
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci., 30 October 2020
Sec. Crop and Product Physiology
Volume 11 - 2020 | https://doi.org/10.3389/fpls.2020.580597
 Xian He1†
Xian He1† Tianxiang Liu1†
Tianxiang Liu1† Ke Ren1†
Ke Ren1† Jie Chen1
Jie Chen1 Gaokun Zhao1
Gaokun Zhao1 Binbin Hu1
Binbin Hu1 Anchuan Xu1
Anchuan Xu1 Yan Jin1
Yan Jin1 Yanmei Zhu2*
Yanmei Zhu2* Congming Zou1*
Congming Zou1*Salicylic acid (SA) can induce plants to actively enhance abiotic stress resistance. Spraying SA to prevent cold stress in flue-cured tobacco fields can provide theoretical support and practical guidance for the actual protection from cold stress in fields at high altitude in Yunnan. The experiment was performed in Jianchuan County Yunnan Province, China. Honghuadajinyuan, a flue-cured tobacco variety with cold resistance, was used as the research object. SA was tested at two concentrations (0.05 [SA-1] and 0.1 [SA-1] mol L–1) relative to an untreated control (Control) to compare the quality of fresh tobacco leaves, curing characteristics, enzyme activity of antioxidants, and quality of the first-cured tobacco leaves. The tissue structure thickness, SPAD, and plastid pigment content of fresh tobacco leaves were least in the control; there was no significant difference between SA-1 and SA-2. The change of moisture content during curing was SA-1 > SA-2 > Control, and the water loss rate was Control > SA-2 > SA-1, and both varied greatly at 38–48°C. In each curing stage, the carbon and nitrogen metabolites and polyphenols changed most rapidly at 38°C, and the sugar metabolites changed as follows: Control > SA-1 > SA-2. The activities of the antioxidant enzymes superoxide dismutase, peroxidase, and catalase in fresh tobacco leaves were SA-1 > SA-2 > Control. Malondialdehyde content and the inactivation rate of antioxidant enzymes during curing was Control > SA-2 > SA-1. The economic character and sensory smoking quality of flue-cured tobacco leaves were SA-1 > SA-2 > Control. In high-altitude tobacco planting areas prone to cold stress in the field, early warning weather forecast and field spraying 0.05 mol L–1 SA are beneficial to protect and improve the quality of fresh tobacco leaves, curing characteristics, antioxidant system enzyme activities, and the quality of flue-cured tobacco leaves.
Cold stress occurs at low but above-zero temperatures, and the agricultural loss caused by cold injury in the world costs hundreds of billions of dollars each year. The damage to crops is mainly reflected in the destruction of the cytoplasmic membrane in the plant’s physiological and biochemical systems and the damage to the structure and activity of protective enzymes (Kumar et al., 2010). Cold stress can also inhibit photosynthesis and respiration (Wang et al., 2008; Yadav, 2010). After cold stress, plants will show phenotypic symptoms such as loss of water, wilting of leaves, fading of green, yellowing, decrease in leaf area, and even tissue death (Hussain et al., 2018).
Many warm season crops are extremely sensitive to cold stress. Cold injury in maize (Zea mays) from emergence to maturity will lead to a decrease in seed germination rate, hinder the growth of functional leaves, inhibit photosynthesis, slow dry matter accumulation and filling rate, and finally reduce yield and quality (Prasad et al., 1994). When cotton (Gossypium hirsutum L.) encounters a short-term low temperature in the later stage of growth, it will experience significant premature aging, with a 30–40% reduction in output and a large-scale outbreak of leaf spot lesions (Zhao et al., 2012). Soybean (Glycine max) suffering from low temperature at flowering will have a rapid decrease in the number of pods and seeds per plant, affecting the formation of seed pods, resulting in a decrease in yield and quality (Yang et al., 2004).
Salicylic acid (o-hydroxybenzoic acid) is a type of phenolic plant growth regulator that can be synthesized in trace amounts and that has a positive regulatory effect on plant stress resistance (Madan and Levitt, 2014). Many studies have shown that salicylic acid can reduce crop cell membrane permeability, increase proline content to maintain cell water content, reduce or eliminate membrane lipid peroxidation, and effectively enhance the cold resistance of crops (Agarwal et al., 2005; Ghanbari and Sayyari, 2018). Salicylic acid is widely used to improve the stress ability of crops, especially to reduce membrane lipid peroxidation and cell membrane permeability during cold stress and to enhance the activity of protective enzymes to help eliminate the accumulation of reactive oxygen species in plants (Achuo et al., 2004; Farooq et al., 2008; Supapvanich and Promyou, 2013). Salicylic acid treatment in low temperature stress could reduce the damage of leaf tissue structure of papaya (Carica papaya L.) seedlings and maintain the integrity of epidermal cell structure (Mandal et al., 2017). Fan et al. (2012) confirmed that applying 1.0 mmol L–1 salicylic acid could effectively increase the chlorophyll content of muskmelon (Cucumis melo L. cv. Yujinxiang) seedlings in low temperature stress. Janda et al. (1999) pretreated maize seedlings in low temperature stress with 0.5 mmol L–1 salicylic acid and found that malondialdehyde (MDA) and O2– in seedlings decreased, whereas the activities of protective enzymes such as superoxide dismutase (SOD) and peroxidase (POD) significantly increased.
Yunnan province is located at a low latitude and high altitude, and the tobacco (Nicotiana tabacum L.) planting area is densely distributed at 1,300–2,000 m above sea level (ASL). In tobacco planting areas with an altitude of more than 2,000 m ASL, fields have a large temperature difference between day and night, and the temperature drop is common. Cold stress has a great influence on the quality of the fresh tobacco leaves, which has a serious effect on the curing of the flue-cured tobacco, resulting in a substantial reduction in tobacco leaf quality (Bolat et al., 2014). Currently, field research of flue-cured tobacco treated with salicylic acid has mainly focused on the physiological and biochemical mechanisms of stress in the seedling stage of flue-cured tobacco exposed to artificial cold injury. Therefore, it is of great interest to explore the efficacy of applying salicylic acid to prevent cold injury stress on leaf quality and curing characteristics of fresh flue-cured tobacco leaves under fields in high-altitude Yunnan tobacco planting areas.
After cold injury of flue-cured tobacco, with the prolongation of cold stress time and the continuous decrease in temperature, the degree of water loss, wilting, shrinkage, and yellowing of flue-cured tobacco leaves increases. On the other hand, cold stress will promote the formation of waxy layer on the surface of flue-cured tobacco leaves, increasing leaf thickness and decreasing tissue ratio. Plasmolysis occurs, and important organelles such as mitochondria deform and disintegrate (Hetherington and Öquist, 2006). The effect of cold stress on the quality of fresh tobacco leaves is also reflected in inhibiting the formation of chloroplasts and reducing the chlorophyll synthesis rate, resulting in decreased photosynthesis of tobacco leaves (Moon et al., 1995; Wang et al., 2006).
The curing characteristics of tobacco leaves inevitably reflect the differences in the quality of fresh tobacco, including two aspects: “ease of curing” and “resistance to flue-curing” (Ke-ai et al., 2006). During curing, the water loss and yellowing of normal tobacco leaves are easily regulated, and a better quality of flue-cured tobacco leaf can be obtained. Cold-injured tobacco leaves lose water with difficulty in the curing process because of the waxy layer and thickened cell wall formed on its surface (Wang et al., 2006). At the same time, because of the decrease in chlorophyll content of cold-injured tobacco leaves, the yellowing rate of tobacco leaves is faster than the rate of water loss, and the two cannot be coordinated, which also brings difficulties to flue-curing. Many enzymatic browning reactions occur easily because of the damage of cell membranes, which leads to the formation of browning tobacco (Chen et al., 2019).
Carbon and nitrogen metabolism are the most basic metabolic process of flue-cured tobacco that has a significant effect on the quality of flue-cured tobacco (Hongbin, 2007). Cold injury inhibits photosynthesis and the activity of carbon and nitrogen metabolic enzymes in tobacco, decreases the content of photosynthates, and then affects the formation and transformation of starch, disaccharide, protein, nicotine, and total nitrogen (Yang et al., 2004). Polyphenols are important secondary metabolites and aroma precursors of tobacco, which play an important role in the growth, development, yield, and quality of tobacco, and are an important factor to measure the quality of tobacco. Some studies have shown that cold stress can promote the synthesis of polyphenols in flue-cured tobacco seedlings (Dagnon and Edreva, 2003; Wang et al., 2008). By enhancing the cold resistance of crops, salicylic acid can reduce or prevent the quality and curing characteristics of fresh tobacco leaves from being affected by cold stress.
In low temperature stress, the antioxidant protective enzymes in flue-cured tobacco can scavenge reactive oxygen species and maintain a low level of reactive oxygen species. Catalase (CAT), POD, and SOD are important antioxidant protective enzymes in tobacco seedlings under low temperature stress (Xue et al., 2009). In normal circumstances, cold injury will increase the activity of antioxidant enzymes for a short time, and then the enzyme activity will decrease. Salicylic acid can improve the stability of cell membrane and induce and enhance the activities of antioxidant enzymes CAT, POD, and SOD to alleviate the cold injury caused by low temperature stress by reducing the MDA content and electrical conductivity of leaves. The appropriate concentration of salicylic acid could enhance resistance to membrane lipid peroxidation of flue-cured tobacco in low temperature stress, thus resisting the harm of low temperature; a concentration of 1.0 mmol L–1 appears best (Agarwal et al., 2005). In addition, there is an important relationship between the metabolism of polyphenols and polyphenol oxidase (PPO) is the key enzyme of enzymatic browning reaction in the curing process. Some studies show that low temperature can significantly reduce PPO activity of fresh tobacco leaves (Dechuan, 2006; Bahari et al., 2015). In the curing stage, because of the increase in temperature and humidity, cold injury cell membrane damage very easily allows enzymatic browning reaction to form browning tobacco. The alleviation of salicylic acid on membrane lipid peroxidation and cell membrane permeability under cold stress can effectively prevent the binding of PPO and polyphenols.
When the high-altitude tobacco-growing areas in Yunnan enter the harvesting and curing period, there are large daily temperature differences between day and night, and cliff cooling often occurs. Periodic rainfall accentuates the cold stress, bringing a great threat to local flue-cured tobacco production. There have been a few recent studies on the application of salicylic acid to prevent cold injury stress of flue-cured tobacco during harvesting and curing period in Yunnan. The mechanism of the effect of salicylic acid on the quality, curing characteristics, chemical composition, yield, and quality of flue-cured tobacco leaves is not clear, so it is of important practical significance to explore the mechanisms by which salicylic acid prevents field cold injury stress of flue-cured tobacco during the harvesting and curing period.
The experiment was performed in 2019 at an altitude of 2,565 m ASL in Laojun Mountain Town, Jianchuan country Yunnan Province (E 99°33′, N 26°31′). The tobacco variety Honghuadajinyuan was used for the experiment and was prepared by floating seedling technology. The young seedlings germinated on membranes were transplanted on April 13, with the row and plant spacing being 120 cm × 60 cm. The seedlings were topped on June 10, during which 15 or 16 leaves were left; the lower leaves were picked on July 03, and curing was completed on September 07. Details of soil nutrient levels at the experiment site are as follows: pH 6.47, organic matter content of 56.19 g kg–1, total nitrogen of 2.76 g kg–1, total phosphorus of 1.11 g kg–1, total potassium of 17.64 g kg–1, soluble nitrogen of 210.8 mg kg–1, available phosphorous of 91.3 mg kg–1, and rapidly available potassium of 285.5 mg kg–1. Precipitation of the field growth period is as follows: April: 36 mm, May: 6 mm, June: 220 mm, July: 569 mm, and August: 423 mm. A compound fertilizer designed for growing tobacco was used as the base fertilizer, having N: P2O5: K2O = 12: 10: 25, with the application rate of 150 kg ha–1, 18 kg ha–1 of nitrogen fertilizer was applied, accompanied by composted farmyard manure (15,000 kg ha–1). During top dressing, the application rates of compound fertilizer designed for tobacco, nitrogen fertilizer, and accompanying potassium sulfate (51%) were 75, 9, and 30 kg ha–1, respectively. The base fertilizers and fertilizers for top dressing were separately applied after transplanting for 15 and 30 days.
For the 2012–2016 period during prophase (early May to mid-June), the average temperature that slowly rose leveled off (mid-June to mid-July), with the late period (mid to late of July) showing volatility in average temperature. On the whole, the diurnal range of temperature in the growing period of field was higher in the early stage (from May to mid-June), lower in the middle stage (from mid-June to early August), and higher in the later stage (from mid– August to late August) with severe irregular fluctuations. Therefore, it can be concluded that in the middle and late periods of flue-cured tobacco production (mid and late August) experienced the greatest temperature fluctuations in Jianchuan, Yunnan (Supplementary Figure 1). Tobacco leaves were affected by high and low temperature, and were vulnerable to cold damage stress, which affects the leaf yield quality. According to the experience of local tobacco farmers and the 2019 weather forecast, August was the main period when the local temperature changes were greatest and the field chilling stress occurs frequently. Therefore, the treatments were applied on August 01. The experiment included three treatments (n = 3): no salicylic acid (Control); 0.05 mol L–1 salicylic acid (SA-1); 0.1 mol L–1 salicylic acid (SA-2). Using a randomized block design, each treatment was 12 m long and 8.5 m wide. Salicylic acid was sprayed every 5 days until field chilling injury occurred, and field sampling and tobacco curing characteristics were analyzed.
A TH12R-EX temperature and humidity recorder (Shenzhen Huahanwei Science and Technology Co., Ltd.) were placed in the test field to record daily temperature and humidity changes, while a WH-2310 wireless weather station (Jiaxing Misu Electronic Co., Ltd.) was placed to record daily temperature, precipitation and wind speed.
According to the regular harvest time of the local middle leaves, the tobacco leaves were picked and poled to ensure that the maturity of the tobacco leaves was balanced and consistent, the same quality was on the same pole, and the density was moderate. The tobacco leaves were cured in the local dense curing barn.
The tobacco is packed in three layers, 100–120 leaves per rod and 150–170 rods per layer. The tobacco curing process was mainly carried out according to the local main curing technology (Figure 1). The whole curing process can be divided into three stages: yellowing stage (35–42°C), leaf-drying stage (42–54°C), and stem-drying stage (60–66°C). In the yellowing stage, the original dry bulb and wet bulb temperatures were 24 and 20°C, respectively. The temperature of the dry bulb and that of the wet bulb were raised to 34 and 32°C with temperature rise rates of 1.1°C h–1 (dry bulb) and 1.33°C h–1 (wet bulb), respectively, and curing for 18 h at a steady temperature. Then, the temperature of the dry bulb and that of the wet bulb were raised to 38 and 36°C at a heating rate of 0.5°C h–1, respectively. After curing for 23.5 h at stable temperature, the leaf-drying stage occurred. In the leaf-drying stage, the dry bulb temperature was increased to 42°C at a heating rate of 0.5°C h–1, whereas the wet bulb temperature was controlled to 37°C, and drying occurred for 16.5 h at stable temperature. The dry bulb temperature was then increased to 48°C at a heating rate of 0.5°C h–1, whereas the wet bulb temperature was unchanged, and curing occurred for 15 h at a stable temperature. Finally, the dry bulb temperature was increased to 54°C at a heating rate of 0.5°C h–1, whereas the temperature of the wet bulb remained unchanged. After curing for 22.5 h at stable temperature, transfer to the stem-drying stage occurred. In the stem-drying stage, the dry bulb temperature was increased to 66°C at a heating rate of 1°C h–1, whereas the wet bulb temperature was controlled to 38°C, and the curing proceeded for 26 h at stable temperature.
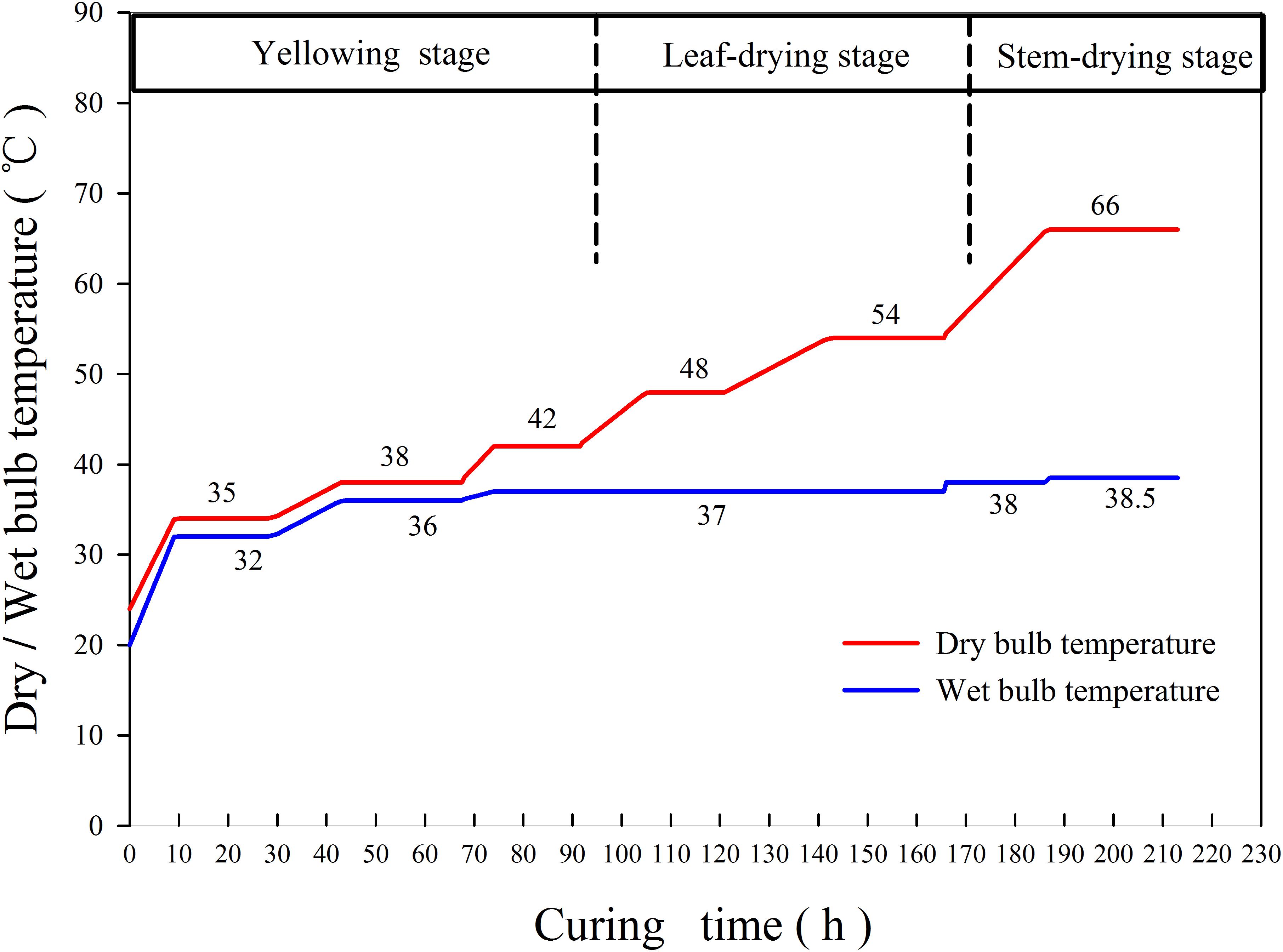
Figure 1. Common curing technology for bulk curing barns in tobacco-growing areas in Laojun Mountain Town, Jianchuan County (variety: Honghuadajinyuan).
The daily meteorological data from August, including temperature, hygrometer, rainfall, and wind speed, were collected by a meteorological station located. The meteorological data collected during the period when local tobacco leaves suffered cold stress were intensively analyzed. All tobacco samples were picked from the middle leaves (5th–10th leaves from bottom to the top) and collected before curing (fresh tobacco leaves), during the critical period of curing process (at the end of 38, 42, 48, and 54°C), and at the end of curing. In each stage, samples comprising 10 tobacco leaves were separately taken for the determination of various indices.
The microstructures of fresh tobacco leaves before harvesting and curing were observed by the following method: small pieces of about 0.5 cm × 0.5 cm between the right leaf tip and the sixth to seventh branch veins of the leaf samples were fixed FAA (Formaldehyde, Acetic acid, and Alcohol) fixing solution. A conventional paraffin section method, with slice thickness of 10 μm, was used for hematoxylin staining and sealed with Canadian gum seal to make permanent sections for observation and determination by an Olympus microscope. A total of two sections were observed, and five visual fields were observed and averaged in each section. The observation indices were leaf thickness, upper epidermis thickness, lower epidermis thickness, palisade tissue thickness and sponge tissue thickness, and the data were statistically analyzed.
A SPAD-502 chlorophyll meter (Konica Minolta Company, Japan) was used to determine the SPAD value of tobacco leaves at each sampling stage; the measuring accuracy was within ±1.0 SPAD unit. The measuring position was from the symmetrical leaf margin and the middle part between the main vein of tobacco leaf, with three parts of the left and right half of the leaf measured, and the average taken as the measure of chlorophyll.
Fresh tobacco leaves dried with surface moisture, and the samples in each stage of the curing process were weighed by electronic balance (American Shuangjie SA-200Y). The weights were recorded as FW0 and FWn (38, 42, 48, and 54°C), respectively. Then the fresh tobacco leaves and the samples of each stage in the curing process were repeatedly put into a blast drying box and dried at 60°C for 1 h at 105°C. The samples were weighed again, which were recorded as DW0 and DWn (38, 42, 48, and 54°C), respectively.
Moisture content of tobacco leaves at different curing stages (%)
Immediately after sampling in each stage, leaves were put into liquid nitrogen and transferred to a freezer at −80°C to determine antioxidant enzymes, including SOD (kit [NBT method], product number: SOD-1-Y); POD (kit, product number: POD-1-Y); CAT (kit [UV absorption method], product number: CAT-1-Y); MDA (kit, product number: MDA-1-Y); PPO (kit, product number: PPO-1-Y). The activity of each enzyme was determined by the kit produced by Suzhou Keming Biotechnology Co., Ltd.
Six main compounds (i.e., starch, total sugar, reducing sugar, total nitrogen, nicotine, and protein) of tobacco samples in different stages were detected using the following methods: total sugar and reducing sugar were analyzed according to Tobacco and Tobacco Products—Determination of Water Soluble Sugars—Continuous Flow Method (YC/T159-2002); total nitrogen was analyzed based on Tobacco and Tobacco Products—Determination of Total Nitrogen—Continuous Flow Method (YC/T161-2002); nicotine was analyzed by utilizing Tobacco and Tobacco Products—Determination of Nicotine—Continuous Flow Method (YC/T160-2002); starches were measured by referring to Tobacco and Tobacco Products–Determination of Starch—Continuous Flow Method (YC/T 216-2014). In terms of polyphenols, the chlorogenic acid, scopoletin, rutin, neochlorogenic acid, caffeic acid, lutein, β-carotene, and total phenols were measured using the method recommended in YC/T 202–2006.
The chloroplast pigment index (chlorophyll and carotenoids) in tobacco leaves at different stages were determined by high-performance liquid chromatography method (YC/T 382-2010). The total chloroplast pigment is the sum of chlorophyll a, chlorophyll b, and carotenoids.
The flue-cured tobacco leaves were graded according to the national standard GB2635-92, and the tested tobacco leaves were evaluated by the grading hands of the local tobacco station. The prices were all local prices in the same year. Statistics were made for the proportion and average price of high and medium flue-cured tobacco leaves after each treatment.
Sensory quality measurement was evaluated by seven experts from Yunnan Tobacco Technology Center. According to the sensory quality evaluation standard of Yunnan Chinese tobacco, the smoking index can be divided into style characteristics (aroma note), aroma characteristics (aroma volume and aroma quality), smoke characteristics (concentration, mixed gas, strength, and irritancy), and taste characteristics (taste, cleanliness, and moisture) for a total of 100 points.
The data were analyzed by the general linear model program, with multiple regression in the SAS 9.3 computer package (SAS Institute Inc., Cary, NC, United States). Salicylic acid level was regarded as a discrete variable and was not analyzed as a quantitative variable because it was not intended to interpolate the effects of salicylic acid over the 0 to 0.1 mol L–1 rate range. Values used for statistical analysis represent the mean of three biological replicates. The significance of statistical analysis of all data was based on P < 0.05. The data were separated by Tukey (honestly significant difference) test in 95% confidence intervals. Charts were produced by Sigma Plot 12.5 (Systat Software Inc., United States) and Origin 6.0 (Microcal Software, Inc., United States).
The experiment site located in Jianchuan village of Laojun Mountain Town showed significant fluctuations in temperature and rainfall in August (Figure 2). The daily temperature fluctuated between 21.5 and 41.9°C (high) and 9.4 to 17.1°C (low). The daily precipitation varied within 0–21.6 mm. The maximum diurnal temperature difference (30.1°C) was recorded on August 12; however, no rainfall occurred thereon. The minimum diurnal temperature difference (8.7°C) was found on August 01, accompanying with 5.9 mm of precipitation. Substantial cooling accompanying meteorological disasters (including rainfall and a small amount of hail) appeared on August 16 and 17; in the next 6 days, tobacco showed a range of symptoms caused by cold stress (Figure 3). The tobacco leaves in the middle and upper parts suffered the most serious damage. The surface color of the tobacco leaves changed from normal to dark green (within 1–2 days), to purple red (within 2–3 days), then dark red (within 3–4 days), and finally off-white (within 4–6 days). Eventually, a large area of scalded leaves was found, showing poor flue-curing availability.
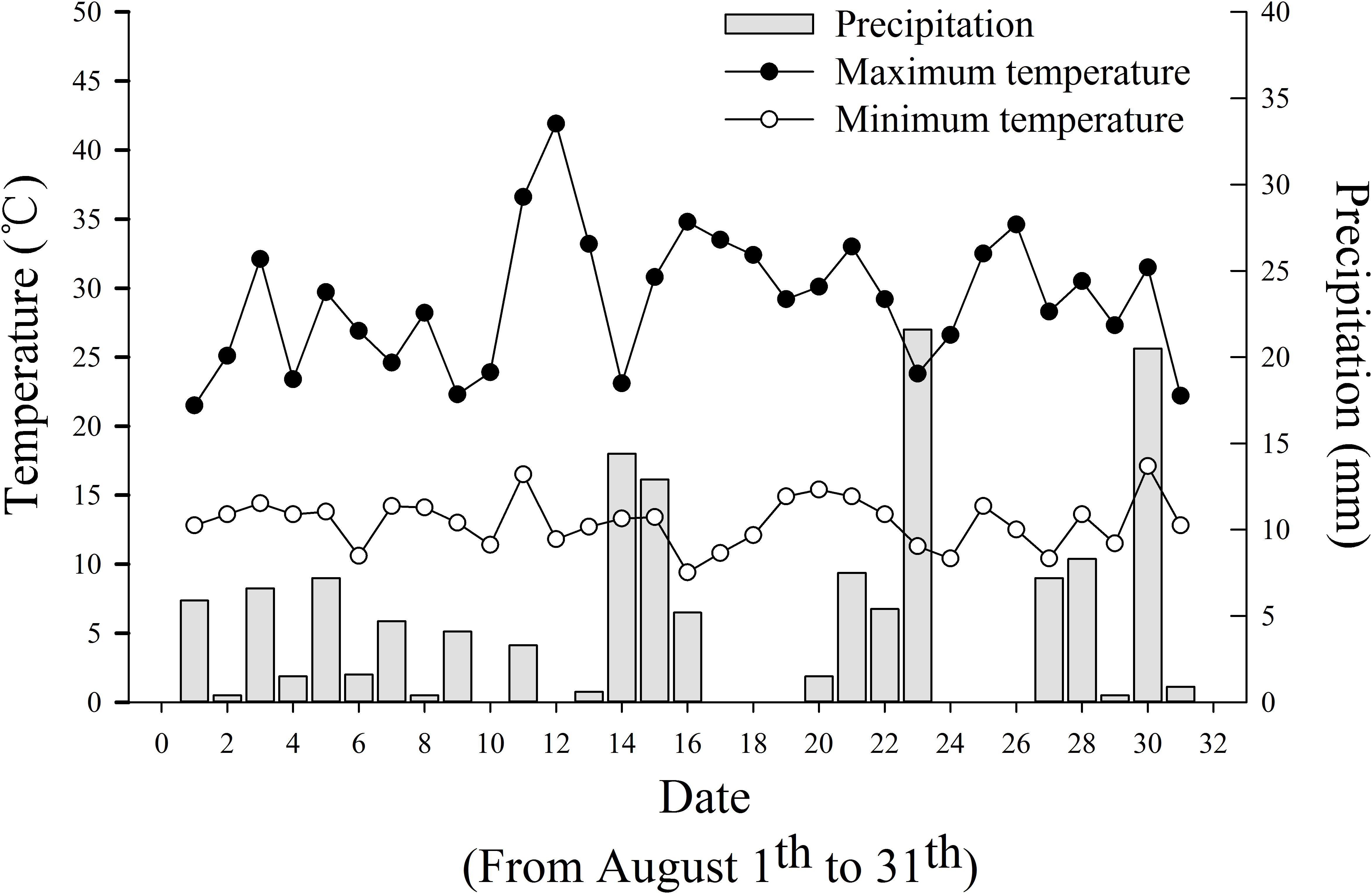
Figure 2. Daily precipitation, the highest and lowest temperatures from August 01 to 31 in Jianji village, Laojun Mountain Town, Jianchuan County.
In the appearance comparison of fresh tobacco leaves treated with different salicylic acid concentrations, there was no significant difference in the color and leaf size of each treatment, but there was a yellow–gray area on the surface of the Control treated tobacco leaves and no such apparent phenomenon in other treatments (Figure 4).

Figure 4. Comparison of leaf appearance in fresh tobacco leaves treated with different salicylic acid concentrations.
There were significant differences in blade thickness, palisade tissue thickness, spongy tissue thickness, and palisade tissue thickness/sponge tissue thickness (tissue ratio) among the treatments (P < 0.05) (Figure 5 and Table 1). The palisade tissue thickness, upper epidermal thickness, and lower epidermal thickness treated by SA-2 and SA-1 were significantly higher than those of the Control. The palisade tissue thicknesses in SA-1 treatment were 16 and 178% higher than those treated with SA-2 and Control, respectively. The upper epidermal and lower epidermal thicknesses in SA-2 treatment were 5, 158, and 110%, 129% higher than those treated with SA-1 and Control, respectively. The spongy tissue thickness and blade thickness were SA-1 > SA-2 > Control.
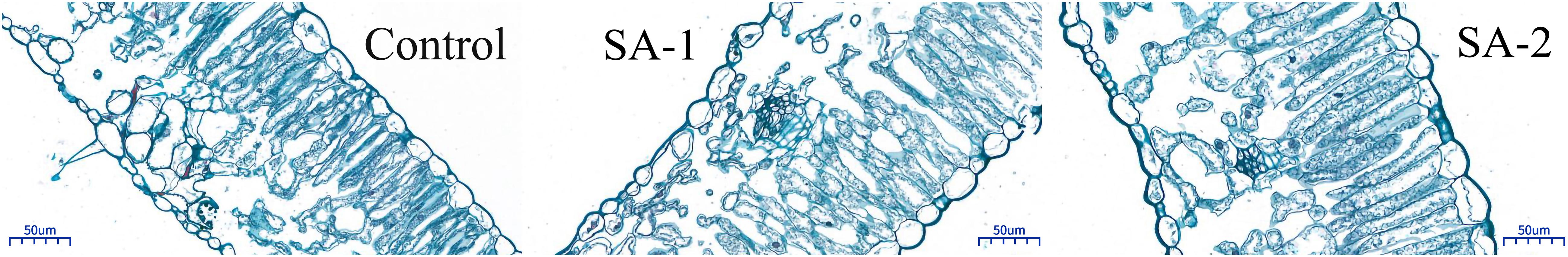
Figure 5. Tissue structure of fresh tobacco leaves treated with different salicylic acid concentration.
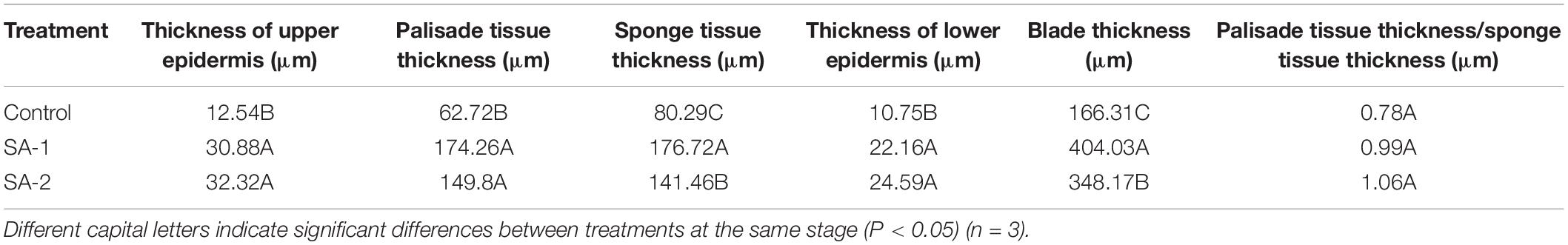
Table 1. Tissue structure parameters of fresh tobacco leaves treated with different salicylic acid concentration.
With the advance of curing time, the moisture content in all treatments declined. There were significant differences (P < 0.05) in water loss and moisture content of tobacco leaves in different curing stages (Figures 6, 7). There are no significant differences in water loss and moisture content of tobacco leaves in the same curing temperature.
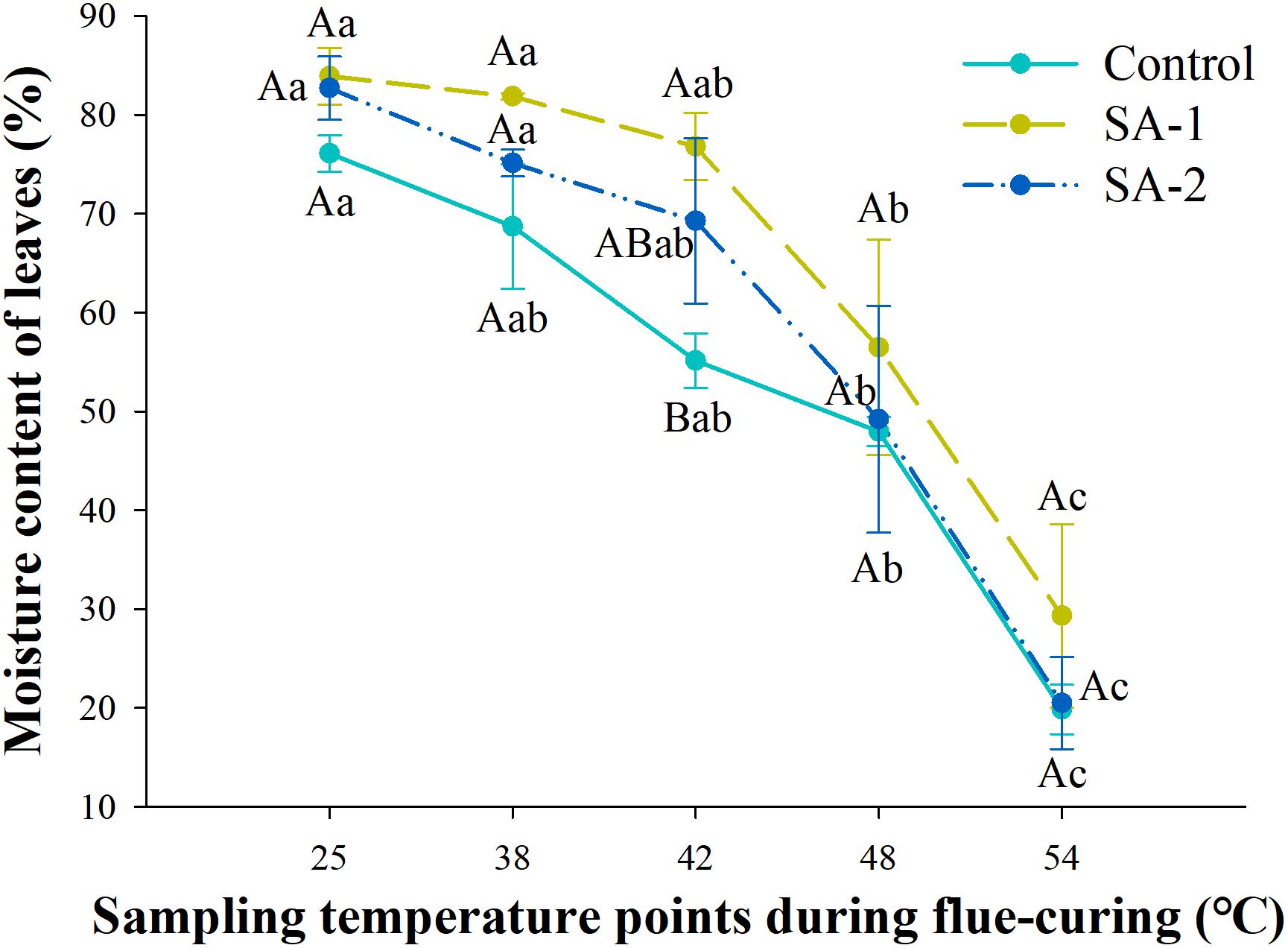
Figure 6. Change of moisture content during bulk curing of flue-cured tobacco with different salicylic acid concentrations. Different capital letters indicate significant differences between treatments at the same stage (P < 0.05). Different lowercase letters indicate significant differences between the same treatments at different curing stages. Data are presented as means ± standard error (n = 3).
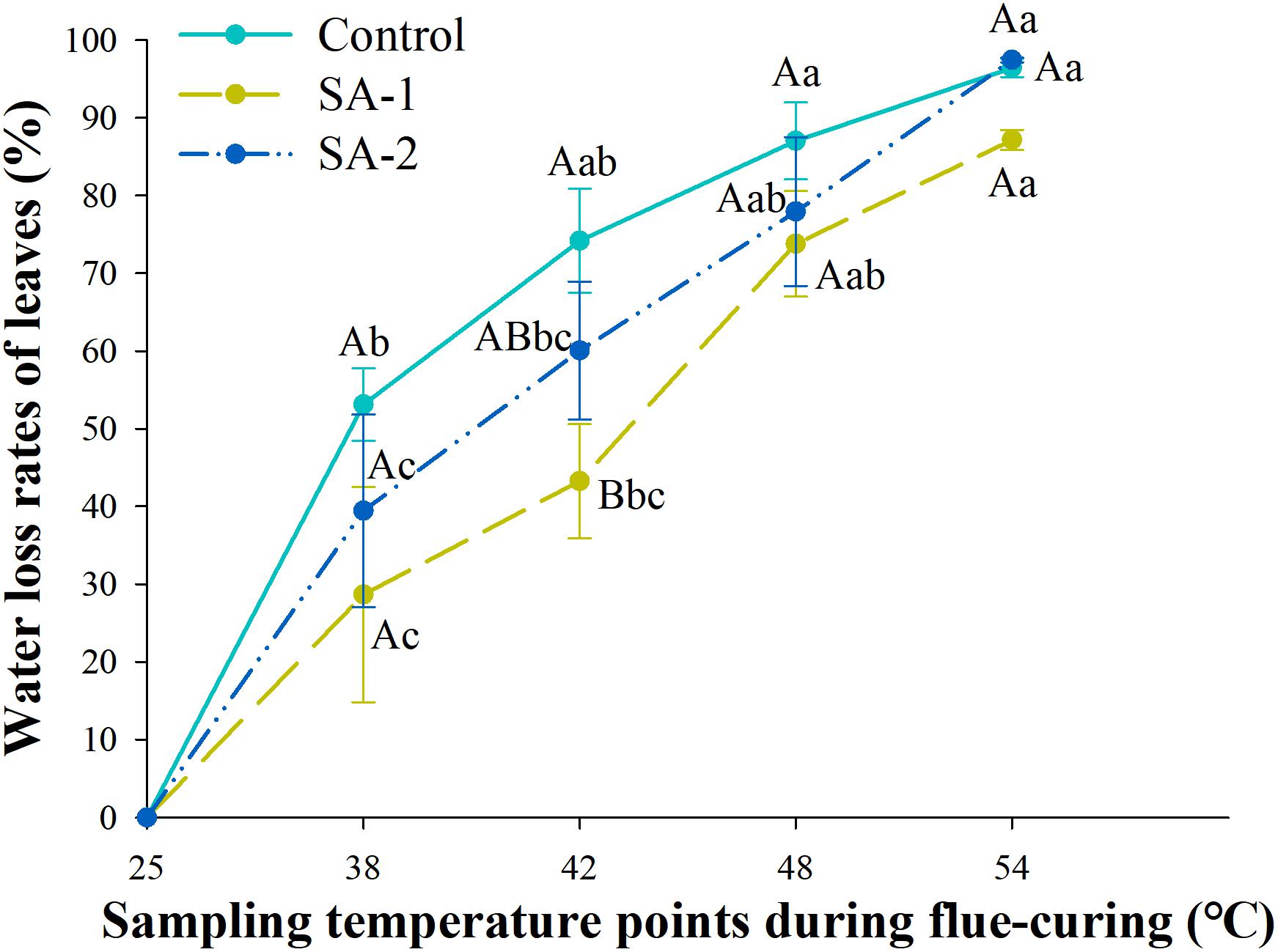
Figure 7. Change in water loss rate during bulk curing of flue-cured tobacco with different salicylic acid concentrations. Different capital letters indicate significant differences between treatments at the same stage (P < 0.05). Different lowercase letters indicate significant differences between the same treatments at different curing stages. Values represent the averages of three biological replicates. Data are presented as means ± standard error (n = 3).
Under the water content index, the change trend was significant between SA-1 and Control and SA-2 at 25–48°C. At 25–48°C, SA-1 was 10–39% higher than Control, whereas SA-2 was 3–26% higher than Control. The SA-1 and SA-2 treatments were significantly higher than Control. The moisture content of SA-1 and SA-2 treatments quickly decreased after 42°C, the moisture content decreased 62 and 70%, respectively, at 54°C compared with 42°C (Figure 6). Under the index of water loss rate, at 38–54°C, the change trend was for a significant difference between Control and SA-1 and SA-2; Control increased by 11–85% and 12–35% compared with SA-1 and SA-2, respectively, and SA-2 increased by 6–39% compared with SA-1. The SA-1 treatments were significantly higher than SA-2 and Control at 42°C. Compared with 38°C, the water loss rate of Control, SA-1, and SA-2 increased by 81, 204, and 147%, respectively (Figure 7).
There were significant differences (P < 0.05) in SPAD, chlorophyll a, and chlorophyll b, among three concentrations of salicylic acid in flue-cured tobacco leaves (Table 2).
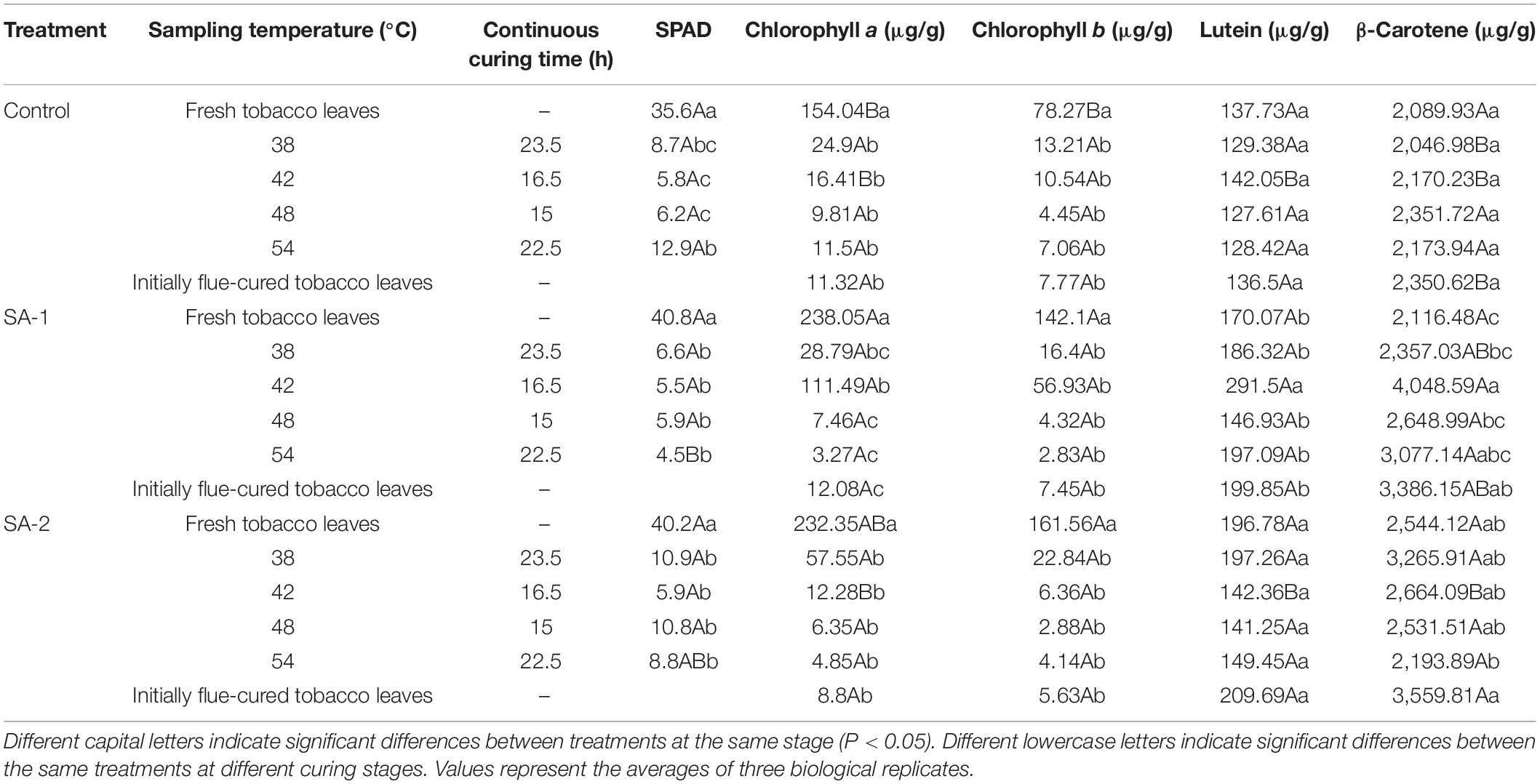
Table 2. Changes of SPAD and chloroplast pigments in different curing stages with different salicylic acid concentrations.
During the curing process, the SPAD, chlorophyll a, and chlorophyll b contents of each treatment declined, and degradation the fastest at 38°C.
For the SPAD index, the content of Control was the lowest in the fresh tobacco stage. For the chlorophyll a and b indices, SA-1 was higher than Control when the curing stage was fresh tobacco to 42°C.
For the SPAD index, SA-1 and SA-2 were 15% and 13% higher than Control in fresh tobacco leaves, respectively. For the chlorophyll a and chlorophyll b index, SA-1 was 16–579% and 24–440% higher than Control in the curing stage of fresh tobacco leaves to 42°C, and the difference was not significant in the later stage. For the lutein index, SA-1 and SA-2 were 15–105% and 0.22–54% higher than Control. For the β-carotene index, SA-1 and SA-2 were 1–87% and 1–60% higher than Control.
The ratios of starch, total sugar, reducing sugar, and sugar alkali in the chemical composition indices of tobacco leaves treated with salicylic acid were significantly different from that of Control (Tables 3, 4). There are significant differences in the ratios of chlorogenic acid and rutin in the polyphenols.
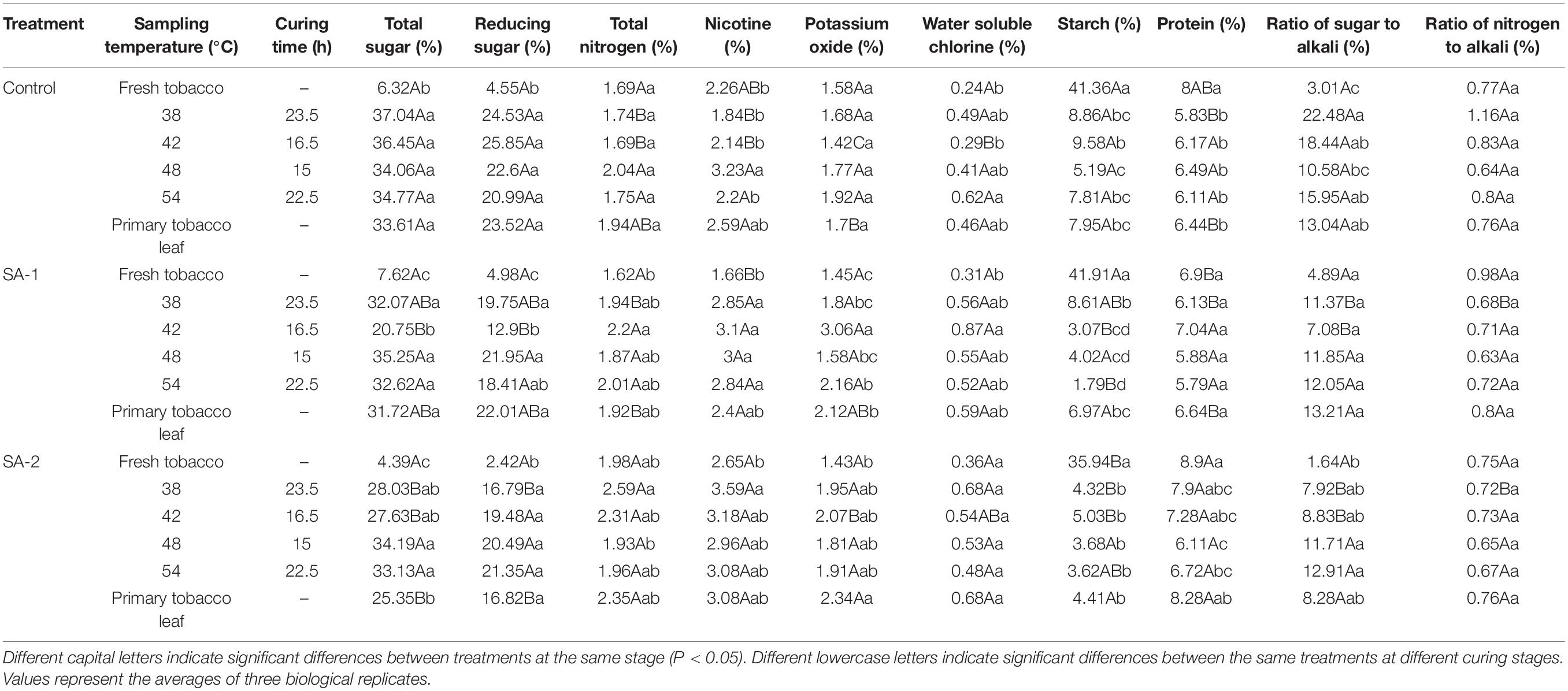
Table 3. Contents of chemical compounds during bulk curing of flue-cured tobacco with different salicylic acid concentrations.
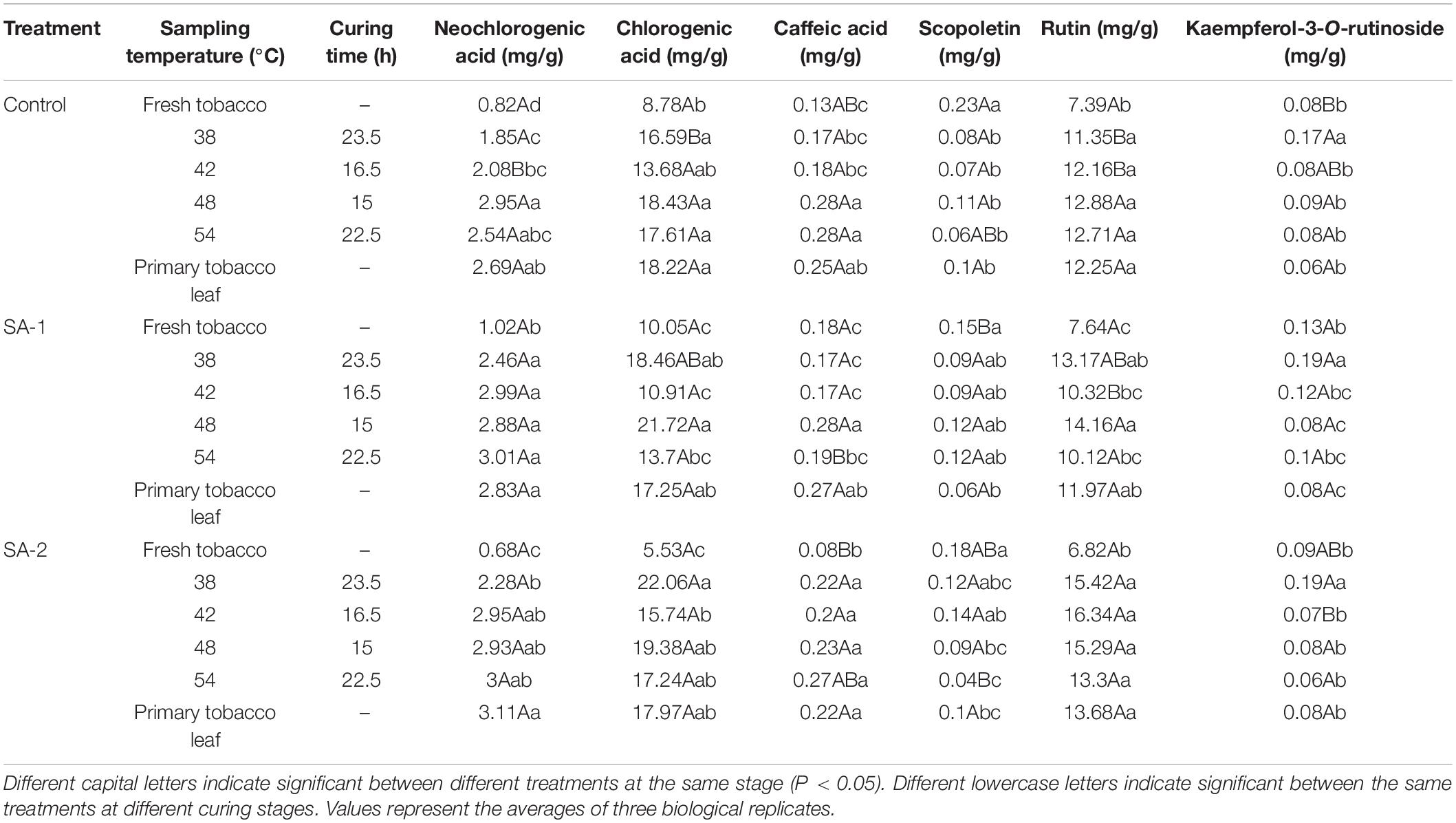
Table 4. Contents of polyphenols during bulk curing of flue-cured tobacco with different salicylic acid concentrations.
With the advance of curing time, the starch content of the treated tobacco leaves decreased; the fastest decline was at 38°C. The ratio of total sugar, reducing sugar, and sugar alkali increased rapidly at first and then decreased slowly, and increased rapidly at 38°C stage of the fresh tobacco leaves.
Under different curing stages, the change trend of carbon metabolite index was Control > SA-1 > SA-2. The total sugar content of Control was 6–76% and 5–44% higher than that of SA-1 and SA-2; the reducing sugar content of Control was 3–100% and 10–88% higher than that of SA-1 and SA-2, and the starch content of Control was 3–336% and 15–116% higher than that of SA-1 and SA-2, respectively. Under different curing stages, the change trend of nitrogen metabolite index was as follows: SA-2 > SA-1 > Control. The total nitrogen content of SA-2 was 12–49% and 3–34% higher than Control and SA-1, the nicotine content of SA-2 was 17–95% and 3–60% higher than CK and SA-1, and the protein content of SA-2 was 10–36% and 3–29% higher than Control and SA-1, respectively.
The chlorogenic acid and rutin of each treatment increased rapidly from fresh tobacco leaves to 38°C and then stabilized gradually. Under the chlorogenic acid index, the 38°C of Control, SA-1, and SA-2 treatment increased 89, 84, and 299%, respectively, compared with the fresh tobacco leaf stage; under the rutin index, the 38°C of Control, SA-1, and SA-2 treatment increased 54, 72, and 126%, respectively, compared with the fresh tobacco leaf stage.
For the SOD and POD activity indices, there is a rule of SA-2 > SA-1 > Control, and under the CAT index, there is a rule of Control > SA-1 > SA-2 (Figure 8). Except for the SA-2 treatment under the CAT index, the three enzymes show a change trend of increasing first and then decreasing. The three enzymes have the highest activity at the curing stage of 25–42°C and then a sharp decline. The MDA content of different treatments increased with the curing process, showing the rule of Control > SA-1 > SA-2. The growth rate and content of Control treatment at 25°C were higher than SA-1 and SA-2; the growth rate and content of SA-2 treatment at 42°C were higher than SA-1. The PPO activity of each treatment was Control > SA-1 > SA-2. The PPO activity of Control and SA-1 increased first and then decreased sharply, reaching the peak at 42°C; the PPO activity of SA-1 at 38–48°C was higher than that at 25 and 54°C.
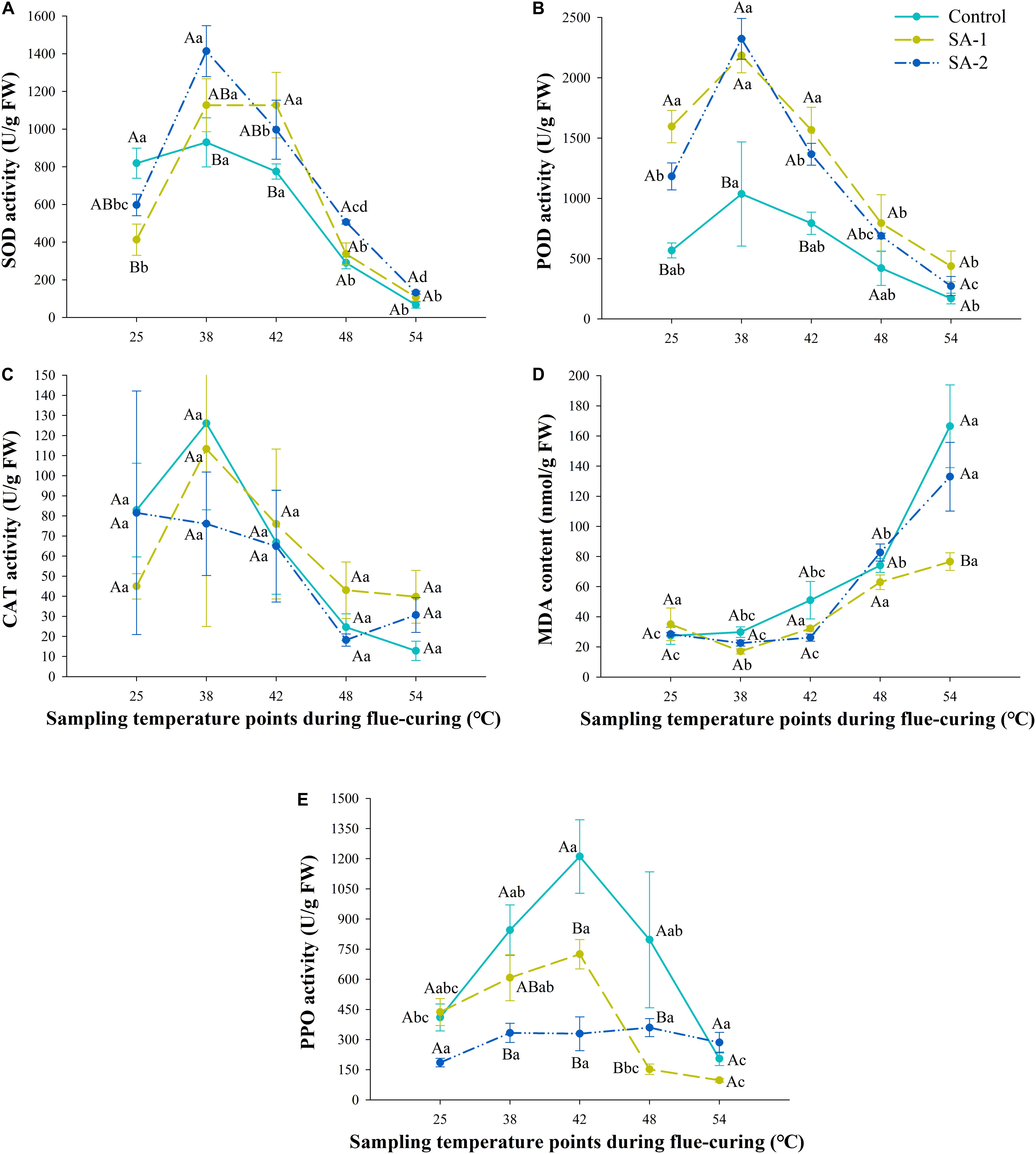
Figure 8. Antioxidant enzyme system and polyphenol oxidase activity during bulk curing of flue-cured tobacco with different salicylic acid concentrations. (A) Effect of treatment with different salicylic acid concentrations on SOD activity at different curing stages. (B) Effect of treatment with different salicylic acid concentrations on POD activity at different curing stages. (C) Effect of treatment with different salicylic acid concentrations on CAT activity at different curing stages. (D) Effect of treatment with different salicylic acid concentrations on the content of MDA at different curing stages. (E) Effect of treatment with different salicylic acid concentrations PPO activity at different curing stages. Different capital letters indicate significant differences between treatments at the same stage (P < 0.05). Different lowercase letters indicate significant differences between the same treatments at different curing stages. Data are presented as means ± standard error (n = 3).
There were significant difference in appearance between CK and SA-1 and SA-2 treated primary flue-cured tobacco leaves; SA-1 and SA-2 treated tobacco leaves were bright in color, without obvious variegation and browning; CK treated tobacco leaves were dark in color, with small leaf opening, and obvious visible variegation and browning on the surface (Figure 9).

Figure 9. Comparison of appearance of flue-cured tobacco after curing with different salicylic acid concentrations.
There were significant differences between Control and SA-1 and SA-2 in the yield, output value, proportion of middle and top grade tobacco, average price, and sensory evaluation quality of primary flue-cured tobacco leaves (Tables 5, 6); Control treatment was worst. The yield, output value, proportion of middle and top grade tobacco, and average price of SA-1 was 21, 63, 53, and 35% higher than Control, and SA-2 was 25, 51, 25, and 22% higher than Control, respectively. In sensory quality, the total score of SA-2 treatment was higher than that of SA-1 treatment by 4%, significantly higher than Control treatment by 22%; SA-1 treatment was higher than Control treatment by 17%.
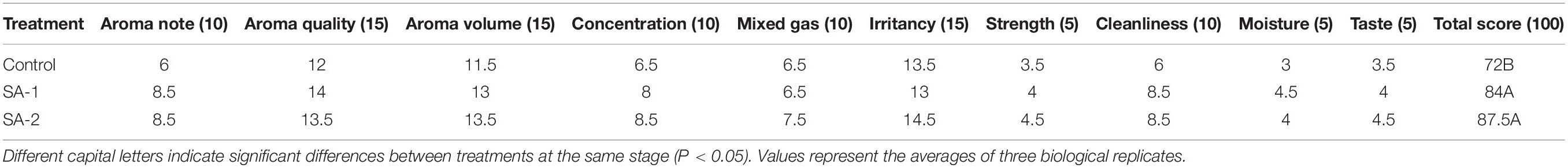
Table 6. Sensory evaluation of smoking quality of flue-cured tobacco with different salicylic acid concentrations.
Spraying salicylic acid can effectively prevent or alleviate the stress effect of cold injury on the tissue structure, photosynthetic system, and yellowing of flue-cured tobacco leaves during the harvesting and curing period (Dat et al., 2001; Zhu et al., 2013). Spraying salicylic acid significantly increased the thickness of palisade tissue, upper epidermal and lower epidermal, compared with the Control. This result could be related to the ability of leaf secretion; SA is shown to alleviate and regulate various cold-induced changes (Saleem et al., 2020). After cold stress, plant leaves will significantly thicken because of the secretion of a metabolic wax layer and a large amount of lignin, and palisade tissue and spongy tissue will significantly shrink to cope with water loss and wilting (Li et al., 2004, 2011). A suitable concentration of salicylic acid could increase the activity of antioxidant enzymes such as SOD and POD in leaves, maintain the stability of membrane system, reduce the loss of leaf tissue cells, alleviate the damage caused by cold damage to leaf tissue structure, and thus improve the cold resistance of flue-cured tobacco in the period of harvesting and curing (Janda et al., 1999; Kang and Saltveit, 2002; Khan et al., 2015). This study showed that the quality of tobacco leaves with high concentration of salicylic acid was not as good as that with low concentration. Existing studies also show that with increasing salicylic acid concentration, the membrane permeability increases rapidly, and the high concentration of salicylic acid increases the damage of low temperature on plants (Wang et al., 2009). Meanwhile, SA plays an important role in improved photosynthetic system and chloroplast pigment content in stressed plants (Fariduddin et al., 2003). In our study, chloroplast pigment content was SA-1, SA-2 > Control in fresh tobacco leaves. This result could be related to membrane system. The chloroplast membrane is protected by spraying salicylic acid and thus increases the content of chloroplast pigment (Huang et al., 2016). The plastid pigment content with a low concentration of salicylic acid spraying was significantly higher than that of high concentration and no spraying of salicylic acid, which shows that low-concentration salicylic acid spraying can effectively guarantee the vigorous light cooperation of tobacco plant use.
The coordination of water loss and yellowing is an important link to determine the quality of tobacco leaves. The fastest water loss rate was at 42–48°C, and the water loss rate of each treatment per unit time was Control > SA-2 > SA-1. The degradation rate of chloroplast pigment in each treatment was Control > SA-2 > SA-1, which indicated that the rate of water loss and yellowing of tobacco leaves without salicylic acid treatment was too fast in the process of tobacco curing, and the time provided for the transformation of contents and the formation of tobacco quality in the yellowing and fixation period was far from enough. This result could be related to the ability of cuticular wax layer. Exogenous salicylic acid can induce the increase in the total amount and component content of cuticular wax in plant leaves, preventing damage of low temperature to the internal structures of plants, and effectively reduce the water loss through transpiration and stomata of cuticle, thus reducing plant water loss rate (Kosma et al., 2009; Ni et al., 2013; Zhu et al., 2017; Wu et al., 2018). And when salicylic acid is not sprayed, the tissue cells of tobacco leaves were ruptured due to the damage of plasma membrane, the water loss was too fast, and it is easy to be browned by enzyme and cause browning tobacco in a large area (Ghanbari and Sayyari, 2018). Therefore, compared with the treatment without spraying, spraying salicylic acid can effectively ensure that the yellowing and water loss can be carried out simultaneously, and the effect is better at a lower concentration, which can ensure the orderly transformation of the internal substances and greatly alleviate the curing difficulty caused by cold injury stress.
By spraying appropriate concentration of salicylic acid, the activity of the antioxidant enzyme system and the accumulation of MDA in flue-cured tobacco can be increased (Cai et al., 2003). Meanwhile, these results showed that the low salicylic acid treatment could ensure the cell integrity of tobacco leaves, maintain water loss and yellowing in the curing process in an orderly manner, and reduce browning tobacco and invertase deactivation caused by cell damage and rapid water loss during the yellowing and fixation period. When flue-cured tobacco receives a low temperature signal, SOD activity in tobacco leaves increases rapidly. It decomposes the O2– and H2O2 produced by superoxide in the plant under cold stress together with CAT and POD. With increasing cold stress, the activity of antioxidant enzymes is inhibited, unable to complete the decomposition function until the oxidation products accumulated in the cell poison the cell membrane and cause damage to plants (Jing et al., 2008). The MDA content reflects the degree of oxidation in plants, and its level is one of the indicators of the degree of stress damage to plants. It can also be used as a reference indicator of the strength of stress tolerance of plants (Liu et al., 2018). In the current study, salicylic acid treatment of flue-cured tobacco seedlings in cold stress can significantly improve the ability to scavenge reactive oxygen species, protect the integrity of cell membrane, and stabilize physiological and biochemical reactions by increasing the activities of POD and SOD, which is consistent with the results of Zhu et al. (2013).
Cold stress can promote the rapid accumulation of polyphenols in tobacco leaves, destroy the integrity of cell membrane, and lead to the combination of PPO in vacuole and polyphenols (Gong, 2011; Ghanbari and Sayyari, 2018). Spraying salicylic acid can effectively alleviate the accumulation of polyphenols, reduce the activity of PPO, and protect the integrity of cell membrane (Han et al., 2017). The PPO activity of the fresh tobacco leaves increased and then decreased in the curing process. The peak value appeared at 42°C, and the PPO activity of each stage was consistent with that of the fresh tobacco leaves. Treatment by low concentrations of salicylic acid could alleviate cold stress in the field, better ensure the orderly enzymatic browning reaction in the tobacco curing process, and reduce the formation of browning tobacco.
Salicylic acid had a positive effect on the inhibition of carbon and nitrogen metabolism and aroma matter accumulation of flue-cured tobacco (Dechuan, 2006). Increasing spraying concentration of salicylic acid decreased carbon metabolism and increased nitrogen metabolism. Chang et al. (2012) show that the soluble sugar content in the leaves of soybean seedlings under cold stress will increase after being treated with salicylic acid, and the activity of various enzymes and the capacity for osmotic regulation will increase. This differs from the results of the current study, which may be because the proteins involved in carbohydrate catabolism were also induced by salicylic acid (Cai et al., 2014). Studies have also shown that lower concentrations of salicylic acid can increase the content of carbon metabolites and stress resistance of plants, which is similar to the results of this study (Zhang, 2006; Cui, 2007; Khan et al., 2015).
In this study, the economic characteristics and sensory evaluation quality of the primary flue-cured tobacco leaves treated with low concentrations of salicylic acid were higher than those of the leaves non-treated and treated with high concentration of salicylic acid. Economic loss could be effectively reduced by spraying low concentration of salicylic acid. Low temperature can promote the accumulation of phenolics in plants, inhibit oxygen free radicals, and protect the integrity of light system and cell membrane, so cold stress can promote the accumulation of polyphenols. Because of the destruction of the cell membrane and the strong peroxidation of membrane lipid, PPO activity was strong under conditions of high temperature and high humidity in the curing process (Farooq et al., 2008). After the cell membrane breaks, it easily reacts with a large amount of accumulated polyphenols forming a large area of browning tobacco, reducing the quality of tobacco (Rivera-Pastrana et al., 2010; Liu et al., 2017). By paying attention to weather forecast and long-term accumulated experience of field climate change in high-altitude tobacco planting areas of Yunnan Province, low-concentration salicylic acid can be sprayed before field cold stress, so as to effectively prevent cold stress and ensure high tobacco quality.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Written informed consent for participation in this study was obtained from the participants.
CZ and YZ designed the experiments and managed the projects. JC, AX, and GZ performed the experiments. YJ, BH, and XH performed the data analysis. XH, TL, and KR wrote the manuscript. All authors contributed to the article and approved the submitted version.
This study was financially supported by several projects from the National Natural Science Foundation of China (No. 41601330), Yunnan Science and Technology Innovation Project (2019HB068), Yunnan Ten Thousand People Program (YNWR-QNBJ-2018-400), Yunnan Applied Basic Research Project (Study of mechanism of tobacco enzymatic browning reaction during flue-curing and its application), and Tobacco Monopoly Bureau China (2017YN09 and 2020530000241025). The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
YZ was employed by the company Tobacco Monopoly Administration of Yunnan Wenshan Prefecture.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors are thankful to Dr. Junying Li for his valuable assistance and advice in the preparation of this paper.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2020.580597/full#supplementary-material
Supplementary Figure 1 | The variation of average temperature and diurnal range in Jianchuan from April 13th to August 31st in 2012 to 2016.
Achuo, E. A., Audenaert, K., Meziane, H., and Höfte, M. (2004). The salicylic acid−dependent defence pathway is effective against different pathogens in tomato and tobacco. Plant Pathol. 53, 65–72. doi: 10.1111/j.1365-3059.2004.00947.x
Agarwal, S., Sairam, R. K., Srivastava, G. C., and Meena, R. C. (2005). Changes in antioxidant enzymes activity and oxidative stress by abscisic acid and salicylic acid in wheat genotypes. Biol. Plant. 49, 541–550. doi: 10.1007/s10535-005-0048-z
Bahari, A. A., Sokhtesaraei, R., Chaghazardi, H. R., Masoudi, F., and Nazarli, H. (2015). Effect of water deficit stress and foliar application of salicylic acid on antioxidants enzymes activity in leaves of Thymus daenensis Subsp. lancifolius. Cercetari Agron. Moldova 48, 57–67. doi: 10.1515/cerce-2015-0017
Bolat, I., Dikilitas, M., Ercisli, S., Ikinci, A., and Tonkaz, T. (2014). The effect of water stress on some morphological, physiological, and biochemical characteristics and bud success on apple and quince rootstocks. ScientificWorldJournal 2014, 769732. doi: 10.1155/2014/769732
Cai, C. J., Chen, S. N., Yin, M., Zhou, H. C., and Wang, Q. (2003). Effects of salicylic acid on some enzyme activities related to stress resistance and content of MDA in Vanilla planifolia. Acta Bot. Yunnanica 25, 700–704.
Cai, H., Yuan, X. Z., Pan, J. J., Li, H., and Wang, Y. (2014). Biochemical and proteomic analysis of grape berries (Vitis labruscana ) during cold storage upon postharvest salicylic acid treatment. J. Agric. Food Chem. 62, 10118–10125. doi: 10.1021/jf503495z
Chang, Y. X., Xu, K. D., Chen, C., and Chen, L. (2012). Salicylic acid mitigating the inhibition of low temperature stress to soybean seedlings. Soybean Sci. 6, 927-931.
Chen, Y. J., Zhou, J. F., Ren, K., Zou, C. M., Liu, J. J., Yao, G. M., et al. (2019). Effects of enzymatic browning reaction on the usability of tobacco leaves and identification of components of reaction products. Sci. Rep. 9, 619–624. doi: 10.1038/s41598-019-54360-2
Cui, J. (2007). Effects of salicylic acid on resisting adversity of plants. Auhui Agric. Sci. Bull. 9, 35–38. doi: 10.16377/j.cnki.issn1007-7731.2007.09.017
Dagnon, S., and Edreva, A. (2003). Application of pattern recognition method for color assessment of Oriental tobacco based on HPLC of polyphenols. Beiträge Tabakforschung Inter. 20, 356–359. doi: 10.2478/cttr-2013-0750
Dat, J. F., Inzé, D., and Van Breusegem, F. (2001). Catalase-deficient tobacco plants: tools for in planta studies on the role of hydrogen peroxide. Redox Rep. 6, 37–42. doi: 10.1179/135100001101536012
Dechuan, W. (2006). Research advances in polyphenol oxidase in tobacco plants and the relations between PPO and tobacco leaf quality. J. Anhui Agri. Sci. 34:1381.
Fan, C., Bi, Y., Wang, Y., Ren, Y., Yang, Z., and Wang, Y. (2012). Effect of salicylic acid dipping on postharvest diseases and phenylpropanoid pathway in muskmelon fruits. Sci. Agri. Sin. 45, 584–589.
Fariduddin, Q., Hayat, S., and Ahmad, A. (2003). Salicylic acid influences net photosynthetic rate, carboxylation efficiency, nitrate reductase activity, and seed yield in Brassica juncea. Photosynthetica 41, 281–284. doi: 10.1023/b:phot.0000011962.05991.6c
Farooq, M., Aziz, T., Basra, S. M. A., Cheema, M. A., and Rehman, H. (2008). Chilling tolerance in hybrid maize induced by seed priming with salicylic acid. J. Agron. Crop Sci. 194, 161–168. doi: 10.1111/j.1439-037x.2008.00300.x
Ghanbari, F., and Sayyari, M. (2018). Controlled drought stress affects the chilling-hardening capacity of tomato seedlings as indicated by changes in phenol metabolisms, antioxidant enzymes activity, osmolytes concentration and abscisic acid accumulation. Sci. Hortic. 229, 167–174. doi: 10.1016/j.scienta.2017.10.009
Han, C., Zuo, J. H., Wang, Q., Dong, H. Z., and Gao, L. P. (2017). Salicylic acid alleviates postharvest chilling injury of sponge gourd (Luffa cylindrica). J. Integr. Agric. 16, 735–741. doi: 10.1016/S2095-3119(16)61390-4
Hetherington, S. E., and Öquist, G. (2006). Monitoring chilling injury: a comparison of chlorophyll fluorescence measurements, post-chilling growth and visible symptoms of injury in Zea mays. Physiol. Plant 72, 241–247. doi: 10.1111/j.1399-3054.1988.tb05829.x
Hongbin, Y. U. E. (2007). Effects of various nitrogen levels on key enzymes activeness of flue-cured tobacco leaves in carbon and nitrogen metabolism. China Tob. Sci. 1, 18–20.
Huang, C. P., Wang, D., Sun, L., and Wei, L. (2016). Effects of exogenous salicylic acid on the physiological characteristics of Dendrobium officinale under chilling stress. Plant Growth Regul. 79, 199–208. doi: 10.1007/s10725-015-0125-z
Hussain, H. A., Hussain, S., Khaliq, A., Ashraf, U., Anjum, S. A., Men, S., et al. (2018). Chilling and drought stresses in crop plants: implications, cross talk, and potential management opportunities. Front. Plant Sci. 9:393. doi: 10.3389/fpls.2018.00393
Janda, T., Szalai, G., Tari, I., and Paldi, E. (1999). Hydroponic treatment with salicylic acid decreases the effects of chilling injury in maize (Zea mays L.) plants. Planta 208, 175–180. doi: 10.1007/s004250050547
Jing, H. Y., Yuan, G., Yan-Man, L., Xiao-Hua, Q., and Zhang, M. F. (2008). Salicylic acid-induced enhancement of cold tolerance through activation of antioxidative capacity in watermelon. Sci. Hortic. 118, 200–205. doi: 10.1016/j.scienta.2008.06.015
Kang, H. M., and Saltveit, M. E. (2002). Chilling tolerance of maize, cucumber and rice seedling leaves and roots are differentially affected by salicylic acid. Physiol. Plant 115, 571–576. doi: 10.1034/j.1399-3054.2002.1150411.x
Ke-ai, M. E. N. G., Rong-bang, N. I. E., Chun-sheng, X. I. A. O., and Chun-gui, T. A. N. G. (2006). Changes of pigment and moisture content in cured tobacco leaves during bulk curing process. J. Hunan Agri. Univ. 2, 144–148.
Khan, M. I. R., Fatma, M., Per, T. S., Anjum, N. A., and Khan, N. A. (2015). Salicylic acid-induced abiotic stress tolerance and under lying mechanisms in plants. Front. Plant Sci. 6:462. doi: 10.3389/fpls.2015.00462
Kosma, D. K., Bourdenx, B., Bernard, A., Parsons, E. P., Lu, S., Joubes, J., et al. (2009). The impact of water deficiency on leaf cuticle lipids of Arabidopsis. Plant Physiol. 151, 1918–1929. doi: 10.1104/pp.109.141911
Kumar, S. P., Kumar, C. V., and Bandana, B. (2010). Effects of salicylic acid on seedling growth and nitrogen metabolism in cucumber (Cucumis sativus L.). J. Stress Phys. Biochem. 6, 102–113.
Li, Q., Yu, B., Gao, Y., Dai, A. H., and Bai, J. G. (2011). Cinnamic acid pretreatment mitigates chilling stress of cucumber leaves through altering antioxidant enzyme activity. J. Plant Phys. 168, 927–934. doi: 10.1016/j.jplph.2010.11.025
Li, X. G., Wang, X. M., Meng, Q. W., and Zou, Q. (2004). Factors limiting photosynthetic recovery in sweet pepper leaves after short-term chilling stress under low irradiance. Photosynthetica 42, 257–262. doi: 10.1023/b:phot.0000040598.48732.af
Liu, B. Y., Lei, C. Y., and Liu, W. Q. (2017). Nitrogen addition exacerbates the negative effects of low temperature stress on carbon and nitrogen metabolism in moss. Front. Plant Sci. 8:1328. doi: 10.3389/fpls.2017.01328
Liu, C. T., Wang, W., Mao, B. G., and Chu, C. C. (2018). Cold stress tolerance in rice: physiological changes, molecular mechanism, and future prospects. Yi Chuan 40, 171–185. doi: 10.16288/j.yczz.18-007
Madan, R. K., and Levitt, J. (2014). A review of toxicity from topical salicylic acid preparations. J. Am. Acad. Dermatol. 70, 788–792. doi: 10.1016/j.jaad.2013.12.005
Mandal, D., Thamawizuali, Z. R., Hazarika, T. K., and Shukla, A. C. (2017). Effect of salicylic acid as papaya post-harvest treatment in ambient condition. Environ. Ecol. 35, 3149–3153.
Moon, B. Y., Higashi, S., Gombos, Z., and Murata, N. (1995). Unsaturation of the membrane lipids of chloroplasts stabilizes the photosynthetic machinery against low-temperature photoinhibition in transgenic tobacco plants. Proc. Natl. Acad. Sci. U.S.A. 92, 6219–6223. doi: 10.1073/pnas.92.14.6219
Ni, Y., Wang, J., Song, C., Xia, R. E., Sun, Z. Y., Guo, Y. J., et al. (2013). Effects of SA induction on leaf cuticular wax and Resistance to Sclerotinia sclerotiorurn in Brassica napus. Acta Agron. Sin. 39, 110–117. doi: 10.3724/SP.J.1006.2013.00110
Prasad, T. K., Anderson, M. D., Martin, B. A., and Stewart, C. R. (1994). Evidence for chilling-induced oxidative stress in maize seedlings and a regulatory role for hydrogen peroxide. Plant Cell 6, 65–74. doi: 10.2307/3869675
Rivera-Pastrana, D. M., Yahia, E. M., and González-Aguilar, G. A. (2010). Phenolic and carotenoid profiles of papaya fruit (Carica papaya L.) and their contents under low temperature storage. J. Sci. Food Agric. 90, 2358–2365. doi: 10.1002/jsfa.4092
Saleem, M., Fariduddin, Q., and Janda, T. (2020). Multifaceted role of salicylic acid in combating cold stress in plants: a review. J. Plant Growth Regul. doi: 10.1007/s00344-020-10152-x [Epub ahead of print].
Supapvanich, S., and Promyou, S. (2013). “Efficiency of salicylic acid application on postharvest perishable crops,” in Salicylic Acid, eds S. Hayat, A. Ahmad, and M. Alyemeni (Dordrecht: Springer), doi: 10.1007/978-94-007-6428-6_15
Wang, D. H., Li, X. X., Su, Z. K., and Ren, H. X. (2009). The role of salicylic acid in response of two rice cultivars to chilling stress. Biol. Plant. 53, 545–552. doi: 10.1007/s10535-009-0099-7
Wang, H., Zhao, M., Yang, B., Jiang, Y., and Rao, G. (2008). Identification of polyphenols in tobacco leaf and their antioxidant and antimicrobial activities. Food Chem. 107, 1399–1406. doi: 10.1016/j.foodchem.2007.09.068
Wang, P., Duan, W., Takabayashi, A., Endo, T., Shikanai, T., Ye, J. Y., et al. (2006). Chloroplastic NAD (P) H dehydrogenase in tobacco leaves functions in alleviation of oxidative damage caused by temperature stress. Plant Phys. 141, 465–474. doi: 10.1104/pp.105.070490
Wu, X., Chen, Y. Y., Shi, X. J., Qi, K. J., Cao, P., Yin, H., et al. (2018). Effects of spraying exogenous hormones on cuticular wax composition, structure and permeability of the leaves in ‘Yuluxiang’ pear. J. Nanjing Agric. Univ. 41, 647–654. doi: 10.7685/jnau.201710007
Xue, T., Li, X., Zhu, W., Wu, C., Yang, G., and Zheng, C. (2009). Cotton metallothionein GhMT3a, a reactive oxygen species scavenger, increased tolerance against abiotic stress in transgenic tobacco and yeast. J. Exp. Bot. 60, 339–349. doi: 10.1093/jxb/ern291
Yadav, S. K. (2010). Cold stress tolerance mechanisms in plants. A review. Agron. Sustain. Dev. 30, 515–527. doi: 10.1051/agro/2009050
Yang, Y. Q., Wang, X. F., and Wang, S. H. (2004). Effect of osmoregulation on the plasma membrane redox activity in chilling injury sensitive soybean seeds. J. Plant Physiol. Mol. Biol. 30, 589–594. doi: 10.1300/J079v30n03_01
Zhang, R. (2006). Effects of salicylic acid on physiological and biochemical character of rice seedlings during chilling stress. Southwest university 1, 1–66.
Zhao, J., Li, S., Jiang, T., Liu, Z., Zhang, W., Jian, G., et al. (2012). Chilling stress—the key predisposing factor for causing Alternaria alternata infection and leading to cotton (Gossypium hirsutum L.) leaf senescence. PLoS One 7:e36126. doi: 10.1371/journal.pone.0036126
Zhu, F., Zhang, P., Meng, Y. F., Xu, F., Zhang, D. W., Cheng, J., et al. (2013). Alpha-momorcharin, a RIP produced by bitter melon, enhances defense response in tobacco plants against diverse plant viruses and shows antifungal activity in vitro. Planta 237, 77–88. doi: 10.1007/s00425-012-1746-3
Keywords: abiotic stress, physiology and biochemistry, carbon metabolites, nitrogen metabolites, yield
Citation: He X, Liu T, Ren K, Chen J, Zhao G, Hu B, Xu A, Jin Y, Zhu Y and Zou C (2020) Salicylic Acid Effects on Flue-Cured Tobacco Quality and Curing Characteristics During Harvesting and Curing in Cold-Stressed Fields. Front. Plant Sci. 11:580597. doi: 10.3389/fpls.2020.580597
Received: 06 July 2020; Accepted: 22 September 2020;
Published: 30 October 2020.
Edited by:
Ling Yuan, University of Kentucky, United StatesReviewed by:
Yongfeng Guo, Tobacco Research Institute (CAAS), ChinaCopyright © 2020 He, Liu, Ren, Chen, Zhao, Hu, Xu, Jin, Zhu and Zou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanmei Zhu, c2h1aW1lbmdjYW50b3VAMTYzLmNvbQ==; Congming Zou, em91Y29uZ21pbmd6Y21AMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.