
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci., 26 June 2020
Sec. Plant Systematics and Evolution
Volume 11 - 2020 | https://doi.org/10.3389/fpls.2020.00942
The typical plastid genome (plastome) of photosynthetic angiosperms comprises a pair of Inverted Repeat regions (IRs), which separate a Large Single Copy region (LSC) from a Small Single Copy region (SSC). The independent losses of IRs have been documented in only a few distinct plant lineages. The majority of these taxa show uncommonly high levels of plastome structural variations, while a few have otherwise conserved plastomes. For a better understanding of the function of IRs in stabilizing plastome structure, more taxa that have lost IRs need to be investigated. We analyzed the plastomes of eight species from two genera of the putranjivoid clade of Malpighiales using Illumina paired-end sequencing, the de novo assembly strategy GetOrganelle, as well as a combination of two annotation methods. We found that all eight plastomes of the putranjivoid clade have lost their IRB, representing the fifth case of IR loss within autotrophic angiosperms. Coinciding with the loss of the IR, plastomes of the putranjivoid clade have experienced significant structural variations including gene and intron losses, multiple large inversions, as well as the translocation and duplication of plastome segments. However, Balanopaceae, one of the close relatives of the putranjivoid clade, exhibit a relatively conserved plastome organization with canonical IRs. Our results corroborate earlier reports that the IR loss and additional structural reorganizations are closely linked, hinting at a shared mechanism that underpins structural disturbances.
Plastids, such as chloroplasts, chromoplasts, and leucoplasts, are the place for photosynthesis and the major organelle for organic product storage in plants. Plastids retain a semi-autonomous genetic system with their own genome (plastome). Typically, the plastome of a photosynthetic angiosperm is a circular molecule, with a length of 120–160 kb (Wicke et al., 2011). Structurally, such plastome comprises a pair of Inverted Repeat regions (hereafter called IRs; ~25 kb), a Large Single Copy region (LSC; ~85 kb), and a Small Single Copy region (SSC; ~15 kb) (Ruhlman and Jansen, 2014; Mower and Vickrey, 2018). IRs may play an important role in maintaining plastome stability (Maréchal and Brisson, 2010), which might be one of the reasons why most autotrophic angiosperms possess canonical IRs. However, IR losses have been documented in a few distinct angiosperm lineages, namely the IR-Lacking Clade (IRLC) of Leguminosae (Palmer and Thompson, 1981; Palmer and Thompson, 1982; but see Choi et al., 2019), two Erodium lineages of Geraniaceae (Guisinger et al., 2011; Ruhlman et al., 2017), Carnegiea gigantea of Cactaceae, and Tahina spectabilis of Arecaceae (Choi et al., 2019). Plastomes of the IRLC, C. gigantea (Sanderson et al., 2015), Tahina spectabilis (Barrett et al., 2016) showed significant higher rearrangement degrees compared to their sister clade, while species in a lineage of Erodium that has lost one IR exhibit an otherwise conserved plastome structure (Blazier et al., 2016). Hence, further comparative study is needed to elucidate the function of IRs in stabilizing plastome structure.
Malpighiales are one of the largest orders of flowering plants. Plants in this order exhibit a remarkable morphological and ecological diversity, with many species of great ecological and economic importance (Xi et al., 2012). Previous studies have revealed significant structural variations in the plastomes of multiple taxa in this order. Rabah et al. (2019) compared plastomes of 15 species of the genus Passiflora (Passifloraceae) and found that this genus has experienced widespread genomic changes, including inversions, gene and intron losses along with multiple independent IR expansions and contractions. Lopes et al. (2018) revealed the contraction and expansion of the IRs altering the size, gene content, and gene order of SC and IRs in the plastome of Linum usitatissimum (Linaceae). Tangphatsornruang et al. (2011) reported a 30-kb inversion between trnE-UUC—trnS-GCU and trnT-GGU—trnR-UCU in Hevea brasiliensis (Euphorbiaceae). Two recent studies detected an inversion in the LSC, significant variation in length reduction of the IRs, gene loss and pseudogenization events in plastomes of Podostemaceae (Bedoya et al., 2019; Jin et al., 2020). An inversion over 50 kb spanning from trnK-UUU to rbcL in the LSC is shared by Cratoxylum cochinchinense (Hypericaceae), Tristicha trifaria, and Marathrum foeniculaceum (Podostemaceae) (Jin et al., 2020). Previous studies suggested that multiple lineages of Malpighiales have experienced plastome structural variations, but knowledge of plastomes evolution in this large order is still limited.
The putranjivoid clade in Malpighiales consists of two families: Lophopyxidaceae and Putranjivaceae (Wurdack and Davis, 2009). Lophopyxidaceae have a single genus, whereas Putranjivaceae contain three genera and ca. 216 species. Containing 209 species, Drypetes is the largest genus in Putranjivaceae. The species in this clade are perennial trees or shrubs, growing primarily in tropical and subtropical areas (Kubitzki, 2014).
As it is unknown to date how plastid genomes evolve in the putranjivoid clade, we here assembled the complete plastome sequences for eight species, as well as two species from the closely related family Balanopaceae, representing one genus each from each family. Our analyses focused on exploring the structural variation of plastomes and revealed that all plastomes of the putranjivoid calde have lost the IRB entirely and experienced extensive additional structural rearrangements. In contrast, the plastomes of the two Balanopaceae species retain a relatively conserved plastome structure, indicating an evolutionary shift after the split of both lineages.
We sampled seven species from the largest genus Drypetes of Putranjivaceae, one species from Lophopyxidaceae, and two species from Balanopaceae as outgroups. Total genomic DNA of all samples was isolated from herbarium specimens or silica gel-dried leaves using the DNeasy Plant Mini Kit (Tiangen Biotech Co., LTD., Beijing, China) or a standardized CTAB-protocol (Doyle and Doyle, 1987). Following quantity checks and library preparations, paired-end sequencing was carried out on Illumina HiSeq 2000 or HiSeq X TEN at the Plant Germplasm and Genomics Center (Kunming Institute of Botany, Chinese Academy of Sciences). A genome skimming sequencing approach was employed. Table S1 provides original collection location, herbarium voucher information, GenBank accession numbers, as well as the read characteristics for all taxa discussed in this study.
Plastomes were assembled using GetOrganelle v1.6.1a with default settings, which filtered plastid-like reads, conducted the de novo assembly, purified the assembly graph, and generated the complete plastomes (Camacho et al., 2009; Bankevich et al., 2012; Langmead and Salzberg, 2012; Jin et al., 2019). K-mer gradients were set according to the sequenced read lengths as “-k 21,31,41,51,65,85,91,95,99,101,111,121,127” for 150 bp reads; “-k 21,31,41,51,61,71,81,85,87” were used for 90 bp reads. Final assembly graphs were visualized in Bandage (Wick et al., 2015) to confirm the automatically generated plastomes. Two configurations of each plastome caused by the flip-flop recombination mediated by the IR or the ~1.2 kb sIR (short Inverted Repeat regions) were obtained, and one of them was arbitrarily selected for downstream analysis (Walker et al., 2015). All plastomes were initially annotated using PGA (Qu et al., 2019) and GeSeq (Tillich et al., 2017), with the annotated plastome of Amborella trichopoda (NC_005086) (Goremykin et al., 2003) selected as the reference. For confirmation, all annotations were compared with the previously published plastome of Byrsonima coccolobifolia (NC_037191; Malpighiaceae; Menezes et al., 2018) and manually examined in Geneious Prime (https://www.geneious.com). All newly sequenced plastomes were deposited in GenBank under accession numbers MN504788–MN504797.
Phylogenetic analysis was performed using 71 protein-coding genes, which were shared by all study species (Table S2). Gene sequences were extracted using get_annotated_regions_from_gb.py (https://github.com/Kinggerm/PersonalUtilities, accessed on July 30, 2019; Zhang et al., 2020), aligned individually using prank v.140603 (Loytynoja and Goldman, 2008), then concatenated into a single aligned dataset using concatenate_fasta.py (https://github.com/Kinggerm/PersonalUtilities, accessed on July 30, 2019; Zhang et al., 2020). To reconstruct the phylogenetic relationships among our taxa, we employed RAxML v.8.2.11 (Stamatakis, 2014) with “-m GTRGAMMA”, which performs tree searches and optimization under the maximum likelihood paradigm. For statistical support, we ran 1,000 bootstrap replicates, and visualized the results in FigTree v.1.4.4 (http://tree.bio.ed.ac.uk). We mapped the events manually, facilitated by the small size of the data set, assuming that pseudogenization, gene loss, and IR loss are irreversible events.
To build whole plastome alignments for the putranjivoid clade, and the two Balanops species, we used the progressiveMauve algorithm in Mauve v2.3.1 (Darling et al., 2010) with default settings. The IRB was removed from plastid genomes with two copies of the large inverted repeats to allow for an optimal homology assessment (Wicke et al., 2013). Based on the strand orientation of the Locally Collinear Blocks (LCBs) identified by the progressiveMauve alignment, strand orientation determines the sign (+/-). Compared with the references, each LCB was numbered. Subsequently, we used GRIMM (Tesler, 2002) to calculate genome rearrangement distances.
Dispersed repeats (including forward, reverse, complement, and palindromic repeats) were identified by REPuter (Kurtz et al., 2001) based on the following criteria: minimum repeat size ≥ 30 bp; sequence identities ≥ 90%; Hamming distance = 3. Again, the IRB was removed, where present. REPuter overestimates the number of repetitive elements in a given sequence by recognizing nested or overlapping repeats within a given region containing multiple repeats (Wang et al., 2018). The FindRepeats plugin of Geneious Prime was also used to identify repeated regions using a minimum repeat length of 30 bp and zero mismatches.
sIR range from 11 bp to several kbs in plastomes and are capable of inducing plastomic inversions and isomer (Martin et al., 2014; Wang et al., 2018). As sIR can potentially induce isomers, we used the library information of paired-end reads to confirm the existence of each potential isomers in Lophopyxis maingayi. We mapped the paired-end reads to the plastome sequence of each isomer, visually inspected the mapped read pairs in Geneious, and verified the existence of properly-mapped read pairs spanning the entire sIR. An isomer with read pairs spanning the entire sIR was supported to exist. Specifically, we firstly conducted read mapping using the evaluate_assembly_using_mapping.py script from the GetOrganelle toolkit, which calls Bowtie2 (Langmead and Salzberg, 2012). Because of the relatively short average insert size (Table S1), most read pairs are too short in insert size for providing confirmation and hampered visual inspection. For better visualization, we filtered the alignment using SAMtools (Li et al., 2009) by keeping records with an insert size between 330 and 600. Finally, we imported the filtered alignment file (*.sam) into Geneious Prime, turn on the “Layout-Link paired reads” mode and checked whether there are read pairs spanning the entire sIR.
Due to the differences in plant materials, the average base coverages of plastomes varied from 72 x to 640 x (Table S1). However, all ten newly assembled plastomes were complete. Plastomes from the putranjivoid clade are relatively small compared to their sister family Balanopaceae (Figure 1; Table 1). Variation in plastome size of the sampled putranjivoid sepcecies was small: Drypetes hainanensis has the smallest plastome with a length of 119,105 bp, while Drypetes lateriflora has the largest plastome with a length of 120,800 bp.
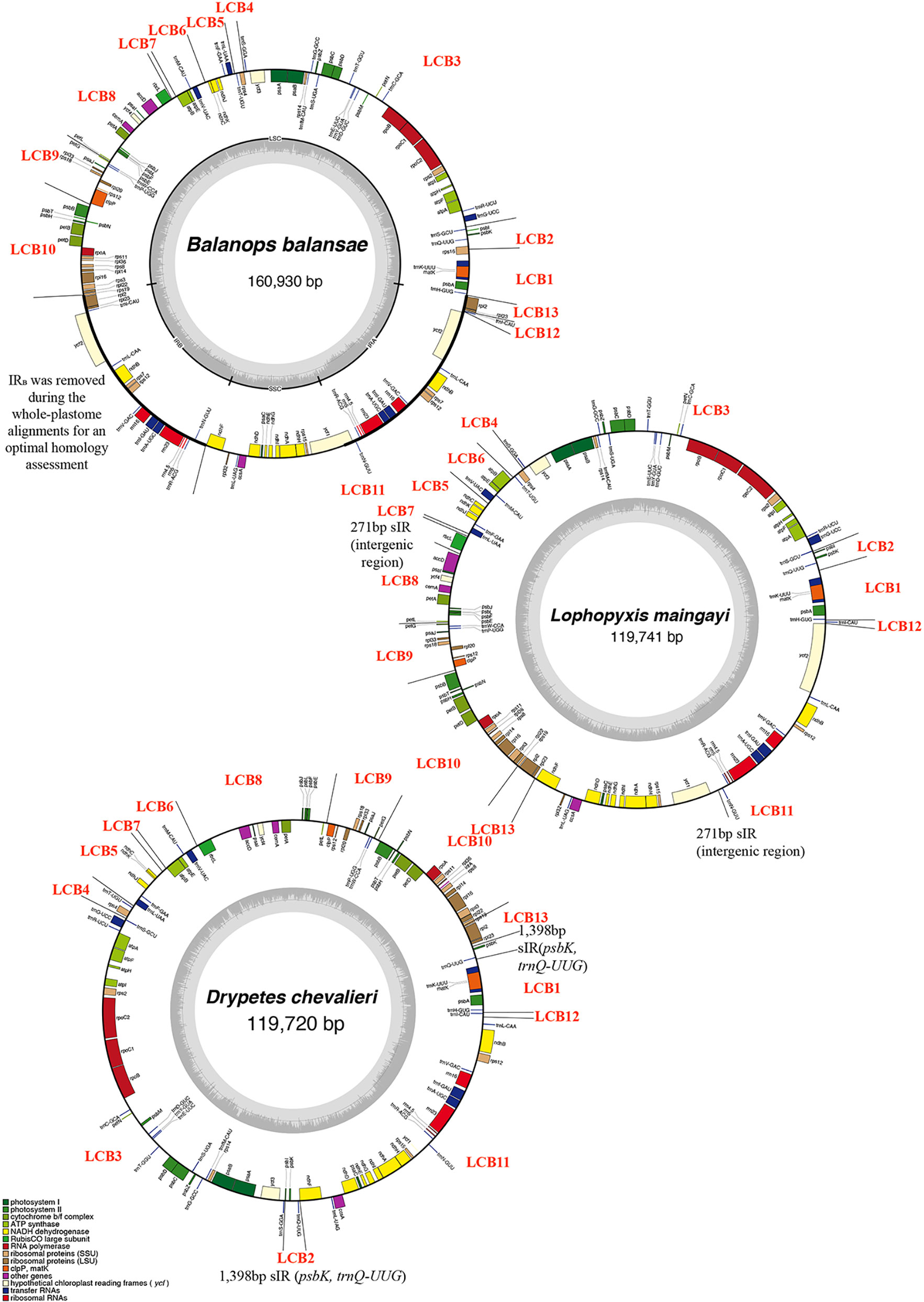
Figure 1 Plastid genomes of three species of Malpighiales representing three genera involved in this study. GC content graphs are shown as dark gray bars toward the center of each diagram. Each Locally Collinear Block (LCB) was indicated in the circular map, as well as the ~12kb short Inverted Repeat (sIR) and 271bp sIR.
Across autotrophic flowering plants, the content of IRs nearly universally includes all 4 rRNA genes, 7 tRNA genes, and a small number of protein genes (Mower and Vickrey, 2018). Plastomes of all studied putranjivoid species have lost a copy of the inverted repeat, namely IRB (Figure 1, Figure 2; Table 1), which led to the observed significant reduction of their overall plastome size. All sampled putranjivoid species have lost the same segment of IRB including 4 rRNA genes, 7 tRNA genes, and several protein coding genes (rps12, rps7, ndhB, ycf2, rpl23, and rpl2). Their plastome sizes were slightly varied due to the differences in intergenic regions. However, not all inversions are shared by L. maingayi and Drypetes species (Figure 1, Table 2).
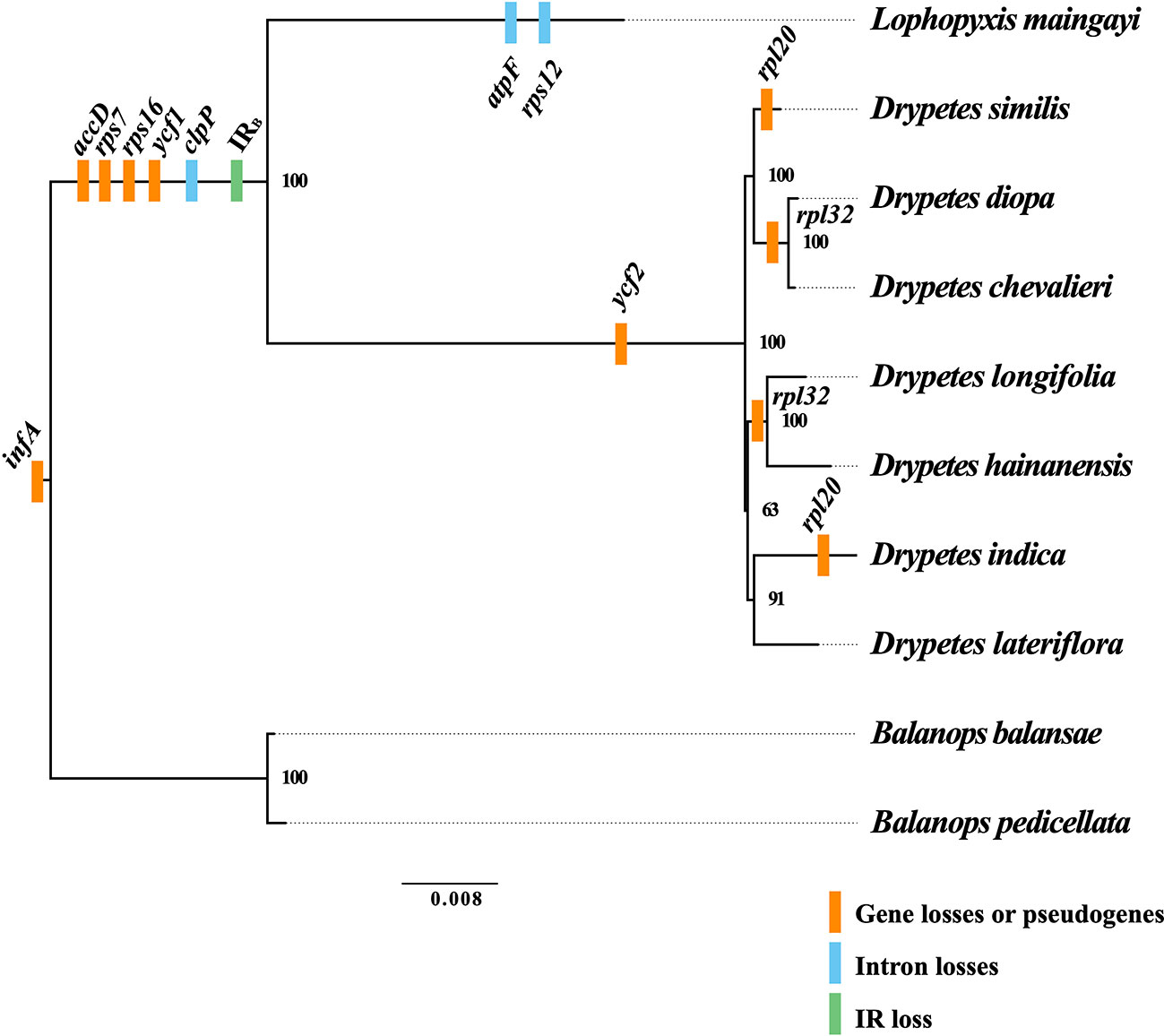
Figure 2 Gene losses or pseudogenes in the plastomes of the putranjivoid clade. Based on the phylogenetic tree, we mapped the events of gene and intron losses, as well as the event of the inverted repeat (IR) loss. Branch lengths correspond to substitutions per site.
To our knowledge, the IR loss event in the putranjivoid clade represents the fifth reported IR loss of autotrophic flowering plants. Among the five IR losses, the putranjivoid clade and Tahina spectabilis have lost IRB (Barrett et al., 2016), while the IR-lacking legumes (Palmer and Thompson, 1981; Palmer and Thompson, 1982), C. gigantea (Sanderson et al., 2015), and some Erodium species (Guisinger et al., 2011; Ruhlman et al., 2017) all have lost their IRA. Which copy of IR has been lost seems to be a stochastic phenomenon. The two identical copies of the IR contain the same genes. None of the IR-lacking lineages, including all putranjivoid species, exhibits an impaired phenotype or habits (Blazier et al., 2016). Therefore, we may conclude that for those lineages one copy per IR-gene seems to be sufficient to support the overall function of the plastid.
The plastomes of Balanopaceae, one of the closest relatives of the putranjivoid clade, possess a canonical IR structure and a relatively conserved gene content and organization, which resembles those of the supposed ancestral angiosperm plastome (Ruhlman and Jansen, 2014). However, the plastomes of the putranjivoid clade have experienced significant gene content changes (Table 1; Figure 2). All examined plastomes from the putranjivoid clade lack intact accD, rps7, rps16, and ycf1 genes (Figure 2), and all examined Putranjivaceae plastomes have one copy of the ycf2 gene lost or became a pseudogene. The rpl20 gene was inferred to be a pseudogene due to the presence of internal stop codons in the plastomes of D. similis and D. indica (Figure S1). Drypetes diopa, D. chevalieri, and D. longifolia lost the rpl32 gene independently, and the rpl32 gene of D. hainanensis was a pseudogene due to internal stop codons (Figure S2). The loss of rps16 is common in angiosperm plastomes (Jansen et al., 2007). A study in Medicago truncatula (Leguminosae) and Populus alba (Salicaceae) showed that the rps16 gene was lost in both species. However, the function of the plastid rps16 was compensated by a nuclear-encoded rps16 in both species (Ueda et al., 2008). The loss of accD in Trifolium species has been achieved by relocation to the nucleus (Magee et al., 2010). Two previous studies (Bedoya et al., 2019; Jin et al., 2020) suggested the uncommon loss or pseudogenization of ycf1 and ycf2 in Podostemaceae. Our results also suggested the loss or pseudogenization of ycf1 in the putranjivoid clade, and the loss or pseudogenization of ycf2 in Putranjivaceae. Moreover, all putranjivoid species lack both clpP introns, and L. maingayi lacks the typical introns in atpF and rps12 (Figure 2). Previous studies indicated the loss of rps12 and clpP introns in various legume lineages (Jansen et al., 2008; Wang et al., 2018). Recent studies on Podostemaceae also found the loss of both introns of clpP in riverweeds (Bedoya et al., 2019; Jin et al., 2020). The loss of the atpF intron was found not only in Lophopyxis maingayi, but also in members of Euphorbiaceae, Phyllanthaceae, Elatinaceae, and Passifloraceae of Malpighiales (Daniell et al., 2008). However, the mechanisms responsible for the intron losses remain elusive.
Plastomes of the putranjivoid clade have experienced notable structural reorganizations. Our progressiveMauve plastomes alignment of the putranjivoid clade with Balanops as references identified 13 syntenic regions (Figures 1 and 3, Figures S3 and S4; Table 2). Genes or intergenic regions located in each LCB were identified (Table 3). Plastomic rearrangement distances were estimated based on the LCB orientations. The plastome of L. maingayi showed fewer rearrangements than those of Putranjivaceae species (Figure 3), as reflected in a lower genome rearrangement distance of 3 for L. maingayi but a higher genome rearrangement distance of 7 for the Drypetes species (Table 1). In L. maingayi, an inversion altered the syntenic blocks (4) (5) (6) (7) into (4) (-6) (-5) (7). LCB (5) and (6) corresponded to a 7.5-kb region between atpB and trnL-UAA. The order of the LCBs (10) (-13), (-13) (11), and the disruption of the adjacency of blocks (12) (13) were also the results of a translocation of LCB (13). LCB (13) corresponds to a 2-kb region spanning from the rpl23 to the rpl2 gene. Alternatively, a reasonable explanation for the changes around LCB (13) is that the rpl23 and rpl2 genes located in IRA were lost, while the identical though inverted copies of these two genes from IRB remained intact. Plastomes of all Drypetes species shared all inversions (Figure 3, Figure S3, and Figure S4). One optimal reversal (means rearrangement event such as inversion) scenario included 7 inversion events, which means the minimum number of inversions required for transforming in gene order from a Drypetes plastome to a Balanops plastome is 7.

Figure 3 Plastid genome variation in the putranjivoid clade. Whole-plastome alignments divide the plastid genome of our study taxa into 13 Locally Collinear Blocks (LCB), which are shown as color-coded representations of syntenic regions. The IRB was removed from plastid genomes with two copies of the large inverted repeats to allow for an optimal homology assessment. Blocks below the horizontal central line represent inversions relative to the references, shown as the upper two taxa. The height of the colored region within a block reflects the average sequence identity relative to the reference. Species names are color-coded to indicate their family: Balanopaceae (black), Lophopyxidaceae (blue), and Putranjivaceae (purple). The pink blocks in both Balanops species indicate the IR regions. Red blocks represent rrn5, rrn4.5, rrn23, and rrn16 genes, green blocks represent trnA-UGC and trnI-GAU genes.
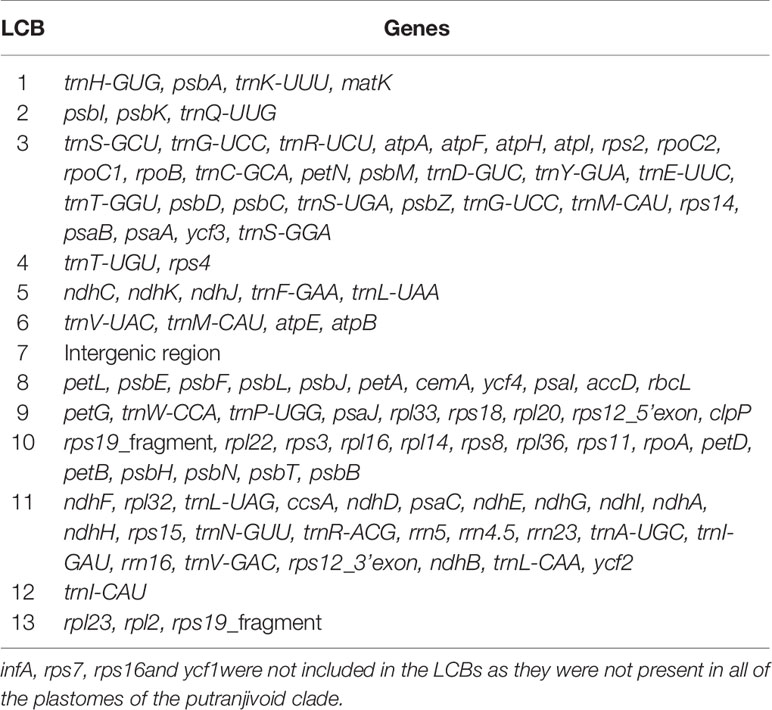
Table 3 Genes in Locally Collinear Blocks (LCB) identified using ProgressiveMauve alignment for plastomes of the putranjivoid clade.
IRs are thought to play a role in stabilizing the plastome (Maréchal and Brisson, 2010). This hypothesis is based on the fact that legume and conifer plastomes, which have no IRs, also show more rearrangements than plastomes containing canonical IRs (Palmer and Thompson, 1982; Hirao et al., 2008; Mower and Vickrey, 2018). The putranjivoid clade is another solid example that increased structural variations coincide with the loss of the IRs. However, species in a lineage of Erodium, which also have no IRs, still exhibit a conserved overall plastome structure, resembling those of IR-containing species (Blazier et al., 2016). In contrast, many species of Geranium and Pelargonium (Chumley et al., 2006; Guisinger et al., 2011; Röschenbleck et al., 2016; Weng et al., 2016) and Campanulaceae (Haberle et al., 2008), some of which have canonical though expanded IRs, possess highly rearranged plastomes. These cases suggest that further comparative study is needed to elucidate the function of IRs in stabilizing plastome structure.
An emerging consensus is that the presence of smaller repeats, rather than the loss of the IRs, is a major driver of plastome rearrangements (Mower and Vickrey, 2018). In the putranjivoid clade, we observed an obvious tendency that plastomes with more genomic rearrangements were also richer in repeats of 30 bp or more (Table 4). The number of short repeats are the largest in the Drypetes plastomes. While the Balanops plastomes, which are the most conserved ones have the fewest number of repeats. Furthermore, more rearrangement events also coincide with the presence of longer repeats (Table 4). Being the most rearranged, all Drypetes plastomes do possess a pair of sIRs with the length of more than 1,000 bp. As the only case that sIR induced gene duplication found in our study, all Drypetes species have two copy of two genes, psbK and trnQ-UUG, due to the ~1.2kb sIR. Typical IRs in plastomes trigger intra-plastomic homologous recombination, which generates two isomeric plastomes in equimolar abundance (Palmer, 1983; Martin et al., 2014). Multiple studies have detected isomeric plastome structures caused by sIR in several conifers and legumes (Tsumura et al., 2000; Wu et al., 2011; Yi et al., 2013; Qu et al., 2017; Wang et al., 2018). We also confirmed the existence of isomers induced by a pair of 271 bp sIRs in L. maingayi (Figure S5). Based on our findings, we conclude that smaller repeats indeed have played a role in enhancing plastome structural variation in the putranjivoid clade.
The datasets generated for this study can be found in the GenBank Database, MN504788–MN504797.
T-SY, J-JJ, and D-MJ designed the study. T-SY, J-BY, and D-MJ contributed to tissue sample collections, experiments, and sequences. D-MJ, J-JJ, and LG assembled the plastomes. D-MJ and J-JJ conducted the analysis. D-MJ, T-SY, SW, and J-JJ wrote and edited the manuscript; all authors commented on the manuscript.
This project was funded by grants from the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB31010000); the Large-scale Scientific Facilities of the Chinese Academy of Sciences (No. 2017-LSF-GBOWS-02); the National Natural Science Foundation of China [key international (regional) cooperative research project No. 31720103903]; and the open research project of “Cross-Cooperative Team” of the Germplasm Bank of Wild Species, Kunming Institute of Botany, Chinese Academy of Sciences.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank the Missouri Botanical Garden for providing specimens and the Molecular Biology Experiment Center, Germplasm Bank of Wild Species in Southwest China for skillful laboratory assistance.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2020.00942/full#supplementary-material
Bankevich, A., Nurk, S., Antipov, D., Gurevich, A. A., Dvorkin, M., Kulikov, A. S., et al. (2012). SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 19 (5), 455–477. doi: 10.1089/cmb.2012.0021
Barrett, C. F., Baker, W. J., Comer, J. R., Conran, J. G., Lahmeyer, S. C., Leebens-Mack, J. H., et al. (2016). Plastid genomes reveal support for deep phylogenetic relationships and extensive rate variation among palms and other commelinid monocots. New Phytol. 209 (2), 855–870. doi: 10.1111/nph.13617
Bedoya, A. M., Ruhfel, B. R., Philbrick, C. T., Madriñán, S., Bove, C. P., Mesterházy, A., et al. (2019). Plastid genomes of five species of riverweeds (Podostemaceae): structural organization and comparative analysis in Malpighiales. Front. Plant Sci. 10, 1035. doi: 10.3389/fpls.2019.01035
Blazier, J. C., Jansen, R. K., Mower, J. P., Govindu, M., Zhang, J., Weng, M.-L., et al. (2016). Variable presence of the inverted repeat and plastome stability in Erodium. Ann. Bot. 117 (7), 1209–1220. doi: 10.1093/aob/mcw065
Camacho, C., Coulouris, G., Avagyan, V., Ma, N., Papadopoulos, J., Bealer, K., et al. (2009). BLAST+: architecture and applications. BMC Bioinf. 10, 421. doi: 10.1186/1471-2105-10-421
Choi, I. S., Jansen, R., Ruhlman, T. (2019). Lost and found: return of the inverted repeat in the legume clade defined by its absence. Genome Biol. Evol. 11 (4), 1321–1333. doi: 10.1093/gbe/evz076
Chumley, T. W., Palmer, J. D., Mower, J. P., Fourcade, H. M., Calie, P. J., Boore, J. L., et al. (2006). The complete chloroplast genome sequence of Pelargonium x hortorum: organization and evolution of the largest and most highly rearranged chloroplast genome of land plants. Mol. Biol. Evol. 23 (11), 2175–2190. doi: 10.1093/molbev/msl089
Daniell, H., Wurdack, K. J., Kanagaraj, A., Lee, S. B., Saski, C., Jansen, R. K. (2008). The complete nucleotide sequence of the cassava (Manihot esculenta) chloroplast genome and the evolution of atpF in Malpighiales: RNA editing and multiple losses of a group II intron. Theor. Appl. Genet. 116 (5), 723–737. doi: 10.1007/s00122-007-0706-y
Darling, A. E., Mau, B., Perna, N. T. (2010). progressiveMauve: multiple genome alignment with gene gain, loss and rearrangement. PloS One 5 (6), e11147. doi: 10.1371/journal.pone.0011147
Doyle, J. J., Doyle, J. L. (1987). A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 19, 11–15.
Goremykin, V. V., Hirsch-Ernst, K. I., Wolfl, S., Hellwig, F. H. (2003). Analysis of the Amborella trichopoda chloroplast genome sequence suggests that Amborella is not a basal angiosperm. Mol. Biol. Evol. 20 (9), 1499–1505. doi: 10.1093/molbev/msg159
Guisinger, M. M., Kuehl, J. V., Boore, J. L., Jansen, R. K. (2011). Extreme reconfiguration of plastid genomes in the angiosperm family Geraniaceae: rearrangements, repeats, and codon usage. Mol. Biol. Evol. 28 (1), 583–600. doi: 10.1093/molbev/msq229
Haberle, R. C., Fourcade, H. M., Boore, J. L., Jansen, R. K. (2008). Extensive rearrangements in the chloroplast genome of Trachelium caeruleum are associated with repeats and tRNA genes. J. Mol. Evol. 66 (4), 350–361. doi: 10.1007/s00239-008-9086-4
Hirao, T., Watanabe, A., Kurita, M., Kondo, T., Takata, K. (2008). Complete nucleotide sequence of the Cryptomeria japonica D. Don. chloroplast genome and comparative chloroplast genomics: diversified genomic structure of coniferous species. BMC Plant Biol. 8, 70. doi: 10.1186/1471-2229-8-70
Jansen, R. K., Cai, Z., Raubeson, L. A., Daniell, H., dePamphilis, C. W., Leebens-Mack, J., et al. (2007). Analysis of 81 genes from 64 plastid genomes resolves relationships in angiosperms and identifies genome-scale evolutionary patterns. Proc. Natl. Acad. Sci. U. S. A. 104 (49), 19369–19374. doi: 10.1073/pnas.0709121104
Jansen, R. K., Wojciechowski, M. F., Sanniyasi, E., Lee, S. B., Daniell, H. (2008). Complete plastid genome sequence of the chickpea (Cicer arietinum) and the phylogenetic distribution of rps12 and clpP intron losses among legumes (Leguminosae). Mol. Phylogenet. Evol. 48 (3), 1204–1217. doi: 10.1016/j.ympev.2008.06.013
Jin, J.-J., Yu, W.-B., Yang, J.-B., Song, Y., dePamphilis, C. W., Yi, T.-S., et al. (2019). GetOrganelle: a fast and versatile toolkit for accurate de novo assembly of organelle genomes. bioRxiv. 265470. doi: 10.1101/256479
Jin, D.-M., Jin, J.-J., Yi, T.-S. (2020). Plastome structural conservation and evolution in the clusioid clade of Malpighiales. Sci. Rep. 10 (1), 9091. doi: 10.1038/s41598-020-66024-7
Kubitzki, K. (2014). Flowering Plants Eudicots: Malpighiales (Heidelberg: Springer: Springer-Verlag Berlin), 247–276.
Kurtz, S., Choudhuri, J. V., Ohlebusch, E., Schleiermacher, C., Stoye, J., Giegerich, R. (2001). REPuter: the manifold applications of repeat analysis on a genomic scale. Nucleic Acids Res. 29 (22), 4633–4642. doi: 10.1093/nar/29.22.4633
Langmead, B., Salzberg, S. L. (2012). Fast gapped-read alignment with Bowtie 2. Nat. Methods 9 (4), 357–359. doi: 10.1038/nmeth.1923
Li, H., Handsaker, B., Wysoker, A., Fennell, T., Ruan, J., Homer, N., et al. (2009). The sequence alignment/map format and SAMtools. Bioinformatics 25 (16), 2078–2079. doi: 10.1093/bioinformatics/btp352
Lopes, A. D., Pacheco, T. G., dos Santos, K. G., Vieira, L. D., Guerra, M. P., Nodari, R. O., et al. (2018). The Linum usitatissimum L. plastome reveals atypical structural evolution, new editing sites, and the phylogenetic position of Linaceae within Malpighiales. Plant Cell Rep. 37 (2), 307–328. doi: 10.1007/s00299-017-2231-z
Loytynoja, A., Goldman, N. (2008). Phylogeny-aware gap placement prevents errors in sequence alignment and evolutionary analysis. Science 320 (5883), 1632–1635. doi: 10.1126/science.1158395
Magee, A. M., Aspinall, S., Rice, D. W., Cusack, B. P., Semon, M., Perry, A. S., et al. (2010). Localized hypermutation and associated gene losses in legume chloroplast genomes. Genome Res. 20 (12), 1700–1710. doi: 10.1101/gr.111955.110
Maréchal, A., Brisson, N. (2010). Recombination and the maintenance of plant organelle genome stability. New Phytol. 186 (2), 299–317. doi: 10.1111/j.1469-8137.2010.03195.x
Martin, G. E., Rousseau-Gueutin, M., Cordonnier, S., Lima, O., Michon-Coudouel, S., Naquin, D., et al. (2014). The first complete chloroplast genome of the Genistoid legume Lupinus luteus: evidence for a novel major lineage-specific rearrangement and new insights regarding plastome evolution in the legume family. Ann. Bot. 113 (7), 1197–1210. doi: 10.1093/aob/mcu050
Menezes, A. P. A., Resende-Moreira, L. C., Buzatti, R. S. O., Nazareno, A. G., Carlsen, M., Lobo, F. P., et al. (2018). Chloroplast genomes of Byrsonima species (Malpighiaceae): comparative analysis and screening of high divergence sequences. Sci. Rep. 8 (1), 2210. doi: 10.1038/s41598-018-20189-4
Mower, J. P., Vickrey, T. L. (2018). “Structural diversity among plastid genomes of land plants,” in Advances in botanical research. Ed. J.R. Chaw, S. M. (Cambridge, MA, USA: Academic Press), 263–292.
Palmer, J. D., Thompson, W. F. (1981). Rearrangements in the chloroplast genomes of mung bean and pea. Proc. Natl. Acad. Sci. U. S. A. 78 (9), 5533–5537. doi: 10.1073/pnas.78.9.5533
Palmer, J. D., Thompson, W. F. (1982). Chloroplast DNA rearrangements are more frequent when a large inverted repeat sequence is lost. Cell 29 (2), 537–550. doi: 10.1016/0092-8674(82)90170-2
Palmer, J. D. (1983). Chloroplast DNA exists in two orientations. Nature 301, 92–93. doi: 10.1038/301092a0
Qu, X.-J., Wu, C.-S., Chaw, S.-M., Yi, T.-S. (2017). Insights into the existence of isomeric plastomes in Cupressoideae (Cupressaceae). Genome Biol. Evol. 9 (4), 1110–1119. doi: 10.1093/gbe/evx071
Qu, X.-J., Moore, M. J., Li, D.-Z., Yi, T.-S. (2019). PGA: a software package for rapid, accurate, and flexible batch annotation of plastomes. Plant Methods 15, 50. doi: 10.1186/s13007-019-0435-7
Röschenbleck, J., Wicke, S., Weinl, S., Kudla, J., Müller, K. F. (2016). Genus-wide screening reveals four distinct types of structural plastid genome organization in Pelargonium (Geraniaceae). Genome Biol. Evol. 9, 64–76. doi: 10.1093/gbe/evw271
Rabah, S. O., Shrestha, B., Hajrah, N. H., Sabir, M. J., Alharby, H. F., Sabir, M. J., et al. (2019). Passiflora plastome sequencing reveals widespread genomic rearrangements. J. Syst. Evol. 57 (1), 1–14. doi: 10.1111/jse.12425
Ruhlman, T. A., Jansen, R. K. (2014). “The Plastid Genomes of Flowering Plants,” in Chloroplast Biotechnology: Methods and Protocols. Ed. Maliga, P. (New York: Springer), 3–38.
Ruhlman, T. A., Zhang, J., Blazier, J. C., Sabir, J. S. M., Jansen, R. K. (2017). Recombination-dependent replication and gene conversion homogenize repeat sequences and diversify plastid genome structure. Am. J. Bot. 104 (4), 559–572. doi: 10.3732/ajb.1600453
Sanderson, M. J., Copetti, D., Burquez, A., Bustamante, E., Charboneau, J. L. M., Eguiarte, L. E., et al. (2015). Exceptional reduction of the plastid genome of saguaro cactus (Carnegiea gigantea): loss of the ndh gene suite and inverted repeat. Am. J. Bot. 102 (7), 1115–1127. doi: 10.3732/ajb.1500184
Stamatakis, A. (2014). RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30 (9), 1312–1313. doi: 10.1093/bioinformatics/btu033
Tangphatsornruang, S., Uthaipaisanwong, P., Sangsrakru, D., Chanprasert, J., Yoocha, T., Jomchai, N., et al. (2011). Characterization of the complete chloroplast genome of Hevea brasiliensis reveals genome rearrangement, RNA editing sites and phylogenetic relationships. Gene 475 (2), 104–112. doi: 10.1016/j.gene.2011.01.002
Tesler, G. (2002). GRIMM: genome rearrangements web server. Bioinformatics 18 (3), 492–493. doi: 10.1093/bioinformatics/18.3.492
Tillich, M., Lehwark, P., Pellizzer, T., Ulbricht-Jones, E. S., Fischer, A., Bock, R., et al. (2017). GeSeq - versatile and accurate annotation of organelle genomes. Nucleic Acids Res. 45 (W1), W6–W11. doi: 10.1093/nar/gkx391
Tsumura, Y., Suyama, Y., Yoshimura, K. (2000). Chloroplast DNA inversion polymorphism in populations of Abies and Tsuga. Mol. Biol. Evol. 17 (9), 1302–1312. doi: 10.1093/oxfordjournals.molbev.a026414
Ueda, M., Nishikawa, T., Fujimoto, M., Takanashi, H., Arimura, S., Tsutsumi, N., et al. (2008). Substitution of the gene for chloroplast rps16 was assisted by generation of a dual targeting signal. Mol. Biol. Evol. 25 (8), 1566–1575. doi: 10.1093/molbev/msn102
Walker, J. F., Jansen, R. K., Zanis, M. J., Emery, N. C. (2015). Sources of inversion variation in the small single copy (SSC) region of chloroplast genomes. Am. J. Bot. 102 (11), 1751–1752. doi: 10.3732/ajb.1500299
Wang, Y.-H., Wicke, S., Wang, H., Jin, J.-J., Chen, S.-Y., Zhang, S.-D., et al. (2018). Plastid genome evolution in the early-diverging legume subfamily Cercidoideae (Fabaceae). Front. Plant Sci. 9, 138. doi: 10.3389/fpls.2018.00138
Weng, M.-L., Ruhlman, T. A., Jansen, R. K. (2016). Expansion of inverted repeat does not decrease substitution rates in Pelargonium plastid genomes. New Phytol. 214 (2), 842–851. doi: 10.1111/nph.14375
Wick, R. R., Schultz, M. B., Zobel, J., Holt, K. E. (2015). Bandage: interactive visualization of de novo genome assemblies. Bioinformatics 31 (20), 3350–3352. doi: 10.1093/bioinformatics/btv383
Wicke, S., Schneeweiss, G. M., dePamphilis, C. W., Muller, K. F., Quandt, D. (2011). The evolution of the plastid chromosome in land plants: gene content, gene order, gene function. Plant Mol. Biol. 76 (3-5), 273–297. doi: 10.1007/s11103-011-9762-4
Wicke, S., Müller, K. F., de Pamphilis, C. W., Quandt, D., Wickett, N. J., Zhang, Y., et al. (2013). Mechanisms of functional and physical genome reduction in photosynthetic and nonphotosynthetic parasitic plants of the broomrape family. Plant Cell 25 (10), 3711–3725. doi: 10.1105/tpc.113.113373
Wu, C.-S., Lin, C.-P., Hsu, C.-Y., Wang, R.-J., Chaw, S.-M. (2011). Comparative chloroplast genomes of Pinaceae: insights into the mechanism of diversified genomic organizations. Genome Biol. Evol. 3, 309–319. doi: 10.1093/gbe/evr026
Wurdack, K. J., Davis, C. C. (2009). Malpighiales phylogenetics: gaining ground on one of the most recalcitrant clades in the angiosperm tree of life. Am. J. Bot. 96 (8), 1551–1570. doi: 10.3732/ajb.0800207
Xi, Z.-X., Ruhfel, B. R., Schaefer, H., Amorim, A. M., Sugumaran, M., Wurdack, K. J., et al. (2012). Phylogenomics and a posteriori data partitioning resolve the Cretaceous angiosperm radiation Malpighiales. Proc. Natl. Acad. Sci. U. S. A. 109 (43), 17519–17524. doi: 10.1073/pnas.1205818109
Yi, X., Gao, L., Wang, B., Su, Y.-J., Wang, T. (2013). The complete chloroplast genome sequence of Cephalotaxus oliveri (Cephalotaxaceae): evolutionary comparison of Cephalotaxus chloroplast DNAs and insights into the loss of inverted repeat copies in gymnosperms. Genome Biol. Evol. 5 (4), 688–698. doi: 10.1093/gbe/evt042
Keywords: plastome evolution, Inverted Repeat region loss, genomic rearrangement, Lophopyxidaceae, Putranjivaceae
Citation: Jin D-M, Wicke S, Gan L, Yang J-B, Jin J-J and Yi T-S (2020) The Loss of the Inverted Repeat in the Putranjivoid Clade of Malpighiales. Front. Plant Sci. 11:942. doi: 10.3389/fpls.2020.00942
Received: 29 January 2020; Accepted: 10 June 2020;
Published: 26 June 2020.
Edited by:
Gerald Matthias Schneeweiss, University of Vienna, AustriaReviewed by:
Sònia Garcia, Botanical Institute of Barcelona, SpainCopyright © 2020 Jin, Wicke, Gan, Yang, Jin and Yi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun-Bo Yang, amJ5YW5nQG1haWwua2liLmFjLmNu; Jian-Jun Jin, amluamlhbmp1bkBtYWlsLmtpYi5hYy5jbg==; Ting-Shuang Yi, dGluZ3NodWFuZ3lpQG1haWwua2liLmFjLmNu
†Present address: Susann Wicke, Institute for Biology, Humboldt-University Berlin, Berlin, Germany
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.