
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci. , 13 August 2020
Sec. Plant Abiotic Stress
Volume 11 - 2020 | https://doi.org/10.3389/fpls.2020.01251
Low temperature stress is one of the major causes of crop yield reduction in agriculture. The alteration of gene expression pattern and the accumulation of stress-related proteins are the main strategies activated by plants under this unfavourable condition. Here we characterize the Arabidopsis thaliana Salt Tolerance Related Protein (STRP). The protein rapidly accumulates under cold treatment, and this effect is not dependent on transcriptional activation of the STRP gene, but on the inhibition of proteasome-mediated degradation. Subcellular localization of STRP was determined by the transient expression of STRP-YFP in A. thaliana protoplasts. STRP is localized into the cytosol, nucleus, and associated to the plasma membrane. Under cold stress, the membrane-associated fraction decreases, while in the cytosol and in the nucleus STRP levels strongly increase. STRP has high similarity with WCI16, a wheat Late Embryogenesis Abundant (LEA)-like protein. Despite no canonical LEA motifs in the STRP sequence are present, physicochemical characterization demonstrated that STRP shares common features with LEA proteins, being a high hydrophilic unstructured protein, highly soluble after boiling and with cryoprotectant activity. To clarify the physiological function of STRP, we characterized the phenotype and the response to low temperature stress of the strp knockout mutant. The mutation causes an equal impairment of plant growth and development both in physiological and cold stress conditions. The strp mutant is more susceptible to oxidative damage respect to the wild type, showing increased lipid peroxidation and altered membrane integrity. Furthermore, the analysis of Abscisic acid (ABA) effects on protein levels demonstrated that the hormone induces the increase of STRP levels, an effect in part ascribable to its ability to activate STRP expression. ABA treatments showed that the strp mutant displays an ABA hyposensitive phenotype in terms of seed germination, root development, stomata closure and in the expression of ABA-responsive genes. In conclusion, our results demonstrate that STRP acts as a multifunctional protein in the response mechanisms to low temperature, suggesting a crucial role for this protein in stress perception and in the translation of extracellular stimuli in an intracellular response.
Low temperature is a major environmental factor that adversely affects survival, growth and development of plants, thus limiting their geographical distribution and affecting productivity and quality of crops (Sanghera et al., 2011). Over the course of evolution, plants have evolved a repertoire of physiological and biochemical responses to cope with cold stress, including alteration of metabolism, changes in membrane lipid composition, production of osmolytes and release of reactive oxygen species (ROS). These responses protect plants and are crucial for the acclimation to the low temperatures. Cold acclimation is a dynamic process that occurs at different levels, including the activation of specific subset of cold-responsive (COR) genes, mainly mediated by C-repeat (CRT)-binding factors (CBFs) transcription factors, also known as dehydration-responsive element (DRE)-binding proteins (DREBs), (Liu et al., 1998; Jia et al., 2016; Zhao et al., 2016). These transcription factors are rapidly induced by low temperature and recognize a specific CRT/DRE cis-element in the promoter of COR genes (Shi et al., 2018), that include genes encoding for enzymes of lipid metabolism and osmo-protectants biosynthesis, late embryogenesis abundant (LEA) proteins, transcription factors and proteins involved in hormone signalling (Liu et al., 2019). However, many COR genes lack the CRT/DRE sequence, supporting the existence of a CBF-independent regulation for these genes, during cold acclimation (Park et al., 2015; Zhao et al., 2016). The CBF-independent pathway is also mediated by both ABA-dependent and ABA-independent mechanisms (Huang et al., 2017). ABA is a key phytohormone in the regulation of many physiological processes and plays a crucial role in stress response (Verslues and Zhu, 2005; Fujii et al., 2009; Kim et al., 2016). Although ABA levels only slightly increase under cold stress (Lang and Palva, 1992), different evidence supports the involvement of this hormone in cold acclimation. About 10% of ABA-responsive genes are also activated by low temperature (Kreps et al., 2002), plants acquire freezing tolerance when treated with exogenous ABA (Kumar et al., 2008; Kim et al., 2016), and the ABA-deficient mutant aba-1 has cold acclimation impaired ability (Koornneef et al., 1982; Heino et al., 1990). In addition, several proteins induced by low temperature are also synthesized in response to ABA (Cattivelli and Bartels, 1992; Palva, 1994), suggesting that hormone biosynthesis and signalling are important for COR genes activation (Gilmour and Thomashow, 1991; Mantyla et al., 1995). In the last years, evidence for COR-independent pathways have been also provided (Liu et al., 2019). Nevertheless, these pathways remain largely uncharacterized.
Cold stress responses involve changes in the cellular protein profile and, in this background, several proteomic studies identified a multitude of differently represented proteins in different plant species subjected to low-temperature regimes (Bae et al., 2003; Amme et al., 2006; Li et al., 2011; Rocco et al., 2013).
Rocco et al. (2013) identified in Arabidopsis thaliana leaves 28 proteins whose levels were affected by short-term cold stress. Among these proteins, our attention was focused on a 16 kDa protein (TAIR accession At1g13930) whose levels are greatly increased (13-fold) under cold stress. The function of this protein is unknown, but it was related with salt tolerance. In fact, the amino acid sequence is 59% identical to ST6-66, a Thellungiella halofila protein that confers salt tolerance when overexpressed in A. thaliana (Du et al., 2008). Accordingly, the loss of function mutant is hypersensitive to salt stress, hence the protein name Salt Tolerance Related Protein (STRP) was proposed (Du et al., 2008).
Different pieces of evidence propose the involvement of STRP in the response mechanisms to abiotic stresses. The protein has a 42% of amino acid sequence similarity with WCI16, a wheat Late Embryogenesis Abundant (LEA)-like protein that plays a role in freezing tolerance (Sasaki et al., 2014). Moreover, STRP was found to interact with DEK3, a nuclear protein involved in chromatin remodelling processes in response to salt stress (Waidmann et al., 2014).
The goal of the present study was to unveil the physiological function of STRP and to clarify its role in the plant response to cold stress. To this purpose, STRP subcellular localization and the mechanism underlying protein accumulation in response to cold stress have been investigated. Moreover, the effect of STRP mutation was analysed both at the biochemical and physiological level, pointing out a crucial role of STRP in cold stress response.
The A. thaliana plants used in this study were of Columbia (Col-0) ecotype. The strp knockout mutant (SALK_076125; Alonso et al., 2003) was obtained from the Nottingham Arabidopsis Stock Center (NASC).
Wild type and strp mutant seedlings were grown in soil in a growth chamber at 22° C, 80% humidity, under a 16/8 h light/dark cycle. Plants were subjected to cold stress by incubation at 4° C for 30 min-18 h periods, maintaining the same light conditions. Hypocotyl length was measured by harvesting at least 30 seedlings after 7 days. Photographs were taken using a digital camera and the acquired images were processed using ImageJ (Schneider et al., 2012). Leaf area was measured by analysing digital pictures with Easy Leaf Area software (Easlon and Bloom, 2014).
For MG132 (Selleckchem, Houston, TX) and ABA treatments, wild type or strp seeds were sterilized with 70% ethanol and a 1% sodium hypochlorite solution containing 0.05% Tween-20, washed with sterile water and sowed on Murashige and Skoog (MS) medium containing 1% sucrose and 0.8% agar, pH 5.7. Seeds were stratified at 4° C in the dark for 3 days, and then transferred in the growth chamber at 22° C, 80% humidity, under a 16/8 h light/dark cycle for 2 weeks. Plants were then transferred on MS supplemented with 100 µM MG132 or different ABA concentrations. Stressed and control plants were then sampled and subjected to further analysis, as described below.
To assess the effects of ABA on strp seeds germination, 200 wild type and strp seeds were sowed on MS medium supplemented with different ABA concentration. After stratification, seeds were transferred to a growth chamber and the germination rate assessed after one week. The germination rate in the absence of stratification was determined on 200 wild type and strp seeds sowed on MS and immediately transferred to a growth chamber.
For ABA-mediated inhibition of primary root elongation, lateral roots formation and plant growth rate, 200 wild type and strp seeds, sowed and germinated on MS, were transferred after 4 days to the same medium supplemented with different ABA concentrations. After one-week, primary root was measured by analysing digital pictures with ImageJ software, while the number of lateral roots was determined by counting the roots with a stereomicroscope. The inhibition of plant growth was assessed after two weeks by measuring the seedling fresh weight with an analytical balance.
For water-loss assay, 25 two-week-old wild type and strp seedlings were transferred in MS plates containing 20 µM ABA. After 18 h, leaves were detached, and their weight was recorded 6 times every 15 min. Stomatal aperture measurements were done in leaves of three-week-old wild type and mutant lines as described by Yu et al. (2016). Leaves were incubated for 2 h with the stomata open buffer (SOB, 10 mM Mes, 50 mM KCl, 0.1 mM CaCl2, pH 6.15) and transferred to the SOB containing 20 µM ABA. After 2 h incubation, the leaf lower epidermis was detached by sticking to adhesive tape (Hansen and van Ooijen, 2016), stained for 20 min with 30 µM propidium iodide and observed under a fluorescence microscope (Nikon TE 2000, Nikon Instruments Inc., Melville, NY). Propidium iodide fluorescence was detected exciting with an Argon laser at 488 nm and emission was detected in the 600–680 nm range. At least 50 stomata were measured in each experiment.
Total RNA was extracted from 100 mg of A. thaliana leaves using the RNeasy Plant Mini Kit RNA from Qiagen (Germantown, MD). Two micrograms of RNA were used for retro-transcription with FastGene Scriptase II cDNA-Kit (Nippon Genetics Europe, Düren, Germany). qPCR was performed in triplicates by using specific qPCR primers (Sigma-Aldrich Corporation, St. Louis, MO), the qPCRBIO SyGreen Mix (PCR Biosystem, London, UK) and the Real-Time PCR Light-Cycler II (Roche Diagnostics, Indianapolis, IN). mRNA levels were normalized to actin (ACT8), GAPDH and tubulin beta chain (TUB8) transcripts, and the relative mRNA levels were determined by using the 2-ΔΔCt method (Pfaffl, 2001). The primer sequences are listed in Supplemental Table S1.
The STRP coding sequence (Accession Number AT1G13930) was cloned upstream of the YFP in a modified pGreen 0029 binary vector (Zottini et al., 2008). STRP cDNAs was inserted in the NcoI restriction site located at the 5’ end of YFP sequence using appropriate primer pairs (Supplemental Table S1). A. thaliana protoplasts were isolated and transformed as described by Yoo et al. (2007). For transient transformation, 20 µg of plasmid and 104 protoplasts were added to an equivalent volume of a solution containing 40% PEG 4000, 0.2 M mannitol, 100 mM CaCl2. Samples were incubated for 30 min at 23° C, diluted with W5 buffer (2 mM MES-KOH, 154 mM NaCl, 125 mM CaCl2, 5 mM KCl, pH 5.7), centrifuged at 800 g for 1 min and resuspended in W5 buffer. After 18 h at 23° C incubation, transformed protoplasts were imaged on GFP fluorescence by confocal microscopy with a laser scanning microscope (Olympus FV1000). Lasers 488 nm (Argon) and 635 nm (diode) were used to detect the green and red fluorescence of YFP and chlorophyll signals, respectively. Cell Mask Orange and Hoechst 33342 (Thermo Fisher Scientific, Waltham, MA), detected with lasers at 554 nm and 361 nm, were used for plasma membrane and nuclei staining, respectively. Images were acquired using a 40× objective.
To produce the recombinant protein, the coding sequence of STRP was amplified by PCR using appropriate primer pairs (Supplemental Table S1) and cloned into the pGEX-2T vector. Recombinant protein was expressed in E. coli as a GST-fusion protein, affinity purified using Glutathione Sepharose 4B (GE Healthcare, Chicago, IL) and the GST was removed by thrombin digestion.
For polyclonal anti-STRP antibodies production, 1 mg purified STRP was mixed with Freund’s complete adjuvant (1:1, v/v) and injected into two rabbits. Two booster injections were given at 2-week intervals. Antisera were then used for Western blot analysis.
The cell free degradation assay was performed accordingly to Osterlund et al. (2000). Two-week-old A. thaliana plants were homogenised to a fine powder with liquid nitrogen and resuspended in 20 ml of degradation buffer (25 mM Tris-HCl, 10 mM NaCl, 10 mM MgCl2, 10 mM ATP, 1 mM PMSF, 5 mM DTT, pH 7.5). Cellular debris were removed by two-centrifugation steps at 17000 g at 4° C for 10 min. The supernatant was recovered, and samples were incubated with MG132 50 µM (Selleckchem, Houston, TX) for 15, 30, and 60 min at 22° C. Samples were then subjected to SDS-PAGE and Western blot analysis.
A. thaliana wild type and strp seedlings were homogenised in liquid nitrogen and the powder was resuspended in 2.5 ml of extraction buffer (25 mM Tris-HCl, 1 mM EDTA, 150 mM NaCl, 10% glycerol, 5 mM DTT, 2 mM PMSF, 2% polyvinylpyrrolidone, 0,1% Nonidet P-40, pH 7.5). Samples were centrifuged at 8,000 g for 30 min at 4° C to completely remove the cell debris. The supernatant was collected, and protein concentration determined with the Bradford method (Bradford, 1976), using BSA as a standard.
Cytosolic fraction and two-phase partitioned plasma membranes were isolated from A. thaliana leaves as previously described (Pallucca et al., 2014). Nuclei were isolated as described by Xu and Copeland (2012). Briefly, 8 g of leaves from three-week-old A. thaliana plants were grinded in a cold mortar. The powder was resuspended in 20 ml of Lysis Buffer (20 mM Tris-HCl, 25% glycerol, 20 mM KCl, 2 mM EDTA, 5 mM MgCl2, 250 mM sucrose, 1 mM DTT, and 1 mM PMSF, pH 7.4). The homogenate was sequentially filtered through a 100 μm and a 40 μm nylon mesh and centrifuged at 1,500 g for 10 min at 4° C. The pelleted nuclei were first washed in 3 ml Nuclei Resuspension Buffer (NRB, 20 mM Tris-HCl, 25% glycerol, 2.5 mM MgCl2, pH 7.4) containing 0.2% Triton X-100, then in 3 ml NRB buffer and finally resuspended in 400 µl NRB containing 440 mM sucrose.
Soluble nuclear protein and chromatin binding fractions were isolated from 400 µl nuclear suspension as described by Barbosa et al. (2010). Pelleted nuclei were treated with 100 μl of Nuclear Lysis Buffer (NLB, 10 mM Tris-HCl, 0.5% sodium deoxycholate, 10 mM NaCl, 3 mM MgCl2, 10 mM NaF, 1% Tween-20, 1.2 mM PMSF). Soluble nuclear proteins were separated from chromatin by centrifugation at 20,000 g for 5 min at 4° C. After 2 washes in NLB, the chromatin fraction was incubated in 50 μl Digestion Buffer (10 mM Pipes, 50 mM NaCl, 300 mM sucrose, 3 mM MgCl2, 1 mM EGTA, 10 mM NaF, 0.5% Triton X-100, 1.2 mM PMSF, 0.6 U/μl DNase I, 50 ng/µL RNase A) for 1 h at 20° C and then treated for 5 min at room temperature with 5 µl ammonium sulphate 2.5 M. Chromatin-associated proteins were separated by centrifugation at 20,000 g for 10 min at 4° C.
The electrolyte leakage assay was performed as described by Visconti et al. (2019). A. thaliana leaves (0.2 g) were cut into 5 mm slices and incubated in 30 ml of deionized water under continuous stirring for 2 h at 25° C. The electrical conductivity of solution was measured by using an electrical conductivity meter. Boiled samples were used to determine the maximum percentage of electrolyte leakage. Lipid peroxidation was evaluated by measuring malondialdehyde (MDA) production, following the procedure described by Taulavuori et al. (2001).
To determine the STRP protein solubility, samples were boiled for 20 min, centrifuged at 12,000 g for 20 min and separated by SDS–PAGE. The cryoprotective activity of recombinant STRP was assayed as described by Sasaki et al. (2014) using L-Lactic Dehydrogenase from rabbit muscle (Sigma-Aldrich Corporation).
For immunoblotting analysis, samples were separated by SDS-PAGE, performed as described by Laemmli (1970) in a Mini Protean apparatus (Bio-Rad, Hercules, CA), and transferred onto a PVDF membrane with a Bio-Rad semi-dry apparatus following the manufacturer’s instructions. Anti-STRP polyclonal antibody 1:10,000 dilution, anti-H3 (Sigma-Aldrich Corporation) 1:2,000 dilution, anti-H+-ATPase (Camoni et al., 2006) 1:2,000 dilution and anti-actin (Sigma-Aldrich Corporation) 1:1,000 were used. Immunoblot detection was performed by incubating the membrane with horseradish peroxidase conjugated anti-rabbit secondary antibody (1:5,000) or anti-mouse secondary antibody (1:5,000) from Bio-Rad.
Hydropathy analysis of STRP, based on Kyte–Doolittle values (Kyte and Doolittle, 1982), was performed by using ExPASy tool (Gasteiger et al., 2005). Protein dynamics was predicted by using the DynaMine webserver (Cilia et al., 2013).
Each experiment was performed at least three times. Statistical significance was assessed by unpaired Student’s t-test. All values are expressed as means ± S.E.M.
The identification of STRP as a cold up-regulated protein (Rocco et al., 2013) points to its possible role in cold stress response mechanisms. To deeper assess the effect of cold stress on STRP levels, three-week-old A. thaliana plants were exposed to 4° C for periods ranging from 30 min to 18 h. Whole cellular extracts were prepared and analysed by western blot using polyclonal anti-STRP antibodies, obtained by immunizing a rabbit with STRP recombinant protein (Figure S1). The time course shows that STRP is rapidly accumulated under cold stress conditions, being protein levels already increased after 30 min of stress (Figure 1A).
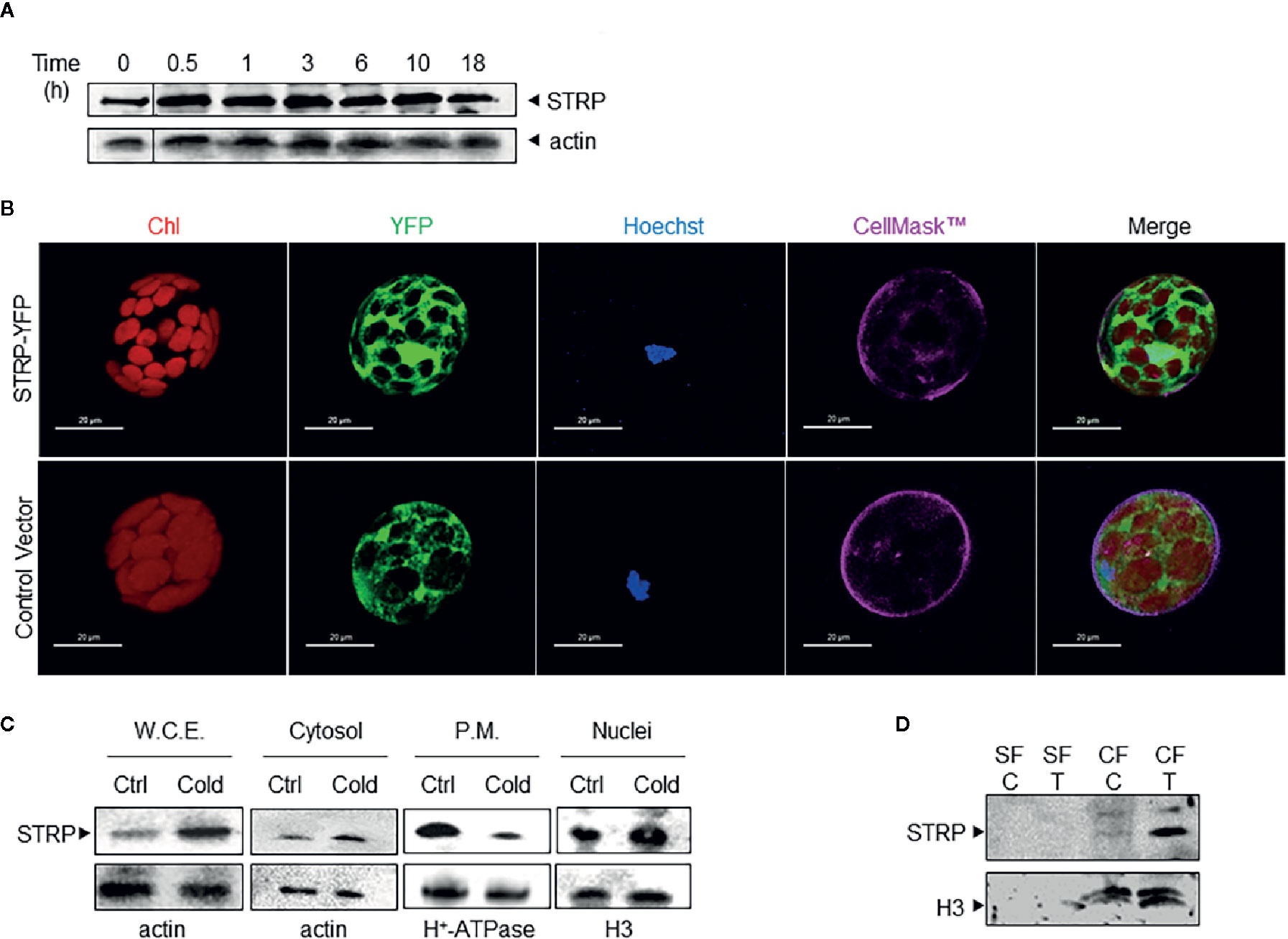
Figure 1 Cold stress affects STRP subcellular localization and levels. (A): time course of STRP levels under cold stress. A. thaliana whole cellular extract was prepared after 0.5, 1, 3, 6, 10, and 18 h of cold stress. 15 μg of samples were separated by SDS-PAGE and subjected to western blotting with anti-STRP antibodies. Actin was used as loading control. (B): STRP subcellular localization in A. thaliana mesophyll protoplasts expressing STRP-YFP fusion protein. CellMask™ Orange and Hoechst 33342 were used as marker for plasma membrane and nuclei, respectively. YFP signal is shown in green, chlorophyll in red, Hoechst 33342 in blue and CellMask™ Orange is pseudo-coloured in magenta. Scale bar, 20 μm. (C): 15 µg of proteins from whole cellular extract (W.C.E.), cytosol, plasma membrane (P.M.) and nuclei were separated by 12% SDS-PAGE and analysed by western blotting with anti-STRP antibodies. Actin, H+-ATPase and H3 were used as loading control. (D): analysis of STRP levels in the soluble nuclear fraction (SF) and in the chromatin fraction (CF) obtained from control (C) and cold treated (T) nuclei. 20 µg of SF and CF were separated by SDS-PAGE, blotted onto PVDF membrane and immunodecorated with anti-STRP antibodies. Histone H3 was used as marker for CF.
In order to study the subcellular localization of STRP, mesophyll protoplasts were purified from A. thaliana leaves and transiently transformed with the 35S-STRP-YFP construct. STRP is localized into the cytosol, in the nucleus and it is also associated to the plasma membrane (Figure 1B). To assess whether cold stress brings about variations on STRP subcellular localization, cytosolic, nuclear and plasma membrane fractions were purified from cold-treated A. thaliana plants, and protein levels analysed by western blot. As shown in Figure 1C, cold stress induces a strong decrease of STRP levels associated to the plasma membrane, whilst protein content in the cytosolic and nuclear fraction is greatly increased.
The recent identification of STRP as an interactor of the DEK Domain-Containing Protein 3 (DEK3), a nuclear protein involved in chromatin remodelling, suggested that in the nucleus STRP can be associated to chromatin. To test this hypothesis, soluble and chromatin-bound proteins were purified from nuclei and analysed by western blot. As shown in Figure 1D, STRP is entirely associated with the chromatin fraction. Interestingly, the amount of STRP bound to chromatin-greatly increases under cold stress.
In order to ascertain whether the increase of STRP levels under cold stress is due to a transcriptional activation of the STRP gene, a RT-qPCR was performed on plants exposed to cold stress for 6 h. As shown in Figure 2A, STRP is not activated by cold stress, whereas the cold responsive gene CBF1, used as positive control, results to be greatly activated. A similar mechanism that leads to STRP accumulation in the absence of gene expression activation has been also observed in response to salt stress (Du et al., 2008; Fiorillo and Camoni, personal communication). This finding suggests that STRP levels could be regulated by post-transcriptional mechanisms leading to protein stabilization under different abiotic stress. In order to ascertain whether STRP can be target of a proteasome-dependent degradation mechanism in response to cold stress, a cell-free degradation assay was performed. Proteins were extracted from two-week-old A. thaliana plants, incubated at 22° C for 15, 30 and 60 min with the 26S proteasome inhibitor MG132 and then analysed by western blotting. As shown in Figure 2B, STRP is rapidly degraded in mock-treated samples, whereas MG132 brings about STRP stabilization. To investigate whether cold stress affects STRP levels by regulating protein stability, A. thaliana seedlings, grown on MS medium, were transferred on MS supplemented with 100 µM MG132 and exposed at 4° C for 18 h. As shown in Figure 2C, STRP levels strongly increase in the presence of MG132 in unstressed samples, equalling the levels of the cold-treated one. This result suggests that, in control conditions, STRP is an unstable protein targeted for the 26S proteasome-dependent proteolysis, and that its degradation is inhibited by cold stress.
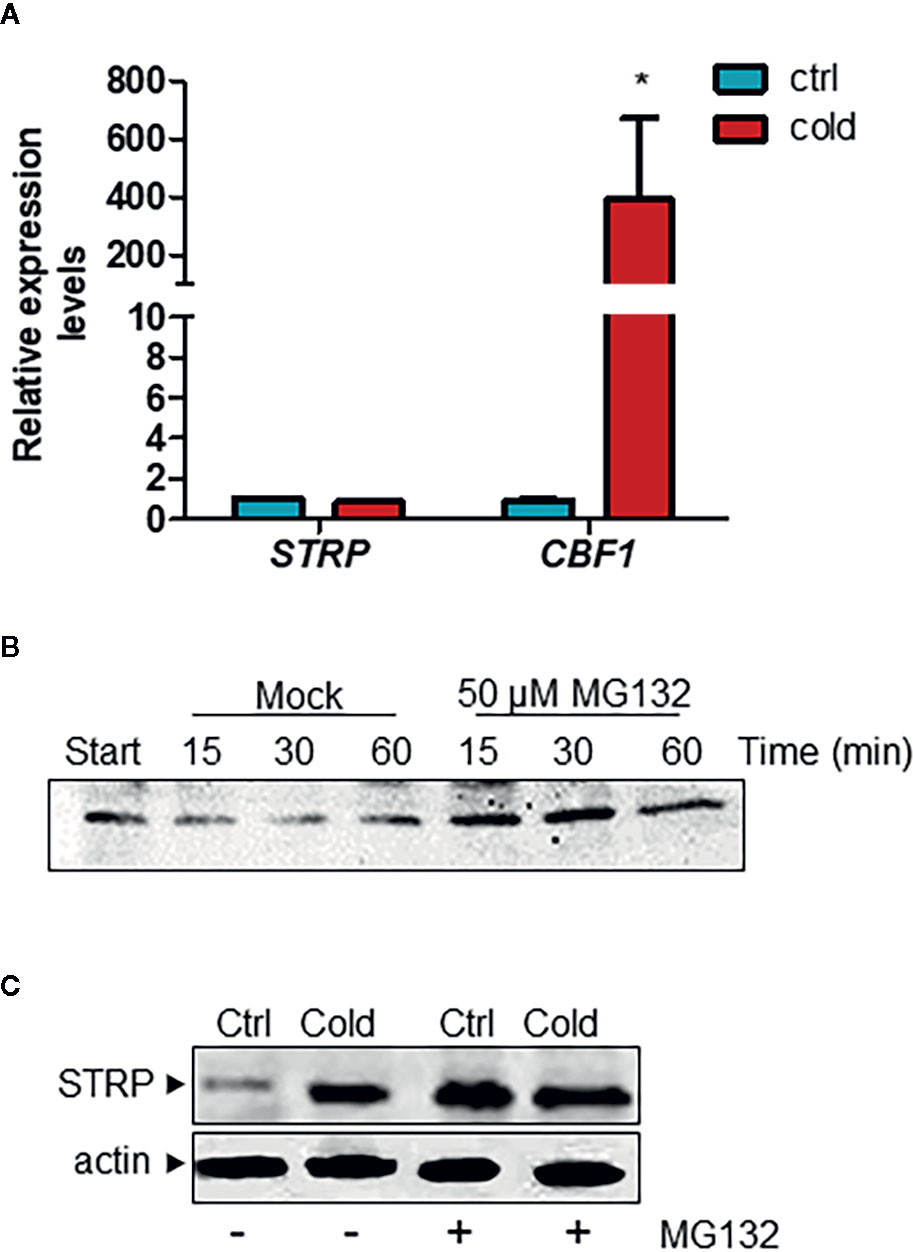
Figure 2 Cold stress stabilizes STRP protein by inhibiting its proteasome-mediated degradation. (A) STRP expression levels under cold stress was determined on total RNA extracted from A. thaliana plants exposed 6 h at 4°C. RNA was retrotranscribed and analysed by RT-qPCR. mRNA levels were normalized to GAPDH mRNA. Error bars are S.E.M. of three independent experiments. *P < 0.01, by Student’s t-test. (B) cell-free degradation assay: 15 µg of total extract were treated with 50 µM MG132 or DMSO for 15, 30 and 60 min and then analysed by western blotting with anti-STRP antibodies. (C) effect of MG132 on STRP levels. 20 µg of cytosol extracted from control (Ctrl) and cold-exposed (Cold) plants treated with MG132 for 18 h were separated by SDS-PAGE, transferred on PVDF membrane and immunodecorated with anti-STRP antibodies. Actin was used as loading control.
STRP has no similarity to other A. thaliana proteins (Du et al., 2008). The hydropathy plot, obtained using the Kyte–Doolittle algorithm (Kyte and Doolittle, 1982) shows that STRP is a highly hydrophilic protein, with few short hydrophobic regions (Figure 3A). Given the amino acid sequence similarity between STRP and the wheat LEA-like protein WCI16 (Sasaki et al., 2014), the possibility that STRP could share some biochemical properties with WCI16 was investigated. The protein backbone dynamics was analysed with the DynaMine prediction system. This system analyses the amino acid sequence of the proteins, in order to identify folded, unfolded and disordered regions. As shown in Figure 3B, STRP is highly unstructured and flexible, with only a small portion able to assume a stable conformation in context-dependent manner. Accordingly, the analysis of conserved protein domains, carried out with the NCBI Conserved Domain Search -NIH (Marchler-Bauer et al., 2017), revealed that the protein has no conserved known domains (data not shown). The lack of a defined secondary structure explains why the STRP apparent molecular mass on SDS–polyacrylamide gels is 25 KDa, greater than the expected mass of 16 KDa (Figure S1). In fact, intrinsically unstructured proteins bind less SDS than other proteins do, and consequently their electrophoretic mobility is reduced (Tompa, 2002).
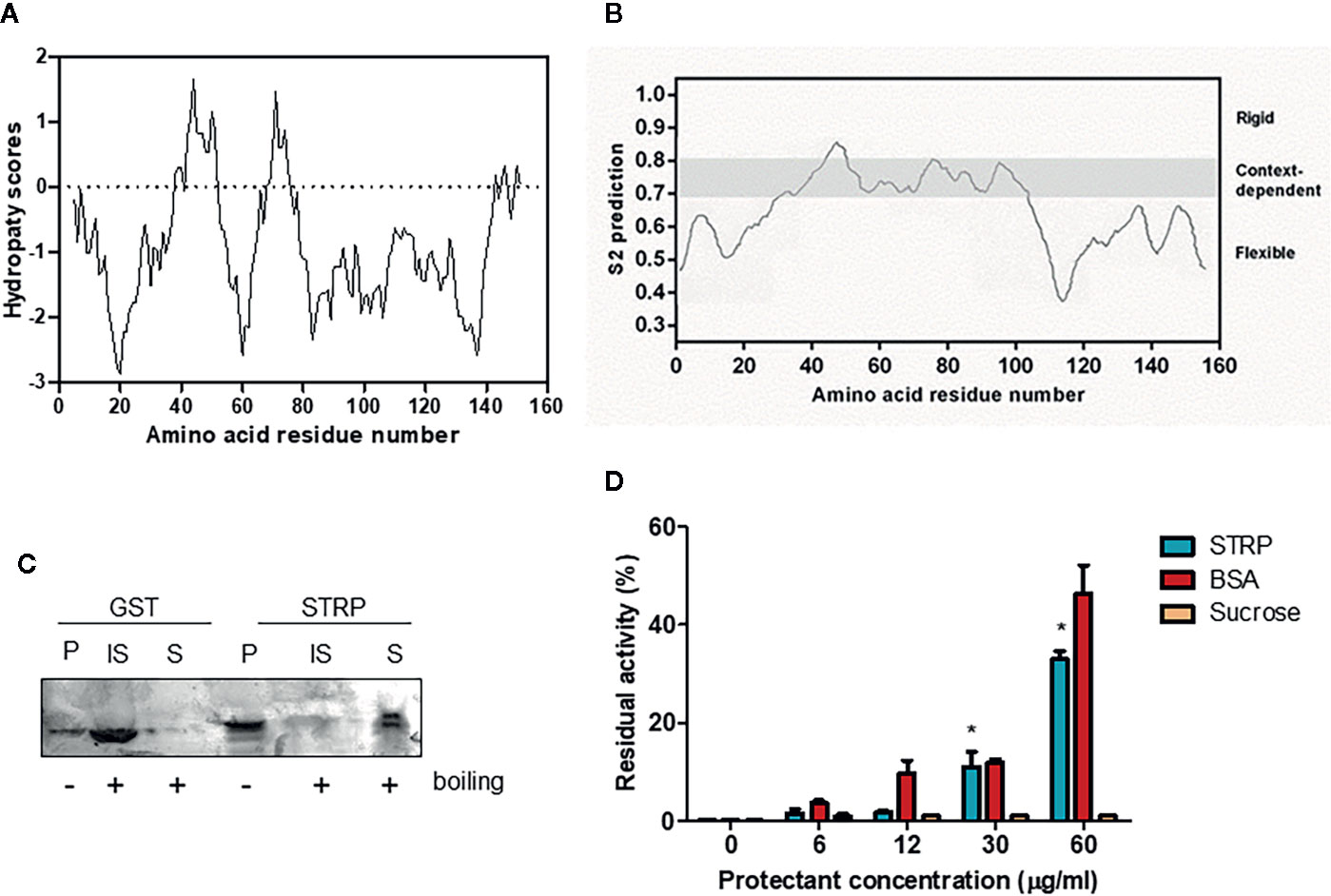
Figure 3 STRP physicochemical properties. (A) hydropathy plot of STRP. The analysis was performed by using Kyte–Doolittle values (Kyte and Doolittle, 1982) with a window size of 9. Negative values indicate the predicted hydrophilic regions. (B) prediction of STRP folding with DynMine system (Cilia et al., 2013). (C) STRP solubility after boiling. Recombinant STRP and GST were treated (+) or not (−) for 20 min at 100° C, cooled and separated by SDS-PAGE. P, purified protein; IS, insoluble fraction; S, soluble fraction. (D) STRP cryoprotection assay on LDH. LDH was frozen for 24 h at −20° C in presence of different concentrations of cryoprotectants. The enzyme was thawed on ice and the residual activity, expressed as a percentage of enzyme initial activity before the freeze-thaw cycle, measured at 340 nm. Error bars are S.E.M. of three independent experiments. *P < 0.01, by Student’s t-test.
Several LEA proteins are comprised in a more widespread group, called hydrophilins (Garay-Arroyo et al., 2000; Battaglia et al., 2008). The physicochemical characteristics that define this group are a Gly content greater than 6% and a hydrophilicity index (Kyte and Doolittle, 1982) greater than 1. STRP has a Gly content of 9% and a hydrophilicity index of 0.88, a value that does not allow to identify STRP as a hydrophilin. However, similar values are in any case typical of some LEA proteins families.
Based on these results, other typical features of LEA proteins, such as the solubility after boiling and the cryoprotectant activity, were investigated. STRP solubility after boiling was assessed on recombinant STRP using GST as a control. Proteins were boiled for 20 min and subsequently cooled on ice. The soluble fraction was separated from the insoluble one, and proteins were analysed by SDS-PAGE. As shown in Figure 3C, STRP is fully soluble after boiling, whereas GST is found in the insoluble fraction. This feature is typical of unstructured and highly hydrophilic proteins, where no hydrophobic regions are exposed to the solvent after boiling. On the contrary, structured proteins, like GST, expose the hydrophobic regions, causing aggregation and precipitation.
The cryoprotectant activity was assessed by measuring the ability of STRP to prevent the inactivation of the freeze-labile enzyme lactate dehydrogenase by freezing treatment. Equivalent concentrations of Bovine Serum Albumin (BSA) and sucrose were used as positive and negative control, respectively. As shown in Figure 3D, 30 and 60 µg/ml STRP preserve LDH activity similarly to BSA. This result highlights a cryoprotectant activity for STRP, thus suggesting the possibility that STRP may function preventing damage of cellular enzymes in stress conditions.
In order to clarify the physiological function of STRP and to ascertain its role in cold stress responses, the characterization of the STRP knock-out mutant was carried out. The strp mutant utilized (SALK_076125) is characterized by a T-DNA insertion into the STRP promoter (Baulcombe et al., 1986). The genotype of the STRP mutant was determined by means of a genomic three-oligonucleotides PCR assay (Du et al., 2008). The mutant is homozygous for the T-DNA insertion (Figure S2A). The absence of STRP expression in the mutant was confirmed by RT-PCR and RT-qPCR (Figure S2B). Western blot analysis shows that STRP protein is undetectable in whole cellular extract from two-week-old plants, thus confirming that the STRP insertional mutant is not able to synthetize the protein (Figure S2C).
To evaluate whether the strp mutation causes an impairment of plant growth and development, the phenotypical analysis in physiological conditions was performed. One-week post-germination, hypocotyls length was measured. As shown in Figure 4A, the strp mutant has shorter hypocotyls compared to wild type plants. Rosette leaf area was measured after two- and three-weeks post- germination. As shown in Figure 4B, the rosette of the strp mutant is smaller compared to the wild type. Hence, STRP loss of function mutation produces a significant impairment of plant growth in physiological conditions.
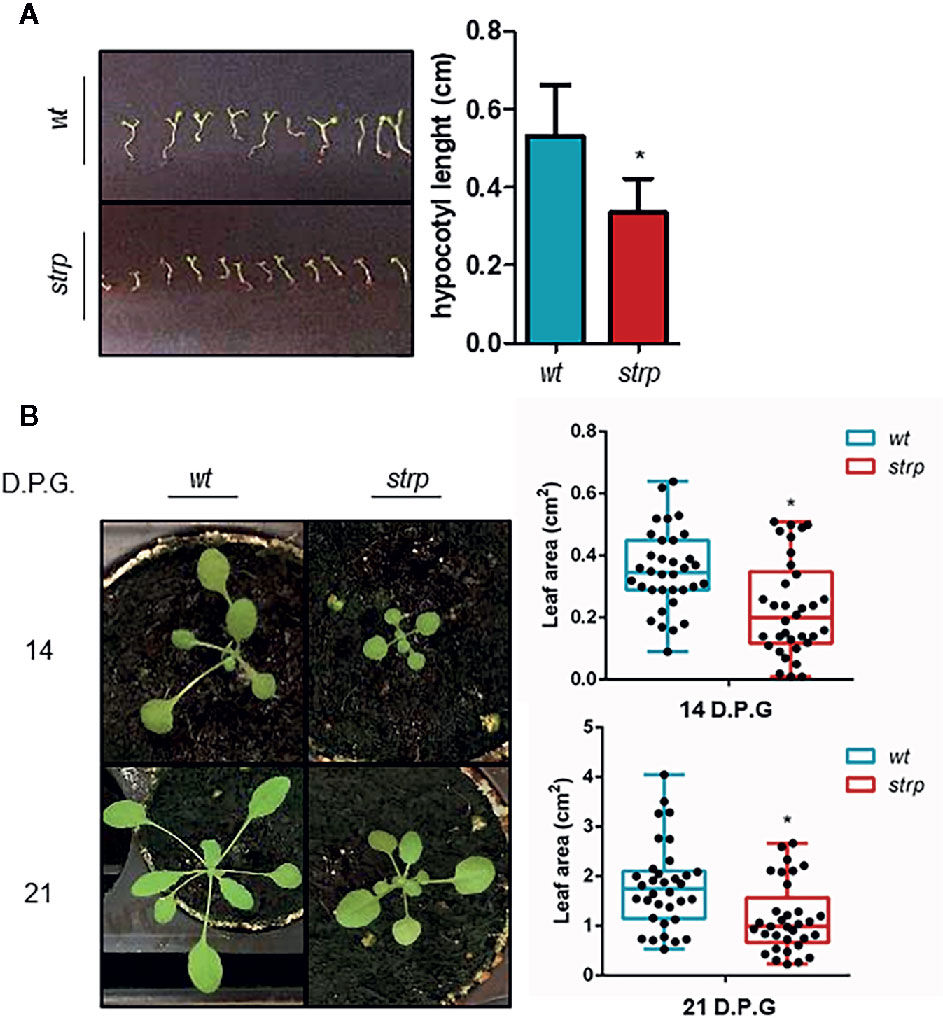
Figure 4 Phenotypical characterization of strp mutant. (A) hypocotyl length of one-week-old seedlings. Hypocotyls were measured by using ImageJ (Schneider et al., 2012). (B) rosette leaf area of plants grown for one, two or three weeks in universal soil. Rosette area was determined with EasyLeaf area software (Easlon and Bloom, 2014). D.P.G., days post-germination. Error bars are S.E.M. of four independent experiments. *P < 0.01, by Student’s t-test.
In order to ascertain whether cold stress conditions can induce more severe growth defects on the strp mutant compared to wild type plants, three-week-old plants were exposed for 5 nights to a 4° C for 8 h, in parallel with the photoperiodic cycle.
As shown in Figure 5, the strp mutant has a smaller rosette as compared to wild type plants, both in control (Figure 5A) and cold stress conditions (Figure 5B). However, the reduction rate of leaf area of the strp mutant is similar both in control and cold stress conditions (Figure 5C). Therefore, overall data highlight that strp mutation brings about a significant growth impairment, which is not however more severe under cold stress.
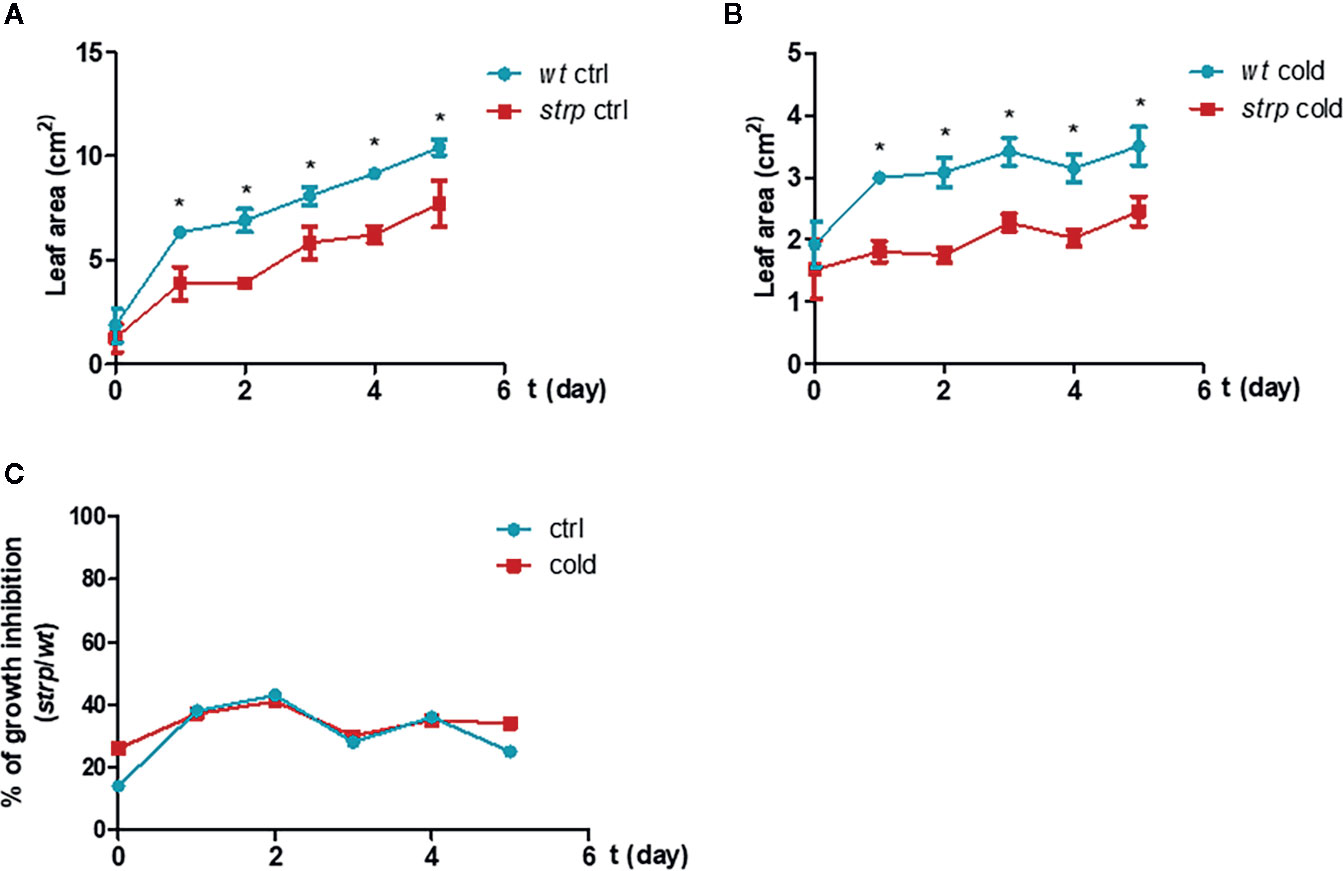
Figure 5 Cold stress affects wild type and strp plant growth. Rosette leaf area was determined on plants grown in universal soil for three weeks and then exposed to low temperature, during the night, for additional 5 days. Rosette area was determined with EasyLeaf area software. Panel A: ctrl; Panel B: cold treated; Panel C: percent ratio of growth inhibition between strp and wild type plants. Error bars are S.E.M. of four independent experiments. *P < 0.01, by Student’s t-test.
Since a strong increase of STRP levels was observed under cold stress conditions, a protective role for this protein was hypothesized. Under cold stress, high amounts of ROS are released, generating a severe oxidative burst (Dreyer and Dietz, 2018). The most common and well-characterized damages caused by ROS occur at the level of biological membranes. Therefore, the content of malondialdehyde (MDA), an oxidation product of the unsaturated membrane lipids, indicator of membrane structural integrity (Hodges et al., 1999), was evaluated. MDA production was measured on two-week-old plants exposed to 4° C for 18 h.
As shown in Figure 6A, MDA content is strongly increased by cold stress both in wild type and in strp plants. However, MDA production is significantly higher in the mutant as compared to wild type.
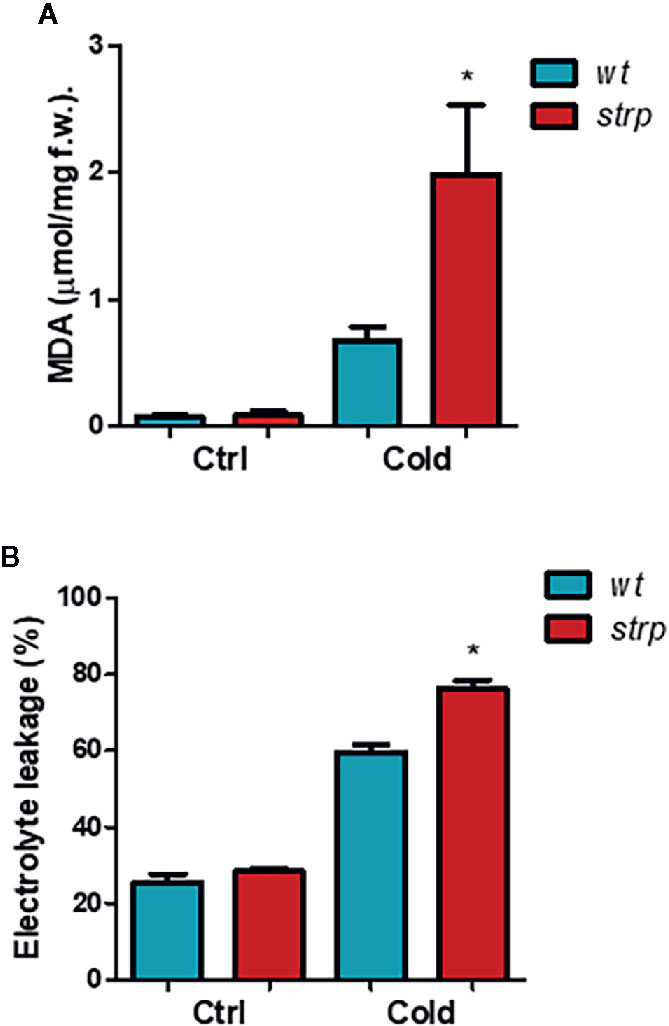
Figure 6 The strp mutation increases the cold-induced oxidative damages in A. thaliana. Wild type and strp mutant plants were exposed to 4°C for 18 h. Leaves were then used to assay MDA production (Panel A) and ion leakage (Panel B). Error bars are S.E.M. of three independent experiments. *P < 0.01, by Student’s t-test.
Besides lipid peroxidation, cold stress also affects membrane integrity, as a result of ROS-induced or mechanical damages. Relative electrolyte conductivity (REC), corresponding to membrane permeability to ions, was measured in leaves of cold-treated wild type and strp plants. As shown in Figure 6B, cold stress induces a significant increase in the ion leakage, both in wild type and strp mutant. Membrane damages are significantly higher in the mutant as compared to wild type. These results demonstrate that cold-induced oxidative damages have led to a more severe loss of membrane functionality in the strp mutant.
It is well known that ABA plays a significant role in plant responses to cold stress (Cutler et al., 2010) and that ABA levels are increased under low temperature conditions (Lång et al., 1994). Hence, the ability of the hormone to influence STRP protein levels was investigated. Two-week-old plants were treated with different ABA concentrations for 18 h, and STRP levels were analysed by western blot. As shown in Figure 7A, all ABA concentrations tested induce a strong increase of STRP levels. To ascertain whether STRP increase depends on gene activation, total RNA was extracted from ABA-treated plants, and STRP expression was evaluated by RT-qPCR. As shown in Figure 7B, STRP expression is only induced at the highest ABA concentration, whereas increase of protein levels occurs also at lower concentrations, suggesting the presence of different mechanisms regulating STRP levels in response to ABA.
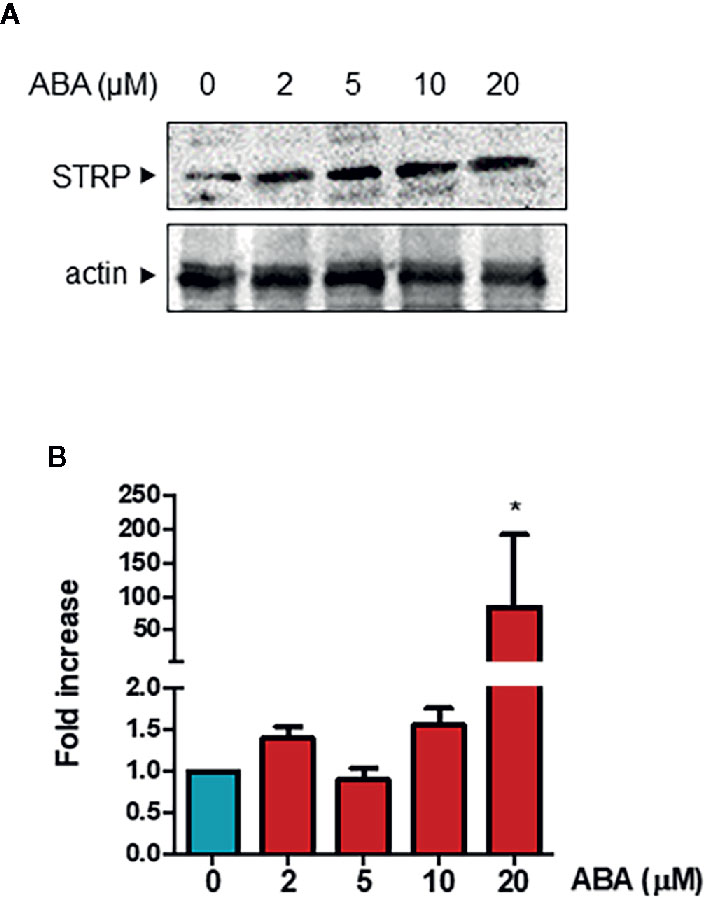
Figure 7 ABA treatment activates STRP expression and increases STRP levels. STRP levels and gene expression were assessed on 2-week-old seedlings treated with 2, 5, 10, and 20 µM ABA for 18 h. STRP levels were determined by western blot (Panel A) on 20 μg of total extract. Samples were separated by SDS-PAGE, transferred on PVDF membrane and incubated with the anti-STRP antibodies. Actin was used as loading control. STRP expression levels (Panel B) were determined on total RNA extracted from A. thaliana, retrotranscribed and analysed by RT-qPCR. mRNA levels were normalized to beta 8-tubulin mRNA (TUB8). Error bars are S.E.M. of four independent experiments. *P < 0.01, by Student’s t-test.
Since ABA treatment strongly increased STRP levels, the effect of STRP mutation on different ABA-regulated processes was investigated. The inhibitory effect of ABA on seed germination was evaluated by treating strp and wild type seeds with different ABA concentrations. As shown in Figure 8A, the germination rate of strp seeds is significantly higher in the presence of 0.5 µM and 1 µM ABA, thus suggesting that the mutant is hyposensitive to ABA. At the highest ABA concentration tested (2 µM), the mutant is no longer able to counteract the inhibitory action of the hormone and responds similarly to the wild type.
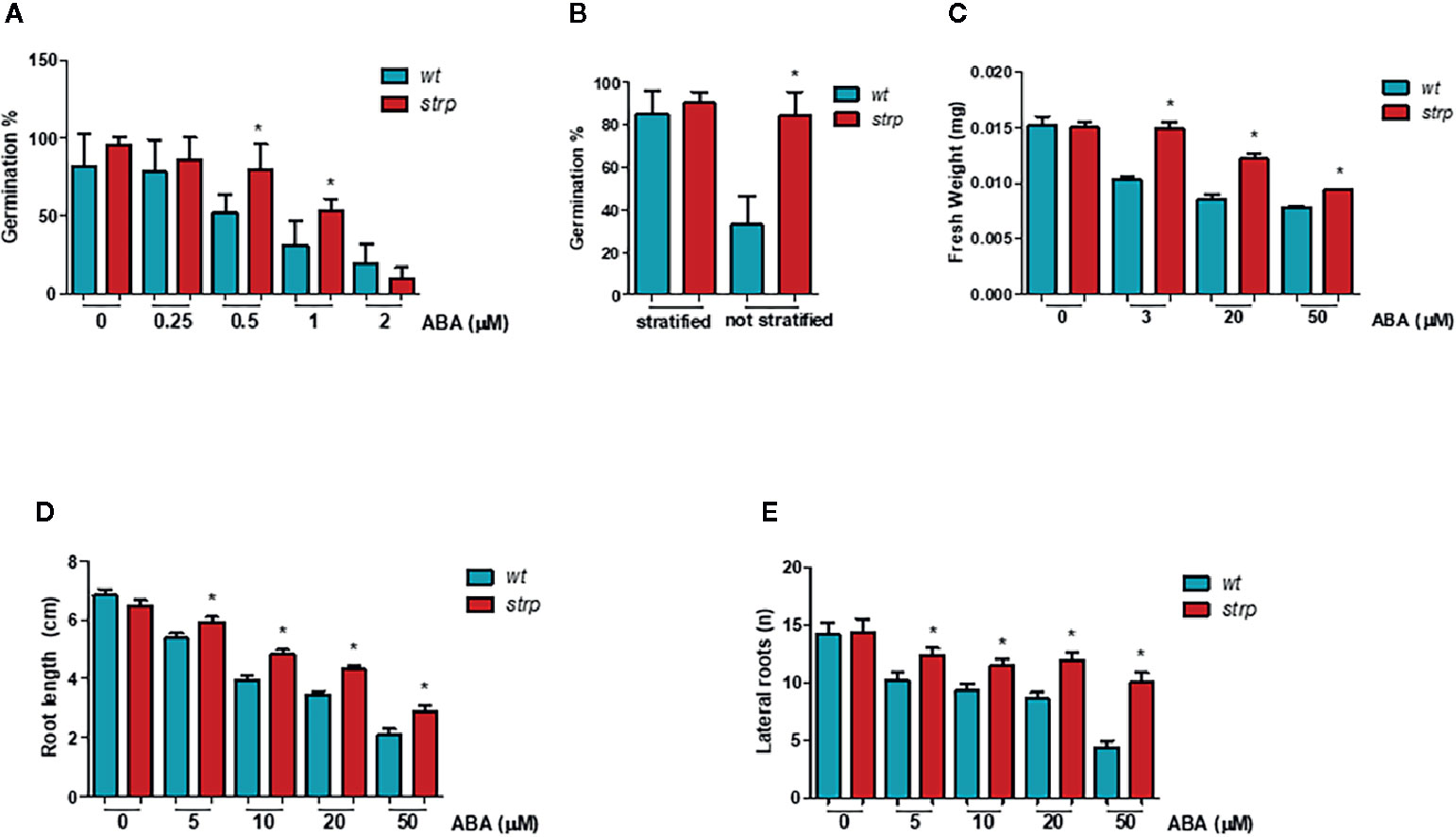
Figure 8 The strp mutant has an ABA-hyposensitive phenotype. (A) germination rate of strp and wild type seeds, assessed on seeds sowed on MS supplemented with 0.25, 0.5, 1, and 2 µM ABA, stratified and transferred for one week in the growth chamber. (B) germination after stratification or not, determined on 200 wild type and strp seeds after one week from sowing on MS. (C) plant growth rate on ABA, analysed on strp and wild type seedlings sowed on MS and transferred after 4 days on MS supplemented with 3, 20, and 50 µM ABA, and grown for additional two weeks. About 50 seedlings for each ABA concentration were used. (D, E) primary root elongation and lateral roots formation, assessed on wild type and strp plants germinated on MS and transferred after 4 days on the same medium supplemented with 5, 10, 20, and 50 µM ABA and grown for one additional week. Error bars are S.E.M. of three independent experiments. *P < 0.01, by Student’s t-test.
To determine whether the strp mutant is also hyposensitive to endogenous ABA, the germination efficiency in the absence of exogenous ABA was analysed. As shown in Figure 8B, the germination rate of wild type seeds is strongly reduced in the absence of stratification. Interestingly, the strp mutant maintains instead a high germination rate. This result suggests that the mutant is also hyposensitive to the inhibition of germination exerted by endogenous ABA.
To test the effect of strp mutation in the ABA-mediated inhibition of plant growth, wild type and strp plants were grown in MS agar plates in the presence of different concentrations of ABA. The weight of the seedling was measured after two weeks, while the length of primary root and the number of lateral roots were measured after one week of ABA treatment. For this experiment, wild type and strp plants of the same weight were utilized. Hormone addition in the growth medium strongly reduces the weight of both wild type and strp plants, with a less pronounced effect in the mutant (Figure 8C). Moreover, roots of the strp mutant are less sensitive than wild type to the inhibitory effect of exogenous ABA, both in terms of primary root growth (Figure 8D) and of lateral root development (Figure 8E). Together, these results indicate that the strp mutant is hyposensitive to ABA effects on plant development.
The ability of ABA to induce stomatal closure was also investigated, using detached leaves from three-week-old wild type and strp plants. Leaves were first incubated with an appropriate solution for stomata opening, then treated for 2 h with 20 µM ABA. Epidermal peels were then isolated and stained with Propidium Iodide (PI). As shown in Figure 9A, ABA-induced stomatal closure is significantly reduced in the strp mutant.
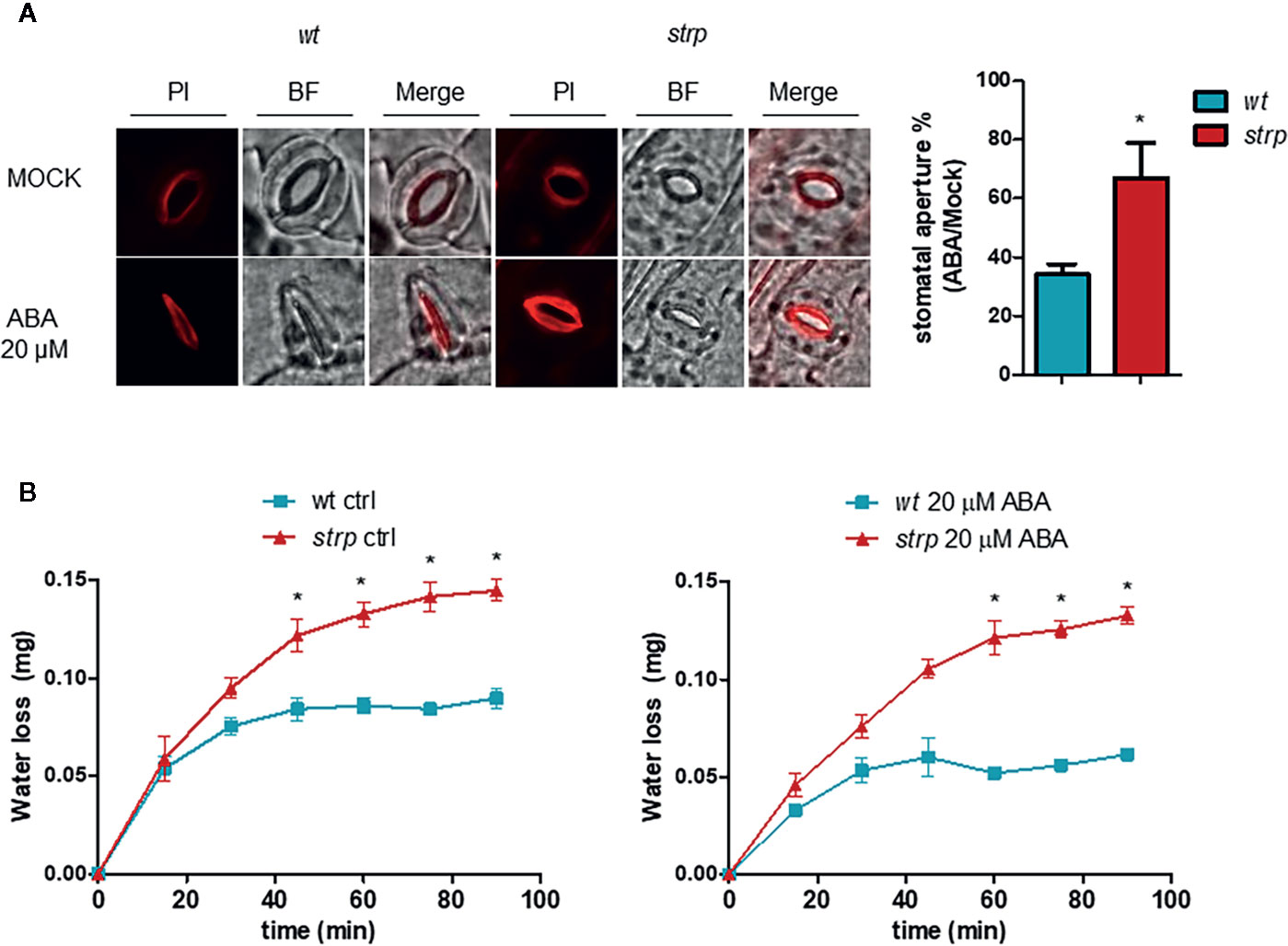
Figure 9 The strp mutation alters the ABA-induced stomatal closure and increases water loss by transpiration. (A) ABA-mediated stomatal closure, evaluated by treating the leaves with 20 µM ABA for 2 h. Epidermis was then isolated and stained with propidium iodide. (B) water loss was detected on strp and wild type detached leaves treated with 20 µM of ABA. Data are represented as variation of fresh weight respect to T0. Error bars are S.E.M. of three independent experiments. *P < 0.01, by Student’s t-test.
A reduced stomatal closure in response to ABA should result in increased water loss through transpiration. Hence, water loss was determined using detached wild type and strp leaves treated with 20 µM ABA. As shown in Figure 9B, strp mutant leaves exhibit an increased leaf water loss relative to the wild type, both in the absence or in the presence of ABA.
Taken together, these results demonstrate that the strp mutant is hyposensitive to all the effects mediated by ABA on plant growth, development and in the responses to drought stress, thus proposing the involvement of STRP in ABA signalling or homeostasis.
To investigate the possible role of STRP in ABA signalling, the transcriptional activation of the ABA-responsive genes KIN1 and RAB18 was assessed by RT-qPCR. As expected, exogenous ABA activates KIN1 and RAB18 expression in wild type plants (Figures 10A, B). As shown in Figures 10A, B, the activation of these genes in the strp mutant is strongly reduced at all the ABA concentrations tested. The impaired activation of the ABA-responsive genes in the mutant suggests that the reduced ABA responsiveness could be ascribable to a downregulation of ABA signalling pathway.
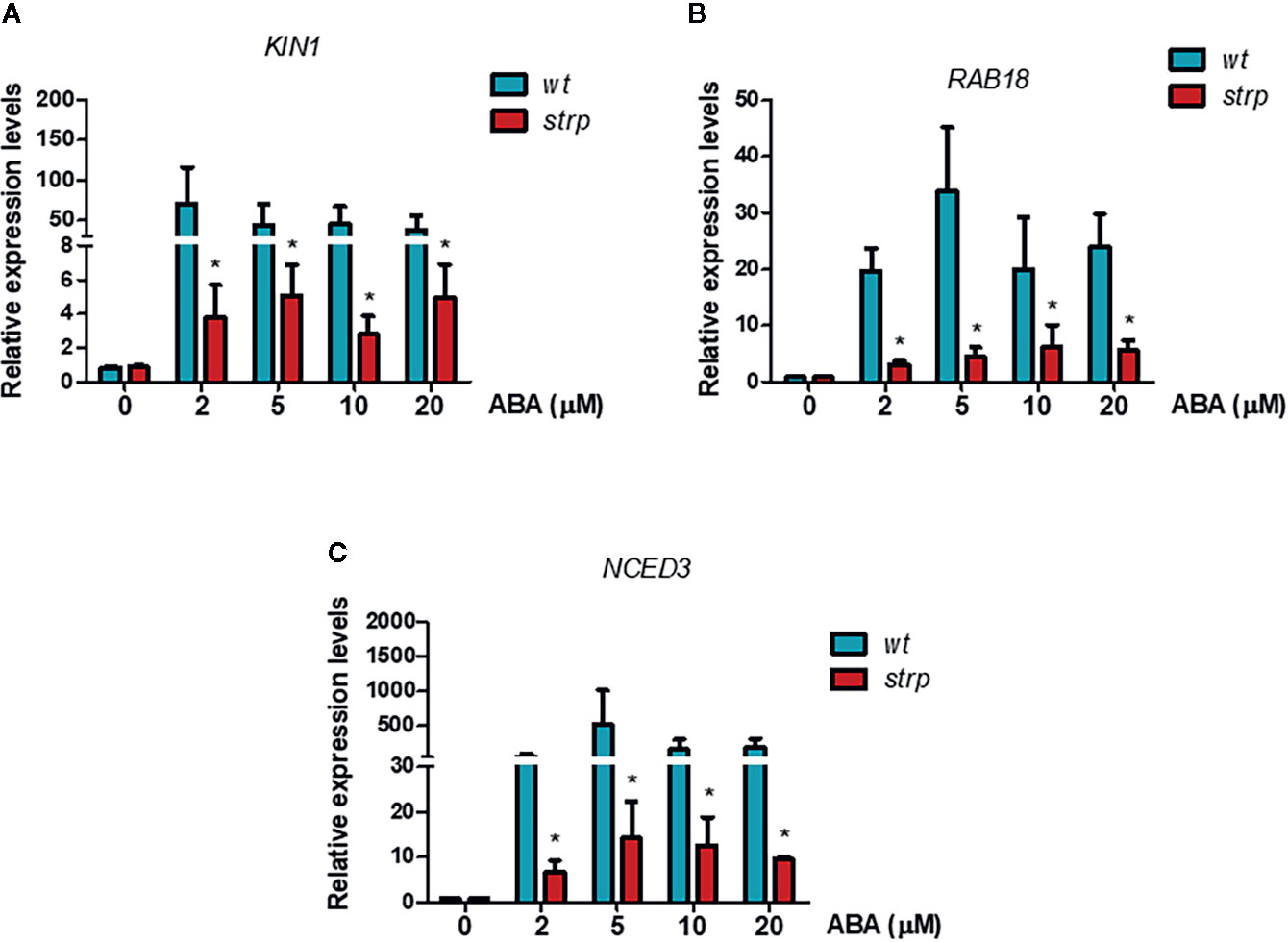
Figure 10 ABA-responsive genes expression is reduced in the strp mutant. Relative expression of KIN1 (A), RAB18 (B), and NCED3 (C) was determined on two-week-old wild type and strp plants treated with 2, 5, 10, and 20 µM ABA for 18 h. mRNA levels were normalized to actin mRNA (ACT8). Error bars are S.E.M. of three independent experiments. *P < 0.01, by Student’s t-test.
A key role in ABA biosynthesis is played by the 9-cis-epoxycarotenoid dioxygenase (NCED). Oxidative cleavage of epoxy-carotenoids is a highly regulated step, and ABA accumulation is the result of the stimulation of NCED expression in response to various stresses (Xiong and Zhu, 2003). Moreover, a positive feedback mechanism for ABA-mediated activation of its biosynthetic genes, including NCED3, is also present. Hence, activation of NCED3 gene in response to exogenous ABA was analysed in the strp mutant by RT-qPCR. As shown in Figure 10C, NCED3 is strongly activated by ABA in wild type plants, whilst the expression of the gene is greatly impaired in the strp mutant, suggesting that also the endogenous levels of ABA can be impaired in the strp mutant.
The aim of the present work was to understand the role played by STRP protein in the response mechanisms to cold stress in A. thaliana. The evidence that STRP levels strongly increased in total cellular extracts of plants exposed to low temperature (Rocco et al., 2013) prompted us to perform an extensive biochemical characterization of the protein. Western blot analysis showed that STRP rapidly increases under cold stress into the cytosol. Confocal microscopy and sub-cellular fractionation experiments suggest that the protein could be also associated to the plasma membrane, and that cold stress brings about a reduction of protein content localized in this compartment. In this scenario, STRP could act as a stress sensor, since plasma membrane-associated proteins can perceive extracellular stimuli (e.g. low temperature), transducing them in an intracellular response. At the plasma membrane level, STRP could be associated to the phospholipid bilayer or, alternatively, to a membrane docking protein. Similar mechanisms are rather recurring in plants: several stress sensors are anchored to plasma membrane proteins or lipids, e.g. to sphingolipids, sterols, and glycosylphosphatidylinositol (Niu and Xiang, 2018). On the other hand, different proteins, such as Heat Shock Proteins, G proteins, and phospholipase C and D, are well-known membrane-linked stress sensors (Plieth et al., 1999; Ruelland et al., 2002; Gupta et al., 2011; Guo et al., 2011). Hence, the release of STRP from the membrane could be part of a transduction pathway leading to cold stress tolerance.
As far as the mechanism underlying the increase of STRP levels, it was found that STRP gene is not induced under cold stress conditions, suggesting a different regulatory mechanism. In non-stressed plants, STRP protein levels increased by a proteasome inhibitor treatment, indicating that STRP is targeted for degradation by the ubiquitin-proteasome system and that cold stress stabilizes the protein by inhibiting this process.
STRP also localizes in the nucleus, as ascertained by western blot and STRP-YFP transient expression in A. thaliana protoplasts. Cold stress determines the increase of STRP nuclear content, promoting protein association to chromatin. It is well known that chromatin organization plays a key role in stress responses (Kim et al., 2010; Zhu et al., 2012; Han and Wagner, 2014). The recent finding that the chromatin-remodelling protein DEK3 interacts with STRP (Waidmann et al., 2014) strongly proposes the involvement of STRP in chromatin remodelling processes under cold stress, modulating the expression of specific cold-activated genes. In addition, cold stress induces oxidative damages to the main cellular structures, including DNA (Apel and Hirt, 2004; Bartoli et al., 2004). Hence, STRP interaction with chromatin could also represent a protection mechanism in response to ROS.
STRP has no similarity with other well-characterized A. thaliana proteins. However, STRP has high similarity with WCI16, a wheat LEA-like protein (Sasaki et al., 2014). Correspondingly, STRP is a high hydrophilic unstructured protein, with just few regions predicted to be folded in a context-dependent manner. Moreover, STRP is highly soluble after boiling and is a good cryoprotectant, able to prevent enzymes inactivation after freezing. Hence, despite the absence of canonical LEA motifs in the STRP sequence, the protein shares some common features with LEA proteins. Consequently, STRP accumulation under cold stress could greatly contribute to stabilize cellular membranes, to prevent protein misfolding and therefore to preserve enzyme activities.
Phenotypical analysis of the strp knockout insertional mutant revealed that the mutant has an impaired growth, with short hypocotyls and reduced leaf area. Cold stress hampered the growth rate of rosette leaves similarly in wt and in strp plants. However, the strp mutant shows an increased stress-induced oxidative damage, suggesting the participation of STRP in ROS-detoxifying mechanisms during cold stress acclimation. Generally, oxidative stress and membrane damage lead to plant growth inhibition (Gechev et al., 2006). It is therefore conceivable that, in the stress conditions used, the production of ROS and the extent of lipid peroxidation is too low to induce a significant growth defect.
Considering the role played by ABA in a range of abiotic stresses, a possible connection between ABA and the STRP-mediated response to cold stress was analysed. Exogenous ABA application on A. thaliana brings about a strong accumulation of STRP protein, and high ABA concentrations induce STRP transcriptional activation. The analysis of ABA effects on strp revealed that the mutant is hyposensitive to all the effects of ABA on plant development. In fact, the mutant has a longer primary root, increased lateral roots formation, high germination rate (0.5 and 1 µM), even without stratification, altered stomatal closure and increased water loss under water deficit. Moreover, down-regulation of the ABA-responsive genes further confirms that ABA responses are impaired in the strp mutant.
The interplay between STRP function and ABA remains to be clarified, being not clear how STRP is correlated to the hormone signalling and perception. Hence, the characterization of STRP-mediated stress responses in ABA-deficient mutant could contribute to clarify this aspect.
In conclusion, results obtained identify STRP as a multitasking protein acting at different levels in the response mechanisms to low temperatures in A. thaliana. Moreover, this work lays the foundation for the clarification of a possible involvement of the protein in the resistance mechanism toward different abiotic and biotic stress.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical review and approval was not required for the animal study because The rabbit antibody production has been carried out following the guidelines of the Institutional Animal Care and Use Committee (IACUC) of the Interdepartmental Service Center - Station for Animal Technology of the University of Rome Tor Vergata, in compliance with the Legislative Decree of 27 January 1992 n. 116, which implements the EEC directive n. 609/86.
LC, SV, and AF formulated the original hypothesis of the article and designed the experiments. AF and SV performed the experiments. MM prepared the anti-STRP antibody. LC and AF wrote the manuscript and prepared the figures. PA analysed the data and revised the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2020.01251/full#supplementary-material
Figure S1 | SDS-PAGE and Western blot analysis on STRP. To test the ability of the anti-STRP polyclonal antibody to recognize STRP, 1 µg of purified recombinant protein and 15 μg of plant whole cell extract (W.C.E.) were separated by 12% SDS-PAGE, transferred on PVDF membrane and incubated with the anti-STRP antibodies. CBB, Coomassie Brilliant Blue; WB, western blot.
Figure S2 | The strp mutant is homozygous for the T-DNA insertion, leading to loss of STRP expression. A: left panel, schematic model of the T-DNA insertion in the STRP promoter; right, genomic three-oligonucleotides PCR for the identification of homozygous mutant lines. Genotype analysis of the strp mutant was carried out on two different plant generations, named T2 and T3, with a genomic PCR using three primers: LP primer 5’-TACACTCACTCGTCACTCCCC-3’, RP primer 5’-TAAATGTCTTTTTCGGCAACG-3’ and LBb1.3 primer 5’-ATTTTGCCGATTTCGGAAC-3’. The genomic DNA extraction was performed with the “DNeasy® Plant Mini Kit” (QIAGEN) according to manufacturer’s instructions. The reaction with LB+RP, complementary to the STRP promoter, and with BP+RP, complementary to the T-DNA insertion and the STRP promoter, were programmed to get the blank for homozygous mutant and for wild type plants, respectively. The complete reaction with the three oligonucleotides (LP+BP+RP) produces one amplified fragment of 900 bp for the wild type and one of 700 bp for the homozygous mutant. B: RT-PCR (left panel) and RT-qPCR (right panel) were used to verify the lack of STRP expression in the strp mutant. The PCR were performed with the following primer pairs: 5’-CCATCTCTTAACTCTTCCATCCAA-3’, and 5’-TCTGGCTCCTCTGGTGTT-3’. mRNA levels were normalized to GAPDH mRNA. Error bars are S.E.M. of three independent experiments. *P < 0.01, by Student’s t-test. C: western blot analysis, performed on 20 µg of W.C.E. of wild type and strp T2 and T3 plants. Samples were separated by 12% SDS-PAGE, transferred on PVDF and incubated with anti-STRP antibodies.
Figure S3 | Densitometric analysis of bands of western blot experiments. Densitometric analysis was performed using the ImageJ image-processing program. Data are expressed as the percentage of the maximum integrated densitometric value (IDV, the product of the area and mean grey value).
Alonso, J. M., Stepanova, A. N., Leisse, T. J., Kim, C. J., Chen, H., Shinn, P., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301 (5633), 653–657. doi: 10.1126/science.1086391
Amme, S., Matros, A., Schlesier, B., Mock, H. P. (2006). Proteome analysis of cold stress response in Arabidopsis thaliana using DIGE-technology. J. Exp. Bot. 57 (7), 1537–1546. doi: 10.1093/jxb/erj129
Apel, K., Hirt, H. (2004). Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55, 373–399. doi: 10.1146/annurev.arplant.55.031903.141701
Bae, M. S., Cho, E. J., Choi, E. Y., Park, O. K. (2003). Analysis of the Arabidopsis nuclear proteome and its response to cold stress. Plant J. 36 (5), 652–663. doi: 10.1046/j.1365-313x.2003.01907.x
Barbosa, L. F., Cerqueira, F. M., Macedo, A. F., Garcia, C. C., Angeli, J. P., Schumacher, R. I., et al. (2010). Increased SOD1 association with chromatin, DNA damage, p53 activation, and apoptosis in a cellular model of SOD1-linked ALS. Biochim. Biophys. Acta 1802 (5), 462–471. doi: 10.1016/j.bbadis.2010.01.011
Bartoli, C. G., Gomez, F., Martinez, D. E., Guiamet, J. J. (2004). Mitochondria are the main target for oxidative damage in leaves of wheat (Triticum aestivum L.). J. Exp. Bot. 55 (403), 1663–1669. doi: 10.1093/jxb/erh199
Battaglia, M., Olvera-Carrillo, Y., Garciarrubio, A., Campos, F., Covarrubias, A. A. (2008). The enigmatic LEA proteins and other hydrophilins. Plant Physiol. 148 (1), 6–24. doi: 10.1104/pp.108.120725
Baulcombe, D. C., Saunders, G. R., Bevan, M. W., Majo, M. A., Harrison, B. D. (1986). Expression of biologically active viral satellite RNA from the nuclear genome of transformed plants. Nature 321, 446–449. doi: 10.1038/321446a0
Bradford, M. M. (1976). A rapid and sensitive method for the quantification of microgram quantities of protein using the principle of protein-dye binding. Anal. Biochem. 72, 248–254. doi: 10.1006/abio.1976.9999
Camoni, L., Marra, M., Garufi, A., Visconti, S., Aducci, P. (2006). The maize root plasma membrane H+-ATPase is regulated by a sugar-induced transduction pathway. Plant Cell Physiol. 47 (6), 743–747. doi: 10.1093/pcp/pcj046
Cattivelli, L., Bartels, D. (1992). “Biochemistry and molecular biology of cold-inducible enzyme and proteins in higher plants,” in Inducible Plant Proteins society for Experimental Biology Seminar Series, vol. 49. Ed. Wray, J. L. (Cambridge, UK: University Press), 267–288.
Cilia, E., Pancsa, R., Tompa, P., Lenaerts, T., Vranken, W. F. (2013). From protein sequence to dynamics and disorder with DynaMine. Nat. Commun. 4, 2741. doi: 10.1038/ncomms3741
Cutler, S. R., Rodriguez, P. L., Finkelstein, R. R., Abrams, S. R. (2010). Abscisic acid: emergence of a core signaling network. Annu. Rev. Plant Biol. 61, 651–679. doi: 10.1146/annurev-arplant-042809-112122
Dreyer, A., Dietz, K.-J. (2018). Reactive Oxygen Species and the Redox-Regulatory Network in Cold Stress Acclimation. Antioxid. (Basel) 7 (11), 169. doi: 10.3390/antiox7110169
Du, J., Huang, Y. P., Xi, J., Cao, M. J., Ni, W. S., Chen, X., et al. (2008). Functional gene- mining for salt- tolerance genes with the power of Arabidopsis. Plant J. 56 (4), 653–664. doi: 10.1111/j.1365-313X.2008.03602.x
Easlon, H. M., Bloom, A. J. (2014). Easy Leaf Area: Automated digital image analysis for rapid and accurate measurement of leaf area. Appl. Plant Sci. 2 (7), apps.1400033. doi: 10.3732/apps.1400033
Fujii, H., Chinnusamy, V., Rodrigues, A., Rubio, S., Antoni, R., Park, S. Y., et al. (2009). In vitro reconstitution of an abscisic acid signalling pathway. Nature 462 (7273), 660–664. doi: 10.1038/nature08599
Garay-Arroyo, A., Colmenero-Flores, J. M., Garciarrubio, A., Covarrubias, A. A. (2000). Highly hydrophilic proteins in prokaryotes and eukaryotes are common during conditions of water deficit. J. Biol. Chem. 275 (8), 5668–5674. doi: 10.1074/jbc.275.8.5668
Gasteiger, E., Hoogland, C., Gattiker, A., Duvaud, S., Wilkins, M. R., Appel, R. D., et al. (2005). “Protein Identification and Analysis Tools on the ExPASy Server,” in The Proteomics Protocols Handbook. Ed. Walker, J. M. (Totowa, NJ: Humana Press), 571–607.
Gechev, T., Van Breusegem, F., Stone, J. M., Denev, I., Laloi, C. (2006). Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. Bioessays 28, 1091–1101. doi: 10.1002/bies.20493
Gilmour, S. J., Thomashow, M. F. (1991). Cold acclimation and cold-regulated gene expression in ABA mutants of Arabidopsis thaliana. Plant Mol. Biol. 17 (6), 1233–1240. doi: 10.1007/bf00028738
Guo, L., Mishra, G., Taylor, K., Wang, X. (2011). Phosphatidic acid binds and stimulates Arabidopsis sphingosine kinases. J. Biol. Chem. 286, 13336–13345. doi: 10.1074/jbc.M110.190892
Gupta, K. J., Hincha, D. K., Mur, L. A. (2011). No way to treat a cold. New Phytol. 189, 360–363. doi: 10.1111/j.1469-8137.2010.03586.x
Han, S.-K., Wagner, D. (2014). Role of chromatin in water stress responses in plants. J. Exp. Bot. 65 (10), 2785–2799. doi: 10.1093/jxb/ert403
Hansen, L. L., van Ooijen, G. (2016). Rapid Analysis of Circadian Phenotypes in Arabidopsis Protoplasts Transfected with a Luminescent Clock Reporter. J. Vis. Exp. 115, 54586. doi: 10.3791/54586
Heino, P., Sandman, G., Lång, V., Nordin, K., Palva, E. T. (1990). Abscisic acid deficiency prevents development of freezing tolerance in Arabidopsis thaliana (L.) Heynh. Theor. Appl. Genet. 79 (6), 801–806. doi: 10.1007/BF00224248
Hodges, D. M., De Long, J. M., Forney, C. F., Prange, R. K. (1999). Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207, 604–611. doi: 10.1007/s004250050524
Huang, X., Shi, H., Hu, Z., Liu, A., Amombo, E., Chen, L., et al. (2017). ABA Is Involved in Regulation of Cold Stress Response in Bermudagrass. Front. Plant Sci. 8, 1613. doi: 10.3389/fpls.2017.01613
Jia, Y., Ding, Y., Shi, Y., Zhang, X., Gong, Z., Yang, S. (2016). The cbfs triple mutants reveal the essential functions of CBFs in cold acclimation and allow the definition of CBF regulons in Arabidopsis. New Phytol. 212 (2), 345–353. doi: 10.1111/nph.14088
Kim, J. M., To, T. K., Nishioka, T., Seki, M. (2010). Chromatin regulation functions in plant abiotic stress responses. Plant Cell Environ. 33 (4), 604–611. doi: 10.1111/j.1365-3040.2009.02076.x
Kim, Y. H., Choi, K. I., Khan, A. L., Waqas, M., Lee, I. J. (2016). Exogenous application of abscisic acid regulates endogenous gibberellins homeostasis and enhances resistance of oriental melon (Cucumis melo var. L.) against low temperature. Sci. Hortic. 207, 41–47. doi: 10.1016/j.scienta.2016.05.009
Koornneef, M., Jorna, M. L., Brinkhorst-van der Swan, D. L. C., Karssen, C. M. (1982). The isolation of abscisic acid (ABA) deficient mutants by selection of induced revertants in non-germinating gibberellin sensitive lines of Arabidopsis thaliana (L) Heynh. Theor. Appl. Genet. 61 (4), 385–393. doi: 10.1007/BF00272861
Kreps, J. A., Wu, Y., Chang, H. S., Zhu, T., Wang, X., Harper, J. F. (2002). Transcriptome changes for Arabidopsis in response to salt, osmotic, and cold stress. Plant Physiol. 130, 2129–2141. doi: 10.1104/pp.008532
Kumar, S., Kaur, G., Nayyar, H. (2008). Exogenous application of Abscisic acid improves cold tolerance in Chickpea (Cicer arietinum L.). J. Agron. Crop Sci. 194, 449–456. doi: 10.1111/j.1439-037X.2008.00335.x
Kyte, J., Doolittle, R. F. (1982). A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157 (1), 105–132. doi: 10.1016/0022-2836(82)90515-0
Laemmli, U. K. (1970). Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 227, 680–685. doi: 10.1038/227680a0
Lång, V., Palva, E. T. (1992). The expression of a rab-related gene, RAB18, is induced by abscisic acid during the cold acclimation process of Arabidopsis thaliana (L.) Heynh. Plant Mol. Biol. 20 (5), 951–962. doi: 10.1007/bf00027165
Lång, V., Mantyla, E., Welin, B., Sundberg, B., Palva, E. T. (1994). Alterations in water status, endogenous Abscisic Acid content, and expression of rab18 gene during the development of freezing tolerance in Arabidopsis thaliana. Plant Physiol. 104, 1341–1349. doi: 10.1104/pp.104.4.1341
Li, T., Xu, S. L., Oses-Prieto, J. A., Putil, S., Xu, P., Wang, R. J., et al. (2011). Proteomics analysis reveals post-translational mechanisms for cold-induced metabolic changes in Arabidopsis. Mol. Plant 4 (2), 361–374. doi: 10.1093/mp/ssq078
Liu, Q., Kasuga, M., Sakuma, Y., Abe, H., Miura, S., Yamaguchi-Shinozaki, K., et al. (1998). Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell. 10 (8), 1391–1406. doi: 10.1105/tpc.10.8.1391
Liu, Y., Dang, P., Liu, L., He, C. (2019). Cold acclimation by the CBF-COR pathway in a changing climate: lesson from Arabidopsis thaliana. Plant Cell Rep. 38 (5), 511–519. doi: 10.1007/s00299-019-02376-3
Mantyla, E., Lång, V., Palva, E. T. (1995). Role of abscisic acid in drought-induced freezing tolerance, cold acclimation, and accumulation of LT178 and RAB18 proteins in Arabidopsis thaliana. Plant Physiol. 107 (1), 141–148. doi: 10.1104/pp.107.1.141
Marchler-Bauer, A., Bo, Y., Han, L., He, J., Lanczycki, C. J., Lu, S., et al. (2017). CDD/SPARCLE: functional classification of proteins via subfamily domain architectures. Nucleic Acids Res. 45 (D), 200–203. doi: 10.1093/nar/gkw1129
Niu, Y., Xiang, Y. (2018). An Overview of Biomembrane Functions in Plant Responses to High-Temperature Stress. Front. Plant Sci. 9, 915 (915). doi: 10.3389/fpls.2018.00915
Osterlund, M. T., Hardtke, C. S., Wei, N., Deng, X. W. (2000). Targeted destabilization of HY5 during light-regulated development of Arabidopsis. Nature 405, 462–466. doi: 10.1038/35013076
Pallucca, R., Visconti, S., Camoni, L., Cesareni, G., Melino, S., Panni, S., et al. (2014). Specificity of ϵ and non-ϵ isoforms of Arabidopsis 14-3-3 proteins towards the H+-ATPase and other targets. PloS One 9 (6), e90764. doi: 10.1371/journal.pone.0090764
Palva, E. T. (1994). “Gene expression under low temperature stress,” in Stress Induced Gene Expression in Plants. Ed. Basra, A. S. (New York: Harwood Academic), 103–130.
Park, S., Lee, C. M., Doherty, C. J., Gilmour, S. J., Kim, Y., Thomashow, M. F. (2015). Regulation of the Arabidopsis CBF regulon by a complex low-temperature regulatory network. Plant J. 82 (2), 193–207. doi: 10.1111/tpj.12796
Pfaffl, M. W. (2001). A new mathematical model for relative quantification in realtime RT-PCR. Nucleic Acids Res. 29 (9), e45. doi: 10.1093/nar/29.9.e45
Plieth, C., Hansen, U. P., Knight, H., Knight, M. R. (1999). Temperature sensing by plants: the primary characteristics of signal perception and calcium response. Plant J. 18, 491–497. doi: 10.1046/j.1365-313x.1999.00471.x
Rocco, M., Arena, S., Renzone, G., Scippa, S., Lomaglio, T., Verrillo, F., et al. (2013). Proteomic analysis of temperature stress-responsive proteins in Arabidopsis thaliana rosette leaves. Mol. Biosyst. 9 (6), 1257–1267. doi: 10.1039/c3mb70137a
Ruelland, E., Cantrel, C., Gawer, M., Kader, J. C., Zachowski, A. (2002). Activation of phospholipases C and D is an early response to a cold exposure in Arabidopsis suspension cells. Plant Physiol. 130, 999–1007. doi: 10.1104/pp.006080
Sanghera, G. S., Wani, S. H., Hussain, W., Singh, N. B. (2011). Engineering Cold Stress Tolerance in Crop Plants. Curr. Genomics 12 (1), 30–43. doi: 10.2174/138920211794520178
Sasaki, K., Christov, N. K., Tsuda, S., Imai, R. (2014). Identification of a novel LEA protein involved in freezing tolerance in wheat. Plant Cell Physiol. 55 (1), 136–147. doi: 10.1093/pcp/pct164
Schneider, C. A., Rasband, W. S., Eliceiri, K. W. (2012). NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9 (7), 671–675. doi: 10.1038/nmeth.2089
Shi, Y., Ding, Y., Yang, S. (2018). Molecular Regulation of CBF Signaling in Cold Acclimation. Trends Plant Sci. 23 (7), 623–637. doi: 10.1016/j.tplants.2018.04.002
Taulavuori, E., Hellstrom, E. K., Tauvaluori, K., Laine, K. (2001). Comparison of two methods used to analyse lipid peroxidation from Vaccinium myrtillus (L.) during snow removal, reacclimation and cold acclimation. J. Exp. Bot. 52 (365), 2375–2380. doi: 10.1093/jexbot/52.365.2375
Tompa, P. (2002). Intrinsically unstructured proteins. Trends Biochem. Sci. 27, 527–533. doi: 10.1016/s0968-0004(02)02169-2
Verslues, P. E., Zhu, J.-K. (2005). Before and beyond ABA: Upstream Sensing and Internal Signals that Determine ABA Accumulation and Response Under Abiotic Stress. Biochem. Soc. Trans. 33 (2), 375–379. doi: 10.1042/BST0330375
Visconti, S., D’Ambrosio, C., Fiorillo, A., Arena, S., Muzi, C., Zottini, M., et al. (2019). Overexpression of 14-3-3 proteins enhances cold tolerance and increases levels of stress-responsive proteins of Arabidopsis plants. Plant Sci. 289, 110215. doi: 10.1016/j.plantsci.2019.110215
Waidmann, S., Kusenda, B., Mayerhofer, J., Mechtler, K., Jonak, C. (2014). A DEK domain-containing protein modulates chromatin structure and function in Arabidopsis. Plant Cell. 26 (11), 4328–4344. doi: 10.1105/tpc.114.129254
Xiong, L., Zhu, J.-K. (2003). Regulation of Abscisic Acid Biosynthesis. Plant Physiol. 133 (1), 29–36. doi: 10.1104/pp.103.025395
Xu, F., Copeland, C. (2012). Nuclear Extraction from Arabidopsis thaliana. Bio-protocol 2 (24), e306. doi: 10.21769/BioProtoc.306
Yoo, S. D., Cho, Y. H., Sheen, J. (2007). Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat. Protoc. 2, 1565–1575. doi: 10.1038/nprot.2007.199
Yu, J., Yang, L., Liu, X., Tang, R., Wang, Y., Ge, H., et al. (2016). Overexpression of Poplar Pyrabactin Resistance-Like Abscisic Acid Receptors Promotes Abscisic Acid Sensitivity and Drought Resistance in Transgenic Arabidopsis. PloS One 11 (12), e0168040. doi: 10.1371/journal.pone.0168040
Zhao, C., Zhang, Z., Xie, S., Si, T., Li, Y., Zhu, J. K. (2016). Mutational evidence for the critical role of CBF transcription factors in cold acclimation in Arabidopsis. Plant Physiol. 171 (4), 2744–2759. doi: 10.1104/pp.16.00533
Zhu, Y., Dong, A., Shen, W. H. (2012). Histone variants and chromatin assembly in plant abiotic stress responses. Biochim. Biophys. Acta 1819 (3-4), 343–348. doi: 10.1016/j.bbagrm.2011.07.012
Keywords: salt tolerance related protein, cold stress, abscisic acid, Arabidopsis thaliana, late embryogenesis abundant proteins
Citation: Fiorillo A, Mattei M, Aducci P, Visconti S and Camoni L (2020) The Salt Tolerance Related Protein (STRP) Mediates Cold Stress Responses and Abscisic Acid Signalling in Arabidopsis thaliana. Front. Plant Sci. 11:1251. doi: 10.3389/fpls.2020.01251
Received: 15 April 2020; Accepted: 29 July 2020;
Published: 13 August 2020.
Edited by:
Alejandra A. Covarrubias, National Autonomous University of Mexico, MexicoReviewed by:
Vivekanand Tiwari, Agricultural Research Organization (ARO), IsraelCopyright © 2020 Fiorillo, Mattei, Aducci, Visconti and Camoni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sabina Visconti, dmlzY29udGlAdW5pcm9tYTIuaXQ=; Lorenzo Camoni, Y2Ftb25pQHVuaXJvbWEyLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.