
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci. , 20 May 2020
Sec. Crop and Product Physiology
Volume 11 - 2020 | https://doi.org/10.3389/fpls.2020.00543
This article is part of the Research Topic Regulation of Fruit Ripening and Senescence View all 13 articles
Nitric oxide (NO), a signaling molecule, participates in defense responses during plant–pathogen interactions. S-Nitrosoglutathione (GSNO) is found to be an active intracellular NO storage center and regulated by S-nitrosoglutathione reductase (GSNOR) in plants. However, the role of GSNOR in NO-induced disease resistance is not clear. In this research, the effects of NO and GSNOR inhibitor (N6022) on the defense response of harvested peach fruit to Monilinia fructicola infection were investigated. It was found that the disease incidence and lesion diameter of peach fruits were markedly (P < 0.05) reduced by NO and GSNOR inhibitor. However, the expression of GSNOR was significantly inhibited (P < 0.05) by NO only during 2–6 h. Analyses using iodo-TMT tags to detect the nitrosylation sites of GSNOR revealed that the sulfhydryl group of the 85-cysteine site was nitrosylated after NO treatment in peach fruit at 6 and 12 h, suggesting that exogenous NO enhances disease resistance via initial inhibition of gene expression and the S-nitrosylation of GSNOR, thereby inhibiting GSNOR activity. Moreover, NO and GSNOR inhibitor enhanced the expression of systemic acquired resistance (SAR)-related genes, such as pathogenesis-related gene 1 (PR1), nonexpressor of PR1 (NPR1), and TGACG-binding factor 1 (TGA1). These results demonstrated that S-nitrosylation of GSNOR protein and inhibition of GSNOR activity contributed to the enhanced disease resistance in fruit.
Peach [Prunus persica (L.) Batsch] fruit is rich in nutrients that provide favorable growth conditions for pathogenic bacteria. In addition, due to the high temperature and environmental humidity during the picking period of the peach fruit, the infection and growth of pathogenic bacteria are accelerated, causing a large amount of decay of the peach fruit, resulting in huge economic losses. Brown rot caused by Monilinia fructicola is severely destructive to stone fruits, involving in cherries, plums, and peaches (Hu et al., 2011).
At present, cold storage and chemical fungicides are widely used to inhibit brown rot development of peach fruit. Studies have shown that cold storage can effectively control M. fructicola disease by delaying spore germination (Sommer, 1985). However, peach fruit is very sensitive to low temperature, resulting in chilling injury during cold storage. Chemical fungicides can inhibit brown rot (Adaskaveg et al., 2005), but long-term use of chemicals may induce several problems such as fungicide resistance, chemicals residues on fruits, and environmental pollution. Therefore, the development of safe and effective methods is necessary to control brown rot in stone fruit.
Induced resistance based on biotic or abiotic activation of certain cellular defense responses is considered a sustainable strategy to inhibit pathogen invasion and reduce postharvest decay (Romanazzi et al., 2016). Nitric oxide (NO) is an active molecule with high fat solubility that can diffuse rapidly through the cell membrane. NO is also a gaseous radical. NO and NO-derived molecules are collectively referred to as reactive nitrogen species (RNS) (Corpas et al., 2007). Less is known about the role of NO-derived molecules in the interactions of plants with pathogens (Chaki et al., 2009). However, the function of NO as an important RNS in plants has been extensively studied (Corpas et al., 2007; Besson-Bard et al., 2008). NO is involved in various physiological processes in plants, including seed germination, growth, development, maturation, senescence, and stress response (Arasimowicz and Floryszak-Wieczorek, 2007). Moreover, during plant–pathogen interactions, NO participates in defense responses (Romero-Puertas et al., 2004). Salicylic acid (SA)-mediated activation of signaling pathways is an important manifestation of NO-induced resistance in plants (Domingos et al., 2015). Studies have shown that exogenous NO treatment has obvious inhibitory effects on pathogens including Penicillium expansum, Botrytis cinerea, and Colletotrichum gloeosporioides of postharvest fruits (Zheng et al., 2011; Lai et al., 2014; Hu et al., 2019). However, mechanisms of disease resistance induced by NO in harvested peach fruit are not well understood.
Stamler (1994) first proposed the concept of protein nitrosylation modification, that is, the NO group is covalently bonded to the cysteine (Cys) residue of the protein to produce S-nitrosothiol (SNO). Nitrosylation can influence the structure, activity, and function of target proteins (Stamler et al., 2001; Chen et al., 2006), thereby affecting the corresponding signal transduction pathways in the cell. Numerous studies confirm that S-nitrosylation plays a wide range of roles in different pathological and physiological processes (Hess and Stamler, 2012). Recently, it is found that NO may accomplish long distance signal transduction by protein nitrosylation in plants (Skelly and Loake, 2013; Kulik et al., 2015). S-nitrosoglutathione reductase (GSNOR), classified as the alcohol dehydrogenase family, which exists in all species from bacteria to humans, is an important regulatory protein in NO turnover (Liu et al., 2001). Feechan et al. (2005) found that GSNOR, associated with protein nitrosylation in Arabidopsis, plays a critical role in disease resistance. GSNOR has attracted more and more attention as an important regulator of nitrosylation of proteins.
S-Nitrosoglutathione (GSNO) is a storage site for the biological activity of NO. GSNOR regulates the levels of GSNO and other S-nitrosothiols (SNOs) and protein nitrosylation in eukaryotic cells (Liu et al., 2001). As a key factor in plant disease resistance response, GSNOR causes changes in intracellular redox status by regulating GSNO content, which in turn leads to S-nitrosylation of defense-related proteins (Sakamoto et al., 2002; Dìaz et al., 2003; Lee et al., 2008). Post-translational modification of these proteins can cause different defense responses in plants (Rustérucci et al., 2007; Kwon et al., 2012). However, the study of GSNOR in post-harvest fruits has not been reported. Therefore, it is of great significance to elucidate the role of GSNOR in the disease resistance of post-harvest peach fruit.
Peach fruit (cultivar “Zhonghuashoutao”) were collected from Yiyuan, Shandong Province, China, and selected for uniform size and no mechanical damage. According to previous methods, peach fruit were soaked in either deionized water (served as control), NO solution (15 μmol L–1; diluted with a saturated NO solution made from NO gas), or GSNOR inhibitor [N6022, bought on MCE official website (MedChemExpress)1 ] solution (60 μmol L–1), respectively, for 20 min and dried at room temperature (Gu et al., 2014). A 3 mm × 3 mm × 3 mm wounded site was made on each fruit and inoculated with 20 μl of 1 × 105 spores ml–1 of brown rot spore suspension. Then, the fruits were stored in a constant temperature and humidity chamber [23°C, 85–90% relative humidity (RH)] to observe the disease development in peach fruit. The disease incidence and lesion diameter were measured at 48 and 72 h, respectively. Six repetitions are set for each treatment, and each repetition contained 30 peaches. Samples of three peach fruits at each time point were taken out from each repetition and cut into small pieces. Then, the pieces were frozen with liquid nitrogen, grinded into power, and stored at −80°C. Moreover, samples of three fruits of each repetition were taken at 0, 6, 12, 24, 48, and 72 h for RNA and protein extraction, enzyme assay, and the content of SNOs, GSNO, glutathione of reduced state (GSH), glutathione disulfide (GSSG), and endogenous NO measurement.
Glutathione reductase (GR) activity was measured according to Knorzer et al. (1996). One half gram peach powder was homogenized in 4 ml of 50 mM phosphate-buffered saline (PBS) [pH 7.0, containing 20% (v/v) glycerol, 2 mM DL-dithiothreitol (DTT), 2 mM ethylene diamine tetraacetic acid (EDTA), 2% polyvinylpyrrolidone (PVP)]. The supernatant obtained after centrifugation at 20,000 × g for 30 min at 4°C was used to determine the enzyme activity. The reaction system included 100 μl supernatant, 3 ml reaction liquid [pH 7.5, containing 50 mM Tris–HCl, 0.5 mM GSSG, 5 mM MgCl2, and 0.2 mM reduced nicotinamide adenine dinucleotide phosphate (NADPH)]. The changes in absorbance at 340 nm were determined.
S-nitrosoglutathione reductase activity was measured following the means of Sakamoto et al. (2002). One half gram peach powder was extracted with 3 ml assay mixture containing 50 mM HEPES (pH 8.0), 20% (v/v) glycerol, 10 mM MgCl2, 1 mM EDTA, 1 mM ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA), 1 mM benzamidine, and 1 mM ε-aminocaproic acid at 4°C and then centrifuged at 16,000 × g for 15 min. Three hundred microliters of supernatant was incubated in 3 ml assay mixture containing 20 mM Tris–HCl (pH 8.0), 0.5 mM EDTA, and 0.2 mM NADH, and the GSNO was mixed to a final concentration of 400 mM to initiate the reaction.
The SNO content was measured according to Frungillo et al. (2013) with some modifications. One half gram peach powder was homogenized in 4 ml of 100 mM phosphate buffer (pH 7.2, containing 100 mM EDTA, 100 mM EGTA), after which centrifugation was carried out at 20,000 × g for 30 min at 4°C to obtain the supernatant. One milliliter supernatant was reacted with solution I [containing 1% sulfanilamide, 0.1% N-(1-naphthyl) ethylene-diamino dihydrochoride] or solution II [containing 1% sulfanilamide, 0.1% N-(1-naphthyl) ethylene-diamino dihydrochoride, 2 mM HgCl2] in a ratio of 1:1. After incubating in the dark for 10 min, the reaction solution was centrifuged at 12,000 × g for 5 min. The SNO content in plants was quantified by determining the absorbance difference between solutions II and I at 540 nm. The absorbance at 540 nm was determined using different concentrations of GSNO instead of the enzyme solution, and a standard curve was prepared. The content of SNOs in the plants was calculated according to the value of the standard curve.
The NO content was performed according to the means of Shi et al. (2015). One half gram peach powder was extracted in 3 ml 50 mM glacial acetic acid buffer [pH 3.6, containing 4% (w/v) zinc acetate], then centrifuged at 10,000 × g for 15 min at 4°C. Equal volumes of extraction and Griess reagent (Sigma-Aldrich, St Louis, United States) were mixed and reacted at 25°C for 30 min. The optical density (OD) value at 540 nm was determined.
The contents of GSNO, GSSG, and GSH were measured according to Hodges and Forney (2000) with minor modifications. One half gram peach powder was dissolved with 5 ml of 5% (w/v) 5-sulfosalicylic acid and then centrifuged at 12,000 × g for 15 min at 4°C. Solution A (containing 100 mM Na2HPO4⋅7H2O, 40 mM Na2HPO4⋅H2O, 1.8 mM 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB), 15 mM EDTA, and 0.04% (w/v) bovine serum albumin (BSA)] and solution B (including 1.0 mM EDTA, 50 mM imidazole, and 0.02% (w/v) BSA] were prepared and then adjusted to pH 7.2. The GSNO content was measured by mixing 800 μl solution A, 640 μl solution B, 800 μl of 1:25 dilution of extract in 0.5 M K2HPO4 buffer (pH 7.0), and 160 μl of 3.0 mM NADPH. The changes in the OD value of the mixed solution at 412 nm were recorded. The GSH content was measured by adding 300 μl of 10 U mol–1 glutathione reductase (GR) (100 U) to the above reaction system for 1 min, and the change of OD value at 412 nm for 5 min was measured. The measurement method of GSSG was as follows. 1.0 ml extract was diluted into 1:10 in 0.5 M K2HPO4 buffer (pH 6.5, containing 20 μl 2-vinylpyridine) at 25°C for 1 h. Then, 400 μl of solution A and 320 μl of solution B was added, and the change in OD value was determined at 412 nm. The standard curve of GSNO, GSSG, and GSH was prepared, and the content of GSNO, GSSG, and GSH in the sample was calculated according to the standard curve.
Total RNA extraction was conducted from samples at various time intervals. One hundred milligrams of the sample was taken, and the RNAprep Pure Polysaccharide Polyphenol Plant Total RNA extraction kit (DP441) produced by Tiangen (Shanghai, China) was used for extraction of the total RNA. The CWBIO HiFiScript cDNA Synthesis Kit (CW2596) was use to synthesize complementary DNA (cDNA) by incubating at 42°C for 50 min, then at 85°C for 5 min. The primers of GR, GSNOR, PR1, NPR1, and TGA1 were designed based on their genetic sequences (Table 1). TEF2 (TC3544) and tubulin-α (DY650410) from peach fruit were used as reference genes (Tong et al., 2009). Real-time (RT-PCR) was performed using of the CWBIO UltraSYBR Mixture (CW0957) kit with reaction volumes of 25 μl.
Some experimental methods involved in the detection of nitrosation sites mainly refer to Gong and Shi (2019) and Xie et al. (2016) with slight modifications. The experimental methods and steps were as follows.
The peach fruit treated with NO, inoculated with M. fructicola then stored for 4, 6, 12, and 24 h, was ground into powder in liquid nitrogen. The sample with lysis buffer [including 8 M urea, 100 mM triethylammonium bicarbonate buffer (TEAB, Sigma-Aldrich, St Louis, MI, United States), 50 mM Iodoacetamide (IAM, Sigma-Aldrich, St Louis, MI, United States), 1% Protease Inhibitor Cocktail (Merck Millipore, Billerica, MA, United States), and 1% Triton X-100] added was sonicated three times on ice. The lysate was incubated at room temperature without light for 30 min, and then, centrifugation was performed at 20,000 × g at 4°C for 10 min to remove the remaining debris. Finally, cold 20% TCA was used to precipitate the protein at −20°C for 2 h. The precipitate obtained by centrifugation was washed three times with cold acetone. HES buffer [50 mM TEAB, 1 mM EDTA, and 0.1% sodium dodecyl sulfate (SDS)] was used to redissolve the protein. The protein concentration was measured according to the instructions of the BCA kit (Beyotime Biotechnology, Shanghai, China).
One milligram protein per treatment was redissolved in 1 ml HES buffer and processed with the iodo-TMT kit (Thermo Fisher Scientific, Waltham, MA, United States). In short, iodo-TMT reagent dissolved in 10 μl MS grade methanol was mixed to the redissolved protein solution; then, 20 μl of 1 M sodium ascorbate was added and mixed briefly. The mixed solution was incubated in the dark at 37°C for 2 h. The reaction was quenched by the addition of 40 μl of 0.5 M DTT (20 mM final concentration) and incubated in darkness at 37°C for 15 min.
Six volumes of prechilled (−20°C) acetone were used to precipitate the mixed labeled protein at −20°C for at least 2 h and then centrifuged to discard the supernatant. The protein precipitate was dissolved in 8 M urea after washing with cold acetone three times. The protein solution after reduction with 5 mM DTT for 30 min at 56°C was alkylated with 11 mM IAM for 15 min at room temperature without light. The protein samples were diluted with 100 mM TEAB, and the final urea concentration was below 2 M. Trypsin was added at a trypsin-to-protein mass ratio of 1:50 overnight, and a second digestion was performed at a mass ratio of 1:100 trypsin to protein for 4 h. Strata X C18 SPE column (Phenomenex, Torrance, CA, United States) was used for desalting of trypsin-digested peptides. Finally, the peptide was dried under vacuum.
Fractionation of the samples was carried out by high pH reverse-phase HPLC (EASY-nLC 1000, Thermo Fisher Scientific, Waltham, MA, United States) applying 300 Extend C18 column (5 μM particles, 4.6 mM ID, and 250 mM length; Agilent Technologies, Santa Clara, CA, United States). First, the peptides were dissociated into 80 fractions with a gradient of 2–60% acetonitrile (ACN) in 10 mM NH4HCO3 (pH 10) over 80 min. Then, the peptides were combined into four fractions and vacuum dried.
Tryptic peptides were dissolved in TBS buffer (pH 7.5, 150 mM NaCl, 250 mM Tris–HCl) and incubated with prewashed anti-TMT antibody beads (Thermo Fisher Scientific, Waltham, MA, United States) at 4°C overnight to enrich cysteine nitrosylation peptides The beads were cleaned once with disinfected deionized water after three times with TBS buffer. The peptide bound to the beads was eluted with elution buffer (0.4% TFA, 50% ACN). The eluted peptides were mixed and vacuum dried. The peptides obtained after C18 ZipTips (Millipore, Boston, MA, United States) washing were analyzed by liquid chromatography tandem mass spectrometry (LC-MS/MS).
Refer to the means of Yang et al. (2018) with minor modifications. The peptides were resuspended in solvent A (containing 0.1% FA and 2% ACN) and separated using an EASY-nLC 1000 UPLC system (Thermo Fisher Scientific, Waltham, MA, United States). The gradient of solvent B (containing 0.1% FA and 98% ACN) included an increase from 6 to 25% over 26 min, 25 to 40% in 8 min, and climbing to 80% in 3 min, then remaining at 80% for the last 3 min. The flowrate was maintained at 400 nl min–1. The peptides were injected into the NSI source for ionization and then analyzed using tandem mass spectrometry (MS/MS) on an Q ExactiveTM Plus (Thermo Fisher Scientific, Waltham, MA, United States) coupled online to the ultraperformance liquid chromatography (UPLC).
The MaxQuant search engine (v.1.5.2.8) was used to analysis the resulting MS/MS data. The UniProtKB Prunus persica (sequences: 28234. Version: 2016.5.30) database was connected to the reverse decoy database to search for tandem mass spectra. IodoTMT-6plex was selected as the quantification method. The fold-change threshold was set when peptides with quantitative ratios over 1.2 or under 1/1.2 are considered significant. Intensive bioinformatic analyses were then carried out to annotate those quantifiable lysine acetylated targets in response to NO solution treatment, including Gene Ontology (GO) annotation, subcellular localization, Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway annotation, etc. Based on the results, further studies following the quantitative cysteine nitrosylation analysis were suggested. Three repetitions were used in nitrosylation measurement. The compilation and mapping of experimental data were performed using Microsoft Excel 2013 and Sigma Plot 10.0 software. IBM SPSS version 20.0 was used for statistical analysis, and significant (P < 0.05) level test was performed using least significant difference (LSD) test.
The disease incidence and lesion diameter of peach fruit inoculated with M. fructicola were significantly (P < 0.05) suppressed by NO and GSNOR inhibitor (N6022), and the inhibitory effect of GSONR inhibitor on M. fructicola was better than that of NO solution treatment (Figure 1). At 36 h, the disease incidence of the control fruit was approximately 15%, and the average lesion diameter was 2.3 mm, however, the disease symptom was not present on GSNOR inhibitor-treated fruit. During the investigated period, the lesion development on NO-treated or GSNOR inhibitor-treated fruit was lower than that on the control (Figure 1A). Moreover, 36 and 48 h, the NO and GSNOR inhibitor-treated fruit exhibited significantly (P < 0.05) lower disease incidence than the control (Figure 1B). The lesion diameter on the control fruit was 2.1, 1.5, and 1.6 times of NO-treated fruit, and even at 72 h, the lesion diameter on the control fruit was almost 3.1 times that of GSNOR inhibitor-treated peach fruit (Figure 1C). These results indicate that treatment with NO and GSNOR inhibitor reduces disease incidence and the extent of lesions. GSNOR inhibitor was the most effective at inhibiting brown rot development.
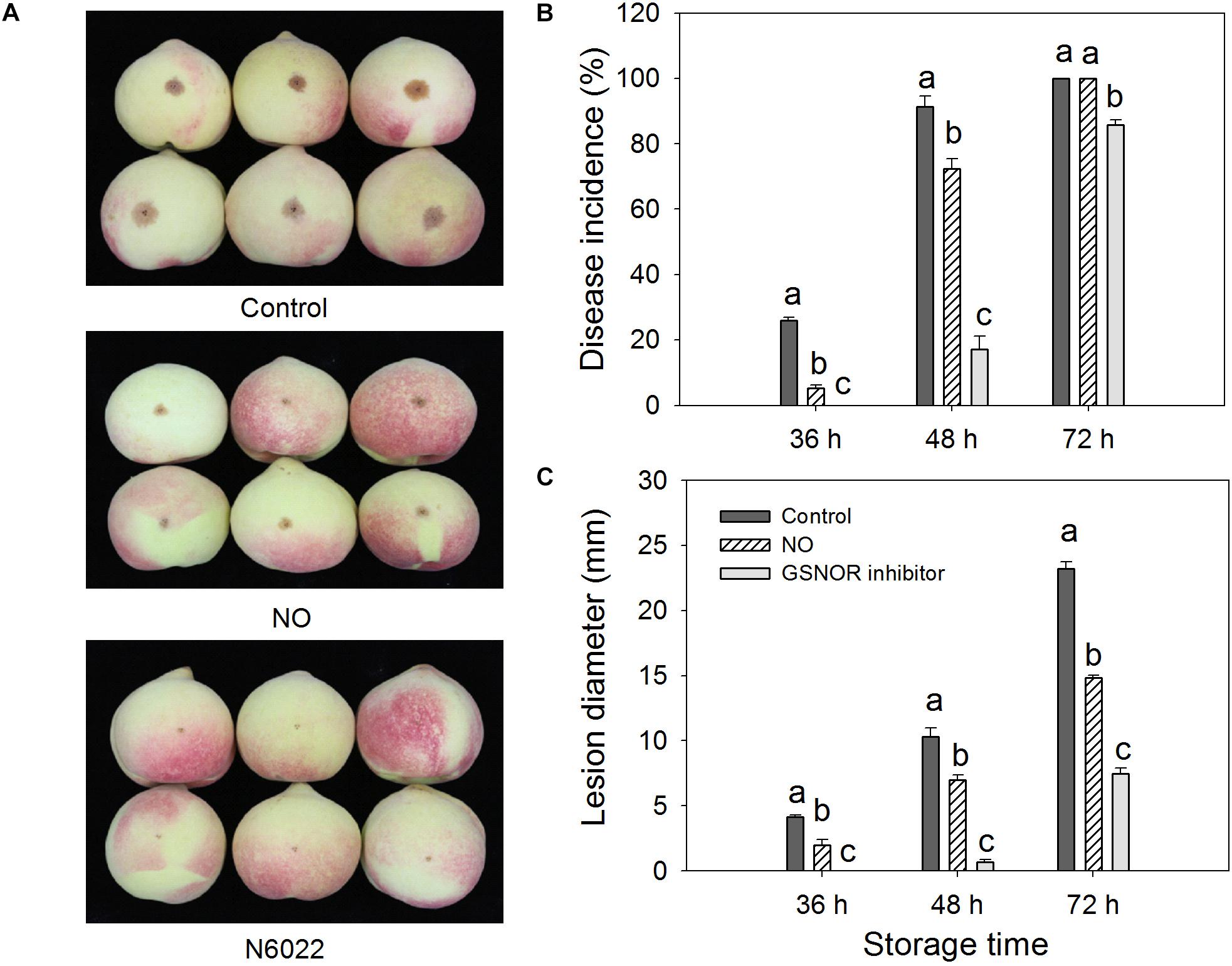
Figure 1. Effect of nitric oxide (NO) or S-nitrosoglutathione reductase (GSNOR) inhibitor treatment on the (A) disease development (at 48 h after inoculation), (B) disease incidence, and (C) lesion diameter of peach fruit after inoculation with M. fructicola spore suspension (1 × 105 spores ml–1) and then stored at 23°C. Bars represent standard deviations of the means (n = 6). Different letters represent significant differences (p < 0.05) according to least significant difference (LSD) test.
The activity and gene expression of GR and GSNOR were measured in peach fruit after inoculation with M. fructicola (Figure 2). Compared with the control, NO and GSNOR inhibitor obviously (P < 0.05) enhanced the activity of GR. The enhancement effect of NO on GR activity was very obvious at 6–24 h, but the GR activity was slightly different from the control at 48 and 72 h. The effect of GSNOR inhibitor on GR activity was more obvious than that of NO and GR activity, showing a downward trend at 0–72 h (Figure 2A). The activity of GSNOR showed a tendency of increase first and then decrease. Both NO and N6022 inhibited the activity of GSNOR, and the inhibitory effect of N6022 treatment was stronger than that of NO treatment (Figure 2B). Furthermore, higher levels of GR expression were observed in both NO-treated and GSNOR inhibitor-treated fruit than the control (Figure 2C). GSNOR expression in peach fruit treated with NO and GSNOR inhibitor was obviously (P < 0.05) lower than the control at 2–6 h. The expression of GSNOR in all three treatments stabilized at 12 h. GSNOR inhibitors have the most marked inhibitory effect on GSNOR gene expression (Figure 2D).
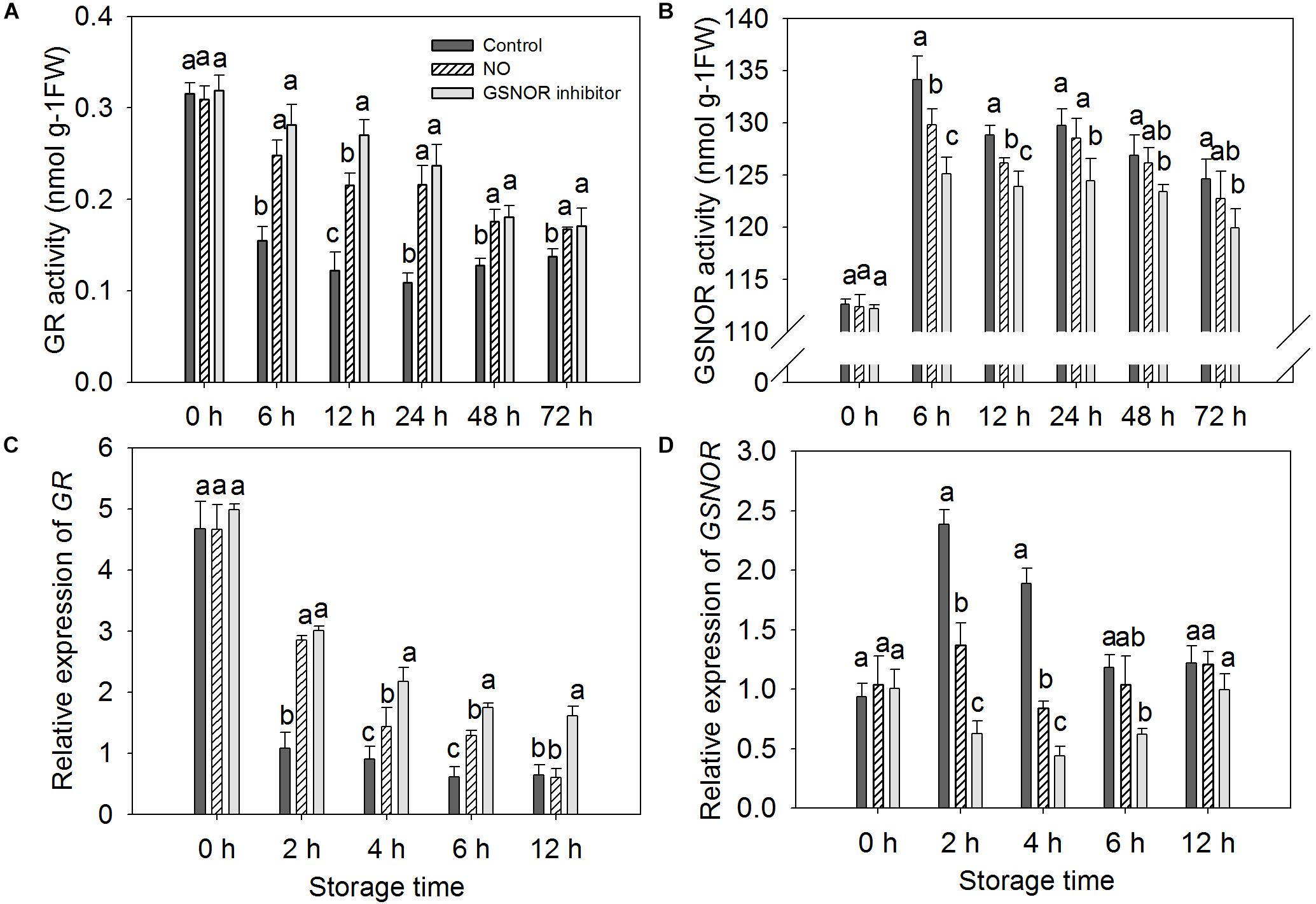
Figure 2. Effect of nitric oxide (NO) or S-nitrosoglutathione reductase (GSNOR) inhibitor treatment on the activity of (A) glutathione reductase (GR) and (B) GSNOR and the (C) gene expression of GR and (D) GSNOR of peach fruit storage at 23°C. Vertical bars represent the standard deviation of the means (n = 6). Different letters represent significant differences (P < 0.05) according to least significant difference (LSD) test.
Nitric oxide-treated and non-treated peach fruits inoculated with M. fructicola were quantitatively measured for nitrosylation using iodo-TMT tags. The sulfydryl in Cys-85 of GSNOR was identified as an S-nitrosylated residue in peach fruit treated with NO at 6 and 12 h (Figure 3A). The peptide sequences of nitrosylation was KILYTALCHTDAYTWGGKD. To get the 3D structural model of GSNOR, find the GSNOR (M5VJ61) information page on the UniProt official website and click M5VJ61 under “Structure” (Figure 3B). From the structure of the model, GSNOR is a dimer containing two subunits, and its ligands include two zinc ion and two nicotinamide adenine dinucleotide.
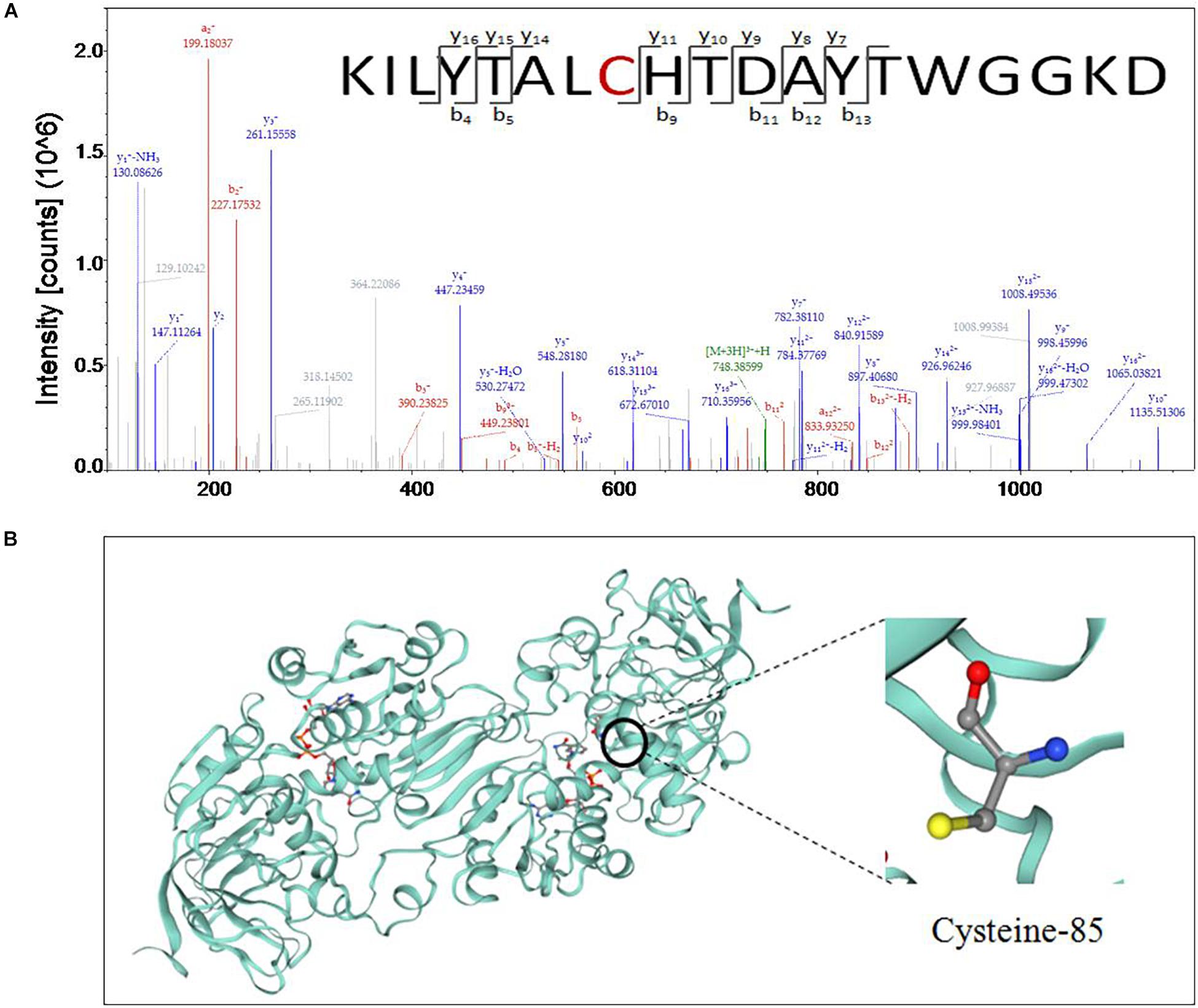
Figure 3. (A) Cys-85 of S-nitrosoglutathione reductase (GSNOR) was identified as an S-nitrosylated residue by site-specific proteomic mass spectrometry, and (B) 3D structure model of GSNOR protein of peach was predicted in Uniprot. Illustrated in the figure is the cysteine located at position 85 of the amino acid sequence.
The content of endogenous NO and SNOs increased from 0 to 12 h and then decreased in both treated and the control fruit from 12 to 48. At 72 h, the content increased slightly but did not change much. The change in endogenous NO content is similar to SNOs (Figure 4). Treatment with NO and GSNOR inhibitor increased the levels of SNOs and NO in peach fruit compared to the control (Figures 4A,B). GSNOR inhibitor treatment had a stronger effect on the content of SNOs and NO than exogenous NO treatment.
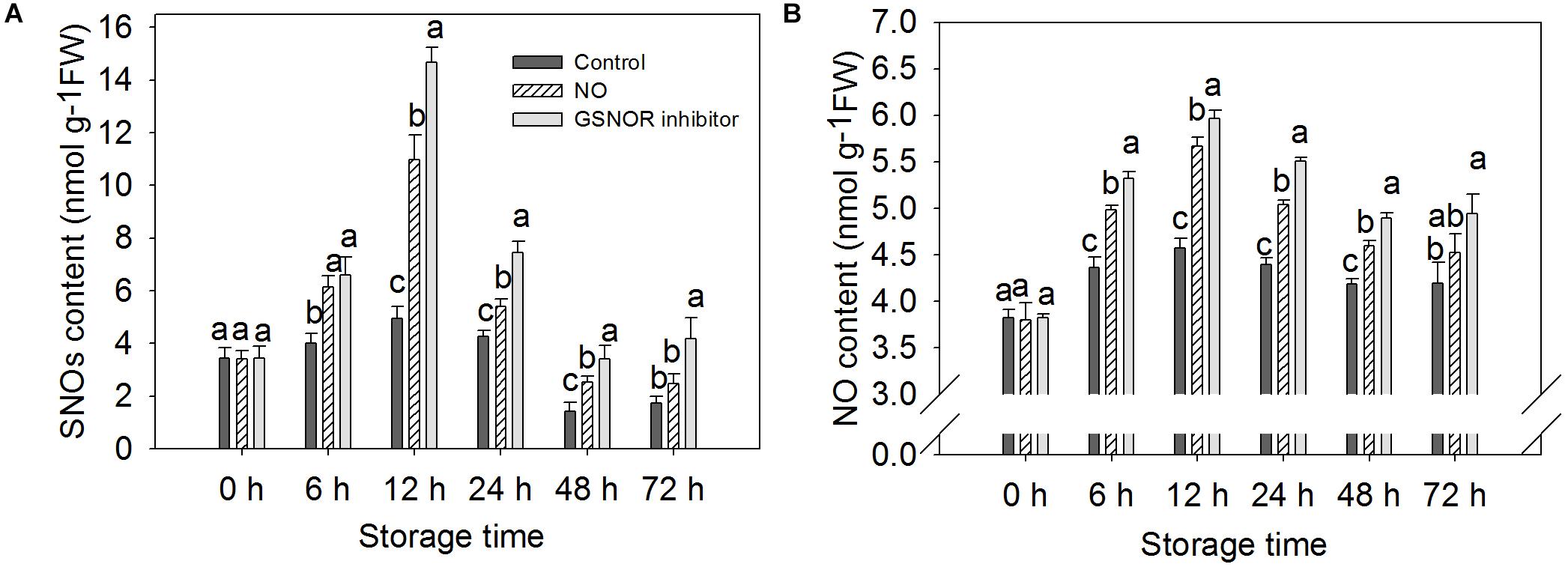
Figure 4. Effect of nitric oxide (NO) or S-nitrosoglutathione reductase (GSNOR) inhibitor treatment on the content of (A) SNOs and (B) NO of peach fruit storage at 23°C. Vertical bars represent the standard deviation of the means (n = 6). Different letters represent significant differences (P < 0.05) according to least significant difference (LSD) test.
The level of GSH increased from 0 to 72 h in the treated fruit. However, GSH levels in control fruits increased from 0 to 48 h and decreased at 72 h (Figure 5A). GSNOR inhibitor treatment enhanced GSH level maximum, NO treatment was the second, and the GSH level of the control peach fruit was relatively low (Figure 5A). The change in GSSG content is opposite to that of GSH. GSSG content showed a downward trend within 0–48 h after inoculation with M. fructicola. The GSSG content of the control fruits increased at 72 h, but a decrease in GSSG levels was still found in the treated peach fruit (Figure 5B). The level of GSNO increased significantly at 0–6 h, and then decreased. It can be seen from the figure that NO and GSNOR inhibitor treatment could obviously (P < 0.05) increase the level of GSNO during 0–24 h. However, GSNO content in the three treatment fruits had no significant difference (P > 0.05) at 48 h. At 72 h, the content of GSNO in peach fruits treated with GSNOR inhibitor was higher than the other two groups (Figure 5C).
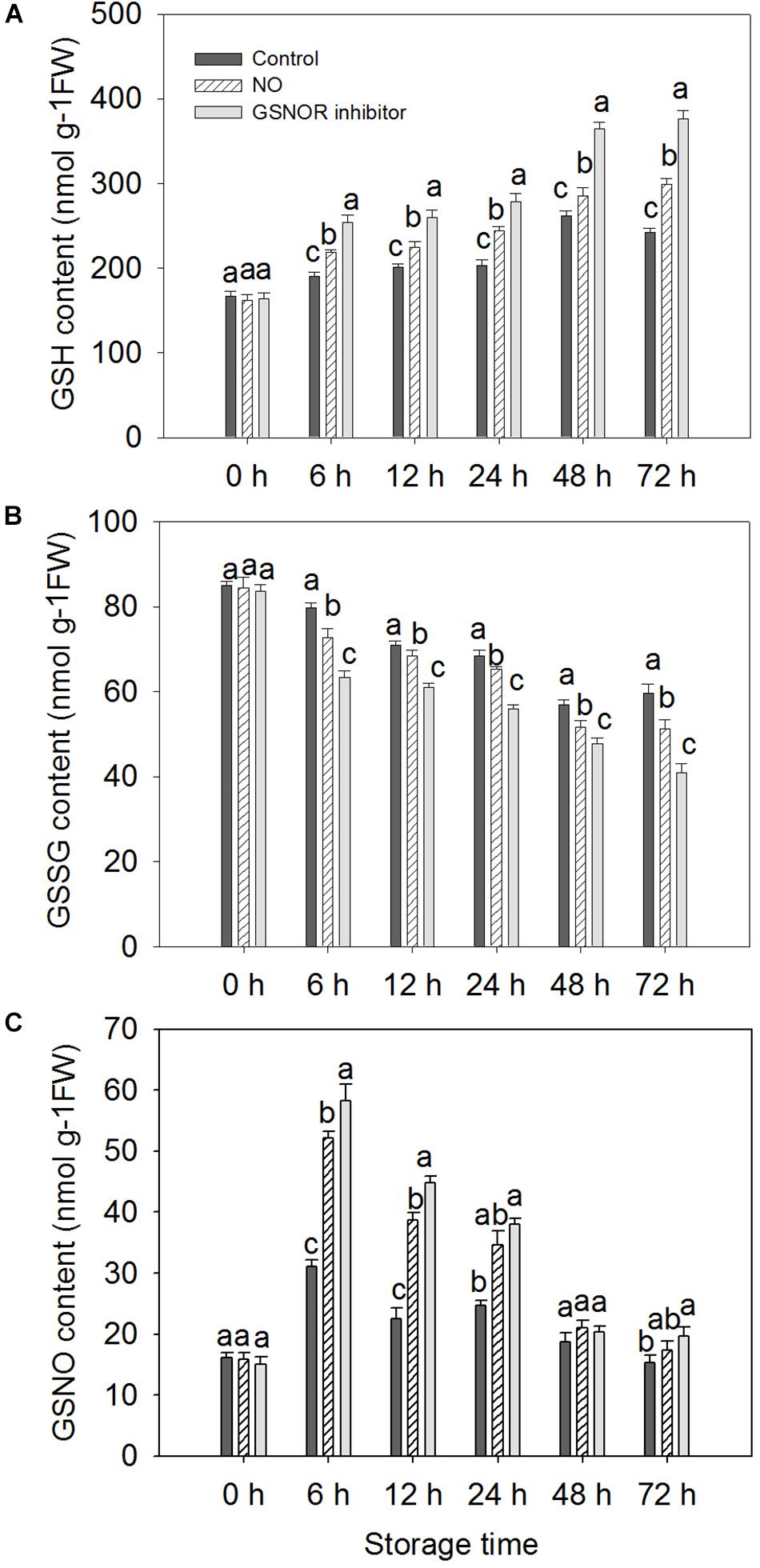
Figure 5. Effect of nitric oxide (NO) or S-nitrosoglutathione reductase (GSNOR) inhibitor treatment on the level of (A) GSH, (B) GSSG, and (C) GSNO of peach fruit during storage at 23°C. Vertical bars represent the standard deviation of the means (n = 6). Different letters represent significant differences (P < 0.05) according to least significant difference (LSD) test.
The expressions of three defense-related genes in peaches pretreated with NO and GSNOR inhibitor were investigated by RT-PCR. NO and GSNOR inhibitor increased the expression levels of PR1, NPR1, and TGA1 genes (Figure 6). The expression trend of PR1 was similar to that of NPR1, gradually increasing during 0–48 h. It decreases when it reaches the maximum at 48 h (Figures 6A,B). The expression level of TGA1 in the control fruit was relatively stable, but NO and GSNOR inhibitor significantly (P < 0.05) induced TGA1 gene expression from 24 to 72 h (Figure 6C).
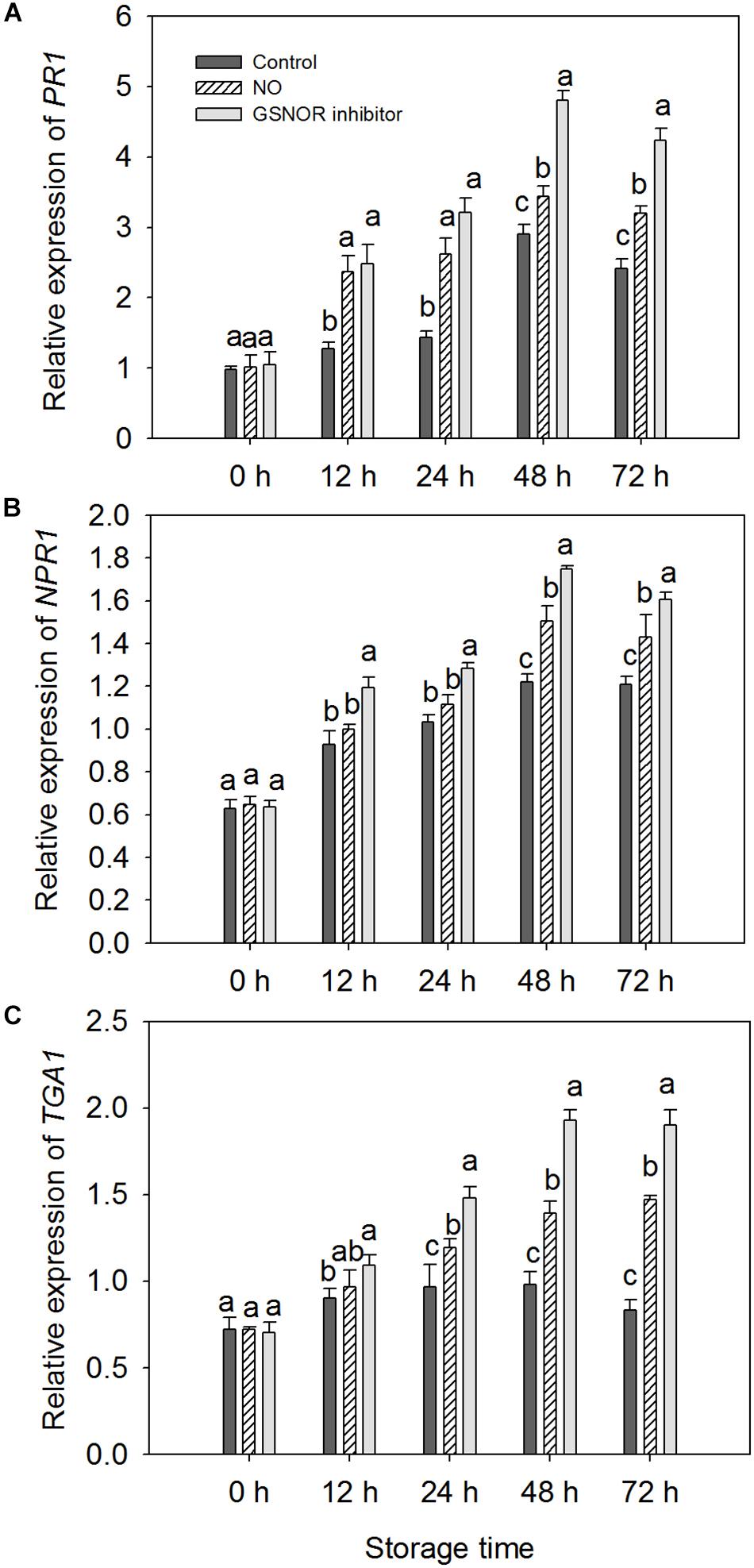
Figure 6. Effects of nitric oxide (NO) or S-nitrosoglutathione reductase (GSNOR) inhibitor treatment on (A) PR1, (B) NPR1, and (C) TGA1 gene expression, determined by real-time qPCR. All experiments were run in triplicate with different complementary DNAs (cDNAs) synthesized from six biological replicates. Vertical bars represent the standard deviation of the means (n = 6). Different letters represent significant differences (P < 0.05) according to least significant difference (LSD) test.
Nitric oxide involves participating in plant responses to biotic and abiotic stresses as an important signaling molecule (Mur et al., 2006; Carreras and Poderoso, 2007). It is reported that NO has a direct and effective inhibitory effect on a variety of microorganisms (Schairer et al., 2012). However, in our previous studies, low concentration of NO solution could effectively restrict disease development but had no significant inhibition against M. fructicola in vitro (Gu et al., 2014). In recent years, more and more studies have shown that NO has obvious inhibitory effects on post-harvest diseases. Treatment of tomato fruit with L-arginine, a precursor of NO, inhibits the expansion of lesion diameter caused by B. cinerea (Zheng et al., 2011). In apple fruit, NO donor sodium nitroprusside (SNP) treatment could inhibit virulence of P. expansum, causing obviously lower disease incidence and smaller lesion diameter compared to the water treatment (Lai et al., 2014). Hu et al. (2019) showed that NO could significantly increase the resistance of pitaya fruit to C. gloeosporioides after harvest, which is consistent with the results observed on the present result in peach fruit.
The pyrrole group located in N6022 is an efficient GSNOR inhibitor with potent inhibitory ability (Sun et al., 2011). The inhibitory effect of N6022 on GSNOR activity has been shown to be safe and effective in animal models of asthma and inflammatory disease. As a tight bonding inhibitor, N6022 is currently under application for early clinical research in humans (Green et al., 2012). However, it has not been used in postharvest fruits. The present results show that the GSNOR inhibitor N6022 reduces disease incidence significantly (P < 0.05) and enhances the resistance of peach fruit to brown rot while reducing the activity of GSNOR of peaches.
S-nitrosoglutathione reductase regulates the levels of GSNO and SNOs in eukaryotic cells by specifically recognizing and degrading GSNO that can be decomposed into GSSG and ammonia (NH3) (Liu et al., 2001). As the most abundant of endogenous intracellular SNOs, GSNO is considered as a potential NO storage site or transport center in the cell (Butler and Rhodes, 1997; Mayer et al., 1998; Liu et al., 2001). It can transfer NO to the target protein to change the function of the protein (Liu et al., 2001). The present results show that NO-treated fruit had markedly (P < 0.05) higher content of GSNO than the control, which contributed to promoting the nitrosylation of the target protein of GSNOR (Figures 3A, 5C). Studies have shown that when plants are infected with pathogenic bacteria, the content of SNOs will increase, suggesting that SNOs play a vital role in signal transduction and host defense (Feechan et al., 2005; Chaki et al., 2009). The present results showed that the content of SNOs increased at 0–12 h, and both NO and GSNOR inhibitor treatments promoted the production of SNOs, which was helpful for the improvement of disease resistance of peach fruit (Figure 4A). It is reported that the concentration of NO in the cells increased, and NO could bound to GSH to form GSNO under stress conditions (Liu et al., 2001). Under the catalysis of GR, the oxidized form of glutathione (GSSG) can be readily converted to the reduced state (GSH). GSH is a major intracellular antioxidant that eliminates reactive oxygen species (ROS) (Macdonald et al., 2003). GR maintains a high GSH/GSSG ratio by reducing GSSG to play a key role in the antioxidant defense process (Foyer and Noctor, 2005; Patel and Patra, 2015). The present result showed that exogenous NO and N6022 treatments promoted the degradation of GSSG by increasing the activity of GR, thereby increasing the content of GSH, thus improving the antioxidant capacity of peach fruit.
The accumulation of endogenous NO after inoculation of Rhizoctonia solani in cucumber plant may be due to a decrease in GSNOR activity (Nawrocka et al., 2019). Our study also found the content of endogenous NO increased when GSNOR activity was inhibited by exogenous NO and GSNOR inhibitor (Figure 4B). Romero-Puertas et al. (2008) has identified some S-nitrosylated proteins in Arabidopsis thaliana during defense response of the plant, which shows that protein nitrosylation plays an indispensable role in the process of defense reaction. Our nitrosylation modification analysis showed that the GSNOR protein in the NO-treated peach fruit was nitrosylated, which indicates that exogenous NO may participate in the disease resistance response by nitrosylation modification of this protein. As GSNOR inhibitor or NO treatment reduced the activity of GSNOR in peach fruit, the degrading activity of GSNOR on GSNO declined, which leads to GSNO and other SNOs accumulation in peach fruit. Studies have shown that nitrosation of cysteine results in reduced GSNOR activity in Arabidopsis, budding yeast, and humans (Guerra et al., 2016). Similar results were shown in this research. Therefore, it can be speculated that NO induces nitrosylation of cysteine-85 in GSNOR, which results in lower GSNOR activity in peach fruit. Lee et al. (2008) found that transcriptional levels or protein abundance levels of GSNOR was not significantly (P < 0.05) regulated under stress conditions in Arabidopsis, suggesting that some mean of redox regulation via cysteine modification may be a mechanism to control its activity. This study indicated that the relative gene expression of GSNOR in the NO-treated peach fruit was not markedly (P > 0.05) different from the control after 6 h, but the GSNOR activity was markedly (P < 0.05) inhibited (Figure 2). Therefore, the nitrosylation of GSNOR may be another main regulation mode besides the transcriptional control that occurred to its enzyme activity changes.
S-nitrosoglutathione reductase is a highly conserved protein rich in cysteine. Through analysis, it is found that the GSNOR homology between peach and Arabidopsis reached 84.17% (Supplementary Figure S1). Xu et al. (2013) analyzed the structure of Arabidopsis GSNOR protein and found that Cys-10, Cys-271, and Cys-370 of AtGSNOR are solvent accessible and can provide conditions for post-translational modification. In addition, it was suggested that these three residues may be conserved in regulating the activity of GSNOR. Moreover, Cys-10, Cys-271, and Cys-370 of AtGSNOR have been identified as S-nitrosylated residues (Guerra et al., 2016; Zhan et al., 2018). However, Cys-85 of GSNOR of peach was found to be an S-nitrosylated residues in our identification results. Sequence analysis of GSNOR revealed that Cys-85 corresponds to Cys-47 in Arabidopsis thaliana, Antrodia camphorata, and tomato (Supplementary Figures S2, S3). At the same time, Cys-85 residue was also found to be conserved in other plant GSNORs. Huang et al. (2009) found that Cys-47 of A. camphorata is closely related to the enzyme activity of GSNOR. This further indicates the possible role of Cys-85 nitrosylation in the control of protein activity.
Pathogenesis-related gene 1 was first identified from Nicotiana tabacum infected with tobacco mosaic virus (TMV) in the 1970s (Van Loon and Van Kammen, 1970). After that, similar PR1 proteins from many species of both mono- and dicotyledonous plants have been reported successively (Ohshima et al., 1987; Tornero et al., 1997; Maleck et al., 2000; Liu and Xue, 2006; Mitsuhara et al., 2008). Studies have confirmed that PR1 is capable to protect plants against abiotic stress (Rauscher et al., 1999; Cutt et al., 1989). Klessig et al. (2000) demonstrated that NO can participate in the induction of PR1 gene expression. Inhibition of Arabidopsis GSNOR1 expression enhances Arabidopsis resistance to Peronospora parasitica and promotes systemic acquired resistance (SAR) and PR1 expression (Rustérucci et al., 2007). Our research has yielded similar results, which indicates that GSNOR may have the parallel role in post-harvest fruits. Gene expression of PR1 is upregulated when GSNOR is inhibited by NO and GSNOR inhibitors. NPR1 and TGA1 are pivotal redox-controlled regulators of SAR in plants. NO could promote the ability of NPR1 to increase DNA binding activity of TGA1 (Lindermayr et al., 2010). When the SA-mediated defense response is activated, changes in intracellular redox status will lead to the revivification of NPR1 to its active monomeric form. The NPR1 monomers interacts with the reduced form of TGA1 to promote the binding of TGA1 to the activation sequence-1 (as-1) element of the promoter region of defense proteins. Studies have confirmed that this interaction promotes the DNA combing activity of TGA1 to the as-1 of the PR-1 gene to motivate its expression (Després et al., 2003; Pieterse and Van Loon, 2004; Lindermayr et al., 2010). Lindermayr et al. (2010) showed that GSNO protects TGA1 from oxygen-mediated modifications and promotes the combing of TGA1 to the as-1 when NPR1 is present. From our results, it can be seen that both NO and GSNOR inhibitor treatment increased the content of GSNO and endogenous NO, and the expressions of NPR1 and TGA1 were obviously higher than the control. Therefore, treatment of peach fruit by NO and GSNOR inhibitors may promote SAR by activating the SA signaling pathway, thereby improving the disease resistance of peach fruit.
S-nitrosoglutathione reductase is critical for GSNO and SNOs homeostasis, as well as protecting against nitrosative stress. GSNOR activity is also a major regulator of intracellular GSNO and SNO levels, playing a key role in regulating plant resistance (Liu et al., 2001; Lindermayr et al., 2006). Salicylic acid (SA), as an immune activator in plants, is controlled by GSNOR1 in its synthesis and signaling transduction, so the regulation of GSNOR activity is closely related to plant disease resistance (Feechan et al., 2005; Malik et al., 2011). In this study, GSNOR inhibitor N6022 was used for soaking postharvest peach fruits that were later inoculated with M. fructicola. It was found that N6022 could significantly (P < 0.05) inhibit the disease spot development of peach fruit. Moreover, the expression of GSNOR was decreased while the expression of PR1 was significantly increased, and the content of endogenous SNOs and glutathione (GSH) in fruits was increased. The results indicate that partial inhibition of GSNOR activity can lead to increase in intracellular SNOs levels, accumulation of GSH, and enhancement of fruit defense capacity.
Plant defense response is a complex network involving the transmission of multiple hormones and signaling molecules. Our research mainly revealed an important defense mechanism for GSNOR to regulate the level of nitrosation and promote the production of SAR by regulating the levels of GSNO and NO (Figure 7). This provides a new orientation for probing the defense mechanism of peach fruit against M. fructicola and lays a foundation for in-depth research on the function of GSNOR in harvested fruit. However, it is unclear whether other signaling pathways have an effect on this mechanism, which requires more extensive research.
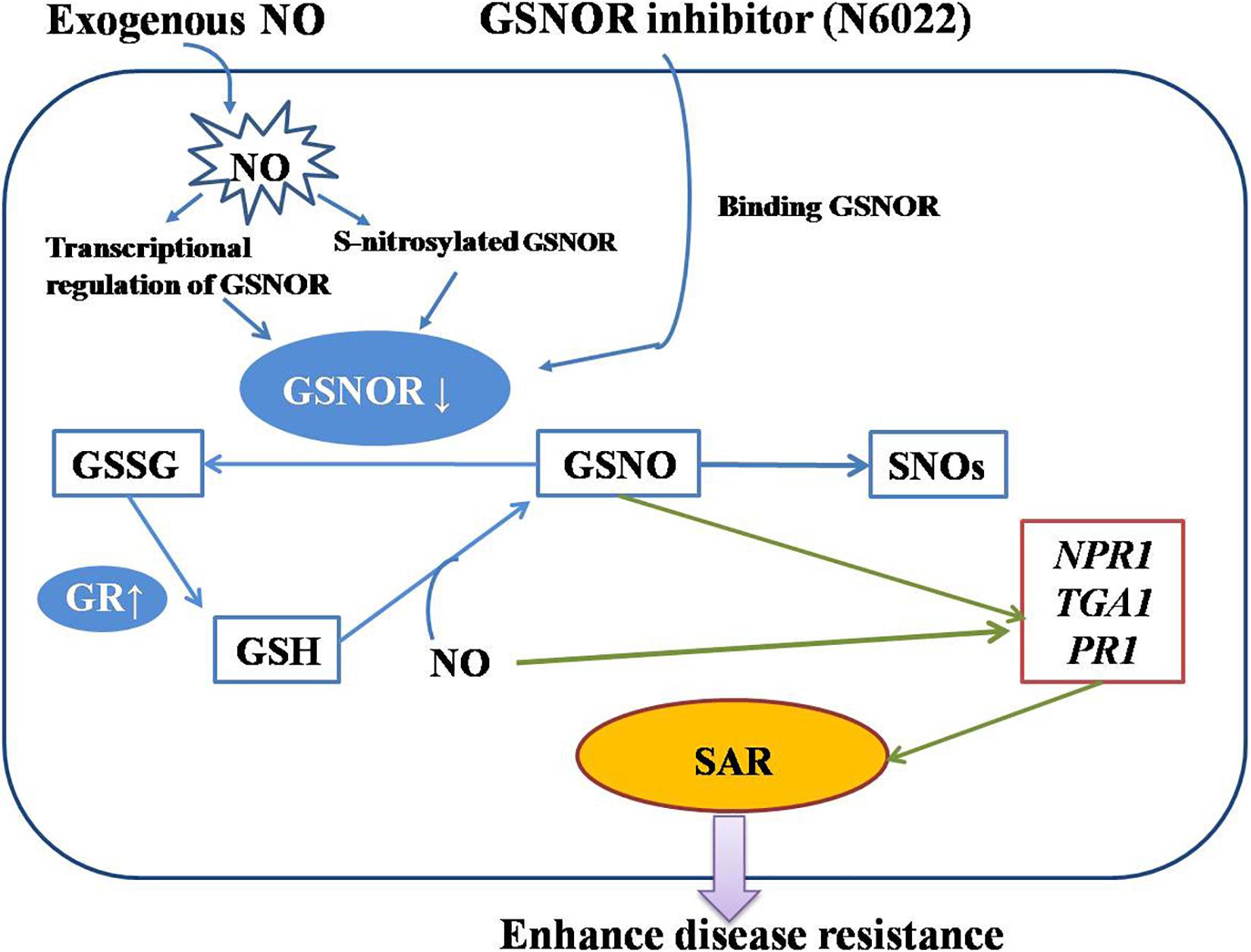
Figure 7. A diagram of the action mechanism of exogenous nitric oxide (NO) and N6022 on improving disease resistance of peach fruits made according to our research.
In conclusion, the results of this study point to the key role of GSNOR in the potential molecular mechanisms of peach fruit resistant to brown rot stress. Studies have found that NO and N6022 have a good inhibitory effect on peach fruit brown rot. They inhibit the GSNOR activity and cause the accumulation of SNO, GSNO, and endogenous NO in peach fruit, and increase the GSH accumulation through enhancing GR activity, leading to the enhancement of antioxidant and defense capabilities of peach fruit. In addition, NO and N6022 activate the SA signaling pathway by promoting the expression of SAR-related genes (such as PR1, NPR1, and TGA1), which are also important components of peach fruit defense mechanism. Interestingly, Cys-85 residue of GSNOR was identified as an S-nitrosylated residue in the NO-treated peach fruit. This has not been reported in heretofore studies. It is speculated that this may be related to GSNOR enzyme activity, but further research is needed to support this conclusion. Collectively, presented data uncover that inhibition of GSNOR activity has a positive role in peach fruit response to M. fructicola infection.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
JS designed the experiments. ZY and JC conducted the experiments and analyzed the data. ZY conceived the manuscript. JS, YP, LZ, and SZ provided the valuable advice and revised the manuscript.
The work was supported by the Projects of National Natural Science Foundation of China (31570688) and Funds of Shandong “Double Tops” Program (SYT2017XTTD04).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2020.00543/full#supplementary-material
Adaskaveg, J. E., Forster, H., Gubler, W. D., Teviotdale, B. L., and Thompson, D. F. (2005). Reduced-risk fungicides help manage brown rot and other fungal diseases of stone fruit. Calif. Agric. 59, 109–114. doi: 10.3733/ca.v059n02p109
Arasimowicz, M., and Floryszak-Wieczorek, J. (2007). Nitric oxide as a bioactive signaling molecule in plant stress responses. Plant Sci. 172, 876–887. doi: 10.1016/j.plantsci.2007.02.005
Besson-Bard, A., Pugin, A., and Wendehenne, D. (2008). New insights into nitric oxide signaling in plants. Annu. Rev. Plant Biol. 59, 21–39. doi: 10.1146/annurev.arplant.59.032607.092830
Butler, A. R., and Rhodes, P. (1997). Chemistry, analysis, and biological roles of S-Nitrosothiols. Anal. Biochem. 249, 1–9. doi: 10.1006/abio.1997.2129
Carreras, M. C., and Poderoso, J. J. (2007). Mitochondrial nitric oxide in the signaling of cell integrated responses. Am. J. Physiol. Cell. Physiol. 292, C1569–C1580. doi: 10.1152/ajpcell.00248.2006
Chaki, M., Fernandez-Ocana, A. M., Valderrama, R., Carreras, A., Esteban, F. J., Luque, F., et al. (2009). Involvement of reactive nitrogen and oxygen species (RNS and ROS) in sunflower-mildew interaction. Plant Cell. Physiol. 50, 665–679. doi: 10.1093/pcp/pcp039
Chen, C., Huang, B., Han, P. W., and Duan, S. J. (2006). S-nitrosation: the prototypic redox-based post-translational modification of proteins. Prog. Biochem. Biophys. 33, 609–615.
Corpas, F. J., Carreras, A., Valderrama, R., Chaki, M., Palma, J. M., del Río, L. A., et al. (2007). Reactive nitrogen species and nitrosative stress in plants. Plant Stress 1, 37–41.
Cutt, J. R., Harpster, M. H., Dixon, D. C., Carr, J. P., Dunsmuir, P., and Klessig, D. F. (1989). Disease response to tobacco mosaic virus in transgenic tobacco plants that constitutively express the pathogenesis-related PR1b gene. Virology 173, 89–97. doi: 10.1016/0042-6822(89)90224-9
Després, C., Chubak, C., Rochon, A., Clark, R., Bethune, T., Desveaux, D., et al. (2003). The Arabidopsis NPR1 disease resistance protein is a novel cofactor that confers redox regulation of DNA binding activity to the basic domain/leucine zipper transcription factor TGA1. Plant Cell 15, 2181–2191. doi: 10.1105/tpc.012849
Dìaz, M., Achkor, H., Titarenko, E., and Martìnez, M. C. (2003). The gene encoding glutathione-dependent formaldehyde dehydrogenase/GSNO reductase is responsive to wounding, jasmonic acid and salicylic acid. FEBS Lett. 543, 136–139. doi: 10.1016/s0014-5793(03)00426-5
Domingos, P., Prado, A., Wong, A., Gehring, C., and Feijo, J. (2015). Nitric oxide: a multitasked signaling gas in plant. Mol. Plant 8, 506–520. doi: 10.1016/j.molp.2014.12.010
Feechan, A., Kwon, E. J., Yun, B. W., Wang, Y., Pallas, J. A., and Loake, G. J. (2005). A central role for S-nitrosothiols in plant disease resistance. Proc. Natl. Acad. Sci. U.S.A. 102, 8054–8059. doi: 10.1073/pnas.0501456102
Foyer, C. H., and Noctor, G. (2005). Oxidant and antioxidant signaling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ. 28, 1056–1071. doi: 10.1111/j.1365-3040.2005.01327.x
Frungillo, L., De Oliveira, J. F. P., Saviani, E. E., Oliveira, H. C., Martínez, M. C., and Salgado, I. (2013). Modulation of mitochondrial activity by S-nitrosoglutathione reductase in Arabidopsis thaliana transgenic cell lines. Biochim. Biophys. Acta 1827, 239–247. doi: 10.1016/j.bbabio.2012.11.011
Gong, B., and Shi, Q. H. (2019). Identifying S-nitrosylated proteins and unraveling S-nitrosoglutathione reductase-modulated sodic alkaline stress tolerance in Solanum lycopersicum L. Plant Physiol. Biochem. 142, 84–93. doi: 10.1016/j.plaphy.2019.06.020
Green, L. S., Chun, L. E., Patton, A. K., Sun, X. C., Rosenthal, G. J., and Richards, J. P. (2012). Mechanism of inhibition for N6022, a first-in-class drug targeting S-Nitrosoglutathione reductase. Biochemistry 51, 2157–2168. doi: 10.1021/bi201785u
Gu, R. X., Zhu, S. H., Zhou, J., Liu, N., and Shi, J. Y. (2014). Inhibition on brown rot disease and induction of defence response in harvested peach fruit by nitric oxide solution. Eur. J. Plant Pathol. 139, 369–378. doi: 10.1007/s10658-014-0393-x
Guerra, D., Ballard, K., Truebridge, I., and Vierling, E. (2016). S-Nitrosation of conserved cysteines modulates activity and stability of S-Nitrosoglutathione reductase (GSNOR). Biochemistry 55, 2452–2464. doi: 10.1021/acs.biochem.5b01373
Hess, D. T., and Stamler, J. S. (2012). Regulation by S-nitrosylation of protein post-translational modification. J. Biol. Chem. 287, 4411–4418. doi: 10.1074/jbc.r111.285742
Hodges, D. M., and Forney, C. F. (2000). The effects of ethylene, depressed oxygen and elevated carbon dioxide on antioxidant profiles of senescing spinach leaves. J. Exp. Bot. 51, 645–655. doi: 10.1093/jexbot/51.344.645
Hu, M. J., Cox, K. D., Schnabel, G., Luo, C. X., and Redfield, R. J. (2011). Monilinia species causing brown rot of peach in china. PLoS One 6:e24990. doi: 10.1371/journal.pone.0024990
Hu, M. J., Zhu, Y. Y., Liu, G. S., Gao, Z. Y., Li, M., Su, Z. H., et al. (2019). Inhibition on anthracnose and induction of defense response by nitric oxide in pitaya fruit. Sci. Hortic. 245, 224–230. doi: 10.1016/j.scienta.2018.10.030
Huang, C. Y., Ken, C. F., Wen, L., and Lin, C. T. (2009). An enzyme possessing both glutathione-dependent formaldehyde dehydrogenase and s-nitrosoglutathione reductase from antrodia camphorata. Food Chem. 112, 795–802. doi: 10.1016/j.foodchem.2008.06.031
Klessig, D. F., Durner, J., Noad, R., Navarre, D. A., Wendehenne, D., Kumar, D., et al. (2000). Nitric oxide and salicylic acid signaling in plant defense. Proc. Natl. Acad. Sci. 97, 8849–8855. doi: 10.1073/pnas.97.16.8849
Knorzer, O. C., Durner, J., and Boger, P. (1996). Alterations in the antioxidative system of suspension-cultured soybean cells (Glycine max) induced by oxidative stress. Physiol. Plant. 97, 388–396. doi: 10.1034/j.1399-3054.1996.970225.x
Kulik, A., Noirot, E., Grandperret, V., Bourque, S., Fromentin, J., Salloignon, P., et al. (2015). Interplays between nitric oxide and reactive oxygen species in cryptogein signaling. Plant Cell Environ. 38, 331–343. doi: 10.1111/pce.12295
Kwon, E., Feechan, A., Yun, B. W., Hwang, B. H., Pallas, J. A., Kang, J. G., et al. (2012). AtGSNOR1 function is required for multiple developmental programs in Arabidopsis. Planta 236, 887–900. doi: 10.1007/s00425-012-1697-8
Lai, T. F., Chen, Y., Li, B. Q., Qin, G. Z., and Tian, S. P. (2014). Mechanism of Penicillium expansum in response to exogenous nitric oxide based on proteomics analysis. J. Proteomics 103, 47–56. doi: 10.1016/j.jprot.2014.03.012
Lee, U., Wie, C., Fernandez, B. O., Feelisch, M., and Vierling, E. (2008). Modulation of nitrosative stress by S-nitrosoglutathione reductase is critical for thermotolerance and plant growth in Arabidopsis. Plant Cell 20, 786–802. doi: 10.1105/tpc.107.052647
Lindermayr, C., Saalbach, G., Bahnweg, G., and Durner, J. (2006). Differential inhibition of Arabidopsis methionine adenosyl transferases by protein S-nitrosylation. J. Biol. Chem. 281, 4285–4291. doi: 10.1074/jbc.M511635200
Lindermayr, C., Sell, S., Müller, B., Leister, D., and Durner, J. (2010). Redox regulation of the NPR1-TGA1 system of Arabidopsis thaliana by nitric oxide. Plant Cell 22, 2894–2907. doi: 10.1105/tpc.109.066464
Liu, L. M., Hausladen, A., Zeng, M., Que, L., Heitman, J., and Stamler, J. S. (2001). A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans. Nature 410, 490–494. doi: 10.1038/35068596
Liu, Q. P., and Xue, Q. Z. (2006). Computational identification of novel PR-1-type genes in Oryza sativa. J. Genet. 85, 193–198. doi: 10.1007/bf02935330
Macdonald, J., Galley, H. F., and Webster, N. R. (2003). Oxidative stress and gene expression in sepsis. Br. J. Anaesth. 90, 221–232. doi: 10.1093/bja/aeg034
Maleck, K., Levine, A., Eulgem, T., Morgan, A., and Dietrich, R. A. (2000). The transcriptome of Arabidopsis thaliana during systemic acquired resistance. Nature 26, 403–410. doi: 10.1038/82521
Malik, S. I., Hussain, A., Yun, B. W., Spoel, S. H., and Loake, G. J. (2011). GSNOR-mediated de-nitrosylation in the plant defence response. Plant Sci. 181, 540–544. doi: 10.1016/j.plantsci.2011.04.004
Mayer, B., Pfeiffer, S., Schrammel, A., Koesling, D., Schmidt, K., and Brunner, F. (1998). A new pathway of nitric oxide/cyclic GMP signaling involving S-Nitrosoglutathione. J. Biol. Chem. 273, 3264–3270. doi: 10.1074/jbc.273.6.3264
Mitsuhara, I., Iwai, T., Seo, S., Yanagawa, Y., Kawahigasi, H., Hirose, S., et al. (2008). Characteristic expression of twelve rice PR1 family genes in response to pathogen infection, wounding, and defense-related signal compounds (121/180). Mol. Genet. Genomics 279, 415–427. doi: 10.1007/s00438-008-0322-9
Mur, L. A. J., Carver, T. L. W., and Prats, E. (2006). NO way to live; the various roles of nitric oxide in plant-pathogen interactions. J. Exp. Bot. 57, 489–505. doi: 10.1093/jxb/erj052
Nawrocka, J., Gromek, A., and Małolepsza, U. (2019). Nitric oxide as a beneficial signaling molecule in Trichoderma atroviride TRS25-induced systemic defense responses of cucumber plants against Rhizoctonia solani. Front. Plant Sci. 10:421. doi: 10.3389/fpls.2019.00421
Ohshima, M., Matsuoka, M., Yamamoto, N., Tanaka, Y., Kano-Murakami, Y., Ozeki, Y., et al. (1987). Nucleotide sequence of the PR-1 gene of Nicotiana tabacum. FEBS Lett. 225, 243–246. doi: 10.1016/0014-5793(87)81166-3
Patel, A., and Patra, D. D. (2015). Effect of tannery sludge amended soil on glutathione activity of four aromatic crops: Tagetes minuta, Pelargonium graveolens, Ocimum basilicum and Mentha spicata. Ecol. Eng. 81, 348–352. doi: 10.1016/j.ecoleng.2015.04.070
Pieterse, C. M., and Van Loon, L. C. (2004). NPR1: the spider in the web of induced resistance signaling pathways. Curr. Opin. Plant Biol. 7, 456–464. doi: 10.1016/j.pbi.2004.05.006
Rauscher, M., Adám, A. L., Wirtz, S., Guggenheim, R., Mendgen, K., and Deising, H. B. (1999). PR-1 protein inhibits the differentiation of rust infection hyphae in leaves of acquired resistant broad bean. Plant J. Cell Mol. Biol. 19, 625–633. doi: 10.1046/j.1365-313x.1999.00545.x
Romanazzi, G., Sanzani, S. M., Bi, Y., Tian, S. P., Gutiérrez Martínez, P., and Alkan, N. (2016). Induced resistance to control postharvest decay of fruit and vegetables. Postharvest Biol. Technol. 122, 82–94.
Romero-Puertas, M. C., Campostrini, N., Mattè, A., Righetti, P. G., and Dr, M. D. P. (2008). Proteomic analysis of S-nitrosylated proteins in Arabidopsis thaliana undergoing hypersensitive response. Proteomics 8, 1459–1469. doi: 10.1002/pmic.200700536
Romero-Puertas, M. C., Perazzolli, M., Zago, E. D., and Delledonne, M. (2004). Nitric oxide signalling functions in plant–pathogen interactions. Cell. Microbiol. 6, 795–803. doi: 10.1111/j.1462-5822.2004.00428.x
Rustérucci, C., Espunya, M. C., Díaz, M., Chabannes, M., and Martínez, M. C. (2007). S-nitrosoglutathione reductase affords protection against pathogens in Arabidopsis, both locally and systemically. Plant Physiol. 143, 1282–1292. doi: 10.1104/pp.106.091686
Sakamoto, A., Ueda, M., and Morikawa, H. (2002). Arabidopsis glutathione dependent formaldehyde dehydrogenase is an S-nitrosoglutathione reductase. FEBS Lett. 515, 20–24. doi: 10.1016/s0014-5793(02)02414-6
Schairer, D. O., Chouake, J. S., Nosanchuk, J. D., and Friedman, A. J. (2012). The potential of nitric oxide releasing therapies as antimicrobial agents. Virulence 3, 271–279. doi: 10.4161/viru.20328
Shi, J. Y., Liu, N., Gu, R. X., Zhu, L. Q., Zhang, C., Wang, Q. G., et al. (2015). Signals induced by exogenous nitric oxide and their role in controlling brown rot disease caused by Monilinia fructicola in postharvest peach fruit. J. Gen. Plant Pathol. 81, 68–76. doi: 10.1007/s10327-014-0562-y
Skelly, M. J., and Loake, G. (2013). Synthesis of redox-active molecules and their signalling functions during the expression of plant disease resistance. Antioxid. Redox Signal. 19, 990–997. doi: 10.1089/ars.2013.5429
Sommer, N. F. (1985). Role of controlled environments in suppression of postharvest diseases. Can. J. Plant Pathol. 7, 331–339.
Stamler, J. S. (1994). Redox signaling: nitrosylation and related target interactions of nitric oxide. Cell 78, 931–936. doi: 10.1016/0092-8674(94)90269-0
Stamler, J. S., Lamas, S., and Fang, F. C. (2001). Nitrosylation: the prototypic redox-based signaling mechanism. Cell 106, 675–683. doi: 10.1016/s0092-8674(01)00495-0
Sun, X. C., Wasley, J. W., Qiu, J., Blonder, J. P., Stout, A. M., Green, L. S., et al. (2011). Discovery of S-nitrosoglutathione reductase inhibitors: potential agents for the treatment of asthma and other inflammatory diseases. ACS Med. Chem. Lett. 2, 402–406. doi: 10.1021/ml200045s
Tong, Z. G., Gao, Z. H., Wang, F., Zhou, J., and Zhang, Z. (2009). Selection of reliable reference genes for gene expression studies in peach using real-time PCR. BMC Mol. Biol. 10:71. doi: 10.1186/1471-2199-10-71
Tornero, P., Gadea, J., Conejero, V., and Vera, P. (1997). Two PR-1 genes from tomato are differentially regulated and reveal a novel mode of expression for a pathogenesis-related gene during the hypersensitive response and development. Mol. Plant Microbe Interact. 10, 624–634. doi: 10.1094/MPMI.1997.10.5.624
Van Loon, L. C., and Van Kammen, A. (1970). Polyacrylamide disc electro-phoresis of the soluble leaf proteins from Nicotiana tabacum var. ‘Samsun’ and ‘Samsun NN’. II. Changes in protein constitution after infection with Tobacco mosaic virus. Virology 40, 199–211. doi: 10.1016/0042-6822(70)90395-8
Xie, L. X., Wang, G. R., Yu, Z. X., Zhou, M. L., Li, Q. M., Huang, H. R., et al. (2016). Proteome-wide lysine glutarylation profiling of the mycobacterium tuberculosis h37rv. J. Proteome Res. 15, 1379–1385. doi: 10.1021/acs.jproteome.5b00917
Xu, S., Guerra, D., Lee, U., and Vierling, E. (2013). S-nitrosoglutathione reductases are low-copy number, cysteine-rich proteins in plants that control multiple developmental and defense responses in Arabidopsis. Front. Plant Sci. 4:430. doi: 10.3389/fpls.2013.00430
Yang, F., Feng, L., Liu, Q., Wu, X., and Yang, W. (2018). Effect of interactions between light intensity and red-to- far-red ratio on the photosynthesis of soybean leaves under shade condition. Environ. Exp. Bot. 150, 79–87. doi: 10.1016/j.envexpbot.2018.03.008
Zhan, N., Wang, C., Chen, L. C., Yang, H. J., Feng, J., Gong, X. Q., et al. (2018). S -nitrosylation targets gsno reductase for selective autophagy during hypoxia responses in plants. Mol. Cell 71, 142–154. doi: 10.1016/j.molcel.2018.05.024
Keywords: Prunus persica, nitric oxide, S-nitrosoglutathione reductase, brown rot, nitrosylation
Citation: Yu Z, Cao J, Zhu S, Zhang L, Peng Y and Shi J (2020) Exogenous Nitric Oxide Enhances Disease Resistance by Nitrosylation and Inhibition of S-Nitrosoglutathione Reductase in Peach Fruit. Front. Plant Sci. 11:543. doi: 10.3389/fpls.2020.00543
Received: 31 January 2020; Accepted: 09 April 2020;
Published: 20 May 2020.
Edited by:
Cai-Zhong Jiang, Crops Pathology and Genetics Research Unit, USDA-ARS, United StatesReviewed by:
David Obenland, San Joaquin Valley Agricultural Sciences Center, United StatesCopyright © 2020 Yu, Cao, Zhu, Zhang, Peng and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Peng, cGVuZ3lvbmd4eXpAc2luYS5jb20=; Jingying Shi, anlzaGk4MEAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.