- 1Department of Agronomy, University of Córdoba, Córdoba, Spain
- 2Department of Plant Pathology, University of California, Davis, Davis, CA, United States
- 3Biogeos, Estudios Ambientales, Centauro, Spain
Verticillium wilt, caused by Verticillium dahliae, challenges olive cultivation and an Integrated Disease Management (IDM) approach is the best-suited tool to combat it. Since 1998, an IDM strategy in an orchard (called Granon, Spain) of the susceptible cv. Picual was conducted by increasing planting density with moderately resistant cv. Frantoio, chemical weed control, and replanting of dead olives with cv. Frantoio following soil solarization. The Verticillium wilt epidemic in Granon orchard was compared to the epidemic in a non-IDM orchard (called Ancla, Spain) with plowed soil and dead Picual olives replanted with the same cultivar. Field evaluations (2012–2013) showed an incidence and severity of the disease as Picual–Ancla > Picual–Granon > Frantoio–Granon. The spatiotemporal dynamics of the Verticillium epidemics from 1998 to 2010 were monitored with digital images using SIG. The annual tree mortalities were 5.6% for Picual olives in Ancla orchard, and 3.1 and 0.7% for Picual and Frantoio olives in Granon orchard, respectively. There was a negative relationship between the mortality of olive trees (%) by the pathogen and the height (m) above sea level. The annual mortality of cv. Picual olives was positively correlated with spring rainfalls. The Index of Dispersion and beta-binomial distribution showed aggregation of Verticillium-dead olives. In conclusion, this IDM strategy considerably reduced the disease in comparison with traditional agronomic practices.
Introduction
The olive tree (Olea europaea L. subsp. europaea) is one of the most important woody crops around the world. Spain accounts for the largest production of both olive oil and table fruit. In this country, the major area of olive groves covers around 1.65 million hectares and is located in Andalusia, Southern region, being one-third concentrated in the province of Jaen (ESYRCE, 2019). Verticillium wilt of olive, caused by the soil-borne pathogen Verticillium dahliae Kleb., is a major concern for the producers of this crop worldwide (Lopez-Escudero and Mercado-Blanco, 2011; Tsror, 2011; Keykhasaber et al., 2017). In Spain, the increase and spread of Verticillium wilt of olive have been mainly driven by (i) the olive colonization of soils historically cropped with cotton, which is a major host of V. dahliae; and (ii) the spread of the defoliating (D) pathotype of the pathogen, which is more virulent than the previously dominant non-defoliating pathotype (ND) (Navas-Cortes et al., 2008; Lopez-Escudero et al., 2010; Milgroom et al., 2016). The D isolates of V. dahliae cause defoliation in olive and cotton, whereas ND isolates cause wilting but no defoliation (Lopez-Escudero et al., 2004; Jimenez-Diaz et al., 2017).
In the disease cycle of Verticillium wilt of olive, the pathogen has a parasitic phase in the host and a non-parasitic phase as microsclerotia (Keykhasaber et al., 2017), which can survive in the field soils for a prolonged period (up to 15 years) (Wilhelm, 1955). Microsclerotia germinate under favorable microbiological and environmental conditions and in the presence of root exudates of olive infect the roots (Prieto et al., 2009; Jimenez-Diaz et al., 2012). Subsequently, the pathogen systemically colonizes the olive tree with hyphae and conidia before symptoms develop (Jimenez-Diaz et al., 2012). Colonized trees show characteristic canopy wilting and ultimately die (Navas-Cortes et al., 2008). However, some infected olive trees can overcome the disease (Levin et al., 2003; Lopez-Escudero and Blanco-Lopez, 2005; Bubici and Cirulli, 2014).
Because no control measures applied singly are completely effective in controlling Verticillium wilt of olive, the disease should be managed according to an Integrated Disease Management (IDM) strategy (Tsror, 2011; Jimenez-Diaz et al., 2012). An IDM strategy should be based on the application of both preplanting and postplanting control measures (Lopez-Escudero and Mercado-Blanco, 2011). Preplanting control measures include (i) the quantification of the pathogen in soil while avoiding planting in infested soils (Perez-Artes et al., 2005); (ii) the use of pathogen-free planting material (Morello et al., 2016); and (iii) the selection of cultivars with elevated resistance to the V. dahliae, because there are no immune ones (Lopez-Escudero et al., 2004; Trapero et al., 2013; Leyva-Perez et al., 2017). After planting, control measures may include (i) the elimination of alternative weed hosts to avoid the increase in pathogen inoculum (Ligoxigakis et al., 2002); (ii) the removal of pruning remains by burning (Morello et al., 2016); (iii) the application of disinfectants to reduce the viability of the pathogen in the irrigation water (Gomez-Galvez et al., 2018; Gomez-Galvez and Rodriguez-Jurado, 2018); or (iv) biofumigation by sowing Brassicaceae species and/or solarization because both strategies reduce pathogen inoculum density in the soil (Tjamos, 1991; Lopez-Escudero and Blanco-Lopez, 2001; Bejarano-Alcazar, 2008). The use of an IDM strategy to control Verticillium wilt of olive has been highly recommended for many authors (Lopez-Escudero and Mercado-Blanco, 2011; Jimenez-Diaz et al., 2012; Keykhasaber et al., 2017; Montes-Osuna and Mercado-Blanco, 2020); however, the examination of an IDM strategy controlling the disease in a commercial olive orchard and the long-term effect in field conditions is needed. While spatiotemporal studies have highlighted their potential in understanding epidemics of soil-borne pathogens (Madden et al., 2007), these remain barely used in Verticillium wilt of olive and limited to the moderately susceptible cultivar Arbequina in experimental plots (Navas-Cortes et al., 2008). Generally, olive trees showing Verticillium wilt symptoms occur in clusters in the field where the disease is driven by the natural inoculum, i.e., the soil microsclerotia (Navas-Cortes et al., 2008), although the use of infected planting stocks could also contribute to increase the heterogeneity of affected olives in the field (Thanassoulopoulos, 1992). The effect of Verticillium wilt management practices on the spatial patterns of dead olives by the pathogen will aid in the understanding of the impact of these control methods on the diseases and how we can improve them.
Thereunder, we conducted an epidemiological study in two commercial olive orchards intending to characterize the impact of a specific IDM strategy on spatiotemporal of V. dahliae–dead olives for 12 years. The IDM described in the present study has contributed to keeping with relatively low levels of Verticillium wilt in a commercial olive orchard.
Materials and Methods
Orchards and Background
The present study was conducted in two adjacent (distance between them 100 m) commercial olive groves (4159393.6N, 457616.1W), called Ancla and Granon, counting 21.5 and 16.2 ha, respectively. Both Ancla and Granon orchards stand 329 m (from 264 to 384 m) and 286 m (from 260 to 312 m) above sea level. The orchards were separated by an 18-m-wide road and located in the province of Jaen, in Andalusia (South of Spain), considered to be the leading olive-growing region in the world, having more than 592,000 ha of this crop (ESYRCE, 2019). This location held an average rainfall of 524 mm and average temperature of 17°C, and a xerofluvent soil, according to Soil Survey Staff (2015). Both orchards originally belonged to the same owner, and they were planted with V. dahliae–susceptible herbaceous crops (alfalfa, cotton, and tomato) for 25 years. Then, the ownership of the orchards was transferred to two olive farmers, just before the starting of the study.
For planting, 1-year-old self-rotten olives cv. Picual were used, which are highly susceptible to both non-defoliating and defoliating pathotypes of the pathogen (Lopez-Escudero et al., 2004; Roca et al., 2016). The olives were established during the fall–winter of 1995–1996 (Ancla orchard) and the fall–winter of 1996–1997 (Granon orchard). In Granon orchard, the spacing was 10 × 10 m2 (100 trees per hectare). In Ancla orchard, the tree spacing was the same (10 × 10 m2), although 11 rows of olive trees were planted at 6 × 3 m2 on the west side of the orchard.
We observed the first Verticillium wilt symptoms in spring 1998, 2 years after plantation, in Ancla orchard. In 2000, the pathogen was isolated on potato dextrose agar (PDA) of 10 symptomatic trees of each orchard as described below. Also, inoculum densities of V. dahliae microsclerotia on the soil of both orchards were estimated by subsampling using the wet sieving technique as described below (Soil Isolation). At this point, the inoculum density in both orchards was similar and ranged from 0.4 to 0.6 microsclerotia per gram of soil. Also, in 2010, an evaluation of Phytophthora species was performed, but with no detection of the pathogen (Moral, unpublished data).
Agronomic Practices
Granon Orchard
From 1998, the agronomic practices applied to the Granon orchard aimed to decrease the Verticillium impact. At that time, we started an IDM strategy based on the combination of diverse control methods that had individually shown their efficacy controlling the pathogen. This IDM strategy has been systematically applied since 1998 and is based on (i) weed control by herbicides (mainly glyphosate) to avoid inoculum production of the pathogen on alternative hosts (Thanassoulopoulos et al., 1981); (ii) burning of the pruning debris to prevent the spread of V. dahliae inoculum (Morello et al., 2016); (iii) annual harvesting of the fallen leaves of the symptomatic olive trees using leaf blowers and eliminating through burning; (iv) uprooting, burning, and solarizing the soil by using a polyethylene sheet (4 × 4 m2) for 50–60 days during the summer (Lopez-Escudero and Blanco-Lopez, 2001); and finally (v) during the fall, the moderately resistant cv. Frantoio was used to replant the solarized sites to replace the dead tree (Lopez-Escudero et al., 2004; Leyva-Perez et al., 2017). In 2000, an olive tree of the moderately resistant cv. Frantoio was planted in the middle of every four olive trees of the cv. Picual increasing the tree density from 100 to 204 olive trees ha–1. Also, a drip irrigation system was installed, consisting of two drippers (8 L h–1) per tree and 1,500 m3 ha–1 yr–1. With variations from one season to another, irrigation was mainly applied during spring and autumn (approximately 80% of the total applied water), whereas more severe water restrictions were applied during the summer. Furthermore, despite that infective propagules of V. dahliae are common in irrigation waters in Andalusia (Garcia-Cabello et al., 2012), no water surveys were conducted to test the presence of the pathogen in the water, and subsequently, no specific management was conducted in this sense.
Ancla Orchard
In this orchard, the management followed the traditional agronomic practices performed by olive growers in Jaen province. The Ancla orchard was rainfed, and the dead olives of cv. Picual were replaced with trees belonging to the same susceptible Picual cultivar. The topsoil layer was mechanically tilled for weed control, and pruning debris were burned.
In both Granon and Ancla orchards, different copper-based fungicides were used within a normal annual treatment schedule (two to three treatments per year) during spring and fall–winter to control different aerial diseases such as Venturia oleaginea and Pseudocercospora cladosporioides (Moral et al., 2018). These compounds have no activity against vascular diseases such as Verticillium wilt (Fodale and Mule, 1999).
Weather Data
Weather data were obtained from the nearby (∼10 km) Linares weather station (443,002.0 N, 4,212,540.0 W, altitude 432 m). Weather data collection included daily measurements of temperature and precipitation. Therefore, we calculated the monthly data for the following parameters: minimum, maximum, and average temperatures and total rainfall. Similarly, we calculated the same parameters for each season, i.e., fall (September–November), winter (December–February), spring (March–May), and summer (June–August).
Field Evaluations
Field evaluations were conducted in 2012 and 2013. For that, we chose three experimental plots in Granon orchard with around 200 olive trees per plot (100 of each, Frantoio and Picual cultivars). Because of the very high disease intensity in the Ancla orchard, we only defined an experimental plot with 200 olive trees cv. Picual in the center of the orchard. All the olive tree positions were marked according to the row number and within the row tree number.
Disease Parameters
The symptoms, in terms of incidence and disease severity, were evaluated in June 2012, October 2012, April 2013, June 2013, and October 2013. The disease severity was assessed with a 0–6-point rating scale where 0 = no symptoms, 1% < 5%, 2 = 5–10%, 3 = 11–20%, 4 = 21–40%, 5 = 41–80% of wilted canopy, 6 = dead tree. We used this rating scale since affected olive trees usually showed a low percentage of wilted canopy, while those showing more than 80% of their canopy wilted were dead. Each tree was gauged by two evaluators, and the mean value of the evaluations was used for the data analysis. The rating scale values helped to calculate (i) the Disease Intensity Index (DII), as the average severity of all the trees (including the no affected ones); ii) the disease severity, as the average severity of all the affected trees; and (iii) dead trees percentage or mortality.
Pathogen Isolation and Identification
During every field evaluation, wilted shoot samples were taken from 15 (Granon orchard, n = 75) and 10 (Ancla orchard, n = 50) symptomatic olive trees cv. Picual to conduct the isolation of the pathogen on acidified PDA culture media (Difco Laboratories, Detroit, MI, United States) (Lopez-Escudero et al., 2004). Similarly, isolations from samples of 10 asymptomatic and two symptomatic (n = 12) trees of the olive cv. Frantoio were conducted.
Pathogen Detection by Quantitative Polymerase Chain Reaction
A previously validated real-time polymerase chain reaction (PCR) protocol with the specific primers VertBt-F and VertBt-R (Atallah et al., 2007) was used (Morello et al., 2016). DNA was isolated using the DNeasy Plant Mini kit (QIAGEN). The quantitative PCR (qPCR) was conducted using SYBR Green PCR Master Mix (Applied Biosystems) using an iCycler (Bio-Rad) (Gramaje et al., 2013; Morello et al., 2016).
Soil Isolation
We estimated the density of V. dahliae microsclerotia on the soil of both orchards at four times during the summer of 2012 and 2013. Microsclerotia were quantified using the wet sieving technique (Huisman and Ashworth, 1974) and the modified sodium polypectate agar medium (MSPA (Butterfield and Devay, 1977). In both orchards, the inoculum density was evaluated four times using 25-g-soil samples collected in June and October 2012 and 2013. Each 25-g-soil sample was constituted by 20–25 subsamples (approximately 1 g/sample) regularly collected in the studied orchards (Lopez-Escudero and Blanco-Lopez, 2007). The detection limit of the pathogen in the soil ranged from 0.2 to 0.02 microsclerotia per gram of soil, depending on the number of Petri dishes (0.25 g of soil/dish) used in each evaluation. Because of the low density of V. dahliae inoculum in the soil, we combined the whole of data to increase the limit of detection of the pathogen.
Pathotype Identification
Fifty V. dahliae isolates from 25 olive trees of each orchard were assigned to defoliating or non-defoliating pathotype by PCR using two couples of specific primers (DF/DR vs. NDF/NDR) (Perez-Artes et al., 2000).
Disease Dynamic Through Digital Orthophotography
Digital color orthophotographs, dated in 1998, 2001, 2004, 2005, 2006, 2008, and 2010 at a 1:5,000 scale, were used to study the Verticillium wilt epidemics in both orchards. All digital orthophotographs were taken during July–August by the Instituto Cartografico de Andalucia, distributed by Red de Information Ambiental de Andalucia (REDIAM)1. The orthophotographs were processed using the gvSIG software (Valencian Generalitat Geographic Information System)2. The spatiotemporal dynamics of the Verticillium epidemic were based on the peer expert interpretation of the digital images. For that, we added a layer of points to each digital orthophotograph, in which each point represents the given position of the tree. Then, we generated a database for every given position with the following information: the binary mortality [alive (= 1) or death tree (= 0)] and absence/presence of tree. Because the projection of the crown size of a developing olive trees is associated with the years after plating and considering that the used images were ortho-rectified (REDIAM)3, the tree age was calculated according to its crown projection (Diaz-Varela et al., 2015). The Ancla orchard layer had 2,138-point olive trees, whereas the Granon orchard layer had 3,139-point olive trees (1664 cv. Picual and 1475 cv. Frantoio).
Data Analysis
We differentiated the following treatments: olives of susceptible cv. Picual growing on Ancla orchard (treatment Picual–Ancla) and olives of moderately resistant cv. Frantoio and the susceptible cv. Picual growing on Granon orchard (treatments: Frantoio–Granon and Picual–Granon, respectively).
Field Data
Zar’s test of multiple comparisons of proportions was used for mean separation of the mortality (%) between treatments (Zar, 2010). Severity and DII data were analyzed using the non-parametric Kruskal–Wallis test, and mean ranks of the cultivars were compared at P = 0.05 using Dunn test with a Bonferroni adjustment at P = 0.05 (Demšar, 2006).
Orthophotograph Data
We stratified the data comparing the accumulated mortality at 8th and 10th years after planting for the three orchard-cultivar combinations to eliminate the planting year factor. Subsequently, we calculated the relative risk (RR, syn. risk ratio) associated with IDM by comparing the cv. Picual growing in both orchards and RR associated with the cultivar resistance by comparing both cultivars in Granon orchard. RRs were calculated dividing the mortalities of the treatments to compare, and χ2-tests were used to evaluate the significance of the RRs at P < 0.05 (Newman, 2001). These RRs are an optimum measure for both IDM and the use of the cultivar with different grade of resistance.
For each treatment, the increase in mortality (%) over time, considering the replanting of dead olive trees (i.e., several trees could die in a specific position throughout the study) and without considering it, was fitted using several linear and non-linear models. The best model was a Weibull model that includes rate and shape parameters, which allowed the generation of a wide variety of response curves according to the equation:
where Y = accumulated mortality (%), a = upper asymptote parameter (i.e., upper limit of Y), b = an intrinsic rate of Y increase over time, c = a shape parameter (a higher c value, the function takes longer to reach the asymptote), t = time (year after planting), and d = a shape parameter associated with the type of curve. In all cases, the R2-values of the Weibull models were > 0.920, and standardized residuals were randomly distributed over predicted Y (Campbell and Madden, 1990). Subsequently, we compared the change rates (ΔY/Δt) between treatments using analysis of variance, followed by the least significant differences at P = 0.05, or t-test considering replanting or no-replanting. Scaled (standardized) area under disease progress curve was calculated by trapezoidal integration of mortality values over time divided by the duration of the observed epidemics (Madden et al., 2007). Survival analyses on the occurrence and timing after plantation of olive death were measured using mortality curves estimated by Kaplan–Meier method (Scherm and Ojiambo, 2004), and the treatments were compared using a log-rank test at P = 0.05 as previously for Verticillium epidemics (Perez-Rodriguez et al., 2016).
The relationship of the height above the sea level (m) and the accumulated olive tree mortality (%) were analyzed according to a logistic regression using the number of olive trees as a weight variable. Significance of the logistic regression was considered according to the P-value and regression coefficient (R2). The mortality (%) of olive trees of each year according to digital orthophotographs was correlated (Pearson correlation) with several weather variables from March of the previous year (n - 1) to June (n) to study the effect of weather variables on Verticillium wilt epidemics. For these correlations, we used the following weather variables: maximum, minimum, and average temperatures; and monthly and seasonal rainfall (mm) [fall (September–November), winter (December–February), spring (March–May), and total]. Data from this and the above experiment were analyzed with SPSS (version 14; SPSS Inc., Chicago, IL, United States) or Statistix 10 (Analytical Software, Tallahassee, FL, United States).
Spatial Analysis of Orthophotograph Data
Exponential kriging, an interpolation technique that was previously selected, was used to generate Verticillium wilt maps of both orchards according to the binary mortality data (Bailey, 1994). For that, a semivariance analysis followed by kriging was achieved by using the module “Sextante” of gvSIG software4. This interpolation technique has previously shown a good fit for Verticillium wilt data (Navas-Cortes et al., 2008). Spatial correlation between neighboring olive trees was conducted using the join-count statistic considering the largest square matrix in the center of each orchard (PASSgE, version 2.0; Rosenberg and Anderson, 2011).
Distance indices (SADIE) was used to study the strength and directionality or orientation of aggregation among quadrants containing dead olive trees by Verticillium wilt. For that, we calculated general clustering index (Ia) and clustering indices of patches (Vi) and gaps (Vj) using the SADIE software (Perry et al., 1996), in which the patches and gaps are considered areas with counts of dead trees greater and less than the mean, respectively (Perry et al., 1999). SADIE analysis was conducted on four and three subplots of the Granon and Ancla orchards, respectively (Supplementary Table S3).
Finally, the Index of Dispersion (Iβ) was calculated as the ratio between the observed variance (Vobs) of the data between quadrants to the expected binomial variance (Vbin). For that, we used an iterative process according to quadrant size (QSn; from n = 16 to 48) using a Visual Basic Excel 2007 macro (Microsoft Office, 2007), in which the hypothesis significance was calculated by χ2-test (P < 0.05) (Hughes and Madden, 1992; Madden and Hughes, 1995). Likewise, we calculated the K-value associated with the Iβ as K = (Iβ – 1)/(n – 1), where n is the quadrant size. K-value close to zero indicates a random spatial arrangement of the disease (Navas-Cortes et al., 2008), whereas positive values of K show its aggregation within a quadrant (Madden and Hughes, 1995). Finally, we fitted Verticillium-dead olive trees from both orchards to a beta (BD) and beta-binomial distributions (BBD), which indicate randomness or heterogeneity (aggregation or overdispersion), respectively, of the binary data of plant mortality (Madden and Hughes, 1995), using the software for MS-DOS, BBD.
Results
Field Evaluation
The impact of the IDM strategy on Verticillium wilt of olive was evaluated by comparing the disease on the susceptible olive cv. Picual, growing in two orchards, Ancla (traditionally managed with no IDM) and Granon (IDM). In Granon orchard, we also compared the Verticillium wilt epidemics of this cultivar and the moderately resistant Frantoio. Under field conditions (from June 2012 to October 2013), two Frantoio olive trees died due to the pathogen (0.60%), while the mortality of Picual olive trees in the Ancla orchard reached 41.5%. The mortality of Picual olives in Ancla orchard was significantly higher (P < 0.05) than the mortality (%) of Frantoio and Picual olives in Granon orchard, which did not show significant (P > 0.05) differences between them. Overall, through the study, significant differences (P < 0.05) were found in the incidence between treatments, as Picual–Ancla > Picual–Granon > Frantoio–Granon of trees died. Both Frantoio and Picual showed similar (P > 0.05) DII and severity in Granon orchard. Conversely, Picual olive trees showed lower (P < 0.05) DII and severity than them in Ancla orchard (Table 1).
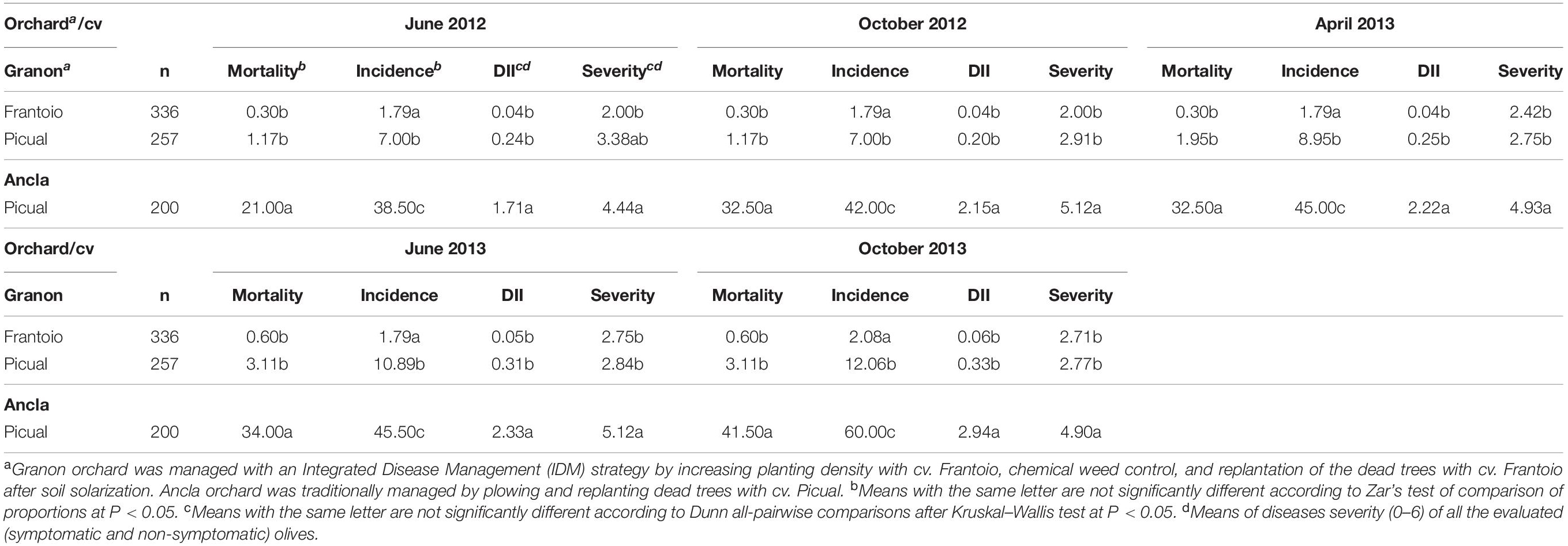
Table 1. Mortality (%), incidence (%), Disease Intensity Index (DII), and severity of olive trees of the cvs. Picual (susceptible) and Frantoio (moderately resistant) affected with Verticillium wilt in two commercial orchards with different disease management practices in Southern Spain.
The species V. dahliae was isolated in cultured media from 20% of the symptomatic olive samples belonging to cv. Picual, from both orchards, but the pathogen was detected by qPCR in the 76% of these samples. Regarding the moderately resistant cv. Frantoio, the pathogen was detected by qPCR only in the two dead olives. Molecular identification conducted using 25 V. dahliae isolates of each orchard showed the following rate among pathotypes (non-defoliating/defoliating): 2/23 (Ancla orchard) and 7/18 (Granon orchard) being this difference not significant (P = 0.066).
In 2002, the average densities of microsclerotia of V. dahliae per gram of soil were 0.4 and 0.5 in Ancla and Granon orchards, respectively (Moral, unpublished data). In 2012–2013, the averages of inoculum considering the four samplings were 0.040 and 0.018 microsclerotia per gram of soil in the Ancla and Granon orchards, respectively. According to these results, the density of V. dahliae propagules decreased around 10-fold (Ancla orchard) and 20-fold (Granon orchard) over 12–13 years.
Disease Dynamic Through Digital Orthophotography
Temporal Analysis
In 1998, we observed the first Picual olive trees with unequivocal symptoms of Verticillium wilt in both Ancla and Granon orchards. Two years later, the pathogen was isolated from 10 olive trees in each orchard. In the case of the moderately resistant cv. Frantoio, the first olive trees showing Verticillium wilt symptoms were observed in 2006, i.e., in year 6 after they were planted. Irrespective of replanting (i.e., considering that only one tree was planted in each position), the accumulated mortalities of Picual olive trees were 71.00 and 32.95% in Ancla and Granon orchards, respectively. In the case of the moderately resistant cv. Frantoio, the accumulated mortality was 6.37% (Figure 1). When we compared the mortality of the olive cv. Picual in both orchards, the RRs due to inadequate agronomic practices were 1.26 and 1.55 in years 8 and 10 after planting, respectively. Likewise, the susceptible cv. Picual, in contrast to the moderately resistant cv. Frantoio, was associated with an around fivefold increase in the mortality risk (χ2-test P < 0.001) (Figure 2). Considering the replantation, the accumulated number of dead Picual olive trees in the Ancla orchard was 3546 (165.86%), whereas it was 8.54% with Frantoio in the Granon orchard (Supplementary Table S1). Interestingly, Picual olives were planted even four times in 171 given positions in the Ancla orchard.
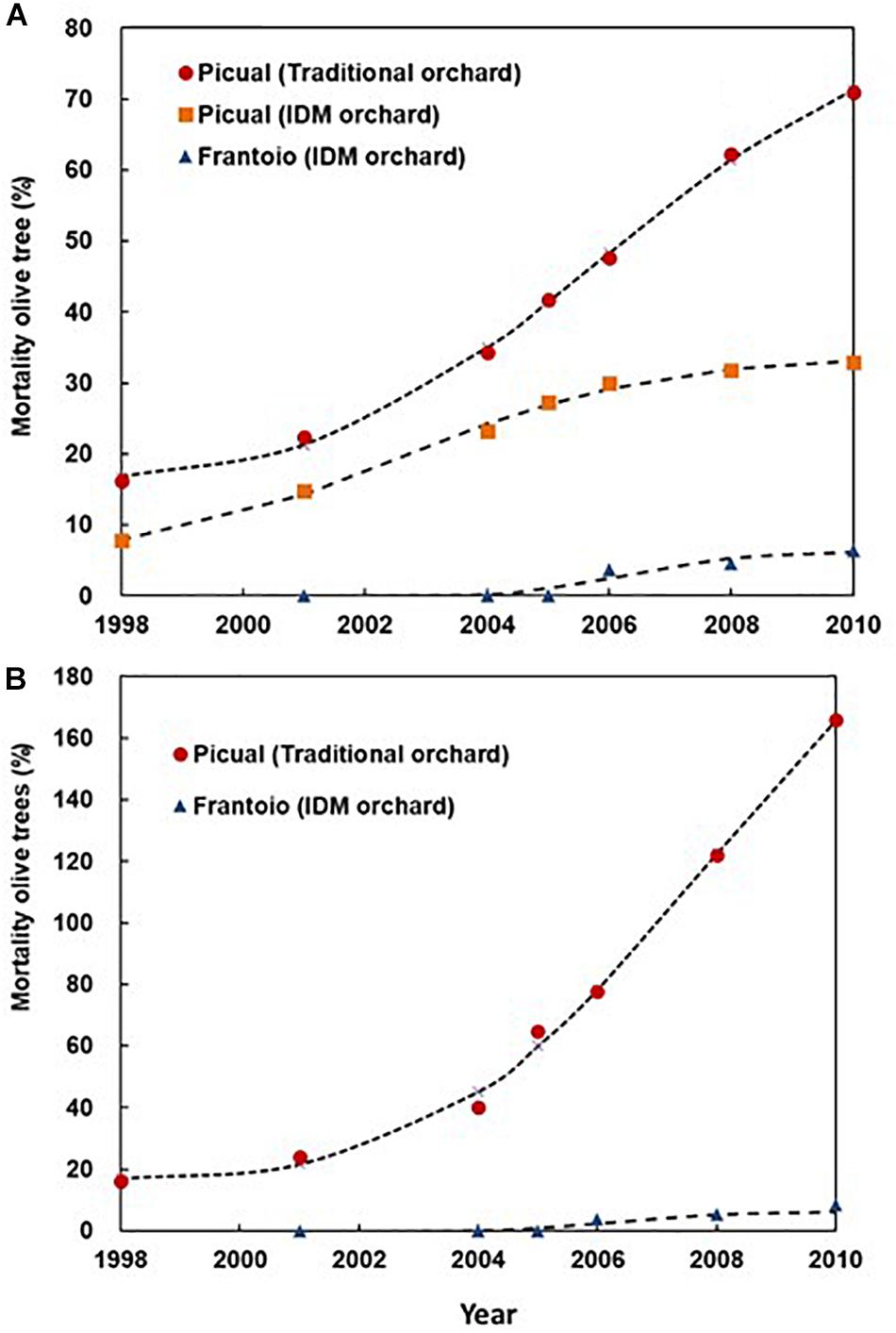
Figure 1. Accumulated mortality of olive trees cvs. Picual (susceptible) and Frantoio (moderately resistant) by Verticillium dahliae in two orchards in Southern Spain. In Granon orchard, an Integrated Disease Management of the Verticillium wilt was applied, while traditional agronomic (no-IDM) practices were applied in Ancla orchard. The lines represent the fitted curves according to a Weibull Model (Supplementary Table S2). (A) Accumulated olive tree mortality without considering plant replanting. (B) Accumulated olive tree mortality considering plant replanting.
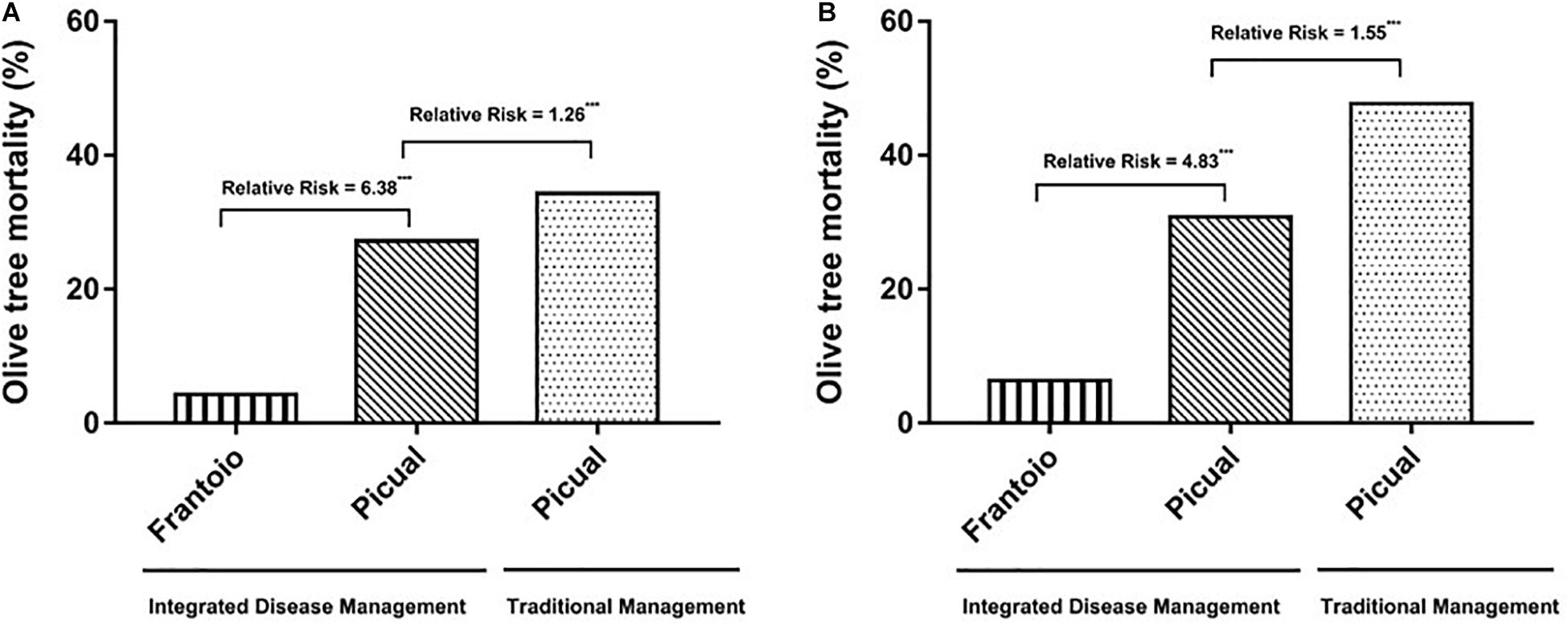
Figure 2. Accumulated mortality of olive trees cvs. Picual (susceptible) and Frantoio (moderately resistant) by Verticillium dahliae in two orchards in Southern Spain in the 8th (A) and 10th (B) years after plantation. In Granon orchard, an Integrated Disease Management (IDM) of the Verticillium wilt was applied, whereas traditional agronomic (no-IDM) practices were applied in Ancla orchard. Relative risk significant according to χ2-test at ***P < 0.001.
The Weibull model adequately (R2 > 0.900) described the increase in dead olives (with or without replanting) over time in both orchards. In all cases, there were significant differences (confidence interval at 95%) among the a and b parameters, i.e., upper asymptote and rate of the accumulated mortality, of the Weibull model for the three treatments; whereas no differences were observed among the shape parameters of the model (c and d) (Figure 1). Attending the average rate of change, there were significant differences (P = 0.001) among the epidemic rates being 5.59, 3.02, and 0.72% of Verticillium-dead olive per year for the treatments Picual–Ancla, Picual–Granon, and Frantoio–Granon without replanting, respectively. Likewise, the epidemic rate was 1.02% yr–1 for the treatment Frantoio–Granon, i.e., 13 times lower than Picual–Ancla (P = 0.008) (Supplementary Table S2). According to the Kaplan–Meier test, Verticillium wilt epidemics took 6 and 10 years to cause the death of 25% of olives cv. Picual in the Ancla and Granon orchards, respectively. Conversely, the pathogen caused a 6.37% of mortality of olives cv. Frantoio over 10 years. There were significant differences (Longrank test, P < 0.001) among the three treatments (Supplementary Figure S1).
During the study term, there was a significant (P < 0.05) and negative relationship between the number Verticillium-dead trees of the susceptible olive cv. Picual and the height above sea level. This relationship was significant (P < 0.05) in both orchards except for the combination Ancla 2004, it was clearer in the Granon orchard than in Ancla orchard (Table 2). Conversely, there was no significant relation between both mortality of the olive trees cv. Frantoio and height above sea level. Finally, we found positive regression (R2 = 0.961; P < 0.001) between total rainfall during March through May and the olive mortality in the Ancla orchard.
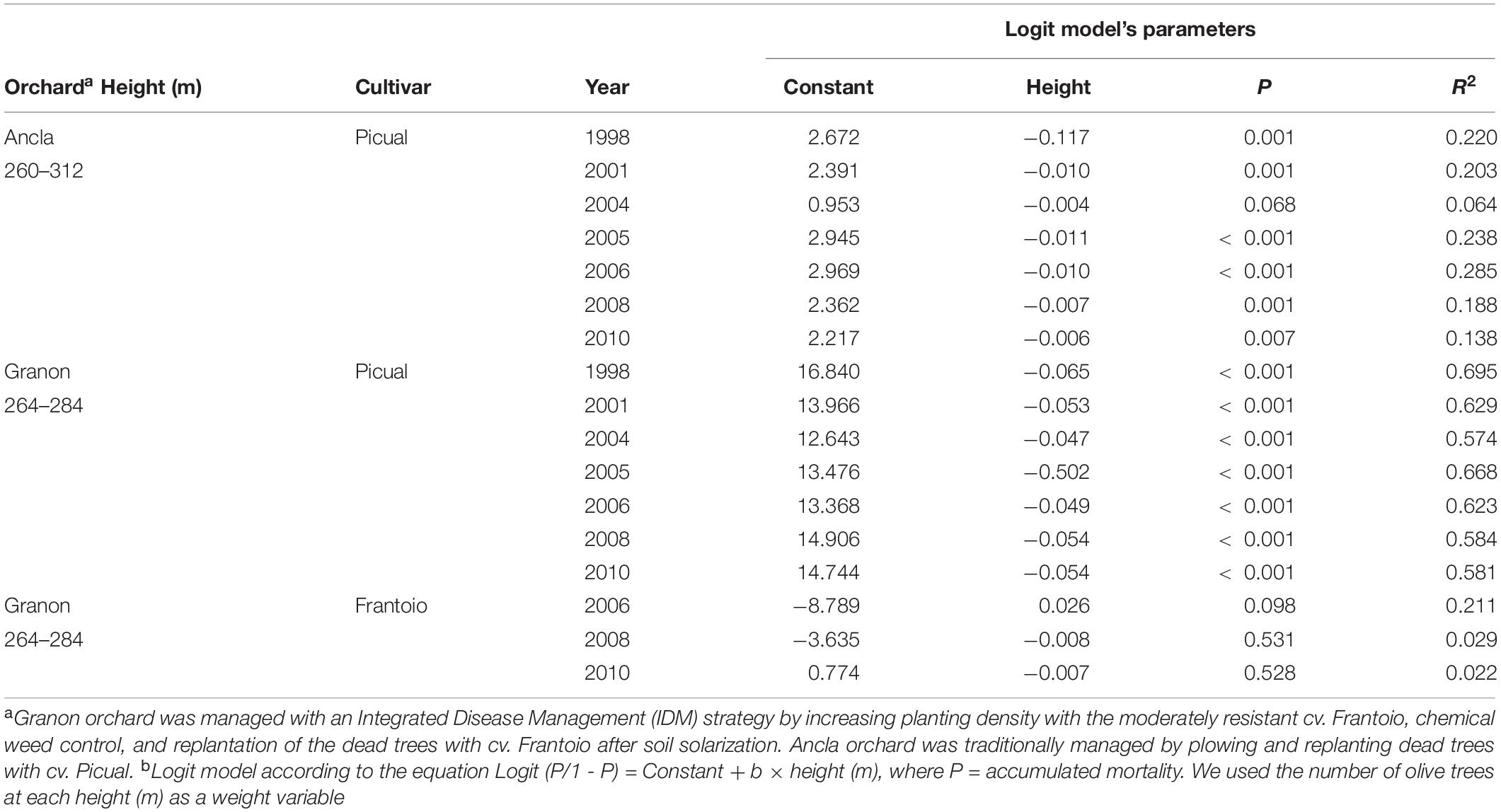
Table 2. Relationship between the height (m) above sea level and the accumulated mortality of olive trees cvs. Picual (susceptible) and Frantoio (moderately resistant) by Verticillium dahliae in two commercial orchards in Southern Spain.
Spatiotemporal Analysis
Figure 3 and Supplementary Figure S2 show the spatiotemporal pattern of the mortality caused by V. dahliae on both orchards. In 1998, olives of the cv. Picual at the southwestern side of the Ancla orchard and the northwestern side of the Granon orchard, respectively, were the most affected by the pathogen. In the Ancla orchard, the size of the principal focus increased over time, whereas new foci mainly developed at the edges, and only a central area of the orchard was Verticillium-free. In the Granon orchard, the Verticillium epidemic affected the entire band of olive cv. Picual at the north side of the orchard at the end of this study. Considering the cv. Frantoio, small foci were observed without an apparent pattern in the sixth years after planting, but new wilt trees appeared more frequently next to original foci of Verticillium-dead olives (Figure 3). At the Granon orchard, the replacement of the dead trees of the susceptible cv. Picual with the moderately resistant cv. Frantoio decreased the aggregation of the disease. Because a higher aggregation of Verticillium-dead olive in the edges of both orchards and the edges were not considered for the join-count statistic because of limitation of the used software, this only showed significant aggregation between dead olive trees (P < 0.05) cv. Frantoio in Granon orchard (from 2006 to 2010) and cv. Picual (P < 0.001) in Ancla orchard in 2010 (data not shown).
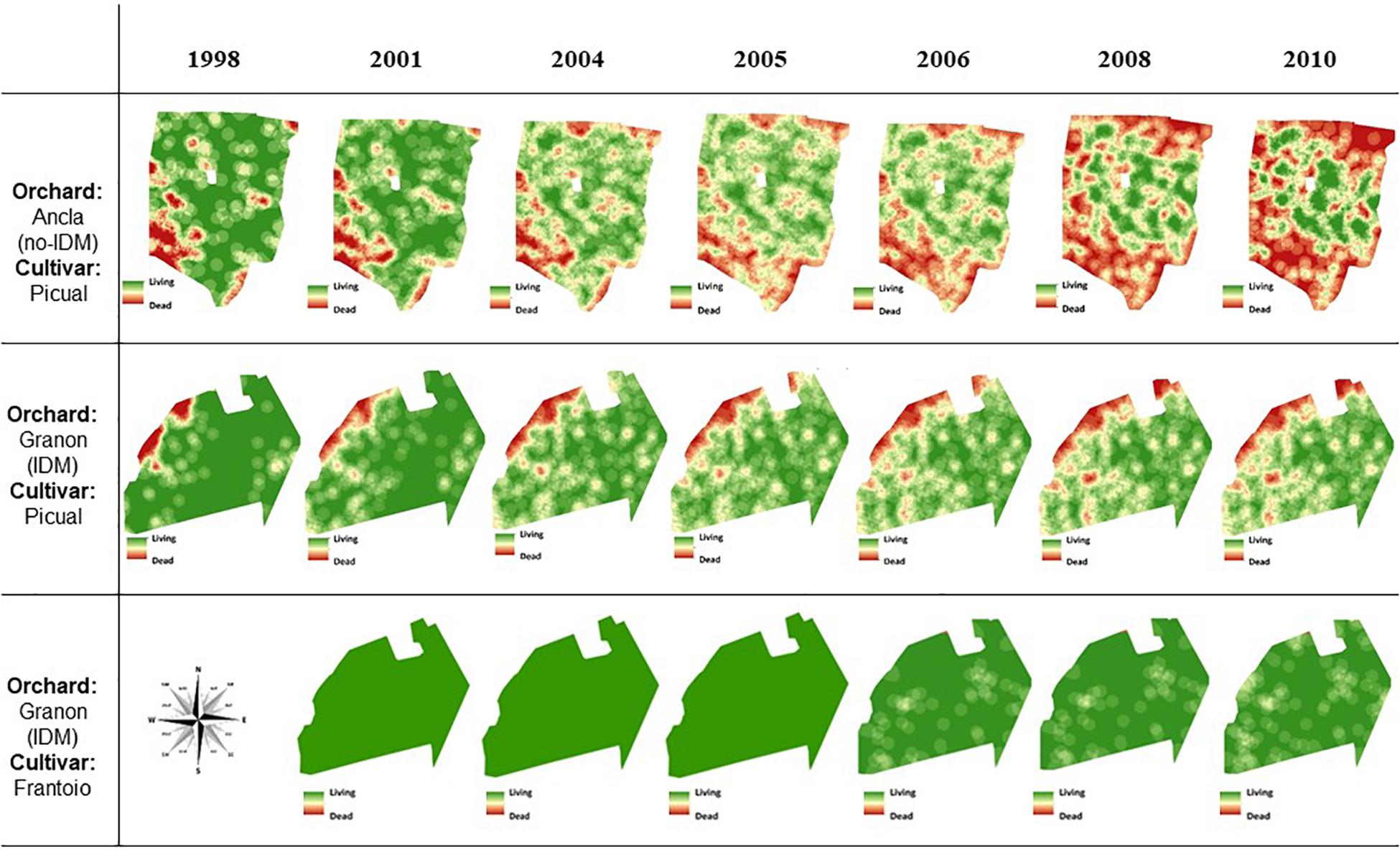
Figure 3. Kriging estimate maps based on Verticillium-dead mortality of olive trees in two commercial orchards in Southern Spain in the summer of each of 1998, 2001, 2004, 2005, 2006, 2008, and 2010 crop seasons. In Granon orchard, an Integrated Disease Management (IDM) of the Verticillium wilt has been applied since 1998. Olive trees of the susceptible cv. Picual were planted during fall–winter 1996–1997 in Granon orchard. In 2000, in the latter orchard, an olive tree of the resistant cv. Frantoio was planted in the middle of every four olive trees of the cv. Picual. Olive trees of the cv. Picual were planted in fall–winter 1995–1996 in Ancla orchard, in which traditional agronomic (no-IDM) practices have been applied since tree planting.
Distance indices analysis (SADIE) was conducted in both orchards using different plots with 200–480 olives each. The clustering index (Ia) and clustering indices of patches (Vi) and gaps (Vj) were correlated. The three SADIE indices were variable among plots and years. In Ancla orchard, we observed a significant aggregated pattern (P < 0.05) in at least one plot during the 12 years of this study. There was a reduction in the degree of aggregation of the disease through the time in the susceptible cv. Picual in the Granon orchard; thus, while the dead-Verticillium olives cv. Picual were aggregated, at least in one plot at the beginning of this study (1998 and 2001), the disease showed a random pattern at the end of this study (2008 and 2010). Conversely, once the first moderately resistant olives cv. Frantoio died in 2006, there was a significant aggregation of the Verticillium wilt according to Ia, Vi, and Vj (Supplementary Table S3). Overall, the replanting of dead olives decreased the heterogeneity of the Verticillium-dead olives in both orchards (data not shown).
Similar results were independently obtained of quadrant size. Thereafter, Iβ was calculated considering a quadrant size of 48 trees. Thus, when Verticillium-dead olives showed a non-random pattern according to the Iβ (P < 0.05), data significantly fitted to the BBD but not to BD. Results of the D and BBD analysis suggested an aggregated pattern of distribution of Verticillium-dead olive trees of the susceptible cv. Picual in both orchards through the 12 years. Likewise, in Granon orchard, Verticillium-dead olives of the moderately resistant cv. Frantoio showed a heterogeneous pattern, but only in 2006 and 2008 (Table 3). In Ancla orchard, there was an important decline of the Iβ from 1998 to 2005 because of the increase in the dead trees, but it subsequently remained stable around 5.5. Likewise, in Granon orchard, the Iβ suggested aggregation of Verticillium-dead olives cv. Picual and a light aggregation of the dead olives cv. Frantoio, given the low number of dead plants. Besides, as expected, the replanting process decreased the aggregation values (D and BBD) of the disease (data not shown).
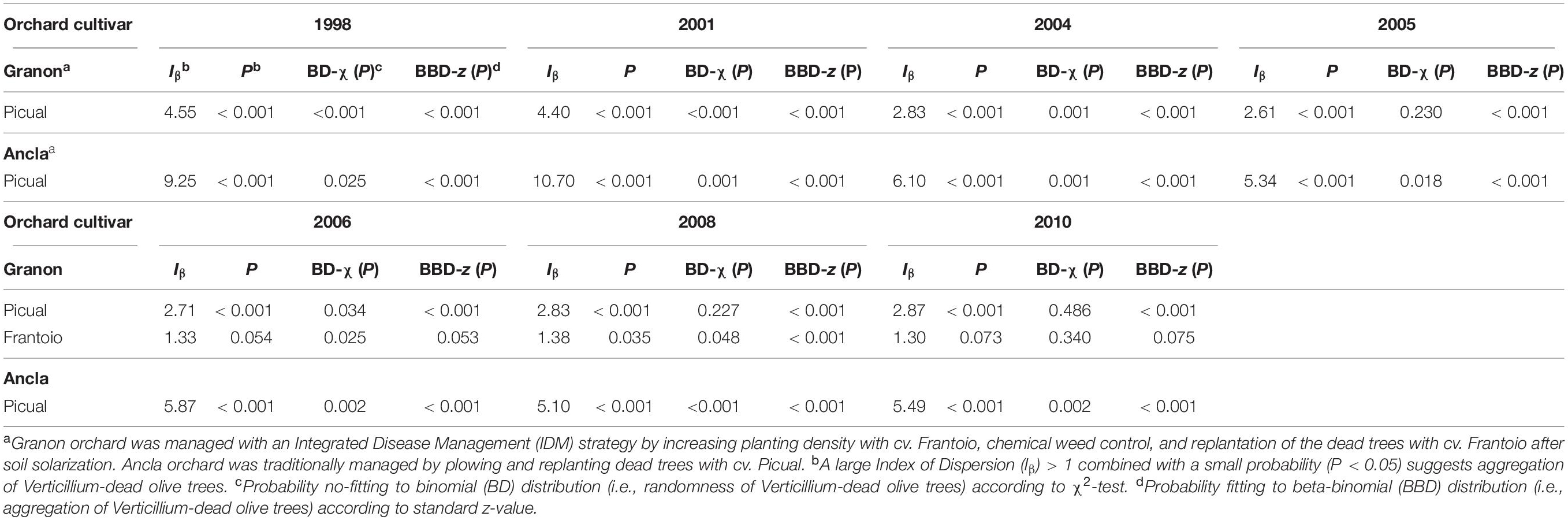
Table 3. Index of Dispersion (Iβ) and probability to no-fitting binomial (BD) and fittings beta-binomial (BBD) of the distributions of dead olive trees cvs. Picual (susceptible) and Frantoio (moderately resistant) by Verticillium dahliae in two commercial orchards with different disease management practices in Southern Spain.
Discussion
Verticillium wilt is the most important disease of olive in many countries of the Mediterranean basin, having a major impact in Spain, the main olive producer worldwide (Lopez-Escudero and Mercado-Blanco, 2011; Jimenez-Diaz et al., 2012). Control of Verticillium wilt of olive depends heavily on the use of resistant cultivars; however, there are no completely resistant cultivars (immune) to the pathogen (Lopez-Escudero et al., 2004; Leyva-Perez et al., 2017). The use of moderately resistant olive cultivars to V. dahliae should be applied in a coordinated manner with other management disease practices to maximize the benefits of this resistance. Here, we studied the effect that an IDM performed in an orchard (called Granon) of the susceptible olive cv. Picual in the spatiotemporal development of Verticillium wilt by comparing with a traditional orchard (called Ancla). This IDM strategy comprised the increasing planting density with moderately resistant cv. Frantoio, chemical weed control, and replanting of the dead tree with cv. Frantoio after soil solarization. Other studies suggest the replacement of wilted olive trees with apple trees, a non-host plant, as an environmentally friendly management practice (Karajeh and Owais, 2012), but little is known about replacement using resistant cultivars of olive.
Generally, the rate of Verticillium-dead olive trees per year was 5.59%, when the susceptible cv. Picual was growing in the orchard with no disease management practices, and 3.02% yr–1 when the same cultivar was at the IDM orchard. When planted in soils with low inoculum densities (<1 microsclerotia/g of soil), the cv. Picual is extremely susceptible to the defoliating pathotype of V. dahliae (Lopez-Escudero and Blanco-Lopez, 2007). When the IDM was performed in orchards planted with the moderately resistance cv. Frantoio, the annual rate of dead olive trees was around sevenfold lower than the observed in the orchard more conducive for the disease, i.e., susceptible cultivar and non–disease management practices. Likewise, according to the RR, the impact of using a moderately resistant cultivar in the reduction of tree mortality was much higher than the application of different agronomic practices between orchards. It is worth mentioning here that two agronomic factors could have favored the epidemic development in Granon orchard. On one side, irrigation water disseminates the pathogen through the olive orchards of Southern Spain (Garcia-Cabello et al., 2012) (Ancla was rainfed during the duration of the study) and increases the olives susceptibility to pathogen infection (Perez-Rodriguez et al., 2016; Santos-Rufo et al., 2017). Second, the plant density, when higher because of the interplanting of the moderately resistant cv. Frantoio, reduced the distance between adjacent trees and increased the potential number of infected olives and, simultaneously, the inoculum source. Roca et al. (2016) observed that non-significant effect of the plating density on the epidemic of Verticillium wilt of olive was not observed, although this study was conducted on the cvs. Arbequina and Picual.
The differences in susceptibility to V. dahliae between cvs. Frantoio (syn. Oblonga) and Picual have been well-known for a long time (Hartmann et al., 1971; Lopez-Escudero et al., 2004). The moderate resistance of the cv. Frantoio is associated with a protein of Bet v I family and a dirigent-like protein involved in lignification and reactive oxygen species stress response (Leyva-Perez et al., 2017). The phenotypic reaction of the cv. Frantoio to the pathogen is well-known under controlled conditions and experimental fields (Lopez-Escudero et al., 2004; Gramaje et al., 2013; Trapero et al., 2013). Unfortunately, few or too short-term studies have been conducted in naturally infested soils (Montes-Osuna and Mercado-Blanco, 2020). For example, Trapero et al. (2013) observed no affected olives of the cv. Frantoio in highly infested soils (21 microsclerotia/g) but only in a 2-year study. Under the conditions of our study, the first Verticillium dead tree of the cv. Frantoio was detected in the sixth year after planting, once the mortality had reached up to 4.27% of the trees of this cultivar, whereas susceptible Picual showed mortality around 30%.
In our study, and although there was no periodic monitoring, there was a 20-fold decrease from 2000 to 2012–2013 in the density of V. dahliae microsclerotia in Granon orchard. This reduction may be because of the immediate burning of fallen leaves of the olive trees affected by the pathogen, which are the major source of new microsclerotia (Jimenez-Diaz et al., 2012). However, in the Ancla orchard, an important decrease (around 10-fold) in the microsclerotia density was also detected. This observation suggests that the balance between microsclerotia production and their loss decreases the inoculum in soil, but more studies are needed. Conversely, in several herbaceous hosts, when affected by V. dahliae, a marked increment of inoculum in the soil is observed at the end of the season (Mol and Termorshuizen, 1995; Ioannou et al., 1977).
In the rainfed orchard, the disease epidemic was driven by the accumulated rainfall during the spring, which is in accordance with the fact that the maximum infection rate of Verticillium wilt of olive occurs at the end of the winter–early spring (Navas-Cortes et al., 2008) and favored by moist soils (Perez-Rodriguez et al., 2016; Santos-Rufo et al., 2017).
The disease cycle, among other factors, such as the cultivar resistance, the agronomic practices, and the climatic variable of the region, determines the spatial pattern of the diseases (Madden et al., 2007). Factors such as the spatial distribution of the microsclerotia (Bejarano-Alcazar et al., 1995; Xiao et al., 1997) and the potential use of infected plating material (Thanassoulopoulos, 1992) have an essential impact on the spatial pattern of the Verticillium wilt of olive during the first years after planting. After this, V. dahliae spreads between, and within, olive orchards through infested soil particles and machinery, irrigation water, and infected plant debris, and thus affecting the spatial pattern of the disease (Lopez-Escudero and Mercado-Blanco, 2011; Jimenez-Diaz et al., 2012). In our study, we observed an aggregated pattern of Verticillium-dead olives in both orchards, Ancla and Granon, although a lower level of aggregation in the latter. The height (m) of the trees explained part of this aggregation since the disease leaned to cluster in the low-lying parts of the orchards as a consequence of higher inoculum density. Similarly, the Verticillium wilt epidemics of lettuce usually start in low-lying fields/areas in California (Vallad et al., 2006). In any case, results shown in the current study agree with many diseases caused by soil-borne pathogens, including the Verticillium wilt of olive (Navas-Cortes et al., 2008), tending to be more aggregated than aerial pathogens (Campbell and Benson, 1994).
Finally, we also observed that the replanting of dead olives decreased aggregation of the disease, mainly when conducted employing the moderately resistant cv. Frantoio. Furthermore, the level of disease aggregation decreased with the accumulated mortality of olive trees because of the homogenization process of the disease in the field (Turechek et al., 2011).
Under the conditions of our study, which include low inoculum pressure of V. dahliae and low winter temperatures that reduce the period of infection (Trapero et al., 2013), the described IDM strategy has allowed an acceptable control of the Verticillium wilt and has kept the profitability of the Granon orchard.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Author Contributions
JM conceived and designed the study. JM, EO, and FL-E performed the evaluation under field conditions. EO and MG-L performed the laboratory works. JM, RP, and EO analyzed the data. JM, MG-L, and TM wrote the manuscript. FL-E, AT, RP, and EO reviewed and discussed the final revised version. All authors contributed to the article and approved the submitted version.
Funding
JM was supported by a FEDER project (UCO-27464) co-funded by European Union FEDER funds and Junta de Andalucia. This research was funded by “Aceites Hijos de Vicente Moral,” and the Spanish Ministry of Science, Innovation and Universities throughout the project AGL2016-76240-R.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer FJGG declared a shared affiliation, with no collaboration, with several of the authors, EO, MG-L, FL-E, AT-C, JM, to the handling editor at the time of the review.
Acknowledgments
We thank Vicente Moral (Granon orchard owner) for his essential support.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2020.584496/full#supplementary-material
Supplementary Figure 1 | Kaplan-Meier survival functions of olive trees cvs. Picual (susceptible) and Frantoio (moderately resistant) affected by Verticillium dahliae in two orchards in Southern Spain. In Granon orchard, an Integrated Diseases Management (IDM) of the Verticillium wilt was applied, while traditional agronomic (no-IDM) practices were maintained in Ancla orchard.
Supplementary Figure 2 | Orthophotographs showing Verticillium-dead mortality of olive trees in two commercial orchards in Southern Spain in the summer of each of 2001, 2006, and 2010 crop seasons. In Granon orchard, an Integrated Diseases Management (IDM) of the Verticillium wilt was applied since 1998. Olive trees of the susceptible cv. Picual were planted during fall-winter 1996–1997 in Granon orchard. In 2000, in the latter orchard, an olive tree of the resistant cv. Frantoio was planted in the middle of every four olive trees of the cv. Picual. Olive trees of the cv. Picual were planted in fall-winter 1995–1996 in Ancla orchard, in which traditional agronomic (no-IDM) practices were applied since tree planting.
Footnotes
- ^ http://www.juntadeandalucia.es/medioambiente/site/rediam
- ^ http://www.gvsig.com/
- ^ http://www.juntadeandalucia.es/medioambiente/site/rediam
- ^ http://www.gvsig.com/
References
Atallah, Z. K., Bae, J., Jansky, S. H., Rouse, D. I., and Stevenson, W. R. (2007). Multiplex real-time quantitative pcr to detect and quantify Verticillium dahliae colonization in potato lines that differ in response to verticillium wilt. Phytopathology 97, 865–872. doi: 10.1094/phyto-97-7-0865
Bailey, T. C. (1994). “A review of statistical spatial analysis in geographical information systems,” in Spatial Analysis and GIS, eds S. Fotheringham and P. Rogerson (London: Taylor and Francis), 13–44.
Bejarano-Alcazar, J. (2008). Efecto de las cubiertas vegetales sobre la epidemiología y control de la verticilosis del olivo. Mercacei 51, 206–208.
Bejarano-Alcazar, J., Melero-Vara, J. M., Blanco-Lopez, M. A., and Jimenez-Diaz, R. M. (1995). Influence of inoculum density of defoliating and nondefoliating pathotypes of Verticillium dahliae on epidemics of Verticillium wilt of cotton in Southern Spain. Phytopathology 85, 1474–1481. doi: 10.1094/phyto-85-1474
Bubici, G., and Cirulli, M. (2014). Natural recovery from Verticillium wilt in olive: can it be exploited in a control strategy? Plant Soil 381, 85–94. doi: 10.1007/s11104-014-2112-y
Butterfield, E. J., and Devay, J. E. (1977). Reassessment of soil assays for Verticillium dahliae. Phytopathology 67, 1073–1078. doi: 10.1094/phyto-67-1073
Campbell, C. L., and Benson, D. M. (eds) (1994). “Spatial aspects of the development of root disease epidemics,” in Epidemiology and Management of Root Diseases, (Berlin: Springer-Verlag), 195–243. doi: 10.1007/978-3-642-85063-9_7
Campbell, C. L., and Madden, L. V. (1990). Introduction to Plant Disease Epidemiology. New York: Wiley.
Demšar, J. (2006). Statistical comparisons of classifiers over multiple data sets. J. Mach. Learn. Res. 7, 1–30. doi: 10.1155/2013/395096
Diaz-Varela, R. A., De la Rosa, R., Leon, L., and Zarco-Tejada, P. J. (2015). High-resolution airborne UAV imagery to assess olive tree crown parameters using 3D photo reconstruction: application in breeding trials. Remote Sens. 7, 4213–4232. doi: 10.3390/rs70404213
ESYRCE (2019). Encuesta Sobre Superficies y Rendimientos Cultivos (ESYRCE), Encuesta de Marco de Áreas de España. Available online at: https://www.mapa.gob.es/es/estadistica/temas/estadisticas-agrarias/agricultura/esyrce/
Fodale, A. S., and Mule, R. (1999). Control of Verticillium dahliae wilt in olive trees by injection of fungicides into the trunk [Olea europaea L.-Sicily]. Infor. Fitopatol. 49, 19–24.
Garcia-Cabello, S., Perez-Rodriguez, M., Blanco-Lopez, M. A., and Lopez-Escudero, F. J. (2012). Distribution of Verticillium dahliae through watering systems in widely irrigated olive growing areas in Andalucia (Southern Spain). Eur. J. Plant Pathol. 133, 877–885. doi: 10.1007/s10658-012-0011-8
Gomez-Galvez, F. J., and Rodriguez-Jurado, D. (2018). Potential efficacy of soil-applied disinfectant treatments against Verticillium wilt of olive. Crop. Protection 106, 190–200. doi: 10.1016/j.cropro.2018.01.002
Gomez-Galvez, F. J., Vargas-Osuna, E., and Rodriguez-Jurado, D. (2018). Suppressive and preventive activity of chemical disinfectants against sclerotia of Verticillium dahliae in water. Crop. Protection 108, 12–22. doi: 10.1016/j.cropro.2018.02.011
Gramaje, D., Perez-Serrano, V., Montes-Borrego, M., Navas-Cortes, J. A., Jimenez-Diaz, R. M., and Landa, B. B. (2013). A comparison of real-time PCR protocols for the quantitative monitoring of asymptomatic olive infections by Verticillium dahliae pathotypes. Phytopathology 103, 1058–1068. doi: 10.1094/phyto-11-12-0312-r
Hartmann, H., Schnathorst, W. C., and Hisler, J. (1971). Oblonga, a clonal olive root-stock resistant to Verticillium wilt. Calif. Agric. 25, 12–15.
Hughes, G., and Madden, L. V. (1992). Aggregation and incidence of disease. Plant Pathol. 41, 657–660. doi: 10.1111/j.1365-3059.1992.tb02549.x
Huisman, O. C., and Ashworth, L. J. J. (1974). Quantitative assessment of Verticillium albo-atrum in field soils: procedural and substrate improvements. Phytopathology 94, 1043–1044. doi: 10.1094/phyto-64-1043
Ioannou, N., Schneider, R. W., Grogan, R. G., and Duniway, J. M. (1977). Effect of water potential and temperature on growth, sporulation, and production of microsclerotia by Verticillium dahliae. Phytopathology 67, 637–644. doi: 10.1094/phyto-67-637
Jimenez-Diaz, R., Cirulli, M., Bubici, G., Jimenez-Gasco, M., Antoniou, P., and Tjamos, E. (2012). Verticillium wilt, a major threat to olive production: current status and future prospects for its management. Plant Dis. 96, 304–329. doi: 10.1094/pdis-06-11-0496
Jimenez-Diaz, R. M., Olivares-Garcia, C., Trapero-Casas, J. L., Jimenez-Gasco, M. M., Navas-Cortes, J. A., Landa, B. B., et al. (2017). Variation of pathotypes and races and their correlations with clonal lineages in Verticillium dahliae. Plant Pathol. 66, 651–666. doi: 10.1111/ppa.12611
Karajeh, M. R., and Owais, S. J. (2012). Reaction of selected apple cultivars to wilt pathogen: Verticillium dahliae. Plant Prot. Sci. 48, 99–104. doi: 10.17221/61/2011-pps
Keykhasaber, M., Pham, K. T. K., Thomma, B. P. H. J., and Hiemstra, J. A. (2017). Reliable detection of unevenly distributed Verticillium dahliae in diseased olive trees. Plant Pathol. 66, 641–650. doi: 10.1111/ppa.12647
Levin, A. G., Lavee, S., and Tsror, L. (2003). Epidemiology and effects of Verticillium wilt on yield of olive trees (cvs. Barnea and Souri) irrigated with saline water in Israel. Phytoparasitica 31, 333–343. doi: 10.1007/bf02979804
Leyva-Perez, M. O., Jimenez-Ruiz, J., Gomez-Lama Cabanas, C., Valverde-Corredor, A., Barroso, J. B., Luque, F., et al. (2017). Tolerance of olive (Olea europaea) cv Frantoio to Verticillium dahliae relies on both basal and pathogen-induced differential transcriptomic responses. New Phytol. 217, 671–686. doi: 10.1111/nph.14833
Ligoxigakis, E. K., Vakalounakis, D. J., and Thanassoulopoulos, C. C. (2002). Weed hosts ofVerticillium dahliae in crete: susceptibility, symptomatology and significance. Phytoparasitica 30, 511–518. doi: 10.1007/bf02979756
Lopez-Escudero, F., del Rio, C., Caballero, J., and Blanco-Lopez, M. A. (2004). Evaluation of olive cultivars for resistance to Verticillium dahliae. Eur. J. Plant Pathol. 110, 79–85. doi: 10.1023/b:ejpp.0000010150.08098.2d
Lopez-Escudero, F. J., and Blanco-Lopez, M. A. (2001). Effect of a single or double soil solarization to control Verticillium wilt in established olive orchards in Spain. Plant Dis. 85, 489–496. doi: 10.1094/pdis.2001.85.5.489
Lopez-Escudero, F. J., and Blanco-Lopez, M. A. (2005). Recovery of young olive trees from Verticillium dahliae. Eur. J. Plant Pathol. 113, 367–375. doi: 10.1007/s10658-005-3145-0
Lopez-Escudero, F. J., and Blanco-Lopez, M. A. (2007). Relationship between the inoculum density of Verticillium dahliae and the progress of Verticillium wilt of olive. Plant Dis. 91, 1372–1378. doi: 10.1094/pdis-91-11-1372
Lopez-Escudero, F. J., and Mercado-Blanco, J. (2011). Verticillium wilt of olive: a case study to implement an integrated strategy to control a soil-borne pathogen. Plant Soil 344, 1–50. doi: 10.1007/s11104-010-0629-2
Lopez-Escudero, F. J., Mercado-Blanco, J., Roca, J. M., Valverde-Corredor, A., and Blanco-Lopez Angel, M. (2010). Verticillium wilt of olive in the guadalquivir valley (Southern Spain): relations with some agronomical factors and spread of Verticillium dahliae. Phytopathol. Mediterr. 49, 370–380.
Madden, L. V., and Hughes, G. (1995). Plant disease incidence?: heterogeneity, and temporal anal ysis. Annu. Rev. Phytopathol. 33, 529–564. doi: 10.1146/annurev.py.33.090195.002525
Madden, L. V., Hughes, G., and van den Bosch, F. (2007). The Study of Plant Disease Epidemics. St. Paul, MN: APS Press.
Milgroom, M. G., Jimenez-Gasco, M. D. M., Olivares-Garcia, C., and Jimenez-Diaz, R. M. (2016). Clonal expansion and migration of a highly virulent, defoliating lineage of Verticillium dahliae. Phytopathology 106, 1038–1046. doi: 10.1094/phyto-11-15-0300-r
Mol, L., and Termorshuizen, A. J. (1995). “Life cycle and ecology of Verticillium dahliae in potato,” in Potato Ecology And Modelling of Crops Under Conditions Limiting Growth, eds A. J. Haverkort and D. K. L. MacKerron (Dordrecht: Springer), 251–263. doi: 10.1007/978-94-011-0051-9_16
Montes-Osuna, N., and Mercado-Blanco, J. (2020). Verticillium wilt of olive and its control: what did we learn during the last decade? Plants 9:735. doi: 10.3390/plants9060735
Moral, J., Agusti-Brisach, C., Agalliu, G., de Oliveira, R., Perez-Rodriguez, M., Roca, L. F., et al. (2018). Preliminary selection and evaluation of fungicides and natural compounds to control olive anthracnose caused by Colletotrichum species. Crop Prot. 114, 167–176. doi: 10.1016/j.cropro.2018.08.033
Morello, P., Diez, C. M., Codes, M., Rallo, L., Barranco, D., Trapero, A., et al. (2016). Sanitation of olive plants infected by Verticillium dahliae using heat treatments. Plant Pathol. 65, 412–421. doi: 10.1111/ppa.12432
Navas-Cortes, J. A., Landa, B. B., Mercado-Blanco, J., Trapero-Casas, J. L., Rodriguez-Jurado, D., and Jimenez-Diaz, R. M. (2008). Spatiotemporal analysis of spread of infections by Verticillium dahliae pathotypes within a high tree density olive orchard in Southern Spain. Phytopathology 98, 167–180. doi: 10.1094/phyto-98-2-0167
Perez-Artes, E., Garcia-Pedrajas, M. D., Bejarano-Alcazar, J., and Jimenez-Diaz, R. M. (2000). Differentiation of cotton-defoliating and nondefoliating pathotypes of Verticillium dahliae by RAPD and Specific PCR Analyses. Eur. J. Plant Pathol. 106, 507–517.
Perez-Artes, E., Mercado-Blanco, J., Ruz-Carrillo, A. R., Rodriguez-Jurado, D., and Jimenez-Diaz, R. M. (2005). Detection of the defoliating and nondefoliating pathotypes of Verticillium dahliae in artificial and natural soils by nested PCR. Plant Soil 268, 349–356. doi: 10.1007/s11104-004-0378-1
Perez-Rodriguez, M., Serrano, N., Arquero, O., Orgaz, F., Moral, J., and Lopez-Escudero, F. J. (2016). The effect of short irrigation frequencies on the development of Verticillium wilt in the susceptible olive cultivar ‘Picual’ under field conditions. Plant Dis. 100, 1880–1888. doi: 10.1094/pdis-09-15-1018-re
Perry, J. N., Bell, E. D., Smith, H. R., and Woiwod, I. P. (1996). SADIE: software to measure and model spatial pattern. Asp. Appl. Biol. 46, 95–102.
Perry, J. N., Winder, L., Holland, J. M., and Alston, R. D. (1999). Red–blue plots for detecting clusters in count data. Ecol. Lett. 2, 106–113. doi: 10.1046/j.1461-0248.1999.22057.x
Prieto, P., Navarro-Raya, C., Valverde-Corredor, A., Amyotte, S. G., Dobinson, K. F., and Mercado-Blanco, J. (2009). Colonization process of olive tissues by Verticillium dahliae and its in planta interaction with the biocontrol root endophyte Pseudomonas fluorescens PICF7. Microb. Biotechnol. 2, 499–511.
Roca, L. F., Moral, J., Trapero, C., Blanco-Lopez, M. A., and Lopez-Escudero, F. J. (2016). Effect of inoculum density on Verticillium wilt incidence in commercial olive orchards. J. Phytopathol. 164, 61–64. doi: 10.1111/jph.12382
Rosenberg, M. S., and Anderson, C. D. (2011). PASSaGE: pattern analysis, spatial statistics and geographic exegesis. version 2. Methods Ecol. Evol. 2, 229–232. doi: 10.1111/j.2041-210x.2010.00081.x
Santos-Rufo, A., Vega, V., Hidalgo, J. J., Hidalgo, J. C., and Rodriguez-Jurado, D. (2017). Assessment of the effect of surface drip irrigation on Verticillium dahliae propagules differing in persistence in soil and on verticillium wilt of olive. Plant Pathol. 66, 1117–1127. doi: 10.1111/ppa.12652
Scherm, H., and Ojiambo, P. S. (2004). Applications of survival analysis in botanical epidemiology. in new applications of statistical tools in plant pathology. Phytopathology 94, 1022–1026. doi: 10.1094/phyto.2004.94.9.1022
Soil Survey Staff. (2015). Keys to Soil Taxonomy, 16th Edn. Washington, DC: United States Department of Agriculture.
Thanassoulopoulos, C. C. (1992). Spread of verticillium wilt by nursery plants in olive groves in the Halkidiki area (Greece), Paper presented at the Joint MPU/EPPO Conference on Olive Diseases, Sounion GR.
Thanassoulopoulos, C. C., Biris, D. A., and Tjamos, E. C. (1981). Weed hosts as inoculum source of Verticillium in olive orchards. Phytopathol. Mediterr. 20, 164–168.
Tjamos, E. C. (1991). Recovery of olive trees with Verticillium wilt after individual application of soil solarization in established olive orchards. Plant Dis. 75:557. doi: 10.1094/pd-75-0557
Trapero, C., Serrano, N., Arquero, O., Del Rio, C., Trapero, A., and Lopez-Escudero, F. J. (2013). Field resistance to Verticillium wilt in selected olive cultivars grown in two naturally infested soils. Plant Dis. 97, 668–674. doi: 10.1094/pdis-07-12-0654-re
Tsror, L. (2011). Epidemiology and control of Verticillium wilt on olive. Israel J. Plant Sci. 59, 59–69. doi: 10.1560/ijps.59.1.59
Turechek, W. W., Madden, L. V., Gent, D. H., and Xu, X. M. (2011). Comments regarding the binary power law for heterogeneity of disease incidence. Phytopathology 101, 1396–1407. doi: 10.1094/phyto-04-11-0100
Vallad, G. E., Qin, Q. M., Grube, R., Hayes, R. J., and Subbarao, K. V. (2006). Characterization of race-specific interactions among isolates of Verticillium dahliae pathogenic on lettuce. Phytopathology 96, 1380–1387. doi: 10.1094/phyto-96-1380
Wilhelm, S. (1955). Longevity of the Verticillium wilt fungus in the laboratory and field. Phytopathology 45, 180–181.
Xiao, C. L., Hao, J. J., and Subbarao, K. V. (1997). Spatial patterns of microsclerotia of Verticillium dahliae in soil and verticillium wilt of cauliflower. Phytopathology 87, 325–331. doi: 10.1094/phyto.1997.87.3.325
Keywords: Verticillium wilt, Integrated Disease Management, olive, Verticillium, plant pathogen control
Citation: Ostos E, Garcia-Lopez MT, Porras R, Lopez-Escudero FJ, Trapero-Casas A, Michailides TJ and Moral J (2020) Effect of Cultivar Resistance and Soil Management on Spatial–Temporal Development of Verticillium Wilt of Olive: A Long-Term Study. Front. Plant Sci. 11:584496. doi: 10.3389/fpls.2020.584496
Received: 17 July 2020; Accepted: 28 September 2020;
Published: 27 October 2020.
Edited by:
Antonieta De Cal, National Institute for Agricultural and Food Research and Technology (INIA), SpainReviewed by:
David Ruano-Rosa, Instituto Tecnológico Agrario de Castilla y León, SpainFrancisco Jesus Gómez Gálvez, University of Córdoba, Spain
Sotiris Tjamos, Agricultural University of Athens, Greece
Copyright © 2020 Ostos, Garcia-Lopez, Porras, Lopez-Escudero, Trapero-Casas, Michailides and Moral. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juan Moral, anVhbi5tb3JhbEB1Y28uZXM=; anVhbm1vcmFsbW9yYWxAeWFob28uZXM=
†These authors have contributed equally to this work
 Eduardo Ostos
Eduardo Ostos María Teresa Garcia-Lopez
María Teresa Garcia-Lopez Rafael Porras3
Rafael Porras3 Francisco J. Lopez-Escudero
Francisco J. Lopez-Escudero Antonio Trapero-Casas
Antonio Trapero-Casas Juan Moral
Juan Moral