of the Arabidopsis calmodulin-like protein CML37 deregulates the jasmonate pathway and enhances susceptibility to herbivory. Mol. Plant 7, 1712–1726. doi: 10.1093/mp/ssu102
Schuurink, R. C., Shartzer, S. F., Fath, A., and Jones, R. L. (1998). Characterization of a calmodulin-binding transporter from the plasma membrane of barley aleurone. Proc. Natl. Acad. Sci. U.S.A. 95, 1944–1949. doi: 10.1073/pnas.95.4.1944
Schwechheimer, C., Serino, G., Callis, J., Crosby, W. L., Lyapina, S., Deshaies, R. J., et al. (2001). Interactions of the COP9 signalosome with the E3 ubiquitin ligase SCFTIR1 in mediating auxin response. Science 292, 1379–1382. doi: 10.1126/science.1059776
Seo, H. S., Yang, J.-Y., Ishikawa, M., Bolle, C., Ballesteros, M. L., and Chua, N.-H. (2003). LAF1 ubiquitination by COP1 controls photomorphogenesis and is stimulated by SPA1. Nature 423, 995–999. doi: 10.1038/nature01696
Serino, G., and Deng, X.-W. (2003). The COP9 signalosome: regulating plant development through the control of proteolysis. Annu. Rev. Plant Biol. 54, 165–182. doi: 10.1146/annurev.arplant.54.031902.134847
Sessa, G., Carabelli, M., Possenti, M., Morelli, G., and Ruberti, I. (2018). Multiple pathways in the control of the shade avoidance response. Plants 7:102. doi: 10.3390/plants7040102
Seybold, H., Trempel, F., Ranf, S., Scheel, D., Romeis, T., and Lee, J. (2014). Ca2+ signalling in plant immune response: from pattern recognition receptors to Ca2+ decoding mechanisms. New Phytol. 204, 782–790. doi: 10.1111/nph.13031
Shafia, A., Sutton, J., Yu, H., and Fletcher, R. (2001). Influence of preinoculation light intensity on development and interactions of Botrytis cinerea and Clonostachys rosea in tomato leaves. Can. J. Plant Pathol. 23, 346–357. doi: 10.1080/07060660109506955
Sharma, M., and Bhatt, D. (2015). The circadian clock and defence signalling in plants. Mol. Plant Pathol. 16, 210–218. doi: 10.1111/mpp.12178
Shen, Q., Fu, L., Su, T., Ye, L., Huang, L., Kuang, L., et al. (2020). Calmodulin HvCaM1 negatively regulates salt tolerance via modulation of HvHKT1s and HvCAMTA4. Plant Physiol. 183, 1650–1662. doi: 10.1104/pp.20.00196
Shi, H., Wang, X., Mo, X., Tang, C., Zhong, S., and Deng, X. W. (2015). Arabidopsis DET1 degrades HFR1 but stabilizes PIF1 to precisely regulate seed germination. Proc. Natl. Acad. Sci. U.S.A. 112, 3817–3822. doi: 10.1073/pnas.1502405112
Shigeyama, T., Tominaga, A., Arima, S., Sakai, T., Inada, S., Jikumaru, Y., et al. (2012). Additional cause for reduced JA-Ile in the root of a Lotus japonicus phyB mutant. Plant Signal. Behav. 7, 746–748. doi: 10.4161/psb.20407
Shin, J., Kim, K., Kang, H., Zulfugarov, I. S., Bae, G., Lee, C.-H., et al. (2009). Phytochromes promote seedling light responses by inhibiting four negatively-acting phytochrome-interacting factors. Proc. Natl. Acad. Sci. U.S.A. 106, 7660–7665. doi: 10.1073/pnas.0812219106
Singh, A., Kanwar, P., Yadav, A. K., Mishra, M., Jha, S. K., Baranwal, V., et al. (2014). Genome-wide expressional and functional analysis of calcium transport elements during abiotic stress and development in rice. FEBS J. 281, 894–915. doi: 10.1111/febs.12656
Singh, A., Sagar, S., and Biswas, D. K. (2017). Calcium dependent protein kinase, a versatile player in plant stress management and development. Crit. Rev. Plant Sci. 36, 336–352. doi: 10.1080/07352689.2018.1428438
Singh, P., and Virdi, A. S. (2013). “Ca 2+, calmodulin and plant-specific calmodulin-binding proteins: implications in abiotic stress adaptation,” in Stress Signaling in Plants: Genomics and Proteomics Perspective, Vol. 1, eds M. Sarwat, A. Ahmad, and M. Abdin (New York, NY: Springer), 1–23. doi: 10.1007/978-1-4614-6372-6_1
Slattery, R. A., Vanloocke, A., Bernacchi, C. J., Zhu, X.-G., and Ort, D. R. (2017). Photosynthesis, light use efficiency, and yield of reduced-chlorophyll soybean mutants in field conditions. Front. Plant Sci. 8:549. doi: 10.3389/fpls.2017.00549
Sobiczewski, P., Iakimova, E., Mikiciński, A., Wêgrzynowicz-Lesiak, E., and Dyki, B. (2017). Necrotrophic behaviour of Erwinia amylovora in apple and tobacco leaf tissue. Plant Pathol. 66, 842–855. doi: 10.1111/ppa.12631
Soto-Pinto, L., Perfecto, I., and Caballero-Nieto, J. (2002). Shade over coffee: its effects on berry borer, leaf rust and spontaneous herbs in Chiapas, Mexico. Agroforestry Syst. 55, 37–45. doi: 10.1023/A:1020266709570
Spalding, E. P., and Harper, J. F. (2011). The ins and outs of cellular Ca2+ transport. Curr. Opin. Plant Biol. 14, 715–720. doi: 10.1016/j.pbi.2011.08.001
Spoel, S. H., and Dong, X. (2012). How do plants achieve immunity? Defence without specialized immune cells. Nat. Rev. Immunol. 12, 89–100. doi: 10.1038/nri3141
Stamm, P., and Kumar, P. P. (2010). The phytohormone signal network regulating elongation growth during shade avoidance. J. Exper. Bot. 61, 2889–2903. doi: 10.1093/jxb/erq147
Stegmann, M., Monaghan, J., Smakowska-Luzan, E., Rovenich, H., Lehner, A., Holton, N., et al. (2017). The receptor kinase FER is a RALF-regulated scaffold controlling plant immune signaling. Science 355, 287–289. doi: 10.1126/science.aal2541
Stracke, R., Werber, M., and Weisshaar, B. (2001). The R2R3-MYB gene family in Arabidopsis thaliana. Curr. Opin. Plant Biol. 4, 447–456. doi: 10.1016/S1369-5266(00)00199-0
Stratmann, J. (2003). Ultraviolet-B radiation co-opts defense signaling pathways. Trends Plant Sci. 8, 526–533. doi: 10.1016/j.tplants.2003.09.011
Strong, A. M., Sherry, T. W., and Holmes, R. T. (2000). Bird predation on herbivorous insects: indirect effects on sugar maple saplings. Oecologia 125, 370–379. doi: 10.1007/s004420000467
Su, H., Van Bruggen, A., and Subbarao, K. (2000). Spore release of Bremia lactucae on lettuce is affected by timing of light initiation and decrease in relative humidity. Phytopathology 90, 67–71. doi: 10.1094/PHYTO.2000.90.1.67
Sun, C., Jin, L., Cai, Y., Huang, Y., Zheng, X., and Yu, T. (2019). L-Glutamate treatment enhances disease resistance of tomato fruit by inducing the expression of glutamate receptors and the accumulation of amino acids. Food Chem. 293, 263–270. doi: 10.1016/j.foodchem.2019.04.113
Suzuki, A., Suriyagoda, L., Shigeyama, T., Tominaga, A., Sasaki, M., Hiratsuka, Y., et al. (2011). Lotus japonicus nodulation is photomorphogenetically controlled by sensing the red/far red (R/FR) ratio through jasmonic acid (JA) signaling. Proc. Natl. Acad. Sci. U.S.A. 108, 16837–16842. doi: 10.1073/pnas.1105892108
Suzuki, N., Rivero, R. M., Shulaev, V., Blumwald, E., and Mittler, R. (2014). Abiotic and biotic stress combinations. New Phytol. 203, 32–43. doi: 10.1111/nph.12797
Szechyńska-Hebda, M., Kruk, J., Górecka, M., Karpińska, B., and Karpiński, S. (2010). Evidence for light wavelength-specific photoelectrophysiological signaling and memory of excess light episodes in Arabidopsis. Plant Cell 22, 2201–2218. doi: 10.1105/tpc.109.069302
Taiz, L., and Zeiger, E. (2006). Secondary metabolites and plant defense. Plant Physiol. 4, 315–344.
Takano, M., Inagaki, N., Xie, X., Kiyota, S., Baba-Kasai, A., Tanabata, T., et al. (2009). Phytochromes are the sole photoreceptors for perceiving red/far-red light in rice. Proc. Natl. Acad. Sci. U.S.A. 106, 14705–14710. doi: 10.1073/pnas.0907378106
Taneja, M., and Upadhyay, S. K. (2018). Molecular characterization and differential expression suggested diverse functions of P-type II Ca 2+ ATPases in Triticum aestivum L. BMC Genom. 19:389. doi: 10.1186/s12864-018-4792-9
Tang, R.-J., Wang, C., Li, K., and Luan, S. (2020). The CBL-CIPK calcium signaling network: Unified paradigm from 20 years of discoveries. Trends Plant Sci. 25, 604–617. doi: 10.1016/j.tplants.2020.01.009
Tao, Y., Xie, Z., Chen, W., Glazebrook, J., Chang, H.-S., Han, B., et al. (2003). Quantitative nature of Arabidopsis responses during compatible and incompatible interactions with the bacterial pathogen Pseudomonas syringae. Plant Cell 15, 317–330. doi: 10.1105/tpc.007591
Teardo, E., Carraretto, L., Wagner, S., Formentin, E., Behera, S., De Bortoli, S., et al. (2017). Physiological characterization of a plant mitochondrial calcium uniporter in vitro and in vivo. Plant Physiol. 173, 1355–1370. doi: 10.1104/pp.16.01359
Thoma, I., Loeffler, C., Sinha, A. K., Gupta, M., Krischke, M., Steffan, B., et al. (2003). Cyclopentenone isoprostanes induced by reactive oxygen species trigger defense gene activation and phytoalexin accumulation in plants. Plant J. 34, 363–375. doi: 10.1046/j.1365-313X.2003.01730.x
Thomma, B. P., Eggermont, K., Penninckx, I. A., Mauch-Mani, B., Vogelsang, R., Cammue, B. P., et al. (1998). Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc. Natl. Acad. Sci. U.S.A. 95, 15107–15111. doi: 10.1073/pnas.95.25.15107
Thor, K., Jiang, S., Michard, E., George, J., Scherzer, S., Huang, S., et al. (2020). The calcium-permeable channel OSCA1. 3 regulates plant stomatal immunity. Nature 585, 569–573. doi: 10.1038/s41586-020-2702-1
Tilbrook, K., Arongaus, A. B., Binkert, M., Heijde, M., Yin, R., and Ulm, R. (2013). The UVR8 UV-B photoreceptor: perception, signaling and response. Am. Soc. Plant Biol. 11:e0164. doi: 10.1199/tab.0164
Toledo-Ortiz, G., Johansson, H., Lee, K. P., Bou-Torrent, J., Stewart, K., Steel, G., et al. (2014). The HY5-PIF regulatory module coordinates light and temperature control of photosynthetic gene transcription. PLoS Genet. 10:e1004416. doi: 10.1371/journal.pgen.1004416
Torres, M. A. (2010). ROS in biotic interactions. Physiol. Plant. 138, 414–429. doi: 10.1111/j.1399-3054.2009.01326.x
Tripathi, S., Hoang, Q. T., Han, Y.-J., and Kim, J.-I. (2019). Regulation of photomorphogenic development by plant phytochromes. Intern. J. Mol. Sci. 20:6165. doi: 10.3390/ijms20246165
Trotta, A., Rahikainen, M., Konert, G., Finazzi, G., and Kangasjärvi, S. (2014). Signalling crosstalk in light stress and immune reactions in plants. Philos. Trans. R. Soc. B Biol. Sci. 369:20130235. doi: 10.1098/rstb.2013.0235
Turner, J. G., Ellis, C., and Devoto, A. (2002). The jasmonate signal pathway. Plant Cell 14, S153–S164. doi: 10.1105/tpc.000679
Vadassery, J., Reichelt, M., Hause, B., Gershenzon, J., Boland, W., and Mithöfer, A. (2012). CML42-mediated calcium signaling coordinates responses to Spodoptera herbivory and abiotic stresses in Arabidopsis. Plant Physiol. 159, 1159–1175. doi: 10.1104/pp.112.198150
Van Bael, S. A., and Brawn, J. D. (2005). The direct and indirect effects of insectivory by birds in two contrasting Neotropical forests. Oecologia 143, 106–116. doi: 10.1007/s00442-004-1774-1
van Loon, L. C., Rep, M., and Pieterse, C. M. (2006). Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 44, 135–162. doi: 10.1146/annurev.phyto.44.070505.143425
Vandenbussche, F., and Van Der Straeten, D. (2004). Shaping the shoot: a circuitry that integrates multiple signals. Trends Plant Sci. 9, 499–506. doi: 10.1016/j.tplants.2004.08.002
VanLaerhoven, S. L., Gillespie, D. R., and Roitberg, B. D. (2003). Diel activity pattern and predation rate of the generalist predator Dicyphus hesperus. Entomol. Exper. Appl. 107, 149–154. doi: 10.1046/j.1570-7458.2003.00050.x
Verhage, A., Vlaardingerbroek, I., Dicke, M., Van Wees, S., and Pieterse, C. (2012). The transcription factor MYC2 shapes plant defense responses in Arabidopsis upon Pieris rapae herbivory. Anim. Behav. 83, 21–25.
Vincent, T. R., Avramova, M., Canham, J., Higgins, P., Bilkey, N., Mugford, S. T., et al. (2017). Interplay of plasma membrane and vacuolar ion channels, together with BAK1, elicits rapid cytosolic calcium elevations in Arabidopsis during aphid feeding. Plant Cell 29, 1460–1479. doi: 10.1105/tpc.17.00136
Vollenweider, S., Weber, H., Stolz, S., Chételat, A., and Farmer, E. E. (2000). Fatty acid ketodienes and fatty acid ketotrienes: michael addition acceptors that accumulate in wounded and diseased Arabidopsis leaves. Plant J. 24, 467–476. doi: 10.1046/j.1365-313x.2000.00897.x
von Arnim, A. G., Osterlund, M. T., Kwok, S. F., and Deng, X.-W. (1997). Genetic and developmental control of nuclear accumulation of COP1, a repressor of photomorphogenesis in Arabidopsis. Plant Physiol. 114, 779–788. doi: 10.1104/pp.114.3.779
Walker, B. J., Drewry, D. T., Slattery, R. A., Vanloocke, A., Cho, Y. B., and Ort, D. R. (2018). Chlorophyll can be reduced in crop canopies with little penalty to photosynthesis. Plant Physiol. 176, 1215–1232. doi: 10.1104/pp.17.01401
Wang, F., Wu, W., Wang, D., Yang, W., Sun, J., Liu, D., et al. (2016). Characterization and genetic analysis of a novel light-dependent lesion mimic mutant, lm3, showing adult-plant resistance to powdery mildew in common wheat. PLoS One 11:e0155358. doi: 10.1371/journal.pone.0155358
Wang, G., Zhang, S., Ma, X., Wang, Y., Kong, F., and Meng, Q. (2016). A stress-associated NAC transcription factor (SlNAC35) from tomato plays a positive role in biotic and abiotic stresses. Physiol. Plant. 158, 45–64. doi: 10.1111/ppl.12444
Wang, W.-X., Lian, H.-L., Zhang, L.-D., Mao, Z.-L., Li, X.-M., Xu, F., et al. (2016). Transcriptome analyses reveal the involvement of both C and N termini of cryptochrome 1 in its regulation of phytohormone-responsive gene expression in Arabidopsis. Front. Plant Sci. 7:294. doi: 10.3389/fpls.2016.00294
Wang, P.-H., Lee, C.-E., Lin, Y.-S., Lee, M.-H., Chen, P.-Y., Chang, H.-C., et al. (2019). The glutamate receptor-like protein GLR3. 7 interacts with 14-3-3ω and participates in salt stress response in Arabidopsis thaliana. Front. Plant Sci. 10:1169. doi: 10.3389/fpls.2019.01169
Wang, Z., Ma, L.-Y., Cao, J., Li, Y.-L., Ding, L.-N., Zhu, K.-M., et al. (2019). Recent advances in mechanisms of plant defense to Sclerotinia sclerotiorum. Front. Plant Sci. 10:1314. doi: 10.3389/fpls.2019.01314
Wang, Y., Kang, Y., Ma, C., Miao, R., Wu, C., Long, Y., et al. (2017). CNGC2 is a Ca2+ influx channel that prevents accumulation of apoplastic Ca2+ in the leaf. Plant Physiol. 173, 1342–1354. doi: 10.1104/pp.16.01222
Woldemariam, M. G., Dinh, S. T., Oh, Y., Gaquerel, E., Baldwin, I. T., and Galis, I. (2013). NaMYC2 transcription factor regulates a subset of plant defense responses in Nicotiana attenuata. BMC Plant Biol. 13:73. doi: 10.1186/1471-2229-13-73
Xu, D., Jiang, Y., Li, J., Lin, F., Holm, M., and Deng, X. W. (2016a). BBX21, an Arabidopsis B-box protein, directly activates HY5 and is targeted by COP1 for 26S proteasome-mediated degradation. Proc. Natl. Acad. Sci. U.S.A. 113, 7655–7660. doi: 10.1073/pnas.1607687113
Xu, D., Zhu, D., and Deng, X. W. (2016b). The role of COP1 in repression of photoperiodic flowering. F1000Research 5:178. doi: 10.12688/f1000research.7346.1
Xu, D., Lin, F., Jiang, Y., Huang, X., Li, J., Ling, J., et al. (2014). The RING-finger E3 ubiquitin ligase COP1 SUPPRESSOR1 negatively regulates COP1 abundance in maintaining COP1 homeostasis in dark-grown Arabidopsis seedlings. Plant Cell 26, 1981–1991. doi: 10.1105/tpc.114.124024
Xu, X., Paik, I., Zhu, L., Bu, Q., Huang, X., Deng, X. W., et al. (2014). PHYTOCHROME INTERACTING FACTOR1 enhances the E3 ligase activity of CONSTITUTIVE PHOTOMORPHOGENIC1 to synergistically repress photomorphogenesis in Arabidopsis. Plant Cell 26, 1992–2006. doi: 10.1105/tpc.114.125591
Xu, H., Martinoia, E., and Szabo, I. (2015a). Organellar channels and transporters. Cell Calc. 58, 1–10. doi: 10.1016/j.ceca.2015.02.006
Xu, X., Paik, I., Zhu, L., and Huq, E. (2015b). Illuminating progress in phytochrome-mediated light signaling pathways. Trends Plant Sci. 20, 641–650. doi: 10.1016/j.tplants.2015.06.010
Xu, X., Kathare, P. K., Pham, V. N., Bu, Q., Nguyen, A., and Huq, E. (2017). Reciprocal proteasome-mediated degradation of PIFs and HFR1 underlies photomorphogenic development in Arabidopsis. Development 144, 1831–1840. doi: 10.1242/dev.146936
Yan, C., Fan, M., Yang, M., Zhao, J., Zhang, W., Su, Y., et al. (2018). Injury activates Ca2+/calmodulin-dependent phosphorylation of JAV1-JAZ8-WRKY51 complex for jasmonate biosynthesis. Mol. Cell 70, 136–149. doi: 10.1016/j.molcel.2018.03.013
Yang, D., Seaton, D. D., Krahmer, J., and Halliday, K. J. (2016). Photoreceptor effects on plant biomass, resource allocation, and metabolic state. Proc. Natl. Acad. Sci. U.S.A. 113, 7667–7672. doi: 10.1073/pnas.1601309113
Yang, D.-L., Shi, Z., Bao, Y., Yan, J., Yang, Z., Yu, H., et al. (2017). Calcium pumps and interacting BON1 protein modulate calcium signature, stomatal closure, and plant immunity. Plant Physiol. 175, 424–437. doi: 10.1104/pp.17.00495
Yang, J., Duan, G., Li, C., Liu, L., Han, G., Zhang, Y., et al. (2019). The crosstalks between jasmonic acid and other plant hormone signaling highlight the involvement of jasmonic acid as a core component in plant response to biotic and abiotic stresses. Front. Plant Sci. 10:1349. doi: 10.3389/fpls.2019.01349
Yang, J., Lin, R., Sullivan, J., Hoecker, U., Liu, B., Xu, L., et al. (2005). Light regulates COP1-mediated degradation of HFR1, a transcription factor essential for light signaling in Arabidopsis. Plant Cell 17, 804–821. doi: 10.1105/tpc.104.030205
Yoshioka, K., Kachroo, P., Tsui, F., Sharma, S. B., Shah, J., and Klessig, D. F. (2001). Environmentally sensitive, SA-dependent defense responses in the cpr22 mutant of Arabidopsis. Plant J. 26, 447–459. doi: 10.1046/j.1365-313X.2001.2641039.x
Yoshioka, K., Moeder, W., Kang, H.-G., Kachroo, P., Masmoudi, K., Berkowitz, G., et al. (2006). The chimeric Arabidopsis CYCLIC NUCLEOTIDE-GATED ION CHANNEL11/12 activates multiple pathogen resistance responses. Plant Cell 18, 747–763. doi: 10.1105/tpc.105.038786
Yu, J.-W., Rubio, V., Lee, N.-Y., Bai, S., Lee, S.-Y., Kim, S.-S., et al. (2008). COP1 and ELF3 control circadian function and photoperiodic flowering by regulating GI stability. Mol. Cell 32, 617–630. doi: 10.1016/j.molcel.2008.09.026
Yu, X., Liu, H., Klejnot, J., and Lin, C. (2010). The cryptochrome blue light receptors. Am. Soc. Plant Biol. 8:e0135. doi: 10.1199/tab.0135
Yuan, F., Yang, H., Xue, Y., Kong, D., Ye, R., Li, C., et al. (2014). OSCA1 mediates osmotic-stress-evoked Ca 2+ increases vital for osmosensing in Arabidopsis. Nature 514, 367–371. doi: 10.1038/nature13593
Zhang, H., Kang, H., Su, C., Qi, Y., Liu, X., and Pu, J. (2018). Genome-wide identification and expression profile analysis of the NAC transcription factor family during abiotic and biotic stress in woodland strawberry. PLoS One 13:e0197892. doi: 10.1371/journal.pone.0197892
Zhang, J. X., Dilantha Fernando, W., and Xue, A. G. (2005). Daily and seasonal spore dispersal by Mycosphaerella pinodes and development of mycosphaerella blight of field pea. Can. J. Bot. 83, 302–310. doi: 10.1139/b05-003
Zhang, M., Liu, Y., He, Q., Chai, M., Huang, Y., Chen, F., et al. (2020). Genome-wide investigation of calcium-dependent protein kinase gene family in pineapple: evolution and expression profiles during development and stress. BMC Genom. 21:72. doi: 10.1186/s12864-020-6501-8
Zhang, S., Pan, Y., Tian, W., Dong, M., Zhu, H., Luan, S., et al. (2017). Arabidopsis CNGC14 mediates calcium influx required for tip growth in root hairs. Mol. Plant 10, 1004–1006. doi: 10.1016/j.molp.2017.02.007
Zhao, Y., Zhou, J., and Xing, D. (2014). Phytochrome B-mediated activation of lipoxygenase modulates an excess red light-induced defence response in Arabidopsis. J. Exper. Bot. 65, 4907–4918. doi: 10.1093/jxb/eru247
Zhou, L., Lan, W., Jiang, Y., Fang, W., and Luan, S. (2014). A calcium-dependent protein kinase interacts with and activates a calcium channel to regulate pollen tube growth. Mol. Plant 7, 369–376. doi: 10.1093/mp/sst125
Zhu, D., Maier, A., Lee, J.-H., Laubinger, S., Saijo, Y., Wang, H., et al. (2008). Biochemical characterization of Arabidopsis complexes containing CONSTITUTIVELY PHOTOMORPHOGENIC1 and SUPPRESSOR OF PHYA proteins in light control of plant development. Plant Cell 20, 2307–2323. doi: 10.1105/tpc.107.056580
Zhu, L., Bu, Q., Xu, X., Paik, I., Huang, X., Hoecker, U., et al. (2015). CUL4 forms an E3 ligase with COP1 and SPA to promote light-induced degradation of PIF1. Nat. Commun. 6, 1–10. doi: 10.1038/ncomms8245
Keywords: biotic stress, dark, defense response, light, plant protection, transcription factor
Citation: Iqbal Z, Iqbal MS, Hashem A, Abd_Allah EF and Ansari MI (2021) Plant Defense Responses to Biotic Stress and Its Interplay With Fluctuating Dark/Light Conditions. Front. Plant Sci. 12:631810. doi: 10.3389/fpls.2021.631810
Received: 21 November 2020; Accepted: 08 February 2021;
Published: 04 March 2021.
Edited by:
Péter Poór, University of Szeged, HungaryReviewed by:
Amarjeet Singh, National Institute of Plant Genome Research (NIPGR), IndiaJing Yang, Yunnan Agricultural University, China
Copyright © 2021 Iqbal, Iqbal, Hashem, Abd_Allah and Ansari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammad Israil Ansari, YW5zYXJpX21pQGxrb3VuaXYuYWMuaW4=
 Zahra Iqbal
Zahra Iqbal Mohammed Shariq Iqbal
Mohammed Shariq Iqbal Abeer Hashem
Abeer Hashem Elsayed Fathi Abd_Allah
Elsayed Fathi Abd_Allah Mohammad Israil Ansari6*
Mohammad Israil Ansari6*