
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci., 08 December 2020
Sec. Plant Pathogen Interactions
Volume 11 - 2020 | https://doi.org/10.3389/fpls.2020.598483
This article is part of the Research TopicPlant-Pest Interactions Volume II: HemipteraView all 9 articles
 Nathan M. Gyan1
Nathan M. Gyan1 Beery Yaakov2
Beery Yaakov2 Nati Weinblum1
Nati Weinblum1 Anuradha Singh3
Anuradha Singh3 Alon Cna’ani3
Alon Cna’ani3 Shiran Ben-Zeev4
Shiran Ben-Zeev4 Yehoshua Saranga4
Yehoshua Saranga4 Vered Tzin2*
Vered Tzin2*Tef (Eragrostis tef), a staple crop that originated in the Horn of Africa, has been introduced to multiple countries over the last several decades. Crop cultivation in new geographic regions raises questions regarding the molecular basis for biotic stress responses. In this study, we aimed to classify the insect abundance on tef crop in Israel, and to elucidate its chemical and physical defense mechanisms in response to insect feeding. To discover the main pests of tef in the Mediterranean climate, we conducted an insect field survey on three selected accessions named RTC-144, RTC-405, and RTC-406, and discovered that the most abundant insect order is Hemiptera. We compared the differences in Rhopalosiphum padi (Hemiptera; Aphididae) aphid performance, preference, and feeding behavior between the three accessions. While the number of aphid progeny was lower on RTC-406 than on the other two, the aphid olfactory assay indicated that the aphids tended to be repelled from the RTC-144 accession. To highlight the variation in defense responses, we investigated the physical and chemical mechanisms. As a physical barrier, the density of non-granular trichomes was evaluated, in which a higher number of trichomes on the RTC-406 than on the other accessions was observed. This was negatively correlated with aphid performance. To determine chemical responses, the volatile and central metabolite profiles were measured upon aphid attack for 4 days. The volatile analysis exposed a rich and dynamic metabolic profile, and the central metabolism profile indicated that tef plants adjust their sugars and organic and amino acid levels. Overall, we found that the tef plants possess similar defense responses as other Poaceae family species, while the non-volatile deterrent compounds are yet to be characterized. A transcriptomic time-series analysis of a selected accession RTC-144 infested with aphids revealed a massive alteration of genes related to specialized metabolism that potentially synthesize non-volatile toxic compounds. This is the first report to reveal the variation in the defense mechanisms of tef plants. These findings can facilitate the discovery of insect-resistance genes leading to enhanced yield in tef and other cereal crops.
The world depends on many crop species to sustain the food supply. However, the commercialization of conventional agriculture has led to concentrating on only a few of these crops, which must be examined critically to ensure reliable food supply even with current population growth and climate change (Awika, 2011; Curtis and Halford, 2014). Approximately 50% of plant-based caloric intake is obtained from three primary grain sources—rice, wheat, and maize, while most traditional species are neglected and underutilized. Examples for underutilized cereals are broomcorn millet (Panicum miliaceum L.), canary seed (Phalaris canariensis L.), and tef [Eragrostis tef (Zuccagni) Trotter], which are monocotyledonous plants in the family of Poaceae (grasses), the same as the abovementioned staple crop (Bekkering and Tian, 2019). Most of these traditional crops offer an opportunity to improve agricultural production and maintain sustainable food security. Furthermore, these crops have a wealth of nutritional qualities and desirable traits that enhance their adaptability to climate change (Padulosi et al., 2012), and much more fundamental research is required to better understand them as a potential source of sustainable food production.
Tef is a small-seeded cereal millet. Tef is an allotetraploid cereal with a chromosome number of 20 (AB; 2n = 4x = 40), and its subgenomes are relatively small (∼300 Mb), with high gene density and low transposable element content (VanBuren et al., 2020). It originated in Ethiopia, where it is considered a staple crop, and the number one cereal produced in the country (Seyfu, 1993). Traditionally, it is grown by small-scale farmers; therefore, thousands of locally adapted accessions have been developed (Report on Area and Production Major Crops, 2012). The available genetic diversity in Ethiopia has driven breeding programs to improve existing varieties and meet market demand and consumers’ specifications (Ayalew et al., 2011; Assefa et al., 2015). The grains are commonly used for the preparation of a fermented sourdough bread known as “injera,” as well as for straw, feed, and to reinforce the walls of mud huts. Tef has more essential amino acids—including lysine, the most limiting amino acid—than barley, and wheat (Jansen et al., 1962; Yigzaw et al., 2004). It has high flour productivity, high market price, and adaptability to a wide range of environmental conditions (Reda, 2014). Recently, tef plants have been introduced to different parts of the world, including the United States, the Netherlands, and Israel (Assefa et al., 2011).
Millets such as tef face several production constraints since they are mostly cultivated in marginal areas with low moisture and limited fertility conditions (Dosad and Chawla, 2018). Inherent characteristics, such as susceptibility to pests and diseases, can cause a significant yield loss (Assefa et al., 2011; Ben-Zeev et al., 2020). One of the main reasons for crop loss is pests, which cause an average 15% reduction in grain quality and yield (Lee et al., 1981; Deutsch et al., 2018). Aphids (Hemiptera: Aphididae), of which there are approximately 5,000 species worldwide, are a dominant pest of cereal crops (Vickerman and Wratten, 1979; Rabbinge et al., 1981). This pest affects plant production through the reduction of nutrients, diminished photosynthetic efficiency, modification of sink-source ratio (Bing et al., 1991; Zhou et al., 2015), and transmission of plant viruses (Fereres et al., 1989; Nault, 1997). The aphids are phloem-feeding insects that use their stylets to penetrate the host tissues, causing minimal tissue damage (Douglas, 2003). Once an aphid finds a suitable feeding site, it can ingest phloem sap for hours or even days and adapt to the phloem sap compound composition (Nalam et al., 2020). There is limited knowledge about tef pests in general and aphids in particular. To reduce pest damage, plants have evolved defense strategies, that can be present constitutively or be induced on demand (Agrawal, 1999; Mithöfer and Boland, 2012). Some of the main strategies commonly present in the Poaceae family plant species include: (i) physical barriers, (ii) metabolic adjustments to modify the food source consumed by aphids, and (iii) chemical defenses and signals (volatiles and non-volatiles). The physical barrier on the leaf surface is the key interface between plants and insects that interrupts insect feeding. Many surface characteristics, including the trichomes, cuticle, epidermis, waxes, and cell walls, can modulate these interactions (Agrawal et al., 2009). The leaf surface of young wheat and barley plants are covered with non-glandular trichomes, specialized epidermal hair-like structures, that might affect aphid movement and reproduction rate (Leybourne et al., 2019; Batyrshina et al., 2020b; Correa et al., 2020). To cope with insect attack, plants adjust their central metabolism by transiently modifying photosynthetic efficiency and remobilizing carbon and nitrogen resources (Meihls et al., 2012; Appel et al., 2014). The Russian wheat aphid (Diuraphis noxia) infestation on wheat leaves has caused significant losses of chlorophyll a and b and carotenoids (Ni et al., 2002). In barley leaves, 30 genes associated with photosynthesis were inhibited after 3 h of feeding (Gutsche et al., 2009). The metabolite content in the phloem sap can be adjusted in response to aphid feeding (Leybourne et al., 2019). For example, the feeding of greenbug aphids (Schizaphis graminum) on wheat leaves enhances the content of essential amino acids in the phloem sap (Dorschner et al., 1987; Sandström et al., 2000).
In response to insect attack, plants adjust not only their central metabolites but also synthesize specialized deterrent metabolites that can affect the insect nervous, digestive, and endocrine systems (Eisner et al., 2000; Meihls et al., 2012; Fürstenberg-Hägg et al., 2013). In the Poaceae family, substrates from the shikimate pathway, mainly indole-, and Tyr-derived compounds, serve as a source for various classes of specialized deterrent metabolites. This includes: (i) benzoxazinoids in wheat and maize (Frey et al., 1997), (ii) gramine in cultivated barley (Grün et al., 2005), (iii) serotonin and melatonin, detected in rice, and Echinochloa esculenta (Japanese barnyard millet) (Ishihara et al., 2008; Lu et al., 2018), and (iv) the cyanogenic glucoside dhurrin in Sorghum (Zhu-Salzman et al., 2004). However, none of these specialized metabolites were previously reported to be synthesized in tef plants. Another chemical response is the biosynthesis and emission of volatile organic compounds (VOCs) (Dicke, 1999). VOCs are released into the atmosphere and act as long-distance cues for herbivore deterrence, natural enemy attraction, or even serve as host-finding signals for the herbivores themselves (Gershenzon and Dudareva, 2007; Bleeker et al., 2009). The VOCs are composed of a blend of metabolites from diverse chemical groups: (i) terpenoids, (ii) fatty acids (FAs) derivatives including methyl jasmonate, and green leaf volatiles (GLVs), (iii) indole- and Phe-derived phenolic products including methyl salicylate, (iv) methanol; and (v) ethylene (Kant et al., 2009). Most studies conducted on plants from the Poaceae family have suggested that the mono-, sesqui-, and di-terpenoids, and FAs are the main VOCs that are modified in response to herbivory (Richter et al., 2015, 2016; Ameye et al., 2018) as well as methyl salicylate (Stepanycheva et al., 2016).
Here, we characterized what are the pests that feed on tef in Israel, and how the plants defend themselves against these pests. Plant genotypes (accessions or lines) can widely differ in their molecular responses to aphids (Song et al., 2017). We hypothesize that tef plants evolved defense mechanisms similar to other Poaceae plant species, that can vary between tef accessions. To reveal the variety and effectiveness of tef defense mechanisms, we used three tef accessions. We started this study by elucidating the overall insect abundance on tef in the field, then focused on one pest, the bird cherry-oat aphid (Rhopalosiphum padi L.), which is among the most agriculturally devastating aphids worldwide (Blackman and Eastop, 2000; Parry, 2013). We analyzed the differences in insect performance and preference, trichome density, and metabolic and transcriptomic changes in response to aphid attack. We discovered that tef plants rely on both physical and chemical defenses and adjust their central metabolism in repose to aphid attack. Our work is the first report to highlight the defense mechanisms of tef plants in response to herbivore attack on the molecular level. These findings could be further utilized to reduce pesticide applications and breed accessions with enhanced resistance.
Three tef accessions, RTC-144, RTC-405, and RTC-406, were selected from the available germplasm (Ben-Zeev et al., 2018). Among 273 tef accessions examined in this field study, both RTC-405 and RTC-406 were found suitable for Mediterranean climate and used as standards in our earlier trials. RTC-144, which is also named “Magna,” is an improved variety that was previously used as a part of 20 tef cultivars panel, for discovering novel, simple sequence repeat (SSR) markers (Cannarozzi et al., 2014). The plant phenotypes, and seed color of the three accessions are presented in Supplementary Figure S1. Field experiments were conducted at two research sites: (i) Sede Boqer campus, southern Israel (30.87417°N, 34.79639°E), and (ii) Revadim, central Israel (31.772576°N, 34.806949°E). The Sede Boqer experiment consisted of three 1 m2 plots of each of the three tef accession, randomly positioned with 1 m distance between plots. Water was provided once a week, either via rainfall or irrigation. Fertilizer was provided as previously described (Batyrshina et al., 2020a), and no pesticides or herbicides were applied during the experiments. The Revadim experimental site included a total of 21 accessions sown in a randomized block design with four replicates. Each plot was 8 m long by 1.93 m wide. Water was applied once a week using a sprinkler irrigation system. All management operations (soil preparation, irrigation, and pesticide application), were conducted according to the commercial growing protocol adopted by local farmers in Israel. Only two accessions were grown in this site, RTC-405 and RTC-406. The insect survey was conducted by holding the VortisTM suction sampler (Burkard Manufacturing Co., Ltd., United Kingdom) above the plants across the 1 m2 plot (Sede Boqer), and along 15.4 m2 (Revadim) and vacuuming at maximum suction power for 30 s into a 50 mL collection tube (Arnold, 1994; Zentane et al., 2016). Sampling was done prior to flowering (late May 2019), and during flowering (late June 2019). Insects were subsequently kept in 2–3 mL of 70% ethanol, transferred to 9 cm diameter Petri dishes, then observed by stereomicroscope (Nikon SMZ745, Nikon Instruments Inc., United States) under 10x magnification. The insects were sorted by order level, using the Key to Insects Orders (extension.colostate.edu/Gardennotes/315.pdf) and family level (Hamilton et al., 2012; Zettler et al., 2016), and normalized for insect order per square meter of tef plants (Supplementary Table S1 and Supplementary Figure S2).
Several dozen tef seeds were sown on moistened soil mix [tuff mixture with vermiculite (2:1) and an N-P-K fertilizer] in 330 cm3 plastic pots, maintained under controlled growth conditions with a light regime of 12 h light/12 h dark photoperiod at a constant room temperature of 26–28°C, relative humidity of 60–70%, and an average light intensity of 300 μmol photons m–2 s–1. After 2 weeks, the seedlings were transplanted into individual plastic pots, and the same growth conditions were maintained.
The bird cherry-oat aphids (Rhopalosiphum padi) were collected from the field in Spring 2017, and the colony was reared on tef plants (accession RTC-144) under controlled conditions, as mentioned above. For the aphid reproduction bioassay, 20 adult aphids were applied onto 1-month-old tef plants for 4 and 7 days (14–15 biological replicates were tested at each time point and accession). The total number of aphids was counted (total nymphs and adults) and divided by the initial number of adults. The bioassays were conducted in a whole cage bioassay where plants were covered with plastic bags (Cryovac Crispac Beutel Super Micro Lochung 15 × 60 cm; Baumann Saatzuchtbedarf, Germany). After infestation time, tissue samples were harvested and flash frozen in liquid nitrogen, then stored at −80°C for further metabolic analysis.
The Y-shape olfactometer was built as previously described (Akol et al., 2003), with several adjustments. It was comprised of a 21 cm-long base with an internal diameter of 3.5 cm and two lateral 15 cm branches at an angle of 75°connected to a 10 L glass beaker in which the odor source was held (see Supplementary Figure S3). The tef plants were held for 1 h in the glass beaker as a source of volatiles, and air was provided at 0.8 L min–1 to both branches of the Y-tube via an air pump. One adult aphid was released within the base of the Y-tube with a paintbrush after being starved for 2 h. The aphid choice was conducted up to 5 min, and an aphid that walked halfway or more toward the Y-tube lateral branches was reported as a responsive individual. A 20W fluorescent light was placed 0.5 m above the Y-tube olfactometer in a controlled environment (25°C and 60% relative humidity) to disable the insect’s vision. The positions of the volatile sources were alternated between replicates to eliminate directional bias. All glassware and Y-tubes were cleaned and sterilized with 70% ethanol before new plants were used to reduce the risk of contamination by previously tested volatiles. Overall, the test was repeated five times for each pair of odor sources, with 30 adult aphids. As a control, aphids were introduced to the same accession (RTC-405) from both sides of the Y-tube, which had shown no significant differences, indicating that the olfactometer system is balanced.
Aphid feeding behavior was monitored on two tef accessions, RTC-144 and RTC-406, using the EPG on a Giga-8dd system (Wageningen, Netherlands). A gold wire (18 μm diameter) was attached to the dorsal surface of each R. padi aphid’s abdomen using silver glue (Salvador-Recatalà and Tjallingii, 2015). One-month-old tef plants were placed into a Faraday cage, electrodes were placed into the soil, and the insect probes were adjusted, allowing for contact between the leaf surface and the insect. Voltage waveforms were digitized at 100 Hz with an A/D converter, and patterns were identified as previously described (Tjallingii, 1978; Tjallingii and Esch Hogen, 1993). Waveform recordings were dissected every 30 s with the EPG analysis software StyletD installed in a computer connected to a Giga direct current amplifier. The parameters measured were comparable to those categorized by Sarria et al. (2009): (i) time until first probing (t_1Pr), (ii) xylem–including duration (s_G), and number of occurrences (n_G); (iii) phloem–including the total duration of E1 followed by E2 (s_E1– > E2), the total duration of E (s_E), number of E1 occurrences (n_E1), and number of E2 occurrences (n_E2); (iv) all tissue–including the total duration of C occurrences (s_C), the total duration of non-probing occurrences (s_NP), the total duration of potential drops occurrences (s_PD), number of probing occurrences (n_Pr), number of non-probing occurrences (n_NP), and number of potential drop occurrences (n_PD). The pathway phase analyzed A, B, and C were not calculated separately. EPG waveforms and results were analyzed using StyletA software as previously described (Nalam et al., 2018), and Excel for automatic parameter workbook calculation (Sarria et al., 2009). The data for the four phases was recorded for 6 h, while after the 4–5 h, plant rejections were observed. Therefore, we analyzed the first 3 h, where the significant possible sequence of feeding differences was detected (Marchetti et al., 2009). Overall, 15 plants from each accession were tested.
Tef plants from the three accessions were grown for 1 month (no aphids were applied on these leaves). Then, 2 cm sections were sampled from the widest part of three leaves: (i) lower leaf (a first leaf from the base), (ii) middle leaf, and (iii) upper leaf. The three leaves were dissected, bleached in 80% (v/v) ethanol, boiled at 90°C for 20 min, and washed with distilled water as previously described (Batyrshina et al., 2020b). For trichome visualization, leaves were mounted on microscope slides with the adaxial side facing up, covered with glass coverslips. A digital camera connected to an Axioplan 2 Upright Light Microscope (Zeiss, Oberkochen, Germany) was used for imaging. For each tef accession, five biological replicates with two pictures per leaf were taken. For density quantification, trichomes were counted using ImageJ software1 and normalized per mm2.
One-month-old plants of the three accessions were infested with R. padi aphids for 4 days, and tissue samples were harvested and immediately frozen in liquid nitrogen and stored at −80oC. Then, 1 g of frozen tissue was ground and added to a 20 mL glass vial (Chrom4, Thüringen, Germany), containing 0.8 μg isobutylbenzene internal standard (Sigma-Aldrich, Israel), 7 mL NaCl (20%), and 1 g NaCl. A divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS 50/30 μm, Supelco/Sigma-Aldrich, Israel) solid-phase micro-extraction (SPME) fiber was used to collect VOCs. Since tef volatiles have not been previously studied, a broad survey to reveal all potential VOCs was selected. C2–C20 n-alkane size standards were added to the samples (Garcia-Esteban et al., 2004). A COMBI PAL-XT (CTC Analytics AG, Switzerland) auto-sampler/robot for Agilent gas chromatography (GC) 7890 connect to mass spectrometry (MS) 5977b was used. Glass vials were heated at 60°C for 15 min prior to sampling, after which the fiber was inserted into the vial headspace for an additional 15 min at the same temperature. The vial needle penetration was 11 mm. The injection volume was 10 μL, the needle penetration was 32 mm, and the injection fiber exposure was 22 mm for an absorption time of 10 min. The analytes were then desorbed by heating the fiber in the injection port of a GC-MS to 250°C for 3 min. The analytes were separated on a VF-5MS + 10 m EZ guard capillary column (30 m × 0.25 mm × 0.25 μm; Agilent CP9013, United States). The oven temperature program was as follows: 40°C initially for 1 min, increased to 250°C at 6°C/min, followed by a post-run 280°C for 5 min. Helium was used as a carrier gas at a constant flow rate of 1 mL min–1. Injection temperature was set to 270°C (splitless mode), the transfer line temperature was 280°C and the ion source was adjusted to 230°C. Mass spectra were collected at 2.1 scans s–1 with a scanning range of 40–400 mass−to−charge (m/z) ratio and electron energy of 70 eV. Extracted compounds were tentatively identified based on Wiley 10 with NIST 2014 mass spectral library data using the MassHunter software package (version B.10.0.368, Agilent, United States). Further compound identification was based on a comparison of mass spectra and retention times with authentic standards (Sigma−Aldrich, Israel) analyzed under similar conditions. Compounds that could not be identified using standards were designated as “Unidentified,” followed by their putative class (Supplementary Tables S2, S3). For each tef accession, 4–5 biological replicates were analyzed.
One-month-old plants of the three accessions were infested with R. padi aphids, or kept uninfested as control, following the non-choice whole cage bioassay as described above. After 4 days, the samples were harvested and immediately flash frozen in liquid nitrogen, and stored at −80°C. Then metabolites were extracted using 100 mg of ground frozen plant tissue mixed with a methanol/water/chloroform solvent at a ratio of 55:23:22 (v/v/v) following a previously described protocol, with minor modifications (Rosental et al., 2016). In brief, the top 300 μL of hydrophilic layer was collected and dried in a vacuum. For derivatization, 40 μL of 20 mg/mL methoxyamine hydrochloride (Sigma-Aldrich, Israel) was added, dissolved in pyridine, and incubated for 2 h in an orbital shaker at 37°C. Next, N-methyl-N-(trimethylsilyl) tri-fluoroacetamide (MSTFA), including an alkane standard mix in a volume of 77 μL, was added to each sample, followed by a 30 min incubation in an orbital shaker at 37°C. Finally, 1 μL of the sample was injected into the Agilent 5977B GC-MS instrument. Data acquisition was conducted using the Mass Hunter software, NIST mass spectral library, and retention index (RI) libraries2 (Lisec et al., 2006; Hochberg et al., 2013). Each metabolite was normalized to D-sorbitol (13C6) as an internal standard and presented as the relative abundance of the ion counts (Supplementary Tables S4, S5). For each tef accession, 4–5 biological replicates were analyzed.
One-month-old RTC-144 tef plants were infested with 20 adult R. padi aphids for 6, 24, and 96 h as well as uninfested control using a non-choice whole cage bioassay as described above. All plants were caged at the beginning of the experiment, and the addition of aphids was staggered so that the leaf tissues for gene expression were harvested at the same time (96 h after the start of the experiment). For each time point, three replicates were generated. Total RNA was extracted using an SV Total RNA Isolation Kit with on-column DNaseI treatment (QIAGEN), then purified and quantified. For next-generation sequencing, 2.5μg of each sample was used. The paired-end (150 bp read length) RNAseq was conducted using an Illumina HiSeq 4000 instrument by GeneWIZ Inc.3 Quality control was performed using FASTQC. Adapters and low-quality sequences were trimmed and excluded using Trimmomatic v0.36. Then, mapping was performed using STAR aligner v2.5.2b against the Eragrostis tef reference transcriptome version 1.0 (Cannarozzi et al., 2014). Reads aligning to exons were retrieved using Subread v1.5.2. Differential gene analysis was performed using DESeq2 v1.22.2 (Love et al., 2014), via a likelihood ratio test to evaluate multiple genotypes at once (adjusted p < 0.05). The data was transformed using rlog (Supplementary Table S6). GO annotations were extracted by comparison with the SwissProt annotation of tef genes provided by Cannarozzi et al. (2014) to functional annotation of SwissProt entries. Gene expression fold change was calculated by dividing each value by the average of the gene control samples. The raw sequence data have been submitted to NCBI Sequence Read Archive (SRA) accession PRJNA623870.
The olfactometer results were examined by chi-square goodness of fit test at p < 0.05. The EPG parameters were compared between the two accessions using a paired Student’s t-test, p < 0.05. Differences in aphid reproduction using a non-choice bioassay and trichome density among accessions at each time or leaf section, were analyzed by two-way ANOVA (analysis of variance), and one-way ANOVA (each time point or leaf section, respectively), followed by a post hoc test using TukeyHSD, corrected with the false discovery method. These analyses were conducted by JMP13 software (SAS)4, and figure presentations were done in Microsoft Excel. For the VOC and central metabolic analysis, the raw data were normalized using the MetaboAnalyst software using the following steps: observations missing more than 50% of value estimation features were removed and replaced by a small value that was calculated as half of the minimum value of the original data, and the interquartile range data was filtered, then normalized to the median, transformed into log scale, and auto-scaled (Xia et al., 2009). The normalized data was used for the heatmap, the two-way ANOVA, and the paired Student’s t-test (p < 0.05) corrected with the false discovery method. These analyses were conducted by MetaboAnalyst. The principal component analysis (PCA) and Venn diagrams were calculated and designed using R. For the heatmap, the Euclidean distance with Ward’s minimum variance method was calculated using the default parameters.
In the first sampling date (May 2019; prior to tef flowering) at the Sede Boqer field insect survey, seven insect orders were detected on tef plants: Coleoptera, Diptera, Hemiptera, Hymenoptera, Lepidoptera, Neuroptera, and Orthoptera (Figure 1). The largest number of insects counted on all three tef accessions were of Hemiptera, including three families: Pentatomoidea, Cicadoidea, and Aphididea, the smallest number belonged to Lepidoptera. The survey indicated differences in insect abundance between the three tef accessions, wherein Orthoptera showed more than twofold differences between the tef accessions (13% to RTC-144 relative to 6% to RTC-406 from the total insects per accession). The Dipteran order, including the superfamily Tachinidea, and families Muscidae and Syrphidae, play an essential role in various trophic levels both as pests of crops, as well as pollinators (Rader et al., 2016). The Dipteran order showed the most diversity between the three tef accessions and was 2.5 times more abundant on RTC-144 (10%) than RTC-405 (4%). A similar trend was detected on the second sampling date (June 2019) in Sede Boqer, as well as in the Revadim site (Supplementary Table S1). Results of both sampling dates and sites emphasize that the insects from the Hemiptera order are highly abundant on tef plants in this geographic region, 23–34% in Sede Boqer, and 30–38% in Revadim from the total insects per accession.
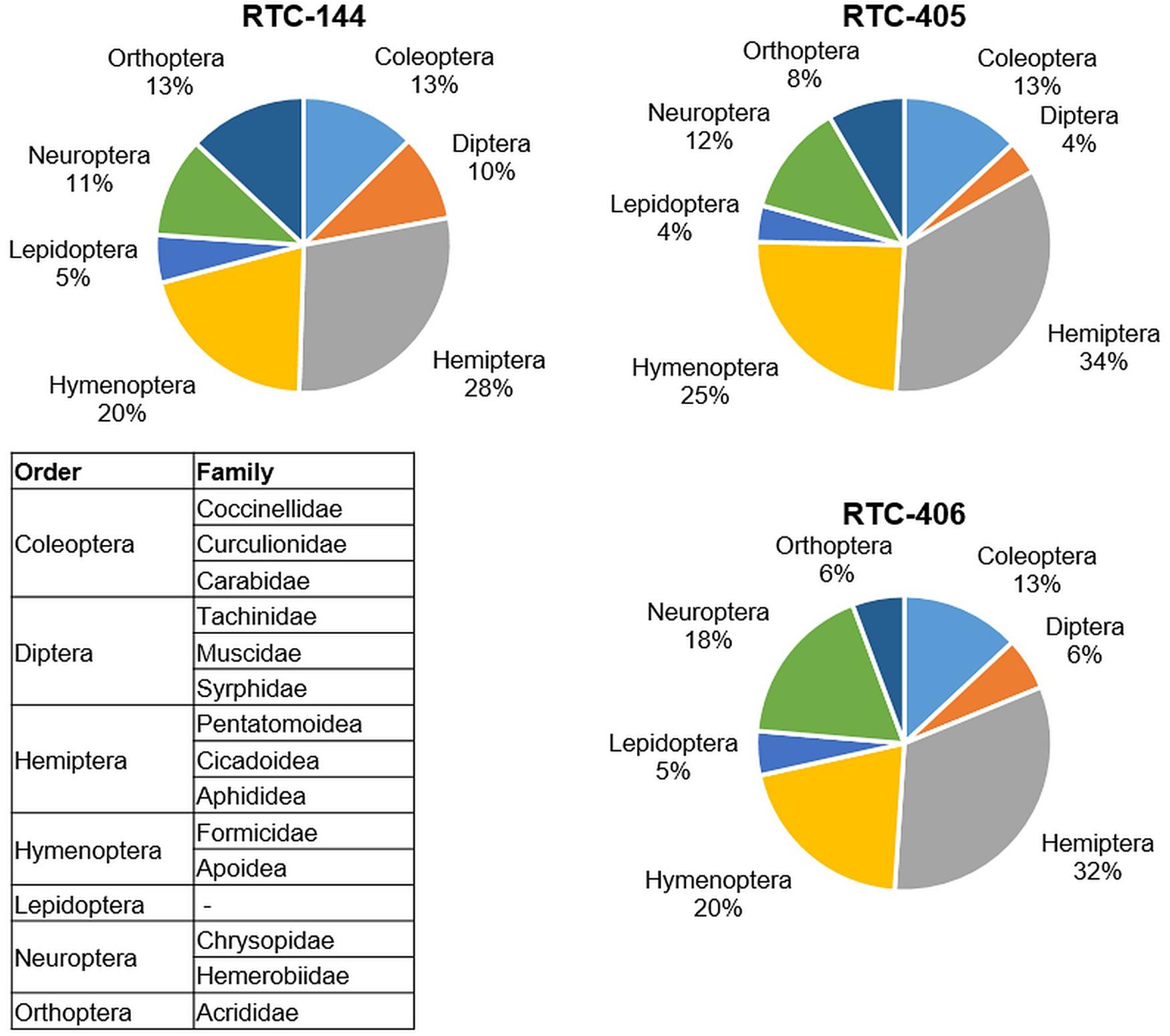
Figure 1. Insect abundance on the three tef accessions from the field survey. Pie chart of the insects that were collected in three locations in the field (total of 3 m2 and normalized to 1 m2) sorted by orders. The insect families monitored in each order are included in the table. Sampling was performed prior to flowering (late May 2019).
The bird cherry-oat aphid (Hemiptera; Aphididae; Rhopalosiphum padi), is highly abundant on host plants from the Poaceae family (Swirski and Amitai, 1999). Thus, we characterized tef defense responses by focusing our laboratory experiments on a single aphid species, R. padi. First, we performed a choice bioassay using a Y-shape olfactometer. The results showed that aphids tended to be repelled by accession RTC-144 compared to either RTC-405 or RTC-406, while no preference between the two later accessions, RTC-405 and RTC-406 were observed (Figure 2A). Additionally, we evaluated the aphid reproduction on the three tef accessions at two infestation time points, 4 and 7 days, using a non-choice bioassay (Figure 2B). The two-way ANOVA suggested a significant difference between the three tef accessions (Faccession 2,86 = 8.44, p = 0.0005), the time of aphid-infestation (Ftime 1,86 = 61.53, p < 0.0001), but no significant interaction between the two factors (Faccession*time 2,86 = 2.45, p = 0.092). A one-way ANOVA of the aphid number at each time point indicated that after a 4 days infestation, the number of aphids was significantly lower in the RTC-406 accession relative to the other two accessions, while after 7 days, there was only a significant difference between RTC-144 and RTC-406.
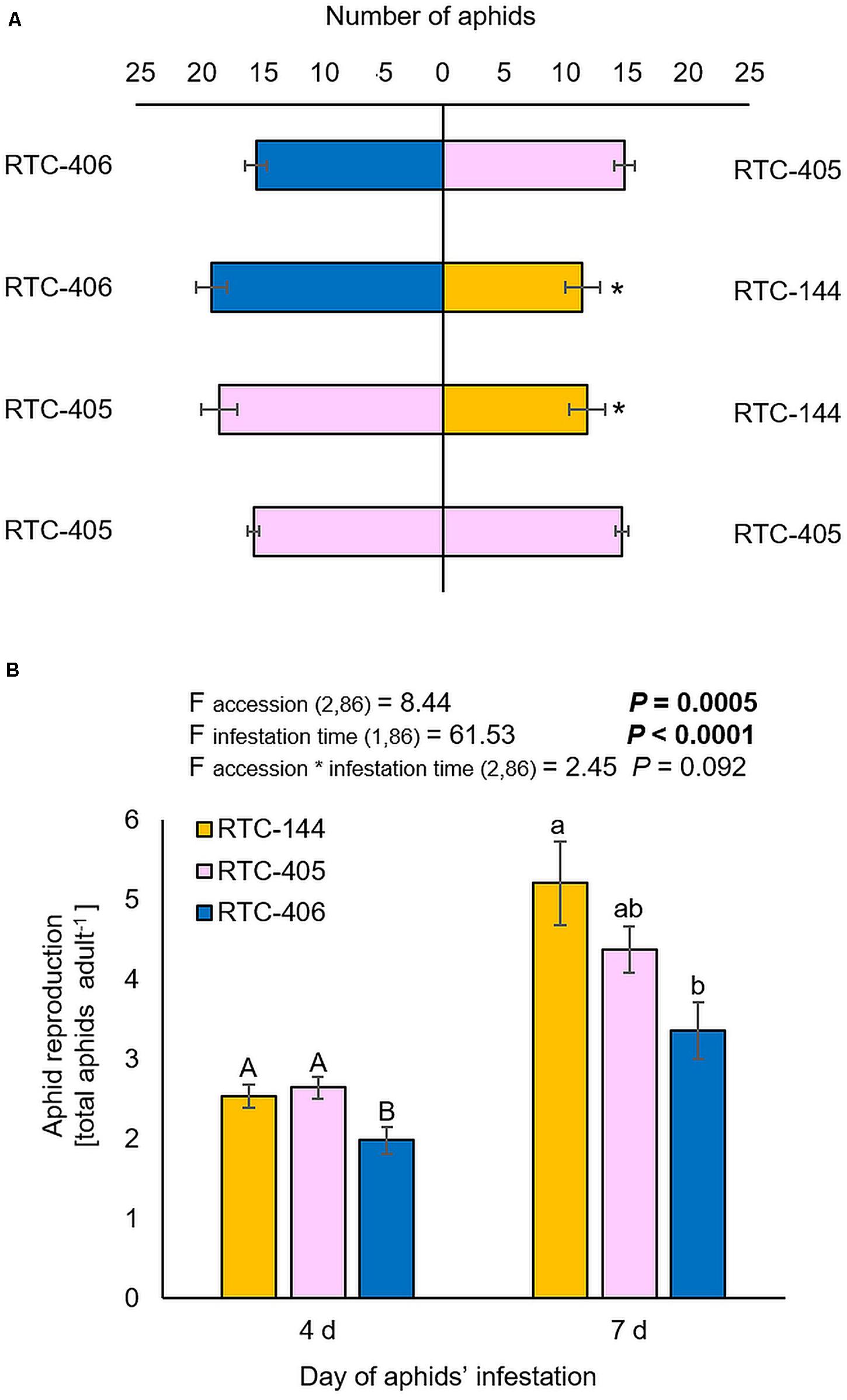
Figure 2. Aphid performance and preference for 1-month-old tef plants. (A) A Y-tube olfactometer choice bioassay was used to determine the aphid preference. Bars represent the average number of aphids (mean ± SE, n = 5). In each replicate, 30 aphids were tested. The asterisk indicates significantly different choices as determined by the chi-square goodness of fit test at P < 0.05. (B) A non-choice bioassay was used to determine the differences in aphid performance between the three tef accessions. The whole-plants were infested with 20 adult R. padi aphids for 4 and 7 days, then the total number of adult and nymphs was counted (mean ± SE, n = 14–15). On the top, a summary of the two-way ANOVA, comparing the aphid reproduction among the three accessions at two infestation time periods 4 and 7 days (p < 0.05). Different letters above the bars indicate significant differences, using one-way ANOVA followed by TukeyHSD test separately for each time point, corrected with the false discovery method.
Lastly, we investigated aphid feeding behavior using the electrical penetration graph (EPG) technique (Tjallingii and Esch Hogen, 1993). We conducted this experiment on two selected accessions, RTC-144 and RTC-406, which possessed opposite trends in performance and preference (Figure 2). Parameters from the four phases were recorded, including epidermis, xylem, phloem, and all tissues (the phases were categorized by Sarria et al., 2009). As shown in Table 1, three variables were significantly different between the tef accessions. The time to first probe from the start of EPG (t_1Pr) was significantly longer in RTC-406 (13.05 min) than RTC-144 (4.59 min). The number of xylem events (n_G) was larger on RTC-144 (2.58 times) than RTC-406 (1.29 times), and the total duration of non-probing (s_NP) was longer for RTC-406 (22.30 min) than RTC-144 (11.82 min). Altogether, the results indicated that the variation in aphid performance and feeding behavior between the tef accessions might be due to multiple factor defense responses. Thus, we performed several experiments to reveal these factors, including evaluating the physical barriers related to the time to first probing, and central and specialized metabolites that might affect reproduction. Additionally, we quantified volatile content, as their potential emission can affect aphid preference from a long distance.
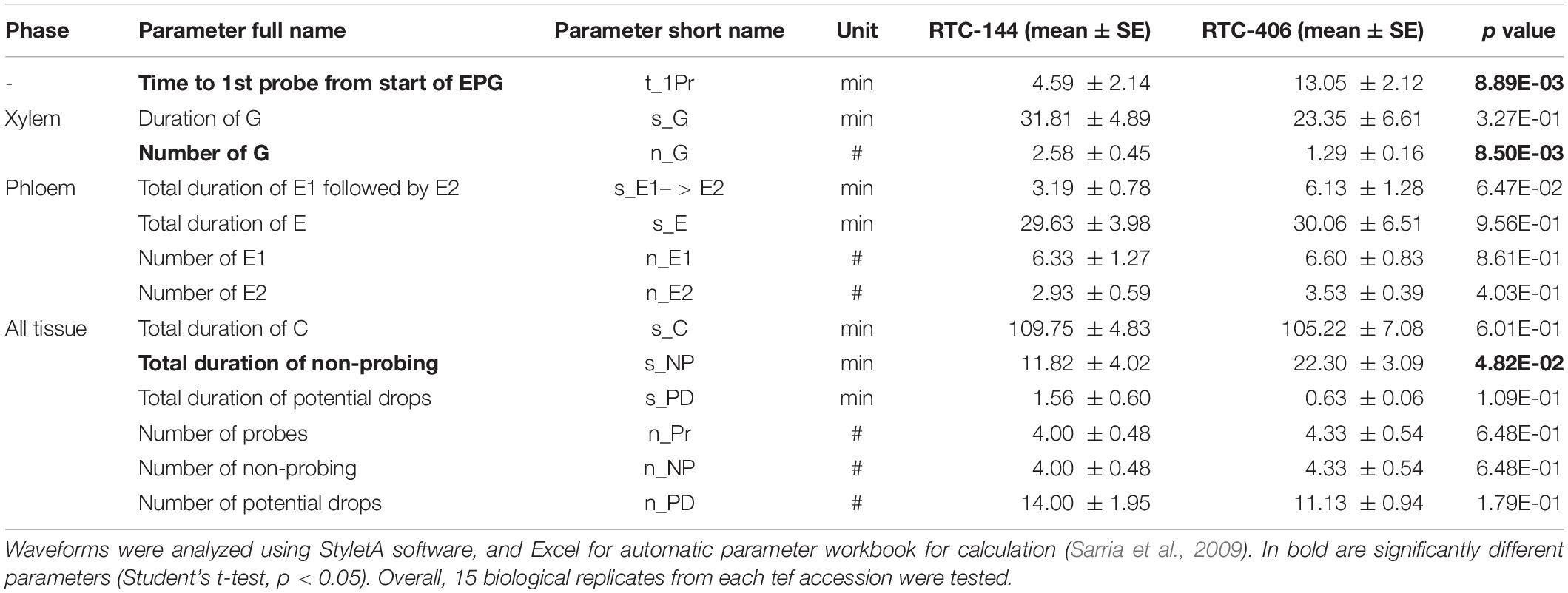
Table 1. Feeding behavior of Rhopalosiphum padi on two tef accessions using the electrical penetration graph (EPG) technique.
The trichome density was evaluated on the lower, middle, and upper leaves of the main tiller. The experiment was conducted on uninfested leaves, and therefore, represent the constitutive trichome levels. As presented in Figure 3, the two-way ANOVA suggested a significant difference in trichome number between tef accessions (F accession (2,89) = 49.74, p < 0.0001), leaf position (F leaf position (2,89) = 261.70, p < 0.0001), and a significant interaction between the two factors (F accession*leaf position (4,89) = 3.99, p = 0.0052). Between three tef accessions, the number of trichomes was significantly higher in the middle leaf than on the lower and upper leaf. Next, we analyzed the differences in trichome density at each leaf position between accessions, using one-way ANOVA. The results revealed that RTC-406 possessed the highest number of trichomes on all three leaves compared to the other two accessions. The high trichome density of RTC-406 can limit aphid feeding and cause a reduction in their reproduction.
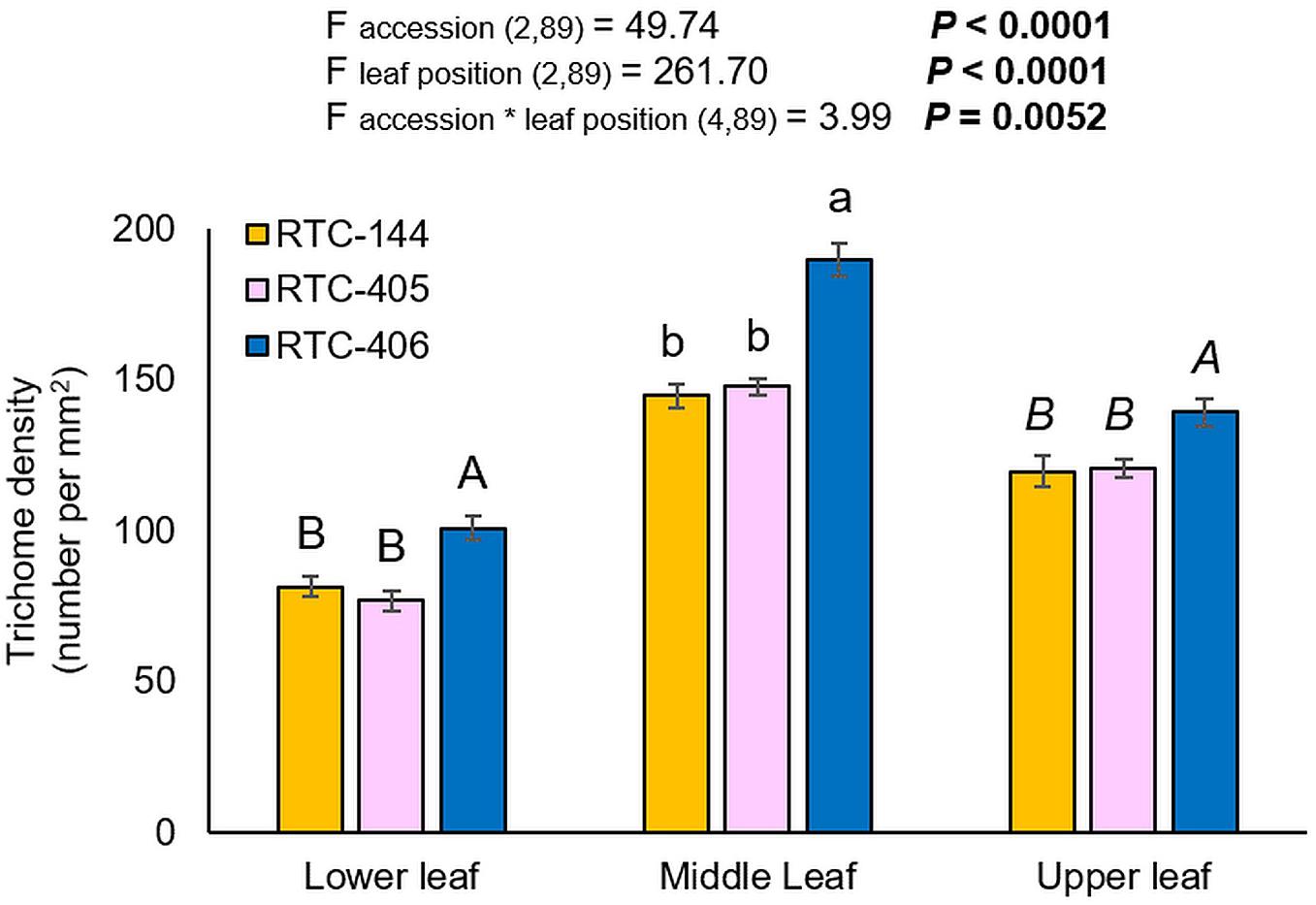
Figure 3. Trichome density of three tef leaves. Bars represent the average number of trichome density per mm2 (mean ± SE, n = 10). On the top, a summary of the two-way ANOVA, comparing the number of trichome among the three accessions at three leaf sections (p < 0.05). Different letters above the bars indicate significant differences, using one-way ANOVA followed by TukeyHSD test separately for each time point, corrected with the false discovery method.
The olfactometer experiment indicated that aphids respond according to the variation in tef’s volatile organic compound (VOC) profile, which conveys long-distance signals. Thus, we analyzed the tef VOC profiles of aphid infested plants using solid-phase micro-extraction (SPME) coupled with GC-MS. In total, 105 VOCs were identified and classified into five main chemical groups: fatty acid (FA) derivatives (including green leaf volatiles; GLVs), furans, terpenoids (mono-, and irregular terpenes), phenylpropanoids and benzenoids, and an unidentified nitrogen-containing compound. A two-way ANOVA analysis revealed 74 metabolites that were different in one of the factors: accession and aphid treatment, or an interaction between the two factors (Supplementary Table S3). A heatmap of the normalized value of these 74 metabolites is presented in Figure 4. The results revealed that treated and untreated RTC-405 and RTC-406 accessions were clustered together, while the aphid-treated and untreated RTC-144 samples were grouped separately. In the RTC-144 accession, almost half of the VOCs, belonging to classes of FA derivatives (aldehydes, ketones, and alcohols), terpenes, and furans, decreased under aphid attack, while the ester FA derivatives increased. The VOC changes were slight in RTC-405 and RTC-406 accessions. To detect the changes induced in response to aphids, paired t-tests were conducted between aphid-treated samples relative to untreated control in each accession separately. Table 2 presents only metabolites with at least twofold changes, and p < 0.05, false discovery rate (FDR) adjusted. RTC-144 showed a massive modification in the VOC levels, including a reduction in aldehyde-, ketone-, and alcohol FA derivatives, furans and phenylpropanoid and benzenoid classes, and induction in the ester FA derivatives. In RTC-405, only three metabolite levels were altered, including 2-methyl-2-butene and ethyl 3-hexanoate and methyl hexanoate, which, together with methyl hexanoate, were the only metabolites significantly increased in all three accessions. Altogether, this suggested that tef leaves possess a rich and unique blend of VOCs, which was largely modified in response to aphid infestation, especially in the RTC-144 accession.
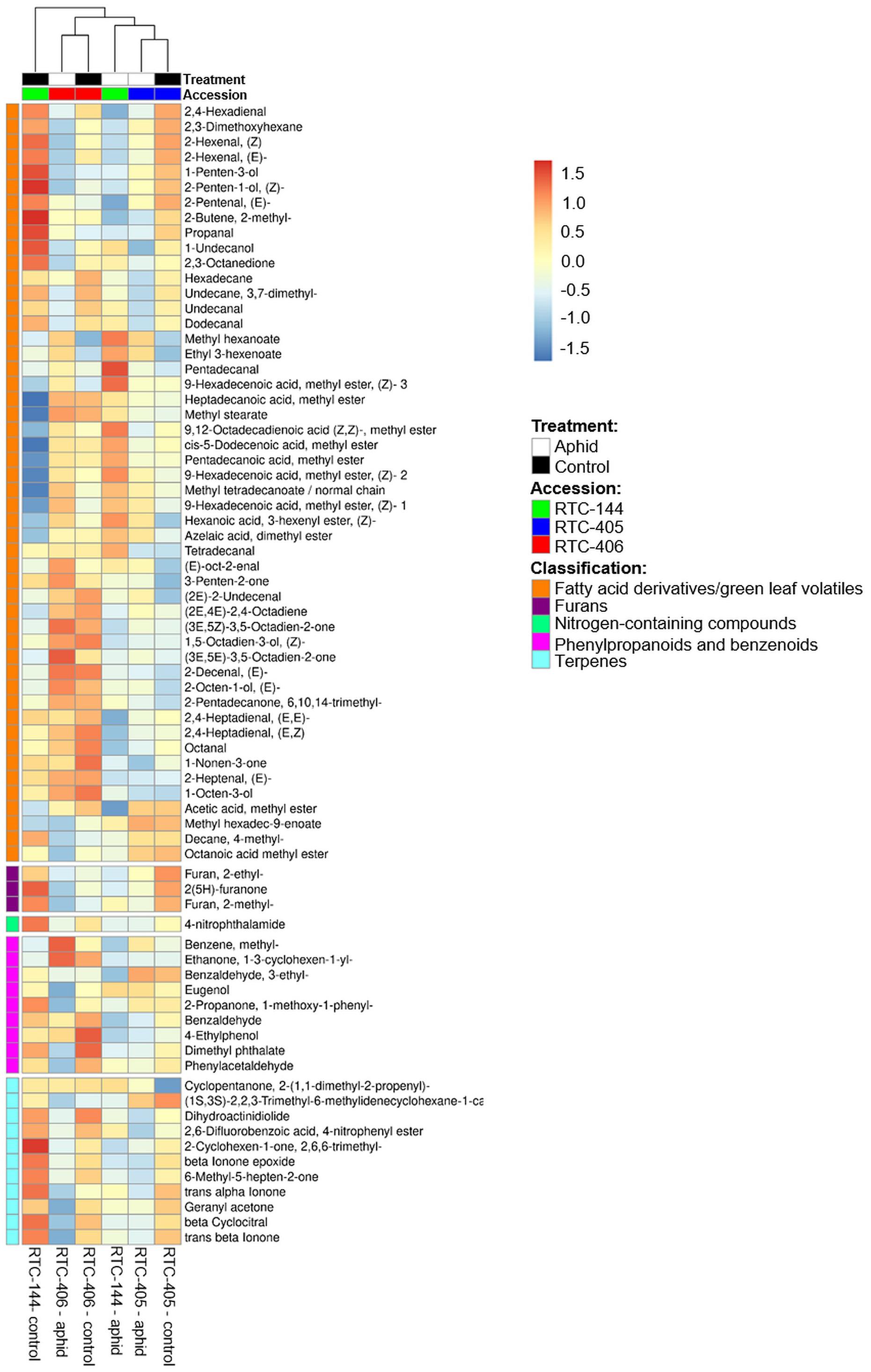
Figure 4. Heatmap of the VOC profile of aphid-infested and untreated control tef plants. The VOCs were selected using two-way ANOVA comparing the three accessions and the aphid treatment. The Euclidean distance with Ward’s minimum variance method was calculated using the default parameters of the MetaboAnalyst software, and the graph was created in R and presented in average values. Colors correspond with concentration values (autoscaled parameters), where red indicates high levels, and blue indicates low levels (n = 4–5 biological replicates).
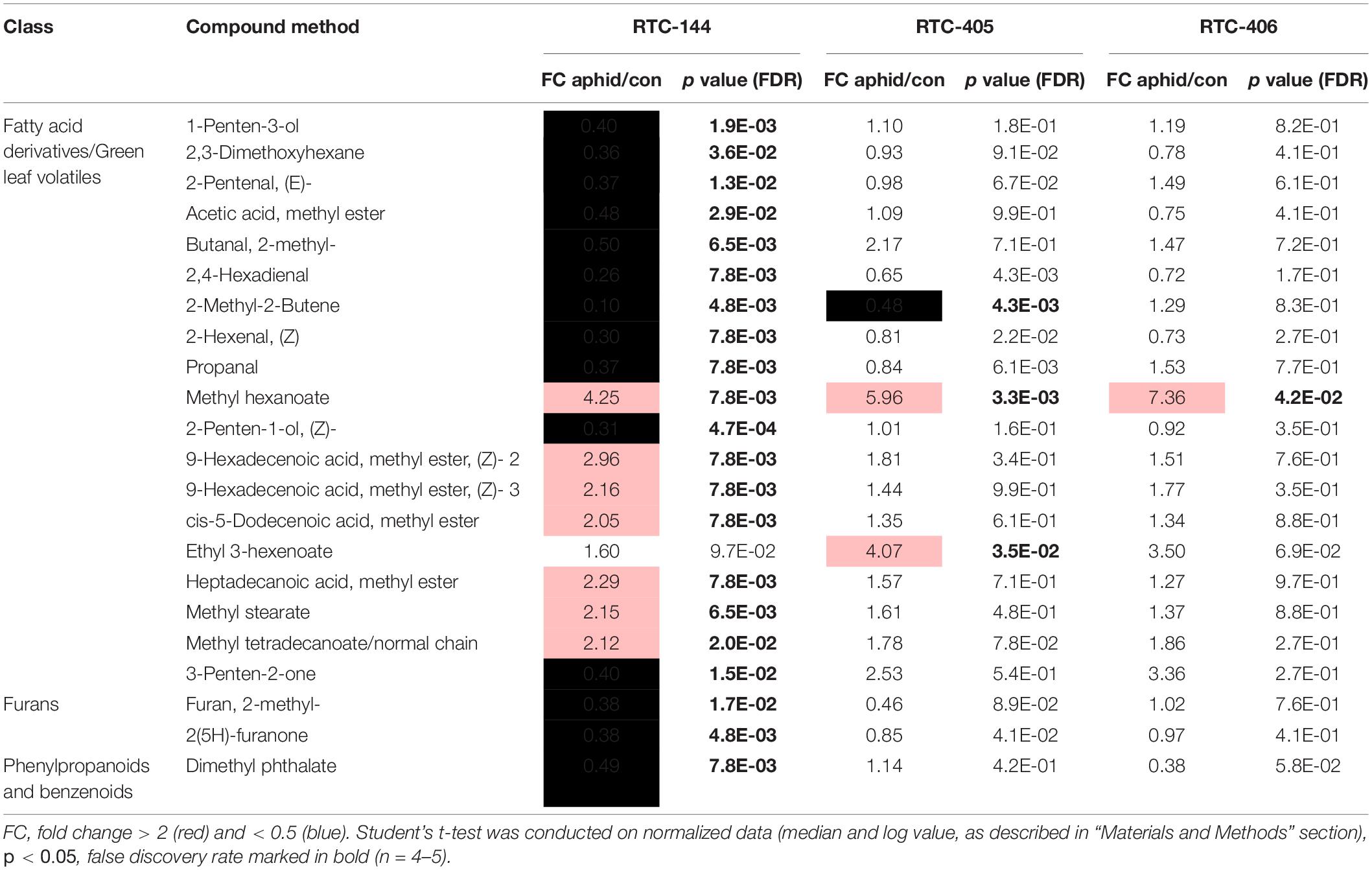
Table 2. Volatile organic compounds significantly modified in response to aphid feeding in at least one tef accession.
We also characterized the central metabolite profiles of the three tef accessions and their adjustment to aphid feeding after 4 days of infestation, using GC-MS. The levels of 65 metabolites were detected, including amino acids, amino alcohols, lipids, nucleotides, organic acids, sugars, and sugar alcohols (Supplementary Table S4). A two-way ANOVA analysis revealed a total of 24 metabolites that were either significantly different between the accessions, in response to aphid infestation, and/or interaction between the two factors (Supplementary Table S4). Figure 5 presents a heatmap of the average value of these 24 metabolites. The levels of most of the sugars and organic acids, as well as glutamate and myo-inositol-2-phosphate, were high in the untreated plants. Upon aphid feeding, the levels of most of the sugars, organic acids, and the amino acid Gln declined, while the levels of most of the amino acids (Gly, Leu, and Val), and the organic acid pyruvate increased. This trend was strongest for accession RTC-406. To determine the inducible effect of aphid infestation, paired t-tests were performed, and FDR adjusted (p < 0.05) on metabolites with at least a twofold change. As presented in Table 3, the RTC-405 accession showed a significant reduction in organic acid, succinic acid, and two sugars (raffinose and xylulose-5-phosphate), while only Val was significantly elevated in RTC-144. RTC-406 showed a significant reduction in cellobiose, laminaribiose, and 2-oxoglutaric acid, while glucose and Val were increased. Altogether, this suggested that the composition of the central metabolites in the tef plants slightly shift from carbon-rich compounds such as sugars and organic acids, toward nitrogen-containing compounds such as amino acids, upon aphid feeding. The metabolic changes are more pronounced in RTC-406 than RTC-144 and RTC-405.
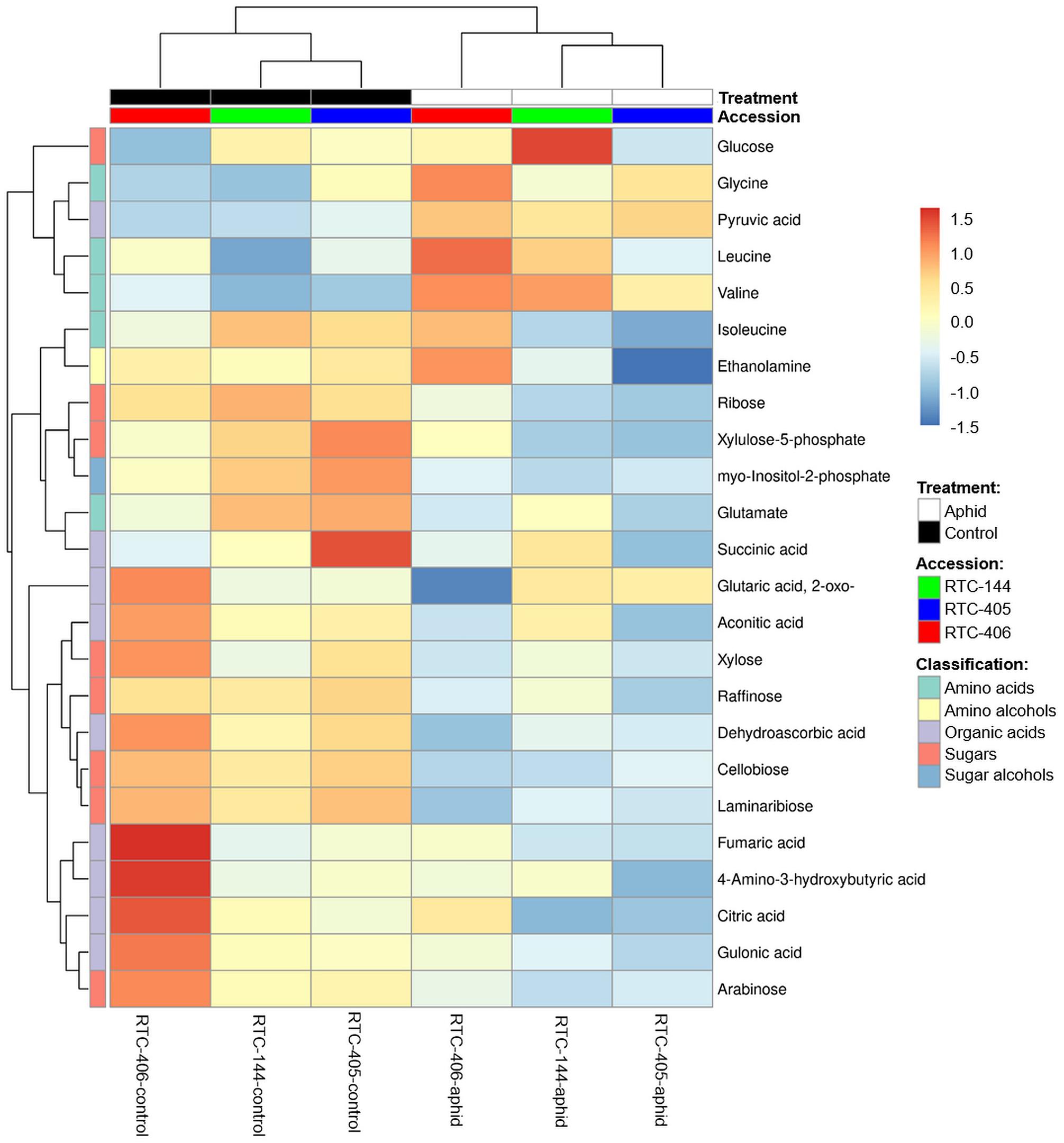
Figure 5. Heatmap of the central metabolites profile of aphid-infested and untreated control tef plants. The Euclidean distance with Ward’s minimum variance method was calculated using the default parameters of the MetaboAnalyst software, and the graph created in R. Colors correspond with concentration values (autoscale parameters), where red indicates high levels and blue indicates low levels (n = 4–5 biological replicates).

Table 3. Central metabolites significantly modified in response to aphid feeding in at least one tef accession.
We searched for the presence of known deterrent molecules, that were previously reported in other Poaceae family species by comparing the GC-MS data to gramine, and serotonin authentic standards, and HPLC to the benzoxazinoids authentic standards (data not shown). None of these indole-derived compounds were detected. Therefore, we performed a transcriptomic analysis and looked for potential specialized metabolite pathways that are modified in response to aphid infestation. The RTC-144 accession was selected due to its massive variation in VOC metabolism that might relate to the production of other non-volatile specialized metabolite pathways (War et al., 2012). A time-course experiment exposing 1-month-old leaves to R. padi for 6, 24, and 96 h, was conducted, and the transcripts were annotated to the gene models found in the Eragrostis tef v1.0 reference genome sequence (Cannarozzi et al., 2014). This analysis revealed a total of 35,284 transcripts (Supplementary Table S6). For an overview of the transcriptomic dataset, a PCA plot was constructed on the total tef transcripts. As presented in Figure 6A, the PCA plot indicated that samples from each infestation point were clustered together, with component 1 (90% variance) showing a separation of control and treated samples. Component 2 (3% variance) showed discrimination between 24 and 96 h, while the 6 h samples were divided between these two.
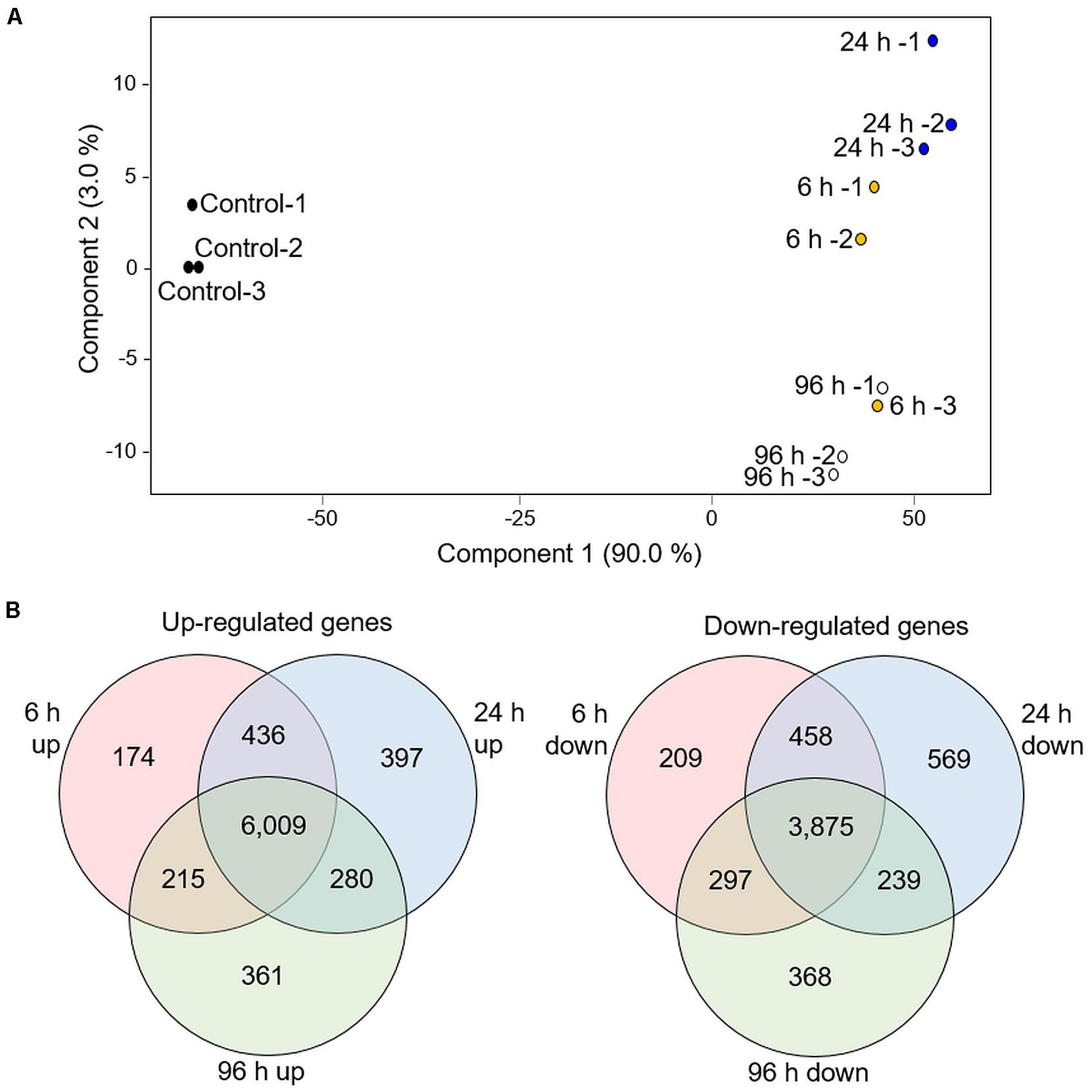
Figure 6. Transcriptomic overview of RTC-144 tef leaves infested with R. padi aphid for different periods. (A) PCA plot was generated using 35,284 genes. (B) Venn diagram illustrating the number of genes up- or down-regulated by aphid infestation in the time course. p < 0.05 FDR, and absolute fold change > 2 (n = 3 biological replicates for each time point).
We selected genes with significant expression differences (p < 0.05, FDR), and at least a twofold change relative to control, for at least one of the time points (Supplementary Table S7). The total number of up-regulated genes was 7,872, and the down-regulated genes was 6,015 (at least in one of the infestation time points). The distribution of up- and down-regulated genes was calculated for each time point and is presented in a Venn diagram (Figure 6B). Although a unique set of genes was modified at each time point, an impressively large number of genes were detected in the overlap between the three time points (6,009 up-regulated and 3,875 down-regulated genes) These set of genes were associated with defense strategies and metabolic adjustments.
To characterize the metabolic changes occurring in response to aphid attack, an over-representation pathway enrichment analysis was performed on the gene list from each Venn diagram group using the MetGenMAP tool (Joung et al., 2009), comparing the rice orthologs (LOC gene ID; Supplementary Table S8). The super-class of each pathway was categorized by RiceCyc output5. Table 4 presents the significantly enriched pathways of up- and down-regulated genes divided into 14 groups. The up-regulated enriched pathways belong to the biosynthesis of specialized metabolites from flavonoids, canavanine, and terpenes. Jasmonic acid biosynthesis, which is a defense-related phytohormone, was enriched upon 24 and 96 h of aphid feeding. In addition, the following pathways were overrepresented: amino acid metabolism (Gly, Cys, Pro, Trp, Asn, Asp, and Arg), nucleoside and nucleotide biosynthesis (purine and pyrimidine), cofactors, prosthetic groups, electron carrier biosynthesis (chlorophyllide a, glutathione), carbohydrate biosynthesis (UDP-D-xylose and dTDP-L-rhamnose) and cell structure biosynthesis (cellulose) (Table 4A). The down-regulated enriched pathways mainly included biosynthesis of specialized metabolites and phytohormones, such as phenylpropanoid biosynthesis, gibberellin, jasmonic acid, cytokinin, and ethylene. Genes from the following pathways were downregulated: carbohydrate biosynthesis (gluconeogenesis and trehalose), carbohydrate degradation (sucrose, starch, and mannose), as well as UDP-glucose conversion and generation of precursor metabolites and energy such as the Calvin cycle, glycolysis and photorespiration were over-represented pathways. Additionally, FA and lipid biosynthesis (acyl-CoA thioesterase and glycolipid), nitrogen metabolism, and Met, Cys, and His amino acid biosynthesis were downregulated (Table 4B). Overall, this suggested massive transcriptomic changes occurring in response to R. padi feeding on tef leaves and indicated few potential specialized metabolite pathways that might be involved in tef chemical defense mechanisms.
Our study is the first report of insect groups associated with tef crops grown in a Mediterranean climate. Seven orders were detected in our survey: Coleoptera, Diptera, Hemiptera, Hymenoptera, Lepidoptera, Neuroptera, and Orthoptera (Figure 1). In Ethiopia, the major insect pests of tef plants are the Wello-bush cricket (Decticoides brevipennis, order Orthoptera), the barley fly (Delia arambourgi; order Diptera), the black tef beetle (Erlangerius niger Weise; order Coleoptera), the Mendi termite (Macrotermes subhyalinus; order Isoptera), and red tef worm (Mentaxya ignicollis; order Lepidoptera). Among the minor pest abundance in Ethiopia are two aphids species from the Hemiptera order, Russian wheat aphid (Diuraphis noxia) and corn leaf aphid (Rhopalosiphum maidis), and desert locust (Schistocerca gregaria; order Orthoptera) (Gebremedhin, 1987; Stallknecht et al., 1993), and Insect Pests of Cereals in Ethiopia database6. There are some similarities between the insect orders in Israel and Ethiopia, but not in the insect abundance. In Israel, the most abundant insects in Sede Boqer (three tef accessions) and Revadim (two accessions) were Hemipterans and their three families, Pentatomidae, Cicadidae, and Aphidoidea (Figure 1).
In Israel, 194 aphid species were reported, and many of them are fed on Poaceae family plant species (Swirski and Amitai, 1999). Interestingly, green lacewings (Chrysopa perla), from the Neuroptera order and Chrysopidae family, were also spotted in the field. Larvae of this species are documented to be voracious predators feeding on aphids and other soft-bodied arthropods, therefore serving as a biocontrol of aphids (Tauber et al., 2000). Increasing vegetation biodiversity in agroecosystems can impact the abundance of insect herbivory and their natural enemies (Knops et al., 1999). Tef is commercially grown in Israel since 2014 at a minor scale and might change the vegetation biodiversity. If tef cultivation expands, it might affect insect pests, depending on the insect’s ability to use a wide range of plants such as wild and cultivated Poaceae plant species as well as alternative hosts.
Aphids are major agricultural pests worldwide and are considered a common pest on Poaceae family plant species such as maize, wheat, barley, and millets (Robinson and Hsu, 1963; Kalaisekar et al., 2017). The non-choice bioassay indicated that aphids reproduced in all three accessions; RTC-406 was the most aphid resistant among the three accessions (Figure 2B), while the choice bioassay revealed that RTC-144 is the most repelling (Figure 2A). The EPG results imply that the aphids settled and started probing more swiftly on the leaf of RTC-144 and spend less time non-probing than RTC-406. A recent study assessed the potential surface resistance of sorghum plants to sugarcane aphids (Melanaphis sacchari) and suggested that the aphids spend approximately twice longer in the non-probing phase in the resistant plants than in the susceptible plants (Tetreault et al., 2019). Barley leaves infested with R. padi showed a shorter time of salivation and ingestion of the phloem on resistant relative to the susceptible plants. Feeding patterns reflect many factors, including mechanical barriers present at the leaf surface, olfactory repellents, and host metabolism (Tetreault et al., 2019). The results highlight the need for conducting multiple bioassays combined with metabolic and transcriptomic methods to expose the mode of defense.
Tef leaves are covered with non-glandular trichomes (epidermal hair-like structures). Similar structures were observed on wheat and barley leaves (Leybourne et al., 2019; Correa et al., 2020). The non-glandular trichomes serve as a physical barrier that can limit insect movement and interrupt the stylet insertion of phloem feeders (Handley et al., 2005; Sato and Kudoh, 2015). Trichome density can vary by leaf position, development stages, genetic backgrounds, and even be induced upon insect attack (Leybourne et al., 2019). The RTC-406 accession possessed the highest trichome levels in all three leaves, suggesting the combined impacts of leaf position and genotype (Figure 3). Trichome density was negatively correlated with aphid reproduction (Batyrshina et al., 2020b), suggesting the role of non-glandular trichomes on tef leaves as a partial defense strategy. The trichome destiny and feeding behavior results emphasize that the high number of trichomes of RTC-406 tef leaves, is one of the factors that might extend the time of aphid penetration to the tef leaf tissue. The time spent by aphids in the phloem stage is linked primarily to feeding as well as acquisition and transmission of viruses and bacteria (Martin et al., 1997). However, we found no significant difference between RTC-406 and RTC-144 in the phloem phase. Aphids are phloem feeders that occasionally feed on xylem fluid (Nalam et al., 2020), possibly to attenuate the high osmotic potential of the phloem sap (Douglas, 2006; Tetreault et al., 2019). The EPG results expose that aphids spent more time ingesting sap in the xylem on RTC-144 than RTC-406. This might be due to differences in the constitutive levels of glucose between the two accessions (Figure 5), which is known to determine the osmotic potential of the phloem sap (van Bel and Hess, 2008). The results suggest that the factors involved in tef resistance are found not only on the surface but also in phloem and xylem composition.
Tef plants synthesized VOCs from five different metabolic classes (Supplementary Table S2). A recent study on two grasses, itchgrass (Rottboellia cochinchinensis) and African star grass (Cynodon nlemfuensis), showed that their VOC profile is composed of metabolites from different classes (Ramírez-Medorio et al., 2019). In contrast, wheat and maize main VOC classes are terpenoids and FA derivatives (including the GLV), which are associated with defenses (Richter et al., 2015, 2016). Although several mono-, and sesqui-terpenes have shown repellent properties to insects (Bleeker et al., 2009), none of the compounds from the terpene class were significantly modified in tef (Table 4), which suggests that other VOC classes might play a defensive role. Furans were only detected in fleshy fruits during ripening stages (Klein et al., 2007), but were not previously reported in vegetative tissues. This class might be unique for tef volatiles and might demonstrate that VOC compositions are species-specific (Nordlund et al., 1977).
Volatile compounds have broad ecological functions as olfactory repellents or attractants (Bernasconi et al., 1998; Jimenez-Martínez et al., 2004; Piesik et al., 2008). For example, (E)-2-pentenal (GLV class), and FA esters are known to have anti-feedant properties to aphids (Hammond et al., 2000; Santana et al., 2012). The VOC profile revealed that untreated RTC-144 plants, produced high levels of GLV, furans, and irregular terpenes and low levels of ester FA derivatives compared to RTC-406 (Figure 4). This accession repelled the R. padi aphids in the olfactometer choice bioassay (Figure 2A), which emphasizes that the VOC composition of RTC-144 has constitutive repellent properties. In response to aphid infestation, ester FA derivative levels increased while some of the aliphatics were reduced in RTC-144 (Figure 3). These results suggest that FA derivatives might have a potential function as attractants of predators and parasitoids (Kessler and Baldwin, 2002; Shiojiri et al., 2006); this requires further investigation. Methyl hexanoate was significantly increased in all three accessions (Table 2). This compound was previously reported to act as insect attractant pheromone of Mediterranean fruit fly (Ceratitis capitate) in peach plants (Prunus persicae), and found in low levels in the least susceptible cultivars (Tabilio et al., 2013). The ecological function of methyl hexanoate produced by the tef plants is yet unknown.
Numerous changes in the central metabolism of plants occur in response to insect herbivory, including the alternation of photosynthetic efficiency, remobilization of carbon and nitrogen resources, and regulation of plant growth rate (Zhou et al., 2015). The metabolic analysis of tef leaves infested with R. padi, indicated a shift in the biosynthesis of carbon-rich compounds (sugars and organic acids), toward nitrogen-containing compounds (amino acids). Modification of the amino acid composition and levels can reduce plant palatability and nutritional quality in the phloem sap, and contribute to increased resistance to aphids (Karley et al., 2002). The infestation of R. padi on barley plants under nitrogen-deficient growth conditions exhibited reduced reproduction rates relative to aphids exposed to plants grown under nitrogen-rich conditions (Ponder et al., 2000, 2001). This can be determined by the composition of amino acids. Previous studies reported that upon aphid infestation, the levels of essential amino acids were elevated in susceptible plants (Vogel and Moran, 2011; Leybourne et al., 2019). Similarly in the three tef accessions, both essential amino acids (Val and Leu), as well as a non-essential amino acid (Gly), were increased upon aphid attack (Table 3 and Figure 5). Cereal aphid species actively remobilize wheat and barley nutrients in the phloem to increase the abundance of amino acids, while R. padi seems to have a slight effect on amino acid composition (Sandström et al., 2000; Leybourne et al., 2019). To better understand the metabolic changes in the tef leaves, further metabolic analysis of the phloem sap is required.
The tef transcriptome was dramatically modified in response to aphid infestation. The effect of insect feeding on plant leaves is a dynamic process that continually changes according to exposure time (Tzin et al., 2015). A recent time-course transcriptomic analysis of wheat leaves infested with S. graminum aphids reported that approximately 10,000 genes were significantly altered (Zhang et al., 2020). In the tef leaves, the expression levels of 13,887 genes were significantly altered within 6 h and continued to change during the entire 96 h experiment (Figure 6). Herbivory causes changes in the expression of genes involved in both central and specialized metabolism (Appel et al., 2014). In the tef transcriptome analysis, the up-regulated enriched pathways included amino acid metabolism, and biosynthesis of nucleosides and nucleotides, cofactors, prosthetic groups, electron carriers, carbohydrates, and cell structures. The downregulated enriched pathways mainly included FAs and lipids, inorganic nitrogen metabolism, and amino acid biosynthesis (Met, Cys, and His), carbohydrate biosynthesis, carbohydrate degradation, glucose conversion and generation of precursor metabolites and energy such as through the Calvin cycle, glycolysis, and photorespiration. The observed reduction in carbohydrate metabolism and generation of precursor metabolites and energy pathways combined with modification in the biosynthesis of phytohormones such as jasmonic acid has been reported as the result of regulation of resource-based trade-offs between growth and defense (Mitra and Baldwin, 2014).
The transcriptomic dataset indicated that the gene expressions of different classes of specialized metabolites were over-represented, including flavonoids, canavanine, and terpenes. The accumulation of flavonoids, including the subclass flavones, flavonols, and anthocyanins, was enhanced in pea seedlings (Pisum sativum) in response to attacks by the pea aphid (Acyrthosiphon pisum) (Morkunas et al., 2016). Canavanine is a non-protein toxic amino acid, structurally related to the amino acid Arg. It is highly abundant in seeds and sprouts of many legumes and possesses insecticidal properties to most insects (Rosenthal, 2001; Mitri et al., 2009; Staszek et al., 2017). Terpenes have defensive properties such as volatile metabolites or non-volatiles such as triterpene saponins (Fürstenberg-Hägg et al., 2013; Liu et al., 2019). These three pathways should be further investigated as potential defensive compounds in tef.
While the world depends on many crop species, the commercialization of conventional agriculture is limited to a few cereal crops, mainly wheat, rice, and maize. Traditional crops, such as tef, are important resources for improving agricultural diversity, production, nutritional qualities, and increasing food security (Padulosi et al., 2012). Therefore, further investigation is required to understand understudied crop plants such as tef and other millets. In this research, we explored the molecular mechanisms involved in the interaction between R. padi and tef by comparing them to the well-studied physical and chemical mechanisms used by other crops such as wheat and barley. We discovered that tef plants use similar defense mechanisms; however, the indole-derived toxic compounds present in these crops were not synthesized by tef leaves. Here, we suggest three potential specialized metabolite pathways that might function as deterrent metabolites, which requires further investigation. Notably, in this research, only three accessions were tested that represent a random sampling of the variation in tef and were not selected based on aphid resistance. The tef germplasm might exhibit stronger resistant and susceptible accessions than the ones that we tested. To fully understand how well tef adapted to aphids, there is a need to conduct a large-scale experiment and exploit the most resistant accessions to better understand deterrent molecules involved in defenses. The overall understanding of tef biotic challenges and their responses are essential for the development of strategies to control pest infestations and reduce yield loss in worldwide cereal crops, supporting global food security.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
NG, BY, YS, and VT conceived and designed the experiments. NG, NW, AS, AC, and SB-Z performed the experiments. NG, BY, NW, AC, and VT analyzed the data. NG, BY, SB-Z, YS, and VT contributed to the writing of the manuscript. All authors contributed to the article and approved the submitted version.
This research was partially funded by Israel’s Chief Scientist of the Ministry of Agriculture number 16-38-0019 and 12-01-0032. NG awarded a master fellowship by the Gordon and Betty Moore Foundation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We are grateful to Ruth Yonah and Jajaw Bimro from The Hebrew University of Jerusalem, and Tamir Rozenberg from the Ben Gurion University of the Negev for identifying the insect species. We also thank Noga Sikron Persi from the Ben Gurion University of the Negev, for helping with the GC-MS analysis, and Anuma Dangol and Zhaniya Batyrshina for comments that greatly improved the manuscript. VT is the chair of Sonnenfeldt-Goldman Career Development in Desert Research. YS is the incumbent of the Haim Gvati Chair in Agriculture. SB-Z is indebted to the Robert H. Smith Foundation for a doctoral fellowship award.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2020.598483/full#supplementary-material
Supplementary Figure 1 | A photo of the three selected tef accessions. The plants are 1-month-old.
Supplementary Figure 2 | Photos of several insects identified in the field survey.
Supplementary Figure 3 | The Y-shape tube olfactometer system used for this experiment.
Supplementary Table 1 | Total insect identified in the two field sites, Sede Boqer and Revadim, at two sampling dates in May and June 2019. The insects were divided into orders and families.
Supplementary Table 2 | Known VOCs identified in leaves of 1-day-old tef plants infested with aphids for 4 days. The metabolites were normalized to the internal standard and presented as the relative abundance of the ion counts.
Supplementary Table 3 | VOCs that were significantly different between tef accession, treatment (with and without aphid infestation), or through the interaction of these factors using two-way ANOVA.
Supplementary Table 4 | Central metabolites identified in leaves of 1-day-old tef plants infested with aphids for 4 days. The metabolites were normalized using GC-MS to the internal standard and presented as the relative abundance of the ion counts.
Supplementary Table 5 | Central metabolites that were significantly different between tef accession, treatment (with and without aphid infestation), or through the interaction of these factors using two-way ANOVA.
Supplementary Table 6 | Total RNA-seq values after rlog normalization. Gene IDs were determined by Eragrostis tef reference transcriptome version 1.0.
Supplementary Table 7 | Distribution of RTC-144 tef genes into the seven Venn diagram groups. The genes were either up-, or down-regulated by aphid infestation in the time course. p< 0.05 false discovery rate, and fold change > 2 or < 0.5.
Supplementary Table 8 | Distribution of rice orthologs of tef genes, divided into the seven Ven diagram groups. The genes were either up-, or down-regulated by aphid infestation in the time course. p < 0.05 false discovery rate, and fold change > 2 or < 0.5.
Agrawal, A. A., Fishbein, M., Jetter, R., Salminen, J.-P., Goldstein, J. B., Freitag, A. E., et al. (2009). Phylogenetic ecology of leaf surface traits in the milkweeds (Asclepias spp.): chemistry, ecophysiology, and insect behavior. New Phytol. 183, 848–867. doi: 10.1111/j.1469-8137.2009.02897.x
Agrawal, A. A. (1999). “Induced plant defense: evolution of induction and adaptive phenotypic plasticity,” in Inducible Plant Defenses Against Pathogens and Herbivores: Biochemistry, Ecology, and Agriculture, eds A. A. Agrawal, S. Tuzun, and E. Bent, (St. Paul, MN: American Phytopathological Society Press), 251–268.
Akol, A. M., Njagi, P. G. N., Sithanantham, S., and Mueke, J. M. (2003). Effects of two neem insecticide formulations on the attractiveness, acceptability and suitability of diamondback moth larvae to the parasitoid, Diadegma mollipla (Holmgren) (Hym., Ichneumonidae). J. Appl. Entomol. 127, 325–331. doi: 10.1046/j.1439-0418.2003.00771.x
Ameye, M., Allmann, S., Verwaeren, J., Smagghe, G., Haesaert, G., Schuurink, R. C., et al. (2018). Green leaf volatile production by plants: a meta-analysis. New Phytol. 220, 666–683. doi: 10.1111/nph.14671
Appel, H. M., Fescemyer, H., Ehlting, J., Weston, D., Rehrig, E., Joshi, T., et al. (2014). Transcriptional responses of Arabidopsis thaliana to chewing and sucking insect herbivores. Front. Plant Sci. 5:565. doi: 10.3389/fpls.2014.00565
Arnold, A. J. (1994). Insect suction sampling without nets, bags or filters. Crop Prot. 13, 73–76. doi: 10.1016/0261-2194(94)90139-2
Assefa, K., Cannarozzi, G., Girma, D., Kamies, R., Chanyalew, S., Plaza-Wüthrich, S., et al. (2015). Genetic diversity in tef [Eragrostis tef (Zucc.) Trotter]. Front. Plant Sci. 6:177. doi: 10.3389/fpls.2015.00177
Assefa, K., Yu, J. K., Zeid, M., Belay, G., Tefera, H., and Sorrells, M. E. (2011). Breeding tef [Eragrostis tef (Zucc.) trotter]: conventional and molecular approaches. Plant Breed. 130, 1–9. doi: 10.1111/j.1439-0523.2010.01782.x
Awika, J. M. (2011). Major cereal grains production and use around the world. ACS Symp. Ser. 1089, 1–13. doi: 10.1021/bk-2011-1089.ch001
Ayalew, A., Kena, K., and Dejene, T. (2011). Application of NP fertilizers for better production of teff (Eragrostis tef (zucc.) trotter) on different types of soils in southern Ethiopia. J. Nat. Sci. Res. 1, 6–15.
Batyrshina, Z. S., Cna’ani, A., Rozenberg, T., Seifan, M., and Tzin, V. (2020a). The combined impacts of wheat spatial position and phenology on cereal aphid abundance. PeerJ 8:e9142. doi: 10.7717/peerj.9142
Batyrshina, Z. S., Yaakov, B., Shavit, R., Singh, A., and Tzin, V. (2020b). Comparative transcriptomic and metabolic analysis of wild and domesticated wheat genotypes reveals differences in chemical and physical defense responses against aphids. BMC Plant Biol. 20:19. doi: 10.1186/s12870-019-2214-z
Bekkering, C. S., and Tian, L. (2019). Thinking outside of the cereal box: breeding underutilized (pseudo)cereals for improved human nutrition. Front. Genet. 10:1289. doi: 10.3389/fgene.2019.01289
Ben-Zeev, S., Bimro, J., Barak, V., and Saranga, Y. (2018). Phenotypic diversity and heritability in Eragrostis tef under irrigated Mediterranean conditions. Isr. J. Plant Sci. 65, 222–231. doi: 10.1163/22238980-00001061
Ben-Zeev, S., Rabinovitz, O., Orlov-Levin, V., Chen, A., Graff, N., Goldwasser, Y., et al. (2020). Less is more: lower sowing rate of irrigated tef (Eragrostis tef) alters plant morphology and reduces lodging. Agronomy 10:570. doi: 10.3390/agronomy10040570
Bernasconi, M. L., Turlings, T. C. J., Ambrosetti, L., Bassetti, P., and Dorn, S. (1998). Herbivore-induced emissions of maize volatiles repel the corn leaf aphid. Rhopalosiphum maidis. Entomol. Exp. Appl. 87, 133–142. doi: 10.1023/A:1003200108763
Bing, J. W., Novak, M. G., Obrycki, J. J., and Guthrie, W. D. (1991). Stylet penetration and feeding sites of Rhopalosiphum maidis (Homoptera: Aphididae) on two growth stages of maize. Ann. Entomol. Soc. Am. 84:549. doi: 10.1093/aesa/84.5.549
Blackman, R., and Eastop, V. (2000). Aphids on the World’s Crops: An Identification and Information Guide. London: John Wiley & Sons.
Bleeker, P. M., Diergaarde, P. J., Ament, K., Guerra, J., Weidner, M., Schütz, S., et al. (2009). The role of specific tomato volatiles in tomato-whitefly interaction. Plant Physiol. 151, 925–935. doi: 10.1104/pp.109.142661
Cannarozzi, G., Plaza-wüthrich, S., Esfeld, K., Larti, S., Wilson, Y. S., and Girma, D. (2014). Genome and transcriptome sequencing identifies breeding targets in the orphan crop tef (Eragrostis tef) Genome and transcriptome sequencing identifies breeding targets in the orphan crop tef (Eragrostis tef). BMC Genomics 15:581. doi: 10.1186/1471-2164-15-581
Correa, L. J., Maciel, O. V. B., Bücker-Neto, L., Pilati, L., Morozini, A. M., Faria, M. V., et al. (2020). A comprehensive analysis of wheat resistance to Rhopalosiphum padi (Hemiptera: Aphididae) in Brazilian wheat cultivars. J. Econ. Entomol. 113, 1493–1503. doi: 10.1093/jee/toaa059
Curtis, T., and Halford, N. G. (2014). Food security: the challenge of increasing wheat yield and the importance of not compromising food safety. Ann. Appl. Biol. 164, 354–372. doi: 10.1111/aab.12108
Deutsch, C. A., Tewksbury, J. J., Tigchelaar, M., Battisti, D. S., Merrill, S. C., Huey, R. B., et al. (2018). Increase in crop losses to insect pests in a warming climate. Science 919, 916–919. doi: 10.1126/science.aat3466
Dicke, M. (1999). “Evolution of induced indirect defense of plants”, in The Ecology and Evolution of Inducible Defenses, eds R. Tollrian and C. D. Harvell (Princeton, NJ: Princeton University Press), 62–88.
Dorschner, K. W., Ryan, J. D., Johnson, R. C., and Eikenbary, R. D. (1987). Modification of host nitrogen levels by the greenbug (Homoptera: Aphididae): its role in resistance of winter wheat to aphids. Environ. Entomol. 16, 1007–1011. doi: 10.1093/ee/16.4.1007
Dosad, S., and Chawla, H. S. (2018). “Genetic transformation of millets: the way ahead,” in Biotechnologies of Crop Improvement, Volume 2: Transgenic Approaches, eds S. S. Gosal and S. H. Wani, (Cham: Springer International Publishing), 249–286. doi: 10.1007/978-3-319-90650-8_11
Douglas, A. E. (2003). The nutritional physiology of aphids. Adv. In Insect Phys. 31, 73–140. doi: 10.1016/s0065-2806(03)31002-1
Douglas, A. E. (2006). Phloem-sap feeding by animals: problems and solutions. J. Exp. Bot. 57, 747–754. doi: 10.1093/jxb/erj067
Eisner, T., Eisner, M., Rossini, C., Iyengar, V. K., Roach, B. L., Benedikt, E., et al. (2000). Chemical defense against predation in an insect egg. Proc. Natl. Acad. Sci. U.S.A. 97, 1634–1639. doi: 10.1073/pnas.030532797
Fereres, A., Lister, R. M., Araya, J. E., and Foster, J. E. (1989). Development and reproduction of the English grain aphid (Homoptera: Aphididae) on wheat cultivars infected with barley yellow dwarf virus. Environ. Entomol. 18, 388–393. doi: 10.1093/ee/18.3.388
Frey, M., Chomet, P., Glawischnig, E., Stettner, C., Grün, S., Winklmair, A., et al. (1997). Analysis of a chemical plant defense mechanism in grasses. Science 277, 696–699. doi: 10.1126/science.277.5326.696
Fürstenberg-Hägg, J., Zagrobelny, M., and Bak, S. (2013). Plant defense against insect herbivores. Int. J. Mol. Sci. 14, 10242–10297. doi: 10.3390/ijms140510242
Garcia-Esteban, M., Ansorena, D., Astiasarán, I., and Ruiz, J. (2004). Study of the effect of different fiber coatings and extraction conditions on dry cured ham volatile compounds extracted by solid-phase microextraction (SPME). Talanta 64, 458–466. doi: 10.1016/j.talanta.2004.03.007
Gebremedhin, T. (1987). The control of red tef worm, Mentaxya ignicollis (Walker) (Lepidoptera: Noctuidae) in Ethiopia. Trop. Pest Manag. 33, 170–172. doi: 10.1080/09670878709371140
Gershenzon, J., and Dudareva, N. (2007). The function of terpene natural products in the natural world. Nat. Chem. Biol. 3, 408–414. doi: 10.1038/nchembio.2007.5
Grün, S., Frey, M., and Gierl, A. (2005). Evolution of the indole alkaloid biosynthesis in the genus Hordeum: distribution of gramine and DIBOA and isolation of the benzoxazinoid biosynthesis genes from Hordeum lechleri. Phytochemistry 66, 1264–1272. doi: 10.1016/j.phytochem.2005.01.024
Gutsche, A., Heng-Moss, T., Sarath, G., Twigg, P., Xia, Y., Lu, G., et al. (2009). Gene expression profiling of tolerant barley in response to Diuraphis noxia (Hemiptera: Aphididae) feeding. Bull. Entomol. Res. 99, 163–173. doi: 10.1017/S0007485308006184
Hamilton, J., Zangerl, A. R., Berenbaum, M. R., Sparks, J. P., Elich, L., Eisenstein, A., et al. (2012). Acta oecologica elevated atmospheric CO 2 alters the arthropod community in a forest understory. Acta Oecol. 43, 80–85. doi: 10.1016/j.actao.2012.05.004
Hammond, D. G., Rangel, S., and Kubo, I. (2000). Volatile aldehydes are promising broad-spectrum postharvest insecticides. J. Agric. Food Chem. 48, 4410–4417. doi: 10.1021/jf000233
Handley, R., Ekbom, B., and Ågren, J. (2005). Variation in trichome density and resistance against a specialist insect herbivore in natural populations of Arabidopsis thaliana. Ecol. Entomol. 30, 284–292. doi: 10.1111/j.0307-6946.2005.00699.x
Hochberg, U., Degu, A., Toubiana, D., Gendler, T., Nikoloski, Z., Rachmilevitch, S., et al. (2013). Metabolite profiling and network analysis reveal coordinated changes in grapevine water stress response. BMC Plant Biol. 13:184. doi: 10.1186/1471-2229-13-184
Ishihara, A., Hashimoto, Y., Tanaka, C., Dubouzet, J. G., Nakao, T., Matsuda, F., et al. (2008). The tryptophan pathway is involved in the defense responses of rice against pathogenic infection via serotonin production. Plant J. 54, 481–495. doi: 10.1111/j.1365-313X.2008.03441.x
Jansen, G., DiMaio, L., and Hause, N. (1962). Cereal proteins, amino acid composition and lysine supplementation of Teff. J. Agric. Food Chem. 10, 62–64. doi: 10.1021/jf60119a021
Jimenez-Martínez, E. S., Bosque-Pérez, N. A., Berger, P. H., Zemetra, R. S., Ding, H., and Eigenbrode, S. D. (2004). Volatile cues influence the response of rhopalosiphum padi (Homoptera: Aphididae) to barley yellow dwarf virus–infected transgenic and untransformed wheat. Environ. Entomol. 33, 1207–1216. doi: 10.1603/0046-225X-33.5.1207
Joung, J.-G., Corbett, A. M., Fellman, S. M., Tieman, D. M., Klee, H. J., Giovannoni, J. J., et al. (2009). Plant MetGenMAP: an integrative analysis system for plant systems biology. Plant Physiol. 151, 1758–1768. doi: 10.1104/pp.109.145169
Kalaisekar, A., Padmaja, P., Bhagwat, V., and Patil, J. V. (2017). Insect Pests of Millets, Systematics, Bionomics, and Managemen, 1st Edn. Cambridge, MA: Academic Press.
Kant, M. R., Bleeker, P. M., van Wijk, M., Schuurink, R. C., and Haring, M. A. (2009). Chapter 14 Plant volatiles in defence. Adv. Bot. Res. 51, 613–666. doi: 10.1016/S0065-2296(09)51014-2
Karley, A. J., Douglas, A. E., and Parker, W. E. (2002). Amino acid composition and nutritional quality of potato leaf phloem sap for aphids. J. Exp. Biol. 205, 3009–3018.
Kessler, A., and Baldwin, I. T. (2002). Plant responses to insect herbivory: the emerging molecular analysis. Annu. Rev. Plant Biol. 53, 299–328. doi: 10.1146/annurev.arplant.53.100301.135207
Klein, D., Fink, B., Arold, B., Eisenreich, W., and Schwab, W. (2007). Functional characterization of enone oxidoreductases from strawberry and tomato Fruit. J. Agric. Food Chem. 55, 6705–6711. doi: 10.1021/jf071055o
Knops, J. M. H., Tilman, D., Haddad, N. M., Naeem, S., Mitchell, C. E., Haarstad, J., et al. (1999). Effects of plant species richness on invasion dynamics, disease outbreaks, insect abundances and diversity. Ecol. Lett. 2, 286–293. doi: 10.1046/j.1461-0248.1999.00083.x
Lee, G., Stevens, D. J., Stokes, S., and Wratten, S. D. (1981). Duration of cereal aphid populations and the effects on wheat yield and breadmaking quality. Ann. Appl. Biol. 98, 169–178. doi: 10.1111/j.1744-7348.1981.tb00750.x
Leybourne, D. J., Valentine, T. A., Robertson, J. A. H., Pérez-Fernández, E., Main, A. M., Karley, A. J., et al. (2019). Defence gene expression and phloem quality contribute to mesophyll and phloem resistance to aphids in wild barley. J. Exp. Bot. 70, 4011–4026. doi: 10.1093/jxb/erz163
Lisec, J., Schauer, N., Kopka, J., Willmitzer, L., and Fernie, A. R. (2006). Gas chromatography mass spectrometry–based metabolite profiling in plants. Nat. Protc. 1, 387–396. doi: 10.1038/nprot.2006.59
Liu, Q., Khakimov, B., Cárdenas, P. D., Cozzi, F., Olsen, C. E., Jensen, K. R., et al. (2019). The cytochrome P450 CYP72A552 is key to production of hederagenin-based saponins that mediate plant defense against herbivores. New Phytol. 222, 1599–1609. doi: 10.1111/nph.15689
Love, M. I., Huber, W., and Anders, S. (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15:550. doi: 10.1186/s13059-014-0550-8
Lu, H., Luo, T., Fu, H., Wang, L., Tan, Y., Huang, J., et al. (2018). Resistance of rice to insect pests mediated by suppression of serotonin biosynthesis. Nat. Plants 4, 338–344. doi: 10.1038/s41477-018-0152-7
Marchetti, E., Civolani, S., Leis, M., Chicca, M., Tjallingii, W. F., Pasqualini, E., et al. (2009). Tissue location of resistance in apple to the rosy apple aphid established by electrical penetration graphs. Bull. Insectol. 62, 203–208.
Martin, B., Collar, J. L., Tjallingii, W. F., and Fereres, A. (1997). Intracellular ingestion and salivation by aphids may cause the acquisition and inoculation of non-persistently transmitted plant viruses. J. Gen. Virol. 78, 2701–2705. doi: 10.1099/0022-1317-78-10-2701
Meihls, L. N., Kaur, H., and Jander, G. (2012). Natural variation in maize defense against insect herbivores. Cold Spring Harb. Symp. Quant. Biol. 77, 269–283. doi: 10.1101/sqb.2012.77.014662
Mithöfer, A., and Boland, W. (2012). Plant defense against herbivores: chemical aspects. Annu. Rev. Plant Biol. 63, 431–450. doi: 10.1146/annurev-arplant-042110-103854
Mitra, S., and Baldwin, I. T. (2014). RuBPCase activase (RCA) mediates growth-defense trade-offs: silencing RCA redirects jasmonic acid (JA) flux from JA-isoleucine to methyl jasmonate (MeJA) to attenuate induced defense responses in Nicotiana attenuata. New Phytol. 201, 1385–1395. doi: 10.1111/nph.12591
Mitri, C., Soustelle, L., Framery, B., Bockaert, J., Parmentier, M.-L., and Grau, Y. (2009). Plant insecticide L-canavanine repels Drosophila via the insect orphan GPCR DmX. PLoS Biol. 7:e1000147. doi: 10.1371/journal.pbio.1000147
Morkunas, I., Woźniak, A., Formela, M., Mai, V. C., Marczak, Ł, Narożna, D., et al. (2016). Pea aphid infestation induces changes in flavonoids, antioxidative defence, soluble sugars and sugar transporter expression in leaves of pea seedlings. Protoplasma 253, 1063–1079. doi: 10.1007/s00709-015-0865-7
Nalam, V., Louis, J., Patel, M., and Shah, J. (2018). Arabidopsis-green peach aphid interaction: rearing the insect, no-choice and fecundity assays, and electrical penetration graph technique to study insect feeding behavior. Bio Protoc. 8:e2950. doi: 10.21769/BioProtoc.2950
Nalam, V., Isaacs, T., Moh, S., Kansman, J., Finke, D., Albrecht, T., et al. (2020). Diurnal feeding as a potential mechanism of osmoregulation in aphids. Insect. Sci. doi: 10.1111/1744-7917.12787
Nault, L. R. (1997). Arthropod transmission of plant viruses: a new synthesis. Ann. Entomol. Soc. Am. 90, 521–541. doi: 10.1093/aesa/90.5.521
Ni, X., Quisenberry, S. S., Heng-Moss, T., Markwell, J., Higley, L., Baxendale, F., et al. (2002). Dynamic change in photosynthetic pigments and chlorophyll degradation elicited by cereal aphid feeding. Entomol. Exp. Appl. 105, 43–53. doi: 10.1046/j.1570-7458.2002.01031.x
Nordlund, D. A., Lewis, W. J., Todd, J. W., and Chalfant, R. B. (1977). Kairomones and their use for management of entomophagous insects. J. Chem. Ecol. 3, 513–518. doi: 10.1007/bf00989072
Padulosi, S., Bergamini, N., and Lawrence, T. (2012). “On-farm conservation of neglected and underutilized species: status, trends and novel approaches to cope with climate change,” in Proceedings of an International Conference (Rome: Bioversity International), 14–16.
Parry, H. R. (2013). Cereal aphid movement: general principles and simulation modelling. Mov. Ecol. 1:14. doi: 10.1186/2051-3933-1-14
Piesik, D., Weaver, D. K., Runyon, J. B., Buteler, M., Peck, G. E., and Morrill, W. L. (2008). Behavioural responses of wheat stem sawflies to wheat volatiles. Agric. For. Entomol. 10, 245–253. doi: 10.1111/j.1461-9563.2008.00380.x
Ponder, K., Pritchard, J., Harrington, R., and Bale, J. (2000). Difficulties in location and acceptance of phloem sap combined with reduced concentration of phloem amino acids explain lowered performance of the aphid Rhopalosiphum padi on nitrogen deficient barley (Hordeum vulgare) seedlings. Entomol. Exp. Appl. 97, 203–210. doi: 10.1046/j.1570-7458.2000.00731.x
Ponder, K. L., Pritchard, J., Harrington, R., and Bale, J. S. (2001). Feeding behaviour of the aphid Rhopalosiphum padi (Hemiptera: Aphididae) on nitrogen and water-stressed barley (Hordeum vulgare) seedlings. Bull. Entomol. Res. 91, 125–130.
Rabbinge, R., Drees, E. M., van der Graaf, M., Verberne, F. C. M., and Wesselo, A. (1981). Damage effects of cereal aphids in wheat. Netherlands J. Plant Pathol. 87, 217–232. doi: 10.1007/BF02084437
Rader, R., Bartomeus, I., Garibaldi, L. A., Garratt, M. P. D., Howlett, B. G., Winfree, R., et al. (2016). Non-bee insects are important contributors to global crop pollination. Proc. Natl. Acad. Sci. U.S.A. 113, 146–151. doi: 10.1073/pnas.1517092112
Ramírez-Medorio, N. J., Hernández-Rosas, F., Osorio-Acosta, F., López-Collado, J., Figueroa-Rodríguez, K. A., and Amante-Orozco, A. (2019). Chemical composition of volatiles from sugarcane leaves and pastures by gas chromatography. Rev. Mex. Ciencias Agrícolas 22, 129–138.
Reda, A. (2014). Achieving food security in Ethiopia by promoting productivity of future world food tef: a review. Adv. Plants Agric. Res. 2, 86–95. doi: 10.15406/apar.2015.02.00045
Report on Area, and Production Major Crops, (2012). Central Statistical Agency, Ministry of Finance and Economic Development - Agricultural Sample Survey (Addis Ababa: Statistical Bulletin), 388pp.
Richter, A., Schaff, C., Zhang, Z., Lipka, A. E., Tian, F., Köllner, T. G., et al. (2016). Characterization of biosynthetic pathways for the production of the volatile homoterpenes DMNT and TMTT in Zea mays. Plant Cell 28, 2651–2665. doi: 10.1105/tpc.15.00919
Richter, A., Seidl-Adams, I., Köllner, T. G., Schaff, C., Tumlinson, J. H., and Degenhardt, J. (2015). A small, differentially regulated family of farnesyl diphosphate synthases in maize (Zea mays) provides farnesyl diphosphate for the biosynthesis of herbivore-induced sesquiterpenes. Planta 241, 1351–1361. doi: 10.1007/s00425-015-2254-z
Robinson, A. G., and Hsu, S.-J. (1963). Host plant records and biology of aphids on cereal grains and grasses in Manitoba (Homoptera: Aphididae). Can. Entomol. 95, 134–137. doi: 10.4039/Ent95134-2
Rosental, L., Perelman, A., Nevo, N., Toubiana, D., Samani, T., Batushansky, A., et al. (2016). Environmental and genetic effects on tomato seed metabolic balance and its association with germination vigor. BMC Genomics 17:1047. doi: 10.1186/s12864-016-3376-9
Rosenthal, G. A. (2001). L-Canavanine: a higher plant insecticidal allelochemical. Amino Acids 21, 319–330. doi: 10.1007/s007260170017
Salvador-Recatalà, V., and Tjallingii, W. F. (2015). A new application of the electrical penetration graph (EPG) for acquiring and measuring electrical signals in phloem sieve elements. JoVE 2:e52826. doi: 10.3791/52826
Sandström, J., Telang, A., and Moran, N. A. (2000). Nutritional enhancement of host plants by aphids — a comparison of three aphid species on grasses. J. Insect Physiol. 46, 33–40. doi: 10.1016/S0022-1910(99)00098-0
Santana, O., Reina, M., Fraga, B. M., Sanz, J., and González-Coloma, A. (2012). Antifeedant activity of fatty acid esters and phytosterols from Echium wildpretii. Chem. Biodivers. 9, 567–576. doi: 10.1002/cbdv.201100083
Sarria, E., Cid, M., Garzo, E., and Fereres, A. (2009). Excel Workbook for automatic parameter calculation of EPG data. Comput. Electron. Agric. 67, 35–42. doi: 10.1016/j.compag.2009.02.006
Sato, Y., and Kudoh, H. (2015). Tests of associational defence provided by hairy plants for glabrous plants of Arabidopsis halleri subsp. gemmifera against insect herbivores. Ecol. Entomol. 40, 269–279. doi: 10.1111/een.12179
Seyfu, K. (1993). Tef (Eragrostis Tef): Breeding, Genetic Resources, Agronomy, Utilization and Role in Ethiopian Agriculture, Vol. 102. Addis Abeba: Institute of Agricultural Research.
Shiojiri, K., Kishimoto, K., Ozawa, R., Kugimiya, S., Urashimo, S., Arimura, G., et al. (2006). Changing green leaf volatile biosynthesis in plants: an approach for improving plant resistance against both herbivores and pathogens. Proc. Natl. Acad. Sci. U.S.A. 103, 16672–16676. doi: 10.1073/pnas.0607780103
Song, J., Liu, H., Zhuang, H., Zhao, C., Xu, Y., Wu, S., et al. (2017). Transcriptomics and alternative splicing analyses reveal large differences between maize lines B73 and Mo17 in response to aphid Rhopalosiphum padi infestation. Front. Plant Sci. 8:1738. doi: 10.3389/fpls.2017.01738
Stallknecht, G. F., Gilbertson, K. M., and Eckoff, J. L. (1993). Teff: Food Crop for Humans and Animal, ed. J. Janick, (New York, NY: New Crops. Wiley and Sons).
Staszek, P., Weston, L. A., Ciacka, K., Krasuska, U., and Gniazdowska, A. (2017). l-Canavanine: how does a simple non-protein amino acid inhibit cellular function in a diverse living system? Phytochem. Rev. 16, 1269–1282. doi: 10.1007/s11101-017-9536-y
Stepanycheva, E. A., Petrova, M. O., Chermenskaya, T. D., and Shamshev, I. V. (2016). Effect of methyl salicylate on behavioral responses of insects in a forest park. Entomol. Rev. 96, 284–287. doi: 10.1134/S0013873816030052
Swirski, E., and Amitai, S. (1999). Annotated list of aphids (Aphidoidea) in Israel. Isr. J. Entomol. 33, 1–120.
Tabilio, M. R., Fiorini, D., Marcantoni, E., Materazzi, S., Delfini, M., De Salvador, F. R., et al. (2013). Impact of the Mediterranean fruit fly (Medfly) Ceratitis capitata on different peach cultivars: the possible role of peach volatile compounds. Food Chem. 140, 375–381. doi: 10.1016/j.foodchem.2013.02.074
Tauber, M. J., Tauber, C. A., Daane, K. M., and Hagen, K. S. (2000). Commercialization of predators: recent lessons from green lacewings (Neuroptera: Chrysopidae: Chrysoperla). Am. Entomol. 46, 26–38. doi: 10.1093/ae/46.1.26
Tetreault, H. M., Grover, S., Scully, E. D., Gries, T., Palmer, N. A., Sarath, G., et al. (2019). Global responses of resistant and susceptible sorghum (Sorghum bicolor) to sugarcane aphid (Melanaphis sacchari). Front. Plant Sci. 10:145. doi: 10.3389/fpls.2019.00145
Tjallingii, W., and Esch Hogen, T. (1993). Fine structure of aphid stylet routes in plant tissues in correlation with EPG signals. Physiol. Entomol. 18, 317–328. doi: 10.1111/j.1365-3032.1993.tb00604.x
Tjallingii, W. F. (1978). Electronic recording of penetration behaviour by aphids. Entomol. Exp. Appl. 24, 721. doi: 10.1111/j.1570-7458.1978.tb02836.x
Tzin, V., Fernandez-Pozo, N., Richter, A., Schmelz, E. A., Schoettner, M., Schäfer, M., et al. (2015). Dynamic maize responses to aphid feeding are revealed by a time series of transcriptomic and metabolomic assays. Plant Physiol. 169, 1727–1743.
van Bel, A. J. E., and Hess, P. H. (2008). Hexoses as phloem transport sugars: the end of a dogma? J. Exp. Bot. 59, 261–272. doi: 10.1093/jxb/erm294
VanBuren, R., Man Wai, C., Wang, X., Pardo, J., Yocca, A. E., Wang, H., et al. (2020). Exceptional subgenome stability and functional divergence in the allotetraploid Ethiopian cereal teff. Nat. Commun. 11:884. doi: 10.1038/s41467-020-14724-z
Vickerman, G. P., and Wratten, S. D. (1979). The biology and pest status of cereal aphids (Hemiptera: Aphididae) in Europe: a review. Bull. Entomol. Res. 69, 1–32. doi: 10.1017/S0007485300017855
Vogel, K. J., and Moran, N. A. (2011). Sources of variation in dietary requirements in an obligate nutritional symbiosis. Proceedings. Biol. Sci. 278, 115–121. doi: 10.1098/rspb.2010.1304
War, A. R., Paulraj, M. G., Ahmad, T., Buhroo, A. A., Hussain, B., Ignacimuthu, S., et al. (2012). Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 7, 1306–1320. doi: 10.4161/psb.21663
Xia, J., Psychogios, N., Young, N., and Wishart, D. S. (2009). MetaboAnalyst: a web server for metabolomic data analysis and interpretation. Nucleic Acids Res. 37, W652–W660. doi: 10.1093/nar/gkp356
Yigzaw, Y., Gorton, L., Solomon, T., and Akalu, G. (2004). Fermentation of seeds of teff (Eragrostis teff), grass-pea (Lathyrus sativus), and their mixtures: aspects of nutrition and food safety. J. Agric. Food Chem. 52, 1163–1169. doi: 10.1021/jf034742y
Zentane, E., Quenu, H., Graham, R. I., and Cherrill, A. (2016). Suction samplers for grassland invertebrates: comparison of numbers caught using Vortis TM and G-vac devices. Insect Conserv. Divers. 9, 470–474. doi: 10.1111/icad.12185
Zettler, J. A., Mateer, S. C., Link-Pérez, M., Bailey, J. B., DeMars, G., and Ness, T. (2016). To key or not to key: a new key to simplify & improve the accuracy of insect identification. Am. Biol. Teach. 78, 626–633. doi: 10.1525/abt.2016.78.8.626
Zhang, Y., Fu, Y., Wang, Q., Liu, X., Li, Q., and Chen, J. (2020). Transcriptome analysis reveals rapid defence responses in wheat induced by phytotoxic aphid Schizaphis graminum feeding. BMC Genomics 21:339. doi: 10.1186/s12864-020-6743-5
Zhou, S., Lou, Y.-R., Tzin, V., and Jander, G. (2015). Alteration of plant primary metabolism in response to insect herbivory. Plant Physiol. 169, 1488–1498. doi: 10.1104/pp.15.01405
Keywords: cereal crop, electrical penetration graph, green leaf volatile, insect behavior, trichome, volatile organic compounds, aphid
Citation: Gyan NM, Yaakov B, Weinblum N, Singh A, Cna’ani A, Ben-Zeev S, Saranga Y and Tzin V (2020) Variation Between Three Eragrostis tef Accessions in Defense Responses to Rhopalosiphum padi Aphid Infestation. Front. Plant Sci. 11:598483. doi: 10.3389/fpls.2020.598483
Received: 24 August 2020; Accepted: 09 November 2020;
Published: 08 December 2020.
Edited by:
Martin De Vos, KWS Saat, GermanyReviewed by:
Eduard Venter, University of Johannesburg, South AfricaCopyright © 2020 Gyan, Yaakov, Weinblum, Singh, Cna’ani, Ben-Zeev, Saranga and Tzin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vered Tzin, dnR6aW5AYmd1LmFjLmls
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.