- 1State Key Laboratory of Biocatalysis and Enzyme Engineering, School of Life Sciences, Hubei University, Wuhan, China
- 2School of Biology and Agricultural Resources, Huanggang Normal University, Huanggang, China
Cytokinins (CKs) can modulate plant immunity to various pathogens, but how CKs are involved in plant defense responses to the necrotrophic pathogen Botrytis cinerea is still unknown. Here, we found that B. cinerea infection induced transcriptional changes in multiple genes involved in the biosynthesis, degradation, and signaling of CKs, as well as their contents, in pathogen-infected Arabidopsis leaves. Among the CKs, the gene expression of CYTOKININ OXIDASE/DEHYDROGENASE 5 (CKX5) was remarkably induced in the local infected leaves and the distant leaves of the same plant without pathogen inoculation. Cis-zeatin (cZ) and its riboside (cZR) accumulated considerably in infected leaves, suggesting an important role of the cis-zeatin type of CKs in the plant response to B. cinerea. Cytokinin double-receptor mutants were more susceptible to B. cinerea infection, whereas an exogenous CK treatment enhanced the expression levels of defense-related genes and of jasmonic acid (JA) and ethylene (ET), but not salicylic acid (SA), resulting in higher resistance of Arabidopsis to B. cinerea. Investigation of CK responses to B. cinerea infection in the JA biosynthesis mutant, jar1-1, and ET-insensitive mutant, ein2-1, showed that CK signaling and levels of CKs, namely, those of isopentenyladenine (iP), isopentenyladenine riboside (iPR), and trans-zeatin (tZ), were enhanced in jar1-1-infected leaves. By contrast, reductions in iP, iPR, tZ, and tZ riboside (tZR) as well as cZR contents occurred in ein2-1-infected leaves, whose transcript levels of CK signaling genes were likewise differentially regulated. The Arabidopsis Response Regulator 5 (ARR5) gene was upregulated in infected leaves of ein2-1 whereas another type-A response regulator, ARR16, was significantly downregulated, suggesting the existence of a complex regulation of CK signaling via the ET pathway. Accumulation of the cis-zeatin type of CKs in B. cinerea-infected leaves depended on ET but not JA pathways. Collectively, our findings provide evidence that CK responds to B. cinerea infection in a variety of ways that are differently modulated by JA and ET pathways in Arabidopsis.
Introduction
To detect and defend themselves against phytopathogens in their environment, plants have evolved a sophisticated innate immune system consisting of multiple layers, which are highly interconnected and tightly regulated (Jones and Dangl, 2006). The innate immunity depends on extensive transcriptional reprogramming in the host, which is activated and controlled by plant hormones, including jasmonic acid (JA), ethylene (ET), and salicylic acid (SA) (Li et al., 2019). Generally, SA is considered to play a central role in defense against biotrophic and hemi-biotrophic pathogens, while JA and ET mainly contribute to host immunity to necrotrophic pathogens including Botrytis cinerea (Glazebrook, 2005; AbuQamar et al., 2017).
Cytokinins (CKs), well known for their functions in controlling plant growth and development, can also affect plant resistance to disease (Pertry et al., 2009; Naseem et al., 2014). Many (hemi)biotrophic pathogens can secrete CKs or cause a local increase of cytokinin production in a host plant, to modulate host immunity and optimize nutrient supply (Spallek et al., 2018). In addition, host-derived CKs can also influence plant immunity to pathogens that do not secrete CKs by interacting with the SA pathway or by increasing the synthesis of antimicrobial phytoalexin independently of SA signaling (Choi et al., 2010; Grosskinsky et al., 2011). Yet the role of CK in plant immunity to necrotrophic pathogens such as B. cinerea, which does not produce detectable CKs (Cooper and Ashby, 1998; Walters and McRoberts, 2006), is far less understood. Expression of the CK biosynthesis gene IPT from Agrobacterium under the control of the SAG12 (senescence-specific gene) promoter resulted in increased resistance of Arabidopsis thaliana to B. cinerea infection (Swartzberg et al., 2008). Arabidopsis histidine kinase 5 (AHK5), a member of the HK family of the two-component systems which involve cytokinin receptors (AHK2, AHK3, and AHK4), contributes to resistance to B. cinerea (Pham et al., 2012), suggesting the function of histidine kinases in regulating resistance against fungal infection. Moreover, transgenic Nicotiana attenuata having increased CK levels had an increased accumulation of JA metabolites (Schäfer et al., 2013). Recently, Gupta et al. (2020) revealed how CK promotes the resistance of tomato to B. cinerea through an SA- and ET-dependent mechanism. These cases provide evidence suggesting the potential contribution of CK to a plant defense response to B. cinerea, as well as the existence of cross talk between CKs and other phytohormone pathways, including those of JA, ET, and SA.
CK metabolism and signaling pathways in Arabidopsis are well elucidated (Kieber and Schaller, 2014). Briefly, CKs’ biosynthesis is initiated as a rate-limiting step by isopentenyl transferases (IPTs). The CK riboside 5’-monophosphate phosphoribohydrolase LONELY GUY (LOG) is responsible for the final step of CKs’ biosynthesis, in forming active CKs, such as isopentenyladenine (iP), trans-zeatin (tZ), cis-zeatin (cZ), and dihydrozeatin (DHZ), and their respective ribosides (∼R) (Kuroha et al., 2009). These CKs, however, can be inactivated by CK OXIDASE/DEHYDROGENASE (CKX). The CK signal is perceived by specific receptors named ARABIDOPSIS HISTIDINE KINASE 2 (AHK2), AHK3, and AHK4/CYTOKININ RESPONSE 1 (CRE1) (Yamada et al., 2001) and subsequently transmitted by Arabidopsis histidine phosphotransfer proteins (AHPs) to nuclear-localized type-B response regulators (type-B ARRs) that essentially function as transcription factors (Mason et al., 2005; Hutchison et al., 2006). The type-A response regulators, targets of type-B ARRs, are thus negative feedback regulators of the CK pathway (To et al., 2004).
Here we analyzed the role of CKs in the Arabidopsis–B. cinerea interactions to understand (i) whether the CK pathway and CKs’ contents are altered during B. cinerea infection; (ii) whether changes in CK levels may affect Arabidopsis resistance to B. cinerea; and (iii) whether the CK response to B. cinerea infection in plant is regulated by JA/ET pathways. Our results demonstrate that CK signaling and levels respond to B. cinerea infection and are under the control of JA and ET pathways.
Materials and Methods
Plant Material and Growth Conditions
All Arabidopsis thaliana plants used in this study were of the Columbia-0 (Col-0) ecotype. Seeds of the cytokinin double receptor mutants (cre1 ahk3, cre1 ahk2; Chang et al., 2015), ARR5:GUS and CKX5:GUS (Chang et al., 2015), were obtained from Dr. Thomas Schmülling (Freie Universität Berlin, Germany). Both the jasmonate-amino acid synthetase mutant JA-resistant 1 (jar1-1) (Staswick et al., 2002) and the ethylene signal mutant ethylene insensitive 2 (ein2-1) (Guzmán and Ecker, 1990) were kindly provided by Dr. Shunping Yan (Huazhong Agricultural University). Seeds of Arabidopsis plants used for B. cinerea infection experiments were surface-sterilized and sown on plates with solid Murashige and Skoog (MS) medium and stratified at 4°C for 3 days in the dark before germination. Then, the plates were transferred to the growth chamber. Two weeks later, the seedlings were transplanted to sterile soil to avoid infection from unspecified pathogens. The plants were grown in designated cabinets (CIMO, QHX-300BSH-III, Shanghai) under conditions of 12 h’ white light (∼100 μmol m–2 s–1) at 23°C/12 h’ darkness at 20°C, with 60% humidity.
Pathogen Bioassays
The B. cinerea strain B05.10 was cultivated on potato dextrose agar (PDA) medium (Coolaber, Beijing) at 22°C for 10 days. Spores were collected, filtered, and resuspended in half-strength potato dextrose broth (1/2 PDB), to a final concentration of 2.5 × 105 spores mL–1. For droplet inoculations, 4 μL of spore suspension was applied to single leaves of 4-weeks-old intact plants; three leaves (5th to 7th rosette leaves) were infected of each plant. Leaves were excised from plants only for rating symptoms’ severity. For the mock treatment (i.e., the control), the same amount of 1/2 PDB alone was used. After the inoculations, all the plants were kept under sealed transparent hoods at high humidity.
Two days after B. cinerea infection, the hoods were removed and the symptoms on the infected leaves were analyzed. Lesion diameters on leaves were measured with Image J software1. Following Van Wees et al. (2013), disease symptoms on inoculated leaves were recorded and grouped into four classes: lesion diameter < 2 mm (class I), 2-mm lesion with chlorosis (class II), 2–4 mm lesion with chlorosis (class III), and lesion with a spread > 4 mm (class IV). Quantification of fungal biomass relative to plant biomass by qPCR was performed as already described by Gachon and Saindrenan (2004). The abundance of cutinase A was quantified in the infected samples and normalized against the Arabidopsis actin2 gene. Primers used for used for B. cinerea growth biomass are listed in Supplementary Table S4.
Trypan Blue and GUS (β-Glucuronidase) Staining of Plant Materials
To visualize fungal tissue and dying plant cells, the leaves were stained with trypan blue as described before (Argueso et al., 2012). The histochemical detection of GUS activity was carried out following the methodology of Chang et al. (2013, 2015).
Quantitative Real-Time Reverse Transcription PCR (qRT-PCR)
Total RNA was extracted from the whole leaves of plants that were either B. cinerea-infected or mock-treated, by using the RNAiso Plus kit (Takara, Beijing, China) and following the manufacturer’s instructions. Quality and integrity of RNA were assessed by gel electrophoresis and A260/A280 and A260/A230 ratios in a NanoDrop photometer (PeqLab, Germany). Pure and highly intact RNA samples were used for cDNA synthesis with HifairTM 1st Stranded cDNA Synthesis SuperMix for qPCR (gDNA digester plus) kit (Yeasen, Shanghai, China). The generated cDNA was then subjected to quantitative PCR (qPCR) with gene-specific primers (Supplementary Table S3), by using the TB Green® Premix Ex TaqTM II (Tli RNaseH Plus) kit (Takara). The qPCRs were implemented in a CFX Connect Real-Time System (Bio-Rad, United States), with three technical replicates in the same run with at least three biological replicates used. For normalization purposes, EXP (At4g26410) was used as an endogenous reference gene because it has high expression stability under varying plant stress conditions (Czechowski et al., 2005; Liu S. et al., 2017). Primers used for reference genes and genes of interest are listed in Supplementary Table S3. The ΔΔCt method (Livak and Schmittgen, 2001) was used to calculate the relative expression levels of genes.
Hormone Treatment
For kinetin’s application, it was dissolved in 0.1 N sodium hydroxide (NaOH), to make a stock solution. The leaves of 4-weeks-old Arabidopsis plants were sprayed with a solution of 0.015% (vol/vol) Silwet L-77 (GE Healthcare, Beijing, China) containing the indicated concentrations of kinetin (Biosharp, Shanghai, China) for 3 days, three times per day. The plants were covered with transparent hoods immediately after their spraying, to retain the humidity. Mock treatments were sprayed with a solution containing only 0.015% (vol/vol) Silwet L-77.
Quantification of Cytokinins (CKs)
After the B. cinerea and mock treatments were completed, the leaves were collected and frozen in liquid nitrogen. Their CKs were then measured as described previously (Liu et al., 2010).
Results
Regulation of CK-Related Genes by B. cinerea Infection
According to the transcriptomic dataset from the microarray platform GENEVESTIGATOR2, CK-related genes were transcriptionally regulated after B. cinerea inoculation (Supplementary Figure S1). To confirm these changes, those highly regulated CK genes identified from the available microarray data were selected and analyzed via qRT-PCR for infected leaves at 14, 24, and 48 h post inoculation (hpi) of B. cinerea (Figure 1). The fungus and dying plant cells at the time-points 0, 12, 14, 24, and 48 hpi were detected with trypan blue (Supplementary Figure S2); spotty blue staining, indicative of dying cells, was visible at 12 hpi and became more pronounced at 14 hpi on infected leaves. The blue staining expanded rapidly, forming a much bigger lesion on leaves at 24 hpi. By 48 hpi, the fungus had grown more aggressively, with nearly half of each leaf now necrotic.
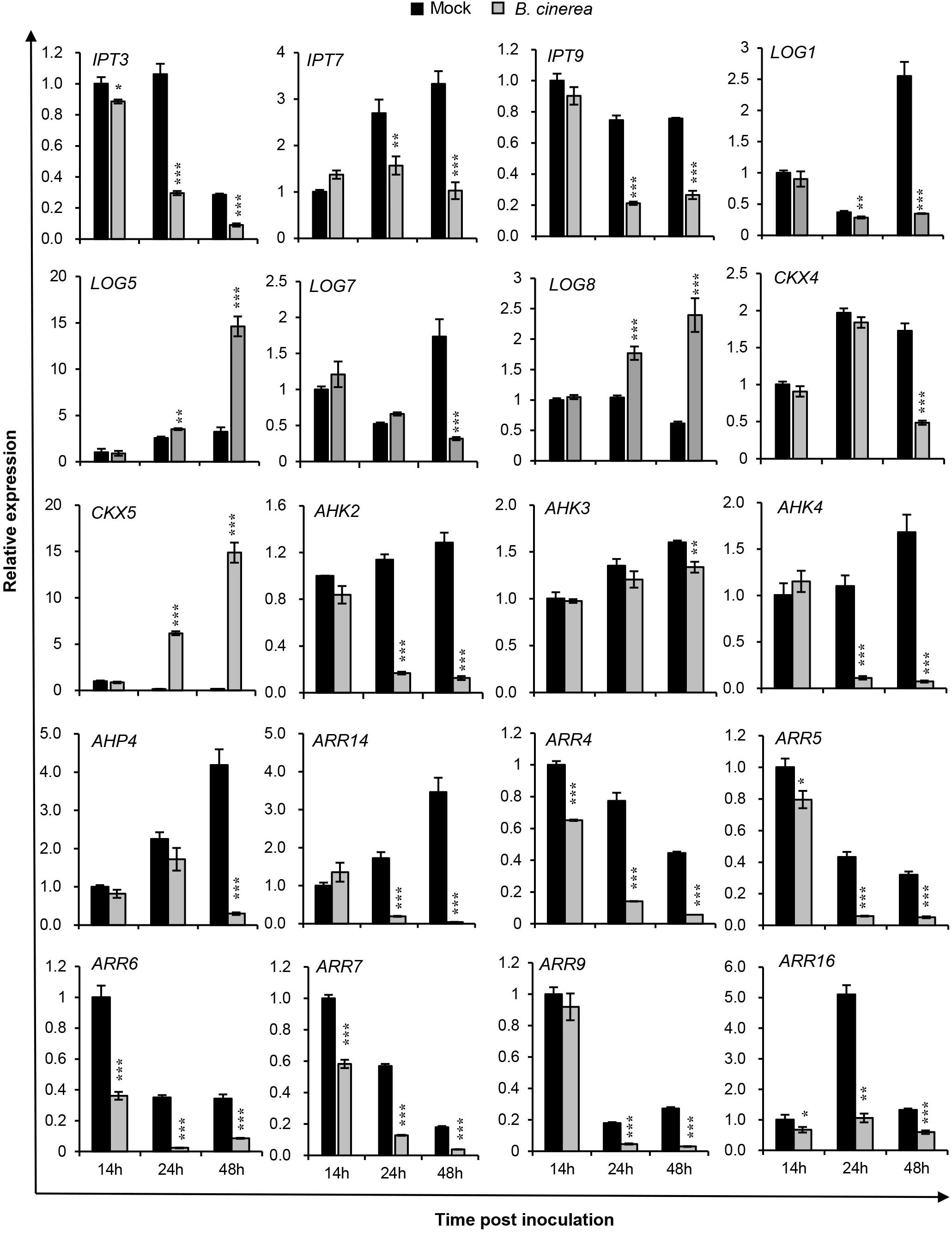
Figure 1. Expression profiles of selected cytokinin-related genes in Arabidopsis wild-type (Col-WT) plants after Botrytis cinerea inoculation. Transcript accumulations of cytokinin metabolism and signaling genes were determined by qRT-PCR in 4-weeks-old WT leaves inoculated with B. cinerea at different time-points, and likewise in corresponding leaves receiving the same amount of 1/2 PDB solution as the mock treatment. Expression levels in the mock leaves at 14 h were set to a value of 1. All data were normalized to the expression of EXP (At4g26410). Asterisks indicate significant differences between B. cinerea and mock-treated samples at the same time-point (two-tailed Student’s t-test: *P < 0.05, **P < 0.01, ***P < 0.001). Error bars are standard deviations (n = 3). Three independent experiments were performed with a similar outcome; results from one representative experiment are shown.
Figure 1 shows the transcript levels of selected CK genes involved in CK metabolism and signaling components that were differentially regulated by B. cinerea infection vs. the mock-treated leaves. Evidently, transcript levels of CK biosynthesis genes IPT3, IPT7, IPT9, LOG1, and LOG7 were strongly repressed from 24 hpi onward, while LOG5 and LOG8 expression levels were upregulated. For CKX4, a CK degradation enzyme gene, its expression was unchanged at 14 and 24 hpi, but reduced at 48 hpi; however, another CK degradation enzyme gene, CKX5, was significantly upregulated after B. cinerea inoculation, at levels 37-fold and 86-fold higher than mock treatments at 24 and 48 hpi, respectively. All three CK receptor genes were reduced in their expression. Compared with AHK3, the suppressed levels of AHK2 and CRE1/AHK4 were more pronounced following the B. cinerea treatment, suggesting their importance in the plant response to infection by this pathogen. The CK signaling genes—AHP4, the type-B response regulator gene ARR14, and the type-A Arabidopsis response regulator genes ARR4, ARR5, ARR6, ARR7, ARR9, and ARR16—were strongly repressed in the 48-h period since the B. cinerea inoculation. Among them, ARR4, ARR6, and ARR16 were regulated more rapidly, being strikingly suppressed at 14 hpi. These qRT-PCR results suggested that B. cinerea infection strongly affects CK levels in Arabidopsis plants.
Next we employed two reporter lines, ARR5:GUS (β-glucuronidase) and CKX5:GUS, to verify the expression pattern of ARR5 and CKX5 in leaves infected with B. cinerea at different time-points (Figure 2). As shown in Figure 2A, in the mock-treated leaves, the activity of ARR5:GUS was mainly detected in their vascular bundle; at 14 hpi, the ARR5:GUS expression was still similar to mock-treated leaves. At 24 and 48 hpi, however, GUS activity clearly diminished in the leaf portion without necrosis, further confirming the repressed CK signaling in B. cinerea-infected leaves. For CKX5:GUS, very weak expression was observed in either the mock leaves (Figure 2B) or mock seedlings (Figure 2C), but CKX5:GUS expression was remarkably induced at the site in the leaves where B. cinerea was inoculated (indicated with arrowheads in Figures 2B,C). Later, at 24 and 48 hpi, GUS activity was very strong and appeared in nearly the entire leaf except its necrotic parts (Figure 2B). Besides those leaves inoculated with B. cinerea, CKX5:GUS also showed very high expression in the distant leaves of the same plant that were not inoculated (Figure 2C), indicating that CKs’ degradation by CKX5 occurred in a systemic manner following B. cinerea infection.
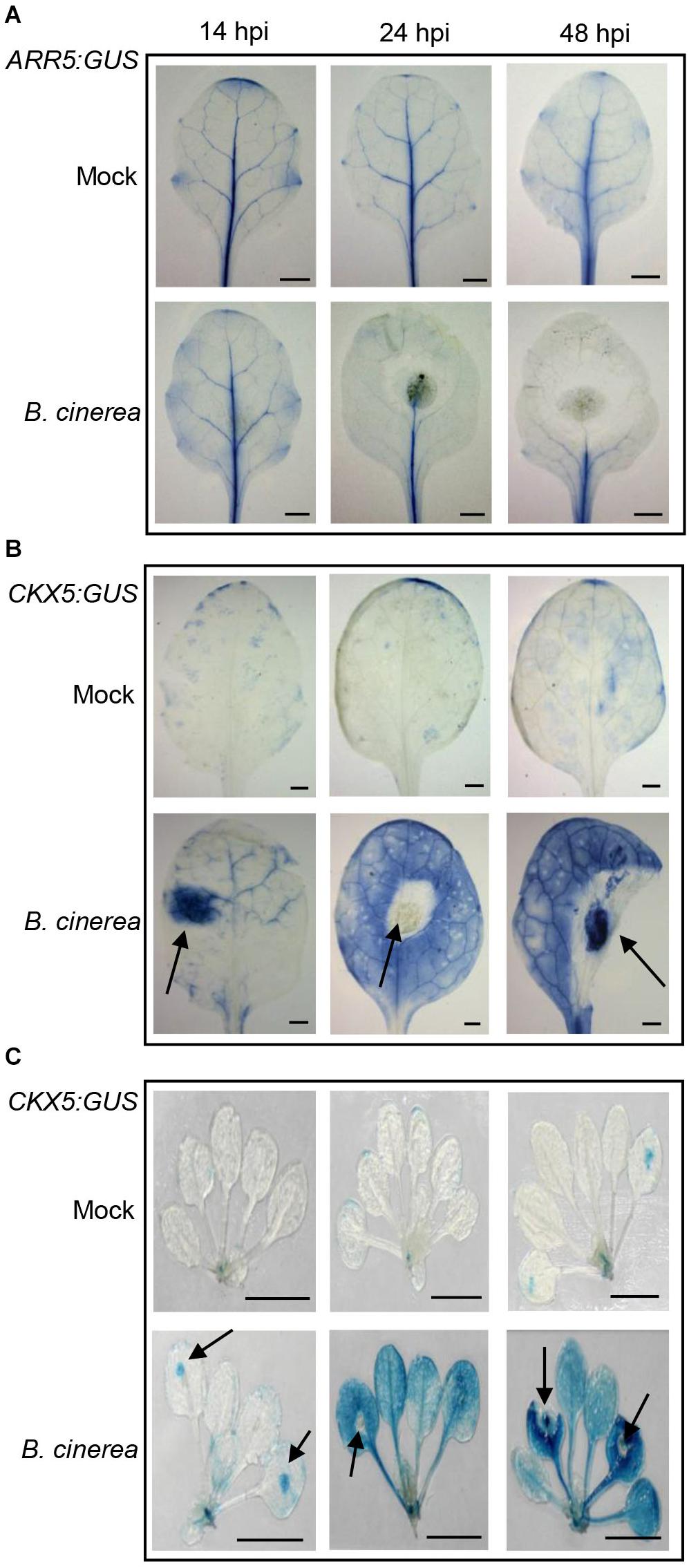
Figure 2. Expression of ARR5:GUS and CKX5:GUS after Botrytis cinerea inoculation. Expression of ARR5:GUS (A) and CKX5:GUS (B) in infected leaves at different time-points (hours) following the drop-inoculation (hpi) with B. cinerea. For mock treatment, the same amount of 1/2 PDB solution was applied. (C) Expression of CKX5:GUS in whole shoots after B. cinerea infection. Representative images are shown. For each treatment at each time-point, at least 10 plants were analyzed; 4 μL of 2.5 × 105 spores mL–1 of B. cinerea were applied to the 5th–7th leaves of each plant. Leaves were detached from the shoot for photographing. The arrowheads in (B,C) point to the inoculation sites of B. cinerea. Scale bars = 1 cm.
CK Levels Are Regulated by B. cinerea Infection
To characterize how the endogenous cytokinin production could be affected during B. cinerea infection, the biological active CKs—isopentenyladenine (iP), trans-zeatin (tZ), cis-zeatin (cZ), and their ribosides (iPR, tZR, cZR)—were quantified for leaves inoculated with B. cinerea at indicated time-points (Figure 3 and Supplementary Table S1). As Figure 3 shows, compared with mock treatments, the iP content was rapidly induced to higher level in leaves post-B. cinerea inoculation. By contrast, the content of riboside-type iPR was significantly reduced at both 24 and 48 hpi. Similarly, the tZ concentration increased considerably in B. cinerea-infected leaves whereas the amount of tZR quickly decreased after B. cinerea infection. However, unlike the iP and tZ types, both cZ and cZR levels were dramatically elevated at 24 and 48 hpi. In mock-treated leaves, the level of cZ was too low to be detected, but it did reach ca. 0.72 ng/g fresh weight of B. cinerea-infected leaves at 48 hpi. Concerning cZR, the B. cinerea application increased its levels by approximately 85% at 24 hpi and 500% at 48 hpi. Compared with the iP and tZ types, the sum of cZ and cZR in B. cinerea-infected leaves rose to 10 times that of mock treated leaves by 48 hpi. The prominent alteration of cZ-type CK levels suggested a crucial role of cZ-type CK in the interaction between Arabidopsis and B. cinerea.
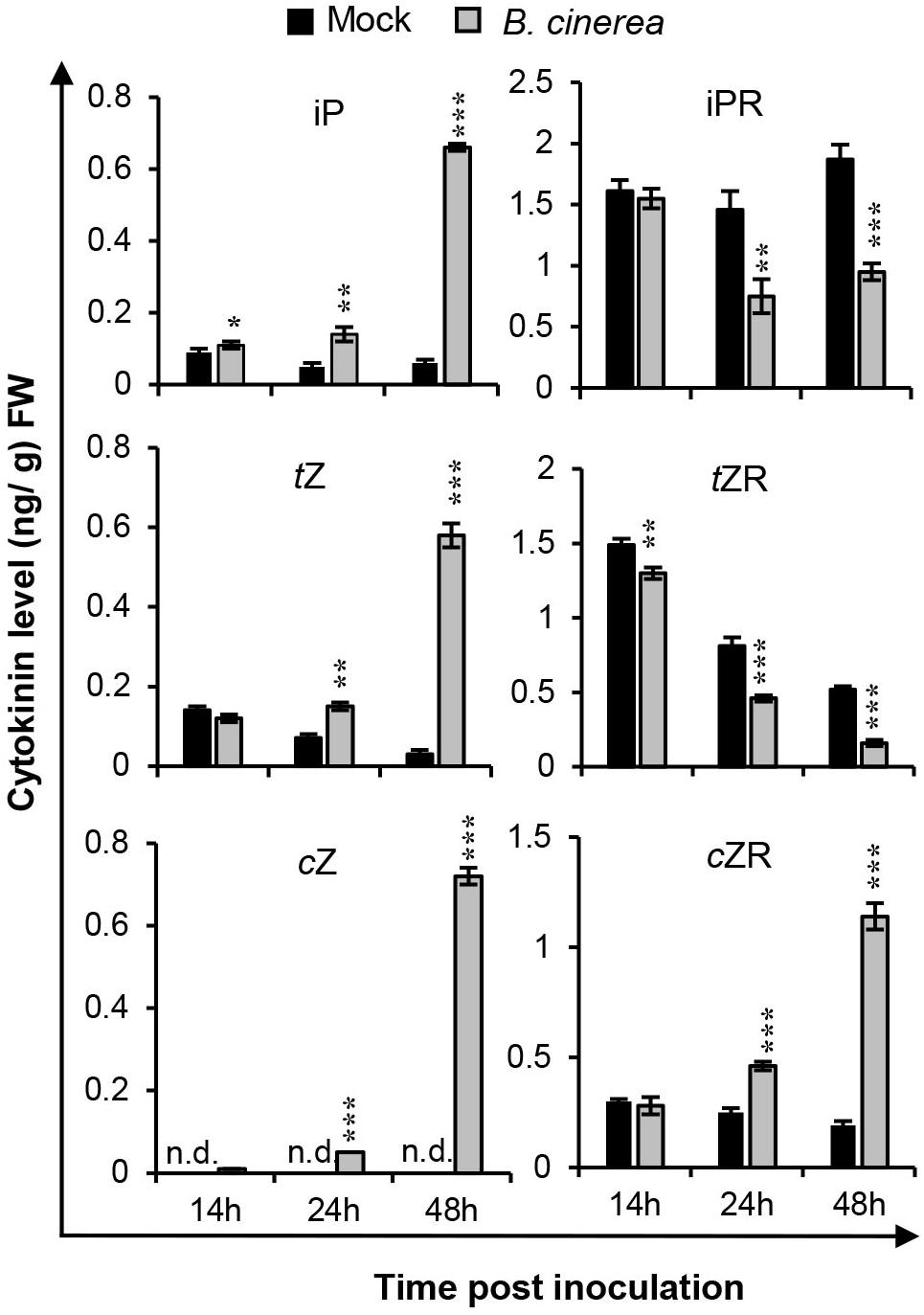
Figure 3. Cytokinin levels were changed after Botrytis cinerea infection. Isopentenyladenine (iP), isopentenyladenosine (iPR), trans-zeatin (tZ), trans-zeatin riboside (tZR), cis-zeatin (cZ), and cis-zeatin riboside (cZR) levels were measured in leaves of 4-weeks-old wild-type plants at different time-points after the B. cinerea inoculation or 1/2 PDB treatment (mock). Significant differences between B. cinerea and mock-treated samples at the same time-point were analyzed by two-tailed Student’s t-test. *P < 0.05; **P < 0.01; ***P < 0.001. Error bars are standard deviations. Three independent experiments were performed with similar results. FW, fresh weight.
Cytokinin Status Affects Arabidopsis Sensitivity to B. cinerea
To further confirm the importance of CK in the plant–pathogen interaction between Arabidopsis and B. cinerea, CK double-receptor mutants (cre1 ahk2 and cre1 ahk3) were inoculated with B. cinerea spores. Disease symptoms were recorded 48 h post-inoculation by measuring their lesion size (diameter) and fungal development with real-time PCR analysis (Figure 4). These results revealed larger lesions and enhanced fungal growth in cre1 ahk2 and cre1 ahk3 mutants than the wild-type, indicating that those plants with repressed CK signaling were more susceptible to B. cinerea.
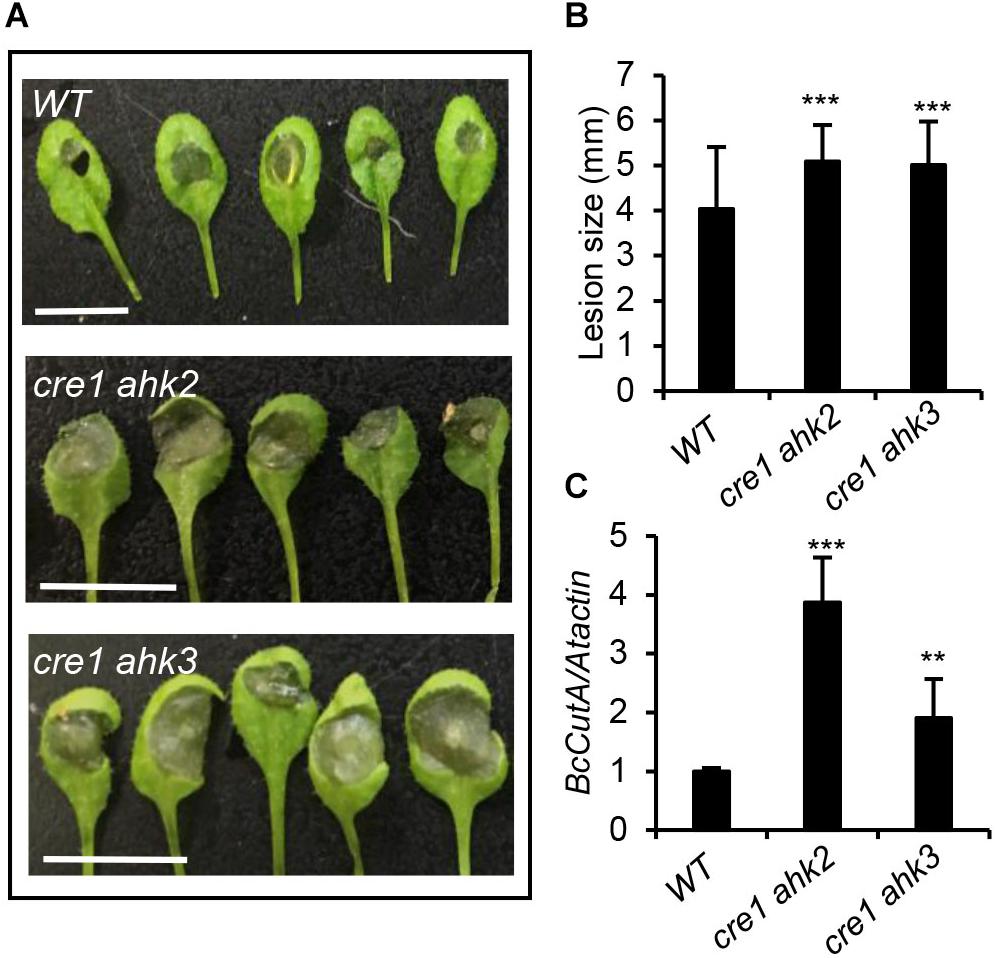
Figure 4. Cytokinin double-receptor mutants were more susceptible to Botrytis cinerea infection. (A) Disease symptoms in 4-weeks-old wild-type (WT) and cytokinin double-receptor mutants (cre1 ahk2 and cre1 ahk3) after infection with B. cinerea. Inoculated leaves were detached for photographing at 2 days post inoculation (dpi). Scale bars = 1 cm. (B) Disease lesion size in the indicated genotypes. The values shown are the means ± SD (n = 50 inoculated leaves). (C) The qPCR analysis of B. cinerea biomass in the leaves of indicated Arabidopsis genotypes. Data shown are the mean values ± SD (n = 3 biological replicates). In (B,C), mean values with statistically significant differences are indicated by asterisks (two-tailed Student’s t-test: **P < 0.01; ***P < 0.001).
Then, a series of concentrations of exogenous kinetin (KT, a type of cytokinin) were sprayed onto 4-weeks-old Arabidopsis leaves, for 3 days, before their inoculation with B. cinerea, followed by an assessment of disease symptoms 2 days later. Results in Figure 5A clearly illustrate that plants pre-treated with concentrations from 5 to 50 μM KT developed significantly less-severe disease symptoms than did mock-treated plants, suggesting that enhanced resistance of Arabidopsis to B. cinerea could be induced by a CK pre-treatment. To investigate whether exogenous CK application could change this susceptibility by influencing the JA/ET and SA pathways, expressions of JA/ET, SA biosynthesis genes and JA/ET-, SA-responsive genes were tested via qRT-PCR in the leaves infected with B. cinerea, with or without a 10-μM KT pre-treatment. As Figure 5B reveals, LOX2 (LIPOXYGENASE 2), encoding a key enzyme in the octadecanoid pathway leading to JA biosynthesis, and ACS6 (ACC Synthase 6), the gene product of which functions in the biosynthesis of the ethylene precursor aminocyclopropane carboxylase (ACC), were both transcriptionally upregulated by the KT pre-treatment. Further, ETHYLENE RESPONSE FACTOR1 (ERF1) and OCTADECANOID-RESPONSE ARABIDOPSIS 59 (ORA59) function as integrators of JA and ET signaling in plant defense responses (Pre et al., 2008), in which PLANT DEFENSIN1.2 (PDF1.2) acts downstream of ERF1/ORA59 and operates as part of the JA-ET-responsive antifungal defense (Spoel et al., 2003). For all three genes, their expression levels were strongly increased after applying the KT pre-treatment (Figure 5C). Taken together, these results indicated that an exogenous CK pre-treatment could promote JA/ET’s biosynthesis and response, pointing to potential crosstalk between the CK pathway and JA/ET in plant immunity. However, as seen in Figure 5D, ICS1 (isochorismate synthase 1), encoding a key enzyme in SA production, and PR1 (PATHOGENESIS RELATED PROTEIN 1), the SA-responsive marker gene, were both not significantly regulated by the KT pre-treatment, whereas PAD4 (PHYTOALEXIN DEFICIENT 4), a lipase-like gene important for SA signaling, was transcriptionally repressed. Since the role of PAD4 in resistance to B. cinerea might be more complicated than that depending on its role as regulator of SA signaling pathways (Nandi et al., 2005), more work will be necessary to show CK-SA crosstalk in the plant immunity to B. cinerea.
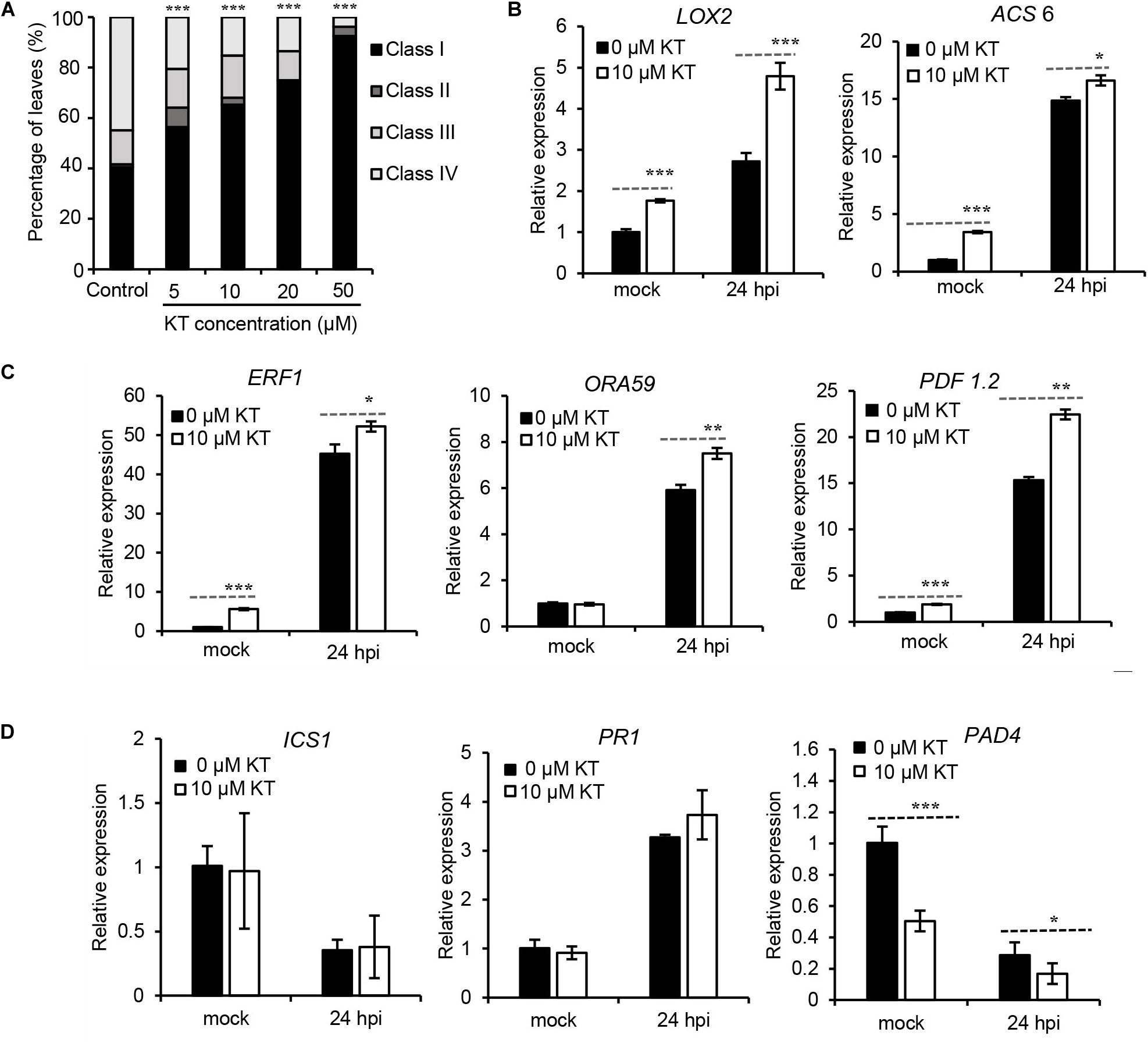
Figure 5. Pre-treatment of kinetin increased Arabidopsis resistance to Botrytis cinerea and transcript levels of JA/ET related genes. (A) Disease symptoms of leaves from 4-weeks-old Arabidopsis pre-treated with different concentrations of kinetin (KT) for 3 days before 2 days B. cinerea infection. Class I, lesion < 2 mm; Class II, 2 mm lesion plus chlorosis; Class III, lesion 2–4 mm plus chlorosis; Class IV, lesion > 4 mm plus chlorosis. The distribution at each kinetin concentration was calculated from 50 leaves. The significance of differences was analyzed by χ2-test. ***P < 0.001. (B) Transcript levels of the JA biosynthesis gene LOX2 and ET biosynthesis gene ACS6, in response to B. cinerea infection following prior treatment with 0 or 10 μM of KT. (C) Differential expression of plant immunity JA/ET-responsive genes ERF1, ORA59, and PDF1.2 in response to B. cinerea infection following the 0 or 10 μM KT treatment. (D) Transcript levels of SA biosynthesis gene ICS1, SA-responsive marker gene PR1, and SA signaling gene PAD4, in response to B. cinerea infection following prior treatment with 0 or 10 μM of KT. In (B–D), expression levels in the mock leaves at 24 h post-inoculation (hpi) with 0 μM KT pre-treatment were set to a value of 1. Error bars are standard deviations (n = 3). Mean values with statistically significant differences are indicated by asterisks (two-tailed Student’s t-test: *P < 0.05; **P < 0.01; ***P < 0.001). Three independent experiments were performed with a similar outcome; results from one representative experiment are shown.
JA and ET Affect CK Levels and Signaling Differentially in the Interaction Between Arabidopsis and B. cinerea
To investigate how JA/ET may influence CK levels or signaling in the Arabidopsis response to B. cinerea, expression levels of CK-related genes and CK contents in B. cinerea-infected leaves were analyzed in the JA biosynthesis mutant jar1-1 and the ET-insensitive mutant ein2-1 (Figures 6, 7). As shown in Figure 6A, the jar1-1 mutant’s leaves showed more pronounced transcript accumulations of AHK2, AHK3, and CRE1/AHK4, ARR5, ARR16, and ARR17 at 24 hpi compared with the wild type, suggesting that impaired JA biosynthesis released CK signaling in Arabidopsis. Higher transcript levels of CK biosynthesis gene IPT7, LOG1, and LOG5, coupled to a lower transcript level of the CK oxidase gene CKX5, indicated that CKs’ contents might be reduced in jar1-1. Figure 6B and Supplementary Table S2 report the contents of iP, iPR, tZ, tZR, tZ7G (trans-zeatin N7-glucoside), and cZR in the wild-type and infected leaves of jar1-1; compared to the former plants, the jar1-1 leaves at 24 hpi of B. cinerea had substantially higher levels of iP, iPR, and tZ, whereas no differences were observed for tZR, tZ7G, and cZR. These results suggested a possibly negative role of the JA pathway acting on CK biosynthesis and signaling during B. cinerea infection.
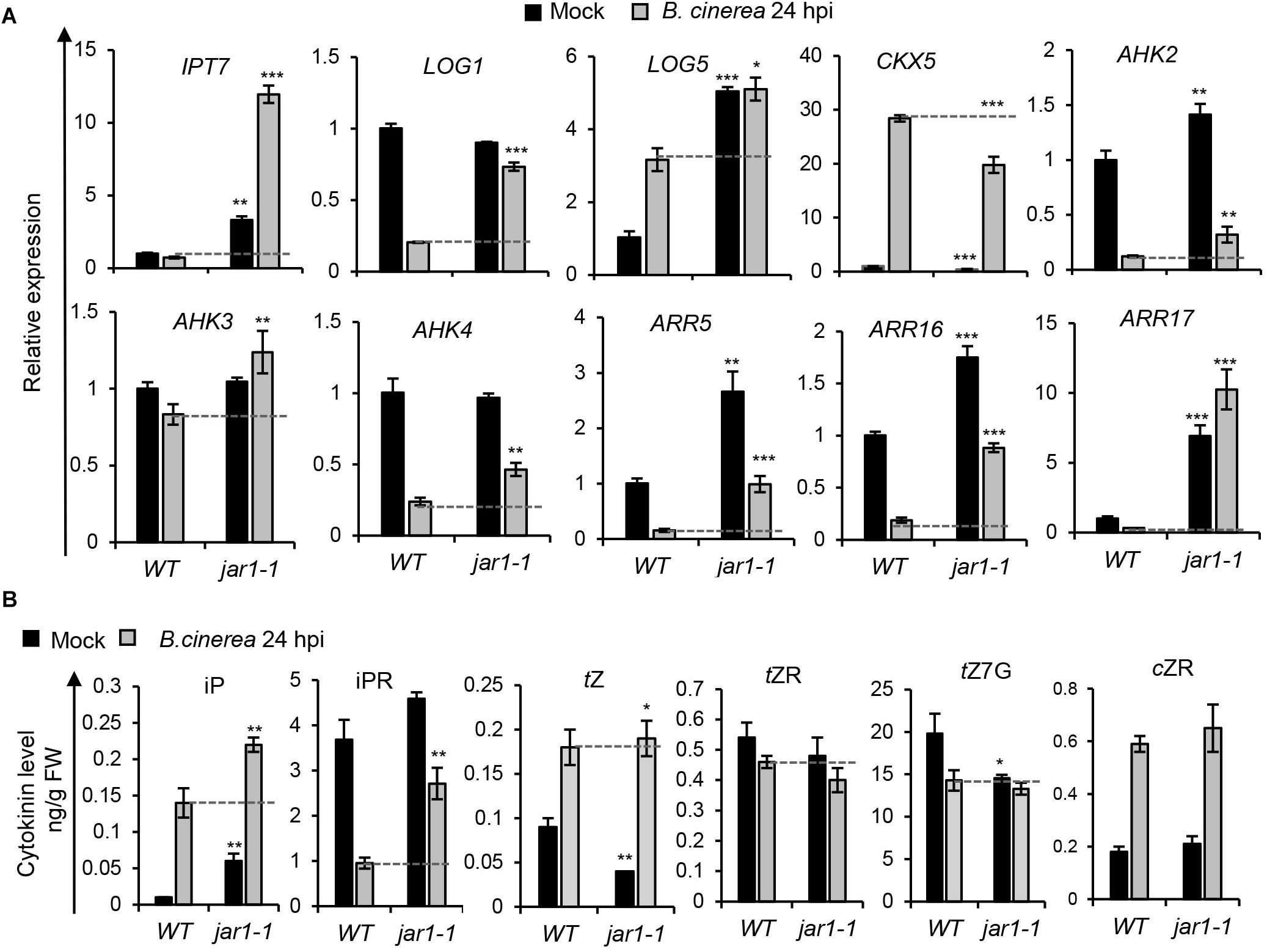
Figure 6. Botrytis cinerea infection induced cytokinin levels and signaling were regulated in the JA biosynthesis mutant. (A) Transcript levels of cytokinin-related genes were analyzed in leaves of the wild-type (WT) and jar1-1 at 24 h post-inoculation (hpi) with B. cinerea or mock solution. The expression values in WT leaves inoculated with mock solution were set to 1. All data were normalized to the expression of EXP (At4g26410). Error bars represent means ± SD (n = 3). Three independent experiments were performed with a similar outcome; results from one representative experiment are shown. Asterisks indicate significant differences between WT and mutants with same treatments (two-tailed Student’s t-test, *P < 0.05; **P < 0.01; ***P < 0.001). (B) Cytokinin contents in leaves of WT and jar1-1 at 24 hpi of B. cinerea or mock solution. Isopentenyladenine (iP), isopentenyladenosine (iPR), trans-zeatin (tZ), trans-zeatin riboside (tZR), trans-zeatin N7-glucoside (tZ7G), and cis-zeatin riboside (cZR) levels were measured. Bars represent means ± SD (n = 3). Three independent experiments were performed. Asterisks indicate significant differences between WT and mutants for the same treatment (two-tailed Student’s t-test, *P < 0.05; **P < 0.01). FW, fresh weight.

Figure 7. Cytokinin contents and signaling induced by Botrytis cinerea were changed in the ET mutant. (A) Transcript levels of cytokinin-related genes were measured in leaves of the wild-type (WT) and ein2-1 at 24 h post-inoculation (hpi) of B. cinerea or mock solution. Expression values in WT leaves inoculated with the mock solution were set to a value of 1. All data were normalized to the expression of EXP (At4g26410). Bars are the means ± SD (n = 3). Three independent experiments were performed with a similar outcome; results from one representative experiment are shown. Asterisks indicate significant differences between WT and mutant plants with same treatments (two-tailed Student’s t-test, **P < 0.01; ***P < 0.001). (B) Cytokinin contents in leaves of WT and ein2-1 at 24 hpi of B. cinerea or mock solution. Isopentenyladenine (iP), isopentenyladenosine (iPR), trans-zeatin (tZ), trans-zeatin riboside (tZR), trans-zeatin N7-glucoside (tZ7G), and cis-zeatin riboside (cZR) levels were measured. Error bars represent means ± SD (n = 3). Three independent experiments were performed. Asterisks indicate significant differences between WT and mutant plants under the same treatment (two tailed Student’s t-test, **P < 0.01; ***P < 0.001). FW, fresh weight.
The effect of mutation in the ET pathway on CK levels and signaling are shown in Figure 7. Evidently, CK metabolism-related genes were affected by the ET pathway (Figure 7A). Specifically, at 24 hpi, the abundance of IPT7 transcript was slightly lower in the ein2-1 mutant than the wild type in the absence of B. cinerea infection; LOG5 and LOG8 transcript levels were strongly repressed in ein2-1 leaves upon challenging them with B. cinerea; and the degradation gene CKX5 was expressed much higher in leaves of ein2-1 than in those of wild-type plants when inoculated with B. cinerea, together suggesting that CK contents might be suppressed in ein2-1 after B. cinerea infection. The responses of CK-signaling genes to B. cinerea infection were also changed in the ein2-1 mutant. Compared with the wild type’s infected leaves, AHK4 and ARR16 transcript levels were significantly repressed and ARR5’s expression was much higher in the infected ein2-1 leaves (Figure 7A). These results suggested a more complex regulation mechanism underpinning the ET pathway’s influence on CK signaling during B. cinerea infection.
We next measured the CKs’ contents at 24 hpi in leaves of wild-type and ein2-1 mutant inoculated with B. cinerea or the mock treatment. As shown in Figure 7B and Supplementary Table S2, in locally infected leaves of the ein2-1 mutant, their iP, tZ, tZR, and cZR contents were dramatically decreased compared with the wild type. Furthermore, without B. cinere applied, the iPR content of the ein2-1 mutant was lower than in the wild type, indicating that mutation in the ET pathway repressed CK biosynthesis.
Combining the Figure 3 results, which demonstrated that cZ-type CK contents were strongly elevated after B. cinerea infection, with those of Figures 6B, 7B, it appears that cZR accumulation was strongly suppressed in the ein2-1 mutant but negligibly changed in the jar1-1 mutant. Expression levels of the genes responsible for cZ-type CK metabolism were further analyzed by qRT-PCR in wild-type plants and ein2-1 mutant, with or without B. cinerea infection, at 24 hpi (Figure 8). This revealed that while transcript levels of IPT9 and CKX1 were slightly reduced after B. cinerea inoculation in the ein2-1 mutant, its CKX7’s expression level increased remarkably. This suggested that the reduction of cZR contents in ein2-1 mutant might be driven mainly by up-regulation of CKX7.
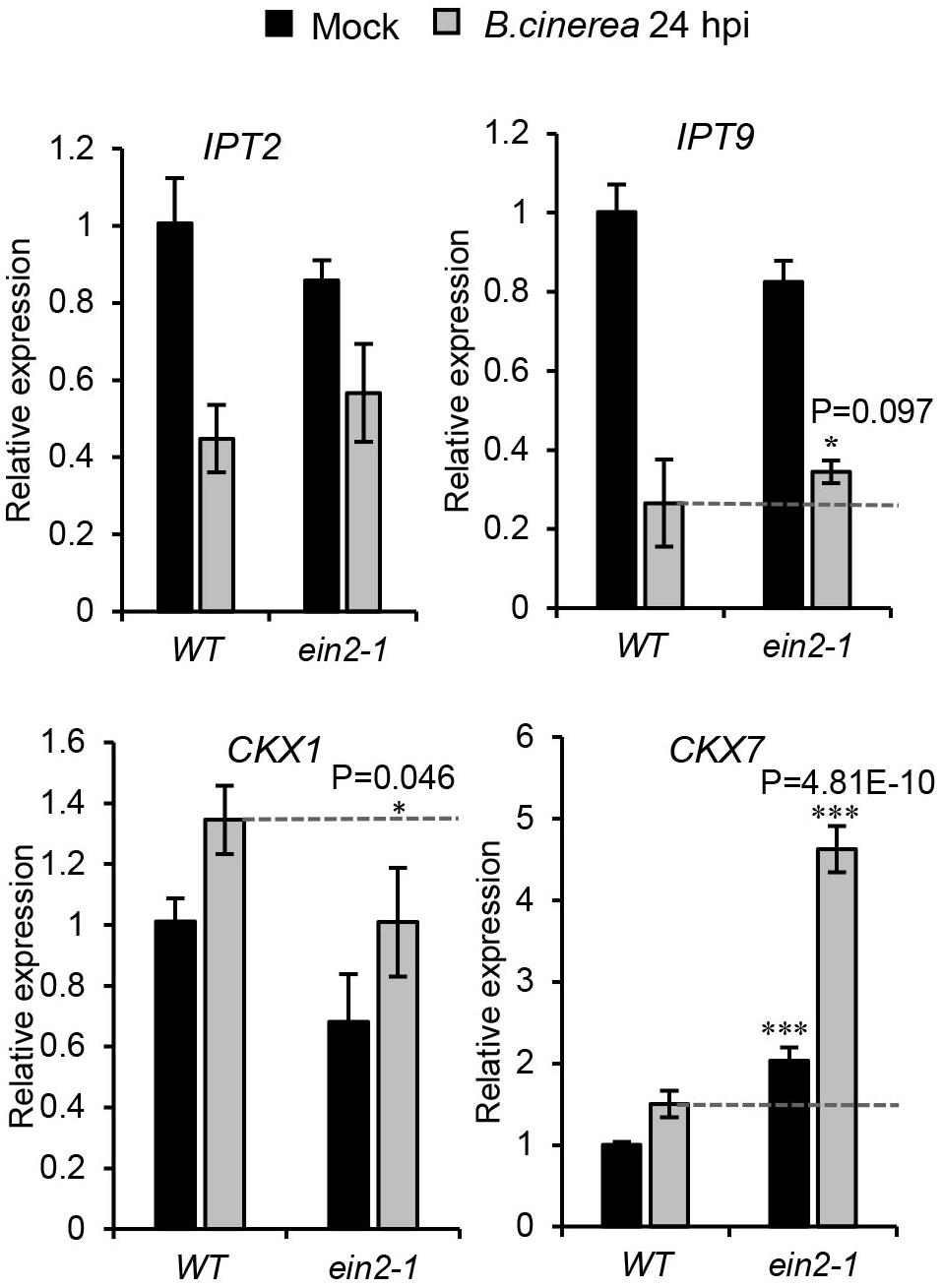
Figure 8. Expression profiles of genes related to cis-zeatin-type cytokinins’ biosynthesis and metabolism in the wild-type (WT) and ein2-1 leaves drop-inoculated with or without Botrytis cinerea spores. Leaves were harvested at 24 h after treatments. The expression values in WT leaves with mock treatment were set to a value of 1. All data were normalized to the expression of EXP (At4g26410). Bars are the mean ± SD from three independent experiments. Asterisks indicate significant differences between WT and mutant plants with the same treatments (two tailed Student’s t-test, *0.01 < P < 0.05; ***P < 0.001).
Discussion
In this paper, we addressed the responsiveness of CKs to B. cinerea infection in Arabidopsis. We showed that the CK pathway did respond to B. cinerea infection and preliminarily demonstrated its interactions with the JA and ET pathways. These results indicated that CKs are plant components crucially involved in their responses to the necrotrophic pathogen B. cinerea.
The CK Pathway Responds to B. cinerea Infection
Several transcriptomics analyses of Arabidopsis treated with B. cinerea have shown that transcripts of CK-related genes are potentially regulated by B. cinerea infection (Argueso et al., 2012; Birkenbihl et al., 2012; Zhang et al., 2017). Here, we confirmed that B. cinerea infection could alter the contents of active CKs (iP, iPR, tZ, tZR, cZ, and cZR) in locally infected Arabidopsis leaves, accompanied by significant alterations of transcript levels of many genes involved in CK metabolism and signaling. We found that, from 24 hpi onward, the B. cinerea-infected leaves partly decayed and their contents of active cis-Zeatin-type CKs (cZ and cZR) were dramatically increased, indicating a key role of cZ-type CKs in how Arabidopsis plants respond to B. cinerea infection (Figure 3). The cisZ-type CKs are thought to act as sensitive regulators of CK responses in plants under growth-limiting conditions and are able to modulate plant defense responses (Gajdošová et al., 2010; Schäfer et al., 2015a). Several pathogens have been identified which produce cZs to modulate their hosts’ physiology for their own benefits (Schäfer et al., 2015a; Han and Kahmann, 2019). Besides pathogens, there is also evidence that cZ-type CKs are also involved in plant–herbivore interactions; for example, Manduca sexta and Tupiocoris notatus herbivory has been shown to increase the levels of cZ-type CKs in N. attenuata (Schäfer et al., 2015c; Brütting et al., 2018). Furthermore, applying cZR to N. attenuate leaves increased MeJA-mediated induction of defense metabolites, such as those associated with the phenolamide pathway and trypsin proteinase inhibitor activity, suggesting that cZs are potentially involved in defense metabolite accumulation after herbivore attack (Schäfer et al., 2015a). Since B. cinerea cannot produce CKs (Cooper and Ashby, 1998; Walters and McRoberts, 2006), the significant increase in levels of cZ-type CKs we found in the infected Arabidopsis leaves (Figure 3) led us to suppose that, to protect themselves, plants might manipulate the content of cZ-type CKs to delay the leaf senescence and defend against B. cinerea attacks (Swartzberg et al., 2008). Further experiments that use Arabidopsis plants with altered cZ-type CKs levels, for example, via impaired cZ-biosynthesis (ipt2 9 mutants, Miyawaki et al., 2006) or increased cZ-degradation (AtCKX7 overexpression, Köllmer et al., 2014) or an overproduction of cZ (via overexpression of cZ biosynthesis gene AtIPT2), are needed to elucidate the role of cZ-type CKs in Arabidopsis responses to B. cinerea.
Connecting specific alterations in transcript levels of genes involved in CK metabolism with concomitant alterations in CK contents upon the infection of B. cinerea is an intricate task. Since CKs have essential roles in leaf senescence, apoptosis, immunity, and complicated forms with differentiated functions (reviewed by Kieber and Schaller, 2014), during Arabidopsis–B. cinerea interactions, CK metabolism might be manipulated by the pathogen and the host in different ways for their own benefit. The transcript levels of CK-related genes could be influenced by pathogen effectors (Hann et al., 2014; Kazan and Lyons, 2014), other induced phytohormones involved in the plant defense response (Naseem et al., 2014), and tissue disruption and CK-mediated feedback regulation (Brenner et al., 2012). More genetic and biochemical experiments are needed to clearly address how CK contents and signaling are changed during the process of B. cinerea infection of plants.
CK Cross Talk With JA and ET Pathways After B. cinerea Infection
CKs can intercommunicate with other phytohormones, including auxins, abscisic acid, and gibberellins, in the modulation of plants’ development and adaptation to stress (Seif El-Yazal et al., 2015). Defense responses to attacks by necrotrophic pathogens and herbivorous insects are considered as mainly mediated by the JA pathway acting together with ET in a coordinated manner. A few reports have investigated CK-JA interplay in plants under conditions of wounding and herbivory (Sano et al., 1996; Dervinis et al., 2010; Schäfer et al., 2015b,c), yet little is known about the possible CK-JA cross talk occurring in plant defense upon challenge with pathogens. As to the interaction between CK and the ET pathway, the correlation between CK and ET in root development or plant response to light or cold stress has been demonstrated (Shi et al., 2012; Zdarska et al., 2015; Liu J. et al., 2017), but how CK and ET interact under biotic stress conditions, including necrotroph infection, is not clear. Here we found that a pre-treatment with kinetin could strongly elevate the transcript levels of JA/ET biosynthesis genes as well as JA/ET response genes (Figures 5B,C), suggesting the positive effect of CKs on the JA/ET pathway. This could explain the Arabidopsis resistance to B. cinerea induced by the exogenous kinetin pre-treatment, which agrees with the reports that the senescence-inducible expression of a CK biosynthesis gene, IPT, could suppress B. cinerea-induced disease symptoms. The effects of kinetin upon the JA pathway in our study are also consistent with the findings of positive effects of CKs on JA levels and JA-mediated defenses to herbivores (Dervinis et al., 2010; Schäfer et al., 2015b).
Furthermore, we investigated the effects of JA and ET pathways on CK signaling and levels upon B. cinerea challenge at the 24-hpi time-point (Figures 6, 7). In the JA biosynthesis mutant, jar1-1, transcript levels of genes involved in CK signaling (including the three CK receptor genes and type-A ARR genes) were much higher in locally B. cinerea-infected leaves than that in mock leaves, which indicated that the repressed JA response could release the CK signaling. Accordingly, we speculated that during B. cinerea infection, the JA pathway may negatively affect CK signaling. The transcript levels of CK metabolism genes in the jar1-1 mutant upon pathogen attack could be associated with changed CK levels (Figure 6). CKs contents in the jar1-1 mutant during B. cinerea infection (Figure 6B) indicate a suppressive effect of JA on the iP, iPR, and tZ accumulation. JAs have been shown to counteract activities of CKs more generally than what our data here suggest. JAs can reduce the transcripts of CK-responsive genes (Brenner et al., 2012), and their effects are opposite those of CKs on various physiological traits, such as senescence (Richmond and Lang, 1957; He et al., 2002), leaf growth, and cell division (Noir et al., 2013; Attaran et al., 2014). In our study, the cZR contents were similar between jar1-1 and the wild type after B. cinerea inoculation (Figure 6B), suggesting that the JA pathway is likely not responsible for the accumulation of cisZ-type CKs during B. cinerea infection in wild-type leaves. However, this contradicts the findings in N. attenuata, in which JA supplementation promoted the accumulation of cZR and the silencing of COI1, a receptor gene in JA signaling, strongly reduced cZR levels, in response to M. sexta oral secretions (Schäfer et al., 2015c). These data indicate that JA-CK cross talk behaves differently in Arabidopsis and N. attenuata.
Different from the situations in jar1-1, CK contents (iP, iPR, tZ, tZR, and cZR) were all significantly reduced, biosynthesis genes (IPT7, LOG5, LOG8) were downregulated, and the CK degradation gene CKX5 was upregulated in the infected leaves of ein2-1 when compared to wild-type leaves (Figure 7). These results suggest that an induced accumulation of CKs in response to B. cinerea infection depended on the ET pathway. Expression of the type-A response regulator gene ARR16 was strongly repressed, but the transcription of ARR5 was upregulated during B. cinerea infection in ein2-1, showcasing the complexity characterizing the regulation of CK signaling by the ET pathway. In root development, the ET signaling pathway involves different receptor clusters having multiple layers of complexity in cross talk with CKs (Liu J. et al., 2017). Ethylene can both positively and negatively regulate CK signaling respectively through ARR5 and ARR2. EIN3 negatively regulates ARR5 in Arabidopsis and downstream ET signaling can positively regulate the CK pathway, in turn modulating the expression of ARR5. In a different way, another ET receptor, ETR1, which has histidine kinase activity, can positively regulate general cytokinin signaling through ARR2, which upregulates CK oxidase. Most recently, ETR1 was shown to regulate CK signaling specifically in the root transition zone, presumably via regulation of ARR10, a positive regulators of the multistep phosphorelay (MSP) pathway, which differs from canonical CTR1/EIN2/EIN3 ethylene signaling and is independent of EIN2 (Zdarska et al., 2019). We find that JA and ET can regulate CK signaling and levels in a rather different manner after B. cinerea infection, though JA and ET pathway are reportedly able to also mediate plant defense responses in a synergistic way (AbuQamar et al., 2017).
Taken together, CK signaling and levels were modulated after B. cinerea infection. CK signaling was repressed, but cis-Zeatin-type CKs (cZ and cZR) contents were strongly increased in locally infected Arabidopsis leaves. Both the JA and ET pathways contribute to changed CK signaling and levels during B. cinerea infection, albeit differently. Based on our results, we propose that the JA pathway can adversely affect CK signaling and the production of iP, iPR, and tZ upon B. cinerea challenge, whereas the ET pathway affects the active CKs’ contents in a positive way following infection by this fungus (Figure 9). Clarifying how JA and ET pathways regulate the response of CKs to B. cinerea will provide new insights into these complex interactions occurring on the battlefield between plant and B. cinerea.
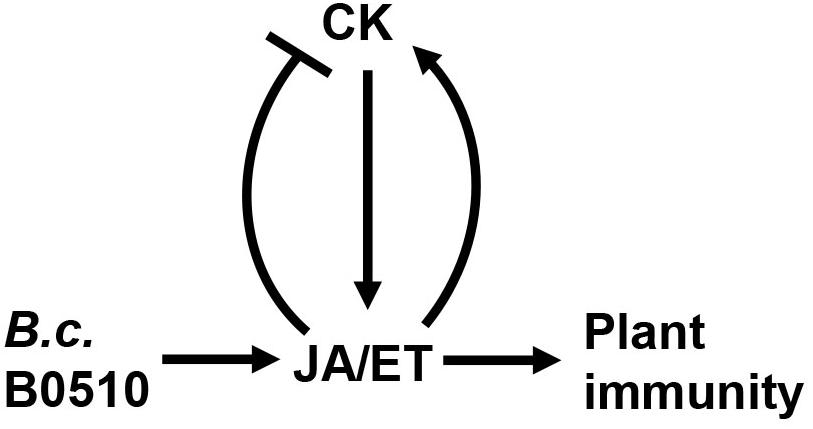
Figure 9. Proposed model showing how CK and JA/ET pathways interact during B. cinerea infection. Upon B. cinerea infection, JA and ET pathways are activated. CK promote resistance of Arabidopsis to B. cinerea by positively regulating JA and ET pathway. Conversely, JA negatively regulates CK signaling and content, while ET interacts positively with the CK pathway.
CK Cross Talk With the SA Pathway After B. cinerea Infection
The function of SA in immune responses to B. cinerea may vary, depending on the plant species. In tomato, applying SA significantly increases its resistance to B. cinerea (Angulo et al., 2015). By contrast, in Arabidopsis, an increase in SA level has either no or only affects resistance at the primary infection site (Ferrari et al., 2003). Tomato NahG plants overexpressing an SA hydroxylase to decrease SA levels in vivo showed high susceptibility to B. cinerea, while tobacco and Arabidopsis NahG plants responded similarly to the wild type to the same pathogen (Ferrari et al., 2003; Asai et al., 2010; El Oirdi et al., 2011). In plants, SA can antagonize the JA signaling pathway and vice versa. Since B. cinerea can produce an exopolysaccharide as an elicitor of the SA pathway, this activated SA pathway antagonizes the JA signaling pathway through NPR1, enabling the pathogen to promote disease development in tomato (El Oirdi et al., 2011). The CK pathway has been proven to have a role in SA-JA cross talk. ARR11, the B-type response regulator of CKs, has been described as a novel negative regulator of SA-JA cross talk, promoting its resistance against B. cinerea (Proietti et al., 2018). Moreover, CK can strengthen the immunity of Arabidopsis to hemi-biotrophs via ARR2, by interacting with the SA transcription factor TGA3 (Choi et al., 2010). In our study here, the KT pre-treatment did not significantly change the transcript levels of SA biosynthesis gene ICS1 or the SA-responsive marker gene PR1 during B. cinerea infection, but it suppressed the level of another SA signaling gene, PAD4 (Figure 5D). In tomato leaves, however, an external CK pre-treatment led to greater internal SA production and their increased resistance to B. cinerea due to CK pre-treatment being SA- and ET-dependent, but the changes in the JA content and pathway were not described (Gupta et al., 2020). Lower SA levels may be correlated with increased B. cinerea resistance in some cases in tomato (Angulo et al., 2015; Mehari et al., 2015), and Gupta et al. (2020) proposed that the severity of B. cinerea disease and the resultant increase in SA levels depend on both host and the pathogen isolate. In plant–B. cinerea interactions, the cross talk that occurs among the phytohormones CK, SA, and JA/ET seems highly complex and a puzzle that can only be solved with more concerted research efforts.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
LC designed the study. LC, BL, RW, and SW performed the experiments and analyzed the data. LC and JZ discussed the results and wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (grant no. 31800223) and the Natural Science Foundation of Hubei Province (grant no. 2018CFB637), both to LC.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. Baodong Cai at Wuhan University for the cytokinins’ measurement.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2021.584042/full#supplementary-material
Footnotes
References
AbuQamar, S., Moustafa, K., and Tran, L. S. (2017). Mechanism and strategies of plant defense against Botrytis cinerea. Crit. Rev. Biotechnol. 37, 262–274. doi: 10.1080/07388551.2016.1271767
Argueso, C. T., Ferreira, F. J., Epple, P., To, J. P., Hutchison, C. E., Schaller, G. E., et al. (2012). Two-component elements mediate interactions between cytokinin and salicylic acid in plant immunity. PLoS Genet. 8:e1002448. doi: 10.1371/journal.pgen.1002448
Angulo, C., Leyva, M. D. L. O., Finiti, I., López-Cruz, J., Fernández-Crespo, E., García-Agustín, P., et al. (2015). Role of dioxygenase α-DOX2 and SA in basal response and in hexanoic acid-induced resistance of tomato (Solanum lycopersicum) plants against Botrytis cinerea. J Plant Physiol. 175, 163–173. doi: 10.1016/j.jplph.2014.11.004
Asai, S., Mase, K., and Yoshioka, H. (2010). A key enzyme for flavin synthesis is required for nitric oxide and reactive oxygen species production in disease resistance. Plant J. 62, 911–924. doi: 10.1111/j.1365-313X.2010.04206.x
Attaran, E., Major, I., Cruz, J., Rosa, B., Koo, A., Chen, J., et al. (2014). Temporal dynamics of growth and photosynthesis suppression in response to jasmonate signaling. Phant Physiol. 165, 1302–1314. doi: 10.1104/pp.114.239004
Birkenbihl, R. P., Diezel, C., and Somssich, I. E. (2012). Arabidopsis WRKR33 is a key transcriptional regulator of hormonal and metabolic response toward Botrytis cinerea infection. Plant Physiol. 159, 266–285. doi: 10.2307/41496262
Brenner, W. G., Ramireddy, E., Heyl, A., and Schmülling, T. (2012). Gene regulation by cytokinin. Front. Plant Sci. 3:8. doi: 10.3389/fpls.2012.00008
Brütting, C., Crava, C. M., Schäfer, M., Schuman, M. C., Meldau, S., Adam, N., et al. (2018). Cytokinin transfer by a free-living mirid to Nicotiana attenuate recapitulates a strategy of endophytic insects. eLife 7:e36268. doi: 10.7554/eLife.36268
Chang, L., Ramireddy, E., and Schmülling, T. (2013). Lateral root formation and growth of Arabidopsis is redundantly regulated by cytokinin metabolism and signaling genes. J. Exp. Bot. 64, 5021–5032. doi: 10.1093/jxb/ert291
Chang, L., Ramireddy, E., and Schmülling, T. (2015). Cytokinin as a positional cue regulating lateral root spacing in Arabidopsis. J. Exp. Bot. 66, 4759–4768. doi: 10.1093/jxb/erv252
Choi, J., Huh, A. U., Kojima, M., Sakakibara, H., Paek, K. H., and Hwang, I. (2010). The cytokinin-activated transcription factor ARR2 promotes plant immunity via TGA3/APR1-dependent salicylic acid signaling in Arabidopsis. Dev. Cell 19, 284–295. doi: 10.1016/j.devcel.2010.07.011
Cooper, S. J., and Ashby, A. M. (1998). Comparison of cytokinin and cytokinin-O-glucoside cleaving beta-glucosidase production in vitro by Venturia inaequalis and other phytopathogenic fungi with differing modes of nutrition in planta. Physiol. Mol. Plant Pathol. 53, 61–72. doi: 10.1006/pmpp.1998.0171
Czechowski, T., Stitt, M., Altmann, T., Udvardi, M. K., and Scheible, W. R. (2005). Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol. 139, 5–17. doi: 10.1104/pp.105.063743
Dervinis, C., Frost, C. J., Lawrence, S. D., Novak, N. G., and Davis, J. M. (2010). Cytokinin primes plant responses to wounding and reduces insect performance. J. Plant Growth Regul. 29, 289–296. doi: 10.1007/s00344-009-9135-2
El Oirdi, M., Abd, E. I., Rahman, T., Rigano, L., Ei Hadrami, A., Rodriguez, M. C., et al. (2011). Botrytis cinerea manipulates the antagonistic effects between immune pathways to promote disease development in Tomato. Plant Cell 23, 2405–2421.
Ferrari, S., Plotnikova, J. M., De Lorenzo, G., and Ausubel, F. M. (2003). Arabidopsis local resistance to Botrytis cinerea involves salicylic acid and camalexin and requires EDS4 and PAD2, but no SID2, EDS5 or PAD4. Plang J. 35, 193–205. doi: 10.1046/j.1365-313x.2003.01794.x
Gachon, C., and Saindrenan, P. (2004). Real-time PCR monitoring of fungal development in Arabidopsis thaliana infected by Alternaria brassicicola and Botrytis cinerea. Plant Physiol. Bioch. 42, 367–371. doi: 10.1016/j.plaphy.2004.04.001
Gajdošová, S., Spíchal, L., Kamínek, M., Hoyerová, K., Novák, O., Dobrev, P. I., et al. (2010). Distribution, biological activities, metabolism, and the conceivable function of cis-zeatin-type cytokinins in plants. J. Exp. Bot. 62, 2827–2840. doi: 10.1093/jxb/erq457
Glazebrook, J. (2005). Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 43, 205–227. doi: 10.1146/annurev.phyto.43.040204.135923
Grosskinsky, D. K., Naseem, M., Abdelmohse, U. R., Plickert, N., Engelke, T., Griebel, T., et al. (2011). Cytokinins mediate resistance against Pseudomonas syringae in Tobacco through increased antimicrobial phytoalexin synthesis independent of salicylic acid signaling. Plant Physiol. 157, 815–830. doi: 10.1104/pp.111.182931
Gupta, R., Pizarro, L., Leibman-Markus, M., Marash, I., and Bar, M. (2020). Cytokinin response induces immunity and fungal pathogen resistance, and modulates trafficking of the PRR LeEIX2 in tomato. Mol. Plant Pathol. 21, 1287–1306. doi: 10.1111/mpp.12978
Guzmán, P., and Ecker, J. R. (1990). Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell 2, 513–523. doi: 10.2307/3869113
Han, X., and Kahmann, R. (2019). Manipulation of phytohormone pathways by effectors of filamentous plant pathogens. Front. Plant Sci. 10:822. doi: 10.3389/fpls.2019.00822
Hann, D. R., Domínguez-Ferreras, A., Motyka, V., Dobrev, P. I., Schornack, S., Jehle, A., et al. (2014). The Pseudomonas type III effector HopQ1 activates cytokinin signaling and interferes with plant innate immunity. New Phytol. 201, 585–598. doi: 10.1111/nph.12544
He, Y. H., Fukushige, H., Hildebrand, D. F., and Gan, S. S. (2002). Evidence supporting a role of jasmonic acid in Arabidopsis leaf senescence. Plant Physiol. 128, 876–884. doi: 10.1104/pp.010843
Hutchison, C. E., Li, J., Argueso, C., Gonzalez, M., Lee, E., Lewis, M. W., et al. (2006). The Arabidopsis histidine phosphotransfer proteins are redundant positive regulators of cytokinin signaling. Plant Cell 18, 3073–3087. doi: 10.2307/20076849
Jones, J. D., and Dangl, J. L. (2006). The plant immune system. Nature 444, 323–329. doi: 10.1038/nature05286
Kazan, K., and Lyons, R. (2014). Intervention of phytohormone pathways by pathogen effectors. The Plant Cell 26, 2285–2309. doi: 10.1105/tpc.114.125419
Köllmer, I., Novak, O., Strnad, M., Schmülling, T., and Werner, T. (2014). Overexpression of the cytosolic cytokinin oxidase/dehydrogenase (CKX7) from Arabidopsis causes specific changes in root growth and xylem differentiation. Plant J. 78, 359–371. doi: 10.1111/tpj.12477
Kuroha, T., Tokunaga, H., Kojima, M., Ueda, N., Ishida, T., Nagawa, S., et al. (2009). Functional analyses of LONELY GUY cytokinin-activating enzymes reveal the importance of the direct activation pathway in Arabidopsis. Plant Cell 21, 3152–3169. doi: 10.1105/tpc.109.068676
Li, N., Han, X., Feng, D., Yuan, D., and Huang, L. J. (2019). Signaling crosstalk between salicylic acid and ethylene/jasmonate in plant defense: do we understand what they are whispering? Int. Journal Mol. Sci. 20:671. doi: 10.3390/ijms20030671
Liu, J., Moore, S., Chen, C., and Lindsey, K. (2017). Crosstalk complexities between auxin, cytokinin and ethylene in Arabidopsis root development: from experiments to systems modeling, and back again. Mol. Plant 10, 1480–1496. doi: 10.1016/j.molp.2017.11.002
Liu, S., Ziegler, J., Zeier, J., Birkenbihl, R. P., and Somssich, I. E. (2017). Botrytis cinerea B05.10 promotes disease development in Arabidopsis by suppressing WRKY33-mediated host immunity. Plant Cell Environ. 40, 2189–2206. doi: 10.1038/sj.mn.7800198
Liu, Z., Wei, F., and Feng, Y. (2010). Determination of cytokinins in plant samples by polymer monolith microextraction coupled with hydrophilic interaction chromatography-tandem mass spectrometry. Anal. Methods 2, 1676–1685. doi: 10.1039/c0ay00334d
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C (T)) method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Mason, M. G., Mathews, D. E., Argyros, D. A., Maxwell, B. B., Kieber, J. J., Alonso, J. M., et al. (2005). Multiple type-B response regulators mediate cytokinin signal transduction in Arabidopsis. Plant Cell 17, 3007–3018. doi: 10.2307/3872426
Mehari, Z. H., Elad, Y., Rav-David, D., Graber, E. R., and Meller Harel, Y. (2015). Induced systemic resistance in tomato (Solanum lycopersicum) against Botrytis cinerea by biochar amendment involves jasmonic acid signaling. Plant Soil 395, 31–44.
Miyawaki, K., Tarkowski, P., Matsumoto-Kitano, M., Kato, T., Sato, S., Tarkowska, D., et al. (2006). Roles of Arabidopsis ATP/ADP isopentenyltransferases and tRNA isopentenyltransferases in cytokinin biosynthesis. Proc. Natl. Acad. Sci. U.S.A. 103, 16598–16603. doi: 10.1073/pnas.0603522103
Nandi, A., Moeder, W., Kachroo, P., Klessig, D. F., and Shah, J. (2005). Arabidopsis ssi2-conferred susceptibility to Botrytis cinerea is dependent on EDS5 and PAD4. Mol. Plant Microbe Interact. 18, 363–370. doi: 10.1094/MPMI-18-0363
Naseem, M., Wölfling, M., and Dandekar, T. (2014). Cytokinins for immunity beyond growth, galls and greenisland. Trend Plant Sci. 19, 481–484. doi: 10.1016/j.tplants.2014.04.001
Noir, S., Bömer, M., Takahashi, N., Ishida, T., Tsui, T. L., Balbi, V., et al. (2013). Jasmonate controls leaf growth by repressing cell proliferation and the onset of endoreduplication while maintain a potential stand-by mode. Plant Physiol. 161, 1930–1951. doi: 10.1104/pp.113.214908
Pertry, I., Václavíková, K., Depuydt, S., Galuszka, P., Spíchal, L., Temmerman, W., et al. (2009). Identification of Rhodococcus fascians cytokinins and their modus operandi to reshape the plant. Proc. Natl. Acad. Sci. U.S.A. 106, 929–934. doi: 10.1073/pnas.0811683106
Pham, J., Liu, J., Bennett, M. H., Mansfield, J. W., and Desikan, R. (2012). Arabidopsis histidine kinase 5 regulates salt sensitivity and resistance against bacterial and fungal infection. New Phytol. 194, 168–180. doi: 10.1111/j.1469-8137.2011.04033.x
Pre, M., Atallah, M., Champion, A., De Vos, M., Pieterse, C. M., and Memelin, J. (2008). The AP2/ERF domain transcription factor ORA59 integrates jasmonic acid and ethylene signals in plant defense. Plant Physiol. 147, 1347–1357. doi: 10.1104/pp.108.117523
Proietti, S., Caarls, L., Coolen, S., Van Pelt, J. A., Van Wees, S. C. M., and Pieterse, C. M. J. (2018). Genome-wide association study reveals novel players in defense hormone crosstalk in Arabidopsis. Plant Cell Environ. 41, 2342–2356. doi: 10.1111/pce.13357
Richmond, A. E., and Lang, A. (1957). Effect of kinetin on protein content and survival of detached Xanthium leaves. Science 125, 650–651. doi: 10.1126/science.125.3249.650-a
Sano, H., Seo, S., Koizumi, N., Niki, T., Iwamura, H., and Ohashi, Y. (1996). Regulation by cytokinins of endogenous levels of jasmonic and salicylic acids in mechanically wounded tobacco plants. Plant Cell Physiol. 37, 762–769. doi: 10.1093/oxfordjournals.pcp.a029011
Schäfer, M., Brütting, C., Gase, K., Reichelt, M., Baldwin, I., and Meldau, S. (2013). ‘Real time’ genetic manipulation: a new tool for ecological field studies. Plant J. 3, 506–518. doi: 10.1111/tpj.12301
Schäfer, M., Brütting, C., Meza-Canales, I. D., Großkinsky, D. K., Vankova, R., Baldwin, I. T., et al. (2015a). The role of cis-zeatin-type cytokinins in plant growth regulation and mediating responses to environmental interactions. J. Exp. Bot. 66, 4873–4884. doi: 10.1093/jxb/erv214
Schäfer, M., Meza-Canales, I. D., Brütting, C., Baldwin, I. T., and Meldau, S. (2015b). Cytokinin concentrations and Chasedomain containing his kinase 2 (NaCHK2)- and NaCHK3-mediated perception modulate herbivory-induced defense signaling and defenses in Nicotiana attenuata. New Phytol. 207, 645–658. doi: 10.1111/nph.13404
Schäfer, M., Meza-Canales, I. D., Navarro-Quezada, A., Brütting, C., Vankova, R., Baldwin, I. T., et al. (2015c). Cytokinin levels and signaling respond to wounding and the perception of herbivore elicitors in Nicotiana attenuata. J. Integr. Plant Biol. 57, 198–212. doi: 10.1111/jipb.12227
Seif El-Yazal, S. A., Seif El-Yazal, M. A., Dwidar, E. F., and Rady, M. M. (2015). Phytohormone crosstalk research: cytokinin and its crosstalk with other phytohormones. Curr. Protein Pept. Sci. 16, 395–405. doi: 10.2174/1389203716666150330141159
Shi, Y., Tian, S., Hou, L., Huang, X., Zhang, X., Guo, H., et al. (2012). Ethylene signaling negatively regulates freezing tolerance by repressing expression of CBF and type-A ARR genes in Arabidopsis. Plant Cell 24, 2578–2595. doi: 10.2307/23264476
Spallek, T., Gan, P., Kadota, Y., and Shirasu, K. (2018). Same tune, different song- cytokinins as virulence factors in plant-pathogen interactions? Curr. Opin. Plant Biol. 44, 82–87. doi: 10.1016/j.pbi.2018.03.002
Spoel, S. H., Koornneef, A., Claessens, S. M. C., Korzelius, J. P., Van Pelt, J., Mueller, M. J., et al. (2003). NPR1 modulates cross-talk between salicylate- and jasmonate-dependent defense pathways through a novel function in the cytosol. Plant Cell 15, 760–770. doi: 10.2307/3871809
Staswick, P. E., Tiryaki, I., and Rowe, M. L. (2002). Jasmonate response locus JAR1 and several related Arabidopsis genes encode enzymes of the firefly luciferase superfamily that show activity on jasmonic, salicylic, and indole-3-acetic acids in an assay for adenylation. Plant Cell 14, 1405–1415. doi: 10.1105/tpc.000885
Swartzberg, D., Kirshner, B., Rav-David, D., Elad, Y., and Granot, D. (2008). Botrytis cinerea induces senescence and is inhibited by autoregulated expression of the IPT gene. Eur. J. Plant Pathol. 120, 289–297. doi: 10.1007/s10658-007-9217-6
To, J. P. C., Haberer, G., Ferreira, F. J., Deruère, J., Mason, M. G., Schaller, G. E., et al. (2004). Type- A Arabidopsis response regulators are partially redundant negative regulators of cytokinin signaling in Arabidopsis. Plant Cell 16, 658–671. doi: 10.1105/tpc.018978
Van Wees, S. C. M., Van Pelt, J. A., Bakker, P. A. H. M., and Pieterse, C. M. J. (2013). Bioassays for assessing jasmonate-dependent defenses triggered by pathogens, herbivorous insects, or beneficial rhizobacteria. Methods Mol. Biol. 1011, 35–49. doi: 10.1007/978-1-62703-414-2_4
Walters, D. R., and McRoberts, N. (2006). Plants and biotrophs: a pivotal role for cytokinins? Trends Plant Sci. 11, 581–586. doi: 10.1016/j.tplants.2006.10.003
Yamada, H., Suzuki, T., Terada, K., Takei, K., Ishikawa, K., Miwa, K., et al. (2001). The Arabidopsis AHK4 histidine kinase is a cytokinin-binding receptor that transduces cytokinin signals across the membrane. Plant Cell Physiol. 42, 1017–1023. doi: 10.1093/pcp/pce127
Zdarska, M., Dobisová, T., Gelová, Z., Pernisová, M., Dabravolski, S., and Hejátko, J. (2015). Illuminating light, cytokinin, and ethylene signaling crosstalk in plant development. J. Exp. Bot. 66, 4913–4931. doi: 10.1093/jxb/erv261
Zdarska, M., Cuyacot, A. R., Tarr, P. T., Yamoune, A., Szmitkowska, A., Hrdinová, V., et al. (2019). ETR1 integrates response to ethylene and cytokinins into a single multistep phosphorelay pathway to control root growth. Mol. Plant 12, 1338–1352. doi: 10.1016/j.molp.2019.05.012
Keywords: cytokinins, Botrytis cinerea, plant immunity, jasmonic acid, ethylene, hormonal crosstalk
Citation: Li B, Wang R, Wang S, Zhang J and Chang L (2021) Diversified Regulation of Cytokinin Levels and Signaling During Botrytis cinerea Infection in Arabidopsis. Front. Plant Sci. 12:584042. doi: 10.3389/fpls.2021.584042
Received: 16 July 2020; Accepted: 06 January 2021;
Published: 10 February 2021.
Edited by:
Essaid Ait Barka, Université de Reims Champagne-Ardenne, FranceReviewed by:
Silvia Proietti, University of Tuscia, ItalySimone Ferrari, Sapienza University of Rome, Italy
Copyright © 2021 Li, Wang, Wang, Zhang and Chang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling Chang, bGluZ2NoYW5nQGh1YnUuZWR1LmNu
†These authors have contributed equally to this work
 Beibei Li
Beibei Li Ruolin Wang1†
Ruolin Wang1† Jiang Zhang
Jiang Zhang Ling Chang
Ling Chang