- 1Oil Crops Research Institute of the Chinese Academy of Agricultural Sciences, Key Laboratory of Biology and Genetic Improvement of Oil Crops, Ministry of Agriculture and Rural Affairs, Wuhan, China
- 2Zhumadian Academy of Agricultural Sciences, Zhumadian, China
- 3Xiangyang Academy of Agricultural Sciences, Xiangyang, China
- 4National Key Laboratory of Crop Genetic Improvement, National Sub-Center of Rapeseed Improvement in Wuhan, Huazhong Agricultural University, Wuhan, China
Leaf size is a crucial component of sesame (Sesamum indicum L.) plant architecture and further influences yield potential. Despite that it is well known that leaf size traits are quantitative traits controlled by large numbers of genes, quantitative trait loci (QTL) and candidate genes for sesame leaf size remain poorly understood. In the present study, we combined the QTL-seq approach and SSR marker mapping to identify the candidate genomic regions harboring QTL controlling leaf size traits in an RIL population derived from a cross between sesame varieties Zhongzhi No. 13 (with big leaves) and ZZM2289 (with small leaves). The QTL mapping revealed 56 QTL with phenotypic variation explained (PVE) from 1.87 to 27.50% for the length and width of leaves at the 1/3 and 1/2 positions of plant height. qLS15-1, a major and environmentally stable pleiotropic locus for both leaf length and width explaining 5.81 to 27.50% phenotypic variation, was located on LG15 within a 408-Kb physical genomic region flanked by the markers ZMM6185 and ZMM6206. In this region, a combination of transcriptome analysis with gene annotations revealed three candidate genes SIN_1004875, SIN_1004882, and SIN_1004883 associated with leaf growth and development in sesame. These findings provided insight into the genetic characteristics and variability for sesame leaf and set up the foundation for future genomic studies on sesame leaves and will serve as gene resources for improvement of sesame plant architecture.
Introduction
Sesame is a traditional and ancient oil crop. It has been cultivated for more than five thousand years in Asia and has been considered a high-quality oil crop and health food for humans (Cheng et al., 2006; Namiki, 2007; Takada et al., 2015). Plant leaf represents the main organ for photosynthesis, and the morphology of leaves, such as leaf angle, leaf length, leaf width, leaf size, and leaf shape are crucial factors determining plant architecture, which thereby influence the yield potential (Govaerts et al., 1996; Bar and Ori, 2014). Yield is the consequence of cumulative effects of various traits and processes throughout the whole plant growth period (Mikołajczak et al., 2016). The development of high-yielding sesame varieties with the high-density population is always limited by the plant architecture mainly.
Early studies in other crops suggested that leaf size traits are quantitative traits that ascribe to combinations of multiple genes with a minor effect for each gene (Bian et al., 2014). Moreover, leaf size traits are also strongly influenced by numerous environmental factors (Kobayashi et al., 2003). Recently, QTL related to flag leaf length (FLL), flag leaf width (FLW), and flag leaf area (FLA) have been detected in cereal crops using various populations (Liu et al., 2015). For instance, using six populations of bread wheat across ten different environments, Ma et al. (2019) identified a total of eight major and stable QTL for FLL, FLW, and FLA. Tang et al. (2018) detected 14 and 8 QTL, respectively, for FLL and FLW in rice using a CSSL population. Among these rice QTL, qFW4-2 was located to a 37-kb region, with the most possible candidate gene which previously detected NAL1. Moreover, 17 and 14 QTL, respectively, for FLL and FLW have been identified in a maize-teosinte BC2S3 RIL population (Fu et al., 2019). Many mutants with leaf size phenotypes have been characterized, and their relevant genes have been cloned in rice (Fujino et al., 2008; Hu et al., 2010; Xiang et al., 2012; Liu et al., 2016). However, few of the identified leaf size QTL have been narrowed down to a small genomic region or cloned (Shen et al., 2012; Takai et al., 2013). In addition, to date, no QTL and candidate genes related to leaf size traits have been reported in sesame.
The genetic mapping in sesame started in 2009 with 220 molecular markers based on RSAMPL (Random Selective Amplification of Microsatellite Polymorphic Loci) (Wei et al., 2009). The recent years witnessed the development and utilization of sesame genomic and genetic resources including a novel genetic map for sesame with 424 SSR genetic markers (Wang et al., 2017a). Genetic markers such as SSR, Indel, AFLP, and RAPD were applied extensively in the QTL mapping of agronomic traits such as yield-related traits, seed, oil quality, and disease resistance (Zhang et al., 2013; Wu et al., 2014; Wang et al., 2016, 2017b). However, it is laborious and time-consuming for genotyping in the mapping population using markers and also hard to directly discover candidate genes and tightly linked markers. The completed genome sequences of cultivated sesame (Wang et al., 2014) enabled the utilization of NGS-based technology in rapid mapping in sesame. The quantitative trait locus (QTL)-seq is a method that combines bulked-segregant analysis (BSA) and high-throughput whole-genome resequencing to detect the major regions of a target quantitative trait in a segregating population (Zhang et al., 2020). Compared with traditional QTL mapping, QTL-seq shows much higher efficiency and accuracy in identifying candidate genomic regions containing QTL affecting target traits in crops. The QTL-seq method has been proved to be successful in many crop populations, such as in the mapping of flowering traits in cucumber (Lu et al., 2014), tomato fruit weight (Illa-Berenguer et al., 2015), rice grain length and weight (Yaobin et al., 2018), purple skin of radish fleshy taproots (Liu et al., 2019), and partial resistance to the fungal rice blast disease and seedling vigor (Takagi et al., 2013).
In this study, we combined QTL-seq, SSR marker mapping, and transcriptome analysis to identify major QTL and candidate genes that shape leaf size in sesame. These results constitute the genetic basis of leaf size traits and will help to improve sesame plant architecture using molecular breeding approaches.
Materials and Methods
Plant Materials and Phenotypic Evaluation
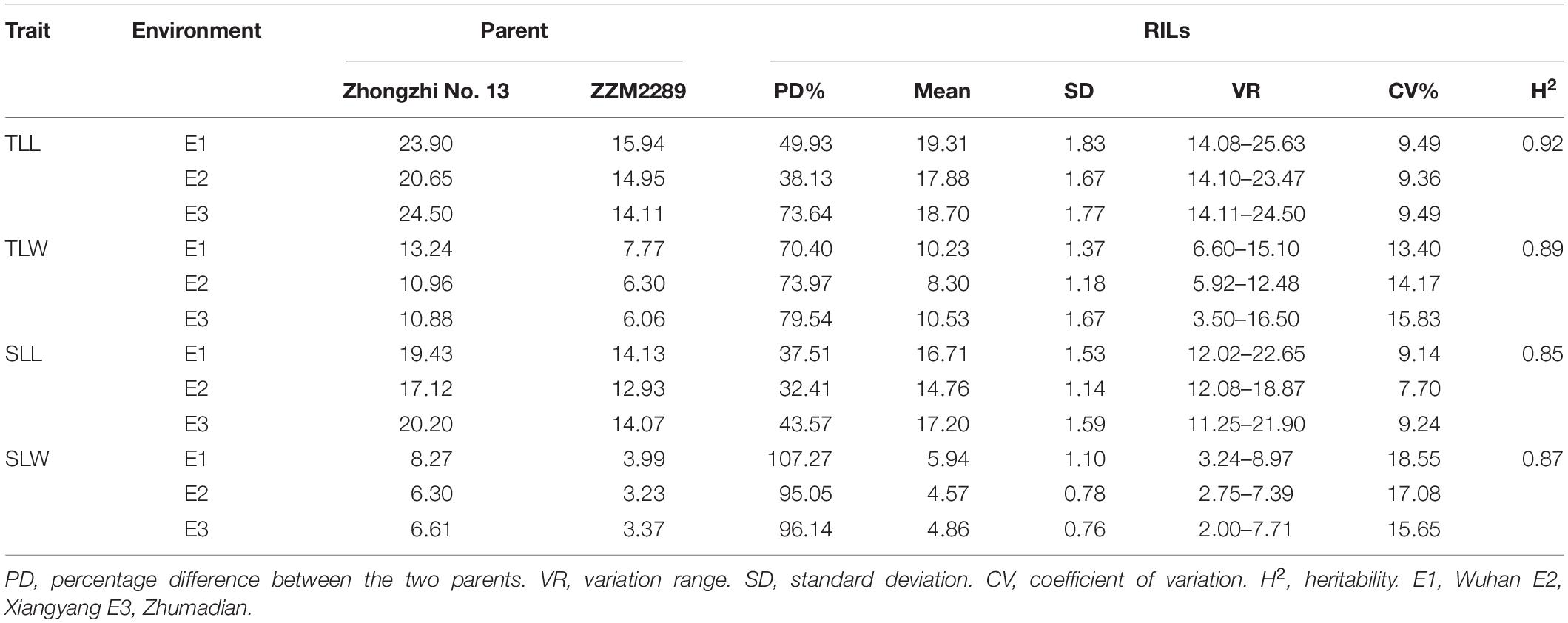
A total of 488 of the RIL population were derived from a cross between Zhongzhi No. 13 (female parent with big leaves, numbered BL) and ZZM2289 (male parent with small leaves, numbered SL). The parents and RIL population were grown in three environments (Wuhan, Xiangyang, and Zhumadian, China) in 2017. The three sites are different in climatic conditions, as Wuhan belongs to the Yangtze River valley (Southern China) with a humid subtropical monsoon climate. Zhumadian belongs to the Huang-Huai River valley (northern China) with a warm temperate climate. Xiangyang is in the middle of Wuhan and Zhumadian with a subtropical monsoon climate. The leaf size-related traits, including length of the leaves at the 1/3 position of plant height (TLL), width of the leaves at the 1/3 position of plant height (TLW), length of the leaves at the 1/2 position of plant height (SLL), and width of the leaves at 1/3 position of plant height (SLW), were investigated. Experiments were conducted in a randomized complete block design with three replications, and normal agronomic practices were used in field management. The leaf size-related traits were evaluated in triplicate at the late blossom period in each environment.
Construction of Extreme Bulks
Flesh leaves of BL, SL, and RIL plants were sampled in the Wuhan field and used for DNA extraction. Each sample DNA was isolated using the CTAB method and used for both QTL-seq and SSR marker analysis. To construct the DNA bulk, 25 top lines were first selected for different traits based on the average phenotypic values from three environments, then each line was checked in different environments to make sure it was included in the extreme groups parallelly. Finally, eight DNA bulks, each had 20 RILs, including long bulk and short bulk for TLL, wide bulk and narrow bulk for TLW, long bulk and short bulk for SLL, and wide bulk and narrow bulk for SLL, were constructed, respectively, by mixing an equal amount of DNAs.
Generation Analysis of NGS Data
Pair-end sequencing libraries (read length 100 bp) with insert sizes of around 500 bp were prepared for sequencing with the Illumina NovaSeq 6000 PE 150 platform. The filtered high-quality reads were extracted from the raw data and used for further analysis based on a Q-score of 30 across > 90% of bulks. The short reads from DNA bulks were aligned to the Zhongzhi No. 13 reference genome sequence1 with BWA software. SNP calling was performed using SAM tools software. Functionally annotated putative SNP detection and candidate polymorphic marker locus annotation were performed with SnpEff software. The SNP-index and Δ(SNP-index) were calculated to identify candidate regions for leaf size QTL. The subtraction of the SNP index of two extreme bulks was used to calculate the Δ(SNP-index). In a given genomic region, an average of SNP-index of SNPs was calculated using a sliding window analysis with 1 Mb window size and 10 kb increment. The Δ(SNP-index) value should not be significantly different from 0 in a genomic region where no major loci were located. Under the null hypothesis of no QTL, the statistical confidence intervals of Δ (SNP-index) for all the SNP positions were conducted with given read depths. Then, they were plotted along with Δ(SNP-index). For each read depth, Δ(SNP-index) in the 95% confidence intervals were obtained following Takagi et al. (2013).
QTL Analyses With SSR Markers
To confirm the candidate regions for leaf size, we conducted traditional QTL analysis based on SSR markers on the 488 RIL population focusing on the genomic regions detected by the QTL-seq method. The SSR markers in the candidate regions were developed according to Zhongzhi No. 13 reference genome sequence (see text footnote 1). The polymorphic SSR markers in the parents were used to genotype the RILs using the polyacrylamide gel electrophoresis method (PAGE). The genetic map was constructed by the software Join Map 4.0, and genetic distances were estimated with the Kosambi mapping function (Kosambi, 1943). The QTL for leaf size traits were identified by composite interval mapping (CIM) analysis using the Windows QTL Cartographer 2.5 software based on a permutation test (1,000 permutation, P = 0.05). Analyses of the QTL interactions across environments were performed using QTLNetwork software version 2.0 with the mixed linear model (MLM) approach (Yang et al., 2008). QTL identified in different environments for the same trait were considered to be the same if their confidence intervals were overlapped. In the present study, a QTL was considered to be major and stable when it was detected in more than two environments and had significant effects accounting for more than 10% of the phenotypic variation.
RNA Isolation and RNA-seq Analysis
To dissect the gene expression difference between BL and SL, the 8th and 20th new leaves from the bottom of the two parents during plant development were sampled for RNA-seq. Total RNA samples were extracted using Trizol reagent. Three biological replicates of two stages of leaves from the BL and SL were used to create 12 independent RNA libraries. RNA-seq libraries were sequenced on an Illumina Hiseq X ten platform. The gene expression levels were normalized to the number of Reads per Kilobase of transcript per Million mapped reads (RPKM) using the HTSeq 6.0 software (Anders and Huber, 2010).
Real-Time Quantitative PCR (qRT-PCR) of RNA-seq
Real-time quantitative PCR was performed using SYBR® Select Master Mix (2X) (Vazyme Biotech, Nanjing, China) on a Light Cycler 480 II (Roche, Basel, Switzerland). Specific primers for the candidate genes identified were designed using Beacon Designer 8.0 (Supplementary Table 5). The internal control was the SIN_1004293, a Histone H3.3 gene which was used to normalize transcript levels. PCR reaction mixtures are as follows: 10 μL mix (Vazyme Biotech, Nanjing, China), 5 μl cDNA, 0.5 μL of each primer, and 4 μL ddH2O. The PCR program was conducted according to the protocol of introduction. Pre-incubation 1 cycle: 95°C for 30 s; amplification 40 cycles: 95°C for 10 s, 60°C for 30 s; melting curve 1 cycle: 95°C for 15 s, 60°C for 60 s, 95°C for 15 s; and cooling 1 cycle: 40°C for 30 s. Real-time assay for each gene was performed with three independent biological replicates under identical conditions. The expression levels of gene were calculated from cycle threshold values through the 2-Δ Δ Ct method (Livak and Schmittgen, 2001).
Identification of Putative Candidate Genes
The most stable and reliable QTL were mapped on the sesame genome, and the candidate genes located in the confidence intervals were figured out in combination with the gene expression profiles and functional annotation.
Results
Phenotypic Variations of Sesame Leaf Size
Four leaf size-related traits, including the length and width of the leaves at the 1/3 (TLL and TLW) and 1/2 (SLL and SLW) positions of plant height from the bottom, were investigated in three environments (Figure 1). The leaves of the parent Zhongzhi No. 13 (BL) were more than 32.41% longer and 70.40% wider than those of ZZM2289 (Table 1 and Figure 1). For the RILs, the length and width of the 1/3 position leaf ranged from 14.08 to 25.63 cm and 3.50 to 16.50 cm in the three environments, respectively. For the 1/2 position leaf, the length and width ranged from 11.25 to 22.65 cm and 2.00 to 8.97 cm, respectively. All the target leaf size-related traits showed a continuous variation with a normal distribution characteristic in the three environments (Figure 2). Positive and negative transgressive segregations were generally observed for the four leaf size-related traits.
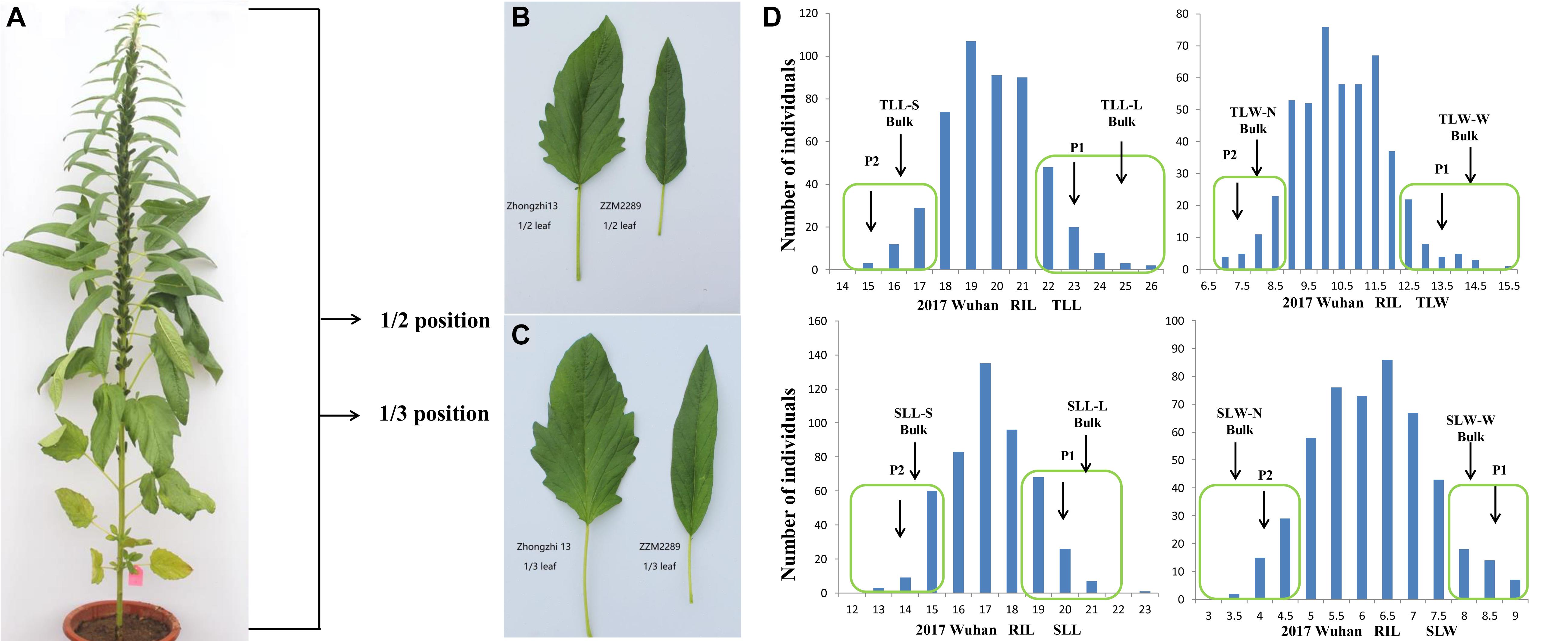
Figure 1. Phenotypic diversity and construction of DNA bulks. (A) The whole sesame plant to explain the 1/2 and 1/3 positions of the plant. (B) Leaf in the position of 1/2 plant height of two parents. (C) Leaf in the position of 1/3 plant height of two parents. (D) Frequency distribution of leaf size traits among RIL populations in Wuhan 2017. DNA of 20 RILs with extreme phenotypes was used to develop bulks (L, long S, short N, narrow W, wide).
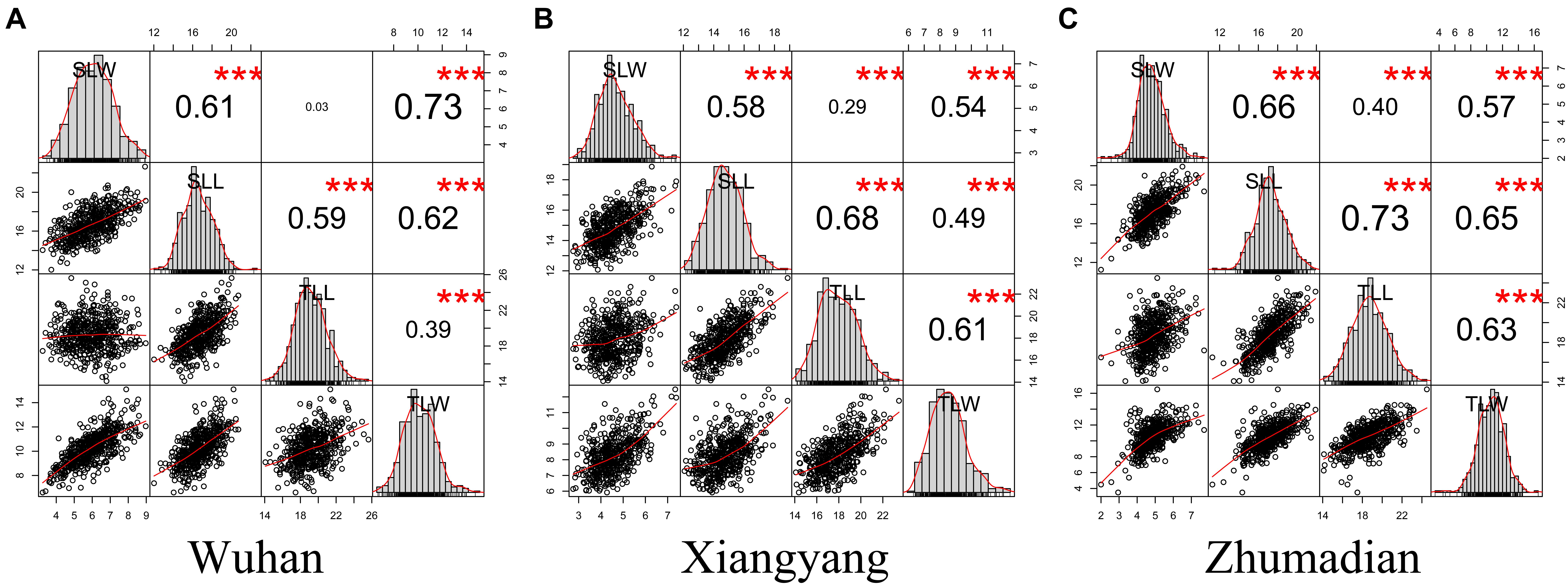
Figure 2. The correlation analysis of leaf size traits. (A–C) were the correlation analyses in three environments. ***P < 0.001.
The four leaf-related traits had significant correlation coefficients with each other in different environments, except for SLW and TLL in Wuhan (Figure 2). All four traits had broad-sense heritability (H2) over 0.85 (Table 1). The ANOVA showed that environment effects were significant for TLL, TLW, SLL, and SLW at P < 0.001.
QTL-seq Identified 77 Candidate Genomic Regions Controlling Sesame Leaf Size
Based on the mean values of leaf length and width in the three environments, four pairs of extreme separated DNA bulks were constructed using the leaves at the 1/3 (TLL and TLW) and 1/2 (SLL and SLW) positions of plant height (Figure 1D). 10 samples in total (two parents and eight extreme bulks) were then analyzed using QTL-seq based on Illumina NovaSeq 6000 PE 150 platform. For each sample, 96,122,878, 46,240,582, 121,519,676, 124,032,266, 127,961,366, 120,295,522, 123,472,918, 118,170,232, 121,852,830, and 120,478,184 clean short reads from different samples were generated (100 bp in length), respectively. Among these short reads, 69.05–81.93% were uniquely and confidently mapped to the reference genome version 1.0 of Zhongzhi No. 13. 580,091, 582,745, 605,167, 585,644, 587,976, 587,264, 584,329, and 584,571 genome-wide SNPs were identified, respectively, for each bulk (Supplementary Table 1).
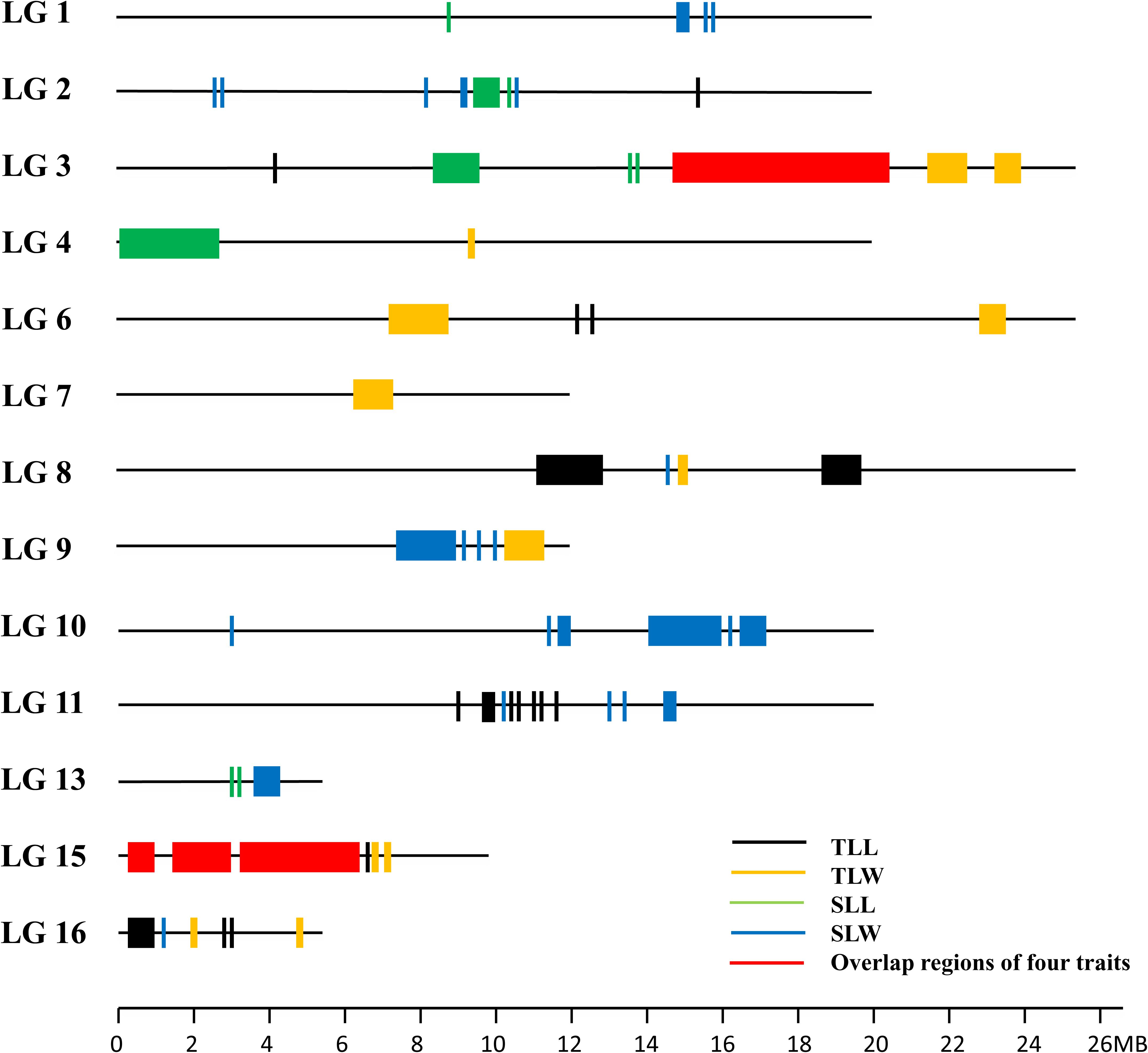
The ΔSNP-index was calculated by combining the information of the SNP index for each pair of extreme bulks. At the 95% significance level, a total of 77 candidate genomic regions that may contain the QTL of leaf size were figured out on the 13 linkage groups (LGs) except for LG5, LG12, and LG14. LG2, LG3, LG10, LG11, LG15, and LG16 contained 6 or more candidate regions (Figure 3). It was worth noting that there were some pleiotropic loci on LG3 and LG15 (Figure 4). Moreover, we detected 823,202 SNPs within the intervals of the 77 candidate genomic regions (Supplementary Table 2). These SNPs were further targeted for sesame leaf size candidate gene identification.
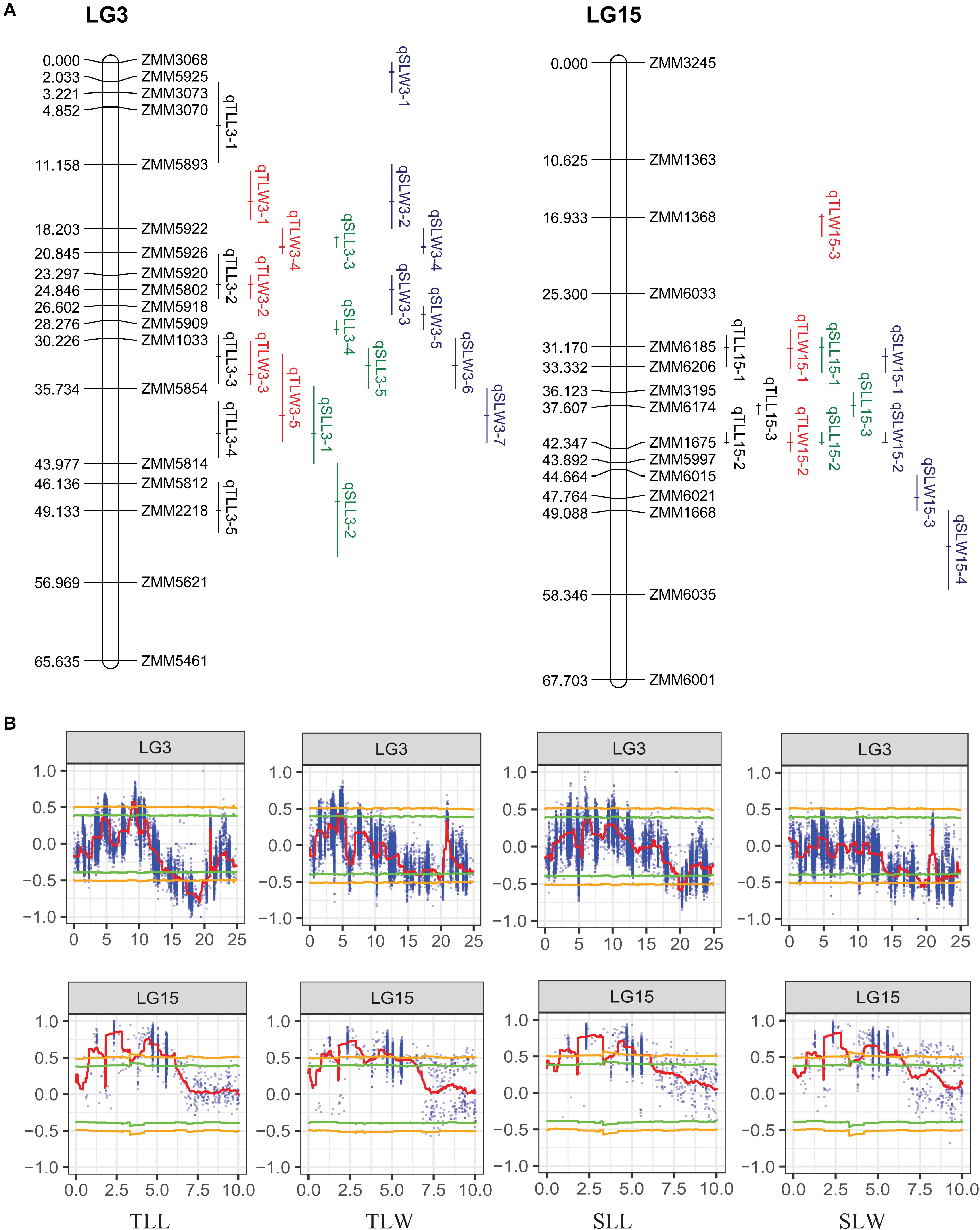
Figure 4. The genomic regions detected by QTL-seq and linkage maps of LG3 and LG15. (A) The genetic linkage map of LG3 and LG15. (B) The genomic regions detected by QTL-seq on LG3 and LG15.
QTL Mapping for Sesame Leaf Size Using SSR Markers
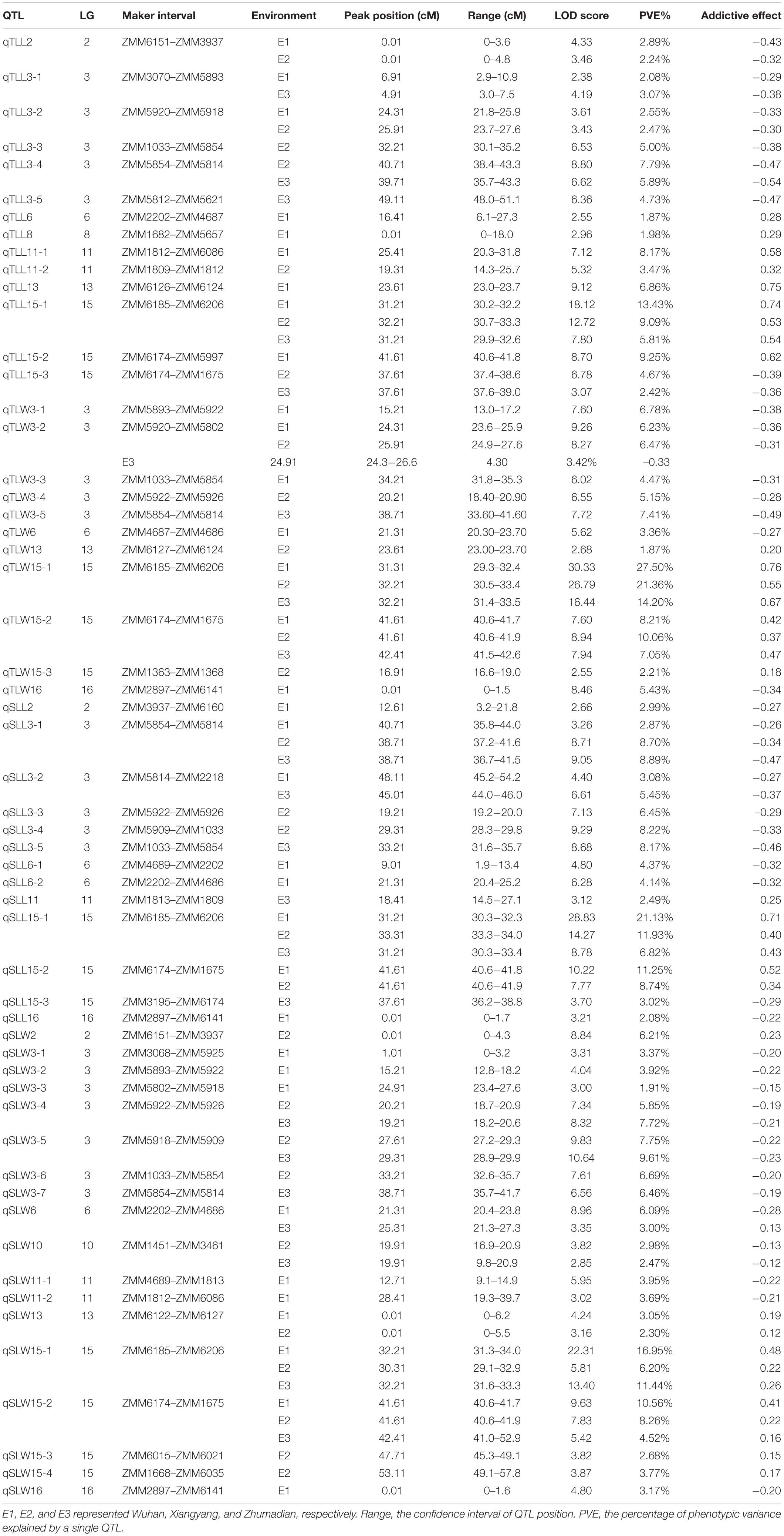
To specifically detect QTL linked to sesame leaf size-related traits, 942 SSR markers (including 62 published and 880 newly developed) in the intervals of the 77 candidate genomic regions revealed by QTL-seq were tested in the two parents. Among the developed SSR markers, 81 were polymorphic between the parents, BL and SL, accounting for 8.6% of the total markers. The 81 polymorphic SSR markers were used to genotype the 488 RILs, and a genetic linkage map composed of 71 SSR markers was constructed using Join map 4.0 for further QTL mapping (Supplementary Table 3). The composite interval mapping (CIM) method detected 56 QTL underlying the four leaf-related traits across three environments (Table 2). Meanwhile, 14 QTL explained 1.87–13.43% of phenotypic variation (PVE) for TLL; 11 QTL explained 1.87–27.50% PVE for TLW; 13 QTL explained 2.08–21.13% of PVE for SLL; and 18 QTL explained each 1.91–16.95% of PVE for SLW. The significant LOD score ranged from 2.38 to a high of 30.33. 15 of the 56 QTL were detected in multiple environments, among which, there were seven major QTL with more than 10% PVE, qTLL15-1, qTLW15-2, qTLW15-1, qSLL15-1, qSLL15-2, qSLW15-1, and qSLW15-2. These seven main effect QTL were all mapped on LG15. Alleles from Zhongzhi No.13, a big leaf parent, at all of mapped major QTL had increasing effects for leaf size.
Although 56 QTL for the leaf related traits were mapped on nine linkage groups, they were not evenly distributed. Among the 56 QTL detected in the RIL population, 35 were clustered on LG3 and LG15, and some pleiotropic loci were also concentrated on these two linkage groups, confirming the result of QTL-seq (Figure 4). The QTL qTLL15-1, qTLW15-1, qSLL15-1, and qSLW15-1 that were clustered in the same region between markers ZMM6185 and ZMM6206 were all detected across three environments with the highest phenotype contribution rates for different traits ranging from 5.81 to 27.50% averaged 13.82%. The pleiotropic region of different leaf size parameters was designated qLS15-1.
Epistasis Analysis
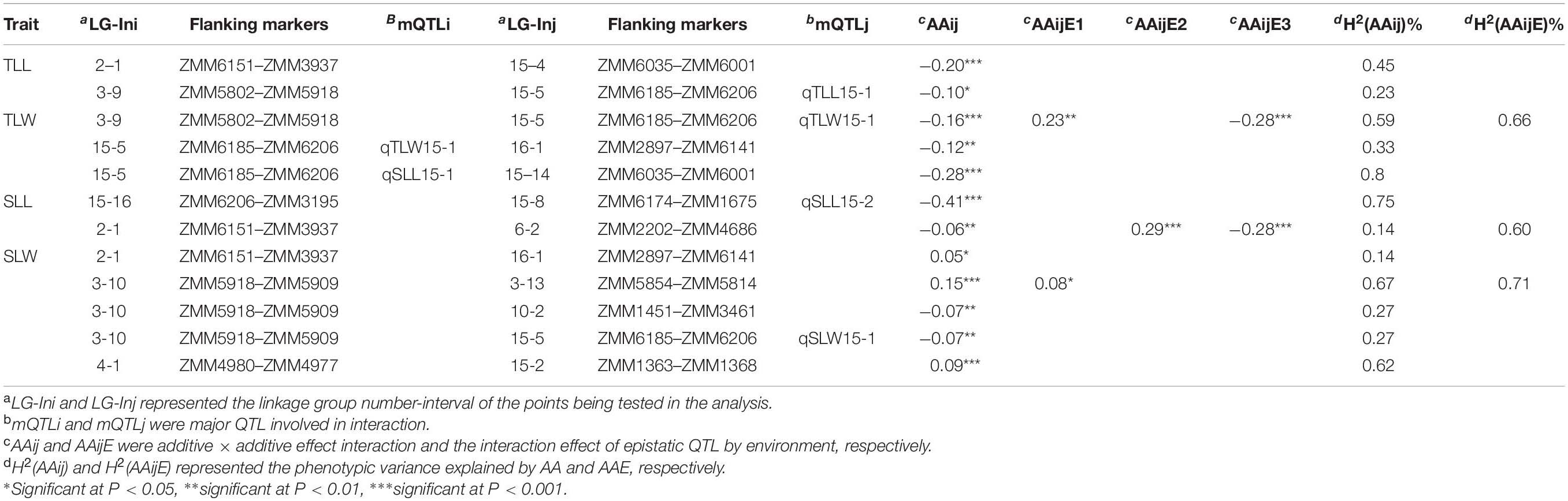
Twelve pairs of loci with significant (P < 0.05) additive × additive epistatic effects regulating four sesame leaf size-related traits were detected (Table 3). The phenotypic variance explained by each pair of epistatic interactions ranged from 0.14% to 0.80%, and the epistatic interactions accounted for 5.26% of the phenotype variation totally. Three interactions occurred between two loci on the same linkage group, while others on separate linkage group. Six of 12 pairs involved major QTL locus with a negative value indicated that the parental two-locus genotypes have a negative effect and that the recombinants had a positive effect. Furthermore, significant epistatic QTL × environment (AAijE) effects for three of the 12 epistatic QTL pairs were identified, and these AAE effects differed considerably in direction under different environments.
The Candidate Genes for the Major Stable Locus qLS15-1
The major stable locus qLS15-1 was mapped in the sesame genome between 2,339,824 and 2,747,783 bp on LG15. The genomic region spanning 408 Kb had a total of 55 annotated or predicted gene models. We examined the 46,464 SNPs and detected that only six missense variant SNPs were located in the qLS15-1 locus. Two genes, SIN_1004921 and SIN_1004909, were found to be under the influence of these six SNPs.
RNA-seq provided the gene expression profiles between two parents during leaf development. Among the 55 genes, 12 expressed lower and 14 expressed higher in the large leaf parent at both the 8th and 20th leaf stages. The gene expressions were validated using qRT-PCR by selecting eight genes. It showed that the expression profiles of the eight genes from qRT-PCR were consistent with RNA-seq with similar trends, which thus supported the validity of RNA-seq analysis (Supplementary Figure 1). The expression profiles of the two genes influenced by missense variant SNPs showed no difference between BL and SL. Therefore, they cannot be included in the list of candidate genes for sesame leaf size.
In combination with the predicted gene function and expression profiles, we selected three genes (SIN_1004875, SIN_1004882, and SIN_1004883) that control sesame plant leaf size for further study (Supplementary Table 5). Both RNA-seq and qRT-PCR showed the differences of the three genes in BL and SL (Figure 5). Function annotation showed that the three genes were known to regulate cell proliferation and expansion by affecting cell wall biosynthesis, auxin transportation, and cell cycle. These results suggested the three candidate genes involved in the regulation of sesame leaf size.
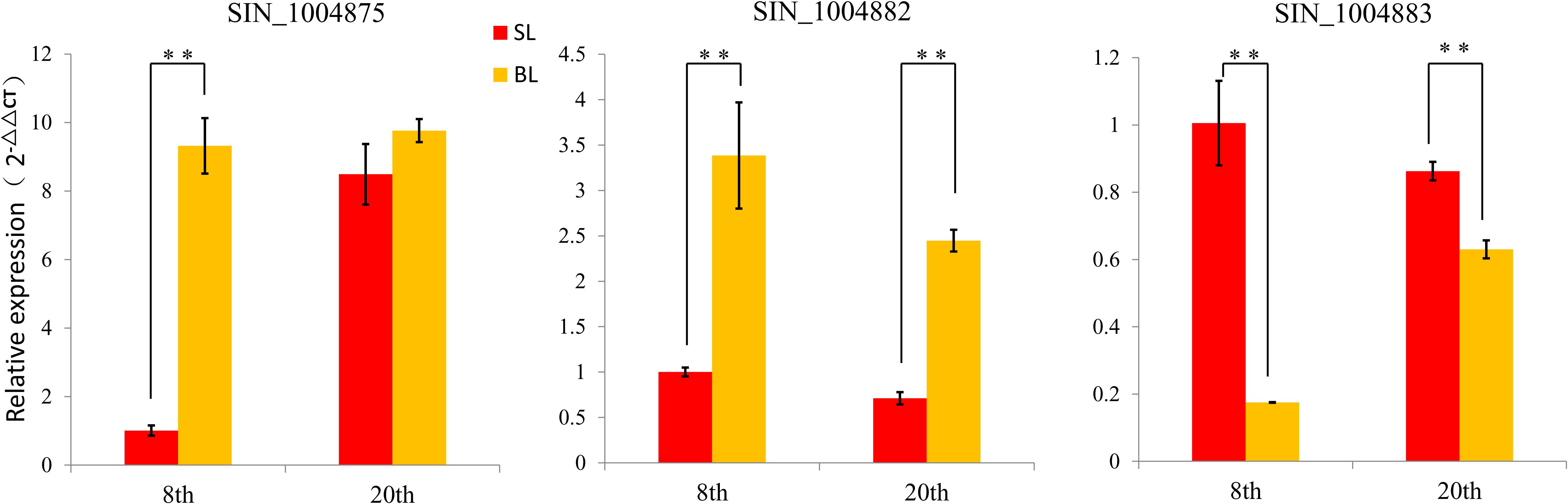
Figure 5. Comparison the relative expression of three candidate genes between BL and SL at different stages by qRT-PCR. Error bars indicate standard deviations (SD) based on two replicates. **P < 0.01, t test.
Discussion
Leaf size is an important component of plant architecture and further influences yield potential (Amalero et al., 2003; Tambussi et al., 2007; Jiang et al., 2013). In rice (Oryza sativa L), the top three leaves were identified as the most essential photosynthesis organs and the main source of carbohydrate accumulation (Li et al., 1998; Khaliq et al., 2008; Liu et al., 2015; Cui et al., 2017). Sesame yield is lower than 1,500 kg/ha usually, and the ideal plant architecture is sure to be the key factor for yield improvement. Like maize, rice, and wheat which went through the “green revolution,” breeding sesame varieties with ideal plant architecture (small leaf size, small leaf angle, and semi-dwarf plant) might improve sesame yield components and resistance to diverse stresses (Baydar, 2005; Wang et al., 2016). In the early study, leaf size traits had been demonstrated to be quantitative traits (Simon, 1999; Himanen et al., 2007). Sesame leaf traits were more complicated and few studies on it had been reported, especially for the large population, in consideration of the difference of the leaf at different positions from bottom to top. In the present study, the leaves at the middle and one-third positions of plant from the bottom were investigated based on sesame specific plant architecture with small leaves at top position but large leaves at middle and lower positions, which were the key determinants of sesame plant architecture and sunlight capture ability. The results showed though that sesame leaf size was easily affected by the environmental conditions and had broad variation but still had high heritability (all the H2 were higher than 0.85; Table 1). It suggested that the detection, validation, and clone of QTL for leaf size-related traits should be based on multiple environments to increase reliability.
QTL-seq combined with conventional marker mapping has been proved to increase the efficiency and accuracy of identification of genomic regions harboring the major QTL in several crops including Chinese cabbage (Li et al., 2020), peanut (Luo et al., 2019), cucumber (Lu et al., 2014), and chickpea (Deokar et al., 2019). To our knowledge, this study represents the first report on the genetic control of leaf size in sesame. QTL-seq firstly predicted 77 candidate genomic regions related to sesame leaf width and length. Targeting these genomic regions, 942 SSR markers were used to scan the two parents, among which 81 (8.6%) of them were polymorphic. The similar low polymorphism of SSR markers in sesame had been reported previously. Zhang found that 6.52% of SSR markers were polymorphic in a mapping population (Zhang et al., 2012). Wang designed and synthesized 1550 SSR markers from transcript sequences, and only 59 (3.8%) showed polymorphism within 36 individuals (Wang L. et al., 2012). Wang also found 6.8% of 7357 SSR markers amplified different bands in two materials (Wang et al., 2017b). The low polymorphism of SSR markers in sesame is likely due to its narrow genetic basis (Dixit et al., 2005; Wei et al., 2008).
We used the 81 polymorphic SSR markers to map the sesame reference genome and identified 56 QTL for leaf size in sesame. The PVE of 56 mapped QTL varied from 1.87 to 27.50%. Some QTL clustered on LG2, LG3, LG6, LG11, LG13, LG15, and LG16, suggesting several loci with a pleiotropic effect for different sesame leaf size traits. Particularly, two noticeable loci on LG15 contained four and six QTL, respectively, with environmentally stable expression and higher PVE. The one in the interval between SSR markers ZMM6185 and ZMM6206, namely, qLS15-1 contained four QTL, and each of them was detected in three environments with the maximum 27.50% of PVE. The other in the interval between ZMM6174 and ZMM1675 contained six QTL, and two of them can be detected in multiple environments with the maximum of 11.25% of PVE. Co-localization may suggest pleiotropy or close linkages whereby a genomic region contains a cluster of QTL that affect some traits indicating the benefit for improvement breeding efficiency for multiple elite traits (Wang P. et al., 2012). Many studies had reported the co-located QTL for leaf size traits (Wu et al., 2016; Hussain et al., 2017; Du et al., 2019). A QTL which consistently expresses in different genetic backgrounds and environments with a large effect is theoretically regarded as a valuable locus for further analysis such as fine mapping and cloning (Bonneau et al., 2013). Further fine mapping and cloning of these QTL to determine their genetic functions and physical relationships will improve our understanding of leaf size development. Clustered QTL regions will be targeted to understand molecular mechanisms involved in sesame leaf development and to improve sesame plant architecture.
Epistatic interactions have been proved to influence the many phenotype of morphology and yield related traits (Wu, 2012; Hou et al., 2015). Twelve pairs of loci with significant additive × additive epistatic effects regulating four sesame leaf size-related traits were detected. Although the effects of epistasis varied considerably among the traits, the total effects of the epistatic QTL (5.26%) were much smaller than those of the major QTL, indicating that additive effects contributing to variation in sesame leaf size are predominated in our population. These results demonstrated that epistasis could be of minor importance to variation in sesame leaf size development.
Various factors, such as plant hormones and transcription factors, can elaborate the morphology and size of the leaf by regulating cell division and cell expansion (Andriankaja et al., 2012; Gonzalez et al., 2012; Powell and Lenhard, 2012). In consideration of the pleiotropic and stable high PVE of qLS15-1, we selected this locus for candidate gene identification by comparing BL and SL transcriptomes at two stages of sesame development. Among the 55 genes in qLS15-1, 26 expressed differently and 29 showed similar expression profiles in the two parents at both the 8th and 20th leaf stages. The 29 similar expressed genes, including the two genes identified by examining the SNPs which may suggest other type mutations such as insert, deletion, structure variation, or mechanisms such as alternative splicing and so on, will be involved in the regulation of sesame leaf size development. Based on their expression profiles and functional annotation, three candidate genes SIN_1004875, SIN_1004882, and SIN_1004883 were identified associated with leaf development in sesame. SIN_1004875 is homologous to IRX14-L (AT5G67230), which encodes a member of the glycosyltransferase 43 family (GT43) involved in the synthesis of the hemicellulose glucuronoxylan, a major component of secondary cell walls (Lee et al., 2010; Wu et al., 2010). This gene may function for cell expansion by synthesizing new cell wall material to counteract the loosening of the cell wall and fuel further leaf growth (Powell and Lenhard, 2012). SIN_1004882 is homologous to Cyclin-D3-3 (AT3G50070), which is a member of D3-type cyclin (CYCD3-1, CYCD3-2, and CYCD3-3). Studies showed that CYCD3 can affect cell number and cell size in developing leaves by regulating the duration of the mitotic phase and timing of the transition to endocycles (Dewitte et al., 2007). SIN_1004883 is homologous of ADP-ribosylation factor 1 (AT1G23490), a gene that was found to correlate with auxin directional transportation by recycling PIN auxin transporters and participating in various auxin-dependent developmental processes in Arabidopsis (Tanaka et al., 2014). Collectively, the three candidate genes were worthy to be further validated by transgenics or gene editing. The study also suggested the reliability of QTL-seq combined with conventional marker mapping in sesame not only is labor-saving and time-saving for sesame QTL mapping but also can detect minor loci.
Conclusion
This study detected 56 QTL controlling sesame leaf length and width with PVE from 1.87 to 27.50% across three environments. The stable pleiotropic locus qLS15-1 presented with the maximum 27.50% phenotype contribution rates for different leaf width and length. Three candidate genes (SIN_1004875, SIN_1004882, and SIN_1004883) that may control sesame leaf development were figured out in combination with the gene function annotation and expression profiles. The study provided the first utility of QTL-seq combined with SSR marker mapping and comparative transcriptome analysis on sesame quantitative traits and revealed the genetic variations and candidate loci and genes underlying sesame leaf sizes. These findings may serve as genetic resources for improvement of sesame plant architecture and yield.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: GeneBank and SRR12341778∼SRR12341789.
Author Contributions
LW, XZ, and JT contributed to the design of the research. CS, XC, XT, YZ, and LW investigated the phenotypes. SS, CS, RZ, YG, and DL prepared the leaf samples. SS and CS participated in locus and gene annotation. LW and CS performed statistical analysis and wrote the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by the Natural Science Foundation of Hubei Province, China (2019CFB574), the Science and Technology Innovation Project of Hubei Province (201620000001048), Wuhan cutting-edge application technology fund (2018020401011303), the Fundamental Research Funds for Central Non-profit Scientific Institution (1610172017003 and Y2019XK15-02), China Agriculture Research System (CARS-14), and the Agricultural Science and Technology Innovation Project of the Chinese Academy of Agricultural Sciences (CAAS-ASTIP-2016-OCRI).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2021.580846/full#supplementary-material
Supplementary Figure 1 | Comparison of the relative expression abundance measured by qRT-PCR and RNA-seq for 8 selected genes between BL and SL at different stages.
Supplementary Table 1 | Statistical table of sequencing in different bulks.
Supplementary Table 2 | Number of SNPs in candidate regions detected by QTL-seq.
Supplementary Table 3 | The information of the 71 SSR markers.
Supplementary Table 4 | Candidate genes in the qLS15-1.
Supplementary Table 5 | The primer sequence for qRT-PCR.
Footnotes
References
Amalero, E. G., Ingua, G. L., Erta, G. B., and Emanceau, P. L. (2003). Review article methods for studying root colonization by introduced. Agronomie 23, 407–418. doi: 10.1051/agro
Anders, S., and Huber, W. (2010). Differential expression analysis for sequence count data. Genome Biol. 11:R106. doi: 10.1186/gb-2010-11-10-r106
Andriankaja, M., Dhondt, S., DeBodt, S., Vanhaeren, H., Coppens, F., DeMilde, L., et al. (2012). Exit from proliferation during leaf development in Arabidopsis thaliana: a not-so-gradual process. Dev. Cell 22, 64–78. doi: 10.1016/j.devcel.2011.11.011
Bar, M., and Ori, N. (2014). Leaf development and morphogenesis. Development 141, 4219–4230. doi: 10.1242/dev.106195
Baydar, H. (2005). Breeding for the improvement of the ideal plant type of sesame. Plant Breed. 124, 263–267. doi: 10.1111/j.1439-0523.2005.01080.x
Bian, J., He, H., Shi, H., Zhu, G., Li, C., Zhu, C., et al. (2014). Quantitative trait loci mapping for flag leaf traits in rice using a chromosome segment substitution line population. Plant Breed. 133, 203–209. doi: 10.1111/pbr.12146
Bonneau, J., Taylor, J., Parent, B., Bennett, D., Reynolds, M., Feuillet, C., et al. (2013). Multi-environment analysis and improved mapping of a yield-related QTL on chromosome 3B of wheat. Theor. Appl. Genet. 126, 747–761. doi: 10.1007/s00122-012-2015-3
Cheng, F. C., Jinn, T. R., Hou, R. C., and Tzen, J. T. (2006). Neuroprotective effects of sesamin and sesamolin on gerbil brain in cerebral ischemia. Int. J. Biomed. Sci. 2, 284–288.
Cui, T. T., He, K. H., Chang, L. G., Zhang, X. H., Xue, J. Q., and Liu, J. C. (2017). QTL mapping for leaf area in maize (Zea mays L.) under multi-environments. J. Integr. Agric. 16, 800–808. doi: 10.1016/S2095-3119(16)61524-1
Deokar, A., Sagi, M., Daba, K., and Tar’an, B. (2019). QTL sequencing strategy to map genomic regions associated with resistance to Ascochyta blight in chickpea. Plant Biotechnol. J. 17, 275–288. doi: 10.1111/pbi.12964
Dewitte, W., Scofield, S., Alcasabas, A. A., Maughan, S. C., Menges, M., Braun, N., et al. (2007). Arabidopsis CYCD3 D-type cyclins link cell proliferation and endocycles and are rate-limiting for cytokinin responses. Proc. Natl. Acad. Sci. U.S.A. 104, 14537–14542. doi: 10.1073/pnas.0704166104
Dixit, A., Jin, M. H., Chung, J. W., Yu, J. W., Chung, H. K., Ma, K. H., et al. (2005). Development of polymorphic microsatellite markers in sesame (Sesamum indicum L.). Mol. Ecol. Notes 5, 736–738. doi: 10.1111/j.1471-8286.2005.01048.x
Du, B., Liu, L., Wang, Q., Sun, G., Ren, X., Li, C., et al. (2019). Identification of QTL underlying the leaf length and area of different leaves in barley. Sci. Rep. 9, 1–8. doi: 10.1038/s41598-019-40703-6
Fu, Y., Xu, G., Chen, H., Wang, X., Chen, Q., Huang, C., et al. (2019). QTL mapping for leaf morphology traits in a large maize-teosinte population. Mol. Breed. 39:103. doi: 10.1007/s11032-019-1012-5
Fujino, K., Matsuda, Y., Ozawa, K., Nishimura, T., Koshiba, T., Fraaije, M. W., et al. (2008). NARROW LEAF 7 controls leaf shape mediated by auxin in rice. Mol. Genet. Genomics 279, 499–507. doi: 10.1007/s00438-008-0328-3
Gonzalez, N., Vanhaeren, H., and Inzé, D. (2012). Leaf size control: complex coordination of cell division and expansion. Trends Plant Sci. 17, 332–340. doi: 10.1016/j.tplants.2012.02.003
Govaerts, Y. M., Jacquemoud, S., Verstraete, M. M., and Ustin, S. L. (1996). Three-dimensional radiation transfer modeling in a dicotyledon leaf. Appl. Opt. 35:6585. doi: 10.1364/ao.35.006585
Himanen, K., Dino, G., and Mieke, A. (2007). Genetic and epigenetic control of leaf size and shape. Int. J. Plant Dev. Biol. 1, 226–238.
Hou, X., Liu, Y., Xiao, Q., Wei, B., and Zhang, X. (2015). Genetic analysis for canopy architecture in an F 2 : 3 population derived from two-type foundation parents across multi-environments. Euphytica 205, 421–440. doi: 10.1007/s10681-015-1401-8
Hu, J., Zhu, L., Zeng, D., Gao, Z., Guo, L., Fang, Y., et al. (2010). Identification and characterization of NARROW AND ROLLED LEAF 1, a novel gene regulating leaf morphology and plant architecture in rice. Plant Mol. Biol. 73, 283–292. doi: 10.1007/s11103-010-9614-7
Hussain, W., Stephen Baenziger, P., Belamkar, V., Guttieri, M. J., Venegas, J. P., Easterly, A., et al. (2017). Genotyping-by-sequencing derived high-density linkage map and its application to QTL mapping of flag leaf traits in bread wheat. Sci. Rep. 7, 1–15. doi: 10.1038/s41598-017-16006-z
Illa-Berenguer, E., Van Houten, J., Huang, Z., and van der Knaap, E. (2015). Rapid and reliable identification of tomato fruit weight and locule number loci by QTL-seq. Theor. Appl. Genet. 128, 1329–1342. doi: 10.1007/s00122-015-2509-x
Jiang, L., Liu, X., Xiong, G., Liu, H., Chen, F., Wang, L., et al. (2013). DWARF 53 acts as a repressor of strigolactone signalling in rice. Nature 504, 401–405. doi: 10.1038/nature12870
Khaliq, I., Irshad, A., and Ahsan, M. (2008). Awns and flag leaf contribution towards grain yield in spring wheat (Triticum aestivum L.). Cereal Res. Commun. 36, 65–76. doi: 10.1556/CRC.36.2008.1.7
Kobayashi, S., Fukuta, Y., Morita, S., Sato, T., and Osaki, M. (2003). Quantitative trait loci affecting flag leaf development in rice (Oryza sativa L.). Breed. Sci. 262, 255–262.
Kosambi, D. D. (1943). The estimation of map distances from recombination values. Ann. Eugen. 12, 172–175. doi: 10.1111/j.1469-1809.1943.tb02321.x
Lee, C., Teng, Q., Huang, W., Zhong, R., and Ye, Z. H. (2010). The Arabidopsis family GT43 glycosyltransferases form two functionally nonredundant groups essential for the elongation of glucuronoxylan backbone. Plant Physiol. 153, 526–541. doi: 10.1104/pp.110.155309
Li, P., Su, T., Zhang, B., Li, P., Xin, X., Yue, X., et al. (2020). Identification and fine mapping of qSB. A09, a major QTL that controls shoot branching in Brassica rapa ssp. chinensis Makino. Theor. Appl. Genet. 133, 1055–1068. doi: 10.1007/s00122-020-03531-1
Li, Z., Pinson, S. R. M., Stansel, J. W., and Paterson, A. H. (1998). Genetic dissection of the source-sink relationship affecting fecundity and yield in rice (Oryza sativa L.). Mol. Breed. 4, 419–426. doi: 10.1023/A:1009608128785
Liu, L., Sun, G., Ren, X., Li, C., and Sun, D. (2015). Identification of QTL underlying physiological and morphological traits of flag leaf in barley. BMC Genet. 16:29. doi: 10.1186/s12863-015-0187-y
Liu, T., Wang, J., Wu, C., Zhang, Y., Zhang, X., Li, X., et al. (2019). Combined QTL-seq and traditional linkage analysis to identify candidate genes for purple skin of radish fleshy taproots. Front. Genet. 10:808. doi: 10.3389/fgene.2019.00808
Liu, X., Li, M., Liu, K., Tang, D., Sun, M., Li, Y., et al. (2016). Semi-rolled leaf2 modulates rice leaf rolling by regulating abaxial side cell differentiation. J. Exp. Bot. 67, 2139–2150. doi: 10.1093/jxb/erw029
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Lu, H., Lin, T., Klein, J., Wang, S., Qi, J., Zhou, Q., et al. (2014). QTL-seq identifies an early flowering QTL located near flowering locus T in cucumber. Theor. Appl. Genet. 127, 1491–1499. doi: 10.1007/s00122-014-2313-z
Luo, H., Pandey, M. K., Khan, A. W., Guo, J., Wu, B., Cai, Y., et al. (2019). Discovery of genomic regions and candidate genes controlling shelling percentage using QTL-seq approach in cultivated peanut (Arachis hypogaea L.). Plant Biotechnol. J. 17, 1248–1260. doi: 10.1111/pbi.13050
Ma, J., Tu, Y., Zhu, J., Luo, W., Liu, H., Li, C., et al. (2019). Flag leaf size and posture of bread wheat: genetic dissection, QTL validation and their relationships with yield-related traits. Theor. Appl. Genet. 133, 297–315. doi: 10.1007/s00122-019-03458-2
Mikołajczak, K., Ogrodowicz, P., Gudyś, K., Krystkowiak, K., Sawikowska, A., Frohmberg, W., et al. (2016). Quantitative trait loci for yield and yield-related traits in spring barley populations derived from crosses between European and Syrian cultivars. PLoS One 11:e0155938. doi: 10.1371/journal.pone.0155938
Namiki, M. (2007). Nutraceutical functions of sesame: a review. Crit. Rev. Food Sci. Nutr. 47, 651–673. doi: 10.1080/10408390600919114
Powell, A. E., and Lenhard, M. (2012). Control of organ size in plants. Curr. Biol. 22, R360–R367. doi: 10.1016/j.cub.2012.02.010
Shen, B., Yu, W. D., Zhu, Y. J., Fan, Y. Y., and Zhuang, J. Y. (2012). Fine mapping of a major quantitative trait locus, qFLL6.2, controlling flag leaf length and yield traits in rice (Oryza sativa L.). Euphytica 184, 57–64. doi: 10.1007/s10681-011-0539-2
Simon, M. R. (1999). Inheritance of flag-leaf angle, flag-leaf area and flag-leaf area duration in four wheat crosses. Theor. Appl. Genet. 98, 310–314.
Takada, S., Kinugawa, S., Matsushima, S., Takemoto, D., Furihata, T., Mizushima, W., et al. (2015). Sesamin prevents decline in exercise capacity and impairment of skeletal muscle mitochondrial function in mice with high-fat diet-induced diabetes. 11, 1319–1330. doi: 10.1113/EP085251
Takagi, H., Abe, A., Yoshida, K., Kosugi, S., Natsume, S., Mitsuoka, C., et al. (2013). QTL-seq: rapid mapping of quantitative trait loci in rice by whole genome resequencing of DNA from two bulked populations. Plant J. 74, 174–183. doi: 10.1111/tpj.12105
Takai, T., Adachi, S., Taguchi-Shiobara, F., Sanoh-Arai, Y., Iwasawa, N., Yoshinaga, S., et al. (2013). A natural variant of NAL1, selected in high-yield rice breeding programs, pleiotropically increases photosynthesis rate. Sci. Rep. 3, 1–11. doi: 10.1038/srep02149
Tambussi, E. A., Bort, J., Guiamet, J. J., Nogués, S., and Araus, J. L. (2007). The photosynthetic role of ears in C3 cereals: metabolism, water use efficiency and contribution to grain yield. CRC Crit. Rev. Plant Sci. 26, 1–16. doi: 10.1080/07352680601147901
Tanaka, H., Nodzyñski, T., Kitakura, S., Feraru, M. I., Sasabe, M., Ishikawa, T., et al. (2014). BEX1/ARF1A1C is required for BFA-sensitive recycling of PIN auxin transporters and auxin-mediated development in Arabidopsis. Plant Cell Physiol. 55, 737–749. doi: 10.1093/pcp/pct196
Tang, X., Gong, R., Sun, W., Zhang, C., and Yu, S. (2018). Genetic dissection and validation of candidate genes for flag leaf size in rice (Oryza sativa L.). Theor. Appl. Genet. 131, 801–815. doi: 10.1007/s00122-017-3036-8
Wang, L., Xia, Q., Zhang, Y., Zhu, X., Zhu, X., Li, D., et al. (2016). Updated sesame genome assembly and fine mapping of plant height and seed coat color QTLs using a new high-density genetic map. BMC Genomics 17:31. doi: 10.1186/s12864-015-2316-4
Wang, L., Yu, S., Tong, C., Zhao, Y., Liu, Y., Song, C., et al. (2014). Genome sequencing of the high oil crop sesame provides insight into oil biosynthesis. Genome Biol. 15:R39. doi: 10.1186/gb-2014-15-2-r39
Wang, L., Zhang, Y., Qi, X., Gao, Y., and Zhang, X. (2012). Development and characterization of 59 polymorphic cDNA-SSR markers for the edible oil crop Sesamum indicum (pedaliaceae). Am. J. Bot. 99, 1–5. doi: 10.3732/ajb.1200081
Wang, L., Zhang, Y., Zhu, X., Zhu, X., Li, D., Zhang, X., et al. (2017a). file:///F:/Shengchen/sesame SSR/development and validation of genic-SSR markers in sesame by RNA-seq-BMCgenomic-2012-32SSR.pdf. Sci. Rep. 7, 1–8.
Wang, L., Zhang, Y., Zhu, X., Zhu, X., Li, D., Zhang, X., et al. (2017b). Development of an SSR-based genetic map in sesame and identification of quantitative trait loci associated with charcoal rot resistance. Sci. Rep. 7:8349. doi: 10.1038/s41598-017-08858-2
Wang, P., Zhou, G., Cui, K., Li, Z., and Yu, S. (2012). Clustered QTL for source leaf size and yield traits in rice (Oryza sativa L.). Mol. Breed. 29, 99–113. doi: 10.1007/s11032-010-9529-7
Wei, L. B., Zhang, H. Y., Zheng, Y. Z., Guo, W. Z., and Zhang, T. Z. (2008). Developing EST-derived microsatellites in sesame (Sesamum indicum L.). Acta Agron. Sin. 34, 2077–2084. doi: 10.1016/S1875-2780(09)60019-5
Wei, L. B., Zhang, H. Y., Zheng, Y. Z., Miao, H. M., Zhang, T. Z., and Guo, W. Z. (2009). A genetic linkage map construction for Sesame (Sesamum indicum l.). Genes Genomics 31, 199–208. doi: 10.1007/BF03191152
Wu, A. M., Hörnblad, E., Voxeur, A., Gerber, L., Rihouey, C., Lerouge, P., et al. (2010). Analysis of the Arabidopsis IRX9/IRX9-L and IRX14/IRX14-L pairs of glycosyltransferase genes reveals critical contributions to biosynthesis of the hemicellulose glucuronoxylan. Plant Physiol. 153, 542–554. doi: 10.1104/pp.110.154971
Wu, K., Liu, H., Yang, M., Tao, Y., Ma, H., Wu, W., et al. (2014). High-density genetic map construction and QTLs analysis of grain yield-related traits in Sesame (Sesamum indicum L.) based on RAD-Seq techonology. BMC Plant Biol. 14:274. doi: 10.1186/s12870-014-0274-7
Wu, Q., Chen, Y., Fu, L., Zhou, S., Chen, J., Zhao, X., et al. (2016). QTL mapping of flag leaf traits in common wheat using an integrated high-density SSR and SNP genetic linkage map. Euphytica 208, 337–351. doi: 10.1007/s10681-015-1603-0
Wu, T. (2012). Mapping QTL for agronomic traits in breeding populations. Theor. Appl. Genet. 125, 201–210. doi: 10.1007/s00122-012-1887-6
Xiang, J. J., Zhang, G. H., Qian, Q., and Xue, H. W. (2012). Semi-rolled leaf1 encodes a putative glycosylphosphatidylinositol-anchored protein and modulates rice leaf rolling by regulating the formation of bulliform cells. Plant Physiol. 159, 1488–1500. doi: 10.1104/pp.112.199968
Yang, J., Hu, C., Hu, H., Yu, R., Xia, Z., Ye, X., et al. (2008). QTLnetwork: mapping and visualizing genetic architecture of complex traits in experimental populations. Bioinformatics 24, 721–723. doi: 10.1093/bioinformatics/btm494
Yaobin, Q., Peng, C., Yichen, C., Yue, F., Derun, H., Tingxu, H., et al. (2018). QTL-Seq identified a major QTL for grain length and weight in rice using near isogenic F2 population. Rice Sci. 25, 121–131. doi: 10.1016/j.rsci.2018.04.001
Zhang, H., Miao, H., Wei, L., Li, C., Zhao, R., and Wang, C. (2013). Genetic analysis and QTL mapping of seed coat color in Sesame (Sesamum indicum L.). PLoS One 8:e63898. doi: 10.1371/journal.pone.0063898
Zhang, H., Wei, L., Miao, H., Zhang, T., and Wang, C. (2012). Development and validation of genic-SSR markers in sesame by RNA-seq. BMC Genomics 13:316. doi: 10.1186/1471-2164-13-316
Keywords: sesame, leaf size, QTL-seq, QTL, Transcriptome, candidate genes
Citation: Sheng C, Song S, Zhou R, Li D, Gao Y, Cui X, Tang X, Zhang Y, Tu J, Zhang X and Wang L (2021) QTL-Seq and Transcriptome Analysis Disclose Major QTL and Candidate Genes Controlling Leaf Size in Sesame (Sesamum indicum L.). Front. Plant Sci. 12:580846. doi: 10.3389/fpls.2021.580846
Received: 07 July 2020; Accepted: 08 January 2021;
Published: 24 February 2021.
Edited by:
Matteo Busconi, Catholic University of the Sacred Heart, ItalyReviewed by:
Xiaodong Wang, Jiangsu Academy of Agricultural Sciences, ChinaByoung-Cheorl Kang, Seoul National University, South Korea
Copyright © 2021 Sheng, Song, Zhou, Li, Gao, Cui, Tang, Zhang, Tu, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Linhai Wang, d2FuZ2xpbmhhaUBjYWFzLmNu; bGluaGFpODI3QDE2My5jb20=; Xiurong Zhang, emhhbmd4ckBvaWxjcm9wcy5jbg==
 Chen Sheng
Chen Sheng Shengnan Song1
Shengnan Song1 Rong Zhou
Rong Zhou Donghua Li
Donghua Li Yanxin Zhang
Yanxin Zhang Jinxing Tu
Jinxing Tu Xiurong Zhang
Xiurong Zhang Linhai Wang
Linhai Wang