Introduction
In the process of growth and development, plants are exposed to various abiotic stresses such as salinity, drought, low temperature, which limit crop yield and quality. During evolution, plants acquire series of resistances to these environmental stresses and survive through physiological, biochemical, and molecular responses. These responses are usually originated by regulating the expression of relevant genes. bZIP (basic leucine zipper) transcription factors, as one of the largest transcription factor regulatory families, play very important roles in responses to these abiotic stresses. bZIP TFs could be activated by drought, high salt and chilling damages. By binding specifically to cis-elements in the promoter region of stress related genes, they can regulate the transcriptional expressions of target genes, thereby regulating stress resistance of plants. This article comprehensively reviews the structural characteristics of bZIPs and their regulation mechanisms on target genes under various abiotic stresses.
Distribution and Classification of bZIP Transcription Factors
Currently, there are at least 64 families of transcription factors have been found in plants (Pérez-Rodriguez et al., 2010). According to their differences in DNA-binding domains, transcription factors can be defined as different families, such as bZIP, NAC, MYB, EREBP/AP2, Zinc-finger, etc. To date, a large number of bZIP transcription factors have been identified in almost all eukaryotes. There are 57, 77, 62, 96, 85, 87, 89, 262, 92, 89, 178, 103, 65, 69, 125, 64, 55, 114 bZIP transcription factors been found in Ananas comosus, Arabidopsis thaliana, Citrullus lanatus, Fagopyum talaricum, Gossypium raimondii, Gossypium arboreum, Oryza sativa, Glycine max, Sorghum bicolor, Hordeum vulgare L, Panicum virgatum L, Olea europaea L, Solanum tuberosum L., Solanum lycopersicum, Zea mays, Cucumis sativus, Vitis vinifera and Malus domestica, respectively (Corrêa et al., 2008; Nijhawan et al., 2008; Wang et al., 2011; Wei et al., 2012; Baloglu et al., 2014; Liu J. Y. et al., 2014; Li et al., 2015; Pourabed et al., 2015; Li et al., 2016; Zhang et al., 2018; Liu M. et al., 2019; Yang W. et al., 2019; Azeem et al., 2020; Liu et al., 2020; Rong et al., 2020; Wang et al., 2020; Zhao et al., 2020). Only 25, 21, and 21 bZIP transcription factors were found in yeast, nematode, and fruit fly, respectively (Riechmann et al., 2000). Compared to other eukaryotes, plants seem to have more bZIP homologous proteins and more conserved amino acid sequences in these homologies (Ali et al., 2016). Studies have shown that the structures of bZIP protein are closely related to its biological function. Jakoby et al. (2002) used MEME (multiple em for motif elicitation) to analyze a large number of bZIP transcription factors in Arabidopsis thaliana. Based on the characteristics of both the bZIP and other conserved motifs, the 75 bZIPs in Arabidopsis thaliana were classified into 10 subfamilies (A, B, C, D, E, F, G, H, I, and S). With similar method, the bZIP transcription factor family genes in other plants have also been categorized. The 131 bZIP transcription factors isolated from the soybean genome were also divided into abovementioned 10 subfamilies A~S (Liao et al., 2008). Though the 89 members of the bZIP transcription factor family in rice were also divided into 10 subfamilies, the subfamily S was replaced with J (Nijhawan et al., 2008). It seems that most of these subfamilies of bZIPs are conserved among different plants. Corrêa et al. (2008) identified the possible non-redundant complete sets of 92 bZIPs in rice and 89 bZIPs in black cottonwood. Based on the similarities of both bZIP and other conserved motifs, these collections of bZIPs together with the 77 bZIPs from Arabidopsis were categorized into 13 subfamilies, including A, B, C, D, E, F, G, H, I, J, K, L, and S. In which, three subgroups including J, K, and L were added.
With the advancement of bioinformatics, more and more conversed motifs, except bZIP, were identified for categorizing bZIP subfamilies. Hence, the classification of bZIP transcription factors has become more and more sophisticated. Due to the advancement of bioinformatics, there are increasing researches provide preliminary analyses on globally identifying bZIP members from the fresh released genomic database of many plants, such as potato, switchgrass, olive, pineapple, cotton, watermelon, and tartaty buckwheat, laying the foundation for subsequent research (Yang W. et al., 2019; Liu M. et al., 2019; Azeem et al., 2020; Liu et al., 2020; Rong et al., 2020; Wang et al., 2020; Zhao et al., 2020). Recent years, there are increasing reports on regulation mechanism of various bZIPs on different stress responses (Liu et al., 2012; Ji et al., 2013; Hwang et al., 2014; van Leene et al., 2016; Tsugama et al., 2016; Zhang C. Y. et al., 2017; Zhang L. N. et al., 2017; Wang et al., 2019). Specific roles of bZIPs in different subgroups might also be categorized into corresponding biological pathways, considering plenty of functional annotated bZIPs been classified into the known subfamilies with those sophisticated bioinformatics.
Architecture Characteristics of bZIP Transcription Factors
Transcription factor, also known as trans-acting factor, is a category of proteins that can specifically bind to cis-acting elements in the promoter region of eukaryotic genes, thereby activating or silencing the expression of related genes with temporal and spatial specificity. The structure of plant transcription factors generally includes at least four functional domains, including the DNA binding domain, the transcriptional regulatory domain, the nuclear localization signal peptide, and the oligomerization site (Du et al., 2012). They work together to regulate various biological processes.
Although the classification of bZIPs varies depending on the researcher’s choice of criterions, there is currently a consensus on this family that their protein sequence contains a conserved bZIP domain with 60~80 amino acids length. This domain is consisted of at least two specific structures. Firstly, the N-terminus is a basic region composed of about 20 basic amino acids, containing a nuclear localization signal (NLS) and a N-x7-R/K structural unit that specifically binds to a DNA sequence. This region is involved in nuclear localization and DNA binding (Lee S. C. et al., 2006). Secondly, the C-terminus, which is a leucine zipper region, a heptad repeat of leucine or other bulky hydrophobic amino acids (Ile, Val, Phe, or Met), creates an amphipathic helix. This region is involved in the dimerization of the bZIP protein before it binds to DNA (Landschulz et al., 1988; Hurst, 1994; Jakoby et al., 2002). In addition to the bZIP domain, the bZIPs also contain other conserved domains with transcriptional activation functions, such as the R/KxxS/T and S/TxxD domains, which are phosphorylation sites of Ca2+ independent protein kinase and casein kinase II (Furihata et al., 2006). Besides, there are also some regions rich in acidic amino acids, which can activate the transcriptional expression of downstream target genes (Liao et al., 2008).
Mechanisms of bZIP on Transcriptional Regulation of Target Genes
Through dimerization, phosphorylation, or interaction with other nuclear proteins, the specificity and affinity of bZIP binding to DNA will change, which will affect the activation of other genes, as well as its own stability and subcellular localization (Schütze et al., 2008). By forming homo- or heterodimers and binding specific promoters in its basic region, the bZIP transcription factor inhibits or activates the expression of target genes.
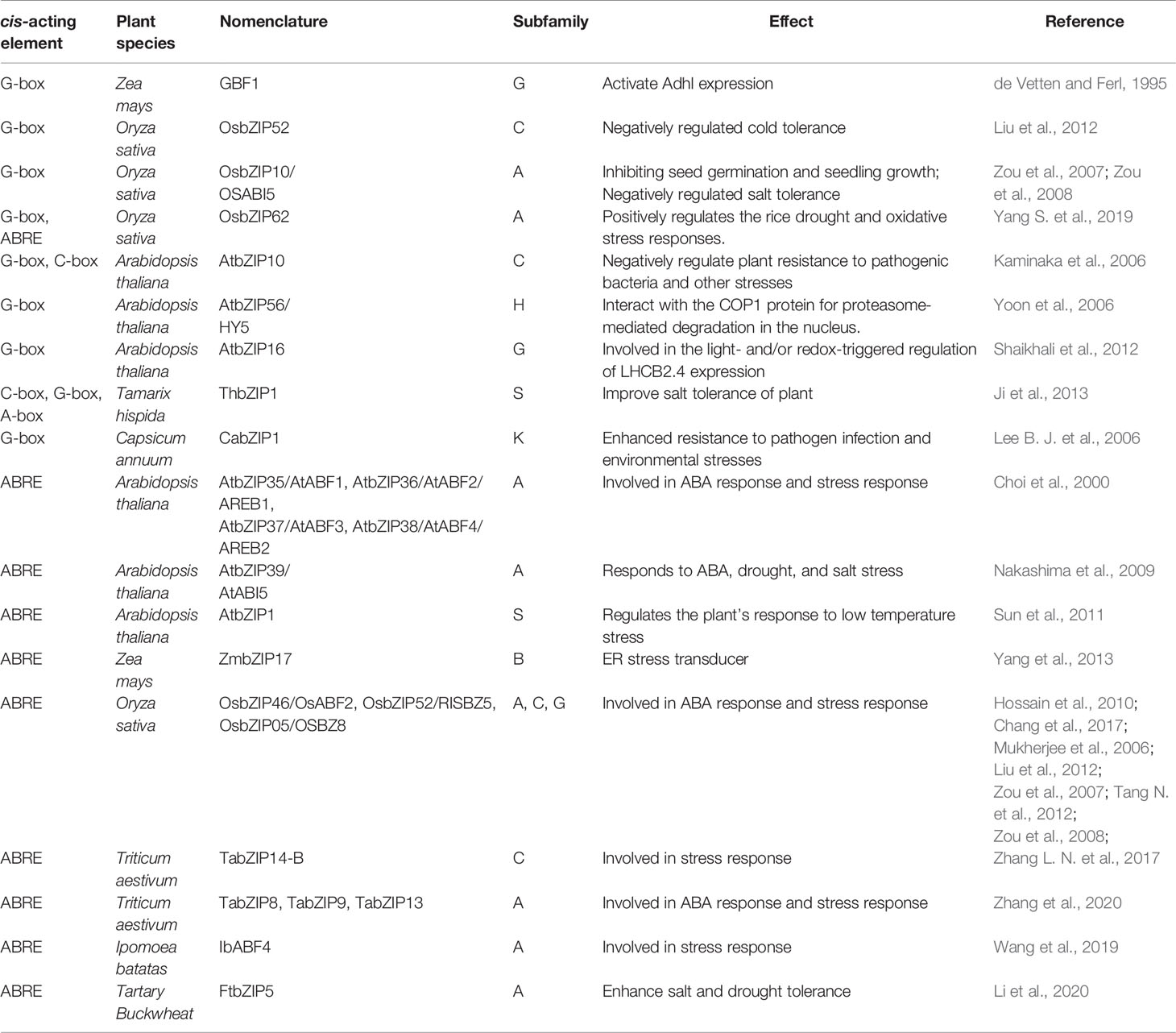
The binding specificity of bZIP factors in plants is mainly determined by three bases flanking the four core nucleotides. Generally, bZIP factors preferentially select ACGT core palindromes or pseudo-palindromic cis-acting elements to bind, such as G-box (CACGTG), C-box (GACGTC), A-box (TACGTA), ABRE (ACGTGGC) (Izawa et al., 1993; Kim et al., 2004). Most of them are located in the ABA hormone-induced promoter region. When the bZIP protein interacts with these cis-acting elements, the N-terminus of its basic domain is inserted into the large groove of the DNA double-strand, and the C-terminus of the leucine zipper is dimerized to form a superimposed curl helix (Landschulz et al., 1988; Ellenberger et al., 1992).
G-box is one of the most common targets of bZIP transcription factors. de Vetten and Ferl (1995) firstly found that corn GBF1 is a basic region leucine zipper protein and could activate Adhl expression by binding to its G-box. After that, series of stress related genes were found to be bound at their G-box and regulated by various bZIPs. Kaminaka et al. (2006) found that Arabidopsis thaliana AtbZIP10 can combine with G-box to negatively regulate plant resistance to pathogenic bacteria and other stresses. Zou et al. (2008) demonstrated that the rice OsbZIP10/OsABI5 could bind to the G-box element for trans-activating stress resistance genes, thereby inhibiting seed germination and seedling growth. Liu et al. (2012) also found that OsbZIP52/RISBZ5 can recognize the G-box on target genes to enhance the low temperature sensitivity of rice. The Arabidopsis thaliana AtbZIP56/HY5 binds directly to the promoters of light responsible element containing the G-box and thus regulates their transcriptional activity (Yoon et al., 2006). Induced by salt, the Tamarix hispida bZIP1 bound to G-box of the stress response genes and regulated their expression (Ji et al., 2013). Using chromatin immunoprecipitation, Lee et al. (2006a) demonstrated that CabZIP1 bound to the G-box elements in native promoter of the hot pepper pathogenesis-related protein 1 (CaPR-1) gene in vivo. Shaikhali et al. (2012) identified the AtbZIP16 as a component binding to the G-box-containing promoter fragment of light-harvesting chlorophyll a/b-binding protein2.4 (LHCB2.4) from nuclear extracts of high light-treated Arabidopsis plants.
The ABRE element is also a favorite target of bZIP transcription factors. Sun et al. (2011) found that AtbZIP1 binds to ABRE active elements and regulates the plant’s response to low temperature stress through ABA-dependent signaling pathways. Yoshida et al. (2015) demonstrated that the Arabidopsis thaliana bZIP transcription factors ABF1, ABF2, ABF3, and ABF4 combined with ABRE and regulated the expression of downstream genes related to salt and drought tolerance. In maize, ZmbZIP17 functions as an ER stress transducer, interacting with ABREs (Yang et al., 2013). Rice OsbZIP46/OsABF2 (Hossain et al., 2010; Tang N. et al., 2012; Chang et al., 2017), OsbZIP52/RISBZ5 (Liu et al., 2012), OsbZIP10/OsABI5 (Zou et al., 2007; Zou et al., 2008; Chang et al., 2019), OsbZIP05/OSBZ8 (Nakagawa et al., 1996; Mukherjee et al., 2006) could all regulate the expression of plant ABA-responsive genes by binding to their ABRE element. Zhang et al. (2017b) proved that wheat TabZIP14-B showed transcriptional activation ability through the transactivation assay and was capable of binding the ABRE in yeast. Zhang et al. (2020) found that, TabZIP8, 9, 13 could combine to the ABREs of TaNCED2 gene to promote ABA biosynthesis in wheat roots in response to salt stress. Wang et al. (2019) isolated the sweet potato bZIP transcription factor IbABF4 gene, and found its cis-acting activity on ABRE in vitro. Liu et al. (2019b) found that the Cassava MeABL5 was able to specifically interact with the ABRE cis-element in the promoter of the major cell wall invertase gene MeCWINV3.
In addition, bZIP transcription factors could target on genes by C-Box and A-box. The C-box of pathogenic responsive genes could bound and negatively regulated by AtbZIP10 in Arabidopsis thaliana (Kaminaka et al., 2006). Induced by ABA and drought, the Tamarix hispida bZIP1 bound to C-box and A-box cis-elements of the stress response gene (Ji et al., 2013).
In summary, bZIP transcription factors regulate the transcriptional expression by interacting with specific cis-regulatory sequences in the promoter region of response genes to regulate plant stress tolerance (Sornaraj et al., 2016). To understand the actual relationship between bZIP subfamilies and their binding cis-regulator motifs (Table 1 and Figure 1), all the functional annotated bZIPs were categorized into 13 known subgroups based on the method described by Corrêa et al. (2008). It seems that the G-Box and ABRE attracts most scientists’ interests and are two most understood cis-elements of bZIP transcription factors (Table 1). The bZIPs that bind to G-Box are most categorized into subfamilies A, C, G, H, K, and S; while those recognize ABRE usually belong to the subgroups A, B, C, G, and S (Table 1). Besides, there are also several reports on mechanisms about how bZIP transcription factors regulate other two cis-elements, C-box and A-box (Table 1). Interestingly, bZIPs that bind to C-box are usually belong to subfamilies C and S; the functional annotated bZIP bind to A-box is classified into subfamily S. Though the number of functional annotated bZIP is limit, their binding activities of different subfamilies to specific cis-elements could also provide directional suggestions for further research on de novo bZIPs and potential targets. However, more evidences are still needed to fulfill the relevance between bZIP subfamilies and corresponding cis-elements.
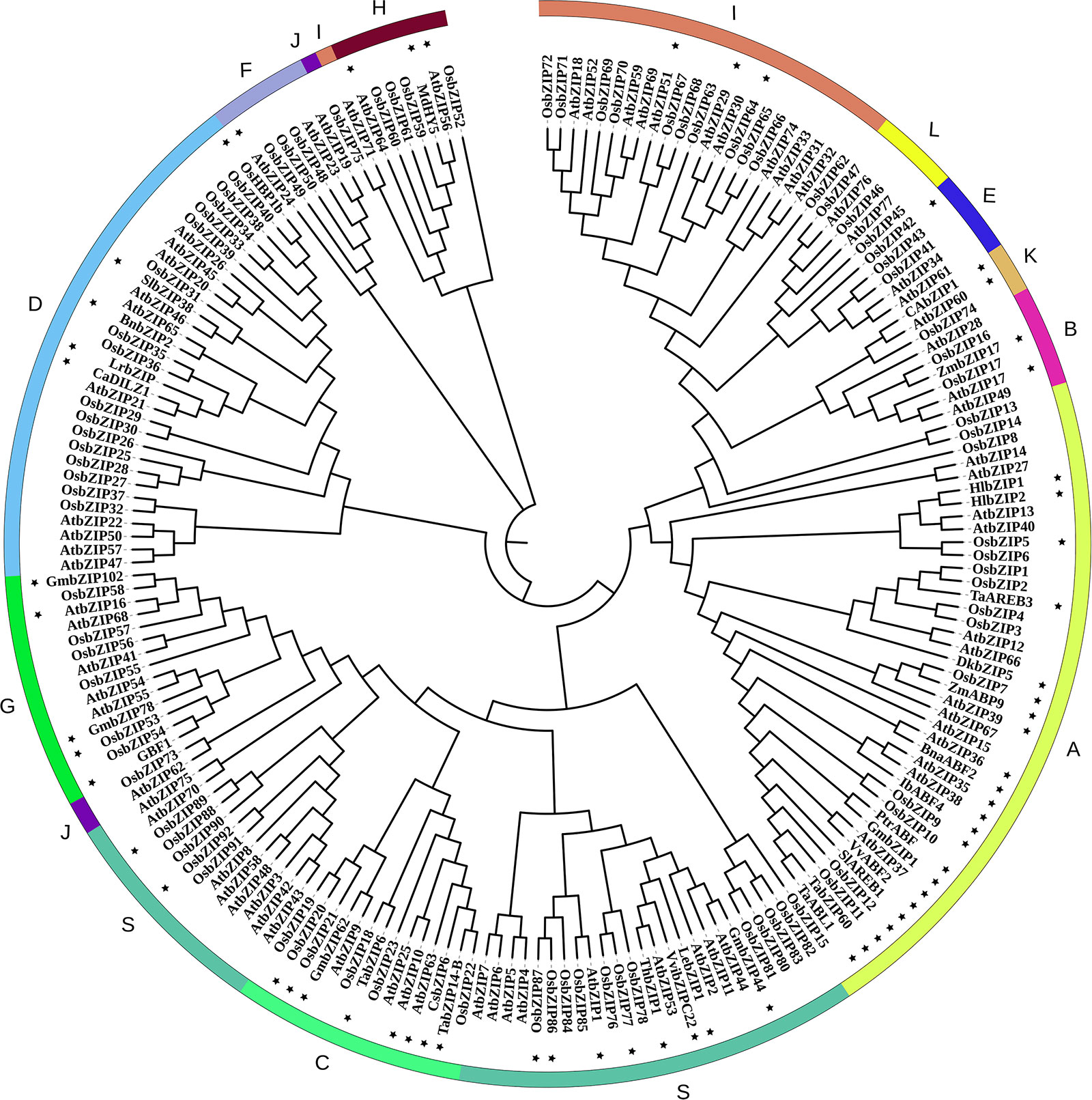
Figure 1 Phylogenetic relationships of bZIP family members. Concatenated sequence of all conserved motifs were used for multiple sequence alignments with the clustalX software. The phylogenetic tree was constructed using MEGA7.0 with the neighbor-joining method and 1000 bootstrap replicates (Saitou and Nei, 1987; Kumar et al., 2016). Then the trees were visualized using iTOL (https://itol.embl.de/). Group names were marked outside the circle. The bZIP protein sequences were downloaded from the JGI (http://www.jgi.doe.gov/) and NCBI (https://www.ncbi.nlm.nih.gov) databases. The Gene and Protein IDs of all these bZIPs are list in Supplemental Tables S1–S3. In this phylogenetic tree, the nomenclature of the Oryza sativa bZIPs is consistent with Corrêa et al., 2008 if there is any conflict with that in other publications (Supplemental Table S1). And the bZIP members that have been functional annotated are labeled with a star marker.
Regulation Mechanism of Plant bZIPS to Various Stresses
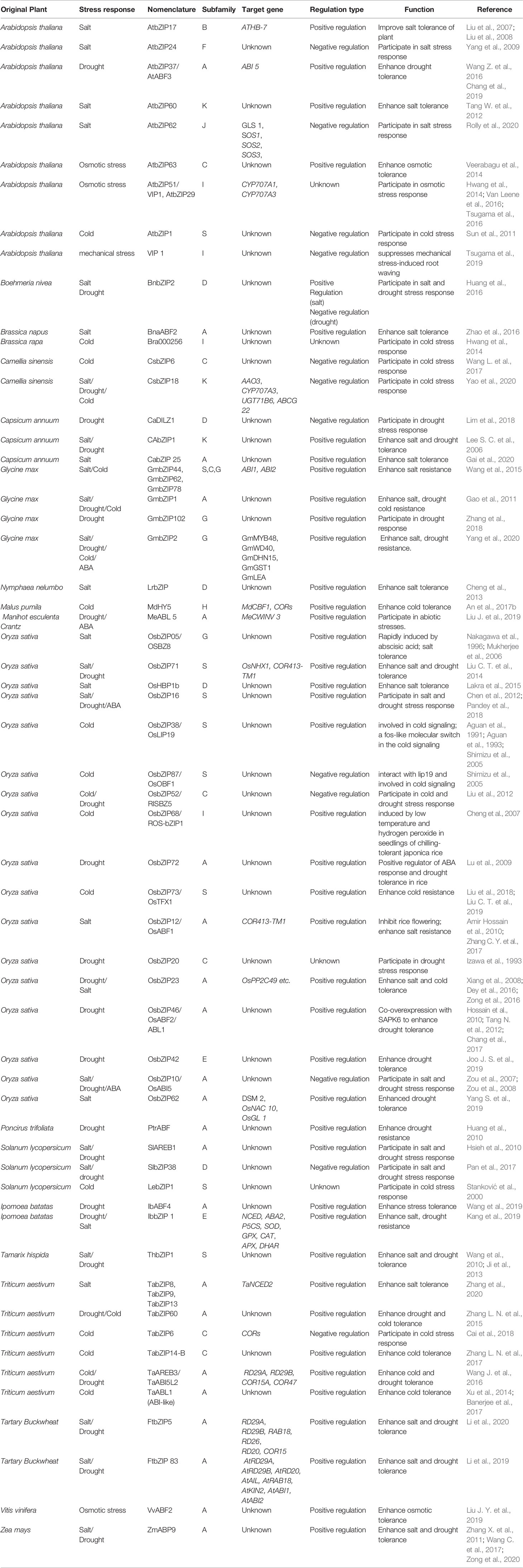
Previous studies have found that bZIPs play important roles in response to a variety of plant stresses, such as salinity, drought, and cold damages (Table 2). Their regulation mechanism varies depending on species of plant and types of stresses.
bZIP TFs Involved in Salt Stress Response
Under salt stress, plant cell should successively face challenges of osmotic stress, ion toxicity and oxidative stress (Munns, 2005; Rozema and Flowers, 2008). In these responses, bZIP transcription factors play key roles in various physiological processes in Arabidopsis thaliana, tomato, tobacco, rice, and soybeans, etc.
In Arabidopsis thaliana, AtbZIP17 was proven as a positive regulator in the processes salt stress responses, it activates both the expression of salt stress response gene ATHB-7 and SES1 (Liu et al., 2007; Liu et al., 2008); while the AtbZIP24 was revealed as a negative regulator in plant tolerance to salinity (Yang et al., 2009). Tang W. et al. (2012) found that heterologously expressing Arabidopsis thaliana AtbZIP60 could increase salt resistance and superoxide dismutase activity of tobacco, rice, and Pinus elliottii. Recently, Rolly et al. (2020) found that AtbZIP62 negatively regulated the transcriptional SOS signaling pathway genes and thus negatively regulates the salt tolerance of Arabidopsis. In Glycine max, overexpression of the GmbZIP1 enhances salt tolerance in transgenic plants (Gao et al., 2011). The overexpression of GmbZIP2 in soybean hairy roots could enhance the expression of the stress responsive genes GmMYB48, GmWD40, GmDHN15, GmGST1, and GmLEA, thereby improving plant resistance to drought and salt stresses (Yang et al., 2020). Besides, heterologously expressing GmbZIP44, GmbZIP62, and GmbZIP78 could significantly increase salt resistance of transgenic Arabidopsis thaliana plants (Wang et al., 2015). In maize, the ABP9 was found as a salinity responsible bZIP gene by Zhang et al. (2011a). Then, Wang et al. (2017a) heterologously expressed it to improve the salt tolerance of transgenic cotton. In Oryza sativa, the OsbZIP05/OSBZ8 firstly found with a higher transcriptional level in salt tolerant cultivar than in salt sensitive cultivar, indicate that OsbZIP05/OSBZ8 might play as a positive role in this stress responses (Mukherjee et al., 2006). After that, OsbZIP12/OsABF1, OsbZIP23, OsbZIP46/OsABF2, OsBZIP71, and OsbZIP72 were successively proven to act as positive regulators in the process of salt tolerance (Xiang et al., 2008; Lu et al., 2009; Amir Hossain et al., 2010; Hossain et al., 2010; Tang N. et al., 2012; Liu C. T. et al., 2014; Chang et al., 2017; Zhang C. Y. et al., 2017). OsbZIP71 can form both homodimers and heterodimers with Group C members of the bZIP gene family, and overexpression of OsbZIP71 can significantly enhance the salt tolerance of transgenic rice (Liu C. T. et al., 2014). On the contrary, the plants overexpressing OsbZIP10/OsABI5 showed more obvious chlorosis than wild type under high salt concentration, indicating that OsbZIP10/OsABI5 participates in the salt stress tolerance response of rice as a negative regulator (Zou et al., 2008).
Recent years, bZIPs in other plants have also been revealed to participate salinity responsive processes. Cheng et al. (2013) isolated a salt responsive transcriptional factor LrbZIP in lotus root and found that transgenic lotus with LrbZIP overexpression could grow with normal root biomass, chlorophyll content, and electrolyte exudation rate under NaCl treatment. Zhao et al. (2016) revealed that Brassica napus bZIP transcription factor BnaABF2 enhanced salt tolerance of plants through the ABA pathway. Gai et al. (2020) demonstrated that overexpression of the pepper CabZIP25 enhanced the germination rate, fresh weight, chlorophyll content, and root lengths under salt stress.
To sum up, many bZIP genes have been excavated in different plants and confirmed that they can significantly enhance the salt tolerance of plants, making the bZIP gene family a gene treasure house for improving the salt tolerance of crops. Therefore, the use of bZIP transcription factors to improve the salt tolerance of crops and breed new salt-tolerant varieties is of great significance for improving agricultural productivity and improving saline soils.
bZIP TFs Involved in Drought Stress Response
Drought is an adverse environmental factor that threatens plant growth and development. Many plant bZIP family members are involved in response to drought stress. Series of studies have shown that several rice bZIP transcription factors are involved in drought resistance. Liu J. Y. et al. (2014) found that rice OsbZIP71 directly binds to the promoters of OsNHX1 and COR413-TM1 and activates their transcription so as to enhance drought resistance of transgenic rice. Yang et al. (2019a) showed that overexpression of OsbZIP62 enhanced the drought tolerance and oxidative stress tolerance of transgenic rice. Except rice, some drought-related bZIP transcription factor genes cloned in other plants also significantly enhanced the drought resistance of transgenic crops. Overexpression of maize ABP9 confers excellent drought tolerance to transgenic Arabidopsis thaliana plant (Wang C. et al., 2017). Under drought stress, the transgenic Arabidopsis plants of IbbZIP1 showed significant upregulation of the genes involved in ABA and proline biosynthesis and reactive oxygen species scavenging system, so as to significantly decrease of H2O2 content (Kang et al., 2019). During seed germination and plant development, transgenic ramie plants overexpressing BnbZIP2 were more sensitive to drought stress than wild-type (Huang et al., 2016). In addition, overexpression of transcription factors such as Arabidopsis thaliana ABF3 (Wang Z. et al., 2016) and wheat TabZIP60 (Zhang L. N. et al., 2015) in plants can significantly improve the drought resistance of transgenic plants. On the contrary, Lim et al. (2018) found that the pepper bZIP transcription factor CaDILZ1 plays a negative regulatory role in response to drought stress.
bZIP TFs Involved in Cold Stress Response
Low temperature stimulation will disturb the normal physiological and metabolic activities and further affect the plant growth and development. The plant mainly responds to low temperature stress through the ICE-CBF-COR pathway. Low temperature induces CBFs (C-repeat-binding Factors) expression by ICE (inducer of CBF expression), which recognizes CRT/DRE (C-repeat/dehydration responsive cis element) located on the promoter of COR (cold regulated) genes (Shi et al., 2018). bZIP transcription factors also play indispensable roles in regulating plant cold stress responses.
The first rice bZIP-like transcription factor identified and reported was OsbZIP38/LIP19 of the H subfamily. As a Fos-like molecular switch, it is involved in the plant’s response to cold signal pathways (Aguan et al., 1991; Aguan et al., 1993; Shimizu et al., 2005). OsbZIP38/LIP19 and OsbZIP87/OsOBF1 are more likely to form heterodimers to participate in the plant’s response to cold signaling (Shimizu et al., 2005). In addition, the rice OsbZIP52/RISBZ5, OsbZIP68/ROS-bZIP1, and OsbZIP73/OsTFX1 were also involved in cold resistance. As a member in the G subfamily, OsbZIP52/RISBZ5 is not induced by drought, salt, PEG, and ABA, but by low temperature. It can form homodimers and specifically bind G-box. However, the survival rate of rice plants over-expressed OsbZIP52/RISBZ5 were significantly lower than those of wild type, indicating that OsbZIP52/RISBZ5 negatively regulates the rice cold tolerance (Liu et al., 2012). Cheng et al. (2007) found that OsbZIP68/ROS-bZIP1 could be induced and responded quickly within 24 h when rice was treated at 10°C. Liu et al. (2018, 2019a) identified eight low temperature resistant bZIP genes in rice, including OsbZIP08, OsbZIP35, OsbZIP38, OsbZIP46, OsbZIP63, OsbZIP72, OsbZIP73, and OsbZIP76.
Except for rice, carrot, soybean, wheat, tomato, and other crops have also been successively excavated bZIP transcription factors in response to low temperature stress. For example, Ito et al. (1999) found that the expression of bZIP-like protein Lip (Low temperature-Induced protein) in the roots of radish was up-regulated under low temperature treatment, thereby enhancing its cold resistance. Soybeans GmbZIP44, GmbZIP62 and GmbZIP78 can regulate and promote the synthesis of proline (plant cold tolerance osmotic regulator) to enhance the plant tolerance to cold stress by activating the expression of downstream genes ERF5, KIN1, CORl5A, and COR78 (Liao et al., 2008). Hwang et al. (2014) treated Brassica rapa with low temperature stress and found that the expression of 27 BrbZIPs were significantly up-regulated, among which Bra000256, Bra003320, Bra004689, Bra011648, Bra020735, and Bra023540 may be the key genes involved in the response to this stress. Compared with wild-type Arabidopsis thaliana, heterologous expression of TabZIP6 in wheat under cold treatment significantly reduced the expression of CBFs, key CORs, and other genes in transgenic plants, making the transgenic plants sensitive to low temperature (Cai et al., 2018). However, the over-expressed wheat TabZIP14-B, TaAREB3, and TabZIP60 in Arabidopsis thaliana can significantly enhance the ability of plants to resist cold stress. In addition, transgenic plants are more sensitive to ABA than wild type, indicating that TabZIP14-B, TaAREB3, and TabZIP60 all enhance the cold resistance of plants through the ABA pathway (Zhang L. N. et al., 2015; Wang J. et al., 2016; Zhang L. N. et al., 2017). Xu et al. (2014) found that over-expression of wheat bZIP transcription factor TaABL 1 (ABI-like) elevated cold tolerance in wheat. Apple bZIP transcription factor MdHY5 can respond to low temperature stress at both the transcriptional and protein levels. Overexpression of MdHY5 can significantly enhance cold stress resistance in apple callus and transgenic Arabidopsis thaliana. EMSA results indicate that MdHY5 can bind to G-Box on the MdCBF1 promoter, thereby increasing its transcription level COR genes independent of CBF (An et al., 2017b). Wang et al. (2017b) found that transgenic Arabidopsis thaliana plants showed reduced survival, increased electrical conductivity, increased malondialdehyde content, and reduced soluble sugar content when overexpressed Camellia sinensis CsbZIP6 in it. Transcriptome analysis found that the expression of low-temperature and drought-responsive genes in over-expressed plants was significantly lower than that of wild type, indicating that CsbZIP6 plays a negative regulatory role in low-temperature stress response. Recently, Yao et al. (2020) also discovered that CsbZIP18 is a negative regulator of freezing tolerance via an ABA-dependent pathway.
bZIP TFs Involved in Osmotic Stress Response
Salinity and drought usually induce secondary damages, such as osmotic stress. Hence, it’s not difficult to understand that plant bZIPs also act as significant roles in response to osmotic stress.
The rice OsbZIP71 transcription factor recognizes and combines with the promoter of the osmo-regulatory gene OsNHX1, and further transports excess Na+ and K+ in the cytoplasm to the vacuole, reducing salt concentration in the cytoplasm to improve rice salt tolerance (Liu C. T. et al., 2014). In Arabidopsis thaliana, the AtbZIP63 can regulate protein-protein interactions to regulate the activity of proline dehydrogenase I, thereby enhancing the ability of the plant to tolerate hypotonic stress (Veerabagu et al., 2014); the VIP1 (AtbZIP51) rapidly accumulates in the nucleus in response to hypotonic stress (Hwang et al., 2014; Tsugama et al., 2016). Actually, VIP1/AtbZIP51 and bZIP29 can form a heterodimer to enhance their binding to the hypotonic response element (AGCTGK) in the promoters of osmotic response genes CYP707A1 and CYP707A3 (Van Leene et al., 2016). Furthermore, Tsugama et al. (2019) found that the VIP1/AtbZIP51 was dephosphorylated by PP2A (protein phosphatase 2A), so as to suppress mechanical stress-induced root waving.
bZIP TFs Involved in Regulating ABA Signaling Pathway
As a ‘emergency hormone’ in plants, ABA is an important signaling molecule in plants. When plants encounter abiotic stress such as salt, drought, or low temperature, they will activate both ABA-dependent and ABA-independent signaling pathways (Shinozaki and Yamaguchi-Shinozaki, 1996; Bray, 1997; Thomashow, 1998; Verslues and Zhu, 2005). Genes involved in the ABA-dependent pathway not only induce ABA biosynthesis, but also regulate the expression of genes containing ABA response element binding factors (AREBs) (Zhu, 2002; Shinozaki and Yamaguchi-Shinozaki, 2007). The bZIP transcription factor family can bind to ABRE elements (Choi et al., 2000; Uno et al., 2000). So far, bZIP transcription factors are proven to participate in ABA-dependent stress signaling in various plants, including Arabidopsis thaliana, rice, soybean, wheat (Casaretto and Ho, 2003; Fujita et al., 2005; Kobayashi et al., 2008; Lu et al., 2009).
The A subfamily bZIP transcription factor in Arabidopsis thaliana is a major regulator of ABA-dependent responses (Satoh et al., 2004). AtbZIP1 regulates ABA signal transduction by binding to the ABREs and alters the expressions of the ABA responsive genes to tolerate the cold stress (Sun et al., 2011). In rice, OsbZIP23 and OsbZIP46 can directly regulate the expression of multiple stress genes through the ABA pathway, thereby significantly improving drought- and salt-resistance of rice (Xiang et al., 2008; Tang N. et al., 2012; Dey et al., 2016; Zong et al., 2016). OsbZIP23/66/72 positively regulates ABA-responsive genes through interacting with OsMFT2and promotes seed germination (Song et al., 2020). In the transgenic plants over-expressing OsbZIP42, it showed a rapid rise of transcriptional expression of ABA responsive LEA3 and Rab16 and increased tolerance to drought stress (Joo H. et al., 2019). In soybeans, GmbZIP44, GmbZIP62, and GmbZIP78 can positively regulate the expression of ABI1 and ABI2 genes and further induce the expression of downstream genes such as ERF5, KIN1, COR15A, and COR78 in response to ABA treatment (Liao et al., 2008). In maize, the transcription factor NCP1 can interact with the ABRE-binding bZIP transcription activator ABP9 and inhibit its activity, then negatively regulating ABA signal and weakening plant tolerance to multiple stresses (Zong et al., 2020).
Recent years, bZIPs are also found with increasing contributions in regulating ABA responses in other plants. Joo H. et al. (2019, 2020) showed that the stability of bZIP transcription factor CaAIBZ1 and CaATBZ1 could be modulated by a RING-type E3 ligase, CaASRF1, so as to positively modulates abscisic acid (ABA) signaling and ABA-mediated drought response in pepper. Liu et al. (2019c) found that overexpression of the ABA-depended grapevine VvABF2 gene could enhance osmotic stress tolerance in Arabidopsis thaliana and thereby reduce the cell membrane damage. Wang et al. (2019) found that sweet potato IbABF4 gene, encodes a bZIP transcription factor, overexpression in Arabidopsis thaliana and sweet potato could enhance their tolerance to multiple abiotic stresses through the ABA signaling pathway. Li et al. (2019, 2020) showed that the tartary buckwheat bZIP genes, FtbZIP83, FtbZIP5 were both positive regulators involved in drought or salt stress via an ABA-dependent signaling pathway. In short, bZIP family members play important roles in the abscisic acid signaling pathway under various stresses. A large number of studies have shown that bZIP transcription factors affect ABA biosynthesis through the ABA-mediated signal transduction pathways and thus improve plant stress resistances.
bZIP TFs Involved in Antioxidant System
Actually, the antioxidant system is an effective way for bZIP transcription factors to respond to abiotic stresses in plants (Miller et al., 2008; Choudhury et al., 2013). Superoxide dismutase (SOD), peroxidase (POD) and catalase (CAT) are three groups of key enzymes that removes active oxygen from plants. Overexpressing the bZIP gene in plants can increase the activity of peroxidase POD and SOD and increase the content of soluble sugars and proteins; it can also increase the elimination of active oxygen, promote the accumulation of soluble penetrants (Choudhury et al., 2013). For example, over expression of pepper CAbZIP1 gene in Arabidopsis thaliana can eliminate the active oxygen by regulating the degradation enzyme POD and CAT, so as to improve the drought resistance and salt resistance of transgenic plants (Lee S. C. et al., 2006). Under stress conditions, POD and SOD activities of transgenic tobacco plants overexpressing Tamarix hispida ThbZIP1 were significantly increased, accompanied by an increase in soluble protein and sugar content. Studies have shown that the ThbZIP1 gene was significant upregulated under high-salt conditions, so as to improve plant salt tolerance by effectively removing reactive oxygen free radicals and accumulating soluble osmotic substances (Ji et al., 2013). Compared with wild-type plants, the transgenic tobacco with OsHBP1b under salt treatment enhanced the SOD activity, which further improved the stability of the vacuolar membrane and the K+/Na+ ratio, and had a stronger anti-oxidative damage function (Lakra et al., 2015). Further, Das et al. (2019) demonstrated that transgenic rice plants over-expressing OsHBP1b exhibit better survival and favorable osmotic parameters under salinity stress than the wild type counterparts. Overexpressing Poncirus trifoliata PtrABF in tobacco can stably promote the expression of nine stress-responsive genes in tobacco, and significantly induce the expression of three antioxidant enzyme genes under drought stress, which can be better removals of active oxygen free radicals and in turn enhances the resistance of transgenic plants to drought (Huang et al., 2010).
To reveal the relevance between bZIP subfamilies and stress types, the functional annotated bZIPs were also classified into 13 verified clades followed the approach used by Corrêa et al. (2008) (Table 2 and Figure 1). There is yet not any functional report on bZIPs in subfamilies H, J, and L on abiotic stresses. Among the rest 10 subfamilies, there are 8, 7, 6, and 3 of which involved in salinity, drought, cold and osmotic stress, respectively. The bZIPs for regulating salinity tolerance are most frequently found in subgroups A, D, G, and S; while for modulating resistances to both drought and osmotic stress are most members in subgroup A; and for controlling cold responses are most those from subgroups A, C and S (Table 2).
Regulation of bZIPs on Metabolism of Flavonoids Involved in Stress Responses
Recently, a plenty of flavonoids show significant contributions to plant tolerances to abiotic stresses (Yamasaki et al., 1997; Agati et al., 2012; Yan et al., 2014; Pi et al., 2016; Pi et al., 2018; Pi et al., 2019). Flavonoids are widely distributed in the plant kingdom and are abundant in flowers, fruits, and leaves of many plants (Du et al., 2010). Based on the different oxygen rings and conformations of the basic molecular structure, flavonoids are generally divided into six categories: flavone, flavonol, isoflavone, flavanone, flavanol, and anthocyanidin (Rice-Evans and Miller, 2010). The starting substrate for plant flavonoid biosynthesis is derived from coumaroyl-CoA of the phenylpropane metabolic pathway and malonyl-CoA from acetyl-coenzymes. Under the action of chalcone synthase (CHS), they first form chalcone (Aoki et al., 2000), and then the naringenin is formed by the catalytic action of chalcone isomerase (CHI) (McKhann and Hirsch, 1994). Under the catalysis of cytochrome P450 monooxygenase (CPM) and other enzymes, naringen can be used as a major intermediate metabolite to synthesize other flavonoids (Akashi et al., 1999; Liu et al., 2003; Falcone Ferreyra et al., 2012; Lam et al., 2014; Uchida et al., 2015).
More than 10,000 plant flavonoids have been discovered (Aoki et al., 2000; Jiang et al., 2010). They play very important roles in plant resistance to various stress (Yamasaki et al., 1997; Agati et al., 2012; Yan et al., 2014). They could remove free radicals under ultraviolet radiation (Li et al., 1993; Treutter, 2005), improve seed storage capacity and prolong life (Debeaujon et al., 2000), change petal color (Mola et al., 1998), interfere with the polar distribution of auxin (Buer and Muday, 2004), and affect the accumulation and composition of fatty acids (Lian et al., 2017).
Early studies on the mechanism of flavonoids involved in stress resistance mainly focused on their regulations on response to ultraviolet radiation (Tattini et al., 2006; Mellway et al., 2009). Later, flavonoids were found with strong antioxidant activity (Treutter, 2006; Agati et al., 2007; Pourcel et al., 2007; Hernández et al., 2009). Since various stresses can cause excessive peroxide to accumulate in plants, the significant role of flavonoids in plants’ stress resistance attracts increasing interests (Qiu et al., 2008; Fasano et al., 2014; Watkins et al., 2014; Rai et al., 2016). Tattini et al. (2004) reported that European privet flavonoids as antioxidants respond to strong light and drought stresses. Li et al. (2011) found a conserved trans-acting element (G-box, CACGTG) in the promoter region of the chalcone synthase family gene (AtCHS) in Arabidopsis thaliana, which regulates the accumulation of H2O2 by responding to cGMP signals (Abu Zahra et al., 2014). Yan et al. (2014) found that the cytochrome P450 monooxygenase GmFNSII/GmCPM in soybean was beneficial to the accumulation of flavonoid aglycones in plants and the reduction of H2O2 content. In previous studies, we found that the content of flavonoids such as quercimeritrin in salt-tolerant soybeans is relatively higher than that of salt-sensitive soybeans, which is beneficial for soybeans to adapt to salt stress (Lu et al., 2013). We further discovered that enzymes related to the flavonoid metabolism pathway are important salt stress response factors, and they can significantly regulate the salt tolerance of plants such as Arabidopsis thaliana and soybean (Pi et al., 2016). We recently found that the salt-triggered phosphorylation of GmMYB173, subsequent elevates the transcription of GmCHS5 for enhancing the accumulation of dihydroxy B-ring flavonoids (such as cyaniding-3-arabinoside chloride) (Pi et al., 2018); while salt-inhibited phosphorylation of GmMYB183 subsequently decreases the transcription of GmCYP81E11 for reducing monohydroxy B-ring flavonoids (such as ononin) (Pi et al., 2019). Actually, both GmMYB173 phosphorylation and GmMYB183 dephosphorylation contribute to soybean salt tolerance.
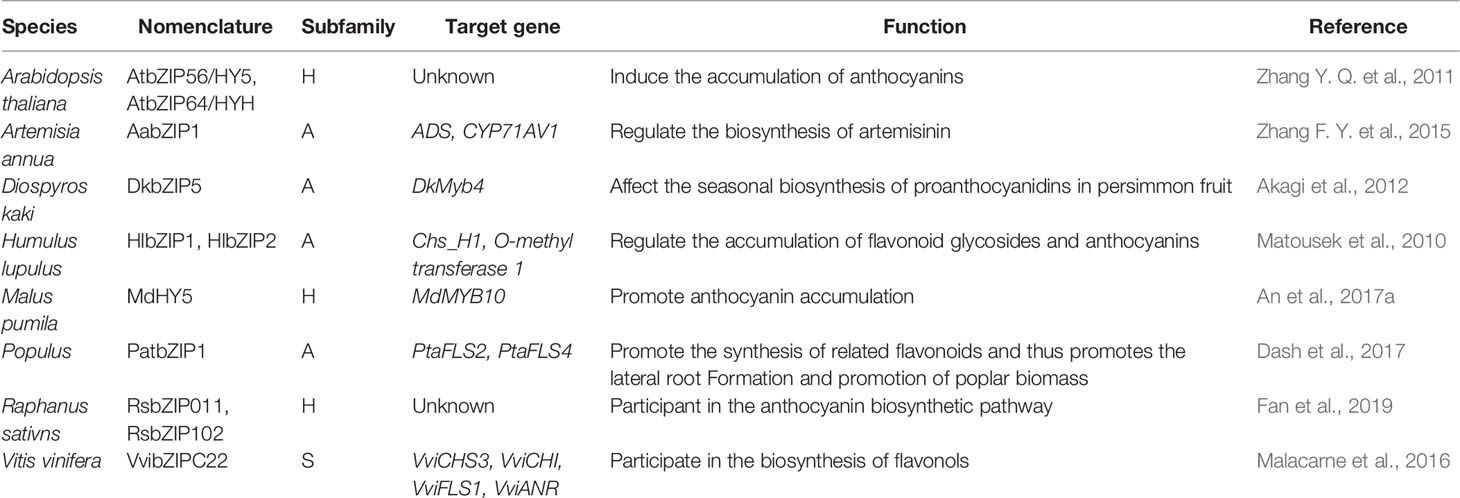
The abovementioned studies showed that flavonoids played very important roles in plant responses to stress. Interestingly, many bZIP transcription factors usually play key regulatory roles in the process of flavonoid biosynthesis. They regulate the expression of key enzyme genes in the synthetic pathway, thereby regulating the metabolism and synthesis of flavonoids.
Matousek et al. (2010) found that both hop HlbZIP1 and HlbZIP2 could activate the expression of chalcone synthase chs_H1 and the O-methyl transferase 1 genes and further regulate the accumulation of flavonoid glycosides and anthocyanins. Akagi et al. (2012) found that ectopic DkbZIP5 overexpression in persimmon calluses could induced the up-regulation of DkMyb4 and then affect the seasonal biosynthesis of proanthocyanidins in persimmon fruit. Malacarne et al. (2016) showed that VvibZIPC22, a member of clade C of the grapevine bZIP family, was able to activate the transcriptional expression of specific genes of the flavonoid pathway including VviCHS3, VviCHI, VviFLS1, and VviANR, alone or together with other factors to participate in the biosynthesis of flavonols during flowering and UV light-mediated induction. Dash et al. (2017) found that the poplar PatbZIP1 transcription factor regulated the expression of two flavonol synthase genes PtaFLS2 and PtaFLS4 and thus promotes the lateral root formation. bZIP transcription factor HY5 plays a multifaceted role in plant growth and development. Apple MdHY5 gene, induced by light and abscisic acid treatments, promoted anthocyanin accumulation by regulating expression of the MdMYB10 gene and downstream anthocyanin biosynthesis genes (An et al., 2017a). Zhang et al. (2011b) found that two bZIP transcription factors AtbZIP56/HY5 and AtbZIP64/HYH in Arabidopsis thaliana induced the accumulation of anthocyanins under low temperature. In addition, ABA can induce the expression of Artemisia annua AabZIP1 to activate the expression of downstream gene ADS and CYP71AV1, thereby regulating the biosynthesis of artemisinin (Zhang F. Y. et al., 2015). Fan et al. (2019) showed that the expression of RsbZIP011 and RsbZIP102 were significantly up-regulated in radish tissue with higher anthocyanin content under heat and salt stress.
So far, the bZIPs that involve in flavonoid synthesis varies from plant species and their target genes (coding for different enzymes in flavonoid metabolism). To uncover the relationship between bZIP subfamilies and flavonoid synthesis, all the functional annotated bZIPs were also categorized into the 13 known subgroups according to Corrêa et al. (2008) (Table 3 and Figure 1). It seems that only bZIPs in subfamilies A, H, and S might regulate flavonoid metabolism.
Concluding Remarks
Due to their significant roles in plant tolerances to various stresses, the bZIP transcription factors have been comprehensively studied, including their categorization and regulatory mechanisms of target genes. However, there is at least one interesting issue worthy of further investigation: whether bZIP transcription factor regulates plant stress tolerance by modulating the synthesis of flavonoids.
To date, plenty of literatures show that bZIPs regulate plant tolerances to various abiotic stresses, such as low temperature, drought, high salt, nitrogen deficiency, zinc deficiency time (Lilay et al., 2020; Ueda et al., 2020). Besides, there are many reports reveal that flavonoids participate in various stress responses. Moreover, a lot of researches have now confirmed that bZIP transcription factors play an important role in the synthesis of flavonoids. Specially, bZIPs in subfamily H could bind to G-box in promoter of cold responsive genes (Tables 1 and 2); members of this subfamily also could modulate the synthesis of some flavonoids (Table 3). Since members in this group shares similar conversed protein motifs (Supplemental Figures S1 and S2), it is reasonable to hypothesize that plant bZIPs in subfamily H could bind to G-box of cold-responsive genes to further regulate the synthesis of flavonoids. Similarly, it also makes sense that bZIPs in subfamily A could regulate the synthesis of flavonoids by binding to G-box or ABRE cis-elements of target genes involved in cold, salinity, drought and osmotic stresses; subfamily S could regulate the synthesis of flavonoids by bind to G-box or C-box or A-box or ABRE of genes involved in cold, salinity, and drought stresses (Tables 1–3). However, these hypotheses are still needed to be further verified.
Author Contributions
YY completed the writing of this article. YQ, MJ, and JY assisted in the data collection and table making. JX, TZ, and LG took charge of the drawing. EP is responsible for the revision of this article.
Funding
This work was supported by the grants 31970286 and 31301053 from the National Science Foundation of China, LY17C020004 from the Natural Science Foundation of Zhejiang Province, 20170432B01 from the Hangzhou Science and Technology Bureau, PF14002004014, PD11002002018001, 2016XJSGWXM27 and 2016XJSGWXM32 from Hangzhou Normal University.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2020.01258/full#supplementary-material
References
Abu Zahra, H., Kuwamoto, S., Uno, T., Kanamaru, K., Yamagata, H. (2014). A cis-element responsible for cGMP in the promoter of the soybean chalcone synthase gene. Plant Physiol. Bioch. 74, 92–98. doi: 10.1016/j.plaphy.2013.10.034
Agati, G., Matteini, P., Goti, A., Tattini, M. (2007). Chloroplast-located flavonoids can scavenge singlet oxygen. New Phytol. 174, 77–89. doi: 10.1111/j.1469-8137.2007.01986.x
Agati, G., Azzarello, E., Pollastri, S., Tattini, M. (2012). Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 196, 67–76. doi: 10.1016/j.plantsci.2012.07.014
Aguan, K., Sugawara, K., Suzuki, N., Kusano, T. (1991). Isolation of genes for low-temperature-induced proteins in rice by a simple subtractive method. Plant Cell Physiol. 32, 1285–1289. doi: 10.1093/oxfordjournals.pcp.a078207
Aguan, K., Sugawara, K., Suzuki, N., Kusano, T. (1993). Low-temperature-dependent expression of a rice gene encoding a protein with a leucine-zipper motif. Mol. Gen. Genet. 240, 1–8. doi: 10.1007/bf00276876
Akagi, T., Katayama-Ikegami, A., Kobayashi, S., Sato, A., Kono, A., Yonemori, K. (2012). Seasonal abscisic acid signal and a basic leucine zipper transcription factor, DkbZIP5, regulate proanthocyanidin biosynthesis in persimmon fruit. Plant Physiol. 158, 1089–1102. doi: 10.1104/pp.111.191205
Akashi, T., Fukuchi-Mizutani, M., Aoki, T., Ueyama, Y., Yonekura-Sakakibara, K., Tanaka, Y., et al. (1999). Molecular cloning and biochemical characterization of a novel cytochrome P450, flavone synthase II, that catalyzes direct conversion of flavanones to flavones. Plant Cell Physiol. 40, 1182–1186. doi: 10.0000/PMID10635120
Ali, Z., Sarwat, S. S., Karim, I., Rabia, F., Jaskani, M. J., Khan, A. A. (2016). Functions of plant’s bZIP transcription factors. Pak. J. Agr. Sci. 53, 303–314. doi: 10.21162/PAKJAS/16.2043
Amir Hossain, M., Lee, Y., Cho, J., II, Ahn, C. H., Lee, S. K., Jeon, J. S., et al. (2010). The bZIP transcription factor OsABF1 is an ABA responsive element binding factor that enhances abiotic stress signaling in rice. Plant Mol. Biol. 72, 557–566. doi: 10.1007/s11103-009-9592-9
An, J. P., Qu, F. J., Yao, J. F., Wang, X. N., You, C. X., Wang, X. F., et al. (2017a). The bZIP transcription factor MdHY5 regulates anthocyanin accumulation and nitrate assimilation in apple. Hortic. Res-England. 4, 17023–17030. doi: 10.1038/hortres.2017.56
An, J. P., Yao, J. F., Wang, X. N., You, C. X., Wang, X. F., Hao, Y. J. (2017b). MdHY5 positively regulates cold tolerance via CBF-dependent and CBF-independent pathways in apple. J. Plant Physiol. 218, 275–281. doi: 10.1016/j.jplph.2017.09.001
Aoki, T., Akashi, T., Ayabe, S. (2000). Flavonoids of leguminous plants: structure, biological activity, and biosynthesis. J. Plant Res. 113, 475–488. doi: 10.1007/pl00013958
Azeem, F., Tahir, H., Ijaz, U., Shaheen, T. (2020). A genome-wide comparative analysis of bZIP transcription factors in G. arboreum and G. raimondii (Diploid ancestors of present-day cotton). Physiol. Mol. Biol. Plants. 26, 433–444. doi: 10.1007/s12298-020-00771-9
Bailey, T. L., Boden, M., Buske, F. A., Frith, M., Grant, C. E., Clementi, L., et al. (2009). MEME Suite: Tools for motif discovery and searching. Nucleic Acids Res. 37, W202–W208. doi: 10.1093/nar/gkp335
Baloglu, M. C., Eldern, V., Hajyzadeh, M., Unver, T. (2014). Genome-wide analysis of the bZIP transcription factors in cucumber. PloS One 9, e96014. doi: 10.1371/journal.pone.0096014
Banerjee, A., Roychoudhury, A. (2017). Abscisic-acid-dependent basic leucine zipper (bZIP) transcription factors in plant abiotic stress. Protoplasma 254, 3–16. doi: 10.1007/s00709-015-0920-4
Bray, E. A. (1997). Plant responses to water deficit. Trends Plant Sci. 2, 48–54. doi: 10.1016/S1360-1385(97)82562-9
Buer, C. S., Muday, G. K. (2004). The transparent testa4 mutation prevents flavonoid synthesis and alters auxin transport and the response of Arabidopsis roots to gravity and light. Plant Cell. 16, 1191–1205. doi: 10.1105/tpc.020313
Cai, W. T., Yang, Y. L., Wang, W. W., Guo, G. Y., Liu, W., Bi, C. L. (2018). Overexpression of a wheat (Triticum aestivum L.) bZIP transcription factor gene, TabZIP6, decreased the freezing tolerance of transgenic Arabidopsis seedlings by down-regulating the expression of CBFs. Plant Physiol. Bioch. 124, 100–111. doi: 10.1016/j.plaphy.2018.01.008
Casaretto, J., Ho, T. H. D. (2003). The transcription factors HvABI5 and HvVP1 are required for the ABA induction of gene expression in burley aleurone cells. Plant Cell. 15, 271–284. doi: 10.1105/tpc.007096
Chang, Y., Nguyen, B. H., Xie, Y. J., Xiao, B. Z., Tang, N., Zhu, W. L., et al. (2017). Co-overexpression of the constitutively active form of OsbZIP46 and ABA-activated protein kinase SAPK6 improves drought and temperature stress resistance in rice. Front. Plant Sci. 8:1102:1102. doi: 10.3389/fpls.2017.01102
Chang, H. C., Tsai, M. C., Wu, S. S., Chang, I. F. (2019). Regulation of ABI5 expression by ABF3 during salt stress responses in Arabidopsis thaliana. Bot. Stud. 60, 16–30. doi: 10.1186/s40529-019-0264-z
Chen, H., Chen, W., Zhou, J. L., He, H., Chen, L. B., Chen, H. D., et al. (2012). Basic leucine zipper transcription factor OsbZIP16 positively regulates drought resistance in rice. Plant Sci. 193–194, 8–17. doi: 10.1016/j.plantsci.2012.05.003
Cheng, C., Yun, K. Y., Ressom, H. W., Mohanty, B., Bajic, V. B., Jia, Y., et al. (2007). An early response regulatory cluster induced by low temperature and hydrogen peroxide in seedlings of chilling-tolerant japonica rice. BMC Genomics 8, 175. doi: 10.1186/1471-2164-8-175
Cheng, L. B., Li, S. Y., Hussain, J., Xu, X. Y., Yin, J. J., Zhang, Y., et al. (2013). Isolation and functional characterization of a salt responsive transcriptional factor, LrbZIP from lotus root (Nelumbo nucifera Gaertn). Mol. Biol. Rep. 40, 4033–4045. doi: 10.1007/s11033-012-2481-3
Choi, H., II, Hong, J. H., Ha, J. O., Kang, J. Y., Kim, S. Y. (2000). ABFs, a family of ABA-responsive element binding factors. J. Biol. Chem. 275, 1723–1730. doi: 10.1074/jbc.275.3.1723
Choudhury, S., Panda, P., Sahoo, L., Panda, S. K. (2013). Reactive oxygen species signaling in plants under abiotic stress. Plant Signal. Behav. 8, e23681. doi: 10.4161/psb.23681
Corrêa, L. G. G., Riaño-Pachón, D. M., Schrago, C. G., dos Santos, R. V., Mueller-Roeber, B., Vincentz, M. (2008). The role of bZIP transcription factors in green plant evolution: adaptive features emerging from four founder genes. PloS One 3, e2944. doi: 10.1371/journal.pone.0002944
Das, P., Lakra, N., Nutan, K. K., Singla-Pareek, S. L., Pareek, A. (2019). A unique bZIP transcription factor imparting multiple stress tolerance in Rice. Rice (N. Y.). 12, 58–74. doi: 10.1186/s12284-019-0316-8
Dash, M., Yordan, Y. S., Georgieva, T., Tschaplinski, T. J., Yordanova, E., Busov, V. (2017). Poplar PtabZIP1-like enhances lateral root formation and biomass growth under drought stress. Plant J. 89, 692–705. doi: 10.1111/tpj.13413
de Vetten, N. C., Ferl, R. J. (1995). Characterization of a maize G-box binding factor that is induced by hypoxia. Plant J. 7, 589–601. doi: 10.1046/j.1365-313X.1995.7040589.x
Debeaujon, I., Léon-kloosterziel, K. M., Koornneef, M. (2000). Influence of the testa on seed dormancy, germination, andlongevity in Arabidopsis. Plant Physiol. 122, 403–414. doi: 10.1104/pp.122.2.403
Dey, A., Samanta, M. K., Gayen, S., Sen, S. K., Maiti, M. K. (2016). Enhanced gene expression rather than natural polymorphism in coding sequence of the OsbZIP23 determines drought tolerance and yield improvement in rice genotypes. PloS One 11, e0150763. doi: 10.1371/journal.pone.0150763
Du, H., Huang, Y. B., Tang, Y. X. (2010). Genetic and metabolic engineering of isoflavonoid biosynthesis. Appl. Microbiol. Biot. 86, 1293–1312. doi: 10.1007/s00253-010-2512-8
Du, H., Yang, S. S., Liang, Z., Feng, B. R., Liu, L., Huang, Y. B., et al. (2012). Genome-wide analysis of the MYB transcription factor superfamily in soybean. BMC Plant Biol. 12, 1–22. doi: 10.1186/1471-2229-12-106
Ellenberger, T. E., Brandl, C. J., Struhl, K., Harrison, S. C. (1992). The GCN4 basic region leucine zipper binds DNA as a dimer of uninterrupted alpha helices: crystal structure of the protein-DNA complex. Cell 71, 1223–1237. doi: 10.1016/s0092-8674(05)80070-4
Falcone Ferreyra, M. L., Rius, S. P., Casati, P. (2012). Flavonoids: biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 3, 222. doi: 10.3389/fpls.2012.00222
Fan, L. X., Xu, L., Wang, Y., Tang, M. J., Liu, L.,. W. (2019). Genome- and transcriptome-wide characterization of bZIP gene family identifies potential members involved in abiotic stress response and anthocyanin biosynthesis in Radish (Raphanus sativus L.). Int. J. Mol. Sci. 20, 6334. doi: 10.3390/ijms20246334
Fasano, R., Gonzalez, N., Tosco, A., Dal Piaz, F., Docimo, T., Serrano, R., et al. (2014). Role of Arabidopsis UV RESISTANCE LOCUS 8 in plant growth reduction under osmotic stress and low levels of UV-B. Mol. Plant 7, 773–791. doi: 10.1093/mp/ssu002
Fujita, Y., Fujita, M., Satoh, R., Maruyama, K., Parvez, M. M., Seki, M., et al. (2005). AREB1 is a transcription activator of novel ABRE-dependent ABA signaling that enhances drought stress tolerance in Arabidopsis. Plant Cell. 17, 3470–3488. doi: 10.1105/tpc.105.035659
Furihata, T., Maruyama, K., Fujita, Y., Umczawa, T., Yoshida, R., Shinozaki, K., et al. (2006). Abscisic acid-dependent multisite phosphorylation regulates the activity of a transcription activator AREB1. P. Natl. Acad. Sci. USA. 103, 1988–1993. doi: 10.1073/pnas.0505667103
Gai, W. X., Ma, X., Qiao, Y. M., Shi, B. H., U. Haq, S., Li, Q. H., et al. (2020). Characterization of the bZIP transcription factor family in pepper (Capsicum annuum L.): CabZIP25 positively modulates the salt tolerance. Front. Plant Sci. 11, 139. doi: 10.3389/fpls.2020.00139
Gao, S. Q., Chen, M., Xu, Z. S., Zhao, C. P., Li, L. C., Xu, H. J., et al. (2011). The soybean GmbZIP1 transcription factor enhances multiple abiotic stress tolerances in transgenic plants. Plant Mol. Biol. 75, 537–553. doi: 10.1007/s11103-011-9738-4
Hernández, I., Alegre, L., Van Breusegem, F., Munné-Bosch, S. (2009). How relevant are flavonoids as antioxidants in plants? Front. Plant Sci. 14, 125–132. doi: 10.1016/j.tplants.2008.12.003
Hossain, M. A., Cho, J., II, Han, M., Ahn, C. H., Jeon, J. S., An, G., et al. (2010). The ABRE-binding bZIP transcription factor OsABF2 is a positive regulator of abiotic stress and ABA signaling in rice. J. Plant Physiol. 167, 1512–1520. doi: 10.1016/j.jplph.2010.05.008
Hsieh, T. H., Li, C. W., Su, R. C., Cheng, C. P., Sanjaya, Tsai, Y. C., et al. (2010). A tomato bZIP transcription factor, SlAREB, is involved in water deficit and salt stress response. Planta 231, 1459–1473. doi: 10.1007/s00425-010-1147-4
Huang, X. S., Liu, J. H., Chen, X. J. (2010). Overexpression of PtrABF gene, a bZIP transcription factor isolated from Poncirus trifoliate, enhances dehydration and drought tolerance in tobacco via scavenging ROS and modulating expression of stress-responsive genes. BMC Plant Biol. 10, 230. doi: 10.1186/1471-2229-10-230
Huang, C. J., Zhou, J. H., Jie, Y. C., Xing, H. C., Zhong, Y. L., Yu, W. L., et al. (2016). A ramie bZIP transcription factor BnbZIP2 is involved in drought, salt, and heavy metal stress response. DNA Cell Biol. 35, 776–786. doi: 10.1089/dna.2016.3251
Hwang, I., Jung, H. J., Park, J., II, Yang, T. J., Nou, I. S. (2014). Transcriptome analysis of newly classified bZIP transcription factors of Brassica rapa in cold stress response. Genomics 104, 194–202. doi: 10.1016/j.ygeno.2014.07.008
Ito, K., Kusano, T., Tsutsumi, K., II (1999). A cold-inducible bZIP protein gene in radish root regulated by calcium- and cycloheximide-mediated signals. Plant Sci. 142, 57–65. doi: 10.1016/S0168-9452(98)00250-7
Izawa, T., Foster, R., Chua, N. H. (1993). Plant bZIP protein DNA binding specificity. J. Mol. Biol. 230, 1131–1144. doi: 10.1006/jmbi.1993.1230
Jakoby, M., Weisshaar, B., Droge-Laser, W., Vicente-Carbajosa, J., Tiedemann, J., Kroj, T., et al. (2002). bZIP transcription factors in Arabidopsis. Trends Plant Sci. 7, 106–111. doi: 10.1016/S1360-1385(01)02223-3
Ji, X. Y., Liu, G. F., Liu, Y. H., Zheng, L., Nie, X. G., Wang, Y. C. (2013). The bZIP protein from Tamarix hispida, ThbZIP1, is ACGT elements binding factor that enhances abiotic stress signaling in transgenic Arabidopsis. BMC Plant Biol. 13, 151. doi: 10.1186/1471-2229-13-151
Jiang, Y. N., Wang, B., Li, H., Yao, L. M., Wu, T. L. (2010). Flavonoid production is effectively regulated by RNA1 interference of two flavone synthase genes from Glycine max. J. Plant Biol. 53, 425–432. doi: 10.1007/s12374-010-9132-9
Joo, H., Lim, C. W., Lee, S. C. (2019). Roles of pepper bZIP transcription factor CaATBZ1 and its interacting partner RING-type E3 ligase CaASRF1 in modulation of ABA signalling and drought tolerance. Plant J. 100, 399–410. doi: 10.1111/tpj.14451
Joo, J. S., Lee, Y. H., Song, S., II (2019). OsbZIP42 is a positive regulator of ABA signaling and confers drought tolerance to rice. Planta 249, 1521–1533. doi: 10.1007/s00425-019-03104-7
Joo, H., Lim, C. W., Lee, S. C. (2020). The pepper RING-type E3 ligase, CaATIR1, positively regulates abscisic acid signalling and drought response by modulating the stability of CaATBZ1. Plant Cell Environ. doi: 10.1111/pce.13789
Kaminaka, H., Nake, C., Epple, P., Dittgen, J., Schütze, K., Chaban, C., et al. (2006). bZIP10-LSD1 antagonism modulates basal defense and cell death in Arabidopsis following infection. EMBO J. 25, 4400–4411. doi: 10.1038/sj.emboj.7601312
Kang, C., Zhai, H., He, S., Zhao, N., Liu, Q. (2019). A novel sweetpotato bZIP transcription factor gene, IbbZIP1, is involved in salt and drought tolerance in transgenic Arabidopsis. Plant Cell Rep. 38, 1373–1382. doi: 10.1007/s00299-019-02441-x
Kim, S., Kang, J. Y., Cho, D. L., Park, J. H., Kim, S. Y. (2004). ABF2, an ABRE-binding bZIP factor, is an essential component of glucose signaling and its overexpression affects multiple stress tolerance. Plant J. 40, 75–87. doi: 10.1111/j.1365-313x.2004.02192.x
Kobayashi, F., Maeta, E., Terashima, A., Takumi, S. (2008). Positive role of a wheat HvABI5 ortholog in abiotic stress response of seedlings. Physiol. Plantarum. 134, 74–86. doi: 10.1111/j.1399-3054.2008.01107.x
Kumar, S., Stecher, G., Tamura, K. (2016). MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874. doi: 10.1093/molbev/msw054
Lakra, N., Nutan, K. K., Das, P., Anwar, K., Singla-Pareek, S. L., Pareek, A. (2015). A nuclear-localized histone-gene binding protein from rice (OsHBP1b) functions in salinity and drought stress tolerance by maintaining chlorophyll content and improving the antioxidant machinery. J. Plant Physiol. 176, 36–46. doi: 10.1016/j.jplph.2014.11.005
Lam, P. Y., Zhu, F. Y., Chan, W. L., Liu, H., Lo, C. (2014). Cytochrome P450 93G1 is a flavone synthase II that channels flavanones to the biosynthesis of tricin O-linked conjugates in rice. Plant Physiol. 165, 1315–1327. doi: 10.1104/pp.114.239723
Landschulz, W. H., Johnson, P. F., McKnight, S. L. (1988). The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science 240, 1759–1764. doi: 10.1126/science.3289117
Lee, B. J., Park, C. J., Kim, S. K., Kim, K. J., Paek, K. H. (2006). In vivo binding of hot pepper bZIP transcription factor CabZIP1 to the G-box region of pathogenesis-related protein 1 promoter. Biochem. Bioph. Res. Co. 344, 55–62. doi: 10.1016/j.bbrc.2006.03.153
Lee, S. C., Choi, H. W., Hwang, I. S., Choi, D. S., Hwang, B. K. (2006). Functional roles of the pepper pathogen-induced bZIP transcription factor, CAbZIP1, in enhanced resistance to pathogen infection and environmental stresses. Planta 224, 1209–1225. doi: 10.2307/23389543
Li, J. Y., Ou-Lee, T. M., Raba, R., Amundson, R. G., Last, R. L. (1993). Arabidopsis flavonoid mutants are hypersensitive to UV-B irradiation. Plant Cell. 5, 171–179. doi: 10.2307/3869583
Li, J. S., Wang, X. M., Zhang, Y. L., Jia, H., Bi, Y. R. (2011). cGMP regulates hydrogen peroxide accumulation in calcium-dependent salt resistance pathway in Arabidopsis thaliana roots. Planta 234, 709–722. doi: 10.1007/s00425-011-1439-3
Li, D. Y., Fu, F. Y., Zhang, H. J., Song, F. M. (2015). Genome-wide systematic characterization of the bZIP transcriptional factor family in tomato (Solanum lycopersicum L.). BMC Genomic. 16, 60–78. doi: 10.1186/s12864-015-1990-6
Li, Y. Y., Meng, D., Li, M. J., Cheng, L. L. (2016). Genome-wide identification and expression analysis of the bZIP gene family in apple (Malus domestica). Tree Genet. Genomes. 12, 1–17. doi: 10.1007/s11295-016-1043-6
Li, Q., Wu, Q., Wang, A., Lv, B., Dong, Q., Yao, Y., et al. (2019). Tartary buckwheat transcription factor FtbZIP83 improves the drought/salt tolerance of Arabidopsis via an ABA-mediated pathway. Plant Physiol. Biochem. 144, 312–323. doi: 10.1016/j.plaphy.2019.10.003
Li, Q., Zhao, H., Wang, X., Kang, J., Lv, B., Dong, Q., et al. (2020). Tartary Buckwheat Transcription Factor FtbZIP5, Regulated by FtSnRK2.6, Can Improve Salt/Drought Resistance in Transgenic Arabidopsis. Int. J. Mol. Sci. 21, 1123. doi: 10.3390/ijms21031123
Lian, J. P., Lu, X. C., Yin, N. W., Ma, L. J., Lu, J., Liu, X., et al. (2017). Silencing of BnTT1 family genes affects seed flavonoid biosynthesis and alters seed fatty acid composition in Brassica napus. Plant Sci. 254, 32–47. doi: 10.1016/j.plantsci.2016.10.012
Liao, Y., Zou, H. F., Wei, W., Hao, Y. J., Tian, A. G., Huang, J., et al. (2008). Soybean GmbZIP44, GmbZIP62 and GmbZIP78 genes function as negative regulator of ABA signaling and confer salt and freezing tolerance in transgenic. Arabidopsis. Planta. 228, 225–240. doi: 10.1007/s00425-008-0731-3
Lilay, G. H., Castro, P. H., Guedes, J. G., Almeida, D. M., Campilho, A., Azevedo, H., et al. (2020). Rice F-bZIP transcription factors regulate the zinc deficiency response. J. Exp. Bot. 71, 3664–3677. doi: 10.1093/jxb/eraa115
Lim, C. W., Baek, W., Lee, S. C. (2018). Roles of pepper bZIP protein CaDILZ1 and its interacting partner RING-type E3 ligase CaDSR1 in modulation of drought tolerance. Plant J. 96, 452–467. doi: 10.1111/tpj.14046
Liu, C. J., Huhman, D., Sumner, L. W., Dixon, R. A. (2003). Regiospecific hydroxylation of isoflavones by cytochrome p450 81E enzymes from Medicago truncatula. Plant J. 36, 471–484. doi: 10.1046/j.1365-313X.2003.01893.x
Liu, J. X., Srivastava, R., Che, P., Howell, S. H. (2007). Salt stress in Arabidopsis utilize a signal transduction pathway related to endoplasmic reticulum stress signaling. Plant J. 51, 897–909. doi: 10.1111/j.1365-313x.2007.03195.x
Liu, J. X., Srivastava, R., Howell, S. H. (2008). Stress-induced expression of an activated form of AtbZIP17 provides protection from salt stress in Arabidopsis. Plant Cell Environ. 31, 1735–1743. doi: 10.1111/j.1365-3040.2008.01873.x
Liu, C. T., Wu, Y. B., Wang, X. P. (2012). bZIP transcription factor OsbZIP52/RISBZ5: a potential negative regulator of cold and drought stress response in rice. Planta 235, 1157–1169. doi: 10.1007/s00425-011-1564-z
Liu, C. T., Mao, B. G., Ou, S. J., Wang, W., Liu, L. C., Wu, Y. B., et al. (2014). OsbZIP71, a bZIP transcription factor, confers salinity and drought tolerance in rice. Plant Mol. Biol. 84, 19–36. doi: 10.1007/s11103-013-0115-3
Liu, J. Y., Chen, N. N., Chen, F., Cai, B., Dal Santo, S., Tornielli, G. B., et al. (2014). Genome-wide analysis and expression profile of the bZIP transcription factor gene family in grapevine (Vitis vinifera). BMC Genomics 15, 1–18. doi: 10.1186/1471-2164-15-281
Liu, C. T., Ou, S. J., Mao, B. G., Tang, J. Y., Wang, W., Wang, H. R., et al. (2018). Early selection of bZIP73 facilitated adaptation of japonica rice to cold climates. Nat. Commun. 9, 3302. doi: 10.1038/s41467-018-05753-w
Liu, C. T., Schläppi, M. R., Mao, B. R., Wang, W., Wang, A. J., Chu, C. C. (2019). The bZIP73 transcription factor controls rice cold tolerance at the reproductive stage. Plant Biotechnol. J. 17, 1834–1849. doi: 10.1111/pbi.13104
Liu, J., Chen, X., Wang, S., Wang, Y., Ouyang, Y., Yao, Y., et al. (2019). MeABL5, an ABA insensitive 5-Like Basic Leucine Zipper transcription factor, positively regulates MeCWINV3 in Cassava (Manihot esculenta Crantz). Front. Plant Sci. 10, 772. doi: 10.3389/fpls.2019.00772
Liu, J. Y., Chu, J. J., Ma, C. J., Jiang, Y. T., Ma, Y. C., Xiong, J. S., et al. (2019). Overexpression of an ABA-dependent grapevine bZIP transcription factor, VvABF2, enhances osmotic stress in Arabidopsis. Plant Cell Rep. 38, 587–596. doi: 10.1007/s00299-019-02389-y
Liu, M., Wen, Y., Sun, W., Ma, Z., Huang, L., Wu, Q., et al. (2019). Genome-wide identification, phylogeny, evolutionary expansion and expression analyses of bZIP transcription factor family in tartaty buckwheat. BMC Genomics 20, 483–493. doi: 10.1186/s12864-019-5882-z
Liu, Y., Chai, M., Zhang, M., He, Q., Su, Z., Priyadarshani, S., et al. (2020). Genome-wide analysis, characterization, and expression profile of the basic leucine zipper transcription factor family in pineapple. Int. J. Genomics 2020, 1–13. doi: 10.1155/2020/3165958
Lu, G. J., Gao, C. X., Zheng, X. N., Han, B. (2009). Identification of OsbZIP72 as a positive regulator of ABA response and drought tolerance in rice. Planta 229, 605–615. doi: 10.1007/s00425-008-0857-3
Lu, Y. H., Lam, H. M., Pi, E. X., Zhan, Q. L., Tsai, S. N., Wang, C. M., et al. (2013). Comparative metabolomics in Glycine max and Glycine soja under salt stress to reveal the phenotypes of their offspring. J. Agr. Food Chem. 61, 8711–8721. doi: 10.1021/jf405681k
Malacarne, G., Coller, E., Czemmel, S., Vrhovsek, U., Engelen, K., Goremykin, V., et al. (2016). The grapevine VvibZIPC22 transcription factor is involved in the regulation of flavonoid biosynthesis. J. Exp. Bot. 67, 3509–3522. doi: 10.1093/jxb/erw181
Matousek, J., Kocabek, T., Patzak, J., Stehlík, J., Füssy, Z., Krofta, K., et al. (2010). Cloning and molecular analysis of HlbZip1 and HlbZip2 transcription factors putatively involved in the regulation of the lupulin metabolome in Hop (Humulus lupulus L.). J. Agr. Food Chem. 58, 902–912. doi: 10.1021/jf9043106
McKhann, H., II, Hirsch, A. M. (1994). Isolation of chalcone synthase and chalcone isomerase cDNAs from alfalfa (Medicago sativa L.): highest transcript levels occur in young roots and root tips. Plant Mol. Biol. 24, 767–777. doi: 10.1007/BF00029858
Mellway, R. D., Tran, L. T., Prouse, M. B., Campbell, M. M., Constabel, C. P. (2009). The wound-, pathogen-, and ultraviolet B-responsive MYB134 gene encodes an R2R3 MYB transcription factor that regulates proanthocyanidin synthesis in poplar. Plant Physiol. 150, 924–941. doi: 10.1104/pp.109.139071
Miller, G., Shulaev, V., Mittler, R. (2008). Reactive oxygen signaling and abiotic stress. Physiol. Plantarum. 133, 481–489. doi: 10.1111/j.1399-3054.2008.01090.x
Mola, J., Grotewold, E., Koesa, R. (1998). How genes paintflowers and seeds. Trends Plant Sci. 3, 212–217. doi: 10.1016/S1360-1385(98)01242-4
Mukherjee, K., Choudhury, A. R., Gupta, B., Gupta, S., Sengupta, D. N. (2006). An ABRE-binding factor, OSBZ8, is highly expressed in salt tolerant cultivars than in salt sensitive cultivars of indica rice. BMC Plant Biol. 6, 1–14. doi: 10.1186/1471-2229-6-18
Munns, R. (2005). Genes and salt tolerance: bringing them together. New Phytol. 167, 645–663. doi: 10.1111/j.1469-8137.2005.01487.x
Nakagawa, H., Ohmiya, K., Hattori, T. (1996). A rice bZIP protein, designated OSBZ8, is rapidly induced by abscisic acid. Plant J. 9, 217–227. doi: 10.1046/j.1365-313X.1996.09020217.x
Nakashima, K., Ito, Y., Yamaguchi-Shinozaki, K. (2009). Transcriptional regulatory networks in response to abiotic stresses in Arabidopsis and grasses. Plant Physiol. 149, 88–95. doi: 10.1104/pp.108.129791
Nijhawan, A., Jain, M., Tyagi, A. K., Khurana, J. P. (2008). Genomic survey and gene expression analysis of the basic leucine zipper transcription factor family in rice. Plant Physiol. 146, 333–350. doi: 10.1104/pp.107.11282
Pan, Y. L., Hu, X., Li, C. Y., Xu, X., Su, C. G., Li, J. H., et al. (2017). SlbZIP38, a tomato bZIP family gene downregulated by abscisic acid, is a negative regulator of drought and salt stress tolerance. Genes 8, 402. doi: 10.3390/genes8120402
Pandey, A. S., Sharma, E., Jain, N., Singh, B., Burman, N., Khurana, J. P. (2018). A rice bZIP transcription factor, OsbZIP16, regulates abiotic stress tolerance when over-expressed in Arabidopsis. J. Plant Biochem. Biot. 27, 393–400. doi: 10.1007/s13562-018-0448-8
Pérez-Rodriguez, P., Riaño-Pachon, D. M., Corrêa, L. G., Rensing, S. A., Kersten, B., Mueller-Roeber, B. (2010). PlnTFDB: updated content and new features of the plant transcription factor database. Nucleic Acids Res. 38 (Database issue), D822–D827. doi: 10.1093/nar/gkp805
Pi, E. X., Qu, L. Q., Hu, J. W., Huang, Y. Y., Qiu, L. J., Lu, H. F., et al. (2016). Mechanisms of soybean roots’ tolerances to salinity revealed by proteomic and phosphoproteomic comparisons between two cultivars. Mol. Cell. Proteomics. 15, 266–288. doi: 10.1074/mcp.M115.051961
Pi, E. X., Zhu, C. M., Fan, W., Huang, Y. Y., Qu, L. Q., Li, Y. Y., et al. (2018). Quantitative phosphoproteomic and metabolomic analyses reveal GmMYB173 optimizes flavonoid metabolism in soybean under salt stress. Mol. Cell. Proteomics. 17, 1209–1224. doi: 10.1074/mcp.RA117.000417
Pi, E. X., Xu, J., Li, H. H., Fan, W., Zhu, C. M., Zhang, T. Y., et al. (2019). Enhanced salt tolerance of rhizobia-inoculated soybean correlates with decreased phosphorylation of the transcription factor GmMYB183 and altered flavonoid biosynthesis. Mol. Cell. Proteomics. 18, 2225–2243. doi: 10.1074/mcp.RA119.001704
Pourabed, E., Golmohamadi, F. G., Monfared, P. S., Razavi, S. M., Shobbar, Z. S. (2015). Basic leucine zipper family in barley: genome-wide characterization of members and expression analysis. Mol. Biotechnol. 57, 12–26. doi: 10.1007/s12033-014-9797-2
Pourcel, L., Routaboul, J. M., Cheynier, V., Lepiniec, L., Debeaujon, I. (2007). Flavonoid oxidation in plants: from biochemical properties to physiological functions. Trends Plant Sci. 12, 29–36. doi: 10.1016/j.tplants.2006.11.006
Qiu, D. Y., Xiao, J., Xie, W. B., Liu, H. B., Li, X. H., Xiong, L. Z., et al. (2008). Rice gene network inferred from expression profiling of plants overexpressing OsWRKY13, a positive regulator of disease resistance. Mol. Plant 1, 538–551. doi: 10.1093/mp/ssn012
Rai, A., Umashankar, S., Rai, M., Kiat, L. B., Bing, J. A., Swarup, S. (2016). Coordinate regulation of metabolite glycosylation and stress hormone biosynthesis by TT8 in Arabidopsis. Plant Physiol. 171, 2499–2515. doi: 10.1104/pp.16.00421
Rice-Evans, C. A., Miller, N. J. (2010). ChemInform Abstract: Structure-antioxidant activity relationships of flavonoids and isoflavonoids. Antioxid. Health Dis. 29, 199–219. doi: 10.1002/chin.199819287
Riechmann, J. L., Heard, J., Martin, G., Reuber, L., Jiang, C. Z., Keddie, J., et al. (2000). Arabidopsis transcription factors genome-wide comparative analysis among eukaryotes. Science 290, 2105–2110. doi: 10.1126/science.290.5499.2105
Rolly, N. K., Imran, Q. M., Lee, I. J., Yun, B. W. (2020). Salinity stress-mediated suppression of expression of salt overly sensitive signaling pathway genes suggests negative regulation by AtbZIP62 transcription factor in Arabidopsis thaliana. Int. J. Mol. Sci. 21, 1726–1739. doi: 10.3390/ijms21051726
Rong, S., Wu, Z., Cheng, Z., Zhang, S., Liu, H., Huang, Q. (2020). Genome-wide identification, evolutionary patterns, and expression analysis of bZIP gene family in olive (Olea europaea L.). Genes (Basel). 11, 510–532. doi: 10.3390/genes11050510
Rozema, J., Flowers, T. (2008). Crops for a salinized world. Science 322, 1478–1480. doi: 10.1126/science.1168572
Saitou, N., Nei, M. (1987). The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4, 406–425. doi: 10.1093/oxfordjournals.molbev.a040454
Satoh, R., Fujita, Y., Nakashima, K., Shinozaki, K., Yamaguchi-Shinozaki, K. (2004). A novel subgroup of bZIP proteins functions as transcriptional activators in hypoosmolarity-responsive expression of the ProDH gene in Arabidopsis. Plant Cell Physiol. 45, 309–317. doi: 10.1093/pcp/pch036
Schütze, K., Harter, K., Chaban, C. (2008). Post-translational regulation of plant bZIP factors. Trends Plant Sci. 13, 247–255. doi: 10.1016/j.tplants.2008.03.002
Shaikhali, J., Norén, L., Dios, de, Barajas-López, J., Srivastava, V., König, J., et al. (2012). Redox-mediated mechanisms regulate DNA binding activity of the G-group of basic region leucine zipper (bZIP) transcription factors in Arabidopsis. J. Biol. Chem. 287, 27510–27525. doi: 10.1074/jbc.M112.361394
Shi, Y. T., Ding, Y. L., Yang, S. H. (2018). Molecular regulation of CBF signaling in cold acclimation. Trends Plant Sci. 23, 623–637. doi: 10.1016/j.tplants.2018.04.002z
Shimizu, H., Sato, K., Berberich, T., Miyazaki, A., Ozaki, R., Imai, R., et al. (2005). LIP19, a basic region leucine zipper protein, is a Fos-like molecular switch in the cold signaling of rice plants. Plant Cell Physiol. 46, 1623–1634. doi: 10.1093/pcp/pci178
Shinozaki, K., Yamaguchi -Shinozaki, K. (2007). Gene networks involved in drought stress response and tolerance. J. Exp. Bot. 58, 221–227. doi: 10.1093/jxb/erl164
Shinozaki, K., Yamaguchi-Shinozaki, K. (1996). Molecular responses to drought and cold stress. Curr. Opin. Biotech. 7, 161–167. doi: 10.1016/B978-0-444-82884-2.50013-3
Song, S., Wang, G., Wu, H., Fan, X., Liang, L., Zhao, H., et al. (2020). OsMFT2 is involved in the regulation of ABA signaling-mediated seed germination through interacting with OsbZIP23/66/72 in rice. Plant J. 103, 532–546. doi: 10.1111/tpj.14748
Sornaraj, P., Luang, S., Lopato, S., Hrmova, M. (2016). Basic leucine zipper (bZIP) transcription factors involved in abiotic stresses: A molecular model of a wheat bZIP factor and implications of its structure in function. BBA-Biomembranes 1860, 46–56. doi: 10.1016/j.bbagen.2015.10.014
Stanković, B., Vian, A., Henry-Vian, C., Davies, E. (2000). Molecular cloing and characterization of a tomato cDNA encoding a systemically wound-inducible bZIP DNA binding protein. Planta 212, 60–66. doi: 10.1007/s004250000362
Sun, X. L., Li, Y., Cai, H., Bai, X., Ji, W., Ji, Z. J., et al. (2011). Arabidopsis bZIP1 transcription factor binding to ABRE cis-element regulates abscisic acid signal transduction. Acta Agronomica Sinica. 37, 612–619. doi: 10.1016/s1875-2780(11)60016-3
Tang, N., Zhang, H., Li, X. H., Xiao, J. H., Xiong, L. Z. (2012). Constitutive activation of transcription factor OsbZIP46 improves drought tolerance in rice. Plant Physiol. 158, 1755–1768. doi: 10.1104/pp.111.190389
Tang, W., Page, M., Fei, Y. J., Liu, L. C., Xu, F., Cai, X. D., et al. (2012). Overexpression of AtbZIP60deltaC gene alleviates salt-induced oxidative damage in transgenic cell cultures. Plant Mol. Biol. Rep. 30, 1183–1195. doi: 10.1007/s11105-012-0437-3
Tattini, M., Galardi, C., Pinelli, P., Massai, R., Remorini, D., Agati, G. (2004). Differential accumulation of flavonoids and hydroxycinnamates in leaves of Ligustrum vulgare under excess light and drought stress. New Phytol. 163, 547–561. doi: 10.1111/j.1469-8137.2004.01126.x
Tattini, M., Remorini, D., Pinelli, P., Agati, G., Saracini, E., Traversi, M. L., et al. (2006). Morpho-anatomical, physiological and biochemical adjustments in response to root zone salinity stress and high solar radiation in two Mediterranean evergreen shrubs, Myrtus communis and Pistacia lentiscus. New Phytol. 170, 779–794. doi: 10.1111/j.1469-8137.2006.01723.x
Thomashow, M. F. (1998). Role of cold-responsive genes in plant freezing tolerance. Plant Physiol. 118, 1–7. doi: 10.1104/pp.118.1.1
Treutter, D. (2005). Significance of flavonoids in plant resistance and enhancement of their biosynthesis. Plant Biol. 7, 581–591. doi: 10.1055/s-2005-873009
Treutter, D. (2006). Significance of flavonoids in plant resistance: a review. Environ. Chem. Lett. 4, 147–157. doi: 10.1007/s10311-006-0068-8
Tsugama, D., Liu, S., Takano, T. (2016). The bZIP protein VIP1 is involved in touch responses in Arabidopsis roots. Plant Physiol. 171, 1355–1365. doi: 10.1104/pp.16.00256
Tsugama, D., Yoon, H. S., Fujino, K., Liu, S., Takano, T. (2019). Protein phosphatase 2A regulates the nuclear accumulation of the Arabidopsis bZIP protein VIP1 under hypo-osmotic stress. J. Exp. Bot. 70, 6101–6112. doi: 10.1093/jxb/erz384
Uchida, K., Akashi, T., Aoki, T. (2015). Functional expression of cytochrome P450 in Escherichia coli: An approach to functional analysis of uncharacterized enzymes for flavonoid biosynthesis. Plant Biotechnol-Nar. 32, 205–213. doi: 10.5511/plantbiotechnology.15.0605a
Ueda, Y., Ohtsuki, N., Kadota, K., Tezuka, A., Nagano, A. J., Kadowaki, T., et al. (2020). Gene regulatory network and its constituent transcription factors that control nitrogen-deficiency responses in rice. New Phytol. 227, 1434–1452. doi: 10.1111/nph.16627
Uno, Y., Furuhata, T., Abe, H., Yoshida, R., Shinozaki, K., Yamaguchi-Shinozaki, K. (2000). Arabidopsis basic leucine zipper transcription factors involved in an abscisic acid-dependent signal transduction pathway under drought and high-salinity conditions. P. Natl. Acad. Sci. USA. 97, 11632–11637. doi: 10.1073/pnas.190309197
Van Leene, J., Blomme, J., Kulkarni, S. R., Cannoot, B., De Winne, N., Eeckhout, D., et al. (2016). Functional Characterization of the Arabidopsis transcription factor bZIP29 reveals its role in leaf and root development. J. Exp. Bot. 67, 5825–5840. doi: 10.1093/jxb/erw347
Veerabagu, M., Kirchler, T., Elgass, K., Stadelhofer, B., Stahl, M., Harter, K., et al. (2014). The interaction of the Arabidopsis response regulator ARR18 with bZIP63 mediates the regulation of PROLINE DEHYDROGENASE expression. Mol. Plant 7, 1560–1577. doi: 10.1093/mp/ssu074
Verslues, P. E., Zhu, J. K. (2005). Before and beyond ABA: Upstream sensing and internal signals that determine ABA accumulation and response under abiotic stress. Biochem. Soc T. 33 (Pt 2), 375–379. doi: 10.1042/BST0330375
Wang, Y. C., Gao, C. Q., Liang, Y. N., Wang, C., Yang, C. P., Liu, G. F. (2010). A novel bZIP gene from Tamarix hispida mediates physiological responses to salt stress in tobacco plants. J. Plant Physiol. 167, 222–230. doi: 10.1016/j.jplph.2009.09.008
Wang, J. Z., Zhou, J. X., Zhang, B. L., Vanitha, J., Ramachandran, S., Jiang, S. Y. (2011). Genome-wide expansion and expression divergence of the basic leucine zipper transcriptionfactors in higher plants with an emphasis on sorghum. J. Intehr. Plant Biol. 53, 212–231. doi: 10.1111/j.1744-7909.2010.01017.x
Wang, Z. H., Cheng, K., Wan, L. Y., Yan, L. Y., Jiang, H. F., Liu, S. Y., et al. (2015). Genome-wide analysis of the basic leucine zipper (bZIP) transcription factor gene family in six legume genomes. BMC Genomics 16, 1053. doi: 10.1186/s12864-015-2258-x
Wang, J., Li, Q., Mao, X. G., Li, A., Jing, R. L. (2016). Wheat transcription factor TaAREB3 participates in drought and freezing tolerances in Arabidopsis. Int. J. Biol. Sci. 12, 257–269. doi: 10.7150/ijbs.13538
Wang, Z., Su, G. X., Li, M., Ke, Q. B., Kim, S. Y., Li, H. B., et al. (2016). Overexpressing Arabidopsis ABF3 increases tolerance to multiple abiotic stresses and reduces leaf size in alfalfa. Plant Physiol. Bioch. 109, 199–208. doi: 10.1016/j.plaphy.2016.09.020
Wang, C. L., Lu, G. Q., Hao, Y. Q., Guo, H. M., Guo, Y., Zhao, J., et al. (2017). ABP9, a maize bZIP transcription factor, enhances tolerance to salt and drought in transgenic cotton. Planta 246, 453–469. doi: 10.1007/s00425-017-2704-x
Wang, L., Cao, H. L., Qian, W. J., Yao, L. N., Hao, X. Y., Li, N. N., et al. (2017). Identification of a novel bZIP transcription factor in Camellia sinensis as a negative regulator of freezing tolerance in transgenic Arabidopsis. Ann. Bot-London. 119, 1195–1209. doi: 10.1093/aob/mcx011
Wang, W., Qiu, X. P., Yang, Y. X., Kim, H. S., Jia, X. Y., Yu, H., et al. (2019). Sweetpotato bZIP transcription factor IbABF4 confers tolerance to multiple abiotic stresses. Front. Plant Sci. 10, 630. doi: 10.3389/fpls.2019.00630
Wang, W., Wang, Y., Zhang, S., Xie, K., Zhang, C., Xi, Y., et al. (2020). Genome-wide analysis of the abiotic stress-related bZIP family in switchgrass. Mol. Biol. Rep. 47, 4439–4454. doi: 10.1007/s11033-020-05561-w
Watkins, J. M., Hechler, P. J., Muday, G. K. (2014). Ethylene-induced flavonol accumulation in guard cells suppresses reactive oxygen species and moderates stomatal aperture. Plant Physiol. 164, 1707–1717. doi: 10.1104/pp.113.233528
Wei, K., Chen, J., Wang, Y., Chen, Y., Chen, S., Lin, Y., et al. (2012). Genome-wide analysis of bZIP-encoding genes in maize. DNA Res. 19, 463–476. doi: 10.1093/dnares/dss026
Xiang, Y., Tang, N., Du, H., Ye, H. Y., Xiong, L. Z. (2008). Characterization of OsbZIP23 as a key player of the basic leucine zipper transcription factor family for conferring abscisic acid sensitivity and salinity and drought tolerance in rice. Plant Physiol. 148, 1938–1952. doi: 10.1104/pp.108.128199
Xu, D. B., Gao, S. Q., Ma, Y. Z., Xu, Z. S., Zhao, C. P., Tang, Y. M., et al. (2014). ABI-like transcription factor gene TaABL1 from wheat improves multiple abiotic stress tolerances in transgenic plants. Funct. Integr. Genomic. 14, 717–730. doi: 10.1007/s10142-014-0394-z
Yamasaki, H., Sakihama, Y., Ikehara, N. (1997). Flavonoid-peroxidase reaction as a detoxification mechanism of plant cells against H2O2. Plant Physiol. Bioch. 115, 1405–1412. doi: 10.1104/pp.115.4.1405
Yan, J. H., Wang, B., Jiang, Y. N., Cheng, L. J., Wu, T. L. (2014). GmFNSII-controlled soybean flavone metabolism responds to abiotic stresses and regulates plant salt tolerance. Plant Cell Physiol. 55, 74–86. doi: 10.1093/pcp/pct159
Yang, O., Popova, O. V., Süthoff, U., Lüking, I., Dietz, K. J., Golldack, D. (2009). The Arabidopsis basic leucine zipper transcription factor AtbZIP24 regulates complex transcriptional networks involved in abiotic stress resistance. Gene 436, 45–55. doi: 10.1016/j.gene.2009.02.010
Yang, Y. G., Lv, W. T., Li, M. J., Wang, B., Sun, D. M., Deng, X. (2013). Maize membrane-bound transcription factor Zmbzip17 is a key regulator in the cross-talk of ER quality control and ABA signaling. Plant Cell Physiol. 54, 2020–2033. doi: 10.1093/pcp/pct142
Yang, S., Xu, K., Chen, S., Li, T., Xia, H., Chen, L., et al. (2019). A stress-responsive bZIP transcription factor OsbZIP62 improves drought and oxidative tolerance in rice. BMC Plant Biol. 19, 260–275. doi: 10.1186/s12870-019-1872-1
Yang, Y., Li, J., Li, H., Yang, Y., Guang, Y., Zhou, Y. (2019). The bZIP gene family in watermelon: genome-wide identification and expression analysis under cold stress and root-knot nematode infection. PeerJ 7, e7878. doi: 10.7717/peerj.7878
Yang, Y., Yu, T. F., Ma, J., Chen, J., Zhou, Y. B., Chen, M., et al. (2020). The soybean bZIP transcription factor gene GmbZIP2 confers drought and salt resistances in transgenic plants. Int. J. Mol. Sci. 21, 670. doi: 10.3390/ijms21020670
Yao, L., Hao, X., Cao, H., Ding, C., Yang, Y., Wang, L., et al. (2020). ABA-dependent bZIP transcription factor, CsbZIP18, from Camellia sinensis negatively regulates freezing tolerance in Arabidopsis. Plant Cell Rep. 39, 553–565. doi: 10.1007/s00299-020-02512-4
Yoon, M. K., Shin, J., Choi, G., Choi, B. S. (2006). Intrinsically unstructured N-terminal domain of bZIP transcription factor HY5. Proteins 65, 856–866. doi: 10.1002/prot.21089
Yoshida, T., Fujita, Y., Maruyama, K., Mogami, J., Todaka, D., Shinozaki, K., et al. (2015). Four Arabidopsis AREB/ABF transcription factors function predominantly in gene expression downstream of SnRK2 kinases in abscisic acid signalling in response to osmotic stress. Plant Cell Environ. 38, 35–49. doi: 10.1111/pce.12351
Zhang, X., Wang, L., Meng, H., Wen, H. T., Fan, Y., Zhao, J. (2011). Maize ABP9 enhances tolerance to multiple stresses in transgenic Arabidopsis by modulating ABA signaling and cellular levels of reactive oxygen species. Plant Mol. Biol. 75, 365–378. doi: 10.1007/s11103-011-9732-x
Zhang, Y. Q., Zheng, S., Liu, Z. J., Wang, L. G., Bi, Y. R. (2011). Both HY5 and HYH are necessary regulators for low temperature-induced anthocyanin accumulation in Arabidopsis seedlings. J. Plant Physiol. 168, 367–374. doi: 10.1016/j.jplph.2010.07.025
Zhang, F. Y., Fu, X. Q., Lv, Z. Y., Lu, X., Shen, Q., Zhang, L., et al. (2015). A basic leucine zipper transcription factor, AabZIP1, connects abscisic acid signaling with artemisinin biosynthesis in Artemisia annua. Mol. Plant 8, 163–175. doi: 10.1016/j.molp.2014.12.004
Zhang, L. N., Zhang, L., Xia, C., Zhao, G. Y., Liu, J., Jia, J. Z., et al. (2015). A novel wheat bZIP transcription factor, TabZIP60, confers multiple abiotic stress tolerances in transgenic Arabidopsis. Physiol. Plantarum 153, 538–554. doi: 10.1111/ppl.12261
Zhang, C. Y., Li, C., Liu, J., Lv, Y. D., Yu, C. S., Li, H. Y., et al. (2017). The OsABF1 transcription factor improves drought tolerance by activating the transcription of COR413-TM1 in rice. J. Exp. Bot. 68, 4695–4707. doi: 10.1093/jxb/erx260
Zhang, L. N., Zhang, L. C., Xia, C., Gao, L. F., Hao, C. Y., Zhao, G. Y., et al. (2017). A Novel Wheat C-bZIP Gene, TabZIP14-B, Participates in Salt and Freezing Tolerance in Transgenic Plants. Front. Plant Sci. 8, 710. doi: 10.3389/fpls.2017.00710
Zhang, M., Liu, Y. H., Shi, H., Guo, M. L., Chai, M. N., He, Q., et al. (2018). Evolutionary and expression analyses of soybean basic leucine zipper transcription factor family. BMC Genomics 19, 159. doi: 10.1186/s12864-018-4511-6
Zhang, L., Xie, J., Wang, L., Si, L., Zheng, S., Yang, Y., et al. (2020). Wheat TabZIP8, 9, 13 participate in ABA biosynthesis in NaCl-stressed roots regulated by TaCDPK9-1. Plant Physiol. Biochem. 151, 650–658. doi: 10.1016/j.plaphy.2020.03.039
Zhao, B. Y., Hu, Y. F., Li, J. J., Yao, X., Liu, K. D. (2016). BnaABF2, a bZIP transcription factor from rapeseed (Brassica napus L.), enhances drought and salt tolerance in transgenic Arabidopsis. Bot. Stud. 57, 12. doi: 10.1186/s40529-016-0127-9
Zhao, P., Ye, M., Wang, R., Wang, D., Chen, Q. (2020). Systematic identification and functional analysis of potato (Solanum tuberosum L.) bZIP transcription factors and overexpression of potato bZIP transcription factor StbZIP-65 enhances salt tolerance. Int. J. Biol. Macromol. 161, 155–167. doi: 10.1016/j.ijbiomac.2020.06.032
Zhu, J. K. (2002). Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 53, 247–273. doi: 10.1146/annurev.arplant.53.091401.143329
Zong, W., Tang, N., Yang, J., Peng, L., Ma, S. Q., Xu, Y., et al. (2016). Feedback regulation of ABA signaling and biosynthesis by a bZIP transcription factor targets drought-resistance-related genes. Plant Physiol. 171, 2810–2825. doi: 10.1104/pp.16.00469
Zong, N., Wang, H., Li, Z., Ma, L., Xie, L., Pang, J., et al. (2020). Maize NCP1 negatively regulates drought and ABA responses through interacting with and inhibiting the activity of transcription factor ABP9. Plant Mol. Biol. 102, 339–357. doi: 10.1007/s11103-019-00951-6
Zou, M. J., Guan, Y. C., Ren, H. B., Zhang, F., Chen, F. (2007). Characterization of alternative splicing products of bZIP transcription factors OsABI5. Biochem. Bioph. Res. Co. 360, 307–313. doi: 10.1016/j.bbrc.2007.05.226
Keywords: plant, basic leucine zipper transcription factor, cis-element, stress tolerance, flavonoid
Citation: Yu Y, Qian Y, Jiang M, Xu J, Yang J, Zhang T, Gou L and Pi E (2020) Regulation Mechanisms of Plant Basic Leucine Zippers to Various Abiotic Stresses . Front. Plant Sci. 11:1258. doi: 10.3389/fpls.2020.01258
Received: 14 May 2020; Accepted: 30 July 2020;
Published: 20 August 2020.
Edited by:
Rosa M. Rivero, Spanish National Research Council, SpainReviewed by:
Yimiao Tang, Beijing Academy of Agricultural and Forestry Sciences, ChinaDayong Li, Zhejiang University, China
Copyright © 2020 Yu, Qian, Jiang, Xu, Yang, Zhang, Gou and Pi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Erxu Pi, MjAxMzAwMTRAaHpudS5lZHUuY24=
 Yan Yu
Yan Yu Yuchen Qian
Yuchen Qian Mengyue Jiang
Mengyue Jiang Jia Xu
Jia Xu Jingting Yang
Jingting Yang Tongyao Zhang
Tongyao Zhang Erxu Pi
Erxu Pi