- 1Royal Botanic Gardens, Kew, Richmond, United Kingdom
- 2Welthungerhilfe, Freetown, Sierra Leone
- 3Department of Agriculture, Health and Environment, Faculty of Engineering and Science, Natural Resources Institute, University of Greenwich, Medway, United Kingdom
Coffea arabica (Arabica) and C. canephora (robusta) almost entirely dominate global coffee production. Various challenges at the production (farm) level, including the increasing prevalence and severity of disease and pests and climate change, indicate that the coffee crop portfolio needs to be substantially diversified in order to ensure resilience and sustainability. In this study, we use a multidisciplinary approach (herbarium and literature review, fieldwork and DNA sequencing) to elucidate the identity, whereabouts, and potential attributes, of two poorly known coffee crop species: C. affinis and C. stenophylla. We show that despite widespread (albeit small-scale) use as a coffee crop species across Upper West Africa and further afield more than 100 years ago, these species are now extremely rare in the wild and are not being farmed. Fieldwork enabled us to rediscover C. stenophylla in Sierra Leone, which previously had not been recorded in the wild there since 1954. We confirm that C. stenophylla is an indigenous species in Guinea, Sierra Leone, and Ivory Coast. Coffea affinis was discovered in the wild in Sierra Leone for the first time, having previously been found only in Guinea and Ivory Coast. Prior to our rediscovery, C. affinis was last seen in the wild in 1941, although sampling of an unidentified herbarium specimen reveals that it was collected in Guinea-Conakry in 2015. DNA sequencing using plastid and ITS markers was used to: (1) confirm the identity of museum and field collected samples of C. stenophylla; (2) identify new accessions of C. affinis; (3) refute hybrid status for C. affinis; (4) identify accessions confused with C. affinis; (5) show that C. affinis and C. stenophylla are closely related, and possibly a single species; (6) substantiate the hybrid C. stenophylla × C. liberica; (7) demonstrate the use of plastid and nuclear markers as a simple means of identifying F1 and early-generation interspecific hybrids in Coffea; (8) infer that C. liberica is not monophyletic; and (9) show that hybridization is possible across all the major groups of key Africa Coffea species (Coffee Crop Wild Relative Priority Groups I and II). Coffea affinis and C. stenophylla may possess useful traits for coffee crop plant development, including taste differentiation, disease resistance, and climate resilience. These attributes would be best accessed via breeding programs, although the species may have niche-market potential via minimal domestication.
Introduction
Coffee is a globally significant crop that supports a multibillion-dollar global industry (International Coffee Organization (ICO), 2019), over a lengthy value chain from farmer to consumer. Coffee farming alone involves the farming activities of around 100 million people worldwide (Vega et al., 2003). Two species dominate global coffee production: Arabica (Coffea arabica) and robusta (C. canephora), providing c. 60% and c. 40% of traded coffee, respectively (International Coffee Organization (ICO), 2019). Liberica coffee is cultivated worldwide in small quantities, and is insignificant in terms of global trade, although production in the Philippines and Malaysia can be substantial. Aside from C. arabica, C. canephora, and C. liberica, there are another 121 coffee species known to science (Davis et al., 2006, 2011, 2019). Some of these are used to make the beverage coffee, such as C. congensis, C. eugenioides, and C. racemosa, some have been used in breeding programs, and others have been used as high performing pest and diseases resistant rootstocks (Davis et al., 2019). Many more are used on a small, local scale, or are harvested directly from the wild, in Africa, Madagascar, and Asia. In previous centuries, and particularly at the end of the 1800s and early 1900s, there was considerable interest in, and use of, a range of beverage producing coffee species, more than there is today (Davis et al., 2019). It is also of note that since those times, a substantial proportion of the world’s coffee species diversity has been discovered and named by science, particularly from the 1960s onward (Bridson, 1988; Davis et al., 2006; Davis and Rakotonasolo, 2009). The decline in the interest in these ‘other’ coffee species has been largely due to the overwhelming success of robusta coffee, which was itself transformed from a wild plant, and minor African crop species, to a major global commodity in around 150 years (Davis et al., 2019). Robusta gained market share against Arabica from the early 1900s onward due to its resistance to coffee leaf rust (CLR; Hemileia vastatrix) (Wrigley, 1988), a broader agroecological envelope (Davis et al., 2006), higher productivity (Wellman, 1961; Wrigley, 1988), lower purchase price (International Coffee Organization (ICO), 2019), and other specific attributes (Davis et al., 2019). Recently, however, there has been renewed interest in underutilized, forgotten, and little known coffee species, both cultivated and wild, due to their potential to counter specific pests and diseases, and provide resilience in an era of accelerated climate change (Davis et al., 2019). There is also an increasing curiosity in lesser known coffee species from the specialty coffee sector, in its quest to discover new and differentiated sensory experiences in coffee.
Among those of particular interest are two West African species: C. stenophylla and C. affinis, mainly due to historical reports of a superior taste, particularly for C. stenophylla (Cheney, 1925) but also C. affinis (De Wildeman, 1904). Given that these two species occur in Upper West Africa at relatively low elevations (see below) there may also be the potential for climate resilience. Both species fall within Coffee Crop Wild Relative Priority Group II, which includes species closely related to the main crop species, for which gene transfer to the crop is proven or assumed (with low to high post-crossing fertility rates) (Davis et al., 2019). Priority Group II includes all African species, apart from the main coffee crop species and their progenitors (C. arabica, C. canephora, C. liberica, and C. eugenioides: Priority Group I) and African species of Priority Group III. Priority Group III includes all the short-styled Coffea species (previously assigned to the genus Psilanthus) from Africa, Asia and Australasia, and all Madagascan species and Mascarene species (Davis et al., 2019).
Our recent knowledge of C. stenophylla and C. affinis is principally limited to germplasm surveys. Coffea stenophylla is recorded as a living plant in several (ex situ) coffee research collections (Anthony et al., 2007; Engelmann et al., 2007; Bramel et al., 2017); C. affinis is included in the most recent of these reviews (Bramel et al., 2017) but only as an entry based on our knowledge of accepted coffee species (Govaerts et al., 2019). Contemporary evaluations of coffee species diversity (Davis et al., 2006, 2011, 2019; Maurin et al., 2007; Hamon et al., 2017) clearly show that our knowledge of C. stenophylla and C. affinis is inadequate. Initial review of literature for C. affinis showed almost no extra knowledge of this species has been gained since 1937 (Portères, 1937a), with the exception of work in Ivory Coast and Guinea in the 1980s (Berthaud, 1983, 1986; Le Pierrès et al., 1989). It is imperative that we improve our knowledge of these two species, both in cultivation (including any commercial production) and in the wild.
In this study, our main objectives were to elucidate: the current cultivated and wild status of C. stenophylla and C. affinis; the taxonomic identity and systematic position of the poorly known C. affinis; and to assemble available information on crop plant attributes. To achieve these objectives we undertook: (1) a literature review; (2) a survey of herbarium and economic botany collections; (3) field surveys in Sierra Leone, visiting farms, research stations and natural forest locations; and (4) DNA sequencing of recently collected material, historical samples (herbarium and economic botany collection samples), known interspecies hybrids, and their analysis incorporating a reference set of previously published Coffea sequences.
Materials and Methods
Literature Review
We examined all key literature pertaining to C. affinis and C. stenophylla. Knowledge of ex situ cultivation in research collections was gleaned from published works (Anthony, 1982, 1992; Anthony et al., 2007; Engelmann et al., 2007; Bramel et al., 2017; Davis et al., 2019), supported by personal observations (A. Davis, J. Haggar) and personal communication.
Review of Herbarium Collections and Economic Botany Collections
Herbarium specimens are well suited to this type of study because they are verifiable in space (location), time (date) and form (species identity), and are often accompanied by additional information on the herbarium label (e.g., ecology, elevation, geology, and uses). We consulted herbarium specimen records from nine herbaria (BM, BR, K, MO, P, UPS, WAG) including those in Sierra Leone (SL, FBC). Herbarium codes follow standard abbreviations (Holmgren et al., 1990; Thiers, 2019). The specimen data was disaggregated into unique records and duplicate specimens. Unique records comprise the combination of collector’s name and number (e.g., Chillou 2381) or collector’s name and date (e.g., Cope s.n., 7 iii 1912); s.n. is an abbreviation for sine numero, and lowercase Roman numerals to represent the month). Duplicate specimens possess the same unique identifier, i.e., they are from the same plant or possibly nearby individuals, but are found on separate herbarium sheets; these may either be found in the same herbarium or across two or more herbaria.
Fieldwork in Sierra Leone
During 2014 and 2016 we made a request for samples of C. affinis and C. stenophylla and any atypical coffee morphotypes, from NGOs and farmer associations representing 10,000 coffee farmers across Kenema, Kailahun, and Kono Districts, which represent the major coffee producing region of Sierra Leone. We also visited the Sierra Leone Agricultural Research Institute (SLARI) research collection at Pendembu, Kailahun District (Table 1), to sample putative examples of C. stenophylla and C. affinis. In addition, 50 A4 posters showing the most obvious morphological differences (leaf shape and size) between the two cultivated coffee species, robusta coffee (C. canephora) and Liberica (C. liberica) and C. stenophylla were printed and distributed to district agriculture offices with coffee farming communities in southern Sierra Leone, between Freetown and Kenema. The aim was to provide an additional means of identifying farms that might be cultivating C. stenophylla or C. affinis. Visits to sites where C. stenophylla had been recorded in cultivation in northern Sierra Leone (based on the herbarium survey) were visited in 2017. In December 2018, we followed up on the poster survey, by visiting five farms that had stated cultivation of C. stenophylla. On the same trip, we visited the last known (1954) forest sites for C. stenophylla in the Kasewe Hills (Southern Province), and several possible locations: around Freetown (Western Area), near Moyamba Junction (Southern Province), and the forest area of Kambui Hills, adjacent to Kenema (Eastern Province). Follow up visits to the Kambui Hills were made throughout 2019 and in early 2020.
Assembly of DNA Reference Collection
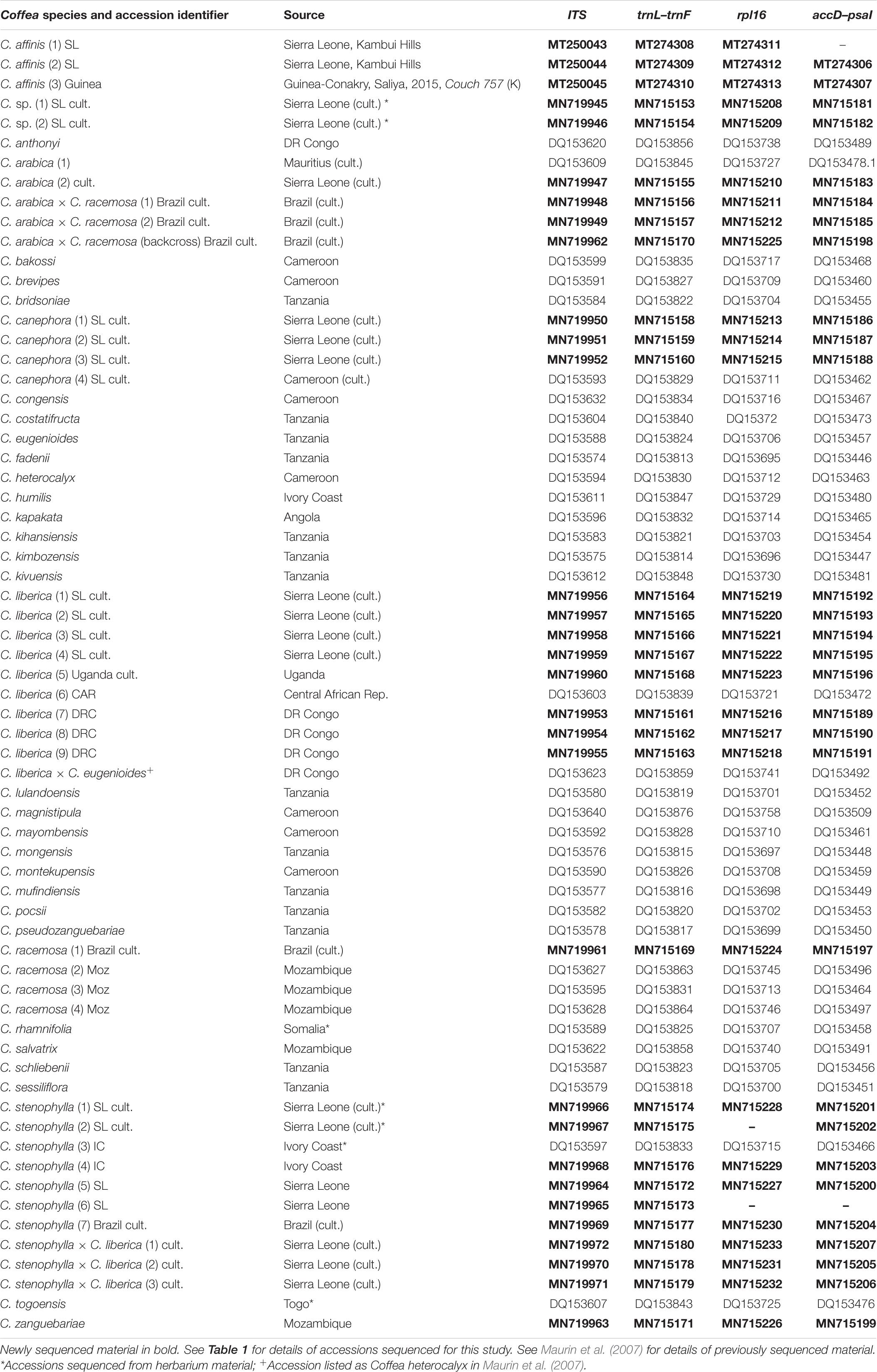
The most taxonomically comprehensive DNA dataset for wild coffee species is for plastid (trnL–F intron, trnL–F intergenic spacer (IGS), rpl16 intron and accD–psaI IGS) and the internal transcribed spacer (ITS) region of nuclear rDNA (ITS 1/5.8S/ITS 2) (Maurin et al., 2007; Davis et al., 2011). These markers have the ability to distinguish between African coffee species, and identify recently formed hybrids via differential inheritance of plastid and nuclear genomes (Maurin et al., 2007). Thirty-one accessions (Tables 1, 2) were sequenced with the four markers: 14 collected by us in Sierra Leone; nine reference samples from the museum collections of RBG Kew (K), including three for C. stenophylla, four for C. affinis (including two farmed accessions), and four other coffee species; five samples associated with the production of the artificial hybrid C. arabica × C. racemosa (Medina Filho et al., 1977a, b); and one unpublished sequence of C. zanguebariae (Table 2). The five museum collections of C. stenophylla and C. affinis were selected to represent authentic material, i.e., that being cultivated at the end of the 19th and beginning of the 20th centuries (respectively) in Sierra Leone. The published reference sequence dataset (Maurin et al., 2007; Davis et al., 2011) included a single verified example of C. stenophylla. Two known interspecific hybrids, as identified in a previous study using the same markers (Maurin et al., 2007), were included in the sampling: C. arabica (C. canephora × C. eugenioides) and C. liberica × C. eugenioides [originally accessioned (Maurin et al., 2007) as C. heterocalyx]. Initial analyses were conducted using the study species (see above) and a global data set of African, Madagascar, Mascarene, and Asian coffee species (Maurin et al., 2007; Davis et al., 2011). Following this analysis and confirming general placement of accessions, this was reduced to African taxa, excluding short-styled Coffea species (former Psilanthus), equating to Coffee Crop Wild Relative (Priority) Groups I and II (Davis et al., 2019).
DNA Extraction, Sequencing, and Data Analysis
Total DNA was extracted from silica dried leaves, fresh seeds, and seeds from herbarium specimens and other archival material (Tables 1, 2) using a modified CTAB method (Doyle and Doyle, 1987) and purified using the QIAquick PCR purification kit (QIAGEN). Genetic variation among the accessions was assessed by employing four regions: nuclear internal transcribed spacers (ITS1 and ITS2), plastid trnL–trnF (trnL intron and trnL–trnF intergenic spacer), rpl16 intron and accD–psaI intergenic spacer. Amplifications were carried out following the protocol of Maurin et al. (2007); PCR products were purified using QIAquick PCR purification kit (QIAGEN) and sequenced following the methods employed by Maurin et al. (2007). Capillary electrophoresis was conducted on an ABI3730 DNA Analyzer (Applied Biosystems). Sequencing results were inspected in GENEIOUS v. 8.1.7 (Kearse et al., 2012). Newly sequenced accessions and unpublished sequences held at RBG Kew were referenced against GenBank accessions of Coffea species (Maurin et al., 2007; Davis et al., 2011). The sequences were aligned using MUSCLE (Edgar, 2004), as implemented in GENEIOUS. Gaps were treated as missing data and ambiguities were scored with IUPAC ambiguity codes. The model of character evolution was assessed in jModelTest v. 2.1.10 (Posada, 2008). Relationships among the taxa were reconstructed in MrBayes v. 3.2.7a (Huelsenbeck and Ronquist, 2001; Ronquist and Huelsenbeck, 2003) as implemented on the CIPRES Scientific Gateway v3.3; C. rhamnifolia was used as the outgroup. Analyses were conducted separately for the ITS and the plastid DNA datasets and regions difficult to align were excluded from the phylogenetic analysis. We also conducted a separate analysis on the concatenated ITS/plastid matrix for the Upper Guinea (UG) clade (including C. togoensis, C. affinis, and C. stenophylla), with C. canephora and C. liberica as outgroups. MCMC sampling was performed with two runs and four chains for 2 × 107 generations, with a sampling frequency of 1,000 and a relative burn-in of 25%; specified model of character evolution was GTR+G. Convergence was visually assessed with Tracer v. 1.6 (Rambaut et al., 2013), by combining trace files to confirm mixing and high effective sampling size (EES). Maximum clade credibility trees were drawn in FigTree v. 1.4.4 (Rambaut, 2018). Clade and species alliance terminology follow Maurin et al. (2007) and Davis et al. (2011).
Results
Literature Review
Coffea stenophylla (Highland Coffee of Sierra Leone, Rio-Nunez Coffee, Senegal Coffee, Sierra Leone Coffee) (Figures 1, 2A)
Knowledge of C. stenophylla and its commercial potential dates back to at least 1794, based on reports by Adam Afzelius (1750–1837), who worked in, and collected plants from, Sierra Leone (Hiern, 1876). Coffea stenophylla was described as new to science in 1834 (Don, 1834) and was characterized on the basis of having narrow leaves (hence the species epithet) and black fruits (most coffee species have red fruits). Don (1834) stated that is was a ‘Native of Sierra Leone, where it is cultivated’… and that ‘The seeds of this species are roasted and used as the common coffee, and are even considered superior to it.’ De Wildeman (1904) reported C. stenophylla as indigenous in Guinea (in the forests that border the Southern Rivers area) and Ivory Coast. From at least the 1850s, the seeds of C. stenophylla were disseminated from Sierra Leone, with the accompanying vernacular names of ‘Highland coffee of Sierra Leone’ or ‘Sierra Leone coffee’ (Hooker, 1896). Commercial (cultivated) samples reached the Royal Botanic Gardens, Kew (England) in 1856 (specimens in the Economic Botany Collection, Kew). According to Chevalier (1929), C. stenophylla was being cultivated in quantity in Sierra Leone in the 1890s (c. 1893), and in Guinea, to the extent that is was exported as a commercial product to France. In France it apparently received an exceptionally favorable market price (Scott Elliot, 1893). Living material (seeds) of C. stenophylla were sent to the Royal Botanic Gardens, Kew in May 1894, and from here it was sent to India, Sri Lanka (then known as Ceylon), Trinidad (Figure 1), and Java (Cheney, 1925; Scott Elliot, 1893). From Guinea is was sent to Vietnam (Chevalier, 1929) and probably other countries under French colonial rule at that time. In 1904, De Wildeman (1904) provided a summary of the cultivation of C. stenophylla in Guinea, where it seems to have been cultivated in some quantity as Rio-Nunez coffee, after the Nunez River (a major river in Guinea). It was also cultivated in Ghana, Senegal (where it was known as Senegal coffee), and Ivory Coast, possibly through early intervention by the Portuguese (Haarer, 1962), and in Uganda (Tothill, 1940). Chevalier (1929), states that the export of C. stenophylla in Sierra Leone and Guinea amounted to around three to five tons (3,000 to 5,000 kg) per year, although this does not include the amount of coffee consumed in these producing countries, which may have been substantial. Coffea stenophylla appears to have been a prominent feature of agriculture in Sierra Leone up until at least the 1920s (Dudgeon, 1922), but it may have been in decline after that time (Chevalier, 1929), perhaps due to a fall in coffee prices (Dudgeon, 1922). Elsewhere, despite all the reports of an excellent flavor (Don, 1834; Scott Elliot, 1893; De Wildeman, 1904, 1906b; Watt, 1908; Macmillan, 1914; Dudgeon, 1922; Cheney, 1925; Chevalier, 1929; Wellman, 1961; Haarer, 1962) and a range of potential agronomic attributes (see below) it did not prevail as a coffee crop species. Chevalier (1929) reported that although it was considered by many to be an exquisite coffee (“Suivant beaucoup de dégustateurs, c’est un café exquis”) it was not widespread in global cultivation, due to low yields. Likewise, despite being introduced to Uganda in 1919 and then again in 1931, small bean size and low yield prevented it from being a sustainable coffee crop plant (Tothill, 1940). Despite this, a good agronomic performance at low elevations (e.g., c. 150 m) has been reported for C. stenophylla (Watt, 1908; Cheney, 1925; Bramel et al., 2017), and there is a report of potential resistance to CLR (Cheney, 1925). Given that the natural, and once-cultivated, environments of these two species in Upper West Africa were at relatively low elevations (150–610 m) (Watt, 1908) and that C. stenophylla is reported to withstand dry conditions (Wellman, 1961; Wrigley, 1988), there may also be some resilience to high temperatures and low rainfall, compared to the main crop species.

Figure 1. Coffea stenophylla, cultivated in Trinidad Botanical Garden, with Demerara sugarcanes, photograph taken around 1900. The man in the photograph is 5 ft. 8 in. (1.72 m) tall. Image: Royal Botanic Gardens, Kew.

Figure 2. Coffea affinis and C. stenophylla. (A) C. stenophylla in fruit, at Centre National de Recherche Agronomique (CNRA), Ivory Coast (image: Charles Denison); (B) C. affinis in flower; (C) C. affinis, fruits and seeds (partially dried); (D) C. affinis, leaves. Images (B–D), from Kambui Hills, Sierra Leone (images: Daniel Sarmu).
Since the 1940s, most of the literature on C. stenophylla has been restricted to the recycling of information from previous publications (Wellman, 1961; Haarer, 1962; Wrigley, 1988; Stoffelen, 1998; Davis et al., 2006). The exceptions to this are the reports of dedicated coffee collection missions in Upper West Africa undertaken in the 1980s. Reporting on various missions to Ivory Coast between 1984 and 1987, Le Pierrès et al. (1989) record two wild populations of C. stenophylla from the main forest block of the Sud-Est region of Ivory Coast (north east of Abidjan); and from Guinea, 114 examples of small scale cultivation of this species in the gardens of local houses (between Boffa and Boke, and around Boke). Berthaud (1986) demonstrated the existence of populations of C. stenophylla in Ivory Coast, from Ira Forest (Forêt L’Ira), three other localities (populations) in the east of the country. In addition, Berthaud (1983) recorded this species at a dry forest site in the Ouellé area in western Ivory Coast. Berthaud (1986) reported C. stenophylla, C. liberica, and C. canephora in Ira Forest (Forêt L’Ira); C. stenophylla was restricted to the upper, drier parts of the hills, whereas the other two species were found in the valley bottoms (lower, wetter areas).
Coffea affinis (Kamaya Coffee)
This species was first reported in c. 1900 from the coffee research garden of M. Boery in Guinea, having been originally collected from nearby native forests (De Wildeman, 1904). On first inspection, De Wildeman considered these plants to be similar in many characteristics to C. stenophylla (i.e., the presence of black fruits, rather than the usual red) but different in other respects and particularly leaf size and shape (De Wildeman, 1904). When De Wildeman visited Guinea (De Wildeman, 1904) to observe these plants, which were being grown collectively as Rio-Nunez coffee, he declared that there were two species, C. stenophylla and another species, which he named as a new to science: C. affinis. According to De Wildeman (1904) C. affinis was akin to C. stenophylla in the color and shape of the fruit, and shape of the seeds, but differed in its vegetative characters (e.g., stems, leaves, stipules) and mainly by its larger leaves. De Wildeman (1904) believed that C. affinis was of considerable importance as new coffee crop species, due to its vigor, the quality (high value) of the coffee, and general resistance to disease (compared to Arabica coffee). A contemporaneous photograph of C. affinis (De Wildeman, 1906b) shows a coffee plant that differs from the narrow-leaved C. stenophylla by having larger, broader leaves.
Contrary to the viewpoints of De Wildeman (1904, 1906b), Chevalier (1905) suggested that C. affinis was native in Sierra Leone, as he had no knowledge of it growing wild in Guinea. Subsequently, Chevalier (1929) considered C. affinis to be a hybrid between C. liberica and C. stenophylla. The potential hybrid status of C. affinis was discussed at length by Portères (1937a), who argued against a hybrid origin, particularly in relation to a new coffee plant he considered indigenous to the Ivory Coast, known locally as ‘Kamaya.’ He drew a close association between ‘Kamaya’ and C. affinis, but owing to the uncertainty over the application of C. affinis, decided to name this plant C. stenophylla var. camaya. Portères (Portères, 1937a) reported that C. stenophylla var. camaya was found as single example in a coffee plantation near Abengourou (Figure 3), but that it originated from the wild at a nearby location close to Niabli (6° 39′ N 3° 16′ W) and that a few small plantations were established in Ivory Coast. A decade later, Chevalier (1947) suggested that C. stenophylla var. camaya and C. affinis were the same species, and in contrast to his earlier report was only found in its wild state in Ivory Coast. Wellman (1961) considered C. affinis to be indigenous to Guinea and Ivory Coast, and suggested that it was a fixed mutation of C. stenophylla. Other workers (Cramer, 1957; Stoffelen, 1998) referred back to the earlier opinion of Chevalier (1905), i.e., that C. affinis is a hybrid between C. liberica and C. stenophylla (Davis et al., 2006).
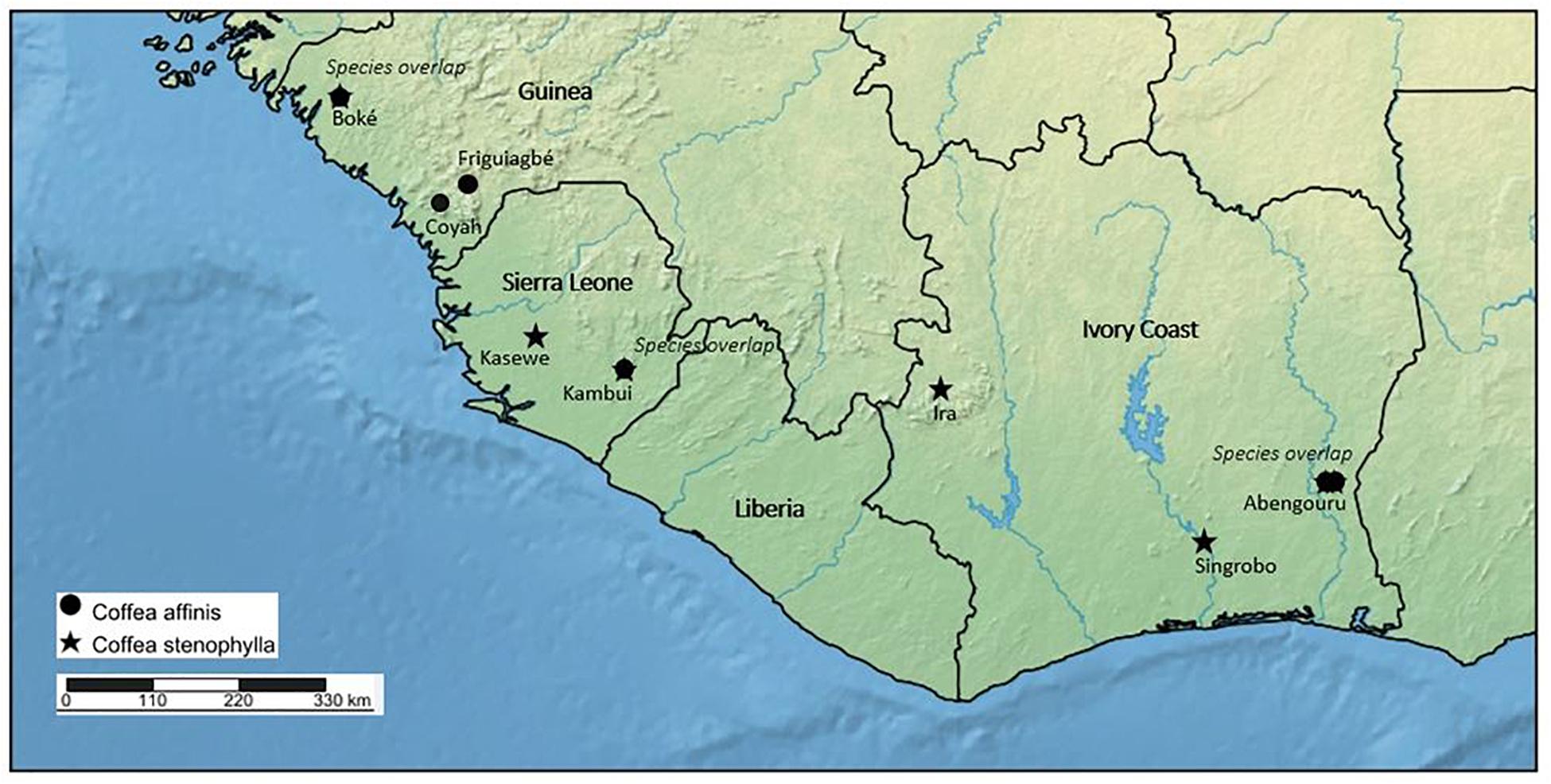
Figure 3. Distribution map of Coffea affinis and C. stenophylla based on herbarium and literature survey, and fieldwork. Labels beneath species symbols indicate general collection sites [Boké, Friguiagbé, Coyah (Saliya Forest Reserve), Kasewe (Kasewe Hills Forest Reserve, near Moyamba), Kambui (Kambui Hills Forest Reserve, near Kenema, Ira [Ira Forest (Forêt L’Ira), north of Man], Abengouru and Singrobo)]. Species overlap, indicates where C. affinis and C. stenophylla occur in the same location.
Herbarium Collection Survey
For C. affinis the herbarium survey yielded 12 unique records (seven cultivated and five wild records) with a total of 28 herbarium specimens (including duplicates), and for C. stenophylla 50 unique records (29 cultivated, 12 wild, and 9 with no data) and 72 herbarium specimens. Herbarium records associated with these species include the hybrid C. liberica × C. stenophylla, of which there were two unique records, both from cultivated material found in research collections. Compared to many other coffee species the number of herbarium specimens is low, especially for C. affinis. We examined two commercial seed collections of C. stenophylla, one with and one without parchment (pre-milling stage, with endocarp attached) and one of clean (green, pre-roasted) coffee (endocarp removed), and one fruit collection (whole, sun-dried fruits) from the Economic Botany Collection of the Royal Botanic Gardens, Kew (K). Examination of herbarium specimens confirms many aspects of the literature survey (see above).
From the herbarium survey, C. stenophylla is confirmed as an indigenous (wild) species of Guinea, Sierra Leone and Ivory Coast (Davis et al., 2006), and that it was also farmed and otherwise cultivated (e.g., research stations and farms) in these countries, as per the literature (see above). Most of the herbarium specimens date from the late 1800s and early 1900s. The most recent collections for these countries are as follows: Guinea (from the wild, 1941; from (small-scale) farm cultivation 1961); Sierra Leone [wild, 1954; from (small-scale) farm cultivation 1963]; Ivory Coast (wild 1932; from farms, no data). By comparison, the literature survey reveals small scale cultivation of C. stenophylla in Guinea and Ivory Coast, in the mid to late 1980s (Berthaud, 1983, 1986; Le Pierrès et al., 1989). Herbarium data also show that this species was cultivated in coffee research collections, and other germplasm collections, in Africa (Ivory Coast, Guinea, Nigeria, Sao Tomé, Sierra Leone, Tanzania, Ghana, and the Democratic Republic of Congo) and in Asia (Vietnam, Java).
The herbarium survey reveals that C. affinis is as an indigenous (wild) species of Guinea and Ivory Coast (De Wildeman, 1904). Chevalier could not find any evidence of its wild status in Guinea, referring only to cultivated material from the gardens of Conakry and Cameyenne (Chevalier, 1905), but herbarium data provide clear evidence of collections from natural forests in Guinea (see below). In contrast to the views of Chevalier (1905), and even more recent opinion (Davis et al., 2006) we could not find any evidence of wild C. affinis in Sierra Leone, which is also the case for the literature survey (but see Fieldwork in Sierra Leone, below). Herbarium specimens exist that were collected from a coffee plantation [Cope s.n., 7 iii 1912 (K)], labeled as C. affinis, which has the key leaf characteristics of this species but the flowers are absent, and the fruit color is not noted. Regarding the native status of C. affinis in Guinea, there are two collections in the Paris herbarium (P) collected from the environs of Boké (Boké Prefecture) that are identifiable as C. affinis and the labels clearly state that the plants were spontaneous (wild): Chillou s.n. 20 xii 1923 (three duplicates); Chillou s.n., 17 xii 1923 (two duplicates); a third specimen collected from Friguiagbé (Kindia Prefecture), by the same collector (Chillou 2381, 3 ii 1941) is also likely to be spontaneous, although the native/cultivated status is not indicated on the specimen. The most recent collections for these countries are as follows: Guinea (from the wild, 1941; from farms, 1905); Sierra Leone (from a coffee plantation, 1912); Ivory Coast (wild 1930; from farms 1934).
Fieldwork in Sierra Leone
A collection of 20 samples (leaf samples, images, and DNA samples) were made between 2014 and 2016 resulting from our request for samples of C. affinis and C. stenophylla and of any atypical coffee morphotypes (see the section “Materials and Methods”). Of these, seven were selected for DNA analysis (Table 1); the remaining samples conformed to regular variants of C. robusta and C. liberica. Three samples of putative C. stenophylla and C. affinis coffee were collected from the SLARI research collection (Table 1). The 10 samples were either considered as potential candidates for C. stenophylla or C. affinis, or hybrids between these species. Visits to sites (in 2017) where C. stenophylla had been recorded in cultivation in northern Sierra Leone failed to produce any coffee sightings. In December 2018, we followed up on the farm survey, visiting five farms that had stated cultivation of C. stenophylla, but no plants of this species or C. affinis were located (only C. canephora). Our visit to Kasewe Hills (Southern Province) resulted in the collection of a single sterile (no flowers or fruits) immature plant, which we preliminary identified as C. stenophylla. We did not find any plants matching C. stenophylla in forest locations within the Western Peninsula National Park (near Freetown) or near Moyamba Junction, but located a small population (with mature trees up to 7 m tall) matching this species in the forested area of Kambui Hills. At both localities the plants were collected in humid evergreen (lowland) forest at c. 400 m elevation — on a ridge top in the case of Kasewe Hills and on the side of a ridge on steeply sloping ground at Kambui Hills. Further visits to Kambui Hills throughout 2019 and early 2020 yielded further C. stenophylla, and trees provisionally identified as C. affinis, in both flower and fruit (Figures 2B–D). DNA samples from the two locations (Kasewe Hills and Kambui Hills) were added to the DNA analyses (see below and Table 1).
DNA Analyses
A total of 120 sequences from four DNA regions, from 31 accessions, were generated for this study and their sequences deposited in GenBank (with NCBI accession numbers; see Table 2); 35 species-level reference sequences were downloaded from GenBank, from two previous studies (Maurin et al., 2007; Davis et al., 2011). The ITS alignment had a length of 804 bp, whereas the concatenated plastid dataset (trnL–trnF, rpl16 and accD–psaI) had a total length of 3253 bp. A merged ITS/plastid matrix for a subset of species containing members of the Upper Guinea (UG) Clade, plus two outgroup species, had a length of 3989 bp. Some sequences were missing due to poor sequencing quality: rpl16 in C. stenophylla (2) SL cult. and C. stenophylla (6) SL; and accD–psaI in C. stenophylla (2) SL cult. and C. affinis (1) SL. Accession information is given in Tables 1, 2.
ITS Analysis (Figure 4)
The results obtained are consistent with previously obtained ITS analysis (Maurin et al., 2007; Davis et al., 2011), in terms of species relationships and their placement into geographically delimited clades. All tetraploid hybrids (4n = 44) between C. arabica and C. racemosa are placed in the East African (EA) Clade (2) with C. racemosa (BS = 1). The diploid hybrid (2n = 22) C. liberica × C. eugenioides is placed (BS = 1) with species of the East-Central Africa (EC-Afr) Clade, in an unresolved position with C. eugenioides. The natural tetraploid hybrid (4n = 44) C. arabica (two accessions) is placed with species of the Lower Guinea/Congolian (LG/C) Clade in the ‘Canephora Alliance’ (BS = 1), sister to two accessions of C. canephora (BS = 1). Specimens of C. stenophylla collected from the wild in Sierra Leone [specimens (5) SL & (6) SL], other wild accessions of this species, and wild accessions of C. affinis from Sierra Leone, all fall within the Upper Guinea (UG) Clade (BS = 0.93) with C. humilis and C. togoensis [specimens (1) SL & (2) SL]. Three cultivated collections from Sierra Leone originally accessioned as C. arabica and C. affinis (×2) were placed in the Upper Guinea (UG) Clade (labeled in Figure 4 as C. liberica × C. stenophylla SL cult. (1), (2), and (3). The historical accessions of farmed C. affinis from Sierra Leone (C. affinis in Table 1) were not placed with C. stenophylla or C. affinis, but in a clade with C. liberica and C. montekupensis (BS = 0.79), within the LG/C Clade [labeled as C. sp. (1) and C. sp. (2) in Figure 4].
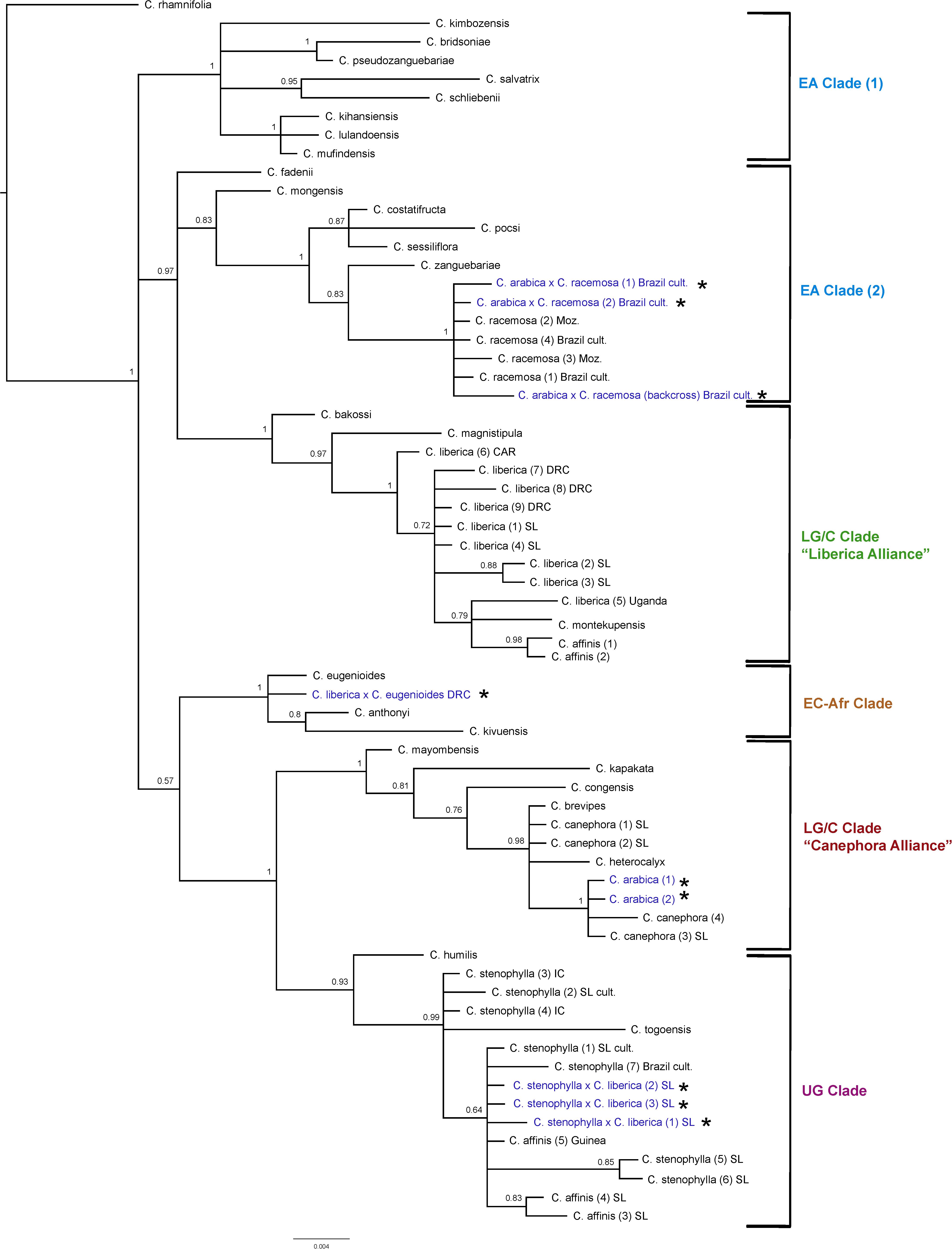
Figure 4. ITS maximum clade credibility tree. Bayesian posterior probabilities are indicated above branches. See Tables 1, 2 for accession information. Country abbreviations: CAR, Central African Republic; DRC, Democratic Republic of Congo; IC, Ivory Coast; Moz, Mozambique; SL, Sierra Leone. Clade terminology follows Maurin et al. (2007) and Davis et al. (2011): EA, East Africa; LG/C, Lower Guinea/Congolian; EC-Afr, East-Central Africa; UG, Upper Guinea. All known and identified interspecies hybrids are marked in blue text and with a star (*).
Plastid Analysis (Figure 5)
The results obtained are consistent with previously obtained plastid analysis using the same markers (Maurin et al., 2007; Davis et al., 2011), in terms of the relationships between species and their placement into geographically delimited clades. The F1 tetraploid hybrids (4n = 44) C. arabica × C. racemosa are placed in the East-Central Africa (EC-Afr) Clade, sister to a clade comprising C. arabica, C. eugenioides, C. kivuensis, and C. anthonyi. In contrast to the ITS analysis, the backcrossed C. arabica × C. racemosa is placed in the EA clade with wild and cultivated C. racemosa accessions. Coffea liberica × C. eugenioides is placed in one of the Lower Guinea/Congolian (LG/C) Clades with two species, C. liberica and C. magnistipula (BS = 0.66) in an unresolved position with three C. liberica accessions (BS = 0.99). The tetraploid (4n = 44) hybrid species C. arabica is placed with species of the EC-Afr Clade, viz. C. eugenioides, C. kivuensis, and C. anthonyi (BS = 0.97). Three cultivated collections from Sierra Leone originally accessioned as C. arabica and C. affinis (×2) were placed within one of the two LG/C clades [labeled in Figure 5 as C. liberica × C. stenophylla SL cult. (1), (2), and (3)] in an unresolved position in a clade with various C. liberica accessions (BS = 0.97), and a subclade of C. liberica × C. eugenioides, C. liberica and C. magnistipula (BS = 0.66).
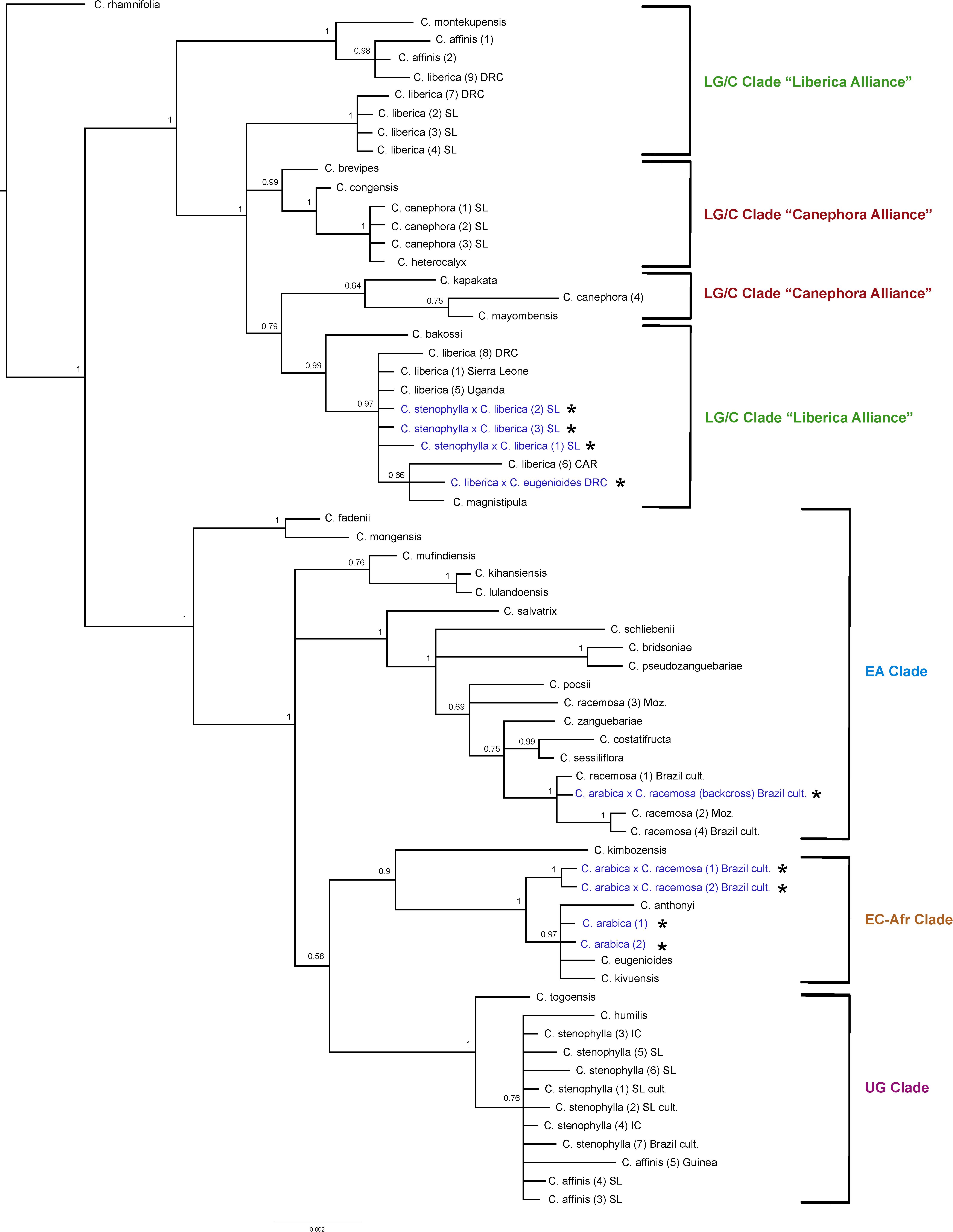
Figure 5. Plastid maximum clade credibility tree. Bayesian posterior probabilities are indicated above branches. See Tables 1, 2 for accession information. Country abbreviations: CAR, Central African Republic; DRC, Democratic Republic of Congo; IC, Ivory Coast; Moz, Mozambique; SL, Sierra Leone. Clade terminology follows Maurin et al. (2007) and Davis et al. (2011): EA, East Africa; LG/C, Lower Guinea/Congolian; EC-Afr, East-Central Africa; UG, Upper Guinea. All known and identified interspecies hybrids are marked in blue text and with a star (*).
All wild and cultivated accessions of C. stenophylla, and wild accessions of C. affinis fall within the UG Clade (BS = 1), which includes C. togoensis and C. humilis. A separate clade containing only all wild and cultivated accessions of C. stenophylla and all wild species of C. affinis, but including C. humilis fall within and in an unresolved clade (BS = 0.76). Our historical accessions of farmed C. affinis (C. affinis in Table 1) from Sierra Leone are not placed with C. stenophylla or C. affinis, but in a clade with C. liberica (BS = 0.98), sister to C. montekupensis (BS = 1), within one of the two LG/C clades [accessions are labeled as C. sp. (1) and C. sp. (2) in Figure 5].
Incongruence Between ITS and Plastid Trees
There are several points of substantial incongruence between the ITS and plastid analyses (Figures 4, 5), including those taxa of known (manmade) or proven (via DNA study) hybrid origin: C. arabica (C. arabica × C. eugenioides (Maurin et al., 2007), C. arabica × C. racemosa (Medina Filho et al., 1977a, b), and C. liberica × C. eugenioides (Maurin et al., 2007). These incongruencies are anticipated based on the knowledge, DNA and otherwise, that they are hybrids. In this study we identified three samples, originally accessioned (Table 1) as C. arabica and C. affinis (× 2), that were substantially incongruent in each analysis. Given the specific positions of these accessions in the analyses (see ITS and plastid results, and Figures 4, 5), and their morphological features, we suggest that they represent the hybrid C. stenophylla × C. liberica. All known and identified interspecies hybrids are marked in the phylogenetic trees (Figures 4, 5) in blue text and with a star (∗).
Combined Analysis for the Upper Guinea (UG) Clade (Figure 6)
Combining ITS and plastid data sets for all members of the Upper Guinea (UG) clade, C. humilis, C. togoensis, C. affinis, and C. stenophylla, produced relationships congruent with previous analyses using the same data (Maurin et al., 2007; Davis et al., 2011). The four species of the UG Clade are monophyletic (BS = 1), with C. humilis sister to C. togoensis, C. affinis and C. stenophylla (BS = 1); C. affinis and C. stenophylla form a clade (BS = 0.88) but each species is not monophyletic. The Kambui Hills accessions of C. affinis [(1) SL and (2) SL] and C. stenophylla accessions from Kasewe Hills and Kambui Hills [(5) SL and (6) SL, respectively] are monophyletic (BS = 0.92), and are sister to two accessions of C. stenophylla [(3) IC and (4) IC] from Ivory Coast (BS = 0.76). The C. affinis accession [(3) Guinea] from Guinea falls (BS = 0.78) with C. stenophylla accessions [(1) SL cult. and (7) Brazil cult], which originate from unknown localities in Sierra Leone.
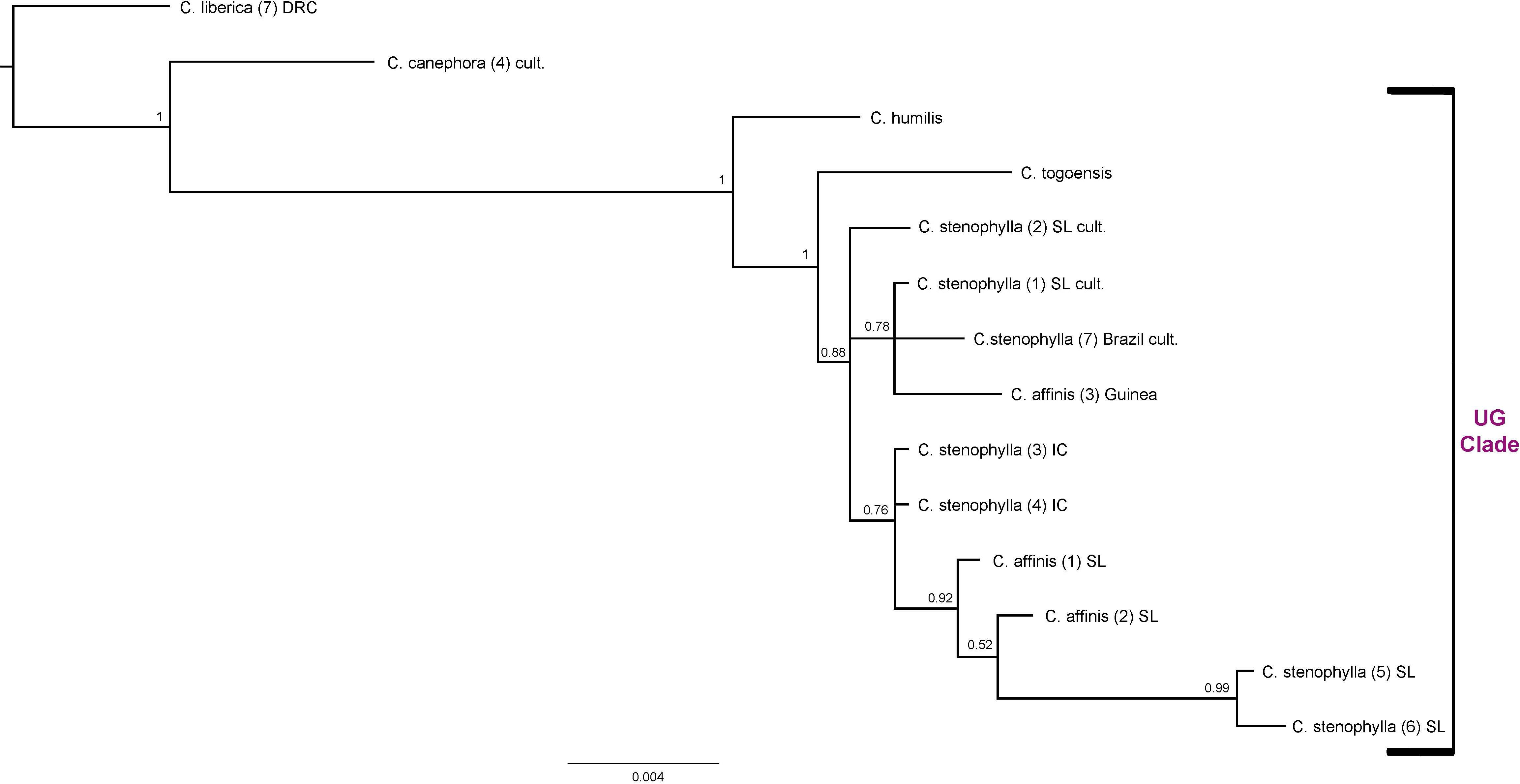
Figure 6. Combined ITS and plastid maximum clade credibility tree. Bayesian posterior probabilities are indicated above branches. See Tables 1, 2 for accession information. Country abbreviations: IC, Ivory Coast; SL, Sierra Leone. Clade terminology follows Maurin et al. (2007) and Davis et al. (2011): UG, Upper Guinea.
Discussion
Historical and Present-Day Status of C. affinis and C. stenophylla
In 2018, we rediscovered C. stenophylla in two locations in Sierra Leone, one from where it been collected before (Kasewe Hills, in 1954) and one a new location (Kambui Hills) (Figure 3). In both locations, C. stenophylla is extremely localized, and seemingly threatened. In the Kasewe Hills, near Moyamba, we were only able to locate a single plant, in an area of high deforestation. In the Kambui Hills, near Kenema, we located a small population, the extent of which is as yet unknown, but there are ongoing threats from logging, human encroachment, and artisanal gold mining. In late 2019, we located C. affinis in the Kambui Hills, not far from the populations of C. stenophylla. This is the first record of this species from the wild in Sierra Leone. The present day status of C. stenophylla and C. affinis in Guinea and Ivory Coast is poorly known. In Guinea, C. stenophylla was recorded as being under limited small scale cultivation in the 1980s (Le Pierrès et al., 1989), but there were no records of wild plants at that time. From a basic survey of remaining forest cover in Guinea, using satellite imagery (Google Earth, 2019), the likelihood of finding C. affinis and C. stenophylla in many of the localities where it was previously recorded as an indigenous plant (see the section “Results”; Figure 3) is limited, although possible. Field survey in more remote locations in Guinea, in appropriate environments and elevations, may reveal wild populations of these species. Indeed, we report here on a recent collection [2015; Couch 757 (K)] of a sterile (no flowers or fruit) coffee specimen from Guinea, which was identified on the basis of our DNA sequencing and morphology as C. affinis (see below). Despite this encouraging find, deforestation rates in Guinea are very high and ongoing (Couch et al., 2019); in 1992 it was calculated that 96% of the original forest had already been destroyed (Sayer et al., 1992). In Ivory Coast, the likelihood of finding more extensive wild populations of C. stenophylla and C. affinis are better, particularly as satellite data (Google Earth, 2019) shows the existence of native remnant vegetation in localities where this species was previously recorded, and where it is likely to be located. That said, one of the best known forest sites for this species in Ivory Coast (Berthaud, 1986), i.e., at Ira Forest (Forêt L’Ira), was largely destroyed around 2008. In summary, C. stenophylla and C. affinis are threatened throughout their indigenous ranges, and particularly in Guinea. On the IUCN Red List C. stenophylla is assessed as Vulnerable (VU); C. affinis is currently Data Deficient (DD) (International Union for Conservation of Nature (IUCN), 2020). On the basis of our literature, herbarium, and field survey, and DNA analysis, C. stenophylla and C. affinis are indigenous species of Guinea, Ivory Coast and Sierra Leone (Figure 3).
Historical data shows that C. stenophylla and C. affinis were farmed in some quantity in Upper West Africa, and especially C. stenophylla in Guinea and Sierra Leone. Coffea stenophylla was also widely cultivated in research stations across Africa and in various Asian countries; the presence of C. affinis in research stations appears to have been restricted to Upper West Africa (Guinea, Ivory Coast) during the early part of the last century. Our field surveys in Sierra Leone indicates that neither C. stenophylla nor C. affinis are under commercial cultivation (i.e., being farmed), or otherwise cultivated, in the present day. The last confirmed record of C. stenophylla production in Sierra Leone may have been the small plantation at Njala University grounds, which was apparently cut down after being abandoned in the 1980s (A. Lebbie pers. comm.). In Guinea, C. stenophylla was recorded as being under limited small scale cultivation in the 1980s (Le Pierrès et al., 1989). Coffea stenophylla has been recorded recently in several coffee research collections including the Centre National de Recherche Agronomique (CNRA), in Ivory Coast (Figure 2A); L’Institut de recherche pour le développement (IRD), France [A. Davis pers. observ.], and Entebbe Botanical Gardens, Uganda [A. Davis pers. observ.]. It is also likely to exist in other ex situ collections. However, across the ex situ coffee germplasm network there is the problem of duplication, i.e., the same genotype(s) being represented in multiple sites (Anthony et al., 2007; Bramel et al., 2017; Davis et al., 2019). There also seems to be some confusion with the narrow-leaved variant of C. arabica ‘Angustifolia,’ which owing to its narrow leaves is sometimes mistakenly accessioned as C. stenophylla. Coffea affinis has not been recorded in coffee research collections since the beginning of the twentieth century. Genotyping by sequencing, or similar methods, are required to make a full assessment of ex situ collections of C. stenophylla, and all other coffee species.
Natural Habitat (Growing Environment) of C. affinis and C. stenophylla
Knowing the location and associated habitat of crop wild relatives is important, as it can provide an initial assessment of environmental suitability as a crop plant. Our literature survey indicates that C. stenophylla may have drought tolerance characteristics (Portères, 1937b; Wellman, 1961; Wrigley, 1988). Both species occur at relatively low elevations (150–700 m) (De Wildeman, 1906a; Watt, 1908; Portères, 1937b). In Ivory Coast (at Ira Forest) C. stenophylla occurs on the upper, drier parts of hills; in the same location, C. canephora and C. liberica was found in the valleys (i.e., the lower, wetter areas). The locations for this species in Ivory Coast are generally drier than Sierra Leone (see below), with rainfall in the region of 1,500–1,700 mm per year, a 3–4 months dry season (Portères, 1937b), and an average annual temperature of c. 25.5°C. In Guinea, De Wildeman (1906a) reported that in its natural state C. affinis occurs in gallery forest (forest associated with rivers) bordering waterfalls and in humid (evergreen) forest, and that it was frequently found at elevations of 400–700 m at a distance of 100 to 300 km from the sea.
In Sierra Leone our fieldwork located C. stenophylla at precisely 400 m at two locations (Kasewe and Kambui Hills), even when there was sufficient forest down to 200 m. We visited higher elevation locations within the Western Peninsula National Park (up to 600 m), but did not locate either of the two species. Sierra Leone is generally not a mountainous country, and most of its land is below 500 m. At Kasewe and Kambui Hills the climate is tropical monsoonal, with an annual rainfall average of 2,350–2,650 mm, an average annual temperature of c. 26°C, and a distinct 3–4 months dry season (November to March/April). It should be noted that there is a considerable difference in rainfall between the wild locations of C. affinis and C. stenophylla in Ivory Coast and Sierra Leone, although this requires careful verification.
Our herbarium survey did not provide a great deal of further information on the habitat of either of these two species. Notes on herbarium specimens for C. stenophylla infrequently state ‘hills,’ one specimen states ‘very common [on] more open places on augite hills,’ and another ‘dans les montagnes de Sierra Leone,’ which suggested an association with high ground topology.
Species Status and Systematic Affinities of C. affinis and C. stenophylla
A recent hybrid origin for C. affinis, as a result of crossing between C. liberica and C. stenophylla (Chevalier, 1929; Cramer, 1957; Stoffelen, 1998) is ruled out on three counts: (1) C. affinis is clearly fertile and productive, as evidenced from literature records (Portères, 1937a), herbarium specimens (with plentiful seed), and recent (2020) field observation (D. Sarmu pers. observ.; Figure 2C). Diploid interspecies hybrids of coffee are usually sterile, and while they may produce flowers, fruit set and production of viable seed are minimal unless fertility is restored via polyploidization (usually tetraploids) (Carvalho and Monaco, 1968; Charrier, 1978). (2) The fruits of C. affinis are always described as black; the hybrid C. liberica × C. stenophylla (see below) has purple fruits. (3) Our samples of C. affinis do not alternate between ITS and plastid markers, as in known hybrids [C. arabica, C. arabica × C. racemosa, C. liberica × C. eugenioides, and C. liberica × C. stenophylla (see below)], but instead are consistently resolved as sister to C. stenophylla. The idea that C. affinis is a fixed mutation of C. stenophylla, (Wellman, 1961) is also ruled out because of the variation evident in this taxon.
Our DNA analyses infer that C. stenophylla and C. affinis are closely related. Separate ITS (Figure 4) and plastid analysis (Figure 5) fail to resolve the systematic positions of the four Upper Guinea (UG) clade species (i.e., including C. humilis and C. togoensis), but a combined analysis of these data sets retrieves monophyly monophyly for C. affinis and C. stenophylla (Figure 6). Coffea affinis and C. stenophylla share specific characters, including: the habit of a small tree, obovate leaves with a distinct apical tip (acumen) and drying green, 2 to 4 flowers per axil, 6- to 8-merous flowers (i.e., six to eight corolla lobes and anthers per flower; and black or black–purple fruits (see Figure 2). Coffea togoensis, from Ghana and Togo, is also a small tree, and has leaves with a distinct apical tip (acumen), 6- to 8-merous flowers and black fruits, but generally has elliptic leaves, drying grayish or gray-green, 1 or 2 flowers per axil, and smaller fruits and seeds. Coffea humilis is unlike C. affinis, C. stenophylla and C. togoensis, as it is a monocaul dwarf (single-stemmed woody plant, up to 1 m high), with large (up to 22 cm long) obovate leaves, 5- to 7-merous flowers and red fruits.
Coffea stenophylla and C. affinis exhibit considerable morphological variation, particularly with regard to leaf size and shape. Some examples of C. stenophylla approach C. affinis in terms of leaf shape and dimensions. Further work, including morphological and more detailed molecular study, is required to determine the precise relationship between these two species. There could be grounds for subsuming C. affinis within C. stenophylla, for example as C. stenophylla var. camaya (Portères, 1937a).
Our historical accession of farmed C. affinis from a coffee plantation in Sierra Leone (two genotypes from a single accession [Cope s.n., 7 iii 1912 (K)] is not related to either C. affinis or C. stenophylla. ITS and plastid marker data place this accession in the Lower Guinea/Congolian Clade. The specimens of this accession are a reasonable morphological match for C. affinis, with leaves of the same dimensions and possessing an acuminate leaf tip, but the seeds are somewhat larger and narrower than C. affinis; flower morphology and fruits color are unknown. The Cope s.n. accession is compelling, as it placed with the Liberica Alliance but does not conform to any of the known species in this alliance. The distinct, elongated leaf tip (acumen) immediately sets it apart from all variants of C. liberica (Stoffelen, 1998).
In both ITS (Figure 4) and plastid (Figure 5) analyses C. liberica is not monophyletic. Further molecular data is required for C. liberica and closely related taxa. As currently circumscribed, C. liberica is a highly polymorphic (Bridson, 1985, 1988; Stoffelen, 1998; Davis et al., 2006) encompassing a broad range of morphological variation.
Identification of Coffea Species Hybrids
Identification of interspecies hybrids or introgressed plants on the basis of incongruence between biparental (e.g., nuclear) and uniparental (plastid) phylogenetic trees is well established (Linder and Rieseberg, 2004). In most flowering plants plastid DNA is maternally inherited, whereas the nuclear DNA and the ITS region of ribosomal DNA is not (Chase et al., 2005). Previous analysis of ITS and plastid markers in Coffea have been used to identify the parents of the allotetraploid C. arabica (C. eugenioides × C. canephora) and the diploid hybrid C. eugenioides × C. liberica (Maurin et al., 2007). As part of this study, we undertook further tests of this method using an additional interspecies hybrids of known crossing history, viz. C. arabica × C. racemosa (Medina Filho et al., 1977a, b), which demonstrated the utility of the method, at least for recently produced hybrids (Figures 4, 5). Using this method, we were able to identify the interspecies hybrid C. liberica × C. stenophylla (Figures 4, 5) from cultivated material collected by us in Sierra Leone (see Table 1). The hybrid C. liberica × C. stenophylla has been reported before (Cramer, 1957), but never authenticated. The accession of this hybrid from Sierra Leone appears to be partially or totally sterile; in some years it produces a few fruits, but neither the viability nor fertility of seeds have been tested. The low level of fruit setting suggests that this hybrid is a diploid (2n = 22) rather than a polyploid; tetraploids generally have higher fertility or restored fertility (Carvalho and Monaco, 1968; Charrier, 1978). We have no means of knowing whether this hybrid was the result of a cross in cultivation or in the wild. Either origin is plausible: both species were grown together in a number of research stations in Africa, and Asia (Cramer, 1957); and in situ crossings have been reported. In Ivory Coast hybrid seedlings (but not mature plants) of C. liberica × C. stenophylla, were detected in the wild (Berthaud, 1986). Generally, interspecific hybridization in natural populations of Coffea is a rare phenomenon (Charrier, 1978; Berthaud, 1986).
Interspecies hybrids, once fertility (and thus yield) is restored via conversion to the tetraploid (4n = 44) state (Carvalho and Monaco, 1968; Nagai et al., 2008), are valuable for coffee crop development, as they provide the possibility of introducing useful traits. For example, CLR resistance (C. canephora × C. arabica; Clarindo et al., 2013; Avelino et al., 2015) and leaf-miner resistance (C. arabica × C. racemosa; Medina Filho et al., 1977b) for Arabica coffee. In our DNA survey of long-styled African species [Coffee Crop Wild Relative Priority Groups I and II (Davis et al., 2019)] we confirm that hybridization is possible across all the major African coffee clades (lineages), indicating the potential to create custom interspecies hybrids across this wide spectrum of Coffea species diversity.
Conclusion
Coffea affinis and C. stenophylla may possess useful attributes for coffee crop plant development, including taste, disease resistance, and climate resilience. These attributes would be best accessed via breeding programs, including those involving interspecies crossing, followed by tetraploidization. Here, we confirm that (initial) hybridization is possible across all the major clades of long-styled African Coffea species (Maurin et al., 2007; Davis et al., 2011; Hamon et al., 2017), i.e., Coffee Crop Wild Relative (Priority) Groups I and II (Davis et al., 2019). For C. stenophylla we confirm via DNA sequencing that a cross can be made with C. liberica, supporting the work of Louarn (1992), who also demonstrated that C. stenophylla can be crossed with C. canephora, C. congensis, and C. pseudozanguebariae. Development of C. stenophylla and C. affinis via minimal domestication (e.g., the selection of trait-specific genotypes) may be possible, although this route would probably only be feasible for high-value markets, such as the upper end of the speciality coffee sector, based on the historical reports of its superior taste. Productivity (green coffee yield) appears to be lower than the major commercial species (C. arabica, C. canephora and C. liberica). A key caveat here, is that C. stenophylla has not undergone sensory or agronomic evaluation in a contemporary setting. Despite the shortfall in our understanding of these species, the available evidence, as summarized and reviewed here, is more than sufficient to warrant further research for C. affinis and C. stenophylla, and to take measures to ensure their survival in the wild (in situ) and in cultivation (ex situ). Deforestation, and other forms of land-use change, are threatening the survival of these species in the wild, in Sierra Leone, Ivory Coast and Guinea.
The decline in the use of C. stenophylla use as a crop plant in Upper West Africa was dramatic, from once widespread (although comparatively small scale) use in the late and early parts of the 19th and 20th centuries, to apparently nothing today. Our survey of local farming communities in Sierra Leone, reported an absence of indigenous knowledge for this species. One of the other main reasons for the decline in its use may have been the considerable agronomic and commercial success of robusta coffee (C. canephora), which was introduced into global cultivation around the same time as C. stenophylla and greatly surpassed the comparatively meagre productivity of other underutilized coffee species (Davis et al., 2019). Following on from our fieldwork in Sierra Leone (2018–2020), wild stock of C. affinis and C. stenophylla is now being propagated in quantity, for sensory and agronomic evaluation, and to safeguard its existence. Field work in Guinea and Ivory Coast are required to further ascertain the present day indigenous and cultivated (farmed) status, respectively, and to provide a conservation management plan to ensure its survival in the wild. An in-depth review of coffee research collections, including genotyping (genome banking), is required to formulate an effective ex situ conservation management strategy for C. affinis and C. stenophylla, and indeed many other coffee species. African coffee species provide key resources for the sustainability of the global coffee sector (Davis et al., 2019) and should receive appropriate conservation measures in the wild (in situ) and in cultivation (ex situ).
Data Availability Statement
The accession numbers for the sequencing data presented in this article can be found in Table 2 within the article.
Author Contributions
AD devised the non-fieldwork elements of the study, undertook the literature and herbarium, participated in fieldwork, and was the lead on writing the manuscript. RG undertook the DNA sequencing and analyses, and contributed toward the writing of the manuscript. MF helped to devise the study, provided assistance with DNA sequencing, and contributed toward the writing of the manuscript. DS undertook the bulk of the fieldwork in Sierra Leone. JH devised the overall framework of the study, devised and undertook substantial fieldwork in Sierra Leone, and contributed toward the writing of the manuscript.
Funding
Funding for this research was provided from different sources including the EU funded Agriculture for Development Programme - Robusta Coffee Development Project contract FED/2013/322-213 administered by the Government of Sierra Leone; and a Darwin Initiative Scoping Project DARSC196 - Conservation and use of native coffee species in Sierra Leone.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We acknowledge the support and collaboration of the Department for Forestry, Ministry of Agriculture, and Sierra Leone Agricultural Research Institute, in Sierra Leone. We would like to offer our sincere gratitude to Welthungerhilfe (WHH), and to staff at their offices in Freetown and Kenema (Sierra Leone) for various support during this project. At WHH we would particularly like to thank Franz Moestl, Manfried Bischofberger, and Derek Wambulwa Makokha. We would also like to thank: Arnaud Havet, at WARC, in Sierra Leone; Martin Cheek at the Royal Botanic Gardens, Kew (UK) for advice on deforestation in Upper West Africa and for showing us project material from Guinea; Charles Denison for providing images of C. stenophylla; and Iris Wiegant and László Csiba for their assistance with lab work at Kew.
References
Anthony, F. (1982). La Conservation des Ressources Génétiques chez les Caféiers Prospection, Gestion et Evaluation du Materiel en Collection en Cote d’Ivoire. Paris: ORSTOM, 15.
Anthony, F. (1992). Les Ressources Génétiques des Caféiers: Collecte, Gestion d’un Conservatoire et Évaluation de la Diversité Génétique. Série TDM 8 I. Paris: ORSTOM.
Anthony, F., Dussert, S., and Dulloo, M. E. (2007). II. Coffee Genetic Resources. Conserving Coffee Genetic Resources: Complementary Strategies for ex Situ Conservation of Coffee (Coffea Arabica L.) Genetic Resources. Rome: CATIE.
Avelino, J., Cristancho, M., Georgiou, S., Imbach, P., Aguilar, L., Bornemann, G., et al. (2015). The coffee rust crises in Colombia and Central America (2008–2013): impacts, plausible causes and proposed solutions. Food Secur. 7, 303–321. doi: 10.1007/s12571-015-0446-9
Berthaud, J. (1983). Liste du Matériel Provenant des Prospections de Côte D’ivoire. Document de Travail. Paris: ORSTOM, 17.
Berthaud, J. (1986). Les Ressources Génétiques Pour L’amélioration des Cafeiers Africains Diploïdes. ORSTOM, Série TDM 188. Paris: ORSTOM.
Bramel, P., Krishnan, S., Horna, D., Lainoff, B., and Montagnon, C. (2017). Global Conservation Strategy For Coffee Genetic Resources. Washington, DC: Crop Trust & World Coffee Research.
Bridson, D. M. (1988). “Coffea,” in Flora of Tropical East Africa, Rubiaceae, Part 2, eds R. M. Polhill, D. M. Bridson, and B. Verdcourt (Avereest: Balkema, Rotterdam/Brookfield), 703–723.
Carvalho, A., and Monaco, L. C. (1968). Relaciones genéticas de especies seleccionadas de Coffea. Cafe 9, 3–19.
Charrier, A. (1978). La Structure Génétique des Caféiers Spontanés de la Région Malgache (Mascarocoffea). Paris: ORSTOM.
Chase, M. W., Salamin, N., Wilkinson, M., Dunwell, J. M., Kesanakurthi, R. P., Haider, N., et al. (2005). Land plants and DNA barcodes: short-term and long-term goals. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 360, 1889–1895. doi: 10.1098/rstb.2005.1720
Cheney, R. H. (1925). A Monograph of the Economic Species of the Genus Coffea L. New York, NY: The New York University Press.
Chevalier, A. (1905). Les caféiers sauvages de la Guinée françiaise. Note de M.A.Chevalier, présentée par M. Ph. van Tieghem. C. R. Hebd. Acad. Sci. 140, 1472–1475.
Chevalier, A. (1929). Les caféiers du globe, fasc. 1: généralités sur les caféiers. Ency. Biol. 5, 1–196.
Chevalier, A. (1947). Les caféiers du globe, fasc. 3: systématique des caféiers et faux-caféiers. Maladies et insectes nuisibles. Ency. Biol. 28:212.
Clarindo, W. R., Carvalho, C. R., Eveline, T. C., and Koehler, A. D. (2013). Following the track of “Híbrido de Timor” origin by cytogenetic and flow cytometry approaches. Genet. Resour. Crop Evol. 60, 2253–2259. doi: 10.1007/s10722-013-9990-3
Couch, C., Cheek, M., Haba, P., Molmou, D., Williams, J., Magassouba, S., et al. (2019). Threatened Habitats and Tropical Important Plant Areas (TIPAs) of Guinea, West Africa. Kew: Royal Botanic Gardens.
Cramer, P. J. S. (1957). A Review of Literature of Coffee Research in Indonesia. Turrialba: Inter-American Institute of Agricultural Sciences.
Davis, A. P., Chadburn, H., Moat, J., O’Sullivan, R., Hargreaves, S., and Nic Lughadha, E. (2019). High extinction risk for wild coffee species and implications for coffee sector sustainability. Sci. Adv. 5:eaav3473. doi: 10.1126/sciadv.aav3473
Davis, A. P., Govaerts, R., Bridson, D. M., and Stoffelen, P. (2006). An annotated taxonomic conspectus of the genus Coffea (Rubiaceae). Bot. J. Linn. Soc. 152, 465–512. doi: 10.1111/j.1095-8339.2006.00584.x
Davis, A. P., and Rakotonasolo, F. (2009). A taxonomic revision of the baracoffea alliance: nine remarkable Coffea species from western Madagascar. Bot. J. Linn. Soc. 158, 355–390. doi: 10.1111/j.1095-8339.2008.00936.x
Davis, A. P., Tosh, J., Ruch, N., and Fay, M. (2011). Growing coffee: Psilanthus (Rubiaceae) subsumed on the basis of plastid and nuclear DNA sequences; implications for the size, morphology, distribution and evolutionary history of Coffea. Bot. J. Linn. Soc. 167, 357–377. doi: 10.1111/j.1095-8339.2011.01177.x
De Wildeman, E. (1904). Noveaux caféiers de la cote occidentale d’afrique. Agric. Prat. Pays Chauds 4, 113–116.
De Wildeman, E. (1906a). Mission Émile Laurent (1903–1904), Vol. 1 (Texte). Brussels: F. Vandenbuggenhoudt.
De Wildeman, E. (1906b). Mission Émile Laurent (1903–1904), Vol. 2 (Planches). Bruxelles: F. Vandenbuggenhoudt.
Don, G. (1834). General System of Gardening and Botany (Vol. 3). London: Gilbert and Rivington Printers, St. John’s Square.
Doyle, J. J., and Doyle, J. L. (1987). A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phyt. Bull. Bot. Soc. Amer. 19, 11–15.
Dudgeon, G. C. (1922). Imperial Institute Handbooks. The Agricultural and Forest Products of British West Africa, 2nd Edn. London: John Murray.
Edgar, R. C. (2004). MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucl. Acid. Res. 32, 1792–1797. doi: 10.1093/nar/gkh340
Engelmann, F., Dulloo, M. E., Astorga, C., Dussert, S., and Anthony, F. (2007). Conserving Coffee Genetic Resources: Complementary Strategies for ex Situ Conservation of Coffee (Coffea Arabica L.) Genetic Resources. Rome: CATIE.
Google Earth (2019). Google Earth Pro, Version 7.3.1. Available online at: https://www.google.com/intl/en_uk/earth/desktop (accessed June 30, 2019).
Govaerts, R., Rhusam, M., Frodin, D., Bridson, D., Dawson, S., and Davis, A. P. (2019). World Checklist of Rubiaceae. Facilitated by the Royal Botanic Gardens, Kew. Published on the Internet. Available online at: http://wcsp.science.kew.org/ (accessed December 18, 2019).
Hamon, P., Grover, C., Davis, A., Rakotomalala, J.-J., Raharimalala, N., Albert, V., et al. (2017). Genotyping-by-sequencing provides the first well-resolved phylogeny for coffee (Coffea) and insights into the evolution of caffeine content in its species. Mol. Phylogenet. Evol. 109, 351–361. doi: 10.1016/j.ympev.2017.02.009
Hiern, W. P. (1876). On the African species of the genus Coffea. Trans. Linn. Soc. London Bot. 1, 169–176. doi: 10.1111/j.1095-8339.1876.tb00033.x
Holmgren, P. K., Holmgren, N. H., and Barnett, L. C. (1990). Index Herbariorum. Part 1: The Herbaria of the World, 8th edn. Regnum Vegetabile. New York, NY: New York Botanical Garden.
Huelsenbeck, J. P., and Ronquist, F. (2001). MrBAYES: bayesian inferenceof phylogenetic trees. Bioinformatics 17, 754–755. doi: 10.1093/bioinformatics/17.8.754
International Coffee Organization (ICO) (2019). Trade Statistics. Available online at: http://www.ico.org/trade_statistics.asp (accessed October 7, 2019).
International Union for Conservation of Nature (IUCN) (2020). The IUCN Red List of Threatened Species. Available online at: http://www.iucnredlist.org/ (accessed November 14, 2018).
Kearse, M., Moir, R., Wilson, A., Stones-Havas, S., Cheung, M., Sturrock, S., et al. (2012). Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28, 1647–1649. doi: 10.1093/bioinformatics/bts199
Le Pierrès, D., Charmetant, P., Yapo, A., Leroy, T., Couturon, E., Bontems, S., et al. (1989). Les Caféiers Sauvages de Côte d’Ivoire et de GUINÉE: Bilan des Missions de Prospection Effectuées de 1984 à 1987. Treizième Colloque Scientifique International sur le Café. Paris: ASIC, 420–428.
Linder, C. R., and Rieseberg, L. H. (2004). Reconstructing patterns of reticulate evolution in plants. Am. J. Bot. 91, 1700–1708. doi: 10.3732/ajb.91.10.1700
Louarn, J. (1992). La fertilite des Hybrides Interspecifiques et Les Relations Genomiques Entre Cafeiers Diploides D’origine Africaine (Genre Coffea l. Sous-Genre Coffea). Ph.D. Thesis. Universite de Paris Sud, Centre d’Orsay, Paris.
Macmillan, H. F. (1914). A Handbook of Tropical Gardening and Planting, 2nd Edn. Colombo: Amen Corner: H.W. Cave & Co.
Maurin, O., Davis, A. P., Chester, M., Mvungi, E. F., Jaufeerally-Fakim, Y., and Fay, M. F. (2007). Towards a phylogeny for Coffea (Rubiaceae): identifying well-supported lineages based on nuclear and plastid DNA sequences. Ann. Bot. 100, 1565–1583. doi: 10.1093/aob/mcm257
Medina Filho, H. P., Carvalho, A., and Medina, D. M. (1977a). Germoplasma de C. racemosa e seu potencial no melhoramento do cafeeiro. Bragantia 36, XLIII–XLVI.
Medina Filho, H. P., Carvalho, A., and Monaco, L. (1977b). Melhoramento do cafeeiro. XXXVII – Observações sobre a resistência do cafeeiro ao bicho mineiro. Bragantia 36, 131–137. doi: 10.1590/s0006-87051977000100011
Nagai, C., Rakotomalala, J.-J., Katahira, R., Li, Y., Yamagata, K., and Ashihara, H. (2008). Production of a new low-caffeine hybrid coffee and the biochemical mechanism of low caffeine accumulation. Euphytica 164, 133–142. doi: 10.1007/s10681-008-9674-9
Portères, R. (1937a). Etude sur les caféiers spontanés de la section “Des Eucoffeae”. Leur répartition, leur habitat, leure mise en culture et leur sélection en Cote d’Ivoire. Deuxième partie: Espèce, variétés et formes. Ann. Agric. Afr. Occid. 1, 219–283.
Portères, R. (1937b). Etude sur les caféiers spontanés de la section “Des Eucoffeae”. Leur répartition, leur habitat, leure mise en culture et leur sélection en Cote d’Ivoire. Première partie: Répartition et habitat. Ann. Agric. Afr. Occid. 1, 68–91.
Posada, D. (2008). jModelTest: phylogenetic model averaging. Molec. Biol. Evol. 25, 1253–1256. doi: 10.1093/molbev/msn083
Rambaut, A. (2018). FigTree, Version 1.4.4. Available online at: http://github.com/rambaut/figtree/ (accessed February 18, 2020).
Rambaut, A., Drummond, A. J., and Suchard, M. (2013). Tracer, Version 1.6. Available online at: http://tree.bio.ed.ac.uk/software/ (accessed February 18, 2020).
Ronquist, F., and Huelsenbeck, J. P. (2003). MrBayes 3: Bayesian phylogeneticinference under mixed models. Bioinformatics 19, 1572–1574. doi: 10.1093/bioinformatics/btg180
Sayer, J. A., Harcourt, C. S., and Colins, N. M. (1992). The Conservation Atlas of Tropial Forests: Africa. Cambridge: IUCN.
Stoffelen, P. (1998). Coffea and Psilanthus in Tropical Africa: A Systematic and Palynological Study, Including a Revision of the West and Central African Species. Leuven: Katholieke Universiteit Leuven.
Thiers, B. (2019). Index Herbariorum: A Global Directory of Public Herbaria and Associated Staff. New York Botanical Garden’s Virtual Herbarium. Available online at: http://sweetgum.nybg.org/science/ih/ (accessed September 11, 2019).
Vega, F. E., Rosenquist, E., and Collins, W. (2003). Global project needed to tackle coffee crisis. Nature 425:343. doi: 10.1038/425343a
Wellman, F. L. (1961). Coffee: Botany, Cultivation and Utilization. New York, NY: Leonard Hill [Books] Limited/Interscience Publishers, Inc.
Keywords: agronomy, climate change, coffee, West Africa, crop wild relatives (CWRs), DNA, Sierra Leone, speciality coffee
Citation: Davis AP, Gargiulo R, Fay MF, Sarmu D and Haggar J (2020) Lost and Found: Coffea stenophylla and C. affinis, the Forgotten Coffee Crop Species of West Africa. Front. Plant Sci. 11:616. doi: 10.3389/fpls.2020.00616
Received: 19 December 2019; Accepted: 21 April 2020;
Published: 19 May 2020.
Edited by:
Petr Smýkal, Palackı University Olomouc, CzechiaReviewed by:
Alan C. Andrade, Brazilian Agricultural Research Corporation (EMBRAPA), BrazilBenoit Bertrand, Centre de Coopération Internationale en Recherche Agronomique pour le Développement (CIRAD), France
Copyright © 2020 Davis, Gargiulo, Fay, Sarmu and Haggar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aaron P. Davis, a.davis@kew.org; Jeremy Haggar, J.P.Haggar@greenwich.ac.uk
 Aaron P. Davis
Aaron P. Davis Roberta Gargiulo
Roberta Gargiulo Michael F. Fay
Michael F. Fay Daniel Sarmu
Daniel Sarmu Jeremy Haggar
Jeremy Haggar